Highlights
-
•
Intravenous iron supplementation provides symptomatic relief in patients with heart failure and concomitant iron deficiency.
-
•
The current definition of iron deficiency based on ferritin <100 ng/ml or transferrin saturation <20% may not accurately reflect the levels of iron within tissue and cells. Therefore, intravenous iron may be administered to heart failure patients who do not require iron supplementation.
-
•
Intravenous administration of iron bypasses essential regulatory mechanisms and can cause endothelial damage via the production of reactive oxygen species.
-
•
Although iron should be given to patients with heart failure and iron deficiency, sufficient consideration should be given to the route of administration and the potential for adverse effects, especially in non–iron deficient patients.
-
•
Further research must be conducted to determine whether changes in the cellular and subcellular distribution of iron in patients with heart failure are compensatory and beneficial or maladaptive and potentiates disease.
Key Words: heart failure, intravenous iron, iron chelation, iron deficiency
Abbreviations and Acronyms: 6MWT, 6-min walk test; CKD, chronic kidney disease; DMT1, divalent metal transporter 1 protein; FCM, ferric carboxymaltose; FGF, fibroblast growth factor; Fpn1, ferroportin 1; Hb, hemoglobin; ID, iron deficiency; I/R, ischemia/reperfusion; IV, intravenous; LVEF, left ventricular ejection fraction; NTBI, non–transferrin-bound iron; NYHA, New York Heart Association; PGA, Patient Global Assessment; RCT, randomized clinical trial; ROS, reactive oxygen species; sTfR, soluble transferrin receptor; TfR1, transferrin receptor protein 1; TSAT, transferrin saturation; VO2, peak oxygen uptake
Summary
To date, 3 clinical trials have shown symptomatic benefit from the use of intravenous (IV) iron in patients with heart failure (HF) with low serum iron. This has led to recommendations in support of the use of IV iron in this population. However, the systemic and cellular mechanisms of iron homeostasis in cardiomyocyte health and disease are distinct, complex, and poorly understood. Iron metabolism in HF appears dysregulated, but it is still unclear whether the changes are maladaptive and pathologic or compensatory and protective for the cardiomyocytes. The serum markers of iron deficiency in HF do not accurately reflect cellular and mitochondrial iron levels, and the current definition based on the ferritin and transferrin saturation values is broad and inclusive of patients who do not need IV iron. This is particularly relevant in view of the potential risks that are associated with the use of IV iron. Reliable markers of cellular iron status may differentiate subgroups of HF patients who would benefit from cellular and mitochondrial iron chelation rather than IV iron.
Iron is an essential micronutrient for a myriad of human biological processes. The average human body contains about 3.5 to 4.5 g of iron, the majority of which is intracellular and is either bound to hemoglobin in red blood cells (∼ 60%) or stored in hepatocytes and macrophages within the liver and spleen (25%), where it is bound to a specialized cytoplasmic protein called ferritin. All human cells also contain a proportion of iron that is incorporated into iron-sulfur (Fe/S) clusters or stored inside mitochondria. The extracellular iron constitutes only about 0.1% of the total body iron and is mainly bound to the iron transport protein in the serum named transferrin (Tf).
Although iron is one of the most abundant elements on earth, the dominant form of iron in the oxygen-rich environment is the oxidized form (Fe+3), which has low solubility and limited bioavailability. Consequently, the human body has evolved to strictly conserve and effectively recycle most of its iron. Moreover, excess iron can be detrimental to human cells through the production of reactive oxygen species (ROS). Thus, sophisticated regulatory mechanisms have evolved in humans to predominantly: 1) control the influx of iron from intestinal epithelial cells and the release of iron from macrophages and hepatocytes in order to maintain a steady-state serum iron concertation at the systemic level; and 2) control cellular iron uptake to maintain a safe and sufficient level of iron at the cellular level. Understanding these regulatory mechanisms is particularly relevant because of the recently more lenient view regarding the use of intravenous (IV) iron in patients with heart failure (HF). Introducing large concentrations of elemental iron into human vessels is nonphysiologic and bypasses systemic as well as cellular iron homeostatic mechanisms.
Systemic Iron Regulation
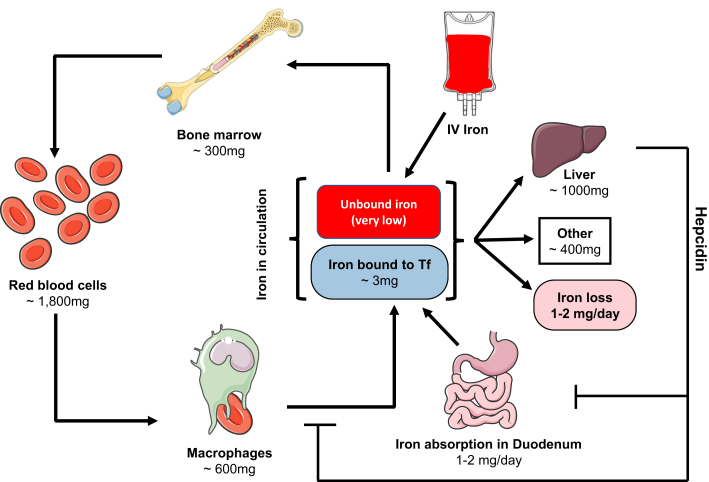
Serum contains 2 to 4 mg nonheme iron that is mainly in the form of transferrin-bound iron. Each day, serum iron turns over several times, and about 20 to 25 mg iron moves through the serum (1). A large part of this iron involves normal recycling of aged erythrocytes. Scavenger macrophages phagocytize senescent and damaged red cells and release their iron into the serum. The released iron is mainly used for the production of new red cells in the bone marrow. Dietary iron is absorbed in the duodenum and proximal jejunum. On average, 1 to 2 mg iron is absorbed every day by the enterocytes and transferred into the serum. Under normal conditions, this amount completely compensates for the daily loss of iron from the body, which is about 1 to 2 mg and is mainly from desquamation of the epithelial cells (2). The intestinal absorption of the iron can increase up to 10-fold in situations of increased demand (3). Because iron loss from the body is not regulated and can only be achieved through desquamation or bleeding, the critical focus of systemic iron regulation is on iron influx into the circulation that involves iron absorption from the enterocytes and iron release from hepatocytes and macrophages. A graphic depiction of systemic iron regulation is presented in Figure 1.
Figure 1.
Systemic Iron Regulation
Dietary iron is absorbed in the duodenum and transported across the epithelia where it is delivered to Transferrin (Tf). The majority of iron is then either delivered to the bone marrow where it is used in hematopoiesis, or it is stored in the liver. Hepcidin is a hormone released by the liver that prevents iron absorption and release from macrophages when iron stores reach sufficient levels. Although iron absorbed by the gut and bound to Tf is mostly redox inactive, iron administered intravenously enters systemic circulation as unbound iron and can be toxic to cells and tissues through the production of reactive oxygen species.
Systemic iron homeostasis is predominantly regulated by the hormone hepcidin and the protein ferroportin 1 (Fpn1), the latter being the only known iron export protein in mammals. The efflux of iron from enterocytes, macrophages, and hepatocytes into the extracellular fluid and, ultimately, into the serum occurs via Fpn1. Hepcidin, which is produced in the liver, binds to Fpn1 and triggers its internalization and lysosomal degradation. Therefore, the ultimate action of hepcidin is to down-regulate the export of iron from enterocytes, hepatocytes, and macrophages into the circulation (Figure 1).
The production of hepcidin is transcriptionally up-regulated in response to an elevated concentration of serum iron, increased tissue iron stores, and in chronic inflammatory states (1). Ganz et al. (4) demonstrated that even a mild transient increase in the serum iron level after the administration of a dose of oral iron is sufficient to dramatically raise serum hepcidin level within 8 h. In chronic inflammatory states, the increased production of hepcidin diminishes iron release from enterocytes and induces iron trapping within macrophages and hepatocytes, a condition of iron restriction that is the hallmark of the anemia of chronic disease. Furthermore, patients with chronic kidney disease (CKD) have elevated levels of hepcidin, which may be associated with increased atherosclerosis and cardiovascular risk in this population, possibly through intracellular iron sequestration and oxidative stress (5, 6, 7, 8). Similarly, obesity has been associated with increased hepcidin levels (9), and the mechanism appears to be through the leptin-mediated increase in the production of hepcidin (10).
Conversely, in conditions of enhanced erythropoiesis or iron deficiency (ID), the production of hepcidin is down-regulated, allowing more iron to be released into the serum from intestinal epithelial cells and from iron stores within macrophages and hepatocytes. In response to endogenous or exogenous erythropoietin (EPO), erythroid precursor cells produce the hormone erythroferrone (ERFE). The main effect of ERFE is the suppression of hepcidin production in the liver to maintain adequate supply of iron to the bone marrow to sustain normal erythropoiesis, which is the most iron-consuming process in the body.
The extracellular iron level is sensed by the hepatocytes and can regulate hepcidin production. After absorption from the intestine and release into the blood through Fpn1, iron binds to Tf. Human cell membrane contains Tf receptors (TfR) that bind to Tf. The Tf–TfR complex is then internalized, iron is released into the cell, and Tf is recycled back into the serum (3). Transferrin receptor protein 1 (TfR1) plays a crucial role in iron uptake in human cells. Transferrin receptor protein 2, which is mostly found in the liver, is a sensor of the extracellular iron level and is thought to play a role in regulating hepcidin production (1,11).
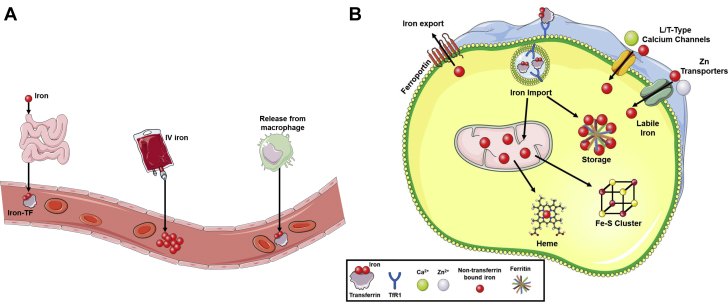
There are 2 main strategies for increasing iron in iron-deficient patients. The first strategy relies on oral iron supplements that can be absorbed naturally in the duodenum and proximal jejunum, a process that is significantly facilitated by the acidic environment of the stomach. The low levels of hepcidin that occur during ID allow the iron to exit the enterocytes and enter the circulation. As serum iron levels are restored to normal, the production of hepcidin in the liver is increased, and further release of iron from the enterocytes is prevented. Because of this feedback mechanism, oral iron supplements are generally safe and rarely, if ever, cause systemic iron overload. The second strategy for iron repletion is via direct IV delivery (Figures 1 and 2A). A benefit of this method is that it allows for rapid and significant correction of iron indexes, particularly in instances in which absorption is compromised (e.g., in patients on proton pump inhibitors). However, this method of administration bypasses the aforementioned regulatory mechanisms and can potentially cause local iron overload in endothelial cells and cardiomyocytes via non–Tf-mediated iron uptake (Figure 2B). This will be discussed in further detail later.
Figure 2.
Cellular Iron Regulation and Systemic Transport
(A) A graphic depicting different forms of iron transport through blood vessels. Under normal physiologic conditions, iron is absorbed from the small intestine or recycled through macrophages and released as Tf-bound iron. However, clinical use of intravenous (IV) iron in patients with iron deficiency introduces a large bolus of non–transferrin-bound iron (NTBI) into the vessel. Although the IV iron can be in colloids based on small spheroidal iron-carbohydrate particles, NTBI is redox active and can form reactive oxygen species, damaging endothelial cells. (B) A graphic depicting the import and fate of cellular iron. The uptake of transferrin (Tf)-bound iron is mediated through binding to transferrin receptor 1 (TfR1) and subsequent internalization by endocytosis. The acidic environment of the lysosome liberates iron from the Tf-TfR1 complex, and iron is transported into the cytosol, whereas the Tf-TfR1 complex is recycled to the cell surface. Upon entry into the cytosol, the majority of iron is bound by the storage molecule ferritin, and a small amount remains as labile iron. Non–Tf-mediated iron uptake can also occur through L/T-type calcium channels and zinc transporters. Once in the cell, iron can be transported into the mitochondria for the synthesis of heme or Fe/S clusters. The only mechanism capable of removing iron from the cell is via export through ferroportin.
Cellular Iron Regulation in the Heart
Cardiomyocytes, like other cell types, have only one pathway for iron export and that is through Fpn1. However, there are several ways that iron can enter cardiomyocytes (described later and depicted in Figure 2B). This makes cardiomyocytes particularly vulnerable to iron overload. Mice with cardiomyocyte-specific deletion of Fpn1 develop a rapid and ultimately fatal dilated cardiomyopathy that is associated with the accumulation of iron inside the cardiomyocytes while the systemic iron level remains unaltered (12). Hepcidin is also produced in cardiomyocytes. Cardiac hepcidin has important autocrine effects and participates in the autonomous regulation of iron in cardiomyocytes that are distinct from systemic iron regulation (13). In contrast to systemic hepcidin, the level of cardiac hepcidin increases in iron deficiency to preserve the cellular iron.
Iron uptake by cardiomyocytes primarily occurs through TfR1. Only Tf-bound iron can enter cardiomyocytes via this pathway. Mice lacking TfR1 in the heart die early from a cardiomyopathy that is associated with cardiac ID (14). Another important pathway for the influx of iron into the cardiomyocytes is through the divalent metal transporter 1 protein (DMT1), which mediates the import of non–transferrin-bound iron (NTBI). Inside the cell, iron can either be stored as ferritin, where it is redox inert; or go through biosynthetic pathways to generate heme or Fe/S clusters; or remain as labile iron (Figure 2B). Under normal conditions, the level of labile iron is kept very low to prevent ROS formation. However, pathological states of iron overload can dramatically increase the labile iron pool.
Cellular iron homeostasis is maintained by iron regulatory proteins (IRPs). When the cellular iron concentration is low, IRPs stabilize the messenger RNA of TfR1 and DMT1 to promote iron influx; at the same time, they inhibit messenger RNA translation of Fpn1 and ferritin to inhibit iron efflux and iron storage, respectively (3,15). Cardiac TfR1 and DMT1 are not regulated by systemic iron (3). In addition to the TfR1- and DMT1-mediated pathways, cardiomyocytes carry L-type and T-type calcium channels as well as zinc transporters, all of which are capable of transporting NTBI into the cardiac cells (3,16, 17, 18). Although the cellular regulatory mechanisms of iron in the heart can modify the import of Tf-bound iron via TfR1 and control the entry of NTBI via DMT1, the influx of NTBI into the cardiomyocytes through calcium channels and zinc transporters are not regulated by these cellular regulatory mechanisms.
Dysregulation of Iron Metabolism in Heart Disease: Excess or Deficiency?
Some studies have shown that patients with HF are “iron deficient” (19, 20, 21, 22), and the current society guidelines recommend iron supplementation in HF patients with reduced ejection fraction for symptomatic benefit, regardless of the presence or absence of anemia (Table 1). However, the etiology of ID in HF and, more importantly, the best method to diagnose this condition in HF are matters of controversy. The current proposed criteria to diagnose ID in HF are serum ferritin <100 ng/ml or serum transferrin saturation (TSAT) <20% (23, 24, 25, 26). This definition was originally used in patients with CKD (27,28). Because clinical trials of IV iron in HF used the same definition and were associated with a symptomatic benefit, subsequent studies and the current HF guidelines adopted these cutoffs to define ID in HF. However, as discussed later, the differential mechanisms regulating systemic and cellular iron and the unanswered questions in the pathophysiology underlying abnormal myocardial iron in HF challenge the accuracy of these simple serum marker cutoffs to diagnose ID in HF.
Table 1.
Guideline Recommendations for Diagnosis and Treatment of ID in HF
| Iron Deficiency in HF | 2017 ACC/AHA/HFSA Focused Update of the U.S. Guideline for Management of HF | 2016 ESC Guidelines for Diagnosis and Treatment of Acute and Chronic HF |
|---|---|---|
| Diagnosis | Ferritin <100 ng/ml or ferritin 100-300 ng/ml if TSAT < 20% | Ferritin <100 ng/ml or ferritin 100-300 ng/ml if TSAT <20% |
| Target HF population | NYHA functional class II and III | Symptomatic HFrEF |
| Recommendations | IV iron replacement might be reasonable to improve functional status and QOL | IV FCM should be considered in order to alleviate HF symptoms and improve exercise capacity and QOL |
| Class of recommendation | IIb | IIa |
| Level of recommendation | B (randomized) | A |
ACC = American College of Cardiology; AHA = American Heart Association; ESC = European Society of Cardiology; FCM = ferric carboxymaltose; HF = heart failure; HFrEF = left ventricular ejection fraction < 40% in ESC; HFSA = Heart Failure Society of America; ID = iron deficiency; IV = intravenous; NYHA = New York Heart Association; QOL = quality of life; TSAT = transferrin saturation.
Because HF is associated with elevated levels of inflammatory cytokines such as interleukin 1, interleukin-6, and tumor necrosis factor-α (29, 30, 31), it was originally postulated that, similar to chronic inflammatory states, HF patients have elevated levels of serum hepcidin and thus are at risk of developing functional ID. However, recent studies in chronic as well as acute HF demonstrated the opposite and showed that the serum hepcidin level is actually diminished in HF (21,32). Thus, the notion that the inflammatory state of HF leads to an elevated level of systemic hepcidin and hence functional ID (defined as the presence of adequate iron stores but insufficient iron availability) is not accurate. Additionally, several mechanisms have been proposed as potential causes of absolute ID (defined as low or absent iron staining in bone marrow) in HF. These include dietary nutritional deficiency of iron, reduced absorption due to bowel edema, reduced absorption due to the use of proton pump inhibitors, and increased iron loss in the gastrointestinal and genitourinary systems due to the use of antiplatelet and anticoagulant agents. However, there is no evidence to support or even suggest a causative association between any of these speculative mechanisms and the development of absolute ID in HF. Thus, it is not clear whether HF as a disease entity causes either functional or absolute ID, and existing evidence does not support this hypothesis.
Unlike systemic iron, cellular iron levels in myocardial tissue appear to be dysregulated in HF. Leszek et al. (33) showed reduced levels of mitochondrial iron in the explanted heart of patients with advanced HF who underwent cardiac transplantation. Interestingly, serum levels of ferritin and TSAT were not associated with myocardial iron, and the only serum marker that showed association was soluble transferrin receptor (sTfR). In a similar study, Melenovsky et al. (34) showed myocardial ID in the explanted hearts of patients with advanced HF, and this was associated with abnormal mitochondrial function. In contrast, our group has shown that mitochondrial iron and total cellular heme levels are elevated in advanced HF (35). We have also shown increased mitochondrial iron in mice after ischemia/reperfusion and in human hearts with ischemic heart disease, suggesting detrimental effects of increased cellular iron by generating ROS and oxidative damage (36). These studies do not demonstrate a cause-and-effect relationship, and more research is needed to determine whether the changes in myocardial iron in patients with HF are pathologic and maladaptive or protective and compensatory.
In a prospective study of 165 patients with a recent episode of acute HF, Jankowska et al. (21) defined ID as the concomitance of low serum hepcidin (as a marker of depleted body iron stores) and elevated sTfR (as a marker of insufficient cellular iron). In multivariable analysis, this definition was strongly predictive of all-cause mortality at 12 months. However, ID based on the definition of ferritin <100 ng/ml or TSAT <20% was not predictive of the outcomes. More importantly, according to the ferritin-TSAT definition, 65% of the patients in this study were categorized as “iron deficient.” However, ID was present in only 37% of the patients based on the hepcidin-sTfR definition, indicating the risk of misclassification of HF patients as “iron deficient” simply based on ferritin and TSAT values (21).
The validity of the ferritin-TSAT definition of ID was also tested in a group of HF patients against the diagnosis of ID on bone marrow samples (taken from the sternum at the time of coronary bypass surgery). The ferritin-TSAT definition had a positive predictive value of 66.7%. Therefore, 33% of the HF patients in this particular cohort who were considered “iron deficient” based on the ferritin-TSAT criteria had an adequate amount of iron stores in their bone marrow. In this study, TSAT <19.8% or simply a serum iron level <72 μg/dl had the best correlation with bone marrow ID (20). Thus, the definition of ID in HF based on a ferritin level <100 ng/ml or TSAT <20% appears lenient and potentially inclusive of patients without ID who do not need any form of iron supplementation and particularly not the IV form.
Iron Supplementation in HF
Over the past decade, the effects of iron supplementation on HF have been tested in several studies (Table 2). In the subsequent sections, we review the major randomized trials of IV iron in HF, the potential risks associated with IV iron, and, ultimately, the role of oral iron in patients with HF.
Table 2.
Major Published Clinical Trials of Iron Therapy in HF
| First Author, Year (Study) (Ref. #) | Design | N | Definition of ID | Inclusion | Intervention | Primary Endpoint | Follow-up | Results |
|---|---|---|---|---|---|---|---|---|
| Toblli et al., 2007 (40) |
|
40 |
|
|
Iron sucrose, 200 mg, weekly for 5 weeks |
|
6 months | ↓ NT-proBNP ↓ CRP |
| Okonko et al., 2008 (FERRIC-HF) (41) |
|
35 |
|
|
Iron sucrose, 200 mg, weekly until ferritin >500 and then monthly until 16 weeks |
|
18 weeks | ↑ VO2 increased only in pre-specified group with baseline Hb <12.5 |
| Anker et al., 2009 (FAIR-HF) (37) |
|
459 |
|
|
FCM, 200 mg, weekly for correction and monthly for maintenance |
|
24 weeks | ↓ NYHA functional class ↑ PGA ↑ 6MWT ↑ KCCQ score |
| Ponikowski et al., 2015 (CONFIRM-HF) (38) |
|
304 |
|
|
FCM at baseline and week 6 for total 500-2,000 mg and then 500 mg at weeks 12, 24, and 36 if ID present |
|
52 weeks | ↑ 6MWT distance ↓ NYHA functional class ↑ PGA ↓ Fatigue score ↑ KCCQ score |
| Van Veldhuisen et al., 2017 (EFFECT-HF) (39) |
|
174 |
|
|
FCM at weeks 0, 6, and 12 |
|
24 weeks | ↔ VO2 ↓ NYHA functional class ↑ PGA |
| Lewis et al., 2017 (IRONOUT-HF) (68) |
|
225 |
|
|
Oral iron polysaccharide, 150 mg BID |
|
16 weeks | ↔ VO2 ↔ 6MWT ↔ KCCQ |
Δ refers to change in the parameter.
6MWT = 6-min walk test; BID = twice a day; BNP = B-type natriuretic peptide; CRP = C-reactive protein; EF = ejection fraction; EFFECT-HF = Exercise Capacity in Patients With Iron Deficiency and Chronic Heart Failure; F = female; FAIR-HF = Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure; Hb = hemoglobin; IRONOUT-HF = Iron Repletion Effects on Oxygen Uptake in Heart Failure; KCCQ = Kansas City Cardiomyopathy Questionnaire; M = male; NT-proBNP = N-terminal pro–B-type natriuretic peptide; PGA = Patient Global Assessment; PVO2 = peak oxygen consumption; other abbreviations as in Table 1.
Intravenous Iron Supplementation in HF: Clinical Trials
To date, the effect of IV iron in HF patients with ID has been evaluated in 3 large randomized clinical trials (RCTs). In all 3 trials, ID was defined as either serum ferritin <100 ng/ml or TSAT <20% if the ferritin value was between 100 and 300 ng/ml.
FAIR-HF (Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure) (2009) was a double-blind, placebo-controlled RCT that enrolled 459 ambulatory HF patients in New York Heart Association (NYHA) functional class II (left ventricular ejection fraction [LVEF] ≤40%) or class III (LVEF ≤45%) (37). Eligible patients had a hemoglobin (Hb) level between 9.5 and 13.5 g/dl and were iron deficient. Participants were randomized in a 2:1 ratio to receive either IV ferric carboxymaltose (FCM) or placebo (normal saline). FCM was administered weekly as an IV bolus injection of 200 mg until iron repletion was achieved (correction phase). This was followed by a maintenance phase during which the FCM injections were continued on a monthly basis. After 24 weeks, treatment with FCM significantly improved the self-reported Patient Global Assessment (PGA) such that 50% of the participants in the treatment group reported that they were “much or moderately improved” compared with only 28% in the placebo arm. There was also significant improvement in the NYHA functional class in the treatment group. Secondary endpoints that included the 6-min walk test (6MWT) distance and health-related quality of life surveys also showed a significant improvement. Patients with anemia at baseline (Hb ≤12 g/dl) showed an increase of 0.9 g/dl in the level of Hb at week 24, but symptomatic benefits from FCM were similar in patients with and without anemia. Although the primary endpoints of PGA and NYHA class provide important patient-centric outcomes, they carry some degree of subjectivity that make them susceptible to individual interpretation and hence bias.
CONFIRM-HF (Ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic Heart Failure) (2015) was the second RCT that enrolled 304 ambulatory HF patients in NYHA functional class II and III with LVEF ≤45% and ID. Participants were randomized in a 1:1 ratio to receive either FCM or placebo (38). In the “therapy phase” of the study, participants received FCM once at baseline and another time at week 6 for a total dose of 500 to 2,000 mg elemental iron. The “maintenance phase” of the study included 3 FCM injections of 500 mg at weeks 12, 24, and 36 if ID was still present. The primary endpoint for the study was the change in the 6MWT distance from baseline to week 24. Treatment with FCM significantly improved the primary endpoint. Additionally, the use of FCM compared with placebo was associated with a significant improvement in the secondary endpoints of NYHA class, PGA score, fatigue score, and the HF quality of life measures up to 52 weeks of follow-up.
EFFECT-HF (Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Iron Deficiency and Chronic Heart Failure) (2017) was an open-label RCT that enrolled 174 HF patients with ID in NYHA functional class II and III with LVEF ≤45% (39). Participants were randomized in a 1:1 ratio to receive either IV FCM or standard of care. FCM was injected once at baseline and another time at week 6 for a total dose of 500 to 2,000 mg elemental iron. At week 12, another dose of FCM was given at a fixed dose of 500 mg if ID was still present. The primary endpoint for the study was the change in peak oxygen uptake (VO2) from baseline to week 24.
At week 24, peak VO2 decreased by 1.19 ± 0.38 ml/kg/min in the standard of care group but was virtually unchanged in the FCM group. During the course of the study, there were 4 deaths in the standard of care group (all before week 24) and zero mortality in the FCM group. The investigators assigned a value of 0 to peak VO2 at week 24 for patients who had died before this time point, which explains the significant decrease in the 24-week peak VO2 compared with baseline in this group. This limitation was acknowledged in the original paper, and the investigators concluded that without the imputation of deaths there was no significant difference between the 2 groups in the primary endpoint.
It is noteworthy that these 3 major clinical trials (FAIR-HF, CONFIRM-HF, and EFFECT-HF) were all sponsored by the same manufacturer of FCM (Ferinject/Injectafer, Vifor Pharma, Zurich, Switzerland). Additionally, in the FAIR-HF and CONFIRM-HF trials, representatives from the sponsor were involved in the design, implementation, and oversight of the trial.
The therapeutic effects of IV iron have also been studied in smaller RCTs. In a single-center study, Toblli et al. (40) randomized 40 HF patients with LVEF ≤35%, NYHA functional class II to IV, Hb ≤13.5 g/dl, and ID to receive either 5 weekly infusions of iron sucrose or placebo. The treatment group showed a significant improvement in virtually all the measured parameters including N-terminal pro–B-type natriuretic peptide and C-reactive protein (which was the primary endpoint) as well as other parameters such as LVEF, 6MWT distance, serum creatinine, and even body mass index. FERRIC-HF (Ferric Iron Sucrose in Heart Failure) randomized 35 HF patients with LVEF ≤45%, NYHA functional class II to III, and ID to receive in a 2:1 ratio either iron sucrose or no iron therapy (41). At week 18, treatment with IV iron resulted in an improvement in peak VO2 and NYHA class. However, these changes were only significant in the pre-specified subgroup of patients with anemia (Hb <12.5 g/dl).
Two meta-analyses were published in follow-up of the previously mentioned studies. The first study included subjects from FAIR-HF and CONFIRM-HF as well as the 2 previously described small RCTs (Toblli et al. [40] and FERRIC-HF [41]) (42). It also included subjects from the IRON-HF (Iron Supplementation in Heart Failure Patients With Anemia) study that enrolled only 23 anemic HF patients with a ferritin cutoff of 500 ng/ml and was a study to compare IV versus oral iron (43). This meta-analysis showed that therapy with IV iron could reduce HF hospitalization in HF patients with anemia, albeit with significant limitations associated with the analysis. The second meta-analysis included subjects from FAIR-HF and CONFIRM-HF as well as 2 small studies, PER-CARS-01 (30 patients received FCM and 15 placebo) and EFFICACY-HF (20 patients received FCM and 14 placebo) (44). This meta-analysis also showed a reduction in HF hospitalization with the use of IV iron.
The previously mentioned studies on the use of IV iron in HF collectively suggest a symptomatic benefit from the use of IV iron in HF patients with ID, as defined by the serum iron indexes. In chronic diseases such as HF, improvements in symptoms and patient-reported outcomes are important. However, unlike therapies that improve mortality and morbidity, interventions that purely improve symptoms require more convincing evidence before widely being incorporated into clinical practice. This is particularly relevant if there is potential for harm with those interventions.
Side Effects of IV Iron: Cause for Concern
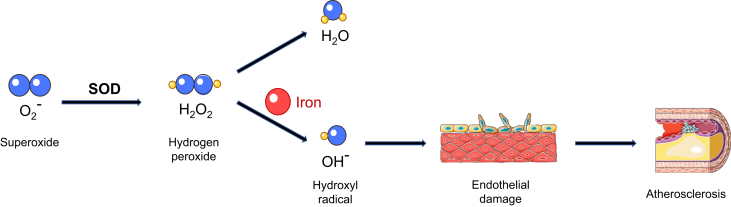
The adverse cardiovascular effects of iron are mainly related to the redox properties of elemental iron that promote the generation of ROS. Excess iron can overwhelm the iron-carrying capacity of transferrin, causing accumulation of NTBI as well as a highly reactive labile iron pool inside the cells. This nonbound iron can react with hydrogen peroxide and generate highly toxic hydroxyl radicals (Figure 3). Moreover, ferroptosis, as a nonapoptotic, iron-mediated form of cell death, has been shown to be involved in several cardiovascular processes such as ischemia/reperfusion (I/R) injury and doxorubicin-induced cardiomyopathy (45,46). Interestingly, the administration of an iron chelator in vivo was protective against I/R-induced cardiomyopathy (46)(Central Illustration).
Figure 3.
Free Iron Promotes the Formation of Reactive Oxygen Species and Atherosclerosis
A graphic depicting the stepwise production of hydroxyl radicals via the Fenton reaction. Hydroxyl radical is a strong reactive oxygen species that can damage cells and tissues by oxidizing lipid and proteins. Oxidative damage to endothelial cells lining the blood vessels can promote the formation of atherosclerotic lesions.
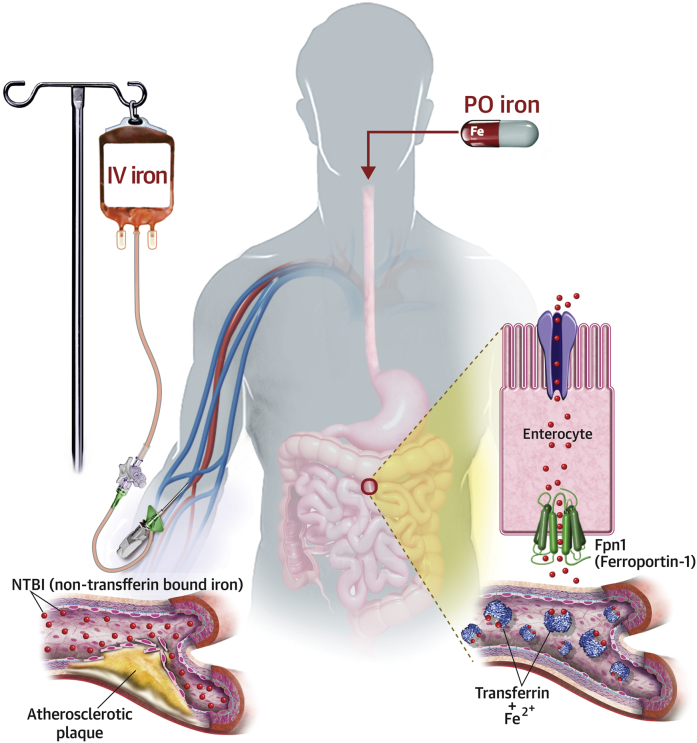
Central Illustration.
Absorption and Distribution of Iron
Iron infusion introduces large amount of non-transferrin bound iron (NTBI) into the vasculature and bypasses homeostatic mechanisms of the body that meticulously regulate influx of iron into the circulation. Accumulation of NTBI in the serum and labile iron pool (LIP) inside the cells can lead to endothelial cell damage and progression of atherosclerosis. In contrast, absorption of oral iron (PO) is tightly controlled by the function of hepcidin on ferroportin-1 (Fpn1) to minimize the reactive unbound iron pool while ensuring a sufficient supply of iron to the body.
The potential toxic effects of increased iron on endothelial cells was demonstrated in a study using an apolipoprotein E–deficient mouse model in which it was shown that the iron load was associated with the progression of atherosclerosis via inducing a proinflammatory state (47). In another study, the restriction of dietary iron in a similar mouse model resulted in significant inhibition of atherosclerosis (48). IV iron has also been shown to be associated with markers of endothelial dysfunction (49, 50, 51). In a human study, the administration of IV iron at therapeutic doses to healthy volunteers resulted in transient endothelial dysfunction and was associated with a significant rise in NTBI and a biomarker of oxidative stress (52). Conversely, treatment with iron chelators can reduce endothelial dysfunction in patients with coronary artery disease (53).
Recent studies have shown an association between the use of IV iron and elevated levels of the biologically active form of fibroblast growth factor (FGF)-23. This hormone is primarily produced by the bone cells and is involved in phosphate homeostasis. Abnormally elevated levels of FGF-23 reduce phosphate absorption in the kidney and result in hypophosphatemia, bone resorption, and ultimately osteomalacia (54). Both hypophosphatemia and osteomalacia have been reported in connection with the use of IV iron (55, 56, 57, 58). In the CKD population, enhanced levels of FGF-23 have been associated with left ventricular hypertrophy, myocardial fibrosis, all-cause mortality, and adverse cardiovascular events, independent of traditional cardiovascular risk factors (59, 60, 61, 62). Moreover, FGF-23 has been shown to be associated with incident HF in the general population (63, 64, 65).
Interestingly, it appears that FCM, which is the only recommended form of IV iron in HF by the European guidelines, has the unique capability of increasing the FGF-23 level as opposed to other forms of IV iron. In a double-blind RCT comparing 2 different forms of IV iron in the treatment of adults with ID anemia (FCM 750 mg vs. ferumoxytol 510 mg at week 0 and week 1), the incidence of severe hypophosphatemia at week 2 was significantly higher in the FCM group (50.8% vs. 0.9%; p < 0.001). This was correlated with the doubling of FGF-23 levels after each infusion of FCM, whereas the level of FGF-23 remained unchanged in the other group. At week 5, about 30% of patients in the FCM group continued to have severe hypophosphatemia (66).
These findings collectively raise concerns about the safety of the prolonged use of IV iron in the HF population. In particular, hypophosphatemia and osteomalacia were not monitored as side effects in the trials of IV iron in HF. The long-term safety of the use of IV iron in HF patients remains to be determined.
Is Oral Iron Effective in HF?
The only study to date to compare IV iron versus oral iron in HF was terminated prematurely because of competing trials and insufficient funding (43). In this study (IRON-HF), 23 anemic HF patients with LVEF <40%, TSAT <20%, and ferritin <500 ng/ml were randomized into 3 groups to receive IV iron (iron sucrose), oral iron (ferrous sulphate), or placebo. Over a 3-month follow-up period, the primary endpoint of change in peak VO2 numerically increased by 3.5 ml/kg/min in the IV group without a detectable increase with the use of oral iron. However, there was a significant increase in the serum ferritin and TSAT levels observed in both the IV and oral iron groups. The use of oral iron in another retrospective study was also associated with a significant increase in the values of serum iron, ferritin, TSAT, and Hb level (all p < 0.001) (67). These 2 studies collectively suggest that the use of oral iron can improve iron indexes. The question remains whether the improvement of iron indexes with oral iron would translate into better clinical status.
IRONOUT-HF (Iron Repletion Effects on Oxygen Uptake in Heart Failure) was a National Institutes of Health–sponsored, double-blind, placebo-controlled RCT to test the hypothesis that oral iron compared with placebo can improve exercise capacity in HF patients with ID (68). In this study, 225 HF patients with LVEF ≤40% and ID (ferritin <100 or TSAT <20%) were randomized to receive oral iron polysaccharide at 150 mg twice daily or placebo. The primary endpoint was change in peak VO2 after 16 weeks of therapy. At week 16, peak VO2 virtually remained unchanged compared with baseline in both groups. Similarly, there was no significant between-group differences in the amount of change in the 6MWT distance, N-terminal pro B-type natriuretic peptide level, or HF-related quality of life score from baseline to week 16. However, compared with placebo, oral iron increased TSAT by 3.3% (p = 0.003) and the ferritin level by 11.3 ng/ml (p = 0.06). The following points should be considered in interpreting the findings of this trial:
-
1.
In this trial, there was no direct comparison between IV and oral iron. As stated earlier, the only clinical trial to date that attempted to conduct such a comparison was terminated prematurely after enrolling 23 patients.
-
2.
The peak VO2 remained virtually unchanged after 16 weeks of therapy with oral iron. However, therapy with IV iron either has not been shown to increase peak VO2 in nonanemic HF patients.
-
3.
The best method to diagnose ID in the HF population is controversial. In the treatment group of this trial, the median ferritin, TSAT, and iron levels at baseline were 75 ng/ml, 19%, and 12.6 g/dl. One could argue that not of all the participants were iron deficient and therefore were unlikely to show any benefit from iron supplementation. Considering the size (N = 225) and duration (16 weeks) of the study, this study might have been underpowered to detect a meaningful benefit in the subgroup of participants with true ID.
-
4.
The pharmacokinetics of oral iron is drastically different from that of IV iron and despite the lack of direct comparison, it is conceivable that IV iron therapy would improve iron indexes faster than oral formulations. Thus, a duration of therapy longer than 16 weeks might have been needed in the IRONOUT-HF trial to detect any beneficial effects of oral iron therapy.
-
5.
Results of the IRONOUT-HF trial suggest that a subset of HF patients with ID who have depressed serum hepcidin may respond favorably to oral iron. Because hepcidin is the main regulator of systemic iron in humans and its level decreases in ID, hepcidin might be a more accurate biomarker of ID in HF rather than ferritin or TSAT. In this study, patients with hepcidin values <6.6 ng/ml (the lower 2 quartiles) responded favorably to oral iron.
New formulations of oral iron
One of the most innovative preparations of oral iron is sucrosomial iron, which is a preparation of ferric pyrophosphate covered by phospholipids and a sucrose matrix. Preliminary studies suggest that the absorption of this formulation is probably independent of hepcidin, and it can improve Hb similar to IV formulations with improved gastrointestinal tolerance (69, 70, 71). Ferric citrate has emerged as another effective oral formulation of iron. A randomized clinical trial in the CKD population with ID anemia showed that 16 weeks of therapy with this oral formulation was associated with significant increase in iron indexes (72).
Dosing schedule of oral iron
Emerging evidence suggests that the conventional schedule of 2 or 3 times daily of oral iron therapy could lead to a rapid and transient response in hepcidin production and result in limited absorption of the subsequent dose of oral iron (73). Intriguing findings from an RCT suggest that oral iron supplementation on alternate days and in single doses can optimize iron absorption and might be the preferred dosing regimen (74).
Iron Chelation in Cardiovascular Disease
There is no mechanism for the removal of excess body iron. In iron overload states, cardiomyocytes are uniquely susceptible to iron-mediated injury because the influx of NTBI can occur through DMT1 and abundant voltage-gated Ca2+ channels, bypassing the cellular regulatory mechanisms of iron entry into the cardiomyocytes. Thus, in iron overload cardiomyopathy, different iron chelators have been used with success to reduce the level of systemic iron, decrease myocardial iron, and improve cardiac function. Additionally, the use of calcium-channel blockers has been shown to effectively reduce myocardial iron and improve cardiac as well as mitochondrial function in iron-overload states (75,76), likely via inhibiting the influx of NTBI through cardiomyocyte Ca2+ channels. In a double-blind, placebo-controlled RCT, the addition of amlodipine to standard chelation therapy in a group of patients with thalassemia significantly reduced the myocardial iron concentration as assessed by cardiac magnetic resonance (77).
Moreover, several studies have explored the application of dietary iron restriction or iron chelators in cardiovascular disease without iron overload. Our group has demonstrated in mice that the reduction of baseline mitochondrial iron, either by the overexpression of a mitochondrial iron export protein named ATP-binding cassette transporter protein B8 (ABCB8) or the use of a cell-permeable iron chelator (2, 2’ bipyridyl), is protective against I/R injury (36). Similarly, Fang et al. (46) showed that pre-treatment with the iron chelator dexrazoxane was protective against a mouse model of I/R injury, as demonstrated by a reduction in the infarct size and the amount of myocardial fibrosis. In mice subjected to myocardial infarction, Eguchi et al. (78) found that dietary iron restriction prevented left ventricular remodeling and improved systolic function. In rats, Phaelante et al. (79) demonstrated that the iron chelator deferoxamine, in combination with the antioxidant n-acetylcysteine, also improved cardiac function after nonreperfused myocardial infarction. In another study, treatment of mouse models of diabetic cardiomyopathy with the iron chelator deferiprone was associated with a significant reduction in biomarkers of oxidative stress, inflammation, and fibrosis, suggesting a potential therapeutic effect of iron chelation in diabetic cardiomyopathy (80). In human subjects, infusion of iron chelators during coronary artery bypass graft surgery has been associated with a reduction of oxygen free-radicals, inhibition of lipid peroxidation, and, more importantly, improvement in the myocardial function up to 12 months post-surgery (81,82). Because serum markers of systemic iron, such as ferritin and TSAT, do not accurately reflect the status of iron in cardiomyocytes, reliable markers of cellular and mitochondrial iron may enable clinicians to differentiate HF patients who do not need IV iron and, on the contrary, may benefit from cellular and mitochondrial iron chelators.
Conclusions
The human body has evolved to conserve iron. It has also developed meticulous regulatory mechanisms to protect cells from the toxic effects of free iron by regulating its absorption and storage. It is still unclear if the apparent dysregulation of iron metabolism in cardiac disease is maladaptive or a regulatory effort of the body to maintain cellular iron homeostasis. While the pursuit of understanding iron metabolism in cardiac health and disease is continued, the use of IV iron should be considered cautiously and limited to patients with true iron deficiency.
Footnotes
Supported by NIH R01 HL127646-01A1, NIH R01 HL140973-01, and NIH R01 HL138982-01A1. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 2.Green R., Charlton R., Seftel H. Body iron excretion in man: a collaborative study. Am J Med. 1968;45:336–353. doi: 10.1016/0002-9343(68)90069-7. [DOI] [PubMed] [Google Scholar]
- 3.Paterek A., Mackiewicz U., Maczewski M. Iron and the heart: a paradigm shift from systemic to cardiomyocyte abnormalities. J Cell Physiol. 2019;234:21613–21629. doi: 10.1002/jcp.28820. [DOI] [PubMed] [Google Scholar]
- 4.Ganz T., Olbina G., Girelli D., Nemeth E., Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 5.Gaweda A.E. Markers of iron status in chronic kidney disease. Hemodial Int. 2017;21 Suppl 1:S21–S27. doi: 10.1111/hdi.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakanishi T., Hasuike Y., Otaki Y., Kida A., Nonoguchi H., Kuragano T. Hepcidin: another culprit for complications in patients with chronic kidney disease? Nephrol Dial Transplant. 2011;26:3092–3100. doi: 10.1093/ndt/gfr410. [DOI] [PubMed] [Google Scholar]
- 7.Kuragano T., Itoh K., Shimonaka Y. Hepcidin as well as TNF-alpha are significant predictors of arterial stiffness in patients on maintenance hemodialysis. Nephrol Dial Transplant. 2011;26:2663–2667. doi: 10.1093/ndt/gfq760. [DOI] [PubMed] [Google Scholar]
- 8.van der Weerd N.C., Grooteman M.P., Bots M.L. Hepcidin-25 is related to cardiovascular events in chronic haemodialysis patients. Nephrol Dial Transplant. 2013;28:3062–3071. doi: 10.1093/ndt/gfs488. [DOI] [PubMed] [Google Scholar]
- 9.Auguet T., Aragones G., Berlanga A. Hepcidin in morbidly obese women with non-alcoholic fatty liver disease. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto K., Kuragano T., Kimura T., Nanami M., Hasuike Y., Nakanishi T. Interplay of adipocyte and hepatocyte: leptin upregulates hepcidin. Biochem Biophys Res Commun. 2018;495:1548–1554. doi: 10.1016/j.bbrc.2017.11.103. [DOI] [PubMed] [Google Scholar]
- 11.Hentze M.W., Muckenthaler M.U., Galy B., Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Lakhal-Littleton S., Wolna M., Carr C.A. Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function. Proc Natl Acad Sci U S A. 2015;112:3164–3169. doi: 10.1073/pnas.1422373112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakhal-Littleton S., Wolna M., Chung Y.J. An essential cell-autonomous role for hepcidin in cardiac iron homeostasis. Elife. 2016;5 doi: 10.7554/eLife.19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu W., Barrientos T., Mao L., Rockman H.A., Sauve A.A., Andrews N.C. Lethal cardiomyopathy in mice lacking transferrin receptor in the heart. Cell Rep. 2015;13:533–545. doi: 10.1016/j.celrep.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao G., Arosio P., Chasteen N.D. Iron(II) and hydrogen peroxide detoxification by human H-chain ferritin. An EPR spin-trapping study. Biochemistry. 2006;45:3429–3436. doi: 10.1021/bi052443r. [DOI] [PubMed] [Google Scholar]
- 16.Lupu M., Tudor D.V., Filip G.A. Influence of mitochondrial and systemic iron levels in heart failure pathology. Heart Fail Rev. 2019;24(5):647–659. doi: 10.1007/s10741-019-09788-z. [DOI] [PubMed] [Google Scholar]
- 17.Tsushima R.G., Wickenden A.D., Bouchard R.A., Oudit G.Y., Liu P.P., Backx P.H. Modulation of iron uptake in heart by L-type Ca2+ channel modifiers: possible implications in iron overload. Circ Res. 1999;84:1302–1309. doi: 10.1161/01.res.84.11.1302. [DOI] [PubMed] [Google Scholar]
- 18.Huang B., Qin D., Deng L., Boutjdir M., El-Sherif N. Reexpression of T-type Ca2+ channel gene and current in post-infarction remodeled rat left ventricle. Cardiovasc Res. 2000;46:442–449. doi: 10.1016/s0008-6363(00)00017-1. [DOI] [PubMed] [Google Scholar]
- 19.Jankowska E.A., Rozentryt P., Witkowska A. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872–1880. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- 20.Grote Beverborg N., Klip I.T., Meijers W.C. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.117.004519. [DOI] [PubMed] [Google Scholar]
- 21.Jankowska E.A., Kasztura M., Sokolski M. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J. 2014;35:2468–2476. doi: 10.1093/eurheartj/ehu235. [DOI] [PubMed] [Google Scholar]
- 22.Klip I.T., Comin-Colet J., Voors A.A. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165:575–582.e3. doi: 10.1016/j.ahj.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Yancy C.W., Jessup M., Bozkurt B. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 25.Ezekowitz J.A., O'Meara E., McDonald M.A. 2017 comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol. 2017;33:1342–1433. doi: 10.1016/j.cjca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 26.von Haehling S., Ebner N., Evertz R., Ponikowski P., Anker S.D. Iron deficiency in heart failure: an overview. J Am Coll Cardiol HF. 2019;7:36–46. doi: 10.1016/j.jchf.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 27.IV. NKF-K/DOQI clinical practice guidelines for anemia of chronic kidney disease: update 2000. Am J Kidney Dis. 2001;37:S182–S238. doi: 10.1016/s0272-6386(01)70008-x. [DOI] [PubMed] [Google Scholar]
- 28.Wish J.B. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1 Suppl 1:S4–S8. doi: 10.2215/CJN.01490506. [DOI] [PubMed] [Google Scholar]
- 29.Mehta J.L., Pothineni N.V. Inflammation in heart failure: the Holy Grail? Hypertension. 2016;68:27–29. doi: 10.1161/HYPERTENSIONAHA.116.07307. [DOI] [PubMed] [Google Scholar]
- 30.Briasoulis A., Androulakis E., Christophides T., Tousoulis D. The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail Rev. 2016;21:169–176. doi: 10.1007/s10741-016-9533-z. [DOI] [PubMed] [Google Scholar]
- 31.Shirazi L.F., Bissett J., Romeo F., Mehta J.L. Role of inflammation in heart failure. Curr Atheroscler Rep. 2017;19:27. doi: 10.1007/s11883-017-0660-3. [DOI] [PubMed] [Google Scholar]
- 32.Jankowska E.A., von Haehling S., Anker S.D., Macdougall I.C., Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. 2013;34:816–829. doi: 10.1093/eurheartj/ehs224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leszek P., Sochanowicz B., Szperl M. Myocardial iron homeostasis in advanced chronic heart failure patients. Int J Cardiol. 2012;159:47–52. doi: 10.1016/j.ijcard.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Melenovsky V., Petrak J., Mracek T. Myocardial iron content and mitochondrial function in human heart failure: a direct tissue analysis. Eur J Heart Fail. 2017;19:522–530. doi: 10.1002/ejhf.640. [DOI] [PubMed] [Google Scholar]
- 35.Khechaduri A., Bayeva M., Chang H.C., Ardehali H. Heme levels are increased in human failing hearts. J Am Coll Cardiol. 2013;61:1884–1893. doi: 10.1016/j.jacc.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang H.C., Wu R., Shang M. Reduction in mitochondrial iron alleviates cardiac damage during injury. EMBO Mol Med. 2016;8:247–267. doi: 10.15252/emmm.201505748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anker S.D., Comin Colet J., Filippatos G. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 38.Ponikowski P., van Veldhuisen D.J., Comin-Colet J. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J. 2015;36:657–668. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Veldhuisen D.J., Ponikowski P., van der Meer P. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017;136:1374–1383. doi: 10.1161/CIRCULATIONAHA.117.027497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toblli J.E., Lombrana A., Duarte P., Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50:1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 41.Okonko D.O., Grzeslo A., Witkowski T. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51:103–112. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 42.Jankowska E.A., Tkaczyszyn M., Suchocki T. Effects of intravenous iron therapy in iron-deficient patients with systolic heart failure: a meta-analysis of randomized controlled trials. Eur J Heart Fail. 2016;18:786–795. doi: 10.1002/ejhf.473. [DOI] [PubMed] [Google Scholar]
- 43.Beck-da-Silva L., Piardi D., Soder S. IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol. 2013;168:3439–3442. doi: 10.1016/j.ijcard.2013.04.181. [DOI] [PubMed] [Google Scholar]
- 44.Anker S.D., Kirwan B.A., van Veldhuisen D.J. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail. 2018;20:125–133. doi: 10.1002/ejhf.823. [DOI] [PubMed] [Google Scholar]
- 45.Stamenkovic A., Pierce G.N., Ravandi A. Phospholipid oxidation products in ferroptotic myocardial cell death. Am J Physiol Heart Circ Physiol. 2019;317:H156–H163. doi: 10.1152/ajpheart.00076.2019. [DOI] [PubMed] [Google Scholar]
- 46.Fang X., Wang H., Han D. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu X., Cai X., Ma R., Fu W., Zhang C., Du X. Iron-load exacerbates the severity of atherosclerosis via inducing inflammation and enhancing the glycolysis in macrophages. J Cell Physiol. 2019;234:18792–18800. doi: 10.1002/jcp.28518. [DOI] [PubMed] [Google Scholar]
- 48.Lee T.S., Shiao M.S., Pan C.C., Chau L.Y. Iron-deficient diet reduces atherosclerotic lesions in apoE-deficient mice. Circulation. 1999;99:1222–1229. doi: 10.1161/01.cir.99.9.1222. [DOI] [PubMed] [Google Scholar]
- 49.Zheng H., Huang X., Zhang Q., Katz S.D. Iron sucrose augments homocysteine-induced endothelial dysfunction in normal subjects. Kidney Int. 2006;69:679–684. doi: 10.1038/sj.ki.5000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamanna V.S., Ganji S.H., Shelkovnikov S., Norris K., Vaziri N.D. Iron sucrose promotes endothelial injury and dysfunction and monocyte adhesion/infiltration. Am J Nephrol. 2012;35:114–119. doi: 10.1159/000334939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuo K.L., Hung S.C., Lee T.S., Tarng D.C. Iron sucrose accelerates early atherogenesis by increasing superoxide production and upregulating adhesion molecules in CKD. J Am Soc Nephrol. 2014;25:2596–2606. doi: 10.1681/ASN.2013080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rooyakkers T.M., Stroes E.S., Kooistra M.P. Ferric saccharate induces oxygen radical stress and endothelial dysfunction in vivo. Eur J Clin Invest. 2002;32 Suppl 1:9–16. doi: 10.1046/j.1365-2362.2002.0320s1009.x. [DOI] [PubMed] [Google Scholar]
- 53.Duffy S.J., Biegelsen E.S., Holbrook M. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation. 2001;103:2799–2804. doi: 10.1161/01.cir.103.23.2799. [DOI] [PubMed] [Google Scholar]
- 54.Zoller H., Schaefer B., Glodny B. Iron-induced hypophosphatemia: an emerging complication. Curr Opin Nephrol Hypertens. 2017;26:266–275. doi: 10.1097/MNH.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 55.Schaefer B., Wurtinger P., Finkenstedt A. Choice of high-dose intravenous iron preparation determines hypophosphatemia risk. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hardy S., Vandemergel X. Intravenous iron administration and hypophosphatemia in clinical practice. Int J Rheumatol. 2015;2015:468675. doi: 10.1155/2015/468675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamamoto S., Okada Y., Mori H., Fukumoto S., Tanaka Y. Fibroblast growth factor 23-related osteomalacia caused by the prolonged administration of saccharated ferric oxide. Intern Med. 2012;51:2375–2378. doi: 10.2169/internalmedicine.51.7450. [DOI] [PubMed] [Google Scholar]
- 58.Sato K., Shiraki M. Saccharated ferric oxide-induced osteomalacia in Japan: iron-induced osteopathy due to nephropathy. Endocr J. 1998;45:431–439. doi: 10.1507/endocrj.45.431. [DOI] [PubMed] [Google Scholar]
- 59.Isakova T., Xie H., Yang W. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scialla J.J., Xie H., Rahman M. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25:349–360. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faul C., Amaral A.P., Oskouei B. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grabner A., Amaral A.P., Schramm K. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 2015;22:1020–1032. doi: 10.1016/j.cmet.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ix J.H., Katz R., Kestenbaum B.R. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study) J Am Coll Cardiol. 2012;60:200–207. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Udell J.A., Morrow D.A., Jarolim P. Fibroblast growth factor-23, cardiovascular prognosis, and benefit of angiotensin-converting enzyme inhibition in stable ischemic heart disease. J Am Coll Cardiol. 2014;63:2421–2428. doi: 10.1016/j.jacc.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akhabue E., Vu T.T., Vaidya A. Fibroblast growth factor-23, heart failure risk, and renin-angiotensin-aldosterone-system blockade in hypertension: the MESA Study. Am J Hypertens. 2019;32:18–25. doi: 10.1093/ajh/hpy142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolf M., Chertow G.M., Macdougall I.C., Kaper R., Krop J., Strauss W. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight. 2018;3:124486. doi: 10.1172/jci.insight.124486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niehaus E.D., Malhotra R., Cocca-Spofford D., Semigran M., Lewis G.D. Repletion of iron stores with the use of oral iron supplementation in patients with systolic heart failure. J Card Fail. 2015;21:694–697. doi: 10.1016/j.cardfail.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 68.Lewis G.D., Malhotra R., Hernandez A.F. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF Randomized Clinical Trial. JAMA. 2017;317:1958–1966. doi: 10.1001/jama.2017.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fabiano A., Brilli E., Fogli S. Sucrosomial® iron absorption studied by in vitro and ex-vivo models. Eur J Pharm Sci. 2018;111:425–431. doi: 10.1016/j.ejps.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 70.Girelli D., Ugolini S., Busti F., Marchi G., Castagna A. Modern iron replacement therapy: clinical and pathophysiological insights. Int J Hematol. 2018;107:16–30. doi: 10.1007/s12185-017-2373-3. [DOI] [PubMed] [Google Scholar]
- 71.Mafodda A., Giuffrida D., Prestifilippo A. Oral sucrosomial iron versus intravenous iron in anemic cancer patients without iron deficiency receiving darbepoetin alfa: a pilot study. Support Care Cancer. 2017;25:2779–2786. doi: 10.1007/s00520-017-3690-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fishbane S., Block G.A., Loram L. Effects of ferric citrate in patients with nondialysis-dependent CKD and iron deficiency anemia. J Am Soc Nephrol. 2017;28:1851–1858. doi: 10.1681/ASN.2016101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moretti D., Goede J.S., Zeder C. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126:1981–1989. doi: 10.1182/blood-2015-05-642223. [DOI] [PubMed] [Google Scholar]
- 74.Stoffel N.U., Cercamondi C.I., Brittenham G. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: 2 open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533. doi: 10.1016/S2352-3026(17)30182-5. [DOI] [PubMed] [Google Scholar]
- 75.Kumfu S., Chattipakorn S., Chinda K., Fucharoen S., Chattipakorn N. T-type calcium channel blockade improves survival and cardiovascular function in thalassemic mice. Eur J Haematol. 2012;88:535–548. doi: 10.1111/j.1600-0609.2012.01779.x. [DOI] [PubMed] [Google Scholar]
- 76.Kumfu S., Chattipakorn S.C., Fucharoen S., Chattipakorn N. Dual T-type and L-type calcium channel blocker exerts beneficial effects in attenuating cardiovascular dysfunction in iron-overloaded thalassaemic mice. Exp Physiol. 2016;101:521–539. doi: 10.1113/EP085517. [DOI] [PubMed] [Google Scholar]
- 77.Fernandes J.L., Loggetto S.R., Verissimo M.P. A randomized trial of amlodipine in addition to standard chelation therapy in patients with thalassemia major. Blood. 2016;128:1555–1561. doi: 10.1182/blood-2016-06-721183. [DOI] [PubMed] [Google Scholar]
- 78.Eguchi A., Naito Y., Iwasaku T. Association of dietary iron restriction with left ventricular remodeling after myocardial infarction in mice. Heart Vessels. 2016;31:222–229. doi: 10.1007/s00380-014-0621-5. [DOI] [PubMed] [Google Scholar]
- 79.Phaelante A., Rohde L.E., Lopes A. N-acetylcysteine plus deferoxamine improves cardiac function in Wistar rats after non-reperfused acute myocardial infarction. J Cardiovasc Transl Res. 2015;8:328–337. doi: 10.1007/s12265-015-9633-5. [DOI] [PubMed] [Google Scholar]
- 80.Zou C., Liu X., Xie R. Deferiprone attenuates inflammation and myocardial fibrosis in diabetic cardiomyopathy rats. Biochem Biophys Res Commun. 2017;486:930–936. doi: 10.1016/j.bbrc.2017.03.127. [DOI] [PubMed] [Google Scholar]
- 81.Menasche P., Antebi H., Alcindor L.G. Iron chelation by deferoxamine inhibits lipid peroxidation during cardiopulmonary bypass in humans. Circulation. 1990;82:IV390–IV396. [PubMed] [Google Scholar]
- 82.Paraskevaidis I.A., Iliodromitis E.K., Vlahakos D. Deferoxamine infusion during coronary artery bypass grafting ameliorates lipid peroxidation and protects the myocardium against reperfusion injury: immediate and long-term significance. Eur Heart J. 2005;26:263–270. doi: 10.1093/eurheartj/ehi028. [DOI] [PubMed] [Google Scholar]