Abstract
Excitotoxicity due to the excessive activation of glutamatergic receptors leads to neuronal dysfunction and death. Excitotoxicity has been implicated in the pathogenesis of a myriad of neurodegenerative diseases with distinct etiologies such as Alzheimer’s and Parkinson’s. Numerous studies link apolipoprotein D (apoD), a secreted glycoprotein highly expressed in the central nervous system (CNS), to maintain and protect neurons in various mouse models of acute stress and neurodegeneration. Here, we used a mouse model overexpressing human apoD in neurons (H-apoD Tg) to test the neuroprotective effects of apoD in the kainic acid (KA)-lesioned hippocampus. Our results show that apoD overexpression in H-apoD Tg mice induces an increased resistance to KA-induced seizures, significantly attenuates inflammatory responses and confers protection against KA-induced cell apoptosis in the hippocampus. The apoD-mediated protection against KA-induced toxicity is imputable in part to increased plasma membrane Ca2+ ATPase type 2 expression (1.7-fold), decreased N-methyl-d-aspartate receptor (NMDAR) subunit NR2B levels (30 %) and lipid metabolism alterations. Indeed, we demonstrate that apoD can attenuate intracellular cholesterol content in primary hippocampal neurons and in brain of H-apoD Tg mice. In addition, apoD can be internalised by neurons and this internalisation is accentuated in ageing and injury conditions. Our results provide additional mechanistic information on the apoD-mediated neuroprotection in neurodegenerative conditions.
Keywords: Apolipoprotein D (apoD), Kainic acid, Seizure, Apoptosis, Excitotoxicity, Cholesterol metabolism, Inflammation, Calcium, Neurodegenerative diseases
Introduction
Apolipoprotein D (apoD) is a secreted glycoprotein and a member of the lipocalin family of proteins, which bind and transport small lipophilic molecules [1]. In humans, this atypical apolipoprotein is highly expressed in several tissues including the central nervous system (CNS), unlike other apolipoproteins which are essentially produced in liver and intestine [1–3]. In rodents, apoD expression is found primarily in the CNS. In the brain, high apoD expression is observed in the hippocampus, the prefrontal cortex and the substantia nigra [4], mainly in glial cells (astrocytes and oligodendrocytes) but also in a few neurons [5–8].
The upregulation of apoD in the ageing brain [9] as well as in multiple neurological conditions, including Alzheimer’s [10], Parkinson’s [11], Niemann-Pick’s type C (NPC) [12] diseases, multiple sclerosis [13] and nervous system injuries including stroke [14–16] raises the possibility that apoD plays a prominent role in neuronal maintenance and protection against injury.
Supporting this hypothesis, studies revealed that overexpression of apoD (or its orthologs) in transgenic mice, Drosophila and Arabidopsis lead to increased resistance to oxidative stress [17–20] and inflammation [21]. Conversely, apoD inactivation in mice, plants and flies resulted in decreased resistance and ultimately reduced survival in response to oxidative stress [17, 18, 22]. It was further established that apoD specifically prevents lipid peroxidation but not protein carbonylation in response to oxidative insults [17]. This anti-oxidant effect of apoD on lipids is mediated through a highly conserved methionine residue (Met93), converting reactive to non-reactive lipid hydroxides [23, 24]. ApoD may also influence inflammatory pathways via the regulation of arachidonic acid (AA) signalling and metabolism [21]. Indeed, it was suggested that apoD could stabilise AA into the cell membrane or sequester AA, reducing the availability of free AA and thus preventing its conversion into pro-inflammatory molecules [25, 26]. Another way for apoD to prevent neurotoxicity lies in its capacity to bind several ligands involved in neurological injuries. ApoD can bind AA, progesterone and sphingomyelin, with high affinity, as well as cholesterol, bilirubin and estradiol, with lower affinity.
Despite increasing evidence indicating that apoD accumulation in neuropathological conditions is potentially protective, little is known on the response of apoD to excitotoxic insults and the pathways involved. Earlier work demonstrated increased apoD levels in the hippocampus of kainic acid-treated rats [27, 28]. Kainic acid (KA), an analogue of glutamate, can induce the overactivation of glutamate receptors and is widely used as a model to explore excitotoxic processes in neurodegenerative injury [29]. Because of the abundance of kainate receptors, the hippocampus is more sensitive to KA-induced neurotoxicity [30]. Also, glutamatergic pathways seem to be particularly affected in apoD knockout mice with a 20 % decrease of kainate receptors in the CA 2–3 subfields of the hippocampus, a global decrease in AMPA receptors and a global increase in muscarinic M2/M4 receptors [31]. These changes may potentially contribute to the impairments in learning and memory and in motor- and orientation-based tasks observed in these animals [17], all of which involve glutamatergic neurotransmission.
Therefore, using transgenic mice overexpressing human apoD in their neurons, we sought to better understand the role of apoD in excitotoxic injury induced by KA treatment. In the present study, we demonstrate that apoD overexpression in neurons protects mice against KA-induced seizures and cellular apoptosis. In addition, we show that apoD protection is conferred not only through its anti-inflammatory properties but also through the regulation of cholesterol distribution in neurons, and by affecting the levels of proteins known for their involvement in limiting excitotoxic effects.
Materials and Methods
Mice
Human apoD transgenic (H-apoD Tg) mice express the human apoD (H-apoD) cDNA under the control of the Thy-1.2 promoter, as previously described [17, 21]. In H-apoD Tg mice, H-apoD is expressed in neuronal cells in all regions of the nervous system [21]. Because the FVB/N mouse strain is more vulnerable to KA lesions [32], all mice were backcrossed into the FVB/N background (Charles River, Canada) for at least ten generations. Genotyping was performed by PCR on tail biopsies as described previously [17]. In all experiments, 12–16 weeks old male wild-type (WT) and H-apoD Tg littermates were used. The animals were housed under standard conditions at constant temperature (20 to 22 °C) and humidity (50 to 60 %), under a 12-h light/dark cycle and had free availability to water and food. All mice were euthanized by CO2 asphyxiation after being anesthetised with Isoflurane (PPC, Richmond Hill, ON, Canada). The experimental procedures were approved by the Animal Care and Use Committee of Université du Québec à Montréal.
Kainate Administration and Assessment of Behavioural Seizures
KA (15 mg/kg, prepared in phosphate-buffered saline (PBS); Sigma-Aldrich, St Louis, MO) or vehicle (PBS) were injected intraperitoneally (i.p.) in WT and H-apoD Tg mice. Results of preliminary dose–response experiments revealed consistent seizures with a mortality of less than 10 % in animals injected with a KA dose of 15 mg/kg. This dose was used in all following experiments. For behavioural assessment, animals (17 mice for each group) were monitored every 5 min for behavioural seizures during 2 h, and the seizure intensity were blind-scored following a modified Racine’s classification [33]: ‘stage 0’, no seizure and normal behaviour; ‘stage 1’, immobilisation; ‘stage 2’, myoclonic jerk and head nodding; ‘stage 3’, bilateral forelimb clonus and rearing; ‘stage 4’, continuous rearing and falling; ‘stage 5’, loss of posture; and ‘stage 6’, running and jumping seizure. For each mouse, the sum of all seizure intensity was also calculated by adding the seizure scores observed in all 5 min intervals during 2 h following KA injection. The latency to stages 2, 3 and 4 seizures was also monitored. Animals that did not present these ‘stage’ seizures were given a value of 120 min.
Protein Extraction and Western Blot Analysis
Non-injected (4–5 WT and 4 H-apoD Tg mice) and 1, 3 or 7 days post-KA injection (4–5 WT and H-apoD Tg mice) animals were euthanized, and hippocampi were collected, frozen in dry ice and conserved at −80 °C until protein extraction. Hippocampi were then homogenised in cold lysis buffer (50 mM Tris–HCl, pH 7.3, 150 mM NaCl, 5 mM EDTA, 0,2 % (v/v) Triton X-100 complemented with 10 % (w/v) complete protease inhibitor (Roche Molecular Diagnostics, Mannheim, Germany). Homogenates were incubated 30 min at 4 °C, sonicated and cleared by centrifugation. Protein concentration was assessed by the Bio-Rad protein assay (Bio-Rad Laboratories, Mississauga, Canada). Proteins (30 mg) were separated on 12 % (w/v) SDS-polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Ontario, Canada). To analyse N-methyl-d-aspartate receptor (NMDAR) subunits and plasma membrane calcium ATPases (PMCA) expression, proteins were separated on 8 % (w/v) SDS-polyacrylamide gels. Membranes were incubated with primary antibodies/human apoD mouse monoclonal antibody (2B9), 1:10,000 [10]; mouse apoD goat polyclonal antibody (Santa Cruz biotechnology, Dallas, USA), 1:1000; GFAP rabbit polyclonal antibody (Cell Signalling, Whitby, Canada), 1:10,000; Mac-2 rat monoclonal antibody (ATCC, Cedarlane, Burlington, Canada), 1:1000; COX-2 rabbit polyclonal antibody (Cell Signaling), 1:1000; synaptophysin rabbit polyclonal antibody (Abcam, Toronto, Canada), 1:10,000, NMDAR1 rabbit monoclonal antibody (Cell Signaling), 1:1000; NMDAR2A rabbit polyclonal antibody (Cell Signaling), 1:1000; NMDAR2B rabbit monoclonal antibody (Cell Signaling), 1:1000; PMCA2 rabbit monoclonal antibody (Sigma-Aldrich), 1:1000; and β-actin mouse monoclonal antibody (Sigma-Aldrich), 1:10,000. Then, membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies and visualised by chemiluminescence (ECL, GE Healthcare, Quebec, Canada) using a Fusion FX7 system (Vilber Lourmat, France).
TUNEL Assay
To detect apoptosis-induced nuclear DNA fragmentation, we used the ApoAlert DNA Fragmentation Assay kit (Clontech Laboratories, Montain View, CA), a fluorescence terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end-labelling (TUNEL)-based assay. Briefly, 3 days post-KA injection, brains of WT (n = 4) and H-apoD Tg mice (n = 4) were dissected and placed in 4 % paraformaldehyde (PFA) at 4 °C for 24 h. The fixed brains were then embedded in OCT freezing medium (Fisher Scientific, Ottawa, Canada). Eight micromoles of coronal sections were cut using a cryostat (Leica, Ontario, Canada) and mounted onto silane-coated slides. The fixed sections were then permeabilized with proteinase K at room temperature for 10 min before being incubated with equilibrium buffer for 10 min at room temperature. The sections were then incubated with equilibrium buffer containing nucleotide mix and TdT Terminal Transferase at 37 °C for 60 min. The reaction was stopped with 2× SSC, and the sections were washed with PBS. The sections were stained with propidium iodide (PI; 2.5 μg/mL, Sigma-Aldrich) for nuclei labelling. TUNEL-positive nuclei were visualised by fluorescence microscopy. Mean pixel TUNEL and PI fluorescence intensity were measured in hippocampus using ImageJ software.
Subcellular Fractionation of Brain Tissue
Whole mouse brains of four non-injected WT and H-apoD Tg mice were homogenised in 10 vol. of cold homogenisation buffer (0.32 M sucrose, 10 mM HEPES, pH 7.4, 2 mM EDTA and 10 % (w/v) complete protease inhibitor (Roche)) using a motorised glass-Teflon homogeniser. The homogenates (H) were centrifuged at 1000×g for 15 min. The nuclear pellets (P1) were removed and the S1 supernatants were centrifuged at 10,000×g for 30 min. Supernatants (S2, cytosol fraction) were separated from pellets (P2). To remove any cytosol contaminations, the pellets P2 were resuspended in homogenisation buffer and centrifuged again at 100,000×g for 30 min. The supernatants (S’2) were removed and the pellets (P’2, membrane fractions) were resuspended in homogenisation buffer.
Cholesterol Quantification
Cholesterol levels were assessed using the Amplex Red cholesterol assay (Life Technologies) in whole brain homogenates (H), membrane (P’2) and cytosol (S2) fractions of mouse brain. Samples, diluted in reaction buffer, were mixed with an equivalent volume of Amplex Red working solution containing 300 μM Amplex Red reagent, 2 U/mL cholesterol oxidase, 0.2 U/mL cholesterol esterase and 2 U/mL horseradish peroxidase (HRP). After incubation at 37 °C for 30 min, absorbance was measured at 568 nm using fluorescence spectroscopy (Tecan Infinite M1000, Tecan US, NC, USA). Cholesterol levels were normalised by protein concentration.
24(S)-Hydroxycholesterol Quantification
Brain and plasma 24(S)-hydroxycholesterol (24-HOC) levels were assessed using a 24(S)-hydroxycholesterol ELISA kit (Enzo Life Sciences, Farmingdale, NY, USA). Brains (100 mg) of four non-injected WT and H-apoD Tg mice were homogenised in ethanol 95 % (1 mL). After centrifugation, the pellets were resuspended in 1 mL of ethanol/dichloromethane (1:1, v/v) and then re-centrifuged. The supernatants (obtained after the two centrifugations) were combined and evaporated to dryness with a rotary evaporator. The samples were then rehydrated at room temperature with ethanol 95 % (16 μL) and assay buffer (484 μL). After diluting samples in assay buffer, 24-HOC quantification in plasma (1:1000) and brain homogenates (1:100) was performed according to manufacturer’s instructions.
Hippocampal Culture Preparation
WT (FVB/N stain) mouse embryos, at 18 days of gestation (from four WT females), were used to prepare primary hippocampal neuronal cultures as previously described [34]. Briefly, hippocampi were dissected in HBSS (without Ca2+ and Mg2+) supplemented with 1 mM sodium pyruvate and 10 mM HEPES and dissociated by trituration with a fire-polished Pasteur pipette. Supernatants were then centrifuged for 1 min at 1000×g. The pellets were resuspended in HBSS. The cell suspensions were plated at a density of 5 × 105/cm2 in neurobasal medium (Invitrogen) supplemented with 0.5 mM l-glutamine and B27 supplement (Invitrogen) and grown on glass coverlips pretreated with poly-d-lysine (Sigma-Aldrich).
Filipin Staining and GAP-43 Immunohistochemistry
Hippocampal primary neurons (10 days) were incubated with purified H-apoD (250 ng/mL; purified from breast cyst fluid) for 24 h. For KA toxicity, neurons, pre-incubated with H-apoD for 24 h, were incubated with or without KA (100 μM) for 3 h. Staining of intracellular cholesterol was achieved using filipin, a fluorescent polyene antibiotic with high affinity for cholesterol [35]. Hippocampal neurons were fixed in 4 % paraformaldehyde (PFA) in PBS containing 2 % sucrose for 15 min, washed with PBS and permeabilized with 0.1 % Triton X-100. The cells were incubated with filipin (125 μg/mL; Sigma-Aldrich) in PBS for 2 h at room temperature. Filipin-cholesterol complexes were visualised by confocal microscopy. For GAP-43 (neuronal marker) immunohistochemistry, neurons were blocked in PBS with 10 % goat serum, 10 % bovin serum albumin (BSA), and 0.1 % triton for 1 h and incubated overnight at 4 °C in humid atmosphere with a primary antibody against GAP-43 (rabbit polyclonal antibody, 1:250, Abcam). After washing in PBS (3× for 10 min), the cells were then exposed to a goat anti-rabbit IgG-Alexa 568 (1:1000; Life Technologies) at room temperature for 1 h, washed and mounted on slide with Prolong Gold antifade (Life Technologies) Gap-43 immunoreactivity was visualised by confocal microscopy.
Cholesterol Treatment
Hippocampal primary neurons (10 days) were pre-incubated for 3 h with water-soluble cholesterol (10 μg/mL; Sigma). The cells were then exposed with or without purified H-apoD (250 ng/mL) for 24 h. Cholesterol uptake was visualised by filipin staining using confocal microscopy. Mean pixel filipin fluorescence intensity was measured in the soma of cells using image J software.
ApoD Internalisation
Purified H-apoD uptake was assessed in hippocampal neurons in ageing and KA-induced excitotoxic conditions. For KA toxicity, hippocampal neurons (at 10 days) were pre-incubated with or without purified H-apoD (250 ng/mL) for 24 h. Then, cells were exposed to KA (100 μM) for 24 h. For ageing conditions, hippocampal neurons were cultured for 28 days and then incubated with purified H-apoD (250 ng/mL) for 24 h.
KA-treated and untreated neurons were fixed in 4 % PFA and processed for immunohistochemistry using an antibody against H-apoD (1:100; mouse monoclonal antibody, 2B9) and a goat anti-mouse IgG-Alexa 488 (1:1000; Life Technologies) as described here above. H-apoD and DAPI fluorescent labellings were visualised by confocal microscopy.
Statistical Analyses
Statistical differences were determined using one-way ANOVAs and two-way ANOVAs followed by Bonferroni post hoc tests and unpaired t test, as indicated in the figure legends. Statistical analyses were performed with GraphPadPrism Software for statistical differences. p < 0.05 was defined as the significance threshold. All data are presented as mean ± SEM.
Results
Endogenous M-apoD Induction in the KA-Lesioned Mouse Hippocampus Coincides with Astrocytic Activation
KA-induced apoD expression has previously been reported in the rat hippocampus [27, 28]. To investigate the time-course of endogenous apoD expression in the mouse hippocampus following KA treatment, WT mice were injected with KA (15 mg/kg; i.p.) and the hippocampus was processed for the detection of apoD protein at days 1, 3 and 7 post-injection. In parallel, the expression of the classic reactive astrocyte marker GFAP, known as a neurotoxicity marker after brain injury [36], was also assessed [37]. Endogenous apoD protein started to accumulate 1 day following KA injection and peaked at day 3 (2-fold induction) compared with non-injected mice (NI) (Fig. 1a). Hippocampal apoD expression returned to baseline levels 7 days post-injection. In a similar manner, the relative hippocampal expression of GFAP protein (Fig. 1b) began to increase on day 1 post-injection, with its highest expression at day 3 (3-fold induction), corresponding to the neurotoxicity peak. GFAP levels, as for apoD, were lower at day 7 although still significantly higher than in non-injected animals. Overall, these data show that KA treatment induces apoD accumulation in the hippocampus of mice, a phenomenon which is concomitant with astrocyte activation.
Fig. 1.
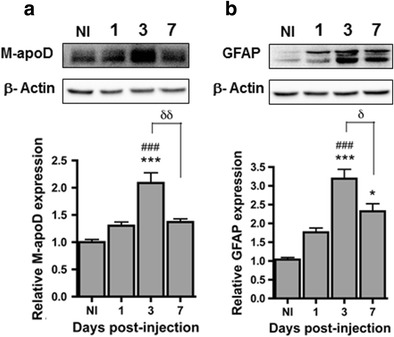
Correlation between M-apoD protein expression and astrogliosis after KA treatment. Endogenous apoD (M-apoD) (a) and astrocyte activation (b) GFAP protein expression were analysed by western blot of hippocampal homogenates from non-injected (NI) and WT mice injected with kainic acid (15 mg/kg) 1, 3 and 7 days post-injection. Values were normalised by β-actin protein expression: the ratio in non-injected mice was given an arbitrary value of 1. Normalised values are presented as mean ± SEM (n = 3 to 5 animals for each group). One-way ANOVA followed by Bonferroni post-test: *p < 0.05; ***p < 0.001 compared with non-injected mice; ### p < 0.001 compared with 1 day post-injected mice; δ p < 0.05; δδ p < 0.01 compared with 3 days post-injected mice
Since endogenous M-apoD appears upregulated after KA insult and as H-apoD overexpression in neurons has demonstrated protective effects against oxidative stress [17, 20], encephalitis [21] and nerve damage [38], we next aimed at evaluating a potential protective role for apoD against KA-induced excitotoxicity.
H-apoD Tg Mice Are More Resistant to KA-Induced Seizures
KA affects the limbic structure and induces epileptic discharges [39]. To investigate the effect of H-apoD overexpression on the generation of KA-induced seizures, WT and H-apoD Tg mice were injected with KA and their behaviour was recorded for 2 h post-injection. Seizure behaviours were assessed according to two scoring systems: the seizure index and the cumulative score. KA injection induced seizures of significantly lower severity in H-apoD Tg mice compared with WT animals (Fig. 2a, left axis). In addition, the sum of all seizure scores (cumulative score) that mice experienced over that time was 2-fold lower in H-apoD Tg mice (26.3 ± 3.06) compared with WT animals (50.12 ± 4.12; Fig. 2a, right axis). We also assessed the latency to reach stage 2 (myoclonic jerks of the head), stage 3 (bilateral forelimb clonus and rearing rearing) and stage 4 (continuous rearing and falling) seizures. If the animal did not present these ‘stage’ seizures, the latency was considered as 120 min. Compared with WT mice, the latency to seizure onset was significantly higher in H-apoD Tg mice (Fig. 2b). This onset was delayed by 34 ± 14.5 min for stage 4. In stage 3 occurrence, this difference was further emphasised. Indeed, the onset of stage 3 seizures was observed 57 ± 13 min later in H-apoD Tg mice than in WT animals. No significant difference was observed for the latency to seizure stage 2 between WT and H-apoD Tg mice. These results suggest that H-apoD overexpression in transgenic mice induces an increased resistance to KA-induced seizures. We thus explored the mechanisms involved in such protection.
Fig. 2.
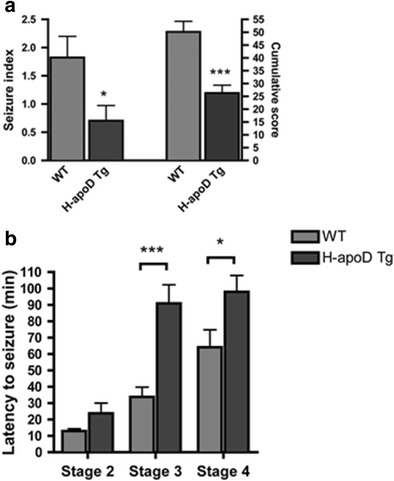
H-apoD Tg mice are more resistant to KA-induced seizures. a Seizure index was evaluated for 2 h, according to the following arbitrary scale [95]: 0, no seizure; 1, one severe seizure (class 4–5); 2, three to five severe seizures; 3, six to ten severe seizures; and 4, more than ten severe seizures or very severe (class 6) seizures, in WT and H-apoD Tg mice injected with kainic acid (15 mg/kg). Cumulative seizure score was assessed as the sum of the scores recorded each 5 min over a 2-h period after KA injection in WT and H-apoD Tg mice. Mann–Whitney U test: *p < 0.05; ***p < 0.001 compared with WT. b The latency to ‘stage 2’, ‘stage 3’ and ‘stage 4’ seizures was measured in WT (grey bars) and H-apoD Tg (dark grey bars) mice. Animals that did not present these ‘stage’ seizures were given a value of 120 min. Two-way ANOVA followed by Bonferroni post-test: *p < 0.05; ***p < 0.001 compared with WT. Values are means ± SEM of 17 animals per group
Transgenic Mice Expressing H-apoD Are More Resistant to KA-Induced Apoptosis
Because H-apoD Tg mice were more resistant to KA-induced seizures, we investigated whether apoD overexpression attenuated the pathological consequences of KA-excitotoxicity at the cellular level. KA-induced cell apoptosis, a consequence of seizures, was assessed in hippocampal sagittal sections from WT and H-apoD Tg mice using TUNEL labelling, at day 3 post-injection (corresponding to the astrogliosis and apoD peaks) (Fig. 3). In WT mice, TUNEL labelling was present in different hippocampal subfields (CA1, CA3, hilus of the dentate gyrus) (Fig. 3c). In contrast, in the hippocampus of H-apoD Tg mice, TUNEL staining was decreased in the different subfields (Fig. 3d). Moreover, at day 3 post-KA injection, the overall level of cell damage, quantified as total TUNEL fluorescence in the H-apoD Tg hippocampus was significantly reduced compared with WT mice (Fig. 3e). These results indicate that H-apoD expression confers protection against KA-induced cell apoptosis.
Fig. 3.
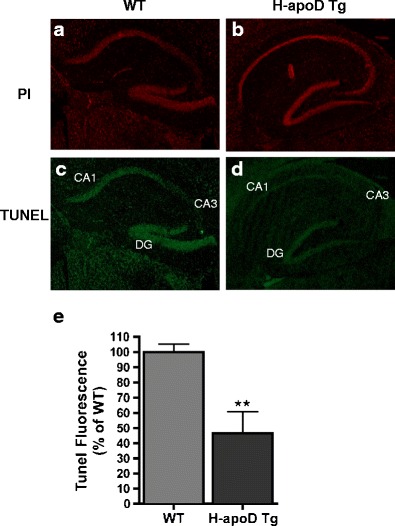
H-apoD Tg mice are more resistant to KA-induced apoptosis. Representative images of nuclear (PI, red) (a, b) and TUNEL staining (green) (c, d) of coronal sections (dorsal hippocampus is shown) from WT (a, c) and H-apoD Tg (b, d) mice, 3 days post-KA-injection. e Quantification of apoptotic cells in the hippocampus of KA-treated mice. The number of TUNEL-positive cells was divided by the number of total cells (PI-positive nuclei) and normalised by the WT, which was given an arbitrary value of 100 %. Normalised values are presented as mean (four animals per group) ± SEM. Unpaired t test: **p = 0.0063 compared with WT
H-apoD Reduces the Activation of Inflammatory Processes in the Hippocampus
KA-induced neurodegeneration is associated with glial activation (microglia and astrocytes) and inflammatory mediators production [40]. Since that apoD production is accompanied by GFAP activation following KA challenge (Fig. 1b) and based on the significant resistance of H-apoD Tg mice against seizures and hippocampal KA-induced apoptosis, we examined the presence of inflammatory processes in the hippocampus of WT and H-apoD Tg mice at day 3 post-injection. In the hippocampus of WT mice, KA injection resulted in significant astrocytic (GFAP; 3-fold) (Figs. 1b and 4a, b) and microglial activation (Mac-2; 3-fold) (Fig. 4a,c). It also induced expression of COX-2, an inflammatory mediator which promotes the recurrence of seizures and neuronal loss [41] (Fig. 4a,d). Interestingly, in response to KA treatment, H-apoD Tg mice display lower levels of Mac-2 and COX-2 than their WT counterparts. However, H-apoD Tg mice also display robust GFAP activation in their hippocampus, comparable with levels detected in WT animals. Together, these data demonstrate that the expression of H-apoD significantly attenuates inflammatory responses consequent to KA-induced excitotoxic neurodegeneration, except for the activation of astrocytes.
Fig. 4.
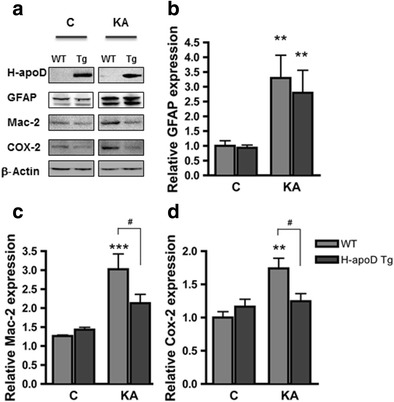
KA-induced inflammation is reduced in H-apoD Tg mice. a Western blot analysis of H-apoD, GFAP (astrocyte marker), Mac-2 (microglia marker) and COX-2 in hippocampal homogenates of vehicle (c) and KA-injected WT and H-apoD Tg mice, 3 days post-injection. β-Actin is used as loading control. Quantification of GFAP (b), Mac-2 (c) and COX-2 (d) protein expression by densitometry. Values were normalised by β-actin protein expression and by vehicle-injected WT mice values, which were given an arbitrary value of 1. Normalised values are presented as mean ± SEM (n = 3 to 5 animals for each group). Two-way ANOVA followed by Bonferroni post-test: **p < 0.01; ***p < 0.001 compared with vehicle-injected WT; # p < 0.05 compared with KA-injected WT mice. C mice injected with PBS (vehicle), KA mice injected with kainate (15 mg/kg)
Effect of H-apoD on PMCA2 Expression in Hippocampus
We then hypothesised that the neuronal expression of H-apoD induces favourable conditions that may attenuate KA-induced lesions. PMCA, major calcium pumps, play an important role in regulating Ca2+ intracellular levels. Kainic acid reduces the levels of PMCA2, a major isoform present in the brain, leading to Ca2+ dyshomeostasis [42]. We thus analyzed the expression of PMCA2 in the hippocampus of WT and H-apoD Tg mice. PMCA2 expression in baseline, non-KA-induced conditions was 1.7-fold higher in H-apoD Tg mice compared with WT animals (Fig. 5a,b). These data reveal that neuronal H-apoD overexpression leads to increased PMCA2 expression.
Fig. 5.
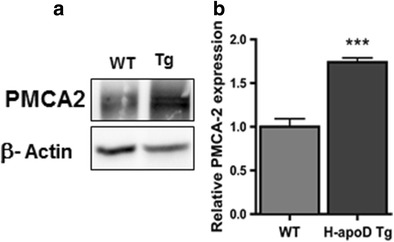
PMCA2 levels are upregulated in H-apoD Tg mice. a Western blot analysis of PMCA2 in hippocampal homogenates of WT and H-apoD Tg mice. β-Actin is used as loading control. b Quantification of PMCA2 protein expression. Values were normalised by β-actin protein expression and by WT values, which were given an arbitrary value of 1. Normalised values are presented as mean ± SEM (n = 3 to 4 animals for each group). Unpaired t test: ***p = 0.0006 compared with WT mice
Effect of H-apoD on NMDA Receptors in Hippocampus
Since the excitotoxicity process is induced through overactivation of glutamate receptors, we assessed the protein level of NMDA receptor subunits (NR1, NR2A and NR2B) in WT and H-apoD Tg mice (Fig. 6). WT and H-apoD Tg animals presented similar hippocampal protein levels of NR1 and NR2A (Fig. 6a–c), although a trend for NR2A downregulation in H-apoD Tg mice was observed (Fig, 6a, c). In contrast, NR2B levels were significantly reduced in H-apoD Tg mice (30 % decrease) (Fig. 6a, d). Because NMDAR are essential mediators of brain plasticity, we also examined the effect of neuronal H-apoD overexpression on synaptophysin, a marker of neuronal terminals reflecting synaptic plasticity processes [43]. Interestingly, synaptophysin levels were not altered in the hippocampus of H-apoD Tg compared with WT animals (Fig. 6a, e). This data indicates that the protection against KA-induced toxicity observed in H-apoD Tg mice might be imputable in part to decreased NR2B levels in these mice.
Fig. 6.
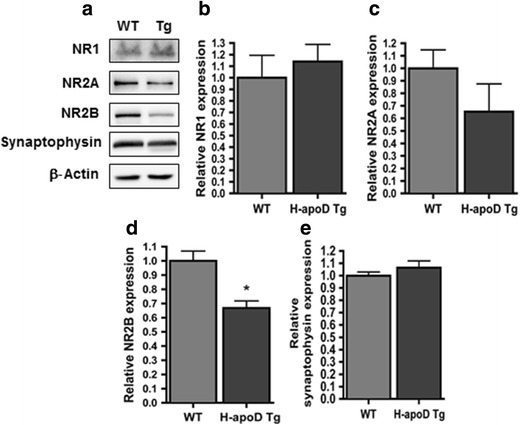
NMDA receptor subunit expression in H-apoD Tg mice. a Western blot analysis of NMDAR subunits (NR1, NR2A, NR2B) and Synaptophysin in hippocampal homogenates of WT and H-apoD Tg mice. β-Actin is used as loading control. Quantification of NR1 (b), NR2A (c), NR2B (d) and synaptophysin (e) protein expression. Values were normalised by β-actin protein expression and by WT values (with an arbitrary value of 1). Note that only NR2B expression in H-apoD Tg was statistically different from WT. Normalised values are presented as mean ± SEM (n = 3 to 4 animals for each group). Unpaired t test: *p = 0.0181 compared with WT mice.
H-apoD Modulates Cholesterol Metabolism in Neurons
A role for apoD in lipid metabolism has been suggested by several studies [44–46]. Defects in cholesterol metabolism are associated with various neurological injuries, such as kainate-induced excitotoxicity [47, 48]. We first evaluated whether neuronal expression of human apoD modulates cholesterol levels in the mouse brain. These were measured in membrane, cytosol and whole brain fractions of WT and H-apoD Tg mice, using the Amplex Red Cholesterol assay. Interestingly, the brain of H-apoD Tg mice contained significantly less cholesterol (40 % decrease) in the cytosolic fraction compared with WT mice (Fig. 7b). However, the cholesterol levels remained unchanged in the whole homogenate (Fig. 7a) and membrane fractions (Fig. 7c).
Fig. 7.
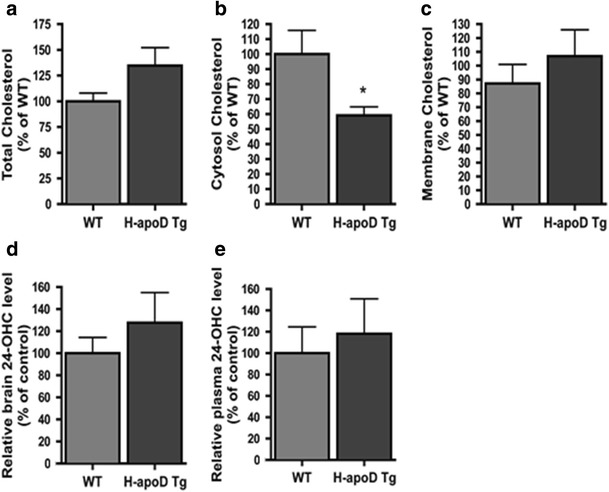
Cholesterol metabolism in H-apoD Tg mice. Cholesterol levels were assessed in whole (a), cytosolic (b) and membrane (c) fractions of brain homogenates in WT and H-apoD Tg mice using the Amplex red assay. Values were normalised by the total protein content of each fractions. Each graph represents the mean values ± SEM (n = 3 to 6 animals for each group), normalised by WT mice, which was given an arbitrary value of 100 %. Note that only the cytosolic fraction of H-apoD Tg brain was statistically different from WT. Unpaired t test: *p = 0.0283 compared with WT mice. d, e 24-Hydroxycholesterol (24-OHC) quantification in brain homogenates (d) and plasma (e) of WT and H-apoD Tg mice. Each graph represents the mean values ± SEM (n = 3 to 4 animals for each group), normalised by WT mice, which was given an arbitrary value of 100 %
Because neurons can convert an excess of cholesterol into 24-HOC which crosses the blood brain barrier to be eliminated to the liver via the plasma [49], we examined if the decrease in cholesterol levels in the cytosolic brain fraction was associated with an increase in 24-HOC levels in the brain and plasma of H-apoD Tg mice. Despite a trend towards an increase, 24-HOC levels in the plasma (Fig. 7e) and brain (Fig. 7d) were not significantly different between WT and H-apoD Tg mice. Thus, the 24-HOC pathway is not involved in the reduction of cholesterol levels in the cytosolic brain fraction (Fig. 7b) of H-apoD Tg mice.
Besides 24-HOC production, neurons can release their cholesterol by efflux pathways [50]. To investigate the effect of apoD on intracellular cholesterol content, primary hippocampal neurons grown for 10 days were treated with purified H-apoD for 24 h. The intracellular cholesterol was then stained with filipin, which can bind cholesterol. H-apoD-treated hippocampal neurons (Fig. 8d, f) showed less cholesterol/filipin staining than untreated neurons (Fig. 8(a, c)), consistent with our observations in the brain of H-apoD Tg mice (Fig. 7). Interestingly, the cholesterol/filipin content of neurites remained unchanged in H-apoD-treated neurons (Fig. 8(c’, f’)). To improve further our understanding of the processes involved in apoD-mediated reduction of cholesterol content, we then assessed the effect of purified H-apoD on the neuronal uptake of exogenous cholesterol. Hippocampal neuronal cultures were pre-incubated for 3 h in the presence of cholesterol and further exposed or not to purified human apoD for 24 h. Cholesterol uptake was stained by filipin. Filipin staining showed cholesterol accumulation in hippocampal neurons pre-incubated with exogenous cholesterol (Fig. 8(g)). Interestingly, this accumulation was decreased by 50 % following purified H-apoD incubation (Fig. 8(h, i)). Overall, these results show that apoD is involved in the regulation of intraneuronal cholesterol content.
Fig. 8.
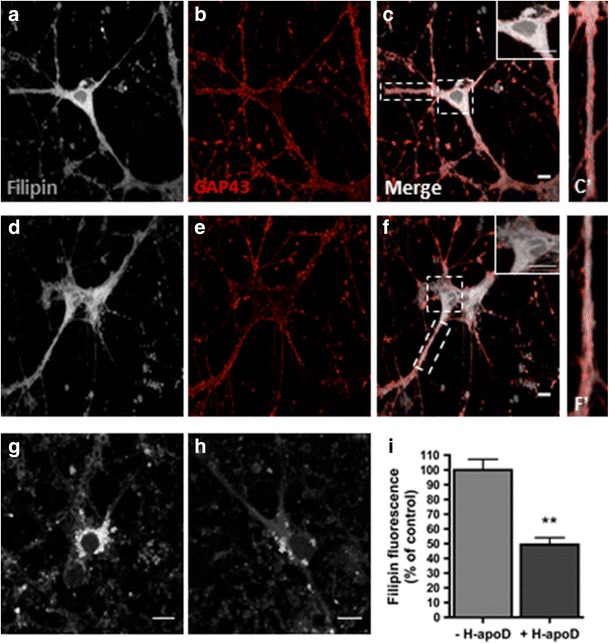
H-apoD affects cholesterol distribution in neurons. Hippocampal neurons (10 days), treated (d–f) or not (a–c) with purified human ApoD for 24 h, were stained with filipin (a, d) (grey) and anti-GAP43 antibodies (red) (b, e) and analysed by confocal microscopy. Enlarged view of boxed segment shows decreased filipin labelling intensity in the soma of neurons treated with purified H-apoD (f) compared with non-treated neurons (c). Note that the filipin staining extended to the neurites and that H-apoD-treated (f’) and non-treated neurons (c’) show similar filipin staining. Scale bar = 10 μm. g–i Filipin staining was assessed in hippocampal neuronal (10 days) pre-incubated for 3 h in the presence of cholesterol and further exposed with (h) or without purified H-apoD (g) for 24 h. Quantification of filipin staining (i). Values were normalised by filipin staining of neurons non-treated with H-apoD (arbitrary value of 100 %). Normalised values are presented as mean ± SEM (n = 3). Unpaired t test: **p = 0.0011 compared with neurons non-treated with H-apoD
H-apoD Internalisation by Neurons Is Increased Following KA Treatment
ApoD is a secreted protein which can be internalised by various cell lines [26, 51–53] and in primary mouse astrocytes [20]. It was also proposed that neurons, which express very little or no apoD, can import it from surrounding glial cells [11]. However, apoD internalisation by neurons in normal and stress conditions was never demonstrated. To address this question, hippocampal neuronal cultures (at 10 days) were pre-incubated for 24 h with purified H-apoD and further exposed to kainic acid (100 μM) or control conditions for 24 h. The exogenous apoD internalised in neurons was detected by immunocytochemistry, using a specific antibody raised against human apoD. Under physiological conditions, purified human apoD was detected mostly in the perinuclear area into vesicular compartments in soma and neurites (Fig. 9a (b)). Kainate treatment provoked an important intracellular and nuclear accumulation of H-apoD (Fig. 9a (c)). This data demonstrates that apoD can be internalised in neurons and that this internalisation is accentuated in stress conditions.
Fig. 9.
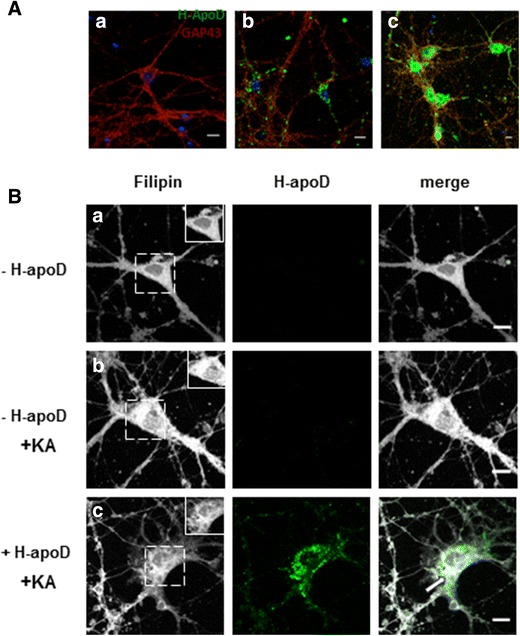
H-apoD internalization is increased in KA-treated neurons. A Hippocampal Neurons (10 days) pre-incubated for 24 h with purified H-apoD and further exposed to control conditions (A (b)) or kainic acid (100 μM) (A (c)) for 24 h were immunostained with anti-H-apoD (green) and anti-GAP43 antibodies (red) and analysed by confocal microscopy. As a control, staining was also assessed in cells without H-apoD pre-incubation, before KA treatment (A (a)). Nuclei were labeled with DAPI (blue). Note that important H-apoD internalization was observed in KA-treated (A (c)) compared to untreated neurons (A (b)). Scale bar = 10 μm. B Hippocampal neurons (10 days) were pre-incubated with (+ H-apoD; B (c)) or without (− H-apoD; B (a, b)) purified human apoD for 24 h and further treated with kainic acid (100 μM) for 3 h (+KA; B (b, c)) or not (B (a)). Cells were subsequently stained with filipin (gray) and anti-H-apoD antibodies (green). Enlarged view of boxed segment (B (a–c)) shows that the filipin staining decreases in the soma of neurons treated with purified H-apoD (B (c)) compared to untreated neurons (B (b)) following kainate treatment. Scale bar = 10 μm
As defects in cholesterol metabolism are associated with various neurological injuries, including kainate-induced excitotoxicity [47, 48, 54], we further evaluated the effect of exogenous apoD on the intracellular levels of cholesterol in the presence of KA-induced toxicity in primary neurons (Fig. 9b). In accordance with previous studies [48], we observed that KA-treated neurons displayed an important accumulation of cholesterol (Fig. 9b (b)) compared with non-treated neurons (Fig. 9b (a)). Interestingly, H-apoD pre-treated hippocampal neurons (Fig. 9b (c)) showed less cholesterol/filipin staining than non-pretreated neurons (Fig. 9b (b)) in response to KA toxicity. As previously demonstrated, KA treatment induced an important uptake of exogenous apoD, which co-localised with cholesterol (Fig. 9b (c)). Overall, our results show that internalised apoD attenuates the increase in intraneuronal cholesterol content induced by KA treatment.
Discussion
The excessive activation of glutamatergic receptors can cause excitotoxicity due to increased Ca2+ influx into cells. This leads to a number of deleterious consequences and ultimately to neuronal dysfunction and death. Excitotoxicity was reported in various neurodegenerative conditions with distinct etiologies such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis and amyotrophic lateral sclerosis [55, 56]. This suggests that excitotoxicity may be a common pathway underlying neurological pathogenesis. Understanding the mechanisms associated with excitotoxicity regulation should provide potential targets for neuroprotective strategies.
In this study, we provide additional evidence of apoD-mediated neuroprotection and clues on the mechanisms involved. We demonstrate that overexpression of human apoD in neurons protects against KA-induced lesions in vivo, decreasing the severity of seizures, cellular apoptosis and reactive gliosis in KA-treated animals. This neuroprotection appears to be linked to the control of calcium levels, glutamate receptor subunits and cholesterol metabolism.
In accordance with previous studies [27, 28], acute kainate treatment induced a transient apoD upregulation in the mouse hippocampus that peaked at day 3 post-injection and was resolved after 7 days. Such peak of apoD production corresponds to that of astrocyte activation and proliferation, as documented by increased GFAP levels. The concordance in time of apoD and GFAP levels is in line with the fact that astrocytes are one of major site of apoD production in the CNS [1, 6, 8]. In addition, increased synthesis of apoD in astrocytosis has been observed following enthorinal cortex lesion [16], coronavirus OC43-induced encephalitis [21], experimental stroke [57] and ageing processes [58]. However, it remains to be determined if apoD production is a cause or a consequence of astrocytosis. Nevertheless, astrocytes may not be the sole source of apoD production as after KA injury, apoD was also detected in pyramidal neurons of the hippocampus [28]. The peak of apoD production also correlated with increased apoptosis, as assessed by TUNEL labelling. Increased apoD expression has been reported in pathologic conditions with increased apoptosis and a potential role of apoD on the death-survival balance was suggested [59].
In rodents, the seizures, induced by systemic or intracerebral injection of KA [60], are generated in the CA3 region of the hippocampus, where pyramidal cell are more sensitive to KA treatment [61], then propagate to other limbic structures [61, 62], involving excitotoxic cell death cascades [29]. However, the molecular mechanisms involved in seizure-induced cell death are not clearly understood. A tight relationship between COX-2 and seizures severity and induced cell death was reported in both in vivo and in vitro models of excitotoxicity [41, 63–65]. KA treatment was shown to immediately induce COX-2 expression thus facilitating the recurrence of hippocampal seizures [41, 66]. Interestingly, the COX-2 induction was reduced in KA-injected H-apoD Tg mice. The effect of apoD on COX-2 downregulation might be mediated through the control of arachidonic acid (AA) levels. First, apoD could influence the availability of free AA, its preferential ligand, in the cell by binding/chelation, limiting its use in enzymatic pathways such as COX-2 [25, 67].
Moreover, apoD could attenuate PLA2 activity triggered by excitotoxic conditions, again reducing free AA levels [21]. Our results further demonstrate that the anti-inflammatory effects of apoD in KA response are mediated by limiting microglial but not astrocytic activation. It has already been established that inhibition of microglial activation exerts neuroprotective actions against KA-induced injury [68, 69]. The effects of apoD on microglia activation might be COX-2-dependent considering that COX-2 contributes to microglial activation [70, 71]. However, as COX-2 regulates both microglia and astrocytic activation, the fact that H-apoD Tg mice display, at day 3 after KA treatment, similar GFAP but decreased Mac-2 levels compared with WT mice suggests that the response to COX-2 reduction due to apoD overexpression is faster in microglia than in astrocytes.
Another pathway by which apoD could be protective is by increasing the Ca2+ buffering capacity of neurons. Excessive influx of calcium into neurons, as generated by the overactivation of glutamate receptors by KA plays a critical role in seizure genesis [72, 73] and triggers diverse cellular death mechanisms [74, 75]. Intracellular calcium homeostasis requires several mechanisms, including Ca2+ efflux, which can be carried out by PMCA. The expression of the most abundant isoform in the CNS, PMCA-2, is decreased after KA exposure [42, 76]. It is likely that, by stimulating the production of PMCA-2, the presence of H-apoD in neurons contributes to lower intracellular calcium levels and consequently the detrimental effects of excitotoxicity in H-apoD Tg mice. However, the mechanism by which apoD influences PMCA-2 levels remains to be determined. As the increase of intracellular Ca2+ content by excitotoxicity stimulates the generation of reactive oxygen species [77] and lipid peroxidation, which in turn downregulates rapidly PMCA levels [78], it is possible that apoD affects PMCA-2 levels through its anti-oxidant properties. Indeed, apoD and its orthologs have been shown to protect from pro-oxidative insults in several organisms [17–19, 22].
Our results also support the hypothesis that apoD limits excitotoxicity by controlling NMDAR subunit composition. It is well established that the activation of NMDAR-mediated neuronal death or survival pathways depends on its subunit composition and subcellular localisation [79–82]. Indeed, activation of NR2B containing NMDARs increases neuronal apoptosis and in contrast, the action of NR2A-containing NMDARs promotes neuronal survival pathways in rat models (in vitro and in vivo) of ischemic stroke [80, 83]. The downregulation of NR2B levels in H-apoD Tg mice hippocampus, with unchanged levels of NR1 and NR2A subunits, might contribute to limit seizure severity and detrimental effects such as apoptosis in response to KA challenge. However, NR2B downregulation in surface membrane at synaptic sites of rat striatal neurons has been reported to produce synaptic plasticity alterations [84]. Of relevance, this NR2B downregulation does not affect the expression of the presynaptic vesicle protein synaptophysin [85–87], suggesting normal synaptic plasticity mechanisms. However, how apoD controls NR2B levels remains unknown and needs further attention.
Finally, apoD-mediated neuroprotection after KA challenge can also be related to its role in lipid metabolism. A direct association between dysregulation of cholesterol homeostasis and neurodegeneration has been clearly demonstrated in several neurodegenerative diseases, such as Alzheimer’s, Huntington’s and Niemann-Pick type C disease [88], pathologies which also show an upregulation of apoD. In accordance with cholesterol dysmetabolism in neurodegenerative diseases, it was reported that intracerebroventricular injections of KA in rats resulted in an increase in immunoreactivity to cholesterol in the affected CA fields of the hippocampus [47]. An accumulation of cholesterol was also demonstrated in our primary neuronal cultures exposed to KA treatment. Interestingly, our results show an important increase in the internalisation of H-apoD in KA-treated primary mouse neurons. Moreover, the addition of exogenous H-apoD to KA-treated primary neurons reduced their cholesterol content. Altogether, our study suggests an important role for apoD in regulating cholesterol metabolism in the injured brain. Cholesterol, an integral component of neural membranes, is crucial for the neuronal functions such as axon guidance and synaptic transmission [89]. Cholesterol is mostly synthesised in astrocytes and transported to neurons by apolipoprotein E-containing lipoproteins even though neurons can also produce cholesterol in their cell bodies [88].
Our results show that H-apoD Tg mice harbour decreased levels of cytosolic cholesterol but similar total cholesterol levels in their brain compared with WT mice. This suggests that apoD favours cholesterol redistribution, namely to membranes, or elimination. In support to this, mice lacking apoD display an increase of specific fatty acids in their brain with a decreased ratio of cholesterol:total phospholipids in the membrane compartment [90]. Excessive intraneuronal cholesterol is eliminated by its conversion to 24-hydroxycholesterol (24-OHC), which can cross the blood brain barrier and reach the liver for elimination [91, 92]. This capacity to convert cholesterol to 24-HOC is specific to neurons [49]. However, in this study, we could not detect significant differences in the levels of membrane-bound cholesterol and we demonstrated that 24-HOC pathway is not involved in the decreased cholesterol levels of the cytosolic brain fraction in transgenic mice that overexpress neuronal apoD. Thus, apoD seems to utilise another pathway to regulate intraneuronal cholesterol levels. Other cholesterol transporters, including the ATP-binding cassette (ABC) transporters and high-density lipoproteins (HDL), are involved in the regulation of cholesterol efflux, namely in cortical neuronal cultures [50]. It is worth mentioning that apoD is primarily associated with high-density lipoproteins in human plasma. However, the relation between apoD and ATP-binding cassette (ABC) transporters has not yet been defined. Moreover, apoD associated with HDL-like particles found in the cerebrospinal fluid [93] could be involved in the cholesterol efflux mediated by the ABC transporter pathway. ApoE is also known to be involved in cholesterol efflux in astrocytes [50] as a compensatory mechanism to maintain the cholesterol homeostasis in the brain [94]. Of relevance, it was reported that apolipoprotein E-deficient mice display an increased expression of both apoD and ABCA1 [94]. In light of this and other studies, we could propose that increased apoD in neurodegenerative diseases is protective against ongoing neuronal stress culminating in neuron loss, although we cannot directly compare the apoD levels present in the brain of transgenic mice with those in patient brain. Thus, any strategy to enhance apoD expression may provide neuroprotection against excitotoxicity associated with neuropathogenesis. Nevertheless, as variations in the levels of apoD might influence the lipid and glucose metabolism at off-target sites, potentially leading to insulin resistance and hepatic steatosis [44], such strategy needs to be highly specific.
Conclusion
In sum, this study provides additional insights into the emerging mechanisms underlying the neuroprotective role of apoD upregulation in CNS injury. As a result of its lipid-binding properties, apoD might reverse the excitotoxicity-induced accumulation of cholesterol and potentially other lipids in neurons and most importantly their presence as free, cytosolic species, limiting their availability for deleterious reactions. In the same way, apoD limits pro-inflammatory reactions such as microglial activation and this might be related to its capacity of binding/chelating free lipids. Apart from its functions in lipid metabolism, apoD could limit the effects of acute excitotoxicity by controlling NMDAR subunit composition and intracellular Ca2+ levels (Fig. 10). Our results suggest apoD as an attractive molecule for potential targeted therapy in the treatment of excitotoxicity associated with many neurodegenerative conditions such as Alzheimer’s and Parkinson’s diseases and stroke.
Fig. 10.
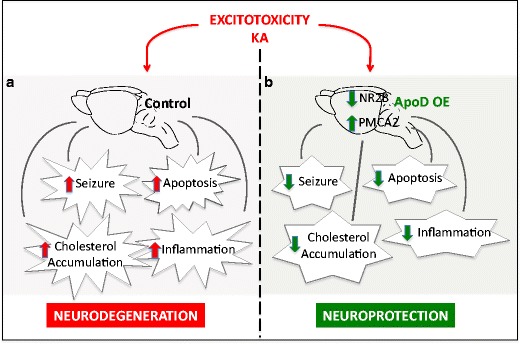
Schematic representation of ApoD protective role in the presence of KA-induced excitotoxicity. a In control wild-type mice brain, KA treatment results in increased apoptosis, seizures, intracellular cholesterol accumulation and inflammatory responses. b apoD overexpression (apoD OE) in H-apoD Tg mice reduces KA-induced seizures and apoptosis and attenuates inflammation. This protection is associated with increased PMCA2 and decreased NR2B levels (30 %) and a downregulation of intracellular cholesterol content
Acknowledgements
We thank Denis Flipo for his help with the confocal microscopy analysis. We also thank Diego Sanchez and Maria Ganfornina for helpful discussions. This work was supported by the Canadian Institutes of Health Research grant MOP-15677 (ER).
Abbreviations
- H-apoD
Human apolipoprotein D
- M-apoD
Mouse apolipoprotein D
- KA
Kainic acid
- NMDAR
N-methyl-d-aspartate receptor
- Tg
Transgenic
- AA
Arachidonic acid
- CNS
Central nervous system
- PMCA
Plasma membrane calcium ATPases
- CA
Cornu ammonis
- NPC
Niemann-Pick’s type C
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labelling
- DAPI
4′,6-diamidino-2-phenylindole
- 24-HOC
24(S)-Hydroxycholesterol
- GAP-43
Growth-associated protein-43
- COX-2
Cyclooxygenase-2
- GFAP
Glial fibrillary acidic protein
- PLA2
Phospholipase A2
Compliance with Ethical Standard
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Rassart E, Bedirian A, Do Carmo S, Guinard O, Sirois J, Terrisse L, Milne R. Apolipoprotein D. Biochim Biophys Acta. 2000;1482(1–2):185–198. doi: 10.1016/S0167-4838(00)00162-X. [DOI] [PubMed] [Google Scholar]
- 2.Drayna D, Fielding C, McLean J, Baer B, Castro G, Chen E, Comstock L, Henzel W, et al. Cloning and expression of human apolipoprotein D cDNA. J Biol Chem. 1986;261(35):16535–16539. [PubMed] [Google Scholar]
- 3.Weech PK, Provost P, Tremblay NM, Camato RN, Milne RW, Marcel YL, Rassart E. Apolipoprotein D—an atypical apolipoprotein. Prog Lipid Res. 1991;30(2–3):259–266. doi: 10.1016/0163-7827(91)90023-X. [DOI] [PubMed] [Google Scholar]
- 4.Elliott DA, Weickert CS, Garner B. Apolipoproteins in the brain: implications for neurological and psychiatric disorders. Clin Lipidol. 2010;51(4):555–573. doi: 10.2217/clp.10.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu CY, Ong WY, Sundaram RK, Chan C, Patel SC. Immunocytochemical localization of apolipoprotein D in oligodendrocyte precursor-like cells, perivascular cells, and pericytes in the human cerebral cortex. J Neurocytol. 2001;30(3):209–218. doi: 10.1023/A:1012797623620. [DOI] [PubMed] [Google Scholar]
- 6.Provost PR, Villeneuve L, Weech PK, Milne RW, Marcel YL, Rassart E. Localization of the major sites of rabbit apolipoprotein D gene transcription by in situ hybridization. J Lipid Res. 1991;32(12):1959–1970. [PubMed] [Google Scholar]
- 7.Rickhag M, Deierborg T, Patel S, Ruscher K, Wieloch T. Apolipoprotein D is elevated in oligodendrocytes in the peri-infarct region after experimental stroke: influence of enriched environment. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2008;28(3):551–562. doi: 10.1038/sj.jcbfm.9600552. [DOI] [PubMed] [Google Scholar]
- 8.Smith KM, Lawn RM, Wilcox JN. Cellular localization of apolipoprotein D and lecithin:cholesterol acyltransferase mRNA in rhesus monkey tissues by in situ hybridization. J Lipid Res. 1990;31(6):995–1004. [PubMed] [Google Scholar]
- 9.Kalman J, McConathy W, Araoz C, Kasa P, Lacko AG. Apolipoprotein D in the aging brain and in Alzheimer’s dementia. Neurol Res. 2000;22(4):330–336. doi: 10.1080/01616412.2000.11740678. [DOI] [PubMed] [Google Scholar]
- 10.Terrisse L, Poirier J, Bertrand P, Merched A, Visvikis S, Siest G, Milne R, Rassart E. Increased levels of apolipoprotein D in cerebrospinal fluid and hippocampus of Alzheimer’s patients. J Neurochem. 1998;71(4):1643–1650. doi: 10.1046/j.1471-4159.1998.71041643.x. [DOI] [PubMed] [Google Scholar]
- 11.Ordonez C, Navarro A, Perez C, Astudillo A, Martinez E, Tolivia J. Apolipoprotein D expression in substantia nigra of Parkinson disease. Histol Histopathol. 2006;21(4):361–366. doi: 10.14670/HH-21.361. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida K, Cleaveland ES, Nagle JW, French S, Yaswen L, Ohshima T, Brady RO, Pentchev PG, et al. Molecular cloning of the mouse apolipoprotein D gene and its upregulated expression in Niemann-Pick disease type C mouse model. DNA Cell Biol. 1996;15(10):873–882. doi: 10.1089/dna.1996.15.873. [DOI] [PubMed] [Google Scholar]
- 13.Reindl M, Knipping G, Wicher I, Dilitz E, Egg R, Deisenhammer F, Berger T. Increased intrathecal production of apolipoprotein D in multiple sclerosis. J Neuroimmunol. 2001;119(2):327–332. doi: 10.1016/S0165-5728(01)00378-2. [DOI] [PubMed] [Google Scholar]
- 14.Boyles JK, Notterpek LM, Anderson LJ. Accumulation of apolipoproteins in the regenerating and remyelinating mammalian peripheral nerve. Identification of apolipoprotein D, apolipoprotein A-IV, apolipoprotein E, and apolipoprotein A-I. J Biol Chem. 1990;265(29):17805–17815. [PubMed] [Google Scholar]
- 15.Franz G, Reindl M, Patel SC, Beer R, Unterrichter I, Berger T, Schmutzhard E, Poewe W, et al. Increased expression of apolipoprotein D following experimental traumatic brain injury. J Neurochem. 1999;73(4):1615–1625. doi: 10.1046/j.1471-4159.1999.0731615.x. [DOI] [PubMed] [Google Scholar]
- 16.Terrisse L, Seguin D, Bertrand P, Poirier J, Milne R, Rassart E. Modulation of apolipoprotein D and apolipoprotein E expression in rat hippocampus after entorhinal cortex lesion. Brain Res Mol Brain Res. 1999;70(1):26–35. doi: 10.1016/S0169-328X(99)00123-0. [DOI] [PubMed] [Google Scholar]
- 17.Ganfornina MD, Do Carmo S, Lora JM, Torres-Schumann S, Vogel M, Allhorn M, Gonzalez C, Bastiani MJ, et al. Apolipoprotein D is involved in the mechanisms regulating protection from oxidative stress. Aging Cell. 2008;7(4):506–515. doi: 10.1111/j.1474-9726.2008.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charron JB, Ouellet F, Houde M, Sarhan F. The plant Apolipoprotein D ortholog protects Arabidopsis against oxidative stress. BMC Plant Biol. 2008;8:86. doi: 10.1186/1471-2229-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muffat J, Walker DW, Benzer S. Human ApoD, an apolipoprotein up-regulated in neurodegenerative diseases, extends lifespan and increases stress resistance in Drosophila. Proc Natl Acad Sci U S A. 2008;105(19):7088–7093. doi: 10.1073/pnas.0800896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajo-Graneras R, Ganfornina MD, Martin-Tejedor E, Sanchez D. Apolipoprotein D mediates autocrine protection of astrocytes and controls their reactivity level, contributing to the functional maintenance of paraquat-challenged dopaminergic systems. Glia. 2011;59(10):1551–1566. doi: 10.1002/glia.21200. [DOI] [PubMed] [Google Scholar]
- 21.Do Carmo S, Jacomy H, Talbot PJ, Rassart E. Neuroprotective effect of apolipoprotein D against human coronavirus OC43-induced encephalitis in mice. J Neurosci. 2008;28(41):10330–10338. doi: 10.1523/JNEUROSCI.2644-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez D, Lopez-Arias B, Torroja L, Canal I, Wang X, Bastiani MJ, Ganfornina MD. Loss of glial lazarillo, a homolog of apolipoprotein D, reduces lifespan and stress resistance in Drosophila. Curr Biol CB. 2006;16(7):680–686. doi: 10.1016/j.cub.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Bhatia S, Knoch B, Wong J, Kim WS, Else PL, Oakley AJ, Garner B. Selective reduction of hydroperoxyeicosatetraenoic acids to their hydroxy derivatives by apolipoprotein D: implications for lipid antioxidant activity and Alzheimer’s disease. Biochem J. 2012;442(3):713–721. doi: 10.1042/BJ20111166. [DOI] [PubMed] [Google Scholar]
- 24.Oakley AJ, Bhatia S, Ecroyd H, Garner B. Molecular dynamics analysis of apolipoprotein-D-lipid hydroperoxide interactions: mechanism for selective oxidation of Met-93. PLoS One. 2012;7(3):e34057. doi: 10.1371/journal.pone.0034057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas EA, Copolov DL, Sutcliffe JG. From pharmacotherapy to pathophysiology: emerging mechanisms of apolipoprotein D in psychiatric disorders. Curr Mol Med. 2003;3(5):408–418. doi: 10.2174/1566524033479681. [DOI] [PubMed] [Google Scholar]
- 26.Thomas EA, George RC, Sutcliffe JG. Apolipoprotein D modulates arachidonic acid signaling in cultured cells: implications for psychiatric disorders. Prostaglandins Leukot Essent Fat Acids. 2003;69(6):421–427. doi: 10.1016/j.plefa.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Montpied P, de Bock F, Lerner-Natoli M, Bockaert J, Rondouin G. Hippocampal alterations of apolipoprotein E and D mRNA levels in vivo and in vitro following kainate excitotoxicity. Epilepsy Res. 1999;35(2):135–146. doi: 10.1016/S0920-1211(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 28.Ong WY, He Y, Suresh S, Patel SC. Differential expression of apolipoprotein D and apolipoprotein E in the kainic acid-lesioned rat hippocampus. Neuroscience. 1997;79(2):359–367. doi: 10.1016/S0306-4522(96)00608-2. [DOI] [PubMed] [Google Scholar]
- 29.Zheng XY, Zhang HL, Luo Q, Zhu J. Kainic acid-induced neurodegenerative model: potentials and limitations. J Biomed Biotechnol. 2011;2011:457079. doi: 10.1155/2011/457079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF. Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J Neurosci. 2003;23(22):8013–8019. doi: 10.1523/JNEUROSCI.23-22-08013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boer S, Sanchez D, Reinieren I, van den Boom T, Udawela M, Scarr E, Ganfornina MD, Dean B. Decreased kainate receptors in the hippocampus of apolipoprotein D knockout mice. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34(2):271–278. doi: 10.1016/j.pnpbp.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 32.McLin JP, Steward O. Comparison of seizure phenotype and neurodegeneration induced by systemic kainic acid in inbred, outbred, and hybrid mouse strains. Eur J Neurosci. 2006;24(8):2191–2202. doi: 10.1111/j.1460-9568.2006.05111.x. [DOI] [PubMed] [Google Scholar]
- 33.Racine RJ. Modification of seizure activity by electrical stimulation II Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 34.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35(5):567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 35.Coxey RA, Pentchev PG, Campbell G, Blanchette-Mackie EJ. Differential accumulation of cholesterol in Golgi compartments of normal and Niemann-Pick type C fibroblasts incubated with LDL: a cytochemical freeze-fracture study. J Lipid Res. 1993;34(7):1165–1176. [PubMed] [Google Scholar]
- 36.Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4(3):229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen Z, Ljunggren HG, Bogdanovic N, Nennesmo I, Winblad B, Zhu J. Excitotoxic neurodegeneration induced by intranasal administration of kainic acid in C57BL/6 mice. Brain Res. 2002;931(2):135–145. doi: 10.1016/S0006-8993(02)02268-0. [DOI] [PubMed] [Google Scholar]
- 38.Ganfornina MD, Do Carmo S, Martinez E, Tolivia J, Navarro A, Rassart E, Sanchez D. ApoD, a glia-derived apolipoprotein, is required for peripheral nerve functional integrity and a timely response to injury. Glia. 2010;58(11):1320–1334. doi: 10.1002/glia.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben-Ari Y, Tremblay E, Riche D, Ghilini G, Naquet R. Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience. 1981;6(7):1361–1391. doi: 10.1016/0306-4522(81)90193-7. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z, Duan RS, Quezada HC, Mix E, Nennesmo I, Adem A, Winblad B, Zhu J. Increased microglial activation and astrogliosis after intranasal administration of kainic acid in C57BL/6 mice. J Neurobiol. 2005;62(2):207–218. doi: 10.1002/neu.20099. [DOI] [PubMed] [Google Scholar]
- 41.Kim EJ, Lee JE, Kwon KJ, Lee SH, Moon CH, Baik EJ. Differential roles of cyclooxygenase isoforms after kainic acid-induced prostaglandin E(2) production and neurodegeneration in cortical and hippocampal cell cultures. Brain Res. 2001;908(1):1–9. doi: 10.1016/S0006-8993(01)02432-5. [DOI] [PubMed] [Google Scholar]
- 42.Kurnellas MP, Li H, Jain MR, Giraud SN, Nicot AB, Ratnayake A, Heary RF, Elkabes S. Reduced expression of plasma membrane calcium ATPase 2 and collapsin response mediator protein 1 promotes death of spinal cord neurons. Cell Death Differ. 2010;17(9):1501–1510. doi: 10.1038/cdd.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W Jr, Kaye J, Manczak M (2005) Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimer’s Dis JAD 7(2):103–117 [DOI] [PubMed]
- 44.Do Carmo S, Fournier D, Mounier C, Rassart E. Human apolipoprotein D overexpression in transgenic mice induces insulin resistance and alters lipid metabolism. Am J Physiol Endocrinol Metab. 2009;296(4):802–811. doi: 10.1152/ajpendo.90725.2008. [DOI] [PubMed] [Google Scholar]
- 45.Perdomo G, Henry Dong H. Apolipoprotein D in lipid metabolism and its functional implication in atherosclerosis and aging. Aging. 2009;1(1):17–27. doi: 10.18632/aging.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perdomo G, Kim DH, Zhang T, Qu S, Thomas EA, Toledo FG, Slusher S, Fan Y, et al. A role of apolipoprotein D in triglyceride metabolism. J Lipid Res. 2010;51(6):1298–1311. doi: 10.1194/jlr.M001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ong WY, Goh EW, Lu XR, Farooqui AA, Patel SC, Halliwell B. Increase in cholesterol and cholesterol oxidation products, and role of cholesterol oxidation products in kainate-induced neuronal injury. Brain Pathol. 2003;13(3):250–262. doi: 10.1111/j.1750-3639.2003.tb00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He X, Jenner AM, Ong WY, Farooqui AA, Patel SC. Lovastatin modulates increased cholesterol and oxysterol levels and has a neuroprotective effect on rat hippocampal neurons after kainate injury. J Neuropathol Exp Neurol. 2006;65(7):652–663. doi: 10.1097/01.jnen.0000225906.82428.69. [DOI] [PubMed] [Google Scholar]
- 49.Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004;24(5):806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Zhang X, Kusumo H, Costa LG, Guizzetti M. Cholesterol efflux is differentially regulated in neurons and astrocytes: implications for brain cholesterol homeostasis. Biochim Biophys Acta. 2013;1831(2):263–275. doi: 10.1016/j.bbalip.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Do Carmo S, Levros LC, Jr, Rassart E. Modulation of apolipoprotein D expression and translocation under specific stress conditions. Biochim Biophys Acta. 2007;1773(6):954–969. doi: 10.1016/j.bbamcr.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Leung WC, Lawrie A, Demaries S, Massaeli H, Burry A, Yablonsky S, Sarjeant JM, Fera E, et al. Apolipoprotein D and platelet-derived growth factor-BB synergism mediates vascular smooth muscle cell migration. Circ Res. 2004;95(2):179–186. doi: 10.1161/01.RES.0000135482.74178.14. [DOI] [PubMed] [Google Scholar]
- 53.Sarjeant JM, Lawrie A, Kinnear C, Yablonsky S, Leung W, Massaeli H, Prichett W, Veinot JP, et al. Apolipoprotein D inhibits platelet-derived growth factor-BB-induced vascular smooth muscle cell proliferated by preventing translocation of phosphorylated extracellular signal regulated kinase 1/2 to the nucleus. Arterioscler Thromb Vasc Biol. 2003;23(12):2172–2177. doi: 10.1161/01.ATV.0000100404.05459.39. [DOI] [PubMed] [Google Scholar]
- 54.Sodero AO, Vriens J, Ghosh D, Stegner D, Brachet A, Pallotto M, Sassoe-Pognetto M, Brouwers JF, et al. Cholesterol loss during glutamate-mediated excitotoxicity. EMBO J. 2012;31(7):1764–1773. doi: 10.1038/emboj.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009;30(4):379–387. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Den Bosch L, Van Damme P, Bogaert E, Robberecht W. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762(11–12):1068–1082. doi: 10.1016/j.bbadis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Ruscher K, Erickson A, Kuric E, Inacio AR, Wieloch T. Effects of chronic Clozapine administration on apolipoprotein D levels and on functional recovery following experimental stroke. Brain Res. 2010;1321:152–163. doi: 10.1016/j.brainres.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 58.del Valle E, Navarro A, Astudillo A, Tolivia J. Apolipoprotein D expression in human brain reactive astrocytes. J Histochem Cytochem. 2003;51(10):1285–1290. doi: 10.1177/002215540305101005. [DOI] [PubMed] [Google Scholar]
- 59.Bajo-Graneras R, Crespo-Sanjuan J, Garcia-Centeno RM, Garrote-Adrados JA, Gutierrez G, Garcia-Tejeiro M, Aguirre-Gervas B, Calvo-Nieves MD, et al. Expression and potential role of apolipoprotein D on the death-survival balance of human colorectal cancer cells under oxidative stress conditions. Int J Color Dis. 2013;28(6):751–766. doi: 10.1007/s00384-012-1616-2. [DOI] [PubMed] [Google Scholar]
- 60.Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14(2):375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 61.Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23(11):580–587. doi: 10.1016/S0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- 62.Miles R, Wong RK. Single neurones can initiate synchronized population discharge in the hippocampus. Nature. 1983;306(5941):371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- 63.Kunz T, Oliw EH. The selective cyclooxygenase-2 inhibitor rofecoxib reduces kainate-induced cell death in the rat hippocampus. Eur J Neurosci. 2001;13(3):569–575. doi: 10.1046/j.1460-9568.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- 64.Sayyah M, Javad-Pour M, Ghazi-Khansari M. The bacterial endotoxin lipopolysaccharide enhances seizure susceptibility in mice: involvement of proinflammatory factors: nitric oxide and prostaglandins. Neuroscience. 2003;122(4):1073–1080. doi: 10.1016/j.neuroscience.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 65.Takemiya T, Suzuki K, Sugiura H, Yasuda S, Yamagata K, Kawakami Y, Maru E. Inducible brain COX-2 facilitates the recurrence of hippocampal seizures in mouse rapid kindling. Prostaglandins Other Lipid mediators. 2003;71(3–4):205–216. doi: 10.1016/S1098-8823(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 66.Takemiya T, Matsumura K, Yamagata K. Roles of prostaglandin synthesis in excitotoxic brain diseases. Neurochem Int. 2007;51(2–4):112–120. doi: 10.1016/j.neuint.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 67.He X, Jittiwat J, Kim JH, Jenner AM, Farooqui AA, Patel SC, Ong WY. Apolipoprotein D modulates F2-isoprostane and 7-ketocholesterol formation and has a neuroprotective effect on organotypic hippocampal cultures after kainate-induced excitotoxic injury. Neurosci Lett. 2009;455(3):183–186. doi: 10.1016/j.neulet.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Byun JS, Lee JW, Kim SY, Kwon KJ, Sohn JH, Lee K, Oh D, Kim SS, et al. Neuroprotective effects of stanniocalcin 2 following kainic acid-induced hippocampal degeneration in ICR mice. Peptides. 2010;31(11):2094–2099. doi: 10.1016/j.peptides.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 69.Penkowa M, Florit S, Giralt M, Quintana A, Molinero A, Carrasco J, Hidalgo J. Metallothionein reduces central nervous system inflammation, neurodegeneration, and cell death following kainic acid-induced epileptic seizures. J Neurosci Res. 2005;79(4):522–534. doi: 10.1002/jnr.20387. [DOI] [PubMed] [Google Scholar]
- 70.Palumbo S, Bosetti F. Alterations of brain eicosanoid synthetic pathway in multiple sclerosis and in animal models of demyelination: role of cyclooxygenase-2. Prostaglandins Leukot Essent Fat Acids. 2013;89(5):273–278. doi: 10.1016/j.plefa.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 71.Vijitruth R, Liu M, Choi DY, Nguyen XV, Hunter RL, Bing G. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. J Neuroinflammation. 2006;3:6. doi: 10.1186/1742-2094-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McNamara JO. The neurobiological basis of epilepsy. Trends Neurosci. 1992;15(10):357–359. doi: 10.1016/0166-2236(92)90178-B. [DOI] [PubMed] [Google Scholar]
- 73.Meyer FB. Calcium, neuronal hyperexcitability and ischemic injury. Brain Res Brain Res Rev. 1989;14(3):227–243. doi: 10.1016/0165-0173(89)90002-7. [DOI] [PubMed] [Google Scholar]
- 74.Zipfel GJ, Lee JM, Choi DW. Reducing calcium overload in the ischemic brain. N Engl J Med. 1999;341(20):1543–1544. doi: 10.1056/NEJM199911113412011. [DOI] [PubMed] [Google Scholar]
- 75.Pellegrini-Giampietro DE, Gorter JA, Bennett MV, Zukin RS. The GluR2 (GluR-B) hypothesis: Ca(2+)-permeable AMPA receptors in neurological disorders. Trends Neurosci. 1997;20(10):464–470. doi: 10.1016/S0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- 76.Tempel BL, Shilling DJ. The plasma membrane calcium ATPase and disease. Sub-cellular Biochem. 2007;45:365–383. doi: 10.1007/978-1-4020-6191-2_13. [DOI] [PubMed] [Google Scholar]
- 77.Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic–ischemic brain damage. Ann Neurol. 1986;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 78.Chiarello DI, Marin R, Proverbio F, Benzo Z, Pinero S, Botana D, Abad C. Effect of hypoxia on the calcium and magnesium content, lipid peroxidation level, and Ca(2)(+)-ATPase activity of syncytiotrophoblast plasma membranes from placental explants. BioMed Res Int. 2014;2014:597357. doi: 10.1155/2014/597357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5(5):405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27(11):2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou M, Baudry M. Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2006;26(11):2956–2963. doi: 10.1523/JNEUROSCI.4299-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47(2):122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Taghibiglou C, Martin HG, Lai TW, Cho T, Prasad S, Kojic L, Lu J, Liu Y, et al. Role of NMDA receptor-dependent activation of SREBP1 in excitotoxic and ischemic neuronal injuries. Nat Med. 2009;15(12):1399–1406. doi: 10.1038/nm.2064. [DOI] [PubMed] [Google Scholar]
- 84.Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, et al. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12(5):602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y, Liang Z, Liu J, Zou W, Li X, Wang Y, An L. Downregulation of caveolin-1 contributes to the synaptic plasticity deficit in the hippocampus of aged rats. Neural Regen Res. 2013;8(29):2725–2733. doi: 10.3969/j.issn.1673-5374.2013.29.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martinez G, Di Giacomo C, Carnazza ML, Sorrenti V, Castana R, Barcellona ML, Perez-Polo JR, Vanella A. MAP2, synaptophysin immunostaining in rat brain and behavioral modifications after cerebral postischemic reperfusion. Dev Neurosci. 1997;19(6):457–464. doi: 10.1159/000111243. [DOI] [PubMed] [Google Scholar]
- 87.Rosenbrock H, Koros E, Bloching A, Podhorna J, Borsini F. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res. 2005;1040(1–2):55–63. doi: 10.1016/j.brainres.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 88.Vance JE. Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis Models Mech. 2012;5(6):746–755. doi: 10.1242/dmm.010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12(2):105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 90.Thomas EA, Yao JK. Clozapine specifically alters the arachidonic acid pathway in mice lacking apolipoprotein D. Schizophr Res. 2007;89(1–3):147–153. doi: 10.1016/j.schres.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 91.Bjorkhem I, Lutjohann D, Breuer O, Sakinis A, Wennmalm A. Importance of a novel oxidative mechanism for elimination of brain cholesterol. Turnover of cholesterol and 24(S)-hydroxycholesterol in rat brain as measured with 18O2 techniques in vivo and in vitro. J Biol Chem. 1997;272(48):30178–30184. doi: 10.1074/jbc.272.48.30178. [DOI] [PubMed] [Google Scholar]
- 92.Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, Russell DW. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J Biol Chem. 2003;278(25):22980–22988. doi: 10.1074/jbc.M303415200. [DOI] [PubMed] [Google Scholar]
- 93.Koch S, Donarski N, Goetze K, Kreckel M, Stuerenburg HJ, Buhmann C, Beisiegel U. Characterization of four lipoprotein classes in human cerebrospinal fluid. J Lipid Res. 2001;42(7):1143–1151. [PubMed] [Google Scholar]
- 94.Jansen PJ, Lutjohann D, Thelen KM, von Bergmann K, van Leuven F, Ramaekers FC, Monique M. Absence of ApoE upregulates murine brain ApoD and ABCA1 levels, but does not affect brain sterol levels, while human ApoE3 and human ApoE4 upregulate brain cholesterol precursor levels. J Alzheimer’s Dis JAD. 2009;18(2):319–329. doi: 10.3233/JAD-2009-1150. [DOI] [PubMed] [Google Scholar]
- 95.Bregola G, Zucchini S, Rodi D, Binaschi A, D’Addario C, Landuzzi D, Reinscheid R, Candeletti S, et al. Involvement of the neuropeptide nociceptin/orphanin FQ in kainate seizures. J Neurosci. 2002;22(22):10030–10038. doi: 10.1523/JNEUROSCI.22-22-10030.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


