Abstract
Objective:
Short sleep duration has been associated with cardiovascular morbidity and mortality. However, previous studies were limited by using subjective sleep measures and treating sleep duration as a sole, independent predictor. Therefore, the role of sleep duration in predicting mortality is still not well understood. We posit that objective sleep duration is an effect modifier of the relationship between hypertension and all-cause mortality.
Methods:
We addressed this question in the Penn State Adult Cohort, a random, general population sample of 1741 men and women (48.7±13.5 years) who were studied in the sleep laboratory and followed up for 15.5±4.1 years. Hypertension was defined on the basis of SBP and DBP (≥140/≥90 mmHg) or use of antihypertensive medication. Polysomnographic sleep duration was classified into three clinically meaningful categories.
Results:
We tested the interaction between hypertension and polysomnographic sleep duration on all-cause mortality using multiple logistic regression while controlling for several potential confounders (P value = 0.03). The odds (95% confidence interval) of all-cause mortality associated with hypertension were 1.77 (1.07–2.92), 2.78 (1.47–5.24), and 3.93 (2.22–6.95) for individuals who slept at least 6, 5–6, and 5 h or less, respectively.
Conclusion:
The risk of mortality associated with hypertension increases in a dose–response manner as a function of shorter sleep duration. Short sleep in hypertensive individuals may be a marker of the degree of central autonomic dysfunction. Future epidemiological studies should examine this effect modification using cause-specific mortality, whereas future clinical trials should examine whether lengthening sleep improves the prognosis of individuals with hypertension.
Keywords: hypertension, mortality, sleep
INTRODUCTION
Approximately 30% of the United States population has hypertension, which is associated with significant cardiovascular morbidity, great economic healthcare cost, and increased mortality [1]. A large amount of literature has linked sleep disorders, particularly sleep-disordered breathing (SDB), to hypertension [2-4] and mortality [5-7]. Furthermore, sleep duration has been recently shown to be associated with hypertension [8,9], other cardiovascular risk factors [10-14], and mortality [15-17]; however, these previous studies have reported modest and inconsistent effects [10,17,18]. This has led to the understanding that measures of impaired sleep, such as sleep duration, may not yet be useful in predicting adverse health outcomes, such as probability of death, in the context of cardiovascular risk.
An important limitation of previous studies is that they relied typically on subjective measures of sleep duration and, thus, the role of polysomnographic sleep duration was not examined, and the potential confounder of SDB not controlled for. Most importantly, these previous studies focused on the sole, independent association of sleep duration with morbidity and mortality [18], and none conceptualized objective sleep duration as an effect modifier of the association of cardiovascular risk with mortality. Support for a potential role of objective sleep duration as an effect modifier of cardiovascular risk comes from previous studies showing that objective short sleep duration modifies the association of insomnia, the most prevalent sleep disorder, with cardiometabolic morbidity [19-25] and its underlying biological mechanisms [26-41]. Thus, to test the novel hypothesis that objective sleep duration may likely modify the increased risk of mortality associated with hypertension, we examined the effect modification of objective sleep duration on the risk of all-cause mortality associated with hypertension in a large, random, general population sample. We hypothesized that the association between hypertension and all-cause mortality was stronger in individuals with shorter objective sleep duration.
METHODS
Participants
The data presented here were collected as part of a population-based study of sleep disorders, which used a two-phase protocol to recruit participants from various age groups [19-21,24,25,42-44]. In the first phase of the study, telephone interviews were conducted with 4364 age-eligible men and 12 219 age-eligible women residing in the sample households, for a total sample of 16 583 with response rates of 73.5 and 74.1%, respectively. In the second phase of this study, a subsample of 741 men and 1000 women, selected randomly from the first phase, were studied in our sleep laboratory, with response rates of 67.8 and 65.8%, respectively. These baseline data were collected between January 1990 and March 1999 [42,43]. After giving a complete description of the study to the participants, written informed consent was obtained. The study protocol was approved by the Institutional Review Board at the Pennsylvania State University College of Medicine.
Mortality follow-up
Death certificates for deceased individuals as of 31 December 2011 in this cohort were retrieved from the U.S. Center for Disease Control and Prevention (CDC). Participants were linked by CDC to death records from the National Death Index for the years 1992–2011, and vital status was determined through a rigorous process of probabilistic matching and death certificate review based on participant social security number, full name, date and state of birth, sex, race/ethnicity, state of residence, and marital status [45,46]. Of the 1741 participants, a total of 1361 participants were alive at follow-up, whereas 380 were deceased. Duration of follow-up was calculated from the time of the baseline evaluation to the date of death for those deceased or to 31 December 2011 for those alive. The average follow-up duration was 15.5±4.1 years. Our primary outcome was all-cause mortality.
Hypertension
Blood pressure (BP) was measured in the evening, about 2 h before the start of the sleep recording, using a pneumoelectric microprocessor-controlled instrument with the appropriately sized cuffs (Welch Allyn; Skaneateles Falls, New York, USA) used for routine clinical care at our hospital at the time of the baseline examination. The accuracy of this monitor is reported to be ±3mmHg; in addition, internal calibration was performed before each use, and the machine was checked against a mercury sphygmomanometer at least annually; if required, devices were sent to clinical engineering for repair. The recorded BP was the average of three consecutive readings during a 5-min period following 10 min of rest in the supine position. BP levels at the time of the sleep laboratory evaluation were categorized as normal (i.e. SBP < 120mmHg and DBP < 80mmHg), prehypertensive (i.e. SBP 120–139mmHg or DBP 80–89mmHg), and hypertensive (SBP ≥ 140mmHg or DBP ≥ 90mmHg). Our primary exposure was hypertension defined by the presence of hypertensive BP levels or use of antihypertensive medication, as compared with individuals with SBP less than 140mmHg and DBP less than 90mmHg and who did not use antihypertensive medication [19,20,44].
Sleep laboratory evaluation
All participants were evaluated for one night in the sleep laboratory in sound-attenuated, light-controlled, and temperature-controlled rooms. During this evaluation, each participant was continuously monitored for 8 h (fixed time period) using 16-channel polysomnography (PSG) including electroencephalogram, electrooculogram, and electromyogram. Bedtimes were adjusted to conform to participants’ usual bedtimes, and participants were recorded between 2200–2300 h and 0600 -0700 h. The sleep recordings were subsequently scored independently, according to Rechtschaffen and Kales [47] criteria. Respiration was monitored throughout the night by use of thermocouples at the nose and mouth and thoracic strain gauges. All night recordings of hemoglobin oxygen saturation were obtained with an oximeter attached to the finger. In this study, the presence of SDB was defined as an obstructive apnea/hypopnea index (AHI) at least 5 [19,20]. According to distribution of polysomnographic sleep duration, we categorized the entire study sample into three ordinal groups: at least 50th percentile (i.e. ≥6 h), 25–50th percentile (i.e. 5–6 h), and 25th percentile or less (i.e. ≤5 h). This cutoff point of 6 h of sleep has been shown in previous studies to be predictive of significant medical morbidity and mortality [14,17,19-21,24,25].
Other measurements
BMI was based on measured height (cm) and weight (kg) during the participants’ sleep laboratory visit. As part of the standardized questionnaire, we also assessed the presence of all sleep disorders. The presence of sleep difficulty was established on three levels of severity: insomnia was defined by a complaint of insomnia with a duration of at least 1 year, poor sleep was defined as a moderate-to-severe complaint (based on a mild-to-severe scale) of difficulty falling asleep, difficulty staying asleep, early final awakening, or unrefreshing sleep, whereas normal sleeping was defined as the absence of either of these two categories [19-25,44]. Additional information obtained from the standardized questionnaire included assessing other physical health conditions, depression, and substance use. The presence of diabetes at baseline was defined as a self-report of receiving treatment for diabetes or having a fasting blood sugar at least 126mg/dl from blood drawn the morning after the participant’s PSG [21]. From this blood drawn, we also ascertained total cholesterol (TC) levels. Baseline information regarding the participant’s history of heart disease, stroke, and depression, including a history of suicidal thoughts or attempts, were also obtained [44]. Participants’ daily consumption of caffeine (number of cups/day), tobacco (number of cigarettes/day), and alcohol (number of drinks/day) was also assessed at baseline.
Statistical analyses
The design of this study included oversampling of those at higher risk for SDB and women with markedly higher BMI to increase the precision of the risk estimates. Because of this sampling strategy, numeric sampling weights were developed for the analysis so that the estimates could be inferred to the original target population [42,43,48-50]. We adjusted for the sampling weight in all of our statistical analyses, including those estimating the rate of all-cause mortality.
Multivariable adjusted logistic regression models were used to assess the association between hypertension and all-cause mortality, and the potential effect modification of objective sleep duration in the association. Sex, age, race, BMI, smoking, sleep apnea, sleep difficulty, heart disease, stroke, TC level, and depression were treated as major covariates and adjusted in the models. Sleep duration was adjusted when investigating the overall effect of hypertension on all-cause mortality in the entire sample. To assess the potential effect modification of objective sleep duration in the association between hypertension and all-cause mortality, the significance of the interaction term between hypertension and objective sleep duration was examined. The association between hypertension and mortality was then evaluated in each objective sleep duration stratum (i.e. ≥6, 5–6, and ≤5 h), without assuming common variance in covariables. The results are presented as the multivariable-adjusted odds ratios (ORs) with their 95% confidence intervals (CIs). To further plot and interpret the risk of all-cause mortality associated with hypertension, we compared the projected mortality rates between hypertensive and normotensive participants across objective sleep duration groups, under the average demographic and clinical characteristics of the sample (i.e. aged 48.8 years; 47.8% men; 90.1% white; 27.6 kg/m2 BMI; 211.5 mg/dl cholesterol; 23% smoker; 14% diabetic; 10% with a history of heart disease; 1.7% with a history of stroke; 10.7% SDB; 7.5% insomnia; and 17.3% depression). More specifically, the log odds of mortality rates were estimated by applying these values, along with objective sleep duration stratum and hypertension status, to the fitted logistic regression model. The inverse logit function was then applied to transform log odds to mortality probabilities. Finally, to examine whether the effect modification by objective short sleep duration on all-cause mortality applied to hypertensive groups regardless of their treatment status, we split the hypertensive participants into clinically meaningful subgroups based on both their treatment status and BP management. Controlled hypertension was defined as receiving treatment for high BP and BP levels less than 140/90mmHg, untreated hypertension as not receiving treatment for high BP and BP levels at least 140/90 mmHg, and uncontrolled hypertension as receiving treatment for high BP and BP levels at least 140/90mmHg. As sample size across these four groups did not permit using the three-level objective sleep duration variable to examine effect modification, we used the cutoff of 6 h (i.e. ≥6 vs. <6 h) to stratify the data; this cutoff has previously shown to be associated with significant cardiometabolic morbidity and mortality [14,17,19-21,24,25]. A P value of 0.05 or less was used to determine the significance for all analyses. All analyses were conducted with SAS version 9.3 (SAS Institute Inc., Cary, North Carolina, USA).
RESULTS
The average demographic characteristics of the study population are presented in Table 1. The mean (SD) age was 48.8 (13.6) years, 47.8% were men and 90% whites, with an overall mortality rate of 19.6%. Overall, participants with hypertension showed a three times higher crude mortality rate than normotensive participants (35.5 vs. 11%, P < 0.01). The mean (SD) objective sleep duration for the entire sample was 5.9 (1.2) h. On average, participants with hypertension slept 0.6 h less than those without hypertension. As anticipated, the hypertensive group showed significantly higher BMI, AHI, and other cardiometabolic risk factors (all P ≤ 0.01).
TABLE 1.
Demographic and clinical characteristics of the study population
| Hypertension | ||||
|---|---|---|---|---|
| Overall | No | Yes | P value | |
| N | 1741 | 783 | 958 | |
| Age (years) | 48.8 (13.6) | 44.7 (14.1) | 56.4 (10.4) | <0.01 |
| Male (%) | 47.8 | 45.4 | 52.1 | <0.01 |
| White (%) | 90.1 | 91.6 | 87.4 | <0.01 |
| BMI (kg/m2) | 27.6 (5.7) | 26.5 (6.0) | 29.5 (5.0) | <0.01 |
| Diabetes (%) | 14.0 | 7.8 | 25.4 | <0.01 |
| Cholesterol (mg/dl) | 211.5 (73.7) | 204.7 (52.1) | 224.2 (86.6) | <0.01 |
| Heart disease (%) | 10.0 | 6.0 | 17.2 | <0.01 |
| Stroke (%) | 1.7 | 1.2 | 2.6 | 0.03 |
| AHI (events/h) | 2.4 (7.6) | 1.6 (7.4) | 3.8 (7.7) | <0.01 |
| Insomnia (%) | 7.5 | 5.2 | 11.8 | <0.01 |
| Depression (%) | 17.3 | 15.6 | 20.4 | 0.01 |
| Smoker (%) | 23.0 | 25.2 | 18.9 | <0.01 |
| Objective sleep duration (h) | 5.9 (1.2) | 6.1 (1.3) | 5.5 (1.0) | <0.01 |
All data are adjusted for sampling weight. AHI, apnea/hypopnea index.
The interaction between hypertension and objective sleep duration was statistically significant (P = 0.03); therefore, the association between hypertension and mortality was evaluated for each objective sleep duration stratum. Overall, hypertensive participants showed a 2.54 (95% CI = 1.81–3.57, P < 0.01) times higher odds of dying compared with normotensive participants after adjusting for major covariables. When we examined the OR for different objective sleep duration groups, we observed that the strength of the association between hypertension and mortality increased in a dose–response manner. Specifically, the odds of all-cause mortality associated with hypertension was 1.77 (95% CI = 1.07–2.92, P = 0.03), 2.78 (95% CI = 1.47–5.24, P < 0.01), and 3.93 (95% CI = 2.22–6.95, P < 0.01) for participants who slept at least 6, 5–6, and 5 h or less, respectively. These data indicated that objective sleep duration is an effect modifier of the association between hypertension and all-cause mortality already at the less than 6 h of sleep threshold, with the strongest association found in the 5 h or less of sleep group.
To graphically illustrate the effect modification of objective sleep duration in the association between hypertension and all-cause mortality, we projected the mortality rates in hypertensive participants vs. normotensive participants across objective sleep duration subgroups with equal demographic and clinical characteristics (‘Methods’ section). As shown in Fig. 1, the expected mortality rate for normotensive participants who slept at least 6 and 5–6 h was 14.0%, whereas the expected mortality rates for hypertensive participants who slept at least 6 and 5–6 h were 22.4 and 31.2%, respectively (P for comparisons <0.05 and <0.01, respectively). Among participants who slept 5 h or less, hypertension showed a significantly larger impact; the expected mortality rate for normotensive participants who slept 5 h or less was 19.4%, whereas the expected mortality rate for hypertensive participants who slept 5 h or less was 49.7% (P < 0.01). The substantial differences with respect to the effect of hypertension on all-cause mortality rates across the three objective sleep duration groups with identical demographic and clinical characteristics shown in Fig. 1 were commensurate with the multivariable-adjusted ORs reported above. It should be noted that the ratio of these expected mortality rates can provide a closer estimate of associated risk than the ORs reported above; specifically, we could estimate a 1.6 (22.4 vs. 14.0%), 2.2 (31.2 vs. 14.0%), and 2.6 times (49.7 vs. 19.4%) higher risk of mortality in hypertensive participants than normotensive participants among those who slept at least 6, 5–6, and 5 h or less, respectively.
FIGURE 1.
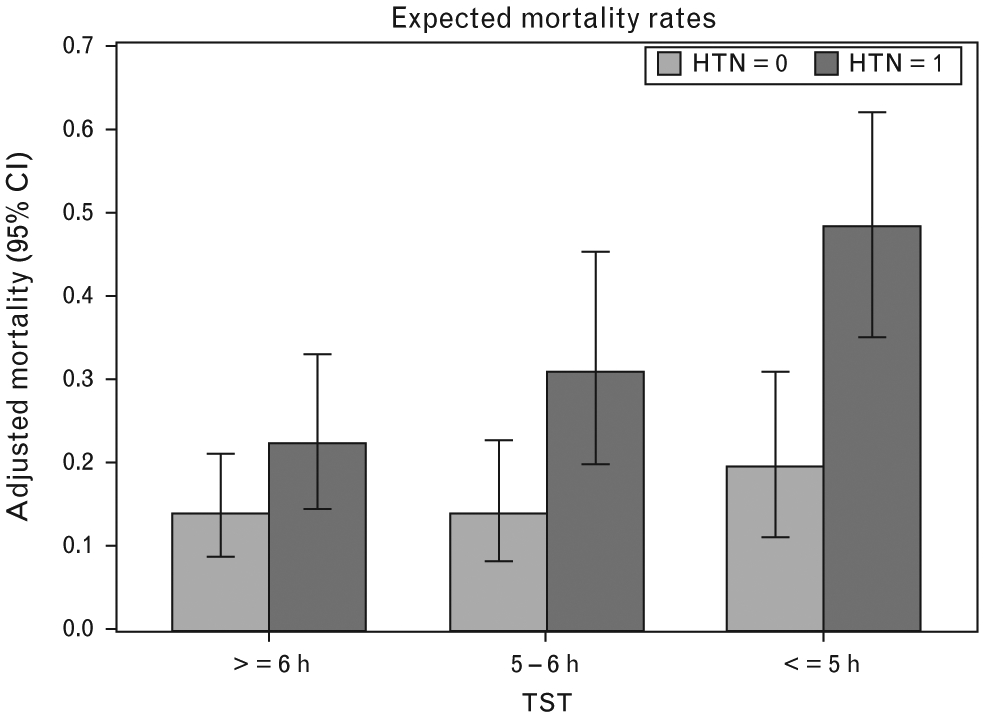
Expected mortality rates associated with hypertension across objective sleep duration groups. Figure depicts the expected probability of death associated with hypertension (HTN) across objective sleep duration groups with identical demographic (e.g. age, sex, and race) and clinical (e.g., prevalence of smoking, obesity, sleep-disordered breathing, diabetes, and depression) characteristics.
Furthermore, we examined other clinically meaningful hypertensive groups based on both their treatment status and BP management. Overall, the odds of all-cause mortality associated with controlled, untreated, and uncontrolled hypertension followed a dose–response pattern (Table 2). We found that the interaction between hypertensive treatment status groups and objective sleep duration on all-cause mortality was also statistically significant (P < 0.05). As shown in Table 2, the odds of all-cause mortality associated with controlled, untreated, and uncontrolled hypertension were significantly elevated among participants who slept less than 6 h, that is, 1.90, 2.21, and 3.06, respectively. In contrast, the odds of all-cause mortality associated with these three hypertensive treatment status groups were not significantly elevated among participants who slept at least 6 h. These data further indicated that the observed dose–response pattern of controlled, untreated, and uncontrolled hypertension was present specifically among participants who slept less than 6 h.
TABLE 2.
Odds ratio (95% confidence interval) associating hypertension status and all-cause mortality
| Hypertension | Overall | ≥6 h | <6 h |
|---|---|---|---|
| No | 1.00 | 1.00 | 1.00 |
| Controlled | 1.60 (0.85–3.01) | 1.22 (0.47–3.18) | 1.90 (0.94–3.85) |
| Untreated | 1.88 (1.25–2.84) | 1.49 (0.79–2.84) | 2.21 (1.30–3.77) |
| Uncontrolled | 2.28 (1.40–3.72) | 1.58 (0.72–3.45) | 3.06 (1.74–5.38) |
Adjusted for age, race, sex, BMI, smoking, SDB, sleep difficulty, heart disease, stroke, cholesterol, depression, and sampling weight.
P value for the interaction between hypertension and objective sleep duration <0.05.
In secondary analyses, we also tested the association of the three-level BP variable with all-cause mortality and its interaction with objective sleep duration. As compared with normotensive participants, the odds of all-cause mortality were significantly increased in hypertensive participants (OR = 2.49, 95% CI = 1.40–4.46, P < 0.01) but not in prehypertensive participants (OR = 1.27, 95% CI = 0.75–2.12, P = 0.36), and the interaction between the three-level BP variable and objective sleep duration was not statistically significant (P = 0.84). These data indicated that the observed effect modification applied to individuals with clinical hypertension, which is significantly associated with all-cause mortality.
DISCUSSION
The current study is the first one to demonstrate that objectively measured short sleep duration modifies the increased odds of all-cause mortality associated with hypertension. This increased odds follows a dose–response pattern, with the largest magnitude of association observed in those who slept 5 h or less, and is independent of other factors frequently associated with hypertension, mortality, or objective sleep duration (e.g. sex, age, race, smoking, obesity, diabetes, SDB, or depression). Objective short sleep duration in persons with hypertension may be biologically driven (e.g. genetics), behaviorally induced (e.g. self-infringed sleep deprivation), or, most likely, a marker of the degree of central autonomic dysfunction (e.g. sleep/arousal mechanisms and sympathetic nervous system dysregulation). Future epidemiological studies should examine whether this effect modification is stronger when cause-specific mortality is ascertained [i.e. cardiovascular disease (CVD) and stroke], whereas future clinical studies should examine whether improving/lengthening sleep reduces the odds of mortality in individuals with hypertension.
Consistent with previous studies, individuals with hypertension were associated with a significant risk of all-cause mortality [1]. When we stratified this risk by objectively measured short sleep duration, we showed a strong and significant dose–response on the association of hypertension with all-cause mortality. Among individuals who slept at least 6 h, hypertensive participants were associated with a significant 1.8-fold odds of all-cause mortality compared with normotensive participants. In contrast, among individuals who slept 5–6 and 5 h or less, hypertensive participants were associated with an increased 2.8 and 3.9-fold odds of all-cause mortality, respectively. Thus, objective short sleep duration had a strong modifying effect in the association between hypertension and all-cause mortality.
In the present study, we did not only calculate the odds of mortality associated with hypertension as modified by objective sleep duration while adjusting for multiple potential confounders, but we also pinpointed the expected mortality rate in this effect modification for populations with equal demographic (e.g. age, sex, and race) and clinical characteristics (e.g. prevalence of smoking, obesity, SDB, diabetes, and depression). The data presented in Fig. 1 can, therefore, be extrapolated to other populations with similar characteristics to our cohort of middle-aged men and women. Furthermore, these findings support the additional consideration of objective short sleep duration to increase the accuracy in estimating the risk of mortality associated with hypertension.
The vast majority of previous studies have focused on examining the independent association of sleep duration, typically subjectively measured, with hypertension, other morbidity and mortality, and the results have been inconsistent and modest [8-18]. In most of those studies, no PSG measures were obtained, and therefore the potential confounder of SDB was not controlled for, and the role of objective short sleep duration not examined. Most importantly, none of the previous studies conceptualized sleep duration as an effect modifier. The strong effect modification presented herein raises the question of what does objective short sleep duration mean in the context of cardiovascular risk.
There are, at least, three potential mechanistic explanations. Objective short sleep duration in persons with hypertension may be behaviorally induced – for example, individuals with short sleep duration in the sleep laboratory may be demonstrating self-infringed sleep deprivation. However, this is unlikely given that individuals were given enough in-lab conditions to satiate any accumulated sleep debt. Alternatively, objective short sleep duration in persons with hypertension may be biologically driven – for example, it may be a genetically determined trait that is unlikely to be subjected to modification. However, short sleep duration on the absence of high BP was not associated with significantly increased mortality rates, as shown in Fig. 1. That objective short sleep duration modified the odds of all-cause mortality in persons with hypertension suggests that objective short sleep is rather a biological marker of the degree of central autonomic dysfunction (e.g. sympathetic nervous system dysregulation), a mechanism that is well known to be associated both with BP and sleep regulation. This view is consistent with recent studies that showed that objective short sleep duration modifies in a similar manner the association of insomnia with significant morbidity, including hypertension [19,20], diabetes and poor glucose regulation [21-23], neurocognitive deficits [24], and mortality [25]. Notably, insomnia with objective short sleep duration has been associated with increased cortisol levels [26-30], catecholaminergic activity [31,32], 24-h metabolic rate [33], daytime alertness [34-36], and heart rate (HR) as well as impaired HR variability [37-39] and faster cardiac preejection period [40] suggesting that objective short sleep duration, as measured by PSG, may be a marker of the biological severity of the disorder [41]. Thus, the results of the present study further expand the proposal that objective short sleep duration in the context of conditions associated with sympathetic nervous system activation (i.e. hypertension and insomnia) is an index of their biological severity and a premorbid, modifiable risk factor for cardiovascular morbidity and mortality.
Future experimental studies should examine the central and peripheral mechanisms that may differentiate individuals with hypertension who sleep at least 6 h vs. those who sleep less than 6 h, given the strong effect modification on all-cause mortality found in this study. Moreover, future epidemiological studies should examine whether the effect modification found herein is stronger when CVD and stroke mortality is ascertained. Furthermore, future clinical trials should examine whether lengthening sleep improves the prognosis of individuals with hypertension.
Some limitations should be taken into account when interpreting our results. First, the objective sleep duration in this study was based on one night of PSG, which may be affected by the ‘first night effect’. However, we and others have recently shown that measures of sleep continuity, such as objective sleep duration based on three consecutive nights or two single-night recordings separated by several years, are stable and reflect a person’s habitual sleep and that a single night in the laboratory is useful for reliably classifying individuals as short sleepers [51,52]. Nevertheless, future studies should explore the association between hypertension, sleep duration, and mortality using multiple night recordings. Second, we only measured BP in the evening, and we did not have morning levels available, which would have provided a more in-depth assessment of hypertension throughout the 24 h. Third, we examined all-cause mortality. As we are currently following up the Penn State Adult Cohort for cause of death, we will explore in the near future the role of objective sleep duration using cause-specific mortality, when sample size permits. Finally, the OR reported herein should be cautiously interpreted and differentiated from relative risk (RR). As mentioned above, the expected mortality rates and their ratio may provide a closer estimate of the RR.
In summary, the odds of all-cause mortality associated with hypertension increases as a function of objective short sleep duration in a dose–response manner, with the greatest odds observed in hypertensive participants who sleep 5 h or less. Given the high prevalence of hypertension in the general population and the need to more accurately predict its prognosis, the introduction of novel, modifiable biological markers should become the target of public health policy. Individuals with hypertension who demonstrate objective short sleep duration may suffer from more severe central autonomic dysregulation and be at greater risk of all-cause mortality. Finally, because PSG measures of sleep are inconvenient and expensive, there is a need for validation of practical, easy to use, and inexpensive methods to measure sleep duration outside of the sleep lab. Meanwhile, our findings indicate that individuals with hypertension should undergo a sleep study, particularly those reporting symptoms of SDB or insomnia.
ACKNOWLEDGEMENTS
The work was performed at the Sleep Research & Treatment Center at the Penn State University Milton S. Hershey Medical Center, and the staff is especially commended for their efforts. Funding provided by American Heart Association (14SDG19830018) and National Institutes of Health (R01 HL51931, R01 HL40916, and R01 HL64415).
Abbreviations:
- 95% CI
95% confidence interval
- AHI
apnea/hypopnea index
- BP
blood pressure
- CDC
U.S. Center for Disease Control and Prevention
- CVD
cardiovascular disease
- NDI
National Death Index
- OR
odds ratio
- PSG
polysomnography
- SDB
sleep-disordered breathing
- SpO2
hemoglobin oxygen saturation
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. , Writing Group Members, On behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. , American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, American Heart Association Stroke Council, American Heart Association Council on Cardiovascular Nursing, American College of Cardiology Foundation. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008; 118:1080–1111. [DOI] [PubMed] [Google Scholar]
- 3.Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res 2013; 36:985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepin JL, Borel AL, Tamisier R, Baguet JP, Levy P, Dauvilliers Y. Hypertension and sleep: overview of a tight relationship. Sleep Med Rev 2014; 18:509–519. [DOI] [PubMed] [Google Scholar]
- 5.Ge X, Han F, Huang Y, Zhang Y, Yang T, Bai C, Guo X. Is obstructive sleep apnea associated with cardiovascular and all-cause mortality? PLoS One 2013; 8:e69432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol 2013; 169:207–214. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Zhang X, Winkelman JW, Redline S, Hu FB, Stampfer M, et al. Association between insomnia symptoms and mortality: a prospective study of U.S. men. Circulation 2014; 129:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res 2012; 35:1012–1018. [DOI] [PubMed] [Google Scholar]
- 9.Guo X, Zheng L, Wang J, Zhang X, Zhang X, Li J, Sun Y. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep Med 2013; 14:324–332. [DOI] [PubMed] [Google Scholar]
- 10.Holliday EG, Magee CA, Kritharides L, Banks E, Attia J. Short sleep duration is associated with risk of future diabetes but not cardiovascular disease: a prospective study and meta-analysis. PLoS One 2013; 8:e82305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 2015; 38:529–537. [DOI] [PubMed] [Google Scholar]
- 12.Magee L, Hale L. Longitudinal associations between sleep duration and subsequent weight gain: a systematic review. Sleep Med Rev 2012; 16:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Zhai L, Zhang D. Sleep duration and obesity among adults: a meta-analysis of prospective studies. Sleep Med 2014; 15:1456–1462. [DOI] [PubMed] [Google Scholar]
- 14.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011; 32:1484–1492. [DOI] [PubMed] [Google Scholar]
- 15.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res 2009; 18:148–158. [DOI] [PubMed] [Google Scholar]
- 16.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 2010; 33:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurina LM, McClintock MK, Chen JH, Waite LJ, Thisted RA, Lauderdale DS. Sleep duration and all-cause mortality: a critical review of measurement and associations. Ann Epidemiol 2013; 23:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandner MA, Patel NP, Gehrman PR, Perlis ML, Pack AI. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev 2010; 14:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 2009; 32:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, Bixler EO. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension 2012; 60:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care 2009; 32:1980–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Crosssectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care 2011; 34:1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasisht KP, Kessler LE, Booth JN 3rd, Imperial JG, Penev PD. Differences in insulin secretion and sensitivity in short-sleep insomnia. Sleep 2013; 36:955–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Mendoza J, Calhoun S, Bixler EO, Pejovic S, Karataraki M, Liao D, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep 2010; 33:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep 2010; 33:1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab 2001; 86:3787–3794. [DOI] [PubMed] [Google Scholar]
- 27.Rodenbeck A, Huether G, Ruether E, Hajak G. Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neurosci Lett 2002; 324:159–163. [DOI] [PubMed] [Google Scholar]
- 28.Shaver JL, Johnston SK, Lentz MJ, Landis CA. Stress exposure, psychological distress, and physiological stress activation in midlife women with insomnia. Psychosom Med 2002; 64:793–802. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Mendoza J, Vgontzas AN, Calhoun SL, Vgontzas A, Tsaoussoglou M, Gaines J, et al. Insomnia symptoms, objective sleep duration and hypothalamic-pituitary-adrenal activity in children. Eur J Clin Invest 2014; 44:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riemann D, Klein T, Rodenbeck A, Feige B, Horney A, Hummel R, et al. Nocturnal cortisol and melatonin secretion in primary insomnia. Psychiatry Res 2002; 113:17–27. [DOI] [PubMed] [Google Scholar]
- 31.Vgontzas AN, Tsigos C, Bixler EO, Stratakis CA, Zachman K, Kales A, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res 1998; 45:21–31. [DOI] [PubMed] [Google Scholar]
- 32.Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and controls subjects. Brain Behav Immun 2003; 17:365–372. [DOI] [PubMed] [Google Scholar]
- 33.Bonnet MH, Arand DL. 24-h metabolic rate in insomniacs and matched normal sleepers. Sleep 1995; 18:581–588. [DOI] [PubMed] [Google Scholar]
- 34.Roehrs TA, Randall S, Harris E, Maan R, Roth T. MSLT in primary insomnia: stability and relation to nocturnal sleep. Sleep 2011; 34:1647–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edinger JD, Means MK, Krystal AS. Does physiological hyperarousal enhance error rates among patients with insomnia? Sleep 2013; 36:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Vgontzas AN, Fernandez-Mendoza J, Bixler EO, Sun Y, Zhou J, et al. Insomnia with physiological hyperarousal is associated with hypertension. Hypertension 2015; 65:644–650. [DOI] [PubMed] [Google Scholar]
- 37.Stepanski E, Glinn M, Zorick F, Roehrs T, Roth T. Heart rate changes in chronic insomnia. Stress Med 1994; 10:261–266. [Google Scholar]
- 38.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med 1998; 60:610–615. [DOI] [PubMed] [Google Scholar]
- 39.Spiegelhalder K, Fuchs L, Ladwig J, Kyle SD, Nissen C, Voderholzer U, et al. Heart rate and heart rate variability in subjectively reported insomnia. J Sleep Res 2011; 20:137–145. [DOI] [PubMed] [Google Scholar]
- 40.de Zambotti M, Covassin N, Sarlo M, De Min Tona G, Trinder J, Stegagno L. Nighttime cardiac sympathetic hyper-activation in young primary insomniacs. Clin Auton Res 2013; 23:49–56. [DOI] [PubMed] [Google Scholar]
- 41.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev 2013; 17:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med 1998; 157:144–148. [DOI] [PubMed] [Google Scholar]
- 43.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 2001; 163:608–613. [DOI] [PubMed] [Google Scholar]
- 44.Bixler EO, Vgontzas AN, Lin HM, Vela-Bueno A, Kales A. Insomnia in central Pennsylvania. J Psychosom Res 2002; 53:589–592. [DOI] [PubMed] [Google Scholar]
- 45.National Center for Health Statistics. 2003 revision of the U.S. standard certificate of death. 2003; Available from: http://www.cdc.gov/nchs/data/dvs/DEATH11-03final-acc.pdf (Accessed 4 April 2016).
- 46.National Center for Health Statistics. Report of the panel to evaluate the U.S. standard certificates. 2000; Available from: http://www.cdc.gov/nchs/data/dvs/panelreport_acc.pdf (accessed 4 April 2016).
- 47.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, MD: National Institutes of Health; 1968. [Google Scholar]
- 48.Waksberg J. Sampling methods for random digit dialing. J Am Stat Assoc 1978; 73:40–46. [Google Scholar]
- 49.Kish L. Survey sampling. New York: John Wiley & Sons, Inc; 1965. [Google Scholar]
- 50.U.S. Department of Health and Human Services (DHHS), National Center for Health Statistics. Third National Health and Nutrition Examination Survey 1988–1994. NHANES III laboratory data file. Hyattsville, MD: Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 51.Wohlgemuth WK, Edinger JD, Fins AI, Sullivan RJ. How many nights are enough? The short-term stability of sleep parameters in elderly insomniacs and normal sleepers. Psychophysiology 1999; 36:233–244. [PubMed] [Google Scholar]
- 52.Gaines J, Vgontzas AN, Fernandez-Mendoza J, Basta M, Pejovic S, He F, Bixler EO. Short- and long-term sleep stability in insomniacs and healthy controls. Sleep 2015; 38:1727–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]


