Abstract
Crumbs (Crb in Drosophila; CRB1-3 in mammals) is a transmembrane determinant of epithelial cell polarity and a regulator of Hippo signalling. Crb is normally localized to apical cell-cell contacts, just above adherens junctions, but how apical trafficking of Crb is regulated in epithelial cells remains unclear. We use the Drosophila follicular epithelium to demonstrate that polarized trafficking of Crb is mediated by transport along microtubules by the motor protein Dynein and along actin filaments by the motor protein Myosin-V (MyoV). Blocking transport of Crb-containing vesicles by Dynein or MyoV leads to accumulation of Crb within Rab11 endosomes, rather than apical delivery. The final steps of Crb delivery and stabilisation at the plasma membrane requires the exocyst complex and three apical FERM domain proteins – Merlin, Moesin and Expanded – whose simultaneous loss disrupts apical localization of Crb. Accordingly, a knock-in deletion of the Crb FERM-binding motif (FBM) also impairs apical localization. Finally, overexpression of Crb challenges this system, creating a sensitized background to identify components involved in cytoskeletal polarization, apical membrane trafficking and stabilisation of Crb at the apical domain.
Graphical abstract
Highlights
-
•
Crumbs is transported apically along microtubules and actin filaments.
-
•
Dynein is the key microtubule motor protein transporting Crumbs.
-
•
Myosin V is the key F-actin motor protein transporting Crumbs.
-
•
Crumbs polarises the cytoskeleton, which then directs further Crumbs delivery.
How epithelial cells polarize is a fascinating unsolved problem in biology. A key determinant of epithelial polarity is the transmembrane protein Crumbs, which localises specifically to the apical domain of epithelial cells and then helps direct the apical-basal polarization of the entire epithelial cell. How Crumbs itself becomes localized apically is still poorly understood. Here we define a key role for two motor proteins which can transport Crumbs apically along microtubules and actin filaments, respectively. Thus, a polarised cytoskeleton directs Crumbs delivery, which then helps define the apical-basal axis, which feeds back to polarize the cytoskeleton - a positive feedback loop.
1. Introduction
Cell polarity is a fundamental characteristic of living organisms. The molecular determinants of cell polarity have been revealed through pioneering genetic screens in the yeasts S. cerevisiae and S. pombe, the worm C. elegans, and the fruit fly Drosophila melanogaster (St Johnston and Ahringer, 2010; Tepass, 2012; Thompson, 2013). In yeast, the small GTPase Cdc42 was discovered to be a fundamental determinant of cell polarity (Adams et al., 1990), localizing to one pole of the cell through a positive feedback loop of self-recruitment (Johnson et al., 2011; Martin, 2015; Slaughter et al., 2009). Two general mechanisms for Cdc42-driven positive feedback were identified in yeast: (1) oligomeric clustering of Cdc42 complexes (Altschuler et al., 2008; Bendezu et al., 2015; Irazoqui et al., 2003) and (2) actin cytoskeleton mediated delivery of Cdc42 containing vesicles by a Myosin motor protein (Lechler et al., 2000; Wedlich-Soldner et al., 2003) in S. cerevisiae or microtubule mediated transport of polarizing factors in S. pombe (Martin and Arkowitz, 2014; Martin et al., 2005; Mata and Nurse, 1997; Minc et al., 2009). In fertilized worm oocytes (zygotes), polarization depends on clustering of Cdc42 and PAR-3 complexes (Dickinson et al., 2017; Gotta et al., 2001; Rodriguez et al., 2017; Sailer et al., 2015). The actin cytoskeleton flows to one pole of the worm zygote, pulling Cdc42-containing PAR-3 complexes along with it via bulk fluid ‘advection’ (Goehring et al., 2011). However, the actin cytoskeleton is not strictly required for polarization of the worm zygote, which can also be triggered by a microtubule-based mechanism (Motegi et al., 2011; Zonies et al., 2010). In Drosophila and mammalian oocytes, the actin cytoskeleton is polarized and plays an important role with Cdc42 in breaking symmetry (Leblanc et al., 2011; Leibfried et al., 2013; Ma et al., 2006; Wang et al., 2013; Yi et al., 2011, 2013; Zhang et al., 2008). In epithelial cells, which of these two mechanisms, oligomeric clustering of determinants versus cytoskeletal transport of determinants, is responsible for directing polarity remains a fundamental unsolved problem.
Drosophila epithelial cells exhibit a more complex polarization than oocytes, with the plasma membrane divided into distinct apical and basolateral domains, separated by a ring of adherens junctions (Gibson and Perrimon, 2003; St Johnston and Ahringer, 2010; Tepass, 2012; Thompson, 2013). Like oocytes, epithelial cells express the cortical polarity determinant Par-3/Bazooka (Baz), which is polarized through oligomeric clustering at the plasma membrane (Benton and St Johnston, 2003b, Benton and St Johnston, 2003a; Harris, 2017; Krahn et al., 2010; McKinley et al., 2012; Mizuno et al., 2003). In addition, epithelial cells express a second apical polarity determinant, Crumbs (Crb) (Bazellieres et al., 2018; Campbell et al., 2009; Grawe et al., 1996; Knust et al., 1993; Tepass, 2012; Tepass and Knust, 1993; Tepass et al., 1990). Baz and Crb act in parallel to maintain the apical domain via recruitment of the Cdc42-Par6-aPKC complex (Fletcher et al., 2012, 2015; Hutterer et al., 2004; Joberty et al., 2000; Petronczki and Knoblich, 2001; Shahab et al., 2015; Tanentzapf and Tepass, 2003). The Cdc42-Par6-aPKC complex promotes Crb polarization, forming a positive feedback loop whose nature is still not fully understood (Fletcher et al., 2012; Harris and Tepass, 2008). The redundancy between Baz and Crb makes it possible to study Crb polarization in isolation, because defects in Crb localization to the apical domain do not disrupt the overall apical-basal polarization of most epithelial cells, and instead disrupts Hippo signalling (Chen et al., 2010; Fletcher et al., 2012, 2015; Ling et al., 2010) – indeed the crucial requirement for Crb in epithelial polarity occurs during embryonic gastrulation (Campbell et al., 2009; Grawe et al., 1996), when Baz is localized to adherens junctions in a planar polarized fashion during germ-band extension (Simoes Sde et al., 2010; Zallen and Wieschaus, 2004).
We previously proposed a model of Crb localization via Cdc42-dependent positive feedback, based on analysis of overexpressed Crb in Drosophila follicle cells (Fletcher et al., 2012). In this model, Crb – a transmembrane protein – is delivered apically from Rab11 endosomes (Blankenship et al., 2007; Li et al., 2007; Roeth et al., 2009) and engages in oligomeric clustering via its extracellular domain, as well as interacting via its cytoplasmic domain with the PDZ domain protein Stardust/PALS1 [Sdt (Bachmann et al., 2001; Hong et al., 2001; Knust et al., 1993; Muller and Wieschaus, 1996; Tepass and Knust, 1993)] and the FERM domain proteins Moesin [Moe (Medina et al., 2002; Polesello et al., 2002; Sherrard and Fehon, 2015b; Speck et al., 2003)] and Expanded [Ex (Chen et al., 2010; Ling et al., 2010)] to regulate Crb localization at the plasma membrane (Fletcher et al., 2012; Thompson et al., 2013). It was further suggested that aPKC phosphorylates the Crb FERM-binding domain (Sotillos et al., 2004) and that this domain might contribute to stabilisation of Crb at the apical domain (Fletcher et al., 2012). Further genetic and structural evidence supports a key role for direct interaction with Sdt/PALS1 in maintaining endogenous Crb at the plasma membrane by preventing Crb endocytosis (Ivanova et al., 2015; Li et al., 2014; Lin et al., 2015) and that aPKC phosphorylation of Crb may antagonize Moe binding in favour of Ex (Sherrard and Fehon, 2015b; Su et al., 2017) or Sdt (Wei et al., 2015). However, recent evidence indicates that deletion of the extracellular domain of endogenously expressed Crb, or mutation of the FERM binding domain of endogenously expressed Crb, does not disrupt epithelial polarity during embryonic gastrulation in Drosophila (Cao et al., 2017; Das and Knust, 2018; Klose et al., 2013), although it can affect Crb localization in the follicle cell epithelium (Sherrard and Fehon, 2015a). Furthermore, FERM domain phosphorylation is also not strictly essential for endogenous Crb localization or function in the embryo (Cao et al., 2017). The reasons for the discrepancy in results between overexpressed Crb (Fletcher et al., 2012; Letizia et al., 2011, 2013; Roper, 2012) and endogenous Crb (Cao et al., 2017; Das and Knust, 2018) remain unclear and are important to resolve. Evidently, mechanisms of positive feedback exist that can recruit Crb to the apical domain in the absence of Crb-Crb oligomeric clustering via the extracellular domain or aPKC phosphorylation of the Crb intracellular domain.
We therefore considered alternative mechanisms of positive feedback that might promote Crb localization to the apical domain. Since Crb is a transmembrane protein, it is conceivable that its exocytic delivery to the plasma membrane could become polarized by directional transport along either microtubules or actin. Microtubule polarity depends on core epithelial polarity determinants, which localise the microtubule minus-end binding proteins Shot (MACF1, ACF7, BPAG1 in mammals) and Patronin (CAMSAP1, CAMSAP2, CAMSAP3 in mammals) for acentrosomal nucleation apically (Khanal et al., 2016; Noordstra et al., 2016; Toya et al., 2016). The actin cytoskeleton is organized by the Rho GTPase and adherens junctions (Cox et al., 1996; Fox et al., 2005; Homem and Peifer, 2008; Muller and Wieschaus, 1996; Peifer et al., 1993) which are ultimately positioned between the core apical and basolateral polarity determinants. The apical determinants include Baz (Muller and Wieschaus, 1996), Crb (Grawe et al., 1996; Tanentzapf et al., 2000; Tepass et al., 1990), Sdt (Bachmann et al., 2001; Hong et al., 2001; Knust et al., 1993; Muller and Wieschaus, 1996; Tepass and Knust, 1993), Cdc42 (Atwood et al., 2007; Eaton et al., 1995; Genova et al., 2000; Harris and Tepass, 2008; Hutterer et al., 2004; Joberty et al., 2000; Peterson et al., 2004) and its effector kinases aPKC (Hutterer et al., 2004; Kim et al., 2009; Wodarz et al., 2000), Pak1 (Aguilar-Aragon et al., 2018; Conder et al., 2007; Harden et al., 1996), Pak4 (Walther et al., 2016), Gek/MRCK (Luo et al., 1997; Zihni et al., 2017) as well as WASP (Leibfried et al., 2008) and the Arp2/3 complex (Georgiou et al., 2008; Leibfried et al., 2013). The basolateral polarity determinants Lgl, Scrib, Dlg and Yurt engage in mutual antagonism with apical polarity determinants and are thus necessary for cytoskeletal polarization (Bilder et al., 2000, 2003; Bilder and Perrimon, 2000; Gamblin et al., 2014; Laprise et al., 2006, 2009; Tanentzapf et al., 2000; Tanentzapf and Tepass, 2003). Despite this wealth of understanding of how the cytoskeleton is polarized along the apical-basal axis in epithelia, whether it might function to direct membrane trafficking of Crb remains poorly understood.
Here we show that the actin motor protein Myosin-V (MyoV), previously implicated in Rab11-mediated apical secretion and found to co-localise with Crb (Li et al., 2007; Pocha et al., 2011), as well as the microtubule motor protein Dynein, previously implicated in transporting crb and sdt mRNA (Cao et al., 2017; Horne-Badovinac and Bilder, 2008; Li et al., 2008), both function in directing membrane trafficking of the Crb protein from Rab11 endosomes (Fletcher et al., 2012; Roeth et al., 2009) to the apical domain of the ovarian follicle cell epithelium. Accordingly, disruption of microtubule polarization and/or the actin cytoskeleton impairs Crb localization to the apical domain. We confirm that the Exocyst complex then promotes the final step in delivery of Crb to the plasma membrane, as previously demonstrated in the embryo (Blankenship et al., 2007). Crb must then interact with apical FERM-domain proteins via its FERM-binding motif to be stabilized at the plasma membrane. Finally, we find that overexpression of Crb challenges this system of polarized transport and exocytic delivery, acting as a sensitized background for the testing of molecular mechanisms involved in Crb polarization.
2. Results
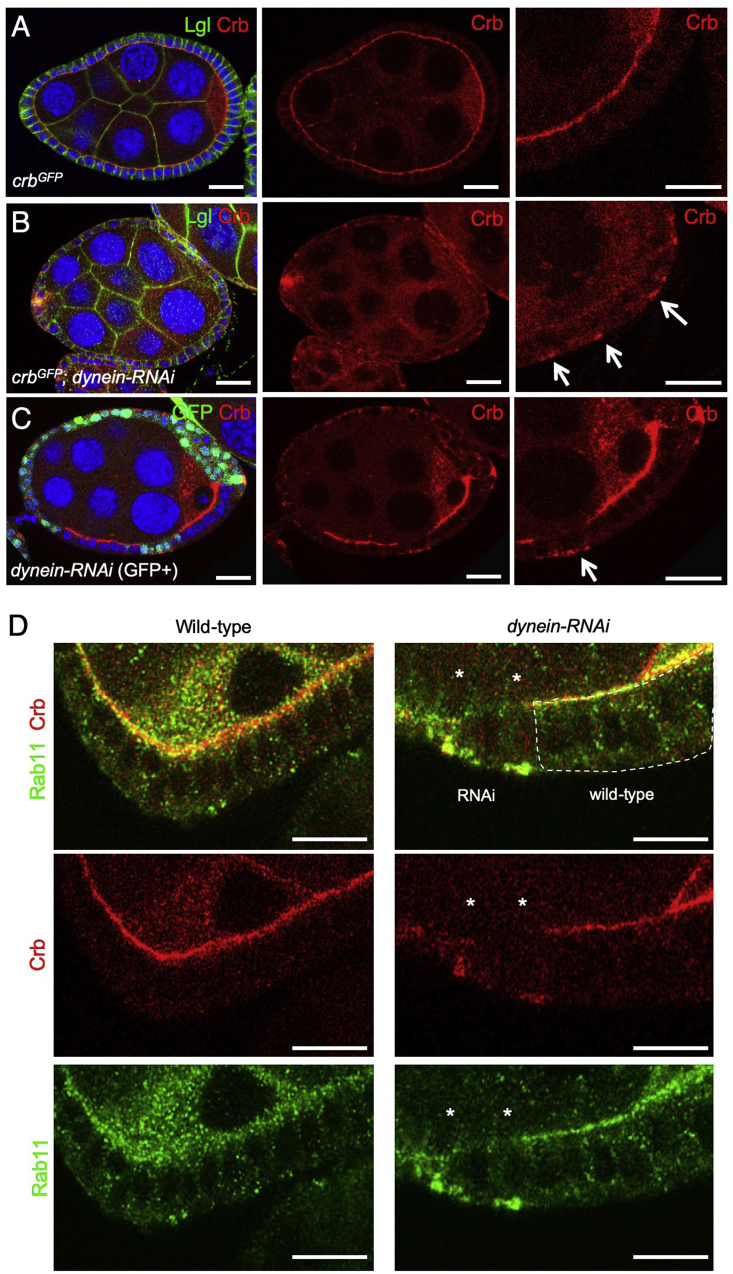
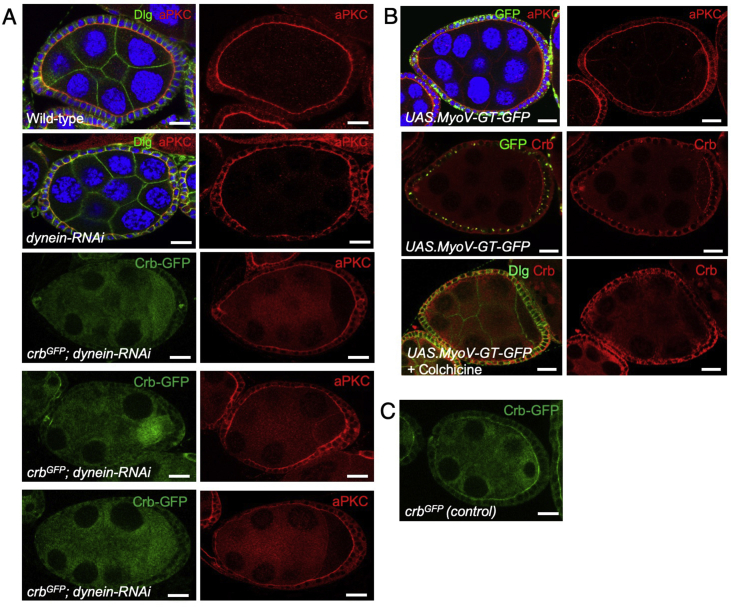
We began by re-examining the requirement for the microtubule motor protein Dynein in regulation of Crb in the Drosophila ovarian follicle cell epithelium. Previously, loss of Dynein was reported to cause decreased levels of Crb protein in follicle cells due to failure of crb or sdt mRNA transport (Cao et al., 2017; Horne-Badovinac and Bilder, 2008; Li et al., 2008). Using a Crb-GFP knockin line, we find that, in addition to reduced Crb protein at the apical membrane domain, Crb also accumulates basally in Rab11 endosomes upon silencing of Dynein expression by conditional expression of UAS.dynein-RNAi hairpins with the follicle cell-specific trafficjam.Gal4 (tj.Gal4) driver (Fig. 1A,B; Fig. 1-S1). We find similar results with antibody staining for Crb, which localises basally upon clonal expression of actin > flipout > Gal4 UAS.dynein-RNAi hairpins but not in neighbouring wild-type follicle cells (Fig. 1C). Co-staining for Crb and Rab11 in wild-type and dynein-RNAi expressing follicle cells reveals that Crb protein accumulates within Rab11-positive endosomes (Fig. 1D). These results suggest that upon loss of the microtubule minus-end directed motor protein Dynein, Rab11 endosomes containing Crb protein are transported basally, rather than apically.
Fig. 1.
Dynein is required to traffic Crumbs protein apically in the Drosophila follicle cell epithelium
A) Endogenously tagged knockin Crb-GFP localises apically in the follicle cell epithelium (stage 7/8 egg chamber; scale bar approximately 10 μm).
B) Expression of Dynein hairpin RNAi throughout the follicle cell epithelium with tj.Gal4 causes relocalisation of Crb-GFP to the basal surface of follicle cells. Anti-GFP antibody staining is shown (stage 7/8 egg chamber; scale bar approximately 10 μm).
C) Clonal expression of Dynein hairpin RNAi, marked by expression of nls-GFP, which causes cell-autonomous relocalisation of Crb to the basal surface of follicle cells within the clone (stage 7/8 egg chamber; scale bar approximately 10 μm).
D) Co-immunostaining for Crb and Rab11 upon clonal expression of Dynein hairpin RNAi, marked by expression of nls-GFP, reveals co-localization within basal endosomes (stage 7/8 egg chamber; scale bar approximately 10 μm). Asterisks indicate a clone of cells expressing UAS.Dynein-RNAi.
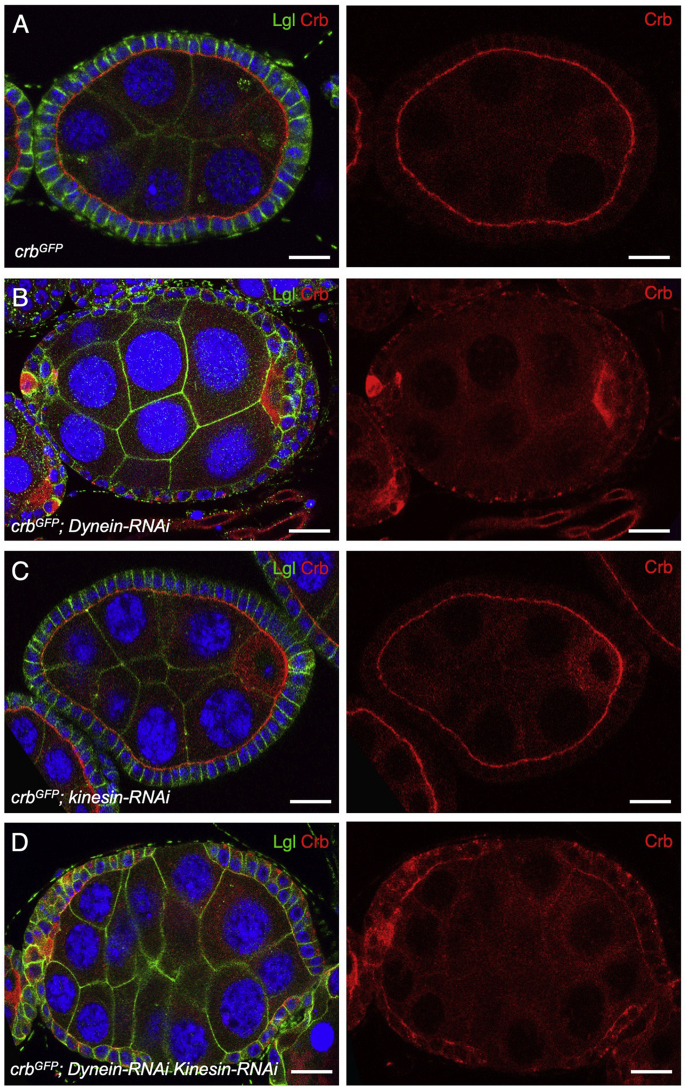
The basal transport of endosomes is normally mediated by the plus-end directed microtubule motor protein Kinesin (Mellman and Nelson, 2008; Mostov et al., 2000; Nelson, 1991, 2003; Rodriguez-Boulan and Macara, 2014; Weisz and Rodriguez-Boulan, 2009). We therefore sought to confirm that, in the absence of Dynein, Kinesin is responsible for basal transport of Crb, using the Gal4-UAS system. We find that the basal transport of Crb-GFP in dynein-RNAi expressing follicle cells is disrupted by simultaneous expression of kinesin-RNAi, leading to an abnormal depolarised localization of Crb (Fig. 2A–D). Note that co-expression of UAS-driven dynein-RNAi with a UAS-driven GFP does not alter the dynein-RNAi phenotype, which rules out a dilution effect of the GAL4 transcription factor. These results confirm that both Dynein and Kinesin are capable of transporting Crb containing Rab11-positive endosomes, but that under normal conditions the Dynein-dependent transport is more efficient, leading to apical localization of Rab11 endosomes and delivery of the Crb protein to the apical plasma membrane domain.
Fig. 2.
Dynein loss is partially rescued by Kinesin-RNAi.
A) Endogenously tagged knockin Crb-GFP localises apically in the follicle cell epithelium (stage 7 egg chamber; scale bar approximately 10 μm).
B) Expression of Dynein hairpin RNAi throughout the follicle cell epithelium with tj.Gal4 causes relocalisation of Crb-GFP to the basal surface of follicle cells (stage 7 egg chamber; scale bar approximately 10 μm).
C) Expression of Kinesin hairpin RNAi throughout the follicle cell epithelium with tj.Gal4 does not affect localization of Crb-GFP in follicle cells (stage 7 egg chamber; scale bar approximately 10 μm).
D) Expression of both Dynein and Kinesin hairpin RNAi throughout the follicle cell epithelium with tj.Gal4 does partially rescues the effect of Dynein RNAi on localization of Crb-GFP in follicle cells. Anti-GFP antibody staining is shown (stage 7 egg chamber; scale bar approximately 10 μm).
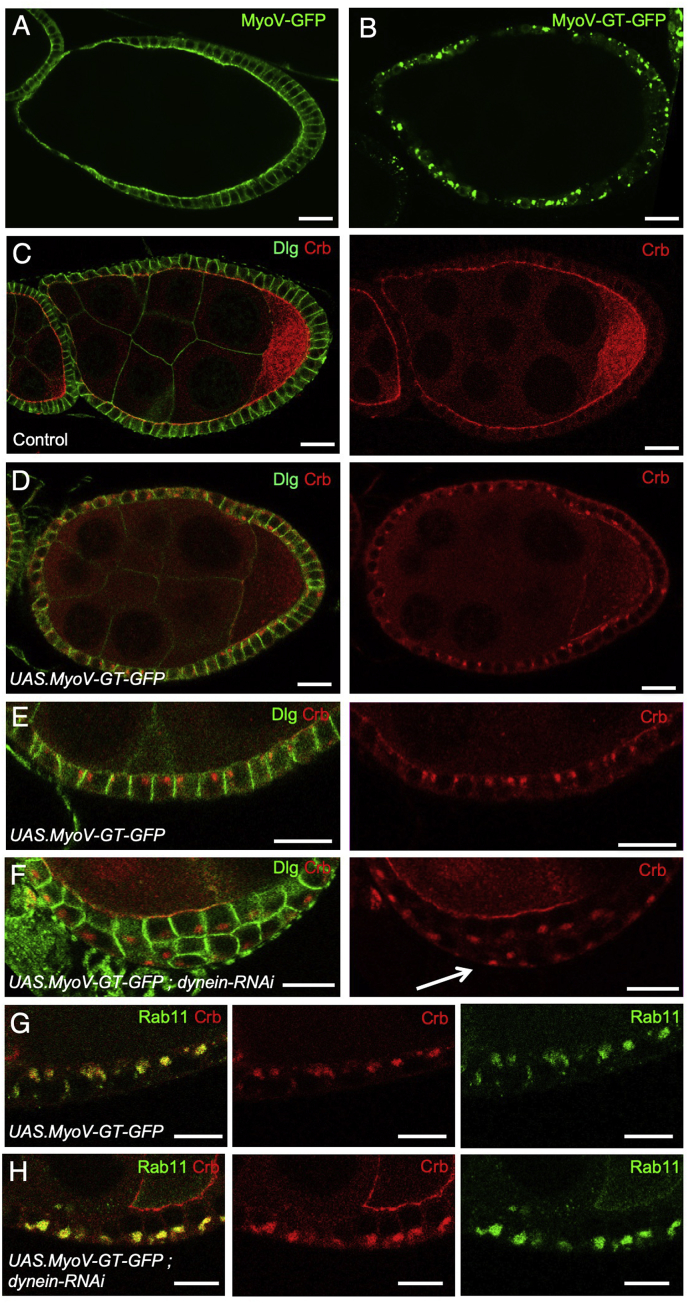
In addition to transport along microtubules by the Dynein motor, it is conceivable that Crb may also be transported along actin filaments by motors such as Myosin V (MyoV), which has been previously shown to form a complex with Crb and to promote apical secretion from Rab11 endosomes in the Drosophila photoreceptor (Li et al., 2007; Pocha et al., 2011). Class V Myosins were originally discovered in the yeast S. cerevisiae to promote polarized transport along F-actin filaments (Johnston et al., 1991; Pruyne et al., 1998, 2004), alongside Class I Myosins (Anderson et al., 1998; Goodson et al., 1996; Lechler et al., 2000, 2001; Wedlich-Soldner et al., 2003), suggesting possible redundancy between Class I and Class V Myosins. We find that ectopically expressed MyoV-GFP primarily localises apically in the follicle cell epithelium, while a dominant-negative form of MyoV named MyoV-GT-GFP, which retains cargo-binding activity but cannot transport it (Krauss et al., 2009), fails to localise apically and instead localises on endosomes (Fig. 3A,B). Use of the dominant-negative MyoV-GT-GFP thus circumvents any possible redundancy between Drosophila Myosins. Accordingly, ectopic expression of MyoV-GT-GFP prevents delivery of Crb to the apical membrane, such that the Crb protein is trapped inside large endosomes that reside just underneath the apical domain (Fig. 3C–E). The apical localization of these enlarged Crb-positive endosomes is completely disrupted upon co-expression of UAS.dynein-RNAi, which leads to basal localization in cells that are still polarized, or to random distribution in those cells that have been extruded (Fig. 3F–H), or by depolymerization of microtubules with Colchicine (Fig. S1B). Co-staining of Crb and Rab11 confirms that these Crb-containing endosomes are indeed Rab11-positive (Fig. 3F,G). These results confirm a dual requirement for transport of the Crb protein by F-actin and microtubule motor proteins.
Fig. 3.
Dominant-negative Myosin V prevents apical delivery of Crumbs, revealing apical transport of entire Crumbs-containing Rab11-positive endosomes by Dynein.
A) GFP-tagged MyoV expressed with tj.Gal4 localises primarily to the apical domain of follicle cells (n > 10 stage 7/8 egg chambers; scale bar approximately 10 μm).
B) GFP-tagged MyoV GT (dominant-negative) localises to endosomes (n > 10 stage 7/8 egg chambers; scale bar approximately 10 μm).
C) Control egg chambers immunostained for Dlg and Crb (n > 12 stage 7/8 egg chambers; scale bar approximately 10 μm).
D) Expression of dominant-negative MyoV GT prevents apical localization of Crb, which instead accumulates in endosomes that localise towards the apical pole of the cell (n > 15 stage 7/8 egg chambers; scale bar approximately 10 μm).
E) High magnification view of C (n > 15 stage 7/8 egg chambers; scale bar approximately 10 μm).
F) Co-expression of Dynein hairpin RNAi with dominant-negative MyoV GT causes accumulation of Crb in endosomes that localise basally (n > 11 stage 7/8 egg chambers; scale bar approximately 10 μm).
G) Co-immunostaining for Rab11 and Crb upon expression of dominant-negative MyoV GT reveals co-localization in enlarged apical endosomes, which are also positive for MyoV-GT-GFP (see Fig. S1B) (n > 8 stage 7/8 egg chambers; scale bar approximately 10 μm).
H) Co-immunostaining for Rab11 and Crb upon expression of dominant-negative MyoV GT and dynein-RNAi hairpins reveals co-localization in enlarged basal endosomes (n > 9 stage 7/8 egg chambers; scale bar approximately 10 μm).
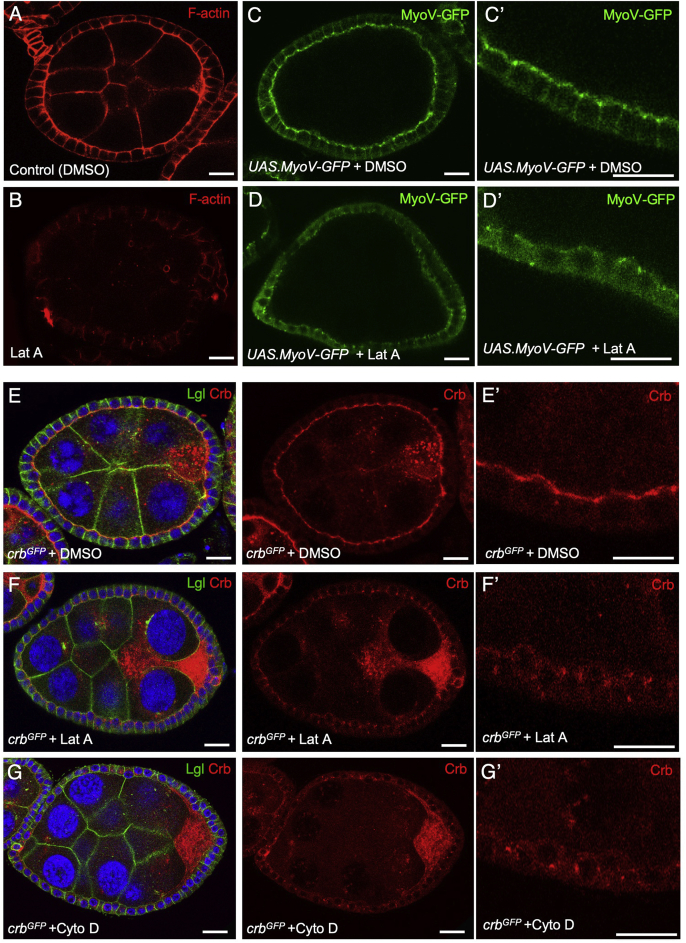
Given our findings with MyoV, we sought to confirm that the F-actin cytoskeleton is also necessary for apical localization of Crb. As expected, disruption of F-actin by acute treatment with Latrunculin A (Lat A) caused a strong loss of MyoV-GFP and Crb-GFP from the apical domain, with Crb-GFP accumulating in endosomes (Fig. 4A–F). Treatment with another F-actin cytoskeleton disrupting compound, Cytochalasin D (Cyto D), had a similar effect on Crb-GFP localization (Fig. 4G). These results confirm the essential requirement for the F-actin cytoskeleton in polarization of Crb.
Fig. 4.
The F-actin cytoskeleton, which concentrates apically, is required for apical localization of both MyoV and Crb.
A) Control (DMSO-treated) egg chamber stained for F-actin with phalloidin (n > 9 stage 7/8 egg chambers; scale bar approximately 10 μm).
B) Latrunculin A treated egg chamber stained for F-actin with phalloidin (n > 10 stage 7/8 egg chambers; scale bar approximately 10 μm).
C) Control (DMSO-treated) egg chamber expressing MyoV-GFP with tj.Gal4 (n > 10 stage 7/8 egg chambers; scale bar approximately 10 μm). C′ show zoom image.
D) Latrunculin A treated egg chamber expressing MyoV-GFP with tj.Gal4 shows loss of MyoV-GFP apical localization (n > 12 stage 7/8 egg chambers; scale bar approximately 10 μm). D′ shows zoom image.
E) Control (DMSO-treated) Crb-GFP egg chamber showing normal apical localiz
ation of Crb in follicle cells (n > 7 stage 7/8 egg chambers; scale bar approximately 10 μm). E′ shows zoom image.
F) Latrunculin A treated Crb-GFP egg chamber showing loss of apical Crb localiz
ation and localization to endosomal punctae (n > 5 stage 7/8 egg chambers; scale bar approximately 10 μm). F′ shows zoom image.
G) Cytochalasin D treated Crb-GFP egg chamber showing loss of apical Crb localiz
ation and localization to endosomal punctae (n > 7 stage 7/8 egg chambers; scale bar approximately 10 μm). G′ shows zoom image.
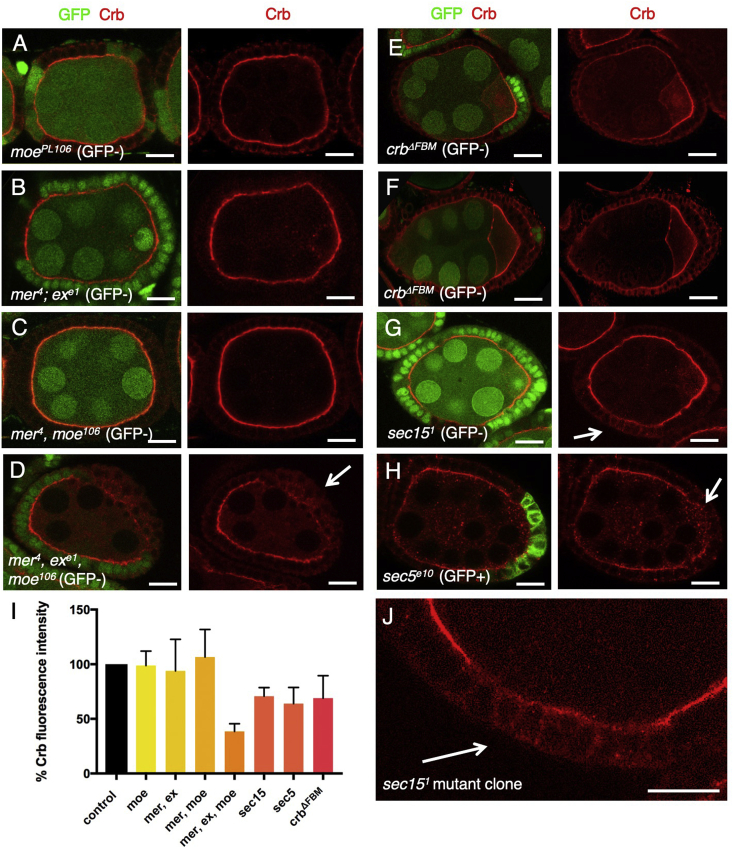
We next sought to examine the role of the apically-localized FERM domain proteins – Merlin (Mer), Expanded (Ex) and Moesin (Moe). FERM domains link the actin cytoskeleton to the plasma membrane (Chishti et al., 1998) and bind directly to spectrins (Baines et al., 2014), which are required to polarize microtubules and regulate the Hippo pathway in Drosophila (Fletcher et al., 2015; Khanal et al., 2016). Mer and Ex are redundantly required to regulate the Hippo signaling pathway in Drosophila, a parallel function that may arise from the different subcellular localizations of Mer, which is found across the apical surface, and Ex, which localises to the sub-apical junction through direct interaction with the Crb intracellular domain (Fletcher et al., 2015; Hamaratoglu et al., 2006; Su et al., 2017). Moe has important roles in linking cortical F-actin to the plasma membrane, particularly during mitotic cell rounding and microvilli formation (Carreno et al., 2008; Fehon et al., 2010; Kunda et al., 2008; Sauvanet et al., 2015). We find that mutation of moe does not affect Crb localization in follicle cells, similar to mer, ex double mutants (Fig. 5A,B). Double mutants of mer, moe also have no effect on Crb (Fig. 5C). However, triple mutant clones of mer, ex, moe do lead to a strong disruption of Crb localization (Fig. 5D). Accordingly, knock-in deletion of the Crb FERM-binding motif (FBM) (Huang et al., 2009) also reduces the apical localization of Crb in follicle cells (Fig. 5E,F). These findings demonstrate that the apical FERM domain proteins are collectively required to promote Crb polarization, a role that is consistent with their molecular functions in polarising the cytoskeleton in epithelia and with their ability to directly bind the Crb intracellular domain.
Fig. 5.
Apical FERM domain proteins and the Exocyst promote apical localisation of Crb.
A) Mutant clones for moesin, marked by absence of GFP, show normal Crb localization (n > 12 stage 6–9 egg chambers; scale bar approximately 10 μm).
B) Double mutant clones for merlin and expanded, marked by absence of GFP, show normal Crb localization (n > 6 stage 6–9 egg chambers; scale bar approximately 10 μm).
C) Double mutant clones for merlin and moesin, marked by absence of GFP, show normal Crb localization (n > 14 stage 6–9 egg chambers; scale bar approximately 10 μm).
D) Triple mutant clones for merlin, expanded and moesin, marked by absence of GFP, show loss of apical Crb localization (n > 4 stage 6–9 egg chambers; scale bar approximately 10 μm).
E) Mutant clones for crbΔFBM, marked by absence of GFP, showing weakly reduced apical Crb localization (n > 3 stage 6–9 egg chambers; scale bar approximately 10 μm).
F) Mutant clones for crbΔFBM, marked by absence of GFP, showing strongly reduced apical Crb localization (n > 6 stage 6–9 egg chambers; scale bar approximately 10 μm).
G) Mutant clones for sec15, marked by absence of GFP, show loss of apical Crb localization (n > 5 stage 6–9 egg chambers; scale bar approximately 10 μm).
H) Mutant clones for sec15, marked positively by expression of GFP, show loss of apical Crb localization in extruded cells (n > 7 stage 6–9 egg chambers; scale bar approximately 10 μm).
I) Quantification of average apical Crb fluorescence pixel intensity, measured with Image J in n > 20 cells per genotype.
J) Zoom image of (G) showing mislocalisation of Crb in sec151 mutant cells.
Once Crb has been successfully trafficked to the apical membrane of the cell, it must be delivered to the plasma membrane through a process of regulated exocytosis. The exocyst complex was discovered to mediate polarized exocytosis in the yeast S. cerevisiae (He and Guo, 2009; Novick et al., 1980; TerBush et al., 1996). The exocyst subunit Sec15 directly interacts with the Cdc42-mediated polarity establishment complex in yeast (France et al., 2006). In mammalian epithelial cells in culture, the exocyst associates with adherens junctions, tight junctions (Yeaman et al., 2004), and Par3 (Ahmed and Macara, 2017). In Drosophila epithelia, the exocyst component Exo84 is required for apical trafficking of Crb from Rab11 endosomes to the plasma membrane in embryos (Blankenship et al., 2007); Sec6 is required for apical exocytosis in Drosophila photoreceptors (Beronja et al., 2005); and Sec5 is required for efficient delivery of E-cadherin to adherens junctions in the pupal notum (Langevin et al., 2005) but is not required for cytoskeletal polarization in follicle cell epithelium (Murthy and Schwarz, 2004). We therefore tested whether Sec15 and Sec5 were required for apical localization of Crb in the follicle cell epithelium. We find that mutants clones for sec15 or sec5 strongly disrupt the apical localization of Crb (Fig. 5G–J). These findings confirm an essential requirement for the exocyst in delivery of Crb to the apical membrane in the follicular epithelium.
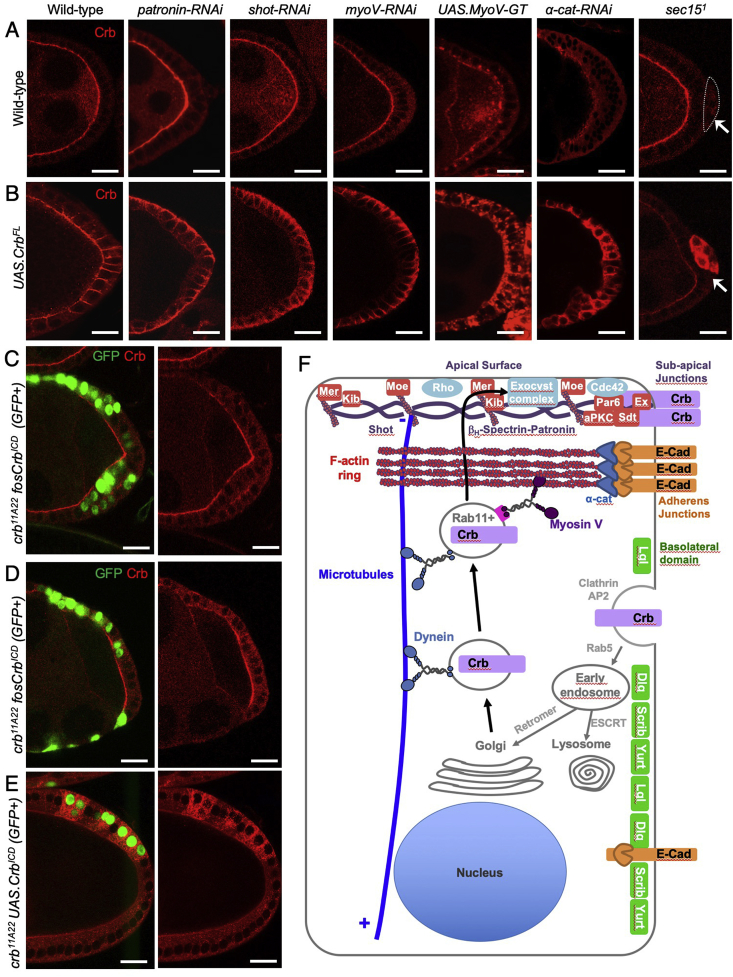
Finally, we sought to challenge the system of polarized Crb exocytosis by overexpressing full-length Crb protein in the follicle cell epithelium. We find that this results in ectopic localization of Crb to lateral membranes, in addition to the apical domain (Fig. 6A,B). Importantly, loss of individual components of the cytoskeletal polarization/trafficking machinery or exocyst dramatically enhances the Crb overexpression phenotype, leading to abnormal accumulation of Crb in membranes at the lateral and basal sides of the cell as well as internal endosomal membranes (Fig. 6A,B). This genetic interaction also occurs for genes whose individual loss-of-function has no detectable effect on Crb in a wild-type background, such as patronin or shot. The assay further highlights the key role of adherens junctions, whose disruption with alpha-catenin-RNAi strongly affects the F-actin cytoskeleton and causes a striking endosomal accumulation of overexpressed Crb (Fig. 6B). The assay similarly reveals a key role for the Crb extracellular domain, whose loss leads to strong accumulation of the overexpressed Crb transmembrane and intracellular domain (CrbICD) in endosomes, even though endogenously-expressed CrbICD exhibits only a moderate reduction in apical localization (Fig. 6C–E). Thus, Crb overexpression in follicle cells is a highly sensitized assay for detecting components of the Crb trafficking and polarization machinery, even where such components exhibit redundancy or degeneracy with others or have pleiotropic phenotypes. Novel genes identified in this assay should then be examined for their effect on endogenous Crb in double and triple mutant combinations with other redundantly required genes. A summary of the key components of cytoskeletal polarization and Crb trafficking is shown schematically in the final diagram (Fig. 6F).
Fig. 6.
Overexpression of Crumbs is a sensitized assay to identify genes required for its apical trafficking and delivery.
A) Crb localization in wild-type posterior follicle cells and those of the indicated genotypes, either transgenes expressed with tj.gal4 or mutant clones of sec15 (arrow) (n > 8 samples per experimental condition; posterior region of stage 7/8 egg chambers; scale bar approximately 10 μm).
B) Overexpressed full-length Crb localization in otherwise wild-type posterior follicle cells and those of the indicated genotypes, either transgenes expressed with tj.gal4 or mutant clones of sec15 (arrow). Note the strong accumulation of overexpressed Crb upon manipulation of key trafficking regulators, including those that disrupt the microtubule cytoskeleton. Thus, although some factors are normally dispensable for endogenous Crb localization, they are essential to localise overexpressed Crb (n > 8 samples per experimental condition; posterior region of stage 7/8 egg chambers; scale bar approximately 10 μm).
C) Null mutant clones for crb expressing Crb intracellular domain (ICD) at endogenous levels from a fosmid (marked by expression of GFP; posterior region of stage 7/8 egg chambers; scale bar approximately 10 μm).
D) Null mutant clones for crb expressing Crb intracellular domain (ICD) at endogenous levels from a fosmid (marked by expression of GFP; posterior region of stage 7/8 egg chambers; scale bar approximately 10 μm).
E) Null mutant MARCM clones for crb expressing Crb intracellular domain (ICD) at high levels with the GAL4/UAS system (marked by expression of GFP; posterior region of stage 7/8 egg chambers; scale bar approximately 10 μm).
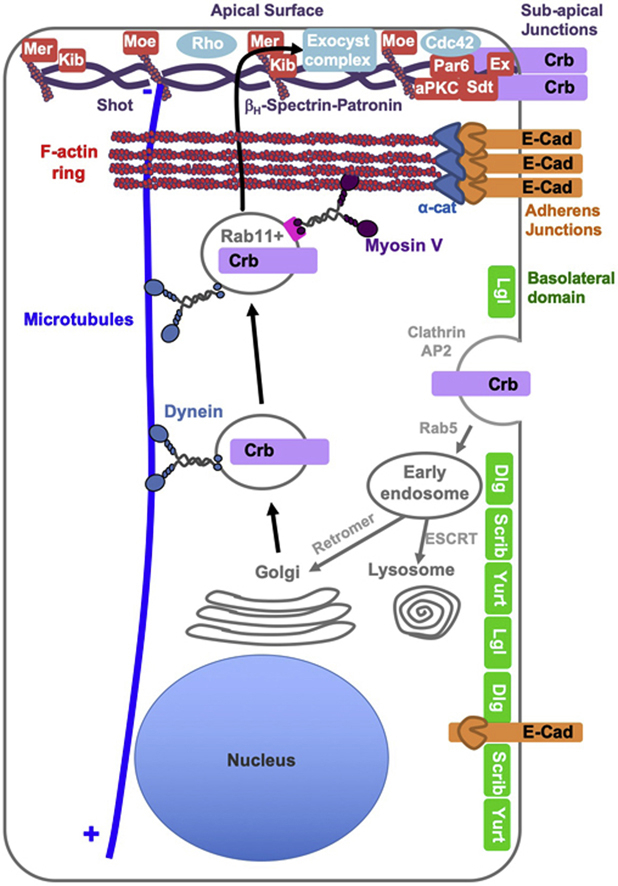
F) Schematic diagram of key components of the cellular cytoskeleton and trafficking machinery and their functions in localising Crb to the apical domain of epithelial cells.
3. Discussion
Polarization of the cytoskeleton is a universal feature of columnar epithelial cells that determines polarized membrane trafficking of many cargo proteins and is known to depend on fundamental determinants of epithelial polarity (Mellman and Nelson, 2008; Mostov et al., 2000; Nelson, 1991, 2003; Rodriguez-Boulan and Macara, 2014; Weisz and Rodriguez-Boulan, 2009). Whether the localization of apical-basal polarity determinants themselves requires cytoskeletal polarization has been uncertain, particularly because apical-basal polarization of the Baz/Par-3 system can arise without cytoskeletal polarization or membrane trafficking in asymmetrically dividing Drosophila neuroblasts or C. elegans zygotes (Halbsgut et al., 2011; St Johnston and Ahringer, 2010; Tepass, 2012; Thompson, 2013). Furthermore, disruption of microtubules or preventing polarization of microtubules does not strongly interfere with polarization of the plasma membrane in S. cerevisiae (Martin and Arkowitz, 2014), in the Drosophila follicle cell epithelium (Khanal et al., 2016) or in cultured mammalian epithelial cells (Noordstra et al., 2016; Toya et al., 2016). In the case of Crb, an apical polarity determinant and transmembrane protein that moves through the secretory pathway via Rab11 endosomes and the exocyst to reach the apical domain (Blankenship et al., 2007; Fletcher et al., 2012; Li et al., 2007; Roeth et al., 2009) (Fig. S1), it was previously unclear whether polarized cytoskeletal transport was in fact required for apical membrane trafficking and localization of the Crb protein, rather than crb or sdt mRNA (Cao et al., 2017; Horne-Badovinac and Bilder, 2008; Li et al., 2008).
Our results demonstrate that cytoskeletal polarization and directed motor transport of Crb protein are necessary for its localization to the apical domain of the Drosophila follicular epithelium. Disruption of either Dynein-mediated microtubule transport or MyoV-mediated actin transport leads to trapping of Crb in endosomes, such that it is unable to reach the apical domain (Fig. 1, Fig. 2, Fig. 3), without obviously affecting overall polarization of aPKC (Fig. S1). Microtubules are polarized along the apical-basal axis of epithelial cells, with minus ends apical, such that loss of the minus-end directed motor Dynein leads to abnormal basally-directed transport of Crb endosomes (Fig. 1). The F-actin cytoskeleton concentrates apically, such that dominant-negative MyoV traps Crb in endosomes that are still transported towards the apical pole of the cell on microtubules, but appear unable to traverse the thick cortical F-actin at the apical surface to reach the plasma membrane (Figs. 3 and 4). Our findings confirm and extend previous work demonstrating that mutation of exocyst complex components also prevents apical delivery of Crb (Fig. 5). Thus, membrane trafficking of Crb occurs by directed motor-driven transport along polarized microtubules and F-actin filaments, followed by exocyst-mediated delivery to the plasma membrane, and is crucial for the apical localization of this key polarity determinant.
Our findings also shed light on the mechanisms of cytoskeletal polarization. We have identified a key role for the apical FERM domain proteins, which link the PIP2-rich plasma membrane with F-actin, apical spectrins and the microtubule minus-end binding proteins Shot and Patronin (Chishti et al., 1998; Fehon et al., 2010; Khanal et al., 2016). This apical cortical meshwork (equivalent to the terminal web in mammalian epithelia) is then responsible for polarizing microtubules and thus ensuring directed apical trafficking of Crb by Dynein. Further work is necessary to understand precisely how the three apical FERM domain proteins become localized, and how the apical meshwork is organized, particularly as Moe and Mer are normally found across the apical surface (with Shot) while Ex is found at the sub-apical junctions (with a βH-Spectrin-Patronin complex) (Su et al., 2017). Importantly, cytoskeletal polarization ultimately depends on the core apical-basal polarity determinants, including Crb itself, which acts redundantly with Baz to organize epithelial polarity (Tepass, 2012; Thompson et al., 2013). Thus, there is a positive feedback loop between apical polarity determinants and cytoskeletal polarization, which then directs further delivery of Crb to the same location on the plasma membrane to reinforce apical identity and maintain a polarized cytoskeleton.
The above mechanism of cytoskeletal polarization is required for localization of Crb, a transmembrane protein, but is not required for polarization of Baz, a cytoplasmic protein that associates with the plasma membrane. Thus, during asymmetric division of Drosophila neuroblasts, which are polarized along the apical-basal axis by Baz, there is no role for either Crb or membrane trafficking (Halbsgut et al., 2011; Hong et al., 2001). Furthermore, during the early establishment of epithelial polarity in the Drosophila embryo, polarity is initiated by Baz before Crb becomes expressed (Blankenship et al., 2007; Harris and Peifer, 2004). It is conceivable that the Baz system is able to polarize more rapidly than the Crb system, which may be an advantage in asymmetric cell division and in early establishment of epithelial polarity, but that the Crb system is advantageous in mature epithelial cells, where stable polarization of the cytoskeleton is fundamental to both cellular structure and function. Importantly, redundancy between Baz and Crb-Sdt in recruiting the Cdc42-Par6-aPKC complex enables one system to maintain apical-basal polarity while the other is deployed in a planar polarized fashion during various episodes of morphogenetic change during development (Campbell et al., 2009; Grawe et al., 1996; Thompson et al., 2013).
Our findings also indicate that overexpression of Crb can saturate this system of polarized transport, such that Crb accumulates abnormally along lateral membranes (Fig. 6A,B). Overexpressed Crb can also accumulate dramatically within endosomes when individual components of the polarized transport machinery are compromised (Fig. 6A,B). The sensitized background caused by Crb overexpression explains the discrepancy between deletion of the conserved extracellular domain in overexpressed Crb, which has dramatic consequences (Fletcher et al., 2012; Letizia et al., 2013; Roper, 2012; Thompson et al., 2013), versus the same experiment in endogenous Crb, which only moderately affects Crb localization (Das and Knust, 2018) likely due to the continued operation of polarized cytoskeletal transport (Fig. 6C–E). Similarly, mutation of the FERM-binding domain of Crb has strong effects upon overexpressed Crb (Fletcher et al., 2012; Letizia et al., 2011) but only causes a moderate reduction of endogenously expressed Crb (Cao et al., 2017; Klose et al., 2013; Sherrard and Fehon, 2015a) (Fig. 5E,F). Thus, important mechanisms of Crb polarization that are obscured through genetic redundancy can be revealed through the study of overexpressed Crb to provide a unifying model of polarization (Fig. 6F).
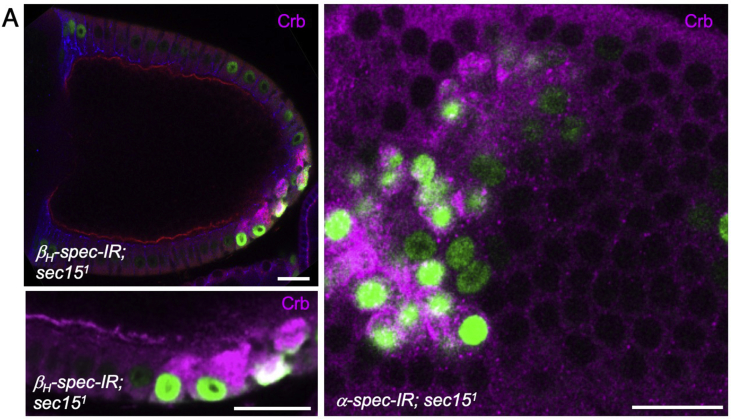
Our results explain the striking genetic interactions we have observed between upstream components of the Hippo signaling pathway, whose disruption leads to increased Crb expression (Fletcher et al., 2018; Genevet et al., 2009; Hamaratoglu et al., 2009; Zhu et al., 2015), and components of the Crb trafficking machinery such as exocyst components (Fletcher et al., 2012, 2015). Specifically, RNAi knockdown of spectrin cytoskeleton components in sec15 mutants or kib mutants, or alternatively double mutants of ex kib, all cause strong Crb accumulation in endosomes in the follicle cell epithelium (Fletcher et al., 2012, 2015) (Fig. S2). Since Crb itself functions with the spectrin cytoskeleton, the FERM domain proteins Merlin and Expanded, and the exocyst-binding partner Kibra to directly regulate Hippo signaling, follicle cells make use of this pathway to sense mechanical stretching of the apical domain to promote increased expression of crb, ex, kib and other target genes to help maintain the apical domain and accommodate mechanical perturbation (Fletcher et al., 2018). Thus, by virtue of being a transmembrane protein, an apical polarity determinant, as well as an upstream component of the Hippo pathway, Crb helps orchestrate cytoskeletal polarization, but is also transported apically, so can act as a sensor of the successful maintenance of cytoskeletal polarity in the face of significant mechanical stress or strain exerted upon the cytoskeleton of epithelial cells during development.
Finally, the model we propose in Drosophila epithelia may be conserved in humans, as many of the components have human orthologs, including CRB3, which localises to sub-apical tight junctions with PALS1 and PAR6, while MYO5A/B, MERLIN, KIBRA, CAMSAPs, spectrins and exocyst components localise across the entire apical surface (www.proteinatlas.org). Notably, CRB3 lacks the homophillic extracellular domain of CRB1 and CRB2, but is still able to localise to tight junctions, suggesting that other tight junction proteins such as JAMs, Occludins or Claudins may mediate extracellular domain clustering, with the entire complex clustered by intracellular multi-PDZ domain proteins such as ZO-1 and MUPP1/PATJ (Bazellieres et al., 2018; Ebnet et al., 2004; Michel et al., 2005). In addition, our findings suggest that polarization of the cytoskeleton in human columnar epithelial cells may also contribute to apically-directed membrane trafficking of CRB1-3 and other transmembrane tight junction components. In future, it will be of interest to test whether the mechanisms of epithelial polarization uncovered in Drosophila are conserved in epithelial organoids, an experimentally tractable model system which forms highly columnar cells similar to those observed in human epithelial tissues.
4. Materials and methods
Drosophila stocks were obtained from the Bloomington Drosophila Stock Centre and are described in FlyBase. Mitotic clones were generated using the FLP/FRT system and were either marked positively (presence of GFP; MARCM) or negatively (absence of GFP). Third instar larvae were heat-shocked once at 37 °C for 1 h and dissected 3 days after eclosion. Expression of UAS-driven transgenic lines was achieved with traffic jam.Gal4 (tj.Gal4) driver, the actin ‘flip-out’ and MARCM systems. The tj.Gal4 line is weakly expressed from the beginning of follicular development and strongly from stage 7 onward. For ‘flip-out’ clones, third instar larvae were heat-shocked at 37 °C for 20 min, and dissected 3 days after eclosion. Fly crosses were kept at a temperature of 25°.
4.1. Immunohistochemistry
Ovaries were dissected in PBS, fixed for 20 min in 4% paraformaldehyde in PBS, washed for 30 min in PBS/0.1% Triton X-100 (PBT) and blocked for 15 min in 5% normal goat serum/PBT (PBT/NGS). Primary antibodies were diluted in PBT/NGS and samples were incubated overnight at 4 °C. Secondary antibodies were used for 2 h at room temperature and then mounted on slides in Vectashield (Vector Labs). Images were taken with a Leica SP5 confocal using 40x oil immersion objective and processed with Adobe Photoshop and ImageJ.
Primary antibodies used were: rat anti-Crumbs (1:200, E. Knust), mouse anti-Crumbs (Cq4) (1:10, DSHB), rat anti-Crb intra (1:500 M.Bhat), rabbit anti-Lgl (1:50, Santa Cruz), mouse anti-Dlg (1:250, DSHB) and FITC-conjugated anti-GFP (1:400, Abcam).
Secondary antibodies used were goat Alexa fluor 488, 546 or 647 (1:500, Invitrogen), Phalloidin (2.5:250, Life Technologies) to stain F-actin and DAPI (1 μg/ml, Life Technologies) to visualize nuclei.
4.2. Inhibitor treatments
Treatment of ovaries expressing Crb:GFP was performed by isolating egg chambers and culturing them as described (Aguilar-Aragon et al., 2018) with Colchicine (0,2 mg/ml), Latrunculin A (0,05 mM), Cytochalasin D (0,05 mM), Jasplakinolide (0,05 mM), Ethanol or DMSO control (all of them from Sigma) for 2 h. After treatment, samples were fixed and processed normally for imaging.
4.3. Statistical analysis
Experiments were performed with at least three biological replicates. Prism software was used to plot the mean of the experimental data and error bars represent the standard deviation. T-test for all conditions tested in the paper was found to be p < 0.01.
4.4. Drosophila genotypes
Fig 1A: w;crb-GFP/+ (knockin allele A; Y. Hong)
Fig 1B: w; tj.Gal4/+; crb-GFP/UAS.dynein-IR(28054 VDRC)
Fig 1C: w hs.flp; actin.FRT.STOP.FRT.Gal4 UAS.GFP/UAS.dynein-IR(28054 VDRC)
Fig 1D: w hs.flp; actin.FRT.STOP.FRT.Gal4 UAS.GFP/UAS.dynein-IR(28054 VDRC)
Fig 2A: w;crb-GFP/+
Fig 2B: w; tj.Gal4/+; crb-GFP/UAS.dynein-IR(28054 VDRC)
Fig 2C: w; tj.Gal4/UAS.kinesin-IR; crb-GFP/
Fig 2D: w; tj.Gal4/UAS.kinesin-IR; crb-GFP/UAS.dynein-IR(28054 VDRC)
Fig 3A: w; tj.Gal4/+; UAS.MyoV-GFP/+
Fig 3B: w; tj.Gal4/+; UAS.GFP-MyoV-GT/+
Fig 3C: w
Fig. 3D–E&G: w; tj.Gal4/+; UAS.GFP-MyoV-GT/+
Fig. 3F&H: w; tj.Gal4/+; UAS.GFP-MyoV-GT/UAS.dynein-IR(28054 VDRC)
Fig. 4A–B: w
Fig. 4C–D: w; tj.Gal4/+; UAS.MyoV-GFP/+
Fig. 4E–G: w;crb-GFP/+
Fig 5A: w hs.flp FRT19A moePL106/FRT19A ubi.RFP
Fig 5B: w hs.flp FRT19A mer4/FRT19A ubi.RFP; FRT40Aexe1/FRT40A GFP
Fig 5C: w hs.flp FRT19A mer4 moePL106/FRT19A ubi.RFP
Fig 5D: w hs.flp FRT19A mer4 moePL106/FRT19A ubi.RFP; FRT40Aexe1/FRT40A GFP
Fig 5E: w hs.flp; FRT82B crbΔFBM/FRT82B ubi.nlsGFP (crbJMM allele, Y Hong)
Fig 5F: w hs.flp; FRT82B crbΔFBM/FRT82B ubi.nlsGFP (crbJMM allele, Y Hong)
Fig 5G: w hs.flp; FRT82Bsec151/FRT82B ubi.nlsGFP
Fig 5H: yw hs.flp tub.Gal4 UAS.GFPnls/+; FRT40Asec5e10/FRT40A tub.Gal80
Fig 6A: w
w; tj.Gal4/UAS.patronin-IR(27654 VDRC)
w; tj.Gal4/+; UAS.shot-IR(41858 BLOOMINGTON)/+
w; tj.Gal4/+; UAS.myoV-IR/+
w; tj.Gal4/+; UAS.GFP-MyoV-GT/+
w; tj.Gal4/+; UAS.α-Cat-IR/+
w hs.flp UAS.GFPnls tub.Gal4/+;FRT82B sec151/FRT82B tub.Gal80
Fig 6B: w; tj.Gal4/UAS.Crb-FL
w; tj.Gal4/UAS.Crb-FL UAS.patronin-IR(27654 VDRC)
w; tj.Gal4/UAS.Crb-FL; UAS.shot-IR(41858 BLOOMINGTON)/+
w; tj.Gal4/UAS.Crb-FL; UAS.myoV-IR/+
w; tj.Gal4/UAS.Crb-FL; UAS.GFP-MyoV-GT/+
w; tj.Gal4/UAS.Crb-FL; UAS.α-Cat-IR/+
w hs.flp UAS.GFPnls tub.Gal4/+; UAS.Crb-FL/+; FRT82B sec151/FRT82B tub.Gal80
yw hs.flp UAS.GFPnls tub.Gal4/+; UAS.Crb-FL/+; FRT82B kib32/FRT82B tub.Gal80
Fig. 6C–E: yw hs.flp tub.Gal4 UAS.GFPnls/+; fosCrbICD/+; FRT82B crb11A22/FRT82B tub.Gal80
Acknowledgments
Funded by the Francis Crick Institute and the Australian National University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ydbio.2019.12.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- Adams A.E., Johnson D.I., Longnecker R.M., Sloat B.F., Pringle J.R. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J. Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Aragon M., Elbediwy A., Foglizzo V., Fletcher G.C., Li V.S.W., Thompson B.J. Pak1 kinase maintains apical membrane identity in epithelia. Cell Rep. 2018;22:1639–1646. doi: 10.1016/j.celrep.2018.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.M., Macara I.G. The Par3 polarity protein is an exocyst receptor essential for mammary cell survival. Nat. Commun. 2017;8:14867. doi: 10.1038/ncomms14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler S.J., Angenent S.B., Wang Y., Wu L.F. On the spontaneous emergence of cell polarity. Nature. 2008;454:886–889. doi: 10.1038/nature07119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B.L., Boldogh I., Evangelista M., Boone C., Greene L.A., Pon L.A. The Src homology domain 3 (SH3) of a yeast type I myosin, Myo5p, binds to verprolin and is required for targeting to sites of actin polarization. J. Cell Biol. 1998;141:1357–1370. doi: 10.1083/jcb.141.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood S.X., Chabu C., Penkert R.R., Doe C.Q., Prehoda K.E. Cdc42 acts downstream of Bazooka to regulate neuroblast polarity through Par-6 aPKC. J. Cell Sci. 2007;120:3200–3206. doi: 10.1242/jcs.014902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann A., Schneider M., Theilenberg E., Grawe F., Knust E. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 2001;414:638–643. doi: 10.1038/414638a. [DOI] [PubMed] [Google Scholar]
- Baines A.J., Lu H.C., Bennett P.M. The Protein 4.1 family: hub proteins in animals for organizing membrane proteins. Biochim. Biophys. Acta. 2014;1838:605–619. doi: 10.1016/j.bbamem.2013.05.030. [DOI] [PubMed] [Google Scholar]
- Bazellieres E., Aksenova V., Barthelemy-Requin M., Massey-Harroche D., Le Bivic A. Role of the Crumbs proteins in ciliogenesis, cell migration and actin organization. Semin. Cell Dev. Biol. 2018;81:13–20. doi: 10.1016/j.semcdb.2017.10.018. [DOI] [PubMed] [Google Scholar]
- Bendezu F.O., Vincenzetti V., Vavylonis D., Wyss R., Vogel H., Martin S.G. Spontaneous Cdc42 polarization independent of GDI-mediated extraction and actin-based trafficking. PLoS Biol. 2015;13 doi: 10.1371/journal.pbio.1002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R., St Johnston D. A conserved oligomerization domain in drosophila Bazooka/PAR-3 is important for apical localization and epithelial polarity. Curr. Biol. : CB. 2003;13:1330–1334. doi: 10.1016/s0960-9822(03)00508-6. [DOI] [PubMed] [Google Scholar]
- Benton R., St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- Beronja S., Laprise P., Papoulas O., Pellikka M., Sisson J., Tepass U. Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J. Cell Biol. 2005;169:635–646. doi: 10.1083/jcb.200410081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D., Li M., Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Bilder D., Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- Bilder D., Schober M., Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat. Cell Biol. 2003;5:53–58. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- Blankenship J.T., Fuller M.T., Zallen J.A. The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J. Cell Sci. 2007;120:3099–3110. doi: 10.1242/jcs.004770. [DOI] [PubMed] [Google Scholar]
- Campbell K., Knust E., Skaer H. Crumbs stabilises epithelial polarity during tissue remodelling. J. Cell Sci. 2009;122:2604–2612. doi: 10.1242/jcs.047183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Xu R., Shi Q., Zhang D., Huang J., Hong Y. FERM domain phosphorylation and endogenous 3’UTR are not essential for regulating the function and subcellular localization of polarity protein Crumbs. J Genet Genomics. 2017;44:409–412. doi: 10.1016/j.jgg.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Carreno S., Kouranti I., Glusman E.S., Fuller M.T., Echard A., Payre F. Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells. J. Cell Biol. 2008;180:739–746. doi: 10.1083/jcb.200709161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.L., Gajewski K.M., Hamaratoglu F., Bossuyt W., Sansores-Garcia L., Tao C., Halder G. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15810–15815. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chishti A.H., Kim A.C., Marfatia S.M., Lutchman M., Hanspal M., Jindal H., Liu S.C., Low P.S., Rouleau G.A., Mohandas N. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem. Sci. 1998;23:281–282. doi: 10.1016/s0968-0004(98)01237-7. [DOI] [PubMed] [Google Scholar]
- Conder R., Yu H., Zahedi B., Harden N. The serine/threonine kinase dPak is required for polarized assembly of F-actin bundles and apical-basal polarity in the Drosophila follicular epithelium. Dev. Biol. 2007;305:470–482. doi: 10.1016/j.ydbio.2007.02.034. [DOI] [PubMed] [Google Scholar]
- Cox R.T., Kirkpatrick C., Peifer M. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J. Cell Biol. 1996;134:133–148. doi: 10.1083/jcb.134.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Knust E. A dual role of the extracellular domain of Drosophila Crumbs for morphogenesis of the embryonic neuroectoderm. Biology open. 2018;7 doi: 10.1242/bio.031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D.J., Schwager F., Pintard L., Gotta M., Goldstein B. A single-cell biochemistry approach reveals PAR complex dynamics during cell polarization. Dev. Cell. 2017;42:416–434 e411. doi: 10.1016/j.devcel.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton S., Auvinen P., Luo L., Jan Y.N., Simons K. CDC42 and Rac1 control different actin-dependent processes in the Drosophila wing disc epithelium. J. Cell Biol. 1995;131:151–164. doi: 10.1083/jcb.131.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K., Suzuki A., Ohno S., Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J. Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- Fehon R.G., McClatchey A.I., Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher G.C., Diaz-de-la-Loza M.D., Borreguero-Munoz N., Holder M., Aguilar-Aragon M., Thompson B.J. vol. 145. Development; 2018. (Mechanical Strain Regulates the Hippo Pathway in Drosophila). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher G.C., Elbediwy A., Khanal I., Ribeiro P.S., Tapon N., Thompson B.J. The Spectrin cytoskeleton regulates the Hippo signalling pathway. EMBO J. 2015;34:940–954. doi: 10.15252/embj.201489642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher G.C., Lucas E.P., Brain R., Tournier A., Thompson B.J. Positive feedback and mutual antagonism combine to polarize Crumbs in the Drosophila follicle cell epithelium. Curr. Biol. : CB. 2012;22:1116–1122. doi: 10.1016/j.cub.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Fox D.T., Homem C.C., Myster S.H., Wang F., Bain E.E., Peifer M. Rho1 regulates Drosophila adherens junctions independently of p120ctn. Development. 2005;132:4819–4831. doi: 10.1242/dev.02056. [DOI] [PubMed] [Google Scholar]
- France Y.E., Boyd C., Coleman J., Novick P.J. The polarity-establishment component Bem1p interacts with the exocyst complex through the Sec15p subunit. J. Cell Sci. 2006;119:876–888. doi: 10.1242/jcs.02849. [DOI] [PubMed] [Google Scholar]
- Gamblin C.L., Hardy E.J., Chartier F.J., Bisson N., Laprise P. A bidirectional antagonism between aPKC and Yurt regulates epithelial cell polarity. J. Cell Biol. 2014;204:487–495. doi: 10.1083/jcb.201308032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevet A., Polesello C., Blight K., Robertson F., Collinson L.M., Pichaud F., Tapon N. The Hippo pathway regulates apical-domain size independently of its growth-control function. J. Cell Sci. 2009;122:2360–2370. doi: 10.1242/jcs.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova J.L., Jong S., Camp J.T., Fehon R.G. Functional analysis of Cdc42 in actin filament assembly, epithelial morphogenesis, and cell signaling during Drosophila development. Dev. Biol. 2000;221:181–194. doi: 10.1006/dbio.2000.9671. [DOI] [PubMed] [Google Scholar]
- Georgiou M., Marinari E., Burden J., Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr. Biol. : CB. 2008;18:1631–1638. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Gibson M.C., Perrimon N. Apicobasal polarization: epithelial form and function. Curr. Opin. Cell Biol. 2003;15:747–752. doi: 10.1016/j.ceb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Goehring N.W., Trong P.K., Bois J.S., Chowdhury D., Nicola E.M., Hyman A.A., Grill S.W. Polarization of PAR proteins by advective triggering of a pattern-forming system. Science. 2011;334:1137–1141. doi: 10.1126/science.1208619. [DOI] [PubMed] [Google Scholar]
- Goodson H.V., Anderson B.L., Warrick H.M., Pon L.A., Spudich J.A. Synthetic lethality screen identifies a novel yeast myosin I gene (MYO5): myosin I proteins are required for polarization of the actin cytoskeleton. J. Cell Biol. 1996;133:1277–1291. doi: 10.1083/jcb.133.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotta M., Abraham M.C., Ahringer J. CDC-42 controls early cell polarity and spindle orientation in C. elegans. Curr. Biol. : CB. 2001;11:482–488. doi: 10.1016/s0960-9822(01)00142-7. [DOI] [PubMed] [Google Scholar]
- Grawe F., Wodarz A., Lee B., Knust E., Skaer H. The Drosophila genes crumbs and stardust are involved in the biogenesis of adherens junctions. Development. 1996;122:951–959. doi: 10.1242/dev.122.3.951. [DOI] [PubMed] [Google Scholar]
- Halbsgut N., Linnemannstons K., Zimmermann L.I., Wodarz A. Apical-basal polarity in Drosophila neuroblasts is independent of vesicular trafficking. Mol. Biol. Cell. 2011;22:4373–4379. doi: 10.1091/mbc.E11-03-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F., Gajewski K., Sansores-Garcia L., Morrison C., Tao C., Halder G. The Hippo tumor-suppressor pathway regulates apical-domain size in parallel to tissue growth. J. Cell Sci. 2009;122:2351–2359. doi: 10.1242/jcs.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F., Willecke M., Kango-Singh M., Nolo R., Hyun E., Tao C., Jafar-Nejad H., Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Harden N., Lee J., Loh H.Y., Ong Y.M., Tan I., Leung T., Manser E., Lim L. A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol. Cell. Biol. 1996;16:1896–1908. doi: 10.1128/mcb.16.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K.P., Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J. Cell Biol. 2008;183:1129–1143. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T.J., Peifer M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J. Cell Biol. 2004;167:135–147. doi: 10.1083/jcb.200406024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T.J.C. Protein clustering for cell polarity: Par-3 as a paradigm. F1000Res. 2017;6:1620. doi: 10.12688/f1000research.11976.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Guo W. The exocyst complex in polarized exocytosis. Curr. Opin. Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homem C.C., Peifer M. Diaphanous regulates myosin and adherens junctions to control cell contractility and protrusive behavior during morphogenesis. Development. 2008;135:1005–1018. doi: 10.1242/dev.016337. [DOI] [PubMed] [Google Scholar]
- Hong Y., Stronach B., Perrimon N., Jan L.Y., Jan Y.N. Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature. 2001;414:634–638. doi: 10.1038/414634a. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S., Bilder D. Dynein regulates epithelial polarity and the apical localization of stardust A mRNA. PLoS Genet. 2008;4:e8. doi: 10.1371/journal.pgen.0040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhou W., Dong W., Watson A.M., Hong Y. Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc. Natl. Acad. Sci. 2009;106:8284–8289. doi: 10.1073/pnas.0900641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutterer A., Betschinger J., Petronczki M., Knoblich J.A. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev. Cell. 2004;6:845–854. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Irazoqui J.E., Gladfelter A.S., Lew D.J. Scaffold-mediated symmetry breaking by Cdc42p. Nat. Cell Biol. 2003;5:1062–1070. doi: 10.1038/ncb1068. [DOI] [PubMed] [Google Scholar]
- Ivanova M.E., Fletcher G.C., O’Reilly N., Purkiss A.G., Thompson B.J., McDonald N.Q. Structures of the human Pals1 PDZ domain with and without ligand suggest gated access of Crb to the PDZ peptide-binding groove. Acta Crystallogr D Biol Crystallogr. 2015;71:555–564. doi: 10.1107/S139900471402776X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joberty G., Petersen C., Gao L., Macara I.G. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- Johnson J.M., Jin M., Lew D.J. Symmetry breaking and the establishment of cell polarity in budding yeast. Curr. Opin. Genet. Dev. 2011;21:740–746. doi: 10.1016/j.gde.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G.C., Prendergast J.A., Singer R.A. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J. Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal I., Elbediwy A., Diaz de la Loza Mdel C., Fletcher G.C., Thompson B.J. Shot and Patronin polarise microtubules to direct membrane traffic and biogenesis of microvilli in epithelia. J. Cell Sci. 2016;129:2651–2659. doi: 10.1242/jcs.189076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Gailite I., Moussian B., Luschnig S., Goette M., Fricke K., Honemann-Capito M., Grubmuller H., Wodarz A. Kinase-activity-independent functions of atypical protein kinase C in Drosophila. J. Cell Sci. 2009;122:3759–3771. doi: 10.1242/jcs.052514. [DOI] [PubMed] [Google Scholar]
- Klose S., Flores-Benitez D., Riedel F., Knust E. Fosmid-based structure-function analysis reveals functionally distinct domains in the cytoplasmic domain of Drosophila crumbs. G3 (Bethesda) 2013;3:153–165. doi: 10.1534/g3.112.005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E., Tepass U., Wodarz A. Crumbs and stardust, two genes of Drosophila required for the development of epithelial cell polarity. Dev. Suppl. 1993:261–268. [PubMed] [Google Scholar]
- Krahn M.P., Klopfenstein D.R., Fischer N., Wodarz A. Membrane targeting of Bazooka/PAR-3 is mediated by direct binding to phosphoinositide lipids. Curr. Biol. : CB. 2010;20:636–642. doi: 10.1016/j.cub.2010.01.065. [DOI] [PubMed] [Google Scholar]
- Krauss J., Lopez de Quinto S., Nusslein-Volhard C., Ephrussi A. Myosin-V regulates oskar mRNA localization in the Drosophila oocyte. Curr. Biol. : CB. 2009;19:1058–1063. doi: 10.1016/j.cub.2009.04.062. [DOI] [PubMed] [Google Scholar]
- Kunda P., Pelling A.E., Liu T., Baum B. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr. Biol. : CB. 2008;18:91–101. doi: 10.1016/j.cub.2007.12.051. [DOI] [PubMed] [Google Scholar]
- Langevin J., Morgan M.J., Sibarita J.B., Aresta S., Murthy M., Schwarz T., Camonis J., Bellaiche Y. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev. Cell. 2005;9:365–376. doi: 10.1016/j.devcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Laprise P., Beronja S., Silva-Gagliardi N.F., Pellikka M., Jensen A.M., McGlade C.J., Tepass U. The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev. Cell. 2006;11:363–374. doi: 10.1016/j.devcel.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise P., Lau K.M., Harris K.P., Silva-Gagliardi N.F., Paul S.M., Beronja S., Beitel G.J., McGlade C.J., Tepass U. Yurt, Coracle, Neurexin IV and the Na(+),K(+)-ATPase form a novel group of epithelial polarity proteins. Nature. 2009;459:1141–1145. doi: 10.1038/nature08067. [DOI] [PubMed] [Google Scholar]
- Leblanc J., Zhang X., McKee D., Wang Z.B., Li R., Ma C., Sun Q.Y., Liu X.J. The small GTPase Cdc42 promotes membrane protrusion during polar body emission via ARP2-nucleated actin polymerization. Mol. Hum. Reprod. 2011;17:305–316. doi: 10.1093/molehr/gar026. [DOI] [PubMed] [Google Scholar]
- Lechler T., Jonsdottir G.A., Klee S.K., Pellman D., Li R. A two-tiered mechanism by which Cdc42 controls the localization and activation of an Arp2/3-activating motor complex in yeast. J. Cell Biol. 2001;155:261–270. doi: 10.1083/jcb.200104094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T., Shevchenko A., Li R. Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization. J. Cell Biol. 2000;148:363–373. doi: 10.1083/jcb.148.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibfried A., Fricke R., Morgan M.J., Bogdan S., Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr. Biol. : CB. 2008;18:1639–1648. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- Leibfried A., Muller S., Ephrussi A. A Cdc42-regulated actin cytoskeleton mediates Drosophila oocyte polarization. Development. 2013;140:362–371. doi: 10.1242/dev.089250. [DOI] [PubMed] [Google Scholar]
- Letizia A., Ricardo S., Moussian B., Martin N., Llimargas M. A functional role of the extracellular domain of Crumbs in cell architecture and apicobasal polarity. J. Cell Sci. 2013;126:2157–2163. doi: 10.1242/jcs.122382. [DOI] [PubMed] [Google Scholar]
- Letizia A., Sotillos S., Campuzano S., Llimargas M. Regulated Crb accumulation controls apical constriction and invagination in Drosophila tracheal cells. J. Cell Sci. 2011;124:240–251. doi: 10.1242/jcs.073601. [DOI] [PubMed] [Google Scholar]
- Li B.X., Satoh A.K., Ready D.F. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J. Cell Biol. 2007;177:659–669. doi: 10.1083/jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wei Z., Yan Y., Wan Q., Du Q., Zhang M. Structure of Crumbs tail in complex with the PALS1 PDZ-SH3-GK tandem reveals a highly specific assembly mechanism for the apical Crumbs complex. Proc. Natl. Acad. Sci. U. S. A. 2014;111:17444–17449. doi: 10.1073/pnas.1416515111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang L., Hays T.S., Cai Y. Dynein-mediated apical localization of crumbs transcripts is required for Crumbs activity in epithelial polarity. J. Cell Biol. 2008;180:31–38. doi: 10.1083/jcb.200707007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.H., Currinn H., Pocha S.M., Rothnie A., Wassmer T., Knust E. AP-2-complex-mediated endocytosis of Drosophila Crumbs regulates polarity by antagonizing Stardust. J. Cell Sci. 2015;128:4538–4549. doi: 10.1242/jcs.174573. [DOI] [PubMed] [Google Scholar]
- Ling C., Zheng Y., Yin F., Yu J., Huang J., Hong Y., Wu S., Pan D. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc. Natl. Acad. Sci. U. S. A. 2010;107:10532–10537. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Lee T., Tsai L., Tang G., Jan L.Y., Jan Y.N. Genghis Khan (Gek) as a putative effector for Drosophila Cdc42 and regulator of actin polymerization. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12963–12968. doi: 10.1073/pnas.94.24.12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Benink H.A., Cheng D., Montplaisir V., Wang L., Xi Y., Zheng P.P., Bement W.M., Liu X.J. Cdc42 activation couples spindle positioning to first polar body formation in oocyte maturation. Curr. Biol. : CB. 2006;16:214–220. doi: 10.1016/j.cub.2005.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S.G. Spontaneous cell polarization: feedback control of Cdc42 GTPase breaks cellular symmetry. Bioessays. 2015;37:1193–1201. doi: 10.1002/bies.201500077. [DOI] [PubMed] [Google Scholar]
- Martin S.G., Arkowitz R.A. Cell polarization in budding and fission yeasts. FEMS Microbiol. Rev. 2014;38:228–253. doi: 10.1111/1574-6976.12055. [DOI] [PubMed] [Google Scholar]
- Martin S.G., McDonald W.H., Yates J.R., 3rd, Chang F. Tea4p links microtubule plus ends with the formin for3p in the establishment of cell polarity. Dev. Cell. 2005;8:479–491. doi: 10.1016/j.devcel.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Mata J., Nurse P. tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell. 1997;89:939–949. doi: 10.1016/s0092-8674(00)80279-2. [DOI] [PubMed] [Google Scholar]
- McKinley R.F., Yu C.G., Harris T.J. Assembly of Bazooka polarity landmarks through a multifaceted membrane-association mechanism. J. Cell Sci. 2012;125:1177–1190. doi: 10.1242/jcs.091884. [DOI] [PubMed] [Google Scholar]
- Medina E., Williams J., Klipfell E., Zarnescu D., Thomas G., Le Bivic A. Crumbs interacts with moesin and beta(Heavy)-spectrin in the apical membrane skeleton of Drosophila. J. Cell Biol. 2002;158:941–951. doi: 10.1083/jcb.200203080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Nelson W.J. Coordinated protein sorting, targeting and distribution in polarized cells. Nat. Rev. Mol. Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel D., Arsanto J.P., Massey-Harroche D., Beclin C., Wijnholds J., Le Bivic A. PATJ connects and stabilizes apical and lateral components of tight junctions in human intestinal cells. J. Cell Sci. 2005;118:4049–4057. doi: 10.1242/jcs.02528. [DOI] [PubMed] [Google Scholar]
- Minc N., Bratman S.V., Basu R., Chang F. Establishing new sites of polarization by microtubules. Curr. Biol. : CB. 2009;19:83–94. doi: 10.1016/j.cub.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Suzuki A., Hirose T., Kitamura K., Kutsuzawa K., Futaki M., Amano Y., Ohno S. Self-association of PAR-3-mediated by the conserved N-terminal domain contributes to the development of epithelial tight junctions. J. Biol. Chem. 2003;278:31240–31250. doi: 10.1074/jbc.M303593200. [DOI] [PubMed] [Google Scholar]
- Mostov K.E., Verges M., Altschuler Y. Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol. 2000;12:483–490. doi: 10.1016/s0955-0674(00)00120-4. [DOI] [PubMed] [Google Scholar]
- Motegi F., Zonies S., Hao Y., Cuenca A.A., Griffin E., Seydoux G. Microtubules induce self-organization of polarized PAR domains in Caenorhabditis elegans zygotes. Nat. Cell Biol. 2011;13:1361–1367. doi: 10.1038/ncb2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H.A., Wieschaus E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 1996;134:149–163. doi: 10.1083/jcb.134.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M., Schwarz T.L. The exocyst component Sec5 is required for membrane traffic and polarity in the Drosophila ovary. Development. 2004;131:377–388. doi: 10.1242/dev.00931. [DOI] [PubMed] [Google Scholar]
- Nelson W.J. Cytoskeleton functions in membrane traffic in polarized epithelial cells. Semin. Cell Biol. 1991;2:375–385. [PubMed] [Google Scholar]
- Nelson W.J. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordstra I., Liu Q., Nijenhuis W., Hua S., Jiang K., Baars M., Remmelzwaal S., Martin M., Kapitein L.C., Akhmanova A. Control of apico-basal epithelial polarity by the microtubule minus-end-binding protein CAMSAP3 and spectraplakin ACF7. J. Cell Sci. 2016;129:4278–4288. doi: 10.1242/jcs.194878. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Peifer M., Orsulic S., Sweeton D., Wieschaus E. A role for the Drosophila segment polarity gene armadillo in cell adhesion and cytoskeletal integrity during oogenesis. Development. 1993;118:1191–1207. doi: 10.1242/dev.118.4.1191. [DOI] [PubMed] [Google Scholar]
- Peterson F.C., Penkert R.R., Volkman B.F., Prehoda K.E. Cdc42 regulates the Par-6 PDZ domain through an allosteric CRIB-PDZ transition. Mol. Cell. 2004;13:665–676. doi: 10.1016/s1097-2765(04)00086-3. [DOI] [PubMed] [Google Scholar]
- Petronczki M., Knoblich J.A. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat. Cell Biol. 2001;3:43–49. doi: 10.1038/35050550. [DOI] [PubMed] [Google Scholar]
- Pocha S.M., Shevchenko A., Knust E. Crumbs regulates rhodopsin transport by interacting with and stabilizing myosin V. J. Cell Biol. 2011;195:827–838. doi: 10.1083/jcb.201105144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesello C., Delon I., Valenti P., Ferrer P., Payre F. Dmoesin controls actin-based cell shape and polarity during Drosophila melanogaster oogenesis. Nat. Cell Biol. 2002;4:782–789. doi: 10.1038/ncb856. [DOI] [PubMed] [Google Scholar]
- Pruyne D., Legesse-Miller A., Gao L., Dong Y., Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 2004;20:559–591. doi: 10.1146/annurev.cellbio.20.010403.103108. [DOI] [PubMed] [Google Scholar]
- Pruyne D.W., Schott D.H., Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 1998;143:1931–1945. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- Rodriguez J., Peglion F., Martin J., Hubatsch L., Reich J., Hirani N., Gubieda A.G., Roffey J., Fernandes A.R., St Johnston D. aPKC cycles between functionally distinct PAR protein assemblies to drive cell polarity. Dev. Cell. 2017;42:400–415. doi: 10.1016/j.devcel.2017.07.007. e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Macara I.G. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 2014;15:225–242. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeth J.F., Sawyer J.K., Wilner D.A., Peifer M. Rab11 helps maintain apical crumbs and adherens junctions in the Drosophila embryonic ectoderm. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper K. Anisotropy of Crumbs and aPKC drives myosin cable assembly during tube formation. Dev. Cell. 2012;23:939–953. doi: 10.1016/j.devcel.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A., Anneken A., Li Y., Lee S., Munro E. Dynamic opposition of clustered proteins stabilizes cortical polarity in the C. elegans zygote. Dev. Cell. 2015;35:131–142. doi: 10.1016/j.devcel.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvanet C., Wayt J., Pelaseyed T., Bretscher A. Structure, regulation, and functional diversity of microvilli on the apical domain of epithelial cells. Annu. Rev. Cell Dev. Biol. 2015;31:593–621. doi: 10.1146/annurev-cellbio-100814-125234. [DOI] [PubMed] [Google Scholar]
- Shahab J., Tiwari M.D., Honemann-Capito M., Krahn M.P., Wodarz A. Bazooka/PAR3 is dispensable for polarity in Drosophila follicular epithelial cells. Biology open. 2015;4:528–541. doi: 10.1242/bio.201410934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard K.M., Fehon R.G. The transmembrane protein Crumbs displays complex dynamics during follicular morphogenesis and is regulated competitively by Moesin and aPKC. Development. 2015;142:1869–1878. doi: 10.1242/dev.115329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard K.M., Fehon R.G. The transmembrane protein Crumbs displays complex dynamics during follicular morphogenesis and is regulated competitively by Moesin and aPKC. Development. 2015;142:2226. doi: 10.1242/dev.126425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes Sde M., Blankenship J.T., Weitz O., Farrell D.L., Tamada M., Fernandez-Gonzalez R., Zallen J.A. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev. Cell. 2010;19:377–388. doi: 10.1016/j.devcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter B.D., Smith S.E., Li R. Symmetry breaking in the life cycle of the budding yeast. Cold Spring Harbor perspectives in biology. 2009;1 doi: 10.1101/cshperspect.a003384. a003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillos S., Diaz-Meco M.T., Caminero E., Moscat J., Campuzano S. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J. Cell Biol. 2004;166:549–557. doi: 10.1083/jcb.200311031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck O., Hughes S.C., Noren N.K., Kulikauskas R.M., Fehon R.G. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature. 2003;421:83–87. doi: 10.1038/nature01295. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141:757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Su T., Ludwig M.Z., Xu J., Fehon R.G. Kibra and merlin activate the Hippo pathway spatially distinct from and independent of expanded. Dev. Cell. 2017;40:478–490. doi: 10.1016/j.devcel.2017.02.004. e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G., Smith C., McGlade J., Tepass U. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J. Cell Biol. 2000;151:891–904. doi: 10.1083/jcb.151.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G., Tepass U. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat. Cell Biol. 2003;5:46–52. doi: 10.1038/ncb896. [DOI] [PubMed] [Google Scholar]
- Tepass U. The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu. Rev. Cell Dev. Biol. 2012;28:655–685. doi: 10.1146/annurev-cellbio-092910-154033. [DOI] [PubMed] [Google Scholar]
- Tepass U., Knust E. Crumbs and stardust act in a genetic pathway that controls the organization of epithelia in Drosophila melanogaster. Dev. Biol. 1993;159:311–326. doi: 10.1006/dbio.1993.1243. [DOI] [PubMed] [Google Scholar]
- Tepass U., Theres C., Knust E. Crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- TerBush D.R., Maurice T., Roth D., Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Thompson B.J. Cell polarity: models and mechanisms from yeast, worms and flies. Development. 2013;140:13–21. doi: 10.1242/dev.083634. [DOI] [PubMed] [Google Scholar]
- Thompson B.J., Pichaud F., Roper K. Sticking together the Crumbs - an unexpected function for an old friend. Nat. Rev. Mol. Cell Biol. 2013;14:307–314. doi: 10.1038/nrm3568. [DOI] [PubMed] [Google Scholar]
- Toya M., Kobayashi S., Kawasaki M., Shioi G., Kaneko M., Ishiuchi T., Misaki K., Meng W., Takeichi M. CAMSAP3 orients the apical-to-basal polarity of microtubule arrays in epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 2016;113:332–337. doi: 10.1073/pnas.1520638113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther R.F., Nunes de Almeida F., Vlassaks E., Burden J.J., Pichaud F. Pak4 is required during epithelial polarity remodeling through regulating AJ stability and bazooka retention at the ZA. Cell Rep. 2016;15:45–53. doi: 10.1016/j.celrep.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.B., Jiang Z.Z., Zhang Q.H., Hu M.W., Huang L., Ou X.H., Guo L., Ouyang Y.C., Hou Y., Brakebusch C. Specific deletion of Cdc42 does not affect meiotic spindle organization/migration and homologous chromosome segregation but disrupts polarity establishment and cytokinesis in mouse oocytes. Mol. Biol. Cell. 2013;24:3832–3841. doi: 10.1091/mbc.E13-03-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Soldner R., Altschuler S., Wu L., Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- Wei Z., Li Y., Ye F., Zhang M. Structural basis for the phosphorylation-regulated interaction between the cytoplasmic tail of cell polarity protein crumbs and the actin-binding protein moesin. J. Biol. Chem. 2015;290:11384–11392. doi: 10.1074/jbc.M115.643791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz O.A., Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J. Cell Sci. 2009;122:4253–4266. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A., Ramrath A., Grimm A., Knust E. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J. Cell Biol. 2000;150:1361–1374. doi: 10.1083/jcb.150.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff K.K., Nelson W.J. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J. Cell Sci. 2004;117:559–570. doi: 10.1242/jcs.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K., Rubinstein B., Unruh J.R., Guo F., Slaughter B.D., Li R. Sequential actin-based pushing forces drive meiosis I chromosome migration and symmetry breaking in oocytes. J. Cell Biol. 2013;200:567–576. doi: 10.1083/jcb.201211068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K., Unruh J.R., Deng M., Slaughter B.D., Rubinstein B., Li R. Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat. Cell Biol. 2011;13:1252–1258. doi: 10.1038/ncb2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen J.A., Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- Zhang X., Ma C., Miller A.L., Katbi H.A., Bement W.M., Liu X.J. Polar body emission requires a RhoA contractile ring and Cdc42-mediated membrane protrusion. Dev. Cell. 2008;15:386–400. doi: 10.1016/j.devcel.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Li D., Wang Y., Pei C., Liu S., Zhang L., Yuan Z., Zhang P. Brahma regulates the Hippo pathway activity through forming complex with Yki-Sd and regulating the transcription of Crumbs. Cell. Signal. 2015;27:606–613. doi: 10.1016/j.cellsig.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Zihni C., Vlassaks E., Terry S., Carlton J., Leung T.K.C., Olson M., Pichaud F., Balda M.S., Matter K. An apical MRCK-driven morphogenetic pathway controls epithelial polarity. Nat. Cell Biol. 2017;19:1049–1060. doi: 10.1038/ncb3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonies S., Motegi F., Hao Y., Seydoux G. Symmetry breaking and polarization of the C. elegans zygote by the polarity protein PAR-2. Development. 2010;137:1669–1677. doi: 10.1242/dev.045823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.