Abstract
In conjunction with an increasing public awareness of infectious diseases, the textile industry and scientists are developing hygienic fabrics by the addition of various antimicrobial and antiviral compounds. In the current study, sodium pentaborate pentahydrate and triclosan are applied to cotton fabrics in order to gain antimicrobial and antiviral properties for the first time. The antimicrobial activity of textiles treated with 3 % sodium pentaborate pentahydrate, 0.03 % triclosan, and 7 % Glucapon has been investigated against a broad range of microorganisms including bacteria, yeast, and fungi. Moreover, modified cotton fabrics were tested against adenovirus type 5 and poliovirus type 1. According to the test results, the modified textile goods attained very good antimicrobial and antiviral properties. Thus, the results of the present study clearly suggest that sodium pentaborate pentahydrate and triclosan solution-treated textiles can be considered in the development of antimicrobial and antiviral textile finishes.
Keywords: Textile, Antimicrobial, Antiviral, Sodium pentaborate pentahydrate, Triclosan
Introduction
Textiles are woven or non-woven products produced by natural and/or synthetic fibers. Textile materials have a wide array of usage in areas such as clothing, food industry, building material, automotive industry, military, medical industry, sports equipment, agriculture, and home furnishings. Properties of textiles such as high-temperature stability, elasticity, waterproofness, biodegradability, or having antimicrobial activity are determined by the basis of use [14].
Increasing public awareness about effects of pathogens on health creates a growing demand about antibacterial materials in various fields such as textile materials, medical devices, hygienic applications, water purification systems, and food packing [36]. Textile materials particularly made of natural fibers supply an outstanding medium for microbial growth due to their large surface area and ability to retain moisture [26, 43]. Moreover, the problem of degeneration of natural products such as natural wood, cotton, or wool by microorganisms or insects has been faced by human beings since they were first started to be used [1]. Therefore, to prevent undesirable effects such as degradation, unpleasant odors, and potential health risks, textiles are treated with antimicrobial agents [4–6, 19]. In order to gain antimicrobial properties, various chemical additives have been applied to textile products such as inorganic salts, organometallics, iodophors (substances that slowly release iodine), phenols and thiophenols, onium salts, antibiotics, heterocyclics with anionic groups, nitro compounds, urea and related compounds, formaldehyde derivatives, amines, and/or other biocidal substances [26].
The misuse and overuse of antibiotics have induced microorganisms to develop antibiotic resistance. Especially methicillin-resistant Staphylococcus aureus (MRSA) [13] and Pseudomonas aeruginosa are creating significant problems in burn and intensive care units despite the improvement in the awareness of nosocomial infections [3, 21, 33]. In the hospital, environment infectious microorganism can be introduced exogenously by other patients or staff members or endogenously from the patients’ own flora. S. aureus has been isolated usually from bedclothes, bed curtains, and the protective clothing of nurses, and they are transmitted by the movement of these goods [2]. The source of nosocomial infections with P. aeruginosa has been found in the wet areas of hospitals and in moist objects, and they are spread by personal contact [39]. Nosocomial infections could be controlled by removing the infection source or preventing the transmission of pathogenic microorganisms from host to patients or materials [38]. In this context, the use of antimicrobial textiles especially in hospitals may prevent microbial growth and reduces the risk of nosocomial infections [34].
Garment and textile goods provide favorable environments for bacterial or fungal growth. Neely and Maley investigated the survival of 22 gram-positive bacteria on five common hospital materials such as smooth 100 % cotton, 100 % cotton terry, 60 % cotton–40 % polyester blend, 100 % polyester, and 100 % polypropylene plastic and reported that all bacterial species could survive on fabric materials between 1 and 90 days in a hospital environment [30]. High survival length of pathogenic microorganisms on textile materials leads to the spreading of diseases in the hospital environment. In order to diminish microbial infections caused by textile goods, antimicrobial textile materials could be used especially in health care facilities [35].
In order to eliminate bacterial load on textile materials, metal salt solutions of copper and zinc (CuSO4 and ZnSO4) were tested against S. aureus, Klebsiella pneumoniae, and MRSA, and antibacterial effect of treated materials was reported by Nakashima et al. [29]. Moreover, zinc pyrithione has recently been used as an antibacterial agent on cotton fabrics and inhibited the growth of S. aureus and Trichophyton mentagrophytes [28]. Nano-sized silver colloidal solutions on cellulosic and synthetic fabrics have been investigated and found to be antibacterial against S. aureus and K. pneumoniae [24].
Besides the prevention of microbial growth, few researchers have attempted to prevent viral presence on textile materials as yet. Park et al. synthesized a new N,N-dodecyl,methyl-polyurethane-based antimicrobial and antiviral material that inhibits S. aureus and Escherichia coli and inactivates influenza viruses on its surface [32]. Also, several sulfated polysaccharides and copolymers of acrylic acid with vinyl alcohol sulfate have been demonstrated to have antiviral activity against human cytomegalovirus (CMV) [31]. However, there is lack of knowledge and study about antiviral textile products so far. Occurrence of polio and vaccinia virus on wool and cotton fabrics has been investigated previously. These viruses were recovered up to 20 and 14 weeks, respectively, from wool fabrics that were exposed to the virus, but they persisted for shorter periods of time on the cotton fabrics [10]. Imai et al. investigated the effect of cotton textiles treated with copper ion-exchanged zeolites on the inactivation of avian influenza virus (AIVs), and they showed that highly pathogenic H5N1 and low pathogenic H5N3 viruses were inactivated on the copper ion-exchanged zeolite textile materials even after short incubations [16].
With increasing health awareness, people have started to pay attention about the removal or minimization of harmful pathogenic organisms from the environment or surfaces; therefore, various kinds of materials are being produced as antimicrobial. Recently, many scientists have investigated antibacterial, antifungal, or antiyeast materials, including textile goods. So far, there have been a number of studies conducted to develop antimicrobial and antiviral textile products, but success is yet to be achieved. This is the first study demonstrating that a novel formulation including triclosan and boron compounds can be used in broad spectrum antimicrobial and antiviral textile products.
Materials and Methods
Microorganisms
In this study, the antibacterial activities of textile products were tested against six bacterial species. In addition, one yeast and two fungi species were tested. The list of microbial species is given in Table 1. The bacteria were obtained from ATCC, and the yeast and fungal species were provided by the culture collection unit of Yeditepe University, Department of Genetics and Bioengineering.
Table 1.
List of microorganisms tested for antimicrobial activity
| Bacteria | Yeast | Fungi |
|---|---|---|
| Escherichia coli (ATCC 10536) | Candida albicans | Aspergillus niger |
| Staphylococcus aureus (ATCC 6538) | Trichophyton mentagrophytes | |
| Salmonella enterica subsp. enterica (ATCC 13311) | ||
| Staphylococcus epidermidis (ATCC 12228) | ||
| Klebsiella pneumoniae (ATCC 13883) | ||
| Methicillin-resistant Staphylococcus aureus (MRSA) (ATCC 33592) |
Viruses and Cell Lines
Adenoid 75 strain of human adenovirus type 5 (ATCC VR-5) and Chat strain of human poliovirus type 1 (ATCC VR-1562) viruses were used for determining textile products’ antiviral activities. HEp-2 (ATCC CCL-23) cells were used for antiviral activity tests.
Chemicals
Triclosan, used as a pharmaceutical secondary standard, was purchased from Sigma-Aldrich (PHR1338). Sodium pentaborate pentahydrate (NaB5O8·5H2O) was obtained from National Boron Research Institute-BOREN (Ankara, Turkey). For an emulsifying agent, Glucapon 215 CS UP was used. Glucapon 215 CS UP was purchased from Veser Chemical Compounds Co., Turkey.
Microbial Tests
The standard NCCLS disk diffusion assay [23] was modified and used to assess antimicrobial activity against each microorganism tested. Briefly, 100 μL of suspensions containing 108 colony-forming unit (CFU)/mL bacteria, 106 CFU/mL yeast, and 104 spore/mL fungi were prepared from freshly grown cultures and spread on tryptic soy agar (TSA), Sabouraud dextrose agar (SDA), and potato dextrose agar (PDA), respectively. The blank disks (6 mm in diameter) were impregnated with 19 μL of 0.03 % triclosan (T) and 3 % sodium pentaborate pentahydrate (SPP) solutions and placed on the inoculated agar. Ofloxacin (5 μg/disk) and nystatin (100 μg/disk) were used as positive controls for bacteria and fungi, respectively. The inoculated plates were incubated for 24 h for bacterial strains and 48 h for yeast strains at 36 ± 1 °C and 72 h at 27 ± 1 °C for fungal species. Antimicrobial activity in the modified disk diffusion assay was evaluated by measuring the zone of inhibition against test microorganisms [18]. Each test was repeated at least twice.
Preparation of Antimicrobial Textile Products
Antimicrobial solution that contained 7 % Glucapon 215 CS UP, 0.03 % T, and 3 % SPP solution was prepared, and pH of the solution was adjusted between 5 and 7 with citric acid or acetic acid. The solution was homogenized by mixing 1–1.5 h. Cotton fabrics were immersed into the solutions and shaken for 30 min; the fabrics were then dried at between 25 and 90 °C.
ICP-MS Analysis
ICP-MS was used to indicate the boron content of the antimicrobial solution-treated textile surfaces. ICP-MS results were obtained by using X Series 2 ICP-MS Thermo Scientific (MA, USA). Treated textile samples (4 × 4 cm (0.28 g)) were mixed with 10 mL nitric acid (65 % HNO3) and poured into microwave sample holders. The microwave was operated at 1600 W, 200 °C, and 600 psi. Samples were filtered through 0.2-μm membrane filters and diluted with 40 mL distilled water. One milliliter of the solution was diluted to 50 mL, and the boron content was measured.
HPLC Analysis
UHPLC was used to indicate the triclosan content of antimicrobial solution-treated textile surfaces. Results were obtained by using Thermo Scientific UltiMate™ 3000 UHPLC systems (MA, USA). The instrument was equipped with an ACQUITY UPLC BEH reversed-phase column of C18 (2.1 × 50 mm, 1.7 μm). The UV wavelength used in the experiment was 230 nm for triclosan. Stock solution of triclosan (0.2 mg/mL) was prepared, and then, standard working solutions were prepared by dilution with a concentration ranging from 0.002 to 0.04 mg/mL in methanol/water (70:30, v/v). These standard samples were run on the HPLC, and a calibration curve was prepared. Treated textile samples were sonicated for 45 min in mobile phase in ultrasonic bath. Sonicated samples were filtered through 0.2-μm regenerated cellulose filters. Then, samples were prepared in appropriate to standard concentration amounts and analyzed by HPLC. The mobile phase of methanol/water (70:30 (v/v)) was delivered at a constant flow rate of 0.3 mL/min. The total run time for an HPLC analysis was 4 min.
Antimicrobial Activity Tests of Textile Surfaces
The antimicrobial activity of the surface-modified cotton textile products were cut as approximately 1 × 1 cm sizes, and antimicrobial activities of these products were investigated for selected microorganisms (Table 1). TSA, SDA or PDA were inoculated with bacteria, yeast, and fungus as described in Microbial tests section, and antimicrobial fabrics were placed onto inoculated medium. Sterile, distilled water-impregnated blank textile products were used as negative controls.
Preparation of Infected HEp-2 Cell Cultures
HEp-2 cells, derived from an epidermoid carcinoma of the larynx, were maintained in minimum essential medium (MEM) (1×), supplemented with 10 % fetal bovine serum (FBS) and 1 % penicillin-streptomycin amphotericin (PSA). After 1 day, passage of HEp-2 cells at 1:4 in plastic flasks was infected by poliovirus type 1 (PV-1) and adenovirus type 5 (AV-5) for preparing viral stocks. Cytopathic effect (CPE) of prepared viral stocks was checked by microscopy. At 50–75 % CPE, the cell line was added to 10 % FBS and frozen at −80 °C. After one freeze thawing of infected cells, supernatant was collected by low-speed centrifugation at 4–8 °C for 30 min at 3300 rpm. All gross debris was discarded, and the supernatant was used as virus stocks in the experiment. To measure virus titer, HEp-2 cells were seeded into 96-well plates at a density of 2 × 104 cells and incubated at 37 °C for 24 h in 5 % CO2 and each individual sample was serially diluted from 10−1 to 10−9 in 10-fold increments. Each dilution was inoculated into HEp-2 cells and incubated for 3 days at 5 % CO2 and 37 °C. PV-1 and AV-5 titers in the cell culture were calculated by Spearman–Karber method [12].
Antiviral Activity Tests of Textile Surfaces
HEp-2 cells were seeded at 2 × 104 in 96-well plates at cells in MEM and incubated at 37 °C in 5 % CO2. Textile surfaces treated with chemical mixture were cut and placed on the top of the 0.5-cm diameter vials. Viral stocks (0.1 mL) were passed through the fabrics, and collected virus stocks in vials were sterilized by passing from filter of pore size 0.22 μm. Then, PV-1 and AV-5 were serially diluted from 10−1 to 10−9 in 10-fold increments. Each dilution was inoculated into HEp-2 and incubated for 3 days at 37 °C in 5 % CO2. PV-1 and AV-5 titers in the cell culture were calculated by Spearman–Karber method [12]. Same procedure was repeated for untreated textiles as control group.
Results and Discussion
Antimicrobial activities of the chemicals and chemical-treated textile goods were investigated against six bacteria, one yeast, and two fungi species based on the disk diffusion assay by evaluating the presence of inhibition zones. Zone diameters measured from the disk diffusion assay are given in Table 2. The disk diffusion assay revealed that both SPP and T solutions displayed variable antimicrobial activities on the microorganisms tested. SPP (3 %) and 0.03 % T solution both showed antimicrobial effect against bacteria, yeast, and fungi. SPP has stronger anticandidal and antifungal activity than T. On the other hand, T had stronger antibacterial activity than SPP and even stronger than the ofloxacin disk that is used as a positive control.
Table 2.
Antimicrobial activities of SPP, T, and in combination of applied cotton textiles on different microorganisms determined based on disk diffusion assay
| Microbial species | SPP 3 % (mm) | T 0.03 % (mm) | Modified cotton textiles (mm) | Untreated cotton textiles (mm) | PC (mm) |
|---|---|---|---|---|---|
| E. coli | – | 30 | 24 | – | 30 |
| S. aureus | – | 21 | 16 | – | 28 |
| S. enterica subsp. enterica | 12 | 35 | 26 | – | 20 |
| S. epidermidis | 18 | 55 | 46 | – | 12 |
| K. pneumoniae | 8 | 38 | 23 | – | 25 |
| MRSA | 17 | 42 | 29 | – | 20 |
| C. albicans | 18 | 8 | 19 | – | 24 |
| A. niger | 25 | – | 23 | – | 25 |
| T. mentagrophytes | 14 | 17 | 24 | – | 23 |
Antimicrobial mixture-treated textile goods antimicrobial test results showed that those fabrics have remarkable antimicrobial (antibacterial, anticandidal, and antifungal) effects against all tested microorganisms. Figure 1 shows the results of the antimicrobial tests concerning microorganisms given in Table 1. The petri dishes that are presented in Fig. 1 correspond to untreated cotton fabric used as negative control, and modified cotton fabrics give qualitative results. In addition, Table 2 showed the inhibition zone diameters of antimicrobial textiles. Fabrics treated with 3 % SPP and 0.03 % T solution showed remarkable antimicrobial activity against all tested microorganisms where untreated fabrics exhibit no antimicrobial performance. Antimicrobial solution loaded on cotton fabrics revealed highest antimicrobial effect against Staphylococcus epidermidis where lowest antimicrobial activity was observed against S. aureus.
Fig. 1.
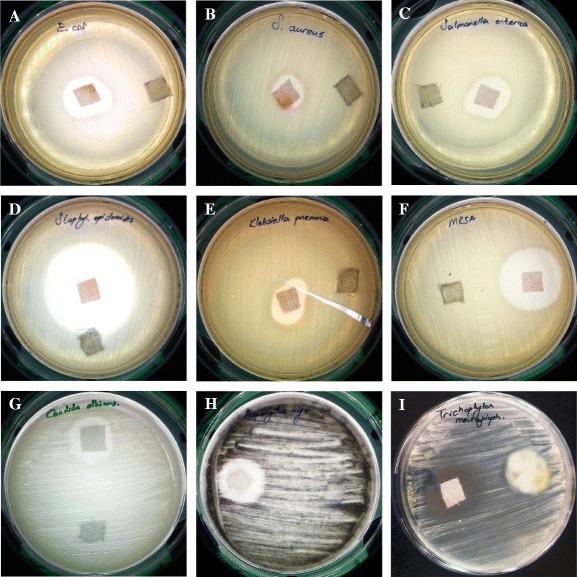
Modified disk diffusion results of treated and untreated cotton fabrics against a E. coli, b S. aureus, c S. enterica subsp. enterica, d S. epidermidis, e K. pneumoniae, f MRSA, g C. albicans, h A. niger, and i T. mentagrophytes
The active ingredient amounts attached to the surface of the cotton fabric were determined quantitatively. According to the ICP-MS analysis, the boron ion contents of the treated textile samples were measured as 0.70 ± 0.050 % (w/w) that has an equivalent value of 3.8 ± 0.272 % SPP. Triclosan amount attached to the surface of the fabric was determined as 0.0243 %. Figure 2 shows the HPLC chromotogram for triclosan recovered from treated textile surface. HPLC chromotogram represents the triclosan peak relevant to the active compound for the retentition time at 2.97 min. Known concentrations of triclosan standards were overlayed with textile samples, and triclosan amounts of samples were obtained.
Fig. 2.
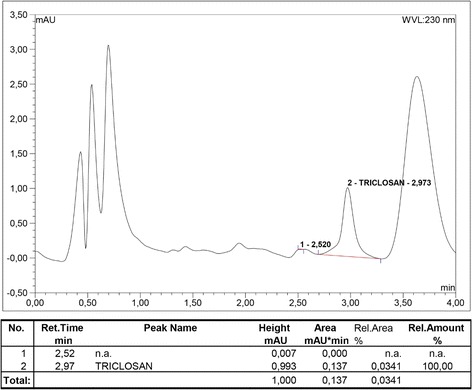
HPLC diagram of triclosan recovered from textile surface. Conditions: C18 column (2.1 × 50 mm, 1.7 μm), UV wavelength 230 nm
Unfortunately, the mechanism of action of boron compounds against microorganisms is not totally understood, but previous reports support the antimicrobial activity. Remarkable antimicrobial activity against a wide range of microorganisms including bacteria (both gram positive and gram negative), fungi, and yeast have been stated [7, 9, 15]. Due to the large surface area and ability to retain moisture, textiles become a favorable medium for the growth of microorganisms on the fabric. Thus, scientists study antimicrobial textiles to control microbial growth, to prevent odors and discolorations caused by microorganisms and moreover to diminish the threat to public health [41]. Despite antimicrobial activity of boron compounds being reported in a few studies, there is no study about antimicrobial textile goods treated with boron compounds up to now.
Contrary to boron, the antimicrobial mechanism of action of triclosan is well understood. It has been reported that triclosan blocks lipid biosynthesis and inhibits microbial growth [11].
The antimicrobial activity of triclosan has been known for decades, and it is used with textiles to obtain antimicrobial properties [11, 25, 27, 37, 42]. In the current study, the antimicrobial activity of triclosan is supported with SPP for the first time on cotton fabrics. Triclosan has no or weak antimicrobial effect against Candida albicans and Aspergillus niger. However, due to the strong antimicrobial effect of SPP against these microorganisms, SPP + T combination-treated cotton textiles have shown remarkable inhibition zones around textile goods.
As mentioned above, the antiviral tests were performed with AV-5 and PV-1. Initial AV-5 and PV-1 virus titers were both 5 log [10]/ml. Collected viral stocks that passed through the treated and untreated textiles were serially diluted and inoculated into HEp-2 cells. After 72 h, the antiviral effects were determined by the 50 % tissue culture-infected dose (TCID50) and the virus-induced cell death was recorded by analyzing cell morphology by inverted light microscope’s brightfield white light mode. Antiviral test results revealed that the rate of decline at virus titer is 3 log [10] for treated cotton textiles while untreated cotton textile causes no decline (Table 3). Rate of decline in virus titers between wells was calculated by observing cell deaths. Figures 3 and 4 showed the view of death and viable cells at 72 h of AV-5 and PV-1 virus titers that were passed through the fabrics and serially diluted in 10-fold increments and the control groups.
Table 3.
Antiviral test results of 3 % SPP + 0.03 % T combination applied in textile materials against adenovirus type 5 (AV-5) and poliovirus type 1 (PV-1)
| AV-5 | PV-1 | ||
|---|---|---|---|
| Virus titera 5.0 | |||
| Modified cotton textiles | Modified textile virus titerb | 2 | 2 |
| The rate of decline in virus titerc | 3 | 3 | |
| Control group (untreated cotton textile) | Untreated textile virus titerb | 5 | 5 |
| The rate of decline in virus titerc | 0 | 0 | |
aLogarithmic TCID50 value of virus per milliliter
bLogarithmic TCID50 value of modified and untreated textile virus in a different time and environment
cLogarithmic TCID50 ratio between virus titer and antiviral textile viral titer
Fig. 3.
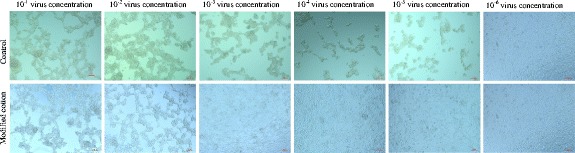
Inverted microscope view of death and viable HEp-2 cell monolayers at 72 h of AV-5 virus titers that were passed through the modified and untreated fabrics
Fig. 4.

Inverted microscope view of death and viable HEp-2 cell monolayers at 72 h of PV-1 virus titers that were passed through the modified and untreated fabrics
Even though antiviral activity of triclosan against a number of viruses has been studied previously [8, 17, 22], there has not been any study about the antiviral activity of triclosan-treated textile products so far. Just like triclosan, boron-containing compounds’ antiviral activity has been indicated in a few studies. The boron-containing antibiotic, boromycin, was found as an antiviral agent against human immunodeficiency virus type 1 (HIV-1) strain in in vitro conditions [20]. Also, boronic acid antiviral activity against hepatitis C virus (HCV) has been indicated previously [40]. However, there is no study that reveals the antiviral activity of textile materials treated with boron compounds, up to now.
In the current study, T and SPP were applied to cotton textile materials for the first time, and these modified textile materials showed antiviral effects against PV-1 and AV-5. The modified textile materials decreased both virus titers 60 %. In this study, antiviral tests performed against both DNA and RNA virus models which are AV-5 and PV-1, respectively. From this aspect, combination of 0.03 % T and 3 % SPP applied on textile materials should be effective against enveloped and non-enveloped DNA viruses such as hepatitis B virus (HBV), and they are assumed to be effective against enveloped and non-enveloped RNA viruses such as HIV, HCV, Ebola, MERS, and SARS. Further studies should be conducted for other types of woven and non-woven textile products.
Conclusion
The examinations and tests performed show that the modification of cotton fabrics with 3 % SPP + 0.03 % T solution makes it possible to obtain antibacterial, anticandidal, antifungal, and antiviral effects as expected. The inhibition zones of microbial growth ranging from 16 to 46 mm obtained in modified disk diffusion assay indicates notable antimicrobial activity of modified cotton fabrics. Modified cotton textile fabrics showed antiviral activity against adenovirus type 5 and poliovirus type 1 and reduced both virus titers 60 %, while untreated fabrics cause no decline in viral titers.
This formulation may be used not only in medical applications but also for manufacturing textile products of daily use and technical textiles. In the future, newly developed antimicrobial textiles are recommended for use in the military, health care, work/uniforms, home fashions and domestic products, and sports apparel. With these new technologies, the growing needs of the consumer in antimicrobial textile related to safety, human health, and environment are fulfilled.
References
- 1.Askew, P. (2014). Efficacy Assessment of Treated Articles: A guidance., 91.
- 2.Babb J, Davies J, Ayliffe G. Contamination of protective clothing and nurses’ uniforms in an isolation ward. Journal of Hospital Infection. 1983;4:149–157. doi: 10.1016/0195-6701(83)90044-0. [DOI] [PubMed] [Google Scholar]
- 3.Bagchi A. Structural characterizations of metal ion binding transcriptional regulator CueR from opportunistic pathogen Pseudomonas aeruginosa to identify its possible involvements in virulence. Applied Biochemistry and Biotechnology. 2015;175:649–656. doi: 10.1007/s12010-014-1304-5. [DOI] [PubMed] [Google Scholar]
- 4.Bagherzadeh R, Montazer M, Latifi M, Sheikhzadeh M, Sattari M. Evaluation of comfort properties of polyester knitted spacer fabrics finished with water repellent and antimicrobial agents. Fibers and Polymers. 2007;8:386–392. doi: 10.1007/BF02875827. [DOI] [Google Scholar]
- 5.Dastjerdi R, Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids and Surfaces B: Biointerfaces. 2010;79:5–18. doi: 10.1016/j.colsurfb.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Dastjerdi R, Montazer M, Shahsavan S. A new method to stabilize nanoparticles on textile surfaces. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2009;345:202–210. doi: 10.1016/j.colsurfa.2009.05.007. [DOI] [Google Scholar]
- 7.De Seta F, Schmidt M, Vu B, Essmann M, Larsen B. Antifungal mechanisms supporting boric acid therapy of Candida vaginitis. Journal of Antimicrobial Chemotherapy. 2009;63:325–336. doi: 10.1093/jac/dkn486. [DOI] [PubMed] [Google Scholar]
- 8.Dellanno C, Vega Q, Boesenberg D. The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. American Journal of Infection Control. 2009;37:649–652. doi: 10.1016/j.ajic.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demirci S, Kaya MS, Doğan A, Kalay Ş, Aaltın NÖ, Yarat A, Akyüz SH, Şahin F. Antibacterial and cytotoxic properties of boron-containing dental composite. Turkish Journal of Biology. 2015;39:417–426. doi: 10.3906/biy-1408-18. [DOI] [Google Scholar]
- 10.Dixon GJ, Sidwell RW, Mcneil E. Quantitative studies on fabrics as disseminators of viruses II. Persistence of poliomyelitis virus on cotton and wool fabrics. Applied microbiology. 1966;14:183–188. doi: 10.1128/am.14.2.183-188.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y, Cranston R. Recent advances in antimicrobial treatments of textiles. Textile Research Journal. 2008;78:60–72. doi: 10.1177/0040517507082332. [DOI] [Google Scholar]
- 12.Hamilton MA, Russo RC, Thurston RV. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environmental Science & Technology. 1977;11:714–719. doi: 10.1021/es60130a004. [DOI] [Google Scholar]
- 13.Hartstein AI, Denny MA, Morthland VH, LeMonte AM, Pfaller MA. Control of methicillin-resistant Staphylococcus aureus in a hospital and an intensive care unit. Infection Control & Hospital Epidemiology. 1995;16:405–411. doi: 10.2307/30141896. [DOI] [PubMed] [Google Scholar]
- 14.Hearle, J. W. and Morton, W. E. (2008) Physical properties of textile fibres. ed. Elsevier.
- 15.Hernandez V, Crépin T, Palencia A, Cusack S, Akama T, Baker SJ, Bu W, Feng L, Freund YR, Liu L. Discovery of a novel class of boron-based antibacterials with activity against gram-negative bacteria. Antimicrobial Agents and Chemotherapy. 2013;57:1394–1403. doi: 10.1128/AAC.02058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai K, Ogawa H, Bui VN, Inoue H, Fukuda J, Ohba M, Yamamoto Y, Nakamura K. Inactivation of high and low pathogenic avian influenza virus H5 subtypes by copper ions incorporated in zeolite-textile materials. Antiviral Research. 2012;93:225–233. doi: 10.1016/j.antiviral.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Jones RD, Jampani HB, Newman JL, Lee AS. Triclosan: a review of effectiveness and safety in health care settings. American Journal of Infection Control. 2000;28:184–196. doi: 10.1067/mic.2000.102378. [DOI] [PubMed] [Google Scholar]
- 18.Kalaycı S, Demirci S, Sahin F. Determination of antimicrobial properties of Picaridin and DEET against a broad range of microorganisms. World Journal of Microbiology and Biotechnology. 2014;30:407–411. doi: 10.1007/s11274-013-1456-4. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi Y, Nakanishi T, Komiyama J. Deodorant properties of wool fabrics dyed with acid mordant dyes and a copper salt. Textile Research Journal. 2002;72:125–131. doi: 10.1177/004051750207200206. [DOI] [Google Scholar]
- 20.Kohno J, Kawahata T, Otake T, Morimoto M, Mori H, Ueba N, Nishio M, Kinumaki A, Komatsubara S, Kawashima K. Boromycin, an anti-HIV antibiotic. Bioscience, Biotechnology, and Biochemistry. 1996;60:1036–1037. doi: 10.1271/bbb.60.1036. [DOI] [PubMed] [Google Scholar]
- 21.Kropec A, Huebner J, Riffel M, Bayer U, Benzing A, Geiger K, Daschner F. Exogenous or endogenous reservoirs of nosocomial Pseudomonas aeruginosa and Staphylococcus aureus infections in a surgical intensive care unit. Intensive Care Medicine. 1993;19:161–165. doi: 10.1007/BF01720533. [DOI] [PubMed] [Google Scholar]
- 22.Lages S, Ramakrishnan M, Goyal S. In-vivo efficacy of hand sanitisers against feline calicivirus: a surrogate for norovirus. Journal of Hospital Infection. 2008;68:159–163. doi: 10.1016/j.jhin.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Lalitha M. Manual on antimicrobial susceptibility testing. Performance standards for antimicrobial testing: Twelfth Informational Supplement. 2004;56238:454–456. [Google Scholar]
- 24.Lee H, Yeo S, Jeong S. Antibacterial effect of nanosized silver colloidal solution on textile fabrics. Journal of Materials Science. 2003;38:2199–2204. doi: 10.1023/A:1023736416361. [DOI] [Google Scholar]
- 25.Levy CW, Roujeinikova A, Sedelnikova S, Baker PJ, Stuitje AR, Slabas AR, Rice DW, Rafferty JB. Molecular basis of triclosan activity. Nature. 1999;398:383–384. doi: 10.1038/18803. [DOI] [PubMed] [Google Scholar]
- 26.Lim S-H, Hudson SM. Application of a fiber-reactive chitosan derivative to cotton fabric as an antimicrobial textile finish. Carbohydrate Polymers. 2004;56:227–234. doi: 10.1016/j.carbpol.2004.02.005. [DOI] [Google Scholar]
- 27.McArthur, J. V., Tuckfield, R. and Baker-Austin, C. (2012). in Antibiotic Resistance, pp. 135–152. Springer.
- 28.Morris CE, Welch CM. Antimicrobial finishing of cotton with zinc pyrithione. Textile Research Journal. 1983;53:725–728. doi: 10.1177/004051758305301202. [DOI] [Google Scholar]
- 29.Nakashima T, Sakagami Y, Ito H, Matsuo M. Antibacterial activity of cellulose fabrics modified with metallic salts. Textile Research Journal. 2001;71:688–694. doi: 10.1177/004051750107100807. [DOI] [Google Scholar]
- 30.Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. Journal of Clinical Microbiology. 2000;38:724–726. doi: 10.1128/jcm.38.2.724-726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neyts J, Snoeck R, Schols D, Balzarini J, Esko JD, Van Schepdael A, De Clercq E. Sulfated polymers inhibit the interaction of human cytomegalovirus with cell surface heparan sulfate. Virology. 1992;189:48–58. doi: 10.1016/0042-6822(92)90680-N. [DOI] [PubMed] [Google Scholar]
- 32.Park D, Larson AM, Klibanov AM, Wang Y. Antiviral and antibacterial polyurethanes of various modalities. Applied Biochemistry and Biotechnology. 2013;169:1134–1146. doi: 10.1007/s12010-012-9999-7. [DOI] [PubMed] [Google Scholar]
- 33.Paul T, Mandal A, Mandal SM, Ghosh K, Mandal AK, Halder SK, Das A, Maji SK, Kati A, Mohapatra PKD. Enzymatic hydrolyzed feather peptide, a welcoming drug for multiple-antibiotic-resistant Staphylococcus aureus: structural analysis and characterization. Applied Biochemistry and Biotechnology. 2015;175:3371–3386. doi: 10.1007/s12010-015-1509-2. [DOI] [PubMed] [Google Scholar]
- 34.Petkova P, Francesko A, Perelshtein I, Gedanken A, Tzanov T. Simultaneous sonochemical-enzymatic coating of medical textiles with antibacterial ZnO nanoparticles. Ultrasonics Sonochemistry. 2016;29:244–250. doi: 10.1016/j.ultsonch.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 35.Qian L, Sun G. Durable and regenerable antimicrobial textiles: synthesis and applications of 3-methylol-2, 2, 5, 5-tetramethyl-imidazolidin-4-one (MTMIO) Journal of Applied Polymer Science. 2003;89:2418–2425. doi: 10.1002/app.12405. [DOI] [Google Scholar]
- 36.Shahidi S, Ghoranneviss M, Moazzenchi B, Rashidi A, Mirjalili M. Investigation of antibacterial activity on cotton fabrics with cold plasma in the presence of a magnetic field. Plasma Processes and Polymers. 2007;4:S1098–S1103. doi: 10.1002/ppap.200732412. [DOI] [Google Scholar]
- 37.Simoncic, B. and Tomsic, B. (2010) Structures of novel antimicrobial agents for textiles—a review. Textile Research Journal.
- 38.Takai K, Ohtsuka T, Senda Y, Nakao M, Yamamoto K, Matsuoka J, Hirai Y. Antibacterial properties of antimicrobial-finished textile products. Microbiology and Immunology. 2002;46:75–81. doi: 10.1111/j.1348-0421.2002.tb02661.x. [DOI] [PubMed] [Google Scholar]
- 39.Tredget EE, Shankowsky HA, Joffe AM, Inkson TI, Volpel K, Paranchych W, Kibsey PC, Alton JM, Burke JF. Epidemiology of infections with Pseudomonas aeruginosa in burn patients: the role of hydrotherapy. Clinical Infectious Diseases. 1992;15:941–949. doi: 10.1093/clind/15.6.941. [DOI] [PubMed] [Google Scholar]
- 40.Trippier PC, McGuigan C. Boronic acids in medicinal chemistry: anticancer, antibacterial and antiviral applications. MedChemComm. 2010;1:183–198. doi: 10.1039/c0md00119h. [DOI] [Google Scholar]
- 41.Xing Y, Yang X, Dai J. Antimicrobial finishing of cotton textile based on water glass by sol–gel method. Journal of Sol-Gel Science and Technology. 2007;43:187–192. doi: 10.1007/s10971-007-1575-1. [DOI] [Google Scholar]
- 42.Yazdankhah SP, Scheie AA, Høiby EA, Lunestad B-T, Heir E, Fotland TØ, Naterstad K, Kruse H. Triclosan and antimicrobial resistance in bacteria: an overview. Microbial Drug Resistance. 2006;12:83–90. doi: 10.1089/mdr.2006.12.83. [DOI] [PubMed] [Google Scholar]
- 43.Yazhini KB, Prabu HG. Antibacterial activity of cotton coated with ZnO and ZnO-CNT composites. Applied Biochemistry and Biotechnology. 2015;175:85–92. doi: 10.1007/s12010-014-1257-8. [DOI] [PubMed] [Google Scholar]


