Abstract
Baxter has developed a new recombinant factor IX (rFIX) drug product (BAX326) for treating patients with hemophilia B, or congenital FIX deficiency. An extensive preclinical program evaluated the pharmacokinetics, efficacy, and safety of BAX326 in different species. The efficacy of BAX326 was tested in three mouse models of primary pharmacodynamics: tail-tip bleeding, carotid occlusion, and thrombelastography. The pharmacokinetics was evaluated after a single intravenous bolus injection in mice, rats, and macaques. Toxicity was assessed in rats and macaques, safety pharmacology in rabbits and macaques, and immunogenicity in mice. BAX326 was shown to be efficacious in all three primary pharmacodynamic studies (P ≤ 0.0076). Hemostatic efficacy was dose related and similar for the three lots tested. Pharmacokinetic results showed that rFIX activity and rFIX antigen concentrations declined in a bi-phasic manner, similar to a previously licensed rFIX product. BAX326 was well tolerated in rabbits and macaques at all dose levels; no thrombogenic events and no adverse clinical, respiratory, or cardiovascular effects occurred. BAX326 was also shown to have a similar immunogenicity profile to the comparator rFIX product in mice. These results demonstrate that BAX326 has a favorable preclinical safety and efficacy profile, predictive of a comparable effect to that of the previously licensed rFIX in humans.
Electronic supplementary material
The online version of this article (doi:10.1007/s12185-013-1448-z) contains supplementary material, which is available to authorized users.
Keywords: Hemophilia B, rFIX, Pharmacokinetics, Safety, Efficacy
Introduction
Hemophilia B, or congenital factor IX (FIX) deficiency, is an X-linked bleeding disorder resulting in a reduced or complete lack of FIX activity in plasma. It is a lifelong condition affecting individuals from birth onwards, and occurring in approximately 1 in 30,000 live male births [1–3]. Clinical manifestations depend on the severity of the disease and are characterized by spontaneous bleeds and/or bleeds of increased severity following accidental or surgical trauma into joints, muscles, or internal organs. Infusion with FIX concentrates is the mainstay of treatment for patients with hemophilia B to prevent or control bleeding episodes.
Plasma-derived (pd) coagulation factors were introduced in the early 1990s and have since had significant medical and quality of life benefits for hemophilia patients. Despite the availability of high-purity, virus-inactivated pd factor VIII and pdFIX products that are considered to be free of known viruses such as human immunodeficiency virus (HIV) and hepatitis B and C, concerns remain about their contamination with new human pathogens, such as West Nile virus and SARS, or non-lipid enveloped infectious agents, such as blood-borne prions causing new variant Creutzfeldt–Jakob disease (vCJD), transmission of hepatitis A virus [4, 5] and parvovirus [6, 7], as current inactivation/removal steps are mainly limited to lipid-coated pathogens [8]. This concern has stimulated intense efforts to develop recombinant human rFVIII and rFIX products for use in persons with hemophilia A and B.
Baxter’s recombinant (r) coagulation FIX (BAX3261) is a glycoprotein that is secreted by genetically engineered mammalian cells derived from a Chinese hamster ovary (CHO) cell line. No materials of human or animal origin are added in the manufacture, purification, or formulation of the final drug product, thus reducing the risk of transmission of adventitious agents. The manufacture of BAX326 includes two independent viral inactivation/reduction steps: solvent/detergent (S/D) treatment and nanofiltration. Here, we describe the pharmacokinetics, safety, and efficacy of BAX326 in several animal models compared with licensed pdFIX and rFIX.
Materials and methods
The rFIX used in the presented studies was synthesized by a recombinant CHO cell clone in suspension culture which coexpresses rFIX and recombinant human wild-type furin. Three lots were tested in the primary pharmacodynamic studies. A commercially available rFIX (Benefix; Wyeth Europe Ltd., South Taplow, UK) and a licensed pdFIX (Mononine; CSL Behring, Nuremberg, Germany) were used as reference items; formulation buffer and/or saline served as negative controls. The following species were chosen to characterize the pharmacokinetic profile of BAX326: FIX knockout (ko) mice, rats, and macaques. Pharmacological activity was shown in in vivo efficacy studies in mice, and in an in vitro activated partial prothrombin time (aPTT) study in rats and macaques. Hence, these species were also considered as suitable to assess pharmacology and toxicity.
All studies complied with national laws governing animal experimentation and were approved by the animal care committees of the respective institutions. Safety pharmacology, pharmacokinetic, toxicology, and immunogenicity studies were performed according to GLP.
Efficacy studies in hemophilia B mice
The efficacy of BAX326, defined as statistically significantly reduced blood loss compared with buffer, was tested in three mouse models of primary pharmacodynamics (FIX ko mice: B6.129P2-F9 tm1Dws). A carotid occlusion model (COM), and thrombelastography (TEG), which has been used in a variety of preclinical models of hemophilia [9, 10], were used to compare the efficacy of BAX326 with that of the previously licensed FIX products. Animals received intravenous (iv; clinical application route) prophylactic treatment with 75 IU/kg of BAX326 or licensed rFIX, and, in the carotid occlusion model, licensed pdFIX. To obtain dose–effect curves, BAX326 was tested at 10, 25, 75, and 100 IU/kg in a tail-tip bleeding (TTB) model.
Animals were anesthetized using 100 mg/kg ketamine and 10 mg/kg xylazine and humanely killed by cervical dislocation immediately after the observation period or blood sampling. Statistical analyses were performed with R (2009) Version 2.8.1 and StatXact (2007) version 8.0.
Carotid occlusion model
10 mice (5m/5f) per group received test or reference item 15 min before exposing the left carotid artery and denuding the endothelium by topical application of 15 % FeCl3 for 3 min [11]. Carotid blood flow was monitored before and after injury using an ultrasound flow probe interfaced with a flow meter (TTFM, Transonic Systems Inc) over 30 min, and time to occlusion (min) assessed.
Thrombelastography
10 mice (5m/5f) received test or reference item 5 min before 1 mL citrated whole blood was drawn by venipuncture of the vena cava caudalis, mixed with CaCl2, and immediately measured by TEG for R-time (min), i.e., the time of latency from when the blood is placed in the analyzer (Haemonetics Corp., Braintree, MA, USA) until initial fibrin formation. This represents clot initiation and resembles the enzymatic portion of coagulation. If no clot was formed, the TEG run was canceled after 3 h.
Tail-tip bleeding model
16 mice (8m/8f) per group received test or reference item 5 min before clipping 2 mm of the tail tip and placing it in warm saline at approximately 37 °C. The total blood loss [mg] was gravimetrically determined over 60 min.
Pharmacokinetic studies in mice, rats, and macaques
The pharmacokinetics of BAX326 after a single iv bolus injection was evaluated and compared with previously licensed rFIX in B6.129P2-F9 tm1Dws mice, Sprague–Dawley rats, and macaques.
Conscious restrained mice received a dose of 75, 200, or 750 of BAX326, 75 IU/kg licensed rFIX, or 75 IU/kg pdFIX; 10 animals (5m/5f) per time point were bled by cardiac puncture 5, 15 min, 1, 3, 6, 10, or 16 h thereafter (serial killing design). Ten Sprague–Dawley rats (5m/5f) per group received 500 μg/kg of test or reference item in a lateral tail vein. Blood samples were drawn from the ventral tail artery at least 24 h before (baseline) and 5, 30, 90 min, 4, 7, and 10 h after dosing. Four conscious restrained macaques (2m/2f) per group received 150 IU/kg of BAX326 or licensed rFIX into the vena saphena; blood samples were drawn at baseline, 5, 30, 90 min, 3, 6, 10, and 16 h after dosing.
The primary end point of these studies was dose-adjusted AUC0–tlast (the area under the curve vs. time curve from 0 to the last time point measured) for human FIX activity in mice and macaques and for human FIX protein (antigen) in all species. Secondary end points were in vivo recovery (IVR), half-life (HL), mean residence time (MRT), total clearance standardized per kg body mass (CLs), and volume of distribution at steady state (Vss). FIX activity was analyzed by chromogenic assay (Biophen Factor IX, Hyphen Biomed, Neuville-sur-Oise, France) in citrated mouse plasma samples, and by FIX 1-stage clotting assay in citrated rat and macaques plasma (see Supplemental Data). FIX protein (antigen) in plasma of all three species was measured by ELISA (see Supplemental Data) using commercially available polyclonal antibodies (Enzyme Research Laboratories, Swansea, UK). Statistical analyses were performed with SAS Version 8.2 for Linux.
Safety pharmacology studies in rabbits and macaques
The thrombogenic potential of BAX326 and its effects on body temperature, and cardiovascular and respiratory function was investigated in rabbits and macaques. The highest dose tested (750 IU/kg) in animals with normal hemostasis reflected at least 10 times the intended human prophylactic dose. Licensed rFIX and pdFIX served as reference items.
Thrombogenic potential in rabbits
In a Wessler rabbit stasis model [12], six animals per treatment group received a single iv bolus injection of BAX326, licensed rFIX, or pdFIX; thrombogenicity was assessed semi-quantitatively using a 4-point thrombus formation scale (blinded reviewer) for isolated jugular vein segments. In a separate study, the thrombogenic potential of BAX326 was evaluated after addition of higher amounts of FIXa (0.1272 IU/mL), similar to those present in the previously licensed rFIX.
Cardiovascular safety in telemetered macaques
Eight male conscious telemetered (integrated radiotelemetry system) macaques received a single iv bolus injection of BAX326 or formulation buffer at 75 or 450 IU/kg into the vena saphena using a Latin square design. Clinical observations, body weight, body temperature, respiratory rate, intra-thoracic pressure changes, ECG parameters, and blood pressure were evaluated.
Toxicology studies in mice, rats, and macaques
Single-dose toxicity in mice and macaques
Single-dose toxicity was assessed in C57BL/6NCrl mice and macaques. 10 mice (5m/5f) per treatment group received an iv bolus injection via tail vein of BAX326, licensed rFIX, pdFIX, buffer, or saline at 750, 4000, or 7500 IU/kg. Immediate effects 1 day after administration and possible long-term effects after a 14-day recovery period were assessed by body weight, clinical condition, hematocrit and platelet count, prothrombin time (PT), aPTT, and macroscopic and microscopic pathology. After a single administration of 200 IU/kg of BAX326 and a 6-day washout period in four (2m/2f) macaques, these animals received a further dose of 750 IU/kg and were assessed for clinical condition, body weight, food consumption, clinical pathology, organ weight, macropathology, and histopathology.
Repeat dose toxicity in rats and macaques
As part of the above-mentioned study in macaques, repeated iv administration of BAX326 was assessed in four (2m/2f) macaques that received a dose of 750 IU/kg every other day for 28 days. Clinical condition, body weight, clinical pathology, organ weight, macropathology and histopathology, clotting function, hematology, blood chemistry, and antibody formation were assessed.
In two further studies, the systemic toxic potential, NOAEL, and safety margin to the clinical dose of BAX326 were compared with previously licensed rFIX administered by iv bolus injection in rats and macaques every other day for 28 days. 30 (15m/15f) rats per treatment group received BAX326 at 200 or 750 IU/kg, licensed rFIX at 200 IU/kg, or buffer at the corresponding fixed volume of 5 mL/kg. Body weight, clinical condition, clinical pathology, food consumption, urinalysis, ophthalmic, organ weight, and macroscopic and microscopic pathology were assessed for reversibility of any toxicological effects during a 2-week recovery period; toxicokinetics and anti-product antibody formation were assessed in satellite animals. Ten (5m/5f) macaques per treatment group received BAX326 at 200 or 750 IU/kg, licensed rFIX at 200 IU/kg, or buffer at the corresponding fixed volume of 5 mL/kg. Clinical signs, body weight, ophthalmoscopy, cardiovascular investigations, clinical pathology, toxicokinetics, analysis of FIX activity in plasma and of neutralizing and binding antibodies in plasma organ weights, necropsy, and histopathology were assessed for reversibility of any effects during a 2-week recovery period.
Local tolerance in rabbits
Local tolerance of BAX326 at potencies of approximately 2000 and 3000 IU/vial was assessed after iv (intended clinical route), and intraarterial and paravenous (possible misapplication routes) application in rabbits. BAX326 or buffer was injected iv or intraarterially (ia) at a volume of 5 mL within 2 min into the right ear of four rabbits (2m/2f). BAX326 or licensed rFIX was injected paravenously (pv) at a volume of 0.5 mL per animal as a bolus injection into the right ear of another four rabbits (2m/2f). An equivalent volume of isotonic saline was administered into the left ear of each rabbit by the same route. The animals’ behavior was observed and injection sites were examined macroscopically for possible changes within 30 min of treatment and thereafter intermittently up to 6 h, and at 24, 48, and 72 h. Histopathological examination was performed at the end of the observation period.
Comparative immunogenicity in mice
The immunogenicity of BAX326 compared with previously licensed rFIX was tested in BALB/c mice. 10 male animals received eight iv injections of test or reference item at doses of 2.5, 5, and 10 μg per mouse at weekly intervals. Plasma samples were taken at baseline, after the fourth and after the last dose, and tested for antibodies against human FIX by ELISA. Development of antibodies against product impurities such as host cell proteins was also analyzed.
Results
Efficacy studies in hemophilia B mice
BAX326 was shown to be efficacious at 75 IU/kg in all three primary pharmacodynamic studies (P ≤ 0.0076) performed. Hemostatic efficacy was similar for all three lots tested and dose related.
Carotid occlusion model
Time to occlusion for all three lots of BAX326 tested was markedly lower (3.3–4.8 min) than for buffer (>30 min = no occlusion; P = 0.00035) (Fig. 1).
Fig. 1.
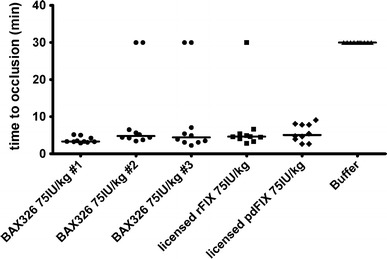
Time to occlusion after treatment with rFIX, pdFIX, or buffer. Three lots of BAX326 (filled circle) were tested at 75 IU/kg to show lot-to-lot consistency. All lots were considered efficacious as median time to occlusion (3.3–4.8 min) was markedly reduced compared with buffer-treated animals (filled triangle >30 min = no occlusion; P = 0.00035, assessed for Lot 2 only). Median time to occlusion was 4.6 min after treatment with 75 IU/kg of the licensed rFIX (filled square) and 5.1 min after treatment with the licensed pdFIX (filled diamond)
Thrombelastography
Median R-time in buffer-treated animals was 98.7 min. Treatment with 75 IU/kg of BAX326 led to a statistically significant reduction of time to clotting (54.5 min; P = 0.00074) (Fig. 2).
Fig. 2.
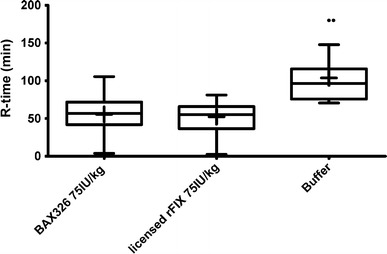
Time to clotting (R-time) of rFIX-treated and buffer-treated FIX ko mice. Median R-time in buffer-treated animals was 98.7 min. Treatment with 75 IU/kg of BAX326 led to a statistically significant reduction of time to clotting (54.5 min; P = 0.00074). Median R-time was similarly reduced to 56.2 min by treatment with 75 IU/kg of the active control item, a previously licensed rFIX
Tail-tip bleeding model
Median total blood loss in buffer-treated animals was 1,036 mg. Median blood loss was reduced to 927 mg with 10 IU/kg of BAX326. Treatment with 75 IU/kg, the dose also used in the COM and TEG models, led to a statistically significantly lower median blood loss (145 mg; P = 0.0085) than with buffer (Fig. 3). Treatment with 100 IU/kg further reduced median blood loss to 32 mg. Statistical evaluation showed a monotone dose–response relation (P = 0.0076).
Fig. 3.
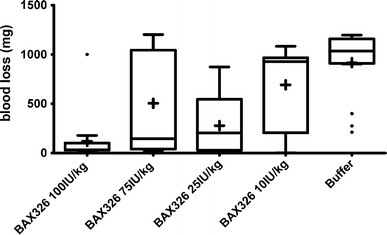
Blood loss in FIX ko mice after treatment with different doses of BAX326 or buffer. Four doses of BAX326 were tested to obtain a dose–effect curve. Buffer-treated animals served as negative controls and showed a median total blood loss of 1,036 mg. Median blood loss was reduced to 927 mg by treatment with 10 IU/kg of BAX326 and to 204 mg by treatment with 25 IU/kg rFIX. Treatment with 75 IU/kg rFIX, the dose also used in the carotid occlusion and TEG studies, led to a median blood loss of 145 mg, which was shown to be statistically different from buffer (P = 0.0085). Treatment with 100 IU/kg further reduced median blood loss to 32 mg. Statistical evaluation proved a monotone dose–response relation (P = 0.0076). The higher inter-individual variation observed for 75 IU/kg than for 25 IU/kg BAX326 is consistent with published results [13–15] and a known and accepted limitation of the model
Pharmacokinetic studies in mice, rats, and macaques
The main pharmacokinetic parameters of BAX326 in FIX ko mice, rats, and macaques are shown in Table 1. The results of these studies showed that both rFIX activity and rFIX antigen concentrations declined in a bi-phasic manner, with no apparent sex-related difference in systemic exposure.
Table 1.
PK variables of BAX326 in FIX ko mice, Sprague–Dawley rats and macaques
| Species | Analyte | AUC0–tlast a | MRT (h) | Terminal HL (h) | IVR (%) | CL (mL/h/kg) | Vss (mL/kg) |
|---|---|---|---|---|---|---|---|
| Mice | Activity | 0.0067 | 7.3 | 5.5 | 9 | 131 | 957 |
| Antigen | 0.0174 | 15.3 | 11.0 | 17 | 37 | 560 | |
| Rats | Antigen | 0.0230 | 7.8 | 3.4 | 21 | 32 | 249 |
| Monkeys | Activity | 0.0805 | 15.6 | 10.4 | 40 | 11 | 170 |
| Antigen | 0.1330 | 17.9 | 12.1 | 69 | 7 | 113 |
a(h*IU/mL/IU/kg) or (h*μg/mL/μg/kg)
In mice, systemic exposure increased disproportionally with dose. The AUC0–tlast for 200 IU/kg was 1.6-fold and the AUC0–tlast for 750 IU/kg was 3.4-fold that of 75 IU/kg. The dose-adjusted AUC0–tlast with 75 IU/kg BAX326 was 1.19-fold that of the same dose of previously licensed rFIX and 0.68 times that of the licensed pdFIX. Data from the pharmacokinetic study in rats confirmed these results.
In macaques, systemic exposure of BAX326 increased in an approximately dose-proportional manner. After iv administration of BAX326 at target doses of 150 (Fig. 4), 300, and 450 IU/kg, plasma concentrations of rFIX antigen and rFIX activity were cleared with an apparent terminal elimination half-life of 9–15 and 9–11 h, respectively. Systemic exposure of rFIX antigen and rFIX activity increased in an approximately dose-proportional manner. The apparent terminal elimination HL, CL, and apparent Vss of rFIX antigen and rFIX activity were dose independent. There was no apparent sex-related difference in the extent of systemic exposure of rFIX antigen and rFIX activity.
Fig. 4.
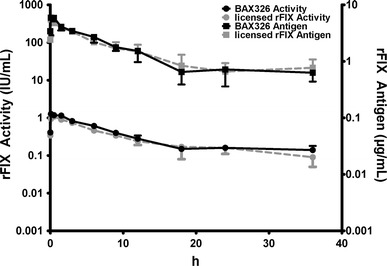
Pharmacokinetics of BAX326 and licensed rFIX (150 IU/kg rFIX) in macaques. The pharmacokinetic profiles of BAX326 (black) and the licensed rFIX (gray) after intravenous bolus administration of 150 IU/kg were similar for rFIX activity and rFIX antigen, declining in a generally bi-phasic manner
Safety pharmacology studies in rabbits and macaques
Thrombogenic potential in rabbits
No thrombogenicity was observed with BAX326: All individual animal scores were 0, and lower than for the previously licensed rFIX. The score for all positive control animals was 4, confirming the validity of the model. Spiking of FIXa into BAX326 to contain an equivalent amount to the comparator rFIX resulted in a mean Wessler score of 0.42 (vs. 0.5 for the previously licensed rFIX). These results showed a clear correlation between the FIXa content of the items tested and the Wessler scores.
Cardiovascular safety in telemetered macaques
All animals survived the experimental period of the study. Treatment with BAX326 did not cause any adverse clinical, respiratory, or cardiovascular effects and was well tolerated at both dose levels (75 and 450 IU/kg).
Toxicology studies in mice, rats and macaques
Single-dose toxicity in mice and macaques
Mice and macaques showed no signs of toxicity at the highest single iv dose tested with a NOAEL of 7500 IU/kg for mice and 750 IU/kg for macaques. These NOAELs indicate a high margin of safety to the human prophylactic dose.
Repeat dose toxicity in rats
Repeated iv administration of 200 or 750 IU/kg of BAX326 for 28 days in rats revealed no treatment-related adverse effects on any parameters investigated. Toxicokinetics confirmed exposure within the whole study period. AUC and C max for BAX326 and the previously licensed rFIX were similar after first and repeated dosing. There was no accumulating effect of repeated dosing at 200 IU/kg; AUC and C max increased marginally after repeated dosing at 750 IU/kg. No binding anti-human FIX antibodies were detectable after treatment in any group. Low titers of binding anti-human FIX antibody titers were detected upon recovery of treatment with 200 IU/kg of BAX326 in 1/10 rats and with 750 IU/kg in 2/10 rats; none of the antibodies had neutralizing activity. Thus, the NOAEL for systemic toxicity in male and female rats following 14 applications of BAX326 every other day is 750 IU/kg, with a high margin of safety to the human prophylactic dose.
Repeat dose toxicity in macaques
Repeated iv administration with 750 IU/kg of BAX326 of every other day for 28 days in macaques was well tolerated and did not reveal any adverse treatment-related changes. In the second repeat dose study in macaques, using 200 and 750 IU/kg of BAX326, there were no mortalities, clinical signs attributable to treatment, and effects on body weight. Toxicokinetics confirmed exposure within the whole study period. Systemic exposure of rFIX activity increased in an approximately dose-proportional manner, with up to 1.6-fold accumulation after bi-daily dosing. The apparent HL, CL, and Vss of rFIX activity were dose- and time independent. No sex-related difference was observed in systemic exposure of rFIX activity. Binding antibodies were detectable in plasma samples of two males (200 IU/kg/day, licensed rFIX), two males and one female (200 IU/kg/day, BAX326), and two males and one female (750 IU/kg/day, BAX326). Analysis of neutralizing antibodies of these eight samples revealed positive titers in plasma samples collected on day 28 for one male (previously licensed rFIX), while all those for all other animals were below the limit of quantification. The NOAEL was 750 IU/kg under the conditions of this study.
Local tolerance in rabbits
Results after iv, ia, or pv administration in rabbits indicated that BAX326 was well tolerated at potencies of approximately 2000 and 3000 IU/vial after iv or ia administration; paravenous administration of both rFIX products led to mild local tissue reactions.
Comparative immunogenicity in mice
No antibody development against potential impurities originating from the producing cell line (CHO protein) was observed after treatment with BAX326 or previously licensed rFIX in BALB/c mice. Anti-human FIX specific antibody development was observed after treatment with either product. The highest anti-human FIX antibody titers were observed with the previously licensed rFIX; differences in anti-human FIX antibody titers between treatment groups were not statistically significant.
Discussion
The presented studies explored the preclinical efficacy, safety pharmacology, pharmacokinetics, toxicology, and immunogenicity of BAX326, a new rFIX drug product, in suitable species.
Efficacy, defined as a significantly reduced hemophilic phenotype compared with negative controls, was demonstrated for BAX326 (all three lots tested), as well as for previously licensed rFIX and pdFIX. The primary pharmacodynamics effects of BAX326 were similar to those of the comparator FIX products. Additionally, the effect of BAX326 was shown to be dose dependent. The higher inter-individual variation observed for 75 IU/kg than for 25 IU/kg BAX326 in tail-tip bleeding assay is consistent with published results [13–15] and a known and accepted limitation of the model.
BAX326 also showed similar pharmacokinetic results to those of the previously licensed rFIX in all species tested. Systemic exposure of rFIX activity and antigen increased in an approximately dose dependent manner in macaques. In rodents, however, dose-adjusted AUC0–tlast increased disproportionally most likely due to a saturated metabolism at high doses. This may be caused by the reduced FIX binding capacity of endothelium and the attainment of steady-state FIX level [16]. Ongoing clinical studies demonstrate BAX326 to have a comparable pharmacokinetic profile in humans to that of the previously licensed rFIX [17].
BAX326 was safe after single and repeated administration in two species at doses at least 10 times higher than the prophylactic clinical dose. The toxicology profile for BAX326 was similar to that of the comparator rFIX product.
The use of FIX products has been associated with the development of thromboembolic complications [18]. BAX326 contains less FIXa than the previously licensed rFIX product, which was demonstrated by a lower thrombogenic potential in a rabbit stasis model. Spiking FIXa into BAX326 to contain an equivalent amount to the comparator rFIX resulted in a similar mean Wessler score to the previously licensed product, showing a clear correlation between the FIXa content of the items tested and the resulting Wessler scores. Therefore, the risk of a thromboembolic event with the use of BAX326 in humans is considered to be similarly low or even lower than for the previously licensed rFIX.
The most challenging complication of replacement therapy is the development of alloantibodies against FIX [19]. FIX inhibitors develop in approximately 1.5–3 % of patients with severe hemophilia B and are commonly associated with the complete absence of FIX antigen due to large gene deletions or nonsense mutations. As a consequence of FIX inhibitor development, minor and major bleeding cannot always be prevented or treated effectively, potentially resulting in increased morbidity and diminished quality of life. Safety evaluations in our studies showed BAX326 to be no more immunogenic than the previously licensed rFIX in BALB/c mice.
Long-term use and treatment of special patient populations were assessed as favorable based on available efficacy and safety data on long-term use of the previously licensed rFIX product [8, 20, 21]. Preliminary pharmacokinetic, safety, and efficacy results of ongoing clinical studies show high accordance of our rFIX product to the previously licensed rFIX product, confirming the predictive preclinical data [17].
BAX326 may provide a further option for treatment and prophylaxis in hemophilia B patients, thereby increase the supply and lower the costs of FIX products available to this population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
All authors are full-time employees of Baxter Innovations GmbH. BD, WH, AS, HR, PLT, HPS, FS, and EMM report having stock options in Baxter.
Footnotes
Licensed in the USA in 2013 (Rixubis; Baxter Healthcare Corp., Westlake Village, CA, USA).
References
- 1.Mukherjee S, Saha A, Biswas P, Mandal C, Ray K. Structural analysis of factor IX protein variants to predict functional aberration causing haemophilia B. Haemophilia. 2008;14:1076–1081. doi: 10.1111/j.1365-2516.2008.01788.x. [DOI] [PubMed] [Google Scholar]
- 2.Bicocchi MP, Pasino M, Rosano C, Molinari AC, Della VE, Lanza T, et al. Insight into molecular changes of the FIX protein in a series of Italian patients with haemophilia B. Haemophilia. 2006;12:263–270. doi: 10.1111/j.1365-2516.2006.01275.x. [DOI] [PubMed] [Google Scholar]
- 3.Giannelli F, Green PM, Sommer SS, Poon M, Ludwig M, Schwaab R, et al. Haemophilia B: database of point mutations and short additions and deletions—eighth edition. Nucleic Acids Res. 1998;26:265–268. doi: 10.1093/nar/26.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mariani G, Di Paolantonio T, Baklaya R, Morfini M, Mannucci PM. Prospective study of the evaluation of hepatitis C virus infectivity in a high-purity, solvent/detergent-treated factor VIII concentrate: parallel evaluation of other markers for lipid-enveloped and non-lipid-enveloped viruses. The Ad Hoc Study Group of the Fondazione dell’Emofilia. Transfusion. 1993;33:814–818. doi: 10.1046/j.1537-2995.1993.331094054617.x. [DOI] [PubMed] [Google Scholar]
- 5.Vermylen J, Peerlinck K. Review of the hepatitis A epidemics in hemophiliacs in Europe. Vox Sang. 1994;67(Suppl 4):8–11. doi: 10.1111/j.1423-0410.1994.tb01290.x. [DOI] [PubMed] [Google Scholar]
- 6.Bartolomei Corsi O, Azzi A, Morfini M, Fanci R. Rossi Ferrini P. Human parvovirus infection in haemophiliacs first infused with treated clotting factor concentrates. J Med Virol. 1988;25:165–170. doi: 10.1002/jmv.1890250206. [DOI] [PubMed] [Google Scholar]
- 7.Yee TT, Cohen BJ, Pasi KJ, Lee CA. Transmission of symptomatic parvovirus B19 infection by clotting factor concentrate. Br J Haematol. 1996;93:457–459. doi: 10.1046/j.1365-2141.1996.5161062.x. [DOI] [PubMed] [Google Scholar]
- 8.Lambert T, Recht M, Valentino LA, Powell JS, Udata C, Sullivan ST, et al. Reformulated BeneFix: efficacy and safety in previously treated patients with moderately severe to severe haemophilia B. Haemophilia. 2007;13:233–243. doi: 10.1111/j.1365-2516.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 9.Landskroner KA, Olson NC, Jesmok GJ. Enhanced factor VIII activity measurements using ROTEG and factor VIII-/- mice whole blood. J Thromb Haemost. 2004;2:2274–2275. doi: 10.1111/j.1538-7836.2004.01074.x. [DOI] [PubMed] [Google Scholar]
- 10.Landskroner KA, Olson NC, Jesmok GJ. Thromboelastography measurements of whole blood from factor VIII-deficient mice supplemented with rFVIII. Haemophilia. 2005;11:346–352. doi: 10.1111/j.1365-2516.2005.01104.x. [DOI] [PubMed] [Google Scholar]
- 11.Day SM, Reeve JL, Myers DD, Fay WP. Murine thrombosis models. Thromb Haemost. 2004;92:486–494. [PubMed] [Google Scholar]
- 12.Wessler S, Reimer SM, Sheps MC. Biologic assay of a thrombosis-inducing activity in human serum. J Appl Physiol. 1959;14:943–946. doi: 10.1152/jappl.1959.14.6.943. [DOI] [PubMed] [Google Scholar]
- 13.Jesmok G, Cui ZH, Canivel D, Frounel M, Pierce G, Landskroner KA. A pharmacologic evaluation of BAY 79-4980 (liposome-formulated kogenate® FS) in the hemophilia A mouse. Blood. 2006;11:108. [Google Scholar]
- 14.Jesmok G, Cui ZH, Canivel D, Lollar P, Parker ET, Landskroner KA. Comparison of human rFVIII and murine rFVIII in a standardized FVIII dependent bleed model in FVIII -/- mice. J Thromb Haemost. 2007;5(Suppl 2):P–030. [Google Scholar]
- 15.Landskroner KA, Cui ZH, Newgren J, Fournel M, Pierce G, Jesmok G. Evaluation of PEG-FVIII molecules with prolonged half-lives in a murine FVIII-dependent bleeding model. Blood. 2006;11:108. [Google Scholar]
- 16.Brinkhous KM, Sigman JL, Read MS, Stewart PF, McCarthy KP, Timony GA, et al. Recombinant human factor IX: replacement therapy, prophylaxis, and pharmacokinetics in canine hemophilia B. Blood. 1996;88:2603–2610. [PubMed] [Google Scholar]
- 17.Windyga J, Lissitchkov T, Stasyshyn O, Mamonov V. Rusen L, Lamas JL et al. Pharmacokinetics, efficacy and safety of BAX326, a novel recombinant factor IX: a prospective, controlled, multicenter phase I/III study in previously treated patients with severe (FIX level < 1 %) or moderately severe (FIX Level ≤ 2%) haemophilia B. Haemophilia. 2013 [Epub ahead of print]. [DOI] [PubMed]
- 18.Gray E, Tubbs J, Thomas S, Oates A, Boisclair M, Kemball-Cook G, et al. Measurement of activated factor IX in factor IX concentrates: correlation with in vivo thrombogenicity. Thromb Haemost. 1995;73:675–679. [PubMed] [Google Scholar]
- 19.Astermark J. Overview of inhibitors. Semin Hematol. 2006;43:S3–S7. doi: 10.1053/j.seminhematol.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Kisker CT, Eisberg A, Schwartz B. Prophylaxis in factor IX deficiency product and patient variation. Haemophilia. 2003;9:279–284. doi: 10.1046/j.1365-2516.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro AD, Di Paola J, Cohen A, Pasi KJ, Heisel MA, Blanchette VS, et al. The safety and efficacy of recombinant human blood coagulation factor IX in previously untreated patients with severe or moderately severe hemophilia B. Blood. 2005;105:518–525. doi: 10.1182/blood-2004-06-2283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


