Abstract
The main protease (Mpro) of severe acute respiratory syndrome coronavirus (SARS-CoV) plays an essential role in the extensive proteolytic processing of the viral polyproteins (pp1a and pp1ab), and it is an important target for anti-SARS drug development. SARS-CoV Mpro is composed of a catalytic N-terminal domain and an α-helical C-terminal domain linked by a long loop. Even though the N-terminal domain of SARS-CoV Mpro adopts a similar chymotrypsin-like fold as that of piconavirus 3C protease, the extra C-terminal domain is required for SARS-CoV Mpro to be enzymatically active. Here, we reported the NMR assignments of the SARS-CoV Mpro N-terminal domain alone, which are essential for its solution structure determination.
Keywords: SARS-CoV, Main protease, N-terminal domain, NMR
Biological context
Severe Acute Respiratory Syndromes Coronavirus (SARS-CoV) was identified as the etiological agent of the pandemic transmissible disease severe acute respiratory syndrome (SARS) (Peiris et al. 2003). SARS-CoV is a positive-sense, single-stranded RNA virus. During infection, two overlapping polyproteins (pp1a and pp1ab) are produced and further cleaved extensively by the main protease (Mpro) and the papain-like protease, yielding the components that assemble into the viral replication complex (Ziebuhr 2004). This makes Mpro essential for the viral life cycle and to be an attractive target for anti-SARS agent development (Anand et al. 2003).
Mpro exists in monomer–dimer equilibrium in solution, and only the dimeric form is the enzymatically active form (Fan et al. 2004). Recently, a super active octamer form of Mpro which is formed by 3D domain-swapping of the C-terminal domains is observed (Zhang et al. 2010, Zhong et al. 2009). Crystal structures revealed that each protomer of Mpro is composed of an N-terminal domain (residues 1–184) and an α-helical C-terminal domain (residues 201–303) linked by a long loop (Yang et al. 2003). Mpro is also known as 3C-like protease (3CLpro) for it has similar substrate specificities to that of picornavirus 3C protease (3Cpro) and its N-terminal domain adopts a similar chymotrypsin-like fold as 3Cpro does. However, Mpro has an extra C-terminal domain which is required for Mpro to be active, and its role in activation remains to be elucidated.
In order to characterize the structural difference for the N-terminal domain of SARS-CoV with and without the C-terminal extra domain, we have expressed the N-terminal domain alone (Mpro-N, residues 1–199) in E. coli, which is well-folded and stable for acquisition of high-quality NMR spectra. Here, we report the nearly complete sequence-specific backbone and side-chain 1H, 13C and 15N resonance assignments of Mpro-N.
Materials and methods
The uniformly 15N/13C-labeled or 15N-labeled Mpro-N protein was cloned and purified as previously described (Zhong et al. 2008). All NMR samples containing ~0.7 mM Mpro-N were in a buffer of 50 mM potassium phosphate (pH 7.0), 30 mM Arginine, 30 mM Glutamine acid, 50 mM NaCl, 1 mM EDTA, 10 mM DTT with 10% D2O.
All NMR experiments were performed on Bruker Avance 500 MHz, 600 MHz NMR, or 800 MHz spectrometers (all with cryoprobes) at 308 K. The backbone resonance chemical shift assignments were based on 2D 1H-15N HSQC, 3D HNCA, HN(CO)CA, HNCACB, CBCA(CO)HN, HNCO, HN(CA)CO and HBHA(CO)NH NMR spectra. The side chain resonance chemical shift assignments were based on 3D HCCH-COSY, HCCH-TOCSY, (H)CCH-COSY, and (H)CCH-TOCSY NMR experiments data. All NMR spectra were processed with NMRPipe (Delaglio et al. 1995), and analyzed using NMRView (Johnson and Blevins 1994). The chemical shift in the 1H dimension was referenced directly to 2, 2-dimethyl-2-silapentanesulfonic acid (DSS), whereas the chemical shifts in the 13C and 15N dimensions were indirectly referenced to DSS.
Assignments and data deposition
SARS-CoV Mpro-N contains 199 residues with a molecular weight of 21.9 kDa. The 2D 1H-15N HSQC spectrum of Mpro-N is shown in Fig. 1, with the assignments indicated. More than 90% of backbone and side-chain resonances are assigned. The backbone NH signals for residues M6, F8, K12, N63 and S139 were missing in the 2D 1H-15N HSQC spectrum, while residues S1, G2, F3, R4, K5, G11 and F140 were completely unassigned. The secondary chemical shifts and chemical shift index (CSI) analysis indicate that the secondary structure of SARS-CoV Mpro-N is mainly in agreement with that in full length Mpro. The assigned 1H, 13C and 15N chemical shifts of the protein have been deposited in BioMagResBank (http://www.bmrb.wisc.edu/) under the accession number 17251 (Fig. 2).
Fig. 1.
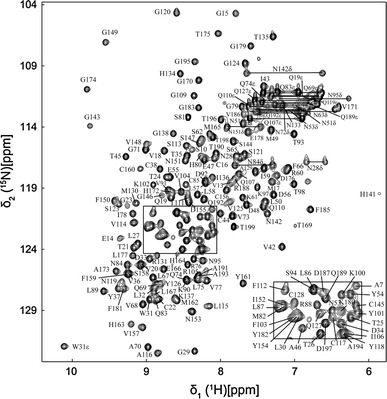
2D 1H-15N HSQC spectrum of Mpro-N. The assignments are annotated by the resonance peaks with one-letter amino acid code and residue number. The side-chain NH2 peaks of Asn and Gln are connected by horizontal lines
Fig. 2.
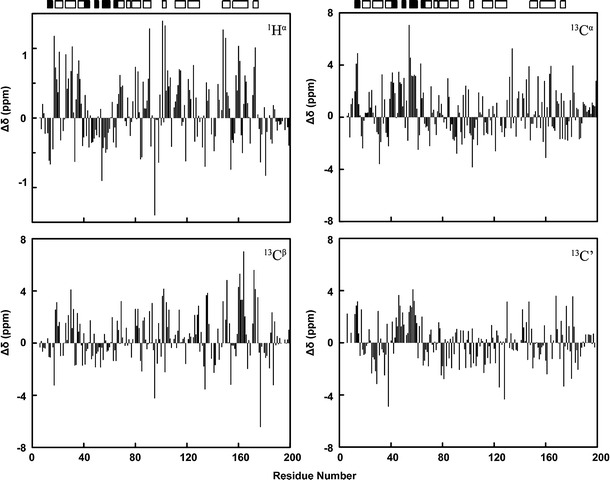
Secondary chemical shifts for 1Hα, 13Cα, 13Cβ and 13C′ resonances versus residue numbers. The random coil chemical shift values were obtained from BMRB database (http://www.bmrb.wisc.edu/published/Ikura_cs_study/index.html). The secondary structural elements of the N-terminal domain in the crystal structure of full length Mpro (PDB code: 2H2Z) (Xue et al. 2007) are indicated on the top of graph. The solid and hollow boxes stand for α-helices and β-strands, respectively
Acknowledgments
All NMR experiments were carried out at the Beijing Nuclear Magnetic Resonance Center (BNMRC), Peking University. This work was supported by Grant 2003CB514104 from the 973 Program of China and Grant 30125009 from NSFC to BX.
References
- Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6(3):277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Fan K, Wei P, Feng Q, Chen S, Huang C, Ma L, Lai B, Pei J, Liu Y, Chen J, Lai L. Biosynthesis, purification, and substrate specificity of severe acute respiratory syndrome coronavirus 3C-like proteinase. J Biol Chem. 2004;279(3):1637–1642. doi: 10.1074/jbc.M310875200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Blevins RA. NMR view: a computer program for the visualization and analysis of NMR data. J Biomol NMR. 1994;4(5):603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Yang H, Shen W, Zhao Q, Li J, Yang K, Chen C, Jin Y, Bartlam M, Rao Z. Production of authentic SARS-CoV Mpro with enhanced activity: application as a novel tag-cleavage endopeptidase for protein overproduction. J Mol Bio. 2007;366:965–975. doi: 10.1016/j.jmb.2006.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yang M, Ding Y, Liu Y, Lou Z, Zhou Z, Sun L, Mo L, Ye S, Pang H, Gao GF, Anand K, Bartlam M, Hilgenfeld R, Rao Z. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc Natl Acad Sci U S A. 2003;100(23):13190–13195. doi: 10.1073/pnas.1835675100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhong N, Xue F, Kang X, Ren X, Chen J, Jin C, Lou Z, Xia B. 3D domain swapping as a mechanism to lock the active conformation in a super-active octamer of SARS-CoV main protease. Protein Cell. 2010;1(4):371–383. doi: 10.1007/s13238-010-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N, Zhang S, Zou P, Chen J, Kang X, Li Z, Liang C, Jin C, Xia B. Without its n-finger, SARS-CoV main protease can form a novel dimer through its C-terminal domain. J Virol. 2008;82(9):4227–4234. doi: 10.1128/JVI.02612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N, Zhang S, Xue F, Kang X, Zou P, Chen J, Liang C, Rao Z, Jin C, Lou Z, Xia B. C-terminal domain of SARS-CoV main protease can form a 3D domain-swapped dimer. Protein Sci. 2009;18(4):839–844. doi: 10.1002/pro.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr J. Molecular biology of severe acute respiratory syndrome coronavirus. Curr Opin Microbiol. 2004;7(4):412–419. doi: 10.1016/j.mib.2004.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


