Abstract
Both replication-incompetent and replication-selective adenoviruses are being developed for the treatment of cancer and other diseases. Concerns have been raised about the safety of intra-vascular adenovirus administration following a patient death on a clinical trial with a replication-defective adenovirus. In addition, the feasibility of vascular delivery to distant tumors has been questioned. dl1520 (ONYX-015) is a replication-selective adenovirus that has previously shown safety and antitumoral activity following intratumoral injection. This is the first report of intra-vascular administration with a genetically engineered, replication-selective virus. A phase I dose-escalation trial was performed in patients with liver-predominant gastrointestinal carcinoma (n = 11 total; primarily colorectal). dl1520 was infused into the hepatic artery at doses of 2 × 108–2 × 1012 particles for two cycles (days 1 and 8). Subsequent cycles of dl1520 were administered in combination with intravenous 5-fluorouracil (5-FU) and leucovorin. No dose-limiting toxicity, maximally tolerated dose or treatment-emergent clinical hepatotoxicity were identified following dl1520 infusion. Mild to moderate fever, rigors and fatigue were the most common adverse events. Antibody titers increased significantly in all patients. Viral replication was detectable in patients receiving the highest two doses. An objective response was demonstrated in combination with chemotherapy in a patient who was refractory to both 5-FU and dl1520 as single agents. Therefore, hepatic artery infusion of the attenuated adenovirus dl1520 was well-tolerated at doses resulting in infection, replication and chemotherapy-associated antitumoral activity.
Keywords: adenovirus, gene therapy, clinical trial, cancer, colorectal carcinoma, replication-competent
Introduction
Novel anticancer agents are being developed with the dual goals of improved efficacy and safety compared with currently available treatments. Viruses are being developed to treat cancer using two contrasting approaches. The initial approach was to use replication-incompetent viruses as delivery vehicles for therapeutic genes.1,2 A contrasting strategy has now emerged to use replication-selective viruses as therapeutic agents themselves.3,4,5,6,7 These viruses replicate in and destroy cancer cells directly, without the addition of exogenous genes. Superior efficacy may eventually be achieved by combining these two strategies and arming replication-selective viruses with therapeutic transgenes.7,8,9 Unfortunately, to date, the published clinical experience with these agents has been limited to intratumoral injection on phase I studies. In addition, data from an adenoviral gene therapy trial for ornithine transcarbamylase deficiency at the University of Pennsylvania has raised questions about the safety of arterial adenovirus administration.10,11,12,13
ONYX-015 is a first-generation replication-selective adenovirus type 2/5 chimera with a deletion in the E1B-55kD gene.14 Because E1B-55kD binds to and inactivates the p53 tumor suppressor gene product, this mutant should theoretically be unable to overcome the p53-mediated blockade of viral replication in a normal cell.15 In a tumor cell lacking p53 function, in contrast, the E1B-55kD protein should be expendable for p53 inhibition and replication should proceed.16 Publications from preclinical studies with different cell systems have reported conflicting data regarding the original McCormick hypothesis.15,16,17,18,19,20 Nevertheless, ONYX-015 has shown promise in phase I and II clinical trials following direct intratumoral injection into recurrent head and neck cancers.21,22,23 Tumor-selective viral replication and necrosis were demonstrated, and the treatment was well-tolerated without dose-limiting toxicities; flu-like symptoms and injection site pain were frequently noted. Although durable responses were rare as a single agent in these advanced refractory tumors,23 a potentially synergistic interaction was subsequently discovered between ONYX-015 and chemotherapy.24,25,26,27 While all recurrent head and neck cancer patients on the combination viral and chemotherapy clinical trial received intravenous chemotherapy (cisplatin and 5-fluorouracil), the response rate of tumors injected with ONYX-015 was significantly higher than in tumors that were not injected.24 Further development of ONYX-015 is indicated in combination with chemotherapy.
Cancers that recur and progress locally (eg head and neck, glioblastoma multiforme) may clearly benefit from intralesional therapy. However, most cancers that fail surgery and/or radiation are multifocal; clinically beneficial intralesional therapy is therefore not feasible in the majority of cancer patients. For viral agents to have significant benefit in these advanced cancer patients, regional or systemic delivery of viruses to tumors through the vasculature is necessary. The intravascular administration of genetically engineered replication-selective viruses in humans has not been previously described.3,4,5,6,7 Potential hurdles to intravascular therapy include virally mediated inflammatory hepatitis, antibody-mediated neutralization of the virus, rapid clearance of the virus from the bloodstream by reticuloendothelial organs and limited viral deposition within tumor masses. Intravenous administration of ONYX-015 in nude mouse–human tumor xenograft models led to infection and growth inhibition of distant tumors in a dose-dependent fashion.28 The dose-limiting toxicity in this model was hepatic necrosis. However, the available animal models could not adequately assess the role of the immune response to the adenovirus, nor the risk of replication-dependent toxicities within normal tissues. Clinical data on intravascular administration of adenoviruses has been eagerly awaited.
Therefore, based on the favorable clinical toxicity and efficacy profile with intratumoral injection, and preclinical efficacy following intravascular administration, we performed a phase I/II dose escalation trial of ONYX-015 administered via the hepatic artery to patients with metastatic gastrointestinal carcinomas (primarily colorectal) to the liver. These patients were selected for intra-arterial treatment for several reasons. First, intrahepatic tumor progression is the cause of death in over 80% of the roughly 50 000 patients dying with colorectal carcinoma in the United States every year, and it is estimated that 30–40% of patients dying of colorectal cancer will have macroscopic disease confined to the liver.29 Second, preferential perfusion of tumor masses (versus normal liver tissue) can be achieved via hepatic artery infusions.29 Finally, new treatments for colorectal carcinoma are needed. At the time of study initiation, the standard first-line therapy for advanced colon cancer was 5-fluorouracil (5-FU) in combination with leucovorin (LV); irinotecan and oxaliplatin were used following 5-FU failure or in combination with 5-FU as first-line therapy. In numerous well-controlled trials, the response rate for 5-FU/LV is in the range of 10 to 25% with few complete responses and little impact on overall survival.30 Therefore, experimental regional therapies utilizing hepatic artery infusion have been developed for colorectal liver metastases.29 The treatment on this trial included two doses of ONYX-015 by hepatic artery infusion as a single agent followed by combination treatment with intravenous 5-FU and LV (Figure 1). We have now demonstrated that intravascular administration of a replication-selective adenovirus is feasible and well-tolerated, both as a single agent and in combination with standard 5-FU and LV chemotherapy. Antitumoral activity was demonstrated in combination with 5-FU in a 5-FU-refractory patient.
Figure 1.
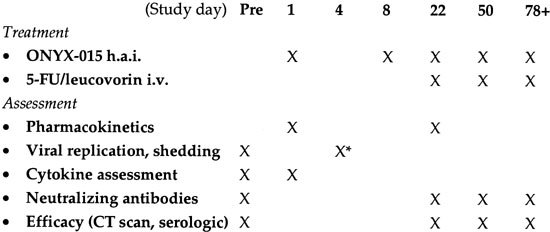
Treatment and assessment schema. The treatment and assessment schema is outlined below by study day. Abbreviations are as follows: h.a.i., hepatic artery infusion; i.v., intravenous; pre, pretreatment. *Day 4 assessment of replication and shedding was based on quantitative PCR of the blood (genomes/ml) approximately 72 h (±24 h) after the first injection. Serologic assessment of response included monitoring of both CEA and lactate dehydrogenase levels. Patients with evidence of antitumoral activity on day 78 were eligible to continue treatment every 28 day for up to four additional cycles (day 78+).
Results
Baseline patient characteristics
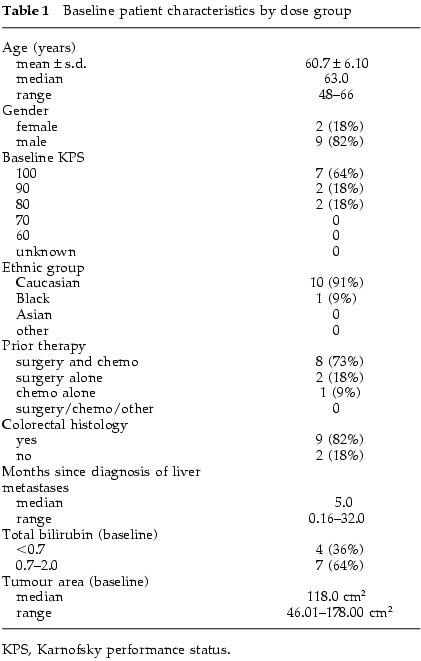
Baseline patient characteristics are described in Table 1. The tumor type was colorectal in 82% and pancreatic in 18%. The median age of patients on study was 61.5 years old (range 39–78), and 18% were female. Prior chemotherapy had been received in 82% of patients. Most patients had a KPS of 90–100%. p53 gene status could be obtained in six patients (55%); three tumors had mutant sequences and three had wild-type gene sequences (exons 2–11).
Table 1.
Baseline patient characteristics by dose group
Treatment characteristics
Treatment characteristics are outlined in Table 2. Eleven patients received at least one cycle of ONYX-015: eight at lower doses (2 × 108–6 × 1011 particles; 107–1011 p.f.u.) and three at high doses (2 × 1012 particles; 1011 p.f.u.). All patients subsequently received at least one cycle of ONYX-015 plus 5-FU and LV.
Table 2.
Treatment characteristics
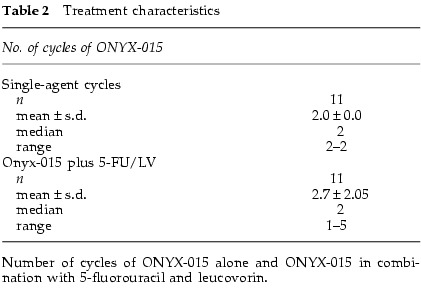
Adverse events and maximally tolerated dose
Dose escalation proceeded from 2 × 108 to 2 × 1012 particles without occurrence of any dose-limiting toxicities. Specifically, no treatment-emergent clinical hepatotoxicity occurred during dose-escalation, despite pre-existing liver abnormalities due to intrahepatic metastases in over half of the patients at baseline. Transient low grade (1–2) transaminitis was documented in three patients (following single agent virus) and was classified by the investigator as ‘possibly attributable’ to ONYX-015 (6 × 1011 and 2 × 1012 particles); the laboratory abnormalities resolved within 12 days and did not reoccur after subsequent treatments. Four patients had liver-related adverse events reported (hyperbilirubinemia) that were classified as ‘unrelated’ to ONYX-015 and were associated with intrahepatic tumor progression. The highest dose administered (2 × 1012 particles) was shown to be well-tolerated in three patients. The 2 × 1012 particle dose level therefore appears to be well-tolerated, and the maximum dose that could be administered based on manufacturing capabilities was the MTD for the study.
Adverse events during cycles 1 and 2 of ONYX-015 (single agent) are reported by grade in Table 3. Table 4 describes the most common adverse events for the combination of ONYX-015 and chemotherapy (cycle 3 and beyond). The adverse event profile was similar between patients receiving low or high doses, although the frequency and severity of these adverse events was greater in the high-dose patients. Nearly all patients reported flu-like symptoms, including fever, myalgias, asthenia and/or chills. Chills, myalgias and flu-like symptoms were mild to moderate (grade 1–2) in most cases, and the duration of these symptoms was typically short (<48 h). No patients discontinued therapy on the basis of flu-like symptoms. Cycles 3 and greater were administered with chemotherapy, and therefore the chemotherapy-related toxicities of leukopenia and mucositis were more common during these cycles (Table 4). The frequency and severity of chemotherapy-related toxicities were within the range expected when these agents are administered without ONYX-015. Therefore, it did not appear from this study that ONYX-015 administration worsened the toxicity associated with chemotherapy.
Table 3.
Adverse events by toxicity grade for cycles 1 and 2: ONYX-015 alone ⩾20% incidence)
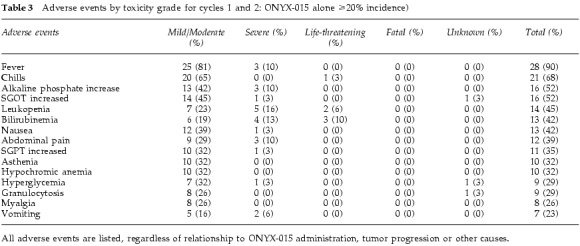
Table 4.
Adverse events by toxicity grade for cycles of ONYX-015 plus 5-fluorouracil and leucovorin (>20% incidence)
In light of the treatment-associated death due to adult respiratory distress syndrome on trial with a replication-defective adenovirus in a patient with ornithine transcarbamylase deficiency, it is notable that no pulmonary symptoms or coagulation abnormalities/disseminated intravascular coagulation were noted in any patients. In addition, no deaths occurred on study; after withdrawal from study, patients have died only as a result of tumor progression. In summary, ONYX-015 has been well-tolerated both as a single agent by hepatic artery infusion and in combination with intravenous 5-FU and leucovorin, at doses of up to 2 × 1012 particles.
Humoral immune response to intravascular ONYX-015
Neutralizing antibody titers to ONYX-015 (Ad5 protein coat) were positive in approximately 55% of patients before treatment. Titers increased and/or became positive in all patients following intravascular administration with ONYX-015, regardless of viral dose (Figure 2); the median antibody titer after a single cycle was approximately 1:10 000. Titers continued to rise after a second cycle of treatment, as well. A formal correlation between antibody levels and the likelihood of toxicity or antitumoral activity could not be made in this study given the heterogeneity in prior chemotherapy and tumor burden in these patients.
Figure 2.
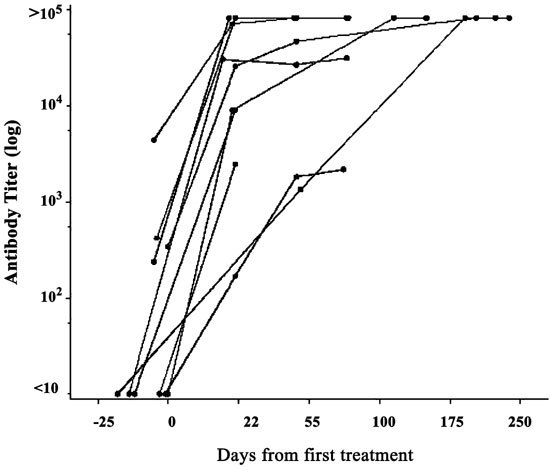
Humoral immune response to ONYX-015 following hepatic arterial infusion: induction of neutralizing antibodies. Neutralizing antibody titers were determined at baseline and on day 22 (after two cycles of therapy). Approximately 50% of patients had positive antibody titers before treatment; all patients had marked increases in antibody levels after treatment regardless of viral dose.
Evidence for viral replication and shedding: viral genomes in blood on day 4 (±1)
Quantitative PCR of the blood was performed on day 4 (±1) to assess viral replication and shedding from tumor tissue. Input virus was rapidly cleared within 6 h after the infusion to levels at or below the level of detection in all patients. In addition, two high-dose patients had assays performed 24–48 h after infusion and neither had genomes detected. Therefore, positivity on day 4 at levels ⩾100-fold above the lower limit of detection was considered strong evidence for replication and shedding. On day 4, no patients had detectable virions at doses of <2 × 1011 particles. In contrast, circulating viral genomes were detected at doses of ⩾2 × 1011 particles (Figure 3). The single patients treated at 2 × 1011 particles and 6 × 1011 particles both had evidence of replication, as did two of three patients at 2 × 1012 particles. Viral genome concentrations in the blood were approximately 1–5 × 106 genomes/ml (ie 0.5–2.5 × 1010 genomes in the total blood volume of 5000 ml).
Figure 3.
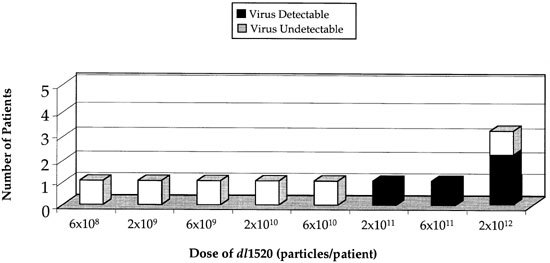
Evidence for viral replication and shedding into the blood: correlation with viral dose and with neutralizing antibody levels. Quantitative PCR of the blood for detection of ONYX-015 was performed on day 4 (±1) of cycle 1. Since ONYX-015 is cleared from the blood within the first 6 h after infusion, the presence of virus at this late time-point is indicative of on-going viral replication and shedding into the blood. Viral replication correlates with viral dose. White boxes, patients PCR (−); black boxes, patients PCR (+).
Biopsy samples from normal and tissues and liver metastases
The vast majority of tumor samples taken after treatment (day 4 after cycle 1) consisted of necrotic debris associated with very few viable cells. Interestingly, this is in contrast to the relatively cellular biopsy samples obtained at baseline. Post-treatment necrosis may therefore be due to ONYX-015 effects, although sampling bias cannot be ruled out. In contrast, post-treatment liver biopsies were uniformly cellular and intact. No cytopathic effects suggesting viral replication were seen in any post-treatment core liver biopsy samples.
Antitumoral activity
This study was not designed to assess single agent activity, since the primary objectives of the study were to determine the safety and feasibility of hepatic arterial administration of adenovirus alone and in combination with chemotherapy. Interestingly, however, radiographic and histologic evidence of acute necrosis induction was seen in some high-dose patients following ONYX-015 alone. After single agent safety was documented, we assessed the efficacy of combination therapy with 5-FU and LV as a secondary endpoint of the study.
Antitumoral activity was demonstrated with ONYX-015 in combination with 5-FU and LV, and activity appeared to be dose-dependent. None of the patients on doses of <6 × 1011 particles had responses in combination with chemotherapy. At high viral doses, however, antitumoral activity was demonstrated. One patient with 5-FU-refractory colorectal carcinoma had a partial response (intrahepatic tumors) following combination therapy with ONYX-015 and 5-FU/LV. In addition, two high-dose patients had stable disease on combination therapy lasting from 7+ to 17+ months.
Additional evidence for combination therapy activity was obtained from a single 5-FU-refractory patient in the low-dose group who was allowed high-dose treatment after tumor progression. This patient had previously failed bolus and continuous infusion 5-FU, as well as irinotecan before study entry. On 2 × 1011 particles plus 5-FU/LV, the tumor remained stable for 2.5 months. ONYX-015 was then discontinued while the patient remained on 5-FU/LV. Disease progression occurred 2.5 months later. At this time-point, ONYX-015 was reinstituted at a 10-fold higher dose (2 × 1012 particles). On resumption of ONYX-015 at this dose, tumor shrinkage occurred alkaline phosphatase decreased (35%) and the patient remained progression-free for 3+ months. Therefore, this patient demonstrates: (1) a dose–response to ONYX-015 in the setting of 5-FU- and irinotecan-refractory disease and (2) ONYX-015 can have antitumoral activity following induction of high neutralizing antibody titers.
Discussion
We performed a phase I dose-escalation trial of the replication-selective adenovirus ONYX-015 administered by hepatic arterial infusion, alone and in combination with intravenous 5-FU and LV, in patients with liver metastases from gastrointestinal carcinomas. The feasibility and safety of this treatment approach was demonstrated. This is the first report describing intravascular administration of genetically engineered, replication-selective virus in patients. The primary study endpoints were to determine the safety of intravascular infusion, alone and in combination with intravenous 5-FU/LV, and the feasibility of infecting metastatic tumors by this route of administration with an attenuated adenovirus. Secondary endpoints included assessment of the humoral immune response and antitumoral activity (assessable on combination therapy only). The hepatic arterial infusion of ONYX-015 was well-tolerated at doses up to 2 × 1012 particles (1011 p.f.u.). The dose of 2 × 1012 particles was the highest practical dose given manufacturing capacity at the time of this study. Mild to moderate fever and rigors were the most common adverse events, and these were transient and not dose-limiting. Despite the dose-limiting liver toxicity identified in mice,28 dose-limiting liver toxicity did not occur in these patients at these doses. Transient, mild to moderate transaminitis was documented occasionally at the highest doses. Therefore, hepatic arterial infusion of this E1B-55kD gene-deleted adenovirus was well-tolerated both as a single agent and in combination with 5-FU. Viral replication occurred at high doses in patients without neutralizing antibodies present at baseline. This study has major implications regarding the safety of adenovirus administration into the blood, particularly in the wake of the unfortunate death of a patient treated with a replication-incompetent adenovirus into the hepatic artery for ornithine transcarbamylase deficiency. Adenovirus administration into the blood does not appear to be inherently dangerous. Importantly, this lack of significant toxicity was demonstrated at doses that resulted in the infection of tumors and in antitumoral activity.
Serious concerns have recently been raised about the safety of intravascular adenovirus following the patient death on a clinical trial for patients with ornithine transcarbamylase (OTC) deficiency at the University of Pennsylvania.10,11,12,13 This patient received a dose of approximately 4 × 1013 particles with a replication-deficient adenovirus expressing the ornithine transcarbamylase gene. By report, in less than 24 h the patient experienced hyperammonemia, adult respiratory distress syndrome (ARDS) and disseminated intravascular coagulation; this was followed over the next few days by multi-organ system failure and death.10 On this trial we observed none of these complications. Several factors may account for these relative differences in safety. One plausible explanation is that the patient populations enrolled on to these two trials differed in their sensitivity to viral infection and the ensuing systemic inflammatory response. It is well-documented that patients with OTC deficiency have a heightened sensitivity to viral and bacterial infections compared with the general population, and that hyperammonemia, ARDS and death can result.31 Unfortunately, the OTC patient reportedly had an elevated blood ammonia level before treatment on the trial. We have observed high-level induction of both interleukin-1 and -6, for example, following dl1520 administration, consistent with an adenovirus-induced systemic inflammatory response (T Reid, personal commu- nication). This sort of inflammation may not be tolerated in OTC patients. A second contributing factor to safety differences may have been that the viral doses tested on this study were approximately 10-fold lower. However, viral replication on this study probably led to longer term adenoviral exposure and shedding into the bloodstream than on the OTC trial with a replication-deficient adenovirus. In addition, five patients on a separate trial have now been treated intravenously with dl1520 at doses of 2 × 1012 to 2 × 1013 particles; these doses are up to 10-fold higher than on this trial and are close to those used before the fatal event in the OTC trial.37 None of the complications that occurred in the OTC patient occurred in these patients. Finally, differences in the viruses themselves may have contributed, although the protein coats of the viral particles were identical on each study. Based on this study, it is clear that adenoviruses, including replication-selective adenoviruses, can be safely administered into the bloodstream at doses that have biological activity against metastatic carcinoma.
The dose-limiting toxicity of serotype 5 adenovirus in mice is hepatic necrosis.28 This hepatotoxicity can be demonstrated with both replication-defective or replication-deficient adenoviruses, although the LD50 with replication-competent adenovirus is significantly lower. This hepatotoxicity is dependent on tumor necrosis factor receptor expression (although tumor necrosis factor may not be the relevant ligand since it is not required for the toxicity to occur). The LD50 seen with dl1520 after tail vein injection in mice was approximately 6 × 1010 particles. Based on allometric scaling, the lethal dose in a human would be predicted to be 6 × 1013 particles. However, rodent toxicity models are suboptimal because these animals are not fully permissive for human adenovirus replication. In addition, the relative expression of coxsackie-adenovirus receptor (CAR) differs between the human and the mouse in some organs.32 Humans have higher relative receptor levels in the heart and lower relative levels in the liver, for example. The lack of clinically significant liver toxicity on this trial was encouraging. Transient low-grade transaminitis was demonstrated in some patients in the high-dose group, as was a single case of transient grade III hyperbilirubinemia. It is possible that clinically significant liver toxicity could occur at higher viral doses. On a separate trial, five patients were treated intravenously with dl1520 at doses of 2 × 1012 to 2 × 1013 particles (ie at doses up to 10-fold higher than on this trial).37 Similar grade I–II transaminitis was demonstrated, but no clinically significant hepatotoxicity or other toxicity was encountered. With improvements in manufacturing, future trials may therefore explore higher doses of adenoviruses in patients with metastatic and treatment-refractory carcinoma in order to determine the maximally tolerated dose by this route of administration; dose escalation would need to proceed cautiously at these high doses, but higher doses may result in enhanced antitumoral efficacy.
This study is the first to describe the replication of an adenovirus following intravascular administration in humans. Evidence of presumptive viral replication and shedding was obtained only at dose levels of ⩾6 × 1011 particles. All patients with circulating viral genomes in the blood on day 4 on cycle 1 were also antibody negative at baseline and had colorectal carcinoma. If future clinical trials demonstrate a correlation between efficacy and neutralizing antibodies, the utility of antibody inhibition prior to and during intravascular viral therapy should be explored.33 In addition, strategies to minimize antibody binding (eg modification of coat with polyethylene glycol) or serotype switching should be explored.34
Although this phase I study was designed to evaluate single agent and combination therapy safety, humoral immune response and feasibility of tumor infection, antitumoral activity of ONYX-015 in combination with chemotherapy was assessed as a secondary endpoint. In combination with 5-FU and LV, dose-related antitumoral activity was demonstrated. Of note, a partial tumor response was demonstrated in a patient who was refractory to both 5-FU-based chemotherapy and to ONYX-015 administered separately; this is suggestive of a potential synergy between these agents. In addition, a patient failing lower dose virus plus chemotherapy subsequently underwent shrinkage (35% decrease) on high-dose virus plus chemotherapy. These findings are consistent with evidence of chemosensitization following intratumoral injection of ONYX-015 in head and neck cancer clinical trials.24 In preclinical studies, we have shown that this interaction is dependent on viral replication and on the sequencing of the agents. Studies are underway to determine the role of inflammatory cytokines and specific viral gene products (eg E1A) in this interaction.35,36 Combination therapy with replication-selective adenoviruses and chemotherapy holds promise. Tumor cross-resistance is unlikely with agents working through such radically different mechanisms, and the combination has been well-tolerated to date due to a lack of overlapping toxicities. Efficacy trials exploring this combination therapy approach are indicated. Now that safety of the combination has been suggested, viral therapy and chemotherapy may be initiated together on cycle 1 in the future.
These findings have major implications for the fields of viral and gene therapies. Arterial delivery is now clearly possible with human adenovirus, and such treatment can result in antitumoral effects. This opens up the possibility of treating a wide range of tumors using this approach. The chemosensitization demonstrated by ONYX-015 on trials of head and neck cancer patients has now been shown with another tumor type and another route of administration. In addition, based on these clinical results, clinical testing of this adenovirus as an intravenous therapy for metastatic tumors is clearly indicated;37 these trials are underway. To achieve equivalent levels of virus within tumors using the intravenous route, however, higher doses may be necessary. Neutralizing antibodies may play a role in these trials and novel methods to inhibit antibody production need to be studied.
Methods
Objectives
The primary objectives of this study were as follows: (1) to determine the safety of repeated infusions of ONYX-015 into the hepatic artery (h.a.i.), alone and in combination with intravenous 5-FU and LV; (2) to determine the maximally tolerated dose and/or dose-limiting toxicity of ONYX-015 administered as described above. Secondary objectives included the following: (3) to determine the humoral immune response to hepatic artery infusions of ONYX-015; (4) to determine the magnitude of viral replication and shedding into the bloodstream as a function of viral dose; (5) to assess the antitumoral efficacy of ONYX-015 infusions alone and in combination with 5-fluorouracil and leucovorin (as above) on metastatic tumors within the liver.
Eligibility criteria
Inclusion criteria included the following: histologically or cytologically confirmed carcinoma of gastrointestinal origin; cancer that was not considered resectable for potential cure; confirmed hepatic artery perfusion of both liver lobes and >50% of all tumor mass(es); Karnofsky performance status (KPS) of ⩾70%; life expectancy of ⩾3 months; ⩾18 years of age; consent form for study participation signed; must have been using a reliable method of contraception if sexually active or of reproductive potential; creatinine <2.0 mg/dl; AST and ALT <3.0-fold upper limit of normal; PT/INR <2.0 and PTT within normal limits; neutrophils >1500/ml, hemoglobin >9 g/dl, platelets >100 000/ml.
Exclusion criteria were as follows: known chronic liver dysfunction before the development of metastatic cancer (eg cirrhosis, chronic hepatitis) which in the estimation of the principal investigator put the patient at high risk for liver complications; >50% liver replacement by tumor (estimated radiographically); history of esophageal variceal bleeding within the preceeding 8 weeks; active infection, including documented human immunodeficiency virus; any viral syndrome diagnosed within the previous 2 weeks; chemotherapy within the previous 3 weeks (6 weeks for nitrosoureas or mitomycin-C); radiotherapy to the target tumor site within the last 4 weeks; concomitant hematological malignancy; chronic immunosuppressive medication; pregnant or lactating females; prior participation in any research protocol which involved administration of adenovirus vectors; treatment with any other investigational therapy within the last 4 weeks. Since no single gold-standard test for p53 function within a tumor existed, p53 tumor status did not affect enrollment. However, the p53 gene status of the tumor was assessed by gene sequencing when sufficient amounts of tissue could be obtained (exons 2–11).
Test article
ONYX-015 (dl1520) is a chimeric human group C adenovirus (Ad2 and Ad5) that does not express the 55 kDa product of the E1B gene; the virus was constructed in the laboratory of Arnold Berk.38 The virus contains a deletion between nucleotides 2496 and 3323 in the E1B region encoding the 55 kDa protein. In addition, a C to T transition at position 2022 in E1B generates a stop codon at the third codon position of the protein. These alterations eliminate expression of the E1B-55kD gene in ONYX-015 infected cells. ONYX-015 was grown and titered on the human embryonic kidney cell line HEK293 as previously described.16
Treatment schedule
The treatment schedule is summarized in Figure 1. The safety and antitumoral activity of single agent ONYX-015 administered via the hepatic artery was determined following single infusions on day 1 and day 8 (cycles 1 and 2). Starting on day 22, treatment cycles were 28 days and consisted of ONYX-015 infusions followed by intravenous chemotherapy within 6 h following virus infusion (see below). After completion of cycle 4, up to four additional cycles were optional based on toxicity and tumor response (see below).
Treatment procedures
A standard hepatic artery catheter was introduced via the femoral artery for the infusion, unless an indwelling hepatic arterial port was already in place. Patient treatment by percutaneous femoral artery catheterization was performed in interventional radiology suites. Selective catheterization of the proper hepatic artery was performed using standard diagnostic catheters and fluoroscopic guidance through a percutaneous femoral arterial approach. Patients were sedated with short-acting opiates and benzodiazepines as necessary for the procedure, and provided analgesics and anti-emetics as necessary afterwards. Patients underwent diagnostic hepatic arteriograms immediately before the initial virus solution infusion to define arterial anatomy and to confirm hepatic arterial supply to intrahepatic tumor(s). Images were obtained by digital subtraction angiography (DSA). The existence of anomalous arterial anatomy, which may occur in up to 40% of the normal patient population, was established and documented. If there was a single branched hepatic artery, the infusion was given into the proper hepatic artery (not the common). In the event of multiple arterial supply, the proportion of the liver supplied by each artery was estimated from the pretreatment CT scan and the arteriogram. After optimal positioning of the catheter to ensure minimal reflux into arterial branches not supplying the liver, a single infusion of 10 ml of viral solution was administered over approximately 5 min followed by a 10 ml normal saline flush given at the same rate and site. The total viral dose administered was specified for the cohort. When multiple hepatic arteries were identified, the total dose was divided and administered into each artery according to the proportion of the liver supplied by that artery in order to provide even and complete distribution throughout the entire liver. After completion of the infusion, the femoral catheter was removed.
Frozen vialed virus solution was warmed and diluted with normal saline to the appropriate titer for each patient's dose level. Vials of ONYX-015 were opened and diluted in biological safety level 2 cabinets. Virus was maintained at 2° to 8°C during dilution and handling, except for warming to room temperature immediately before administration. The virus solution was then further diluted to a final volume of 10 ml. Dilutions were performed immediately before tumor injection using plastic syringes. ONYX-015 infusions were given via intra-arterial catheter over 3–5 min. This infusion was followed immediately by a 10 ml D5W/Electrolyte 48 solution flush given in identical fashion. Vital sign assessments were taken at baseline, at the conclusion of the infusion, and then every 30 min for a total of 2 h. Patients were observed overnight in the hospital after injection at the discretion of the principal investigator.
For cycles 3 and 4 (starting on days 22 and 50), chemotherapy was given for 5 consecutive days starting within 6 h after the ONYX-015 infusion. Leucovorin 20 mg/m2 i.v. was followed by 5-FU 425 mg/m2/day by intravenous bolus. Chemotherapy dose modifications due to toxicity were made according to guidelines at each study center. Up to four additional cycles of combination ONYX-015 and chemotherapy were allowable if treatment was well-tolerated and symptomatic tumor progression had not been documented.
Definitions of maximum-tolerated dose (MTD) and dose-limiting toxicity (DLT)
The MTD was defined as the dosage immediately preceding (lower than) the dose at which two patients experienced a DLT after the first treatment with ONYX-015, or the maximally administered dose based on virus production (the 2 × 1012 particle dose). DLT was defined as any one of the following: grade 4 toxicity of any duration attributed to ONYX-015 treatment, or grade 3 toxicity lasting >5 days attributed to ONYX-015 treatment (excluding flu-like symptoms).
Dose-escalation scheme
One patient was entered per dose level until the maximum-tolerated dose was defined as the highest dose administered (2 × 1012 particles; 1011 p.f.u.); three patients were treated at this dose level. Dose escalation was planned as follows. Each patient was enrolled sequentially into treatment cohorts with at least a 10-day interval between each cohort. Dose escalation was to be based on the toxicity seen with the first two cycles of therapy. If a DLT were to have been observed, up to six patients would have been enrolled at that dose level. Patients would have been enrolled until a second DLT occurred (which defined the toxic dose) or until six patients were enrolled at that dose level without DLT, after which enrollment could continue. All subsequent cohorts after a DLT was observed would have enrolled at least three patients. If one DLT had been observed in a three-patient cohort, enrollment would have continued until up to three additional patients (for a total of up to six) were enrolled at the same dose level. If none of the additional patients had a DLT following treatment, then patients were to be enrolled at the next dose level.
Toxicity assessment on study
The schedule of activities for patients on study is outlined in Figure 1. Blood was drawn on day 8 of each cycle for serum chemistry, complete blood count, PT/PTT, liver function tests (AST, ALT, total and direct bilirubin, alkaline phosphatase) and lactate dehydrogenase. Toxicity, including reports of adverse events, was assessed throughout treatment and for at least 28 days following treatment completion. The NCIC-Common Toxicity Criteria were used to categorize and grade toxicities.
Quantitative PCR testing of blood for viral genomes
Blood was tested for the presence of ONYX-015 by quantitative-PCR on day 1 (pharmacokinetic draws, described above) and on day 4 (±1) of cycle 1. PCR for ONYX-015 was performed using the TaqMan assay which quantitates the number of ONYX–015 genomes in human plasma (the amplicon overlaps the E1B region deletion and does not detect wild-type adenovirus sequences). Viral DNA is extracted from patient samples, standards and controls using QIAamp DNA mini kit. The lower limit of detection is 1.05 × 104 particles of ONYX–015 per ml of plasma. The presence of PCR inhibitors in the sample is monitored using an independent PCR reaction. This PCR test is specific for the ONYX-015 genome and will not detect wild-type adenovirus genomes.
Neutralizing antibody level assessment
Neutralizing antibodies to ONYX-015 were assessed at baseline, day 22 and day 50. Titers against ONYX-015 were determined on blood samples as follows. Patient and control samples were incubated at 55°C for 30 min to inactivate complement. Clinical plasma samples previously determined to produce high, mid-range and negative titers were designated as plasma controls. Each dilution was mixed with adenovirus stock at a titer pre-qualified to produce 15–20 plaques per well of a 12-well dish in DMEM growth medium. The patient's samples and controls were inoculated for 1 h at room temperature, and applied to 70–80% confluent JH393 cells in 12-well dishes. After 2 h of incubation at 37°C, 5% CO2 plasma–virus mix was removed and 2 ml of 1.5% agarose in DMEM was added to each well. Plates were read on day 7 after inoculation by counting the number of p.f.u. per well. The titer of neutralizing antibody for each sample was reported as the dilution of plasma that reduced the number of plaques to 60% of the number of plaques in the virus control without antibody.
Tumor and normal liver biopsy assessment
Pretreatment tumor biopsies were taken for p53 sequencing from an intrahepatic tumor mass that was to be perfused. Exons 2–11 were sequenced completely if adequate tissue was obtained. Core biopsies of tumor (n = 1–2) and normal liver (n = 1) were obtained at baseline and on day 4 of cycle 1. Biopsies were assessed for the degree of tumor necrosis and for evidence of viral replication. Tumor samples were considered evaluable for viral replication if at least 50 viable cells were present on the slide.
Tumor response assessment
The antitumoral activity of single agent ONYX-015 was determined after cycles 1 and 2. Combination therapy efficacy was determined after cycles 3 and 4 (and beyond if treatment extended). Antitumoral efficacy was determined by contrast-enhanced CT scans (performed after each cycle of therapy) and serial CEA and LDH blood levels. Response was assessed separately on the intrahepatic and extrahepatic tumor foci (if present). All intrahepatic masses were measured and included in the response assessment. Complete response (CR) was defined as complete disappearance of all tumor at the assessed site; partial response (PR) as regression of the overall tumor mass by ⩾50% but <100%; stable disease (SD) as tumor decrease or increase in size by <25%; progressive disease (PD) as ⩾25% increase in overall tumor cross-sectional area.
Acknowledgements
We would like to thank the following individuals for their assistance: Margaret Uprichard, Sherry Toney, Amy Waterhouse, Patrick Trown, Deborah Hahn, Ellen Morgan, Phil Custodio.
References
- 1.Kozarsky KF, Wilson JM. Gene therapy: adenovirus vectors. Curr Opin Genet Dev. 1993;3:499–503. doi: 10.1016/0959-437X(93)90126-A. [DOI] [PubMed] [Google Scholar]
- 2.Roth J, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89:21–39. doi: 10.1093/jnci/89.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Kirn D. Replication-selective micro-organisms: fighting cancer with targeted germ warfare. J Clin Invest. 2000;105:836–838. doi: 10.1172/JCI9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heise C, Kirn D. Replication-selective adenviruses as oncolytic agents. J Clin Invest. 2000;105:847–851. doi: 10.1172/JCI9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martuza RL. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 6.Kirn DH. Replicating oncolytic viruses: an overview. Expt Opin Invest Drugs. 1996;5:753–762. doi: 10.1517/13543784.5.6.753. [DOI] [Google Scholar]
- 7.Kirn D . Selectively-replicating viruses as therapeutic agents against cancer. In: Gene Therapy of Cancer, Vol. 1. Academic Press: San Diego 1998 pp 235–248
- 8.Freytag SO. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy (see comments) Hum Gene Ther. 1998;9:1323–1333. doi: 10.1089/hum.1998.9.9-1323. [DOI] [PubMed] [Google Scholar]
- 9.Hawkins L. Replicating adenoviral gene therapy. Proc Am Assoc Cancer Res. 1999;40:476–477. [Google Scholar]
- 10.Marshall E. Clinical trials: gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 11.Beardsley T. Gene therapy setback. Sci Am. 2000;282:36–37. doi: 10.1038/scientificamerican0200-36. [DOI] [PubMed] [Google Scholar]
- 12.Jenks S. Gene therapy death – everyone has to share in the guilt. J Natl Cancer Inst. 2000;92:98–100. doi: 10.1093/jnci/92.2.98. [DOI] [PubMed] [Google Scholar]
- 13.Miller H. Gene therapy on trial (letter) Science. 2000;287:591. doi: 10.1126/science.287.5453.591c. [DOI] [PubMed] [Google Scholar]
- 14.Barker DD, Berk AJ. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987;156:107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- 15.Bischoff JR. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells (see comments) Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 16.Heise C. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents (see comments) Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 17.Harada J, Berk A. p53-independent and -dependent requirements for E1B-55kD in adenovirus type 5 replication. J Virol. 1999;73:5333–5344. doi: 10.1128/jvi.73.7.5333-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothmann T. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J Virol. 1998;72:9470–9478. doi: 10.1128/jvi.72.12.9470-9478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodrum FD, Ornelles DA. p53 status does not determine outcome of E1B 55-kilodalton mutant adenovirus lytic infection. J Virol. 1998;72:9479–9490. doi: 10.1128/jvi.72.12.9479-9490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogulski K. In vivo antitumor activity of ONYX-015 is influenced by p53 status and is augmented by radiotherapy. Cancer Res. 2000;60:1193–1196. [PubMed] [Google Scholar]
- 21.Kirn D, Hermiston T, McCormick F. ONYX-015: clinical data are encouraging. Nat Med. 1998;4:1341–1342. doi: 10.1038/3902. [DOI] [PubMed] [Google Scholar]
- 22.Kirn D. A phase II trial of intratumoral injection with an E1B-deleted adenovirus, ONYX-015, in patients with recurrent, refractory head and neck cancer. Proc Am Soc Clin Oncol. 1998;17:391a. [Google Scholar]
- 23.Nemunaitis J. Selective replication and oncolysis in p53 mutant tumors with Onyx-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359–6366. [PubMed] [Google Scholar]
- 24.Khuri F. A controlled trial of Onyx-015, an E1B gene-deleted adenovirus, in combination with chemotherapy in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 25.Kirn DH. A phase II trial of ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Proc Am Soc Clin Oncol. 1999;18:1505 (Abstr.). [Google Scholar]
- 26.Heise C. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents (see comments) Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 27.Reid A. A phase I/II trial of ONYX-015 administered by hepatic artery infusion to patients with colorectal carcinoma. 1999. [Google Scholar]
- 28.Heise C. Intravenous administration of ONYX-015, a selectively-replicating adenovirus, induces antitumoral efficacy. Cancer Res. 1999;59:2623–2628. [PubMed] [Google Scholar]
- 29.Kemeny N. Hepatic arterial infusion of chemotherapy following resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 30.Abbruzzese J, Evans D, Rich T . Cancer of the pancreas. In: DeVita V, Hellman S, Rosenberg S (eds). Cancer: Principles and Practice of Oncology, 5th edn Lippincott-Raven: Philadelphia 1997 pp 1054–1086
- 31.Brusilow S, Maestri N. Urea cycle disorders: diagnosis, pathophysiology and therapy. Adv Pediatr. 1996;43:127–170. [PubMed] [Google Scholar]
- 32.Roelvink P. Identification of a conserved reseptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286:1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Trinchieri G, Wilson JM. Recombinant IL-12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung (see comments) Nat Med. 1995;1:890–893. doi: 10.1038/nm0995-890. [DOI] [PubMed] [Google Scholar]
- 34.Mastrangeli A. Sero-switch adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum Gene Ther. 1996;7:79–87. doi: 10.1089/hum.1996.7.1-79. [DOI] [PubMed] [Google Scholar]
- 35.Heise C. An adenovirus E1A mutant that demonstrates potent and selective antitumoral efficacy. Nat Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 36.Heise C, Lemmon M, Kirn D. Replication-selective adenovirus plus cisplatin chemotherapy efficacy is dependent on sequencing but independent of p53 status. Clin Cancer Res. 2000;6:4908–4914. [PubMed] [Google Scholar]
- 37.Nemunaitis J, Cunningham C, Randlev B, Kirn D. Intravenous infusion of a replication-selective adenovirus (Onyx-015) in cancer patients: safety feasibility and biological activity. Gene Therapy. 2001;8:746–759. doi: 10.1038/sj.gt.3301424. [DOI] [PubMed] [Google Scholar]
- 38.Barker DD, Berk AJ. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987;156:107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]


