Abstract
Approximately one third of patients with advanced human epidermal growth factor receptor 2 (HER-2)/neu-positive breast cancer respond to trastuzumab monotherapy, a humanized anti-HER-2/neu antibody. However, de novo and acquired antibody resistance is one of the major limitations of trastuzumab therapy warranting the search for other therapeutic strategies. One of the most remarkable features of adenovirus (AdV)-based vaccine is its ability to induce exceptionally high and sustained frequencies of transgene product-specific CD8+ T-cell responses. In this study, we constructed two recombinant AdVs (AdVOVA and AdVHER-2) expressing ovalbumin (OVA) and HER-2/neu, and assessed AdV-induced antigen-specific cellular immune responses and preventive/therapeutic antitumor immunity. We demonstrate that AdVOVA stimulates efficient OVA-specific CD8+ cytotoxic T lymphocyte (CTL) and natural killer responses, leading to preventive long-term immunity against OVA-expressing BL6-10ova melanoma in wild-type C56BL/6 mice. We further demonstrate that AdVHER-2 stimulates HER-2/neu-specific CD8+ CTL responses, leading to a significant reduction in breast carcinogenesis in transgenic FVBneuN mice (P<0.05), but has little therapeutic effect on pre-existing Tg1-1 tumor even at early stage (15 mm3). In contrast, the anti-HER-2/neu antibody therapy is capable of completely inhibiting Tg1-1 tumor growth at early stage, but fails to eradicate well-established Tg1-1 breast tumor (100 mm3). Interestingly, a combinatorial immunotherapy of anti-HER-2/neu antibody with AdVHER-2 vaccine was capable of curing 4 of 10 studied mice bearing well-established Tg1-1 breast tumors and significantly delaying in death of the remaining six tumor-bearing mice (P<0.05). Taken together, our results suggest an adjuvant effect of AdVHER-2 on anti-HER-2/neu antibody therapy for well-established breast tumor in transgenic FVBneuN mice, and this combinatorial immunotherapy of trastuzumab with AdVHER-2 vaccine may be used as a new therapeutic strategy for treatment of advanced HER-2/neu-positive breast cancer.
Keywords: adenoviral vaccine, antibody therapy, HER-2/neu, breast cancer, CD8+ CTL, NK
Subject terms: Viral vectors, Antibody therapy, Cancer immunotherapy, Breast cancer
Introduction
The proto-oncogene human epidermal growth factor receptor (HER)-2 with 80% sequence homology to the rat neu gene-coded protein neu, originally detected on rat neuroblastoma cells,1 is a tyrosine kinase receptor belonging to the epidermal growth factor receptor family.2 HER-2/neu is composed of extracellular, transmembrane and intracellular domains.3 Breast cancer is the most common cancer among women in the Western world. The HER-2/neu protein is overexpressed in about 20% cases of breast cancer.2 HER-2/neu has become an attractive target antigen (Ag), because (i) it is selectively overexpressed in malignant cells and (ii) the immune responses to HER-2/neu are frequently found in patients with HER-2/neu-positive breast cancer,4, 5, 6 indicating that the self-tolerance to HER-2/neu can be broken in humans. Although overexpression of HER-2/neu is often associated with poor prognosis,7 it permits novel therapies directed against HER-2/neu, of which the humanized monoclonal antibody (Ab) trastuzumab is possibly the best known.8 However, most women sooner or later develop resistance against trastuzumab,9, 10 warranting the search for other therapeutic strategies.11
CD8+ cytotoxic T lymphocytes (CTLs) have an important role in host defense against viruses, intracellular bacteria and tumors.12, 13, 14, 15, 16 For this reason, a vaccine capable of inducing a strong CD8+ CTL response becomes a major goal in the field of tumor immunity. Replication-deficient adenoviruses (AdVs) have been found to be very effective vaccine vectors, as they mimic a natural infection and stimulate the innate immune responses, leading to development of an effective CD4+ and CD8+ T-cell responses to the vaccine-encoded Ag.17, 18, 19, 20 Therefore, genetic vaccines based upon recombinant AdVs has been used to immunize against infectious diseases such as Ebola,21 SARS22 and human immunodeficiency virus.23, 24 Vaccination with ovalbumin (OVA)-expressing recombinant AdVs stimulates efficient OVA-specific CTL responses,25, 26, 27 leading to protection against virus challenge.28 In addition, recombinant AdVs have also been applied for induction of antitumor immunity. In most of the studies, recombinant AdV vaccines have been shown to induce efficient prophylactic antitumor immunity. For example, vaccination with tyrosinase-related protein-expressing AdVs induced Gp100- and tyrosinase-related protein-specific CD8+ T-cell responses, leading to preventive immunity against Gp100- and tyrosinase-related protein-expressing melanomas29, 30, 31, 32 and reduction in melanoma relapse after surgery.33 HER-2/neu-expressing AdV (AdVHER-2) vaccination stimulates both HER-2/neu-specific Ab and CD8+ CTL responses, leading to preventive antitumor immunity in wild-type mice.34, 35, 36 However, the therapeutic efficacies remain controversial. For example, ineffectiveness of AdV vaccine has been demonstrated even if it was administered as early as only one33 or two days34 after the tumor cells seeding in wild-type mice. Conversely, other investigators have shown that recombinant AdVHER-2 vaccine efficiently eradicates advanced established murine breast cancer in wild-type mice.37
In this study, we assessed CD4+ and CD8+ T-cell responses and antitumor immunity derived from vaccination with two recombinant AdVs (AdVOVA and AdVHER-2) expressing OVA and HER-2/neu against OVA-expressing melanoma and HER-2/neu-expressing breast cancer, respectively. In addition, we compared the therapeutic efficiency of anti-HER-2/neu Ab therapy, HER-2/neu-expressing AdVHER-2 vaccination and a combination of the two on well-established HER-2/neu-expressing breast cancer in HER-2/neu transgenic (Tg) mice.
Materials and methods
Reagents, cell lines and animals
Monoclonal Ab 7.16.4, a mouse IgG2a Ab reactive with the rat HER-2/neu oncogen-encoded p185 molecule was obtained from American Tissue Type Collection (Rockville, MD).38 The biotin-labeled anti-CD69 Ab was obtained from PharMingen Canada (Mississauga, Ontario, Canada). The fluorescein isothiocyanate (FITC)-conjugated Abs specific for CD4 and CD8 and phycoerythrin (PE)-labeled H-2Kb/OVA257–264 tetramer and PE-labeled H-2Dq/HER-2 peptide (PDSLRDLSVF) tetramer were obtained from Beckman Coulter (San Diego, CA) and NIH Tetramer Facility (Bethesda, MD), respectively. Major histocompatibility complex class I (H-2Kb)-restricted OVAI (OVA257-264, SIINFEKL) peptide and irrelevant Mut1 peptide (FEQNTAQP) were synthesized by Multiple Peptide Systems (San Diego, CA). The highly lung metastatic OVA-transfected B16 melanoma cell line BL6-10ova was generated in our laboratory.39 The mouse breast cancer cell line Tg1-1 (H-2Kq) derived from a spontaneous HER-2/neu-expressing breast cancer tumor was obtained from Dr T Kipps, University of California, San Diego, CA. The natural killer (NK)-sensitive tumor cell line Yac-1 was obtained from American Tissue Cell Collection (Rockville, MD). Female wild-type C57BL/6 (B6, H-2Kb) mice and FVB/NJTgN(MMTVneu)202Mul (FVBneuN) (H-2Kq) Tg mice expressing the rat neu under the control of a mouse mammary tumor virus promoter were obtained from Jackson Laboratories (Bar Harbor, ME). All mice were housed in the animal facility at the Saskatoon Cancer Center; with all animal experiments carried out in accordance to the Canadian Council for Animal Care guidelines.
Construction of recombinant adenovirus AdVOVA
Construction of recombinant AdV-expressing OVA (AdVOVA) was performed by insertion of OVA gene cloned from pAc-OVA vector obtained from Dr M Bevan, University of Washington (Seattle, Washington) into pShuttle vector (Stratagene, La Jolla, CA) to form pLpAOVA-expressing OVA gene (Figure 1). The PmeI-digested shuttle vector was then cotransformed into BJ5183 Escherichia coli cells already containing the backbone vector for increased efficiency of homologous recombination to form the recombinant AdVOVA. The recombinant AdVOVA vector was then linearized by PacI digestion, and then transfected into 293 cells using Lipofectamine (Gibco/BRL, Burlington, Ontario, Canada) to generate AdVOVA. Recombinant AdVHER-2 expressing the rat neu gene and the control AdVNull without any inserted transgene (Figure 1) were previously constructed in our laboratory.40 All recombinant AdVs were amplified in 293 cells, purified by a series of cesium chloride ultracentrifugation gradients, and stored at −80 °C until use.
Figure 1.
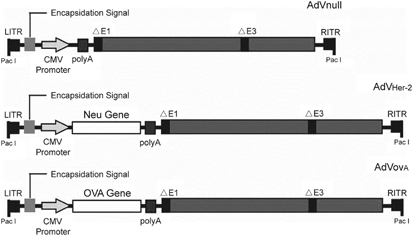
Schematic representation of adenovirus (AdV) vectors. The E1/E3-deleted replication-deficient AdV vectors are under the regulation of the cytomegalovirus (CMV) early/immediate promoter/enhancer. The AdV vectors include AdVnull without any transgene expression, AdVOVA-expressing transgene ovalbumin (OVA) and AdVHER-2-expressing transgene HER-2/neu. HER, human epidermal growth factor receptor; ITR, inverted terminal repeat.
Characterization of NK cell activity
Lymphocytes of the drainage lymph nodes of C57BL/6 mice with s.c. immunization of AdVova (1 × 107 p.f.u. per mouse) were harvested 2 days after the immunization, stained with FITC-anti-CD69 Ab and PE-anti-NK1.1 Ab and then analyzed by flow cytometry. To assess their killing activity, we performed chromiun 51-release assay, in which 51Cr-Yac-1 and lymphocytes derived from immunized mouse drainage lymph nodes were used as target and effector cells, respectively. The target cells were radiolabeled by culturing these cells for 1 h in the culture medium in the presence of 50 μl of sodium [51Cr]-chromate (36 mCi ml–1; Amersham, Arlington Heights, IL), then washed twice with phosphate-buffered saline. Approximately 1 × 105 labeled target cells per triplicate wells were mixed with effector cells at various effector (E): target (T) cell ratios, and then incubated for 6 h. The percentage of specific lysis was calculated as: 100 × [(experimental c.p.m.−spontaneous c.p.m.)/(maximal c.p.m.−spontaneous c.p.m.)].41 Spontaneous c.p.m. release in the absence of effector cells was <10% of specific lysis. The maximal c.p.m. release was determined by lysis of the target cells with 0.25% Triton X-100.
Tetramer staining
C57BL/6 mice were immunized by i.v. injection of AdVOVA (1 × 107 p.f.u. per mouse). At different days after the immunization, 100 μl of mouse peripheral blood was stained with FITC-conjugated anti-CD8 Ab and PE-conjugated H-2Kb/OVA257–264 tetramer for 30 min at room temperature and analyzed by flow cytometry. FVBneuN Tg mice were immunized by i.v. injection of AdVHER-2 (1 × 107 p.f.u. per mouse). Eleven days after the immunization, 100 μl of mouse peripheral blood was incubated with FITC-conjugated anti-CD8 Ab and PE-labeled H-2Dq/HER-2 peptide tetramer for 30 min at room temperature and analyzed by flow cytometry.
Cytotoxicity assay
In in vivo cytotoxicity assay, C57BL/6 mouse spleen cells pulsed with OVAI peptide were strongly labeled with carboxyl-fluorescein succinimidyl ester (CFSE) (3.0 μM, CFSEhigh) and served as OVA-specific target cells, whereas spleen cells pulsed with irrelevant Mut1 peptide were weakly labeled with CFSE (0.6 μM, CFSElow) and served as nonspecific control target cells, respectively. Eleven days following the immunization with AdVOVA, the immunized mice were then i.v. injected with a 1:1 (CFSEhigh:CFSElow) mixture of splenocytes targets. Sixteen hours after target cell delivery, spleens of the recipient mice were removed, and the relative proportions of CFSEhigh and CFSElow target cells remaining in the spleens were analyzed by flow cytometry.
Animal studies
Three types of animal studies were conducted. The first type of animal study was performed for evaluation of the preventive antitumor immunity. Wild-type C57BL/6 mice (10 mice per group) were s.c. vaccinated with AdVOVA (1 × 107 p.f.u. per mouse). Eleven days after the immunization, C57BL/6 mice were s.c. injected in their right thighs with BL6-10OVA tumor cells (0.3 × 106 cells per mouse). To assess the cellular mechanism of AdVOVA-induced antitumor immunity, C57BL/6 mice were i.p. injected with anti-CD8 (53.6.7) or anti-NK1.1 (PK136) Ab (0.3 mg per mouse) to deplete CD8+ T cells or NK cells 10 days after AdVOVA immunization. One day after the Ab treatment, C57BL/6 mice were challenged by s.c. injection of BL6-10OVA (0.3 × 106 cells per mouse). The Ab treatment was repeated once every 3 days for a total five times. To assess the long-term immunity, C57BL/6 mice were also s.c. inoculated with BL6-10OVA (0.3 × 106 cells per mouse) 60 days after the immunization. The second type of animal study was performed to evaluate the prevention of breast carcinogenesis, the Tg FVBneuN mice (10 mice per group) at age of 2 months were vaccinated s.c. with AdVHER-2 (1 × 107 p.f.u. per mouse) at 1-month interval for a total of five vaccinations. Spontaneous breast tumor development was monitored weekly for up to 12 months.40 The third type of animal study was designed to evaluate therapeutic antitumor immunity. FVBneuN mice (10 mice per group) were s.c. injected with Tg1-1 (1 × 106 cells per mouse) cells. Each mouse was monitored weekly for tumor growth measured in two perpendicular diameters using a caliper. Tumor volume (mm3) was calculated using the formula V=a × b2/2, where a is the largest and b is the smallest diameter,42 and represented as mean±s.d. When Tg1-1 tumors grew to a size of ∼15 mm3 (early stage, around 6 days after tumor cell injection) or ∼100 mm3 (well established, around 12 days after tumor cell injection), tumor-bearing FVBneuN Tg mice were s.c. injected with AdVHER-2 (1 × 107 p.f.u. per mouse) at 5-day interval for a total of three times. To mimic the clinical administration of trastuzumab in patients with a loading dose of 4 mg (trastuzumab) per kg (patient body weight),43 Tg1-1 tumor-bearing mice were also i.p. injected with the anti-HER-2/neu Ab at a dose of 4 mg kg–1 (mouse body weight) once every 3 days for a total of five times. In another Tg1-1 tumor-bearing mouse group, mice were treated with both AdVHER-2 and anti-HER-2 Ab. Tumor growth was monitored daily for 30 days; for ethical reason, all mice with tumors that achieved a size of 1000 mm3 in volume were killed.
Statistical analyses
Statistical analyses were conducted using Prism software (GraphPad Software, San Diego, CA) to perform log-rank test for comparing mouse survival between groups. To determine the significance of differences between groups, Student's t-tests were performed. P-values <0.05 were considered statistically significant.40
Results
AdVOVA stimulates OVA-specific functional CD8+ CTL and NK responses in wild-type C57BL/6 mice
To assess the cellular immune responses, we i.v. immunized wild-type C57BL/6 mice with a recombinant OVA-expressing adenovirus AdVOVA and then evaluated OVA-specific CD8+ T-cell responses in mouse peripheral blood using FITC-anti-CD8 Ab and PE-H-2Kb/OVA257–264 tetramer staining by flow cytometry. We found that AdVOVA vaccine stimulated a sustained OVA-specific CD8+ T-cell response accounting for 18.6% of the total CD8+ T-cell population (Figure 2a), which is significantly larger than 0.07% in mice immunized with the control AdVNull (P<0.05). The OVA-specific CD8+ T-cell responses had a peak on day 11 after the immunization and then declined slowly. To assess the functional effect of CD8+ T cells, we performed in vivo cytotoxicity assay. We adoptively transferred OVAI peptide-pulsed splenocytes that had been strongly labeled with CFSE (CFSEhigh), as well as the control peptide Mut1-pulsed splenocytes that had been weakly labeled with CFSE (CFSElow), into recipient mice that had been vaccinated with AdVOVA. As expected, there was a substantial loss (85%) of the CFSEhigh (OVAI peptide-pulsed) cells in the AdVOVA-immunized mice, whereas little cytotoxicity (8%) was induced in mice immunized with the control AdVNull (Figure 2b) (P<0.05), indicating that AdVOVA vaccine efficiently stimulates CD8+ T-cell differentiation into functional OVA-specific CTL effectors. To assess the potential AdV-stimulated NK responses, lymphocytes of C57BL/6 mice with s.c. immunization of AdVova were harvested from the drainage lymph nodes 2 days after the immunization and analyzed for NK activation and killing activity by flow cytometry and in vitro cytotoxicity assay, respectively. As shown in Figure 3a, the proportion of active CD69+NK1.1+ NK cells was significantly greater in AdV (AdVnull and AdVova)-treated mice (∼60%) compared with the control mice (∼10%) (P<0.05). In addition, NK cells derived from AdV (AdVnull and AdVova)-treated mice displayed stronger killing activity against Yac-1 tumor cells than NK cells from the control wild-type C57BL/6 mice (P<0.05) (Figure 3b), indicating that AdVova stimulates nonspecific NK cell responses.
Figure 2.
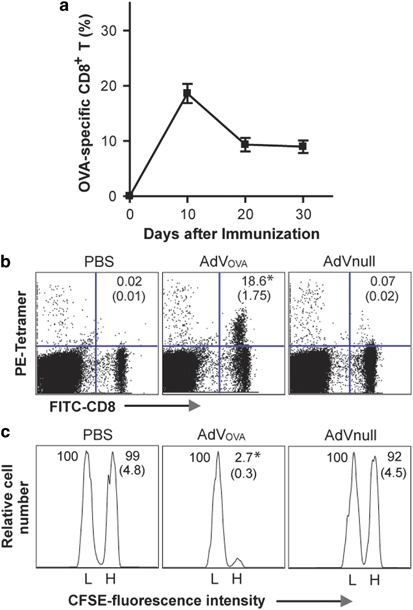
AdVOVA stimulates ovalbumin (OVA)-specific CD8+ cytotoxic T lymphocyte (CTL) responses. (a) The AdVova-immunized C57BL/6 mouse tail blood samples harvested on different days after the immunization of OVA-Texo were stained with PE-H-2Kb/OVAI peptide tetramer and fluorescein isothiocyanate (FITC)-anti-CD8 antibody (Ab), and then analyzed by flow cytometry. (b) The tail blood samples of AdVova-immunized C57BL/6 mice were harvested on day 11 after the immunization and stained with PE-H-2Kb/OVAI peptide tetramer and FITC-anti-CD8 Ab, and then analyzed by flow cytometry. The value in each panel represents the percentage of OVA-specific (tetramer-positive) CD8+ T cells vs the total CD8+ T-cell population. The value in parenthesis represents s.d. (c) In vivo cytotoxicity assay. Six days after the immunization, the immunized mice were i.v. injected with 2 × 106 cells containing a 1:1 mixture of CFSEhigh- and CFSElow-labeled splenocytes that had been pulsed with OVAI or Mut1 peptides, respectively. After 16 h, the spleens of immunized mice were removed and the percentages of the residual CFSEhigh (H) and CFSElow (L) target cells remaining in the recipients’ spleens were analyzed by flow cytometry. The value in each panel represents the percentage of CFSEhigh vs CFSElow target cells remaining in the spleen. The value in parenthesis represents the s.d. *P<0.05 vs cohorts of the control AdVnull group (Student's t-test). One representative experiment of three is shown. AdV, adenovirus; CFSE, carboxyl-fluorescein succinimidyl ester.
Figure 3.
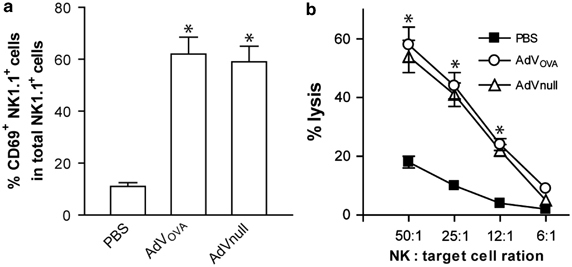
AdVOVA stimulates natural killer (NK) cell responses. (a) Lymphocytes from AdVova-immunized mouse drainage lymph nodes were stained with fluorescein isothiocyanate (FITC)-anti-CD69 antibody and PE-anti-NK1.1 antibody, and then analyzed by flow cytometry. (b) In vitro cytotoxicity assay. NK and 51Cr-Yac-1 cells were used as effector (E) and target (T) cells in a chromiun-release assay. *P<0.05 vs cohorts of the control phosphate-buffered saline (PBS) group (Student's t-test). One representative experiment of three is shown. AdV, adenovirus; OVA, ovalbumin.
AdVOVA stimulates CD8+ CTL-mediated antitumor immunity and long-term T-cell memory in wild-type C57BL/6 mice
To assess preventive antitumor immunity, the above-immunized mice were s.c. challenged with OVA-expressing B16 melanoma BL6-10ova on day 11 subsequent to the immunization. We found that all (10/10) mice immunized with the control AdVNull died of tumor within 21 days subsequent to tumor cell challenge, whereas all (10/10) mice immunized with AdVOVA were tumor free (Figure 4a), indicating that AdVOVA stimulates a preventive antitumor immunity in C57BL/6 mice. To assess the cellular mechanism of the antitumor immunity, we treated immunized mice with anti-CD8 and anti-NK1.1 Ab to deplete CD8+ T and NK cells, respectively, before tumor cell challenge. We demonstrated that all (10/10) mice with treatment of anti-CD8 Ab, but not anti-NK Ab lost their antitumor protection, indicating that AdVOVA-induced antitumor immunity is mainly mediated by CD8+ CTLs. To assess the long-term immunity, AdVOVA-immunized C57BL/6 mice were challenged by s.c. inoculation of BL6-10OVA tumor cells 60 days after the immunization. We found that none of the immunized mice (0/10) grew tumor (Figure 4b), indicating that AdVOVA vaccination can also induce a long-term antitumor immunity.
Figure 4.
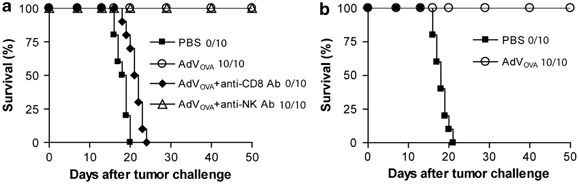
AdVova stimulates preventive ovalbumin (OVA)-specific antitumor immunity in wild-type C57BL/6 mice. (a) AdVova-immunized C57BL/6 mice were s.c. challenged with BL6-10ova tumor cells on day 11 after the immunization. One day before tumor challenge, mice were i.p. injected with anti-CD8 or anti-NK1.1 antibody (0.3 mg per mouse). The antibody treatment was repeated once every 3 days for a total of five times. (b) AdVova-immunized C57BL/6 mice were s.c. challenged with BL6-10ova tumor cells on day 60 after the immunization. Tumor growth was monitored. One representative experiment of two is shown. AdV, adenovirus; NK, natural killer.
AdVHER-2 stimulates functional CD8+ CTL responses leading to reduction in breast carcinogenesis in Tg FVBneuN mice
Tg FVBneuN mice that have neu-specific self-immune tolerance44, 45 spontaneously develop multiple HER-2/neu-expressing breast cancers with different sizes at different ages (Figures 5a and b).46 These Tg mice have been extensively used for evaluation of HER-2/neu-specific immunotherapeutics.47, 48, 49 To assess CD8+ T-cell responses, the peripheral blood samples of the mice immunized with AdVneu were harvested on day 11 after immunization, stained with FITC-anti-CD8 Ab and PE-anti-H-2Kq/HER-2/neu peptide tetramer, and analyzed by flow cytometry. We found that AdVHER-2-stimulated HER-2/neu-specific CD8+ T-cell responses accounted for 0.8% of the total CD8+ T-cell population (Figure 5c), indicating that AdVHER-2 stimulates HER-2/neu-specific CD8+ T-cell responses in Tg FVBneuN mice with self-HER-2/neu-specific immune tolerance. To determine whether AdVHER-2-induced cellular immune responses could reduce breast carcinogenesis, the Tg FVBneuN mice at the age of 2 months were vaccinated s.c. with AdVHER-2 at 1-month interval, for a total of four vaccinations. As shown in Figure 5d, AdVHER-2 vaccination protected 3/10 of the mice from breast carcinogenesis and induced a significant delay in tumor formation in 7/10 of the mice compared with the control AdVNull vaccination (P<0.05), indicating that AdVHER-2 vaccination can partly overcome self-HER-2/neu-specific immune tolerance and reduce breast carcinogenesis in Tg FVBneuN mice.
Figure 5.
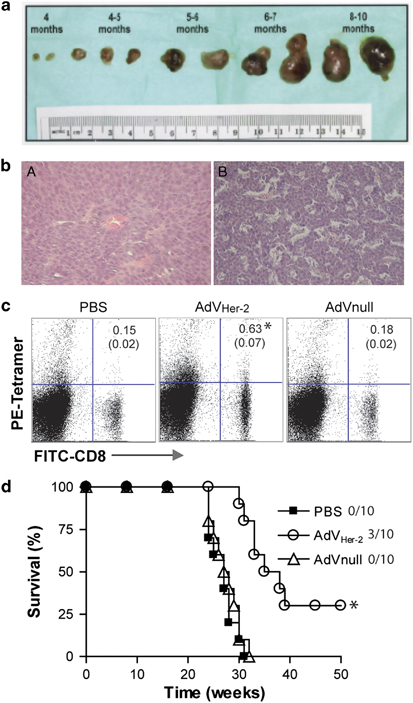
AdVHER-2 stimulates HER-2/neu-specific CD8+ cytotoxic T lymphocyte (CTL) responses and reduces breast carcinogenesis in transgenic FVBneuN mice. (a) Photographs of representative samples of spontaneous breast tumors taken from FVBneuN transgenic mice at various time points ranging from 4 to 10 months of age and ordered in progressing size and time frame. (b) Histologic photomicrographs of breast tumors at (A) 4 and (B) 10 months of age. Magnifications are × 100. (c) The tail blood samples of AdVHER-2-immunized FVBneuN mice were harvested on day 11 after the immunization and stained with PE-H-2Kq/HER-2 peptide tetramer (PE-tetramer) and fluorescein isothiocyanate (FITC)-anti-CD8 antibody (Ab) (FITC-CD8), and then analyzed by flow cytometry. The value in each panel represents the percentage of HER-2/neu-specific (tetramer-positive) CD8+ T cells vs the total CD8+ T-cell population. The value in parenthesis represents the s.d. *P<0.05 vs cohorts of the control AdVnull group (Student's t-test). (d) The transgenic FVBneuN mice at the age of 2 months were vaccinated s.c. with AdVHER-2 at 1-month interval, for a total of four vaccinations. Spontaneous formation of breast tumors was monitored weekly. *P<0.05 vs cohorts of the control groups (phosphate-buffered saline (PBS) and AdVnull) (log-rank test). One representative experiment of two is shown. AdV, adenovirus; HER, human epidermal growth factor receptor.
AdVHER-2 is adjuvant to Ab therapy of well-established tumors in Tg FVBneuN mice
To assess the potential therapeutic effect of HER-2/neu-expressing AdVHER-2 in Tg mice, we repeated the above experiments using AdVHER vaccination in HER-2/neu-expressing Tg1-1 tumor-bearing Tg FVBneuN mice with HER-2/neu-specific self-immune tolerance. We found that all (10/10) of the vaccinated mice bearing early stage (15 mm3) Tg1-1 breast cancer died of cancer within 17 days after the initial vaccination though mouse survival was prolonged (P<0.05) (Figure 5a). It has been demonstrated that the anti-HER-2/neu Ab had potent therapeutic effect on established HER-2/neu-expressing tumors in mice.50, 51 To assess its therapeutic efficiency, we repeated the above experiments using anti-HER-2/neu Ab in HER-2/neu-expressing Tg1-1 tumor-bearing Tg FVBneuN mice. Tg1-1 tumor cells grew aggressively (Figure 6a). We found that all (10/10) of the vaccinated mice bearing early stage (15 mm3) Tg1-1 breast cancer became tumor free (Figure 6b), whereas 0/10 of the mice bearing well-established (100 mm3) tumors survived though these mice had a longer survival (P<0.05) (Figure 6c). Interestingly, we found that 4/10 of the mice bearing well-established tumor were free of tumor when these mice were treated with AdVHER-2 vaccination in combination with anti-HER-2/neu Ab therapy, whereas the remaining 6/10 tumor-bearing mice had a longer survival (P<0.05), indicating that vaccination with recombinant HER-2/neu-expressing adenoviral vector can not only reduce breast carcinogenesis, but also provide adjuvant effect on anti-HER-2/neu Ab therapy for eradication of well-established breast cancers in Tg FVBneuN mice with self-HER-2/neu-specific immune tolerance.
Figure 6.
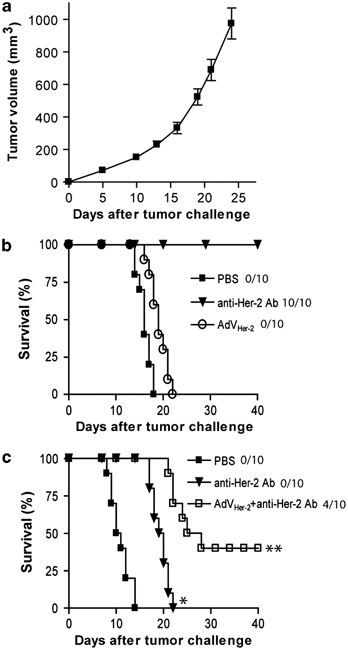
AdVHER-2 provides adjuvant effect on anti-HER-2/neu antibody therapy for well-established tumor in transgenic (Tg) FVBneuN mice. (a) Tg1-1 tumor growth curve. (b) When Tg1-1 tumors grew to a size of ∼15 mm3 (early stage), tumor-bearing FVBneuN Tg mice were s.c. injected with AdVHER-2 at 5-day interval for a total of three times or i.p. injected with the mouse anti-HER-2/neu antibody once every 3 days for a total of five times. (c) When Tg1-1 tumors grew to a size of ∼100 mm3 (well-established), tumor-bearing FVBneuN Tg mice were treated with the anti-HER-2/neu antibody alone or with both AdVHER-2 and the anti-HER-2 antibody in a similar manner as described above. Tumor growth was monitored daily for 40 days. *P<0.05 and **P<0.05 vs cohorts of the control group (phosphate-buffered saline (PBS) and anti-HER-2/neu antibody alone), respectively (log-rank test). One representative experiment of two is shown. AdV, adenovirus; HER, human epidermal growth factor receptor.
Discussion
Conventional cancer therapies including surgery, radiation therapy and chemotherapy have demonstrated a considerable clinical success over the past years. However, tumor-free survival is not always accomplished. For example, surgery and radiation therapy are quite effective in treatment of localized tumors, but they often have only a palliative role in treatment of disseminated diseases. Chemotherapy remains the treatment modality of choice, but severe toxic side-effects often limit its use. The identification of tumor-associated Ags and tumor-specific T-cell responses in cancer patients led to the development of immunotherapies aimed at augmenting antitumor immune responses. Antitumor immunotherapies include the active immunotherapy such as the use of various antitumor vaccines52 to stimulate the patients’ antitumor CD8+ CTL responses and the adoptive immunotherapy such as infusion of antitumor monoclonal Ab trastuzumab8 or tumor-specific tumor-infiltrating lymphocytes.53
The original anti-HER-2/neu murine monoclonal antibodies inhibited HER-2/neu-positive tumor growth in vivo.50, 51, 54 Trastuzumab is a humanized monoclonal Ab directed against the extracellular domain of HER-2/neu and its use, in combination with chemotherapy, was approved by the FDA in 1998 for metastatic HER-2/neu overexpressing breast cancer.43 Preclinical studies demonstrated interesting properties of trastuzumab, including55 internalization and degradation of the HER-2 protein,56 inhibition of cell-cycle progression via inhibition of the mitogen-activated protein kinase pathway,57, 58 suppression of the antiapoptotic phosphatidylinositol 3-kinase and Akt pathway59, 60 and Ab-dependent cellular cytotoxicity.61, 62 Clinical studies have shown that approximately one third of patients with advanced HER-2/neu-positive breast cancer will respond to trastuzumab monotherapy.63, 64 Trastuzumab-based therapy has also been shown to be effective in both adjuvant and neoadjuvant setting in the management of early stage HER-2/neu-positive breast cancer.65, 66 However, one of the major limitations of trastuzumab immunotherapy is the development of Ab resistance usually within 1 year from the beginning of the treatment in the metastatic setting.9, 10 Schematically, the resistance to trastuzumab may be derived from (i) a truncated and active form of receptor that lacks the trastuzumab-binding extracellular domain, (ii) constitutive activation of downstream elements, making activation of the pathway independent of the HER-2neu receptor and/or (iii) bypassing the HER-2/neu-receptor through activation of another transmembrane receptor.67, 68 Additionally, the risk of cardiac toxicity, especially in patients previously treated with anthracyclines,64, 69 may also limit the use of trastuzumab.
Since 1993, immune responses to HER-2/neu have been frequently found in patients with HER-2/neu-positive breast cancer.4, 5 As shown in preclinical model, such immune responses are associated with slower tumor development at early stages of the disease.70 These observations, together with reports about the efficacy and the resistance of passive trastuzumab therapy, motivated the development of various combinatorial immunotherapies. Among them, the combining therapeutic monoclonal antibodies (that is trastuzumab) with various antitumor vaccines (tumor cells, dendritic cells, DNA, peptides, proteins and viral vectors) is a promising avenue for combination immunotherapy.71, 72 When compared with passive immunotherapy with trastuzumab, anti-HER-2/neu vaccines that stimulate a pre-existing anti-HER-2/neu immune responses offer several advantages including (i) fewer iterative injections, (ii) potentially broader use in patients expressing different levels of HER-2/neu and (iii) establishment of a memory immune response capable of preventing diseases recurrence. In clinical trials, it has been demonstrated that anti-HER-2/neu vaccines could be incorporated into trastuzumab therapy.73, 74 These results represent the first clinical evidence of the potential benefits of minimal toxicity that may be derived from combination immunotherapy. It has also been demonstrated that the combination of trastuzumab followed by HER-2/neu peptide vaccine was safe and beneficial.75
One of the most remarkable features of AdV-based vaccines is their ability to induce exceptionally high and sustained frequencies of transgene product-specific CD8+ T-cell responses, which, unlike those induced by other subunit vaccine carriers such as DNA vaccines or poxvirus vectors, do not contract after the initial CTL activation.76, 77 In this study, we also demonstrated that AdVOVA vaccination induces a sustained CD8+ CTL responses due to persistent Ag stimulation, which is consistent with some previous reports by others.27, 28 It has been elucidated that replication-defective AdV vector genomes similar to those of wild-type AdV vectors acquired by natural infections persist.78, 79 These replication-defective AdV vectors have been found in the muscle at the site of inoculation, in the liver and in the lymphatic tissues of experimental animals.27, 28 It has been demonstrated that AdVHER-2 vaccination can stimulate both HER-2/neu-specific Ab and CD8+ CTL responses and preventive antitumor immunity in wild-type mice.34, 35, 36 However, it was not able to reduce breast carcinogenesis in Tg mice with self-immune tolerance though their survival was prolonged.26, 32 In this study, we have demonstrated that AdVHER-2 not only induce both HER-2/neu-specific functional CD8+ T cell, but also NK responses, leading to protective antitumor immunity. However, CD8+ T cells, but not NK cells have a major role in the immunity, which is consistent with a recent report by Wan and colleagues.80 We previously have demonstrated that vaccination with dendritic cells engineered to express HER-2/neu, resulted in a significant delay in tumor formation, however, despite stronger HER-2/neu-specific immune responses than DNA vaccine, it did not reduce breast carcinogenesis.81 In the present study, we demonstrated that AdVHER-2 vaccination significantly reduced breast carcinogenesis in Tg FVBneuN mice with self-immune tolerance, which is consistent with another recent report by Berzofsky et al.33
The therapeutic efficacies of AdV in cancer remain controversial. For example, AdV vaccine was found to be ineffective even when it was administered as early as only one33 or two days34 after seeding the tumor cells in wild-type mice. Conversely, it has also been demonstrated that recombinant AdVHER-2 vaccine efficiently eradicated advanced established murine breast cancer in wild-type mice.37 In this study, we showed that AdVHER-2 vaccine had little therapeutic effect on pre-existing tumors. AdVHER-2 only slightly delayed HER-2/neu-expressing Tg1-1 breast tumor growth, but did not cure any of Tg1-1 tumors in Tg FVBneuN mice even though they were in early stage (15 mm3). The difference in AdVHER-2-mediated therapeutic effects seen in our study and a previous report37 may be derived from the use of different types of mice. In this study, we used Tg FVBneuN mice with self-HER-2/neu-specific immune tolerance, whereas Myun et al. used wild-type mice.37 In comparison, however, the anti-HER-2/neu Ab therapy was much effective since it cured all early stage (15 mm3) Tg1-1 tumors in Tg FVBneuN mice, but failed in eradication of well-established Tg1-1 tumors (100 mm3). Bocangel et al.82 have demonstrated that a combinatorial synergy can be induced by AdVmda-7 vaccine expressing a tumor suppressor gene (melanoma differentiation-associated gene-7) and trastuzumab in delaying growth of HER-2/neu-expressing human breast cancer in nude mice. Interestingly, for the first time, we demonstrated that a combinatorial immunotherapy of anti-HER-2/neu Ab and HER-2/neu-specific AdVHER-2 vaccine was capable of eradicating 4/10 Tg FVBneuN mice bearing well-established HER-2/neu-expressing breast cancer Tg1-1 (100 mm3) and significantly prolonging the survival of the remaining 6/10 tumor-bearing mice, indicating that the synergistic effect of AdVHER-2 vaccine is capable of stimulating both HER-2/neu-specific CD8+ CTL and nonspecific NK cell responses on anti-HER-2/neu Ab therapy for well-established tumors in Tg FVBneuN mice with self-HER-2/neu-specific immune tolerance.
Taken together, we demonstrate that adenoviral vector vaccine can stimulate both CD8+ CTL and NK cell responses and provide an adjuvant effect on anti-HER-2/neu Ab therapy. Our results suggest an adjuvant effect of AdVHER-2 on anti-HER-2/neu Ab therapy, and this combinatorial immunotherapy with trastuzumab and AdVHER-2 vaccine may be used as a new therapeutic strategy for treatment of advanced HER-2/neu-positive breast cancer.
Acknowledgements
This study was supported by a research grant (406991) from Canadian Breast Cancer Foundation.
Competing interests
The authors declare no conflict of interest.
Footnotes
Y Chen and Y Xie: These two authors contributed equally to this work.
References
- 1.Schechter AL, Hung MC, Vaidyanathan L, Weinberg RA, Yang-Feng TL, Francke U. The neu gene: an erbB-homologous gene distinct from and unlinked to the gene encoding the EGF receptor. Science. 1985;229:976–978. doi: 10.1126/science.2992090. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986;319:226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- 4.Pupa SM, Menard S, Andreola S, Colnaghi MI. Antibody response against the c-erbB-2 oncoprotein in breast carcinoma patients. Cancer Res. 1993;53:5864–5866. [PubMed] [Google Scholar]
- 5.Disis ML, Knutson KL, Schiffman K, Rinn K, McNeel DG. Pre-existent immunity to the HER-2/neu oncogenic protein in patients with HER-2/neu overexpressing breast and ovarian cancer. Breast Cancer Res Treat. 2000;62:245–252. doi: 10.1023/a:1006438507898. [DOI] [PubMed] [Google Scholar]
- 6.Peoples GE, Goedegebuure PS, Smith R, Linehan DC, Yoshino I, Eberlein TJ. Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc Natl Acad Sci USA. 1995;92:432–436. doi: 10.1073/pnas.92.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Angulo AM, Hortobagyi GN, Esteva FJ. Adjuvant therapy with trastuzumab for HER-2/neu-positive breast cancer. Oncologist. 2006;11:857–867. doi: 10.1634/theoncologist.11-8-857. [DOI] [PubMed] [Google Scholar]
- 9.Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer Lett. 2006;232:123–138. doi: 10.1016/j.canlet.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho WY, Yee C, Greenberg PD. Adoptive therapy with CD8(+) T cells: it may get by with a little help from its friends. J Clin Invest. 2002;110:1415–1417. doi: 10.1172/JCI17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lara-Tejero M, Pamer EG. T cell responses to Listeria monocytogenes. Curr Opin Microbiol. 2004;7:45–50. doi: 10.1016/j.mib.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–1525. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serbina N, Pamer EG. Quantitative studies of CD8+ T-cell responses during microbial infection. Curr Opin Immunol. 2003;15:436–442. doi: 10.1016/s0952-7915(03)00071-2. [DOI] [PubMed] [Google Scholar]
- 17.Bonnet MC, Tartaglia J, Verdier F, Kourilsky P, Lindberg A, Klein M. Recombinant viruses as a tool for therapeutic vaccination against human cancers. Immunol Lett. 2000;74:11–25. doi: 10.1016/s0165-2478(00)00244-3. [DOI] [PubMed] [Google Scholar]
- 18.Harrop R, John J, Carroll MW. Recombinant viral vectors: cancer vaccines. Adv Drug Deliv Rev. 2006;58:931–947. doi: 10.1016/j.addr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Muruve DA, Cotter MJ, Zaiss AK, White LR, Liu Q, Chan T. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng P, Parks RJ, Cummings DT, Evelegh CM, Sankar U, Graham FL. A high-efficiency Cre/loxP-based system for construction of adenoviral vectors. Hum Gene Ther. 1999;10:2667–2672. doi: 10.1089/10430349950016708. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, Roederer M. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424:681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao W, Tamin A, Soloff A, D’Aiuto L, Nwanegbo E, Robbins PD. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362:1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumida SM, Truitt DM, Kishko MG, Arthur JC, Jackson SS, Gorgone DA. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J Virol. 2004;78:2666–2673. doi: 10.1128/JVI.78.6.2666-2673.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santra S, Seaman MS, Xu L, Barouch DH, Lord CI, Lifton MA. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J Virol. 2005;79:6516–6522. doi: 10.1128/JVI.79.10.6516-6522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang TC, Dayball K, Wan YH, Bramson J. Detailed analysis of the CD8+ T-cell response following adenovirus vaccination. J Virol. 2003;77:13407–13411. doi: 10.1128/JVI.77.24.13407-13411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millar J, Dissanayake D, Yang TC, Grinshtein N, Evelegh C, Wan Y. The magnitude of the CD8+ T cell response produced by recombinant virus vectors is a function of both the antigen and the vector. Cell Immunol. 2007;250:55–67. doi: 10.1016/j.cellimm.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007;110:1916–1923. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang TC, Millar J, Groves T, Grinshtein N, Parsons R, Takenaka S. The CD8+ T cell population elicited by recombinant adenovirus displays a novel partially exhausted phenotype associated with prolonged antigen presentation that nonetheless provides long-term immunity. J Immunol. 2006;176:200–210. doi: 10.4049/jimmunol.176.1.200. [DOI] [PubMed] [Google Scholar]
- 29.Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC. Vaccination with a recombinant vaccinia virus encoding a ‘self’ antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci USA. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lane C, Leitch J, Tan X, Hadjati J, Bramson JL, Wan Y. Vaccination-induced autoimmune vitiligo is a consequence of secondary trauma to the skin. Cancer Res. 2004;64:1509–1514. doi: 10.1158/0008-5472.can-03-3227. [DOI] [PubMed] [Google Scholar]
- 31.Leitch J, Fraser K, Lane C, Putzu K, Adema GJ, Zhang QJ. CTL-dependent and -independent antitumor immunity is determined by the tumor not the vaccine. J Immunol. 2004;172:5200–5205. doi: 10.4049/jimmunol.172.9.5200. [DOI] [PubMed] [Google Scholar]
- 32.Kianizad K, Marshall LA, Grinshtein N, Bernard D, Margl R, Cheng S. Elevated frequencies of self-reactive CD8+ T cells following immunization with a xenoantigen are due to the presence of a heteroclitic CD4+ T-cell helper epitope. Cancer Res. 2007;67:6459–6467. doi: 10.1158/0008-5472.CAN-06-4336. [DOI] [PubMed] [Google Scholar]
- 33.Grinshtein N, Bridle B, Wan Y, Bramson JL. Neoadjuvant vaccination provides superior protection against tumor relapse following surgery compared with adjuvant vaccination. Cancer Res. 2009;69:3979–3985. doi: 10.1158/0008-5472.CAN-08-3385. [DOI] [PubMed] [Google Scholar]
- 34.Gallo P, Dharmapuri S, Nuzzo M, Maldini D, Iezzi M, Cavallo F. Xenogeneic immunization in mice using HER2 DNA delivered by an adenoviral vector. Int J Cancer. 2005;113:67–77. doi: 10.1002/ijc.20536. [DOI] [PubMed] [Google Scholar]
- 35.Park JM, Terabe M, Sakai Y, Munasinghe J, Forni G, Morris JC. Early role of CD4+ Th1 cells and antibodies in HER-2 adenovirus vaccine protection against autochthonous mammary carcinomas. J Immunol. 2005;174:4228–4236. doi: 10.4049/jimmunol.174.7.4228. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Wang JP, Rao XM, Price JE, Zhou HS, Lachman LB. Prime-boost vaccination with plasmid and adenovirus gene vaccines control HER2/neu+ metastatic breast cancer in mice. Breast Cancer Res. 2005;7:R580–R588. doi: 10.1186/bcr1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park JM, Terabe M, Steel JC, Forni G, Sakai Y, Morris JC. Therapy of advanced established murine breast cancer with a recombinant adenoviral ErbB-2/neu vaccine. Cancer Res. 2008;68:1979–1987. doi: 10.1158/0008-5472.CAN-07-5688. [DOI] [PubMed] [Google Scholar]
- 38.Drebin JA, Link VC, Greene MI. Monoclonal antibodies reactive with distinct domains of the neu oncogene-encoded p185 molecule exert synergistic anti-tumor effects in vivo. Oncogene. 1988;2:273–277. [PubMed] [Google Scholar]
- 39.Umeshappa CS, Huang H, Xie Y, Wei Y, Mulligan SJ, Deng Y. CD4+ Th-APC with acquired peptide/MHC class I and II complexes stimulate type 1 helper CD4+ and central memory CD8+ T cell responses. J Immunol. 2009;182:193–206. doi: 10.4049/jimmunol.182.1.193. [DOI] [PubMed] [Google Scholar]
- 40.Sas S, Chan T, Sami A, El-Gayed A, Xiang J. Vaccination of fiber-modified adenovirus-transfected dendritic cells to express HER-2/neu stimulates efficient HER-2/neu-specific humoral and CTL responses and reduces breast carcinogenesis in transgenic mice. Cancer Gene Ther. 2008;15:655–666. doi: 10.1038/cgt.2008.18. [DOI] [PubMed] [Google Scholar]
- 41.Wright P, Zheng C, Moyana T, Xiang J. Intratumoral vaccination of adenoviruses expressing fusion protein RM4/tumor necrosis factor (TNF)-alpha induces significant tumor regression. Cancer Gene Ther. 1998;5:371–379. [PubMed] [Google Scholar]
- 42.Carlsson G, Gullberg B, Hafstrom L. Estimation of liver tumor volume using different formulas—an experimental study in rats. J Cancer Res Clin Oncol. 1983;105:20–23. doi: 10.1007/BF00391826. [DOI] [PubMed] [Google Scholar]
- 43.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 44.Miller F, Jones RF, Jacob J, Kong YC, Wei WZ. From breast cancer immunobiology to her-2 DNA vaccine and autoimmune sequelae. Breast Dis. 2004;20:43–51. doi: 10.3233/bd-2004-20106. [DOI] [PubMed] [Google Scholar]
- 45.Reilly RT, Gottlieb MB, Ercolini AM, Machiels JP, Kane CE, Okoye FI. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 2000;60:3569–3576. [PubMed] [Google Scholar]
- 46.Czerniecki BJ, Koski GK, Koldovsky U, Xu S, Cohen PA, Mick R. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67:1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 47.Ambrosino E, Spadaro M, Iezzi M, Curcio C, Forni G, Musiani P. Immunosurveillance of Erbb2 carcinogenesis in transgenic mice is concealed by a dominant regulatory T-cell self-tolerance. Cancer Res. 2006;66:7734–7740. doi: 10.1158/0008-5472.CAN-06-1432. [DOI] [PubMed] [Google Scholar]
- 48.Wall EM, Milne K, Martin ML, Watson PH, Theiss P, Nelson BH. Spontaneous mammary tumors differ widely in their inherent sensitivity to adoptively transferred T cells. Cancer Res. 2007;67:6442–6450. doi: 10.1158/0008-5472.CAN-07-0622. [DOI] [PubMed] [Google Scholar]
- 49.Whittington PJ, Piechocki MP, Heng HH, Jacob JB, Jones RF, Back JB. DNA vaccination controls Her-2+ tumors that are refractory to targeted therapies. Cancer Res. 2008;68:7502–7511. doi: 10.1158/0008-5472.CAN-08-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harwerth IM, Wels W, Schlegel J, Muller M, Hynes NE. Monoclonal antibodies directed to the erbB-2 receptor inhibit in vivo tumour cell growth. Br J Cancer. 1993;68:1140–1145. doi: 10.1038/bjc.1993.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knutson KL, Almand B, Dang Y, Disis ML. Neu antigen-negative variants can be generated after neu-specific antibody therapy in neu transgenic mice. Cancer Res. 2004;64:1146–1151. doi: 10.1158/0008-5472.can-03-0173. [DOI] [PubMed] [Google Scholar]
- 52.Antonia S, Mule JJ, Weber JS. Current developments of immunotherapy in the clinic. Curr Opin Immunol. 2004;16:130–136. doi: 10.1016/j.coi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 53.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54:721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9:1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nahta R, Esteva FJ. HER-2-targeted therapy: lessons learned and future directions. Clin Cancer Res. 2003;9:5078–5084. [PubMed] [Google Scholar]
- 56.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]
- 57.Le XF, Claret FX, Lammayot A, Tian L, Deshpande D, LaPushin R. The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J Biol Chem. 2003;278:23441–23450. doi: 10.1074/jbc.M300848200. [DOI] [PubMed] [Google Scholar]
- 58.Jackson JG, St Clair P, Sliwkowski MX, Brattain MG. Blockade of epidermal growth factor- or heregulin-dependent ErbB2 activation with the anti-ErbB2 monoclonal antibody 2C4 has divergent downstream signaling and growth effects. Cancer Res. 2004;64:2601–2609. doi: 10.1158/0008-5472.can-03-3106. [DOI] [PubMed] [Google Scholar]
- 59.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 60.Mohsin SK, Weiss HL, Gutierrez MC, Chamness GC, Schiff R, Digiovanna MP. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J Clin Oncol. 2005;23:2460–2468. doi: 10.1200/JCO.2005.00.661. [DOI] [PubMed] [Google Scholar]
- 61.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 62.Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 63.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 64.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 65.Baselga J, Gianni L, Geyer C, Perez EA, Riva A, Jackisch C. Future options with trastuzumab for primary systemic and adjuvant therapy. Semin Oncol. 2004;31:51–57. doi: 10.1053/j.seminoncol.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 66.Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 67.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977–984. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 68.Whenham N, D’Hondt V, Piccart MJ. HER2-positive breast cancer: from trastuzumab to innovatory anti-HER2 strategies. Clin Breast Cancer. 2008;8:38–49. doi: 10.3816/CBC.2008.n.002. [DOI] [PubMed] [Google Scholar]
- 69.Bengala C, Zamagni C, Pedrazzoli P, Matteucci P, Ballestrero A, Da Prada G. Cardiac toxicity of trastuzumab in metastatic breast cancer patients previously treated with high-dose chemotherapy: a retrospective study. Br J Cancer. 2006;94:1016–1020. doi: 10.1038/sj.bjc.6603060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Disis ML, Bernhard H, Shiota FM, Hand SL, Gralow JR, Huseby ES. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood. 1996;88:202–210. [PubMed] [Google Scholar]
- 71.Ladjemi MZ, Jacot W, Chardes T, Pelegrin A, Navarro-Teulon I. Anti-HER2 vaccines: new prospects for breast cancer therapy. Cancer Immunol Immunother. 2010;59:1295–1312. doi: 10.1007/s00262-010-0869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baxevanis CN, Perez SA, Papamichail M. Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer Immunol Immunother. 2009;58:317–324. doi: 10.1007/s00262-008-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Investigating the combination of trastuzumab and HER2/neu peptide vaccines for the treatment of breast cancer. Ann Surg Oncol. 2006;13:1085–1098. doi: 10.1245/ASO.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 74.Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benavides LC, Gates JD, Carmichael MG, Patil R, Holmes JP, Hueman MT. The impact of HER2/neu expression level on response to the E75 vaccine: from U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2009;15:2895–2904. doi: 10.1158/1078-0432.CCR-08-1126. [DOI] [PubMed] [Google Scholar]
- 76.Bruna-Romero O, Gonzalez-Aseguinolaza G, Hafalla JC, Tsuji M, Nussenzweig RS. Complete, long-lasting protection against malaria of mice primed and boosted with two distinct viral vectors expressing the same plasmodial antigen. Proc Natl Acad Sci USA. 2001;98:11491–11496. doi: 10.1073/pnas.191380898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hassett DE, Slifka MK, Zhang J, Whitton JL. Direct ex vivo kinetic and phenotypic analyses of CD8(+) T-cell responses induced by DNA immunization. J Virol. 2000;74:8286–8291. doi: 10.1128/jvi.74.18.8286-8291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garnett CT, Erdman D, Xu W, Gooding LR. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol. 2002;76:10608–10616. doi: 10.1128/JVI.76.21.10608-10616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gu Z, Belzer SW, Gibson CS, Bankowski MJ, Hayden RT. Multiplexed, real-time PCR for quantitative detection of human adenovirus. J Clin Microbiol. 2003;41:4636–4641. doi: 10.1128/JCM.41.10.4636-4641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karimi K, Boudreau JE, Fraser K, Liu H, Delanghe J, Gauldie J. Enhanced antitumor immunity elicited by dendritic cell vaccines is a result of their ability to engage both CTL and IFN gamma-producing NK cells. Mol Ther. 2008;16:411–418. doi: 10.1038/sj.mt.6300347. [DOI] [PubMed] [Google Scholar]
- 81.Chan T, Sami A, El-Gayed A, Guo X, Xiang J. HER-2/neu-gene engineered dendritic cell vaccine stimulates stronger HER-2/neu-specific immune responses compared to DNA vaccination. Gene Ther. 2006;13:1391–1402. doi: 10.1038/sj.gt.3302797. [DOI] [PubMed] [Google Scholar]
- 82.Bocangel D, Zheng M, Mhashilkar A, Liu Y, Ramesh R, Hunt KK. Combinatorial synergy induced by adenoviral-mediated mda-7 and Herceptin in Her-2+ breast cancer cells. Cancer Gene Ther. 2006;13:958–968. doi: 10.1038/sj.cgt.7700972. [DOI] [PubMed] [Google Scholar]


