Abstract
Patients with repaired tetralogy of Fallot have a reduced percentage of predicted peak oxygen consumption (VO2) and percentage of oxygen pulse (O2P%) compared to healthy controls. Because data regarding the progression of exercise intolerance in these patients is limited, we sought to analyze the serial exercise data from patients with Tetralogy of Fallot to quantify the changes in their exercise capacity over time and to identify associations with clinical and cardiac magnetic resonance imaging variables. The data from cardiopulmonary exercise tests (CPXs) from 2002 to 2010 for patients with repaired tetralogy of Fallot with ≥2 CPXs separated by ≥12 months were analyzed. Tests occurring after interventional catheterization or surgery were excluded. A total of 70 patients had 179 CPXs. They had a median age at the initial study of 23.6 years and an interval between the first and last CPX of 2.8 years. At the initial CPX, the peak VO2 was 27.6 ± 8.8 ml/kg/min (78 ± 19% of predicted), and the peak 27.6 ± 8.8 ml/kg/min (78 ± 19% of predicted), and the peak O2P% was 89 ± 22% of predicted. At the most recent study, the peak VO2 averaged 25.0 ±7.4 ml/kg/min (73 ± 16% of predicted), and the peak 27.6 ± 8.8 ml/kg/min (78 ± 19% of predicted), and the peak O2P% averaged 83 ± 20% (p <0.01) for each versus the initial CPX. The decrease in the peak VO2 was strongly associated with a decrease in O2P% and an increase (worsening) in the slope of the minute ventilation-versus-carbon dioxide production relation. Changes in the peak VO2 did not correlate with concomitant changes in any other CPX variable. The rate of decrease was not related to a history of shunt palliation, age at CPX, or any other baseline clinical parameter, including cardiac magnetic resonance measurements. In conclusion, the exercise capacity of patients with repaired tetralogy of Fallot tends to decrease over time. This deterioration is variable and unpredictable and is primarily related to a decrease in the forward stroke volume at peak exercise.
Currently, information regarding the change in the exercise capacity over time in patients with congenital heart disease in general, and those with repaired tetralogy of Fallot (rTOF) in particular, is limited. In this population, several cross-sectional studies have reported an average peak oxygen consumption (VO2) of 51% to 95% predicted.1–8 Given the greatly increased hemodynamic demands imposed on the right ventricle during exercise, the low peak VO2 of these patients with residual right-sided heart disease is not surprising. The potential factors responsible for the depressed exercise capacity of patients with rTOF are numerous; past studies have implicated residual pulmonary regurgitation, pulmonary artery distortion, impaired lung function, chronotropic impairment, and ventricular dysfunction.4,5,9–16 However, a study of the natural history of the exercise function of patients with rTOF, according to assessments using modern cardiopulmonary exercise testing (CPX) technology, has not been undertaken. The purpose of the present study was to analyze the serial CPX data from patients with rTOF to quantify the changes in their exercise capacity over time. We also sought to identify the clinical and cardiac magnetic resonance imaging variables16 associated with any observed changes in exercise function.
Methods
We identified all patients with rTOF who had undergone CPX testing at our institution from 2002 to 2010. The patients were included if they had undergone ≥2 symptom-limited, progressive CPX tests,17 separated by ≥12 months, without an intervening cardiac surgical operation or interventional cardiac catheterization procedure. Patients with TOF and pulmonary atresia and patients with significant residual right ventricular outflow tract obstruction (mean gradient >30 mm Hg on the most recent echocardiogram) were excluded. To avoid confounding the present study of the natural history of the patient with rTOF with the complicated natural history of artificial conduits, we excluded the studies from patients with right ventricle to pulmonary artery conduits. To minimize the potential confounding effects of inadequate patient effort, we excluded the data from studies in which the patient did not achieve a respiratory exchange ratio at peak exercise of ≥1.09.
Doppler estimates of right ventricular outflow tract obstruction and data from cardiac magnetic resonance studies performed within 12 months of the first CPX, when available, were included in the present analysis. Additionally, co-morbidities were recorded from the closest clinic visit records.
During the exercise tests, electrocardiographic monitoring and breath-by-breath expiratory gas analysis were performed using the CardiO2 exercise testing system (Medical Graphics, Minneapolis, Minnesota). Cuff blood pressure determinations and complete 12-lead electrocardiograms were obtained at 2- to 3-minute intervals during exercise, at peak exercise, and at 1, 3, and 5 minutes after exercise. Pulse oximetry oxygen saturation was monitored throughout the study. Immediately before each exercise test, spirometric measurements of the patients’ forced vital capacity and volume of air exhaled in the first second of forced expiration were also obtained.
The temporal changes in the peak VO2 (the most widely used index of exercise function) and the oxygen pulse at peak exercise (O2P, a surrogate for the forward stroke volume at peak exercise18) were the primary outcome variables for the present study. Because of the variation in patient age, size, and gender in our cohort, and because many of our subjects grew significantly and underwent pubertal-related changes in stature and body habitus during the study period, our analyses focused on the changes in the percentage of the predicted values (VO2% and O2P%), rather than the changes in the absolute magnitude or weight-normalized values of these variables.19 The secondary outcome parameters of exercise performance included changes in the percentage of predicted peak heart rate, the slope of the minute ventilation-versus-carbon dioxide production relation (VE/VCO2 slope, an index of the efficiency of gas exchange during exercise20), oxygen saturation, and spirometric measurements. We also calculated the body mass index (weight in kilograms divided by the height in square meters) at each exercise test.
The clinical variables included gender, anatomic diagnoses, type of previous surgical procedures (e.g.,early shunt vs primary intracardiac repair), age at repair and at CPXs, and Doppler-estimated right ventricular outflow tract gradients. When available, the pulmonary regurgitation fraction, indexed right ventricular end-diastolic and systolic volume, right ventricular ejection fraction, indexed left ventricular end-diastolic and systolic volumes, and left ventricular ejection fraction were collected from the cardiac magnetic resonance studies.
Continuous variables are presented as the mean ± SD and the categorical variables as the counts and percentages. For continuous variables with non-normal distributions, we report the medians and ranges. Paired t tests were used to compare the initial and final values for each CPX variable. To identify the factors associated with a steeper decrease in exercise function, the linear regression line of each exercise test variable against time served as each patient’s measure of the rate of change over time. One-sample t tests were used to test for significant changes over time. In this and subsequent analyses, observations were weighted by the interval between the first and last exercise tests to account for the varying lengths of follow-up. We used Pearson’s correlation coefficient to estimate the association between the rate of change in the VO2% (ΔVO2%) and the concomitant change in other exercise variables, the initial values of each exercise variable, and the cardiac magnetic resonance variables. We used Spearman’s rank correlation coefficient to estimate the association between ΔVO2% and age at initial CPX, age at surgery, and right ventricular outflow tract gradient. Comparisons of the mean ΔVO2% by gender, transannular patch status, and previous shunt palliation were made with 2-sample t tests. Multivariate regression analysis was used to identify the independent predictors of ΔVO2%. We compared the clinical characteristics of the patients with high rates of decrease in the peak VO2% (>4% point decrease/year) to those whose demonstrated a >1% point increase/year using chi-square tests and Wilcoxon rank sum tests. The cardiac magnetic resonance variables in these 2 groups were compared using 2-sample t tests. Analyses were performed using SAS software, version 9.2, SAS System for Windows (SAS Institute, Cary, North Carolina).
Results
We identified 70 patients (53% male) with a total of 179 CPXs (mean 2.6 studies/patient) who met the inclusion criteria. The age at the first CPX was 27.8 ± 15 years (range 8.2 to 61.4). The interval between the first and last CPX was 2.7 ± 1.5 years (range 1.0 to 7.2). The patients’ initial TOF repairs were undertaken at a median age of 2.3 years (range 0.1 to 21.6). Of the 70 patients, 44 had undergone transannular repairs and 26 had nontransannular right ventricular outflow patches. The mean residual right ventricular outflow tract gradient was 8.6 ± 13.0 mm Hg. Although most patients had undergone primary complete TOF repair, 17 (24%) had had a palliative shunt placed before the full repair.
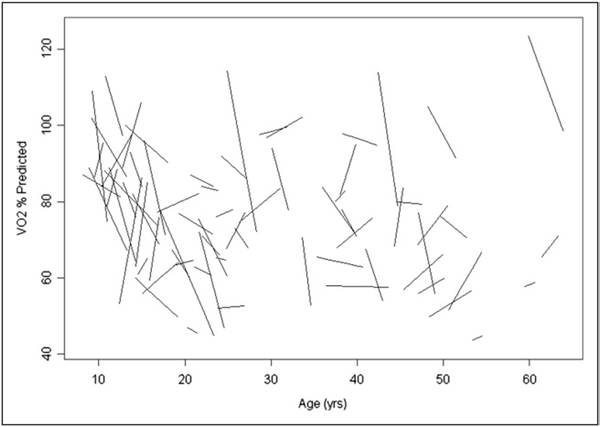
The CPX data from the first and last tests are summarized in Table 1. The peak VO2 on the initial CPX was mild to moderately depressed (27.6 ± 8.8 ml/kg/min; 78 ± 19% of predicted). The peak VO2 on the patients’ final CPX averaged 25.0 ± 7.4 ml/kg/min (73 ± 16% of predicted; p ≤0.01 compared to the initial CPX). The decrease in the O2P was of a similar magnitude: 89 ± 22% of predicted at the initial CPX and 83 ± 20% of predicted at the final CPX (p <0.01). Statistically significant changes over time were not observed in the heart rate at peak exercise, the VE/VCO2 slope, or the baseline spirometric measurements. A small, but statistically significant, increase in the body mass index was observed during the study course; however, the body mass index Z score did not change (Table 1). The mean annual change in VO2% and O2P% (ΔVO2% and ΔO2P%) was −1.4 ± 9.2% and −1.8% ± 11.4% points annually, respectively (Table 2). However, a wide variation was seen in the response over time (Figure 1). In 20 patients (29%), the peak VO2 decreased by >4% annually. In contrast, the peak VO2 increased by ≥1% annually in 23 patients (33%).
Table 1.
Comparison of first and last cardiopulmonary exercise (CPX) test measures
| Variable | First Test | Last Test | Difference | p Value* |
|---|---|---|---|---|
| Peak oxygen consumption (ml/kg/min) | 27.6 ± 8.8 | 25.0 ± 7.4 | −2.5 ± 4.5 | <0.0001 |
| Peak percentage of predicted oxygen consumption (%) | 78 ± 19 | 73 ± 16 | −4 ± 14 | 0.01 |
| Oxygen pulse (ml/beat) | 10.6 ± 3.3 | 10.8 ± 3.1 | 0.1 ± 2.4 | 0.65 |
| Peak percentage of predicted oxygen pulse (%) | 89 ± 22 | 83 ± 20 | −6 ± 18 | 0.01 |
| Slope of minute ventilation vs carbon dioxide production relation | 28.2 ± 4.6 | 27.7 ± 4.1 | −0.5 ± 4.3 | 0.37 |
| Oxygen saturation at rest (%) | 98 ± 2 | 98 ± 1 | −0.5 ± 0.2 | 0.59 |
| Peak oxygen saturation (%) | 97 ± 2 | 97 ± 3 | −0.6 ± 0.2 | 0.46 |
| Percentage of predicted forced vital capacity (%) | 82 ± 17 | 81 ± 17 | −1 ± 9 | 0.50 |
| Percentage of predicted volume of air exhaled in first second of forced exhalation (%) | 81 ± 16 | 81 ± 16 | 0 ± 7 | 0.99 |
| Heart rate at peak exercise (beats/min) | 160.9 ± 21.1 | 160.0 ± 24.4 | −0.9 ± 18.2 | 0.67 |
| Body mass index (kg/m2) | 24.0 ± 5.6 | 25.2 ± 6.0 | 1.3 ± 2.0 | <0.0001 |
| Body mass index Z score | 0.05 ± 1.12 | 0.11 ± 1.18 | 0.06 ± 0.44 | 0.26 |
Data are presented as mean ± SD.
p Value calculated using paired t test.
Table 2.
Rates of change per year
| Variable | Rate of Change per Year of Follow-Up (Mean ± SD) | p Value* |
|---|---|---|
| Peak oxygen consumption (ml/kg/min/year) | −0.8 ± 3.1 | <0.001 |
| Peak percentage of predicted oxygen consumption (% points/year) | −1.4 ± 9.2 | 0.04 |
| Peak percentage of predicted oxygen pulse (% points/year) | −1.8 ± 11.4 | 0.02 |
| Slope of minute ventilation vs carbon dioxide production relation (change/year) | −0.2 ± 3 | 0.38 |
| Percentage of predicted forced vital capacity (% points/year) | −0.3 ± 5.5 | 0.49 |
| Percentage of predicted volume of air exhaled in first second of forced exhalation (% points/year) | −0.1 ± 4.6 | 0.86 |
| Heart rate at peak exercise (beats/min/year) | −0.4 ± 11.3 | 0.60 |
| Peak percentage of predicted heart rate (% points/year) | 0.3 ± 5.8 | 0.40 |
| Body mass index (kg/m2 per year) | 0.4 ± 1.4 | <0.001 |
| Body mass index Z score (change/year) | 0.02 ± 0.3 | 0.37 |
p Value from 1-sample t test comparing mean slope to 0.
Figure 1.
Change in percentage of predicted peak VO2 over time. Each line represents regression line from single patient, using data from all of that patient’s exercise tests.
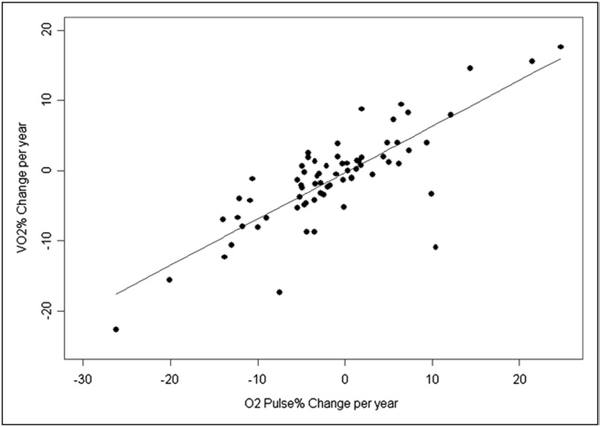
The bivariate correlation analysis revealed a strong association between the ΔVO2% and the ΔO2P% (Figure 2 and Table 3). A more rapid decrease in peak VO2 was also strongly associated with a concurrent increase (worsening) in the VE/VCO2 slope. Changes in the peak VO2 did not correlate with concomitant changes in any other CPX variable. Patients with the greatest initial peak VO2 and greatest initial O2P tended to have a steeper decrease in the peak VO2 during the follow-up period (Table 3). However, no association was found between the rate of decrease and age at CPX, age at reparative surgery, gender, use of a transannular patch, history of previous shunt palliation, or any other baseline clinical parameter, including heart rate, VE/VCO2, forced vital capacity, volume of air exhaled in the first second of forced expiration, or body mass index (Table 4). Multivariate analysis revealed that only the ΔO2P% (p < 0.001) and, to a lesser extent, the initial peak VO2% (p = 0.03) correlated significantly with the ΔVO2%.
Figure 2.
Correlation between change in percentage of predicted peak VO2 and O2P% during follow-up period.
Table 3.
Relation between rate of change in percentage of predicted oxygen consumption (VO2%) with change in exercise test parameters, initial exercise test parameters, and demographic and clinical variables*
| Variable | Correlation | p Value |
|---|---|---|
| Rate of change in exercise test parameter | ||
| Peak percentage of predicted oxygen pulse (%) | 0.79 | <0.0001 |
| Slope of minute ventilation vs carbon dioxide production relation | −0.38 | 0.001 |
| Percentage of predicted forced vital capacity (%) | 0.02 | 0.88 |
| Percentage of predicted volume of air exhaled in first second of forced exhalation (%) | 0.04 | 0.72 |
| Percentage of predicted heart rate (%) | 0.14 | 0.26 |
| Body mass index (kg/m2) | 0.13 | 0.27 |
| Body mass index Z score | 0.15 | 0.21 |
| Initial exercise test parameter | ||
| Peak percentage of predicted oxygen consumption (%) | −0.49 | <0.0001 |
| Peak percentage of predicted oxygen pulse (%) | −0.38 | 0.001 |
| Slope of minute ventilation vs carbon dioxide production relation | 0.2 | 0.09 |
| Percentage of predicted forced vital capacity (%) | −0.09 | 0.46 |
| Percentage of predicted volume of air exhaled in first second of forced exhalation (%) | −0.11 | 0.38 |
| Percentage of predicted heart rate (%) | −0.19 | 0.11 |
| Body mass index (kg/m2) | −0.01 | 0.95 |
| Body mass index Z score | −0.08 | 0.52 |
Weighted by length of follow-up.
Table 4.
Patient characteristics and relation between rate of change in percentage of predicted oxygen consumption (VO2%) with change in exercise test parameters, initial exercise test parameters and demographic and clinical variables*
| Characteristic | Correlation or Mean ± SD | p Value |
|---|---|---|
| Age at initial exercise test (years) | 0.09 | 0.44 |
| Age at surgery (years) | −0.16 | 0.18 |
| Right ventricular outflow tract gradient (mm Hg) | 0.06 | 0.63 |
| Gender | 0.51 | |
| Female (% point change in peak percentage of predicted oxygen consumption/year) | −0.9 ± 9.4 | |
| Male (% point change in peak percentage of predicted oxygen consumption/year) | −1.8 ± 9.1 | |
| Transannular patch | 0.14 | |
| No (% point change in peak percentage of predicted oxygen consumption/year) | −2.6 ± 9.1 | |
| Yes (% point change in peak percentage of predicted oxygen consumption/year) | −0.7 ± 9.2 | |
| Previous shunt palliation | 0.05 | |
| No (% point change in peak percentage of predicted oxygen consumption/year) | −2.2 ± 9.2 | |
| Yes (% point change in peak percentage of predicted oxygen consumption/year) | 0.7 ± 9.0 |
Weighted by length of follow-up.
A subset of 37 patients had cardiac magnetic resonance studies within 12 months of their initial CPX (Table 5). No cardiac medication changes were made between the magnetic resonance study and the first CPX. The mean right ventricular end-diastolic volume Z score was 4.3 ± 2.4; the right ventricular ejection fraction was 51 ± 7%. The pulmonary regurgitation fraction averaged 35 ± 18% (range 1% to 67%). The mean left ventricular end-diastolic volume was normal, and the left ventricular ejection fraction was low-normal at 59 ± 7%. Changes in the peak VO2 during the study course did not correlate with any of the baseline cardiac magnetic resonance measurements.
Table 5.
Baseline magnetic resonance imaging parameters and correlation with change in peak percentage of predicted oxygen consumption (VO2%)
| Parameter | Patients (n) | Mean ± SD | Correlation With ΔVO2% | p Value |
|---|---|---|---|---|
| Right ventricular end-diastolic volume (ml/m2) | 36 | 254.1 ± 80.9 | 0.04 | 0.81 |
| Right ventricular end-diastolic volume Z score | 36 | 4.3 ± 2.4 | 0.09 | 0.60 |
| Right ventricular end-systolic volume (ml/m2) | 36 | 127.1 ± 50.6 | 0.04 | 0.83 |
| Right ventricular ejection fraction (%) | 36 | 51 ± 7 | −0.04 | 0.83 |
| Left ventricular end-diastolic volume (ml/m2) | 36 | 150.6 ± 52.2 | −0.17 | 0.33 |
| Left ventricular end-diastolic volume Z score | 35 | 0.4 ± 1.2 | −0.10 | 0.58 |
| Left ventricular end-systolic volume (ml/m2) | 36 | 64.3 ± 26.0 | −0.07 | 0.69 |
| Left ventricular ejection fraction (%) | 36 | 59 ± 7 | −0.01 | 0.95 |
| Pulmonary regurgitation fraction (%) | 37 | 35 ± 18 | 0.26 | 0.12 |
ΔVO2% = rate of change of percentage of predicted peak oxygen consumption during follow-up period.
The clinical characteristics of the subgroup of patients who had a large (>4% point/year) rate of decrease in the peak VO2% did not differ from those of the subgroup with a >1% point/year increase in the peak VO2% (Table 6). Similarly, a comparison of these 2 groups’ cardiac magnetic resonance data (Table 7) did not identify any statistically significant differences, although the number of patients in these subgroups who also had cardiac magnetic resonance data was small (9 and 10 patients, respectively).
Table 6.
Association between clinical variables and rate of change of peak percentage of predicted oxygen consumption: comparison between 2 groups with largest difference
| Characteristic | >4% Point Loss/Year (n = 20) | >1% Point Gain/Year (n = 23) | p Value |
|---|---|---|---|
| Male gender (n) | 11 (55%) | 10 (44%) | 0.46 |
| Age at first exercise test (year) | 0.55 | ||
| Median | 21.7 | 26.3 | |
| Range | 8.9–59.9 | 9.5–61.4 | |
| Age ≥18 years (n) | 11 (55%) | 13 (57%) | 0.92 |
| Age ≥24 years (n) | 9 (45%) | 13 (57%) | 0.45 |
| Interval from first to last exercise test (year) | 0.78 | ||
| Median | 2.3 | 3.1 | |
| Range | 1.0–6.4 | 1.0–4.9 | |
| Transannular patch (n) | 9 (45%) | 14 (61%) | 0.30 |
Table 7.
Association between cardiac magnetic resonance (CMR) variables and rate of change in peak percentage of predicted oxygen consumption (VO2%)
| Baseline Parameter | >4% Point Loss/Year (n = 9) | >1% Point Gain/Year (n = 10) | p Value |
|---|---|---|---|
| Pulmonary regurgitation fraction ≥40% (n) | 4 (44%) | 7 (70%) | 0.36 |
| Right ventricular end-diastolic volume (ml/m2) | 248.1 ± 94.1 | 254.7 ± 65.5 | 0.63 |
| Right ventricular end-diastolic volume Z score | 4.2 ± 2.6 | 4.9 ± 1.8 | 0.37 |
| Right ventricular end-systolic volume (ml/m2) | 119.8 ± 60.4 | 124.2 ± 45.8 | 0.59 |
| Right ventricular ejection fraction (%) | 54 ± 8 | 53 ± 8 | 0.51 |
| Left ventricular end-diastolic volume (ml/m2) | 167.8 ± 57.4 | 141.3 ± 68.7 | 0.26 |
| Left ventricular end-diastolic volume Z score | 0.99 ± 1.05 | 0.76 ± 1.28 | 0.34 |
| Left ventricular end-systolic volume (ml/m2) | 69.9 ± 26.2 | 67.1 ± 35.2 | 0.56 |
| Left ventricular end-systolic volume Z score | 1.72 ± 0.90 | 2.00 ± 2.97 | 0.86 |
| Left ventricular ejection fraction (%) | 58.7 ± 4.8 | 57.4 ± 6.5 | 0.96 |
Discussion
In normal subjects, the peak VO2 typically reaches a maximum value during adolescence; thereafter, it decreases by approximately 0.7%/year.18 In the present study, by focusing on the percentage of predicted values, it was possible to take into account these normal age-related changes and to observe how the exercise function of patients with rTOF evolves relative to a normal population. The present study found that on the initial exercise tests, the peak VO2 was significantly depressed. During the almost 3-year median follow-up period, a small, but statistically significant, additional decrease was observed. If a patient’s peak VO2 were to decrease in parallel with the normal population, the percentage of predicted values would be expected to remain virtually unchanged. The observed decrease in the percentage of predicted values indicates that our patients’ exercise function deteriorated in excess of the normal age-related decrease. However, the variability in the change in exercise function over time was great. Although 1/3 of the group decreased steeply (>4% points/year), another 1/3 had a >1% point/year increase in the percentage of the predicted peak VO2.
The ΔVO2% did not correlate with the change in the percentage of predicted heart rate at peak exercise during the follow-up period. However, a very strong correlation was found between the ΔVO2% and the ΔO2P%. The peak VO2 is the product of the O2P (i.e., the amount of oxygen consumed per heart beat) and the heart rate at peak exercise. Using the Fick equation, the O2P is also equal to the forward stroke volume at peak exercise times the oxygen extraction at peak exercise. The oxygen extraction at peak exercise is maximized and varies little across subjects.18 Furthermore, our CPX data measurements were derived solely from studies in which the patients expended a good effort (respiratory exchange ratio ≥1.09). Consequently, it is unlikely that the changes in O2P were related to changes in oxygen extraction at peak exercise. Hence, our data suggest that our patients’ decrease in exercise function was associated with deterioration in their ability to maintain forward stroke volume at peak exercise.
A statistically significant negative correlation (r =−0.38; p <0.001) also existed between the ΔVO2% and the ΔVE/VCO2 slope. An elevated VE/VCO2 slope indicates that a patient must breathe more to eliminate a given amount of carbon dioxide (i.e., the gas exchange within the patient’s lungs is inefficient). In patients with rTOF, a strong negative correlation exists between exercise dysfunction and the degree of slope elevation.10,21 An elevated VE/VCO2 slope has also been strongly associated with an increased risk of cardiac-related mortality and hospitalization in these patients22 (and in patients with congestive heart failure23–25). The correlation between the ΔVO2% and ΔVE/VCO2 slope observed in the present study is consistent with these previous observations. The factors responsible for the elevated VE/VCO2 slope in our patients are probably varied and likely included pulmonary blood flow maldistribution and ventilatory/perfusion mismatch secondary to pulmonary artery stenoses, pulmonary vascular disease, and/or congestive heart failure.
A greater baseline peak VO2 was associated with a greater decrease in peak VO2 during the follow-up period. This suggests that, although the exercise function of a cross section of patients with TOF might vary greatly, the physiology of the patient with rTOF is usually unable to sustain normal exercise function in the long term. Paradoxically, a history of a transannular patch was not associated with a steeper decrease in the peak VO2. We believe this unexpected finding might have been because patients who had undergone pulmonary valve replacement were excluded from our study. This excluded group likely had a disproportionate number of poorly functioning patients with transannular patches. Consequently, among the transannular patients who were included, there might have been a selection bias in favor of healthier subjects.
None of the other baseline clinical or demographic variables studied was associated with a more rapid deterioration in exercise function. Similarly, within the subset of patients with baseline magnetic resonance imaging data, none of measurements correlated with the ΔVO2% during the follow-up periode, predicting which patients will deteriorate or when the deterioration will occur is difficult.
Several cross-sectional studies of patients with rTOF have reported that older patients tend to have more compromised exercise function than their younger peers. In a study of 99 adults with rTOF, Samman et al6 documented the %VO2 peak to be depressed at 66 ± 13% of predicted. They found that older age, age at rTOF, chronotropic incompetence, and abnormal lung function were associated with limited exercise capacity. In a larger single-center study of 168 adults with rTOF, Fredriksen et al7 documented a very low %VO2 peak of 51% predicted, with the lowest VO2 values in older patients and those with history of later surgical repair. Diller et al8 studied a cohort of 107 adults at a mean age of 32 years (with rTOF at a mean age of 6 years) and reported a mean peak VO2 of 25.5 ml/kg/ min, or 56% of the normal adult value. In contrast, in a study of 50 children and adolescents (mean age at CPX 12.5 years; mean age at repair 11 months), Mahle et al1 reported that although 16% of the patients had a peak VO2 ≤80% of predicted, the overall peak VO2 averaged 95% of predicted. Additionally, in this young population, they found that older age was associated with a greater peak VO2. These cross-sectional studies do not permit one to determine, however, to what extent the reported differences between older and younger patients with rTOF resulted from an era effect, the age at surgery, or changes in exercise function over time. To our knowledge, our study is the first to establish, on the basis of serial metabolic CPX data, that the inferior exercise capacity commonly encountered in older patients with rTOF is at least partly due to progressive deterioration in exercise function beyond that expected to occur solely from the normal aging process.
Our observation that the decrease in peak VO2% was not different in the 15 patients who had undergone previous shunt palliation and delayed rTOF compared to the other early-repair patients is consistent with earlier work by Rowe et al,2 who studied an older cohort with the average age at complete repair of 8 years. Those investigators found that the age at repair or previous palliative shunt had no influence on indexes of exercise function.2 In contrast to our findings, others have found a correlation between exercise dysfunction and pulmonary regurgitation fraction, right ventricular dilation, and exercise dysfunction.2,16,26,27 However, these cross-sectional studies do not allow us to determine whether the presence of these structural/functional abnormalities were associated with future deterioration in exercise function.
To have a more homogenous population, we elected to exclude patients with pulmonary atresia. Furthermore, to avoid the potential confounding effects of artificial conduits, we excluded data from the patients with right ventricle to pulmonary artery conduits. Hence, we recognize that our patient population is not representative of the entire spectrum of those with rTOF and was limited to patients with native right ventricular outflow tracts. Our study was also constrained to relatively healthy patients with rTOF capable of performing serial exercise tests. However, it is also possible that patients with serial exercise tests were more likely to have reported symptoms (prompting referral for a follow-up exercise test) than patients with only 1 exercise test. This selection bias might have magnified the decrease in VO2%. The observed decrease was nevertheless relatively small (1.4% points/year). Despite these limitations, we believe our patient population was probably representative of most patients with rTOF encountered in most centers.
Acknowledgment
The authors gratefully acknowledge the expert technical assistance of Tracy J. Curran, MS, Julieann O’Neill, MS, Jennifer L. Smith, MS, and Kathleen M. Solly, MS.
References
- 1.Mahle WT, McBride MG, Paridon SM. Exercise performance in tetralogy of Fallot: the impact of primary complete repair in infancy. Pediatr Cardiol 2002;23:224–229. [DOI] [PubMed] [Google Scholar]
- 2.Rowe SA, Zahka KG, Manolio TA, Horneffer PJ, Kidd L. Lung function and pulmonary regurgitation limit exercise capacity in postoperative tetralogy of Fallot. J Am Coll Cardiol 1991;17:461–466. [DOI] [PubMed] [Google Scholar]
- 3.Wessel HU, Cunningham WJ, Paul MH, Bastanier CK, Muster AJ, Idriss FS. Exercise performance in tetralogy of Fallot after intracardiac repair.J Thorac Cardiovasc Surg 1980;80:582–593. [PubMed] [Google Scholar]
- 4.Gatzoulis MA, Clark AL, Cullen S, Newman CG, Redington AN. Right ventricular diastolic function 15 to 35 years after repair of tetralogy of Fallot: restrictive physiology predicts superior exercise performance. Circulation 1995;91:1775–1781. [DOI] [PubMed] [Google Scholar]
- 5.Sutton NJ, Peng L, Lock JE, Lang P, Marx GR, Curran TJ, O’Neill JA, Picard ST, Rhodes J. Effect of pulmonary artery angioplasty on exercise function after repair of tetralogy of Fallot. Am Heart J 2008;155: 182–186. [DOI] [PubMed] [Google Scholar]
- 6.Samman A, Schwerzmann M, Balint OH, Tanous D, Redington A, Granton J, Siu SC, Silversides CK. Exercise capacity and biventricular function in adult patients with repaired tetralogy of Fallot. Am Heart J 2008;156:100–105. [DOI] [PubMed] [Google Scholar]
- 7.Fredriksen PM, Therrien J, Veldtman G, Ali WM, Liu P, Thaulow E, Webb G. Aerobic capacity in adults with tetralogy of Fallot. Cardiol Young 2002;12:554–559. [DOI] [PubMed] [Google Scholar]
- 8.Diller GP, Dimopoulos K, Okonko D, Li W, Babu-Narayan SV, Broberg CS, Johansson B, Bouzas B, Mullen MJ, Poole-Wilson PA, Francis DP, Gatzoulis MA. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation 2005;112:828–835. [DOI] [PubMed] [Google Scholar]
- 9.Roest AAW, Helbing WA, Kunz P, van den Aardweg JG, Lamb HJ, Vliegen HW, van der Wall EE, de Roos A. Exercise MR imaging in the assessment of pulmonary regurgitation and biventricular function in patients after tetralogy of Fallot repair. Radiology 2002;223:204–211. [DOI] [PubMed] [Google Scholar]
- 10.Rhodes J, Dave A, Pulling MC, Geggel RL, Marx GR, Fulton DR, Hijazi ZM. Effect of pulmonary artery stenoses on the cardiopulmonary response to exercise following repair of tetralogy of Fallot. Am J Cardiol 1998;81:1217–1219. [DOI] [PubMed] [Google Scholar]
- 11.Diller GP, Dimopoulos K, Okonko D, Uebing A, Broberg CS, Babu Narayan S, Bayne S, Poole-Wilson PA, Sutton R, Francis DP, Gatzoulis MA. Heart rate response during exercise predicts survival in adults with congenital heart disease. J Am Coll Cardiol 2006;48:1250–1256. [DOI] [PubMed] [Google Scholar]
- 12.Frigiola A, Tsang V, Nordmeyer J, Lurz P, van Doorn C, Taylor AM, Bonhoeffer P, de Leval M. Current approaches to pulmonary regurgitation. Eur J Cardiothorac Surg 2008;34:576–580. [DOI] [PubMed] [Google Scholar]
- 13.Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol 2004;17:1068–1074. [DOI] [PubMed] [Google Scholar]
- 14.Ghai A, Silversides C, Harris L, Webb GD, Siu SC, Therrien J. Left ventricular dysfunction is a risk factor for sudden cardiac death in adults late after repair of tetralogy of Fallot. J Am Coll Cardiol 2002;40:1675–1680. [DOI] [PubMed] [Google Scholar]
- 15.Meijboom F, Szatmari A, Deckers JW, Utens EM, Roelandt JR, Bos E, Hess J. Cardiac status and health-related quality of life in the long term after surgical repair of tetralogy of Fallot in infancy and childhood. J Thorac Cardiovasc Surg 1995;110:883–891. [DOI] [PubMed] [Google Scholar]
- 16.Meadows J, Powell AJ, Geva T, Dorfman A, Gauvreau K, Rhodes J.Cardiac magnetic resonance imaging correlates of exercise capacity in patients with surgically repaired tetralogy of Fallot. Am J Cardiol 2007;100:1446–1450. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes SM, McElhinney DB, Khairy P, Graham DA, Landzberg MJ, Rhodes J. Serial cardiopulmonary exercise testing in patients with previous Fontan surgery. Pediatr Cardiol 2010;31:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones NL. Clinical Exercise Testing. 4th ed. Philadelphia: WB Saunders; 1997:132–135. [Google Scholar]
- 19.Cooper DM, Weiler-Ravell D. Gas exchange response to exercise in children. Am Rev Respir Dis 1984;129:S47–S48. [DOI] [PubMed] [Google Scholar]
- 20.Wasserman K, Hansen JE, Sue DY, Casaburi R, Whipp BJ. Principles of Exercise Testing and Interpretation. 3rd ed. Philadelphia: Lippincott, 1999:173–174. [Google Scholar]
- 21.Clark AL, Gatzoulis MA, Redington AN. Ventilatory responses to exercise in adults after repair of tetralogy of Fallot. Br Heart J 1995;73:445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giardini A, Specchia S, Tacy TA, Coutsoumbas G, Gargiulo G, Donti A, Formigari R, Bonvicini M, Picchio FM. Usefulness of cardiopulmonary exercise to predict long-term prognosis in adults with repaired tetralogy of Fallot. Am J Cardiol 2007;99:1462–1467. [DOI] [PubMed] [Google Scholar]
- 23.Chua TP, Ponikowski P, Harrington D, Anker SD, Webb-Peploe K, Clark AL, Poole-Wilson PA, Coats AJ. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol 1997;29:1585–1590. [DOI] [PubMed] [Google Scholar]
- 24.Ponikowski P, Francis DP, Piepoli MF, Davies LC, Chua TP, Davos CH, Florea V, Banasiak W, Poole-Wilson PA, Coats AJ, Anker SD. Enhanced ventilatory response to exercise in patients with chronic heart failure and preserved exercise tolerance: marker of abnormal cardiorespiratory reflex control and predictor of poor prognosis. Circulation 2001;103:967–972. [DOI] [PubMed] [Google Scholar]
- 25.Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, Coats AJ. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2). Eur Heart J 2000;21:154–161. [DOI] [PubMed] [Google Scholar]
- 26.Wald RM, Haber I, Wald R, Valente AM, Powell AJ, Geva T. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of Fallot. Circulation 2009;119: 1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marx GR, Hicks RW, Allen HD, Goldberg SJ. Noninvasive assessment of hemodynamic responses to exercise in pulmonary regurgitation after operations to correct pulmonary outflow obstruction. Am J Cardiol 1988;61:595–601. [DOI] [PubMed] [Google Scholar]