Abstract
Quinine was first recognized as a potent antimalarial agent hundreds of years ago. Since then, the beneficial effects of quinine and its more advanced synthetic forms, chloroquine and hydroxychloroquine, have been increasingly recognized in a myriad of other diseases in addition to malaria. In recent years, antimalarials were shown to have various immunomodulatory effects, and currently have an established role in the management of rheumatic diseases, such as systemic lupus erythematosus and rheumatoid arthritis, skin diseases, and in the treatment of chronic Q fever. Lately, additional metabolic, cardiovascular, antithrombotic, and antineoplastic effects of antimalarials were shown. In this review, we discuss the known various immunomodulatory mechanisms of antimalarials and the current evidence for their beneficial effects in various diseases and in potential novel applications.
Keywords: Chloroquine, Hydroxychloroquine, Antimalarial, Novel, Therapy, Lupus
Hydroxychloroquine (HCQ) belongs to the group of antimalarial agents, which recognition of its benefit in other diseases except malaria dates back to 1894, when Payne reported that quinine was effective in cutaneous lupus [1]. Since then, therapeutic effects of antimalarial agents were shown in a myriad of other diseases and the evidence for its potential benefit continues to grow.
The first use of quinine as a drug is traditionally attributed to the Incan descendents in Peru, who cured the countess of Chinchon from a febrile illness with a mysterious bark powder in 1630 [2, 3]. Hundreds of years later, the alkaloids from the bark were extracted when chloroquine was one of a large series of 4-aminoquinolines which were investigated, beginning in 1943, as part of the extensive cooperative program of antimalarial research in the USA during World War II when thousands of compounds were synthesized and tested for activity. Chloroquine eventually proved most promising and was released for field trial. When hostilities ceased, it was discovered that the compound had been synthesized and studied as early as 1934 by the Germans but rejected because of toxicity in avian models.
During the Second World War, millions of soldiers took antimalarial prophylaxis, and the observation that antimalarial improved the soldiers’ rashes and inflammatory arthritis led to the first trial that showed the efficacy of antimalarials in systemic lupus erythematosus (SLE) [4]. Since then, the role of antimalarials in rheumatic diseases was established and today its use is widespread in SLE and in rheumatoid arthritis (RA) [5–7]. The synthetic form of HCQ was introduced in 1955 and differs from chloroquine only by a hydroxyl group, decreasing its toxicity while conserving its efficacy.
Hydroxychloroquine has numerous known immunomodulatory effects [8], but its specific mechanism in individual diseases is not clear. The major proposed mechanisms of action of antimalarials on the immune system include: (1) interference with lysosomal acidification and inhibition of proteolysis, chemotaxis, phagocytosis, and antigen presentation [9, 10]; (2) decreasing macrophage-mediated cytokine production, especially interleukin (IL)-1 and IL-6 [11]; (3) inhibition of phospholipase A2 and thereby antagonizing the effects of prostaglandins [12, 13]; (4) absorption and blocking of UV light cutaneous reactions [14]; (5) binding and stabilizing DNA [15]; (6) inhibition of T and B-cell receptors calcium signaling [16]; and (7) inhibition of matrix metalloproteinases [17]. It was recently recognized that HCQ also inhibits toll-like receptors signaling [18], highlighting a new and important effect, which might play a part in numerous pathways and may contribute to our understanding of its usefulness in many autoimmune and other diseases. The various major known immunomodulatory mechanisms of HCQ is schematically drawn in Fig. 1.
Fig. 1.
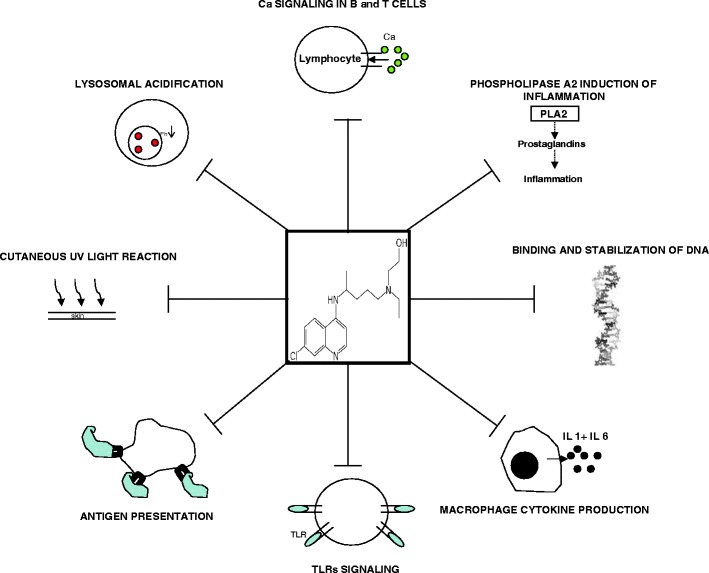
The various inhibitory and immunomodulatory effects of HCQ are schematically drawn. PLA2 phospholipase A2, IL interleukin, TLR toll like receptor, UV ultraviolet
In addition to its established efficacy in SLE and RA, antimalarials were also found to be beneficial in other rheumatic and skin diseases, including palindromic rheumatism [19], eosinophilic fasciitis [20], dermatomyositis [21], Sjögren’s syndrome [22], porphyria cutanea tarda [23, 24], polymorphous light eruption [25], granuloma annulare [26], lichen planus [27], lupus panniculitis [28], and discoid lupus [29]. The traditional indications for the use of HCQ are summarized in Table 1.
Table 1.
Traditional indications and doses for the use of hydroxychloroquine
| Indication | Dose | Study |
|---|---|---|
| Malaria (acute) | 800 mg followed by 400 mg at 6, 24, and 48 h | CDC guidelines[111] |
| Rheumatoid arthritis | 400–600 mg/day | Clark et al. [5] |
| SLE | 200–400 mg/day | Tsakonas et al. [7], Molad et al. [6] |
| Palindromic rheumatism | 200–400 mg/day | Youssef et al. [19] |
| Eosinophilic fasciitis | 400 mg/day | Lakhanpal et al. [20] |
| Dermatomyositis | 400 mg/day | Woo et al. [21] |
| Sjögren’s syndrome | 6–7 mg/kg/day | Fox et al. [22] |
| Porphyria cutanea tarda | 250–500 mg/week | Ashton et al. [23], Valls et al. [24] |
| Polymorphous light eruption | 200–400 mg/day | Murphy et al. [25] |
| Granuloma annulare | 2–9 mg/gk/day | Cannistraci et al. [26] |
| Lichen planus | 200–400 mg/day | Eisen [27] |
| Lupus panniculitis | 200–400 mg/day | Chung et al. [28] |
| Discoid lupus | 400 mg/day | Jessop et al. [29] |
SLE systemic lupus erythematosus
Recently, antimalarials were shown to have potential beneficial effects in many other diseases, including diseases of the immune, cardiovascular, and hematological systems, as well as malignant and infectious diseases other than malaria. Here, we review the current evidence for novel applications of the antimalarials, mainly chloroquine and HCQ.
Antimicrobial Effects
In addition to its antimalarial effect, HCQ was found to be effective against bacterial and viral infections. Its antibacterial and antiviral effects are attributed to the alkalinization of intracellular acidic organelles infected by bacteria and to the inhibition of entry steps and viral proteins glycosylation [30]. The most recognized antibacterial effect of HCQ is in Coxiella burnetii infections, where the treatment of choice for chronic Q-fever endocarditis consists of HCQ together with doxycycline for 18–36 months.
In the human immunodeficiency virus (HIV), HCQ was shown to inhibit virus replication in T cells, by inhibiting the surface envelope glycoprotein 120 [31–34]. This anti-HIV mechanism lacks cross-resistance with other antiretroviral drugs and was novel in the armamentarium of anti-HIV drugs. Paton et al. [35] showed that combined treatment with HCQ, hydroxyurea, and didanosine in antiretroviral naïve HIV patients with low viral load, decreased viral replication, and increased the CD4 count. However, in the current era of the highly active anti-retroviral therapy for HIV, the use of non anti-retroviral drugs such as HCQ is probably redundant.
Human corona virus (hCoV) caused a near pandemic of severe acute respiratory syndrome in 2002–2003. To date, no specific antiviral drugs for the prevention or treatment of hCoV infection are available. Recently, chloroquine was shown to inhibit the replication and spread of corona virus in vitro [36, 37] and to prevent infection with hCoV in newborn mice [38], showing promise as a potential therapy of this resistant virus.
Metabolic and Cardiovascular Effects
The hypoglycemic effect of chloroquine was first demonstrated in a patient with insulin resistance in 1984 [39]. Later on, a clinical trial of non-insulin dependent diabetic patients who were treated with a short course of chloroquine exhibited a significant improvement in glucose tolerance in these patients [40]. When HCQ was combined with insulin for 6 months, the glycated hemoglobin decreased significantly compared to placebo, and the insulin dose had to be reduced by 30% in the HCQ group [41]. In another study, diabetic patients who did not respond to sulfonylurea were successfully treated with HCQ and their glycated hemoglobin was reduced by 1% [42]. The presumed anti-diabetic mechanism of chloroquine is by a decrease in the insulin clearance and degradation rate and an increase in the secretion of C-peptide [43, 44]. Although HCQ was shown to have hypoglycemic effects, it should be noted the current treatment options for diabetes mellitus (DM) are varied and effective, so a role for antimalarials in diabetes treatment is unlikely.
The use of HCQ may also be beneficial for reducing cardiovascular risk by improving glycemic control in RA and SLE patients; Shojania et al. [45] described a case of induction of hypoglycemia in a patient with DM and polyarthritis treated with HCQ. Later, in a cohort of patients with SLE or RA, treatment with HCQ was associated with lower levels of fasting blood glucose [46] and significantly lowered the risk to develop DM in RA patients [47]. It was recently shown that HCQ significantly reduces glycosylated hemoglobin in diabetes patients [48]. This observed association between HCQ treatment and lower risk of DM in rheumatic patients needs further clinical and pharmacological studies to assess HCQ’s effect on blood sugar before becoming common clinical practice.
Hydroxychloroquine was also shown to have a favorable effect on lipid profile in patients with rheumatic diseases. Wallace et al. [49] showed that in RA and SLE, treatment with HCQ lowered the levels of cholesterol, triglycerides and LDL, irrespective of concomitant steroid administration, diet, or weight. Later studies confirmed this observation and suggested that this should be taken into account in rheumatic patients with significant cardiovascular risk factors and in ameliorating the adverse effects of corticosteroid therapy on lipid profile [50–52]. However, a different study in Chinese SLE patients did not show significant effect on serum lipid profile, but these patients had mild or absent disease-activity and used low corticosteroid dose [53]. Of note, in addition to its metabolic effects on blood sugar and lipids, HCQ was recently also shown to improve endothelial function and to protect against thrombovascular events in lupus patients [54–56].
Antithrombotic Effects
Approximately 30 years ago, HCQ was shown to prevent thromboembolic events in patients going total hip replacement [57]. The mechanism was unknown at the time, but it was later shown that HCQ inhibits platelet aggregation and the release of arachidonic acid from stimulated platelets [58]. Treatment with HCQ was also shown to be protective against thrombosis in lupus patients with antiphospholipid syndrome (APS) [59, 60] and to reduce the risk of plaque formation in these patients [61]. Furthermore, HCQ seemed to be protective against thrombus formation in a group of anti-phospholipid (aPL) antibodies carriers without lupus [62].
The antithrombotic mechanism of HCQ in APS is not completely understood, but some novel observations were recently made; Edwards et al. [63] found that HCQ decrease thrombus size and shortens the time to thrombus clearance in mice injected with human aPL antibodies. More recently, Espinola et al. [64] showed that HCQ inhibits platelets activation by aPL antibodies and Rand et al. showed that HCQ significantly reduces the binding of aPL-beta2-glycoprotein I complexes to phospholipid surfaces and also protects against the disruption of the potent anticoagulant annexin A5 by aPL antibodies in vitro and in patients’ plasma [65, 66]. These finding suggest that HCQ should be an integral part of the treatment of APS, especially in patients with concomitant SLE.
Antineoplastic Effects
The antineoplastic effect of antimalarials was first observed when a program of chloroquine prophylaxis against malaria in Tanzania was associated with a reduction in the incidence of Burkitt lymphoma [67]. Later, chloroquine was shown to prevent spontaneous lymphoma development in mice models of human Burkitt lymphoma and ataxia telangiectasia, through the suppression of autophagy and an induction of p53-dependent cell death pathway [68]. Hydroxychloroquine was also shown to induce apoptosis of malignant B-cells from patients with chronic lymphocytic leukemia (CLL), through the activation of caspase-3 and modulation of Bcl-2/bax ratio [69, 70]. Recently, a biodegradable nanoparticle drug delivery system loaded with HCQ was shown to produce a strong apoptotic effect on B-CLL cells in vitro [71].
Except for its effect on hematological malignancies, HCQ was also shown to have antineoplastic effects on solid tumors: (1) An anti breast-cancer effect was shown by demonstration of its antiproliferative effect on the human breast tumor cell models MCF-7 and Bcap-37 [72–74]. (2) An antiproliferative effect on mouse colon cancer cell line was shown, as well as a reduction in tumor volume in vivo with chloroquine treatment [75]. Chloroquine was also shown to potentiate the antiproliferative effect of 5-fluorouracil on the human colorectal cell line HT-29 [76]. (3) Chloroquine was shown to inhibit growth and induce cell death of A549 lung cancer cells [77]. (4) In a randomized, double-blind, placebo-controlled trial, patients with glioblastoma multiforme who were treated with chloroquine had a much better survival rate compared with non-treated patients [78].
In addition to its independent antineoplastic effects, chloroquine was shown to effectively sensitize cancer cells to radiation and chemotherapy, without enhancing normal cells vulnerability [79, 80]. Although these preliminary studies look promising, the potential role for antimalarials in the treatment paradigm of malignancies still remains to be established.
Additional Effects
In addition to its immunomodulatory effects described above, HCQ was also shown to inhibit the T cell response to foreign minor and major histocompatibility antigens and therefore inhibit the development of graft versus host disease (GVHD) in mice [81]. A phase II trial in patients who received bone marrow transplantation, showed better GVHD free survival and lower incidence of high-grade GVHD in patients who were treated with HCQ in addition to the traditional immunosuppressive drugs [82]. However, a phase III trial failed to exhibit additional benefit in GVHD prevention when HCQ was added to monotherpay with cyclosporine A [83].
Additional beneficial effects of HCQ were also shown anecdotally in other diseases: (1) Hydroxychloroquine was shown to be very effective in Kikuchi–Fujimoto disease, a benign disease characterized by lymphadenopathy and fever, usually occurring in young women [84, 85]. (2) Skeletal and metabolic effects of sarcoidosis were successfully treated with HCQ in patients who were not candidates for glucocorticoid treatment [86–88]. (3) Hydroxychloroquine enabled steroid cessation in patients with subglottic stenosis who were steroid dependent due to edema and granulation tissue [89]. (4) Hydroxychloroquine in combination with azathioprine prompted a major recovery in a patient with sensory neuropathy syndrome [90]. (5) Exposure to HCQ during pregnancy in mothers with SLE who carried anti-Ro/La antibodies was associated with a decreased risk of fetal development of cardiac neonatal lupus, compared to mothers who were not treated with HCQ [91, 92]. (6) Hydroxychloroquine was shown to enhance the production of hemoglobin F, showing a therapeutic potential for hemoglobinopathies [93].
Antimalarials Toxicity
Antimalarials are contraindicated in known hypersensitivity and in patients with a history of retinopathy. Taken in proper doses, chloroquine is an extraordinarily safe drug; however, its safety margin is narrow, and a single dose of 30 mg/kg may be fatal [94]. Caution should be used in patients with neuromuscular disorders such as myasthenia gravis and in patients with psychotic disorders. Patients with glucose-6-phosphate deficiency should be carefully monitored for hemolysis, although this is extremely rare in therapeutic doses.
Acute chloroquine toxicity is encountered most frequently when therapeutic or high doses are administered too rapidly by parenteral routes. Toxic manifestations relate primarily to the cardiovascular system and the central nervous system (CNS). Cardiovascular effects include hypotension, vasodilation, suppressed myocardial function, cardiac arrhythmias, and eventual cardiac arrest. CNS side-effects may include confusion, convulsions, and coma. Doses of chloroquine used for oral therapy of acute malarial attack and for rheumatic and skin diseases may cause GI upset, headache, visual disturbances, urticaria, and pruritus. Prolonged use may occasionally cause headache, blurred vision, diplopia, confusion, convulsions, lichenoid skin eruptions, bleaching of hair, widening of the QRS interval, and T wave abnormalities. These complications are rare and usually disappear soon after the drug is withheld. Rare instances of hemolysis and blood dyscrasias have been reported [95]. Chloroquine may also cause discoloration of nail beds and mucous membranes [96].
Irreversible retinopathy and ototoxicity can result from high daily doses (>250 mg) of chloroquine or HCQ that lead to cumulative total doses of more than 1 g of base per kilogram body weight [97–99]. Retinopathy presumably is related to drug accumulation in melanin-rich tissues and can be avoided if the daily dose is 250 mg or less. Prolonged therapy with high doses can also cause toxic myopathy, cardiopathy, and peripheral neuropathy [100–103]; these reactions improve if the drug is withdrawn promptly [104].
Hydroxychloroquine crosses the placenta as fetus levels were shown to be equivalent to maternal levels [105]. However, no fetal toxicity was evident in subsequent studies and no congenital abnormalities were seen in pregnant patients treated with HCQ during the first trimester of pregnancy [106, 107]. During lactation, the amount of HCQ transferred to children seems very low and does not confer a risk of toxicity to the child [108, 109]. Accordingly, continuation of HCQ use during pregnancy is recommended and there’s no evidence to advice against breastfeeding during treatment [110]. A summary of antimalarials toxicity and main adverse effects is presented in Table 2.
Table 2.
Main adverse effects of chloroquine and HCQ treatment
| Type | |
|---|---|
| Ocular | Retinopathy (early changes reversible, may progress despite discontinuation if advanced), blurred vision, corneal changes/deposits |
| Neuromuscular and skeletal | Myopathy, palsy, or neuromyopathy leading to progressive weakness and atrophy of proximal muscle groups (may be associated with mild sensory changes, loss of deep tendon reflexes, and abnormal nerve conduction) |
| Cardiovascular | Cardiomyopathy (rare, relationship to HCQ unclear) |
| Central nervous system | Ataxia, dizziness, emotional changes, headache, irritability, lassitude, nervousness, nightmares, psychosis, seizure, vertigo |
| Gastrointestinal | Abdominal cramping, anorexia, nausea, vomiting, diarrhea, abnormal liver function |
| Cutaneous | Alopecia, angioedema, bleaching of hair, pigmentation changes (skin and mucosal; black-blue color), rash, pruritus |
| Otic | Deafness, tinnitus |
| Respiratory | Bronchospasm, respiratory failure (myopathy-related) |
| Hematological | Agranulocytosis, aplastic anemia, hemolysis (in patients with glucose-6-phosphate deficiency), leukopenia, thrombocytopenia |
| Other | Exacerbation of psoriasis |
In conclusion, since the first use of antimalarial agents nearly a century ago, their effects on diseases in nearly all major branches of medicine have been increasingly recognized, including the fields of immunology, oncology, hematology, dermatology, cardiology, and infectious diseases. A summary of the effects of antimalarials is shown in Table 3. To date, antimalarials have an established role in treatment of SLE, RA, chronic Q fever, and various skin diseases. They may also have a role in the treatment of antiphospholipid syndrome. There are currently many ongoing clinical trials studying the effects of antimalarials in other diseases, such as malignant neoplasms of the lung, breast, prostate, pancreas and colon, melanoma, renal cell carcinoma, CLL, multiple myeloma, influenza, HIV infection, osteoarthritis, and the metabolic syndrome, as can be seen by browsing the website clinicaltrials.gov. The mechanism of action of HCQ and its effectiveness in these diseases and in other potential conditions still remains to be demonstrated in the future.
Table 3.
A summary of the effects of antimalarials in various conditions
| Effect | Disease/state | Mechanism |
|---|---|---|
| Antimicrobial | Chronic Q fever endocarditis | Alkalinization of phagosome |
| Human immunodeficiency virus | Inhibition of virus replication | |
| Human corona virus | Inhibition of replication | |
| Metabolic and cardiovascular | Hypoglycemic in diabetes mellitus | Decreasing insulin clearance and increasing secretion of C-peptide |
| Improves lipid profile in SLE and RA and in steroid use | ||
| Improve endothelial function | ||
| Antithrombotic | Prevention of thromboembolism in immobilized patients | Inhibition of platelet aggregation and arachidonic acid release |
| Prevention of thrombosis in antiphospholipid syndrome | ||
| Inhibition of platelets activation by aPL antibodies, reducing binding of aPL-beta2-GPI to antiphospholipids and disruption of annexin A5 by aPL antibodies | ||
| Antineoplastic | Prevention of lymphoma in mice | Induction of p53-dependentcell death |
| Induction of apoptosis in CLL cells | Activation of caspase-3 | |
| Solid tumors: e.g., breast, colon, glioblastoma multiforme, lung | ||
| Sensitization of cancer cells to radiation and chemotherapy | ||
| Other | Prevention of GVHD disease | Inhibition of T cell response to MHC antigens |
| Kikuchi–Fujimoto disease | ||
| Sarcoidosis | ||
| Subglottic stenosis | ||
| Sensory neuropathy | ||
| Decreases risk of fetal cardiac lupus |
Where known, the mechanism is described
aPL antiphospholipid, GP glycoprotein, CLL chronic lymphocytic leukemia, GVHD graft versus host disease, MHC major histocompatibility complex
References
- 1.Smith CD, Cyr M. The history of lupus erythematosus. From Hippocrates to Osler. Rheum Dis Clin North Am. 1988;14(1):1–14. [PubMed] [Google Scholar]
- 2.Mates M, Nesher G, Zevin S. Quinines—past and present. Harefuah. 2007;146(7):560–562. [PubMed] [Google Scholar]
- 3.Wallace DJ. The history of antimalarials. Lupus. 1996;5(Suppl 1):S2–S3. doi: 10.1177/096120339600500102. [DOI] [PubMed] [Google Scholar]
- 4.Page F. Treatment of lupus erythematosus with mepacrine. Lancet. 1951;2(6687):755–758. doi: 10.1016/S0140-6736(51)91643-1. [DOI] [PubMed] [Google Scholar]
- 5.Clark P, Casas E, Tugwell P, Medina C, Gheno C, Tenorio G, et al. Hydroxychloroquine compared with placebo in rheumatoid arthritis. A randomized controlled trial. Ann Intern Med. 1993;119(11):1067–1071. doi: 10.7326/0003-4819-119-11-199312010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Molad Y, Gorshtein A, Wysenbeek AJ, Guedj D, Majadla R, Weinberger A, et al. Protective effect of hydroxychloroquine in systemic lupus erythematosus. Prospective long-term study of an Israeli cohort. Lupus. 2002;11(6):356–361. doi: 10.1191/0961203302lu203ra. [DOI] [PubMed] [Google Scholar]
- 7.Tsakonas E, Joseph L, Esdaile JM, Choquette D, Senecal JL, Cividino A, et al. A long-term study of hydroxychloroquine withdrawal on exacerbations in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. Lupus. 1998;7(2):80–85. doi: 10.1191/096120398678919778. [DOI] [PubMed] [Google Scholar]
- 8.Kalia S, Dutz JP. New concepts in antimalarial use and mode of action in dermatology. Dermatol Ther. 2007;20(4):160–174. doi: 10.1111/j.1529-8019.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA. 1978;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler HK, Unanue ER. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA. 1982;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperber K, Quraishi H, Kalb TH, Panja A, Stecher V, Mayer L. Selective regulation of cytokine secretion by hydroxychloroquine: inhibition of interleukin 1 alpha (IL-1-alpha) and IL-6 in human monocytes and T cells. J Rheumatol. 1993;20(5):803–808. [PubMed] [Google Scholar]
- 12.Loffler BM, Bohn E, Hesse B, Kunze H. Effects of antimalarial drugs on phospholipase A and lysophospholipase activities in plasma membrane, mitochondrial, microsomal and cytosolic subcellular fractions of rat liver. Biochim Biophys Acta. 1985;835(3):448–455. doi: 10.1016/0005-2760(85)90114-6. [DOI] [PubMed] [Google Scholar]
- 13.Manku MS, Horrobin DF. Chloroquine, quinine, procaine, quinidine, tricyclic antidepressants, and methylxanthines as prostaglandin agonists and antagonists. Lancet. 1976;2(7995):1115–1117. doi: 10.1016/S0140-6736(76)91090-4. [DOI] [PubMed] [Google Scholar]
- 14.Lester RS, Burnham TK, Fine G, Murray K. Immunologic concepts of light reactions in lupus erythematosus and polymorphous light eruptions. I. The mechanism of action of hydroxychloroquine. Arch Dermatol. 1967;96(1):1–10. doi: 10.1001/archderm.1967.01610010007001. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SN, Yielding KL. Spectrophotometric studies of the interaction of chloroquine with deoxyribonucleic acid. J Biol Chem. 1965;240:3123–3131. [PubMed] [Google Scholar]
- 16.Goldman FD, Gilman AL, Hollenback C, Kato RM, Premack BA, Rawlings DJ. Hydroxychloroquine inhibits calcium signals in T cells: a new mechanism to explain its immunomodulatory properties. Blood. 2000;95(11):3460–3466. [PubMed] [Google Scholar]
- 17.Lesiak A, Narbutt J, Sysa-Jedrzejowska A, Lukamowicz J, McCauliffe DP, Wozniacka A. Effect of chloroquine phosphate treatment on serum MMP-9 and TIMP-1 levels in patients with systemic lupus erythematosus. Lupus. 2010;19(6):683–688. doi: 10.1177/0961203309356455. [DOI] [PubMed] [Google Scholar]
- 18.Kyburz D, Brentano F, Gay S. Mode of action of hydroxychloroquine in RA—evidence of an inhibitory effect on toll-like receptor signaling. Nat Clin Pract Rheumatol. 2006;2(9):458–459. doi: 10.1038/ncprheum0292. [DOI] [PubMed] [Google Scholar]
- 19.Youssef W, Yan A, Russell AS. Palindromic rheumatism: a response to chloroquine. J Rheumatol. 1991;18(1):35–37. [PubMed] [Google Scholar]
- 20.Lakhanpal S, Ginsburg WW, Michet CJ, Doyle JA, Moore SB. Eosinophilic fasciitis: clinical spectrum and therapeutic response in 52 cases. Semin Arthritis Rheum. 1988;17(4):221–231. doi: 10.1016/0049-0172(88)90008-X. [DOI] [PubMed] [Google Scholar]
- 21.Woo TY, Callen JP, Voorhees JJ, Bickers DR, Hanno R, Hawkins C. Cutaneous lesions of dermatomyositis are improved by hydroxychloroquine. J Am Acad Dermatol. 1984;10(4):592–600. doi: 10.1016/S0190-9622(84)80263-7. [DOI] [PubMed] [Google Scholar]
- 22.Fox RI, Dixon R, Guarrasi V, Krubel S. Treatment of primary Sjogren’s syndrome with hydroxychloroquine: a retrospective, open-label study. Lupus. 1996;5(Suppl 1):S31–S36. doi: 10.1177/096120339600500108. [DOI] [PubMed] [Google Scholar]
- 23.Ashton RE, Hawk JL, Magnus IA. Low-dose oral chloroquine in the treatment of porphyria cutanea tarda. Br J Dermatol. 1984;111(5):609–613. doi: 10.1111/j.1365-2133.1984.tb06632.x. [DOI] [PubMed] [Google Scholar]
- 24.Valls V, Ena J, Enriquez-De-Salamanca R. Low-dose oral chloroquine in patients with porphyria cutanea tarda and low–moderate iron overload. J Dermatol Sci. 1994;7(3):169–175. doi: 10.1016/0923-1811(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 25.Murphy GM, Hawk JL, Magnus IA. Hydroxychloroquine in polymorphic light eruption: a controlled trial with drug and visual sensitivity monitoring. Br J Dermatol. 1987;116(3):379–386. doi: 10.1111/j.1365-2133.1987.tb05852.x. [DOI] [PubMed] [Google Scholar]
- 26.Cannistraci C, Lesnoni La Parola I, Falchi M, Picardo M. Treatment of generalized granuloma annulare with hydroxychloroquine. Dermatology. 2005;211(2):167–168. doi: 10.1159/000086452. [DOI] [PubMed] [Google Scholar]
- 27.Eisen D. Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol. 1993;28(4):609–612. doi: 10.1016/0190-9622(93)70082-5. [DOI] [PubMed] [Google Scholar]
- 28.Chung HS, Hann SK. Lupus panniculitis treated by a combination therapy of hydroxychloroquine and quinacrine. J Dermatol. 1997;24(9):569–572. doi: 10.1111/j.1346-8138.1997.tb02294.x. [DOI] [PubMed] [Google Scholar]
- 29.Jessop S, Whitelaw DA, Delamere FM (2009) Drugs for discoid lupus erythematosus. Cochrane Database Syst Rev. (4):CD002954 [DOI] [PubMed]
- 30.Rolain JM, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30(4):297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savarino A, Gennero L, Sperber K, Boelaert JR. The anti-HIV-1 activity of chloroquine. J Clin Virol. 2001;20(3):131–135. doi: 10.1016/S1386-6532(00)00139-6. [DOI] [PubMed] [Google Scholar]
- 33.Savarino A, Lucia MB, Rastrelli E, Rutella S, Golotta C, Morra E, et al. Anti-HIV effects of chloroquine: inhibition of viral particle glycosylation and synergism with protease inhibitors. J Acquir Immune Defic Syndr. 2004;35(3):223–232. doi: 10.1097/00126334-200403010-00002. [DOI] [PubMed] [Google Scholar]
- 34.Sperber K, Kalb TH, Stecher VJ, Banerjee R, Mayer L. Inhibition of human immunodeficiency virus type 1 replication by hydroxychloroquine in T cells and monocytes. AIDS Res Hum Retroviruses. 1993;9(1):91–98. doi: 10.1089/aid.1993.9.91. [DOI] [PubMed] [Google Scholar]
- 35.Paton NI, Aboulhab J. Hydroxychloroquine, hydroxyurea and didanosine as initial therapy for HIV-infected patients with low viral load: safety, efficacy and resistance profile after 144 weeks. HIV Med. 2005;6(1):13–20. doi: 10.1111/j.1468-1293.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 36.Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323(1):264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keyaerts E, Li S, Vijgen L, Rysman E, Verbeeck J, Van Ranst M, et al. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob Agents Chemother. 2009;53(8):3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blazar BR, Whitley CB, Kitabchi AE, Tsai MY, Santiago J, White N, et al. In vivo chloroquine-induced inhibition of insulin degradation in a diabetic patient with severe insulin resistance. Diabetes. 1984;33(12):1133–1137. doi: 10.2337/diabetes.33.12.1133. [DOI] [PubMed] [Google Scholar]
- 40.Smith GD, Amos TA, Mahler R, Peters TJ. Effect of chloroquine on insulin and glucose homoeostasis in normal subjects and patients with non-insulin-dependent diabetes mellitus. Br Med J (Clin Res Ed) 1987;294(6570):465–467. doi: 10.1136/bmj.294.6570.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quatraro A, Consoli G, Magno M, Caretta F, Nardozza A, Ceriello A, et al. Hydroxychloroquine in decompensated, treatment-refractory noninsulin-dependent diabetes mellitus. A new job for an old drug? Ann Intern Med. 1990;112(9):678–681. doi: 10.7326/0003-4819-112-9-678. [DOI] [PubMed] [Google Scholar]
- 42.Gerstein HC, Thorpe KE, Taylor DW, Haynes RB. The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas—a randomized trial. Diabetes Res Clin Pract. 2002;55(3):209–219. doi: 10.1016/S0168-8227(01)00325-4. [DOI] [PubMed] [Google Scholar]
- 43.Emami J, Pasutto FM, Mercer JR, Jamali F. Inhibition of insulin metabolism by hydroxychloroquine and its enantiomers in cytosolic fraction of liver homogenates from healthy and diabetic rats. Life Sci. 1999;64(5):325–335. doi: 10.1016/S0024-3205(98)00568-2. [DOI] [PubMed] [Google Scholar]
- 44.Powrie JK, Smith GD, Shojaee-Moradie F, Sonksen PH, Jones RH. Mode of action of chloroquine in patients with non-insulin-dependent diabetes mellitus. Am J Physiol. 1991;260(6 Pt 1):E897–E904. doi: 10.1152/ajpendo.1991.260.6.E897. [DOI] [PubMed] [Google Scholar]
- 45.Shojania K, Koehler BE, Elliott T. Hypoglycemia induced by hydroxychloroquine in a type II diabetic treated for polyarthritis. J Rheumatol. 1999;26(1):195–196. [PubMed] [Google Scholar]
- 46.Penn SK, Kao AH, Schott LL, Elliott JR, Toledo FG, Kuller L, et al. Hydroxychloroquine and glycemia in women with rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol. 2010;37(6):1136–1142. doi: 10.3899/jrheum.090994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wasko MC, Hubert HB, Lingala VB, Elliott JR, Luggen ME, Fries JF, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298(2):187–193. doi: 10.1001/jama.298.2.187. [DOI] [PubMed] [Google Scholar]
- 48.Rekedal LR, Massarotti E, Garg R, Bhatia R, Gleeson T, Lu B, et al. Changes in glycosylated hemoglobin after initiation of hydroxychloroquine or methotrexate treatment in diabetes patients with rheumatic diseases. Arthritis Rheum. 2010;62(12):3569–3573. doi: 10.1002/art.27703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallace DJ, Metzger AL, Stecher VJ, Turnbull BA, Kern PA. Cholesterol-lowering effect of hydroxychloroquine in patients with rheumatic disease: reversal of deleterious effects of steroids on lipids. Am J Med. 1990;89(3):322–326. doi: 10.1016/0002-9343(90)90345-E. [DOI] [PubMed] [Google Scholar]
- 50.Hodis HN, Quismorio FP, Jr, Wickham E, Blankenhorn DH. The lipid, lipoprotein, and apolipoprotein effects of hydroxychloroquine in patients with systemic lupus erythematosus. J Rheumatol. 1993;20(4):661–665. [PubMed] [Google Scholar]
- 51.Munro R, Morrison E, McDonald AG, Hunter JA, Madhok R, Capell HA. Effect of disease modifying agents on the lipid profiles of patients with rheumatoid arthritis. Ann Rheum Dis. 1997;56(6):374–377. doi: 10.1136/ard.56.6.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahman P, Gladman DD, Urowitz MB, Yuen K, Hallett D, Bruce IN. The cholesterol lowering effect of antimalarial drugs is enhanced in patients with lupus taking corticosteroid drugs. J Rheumatol. 1999;26(2):325–330. [PubMed] [Google Scholar]
- 53.Tam LS, Li EK, Lam CW, Tomlinson B. Hydroxychloroquine has no significant effect on lipids and apolipoproteins in Chinese systemic lupus erythematosus patients with mild or inactive disease. Lupus. 2000;9(6):413–416. doi: 10.1191/096120300678828541. [DOI] [PubMed] [Google Scholar]
- 54.Bengtsson C, Andersson SE, Edvinsson L, Edvinsson ML, Sturfelt G, Nived O. Effect of medication on microvascular vasodilatation in patients with systemic lupus erythematosus. Basic Clin Pharmacol Toxicol. 2010;107(6):919–924. doi: 10.1111/j.1742-7843.2010.00604.x. [DOI] [PubMed] [Google Scholar]
- 55.Jung H, Bobba R, Su J, Shariati-Sarabi Z, Gladman DD, Urowitz M, et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 2010;62(3):863–868. doi: 10.1002/art.27289. [DOI] [PubMed] [Google Scholar]
- 56.Tanay A, Leibovitz E, Frayman A, Zimlichman R, Shargorodsky M, Gavish D. Vascular elasticity of systemic lupus erythematosus patients is associated with steroids and hydroxychloroquine treatment. Ann NY Acad Sci. 2007;1108:24–34. doi: 10.1196/annals.1422.003. [DOI] [PubMed] [Google Scholar]
- 57.Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;144:174–177. [PubMed] [Google Scholar]
- 58.Nosal R, Jancinova V, Petrikova M. Chloroquine inhibits stimulated platelets at the arachidonic acid pathway. Thromb Res. 1995;77(6):531–542. doi: 10.1016/0049-3848(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 59.Petri M. Thrombosis and systemic lupus erythematosus: the Hopkins Lupus Cohort perspective. Scand J Rheumatol. 1996;25(4):191–193. doi: 10.3109/03009749609069986. [DOI] [PubMed] [Google Scholar]
- 60.Tektonidou MG, Laskari K, Panagiotakos DB, Moutsopoulos HM. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum. 2009;61(1):29–36. doi: 10.1002/art.24232. [DOI] [PubMed] [Google Scholar]
- 61.Belizna CC, Richard V, Thuillez C, Levesque H, Shoenfeld Y. Insights into atherosclerosis therapy in antiphospholipid syndrome. Autoimmun Rev. 2007;7(1):46–51. doi: 10.1016/j.autrev.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Erkan D, Yazici Y, Peterson MG, Sammaritano L, Lockshin MD. A cross-sectional study of clinical thrombotic risk factors and preventive treatments in antiphospholipid syndrome. Rheumatology (Oxford) 2002;41(8):924–929. doi: 10.1093/rheumatology/41.8.924. [DOI] [PubMed] [Google Scholar]
- 63.Edwards MH, Pierangeli S, Liu X, Barker JH, Anderson G, Harris EN. Hydroxychloroquine reverses thrombogenic properties of antiphospholipid antibodies in mice. Circulation. 1997;96(12):4380–4384. doi: 10.1161/01.cir.96.12.4380. [DOI] [PubMed] [Google Scholar]
- 64.Espinola RG, Pierangeli SS, Gharavi AE, Harris EN. Hydroxychloroquine reverses platelet activation induced by human IgG antiphospholipid antibodies. Thromb Haemost. 2002;87(3):518–522. [PubMed] [Google Scholar]
- 65.Rand JH, Wu XX, Quinn AS, Ashton AW, Chen PP, Hathcock JJ, et al. Hydroxychloroquine protects the annexin A5 anticoagulant shield from disruption by antiphospholipid antibodies: evidence for a novel effect for an old antimalarial drug. Blood. 2010;115(11):2292–2299. doi: 10.1182/blood-2009-04-213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rand JH, Wu XX, Quinn AS, Chen PP, Hathcock JJ, Taatjes DJ. Hydroxychloroquine directly reduces the binding of antiphospholipid antibody-beta2-glycoprotein I complexes to phospholipid bilayers. Blood. 2008;112(5):1687–1695. doi: 10.1182/blood-2008-03-144204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geser A, Brubaker G, Draper CC. Effect of a malaria suppression program on the incidence of African Burkitt’s lymphoma. Am J Epidemiol. 1989;129(4):740–752. doi: 10.1093/oxfordjournals.aje.a115189. [DOI] [PubMed] [Google Scholar]
- 68.Maclean KH, Dorsey FC, Cleveland JL, Kastan MB. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. J Clin Invest. 2008;118(1):79–88. doi: 10.1172/JCI33700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lagneaux L, Delforge A, Carlier S, Massy M, Bernier M, Bron D. Early induction of apoptosis in B-chronic lymphocytic leukaemia cells by hydroxychloroquine: activation of caspase-3 and no protection by survival factors. Br J Haematol. 2001;112(2):344–352. doi: 10.1046/j.1365-2141.2001.02553.x. [DOI] [PubMed] [Google Scholar]
- 70.Lagneaux L, Delforge A, Dejeneffe M, Massy M, Bernier M, Bron D. Hydroxychloroquine-induced apoptosis of chronic lymphocytic leukemia involves activation of caspase-3 and modulation of Bcl-2/bax/ratio. Leuk Lymphoma. 2002;43(5):1087–1095. doi: 10.1080/10428190290021506. [DOI] [PubMed] [Google Scholar]
- 71.Mansilla E, Marin GH, Nunez L, Drago H, Sturla F, Mertz C, et al. The lysosomotropic agent, hydroxychloroquine, delivered in a biodegradable nanoparticle system, overcomes drug resistance of B-chronic lymphocytic leukemia cells in vitro. Cancer Biother Radiopharm. 2010;25(1):97–103. doi: 10.1089/cbr.2009.0655. [DOI] [PubMed] [Google Scholar]
- 72.Jiang PD, Zhao YL, Shi W, Deng XQ, Xie G, Mao YQ, et al. Cell growth inhibition, G2/M cell cycle arrest, and apoptosis induced by chloroquine in human breast cancer cell line Bcap-37. Cell Physiol Biochem. 2008;22(5–6):431–440. doi: 10.1159/000185488. [DOI] [PubMed] [Google Scholar]
- 73.Rahim R, Strobl JS. Hydroxychloroquine, chloroquine, and all-trans retinoic acid regulate growth, survival, and histone acetylation in breast cancer cells. Anticancer Drugs. 2009;20(8):736–745. doi: 10.1097/CAD.0b013e32832f4e50. [DOI] [PubMed] [Google Scholar]
- 74.Zhou Q, McCracken MA, Strobl JS. Control of mammary tumor cell growth in vitro by novel cell differentiation and apoptosis agents. Breast Cancer Res Treat. 2002;75(2):107–117. doi: 10.1023/A:1019698807564. [DOI] [PubMed] [Google Scholar]
- 75.Zheng Y, Zhao YL, Deng X, Yang S, Mao Y, Li Z, et al. Chloroquine inhibits colon cancer cell growth in vitro and tumor growth in vivo via induction of apoptosis. Cancer Invest. 2009;27(3):286–292. doi: 10.1080/07357900802427927. [DOI] [PubMed] [Google Scholar]
- 76.Sasaki K, Tsuno NH, Sunami E, Tsurita G, Kawai K, Okaji Y, et al. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer. 2010;10:370. doi: 10.1186/1471-2407-10-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan C, Wang W, Zhao B, Zhang S, Miao J. Chloroquine inhibits cell growth and induces cell death in A549 lung cancer cells. Bioorg Med Chem. 2006;14(9):3218–3222. doi: 10.1016/j.bmc.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 78.Sotelo J, Briceno E, Lopez-Gonzalez MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144(5):337–343. doi: 10.7326/0003-4819-144-5-200603070-00008. [DOI] [PubMed] [Google Scholar]
- 79.Hu C, Solomon VR, Ulibarri G, Lee H. The efficacy and selectivity of tumor cell killing by Akt inhibitors are substantially increased by chloroquine. Bioorg Med Chem. 2008;16(17):7888–7893. doi: 10.1016/j.bmc.2008.07.076. [DOI] [PubMed] [Google Scholar]
- 80.Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol. 2009;625(1–3):220–233. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 81.Schultz KR, Gilman AL. The lysosomotropic amines, chloroquine and hydroxychloroquine: a potentially novel therapy for graft-versus-host disease. Leuk Lymphoma. 1997;24(3–4):201–210. doi: 10.3109/10428199709039008. [DOI] [PubMed] [Google Scholar]
- 82.Khoury H, Trinkaus K, Zhang MJ, Adkins D, Brown R, Vij R, et al. Hydroxychloroquine for the prevention of acute graft-versus-host disease after unrelated donor transplantation. Biol Blood Marrow Transplant. 2003;9(11):714–721. doi: 10.1016/j.bbmt.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 83.Fong T, Trinkaus K, Adkins D, Vij R, Devine SM, Tomasson M, et al. A randomized double-blind trial of hydroxychloroquine for the prevention of chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(10):1201–1206. doi: 10.1016/j.bbmt.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 84.Chen PH, Huang YF, Tang CW, Wann SR, Chang HT. Kikuchi–Fujimoto disease: an amazing response to hydroxychloroquine. Eur J Pediatr. 2010;169(12):1557–1559. doi: 10.1007/s00431-010-1256-x. [DOI] [PubMed] [Google Scholar]
- 85.Rezai K, Kuchipudi S, Chundi V, Ariga R, Loew J, Sha BE. Kikuchi–Fujimoto disease: hydroxychloroquine as a treatment. Clin Infect Dis. 2004;39(12):e124–e126. doi: 10.1086/426144. [DOI] [PubMed] [Google Scholar]
- 86.DeSimone DP, Brilliant HL, Basile J, Bell NH. Granulomatous infiltration of the talus and abnormal vitamin D and calcium metabolism in a patient with sarcoidosis: successful treatment with hydroxychloroquine. Am J Med. 1989;87(6):694–696. doi: 10.1016/S0002-9343(89)80409-7. [DOI] [PubMed] [Google Scholar]
- 87.O’Leary TJ, Jones G, Yip A, Lohnes D, Cohanim M, Yendt ER. The effects of chloroquine on serum 1, 25-dihydroxyvitamin D and calcium metabolism in sarcoidosis. N Engl J Med. 1986;315(12):727–730. doi: 10.1056/NEJM198609183151203. [DOI] [PubMed] [Google Scholar]
- 88.Shimoni A, Hershcovici T, Mekhmandarov S, Maor-Kendler Y, Beigel Y. Skeletal sarcoidosis: successful treatment with hydroxychloroquine. Isr Med Assoc J. 2000;2(7):558–559. [PubMed] [Google Scholar]
- 89.Hirshoren N, Eliashar R, Weinberger JM. Hydroxychloroquine for subglottic stenosis: a novel therapy in the battle for air. Laryngoscope. 2010;120(4):743–744. doi: 10.1002/lary.20848. [DOI] [PubMed] [Google Scholar]
- 90.Hussain A, Scelsa SN. Sensory neuronopathy with Ro antibodies: response to combination immunosuppression. J Clin Neuromuscul Dis. 2010;11(3):120–123. doi: 10.1097/CND.0b013e3181d05980. [DOI] [PubMed] [Google Scholar]
- 91.Izmirly PM CP, Kim MY DF, Llanos C, C-C N, et al. Hydroxychloroquine and prevention of anti-SSA/Ro associated cardiac disease in mothers with a previous child with Neonatal Lupus. Arthritis Rheum. 2010;62(10):S306. [Google Scholar]
- 92.Izmirly PM, Kim MY, Llanos C, Le PU, Guerra MM, Askanase AD, et al. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann Rheum Dis. 2010 doi: 10.1136/ard.2009.119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iyamu E, Perdew H, Woods G. Growth inhibitory and differentiation effects of chloroquine and its analogue on human leukemic cells potentiate fetal hemoglobin production by targeting the polyamine pathway. Biochem Pharmacol. 2009;77(6):1021–1028. doi: 10.1016/j.bcp.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 94.Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf. 2004;27(1):25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- 95.Hochstein P. Glucose-6-phosphate dehydrogenase deficiency: mechanisms of drug-induced hemolysis. Exp Eye Res. 1971;11(3):389–395. doi: 10.1016/S0014-4835(71)80051-9. [DOI] [PubMed] [Google Scholar]
- 96.Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(2):189–194. doi: 10.1067/moe.2000.106340. [DOI] [PubMed] [Google Scholar]
- 97.Bortoli R, Santiago M. Chloroquine ototoxicity. Clin Rheumatol. 2007;26(11):1809–1810. doi: 10.1007/s10067-007-0662-6. [DOI] [PubMed] [Google Scholar]
- 98.Tehrani R, Ostrowski RA, Hariman R, Jay WM. Ocular toxicity of hydroxychloroquine. Semin Ophthalmol. 2008;23(3):201–209. doi: 10.1080/08820530802049962. [DOI] [PubMed] [Google Scholar]
- 99.Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010;62(6):775–784. doi: 10.1002/acr.20133. [DOI] [PubMed] [Google Scholar]
- 100.Costedoat-Chalumeau N, Hulot JS, Amoura Z, Delcourt A, Maisonobe T, Dorent R, et al. Cardiomyopathy related to antimalarial therapy with illustrative case report. Cardiology. 2007;107(2):73–80. doi: 10.1159/000094079. [DOI] [PubMed] [Google Scholar]
- 101.Keating RJ, Bhatia S, Amin S, Williams A, Sinak LJ, Edwards WD. Hydroxychloroquine-induced cardiotoxicity in a 39-year-old woman with systemic lupus erythematosus and systolic dysfunction. J Am Soc Echocardiogr. 2005;18(9):981. doi: 10.1016/j.echo.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 102.Kwon JB, Kleiner A, Ishida K, Godown J, Ciafaloni E, Looney RJ., Jr Hydroxychloroquine-induced myopathy. J Clin Rheumatol. 2010;16(1):28–31. doi: 10.1097/RHU.0b013e3181c47ec8. [DOI] [PubMed] [Google Scholar]
- 103.Siddiqui AK, Huberfeld SI, Weidenheim KM, Einberg KR, Efferen LS. Hydroxychloroquine-induced toxic myopathy causing respiratory failure. Chest. 2007;131(2):588–590. doi: 10.1378/chest.06-1146. [DOI] [PubMed] [Google Scholar]
- 104.Estes ML, Ewing-Wilson D, Chou SM, Mitsumoto H, Hanson M, Shirey E, et al. Chloroquine neuromyotoxicity. Clinical and pathologic perspective. Am J Med. 1987;82(3):447–455. doi: 10.1016/0002-9343(87)90444-X. [DOI] [PubMed] [Google Scholar]
- 105.Costedoat-Chalumeau N, Amoura Z, Aymard G, Le TH, Wechsler B, Vauthier D, et al. Evidence of transplacental passage of hydroxychloroquine in humans. Arthritis Rheum. 2002;46(4):1123–1124. doi: 10.1002/art.10150. [DOI] [PubMed] [Google Scholar]
- 106.Clowse ME, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 2006;54(11):3640–3647. doi: 10.1002/art.22159. [DOI] [PubMed] [Google Scholar]
- 107.Levy M, Buskila D, Gladman DD, Urowitz MB, Koren G. Pregnancy outcome following first trimester exposure to chloroquine. Am J Perinatol. 1991;8(3):174–178. doi: 10.1055/s-2007-999371. [DOI] [PubMed] [Google Scholar]
- 108.Klinger G, Morad Y, Westall CA, Laskin C, Spitzer KA, Koren G, et al. Ocular toxicity and antenatal exposure to chloroquine or hydroxychloroquine for rheumatic diseases. Lancet. 2001;358(9284):813–814. doi: 10.1016/S0140-6736(01)06004-4. [DOI] [PubMed] [Google Scholar]
- 109.Motta M, Tincani A, Faden D, Zinzini E, Lojacono A, Marchesi A, et al. Follow-up of infants exposed to hydroxychloroquine given to mothers during pregnancy and lactation. J Perinatol. 2005;25(2):86–89. doi: 10.1038/sj.jp.7211208. [DOI] [PubMed] [Google Scholar]
- 110.Costedoat-Chalumeau N, Amoura Z, Huong DL, Lechat P, Piette JC. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases. Review of the literature. Autoimmun Rev. 2005;4(2):111–115. doi: 10.1016/j.autrev.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 111.cdc.gov [online] [database on the Internet]. Available from: http://www.cdc.gov/malaria/resources/pdf/treatmenttable73109.pdf. Accessed 22 Nov 2010


