Abstract
Angelica keiskei (Miq.) Koidz. (Umbelliferae) has traditionally been used to treat dysuria, dyschezia, and dysgalactia as well as to restore vitality. Recently, the aerial parts of A. keiskei have been consumed as a health food. Various flavonoids, coumarins, phenolics, acetylenes, sesquiterpene, diterpene, and triterpenes were identified as the constituents of A. keiskei. The crude extracts and pure constituents were proven to inhibit tumor growth and ameliorate inflammation, obesity, diabetics, hypertension, and ulcer. The extract also showed anti-thrombotic, anti-oxidative, anti-hyperlipidemic, anti-viral, and anti-bacterial activities. This valuable herb needs to be further studied and developed not only to treat these human diseases but also to improve human health. Currently A. keiskei is commercialized as a health food and additives in health drinks. This article presents a comprehensive review of A. keiskei and its potential place in the improvement of human health.
Keywords: Angelica keiskei, Umbelliferae, Chemical constituent, Biological activity
Introduction
Angelica keiskei (Miq.) Koidz. is a hardy perennial, belonging to the family Umbelliferae. A. keiskei has traditionally been used as a diuretic, mild cathartic, tonic, and galactagogue (Kimura and Baba 2003). The herb is called ‘Myeong-Il Yeob’ in Korea and ‘Ashitaba’ in Japan, both literally meaning ‘Tomorrow’s Leaf’ (Baba et al. 1998). Another common name for A. keiskei is ‘Shin-Sun Cho’, which means ‘a precious herb used by God’. A. keiskei is mainly distributed in Asian countries including Korea and Japan (Park 2013). The aerial parts are mainly used as a source of green vegetable juice as a daily health food and the young leaves of the aerial parts are used by parboiling or deep-frying (Imai and Imai 2008). The aerial parts are also used in tea, flour, wine, and cosmetics (Bao 2014; Su 2014; Ku et al. 2014; Liu et al. 2014). This review paper summarizes botanical characteristics, phytochemical research, as well as biological studies on the extracts and pure constituents of A. keiskei. The extracts have been reported to have anti-inflammatory, anti-obesity, anti-oxidative, anti-coagulant, anti-tumor, anti-mutagenic, anti-diabetic, anti-bacterial, and hepato-protective activities. The biological activities of each pure compounds are also separately summarized in this review.
Botanical characteristics of A. keiskei
Angelica keiskei has glossy leaves and flowers from summer to fall. The leaves are harvested from spring to fall before they lose the gloss. A yellow exudate is squeezed out of the stems when the leaves are harvested (Imai and Imai 2008). This yellow color makes it easy to distinguish A. keiskei from A. japonica, an exudate of which shows a nearly white color (Kimura et al. 2008). The characteristic botanical features of A. keiskei are described in Flora of Japan:
Rhizomes thick and short, with few elongate roots. Stems stout, branched above, 80–120 cm long, glabrous. Leaves radical and lower cauline, usually evergreen, 2–3-ternate-pinnate, obtusely deltoid in outline, 20–60 cm. long, glabrous; leaflets rather thick, usually deeply 2–3 cleft of parted; segments usually ovate, 5–10 cm long, 3–6 cm wide, acute, toothed, with impressed veinlets on upper side; sheaths on the upper part elliptic, inflated. Inflorescence umbels rather numerous; peduncles in upper part; the inner side of rays and pedicels puberulent; rays 10–20; pedicels 20–40; bracteoles of involucel several, linear or slightly broaden at base, caudately elongate, slightly longer or shorter than pedicels; styles short, as long as stylopodium. Fruit oblong, glabrous, 6–8 mm long, flat; dorsal ribs short and rather thick; lateral ribs winged; vittae 1 in intervals, 4 on the commissure (Oi et al. 1965).
Phytochemical investigations on A. keiskei
Previous phytochemical investigations on A. keiskei have led to the identification of over 100 compounds including various types of flavonoids (1–31, 41–56), coumarins (57–92), phenolic (96), acetylenes (98–102), sesquiterpene (103), diterpene (104), and triterpenes (105–106). Structures of the reported constituents listed in Tables 1, 2 and 3 are shown in Fig. 1. In our recent study on the aerial parts of A. keiskei, eight constituents, 4,2′,4′-trihydroxy-3′-[(2E,5E)-7-methoxy-3,7-dimethyl-2,5-octadienyl]chalcone (32), (±)-4,2′,4′-trihydroxy-3′-[(2E)-6-hydroxy-7-methoxy-3,7-dimethyl-2-octenyl]chalcone (33), 4,2′,4′-trihydroxy-3′-[(2E)-3-methyl-5-(1,3-dioxolan-2-yl)-2-pentenyl]chalcone (34), 2′,3′-furano-4-hydroxy-4′-methoxychalcone (35), (±)-4-hydroxy-2′,3′-(2,3-dihydro-2-methoxyfurano)-4′-methoxychalcone (36), 3′-carboxymethyl-4,2′-dihydroxy-4′-methoxychalcone (37), (±)-4,2′,4′-trihydroxy-3′-[(3-hydroxy-2,2-dimethyl-6-methylenecyclohexyl)methyl]chalcone (38), and 2″-hydroxyangelichalcone (39) were isolated and reported as new compounds from an ethyl acetate extract along with eight known compounds, xanthoangelol (1), xanthoangelol F (2), 4-hydroxyderricin (3), xanthoangelol G (6), xanthoangelol D (9), xanthoangelol E (10), xanthoangelol H (12), (±)-4,2′,4′-trihydroxy-3′-[(6E)-2-hydroxy-7-methyl-3-methylene-6-octenyl]chalcone (27), artocarmitin A (40), (+)-cis-(3′R,4′R)-methylkhellactone (93), (-)-trans-(3′R,4′S)-methylkhellactone (94), 3,4-dihydroxanthotoxin (95), and (Z)-p-coumaryl alcohol (97) (Kil et al. 2015a, 2016). In particular, xanthoangelol (1) and 4-hydroderricin (3) were isolated in bulk (1: 5 g, 2: 1.8 g), which supported that the two chalcones are the predominant constituents in this herb. As a part of our research on the aerial parts of A. keiskei, an effective method was developed to prepare two chalcones from the crude extract using high-speed counter-current chromatography (two-phase solvent system: n-hexane–ethyl acetate–methanol–water 9:5:9:4, revolution rate: 800 rpm, flow rate: 1.5 mL/min) (Kil et al. 2015b).
Table 1.
The reported biological activities of xanthoangelol (1)
| Parta | Biological activity | Reference(s) |
|---|---|---|
| RT | First isolation from the part | Kozawa et al. (1977) |
| RT | Anti-ulcer activity: gastric H+–K+ ATPase | Murakami et al. (1990) |
| RT | Anti-bacterial activity: gram-positive bacteria | Inamori et al. (1991) |
| Syn | Anti-bacterial activity: gram-positive bacteria | Sugamoto et al. (2011) |
| RT | Anti-tumor-promoter activity: TPA-stimulated 32Pi-incorporation, Ca+-calmodulin system | Okuyama et al. (1991) |
| EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2003) |
| RT | Anti-tumor and anti-metastatic activities: DNA synthesis, tumor-induced neovascularization, binding of VEGF to HUVECs | Kimura and Baba (2003) |
| EXD | Anti-tumor activity: apoptosis (caspase-3) in human neuroblastoma (IMR-32), leukemia (Jurkat) cells | Tabata et al. (2005) |
| EXD | Anti-tumor activity: apoptosis (caspase-9, cytochrome c), ROS, DJ-1 protein in human neuroblastoma (IMR-32) cells | Motani et al. 2008) |
| EXD, ST | Cytotoxicities: human neuroblastoma cells (LA-N-1, NB-39: drug-resistant; IMR-32, SK-N-SH: drug-sensitive) | Motani et al. (2008), Nishimura et al. (2007) |
| ST | Anti-tumor activity: apoptosis (DNA fragmentation) in human stomach cancer (KATO III) cells | Takaoka et al. (2008) |
| AP | Anti-diabetic activity: insulin-like activity | Enoki et al. (2007) |
| RT | Anti-diabetic activity: GLUT4 translocation | Kawabata et al. (2011) |
| AP | Anti-diabetic activity: α-glucosidase | Luo et al. (2012) |
| ST | Anti-diabetic activity: PTP1B | Li et al. (2015) |
| RT | Anti-hypertensive activity: vasoconstriction in rat aortic rings | Matsuura et al. (2001) |
| RT | Preventive effect against metabolic syndrome: adiponectin production | Ohnogi et al. (2012b) |
| RT | Preventive effect against metabolic syndrome: adipocytes differentiation (AMPK, MAPK pathways; C/EBPs, PPARγ) | Zhang et al. (2013) |
| EXD | Anti-inflammatory activity (details in the patent) | Akihisa et al. (2007) |
| RT | Anti-inflammatory activity: NO, TNF-α, iNOS, COX-2, AP-1 | Yasuda et al. (2014) |
| LF | Anti-inflammatory activity: IL-6 in TNF-α-stimulated osteosarcoma cells | Shin et al. (2011) |
| AP | Anti-oxidative activity: NQO1 induction, DPPH radical scavenging | Luo et al. (2012) |
| ST | Anti-oxidative activity: XO | Kim et al. (2014) |
| ST | Anti-thrombotic activity: PAI-1 | Ohkura et al. (2011) |
| RT | Anti-thrombotic activity: platelet aggregation (induced by collagen, platelet-activating factor, phorbol 12-myristate 13-acetate, not thrombin) | Son et al. (2014) |
| ST | Whitening activity: melanin formation in B16 melanoma cells | Arung et al. (2012) |
| AP | Anti-depressant activity: MAO (nonselective), DBH | Kim et al. (2013) |
| WP | Anti-viral activity: SARS-CoV (viral proteases: 3CLpro, PLpro) | Park et al. (2015) |
aPlant part code: WP whole plant, RT root, AP aerial part (including stem and leaf), ST stem, LF leaf, EXD exudate; other source code: Syn synthetic
Table 2.
The reported biological activities of 4-hydroxyderricin (3)
| Parta | Biological activity | Reference(s) |
|---|---|---|
| RT | First isolation from the part | Kozawa et al. (1977) |
| RT | Anti-ulcer activity: gastric H+–K+ ATPase | Murakami et al. (1990) |
| RT | Anti-bacterial activity: gram-positive bacteria | Inamori et al. (1991) |
| Syn | Anti-bacterial activity: gram-positive bacteria | Sugamoto et al. (2011) |
| Syn | Anti-bacterial activity: S. aureus seryl-tRNA synthetase | Battenberg et al. (2013) |
| RT | Anti-tumor-promoter activity: TPA-stimulated 32Pi-incorporation, Ca+-calmodulin system | Okuyama et al. (1991) |
| EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2003) |
| ST | Anti-tumor activity: apoptosis (DNA fragmentation) in human stomach cancer (KATO III) cells | Takaoka et al. (2008) |
| RT | Anti-tumor and anti-metastatic activities: DNA synthesis, tumor-induced neovascularization, lymphocytes, CD4+, CD8+, NK-T cells | Kimura et al. (2004) |
| RT |
Anti-tumor activity: apoptosis (DNA topoisomerase, procasparases-3, -8, -9 in human leukemia (HL60) cells Cytotoxicities: human leukemia (HL60), melanoma (CRL1579), lung cancer (A549), stomach cancer (AZ521) cells |
Akihisa et al. (2011) |
| ST | Cytotoxicities: human neuroblastoma cells (IMR-32, NB-39) | Nishimura et al. (2007) |
| RT | Anti-hypertensive activity: vasoconstriction in rat aortic rings | Matsuura et al. (2001) |
| ST |
Anti-hypertensive activity: systolic blood pressure in SHRSP Anti-hyperlipidemic activity: serum VLDL (microsomal TG transfer-protein), hepatic TG (differentiation factor 1, fatty acid synthase) levels in SHRSP |
Ogawa et al. (2005b) |
| AP | Anti-diabetic activity: insulin-like activity | Enoki et al. (2007) |
| RT | Anti-diabetic activity: chronic ingestion on borderline mild hyperglycemia (adiponectin) | Ohnogi et al. (2007) |
| RT | Anti-diabetic activity: GLUT4 translocation | Kawabata et al. (2011) |
| AP | Anti-diabetic activity: α-glucosidase | Luo et al. (2012) |
| ST | Anti-diabetic activity: PTP1B | Li et al. (2015) |
| EXD | Anti-inflammatory activity (details in the patent) | Akihisa et al. (2007) |
| LF | Anti-inflammatory activity: IL-6 in TNF-α-stimulated osteosarcoma cells | Shin et al. (2011) |
| RT, AP | Anti-inflammatory activity: NO, TNF-α, iNOS, COX-2, AP-1 | Yasuda et al. (2014), Chang et al. (2014) |
| AP | Anti-inflammatory activity: I-κB degradation, NF-κB nuclear translocation | Chang et al. (2014) |
| RT | Anti-thrombotic activity: platelet aggregation (induced by collagen, platelet-activating factor, phorbol 12-myristate 13-acetate, not thrombin) | Son et al. (2014) |
| LF | Anti-viral activity: influenza virus neuraminidase | Park et al. (2011) |
| WP | Anti-viral activity: SARS-CoV (viral proteases: 3CLpro, PLpro) | Park et al. (2015) |
| RT | Preventive effect against metabolic syndrome: adiponectin production | Ohnogi et al. (2012b) |
| RT | Preventive effect against metabolic syndrome: adipocytes differentiation (AMPK, MAPK pathways; C/EBPs, PPARγ) | Zhang et al. (2013) |
| AP | Anti-oxidative activity: NQO1 induction | Luo et al. (2012) |
| WP | Anti-oxidative activity: XO | Kim et al. (2014) |
| ST | Whitening activity: melanin formation in B16 melanoma cells | Arung et al. (2012) |
| AP | Anti-depressant activity: inhibition of MAO-B (selective), DBH | Kim et al. (2013) |
aPlant part code: WP whole plant, RT root, AP aerial part (including stem and leaf), ST stem, LF leaf, EXD exudate; other source code: Syn synthetic
Table 3.
Constituents from A. keiskei and their reported biological activities
| Compound class/name | Parta | Biological activity | Reference(s) |
|---|---|---|---|
| Flavonoids | |||
| Chalcones | |||
| Xanthoangelol F (2) | RT | First isolation from the part | Nakata et al. (1999) |
| RT | Anti-hypertensive activity: phenylephrine-induced vasoconstriction in rat aortic rings | Matsuura et al. (2001) | |
| EXD |
Anti-tumor-promoter activity: EBV-EA by TPA Anti-tumor-initiator activity: NOR 1 |
Akihisa et al. (2003) | |
| EXD | Anti-inflammatory activity (details in the patent) | Akihisa et al. (2007) | |
| ST | Cytotoxicities: human neuroblastoma (IMR-32, NB-39) | Nishimura et al. (2007) | |
| Syn | Anti-bacterial activity: Gram-positive bacteria | Sugamoto et al. (2011) | |
| AP | Anti-viral activity: influenza virus neuraminidase | Park et al. (2011) | |
| WP | Anti-viral activity: SARS-CoV (3CLpro, PLpro) | Park et al. (2015) | |
| WP | Anti-oxidative activity: XO | Kim et al. (2014) | |
| ST | Anti-diabetic activity: PTP1B | Li et al. (2015) | |
| Isobavachalcone (4) | RT | First isolation from the part | Nakata et al. (1999) |
| EXD | Anti-tumor-promoter activity: EBV-EA by TPA, in vivo | Akihisa et al. (2003) | |
|
Two-stage mouse skin carcinogenesis test Anti-tumor-initiator activity: NOR 1 |
Akihisa et al. (2006) | ||
| ST | Anti-tumor activity: apoptosis (procaspase-3, -9, Bax) in human neuroblastoma cells (IMR-32, NB-39) | Nishimura et al. (2007) | |
| EXD | Anti-inflammatory activity (details in the patent) | Akihisa et al. (2007) | |
| Com | Anti-inflammatory activity: iNOS | Shin et al. (2013) | |
| Syn | Anti-bacterial activity: Gram-positive bacteria | Sugamoto et al. (2011) | |
| AP | Anti-oxidative activity: NQO1 induction, DPPH radical scavenging | Luo et al. (2012) | |
| WP | Anti-oxidative activity: XO | Kim et al. (2014) | |
| AP | Anti-diabetic activity: α-glucosidase | Luo et al. (2012) | |
| WP | Anti-viral activity: SARS-CoV (3CLpro, PLpro) | Park et al. (2015) | |
| Xanthoangelol B (5) | RT | First isolation from the part | Baba et al. (1990b) |
| RT | Anti-hypertensive activity: phenylephrine-induced vasoconstriction in rat aortic rings | Matsuura et al. (2001) | |
| RT | Anti-allergic activity: compound 48/80-induced histamine release | Nakata and Baba (2001) | |
| ST | Anti-oxidative activity: superoxide-scavenging | Aoki et al. (2008) | |
| WP | Anti-oxidative activity: XO | Kim et al. (2014) | |
| LF | Anti-inflammatory activity: IL-6 in TNF-α-stimulated osteosarcoma cells | Shin et al. (2011) | |
| AP | Anti-inflammatory activity: NO, iNOS, COX-2 (IκB degradation, NF-κB nuclear translocation) | Chang et al. (2014) | |
| AP | Anti-viral activity: influenza virus neuraminidase | Park et al. (2011) | |
| ST | Anti-thrombotic activity: TNFα-induced PAI-1 | Ohkura et al. (2011) | |
| WP | Anti-viral activity: SARS-CoV (3CLpro, PLpro) | Park et al. (2015) | |
| Xanthoangelol G (6) | RT | First isolation from the part | Nakata et al. (1999) |
| AP | Anti-viral activity: influenza virus neuraminidase | Park et al. (2011) | |
| WP | Anti-viral activity: SARS-CoV (3CLpro, PLpro) | Park et al. (2015) | |
| 4,2′-Dihydroxy-3′-[(2E)-6-hydroperoxy-3-methyl-7-methylene-2-octenyl]-4′-methoxy chalcone (7) | RT | Preventive effect against metabolic syndrome: adiponectin production | Ohnogi et al. (2012b) |
| Xanthoangelol C (8) | RT | Baba et al. (1990b) | |
| RT | Anti-allergic activity: compound 48/80-induced histamine | Nakata and Baba (2001) | |
| Xanthoangelol D (9) | RT | First isolation from the part | Baba et al. (1990b) |
| RT | Anti-inflammatory activity: TNF-α induced ET-1 (NF-κB) | Sugii et al. (2005) | |
| AP | Anti-viral activity: influenza virus neuraminidase | Park et al. (2011) | |
| ST | Anti-thrombotic activity: TNFα-induced PAI-1 | Ohkura et al. (2011) | |
| WP | Anti-viral activity: SARS-CoV (3CLpro, PLpro) | Park et al. (2015) | |
| ST | Anti-diabetic activity: PTP1B | Li et al. (2015) | |
| Xanthoangelol E (10) | RT | First isolation from the part | Baba et al. (1990b) |
| RT | Anti-ulcer activity (details in the patent) | Murakami et al. (1992) | |
| RT | Anti-thrombotic activity: arachidonic acid metabolism in platelets | Fujita et al. (1992) | |
| RT | Anti-hypertensive activity: phenylephrine-induced vasoconstriction in rat aortic rings | Matsuura et al. (2001) | |
| RT | Anti-allergic activity: compound 48/80-induced histamine | Nakata and Baba (2001) | |
| LF | First isolation from the part | Shin et al. (2011) | |
| AP | Anti-inflammatory activity: NO, iNOS, COX-2 (IκB degradation, NF-κB nuclear translocation) | Chang et al. (2014) | |
| WP | Anti-viral activity: SARS-CoV (3CLpro, PLpro) | Park et al. (2015) | |
| ST | Anti-diabetic activity: PTP1B | Li et al. (2015) | |
| Ashitaba-chalcone (11) | RT | Anti-tumor-promoter activity: TPA-stimulated 32Pi-incorporation | Okuyama et al. (1991) |
| Xanthoangelol H (12) | RT | First isolation from the part | Nakata et al. (1999) |
| EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2003) | |
| ST | Cytotoxicities: human neuroblastoma (IMR-32, NB-39) | Nishimura et al. (2007) | |
| ST | Whitening activity: melanin formation in melanoma cells | Arung et al. (2012) | |
| Deoxyxanthoangelol H (13) | EXD | First isolation from the part | Akihisa et al. (2006) |
| EXD | Anti-inflammatory activity (details in the patent) | Akihisa et al. (2007) | |
| ST | Whitening activity: melanin formation in melanoma cells | Arung et al. (2012) | |
| Xanthoangelol I (14) | EXD |
Anti-tumor-promoter activity: EBV-EA by TPA Anti-tumor-initiator activity: NOR 1 |
Akihisa et al. (2006) |
| ST | Cytotoxicities: human neuroblastoma (IMR-32, NB-39) | Nishimura et al. (2007) | |
| 2″-Hydroxy-xanthoangelol I (15) | RT | Protective effect on nerve cells (details in the patent) | Onogi et al. (2004) |
| RT | Preventive effect against metabolic syndrome: adiponectin | Ohnogi et al. (2012b) | |
| Xanthoangelol J (16) | EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2006) |
| Anti-tumor-initiator activity: NOR 1 | |||
| Xanthoangelol K (17) | ST | Anti-diabetic activity: PTP1B | Li et al. (2015) |
| Xanthoangelol L (18) | ST | First isolation from the part | Li et al. (2015) |
| Jejuchalcone D (19) | ST | First isolation from the part | Li et al. (2015) |
| Xanthoangelol M (20) | ST | Anti-diabetic activity: PTP1B | Li et al. (2015) |
| 4-Hydroxy-2′,3′-(2,3-dihydro-2-hydroxy isopropylfurano)-4′-methoxychalcone (21) | RT | Preventive effect against metabolic syndrome: adiponectin production | Ohnogi et al. (2012b) |
| Jejuchalcone E (22) | ST | First isolation from the part | Li et al. (2015) |
| Dorsmannin A (23) | EXD | First isolation from the part | Akihisa et al. (2006) |
| Xanthokeismin A (24) | ST | Anti-oxidative activity: superoxide-scavenging | Aoki et al. (2008) |
| AP | Anti-inflammatory activity: NO, iNOS, COX-2 (IκB degradation, NF-κB nuclear translocation) | Chang et al. (2014) | |
| Xanthokeismin B (25) | ST | Anti-oxidative activity: superoxide-scavenging | Aoki et al. (2008) |
| Xanthokeismin C (26) | ST | Anti-oxidative activity: superoxide-scavenging | Aoki et al. (2008) |
| 4,2′,4′-Trihydroxy-3′-[(6E)-2-hydroxy-7-methyl-3-methylene-6-octenyl]chalcone (27) |
LF AP |
Anti-inflammatory activity: IL-6 in TNF-α-stimulated osteosarcoma cells Protective activity on damaged normal cells: HSPs |
Shin et al. (2011) Kil et al. (2015a) |
| Xanthokeistal A (28) |
AP WP |
Anti-viral activity: influenza virus neuraminidase Anti-viral activity: SARS-CoV (3CLpro, PLpro) |
Park et al. (2011) Park et al. (2015) |
| 4,2′4′-Trihydroxy-3′-[(2E)-6,7-dihydroxy-3,7-dimethyl-2-octenyl] chalcone (29) |
RT AP |
First isolation from the part First isolation from the part |
Ohnogi et al. (2012b) Luo et al. (2012) |
|
4,2′-Dihydroxy-3′,4′-{2,3-dihydro-2-[(4E)-1-hydroxy-1,5-dimethyl-4-hexenyl]furano} chalcone (30) |
RT | Preventive effect against metabolic syndrome: adiponectin production | Ohnogi et al. (2012b) |
| Angelichalcone (31) |
RT RT |
Protective effect on nerve cells (details in the patent) Osteogenesis promotive activity (details in the patent) |
Onogi et al. (2004) Ohnogi et al. (2004) |
| 4,2′,4′-Trihydroxy-3′-[(2E,5E)-7-methoxy-3,7-dimethyl-2,5-octadienyl] chalcone (32) | AP | First isolation from the part | Kil et al. (2015a) |
| (±)-4,2′,4′-Trihydroxy-3′-[(2E)-6-hydroxy-7-methoxy-3,7-dimethyl-2-octenyl]chalcone (33) | AP | First isolation from the part | Kil et al. (2015a) |
| 4,2′,4′-Trihydroxy-3′-[(2E)-3-methyl-5-(1,3-dioxolan-2-yl)-2-pentenyl]chalcone (34) | AP | First isolation from the part | Kil et al. (2015a) |
| 2′,3′-Furano-4-hydroxy-4′-methoxychalcone (35) | AP | First isolation from the part | Kil et al. (2015a) |
| (±)-4-Hydroxy-2′,3′-(2,3-dihydro-2-methoxyfurano)-4′-methoxychalcone (36) | AP | First isolation from the part | Kil et al. (2015a) |
| 3′-Carboxymethyl- 4,2′-dihydroxy-4′-methoxy chalcone (37) | AP | First isolation from the part | Kil et al. (2016) |
| (±)-4,2′,4′-Trihydroxy-3′-[(3-hydroxy-2,2-dimethyl-6-methylenecyclohexyl)methyl]chalcone (38) | AP | First isolation from the part | Kil et al. (2016) |
| 2″-Hydroxy angelichalcone (39) | AP | First isolation from the part | Kil et al. (2016) |
| Artocarmitin A (40) | AP | First isolation from the part | Kil et al. (2016) |
| Dihydrochalcones | |||
| Deoxydihydroxanthoan | EXD | First isolation from the part | Akihisa et al. (2006) |
| Gelol H (41) | ST | whitening activity: melanin formation in melanoma cells | Arung et al. (2012) |
| Erioschalcone A (42) | AP | First isolation from the part | Luo et al. (2012) |
| Flavones | |||
| Cynaroside (43) (=luteolin-7-O-β-d-glucopyranoside) | AP | Anti-hyperlipidemic acitivity | Park et al. (1995) |
| AP | Anti-cholesterogenic activity: HMG-CoA reductase | Park et al. (1997b) | |
| AP | Anti-mutagenic activity (AFB1) | Park et al. (1997a) | |
| AP | Anti-oxidative activity: epoxide hydrolase in liver | Park et al. (2002) | |
| AP | Anti-oxidative activity: DPPH radical scavenging | Kim et al. (2005) | |
| AP | Anti-depressant activity: DBH | Kim et al. (2013) | |
| ST | Anti-diabetic activity: PTP1B | Li et al. (2015) | |
| Luteolin-7-O-β-d-rutinoside (44) | AP | First isolation from the part | Park et al. (1995) |
| AP | Anti-mutagenic activity (AFB1) | Park et al. (1997a) | |
| AP | Anti-oxidative activity: DPPH radical scavenging | Kim et al. (2005) | |
| Luteolin (45) | LF | First isolation from the part | Sugamoto et al. (2008) |
| Flavonol glycosides | |||
| Kaempferol-3-O-α-d-arabinopyranoside (46) | AP | Anti-oxidative activity: DPPH radical scavenging | Kim et al. (2005) |
| Isoquercitrin (=quercetin-3-O-β-d-glucopyranoside) (47) | AP | Anti-oxidative activity: DPPH radical scavenging | Kim et al. (2005) |
| Hyperoside (=quercetin-3-O-β-d-galactopyranoside) (48) | AP | First isolation from the part | Park et al. (1996) |
| AP | Anti-cholesterogenic activity: HMG-CoA reductase | Park et al. (1997b) | |
| AP | Anti-mutagenic activity (AFB1) | Park et al. (1997a) | |
| AP | Anti-oxidative activity: DPPH radical scavenging | Kim et al. (2005) | |
| Quercitrin (49) (=quercetin-3-O-β-d-rhamnopyranoside) | LF | First isolation from the part | Sugamoto et al. (2008) |
| Quercetin-3-O-α-d-arabinopyranoside (50) | AP | Anti-oxidative activity: DPPH radical scavenging | Kim et al. (2005) |
| Flavanones | |||
| Munduleaflavanone A (51) | EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2003) |
| AP | Anti-diabetic activity: α-glucosidase | Luo et al. (2012) | |
| Munduleaflavanone B (52) | EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2006) |
| Anti-tumor-initiator activity: NOR 1 | |||
| EXD | anti-inflammatory activity: TPA-induced mouse ear edema | Akihisa et al. (2007) | |
| Isobavachin (53) | EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2006) |
| Prostratol F (54) | EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2003) |
| Sophoraflavanone A (55) (=8-geranylnaringenin) | EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2006) |
| Anti-tumor-initiator activity: NOR 1 | |||
| EXD | Anti-inflammatory activity: TPA-induced mouse ear edema | Akihisa et al. (2007) | |
| 4′-O-Geranylnaringenin (56) | EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2003) |
| Coumarins | |||
| Umbelliferone (57) | RT | First isolation from the part | Baba et al. (1990b) |
| Scopoletin (58) | RT | First isolation from the part | Baba et al. (1990b) |
| Demethylsuberosin (59) | AP | Anti-diabetic activity: α-glucosidase | Luo et al. (2012) |
| Osthenol (60) | EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2006) |
| Anti-tumor-initiator activity: NOR 1 | |||
| EXD | Anti-inflammatory activity (details in the patent) | Akihisa et al. (2007) | |
| 7-O-β-d-Glucopyranosyloxy-8-prenyl coumarin (61) | RT | NGF production enhancive effect on in mouse fibroblasts (details in the patent) | Ohnogi et al. (2002) |
| Psoralen (62) | RT | First isolation from the part | Hata and Kozawa (1961) |
| AP | First isolation from the part | Luo et al. (2012) | |
| Ergapten (63) | RT | First isolation from the part | Hata and Kozawa (1961) |
| FR | First isolation from the part | Baba et al. (1990a) | |
| AP | First isolation from the part | Luo et al. (2012) | |
| ST | Anti-diabetic activity: PTP1B | Li et al. (2015) | |
| Xanthotoxin (64) | RT | First isolation from the part | Hata and Kozawa (1961) |
| FR | First isolation from the part | Baba et al. (1990a) | |
| EXD | First isolation from the part | Akihisa et al. (2006) | |
| AP | First isolation from the part | Luo et al. (2012) | |
| Isopimpinellin (65) | RT | First isolation from the part | Baba et al. (1990b) |
| FR | First isolation from the part | Nakata et al. (1997) | |
| EXD | First isolation from the part | Akihisa et al. (2006) | |
| Imperatorin (66) | FR | First isolation from the part | Baba et al. (1990a) |
| RT | First isolation from the part | Nakata et al. (1997) | |
| AP | First isolation from the part | Luo et al. (2012) | |
| Isogosferol (67) | FR | First isolation from the part | Baba et al. (1990a) |
| Isoimperatorin (68) | FR | First isolation from the part | Baba et al. (1990a) |
| RT | First isolation from the part | Nakata et al. (1997) | |
| Saxaline (69) | FR | First isolation from the part | Nakata et al. (1997) |
| Marmesin (70) | RT | First isolation from the part | Baba et al. (1990b) |
| (−)-Oxypeucedanin (71) | FR | First isolation from the part | Baba et al. (1990a) |
| RT | First isolation from the part | Nakata et al. (1997) | |
| (+)-Oxypeucedanin hydrate (72) | FR, | First isolation from the part | Nakata et al. (1997) |
| RT | First isolation from the part | ||
| (−)-Oxypeucedanin hydrate (73) | FR | First isolation from the part | Baba et al. (1990a) |
| (−)-Tert-O-methoxy peucedanin hydrate (74) | FR | First isolation from the part | Baba et al. (1990a) |
| (−)-Sec-O-acetoxy peucedanin hydrate(75) | FR | First isolation from the part | Baba et al. (1990a) |
| Pseudoisopsoralen (76) | AP | First isolation from the part | Luo et al. (2012) |
| Angelicin (77) | RT | First isolation from the part | Hata and Kozawa (1961) |
| Columbianadin (78) | RT | First isolation from the part | Kozawa et al. (1978) |
| FR | First isolation from the part | Baba et al. 1990a) | |
| (3′R)-Hydroxy columbianadin (79) (=(8S,9R)-8-angeloyl oxy-8,9-dihydrooroselol) | EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2003) |
| RT | First isolation from the part | Sugamoto et al. (2009) | |
| (8S,9R)-9-Angeloyloxy-8,9-dihydrooroselol (80) | RT | First isolation from the part | Kozawa et al. (1978) |
| RT | Anti-tumor-promoter activity: TPA-stimulated 32Pi-incorporation | Okuyama et al. (1991) | |
| Archangelicin (81) | RT | First isolation from the part | Kozawa et al. (1978) |
| FR | First isolation from the part | Baba et al. (1990a) | |
| RT | Anti-tumor-promoter activity: TPA-stimulated 32Pi-incorporation | Okuyama et al. (1991) | |
| Lomatin (82) | FR | First isolation from the part | Baba et al. (1990a) |
| Selinidin (83) | RT | First isolation from the part | Baba et al. (1990b) |
| FR | First isolation from the part | Baba et al. (1990a) | |
| EXD | Anti-tumor-initiator activity: NOR 1 | Akihisa et al. (2003) | |
| EXD | Anti-allergic activity: lgE-mediated mast cell activation (β-hexosaninidase, LTC4, TNF-α, FcεRI β-chain PLCγ1, p38 MAPK, IκB-α) | Kishiro et al. (2008) | |
| AP | First isolation from the part | Luo et al. (2012) | |
| ST | Anti-diabetic activity: PTP1B | Li et al. (2015) | |
| Selidinin (84) | EXD | Anti-inflammatory activity (details in the patent) | Akihisa et al. (2007) |
| O-Isobutyroyllomatin (85) | ST | Anti-diabetic activity: PTP1B | Li et al. (2015) |
| Khellactone (86) | AP | First isolation from the part | Luo et al. (2012) |
| ST | Anti-diabetic activity: PTP1B | Li et al. (2015) | |
| 3′-Senecioyl khellactone (87) | EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2003) |
| Anti-tumor-initiator activity: NOR 1 | |||
| AP | Anti-diabetic activity: α-glucosidase | Luo et al. (2012) | |
| Peguangxienin (88) | EXD | Anti-inflammatory activity: TPA-induced mouse ear edema | Akihisa et al. (2007) |
| (3′R,4′R)-3′-Angeloyl khellactone (89) (miswritten as isolaserpitin) | RT | First isolation from the part | Baba et al. (1990b) |
| FR | First isolation from the part | Baba et al. (1990a) | |
| EXD |
Anti-tumor-promoter activity: EBV-EA by TPA Anti-tumor-initiator activity: NOR 1 |
Akihisa et al. (2003) | |
| EXD | Anti-inflammatory activity (details in the patent) | Akihisa et al. (2007) | |
| 4′-Senecioyl khellactone (90) | EXD | Anti-tumor-promoter activity: EBV-EA by TPA | Akihisa et al. (2003) |
| Anti-tumor-initiator activity: NOR 1 | |||
| Isolemanidin (91) (miswritten as laserpitin) | RT | First isolation from the part | Baba et al. (1990b) |
| FR | First isolation from the part | Baba et al. (1990a) | |
| EXD |
Anti-tumor-promoter activity: EBV-EA by TPA Anti-tumor-initiator activity: NOR 1 |
Akihisa et al. (2003) | |
| ST | Anti-hyperlipidemic activity: serum HDL↑, hepatic TG↓ (hepatic TG lipase, differentiation factor 1) | Ogawa et al. (2005b) | |
| EXD | Anti-inflammatory activity: TPA-induced mouse ear edema | Akihisa et al. (2007) | |
| Pteryxin (92) | EXD |
Anti-tumor-promoter activity: EBV-EA by TPA Anti-tumor-initiator activity: NOR 1 |
Akihisa et al. (2003) |
| EXD | Anti-inflammatory activity (details in the patent) | Akihisa et al. (2007) | |
| (+)-Cis-(3′R,4′R)-methylkhellactone (93) | AP | First isolation from the part | Kil et al. (2016) |
| (−)-Trans-(3′R,4′S)-methylkhellactone (94), | AP | First isolation from the part | Kil et al. (2016) |
| Dihydrocoumarin | |||
| 3,4-Dihydroxanthotoxin (95) | AP | First isolation from the part | Kil et al. (2016) |
| Phenolics | |||
| 5-n-Pentadecylresorcinol (96) | EXD | First isolation from the part | Akihisa et al. (2003) |
| (Z)-p-Coumaryl alcohol (97) | AP | First isolation from the part | Kil et al. (2016) |
| Acetylenes | |||
| Falcarindiol (98) | EXD | First isolation from the part | Akihisa et al. (2003) |
| AP | Anti-diabetic activity: α-glucosidase | Luo et al. (2012) | |
| ST | Anti-diabetic activity: PTP1B | Li et al. (2015) | |
| Acetylenes | |||
| (11S,16R,Z)-11,16-Dihydroxyoctadeca-9,17-dien-12,14-diynyl acetate (99) | EXD | First isolation from the part | Akihisa et al. (2006) |
| (10S,15R,Z)-10,15-Dihydroxyheptadeca-8,16-dien-11,13-diynyl acetate (100) | AP | Anti-diabetic activity: α-glucosidase | Luo et al. (2012) |
| (11S,16R,Z)-Octadeca-9,17-dien-12,14-diyne-1,11,16-triol (101) | AP | First isolation from the part | Luo et al. (2012) |
| Angelicol B (102) | AP | First isolation from the part | Luo et al. (2012) |
| Sesquiterpene | |||
| Ashitabaol A (103) | SD | Anti-oxidative: free radical scavenging | Aoki and Ohta (2010) |
| Diterpene | |||
| Steviol-13-O-β-d-glucopyranoside 19-β-d-glucopyranosyl ester (104) | LF | First isolation from the part | Zhou et al. (2012) |
| Triterpenes | |||
| Daucosterol (105) | LF | First isolation from the part | Zhou et al. (2012) |
| Stigmasterol (106) | LF | First isolation from the part | Zhou et al. (2012) |
| Triterpenes | |||
| Pregnenolone (107) | AP | Anti-oxidative activity: DPPH radical scavenging | Luo et al. (2012) |
| Nucleoside | |||
| Adenosine (108) | AP | First isolation from the part | Park et al. (1996) |
| Saccharide | |||
| Sucrose (109) | AP | First isolation from the part | Park et al. (1996) |
| AP | Anti-mutagenic activity (AFB1) | Park et al. (1997a) | |
| Acids | |||
| Angelic acid (110) | RT | First isolation from the part | Hata and Kozawa (1961) |
| Behenic acid (111) | RT | First isolation from the part | Hata and Kozawa (1961) |
| Others | |||
| 1-Cerotol (112) | LF | First isolation from the part | Zhou et al. (2012) |
| (E)-2,6-Dimethylocta-5,7-diene-2,3-diol (113) | AP | First isolation from the part | Luo et al. (2012) |
| Angelicol A (114) | AP | Anti-oxidative activity: DPPH radical scavenging | Luo et al. (2012) |
aPlant part code: WP whole plant, RT root, AP aerial part (including stem and leaf), ST stem, LF leaf, EXD exudate, FR fruit, SD seed; other source code: Syn synthetic, Com commercial
Fig. 1.
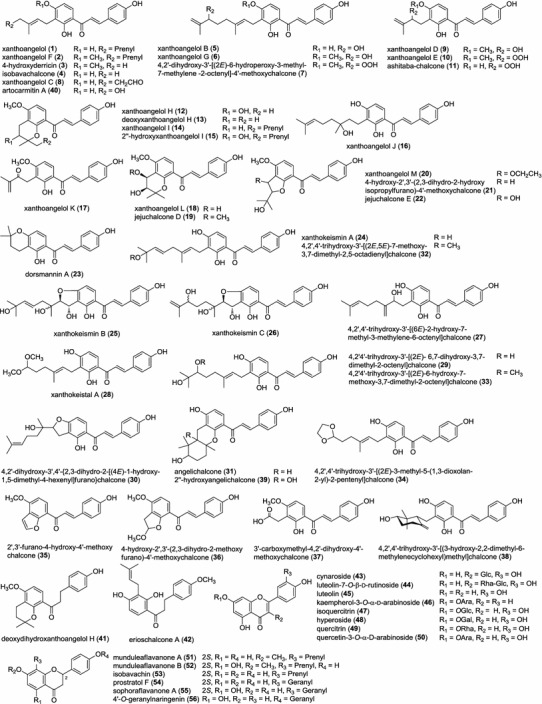
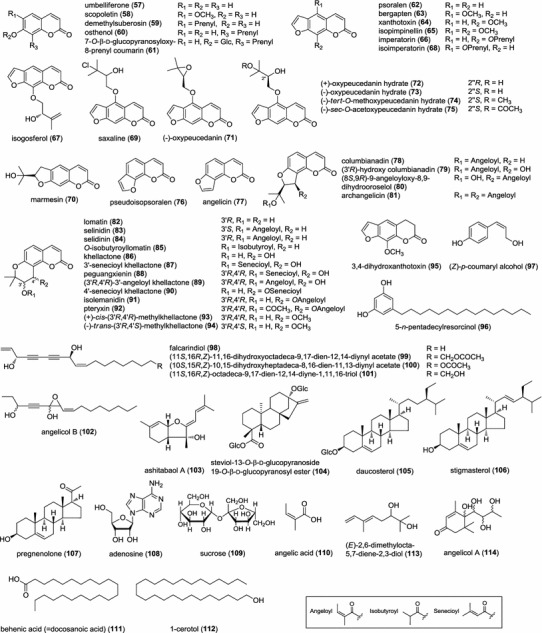
Chemical structures of compounds isolated from A. keiskei
Biological activities of extracts
Anti-inflammatory activity
An n-hexane fraction from an ethanol extract of the aerial parts of A. keiskei showed inhibitory effects on production of lipopolysaccharide (LPS)-induced nitric oxide (NO) and prostaglandin E2 (PGE2) by downregulating inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) protein and mRNA (Lee et al. 2010). An ethyl acetate fraction also inhibited production of NO in LPS-activated RAW 264.7 cells (Chang et al. 2014).
Anti-obesity activity
In a study in mice, an ethyl acetate extract was reported to prevent high-fat diet induced adiposity by modulating lipid metabolism through increasing phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) in adipose tissue and liver (Zhang et al. 2015). A patent has been filed on the anti-obesity activity of the ethanol extract of the aerial parts of A. keiskei in mice (Lee et al. 2012a).
Anti-hyperlipidemic activity
Dietary A. keiskei as an ethyl acetate extract from yellow exudates produced elevation of serum high-density lipoprotein (HDL) levels containing apolipoprotein A1 (ApoA1) and ApoE and reduction of liver triglyceride (TG) levels with decreased mRNA expression of hepatic Acyl-coenzyme A (CoA) synthetase in stroke-prone spontaneously hypertensive rats (SHRSP) (Ogawa et al. 2003). An ethyl acetate fraction from a methanol extract showed inhibitory effects against 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase, a crucial and rate limiting enzyme in biosynthesis of cholesterol (Park et al. 1997b). The impact of A. keiskei was examined in rats fed a high fat diet, and it was shown that liver weight was decreased and gene expression of the anti-oxidant enzymes, catalase and glutathione-s-reductase, in the liver was increased (Kim et al. 2012). In addition, treatment of the ethanol extract reduced increased levels of serum insulin and triglycerides in rats after drinking fructose. The ethanol extract enhanced expression of genes related to fatty acid β-oxidation and HDL production, acyl-CoA oxidase 1 (ACO1), medium-chain acyl-CoA dehydrogenase (MCAD), ATP-binding membrane cassette transporter A1 (ABCA1), and ApoA1 genes in the fructose fed rats (Ohnogi et al. 2012a).
Anti-oxidative activity
The alcohol extracts of A. keiskei were evaluated for their scavenging capability against superoxide anion and 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals, and resulted in decreases of free radical formations. The scavenging effects of the leaves extract were greater than those of the stems extract (Qin et al. 2014; Guo et al. 2013).
Anti-thrombotic activity
Exudates of A. keiskei resulted in anti-coagulation by inhibiting elevated plasma plasminogen activator inhibitor 1 (PAI-1) under inflammatory conditions induced by LPS (Ohkura et al. 2011). A methanol extract of its roots showed anti-platelet activity when evaluated in a washed rabbit platelets model system (Son et al. 2014).
Anti-tumor and anti-mutagenic activity
The extracts induced apoptotic cell death in various human cancer cell lines including breast cancer (MDA-MB-231) (Jeong and Kang 2011), leukemia (HL60), melanoma (CRL1579), lung cancer (A549), stomach cancer (AZ521) (Akihisa et al. 2011), prostate cancer (LNCaP) (Lee et al. 2014). In these cancer cell lines studies, increasing expression level of the proapoptotic gene, Caspase-3, and apoptotic inducer gene, B cell lymphoma 2 (Bcl-2), and decreasing expression level of the apoptotic suppressor gene, Bcl-2 associated x (Bax), were presented as the mechanism of action of anti-cancer effects of A. keiskei (Jeong and Kang 2011). The methanol extract showed an anti-mutagenic activity against aflatoxin B1 (AFB1), N-methyl-N′-nitro-N-nitrosoguanidine, and 4-nitroquinoline-1-oxide in a salmonella assay system (Park et al. 1997a).
Anti-diabetic activity
The ethanol extract exhibited insulin-like activities through a pathway independent of peroxisome proliferator-activated receptor-γ (PPAR-γ) activation (Enoki et al. 2007). Long-term ingestion of a powder of A. keiskei improved blood glucose control and moderately and safely reduced blood glucose (Ohnogi et al. 2007). A patent is filed on the beneficial effects of A. keiskei on diabetes (Lee et al. 2013).
Anti-bacterial activity
An exudate of A. keiskei showed anti-bacterial activity against Helicobacter pylori, suggesting A. keiskei as a preventive agent for gastritis (Fukuo et al. 2005). A patent on this exudate is filed for controlling mouth odor by an anti-bacterial activity against Fusobacterium nucleatum (Saeki et al. 2013).
Hepato-protective activity
A clinical trial for habitual alcohol drinkers with abnormal liver function tests also showed that intake of the extracts significantly reduced γ-glutamyl transferase (GGT) levels (Noh et al. 2015). A patent is filed on the beneficial activity of an ethanol extract of the roots to improve liver functions and relieve fatigue (Kim et al. 2006).
Other biological activities
In addition to these activities, an ethanol extract also showed an anti-hypertensive activity by inhibiting angiotensin 1-converting enzyme (ACE) in spontaneously hypertensive rats, similar to nicotianamine (Shimizu et al. 1999). Green juice of A. keiskei was proven to protect lymphocytic DNA damage of smokers, which was reported to potentially reduce the risk of lung cancer in a clinical trial (Kang et al. 2004). In another clinical study, consuming a drink powder of the leaves three times a day for 2 weeks alleviated mild constipation and improved defecation (Kusaba et al. 2008). Deficit of learning and memory caused by Alzheimer’s disease and aging may be alleviated by A. keiskei since it has been shown that the plant is capable of restoring scopolamine-reduced phosphorylation of cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB) and expression of brain-derived neurotrophic factor (BDNF) in the mouse hippocampus (Oh et al. 2013). Topical uses of the extracts were shown to whiten skin with tyrosinase inhibitory effect (Higa 1985; Lee et al. 2012b) and promote hair growth (Sakaguchi et al. 1996) in patent applications.
Biological activities of isolates from A. keiskei
Isolates from A. keiskei have showed diverse biological activities. Especially two major chalcones, xanthoangelol (1) and 4-hydroxyderricin (3), have been extensively studied and reported to have anti-tumor, anti-inflammatory, anti-depressant, anti-obesity, anti-thrombotic, anti-diabetic, anti-hyperlipidemic, anti-hypertensive, and anti-ulcer effects. The various effects of isolates will be discussed in the subsequent sections and summarized in Tables 1, 2 and 3. In our recent research, several compounds from A. keiskei were evaluated for their effects on heat shock protein (HSP) expression. HSPs are known to have protective effects on damaged cells, and HSP-inducible agents may act as prospective agents to protect normal cells from chemotherapy induced adverse effects. (±)-4,2′,4′-Trihydroxy-3′-[(6E)-2-hydroxy-7-methyl-3-methylene-6-octenyl] chalcone (27) of 2 μM increased expressions of HSF1 (4.3-fold), HSP70 (1.5-fold), and HSP27 (4.6-fold) proteins at 6 h of treatment and mRNA levels of HSP70 (5.0-fold) and HSP27 (1.5-fold) at 3 h with lower cytotoxicity than celastrol, a positive control (Kil et al. 2015a).
Anti-ulcer activity
Xanthoangelol (1) and 4-hydroxyderricin (3) inhibited H+, K+-ATPase of gastric microsomal vesicles which were prepared from pig fundic mucosa. Additionally, they downregulated K+-stimulated p-nitrophenyl phosphatase. Xanthoangelol (1) reduced the formation of stress-induced gastric lesion as well as acid secretion in rats (Murakami et al. 1990). Xanthoangelol E (10) was also found to have the anti-ulcer activity as patented by Murakami et al. (Murakami et al. 1992).
Anti-bacterial activity
Xanthoangelol (1) and 4-hydroxyderricin (3) showed antibacterial activities against gram-positive bacteria, Bacillus subtilis PCI-219, B. subtilis ATCC-6633, B. cereus FDA-5, Staphylococcus aureus 209-P, S. aureus IFO-3060, S. aureus IFO-3762, and Micrococcus luteus IFO-12708, but not gram-negative bacteria. Although the anti-bacterial activities were lower than gentamicin for the aforementioned bacteria, the anti-bacterial activity of xanthoangelol (1) against M. luteus IFO-12708 was as strong as that of gentamicin (MIC 0.76 μg/mL) (Inamori et al. 1991). Mechanism of anti-bacterial activity of 4-hydroxyderricin (3) against S. aureus was elucidated by utilizing a chemical cleavable linker due to its heat sensitivity, reporting that the isolate inhibits amino acylation of tRNAs catalyzed by S. aureus seryl-tRNA synthetase which is an essential enzymatic pathway for bacterial viability (Battenberg et al. 2013). The two chalcones exhibited anti-bacterial activities against plant-pathogenic bacteria, Agrobacteriu tumefaciens IFO-3058, Pseudomonas syringae pv. phaseolicola IFO-12656, P. syringae pv. tabaci IFO-3508, and P. stutzeri IFO-12510 (Inamori et al. 1991). Sumanoto et al. reported anti-bacterial activities of xanthoangelol (1), xanthoangelol F (2), 4-hydroxyderricin (3), and isobavachalcone (4), against gram-positive bacteria, Escherichia coli NBRC 3301, P. mirabilis NBRC 13300, P. fluorescens NBRC 3757, B. subtilis NBRC 3757, S. epidermidis NBRC 12993, and M. luteus NBRC 3333 (Sugamoto et al. 2011).
Chemopreventive activity
Bioactive compounds with anti-tumor-promoter, anti-tumor-initiator, and anti-mutagenic activities may be considered as chemopreventive therapeutic agents.
Two angular furanocoumarin, (8S,9R)-8-angeloxy-8,9-dihydrooroseol (79, (3′R)-hydroxycolumbianadin) and archangelicin (81), and three chalcones, xanthoangelol (1), 4-hydroxyderricin (3), and ashitaba-chalcone (11) inhibited 12-O-tetradecanoylphorbol-13-acetate (TPA)-stimulated 32Pi-incorporation of phospholipids of cultured cells. Xanthoangelol (1) and 4-hydroxyderricin (3) showed anti-tumor-promoting activities in mouse skin carcinogenesis induced by 7,12-dimethylbenz[α]anthracene (DMBA) plus TPA via the modulation of Ca+-calmodulin system (Okuyama et al. 1991). Isobavachalcone (4) exhibited inhibitory effects on skin tumor promotion in in vivo two-stage mouse skin carcinogenesis test (Akihisa et al. 2006). Inhibitory effects on the induction of Epstein-Barr virus early antigen (EBV-EA) by TPA in Raji cells were found for xanthoangelol (1), xanthoangelol F (2), 4-hydroxyderricin (3), isobavachalcone (4), xanthoangelol H (12), xanthoangelol I (14), xanthoangelol J (16), munduleaflavanone A (51), munduleaflavanone B (52), isobavachin (53), prostratol F (54), 8-geranylnaringenin (55, 8-geranulnaringenin), 4′-O-geranylnaringenin (56), osthenol (60), (3′R)-hydroxycolumbianidin (79, (8S,9R)-8-angeloxy-8,9-dihydrooroseol), 3′-senecioyl khellactone (87), (3′R,4′R)-3′-angeloyl khellactone (89, miswritten as “isolaserapitin” in the article), 4′-senecioyl khellactone (90), isolemanidin (91, miswritten as “laserapitin” in the article), and pteryxin (92) (Akihisa et al. 2003, 2006). Xanthoangelol F (2), isobavachalcone (4), xanthoangelol I (14), xanthoangelol J (16), munduleaflavanone B (52), 8-geranylnaringenin (55, sophoraflavanone A), osthenol (60), selinidin (83), 3′-senecioyl khellactone (87), (3′R,4′R)-3′-angeloyl khellactone (89, miswritten as “isolaserapitin” in the article), 4′-senecioyl khellactone (90), isolemanidin (91, miswritten as “laserapitin” in the article), and pteryxin (92) inhibited activation of (±)-(E)-methyl-2-[(E)-hydroxy-imino]-5-nitro-6-methoxy-3-hexemide (NOR 1), a NO donor (Akihisa et al. 2003, 2006). Cynaroside (43, luteolin-7-O-β-d-glucopyranoside), luteolin-7-O-β-d-rutinoside (44), hyperoside (48, quercetin-3-O-β-d-galactopyranoside), and sucrose (109) showed anti-mutagenic activities against aflatoxin B1 (AFB1) (Park et al. 1997a).
Anti-tumor activity
In a previous study, xanthoangelol (1) and 4-hydroxyderricin (3) were identified as active compounds to inhibit tumor growth in Lewis lung carcinoma (LLC)-bearing mice and to prolong survival time and to suppress metastasis to the lung after surgical removal of primary tumors from an active ethyl acetate fraction of a 50% ethanol extract (Kimura and Baba 2003; Kimura et al. 2004). The anti-tumor activities of xanthoangelol (1) might be derived from its inhibitory effects on DNA synthesis in LLC cells and on tumor-induced neovascularization by reducing formation of capillary-like tubes by vascular endothelial cells and adhesion of vascular endothelial growth factor (VEGF) to human umbilical vein endothelial cells (HUVECs) (Kimura and Baba 2003). 4-Hydroxyderricin (3) also inhibited the DNA synthesis in LLC cells and the formation of capillary-like tubes, but did not reduce adhesion of vascular endothelial growth factor (VEGF) to HUVECs. Instead, it suppressed reduction of lymphocytes, CD4+, CD8+, and natural killer (NK)-T cells in spleen of tumor-removed mice (Kimura et al. 2004). Xanthoangelol (1) induced apoptotic cell death by activation of caspase-3 in neuroblastoma (IMR-32) and leukemia (Jurkat) cells through a mechanism, independent of Bax/Bcl-2 signal transduction (Tabata et al. 2005). Their ongoing study revealed details of the mechanism of action of xanthoangelol (1) inducing apoptosis by triggering oxidative stress through generating reactive oxygen species and downregulating antioxidant DJ-1 protein in IMR-32 cells (Motani et al. 2008). Xanthoangelol (1) showed cytotoxic effects on several neuroblastoma cell lines including drug-resistant LA–N-1 and NB-39 cells as well as drug-sensitive IMR-32 and SK-N-SH cells (Nishimura et al. 2007; Motani et al. 2008). Xanthoangelol F (2), 4-hydroxyderricin (3), isobavachalcone (4), xanthoangelol H (12), and xanthoangelol I (14) were cytotoxic to the NB-39 and IMR-32 cells. Isobavachalcone (4) was found to induce apoptotic cell death in the neuroblastoma cells via the mitochondrial pathway including reduction of procaspase-3 and procaspase-9 and induction of Bax level with no cytotoxicity against normal cells (Nishimura et al. 2007). Xanthoangelol (1) and 4-hydroxyderricin (3) inhibited growth of human stomach cancer (KATO III) cells through induction of apoptosis by fragmenting DNA (Takaoka et al. 2008). 4-Hydroxyderricin (3) exhibited cytotoxic activities in human leukemia (HL60), melanoma (CRL1579), lung cancer (A549), and stomach cancer (AZ521) cells. The cytotoxicity to HL60 cells was caused by induction of apoptosis through inhibiting DNA topoisomerase and reducing procasparases-3, -8, and -9 (Akihisa et al. 2011).
Anti-diabetic activity
In a genetically diabetic KK-Ay mouse model, xanthoangelol (1) and 4-hydroxyderricin (3) showed insulin-like activities to enhance glucose uptake and induce preadipocyte differentiation into adipocytes without activating PPAR-γ. 4-Hydroxyderricin (3) also prevented the progression of diabetes in these mice (Enoki et al. 2007). On the basis of the preventive effect, a clinical trial was performed with A. keiskei powder containing 4-hydroxyderricin (3), resulting in reduction of blood glucose and improvement of blood glucose control through increasing adiponectin in subjects with borderline or mild hyperglycemia after 12 weeks of consumption of A. keiskei (Ohnogi et al. 2007). Xanthoangelol (1), 4-hydroxyderricin (3), isobavachalcone (4), munduleaflavanone A (51), demethylsuberosin (59), 3′-senecioyl khellactone (87), falcarindiol (98), and (10S,15R,Z)-10,15-dihydroxyheptadeca-8,16-dien-11,13-diynyl acetate (100) appeared to inhibit α-glucosidase, which involved in glucose metabolism (Luo et al. 2012). Xanthoangelol (1) and 4-hydroxyderricin (3) stimulated skeletal muscle-associated glucose uptake by inducing glucose transporter 4 (GLUT-4) translocation, resulting in normal blood glucose level (Kawabata et al. 2011). Recently, protein tyrosine phosphatase 1B (PTP1B) has been suggested as a therapeutic target for treatment of type 2 diabetes based on its downregulating effect on insulin signaling. Li et al. evaluated isolates from the stems of A. keiskei for their PTP1B inhibitory effects using oleanolic acid as a positive control. Xanthoangelol (1), xanthoangelol F (2), 4-hydroxyderricin (3), xanthoangelol D (9), xanthoangelol E (10), xanthoangelol K (17), xanthoangelol M (20) and bergapten (63, 5-methoxypsoralen; miswritten as “methoxsalen” in the article) showed strong PTP1B inhibitory effect (IC50 0.82–4.42 μg/mL), together with moderately active four compounds (selinidin, 83; O-isobutyroyllomatin, 85; khellactone, 86; falcarindiol, 98, IC50 4.42–11.55 μg/mL) and a weakly active one (cynaroside, 43, IC50 17.49 μg/mL). Furthermore, in a kinetic study, xanthoangelol (1) with IC50 values of 0.82 µg/mL exhibited typical characteristics of the competitive PTP1B inhibitor and in molecular docking simulations, it was suggested that the ring B of the chalcone skeleton may anchor in a pocket of PTP1B (Li et al. 2015).
Nerve cell protective activity
Onogi et al. filed a patent application presenting angelichalcone (31) with its nerve cell protective effect (Onogi et al. 2004). In addition, 7-O-β-d-glucopyranosyloxy-8-prenyl coumarin (61) was reported for its enhancing effect on nerve growth factor (NGF) production in mouse fibroblast L-M cells in a patent (Ohnogi et al. 2002).
Anti-hyperlipidemic activity
Cynaroside (43, luteolin-7-O-β-d-glucopyranoside) showed hypolipidemic activity when administrated by intraperitoneal injection in rats (Park et al. 1995). Cynaroside (43) and hyperoside (48, quercetin-3-O-β-d-galactopyranoside) exhibited anti-cholesterogenic activity by inhibiting HMG-CoA reductase (Park et al. 1997b). When SHRSP was fed diets containing 4-hydroxyderricin for 7 weeks, serum very low-density lipoprotein (VLDL) and hepatic triglycerides (TG) levels were significantly reduced, without any effect on serum HDL levels. It was considered that microsomal TG transfer-protein may be responsible for the decrease in serum VLDL levels and differentiation factor 1 and fatty acid synthase may induce the decrease in hepatic TG content (Ogawa et al. 2005b). In case of isolemanidin (91, miswritten as “laserapitin” in the article), the serum HDL levels, especially apoE-HDL, increased and the hepatic TG content decreased, which might be relevant with a decrease in hepatic TG lipase and differentiation factor 1, respectively (Ogawa et al. 2005a).
Preventive effects against metabolic syndrome
Ohnogi et al. isolated six new chalcones, 4,2′-dihydroxy-3′-[(2E)-6-hydroperoxy-3-methyl-7-methylene-2-octenyl]-4′-methoxychalcone (7), 2″-hydroxyxanthoangelol I (15), 4-hydroxy-2′,3′-(2,3-dihydro-2-hydroxyisopropylfurano)-4′-methoxychalcone (21), 4,2′4′-trihydroxy-3′-[(2E)-6,7-dihydroxy-3,7-dimethyl-2-octenyl]chalcone (29), 4,2′-dihydroxy-3′,4′-{2,3-dihydro-2-[(4E)-1-hydroxy-1,5-dimethyl-4-hexenyl]furano}chalcone (30), and angelichalcone (31) together with xanthoangelol (1) and 4-hydroxyderricin (3), and evaluated their effects on adiponectin mRNA and protein levels. As a result, all isolates, except for angelichalcone (31), increased expression of adiponectin mRNA and production of adiponectin in 3T3-L1 adipocytes. The effects on adiponectin could be expected to prevent metabolic syndrome through improving insulin resistance and dyslipidemia (Ohnogi et al. 2012b). Xanthoangelol (1) and 4-hydroxyderricin (3) suppressed adipocytes differentiation via AMPK and mitogen-activated protein kinase (MAPK) pathways, which resulted in downregulating expression of adipocyte-specific transcription factors, CCAAT/enhancer-binding protein-β (C/EBP-β), C/EBP-α, and PPAR-γ. Inhibitory effects on adipocytes differentiation could provide preventive effects against obesity and obesity-related disorders (Zhang et al. 2013).
Anti-hypertensive activity
Xanthoangelol (1), xanthoangelol F (2), 4-hydroxyderricin (3), xanthoangelol B (5), and xanthoangelol E (10) inhibited phenylephrine-induced vasoconstriction. Among them, xanthoangelol (1), xanthoangelol F (2), 4-hydroxyderricin (3), and xanthoangelol E (10) showed the inhibitory effects through endothelium-dependent production of endothelium-derived relaxing factor (EDRF)/NO. On the other hand, xanthoangelol B (5) directly inhibited smooth muscle functions through reducing intracellular free calcium [Ca2 +]i elevation induced by phenylephrine without affecting EDRF/NO production. 4-Hydroxyderricin (3) also suppressed elevation of [Ca2 +]i induced by phenylephrine (Matsuura et al. 2001). Dietary 4-hydroxyderricin (3) reduced elevation of systolic blood pressure in SHRSP, which also supported its anti-hypertensive activity (Ogawa et al. 2005b).
Anti-allergic activity
Xanthoangelol B (5), xanthoangelol C (8), and xanthoangelol E (10) inhibited the compound 48/80-induced histamine release from rat peritoneal mast cells, whereas, xanthoangelol (1) and 4-hydroxyderricin (3) enhanced it. Compound 48/80 is one of immunological stimulators, which activates the mast cell to induce allergic responses by mediates (Nakata and Baba 2001). Selinidin (83) was found to suppress degranulation, leukotriene C4 (LTC4) synthesis, and tumor necrosis factor-α (TNF-α) production without affecting binding of immunoglobulin E (IgE) with high affinity IgE receptor (FcεRI) and expression of cell surface FcεRI in antigen-stimulated primary mouse bone marrow-derived mast cells (BMMCs). Selinidin (83) inhibited tyrosine phosphorylation of FcεRI β-chain and phospholipase C γ1 (PLCγ1) not FcεRI γ-chain in BMMCs upon FcεRI stimulation, demonstrating its inhibitory effects on FcεRI-dependent signaling pathway. Phosphorylation of p38 MAPK and inhibitory κB-α (IκB-α) were also downregulated by selinidin (83) (Kishiro et al. 2008).
Anti-inflammatory activity
Xanthoangelol D (9) inhibited activation of nuclear factor-κB (NF-κB), which was involved in attenuation of basal and TNF-α-induced endothelin-1 (ET-1), a potent vasoconstrictor peptide. Its inhibitory effect against NF-κB inflammatory signaling pathway could be useful for treating vascular and inflammatory diseases because many vascular diseases are closely related to the inflammatory processes (Sugii et al. 2005). A patent covers the anti-inflammatory activities of seven flavonoids, xanthoangelol (1), xanthoangelol F (2), 4-hydroxyderricin (3), isobavachalcone (4), deoxyxanthoangelol H (13), munduleaflavanone B (52), and sophoraflavanone A (55, 8-geranylnaringenin), and six coumarins, osthenol (60), selidinin (84), peguangxienin (88), (3′R,4′R)-3′-angeloyl khellactone (89), isolemandin (91), and pteryxin (92) from exudates of A. keiskei (Akihisa et al. 2007). Shin et al. reported that xanthoangelol (1), 4-hydroxyderricin (3), xanthoangelol B (5), and 4,2′,4′-trihydroxy-3′-[(6E)-2-hydroxy-7-methyl-3-methylene-6-octenyl]chalcone (27) showed inhibitory activities of IL-6 production in TNF-α-stimulated MG-63 osteosarcoma cells and they have C-4′ hydroxyl groups in common (Shin et al. 2011). Toll-like receptors (TLRs) are essential for induction of innate immune responses against invading pathogens, however, overexpression of transcription factors and pro-inflammatory genes by activation of TLR signaling pathway might cause certain inflammatory diseases. Isobavachalcone (4) reduced overexpression of iNOS induced by TLR agonists (lipopeptide 2-kDa, polyriboinosinic polyribocytidylic acid, or LPS) in murine macrophages (Shin et al. 2013). Xanthoangelol (1) and 4-hydroxyderricin (3) reduced production of LPS-induced NO and TNF-α, expression of iNOS and COX-2 in RAW264 mouse macrophages. Moreover, both suppressed DNA binding activities of activator protein-1 (AP-1) and NF-κB and phosphorylation level of p65 subunit of NF-κB. However, two chalcones downregulated only AP-1 not NF-κB (Yasuda et al. 2014). Inhibitory effects on LPS-induced NO and expression of iNOS and COX-2 genes were also found for xanthoangelol B (5), xanthoangelol E (10), and xanthokeismin A (24) as well as 4-hydroxyderricin (3) and their mechanisms involved inhibition of IκB degradation and NF-κB nuclear translocation (Chang et al. 2014).
Anti-oxidative activity
Cynaroside (43, luteolin-7-O-β-d-glucopyranoside) inhibited bromobenzene-induced hepatic lipid peroxidation by restoring epoxide hydrolase activity in liver of rats (Park et al. 2002). Xanthoangelol B (5), xanthokeismin A (24), xanthokeismin B (25), and xanthokeismin C (26) showed strong superoxide-scavenging activities. Xanthoangelol (1), 4-hydroxyderricin (3), and isobavachalcone (4) induced reduced nicotinamide adenine dinucleotide (phosphate) (NAD(P)H):quinone oxidoreductase 1 (NQO1) which protects against quinone-mediated oxidative damage. In addition, xanthoangelol (1), isobavachalcone (4), cynaroside (43, luteolin-7-O-β-d-glucopyranoside), luteolin-7-O-β-d-rutinoside (44), kaempferol-3-O-α-d-arabinopyranoside (46), isoquercitrin (47, quercetin-3-O-β-d-glucopyranoside), hyperoside (48, quercetin-3-O-β-d-galactopyranoside), quercetin-3-O-α-d-arabinopyranoside (50), pregnenolone (107), and angelicol A (114) showed DPPH radical scavenging activities (Luo et al. 2012; Kim et al. 2005). Ashitabaol A (103), an isolate from the seeds of A. keiskei, was found to be more abundant in the seed coat during germination through HPLC analysis. Ashitabaol A (103) exhibited 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) free radical scavenging activity and was expected to protect the seed against oxidative damage induced by free radicals during germination (Aoki and Ohta 2010). Xanthine oxidase (XO) leads to formation of free radicals, and cause oxidative damage to living tissues. Xanthoangelol (1), xanthoangelol F (2), 4-hydroxyderricin (3), isobavachalcone (4), and xanthoangelol B (5) showed inhibitory effects on XO activity, which could be relevant with attenuation of inflammation (Kim et al. 2014).
Anti-thrombotic activity
Xanthoangelol E (10) was evaluated for effects on arachidonic acid metabolism in gastric antral mucosa and platelet of rabbit. Xanthoangelol E (10) showed no significant effect on production of PGE2, PGF2α, and their metabolites in gastric antral mucosal slices, whereas, suppressed production of thromboxane B2 (TXB2) and 12-hydroxy-5,8,10-heptadecatrienoic acid (HHT) from exogenous arachidonic acid in platelets (Fujita et al. 1992). Ohkura et al. reported that xanthoangelol (1), xanthoangelol B (5), and xanthoangelol D (9) isolated from the exudates of A. keiskei suppressed production of TNFα-induced PAI-1, an inhibitor of fibrinolysis, in human umbilical vein endothelial cells (HUVECs) (Ohkura et al. 2011). Regarding to anti-thrombotic activities, xanthoangelol (1) and 4-hydroxyderricin (3) also exhibited inhibitory effects on platelet aggregation induced by collagen, platelet-activating factor, and phorbol 12-myristate 13-acetate not thrombin (Son et al. 2014).
Anti-depressant activity
Xanthoangelol (1) was compared to iproniazid, an anti-depressant nonselective MAO inhibitor. Xanthoangelol (1) inhibited monoamine oxidase (MAO) in a nonselective manner and suppressed dopamine β-hydroxylase (DBH). On the other hands, 4-hydroxyderricin selectively inhibited MAO-B and mildly suppressed DBH, which was higher than deprenyl, a selective MAO-B inhibitor. Because MAO inhibitors have been widely applied for treating depression, xanthoangelol (1) and 4-hydroxyderricin (3) were suggested as candidates for developing combined antidepressant drug. Additionally, cynaroside (43, luteolin-7-O-β-d-glucopyranoside) showed strong inhibitory effect against DBH (Kim et al. 2013).
Anti-viral activity
Park et al. reported that xanthoangelol F (2), 4-hydroxyderricin (3), xanthoangelol B (5), xanthoangelol G (6), xanthoangelol D (9), and xanthokeistal A (28) have inhibitory effects against hydrolysis of influenza virus neuraminidase (NA) which has been an effective target to develop drugs treating patients infected with influenza virus. All active compounds showed reversible nonselective characteristics and xanthoangelol D (9) was the most potent inhibitor. Furthermore, it was suggested that the influenza virus NA inhibitory activities of the alkylated chalcones are associated with their alkyl groups (Park et al. 2011). Because enzymatic activities of a chymotrypsin-like protease (3CLpro) and a papain-like protease (PLpro) are essential for a viral life cycle of severe acute respiratory syndrome corovirus (SARS-CoV), the two viral cysteine proteases are attractive targets for development of anti-SARS-CoV drugs. Xanthoangelol (1), xanthoangelol F (2), 4-hydroxyderricin (3), isobavachalcone (4), xanthoangelol B (5), xanthoangelol G (6), xanthoangelol D (9), xanthoangelol E (10), and xanthokeistal A (28) showed competitive inhibitory characteristics to 3CLpro and noncompetitive ones to PLpro, except for mixed inhibition manner of isobavachalcone (4) against PLpro. Especially, xanthoangelol E (10) exhibited the most potent inhibitory activity, and docking simulation of xanthoangelol E (10) to both protease revealed that its hydroperoxy group plays a role in enzyme binding by forming hydrogen bonding (Park et al. 2015).
Osteogenesis promotive activity
Angelichalcone (31) was presented as an osteogenesis promotive agent in a patent application (Ohnogi et al. 2004).
Whitening activity
Xanthoangelol (1), 4-hydroxyderricin (3), xanthoangelol H (12), deoxyxanthoangelol H (13), and deoxydihydroxanthoangelol H (41) exhibited inhibitory effects against melanin formation in B16 melanoma cells with low cytotoxicity (Arung et al. 2012).
Discussion
As presented in this review, the pharmacological studies on A. keiskei and its putative active compounds support a number of biological activities that can potentially impact human health. In spite of these reported biological activities and a number of filed patent applications on this plant, A. keiskei has not been developed as a pharmaceutical agent and is currently available on the market as health drinks. This review presented a summary of the studies published to date on this promising plant. Although the majority of these studies are mechanistic, but they can be used as a solid platform to design and conduct clinical studies.
Acknowledgements
This work is supported by Ewha Womans University (collaborative research grant for Ewha-UC Irvine).
Compliance with ethical standards
Conflict of interest
The co-authors have declared no conflict of interest.
Contributor Information
Eun Kyoung Seo, Phone: +82-2-3277-3047, Email: yuny@ewha.ac.kr.
Mahtab Jafari, Phone: +1 (949) 824-0145, Email: mjafari@uci.edu.
References
- Akihisa T, Tokuda H, Ukiya M, Iizuka M, Schneider S, Ogasawara K, Mukainaka T, Iwatsuki K, Suzuki T, Nishino H. Chalcones, coumarins, and flavanones from the exudate of Angelica keiskei and their chemopreventive effects. Cancer Lett. 2003;201:133–137. doi: 10.1016/S0304-3835(03)00466-X. [DOI] [PubMed] [Google Scholar]
- Akihisa T, Tokuda H, Hasegawa D, Ukiya M, Kimura Y, Enjo F, Suzuki T, Nishino H. Chalcones and other compounds from the exudates of Angelica keiskei and their cancer chemopreventive effects. J Nat Prod. 2006;69:38–42. doi: 10.1021/np058080d. [DOI] [PubMed] [Google Scholar]
- Akihisa T, Yasukawa T, Ukiya M, Hasegawa D (2007) Anti-inflammatory agents containing natural products from Angelica keiskei. (Nihon University, Japan). Application: JP
- Akihisa T, Kikuchi T, Nagai H, Ishii K, Tabata K, Suzuki T. 4-Hydroxyderricin from Angelica keiskei roots induces caspase-dependent apoptotic cell death in HL60 human leukemia cells. J Oleo Sci. 2011;60:71–77. doi: 10.5650/jos.60.71. [DOI] [PubMed] [Google Scholar]
- Aoki N, Ohta S. Ashitabaol A, a new antioxidative sesquiterpenoid from seeds of Angelica keiskei. Tetrahedron Lett. 2010;51:3449–3450. doi: 10.1016/j.tetlet.2010.04.122. [DOI] [Google Scholar]
- Aoki N, Muko M, Ohta E, Ohta S. C-Geranylated chalcones from the stems of Angelica keiskei with superoxide-scavenging activity. J Nat Prod. 2008;71:1308–1310. doi: 10.1021/np800187f. [DOI] [PubMed] [Google Scholar]
- Arung ET, Furuta S, Sugamoto K, Shimizu K, Ishikawa H, Matsushita Y-I, Kondo R. The inhibitory effects of representative chalcones contained in Angelica keiskei on melanin biosynthesis in B16 melanoma cells. Nat Prod Commun. 2012;7:1007–1010. [PubMed] [Google Scholar]
- Baba K, Kido T, Yoneda Y, Taniguchi M, Kozawa M. Chemical components of Angelica keiskei Koidzumi. (V). Components of the fruits, and comparison of coumarins and chalcones in the fruits, roots and the leaves. Shoyakugaku Zasshi. 1990;44:235–239. [Google Scholar]
- Baba K, Nakata K, Taniguchi M, Kido T, Kozawa M. Chemical components of Angelica keiskei. Part 7. Chalcones from Angelica keiskei. Phytochemistry. 1990;29:3907–3910. doi: 10.1016/0031-9422(90)85357-L. [DOI] [Google Scholar]
- Baba K, Taniguchi M, Nakata K. Studies on Angelica keiskei “Ashitaba”. Foods Food Ingred J Jpn. 1998;178:52–60. [Google Scholar]
- Bao G (2014) Angelica keiskei-containing nutritional and health-protecting flour and its preparation method (Qingyang county nanyang rice industry Co., Ltd, Peop. Rep. China). Application: CN
- Battenberg OA, Yang Y, Verhelst SHL, Sieber SA. Target profiling of 4-hydroxyderricin in S. aureus reveals seryl-tRNA synthetase binding and inhibition by covalent modification. Mol BioSyst. 2013;9:343–351. doi: 10.1039/c2mb25446h. [DOI] [PubMed] [Google Scholar]
- Chang HR, Lee HJ, Ryu J-H. Chalcones from Angelica keiskei attenuate the inflammatory responses by suppressing nuclear translocation of NF-κB. J Med Food. 2014;17:1306–1313. doi: 10.1089/jmf.2013.3037. [DOI] [PubMed] [Google Scholar]
- Enoki T, Ohnogi H, Nagamine K, Kudo Y, Sugiyama K, Tanabe M, Kobayashi E, Sagawa H, Kato I. Antidiabetic activities of chalcones isolated from a Japanese herb, Angelica keiskei. J Agric Food Chem. 2007;55:6013–6017. doi: 10.1021/jf070720q. [DOI] [PubMed] [Google Scholar]
- Fujita T, Sakuma S, Sumiya T, Nishida H, Fujimoto Y, Baba K, Kozawa M. The effects of xanthoangelol E on arachidonic acid metabolism in the gastric antral mucosa and platelet of the rabbit. Res Commun Chem Pathol Pharmacol. 1992;77:227–240. [PubMed] [Google Scholar]
- Fukuo Y, Ogasawara K, Satoh K (2005) Antimicrobial agent/Antimicrobial agent against Helicobacter pylori comprising a material derived from Angelica keiskei (Japan Bio Science Laboratory Co., Ltd., Japan). Application: US
- Guo X-Q, Wu J-H, Liu C, Li J-Q, Wang Z-W, Yang K-F. Comparison of antioxidant content and oxidation resistance of stems and leaves from Angelica keiskei Koidzmi. Shipin Yu Fajiao Gongye. 2013;39:122–127. [Google Scholar]
- Hata K, Kozawa M. Pharmacognostical studies on umbelliferous plants. XVIII. Constituents of the roots of Angelica keiskei. Yakugaku Zasshi. 1961;81:1647–1649. doi: 10.1248/yakushi1947.81.11_1647. [DOI] [PubMed] [Google Scholar]
- Higa Y (1985) Skin-whitening cosmetic containing Angelica keiskei Koidzumi extract and having tyrosinase inhibiting effect (Sansei Pharmaceutical Co., Ltd., Japan). Application: JP
- Imai K, Imai M. Encyclopedia of wild herbs and vegetables. Seoul: Green Home; 2008. [Google Scholar]
- Inamori Y, Baba K, Tsujibo H, Taniguchi M, Nakata K, Kozawa M. Chemical components of Angelica keiskei. VI. Antibacterial activity of two chalcones, xanthoangelol and 4-hydroxyderricin, isolated from the root of Angelica keiskei Koidzumi. Chem Pharm Bull. 1991;39:1604–1605. doi: 10.1248/cpb.39.1604. [DOI] [PubMed] [Google Scholar]
- Jeong Y-J, Kang KJ. Effect of Angelica keiskei extract on apoptosis of MDA-MB-231 human breast cancer cells. Han’guk Sikp’um Yongyang Kwahak Hoechi. 2011;40:1654–1661. [Google Scholar]
- Kang M-H, Park YK, Kim H-Y, Kim TS. Green vegetable drink consumption protects peripheral lymphocytes DNA damage in Korean smokers. BioFactors. 2004;22:245–247. doi: 10.1002/biof.5520220149. [DOI] [PubMed] [Google Scholar]
- Kawabata K-I, Sawada K, Ikeda K, Fukuda I, Kawasaki K, Yamamoto N, Ashida H. Prenylated chalcones 4-hydroxyderricin and xanthoangelol stimulate glucose uptake in skeletal muscle cells by inducing GLUT4 translocation. Mol Nutr Food Res. 2011;55:467–475. doi: 10.1002/mnfr.201000267. [DOI] [PubMed] [Google Scholar]
- Kil Y-S, Choi S-K, Lee Y-S, Jafari M, Seo E-K. Chalcones from Angelica keiskei: evaluation of their heat shock protein inducing activities. J Nat Prod. 2015;78:2481–2487. doi: 10.1021/acs.jnatprod.5b00633. [DOI] [PubMed] [Google Scholar]
- Kil Y-S, Nam J-W, Lee J, Seo EK. Separation of two major chalcones from Angelica keiskei by high-speed counter-current chromatography. Arch Pharmacal Res. 2015;38:1506–1511. doi: 10.1007/s12272-014-0530-2. [DOI] [PubMed] [Google Scholar]
- Kil Y-S, Kwon J, Lee D, Seo EK. Three new chalcones from the aerial parts of Angelica keiskei. Helv Chim Acta. 2016;99:393–397. doi: 10.1002/hlca.201500519. [DOI] [Google Scholar]
- Kim S-J, Cho J-Y, Wee J-H, Jang M-Y, Kim C, Rim Y-S, Shin S-C, Ma S-J, Moon J-H, Park K-H. Isolation and characterization of antioxidative compounds from the aerial parts of Angelica keiskei. Food Sci Biotechnol. 2005;14:58–63. [Google Scholar]
- Kim TS, Shim JS, Kim SD, Yeo IH (2006) Health-aid food, drink and food composition having hepato-protective effect, which comprises Angelica keiskei root extract and hydroxyderricin and xanthoangelol isolated therefrom (Pulmuone Co., Ltd., S. Korea). Application: KR
- Kim E, Choi J, Yeo I. The effects of Angelica keiskei Koidz on the expression of antioxidant enzymes related to lipid profiles in rats fed a high fat diet. Nutr Res Pract. 2012;6:9–15. doi: 10.4162/nrp.2012.6.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Son YK, Kim GH, Hwang KH. Xanthoangelol and 4-hydroxyderricin are the major active principles of the inhibitory activities against monoamine oxidases on Angelica keiskei K. Biomol Ther. 2013;21:234–240. doi: 10.4062/biomolther.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Curtis-Long MJ, Yuk HJ, Wang Y, Song YH, Jeong SH, Park KH. Quantitative analysis of phenolic metabolites from different parts of Angelica keiskei by HPLC-ESI MS/MS and their xanthine oxidase inhibition. Food Chem. 2014;153:20–27. doi: 10.1016/j.foodchem.2013.12.026. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Baba K. Antitumor and antimetastatic activities of Angelica keiskei roots, part 1: isolation of an active substance, xanthoangelol. Int J Cancer. 2003;106:429–437. doi: 10.1002/ijc.11256. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Taniguchi M, Baba K. Antitumor and antimetastatic activities of 4-hydroxyderricin isolated from Angelica keiskei roots. Planta Med. 2004;70:211–219. doi: 10.1055/s-2004-815537. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Sumiyoshi M, Baba K. Anti-tumor actions of major component 3′-O-acetylhamaudol of Angelica japonica roots through dual actions, anti-angiogenesis and intestinal intraepithelial lymphocyte activation. Cancer Lett. 2008;265:84–97. doi: 10.1016/j.canlet.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Kishiro S, Nunomura S, Nagai H, Akihisa T, Ra C. Selinidin suppresses IgE-mediated mast cell activation by inhibiting multiple steps of FcεRI signaling. Biol Pharm Bull. 2008;31:442–448. doi: 10.1248/bpb.31.442. [DOI] [PubMed] [Google Scholar]
- Kozawa M, Morita N, Baba K, Hata K. The structure of xanthoangelol, a new chalcone from the roots of Angelica keiskei Koidzumi (Umbelliferae) Chem Pharm Bull. 1977;25:515–516. doi: 10.1248/cpb.25.515. [DOI] [Google Scholar]
- Kozawa M, Morita N, Baba K, Hata K. Chemical components of the roots of Angelica keiskei Koidzumi. III. The structure of a new dihydrofurocoumarin. Yakugaku Zasshi. 1978;98:636–638. doi: 10.1248/yakushi1947.98.5_636. [DOI] [PubMed] [Google Scholar]
- Ku CS, Kim MJ, Jung YJ, Jang DI (2014) Cosmetic compositions containing Angelica keiskei extracts (COTDE Co., Ltd., S. Korea). Application: KR
- Kusaba N, Kamiya T, Ikeguchi M, Ono H, Takagaki K, Hayashi M, Ogasawara T, Sugawa-Katayama Y. Effects of AOJIRU drink powder of Angelica keiskei leaf containing indigestible dextrin on defecation in volunteers with constipation. Jpn Pharmacol Ther. 2008;36:1159–1165. [Google Scholar]
- Lee HJ, Choi TW, Kim HJ, Nam D, Jung SH, Lee EH, Lee HJ, Shin EM, Jang H-J, Ahn KS, Shim BS, Choi S-H, Kim S-H, Sethi G, Ahn KS. Anti-inflammatory activity of Angelica keiskei through suppression of mitogen-activated protein kinases and nuclear factor-κB activation pathways. J Med Food. 2010;13:691–699. doi: 10.1089/jmf.2009.1271. [DOI] [PubMed] [Google Scholar]
- Lee MS, Jung MG, Choi GB, Kim TS, Yeo IH, Nam SU (2012a) Angelica keiskei extracts for preventing and treating obesity (Pulmuone Holdings Co., Ltd., S. Korea). Application: KR
- Lee MS, Kim BG, Choi GB, Kim TS, Yeo IH, Nam SU (2012b) Skin whitening composition containing Angelica keiskei extracts (Pulmuone Holdings Co., Ltd., S. Korea). Application: KR
- Lee MS, Lee EJ, Choi GB, Kim TS, Yeo IH, Nam SU (2013) Angelica keiskei extracts for preventing and alleviating diabetes mellitus (Pulmuone Holdings Co., Ltd., S. Korea). Application: KR
- Lee MS, Choi GB, Kim TS, Yeo IH, Nam SU (2014) Angelica keiskei root extracts for inducing apoptosis of prostate cancer cells (Pulmuone Co., Ltd., S. Korea). Application: KR
- Li J-L, Gao L-X, Meng F-W, Tang C-L, Zhang R-J, Li J-Y, Luo C, Li J, Zhao W-M. PTP1B inhibitors from stems of Angelica keiskei (Ashitaba) Bioorg Med Chem Lett. 2015;25:2028–2032. doi: 10.1016/j.bmcl.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Z, Sun J, Wan Y, Rong Y (2014) Coix seed-Angelica keiskei nutritional health-care biscuits and production method thereof (Shanghai Jiao Tong University, Peop. Rep. China). Application: CN
- Luo L, Wang R, Wang X, Ma Z, Li N. Compounds from Angelica keiskei with NQO1 induction, DPPH· scavenging and α-glucosidase inhibitory activities. Food Chem. 2012;131:992–998. doi: 10.1016/j.foodchem.2011.09.099. [DOI] [Google Scholar]
- Matsuura M, Kimura Y, Nakata K, Baba K, Okuda H. Artery relaxation by chalcones isolated from the roots of Angelica keiskei. Planta Med. 2001;67:230–235. doi: 10.1055/s-2001-12011. [DOI] [PubMed] [Google Scholar]
- Motani K, Tabata K, Kimura Y, Okano S, Shibata Y, Abiko Y, Nagai H, Akihisa T, Suzuki T. Proteomic analysis of apoptosis induced by xanthoangelol, a major constituent of Angelica keiskei, in neuroblastoma. Biol Pharm Bull. 2008;31:618–626. doi: 10.1248/bpb.31.618. [DOI] [PubMed] [Google Scholar]
- Murakami S, Kijima H, Isobe Y, Muramatsu M, Aihara H, Otomo S, Baba K, Kozawa M. Inhibition of gastric H+, K+-ATPase by chalcone derivatives, xanthoangelol and 4-hydroxyderricin, from Angelica keiskei Koidzumi. J Pharm Pharmacol. 1990;42:723–726. doi: 10.1111/j.2042-7158.1990.tb06568.x. [DOI] [PubMed] [Google Scholar]
- Murakami S, Kotomo S, Ozawa M, Baba K (1992) Novel chalcone derivative as antiulcer agent (Taisho Pharmaceutical Co., Ltd., Japan). Application: JP
- Nakata K, Baba K. Histamine release-inhibiting activity of Angelica keiskei. Nat Med. 2001;55:32–34. [Google Scholar]
- Nakata K, Katsumata H, Taniguchi M, Kita S, Baba K. Comparison of the characteristics and the constituents of Angelica keiskei KOIDZUMI growing in Hachijo and Oshima. Nat Med. 1997;51:532–536. [Google Scholar]
- Nakata K, Taniguchi M, Baba K. Three chalcones from Angelica keiskei. Nat Med. 1999;53:329–332. [Google Scholar]
- Nishimura R, Tabata K, Arakawa M, Ito Y, Kimura Y, Akihisa T, Nagai H, Sakuma A, Kohno H, Suzuki T. Isobavachalcone, a chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma. Biol Pharm Bull. 2007;30:1878–1883. doi: 10.1248/bpb.30.1878. [DOI] [PubMed] [Google Scholar]
- Noh H-M, Ahn E-M, Yun J-M, Cho B-L, Paek Y-J. Angelica keiskei Koidzumi extracts improve some markers of liver function in habitual alcohol drinkers: a randomized double-blind clinical trial. J Med Food. 2015;18:166–172. doi: 10.1089/jmf.2014.3222. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Nakashima S, Baba K. Effects of dietary Angelica keiskei on lipid metabolism in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2003;30:284–288. doi: 10.1046/j.1440-1681.2003.03830.x. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Nakamura R, Baba K. Beneficial effect of laserpitin, a coumarin compound from Angelica keiskei, on lipid metabolism in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2005;32:1104–1109. doi: 10.1111/j.1440-1681.2005.04306.x. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Ohno M, Baba K. Hypotensive and lipid regulatory actions of 4-hydroxyderricin, a chalcone from Angelica keiskei, in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2005;31:19–23. doi: 10.1111/j.1440-1681.2005.04147.x. [DOI] [PubMed] [Google Scholar]
- Oh SR, Kim S-J, Kim DH, Ryu JH, Ahn E-M, Jung JW. Angelica keiskei ameliorates scopolamine-induced memory impairments in mice. Biol Pharm Bull. 2013;36:82–88. doi: 10.1248/bpb.b12-00681. [DOI] [PubMed] [Google Scholar]
- Ohkura N, Nakakuki Y, Taniguchi M, Kanai S, Nakayama A, Ohnishi K, Sakata T, Nohira T, Matsuda J, Baba K, Atsumi G-I. Xanthoangelols isolated from Angelica keiskei inhibit inflammatory-induced plasminogen activator inhibitor 1 (PAI-1) production. BioFactors. 2011;37:455–461. doi: 10.1002/biof.187. [DOI] [PubMed] [Google Scholar]
- Ohnogi H, Shiraga M, Sagawa H, Kato I (2002) Nerve growth factor enhancers for treatment of disease containing coumarin and/or chroman derivatives (Takara Bio Inc., Japan). Application: WO
- Ohnogi H, Sugiyama K, Muraki N, Sagawa H, Kato I (2004) Plant components for promoting osteogenesis (Takara Bio Inc., Japan). Application: WO
- Ohnogi H, Enoki T, Hino F, Kato I. Antidiabetic effect and safety of long-term ingestion of Ashitaba (Angelica keiskei) powder containing Chalcone (4HD) on borderline mild hyperglycemia. Jpn Pharmacol Ther. 2007;35:647–660. [Google Scholar]
- Ohnogi H, Hayami S, Kudo Y, Deguchi S, Mizutani S, Enoki T, Tanimura Y, Aoi W, Naito Y, Kato I, Yoshikawa T. Angelica keiskei extract improves insulin resistance and hypertriglyceridemia in rats fed a high-fructose drink. Biosci Biotechnol Biochem. 2012;76:928–932. doi: 10.1271/bbb.110927. [DOI] [PubMed] [Google Scholar]
- Ohnogi H, Kudo Y, Tahara K, Sugiyama K, Enoki T, Hayami S, Sagawa H, Tanimura Y, Aoi W, Naito Y, Kato I, Yoshikawa T. Six new chalcones from Angelica keiskei inducing adiponectin production in 3T3-L1 adipocytes. Biosci Biotechnol Biochem. 2012;76:961–966. doi: 10.1271/bbb.110976. [DOI] [PubMed] [Google Scholar]
- Oi J, Meyer FG, Meyer FG, Walker EH. Flora of Japan: in English: combined, much revised and extended translation/by the author of his Flora of Japan (1953) and Flora of Japan, Pteridophyta (1957); edited by Frederick G. Meyer and Egbert H. Walker. Smithsonian Institution: Washington; 1965. [Google Scholar]
- Okuyama T, Takata M, Takayasu J, Hasegawa T, Tokuda H, Nishino A, Nishino H, Iwashima A. Anti-tumor-promotion by principles obtained from Angelica keiskei. Planta Med. 1991;57:242–246. doi: 10.1055/s-2006-960082. [DOI] [PubMed] [Google Scholar]
- Onogi H, Sugiyama K, Muraki N, Enoki T, Sagawa H, Kato I (2004) Chalcone derivatives from Angelica and pharmacological activities thereof (Takara Bio Inc., Japan). Application: WO
- Park J-C. The medicinal herbs, teas, and alcoholic beverages in Donguibogam, proven by patents. Goyang: Pureun-Hyeongbok Publisher; 2013. [Google Scholar]
- Park JC, Cho YS, Park SK, Park JR, Chun SS, Ok KD, Choi JW. Isolation of flavone-7-O-glycosides from the aerial parts of Angelica keiskei and antihyperlipidemic effects. Saengyak Hakhoechi. 1995;26:337–343. [Google Scholar]
- Park JC, Yu YB, Lee JH, Choi MR, Ok KD. Chemical components from the aerial parts of Angelica keiskei. Saengyak Hakhoechi. 1996;27:80–82. [Google Scholar]
- Park J-C, Park J-R, Chung S-K, Yu Y-B, Ha J-O, Park K-Y. Antimutagenic activity of the methanol extract and compounds of Angelica keiskei in the Salmonella assay system. Saengyak Hakhoechi. 1997;28:80–83. [Google Scholar]
- Park J-R, Park J-C, Choi S-H. Screening and characterization of anticholesterogenic substances from food plant extracts. Han’guk Sikp’um Yongyang Kwahak Hoechi. 1997;26:236–241. [Google Scholar]
- Park JC, Park JG, Kim HJ, Hur JM, Lee JH, Sung NJ, Chung SK, Choi JW. Effects of extract from Angelica keiskei and its component, cynaroside, on the hepatic bromobenzene-metabolizing enzyme system in rats. Phytother Res. 2002;16:S24–S27. doi: 10.1002/ptr.783. [DOI] [PubMed] [Google Scholar]
- Park J-Y, Jeong HJ, Kim YM, Park S-J, Rho M-C, Park KH, Ryu YB, Lee WS. Characteristic of alkylated chalcones from Angelica keiskei on influenza virus neuraminidase inhibition. Bioorg Med Chem Lett. 2011;21:5602–5604. doi: 10.1016/j.bmcl.2011.06.130. [DOI] [PubMed] [Google Scholar]
- Park JY, Ko Jin A, Kim Dae W, Kim Young M, Kwon HJ, Jeong Hyung J, Kim Cha Y, Park Ki H, Lee Woo S, Ryu Young B (2015) Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J Enzym Inhib Med Chem: 1–8 [DOI] [PubMed]
- Qin LQ, Luo SY, Zhan ZH, Liu XX, Wang K. Determination of antioxidant compounds from leaf and stem of Angelica keiskei by gas chromatography-mass spectrometry. Asian J Chem. 2014;26:5097–5099. [Google Scholar]
- Saeki Y, Asada S, Kikuchi S, Itoh S (2013) Deodorant composition containing extracts of Angelica keiskei for prevention of mouth odor (Lotte Co., Ltd., Japan). Application: WO; Chemical Indexing Equivalent to 158:544360 (JP)
- Sakaguchi Y, Sakaguchi M, Sakaguchi T (1996) Hair growth promoter comprising Angelica keiskei extract, and preparation method thereof (Japan). Application: JP
- Shimizu E, Hayashi A, Takahashi R, Aoyagi Y, Murakami T, Kimoto K. Effects of angiotensin 1-converting enzyme inhibitor from Ashitaba (Angelica keiskei) on blood pressure of spontaneously hypertensive rats. J Nutr Sci Vitaminol. 1999;45:375–383. doi: 10.3177/jnsv.45.375. [DOI] [PubMed] [Google Scholar]
- Shin JE, Choi EJ, Jin Q, Jin H-G, Woo E-R. Chalcones isolated from Angelica keiskei and their inhibition of IL-6 production in TNF-α-stimulated MG-63 cell. Arch Pharmacal Res. 2011;34:437–442. doi: 10.1007/s12272-011-0311-0. [DOI] [PubMed] [Google Scholar]
- Shin H-J, Shon D-H, Youn H-S. Isobavachalcone suppresses expression of inducible nitric oxide synthase induced by Toll-like receptor agonists. Int Immunopharmacol. 2013;15:38–41. doi: 10.1016/j.intimp.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Son DJ, Park YO, Yu C, Lee SE, Park YH. Bioassay-guided isolation and identification of anti-platelet-active compounds from the root of Ashitaba (Angelica keiskei Koidz.) Nat Prod Res. 2014;28:2312–2316. doi: 10.1080/14786419.2014.931389. [DOI] [PubMed] [Google Scholar]
- Su L (2014) A method for preparing Angelica keiskei wine (Nanjing Zelang Agriculture Development Co., Ltd., Peop. Rep. China). Application: CN
- Sugamoto K, Matsushita Y-I, Matsui T, Kurogi C, Park JC. Isolation and analysis components of Ashitaba (Angelica keiskei koidzumi) leaves growing in Miyazaki and Korea. Mem Fac Eng Miyazaki Univ. 2008;37:75–80. [Google Scholar]
- Sugamoto K, Matsushita Y-I, Matsui T, Hikita H, Park JC. Isolation and analysis components of Ashitaba (Angelica keiskei Koidzumi) roots, stems and leaves. Mem Fac Eng Miyazaki Univ. 2009;38:91–96. [Google Scholar]
- Sugamoto K, Matsusita Y-I, Matsui K, Kurogi C, Matsui T. Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron. 2011;67:5346–5359. doi: 10.1016/j.tet.2011.04.104. [DOI] [Google Scholar]
- Sugii M, Ohkita M, Taniguchi M, Baba K, Kawai Y, Tahara C, Takaoka M, Matsumura Y. Xanthoangelol D isolated from the roots of Angelica keiskei inhibits endothelin-1 production through the suppression of nuclear factor-κB. Biol Pharm Bull. 2005;28:607–610. doi: 10.1248/bpb.28.607. [DOI] [PubMed] [Google Scholar]
- Tabata K, Motani K, Takayanagi N, Nishimura R, Asami S, Kimura Y, Ukiya M, Hasegawa D, Akihisa T, Suzuki T. Xanthoangelol, a major chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma and leukemia cells. Biol Pharm Bull. 2005;28:1404–1407. doi: 10.1248/bpb.28.1404. [DOI] [PubMed] [Google Scholar]
- Takaoka S, Hibasami H, Ogasawara K, Imai N. Chalcones from Angelica keiskei induced apoptosis in stomach cancer cells. J Herbs, Spices Med Plants. 2008;14:166–174. doi: 10.1080/10496470802598727. [DOI] [Google Scholar]
- Yasuda M, Kawabata K, Miyashita M, Okumura M, Yamamoto N, Takahashi M, Ashida H, Ohigashi H. Inhibitory effects of 4-hydroxyderricin and xanthoangelol on lipopolysaccharide-induced inflammatory responses in RAW264 macrophages. J Agric Food Chem. 2014;62:462–467. doi: 10.1021/jf404175t. [DOI] [PubMed] [Google Scholar]
- Zhang T, Sawada K, Yamamoto N, Ashida H. 4-Hydroxyderricin and xanthoangelol from Ashitaba (Angelica keiskei) suppress differentiation of preadiopocytes to adipocytes via AMPK and MAPK pathways. Mol Nutr Food Res. 2013;57:1729–1740. doi: 10.1002/mnfr.201300020. [DOI] [PubMed] [Google Scholar]
- Zhang T, Yamashita Y, Yasuda M, Yamamoto N, Ashida H. Ashitaba (Angelica keiskei) extract prevents adiposity in high-fat diet-fed C57BL/6 mice. Food Funct. 2015;6:134–145. doi: 10.1039/C4FO00525B. [DOI] [PubMed] [Google Scholar]
- Zhou X, Liang C, Xu Q, Zhang X, Liang X, Huang Q, Su X. Chemical constituents of Angelica keiskei. Zhongguo Shiyan Fangjixue Zazhi. 2012;18:103–105. [Google Scholar]


