Summary:
Respiratory viruses (RVs) frequently cause severe respiratory disease in bone marrrow transplant (BMT) recipients. To evaluate the frequency of RV, nasal washes were collected year-round from BMT recipients with symptoms of upper respiratory tract infection (URI). Direct immunofluorescence assay was performed for respiratory syncytial virus (RSV), influenza (Flu) A and B, adenovirus and parainfluenza (Paraflu) virus. Patients with RSV pneumonia or with upper RSV infection, but considered at high risk for developing RSV pneumonia received aerosolized ribavirin. Oseltamivir was given to patients with influenza. A total of 179 patients had 392 episodes of URI. In all, 68 (38%) tested positive: RSV was detected in 18 patients (26.4%), Flu B in 17 (25%), Flu A in 11 (16.2%) and Paraflu in 7 (10.3%). A total of 14 patients (20.6%) had multiple RV infections or coinfection. RSV pneumonia developed in 55.5% of the patients with RSV-URI. One of the 15 patients (6.6%) with RSV pneumonia died. Influenza pneumonia was diagnosed in three patients (7.3%). RSV and influenza infections peaked in fall–winter and winter–spring months, respectively. We observed decreased rates of influenza and parainfluenza pneumonia and low mortality because of RSV pneumonia. The role of antiviral interventions such as aerosolized ribavirin and new neuraminidase inhibitors remains to be defined in randomized trials.
Keywords: respiratory virus, BMT, immunofluorescence, RSV, influenza, parainfluenza, mortality rate
Introduction
Community respiratory virus infections (RVI) represent a major cause of morbidity in immunocompromised hosts, mainly during fall and winter months.1,2 Respiratory syncytial virus (RSV) causes around 50% of the cases of RVI. The incidence of influenza A and B, parainfluenza 1, 2 and 3, and rhinovirus varies among different BMT centers and also between seasons, oscillating from 10 to 20%. Adenovirus and coronavirus infections occur less frequently, the former without the sharp seasonality observed for other respiratory viruses.1,2,3
Bone marrow transplant (BMT) recipients with upper respiratory RSV infection have an estimated 50% risk of developing RSV pneumonia, and mortality rates ranging from 50 to 80% have been described.1,3,4 Patients who acquire RSV infection before engraftment are at higher risk of pneumonia.3,5 Influenza and parainfluenza upper respiratory infections also have a great risk of progression to pneumonia, but data varies greatly among centers and authors.1,6,7 Thus, incidences as high as 70% or as low as 17% of influenza and parainfluenza pneumonias have been described.1,6
The seasonality of RVI in the southern hemisphere countries has been shown to present a different pattern as compared to the northern hemisphere regions. Thus, in some subtropical areas of Brazil, influenza viruses can cause outbreaks throughout the year.8 This information is relevant to establish the policies of influenza control in the southern hemisphere countries, such as the definition of the best time to start annual influenza vaccination.
The consequences of RVI outbreaks in BMT units can be dramatic. This prospective study was conducted to evaluate the prevalence of RVI in BMT recipients, the morbidity and mortality associated to these infections, and the seasonal trends of RVI in this population.
Patients and methods
Patients
Winter in the southern hemisphere starts on June 21st. From April 27, 2001 to April 30, 2002, nasal washes (NW) were taken from BMT recipients with respiratory symptoms to diagnose RVI. In patients who tested positive, weekly samples were taken to evaluate the duration of respiratory virus excretion. Another NW was taken from patients who tested negative but remained symptomatic, 1 week later.
Definitions
Upper respiratory infection was defined as the presence of coryza, pharyngitis, sinusitis and/or cough with or without fever and a clear chest radiograph. Lower respiratory infection was defined as the presence of wheezing, hypoxia or pneumonia with a new radiographic infiltrate. Respiratory virus coinfection was defined when more than one RV was identified in NW taken within a 6-day interval. Multiple RVI was defined when different RVs were identified in different episodes of RVI. Recurrent RVI was defined when the same RV was identified in different episodes of RVI. When clinically indicated, patients with radiological signs suggestive of viral infection and with a negative NW were submitted to bronchoalveolar lavage (BAL).
Specimen
Clinical specimens were collected as described by Englund et al9 (1996), kept on ice and processed within 3 h from sampling.
Antigen detection
Direct immunofluorescence assay for antigen detection of RSV, influenza A and B, parainfluenza and adenovirus was performed according to the manufacturer's instructions (Imagen® DAKO, Cambridgeshire, UK).
RVI control interventions
(1) Contact precautions adopted for all patients with respiratory symptoms until diagnosis. (2) Extended contact precautions for all patients with confirmed RSV infection for 2 weeks or until two negative samples taken 1 week apart or for the duration of respiratory symptoms if no agent was identified. (3) Placement of patients in single-bed rooms or cohorting patients on specific precautions to the same room. (4) RVI testing of healthcare workers with respiratory symptoms. (5) Restriction of visits for adults with respiratory symptoms and for children. (6) Annual influenza vaccination recommended to BMT recipients transplanted 6 months or longer as well as to all healthcare providers. Influenza vaccination for immunocompromised patients and health personnel starts in March.
Treatment of RVI
Patients with upper RSV infection diagnosed before engraftment, with RSV pneumonia or those with severe acute or chronic graft-versus-host-disease (GVHD) received ribavirin aerosolized via a small particle aerosol generator (SPAG-2) and administered through a face mask at a dose of 6 g/day (20 mg/ml for 18 h/day) for 7 days. Intravenous immunoglobulin (IVIg) at 500 mg/kg every other day was added to ribavirin.10 Patients with influenza A or B received oseltamivir (75 mg twice a day) for 5 days. Three patients with influenza A received amantadine (100 mg twice a day).
Statistical analysis
χ2 method (Microsoft Excel) was used to determine the significance concerning the duration of shedding between RSV and other viruses (influenza and parinfluenza).
Results
A total of 179 patients had 392 episodes of upper respiratory infection during the study period (mean 2.2 episodes, ranging from one to seven episodes per patient). Respiratory symptoms were present throughout the year and the number of symptomatic patients ranged from 24 to 49 per month (median 38 patients, Figure 1). A total of 777 samples were taken, mean 4.3 (1–19) per patient. Patient characteristics are shown in Table 1.
Figure 1.
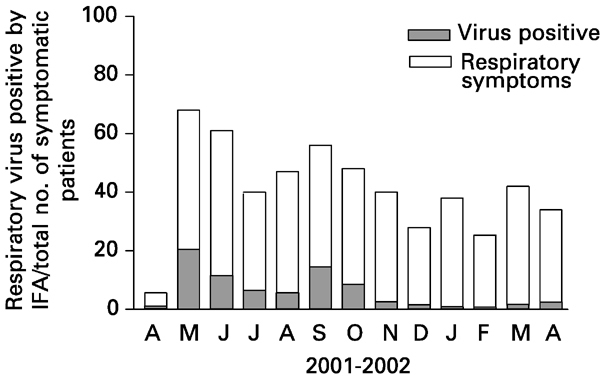
RV infection in patients with respiratory symptoms according to the month of diagnosis.
Table 1.
Patient characteristics (N=179)
| Variable | No. of patientsa (%) |
|---|---|
| Type of BMT | |
| Allogeneic | 128 (71.5) |
| Autologous | 51 (28.5) |
| Underlying diseases | (%) |
| CML | 46 (25.7) |
| AML | 31 (17.3) |
| SAA | 23 (12.8) |
| NHL | 22 (12.3) |
| ALL | 19 (10.6) |
| MM | 19 (10.6) |
| HL | 11 (6.1) |
| Other | 8 (4.4) |
| No. of patients with RVI | 68 (38) |
| Pre-engraftment | 22 (32.3) |
| Postengraftment | 46 (67.6) |
Of 179, 68 patients with upper respiratory infection (38%) tested positive. Distribution frequencies of RVI are described in Table 2.
Table 2.
Distribution frequencies of respiratory virus infections in 68 recipients with respiratory symptoms and positive NW
| RV identified | No. of patients (%) |
|---|---|
| RSV | 18 (26.4) |
| RSV+flu A | 04 (5.8) |
| RSV+flu B | 02 (2.9) |
| RSV+parainfluenza | 02 (2.9) |
| RSV+flu A+flu B | 01 (1.4) |
| Influenza B | 17 (25) |
| Influenza A | 11 (16.2) |
| Influenza A+influenza B | 03 (4.4) |
| Parainfluenza | 07 (10.3) |
| Parainfluenza+influenza B | 02 (2.9) |
| Parainfluenza+influenza A | 01 (1.4) |
| Total | 68 (100) |
RV=respiratory virus; Flu A=influenza A; Flu B=influenza B.
A total of 15 patients (22%) had RV coinfections and/or multiple infections. Five patients (7.3%) had RV coinfection, Flu A/Flu B and RSV/Flu B being the most prevalent associations (two Flu A/Flu B, two RSV/Flu B, 66.6%). Multiple RVI were seen in nine patients (13.2%) during the study period and the interval between the episodes of RVI varied from 2 to 7 months (median 3 months). One patient had an episode of RSV/Flu B coinfection followed by an episode of Flu A 3 months later. No cases of adenovirus respiratory infection were detected by IFA ( Table 2).
RSV infection
A total of 27 patients had upper RSV infection (39.7%). In all, 21 of them (77.7%) were allogeneic BMT recipients. The median time for the occurrence of upper RSV infection was 25 days (day −5 to day +1947) after transplantation and the mean duration of virus excretion was 6 days (ranging from 4 to 30 days). Two patients had recurrent RSV infection within a 4- and 8-month interval between episodes, respectively. A total of 12 patients (44.4%) had only upper RSV infection and 15 patients (55.5%) developed RSV pneumonia (Table 3). All patients with RSV pneumonia were allogeneic BMT recipients and five of them (33.3%) acquired RSV pneumonia during the pre-engraftment period. The median time for the development of pneumonia was 20 days (days –5 to + 150) after BMT. Aerosolized ribavirin was administered to 16 of the 27 patients with RSV infection. According to the protocol, five patients with upper RSV infection considered at risk for developing pneumonia and 11 of the 15 patients with established RSV pneumonia were treated. Three of the remaining four patients with pneumonia improved before ribavirin administration and were not treated. The remaining one could not be treated because the drug was not available at the hospital when the diagnosis was made (day +25). This patient died of RSV pneumonia (6.6%).
Table 3.
Illnesses and deaths in BMT recipients with respiratory virus infection
| Etiologic agent | No. (%) with respiratory illness | No. (%) who died of pneumonia | |
|---|---|---|---|
| URI | Pneumonia | ||
| RSV (n=27) | 27 (100) | 15 (55.5) | 1 (6.6) |
| Influenza (n=41) | 41 (100) | 3 (7.3) | 0 |
| Parainfluenza (n=12) | 12 (100) | 0 0 | |
Influenza infection
Influenza A or B infections were seen in 41 of the 68 patients (60.3%). who tested positive by immunofluorescence assay. Influenza A was diagnosed in 19 patients and influenza B in 25 patients. Three patients had both influenza A and B infection (one coinfection, two multiple infections). The median time for the occurrence of influenza infection was 96 days (ranging from −9 to +1357). Three of the 41 patients (7.3%) with upper influenza infection developed interstitial pneumonia as seen on tomographic images and had a positive BAL for influenza viruses ( Table 3). Two patients had RV coinfection (influenza A/B and influenza B/RSV). The remaining patient had influenza A pneumonia 4 months after an episode of upper RSV infection.
Parainfluenza infection
A total of 12 patients had upper respiratory parainfluenza infection during follow-up. In seven patients, parainfluenza was the only RV detected. The remaining five patients (41.6%) had coinfection (one patient) or multiple infections (four patients). The median time for the occurrence of parainfluenza infection was 74 days (ranging from day −5 to day +999). No patient developed parainfluenza pneumonia during follow-up.
Distribution of RVI and duration of virus shedding
The distribution of RVI according to month of diagnosis showed a higher incidence of RSV in fall months. The majority of RSV cases were detected in May. Influenza A and B predominated during winter and early spring months of the southern hemisphere (Figure 2).
Figure 2.
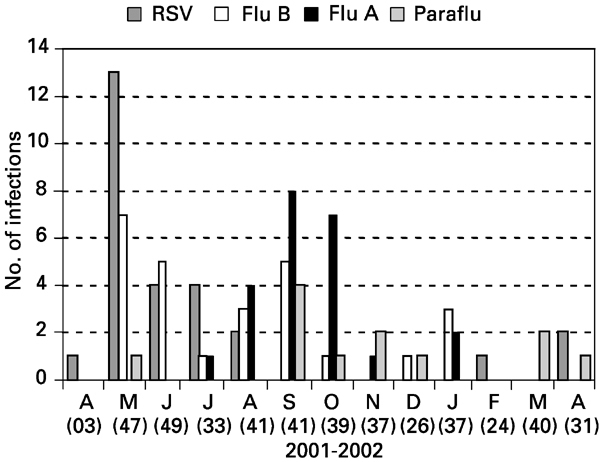
Distribution of RV infection according to the month of diagnosis.
In 56 patients with two or more NW taken within a 7-day interval, RSV shedding was significantly longer than influenza and parainfluenza shedding (χ2 test, P=0.003). The duration of RV shedding according to the virus detected is shown in Table 4.
Table 4.
Respiratory viruses and duration of shedding
| RV | ⩽7 days | > 7 days | Total a |
|---|---|---|---|
| RSV | 13 | 12 | 25 |
| Influenza | 21 | 02 | 23 |
| Parainfluenza | 07 | 01 | 08 |
| Total | 41 | 15 | 56 |
aIn 56 patients with ⩾2 NW taken within a 7-day interval.
Discussion
Few data are available concerning the prevalence of respiratory viruses in immunocompromised patients of the southern hemisphere. Except for some reports on AIDS patients, most of the published papers have analyzed the seasonal trends and clinical patterns of RVI in neonates and immunocompetent children of developing countries.8,11,12,13,14,15
In the present study, we observed a 38% prevalence of RVI in BMT recipients with respiratory symptoms. Prevalence rates varying from 1.8 to 36% have been described in BMT recipients from developed countries.1,6,7 Probably, even higher prevalence would be expected in this series if we had performed virus culture and/or molecular assays to identify adenovirus, rhinovirus and coronavirus in these samples.
The distribution frequencies of RV through the year showed that RSV infection predominates in fall–winter months, while influenza A and B predominates in winter–spring months. This seasonal distribution is similar to what is observed in the US1,3,16 and somewhat different from Europe, where RSV has its peak during winter–spring months. An interesting observation concerns a few cases of influenza A and B seen in summer months, as previously observed by other authors in healthy children of Rio de Janeiro.8 This finding raises a question about ‘seasonal’ influenza vaccination restricted to the autumn. BMT recipients not eligible to receive flu vaccine (those within the first 6 months after BMT) during seasonal vaccination will continue ‘at risk’ of acquiring influenza until the next season according to the current recommendations. These patients would probably benefit of influenza vaccination at any period of the year.
During the study, influenza and RSV were the viruses most frequently identified, accounting for 89.7% of all RVI. Several authors have reported similar findings in the last two decades.1,6
An unexpected finding of this study was that around 15% of the patients had multiple RVI with a median 3-month interval between episodes. This observation has been poorly addressed in previous studies. The present data suggest that RVI sampling should be performed continuously throughout the year in all BMT recipients with respiratory symptoms.
In the present study, we also observed that respiratory symptoms were frequently present during the whole year. These data suggest that other RVs such as rhinovirus (not tested in the present study) and nonviral infections (mainly bacterial and fungal sinusitis) are probably the cause of upper respiratory symptoms especially in nonwinter months (Figure 1).
Besides multiple infections, we observed that six of the 68 BMT recipients (8.8%) had RV coinfection (five coinfection, one coinfection/multiple-infection) as diagnosed by the direct immunofluorescence assay. Recently, in children with bronchiolitis, Andreoletti et al17 and Ong et al18 have observed 21.4 and 10% of dual RVI, respectively, when molecular assays were added to the diagnostic repertoire of clinical laboratories. Thus, combining new techniques to improve the diagnosis of RVI, even higher rates of RV coinfections could be expected in the setting of BMT. Further studies are needed to address this issue.
In the present study, we observed that 55.5% of the patients with upper RSV infection developed pneumonia. Other BMT centers have reported rates varying from 32 to 75%.2,3,10
The outcome of patients with pneumonia has been historically and universally poor with mortality rates ranging from 50 to 83%.3,4,20 Although not yet specifically addressed, we observed, reviewing the literature, that the same centers previously reporting high mortality rates have referred a significant decrease in the deaths related to RSV pneumonia, even though the better outcome of these patients could not be consistently attributed to any interventional strategy. Thus, historic rates of approximately 80% have decreased to 19 and 29% in the MD Anderson Cancer Center and in the Fred Hutchinson Cancer Research Center, respectively.3,10,21
The present study was not designed to evaluate antiviral interventions. Antiviral therapy (aerosolized ribavirin and IVIg) was introduced in those patients considered at higher risk for pneumonia or death. Although no decrease in the incidence of RSV pneumonia was noted, we observed a small incidence of RSV-related death (6.6%) in this series. The role of the strategies adopted in this and the above-mentioned studies, as well as the risk factors for severe disease need to be definitely evaluated in large randomized trials.
The use of IVIg in patients with RSV infections might have decreased the frequency of coinfections with adenovirus since recent clinical and in vitro studies have shown evidence that humoral immunity play an important role in the control of adenovirus infections.22
Similarly, we noted a low rate of influenza and parainfluenza pneumonias. No patient with parainfluenza upper respiratory infection and only three of the 41 patients (7.3%) with influenza A or B infection developed pneumonia. Previous studies have reported parainfluenza pneumonia rates ranging from 17 to 41.2%1,3,7,15 and influenza pneumonia rates ranging from 9.5 to 75%.3,6,7,23
Two interventions could be responsible for the low rates of influenza complications observed in the present study. First, influenza vaccination and second, antiviral therapy. Influenza vaccine is poorly immunogenic within the first 6 months of transplantation and is therefore not considered to be recommended during this period. The patients' influenza vaccination data were not evaluated in the present study, but only 12 of the 41 patients (29.3%) acquired the infection after the 6 month of BMT and could have received influenza vaccine. Therefore, we could argue that oseltamivir, introduced early in the course of the infection (up to 48 h from initial symptoms), may have played a role in preventing influenza complications in the remaining 70% of the patients who certainly did not receive influenza vaccine.
In general, when a safe and effective antiviral agent is available, the benefit of early intervention is more evident in immunocompromised patients than in otherwise healthy persons, as observed with the treatment of CMV infection after BMT. There is no data in the literature concerning the effectiveness of the new neuraminidase inhibitors, zanamivir and oseltamivir, in the treatment of influenza or in the prevention of influenza complications after BMT. Randomized controlled trials of neuraminidase inhibitors in BMT recipients unexposed to vaccination are needed to confirm these findings.
Data on duration of shedding are important to establish precise precautions in the control of hospital infections. In this study, we confirmed the occurrence of prolonged shedding in patients with RSV infections. However, there are no data in the literature concerning the duration of influenza shedding in BMT recipients receiving oseltamivir. We observed that around 10% of these patients shed virus for more than 7 days. These data suggest that control NW should be taken before abolishing specific precautions. Hospital epidemiologists must be aware of this fact.
We conclude that the seasonal distribution of RVI in the southern hemisphere is similar to other countries in the northern hemisphere. However, as a consequence of the occurrence of influenza cases during summer, influenza vaccination available year-round should be considered in regions with the same pattern distribution.
The low mortality rates in BMT recipients with RVI observed in the present study may reflect the progress in the management of the infectious complications after BMT in the last decade. The real contribution of antiviral interventions in the improvement of patient outcome must be established in randomized controlled studies.
Acknowledgements
We thank the BMT attending doctors MCA Macedo, RL Silva and RS Saboya for providing excellent patient care. We appreciate the expert technicians of the Virology Laboratory of Instituto de Medicina Tropical de São Paulo, without whose support this study would not have been possible.
References
- 1.Whimbley E, Chaplin RE, Couch RB. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis. 1996;22:778–792. doi: 10.1093/clinids/22.5.778. [DOI] [PubMed] [Google Scholar]
- 2.Whimbley E, Englund J, Couch RB. Community respiratory virus infections in immunocompromised patients with cancer. Am J Med. 1997;102:10–18. doi: 10.1016/S0002-9343(97)80004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowden RA. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102:27–30. doi: 10.1016/S0002-9343(97)00007-7. [DOI] [PubMed] [Google Scholar]
- 4.Englund J, Sullivan CJ, Jordan C. Respiratory syncytial virus infection in immunocompromised adults. Ann Int Med. 1988;109:203–208. doi: 10.7326/0003-4819-109-3-203. [DOI] [PubMed] [Google Scholar]
- 5.Harrington RD, Hooton TM, Hackman RC. Na outbreak of respiratory syncytial virus in a bone marrow transplant center. J Infect Dis. 1992;165:987–993. doi: 10.1093/infdis/165.6.987. [DOI] [PubMed] [Google Scholar]
- 6.Cough RB, Englund JA, Whimbley E. Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med. 1997;102:2–9. doi: 10.1016/S0002-9343(97)00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ljungman P, Ward KN, Crooks BNA. Respiratory virus infection after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28:479–484. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 8.Nascimento JP, Siqueira MM, Sutmoller F. Longitudinal study of acute respiratory diseases in Rio de Janeiro: occur-rence of respiratory viruses during four consecutive years. Rev Inst Med trop São Paulo. 1991;33:287–296. doi: 10.1590/S0036-46651991000400008. [DOI] [PubMed] [Google Scholar]
- 9.Englund JA, Piedra PA, Jewell A. Rapid diagnosis of respiratory syncytial virus infections in immunocompromised adults. J Clin Microbiol. 1996;34:1649–1653. doi: 10.1128/jcm.34.7.1649-1653.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh S, Champlin RE, Englund JA. Respiratory syncytial virus upper respiratory tract illnesses in adult blood and marrow transplant recipients: combination therapy with aerosolized ribavirin and intravenous immunoglobulin. Bone Marrow Transplant. 2000;25:751–755. doi: 10.1038/sj.bmt.1702228. [DOI] [PubMed] [Google Scholar]
- 11.Madhi AS, Schoub B, Simmank K. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1. J Pediatr. 2000;137:78–84. doi: 10.1067/mpd.2000.105350. [DOI] [PubMed] [Google Scholar]
- 12.Madhi AS, Ramasamy N, Petersen K, Madhi A, Klugman KP. Severe lower respiratory tract infections associated with human parainfluenza viruses 1-3 in children infected and non-infected with HIV type 1. Eur J Clin Microbiol Infect Dis. 2002;21:499–505. doi: 10.1007/s10096-002-0754-9. [DOI] [PubMed] [Google Scholar]
- 13.Vieira SE, Stewien KE, Queiroz DAO. Clinical patterns and seasonal trends in respiratory syncytial virus hospitalizations in São Paulo, Brazil. Rev Inst Med trop S. Paulo. 2001;43:125–131. doi: 10.1590/S0036-46652001000300002. [DOI] [PubMed] [Google Scholar]
- 14.De Arruda E, Hayden FG, McAuliffe JF. Acute respiratory viral infection in ambulatory children of urban northeast Brazil. J Infect Dis. 1991;164:252–258. doi: 10.1093/infdis/164.2.252. [DOI] [PubMed] [Google Scholar]
- 15.Cintra AO, Owa MA, Machado AA. Occurrence and severity of infections caused by subgroup A and B respiratory syncytial virus in children in southeast Brazil. J Med Virol. 2001;65:408–412. doi: 10.1002/jmv.2049. [DOI] [PubMed] [Google Scholar]
- 16.Luján-Zilbermann J, Benaim E, Tong X. Respiratory virus infections in pediatric hematopoietic stem cell transplantation. Clin Infect Dis. 2001;33:962–968. doi: 10.1086/322628. [DOI] [PubMed] [Google Scholar]
- 17.Andreoletti L, Lesay M, Deschildre A. Differential detection of rhinoviruses and enteroviruses RNA sequences associated with classical immunofluorescence assay detection of respiratory virus antigens in nasopharyngeal swabs from infants with bronchiolitis. J Med Virol. 2000;61:341–346. doi: 10.1002/1096-9071(200007)61:3<341::AID-JMV10>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong GM, Wyatt DE, O'Neill HJ. A comparison of nested polymerase chain reaction and immunofluorescence for the diagnosis of respiratory infections in children with bronchiolitis, and the implications for a cohorting strategy. J Hosp Infect. 2001;49:122–128. doi: 10.1053/jhin.2001.1044. [DOI] [PubMed] [Google Scholar]
- 19.Straliotto SM, Siqueira MM, Muller RL. Viral etiology of acute respiratory infections among children in Porto Alegre, RS, Brazil. Rev Soc Bras Med Trop. 2002;35:283–291. doi: 10.1590/S0037-86822002000400002. [DOI] [PubMed] [Google Scholar]
- 20.Whimbey E, Cough RB, Englund J. Respiratory syncytial virus pneumonia in hospitalized adult patients with leukemia. Clin Infect Dis. 1995;21:376–379. doi: 10.1093/clinids/21.2.376. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh S, Whimbey E, Bodey GP. Community respiratory virus infections in the immunocompromised host. Int J Infect Dis. 2002;6(Suppl. 2):23. doi: 10.1016/S1201-9712(02)90220-1. [DOI] [Google Scholar]
- 22.Lord A, Bailey AS, Klapper PE. Impaired humoral responses to subgenus D adenovirusenovirus infections in HIV-positive patients. J Med Virol. 2000;62:405–409. doi: 10.1002/1096-9071(200012)62:4<405::AID-JMV2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Whimbey E, Elting LS, Cough RB. Influenza A virus infections among hospitalized adult bone marrow transplant recipients. Bone Marrow Transplant. 1994;13:437–440. [PubMed] [Google Scholar]


