Abstract
We administered an adenoviral vector, Onyx-015, into the hepatic artery of patients with metastatic colorectal cancer involving the liver. Thirty-five patients enrolled in this multi-institutional phase I/II trial received up to eight arterial infusions of up to 2 × 1012 viral particles. Hepatic toxicity was the primary dose-limiting toxicity observed in preclinical models. However, nearly 200 infusions of this adenoviral vector were administered directly into the hepatic artery without significant toxicity. Therefore, we undertook this analysis to determine the impact of repeated adenoviral exposure on hepatic function. Seventeen patients were treated at our institution, providing a detailed data set on the changes in hepatic function following repeated exposure to adenovirus. No changes in hepatic function occurred with the first treatment of Onyx-015 among these patients. Transient increases in transaminase levels occurred in one patient starting with the second infusion and transient increases in bilirubin was observed in two patients starting with the fifth treatment. These changes occurred too early to be explained by viral-mediated lysis of hepatocytes. In addition, viremia was observed starting 3–5 days after the viral infusion in half of the patient, but was not associated with hepatic toxicity. To further understand the basis for the minimal hepatic toxicity of adenoviral vectors, we evaluated the replication of adenovirus in primary hepatocytes and tumor cells in culture and the expression of the coxsackie-adenoviral receptor (CAR) in normal liver and colon cancer metastatic to the liver. We found that adenovirus replicates poorly in primary hepatocytes but replicates efficiently in tumors including tumors derived from hepatocytes. In addition, we found that CAR is localized at junctions between hepatocytes and is inaccessible to hepatic blood flow. CAR is not expressed on tumor vasculature but is expressed on tumor cells. Spatial restriction of CAR to the intercellular space in normal liver and diminished replication of adenovirus in hepatocytes may explain the minimal toxicity observed following repeated hepatic artery infusions with Onyx-015.
Keywords: colon cancer, gene therapy, oncolytic virus, clinical trial, adenovirus, Onyx-015
Introduction
The survival of patients with recurrent metastatic colorectal cancer is poor. Despite the recent introduction of new cytotoxic agents and targeted therapies, less than 10% of patients are alive at 5 years.1, 2, 3 New approaches are needed to treat patients with metastatic colorectal cancer. Oncolytic viruses have been engineered to exploit mutations in malignant cells and can destroy tumors by cytolytic and immune-based mechanisms that are distinct from the cytotoxic mechanisms of chemotherapeutic agents.4, 5, 6 Onyx-015 is the prototype for this class of therapeutic agents. In this viral vector, the E1b-55K gene, which is essential for efficient viral replication, has been deleted.5, 7, 8 The E1b-55K gene product binds and inactivates p53, thereby overcoming p53-mediated checkpoint arrest and apoptosis that would limit viral replication. As E1b-55K binds and inactivates p53, it was hypothesized that this gene would be unnecessary for efficient viral replication in tumor cells with inactivating mutations in p53 or the p53 pathway.7, 8 The tumor selectivity of this oncolytic strategy is based on the premise that viral replication and lysis of cells would occur in cells with aberrant p53 but not in cells with wild-type p53. Whereas the mechanism of tumor-selective viral replication is controversial, Onyx-015 has shown promise in Phases I and II clinical trials.9, 10, 11, 12 Viral replication was observed in the tumor cells from patients with head and neck cancer and necrosis of the tumor was demonstrated following direct intratumoral injection.13, 14, 15
Based on encouraging results from the head and neck cancer trials, we treated 35 patients with advanced GI malignancies on a phase I/II protocol using hepatic artery infusion of Onyx-015.12, 16 Dose escalation proceeded from 2 × 108 to 2 × 1012 particles and no dose-limiting toxicities, including hepatic toxicities, were observed.16 Adverse events included flu-like symptoms that were observed in >90% of the patients and transient grade I/II hepatic toxicities were observed in about 20% of the patients. As in the head and neck studies, there was evidence of viral replication, tumor necrosis and clinical benefit.12 Quantitative polymerase chain reaction (PCR) of the blood was performed following administration of Onyx-015 and the input virus became undetectable within 6 h after the infusion. Nevertheless, half of the patients treated at doses of ⩾2 × 1011 particles had a secondary peak of detectable virus in the blood starting 3–5 days post-infusion that lasted for up to 10 days after the infusion.12 Despite evidence of ongoing replication of Onyx-015, there were no clinical symptoms of infection of normal tissues such as cough, conjunctivitis or diarrhea; however, necrosis of tumor masses was demonstrated radiographically.12, 17 Taken together, these findings are consistent with tumor-selective replication of Onyx-015.
The patients treated with Onyx-015 had failed an average of three prior chemotherapeutic regimens before enrolling on the study.17 The objective response rate for patients in this study was 8% and the median survival was 11 months, exceeding the expected response rate of 2–5% and survival of 4–6 months for patients with refractory colorectal cancer.18, 19, 20, 21, 22 Although the objective response rate was encouraging, standard radiographic response criteria may not have captured important clinical responses with this biologically active agent.12, 17, 23 Forty-six percent of the patients in the phase II study demonstrated transient enlargement of the tumor masses 3 weeks after administration of Onyx-015 followed by regression of the tumors starting 2–3 months after initiation of therapy.17 Transient enlargement is consistent with inflammation of the tumor masses and this effect, coupled with extensive necrosis and slow resolution of the tumor, may have obscured the actual response rate in this study. Because of the biphasic response curve, patients with clinical benefit as shown by prolonged survival, were not classified as responders by standard radiographic response criteria. By radiographic response criteria, the transient enlargement in many patients would be considered progressive disease. In fact, in the early period of the trial patients were removed for presumed disease progression before the transient nature of the tumor enlargement was appreciated. Partial responses are defined radiographically as >50% reduction in the greatest diameter of the tumor. However, because of extensive necrosis and delayed resolution of these masses, partial responses were not achieved during the period of the study among patients that nevertheless benefited from treatment. The median survival of patients with transient enlargement of the tumors was 19 months. In contrast, the median survival for patients not demonstrating this response was only 6 months and was consistent with the expected survival of 4–6 months for patients who have failed one or more prior therapies. Based on the clinical benefit observed among the patients on this study, additional clinical trials of oncolytic adenoviral vectors are warranted.
Although oncolytic viruses may prove to be important therapeutic agents, concerns about the safety of intravascular administration of adenovirus arose following the patient death on a clinical trial for treatment of ornithine transcarbanoylase (OTC) deficiency.24 This patient received a dose of approximately 4 × 1013 particles with a replication-deficient adenovirus expressing the OTC gene. Within 24 h, the patient experienced hyperammonemia, adult respiratory distress syndrome (ARDS), disseminated intravascular coagulation and liver failure. The cause of death is uncertain but may be related to overexpression of inflammatory cytokines in the context of a compromised hepatic function due to the OTC deficiency.
The potential toxicity of oncolytic adenoviral vectors has been studied in experimental systems. These systems rely primarily on human tumor xenograft models in immunodeficient mice. In these rodent models, adenovirus constructs were rapidly cleared from the systemic circulation primarily by the liver and spleen,25 and liver toxicity was the predominant dose-limiting toxicity.26, 27 The cause of hepatic toxicity includes both direct viral effects and immune-mediated effects. The direct cyctotoxic effects of adenovirus are due to viral replication, inhibition of host protein synthesis, expression of the adenoviral death protein (ADP) and ultimately lysis of the infected cell.28, 29, 30, 31 Immune-mediated mechanisms of hepatic toxicity include the induction of inflammatory cytokines, especially tumor necrosis factor (TNF), which can cause transient increases in transaminase levels. Some adenoviral proteins are also highly immunogenic, resulting in immune-mediated destruction of infected cells. However, genes encoded in the adenoviral E3 region prevent T-cell recognition and block TNF-mediated killing of infected cells.32, 33, 34, 35, 36 Injection of mice with adenoviral vectors with deletions of the E3 region permitted immune recognition of infected cells but limited the expression of transgenes and increased immune-mediated hepatoxicity.37
Hepatic toxicities resulting from exposure to adenovirus can have different times of onset, degree of hepatic damage and duration depending on the mechanism causing the toxicity. For example, induction of high levels of inflammatory cytokines can cause increases in serum levels of hepatic transaminases. Induction of inflammatory cytokines including TNF, interferon, interleukin (IL)-1 and IL-6 was observed within 12–24 h after exposure to Onyx-015 and became more prominent with repeated viral exposure.12 Delayed hepatic toxicity could occur following treatment with replication competent viruses, as viral replication proceeds for 72–96 h post infection before cell lysis. Immune-mediated hepatitis would be expected to result in increased hepatic toxicity after repeated viral exposures. Finally, in patients with cancer, we demonstrated that transient enlargement of tumor masses occurs in the first few weeks after treatment with Onyx-015.17, 23 Enlargement of the tumor masses could cause increased intrahepatic pressure resulting in bile duct obstruction and a rise in blood levels of bilirubin and alkaline phosphatase. We reviewed the data from the Onyx-015 hepatic artery infusion study to determine if repeated infusions of adenovirus into the hepatic artery of patients with metastatic colorectal cancer caused increased or idiosyncratic hepatic toxicity.
Materials and methods
Eligibility criteria
The eligibility and trial design have been previously described.12 Briefly, the inclusion criteria included the following: histologically or cytologically confirmed carcinoma of gastrointestinal origin; cancer that was not considered resectable for potential cure; confirmed hepatic artery perfusion of both liver lobes and >50% of all tumor mass(es); Karnofsky performance status of ⩾70%; life expectancy of ⩾3 months; ⩾18 years of age; consent form for study participation signed; using a reliable method of contraception if sexually active or of reproductive potential; creatinine <2.0 mg/dl; aspertate aminotransferase (AST) and alanine aminotransferase (ALT) <3.0-fold upper limit of normal; total bilirubin <2.0 mg/dl; PT/INR <2.0 and PTT within normal limits; neutrophils >1500/ml, hemoglobin >9 gm/dl, platelets >100 000/ml. Exclusion criteria were as follows: known chronic liver dysfunction before the development of metastatic cancer (e.g. cirrhosis, chronic hepatitis), which in the estimation of the Principal Investigator put the patient at high risk for liver complications; >50% liver replacement by tumor (estimated radiographically); history of esophageal variceal bleeding within the preceding 8 weeks; active infection, including documented human immunodeficiency virus; any viral syndrome diagnosed within the previous 2 weeks.
Burst assay
Cell lines tested were malignant hepatocytes (Hep3B and HepG2) and colorectal carcinoma (HCT 116), obtained from ATCC, Manassas, VA, USA. In addition, normal human hepatocytes (nHep) and Normal Human Bronchial Epithelial (NHBE) cells were obtained as primary cultures from Cambrex (New Jersey, USA). These were grown to 80–90% confluency, before infection with wild-type Ad5 at a multiplicity of infection (MOI) of 1.0 infectious units (IU)/cell. Infection times of 90 minutes in media with 2% fetal calf serum (FCS) were used, before replacement with fresh media with 2% FCS. At 72 h post infection, cells were scraped into the media and this soup was collected and the cells lysed by three rounds of freezing in liquid nitrogen and thawing at 37°C. Cellular fragments were removed by gentle centrifugation and the numbers of infectious viral units in the supernatant were assayed.
Infectious unit assay was performed by the standard 50% tissue culture infective dose (TCID50) assay. Briefly, 96-well plates were seeded with 103 HEK 293 cells in 200 μl of media and allowed to attach overnight. In total, 20 μl of the viral suspension to be tested, or a dilution of this, was inoculated into each of the top 12 wells of the plate, before serial dilutions (transfers of 22 μl) were performed down successive rows. Each well was then scored as positive if there was any viral cytopathic effect (CPE) evident after 10 days incubation. This produces a TCID50 titer for the virus, which is expressed as IU/ml.
Immunohistochemistry
Staining for CAR protein by immunohistochemistry was performed on standard 5 μm sections of formalin-fixed, paraffin-embedded tissue sections. The staining protocol has been described before,38 with the only difference that for the current study a new anti-CAR rabbit polyclonal antibody (CAR H300, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used at a dilution of 1:50.
Results
Patients enrolled
Thirty-five patients were enrolled on the combined Phase I and Phase II studies. Of these, 17 patients were enrolled at the Palo Alto Veteran's Health Care System (PAVAHCS) and had detailed data on hepatic function available during the entire course of treatment. This analysis is restricted to those 17 patients. All 17 patients completed two cycles of viral therapy. Up to eight infusions of Onyx-015 were administered to patients without evidence of progressive disease.
Impact of arterial infusion of Onyx-015 on baseline plasma levels of AST, ALT, bilirubin and alkaline phosphatase
The liver transaminases, AST and ALT, are cytosolic and mitochondrial enzymes that are released into the blood when hepatocytes are damaged.39 Bilirubin is a metabolite of hemoglobin breakdown and increases in total bilirubin are an indication of biliary obstruction. Increases in unconjugated bilirubin occur when hepatic function is insufficient to metabolize the hemoglobin produced.39 Increases in alkaline phosphatase also occur due to hepatic obstruction or stasis of biliary flow. Alkaline phosphatase levels are frequently increased when there is significant tumor involvement of the liver. The median levels of AST, ALT, bilirubin and alkaline phosphatase in the blood were not significantly different before each of the four planned viral infusions (Figure 1a–d). One patient was noted to have an elevated bilirubin (5.3 mg/dl) before the third infusion (Figure 1c). This patient had progressive disease that was shown by computed tomography (CT) scan to result in bile duct compression. Stent placement relieved the blockage.
Figure 1.
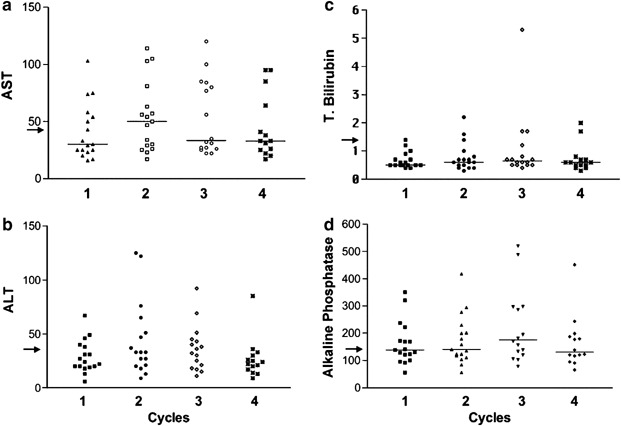
The baseline levels of AST, ALT, alkaline phosphatase and total and direct bilirubin are shown before each of the first four infusion. The median for each group is shown. There was no statistically significant difference between these groups, P>0.05. The arrow indicates the upper end of the normal range for the indicated test.
To obtain a more detailed understanding of the impact of viral exposure on hepatic function, the levels of AST, ALT, bilirubin and alkaline phosphatase in the blood were measured over time following the infusion of Onyx-015. The plasma levels of AST measured during the 7 days following the first four viral infusions for all patients treated at our institution are shown in Figure 2a. No increases in plasma AST above baseline levels were observed with the first viral infusion; however, a transient increase in plasma AST levels was observed in one patient following the second and subsequent infusions of Onyx-015. The increase was evident 12 h after the infusion and had normalized by 48–72 h after the infusion (triangle with connecting line). A similar pattern is observed for ALT in the same patient. These results demonstrate that no significant transaminitis occurred with the first exposure to the virus; however, transient increases in AST and ALT occurred in one patient with the second and subsequent treatments.
Figure 2.
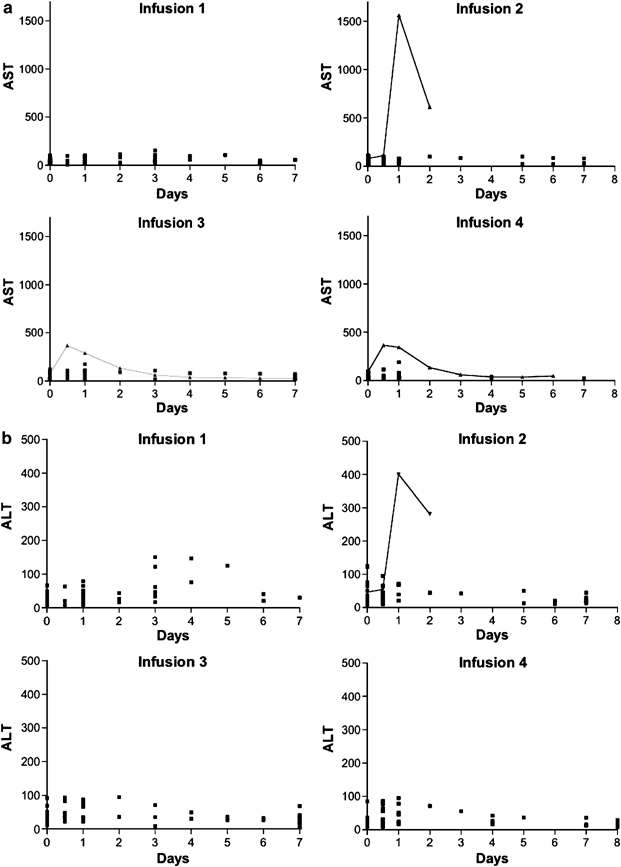
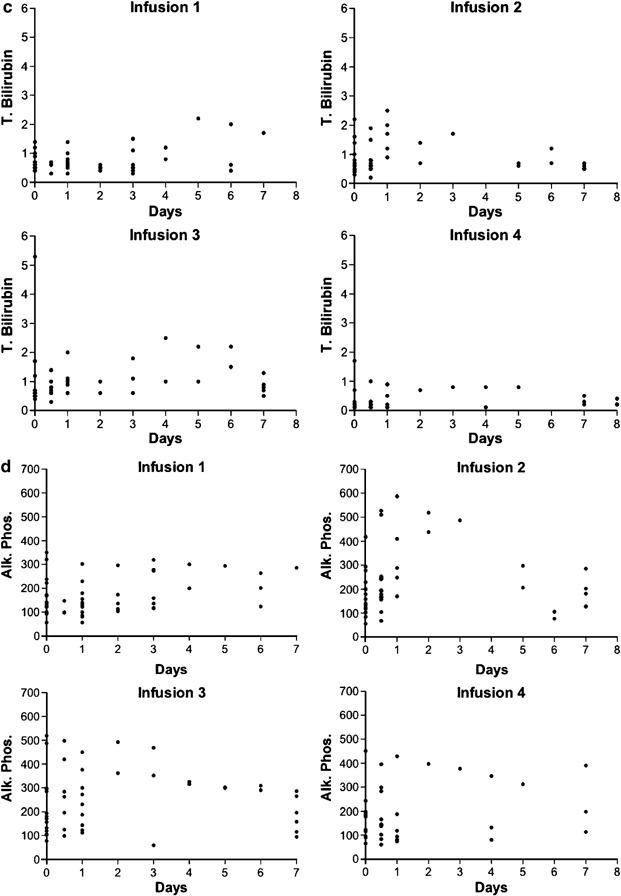
A time course of all AST, ALT, alkaline phosphatase and total and direct bilirubin measurements taken following each of the first four infusions is shown for all 17 patients. Stable patients were discharged after the 24 h measurement and not all patients have measurement on subsequent days.
The levels of bilirubin and alkaline phosphatase were analyzed. Compared to baseline, there were no significant changes in either bilirubin (Figure 2c) or alkaline phosphatase (Figure 2d) levels for up to 7 days following the first four viral treatments. Therefore, only one of 17 patients demonstrated changes in transamainase levels and no patient demonstrated evidence of biliary obstruction or stasis with the first four viral treatments.
We have previously reported that the half-life of Onyx-015 in the serum is 12 min and that viral genomes become undetectable by PCR by 6 h following the infusion.12, 16 About half of the patients demonstrated secondary peaks of viral genomes in the blood that occurred 3–4 days following the infusion of the vector. These secondary peaks are consistent with the time required for viral replication, lysis and release of the viral particles from infected cells. All patients had normal transaminase level 3–4 days following the viral infusion when cell lysis would be expected to occur. Despite viremia with 10 000–100 000 viral genomes/ml, the hepatic enzyme levels were normal in these patients during the period of viremia.
A detailed analysis of the changes in hepatic function tests for the single patient with an acute rise in transaminase levels is shown (Figure 3). The top panel demonstrates that no increases in transaminase levels were observed following the first treatment; however, transient increases in transaminases levels were observed with cycles 2–4. Notably, the largest increases in transaminase levels occurred with the second treatment. The increase in AST and ALT was observed in the first 12–18 h following the infusion and resolved by 48–72 h after the infusion. No significant increase in total or direct bilirubin or alkaline phosphatase was observed following the four viral treatments in this patient, indicating that no significant biliary obstruction occurred in this patient. Transient, early increases in AST and ALT levels without a concurrent increase in alkaline phosphatase or bilirubin levels are consistent with a cytokine-mediated response.
Figure 3.
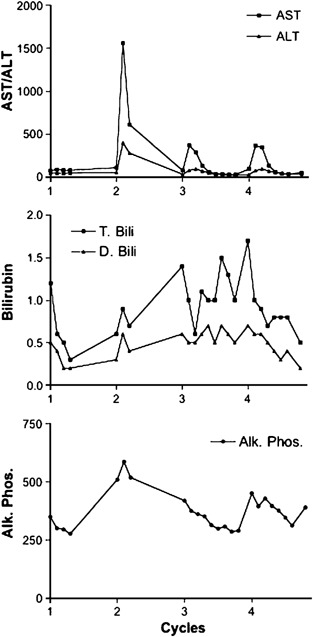
One patient demonstrated elevated AST and ALT levels following the second viral infusion. A detailed analysis of the changes in this patient is shown for four cycles of treatment.
Seven patients had stable to improving disease at the completion of the planned four infusions and received up to four additional treatments with Onyx-015. Two of these seven patients had transient increases in bilirubin with the additional treatments. A detailed analysis of these two patients with these changes is shown in Figure 4a and b. Both patients had transient grade 1/2 increases in bilirubin starting with the fifth infusion. The increase was mainly in the unconjugated form of bilirubin. The transient increase in unconjugated bilirubin is consistent with transient hepatic dysfunction rather than obstruction as the cause of the increased bilirubin. No significant effects were noted on the plasma levels of alkaline phosphatase during the course of treatment, further supporting the absence of hepatic obstruction as the cause of the transient rise in unconjugated bilirubin.
Figure 4.
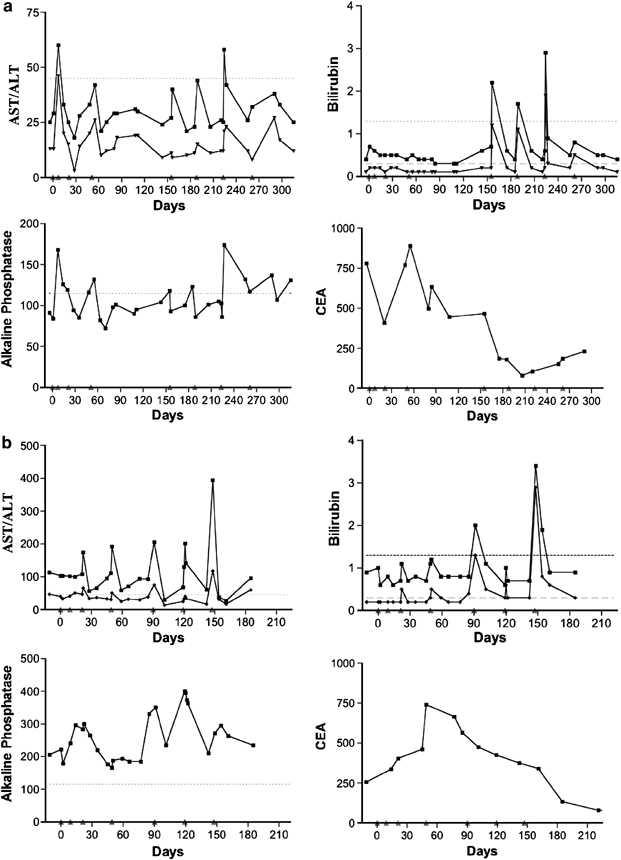
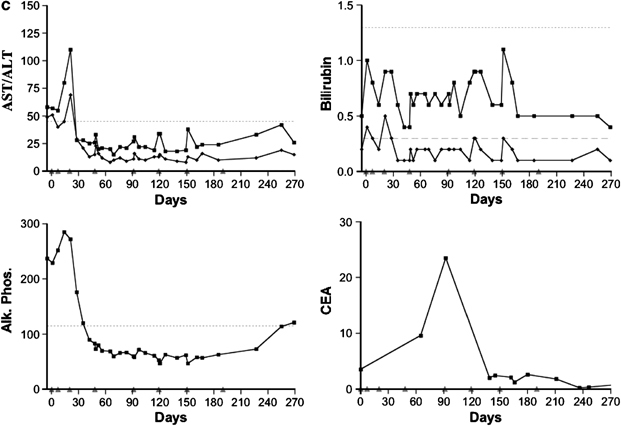
A time course of all AST, ALT, alkaline phosphatase and total and direct bilirubin measurements taken following up to eight infusions in three patients achieving partial responses. The upper limit of the normal range is shown by the horizontal dashed line. (a) This patient had a significant decline in the tumor marker, carcinoembryonic antigen (CEA), during the course of treatment. All measurement of AST and ALT were within the normal range except for transient increases following the second and seventh infusion. These increases resolved rapidly without intervention. Notably, there are small increases in AST and ALT levels following most infusions; however, these remain within the normal range. Transient increases in total and direct bilirubin are observed starting with the fifth infusion. These increases are transient and predominantly indirect bilirubin and no increase exceeds three times the upper limit of normal. (b) This patient had extensive replacement of the liver with metastatic disease and had elevated levels of AST, ALT and alkaline phosphatase at baseline. CEA levels trended upward following the first three infusion of the vector, but demonstrated a marked decline following the fourth infusion. Transient increases in AST and ALT were observed starting with the third infusion but these resolved rapidly. Transient increases in total and direct bilirubin were observed following the fifth infusion with the levels transiently exceeding three times the upper limit of normal following only the seventh infusion. (c) This patient had extensive replacement of the liver with metastatic cancer and had elevated levels of AST, ALT and alkaline phosphatase at baseline. The CEA levels trended upward following the first four infusion of the vector, but demonstrated a marked decline following the fifth infusion. AST, ALT and alkaline phosphatase levels were elevated at baseline and demonstrated a transient rise following the third infusion followed by normalization of these values. All subsequent tests were in the normal range despite repeated viral infusions.
One patient had abnormal levels of serum transaminase and alkaline phosphatase levels at baseline that normalized following the third treatment with Onyx-015. Following normalization of these parameters, the transaminase, bilirubin and alkaline phosphatase levels remained within the normal range for the remainder of the study despite repeated administration of Onyx-015. The blood levels of carcinoembryonic antigen (CEA) also normalized in this patient following treatment indicating an antitumor effect from the treatment.
Radiographic evidence of tumor responses occurred in some patients. Baseline and end of treatment CT scans for three patients (Figure 4) are shown in Figure 5. Nearly complete resolution of extensive disease within the liver of the patient in Figure 4a is shown in Figure 5a. The CT scans for patient in Figure 4b have been previously published and demonstrate nearly complete resolution of extensive intrahepatic metastatic colorectal cancer.23 In Figure 5b, which corresponds to the patient with transaminase data shown in Figure 4c, the tumor masses decreased markedly in size and have a radiographic appearance consistent with extensive necrosis. A positron emission tomography (PET) scan obtained after the sixth treatment demonstrated that these residual masses were metabolically inactive (data not shown). Therefore, radiographic evidence of tumor responses were observed among patients while minimal hepatic toxicity occurred due to the treatment.
Figure 5.
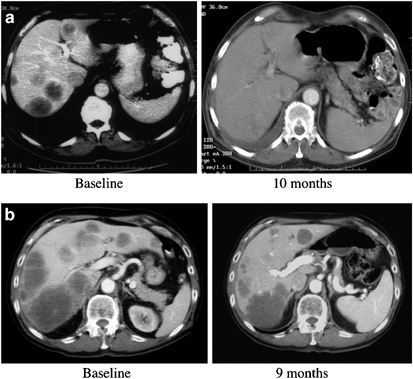
(a) CT images at the level of the largest hypoattenuating lesion before commencement of viral infusions. Baseline and 10 months (end of treatment) CT images are shown. The scan demonstrates regression of the tumor masses. (b) CT images at the level of the largest hypoattenuating lesion before commencement of viral infusions. Baseline and 9 month (end of treatment) scans are shown. Following treatment, the masses became significantly smaller and have decreased heterogeneity, increased margination and decreased attenuation.
Examination of adenoviral interaction with human hepatocytes
It is of interest that preclinical studies in mice have found the major dose-limiting toxicity of adenoviral delivery to be liver toxicity. We have also found that AST and ALT levels are significantly increased in mice plasma following systemic delivery of even low doses of adenovirus (data not shown). However, only limited hepatic toxicity was observed in a small subset of the patients on this trial following intrahepatic viral delivery. These results suggest that the metabolism of adenovirus may differ between mice and humans. Therefore, the replication of adenovirus was evaluated in both normal and malignant hepatocytes in culture (Figure 6). Cells were infected with one viral particle per cell of Ad5 and viral replication was determined after 72 h in culture. No viral replication was observed in the normal hepatocytes, whereas viral replication proceeded effectively in malignant hepatocytes, colorectal carcinoma and normal bronchial epithelial cells. These results are consistent with our clinical findings of minimal hepatic toxicity despite the administration of up to 2 × 1012 particles directly into the hepatic artery.
Figure 6.
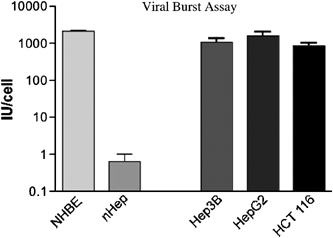
Burst assay following infection of different human cells with wild-type Ad5. Cells were grown to 80% confluent, infected at an MOI of 1.0 with Ad5. Cells and media were then collected after 72 h, cells lysed by freeze/thaw and viral IU/ml measured by TCID50 assay. Cell lines used included two primary cell types; normal hepatocytes (nHep) and normal human bronchial epithelial (NHBE) cells. In addition, three transformed cell lines were tested, two hepatocellular (Hep3B and HepG2) and one colorectal carcinoma (HCT 116).
To further evaluate the basis for the limited hepatic toxicity observed in these patients, we evaluated the expression and localization of the adenoviral receptor (CAR) in normal liver by immunohistochemistry (Figure 7a). We found that CAR is expressed at cell junctions between hepatocytes, in particular surrounding the bile canaliculi, a known location of tight junctions that are preventing bile leaks. In contrast, no CAR expression was detected at the surface of hepatocytes that is bordering the space of Disse, suggesting that CAR is not accessible to adenovirus particles in the blood. In addition, we found that CAR is expressed in the tumor cells but is low or absent in the vasculature of the tumor (Figure 7b). Together, these results indicate that limited access to the CAR receptor and poor viral replication in normal human hepatocytes may limit liver toxicity following delivery of oncolytic adenovirus in human subjects but may permit infection of tumor cells.
Figure 7.
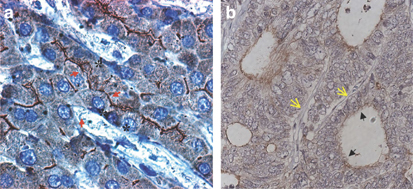
(a) Detection of CAR protein by immunohistochemistry in normal human liver tissue (magnification: × 1000). Arrows point to areas of typical CAR staining, which is particularly strong around the bile canaliculi. Hepatic sinosoids are marked by asterisks. (b) Detection of CAR protein expression in colorectal cancer liver metastasis by immunohistochemistry. Black filled arrows point at CAR staining in tumor pseudo-glands. Yellow open arrows indicate small intra-tumoral and CAR-negative blood vessels (magnification: × 400).
Discussion
We treated 35 patients with advanced gastrointestinal (GI) malignancies on a phase I/II protocol using hepatic artery infusion of an oncolytic vector, Onyx-015.12, 16, 17, 23 The patients received up to eight infusions of up to 2 × 1012 viral particles. Nearly 200 infusions were administered to patients with metastatic colorectal cancer and no dose-limiting toxicities were observed. As hepatic toxicity was dose limiting in preclinical models, we undertook this review to determine if there was evidence for increased hepatic toxicity due to repeated exposure of the patients to Onyx-015. In addition, as hepatic toxicity could arise from several different mechanisms with different patterns of clinical presentation, an analysis of the time of onset, extent and duration of any hepatic abnormalities would provide important information to help guide the safe administration of adenoviral vectors in future clinical trials. This analysis is restricted to the 17 patients treated at the Palo Alto Veteran's Health Care System where we had access to all results of hepatic function tests obtained during the entire course of the study.
No significant changes in serum level of hepatic enzymes were observed following the initial treatment with Onyx-015 in these patients. This finding indicates that the administration of this adenoviral vector at doses of up to 2 × 1012 particles has minimal hepatic toxicity. One patient developed transient increases in serum levels of AST and ALT starting with the second treatment. These changes were observed by 18 h after the treatment and resolved by 48–72 h after treatment. Not only was the duration of this increase in transaminase levels brief, but the peak transaminase levels were less than 10% of the peak levels commonly seen during other causes of reversible hepatic toxicity including viral hepatitis, chemical toxicity or infusion of chemotherapy into the hepatic artery. The transient rise in AST and ALT levels observed in this single patient with the second and subsequent infusions, occurred too early to be explained by viral-mediated lysis of hepatocytes. The rapid onset and resolution of these mild increases in AST and ALT levels are consistent with a cytokine-mediated effect.
Viremia (10 000–100 000 viral particles/ml) was observed in about half of the patients starting 3–4 days after the infusions of Onyx-015 and lasted up to 10 days post infusion. However, the serum transaminase levels were normal in all patients at the time of the viremia despite high titers of circulating virus and clinical symptoms of viral infection including fever and myalgia. Viremia for several days without evidence of hepatic toxicity provides further evidence that exposure to adenovirus has minimal hepatic toxicity.
Seven of the 17 patients had stable to improving disease at the completion of four viral infusions and were treated with up to four additional treatments with Onyx-015. Two of the seven patients had grade I/II increases in bilirubin starting with the fifth treatment. The increase in bilirubin was predominantly unconjugated bilirubin. This finding suggests transient hepatic dysfunction affecting the metabolism of bilirubin rather than biliary stasis or obstruction was the primary cause of the rise in the serum levels of bilirubin. The rapid resolution of the elevated bilirubin levels further argues against obstruction as the cause of the rise. Transient increases in the levels of unconjugated bilirubin are seen during acute infections and are mediated by cytokines including TNF.40, 41, 42, 43, 44
We have previously reported that patients infused with Onyx-015 have more robust cytokine responses with repeated viral infusions.12 Circulating levels of these inflammatory cytokines including IL-1, IL-6, TNF and interferon were induced within 6–18 h of the viral infusion and were amplified 10–1000-fold following repeated viral infusions.12 Transient increases in transaminase levels occur during systemic infections and high levels of inflammatory cytokines, particularly interferon and TNF, contribute to the rise in transaminase levels.43, 44, 45, 46 In experimental systems, the administration of neutralizing antibodies to TNF has been shown to blunt the impact of inflammation on changes in transaminase levels.40, 42 Hepatic dysfunction due to the induction of high levels of inflammatory cytokines is consistent with the time course of these changes and the requirement for repetitive treatments. While all patients had elevated levels of inflammatory cytokines following treatment with Onyx-015, only one patient had significant increases in AST and ALT levels, suggesting that factors in addition to elevated cytokines levels contribute to the observed transaminitis.
Alkaline phosphatase is an enzyme that is elevated in the blood due to obstruction of biliary flow. Patients with significant hepatic involvement with tumor frequently have elevated levels of circulating alkaline phosphatase due to hepatic obstruction and stasis of bililary flow in the regions near the tumors. All the patients on this study had metastatic cancer and many of the patients had elevated circulating levels of alkaline phosphatase at baseline. Treatment with Onyx-015 caused no significant increase in alkaline phosphatase levels among these patients. These findings, coupled with the lack of significant changes in bilirubin levels, indicate that the infusions of Onyx-015 administered in this study did not exacerbate biliary obstruction among these patients.
To further evaluate the basis for this limited hepatitic toxicity, we compared the replication of adenovirus in normal hepatocytes and transformed human cells in vitro. We demonstrate that adenovirus replicates poorly in primary hepatocytes. The cells were infected with one viral particle per cell and after 72 hours no increase in viral particles per cell was detected in the normal hepatocytes. In contrast, 500–2500 adenoviral particles per cell were produced in the malignant hepatocytes, colorectal carcinoma and normal human bronchial epithelial cells. These findings indicate that adenovirus does not effectively infect or replicate in hepatocytes. Diminished infectivity and diminished replication could account for the low level of replication observed in hepatocytes infected with adenovirus.
We have previously demonstrated that the receptor for adenovirus (CAR) is localized to the sites of cell–cell junctions in phenotypically normal mammary epithelial cells grown in three-dimensional cell culture, where it was inaccessible to adenovirus in the media.38 We hypothesized that CAR may be restricted to areas of cell–cell contact in the normal liver and thus may be inaccessible to the vascular flow. Spatial restriction of CAR could explain the minimal hepatic toxicity observed among these patients. We used immunohistochemistry to evaluate the expression and localization of CAR in normal liver. We found that CAR is localized at sites of hepatocellular junctions, surrounding the bile canaliculi, which are sealed off from their surroundings by tight junctions. This is in agreement with CAR's physiological function as a cell-adhesion molecule that participates in the formation of tight junctions where it physically interacts with the tight-junction protein ZO-1.47 No CAR was detected at the hepatocyte surface facing the space of Disse or the hepatic sinusoids. Thus, CAR appears not to be accessible for circulating adenoviral particles passing through the liver. We further found that CAR is not expressed on the endothelium of tumor cells but is expressed on tumor cells. Therefore, the limited hepatic toxicity observed among the patients treated in this study may be related to spatial restriction of CAR in the liver. In addition, hepatic toxicity may be limited by the inability of Onyx-015 to replicate in normal hepatocytes. Taken together, these results indicate that tumor cells metastatic to the liver may be selectively susceptible to infection with adenovirus. Therefore, it is possible that CAR expression on tumor cells may predict response to treatment with oncolytic adenoviral vectors.
Hepatic toxicity was observed as the primary dose-limiting toxicity in rodent systems,48 but was observed at approximately 10-fold higher virus/kg doses than administered to patients in study with Onyx-015. Consequently, hepatic toxicity may have been observed among the patients if higher doses of Onyx-015 were administered. However, direct comparisons between rodents and humans are difficult especially with regard to the impact of hepatic artery infusion of adenovirus. The hepatic artery in rodents is very difficult to canulate. Consequently, hepatic toxicity has been evaluated after intravenous or intraperitoneal administration of adenovirus in mice.48 Dilution of the virus into the systemic circulation and uptake of virus in the spleen and other organs after intravenous or intraperitoneal administration will reduce the amount of virus delivered to the liver making comparison between human and rodent data difficult. However, a dose-limiting toxicity in humans was reached in a related trial using hepatic artery infusion of an adenovirus expressing p53.49 Dose-limiting cardiac output suppression rather than hepatic toxicity was observed following hepatic artery infusion of 7.5 × 1013 particles of an adenoviral vector expressing p53. Accounting for the difficulties in comparing human and rodent systems, similar if not higher doses of adenovirus have been administered to the hepatic artery of humans without the hepatic toxicity observed in rodents. In addition, as human adenovirus does not replicate in mouse tissues, the potential hepatic toxicity of replication competent viruses cannot be assessed in the rodent model but was not observed in the human trials.
Unlike preclinical models, patients present with metastatic cancer in the context of a variety of associated comorbidies that may significantly alter the toxicity profile for these viral vectors. Variability was observed between patients and even within the same patient with repeated dosing, emphasizing the importance of careful evaluation and close monitoring of these patients. Further study of the immune response to adenoviral vectors is essential, especially if repetitive treatment is needed. Although thousands of patients have been safely treated with adenoviral vectors on various gene-therapy protocols and millions of people experience adenoviral infections each year without overt symptoms of hepatic dysfunction, idiosyncratic toxicities have occurred. Adenoviral vectors can be used to deliver unique and targeted therapies and can be a useful tool for the treatment of disease. Although additional research is needed to unravel the molecular basis for these idiosyncratic toxicities, it is likely that these toxicities can be avoided or effectively managed and that adenoviral vectors can be used with an acceptable level of risk, particularly among patients with incurable malignancies. With respect to the design of further clinical trials using adenoviral vectors, this study demonstrates that Onyx-015 can be delivered into the hepatic artery with minimal hepatic toxicity at doses where tumor responses were observed.
References
- 1.Rougier P, Bugat R, Douillard JY, Culine S, Suc E, Brunet P. Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy-naive patients and patients pretreated with fluorouracil-based chemotherapy. J Clin Oncol. 1997;15:251–260. doi: 10.1200/JCO.1997.15.1.251. [DOI] [PubMed] [Google Scholar]
- 2.Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 3.de Gramont A, Tournigand C, Louvet C, Maindrault-Goebel F, Andre T. First-line therapy for advanced colorectal cancer. Curr Oncol Rep. 2005;7:167–172. doi: 10.1007/s11912-005-0069-y. [DOI] [PubMed] [Google Scholar]
- 4.McCormick F. Cancer-specific viruses and the development of ONYX-015. Cancer Biol Ther. 2003;2:S157–S160. doi: 10.4161/cbt.216. [DOI] [PubMed] [Google Scholar]
- 5.Kirn DH, McCormick F. Replicating viruses as selective cancer therapeutics. Mol Med Today. 1996;2:519–527. doi: 10.1016/S1357-4310(97)81456-6. [DOI] [PubMed] [Google Scholar]
- 6.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 7.McCormick F. Interactions between adenovirus proteins and the p53 pathway: the development of ONYX-015. Semin Cancer Biol. 2000;10:453–459. doi: 10.1006/scbi.2000.0336. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 9.Harada JN, Berk AJ. p53-Independent and -dependent requirements for E1B-55K in adenovirus type 5 replication. J Virol. 1999;73:5333–5344. doi: 10.1128/jvi.73.7.5333-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Therapy. 2001;8:89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- 11.McCormick F. ONYX-015 selectivity and the p14ARF pathway. Oncogene. 2000;19:6670–6672. doi: 10.1038/sj.onc.1204096. [DOI] [PubMed] [Google Scholar]
- 12.Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62:6070–6079. [PubMed] [Google Scholar]
- 13.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 14.Morley S, MacDonald G, Kirn D, Kaye S, Brown R, Soutar D. The dl1520 virus is found preferentially in tumor tissue after direct intratumoral injection in oral carcinoma. Clin Cancer Res. 2004;10:4357–4362. doi: 10.1158/1078-0432.CCR-03-0443. [DOI] [PubMed] [Google Scholar]
- 15.Ganly I, Kirn D, Eckhardt G, Rodriguez GI, Soutar DS, Otto R. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 16.Reid T, Galanis E, Abbruzzese J, Sze D, Andrews J, Romel L. Intra-arterial administration of a replication-selective adenovirus (dl1520) in patients with colorectal carcinoma metastatic to the liver: a phase I trial. Gene Therapy. 2001;8:1618–1626. doi: 10.1038/sj.gt.3301512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reid TR, Freeman S, Post L, McCormick F, Sze DY. Effects of Onyx-015 among metastatic colorectal cancer patients that have failed prior treatment with 5-FU/leucovorin. Cancer Gene Ther. 2005;12:673–681. doi: 10.1038/sj.cgt.7700819. [DOI] [PubMed] [Google Scholar]
- 18.Falcone A, Allegrini G, Lencioni M, Pfanner E, Brunetti I, Cianci C. Protracted continuous infusion of 5-fluorouracil and low-dose leucovorin in patients with metastatic colorectal cancer resistant to 5-fluorouracil bolus-based chemotherapy: a phase II study. Cancer Chemother Pharmacol. 1999;44:159–163. doi: 10.1007/s002800050961. [DOI] [PubMed] [Google Scholar]
- 19.Falcone A, Cianci C, Pfanner E, Bertuccelli M, Brunetti I, Muttini MP. Continuous-infusion 5-fluorouracil in metastatic colorectal cancer patients pretreated with bolus 5-fluorouracil: clinical evidence of incomplete cross-resistance. Ann Oncol. 1994;5:291. doi: 10.1093/oxfordjournals.annonc.a058813. [DOI] [PubMed] [Google Scholar]
- 20.Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet. 1998;352:1407–1412. doi: 10.1016/S0140-6736(98)03085-2. [DOI] [PubMed] [Google Scholar]
- 21.Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003;21:2059–2069. doi: 10.1200/JCO.2003.11.126. [DOI] [PubMed] [Google Scholar]
- 22.Mori A, Bertoglio S, Guglielmi A, Aschele C, Bolli E, Tixi L. Activity of continuous-infusion 5-fluorouracil in patients with advanced colorectal cancer clinically resistant to bolus 5-fluorouracil. Cancer Chemother Pharmacol. 1993;33:179–180. doi: 10.1007/BF00685339. [DOI] [PubMed] [Google Scholar]
- 23.Sze DY, Freeman SM, Slonim SM, Samuels SL, Andrews JC, Hicks M. Becker Young Investigator Award: intraarterial adenovirus for metastatic gastrointestinal cancer: activity, radiographic response, and survival. J Vasc Interv Radiol. 2003;14:279–290. doi: 10.1097/01.RVI.0000058422.01661.1E. [DOI] [PubMed] [Google Scholar]
- 24.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Worgall S, Wolff G, Falck-Pedersen E, Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 26.Duncan SJ, Gordon FC, Gregory DW, McPhie JL, Postlethwaite R, White R. Infection of mouse liver by human adenovirus type 5. J Gen Virol. 1978;40:45–61. doi: 10.1099/0022-1317-40-1-45. [DOI] [PubMed] [Google Scholar]
- 27.Bao JJ, Zhang WW, Kuo MT. Adenoviral delivery of recombinant DNA into transgenic mice bearing hepatocellular carcinomas. Hum Gene Ther. 1996;7:355–365. doi: 10.1089/hum.1996.7.3-355. [DOI] [PubMed] [Google Scholar]
- 28.Tollefson AE, Ryerse JS, Scaria A, Hermiston TW, Wold WS. The E3-11.6-kDa adenovirus death protein (ADP) is required for efficient cell death: characterization of cells infected with adp mutants. Virology. 1996;220:152–162. doi: 10.1006/viro.1996.0295. [DOI] [PubMed] [Google Scholar]
- 29.Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE, Wold WS. Overexpression of the ADP (E3-11.6 K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- 30.Tollefson AE, Scaria A, Ying B, Wold WS. Mutations within the ADP (E3-11.6 K) protein alter processing and localization of ADP and the kinetics of cell lysis of adenovirus-infected cells. J Virol. 2003;77:7764–7778. doi: 10.1128/JVI.77.14.7764-7778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lichtenstein DL, Toth K, Doronin K, Tollefson AE, Wold WS. Functions and mechanisms of action of the adenovirus E3 proteins. Int Rev Immunol. 2004;23:75–111. doi: 10.1080/08830180490265556. [DOI] [PubMed] [Google Scholar]
- 32.Wold WS, Gooding LR. Adenovirus region E3 proteins that prevent cytolysis by cytotoxic T cells and tumor necrosis factor. Mol Biol Med. 1989;6:433–452. [PubMed] [Google Scholar]
- 33.Gooding LR, Sofola IO, Tollefson AE, Duerksen-Hughes P, Wold WS. The adenovirus E3-14.7 K protein is a general inhibitor of tumor necrosis factor-mediated cytolysis. J Immunol. 1990;145:3080–3086. [PubMed] [Google Scholar]
- 34.Gooding LR, Aquino L, Duerksen-Hughes PJ, Day D, Horton TM, Yei SP. The E1B 19 000-molecular-weight protein of group C adenoviruses prevents tumor necrosis factor cytolysis of human cells but not of mouse cells. J Virol. 1991;65:3083–3094. doi: 10.1128/jvi.65.6.3083-3094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wold WS. Adenovirus genes that modulate the sensitivity of virus-infected cells to lysis by TNF. J Cell Biochem. 1993;53:329–335. doi: 10.1002/jcb.240530410. [DOI] [PubMed] [Google Scholar]
- 36.Tollefson AE, Toth K, Doronin K, Kuppuswamy M, Doronina OA, Lichtenstein DL. Inhibition of TRAIL-induced apoptosis and forced internalization of TRAIL receptor 1 by adenovirus proteins. J Virol. 2001;75:8875–8887. doi: 10.1128/JVI.75.19.8875-8887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christ M, Louis B, Stoeckel F, Dieterle A, Grave L, Dreyer D. Modulation of the inflammatory properties and hepatotoxicity of recombinant adenovirus vectors by the viral E4 gene products. Hum Gene Ther. 2000;11:415–427. doi: 10.1089/10430340050015888. [DOI] [PubMed] [Google Scholar]
- 38.Anders M, Hansen R, Ding RX, Rauen KA, Bissell MJ, Korn WM. Disruption of 3D tissue integrity facilitates adenovirus infection by deregulating the coxsackievirus and adenovirus receptor. Proc Natl Acad Sci USA. 2003;100:1943–1948. doi: 10.1073/pnas.0337599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223–2230. [PubMed] [Google Scholar]
- 40.Costelli P, Aoki P, Zingaro B, Carbo N, Reffo P, Lopez-Soriano FJ. Mice lacking TNFalpha receptors 1 and 2 are resistant to death and fulminant liver injury induced by agonistic anti-Fas antibody. Cell Death Differ. 2003;10:997–1004. doi: 10.1038/sj.cdd.4401281. [DOI] [PubMed] [Google Scholar]
- 41.Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27:63–68. doi: 10.1016/S0741-8329(02)00215-X. [DOI] [PubMed] [Google Scholar]
- 42.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 43.Orange JS, Salazar-Mather TP, Opal SM, Biron CA. Mechanisms for virus-induced liver disease: tumor necrosis factor-mediated pathology independent of natural killer and T cells during murine cytomegalovirus infection. J Virol. 1997;71:9248–9258. doi: 10.1128/jvi.71.12.9248-9258.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simeonova PP, Gallucci RM, Hulderman T, Wilson R, Kommineni C, Rao M. The role of tumor necrosis factor-alpha in liver toxicity, inflammation, and fibrosis induced by carbon tetrachloride. Toxicol Appl Pharmacol. 2001;177:112–120. doi: 10.1006/taap.2001.9304. [DOI] [PubMed] [Google Scholar]
- 45.Sheron N, Lau J, Daniels H, Goka J, Eddleston A, Alexander GJ. Increased production of tumour necrosis factor alpha in chronic hepatitis B virus infection. J Hepatol. 1991;12:241–245. doi: 10.1016/0168-8278(91)90945-8. [DOI] [PubMed] [Google Scholar]
- 46.Mookerjee RP, Sen S, Davies NA, Hodges SJ, Williams R, Jalan R. Tumour necrosis factor alpha is an important mediator of portal and systemic haemodynamic derangements in alcoholic hepatitis. Gut. 2003;52:1182–1187. doi: 10.1136/gut.52.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heise CC, Williams AM, Xue S, Propst M, Kirn DH. Intravenous administration of ONYX-015, a selectively replicating adenovirus, induces antitumoral efficacy. Cancer Res. 1999;59:2623–2628. [PubMed] [Google Scholar]
- 49.Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther. 2002;9:979–986. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]


