Summary:
The risk of Epstein–Barr virus lymphoproliferative disease (EBV-LPD) increases with the use of highly immunosuppressive therapies. Allogeneic BMT, especially supported by T-cell-depleted stem cell products, is a risk factor for EBV-LPD. Although the risk of EBV-LPD after autologous transplantation is low, case reports of this complication in the autologous setting exist. We report a higher incidence than previously described of EBV-LPD in children undergoing sequential high-dose chemotherapy supported with CD34 selected peripheral blood stem cells (CD34+ PBSC). The median time to LPD after tandem transplant was 3 months (range 1–5 months). Five patients out of 156 (3.5%) developed EBV-LPD while enrolled on two trials of tandem autologous SCT in high-risk pediatric malignancies. Both studies employed five cycles of induction therapy, followed by tandem autologous PBSC transplants. In all, 108 out of 156 patients received CD34+ PBSC; 48 received unselected PBSC. All patients contracting LPD were from the CD34 selected group. Treatment of EBV-LPD included rituximab in four out of five patients, IVIg in two out of five patients, and gancyclovir in two out of five patients. EBV-LPD resolved in four out of five patients. We conclude that the combination of tandem SCT and CD34 selection may have increased immunosuppression in these patients to a point where there is an elevated risk of EBV-LPD.
Keywords: EBV, lymphoproliferative disease, autologous peripheral blood stem cell transplant, CD34 selection, neuroblastoma
Main
Epstein–Barr virus lymphoproliferative disease (EBV-LPD) is a well-described disorder consisting of a spectrum of diseases ranging from significant transient polyclonal B-cell lymphoproliferation to overt lymphoma. It is seen with immunodeficiency states, including post solid organ transplant,1 and with immune-deficient syndromes including HIV/AIDS infection,2 Wiskott–Aldrich syndrome,3,4 and ataxia-telangiectasia.5 It has also been noted to occur in approximately 1% of adult patients after allogeneic bone marrow transplantation.6
A variety of risk factors for EBV-LPD post allogeneic bone marrow transplantation have been recognized, many of which are linked to the depth of immune suppression. Autologous SCT is less immunosuppressive than allogeneic SCT, although EBV-LPD following autologous bone marrow transplantation has been reported, primarily in adults.7,8,9,10,11 Recently, a few single case reports have appeared in which children undergoing autologous peripheral blood stem cell rescue have developed the disorder.12,13 Here, we discuss the incidence of EBV-LPD found in our cohort of pediatric patients after they underwent tandem high-dose chemotherapy supported with CD34 selected peripheral blood stem cells (CD34+ PBSC).
Patients and methods
Treatment of patients
All EBV-LPD patients had been undergoing treatment for high-risk neuroblastoma. The clinical characteristics of the five patients are shown in Table 1. Patients 1 and 2 were enrolled on CHP-594 and patients 3–5 were enrolled on CHP-667. These two studies were limited institutional trials of tandem transplant in high-risk pediatric malignancies. All studies were approved by the Institutional Review Board of the Children's Hospital of Philadelphia.
Table 1.
Summary of patients with EBV-related LPD post transplant
| Pt. no./study no. | Age at dx (year) | Prior EBV status | Single vs tandem SCT | CD 34 selection device | Stem cell dose SCT 1 and 2 (CD34+ × 106/kg) | Time from last SCT to LPD onset (months) | % Periph. atyp. lymphs | EBV copy no. at LPD dx (by PCR) | Rx of LPD | LPD outcome | NB outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (594) | 5 | U | T | Isolex 300i v1.12 | 3.9 (3.9) | 2.5 | 54% | 1.47 × 106 cp/mg tissue, 5.9 × 105 cp/ml blood | Sp | D | N/A |
| 2 (594) | 4.5 | IgG+ | T | Isolex 300i v.1.12 | 2.0 (2.2) | 5 | U | U | R | R | NED |
| IgM+* | |||||||||||
| 3 (667) | 3 | U | T | Isolex 300i v.2.5 | 5.7 (5.0) | 1 | 3% (78% typical lymphs) | 5.86 × 105 cp/ml blood | G | R | NED |
| IVIg | |||||||||||
| R | |||||||||||
| 4 (667) | 3.5 | IgG+ | S | Isolex 300i v.2.5 | 1.1 (N/A) | 4 | 1% (8% typical lymphs) | PCR+, QNS for quant | G | R | D–PD |
| IVIg | |||||||||||
| R | |||||||||||
| 5 (667) | 3 | IgG+ | T | Isolex 300i v.2.5 | 1.1 (1.1 ) | 2.5 | 17% | 9.61 × 105 cp/mg tissue, 1.72 × 105 cp/ml blood | R | R | PR |
All children stage 4 at diagnosis. U, unavailable; S, single transplant; T, tandem transplant; Sp, supportive care; R, rituximab; G, gancyclovir; RS, resolution; D, death; NED, no evidence of disease; PD, progressive disease; PR, partial remission; cp, copies.
*IgM positivity found immediately prior to first stem cell transplant.
Both CHP-594 and CHP-667 employ 5 cycles of standard induction therapy, followed by tandem autologous PBSC transplants. The treatment schema for each study is shown in Figure 1. The major differences between the two studies include: (1) a total dose of cyclophosphamide on CHP-594 of 11.2 vs 16.2 g on CHP-667; (2) collection of PBSC after three cycles of induction therapy (CHP-594) compared to post cycle 2 (CHP-667); (3) differing conditioning regimens on the two studies (Figure 1), with total body irradiation on the earlier study only (CHP-594). In all, 108 out of 156 patients were supported with CD34+ PBSC during tandem transplant. The total patient numbers as well as diagnoses and stem cell products used are summarized in Table 2. Statistical analysis to test for differences between groups was performed using χ2 test for association (Stata 7.0).
Figure 1.
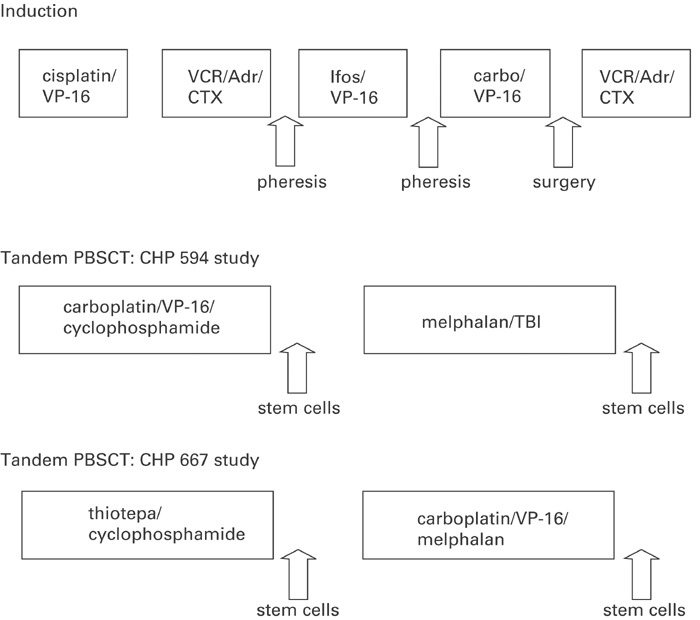
Design of tandem PSCT studies CHP-594 and CHP-667. 5 cycles of induction chemotherapy were followed by tandem PBSCT as shown. Induction chemotherapy doses were similar with the exception of cyclophosphamide, given at 2.1 gm/m2/cycle on 594 and 4.2 g/m2/cycle on 667. Local radiotherapy was given prior to PBSCT on the 594 study and after tandem PBSCT on the 667 study. Carbo=carboplatin Cisplat=Cisplatin Cytox=cytoxan Dox=doxorubicin Ifos=ifosfamide TBI=total body irradiation Vcr=vincristine VP=etoposide XRT=x-ray therapy.
Table 2.
Patients treated on CHP-594 and CHP-667 studies
| Study | Neuroblastoma | Ewing sarcoma | Other sarcoma | Total | Unselected PBSC | CD34+ PBSCT | EBV-LPD |
|---|---|---|---|---|---|---|---|
| 594 | 97 | 23 | 8 | 128 | 47 | 81 | 2 |
| 667 | 22 | 6 | 0 | 28 | 1 | 27 | 3 |
| Both studies | 119 | 29 | 8 | 156 | 48 | 108 | 5 |
| EBV-LPD | 5 | 0 | 0 | 5 | 0 | 5 |
Details of stem cell processing
During the 8 years during which these two trials were conducted, PBSC were either infused without processing, or were CD34 selected using one of four techniques. A total of 48 patients received unprocessed PBSC, 26 received CD34+ PBSC processed on the Cellpro Ceprate device, 82 received CD34+ PBSC processed on the Nexell Isolex, software versions 1.12 or 2.5, and one received CliniMacs selected cells. The collection goals were 2–5 × 106 CD34+ cells/kg for each SCT. CD3+ T cells were <1% of CD34+ PBSC, and CD34 selection is reported to provide ⩾3 logs of T-cell depletion of PBSC.14
Case reports
Patient 1, a female, was diagnosed at 5 years of age, and presented with a left adrenal primary and metastases to her bone and bone marrow. She received local radiotherapy (1080 cGy) prior to stem cell transplant, which occurred 5 months after her initial presentation. She had one episode of sepsis during stem cell transplant (SCT) #1, but had an otherwise uneventful course. Approximately 2.5 months after SCT#2, she presented to an outside hospital with fevers and a dental abscess. Over the course of the next week, she developed a new transfusion dependency, bloody stools, and diffuse lymphadenopathy, followed by respiratory distress and coagulopathy. Her condition continued to deteriorate, and she died 13 days after being admitted. Her autopsy showed EBV genome-positive lymphoproliferative disease (from the lung, 1.4 × 106 copies EBV DNA/mg tissue; from the blood 5.9 × 105 copies EBV DNA/ml). She had marked diffuse lymphadenopathy with architecture effaced by monomorphic lymphoma cells.
Patient 2, a female, was diagnosed at 4.5 years of age with a large left adrenal mass, bony and bone marrow metastases. At 6 months after initial presentation, she underwent SCT#1, which she tolerated without significant complication. SCT#2 occurred 2 months after her first. At 1 month after SCT#2, she developed adenoviral pneumonia and ARDS, from which she recovered. Adenopathy was noted 2 months after SCT#2, and a CT scan revealed multiple peri-intestinal masses. Biopsy showed EBV-LPD with positive EBV PCR (copy number unavailable). She was treated twice with rituximab. GI bleeding complicated rituximab therapy, likely due to lysis of LPD in the gastrointestinal tract. The bleeding stopped, and her lymphadenopathy cleared. She ultimately did well and currently remains neuroblastoma and LPD free 3 years post SCT.
Patient 3, a male, was 3 years old at diagnosis. He presented with a right upper quadrant mass and metastases to his bone and bone marrow. His first SCT was uncomplicated. SCT#2 occurred 6 weeks later. At 1 month after SCT#2, he developed an interstitial pneumonitis with an oxygen requirement, fevers, and hepatosplenomegaly. Peripheral blood PCR testing revealed 5.9 × 105 copies of the EBV genome. He had no significant lymphadenopathy, so a biopsy of nodal tissue was not possible. It was not clear whether this event represented a primary EBV infection or EBV reactivation. Owing to this, the patient was treated with gancyclovir, rituximab, and intravenous gamma globulin (IVIg). The etiology of his pneumonitis was not clear, but the patient did not require mechanical ventilation. All the clinical abnormalities resolved with this therapy, and he remains neuroblastoma free and without evidence of EBV disease, 2 years post therapy.
Patient 4, a male, was 3.5 years old at diagnosis. His neuroblastoma presented as an abdominal mass with bony metastases, but without bone marrow involvement. He was initially treated on CHP-667, but progressed on induction therapy. He was taken off protocol, and experienced a partial response to salvage chemotherapy. At 6 months after initial diagnosis, he was treated on a phase I trial of 131I-meta-iodo-benzyl-guanine (MIBG), followed by high-dose chemotherapy and stem cell rescue. The conditioning for his stem cell rescue included carboplatin, etoposide, and melphalan. Almost 4 months after this investigational SCT, he presented with symptoms of sore throat, fever, and cervical lymphadenopathy, which initially improved with oral antibiotics. His symptoms progressed to increasing abdominal girth and systemic lymphadenopathy. Lymph node biopsy revealed EBV-LPD; the cells were positive for EBV genome by PCR (PCR+, clinical sample not sufficient to determine copy number). He was treated with rituximab, experiencing a prompt clinical response. After the resolution of EBV-LPD, he experienced a recurrence of his neuroblastoma. He expired 6 months after his stem cell rescue of progressive neuroblastoma, without evidence of EBV-LPD.
Patient 5, a male, was 3 years old at diagnosis of neuroblastoma. He presented with a right-sided adrenal mass, bone marrow involvement, and multiple bony lesions. His first SCT occurred 6 months after diagnosis, and his second occurred 6 weeks later. He tolerated both SCT procedures well. Soon after transplant, he experienced symptoms of sinusitis followed by cervical lymphadenopathy; PCR for EBV genome in tissue and blood was positive, and he was diagnosed with EBV-LPD (1.7 × 105 copies/ml blood; 9.6 × 105 copies/mg tissue). Rituximab was administered over 4 weeks. As with other patients, he experienced a rapid defervescence and resolution of his lymphadenopathy. He experienced a relapse of his neuroblastoma, and expired 10 months after second SCT.
Incidence of LPD after tandem SCT
We retrospectively reviewed this cohort of patients to assess the risk of EBV-LPD in patients who had undergone tandem transplant.
A total of 156 total patients were treated on either CHP-667 or CHP-594 (see Figure 1 and above, ‘Treatment of Patients’, for details of and differences between the studies) at five different institutions from 1994 to 2002. As can be seen in Table 2, 119 children were treated for neuroblastoma, 29 for Ewing sarcoma, and eight for other sarcomas. In total, 108 out of 156 patients received CD34+ PBSC, with 48 receiving unselected PBSC. The average dose of CD34 selected cells infused to patients on CHP-594 was 7 × 106 CD34+ cells/kg; average dose on CHP-667 was 8.7 × 106/kg. CD34+ PBSC were given to 81 out of 128 CHP-594 patients; 27 out of 28 patients on CHP-667 received CD34+ PBSC. Five patients developed EBV-LPD for an overall incidence of 3.2%. Incidence on the two studies was 1.6% on CHP-594 and 10.7% in the limited number of patients on CHP-667 (P=0.41). The median time to develop EBV-LPD after transplant was 3 months (range 1–5 months).
All patients who contracted LPD were in the CD34 selected group (P=0.13, χ2 test for association) and all had neuroblastoma (P=0.21, χ2 test), with an incidence of 4.6%. Four of five patients experienced a CR of their EBV disease to treatment; two out of five are currently living (one death due to LPD, two due to progressive neuroblastoma).
Lymphocyte recovery data from the patients are presented in Table 3. Patient 3 did not reach 30 days before onset of EBV disease; therefore data are presented pretransplant and at the onset of the disease. Limited data on lymphocyte recovery are available from other patients on these two studies. There is no statistical difference between the absolute lymphocyte counts between those patients who developed EBV-LPD and those who did not (data not shown).
Table 3.
Lymphocyte recovery post stem cell transplantation (/μl blood)
| Pt. no. | ALC pre-SCT | ALC 30 days post SCT | ALC 60 days post SCT | ALC at onset of LPD |
|---|---|---|---|---|
| 1 | 57.2 | 1155 | 2262 | 2408 |
| 2 | 217 | 399 | 1381 | 1381 |
| 3 | 902 | N/A | N/A | 3321 |
| 4 | 525 | 728 | 1249 | 986 |
| 5 | 1512 | 560 | 660 | 442 |
Discussion
Increasing dose intensity of therapy has had an impact in curing pediatric tumors, including neuroblastoma. The Children's Cancer Group 3891 study demonstrated superior 3 year EFS in children with high-risk neuroblastoma randomized to autologous BMT.15 In order to extend this concept, we have been using a strategy of tandem PBSCT to allow further dose intensification. Although this approach has produced a promising 3-year EFS of 56% and an overall treatment-related mortality similar to single transplant studies,16,17 our experience here suggests that the treatment is highly immunosuppressive. We have experienced an overall incidence of severe EBV complications including LPD of 3.2% in our experience with tandem PBSCT for high-risk pediatric solid tumors. All EBV cases occurred in children who had received CD34+ PBSC, for an incidence of 4.6% in the CD34 selected group.
Epstein–Barr virus is a transforming herpes virus, which shows tropism for B lymphocytes, possessing the ability to immortalize them in vivo. B lymphocytes preferentially maintain EBV genome in a nonreplicating, latent form.18 In the normal host, primary infection results in a transient lymphoproliferative disorder commonly known as infectious mononucleosis. The immune response to EBV is both humoral and cellular. The humoral response is useful for the diagnosis of EBV initial infection and antibodies to EBV proteins have neutralizing properties. These antibodies may also render infected individuals immune to further infection with exogenously transmitted virus, although this is likely aided by further cellular immune-mediated responses as well.19,20 Control of EBV-infected B-cell proliferation is largely mediated through natural killer, CD4+, and CD8+ cytotoxic T cells.21 After recovery, HLA-restricted cytotoxic T cells play an important role in controlling EBV reactivation.22 Cytotoxic T cells undergo antigen-driven polyclonal expansion of up to 30% of cells. Resting memory B cells form the reservoir through which the virus persists throughout life in its host. In the immunocompromised host, suppression by cytotoxic lymphocytes is hampered or unavailable, leading to unchecked replication of B lymphocytes. This replication can behave in a malignant fashion, either leading to invasive polyclonal B-cell hyperplasia or to a monoclonal proliferation of lymphomatous B cells.23
EBV-LPD is well recognized as a consequence of immunosuppressive therapy after solid organ transplantation.24 The first reports of post-BMT EBV-LPD were in leukemic patients who received allogeneic transplants; these patients had developed graft-versus-host disease treated with anti-T-cell monoclonal antibodies,25 once more leading to severely decreased ability to curb EBV-transformed cells. When prospectively studied, the predictors of patients at the greatest risk of developing LPD reflect the degree of suppression to which the patient has been exposed. T-cell depletion of the donor graft, severe GVHD, prophylaxis and/or treatment of GVHD with immunophilins, anti-T-cell antibodies, and HLA-mismatched marrow grafts are the most important and consistent predictors of EBV-LPD.6,26,27
EBV-LPD has been described after autologous BMT using T-cell-depleted marrows28,29 and in patients receiving unmanipulated marrow.7,8,9 EBV lymphoproliferative disease after autologous PSCT has also been reported.10,11 Peripheral stem cells are widely used for autologous stem cell transplant, resulting in faster engraftment. Allogeneic bone marrow transplants have been shown to have a <1% overall complication rate for lymphoproliferative disease;30 however, higher risk groups have been shown to have an incidence of between 8 and 22%.31 Gross et al32 report no cases of EBV-LPD in their review of 853 autologous stem cell transplants. Our review of the literature reveals only three reports of EBV-LPD in children after autologous PSCT. Of these three, one child was transplanted for neuroblastoma, one for retinoblastoma, and one for a nonmalignant disorder.12,13 All occurred within 6 months of transplant, similar to the time frame we report here.
Treatment with rituximab appears to have been curative for EBV-LPD in our patients. Four of our five patients were treated with rituximab, and a complete clinical response was observed in all four. Patients 3 and 4 also received IVIg and gancyclovir. Although both IVIg and gancyclovir have been used in the treatment of EBV-LPD, their efficacy is uncertain. Rituximab appears to offer a well-tolerated and effective addition to the treatment of EBV-LPD in the setting of both solid organ and bone marrow transplantations.33
As supportive care has improved, including the use of PBSC and hematopoietic growth factors, more dose-intensive therapies have come into wider application. An early attempt to perform tandem transplantation in children with neuroblastoma using autologous bone marrow was unsuccessful due to excess treatment-related mortality.34 We have shown that tandem transplantation can be performed successfully in children, using CD34+ PBSC as a stem cell source,16,17 but this therapy is complicated by slower immune recovery. The role that the CD34 selection, which results in >3 log T-cell depletion, plays in the significant immunosuppression we have observed in these patients is unclear. We note that all of the patients who developed EBV-LPD had received a CD34+ PBSC processed on the Isolex device. However, the apparently increased risk of EBV-LPD in the CD34-selected group was not statistically significant.
Prediction of those patients most at risk for EBV-LPD continues to be difficult. While it is clear that patients are at increased risk of EBV-LPD post BMT or SCT, elucidation of subgroups potentially at the highest risk has not been possible. Consistent with the prevailing risk factors listed above, our finding that all the five of our patients who have EBV-LPD received CD34 selected grafts suggests that depth of immune suppression is indeed important. Previous attempts to predict EBV-LPD by measuring total EBV-DNA in peripheral blood have been shown to have a predictive value of about 40%. Meij et al have recently described 25 patients who received partially T-cell depleted SCTs and subsequently developed high EBV-DNA loads. Of these patients, all of those who developed high levels of EBV-DNA prior to the recovery of EBV-specific T cells ultimately developed LPD. For them, this resulted in an increase to a predictive value of 100% for this subset of patients.35 Although their findings need to be replicated, these results may ultimately have defined those patients who need to be followed most closely for EBV-LPD. Further, they suggest the importance of future investigation into the role of T-cell re-infusion for those patients at highest risk of LPD.
Mackall et al36,37 have examined the issue of T-cell dose infused during transplantation after multiple-cycle PBSCT, and found no difference in T-cell recovery between patients supported with autologous PBSC and CD34+ PBSC. Meijer et al38 report that, by maintaining a T/B cell ratio in the graft of ⩾0.25, the risk of EBV-LPD can be significantly reduced in matched unrelated donor BMT. The role of this in autologous stem cell transplantation must be investigated. While transfer of mature T cells in the graft may permit short-term immune function, ultimately, the presence of immune function at 1 year or later correlates with the number of CD4+CD45RA+ (naïve) T cells.39 It is clear from Mackall's careful analyses that multiple-cycle PBSCT is more immunosuppressive, potentially on the basis of greater damage to the thymic epithelium.
Despite dose intensification, relapse remains the principle cause for treatment failure in patients with high-risk neuroblastoma. In general, the incidence of EBV-LPD after autologous transplantation is very low. Our observation of five cases of EBV-LPD suggests that there may be a threshold of treatment intensity that may result in significant enough immunosuppression to increase the risk of this complication. Each component of these highly dose-intensified protocols: induction chemotherapy employing high doses of cyclophosphamide, multiple cycle high-dose chemotherapy with PBSC rescue, and use of CD34+ PBSC, may add to immune suppression and, thus, the EBV-LPD risk.
Clearly, our experience suggests the importance of short-term (6-month) EBV surveillance using available PCR techniques for detecting viral genome, coupled with an awareness that this complication may be more common in children who have been treated with such highly intensified regimens. Our experience suggests that early diagnosis followed by prompt initiation of rituximab therapy may successfully manage EBV-LPD. The current Children's Oncology Group phase III study A3973 will answer the question of whether purging tumor cells from PBSC products impacts the outcome in children with neuroblastoma. If purging does improve EFS, then the use of CD34 selection in the setting of tandem PBSCT may best be pursued with strategies to improve immune reconstitution, such as a plan to return T cells to the patient.40
References
- 1.Leblond V, Sutton L, Dorent R, et al. Lymphoproliferative disorders after organ transplantation: a report of 24 cases observed in a single center. J Clin Oncol. 1995;13:961–968. doi: 10.1200/JCO.1995.13.4.961. [DOI] [PubMed] [Google Scholar]
- 2.Feigel EG. AIDS-associated malignancies: research perspectives. Biochim Biophys Acta. 1999;1423:C1–C9. doi: 10.1016/s0304-419x(98)00033-x. [DOI] [PubMed] [Google Scholar]
- 3.Saemundsen AK, Purtilo DT, Sakamoto K, et al. Documentation of Epstein–Barr virus infection in immunodeficient patients with life-threatening lymphoproliferative diseases by Epstein–Barr virus complementary RNA/DNA and viral DNA/DNA hybridization. Cancer Res. 1981;41(Part 1):4237–4242. [PubMed] [Google Scholar]
- 4.Motohiko O, Gross TG. A review of Epstein–Barr virus infection in patients with immunodeficiency disorders. Am J Med Sci. 2000;319:392–396. doi: 10.1097/00000441-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Saemundsen AK, Berkel AI, Henle W, et al. Epstein–Barr virus carrying lymphoma in a patient with ataxia telangiectasia. Br J Haematol. 1981;282:425–427. doi: 10.1136/bmj.282.6262.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis RE, Travis LB, Rowlings PA, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood. 1999;94:2208–2216. [PubMed] [Google Scholar]
- 7.Shepherd JD, Gascoyne RD, Barnett MJ, et al. Polyclonal Epstein–Barr virus-associated lymphoproliferative disorder following autografting for chronic myeloid leukemia. Bone Marrow Transplant. 1995;15:639–641. [PubMed] [Google Scholar]
- 8.Chao NJ, Berry GJ, Advani R, et al. Epstein–Barr virus-associated lymphoproliferative disorder following autologous bone marrow transplantation for non-Hodgkin's lymphoma. Transplantation. 1993;55:1425–1428. [PubMed] [Google Scholar]
- 9.Hauke RJ, Greiner TC, Smir BN, et al. Epstein–Barr virus-associated lymphoproliferative disorder after autologous bone marrow transplantation: report of two cases. Bone Marrow Transplant. 1998;21:1271–1274. doi: 10.1038/sj.bmt.1701258. [DOI] [PubMed] [Google Scholar]
- 10.Yufu Y, Kimura M, Kawano R, et al. Epstein–Barr virus-associated T cell lymphoproliferative disorder following autologous blood stem cell transplantation for relapsed Hodgkin's disease. Bone Marrow Transplant. 2000;26:1339–1341. doi: 10.1038/sj.bmt.1702721. [DOI] [PubMed] [Google Scholar]
- 11.Peniket AJ, Perry AR, Williams CD, et al. A case of EBV-associated lymphoproliferative disease following high-dose therapy and CD34-purified autologous peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 1998;22:307–309. doi: 10.1038/sj.bmt.1701335. [DOI] [PubMed] [Google Scholar]
- 12.Lones MA, Kirov I, Said JW, et al. Post-transplant lymphoproliferative disorder after autologous peripheral stem cell transplantation in a pediatric patient. Bone Marrow Transplant. 2000;25:1021–1024. doi: 10.1038/sj.bmt.1702593. [DOI] [PubMed] [Google Scholar]
- 13.Heath J, Broxson E, Dole M, et al. Epstein–Barr virus-associated lymphoma in a child undergoing an autologous stem cell rescue. J Pediatr Hematol Oncol. 2002;24:160–163. doi: 10.1097/00043426-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Stainer CJ, Miflin G, Anderson S, et al. A comparison of two different systems for CD34+ selection of autologous or allogeneic PBSC collections. J Hematother. 1998;7:375–383. doi: 10.1089/scd.1.1998.7.375. [DOI] [PubMed] [Google Scholar]
- 15.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-Cis retinoic acid. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 16.Grupp SA, Stern JW, Bunin N, et al. Rapid-sequence tandem transplant for children with high-risk neuroblastoma. Med Pediatr Oncol. 2000;35:696–700. doi: 10.1002/1096-911x(20001201)35:6<696::aid-mpo46>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Grupp SA, Stern JW, Bunin N, Nancarrow C. Tandem high-dose therapy in rapid sequence for children with high-risk neuroblastoma. J Clin Oncol. 2000;18:2567–2575. doi: 10.1200/JCO.2000.18.13.2567. [DOI] [PubMed] [Google Scholar]
- 18.Savoie A, Perpete C, Carpentier L, et al. Direct correlation between the load of Epstein–Barr virus-infected lymphocytes in the peripheral blood of pediatric transplant patients and risk of lymphoproliferative disease. Blood. 1994;83:2715–2722. [PubMed] [Google Scholar]
- 19.Rickinson AB, Moss DJ. Human cytotoxic T lymphocyte responses to Epstein–Barr virus infection. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 20.Yao QY, Tierney RJ, Croom-Carter D, et al. Frequency of multiple Epstein–Barr virus infections from T cell-immunocompromised individuals. J Virol. 1996;70:4884–4894. doi: 10.1128/jvi.70.8.4884-4894.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen JI. Epstein–Barr virus infection. N Engl J Med. 2000;343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 22.Rickinson AB, Moss DJ. Human cytotoxic T lymphocyte responses to Epstein–Barr virus infection. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 23.List AF, Greco A, Vogler LB. Lymphoproliferative diseases in immunocompromised hosts; the role of Epstein–Barr virus. J Clin Oncol. 1987;5:1673–1689. doi: 10.1200/JCO.1987.5.10.1673. [DOI] [PubMed] [Google Scholar]
- 24.Penn I. Cancers complicating organ transplantation. N Engl J Med. 1990;323:1767–1769. doi: 10.1056/NEJM199012203232510. [DOI] [PubMed] [Google Scholar]
- 25.Martin PJ, Shulman HM, Schubach WH, et al. Fatal Epstein–Barr-virus-associated proliferation of donor B cells after treatment of acute graft-versus-host disease with a murine anti-T cell antibody. Ann Intern Med. 1984;101:310–315. doi: 10.7326/0003-4819-101-3-310. [DOI] [PubMed] [Google Scholar]
- 26.Chiang KY, Hazlett LJ, Godder KT, et al. Epstein–Barr virus-associated B cell lymphoproliferative disorder following mismatched related T cell-depleted bone marrow transplantation. Bone Marrow Transplant. 2001;28:1117–1123. doi: 10.1038/sj.bmt.1703311. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro R, McClain K, Frizzera G, et al. Epstein–Barr virus-associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood. 1988;71:1234. [PubMed] [Google Scholar]
- 28.Briz M, Fores R, Regidor C, et al. Epstein–Barr virus associated B cell lymphoma after autologous bone marrow transplantation for T cell acute lymphoblastic leukaemia. Br J Haematol. 1997;98:485–487. doi: 10.1046/j.1365-2141.1997.2153034.x. [DOI] [PubMed] [Google Scholar]
- 29.Young L, Alfieri C, Hennessy K, et al. Expression of Epstein–Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989;321:1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]
- 30.Zutter MM, Martin PJ, Sale GE, et al. Epstein–Barr virus lymphoproliferation after bone marrow transplantation. Blood. 1988;72:520–529. [PubMed] [Google Scholar]
- 31.Faye A, Quartier P, Requerre Y, et al. Chimaeric anti-CD20 monoclonal antibody (rituximab) in post-transplant B-lymphoproliferative disorder following stem cell transplantation in children. Br J Haematol. 2001;115:112–118. doi: 10.1046/j.1365-2141.2001.03041.x. [DOI] [PubMed] [Google Scholar]
- 32.Gross TG, Steinbuch M, DeFor T, et al. B cell lymphoproliferative disorders following hematopoietic stem cell transplantation: risk factors, treatment and outcome. Bone Marrow Transplant. 1999;23:251–258. doi: 10.1038/sj.bmt.1701554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garnier JL, Stevenson G, Blanc-Brunat N, et al. Treatment of post-transplant lymphomas with anti-B cell monoclonal antibodies. Recent Results Cancer Res. 2002;159:113–122. doi: 10.1007/978-3-642-56352-2_14. [DOI] [PubMed] [Google Scholar]
- 34.Philip T, Landstein R, Zucker JM, et al. Double megatherapy and autologous bone marrow transplantation for advanced neuroblastoma: the LMCE2 Study. Br J Cancer. 1993;67:119–127. doi: 10.1038/bjc.1993.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meij P, van Esser JWJ, Niesters HGM, et al. Impaired recovery of Epstein–Barr virus (EBV)-specific CD8+ T lymphocytes after partially T-depleted allogeneic stem cell transplantation may identify patients at very high risk of progressive EBV reactivation and lymphoproliferative disease. Blood. 2003;101:4290–4297. doi: 10.1182/blood-2002-10-3001. [DOI] [PubMed] [Google Scholar]
- 36.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis and CD4+ T lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 37.Mackall CL, Stein D, Fleisher TA, et al. Prolonged CD4 depletion after sequential autologous peripheral blood progenitor cell infusions in children and young adults. Blood. 2000;98:754–762. [PubMed] [Google Scholar]
- 38.Meijer E, Slaper-Coretenbach ICM, Thijsen SFT, et al. Increased incidence of EBV-associated LPD after allogeneic stem cell transplantation from matched unrelated donors due to a change of T cell depletion technique. Bone Marrow Transplant. 2002;29:325–329. doi: 10.1038/sj.bmt.1703362. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg K, Blazar BR, Wagner JE, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97:1458–1466. doi: 10.1182/blood.v97.5.1458. [DOI] [PubMed] [Google Scholar]
- 40.Laport GG, Levine BL, Stadtmauer EA, et al. Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin's lymphoma following CD34-selected hematopoietic cell transplantation. Blood. 2003;102:2004–2013. doi: 10.1182/blood-2003-01-0095. [DOI] [PubMed] [Google Scholar]


