Abstract
Somatic gene transfer to the pulmonary endothelium may be a useful strategy for modifying the phenotype of endothelium and/or vascular smooth muscle in disorders such as primary pulmonary hypertension, ARDS or pulmonary metastatic disease. Adenoviral (Ad) vectors, although highly efficient in liver gene transfer, have proven to be limited for pulmonary gene transfer with respect to efficiency, in part because of difficulty in assuring significant residence time in the lung and/or paucity of receptors for adenovirus on the endothelium. A recent study has shown that the use of a bispecific antibody to endothelial cells and Ad vectors efficiently redirects Ad vectors to pulmonary endothelium and improves gene expression in the lung. In this study, we report that pulmonary gene transfer by Ad vectors can also be improved significantly via the use of cationic liposomes. Preinjection of cationic liposomes followed by adenovirus led to a significant increase in the level of gene expression in the lung. The improvement in pulmonary gene transfer was associated with a decrease in the level of gene expression in the liver. Gene expression in the lung lasted for up to 2 weeks. This protocol, together with genetic modification of adenovirus, may prove to be useful for pulmonary gene transfer for the treatment of pulmonary diseases. This method may also be extended to pulmonary gene transfer using other types of viral vectors via vascular route.
Keywords: adenovirus, gene therapy, targeting, liposome, pulmonary endothelium
Introduction
Adenoviral (Ad) vectors possess many attributes that favor their use in gene therapy, particularly their high efficiency.1 One of the key limitations to the use of Ad vectors is the restricted tropism of the virus. Systemic administration of Ad vectors leads to gene expression mainly in the liver.2 Pulmonary gene transfer by Ad vectors via the vascular route has proven to be limited with respect to efficiency in part because of difficulty in assuring significant residence time in the lung and/or paucity of receptors for adenovirus on the endothelium. Two approaches have been proposed to overcome the problem of restricted tropism: one is to genetically modify Ad vectors to render them target cell-specific3,4,5 and the other one is to use bispecific conjugates to redirect Ad vectors to target cells.6,7,8,9 Several groups have reported selective delivery of Ad vectors to various types of cells via genetic modifications of Ad vectors.3,4,5 Thus far, there is no successful report of targeted delivery of Ad vectors to pulmonary endothelium using this approach. The concept of targeted delivery of Ad vectors to pulmonary endothelium has been nicely demonstrated in a study by Reynolds et al8 using a bispecific antibody. The complexing of Ad vectors with a bispecific antibody to Ad vectors and angiotensin-converting enzyme (ACE) leads to a significant improvement in the infection of endothelial cells that overexpress ACE receptors. Systemic administration of the complexed Ad vectors also leads to improvement in pulmonary gene transfer.8 More importantly, the enhancement in pulmonary gene transfer was associated with a decrease in gene expression in the liver, although the level of gene expression in the liver is still substantially higher than that in the lung.8 In this study, we have shown that pulmonary gene transfer by Ad vectors can also be significantly improved via preinjection of cationic liposomes followed by Ad vectors. This protocol, together with genetic modification of adenovirus, may prove to be useful for pulmonary gene transfer for the treatment of pulmonary diseases. This method may also be extended to pulmonary gene transfer using other types of viral vectors via the vascular route.
Results
Pre-injection of cationic liposomes improves Ad vector-mediated pulmonary gene transfer
We recently showed that preinjection of cationic liposomes followed by plasmid DNA led to efficient gene expression in the lung.10 To test whether preinjection of cationic liposomes can also enhance Ad vector-mediated pulmonary gene transfer, groups of six mice received tail vein injection of various amounts of DOTAP:cholesterol liposomes followed by injection of AdCMVLuc. Gene expression was assayed 3 days following the injection. When AdCMVLuc alone was used, the liver had the highest level of gene expression (Figure 1b). A low level of gene expression was found in the lung and other organs. There was about a 100 to 1000-fold difference in the level of gene expression between the liver and lung Figure 1b. Preinjection of cationic liposomes significantly improved gene expression in the lung. As shown in Figure 1a, increasing the amount of cationic liposomes from 0.3 to 0.9 μmol/mouse led to a steady increase in the level of gene expression in the lung. At the dose of 0.9 μmol DOTAP per mouse, a range of eight- to 40-fold increase in gene expression was observed in seven different experiments. Continuous increase in DOTAP dose was not associated with a further increase in gene expression in the lung. Interestingly, the improvement in gene expression in the lung was associated with a dramatic decrease in gene expression in the liver Figure 1b.
Figure 1.
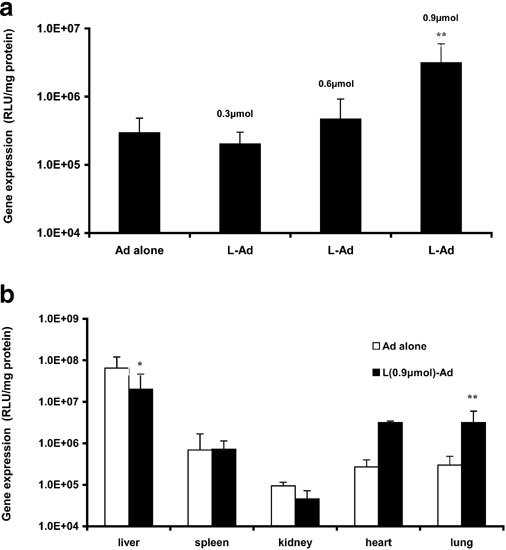
Gene expression in mice following tail vein injection of Ad virus containing luciferase cDNA (AdCMVLuc) or DOTAP:cholesterol liposomes followed by AdCMVLuc (sequential protocol). DOTAP:cholesterol liposomes (1:1; m/m) were prepared by the extrusion method with a final size of 150 nm and the amount of AdCMVLuc injected was 4 × 1010 particles/mouse. Gene expression was assayed 72 h following the injection. (n = 6). Panel (a) shows the level of gene expression in the lung with varying doses of DOTAP:cholesterol liposomes and panel (b) shows the gene expression in major organs at a dose of 0.9 μmol DOTAP/mouse. *P < 0.05; **P < 0.01 (versus Ad alone).
Premixing of cationic liposomes with Ad vectors leads to decreased in vivo gene expression
Several studies have shown that complexation of Ad vectors with cationic lipids/polymers improves infection of a number of cell lines in vitro11,12,13,14,15,16 and airway epithelial cells following intratracheal instillation in mice.12 To examine whether lipid complexation can similarly enhance pulmonary gene transfer by Ad vectors via the vascular route, AdCMVLuc was mixed with various amounts of DOTAP:cholesterol liposomes and gene expression was assayed 3 days following the injection of the complexed AdCMVLuc. In contrast to sequential injection, premixing of cationic liposomes with AdCMVLuc resulted in a decreased gene expression in all major organs examined (Figure 2). Complexation of cationic lipids with AdCMVLuc had minimal effects on infection of cultured mouse lung endothelial cells (MLEC) at low doses and inhibited gene expression at relatively high doses (data not shown).
Figure 2.
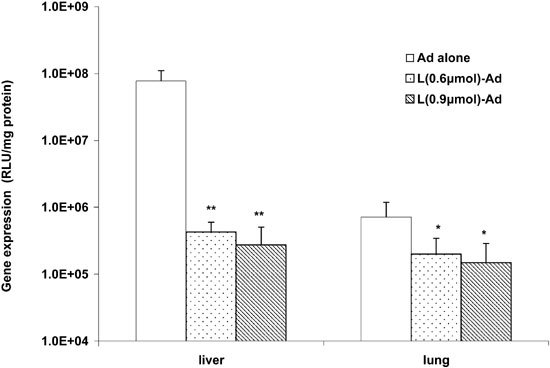
Premixing of AdCMVLuc with DOTAP:cholesterol liposomes resulted in a decrease in gene expression in a dose-dependent manner. AdCMVLuc was mixed with varying amounts of DOTAP:cholesterol liposomes and incubated at room temperature for 10 min before injection. Gene expression in lung and liver was assayed 72 h following the injection (n = 6). *P < 0.05; **P < 0.01 (versus Ad alone).
Pulmonary gene transfer via sequential protocol is Ad vector dose-dependent
Figure 3 shows gene expression in liver and lung 3 days following injection of various amounts of AdCMVLuc, alone or following preinjection of DOTAP:cholesterol liposomes (0.9 μmol/mouse). Increasing the amount of AdCMVLuc from 1 to 4 × 1010 particles/mouse led to a significant increase in gene expression in the lung. Further increase in AdCMVLuc dose was associated with a much smaller increase in gene expression in the lung. Preinjection of cationic liposomes improved gene expression in the lung at all AdCMVLuc doses examined. Increasing the virus dose also led to an increase in gene expression in the liver. However, in contrast to the lung, preinjection of cationic liposomes either had no effect or inhibited gene expression in the liver.
Figure 3.
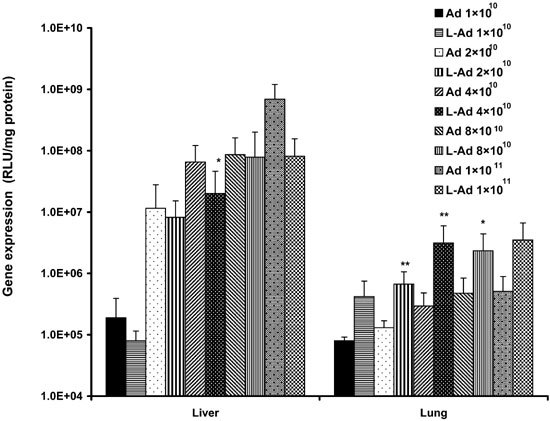
In vivo gene transfer via sequential protocol is virus dose-dependent. Mice received tail vein injection of DOTAP:cholesterol liposomes (0.9 μmol DOTAP/mouse) followed by various amounts of AdCMVLuc. Gene expression in lung and liver was assayed 72 h following the injection (n = 7). *P < 0.05; **P < 0.01 (versus Ad alone).
Time course of gene expression following sequential injection of cationic liposomes and AdCMVLuc
Figure 4 shows gene expression in lung and liver at days 3, 7 and 14 following sequential injection of DOTAP:cholesterol liposomes (0.9 μmol/mouse) and AdCMVLuc (4 × 1010 particles/mouse). Gene expression appears to peak at day 7 and decline gradually thereafter. However, a significant level of expression could still be detected at day 14. A similar pattern was noticed when AdCMVLuc alone was injected into mice, although the level of gene expression in the lung was lower compared with the sequential protocol (data not shown).
Figure 4.
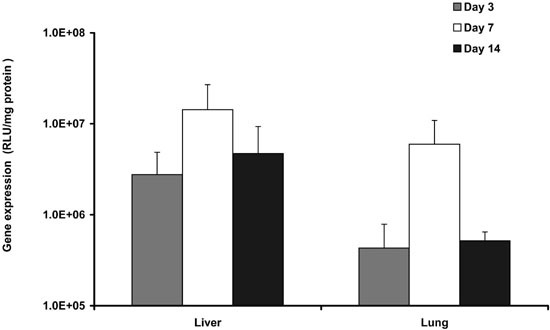
Time course of gene expression following in vivo gene transfer via the sequential protocol. Mice received tail vein injection of DOTAP:cholesterol liposomes and AdCMVLuc as described in the legend to Figure 1. Gene expression in lung and liver was assayed at indicated times following the injection (n = 3).
Identification of transfected cell type
Having defined the optimal conditions with AdCMVLuc, the sequential injection protocol was further evaluated using AdCMVLacZ as a reporter gene. Mice received intravenous injections of DOTAP:cholesterol liposomes followed by AdCMVLacZ. Seventy-two h after injection, mice were killed and lungs were fixed and stained for β-galactosidase activity using X-gal at 37°C. No blue cells were observed in the lungs of mice treated with a control vector (AdCMVLuc) (Figure 5a). In contrast, there was localized gene expression throughout the distal lung of mice that received AdCMVLacZ Figure 5b. The primary loci of LacZ expression appeared to be the capillary endothelium located within the alveolar septum Figure 5b. No sign of inflammation was noticed in any of the lung sections examined. The cell type of transfected cells was further analyzed by anti-platelet endothelial cell adhesion molecule (PECAM) immunofluorescence staining of lung sections of mice receiving cationic liposomes followed by AdCMVEGFP. PECAM is a transmembrane adhesion molecule expressed at high levels on endothelial cells. As shown in Figure 6a and b, EGFP signal was substantially co-localized with PECAM labeling, confirming that endothelial cells were the major cell type transfected.
Figure 5.
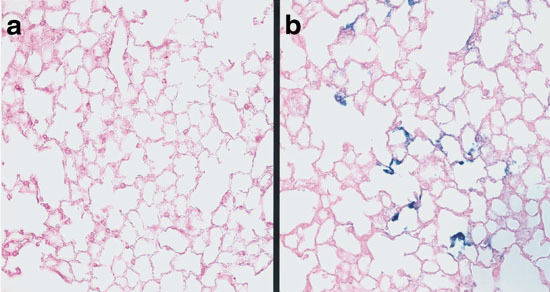
Light photomicrograph of lung sections of mice injected with DOTAP:cholesterol liposomes followed by Ad virus containing LacZ cDNA. Three days following the injection, mice were killed and lungs were perfused intravascularly with 2% paraformaldehyde and incubated in X-gal solution overnight at 37°C. The lungs were then embedded in paraffin and thin sections were prepared. The sections were counterstained with eosin and viewed with a Nikon light microscope. There was no detectable LacZ expression in the lung of control mouse injected with AdCMVLuc (a). In contrast, there was localized expression throughout the distal lung of mice injected with AdCMVLacZ (b). Expression was seen in structures within the alveolar septum that appeared to be capillary endothelium.
Figure 6.
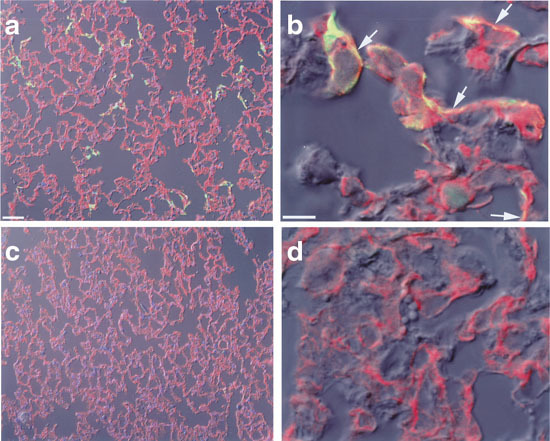
Immunofluorescence detection of transgene-expressing cells. Mice received tail vein injection of DOTAP:cholesterol liposomes followed by AdCMVEGFP. Three days later mice were killed, lungs were collected, and cryosections were prepared. Immunofluorescence staining of PECAM in lungs was performed as described in Materials and methods. (a) Representative lung sections of mice receiving AdCMVEGFP. (b) Confocal microscopic images of lung sections of mice receiving AdCMVEGFP. (c) Lungs of control mice receiving AdCMVLuc. (d) Confocal microscopic images of lungs of control mice.
Sequential injection of cationic liposomes and Ad vectors leads to an increased uptake of ad vectors by the lung
We hypothesized that the improved pulmonary gene transfer via the sequential injection protocol might be due to an increased uptake of Ad vectors by the lung compared with i.v. injection of Ad vectors alone. To test this hypothesis, mice received tail vein injections of AdCMVLuc, alone or following the injection of DOTAP:cholesterol liposomes. At days 1 and 7 following the injection, mice were killed and lungs were perfused and collected. The amount of luciferase cDNA in mouse lungs was evaluated by a semi-quantitative PCR. As shown in Figure 7, the amount of luciferase cDNA was slightly decreased from day 1 to day 7. However, at both time-points, greater amounts of luciferase cDNA were detected in the lungs of mice that received sequential injections of cationic liposomes and AdCMVLuc, than in the lungs of mice that received AdCMVLuc alone.
Figure 7.
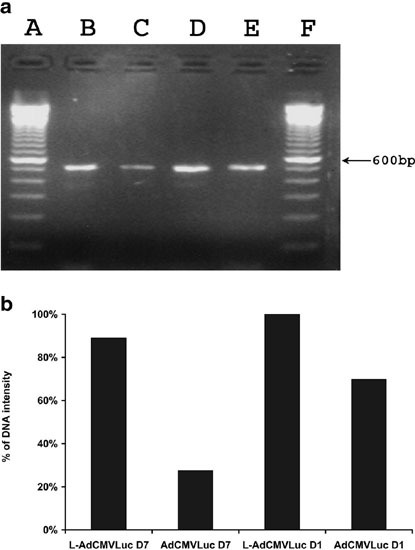
PCR analysis of the amount of viral DNA in lungs at days 1 and 7 following tail vein injection of AdCMVLuc alone or DOTAP:cholesterol liposomes followed by AdCMVLuc. Mice received tail vein injection of AdCMVLuc, alone or following the injection of DOTAP:cholesterol liposomes. At days 1 and 7, mice were killed and lungs were collected. Genomic DNA was extracted from the lungs and used as the template. Luciferase cDNA-specific primers used in the PCR amplification were LUC-1 (5′-CGTCACATCTCATCTACCTC-3′) and LUC-2 (5′-GTATCCCTGGAAGATGGAAG-3’), which generated a 510-bp fragment as shown in (a). (a and f) 100 bp DNA marker; (b) L-AdCMVLuc (day 7); (c) AdCMVLuc (day 7); (d) L-AdCMVLuc (day 1); (e) AdCMVLuc (day 1). The results of densitometry analysis of the amplified fragments were shown in (b) and were expressed as the percentage of the intensity of the DNA fragment on lane D.
Sequential injection of cationic liposomes and Ad vectors induces minimal proinflammatory cytokine response at early time-points
Recent studies have shown that systemic administration of cationic liposome/DNA complexes is associated with an acute proinflammatory cytokine response,17,18,19,20 which can be partially overcome via the sequential protocol.10 To examine whether a similar cytokine response also occurs in Ad vector-mediated pulmonary gene transfer, mice received tail vein injections of AdCMVLuc, alone or following the injection of DOTAP:cholesterol liposomes. Mice were killed 4 h following the injections and cytokine levels in serum or bronchoalveolar lavage fluid were examined. As shown in Figure 8, only a minimal level of cytokine response was observed in mice receiving AdCMVLuc, alone or following the injection of DOTAP:cholesterol liposomes. The levels of three cytokines examined (TNF-α, IL-6 and IL-12) in serum Figure 8aor bronchoalveolar lavage fluid Figure 8bwere comparable to those in control mice injected with PBS.
Figure 8.
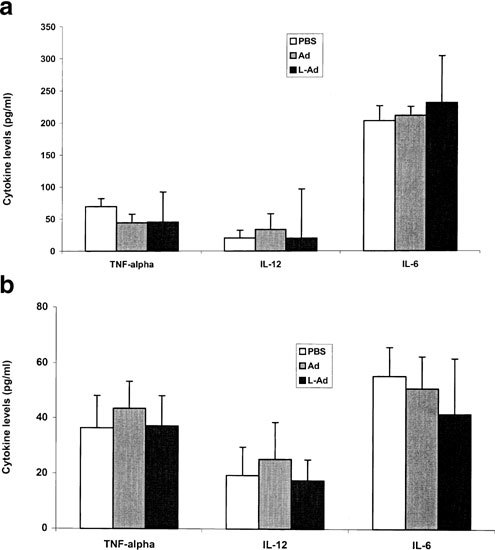
Cytokine levels in mouse serum (a) or bronchoalveolar lavage (b) 4 h following tail vein injection of AdCMVLuc alone or DOTAP:cholesterol liposomes followed by AdCMVLuc (n = 6).
Discussion
We have shown in this study that preinjection of cationic liposomes followed by Ad vectors significantly improves gene expression in the lung Figure 1a. Cationic liposomes enhance pulmonary gene transfer by Ad vectors in a dose-dependent manner. The major cells that are infected appear to be endothelial cells Figures 5 and 6Figures 5 and 6and gene expression lasts for up to 2 weeks Figure 4. The improvement in pulmonary gene transfer is associated with a decrease in the level of gene expression in the liver Figure 1b. Based on cytokine response, this protocol is safe and the liposomes used do not add to the Ad vector-related toxicities Figure 8.
The sequential injection protocol was initially described by Song and colleagues21 to address the roles of cationic liposomes in cationic lipid-mediated pulmonary gene transfer via the vascular route. Recently Tan et al10 have extended these studies by showing that pulmonary gene transfer via sequential injection protocol is even more efficient than that via cationic liposome/DNA complexes. Moreover, the sequential injection protocol overcomes, at least partially, the CpG-related proinflammatory cytokine response that is associated with systemic administration of cationic liposome/DNA complexes. The mechanism of pulmonary gene transfer via sequential protocol is not clearly understood at present. It might be due to a transient slow-down of pulmonary microcirculation by preinjected liposomes, which allows for efficient interaction of subsequently injected DNA with the target cells (endothelial cells). The improved lung gene transfer by Ad vectors via the sequential protocol might be due to a similar mechanism. This was supported by the observation that more viral DNA was detected in lungs of mice that received sequential injection of cationic liposomes and Ad vectors than in lungs of mice that received Ad vectors alone Figure 7. The increased pulmonary uptake of Ad vectors may play an important role in improving the efficiency of gene expression in the lung. Ad vectors are likely to be taken up by endothelial cells in free form.
A number of studies have shown that complexation of Ad vectors with cationic lipids improves infection of cultured cells, especially those cells that lack the receptors for Ad virus.11,12,13,14,15,16 Complexation of Ad vectors with cationic liposomes has also been shown to enhance the infection of airway epithelial cells upon instillation into mouse trachea.12 Cationic liposomes enhance Ad vector-mediated infection mainly by improving the cellular uptake. However, mixing of AdCMVLuc with DOTAP:cholesterol liposomes had minimal effect on infection of cultured mouse lung endothelial cells (MLEC) despite an increased cellular uptake of Ad vectors (data not shown). At high doses, DOTAP:cholesterol liposomes inhibited infection of MLEC in a dose-dependent manner (data not shown). Similarly, mixing of DOTAP:cholesterol liposomes with AdCMVLuc led to a decreased level of gene expression in the lung Figure 2. These differences might reflect the different cellular barriers in Ad-mediated infection in different cell types. MLEC might lack an efficient mechanism in mediating the release of Ad vectors into cytosol following cellular uptake of cationic lipid-complexed Ad vectors. Despite an improved cellular uptake, cationic lipids may inhibit the subsequent release of Ad vectors into cytosol, thus resulting in an overall decreased level of gene expression. More studies are needed to better understand the mechanism of infection of MLEC via Ad vectors, alone or complexed with cationic liposomes.
Several approaches have been proposed to redirect Ad vectors to pulmonary endothelium. These include genetic modification to render the vectors endothelium-specific and the use of bispecific conjugate. Thus far, there has been no successful report of improving Ad-mediated infection of pulmonary endothelium via genetic modification of Ad vectors. Redirecting Ad vectors to pulmonary endothelium has been demonstrated using a bispecific conjugate.8 Our study provides a different approach that is also highly efficient in improving pulmonary gene transfer by Ad vectors via the vascular route. The advantages of our method lie in its simplicity. Cationic liposomes can be prepared in large quantities in a cost-effective manner. This protocol requires no modification of Ad vectors, therefore it can be used for different Ad vectors containing different therapeutic genes. This protocol might be combined with other approaches to achieve a synergistic effect in lung targeting. This method may also be extended to pulmonary gene transfer using other types of viral vectors that carry fewer side-effects than Ad vectors.
It should be noted that while the sequential protocol brings about a significant improvement in pulmonary gene transfer and a concomitant decrease in the level of gene expression in the liver, the absolute level of gene expression in the lung is still relatively lower than that in the liver. A similar phenomenon was also observed in the study by Reynolds et al8 using a bispecific antibody. Further improvements in lung targeting may require the combination of several different approaches and/or the inclusion of an endothelium-specific promoter. These possibilities are currently under investigation in this laboratory.
Materials and methods
Chemicals
1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) and dioleoylphosphatidyl-ethanolamine (DOPE) were purchased from Avanti Lipids (Alabaster, AL, USA). Cholesterol was obtained from Sigma (St Louis, MO, USA). Luciferase assay kit was from Promega (Madison, WI, USA). Other chemicals were of reagent grade.
Animals
For in vivo experiments, female CD-1 mice aged 4–6 weeks (16–18 g) were purchased from Charles River Laboratories (Wilmington, MA, USA). The animals were kept at the University of the Pittsburgh Animal Facilities. All experiments were conducted under protocols approved by the Institutional Animal Care and Use Committee.
Adenoviral vectors
AdCMVLuc is a recombinant E1-, E3-deleted Ad vector expressing firefly luciferase under the control of the cytomegalovirus (CMV) promoter. AdCMVLacZ is a recombinant E1-, E3-deleted Ad vector expressing Escherichia coli β-galactosidase (LacZ) under the control of the CMV promoter. AdCMVEGFP is a recombinant E1-, E3-deleted Ad vector expressing enhanced green fluorescence protein (EGFP). They were propagated in a permissive 293 cell line, purified by centrifugation through cesium chloride gradients and plaque titered on 293 cells by standard techniques.
Preparation of liposomes
Liposomes containing DOTAP and cholesterol in a 1:1 molar ratio were prepared as follows. The lipid mixture in chloroform was dried under a stream of nitrogen as a thin layer in a 100-ml round-bottomed flask, which was further desiccated under vacuum for 2 h. The lipid film was hydrated in 5% dextrose in water to give a final concentration of 10 mg DOTAP/ml. Preparation of small unilamellar vesicles by extrusion was performed as follows. The lipid solution was briefly sonicated, followed by incubation at 50°C for 10 min and then sequentially extruded through polycarbonate membranes with the following pore sizes: 1.0, 0.6 and 0.2 μm. The size of liposomes was around 150 nm as measured by dynamic laser scattering using a Coulter N4SD particle sizer (Hialeah, FL, USA).
Sequential injection of cationic liposomes and Ad vectors
If not otherwise indicated, the amounts of DOTAP:cholesterol liposomes and Ad vector per mouse were 900 nmol and 4 × 1010 particles, respectively. All of the dilutions were made in saline. For sequential injection groups of six mice received first an injection of 150 μl liposome and then 150 μl adenovirus via the tail vein. Control mice received adenovirus in 300 μl saline. In a separate experiment, adenovirus was mixed with various amounts of DOTAP:cholesterol liposomes and the mixtures were incubated at room temperature (RT) for 10 min before injection.
Assay for luciferase activity
At 3 days or the indicated times following injection, mice were killed and organs were homogenized in 1 ml of ice-cold lysis buffer (0.05% Triton X-100, 2 mM EDTA, and 0.1 M Tris, pH 7.8) with a tissue tearer (BioSpec Products, Bartlesvile, OK, USA) for 20 s at high speed. The homogenates were then centrifuged at 14 000 g for 10 min at 4°C. Ten μl of the supernatant was analyzed with the luciferase assay system (Promega) using an automated LB953 luminometer (Berthod, Bad Wildbad, Germany). The protein content of the supernatant was measured with the BioRad protein assay system (BioRad, Hercules, CA, USA). Luciferase activity was expressed as relative lights units (RLU) per milligram of tissue protein.
X-gal staining
Mice received tail vein injection of DOTAP:cholesterol liposomes followed by AdCMVLacZ. Three days later, mice were killed and lungs were perfused intravascularly with 2% paraformaldehyde in PBS and inflated with this fixative to near total lung capacity for 1 h at room temperature. After rinsing with cold PBS, the lungs were incubated in X-gal staining solution (Invitrogen, Carlsbad, CA, USA) overnight at 37°C. The lungs were then embedded in paraffin and thin sections were prepared. The sections were counterstained with eosin and viewed with a Nikon light microscope.
Immunofluorescence staining
Mice were injected with DOTAP:cholesterol liposomes followed by AdCMVEGFP. Three days later, mice were killed and lungs were perfused intravascularly with PBS followed by 2% paraformaldehyde in PBS, and inflated with this fixative to near total lung capacity. The lungs were rinsed with cold PBS and immersed in 30% sucrose in PBS at 4°C overnight. The lungs were then quickly frozen in OCT with dry ice. Five-micrometer lung cryosections were then cut. Following three washes in PBS containing 0.5% bovine serum albumin and 0.15% glycine (PBG buffer) sections were incubated in a 1:100 dilution of rat anti-mouse PECAM (PharMingen, San Diego, CA, USA) for 1 h at RT, washed with PBG three times, and labeled with Cy3-labeled goat anti-rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h at RT. Following three further washes with PBG the sections were stained with Hoescht dye 33258 (Sigma) for 30 s and mounted in Gelvatol (Monsanto, St Louis, MO, USA). Cells were visualized using an Olympus Provis microscope (Olympus, Tokyo, Japan) using a triple pass (blue/green/red) cube, which allows excitation at 384 nm and collection at 540 nm. Images were collected using an Optronics Magnifier Camera (Santa Barbara, CA, USA) or with a Leica TCS NT confocal microscope with a 60× oil immersion objective at 1024 × 1024 pixel resolution.
PCR analysis
Mice received tail vein injections of AdCMVLuc, alone or following DOTAP:cholesterol liposomes. At days 1 and 7 following injection, the mice were killed and the lungs were collected. Genomic DNA in lungs was extracted using the DNeasy Tissue Kit (Qiagen, Valencia, CA, USA). Six hundred ng of DNA was analyzed by the Mastercycle Gradient PCR (Eppendorf, Westbury, NY, USA), using primers specific for the luciferase gene that generate a 510-bp fragment. Primers were synthesized by MWG-Biotech (High Point, NC, USA) and their sequences were CGTCACATCTCATCTACCTC (LUC-1) and GTATCCCTGGAAGATGGAAG (LUC-2), respectively. After an initial denaturation for 5 min at 95°C, 30 cycles of amplification were performed using cycle times of 45 s at 95°C, 45 s at 58°C and 1 min at 72°C. A 5-min extension step at 68°C followed the PCR amplification. Amplification products were analyzed by electrophoresis on a 2% agarose gel.
Immunoassays
At 4 h following injection, mice were bled from the retroorbital sinuses under anesthesia. The blood was allowed to stay at 4°C for 4 h and then centrifuged at 14000 g for 10 min at 4°C. Serum was collected and stored at –80°C until analyzed. In addition, the lungs of the mice were perfused with PBS intratracheally and bronchoalveolar lavage fluid was then collected. The concentrations of cytokines (TNF-α, IL-12 and IL-6) in serum or bronchoalveolar lavage fluid were determined with the specific cytokine immunoassay kit (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
Data were expressed as means ± standard derivations and analyzed by the two-tailed unpaired Student's t test using the PRISM software program (GraphPad Software, San Diego, CA, USA). Data were considered significant if P < 0.05 (*) and very significant if P < 0.01 (**).
Acknowledgements
This work was supported by NIH grants HL63080 (to S Li), HL32154 and GM53789 (to B Pitt), and HL66949 and AR62225 (to P Robbins).
References
- 1.Benihoud K, Yeh P, Perricaudet M. Adenovirus vectors for gene delivery. Curr Opin Biotechnol. 1999;10:440. doi: 10.1016/S0958-1669(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 2.Connelly S. Adenoviral vectors for liver-directed gene therapy. Curr Opin Mol Ther. 1999;1:565. [PubMed] [Google Scholar]
- 3.Krasnykh VN, Douglas JT, van Beusechem VW. Genetic targeting of adenoviral vectors. Mol Ther. 2000;1:391. doi: 10.1006/mthe.2000.0062. [DOI] [PubMed] [Google Scholar]
- 4.Krasnykh V. Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J Virol. 2001;75:4176. doi: 10.1128/JVI.75.9.4176-4183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shayakhmetov DM, Papayannopoulou T, Stamatoyannopoulos G, Lieber A. Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. J Virol. 2000;74:2567. doi: 10.1128/JVI.74.6.2567-2583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wickham TJ. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J Virol. 1996;70:6831. doi: 10.1128/jvi.70.10.6831-6838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas JT. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1548. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds PN. A targetable injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Mol Ther. 2000;2:562. doi: 10.1006/mthe.2000.0205. [DOI] [PubMed] [Google Scholar]
- 9.Trepel M, Grifman M, Weitzman MD, Pasqualini R. Molecular adaptors for vascular-targeted adenoviral gene delivery. Hum Gene Ther. 2000;11:1971. doi: 10.1089/10430340050143408. [DOI] [PubMed] [Google Scholar]
- 10.Tan Y. Sequential injection of cationic liposome and plasmid DNA effectively transfects the lung with minimal inflammatory toxicity. Mol Ther. 2001;3:673. doi: 10.1006/mthe.2001.0311. [DOI] [PubMed] [Google Scholar]
- 11.Hodgson CP, Solaiman F. Virosomes: cationic liposomes enhance retroviral transduction. Nat Biotechnol. 1996;14:339. doi: 10.1038/nbt0396-339. [DOI] [PubMed] [Google Scholar]
- 12.Fasbender A. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo. J Biol Chem. 1997;272:6479. doi: 10.1074/jbc.272.10.6479. [DOI] [PubMed] [Google Scholar]
- 13.Arcasoy SM. Polycations increase the efficiency of adenovirus-mediated gene transfer to epithelial and endothelial cells in vitro. Gene Therapy. 1997;4:32. doi: 10.1038/sj.gt.3300349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu C, DeYoung MB, Finn A, Dichek DA. Cationic liposomes enhance adenovirus entry via a pathway independent of the fiber receptor and alpha(v)-integrins. Hum Gene Ther. 1998;9:507. doi: 10.1089/hum.1998.9.4-507. [DOI] [PubMed] [Google Scholar]
- 15.Clark PR. Polycations and cationic lipids enhance adenovirus transduction and transgene expression in tumor cells. Cancer Gene Ther. 1999;6:437. doi: 10.1038/sj.cgt.7700074. [DOI] [PubMed] [Google Scholar]
- 16.Chillon M, Lee JH, Fasbender A, Welsh MJ. Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene Therapy. 1998;5:995. doi: 10.1038/sj.gt.3300665. [DOI] [PubMed] [Google Scholar]
- 17.Freimark BD. Cationic lipids enhance cytokine and cell influx levels in the lung following administration of plasmid: cationic lipid complexes. J Immunol. 1998;160:4580. [PubMed] [Google Scholar]
- 18.Li S. Effect of immune response on gene transfer to the lung via systemic administration of cationic lipidic vectors. Am J Physiol. 1999;276:796. doi: 10.1152/ajpcell.1999.276.4.C796. [DOI] [PubMed] [Google Scholar]
- 19.Yew NS. Contribution of plasmid DNA to inflammation in the lung after administration of cationic lipid:pDNA complexes. Hum Gene Ther. 1999;10:223. doi: 10.1089/10430349950019011. [DOI] [PubMed] [Google Scholar]
- 20.Lanuti M. Cationic lipid:bacterial DNA complexes elicit adaptive cellular immunity in murine intraperitoneal tumor models. Cancer Res. 2000;60:2955. [PubMed] [Google Scholar]
- 21.Song YK, Liu F, Liu D. Enhanced gene expression in mouse lung by prolonging the retention time of intravenously injected plasmid DNA. Gene Therapy. 1998;5:1531. doi: 10.1038/sj.gt.3300770. [DOI] [PubMed] [Google Scholar]


