Abstract
The prebiotics and probiotics market is constantly growing due to the positive effects of its consumption on human health, which extends beyond the digestive system. In addition, the synbiotic products market is also expanding due to the synergistic effects between pre- and probiotics that provide additional benefits to consumers. Pre- and probiotics are being evaluated for their effectiveness to treat and prevent infectious diseases in other parts of the human body where microbial communities exist. This review examines the scientific data related to the effects of pre- and probiotics on the treatment of diseases occurring in the skin, female urogenital tract, and respiratory tract. The evidence suggests that probiotics consumption can decrease the presence of eczema in children when their mothers have consumed probiotics during pregnancy and lactation. In women, probiotics consumption can effectively prevent recurrent urinary tract infections. The consumption of synbiotic products can reduce respiratory tract infections and their duration and severity. However, the outcomes of the meta-analyses are still limited and not sufficiently conclusive to support the use of probiotics to treat infectious diseases. This is largely a result of the limited number of studies, lack of standardization of the studies, and inconsistencies between the reported results. Therefore, it is advisable that future studies consider these shortcomings and include the evaluation of the combined use of pre- and probiotics.
Keywords: Probiotics, Prebiotics, Skin, Female urogenital system, Respiratory tract
Introduction
Prebiotics and probiotics have been widely studied and applied on the pharmaceutical and food industries due to their health benefits. The global prebiotics and probiotics market is constantly growing partly attributed to consumer awareness of the health benefits of their consumption and, therefore, demand nutritional and therapeutic value of food products.
The activity of the beneficial microbial community has enormous impact on human well-being, including host metabolism, physiology, nutrition, and immune function. Thus, the consumption of probiotics is purported to maintain the balance of the gut flora and to inhibit the growth of pathogenic bacteria. The consumption of nutrients exerting selective and positive effects on the beneficial gut bacterial flora (i.e., prebiotics) is another usually adopted strategy.
Probiotics can be considered as a promising alternative to antibiotics since they can displace harmful bacteria through various mechanisms. In this regard, several studies have explored the effects of probiotic and prebiotics use on microbiota of other parts of the body, to treat halitosis (Suzuki et al. 2014), atopic dermatitis (Wu et al. 2012), and allergies related to the respiratory system (Watts et al. 2016) and to restore the vaginal microbiota (Gabrielli et al. 2018), among others.
This review focuses on the scientific knowledge regarding the effect of probiotics and prebiotics on the prevention and treatment of infectious diseases occurring on the skin, female urogenital tract, and respiratory tract.
Probiotics
Probiotics have been defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (FAO/WHO 2001). However, microorganism classified as a probiotic must comply with the “Guidelines for the Evaluation of Probiotics in Food” (FAO/WHO 2002). These microorganisms should be biologically and genetically stable, have good sensory properties, low cost, maintain viability during processing and storage, and resist physicochemical processing of the food when used as food additives (Collins and Gibson 1999; Prado et al. 2008; Sanders 2008).
The scientific literature shows differing degrees of evidence supporting the beneficial effects of probiotics on human health: some improve lactose digestion and reduce the symptoms of lactose intolerance, reduce intestinal pH and blood cholesterol, produce vitamin B, and improve dietary calcium bioavailability (Nagpal et al. 2012; Prado et al. 2008). In addition, probiotics inhibit the onset of allergic diseases and prevent and treat ischemic heart syndromes (FAO/WHO 2001). However, other health benefits require additional studies, such as cancer prevention, treatment of acute diarrheal diseases, and anticariogenic properties (Kechagia et al. 2013; Nagpal et al. 2012).
Prebiotics
Gibson and Roberfroid introduced the prebiotic concept in 1995 (Gibson and Roberfroid 1995). Most recently, prebiotic has been defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit.” This new definition allows inclusion of non-carbohydrate substances and applications in body sites other than the gastrointestinal tract. This definition also applies to prebiotics for animal use (Gibson et al. 2017).
The prebiotic activity of an ingredient should be evaluated by a series of in vitro and in vivo assays. The following methodologies have recently been proposed (Bajury et al. 2017): (1) in vitro method, which includes in vitro digestion and in vitro fermentation; (2) in vivo method, including animal studies and human clinical studies; (3) analysis of fermentation products; and (4) validation of in vitro with in vivo studies.
The best-known and most frequently studied prebiotics are inulin-type fructans, including native inulin, oligofructose, and synthetic fructooligosaccharides (FOS). Other examples are galactooligosaccharides (GOS), arabinose, raffinose, lactulose, pyrodextrins, soya oligosaccharides, and xylooligosaccharides (de Souza et al. 2011; Gibson and Fuller 2000; Pineiro et al. 2008). They can be extracted from natural sources, such as chicory root, dahlia tubers, artichoke, yacon, agave, garlic, onions, asparagus, leeks, flaxseed, and soya beans, as well as in human breast milk and cow's milk (Campos et al. 2012; Gibson et al. 2017; Gomez et al. 2010).
The configuration of the glycosidic bonds of prebiotics is resistant to hydrolysis by intestinal acid and digestive enzymes. Therefore, prebiotics are not degraded in the upper regions of the gastrointestinal tract and can reach the colon intact, where it is degraded by the gut microbiota to produce acetic, propionic, butyric, and lactic acids as well as gasses (Anadón et al. 2010).
Synbiotic relationship between probiotics and prebiotics
The development of pharmaceutical and food products containing both prebiotics substances and probiotics microorganisms aiming to modulate the gut microbiota is a prominent strategy to control and treat some infectious diseases. These products are called synbiotics, due to synergistic effects between pre- and probiotics. In theory, a synbiotic product improves the survival and implantation of live probiotics in the gastrointestinal tract through the selective stimulation of the growth and/or metabolic activity of a limited number of health-promoting microorganisms (Pandey et al. 2015). Probiotics used in synbiotic formulations usually include strains belonging to the Lactobacillus, Bifidobacterium, Enterococcus, and Streptococcus genera. Main prebiotics in these formulations include inulin and FOS (from different sources), GOS, xylose oligosaccharide, transgalactooligosaccharides, lactulose, lactilol, and lactosucrose (Markowiak and Śliżewska 2017; Pandey et al. 2015; Sekhon and Jairath 2010).
The effects of the synbiotic relationship on human health have been addressed in several studies, most aimed at evaluating the effects of synbiotic products on the metabolic status of patients with type 2 diabetes mellitus (DM) (Tajadadi-Ebrahimi et al. 2014). Other studies focused on health-related quality of life and irritable bowel syndrome symptoms (Šmid et al. 2016), weight loss and maintenance in obese patients (Sanchez et al. 2014), and glucose metabolism and gut microbiome (Liu et al. 2017).
Some studies have suggested that the synbiotic relationship would also be useful in other parts of the human body where beneficial microbial communities for the host also exist (Luoto et al. 2014; Suzuki et al. 2014; Wu et al. 2012).
Probiotics and prebiotics applied in human infectious diseases
The effects of diet on human health have garnered the interest of consumers, researchers, and pharmaceutical and functional food industries. Strategy for enhancing consumer’s health usually promoted by pharmaceutical and food industries includes the combined used of pre- and probiotics due to their synbiotic association (Gibson and Roberfroid 1995). In this context, most studies relate to the treatment/prevention of gastrointestinal diseases, widely discussed in other works reported in the current literature (Folch 2018; Whyand and Caplin 2014). However, probiotics can also confer some benefits in the treatment of halitosis, allergies related to the respiratory system, immune system, prevention of systemic infections, among others. In this context, Braga et al. (2017) evaluated the preventive effects of probiotics in clinical practice. This analysis included 16 Cochrane systematic reviews that used probiotics as preventive interventions compared with placebo, or with any pharmacological or non-pharmacological interventions, or without comparison. These clinical interventions were classified into numbers of systematic reviews addressing the following: prevention of respiratory diseases (2), prevention of gastroenterological diseases (9), gynecological and obstetric diseases (3), urological diseases (1), and immunological/allergic diseases (1). The analysis concluded that there is little scientific evidence to support the use of probiotics. In addition, the quality of evidence provided was low or very low.
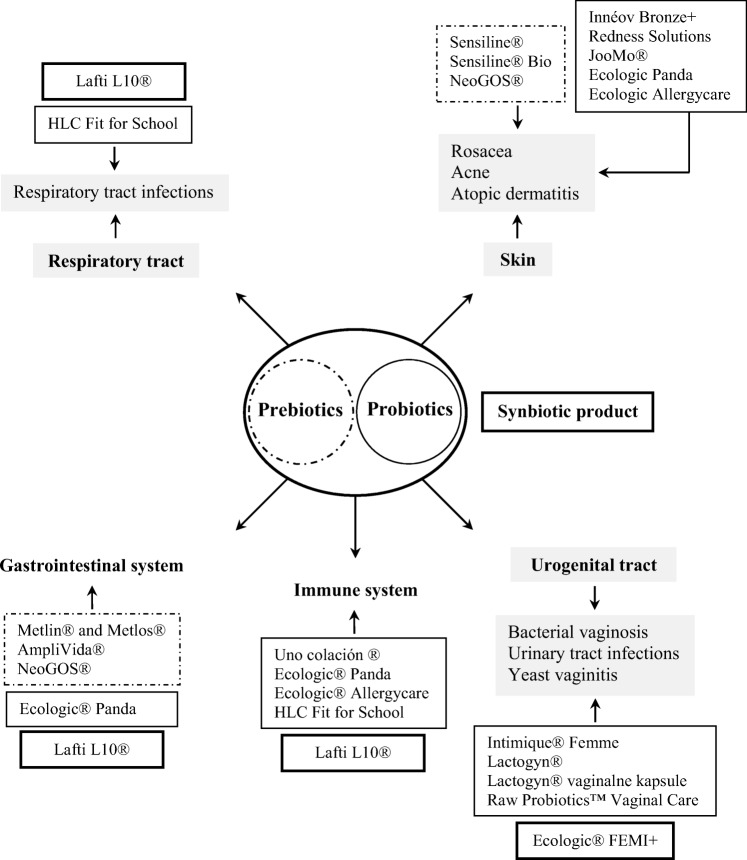
Here, we present some evidence or recent research aimed to implement probiotics and/or prebiotics in clinical dermatology and cosmetics or to develop products that can be applied to parts of the body where microbial communities exist, such as the skin, respiratory tract, and urogenital tract (Fig. 1).
Fig. 1.
Synbiotic relationship between probiotics and prebiotics that can prevent or treat diseases in the human body and commercially available prebiotics and/or probiotics products in the market. ▬, synbiotic product; ─, probiotic products; − ∙−∙ −, prebiotic products
Potential mechanisms of probiotics action
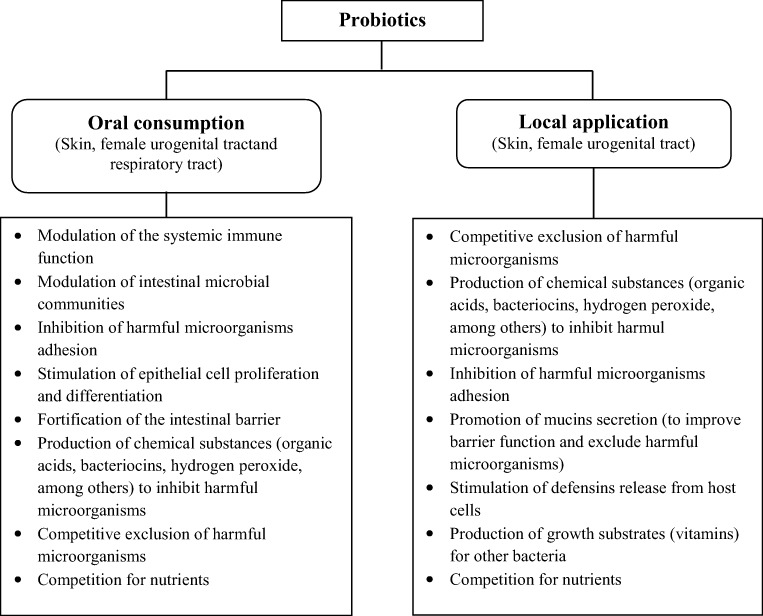
Although probiotics have been widely studied, the mechanisms of action in the prevention or treatment of bacterial infections on other parts of human body, such as skin, female urogenital tract, and respiratory tract, are so far unknown. However, it has been postulated that similar mechanisms than in intestinal epithelium may exist (Tapiovaara et al. 2016). As shown in Fig. 2, probiotics can improve human health by oral consumption and local application. This figure also outlines possible mechanisms of probiotics action in skin, female urogenital tract, and respiratory tract. It includes modulation of the systemic immune function, competitive exclusion of harmful microorganisms, production of antimicrobial substances and growth substrates, inhibition of bacterial toxin production, among others (Bermudez-Brito et al. 2012; Gogineni et al. 2013; Markowiak and Śliżewska 2017; Rahmati Roudsari et al. 2013).
Fig. 2.
Possible mechanisms of probiotic action in the skin, female urogenital tract, and respiratory tract. Adapted from Rahmati Roudsari et al. (2013)
Probiotics and prebiotics effects on skin health
The skin is human body’s largest organ. The skin, which acts as a physical and chemical barrier to protect the body against external harm, covers all parts of the human's body. The skin regulates body’s temperature, controls the evaporation rate, and accounts for lipids and water storage (Grice and Segre 2011). The microorganisms present in the skin include bacteria, fungi, and viruses, which are classified in the following three groups: residents, temporary, and transient species (Holland and Bojar 2002). The resident microorganisms possess beneficial skin functions, such as inhibiting pathogenic species and processing skin proteins, free fatty acids, and sebum (Roth and James 1988). Transient species are defined as contaminants with little or no capacity for sustained growth or reproduction in the cutaneous environment (Krutmann 2009), remaining briefly on the skin (Holland and Bojar 2002).
The development of molecular techniques has established that most skin bacteria belong to one of the following four different phyla: Actinobacteria, Firmicutes, Bacteroidetes, and Proteobacteria (Grice and Segre 2011). Non-bacterial microorganisms have also been isolated, such as Malassezia spp. (fungal species, prevalent in sebaceous areas) and Demodex mites (microscopic arthropods) (Grice and Segre 2011). Studies with 16S rRNA have determined that Staphylococcus epidermidis and Propionibacterium acnes represent fewer than 6% of the captured microbiota (Krutmann 2009). S. epidermidis and P. acnes are considered normal skin commensals that help fight pathogens and regulate the microbiome homeostasis. The skin topography is another factor that influences its microbiome. For instance, partially occluded areas, such as toes or behind the ears with elevated temperature and humidity, allow the growth of Staphylococcus spp. and Corynebacterium spp., both residents on healthy human skin (Grice 2014). Furthermore, areas with a high density of sebaceous glands, such as the face, chest, or back, encourage the growth of lipophilic microorganisms, such as Propionibacterium spp., fungal Malassezia spp. (Grice 2014), and Corynebacterium spp. (Scharschmidt and Fischbach 2013). In addition, two commensal viral groups, anelloviruses and GBV-C, have been identified alerting to the possibility of a larger human virome (Delwart 2007).
Keeping a resident microflora unaltered might be an effective way to maintain healthy normal skin functions. Skin diseases, however, can change microbial ecology (Krutmann 2009).
Rosacea, acne, and atopic dermatitis (AD) are the most frequent skin disorders. Rosacea is a chronic skin condition that primarily affects the face of middle aged and older people. Although the pathogenesis of rosacea is still unknown, its development is presumably associated with the presence of certain microorganisms, such as Helicobacter pylori, S. epidermidis (β-hemolytic), and Demodex mite (Murillo and Raoult 2013). Studies on the use of probiotics and/or prebiotics for rosacea treatment are still limited (Table 1).
Table 1.
Effects of probiotics and/or prebiotics on human infectious diseases
| Part of body | Disease | Probiotic | Prebiotica | Dose | Result | Reference |
|---|---|---|---|---|---|---|
| Skin | Atopic dermatitis | B. breve M-16V | GOS/FOS mixture |
- Ninety infants - Aged 0–7 months - Formula-fed: 1.3 × 109 CFU/100 mL GOS/FOS (0.8 mg/100 mL) - 12 months |
- Synbiotic mixture has no beneficial effect on atopic dermatitis severity in infants. - Synbiotic mixture successfully modulates their intestinal microbiota. |
van der Aa et al. (2010) |
| L. salivarius | FOS |
- Sixty children aged 2–14 years - Capsules: two daily - 8 weeks |
- A synbiotic combination of L. salivarius plus FOS is superior to the prebiotic alone for treating moderate to severe childhood atopic dermatitis. | Wu et al. (2012) | ||
|
Combination of: B. bifidum, L. acidophilus, L. casei, L. salivarius |
– |
- Forty children aged 1–13 years - Probiotic bags 2 × 109 CFU - Two bags daily - 8 weeks |
- Probiotic combination effectively reduced the SCORAD in the treatment of atopic dermatitis. | Yeşilova et al. (2012) | ||
| Acne |
L. acidophilus L. delbrueckii sub. bulgaricus B. bifidum |
– |
- Female - Aged 18–35 years - Two probiotics capsules per day: one in the morning and one in the evening. - Group A: Only probiotic supplementation (two per day). - Group B: Only minocycline (antibiotic) supplementation. - Group C: Treated with both probiotic and minocycline. This group also takes the probiotic at least 2 h after their minocycline dosage. |
- Probiotics may be considered a therapeutic option or adjunct for acne vulgaris by providing a synergistic anti-inflammatory effect with systemic antibiotics. | Jung et al. (2013) | |
| GMH |
- Female volunteers - Aged 18–39 years - Spray formulation of GMH at 5% (w/v). |
- Data indicate that the GMH could be used as a prophylactic or novel topical therapeutic product for acne vulgaris and to improve skin health more generally. | Bateni et al. (2013) | |||
| Female urogenital tract | Urinary tract infection | L. crispatus CTV-05 | – |
- Women - Aged 18–40 - Vaginal suppositories - Once daily for 5 days |
- Treatment with Lactin-V was associated with reduced recurrent urinary tract infections. | Stapleton et al. (2011) |
| Female urogenital tract | Urinary tract infection |
L. rhamnosus GR-1 L. reuteri RC-14 |
– |
- Postmenopausal women - 12 months - Trimethoprim-sulfamethoxazole, 480 mg, once daily - Oral probiotic capsules with 1 × 109CFU, twice daily. |
- L. rhamnosus GR-1 and L. reuteri RC-14 do not meet the noninferiority criteria in the prevention of urinary tract infections compared with trimethoprim-sulfamethoxazole. - Lactobacilli do not increase antibiotic resistance. |
Beerepoot et al. (2012) |
| Respiratory tract | Respiratory tract infections |
L. rhamnosus LGG® B. animalis ssp. lactis BB-12® |
– |
- College students - Probiotic powder with 1 billion CFU of each, stick of 5 g - 12 weeks |
- LGG® and BB-12® may be beneficial among college students with upper respiratory infections for mitigating reductions in health-related quality of life. | Smith et al. (2013) |
|
L. acidophilus spp., L. delbrueckii sub. bulgaricus, B. bifidum, S. salivarius thermophilus |
– |
- Girl swimmers - Aged 13.8 ± 1.8 years - Probiotic yogurt (400 mL, 4 × 1010 CFU/mL) or normal yogurt - Daily for 8 weeks. |
- A reduction in the number of episodes of respiratory infections and duration of some symptoms, such as dyspnea and ear pain, were observed. | Salarkia et al. (2013) | ||
|
B. animalis subsp lactis, L. rhamnosus |
– |
- Danish infants - Sachets with 1 × 109 CFU of each probiotic - Aged 8–14 months - For a 6-month period |
- Daily administration of a combination of B. animalis subsp lactis and L. rhamnosus for 6 months did not reduce the number of days absent from child care in healthy infants at the time of enrollment in child care. | Laursen et al. (2017) | ||
| Virus-associated respiratory tract infections | L. rhamnosus GG ATCC 53103 | GOS/polydextrose (1:1) |
- Preterm infants - Oral probiotic-prebiotic mixture - Probiotic dose: 1 × 109 CFU/day for 1 to 30 days and 2 × 109 CFU/day for 31 to 60 days. - Prebiotic dose: 1 × 3600 mg/day for 1 to 30 days and 2 × 600 mg/day for 31 to 60 day. |
- Lower incidence of respiratory tract infections in infants receiving prebiotics or L. rhamnosus GG compared with those receiving a placebo. | Luoto et al. (2014) | |
aGOS, galactooligosaccharides; FOS, fructooligosaccharides; GMH, glucomannan hydrolysates
Acne vulgaris is a common skin disease that mainly affects teenagers. The factors that can cause acne vulgaris are hyperkeratinization, obstruction of sebaceous follicles resulting from abnormal keratinization of the infundibular epithelium, stimulation of sebaceous gland secretion by androgens, and microbial colonization of pilosebaceous units by P. acnes, renamed recently to Cutibactterium acnes (C. acnes) (Platsidaki and Dessinioti 2018), which promotes perifollicular inflammation (Toyoda and Morohashi 2001). P. acnes, an anaerobic Gram-positive bacteria forms part of the normal skin flora; it can metabolize sebaceous triglycerides into fatty acids (Toyoda and Morohashi 2001; Webster et al. 1980). Meanwhile, AD is a common chronic or relapsing inflammatory disease of the skin that often precedes asthma and allergic disorders (Boguniewicz and Leung 2011). AD is prevalent among children and often resolves in adolescence or adulthood (Huang and Tang 2015). A dysfunctional skin barrier and an imbalanced immune system (Boguniewicz and Leung 2011) are two of several causes of these skin disorders.
Patients with AD are characterized by overexpression of the cytokine T-helper cell 2 (Th2) or an imbalance of T-helper cell 1 (Th1) and Th2. The skin lesions in these patients show T cell infiltrates, which affect the skin barrier functions and can induce keratinocytes apoptosis. This condition stimulates the progress of infection and complications (Rahman et al. 2011). Patients with AD usually acquire cutaneous infections by Staphylococcus aureus, the main colonizing organism (Huang and Tang 2015).
Rosacea, acne and AD are mostly treated with antibiotics, which greatly disrupt the skin's normal microbiome (Reid et al. 2011). Topical and oral treatments only control the symptoms. Topical instead of systemic treatments are preferred for the use of antibiotics or products with anti-inflammatory and antimicrobial effects (Dahl 2014).
Topical application of probiotics and/or prebiotics for the treatment of skin diseases
In general, studies in the field of skin diseases refer mainly to the effects of probiotics and prebiotics ingestion, while the effect of topical application has been barely investigated (Lee et al. 2019).
An innovative line of cosmetics has recently been developed, the so-called prebiotic cosmetics. The strategy is to rebalance the composition of the skin microflora by inhibiting the growth of transient or pathogen species while promoting the growth of beneficial bacteria as the resident microorganisms (Krutmann 2009). Al-Ghazzewi and Tester (2010) showed that konjac glucomannan hydrolysate and probiotic strains (Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus gasseri, and Lactococcus lactis ssp. lactis) inhibited P. acnes NCTC 737 growth in vitro. Bateni et al. (2013) determined that a spray formulation containing konjac glucomannan hydrolysate (5%, w/v) significantly improved the skin's health with acne vulgaris of young women after twenty and forty days treatment.
Kang et al. (2009) studied the therapeutic effect on P. acnes of a lotion prepared with concentrated powder (CBT SL-5) from the cell-free culture supernatant of Enterococcus faecalis SL-5. The randomized, double-blind, placebo-controlled trial included 70 patients (33 in placebo group and 37 in test group) diagnosed with acne vulgaris in the face. Patients (≥ 12 years old) were instructed to apply a thin layer of CBT SL-5 lotion twice a day on affected areas. The results showed that the lotion significantly (P < 0.05) reduced the inflammatory lesion relative to the placebo lotion. Muizzuddin et al. (2012) observed a reduction in acne lesion size during a clinical study of L. plantarum extract (5%); however, no size reduction occurred at 1%.
Lopes et al. (2016) determined, in an in vitro assay, that Propioniferax innocua, L. acidophilus La-5, Lactobacillus delbrueckii, L. acidophilus La-10, Lactobacillus paracasei La-26, Bifidobacterium lactis B-94, and Bifidobacterium animalis Bb-12 exhibited the highest adhesion values to human keratin. In addition, the probiotic P. innocua significantly (P < 0.05) reduced the adherence of P. acnes. On the other hand, L. acidophilus La-5, L. delbrueckii, L. acidophilus La-10, L. paracasei La-26, and B. animalis Bb-12 exhibited important zone of inhibition (0.8 to 2.3 mm) against P. acnes. Lopes et al. (2016) also found that all probiotics of the assay decreased P. acnes biofilm formation.
Blanchet-Réthoré et al. (2017) studied the effect of a lotion containing heat-treated Lactobacillus johnsonii NCC 533 on S. aureus colonization in AD patients. The open-label, multicenter study consisted of the following two stages: (1) an assessment of S. aureus sampling and quantification methods and (2) evaluation of a cosmetic lotion containing a heat-treated probiotic. The first part of the study included 31 patients, while the second part of the study included 21 patients, carriers of S. aureus at the start of the study and with clinically visible lesions on day 8. Patients (male and female, aged 18–75 years) diagnosed with AD of mild-to-moderate severity were instructed to apply the probiotic lotion (3.1 × 1011 CFU/g) on lesional skin twice daily for 3 weeks. Application of the probiotic lotion controlled S. aureus colonization and was associated with local clinical improvement of the Scoring Atopic Dermatitis Index (SCORAD).
Oral administration of probiotics and prebiotics for the treatment of skin diseases
Rosacea has been associated with certain gastrointestinal diseases, such as small intestinal bacterial overgrowth (SIBO), irritable bowel syndrome, and others (Egeberg et al. 2016). However, the possible pathogenic link is unknown (Egeberg et al. 2016). Furthermore, SIBO treatment with antibiotics leads to the eradication of rosacea which was maintained for over nine months (Parodi et al. 2008).
Manzhalii et al. (2016) evaluated a probiotic therapy on intestinal-borne dermatoses. In that study, the placebo group (57 patients) received a vegetarian diet and conventional topical dermatoses therapy (i.e., ointments with tetracycline, steroids, and retinoids). In addition, the test group (37 patients) received an additional therapy consisting of oral Escherichia coli Nissle 1917 administration (one capsule for 4 days, then 2 capsules daily for the following month, each contained 2.5–25 × 109 live bacteria (CFU)). The administration of probiotics capsule significantly (P < 0.01) ameliorated dermatoses, as rosacea and acne, or caused complete recovery in contrast to the placebo group. This improvement was associated with significant increase in IgA levels in serum and cyokime IL-8 suppression.
Daily consumption of fermented milk containing Lactobacillus bulgaricus and Streptococcus thermophilus improved the clinical grade of acne over 12 weeks in a clinical study (Kim et al. 2010). This condition improved by lactoferrin (an anti-inflammatory milk protein) addition, i.e., lactoferrin-enriched fermented milk decreased acne vulgaris with reduced triacylglycerols in skin surface lipids. Additionally, neither of the probiotic-fermented milks produced alterations in skin hydratation or pH.
Jung et al. (2013) evaluated whether probiotics could work synergistically with antibiotics to treat inflammatory acne while reducing side effects of antibiotic administration. This open-label study considered forty-five females (aged 18–35 years) with mild to moderate acne vulgaris. Patients were randomly assigned to one of the following three groups: The probiotic group was advised to take one capsule in the morning and once in the evening. The minocycline group was advised to take the antibiotic once after dinner. Those patients with combined treatment, probiotic plus antibiotic were instructed to take the probiotic at least 2 h after their antibiotics dosage. In addition, all patients received a standard topical acne medication and a facial cleaner applied twice daily. Probiotic capsule included L. acidophilus and L. delbrueckii sp. bulgaricus each 5-billion CFU/capsule and Bifidobacterium bifidum 20 billon CFU/capsule. Clinical evaluation was performed at baseline and during follow-up visits 2, 4, 8, and 12 weeks after treatment initiation. After the first 4 weeks, all patients demonstrated a significant (P < 0.001) improvement in total lesion count. After 8 and 12 weeks, total lesion count of the group with combined treatment decreased significantly compared to the other two groups. Jung et al. (2013) showed that probiotics reduced potential side effects to antibiotic use while also augmenting antibiotic therapy.
In recent decades, the incidence of AD in children has increased. It is a chronic disease causing itchy skin (Chamlin et al. 2004). Several studies evaluated the effect of probiotics combined with prebiotics; however, the results are still inconclusive and may depend on the severity of the disease and probiotics applied. Wu et al. (2012) studied the effect of Lactobacillus salivarius and FOS on infants with AD. Sixty children (aged 2–14 years) received two capsules containing either L. salivarius plus FOS (treatment) or FOS only (control) daily for 8 weeks. The AD intensity decreased significantly (P ≤ 0.013) in children administered the synbiotic treatment compared to the control group. A study evaluated the effect of probiotics on the treatment of children with AD. Forty pediatric patients (aged 1–13 years) were assigned to test or placebo group. Both groups consumed two bags a day for a total of eight weeks. The test bags contained 2 × 109 CFU of four types of probiotic bacteria (B. bifidum, L. acidophilus, L. casei, and L. salivarius), while the placebo bags contained skim milk powder and dextrose. The combination of probiotic strains was effective in reducing the SCORAD in the treatment of AD in pediatric patients (Yeşilova et al. 2012). Recently, the effect of L. plantarum IS-10506 was also evaluated in children with mild and moderate AD in a randomized, double-blind, placebo-controlled trial. The probiotic function was evaluated by its ability to decrease the SCORAD and levels of serum immunoglobulin. In this study, twenty-two AD patients (aged 0–14 years), included in the test group (7 girls, 5 boys), received microencapsulated probiotic L. plantarum IS-10506 at 1010 CFU/day, and the placebo group (3 girls, 7 boys) received skim milk, twice daily for 12 weeks. The SCORAD reduction in the test group was significantly (P = 0.0001) higher than that in the placebo group at the end of the study. Levels of IL-4 (P = 0.0001), IFN-γ (P = 0.006), and IL-17 (P = 0.0001) were significantly lower in the probiotic group than those in the placebo group, while the IgE levels were not significantly changed. Thus, L. plantarum IS-10506 was able to reduce clinical symptoms in children diagnosed with AD and therefore with potential to treat this disease (Prakoeswa et al. 2017).
Cabana et al. (2017) evaluated the effectiveness of daily L. rhamnosus GG supplementation for the first 6 months of life to decrease eczema incidence, a potential early marker of asthma. The intervention infants received a daily dose of L. rhamnosus GG (1 × 109 CFU) and inulin (225 mg), and the control infants received inulin (325 mg) alone. The results showed that the early probiotic supplementation for the first 6 months of life did not prevent the development of either eczema or asthma at 2 years of age in infants with high risk of allergic diseases.
Meta-analysis of the probiotic effectiveness on skin diseases
A meta-analysis on the effect of probiotics and/or prebiotics for the prevention of eczema in infants determined that probiotics or synbiotics may reduce the eczema incidence in infants (< 2 years old). Systemic sensitization did not change following probiotic administration (Danq et al. 2013). A meta-analysis on the effect of prenatal and postnatal probiotic supplementation to prevent infantile and childhood eczema established that the pooled relative risk of all the studies was 0.74 (95% confidence interval 0.67 to 0.82); the authors concluded that the use of probiotic supplements during pregnancy and/or during infancy significantly reduced eczema incidence (Mansfield et al. 2014).
In another meta-analysis, Cuello-Garcia et al. (2015) evaluated the use of probiotics to prevent allergies, such as AD (eczema). The study included randomized controlled trials with a minimum follow-up of 4 weeks that compared any type of probiotic with placebo. The selected studies included pregnant women, breastfeeding mothers, infants, and children. The studies considered the use of probiotics from newborn infants to preschool and school-aged children (up to 9 years of age). The meta-analysis showed that the use of probiotics by pregnant women or breastfeeding mothers and/or given to infants reduced the risk of eczema in infants. However, the certainty in the evidence was low or very low because of the risk of bias, inconsistency and imprecision of results, and the indirectness of available information. In addition, the evidence did not support effect on other allergies, nutritional status, or incidence of adverse effects.
Recently, Yu et al. (2019) evaluated whether the clinical data support the use of oral and topical probiotics for certain dermatologic diseases. The results showed that few clinical trials exist that evaluate the utility of probiotics for the prevention and treatment of dermatologic diseases, with the exception of AD. Most studies investigated oral probiotic administration, and, of those utilizing topical probiotics, few included skin commensals. However, oral and topical probiotics appear to be effective for the treatment of certain inflammatory skin diseases.
Probiotics and prebiotics on the health of the female urogenital tract
The female urogenital tract consists of all the organs involved in reproduction and formation and release of urine. The reproduction system includes the uterus, ovaries, fallopian tubes, and vagina, while the kidneys, ureters, bladder, and urethra belong to the urinary system. Urogenital infections are a major health problem due to the number of women affected annually. These infections are common in women because the urethra is fairly short and straight, making it easier for germs to travel into the bladder. Nonsexually transmitted urogenital infections include bacterial vaginosis (BV), urinary tract infections (UTIs), and yeast vaginitis (Reid and Bruce 2003).
The resident microflora in the vagina includes anaerobic and aerobic microorganisms (Farage et al. 2010). The species of the Lactobacillus genus predominate in the microbiota of a normal vagina (Bik et al. 2017; Reid and Bruce 2003; Waigankar and Patel 2011) and consist of L. crispatus, L. jensenii, L. iners, and L. gasseri (Antonio et al. 1999; Bik et al. 2017; Falagas et al. 2006). Other Lactobacillus present are L. acidophilus, L. fermentum, L. plantarum,L. brevis, L. casei, L. vaginalis, L. delbrueckii, L. salivarius, L. reuteri and L. rhamnosus (Waigankar and Patel 2011). Lactobacilli create a biofilm on the surface of the vaginal mucosa protecting the vagina against pathogenic microorganisms by different mechanisms, such as secretion of organic acids, production of antimicrobial substances (H2O2 and bacteriocins), competition for nutrients, co-aggregation, stimulation of immune system, and exclusion of epithelium adhesion (Reid and Burton 2002).
A stable vaginal microbiota maintains health and can prevent the development of urogenital infections in the host (Barrons and Tassone 2008; Ma et al. 2012). However, this stability can be lost through antibiotics use, sexual activity, and hormonal alteration, among others (Barrons and Tassone 2008). BV is an overgrowth of anaerobic or Gram-negative organisms predominated with Gardnerella vaginalis, Atopobium vaginae, Megasphaera species, Mycoplasma hominis, Mobiluncus species, Ureaplasma urealyticum, Prevotella, and Peptostreptococcus species (Falagas et al. 2007; Fedricks et al. 2005). The common symptoms are malodorous vaginal discharge, and some patients may have vulvovaginal irritation (Livengood et al. 1990). Treatment of these infections involves different antibiotics, such as metronidazole, clindamycin, tinidazole, and secnidazole, administered orally or intra-vaginally (Menard 2011). However, due to its high morbidity and recurrences of BV after antibiotic treatment, alternative therapeutic agents are necessary (Table 1).
Yeast vaginitis is the main source of fungal-infecting organisms; the symptoms are white vaginal discharge characterized by its malodorous, non-homogenous caseous appearance, accompanied by vaginal and introital itch and irritation and evidence of vaginal inflammatory reaction (Abbott 1995). Vulvovaginal candidiasis (VVC) is an infection caused by Candida species with signs and symptoms of inflammation in the absence of other infectious agents (Achkar and Fries 2010; Köhler et al. 2012).
Evidence suggests that restoration of the vaginal microbiota and/or modulation of the local mucosal immune response can be achieved via supplementation with probiotics, which can be administered orally as a probiotic food supplement, intra-vaginally as vaginal suppositories, or applied topically as a gel (Gille et al. 2016; Stapleton et al. 2011; Zeng et al. 2010). Bertuccini et al. (2017) evaluated the effects of L. rhamnosus HN001 and L. acidophilus GLA-14 on bacterial vaginal pathogens (G. vaginalis and A. vaginae) and aerobic vaginitis (AV) (S. aureus and E. coli). In vitro assays demonstrated that probiotic bacteria possess inhibitory activity toward BV and AV pathogenic bacteria, with L. acidophilus GLA-14 generally exerting the highest antagonistic effect against anaerobic strains.
Topical application of probiotics or prebiotics
Zeng et al. (2010) compared the efficacy of a non-antibiotic sucrose gel with an antibiotic metronidazole gel for the treatment of BV. The study considered 560 subjects with clinically diagnosed BV. Patients applied the gel twice daily for five consecutive days. After the treatment, the therapeutic cure rates of the sucrose, metronidazole, and placebo gel groups were 83.1, 71.3, and 0.9% after 7–10 days, respectively. A second evaluation after 21–35 days of treatment showed 61.0, 66.7, and 7.3% cure rates for the sucrose, metronidazole, and placebo gel groups, respectively. According to these results, the researchers suggest that sucrose gel can restore the normal vaginal flora and used as a novel treatment for BV. Moreover, sucrose gel carries less risk of inducing pathogenic bacteria-resistant variants than the currently used antibiotics, including metronidazole. The authors argue that sucrose gel and metronidazole gel have different mechanisms of action in treating BV. The treatment with sucrose gel mediates a shift of vaginal bacterial flora, thereby promoting the growth of lactobacilli, which in turn generate lactic acid that reduce the vaginal pH and secrete antibacterial substances, inhibiting the adhesion and replication of the pathogenic anaerobic bacteria. In contrast, metronidazole gel appears to inhibit the growth of both the pathogenic bacteria and the beneficial lactobacilli. However, researchers believe that lactobacillus will recover faster than the pathogenic bacteria once the metronidazole gel treatment is complete because lactobacillus is more resistant to metronidazole than anaerobic bacteria.
Hemmerling et al. (2010) evaluated the colonization efficiency, safety, tolerability, and acceptability of L. crispatus CTV-05 (Lactin-V) administered by a vaginal applicator. The phase 2a Lactin-V was a randomized, double-blind, placebo-controlled trial in premenopausal women diagnosed with BV. Twenty-four women (aged 18–50 years) were randomized to test or control group. The test group (18 women) received Lactin-V (2 × 109 CFU/dose of L. crispatus CTV-05), while the control group (6 women) received a placebo for 5 initial consecutive days, followed by a weekly application over two weeks. Women returned for evaluation on days 10 and 28. The results showed that L. crispatus CTV-05 colonized eleven women of the test group at day 10 or day 28. The preexisting endogenous Lactobacillus flora at baseline line did not influence colonization with L. crispatus CTV-05. In addition, women who followed the protocol (7 doses at the recommended time) had better colonization than those who used a lower dose of Lactin-V. Finally, the product was well tolerated and accepted.
Coste et al. (2012) evaluated the efficacy and safety of an intravaginal prebiotic gel in the balance recovery of the vaginal flora in women previously treated for BV. The double-blind, parallel placebo-controlled, randomized clinical trial included 42 premenopausal, nonpregnant women aged 18–50 years. Patients confirmed with BV received oral antibiotic treatment with metronidazole during 7 days. Then, the test group (20 patients) were randomized to receive the APP-14 gel inside a small tube containing 7 mL of product with the glucooligosaccharides-alpha prebiotic (6%, equivalent to a minimum of 300 mg of oligosaccharide) and the Trifolium pratense extract (2%). Meanwhile, the control group (22 patients) received placebo gel without the active ingredients. The intravaginal-gel was self-administered once a day for 16 consecutive days. The results showed that after the antibiotic treatment there was no significant (P = 0.296) difference between the groups and the normal vaginal flora was not completely restored. After 8 days of treatment, all women who received prebiotic treatment had normal vaginal flora, while 33% of women in the control group did not completely restore normal vaginal flora. After 16 days treatment, all women treated with the prebiotic gel maintained normal vaginal flora, whereas in the placebo group 24% had not completely restored vaginal flora.
Oral administration of probiotics
The efficacy of orally administered capsules containing probiotics (L. rhamnosus GR-1 and L. reuteri RC-1) was evaluated in a randomized, double-blind, multicentric, placebo-controlled trial including 544 women older than 18 years of age diagnosed with vaginal infection (Vujic et al. 2013). The test group (395 women) and placebo group (149 women) received either two probiotic capsules (each containing >109 CFU of each Lactobacillus strain) or two placebo capsules per day for 6 weeks. Women underwent two gynecological examinations after 6 and 12 weeks of the study. Differences between groups were significant (P < 0.001) at forty-four days after the initial visit; the vaginal microbiota was restored in 243 women in the probiotic group compared to 40 women in the placebo group. After the additional 6 weeks of follow-up, normal vaginal microbiota was still present in more than half of the women in the probiotic group compared to only about one-fifth of those in the placebo group (P < 0.001).
Gille et al. (2016) studied the effect of an oral probiotic food supplement in maintaining or restoring the normal vaginal microbiota during pregnancy. Three hundred and twenty women with less than 12 completed weeks of pregnancy participated in the study. Patients consumed a probiotic capsule containing L. rhamnosus GR-1® and L. reuteri RC-14® (1 × 109 CFU of each strain per capsule) daily. The results showed that oral probiotics may be suitable for implementation in antenatal care but had no effect on vaginal health during mid-gestation.
Recently, Laue et al. (2018) evaluated the effect of yoghurt containing Lactobacillus strains on BV in a single-center, double-blind, placebo-controlled, randomized clinical trial with two parallel arms. The strains were isolated from healthy pregnant women and selected for acidification capacity, H2O2 production, glycogen utilization, bile salt tolerance, and pathogen inhibition. Thirty-six female volunteers were selected at reproductive age (≥ 18 years) with stable menstrual cycles or postmenopausal women diagnosed with BV. The test group (18 patients) received yoghurt (125 g/daily) containing live strains of L. crispatus, L. gasseri, L. rhamnosus, and L. jensenii and the placebo group (18 patients) received chemically acidified milk (125 g/daily) without bacterial strains. Patients were treated with oral metronidazole for 7 days (2 × 500 mg/daily). Starting with the treatment, women consumed twice daily either yoghurt or placebo for 4 weeks. After 4 weeks of intervention, 0 of 17 had BV in the test group vs. 6 of 17 in the placebo group. Additional intake of yoghurt containing these Lactobacillus strains improved the recovery rate, BV symptoms, and vaginal microbial pattern.
Intravaginal administration of probiotics
A recent study evaluated the probiotic capacity of Saccharomyces cerevisiae CNCM I-3856 to modulate the expression of pathogenicity determinants by Candida albicans in vivo during vaginal candidiasis in experimentally infected mice. Besides, the administration of S. cerevisiae was evaluated for its influence on the number and functions of a landmark sign of inflammation in the host. In general, the study determined that daily intravaginal administration of S. cerevisiae CNCM I-3856 leads to the suppression of several fungal components that play critical roles in fungal virulence of C. albicans at the vaginal level. S. cerevisiae modulated the expression of aspartyl proteinases as well as hyphal growth-associated genes, Hwp1 and Ece1, in the vaginal cavity. The probiotic effects on the host and the probiotic suppression of neutrophils influx caused by the fungus into the vaginas of the mice are likely related to lower interleukin-8 (cytokine associated with inflammatory responses) production and inhibition of aspartyl proteinase expression. Besides, there was no evidence of any cytotoxic effect either by the probiotic, when used in vivo, on vaginal epithelial cell and organ architecture, or in in vitro in human vaginal epithelium (Gabrielli et al. 2018).
Handalishy et al. (2014) compared the efficacy of vaginally administered probiotic vaginal tampons with oral metronidazole for the treatment of BV. This single blinded, randomized clinical trial included healthy women diagnosed with BV. The patients, aged 20–40 years, were randomly assigned to two groups (antibiotic and probiotic groups). The antibiotic group (16 patients) received oral metronidazole (500 mg twice daily for 7 days), while the probiotic group (15 patients) used lactobacilli-impregnated vaginal tampons for 5 days during menstruation. The probiotic group had significantly lower incidence of discharge than metronidazole group (P = 0.024) and higher cure rate than metronidazole group, but this difference was not significant (P = 0.269).
Verdenelli et al. (2016) evaluated the effect of probiotic suppositories, SYNBIO® gin, on vaginal health. The study included 35 apparently healthy women (aged 18–48 years), who were instructed to receive daily for 7 days the probiotic suppositories SYNBIO® gin, containing at least 109 CFU of viable lactobacilli, a combination of L. rhamnosus IMC 501® and L. paracasei IMC 502®. Women were examined three times during the study. The results revealed the presence of the two strain originating from SYNBIO® gin in 100% of women at visit 2 and 34% at visit 3. In addition, the probiotic suppositories were well-tolerated and had no adverse effects, and no significant changes were registered for pH between visits.
Meta-analysis of the probiotic effectiveness on female urogenital tract diseases
A meta-analysis evaluating the efficacy of Lactobacillus found that probiotic strains of Lactobacillus are safe and effective in preventing recurrent UTIs in adult women. However, more randomized clinical trials are required before providing a definitive recommendation since only 127 patients contributed data to this meta-analysis (Grin et al. 2013).
Schwenger et al. (2015) evaluated the potential therapeutic advantage of probiotics in a meta-analysis to prevent UTIs in susceptible patients in terms of morbidity and mortality compared to a placebo and other prophylactic interventions. The meta-analysis showed no significant reduction (P = 0.27) in the risk of recurrent symptomatic bacterial UTIs between patients treated with probiotics and a placebo. Furthermore, risk reduction of recurrent symptomatic bacterial UTIs was insignificant between probiotic and antibiotic-treated patients (1 study, 223 participants: RR 1.12, 95% CI 0.95 to 1.33). However, a benefit cannot be excluded as the data were few and derived from small studies with poor methodological reporting.
In a recent meta-analysis, three double-blind randomized clinical trials evaluated the effect of oral administration of a mixture of four Lactobacillus strains (L. crispatus LbV 88 (DSM 22566), L. gasseri LbV 150N (DSM 22583), L. jensenii LbV 116 (DSM 22567), and L. rhamnosus LbV96 (DSM 22560) on vaginal dysbiosis. Selected clinical trials included 60 male-to-female transsexual women with neovagina, 36 women with bacterial vaginosis, and 22 postmenopausal breast cancer patients receiving chemotherapy. The meta-analysis showed that oral intake of a probiotic product containing Lactobacillus strains either as yoghurt or in capsule form may improve the microbial pattern in different forms of vaginal dysbiosis (de Vrese et al. 2019).
Probiotics and prebiotics on respiratory tract health
The respiratory tract is a complex system whose function in human physiology is the exchange of oxygen and carbon dioxide (Man et al. 2017). It consists of the upper respiratory tract (URT) and the lower respiratory tract (LRT). The URT filters, humidifies, and heats inhaled air before it reaches the lungs, i.e., protects the lower respiratory tract (Watelet and Van Cauwenberge 1999). The URT includes the nostrils, nasal passages, paranasal sinuses, nasopharynx, and oropharynx, and the portion of the larynx above the vocal cords. The LRT is formed by the larynx portion below the vocal cords, trachea, smaller airways, and alveoli (Man et al. 2017). These structures inhale air from the upper respiratory system, absorb the oxygen, and release carbon dioxide in exchange.
Several microbial communities, such as bacteria, viruses, and fungi, which change according to the individual age and health, inhabit the healthy respiratory tract. These microbial communities will resist the colonization of pathogenic microorganisms, benefiting the host health. An imbalance or perturbations in these communities will allow bacterial overgrowth and development of respiratory tract infections.
In the first week of life, the healthy URT contains Staphylococcus spp., Corynebacterium spp., Dolosigranulum spp., and Moraxella spp. (Bosch et al. 2016). In adults, these microorganisms colonize the healthy URT in addition to the Propionibacterium, Streptococcus, Haemophilus, Prevotella, and Bifidobacterim species (Man et al. 2017; Schenck et al. 2016). Advances in high-throughput sequencing methods have enabled to determine that a healthy child's URT includes viruses, such as human rhinovirus, bocavirus, adenovirus, and coronavirus, among others (Bogaert et al. 2011). Furthermore, the URT contains mycobiota that include Aspergillus, Penicillium, Candida, and Alternaria species (Eidi et al. 2016).
Studies in healthy LRT have shown that their microbial composition is age-dependent. The microbiota of premature children is composed of Staphylococcus, Ureaplasma, or Acinetobacter species, while, in adults, the preceding species are present as well as Moraxella, Haemophilus, Staphylococcus, and Streptococcus species. Moreover, the healthy LRT includes viruses, such as members of the Anelloviridae family and bacteriophages (Young et al. 2015). The healthy lung mycobiome is composed of the Eremothecium, Systenotrema, and Malassezia genera (Charlson et al. 2012; van Woerden et al. 2013).
Respiratory tract infections (RTIs) are a common human illness caused by infections of mucosal surfaces in the nose, sinuses, pharynx, and/or larynx (Gerritsen and Ormel 2016). Disease treatment depends on the cause of the infection; however, sometimes, antibiotics or antivirals are administered. Nevertheless, the increased bacterial resistance resulting from inappropriate and extensive antibiotics use and the reduced availability of vaccines for most viruses and bacteria necessitate the search for new, safe, efficient, and suitable therapies to treat RTIs, and the use of pre- and probiotics is a prominent innovative strategy.
Several studies have evaluated the effect of probiotics on RTI prevention (Table 1), but the results are inconclusive so far. Smith et al. (2013) assessed the effect of oral probiotic consumption on health-related quality of life during upper respiratory infections in apparently healthy college students living on campus residence. The prospective, randomized, double-blind, placebo-controlled trial included 198 students (27 males and 70 females in placebo group and 20 males and 81 females in test group) aged 18–24 years (median age 19 years). The students were randomized to receive a placebo- or probiotic-containing powder (5 g) daily, loaded with 1 × 109 CFU of both L. rhamnosus LGG® and B. animalis ssp. lactis Bb-12®. After 12 weeks, the median duration of upper respiratory infections was significantly shorter by 2 days, and the median severity score was significantly lower (34%) for probiotics vs. placebo groups, indicative of a higher health-related quality of life during upper respiratory infections.
On the other hand, Salarkia et al. (2013) evaluated the effects of probiotic yoghurt on performance and health status of young adult female endurance swimmers (mean age 13.8 ± 1.8 years, weight 48.6 ± 7.5 kg, and height 159 ± 5.6 cm). Swimmers were randomly assigned to test or control group each with 23 volunteers. The test group received probiotic yoghurt (400 mL) containing 4 × 1010 CFU/mL of L. acidophilus spp., L. delbrueckii bulgaricus, B. bifidum, and Streptococcus salivarius thermophilus daily for 8 weeks, while the control group received an ordinary yoghurt. The daily consumption of probiotic yoghurt significantly reduced the number of respiratory infection episodes (P = 0.009) and duration of some symptoms, such as dyspnea (P = 0.024) and ear pain (P = 0.024), in female swimmers.
Michalickova et al. (2016) evaluated the influence of Lactobacillus helveticus as Lafti L10® supplementation on the duration, severity, and incidence of URT illness and different immune parameters in the population of elite athletes. Thirty-nine elite athletes were randomized either to the test (15 males, 5 females) or placebo (14 males, 5 females) group in a double-blind, placebo-controlled trial. The test group received the probiotic capsules of L. helveticus as Lafti® L10 (2 × 1010 CFU) daily for 14 weeks. The control group received placebo capsules, identical in taste and appearance as the probiotic capsules. Lafti L10® significantly reduced the URT illness episode duration (P = 0.047) and the number of symptoms (P = 0.035).
Another study determined that daily administration of a probiotic combination (B. animalis subsp lactis and L. rhamnosus GG) for 6 months did not reduce the number of days absent due to infections in Danish infants aged 8 to 14 months at enrollment time in child care (Laursen et al. 2017).
Studies on the use of probiotics and prebiotics for RTIs are scarce. Luoto et al. (2014) evaluated the potential of early prebiotic or probiotic supplementation to reduce the risk of virus-associated RTIs during the first year of life in preterm infants. The study period (12 months) was completed by 68 (44 males, 24 females) of the 94 babies, who participated in the randomized, double-blind, placebo-controlled trial. The study included infants with a mean gestational age of 35 weeks (range, 32 to 36 + 6 weeks) and a mean birth weight of 2393 g (range, 1550–3965 g). Preterm infants received oral prebiotics (GOS:polydextrose in a 1:1 ratio at 1 × 600 mg/day for 1 to 30 days and 2 × 600 mg/day for 31 to 60 days) or a probiotic (L. rhamnosus GG, ATCC 53103; at 1 × 109 CFU/day for 1 to 30 days and 2 × 109 CFU/day for 31 to 60 days). The placebo group received microcrystalline cellulose and dextrose anhydrate between 3 and 60 days. Incidence of RTIs was significantly lower in infants receiving prebiotics (P < 0.001) or L. rhamnosus GG (P = 0.022) compared with those receiving placebo. Furthermore, the incidence of rhinovirus-induced episodes was significantly lower in the prebiotic (P = 0.003) and probiotic (P = 0.051) groups than the placebo group.
Lau et al. (2018) studied the effects of Bifidobacterium longum BB536 on diarrhea and/or upper respiratory illnesses. Two hundred and nineteen pre-school children aged 2–6 years old completed this randomized, double-blind, and placebo-controlled trial. The children received a sachet (1 g) containing either B. longum (5 × 109 CFU) or placebo daily. After 10 months, the probiotic strain did not exert significant effect against diarrhea in children. However, the duration of sore throat decreased (46%), with marginal reduction for fever duration (27%), runny nose (15%), and cough (16%), compared to the placebo. In addition, the abundance of the genus Faecalibacterium often associated with anti-inflammatory and immuno-modulatory properties was significantly higher in the test group (P < 0.05) compared to the placebo group.
A recent study evaluated the direct antipathogenic effects of Lactobacillus species on Moraxella catarrhalis, a URT pathogen linked to otitis media, sinusitis, and chronic obstructive pulmonary disease (van den Broek et al. 2018). The study included agar-based assays, time course analysis, biofilm assays, and minimal inhibitory concentration performed using spent culture supernatants at two pH values (4.3 and 7) and D- and/or L-lactic acid at three pH values (2, 4, and 7). Additionally, cell line assays for adhesion competition and immunomodulation were used to substantiate the inhibitory effect of twelve Lactobacillus strains against M. catarrhalis. The positive control was hexetidine (0.1%), a common oral antiseptic agent for oropharyngeal infections. All lactobacilli tested demonstrated strong activity against M. catarrhalis. Strains, such as L. rhamnosus GG, L. casei Shirota, L. casei DN-114001, L. plantarum LMG1284, and L. plantarum WCFS1, presented clear inhibition zones in the range of the positive control. Screening of the activity of the spent culture supernatants after different treatments demonstrated that lactic acid exerted strong antimicrobial activity against this pathogen. In in vitro test, minimal inhibitory concentration values for D- and L-lactic acid ranged from 0.5 to 27 g/L depending on pH. L. rhamnosus GG also reduced M. catarrhalis adhesion (> 50%) to human airway epithelial cells and the expression of mucin MUC5AC, pro-inflammatory cytokines, interleukin, and tumor necrosis factor-α at least 1.2 fold.
One of the benefits of probiotics consumption is the stimulation of the immune system in host health. The immune system is complex and must be maintained and constantly stimulated to recognize and efficiently neutralize pathogens. Probiotics play an important role in balancing the host defense mechanism, including immune responses. However, the mechanism of action of probiotics on the immune system is still not fully elucidated. In this context, Zhang et al. (2018) evaluated the effect of a probiotic supplementation on volunteers who contracted the common cold four or more times in the past year. This single-center, double-blind, randomized, controlled, prospective trial included 136 adults (male and female) between 25 and 45 years old. Adults received a probiotic drink (L. paracasei and L. casei 431® (at least 3 × 107 CFU/mL) and Lactobacillus fermentium PCC® (at least 3 × 106 CFU/mL)) or a placebo for a 12-week study. Probiotic consumption significantly reduced the incidence of upper respiratory infections (P < 0.023) and flu-like symptoms (P < 0.034) compared to placebo. Moreover, the test group showed a significantly higher level of serum IFN-γ (P < 0.001) and gut sIgA (P < 0.0010) compared to the placebo group and their baseline test results. Therefore, the combined consumption of these three probiotic strains can reduce the incidence of the upper respiratory infection, probably by increasing the level of IFN-γ in the blood and sIgA in the gut.
Meta-analysis of the probiotic effectiveness on respiratory tract diseases
King et al. (2014) studied the effects of probiotics on the duration of illness in healthy children and adults who developed common acute respiratory infections. This meta-analysis included twenty randomized controlled trials of varied durations that compared Lactobacillus and/or Bifidobacterium strains consumed orally with placebo or with “untreated” in apparently healthy children or adults who developed acute RTI at some period during the trial. Ten trials investigated probiotics use in children (aged 1–12 years) and ten were conducted in adults (aged 18–67 years), two of which were conducted in elderly free-living adults. This meta-analysis determined that probiotics consumption significantly reduced the duration of illness episodes, number of days of illness per person, and number of days absent from day care/work/school.
Hao et al. (2011) evaluated probiotics effects to prevent acute URT infections in randomized controlled trials, excluding all crossover studies due to potential residual treatment effects. The participants were children and adults of all ages excluding those vaccinated against influenza or other acute URTIs within the last 12 months, taking immune-stimulating medications, undertaking abnormal physical exercise, or with known congenital or acquired immune defects or allergies. The trials included different types of probiotics usually compared with placebo. This meta-analysis showed that compared to placebo, probiotics may be more beneficial for preventing acute URT infections in people of all ages. However, the quality of the evidence was low or very low, because some studies had small sample sizes and the quality of the methods was unsatisfactory in some studies. In contrast, another meta-analysis determined that probiotic consumption appears to be a feasible way to decrease RTI incidence in children from newborn to 18 years (Wang et al. 2016).
Laursen and Hojsak (2018) evaluated the strain-specific probiotic effects on RTIs in children attending day care. This meta-analysis included fifteen randomized controlled trials with 5121 children in day care settings (aged 3 months to 7 years). Twelve randomized controlled trials, with high diversity in reported outcomes, showed that L. rhamnosus GG may be moderately effective in reducing RTI duration and that B. animalis ssp. lactis Bb-12 had no effect on RTI duration or on absence from day care. Besides, the potential effect of other probiotic strains or their combination was impossible to evaluate due to limited available data.
Commercial probiotic or prebiotic products on the market
A series of commercial products containing prebiotics and/or probiotics are available in the market as a complementary approach to manage infections occurring on the skin, female urogenital system, and respiratory tract or to strengthen the immune system, among others (Table 2, Fig. 1).
Table 2.
Prebiotics and/or probiotic products commercially available on the market as a complementary approach to manage infections occurring on the skin, female urogenital, and respiratory tract
| Product name | Distributor or producer | Probiotic and/or prebiotic | Effect | Reference |
|---|---|---|---|---|
|
Metlin® Metlos® |
Nekutli S.A. de C.V. (Mexico) | - Fructans extracted from Blue Agave (Agave tequilana Weber). |
- Both promote changes of microbiota (bifidogenic effect) and exert benefits to host health. - Metlin® and Metlos® are long and short chain fiber, respectively. - Both are sold as syrup (75° Brix) or powder (4% moisture). |
López-Velázquez et al. (2015), Nekutli (2018) |
| AmpliVida® | Prenexus Health. Distributed by DSM—Bright Science. Brighter Living™ (USA) | - Xylooligosaccharide derived from non-genetically modified sugar cane. | - AmpliVida® is targeted to beneficial microbes that are uniquely able to process 5-carbon sugars. | Menayang (2018) |
| NeoGOS® | Neo Cremar Co. Ltd. (South Korea) | - Galacto-oligosaccharides |
- NeoGOS® is a non-digestible food ingredient that benefits the host by selectively stimulating the growth and activity of a limited number of beneficial bacteria in the colon. - NeoGOS® is found naturally in human milk. - Is a product that might reduce wrinkles and increase skin hydration on a span of 8 to 12 weeks. |
Prince (2018a), Schultz (2018) |
|
Sensiline® Sensiline® Bio |
Silab (France) | - Hydrolyzed flaxseed extract. Enriched with acid polysaccharides and peptidoglycans |
- Aqueous solution that prevents the stimulation of cells involved in inflammatory processes. - It is applied to soothe sensitive skin. |
Shim et al. (2015), Silab (2018a, b) |
| Innéov Bronze+ | Innéov (Spain) |
- L. johnsonii La1 - Lycopene and β-carotene |
- Provides to the skin a more intense, lasting and homogeneous tan. Decreases the appearance of sunspots. | Innéov (2019) |
| Redness Solutions | Clinique Laboratories LLC (New York, USA) | - Lactobacillus ferment |
- This product is formulated with probiotic technology to help strengthen skin's moisture. - Clinique also has three other products with probiotic technology. |
Clinique (2019) |
| JooMo® | JooMo HQ (United Kingdom) | - It is 100% natural and preservative free products. |
- Cosmetic line for face and body wash. - These products based on Microbiota Immune Response Regulation (MIRR)® technology restore, rebuild, repair and stimulate the skin’s natural immune environment, keeping the skin’s natural defenses intact. - JooMo® uses the synergistic effect of totally natural ingredients to create the conditions for thriving and biodiverse the ecosystem. |
Whitehouse (2018) |
| Ecologic® Panda | Winclove Probiotics (The Netherlands) |
- B. bifidum W23 B. lactis W51 B. lactis W52 Lc. lactis W58 - Dry powder with 1 × 109 CFU/g. |
- Ecologic® Panda is a multispecies probiotic formulation, which have been selected for their capacity to strengthen the intestinal barrier function, and influence the immune system. - Ecologic® Panda reduces the development of eczema in children and maintained the effect up to two years after delivery, when pregnant women received Ecologic® Panda during pregnancy and after delivery. |
Winclove Probiotics (2018a) |
| Ecologic® Allergycare | Winclove Probiotics (The Netherlands) |
- B. bifidum W23 B. lactis W51 L. acidophilus W55 L. casei W56 L. salivarius W57 Lc. lactis W58 - Dry powder with 1 × 109 CFU/g. - Supplemented with vitamin B2 and biotin. |
- The bacterial strains of Ecologic® Allergycare have been selected to influence the immune system. - Ecologic® Allergycare reduces the SCORAD in children between 1–13 years of age suffering from atopic dermatitis. |
Winclove Probiotics (2018b) |
| Intimique® Femme | Roelmi HPC (Italy) |
- L. plantarum PBS067, L. rhamnosus LRH020, B. lactis BL050. - Capsules contain at least 3 × 109 CFU of each strain. |
- Capsules of a novel food supplement for women intimate healthcare. - In the clinical trials, the product colonizes human vaginal epithelium after oral administration. |
TKS (2018) |
|
Lactogyn® Lactogyn® vaginalne kapsule |
Jadran–Galenski Laboratorij d.d. (JGL d.d.). |
- Both products contain: L. rhamnosus, GR-1® L. reuteri RC-14® |
- Lactogyn® oral capsules is a nutritional supplement with bacterial cultures of two probiotics for girls, women, pregnant women, and menopausal women. - The product restores the balance of the vaginal flora and thereby, naturally, helps establish and maintain urogenital health. - Lactogyn® vaginalne kapsule are used to rebuild and maintain the natural balance of vaginal flora and are applied together with Lactogyn® oral capsules. |
Jgl (2018a, b) |
| Raw probiotics™ vaginal care | Garden of Life® LLC (USA) |
- Each capsule contains thirty-eight probiotic strains - 50 billion CFU/capsule |
- Raw Probiotics™ Vaginal Care maintains a healthy bacterial balance promotes vaginal and urinary tract health. | Garden of Life (2019) |
| Ecologic® FEMI+ | Winclove Probiotics (The Netherlands) |
- B. bifidum W28 L. acidophilus W70 L. helveticus W74 L. brevis W63 L. plantarum W21 L. salivarius W24 - Capsules contain ~ 1.5 × 109 CFU - Supplemented with Lactoferrein |
- Ecologic® FEMI+ is a multispecies probiotic specifically developed to enhance the natural vaginal microbiota and thereby prevent the occurrence and/or recurrence of vaginal Candida infections. | Winclove Probiotics (2018c) |
| Uno Colación® | Soprole (Chile) |
- L. rhamnosus (LR activ®) - Product contain 1 × 107 CFU/g |
- Uno Colación® is a novel Chilean product designed to strengthen the immune system of children. It is a milk drink packaged in an 80 mL Tetra Pak® container. - This product is available in strawberry and multi-fruit flavors. It is low in sodium, sugar- and fat-free. |
Daniells (2018) |
| Lafti L10® | Lallemand Health Solutions Inc. (Canada) |
- L. helveticus - Capsules contain 2 × 1010 CFU - Inulin (200 mg) |
- Lafti L10® capsule promotes gastrointestinal health in physically active adults and helps reduce the incidence of cold-like symptoms in adults with exercise-induced stress. - Clinical studies have shown that Lafti L10® exert immune-health benefits. |
Prince (2018b), Michalickova et al. (2016) |
| HLC Fit for School—Pharmax™ |
Made in UK for Seroyal USA. Pharmax™ is a brand of Seroyal. |
- Each chewable tables contain 12.5 billion CFU of HCL® consortium - L. acidophilus (CUL-21) L. acidophilus (CUL-60) B. animalis subsp. lactis (CUL-34) B. bifidum (CUL-20) |
- Pharmax™—HLC Fit for School includes a combination of research-driven probiotic strains and vitamin C that helps support upper respiratory tract health and immune function in children. | Seroyal (2019) |
Conclusions and future trends
Probiotics and/or prebiotics can be beneficial in preventing and treating certain infectious diseases of the skin, female urogenital tract, and respiratory tract. The evidence suggests that probiotics consumption can decrease the presence of eczema in children when their mothers have consumed probiotics during pregnancy and lactation. In women, probiotics consumption can be effective in preventing recurrent urinary tract infections. The consumption of synbiotic products can reduce respiratory tract infections and their duration and severity. However, the in vitro, in vivo, and clinical studies present certain shortcomings, such as the wide variety of probiotics considered in studies, which makes it difficult to identify the individual effect of each probiotic strain. On the other hand, the studies lack standardization, the number of patients or volunteers required in the trials is small, and the cost-effectiveness of probiotics is another factor to analyze. These issues mean that the information reported in the current literature is limited and the evidence is inconclusive. However, several probiotic and prebiotic products aiming to improve consumers’ quality of life are already on the market, such as Sensiline®, JooMo®, Lafti L10®, NeoGOS®, and others.
Advances in next-generation analytical tools have enabled the exploration of specific probiotics that may positively affect the health of the immune system, cognition, and mental health, among others. Moreover, the advances in studies related to microbiome sequencing when individuals are in different states of activity have resulted in the identification of bacteria associated with peak performance and recovery. This will enable identification of specific probiotics that can promote recovery and energy metabolism and novel probiotic strains that can recover lipid metabolism, reduce symptoms of psychological distress during and after pregnancy, positively impact on children’s behavioral, cognitive, and emotional development, and enhance performance in athletes, among others. Therefore, well-defined and multidisciplinary clinical studies must be developed. Recent publications open new lines of research on novel probiotic and synbiotic formulations that can prolong longevity through mechanisms of gut–brain axis communication related to chronic disease management. This shows that there are still many research questions to answer and hypotheses to confirm regarding the benefit of probiotics on health and quality of life.
Abbreviatons
- AD
Atopic dermatitis
- AV
Aerobic vaginitis
- BMI
Body mass index
- BV
Bacterial vaginosis
- DF
Dietary fiber
- DM
Diabetes mellitus
- FOS
Fructooligosaccharides
- GMH
Glucomannan hydrolysates
- GOS
Galactooligosaccharides
- LRT
Lower respiratory tract
- MJ
Megajoule
- QoL
Quality of life
- RTIs
Respiratory tract infections
- SCORAD
Scoring Atopic Dermatitis Index
- SDG
Secoisolariciresinol diglucoside
- SIBO
Small intestinal bacterial overgrowth
- Th
T-helper cell
- URT
Upper respiratory tract
- UTIs
Urinary tract infections
- VVC
Vulvovaginal candidiasis
Authors’ contribution
Mariela Bustamante conceived, designed, and wrote the review. B. Dave Oomah, Wanderley P. Oliveira, César Burgos-Díaz, Mónica Rubilar, and Carolina Shene provided ideas and contributed to writing the manuscript.
Funding information
This research was supported by CONICYT through FONDECYT project 11160249.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbott J. Clinical and microscopic diagnosis of vaginal yeast infection: a prospective analysis. Ann Emerg Med. 1995;25:587–591. doi: 10.1016/s0196-0644(95)70168-0. [DOI] [PubMed] [Google Scholar]
- Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23:253–273. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghazzewi FH, Tester RF. Effect of konjac glucomannan hydrolysates and probiotics on the growth of the skin bacterium Propionibacterium acnes in vitro. Int J Cosmet Sci. 2010;32:139–142. doi: 10.1111/j.1468-2494.2009.00555.x. [DOI] [PubMed] [Google Scholar]
- Anadón Arturo, Martínez-Larrañaga Maria Rosa, Caballero Virginia, Castellano Victor. Bioactive Foods in Promoting Health. 2010. Assessment of Prebiotics and Probiotics; pp. 19–41. [Google Scholar]
- Antonio M, Hawes S, Hillier S. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristic of women colonized by these species. J Infect Dis. 1999;180:1950–1956. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- Bajury DM, Nashri SM, Hung PKJ, et al. Evaluation of potential prebiotics: a review. Food Rev Int. 2017;34:639–664. [Google Scholar]
- Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clin Ther. 2008;30:453–468. doi: 10.1016/j.clinthera.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Bateni E, Tester R, Al-Ghazzewi F, et al. The use of konjac glucomannan hydrolysates (GMH) to improve the health of the skin and reduce acne vulgaris. Am J Dermatol Venereol. 2013;2:10–14. [Google Scholar]
- Beerepoot MA, ter Riet G, Nys S, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704–712. doi: 10.1001/archinternmed.2012.777. [DOI] [PubMed] [Google Scholar]
- Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, et al. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61:160–174. doi: 10.1159/000342079. [DOI] [PubMed] [Google Scholar]
- Bertuccini L, Russo R, Iosi F, et al. Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int J Immunopathol Pharmacol. 2017;30:163–167. doi: 10.1177/0394632017697987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bik EM, Bir SW, Bustamante JP et al (2017) A new sequencing-based women’s health assay combining self-sampling, HPV detection and genotyping, STI detection, and vaginal microbiome analysis. bioRxiv. 10.1101/217216 [DOI] [PMC free article] [PubMed]
- Blanchet-Réthoré S, Bourdès V, Mercenier A, et al. Effect of a lotion containing the heat-treated probiotic strain. Clin Cosmet Investig Dermatol. 2017;10:249–257. doi: 10.2147/CCID.S135529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert D, Keijser B, Huse S, et al. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS ONE. 2011;6:e17035. doi: 10.1371/journal.pone.0017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguniewicz M, Leung DYM. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch AATM, Levin E, van Houten MA, et al. Development of upper respiratory tract microbiota in infancy is affected by mode of delivery. EBioMedicine. 2016;9:336–345. doi: 10.1016/j.ebiom.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VL, Rocha LPDS, Bernardo DD, et al. What do Cochrane systematic reviews say about probiotics as preventive interventions? Sao Paulo Med J. 2017;135:578–586. doi: 10.1590/1516-3180.2017.0310241017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabana MD, McKean M, Caughey AB, et al. Early probiotic supplementation for eczema and asthma prevention: a randomized controlled trial. Pediatrics. 2017;140:1–11. doi: 10.1542/peds.2016-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos D, Betalleluz-Pallardel I, Chirinos R, et al. Prebiotic effects of yacon (Smallanthus sonchifolius Poepp. & Endl), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food Chem. 2012;135:1592–1599. doi: 10.1016/j.foodchem.2012.05.088. [DOI] [PubMed] [Google Scholar]
- Chamlin SL, Frieden IJ, Williams ML, et al. Effects of atopic dermatitis on young American children and their families. Pediatrics. 2004;114:607–611. doi: 10.1542/peds.2004-0374. [DOI] [PubMed] [Google Scholar]
- Charlson ES, Diamond JM, Bittinger K, et al. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med. 2012;186:536–545. doi: 10.1164/rccm.201204-0693OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinique (2019) Redness Solutions makeup broad spectrum SPF 15 with probiotic technology. Available at https://www.clinique.com/product/1689/13188/skin-care/redness/redness-solutions-makeupbroad-spectrum-spf-15with-probiotic-technology#details. (Accessed 01 March 2019)
- Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69:1052S–1057S. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- Coste Isabelle, Judlin Philippe, Lepargneur Jean-Pierre, Bou-Antoun Sami. Safety and Efficacy of an Intravaginal Prebiotic Gel in the Prevention of Recurrent Bacterial Vaginosis: A Randomized Double-Blind Study. Obstetrics and Gynecology International. 2012;2012:1–7. doi: 10.1155/2012/147867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello-Garcia CA, Brozek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952–961. doi: 10.1016/j.jaci.2015.04.031. [DOI] [PubMed] [Google Scholar]
- Dahl MV. A treatment strategy for rosacea. In: Zouboulis CC, Katsambas AD, Kligman AM, editors. Pathogenesis and treatment of acne and rosacea. Berlin: Springer; 2014. [Google Scholar]
- Daniells S (2018) Soprole works with Tetra Pak to launch new probiotic format for children. Available at https://www.nutraingredients.com/Article/2018/05/24/Soprole-works-with-Tetra-Pak-to-launch-new-probiotic-format-for-children. (Accessed 04 June 2018)
- Danq D, Zhou W, Lun ZJ, et al. Meta-analysis of probiotics and/or prebiotics for the prevention of eczema. J Int Med Res. 2013;41:1426–1436. doi: 10.1177/0300060513493692. [DOI] [PubMed] [Google Scholar]
- de Souza VMC, dos Santos EF, Sgarbieri VC. The importance of prebiotics in functional foods and clinical practice. Food Nutr Sci. 2011;2:133–144. [Google Scholar]
- de Vrese M, Laue C, Papazova E, et al. Impact of oral administration of four Lactobacillus strains on Nugent score—systematic review and meta-analysis. Benef Microbes. 2019;10:483–496. doi: 10.3920/BM2018.0129. [DOI] [PubMed] [Google Scholar]
- Delwart EL. Viral metagenomics. Rev Med Virol. 2007;17:115–131. doi: 10.1002/rmv.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders—a population-based cohort study. Br J Dermatol. 2016;176:100–106. doi: 10.1111/bjd.14930. [DOI] [PubMed] [Google Scholar]
- Eidi S, Kamali SA, Hajari Z, et al. Nasal and indoors fungal contamination in healthy subjects. Health Scope. 2016;5:e30033. [Google Scholar]
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266–272. doi: 10.1093/jac/dkl246. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for the treatment of women with bacterial vaginosis. Clin Microbiol Infect. 2007;13:657–664. doi: 10.1111/j.1469-0691.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- FAO/WHO . Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Córdoba: Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; 2001. [Google Scholar]
- FAO/WHO . Guidelines for the evaluation of probiotics in Food, Food and Agriculture Organization of the United. London: Nations/World Health Organization; 2002. [Google Scholar]
- Farage MA, Miller KW, Sobel JD. Dynamics of the vaginal ecosystem—hormonal influences. Infect Dis Res Treatment. 2010;3:1–15. [Google Scholar]
- Fedricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- Folch MH. The role of prebiotics and probiotics in gastrointestinal disease. Gastroenterol Clin North Am. 2018;47:179–191. doi: 10.1016/j.gtc.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Gabrielli E, Pericolini E, Ballet N, et al. Saccharomyces cerevisiae-based probiotic as novel anti-fungal and anti-inflammatory agent for therapy of vaginal candidiasis. Benef Microbes. 2018;9:219–230. doi: 10.3920/BM2017.0099. [DOI] [PubMed] [Google Scholar]
- Garden of Life (2019) Raw probiotics™ vaginal care. Available at https://www.gardenoflife.com/raw-probiotics-vaginal-care-30-vegetarian-capsules-658010116633. (Accessed 04 February 2019)
- Gerritsen C, Ormel G. Probiotics to prevent upper respiratory tract infections. Agro Food Ind Hi Tech. 2016;27:1–4. [Google Scholar]
- Gibson GR, Fuller R. Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. J Nutr. 2000;130:391S–395S. doi: 10.1093/jn/130.2.391S. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Gille C, Böer B, Marschal M, et al. Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial. Am J Obstet Gynecol. 2016;215:608.e1–608.e7. doi: 10.1016/j.ajog.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Gogineni VK, Morrow LE, Malesker MA. Probiotics: mechanisms of action and clinical applications. J Probiotics Health. 2013;1:1–11. [Google Scholar]
- Gomez E, Touhy KM, Gibson GR, et al. In vitro evaluation of the fermentation properties and potential prebiotic activity of Agave fructans. J Appl Microbiol. 2010;108:2114–2121. doi: 10.1111/j.1365-2672.2009.04617.x. [DOI] [PubMed] [Google Scholar]
- Grice EA. The skin microbiome: potential for novel diagnostic and therapeutic approaches to cutaneous disease. Semin Cutan Med Surg. 2014;33:98–103. doi: 10.12788/j.sder.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grin PM, Kowalewska PM, Alhazzan W, et al. Lactobacillus for preventing recurrent urinary tract infections in women: meta-analysis. Can J Urol Int. 2013;20:6607–6614. [PubMed] [Google Scholar]
- Handalishy II, Behery MA, Elkhouly M, et al. Comparative study between probiotic vaginal tampons and oral metronidazole in treatment of bacterial vaginosis. Al Azhar Assiut Med J. 2014;12(Suppl 2):185–203. [Google Scholar]
- Hao Q, Lu Z, Dong BR, et al. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2011;9:CD006895. doi: 10.1002/14651858.CD006895.pub2. [DOI] [PubMed] [Google Scholar]
- Hemmerling A, Harrison W, Schroeder A, et al. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. Sex Transm Dis. 2010;37:745–750. doi: 10.1097/OLQ.0b013e3181e50026. [DOI] [PubMed] [Google Scholar]
- Holland KT, Bojar RA. Cosmetics: what is their influence on the skin microflora? Am J Clin Dermatol. 2002;3:445–449. doi: 10.2165/00128071-200203070-00001. [DOI] [PubMed] [Google Scholar]
- Huang MCJ, Tang J. Probiotics in personal care products. Microbiology Discovery. 2015;3:1–9. [Google Scholar]
- Innéov (2019) Innéov Bronzer+. Available at https://www.inneov.es/productos/inneov-bronze#ingredientes. (Accessed 01 March 2019)
- Jgl (2018a) Lactogyn®. Jadran–Galenski Laboratorij d.d. Available at https://www.jgl.hr/en/proizvod/lactogyn-kapsule. (Accessed 10 June 2018)
- Jgl (2018b) Lactogyn® vaginalne kapsule. Jadran–Galenski Laboratorij d.d. Available at https://www.jgl.hr/en/proizvod/lactogyn-vaginalne-kapsule. (Accessed 10 June 2018)
- Jung GW, Tse JE, Guiha I, et al. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J Cutan Med Surg. 2013;17:114–122. doi: 10.2310/7750.2012.12026. [DOI] [PubMed] [Google Scholar]
- Kang BS, Seo JG, Lee GS, et al. Antimicrobial activity of Enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J Microbiol. 2009;47:101–109. doi: 10.1007/s12275-008-0179-y. [DOI] [PubMed] [Google Scholar]
- Kechagia M, Basoulis D, Konstantopoulou S, et al. Health benefits of probiotics: a review. ISRN Nutr. 2013;2013:1–7. doi: 10.5402/2013/481651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ko Y, Park YK, et al. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition. 2010;26:902–909. doi: 10.1016/j.nut.2010.05.011. [DOI] [PubMed] [Google Scholar]
- King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41–54. doi: 10.1017/S0007114514000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler GA, Assefa S, Reid G. Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect Dis Obstet Gynecol. 2012;2012:1–14. doi: 10.1155/2012/636474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutmann J. Pre- and probiotics for human skin. J Dermatol Sci. 2009;54:1–5. doi: 10.1016/j.jdermsci.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Lau ASY, Yanagisawa N, Hor YY, et al. Bifidobacterium longum BB536 alleviated upper respiratory illnesses and modulated gut microbiota profiles in Malaysian pre-school children. Benef Microbes. 2018;9:61–70. doi: 10.3920/BM2017.0063. [DOI] [PubMed] [Google Scholar]
- Laue C, Papazova E, Liesegang A, et al. Effect of a yoghurt drink containing Lactobacillus strains on bacterial vaginosis in women—a double-blind, randomised, controlled clinical pilot trial. Benef Microbes. 2018;9:35–50. doi: 10.3920/BM2017.0018. [DOI] [PubMed] [Google Scholar]
- Laursen RP, Hojsak I. Probiotics for respiratory tract infections in children attending day care centers-a systematic review. Eur J Pediatr. 2018;177:979–994. doi: 10.1007/s00431-018-3167-1. [DOI] [PubMed] [Google Scholar]
- Laursen RP, Larnkjær A, Ritz C, et al. Probiotics and child care absence due to infections: a randomized controlled trial. Pediatrics. 2017;140:1–11. doi: 10.1542/peds.2017-0735. [DOI] [PubMed] [Google Scholar]
- Lee GR, Maarouf M, Hendricks AJ, et al. Topical probiotics: the unknowns behind their rising popularity. Dermatol Online J. 2019;25:5. [PubMed] [Google Scholar]
- Liu F, Li P, Chen M, et al. Fructooligosaccharide (FOS) and galactooligosaccharide (GOS) increase Bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci Rep. 2017;7:11789. doi: 10.1038/s41598-017-10722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livengood CH, 3rd, Thomason JL, Hill GB. Bacterial vaginosis: diagnostic and pathogenetic findings during topical clindamycin therapy. Am J Obstet Gynecol. 1990;163:515–520. doi: 10.1016/0002-9378(90)91187-h. [DOI] [PubMed] [Google Scholar]
- Lopes EG, Moreira DA, Gullón P, et al. Topical application of probiotics in skin: adhesion, antimicrobial and antibiofilm in vitro assays. J Appl Microbiol. 2016;122:450–446. doi: 10.1111/jam.13349. [DOI] [PubMed] [Google Scholar]
- López-Velázquez G, Parra-Ortiz M, De la Mora-De la Mora I, et al. Effects of fructans from Mexican Agave in newborns fed with infant formula: a randomized controlled trial. Nutrients. 2015;7:8939–8951. doi: 10.3390/nu7115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoto R, Ruuskanen O, Waris M, et al. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2014;133:405–413. doi: 10.1016/j.jaci.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Forney LJ, Ravel J. Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol. 2012;66:371–389. doi: 10.1146/annurev-micro-092611-150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man WH, de Steenhuijsen Piters WAA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15:259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield JA, Bergin SW, Cooper JR, et al. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med. 2014;179:580–592. doi: 10.7205/MILMED-D-13-00546. [DOI] [PubMed] [Google Scholar]
- Manzhalii E, Hornuss D, Stremmel W. Intestinal-borne dermatoses significantly improved by oral application of Escherichia coli Nissle 1917. World J Gastroenterol. 2016;22:5415–5421. doi: 10.3748/wjg.v22.i23.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowiak P, Śliżewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 10.3390/nu9091021 [DOI] [PMC free article] [PubMed]
- Menard JP. Antibacterial treatment of bacterial vaginosis: current and emerging therapies. Int J Womens Health. 2011;3:295–305. doi: 10.2147/IJWH.S23814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menayang A (2018) DSM and Prenexus Health market sugar cane-derived prebiotic developed to selectively feed “friendly” microbes. Available at https://www.nutraingredients-usa.com/Article/2018/05/14/DSM-Prenexus-market-sugar-cane-xylooligosaccharide-prebiotic-AmpliVida. (Accessed 04 June 2018)
- Michalickova D, Minic R, Dikic N, et al. Lactobacillus helveticus Lafti L10® supplementation reduces respiratory infection duration in a cohort of elite athletes: a randomized, double-blind, placebo-controlled trial. Appl Physiol Nutr Metab. 2016;41:782–789. doi: 10.1139/apnm-2015-0541. [DOI] [PubMed] [Google Scholar]
- Muizzuddin N, Maher W, Sullivan M, et al. Physiological effect of a probiotic on skin. J Cosmet Sci. 2012;63:385–395. [PubMed] [Google Scholar]
- Murillo N, Raoult D. Skin microbiota: overview and role in the skin diseases acne vulgaris and rosacea. Future Microbiol. 2013;8:209–222. doi: 10.2217/fmb.12.141. [DOI] [PubMed] [Google Scholar]
- Nagpal R, Kumar A, Kumar M, et al. Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS Microbiol Lett. 2012;334:1–15. doi: 10.1111/j.1574-6968.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- Nekutli (2018) Fibra orgánica soluble de agave, Metlin y Metlos. Available at http://www.alior.com/nekutli.php?l=es. (Accessed 01 May 2018)
- Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics—a review. J Food Sci Technol. 2015;52:7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in Rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759–764. doi: 10.1016/j.cgh.2008.02.054. [DOI] [PubMed] [Google Scholar]
- Pineiro M, Asp NG, Reid G, et al. FAO technical meeting on prebiotics. J Clin Gastroenterol. 2008;42:S156–S159. doi: 10.1097/MCG.0b013e31817f184e. [DOI] [PubMed] [Google Scholar]
- Platsidaki Eftychia, Dessinioti Clio. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research. 2018;7:1953. doi: 10.12688/f1000research.15659.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado FC, Parada JL, Pandey A, et al. Trends in non-dairy probiotic beverages. Food Res Int. 2008;41:111–123. [Google Scholar]
- Prakoeswa CRS, Herwanto N, Prameswari R, et al. Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef Microbes. 2017;8:833–840. doi: 10.3920/BM2017.0011. [DOI] [PubMed] [Google Scholar]
- Prince J (2018a) AIDP Now exclusive distributor for Neo Cremar prebiotic oligosaccharide ingredients. Available at http://www.nutritionaloutlook.com/digestive-health/aidp-now-exclusive-distributor-neo-cremar-prebiotic-oligosaccharide-ingredients. (Accessed 25 June 2018)
- Prince J (2018b) Lallemand probiotic gains sports nutrition, active nutrition claims in Canada. Available at http://www.nutritionaloutlook.com/digestive-health/lallemand-probiotic-gains-sports-nutrition-active-nutrition-claims-canada. (Accessed 01 June 2018)
- Rahman S, Collins M, Williams C, et al. The pathology and immunology of atopic dermatitis. Inflamm Allergy Drug Targets. 2011;10:486–496. doi: 10.2174/187152811798104935. [DOI] [PubMed] [Google Scholar]
- Rahmati Roudsari M, Karimi R, Mortazavian AM. Health effects of probiotics on the skin. Crit Rev Food Sci Nutr. 2013;55:1219–1240. doi: 10.1080/10408398.2012.680078. [DOI] [PubMed] [Google Scholar]
- Reid G, Bruce AW. Urogenital infections in women: can probiotics help? Postgrad Med J. 2003;79:428–432. doi: 10.1136/pmj.79.934.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Burton J. Use of Lactobacillus to prevent infection by pathogenic bacteria. Microb Infect. 2002;4:319–324. doi: 10.1016/s1286-4579(02)01544-7. [DOI] [PubMed] [Google Scholar]
- Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9:27–38. doi: 10.1038/nrmicro2473. [DOI] [PubMed] [Google Scholar]
- Roth RR, James WD. Microbial ecology of the skin. Annu Rev Microbiol. 1988;42:441–464. doi: 10.1146/annurev.mi.42.100188.002301. [DOI] [PubMed] [Google Scholar]
- Salarkia N, Ghadamli L, Zaeri F, et al. Effects of probiotic yogurt on performance, respiratory and digestive systems of young adult female endurance swimmers: a randomized controlled trial. Med J Islam Repub Iran. 2013;27:141–146. [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, Darimont C, Drapeau V, et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr. 2014;111:1507–1519. doi: 10.1017/S0007114513003875. [DOI] [PubMed] [Google Scholar]
- Sanders ME. Probiotics: definitions, sources, selections, and uses. Clin Infect Dis. 2008;46:S58–S61. [Google Scholar]
- Scharschmidt TC, Fischbach MA. What lives on our skin: ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discov Today. Dis Mech. 2013;10:e83–e89. doi: 10.1016/j.ddmec.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck LP, Surette MG, Bowdish DME. Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett. 2016;590:3705–3720. doi: 10.1002/1873-3468.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz H (2018) AIDP aims new prebiotic toward novel beauty-from-within positioning. Available at https://www.nutraingredients-usa.com/Article/2018/05/29/AIDP-aims-new-prebiotic-toward-novel-beauty-from-within-positioning. (Accessed 04 June 2018)
- Schwenger EM, Tejani AM, Loewen PS. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database Syst Rev. 2015;12:CD008772. doi: 10.1002/14651858.CD008772.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon BS, Jairath S. Prebiotics, probiotics and synbiotics: an overview. J Pharm Educ Res. 2010;1:13–36. [Google Scholar]
- Seroyal (2019) HLC Fit for School. Available at https://www.seroyal.com/hlc-fit-for-school.html. (Accessed 01 March 2019)
- Shim YY, Gui B, Wang Y, et al. Flaxseed (Linum usitatissimum L.) oil processing and selected products. Trends Food Sci Technol. 2015;43:162–177. [Google Scholar]
- Silab (2018a) Sensiline®. Available at https://www.silab.fr/produit-44-sensiline_usa.html. (Accessed 01 May 2018)
- Silab (2018b) Sensiline® Bio. Avaiable at https://www.silab.fr/produit-63-sensiline-bio_usa.html. (Accessed 01 May 2018)
- Šmid A, Strniša L, Bajc K, et al. Randomized clinical trial: The effect of fermented milk with the probiotic cultures Lactobacillus acidophilus La-5® and Bifidobacterium BB-12® and Beneo dietary fibres on health-related quality of life and the symptoms of irritable bowel syndrome in adults. J Funct Foods. 2016;24:549–557. [Google Scholar]
- Smith TJ, Rigassio-Radler D, Denmark R, et al. Effect of Lactobaillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections. Br J Nutr. 2013;109:1999–2007. doi: 10.1017/S0007114512004138. [DOI] [PubMed] [Google Scholar]
- Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212–1217. doi: 10.1093/cid/cir183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Yoneda M, Tanake K, et al. Lactobacillus salivarius WB21-containing tablets for the treatment of oral malodor: a double-blind, randomized, placebo-controlled. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:462–470. doi: 10.1016/j.oooo.2013.12.400. [DOI] [PubMed] [Google Scholar]
- Tajadadi-Ebrahimi M, Bahmani F, Shakeri H, et al. Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. Ann Nutr Metab. 2014;65:34–41. doi: 10.1159/000365153. [DOI] [PubMed] [Google Scholar]
- Tapiovaara L, Pitkaranta A, Korpela R (2016) Probiotics and the upper respiratory tract—a review. Pediatric Infect Dis. 10.21767/2573-0282.100019
- TKS (2018) Intimique™ Femme. The intimate ally for uro-genital infections. Available at http://www.teknoscienze.com/intimiquetm-femme-the-intimate-ally-for-uro-genital-infections/. (Accessed 10 May 2018)
- Toyoda M, Morohashi M. Pathogenesis of acne. Med Electron Microsc. 2001;34:29–40. doi: 10.1007/s007950100002. [DOI] [PubMed] [Google Scholar]
- van den Broek MFL, De Boeck I, Claes IJJ, et al. Multifactorial inhibition of lactobacilli against the respiratory tract pathogen Moraxella catarrhalis. Benef Microbes. 2018;9:429–439. doi: 10.3920/BM2017.0101. [DOI] [PubMed] [Google Scholar]
- van der Aa LB, Heymans HS, van Aalderen WM, et al. Effect of a new synbiotic mixture on atopic dermatitis in infants: a randomized-controlled trial. Clin Exp Allergy. 2010;40:795–804. doi: 10.1111/j.1365-2222.2010.03465.x. [DOI] [PubMed] [Google Scholar]
- van Woerden HC, Gregory C, Brown R, et al. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: a community based case control study. BMC Infec Dis. 2013;13:1–6. doi: 10.1186/1471-2334-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdenelli MC, Cecchini C, Coman MM, et al. Impact of probiotic SYNBIO® administered by vaginal suppositories in promoting vaginal health of apparently healthy women. Curr Microbiol. 2016;73:483–490. doi: 10.1007/s00284-016-1085-x. [DOI] [PubMed] [Google Scholar]
- Vujic G, Knez AJ, Stefanivc VD, et al. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: a double-blind, randomized, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2013;168:75–79. doi: 10.1016/j.ejogrb.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Waigankar SS, Patel V. Role of probiotics in urogenital healthcare. J Midlife Health. 2011;2:5–10. doi: 10.4103/0976-7800.83253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li X, Ge T, et al. Probiotics for prevention and treatment of respiratory tract infections in children. A systematic review and meta-analysis of randomized controlled trials. Medicine. 2016;95:e4509. doi: 10.1097/MD.0000000000004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watelet JB, Van Cauwenberge P. Applied anatomy and physiology of the nose and paranasal sinuses. Allergy. 1999;54:14–25. doi: 10.1111/j.1398-9995.1999.tb04402.x. [DOI] [PubMed] [Google Scholar]
- Watts AM, West NP, Smith PK, et al. Probiotics and allergic rhinitis: a Simon Two-Stage Design to determine effectiveness. J Altern Complement Med. 2016;22:1007–1012. doi: 10.1089/acm.2016.0115. [DOI] [PubMed] [Google Scholar]
- Webster GF, Leyden JJ, Tsai CC, et al. Polymorphonuclear leukocyte lysosomal release in response to Propionibacterium acnes in vitro and its enhancement by sera from inflammatory acne patients. J Investig Dermatol. 1980;74:398–401. doi: 10.1111/1523-1747.ep12544494. [DOI] [PubMed] [Google Scholar]
- Whitehouse L (2018) How do JooMo products encourage natural microbial diversity of the skin? Available at https://www.cosmeticsdesign-europe.com/Article/2018/04/06/How-do-JooMo-products-encourage-natural-microbial-diversity-of-the-skin. (Accessed 10 May 2018)
- Whyand TL, Caplin ME. Review of the evidence for the use of probiotics in gastrointestinal disorders. Gastroenterol Pancreatol Liver Disord. 2014;1(4):1–9. [Google Scholar]
- Winclove Probiotics (2018a) Prevention of early-onset eczema. Available at https://www.wincloveprobiotics.com/sites/default/files/headerpics/ecologic_panda_1.pdf. (Accessed 10 May 2018)
- Winclove Probiotics (2018b) Management of allergic symptoms. Available at https://www.wincloveprobiotics.com/sites/default/files/headerpics/sheet_ecol_allergycare_2018.pdf. (Accessed 10 May 2018)
- Winclove Probiotics (2018c) Prevention of (recurrent) vaginal Candida infections. Available at https://www.wincloveprobiotics.com/sites/default/files/headerpics/ecologic_femi_0.pdf. (Accessed 10 May 2018)
- Wu KG, Li TH, Peng HJ. Lactobacillus salivarius plus fructo-oligosaccharide is superior to fructo-oligosaccharide alone for treating children with moderate to severe atopic dermatitis: a double-blind, randomized, clinical trial of efficacy and safety. Br J Dermatol. 2012;166:129–136. doi: 10.1111/j.1365-2133.2011.10596.x. [DOI] [PubMed] [Google Scholar]
- Yeşilova Y, Çalka O, Akdeniz N, et al. Effect of probiotics on the treatment of children with atopic dermatitis. Ann Dermatol. 2012;24:189–193. doi: 10.5021/ad.2012.24.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Chehoud C, Bittinger K, et al. Viral metagenomics reveal blooms of anelloviruses in the respiratory tract of lung transplant recipients. Am J Transplant. 2015;15:200–209. doi: 10.1111/ajt.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Dunaway S, Champer J et al (2019) Changing our microbiome: probiotics in dermatology. Br J Dermatol. 10.1111/bjd.18088 [DOI] [PubMed]
- Zeng ZM, Liao QP, Yao C, et al. Directed shift of vaginal flora after topical application of sucrose gel in a phase III clinical trial: a novel treatment for bacterial vaginosis. Chin Med J. 2010;123:2051–2057. [PubMed] [Google Scholar]
- Zhang H, Yeh C, Jin Z, et al. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth Syst Biotechnol. 2018;3:113–120. doi: 10.1016/j.synbio.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]