Abstract
Asthma is a chronic disease that has a significant impact on quality of life and is particularly important in children and adolescents, in part due to the higher incidence of allergies in children. The incidence of asthma has increased dramatically during this time period, with the highest increases in the urban areas of developed countries. It seems that the incidence in developing countries may follow this trend as well. While our knowledge of the pathophysiology of asthma and the available of newer, safer medication have both improved, the mortality of the disease has undergone an overall increase in the past 30 years. Asthma treatment goals in children include decreasing mortality and improving quality of life. Specific treatment goals include but are not limited to decreasing inflammation, improving lung function, decreasing clinical symptoms, reducing hospital stays and emergency department visits, reducing work or school absences, and reducing the need for rescue medications. Non-pharmacological management strategies include allergen avoidance, environmental evaluation for allergens and irritants, patient education, allergy testing, regular monitoring of lung function, and the use of asthma management plans, asthma control tests, peak flow meters, and asthma diaries. Achieving asthma treatment goals reduces direct and indirect costs of asthma and is economically cost-effective. Treatment in children presents unique challenges in diagnosis and management. Challenges in diagnosis include consideration of other diseases such as viral respiratory illnesses or vocal cord dysfunction. Challenges in management include evaluation of the child’s ability to use inhalers and peak flow meters and the management of exercise-induced asthma.
Keywords: Asthma, Allergies, Exercise-induced asthma, Pediatric asthma, Atopic march, Wheezing, Viral respiratory infections
Introduction
The incidence of allergies and asthma in the Western world has been increasing over the past 30 years. However, more recent data suggest that over the past 5–10 years, the overall global trends of asthma incidence have begun to stabilize [1]. Urbanization and industrialization has contributed to the increase in developed countries, but the reasons for this are still unclear. Asthma is estimated to be responsible for one in every 250 deaths worldwide. Many of these deaths are preventable, and specific issues have been identified that may contribute to this high mortality rate. Factors that contribute to high mortality and morbidity include slow access to care and medications, inadequate environmental control of allergens and irritants, dietary changes, genetic variations, cultural barriers, lack of education among patients and providers, insufficient resources, and improper use of health care dollars.
The Global Initiative for Asthma, initiated in 1989 for the World Health Organization and the US National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health (NIH) periodically establish guidelines for the diagnosis and treatment of asthma [2–4]. The most recent significant update to these recommendations appeared in the Expert Panel Review 3 (EPR-3) published by the National Education for Asthma Prevention Program (NAEPP) coordinating committee of the NHLBI of the NIH [4]. A historical timeline of these revisions is shown in Fig. 1.
Fig. 1.
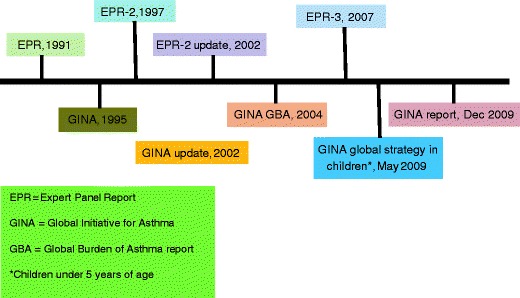
Historical chart showing recommendations in asthma treatment worldwide
The development of newer medications and delivery devices over the past 25 years has made a significant impact on our ability to decrease morbidity of childhood asthma. Hospitalizations for asthma have clearly decreased as a result of the use of controller medications. Quality of life has been identified as a significant metric to measure asthma treatment success. On the other hand, despite our improved knowledge of the pathogenesis of asthma, newer medications with fewer adverse effects, and increased standardization of treatment protocols, there has been a paradoxical increase in asthma mortality. The reasons for this observation are debatable but may include lifestyle changes [5], dietary changes [6], the increase in obesity rates in the Western hemisphere [7], coding anomalies [8], poor patient and/or caregiver education, and the overall increase in the incidence of allergies and asthma. What is clear, however, is that the development and introduction of new pharmaceuticals is not by itself the answer to improving outcomes in children with asthma. Patient education, environmental avoidance measures, proper use of medications, and immunotherapy are all equally important in the successful treatment of the pediatric asthmatic. The good news is that with our awareness of these factors, mortality has at least stabilized over the past 5 years.
Asthma in children has a unique set of characteristics the merit discussion (Table 1). The diagnosis of asthma in children may be difficult to make in the infant because of the prevalence of viral-associated wheezing in this age group of patients. The impact of viral illness on the development of asthma in later years is addressed below, along with the role of allergies in childhood asthma. Certain medications are not always appropriate for all ages, and devices that are used to evaluate asthma status may not be usable by young children. Exercise-induced asthma (EIA) is particularly important in childhood as physical activity is critical to controlling the epidemic of obesity in developed countries, especially the USA. Lifestyle changes, including the use of television, Internet, and other video devices, may play a role in childhood asthma. Finally, the “hygiene hypothesis” has introduced the concept that early exposure to animals, foods, or endotoxin may actually be protective against allergic sensitization. While this is still a controversial issue, it illustrates the complexity of asthma as a heterogeneous disease, with genetic and environmental influences. Indeed, we now know that there is no single asthma gene, rather that there are multiple genetic variants that, under the proper environmental conditions, can result in asthma.
Table 1.
Special considerations for asthma in children
| The increased significance of allergies in childhood asthma |
| The role of passive smoking (ETS exposure) in infancy and pregnancy in the development of asthma |
| The role of RSV and other viral bronchiolitis in pediatric asthma |
| Genetics (host factors) versus environmental exposures in childhood asthma |
| Vocal cord dysfunction in teenage athletes with or without concurrent asthma |
| The increase in obesity in children and its impact on childhood asthma |
| Gender predisposition for asthma in children is reversed that in adults |
| Availability of asthma medications and indications in the pediatric age group |
| The role of immunotherapy in the very young child |
| The use of biologics in children (omalizumab and new drugs) |
| Corticosteroids and growth retardation |
| Exercise-induced asthma and sports in children |
Epidemiology and the Prevalence of Childhood Asthma
The prevalence of asthma is now estimated to be more than 300 million worldwide, or about 5% of the global population. The incidence of asthma has been steadily increasing since the 1970s, with the greatest increase occurring in modern, developed countries. Asthma accounts for about one in every 250 deaths worldwide. The national prevalence of asthma in different countries varies between 1% and 19% (Table 2). It has been observed that developed countries have the higher incidences, while third world countries have the lower rates, but in recent years, the gap is decreasing due to an increasing incidence of asthma in Asia, South America, and Africa.
Table 2.
Asthma prevalence by country (selected countries)
| Country | Prevalence (% population) |
|---|---|
| Scotland | 18.5 |
| Wales | 16.8 |
| England | 15.3 |
| New Zealand | 15.1 |
| Australia | 14.7 |
| Republic of Ireland | 14.6 |
| Canada | 14.1 |
| Peru | 13.0 |
| Trinidad and Tobago | 12.6 |
| Costa Rico | 11.9 |
| Brazil | 11.4 |
| United States | 10.9 |
| Fiji | 10.5 |
| Paraguay | 9.7 |
| Uruguay | 9.5 |
| Israel | 9.0 |
| Panama | 8.8 |
| Kuwait | 8.5 |
| Ukraine | 8.3 |
| Ecuador | 8.2 |
| South Africa | 8.1 |
| Finland | 8.0 |
| Czech Republic | 8.0 |
| Columbia | 7.4 |
| Turkey | 7.4 |
| Germany | 6.9 |
| France | 6.8 |
| Norway | 6.8 |
| Japan | 6.7 |
| Hong Kong | 6.2 |
| United Arab Emirates | 6.2 |
| Spain | 5.7 |
| Saudi Arabia | 5.6 |
| Argentina | 5.5 |
| Chile | 5.1 |
| Italy | 4.5 |
| South Korea | 3.9 |
| Mexico | 3.3 |
| Denmark | 3.0 |
| India | 3.0 |
| Cyprus | 2.4 |
| Switzerland | 2.3 |
| Russia | 2.2 |
| China | 2.1 |
| Greece | 1.9 |
| Georgia | 1.8 |
| Romania | 1.5 |
| Albania | 1.3 |
| Indonesia | 1.1 |
The gender predominance is reversed in children from that in adults. In children under the age of 14, there is a 2:1 male-to-female prevalence of asthma, approximately opposite that in adults. Obesity appears to be a risk factor for asthma. Diet is more complicated, and the initial observation that breast feeding protects against asthma is being challenged. Exposure to Western diets comprising high levels of processed foods with increased levels of n-6 polyunsaturated fatty acids, decreased antioxidant levels, and decreased n3-polyunsaturated fatty acids has been associated with the increase in asthma and allergies observed over the past few decades.
Genetics and Asthma
Asthma is heritable. Children born to asthmatic parents have an increased chance of developing asthma as well. Asthma is polygenic, and multiple phenotypes exist. It is estimated that up to 70% of asthma in children is associated with atopy, or allergies. Airway hyperresponsiveness, serum IgE levels, inflammatory mediator expression, and Th1/Th2 balance are four areas that may be influenced by genetics. The genes involved in regulating these processes may differ between ethnic groups. Some of these processes, such as airway hyperresponsiveness and serum IgE levels, may be co-inherited in some individuals, possibly as a result of close proximity of genes affecting both processes (chromosome 5q).
Genes can also affect an asthmatic child’s response to medications. This has been found to be the case for β-adrenergic agonists, glucocorticoids, and leukotriene modifiers. While studies of pharmacogenetics have so far produced more questions than answers, this is an exciting area of research because it promises the capability of generating customized care plans, or personalized medicine, that will match optimal treatment with each individual asthmatic child. Genes that are involved in asthma are now too numerous to count. An example of genes associated with asthma is shown in Table 3.
Table 3.
A few selected candidate genes for asthma and their impact on disease or treatment
| Gene | Target response/asthma phenotype | Population/location |
|---|---|---|
| CTLA-4 [9] | Response to corticosteroids | European |
| Arginase 1 and 2 [10] | Response to bronchodilators | The Netherlands |
| Glutathione S-transferase [11, 12] | With air pollution as interactive risk factors | Italy |
| ADAM-33 [13] | Risk for asthma, elevated IgE and increased specific IgE to dust mite species | Columbia |
| IL-4R [14] | Specific asthma phenotype, eczema and allergic rhinitis | Sweden |
| DENNB1B [15] | Increased susceptibility to asthma (GWAS study) | North America |
| LTA4H and AOX5AP [16] | Gene–gene interactions convey variants in asthma susceptibility | Latinos (Mexico and Puerto Rico) |
| TLR4 [17] | Gene polymorphisms convey risk of asthma (IRAK1, NOD1, MAP3K7IP1 gene–gene interactions) | The Netherlands |
| PHF11 and DPP10 [18] | Risk for asthma | Chinese, European, and Latin American |
| NOS1 [19] | Increased IgE levels, increase in frequency of asthma phenotype | Taiwanese |
| ECP [20] | Allergy and asthma symptoms, smoking | From the European Community Respiratory Health Survey |
| TSLP [21] | Higher risk of childhood and adult asthma | Japan |
| RANTES [22] | Higher risk of asthma in subgroup analysis by atopic status | Global |
TLR Toll-like receptor, PHF11 plant homeodomain zinc finger protein 11, DPP10 dipeptidyl-peptidase 10, ADAM-33 a disintegrin and metalloprotein 33, LTA4H leukotriene A (4) hydrolase, ALOX5AP arachidonate 5 lipooxygenase activating protein, IL-4R interleukin 4 receptor, CTLA-4 cytotoxic T-lymphocyte antigen 4, NOS-1 nitric oxide synthase 1, ECP eosinophilic cationic protein, TSLP thymic stromal lymphopoietin, RANTES regulated upon activation, normal T cell expressed, and secreted, GWAS genome-wide associations studies
Diagnosis and Differential Diagnosis of Childhood Asthma
Making the diagnosis of childhood asthma requires taking a thorough history and doing a complete physical examination on the patient. There are many conditions which may mimic asthma. Some of these diagnoses are age dependent. A complete list of conditions in the differential diagnosis is shown in Table 4. Asthma is still a clinical diagnosis, as there is no pathognomonic marker for diagnosing asthma. Taking a good history is critical. A good history includes current or past history of cough, wheezing, viral respiratory diseases, accompanying allergy symptoms or signs, shortness of breath, and sinus problems. It is also important to ask an exposure history, to try and determine triggers for the episodes. This can include indoor allergen exposures, outdoor pollens, viral upper respiratory infections and exercise, exposure to foods that may be causing symptoms, and an occupational history of both the child (if old enough to be working) and the parents or caregivers. Exposure to day care is also important and environmental tobacco smoke and pollution exposure should also be documented. A past medical history asking about previous hospitalizations, doctor visits, frequent otitis media, sinusitis or pneumonia, and a complete medication history, including use of nebulizers or inhalers and any other pertinent medications, should be elicited. The family history is also important, as asthma has a genetic component.
Table 4.
Differential diagnosis of cough, wheezing, and other bronchial sounds
| Asthma |
| Foreign body aspiration |
| Aspiration pneumonia |
| Bronchopulmonary dysplasia |
| Heart disease |
| Infections (may be viral, bacterial, fungal, or mycobacterial) |
| Pneumonia |
| Bronchitis |
| Bronchiolitis |
| Epiglottitis (stridor, respiratory distress) |
| Sinusitis |
| Exposures |
| Allergies |
| Smoke inhalation |
| Toxic inhalations |
| Hypersensitivity pneumonitis |
| Gastroesophageal reflux |
| Genetic disorders |
| Cystic fibrosis |
| Iatrogenic |
| ACE inhibitor-related cough |
| Anatomical abnormalities |
| Vocal cord dysfunction |
| Vocal cord anomalies (nodules) |
| Subglottic stenosis (stridor) |
| Laryngotracheal malacia (in infants) |
| Vascular anomalies of the chest |
| Endotracheal fistulas and tracheal anomalies |
| Other |
| Immunodeficiency syndromes |
| Obesity |
In addition to taking a good history, a complete physical examination should also be conducted to rule out any other possible diagnoses. The intensity and characteristics of any breathing sounds should be determined. While the presence of a wheeze may suggest asthma, foreign body aspiration may also be a possibility if the wheeze is unilateral. The presence of a heart murmur may suggest a coarctation with compression of the trachea. Clubbing of the fingers may be suggested of a more chronic condition such as cystic fibrosis. These are only some of the many observations one may glean from a physical examination that can help in establishing or ruling out the diagnosis of asthma.
Procedures and laboratory tests have a role in the diagnosis of asthma. Allergy testing may be indicated if the history is consistent with an allergic component or trigger. Spirometry should certainly be done, with the response to bronchodilators examined as well. Exercise challenge tests can be done in the child or adolescent who suspects exercise-induced asthma or bronchospasm. Other tests that may be helpful in monitoring the condition of an asthmatic may be fractional inhaled nitric oxide. A complete blood count, chest X-ray, or sinus CT scan may also be indicated in some cases. Components of a diagnostic scheme and clinical assessment of asthma are shown in Fig. 2.
Fig. 2.
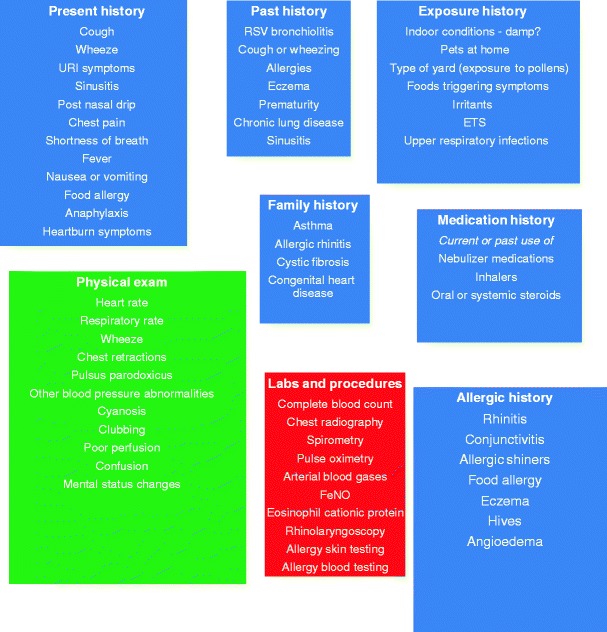
Diagnosis and clinical assessment of the asthmatic child—the appropriate parts of the history and physical examination should be performed depending on the circumstances (e.g., is this a new patient with a history of cough presenting to the office as a consult, or is this a patients with known asthma who is in the midst of an asthma exacerbation presenting to the emergency room)
While we think of wheezing as a hallmark of asthma, it can be present in children without asthma and absent in those with asthma. In children under 5 years of age, wheezing can be categorized into three groups: transient early wheezing, persistent early-onset wheezing, and late-onset wheezing/asthma. These are discussed in more detail below.
Triggers of Asthma in Children
Allergens
It is estimated that between 60% and 70% of asthma in children is allergic asthma. Conversely, children with allergies have a 30% chance of having asthma as well. The atopic march describes a commonly seen paradigm in which children who are atopic (genetically predisposed to developing allergies) present early in life with atopic dermatitis, then asthma, and finally allergic rhinitis and conjunctivitis. Common allergens can be categorized into either indoor or outdoor allergens. The outdoor allergens are mostly linked to seasonal allergic rhinitis, while indoor allergens are linked to perennial allergic rhinitis. There are geographical differences in the seasonal distribution of pollen allergens. Recently, it has been suggested that climate change may be impacting the prevalence of various outdoor allergens through different mechanisms. In addition, environmental exposures and host factors can lead to changes in an individual’s sensitivities throughout life. Certain allergens, such as dust mite, cat and dog dander, and Aspergillus, are independent risk factors for the development of symptoms of asthma in children under 3 years of age [23].
On the other hand, the “hygiene hypothesis” has been used to explain why in some cases exposure to dogs and/or cats leads to a decrease in allergic sensitization [24]. These inconsistencies in studies on allergen sensitivity have not been adequately resolved, and it is likely that other factors, possibly related to the timing of exposure or host factors, play a significant role in the effect of allergen exposure on sensitization. In addition to cats and dogs, other pets such as guinea pig, gerbils, hamsters, mice, rats, and rabbits can also trigger asthma attacks in susceptible individuals.
It has been suggested that exposure to endotoxin may play a protective role in allergen sensitization, though this is not universally observed. Cockroach and mouse allergen have both been found to play a significant role in allergic sensitization in children living in inner city environments [25]. Both early- and late-phase reactions have occurred in places where cockroach infestation is a problem, such as highly populated urban areas in warm climates.
Food allergens can be a significant cause of allergic sensitization in children, especially in the younger age range. Foods are typically associated with eczema in infants and toddlers, but eczema is a feature of atopy and the earliest manifestation of the “atopic march.” These patients can subsequently develop asthma or allergic rhinoconjunctivitis as they grow older.
In order to treat allergic asthma effectively, it is therefore important to identify a child’s current allergic sensitization patterns. This can be done by skin testing or by a blood test that measures specific IgE. There are advantages and disadvantages to both forms of testing. Skin testing is more sensitive and specific, although the blood test methodology is improving. The blood test can be done if there are contraindications to skin testing, such as in the very young child who may not be able to tolerate skin testing, a patient on antihistamines or β-blockers, or a patient with a severe rash. Available now are “multi-test” devices that facilitate skin testing and make it possible to perform in the very young child. A list of common environmental allergens is shown in Table 5. While food allergens can also trigger asthma, these are less likely triggers, and testing for food allergens is not as sensitive or specific as environmental allergens.
Table 5.
Common allergenic determinants
| Determinant | Source | Source scientific name | Protein class/function |
|---|---|---|---|
| Der p 1 | Dust mite | Dermatophagoides pteronyssinus | Cysteine protease |
| Der p 2 | Dust mite | Dermatophagoides pteronyssinus | Serine protease |
| Der f 1 | Dust mite | Dermatophagoides farina | Cysteine protease |
| Der f 2 | Dust mite | Dermatophagoides farina | Serine protease |
| Der m 1 | Dust mite | Dermatophagoides microcerax | Cysteine protease |
| Blo t 1 | Dust mite | Blomia tropicalis | Cysteine protease |
| Fel d 1 | Cat | Felis domesticus | Salivary glycoprotein |
| Can f 1 | Dog | Canis familiaris | Salivary lipocalin proteins |
| Bla g 1 | Cockroach | Blatella germanica | Unknown |
| Bla g 2 | Cockroach | Blatella germanica | Aspartic proteinase |
| Rat n 1 | Rat | Rattus norvegicus | Major urinary protein |
| Mus m 1 | Mouse | Mus muscularis | Major urinary protein |
| Per a 7 | Cockroach | Periplaneta americana | Tropomyosin |
| Lol p 1 | Ryegrass | Lolium perenne | Unknown |
| Amb a 1 | Ragweed | Ambrosia artemistifolia | Polysaccharide lyase 1 family |
| Aln g 1 | Alder | Alnus glutinosa | Pathogenesis-related protein |
| Bet v 1 | Birch | Betula verrucosa | Pathogenesis-related protein |
| Que a 1 | Oak | Quercus alba | Pathogenesis-related protein |
| Ole e 1 | Olive | Olea europea | Unknown |
| Cyn d 1 | Bermuda grass | Cynodon dactylon | Expansin family |
| Art v 1 | Mugwort | Artemisia vulgaris | Unknown |
| Dac g 3 | Orchard grass | Datylus glomerata | Expansin family |
Irritants
Most air pollutants are irritants, although some can have immunomodulatory effects. The most extensively studied airborne pollutant is environmental cigarette smoke. Gaseous irritants include nitric oxide, sulfur dioxide, formaldehyde, ozone, and other volatile organic compounds. Airborne particulates can vary in size, ranging from course particulates to fine particles to ultrafine particles or nanoparticles. The smaller the particle, the greater the penetration into the airway. Particles greater than 10 μm in diameter are generally cleared in the upper airway. Although these larger particles can indirectly trigger early- or late-phase asthma reactions by virtue of their action in the upper airways, fine and ultrafine particles present a more significant problem due to their deeper penetration into the smaller airways [26]. Recently, evidence has surfaced that these ultrafine particles indeed possess effects on inflammatory cells such as neutrophils or eosinophils that can lead to asthma symptoms. This can occur through several different mechanisms and is reviewed extensively elsewhere [26].
Exercise-Induced Asthma or Bronchospasm
Exercise is a common trigger for asthma in children [27]. However, exercise is essential to the physical and psychological development of all children. Lack of exercise also promotes obesity, which has been linked to asthma [28]. Therefore, we no longer recommend that children with asthma stop exercising. In fact, exercise can help to build lung reserve. With proper control of asthma using controller medications, a patient with exercise-induced asthma can almost always participate in regular cardiovascular training without adverse effects. Asthma can even be managed successfully in the elite athlete [29]. The severity of a patient’s EIA can be evaluated using an exercise challenge test. Historically, the methodology of exercise challenge tests has been very inconsistent, but recently the American Thoracic Society has proposed a set of criteria that should be met in order to ensure an accurate and valid test [30]. These criteria and a typical protocol are illustrated in Fig. 3.
Fig. 3.

a An exercise challenge protocol. b Exercise challenge test results sheet—sample. c Guidelines/criteria for exercise challenge testing
Viral Respiratory Illness and Asthma
The link between viral respiratory infections and asthma has been well established [31–33]. In infants, the most well-known viral respiratory illness associated with wheezing is respiratory syncytial virus (RSV). However, rhinovirus is the more common virus in children [34, 35], and wheezing can be associated with other viruses as well, including parainfluenza virus, influenza virus, metapneumonia virus, adenovirus, coronavirus, and picornavirus. Rhinovirus is the major pathogen associated with hospital admissions for asthma in children [36].
Transient early wheezing develops in infancy and usually resolves by 3 years of age. Risk factors include low birth weight, prematurity, maternal exposure to tobacco smoke, male gender, and upper respiratory viral infections. A second phenotype is that of the atopic wheezer. These are school age children who have a history or family history of atopy. Their wheezing may or may not be triggered by a viral infection, but they have a strong probability of continued wheezing into adolescence or adulthood. It is in this group that one would be able to detect chronic inflammatory changes in the airways. A third category, persistent early-onset wheezing, includes pre-school aged children and is usually not associated with a family history of asthma or atopy but instead is associated with acute viral respiratory infections. This group has a better long-term prognosis and symptoms may disappear when they reach school age.
Bronchiolitis in infancy can lead to decreased FEV1 and FEF25–75% in childhood [37]. A connection between asthma and RSV bronchiolitis was supported by an observation that there is elevated ECP and leukotriene C4 in the nasal lavage fluid of infants with RSV bronchiolitis [38]. It is not clear whether the occurrence of RSV bronchiolitis as an infant significantly increases the risk of developing asthma at a later age, although one prospective study of 47 children hospitalized for RSV bronchiolitis in infancy showed a higher incidence of airway hyperreactivity at age 13 compared to 93 matched controls [39]. Continued follow-up of these patients revealed a persistent increase in allergic asthma into early adulthood among patients who had RSV bronchiolitis in infancy [40].
Interpreting the New Asthma Guidelines in Children
The new EPR guidelines were released in 2007 [4]. These guidelines contain revisions that were aimed at improving the overall care of asthmatics. There are several important changes. Firstly, the main goal of asthma treatment is control of symptoms and disease. A list of specific goals to target control is shown in Table 6. Better distinction is made between monitoring asthma control and classifying asthma severity. Severity is defined as the intrinsic intensity of asthma and is still grouped into the original classification of mild intermittent, mild persistent, moderate persistent, or severe persistent. Categorizing severity in this manner is helpful for initiating therapy. Control is defined as the response to therapy, in terms of the degree to which manifestations of asthma are kept to a minimum. Therapy should be adjusted periodically in order to maintain control.
Table 6.
Treatment goals in asthma
| Decreasing mortality |
| Decreasing morbidity and improving quality of life |
| Fewer nighttime awakenings |
| Ability to participate in sports with no limitations |
| Fewer school or work days lost |
| Reduction of symptoms of cough or wheezing |
| Reduction in the need for rescue medications |
| Easy compliance with medications with minimal disruptions in daily life |
| Reduction in side effects of asthma medications |
| Reduction in the number and severity of asthma exacerbations |
| Reduction in emergency or unscheduled office or clinic visits |
| Reduction in the need for systemic steroids |
| Prevention of “airway remodeling” and long-term sequelae of asthma |
| Economic goals |
| Reducing costs of treating asthma by improving preventative measures |
The second major change is the focus on impairment and risk. These are the two key domains of control and severity and provide additional information or parameters to assess response to treatment. Impairment is defined as the extent to which standard goals of asthma treatment are maintained, so this includes the frequency and intensity of symptoms and interference with good quality of life, such as an inability to conduct normal daily activities. Risk can include several parameters—the likelihood of developing an asthma exacerbation, the risk of side effects of medications, and the risk of declining lung function or lung growth.
In order to address the change in focus, the treatment recommendations have also been adjusted. The stepwise approach is still utilized, but now there are six steps, with clearly defined actions, instead of having progressive actions within each step. The treatment recommendations have also been divided into three groups depending on age, a group for children 0–4 years of age, another group addressing children 5–11 years of age, and the third group consisting of adults and children 12 and over. This was done because the evidence for the various treatment modalities may be different between age groups.
Other important recommendations address environmental control, with the recommendation for these actions being present in all age groups. Inhaled corticosteroids are the first-line control drug for all ages. The use of combination inhaled steroid and long-acting β-agonists is considered an equal alternative to increasing the dose of inhaled corticosteroids in patients 5 years of age and older, although the FDA has recently issued a black box warning on the use of long-acting beta agonists. Omalizumab is also recommended in patients with allergic asthma who are 12 years of age and older who require step 5 or 6 therapy. A black box warning for anaphylaxis accompanies omalizumab. The breakdown of the stepwise approach for children under 12 is shown in Tables 7 and 8.
Table 7.
EPR-3 guidelines for the diagnosis and treatment of asthma in children (0–4 years of age)

The above table was adapted from NAEPP EPR-3 guidelines [4]
Table 8.
EPR-3 guidelines for the diagnosis and treatment of asthma in children (5–11 years of age)

The above table was adapted from NAEPP EPR-3 guidelines [4]
Non-pharmacologic Management of Childhood Asthma
An asthma management plan involves approaching the problem from three different angles—environmental control, pharmacologic intervention, and immunotherapy. In addition, objective measurement of asthma status is important, and ongoing monitoring is also of benefit. It is clear that the development of new drugs is only a part of a more comprehensive strategy to treat asthma. In addition to drugs, non-pharmaceutical modes of treatment need to be incorporated into the asthmatic child’s treatment plan. Non-pharmaceutical modes of therapy for asthma are discussed below and listed in Table 9.
Table 9.
Ancillary treatment modalities of asthma
| Objective measurement of asthma status |
| Peak flow monitoring |
| Pulmonary function testing |
| Spirometry |
| Environmental control and identifying sensitivities |
| Allergy skin testing |
| Environmental exposure assessment |
| Allergen avoidance |
| Monitoring and prevention |
| Asthma diary sheets |
| Asthma action or management plans |
| Education |
| Asthma education |
| Exercise regimen |
| Asthma camps for children |
| Dietary assessment |
| Tobacco prevention counseling for parents |
| Internet |
| Printed material |
| Special help for children |
| Use of spacer devices |
| Small volume nebulizer machines |
Environmental Control
Allergen challenge studies have shown that exposure to an allergen to which an asthmatic has been sensitized is likely to bring about an asthma exacerbation [41–43]. Conversely, avoidance of such allergens may lead to resolution of the exacerbation. Thus, allergen avoidance has been recognized as an important part of an asthma management plan. The effectiveness of an allergen avoidance plan requires knowledge of the patient’s sensitivities and exposure pattern.
Avoidance of seasonal allergens, mainly pollens, is difficult without making unreasonable changes to one’s lifestyle because these allergens are windborne and can travel for miles. On the other hand, there are well-established strategies to avoidance of indoor allergens. Because we spend up to a third of our time sleeping in close quarters with dust mites, the bedroom should have a high priority when developing an indoor allergen avoidance program. Dust mites require water to survive, and damp environments allow them to reproduce and proliferate. Keeping the relative humidity of the home around 50–55% will help keep dust mite concentrations down. In addition, the use of mattress and pillow encasings, high-efficiency particulate air filters are additional control measures that may provide benefit. The child with asthma should also probably not be the one to vacuum as the action of vacuuming can disturb dust mite reservoirs and release these particulates into the breathing zone. Good ventilation and filtration systems in the home can also help to reduce exposure. Additional measures of allergen avoidance are illustrated in Fig. 4.
Fig. 4.

Avoidance measures for common allergens
Avoidance of pet allergen is best accomplished by getting rid of the pet altogether. This is often an insurmountable task because of the emotional attachment that patients, especially children, have toward their pets. If getting rid of the pet is not possible, then at least keeping the pet out of the bedroom may help. Washing the pet regularly is of questionable benefit. Numerous “denaturing” preparations are also available, but again, their effectiveness is controversial.
Molds are common allergens that originate from the outdoor environment and are particularly prevalent in moist climates. The presence of a high indoor to outdoor mold count ratio is probably indicative of a water leak or at least of excessive humidity indoors. As in the case of dust mites, keeping the relative humidity of the indoor environment at around 50% helps to reduce mold spore exposure. Substrates for mold growth include decaying living material, damp paper or books, or household plants. Removal of these substrates may reduce indoor mold spore levels.
As mentioned above, pollens are more difficult to avoid, but patients and their parents can glean information regarding outdoor exposures by accessing the American Academy of Allergy, Asthma and Immunology National Allergy Bureau website (see Table 22). The site contains information on pollen and mold counts derived from counting stations run by certified counters. As of Oct 2010, there were 85 counting stations throughout the USA, as well as two in Canada and two in Argentina.
Table 22.
Asthma resources for physicians, patients and parents
| World Allergy Organization | www.worldallergy.org |
| American Academy of Allergy, Asthma and Immunology | www.aaaai.org |
| American College of Allergy, Asthma and Immunology | www.aaaai.org |
| World Health Organization | www.who.org |
| American Lung Association | www.lungusa.org |
| American Thoracic Society | www.thoracic.orgg |
| Asthma and Allergy Foundation of America | www.aafa.org |
| National Technical Information Service | www.ntis.gov |
| National Asthma Education and Prevention Program | www.nhlbi.nih.gov/about/naepp/ |
| National Heart, Lung and Blood Institute | www.nhlbi.nih.gov |
| Allergy and Asthma Network/Mothers of Asthmatics | www.aanma.org |
| Center for Disease Control | www.cdc.gov |
| Global Initiative for Asthma | www.ginaasthma.com |
| National Allergy Bureau | http://pollen.aaaai.org/nab/ |
| Kidshealth | www.kidshealth.org |
Spirometry in Children
Spirometry provides information on lung mechanics. Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), the FEV1/FVC ratio, and peak expiratory flow (PEF) are the four main parameters measured during spirometry. Measurement of spirometry before and after bronchodilation treatment can help determine if there is reversibility of lung function. Because sometimes patient history, especially in children, can be inaccurate, spirometry provides an objective assessment of the child’s condition. Spirometry can be attempted at about 4 to 5 years of age, but at these very young ages, obtaining reliable results depends on the child’s ability to follow instructions and their physical coordination. Data on predicted values of spirometry parameters have been obtained by several investigators and are dependent on age, gender, height, and ethnic background. However, as in the case of peak flow measurement, there is significant individual variability, and it is important to establish each child’s baseline spirometry. Obviously, as the child grows, this will change, so frequent updates may be needed. While spirometry is not diagnostic of asthma, it serves as a complementary test to the history and physical that can be used to support the diagnosis of asthma.
The Use of Peak Flow Meters in Childhood Asthma
Measurement of peak flow should be part of an asthma management plan. Peak flow measurements provide objective evaluation of an asthmatic’s condition. With the proper teaching, even a 5-year old can learn to use a peak flow meter effectively (Fig. 5). Usually, peak flow measurements are done in the morning and in the evening, but the peak flow meter can be used throughout the day or night, whenever necessary. Most new peak flow meters are small enough to fit in a pocket or a purse. Traditional peak flow meters are available for children and adults. The low range peak flow meters usually measure up to about 450 l/m, while the high range measure up to 800 l/m. The peak flow zonal system, based on the child’s personal best peak flow, is a convenient and simple method for parents and patients to assess how they are doing, whether to administer a breathing treatment, and whether to seek additional help. Electronic versions of peak flow meters are also available. These have the advantage of being able to record and store data that can be analyzed and trended on a computer. Some of the newer electronic versions can also measure FEV1, which is considered to be a more reliable measurement of airway obstruction as it is not as dependent on patient technique or effort. An assortment of peak flow meters and spacers is shown in Fig. 6.
Fig. 5.
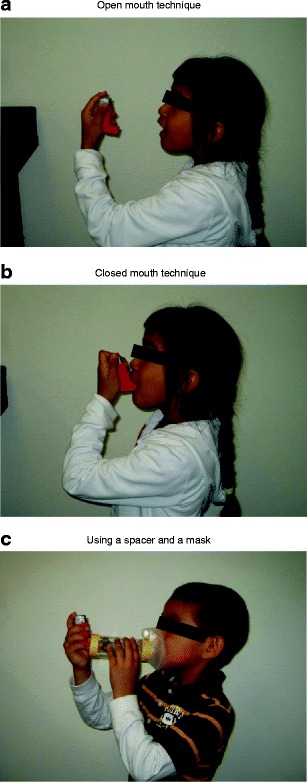
Inhaler technique. a Open mouth technique. b Closed mouth technique. c Using a spacer and a mask
Fig. 6.
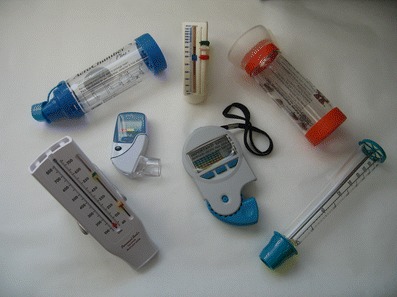
A sampling of peak flow meters and spacer devices. Clockwise from upper left Aerochamber Plus spacer device, Pocket Peak peak flow meter, Aerochamber with mask, Truzone peak flow meter, Airwatch electronic peak flow meter, Piko-1 electronic lung health meter, Personal Best adult range peak flow meter
Asthma Diary Sheets and Asthma Assessment Questionnaires
Asthma diary sheets (illustrated in Fig. 7) provide patients with a means to keep track of their symptoms and their peak flows. The recent availability of electronic peak flow meters with memory is an alternative way to monitor a patient’s asthma status, which is similar to monitoring blood pressure with an automatic blood pressure cuff or diabetes with a home glucose monitoring kit. Monitoring of peak flows not only gives a continuous assessment of the patient’s condition but also may help as a reminder for patients to take their control medications, thus improving compliance. Patients should be instructed to bring their asthma diary sheets to their doctor visit, so that their progress can be reviewed. Besides symptoms and peak flows, there is space to record other pertinent information, such as β-agonist use, exposures that are out of the ordinary, addition of new medications, etc.
Fig. 7.

An asthma diary
In addition to home monitoring, patients should complete an asthma assessment questionnaire each time they visit their asthma care provider (Fig. 8). From the asthma assessment questionnaire, a great deal of important information can be obtained, such as whether the patient is compliant with medications, if they are overusing their rescue inhaler, are they having too many nighttime awakenings, is their daily activity restricted, and so on, all of which speaks to asthma control.
Fig. 8.

A sample asthma assessment tool
Asthma Action Plans
The components of an asthma management plan are shown in Fig. 9. Asthma action plans are an important part of successful asthma management. They provide written guidance for parents and patients once they leave the doctor’s office or hospital. This is important because sometimes asthma treatment can be complicated for patients, and parents and patients can become overwhelmed with all the instructions about multiple medications, how to use a peak flow meter, and what to do in an emergency. The asthma action plan can also serve as a refresher course for patients, who have recently been discharged from the hospital after an admission for an asthma exacerbation [44]. Involving schoolteachers in a child’s asthma action plan can also help to improve the overall asthma control and quality of life [45].
Fig. 9.

An asthma management plan. An asthma action plan must include information on how to assess the child’s condition. Known triggers should be listed and the PEF zonal system can be used to provide easy instructions for patients and parents. The form also allows for entering medication doses
One of the reasons for the persistently high asthma morbidity in the face of newly developed asthma medications is that patients do not usually follow all the instructions to ensure effective asthma control. Written action plans help circumvent this problem, making it easier for patients to be compliant. Besides medication instructions, asthma action plans can also give instructions on environmental control and avoidance of triggers. Instructions on when to seek professional help is also entered on an asthma action plan.
Objective Methods of Assessing Airway Inflammation
Nitric Oxide
Fractional exhaled nitric oxide (FeNO) is elevated in children with asthma [46]. It has been demonstrated to be a marker of eosinophilic airway inflammation in children with asthma, and it also responds to glucocorticoid therapy [47–49]. Measurement of FeNO may be an effective way of monitoring airway inflammation and bronchial hyperresponsiveness [50]. Recently, increased levels of FENO have been found to correlate with risk of asthma in children [51]. Equipment to measure FeNO is currently available [52], but this test has not yet been widely adopted, mainly because there is still controversy regarding its value in managing the asthmatic child [53–55] but also because insurance companies have been slow to reimburse for this test adequately. As these issues are sorted out, FeNO may yet prove to be a valuable tool to assess airway inflammation.
Eosinophil Cationic Protein
Elevated eosinophil cationic protein (ECP) levels in cord blood are predictive of atopy. ECP is a marker of eosinophil activation. Serum ECP levels correlate with airway inflammation in wheezing children [56]. In a retrospective study of 441 patients with respiratory disease, the sensitivity and specificity for asthma were 70% and 74%, respectively. ECP was not predictive for any other respiratory disease [57]. When patients with asthma are bronchial challenged with allergen, activation of eosinophils and generation of specific eosinophilic mediators result. Evaluation and continued monitoring of eosinophil and ECP may be a way to assess efficacy of asthma therapy and airway inflammation in children with allergic asthma [58]. Both leukotriene receptor antagonists and inhaled corticosteroids have been associated with a reduction in sputum ECP levels in patients with mild to moderate persistent asthma [59, 60]. A response of serum ECP levels to glucocorticoid treatment has also been observed [61].
Pharmacologic Management
Controller Medications
Inhaled Corticosteroids
The beneficial effect of ACTH in the treatment of asthma was shown in 1949 [62, 63]. Subsequently, oral corticosteroids were also shown to be beneficial but side effects limited their widespread use. The introduction of inhaled corticosteroids in 1972 heralded a new age in asthma treatment, and inhaled corticosteroids have been the first-line treatment in the control of asthma since then. Corticosteroids work by switching off inflammatory genes through their interaction with the glucocorticoid receptor and recruitment of histone deactylase-2. By regulation of transcription of inflammatory genes or their promoters, they exert a number of anti-inflammatory effects (as illustrated in Fig. 10).
Fig. 10.
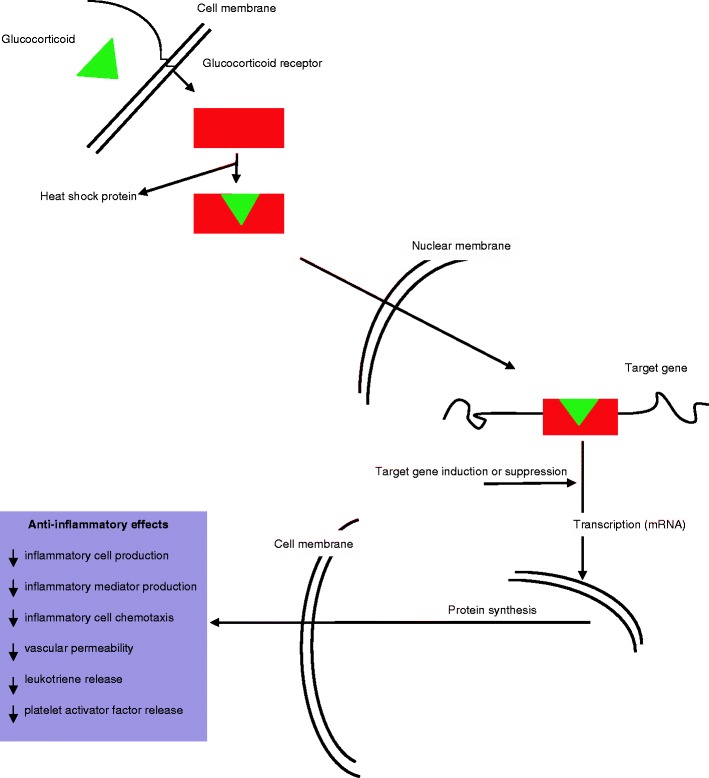
Mechanism of action of glucocorticoids
All of the different inhaled corticosteroids can be used in children. Budesonide is also available in nebulized form, in three strengths, 0.25, 0.5, and 1.0 mg. A multicenter study of 481 children demonstrated improvement in daytime and nighttime symptoms when treated with nebulized budesonide. Inhaled corticosteroids are now the first-line drug for treatment of mild, moderate, and severe persistent asthma [64] (Table 10).
Table 10.
Steroid dose equivalency
| Scientific name | Dose equivalency (mg) | Relative potency | Half-life (h) | Comment |
|---|---|---|---|---|
| Cortisone | 25 | 0.8 | 8–12 | |
| Hydrocortisone | 20 | 1 | 8–12 | |
| Prednisone | 5 | 4 | 12–36 | Available in liquid or tablet form |
| Prednisolone | 5 | 4 | 12–36 | Available in liquid or tablet form |
| Methylprednisolone | 4 | 5 | 12–36 | Used in ED or hospitalized patients |
| Triamcinolone | 4 | 5 | 12–36 | |
| Paramethasone | 2 | 10 | 36–72 | |
| Dexamethasone | 0.75 | 26.67 | 36–72 | |
| Betamethasone | 0.6 | 33.34 | 36–72 |
The issue of adverse effects of inhaled steroids in children has been extensively studied. Steroids are associated with numerous side effects (see Table 11). Most of these have been attributed to oral or parenteral steroids. In children with asthma, the major concerns regarding inhaled or nebulized steroids have been the effect on growth [65–67]. Studies to determine if inhaled corticosteroids indeed have such an effect are difficult to conduct because asthma itself has been associated with growth retardation [68]. Results have therefore been inconsistent; however, the bulk of the evidence suggests that even if there is growth retardation, this is usually reversible and there is a period of “catch-up” growth. Moreover, even if corticosteroids do indeed affect growth, the extent of growth retardation is minimal. Thus, the risk of growth retardation is small compared to the potential for serious asthma exacerbations. Adrenal suppression in children on inhaled steroids is also not a significant problem. In a study of 14 children on a dry powder beclomethasone dipropionate inhaler, there was no suppression of the hypothalamic–pituitary–adrenal (HPA) axis [69]. The dose of beclomethasone was 12–25 μg/kg/day. Other studies have failed to demonstrate adverse effects on the HPA axis [70, 71]. On the other hand, use of high doses of fluticasone has been shown to cause HPA axis suppression [72]. It is not known if there is any clinical significance to these observed effects. A list of the available inhaled corticosteroids and their daily dosing regimens in pediatrics is shown in Table 12.
Table 11.
Adverse effects of asthma medications
| β-Agonists | Inhaled steroids | Systemic steroids | Anticholinergics | Leukotriene pathway modifiers | Theophylline | Anti-IgE |
|---|---|---|---|---|---|---|
| Tremors | Dysphonia | Hyperglycemia | Dry mouth | Elevated liver enzymes | Gastritis | Anaphylaxis |
| Tachycardia | Oral thrush | Hypertension | Blurry vision | Churg–Strauss syndrome | Seizures | |
| Muscle spasmsa | Growth retardationa | Osteonecrosis | Increased wheezing | Risk of suicide | Tremorsa | |
| Hypokalemia | Adrenal suppressiona | Osteoporosis | Insomnia | |||
| Tachyphylaxis | Cushing’s syndrome | Nausea/vomiting | ||||
| Hyperglycemia | Adrenal suppressiona | Tachycardia | ||||
| Headache | Moon faciesa | Hypokalemia | ||||
| Hyperactivitya | Gastritisa | Hypoglycemia | ||||
| Increase in asthma mortalityb | Psychological disturbancesa | Central nervous system stimulation | ||||
| Acnea | Headache | |||||
| Cataracts | Hyperactivitya | |||||
| Hirsutisma | ||||||
| Decreased platelet function | ||||||
| Growth retardationa |
aOf particular importance in children
bNot clearly established, may be related to other confounding issues
Table 12.
Daily pediatric doses of inhaled corticosteroidsb
| Medication | Pediatric indication | Dose/actuation (μg) | Dosing frequency | Mild persistent | Moderate persistent | Severe persistent |
|---|---|---|---|---|---|---|
| Number of actuations/day | Number of actuations/day | Number of actuations/day | ||||
| Beclomethasone dipropionate | 5–11 years | 40 | Bid | 2 | 2–4 | |
| 80 | Bid | 2 | 2 | |||
| Triamcinolonea | 6–12 years | 100 | bid to qid | 4–8 | 8–12 | 8–12 |
| Flunisolide | 6–15 years | 250 | Bid | 4 | 4 | 4 |
| Budesonide | 6 years and older | 200 | Bid | 1 | 2 | 4 |
| Nebulized budesonide | 12 months to 8 years | Ampules of 250, 500 and 1,000 mg | Bid | 1 mg total daily dose | ||
| Fluticasone | 12 years and older | 44 | Bid | 2–4 | 4–10 | |
| 110 | 2–4 | 4–8 | ||||
| 220 | 2–4 | 4–8 | ||||
| Fluticasone diskus | 4–11 years | 50 | Bid | 2–4 | ||
| 100 | 1–4 | 2–4 | ||||
| 250 | 1–4 | 2–4 | ||||
| Mometasone furoate | 4–11 years | 110 | Bid | 1 | 2 | 4 |
| 220 | Bid | 1 | 2 | |||
aTriamcinolone inhaler is no longer commercially available
bThese are suggested doses modified from the package inserts of each drug
Long-Acting β-Agonists
Long-acting β-agonists (LABAs) are available either alone or in combination with an inhaled corticosteroid. The two available long-acting β-agonists currently available are salmeterol xinafoate and formoterol fumarate. Salmeterol xinafoate possesses a long hydrocarbon chain connecting the binding site with the active site of the molecule. Theoretically, this conformation allows repetitive interaction between the active site and the target receptor, as the binding site is firmly attached to an alternate site on the cell membrane and the long chain acts as a tether. Salmeterol is indicated down to age 4. It used to be available as an MDI and the diskus, but now only the diskus is available. The dose per puff in the diskus is 50 μg and should be taken twice daily. The terminal elimination half-life of salmeterol is 5.5 h. Formoterol is available in an aerolizer, a dry powder device in which a capsule must be punctured in a specialized chamber. A total of 12 μg of drug is contained in one capsule. Formoterol is also dosed twice daily. The mean elimination half-life of formoterol in healthy subjects is 10 h. The structures of salmeterol and formoterol are illustrated in Fig. 11.
Fig. 11.
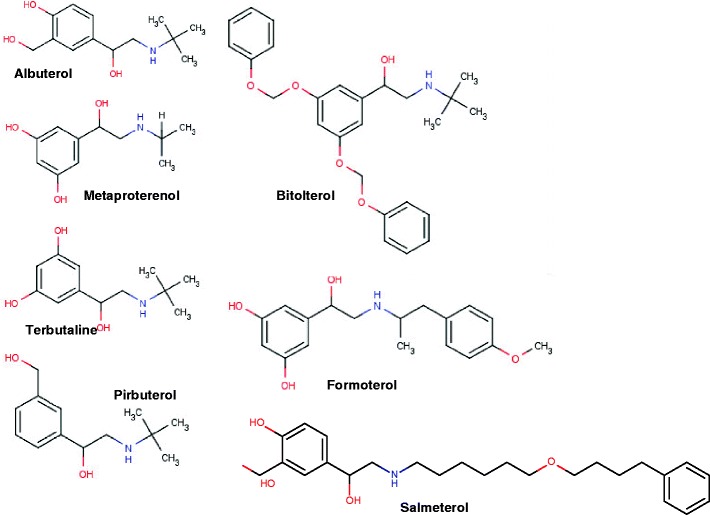
Structure of the β-adrenergic agonists. Comparison of the structures of albuterol and salmeterol helps to explain the long half-life of salmeterol. The long chain connects the binding site to the active site of the molecule. Once bound at the binding site, the long chain is theorized to swing back and forth, allowing the active site to repeatedly come in contact with the receptor site, prolonging the action of the drug
The LABAs are not generally considered first-line treatment for persistent asthma, and the current recommendation is that it be used as add-on therapy. Recently, case reports appeared in the literature of asthma related deaths associated with salmeterol use. The FDA subsequently attached a black box warning on increased asthma related deaths to the LABA class of drugs. The issue is, however, still under significant debate, due to the presence of other confounding variables that may or may not have been taken into account in the studies. The recommendation for the use of LABAs has now changed, whereby once the patient is stabilized on combination therapy, he or she should be weaned off the LABA. It remains to be seen if there will be adverse consequences of such a recommendation [73].
Cromolyn and Nedocromil
These two unrelated compounds have an excellent safety profile. Their chemical structures are illustrated in Fig. 12. Both are mast cell stabilizers, and both also inhibit the activation and release of inflammatory mediators from eosinophils. This appears to be mediated through blockage of chloride channels [74]. Both early- and late-phase reactions to allergen challenge are inhibited. Cromolyn is derived from the plant, Ammi visnaga, or bishop’s weed. The commercial product can be administered in either nebulized form or by MDI. The dose of cromolyn via MDI is 1 mg per actuation, where as the dose of nedocromil is 2 mg per actuation delivered from the valve and 1.75 mg per actuation delivered from the mouth piece of the inhaler. The dose of cromolyn delivered via nebulizer is 20 mg per treatment. The terminal elimination half-life of nedocromil sodium is 3.3 h. Nedocromil sodium is indicated in children 6 years of age or older. Cromolyn sodium is regularly used in very young children via nebulizer.
Fig. 12.
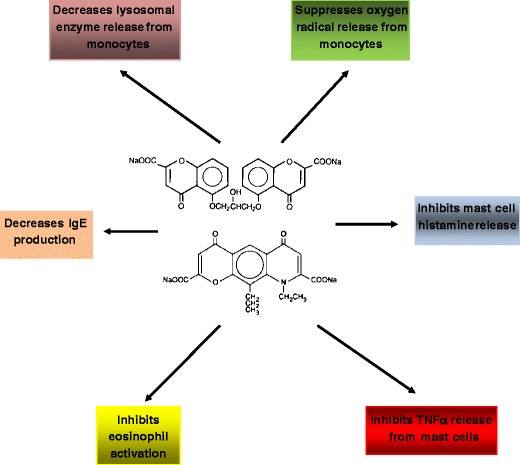
Structure and anti-inflammatory effects of cromolyn and nedocromil
Because of the unfavorable dosing schedule, cromolyn, a previously widely used medication, has given way to other nebulized anti-inflammatory medications, such as the glucocorticoids. Nedocromil has an unpleasant taste and, along with cromolyn, has fallen out of favor recently.
Leukotriene Pathway Drugs
Drugs that block the effects of leukotrienes were first introduced in the early 1990s. Two strategies were used in the development of these drugs, inhibiting their synthesis or blocking their action at the Cys-LT receptor level. Drugs that block leukotriene synthesis, such as zileuton, have been associated with liver toxicity. The leukotriene receptor antagonists have a much better safety profile and dosing schedule and have been the more widely used medications. The mechanism of action of leukotrienes is shown in Fig. 13.
Fig. 13.
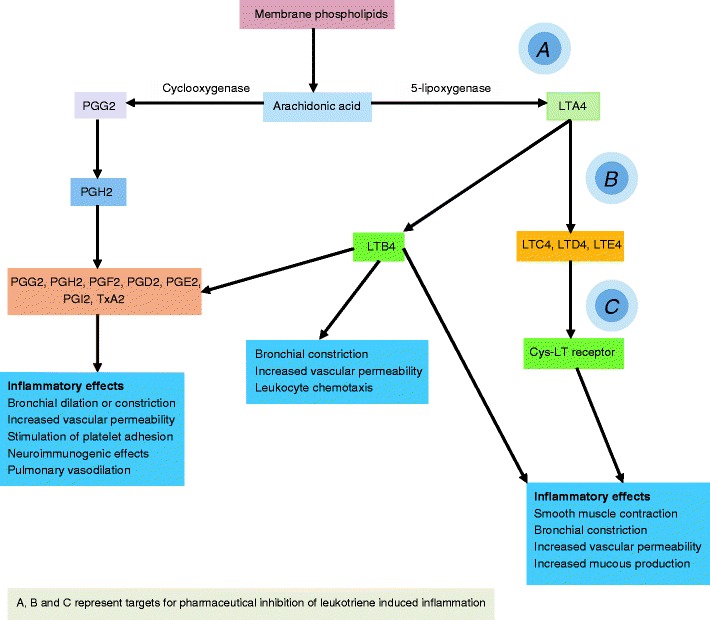
Mechanism of action of leukotriene pathway modifiers
As an inflammatory mediator in asthma, leukotrienes are 1000 times more potent than histamine [75]. The effects of LTC4, LTD4, and LTE4 on the Cys-LT receptor include an increase in mucous production, constriction of bronchial smooth muscle, augmentation of neutrophil and eosinophil migration, and stimulation of monocytes aggregation. In general, side effects of the leukotriene receptor antagonists are mild, with the exception of Churg–Strauss syndrome [76], a vasculitis associated with peripheral eosinophilia, elevated serum total IgE, patchy pulmonary infiltrates, cutaneous purpuric lesions, and pleural effusions. Leukotriene pathway modifiers can also affect metabolism of theophylline and a number of other drugs.
Montelukast, the most commonly used leukotriene pathway drug, is approved in children 1 year and older for asthma, 6 months and older for perennial allergic rhinitis, and 24 months and older for seasonal allergic rhinitis. Montelukast has been particularly useful in the treatment of cough variant asthma in children [77]. Early reports of an association between suicide and montelukast have been re-assessed, and the conclusion is that the risk of suicidal ideation in montelukast use is low. However, it was recommended that patients should be screened for behavioral anomalies including suicide ideation, which are generally more common in adolescents and the elderly [78].
Antihistamines
Whether to use antihistamines in children with asthma has been a hotly debated topic. The FDA originally had a warning on using antihistamines in asthma which was a class effect, so any newer antihistamines that were introduced all carried the same warning. However, while the first generation antihistamines had side effects that could potentiate an asthma exacerbation, such as the anticholinergic effects of drying, as well as the sedative effects, the second-generation antihistamines have much less of these adverse effects and should be safe in asthmatics. They should also provide some benefit, especially in children, where the greater proportion of asthma is associated with allergies [79]. The currently available second-generation antihistamines in the USA are cetirizine, levocetirizine, loratadine, desloratadine, and fexofenadine. These drugs block the allergic effect of environmental allergens, but cetirizine also inhibits leukocyte recruitment and activation and eosinophil migration [80] and has been shown to decreases late leukocyte migration into antigen-challenge skin blister fluid chambers [81]. All three inflammatory cell lines, including neutrophils, eosinophils, and basophils, were affected.
Theophylline in Childhood Asthma
Theophylline and aminophylline had their heyday in the 1980s, when almost every child with an asthma exacerbation requiring hospital admission was started on an aminophylline drip. Similarly, most patients with asthma were placed on theophylline as a maintenance therapy. The use of this class of medications has decreased significantly since then, due to its narrow therapeutic window, and potentially severe side effects (Table 11). Aminophylline is metabolized to theophylline, which is then metabolized to caffeine.
Theophylline acts as a phosphodiesterase inhibitor (Fig. 14). Its efficacy in improving symptom scores and pulmonary function test parameters are similar to inhaled steroids. Therefore, despite the undesirable effects of theophylline, there may still be role for its use as a steroid sparing agent in children with severe persistent asthma, especially those on systemic steroids. Theophylline levels should be monitored regularly every 2 to 3 months or more frequently if there are dosage changes, signs of adverse effects, or lack of efficacy. A list of factors and agents that influence theophylline levels and their effect is shown in Table 13.
Fig. 14.
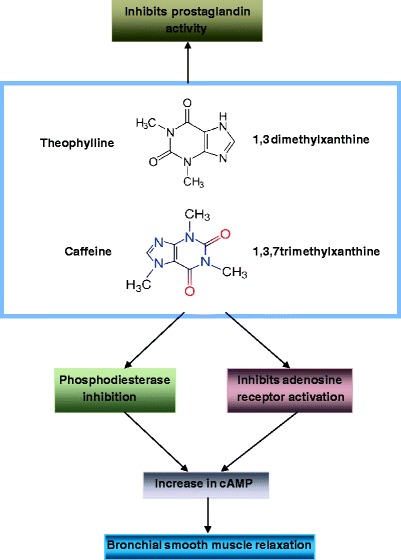
Structure and bronchodilatory effects of theophylline and known actions of theophylline and caffeine. Actual mechanism for the bronchodilatory effect of methylxanthines is not completely understood. Phosphodiesterase inhibition appears to be the most likely mechanism, but theophylline is known to have other activity, as shown
Table 13.
Factors affecting theophylline metabolism
| Factor or drug | Effect on theophylline levels |
|---|---|
| Antibiotics | |
| Ketolides | Increase |
| Ciprofloxacin | Increase |
| Rifampin | Decrease |
| Macrolides: erythromycin, clarithromycin | Increase |
| Antiepileptics | |
| Phenobarbital | Decrease |
| Carbamazepine | Decrease |
| Phenytoin | Decrease |
| Other drugs | |
| Aminoglutethimide | Decrease |
| Disulfiram | Increase |
| Ticlopidine | Increase |
| Propranolol | Increase |
| Cimetidine | Increase |
| Allopurinol | Increase |
| Calcium channel blockers | Increase |
| Methotrexate | Increase |
| Other factors | |
| Diet | Increase/decrease |
| Obesity | Increase |
| Hypoxia | Increase |
| Smoking | Decrease |
| Viral illness | Usually increase |
| Pediatric and geriatric population | Usually increase |
Monoclonal Anti-IgE
Omalizumab (Xolair) is a recombinant DNA-derived humanized IgG1a monoclonal antibody, which binds specifically to human IgE. Binding of IgE by omalizumab inhibits both early- and late-phase reactions of asthma. Effects of omalizumab include a reduction in serum IgE levels and a decrease in allergen-induced bronchoconstriction [82]. Omalizumab is indicated for patients 12 years of age or older who have moderate to severe persistent allergic asthma with a positive skin or blood allergy test, who have IgE levels between 30 and 700 IU/ml. Table 14 shows the dosing schedule for omalizumab. Side effects include malignancies, anaphylactic reactions, and local injection reactions. The high cost of Xolair can be potentially offset by savings in the cost of asthma exacerbations, e.g., hospital costs, outpatient emergency department visits, rescue medications, and indirect costs from loss of productivity by the patient [83].
Table 14.
Dosing schedule for omalizumab
| Pretreatment serum IgE (IU/ml) | Body weight (kg) | |||
|---|---|---|---|---|
| 30–60 | >60–70 | >70–90 | >90–150 | |
| Every 4-week dosing | ||||
| ≥30–100 | 150 | 150 | 150 | 150 |
| >100–200 | 300 | 300 | 300 | See below |
| >200–300 | 300 | See below | See below | |
| >300–400 | See below | |||
| >400–500 | ||||
| >500–600 | ||||
| Every 2-week dosing | ||||
| ≥30–100 | See above | See above | See above | See above |
| >100–200 | 225 | |||
| >200–300 | 225 | 225 | 300 | |
| >300–400 | 300 | 300 | 375 | Do not dose |
| >400–500 | 300 | 375 | Do not dose | |
| >500–600 | 375 | Do not dose | ||
Adapted from omalizumab package insert. Omalizumab is FDA approved in children over 12 years of age
Reliever Medications (Rescue Medications)
Short-Acting β-Agonists
The mechanism of action of the β-agonists is through activation of the β2-adrenergic receptors on airway smooth muscle cells, which leads to activation of adenyl cyclase. This, in turn, leads to an increase in the intracellular concentration of cyclic adenosine monophosphate (cAMP). cAMP activates protein kinase A, causing inhibition of phosphorylation of myosin and lowering of intracellular calcium concentrations, which then results in relaxation of bronchial smooth muscle. β2-Adrenergic receptors are present in all airways, from the trachea to the terminal bronchioles. Another effect of the increase in cAMP concentration is the inhibition of mediator release from mast cells. Adverse effects of β-agonists include paradoxical bronchospasm, cardiovascular effects, central nervous system stimulation, fever, tremors, nausea, vomiting, and an unpleasant taste (Table 11).
The short-acting β-agonist (SABA) used include albuterol, levalbuterol, pirbuterol, bronkosol, isoproterenol, metaproterenol, and terbutaline (Fig. 11). The more recently developed β-agonists are more specific to β2-adrenergic receptors, optimizing the effects on bronchial smooth muscle while reducing cardiac side effects, and have made older less-specific drugs such as metaproterenol and isoproterenol obsolete. Dosing recommendations for short-acting β-agonist inhalers are shown in Table 15. Albuterol, the most common used β-agonist, is available as a 0.083% nebulization solution. The use of β-blockers is a relative contraindication in children with asthma. β-Blockers have been associated with worsening asthma [84].
Table 15.
Characteristics of inhaled or nebulized bronchodilator preparations
| Generic name | Dosage/inhalations or puff | Available delivery devices | Dosing frequency | Max puffs/d | Half-life (h) | Onset of action (min) | Time to peak effect (min) | Duration of action (h) |
|---|---|---|---|---|---|---|---|---|
| Albuterol | 90 μg/puff or 2.5 mg per nebulization | MDI, D, C, N | 2 puffs q4h prn, 1 tmt q4h prn | 12 | 1.5 | 6 | 55–60 | 3 |
| Levalbuterol | 0.31, 0.63, or 1.25 mg per nebulization or 90 μg/MDI puff | MDI, N | 2 puffs q4h prn, 1 tmt q4h prn | 12 | 3.3 | 10–17 | 90 | 8 |
| Metaproterenol | 630 μg/puff or 15 mg nebulization | MDI, N | 2 puffs q4h prn or 1 tmt q4h prn | 12 | N/A | 5–30 | 60–75 | 1–2.5 |
| Pirbuterol | 200 μg | A | 2 puffs q4–6 h prn | 12 | N/A | 5 | 50 | 5 |
| Bitolterol | 370 μg | MDI, N | 2 puffs q6h prn | 12 | N/A | 3–4 | 30–60 | 5–8 |
| Formoterol | 12 μg | D | 2 puff q12h | 2 | 10 | 5 | 60 | 12 |
| Salmeterol | 25 μg | MDI, D | 2 puffs q12h | 4 | 5.5 | 10–20 | 45 | 12a |
| Ipratropium | 18 μg/puff or 500 μg per nebulization | MDI, C, N | 2 puffs q6h prn or 1 tmt q6h | 12 | 2 | 15 | 60–120 | 3–4 |
Some of these are no longer commercially available
MDI metered dose inhaler (only HFA available now), A autoinhaler, D dry powder inhaler, C combination inhaler, N solution for small volume nebulizer
aLate phase reaction may be inhibited up to 30 h
Albuterol is a 50–50 racemic mixture of the stereoisomers R-albuterol (levalbuterol) and S-albuterol. Levalbuterol is available in both inhaler and nebulizer solution form. There are three available doses of levalbuterol, 0.31, 0.63, and 1.25 mg for nebulization. Levalbuterol increases mean FEV1 by 31–37% in children between the ages of 6 and 11 [85]. The elimination half-life of levalbuterol is 3.3 h compared to 1.5 h for albuterol.
Albuterol is also available in oral form, either as a 2-mg/5-ml syrup or a sustained release 4-mg tablet. The oral dose in children is 0.03–0.06 mg/kg/day in three divided doses (no more than 8 mg per day). Terbutaline is also available orally in 2.5 and 5 mg tablets and is indicated for use in children over 12 years of age.
Anticholinergics
Anticholinergic inhalers are indicated for the treatment of chronic obstructive pulmonary disease but may be of some value in the treatment of the asthmatic during an exacerbation. The mechanism of action of ipratropium bromide is through competitive inhibition of M2 and M3 muscarinic cholinergic receptors. This leads to a decrease in airway vagal tone and decreased mucous gland secretion. Bronchoconstriction is also inhibited by anticholinergic agents [86]. Ipratropium bromide is available in nebulized form (2.5 ml of a 0.02% solution = 500 μg), or by HFA MDI (17 μg/dose from the mouthpiece). Ipratropium bromide is not well absorbed in the gastrointestinal tract. The elimination half-life of ipratropium bromide is 1 h when taken by MDI or administered intravenously.
Mucolytics
The use of mucolytics, such as N-acetylcysteine and S-carboxymethycysteine, in childhood asthma is controversial. Mucolytics exert their action by breaking up the disulfide bonds between mucin chains and allowing for easier clearance of mucous. On the other hand, they can cause bronchoconstriction. Although animal studies have demonstrated that N-acetylcysteine can improve gas exchange after methacholine challenge [87], there is currently no clinical indication for the use of mucolytics in the treatment of childhood asthma.
Oral or Parenteral Steroids
Fortunately, the use of systemic steroids in the treatment of asthma has decreased in countries where access to preventative, controller medications is easy and unrestricted. Systemic steroids, administered orally or parenterally on a chronic basis, are associated with a long list of adverse effects, many of which are potentially more serious than the disease they are being used to treat. These side effects are listed in Table 11. One important side effect that is sometimes forgotten is osteonecrosis. While corticosteroid-induced osteonecrosis is more common in autoimmune diseases and transplant patients than in asthma, one should still have a high index of suspicion when treating an asthmatic child who has been on steroids for a long time [88]. Generally speaking, a short course of steroids to treat an asthma exacerbation is acceptable from a risk benefit standpoint. In this case, if the corticosteroid course is less than 7 days, no tapering of dose is needed. A tapering schedule should be formulated for those patients in whom steroids are being used for longer than 1 week. If the patient requires multiple courses of steroids, then the possibility of developing serious side effects should be considered.
There are several corticosteroids available to treat asthma exacerbations. These are shown in Table 10. Many of the oral preparations have a very bad taste and may need to be disguised in foods in order to be able to administer them to young children. There is also available at least one form in an oral disintegrating tablet, which will facilitate compliance in young children.
Treatment of Exercise-Induced Asthma
Exercise is a common trigger for asthma and is particularly relevant in children, as many children are active in sports. Short-acting β-agonists are widely used, whereby the child takes two puffs of an albuterol inhaler prior to exercising. This has the effect of shifting the stimulus response curve to the right. Inhaled albuterol or terbutaline provide relief for up to 1 h during exercise [89]. Other short-acting bronchodilators that have been used in EIA include fenoterol [90] and bitolterol [91]. Oral bronchodilators have provided longer relief, up to 6 h for albuterol [92] and 2–5 h for terbutaline [93]. Cromolyn and nedocromil have been found to protect against EIA for 120 and 300 min, respectively [94, 95]. Theophylline has also been used in EIA, but the narrow therapeutic window and the lack of benefit observed at lower doses has hindered its widespread use [96]. The use of ipratropium bromide in EIA has not produced consistent results [97]. Controller medications that have played a role in preventing EIA include inhaled corticosteroids and the long-acting bronchodilators salmeterol [98] and formoterol. Leukotriene receptor antagonists have also been shown to be of some value in preventing EIA in children [99]. The data on ketotifen [100], calcium channel blockers [101–103], and antihistamines [104] in the treatment of EIA are conflicting.
Inhalation Devices in Children
Metered dose inhalers were first introduced in 1955 to deliver a predetermined amount of drug to the airways. The devices have undergone significant evolution since then and now are the most common device to carry drugs to treat asthma. Dry powder inhalers (DPI) are an alternative to metered dose inhalers. While the ability to deliver drug straight to the airways has revolutionized asthma treatment, the use of these devices in children presents some special considerations. The most important of these is the ability of young children to use these devices effectively. Specifically, this is the ability of the child to (1) understand how to use them and (2) be coordinated enough to use them accurately and effectively. Metered dose inhalers require considerable more coordination than DPIs, although spacer devices do help. If a child is found to be unable to effectively use one of these devices, then it would be much more beneficial to the asthmatic child to continue with the use of nebulizers. A comparison of the various drug delivery devices is shown in Table 16. The table also shows the most common age at which these inhalers can typically be used, although it is important to appreciate that there is a significant variability to these ages.
Table 16.
Comparison of inhaler devices
| CFC inhalers | HFA inhalers | Autoinhalers | Dry powder inhalers | Spacer devices | Nebulizers | |
|---|---|---|---|---|---|---|
| Availability | No longer available | Widespread | Rare | Increasing | Common | Widespread |
| Portability | Easy | Easy | Easy | Easy | Some are cumbersome | Smaller devices are available |
| Ease of use | Difficult | Difficult | No need for coordination | Need for adequate breath actuation | Improves effectiveness of MDI | No coordination necessary |
| Age range of use | 5 years and older | 5 years and older | 4 years and older | 4 years and older | May allow for use of MDI at an earlier age | Any age |
| Available for | Not available | SABA, LABA, Corticosteroids, Ipratropium bromide | SABA (pirbuterol) | LABA, Corticosteroids, Combination products | N/A | SABA, cromolyn, nedocromil, ipratropium bromide, corticosteroids |
| Cost/value | N/A | Expensive/good | Expensive/fair | Expensive/good | Moderate/good | Expensive/good |
| Comments | CFCs no longer available | The standard for MDI devices | Difficult to find | Dose lost if child exhales through device | Improves drug delivery to airways | Most reliable way to deliver drug—less dependent on patient technique |
Immunotherapy in Childhood Asthma
Also referred to as hyposensitization, desensitization, or allergy shots, immunotherapy plays a significant role in the treatment of pediatric asthma. Studies done in children who were allergic to dust mite [105], cat [106], dog [106–110], mold [111], grass [112–114], ragweed [115, 116], olive tree [117], and other allergens have demonstrated a beneficial effect of subcutaneous immunotherapy (SCIT). A study of 215 patients with dust mite allergy demonstrated that those patients with an FEV1 greater than 90% were four times as likely to benefit from immunotherapy to house dust mite, compared with patients with FEV1 less than 60%. Moreover, patients under the age of 20 years were three times more likely to improve than those more than 51 years of age [118]. Indications for immunotherapy include clear evidence of symptom–exposure relationship, perennial symptoms, and inadequate control with medications. Recent advances in sublingual immunotherapy (SLIT) may provide another option for hyposensitization in children with allergic asthma.
Emergency Treatment of Status Asthmaticus
A child may present to an emergency room or urgent care setting in respiratory distress but without a diagnosis of asthma. It is important for the emergency room physician or provider to be able to quickly formulate a differential diagnosis in order to administer the correct treatment. Assessment of respiratory distress involves the evaluation of patient symptoms and signs including heart rate, respiratory rate, retractions, mental status changes, presence of cough or wheezing, pulsus paradoxicus, and, if quickly available, peak flow measurement and pulse oximetry. A differential diagnosis of asthma is shown in Table 4. If wheezing is present, other causes must be ruled out, including foreign body aspiration or bronchiolitis. A chest radiograph may help in this case and also in identifying potential comorbidities of asthma, such as atelectasis or pneumothorax.
A comprehensive initial evaluation of the patient in respiratory distress can be done fairly quickly, and if the respiratory distress is severe, then treatment must be initiated promptly. In an asthmatic in status asthmaticus, a short-acting β-agonist such as albuterol or levalbuterol should be administered quickly, preferably via a small volume nebulizer. If there is time, measurement of peak flow or spirometry done prior to and after the treatment can help to evaluate the effectiveness of the treatment, but one should not delay treatment if the child’s condition is serious. If the child appears dyspneic, the patient should be placed on a cardiac monitor that can provide a rhythm and oxygen saturation. Intravenous (IV) access should be established in patients who are in respiratory distress, or to maintain hydration status. Parenteral steroids may be initiated for moderate to severe asthma exacerbations. Methylprednisolone 1 to 2 mg/kg can be given intravenously or intramuscularly. This can be continued every 6 h if the child requires admission. If the child’s condition improves quickly and he or she remains stable, the child may be discharged home on a short course of oral steroids (prednisone 1 to 2 mg/kg/day) with close follow-up and an action plan with detailed instructions. Measurement of peak flow and oxygen saturation should be done prior to discharge.
Epinephrine, although always important to consider, is less commonly used because of the abundance of other medications with lesser side effects. Side effects of epinephrine include tremors, hypertension, tachycardia, neutrophil demargination, and cardiac stimulation. In very severe cases, subcutaneous epinephrine (1:1,000) has been used in the treatment of the asthmatic child. The dose is 0.01 ml/kg to a maximum of 0.3 ml. The dose can be repeated if the response is inadequate. Having an epinephrine autoinjector available obviates the need to measure out the dose and saves time.
Dehydration can be a factor in the successful treatment of the asthmatic child because it results in drying up of bronchial mucous and/or electrolyte imbalances, making the treatment of the asthmatic more difficult. Concomitant infection, such as pneumonia or sinusitis, should be treated with antibiotics. Other medications used in the treatment of the acute asthmatic include nebulized corticosteroids, nebulized cromolyn, leukotriene receptor antagonists, theophylline or aminophylline, and nebulized anticholinergic agents. The doses of emergency medications and the size of emergency equipment that is used in the pediatric population are summarized in Table 17.
Table 17.
Emergency equipment and medication doses in children
| Emergency medications | |||||
| Age | Weight (kg) | Epinephrine SQ 1:1,000 (ml) | Epinephrine IV 1:10,000 (ml) | Atropine 0.1 mg/ml (ml) | Sodium bicarbonate 0.5 mEq/ml (4.2%) for children <3 m, 1.0 mEq/l >3 m (ml) |
| Newborn | 3.0 | 0.03 | 0.3 | 1.0 | 6.0 |
| 1 m | 4.0 | 0.04 | 0.4 | 1.0 | 8.0 |
| 3 m | 5.5 | 0.055 | 0.55 | 1.1 | 11.0 |
| 6 m | 7.0 | 0.07 | 0.7 | 1.4 | 7.0 |
| 1 years | 10.0 | 0.1 | 1.0 | 2.0 | 10.0 |
| 2 years | 12.0 | 0.12 | 1.2 | 2.4 | 12.0 |
| 3 years | 14.0 | 0.14 | 1.4 | 2.8 | 14.0 |
| 4 years | 16.0 | 0.16 | 1.6 | 3.2 | 16.0 |
| 5 years | 18.0 | 0.18 | 1.8 | 3.6 | 18.0 |
| 6 years | 20.0 | 0.20 | 2.0 | 4.0 | 20.0 |
| 7 years | 22.0 | 0.22 | 2.2 | 4.4 | 22.0 |
| 8 years | 25.0 | 0.25 | 2.5 | 5.0 | 25.0 |
| 9 years | 28.0 | 0.28 | 2.8 | 5.6 | 28.0 |
| 10 years | 34.0 | 0.34 | 3.4 | 6.8 | 34.0 |
| Emergency equipment sizes | |||||
| Age | Weight (kg) | Self-inflating bag size | O2 ventilation mask size | Endotracheal tube size | Laryngoscope blade size |
| Premature newborn | <2.5 | Infant | Newborn small | <3.0 | 0 |
| Newborn | 2.5–4.0 | Infant | Newborn | 3.0–3.5 | 0–1 |
| 6 m | 7.0 | Child | Child | 3.5–4.0 | 1 |
| 1–2 years | 10–12 | Child | Child | 4.0–4.5 | 1–2 |
| 2–5 years | 12–18 | Child | Child | 4.5–5.0 | 2 |
| 5–8 years | 18–24 | Child | Child | 5.0–5.5 | 2 |
| 8–10 years | 24–30 | Child/adult | Small adult | 5.5–6.0 | 2–3 |
Currently, theophylline is much less commonly used in the treatment of the acute asthmatic. However, if β-agonist nebulization is not effective in resolving respiratory distress, theophylline can be administered first as an intravenous bolus followed by a continuous intravenous drip. Once theophylline is started, the child should be admitted to the hospital. Each milligram per kilogram of theophylline IV bolus results in a 2-mg/dl rise in theophylline levels. The therapeutic window of theophylline serum levels is between 10 and 20 mg/dl. Thus, a commonly used bolus of 6 mg/kg results in a level that should fall well within the therapeutic window. An intravenous theophylline drip of 0.8–1 mg/kg/h will result in a steady-state serum level. Theophylline levels must be monitored carefully because of the serious side effects that can occur at higher serum levels (Table 11). Another disadvantage of using theophylline is that multiple factors can affect theophylline metabolism, sometimes leading to unpredictable serum levels. These factors are shown in Table 13.
The use of leukotriene receptor antagonists in the treatment of an asthma exacerbation has been reported [119]. In a recent study of 201 patients, montelukast administered intravenously led to a significantly improved FEV1 after 20 min when compared to patients who were given placebo [120]. The effect lasted longer than 2 h, and patients in the treatment group received less β-agonist and had fewer treatment failures compared to the placebo group. Some success with the use of oral montelukast in the treatment of asthma exacerbations has also been reported [121, 122].
Inpatient Management of Childhood Asthma
The decision to admit a child with an asthma exacerbation to the hospital or intensive care depends on several factors. These include the efficacy of treatment in the emergency room and the original severity of the asthma exacerbation. Persistent wheezing and retractions, dyspnea, reduced oxygen saturation, and abnormal blood gas parameters can all be indications for admission. Treatment that has been initiated in the emergency room can also lead to an admission, such as supplemental oxygen, theophylline drip, intubation, IV rehydration, or IV antibiotics. Intubation should not be delayed if the patient is in impending respiratory arrest because resuscitation is more difficult in patients who are in respiratory failure. If intubation is performed, arterial blood gas measurement and chest radiography to document placement of the endotracheal tube must be done.
Oxygen, nebulized steroids, oral or parenteral steroids, leukotriene receptor antagonists, and theophylline can be continued as indicated, until the patient condition allows for weaning of medications. Oxygen saturation should be monitored either continuously or intermittently depending on the child’s status. Particular attention should be paid to hydration status, fever, or signs of adverse effects of medications, such as tremors or electrolyte imbalances from nebulized steroids. Infections should be treated appropriately (Fig. 15).
Fig. 15.
Algorithm for the treatment of the acute asthmatic child
As the child improves, treatment can be weaned and discharge planning initiated. Children should be sent home with an asthma management plan and instructions to return if conditions worsen. Discharge medications depend on the patient’s history of present illness and past history of asthma. Close follow-up as an outpatient by an asthma specialist is preferable to review ongoing treatment and preventative measures. Table 18 shows pediatric indications for the various asthma medications.
Table 18.
Pediatric indications for asthma drugs
| Drug name (trade name) | Category | Components (scientific names) | Pediatric indicationa |
|---|---|---|---|
| Accolate | Leukotriene receptor antagonist | Zafirlukast | 5 years and older |
| Advair Diskus | Combination (ICS + LABA) | Fluticasone propionate | 4 years and older |
| Salmeterol xinafoate | |||
| Advair HFA | Combination (ICS + LABA) | Fluticasone propionate | 12 years and older |
| Salmeterol xinafoate | |||
| Albuterol oral syrup | SABA | Albuterol sulfate | 2 years and older |
| Alvesco | Inhaled ICS | Ciclesonide | 12 years and older |
| Asmanex Twisthaler | Inhaled ICS | Mometasone furoate | 4 years and older |
| Atrovent HFA | Anticholinergic | Ipratropium bromide | Not established |
| Dulera | Combination (ICS + LABA) | Mometasone furoate | 12 years and older |
| Formoterol fumarate dihydrate | |||
| Foradil | LABA | Formoterol fumarate | 5 years and older |
| Intal inhaler | Anti-inflammatory | Cromolyn | Discontinued in USA, available still in other countries |
| Intal nebulization solution | Anti-inflammatory | Cromolyn | 2 years and older |
| Pro-Air HFA | SABA | Albuterol sulfate | 4 years and older |
| Proventil HFA | SABA | Albuterol sulfate | 4 years and older |
| Pulmicort Flexhaler | Inhaled ICS | Budesonide | 6 years and older |
| Pulmicort respules | Inhaled (nebulized) ICS | Budesonide | 12 months to 8 years |
| QVAR | Inhaled ICS | Beclomethasone dipropionate | 5 years and older |
| Seretide Accuhaler | Combination (ICS + LABA) | Fluticasone propionate | 4 years and older (Australia) |
| Salmeterol xinafoate | |||
| Seretide MDI | Combination (ICS + LABA) | Fluticasone propionate | 4 years and older (Australia) |
| Salmeterol xinafoate | |||
| Serevent Accuhaler | LABA | Salmeterol xinafoate | 4 years and older (Australia) |
| Serevent Diskus | LABA | Salmeterol xinafoate | 4 years and older (USA) |
| Serevent Inhaler | LABA | Salmeterol xinafoate | 4 years and older (Australia) |
| Singulair | Leukotriene receptor antagonist | Montelukast | 12 months and older (for asthma) |
| Spiriva HFA | Anticholinergic | Tiotropium bromide | Not indicated in children |
| Symbicort HFA | Combination (ICS + LABA) | Budesonide | 12 years and older |
| Formoterol fumarate dihydrate | |||
| Tilade CFC free | Anti-inflammatory | Nedocromil sodium | 2 years and older |
| Ventolin HFA | SABA | Albuterol sulfate | 4 years and older |
| Xolair | Monoclonal anti-IgE | Omalizumab | 12 years and older |
| Xopenex HFA | SABA | Levalbuterol tartrate | 4 years and older |
| Xopenex nebulization solution | SABA | Levalbuterol HCl | 6 years and older |
| Zyflo | Leukotriene receptor antagonist | Zileuton | 12 years and older |
aThese are current pediatric age indications by the FDA or corresponding regulatory agency if drug is available elsewhere
Integrative Medicine in Pediatric Asthma
About one third of the population of the USA uses some form of alternative or complementary medicine. Techniques that have been utilized by patients to treat asthma include acupuncture [123], herbal medicines, homeopathy, massage therapy, Ayurvedic medicine, yoga, relaxation techniques, breathing exercises, and meditation [124–126] (Table 19). While popular, these modes of therapy have not been well studied, and at the present time, there is no scientific evidence to support their efficacy in the treatment of asthma. While the majority of these techniques are themselves harmless, using them as a substitute for established asthma management may deny the pediatric asthmatic the proper care that they should be receiving. Special warning should be given to herbal medications, which in addition to the lack of evidence for efficacy may actually be harmful either by themselves or in interaction with concurrent asthma medications. The use of these preparations in children should be especially discouraged until further evidence of safety and efficacy are available.
Table 19.
Important cytokines targets in asthma
| Cytokine | Cellular expression (with particular relevance to asthma) | Cell targets | Function | New drug or drug target for asthma treatment | Results |
|---|---|---|---|---|---|
| IL-3 | Activated T cells | Bone marrow progenitors | Increases lifespan of eosinophils, stimulates differentiation of multiple cell types | None | N/A |
| IL-4 | Macrophages, Th2 cells | Naïve T cells, B cells, T cells | Upregulation of immunoglobulin E synthesis, Th2 lymphocyte differentiation, production of VCAM-1, effects low-affinity CD23 IgE receptors | Soluble IL-4 receptors | Phase II trials show significant improvement in asthma [127] |
| Pitrakinra [128] | Successful asthma treatment in a monkey model | ||||
| IL-5 | T helper 2 cells and mast cells | Eosinophils, B cells | Stimulates differentiation and activation of eosinophils | Monoclonal antibody to IL-5 [129] | Blockage of eosinophils, reduces eosinophil numbers |
| IL-6 | T cells, macrophages, fibroblasts | T cells, B cells, liver cells, mature B cells | Downregulation of inflammatory cell infiltration and enhancement of airway remodeling [130] | None | N/A |
| IL-8 | Macrophages, epithelial cells, platelets | Neutrophils, macrophages, endothelial cells, keratinocytes, mast cells | Chemokine, angiogenic factor, may have a role in bronchiolitis, also known as neutrophil chemotactic factor | None | N/A |
| IL-9 | T helper cells | T helper cells, B cells | Th2 cytokine, activity in conjunction with IL-4, IL-1 and IL-3 | Anti-IL-9 | Inhibits asthma related features in antigen stimulated mice [131] |
| IL-10 | Monocytes, lymphocytes, mast cells, Th2 cells, Treg cells, activated macrophages | T cells, mast cells, B cells | Inhibits allergen-induced airway hyperresponsiveness and inflammation | None | N/A |
| IL-12 | Activated macrophages and dendritic cells | Th cells, Tc cells, NK cells | Immunomodulatory cytokine | R848 (Resiquimod) [132, 133] | Reduces airway inflammation after antigen challenge |
| IL-13 | T h2 cells + many other cell types | B cells + others | Proinflammatory cytokine, Th1/Th2 balance, mediator of allergic inflammation | Anti-IL-13 [134] | Antibody to IL-13 suppressed AHR, eosinophil infiltration, proinflammatory cytokine production, serum IgE in mice, poor results clinically |
| IL-17 | Th cells, NK cells, Treg cells, mast cells | Release of cytokines from many cells | Proinflammatory cytokine, chemokines, differentiation of Th17 cells, airway remodeling | Possibly under investigation for asthma | Unknown |
| IL-19 | Epithelial cells, endothelial cells, macrophages, monocytes | NK cells, T cells, B cells, monocytes | Function unknown, may alter balance of Th1/Th2 cells in favor of Th2 cells, increases proportion of IL-4 producing cells [135] | None | N/A |
| IL-25 | Helper T cells, mast cells | Eosinophils (via stimulation of production of IL-4, IL-5, and IL-13) | Inflammatory bowel disease, mucosal immunity | None | N/A |
| IL-33 | Mast cells, bronchial smooth muscle cells, epithelial cells | Helper T cells, mast cells, eosinophils, basophils | Role in atopic dermatitis and asthma, gene | None | N/A |
| GM-CSF | Macrophages, T cells, endothelial cells, fibroblasts, mast cells | Stem cells | Proinflammatory cytokine, potentially leading to increase in inflammatory cells | None | N/A |
| Interferon-γ | Th1 cells, Tc cells, dendritic cells, NK cells | Many cell types | Suppresses Th2 activity, activates inducible NOS | None | N/A |
| TNF-α | Macrophages, T cells + many other cells | Neutrophils, macrophages, T cells, B cells + others | Proinflammatory cytokine, activates neutrophils, stimulates phagocytosis, acute phase reactant | Has been studied extensively in asthma [136] | Generally poor results |
| ADAM-33 | Vascular smooth muscle cells, fibroblasts, lung mesenchymal cells | Unknown | Type 1 transmembrane protein implicated in asthma and eczema | None | N/A |
| RANTES | Airway smooth muscle cells, mast cells, macrophages | T cells, basophils eosinophils | Chemotactic, leukocyte recruitment | None | N/A |
| CCR3 | Eosinophils, basophils, Th1 cells, Th2 cells, airway epithelial cells | Eosinophils and other inflammatory cells | Eosinophil chemotaxis | None | N/A |
| CXCR2 | Mast cells, human mesenchymal stem cells, endothelial cells | Endothelial cells, neutrophils | Neutrophil and monocytes chemotaxis [137] | None | N/A |
| Matrix metalloproteinase-12 (metalloelastase) | Lung and alveolar macrophages | Extracellular matrix | Repair cycles influence airway changes in asthma, reduction of levels of chemotactic factors and other proinflammatory cytokines [138] | MMP-12 specific inhibitor [139] | Attenuates early airway response, blocks late airway response |
Natural History and Prognosis of Childhood Asthma
While many patients believe that they have outgrown their asthma, this is never really the case because asthma is at least partially genetically determined. On the other hand, a child’s asthma can vary during their childhood and even into adulthood. Asthma is dependent on having a genetic predisposition and, clinically, is modulated by the environment. Environmental modulation can stem from allergenic exposure, exposure to other agents such as endotoxin, irritants, ozone, particulates, and even temperature. Hormones can also play a role in asthma, as suggested by the interesting observation that in pregnancy, women who have asthma have an equal chance of their asthma worsening, improving, or remaining unchanged.
What is known, however, is that asthma is an inflammatory disease that results in bronchoconstriction. Successful treatment of the asthmatic means control of the inflammation. Failure to control inflammation may result in chronic obstructive lung disease, as in the case of the cigarette smoker. The introduction of inhaled corticosteroids over the past few decades has greatly improved asthma care and may have a beneficial effect on long-term sequelae of asthma. The persistently high mortality from asthma is probably more related to patient and provider education and compliance than anything else. This is why treatment of asthma requires a comprehensive management plan that incorporates all facets of treatment, including environmental control, medications, education, and immunotherapy (Fig. 16). Regular monitoring is important to minimize the morbidity of chronic asthma.
Fig. 16.
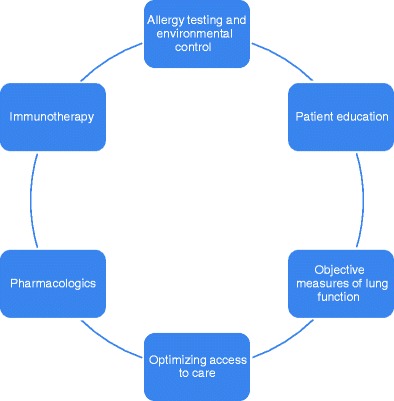
A comprehensive asthma management program
Future Directions
New Medications
Targets for new treatment modalities in asthma include IgE, eosinophils, cytokines, chemokines, cell-signaling pathways, adhesion molecules, and inflammatory mediators such as leukotrienes, prostaglandins, and platelet-activating factor. The development of airway inflammation is under the control of many biological modulators, many of which are targets of asthma research. Currently, phosphodiesterase-4 inhibitors, peroxisome proliferator-activated receptor-γ agonists, nuclear factor κB, phosphoinisotide-3-kinase γ, lipoxins, and p38 mitogen-activated protein kinase inhibitors are anti-inflammatory drugs that are being investigated in the treatment of asthma. It is unlikely that a single agent will be identified that will be a primary target for the treatment of asthma due to the redundancy of the immune system. A list of biologically active molecules that may have a role in asthma is shown in Table 20.
Table 20.
Integrative medicine and asthma
| Acupuncture |
| Herbal medicines |
| Homeopathy |
| Yoga |
| Ayurvedic medicine |
| Massage therapy |
| Relaxation techniques |
| Breathing exercises |
Phosphodiesterase-4 inhibitors are currently being studied in the treatment of asthma. A study of airway responses to allergen challenge in 24 mild asthmatics of an inhaled phosphodiesterase-4 inhibitor showed an inhibition in the fall of minimum and weighted FEV1 compared to placebo [140]. Other phosphodiesterase-4 inhibitors are also under investigation.
Platelet-activating factor has been associated with EIA and allergen-induced asthma. Inhibition of platelet activation diminishes the late-phase reaction of asthma [141–143]. Several medications currently used to treat asthma, including the glucocorticoids and ketotifen, normalize platelet survival times [144]. Platelets also secrete platelet factor 4 [145], platelet-derived growth factor [146, 147], arginine–glycine–aspartic acid, thrombospondin, transforming growth factor-α and transforming growth factor-β [148], 5-hydroxytryptamine, thromboxane A2, 12-hydroxyeicosatetranoic acid, β-lysin, adenosine diphosphate, and platelet-derived histamine releasing factor, all of which may play a role in airway inflammation.
Platelets possess low-affinity receptors for IgG and IgE on their surface and release adhesion molecules and inflammatory cell chemoattractants such as RANTES [149]. Cromolyn sodium [150], nedocromil sodium [151], and cetirizine [152] can all inhibit IgE-induced platelet activation. Platelet activation is associated with increased airway eosinophils. Despite the numerous inflammatory effects of platelets and the potential for new drugs, there are currently no available platelet-related drugs for the treatment of asthma. Interesting, the recent furor over vitamin D has led to the identification of vitamin D as an inhibitor of thrombin and platelet-derived growth factor-induced airway smooth muscle proliferation [153], suggesting that improvement in nutrition in young children may lead to normalization of vitamin D levels and a decreased incidence of asthma.
Other targets for the future treatment of childhood asthma include the prostaglandins, specifically PGD2, a tyrosine kinase inhibitor (masitinib), and a number of potential cytokine based therapies targeting Th2 cytokines such as IL-4, IL-5, IL-9, IL-13, IL-17, and TNFα [154]. Other drugs that are continually being developed for asthma are anticholinergics and new glucocorticoid agents. There are many other potential targets that have not been mentioned here that are beyond the scope of this chapter.
New Forms of Immunotherapy
SLIT has been used in Europe for several years. Early experience in the USA suggests that it is of comparable efficacy to SCIT, but with significantly diminished side effects or risk of anaphylaxis. One potential drawback of SLIT compared to SCIT is the lack of supervision associated with self-administration of oral or sublingual preparations of extracts [155]. Better patient education may circumvent this objection, however, and SLIT may yet become widely used in the treatment of asthma. The other potential road block with SLIT is the absence of an established regimen for prescribing SLIT in polysensitized individual. A study of 51 polysensitized children with allergic rhinitis and/or mild to moderate asthma treated with SLIT for 1 year showed an improvement in symptoms of allergic rhinitis severity and classification, nasal, ocular, and bronchial symptoms and medication use. While the majority (42) of these children was treated with a single allergen, seven were treated with two or more extracts and experienced benefit as well [156]. Studies on the efficacy of SLIT have been done for dust mite [157, 158], Olea [159], grass pollen [160], and others.
Other novel forms of immunotherapy include the development of allergen vaccines based on allergen-derived T cell peptides, recombinant allergens, and hypoallergenic allergen derivatives [161]. Another new chimeric Fc-γ allergen protein immunotherapy is being evaluated for cat and peanut allergy. Studies of these new forms of immunotherapy are still in the early phases and are not currently used clinically.
Genetics Based Therapies in Asthma
Asthma is a polygenic disease with a great deal of heterogeneity. Multiple genes have been identified that convey risk of asthma. The list of asthma genes continues to grow. Single nucleotide polymorphisms have been found that play a role in asthma severity or response to medications. A current goal is matching the asthma phenotype with an existing drug or a drug in development to maximize the response in an individual patient.
The ability to perform genome-wide association studies provides a technology to rapidly analyze and compare genomes of many people to determine variations associated with specific diseases. Identification of genes that may play a role in multiple diseases or conditions has also been made possible, as in the analysis of the relationship between obesity and asthma [162]. This has opened the door to an infinite amount of research on asthma genetics.
Childhood asthma and Healthcare Systems
Delivery of care for allergies and asthma is highly dependent on the existing health care system within each country. There are clearly advantages and disadvantages to socialized medicine versus fee for service medicine. Every country has its own health care system, and outcomes vary as a result of the efficiency and effectiveness of delivery of care.
In the USA, the issue of whether asthma should be managed by a generalist or a specialist has always been hotly debated. Multiple studies have shown that management by an asthma specialist (allergist or pulmonologist) leads to reduced morbidity and mortality and improved quality of life. Treatment by a specialist leads to fewer hospitalizations, fewer exacerbations requiring emergency care, better quality of life, and better outcomes [163]. However, the availability of financial resources and, more important, the failure to properly allocate such resources can be such an economic burden that optimal systems of healthcare are not implemented. As a result of this, many asthmatic children receive the bulk of their care from generalists and even mid-level practitioners, even in those cases where referral to a specialist may be indicated.
Unfortunately, in today’s healthcare climate, generalists are being asked to see more and more patients, and they simply do not have the time to formulate a comprehensive asthma treatment program as illustrated in Fig. 16 [164]. Asthma educators who may be nurses or even medical assistants who are specially trained may help, but no financial resources are actually devoted to this form of medical care. Too many patients with asthma are not well controlled and eventually end up in an urgent care or emergency department, at many times the cost of prevention [165]. A list of recommendations for referral to a specialist is shown in Table 21. These should be considered the very minimum requirements for referral to a specialist.
Table 21.
Indications for referral to a specialist
| Children requiring step 3 care of higher (step 2 for children under 4 years of age) |
| Children on or those who may be candidates for immunotherapy |
| Uncontrolled patients not meeting goals of therapy within 3 months of after initiation of treatment |
| Children who have had a life-threatening asthma exacerbation |
| Children in whom symptoms are atypical or if the diagnosis has not been established |
| Children with comorbid or complicating factors, including chronic sinusitis, nasal polyps, gastroesophageal reflux, allergic rhinitis, allergic bronchopulmonary aspergillosis, etc. |
| Children in whom additional diagnostic testing is needed, such as allergy testing, pulmonary function tests, rhinolaryngoscopy, provocation challenge, or bronchoscopy |
| Children who require systemic corticosteroids on a chronic basis or who have more than two steroid bursts in 1 year |
| Children who have been hospitalized for asthma |
| Children with exercise-induced asthma or other special circumstances |
| Children and/or parents who require or desire counseling on issues related to compliance, environmental evaluation and control, medication usage, device usage, peak flow meter usage, or any other additional asthma education |
| Children who may have an unusual exposure which may be provoking or contributing to asthma |
Conclusions
Since our last edition, the treatment of asthma in children has become more standardized, at least among allergy and asthma specialists. Inhaled corticosteroids have become the first-line medical treatment of asthma in all age groups. There is still debate on the preferred add-on therapy, the options being increasing the dose of steroids, adding a leukotriene receptor antagonists, or adding a long-acting β-agonist, but all of these options are acceptable and should be tailored and customized for each individual child with asthma. Future research may be able to identify which treatment might be preferred based on the patient’s pharmacogenetics, but we have not yet reached this point. Environmental control has become a mainstay in asthma treatment, and new modes of immunotherapy have contributed to a significant less morbidity over the past two or three decades. Hospitalization rates of asthmatic children have decreased, drugs with high rates of side effects such as theophylline have been replaced, and quality of life has improved. Most children with asthma are able to enjoy very normal lives, compete in sports at a high level, and have very few missed school or work days, as long as they are compliant with their asthma management plan.
The educational component of asthma is continually being improved upon. Multiple resources are now available to physician, provider, ancillary staff, parents, and patients. Many of these are available on the internet (Table 22). Publications for children to make it easier for them to understand their disease are now ubiquitous. With better education comes better compliance and, hopefully, better outcomes.
Some problems remain. Mortality has not decreased significantly since the last edition. One of the main remaining issues is related to patient education and compliance. It is now not a matter of availability of education materials, but acceptance and utilization. Another main issue, which may become even more of a problem in the face of cost cutting measures associated with a variety of health care reform ideas, involves the access to specialist care.
The future of pediatric asthma is bright. Research is ongoing to better understand the pathophysiology of asthma and, in doing so, developing new pharmaceuticals to treat asthma. Knowledge of the genetic basis for asthma and how children react to asthma medications will help guide us in developing a personal care plan for the treatment of each child with asthma. Hopefully, this will lead to further improvements in the outcomes of asthmatic children.
References
- 1.Pearce N, Douwes J. The global epidemiology of asthma in children. Int J Tuberc Lung Dis. 2006;10:125–132. [PubMed] [Google Scholar]
- 2.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 3.New NHLBI. Guidelines for the diagnosis and management of asthma. National Heart, Lung and Blood Institute. Lippincott Health Promot Lett. 1997;2(1):8–9. [PubMed] [Google Scholar]
- 4.Expert Panel Report 3 (EPR-3) Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Priftis KN, Mantzouranis EC, Anthracopoulos MB. Asthma symptoms and airway narrowing in children growing up in an urban versus rural environment. J Asthma. 2009;46:244–251. doi: 10.1080/02770900802647516. [DOI] [PubMed] [Google Scholar]
- 6.Chang CC, Phinney SD, Halpern GM, Gershwin ME. Asthma mortality: another opinion—is it a matter of life and … bread? J Asthma. 1993;30:93–103. doi: 10.3109/02770909309054503. [DOI] [PubMed] [Google Scholar]
- 7.Dixon AE, Holguin F, Sood A, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 8.Vollmer WM, Buist AS, Osborne ML. Twenty year trends in hospital discharges for asthma among members of a health maintenance organization. J Clin Epidemiol. 1992;45:999–1006. doi: 10.1016/0895-4356(92)90115-4. [DOI] [PubMed] [Google Scholar]
- 9.Berce V, Potocnik U. Functional polymorphism in CTLA4 gene influences the response to therapy with inhaled corticosteroids in Slovenian children with atopic asthma. Biomarkers. 2010;15:158–166. doi: 10.3109/13547500903384318. [DOI] [PubMed] [Google Scholar]
- 10.Vonk JM, Postma DS, Maarsingh H, Bruinenberg M, Koppelman GH, Meurs H. Arginase 1 and arginase 2 variations associate with asthma, asthma severity and beta2 agonist and steroid response. Pharmacogenet Genomics. 2010;20:179–186. doi: 10.1097/FPC.0b013e328336c7fd. [DOI] [PubMed] [Google Scholar]
- 11.Polimanti R, Piacentini S, Moscatelli B, Pellicciotti L, Manfellotto D, Fuciarelli M. GSTA1, GSTO1 and GSTO2 gene polymorphisms in Italian asthma patients. Clin Exp Pharmacol Physiol. 2010;37:870–872. doi: 10.1111/j.1440-1681.2010.05385.x. [DOI] [PubMed] [Google Scholar]
- 12.Piacentini S, Polimanti R, Moscatelli B, et al. Glutathione S-transferase gene polymorphisms and air pollution as interactive risk factors for asthma in a multicentre Italian field study: a preliminary study. Ann Hum Biol. 2010;37:427–439. doi: 10.3109/03014461003636419. [DOI] [PubMed] [Google Scholar]
- 13.Vergara CI, Acevedo N, Jimenez S, et al. A six-SNP haplotype of ADAM33 is associated with asthma in a population of Cartagena, Colombia. Int Arch Allergy Immunol. 2010;152:32–40. doi: 10.1159/000260081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hesselmar B, Bergin AM, Park H, et al. Interleukin-4 receptor polymorphisms in asthma and allergy: relation to different disease phenotypes. Acta Paediatr. 2010;99:399–403. doi: 10.1111/j.1651-2227.2009.01631.x. [DOI] [PubMed] [Google Scholar]
- 15.Sleiman PM, Flory J, Imielinski M, et al. Variants of DENND1B associated with asthma in children. N Engl J Med. 2010;362:36–44. doi: 10.1056/NEJMoa0901867. [DOI] [PubMed] [Google Scholar]
- 16.Via M, De Giacomo A, Corvol H, et al. The role of LTA4H and ALOX5AP genes in the risk for asthma in Latinos. Clin Exp Allergy. 2010;40:582–589. doi: 10.1111/j.1365-2222.2009.03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reijmerink NE, Bottema RW, Kerkhof M, et al. TLR-related pathway analysis: novel gene-gene interactions in the development of asthma and atopy. Allergy. 2010;65:199–207. doi: 10.1111/j.1398-9995.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- 18.Gao J, Li W, Willis-Owen SA, et al. Polymorphisms of PHF11 and DPP10 are associated with asthma and related traits in a Chinese population. Respiration. 2010;79:17–24. doi: 10.1159/000235545. [DOI] [PubMed] [Google Scholar]
- 19.Wang TN, Tseng HI, Kao CC, et al. The effects of NOS1 gene on asthma and total IgE levels in Taiwanese children, and the interactions with environmental factors. Pediatr Allergy Immunol. 2010;21:1064–1071. doi: 10.1111/j.1399-3038.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson UB, Hakansson LD, Jogi R, Janson C, Venge P. Associations of ECP (eosinophil cationic protein)-gene polymorphisms to allergy, asthma, smoke habits and lung function in two Estonian and Swedish sub cohorts of the ECRHS II study. BMC Pulm Med. 2010;10:36. doi: 10.1186/1471-2466-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harada M, Hirota T, Jodo AI, et al (2011) TSLP promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol. doi:10.1165/rcmb.2009-0418OC [DOI] [PMC free article] [PubMed]
- 22.Zhang YG, Huang J, Zhang J, et al. RANTES gene polymorphisms and asthma risk: a meta-analysis. Arch Med Res. 2010;41:50–58. doi: 10.1016/j.arcmed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Polk S, Sunyer J, Munoz-Ortiz L, et al. A prospective study of Fel d1 and Der p1 exposure in infancy and childhood wheezing. Am J Respir Crit Care Med. 2004;170:273–278. doi: 10.1164/rccm.200310-1348OC. [DOI] [PubMed] [Google Scholar]
- 24.Wennergren G, Ekerljung L, Alm B, Eriksson J, Lotvall J, Lundback B. Asthma in late adolescence—farm childhood is protective and the prevalence increase has levelled off. Pediatr Allergy Immunol. 2010;21:806–813. doi: 10.1111/j.1399-3038.2010.01057.x. [DOI] [PubMed] [Google Scholar]
- 25.Sheehan WJ, Rangsithienchai PA, Wood RA, et al. Pest and allergen exposure and abatement in inner-city asthma: a work group report of the American Academy of Allergy, Asthma & Immunology Indoor Allergy/Air Pollution Committee. J Allergy Clin Immunol. 2010;125:575–581. doi: 10.1016/j.jaci.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang C. The immune effects of naturally occurring and synthetic nanoparticles. J Autoimmun. 2010;34:J234–J246. doi: 10.1016/j.jaut.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Randolph C. The challenge of asthma in adolescent athletes: exercise induced bronchoconstriction (EIB) with and without known asthma. Adolesc Med State Art Rev. 2010;21:44–56. [PubMed] [Google Scholar]
- 28.Peroni DG, Pietrobelli A, Boner AL. Asthma and obesity in childhood: on the road ahead. Int J Obes (Lond) 2010;34:599–605. doi: 10.1038/ijo.2009.273. [DOI] [PubMed] [Google Scholar]
- 29.Lund TK. Asthma in elite athletes: how do we manage asthma-like symptoms and asthma in elite athletes? Clin Respir J. 2009;3:123. doi: 10.1111/j.1752-699X.2008.00111.x. [DOI] [PubMed] [Google Scholar]
- 30.Stickland MK, Spooner CH, Dryden DM, Rowe BH. The need for standardization in exercise challenge testing for exercise-induced asthma/bronchoconstriction. J Allergy Clin Immunol. 2010;126:878–880.e6. doi: 10.1016/j.jaci.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal LA, Avila PC, Heymann PW, et al. Viral respiratory tract infections and asthma: the course ahead. J Allergy Clin Immunol. 2010;125:1212–1217. doi: 10.1016/j.jaci.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol. 2010;125:1178–1187. doi: 10.1016/j.jaci.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sly PD, Kusel M, Holt PG. Do early-life viral infections cause asthma? J Allergy Clin Immunol. 2010;125:1202–1205. doi: 10.1016/j.jaci.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Bizzintino J, Lee WM, Laing IA et al (2011) Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J. doi:10.1183/09031936.00092410 [DOI] [PMC free article] [PubMed]
- 35.Jackson DJ. The role of rhinovirus infections in the development of early childhood asthma. Curr Opin Allergy Clin Immunol. 2010;10:133–138. doi: 10.1097/ACI.0b013e3283352f7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnston SL, Pattemore PK, Sanderson G, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 37.McBride JT. Pulmonary function changes in children after respiratory syncytial virus infection in infancy. J Pediatr. 1999;135:28–32. [PubMed] [Google Scholar]
- 38.Dimova-Yaneva D, Russell D, Main M, Brooker RJ, Helms PJ. Eosinophil activation and cysteinyl leukotriene production in infants with respiratory syncytial virus bronchiolitis. Clin Exp Allergy. 2004;34:555–558. doi: 10.1111/j.1365-2222.2004.1918.x. [DOI] [PubMed] [Google Scholar]
- 39.Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 40.Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 41.Naclerio RM, Meier HL, Kagey-Sobotka A, et al. Mediator release after nasal airway challenge with allergen. Am Rev Respir Dis. 1983;128:597–602. doi: 10.1164/arrd.1983.128.4.597. [DOI] [PubMed] [Google Scholar]
- 42.Gelfand EW, Cui ZH, Takeda K, Kanehiro A, Joetham A. Fexofenadine modulates T-cell function, preventing allergen-induced airway inflammation and hyperresponsiveness. J Allergy Clin Immunol. 2002;110:85–95. doi: 10.1067/mai.2002.124770a. [DOI] [PubMed] [Google Scholar]
- 43.Gelfand EW, Cui ZH, Takeda K, Kanehiro A, Joetham A. Effects of fexofenadine on T-cell function in a murine model of allergen-induced airway inflammation and hyperresponsiveness. J Allergy Clin Immunol. 2003;112:S89–S95. doi: 10.1016/s0091-6749(03)01882-7. [DOI] [PubMed] [Google Scholar]
- 44.Ducharme FM, Zemek RL, Chalut D, et al. Written action plan in pediatric emergency room improves asthma prescribing, adherence and control. Am J Respir Crit Care Med. 2011;183:195–203. doi: 10.1164/rccm.201001-0115OC. [DOI] [PubMed] [Google Scholar]
- 45.Sapien RE, Fullerton-Gleason L, Allen N. Teaching school teachers to recognize respiratory distress in asthmatic children. J Asthma. 2004;41:739–743. doi: 10.1081/jas-200027983. [DOI] [PubMed] [Google Scholar]
- 46.Byrnes CA, Dinarevic S, Shinebourne EA, Barnes PJ, Bush A. Exhaled nitric oxide measurements in normal and asthmatic children. Pediatr Pulmonol. 1997;24:312–318. doi: 10.1002/(sici)1099-0496(199711)24:5<312::aid-ppul2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 47.Mattes J, Storm van’s Gravesande K, Reining U, et al. NO in exhaled air is correlated with markers of eosinophilic airway inflammation in corticosteroid-dependent childhood asthma. Eur Respir J. 1999;13:1391–1395. [PubMed] [Google Scholar]
- 48.Warke TJ, Fitch PS, Brown V, et al. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax. 2002;57:383–387. doi: 10.1136/thorax.57.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baraldi E, Azzolin NM, Zanconato S, Dario C, Zacchello F. Corticosteroids decrease exhaled nitric oxide in children with acute asthma. J Pediatr. 1997;131:381–385. doi: 10.1016/s0022-3476(97)80062-5. [DOI] [PubMed] [Google Scholar]
- 50.del Giudice MM, Brunese FP, Piacentini GL, et al. Fractional exhaled nitric oxide (FENO), lung function and airway hyperresponsiveness in naive atopic asthmatic children. J Asthma. 2004;41:759–765. doi: 10.1081/jas-200027862. [DOI] [PubMed] [Google Scholar]
- 51.Bastain TM, Islam T, Berhane KT et al (2011) Exhaled nitric oxide, susceptibility and new-onset asthma in the children’s health study. Eur Respir J. doi:10.1183/09031936.00021210 [DOI] [PMC free article] [PubMed]
- 52.Korn S, Telke I, Kornmann O, Buhl R. Measurement of exhaled nitric oxide: comparison of different analysers. Respirology. 2011;15:1203–1208. doi: 10.1111/j.1440-1843.2010.01847.x. [DOI] [PubMed] [Google Scholar]
- 53.Caudri D, Wijga AH, Hoekstra MO, et al. Prediction of asthma in symptomatic preschool children using exhaled nitric oxide, Rint and specific IgE. Thorax. 2010;65:801–807. doi: 10.1136/thx.2009.126912. [DOI] [PubMed] [Google Scholar]
- 54.Pedrosa M, Cancelliere N, Barranco P, Lopez-Carrasco V, Quirce S. Usefulness of exhaled nitric oxide for diagnosing asthma. J Asthma. 2010;47:817–821. doi: 10.3109/02770903.2010.491147. [DOI] [PubMed] [Google Scholar]
- 55.Sandrini A, Taylor DR, Thomas PS, Yates DH. Fractional exhaled nitric oxide in asthma: an update. Respirology. 2010;15:57–70. doi: 10.1111/j.1440-1843.2009.01616.x. [DOI] [PubMed] [Google Scholar]
- 56.Shields MD, Brown V, Stevenson EC, et al. Serum eosinophilic cationic protein and blood eosinophil counts for the prediction of the presence of airways inflammation in children with wheezing. Clin Exp Allergy. 1999;29:1382–1389. doi: 10.1046/j.1365-2222.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- 57.Peona V, De Amici M, Quaglini S, et al. Serum eosinophilic cationic protein: is there a role in respiratory disorders? J Asthma. 2010;47:131–134. doi: 10.3109/02770900903497170. [DOI] [PubMed] [Google Scholar]
- 58.Ahlstedt S. Clinical application of eosinophilic cationic protein in asthma. Allergy Proc. 1995;16:59–62. doi: 10.2500/108854195778771426. [DOI] [PubMed] [Google Scholar]
- 59.Perng DW, Huang HY, Lee YC, Perng RP. Leukotriene modifier vs inhaled corticosteroid in mild-to-moderate asthma: clinical and anti-inflammatory effects. Chest. 2004;125:1693–1699. doi: 10.1378/chest.125.5.1693. [DOI] [PubMed] [Google Scholar]
- 60.Basyigit I, Yildiz F, Kacar Ozkara S, Boyaci H, Ilgazli A, Ozkarakas O. Effects of different anti-asthmatic agents on induced sputum and eosinophil cationic protein in mild asthmatics. Respirology. 2004;9:514–520. doi: 10.1111/j.1440-1843.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 61.Kocak AK, Bor O, Yildiz B, Erdogan L, Us T. T-lymphocyte activation and the levels of eosinophilic cationic protein and interleukin-5 in asthmatic children with acute exacerbation and effect of glucocorticoid treatment. Allergy Asthma Proc. 2006;27:371–377. doi: 10.2500/aap.2006.27.2885. [DOI] [PubMed] [Google Scholar]
- 62.Randolph TG, Rollins JP. Adrenocorticotropic hormone (ACTH) its effect in bronchial asthma and ragweed hay fever. Ann Allergy. 1950;8:149–162. [PubMed] [Google Scholar]
- 63.Rose B, Pare JA, et al. Preliminary report on adrenocorticotrophic hormone in asthma. Can Med Assoc J. 1950;62:6–9. [PMC free article] [PubMed] [Google Scholar]
- 64.Mellon M, Leflein J, Walton-Bowen K, Cruz-Rivera M, Fitzpatrick S, Smith JA. Comparable efficacy of administration with face mask or mouthpiece of nebulized budesonide inhalation suspension for infants and young children with persistent asthma. Am J Respir Crit Care Med. 2000;162:593–598. doi: 10.1164/ajrccm.162.2.9909030. [DOI] [PubMed] [Google Scholar]
- 65.Allen DB, Mullen M, Mullen B. A meta-analysis of the effect of oral and inhaled corticosteroids on growth. J Allergy Clin Immunol. 1994;93:967–976. doi: 10.1016/s0091-6749(94)70043-5. [DOI] [PubMed] [Google Scholar]
- 66.Balfour-Lynn L. Growth and childhood asthma. Arch Dis Child. 1986;61:1049–1055. doi: 10.1136/adc.61.11.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balfour-Lynn L. Effect of asthma on growth and puberty. Pediatrician. 1987;14:237–241. [PubMed] [Google Scholar]
- 68.Russell G. Childhood asthma and growth—a review of the literature. Respir Med. 1994;88 Suppl A:31–36. doi: 10.1016/s0954-6111(05)80038-1. [DOI] [PubMed] [Google Scholar]
- 69.Chang CC, Tam AY. Suppression of adrenal function in children on inhaled steroids. J Paediatr Child Health. 1991;27:232–234. doi: 10.1111/j.1440-1754.1991.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 70.Doull IJ, Freezer NJ, Holgate ST. Growth of prepubertal children with mild asthma treated with inhaled beclomethasone dipropionate. Am J Respir Crit Care Med. 1995;151:1715–1719. doi: 10.1164/ajrccm.151.6.7767512. [DOI] [PubMed] [Google Scholar]
- 71.Goldstein DE, Konig P. Effect of inhaled beclomethasone dipropionate on hypothalamic–pituitary–adrenal axis function in children with asthma. Pediatrics. 1983;72:60–64. [PubMed] [Google Scholar]
- 72.Boorsma M, Andersson N, Larsson P, Ullman A. Assessment of the relative systemic potency of inhaled fluticasone and budesonide. Eur Respir J. 1996;9:1427–1432. doi: 10.1183/09031936.96.09071427. [DOI] [PubMed] [Google Scholar]
- 73.Lemanske RF, Jr, Busse WW. The US Food and Drug Administration and long-acting beta2-agonists: the importance of striking the right balance between risks and benefits of therapy? J Allergy Clin Immunol. 2010;126:449–452. doi: 10.1016/j.jaci.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 74.Alton EW, Norris AA. Chloride transport and the actions of nedocromil sodium and cromolyn sodium in asthma. J Allergy Clin Immunol. 1996;98:S102–S105. [PubMed] [Google Scholar]
- 75.Taylor IK, O’Shaughnessy KM, Fuller RW, Dollery CT. Effect of cysteinyl-leukotriene receptor antagonist ICI 204.219 on allergen-induced bronchoconstriction and airway hyperreactivity in atopic subjects. Lancet. 1991;337:690–694. doi: 10.1016/0140-6736(91)90277-v. [DOI] [PubMed] [Google Scholar]
- 76.Martin RM, Wilton LV, Mann RD. Prevalence of Churg–Strauss syndrome, vasculitis, eosinophilia and associated conditions: retrospective analysis of 58 prescription-event monitoring cohort studies. Pharmacoepidemiol Drug Saf. 1999;8:179–189. doi: 10.1002/(SICI)1099-1557(199905/06)8:3<179::AID-PDS414>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 77.Kopriva F, Sobolova L, Szotkowska J, Zapalka M. Treatment of chronic cough in children with montelukast, a leukotriene receptor antagonist. J Asthma. 2004;41:715–720. doi: 10.1081/jas-200027851. [DOI] [PubMed] [Google Scholar]
- 78.Kelsay K. Assessing risk: data from montelukast clinical trials. J Allergy Clin Immunol. 2009;124:697–698. doi: 10.1016/j.jaci.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 79.Ling M, Long AA. Pet dander and difficult-to-control asthma: therapeutic options. Allergy Asthma Proc. 2010;31:385–391. doi: 10.2500/aap.2010.31.3390. [DOI] [PubMed] [Google Scholar]
- 80.Shimizu T, Nishihira J, Watanabe H, Abe R, Ishibashi T, Shimizu H. Cetirizine, an H1-receptor antagonist, suppresses the expression of macrophage migration inhibitory factor: its potential anti-inflammatory action. Clin Exp Allergy. 2004;34:103–109. doi: 10.1111/j.1365-2222.2004.01836.x. [DOI] [PubMed] [Google Scholar]
- 81.Charlesworth EN, Massey WA, Kagey-Sobotka A, Norman PS, Lichtenstein LM. Effect of H1 receptor blockade on the early and late response to cutaneous allergen challenge. J Pharmacol Exp Ther. 1992;262:964–970. [PubMed] [Google Scholar]
- 82.D’Amato G, Liccardi G, Noschese P, Salzillo A, D’Amato M, Cazzola M. Anti-IgE monoclonal antibody (omalizumab) in the treatment of atopic asthma and allergic respiratory diseases. Curr Drug Targets Inflamm Allergy. 2004;3:227–229. doi: 10.2174/1568010043343615. [DOI] [PubMed] [Google Scholar]
- 83.Gendo K, Lodewick MJ. Asthma economics: focusing on therapies that improve costly outcomes. Curr Opin Pulm Med. 2005;11:43–50. doi: 10.1097/01.mcp.0000146782.11092.d6. [DOI] [PubMed] [Google Scholar]
- 84.Lewis RV, Lofthouse C. Adverse reactions with beta-adrenoceptor blocking drugs. An update. Drug Saf. 1993;9:272–279. doi: 10.2165/00002018-199309040-00005. [DOI] [PubMed] [Google Scholar]
- 85.Gawchik SM, Saccar CL, Noonan M, Reasner DS, DeGraw SS. The safety and efficacy of nebulized levalbuterol compared with racemic albuterol and placebo in the treatment of asthma in pediatric patients. J Allergy Clin Immunol. 1999;103:615–621. doi: 10.1016/s0091-6749(99)70233-2. [DOI] [PubMed] [Google Scholar]
- 86.Ihre E, Larsson K. Airways responses to ipratropium bromide do not vary with time in asthmatic subjects. Studies of interindividual and intraindividual variation of bronchodilatation and protection against histamine-induced bronchoconstriction. Chest. 1990;97:46–51. doi: 10.1378/chest.97.1.46. [DOI] [PubMed] [Google Scholar]
- 87.Ueno O, Lee LN, Wagner PD. Effect of N-acetylcysteine on gas exchange after methacholine challenge and isoprenaline inhalation in the dog. Eur Respir J. 1989;2:238–246. [PubMed] [Google Scholar]
- 88.Powell C, Chang C, Naguwa SM, Cheema G, Gershwin ME. Steroid induced osteonecrosis: an analysis of steroid dosing risk. Autoimmun Rev. 2010;9:721–743. doi: 10.1016/j.autrev.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Campbell LM. From adrenaline to formoterol: advances in beta-agonist therapy in the treatment of asthma. Int J Clin Pract. 2002;56:783–790. [PubMed] [Google Scholar]
- 90.Konig P, Hordvik NL, Serby CW. Fenoterol in exercise-induced asthma. Effect of dose on efficacy and duration of action. Chest. 1984;85:462–464. doi: 10.1378/chest.85.4.462. [DOI] [PubMed] [Google Scholar]
- 91.Walker SB, Bierman CW, Pierson WE, Shapiro GG, Furukawa CT, Mingo TS. Bitolterol mesylate in exercise-induced asthma. J Allergy Clin Immunol. 1986;77:32–36. doi: 10.1016/0091-6749(86)90318-0. [DOI] [PubMed] [Google Scholar]
- 92.Francis PW, Krastins IR, Levison H. Oral and inhaled salbutamol in the prevention of exercise-induced bronchospasm. Pediatrics. 1980;66:103–108. [PubMed] [Google Scholar]
- 93.Sly RM, O’Brien SR. Effect of oral terbutaline on exercise-induced asthma. Ann Allergy. 1982;48:151–155. [PubMed] [Google Scholar]
- 94.Debelic M, Stechert R. Exercise-induced asthma—protection with disodium cromoglycate alone and in combination with fenoterol. Monatsschr Kinderheilkd. 1988;136:448–452. [PubMed] [Google Scholar]
- 95.Patel KR, Wall RT. Dose-duration effect of sodium cromoglycate aerosol in exercise-induced asthma. Eur J Respir Dis. 1986;69:256–260. [PubMed] [Google Scholar]
- 96.Merland N, Cartier A, L’Archeveque J, Ghezzo H, Malo JL. Theophylline minimally inhibits bronchoconstriction induced by dry cold air inhalation in asthmatic subjects. Am Rev Respir Dis. 1988;137:1304–1308. doi: 10.1164/ajrccm/137.6.1304. [DOI] [PubMed] [Google Scholar]
- 97.Thomson NC, Patel KR, Kerr JW. Sodium cromoglycate and ipratropium bromide in exercise-induced asthma. Thorax. 1978;33:694–699. doi: 10.1136/thx.33.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Newnham DM, Ingram CG, Earnshaw J, Palmer JB, Dhillon DP. Salmeterol provides prolonged protection against exercise-induced bronchoconstriction in a majority of subjects with mild, stable asthma. Respir Med. 1993;87:439–444. doi: 10.1016/0954-6111(93)90070-g. [DOI] [PubMed] [Google Scholar]
- 99.Kemp JP. Exercise-induced bronchoconstriction: the effects of montelukast, a leukotriene receptor antagonist. Ther Clin Risk Manag. 2009;5:923–933. doi: 10.2147/tcrm.s4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tanser AR, Elmes J. A controlled trial of ketotifen in exercise-induced asthma. Br J Dis Chest. 1980;74:398–402. [PubMed] [Google Scholar]
- 101.Barnes PJ, Wilson NM, Brown MJ. A calcium antagonist, nifedipine, modifies exercise-induced asthma. Thorax. 1981;36:726–730. doi: 10.1136/thx.36.10.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foresi A, Corbo GM, Ciappi G, Valente S, Polidori G. Effect of two doses of inhaled diltiazem on exercise-induced asthma. Respiration. 1987;51:241–247. doi: 10.1159/000195208. [DOI] [PubMed] [Google Scholar]
- 103.Patel KR. Calcium antagonists in exercise-induced asthma. Br Med J (Clin Res Ed) 1981;282:932–933. doi: 10.1136/bmj.282.6268.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zielinski J, Chodosowska E. Exercise-induced bronchoconstriction in patients with bronchial asthma. Its prevention with an antihistaminic agent. Respiration. 1977;34:31–35. doi: 10.1159/000193773. [DOI] [PubMed] [Google Scholar]
- 105.Turner MW, Yalcin I, Soothill JF, et al. In vitro investigations in asthmatic children undergoing hyposensitization with tyrosine-adsorbed Dermatophagoides pteronyssinus antigen. Clin Allergy. 1984;14:221–231. doi: 10.1111/j.1365-2222.1984.tb02201.x. [DOI] [PubMed] [Google Scholar]
- 106.Hedlin G, Heilborn H, Lilja G, et al. Long-term follow-up of patients treated with a three-year course of cat or dog immunotherapy. J Allergy Clin Immunol. 1995;96:879–885. doi: 10.1016/s0091-6749(95)70223-7. [DOI] [PubMed] [Google Scholar]
- 107.Hedlin G, Graff-Lonnevig V, Heilborn H, et al. Immunotherapy with cat- and dog-dander extracts. V. Effects of 3 years of treatment. J Allergy Clin Immunol. 1991;87:955–964. doi: 10.1016/0091-6749(91)90417-m. [DOI] [PubMed] [Google Scholar]
- 108.Sundin B, Lilja G, Graff-Lonnevig V, et al. Immunotherapy with partially purified and standardized animal dander extracts. I. Clinical results from a double-blind study on patients with animal dander asthma. J Allergy Clin Immunol. 1986;77:478–487. doi: 10.1016/0091-6749(86)90183-1. [DOI] [PubMed] [Google Scholar]
- 109.Lowenstein H, Graff-Lonnevig V, Hedlin G, et al. Immunotherapy with cat- and dog-dander extracts. III. Allergen-specific immunoglobulin responses in a 1-year double-blind placebo study. J Allergy Clin Immunol. 1986;77:497–505. doi: 10.1016/0091-6749(86)90185-5. [DOI] [PubMed] [Google Scholar]
- 110.Hedlin G, Graff-Lonnevig V, Heilborn H, et al. Immunotherapy with cat- and dog-dander extracts. II. In vivo and in vitro immunologic effects observed in a 1-year double-blind placebo study. J Allergy Clin Immunol. 1986;77:488–496. doi: 10.1016/0091-6749(86)90184-3. [DOI] [PubMed] [Google Scholar]
- 111.Horst M, Hejjaoui A, Horst V, Michel FB, Bousquet J. Double-blind, placebo-controlled rush immunotherapy with a standardized Alternaria extract. J Allergy Clin Immunol. 1990;85:460–472. doi: 10.1016/0091-6749(90)90156-x. [DOI] [PubMed] [Google Scholar]
- 112.Frankland AW, Augustin R. Prophylaxis of summer hay-fever and asthma: a controlled trial comparing crude grass-pollen extracts with the isolated main protein component. Lancet. 1954;266:1055–1057. doi: 10.1016/s0140-6736(54)91620-7. [DOI] [PubMed] [Google Scholar]
- 113.Frankland AW, Augustin R. Grass pollen antigens effective in treatment. Clin Sci. 1962;23:95–102. [PubMed] [Google Scholar]
- 114.Leynadier F, Banoun L, Dollois B, et al. Immunotherapy with a calcium phosphate-adsorbed five-grass-pollen extract in seasonal rhinoconjunctivitis: a double-blind, placebo-controlled study. Clin Exp Allergy. 2001;31:988–996. doi: 10.1046/j.1365-2222.2001.01145.x. [DOI] [PubMed] [Google Scholar]
- 115.Johnstone DE. Study of the role of antigen dosage in the treatment of pollenosis and pollen asthma. AMA J Dis Child. 1957;94:1–5. doi: 10.1001/archpedi.1957.04030020003001. [DOI] [PubMed] [Google Scholar]
- 116.Mirone C, Albert F, Tosi A, et al. Efficacy and safety of subcutaneous immunotherapy with a biologically standardized extract of Ambrosia artemisiifolia pollen: a double-blind, placebo-controlled study. Clin Exp Allergy. 2004;34:1408–1414. doi: 10.1111/j.1365-2222.2004.02056.x. [DOI] [PubMed] [Google Scholar]
- 117.Gonzalez P, Florido F, Saenz de San Pedro B, de la Torre F, Rico P, Martin S. Immunotherapy with an extract of Olea europaea quantified in mass units. Evaluation of the safety and efficacy after one year of treatment. J Investig Allergol Clin Immunol. 2002;12:263–271. [PubMed] [Google Scholar]
- 118.Bousquet J, Hejjaoui A, Clauzel AM, et al. Specific immunotherapy with a standardized Dermatophagoides pteronyssinus extract. II. Prediction of efficacy of immunotherapy. J Allergy Clin Immunol. 1988;82:971–977. doi: 10.1016/0091-6749(88)90133-9. [DOI] [PubMed] [Google Scholar]
- 119.Ferreira MB, Santos AS, Pregal AL, et al. Leukotriene receptor antagonists (Montelukast) in the treatment of asthma crisis: preliminary results of a double-blind placebo controlled randomized study. Allerg Immunol (Paris) 2001;33:315–318. [PubMed] [Google Scholar]
- 120.Camargo CA, Jr, Smithline HA, Malice MP, Green SA, Reiss TF. A randomized controlled trial of intravenous montelukast in acute asthma. Am J Respir Crit Care Med. 2003;167:528–533. doi: 10.1164/rccm.200208-802OC. [DOI] [PubMed] [Google Scholar]
- 121.Ramsay CF, Pearson D, Mildenhall S, Wilson AM. Oral montelukast in acute asthma exacerbations: a randomised, double-blind, placebo-controlled trial. Thorax. 2011;66:7–11. doi: 10.1136/thx.2010.135038. [DOI] [PubMed] [Google Scholar]
- 122.Nelson KA, Smith SR, Trinkaus K, Jaffe DM. Pilot study of oral montelukast added to standard therapy for acute asthma exacerbations in children aged 6 to 14 years. Pediatr Emerg Care. 2008;24:21–27. doi: 10.1097/pec.0b013e31815f3968. [DOI] [PubMed] [Google Scholar]
- 123.Davis PA, Chang C, Hackman RM, Stern JS, Gershwin ME. Acupuncture in the treatment of asthma: a critical review. Allergol Immunopathol (Madr) 1998;26:263–271. [PubMed] [Google Scholar]
- 124.Torres-Llenza V, Bhogal S, Davis M, Ducharme F. Use of complementary and alternative medicine in children with asthma. Can Respir J. 2010;17:183–187. doi: 10.1155/2010/682142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li XM. Complementary and alternative medicine in pediatric allergic disorders. Curr Opin Allergy Clin Immunol. 2009;9:161–167. doi: 10.1097/ACI.0b013e328329226f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Davis PA, Gold EB, Hackman RM, Stern JS, Gershwin ME. The use of complementary/alternative medicine for the treatment of asthma in the United States. J Investig Allergol Clin Immunol. 1998;8:73–77. [PubMed] [Google Scholar]
- 127.Corren J, Busse W, Meltzer EO, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181:788–796. doi: 10.1164/rccm.200909-1448OC. [DOI] [PubMed] [Google Scholar]
- 128.Tomkinson A, Tepper J, Morton M, et al. Inhaled vs subcutaneous effects of a dual IL-4/IL-13 antagonist in a monkey model of asthma. Allergy. 2010;65:69–77. doi: 10.1111/j.1398-9995.2009.02156.x. [DOI] [PubMed] [Google Scholar]
- 129.Holgate ST. Cytokine and anti-cytokine therapy for the treatment of asthma and allergic disease. Cytokine. 2004;28:152–157. doi: 10.1016/j.cyto.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 130.Qiu Z, Fujimura M, Kurashima K, Nakao S, Mukaida N. Enhanced airway inflammation and decreased subepithelial fibrosis in interleukin 6-deficient mice following chronic exposure to aerosolized antigen. Clin Exp Allergy. 2004;34:1321–1328. doi: 10.1111/j.1365-2222.2004.02013.x. [DOI] [PubMed] [Google Scholar]
- 131.Antoniu SA. MEDI-528, an anti-IL-9 humanized antibody for the treatment of asthma. Curr Opin Mol Ther. 2010;12:233–239. [PubMed] [Google Scholar]
- 132.Quarcoo D, Weixler S, Joachim RA, et al. Resiquimod, a new immune response modifier from the family of imidazoquinolinamines, inhibits allergen-induced Th2 responses, airway inflammation and airway hyper-reactivity in mice. Clin Exp Allergy. 2004;34:1314–1320. doi: 10.1111/j.1365-2222.2004.02023.x. [DOI] [PubMed] [Google Scholar]
- 133.Randolph AG, Lange C, Silverman EK, et al. The IL12B gene is associated with asthma. Am J Hum Genet. 2004;75:709–715. doi: 10.1086/424886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang G, Volk A, Petley T, et al. Anti-IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine. 2004;28:224–232. doi: 10.1016/j.cyto.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 135.Gallagher G, Eskdale J, Jordan W, et al. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. Int Immunopharmacol. 2004;4:615–626. doi: 10.1016/j.intimp.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 136.Babu KS, Davies DE, Holgate ST. Role of tumor necrosis factor alpha in asthma. Immunol Allergy Clin North Am. 2004;24:583–597. doi: 10.1016/j.iac.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 137.Forssmann U, Hartung I, Balder R, et al. n-Nonanoyl-CC chemokine ligand 14, a potent CC chemokine ligand 14 analogue that prevents the recruitment of eosinophils in allergic airway inflammation. J Immunol. 2004;173:3456–3466. doi: 10.4049/jimmunol.173.5.3456. [DOI] [PubMed] [Google Scholar]
- 138.Warner RL, Lukacs NW, Shapiro SD, et al. Role of metalloelastase in a model of allergic lung responses induced by cockroach allergen. Am J Pathol. 2004;165:1921–1930. doi: 10.1016/S0002-9440(10)63244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mukhopadhyay S, Sypek J, Tavendale R, et al. Matrix metalloproteinase-12 is a therapeutic target for asthma in children and young adults. J Allergy Clin Immunol. 2010;126:70–76.e16. doi: 10.1016/j.jaci.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 140.Singh D, Petavy F, Macdonald AJ, Lazaar AL, O’Connor BJ. The inhaled phosphodiesterase 4 inhibitor GSK256066 reduces allergen challenge responses in asthma. Respir Res. 2010;11:26. doi: 10.1186/1465-9921-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Coyle AJ, Page CP, Atkinson L, Sjoerdsma K, Touvay C, Metzger WJ. Modification of allergen-induced airway obstruction and airway hyperresponsiveness in an allergic rabbit model by the selective platelet-activating factor antagonist, BN 52021. J Allergy Clin Immunol. 1989;84:960–967. doi: 10.1016/0091-6749(89)90395-3. [DOI] [PubMed] [Google Scholar]
- 142.Knauer KA, Lichtenstein LM, Adkinson NF, Jr, Fish JE. Platelet activation during antigen-induced airway reactions in asthmatic subjects. N Engl J Med. 1981;304:1404–1407. doi: 10.1056/NEJM198106043042307. [DOI] [PubMed] [Google Scholar]
- 143.Lupinetti MD, Sheller JR, Catella F, Fitzgerald GA. Thromboxane biosynthesis in allergen-induced bronchospasm. Evidence for platelet activation. Am Rev Respir Dis. 1989;140:932–935. doi: 10.1164/ajrccm/140.4.932. [DOI] [PubMed] [Google Scholar]
- 144.Szczeklik A, Schmitz-Schumann M, Krzanowski M, Virchow C., Sr Delayed generation of thrombin in clotting blood of atopic patients with hayfever and asthma. Clin Exp Allergy. 1991;21:411–415. doi: 10.1111/j.1365-2222.1991.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 145.Hayashi N, Chihara J, Kobayashi Y, et al. Effect of platelet-activating factor and platelet factor 4 on eosinophil adhesion. Int Arch Allergy Immunol. 1994;104(Suppl 1):57–59. doi: 10.1159/000236754. [DOI] [PubMed] [Google Scholar]
- 146.Deuel TF, Huang JS. Platelet-derived growth factor. Structure, function, and roles in normal and transformed cells. J Clin Invest. 1984;74:669–676. doi: 10.1172/JCI111482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- 148.Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kameyoshi Y, Dorschner A, Mallet AI, Christophers E, Schroder JM. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992;176:587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tsicopoulos A, Lassalle P, Joseph M, et al. Effect of disodium cromoglycate on inflammatory cells bearing the Fc epsilon receptor type II (Fc epsilon RII) Int J Immunopharmacol. 1988;10:227–236. doi: 10.1016/0192-0561(88)90053-7. [DOI] [PubMed] [Google Scholar]
- 151.Thorel T, Joseph M, Tsicopoulos A, Tonnel AB, Capron A. Inhibition by nedocromil sodium of IgE-mediated activation of human mononuclear phagocytes and platelets in allergy. Int Arch Allergy Appl Immunol. 1988;85:232–237. doi: 10.1159/000234508. [DOI] [PubMed] [Google Scholar]
- 152.De Vos C, Joseph M, Leprevost C, et al. Inhibition of human eosinophil chemotaxis and of the IgE-dependent stimulation of human blood platelets by cetirizine. Int Arch Allergy Appl Immunol. 1989;88:212–215. doi: 10.1159/000234789. [DOI] [PubMed] [Google Scholar]
- 153.Clifford RL, Knox AJ. Vitamin D—a new treatment for airway remodelling in asthma? Br J Pharmacol. 2009;158:1426–1428. doi: 10.1111/j.1476-5381.2009.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Palma-Carlos AG, Palma-Carlos ML, Santos MC, Melo A. Cytokines and adhesion molecules in respiratory allergy. Allerg Immunol (Paris) 1995;27:178–181. [PubMed] [Google Scholar]
- 155.Scurati S, Frati F, Passalacqua G, Puccinelli P, Hilaire C, Incorvaia C. Adherence issues related to sublingual immunotherapy as perceived by allergists. Patient Prefer Adherence. 2010;4:141–145. doi: 10.2147/ppa.s10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ciprandi G, Cadario G, Di Gioacchino GM, et al. Sublingual immunotherapy in children with allergic polysensitization. Allergy Asthma Proc. 2010;31:227–231. doi: 10.2500/aap.2010.31.3337. [DOI] [PubMed] [Google Scholar]
- 157.D’Anneo RW, Bruno ME, Falagiani P. Sublingual allergoid immunotherapy: a new 4-day induction phase in patients allergic to house dust mites. Int J Immunopathol Pharmacol. 2010;23:553–560. doi: 10.1177/039463201002300217. [DOI] [PubMed] [Google Scholar]
- 158.Ferres J, Justicia JL, Garcia MP, Munoz-Tuduri M, Alva V (2011) Efficacy of high-dose sublingual immunotherapy in children allergic to house dust mites in real-life clinical practice. Allergol Immunopathol (Madr). doi:10.1016/j.aller.2010.01.008 [DOI] [PubMed]
- 159.Leonardi S, Arena A, Bruno ME, et al. Olea sublingual allergoid immunotherapy administered with two different treatment regimens. Allergy Asthma Proc. 2010;31:e25–e29. doi: 10.2500/aap.2010.31.3316. [DOI] [PubMed] [Google Scholar]
- 160.Bufe A, Eberle P, Franke-Beckmann E, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123:167–173.e7. doi: 10.1016/j.jaci.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 161.Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010;40:385–397. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- 162.Melen E, Himes BE, Brehm JM, et al. Analyses of shared genetic factors between asthma and obesity in children. J Allergy Clin Immunol. 2010;126:631–637.e1–8. doi: 10.1016/j.jaci.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vollmer WM, Swain MC. Role of the specialist in the treatment of asthma. Curr Opin Allergy Clin Immunol. 2002;2:189–194. doi: 10.1097/00130832-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 164.Stanford RH, Gilsenan AW, Ziemiecki R, Zhou X, Lincourt WR, Ortega H. Predictors of uncontrolled asthma in adult and pediatric patients: analysis of the Asthma Control Characteristics and Prevalence Survey Studies (ACCESS) J Asthma. 2010;47:257–262. doi: 10.3109/02770900903584019. [DOI] [PubMed] [Google Scholar]
- 165.Vollmer WM, Markson LE, O’Connor E, Frazier EA, Berger M, Buist AS. Association of asthma control with health care utilization: a prospective evaluation. Am J Respir Crit Care Med. 2002;165:195–199. doi: 10.1164/ajrccm.165.2.2102127. [DOI] [PubMed] [Google Scholar]


