Abstract
Purpose
Recent evidence suggests that improving the transitional care process may reduce 30-day readmissions and hospital length of stay (LOS). The objective of this study was to evaluate the impact of a pharmacist-led transitions-of-care (TOC) program on 30- and 90-day all-cause readmissions and LOS for patients discharged from the hospital acute care setting.
Methods
A retrospective cohort study was conducted using a difference-in-difference (DID) approach. Patients who were at least 18 years old with any of the following primary diagnoses were included: acute myocardial infarction, chronic obstructive pulmonary disease, congestive heart failure (CHF), and pneumonia. Outcome measures were all-cause 30- and 90-day readmission and LOS for the index admission.
Results
From October 2013 through September 2017, 1,776 patients were discharged from the intervention site, and 2,969 patients were discharged from 3 control sites. Only 33.3% of eligible patients at the intervention site actually received the intervention. The DID analysis showed that the odds ratio (OR) for 30-day readmission was 0.65 [P = 0.035] at the intervention site following TOC program initiation. The OR for 90-day readmission was 0.75 [P = 0.070]. Among all diagnosis groups, the CHF subgroup had the highest proportion of patients who actually received the TOC intervention (57.2%). Within that CHF subgroup, the ORs for 30- and 90-day readmissions were 0.52 [P = 0.056] and 0.47 [P = 0.005], respectively. The mean LOS did not change significantly in either analysis.
Conclusion
This pharmacist-led transitional care intervention was associated with significantly decreased inpatient readmissions. The analysis indicates that pharmacist interventions can significantly reduce 30-day readmissions for high-risk populations and 90-day readmissions in patients with CHF.
Keywords: acute care, pharmacist-led services, readmissions, transition of care
KEY POINTS.
A pharmacist-led transition of care (TOC) program effectively lowered the 30-day readmissions among adult patients hospitalized for acute myocardial infarction, chronic obstructive pulmonary disease, congestive heart failure (CHF), or pneumonia.
Among the patients hospitalized for CHF, the TOC program significantly decreased 90-day readmissions.
There was no difference in the mean length of stay before and after the intervention.
Preventing hospital readmissions has been identified as one of the key areas where policy makers and stakeholders can focus their efforts to reduce the United States’ ever-increasing healthcare costs.1 It is estimated that approximately 27% of hospital readmissions are potentially avoidable, which translates into an estimated economic burden of $25 billion to $45 billion annually.2-4 The Hospital Readmissions Reduction Program (HRRP) is one policy aimed at reducing rising healthcare costs and improving both patient care quality and outcomes. The HRRP, a component of the 2012 Affordable Care Act, requires the Centers for Medicare and Medicaid Services to reduce inpatient prospective payment system payments to those hospitals with excess readmissions. The excess readmission ratio is calculated as a separate performance measure for each of the following primary diagnoses: acute myocardial infarction (AMI), heart failure (HF), pneumonia, chronic obstructive pulmonary disease (COPD), coronary artery bypass graft (CABG) surgery, and elective primary total hip and/or total knee arthroplasty.5 In response to the establishment of these quality measures, hospitals have implemented significant efforts to improve their process of care with the aim of reducing the rate of readmissions. Recent literature suggests that improving the transitions-of-care (TOC) process by educating patients, coordinating with postacute care providers, and reducing medication-related complications during an inpatient stay can reduce 30-day readmissions.6-12
A health system of community hospitals in northern California designed a pharmacist-led TOC program that focuses on improving and optimizing drug therapy and implemented that program at one of its sites in 2015. The intervention aimed to maintain continuous, optimal medication therapy during and after hospitalization by incorporating strategies shown in the literature to be effective plus additional components identified as necessary by the transitional care providers themselves. The purpose of this study was to evaluate the real-world impact of this TOC program on 30- and 90-day readmissions and index admission length of stay (LOS).
Methods
Study design
This was a retrospective cohort study using hospital electronic health records from the intervention and control sites spanning the period October 2013 through September 2017. Pre- and postintervention outcome measures were compared using a difference-in-difference (DID) approach that adjusted for time trends using neighboring control hospitals within the same health system that did not implement any similar intervention during the study period but were otherwise well matched for size, average acuity, and other patient factors (Appendix A).
Subjects
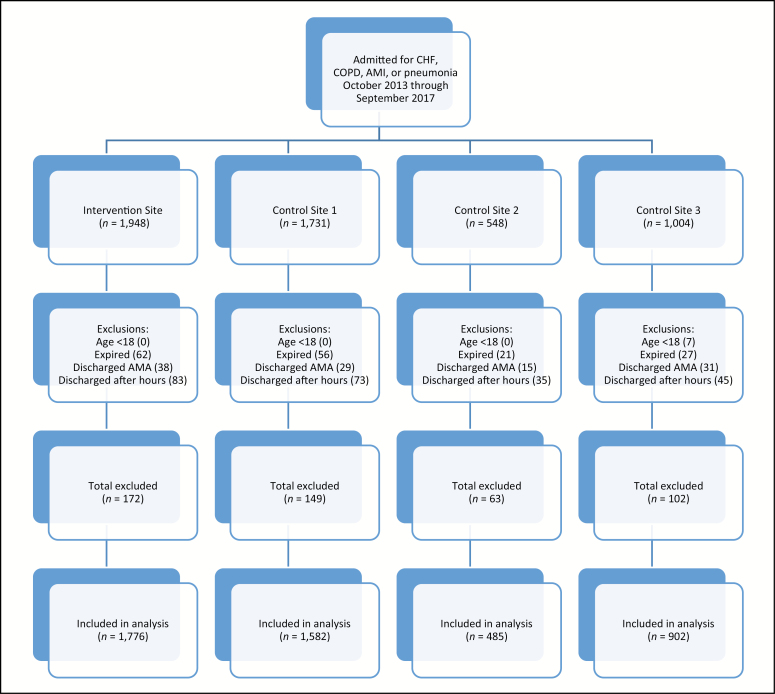
The analysis cohort included all patients who were at least 18 years of age and admitted with a primary diagnosis of HF, AMI, pneumonia, or COPD during the study period. These 4 conditions were selected out of the 6 primary diagnoses subject to the HRRP based on the patient population profile of the intervention site, which did not perform any CABG procedures and had few if any orthopedic patients hospitalized for surgery on-site. Patients were excluded if they (1) died during the index hospitalization, (2) were discharged against medical advice, (3) were electively admitted, or (4) were both admitted and discharged outside of the TOC program hours (Monday through Friday 8 am to 5 pm). A CONSORT diagram showing the number of patients who met our inclusion and/or exclusion criteria and the final sample size is shown in Figure 1.
Figure 1.
CONSORT flow diagram of sample selection by site. AMA indicates against medical advice; AMI, acute myocardial infarction; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease.
Intervention
The TOC program was a multifaceted intervention aimed at improving the outcome of patients’ medication therapy. The intervention incorporated the following specific components: (1) identifying and correcting drug-related issues upon admission, (2) continually monitoring drug therapies for optimization during a hospital stay, (3) preordering postdischarge medications for patients and resolving any insurance conflicts prior to discharge to ensure uninterrupted therapy, (4) educating patients upon discharge on medication therapies and their follow-up appointments, if any, and (5) making follow-up phone calls to address drug-related issues, including barriers to medication adherence, after discharge. The intervention site was chosen from among others because it had in place most of the required elements to deploy the TOC program promptly and efficiently. Those elements included the necessary pharmacist staffing and competency levels plus other essential capabilities such as medication reconciliation and postdischarge follow-up.
At the intervention hospital, patients were eligible to receive the intervention if they had a primary diagnosis of congestive HF (CHF), AMI, pneumonia, or COPD. However, due to limited resources and pharmacist availability, the intervention was not provided to every eligible patient. Patients were therefore prioritized based on perceived patient factors such as medication regimen complexity and comorbid health conditions, leaving some eligible patients in the intervention group untouched by the TOC program. Although every effort was made to expose patients enrolled in the program to all 5 components of the intervention, some participants did not receive all components (eg, because the patient could not be reached by phone after being discharged). Nonetheless, patients were considered as having received the intervention if they were touched by any of the 5 components.
Outcome measures
The primary outcome measures were all-cause 30-day and 90-day hospital readmissions following the index hospitalization. The 90-day hospital readmission measure was included to examine whether the impact of the intervention on 30-day readmissions persisted beyond 30 days. The secondary outcome measure was LOS during the index hospitalization; this outcome was designed to evaluate whether the intervention, which incorporated patient medication reconciliation at the time of admission, would have relatively immediate effects on the time to discharge.
Analysis
The baseline characteristics of patients at the intervention and control sites were compared using t tests for continuous data, and χ 2 tests for discrete data. The level of significance was set, a priori, at 0.05. The impact of the intervention was assessed using a DID design by comparing the outcome measures before and after implementation of the intervention (in January 2015) after adjusting for time trends at the control sites during the study period (October 2013 through September 2017). The DID approach was based on the assumption that there was an otherwise common trend in the outcome measures during the study period among the intervention and 3 neighboring control hospitals. More specifically, the imposed assumption was that the important unmeasured confounding factors varying across the groups (such as individual hospital characteristics) were time invariant, and that the time-varying confounders (such as the time trend of a measured outcome) were group invariant.13 If that assumption held, then any pre- versus postintervention differences in outcomes at the intervention site would be attributable to the TOC program. Each admission episode was entered into the cohort data as a patient-level observation. If a patient had more than 1 admission episode, only the first observed admission record was analyzed for treatment effectiveness. Because (as already noted) not all patients in the intervention group received the intervention, an intention-to-treat (ITT) analysis was applied: the “treatment” group included all patients at the intervention hospital who were eligible for the intervention based on their primary diagnosis during the period when the TOC intervention was available. Multivariate logistic regression was also used to adjust for other individual patient demographic and clinical characteristics, such as age, gender, marital status, race, payer type, diagnosis related group weight, LOS, Elixhauser comorbidity index, discharge status, number of medications at discharge, and history of inpatient and emergency department (ED) visits in the 6 months prior to admission. An interaction term for binary indicators (ie, pre- vs postintervention and intervention vs control site) was also included in the regressions to account for the DID effect on the outcome measures. All analyses were conducted using SAS statistical software, version 9.4 (SAS Institute, Cary, NC). This study was approved by the Sutter Health and the University of Southern California Health Sciences institutional review boards.
Results
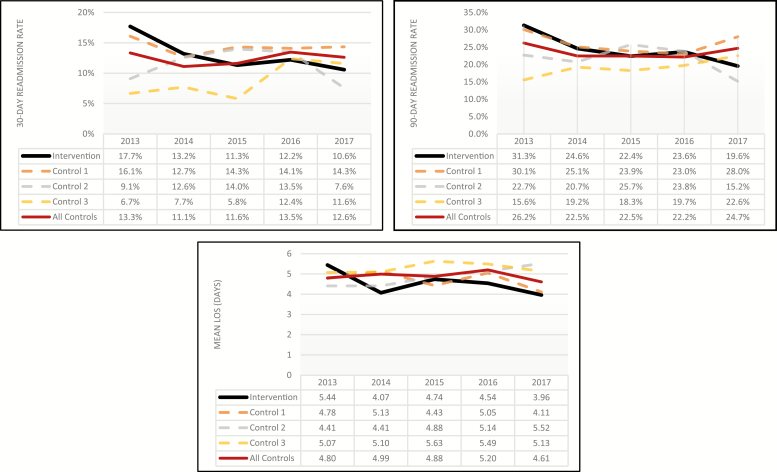
A total of 1,776 and 2,969 patients from the intervention site and the control sites, respectively, were included in the analysis. Of those patients, 655 patients from the intervention group and 958 patients from the control group were admitted prior to implementation of the TOC program in 2015. Patient demographics and clinical characteristics for index admissions are shown in Table 1. For the 3 years of the study period (2015, 2016, and 2017), the proportions of patients who actually received the intervention among the intervention group patients were 27%, 36%, and 39%, respectively (33.3% in aggregate over the 3 years). The unadjusted 30- and 90-day readmission rates and LOS data for each site for each year during the study period are shown in Figure 2. The annual 30-day readmission rates ranged from 13.2% to 17.7% during the preintervention period, compared to a range of 10.6% to 12.2% in the postintervention period. Regression estimation was applied to calculate least-squares means for each outcome measure for the preintervention period and postintervention period. Figure 3 shows the change of the outcome measures before and after the TOC program at the intervention site and the control sites, with 95% confidence intervals (CIs). For 30- and 90-day readmission rates, outcomes were estimated in log-odds while using linear models. These estimates were then used to produce the DID results, which compared the outcome measures before and after the intervention after subtracting the changes observed in the 3 neighboring control hospitals. The resulting odds ratio (OR) for 30-day readmission for patients admitted after the implementation of the TOC program, compared to those who were admitted prior to the program, was 0.654 (P = 0.035). The 90-day readmission OR was 0.752 (P = 0.070) for patients admitted in the postintervention vs the preintervention period (Table 2). There was no statistically significant change in the mean LOS from the preintervention to the postintervention period (–0.15 day; P = 0.662).
Table 1.
Baseline Patient Characteristics by Site
| Characteristic | Intervention (n = 1,776) | Control 1 (n = 1,582) | Control 2 (n = 485) | Control 3 (n = 902) |
|---|---|---|---|---|
| Age, mean (SD), y | 72.5 (16.4) | 71.1 (16.0) | 70.9 (15.7) | 70.7 (16.0) |
| Gender, % female | 53 | 52 | 43 | 51 |
| Race, % | ||||
| White | 63 | 43 | 62 | 27 |
| Black | 16 | 39 | 16 | 23 |
| Asian | 12 | 9 | 13 | 26 |
| Other | 9 | 10 | 9 | 24 |
| Marital status, % | ||||
| Single | 25 | 34 | 46 | 42 |
| Married | 33 | 30 | 25 | 28 |
| Widowed | 29 | 23 | 19 | 22 |
| Divorced | 13 | 12 | 7 | 7 |
| Other | 1 | 1 | 4 | 2 |
| English speaking, % | 90 | 93 | 86 | 64 |
| Discharged to SNF, % | 25 | 18 | 18 | 12 |
| Primary payer, % | ||||
| Medicare | 58 | 54 | 52 | 53 |
| Medicaid | 4 | 3 | 3 | 6 |
| DRG weight, mean (SD) | 1.3 (0.7) | 1.3 (0.7) | 1.2 (0.5) | 1.2 (0.6) |
| Elixhauser comorbidity index score, mean (SD) | 4.3 (2.0) | 4.4 (2.0) | 4.3 (2.1) | 4.6 (2.1) |
| No. medications at discharge, mean (SD) | 11.8 (6.7) | 11.3 (6.7) | 10.0 (7.1) | 9.2 (6.3) |
| Inpatient stay in past 6 mo, % | 37 | 33 | 31 | 28 |
Abbreviations: DRG, diagnosis related group; SNF, skilled nursing facility.
Figure 2.
Unadjusted 30- and 90-day readmission rates (top 2 panels) and mean length of stay (LOS) by site and year.
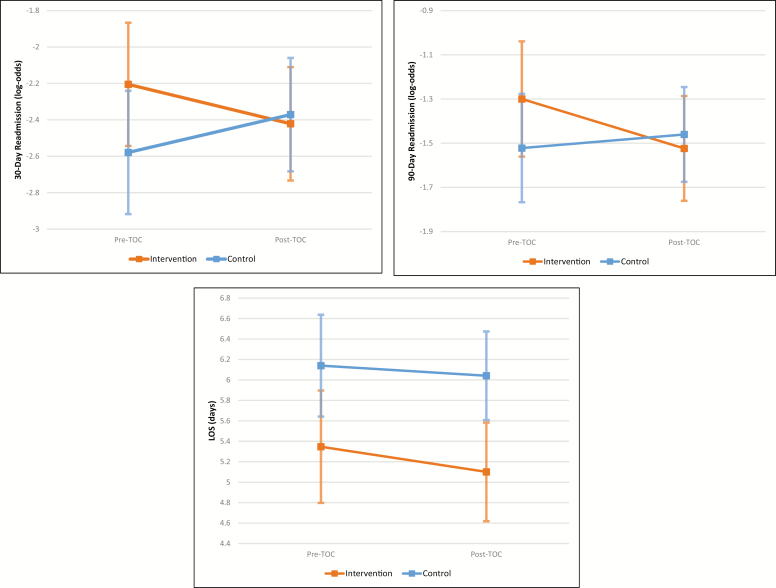
Figure 3.
Least squares estimates (with 95% confidence intervals) from difference-in-difference analysis of changes in 30-day readmission rate (top left), 90-day readmission rate (top right), and mean length of stay (LOS), adjusted by multivariate logistic regression, from the period before to the period after implementation of the transitions-of-care (TOC) program.
Table 2.
Adjusted Difference-in-Difference Results for 30- and 90-Day Readmissions and LOS
| Cohort | DID Estimate (SE) | P Value | Odds Ratio |
|---|---|---|---|
| All patients | |||
| 30-day readmission (log-odds) | –0.424 (0.201) | 0.035a | 0.654 |
| 90-day readmission (log-odds) | –0.285 (0.157) | 0.070 | 0.752 |
| LOS, days | –0.148 (0.337) | 0.662 | NA |
| Patients with CHF | |||
| 30-day readmission (log-odds) | –0.641 (0.336) | 0.056 | 0.527 |
| 90-day readmission (log-odds) | –0.747 (0.269) | 0.0054a | 0.474 |
| LOS, days | 0.620 (0.447) | 0.165 | NA |
Abbreviations: DID, difference-in-difference; CHF, congestive heart failure; LOS, length of stay; NA, not applicable.
aStatistically significant.
The adjusted change in the probability of 30-day readmission at the intervention site was calculated using least-squares estimates for that outcome measure obtained from the multivariate regressions for the intervention and control sites before and after the intervention. Resulting log-odds estimates were converted to mean probabilities (as shown in Appendix C). The difference in the probabilities of 30-day readmission at the intervention site before and after the intervention was –0.0178 (95% CI, –0.0562 to –0.0206). After adjusting for the trends in the control sites, the change in probability was –0.0327 (95% CI, –0.0884 to –0.0231).
Subgroup analysis
In the intervention group, patients admitted with a primary diagnosis of CHF were more likely than those in other diagnosis categories to receive the intervention, likely because of the more complex role that medication therapy usually plays in treating that condition, as opposed to AMI, pneumonia, and COPD. That result was aligned with existing literature demonstrating pharmacists’ impact on clinical outcomes among patients with CHF.14-17 Thus, patients with CHF were often prioritized to receive the intervention before those in other diagnosis groups due to limited pharmacist time and hospital resources. The proportion of patients with CHF who were touched by the intervention over the postintervention period was 57.2%, compared to only 33.3% in the overall intervention group. Therefore, a subgroup analysis focusing only on patients with CHF was performed. There were 607 patients with CHF at the intervention site (226 from the preintervention period) and 1,177 patients with CHF at the control sites (366 from the preintervention period). The annual unadjusted 30-day readmission rates during the preintervention period ranged from 14.0% to 20.0%, compared to rates of 12.1% to 12.6% in the postintervention years. The DID analysis, which accounted for both individual patient characteristics and the time trends at control sites, showed that the adjusted OR was 0.53 (P = 0.056) for 30-day readmissions and 0.47 (P = 0.0054) for 90-day readmissions. However, there was no significant change in mean LOS, paralleling the result in the all-diagnoses group (0.62 day; P = 0.165) (Table 2).
Discussion
This study estimated the impact of a pharmacist-led TOC program on 30- and 90-day readmission rates and LOS in a hospital acute care setting among patients who were admitted for CHF, AMI, pneumonia, or COPD. The multivariate regression results demonstrated that there was a significant reduction in both 30- and 90-day readmission rates at the intervention site following implementation of the intervention, compared to readmission rates in the preintervention period. The DID estimate, which additionally adjusted the results for time trends in outcome measures at 3 neighboring control hospitals, showed that there was a statistically and clinically significant reduction in the odds of 30-day readmission. As discussed previously in the Methods section, the validity of these results relies on the account that the common-trends assumption across the intervention and control sites holds. By visual inspection of unadjusted yearly trends of outcome measures for each hospital presented in Figure 2, the parallel trends in the years prior to 2015 can be confirmed for the intervention site and the control sites in aggregate (for both 30- and 90-day readmissions). The 2 lines suggest that rates were trending downward in the years prior to TOC implementation in 2015. In addition, examination of the group-variant predictors (hospital-level characteristics in this study) revealed no significant changes over the years constituting the study period and confirmed that they remained time invariant. These facility-level characteristics before and after the intervention are provided in Appendixes A and B. It should be noted that due to resource constraints, only a small proportion (33.3%) of the eligible patients actually received the intervention; therefore, the study employed an ITT analysis approach to minimize the bias of potential confounders that arise when restricting such an analysis to treated patients in quasi-experimental designs. This likely biased the analysis against finding a benefit. If a greater proportion of eligible patients had received the TOC intervention, potentially even larger effects might have been observed.
The subgroup analysis, focusing on CHF patients, showed trends of reduced 30- and 90-day readmission rates at the intervention hospital, paralleling the 30-day readmission results seen in the base-case model. For CHF, the DID estimate was statistically significant for 90-day readmissions, with an OR of <1, suggesting that CHF patients may experience a benefit from the intervention after some time has elapsed after discharge. It is reasonable to expect that the TOC program may have different effects on these outcome measures, depending on the patient’s specific diagnosis, since each diagnosis has a different disease course, and each disease is impacted differently by drug therapy. As such, one would expect patient groups with conditions heavily managed by medication therapy to greatly benefit from interventions similar to the TOC program. When adopting a similar TOC intervention, appropriate target patient population selection may decide observed effect size, especially when there are limited resources and providing the intervention to all admitted patients is not feasible.
Several limitations of the study should be noted. As with any retrospective cohort analysis, treatment assignment bias and unmeasured differences between the treatment and control patients (and hospitals) could have skewed the intervention’s estimated effect size. The chosen research approach (the DID approach, which also incorporates patient-level risk factor adjustments) should have minimized those biases. Despite these limitations, our study served as a test of an intervention deployed for quality improvement under real-world conditions, strengthening the external validity of our results for application in similar settings elsewhere (characteristics of the intervention and control hospitals are provided in Appendixes A and B).
In another study limitation, preintervention data were available for less than 2 years, limiting comparison of trends between sites prior to the intervention’s implementation. Specifically, data from 2013 only encompasses the last 3 months of that year, so the 2013 “annual” readmission rate may not reflect an accurate estimate for the entire year. Although the longer study period could have improved the robustness of our results, to our knowledge there were no major unmeasured variables. Because the data included detailed information on patients’ clinical presentation during the hospital stay, our analysis was able to account for potential confounders that would have otherwise led to less accurate estimations. Other analytic approaches, such as propensity score matching, were also applied as a robustness check but suffered from unacceptable endogeneity issues.
As discussed earlier, the intervention only reached about a third of eligible patients at the intervention site. Therefore, the analysis used an ITT approach to provide the most conservative possible estimates, which may have significantly attenuated the estimated intervention effectiveness. Those factors, combined with the (expected) variability in individual outcomes, forced us to reject as nonsignificant some observed effect trends that only achieved P values slightly higher than 0.05.
Overall, our analysis showed that this pharmacist-led transitional care intervention had a positive impact, significantly decreasing inpatient readmissions. The TOC program resulted in a significant reduction in the odds of 30-day readmission for high-risk patient groups in acute care settings. Subgroup analysis also indicated that the intervention significantly reduced 90-day readmissions among CHF patients. This represents a very significant clinical benefit, all the while implying significant opportunity for cost reduction, considering that average hospital expenses per inpatient day in the United States total about $2,424.18 The analysis presented here strongly suggests that the benefits of a TOC program justify deploying such programs more widely. However, randomized controlled studies, testing similar pharmacist-led interventions on high risk populations, may help to further support and refine our findings.
Acknowledgments
The authors acknowledge the following: Fred Hom, PharmD, Christine Chan, PharmD, Roma Bhandarkar, PharmD, and the pharmacy staff at Sutter Eden Medical Center for their contribution to the transitions of care program; and Alan Yee, Nick Digiorgio, Keiko Trenholm, and Catherine N. Trinh, PharmD, for providing data management support. A special acknowledgment to Brian Harris for his executive leadership and support of clinical programs in the North & East Bay at Sutter Health.
Appendix A—Hospital and patient characteristics at intervention and control sites (before TOC program)
| Characteristic | Intervention | Control 1 | Control 2 | Control 3 |
|---|---|---|---|---|
| Hospital characteristics | ||||
| Number of IP discharges | 10,278 | 21,547 | 3,880 | 4,341 |
| Number of ED visits | 43,747 | 45,269 | 13,508 | 23,573 |
| Patient characteristics | ||||
| Age, mean (SD), y | 49.7 (28.5) | 31.3 (27.8) | 60.3 (17.9) | 42.6 (30.1) |
| Gender, % female | 58.5 | 69.0 | 43.1 | 63.0 |
| Race | ||||
| White | 57.6 | 37.0 | 67.1 | 38.5 |
| Black | 13.8 | 23.6 | 9.9 | 14.4 |
| Asian | 14.2 | 16.1 | 10.6 | 19.6 |
| Other | 14.4 | 23.3 | 12.3 | 27.4 |
| Ethnicity | ||||
| Non-Hispanic | 74.1 | 72.6 | 87.5 | 63.6 |
| Mexican | 5.1 | 4.3 | 1.4 | 3.0 |
| Other Hispanic origin | 19.8 | 20.1 | 7.3 | 31.6 |
| English as primary language, % | 85.6 | 80.1 | 90.0 | 71.7 |
| Length of stay, mean (SD), days | 3.9 (5.5) | 4.1 (7.1) | 8.2 (11.4) | 7.9 (74.8) |
| Payor, % | ||||
| Medicare | 30.2 | 14.1 | 37.0 | 26.0 |
| Medi-Cal | 14.4 | 22.8 | 6.5 | 20.4 |
| DRG weight, mean (SD) | 1.37 (1.49) | 0.99 (1.04) | 1.64 (1.36) | 1.13 (0.98) |
| APR-DRG severity of illness category, % | ||||
| 0 | 0 | 0 | 0 | 3.6 |
| 1 | 30.4 | 41.3 | 23.3 | 31.1 |
| 2 | 33.8 | 30.6 | 32.5 | 36.7 |
| 3 | 23.5 | 16.0 | 32.0 | 21.9 |
| 4 | 9.0 | 3.3 | 11.4 | 6.2 |
| Not entered | 3.3 | 8.8 | 0.8 | 0.6 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 |
| No. medications on admission, mean (SD) | 7.6 (7.8) | 4.2 (6.4) | 7.9 (7.1) | 5.2 (6.1) |
| No. medications at discharge, mean (SD) | 6.9 (6.5) | 4.3 (5.5) | 6.8 (6.5) | 4.5 (5.5) |
| Patient class profile, % | ||||
| Inpatient | 86.4 | 68.0 | 88.6 | 72.0 |
| Newborn | 13.6 | 32.0 | 0.0 | 20.4 |
| SNF inpatient | 0 | 0 | 11.4 | 7.6 |
| Total | 100 | 100 | 100 | 100 |
Abbreviations: APR-DRG, All Patient Refined DRG (3M Company, St. Paul, MN); DRG, diagnosis related group; ED, emergency department; IP, inpatient; SNF, skilled nursing facility.
Appendix B—Hospital and patient characteristics at intervention and control sites (after TOC program)
| Characteristic | Intervention | Control 1 | Control 2 | Control 3 |
|---|---|---|---|---|
| Hospital characteristics | ||||
| Number of IP discharges | 9,168 | 19,757 | 4,662 | 5,054 |
| Number of ED visits | 47,939 | 46,429 | 15,926 | 26,299 |
| Patient characteristics | ||||
| Age, mean (SD), y | 52.5 (27.4) | 33.1 (28.2) | 60.7 (18.5) | 45.8 (29.3) |
| Gender, % female | 55.8 | 68.4 | 43.8 | 60.3 |
| Race | ||||
| White | 54.2 | 34.3 | 62.1 | 33.2 |
| Black | 13.4 | 22.7 | 9.4 | 15.0 |
| Asian | 15.0 | 16.0 | 16.0 | 18.8 |
| Other | 17.5 | 27.0 | 12.5 | 33.0 |
| Ethnicity | ||||
| Non-Hispanic | 75.9 | 71.8 | 86.2 | 62.7 |
| Mexican | 6.8 | 4.1 | 1.7 | 3.5 |
| Other Hispanic origin | 15.6 | 19.9 | 8.9 | 33.0 |
| English as primary language, % | 87.2 | 79.8 | 85.1 | 68.0 |
| Length of stay, mean (SD), days | 4.6 (5.9) | 4.0 (6.7) | 8.7 (13.5) | 8.1 (47.9) |
| Payor, % | ||||
| Medicare | 34.7 | 15.5 | 37.8 | 27.9 |
| Medi-Cal | 9.6 | 16.5 | 5.4 | 17.7 |
| DRG weight, mean (SD) | 1.68 (1.86) | 1.13 (1.11) | 1.72 (1.42) | 1.23 (1.06) |
| APR-DRG severity of illness category, % | ||||
| 0 | 0 | 0 | 0.2 | 0.3 |
| 1 | 23.4 | 41.0 | 20.0 | 31.4 |
| 2 | 31.0 | 33.2 | 34.8 | 35.7 |
| 3 | 31.6 | 20.8 | 33.3 | 24.2 |
| 4 | 13.9 | 4.9 | 11.6 | 8.4 |
| Not entered | 0 | 0 | 0.1 | 0.1 |
| Total | 100 | 100 | 100 | 100 |
| No. medications on admission, mean (SD) | 8.9 (8.4) | 4.6 (6.4) | 8.0 (7.0) | 6.3 (6.9) |
| No. medications at discharge, mean (SD) | 7.7 (6.8) | 4.6 (5.7) | 6.9 (6.5) | 5.1 (6.1) |
| Patient class profile, % | ||||
| Inpatient | 88.5 | 69.6 | 89.7 | 71.5 |
| Newborn | 11.5 | 30.4 | 0 | 17.6 |
| SNF inpatient | 0 | 0 | 10.3 | 10.9 |
| Total | 100 | 100 | 100 | 100 |
Abbreviations: APR-DRG, All Patient Refined DRG (3M Company, St. Paul, MN); DRG, diagnosis related group; ED, emergency department; IP, inpatient; SNF, skilled nursing facility.
Appendix C—Least-squares estimates for outcome measures, adjusted by multivariate logistic regression
| Cohort | 30-Day Readmission | 90-Day Readmission | LOS, mean (SE), days | ||
|---|---|---|---|---|---|
| Log-odds, mean (SE) | Converted Probability, mean (SE) | Log-odds, mean (SE) | Converted Probability, mean (SE) | ||
| Intervention Pre-TOC | –2.20 (0.17) | 0.099 (0.015) | –1.30 (0.13) | 0.214 (0.022) | 5.35 (0.28) |
| Intervention Post-TOC | –2.42 (0.16) | 0.082 (0.012) | –1.52 (0.12) | 0.179 (0.018) | 5.10 (0.25) |
| Control Pre-TOC | –2.58 (0.17) | 0.071 (0.011) | –1.52 (0.13) | 0.179 (0.018) | 6.14 (0.25) |
| Control Post-TOC | –2.37 (0.14) | 0.085 (0.011) | –1.46 (0.11) | 0.188 (0.017) | 6.04 (0.22) |
Abbreviations: LOS, length of stay; TOC, transitions of care.
Disclosures
This study was supported by research funding from Sutter Health System, Bay Area. The authors have declared no potential conflicts of interest.
References
- 1. A path to bundled payment around a rehospitalization. In: Report to the Congress: Reforming the Delivery System. Washington, DC: Medicare Payment Advisory Commission, June 2005:83-103. [Google Scholar]
- 2. Van Walraven C, Bennett C, Jennings A, et al. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2001;183:E391-E402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burton R. Health policy brief: improving care transitions. Health Affairs.http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=76. Published September 2012. Accessed October 30, 2018.
- 4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Medicare & Medicaid Services. Hospital Readmissions Reduction Program (HRRP).https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Accessed January 20, 2019.
- 6. Dedhia P, Kravet S, Bulger J, et al. A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes. J Am Geriatr Soc. 2009. Sep; 57(9):1540-1546. [DOI] [PubMed] [Google Scholar]
- 7. Zemaitis CT, Marris G, Cabie M, et al. Reducing readmission at an academic medical center: results of a pharmacy-facilitated discharge counseling and medication reconciliation program. Hosp Pharm. 2016. Jun; 51(6):468-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phatak A, Prusi R, Ward B, et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and postdischarge call-backs (IPITCH Study). J Hosp Med. 2016. Jan; 11(1):39-44. [DOI] [PubMed] [Google Scholar]
- 9. Jackson CT, Trygstad TK, DeWalt DA, DuBard CA. Transitional care cut hospital readmissions for North Carolina Medicaid patients with complex chronic conditions. Health Aff (Millwood). 2013. Aug; 32(8):1407-1415. [DOI] [PubMed] [Google Scholar]
- 10. Rodriques CR, Harrington AR, Murdock N, et al. Effect of pharmacy-supported transition-of-care interventions on 30-day readmissions: a systematic review and meta-analysis. Ann Pharmacother. 2017. Oct; 51(10):866-889. [DOI] [PubMed] [Google Scholar]
- 11. Anderegg SV, Wilkinson ST, Couldry RJ, et al. Effects of a hospitalwide pharmacy practice model change on readmission and return to emergency department rates. Am J Health Syst Pharm. 2014. Sep 1; 71(17):1469-1479. [DOI] [PubMed] [Google Scholar]
- 12. Ni W, Colayco D, Hashimoto J, et al. Impact of a pharmacy-based transitional care program on hospital readmissions. Am J Manag Care. 2017. Mar; 23(3):170-176. [PubMed] [Google Scholar]
- 13. Wing C, Simon K, Bello-Gomez RA. Designing difference in difference studies: best practices for public health policy research. Annu Rev Public Health. 2018. Apr 1; 39:453-469. [DOI] [PubMed] [Google Scholar]
- 14. Hale GM, Hassan SL, Hummel SL, et al. Impact of a pharmacist-managed heart failure postdischarge (bridge) clinic for veterans. Ann Pharmacother. 2017. Jul; 51(7):555-562. [DOI] [PubMed] [Google Scholar]
- 15. Milfred-Laforest SK, Gee JA, Pugacz AM, et al. Heart failure transition of care: a pharmacist-led post-discharge pilot experience. Prog Cardiovasc Dis. 2017. Sep-Oct; 60(2):249-258. [DOI] [PubMed] [Google Scholar]
- 16. Jackevicius CA, de Leon NK, Lu L, et al. Impact of a multidisciplinary heart failure post-hospitalization program on heart failure readmission rates. Ann Pharmacother. 2015. Nov; 49(11):1189-1196. [DOI] [PubMed] [Google Scholar]
- 17. Koshman SL, Charrois TL, Simpson SH, et al. Pharmacist care of patients with heart failure: a systematic review of randomized trials. Arch Intern Med. 2008. Apr 14; 168(7):687-694. [DOI] [PubMed] [Google Scholar]
- 18. Kaiser Family Foundation. Hospital adjusted expenses per inpatient day.https://www.kff.org/state-category/health-costs-budgets/hospital-inpatient-day-expenses/. Accessed May 21, 2019.