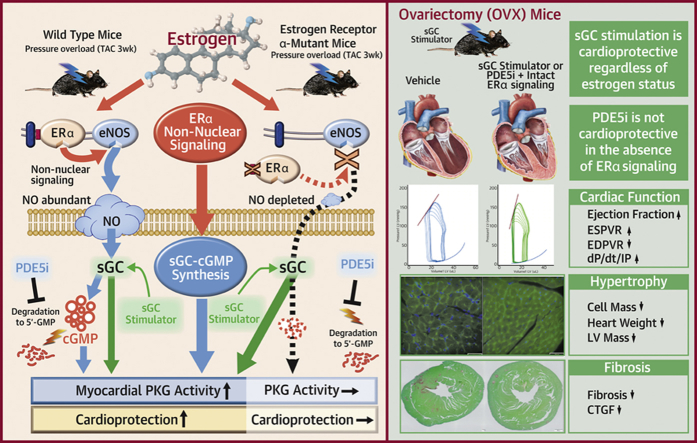
Visual Abstract
Key Words: cyclic GMP, estradiol, heart failure, non-nuclear signaling, sGC stimulator
Abbreviations and Acronyms: cGMP, cyclic guanosine monophosphate; E2, estradiol; ECs, endothelial cells; EDC, estrogen dendrimer conjugate; eNOS, endothelial nitric oxide synthase; ER, estrogen receptor; LV, left ventricular; NO, nitric oxide; PaPE, pathway-preferential estrogen; PDE5i, phosphodiesterase 5 inhibitor; PKG, cGMP-dependent protein kinase G; sGC, soluble guanylate cyclase; TAC, transverse aortic constriction; VO2, oxygen consumption rate
Highlights
-
•
The role of ERα non-nuclear signaling in female heart failure was investigated, using a novel mouse line in which ERα non-nuclear signaling was selectively disrupted by inhibiting the interaction between ERα and striatin, a scaffold protein residing at caveolae.
-
•
ERα non-nuclear signaling was linked to myocardial PKG activity in female hearts and ameliorated cardiac maladaptive remodeling induced by pressure overload.
-
•
ERα non-nuclear signaling was indispensable to the therapeutic efficacy of cGMP-PDE5 inhibition in heart failure but not to that of sGC stimulation.
-
•
sGC stimulation potently ameliorated cardiac remodeling, regardless of estrogen conditions, in sharp contrast to PDE5 inhibition.
-
•
The study provided the first in vivo evidence for the role of ERα non-nuclear signaling in heart failure, linking it to cGMP-PKG pathways. The data also supported the advantage of sGC stimulation over PDE5 inhibition as a potential therapeutic strategy in treating heart failure in post-menopausal women, highlighting the need for female-specific therapeutic strategies.
Summary
Using genetically engineered mice lacking estrogen receptor-α non-nuclear signaling, this study demonstrated that estrogen receptor−α non-nuclear signaling activated myocardial cyclic guanosine monophosphate-dependent protein kinase G and conferred protection against cardiac remodeling induced by pressure overload. This pathway was indispensable to the therapeutic efficacy of cyclic guanosine monophosphate−phosphodiesterase 5 inhibition but not to that of soluble guanylate cyclase stimulation. These results might partially explain the equivocal results of phosphodiesterase 5 inhibitor efficacy and also provide the molecular basis for the advantage of using a soluble guanylate cyclase simulator as a new therapeutic option in post-menopausal women. This study also highlighted the need for female-specific therapeutic strategies for heart failure.
Heart failure is one of the leading causes of death in many developed countries, including the United States, and the number of patients continues to increase worldwide. Despite decades of advances in the standard of care for treating heart failure, its mortality remains high (1). The cyclic guanosine monophosphate (cGMP) signaling pathway plays a central role in the maintenance of cardiovascular homeostasis; its aberrant regulation has been implicated in the pathophysiology of heart failure. Restoring or augmenting cGMP signaling has emerged as new therapeutic strategy, including enhancing its synthesis and inhibiting its degradation. The former is achieved by soluble guanylate cyclase (sGC) stimulation or neprilysin inhibition, and the latter occurs by cGMP−phosphodiesterase (PDE) inhibition.
Among the small molecules that activate cGMP signaling pathways, PDE5 inhibitors (PDE5i) have long been clinically used to treat pulmonary hypertension. Although the PDE5i sildenafil provides cardiac benefits in various experimental models (2, 3, 4, 5, 6), clinical studies testing the efficacy of PDE5i in patients with heart failure have shown equivocal results (7, 8, 9). Negative data come from mostly female patients and positive data come from mostly male patients. This may be attributable to attenuated nitric oxide (NO) production due to reduced levels of estrogen in older adult patients. We previously demonstrated that reduced levels of estrogen critically hampered the anti-remodeling efficacy of PDE5i in female rodent heart failure, which suggested that estrogen-coupled cGMP signaling is essential to the efficacy of PDE5 inhibition (10). In contrast, sGC stimulators have been gaining attention as a potential therapeutic approach for heart failure by enhancing the cGMP signaling pathway, and a new sGC stimulator Vericiguat (Bayer and Merck Sharp & Dohme, Branchburg, New Jersey) is being tested for heart failure in a Phase III clinical study (11). It is of significant clinical importance to determine whether its efficacy is affected by estrogen conditions.
Estrogen plays a significant role in maintaining homeostasis of the cardiovascular system and vascular protection in women (12, 13, 14, 15, 16, 17, 18, 19). Estrogen and its receptors classically bind and translocate to the nucleus, where they directly modulate transcription. Estrogen receptors (ERs) also have extra-nuclear actions that involve activation of kinase signaling, which leads to rapid cellular responses, known as non-nuclear signaling (20). ERα is one of the predominant ER subtypes, which has both nuclear and non-nuclear actions. The roles for ERα nuclear actions have been clarified using unique mouse models of ERα, including a model lacking AF-1 function (ERα AF-10 mice) and a model lacking AF-2 function (ERα AF-20 mice), as well as a complete gene deletion model (21,22). In particular, the dissection of ERα AF-20 that lacks nuclear function has provided evidence that the nuclear actions of ERα mediate protective effects against atherosclerosis, diabetes, and bone demineralization, which are conferred by estrogen (22, 23, 24, 25).
In contrast, non-nuclear actions of ERα have been shown to mediate endothelial NO production, vasodilatation, and endothelial healing (re-endothelialization) from the study of a mouse that had a point mutation at its palmitoylation site (C451A- ERα), and therefore, a membrane-specific loss of function (20,26). Other studies used pharmacological tools to study non-nuclear actions, including estrogen dendrimer conjugates (EDCs) or pathway-preferential estrogens (PaPEs) (20), and demonstrated the role for vasculo-protection and endothelial NO production without growing uterine or breast cancer cells (21,27, 28, 29, 30). However, in light of chronic arterial protection, Guivarc’h et al. (31) performed a study that compared nuclear actions versus non-nuclear actions of ERα in models of chronic hypertension and atherosclerosis using EDCs, PaPEs, C451A-ERα, and ERα AF-20, and demonstrated the prominent protective role of the former. However, non-nuclear actions of ERα might play a pivotal role in the heart. Menazza et al. (26) used an ex vivo model of myocardial ischemia-reperfusion and found that EDCs ameliorated the injury via mechanisms that potentially involved NO and protein S-nitrosylation (26). We hypothesized that non-nuclear actions of ERα might play a role in pathological cardiac remodeling because of the coupling of the non-nuclear actions to endothelial nitric oxide synthase (eNOS) and thus potentially to cGMP-dependent protein kinase G (cGMP-PKG) signaling in the heart.
The present study used a novel mouse line, which we recently reported, in which ERα non-nuclear signaling was disrupted by inhibiting the interaction between ERα and striatin while maintaining intact genomic signaling (32). We investigated the role of ERα non-nuclear signaling in heart failure remodeling and in the cGMP-PKG enhancing therapeutic interventions. We found that ERα non-nuclear signaling protected against cardiac remodeling and was essential to the therapeutic efficacy of cGMP-PDE5 inhibition but not to that of sGC stimulation.
Methods
Animals
All animal procedures were approved by Institutional Animal Care and Use Committee at the University of Tokyo. The animal care and experiments were in accordance with the guidelines of the National Institutes of Health. Animals were maintained with food and water ad libitum and kept in cages with a 12-h light-dark cycle in a temperature-controlled laboratory. Sildenafil citrate (100 mg/kg/day) was fed mixed with soft rodent chow (Transgenic Dough Diet, Bio-Serv, Flemington, New Jersey) for 3 weeks as described previously (6,33). The sGC stimulator (Riociguat: BAY 632521 [Irvine, California]; 3 mg/kg/day) was prepared in specific solution (Transcutol 10%, Cremophor 20%, water 70%) and given by oral gavage every day for 3 weeks as described previously (34).
The KRRKI/KI mice (KRR knock-in mutant ERα) were generated by GenOway (Lyon, France) as previously described (35). The mutations were introduced in the exon 3 of ESR1, replacing amino acids 235K, 237R, and 238R: GCC, GCT, and GCA of mouse ERα (corresponding 231K, 233R, and 234R of human ERα) with alanine. These mutations were located in region C, the DNA-binding domain of ERα. Heterozygous male and female animals were mated, and all of the offspring were genotyped by polymerase chain reaction as previously described (35). Homozygous mutants (KRRKI/KI) and their littermate controls (KRRWT/WT, wild type littermate) were used in this study (10).
Ovariectomy and estrogen replacement
Ovariectomy
Ovariectomy was performed in female mice at the age of 6 to 8 weeks by a standard bilateral back approach procedure (36,37). Briefly, animals were anesthetized with an intraperitoneal injection of 100 mg/kg of etomidate and placed in a prone position. Two small skin incisions (1 cm in length) were made on both lateral back sides, caudal to the last rib and 1 cm lateral from the vertebra. To reach the peritoneal space, the body wall and peritoneal muscle layer were incised. The ovary was identified with oviducts, uterus, and fat tissues, and then exteriorized with forceps. After a ligature was applied between the ovary and the edge of uterine horn for hemostasis, the ovary was carefully excised.
Estrogen replacement
One week after ovariectomy, 60-day time-release estradiol (E2) pellets (0.25 mg, Innovative Research of America, Sarasota, Florida) or placebo-containing pellets were implanted subcutaneously as previously described (10). The size of the uterus was checked to confirm the successful excision of ovaries with or without E2 supplementation, as previously described (31).
Transverse aortic constriction
Animals underwent transverse aortic constriction (TAC) or sham surgery (sham) at 8 to 10 weeks as previously described (6,33). Briefly, animals were anesthetized with isoflurane (1% to 1.5%) and 100 mg/kg of etomidate, intubated, and mechanically ventilated. The transverse aorta was constricted with a 27-gauge needle using 7-0 prolene suture, and animals’ chests were closed. Animals were killed 3 weeks after TAC. Total heart weight was measured, and the left ventricular (LV) tissue was harvested. Snap frozen heart samples are stored at −80°C until analyses.
Echocardiographic study
Transthoracic echocardiography was performed (VEVO2100, 9 to 18 MHz transducer, Visualsonics Inc., Toronto, Ontario, Canada) with conscious mice. M-mode LV end-systolic and end-diastolic dimensions were measured, and the percentage of LV fractional shortening was calculated as previously described (10). Studies were performed by investigators blinded to genotypes and heart conditions. Serial studies were performed on the day of and at 1 and 3 weeks after TAC surgery.
Hemodynamic study using pressure volume analysis
In vivo LV function was assessed by pressure−volume analysis in anesthetized mice as previously described (6,33). The LV apex was exposed through an incision between the seventh and eighth ribs. A 1.4-F pressure−volume catheter (SPR-839, Millar Instruments; Houston, Texas) was inserted from the LV apex and advanced into the LV lumen to lie along the longitudinal axis. The absolute volume was calibrated and pressure−volume data were assessed at the steady state and during the pre-load reduction phase. Data were analyzed with the LabChart application (AD Instruments, Dunedin, New Zealand).
Cardiomyocyte isolation and NOS activity measurement
Cardiomyocytes were isolated from KRRWT/WT and KRRKI/KI mouse hearts as previously described (38). Briefly, the heart was quickly excised and retroperfused through the ascending aorta with modified Tylode’s solution (130 mM sodium chloride, 5.4 mM potassium chloride, 0.33 mM sodium dihydrogen phosphate (NaH2PO4), 0.5 mM magnesium chloride (MgCl2), 22 mM glucose) with 1 mg/ml of collagenase type 2 (Worthington Biochemical, Lakewood, New Jersey) and 0.05 mg/ml of protease (Sigma-Aldrich, St. Louis, Missouri). Cardiomyocytes were incubated in modified Tylode’s solution with 1 nM of E2 (Sigma-Aldrich) or without E2 for 40 min at 37°C, then cells were lysed and NOS activity was measured using an enzyme immunoassay kit (Ultrasensitive NOS Assay Kit; Oxford Biomedical Research, Rochester Hills, Michigan) according to the manufacturer’s protocol.
Measurement of E2 concentration in serum and heart by enzyme immunoassay
Serum and heart E2 levels were measured using an E2 enzyme immunoassay kit (Arbor Assays Estradiol Serum EIA kit [Arbor Assays, Inc., Ann Arbor, Michigan]) according to the manufacturer protocols. For serum E2 measurement, blood samples were centrifuged at 4°C, and supernatants were collected.
For measuring E2 concentration in heart tissue, we followed the published protocol by Iorga et al. (39). LV samples were trimmed in 80 to 100 mg of tissues. Samples were then snap frozen, pressed into powders, and were diluted in E2 enzyme immunoassay buffer at a concentration of 100 mg powder/ml buffer. Amounts per 100 mg heart tissue were calculated.
RNA and protein analysis
Total RNA was extracted from mouse LV heart samples by using TRIreagent (Molecular Research Center Inc., Cincinnati, Ohio). The mRNA was reverse transcribed into cDNA using a high-capacity RNA-to-cDNA Kit (Applied Biosystems, Life Technologies, Rockville, Maryland).
The primer sequences were as follows:
CTGF: forward AGCCTCAAACTCCAAACACC, reverse CAACAGGGATTTGACCAC
Nppb: forward AAGTCCTAGCCAGTCTCCAGA, reverse GAGCTGTCTCTGGGCCATTTC
GAPDH: forward CATGGCCTTCCGTGTTCCTA, reverse CCTGCTTCACCACCTTCTTGAT
GREB1: forward GACCGTCTACTACCTCGTCCA, reverse GCCAGGAGCGTAGGAAGAT
AGT: forward CGGAGGCAAATCTGAACAAC, reverse TCCTCCTCTCCTGCTTTGAG
C3: forward CGGCATAGAGAAGAGGCAAG, reverse AAGGCAGCATAGGCAGAGC
cDNAs were amplified with Thunderbird qPCR Mix (Toyobo Inc., Osaka, Japan), and relative expression levels of target genes were measured using Light Cycler480 (Roche Inc., Basel, Switzerland) as previously described (38,40). Each sample was run in duplicate, and the results were normalized to glyceraldehyde 3-phosphate dehydrogenase.
PKG activity analysis
An enzyme immunoassay colorimetric assay (CycLex, MBL International Corporation, Woburn, Massachusetts) was performed to evaluate PKG activity as previously described. Proteins were extracted from whole heart samples according to the previous description (2).
Histology
Heart samples were fixed with 10% formalin and embedded in paraffin. Samples were sliced into 4- to 5-mm slices. Sections were stained with Picrosirius red for interstitial fibrosis and fast-green for counterstaining. The LV short axis at the levels of papillary muscle was analyzed. Cardiomyocyte cell size was measured using wheat germ agglutinin (Biotin Conjugated Triticum vulgare Lectin, EY Laboratories, Inc., San Mateo, California) stained sections. Forty to 50 cells per slide were measured, and the average value in each sample was calculated.
Analyses of metabolic status
To assess the metabolic status of KRRKI/KI mice after pressure overload and cGMP pathway stimulation, the oxygen consumption rate (VO2) and locomotor activities were measured using an oxygen/carbon dioxide metabolic measurement system (MK-5000, Muromachi, Chuo-ku, Tokyo, Japan), as previously described (41). The VO2 was normalized to body weight.
Statistical analysis
All data are presented as mean ± SEM. D'Agostino and Pearson’s normality test was applied for determining whether sample distributions were normally distributed or skewed. If the values were normally distributed, a 1- or 2-way analysis of variance was applied, with Tukey’s post hoc method used for pairwise comparisons. The Kruskal–Wallis test with Dunn's post hoc method for pairwise comparisons was done if data were not normally distributed. A p value <0.05 was considered statistically significant. Statistical analyses were performed with GraphPad Prism 7 (GraphPad Software, La Jolla, California).
Results
Loss of E2-stimulated NOS activation in cardiac myocytes with preserved nuclear actions in KRRKI/KI animals
To confirm loss of non-nuclear actions in KRRKI/KI hearts, we assessed E2-simulated NOS activity in cardiac myocytes isolated from KRRKI/KI animals. As expected, short-term E2 stimulation failed to activate NOS in KRRKI/KI myocytes, whereas increased NOS activity was found in wild-type myocytes (Supplemental Figure 1), which indicated that the non-nuclear signaling was ablated in cardiac myocytes by the introduction of KRR mutations as reported in endothelial cells (ECs) (30). To exclude the possibility of potential alteration of nuclear actions by introducing the point mutations in the C domain, we examined myocardial expression levels of estrogen responsive element−containing genes including Greb1, C3, and Agt in the presence or absence of E2 (E2 vs. ovariectomy) (35,42). These genes were significantly up-regulated in response to E2 in KRRKI/KI hearts, similar to KRRWT/WT hearts (Supplemental Figure 2), which suggested that the nuclear actions were preserved in KRRKI/KI hearts. Uterus phenotype was consistently comparable between the genotypes (Supplemental Figures 3A and 3B). Ovariectomy led to uterine atrophy in both genotypes, and E2 replacement induced hypertrophy in KRRKI/KI mice, similar to KRRWT/WT mice.
ERα non-nuclear signaling plays a pivotal role in estrogen-conferred cardio-protection and is essential to cGMP-PDE5 benefits
To determine the role of ERα non-nuclear signaling in cardiac remodeling and in the therapeutic efficacy of cGMP-PDE5 inhibition, animals lacking ERα non-nuclear signaling (KRRKI/KI) were ovariectomized, supplemented with or without E2, and exposed to 3 weeks of LV pressure overload by TAC surgery.
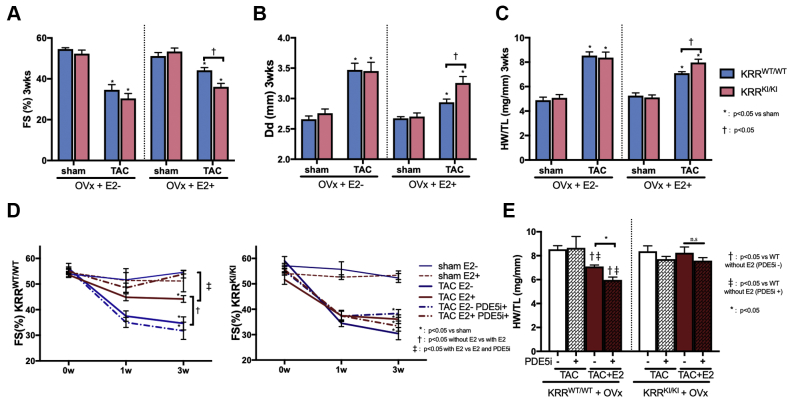
The effects of concomitant PDE5i treatment were then determined. KRRKI/KI mice showed a baseline cardiac phenotype that was indistinguishable from littermates KRRWT/WT at this age, regardless of estrogen conditions, as assessed with echocardiography and terminal heart weight measurement (Figures 1A to 1C). However, in the presence of E2, KRRKI/KI hearts that underwent TAC revealed lower cardiac function (percent of fractional shortening), a larger LV cavity, end-diastolic dimension (Dd), and more of an increase in heart weight normalized to tibial length (HW/TL) compared with KRRWT/WT mice, whereas both genotypes developed severe and dilative LV remodeling without E2 supplementation (Figures 1A to 1C). This suggested that ERα non-nuclear signaling was necessary for E2-conferred cardioprotection against LV remodeling after pressure overload. To rule out the possibility that these physiological impacts might result from potential differential estrogen regulations between the genotypes, we measured estrogen levels in serum and in the myocardium. The serum estrogen levels were approximately 120 pg/ml with E2 pellet implantation in both genotypes, whereas the removal of ovaries resulted in serum estrogen levels of approximately 10 pg/ml (Supplemental Figures 4A and 4B). The levels achieved with the pellet were in the super-physiological range because 20 to 60 pg/ml is the range of the mouse estrous cycle. However, these levels were consistent with previous studies that used the same dose of estrogen pellets (0.25 mg, 60-day release) to study the effects of estrogen on the cardiovascular system (35, 36, 37,43). TAC did not significantly alter serum estrogen levels. We further determined myocardial estrogen levels, which were approximately 15 pg/100 mg heart in either genotype after ovariectomy, and increased to approximately 40 pg/100 mg heart with estrogen supplementation without any significant change by TAC (Supplemental Figures 4C and 4D).
Figure 1.
Cardiac Phenotype of KRRKI/KI Mouse and PDE5i Efficacy
Cardiac echocardiography assessed with (A) the percentage of fractional shortening (FS) and (B) end-diastolic dimension (Dd) (mm) at 3 weeks. (C) Heart weight (HW) (mg) normalized to tibial length (TL) (mm) at 3 weeks after transverse aortic constriction (TAC). (D) Effect of a phosphodiesterase 5 inhibitor (PDE5i) on percentage of FS time course after TAC (n = 5 to 8 per group) and (E) HW/TL (mg/mm) at 3 weeks (n = 6 to 8 per group). E2 = estradiol, KRRKI/KI = KRR knock-in mutant ERα; KRRWT/WT = wild-type littermate; OVx = ovariectomy.
Serial measurement of the percentage of fractional shortening by echocardiography and terminal heart weight showed that concomitant PDE5i treatment significantly improved global LV systolic function and inhibited a heart weight increase in KRRWT/WT TAC mice with E2 supplementation. However, PDE5i failed to provide these effects without E2, which was consistent with our previous report (10). Importantly, PDE5i no longer had such anti-remodeling effects on KRRKI/KI TAC hearts despite E2 supplementation (Figures 1D and 1E), which suggested the pivotal role of ERα non-nuclear signaling in the therapeutic efficacy of PDE5 inhibition in failing hearts.
In contrast, the cardioprotective effects of PDE5i in KRRKI/KI male mice were not abrogated as assessed with the percentage of fractional shortening and heart weight (mg/mm) (Supplemental Figures 5A to 5C), which indicated that ERα non-nuclear signaling in males was not essential for cardioprotection provided by cGMP-PDE5 signaling.
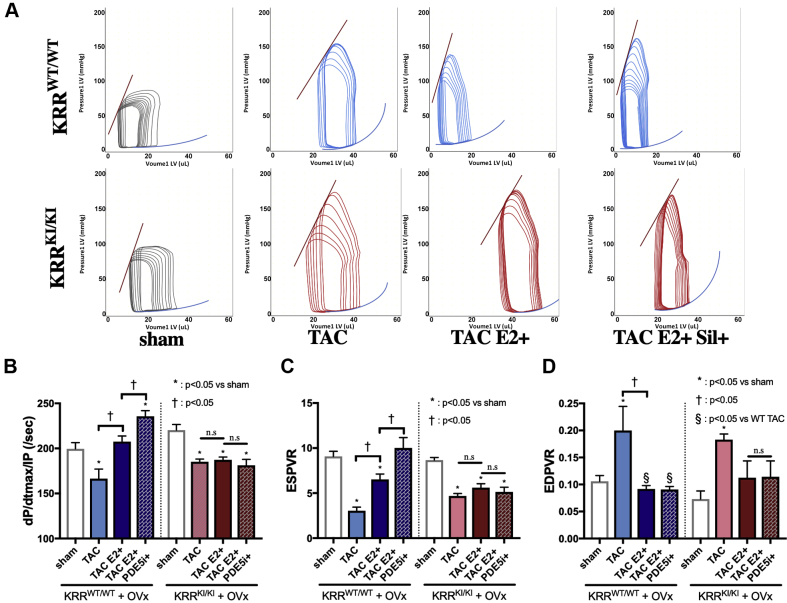
Cardiac functional assessment by pressure−volume loop analysis
We performed invasive pressure−volume loop analysis to assess LV cardiac functions (Figure 2A). E2 alone ameliorated cardiac systolic function assessed with dP/dt max/IP (dP/dt max normalized to instantaneous pressure), end-systolic pressure volume relationship (ESPVR), and ejection fraction (Figures 2B and 2C, Supplemental Figure 6A), as well as diastolic function assessed with end-diastolic pressure volume relationship (EDPVR) (Figure 2D) in KRRWT/WT. Concomitant PDE5i treatment provided further improvement in systolic function (Figures 2B and 2C). In contrast, in KRRKI/KI mice, neither E2 nor PDE5i improved cardiac performance. Among all groups, heart rate was comparable (approximately 600 beats/min) (Supplemental Figure 6B), and TAC groups showed increased LV afterload (Ea: effective arterial elastance, 8 to 10 mm Hg/μl in TAC groups compared with 5 mm Hg/μl in sham groups) (Supplemental Figure 6C). In line with echocardiographic data, these physiological results supported the necessity of ERα non-nuclear signaling in heart failure remodeling and in the efficacy of PDE5i.
Figure 2.
Cardiac Functional Assessment by PV Loop Analysis
(A) Representative pressure−volume (PV) loops during pre-load reduction. (B) dP/dt max/IP (per second). (C) ESPVR. (D) EDPVR (n = 6 to 8 per group). dP/dt max/IP = dP/dt max normalized to instantaneous pressure; EDPVR = end-diastolic pressure volume relationship; ESPVR = end-systolic pressure volume relationship; WT = wild-type. other abbreviations as Figure 1.
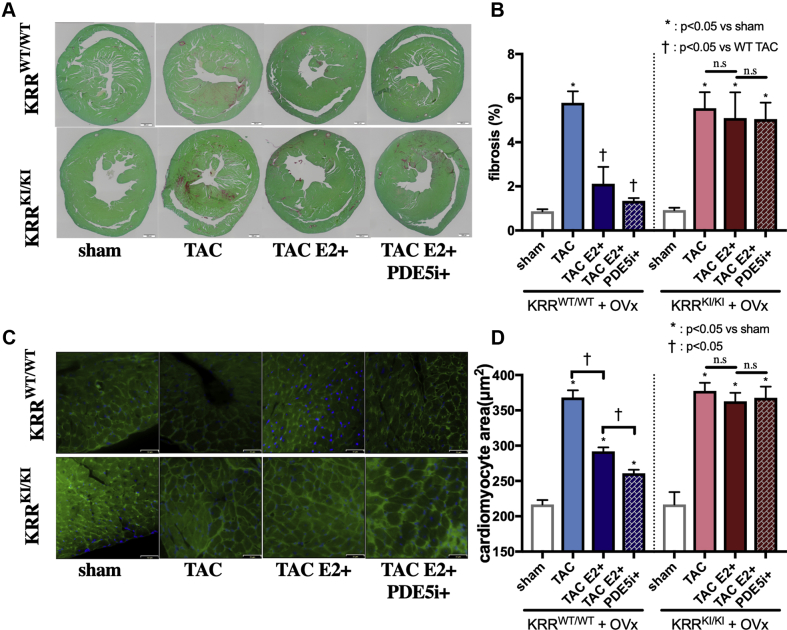
Histological assessment revealed the role of the ERα non-nuclear pathway in cardiac remodeling
We performed histological analyses to assess fibrosis and hypertrophy after TAC surgery. Picrosirius red staining revealed that TAC-induced fibrosis was significantly reduced by E2 alone or by PDE5i in the presence of E2 in KRRWT/WT hearts but was unaltered by either treatment in KRRKI/KI hearts (Figures 3A and 3B). Cell size assessment using wheat germ agglutinin (WGA) staining demonstrated E2 alone attenuated cell size increase by TAC, which was further reduced by PDE5i. Again, neither E2 nor PDE5i with E2 had an impact on cardiomyocyte size in KRRKI/KI TAC hearts (Figures 3C and 3D).
Figure 3.
Histological Analysis of PDE5i Efficacy
(A) Representative slides of Picrosirius-red staining (scale bars: 500 μm). (B) Quantification of fibrosis (%) (n = 5 to 8 per group). (C) Representative slides of heart cross-sectional area stained with WGA (green) and Hoechst (blue) (scale bars: 32 μm). (D) Quantification of cell size (μm2) (n = 5 to 8 per group). WGA = wheat germ agglutinin; other abbreviations as Figure 1.
E2 and PDE5i failed to activate myocardial PKG without the membrane-initiated ERα non-nuclear pathway
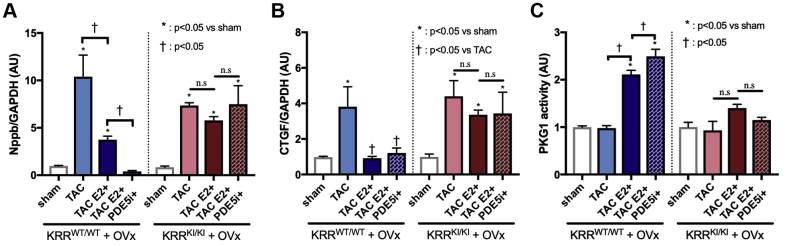
We examined molecular marker genes for hypertrophy (Nppb) and fibrosis (CTGF) in these hearts. Although TAC-induced Nppb (brain natriuretic peptide (BNP)) upregulation was attenuated by E2 alone and was further inhibited by PDE5i + E2 in KRRWT/WT hearts, Nppb induction was not inhibited by either in KRRKI/KI hearts (Figure 4A). The CTGF induction by TAC was also inhibited by E2 or PDE5i + E2 in KRRWT/WT hearts. Interestingly, in KRRKI/KI hearts, CTGF induction by TAC was significantly attenuated by E2 alone, with no additional effects from PDE5i (Figure 4B), which suggested that estrogen’s genomic effects might contribute to the regulation of CTGF.
Figure 4.
Molecular Marker Genes and PKG1 Activity
Myocardial fetal gene expressions of (A) Nppb and (B) CTGF (n = 5 to 8 per group). (C) PKG1 activity (n = 5 to 8 per group). PKG = cyclic guanosine monophosphate (cGMP)−dependent protein kinase G. Abbreviations as Figure 1.
To assess the impact of the non-nuclear signaling of estrogen signaling on the cGMP signaling pathway, we next determined myocardial PKG activity. Importantly, myocardial PKG activity remained at baseline levels with E2 or with E2 + PDE5i in KRRKI/KI hearts, whereas PKG was activated by E2 alone and further augmented by co-treatment with PDE5i in KRRWT/WT mice (Figure 4C). These results indicated that estrogen’s non-nuclear pathway via ERα critically affected myocardial PKG levels and was essential to the PKG activation elicited by PDE5i in female hearts after pressure overload.
sGC stimulation ameliorates cardiac function, regardless of E2 status
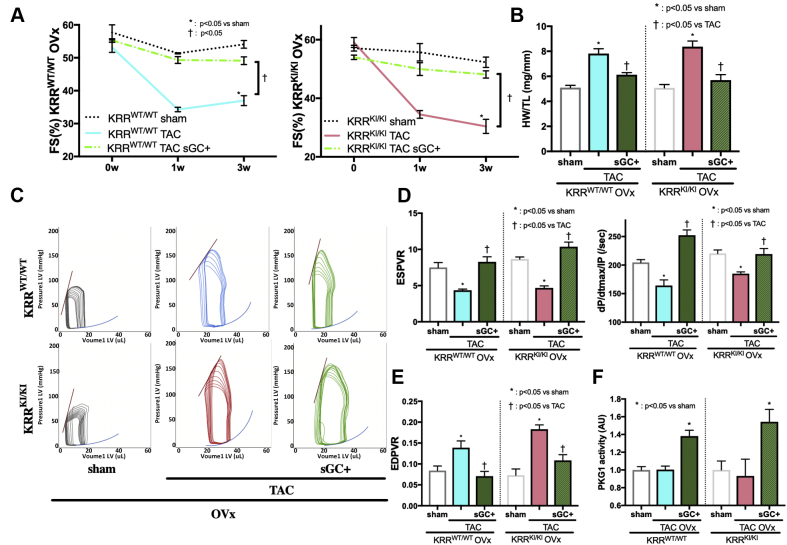
Although PDE5i blocks degradation of cGMP that is coupled to the NO-sGC pathway to activate the cGMP signaling pathway, cGMP production is enhanced independently of NO by sGC stimulators. One of them, vericiguat, is currently being tested in a Phase III clinical study. We tested if the efficacy of sGC stimulation was affected by E2 conditions using the same protocol but without E2 supplementation. The sGC stimulator potently ameliorated cardiac remodeling induced by 3-week TAC in mice that underwent ovariectomy in either genotype, as assessed by serial echocardiographic studies assessed with the percentage of fractional shortening FS (%) and Dd (mm) (Figure 5A, Supplemental Figure 7). Terminal heart weight assessment revealed a TAC-induced increase in heart weight was potently inhibited by sGC stimulation without E2 in either genotype (Figure 5B) and improved the expression profiles of Nppb and CTGF (Supplemental Figures 8A and 8B), as well as histological findings (Supplemental Figures 9A to 9D). Cardiac systolic and diastolic performance (Figures 5C to 5E, Supplemental Figure 10A) was also potently improved by sGC stimulation without altering heart rate or LV afterload (Ea) (Supplemental Figures 10B and 10C). Importantly, the anti-remodeling benefits from sGC stimulator treatment were associated with a marked increase in myocardial PKG activity (Figure 5F). Serum or myocardial levels of estrogen were unaltered by co-treatment of an sGC stimulator (serum E2 concentration mean ± SEM [pg/ml]: ovariectomized KRRWT/WT TAC and ovariectomized KRRWT/WT TAC + sGC treatment 12.4 ± 4.3 vs. 16.5 ± 7.6; p = 0.989, ovariectomized KRRKI/KI TAC vs. ovariectomized KRRKI/KI TAC + sGC treatment 10.0 ± 3.2 vs. 15.1 ± 5.2; p = 0.974).
Figure 5.
Efficacy of sGC Stimulation in Estrogen-Deprived Models
Effect of soluble guanylate cyclase (sGC) stimulation on (A) percentage of FS time course and (B) HW/TL (mg/mm) at 3 weeks (n = 5 to 8 per group). (C) Representative PV loops during pre-load reduction. (D) ESPVR, dP/dt max/IP (per second) and (E) EDPVR (n = 6 to 8 per group). (F) PKG1 activity after sGC stimulator treatment (n = 6 to 8 per group). Abbreviations as in Figures 1, 2, and 3.
Metabolic status was unaffected with cGMP signaling pathway stimulation in KRRWT/WT and KRRKI/KI mice
Because of the reported metabolic phenotype of estrogen’s non-nuclear signaling, we assessed body weight after TAC (Supplemental Table 1), daytime and night VO2 (ml/h/kg), and locomotor activities (counts/min) to check whether cGMP pathway stimulation by sGC stimulator affected metabolic status.
KRRKI/KI and KRRWT/WT animals started to show body weight differences at around 8 weeks of age, when reaching sexual maturity, and the difference became more evident with age (35). In the present study, we used 8- to 10-week old animals to induce pressure overload when we observed an approximate10% nonsignificant borderline increase in body weight in KRRKI/KI mice compared with KRRWT/WT mice (Supplemental Table 1). KRRKI/KI mice showed lower locomotor activities at night and lower VO2 at daytime than KRRWT/WT mice at baseline. In both genotypes, TAC groups showed significantly lower VO2 and locomotor activities, but sGC stimulation did not change either parameter despite the improvement of heart failure phenotype (Supplemental Figures 11 and 12). These results suggested that enhancing cGMP pathways with a sGC stimulator improved cardiac remodeling during pressure overload without affecting locomotor activities or VO2.
Discussion
In the present study, we used a novel genetically engineered mouse model and demonstrated that ERα non-nuclear signaling is a pivotal contributor to the protection against cardiac remodeling conferred by estrogen and is essential to the therapeutic efficacy of cGMP-PDE5i in heart failure but not to that of sGC stimulation (Supplemental Figure 13).
Extensive research on estrogen identified estrogen’s non-nuclear signaling as a significant contributor to the beneficial effects of estrogen on the cardiovascular system (44). ERα is 1 of the major ERs that mediates estrogen’s protective effects against cardiac ischemic injury or vascular injury (45,46); its endothelial non-nuclear action is coupled to the NO-cGMP signaling pathway and/or S-nitrosylation of proteins (27,32,47,48). Previous studies used non-nuclear selective modulators to target this signaling, including EDCs and structurally novel estrogens named PaPEs. Chambliss et al. (29) reported that EDCs activate eNOS via ERα and stimulate EC proliferation and migration, providing vascular benefit (29). Recently, Madak-Erdogan et al. (49) developed PaPEs, which interacted with ERs to activate the non-nuclear pathway preferentially over the nuclear pathway and demonstrated the repair of vascular endothelium following carotid artery injury by PaPEs. Such effects on the vasculature by the non-nuclear pathway was also demonstrated by a study that used a genetically engineered mouse model that had a point mutation of the palmitoylation site of the ERα (C451A-ERα), and therefore, lacked non-nuclear signaling (26). Importantly, a recent work by Guivarc’h et al. (31) used PaPEs and C451A-ERα mice and demonstrated that chronic vasculo-protection of estrogen against hypertension or atherosclerosis was attributable to nuclear actions of estrogen rather than non-nuclear actions. These results suggested the exquisite balance of complexity for the nuclear and non-nuclear actions of estrogen, which provides cardiovascular benefits.
This was the first study to demonstrate the protective role of ERα non-nuclear signaling in cardiac function and remodeling to pathological stressors by using pathway-specific deletion animals. It was reasonable to postulate that myocardial NO-cGMP-PKG might primarily contribute to the benefits from this signaling; however, it remains to be determined which cell type is the key to the estrogen-elicited NO-cGMP-PKG in the heart. Our previous work demonstrated that short-term E2 application to isolated cardiomyocytes induced a cGMP increase in cardiac myocytes (10). In the present study, we found that estrogen-stimulated NOS activity was absent in cardiomyocytes of KRRKI/KI mutants. These findings supported the potential role of cardiomyocyte non-nuclear ERα signaling in this regulation. In contrast, a work by Menazza et al. (47) supported the role of endothelial ERα non-nuclear function. Using tissue-specific ERα deletion models, they observed that EDCs ameliorated ischemia-reperfusion injury in cardiac-specific, but not in endothelial-specific, deletion models, which could involve protein S-nitrosylation. It was also possible that ERα non-nuclear signaling of other diverse cell types might be involved in the observed cardiac benefits other than cardiac myocytes and ECs. Our previous work showed that KRRKI/KI mutants gained more body weight and were metabolically deranged when growing old, via a brain-mediated mechanism (35). Lu et al. (32) showed that ERα non-nuclear signaling regulated various genes involved in cell migration, proliferation, and regulation of inflammatory cells (32). All these cell types could have significant impact on cardiac remodeling.
We previously demonstrated that the therapeutic efficacy of PDE5i is estrogen-dependent in female hearts (10). The present study further unraveled the specific role of ERα non-nuclear signaling in this regulation. PDE5i-elicited myocardial PKG activity in females required intact ERα non-nuclear signaling. Importantly, our data revealed myocardial PKG activity was increased by estrogen alone in an ERα non-nuclear pathway-dependent fashion that was associated with anti-remodeling benefits. These results suggested that cGMP-PKG coupled to ERα non-nuclear signaling might play a role in the cardiac benefits from estrogen in female hearts. In contrast, KRRKI/KI male hearts showed similar LV remodeling to KRRWT/WT male hearts. More importantly, the cardioprotective effects of PDE5i were not abrogated in KRRKI/KI male hearts. These results suggested that ERα non-nuclear signaling hearts might not play a significant role in LV remodeling in males and was dispensable for the anti-remodeling efficacy of PDE5i, in sharp contrast to female hearts. The mechanism for this difference between males and females remains an open question and warrants future studies. However, we speculate that androgens might function in male hearts, as do estrogens in female hearts. For example, a previous study by Sieveking et al. (50) revealed that androgens played an important role in angiogenesis under ischemic stress of hind limbs in male mice but not in female mice. Yoshida et al. (51) reported that androgen signaling activated the Akt/eNOS signaling pathway.
As a translational insight, our data could partially explain the negative results of clinical studies of PDE5i, in which significant numbers of older adult women were enrolled. It was likely that impaired cGMP production in low-estrogen conditions in older adult women might have critically limited the therapeutic efficacy of PDE5i. Because estrogen’s non-nuclear signaling pathway does not reportedly induce endometrial carcinoma cell growth or proliferation of breast cancer cells (29), a pathway-selective future hormone therapy could make PDE5i an attractive treatment option for heart failure in women. Other PDE families could also work for PKG activation with non-nuclear signaling. Inhibition of PDE9 was also demonstrated as one of the key regulators for cGMP signaling, independent of NOS activity (52). Activating estrogen non-nuclear signaling and PDE9 inhibition might work simultaneously with additive PKG activation in female hearts.
Thus far, the effects of the sGC stimulator were mainly examined in male animal models. In the present study, we tested the efficacy of sGC stimulation for the first time in female animals and found that sGC stimulation potently ameliorated female ovariectomized heart failure without estrogen supplementation, in sharp contrast to PDE5i. The difference was attributable to their NO dependency for cGMP elevating function, wherein sGC stimulation did not require NO to activate cGMP-PKG. In a SOCRATES-REDUCED (Phase IIb Safety and Efficacy Study of Four Dose Regimens of BAY1021189 in Patients With Heart Failure With Reduced Ejection Fraction Suffering From Worsening Chronic Heart Failure) Phase II study, a sGC stimulator (vericiguat) was administered to 456 patients with heart failure reduced ejection fraction (<45%) for 12 weeks, and the promising results were obtained with high-dose groups, including better composite endpoints and improved N-terminal pro–B-type natriuretic peptide (53). Currently, the international Phase III VICTORIA (Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction) trial is ongoing to test the efficacy of high doses (11). Our findings had important clinical implications, indicating that the sGC stimulator provided potent cardioprotective benefits in post-menopausal patients.
Study limitations
First, direct enzyme immunoassay did not provide accurate measurement, particularly at the lower concentration range; therefore, it might not be reliable for measuring estrogen levels in mice, considering the lower levels of estrogen in this species versus humans (54). The limited specificity of enzyme immunoassays also might be due to the nonspecific binding of C-reactive protein factors and another “ill-defined matrix” (55). However, enzyme immunoassays have been used in many rodent studies, including the recent one by Iorga et al. (39), due to its simple straight-forwardness. However, an ideal method is mass spectrometry, which requires a larger amount of specimens and a group of experts devoted to measuring estrogen levels. The numbers we obtained were in line with previous publications that used enzyme immunoassays. Most importantly, our conclusions were not affected by these measurement results by design because all the animals underwent ovariectomy and were supplemented with or without exogenous E2.
Second, we did not examine whether all the nuclear signaling pathways were intact in KRRKI/KI mice. Because of uterus morphology and its response to estrogen, as well as several estrogen response element (ERE)-containing gene inductions, were preserved, genomic actions were reasonably preserved. However, we could not completely exclude the possibilities of some interference on other genomic actions.
Third, cell type−specific analyses remain to be assessed because we studied a whole body knocked-in mouse model. Because ERα non-nuclear signaling might be different in ECs, cardiomyocytes, and other cell types, future studies using cell type−specific mouse models should clarify the key cell types to this regulation. Because the estrogen signal network is complicated, disruption of ERα non-nuclear signals might affect other ER signaling or genomic pathways. In this context, ERα non-nuclear signaling might cooperate with ERβ because of the protective role of ERβ in cardiomyocytes under pathological stress (56). Thus far, estrogen-signaling interactions between subtypes or genomic and non-nuclear signals remain to be clarified.
Conclusions
The ERα non-nuclear pathway plays a pivotal role in the cardioprotective mechanism by estrogen and is indispensable to the therapeutic efficacy of cGMP-PDE5 inhibition. In contrast, sGC stimulation potently ameliorates heart failure remodeling, regardless of estrogen status.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Estrogen exerts physiological effects via genomic and non-genomic pathways. The non-nuclear pathway of ER α plays a pivotal role in heart failure remodeling. An intact non-nuclear pathway is required for the therapeutic efficacy of cGMP-PDE5 inhibition in females, whereas estrogen status does not affect the efficacy of cGMP-sGC stimulation.
TRANSLATIONAL OUTLOOK: The study might provide a potential explanation for the negative results of PDE5 inhibition in heart failure and might also provide a molecular basis for the advantage of using a sGC simulator as a new therapeutic option in post-menopausal women. This study also highlighted the need for a female-specific therapeutic strategy.
Footnotes
This work was supported by the National Institutes of Health (grant HL-093432), the American Heart Association (grant-in-aid 11GRNT7700071), the SENSHIN Medical Research Foundation; and the TAKEDA Science Foundation. Dr. Takimoto was supported by a Grant-in-Aid for Scientific Research (25893044). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
For supplemental figures, please see the online version of this paper.
Contributor Information
Eiki Takimoto, Email: etakimoto-tky@umin.ac.jp.
Issei Komuro, Email: komuro-tky@umin.ac.jp.
Appendix
References
- 1.Roger V.L. Epidemiology of heart failure. Circ Res. 2013;113:646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takimoto E., Champion H.C., Belardi D. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res. 2005;96:100–109. doi: 10.1161/01.RES.0000152262.22968.72. [DOI] [PubMed] [Google Scholar]
- 3.Fisher P.W., Salloum F., Das A., Hyder H., Kukreja R.C. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 4.Salloum F.N., Chau V.Q., Hoke N.N. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase G-dependent generation of hydrogen sulfide. Circulation. 2009;120:S31–S36. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim K.H., Kim Y.J., Ohn J.H. Long-term effects of sildenafil in a rat model of chronic mitral regurgitation: benefits of ventricular remodeling and exercise capacity. Circulation. 2012;125:1390–1401. doi: 10.1161/CIRCULATIONAHA.111.065300. [DOI] [PubMed] [Google Scholar]
- 6.Takimoto E., Koitabashi N., Hsu S. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest. 2009;119:408–420. doi: 10.1172/JCI35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannetta E., Isidori A.M., Galea N. Chronic inhibition of cGMP phosphodiesterase 5A improves diabetic cardiomyopathy: a randomized, controlled clinical trial using magnetic resonance imaging with myocardial tagging. Circulation. 2012;125:2323–2333. doi: 10.1161/CIRCULATIONAHA.111.063412. [DOI] [PubMed] [Google Scholar]
- 8.Hoendermis E.S., Liu L.C., Hummel Y.M. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J. 2015;36:2565–2573. doi: 10.1093/eurheartj/ehv336. [DOI] [PubMed] [Google Scholar]
- 9.Guazzi M., Vicenzi M., Arena R., Guazzi M.D. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4:8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki H., Nagayama T., Blanton R.M. PDE5 inhibitor efficacy is estrogen dependent in female heart disease. J Clin Invest. 2014;124:2464–2471. doi: 10.1172/JCI70731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong P.W., Roessig L., Patel M.J. A multicenter, randomized, double-blind, placebo-controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA trial. J Am Coll Cardiol HF. 2018;6:96–104. doi: 10.1016/j.jchf.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Regitz-Zagrosek V. Therapeutic implications of the gender-specific aspects of cardiovascular disease. Nat Rev Drug Discov. 2006;5:425–438. doi: 10.1038/nrd2032. [DOI] [PubMed] [Google Scholar]
- 13.Hsich E.M., Pina I.L. Heart failure in women: a need for prospective data. J Am Coll Cardiol. 2009;54:491–498. doi: 10.1016/j.jacc.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 14.O'Meara E., Clayton T., McEntegart M.B. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;115:3111–3120. doi: 10.1161/CIRCULATIONAHA.106.673442. [DOI] [PubMed] [Google Scholar]
- 15.Adams K.F., Jr., Sueta C.A., Gheorghiade M. Gender differences in survival in advanced heart failure. Insights from the FIRST study. Circulation. 1999;99:1816–1821. doi: 10.1161/01.cir.99.14.1816. [DOI] [PubMed] [Google Scholar]
- 16.Stampfer M., Grodstein F. Cardioprotective effect of hormone replacement therapy. Is not due to selection bias. BMJ. 1994;309:808–809. doi: 10.1136/bmj.309.6957.808b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grodstein F., Stampfer M.J., Manson J.E. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453–461. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- 18.Reis S.E., Holubkov R., Young J.B., White B.G., Cohn J.N., Feldman A.M. Estrogen is associated with improved survival in aging women with congestive heart failure: analysis of the vesnarinone studies. J Am Coll Cardiol. 2000;36:529–533. doi: 10.1016/s0735-1097(00)00738-5. [DOI] [PubMed] [Google Scholar]
- 19.Brouchet L., Krust A., Dupont S., Chambon P., Bayard F., Arnal J.F. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation. 2001;103:423–428. doi: 10.1161/01.cir.103.3.423. [DOI] [PubMed] [Google Scholar]
- 20.Arnal J.F., Lenfant F., Metivier R. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol Rev. 2017;97:1045–1087. doi: 10.1152/physrev.00024.2016. [DOI] [PubMed] [Google Scholar]
- 21.Billon-Gales A., Fontaine C., Filipe C. The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17beta-estradiol. Proc Natl Acad Sci U S A. 2009;106:2053–2058. doi: 10.1073/pnas.0808742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handgraaf S., Riant E., Fabre A. Prevention of obesity and insulin resistance by estrogens requires ERalpha activation function-2 (ERalphaAF-2), whereas ERalphaAF-1 is dispensable. Diabetes. 2013;62:4098–4108. doi: 10.2337/db13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borjesson A.E., Windahl S.H., Lagerquist M.K. Roles of transactivating functions 1 and 2 of estrogen receptor-alpha in bone. Proc Natl Acad Sci U S A. 2011;108:6288–6293. doi: 10.1073/pnas.1100454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontaine C., Abot A., Billon-Gales A. Tamoxifen elicits atheroprotection through estrogen receptor alpha AF-1 but does not accelerate reendothelialization. Am J Pathol. 2013;183:304–312. doi: 10.1016/j.ajpath.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Billon-Gales A., Krust A., Fontaine C. Activation function 2 (AF2) of estrogen receptor-alpha is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci U S A. 2011;108:13311–13316. doi: 10.1073/pnas.1105632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adlanmerini M., Solinhac R., Abot A. Mutation of the palmitoylation site of estrogen receptor alpha in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci U S A. 2014;111:E283–E290. doi: 10.1073/pnas.1322057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernelot Moens S.J., Schnitzler G.R., Nickerson M. Rapid estrogen receptor signaling is essential for the protective effects of estrogen against vascular injury. Circulation. 2012;126:1993–2004. doi: 10.1161/CIRCULATIONAHA.112.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell K.S., Haynes M.P., Sinha D., Clerisme E., Bender J.R. Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc Natl Acad Sci U S A. 2000;97:5930–5935. doi: 10.1073/pnas.97.11.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chambliss K.L., Wu Q., Oltmann S. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendelsohn M.E., Karas R.H. Rapid progress for non-nuclear estrogen receptor signaling. J Clin Invest. 2010;120:2277–2279. doi: 10.1172/JCI43756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guivarc'h E., Buscato M., Guihot A.L. Predominant role of nuclear versus membrane estrogen receptor alpha in arterial protection: implications for estrogen receptor alpha modulation in cardiovascular prevention/safety. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Q., Schnitzler G.R., Ueda K. ER alpha rapid signaling is required for estrogen induced proliferation and migration of vascular endothelial cells. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takimoto E., Champion H.C., Li M. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 34.Wilck N., Marko L., Balogh A. Nitric oxide-sensitive guanylyl cyclase stimulation improves experimental heart failure with preserved ejection fraction. JCI Insight. 2018;3 doi: 10.1172/jci.insight.96006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueda K., Takimoto E., Lu Q. Membrane-initiated estrogen receptor signaling mediates metabolic homeostasis via central activation of protein phosphatase 2A. Diabetes. 2018;67:1524–1537. doi: 10.2337/db17-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patten R.D., Pourati I., Aronovitz M.J. 17 Beta-estradiol differentially affects left ventricular and cardiomyocyte hypertrophy following myocardial infarction and pressure overload. J Card Fail. 2008;14:245–253. doi: 10.1016/j.cardfail.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donaldson C., Eder S., Baker C. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ Res. 2009;104:265–275. doi: 10.1161/CIRCRESAHA.108.190397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koitabashi N., Danner T., Zaiman A.L. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–2312. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iorga A., Li J., Sharma S. Rescue of pressure overload-induced heart failure by estrogen therapy. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagayama T., Hsu S., Zhang M. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol. 2009;53:207–215. doi: 10.1016/j.jacc.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oike Y., Akao M., Yasunaga K. Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat Med. 2005;11:400–408. doi: 10.1038/nm1214. [DOI] [PubMed] [Google Scholar]
- 42.Bourdeau V., Deschenes J., Metivier R. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol. 2004;18:1411–1427. doi: 10.1210/me.2003-0441. [DOI] [PubMed] [Google Scholar]
- 43.Pedram A., Razandi M., Lubahn D., Liu J., Vannan M., Levin E.R. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-beta to inhibit calcineurin. Endocrinology. 2008;149:3361–3369. doi: 10.1210/en.2008-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall J.M., Couse J.F., Korach K.S. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 45.Menazza S., Murphy E. The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ Res. 2016;118:994–1007. doi: 10.1161/CIRCRESAHA.115.305376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahmoodzadeh S., Leber J., Zhang X. Cardiomyocyte-specific estrogen receptor alpha increases angiogenesis, lymphangiogenesis and reduces fibrosis in the female mouse heart post-myocardial infarction. J Cell Sci Ther. 2014;5:153. doi: 10.4172/2157-7013.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menazza S., Sun J., Appachi S. Non-nuclear estrogen receptor alpha activation in endothelium reduces cardiac ischemia-reperfusion injury in mice. J Mol Cell Cardiol. 2017;107:41–51. doi: 10.1016/j.yjmcc.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morales D.E., McGowan K.A., Grant D.S. Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation. 1995;91:755–763. doi: 10.1161/01.cir.91.3.755. [DOI] [PubMed] [Google Scholar]
- 49.Madak-Erdogan Z., Kim S.H., Gong P. Design of pathway preferential estrogens that provide beneficial metabolic and vascular effects without stimulating reproductive tissues. Sci Signal. 2016;9:ra53. doi: 10.1126/scisignal.aad8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sieveking D.P., Lim P., Chow R.W. A sex-specific role for androgens in angiogenesis. J Exp Med. 2010;207:345–352. doi: 10.1084/jem.20091924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida S., Aihara K., Ikeda Y. Androgen receptor promotes sex-independent angiogenesis in response to ischemia and is required for activation of vascular endothelial growth factor receptor signaling. Circulation. 2013;128:60–71. doi: 10.1161/CIRCULATIONAHA.113.001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee D.I., Zhu G., Sasaki T. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature. 2015;519:472–476. doi: 10.1038/nature14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gheorghiade M., Greene S.J., Butler J. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: The SOCRATES-REDUCED Randomized Trial. JAMA. 2015;314:2251–2262. doi: 10.1001/jama.2015.15734. [DOI] [PubMed] [Google Scholar]
- 54.Nilsson M.E., Vandenput L., Tivesten A. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology. 2015;156:2492–2502. doi: 10.1210/en.2014-1890. [DOI] [PubMed] [Google Scholar]
- 55.Lee J.S., Ettinger B., Stanczyk F.Z. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab. 2006;91:3791–3797. doi: 10.1210/jc.2005-2378. [DOI] [PubMed] [Google Scholar]
- 56.Pedram A., Razandi M., Narayanan R., Dalton J.T., McKinsey T.A., Levin E.R. Estrogen regulates histone deacetylases to prevent cardiac hypertrophy. Mol Biol Cell. 2013;24:3805–3818. doi: 10.1091/mbc.E13-08-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.