Abstract
A global health emergency has been declared by the World Health Organization as the 2019-nCoV outbreak spreads across the world, with confirmed patients in Canada. Patients infected with 2019-nCoV are at risk for developing respiratory failure and requiring admission to critical care units. While providing optimal treatment for these patients, careful execution of infection control measures is necessary to prevent nosocomial transmission to other patients and to healthcare workers providing care. Although the exact mechanisms of transmission are currently unclear, human-to-human transmission can occur, and the risk of airborne spread during aerosol-generating medical procedures remains a concern in specific circumstances. This paper summarizes important considerations regarding patient screening, environmental controls, personal protective equipment, resuscitation measures (including intubation), and critical care unit operations planning as we prepare for the possibility of new imported cases or local outbreaks of 2019-nCoV. Although understanding of the 2019-nCoV virus is evolving, lessons learned from prior infectious disease challenges such as Severe Acute Respiratory Syndrome will hopefully improve our state of readiness regardless of the number of cases we eventually manage in Canada.
Keyword: COVID-19
Résumé
Une urgence sanitaire mondiale a été déclarée par l’Organisation mondiale de la Santé alors que l’épidémie de 2019-nCoV se répand dans le monde et que des cas ont été confirmés au Canada. Les patients infectés par le 2019-nCoV sont à risque d’insuffisance respiratoire et peuvent nécessiter une admission à l’unité de soins intensifs. Lors d’une prise en charge optimale de ces patients, il est indispensable de prendre soin d’exécuter rigoureusement les mesures de contrôle des infections afin de prévenir la transmission nosocomiale aux autres patients et aux travailleurs de la santé prodiguant les soins. Bien que les mécanismes précis de transmission ne soient pas encore connus, la transmission d’humain à humain peut survenir, et le risque de dissémination aérienne pendant les interventions médicales générant des aérosols est préoccupant dans certaines circonstances spécifiques. Cet article résume des considérations importantes en ce qui touche au dépistage des patients, aux contrôles environnementaux, au matériel de protection personnelle, aux mesures de réanimation (y compris l’intubation), et à la planification des activités à l’unité de soins intensifs alors que nous nous préparons à la possibilité de nouveaux cas importés ou d’éclosions locales du 2019-nCoV. Bien que la compréhension du virus 2019-nCoV continue d’évoluer, nous espérons que les leçons retenues des éclosions précédentes de maladies infectieuses telles que le syndrome respiratoire aigu sévère nous permettront d’améliorer notre degré de préparation, indépendamment du nombre de cas que nous traiterons au Canada.
Overview of the current state
A new coronavirus, 2019-nCoV, has emerged as the cause of an unusual cluster of viral pneumonia cases in China.1,2 The situation has rapidly evolved into a global health emergency as declared by the World Health Organization.3 Canada has joined the list of countries with patients identified as confirmed or suspected active 2019-nCoV infection in two provinces. Repatriation of Canadian citizens from China is expected to result in hundreds of patients requiring quarantine while monitoring for symptoms and signs of disease.4
This single-strand, positive-sense RNA virus has been fully sequenced, and appears to be distinct from but related to other coronaviruses causing Severe Acute Respiratory Syndrome (SARS-CoV) and Middle East Respiratory Syndrome (MERS-CoV).2,5 Mechanisms of transmission are believed to include contact, droplet, and possibly airborne under certain circumstances based on historical experiences related to SARS-CoV outbreaks.6–10 The basic reproduction number (R0) for this infection, given variable host and environmental factors, is estimated in the initial outbreak to be between 2.2 and 3.6 (number of cases generated after exposure to one patient),11 which is similar to SARS-CoV but higher than MERS-CoV. The case fatality rate is estimated to be approximately 2% based on latest reported data,12 less than SARS-CoV (approximately 10%)13 and MERS-CoV (approximately 40%)14 but significantly higher than the 2009 pH1N1 (0.026%).15 Over 20,000 confirmed cases have been reported worldwide in 25 countries across multiple continents.16 At the time of writing, there are over 2,500 cases considered severe, and over 400 reported deaths.12 These figures are considered by many as gross underestimates of the disease prevalence and are rapidly changing.
The mean incubation period for this infection is not known, but ranges reported by the World Health Organization are from two to ten days, with typical presentations having incubation periods of four to seven days before onset of symptoms.1 One published case17 suggests that transmission may be possible during the asymptomatic period, which could present additional challenges for control of the outbreak; however, some controversy has been raised about whether this risk is real.18 Given the potential for rapid spread of the virus internationally,19 unprecedented measures have been instituted to try and contain the spread of 2019-nCoV, including severe restrictions on millions of people in China and widespread cancellation of international flights in and out of China.20 Despite likely originating from an animal source, the virus appears to have ability for sustained human-to-human transmission.21 Transmission within healthcare facilities to healthcare workers has been documented, with the first published deaths reported of physicians who acquired 2019-nCoV while caring for infected patients.22,23
In a recent study describing a cohort of 99 patients infected with 2019-nCoV,24 the average patient age of the predominantly male cohort was 56 yr, with half of the patients having some significant chronic co-morbid illness (e.g., cardiovascular or cerebrovascular disease). The most frequent clinical manifestations included fever, cough, and shortness of breath; notably, 17% did not have a fever on presentation. Imaging typically showed changes consistent with bilateral pneumonia and 17% of patients met criteria for acute respiratory distress syndrome. Twenty-three percent of patients required admission to an intensive care unit (ICU), and the mortality rate was 11% during the 25-day observation period. Thirteen percent of patients were treated with non-invasive ventilation, 4% required mechanical ventilation, and 3% required extracorporeal membrane oxygenation. Importantly, the limited ability to test the thousands of patients in China with potential (but mild) infection means the overall denominator and subsequent calculated rates of any of these consequences are likely to be over-estimates and thus we expect the final rates of complications and deaths to be lower.
The risk of respiratory failure requiring critical care support in patients infected with 2019-nCoV is significant, so critical care and anesthesiology teams must be prepared for the arrival and sustained care of patients infected with 2019-nCoV. This paper provides a brief and pragmatic overview of key recommendations to help provide optimal care to critically ill patients infected with 2019-nCoV, while maximizing the safety of healthcare staff, other patients, and the public.
Screening and preparation for potential cases
Currently, suspicion for infection with 2019-nCoV requires two elements in the case presentation: presence of fever and symptoms of respiratory illness, and epidemiological link to the virus.25 This link may be travel within 14 days to affected areas, community or nosocomial close contact within 14 days of illness onset with an ill person having confirmed or probable 2019-nCoV infection, close contact with a person having acute respiratory illness and travel to an affected area within 14 days of illness onset, or laboratory exposure to 2019-nCoV biological material. Based on the latest cohort data, most, but not all patients present with fever,24 and therefore appropriate infectious control precautions should be in place even in the absence of all elements of the case definition (e.g., patient presents with bilateral pneumonia with no alternate explanation, recent travel history to an affected area, but no fever). Although Wuhan City is the epicentre for this virus and represents a key affected area, many other areas within China and beyond may have a sufficient virus prevalence to trigger a potential epidemiological link. Case definitions are dynamic, and therefore clinicians are strongly urged to carefully follow updates from public health and infection control authorities as our understanding of this illness matures. Nevertheless, a high index of suspicion and caution is warranted as the lack of an identifiable epidemiological link, or failure to recognise a link, has been well documented to lead to cases of unabated disease transmission.26 As the spread of this virus expands to different countries, the ability to use travel history as a flag for potential 2019-nCoV infection may weaken. Once a few generations of spread have occurred in a new country, there may not be an obvious travel history link for individual patients to flag potential risk. If the situation progresses to more widespread prevalence of the virus, the trigger for appropriate enhanced isolation and other infection control precautions may broaden to include unexplained febrile respiratory illness. Regardless, strict compliance with routine precautions for febrile respiratory illness in general should reduce the potential risk posed by a patient with 2019-nCoV even without a confirmed diagnosis.
Potential cases requiring critical care support may present directly to the emergency department from the community, or through interfacility transfer. In both cases, routine careful questioning about risk for 2019-nCoV exposure through screening is critical to ensure appropriate infection control precautions (including airborne isolation and personal protective equipment [PPE]) are used to manage the patient from initial contact with emergency medical services (EMS), emergency departments, and in-patient units including the ICU. Inaccurate or incomplete information about potential risk can occur when EMS are called for patients in medical crisis, or during discussions about interfacility transfer. If in doubt, patients with febrile respiratory illness of unknown etiology should be treated with contact/droplet ± airborne precautions (if undertaking high-risk procedures) until infection control and public health staff can provide further clarification about the history. Front-line staff should be empowered to treat patients as possible 2019-nCoV cases based on the best information available, rather than waiting for authorization to isolate.8 Every emergency department and ICU should have a plan for a “ready bed” with airborne isolation capacity where a suspect unwell 2019-nCoV patient can be placed in immediate isolation. Regional healthcare systems may want to designate specific hospitals with better isolation capacity as preferred destinations for EMS and the public. Telephone or telemedicine screening of patients to direct to the most appropriate facility may be helpful, and appropriate screening for 2019-nCoV risk must be incorporated into EMS call-taking and dispatch.
Infection control precautions: environmental
The Public Health Agency of Canada has released interim infection control recommendations related to 2019-nCoV.27 Although the predominant mechanism of transmission is thought to be contact/droplet spread related to respiratory secretions, under circumstances relevant to critical care and anesthesia clinicians, airborne transmission may occur. Isolated reports detecting 2019-nCoV in stool have also raised concerns about the potential risk of fecal-oral transmission.28 Risk factors for potential airborne transmission are dependent on the patient and the nature of interventions anticipated. Critically ill patients may have a greater extent of viral shedding, and some patients may act as “supershedders” with enhanced ability to transmit. Certain medical interventions, such as bag-valve-mask ventilation, non-invasive ventilation, and intubation (in spontaneously breathing patients), may create localized aerosol generation that can allow airborne transmission to those closely involved in the procedure.27
Recommendations at present for routine airborne isolation of stable patients with suspect or confirmed 2019-nCoV infection in addition to contact/droplet isolation varies between provinces at this time. These recommendations are based upon relatively limited data on transmission of this novel virus combined with the intent to limit the spread of imported cases. As a result, this guidance may change as more becomes known about the disease and if local community-based transmission becomes prevalent.
Because of the potential need for aerosol-generating medical procedures, the Public Health Agency of Canada guidelines support placing unwell suspect or confirmed 2019-nCoV patients in airborne isolation.27 These patients should be immediately isolated in an airborne isolation room (i.e., single room, negative pressure, frequent air exchange) if available. In the event an airborne isolation room is not available, the patient should be placed in a single room with closed doors. Strategies short of meeting these airborne isolation standards, such as portable HEPA filters or negative air flow, can be considered to reduce risk in a single room. Anterooms with sufficient space to put on and remove PPE should be available adjacent to airborne isolation rooms; if not available, makeshift anterooms can be constructed.
Airflow within hospital wards can dramatically affect the risk of nosocomial transmission of some coronavirus strains, such as SARS.29 During the prior SARS outbreak, hospital engineers were able to create negative airflow isolation rooms to modify existing hospital systems when airborne isolation capacity was overwhelmed.30 In some cases, entire ICUs were converted to negative pressure/airflow wards rather than individual patient rooms. In such cases, full airborne/contact/droplet PPE would be worn in the patient room without independent airborne isolation (“hot zone”) that can be removed on exit. Clean N95 masks, gowns, and gloves should be worn in the makeshift negative pressure ICU outside of patient rooms (“warm zone”) because of potential airborne spread of the virus into adjacent common areas from patient rooms that did not have airborne isolation capacity. Personal protective equipment would not be required outside of the ICU (“cold zone”). These strategies used during the SARS experience could be duplicated if necessary when managing 2019-nCoV surge scenarios, considering local operational restrictions and capabilities.
Infection control precautions: PPE
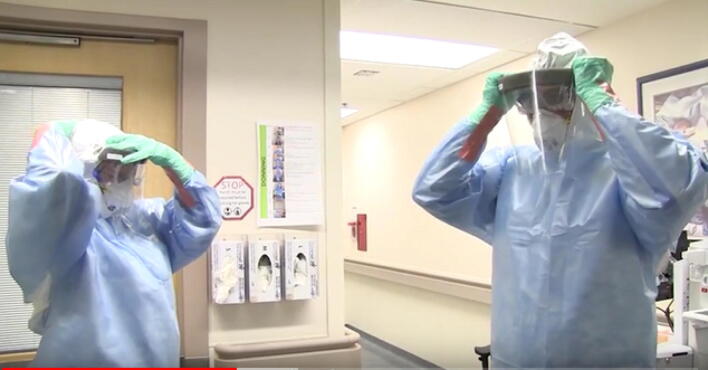
Recommended PPE for contact with critically ill patients with confirmed or suspected 2019-nCoV infection include fluid-resistant gown, gloves, eye protection, full face shield and fit-tested N95 respirators (Fig. 1).27 Hair covers or hoods should also be worn. Longer sleeved gloves are preferred (if available) to prevent exposure of the wrists with glove slippage. Alternately, vertical tape strips can be used to help keep gloves secured to the gown. Circumferential taping of gloves to the gown, such as used when wearing chemical PPE, is not necessary and makes gown and glove removal more challenging. Eye protection should include protection from side exposure with side shields or goggles. Full face shields can provide both eye protection and avoid facial and respirator contamination. Some disposable shoe covers may increase the risk of self-contamination during removal of protection clothing. Shoes worn should be impermeable to fluids and able to be decontaminated. Staff should wear operating room scrub suits or full coveralls under the PPE. Coveralls with an integrated hood may simplify the underlayer worn in conjunction with PPE, however the choice of product should be assessed for ease of removal to avoid contamination during removal. Hand hygiene must be performed after removing PPE, and in the event of inadvertent contamination of the hands by touching dirty surfaces during PPE removal.
Fig. 1.
Example of enhanced droplet/airborne personal protective equipment for intubation of patient with suspected or confirmed novel coronavirus (2019-nCoV) incorporating fit-tested N95 mask. Healthcare staff preparing to enter a room to intubate a patient with suspected or confirmed 2019-nCoV. Note use of fluid-resistant gown, covering of head and neck plus face shield to minimize skin exposure to droplet contamination. Additional eye protection worn under the face shield may help to avoid conjunctival exposure from spray around the shield. Fit-tested N95 mask is worn to protect against inhalation of airborne virus. Strips of tape securing gloves to the gown help prevent gloves from slipping during patient care and exposing wrists to contamination
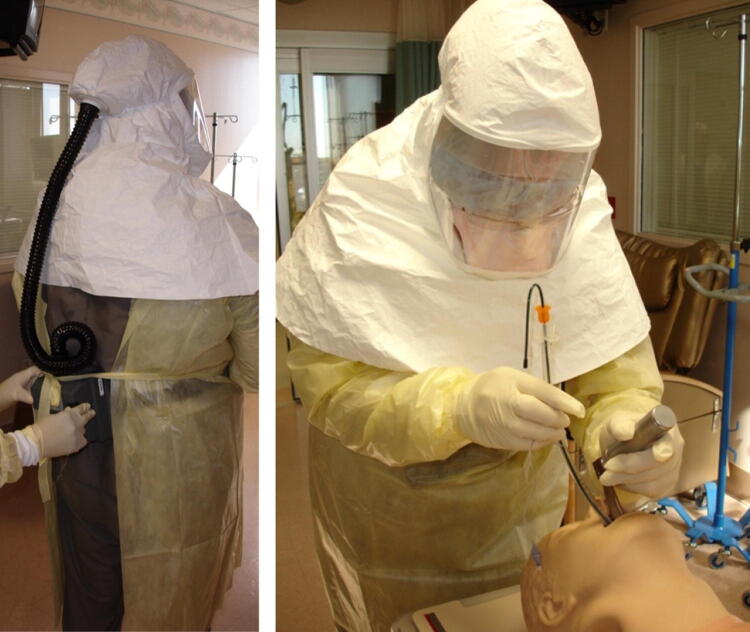
One area of controversy relates to the use of powered air purifying respirators (PAPRs) instead of N95 masks for aerosol-generating procedures.31 Although PAPRs have a higher protective factor compared with N95 respirators, there is no definitive evidence that PAPRs reduce the likelihood of viral transmission in the setting of potential airborne spread. Nonetheless, PAPRs may be more comfortable to wear for prolonged resuscitations, eliminate concerns of unexpected poor N95 respirator fit, and are less likely to be dislodged when managing an agitated patient. PAPRs with hoods covering the entire head and neck (Fig. 2) may also provide additional protection against contamination compared with the typical gear worn in conjunction with an N95 mask.32 Given that healthcare workers became infected during resuscitation of patients with SARS despite wearing N95 masks,10 the use of PAPRs is reasonable for high-risk resuscitation scenarios performed on patients with confirmed or suspected 2019-nCoV infection. Objections to incorporating PAPRs into supported PPE strategies may include challenges in training clinicians to safely remove the equipment without contamination, need for explicit protocols on cleaning of the devices for next use, and concerns about creating a two-tiered approach to PPE that excludes some healthcare workers from access to PAPRs. Considerations regarding decisions on whether or not to use PAPRs as part of enhanced contact/droplet/airborne PPE are summarized in Table 1.
Fig. 2.
Example of enhanced droplet/airborne personal protective equipment incorporating use of powered air purifying respirator (PAPR) for intubation of a simulated patient with 2019-nCoV. Healthcare staff wearing PAPR blower unit with incorporated filter on belt (rear view on left), attached to full hood with hose. Gown and gloves used to avoid droplet or contact contamination. Note that in this case, a fit-tested N95 respirator is being worn under the PAPR hood to protect against inhalation of airborne viral particles during removal of personal protective equipment (PPE), helpful in settings without appropriate individual airborne isolation rooms with anterooms
Table 1.
Considerations in deciding to use powered air purifying respirators vs N95 mask as part of personal protective equipment for novel coronavirus (2019-nCoV) patients
| Potential advantages of PAPR | Potential disadvantages of PAPR |
|---|---|
| Higher protection factor | May be more complicated than required for mode of transmission, leading to greater risk of contamination when removing PAPR |
| Full facial and head coverage | Higher cost compared with N95 respirators |
| More comfortable for prolonged resuscitations or transports and resistance to being accidentally dislodged | Inability to reuse disposable filters between patients, need large supply of filters |
| Eliminates N95 fit testing concerns (especially for those who cannot be successfully fit tested because of facial features) | Need explicit procedures for decontamination and recycling of blower units for next use |
| No need to maintain supply of variety of N95 respirators to meet fit testing requirements | Potential compromise of disposable components (e.g., hoods, hoses) through inappropriate attempts to sterilize and reuse if supplies run low, leading to infection risk |
| Can be used with facial hair or for staff who cannot be successfully fit tested | Communication challenges between staff due to fan noise |
| Need for recurrent training of staff to maintain competence if not frequently used |
PAPR = powered air purifying respirators
Non-invasive oxygenation support and nebulized medications
In patients with mild respiratory illness due to 2019-nCoV infection, supplemental oxygen can be provided with usual delivery devices. During the Toronto SARS outbreak, humidified oxygen was avoided to reduce potential viral spread, although appropriate airborne isolation may obviate this concern. Support of patients prior to being placed in airborne isolation, or during transport within or between facilities, may warrant changes in practice to minimize risk of viral transmission. When wearing nasal prongs, a surgical mask can be worn by the patient over the prongs to reduce droplet spread. Should higher oxygen requirements necessitate use of a mask, non-rebreather masks with an attached exhalation filter can be used; however, the infection control efficacy of many mask/filter units has not been well evaluated, so must not lead to reduced isolation and PPE practices. High-flow nasal cannula (HFNC) delivery systems have become much more frequently used in the years since the SARS outbreak. Nevertheless, these may cause an increase in the risk of viral spread through aerosol generation. Although one recent study suggested that bacterial droplet spread may not be increased with use of HFNC, the potential for viral transmission was not examined.33 Use of HFNC should be limited to patients in appropriate airborne isolation. Nebulization of medications should also be avoided, particularly outside of airborne isolation, because of the risk of viral aerosolization and spread. Bronchodilators should be administered using metered-dose inhalers.
Although some centres that managed patients with SARS reported safe use of continuous positive airway pressure/bilevel positive airway pressure (CPAP/BiPAP),34 there are case reports of considerable SARS transmission risk with the use of BiPAP to many patients over extended distances.29 In theory, CPAP/BiPAP units with an exhalation filter could be used to support 2019-nCoV patients with respiratory failure in appropriate airborne isolation; however, the high incidence of CPAP/BiPAP mask leak may render filtration incomplete. The use of CPAP/BiPAP may increase the risk of delayed deterioration leading to need for emergent intubation and increased risk of mistakes in donning PPE due to time pressures to resuscitate. In general, CPAP/BiPAP should be avoided in patients with 2019-nCoV and should never be used outside of appropriate airborne/droplet isolation.
Airway management and ventilatory support
Intubation of critically ill patients with SARS-CoV was associated with episodes of healthcare worker transmission. The reasons for this are likely multifactorial, including high-level viral shedding due to severity of patient illness, procedures associated with resuscitation or intubation that may generate aerosols, and healthcare worker use of PPE (high-risk patient + high-risk procedure = higher level of precautions). As a result, management of patients requiring intubation or resuscitation warrant specific caution and should be undertaken in an airborne isolation room. All personnel in the room must be using appropriate airborne/droplet PPE, including either a fit-tested N95 mask or a PAPR. Careful planning of this intervention is required. The procedure should be attempted by the most skilled person at intubation using a rapid sequence intubation technique, to optimize first attempt success. Recurrent traffic of people bringing equipment into the room may increase the risk of viral transmission. All necessary equipment and medications should be available in the room at the time of intubation attempt. The number of personnel in the room at the time of intubation should be minimized to essential team members only.
Bag-mask ventilation prior to intubation can generate aerosols, as can the patient coughing during laryngoscopy. An exhalation filter should also be present attached to the resuscitation bag, typically between the mask or endotracheal tube and the bag. Inadequate sedation can also place the intubator at risk if the patient becomes agitated and dislodges PPE. With adequate pre-oxygenation, bag-mask ventilation can ideally be avoided before laryngoscopy. Video laryngoscopy should be used, ideally with a display separate from the blade, to avoid placing the face of the intubator close to the patient. If a difficult airway is anticipated, a flexible bronchoscopic intubation can be performed using a video bronchoscope with the display away from the patient. Endotracheal tube placement must be confirmed with end-tidal carbon dioxide detection. Personal protective equipment, especially PAPRs, may preclude auscultation to help confirm correct tube placement. Careful observation of bilateral chest rise should help ensure correct depth of tube placement pending a portable radiograph. Alternately, ultrasound may be used to assist in determining endotracheal tube depth.35
Once intubated, lung protective mechanical ventilation strategies should be used (target tidal volume 6 mL·kg−1 predicted body weight, plateau pressure ≤30 cm H20, target SaO2 88–95% and pH ≥ 7.25).36 All exhaled gas from the ventilator should be filtered. Pneumothorax was noted in some ventilated patients affected with SARS. Extrapolating to 2019-nCoV infected patients, clinicians should strongly consider pneumothorax in any ventilated patient with sudden respiratory deterioration. Given the potential delay in obtaining a chest x-ray for a patient in airborne isolation, portable ultrasound may be used to quickly assist in the diagnosis of a pneumothorax.
Surgical/anesthesia considerations for 2019-nCoV patients
Unfortunately, the positive pressure airflow environment of the operating room can create risk of viral spread when managing a patient infected with 2019-nCoV. Hospitals should consult with their biomedical engineers to see if any operating rooms can be converted to negative pressure environments with airflow changes. High-risk aerosol-generating procedures, such as intubation, should not be performed in a positive pressure environment. During the SARS outbreak, surgical procedures were performed within airborne isolation ICU rooms, which eliminated the risk of intra-facility transport and avoided the need to make environmental modifications in the operating room.37 Use of intravenous anesthesia would be preferred to the use of a volatile gas anesthetic machine in the ICU environment, especially given that these patients are not going to be rapidly recovered and extubated following the procedure.
Prevention and management of resuscitation crises/respiratory or cardiac arrest: Protected Code Blue (PCB)
Patients infected with 2019-nCoV should be monitored for early signs of respiratory deterioration and intubated electively rather than emergently. If possible, patients isolated with 2019-nCoV should be monitored in a critical care area with airborne isolation and continuous physiologic monitoring, given that isolation is known to reduce the frequency of nursing and physician assessment in a ward environment.38
During the SARS outbreak, the concept of “Protected Code Blue” was created to distinguish usual resuscitation from those requiring special procedures and precautions.39 A demonstration of the PCB procedures can be found online at http://sars.medtau.org/simulatedprotectedcodeblue.pps and is discussed in a presentation at https://emergencymedicinecases.com/biohazard-preparedness-protected-code-blue/. Once designated as requiring 2019-nCoV isolation, these procedures should be used to ensure safety of the responding resuscitation team. Resuscitation should take place in an airborne isolation room (if possible) given the need for aerosol-generating medical procedures. All members of the resuscitation team must be wearing appropriate airborne/droplet/contact PPE. Given the greater risk of infection during a dynamic resuscitation,10 use of PAPRs by specially trained resuscitation teams should be strongly considered. Initial resuscitation efforts by first responders wearing usual airborne/droplet/contact PPE to an acute crisis should focus on measures that are most likely to help the patient and have low risk for viral transmission (summarized in Table 2). Once the PCB team has donned PPE and been checked by an infection control coach, they can enter the room. Team size should be minimal to avoid unnecessary viral exposure during resuscitation—i.e., typically four people with designated roles. Rather than bring an entire resuscitation cart into a room, which will present serious challenges for equipment, cart, and supply decontamination, a specialized cart containing modular packs of equipment can be considered, with PCB team members bringing in the necessary defibrillator and packs rather than the cart. Following resuscitation, team members can exit when appropriate and should remove PPE under careful supervision of an infection control coach using a checklist to avoid self-contamination.
Table 2.
Risk consideration for resuscitation procedures during novel coronavirus (2019-nCoV) Protected Code Blue
| Lower risk resuscitation interventions | Higher risk resuscitation interventions more likely to generate aerosol and/or increase risk of viral transmission to staff |
|---|---|
| Placement of an oral airway | High-flow nasal cannula |
| Placement of an oxygen mask with exhalation filter on patient (if available) | Bag-mask ventilation |
| Chest compressions | CPAP/BiPAP |
| Defibrillation, cardioversion, transcutaneous pacing | Endotracheal intubation/surgical airway |
| Obtaining intravenous or intraosseous access | Bronchoscopy |
| Administration of intravenous resuscitation drugs | Gastrointestinal endoscopy |
CPAP/BiPAP = continuous positive airway pressure/bilevel positive airway pressure
Additional considerations
Critical care services in Canada must remain on high alert in the coming weeks/month for a rapid increase in the demand on resources should the containment of 2019-nCoV fail. This situation is evolving rapidly. Now is the time for every hospital and other organization involved in healthcare delivery to review their protocols and supplies to be ready for any patient presenting with minimal notice. Regional, provincial, and federal authorities should be aware of real-time critical care capacity, including the number of airborne isolation critical care rooms. Surge plans to create additional ICU capacity should be reviewed and refreshed to prepare for the possibility that 2019-nCoV becomes a true pandemic with substantial spread within Canada. Cooperation with researchers developing potential registries can assist with the better understanding of the nature of this disease in a Canadian context. Our national history as a centre of excellence for research in critical care should allow a more rapid evaluation of proposed treatment strategies. The psychologic effects of perceived risk to healthcare providers and the public, especially for those with confirmed or suspected 2019-nCoV infection, cannot be ignored. Clear and transparent communication from governments and healthcare facilities to staff and public will be essential. The Canadian experience with SARS taught many lessons, and hopefully those lessons will serve in keeping healthcare workers safe and providing optimal care to patients infected with 2019-nCoV.
Acknowledgments
Author contributions
Randy S. Wax and Michael D. Christian contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article.
Acknowledgements
Considerable thanks to Vagia T. Campbell, MSc, BHA, RRT for her review and feedback on this manuscript from her perspective as a biohazard education expert, current healthcare leader, and as a former front-line healthcare worker infected while caring for patients during the previous SARS-CoV outbreak in Toronto.
Conflicts of interest
None.
Funding statement
None.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). Available from URL: https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) (accessed February 2020).
- 4.Government of Canada. Government of Canada evacuating Canadians from Wuhan, China. Available from URL: https://www.canada.ca/en/global-affairs/news/2020/02/government-of-canada-evacuating-canadians-from-wuhan-china.html (accessed February 2020).
- 5.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 doi: 10.1016/s0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu IT, Li Y, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 7.Scales DC, Green K, Chan AK, et al. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerg Infect Dis. 2003;9:1205–1210. doi: 10.3201/eid0910.030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller MP, McGeer A. Febrile respiratory illness in the intensive care unit setting: an infection control perspective. Curr Opin Crit Care. 2006;12:37–42. doi: 10.1097/01.ccx.0000198056.58083.a1. [DOI] [PubMed] [Google Scholar]
- 9.Fowler RA, Lapinsky SE, Hallett D, et al. Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 10.Christian MD, Loutfy M, McDonald LC, et al. Possible SARS coronavirus transmission during cardiopulmonary resuscitation. Emerg Infect Dis. 2004;10:287–293. doi: 10.3201/eid1002.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao S, Lin Q, Ran J, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis 2020; DOI: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed]
- 12.World Health Organization. Novel Coronavirus (2019-nCoV). WHO Bull 2020 - Data as reported by January 30, 2020. Available from URL: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200130-sitrep-10-ncov.pdf?sfvrsn=d0b2e480_2 (accessed February 2020).
- 13.Christian MD, Poutanen SM, Loutfy MR, Muller MP, Low DE. Severe acute respiratory syndrome. Clin Infect Dis. 2004;38:1420–1427. doi: 10.1086/420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majumder MS, Rivers C, Lofgren E, Fisman D. Estimation of MERS-coronavirus reproductive number and case fatality rate for the Spring 2014 Saudi Arabia outbreak: insights from publicly available data. PLoS Curr 2014. Available from URL: http://currents.plos.org/outbreaks/index.html%3Fp=40801.html (accessed February 2020). [DOI] [PMC free article] [PubMed]
- 15.Donaldson LJ, Rutter PD, Ellis BM, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ 2010; DOI:10.1136/bmj.b5213 (accessed February 2020). [DOI] [PMC free article] [PubMed]
- 16.World Health Organization. Novel coronavirus (nCoV 2019) situation as of 07 February 2020, 10:00 (CET). Available from URL: http://who.maps.arcgis.com/apps/opsdashboard/index.html#/c88e37cfc43b4ed3baf977d77e4a0667 (accessed February 2020).
- 17.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020 doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kupferschmidt K. Study claiming new coronavirus can be transmitted by people without symptoms was flawed. Science (February 3, 2020). Available from URL: https://www.sciencemag.org/news/2020/02/paper-non-symptomatic-patient-transmitting-coronavirus-wrong (accessed February 2020).
- 19.Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MU, Khan K. Potential for global spread of a novel coronavirus from China. J Travel Med. 2020 doi: 10.1093/jtm/taaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nkengasong J. China’s response to a novel coronavirus stands in stark contrast to the 2002 SARS outbreak response. Nat Med. 2020 doi: 10.1038/s41591-020-0771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020 doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brest M. Wuhan doctor treating coronavirus patients dies after contracting disease. Washington Examiner. Available from URL: https://www.washingtonexaminer.com/news/wuhan-doctor-treating-coronavirus-patients-dies-after-contracting-disease (accessed February 2020).
- 23.Buckley C. Chinese Doctor, Silenced After Warning of Outbreak, Dies From Coronavirus. The New York Times. Available from URL: https://www.nytimes.com/2020/02/06/world/asia/chinese-doctor-Li-Wenliang-coronavirus.html (accessed February 2020).
- 24.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Government of Canada. Interim national case definition: novel coronavirus (2019-nCoV). Available from URL: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/national-case-definition.html (accessed February 2020).
- 26.McDonald LC, Simor AE, Su IJ, et al. SARS in healthcare facilities, Toronto and Taiwan. Emerg Infect Dis. 2004;10:777–781. doi: 10.3201/eid1005.030791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Government of Canada. Infection prevention and control for novel coronavirus (2019-nCoV): interim guidance for acute healthcare settings. Available from URL: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/interim-guidance-acute-healthcare-settings.html (accessed Februay 2020).
- 28.XINHUANET. Novel coronavirus may spread via digestive system: experts. | English.news.cn. Available from URL: http://www.xinhuanet.com/english/2020-02/02/c_138749620.htm (accessed February 2020).
- 29.Li Y, Huang X, Yu IT, Wong TW, Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005;15:83–95. doi: 10.1111/j.1600-0668.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- 30.Loutfy MR, Wallington T, Rutledge T, et al. Hospital preparedness and SARS. Emerg Infect Dis. 2004;10:771–776. doi: 10.3201/eid1005.030717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novak D. Why, where, and how PAPRs are being used in health care. In: Institute of Medicine. The Use and Effectiveness of Powered Air Purifying Respirators in Health Care: Workshop Summary - 2015. Washington, DC: The National Academies Press. Available from URL: https://www.ncbi.nlm.nih.gov/books/NBK294225/#_NBK294225_pubdet (accessed February 2020). [PubMed]
- 32.Zamora JE, Murdoch J, Simchison B, Day AG. Contamination: a comparison of 2 personal protective systems. CMAJ. 2006;175:249–254. doi: 10.1503/cmaj.060094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung CC, Joynt GM, Gomersall CD, et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect. 2019;101:84–87. doi: 10.1016/j.jhin.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Cheung TM, Yam LY, So LK, et al. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest. 2004;126:845–850. doi: 10.1378/chest.126.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottlieb M, Holladay D, Burns KM, Nakitende D, Bailitz J. Ultrasound for airway management: an evidence-based review for the emergency clinician. Am J Emerg Med. 2019 doi: 10.1016/j.ajem.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 37.Tien HC, Chughtai T, Jogeklar A, Cooper AB, Brenneman F. Elective and emergency surgery in patients with severe acute respiratory syndrome (SARS) Can J Surg. 2005;48:71–74. [PMC free article] [PubMed] [Google Scholar]
- 38.Stelfox HT, Bates DW, Redelmeier DA. Safety of Patients Isolated for Infection Control. JAMA. 2003;290:1899–1905. doi: 10.1001/jama.290.14.1899. [DOI] [PubMed] [Google Scholar]
- 39.Abrahamson SD, Canzian S, Brunet F. Using simulation for training and to change protocol during the outbreak of severe acute respiratory syndrome. Crit Care. 2005 doi: 10.1186/cc3916. [DOI] [PMC free article] [PubMed] [Google Scholar]