Summary:
We evaluated 40 patients undergoing high-dose chemo/radiotherapy (HDCT) and hematopoietic stem cell transplantation (HSCT) (allogeneic (22), autologous (18)) to determine the safety and feasibility of administering low molecular weight heparin (LMWH) as hepatic veno-occlusive disease (VOD) prophylaxis. Patients received a once daily subcutaneous injection of dalteparin 2500 anti-Xa i.u. commencing the day prior to starting HDCT, and continuing until day +30 post HSCT or hospital discharge, whichever came first. Dosage adjustments were made for patients developing renal failure. All bleeding episodes were recorded and graded and VOD was diagnosed and graded according to Seattle criteria. At 100 days of follow-up, the overall survival and probability of regimen-related mortality were 85 and 15%, respectively. Nine patients developed VOD. The probability of developing VOD post allogeneic and autologous HSCT was 28% (95% CI, 6–45) and 17% (95% CI, 0–32), respectively. VOD was graded as moderate (n=8) and severe (n=1). VOD resolved in all cases except for one patient who died secondary to severe VOD and multiorgan failure. Clinically significant bleeding episodes occurred in three patients; 24 patients developed minor bleeding not requiring specific therapy. All bleeding episodes resolved. These results suggest that LMWH for VOD prophylaxis is safe with a low incidence of serious bleeding events. Whether it is superior to unfractionated heparin, however, is unknown and should be addressed within the context of a randomized controlled trial.
Keywords: low molecular weight heparin, VOD
High-dose chemo/radiotherapy (HDCT) and hematopoietic stem cell transplantation (HSCT) are widely used to treat many malignant and nonmalignant conditions. Regimen-related toxicity (RRT) remains a frequent and major complication of HSCT.1 Hepatic veno-occlusive disease (VOD) is one of the most common life-threatening RRTs reported to occur in 5–60% of patients undergoing HSCT.2,3,4,5,6,7,8
Clinically, VOD is characterized by jaundice, painful hepatomegaly, ascites and weight gain from fluid retention. In its most severe form, it often leads to multiorgan failure and death.6,7,8 Hepatic VOD is thought to result from injury to hepatocytes surrounding the central veins in zone 3 of the liver acinus. Early pathologic changes include edema of the subendothelium of hepatic venules, followed by thrombosis of the venular lumen.9 Risk factors for VOD have been reported to include a previous history of viral hepatitis, abnormal liver function tests at the time of HDCT and HSCT, the use of high-dose preparative regimens, mismatched or unrelated donor HSCTs, and the use of vancomycin or amphotericin B.2,3,4,5,6,7,8,10,11,12,13
A number of prophylactic strategies have been explored in an attempt to prevent the injury and subsequent thrombosis of hepatic venules and therefore decrease the risk of VOD. They include the administration of prostaglandin E1, pentoxifylline, ursodeoxycholic acid and low-dose standard unfractionated heparin (UH).14,15,16,17,18,19,20,21,22,23 The results of clinical trials using these prophylactic agents have been quite variable and thus their role in VOD prophylaxis remains controversial.
Low-molecular weight heparin (LMWH) is formed by depolymerization of UH.24 In several large clinical trials, when administered as treatment or prophylaxis of deep venous thrombosis (DVT), it has been found to be as efficacious as UH with a low incidence of bleeding complications.25,26,27 There are, however, few published reports specifically examining the safety and efficacy of LMWH as VOD prophylaxis. Our centre therefore undertook a prospective pilot study of LMWH as VOD prophylaxis for patients undergoing HDCT and HSCT.
Patients and methods
Patients
Between October 2000 and November 2001, 40 patients underwent HDCT and HSCT (allogeneic (22), autologous (18)) at the Queen Elizabeth II Health Sciences Centre (QEII HSC), the quaternary referral centre for Atlantic Canada. Patient characteristics are listed in Table 1. Eligibility criteria included: (1) all patients undergoing HSCT for malignant or nonmalignant disease excluding aplastic anemia; (2) age ⩾15 and ⩽65 years; and (3) satisfactory end-organ function. Exclusion criteria were: (1) significant renal dysfunction (measured 24 h creatinine clearance <60 ml/min); (2) previous history of heparin-induced thrombocytopenia (HIT); (3) active bleeding or lesions at risk of bleeding at study enrollment where systemic anticoagulation was felt to be contraindicated; and (4) the use of any other anticoagulant or antithrombotic drugs during the first 30 days post-HSCT except for locally administered anticoagulants or thrombolytic agents in central venous catheters to maintain patency.
Table 1.
Patient characteristics
| No. of patients | 40 |
| Sex (M/F) | 27/13 |
| Median age (range) | 49 years (15–64) |
| Diagnosis | |
| Non-Hodgkin's lymphoma | 13 |
| Multiple myeloma | 9 |
| Acute myeloid leukemia | 6 |
| Myelodysplastic syndrome | 4 |
| Myelofibrosis | 2 |
| Chronic myeloid leukemia | 2 |
| Hodgkin's disease | 2 |
| Acute lymphoblastic leukemia | 2 |
| Median time from diagnosis to HSCT (range) | 10 months (3–60) |
| Conditioning regimen | |
| Autologous HSCT | |
| VP16-MEL | 8 |
| MEL-TBI | 7 |
| MEL | 2 |
| VP16-CY-TBI | 1 |
| Allogeneic HSCT | |
| BU-CY | 18 |
| CY-TBI | 4 |
| Prior liver disease | 4 |
| Hepatic lymphomatous involvement | |
| Anti-HCV+ | |
| Anti-HBs+ | |
| History of chemical hepatitis |
M=male; F=female; HSCT=hematopoietic stem cell transplant; VP16=etoposide; MEL=melphalan; TBI=total body irradiation; BU=busulfan; CY=cyclophosphamide.
The study protocol was approved by the Institutional Review Board at the QEII HSC and all patients provided written informed consent for study participation.
Conditioning regimens
The majority of patients received either busulfan or total body irradiation-based conditioning regimens. They included: busulfan 16 mg/kg and cyclophosphamide 120 mg/kg (n=18); etoposide 60 mg/kg and melphalan 180 mg/m2 (n=8); melphalan 140 mg/m2 and fractionated total body irradiation (fTBI) 200 cGy × 4 (n=7); cyclophosphamide 180 mg/kg and fTBI 200 cGy × 6 (n=3); melphalan 200 mg/m2 (n=2); etoposide 1.8 g/m2, cyclophosphamide 150 mg/kg and fTBI 200 cGy × 6 (n=1); and cyclophosphamide 120 mg/kg and fTBI 200 cGy × 6 (n=1).
Type of transplant
Twenty two patients underwent an allogeneic HSCT. The stem cell source for these 22 patients was a histocompatible sibling in 19 patients and an HLA A, B, C and DR-matched unrelated donor (using high-resolution molecular DNA typing) in three patients. Eighteen patients underwent autologous HSCT. Stem cells were collected in all autologous HSCT recipients and from all sibling donors by apheresis of G–CSF mobilized peripheral blood; stem cells were collected from unrelated donors by standard bone marrow harvest. The median absolute CD34 cell count infused to allogeneic recipients was 5.4 × 106/kg (range 2.1–20.8) and to autologous recipients was 7.1 × 106/kg (range 1.9–33.9).
Graft-versus-host disease (GVHD) prophylaxis
GVHD prophylaxis for allogeneic hematopoietic stem cell recipients consisted of intravenous cyclosporine A (CsA) and short-course methotrexate (MTX). Treatment of acute GVHD consisted of high-dose corticosteroids, with the addition of antithymocyte globulin (ATG) in those patients not responding to corticosteroids.
Supportive care
All patients were nursed in high-efficiency particulate air (HEPA) filtered, positive-pressure rooms until engraftment (absolute neutrophil count (ANC) >0.5 × 109/l). Tunneled triple lumen Hickman central venous catheters were employed. Irradiated blood products were administered to keep the morning hemoglobin (Hgb) concentration >80 g/l and morning platelet count ⩾10 × 109/l. CMV-negative blood products were administered to CMV seronegative donor/recipient pairs. Infection prophylaxis included low-dose acyclovir in all patients; fluconazole prophylaxis was administered only to those patients undergoing allogeneic HSCT; antibacterial prophylaxis was not used, but intravenous antibiotics were initiated for febrile neutropenia. Trimethaprim/sulphamethoxazole was commenced as PCP prophylaxis in allogeneic stem cell recipients from the point of engraftment until immunosuppression was discontinued. Fluconazole or amphotericin B was used as required for documented or suspected fungal infection.
Study design
The primary objective of the study was to determine the safety and feasibility of LMWH administration to HSCT recipients. Therefore, careful assessments of bleeding events, red cell and platelet transfusion requirements, time to white cell and platelet engraftment and adverse events were made. The secondary objective was to determine the incidence and severity of VOD for patients undergoing HSCT. Once daily at 0800 h, patients received a single subcutaneous injection of dalteparin 2500 anti-Xa i.u. (supplied as prefilled, single-dose syringes from Pharmacia, Canada) commencing the day prior to starting HDCT and continuing until day +30 post HSCT or hospital discharge, whichever came first. Dosage adjustments were made in cases of renal failure as follows: patients developing moderate to severe renal insufficiency (calculated creatinine clearance ⩽20 ml/min) received a 50% dose reduction of dalteparin (ie, 1250 anti-Xa i.u.). In those patients requiring dialysis for renal failure, dalteparin was not administered. If renal function subsequently improved, then dalteparin administration was recommenced. Patients were reevaluated at day +100 to determine HSCT outcome and whether cases of documented VOD had resolved.
Data collection
Daily patient evaluation while receiving dalteparin included patient weight, oxygen saturation, liver size, presence of right upper quadrant (RUQ) abdominal pain, ascites or pleural effusions, complete blood count (CBC), serum creatinine, assessment of bleeding and GVHD; adverse events, grading of RRT and the number of red cell and platelet transfusions were recorded; liver function tests (bilirubin, AST, ALT, alkaline phosphatase), INR, PTT and fibrinogen were measured twice weekly. Patient evaluation at day +100 included patient weight, liver size, presence of RUQ abdominal pain, CBC, PTT, INR, fibrinogen, serum creatinine and liver function tests.
VOD evaluation
VOD was diagnosed according to Seattle criteria6 requiring the presence of at least two of the following features before day +20 following stem cell reinfusion: (1) hyperbilirubinemia (total serum bilirubin >34 μmol/l) and (2) the presence of hepatomegaly or RUQ abdominal pain or (3) unexplained weight gain (>2% baseline body weight) or ascites in the absence of other identifiable causes. The severity of VOD was graded as mild, moderate or severe according to modified Seattle criteria (1): (1) mild=total serum bilirubin >34 μmol/l but <100 μmol/l and weight gain >2% over baseline but <5% or hepatomegaly or RUQ abdominal pain; (2) moderate=total serum bilirubin ⩾100 μmol/l but <340 μmol/l or weight gain ⩾5% over baseline or documented ascites; (3) severe=total serum bilirubin ⩾340 μmol/l or ascites compromising respiratory function or hepatic encephalopathy or death from VOD.
Grading of bleeding episodes
All bleeding episodes were recorded and graded according to the following criteria: grade 0=no bleeding; grade 1=minor mucosal bleeding or petechiae not requiring packed red blood cell (PRBC) transfusion; grade 2=any bleeding episode requiring transfusion of 1–2 U of PRBCs/episode in a 24-h period; grade 3=any bleeding episode requiring transfusion of >2 U of PRBCs/episode, but <4 U in a 24-h period or retroperitoneal bleeding; grade 4=any bleeding causing hemodynamic instability or requiring transfusion of ⩾4 U of PRBCs in a 24-h period or any CNS bleeding.
Discontinuation criteria
Treatment was discontinued or interrupted for patients meeting the following criteria: (1) 30 days post-HSCT limit; (2) discharge from hospital; (3) development of dialysis-dependent renal failure; (4) patient request; (5) serious bleeding episode (⩾grade 2) at the discretion of the investigator; (6) development of a thrombotic event necessitating treatment with another anticoagulant or higher dose of LMWH.
Statistical analysis
Estimates of overall survival, regimen-related mortality and the probability of VOD were calculated using the methods of Kaplan and Meier.28
Results
Patients
In all, 40 patients were enrolled in the study. There were 27 males and 13 females. The median age at HSCT was 49 y (range 15–64) and the median time from diagnosis to HSCT was 10 months (range 3–60). Diagnoses included: non-Hodgkin's lymphoma (13), multiple myeloma (9), acute myeloid leukemia (6), myelodysplastic syndrome (4), myelofibrosis (2), chronic myeloid leukemia (2), Hodgkin's disease (2) and acute lymphoblastic leukemia (2). Of these, 22 patients underwent allogeneic HSCT (related donor (19), unrelated donor (3)) and 18 patients underwent autologous HSCT. All patients had normal liver function tests (LFTs) prior to commencing HDCT except for two patients (chronic active hepatitis C infection (n=1), lymphomatous involvement of the liver (n=1)); two other patients had a history of hepatic disease prior to HSCT, but their LFTs were normal at the time of HDCT. The median duration of dalteparin administration was 25 days (range 17–39); two patients required dalteparin dose reduction for renal impairment. All 40 patients completed the study as per the protocol; early termination (before day +30 or hospital discharge) occurred in three patients because of dialysis-dependent renal failure (n=1) or death (n=2).
Transplant outcome
At 100 days of follow-up, the overall survival and the probability of regimen-related mortality was 85% (95% CI, 75–97) and 15% (95% CI, 3–25) respectively. Six patients have died (cardiac arrest (1), multiorgan failure (1), infection (2), respiratory failure (1) and demyelinating peripheral neuropathy (1)) on day +9, +16, +45, +92, +56 and +80 post HSCT respectively. Two patients (UPN 401, 448) had disease progression on or before day +100. In total, 38 patients engrafted with a median time to ANC >0.5 × 109/l and sustained platelet >20 × 109/l of 18 days (range 11–32) and 13 days (range 6–45), respectively; two patients (UPN 403, 406) died before engraftment on days +9 and +16, respectively.
RRT
RRT was ⩾grade III in one or more organ systems in five patients (12.5%). Two patients (UPN 414 and 432) developed lung toxicity requiring intubation and mechanical ventilation because of idiopathic interstitial pneumonitis; UPN 414 improved with high-dose corticosteroid therapy and was extubated, but he eventually died day +92 secondary to abdominal sepsis; UPN 432 continued to deteriorate despite treatment with high-dose corticosteroids and died of respiratory failure on day +56. UPN 409 developed acute renal failure (ARF) secondary to drug-induced acute tubular necrosis necessitating a brief course of dialysis before renal function recovered. UPN 403 died of sudden cardiac arrest on day +9. Autopsy failed to reveal a specific cause of death. Finally UPN 406 died on day +16 of ARF, hepatic VOD and acute respiratory distress syndrome (ARDS). All five patients who developed ⩾grade III toxicity had undergone allogeneic HSCT (sibling (4), unrelated donor (1)). RRT was ⩽grade II for all autologous HSCT recipients.
Incidence of VOD
Nine patients developed VOD (autologous (3), allogeneic (6)). The probability of developing VOD post allogeneic and autologous HSCT was 28% (95% CI, 6–45) and 17% (95% CI, 0–32), respectively (Figure 1). Patient characteristics and outcome are detailed in Table 2. Five of the nine patients had been heavily pre-treated with ⩾3 lines of prior chemotherapy with or without concomitant radiotherapy. Five patients had received TBI in the conditioning regimen, three of which underwent unrelated donor allogeneic HSCT. One patient had chronic active hepatitis C infection, one patient had positive viral serology for hepatitis B antibody indicating prior hepatitis B infection, one patient had lymphomatous involvement of the liver at the time of HSCT and one other patient had a history of drug-induced hepatitis that had resolved prior to commencing HDCT. VOD was graded as moderate in eight patients and severe in one patient (UPN 406). VOD was diagnosed a median of 9 days (range 6–12) post HSCT and the median peak serum bilirubin was 117 mol/l (range 36–264). VOD resolved in all cases before day 100 post HSCT except for UPN 406 who died secondary to severe VOD and subsequent multiorgan failure 16 days following HSCT.
Figure 1.
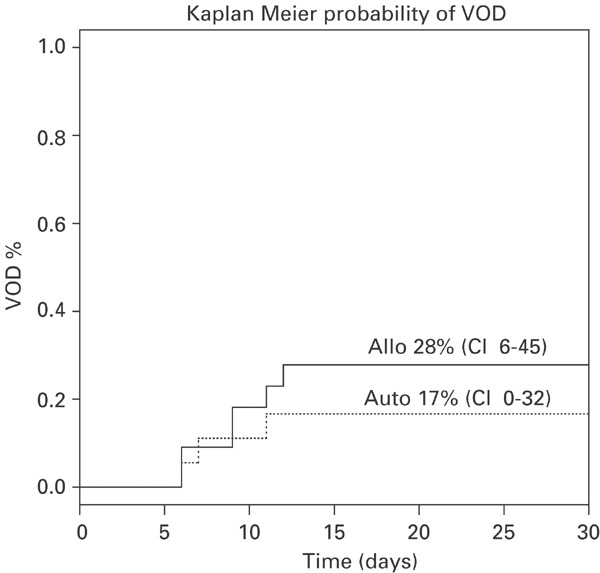
Kaplan–Meier estimates of the cumulative incidence of hepatic veno-occlusive disease following allogeneic hematopoietic stem cell transplantation (solid line) and autologous hematopoietic stem cell transplantation (dotted line).
Table 2.
Characteristics of patients who developed VOD
| UPN | Age/sex | Diagnosis/disease status | No. of lines of prior therapy | Prior hepatic disease | Type of HSCT | Conditioning regimen | Severity of VOD | Peak serum bilirubin (μmol) | Weight gain (% over baseline) | RUQ pain or hepatomegaly | Outcome at day +100 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 396 | 64/M | MM/2PR | 4 | Anti HCV+ | Auto | MEL-TBI | MOD | 36 | 13 | + | Alive; CR |
| 397 | 64/M | NHL/1REL | 3 | Tumor involvement | Auto | VP16-MEL | MOD | 136 | 15 | + | Alive; CRU |
| 401 | 49/M | NHL/1REL | 5 | Auto | VP16-MEL | MOD | 38 | 5 | Alive; relapse day +76 | ||
| 404 | 34/F | NHL/1REL | 4 | Allo MUD | CY-TBI | MOD | 177 | 9 | Alive; CR | ||
| 406 | 48/F | AML/2CR | 2 | Allo MUD | CY-TBI | SEVERE | 117 | 8 | + | Died day +16, MOF | |
| 409 | 39/F | NHL/1REL | 2 | Allo Sib | CY-TBI | MOD | 116 | 7 | + | Alive; CR | |
| 432 | 48/F | AML/1CR | 1 | Allo Sib | BU-CY | MOD | 264 | 4 | Died day +56, ARDS | ||
| 451 | 35/F | ALL/1CR | 1 | Prior chemical hepatitis | Allo MUD | CY-TBI | MOD | 68 | 8 | + | Alive; CR |
| 453 | 49/M | MDS/SD | 0 | Anti-HBs+ | Allo Sib | BU-CY | MOD | 154 | 8 | Alive; CR |
UPN=unique patient number; M=male; F=female; MM=multiple myeloma; PR=partial remission; NHL=non-Hodgkin's lymphoma; REL=relapse; AML=acute myeloid leukemia; CR=complete remission; ALL=acute lymphoblastic leukemia; MDS=myelodysplastic syndrome; SD=stable disease; HCV=hepatitis C virus; Hbs=Hepatitis B surface antibody; HSCT=hematopoietic stem cell transplant; Auto=autologous; Allo=allogeneic; MUD=matched unrelated donor; Sib=sibling; MEL=melphalan; TBI=total body irradiation; VP16=etoposide; CY=cyclophosphamide; BU=busulfan; MOD=moderate; CRU=unconfirmed complete remission; RUQ=right upper quadrant; MOF=multiorgan failure; ARDS=acute respiratory distress syndrome.
Hemorrhagic manifestations
Clinically significant bleeding episodes (⩾grade II) occurred in three patients (grade II (2), grade IV (1)), none of whom had concomitant VOD. UPN 399 developed vaginal bleeding on day +8 following a sibling allogeneic HSCT for relapsed non-Hodgkin's lymphoma. CBC revealed a Hgb of 78 g/l and a platelet count of 7 × 109/l. She was transfused with 2 U of PRBCs and 4 U of random donor platelets. The bleeding resolved in 24 h and no further bleeding occurred. UPN 437 developed melena stool day +14 following a sibling allogeneic HSCT for myelofibrosis. The CBC at the time of bleeding showed a Hgb of 63 g/l and platelet count of 18 × 109/l. He was transfused with 2 U of PRBCs and another 4 U of PRBCs 24 h later. The bleeding subsequently resolved and no further gastrointestinal bleeding was noted. UPN 442 developed bleeding per rectum secondary to hemorrhoids on day +4 following a sibling allogeneic HSCT for myelodysplastic syndrome. CBC at this time revealed a Hgb of 72 g/l and platelet count of 42 × 109/l. She was transfused with 2 U of PRBCs and the bleeding resolved.
A total of 24 patients developed minor bleeding (grade 1) consisting of petechiae, purpura or minor mucosal bleeding. These episodes did not require specific therapy and all resolved. Platelet transfusion thresholds remained at 10 × 109/l for all patients in the study, with the exception of the three patients, with clinically significant bleeding episodes. For these three patients, an attempt was made to keep the platelet count greater than 30 × 109/l until the bleeding episode resolved, at which point the threshold was reduced to 10 × 109/l. None of the bleeding episodes, including the three that were ⩾grade II, were considered severe enough to contraindicate further anticoagulant prophylaxis for VOD and dalteparin administration was continued in all cases.
Discussion
Hepatic VOD is one of the most common life-threatening complications of HDCT and HSCT. The incidence in the literature varies from 5–60% depending on a number of patient characteristics such as disease status at the time of HSCT, previous therapy, pre-existing liver disease etc as well as the type of transplant and prophylactic strategy utilized.2,3,4,5,6,7,8,10,11,12,13,14,15,16,17,18,19,20,21,22,23 In its most severe form, it often leads to multiorgan failure and death6. Despite encouraging reports utilizing defibrotide for established severe VOD following HSCT,29 the mortality remains high and effective prophylactic strategies are needed.
Hepatic VOD is believed to result from injury to the vascular endothelium of small hepatic venules, induced by the HDCT conditioning regimen.1,7 The endothelial injury is often followed by superimposed thrombosis and progressive fibrosis of the venular lumens.9 A number of prophylactic strategies have been explored in an attempt to prevent the endothelial damage and subsequent thrombosis including antioxidants, antiinflammatory agents, hydrophilic bile salts and various anticoagulants with varying success13,14,15,16,17,18,19,20,21,22,23. A recent randomized prospective trial has suggested that ursodeoxycholic acid administration postallogeneic HSCT decreases the incidence of hepatic complications.18 This benefit, however, appears to be because of a reduction in the incidence of acute GVHD and nonrelapse mortality and not because of a reduction in the incidence of VOD.18 Therefore, its specific role in VOD prophylaxis may be limited.
The use of standard low-dose UH as VOD prophylaxis has been the subject of several early studies,13,19,20,21,22,23 only one of which was a prospective randomized controlled trial.22 Two of these uncontrolled trials showed an apparent benefit for UH,19,23 however, the study by Bearman et al using various doses of heparin resulted in a high incidence of bleeding or anticipated bleeding (50%) and a high incidence of VOD (71%), bringing in question the efficacy of UH in VOD prophylaxis.20
There has been only one prospective randomized controlled trial of UH in VOD prophylaxis reported by Attal et al.22 In that study, patients undergoing HDCT and autologous or allogeneic HSCT were randomly assigned to receive UH 100 U/kg/day as a continuous intravenous infusion commencing day −8 until day +30 post HSCT or no prophylaxis. UH administration appeared to be efficacious with a reduction in the incidence of VOD from 13.7% in the patients receiving no prophylaxis to 2.5% in the patients receiving UH, without an increase in bleeding episodes. This trial has been criticized, however, for the small number of patients (11%) at high risk of VOD before commencing HDCT, none of whom developed VOD irrespective of whether they received heparin or not. Therefore, despite the apparent overall positive results of the Attal trial, the role of UH remains controversial and the majority of subsequent studies examining the role of UH in VOD prophylaxis have not shown a clear benefit for UH.13,21
LMWH has a number of potential advantages when compared to UH including greater bioavailability and longer plasma half-life because of less plasma protein binding, a more predictable anticoagulant response, no need for laboratory monitoring, a low incidence of HIT and in some studies, a lower incidence of bleeding.24,25,26,27,30 There have been a number of clinical trials comparing the efficacy and safety of LMWH to UH in the prophylaxis and treatment of DVT in medical and surgical patients.24,25,26,27 These studies have consistently shown that LMWH is both efficacious and safe with a low incidence of bleeding complications. All of these qualities potentially make LMWH an attractive anticoagulant agent in the hematopoietic stem cell transplant setting where the majority of patients experience a variable period of thrombocytopenia and increased risk of bleeding.
There are few published studies, however, specifically examining the role of LMWH as VOD prophylaxis for patients undergoing HDCT and HSCT. In a recent pilot study by Or et al,31 61 patients undergoing HSCT were randomized to receive LMWH (enoxaparin 40 mg subcutaneous daily) or placebo.31 The duration of hyperbilirubinemia and the incidence of hepatomegaly were lower in the enoxaparin group compared to placebo. In another study by the EBMT examining the incidence and outcome of VOD following HDCT and HSCT, 66 of 1652 patients received LMWH as VOD prophylaxis.13 The low number of patients receiving LMWH precluded an adequate evaluation of its prophylactic effect.
Our pilot study has shown that LMWH administration to patients undergoing HSCT is safe with a low number of serious bleeding episodes. Only three of 40 (7.5%) patients developed significant bleeding requiring transfusion of PRBCs and 24 (60%) patients developed minor mucosal bleeding or petechiae. No patients died of hemorrhage or suffered any permanent sequelae because of bleeding. These results are in keeping with the 7–23% incidence of serious bleeding reported in the literature for patients undergoing HDCT and HSCT irrespective of whether they are receiving low doses of systemic anticoagulants.20,31,32 In the pilot study reported by Bearman et al comparing the incidence of VOD and severity of bleeding between a cohort of patients receiving low-dose UH and a control group of patients, the incidence of major bleeding in those patients receiving UH was 7% and all but one patient displayed evidence of minor bleeding.20 The site and severity of bleeding, however, was not statistically different between the UH and control groups. Similarly, in the study reported by Or et al31 where patients undergoing HSCT were randomized between LMWH and placebo, the incidence and duration of bleeding was in fact lower in those patients receiving LMWH. This would support the fact that patients undergoing HDCT and HSCT are at an inherent risk of bleeding because of the development of obligate thrombocytopenia and damaged mucosal surfaces. The administration of low-dose UH or LMWH does not appear to further increase the risk of serious hemorrhage in this patient population and the need for maintaining higher platelet transfusion thresholds in those patients receiving low-dose systemic anticoagulants does not appear to be warranted.
Our pilot study was designed to primarily examine the safety and feasibility of LMWH (dalteparin 2500 IU daily) administration to patients undergoing HSCT. The small number of patients, the lack of randomization and risk stratification made a determination of efficacy difficult. Despite these limitations, the incidence of VOD in our study was 22% and only one patient developed severe VOD. These results are encouraging when compared to the 20–50% reported incidence rates from large published studies where no specific VOD prophylaxis was utilized.2,3,6 Three of the 18 patients (17%) undergoing autologous HSCT is this study developed moderate VOD. While this incidence is higher than what is commonly reported in the literature for this patient population, our results may have been affected by the small number of patients undergoing autologous HSCT, in addition to specific patient characteristics, which may influence the incidence of VOD within individual studies. Finally, the diagnosis of mild–moderate VOD, accounting for eight of the nine patients in our study, can sometimes be difficult to distinguish from other causes of mild hepatic dysfunction such as infection, GVHD and various medications, and this may lead to an overestimate of the true incidence of VOD in this patient population.7,8
In summary, this pilot study has demonstrated that the administration of LMWH (dalteparin 2500 anti-Xa i.u. daily) to patients undergoing HDCT and HSCT is well tolerated and safe with a low incidence of serious bleeding events. The efficacy of LMWH compared to UH for VOD prophylaxis, however, remains controversial and whether either preparation is superior to no prophylaxis is unknown. This should be addressed within the context of a prospective randomized placebo controlled trial.
Acknowledgements
We acknowledge the contribution of the medical and nursing staff of 8A/B Ward and Medical Day Unit at the QEII Health Sciences Centre.
References
- 1.Bearman SI, Appelbaum FR, Buckner CD. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6:1562. doi: 10.1200/JCO.1988.6.10.1562. [DOI] [PubMed] [Google Scholar]
- 2.McDonald GB, Sharma P, Matthews DE. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984;4:16. doi: 10.1002/hep.1840040121. [DOI] [PubMed] [Google Scholar]
- 3.Jones RJ, Lee KSK, Beschorner WE. Venocclusive disease of the liver following bone marrow transplantation. Transplant. 1987;44:778. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Dulley FL, Kanfer EJ, Appelbaum FR. Venocclusive disease of the liver after chemoradiotherapy and autologous bone marrow transplantation. Transplant. 1987;43:870. doi: 10.1097/00007890-198706000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Ayash LJ, Hunt M, Antman K. Hepatic venocclusive disease in autologous bone marrow transplantation of solid tumors and lymphomas. J Clin Oncol. 1990;8:1699. doi: 10.1200/JCO.1990.8.10.1699. [DOI] [PubMed] [Google Scholar]
- 6.McDonald GB, Hinds MS, Fisher LD. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85:3005. [PubMed] [Google Scholar]
- 8.Richardson P, Guinan E. The pathology, diagnosis, and treatment of hepatic veno-occlusive disease: current status and novel approaches. Br J Haematol. 1999;107:485. doi: 10.1046/j.1365-2141.1999.01680.x. [DOI] [PubMed] [Google Scholar]
- 9.Shulman HM, Fisher LB, Schoch HG. Venoocclusive disease of the liver after marrow transplantation: histological correlates of clinical signs and symptoms. Hepatology. 1994;19:1171. doi: 10.1002/hep.1840190515. [DOI] [PubMed] [Google Scholar]
- 10.Shulman HM, Hinterberger W. Hepatic veno-occlusive disease—liver toxicity syndrome after bone marrow transplantation. Bone Marrow Transplant. 1992;10:197. [PubMed] [Google Scholar]
- 11.Rozman C, Carreras E, Qian C. Risk factors for hepatic veno-occlusive disease following HLA-identical sibling bone marrow transplants for leukemia. Bone Marrow Transplant. 1996;17:75. [PubMed] [Google Scholar]
- 12.Kami M, Mori S, Tanikawa S. Risk factors for hepatic veno-occlusive disease after bone marrow transplantation: retrospective analysis of 137 cases at a single institution. Bone Marrow Transplant. 1997;20:397. doi: 10.1038/sj.bmt.1700895. [DOI] [PubMed] [Google Scholar]
- 13.Carreras E, Bertz H, Arcese W, On behalf of the European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European group for blood and marrow transplantation. Blood. 1998;92:3599. [PubMed] [Google Scholar]
- 14.Gluckman E, Jolivet I, Scrobohaci ML. Use of prostaglandin E1 for prevention of liver veno-occlusive disease in leukaemic patients treated by allogeneic bone marrow transplantation. Br J Haematol. 1990;74:277. doi: 10.1111/j.1365-2141.1990.tb02583.x. [DOI] [PubMed] [Google Scholar]
- 15.Attal M, Huguet F, Rubie H. Prevention of regimen-related toxicities after bone marrow transplantation by pentoxifylline: a prospective, randomized trial. Blood. 1993;82:732. [PubMed] [Google Scholar]
- 16.Clift RA, Bianco JA, Appelbaum FR. A randomized controlled trial of pentoxifylline for the prevention of regimen-related toxicities in patients undergoing allogeneic marrow transplantation. Blood. 1993;892:2025. [PubMed] [Google Scholar]
- 17.Essell JH, Schroeder MT, Harman GS. Ursodiol prophylaxis against hepatic complications of allogeneic bone marrow transplantation: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:975. doi: 10.7326/0003-4819-128-12_Part_1-199806150-00002. [DOI] [PubMed] [Google Scholar]
- 18.Ruutu T, Eriksson B, Remes K. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977. doi: 10.1182/blood-2001-12-0159. [DOI] [PubMed] [Google Scholar]
- 19.Rio BY, Lamy T, Zittoun R. Preventive role of heparin for liver veno-occlusive disease (VOD) Bone Marrow Transplant. 1988;3:266. [Google Scholar]
- 20.Bearman SI, Hinds MS, Wolford JL. A pilot study of continuous infusion heparin for the prevention of hepatic veno-occlusive disease after bone marrow transplantation. Bone Marrow Transplant. 1990;5:407. [PubMed] [Google Scholar]
- 21.Marsa-Vila L, Gorin NC, Laporte JP. Prophylactic heparin does not prevent liver veno-occlusive disease following autologous bone marrow transplantation. Eur J Haematol. 1991;47:346. doi: 10.1111/j.1600-0609.1991.tb01859.x. [DOI] [PubMed] [Google Scholar]
- 22.Attal M, Huguet F, Rubie H. Prevention of hepatic veno-occlusive disease after bone marrow transplantation by continuous infusion of low-dose heparin: a prospective randomized trial. Blood. 1992;79:2834. [PubMed] [Google Scholar]
- 23.Cahn JY, Flesch M, Brion A. Prevention of veno-occlusive disease of the liver after bone marrow transplantation: heparin or no heparin? Blood. 1992;80:2149. [PubMed] [Google Scholar]
- 24.Hirsh J, Levine MN. Low molecular weight heparin. Blood. 1992;79:1. [PubMed] [Google Scholar]
- 25.Bergqvist D, Matzsch T, Burmark US. Low molecular weight heparin given the evening before surgery compared with conventional low dose heparin in prevention of thrombosis. Br J Surg. 1988;75:888. doi: 10.1002/bjs.1800750920. [DOI] [PubMed] [Google Scholar]
- 26.Caen JP. A randomized double-blind study between a low molecular weight heparin Kabi 2165 and standard heparin in the prevention of deep vein thrombosis in general surgery. Thromb Haemost. 1988;59:216. doi: 10.1055/s-0038-1642757. [DOI] [PubMed] [Google Scholar]
- 27.Koopman MMW, Prandoni P, Piovella F. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. N Engl J Med. 1996;334:682. doi: 10.1056/NEJM199603143341102. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 29.Richardson PG, Murakami C, Jin Z. Multi-institutional use of defibrotide in 88 patients after stem cell transplantation with severe veno-occlusive disease and multisystem organ failure: response without significant toxicity in a high-risk population and factors predictive of outcome. Blood. 2002;100:4337. doi: 10.1182/blood-2002-04-1216. [DOI] [PubMed] [Google Scholar]
- 30.Warkentin TE, Levine MN, Hirsh J. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995;332:1330. doi: 10.1056/NEJM199505183322003. [DOI] [PubMed] [Google Scholar]
- 31.Or R, Nagler A, Shpilberg O. Low molecular weight heparin for the prevention of veno-occlusive disease of the liver in bone marrow transplantation patients. Transplant. 1996;61:1067. doi: 10.1097/00007890-199604150-00014. [DOI] [PubMed] [Google Scholar]
- 32.Nevo S, Enger C, Hartley E. Acute bleeding and thrombocytopenia after bone marrow transplantation. Bone Marrow Transplant. 2001;27:65. doi: 10.1038/sj.bmt.1702717. [DOI] [PubMed] [Google Scholar]


