Summary
Amyotrophic lateral sclerosis (ALS) is a fatal, progressive neurodegenerative disease resulting from a complex interplay between genetics and environment. Impairments in axonal transport have been identified in several ALS models, but in vivo evidence remains limited, thus their pathogenetic importance remains to be fully resolved. We therefore analyzed the in vivo dynamics of retrogradely transported, neurotrophin-containing signaling endosomes in nerve axons of two ALS mouse models with mutations in the RNA processing genes TARDBP and FUS. TDP-43M337V mice, which show neuromuscular pathology without motor neuron loss, display axonal transport perturbations manifesting between 1.5 and 3 months and preceding symptom onset. Contrastingly, despite 20% motor neuron loss, transport remained largely unaffected in FusΔ14/+ mice. Deficiencies in retrograde axonal transport of signaling endosomes are therefore not shared by all ALS-linked genes, indicating that there are mechanistic distinctions in the pathogenesis of ALS caused by mutations in different RNA processing genes.
Keywords: amyotrophic lateral sclerosis, ALS, intravital imaging, motor neuron disease, MND, RNA-binding protein, TARDBP
Graphical Abstract
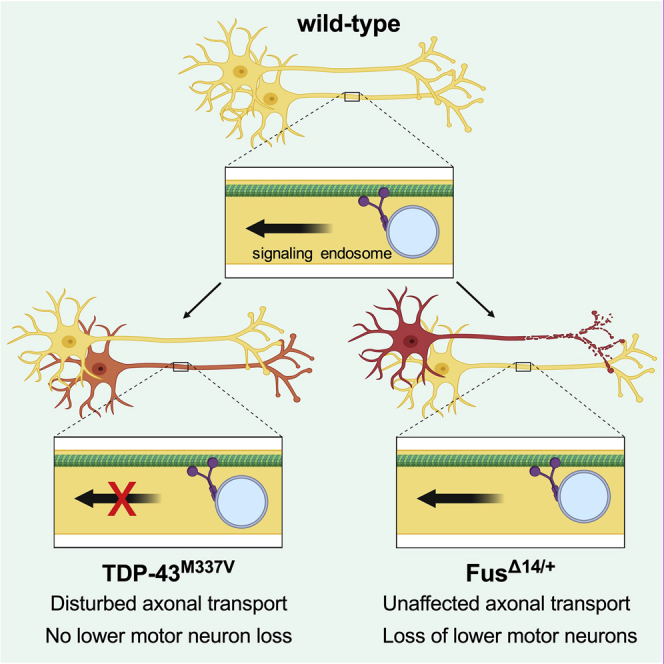
Highlights
-
•
Mutant TDP-43 mice display in vivo defects in signaling endosome axonal transport
-
•
Despite motor neuron loss, mutant FUS mice display no such deficiency
-
•
Axonal transport disruption is thus not common to all ALS mouse models
Sleigh et al. address the importance of disturbances in axonal transport in two mouse models for ALS. They find that deficiencies in the in vivo axonal delivery of target tissue-derived survival factors are not a common feature of all ALS mouse models, suggesting mechanistic distinctions in different ALS-linked genes.
Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive neurodegenerative disorder that results from upper and lower motor neuron loss, leading to muscle wasting, atrophy, and, ultimately, death most often due to respiratory failure (Brown and Al-Chalabi, 2017). Treatment options for ALS patients are severely limited, but gene therapy approaches hold great promise (Tosolini and Sleigh, 2017). ALS is thought to manifest through a multi-step process encompassing additive effects from genetic predispositions and environmental insults (Al-Chalabi and Hardiman, 2013); however, ∼10% of cases show clear monogenic heritability (familial ALS [fALS]), while known causative genetic mutations underlie ∼68% of fALS and ∼11% of the remaining sporadic cases of ALS (Renton et al., 2014). Mutations in numerous genes are linked to the disease, the four most common of which, in ascending order, are dominant mutations in fused in sarcoma (FUS), transactive-region DNA-binding protein (TARDBP encoding TDP-43), superoxide dismutase 1 (SOD1), and large, intronic hexanucleotide repeat expansions in chromosome 9 open reading frame 72 (C9orf72) (Brown and Al-Chalabi, 2017).
Many genes associated with ALS encode proteins important in all cells, and as such, it remains unknown why motor neurons and certain brain regions, such as the frontotemporal cortex, are selectively affected. Nonetheless, impairments in cytoskeletal dynamics and axonal transport are emerging as a central theme based on ALS-linked gene function (Clark et al., 2016, De Vos and Hafezparast, 2017, Sleigh et al., 2019). Axonal transport is the fundamental, bi-directional process whereby cargoes (e.g., organelles and proteins) are actively transported from one end of an axon to the other, along polarized microtubules (Maday et al., 2014). Anterograde transport, which is from the cell body to axon terminal, is dependent on the kinesin family of molecular motors, while the cytoplasmic dynein complex is responsible for retrograde axonal transport in the opposite direction. Patient post-mortem studies provided the first evidence for involvement of impaired transport in ALS, which has since been consolidated by results from a plethora of disease models implicating various cargoes (De Vos and Hafezparast, 2017). Transport deficits have been linked to all four major ALS genes through in vitro, ex vivo, and Drosophila melanogaster experiments; however, these experimental models do not necessarily replicate the complex environment found in mammals, which is required for efficient, rapid axonal transport (Sleigh et al., 2017). In vivo results from mammals in which individual cargoes are tracked in real time, rather than en masse, have been generated in SOD1G93A and TDP-43A315T ALS mice (Table S1; Bilsland et al., 2010, Fellows et al., 2020, Gibbs et al., 2018, Magrané et al., 2014). Axonal transport is disrupted in both models at early disease stages, consistent with a potential causative role in neuromuscular dysfunction and motor neuron degeneration; nonetheless, it is unclear whether these ALS mice, which express disease-causing mutant proteins at supra-physiological levels, are reflective of the full disease spectrum.
We have thus performed pseudolongitudinal assessments of in vivo axonal transport in two recently engineered mouse models of ALS with mutations in genes encoding DNA/RNA-binding proteins instrumental to RNA processing, TDP-43 (Gordon et al., 2019) and Fus (Devoy et al., 2017). Transgenic TDP-43M337V and humanized, knockin FusΔ14/+ mice, which both express mutant protein at physiologically relevant levels, have been used to address the importance of altered axonal transport to ALS neuropathology. Although mitochondria are the most frequently analyzed axonal cargo, in this study we opted to assess the trafficking of signaling endosomes, which are essential to the long-range delivery of signals critical to neuronal survival.
Results
Imaging In Vivo Axonal Transport in Motor Neurons
To assess in vivo dynamics of axonal transport, we used a fluorescently labeled, binding fragment of tetanus neurotoxin (HCT), which is retrogradely transported along axons within neurotrophin-containing signaling endosomes toward neuronal cell bodies (Surana et al., 2018, Villarroel-Campos et al., 2018). Impairments in long-range neurotrophic signaling have been implicated in several neurodegenerative conditions, including ALS (Bronfman et al., 2007, Sleigh et al., 2019). By injecting HCT into the gastrocnemius and tibialis anterior muscles of the lower leg, and exposing the sciatic nerve at mid-thigh level 4–8 h post-injection, individual, fluorescently labeled endosomes being retrogradely transported can be imaged and tracked in the peripheral nerve axons of live, anesthetized mice (Figure S1A; Gibbs et al., 2016; J.N.S., A.P.T., and G.S., unpublished data).
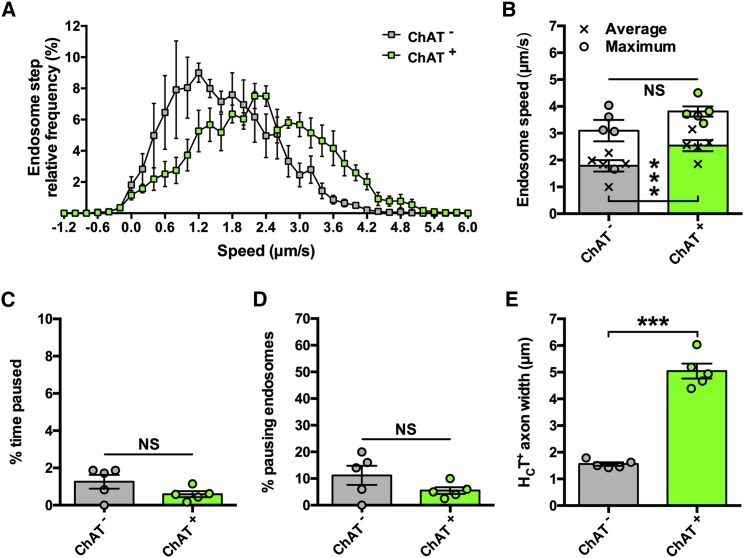
Post-intramuscular injection, about 80% of HCT-positive (HCT+) axons stain for choline acetyltransferase (ChAT) (Bilsland et al., 2010), suggesting that the probe is preferentially transported in motor neurons. Nevertheless, assessing transport in a mixed motor and sensory population may weaken the ability to identify motor-specific trafficking perturbations. Therefore, before analyzing transport in ALS mice, we compared endosome dynamics in motor versus sensory neurons using ChAT-eGFP mice, which permit visual differentiation of peripheral nerve types because motor axons are specifically labeled with eGFP (Figure S1A). Mean endosome transport speeds were greater in ChAT+ motor neurons compared with ChAT− sensory neurons (Figures 1A and 1B), and this was not due to major pausing differences (Figures 1C and 1D). Interestingly, motor axons had clearly larger calibers than sensory axons (Figure 1E), suggesting that by imaging thicker axons, HCT transport can be measured in motor neurons with greater certainty than if randomly selecting an axon (i.e., >80%; Bilsland et al., 2010). To confirm this, transport dynamics were compared between ChAT+ axons and thicker axons from non-fluorescent control mice, and no differences were observed (Figure S1). The bell-shaped, rather than bi-modal, speed frequency distribution generated from non-fluorescent mice (Figure S1B) indicates that in vivo axonal transport of endosomes can be assessed predominantly in motor neurons by selecting large-caliber axons. This approach was thus used to analyze axonal transport in ALS mice.
Figure 1.
Retrograde Axonal Transport of Signaling Endosomes Is Faster in Motor Neurons Than Sensory Neurons
(A) Speed distribution curves of signaling endosome frame-to-frame movements in motor (ChAT+, green) and sensory axons (ChAT−, gray) indicate that axonal transport is faster in motor neurons.
(B) Mean (crosses), but not maximum (circles), endosome transport is faster in motor neurons when calculated per animal.
(C and D) There is no difference between motor and sensory nerves in the percentage of time endosomes paused for (C) or the percentage of endosomes that paused (D).
(E) HCT-containing axons that are ChAT+ have a larger caliber than ChAT− axons.
∗∗∗p < 0.001 and NS, not significant, paired t test. n = 5. Means ± standard error of the mean (SEM) plotted for all graphs.
See also Figure S1.
In Vivo Axonal Transport Is Pre-symptomatically Impaired in Mutant TDP-43 Mice
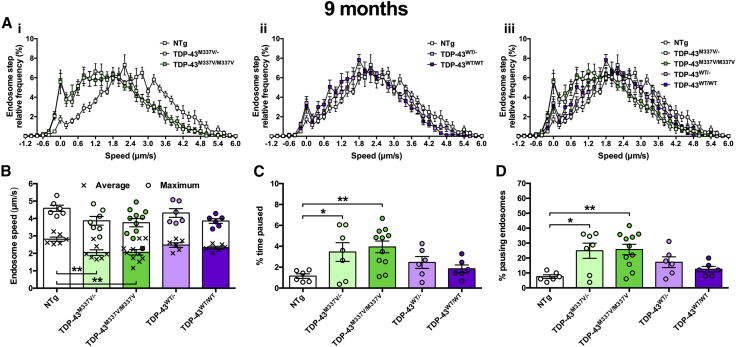
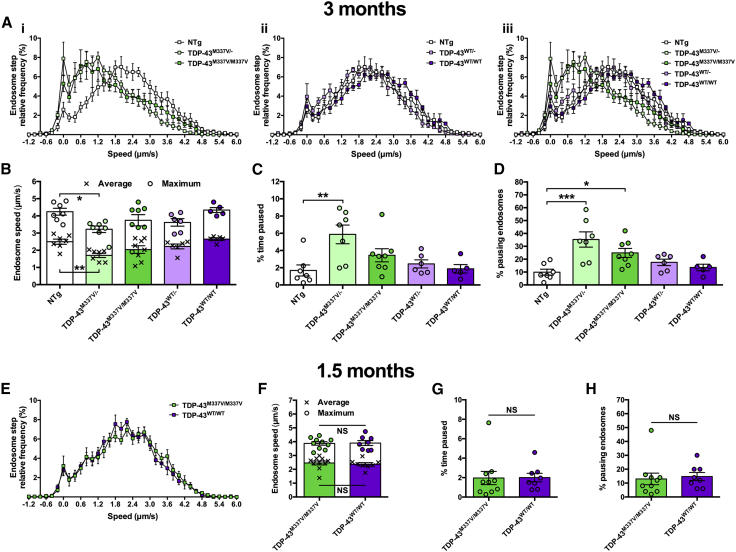
Recently reported transgenic TDP-43M337V mice display impairments in motor function and neuromuscular junction abnormalities beginning at 9 months in homozygous mutants without motor neuron loss up to 12 months (Gordon et al., 2019, Williamson et al., 2019). We therefore first assessed retrograde transport of signaling endosomes at 9 months of age in hemizygous and homozygous TDP-43M337V and TDP-43WT mice and non-transgenic (NTg) controls (Figure 2). The frequency histograms of frame-to-frame endosome speeds of both TDP-43M337V/− and TDP-43M337V/M337V animals are shifted to the left compared with NTg mice, indicative of slower transport, whereas TDP-43WT transport was unaffected because it overlaps with the curve obtained using NTg controls (Figure 2A). When compared, both mutants showed a statistically significant reduction in mean endosome speed (Figure 2B), which was at least partially due to increased pausing (Figures 2C and 2D). Mutant TDP-43 mice do not show clear behavioral phenotypes at 3 months (Gordon et al., 2019, Williamson et al., 2019); we therefore assessed transport at this early time point to see whether axonal transport defects precede symptom onset and thus may contribute to motor neuron pathology. Indeed, a similar deficiency in mutant TDP-43 transport was observed at 3 months, while TDP-43WT transport remained unperturbed (Figures 3A–3D). Finally, to determine at what stage transport becomes affected, we assessed endosomal trafficking at 1.5 months in TDP-43M337V/M337V and TDP-43WT/WT mice. We found no difference between genotypes (Figures 3E–3H) or from NTg control mice (not shown). These data indicate that TDP-43M337V, but not TDP-43WT, mice display a pre-symptomatic, non-developmental in vivo impairment in axonal transport of signaling endosomes that manifests between 1.5 and 3 months of age (Figure S2).
Figure 2.
Mutant TDP Mice Display Perturbed Axonal Transport of Endosomes at 9 Months
(A) Endosome frame-to-frame speed distribution curves indicate that hemizygous and homozygous TDP-43M337V mice (i, iii, green) transport endosomes more slowly than control, non-transgenic (NTg) mice (white), whereas transport is unaffected in TDP-43WT controls (ii, iii, purple).
(B) Mean endosomal speeds (crosses) are significantly reduced in TDP-43M337V/− and TDP-43M337V/M337V mice, but not TDP-43WT controls (p = 0.006, one-way ANOVA), while maximum speeds (circles) remained unchanged (p = 0.0891, one-way ANOVA).
(C) Endosomes in mutant TDP-43M337V hemizygous and homozygous mice paused for longer periods of time compared with NTg mice (p = 0.0139, one-way ANOVA).
(D) TDP-43M337V mice had a greater percentage of endosomes that paused (p = 0.0046, one-way ANOVA).
∗p < 0.05 and ∗∗p < 0.01, Dunnett’s multiple-comparisons test. n = 6–11. Means ± SEM are plotted for all graphs.
See also Figures S2 and S4.
Figure 3.
TDP-43M337V Transport Disruption Occurs between 1.5 and 3 Months
(A) Endosome frame-to-frame speed distribution curves show that at 3 months TDP-43M337V (i, iii, green), but not TDP-43WT (ii, iii, purple), mice transport endosomes more slowly than control, non-transgenic (NTg) mice (white).
(B) Mean (crosses, p = 0.004, one-way ANOVA) and maximum (circles, p = 0.0222, one-way ANOVA) endosomal speeds are significantly reduced in TDP-43M337V/− mice.
(C and D) TDP-43M337V hemizygotes and homozygotes show increased endosome pausing as assessed by calculating the percentage of time paused (C) (p = 0.0171, Kruskal-Wallis test) and the percentage of pausing endosomes (D) (p < 0.001, one-way ANOVA).
(E–H) At 1.5 months, there is no difference in endosome frame-to-frame speed distribution curves (E), average or maximum endosome transport speeds (F), the percentage of time that endosomes were paused (G), or the percentage of pausing endosomes (H) between TDP-43M337V/M337V (green) and TDP-43WT/WT (purple) mice. This suggests that transport disruption occurs between 1.5 and 3 months. Presented 1.5 month data are not significantly different from NTg control (not shown).
∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001, Dunnett’s/Dunn’s multiple-comparisons test. NS, not significant, unpaired t test/Mann-Whitney U test. n = 5–10. Means ± SEM are plotted for all graphs.
See also Figures S2 and S4.
Axonal Transport Remains Largely Unaffected in Mutant Fus Mice Even at Late Stages
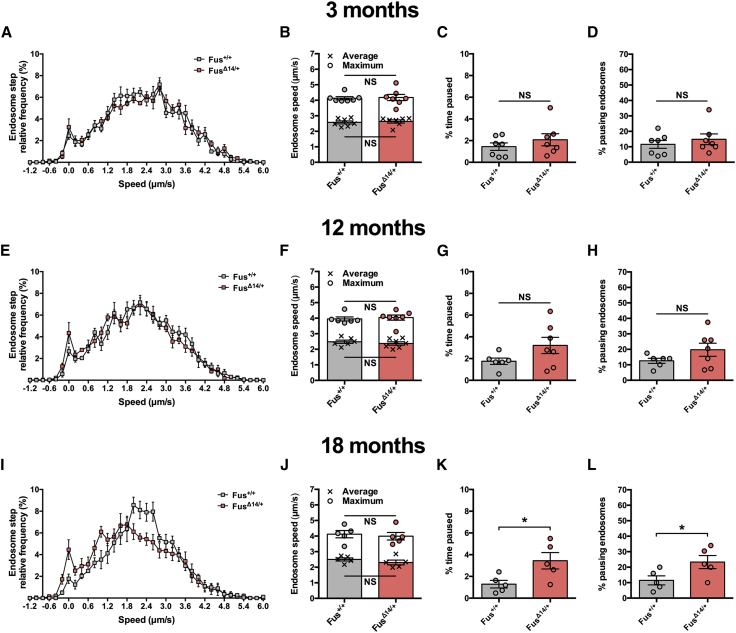
Deficient in vivo trafficking of signaling endosomes has now been observed in SOD1G93A mice (Bilsland et al., 2010, Gibbs et al., 2018) and the TDP-43M337V model reported here. To assess whether this phenotype is common to mouse models of ALS, we assessed in vivo transport in knockin mutant FusΔ14/+ mice. This model displays loss of neuromuscular integrity and progressive degeneration of lumbar spinal motor neurons; at 3 months, mutant Fus mice show no motor neuron loss, which becomes overt by 12 (14% reduction) and 18 (20% reduction) months of age (Devoy et al., 2017). We therefore assessed endosome transport at 3, 12, and 18 months in this ALS model (Figure 4). At 3 and 12 months, there was no significant difference in axonal kinetics of these organelles (Figures 4A–4H), and, despite an increase in pausing (Figures 4K and 4L), there was no significant change in signaling endosome mean or maximum speeds at the late disease stage of 18 months (Figures 4I and 4J). Consistent with this, no significant changes in transport were observed across time points for Fus+/+ or FusΔ14/+ mice, although Fus mutants show a subtle, progressive, yet non-significant, decline (Figure S3).
Figure 4.
FusΔ14/+ Mice Display a Minor Impairment in Axonal Transport of Endosomes, but Only at a Late Disease Stage
(A–H) The axonal dynamics of signaling endosomes are similar between Fus+/+ (gray) and FusΔ14/+ (red) mice at 3 months (A–D) and 12 months (E–H) of age.
(I–L) At 18 months, retrograde axonal transport speed of endosomes is unaffected in FusΔ14/+ mice (I and J); however, mutant Fus mice do show a significant increase in the percentage of time that endosomes pause for (K) and the percentage of endosomes that paused (L).
∗p < 0.05; NS, not significant, unpaired t test. n = 5–7. Means ± SEM are plotted for all graphs.
See also Figures S3 and S4.
We have previously shown that endosome transport remains stable in wild-type mice from 1 to 13–14 months of age (Sleigh and Schiavo, 2016), suggesting that a natural, aging-related decline in transport does not compound the mutant TDP-43 transport defect. To ensure that this remains true up to 18 months, we compared axonal transport in all control mice aged 3–18 months. There were no significant changes in cargo dynamics (Figure S4), suggesting that the mild pausing defect of 18-month-old mutant Fus mice is unlikely to be a direct consequence of aging and that axonal transport of signaling endosomes remains unaltered in wild-type mice up to 18 months.
Discussion
Here, we show that an ALS mouse model of mutant TDP-43 displays a pre-symptomatic, in vivo deficit in axonal transport of signaling endosomes in peripheral axons, which may contribute to motor function deficits and impaired neuromuscular integrity. This defect is specific to the M337V mutation, as TDP-43WT protein, which is expressed at a similar, low level as TDP-43M337V relative to endogenous mouse TDP-43 (Gordon et al., 2019), had no effect on retrograde transport rates. Counter to observations of several, but not all (Gordon et al., 2019), reported TDP-43-linked pathologies (Fratta et al., 2018, White et al., 2018), hemizygous and homozygous TDP-43 mutant mice show similar deficiencies in trafficking, suggesting that once a threshold level of mutant TDP-43 is present, no further transport exacerbation occurs. This may be due to the heterozygous mutant TDP-43 transport defect being caused by a loss of signaling or alterations of the axonal proteome that precipitate a maximum physiological reduction in endosome transport speeds, which cannot be further affected by additional pathological protein. Furthermore, once manifested, the transport defect does not appear to get progressively worse between 3 and 9 months, indicating that a disturbance in retrograde transport of signaling endosomes may underlie the subsequent progressive reduction in neuromuscular integrity and motor function, yet is compatible with motor neuron survival at the level of the spinal cord (Gordon et al., 2019).
Alterations in the population of sciatic nerve axons over the imaging period are unlikely to account for the different rates of TDP-43M337V transport, as there is no spinal cord motor neuron loss at the time points assessed (Gordon et al., 2019). Importantly, loss of neuromuscular junction connectivity does not occur until after 3 months, once again indicating that altered sciatic nerve axon profiles do not cause the transport disruption. Furthermore, loss of motor axons does not necessarily result in impaired axonal endosome dynamics, as revealed by our experiments in the FusΔ14/+ mouse, likely reflecting the robustness of our technique to accurately differentiate between motor and sensory axons. All genotypes assessed showed plentiful uptake and retrograde transport of the fluorescent probe, with no overt changes in endosome number between mutant and wild-type mice, despite endocytosis dysfunction being previously linked to both TDP-43 and FUS toxicity (Liu et al., 2020). Moreover, heterogeneity in axonal endosome-associated Rab proteins, in particular Rab5 and Rab7, is unlikely to cause the observed transport distinctions because Rab7 is the predominant Rab associated with HCT-positive axonal endosomes (Deinhardt et al., 2006).
Nonetheless, the TDP-43M337V mouse data add to the impaired mitochondrial transport reported in TDP-43A315T mice and defective mitochondria and signaling endosome trafficking in SOD1G93A mice (Table S1). ALS-linked mutations in SOD1 and TARDBP may thus cause early/pre-symptomatic, generalized defects in axonal transport in motor neurons (rather than cargo-specific deficits), leading to dysfunction and degeneration (Gordon et al., 2019). This may be caused by cargo-independent impairments in the cytoskeleton or motor proteins (e.g., the cytoskeletal regulator HDAC6 is a known target of TDP-43; Fiesel et al., 2010), or by aberrant binding of mutant ALS proteins to motor complexes (Tateno et al., 2009, Zhang et al., 2007); however, this will have to be directly confirmed in the TDP-43M337V model.
Contrastingly, signaling endosome transport in FusΔ14/+ mice remained largely unaffected even during latter disease stages, despite a 20% loss of spinal cord motor neurons at 18 months (Devoy et al., 2017), confirming that degenerating axons do not always have altered transport kinetics (Malik et al., 2011). This implies that transport disturbances are not necessarily a non-specific by-product of neurodegeneration, at least during earlier disease stages, and thus emphasizes the specificity of transport defects in mutant TDP-43 and SOD1 mice. However, it remains possible that motor neuron loss proceeds very rapidly and targets only specific subpools of motor neurons in FusΔ14/+ mice, such that any preceding defect in transport was missed (Nijssen et al., 2017). Alternatively, mutant Fus mice may display cargo-specific (e.g., mitochondria, RNA granules) or anterograde transport defects, some of which have been reported in other ALS models (Alami et al., 2014, Baldwin et al., 2016), thus additional cargoes should also be assessed in FusΔ14/+ mice. Nevertheless, altogether our findings indicate that pre-symptomatic abnormalities in retrograde axonal transport of neurotrophin-containing signaling endosomes may not be common to all ALS-linked genes and that there are inherent distinctions in the pathomechanism of ALS caused by mutations in different RNA processing genes. Although TDP-43 and FUS are both RNA/DNA-binding proteins that process RNA predominantly in the nucleus, they regulate the expression and splicing of largely distinct gene sets (Colombrita et al., 2012, Lagier-Tourenne et al., 2012) and show neuropathological idiosyncrasies when mutated (Bäumer et al., 2010), which could account for these discrepancies in axonal transport deficits. As could the observation that wild-type TDP-43 and SOD1 proteins consistently associate with motor neuron signaling endosomes, whereas FUS does not (Debaisieux et al., 2016).
Disruptions in axonal transport have been linked to the M337V TARDBP mutation in a range of in vitro and Drosophila larval models (Alami et al., 2014, Baldwin et al., 2016, Wang et al., 2013). Although the severe frameshift FUS mutation modeled in FusΔ14/+ mice has not previously been assessed, transport perturbations have been reported in several mutant FUS models, including Drosophila larvae (Baldwin et al., 2016), isolated squid axoplasm (Sama et al., 2017), and human motor neurons derived from induced pluripotent stem cells (iPSCs) (Guo et al., 2017).
Why then do FusΔ14/+ mice not show impaired signaling endosome transport, at least until a late disease stage? In addition to the possibilities mentioned above, there are other potential explanations. First, distinctions may arise because of the different FUS mutations being analyzed and their expression in the presence or absence of the wild-type allele. Second, although Drosophila is an excellent model that has provided instrumental insights into neurobiology, as well as neurological diseases (Grice et al., 2011, Walters et al., 2019, Yamaguchi and Takashima, 2018), in vivo transport analyses are conducted in larvae in which organs have been removed, so there is considerable disruption to the organism, which is being analyzed during development and is thus perhaps not the best model for age-related neurodegeneration. Moreover, the complex, long-range neurotrophin signaling program is not conserved in Drosophila, while mutant ALS transgenes are often overexpressed to above physiological levels, which can induce phenotypes even with wild-type FUS transgenes (Baldwin et al., 2016). In vitro axonal transport dynamics differ from in vivo trafficking (Bilsland et al., 2010, Gibbs et al., 2016), possibly because of cultured neurons lacking the complete series of necessary cellular and chemical interactions (e.g., myelination and target muscle cells in the case of motor neurons) (Sleigh et al., 2017), which is particularly important for ALS as both cell- and non-cell-autonomous pathomechanisms contribute to disease onset and progression (Nijssen et al., 2017). In addition to variability inherent to iPSC differentiation, it remains unknown how closely motor neuron developmental stages in culture correlate with age-related degeneration in vivo. By imaging axonal transport of signaling endosomes in intact sciatic nerves of anesthetized mice, we are instead certain of the disease stage and physiological environment of the peripheral axons under investigation.
In summary, we have assessed in vivo retrograde axonal transport of signaling endosomes in two mouse models of ALS that express disease-causing mutant proteins at near endogenous levels. Mutant TDP-43, but not mutant Fus, mice displayed a pre-symptomatic deficiency in endosome transport, suggesting that reduced neurotrophin signaling may contribute to mutant TDP-43-mediated neuropathology and that general defects in axonal transport are specific to a subset of ALS-linked genes in an in vivo mammalian setting.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| HCT441 | Restani et al., 2012 | N/A |
| AlexaFluor555 C2 maleimide | Life Technologies | A-20346 |
| recombinant human brain-derived neurotrophic factor | Peprotech | 450-02 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Tg(Chat-EGFP)GH293Gsat/Mmucd (ChAT-eGFP) | Mutant Mouse Resource and Research Center | MMRRC: 000296-UCD |
| Mouse: B6;129S6-Gt(ROSA)26Sorm1(TARDBP∗M337V/Ypet)Tlbt/J (WT and M337V TDP43) | The Jackson Laboratory | JAX: 029266 |
| Mouse: B6N;B6J-Fustm1Emcf./H (Fus+/+ and FusΔ14/+) | Devoy et al., 2017 | N/A |
| Software and Algorithms | ||
| Tracker (Version 2.0.0.26) | Kinetic Imaging Ltd. | N/A |
Lead Contact and Materials Availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Giampietro Schiavo (giampietro.schiavo@ucl.ac.uk). This study did not generate new unique reagents.
Experimental Model and Subject Details
Animals
All mouse handling and experiments were performed under license from the United Kingdom Home Office in accordance with the Animals (Scientific Procedures) Act (1986) and approved by the University College London – Queen Square Institute of Neurology Ethics Committee. All mice were maintained in individually ventilated cages and on a standard diet. Tg(Chat-EGFP)GH293Gsat/Mmucd mice (MMRRC Stock Number 000296-UCD), referred to as ChAT-eGFP mice, were maintained and imaged as heterozygotes on a CD-1 background. The male to female ratio used for these experiments was 2:3. B6;129S6-Gt(ROSA)26Sorm1(TARDBP∗M337V/Ypet)Tlbt/J (WT and M337V TDP43, Jackson Laboratory strain #029266, https://www.jax.org/strain/029266) and B6N;B6J-Fustm1Emcf./H (Fus+/+ and FusΔ14/+) mice were maintained on a C57BL/6 background and genotyped as detailed (Devoy et al., 2017, Gordon et al., 2019). ChAT-eGFP mice used for motor versus sensory analyses were 79-134 days old. Non-transgenic (NTg) control and TDP43 mice sacrificed for 1.5, 3, and 9 month time points were 55-57, 102-125, and 249-71 days old, respectively. The male to female ratio used for these time points were 1:1, 2:15 and 35:6, respectively. Fus mice sacrificed for 3, 12, and 18 month time points were 104-115, 365-368, and 568-588 days old, respectively. The male to female ratio used for these time points were 4:3, 6:7 and 3:7, respectively. Littermates of either sex were pooled in all analyses because no significant differences in transport were observed between males and females independent of the genotype (Figures S4E–S4H, and data not shown). Sample sizes of 5 or above were chosen based on two-sample, two-sided power calculations, with standard power of 0.8 (1−β) and type I error rate of 5% (α). Estimated mean and standard deviation of average signaling endosome transport speeds per animal were calculated from previous data generated from mice modeling neuromuscular diseases.
Method Details
Axonal transport imaging
In vivo kinetics of signaling endosomes labeled with atoxic binding fragment of tetanus neurotoxin (HCT) were assessed as previously described (Gibbs et al., 2016; J.N.S., A.P.T., and G.S., unpublished data). Briefly, HCT (HCT441, residues 875-1315) fused to an improved cysteine-rich tag and a human influenza haemagglutinin epitope was bacterially expressed as a glutathione-S-transferase fusion protein (Restani et al., 2012), and labeled with AlexaFluor555 C2 maleimide (Life Technologies, A-20346). On the morning of analysis, HCT was pre-mixed with recombinant human brain-derived neurotrophic factor (BDNF, Peprotech, 450-02) in phosphate buffered saline. Under isofluorane-induced anesthesia and on a heat-pad to maintain body temperature, two 1-2 mm long incisions were made in the skin above the gastrocnemius and tibialis anterior muscles of the right leg. 3.5-5 μg of HCT with 25 ng BDNF was then injected in a volume of ≈1.5 μL per muscle into the motor end plate region as per Mohan et al. (2014). The incisions were closed by suturing and the animal allowed to fully recover. 4-8 h post-injection, animals were terminally anaesthetised using isofluorane and 1–1.5 cm of the right sciatic nerve was exposed by removal of the overlying skin and musculature. Curved forceps were then used to cautiously separate the sciatic nerve from the underlying connective tissue, such that a small piece of magic tape could be placed between the two to aid imaging. Still under anesthesia, the mouse was transferred to an inverted LSM780 laser scanning microscope (Zeiss) within an environmental chamber pre-warmed and set throughout the experiment to 37°C. The body weight of the animal and careful positioning ensures that the sciatic nerve remains stationary on the coverslip overlying the 63x Plan-Apochromat oil immersion objective lens (Zeiss). An area containing several axons retrogradely transporting the fluorescent HCT probe was selected and imaged every 2.4-3.2 s at 100x digital zoom (1024x1024, 1% laser power). All imaging was completed within 1 h of initiating terminal anesthesia.
Axonal transport analysis
Confocal image series were converted into .avi files and individual endosome dynamics manually tracked using Tracker (Kinetic Imaging Ltd.) (Figure S1A). Endosomes were included in the analysis if they could be observed for ≥ 5 consecutive frames and did not pause for > 10 consecutive images. Endosomes that were tracked and then paused for long periods were not included for fear of issues associated with phototoxicity. Nevertheless, on average fewer than one endosome per animal did this and the phenotype was not linked to a particular genotype. Moreover, endosomes moving solely in the anterograde direction were also not included as they were similarly infrequent. All individual frame-to-frame step speeds are included in the presented speed frequency histograms (459.6 ± 11.4 frame-to-frame speeds per animal were calculated), meaning that an endosome tracked across 11 consecutive frames will generate 10 frame-to-frame speeds to be included in the frequency histogram. To determine the mean endosome speed per animal, the speeds of individual endosomes were calculated and then an average of these speeds determined (50.3 ± 0.8 endosomes per animal were tracked). The fastest endosome speed per animal is reported as the ‘maximum speed’. All speed analyses include frames and time during which endosomes may have been paused, i.e., we report the speed across the entire tracked run length and not the speed solely when motile. An endosome was considered to have paused if it remained in the same position for two consecutive images. The ‘% time paused’ is a calculation of the length of time all tracked endosomes remained stationary, while the ‘% pausing endosomes’ details the proportion of endosomes that displayed at least one pause while being tracked. At least six endosomes from at least two individual, thick axons were assessed per animal.
Axon calibre analysis
Axon calibres were determined from images taken for endosome transport analyses by measuring the distance between the upper and lower margins of transported fluorescent signaling endosomes orthogonally from the direction of transport. A minimum of ten measurements were made along the length of the axon to calculate average widths per axon, and three different axons per animal were used to calculate a per animal mean width.
Quantification and Statistical Analysis
Data were assumed to be normally distributed unless evidence to the contrary could be provided by the D’Agostino and Pearson omnibus normality test. Normally distributed data were statistically analyzed using a t test or one-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons test, and non-normally distributed data with a Mann-Whitney U test or Kruskal-Wallis test with Dunn’s multiple comparisons test. Paired t tests were used to compare transport kinetics in ChAT+ versus ChAT- axons as data were generated from the same animals. Endosomes were tracked from videos in which the genotype of the animal was blinded. All tests were two-tailed and an α-level of p < 0.05 was used to determine significance. GraphPad Prism 6 software was used for all statistical analyses and figure production. All figure legends contain details of statistical tests and sample sizes (i.e. number of animals) used, with dispersion and precision measures.
Data and Code Availability
This study did not generate/analyze datasets/code.
Acknowledgments
We would like to thank Robert M. Brownstone (UCL Queen Square Institute of Neurology, University College London) for sharing the ChAT-eGFP mice. This work was supported by the Wellcome Trust Sir Henry Wellcome Postdoctoral Fellowship (103191/Z/13/Z) (J.N.S.), the Medical Research Council Career Development Award (MR/S006990/1) (J.N.S.), the Motor Neurone Disease Association (D.G., A.D., P.F., E.M.C.F., and K.T.), the Medical Research Council (A.D., P.F., and E.M.C.F.), the American Amyotrophic Lateral Sclerosis Association (A.D. and E.M.C.F.), the Rosetrees Trust (P.F. and E.M.C.F.), the Wellcome Trust Senior Investigator Award (107116/Z/15/Z) (G.S.), the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement 739572 (G.S.), and a UK Dementia Research Institute Foundation award (G.S.).
Author Contributions
J.N.S. and A.P.T. performed the research and analyzed the data. D.G., A.D., P.F., E.M.C.F., and K.T. provided mice and expertise. J.N.S., A.P.T., and G.S. wrote the paper with input from all authors. All authors approved submission of this manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: March 17, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.02.078.
Contributor Information
James N. Sleigh, Email: j.sleigh@ucl.ac.uk.
Giampietro Schiavo, Email: giampietro.schiavo@ucl.ac.uk.
Supporting Citations
The following reference appears in the Supplemental Information: Gurney et al., 1994, Wegorzewska et al., 2009.
Supplemental Information
References
- Al-Chalabi A., Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat. Rev. Neurol. 2013;9:617–628. doi: 10.1038/nrneurol.2013.203. [DOI] [PubMed] [Google Scholar]
- Alami N.H., Smith R.B., Carrasco M.A., Williams L.A., Winborn C.S., Han S.S.W., Kiskinis E., Winborn B., Freibaum B.D., Kanagaraj A. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014;81:536–543. doi: 10.1016/j.neuron.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin K.R., Godena V.K., Hewitt V.L., Whitworth A.J. Axonal transport defects are a common phenotype in Drosophila models of ALS. Hum. Mol. Genet. 2016;25:2378–2392. doi: 10.1093/hmg/ddw105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumer D., Hilton D., Paine S.M.L., Turner M.R., Lowe J., Talbot K., Ansorge O. Juvenile ALS with basophilic inclusions is a FUS proteinopathy with FUS mutations. Neurology. 2010;75:611–618. doi: 10.1212/WNL.0b013e3181ed9cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland L.G., Sahai E., Kelly G., Golding M., Greensmith L., Schiavo G. Deficits in axonal transport precede ALS symptoms in vivo. Proc. Natl. Acad. Sci. USA. 2010;107:20523–20528. doi: 10.1073/pnas.1006869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfman F.C., Escudero C.A., Weis J., Kruttgen A. Endosomal transport of neurotrophins: roles in signaling and neurodegenerative diseases. Dev. Neurobiol. 2007;67:1183–1203. doi: 10.1002/dneu.20513. [DOI] [PubMed] [Google Scholar]
- Brown R.H., Al-Chalabi A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2017;377:162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- Clark J.A., Yeaman E.J., Blizzard C.A., Chuckowree J.A., Dickson T.C. A case for microtubule vulnerability in amyotrophic lateral sclerosis: altered dynamics during disease. Front. Cell. Neurosci. 2016;10:204. doi: 10.3389/fncel.2016.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombrita C., Onesto E., Megiorni F., Pizzuti A., Baralle F.E., Buratti E., Silani V., Ratti A. TDP-43 and FUS RNA-binding proteins bind distinct sets of cytoplasmic messenger RNAs and differently regulate their post-transcriptional fate in motoneuron-like cells. J. Biol. Chem. 2012;287:15635–15647. doi: 10.1074/jbc.M111.333450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos K.J., Hafezparast M. Neurobiology of axonal transport defects in motor neuron diseases: Opportunities for translational research? Neurobiol. Dis. 2017;105:283–299. doi: 10.1016/j.nbd.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaisieux S., Encheva V., Chakravarty P., Snijders A.P., Schiavo G. Analysis of signaling endosome composition and dynamics using SILAC in embryonic stem cell-derived neurons. Mol. Cell. Proteomics. 2016;15:542–557. doi: 10.1074/mcp.M115.051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhardt K., Salinas S., Verastegui C., Watson R., Worth D., Hanrahan S., Bucci C., Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Devoy A., Kalmar B., Stewart M., Park H., Burke B., Noy S.J., Redhead Y., Humphrey J., Lo K., Jaeger J. Humanized mutant FUS drives progressive motor neuron degeneration without aggregation in ‘FUSDelta14’ knockin mice. Brain. 2017;140:2797–2805. doi: 10.1093/brain/awx248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows A.D., Rhymes E.R., Gibbs K.L., Greensmith L., Schiavo G. IGF1R regulates retrograde axonal transport of signalling endosomes in motor neurons. EMBO Rep. 2020 doi: 10.15252/embr.201949129. Published online February 7, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiesel F.C., Voigt A., Weber S.S., Van den Haute C., Waldenmaier A., Görner K., Walter M., Anderson M.L., Kern J.V., Rasse T.M. Knockdown of transactive response DNA-binding protein (TDP-43) downregulates histone deacetylase 6. EMBO J. 2010;29:209–221. doi: 10.1038/emboj.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratta P., Sivakumar P., Humphrey J., Lo K., Ricketts T., Oliveira H., Brito-Armas J.M., Kalmar B., Ule A., Yu Y. Mice with endogenous TDP-43 mutations exhibit gain of splicing function and characteristics of amyotrophic lateral sclerosis. EMBO J. 2018;37:e98684. doi: 10.15252/embj.201798684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs K.L., Kalmar B., Sleigh J.N., Greensmith L., Schiavo G. In vivo imaging of axonal transport in murine motor and sensory neurons. J. Neurosci. Methods. 2016;257:26–33. doi: 10.1016/j.jneumeth.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs K.L., Kalmar B., Rhymes E.R., Fellows A.D., Ahmed M., Whiting P., Davies C.H., Greensmith L., Schiavo G. Inhibiting p38 MAPK alpha rescues axonal retrograde transport defects in a mouse model of ALS. Cell Death Dis. 2018;9:596. doi: 10.1038/s41419-018-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Dafinca R., Scaber J., Alegre-Abarrategui J., Farrimond L., Scott C., Biggs D., Kent L., Oliver P.L., Davies B. Single-copy expression of an amyotrophic lateral sclerosis-linked TDP-43 mutation (M337V) in BAC transgenic mice leads to altered stress granule dynamics and progressive motor dysfunction. Neurobiol. Dis. 2019;121:148–162. doi: 10.1016/j.nbd.2018.09.024. [DOI] [PubMed] [Google Scholar]
- Grice S.J., Sleigh J.N., Liu J.L., Sattelle D.B. Invertebrate models of spinal muscular atrophy: insights into mechanisms and potential therapeutics. BioEssays. 2011;33:956–965. doi: 10.1002/bies.201100082. [DOI] [PubMed] [Google Scholar]
- Guo W., Naujock M., Fumagalli L., Vandoorne T., Baatsen P., Boon R., Ordovás L., Patel A., Welters M., Vanwelden T. HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat. Commun. 2017;8:861. doi: 10.1038/s41467-017-00911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney M.E., Pu H., Chiu A.Y., Dal Canto M.C., Polchow C.Y., Alexander D.D., Caliendo J., Hentati A., Kwon Y.W., Deng H.X. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Lagier-Tourenne C., Polymenidou M., Hutt K.R., Vu A.Q., Baughn M., Huelga S.C., Clutario K.M., Ling S.-C., Liang T.Y., Mazur C. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nat. Neurosci. 2012;15:1488–1497. doi: 10.1038/nn.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Byrd A., Warner A.N., Pei F., Basha E., Buchanan A., Buchan J.R. Cdc48/VCP and endocytosis regulate TDP-43 and FUS toxicity and turnover. Mol. Cell. Biol. 2020;40 doi: 10.1128/MCB.00256-19. e00256-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S., Twelvetrees A.E., Moughamian A.J., Holzbaur E.L.F. Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron. 2014;84:292–309. doi: 10.1016/j.neuron.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrané J., Cortez C., Gan W.-B., Manfredi G. Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Hum. Mol. Genet. 2014;23:1413–1424. doi: 10.1093/hmg/ddt528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik B., Nirmalananthan N., Bilsland L.G., La Spada A.R., Hanna M.G., Schiavo G., Gallo J.-M., Greensmith L. Absence of disturbed axonal transport in spinal and bulbar muscular atrophy. Hum. Mol. Genet. 2011;20:1776–1786. doi: 10.1093/hmg/ddr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan R., Tosolini A.P., Morris R. Targeting the motor end plates in the mouse hindlimb gives access to a greater number of spinal cord motor neurons: an approach to maximize retrograde transport. Neuroscience. 2014;274:318–330. doi: 10.1016/j.neuroscience.2014.05.045. [DOI] [PubMed] [Google Scholar]
- Nijssen J., Comley L.H., Hedlund E. Motor neuron vulnerability and resistance in amyotrophic lateral sclerosis. Acta Neuropathol. 2017;133:863–885. doi: 10.1007/s00401-017-1708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton A.E., Chiò A., Traynor B.J. State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 2014;17:17–23. doi: 10.1038/nn.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restani L., Giribaldi F., Manich M., Bercsenyi K., Menendez G., Rossetto O., Caleo M., Schiavo G. Botulinum neurotoxins A and E undergo retrograde axonal transport in primary motor neurons. PLoS Pathog. 2012;8:e1003087. doi: 10.1371/journal.ppat.1003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sama R.R.K., Fallini C., Gatto R., McKeon J.E., Song Y., Rotunno M.S., Penaranda S., Abdurakhmanov I., Landers J.E., Morfini G. ALS-linked FUS exerts a gain of toxic function involving aberrant p38 MAPK activation. Sci. Rep. 2017;7:115. doi: 10.1038/s41598-017-00091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh J., Schiavo G. Older but not slower: aging does not alter axonal transport dynamics of signalling endosomes in vivo. Matters. 2016 https://sciencematters.io/articles/201605000018 [Google Scholar]
- Sleigh J.N., Vagnoni A., Twelvetrees A.E., Schiavo G. Methodological advances in imaging intravital axonal transport. F1000Res. 2017;6:200. doi: 10.12688/f1000research.10433.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh J.N., Rossor A.M., Fellows A.D., Tosolini A.P., Schiavo G. Axonal transport and neurological disease. Nat. Rev. Neurol. 2019;15:691–703. doi: 10.1038/s41582-019-0257-2. [DOI] [PubMed] [Google Scholar]
- Surana S., Tosolini A.P., Meyer I.F.G., Fellows A.D., Novoselov S.S., Schiavo G. The travel diaries of tetanus and botulinum neurotoxins. Toxicon. 2018;147:58–67. doi: 10.1016/j.toxicon.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Tateno M., Kato S., Sakurai T., Nukina N., Takahashi R., Araki T. Mutant SOD1 impairs axonal transport of choline acetyltransferase and acetylcholine release by sequestering KAP3. Hum. Mol. Genet. 2009;18:942–955. doi: 10.1093/hmg/ddn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosolini A.P., Sleigh J.N. Motor neuron gene therapy: lessons from spinal muscular atrophy for amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2017;10:405. doi: 10.3389/fnmol.2017.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel-Campos D., Schiavo G., Lazo O.M. The many disguises of the signalling endosome. FEBS Lett. 2018;592:3615–3632. doi: 10.1002/1873-3468.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R., Manion J., Neely G.G. Dissecting motor neuron disease with Drosophila melanogaster. Front. Neurosci. 2019;13:331. doi: 10.3389/fnins.2019.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Li L., Lin W.-L., Dickson D.W., Petrucelli L., Zhang T., Wang X. The ALS disease-associated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons. Hum. Mol. Genet. 2013;22:4706–4719. doi: 10.1093/hmg/ddt319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegorzewska I., Bell S., Cairns N.J., Miller T.M., Baloh R.H. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc. Natl. Acad. Sci. U S A. 2009;106:18809–18814. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M.A., Kim E., Duffy A., Adalbert R., Phillips B.U., Peters O.M., Stephenson J., Yang S., Massenzio F., Lin Z. TDP-43 gains function due to perturbed autoregulation in a Tardbp knock-in mouse model of ALS-FTD. Nat. Neurosci. 2018;21:552–563. doi: 10.1038/s41593-018-0113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M.G., Finelli M.J., Sleigh J.N., Reddington A., Gordon D., Talbot K., Davies K.E., Oliver P.L. Neuronal over-expression of Oxr1 is protective against ALS-associated mutant TDP-43 mislocalisation in motor neurons and neuromuscular defects in vivo. Hum. Mol. Genet. 2019;28:3584–3599. doi: 10.1093/hmg/ddz190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Takashima H. Drosophila Charcot-Marie-Tooth disease models. Adv. Exp. Med. Biol. 2018;1076:97–117. doi: 10.1007/978-981-13-0529-0_7. [DOI] [PubMed] [Google Scholar]
- Zhang F., Ström A.-L., Fukada K., Lee S., Hayward L.J., Zhu H. Interaction between familial amyotrophic lateral sclerosis (ALS)-linked SOD1 mutants and the dynein complex. J. Biol. Chem. 2007;282:16691–16699. doi: 10.1074/jbc.M609743200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets/code.