Abstract
Sponsorship Statement: Publication of this supplement is sponsored by the European Society for Blood and Marrow Transplantation.
Copyright: Modified and published with permission from http://www.ebmt2018.org/
PHYSICIANS — ORAL SESSION
Acute leukemia
O009 EBMT ALWP study: myeloablative versus reduced intensity conditioning allogeneic haematopoietic stem cell transplantation in patients with acute myeloid leukaemia in second complete remission
Maria Gilleece1,2, Myriam Labopin3,4,5, Ibrahim Yakoub-Agha6, Gerard Socié7, Tobias Gedde-Dahl8, Didier Blaise9, Nigel H. Russell10, Charles Craddock11, Jan.J. Cornelissen12, William Arcese13, Edouard Forcade14, Charles Crawley15, Emanuelle 'Polge16, Mohamad Mohty4,5, Bipin Savani17, Arnon Nagler18,19
1Leeds Cancer Institute, Leeds Teaching Hospitals Trust, Department of Haematology, Leeds, United Kingdom; 2University of Leeds, Leeds, United Kingdom; 3EBMT Paris Study Office / CEREST-TC, Paris, France; 4Hôpital Saint Antoine, INSERM UMR 938, Paris, France; 5Université Pierre et Marie Curie, Paris, France; 6Hôpital HURIEZ UAM allo-CSH, CHRU, Lille, France; 7Hôpital Saint-Louis, Assistance Publique Hôpitaux de Paris, Service d'Hématologie Greffe, Paris, France; 8Clinic for Cancer, Surgery and Transplantation, Oslo University Hospital, Rikshospitalet, Department of Hematology, Oslo, Norway; 9Centre de Recherche en Cancérologie de Marseille, Institut Paoli Calmettes, Programme de Transplantation &Therapie Cellulaire, Marseille, France; 10Nottingham University Hospitals NHS Trust, Nottingham, United Kingdom; 11Queen Elizabeth Medical Centre, University Hospital Birmingham NHS Trust, Department of Haematology, Birmingham, United Kingdom; 12Erasmus MC Cancer Institute, University Medical Center Rotterdam, Department of Haematology, Rotterdam, Netherlands; 13¨Tor Vergata¨ University of Rome, Policlinico Universitario Tor Vergata, Stem Cell Transplant Unit, Rome, Italy; 14CHU Bordeaux Hôpital Haut-leveque, Pessac, Bourdeaux, France; 15Addenbrookes Hospital, Department of Haematology, Cambridge, United Kingdom; 16Acute Leukemia Working Party, European Society for Blood and Marrow Transplantation Paris Study Office/European Center for Biostatistical and Epidemiological Evaluation in Hematopoietic Cell Therapy (CEREST-TC), Paris, France; 17Vanderbilt University Medical Center, Division of Hematology/Oncology, Department of Internal Medicine, Nashville, TN, United States; 18Chaim Sheba Medical Center, Tel-Hashomer, Israel, Hematology Division BMT and Cord Blood Bank, Ramat Gan, Israel; 19Tel Aviv University, Tel Aviv, Israel
Background: Allogeneic haematopoietic cell transplant (allo-HCT) is the only therapeutic modality to offer cure to patients with relapsed acute myeloid leukaemia (AML) achieving second complete remission (CR2). Few studies have focused on allo-HCT outcomes in AML CR2 regarding the impact of myeloablative (MAC) versus reduced intensity (RIC) conditioning.
Methods: This is a multicentre, retrospective registry study by the Acute Leukemia Working Party of the European Society for Blood & Bone Marrow Transplantation in a large cohort of AML CR2 patients. Eligibility: Age ≥18y, first allo-HCT 2007–16, diagnosis AML CR2, cytogenetic profile at diagnosis, peripheral blood stem cells (PBSC) or bone marrow (BM) from a matched related (MRD), volunteer unrelated with HLA match 10/10 (VUD) or 9/10 (MMVUD), or haplo-identical (haplo) donor. Univariate and Cox Regression multivariate analyses (MVA) were undertaken. Measured outcomes included 2y OS, leukemia free survival (LFS), non-relapse mortality (NRM), graft vs host disease (GVHD), chronic GVHD (cGVHD) and GVHD-free/relapse-free survival (GRFS).
Results: A total of 1879 patients, 1013 male, were eligible and 1010 (54%) received MAC allo-HCT. Donors were MRD (36%), VUD (39%), MMVUD (15%) or haplo (10%). Allocation to MAC allo-HCT was 37% MRD, 36% VUD, 14% MMVUD and 13% haplo (P < 10−3).
MAC versus RIC allo-HCT groups were equivalent for de novo AML (95%), year of HCT, median follow-up (24.8 vs 30.53m), reported FLT3 mutations (25.63 vs 24.4%), NPM1 mutations (48.67 vs 50.16%) and confirmed measurable residual disease at HCT (33%). Recipient-donor pairs were similar for sex-matching and CMV sero-status. Karnofsky performance status was ≥80% in 97.21% MAC and 93.07% RIC allo-HCT recipients (P < 10−3).
At 2y, overall outcomes were LFS 52% (CI: 49.5–54.5), OS 58.7% (CI: 56.2–61.2), RI 28.9% (CI: 26.7–31.2), NRM 19% (CI: 17.2–21), GRFS 38.7% (CI: 36.2–41.1), acute GVHD II-IV 24.3% (CI: 22.3–26.3), cGVHD 37.2% (CI: 34.7–39.7) and extensive cGVHD 15.9% (CI: 14.1–17.8).
In MVA, in < 50y, RIC vs MAC were equivalent for all outcomes.
In ≥50y, RIC vs MAC decreased NRM (HR 0.535, CI 0.378–0.758) with worse cGVHD (HR 1.377, CI 1.027–1.845) but no impact on RI, LFS or OS.
Independent of conditioning intensity, intermediate and adverse cytogenetics increased RI (< 50y HR 1.52 CI 1.115–2.071, HR 3.347 CI 2.26–4.958; ≥50y HR1.436 CI 1.006–2.049, HR 1.79 CI 1.035–3.096) with concomitant effects on OS (< 50y HR 1.318 CI 1.026–1.692, HR 2.417 CI 1.708–3.421; ≥50y HR 1.202 CI 0.903–1.6, HR 1.607 CI 1.042–2.479).
Conclusions: Allo-HCT rescues more than 50% of AML patients achieving CR2 post-relapse. Results of allo-HCT for the select group of relapsed AML patients achieving CR2 appear similar to those reported in the literature for AML patients who received allo-HCT in CR1. In patients with AML CR2, RIC allo-HCT reduces procedural mortality in patients ≥ 50y without increasing RI and provides equivalent outcomes to MAC allo-HCT in patients < 50y.
Standard approaches to MAC allo-HCT in the < 50y need prospective reappraisal.
Conflict of interest: The authors have nothing to disclose
O010
Abstract previously published
O011 Superior Outcomes with Myeloablative versus Reduced-Intensity Conditioning Allogeneic Hematopoietic Cell Transplantation for Secondary Acute Myeloid Leukemia with Prior Solid Tumor: An ALWP of EBMT Study
Catherine Lee1, Myriam Labopin2, Dietrich Beelen3, Jürgen Finke4, Didier Blaise5, Arnold Ganser6, Maija Itälä-Remes7, Patrice Chevallier8, Hélène Labussière-Wallet, MD, PhD9, Johan Maertens10, Ibrahim Yakoub-Agha11, Jean-Henri Bourhis12, Audrey Mailhol13, Mohamed Mohty14, Bipin Savani15, Arnon Nagler16,17
1Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, United States; 2Acute Leukemia Working Party of EBMT, Paris, France; 3University Hospital Essen, Essen, Germany; 4University Medical Center, Department of Hematology and Oncology, Freiburg, Germany; 5Institut Paoli Calmettes, Centre de Recherche en Cancérologie de Marseille (CRCM), Department of Hematology, Marseille, France; 6Hannover Medical School, Department of Haematology, Hemostasis, Oncology and Stem Cell Transplantation, Hannover, Germany; 7Turku University Hospital, Turku, Finland; 8Nantes University Hospital, Nantes, France; 9Centre Hospitalier Lyon Sud, Pavillon Marcel Bérard -Bat 1G, Service Hematologie, Lyon, France; 10University Hospital Gasthuisberg, Department of Hematology, Leuven, Belgium; 11University of Lille Nord de France, Lille, France; 12Gustave Roussy Cancer Center, Department of Hematology, Villejuif, France; 13EBMT Paris Study Office, Paris, France; 14Hospital Saint-Antoine, Paris University UPMC, INSERM U 938, Paris, France; 15Vanderbilt University Medical Center, Nashville, TN, United States; 16Hopital Saint-Antoine, EBMT, Acute Leukemia Working Party and Registry, Paris, France; 17Chaim Sheba Medical Center, Tel Hashomer, Israel
Background: Secondary acute myeloid leukemia (sAML) occurs as a late complication following an antecedent myeloid disease or solid tumor (ST) and their associated treatments, including cytotoxic chemotherapy and/or radiation. sAML has traditionally been associated with inferior outcomes compared with de novo AML. Allogeneic hematopoietic cell transplantation (HCT) remains the most potent post-remission therapy for sAML, however, there are limited data comparing outcomes between ablative (MAC) vs. reduced-intensity conditioning (RIC) HCT for sAML. Therefore, the Acute Leukemia Working Party (ALWP) of the EBMT performed a large registry analysis to study this question.
Methods: We studied 535 patients with sAML with prior ST who received MAC [223 (41.68%)] or RIC [312 (58.32%)] HCT between years 2000–2016. A prior ST diagnosis of breast cancer was most common [MAC: 127 (56.95%); RIC: 191 (61.22%)]. The median time (months) from diagnosis of ST to sAML and from sAML to HCT was 39.5 (range, 1–475.6) and 4.8 (0.6–65.1) in MAC; and 47.6 (range, 1–434.7) and 5.4 (0.3–45.2) in RIC. Median age (years) at HCT was 48.1 (range, 18.8–72) and 58 (19–74.1) for MAC and RIC, respectively. Median follow-up (months) of surviving patients for MAC and RIC was 50.02 (range, 1.87–166.08) and 36.99 (3.22–182.38), respectively. Cohorts were balanced across cytogenetic risk and disease state. Donor sources included matched sibling [MAC: 109 (48.88%); RIC: 121 (38.78%)] and unrelated [MAC: 114 (51.12%); RIC: 191 (61.22%)]. Peripheral blood grafts were used most frequently [MAC: 167 (74.89%); RIC: 288 (92.31%)]. 96% of patients received calcineurin inhibitor-based GVHD prophylaxis and 61.70 % received in vivo T cell depletion (TCD; majority rATG).
Results: Engraftment occurred in 211 (96.35%) of MAC and 301 (97.10%) RIC patients. In univariate analysis, 3-year RI, NRM, OS and GRFS were not significantly different between cohorts, however, 3-year LFS was superior in MAC compared to RIC [48.7% (95% CI 41.9%-55.5%) vs. 36.9% (CI 31.1%-42.6%), p = 0.027]. There were no differences in grade II-IV/III-IV aGVHD at 100 days, nor cGVHD at 3 years between groups. In multivariate analysis, adjusted for all factors differing between the 2 groups or associated with one outcome, patients receiving RIC regimens had increased RI (HR 1.52, 95% CI 1.02–2.26, p = 0.04), lower LFS (HR 1.52, CI 1.12–2.05, p = 0.007) and OS (HR 1.51, CI 1.09–2.09, p = 0.012). There were no differences in NRM and GRFS. Active disease at HCT was associated with inferior LFS and OS. Use of unrelated donors contributed significantly to decreased relapse, and increased grade II-IV aGVHD and NRM. In-vivo TCD had no impact on relapse or survival, and favorably impacted cGVHD (HR 0.55, 95% CI 0.37–0.81, p = 0.002).
Conclusions: This registry-based study evaluated outcomes following MAC vs. RIC HCT for sAML with prior ST. Despite prior therapy for ST, no difference was demonstrated in NRM between conditioning intensity. Importantly, OS and LFS were superior in patients receiving ablative regimens due to a decrease in RI. As NRM continues to decline in the current era, it is conceivable that outcomes of HCT for sAML with prior ST may be improved by careful patient selection for MAC regimens.
Conflict of interest: None of the authors has anything to disclose
O012 First-in-human study with UCART19, an allogeneic anti-CD19 CAR T-cell product, in high risk adult patients with CD19+ R/R B-cell ALL: Preliminary results of CALM study
Charlotte Graham1,2, Deborah Yallop1, Agnieszka Jozwik2, Piers Patten1,2, Alan Dunlop1, Rose Ellard1, Orla Stewart1, Victoria Potter1, Victoria Metaxa1, Shireen Kassam1, Farzin Farzaneh2, Stephen Devereux1, Antonio Pagliuca1, Amina Zinai3, Florence Binlich3, Sandra Dupouy3, Anne Philippe3, Svetlana Balandraud3, Camille Poirot3, Flavie Simon3, Cyril Konto4, Candy Bermingham4, Ghulam Mufti1,2, Reuben Benjamin1,2
1King's College Hospital NHS Foundation Trust, London, United Kingdom; 2King's College London, London, United Kingdom; 3Institut de Recherches Internationales Servier, Paris, France; 4Pfizer Inc, San Francisco, CA, United States
Background: UCART19 (anti-CD19 scFv- 41BB- CD3ζ) is a genetically modified CAR T-cell product manufactured from healthy donor cells, in which TRAC and CD52 genes have been knocked out to allow its administration in non-HLA matched patients. We report preliminary results of the CALM trial, a first-in-human phase 1 dose finding study of UCART19 in adult patients with relapsed/refractory (R/R) B-ALL.
Methods: Adult R/R B-ALL patients (age ≥16 years) with morphological disease or minimal residual disease (MRD) level ≥1x10−3 and who had exhausted available treatment options were eligible for the study. A lymphodepletion regimen combining cyclophosphamide and fludarabine, with or without alemtuzumab was administered prior to UCART19 single dose infusion. Safety and anti-leukemic activity were assessed as primary and secondary objectives, respectively. Exploratory objectives included evaluation of the proportion of patients who proceeded to a transplant as well as the time to transplant following UCART19 treatment.
Results: As of early December 2017, 7 patients have been treated in the dose escalation phase (6 at dose level 1 (DL1) and 1 at DL2 with 6x106 and 8x107 UCART19 respectively). Median age was 23 years (range 18–49). Patients received a median of 4 prior treatment lines (range 1–5). 6 patients had undergone a previous MUD allogeneic SCT (allo-SCT) and had relapsed at a median of 7.8 months post transplant (range 4–11).
All patients experienced cytokine release syndrome (CRS): 1 G1, 5 G2 and 1 G4. The patient with CRS G4 also developed neutropenic sepsis leading to multiple organ failure and death at Day(D)15. Tociluzumab was administered in 4/7 patients. Median time to onset of CRS was 8 days (range 5–12). CRS correlated with serum cytokine increase (IL-6; IL-10 and IFNg) and UCART19 expansion in blood. One patient developed a G1 skin GvHD. G1 neurotoxicity was observed in 2 patients. Asymptomatic viral reactivations (CMV and/or adenovirus) were seen in 3 patients and resolved with antiviral therapy. 3/7 patients developed prolonged G4 cytopenia.
5 out of 7 patients achieved molecular remission (MRD-ve) at D28, 1 had refractory disease at D28 and 1 died at D15. Of the 5 patients who achieved MRD negativity, 1 relapsed with CD19+ve disease at D61, received a 2nd identical dose of UCART19 and became MRD-ve again. All 5 patients who achieved MRD negativity proceeded to a subsequent allo-SCT at a median of 66 days (range 51–140) post UCART19 treatment with all but one transplanted with a different MUD donor.
Post allo-SCT, 4 patients remain alive and 1 early death occurred at D17 from transplant related infections. 2/4 patients became MRD+ve at 52 and 100 days respectively but reverted to MRD negativity following withdrawal of immunosuppression. Currently 3 patients remain in MRD-ve remission and 1 relapsed with CD19+ extramedullary disease.
Conclusions: Allogeneic CAR-T product UCART19 shows an acceptable safety profile and promising results with 5 over 7 patients achieving molecular remission and proceeding to a second stem cell transplant. Recruitment is on-going at DL2.
Clinical Trial Registry: NCT 02746952
Conflict of interest:
C. Graham: research funding: Servier; educational meeting attendance: Pfizer, Gilead, Sanofi
D. Yallop: honoraria: Jazz Pharmaceuticals, Amgen; advisory board: Pfizer
A. Jozwik: research funding: Servier
P. Patten: honoraria and research funding: Gilead Inc; honoraria: Roche, Abbvie
R. Ellard: honoraria: Moldmed
A. Dunlop, O. Stewart, G. Mufti, V. Metaxa, S. Kassam, F. Farzaneh: Nothing to disclose
V. Potter: advisory board: Pfizer, honoraria: Jazz
S. Devereux: consultancy, honoraria, travel expenses and speakers bureau: Janssen, Gilead; consultancy: GSK; consultancy and travel expenses: Roche; consultancy and honoraria: MSD, AbbVie; advisory board: Servier
A. Pagliuca: honoraria and research funding: Merck; honoraria: Jazz, Gilead, Bluebird, Basilea, Pfizer ; consultancy and speakers bureau: Astellas
A. Zinai, F. Binlich, S. Dupouy:, A. Philippe:, S. Balandraud:, C. Poirot:, F. Simon: employment: Servier
C. Konto: employment and equity ownership: Pfizer, BMS
C. Bermingham: employment and equity ownership: Pfizer
R. Benjamin: participated in Adboard meeting and research funding: Pfizer; research funding: Servier; honoraria: Celgene
O013 Exploiting Cutting-Edge Technologies to Analyze Loss of HLA in a Global Multicentric Cohort of Post-Transplantation Relapses: Preliminary Results from the HLALOSS Collaborative Study
Luca Vago1,2, Cristina Toffalori1, Müberra Ahci3, Vinzenz Lange4, Kathrin Lang4, Sonia Todaro1,2, Karin Stempelmann3, Andreas Heinold5, Friedrich Stölzel6, Miguel Waterhouse7, Rainer Claus7, Ketevan Gendzekhadze8, Masahiro Onozawa9, Raynier Devillier10, Ruoping Tang11, Maayan Ulman12, Dejan Lazarevic13, Maria Teresa Lupo Stanghellini2, Jacopo Peccatori2, Nina Kristin Steckel14, Peter Horn5, Alessandra Picardi15, Sara Manetta16, Jose Luis Pinana17, Jaimie Sanz17, Brian Schaffer18, William Arcese15, Guillermo Sanz17, Benedetto Bruno16, Massimo Pini19, Guido Kobbe20, Katherine C. Hsu18, Monzr Al Malki21, Takanori Teshima9, Nicolaus Kroeger22, Jurgen Finke7, Arnon Nagler12, Didier Blaise10, Mohamad Mohty11, Martin Bornhauser6, Dietrich W. Beelen14, Alexander Schmidt4, Fabio Ciceri2, Katharina Fleischhauer3
1San Raffaele Scientific Institute, Unit of Immunogenetics, Leukemia Genomics and Immunobiology, Milano, Italy; 2San Raffaele Scientific Institute, Hematology and Bone Marrow Transplantation Unit, Milano, Italy; 3Essen University Hospital, Institute for Experimental Cellular Therapy, Essen, Germany; 4DKMS Life Science Lab, Dresden, Germany; 5Essen University Hospital, Institute for Transfusion Medicine, Essen, Germany; 6Technical University Dresden, Department of Hematology/Oncology, Medical Clinic and Policlinic I, Dresden, Germany; 7University Medical Center Freiburg, Department of Hematology, Oncology and Stem Cell Transplantation, Freiburg, Germany; 8City of Hope National Medical Center, HLA Laboratory, Duarte, CA, United States; 9Hokkaido University, Faculty of Medicine, Department of Hematology, Sapporo, Japan; 10Institut Paoli-Calmettes, Department of Hematology, Marseille, France; 11Hopital Saint Antoine, Service d'Hématologie Clinique et de Thérapie Cellulaire, Paris, France; 12Chaim Sheba Medical Center, Tel-Hashomer and Tel-Aviv University, Department of Bone Marrow Transplantation, Tel Aviv, Israel; 13San Raffaele Scientific Institute, Center for Translational Genomics and Bioinformatics, Milano, Italy; 14Essen University Hospital, Department of Bone Marrow Transplantation, West German Cancer Center, Essen, Germany; 15Tor Vergata University, Stem Cell Transplant Unit, Rome Transplant Network, Roma, Italy; 16A.O.U. Citta della Salute e della Scienza di Torino, S.S.D. Trapianto di Cellule Staminali, Torino, Italy; 17Hospital Universitari i Politècnic La Fe, Hematology Department, Valencia, Spain; 18Memorial Sloan Kettering Cancer Center, Department of Medicine, New York, NY, United States; 19A.O. SS Antonio e Biagio e C. Arrigo, Hematology Department, Alessandria, Italy; 20Heinrich-Heine University, Department of Hematology, Oncology and Clinical Immunology, Dusseldorf, Germany; 21City of Hope National Medical Center, Department of Hematology and Hematopoietic Cell Transplantation, Duarte, CA, United States; 22University Hospital Eppendorf, Department of Stem Cell Transplantation, Hamburg, Germany
Background: Genomic loss of the patient-specific HLA has been described in previous single-center studies as a frequent mechanism by which leukemic cells evade immune control and outgrow into a clinically evident relapse. HLA loss is deemed to account for up to 30% of relapses after HLA-haploidentical transplants, but the actual frequency and clinical relevance of this phenomenon in other transplantation settings is largely unknown. Here we present the first global collaborative study to investigate the incidence of this phenomenon across transplant platforms.
Methods: Twenty-seven transplant centers from across the globe (Europe n = 22, North America n = 4, Asia n = 1) joined to form the HLALOSS consortium. To date, we collected a total of 634 cases of relapse from adult patients with acute leukemias, myelodysplastic syndromes or myeloproliferative neoplasms after allogeneic HSCT from HLA-haploidentical relatives (29.3%), HLA-mismatched unrelated donors (MMUD, 25.9%), 10/10-matched unrelated donors (MUD, 35.8%), or unrelated cord blood units (UCB, 9.0%). Where available, the donor and patient germlines and the patient pre-transplant disease were tested in parallel. Cases were analyzed using conventional HLA typing of sorted leukemic blasts, the recently developed HLA-KMR assay (Ahci and Toffalori, Blood, 2017) or a novel Next-Generation Sequencing method developed to cover all possible HLA-A,B,C,DRB1,DQB1 and DPB1 alleles and to analyze multiple samples in a single run. Briefly, for each sample exon 2 and 3 of the six HLA loci are amplified by PCR, barcoded to univocally identify samples and sequenced on an Illumina MiseqV3. Analysis is performed by the DKMS proprietary software NexType to detect and quantify the patient-specific, donor-specific and shared HLA alleles.
Results: To date, we analyzed 222 cases of post-transplantation relapse after haploidentical (n = 104), MMUD (n = 61), 10/10-matched, HLA-DPB1 mismatched MUD (n = 49), or UCB (n = 8) HSCTs. Of these, 127 cases were analyzed using the newly developed HLA sequencing platform. This method resulted particularly robust, reliable and sensitive in analyzing large sample series: with a minimum read-depth of 1000x, up to 0.5% of target DNA could be detected in artificial chimerism curves. False positive reads for patient-specific HLA alleles were detected in 34/73 donor samples, but they were as low as 0.5% on average, and always restricted to one single exon of one or two loci. Ten relapse samples tested in parallel via the sequencing platform and HLA-KMR showed remarkable concordance between the two methods (R2 = 0.86, p < 0.0001). In the total 222 cases analyzed to date by the different methods, we have detected 35 HLA loss post-transplantation relapses, 27 of which after haploidentical HSCT (26.0% of relapses in this setting), 7 after MMUD HSCT (11.5%), 1 after 10/10 MUD HSCT (2%) and none after UCB HSCT. Analysis of the remaining 412 collected samples is ongoing, and will be presented at the meeting.
Conclusions: The present data, obtained from the largest collaborative study on the immunobiology of relapse to date, confirm the clinical relevance of HLA loss as a major mechanism of immune evasion and post-transplantation relapse, including after HSCT from partially HLA-incompatible unrelated donors.
Conflict of interest: This study was supported by the European Commission (ERA-NET TRANSCAN JTC2012 Cancer12-045-HLALOSS), to LV, AN, DB, MM, MB, DWB and KF; by the Deutsche José Carreras Leukämie Stiftung (DJCLS R 15/02) to DWB and KF; by the Dr. Werner Jackstädt Stiftung to KF; by the Joseph-Senker Stiftung to KF; by the Italian Ministry of Health RF-2011-02351998 to FC and LV and RF-2011-02348034 to LV; by the Associazione Italiana per la Ricerca sul Cancro Start-Up Grant #14162 to LV; and by the DKMS Mechtild Harf Research Grant to LV. LV and KF received research funding from GenDx (Utrecht, The Netherlands), all other Authors declare no relevant conflicts of interest to disclose.
O014 Transplant Outcomes for Patients with Secondary Acute Myeloid Leukemia after a Prior Hematologic Disease
Salyka Sengsayadeth1, Myriam Labopin2, Juergen Finke3, Gerard Socie4, Dietrich Beelen5, Ibrahim Yakoub-Agha6, Patrice Chevallier7, Arnold Ganser8, Didier Blaise9, Noel Milipied9, Bruno Lioure10, Audrey Mailhol2, Mohamad Mohty11, Bipin Savani1, Arnon Nagler12
1Vanderbilt University Medical Center, Medicine/Hematology-Oncology, Nashville, TN, United States; 2EBMT Paris Study Office / CEREST-TC, Paris, France; 3University of Freiburg, Medicine-Hematology/Oncology, Freiburg, Germany; 4Hopital St. Louis, Dept.of Hematology - BMT, Paris, France; 5University Hospital, Dept. of Bone Marrow Transplantation, Essen, Germany; 6CHU de Lille, LIRIC, INSERM U 995, Université de Lille, Lille, France; 7CHU Nantes, Dept. D'Hématologie, Nantes, France; 8Hannover Medical School, Department of Haematology, Hemostasis, Oncology, and Stem Cell Transplantation, Hannover, Germany; 9Programme de Transplantation & Therapie Cellulaire, Centre de Recherche en Cancérologie de Marseille, Institut Paoli Calmettes, Marseille, France; 10Hôpitaux Universitaires de Strasbourg, Hematology Department, Strasbourg, France; 11Hopital Saint Antoine, Universite Pierre & Marie Curie, INSERM, UMRs 938, Paris, France; 12Chaim Sheba Medical Center, Hematology Division, Tel Hashomer, Israel
Background: Allogeneic hematopoietic cell transplant (HCT) is curative therapy for patients with secondary acute myeloid leukemia (sAML) though the impact of conditioning regimens and other variables on outcomes after HCT for patients with antecedent hematologic malignancy is largely unknown.
Methods: To study outcomes of patients with sAML with prior hematologic malignancy, this multicentre, retrospective registry study of the Acute Leukemia Working Party of the European Society of Blood and Bone Marrow Transplantation was undertaken. Patients older than 18 y with a diagnosis of sAML with an antecedent hematologic malignancy treated with first allograft between 2000–2016 with matched related (MRD), matched unrelated (MUD), haploidentical donor or cord blood transplant with available cytogenetic profiles were included. Univariate and Cox Regression multivariate analysis (MVA) were undertaken. Measured outcomes included overall survival (OS), leukemia-free survival (LFS), non-relapse mortality (NRM), incidence of graft versus host disease (GVHD) and GVHD-free/relapse free survival (GFRS).
Results: A total of 549 patients were eligible. Myeloablative (MAC) and reduced conditioning (RIC) was given in 258 (47%) and 291 (53%), respectively. No difference was seen between the 2 groups in regards to prior hematologic diagnosis, cytogenetics, disease status at transplant, history of prior autologous HCT (auto-HCT), KPS, donor gender, CMV seropositivity, engraftment rates, and acute GVHD (aGVHD). Differences between MAC and RIC groups were seen in age at transplant MAC median age of 47.8 vs 55.9 y for RIC (P = < 10-3). URDs were used less in MAC vs. RIC (49% vs 60%; P = 0.038). In RIC-HCT, there was increased use of in vivo TCD (65% vs 53%, (P = 0.005).
The entire population had a 2y LFS of 31.7% (95% CI, 27.5–35.9), OS of 37.4% (95% CI, 33–41.8), RI of 39.1% (95% CI, 34.8–43.4), NRM of 28.9% (95%CI, 25–33), and GRFS of 22.8% (95%, CI 19–26.6). Grades III-IV aGVHD occurred in 13.7% (95% CI, 10.9–16.8) and incidence of chronic GVHD (cGVHD) of 27% (95% CI, 23–31.1) with extensive cGVHD occurring in 12.8% (95% CI, 9.9–16).
Univariate analysis identified factors suitable for MVA of RI, NRM, LFS, OS, GFRS, acute GVHD grades II-IV, and extensive cGVHD. Patients who received RIC compared to MAC had lower risk of NRM (HR: 0.58, CI: 0.40–0.83, P = 0.003), and improved LFS (HR:0.67, CI, 0.52–0.85, P = 0.001), OS (HR: 0.69, CI: 0.53–0.89, P = 0.004), and GFRS (HR: 0.79, CI: 0.62–0.99, P = 0.045) (Figure1). Increasing age had increased NRM (HR: 1.19, CI: 1.03–1.38, P = 0.02). Increased RI was seen in patients with active disease at transplant (HR: 2.25, CI: 1.62–3.13, P = < 10-5). Inferior NRM (HR: 4.64, CI: 1.05–20.5, P = 0.04), LFS (HR: 3.15, CI: 1.35–7.37, P = 0.008), OS (HR: 6.61, CI: 2–21.85, P = 0.001), and GFRS (HR: 2.82, CI: 1.29–6.19, P = 0.009) was seen in patients who had adverse cytogenetics. Patients with prior auto-HCT had inferior LFS (HR: 1.30, CI: 1.01–1.67, P = 0.01). Prior hematologic diagnosis and donor type had no impact on outcomes.
Conclusions: Patients with sAML with prior hematologic malignancy treated with RIC HCT have lower risk of NRM and improved LFS, OS, and GFRS. Other variables associated with inferior outcomes include older age, active disease, adverse cytogenetics, and prior auto-HCT.
Conflict of interest: All authors have nothing to disclose.
[O014 Figure].
Outcomes after HCT for sAML with Antecedent Hematologic Malignancy based on Conditioning Regimen Typ]
O015 Heterochronic stem cell transplantation to model infant mixed lineage leukemia
R. Grant Rowe, Edroaldo Lummertz da Rocha, Patricia Sousa, Pavlos Missios, Michael Morse, William Marion, Jessica Barragan, Ronald Mathieu, Trista North, George Q. Daley
Boston Children's Hospital, Boston, MA, United States
Background: B-cell acute lymphoblastic leukemia (B-ALL) of infancy, diagnosed before 12 months of age, is a unique entity that often shows hybrid B-lymphoid/myeloid differentiation and bears a poor prognosis compared to B-ALL of later childhood. We hypothesized that the young developmental age of the hematopoietic microenvironment drives the unique biology of infant B-ALL.
Methods: To test this hypothesis, we used heterochronic transplantation in mice to vary the relative age of the cell-of-origin relative to the hematopoietic microenvironment. We transduced adult BM hematopoietic stem and progenitor cells (HPSCs) with the MLL-AF9 oncogene and transplanted these cells into either neonates (P0-P1) or adults. We analyzed differentiation by flow cytometry and morphology. We analyzed gene expression profiles in leukemic cells by RNA sequencing.
Results: We found that transplantation of MLL-AF9-transformed BM HSPCs into neonatal recipients resulted in leukemia containing a small (3.5 ± 1%) population of cells expressing the B-cell marker B220 with morphologic lymphoid differentiation, with transplantation into adults yielded pure acute myeloid leukemia (AML). Serial transplantation of this neonatal leukemia through neonatal recipients decreased the latency of disease onset to as short as 20 days, coincident with expansion of the B220+ component (to 45 ± 4% in tertiary recipients) and infiltration of thymus, lymph nodes, and testes. This mixed B-lymphoid/myeloid leukemia bore an immunophenotype and transcriptional profile paralleling human MLL-AF9-driven B-ALL.
Serial transplantation of neonatal leukemia near limiting dilution suggested the existence of a bipotent LSC capable of producing both leukemic B-lymphoblasts and myeloblasts. By using fluorescence activated cell sorting, we identified neonatal leukemia cells expressing Flk2 - a marker of lymphoid commitment - within the LSC-enriched LGMP compartment (Lin- kit-lo CD16/32+ CD34+). Flk2+ LGMPs are primed to produce both B-lymphoid and myeloid cells in clonogenicity assays, while Flk2- LGMPs are strongly myeloid-biased (P < 0.05). By screening for differentially expressed cytokines between adult and neonatal BM stroma, we implicated the chemokine Ccl5 as a factor restraining B-lymphoid commitment in pro-myeloid adult niches. We further found that Ccl5 regulates leukemia lineage fate by interfacing with the GSK3 signaling pathway.
Conclusions: Our data show that the neonatal hematopoietic microenvironment supports infant-like B-ALL, while an adult niche promotes pure AML from identical cells of origin, demonstrating the importance of the age of the hematopoietic microenvironment in defining leukemia lineage. These results extend previous observations that the lineage fate of MLL-driven leukemia is sensitive to cytokine signals (Cancer Cell 13: 483 [2008] and Science 316:600 [2007]). Moreover, we have derived a novel model of infant B-ALL that recapitulates the lineage phenotype and transcriptome of the human disease, with onset of leukemia prior to weaning age in mice.
Conflict of interest: All authors have nothing to disclose
O016 Trends in allogeneic stem cell transplantation outcomes for acute myeloid Leukemia patients. The experience of the Acute Leukemia Working Party of the EBMT
Jonathan Canaani1, Eric Beohou2, Myriam Labopidn2, Ardeshir Ghavamzadeh3, Dietrich Beelen4, Rose-Marie Hamladji5, Dietger Niederwieser6, Liisa Volin7, Miroslaw Markiewicz8, Renate Arnold9, Ghulam Mufti10, Gerhard Ehninger11, Gerard Socié12, Nicolaus Kröger13, Mohamad Mohty2, Arnon Nagler2,14
1Chaim Sheba Medical Center, Hematology Division, Ramat Gan, Israel; 2EBMT Paris Study Office, Paris, France; 3Shariati Hospital, Hematology-Oncology and BMT Research, Teheran, Iran, Islamic Republic of; 4Essen University Hospital, Hematology Division, Essen, Germany; 5Centre Pierre et Marie Curie, Service Hématologie Greffe de Moelle, Alger, Algeria; 6University Hospital Leipzig, Leipzig, Germany; 7HUCH Comprehensive Cancer Center, Helsinki, Finland; 8Silesian Medical Academy, Katowice, Poland; 9Charité Universitaetsmedizin Berlin, Berlin, Germany; 10GKT School of Medicine, London, United Kingdom; 11Universitaetsklinikum Dresden, Dresden, Germany; 12Hopital St. Louis, Hematology Division, Paris, France; 13University Hospital Eppendorf, Hamburg, Germany; 14Chaim Sheba Medical Center, Ramat Gan, Israel
Background: Outcomes for patients (pts) with acute myeloid leukemia (AML) have significantly improved over the past three decades resulting from both improved supportive care and the introduction of allogeneic stem cell transplantation (allo-SCT) into the routine care of AML patients in the early 1990s. In this analysis we wanted to assess the incremental improvement of transplanted AML pts in the last two decades and determine whether the year of transplant was an independent prognosticator of outcome in this patient population.
Methods: Patients included in this analysis were adult AML pts who underwent allo-SCT from a HLA matched sibling donor or matched unrelated donor in first remission and whose clinical outcomes were captured by the multicenter registry of the Acute Leukemia Working Party of the EBMT. Patients were grouped into three cohorts according to the year of transplant (1993–2002, 2003–2007, and 2008–2012) and respective outcomes were compared between groups.
Results: The analysis comprised a total of 20188 pts of whom 4763 were transplanted between 1993–2002, 5853 in 2003–2007, and 9590 in 2008–2012. Pts transplanted in the 1990s were more likely to be younger compared to the more recent counterparts (median age of 38 years compared to 45 and 50; P < 0.0001). Conversely, the donors of pts transplanted in the 1990s also tended to be younger (median age of 38 years compared to 41 and 40; P < 0.0001). In addition, this group was characterized almost exclusively by the use of matched sibling donors (90% compared to 73% and 57%; P < 0.0001). In multivariate analysis, leukemia free survival rates were significantly improved in more recently transplanted pts compared to pts transplanted in 1993–2002 [Hazard ratio (HR) = 0.84, confidence interval (CI) 95%, 0.77–0.92; P = 0.003], a benefit which also extended to improved overall survival (OS) (HR = 0.8, CI 95%, 0.73–0.89; P < 0.0001), and decreased non-relapse mortality (NRM) rates (HR = 0.65, CI 95%, 0.56–0.75; P < 0.0001). Notably, the incidence of relapse has not significantly changed over the past 2 decades in transplanted AML pts (HR = 0.99, CI 95%, 0.88–1.12; P = 0.8). Finally, the rates of acute graft versus host disease (GVHD) (HR = 0.74, CI 95%, 0.59–0.92; P = 0.008) as well as those of GVHD-free, relapse-free survival (GRFS) (HR = 0.83, CI 95%, 0.76–0.9; P < 0.0001) significantly improved in more recently transplanted pts.
Conclusions: Outcome of allo-SCT for AML patients has markedly improved in the last two decades owing to decreased non-relapse mortality and improved rates of leukemia-free survival resulting in significantly longer survival. In contrast, the incidence of relapse has not significantly changed and it remains the main obstacle for a successful allo-SCT in AML. Future efforts should thus focus on preventing leukemic relapse post transplantation by targeted agents or cellular based therapies.
Clinical Trial Registry: N/A
Conflict of interest: All authors declare they have nothing to disclose.
O017 Gene-edited allogeneic CAR19 T cells (UCART19) induce molecular remission ahead of allo-SCT in high risk pediatric patients with CD19+ relapsed/refractory B-cell acute lymphoblastic leukemia
Waseem Qasim1, Oana Ciocarlie1, Stuart Adams1, Sarah Inglott1, Claire Murphy1, Christine Rivat1, Gary Wright1, Giovanna Lucchini1, Juliana Silva1, Kanchan Rao1, Amina Zinaï2, Florence Binlich2, Sandra Dupouy2, Jeanne Pauly2, Svetlana Balandraud2, Flavie Simon2, Cyril Konto3, Candy Bermingham3, Robert Chiesa1, Sujith Samarasinghe1, Havinder Hara1, Alayna Boyle1, Jan Chu1, Danielle Pinner1, Persis J Amrolia1, Ajay Vora1, Anupama Rao1, Philip Ancliffe1, Paul Veys1
1Great Ormond Street Hospital NHS Trust, London, United Kingdom; 2Institut de Recherches Internationales Servier, Suresnes, France; 3Pfizer Inc, San Francisco, CA, United States
Background: UCART19 (anti-CD19 scFv- 41BB- CD3ζ) is a genetically modified CAR T-cell product manufactured from healthy donor cells, in which TRAC and CD52 genes have been disrupted to allow administration in non-HLA matched patients. Preliminary results of an ongoing Phase I study in CD19+ R/R B-ALL pediatric pts (PALL) are presented.
Methods: Pediatric pts (≥ 6 months to <18 years) who had exhausted treatment options and exhibited morphological disease or minimal residual disease (MRD) ≥1x10−3 were lymphodepleted using a regimen comprising high-intensity fludarabine-cyclophosphamide, and alemtuzumab. A fixed dose of UCART19 (2x107 total cells or 1.1 to 2.3x106 cells/kg) in 4 different weight-bands was infused on Day 0. Safety and ability of UCART19 to achieve molecular remission at Day 28 were assessed as primary and secondary objectives, respectively.
Results: As of October 13 2017, 5 children (3 males and 2 females) between 10 months and 16.4 years have been treated. Of the 5 pts, two had previously undergone allo-SCT but had relapsed with CD19+ B-ALL. Prior to lymphodepletion, 4 patients exhibited < 10% blasts and 1 had 80% blasts, albeit with a hypoplastic marrow. All pts experienced reversible cytokine release syndrome (CRS) between D4-D8 (1 grade (G) 1, 3 G2, 1 G3). CRS G3 required 2 doses of tocilizumab. Acute skin GvHD G1 was confirmed by biopsy and recovered with topical steroids in one patient. Four children experienced viral reactivation (CMV, ADV, BK, Metapneumovirus) after lymphodepletion and 2/5 pts remained neutropenic by D28.
All patients achieved a CRi at D28-D42 with 5/5 confirmed MRD negative (< 0.01%) by flow cytometry and 3/5 MRD negative by PCR. All underwent a subsequent allo-SCT, between 49 and 62 days after UCART19 infusion with conditioning incorporating TBI (2–14.4Gy), fludarabine, +/- cyclophosphamide with or without ATG. All pts also received a single dose of rituximab, to target any remaining UCART19 cells. Two children relapsed 3 months after transplantation (one CD19- and one CD19+; both MRD positive by PCR prior to SCT), and died 7 and 8 months after UCART19 infusion, respectively. One patient died 2.5 months after MSD allo-SCT from transplant-related complications (thrombotic microangiopathy, BK hemorrhagic cystitis and nephritis). Two children remain in CR > 5 months post-transplant and continue to be monitored.
Conclusions: In addition to the two patients successfully treated under special access scheme before the trial and now followed for > 24 months, a further five children with high risk R/R B-ALL have been treated in this study with UCART19 before proceeding to allo-SCT. Preliminary safety data were within expectations, and 2/5 trial patients are in remission. The study is open and recruiting at multiple sites.
Clinical Trial Registry: NCT02808442
Conflict of interest:
W. Qasim: Autolus Ltd, consultancy and equity ownership; Orchard Therapeutics, consultancy and equity ownership; Servier, research funding; Cellectis, research funding; Bellicum, research funding; Miltenyi, research funding; NlHR, research funding
G. Lucchini: Alexion, membership on an entity’s board of directors or advisory committees
A. Zinaï, F. Binlich, S. Dupouy, J. Pauly, S. Balandraud, F. Simon: Servier, employment
C. Konto: Pfizer: employment and equity ownership; Bristol-Myers Squibb, employment and equity ownership
C. Bermingham: Pfizer: employment and equity ownership
O. Ciocarlie, S. Adams, S. lnglott, C. Murphy, C. Rivat, G. Wright, J. Silva, K. Rao, R. Chiesa, S. Samarasinghe, H. Hara, A. Boyle, J. Chu,D. Pinner, P. J. Amrolia, A. Vora, A. Rao, P. Ancliffe: nothing to disclose
P. Veys: Servier, research funding; Bellicum, research funding
O018
Abstract previously published
O019
Abstract previously published
O020
Abstract previously published
O021 Allo-HSCT for Core Binding Factor AML (t(8;21) or inv(16)/t(16;16)) in Second Complete Remission: Report from the Acute Leukemia Working Party of the EBMT
Kazimierz Halaburda1, Myriam Labopin2, Audrey Mailhol2, Gerard Socié3, Charles Craddock4, Mahmoud Aljurf5, Dietrich Beelen6, Jan Cornelissen7, Jean-Henri Bouhris8, Hélène Labussière-Wallet9, Didier Blaise10, Tobias Gedde-Dahl11, Maria Gilleece12, Ibrahim Yakoub-Agha13, Ghulam Mufti14, Jordi Esteve15, Arnon Nagler16
1Institute of Haematology and Transfusion Medicine, Stem Cell Transplantation, Warsaw, Poland; 2Universite Pierre et Marie Curie, Hopital Saint Antoine, ALWP of the EBMT, Paris, France; 3Hopital St. Louis, Paris, France, APHP, Paris, France; 4Queen Elizabeth Hospital Birmingham, Birmingham, United Kingdom; 5King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia; 6University of Essen, Essen, Germany; 7Erasmus MC Cancer Institute, University Medical Center, Rotterdam, Netherlands; 8Gustave Roussy Cancer Center, Villejuif, France; 9Centre Hospitalier Lyon Sud, Lyon, France; 10Institut Paoli Calmettes, Marseille, France; 11Oslo University Hospital, Oslo, Norway; 12Leeds Teaching Hospital, Leeds, United Kingdom; 13Hospital CHU de Lille, LIRIC, INSERM U 995, Université de Lille, Lille, France; 14GKT School of Medicine, London, United Kingdom; 15Hospital Clinic,Institute of Hematology & Oncology, Barcelona, Spain; 16Chaim Sheba Medical Center, ALWP of the EBMT, Tel Hashomer, Israel
Background: In CBF AML, AlloHSCT is considered for patients beyond CR1. The aim of the study was to evaluate results of AlloHSCT in CR2 and factors influencing outcomes.
Methods: We analyzed 631 patients transplanted between 2000 and 2014 from related(42%) or unrelated donors(58%) reported to the EBMT. The primary end-point was 2-year LFS. The secondary end-points were OS, RI, NRM and GRFS.
Results: The median age of patients was 42(18–75); 366(58%) were diagnosed with inv(16)/t(16;16), 265(42%) with t(8;21) AML and in 134 additional chromosome abnormalities were reported with ≥3 abnormalities(≥3 abn) being most frequent (32pts, 5%). The median duration of CR1 before relapse was 318 days(6–2380) and time from diagnosis to transplantation was 17 months(4–223). Transplants from matched related and unrelated donors constituted 264(42%) and 367(58%) cases respectively and were performed after MAC (424-68%) or RIC (204-32%) with PBSC (514-81%) or BM (117-19%). At transplantation Karnofsky performance score(KPS) was < 80 in 16 patients. In vivo T-cell depletion(TCD) was given to 325 patients. All patients were in hematological CR2 with < 5% blasts in BM. Molecular remission(MR) was achieved in 343(73%), while 125(27%) were transplanted with no MR (163-missing data). The median follow-up for surviving patients was 60 months (0.9–201). 2y-LFS probability was 59.1%, OS 65%, RI 19.8%, NRM 20.9%, GRFS 40.2%, aGvHD gII-IV 28%, aGvHD gIII-IV 9.5% cGvHD 46.7%. Respective 5-y probabilities for LFS, OS, RI, NRM, GRFS and cGvHD were 54.1%, 58.2%, 22.5%, 23.3%, 34.6% and 48.3%. In multivariate analysis independent significant factors for LFS were type of AML (t(8;21) vs. inv(16)/t(16;16)), presence or absence of ≥3 abn(p = 0.022, HR = 1.398 and p = 0.004, HR = 2.089 respectively) and KPS > vs.≤80(p = 0.006, HR = 0.32) while for MR vs. noMR a trend was seen(p = 0.08, HR = 0.755). Factors influencing OS were: t(8;21) vs. inv(16)/t(16;16)(p = 0.00002, HR = 1.755), ≥3 abn vs. no≥3 abn (p = 0.037, HR = 1.675) and KPS> vs.≤80(p = 0.002, HR = 0.359. Independent factors for RI were t(8;21) vs. inv(16)/t(16;16)(p = 0.002. HR = 1.89), ≥3 abn vs. no≥3 abn (p = 0.011, HR = 2.311), time from diagnosis to transplant > vs. ≤median(p = 0.023, HR = 0.967), RIC vs. MAC(p = 0.017, HR = 1.64) and MR vs. noMR(p = 0.043, HR = 0.646). NRM depended on KPS(p = 0.001, HR 0.288). GRFS was significantly influenced by ≥3 abn vs. no≥3 abn (p = 0.031, HR = 1.06) and TCD vs. noTCD(p = 0.027, HR = 0.763) while there was a trend for increased GRFS in patients with MR and decreased in case of donor CMV seropositivity. Type of conditioning (RIC vs. MAC) was significant for aGvHD gII-IV(p = 0.011, HR = 0.637). For cGvHD important factors were: TCD vs. noTCD(p < 0.00001, HR = 0.555), PB vs. BM(p = 0.003, HR 1.72) and donor CMV seropositivity(p = 0.004, HR 1.45).
Conclusions: Favorable outcomes seen in the study support postponing AlloHSCT in CBF AML until CR2. Better results are seen in patients with inv(16)/t(16;16) compared to t(8;21). The outcomes were most strongly dependent on type of CBF AML, performance status and presence of ≥3 abn. RI was additionally affected by conditioning intensity and time from diagnosis to transplant. KPS was significant for LFS, OS and NRM. MR decreased risk of relapse with a trend for improved LFS and GRFS without affecting OS, indicating effectiveness of alloHSCT even in patients not achieving MR before transplantation.
Conflict of interest: None of the authors has anything to disclose.
O022 The Superiority of Haplo-HSCT over Chemotherapy for AML Patients with Intermediate Risk Cytogenetics in CR1—Prospective, Landmark Analysis of the Results from ChiCTR-OCH-10000940 Trial
Meng Lv, Yu Wang, Ying-Jun Chang, Xiao-Hui Zhang, Lan-Ping Xu, Xiao-Jun Huang
Peking University People's Hospital, Beijing, China
Background: In the present prospective trial we investigated whether human leukocyte antigen (HLA) haplo-HSCT has a favorable impact on survival as post remission treatment for acute myeloid leukemia (AML) patients with intermediate (int)-risk cytogenetics in the first complete remission (CR1) by comparing with chemotherapy alone.
Methods: For AML patients with int-risk cytogenetics in CR1, HLA matched sibling (MSD) was the first choice for allogeneic HSCT. If MSD was unavailable, subjects without a suitable HLA-matched unrelated donor (≥8/10 URD) after 2 cycles of consolidation were eligible for haplo-HSCT or further chemotherapy. To exclude bias that may arise from including patients who relapsed or died too early to receive haplo-HSCT in CR1, landmark analysis (4 months after CR1) was used when comparing the outcomes of patients receiving haplo-HSCT vs. chemotherapy. The survival functions were estimated using Kaplan-Meier method with log-rank test, cumulative incidence of relapse (CIR) and treatment related mortality (TRM) were calculated using competing risks, Cox model was tested with patient age, sex, WBC count at diagnosis, cytogenetic (normal or other int risk), courses to achieving CR1 (≤2 courses or not), molecular risk group (NCCN favorable, int or poor).
Results: 355 newly diagnosed AML patients (age 15–60 years old) with int-risk cytogenetics were consecutively enrolled at Peking University People's Hospital between July 2010 and June 2014. Patients were excluded in survival analysis: 1) induction failure or death during induction (n = 36); 2) early TRM (n = 2), relapse (n = 32) or withdraw from trial (n = 16) within 4 months after CR1; 3) MSD-HSCT (n = 49) or URD-HSCT (n = 7). The remaining patients (n = 213) were divided into haplo-HSCT group (n = 99) or chemotherapy group (n = 114). The 5-year overall survival (OS) and Leukemia-free survival (LFS) of the Haplo-HSCT group was significantly higher than the chemotherapy group (73.1% ± 6.9% vs 35.7% ± 5.6%, p < 0.0001; 71.1% ± 7.0% vs 34.8% ± 5.7%, p < 0.0001) (Figure). In multivariate analysis, the risk ratios of OS were 1.958 (95% CI, 1.211–3.163; p < 0.001) for chemotherapy compared with Haplo-HSCT, 1.858 for other cytogenetic abnormalities vs normal (95% CI,1.100–3.136; p = 0.02) and 1.712 for age>40 vs ≤40 (95% CI, 1.034–2.836; p = 0.037). Chemotherapy vs Haplo-HSCT and/or cytogenetic abnormalities were also independent factors affecting LFS, relapse and TRM (Table). In multivariate analysis, patient age, sex, WBC count at diagnosis, courses to achieving CR1, NCCN molecular risk group did not influence the OS, LFS, relapse rate and TRM.
Conclusions: Our results suggest that Haplo-HSCT is superior to chemotherapy alone as post-remission treatment for AML patients with int-risk cytogenetics in CR1.
Clinical Trial Registry: ChiCTR-OCH-10000940;http://www.chictr.org.cn/showprojen.aspx?proj=8598
Conflict of interest: All authors have nothing to disclose.
| Parameter | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
| OS | Chemotherapy vs Haplo-HSCT | 2.447 | 1.532–3.908 | p<0.001 | 1.958 | 1.211–3.163 | p = 0.006 |
| Age > 40 vs <40 | 2.344 | 1.492–3.683 | p<0.001 | 1.712 | 1.034–2.836 | p = 0.037 | |
| Cytogenetic Other vs Normal | 2.091 | 1.296–3.373 | p = 0.002 | 1.858 | 1.100–3.136 | p = 0.02 | |
| DFS | Chemotherapy vs Haplo-HSCT | 2.595 | 1.657–4.064 | p<0.001 | 2.529 | 1.612–3.965 | p<0.001 |
| Cytogenetic Other vs Normal | 1.802 | 1.133–2.865 | p = 0.013 | 1.688 | 1.06–2.688 | p = 0.027 | |
| Relapse | Chemotherapy vs Haplo-HSCT | 9.399 | 4.26–20.737 | p<0.001 | 9.399 | 4.26–20.737 | p<0.001 |
| TRM | Chemotherapy vs Haplo-HSCT | 0.321 | 0.136–0.756 | p = 0.009 | 0.300 | 0.127–0.709 | p = 0.006 |
| Cytogenetic Other vs Normal | 2.596 | 1.193–5.651 | p = 0.016 | 2.840 | 1.304–6.185 | p = 0.009 | |
[ [O022 Table] Table]
[O022 Figure].
[Overview of patients and OS/DFS]
O023 Influence of patient, disease and transplant characteristics on post transplant outcomes in FLT3 mutated AML: a report from the EBMT acute leukemia working party
Ali Bazarbachi1,2, Myriam Labopin3,4,5, Giorgia Battipaglia3, Azedine Djabali3,4, Edouard Forcade6, William Arcese7, Gerard Socié8, Didier Blaise9, Jakob Passweg10, Jan J Cornelissen11, Patrice Chevallier12, Johan Maertens13, Nicolaas Schaap14, Khowla Hashaishi4, Jean El Cheikh1, Jordi Esteve4,15, Arnon Nagler4,16, Mohamad Mohty3,4,5
1Bone Marrow Transplantation Program, Department of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon; 2American University of Beirut, Department of Cell Biology, Anatomy and Physiological Sciences, Beirut, Lebanon; 3Hôpital Saint Antoine, Service d'Hématologie et Thérapie Cellulaire, Hematology Department, Paris, France; 4Acute Leukemia Working Party of EBMT, Paris, France; 5Hopital Saint Antoine, Universite Pierre & Marie Curie, INSERM, UMRs 938, Paris, France; 6CHU Bordeaux Hôpital Haut-leveque, Pessac, France; 7Tor Vergata University of Rome, Stem Cell Transplant Unit, Policlinico Universitario Tor Vergata, Rome, Italy; 8Hopital St. Louis, Dept.of Hematology - BMT, Paris, France; 9Centre de Recherche en Cancérologie de Marseille, Institut Paoli Calmettes, Programme de Transplantation & Therapie Cellulaire, Marseille, France; 10University Hospital, Hematology, Basel, Switzerland; 11Erasmus MC Cancer Institute, University Medical Center Rotterdam, 12. Department of Hematology, Rotterdam, Netherlands; 12CHU Nantes, Departement d'Hematologie, Nantes, France; 13University Hospital Gasthuisberg, Dept. of Hematology, Leuven, Belgium; 14Nijmegen Medical Centre, Department of Hematology, Nijmegen, Netherlands; 15IDIBAPS, Hospital Clinic, Hematology Department, Barcelona, Spain; 16Chaim Sheba Medical Center, Department of Bone Marrow Transplantation, Tel Hashomer, Israel
Background: FLT3 gene mutations represent one of the most frequently observed genetic alterations in AML, with an incidence of approximately 30%. The prognosis of AML patients with FLT3-ITD is generally dismal. It is recommended that patients harboring this mutation undergo an allogeneic hematopoietic cell transplantation (allo-HCT) in first complete remission whenever possible. However, long-term survival remains poor as a result of the high rate of early relapse and the lack of response to further treatment. The purpose of this study was to assess the influence of patients, disease and transplant characteristics on post-transplant outcomes in FLT3 mutated AML.
Methods: We identified 462 adult patients (49% females; median age 50 years; range 18–75) with FLT3 mutated AML (FLT3 ITD-437; FLT3 TKD-11; 14 both) allografted between 2010 and 2015 from a matched related (40%), matched unrelated (49%) or haploidentical donor (11%) at EBMT participating centers. Karyotype was intermediate risk in 82% and NPM1 was mutated in 55% of patients with available data. Most patients (71.5%) were transplanted in first complete remission (CR1), 10.5% in CR2 and 18% with active disease. A second induction was given in 38% of patients and 75% received consolidation therapy. At time of transplant, for patients in CR, 61 were minimal residual disease (MRD) positive, 150 MRD negative, 150 not evaluated and 16 missing. Conditioning was myeloablative (MAC) in 53% of patients and reduced intensity (RIC) in 47%. In vivo T cell depletion graft was given to 285 (62%) of patients and 83% received peripheral blood stem cells. Most patients (63%) and donors (55%) were CMV positive. Nineteen percent of patients were males with a female donor. Pre-transplant sorafenib was given to 9 patients during induction, 10 patients during consolidation and 8 patients for salvage whereas 28 patients received post-transplant sorafenib maintenance. Median follow-up of alive patients was 39 months (range 1–87).
Results: Day 100 acute GVHD grade II-IV and grade III-IV were encountered in 26% and 9% of patients, respectively whereas the 2 year cumulative incidence of chronic and extensive chronic GVHD were 34% and 16%, respectively. The 2-year cumulative incidence of relapse (CIR) and non-relapse mortality (NRM) was 34% and 15%, respectively. The 2-year leukemia free survival (LFS), overall survival (OS) and GVHD relapse free survival (GRFS) was 51%, 59% and 38%, respectively. In multivariate Cox analysis, NPM1 mutation, transplantation in CR1, in vivo T cell depletion, and sorafenib maintenance significantly improved OS whereas the need for more than one induction negatively affected OS. Similarly, NPM1 mutation (HR = 0.66; p = 0.002), the use of a haploidentical donor compared to matched sibling donors (HR = 0.61; p = 0.04), in vivo T cell depletion (HR = 0.55; p = 0.00001), and sorafenib maintenance (HR = 0.44; p = 0.02) significantly improved GRFS whereas the need for more than one induction (HR = 1.5; p = 0.005) and active disease at transplant (HR = 2.5; p < 10−5) negatively affected it.
Conclusions: FLT3 mutated AML remains a challenge even following allo-HCT. Post- transplant maintenance with sorafenib appears to significantly improve OS and GRFS, and may be considered as standard of care in that setting.
Conflict of interest: nothing to disclose
Arnon Nagler and Mohamad Mohty are equal contributors.
O024 Alternative donor transplantation in patients with active acute leukemia at transplant (GANDALF): final analysis of a prospective Study from Gruppo Italiano Trapianto Midollo Osseo (GITMO)
Fabio Ciceri1, Paolo Bernasconi2, Alessandra Picardi3, Anna Paola Iori4, Maria Teresa Van Lint5, Cristina De Pau6, Stefano Guidi7, Alessandro Busca8, Angelo Michele Carella9, Irene Cavattoni10, Jacopo Peccatori11, Fabio Giglio11, Daniela Cilloni12, Giuseppe Console13, Paolo Corradini14, Francesco Onida15, Giovanni Grillo16, Anna Proia17, Giuseppe Milone18, Roberto Sorasio19, Alessandra Crescimanno20, Franco Narni21, Francesca Patriarca22, Francesco Zallio23, Matteo Parma24, Giovanni Pisapia25, Stella Santarone26, Rosanna Scimè27, Simona Sica28, Paola Carluccio29, Adriana Vacca30, Daniele Vallisa31, Arianna Masciulli32, Chiara Pavoni33, Sonia Mammoliti34, Corrado Girmenia4, Francesca Bonifazi35, Alessandro Rambaldi33
1San Raffaele Scientific Institute, Hematology, Milan, Italy; 2IRCCS Policlinico S Matteo, Pavia, Italy; 3Policlinico Tor Vergata, Roma, Italy; 4Policlinico Umberto I, Roma, Italy; 5IRCCS AUO IST- San Martino, Genova, Italy; 6Ospedale Oncologico Businco, Cagliari, Italy; 7Ospedale Careggi, Firenze, Italy; 8AO Citta della Salute e della Scienza, Torino, Italy; 9Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy; 10Comprensorio Sanitario di Bolzano, Bolzano, Italy; 11IRCCS San Raffaele Scientific Institute, Milano, Italy; 12Centro Trapianti Metropolitano, Torinio, Italy; 13AZ. Osp. Bianchi Melacrino Morelli, Reggio Calabria, Italy; 14Fondazione IRCCS Istituto Nazionale dei Tumori, Milano, Italy; 15IRCCS AUO IST- San MartinoIRCCS Ca' Granda Ospedale Maggiore Policlinico, Milano, Italy; 16Ospedale Niguarda, Milano, Italy; 17AO San Camillo Forlanini, Roma, Italy; 18Ospedale Ferrarotto - Università degli Studi di Catania, Catania, Italy; 19Ospedale Cuneo, Cuneo, Italy; 20Ospedale La Maddalena, Palermo, Italy; 21Università Modena, Modena, Italy; 22Azienda Ospedaliero-Universitaria 'Santa Maria della Misericordia', Udine, Italy; 23AO SS Antonio e Biagio, Alessandria, Italy; 24Ospedale San Gerardo, Clinica Ematologica dell`Universita Milano-Bicocca, Monza, Italy; 25Ospedale San Giuseppe Moscato, Taranto, Italy; 26Ospedale Civile, Pescara, Italy; 27AO Ospedali Riuniti Villa Sofia - Cervello, Palermo, Italy; 28Policlinico Gemelli, Univ. Cattolica Sacro Cuore, Roma, Italy; 29Policlinico Consorziale di Bari, Bari, Italy; 30PO R. Binaghi, Cagliari, Italy; 31Ospedale G. Da Saliceto di Piacenza, Piacenza, Italy; 32AO Papa Giovanni XXIII, Bergamo, Italy; 33Ospedale Papa Giovanni XXIII, Bergamo, Italy; 34IRCCS H San Martino, Genova, Italy; 35Istituto Seragnoli, Bologna, Italy
Background: Patients with acute leukemia refractory to initial or salvage chemotherapy have dismal prognoses if they do not undergo allogeneic hematopoietic stem-cell transplantation (allo-HSCT). However, the real benefit of transplantation for patients not in complete remission (CR) at time of transplant is still controversial.
Methods: Across GITMO centres, a treatment algorithm was promoted including an alternative donor search early in the treatment plan of adults patients with Primary Induction Failure (PIF) or relapsed acute leukemia (rel). Patients without an available unrelated donor received a transplant from CBT or family haploidentical donor (GANDALF-01 trial, Gitmo Against Non-responding anD Acute Leukemia Failures; Eudract 2012-004008-37). Adult patients with acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), were candidate to receive allo-HSCT from alternative donor for active leukemia. Conditioning regimen was planned homogeneous in all 3 donor sources, based on Thiotepa 10 mg/kg, Busulfan iv 9,6 mg/kg, fludarabine (150 mg/m2) (TBF regimen). The primary study end-point was 2-years overall survival with an intention-to-treat analysis.
Results: From July 2013 to October 2014, 101 patients (93 AML, 8 ALL with 48 PIF, 53 rel status) were prospectively enrolled and an unrelated donor search started. Median age was 54 (16–69), median time diagnosis-inclusion was 51 days; 87/101 received allo-HSCT and median time inclusion-transplant was 18 days (5–179). Donor was 10/10 matched unrelated in 27, mismatched unrelated in 24, CB in 6 and haplo in 33. Source of graft was BM in 37, PB in 44, CB in 6.
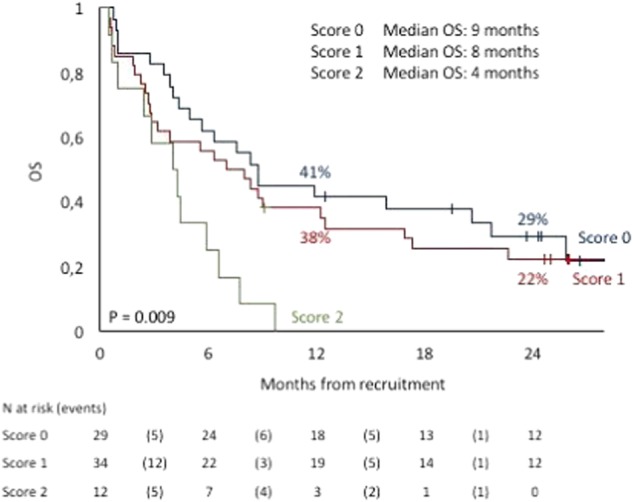
Kaplan-Meyer estimates for Overall Survival were 36% at 1 y and 18% at 2 years for the entire patient population. Relapse incidence was 38% (1y) and 50% (2y); overall transplant-related mortality 35% at 2y. All figures were with no difference according to donor sources. Outcome data analyzed according to GITMO score (Todisco et al, BMT 2017), showed a dismal prognosis in patients with score-2 (Figure 1), with an OS of 22%, 29% at 2 years, 38% and 41% at 1 year in patients with score-1 and score-0 respectively.
Conclusions: This prospective multicenter study confirms that allo-HSCT is a potential curative option in patients with acute leukemia active at transplant. An early registry search and an algorithm including haploidentical and CB provide a suitable donor in a large proportion of patients in need in a due time for transplantation. CIBMTR score >2 at transplant identify patients without a clinical benefit.
Clinical Trial Registry: Eudract 2012-004008-37
Conflict of interest: nothing to disclose
[ [O024 Figure] Figure 1]
O025 Stem cell transplantation from unrelated cord blood or haploidentical donor grafts in adult patients with secondary acute myeloid leukemia, comparative study from Eurocord and ALWP-EBMT
Annalisa Ruggeri1, Myriam Labopin1, Bipin Savani2, Didier Blaise3, Fernanda Volt4, Fabio Ciceri5, Andrea Bacigalupo6, Johanna Tischer7, Patrice Chevallier8, Yener Koc9, Jan Cornelissen10, Gerhard Ehninger11, Guillermo Sanz12, Eric Deconinck13, Annalisa Paviglianiti4, Vanderson Rocha4, Frederic Baron14, Mohamad Mohty1, Eliane Gluckman4, Arnon Nagler15
1Saint Antoine, Paris, France; 2BMT, Nashville, TN, United States; 3IPC, Marseille, France; 4Eurocord, Paris, France; 5San Raffaele Scientific Institute, Milano, Italy; 6Ospedale San Martino, Genova, Italy; 7BMT, Muenchen, Germany; 8BMT, Nantes, France; 9BMT, Antalya, Turkey; 10BMT, Rotterdam, Netherlands; 11BMT, Dresden, Germany; 12BMT, Valencia, Spain; 13BMT, Besancon, France; 14BMT, Liege, Belgium; 15Sheba University, Tel Aviv, Israel
Background: Survival of patients with secondary acute myeloid leukemia (sAML) is poor and allogeneic hematopoietic cell transplantation (HSCT) is a curative option.
For patients who do not have an HLA matched sibling or unrelated donor, cord blood transplantation (UCBT) and non T-cell depleted stem cell transplantation from haploidentical donors (HAPLO) are possible alternatives. Both strategies have shown encouraging results in recent years.
Methods: We retrospectively compared outcomes of 409 adults with sAML (secondary to other haematological malignancies) receiving either UCBT (n = 163) or HAPLO (n = 246) in EBMT centers from 2007 to 2016.
Results: Median follow-up was 24 months for UCBT and 17 months for HAPLO. Myelodysplastic syndrome (MDS) or myeloproliferative disorder (MPD) was the primary diagnosis in 79% of UCBT and 85% of HAPLO recipients (p = 0.07). For HAPLO, the stem cell source was bone marrow (BM) in 93 and peripheral blood stem cells (PBSC) in 153. For UCBT, 59 patients received single and 104 doubleUCBT. Compared to UCBT, HAPLO were performed more recently (2014 vs. 2011, p < 0.001), recipients were older (p = 0.003) and in more advanced disease status at HSCT (active disease 50% vs. 37%, p = 0.03). Anti-thymocyte globulin (ATG) was more frequently used in UCBT (28% vs. 43%, p = 0.002).
Reduced intensity conditioning regimen was used in 59% of HAPLO and 66% UCBT. GVHD prophylaxis varied according to HSCT strategy and consisted mostly of post-transplant cyclophosphamide (PT-CY) in 66% of HAPLO and CyclosporineA+ mycophenolate-mofetil in 72% of UCBT. Cumulative incidence (CI) of neutrophil engraftment was 88% for HAPLO and 84% for UCBT (p = 0.003). CI of grade II-IV acute-GVHD was 23% and 33% (p = 0.018) and CI of chronic-GVHD was 26% and 26% (p = 0.706) for HAPLO and UCBT, respectively. CI of relapse incidence (RI) was 30% in HAPLO and 33% in UCBT (p = 0.380); while non-relapse mortality (NRM) was 34% and 41%, p = 0.711, for HAPLO and UCBT, respectively. The 2-year leukemia-free-survival (LFS), and overall survival (OS) was not different among the 2 groups, being 36% and 41% for HAPLO and 26% and 29% for UCBT recipients, respectively (p = 0.235 and 0.246).
In multivariate analysis (adjusted for age, disease status, year of HSCT, recipient and donor CMV serology, conditioning regimen, use of ATG, and center), UCBT was associated with higher risk of grade II-IV acute GVHD (HR 1.9, p = 0.009) and lower GHVD-free-relapse-free-survival (GRFS) (HR 1.57, p = 0.007) compared to HAPLO. Other outcomes namely, chronic-GVHD, RI, NRM, LFS and OS, were not statistically different between the two groups.
Early disease stage at transplant was independently associated with lower RI and NRM and higher OS and LFS.
These results were confirmed also in a further multivariate model restricted to patients receiving Haplo only with PT-Cy versus UCBT.
Conclusions: In this study focusing on patients with sAML, HAPLO was associated with lower acute GVHD and better GRFS. Other outcome were no statistically significant between HAPLO and UCBT. Disease status at HSCT remains an important factor associated with outcomes. Both transplant approaches are a valid option for patients with sAML.
Conflict of interest: No conflict of interest to disclose
Aplastic anaemia
O026 Updated Treatment Algorism for Children with Acquired Aplastic Anemia: A Report from the Japan Childhood Aplastic Anemia Study Group
Nao Yoshida1, Atsushi Narita2, Hideki Muramatsu2, Ryoji Kobayashi3, Hiromasa Yabe4, Kazuko Kudo5, Hiroshi Yagasaki6, Kenichiro Watanabe7, Akira Morimoto8, Etsuro Ito9, Shouichi Ohga10, Akira Ohara11, Yoshiyuki Takahashi2, Seiji Kojima2
1Japanese Red Cross Nagoya First Hospital, Children's Medical Center, Department of Hematology and Oncology, Nagoya, Japan; 2Nagoya University Graduate School of Medicine, Department of Pediatrics, Nagoya, Japan; 3Sapporo Hokuyu Hospital, Department of Pediatrics, Sapporo, Japan; 4Tokai University School of Medicine, Department of Cell Transplantation and Regenerative Medicine, Isehara, Japan; 5Fujita Health University School of Medicine, Department of Pediatrics, Toyoake, Japan; 6Nihon University School of Medicine, Department of Pediatrics, Tokyo, Japan; 7Shizuoka Children's Hospital, Division of Hematology and Oncology, Shizuoka, Japan; 8Jichi Medical University School of Medicine, Department of Pediatrics, Shimotsuke, Japan; 9Hirosaki University Graduate School of Medicine, Department of Pediatrics, Hirosaki, Japan; 10Graduate School of Medical Sciences, Kyushu University, Department of Pediatrics, Fukuoka, Japan; 11Toho University School of Medicine, Department of Pediatrics, Tokyo, Japan
Background: Treatment for acquired aplastic anemia (AA) consists of immunosuppressive therapy (IST) or hematopoietic cell transplantation (HCT), both of which have improved outcomes over decades. We previously identified a combination of the absence of minor paroxysmal nocturnal hemoglobinuria clones (PNH-) and a short telomere length (sTL) as a strong predictor of poor IST response. For the patients who have small chance to respond to IST, HCT can be expanded to include donors other than matched related donors (MRD). Here, we propose an updated treatment algorithm for children with AA.
Methods: To resolve questions regarding treatment of choice for children with AA, we conducted 6 retrospective studies analyzing the outcomes of children with AA who received IST within prospective trials conducted by the Japan Childhood Aplastic Anemia Study Group or who underwent HCT registered in the Japan Society for Hematopoietic Cell Transplantation Registry.
Results: The first study confirmed an advantage for children receiving bone marrow transplantation (BMT) from MRD (n = 213) than IST (n = 386) as first-line therapy; the overall survival (OS) did not differ (92% vs. 88%), whereas failure-free survival (FFS) was significantly inferior after IST (87% vs. 56%; P < 0.001). The OS in patients receiving HCT from unrelated donors (UD) after failed IST (n = 113) was 79%. The second study indicated that BMT from 1-locus mismatched related donors (1MMRD) (n = 55) provided a comparable OS to BMT from MRD (n = 399) (94% vs. 92%). The third demonstrated excellent outcomes after upfront BMT from UD (n = 33); the OS and FFS was 97% and 86%, respectively. In the fourth study, BMT with fludarabine (FLU)/melphalan (MEL)-based regimen (n = 36) gave a better FFS than FLU/cyclophosphamide-based regimen (n = 270) (100% vs. 86%; P = 0.07). The others showed that cord blood transplantation (CBT) and haploidentical (Haplo)-HCT provided promising outcomes if the FLU/MEL-based regimen was applied; the OS was 100% in the both settings. Taking into account the predictor of IST response, we propose an algorism (Figure). Briefly, BMT from MRD/1MMRD is the treatment of choice. When a MRD/1MMRD is not available, patients with PNH- and sTL are recommended to receive upfront BMT from UD, but not IST. CBT and Haplo-HCT can be promising options.
Conclusions: The updated treatment algorithms will lead to improve overall outcomes in children with AA.
Conflict of interest: None of the authors has anything to disclose.
[O026 Figure].
TREATMENT ALGORISM FOR CHILDREN WITH AA]
O027
Abstract previously published
O028 Ex vivo T cell-depleted haploidentical HCT in children and adolescents with acquired SAA: Less graft failure in TCRαβ-depleted transplant compared to CD3-depleted transplant
Ho Joon Im, Hyery Kim, Kyung-Nam Koh, Sung Han Kang, Jae Won Yoo, Eun Seok Choi, Jong Jin Seo
University of Ulsan College of Medicine, Pediatrics, Seoul, Korea, Republic of
Background: Hematopoietic cell transplantation (HCT) is a curative therapy for acquired severe aplastic anemia (SAA). Haploidentical HCT (HHCT) from a family donor is a possible alternative for patients with SAA lacking a matched related or unrelated donor. We evaluated the outcomes of children and adolescents with acquired SAA who received haploidentical HCT (HHCT) with ex vivo T cell-depleted peripheral blood stem cells and compared their outcomes according to depletion methods.
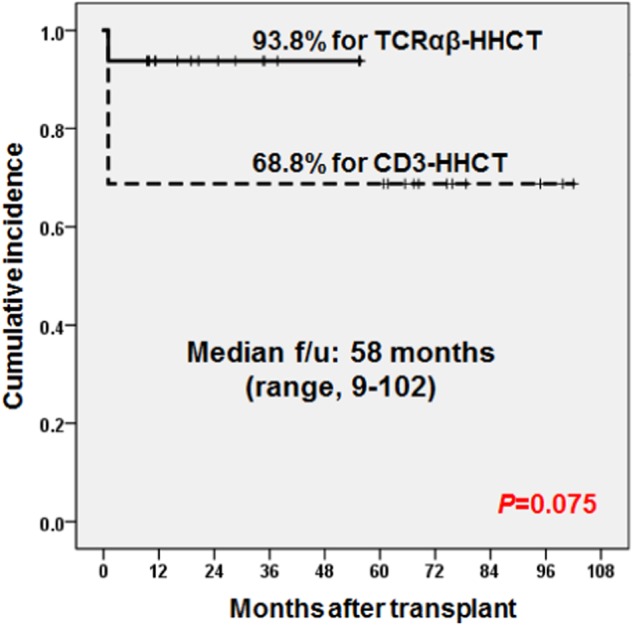
Methods: Between July 2008 and March 2017, 32 patients with acquired SAA received haploidentical hematopoietic cell transplantation (HHCT) at Asan Medical Center Children's Hospital. Sixteen patients received CD3-depleted HHCT (CD3-HHCT) and 16 received TCRαβ-depleted graft (TCRαβ-HHCT). The median age at transplant was 13 years (range 1–22 years). Failures included graft failure, transfusion dependency and death.
Results: One patient, who received CD3-HHCT, experienced primary graft failure (GF) and the remaining 31 achieved engraftment of neutrophil at a median of 10 days (range, 9–13 days). Of 16 patients who received CD3-HHCT, one patient failed to achieve primary engraftment and four experienced graft rejection (GR) soon after engraftment. All five patients who experienced early graft failure (GF) received a second HHCT and achieved sustained engraftment. No patients out of 16 who received TCRαβ-HHCT developed GF/GR. Graft failure rate was higher in CD3-HHCT than in TCRαβ-HHCT (31% vs 0%, P = 0.017). The cumulative incidences of grades 2–4 and grades 3–4 acute GVHD were 30% and 14%, respectively, which were not different between TCRαβ-HHCT and CD3-HHCT. Two patients died at 5.2 (CMV pneumonia) and 15.2 months (Pure red cell aplasia with autoimmune hemolytic anemia) post-transplant, leading to TRM of 3.1% at 6 months and 6.9% at 1 year, respectively. All survived 30 patients were transfusion independent. At a median follow-up of 58 months (range, 9–102 months), failure free survival (FFS) and overall survival (OS) at 3 years of total patients were 81.3 ± 6.9% and 93.1 ± 4.7%, respectively. FFS was better in TCRαβ-HHCT than that of CD3-HHCT (93.8 ± 11.6% vs 68.8 ± 7.4%, P = 0.075). FFS seemed to be better in TCRαβ-HHCT than that of CD3-HHCT, but OS was same regardless of depletion method.
Conclusions: Our study suggests that HCT from haploidentical family donors using ex vivo TCRαβ-depleted graft is a realistic therapeutic option for children and adolescents with acquired SAA.
Clinical Trial Registry: NCT02014506,
ClinicalTrials.gov
Conflict of interest: The authors have no conflicts of interest to declare.
[[O028 Figure] Failure-free survival according to depletion method]
O029 Allogeneic hematopoietic cell transplantation for paroxysmal nocturnal hemoglobinuria - multicenter analysis by Polish Adult Leukemia Group
Mirosław Markiewicz1, Joanna Drozd-Sokołowska2, Anna Koclęga1, Monika Dzierżak-Mietła1, Piotr Boguradzki2, Beata Piątkowska-Jakubas3, Agnieszka Piekarska4, Magdalena Tormanowska5, Kazimierz Hałaburda5, Marek Ussowicz6, Grzegorz Basak2, Anna Waszczuk-Gajda2, Łukasz Bołkun7, Justyna Rybka8, Maria Saduś-Wojciechowska9, Sebastian Giebel9, Jadwiga Dwilewicz-Trojaczek2
1Medical University of Silesia, Department of Hematology and Bone Marrow Transplantation, Katowice, Poland; 2Medical University of Warsaw, Department of Hematology, Oncology and Internal Diseases, Warsaw, Poland; 3Jagiellonian University, Department of Hematology, Cracow, Poland; 4Medical University of Gdansk, Department of Hematology and Transplantology, Gdansk, Poland; 5Institute of Hematology and Transfusion Medicine, Department of Haematopoietic Stem Cell Transplantation, Warsaw, Poland; 6Wroclaw Medical University, Department of Paediatric Bone Marrow Transplantation, Oncology and Hematology, Wroclaw, Poland; 7Medical University of Białystok, Department of Hematology, Białystok, Poland; 8Wroclaw Medical University, Department and Clinic of Hematology, Blood Neoplasms, and Bone Marrow Transplantation, Wroclaw, Poland; 9Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Department of Bone Marrow Transplantation and Hematology-Oncology, Gliwice, Poland
Background: Paroxysmal nocturnal hemoglobinuria (PNH) is a rare acquired hematopoietic stem cell disorder associated with somatic mutation in PIG-A gene, leading to deficiency of glycosyl phosphatidylinositol-anchored proteins. Clinical phenotype encompasses hemolytic anemia, bone marrow failure syndrome and thrombotic events, allowing for the recognition of two major entities: classical paroxysmal nocturnal hemoglobinuria (cPNH) and bone marrow failure associated paroxysmal nocturnal hemoglobinuria (BMF/PNH). While allogeneic hematopoietic cell transplantation (alloHCT) remains the backbone of BMF/PNH treatment, it is disputable in cPNH in the era of C5-inhibitors.
Methods: This retrospective analysis covers 77 patients, 45 males, with PNH from 9 Polish hematological centers. 38 patients suffered from classical PNH, 39 - from PNH associated with bone marrow failure. Median age at transplantation was 33 years (range 16–52) in cPNH, 27 (range 12–65) in BMF/PNH. 35 cPNH and 37 BMF/PNH patients received reduced intensity conditioning. The donor was identical sibling in 9 cPNH, and 10 BMF/PNH cases; matched unrelated donor in 24 cPNH and BMF/PNH cases each. 10 patients received hematopoietic cells from mismatched unrelated donors. The source of stem cells was either peripheral blood (28 cPNH and BMF/PNH each), bone marrow (10 cPNH and BMF/PNH each) or a combination of both (1 BMF/PNH). Altogether 9 patients suffered from previous thrombotic episodes (5 in cPNH, 4 in BMF/PNH). No patient was treated with C5-inhibitor prior to alloHCT. Clone size at transplantation amounted at median 80% and 10% for cPNH and BMF/PNH.
Results: Engraftment was reported for 37 out of 38 cPNH, and for 37 out or 39 BMF/PNH patients. With the median survival of surviving patients of 4.5 years, the 5-year overall survival (OS) reached 94.2% (95% CI, 78.8–98.5) for cPNH, and 76.1% (95% CI, 57.9–87.3) for BMF/PNH. Patients with cPNH and history of thrombosis had tendency to inferior survival in comparison to patients without thrombosis i.e. 5-yrs OS reached 77.8% (95% CI, 16.6–96.5) vs 96.7% (95% CI, 78.6–99.5) - the difference was not significant (p>0,05). Type of conditioning, source of stem cells, donor type, history of thrombosis or hemolysis did not impact survival in univariate analysis. Acute graft versus host disease grade 2–4 was observed in 6 cPNH, and in 9 BMF/PNH patients, while chronic graft versus disease in 12 cPNH patients (including one severe), and 10 BMF/PNH patients (inc. 4 severe).
Conclusions: Allogeneic hematopoietic cell transplantation is a potent method of treatment for patients with both classic and bone marrow failure associated paroxysmal nocturnal hemoglobinuria. It offers very high long-term survival with acceptable toxicity.
Clinical Trial Registry: not applicable
Conflict of interest: none
Autoimmune diseases
O030 Autologous Stem cell transplantation for progressive systemic sclerosis: an EBMT Autoimmune Disease Working Party prospective non-interventional approach
Joerg Henes1, Maria Carolina Oliveira2, Myriam Labopin3, Manuela Badoglio4, Hans Ulrich Scherer5, Nicoletta Del Papa6, Thomas Daikeler7, Marc Schmalzing8, Roland Schroers9, Thierry Martin10, Gregory Pugnet11, Belinda Simoes12, Régis Peffault de Latour13, Bruno Lioure14, Jacques Olivier Bay15, John A Snowden16, Montserrat Rovira17, Anne Huynh18, Onida Francesco19, Lohar Kanz20, Zora Marjanovic21, Dominique Farge22
1University of Tuebingen, Department of Internal Medicine II, Tuebingen,, Germany; 2Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil; 3EBMT Paris Study Office, Saint Antoine Hospital, Université Pierre et Marie Curie, INSERM UMR 938,, Department of Haematology, Paris, France; 4EBMTParis Study Office, Hôpital St Antoine, INSERM UMR 938,, Paris, France; 5Leiden University Medical Center, Department of Rheumatology, Leiden, Netherlands; 6Scleroderma Clinic, Osp. G. Pini, Department of Rheumatology, Milan, Italy; 7University and University Hospital of Basel, Department of Rheumatology, Basel, Switzerland; 8University of Wuertzburg, Centre of Internal Medicine, Wuertzburg, Germany; 9Universitäts Klinik of Bochum, Med. Klinik, Bochum, Germany; 10Hôpital Civil, Service de Médecine Interne et Immunologie Clinique, Strasbourg, France; 11CHU de Toulouse, Hôpital Purpan, Service de Médecine Interne, Toulouse, France; 12Ribeirão Preto Medical School, University of São Paulo, Division of Hematology, Ribeirão Preto, Brazil; 13Hôpital Saint Louis & Université Paris 7, Denis Diderot, Department of Hematology, Paris, France; 14Strasbourg University Hospital, Department of Hematology, Strasbourg, France; 15CHU de Clermont Ferrand, Department of Hematology, Clermont Ferrand, France; 16Sheffield Teaching Hospitals, NHS Foundation Trust, Department of Haematology, Sheffield, United Kingdom; 17Hospital Clínic of Barcelona, Department of Haematology, Barcelona, Spain; 18IUCT Oncopole, Department of Haematology, Toulouse, France; 19Fondazione IRCCS Ca Granda Ospedale Maggiore Policlinico, University of Milan, Department of Oncology and Hemato-Oncology, Milan, Italy; 20Centre for Interdisciplinary Clinical Immunology, Rheumatology and Auto-inflammatory Diseases, University Hospital Tuebingen, Department of Internal Medicine II (Oncology, Haematology, Immunology, Rheumatology, Pulmonology), Tuebingen, Germany; 21Saint Antoine Hospital, Department of Haematology, Paris, France; 22Hôpital Saint-Louis, AP-HP Assistance Publique des Hôpitaux de Paris, CRMR Centre de Référence des Maladies Auto-Immunes Systémiques Rares d'Ile-de-France, Filière 'FAI2R', Paris Denis Diderot University, Department of Internal Medicine, Unité Clinique de Médecine Interne, Maladies Auto-Immunes et Pathologie Vasculaire, UF 04, Paris, France
Background: Following 3 adequate randomized trials using non-myeloablative (ASTIS, ASSIT) or TBI-myeloablative regimen (SCOTT) plus regularly updated EBMT guidelines, autologous stem cell transplantation (AHSCT) became the best proven effective therapy (EBMT evidence level grade 1) for systemic sclerosis (SSc) with organ involvement within 5 years of disease onset, which otherwise carries dreadful outcome. Nonetheless, different patient selection criteria and treatment protocols are used in various centres and it is not clear which approach is the safest and most efficient.
Methods: An EBMT-ADWP open, multi-center, prospective non-interventional study analysed routinely collected clinical and biological data for all consecutive AHSCT performed in SSc patients, diagnosed according to ACR-criteria and aged 18 to 65 years at transplant, from 2013 to 2015. Endpoints were: Response to treatment ( = increase in modified Rodnan skin score (mRSS) ≥25% and/or in Forced Vital Capacity (FVC) and/or Carbon Monoxide Lung diffusing capacity (DLCO) ≥10% without need of further immunosuppression), Relapse Incidence (RI), Progression free survival (PFS, survival with/without relapse or progression), Overall survival (OS) and Non Relapse Mortality (NRM). Univariate analyses using log-rank test for OS and PFS, Gray's test for RI and TRM were used to study possible impact of prognostic factors (age, gender, mRRS, lung, cardiac and kidney abnormalities). Multivariate analysis was performed using Cox proportional hazards model.
Results: (median, range): 82 patients (57 female) from 12 centres in 7 countries, aged 43y (20–66) at AHSCT, were followed for 24.11 months (5.97–59.84). Baseline patient's characteristics at AHSCT, after 23.77 months (5.28–103.7) disease duration, were: mRSS at 24 (2–49), Body Mass Index 23.4 (15.4–35.2), 41(50%) with lung crepitations, 33(66%) with Pulmonary Hypertension, 14 (18%) with FVC < 60%, 8 (10.5 %) with DLCO < 40%, 67(87%) with abnormal lung CT scan, 10 (12%) with cardiac involvement, 13(16.67%) with kidney abnormalities. All patients were mobilized with 2g/sqm (1–4) cyclophosphamide and G-CFS for 77 (98%). Conditioning used cyclophosphamide alone in 78 pts (95%) at 200 mg/kg (50–240) or cyclophosphamide at100 mg/kg and thiotepa 10 mg/kg in 4 patients. All patients received ATG 7.50 mg/kg (2–41) over 4 days (1–9) and 67 pts (82%) methylprednisolone at 400mg (10–3000). (43.2%) patients received G-CSF following AHSCT. Time to neutrophils > 0.5 x 10^9/L was 11.5d (8–24), 9d (1–25) for platelets > 20 x 10^9/L. At 2 years, cumulative incidence (CI) of response was 85.4 % (95% CI: 75.3–91.5), RI 24.3 % (95% CI: 15.1–34.7), PFS 69.6 % (95% CI: 59–80.1) and OS 90.2 % (95% CI: 83.7–96.6). The 100d and 2yrs NRM was 6.2 % (95% CI: 2.3–12.9). Figure 1 shows mRSS improvement over 2 yrs. On multivariate analysis, no factors were significantly associated with response, RI or OS, only lung crepitation at baseline was significantly associated with PFS [HR = 0.43 (95% CI: 0.19–0.97) p = 0.042].
Conclusions: This largest prospective study with 2 years follow-up after AHSCT in 82 SSc patients highlights improved practices over time with decreased TRM at 6.2% (versus 10% in ASTIS) and sustained skin and lung fibrosis regression after transplant.
Clinical Trial Registry: NCT 02516124
Conflict of interest: The authors declare no conflict of interest
[ [O030 Figure] Figure1. Improvement of mRSS during the 2 yrs follow-up after AHSCT]
O031 The use of Autologous Haematopoietic Stem Cell Transplantation in treatment naïve patients with severe multiple sclerosis
Joyutpal Das1, John Snowden2,3, Sona Mistry4, Helen Jessop2, Marjorie Bowman5, Azza Ismail1, Simon Bell6, Mark Freedman5, Harry Atkins5, Joachim Burman7, Basil Sharrack1,6
1Sheffield Teaching Hospitals NHS Foundation Trust, Academic Department of Neurology, Sheffield, United Kingdom; 2Sheffield Teaching Hospitals NHS Foundation Trust, Department of Haematology, Sheffield, United Kingdom; 3University of Sheffield, Department of Oncology & Metabolism, Sheffield, United Kingdom; 4University of Sheffield, Sheffield Medical School, Sheffield, United Kingdom; 5Ottawa Hospital Research Institute, Department of Neurology, Ottawa, Canada; 6University of Sheffield, Sheffield Institute for Translational Neuroscience, Sheffield, United Kingdom; 7Uppsala University Hospital, Department of Neurology, Uppsala, Sweden
Background: Autologous haematopoietic stem cell transplantation (AHSCT) is a very effective treatment in patients with highly active relapsing remitting multiple sclerosis (MS) who failed to respond to standard disease modifying therapies (DMTs). International guidelines advocate its use as a first line treatment in patients with malignant MS (1). There is no universally accepted definition of malignant MS, but it commonly refers to an aggressive form of MS with a rapid progressive course, often leading to significant disability over a relatively short period. Rapidly evolving severe MS (RESMS) is another form of aggressive MS designated by two or more disabling relapses associated with one or more gadolinium enhancing magnetic resonance imaging (MRI) lesions during the preceding 12 months (2). Here we describe the experiences of three centres in the use of AHSCT as a first line treatment in patients with severe MS.
Methods: Seven patients from Sheffield (UK), seven patients from Uppsala (Sweden) and four patients from Ottawa (Canada) with severe MS received AHSCT between May 2014 and May 2017. None of these patients received standard DMTs before AHSCT. BEAM (carmustine, etoposide, cytarabine, melphalan) with antithymocyte globulin (ATG), cyclophosphamide with ATG and combination of cyclophosphamide, ATG and busulphan were used as conditioning regimens in 3, 11 and 4 patients respectively.
Results: The median age of patients at diagnosis was 28 (range, 19–47) years. Ten patients had 'malignant' MS and eight had RESMS. All patients had rapidly progressive inflammatory disease course with poor prognostic indicators. Pre-treatment MRI scans showed multiple gadolinium enhancing cranial and spinal cord lesions over multiple time points. Median time between symptom onset and AHSCT was 9 (2–52) months. Patients were followed up clinically at 3, 6, and 12 months, and annually thereafter. Follow up MRI scans were obtained at 6 and 12 months, and annually thereafter. Median pre-treatment Expanded Disability Status Scale (EDSS) score was 6.5 (2.0–9.5). Median follow up was 29.5 (6–118) months. Median EDSS score at the last follow up was 2.0 (0–6.5). There was a significant improvement between pre-treatment and last follow up EDSS scores (p < 0.05, Wilcoxon signed-rank test). No clinical relapse was observed post-AHSCT in these patients. Three patients had new T2 lesions with or without gadolinium enhancement during the first six months following treatment, but no further new or enhancing lesions were observed in any subsequent scans. There were routine toxicities, but no treatment related mortality.
Conclusions: AHSCT was safe and highly effective in inducing remission in this cohort of treatment naïve patients with severe forms of MS. Long-term remission was achieved in all patients and none had further clinical or imaging evidence of disease activity. The treatment was associated with a significant improvement of their disability. Further studies are required to establish long-term safety, efficacy and cost-effectiveness of this treatment compare to standard DMTs.
References:
1. Snowden JA et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. BMT. 2012
2. Muraro et al. AHSCT for treatment of MS. Nature. 2017
Clinical Trial Registry: not applicable
Conflict of interest: Authors declared no conflict of interest.
O032 An Open-label Proof-of-principle phase 2a study to evaluate autologous hematopoietic stem cell transplantation for allogeneic organ transplant tolerance (ASCOTT)
Harold Atkins1, Andrzej Chruscinski2, Anne Marie Clement1, David Grant2, Christopher Bredeson1, Eberhart Renner3, Les Lilly2, Nazia Selzner2, Oyedele Adeyi2, Rob Smith2, Atul Humar2, Kathryn Tinckam2, Stephen Juvet2, Gary Levy2
1Ottawa Hospital Research Institute, Ottawa, Canada; 2University Health Network, Toronto, Canada; 3University of Manitoba, Winnepeg, Canada
Background: Long-term survival of recipients of solid organ transplants is hampered by chronic allograft rejection, disease recurrence and immunosuppression-induced toxicity. Self-tolerance is re-established after autologous hematopoietic stem cell transplantation (aHSCT) for patients with autoimmune disease. We are testing whether aHSCT can induce tolerance in liver transplant recipients.
Methods: Patients, 18 to 55 years old, with autoimmune liver disease treated with liver transplantation who are > 3 months post-liver transplant were eligible for this proof-of-principle trial. Following baseline evaluation, autologous hematopoietic stem cells were mobilized using cyclophosphamide (CTX) and filgrastim, collected by leukopheresis and cryopreserved after auto- and allo-reactive lymphocytes were removed using CD34 cell immunomagnetic selection. Busulfan, CTX and rabbit αnti-thymocyte globulin were administered to ablate auto- and allo-reactivity followed by aHSCT for marrow and immune reconstitution. Immunosuppressive medications were discontinued at the time of aHSCT and everolimus was to be given to expand regulatory T cells for the first 6 months following HSCT. Patients were followed for evidence of tolerance or rejection.
Results: 75 liver transplant recipients were screened. 12 patients were potentially eligible and selected for closer evaluation. Liver damage was too advanced in 3 patients, too mild in 2 patients, 1 patient declined participation and 1 patient remains eligible but has not undergone aHSCT. aHSCT was carried out on 5 pts with evidence of recurrent primary sclerosing cholangitis (PSC) with moderate to severe ductopenia and fibrosis at a median of 98 months (15–233 mo.) after liver transplantation. The median age was 40 (36–44) years. 4 patients were male. 3 patients received living and 2 patients received cadaveric liver grafts. One patient had undergone two cadaveric liver transplants. Ulcerative colitis (UC) was a comorbidity in 4 patients. Patients received a mean 7.21x106 (3.41–11.79) purified CD34 cells/kg. Neutrophil engraftment occurred at d11 (d10-12) and platelet engraftment at d17 (d13-24). Grade 3–4 non-hematologic toxicity was seen in all patients. 3 patients required ICU care. Two patients are alive and off all immunosuppressive medication 406 and 518 days after HSCT. One patient is off immunosuppression but has VOD, 140 days after HSCT. One patient was off immune suppression with no evidence of ongoing liver damage on biopsy but died of cardiac issues 212 days after HSCT. One patient died 87 days after HSCT from sepsis and multi-organ failure.
Conclusions: These results provide evidence that aHSCT can induce tolerance in liver transplant recipients with autoimmune liver disease, controlling both the autoimmune PSC and allograft rejection, and eliminating the need for ongoing immunosuppressive medication. Toxicity is a significant problem that needs to be addressed in any future study.
Clinical Trial Registry: NCT02549586
Conflict of interest: This study is funded by a grant from the Canadian Stem Cell Network. Everolimus is provided by Novartis.
Cell therapy / cellular therapy
O033 A novel pharmacologic 'remote control' to modulate CAR-T cell function and prevent cytokine release syndrome in vivo
Katrin Mestermann, Julian Rydzek, Silke Frenz, Hermann Einsele, Michael Hudecek
Universitätsklinikum Würzburg, Medizinische Klinik und Poliklinik II, Würzburg, Germany
Background: Immunotherapy with CAR-T cells (CAR-T) is a powerful novel treatment for hematologic malignancies, but also bound with significant acute and chronic side effects, including potentially life-threatening cytokine release syndrome (CRS). This toxicity limits clinical utility and is at least in part caused by the inability to effectively control CAR-T function following infusion. Here, we present a novel strategy of pharmacologic 'remote control' to precisely control CAR-T function in real-time.
Methods: We considered that an effective way for controlling CAR-T function was to interfere with signal transduction though the CAR. We assembled a library of clinically approved drug compounds and screened for their ability to reversibly block CAR-T function without affecting CAR-T viability. We performed functional testing with CD8+ and CD4+ CAR-T (n = 3 donors) in the presence of titrated doses of the lead compound, and employed CD19- and ROR1-specific CARs comprising 4-1BB or CD28 costimulatory moieties.
Results: We identified a lead compound, TCI-1, that stood out through its ability to confer a dose-dependent (partial at lower, complete at higher doses) blockade of all CAR-T effector functions, i.e. cytolytic activity, cytokine secretion and proliferation. We confirmed that TCI-1 was effective in both CD8+ and CD4+ T cells, and with each of the three CAR constructs. The onset of CAR-T blockade was immediate after exposure to TCI-1 and was caused by interference with early phosphorylation events in the CAR signaling cascade as demonstrated by Western blot, and interference with the induction of transcription factors, as demonstrated with an NFAT-inducible reporter gene. Intriguingly, blockade of CAR-T function was effective for several days if exposure to TCI-1 was sustained, and instantaneously and fully reversible after removal of the compound. Short- and long-term exposure to TCI-1 did not reduce CAR-T viability, and did not hinder the subsequent ability of CAR-T to exert their functions. We considered that in patients with CRS, CAR-T are in an activated state, and performed comprehensive testing to show that TCI-1 was able to arrest CAR-T that are in the process of executing their effector functions. In addition, we employed a xenograft model in immunodeficient mice (NSG/Raji) to determine whether TCI-1 was capable of controlling the function of CD19 CAR-T cells in vivo. Indeed, we demonstrate that administration of TCI-1 conferred a functional arrest of CAR-T and prevented CRS; and that CAR-T resumed their antitumor function once administration of TCI-1 was discontinued.
Conclusions: Our data show that TCI-1 is capable to exert real-time, on/off control over CAR-T function, suggesting the potential to prevent or mitigate side-effects of CAR-T therapy in a clinical setting. The complete and reversible inhibition of CAR-T function through TCI-1 without compromise to CAR-T viability surpasses the qualities of steroids that are toxic to T cells and provide only incomplete functional control, and complement suicide-gene strategies that effectively control chronic side effects but also abrogate the antitumor effect of CAR-T.
Conflict of interest: Katrin Mestermann and Michael Hudecek are co-inventors on a patent related to TCI-1 that has been filed by the University of Würzburg.
O034
Abstract previously published
O035 An analysis of the role of donor NK cell alloreactivity in HLA-haploidentical transplantation: A Non-interventional Prospective Study on behalf of the CTIWP of the EBMT
Loredana Ruggeri1, Luca Vago2, Diderik-Jan Eikema3, Simona Iacobelli4, Fabio Ciceri2, Attilio Bondanza2, Miguel Angel Diaz5, Franco Locatelli6, Pavel Jindra7, Giuseppe Milone8, J.L. Diez-Martin9, Ali Unal10, Jose Antonio Pérez-Simón11, Linda Koster3, Steffie van der Werf3, Mara Merluzzi12, Maddalena Noviello2, Anja van Biezen3, Antoine Toubert13, Aron Nagler14, Christian Chabannon15, Chiara Bonini2, Andrea Velardi12
1Ospedale Santa Maria della Misericordia, Perugia, Italy; 2Ospedale San Raffaele, Milan, Italy; 3EBMT Data Office, Leiden, Netherlands; 4Centro Interdipartimentale di Biostatistica e Bioinformatica, Università Tor Vergata, Rome, Italy; 5Niño Jesus Children`s Hospital, Madrid, Spain; 6IRRCS Ospedale Pediatrico Bambino Gesù, Rome, Italy; 7Charles University Hospital, Pilsen, Czech Republic; 8Ospedale Ferrarotto, Catania, Italy; 9Hospital Gregorio Marañón, Madrid, Spain; 10Erciyes Medical School, Kayseri, Turkey; 11Hospital Universitario Virgen del Rocío, Sevilla, Spain; 12University of Perugia, Perugia, Italy; 13Université Paris Diderot, Sorbonne Paris Cité, Institut Universitaire d'Hématologie, Paris, France; 14Chaim Sheba Medical Center, Tel Hashomer, Israel; 15Institut Paoli Calmettes, Marseille, France
Background: In T-cell depleted haploidentical transplantation, the absence of post transplant pharmacological GvHD prophylaxis favors the emergence of Natural Killer (NK) cell-mediated alloreactions that contribute to reduce relapse rates and improved survival in adult and pediatric acute leukemia patients (Ruggeri et al. Science 2002, Blood 2007; Handgretinger et al. JI 2005; Bernardo et al. EBMT 2011). The results obtained in T cell-depleted haploidentical transplantation were not Always confirmed in unmanipulated, unrelated, KIR-ligand mismatched donor transplantation (Davies et al. Blood 2002; Blood 2003, Farag et al. BBMT 2006): the presence of T cells in the graft and post-transplant GVHD prophylaxis might antagonize the benefits of donor-vs-recipient NK cell alloreactivity. In recent years, the haploidentical transplantation field saw the development of unmanipulated grafts combined with new strategies to prevent GVHD. Here, we performed a non-interventional, prospective study on the role of NK cell alloreactivity in haploidentical transplantation performed under a variety of protocols that included T-cell depleted as well as unmanipulated grafts.
Methods: A non-interventional prospective multicenter study was performed in a combined series of adult (n = 93) and pediatric (n = 47) patients with acute leukemia (89 AML and 51 ALL) who received a transplant from an HLA haploidentical family donor. One hundred and two patients were in I or II complete remission (CR), 34 were in chemo-resistant relapse or in > II CR. Fifty-two patients received ex-vivo T cell depleted transplants, the remaining patients received an unmanipulated transplant or a T-cell depleted transplant followed by T cell add-backs (i.e adoptive immune therapy with regulatory and conventional T cells or suicide gene engineered T cells). Fifty-two patients were transplanted from NK alloreactive donors, 88 from non-NK alloreactive donors.
Results: In the whole cohort of patients, at a median follow up of 24 months, the probability of leukemia-free survival (LFS) was 42% (95% CI: 24–51). The 24 month cumulative incidence (CI) of relapse was 35% (95% CI: 26–43). The 24 month CI of non-relapse mortality (NRM) was 23% (95% CI:16–30). The 100 day CI of aGvHD was 18 % (95% CI: 18–24). The 24 month CI of cGvHD was 26% (95% CI: 18–34). Transplantation from NK alloreactive donors did not impact on cGvHD/Leukemia-free survival in the whole cohort of patients. Univariate analyses showed younger age (< 18y), disease status (complete remission) at transplant and T cell depletion were associated with LFS. Forty-seven patients were younger than 18 years and all received a T cell depleted graft. In these patients, transplantation from NK alloreactive donors was associated with reduced incidences of leukemia relapse and cGvHD (Fig 1, panels A-B) this resulting into better cGvHD/Leukemia-free survival. It was 64% in patients transplanted from NK alloreactive donors vs 34% in patients transplanted from non-NK alloreactive donors (P = 0.09) (Fig. 1, Panel C).
Conclusions: This study shows that NK cell alloreactivity does not play a role in haploidentical unmanipulated transplants and in haploidentical T-cell depleted transplants followed by T cell addbacks. However, the present data confirm previous observations on the beneficial role of NK cell alloreactivity in T-cell depleted haploidentical transplantation.
Conflict of interest: None of the authors has anything to disclose.
[O035 Figure].
Survival]
O036 Long-lived donor-derived human memory-like NK cells with potent anti-leukemia activity as a novel cell therapy approach to control leukemia relapse after haploidentical HSCT
Matteo Tanzi1, Michela Consonni2, Federica Ferulli1, Ilaria Turin1, Enrica Montini1, Stella Boghen3, Gloria Acquafredda1, Francesca Compagno3, Paolo Dellabona2, Giulia Casorati2, Marco Zecca3, Daniela Montagna1,4
1Fondazione IRCCS Policlinico San Matteo, Laboratorio Immunologia e Trapianti, Pavia, Italy; 2San Raffaele Scientific Institute, Division of Immunology, Transplantation and Infectious Diseases, Milano, Italy; 3Fondazione IRCCS Policlinico San Matteo, Oncoematologia Pediatrica, Pavia, Italy; 4University of Pavia, Pavia, Italy
Background: Adoptive transfer of allogeneic natural killer (NK) cells has great potential to sustain anti-leukemia surveillance early after haploidentical (haplo)-HSCT without inducing GVHD. However, the efficacy of current NK cell-based adoptive immunotherapy is limited by short-term persistence of the transferred cells. Studies in mice have shown that in vitro activation with appropriate cytokines results in differentiation of long-lived NK lymphocytes with memory-like properties. The aim of this study was to investigate the generation in vitro of donor-derived human memory-like NK cells endowed with potent anti-leukemia cytotoxicity and long term persistence.
Methods: We have analyzed the feasibility of inducing donor-derived memory-like NK cells in 5 donor/recipient pairs, in which patients were children affected by acute lymphoblastic leukemia (ALL) (n = 2), or acute myeloid leukemia (AML) (n = 3), while the donor was haploidentical family donor. Memory-like NK cells were also isolated from buffy-coats of 5 healthy donors. Purified NK cells were pre-activated for 16 hrs with IL-2/IL-18/IL-15, then washed and cultured in CellGro medium supplemented with IL-15/IL-2 to support survival and expansion. Cells were cultured for 7 days and then re-stimulated or not with IL-12/IL-15. Alternatively, purified NK cells were stimulated ON with IL-2 (control NK). At the end of culture, cell recovery, NK receptors expression and cytotoxic activity against patient leukemia blasts (LB) and the AML cell line THP-1, were evaluated. Persistence in vivo was assessed by injecting 7x106 cells in NSG mice and enumerating circulating human NK cells by flow cytometry over time.
Results: Optimal culture conditions, in terms of cytokine combination and concentration, to differentiate substantial numbers of memory-like NK cells exhibiting potent anti-tumor cytotoxic function in vitro, were defined. Memory-like NK cells from an haploidentical donor or from healthy donors efficiently killed both patient LBs and the THP-1 cell line, compared with control NK cells (memory-like NK: mean: 53.7%, and SD 18%, against LB; and mean 36%, and SD 7% against THP-1. Control NK: mean: 16%, and SD 8%, against LB; and mean 9.6%, and SD 7.6% against THP-1. These data refer to levels of cytotoxic activity at an E:T ratio of 30:1). Lysis of patients' non-malignant cells was always less than 10%. In agreement with published data, human memory-like NK cells expressed higher levels of CD94, NKG2A, NKp46 and CD69 compared with control NK cells. Transfer experiments in NSG mice demonstrated that, whereas control NK cells remained detectable only for three days in the peripheral blood of mice, memory-like NK cells were found up to 21 days after their transfer. Biological properties of memory like NK cells were not affected by cryopreservation.
Conclusions: We have consistently generated long-lived, donor-derived human memory-like NK cells with potent anti-leukemia activity. After confirming that these cells can maintain their effector functions in vivo and considering the low risk of allogeneic NK cells to induce GVHD, this approach could be rapidly translated to clinical adoptive cell therapy trials to control leukemia relapse in high risk patients in the early post haplo-HSCT period.
Conflict of interest: nothing to disclose
O037
Abstract previously published
O038 A minority of tumor associated antigen specific T cells restricted to self-HLA alleles is of sufficient avidity to recognize overexpressed endogenously processed antigen
Marthe C.J. Roex1, Lois Hageman1, Esther van Egmond1, Sabrina A.J. Veld1, Conny Hoogstraten1, Lothar Germeroth2, J.H.Frederik Falkenburg1, Inge Jedema1
1Leiden University Medical Center, Hematology, Leiden, Netherlands; 2Juno Therapeutics, Goettingen, Germany
Background: Tumor associated antigens (TAA) have been proposed as targets for a graft versus leukemia effect in the HLA-matched allogeneic stem cell transplantation setting. As TAA like NY-eso, WT1, RHAMM, Proteinase 3 and PRAME are monomorphic self-antigens that are also expressed at low levels in normal, non-malignant cells, T cells recognizing these TAA peptides with high avidity in self-HLA are anticipated to be eliminated from the T cell repertoire by negative thymic selection. In malignant cells, the genes encoding TAA can be aberrantly upregulated resulting in an increased antigen density on the cell surface. In this study, we investigated whether TAA specific T cells with sufficient avidity to recognize overexpressed endogenously processed antigen in self-HLA can be found in healthy donors.
Methods: T cells directed against the TAA peptides NY-eso-1-SLL/A*02:01, WT1-RMF/A*02:01, RHAMM-ILS/A*02:01, Proteinase-3-VLQ/A*02:01 and PRAME-VLD/A*02:01 were enriched from ≥500*10^6 PBMC of HLA-A*02:01+ donors using the MHC-I-Streptamer isolation technology. Tetramer+/CD8+ T cells were clonally FACS sorted, expanded and screened for antigen specific reactivity measured by cytokine release after overnight stimulation with TAP-deficient T2 cells exogenously loaded with 10^-10M to 10^-5M of the respective peptide. The recognition of overexpressed endogenously processed antigen was analyzed using two HLA-A*02:01+ EBV-LCL transduced with a retroviral vector encoding the full protein sequence of the corresponding TAA.
Results: Over 900 TAA specific T cell clones were isolated from 18 HLA-A*02:01+ donors. To avoid redundancy in the functional analysis, the minimal number of unique clones per TAA specificity was determined based on T cell receptor Vbeta-family analysis per donor which illustrated 15 unique NY-eso-1-SLL/A*02:01 clones from 5 donors, 33 unique WT1-RMF/A*02:01 clones from 17 donors, 15 unique RHAMM-ILS/A*02:01 clones from 9 donors, 8 unique Proteinase-3-VLQ/A*02:01 clones from 5 donors and 14 unique PRAME-VLD/A*02:01 clones from 9 donors. High tetramer staining in the FACS analysis was comparable for all clones, but functional screening revealed a wide variety in functional avidities as defined by the minimal concentration of peptide exogenously loaded onto T2 cells needed for T cell activation measured by cytokine production. 43/85 clones were non-functional or classified as low avidity with an activation threshold ≥10^-6M peptide, 29/86 clones as intermediate avidity with an activation threshold between 10^-7M and 10^-8M peptide, and 13/86 clones were classified as high avidity with an activation threshold ≤10^-9M peptide including 5 WT1-RMF/A*02:01 clones, 2 RHAMM-ILS/A*02:01 clones, 1 Proteinase-3-VLQ/A*02:01 clone, 5 PRAME-VLD/A*02:01 clones. TAA clones of intermediate and high avidity were subsequently tested for recognition of EBV-LCL transduced to overexpress the full protein sequence of the respective TAA. 2/6 NY-eso-1-SLL/A*02:01 clones, 6/13 tested WT1-RMF/A*02:01 clones, 0/4 tested RHAMM-ILS/A*02:01 clones, 0/1 Proteinase-3-VLQ/A*02:01 clone and 4/12 PRAME-VLD/A*02:01 clones showed recognition of overexpressed endogenously processed antigen.
Conclusions: These results illustrate that self-HLA restricted TAA specific T cells can be easily isolated from peripheral blood of healthy individuals, but that only a minority of the TAA specific T cells are capable of recognizing overexpressed endogenously processed antigen. Classification of functional TAA specific T cells by only high tetramer staining and peptide specificity leads to overestimation of relevant avidity of these T cells.
Conflict of interest: Lothar Germeroth: employee of Juno Therapeutics GmbH, Goettingen, Germany, and member of the executive committee of Juno Therapeutics Inc, Seattle, United States. The remaining authors have nothing to disclose.
O039 Alpha/beta T-cell depleted Haploidentical HSCT followed by infusion of donor lymphocytes transduced with inducible caspase9 gene is safe and effective for patients with erythroid disorders
Federica Galaverna1, Giuseppina Li Pira1, Mattia Algeri1, Luisa Strocchio1, Letizia Pomponia Brescia1, Daria Pagliara1, Valentina Bertaina1, Matilde Sinibaldi1, Valentina Cirillo1, Mauro Montanari1, Giovanna Leone1, Katia Girardi1, Simone Biagini1, Alice Bertaina1, Alan Foster2, Paul Woodard2, Pietro Merli1, Franco Locatelli1
1Ospedale Pediatrico Bambino Gesù IRCCS, Pediatric Hematology and Oncology, Rome, Italy; 2Bellicum Pharmaceutical Company, Houston, TX, United States
Background: Allogeneic HSCT is the only well-established curative therapy for patients with erythroid disorders [including thalassemia, sickle cell disease (SCD) and Diamond-Blackfan anemia (DBA)]. However, use of allogeneic HSCT is largely limited by the availability of an HLA-matched, either related or unrelated, donor.
Methods: We conducted a prospective clinical trial on the use of alpha/beta T-cell and B-cell depleted HLA-haploidentical HSCT followed by infusion of donor lymphocytes genetically transduced with the inducible caspase 9 (iC9) suicide gene (BPX-501 cells). Enrolled in the study were 24 patients (20 with thalassemia, 3 with DBA and one with SCD). Twelve patients were males and 12 females. Median age at HSCT was 8.6 years (range 2.1–14.3). The conditioning regimen consisted of a combination of busulfan, thiotepa and fludarabine in all patients. To prevent graft rejection, all patients received anti-thymocyte globulins (ATLG Neovii® 4 mg/kg/day on days -4, -3, -2). The donor was the mother in 15 patients and the father in the remaining 9. The median number of infused CD34+ cells, gamma/delta T lymphocytes and NK cells/kg recipient b.w. was 28.6, 15, 49.9x106/kg, respectively. The median number of alpha/beta T cells infused with the graft was 2.7x104/kg.
Results: Twenty patients had sustained donor engraftment, one thalassemia patient experienced secondary graft failure and was successfully re-transplanted from the same donor. The remaining 3 patients (all with thalassemia) experienced primary graft failure; 2 of them underwent a successful second alpha/beta/CD19-depleted haplo-HSCT from the other parent without receiving BPX-501 cells. Parents of the last patient refused a second allograft. BPX-501 cells were infused in the 20 patients with sustained donor engraftment at a median time of 16 days (range 10–113). In patients with primary sustained engraftment, median time to neutrophil and platelet recovery were 15 and 11 days, respectively. Five patients experienced grade I-II acute GVHD involving only the skin in all children but one; the cumulative incidence of this complication was 23%. One of these patients who had steroid-resistant acute GvHD received an infusion of the dimerizing agent (rimiducid) activating iC9, with complete response. One of the 19 patients at risk developed mild chronic GVHD. All 24 patients are alive. With a median follow-up of 17 months (range 1–30), the probability of disease-free survival (DFS) of patients given BPX-501 cells is 100%. In an intention-to-treat analysis, the event-free survival probability is 82.6%, counting graft failure as an event, while the DFS is 95.7%, considering as an event the persistence of transfusion-dependence. Last erythrocyte transfusion was administered at a median time of 9 days after haplo-HSCT (range 4–67). At last evaluation, median donor chimerism is 100% (range 75–100) and median Hb level is 11.8 gr/dL (range 9.4–13.7).
Conclusions: These data indicate that this approach is associated with high engraftment rate and fast neutrophil and platelet recovery. Transfusion independence was reached soon after haplo-HSCT. BPX-501 infusion is safe and well tolerated with a low incidence of both acute and chronic GVHD despite high number of HLA-disparate donor lymphocytes infused. Rimiducid infusion is able to rapidly control acute GVHD.
Clinical Trial Registry: ClinicalTrials.gov identifier: NCT02065869
EUDRACT number:2014-000584-41
Conflict of interest:
A. Foster, P. Woodward: employed at Bellicum Pharmaceutical. All other authors have nothing to disclose.
O040
Abstract previously published
O041
Abstract previously published
O042 Phase I/II clinical trial demonstrates feasibility, safety and effectivity of CMV-pp65-specific donor T-cells for the treatment of refractory CMV reactivation after allogeneic stem cell transplantation
Inge Jedema1, Peter van Balen1, Constantijn J.M. Halkes1, Esther H.M. van Egmond1, Ellis A.G. van Liempt1, S.A.J. Veld1, C. Hoogstraten1, Pauline Meij2, Sabina Kersting1, Jaap Jan Zwaginga3, Georg Rauser4, Mario Assenmacher4, J.H. Frederik Falkenburg1
1Leiden University Medical Center, Hematology, Leiden, Netherlands; 2Leiden University Medical Center, Clinical Pharmacology and Toxicology, Leiden, Netherlands; 3Leiden University Medical Center, Immunohematology and Blood Transfusion, Leiden, Netherlands; 4Miltenyi Biotec GmbH, Bergisch Gladbach, Germany
Background: Uncontrolled reactivation of latent viruses like cytomegalovirus (CMV) can cause major complications in immune compromised patients after allogeneic stem cell transplantation (alloSCT). Infusion of in-vitro selected populations of virus-specific donor T-cells may be effective in restoring anti-viral immunity without coinciding induction of graft versus host disease (GvHD).
Methods: 0.5-2x10^9 PBMC of the CMV-seropositive donors were stimulated overnight with 10^-6M of CMV-pp65 protein-spanning overlapping 15-mer peptide pool (MACS GMP PepTivator). CMV-specific CD4 and CD8 T-cells were isolated based on their secretion of interferon gamma using the CliniMACS Cytokine Capture System, and directly infused into the patient at the day of isolation. Frequencies of CMV-pp65-specific CD4 and CD8 T-cells were assessed in peripheral blood samples drawn at different time points after infusion of the CMV-pp65-specific T-cell product.
Results: In this phase I/II clinical trial, we investigated the feasibility and safety of the generation and administration of in-vitro selected CMV-pp65-specific donor T-cells to patients with CMV reactivation or CMV disease failing anti-viral therapy after alloSCT with a CMV-seropositive donor. 17 patients were included in this trial, of which 15 received a CMV-pp65-specific T-cell product at 35–104 days (median 79 days) after transplantation. Two products did not meet the release criteria. 5/15 of the patients were CMV- seronegative prior to transplantation. 4/15 patients were matched for ≥9/10 HLA alleles with the donor, whereas 1 patient was transplanted with a 6/12 matched haploidentical donor. The infused T-cell products contained 0.2–53.4x10^6 T-cells (median 0.7x10^6). No transfusion related complications occurred and no serious adverse events (SAEs) associated with the infusion of the CMV-pp65-specific T-cell products were reported. 1 patient died from a relapse of lymphoma early after the infusion of virus-specific T-cells, hampering evaluation of the effect of the transferred T-cells. 9/14 patients cleared the virus within 2–8 weeks after adoptive transfer of the CMV-pp65-specific T-cell product. 2/14 patients showed initial viral clearance, followed by a period of reactivation and ultimate clearance 4–7 months after the infusion of virus-specific T-cells. 1/14 patients who received virus-specific T-cells for CMV pneumonitis initially cleared the virus, but reactivated after start of immune suppression (prednisolone and cyclosporine) for reactivating GvHD and died 60 days after the infusion of virus-specific T-cells. 2/14 patients who did not clear the virus after the first infusion received a second infusion of CMV-pp65-specific T-cells. One patient cleared the virus after this second infusion, whereas the other patient developed CMV encephalitis and died 50 days after the second infusion. In some patients a clear appearance and/or increase in the numbers of circulating CMV-pp65-specific T-cells was observed after infusion of the CMV-pp65-specific T-cell product, whereas in other patients no, or only a subtle increase in the numbers of circulating CMV-pp65-specific T-cells was seen.
Conclusions: In this clinical study, we show that the adoptive transfer of in-vitro generated CMV-pp65-specific donor T-cell products is feasible and safe and can be used as a strategy to restore anti-viral immunity after alloSCT, although a definite causal correlation between the transfer of the product and clinical response cannot always be substantiated.
Clinical Trial Registry: EudraCT number 2010-024307-27
Conflict of interest: G. Rauser and M. Assenmacher are employees of Miltenyi Biotec.
Chronic leukaemia and other myeloproliferative disorders
O043 Allogeneic stem cell transplantation in patients with CML-CP in the era of third generation tyrosine kinase inhibitors: a study by the CMWP of the EBMT
Yves Chalandon1,2, Giulia Sbianchi3, Jennifer Hoek4, Jane Apperley5, Maija Itälä-Remes6, Miroslaw Markiewicz7, Jenny Byrne8, Henrik Sengeloev9, Naeem Chaudri10, Ram Malladi11, Péter Reményi12, Marco de Groot13, John Snowden14, Marie Robin15, Per Ljungman16, Nicolaas Schaap17, Stig Lenhoff18, Jakob Passweg19, Johan Maertens20, Jan Cornelissen21, Ibrahim Yakoub-Agha22, Francis Ayuk23, Boris Afanasyev24, Nicolaus Kroeger23
1HUG, Hematology Division, Oncology Department, Genève, Switzerland; 2Faculty of Medicine/Univesity of Geneva, Geneva, Switzerland; 3Tor Vergata University, Rome, Italy; 4EBMT Data Office Leiden, Leiden, Netherlands; 5Imperial College, Hammersmith Hospital,, London, United Kingdom; 6HUCH Comprehensive Cancer Center, Helsinki, Finland; 7Medical University of Silesia, Katowice, Poland; 8Nottingham University, Nottingham, United Kingdom; 9Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark; 10King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia; 11Queen Elizabeth Hospital, Birmingham, United Kingdom; 12St. István & St. Laszlo Hospital, Budapest, Hungary; 13University Medical Centre Groningen, Groningen, Netherlands; 14Royal Hallamshire Hospital, Sheffield, United Kingdom; 15Hôpital St. Louis, Paris, France; 16Karolinska University Hospital, Stockholm, Sweden; 17Radboud University Medical Centre, Nijmegen, Netherlands; 18Skanes University Hospital, Lund, Sweden; 19University Hospital Basel, Basel, Switzerland; 20University Hospital Gasthuisberg, Leuven, Belgium; 21Erasmus MC Cancer Institute, Rotterdam, Netherlands; 22CHU de Lille, LIRIC, INSERM U 995, Université de Lille, Lille, France; 23University Hospital Eppendorf, Hamburg, Germany; 24First State Pavlov Medical University of St. Petersburg, St. Petersburg, Russian Federation
Background: Following the introduction of TKI in the early 2000's the use of alloHSCT for CML has dramatically decreased. Imatinib was the first TKI introduced and is mostly used as 1st treatment. In case of insufficient response, resistance or intolerance, CML patients can be treated with a 2nd or 3rd generation TKI. Nevertheless there is still a role for alloHSCT for CML patients. We analysed the impact of the use of 1, 2 or 3 TKI prior to alloHSCT on transplant outcomes in patients who received alloHSCT for CML in CP between 2006 and 2016.
Methods: 3614 patients underwent an alloHSCT for CML, of whom 932 had information on the use of TKI prior to alloHSCT for CML in CP. 802 patients had Imatinib, 530 dasatinib, 362 nilotinib, 12 bosutinib and 44 ponatinib. 331 patients had 1, 383 2 and 218 3 TKI prior to transplant. The majority had imatinib as first TKI (n = 771, 82.7%).
Results: The number of TKI given prior to alloHSCT has been increasing over the years, median 1 in 2008, 2 in 2010 and 3 in 2012. Patients with only 1 TKI prior to alloHSCT were more likely to have BC at diagnosis (30%) compared with 2 TKI (11%) or 3 TKI (4%). The interval from diagnosis to alloHSCT was longer for patients with 3 TKIs, p < 0.001.Transplants were performed in CP1, n = 549, CP2, n = 306, and CP3, n = 77, from HLA identical siblings (n = 348), MUD (n = 532) or others (n = 52). Median age at transplant was 45 (18–71) years, 573 pts (61.5%) were male.
Median follow-up was 47.5 (0.5–138) months. OS for the entire population at 5 yrs was 64.1% (95% CI 60.7–67.6%), PFS 50% (95% CI 46.3.7–53.6%), RI 28.1% (95% CI 24.9–31.3%) and NRM 21.9% (95% CI 19–24.9%). In univariate analysis there was no difference on OS, PFS and RI related to the number of TKI prior to alloHSCT or to the type of TKI given (p = ns), although there was a tendency for worse OS, PFS or RI for pts who had bosutinib or ponatinib.
In multivariable analysis for OS, the number of TKI given did not have any impact. Factors influencing OS were CP2 vs CP1, HR 1.48 (1.14–1.94), p = 0.004, a tendency for CP3 vs CP1, HR 1.37 (0.88–2.12), p = 0.161, patients-donor sex combination with male patients-female donors having a worse outcome, HR 0.74 (0.55–1.0), p = 0.047 and Karnovsky score >= 90 vs < 90 HR 0.64 (0.5–0.83), p = 0.001. In multivariable analysis for RFS the number of TKI given prior to alloHSCT did not have any impact. Factors influencing RFS were CP2 vs CP1, HR 1.5 (1.19–1.88), p = 0.001, and Karnovsky score HR 0.76(0.62–0.95), p = 0.015.
Conclusions: These data suggest that the number of TKI given prior to alloHSCT has no impact on post-transplant outcomes. Patients receiving 3rd Generation TKI might have worse outcomes. Patients in CP1 have better survival than more advanced CML patients. The performance status at transplant remains as an important predictive factor in the era of 3rd generation TKI.
Conflict of interest:
Y. Chalandon: advisory board, Novartis, BMS, Pfizer, Incyte
J. Snowden: honoraria for speaking from Sanofi and Jazz
F. Ayuk: advisory board, Novartis
All other authors have nothing to disclose.
| All patients | 1 TKI | 2 TKI | 3 TKI | p | |
|---|---|---|---|---|---|
| 5-yr OS | 64.1% (60.7–67.6%) | 66.7% (61.1–72.3%) | 60.4% (55–65.8%) | 67.6% (60.3–75%) | 0.2265 |
| 5-yr PFS | 50% (46.3–53.6%) | 53.5% (47.5–59.5%) | 44.8% (39.1–50.5%) | 54.6% (46.9–62.4%) | 0.1287 |
| 5-yr RI | 28.1% (24.9–31.3%) | 26.6% (21.3–31.8%) | 31.1% (25.9–36.2%) | 24.1% (18–30.1%) | 0.3106 |
| 5-yr NRM | 21.9% (19–24.9%) | 19.9% (15.2–24.6%) | 24.1% (19.3–28.9%) | 21.3% (14.8–27.9%) | 0.6523 |
[ [O043 Table] Table 1]
O044 Transplant-related prediction of survival in primary myelofibrosis: a study by the EBMT
Nico Gagelmann1, Yves Chalandon2, Junfeng Wang3, Liesbeth de Wreede4, Christine Wolschke1, Maria Teresa Van Lint5, Dietrich Beelen6, Gerard Socié7, Gernot Stuhler8, Antonin Vitek9, Gerhard Ehninger10, Dietger Niederwieser11, Herman Einsele12, Miroslaw Markiewicz13, Renate Arnold14, Ibrahim Yakoub-Agha15, Stephen Robinson16, Péter Reményi17, Jakob Passweg18, Henrik Sengeloev19, Xavier Poiré20, Eefke Petersen21, Peter Dreger22, Nicolaus Kröger1, Marie Robin7
1University Medical Center Hamburg Eppendorf, Hamburg, Germany; 2Hôpitaux Universitaires de Genève, Geneva, Switzerland; 3EBMT Data Office, Leiden, Netherlands; 4LUMC and DKMS CTU, Dresden, LUMC Department of Medical Statistics and Bioinformatics, Department of Medical Statistics and Bioinformatics, Leiden, Netherlands; 5Ospedale San Martino, Genove, Italy; 6University Hospital, Essen, Germany; 7Hopital St. Louis, Paris, France; 8Deutsche Klinik fuer Diagnostik, Wiesbaden, Germany; 9Institute of Hematology and Blood Transfusion, Prague, Czech Republic; 10Universitaetsklinikum Dresden, Dresden, Germany; 11University Hospital Leipzig, Leipzig, Germany; 12Universitätsklinikum Würzburg, Würzburg, Germany; 13Medical University of Silesia, Katowice, Poland; 14Charité Universitaetsmedizin Berlin, Berlin, Germany; 15CHU de Lille, LIRIC, INSEM U 995, Université Lille2, Lille, France; 16Bristol Royal Hospital for Children, Bristol, United Kingdom; 17St. István & St. Laszlo Hospital, Budapest, Hungary; 18University Hospital, Basel, Switzerland; 19Rigshospitalet, Copenhagen, Denmark; 20Cliniques Universitaires St. Luc, Brussels, Belgium; 21University Medical Centre, Utrecht, Netherlands; 22University of Heidelberg, Heidelberg, Germany
Background: Current prognosis of patients with primary myelofibrosis (PMF) is based on disease-specific systems. However, outcome after allogeneic stem-cell transplantation (allo-SCT) is also influenced by patient- and transplant-specific factors. Here we aim to validate currently used prognostic scores and to evaluate all available variables at transplant that might be candidates for a transplant-related prognostic system.
Methods: Within the EBMT registry, we identified 585 PMF patients who received allo-SCT from an HLA-identical sibling or unrelated donor between 2007 and 2015 with full data on hemoglobin, white blood cell and platelet counts at transplant. Data on existing scores were calculated and thus available in 544 (93%, Karnofsky), 350 (60%, comorbidity index [HCT-CI]), 480 (82%, IPSS), 482 (82%, DIPSS), and 585 (100%, Lille). The primary outcome was overall survival (OS) up to three years after allo-SCT. The univariate impact on OS was investigated by using the Kaplan-Meier method. The discriminatory power of each score was evaluated by using concordance (C-) indices.
Results: Most patients were male (71%) and median age of the total cohort was 57 years (range, 24–75). Median time between diagnosis and transplant was 20 months (range, 1–501) while 203 (32%) transplants were received from an HLA-identical sibling. Peripheral blood was the most common graft source (n = 565, 88%). Median hemoglobin level was 9.4 g/dL(range, 3.7–17.9), median white blood cell and platelet counts were 7.9 g/dL (range, 0.1–365) and 109 x109/L (range, 1.3–1644). Mutation status was available in 331 (52%) patients.
Overall survival at three years after allo-SCT was 55.3% (95% CI, 51.0–59.6). Median follow-up of patients alive at last follow-up was 37 months. Patient- and transplant-related factors that showed a significant impact on OS until three years were: age < 60 vs. ≥60 years (p = 0.01), platelets ≤125 vs. >125 and hemoglobin ≤10 vs. >10 at transplant (p < 0.001, respectively), JAK2 vs. CALR vs. MPL mutation (p = 0.03), total body irradiation (p = 0.03), blast count < 10 vs. ≥10 (p = 0.03), white blood cell count < 25 x109/L (p = 0.04), the combination of a male recipient and female donor vs. other combinations (p = 0.01) as well as the combination of a recipient with a positive cytomegalovirus (CMV) serostatus and a CMV negative donor (p < 0.001). Donor type, graft source as well as whether a splenectomy was performed pre-transplant were not significant (p = 0.10, p = 0.27, and p = 0.14).
In the univariate analysis, scores such as Lille, Karnofsky, HCT-CI (p < 0.001, respectively) as well as DIPSS (p = 0.05) were associated with OS, in contrast to the IPSS (p = 0.33). The corresponding ranking ability showed C-indices of 0.57 (Lille), 0.57 (Karnofsky), 0.54 (HCT-CI), 0.55 (DIPSS), and 0.53 (IPSS).
Conclusions: First, currently available scores provide moderate prognostic ability to predict outcome of PMF patients undergoing stem-cell transplantation. Second, we identified eight factors that showed impact on OS which will be integrated in an augmented patient- and transplant-related score in further analyses.
Conflict of interest: No conflicts of interest related to the abstract.
O045 Outcome of Myeloablative and Reduced-intensity conditioned Allogeneic Haematopoietic Stem Cell Transplantation in Myelofibrosis: A Retrospective Study by the Chronic Malignancies Working Party of the EBMT
Donal McLornan1,2, Richard Szydlo3, Marie Robin4, Anja van Biezen5, Linda Koster6, Henrik Jan P Blok6, Dietrich Beelen7, Arne Brecht8, Gerard Socie4, Gerhard Ehninger9, Maria Teresa Van Lint10, Dietger Niederwieser11, Renate Arnold12, Jurgen Finke13, Antonin Vitek14, Ibrahim Yakoub-Agha15, Liisa Volin16, Peter Dreger17, Lothar Kanz18, Eefke Petersen19, Patrice Chevallier20, Matthias Stelljes21, Stephen Robinson22, E Klyuchnikov23, Yves Chalandon24, Nicolaus Kroger23
1Guy's and St.Thomas NHS Foundation Trust, Haematology, London, United Kingdom; 2King's College Hospital/King's College London, London, United Kingdom; 3Imperial College Healthcare NHS Trust, Department of Clinical Sciences, London, United Kingdom; 4Hôpital Saint-Louis, Service d'Hématologie-Greffe, Paris, France; 5EBMT Data Office, Leiden, Netherlands; 6University Medical Center, Statistics; EBMT Data Office, Leiden, Netherlands; 7Univeristy Hospital Essen, Haematology, Essen, Germany; 8Deutsche Klinik für Diagnostik, Wiesbade, Germany; 9Universitaetsklinikum Dresden, Dresden, Germany; 10Ospedale San Martino, Genova, Italy; 11University Hospital Leipzig, Division of Haematology & Oncology, Leipzig, Germany; 12Charité Universitaetsmedizin Berlin, Berlin, Germany; 13University of Freiburg, Department of Medicine - Hematology, Oncology, Freiburg, Germany; 14Institute of Hematology and Blood Transfusion, Prague, Czech Republic; 15CHU de Lille, LIRIC, INSERM U 995, Universite de Lille, Lille, France; 16HUCH Comprehensive Cancer Center, Helsinki, Finland; 17University of Heidelberg, Medizinische Klinik u. Poliklinik V, Heidelberg, Germany; 18Universitat Tübingen, Tübingen, Germany; 19University Medical Centre, Utrecht, Netherlands; 20CHU Nantes, Departement d’Hematologie, Nantes, France; 21University of Münster, Munster, Germany; 22University Hospital Bristol, Bristol, United Kingdom; 23University Hospital Eppendorf, Hamburg, Germany; 24Hôpitaux Universitaires de Genève, Geneva, Switzerland
Background: There has been a significant increase in the number of patients with Myelofibrosis (MF) undergoing Allogeneic Haematopoietic Stem Cell Transplantation (allo-HSCT). Historically, conditioning regimens-myeloablative (MAC) and reduced intensity (RIC)- have been heterogeneous in nature and direct comparisons limited. We conducted a retrospective, EBMT-registry analysis of a large cohort of MF allo-HSCT recipients.
Methods: All patients who underwent allo-HSCT for MF between 2000–2014 (RIC or MAC) utilising either bone marrow or peripheral blood stem cells and reported to the EBMT were selected. Statistical analyses were performed with SPSS 22 (SPSS Inc./IBM, Armonk, NY). All patients provided informed consent according to the Declaration of Helsinki.
Results: A total of 2183 patients were analysed and regimen intensity assessed by standard EBMT criteria. MAC regimens were utilised in 760 patients (Primary Myelofibrosis (PMF; n = 631); secondary MF (sMF; n = 129) while 1423 received RIC (PMF; n = 1423 and sMF; n = 369). Median age at allo-HSCT was 53 yrs (range (r), 18–74) in MAC and 58 (r,21–76) in RIC cohorts respectively. Median time from diagnosis to allo-HSCT in the MAC and RIC cohorts = 22 months (r, 1–324) and 31 months (r,1–526). Median follow up for surviving patients for MAC was 35 months (r,1–198) and 43 months (r,1–191) for RIC. Similar proportions of patients received prior therapy (MAC; n = 423 (56%) versus RIC; n = 783 (55%)). Lille score was documented for only 19% of the cohort. Donor source for both cohorts was similar: MAC; matched sibling donors (MSD) n = 309 (41%) and unrelated donors (URD) n = 451 (59%) and RIC cohort MSD; n = 543 (38%) and URD; n = 880 (62%). No significant differences existed in donor age or CMV serostatus (Donor/Recipient) between the conditioning cohorts. Time to neutrophil engraftment was documented for 94% of MAC and 93% of RIC cohorts with a median time of 18 and 17 days respectively. Median time to platelet engraftment was identical in both groups (19 days). Acute (a) and Chronic (c) Graft Versus Host Disease (GVHD) status was documented in 97% and 76% of entire cohort. Rates of any grade aGVHD were 29% in the MAC cohort and 32% in the RIC cohort whereas cGVHD rates (limited/extensive) were 23%/27% in the MAC cohort versus 20%/32% in the RIC cohort (p = 0.10). Non-relapse mortality (NRM) probabilities at 1,3 and 5-years were similar: 27.9%, 35.5% and 37.5% (MAC) and 27.4%, 35.9% and 39.1 % (RIC). Cumulative incidence of relapse (CIR) at 1,3 and 5-years was 13.8%, 23.3% and 27.7% (MAC) and 16.8%, 25.4% and 30.6% (RIC) (p = 0.10). Importantly, no significant difference in median Overall Survival (OS) between either approach was noted: MAC median OS = 6.6 years (95% confidence intervals (CI) 4.4–8.7) and RIC cohort 5.3 years (95% CI 3.9–6.7); p = 0.93). Moreover, no significant difference in progression free survival was evident.
Conclusions: This EBMT registry study is the largest cohort reported to date. Historically, the impact of conditioning intensity on outcome in MF patients has been unclear. We observed no statistically significant differences between engraftment, GVHD rates, NRM, PFS and OS between two large RIC and MAC cohorts. Multivariate and risk-adjusted subgroup analyses will be presented.
Clinical Trial Registry: Not relevant
Conflict of interest: No conflicts of interest or relevant financial disclosures from authors for this work.
O046 Final results of a multicentre phase II randomized study comparing fludarabine-busulfan versus fludarabine-thiotepa as reduced intensity preparative regimen for allogeneic transplantation in patients with myelofibrosis
Francesca Patriarca1, Arianna Masciulli2, Andrea Bacigalupo3, Chiara Pavoni2, Maria Chiara Finazzi2, Alberto Bosi4, Domenico Russo5, Franco Narni6, Giuseppe Messina7, Emilio Paolo Alessandrino8, Nicola Cascavilla9, Giuseppe Milone10, Benedetto Bruno11, Sonia Mammoliti12, Barbara Bruno12, Renato Fanin1, Francesca Bonifazi13, Alessandro Rambaldi2,14
1University of Udine-Azienda Sanitaria Universitaria, DAME, Udine, Italy; 2ASST Papa Giovanni XXIII, Bergamo, Italy; 3IRCSS San Martino, Genova, Italy; 4Ospedale Carreggi, Firenze, Italy; 5Spedali Civili, Brescia, Italy; 6Azienda Ospedaliera Universitaria, Modena, Italy; 7Azienda Ospedaliera, Reggio Calabria, Italy; 8IRCSS Policlinico S. Matteo, Pavia, Italy; 9IRCSS, San Giovanni Rotondo (FG), Italy; 10Ospedale Ferrarotto, Catania, Italy; 11Azienda Ospedaliera Universitaria, Città della Salute e della Scienza, Torni, Italy; 12Ufficio Sperimentazioni Cliniche, Gruppo Trapianti di Midollo Osseo (G.I.T.M.O.), Genova, Italy; 13Istituto Seragnoli, Bologna, Italy; 14Universiy of Milan, Hematology-Oncology, Milano, Italy
Background: Allogeneic haematopoietic stem cell transplantation (HSCT) remains the sole curative option for patients with myelofibrosis (MF). Although a spectrum of conditioning regimens has been used, the optimal preparative treatment before HSCT remains to be defined.
Methods: We conducted a phase II randomized study at 21 transplant centers comparing the reduced intensity conditioning (RIC) fludarabine-busulfan (FB) (conventional arm), that had been already tested in the prospective EBMT study (Kroeger N. et al, Blood 2009) with the RIC fludarabine-thiotepa (FT) (experimental arm), that has been widely used in Italy in the last 2 decades (Patriarca F. et al, Haematologica 2008). Eligible to this study were: patients with primary or secondary MF, age ≤ 70 years, Karnofsky performance status ≥ 60, comorbidity index ≤ 5 and with at least one of the following unfavorable prognostic factors: anemia (Hb < 10 g/dL), leukocytosis (25x109^/L), circulating blasts>1% or constitutional symptoms. Patients were randomized to receive intravenous busulfan 8 mg/kg or thiotepa 12 mg/kg associated to fludarabine 180 mg/mq. Anti-thymocyte globulin 7 mg/kg was administered in case of unrelated donors. The primary study endpoint was PFS. The sample size was defined on the basis of feasibility reasons and did not follow the statistical power estimate in order to demonstrate differences between the 2 arms, that are postponed to an eventual future phase III randomized study.
Results: From July 2011 to November 2015, 60 patients with a median age of 56 years (36–66) were enrolled . DIPSS score was intermediate-1, intermediate- 2 and high in 21 (35%), 36 (60%), and 3 (5%) patients, respectively. Donors were HLA-identical sibling (25), HLA-matched unrelated (25) or mismatched for a single class I HLA allele (10). At day + 30, cumulative incidence of neutrophil and platelets engrafment was 91% and 77%, respectively, without differences between the 2 arms. Patients with splenomegaly had a significantly slower neutrophil engrafment [HR 0.51 (95%CI 0.27–0.94) p = 0.032], while patients who had been splenectomized before HSCT had a significantly faster engrafment [HR 2.28 (95%CI 1.16–4.51) p = 0.017]. Overall, 5 patients had primary graft failure. Cumulative incidence of grade II-IV acute GVHD was 20% (including 8% grade III-IV) and cumulative incidence of mild of moderate chronic GVHD was 15%. With a median follow-up of 22 months (0–82), on an intention-to-treat basis the following outcomes were observed at 2 years after HSCT in the FB vs. the FT arm: PFS was 43% vs 55%, (p = 0.29), OS was 54% vs. 70% (p = 0.17), cumulative incidence of relapse and progression was 36% vs 24% (Gray's test p = 0.24) and NRM was 21% in both arms (Gray's test p = 0.99). A trend for a prolonged PFS was observed in patients with intermediate I DIPSS score, while donor type did not have a significant impact on the outcome.
Conclusions: We conclude that the experimental FT preparative treatment showed an higher disease control in comparison with FB treatment, although this advantage in small and does not support future phase 3 studies. Both RIC regimen were safe for transplants from sibling and unrelated donors. Spleen size significantly impaired the engrafment.
Clinical Trial Registry: ClinicalTrials.gov, number NCT01814475
Conflict of interest: No conflict of interest to disclose.
O047 In vivo T-cell depletion in patients with myelofibrosis transplanted from an HLA matched sibling donor: an EBMT study
Marie Robin1, Sylvie Chevret2, Linda Koster3, Christine Wolschke4, Ibrahim Yakoubagha5, Jean-Henri Bourhis6, Patrice Chevallier7, J.J. Cornelissen8, Péter Reményi9, Johan Maertens10, Xavier Poire11, Charles Craddock12, Gerard Socié13, Maija Itälä-Remes14, Harry Schouten15, Tony Marchand16, Jakob Passweg17, Didier Blaise18, Gandhi Damaj19, Zubeyde Nur Ozkurt20, Tsila Zuckerman21, Pierre-Simon Rohrlich22, Hélène Labussière23, Jörg Cammenga24, Yves Chalandon25, Nicolaus Kroger4
1Hopital Saint-Louis, Hematology / Transplantation, Paris, France; 2University Paris 7, Paris, France; 3EBMT Data Office, Leiden, Netherlands; 4University Hospital Eppendorf, Hamburg, Germany; 5CHU de Lille, INSERM U 995, Lille, France; 6Gustave Roussy, institut de cancérologie, Villejuif, France; 7CHU Nantes, Nantes, France; 8Erasmus MC Cancer Institute, University Medical Center, Rotterdam, Netherlands; 9St. István & St. Laszlo Hospital, Budapest, Hungary; 10iversity Hospital Gasthuisberg, Leuven, Belgium; 11Cliniques Universitaires St. Luc, Brussels, Belgium; 12Queen Elizabeth Hospital, Birmingham, United Kingdom; 13Hopital Saint-Louis, APHP, Paris, France; 14HUCH Comprehensive Cancer Center, Helsinki, Finland; 15University Hospital Maastricht, Maastricht, Netherlands; 16Centre Hospitalier Universitaire de Rennes, Rennes, France; 17University Hospital Basel, Basel, Switzerland; 18Institut Paoli Calmettes, Marseille, France; 19CHU Caen, Caen, France; 20Gazi University Faculty of Medicine, Ankara, Turkey; 21Rambam Medical Center, Haifa, Israel; 22CHU Nice - Hôpital de l`ARCHET I, Nice, France; 23Centre Hospitalier Lyon Sud, Lyon, France; 24University Hospital, Linköping, Sweden; 25Hôpitaux Universitaires de Genève, Genova, Switzerland
Background: Allogeneic hematopoietic stem cell transplant is the only curative treatment in patients with myelofibrosis. A recent prospective randomized study (NEJM 2016 Kröger) has reported that anti-human T lymphocyte immune globulin (ATG) was efficient to prevent chronic GVHD in the setting of HLA matched sibling transplant in patients with acute leukemia without increasing relapse risk. Regarding myelofibrosis, very few data are available for the potential effect of in vivo T-cell depletion (TCD) in this rare disease. We report here the outcome of myelofibrosis patients after HLA matched sibling donor with or without TCD.
Methods: Patients with primary or secondary myelofibrosis transplanted between 2007 and 2015 from an HLA matched sibling donor and registered in the European registry Promise were included. Patients who had no data regarding blood cell count at transplant were excluded. Three hundred patients were identified and 148 (49%) had received TCD during their regimen of whom 13 had received alemtuzumab and 135 received ATG. GRFS was defined as a survival without relapse and without chronic extensive GVHD and no previous grade III-IV acute GVHD. Multivariable models based on Cox proportional hazard were performed to test potential predictors and ATG effect was thus included in the models. Statistical analysis was performed using R software.
Results: Patients who received a TCD were younger (56 vs. 58 years), had less often splenectomy before transplant (10% vs. 28%), received more frequently a myelo-ablative regimen (24 vs 16%) and marrow as source of stem cell (17% vs. 2%). Lille score was intermediate/high in 71% and 67% of patients treated with or without TCD, respectively. Cumulative incidence for grade II-IV acute GVHD was higher without TCD, (41 vs 24%, p = 0.0023) (Figure 1a). Incidence of grade III-IV acute GVHD was similar. Chronic GVHD incidence was at 61% and 53% with and without TCD (p = 0.28) and incidence of chronic extensive GVHD was not significantly different. Relapse incidence was higher after TCD, (19% vs. 25%, p = 0.06) (Figure 1b). Overall survival and non-relapse mortality were superimposable in the 2 groups while EFS and GRFS were non significantly better in favor of the group without ATG (Figure 1c & 1d). Multivariable analyses for all outcomes failed to demonstrate any significant difference with or without ATG after adjustment for age, DIPSS, Karnofsky, conditioning regimen intensity and previous splenectomy: hazard ratio were 1.39 for relapse (95%CI: 0.78–2.47, more relapse with TCD), 1.125 (95%CI: 0.79–1.98) for chronic extensive GVHD, 0.75 (95%CI: 0.51–1.11) for OS (less mortality with TCD), 0.93 (95%CI: 0.65–1.34) for EFS, 0.93 (95%CI: 0.65–1.34) for GRFS, 0.70 (0.44–1.13) in favor of TCD.
Conclusions: In myelofibrosis patients, TCD taken all together (campath®, grafalon®, thymoglobuline®) prevent grade II-IV acute GVHD without decreasing incidence of grade III-IV acute GVHD and chronic GVHD without impacting significantly survivals. More deeply analyses will be done to analyze the role of TCD brand and dosage.
Conflict of interest: there is no conflict of interest in this study.
[[O047 Figure].
Figure 1]
O048 Contemporary role of maintenance tyrosine kinase inhibitors following allogeneic hematopoietic cell transplantation for chronic myeloid leukemia: a CIBMTR analysis
Zachariah DeFilipp1, Richard Ancheta2, Ying Liu3, Kwang Woo Ahn3, Zhen Huan Hu3, Edwin Alyea4, Uday Popat5, Ronald Sobecks6, Wael Saber3
1Massachusetts General Hospital, Blood and Marrow Transplant Program, Boston, MA, United States; 2Scripps Blood & Marrow Transplant Program, La Jolla, CA, United States; 3Medical College of Wisconsin, Milwaukee, WI, United States; 4Dana Farber and Boston Children's Cancer and Blood Disorders Center, Boston, MA, United States; 5MD Anderson Cancer Center, Houston, TX, United States; 6Cleveland Clinic Taussig Cancer Institute, Cleveland, OH, United States
Background: Tyrosine kinase inhibitors (TKIs) targeting BCR-ABL have changed the therapeutic landscape for chronic myeloid leukemia (CML). Currently, allogeneic hematopoietic cell transplantation (HCT) remains central to the management of accelerated (AP) and blast phase (BP) CML and is reserved for TKI failure in chronic phase (CP) disease. We sought to determine whether maintenance therapy with TKIs following allogeneic HCT improves disease control and survival for patients with CML.
Methods: The primary aims were to examine the impact of maintenance TKI therapy on disease-free survival (DFS) and overall survival (OS) in 390 patients with CML receiving allogeneic HCT between 2007 and 2014, as reported to the CIBMTR. All patients received TKI therapy prior to HCT. We analyzed transplant outcomes for two cohorts of patients: a) those who received post-HCT maintenance TKI and b) those who did not receive post-HCT maintenance TKI. Maintenance therapy was determined based on data recorded from post-transplant disease specific forms, which included the choice of TKI but not the date of initiation or the duration of maintenance therapy. Relapse was defined by report of molecular, cytogenetic, and/or hematologic disease post-HCT. Multivariate regression analysis was performed using Cox proportional hazards model to evaluate the association between maintenance TKI therapy with chronic GVHD, relapse, TRM, DFS and OS, with adjustment for patient-related, disease-related, and transplant-related values. In an attempt to correct for selection bias, this study was conducted as a landmark analysis that excluded patients that died, relapsed, had cGVHD or were censored prior to day 100 post-HCT.
Results: Disease status at transplant included CP1 (n = 109, 28%), CP2+ (n = 99, 25%), AP (n = 55, 14%), BP (n = 23, 6%), or complete hematologic response (n = 104, 27%). The most common potential indication for HCT amongst patients in CP1 or CHR was the failure to achieve cytogenetic or molecular CR (n = 140, 66%). The majority of patients received MAC (n = 326, 84%), as compared to RIC (n = 48, 12%) or NMA (n = 15, 4%). Graft sources included peripheral blood (n = 258, 66%), bone marrow (n = 82, 21%), and cord blood (n = 50, 13%). The number of transplants decreased over time (2007–2010, n = 296; 2011–2014, n = 94). Eighty-nine patients received post-HCT maintenance TKIs, while 301 received no maintenance. The most common TKIs used for maintenance were dasatinib (n = 38), nilotinib (n = 22), and imatinib (n = 17), with an additional 12 patients receiving multiple TKIs. On univariate analysis, maintenance therapy did not significantly impact 5-year DFS (41% v 45%, p = 0.96) or OS (58% v 58%, p = 0.56). In multivariate analysis (Figure), CP2+ disease was associated with an increased risk for relapse and inferior DFS and OS while BP disease was associated with inferior OS as compared to patients transplanted in CP1. In multivariate analysis, maintenance therapy following HCT did not significantly impact relapse,DFS, or OS, regardless of disease status at transplant.
Conclusions: Allogeneic HCT remains a curative therapy for patients with CML with encouraging disease control and survival. Our data did not demonstrate a significant impact of maintenance TKI therapy on relapse, DFS or OS. The optimal approach to TKI administration in the post-transplant setting remains undetermined.
Conflict of interest: None of the authors has anything to disclose.
| Relapse | DFS | OS | |
|---|---|---|---|
| Disease status | |||
| CP1 (reference) | – | — | — |
| AP | 1.5 (0.8–2.9), p = 0.254 | 1.2 (0.7–2.0), p = 0.438 | 1.0 (0.6–1.7), p = 0.947 |
| BP | 2.2 (1.0–4.7), p = 0.051 | 1.8 (1.0–3.1), p = 0.054 | 2.5 (1.3–4.6), p = 0.004 |
| CP2+ | 2.3 (1.3–4.0), p = 0.004 | 1.9 (1.3.-2.8), p = 0.002 | 1.7 (1.1–2.7), p = 0.013 |
| Hematologic CR | 1.0 (0.5–1.9), p = 0.957 | 1.1 (0.7–1.7), p = 0.619 | 1.2 (0.7–1.8), p = 0.552 |
| Maintenance therapy | |||
| No (reference) | — | — | — |
| Yes | 1.2 (0.8–1.9),p = 0.380 | 0.9 (0.7–1.3), p = 0.724 | 0.8 (0.5–1.2), p = 0.233 |
[ [O048 Figure] Table 1]
Conditioning regimen
O049
Abstract previously published
O050
Abstract previously published
O051 Comparison of sequential versus myeloablative, reduced intensity and non-myleoablative conditioning for patients with myelodysplastic syndrome: An analysis of the CMWP of the EBMT
Victoria Potter1, Jufeng Wang2, Gerhard Ehninger3, Francis Auyk4, Linda Koster5, Juergen Finke6, Dietrich Beelen7, Gerard Socie8, Arnold Ganser9, Henrik Sengeloev10, Helene Labussière-Wallet11, Maija Itala-Remes12, Lisbeth de Wreede13, Marie Cascon14, Pavel Jindra15, Ghulam Mufti16, Johanna Tischer17, Yener Koe18, Mutlu Arat19, Martin Bornhauser3, Maximillian Christopeit4, Hartmut Bertz6, Nina-Kristina Steckel7, Nicolaus Kroeger4, Marie Robin8
1Kings College Hospital, NHS Foundation Trust, Hematological Medicine, London, United Kingdom; 2EBMT Statistical Unit Data Office Leiden, Leiden, Netherlands; 3Universitaetsklinikum Dresden, Dresden, Germany; 4University Hospital Eppendorf, Hamburg, Germany; 5EBMT Data Office Leiden, Leiden, The Netherlands, Leiden, Netherlands; 6University of Freiburg, Freiburg, Germany; 7University Hospital, Essen, Germany; 8Hopital St. Louis, Paris, France; 9Hannover Medical School, Hannover, Germany; 10Rigshospitalet, Copenhagen, Denmark; 11Centre Hospitalier Lyon Sud, Lyon, France; 12HUCH Comprehensive Cancer Center, Heksinki, Finland; 13Leiden University Medical Centre, Leiden, Netherlands; 14Hospital Regional de Málaga, Malaga, Spain; 15Charles University Hospital, Pilsen, Czech Republic; 16Kings College Hospital and Kings College London, London, United Kingdom; 17Klinikum Grosshadern, Munich, Germany; 18Medical Park Hospitals, Antayla, Turkey; 19Florence Nightingale Sisli Hospital, Istanbul, Turkey
Background: Outcomes for the majority of patients who receive stem cell transplantation (SCT) for myelodysplastic syndrome (MDS) remain suboptimal with less than 50% of patients surviving in the longer-term. In recent years the development of the sequential approach (Seq) whereby transplant conditioning is delivered immediately after a course of chemotherapy to eradicate pre-transplant disease burden has appeared promising. No prospective data exists that comparing this approach with standard conditioning including myeloablative (MA), reduced intensity (RIC) or non-myeloablative (NMA) protocols. In this context the CMWP of the EBMT conducted a retrospective analysis of patients receiving SCT for MDS to assess the optimal conditioning protocol.
Methods: Records were identified from all registry patients with a diagnosis of MDS included in the data quality initiative from 2007 to 2014. Data collected included demographics and disease variables. Survival analyses were done via Kaplan-Meier and multivariate analysis performed for relevant variables.
Results: 767 patients were identified receiving Seq (n = 158), NMA (n = 36), RIC (n = 423) and MA (n = 144) protocols. Median follow-up was 62 months and median age at SCT was 59 (range 18–79) years. Disease types at SCT were RA/del5q/RCMD (n = 128), RAEB (n = 360), MDS unclassifiable (n = 48), and transformed to AML (n = 228). IPSS stage was low (n = 54), int-1 (n = 217), int-2 (173) and high (124). Karnofsky performance status (KPS) was 90–100 in 68% of patients and < 90 in 32%. Complete remission was reported in 24% at time of SCT. Donors were identical sibling in 43% (n = 329) and 8/8 HLA matched unrelated in 57% (n = 438). The majority of patients (90%, n 693) received peripheral blood stem cells as graft source and t-cell depletion was included as part of protocol in 56% (n = 428). Overall survival (OS) was 54% (95%CI 51–58%) for the entire cohort at 3yrs. Significant differences were noted per protocol (p = 0.02) with those receiving MA having the best OS: MA 3yr OS 64% (95%CI: 56–72%), NMA 3yrOS 58% (95%CI: 42–72%), RIC 3yr OS 52% (95%CI: 47–57%) and Seq 3yr OS 50%(95%CI: 42–58%). Inferior outcomes were reported for those with KPS < 90 (3yrOS 41% vs 61% for KPS 90–100 (p < 0.001)), and male sex (3yrOS 52% for male vs 58% for female). For relapse free survival (RFS) significant differences were reported for KPS (p < 0.001) and conditioning protocol (p = 0.04) with those receiving MA having the best outcome. RFS at 3yrs for MA was 59% (95%CI: 51–67%), RIC 47% (95%CI: 42–52%) and Seq 44% (95%CI: 36–52%). For non-relapse mortality (NRM) per protocol no significant difference was observed. On multivariate analysis conditioning protocol was not significant after adjustment for other factors. Significant differences were found for KPS (HR 1.7, p < 0.001), age at time of SCT (HR 1.2, p = 0.002), and disease transformed to AML (HR 1.6, p = 0.003).
Conclusions: This report is the largest analysis to date of different conditioning protocols focussed on patients with receiving SCT for MDS. Notably those receiving myeloablative protocols do well. However on multivariate analysis this advantage is mitigated by other factors and outcomes are more affected by performance status, age and disease status having transformed to AML.
Conflict of interest: No relevant conflicts of interest for this abstract
O052
Abstract previously published
O053 Thiotepa, Fludarabine and Busulfan conditioning regimen before T-cell replete haploidentical transplantation with post-transplant cyclophosphamide for AML and MDS : a bicentric experience of 109 patients
Thomas Pagliardini1, Luca Castagna2, Samia Harbi1, Jérome Rey1, Sabine Furst1, Stefania Bramanti2, Faezeh Legrand1, Valerio Maisano1, Catherine Faucher1, Angela Granata1, Pierre-Jean Weiller1, Boris Calmels1, Claude Lemarié1, Christian Chabannon1, Norbert Vey1, Didier Blaise1, Raynier Devillier1
1Institut Paoli Calmettes, Marseille, France; 2Humanitas Cancer Center, Milan, Italy
Background: Haploidentical transplantation (Haplo-SCT) with post-transplant cyclophosphamide (PT-Cy) results in low rates of both GVHD and non-relapse mortality (NRM), especially using a non-myeloablative conditioning (NMAC) regimen (Cyclophosphamide, Fludarabine (Flu) and 2 Gy TBI, Luznik BBMT 2008). In the setting of HLA-identical transplantation, we previously reported that more intensive regimen including busulfan (Bu) had better anti-tumor effect than NMAC (Blaise, Cancer 2013). Thus, to improve disease control after Haplo-SCT, we have progressively replaced NMAC by a thiotepa (TT), Flu and Bu conditioning platform (TBF), especially for myeloid malignancies. Here, we report the outcome of TBF conditioning Haplo-SCT in AML or MDS patients. In addition, we analyzed the prevalence of GVHD and immunosuppressive therapy after Haplo-SCT, and the early toxicities that may be related to TBF with PT-Cy.
Methods: We included patients with following criteria: (1) adult patients with AML or MDS; (2) Haplo-SCT with PT-Cy between 2011 and 2016 in 2 collaborative transplantation centers; and (3)TBF conditioning regimen. Fludarabine total dose was 120–160 mg/m² whatever the conditioning intensity. The association of TT at 5 mg/kg with intravenous busulfan at the total dose of 260 mg/m² was defined as RIC-TBF. More intensive combinations (TT at 5–10 mg/kg with Bu total dose ≥390 mg/m²) were defined as MAC-TBF.
Results: We analyzed 109 patients (MDS n = 27; AML n = 82) with a median age of 61 years (22–72). The median follow up period was 18 months (4–60). 45 (41%) and 33 (31%) patients underwent Haplo-SCT in first complete remission (CR1) and advanced CR (CR≥2), respectively, while 31 (29%) patients had refractory disease. Cytogenetic risk was favorable, intermediate and unfavorable in 7 (6%), 70 (64%), and 30 (30%) patients, respectively. Peripheral blood stem cell grafts were used in 95 (87%) patients. 85 (78%) and 24 (22%) patients received RIC-TBF and MAC-TBF, respectively. Cumulative incidences of grade II-IV, grade III-IV acute GVHD, and moderate or severe chronic GVHD were 18%, 7%, and 10%, respectively. At 1 year after Haplo-SCT, most disease-free patients were living with no immunosuppressive treatment (80%) and no GVHD (82%) (Figure 1)
We observed 17% of grade 3/4 liver toxicity and 14% of hemorrhagic cystitis. Non-relapse mortality at 2 years (NRM) was 30%. Non-relapse deaths were related to sepsis (50%), GVHD (31%), neurologic failure (13%) and others causes (6%). We observed higher NRM in CR≥2 patients (CR1 vs. CR≥2 vs. refractory: 20% vs. 42% vs. 29%; p = .242). No difference in NRM was observed between MAC (26%) and RIC (31%) (p = .763). At 2 years, the cumulative incidence of relapse (CIR) was 11%, 23% and 23% in patients transplanted in CR1, CR≥2 and with refractory disease, respectively. OS, PFS and GRFS were 57%, 52%, and 43%, respectively.
Conclusions: We conclude that TBF conditioning regimen for Haplo-SCT is highly effective for AML/MDS patients (CIR for CR1: 11%; for advanced disease: 23%). However, high NRM was observed in patients who were heavily pretreated before transplantation (i.e. CR≥2). This result suggests that the risk of NRM has to be better evaluated for patients with advanced diseases.
Conflict of interest: None of the authors has anything to disclose.
[O053 Figure].
![[O053 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/67ec8f842cd0/41409_2018_318_Figi_HTML.jpg)
Prevalence of immunosupressive therapy and GVHD after haplo-HSCT for patients without relapse]
O054
Abstract previously published
O055
Abstract previously published
O056 Phase I Study of Escalating Doses of Total Marrow and Lymphoid Irradiation for Haploidentical Hematopoietic Cell Transplantation Combined with Post-Transplant Cyclophosphamide Myelodysplasia or Acute Leukemia
Monzr Al Malki1, Joycelynne Palmer2, Jeffrey Wong3, Susanta Hui3, Ni-Chun Tsai2, Chatchada Karanes Chatchada Karanes1, Sally Mokhtari4, Ketevan Gendzekhadze5, David Senitzer5, Anthony Stein1, Ryotaro Nakamura1, Stephen J. Forman1, Joseph Rosenthal6
1City of Hope, Hematology/HCT, Duarte, CA, United States; 2City of Hope, Information Sciences - BRI, Duarte, CA, United States; 3City of Hope, Radiation Oncology, Duarte, CA, United States; 4City of Hope, Clinical Translational Program Developement, Duarte, CA, United States; 5City of Hope, Histocompatibility Lab, Duarte, CA, United States; 6City of Hope, Pediatric HCT, Duarte, CA, United States
Background: While utilizing T cell replete graft followed by PTCy has demonstrated acceptably low incidences of non-relapse mortality (NRM) and graft-versus-host disease (GvHD) in patients receiving HaploHCT, relapse rate, especially for high-risk patients, remains high mainly due to limited disease control. Thus, optimization of preparative regimen could improve transplant outcomes. Targeted conformal total marrow and lymphoid irradiation (TMLI) allows for delivery of precisely focused radiation to the major marrow sites and other targeted structures, without increasing off-target radiation exposure and toxicity to vital organs. Here, we report the results of a phase I trial, evaluating the safety and determining the maximum tolerated dose (MTD) of pre-HCT TMLI combined with PTCy.
Methods: Patients with intermediate/high-risk myelodysplastic syndrome, high-risk acute lymphoblastic or myeloid leukemia were assessed. The transplant preparative regimen was Fludarabine 25mg/m2/day (days -7 to -3), cyclophosphamide 14.5 mg/kg/day (days -7 and -6) and TMLI (days -7 to -3). Radiation dose was escalated in increments of 200cGy (1200 to 2000cGy) in cohorts of 3–6 patients until dose limiting toxicity (DLT) was reached (Bearman and CTCAE 4.0 scales). Liver and brain radiation doses were kept at 1200cGy. Mean dose to normal organs were 13–67% of the marrow dose (lung 45%, esophagus 34% and oral cavity 21%). All patients received peripheral blood stem cells. GvHD prophylaxis consisted of PTCy at 50 mg/kg (days +3 and +4) combined with Tacrolimus and MMF(starting day +5).
Results: From 7/6/2015 to 8/29/2017, 18 patients underwent HaploHCT (Table). Median follow-up for surviving patients was 11.8 months (2.3–24.4). Six patients were treated at 1800 cGy, the current dose level being tested, without experiencing DLT. The 1 year overall-survival and cumulative incidence of relapse/progression were 86.2% (95%CI: 55.0–96.4) and 13.3% (95%CI: 3.7–48.4), respectively. All evaluable patients (n = 15) achieved CR/CRi at day 30. Eleven patients (out of 15 fully evaluable) (61%) developed aGvHD (maximum grade is 2). The day 30 and 100 NRM rates were 0% and 6.7%, respectively. The most common toxicities, across all dose levels tested, were grade 2 Hepatic (n = 3) and gastrointestinal (n = 3) (Bearman). One patient, treated at 1800cGy experienced grade 3 pulmonary toxicity DLT (Bearman). Causes of death were veno-occlusive disease (n = 1) and infection (n = 1).
Conclusions: TMLI doses can be safely escalated to 1800cGy (No MTD, thus dose escalation will continue to 2000cGy). Compared to current published reports, a reduction in relapse/progression was achieved without increasing NRM.
Clinical Trial Registry: NCT02446964
https://clinicaltrials.gov/ct2/show/NCT02446964
Conflict of interest: Authors do not have any relevant conflicts of interest.
| Variable | Median (range)/N | Variable | Median (range)/N |
|---|---|---|---|
| Age | 39 (21–58) | Cytogenetic Risk | |
| Diagnosis | Favorable | 6 | |
| AML | 10 | Intermediate | 3 |
| ALL | 7 | Unfavorable | 7 |
| MDS | 1 | Unavailable | 2 |
| Disease status at HCT | KPS at HCT | 80 (70–100) | |
| CR1 | 6 | WBC at HCT | 2.9 (0.1–7.7) |
| CR2 | 5 | % Blasts at HCT : Blood/BM | 0 (0–6)/1 (0–11) |
| CR3/IF | 2/5 | extramedullary disease at HCT | 0 |
[ [O056 Table] Table. Patient Characteristics]
Early complications/late effects and quality of life
O057 Subsequent Malignancies and Mortality in Children Undergoing Allogeneic Hematopoietic Cell Transplantation for Non-Malignant Diseases: A Report from the Late Effects Working Committee of the CIBMTR
Justine Kahn1, Bronwen Shaw2, Ruta Brazauskas2, Heather Millard2, Minoo Battiwalla3, Bipin Savani4, Mary Flowers5, Prakash Satwani6
1Columbia University Medical Center, Pediatric Hematology/Oncology/Stem Cell Transplantation, New York, NY, United States; 2Medical College of Wisconsin, Milwaukee, WI, United States; 3National Heart, Lung and Blood Institute, Bethesda, MD, United States; 4Vanderbilt University Medical Center, Brentwood, TN, United States; 5Fred Hutchinson Cancer Research Center, Seattle, WA, United States; 6Columbia University Medical Center, New York, NY, United States
Background: Allogeneic hematopoietic cell transplantation (alloHCT) is a curative treatment option for children with non-malignant diseases (NMD). Given increasing use of alloHCT for NMD, data regarding late sequelae and long-term outcomes are of high importance. We assessed the risk of subsequent neoplasms (SNs) and mortality in children undergoing alloHCT for NMD, and examined whether they had 1) increased risk for SNs, and 2) higher mortality relative to the general population of age-matched controls.
Methods: We studied subjects < 21 years of age after first alloHCT for NMD, reported to the CIBMTR from 237 centers. Survival was determined from transplantation until last contact. Comparison of mortality rates with the general population included only the U.S. and Canada; 20,370 person-years contributed to the analysis. Confirmed SNs were used for comparisons of site-specific cancer incidence to the general population.
Results: From 1995–2012, 6,028 subjects met criteria for inclusion in this study. Median age at transplantation was 6 years, median follow-up was 7.8 years. Severe aplastic anemia (SAA) was the most common NMD, N = 1456 (24%). Fanconi anemia (N = 598), thalassemia (N = 574) and severe combined immunodeficiency (SCID) (N = 583) each comprised 10%; 3731 (62%) patients received myeloablative conditioning (17% total-body irradiation); 2298 (38%) underwent matched sibling, and 1023 (17%) underwent matched unrelated donor transplants.
Mortality: Overall survival was 72% (95% confidence interval: 71% to 73%). Transplant patients had a 213-fold increased mortality risk compared to the general population (standardized mortality ratio [SMR] = 213.61, [95% confidence interval] 197.55–230.58; p < 0.0001). Leading causes of death were infection (20%) and organ failure (23%).
Subsequent neoplasms: A total of 71 SNs were reported, 40 confirmed. Compared to the general population, the studied cohort had an 11-fold increased risk of developing SNs (standardized incidence ratio [SIR], 11). Rates of SN were higher in children with Fanconi anemia (n = 31, 5.2%), SAA (n = 16, 1%) and marrow failure (n = 16, 1.7%), than in children with immunodeficiency syndromes including SCID, (n = 5, 0.3%). Oropharyngeal neoplasms, (n = 18; 25%) were the most common SNs, followed by hematologic malignancies (n = 19; 27%) including leukemia and myelodysplastic syndrome (MDS). Skin cancers, including melanoma, accounted for 13% (n = 9). Considering confirmed cases only, there were significantly higher than expected rates of: myeloid leukemia (SIR, 25.2), MDS (SIR, 772.3), oropharyngeal, including tongue (SIR, 752.3) and mouth (SIR, 143.3), liver (SIR, 45.3) and thyroid (SIR, 22.1) cancers. All cases of acute leukemia developed before 5-years post-alloHCT; MDS risk persisted past 10 years. Oropharyngeal and thyroid cancers were all diagnosed after 5 years.
Conclusions: Long-term follow-up of this large cohort of children with NMD reveals higher than expected occurrence of SNs after alloHCT, and higher than expected mortality compared with the general population. In addition to early leukemia and skin cancer, there is a later risk for radiation-associated cancers (thyroid, oropharyngeal), liver cancer as well as a persistent risk for MDS. The majority of non-leukemic SNs occurred 5 or more years post-transplant, further highlighting the need for long-term surveillance and close multidisciplinary follow-up in these at-risk patients.
Conflict of interest: None of the authors has anything to disclose
[O057 Figure].
Table 1 Standardized incidence ratios of development of subsequent neoplasms in the US and Canada Tab1
.
O058 Comparable Long-Term Outcome after Allogeneic Stem-Cell Transplantation from Sibling and Matched-Unrelated Donors in AMLpatients older than 50 years. A Report from the ALWP of EBMT
Avichai Shimoni1, Myriam Labopin2, Bipin Savani3, Liisa Volin4, Jürgen Finke5, Dieter Niederwieser6, Gerhard Ehninger7, Didier Blaise8, Dietrich Beelen9, Reza Tabrizi10, Henrik Sengeloev11, Arnold Ganser Ganser12, Jan Cornelissen13, Mohamad Mohty2, Arnon Nagler1
1Chaim Sheba Medical Center, Tel-Hashomer, Israel; 2Hôpital Saint Antoine, Paris, France; 3Vanderbilt University, Nashville, TN, United States; 4HUCH Comprehensive Cancer Center, Helsinki, Finland; 5University of Freiburg, Freiburg, Germany; 6University Hospital Leipzig, University Hospital Leipzig, Germany; 7Universitaetsklinikum Dresden, Dresden, Germany; 8Centre de Recherche en Cancérologie de Marseille, Marseille, France; 9University Hospital Essen, Essen, Germany; 10CHU Bordeaux, Bordeaux, France; 11National University Hospital, Copenhagen, Copenhagen, Denmark; 12Hannover Medical School, Hannover, Germany; 13Erasmus MC Cancer Institute, Rotterdam, Netherlands
Background: Allogeneic stem-cell transplantation (SCT) is curative therapy in AML. Most deaths after SCT occur within the first 2 years. Marked improvement has been achieved in SCT from unrelated donors (UD) in recent years. Prior large cohort studies showed that patients surviving leukemia-free 2 years after SCT have high probability of survival at 10 years. Most of these studies were done in younger patients following myeloablative conditioning (MAC). However, there is relatively limited data on the comparison of long-term outcomes (beyond 10 years) of SCT in older AML patients (age ≥50 years) from sibling and UD.
Methods: We analyzed long-term outcomes in a relatively large cohort of patients with de-novo AML (n = 1134), age ≥50 years, who were alive and leukemia-free 2 years after SCT from matched siblings (n = 848) or UD (n = 286), in the years 2000–2007, median follow up 8.6 years (2–16.4).
Results: The median patient age was 56 in the sibling group and 58 years in the UD group (P = 0.005). 77%, 12% and 11% in the sibling group were in CR1, CR2 and active leukemia at SCT compared to 50%, 25% and 25% in the UD group, respectively (P < 0.001). 37% and 38% had reduced-intensity conditioning (P = 0.78). 27% and 70% had in-vivo T-cell depletion (TCD, P < 0.001). Chronic GVHD occurred in 61% and 53%, respectively (P = 0.02). The 10-year leukemia-free survival (LFS) of patients surviving leukemia-free 2 years after SCT was 72% (68–75) and 62% (55–70), respectively (P = 0.30). Multivariate-analysis identified active leukemia at SCT (HR 1.8, P < 0.001), CR2 (HR 1.5, P = 0.02) compared to CR1 and female recipient (HR 0.7, P = 0.005) as independent factors predicting LFS. The donor type, conditioning regimen, age, cytogenetics and prior acute or chronic GVHD were not significant. Relapse occurred in 15% (13–18%) and 17% (12–22%, P = 0.97), respectively. SCT in active disease (HR 2.2, P < 0.001), CR2 (HR 1.9, P = 0.006), poor cytogenetics (HR 5.8, P = 0.02), in-vivo TCD (HR 5.5, P = 0.03) and female gender (HR 0.6, P = 0.03) were risk-factors for late relapse. Non-relapse mortality (NRM) occurred in 13% (11–16%) and 21% (15–28%, P = 0.15), respectively. Advanced age was the only risk-factor for late NRM (HR, 1.6, P = 0.03). Donor and conditioning type were not predictive of late relapse or NRM. There were 209 late deaths after sibling and 72 after UD SCT. Relapse was the cause of death in 53% and 37% of late deaths, respectively (P = 0.06). GVHD was the cause of death in 16% and 22% and infection in 9% and 19%, respectively (P = 0.05). Second malignancy was the cause of death in 13% and 12% of late deaths, respectively.
Conclusions: Long-term outcome is similar after SCT form sibling or UD in AML patients older than 50 years. Patients who are leukemia-free 2 years after SCT can expect good and similar subsequent outcome with both donor types. Disease status was the major predictor of subsequent LFS while conditioning intensity had no effect. Whereas relapse is the major cause of late death after both donor types, NRM and in particular GVHD and infections are more common causes of late death after SCT from UD.
Conflict of interest: The authors report no conflicts of interest.
O059 Graft failure after reduced intensity conditioning - a retrospective study of the Transplant Complications Working Party EBMT
Bernd Hertenstein1, Eric Beohou2, Steffie van der Werf3, Didier Blaise4, Renate Arnold5, Boris Afanasyev6, Peter Dreger7, Gerard Socie8, Jürgen Finke9, Dietger Niederwieser10, Nigel Russell11, J. Cornelissen12, Christof Scheid13, Martin Bornhäuser14, Ernst Holler15, Johanna Tischer16, Ellen Meijer17, Michael Potter18, Henrik Sengeloev19, Nicolaus Kröger20, Silvia Montoto21, Arnon Nagler22, Mohamad Mohty23, Grzegorz Basak24, Rafael Duarte25, Tapani Ruutu26
1Klinikum Bremen-Mitte, Bremen, Germany; 2Paris Study Office/CEREST-TC, Paris, France; 3Data Office, Leiden, Netherlands; 4Institut Paoli Calmettes, Cedex Marseille, France; 5Charité Universitaetsmedizin Berlin, Berlin, Germany; 6First State Pavlov Medical University of Saint Petersburg, Raisa Gorbacheva Memorial Institute for Children Oncology, Hematology and Transplantation, Saint Petersburg, Russian Federation; 7University of Heidelberg, Heidelberg, Germany; 8Hospital St. Louis, Paris, France; 9University of Freiburg, Freiburg, Germany; 10University Hospital Leipzig, Leipzig, Germany; 11Nottingham City Hospital, Nottingham, United Kingdom; 12University Medical Center Rotterdam, Rotterdam, Netherlands; 13University of Cologne, Cologne, Germany; 14Universitätsklinikum Dresden, Dresden, Germany; 15University Regensburg, Regensburg, Germany; 16Klinikum Grosshadem, Munich, Germany; 17VU University Medical Center, Amsterdam, Netherlands; 18Royal Marsden Hospital, London Surrey, United Kingdom; 19Rigshospitalet, Copenhagen, Denmark; 20University Hospital Eppendorf, Hamburg, Germany; 21St. Bartholomew`s and The Royal London NHS Trust, London, United Kingdom; 22Chaim Sheba Medical Center, Tel Hashomer, Israel; 23Hospital Saint Antoine, Paris, France; 24Medical University of Warsaw, Warsaw, Poland; 25Hospital Universitario Puerta de Hierro, Madrid, Spain; 26HUCH Comprehensive Cancer Center, Helsinki, Finland
Background: Graft failure is a rare complication in patients after transplantation using myeloablative conditioning. We were interested in the incidence and potential risk factors for graft failure in adult patients receiving a transplant from HLA-identical donors after reduced intensity conditioning (RIC).
Methods: A total of 23275 patients transplanted between 2010 and 2016 after RIC according to the EBMT definition were analysed. Median age of the patients at transplant was 57.3 ys. (18.0–79.7). Donors were related in 39.5% and unrelated in 60.5% of the patients. Transplants were performed for acute leukemias (39.8%), chronic leukemias (8.3%) lymphoma (19.5%) plasma cell disorders (8.8%) and myelodysplastic/myeloproliferative disorders (23.6%). The conditioning regimens used were Flu/Bu in 33.7%, Flu/Mel in 25.7%, TBI/Flu in 12%, TBI+others in 7.9%, Flu only in 5.1% and Flu/Cy in 3.4% of the patients. In 11.8% other RIC regimens were used. ATG was given in 47.4% of the transplants.
Results: Graft failure was reported in 975 patients (4.2%). It occurred more frequently in male (4.4%) than in female patients (3.8%; p = 0.03) and was seen more frequently in patients transplanted in years 2010–2011 (4.7 %) than in 2012–2016 (3.9%). There was no difference in frequency of graft failure between patients aged 18–49ys (3.9%) and ≥50ys. (4.3%; p = 0.25). The highest incidence was seen in patients transplanted for myelodysplastic/myeloproliferative disorders (6.94%), followed by chronic leukemias (4.7%), acute leukemias (3.7%), plasma cell disorders (3.1%) and lymphomas (2.2%; p < 0.001). The risk of graft failure was increased in patients transplanted not in CR compared to patients in CR (5.5 vs. 2.9; p < 001). It was more common in patients receiving bone marrow than PBSC as stem cell graft (8.6% vs. 3.8%; p < 0.001). There was no difference between transplants from related vs. unrelated donors (4.0% vs. 4.3%; p = 0.26) Regarding the conditioning regimen, the use of fludarabine and melphalan was associated with a lower incidence of graft failure (2.8%) compared to Flu/Cy (3.9%), Flu (4.9%), Flu/TBI (4.9%), Flu/Bu (4.9%), TBI+others (4.9%) and other regimens (3.8%; p < 0.0001). The above-mentioned risk factors were also significant in multivariate analysis (Table 1). The kind of GVHD-prophylaxis used (CSA vs. CSA+MTX vs. CSA+MMF vs. others), the use of ATG, sex mismatch and CMV-status had no significant influence on the incidence of graft failure in multivariate analysis.
Conclusions: This analysis of graft failure in a large number of patients receiving reduced intensity conditioning according to the EBMT definition shows a low incidence of this complication. Major risk factors were myelodysplastic/myeloproliferative disorders as underlying disease, the use bone marrow vs. PBSC as graft source and transplantation in patients not in CR. The use of a reduced conditioning regimen with Flu/Mel was associated with the lowest incidence of graft failure. The kind of GvHD-Prophylaxis, CMV and sex mismatch, recipient age as well as the use of related or unrelated donors had no influence.
Conflict of interest: Hertenstein: nothing to disclose
[[O059 Figure] table 1].
Table 1 Multivariate analysis of factors affecting graft failure after alloHCT.
O060 Exceptionally high mortality in hematopoietic stem cell transplantation-associated thrombotic microangiopathy and concomitant acute graft-versus-host disease
Sarah Kraft1, Noemi Bollinger1, Benjamin Bodenmann1, Dominik Heim1, Jakob Passweg1, Christoph Bucher1, Martina Kleber1, Claudia Lengerke1, Dimitrios Tsakiris1, Alexandar Tzankov2, Michael Medinger1
1Hematology, University Hospital Basel, Basel, Switzerland; 2Institute of Medical Genetics and Pathology, Basel, Switzerland
Background: Steroid refractory acute graft-versus-host disease (GvHD) remains a major complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT). GvHD has been associated with transplant-associated (thrombotic) microangiopathy (TA-TMA). We hypothesize that TA-TMA correlates with steroid-refractory acute GvHD and conducted a study to explore the possible risk factors for the occurrence and mortality of TA-TMA with concomitant aGvHD and to investigate outcomes and treatments of this disorder after allo-HSCT.
Methods: A retrospective analysis of 660 consecutive patients with hematological diseases receiving an allo-HSCT at the University Hospital Basel in the period from 2006 to 2016 was performed. Data on the occurrence, risk factors and outcome of patients with TA-TMA and the correlation with acute GvHD was collected.
Results: 660 patients suffering from either AML n = 248 (37.6%), ALL n = 79 (12.0%), CML n = 23 (3.5%), CLL n = 36 (5.5%), lymphoma/myeloma n = 127 (19.2%), MDS/MPN n = 124 (18.8%) or bone marrow failure n = 22 (3.3%) underwent a myeloablative (n = 518; 78.5%) and non-myeloablative (n = 140; 21.2%) allo-HSCT at a median age of 47 years (range 19–71 years). Sixty-five (9.8%) patients matched the established diagnostic criteria for TA-TMA (increased LDH, platelet count < 50G/L or < 50% of normal baseline, schistocytes >2 per high power field, >1.5x creatinine increase, haptoglobin decrease). The median time to onset of TA-TMA was 36 days post-transplant (range 22 to 67 days). Subjects with TA-TMA had significantly higher non-relapse mortality (NRM) compared to those without. TA-TMA was a very relevant independent risk factor for mortality (RR 3.265; 95% CI 2.066–5.161). Patients with TA-TMA and concomitant aGvHD had a markedly lower overall survival (OS) compared to patients with TA-TMA or aGvHD alone (median 5.6 months versus 7.6 months versus 55.4 months; p < 0.0001).
Patients with clinically relevant aGvHD (≥ grade 2) more frequently developed TA-TMA than patients without aGvHD [46/337 (13.65%) versus 19/286 (6.64%); p = 0.003].
Conclusions: TA-TMA, with or without concomitant aGvHD is a significant complication after allo-HSCT and is a high risk factor for dismal outcome considering both OS and NRM. There are specific risk factors associated with occurrence and mortality of this complication. Allo-HSCT recipients with grades 2 to 4 aGvHD or CMV viremia should be closely monitored for the presence of TA-TMA.
Independent risk factors for development of TA-TMA were clinically relevant aGvHD ≥ grade 2, higher GvHD grade, steroid-refractory aGvHD, CMV reactivation, but not the conditioning regimen (RIC or MAC), usage of TBI or TBI dose, underlying disease, age and sex.
Conflict of interest: None of the authors has anything to disclose.
[[O060 Figure].
![[[O060 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/bf7c67d1279c/41409_2018_318_Figl_HTML.jpg)
Figure 1: Cumulative survival curves for patients with/without TA-TMA and aGvHD.]
| All n = 660 (100%) | no TA-TMA n = 576 (87.3%) | TA-TMA n = 65 (9.8%) | p | |
|---|---|---|---|---|
| CMV reactivation | no | 361 (74%) | 26 (49%) | 0.000248 |
| ves | 128 (26%) | 27 (51%) | ||
| Acute GvHD steroid-responsive | no | 29(9%) | 16(36%) | p<0.00001 |
| yes | 278 (91%) | 29 (64%) | ||
| Acute GvHD ≥ grade 2 | no | 267 (48%) | 19 (29%) | 0.003 |
| yes | 291 (52%) | 46 (71%) | ||
| Conditioning regimen | RIC | 125 (22%) | 14 (22%) | 0.555 |
| MAC | 449 (78%) | 51 (78%) | ||
| TBI dose, Gy | 6.78 ± 4.91 | 5.04 ± 4.54 | 0.065 |
[ [O060 Table] Comparison of patients with and without TA-TMA]
O061 Serum Interleukin-6 Predicts the Outcomes of Allogeneic Transplant with Post-Transplant Cyclophosphamide
Raffaella Greco, Francesca Lorentino, Rosamaria Nitti, Maria Teresa Lupo Stanghellini, Elisabetta Xue, Fabio Giglio, Daniela Clerici, Simona Piemontese, Andrea Assanelli, Sarah Marktel, Consuelo Corti, Massimo Bernardi, Fabio Ciceri, Jacopo Peccatori
IRCCS San Raffaele Scientific Institute, Hematology and Bone Marrow Transplant Unit, Milan, Italy
Background: Although the outcome of allo-HSCT has dramatically improved in the past decade, it is still compromised by TRM mainly caused by GvHD. We previously reported a correlation between IL6 and the risk of acute GvHD and TRM (Greco R et al, BMT, vol 52, 2017); herein we studied the same feature in the setting of PT-Cy.
Methods: We collected samples from 166 consecutive patients (105 males; median age 48.5) who underwent allo-HSCT with PT-Cy between April 2014 and June 2017. Most patients were affected by myeloid malignancies (AML = 55%). All patients received a treosulfan-based conditioning regimen (myeloablative in 81%) and 91% PBSC. Stem cell donors were unrelated (n = 41), haploidentical (n = 89), sibling (n = 36). GvHD prophylaxis was based on PT-Cy, sirolimus and MMF. All patients included in this analysis were tested for IL6 levels in blood samples before conditioning (baseline) and 7 days after allo-HSCT.
Results: Median follow-up on survivors was 469 days (range 69–1269). The cumulative incidence (CI) of grade 2–4 acute GvHD was 29% (16% grade 3–4). The 100-d CI of TRM was of 8% with an OS of 70% at last follow-up.
Pre-transplant IL6 levels showed a strong correlation with TRM, identifying a threshold of 2.5 pg/ml (AUC 0.74; sens 71%, spec 72%, p < 0.01) by ROC analysis.
Post-transplant IL6 was able to predict TRM and acute GvHD. ROC analysis identified a threshold of 16.5 pg/ml as predictor of grade II-IV acute GvHD, grade III-IV acute GvHD and TRM (AUC 0.74, sens 76%, spec 67%, p < 0.01; AUC 0.81, sens 90%, spec 63%, p < 0.01; AUC 0.69, sens 76%, spec 57%, p 0.005).
Moreover, we divided patients into groups according to whether biomarker concentrations were above (high) or below (low) the identified thresholds. Survival analysis confirmed decreased OS in patients with high baseline IL6 (38% vs 79%; p < 0.01) and/or high post-transplant IL6 (47% vs 82%; p < 0.01). We found a trend towards a worse TRM in patients presenting high post-transplant IL6 (p = 0.06).
Rates of grades 2–4 and 3–4 acute GvHD were higher in patients with high post-transplant IL6 levels (46% versus 13 %, p < 0.01; 31% versus 0.03%, p < 0.01, respectively). Also high baseline IL6 levels were associated with grade 2–4 aGvHD (p = 0.03).
Moreover, multivariate analysis (adjusting for age, DRI, Sorror-CI, donor, source of stem cells, CMV-status) was performed. Pre-transplant IL6 concentrations were significantly associated to grade 2–4 aGvHD (HR 1.8, 95% CI 1–3.3; p 0.04), TRM (HR 6.7, 95% CI 2.2–20.2; p < 0.01), and OS (HR 4.3, 95% CI 2.2–8.1; p < 0.01). Post-transplant IL6 levels correlated with grade 2–4 aGvHD (HR 5, 95% CI 2.6–9.5; p < 0.01), grade 3–4 aGvHD (HR 10.2, 95% CI 3.4–29.9; p < 0.01), TRM (HR 3.5, 95% CI 1.2–10.5; p = 0.02), and OS (HR 3.3, 95% CI 1.7–6.4; p < 0.01).
Conclusions: Plasma IL6 levels, both pre and post transplant, resulted a valuable biomarker with a significant impact on clinical outcomes in this large series of allo-HSCT treated with Treosufan-based conditioning and PT-Cy.
Clinical Trial Registry: NA
Conflict of interest: Nothing to disclose
O062 Slow Off-rate Modified Aptamer (SOMAmer) Based Proteomics Profiling and Machine Learning Applied to Premature Coronary Heart Disease in Hematopoietic Cell Transplant Survivors
Mindo Battiwalla1, Xin Tian1, Kimberly Doucette1,2, Marcus Chen1, Robert Le1, Natasha Jain1,3, Upneet Chawla1,4, Angelique Biancotto5, Katie Stagliano5, Eleftheria Koklanaris1, Richard Childs1, Sawa Ito1, John Barrett1
1National Institutes of Health, NHLBI, Bethesda, MD, United States; 2Washington Hospital Center, Internal Medicine, Washington D.C., DC, United States; 3Ohio State University, Columbus, OH, United States; 4Mercy St. Vincent Medical Center, Medicine, Toledo, OH, United States; 5National Institutes of Health, NIAID/CHI, Bethesda, MD, United States
Background: Premature coronary heart disease (CHD) is a leading contributor to late mortality in allogeneic hematopoietic cell transplantation (HCT) survivors. Unique risks, such as irradiation, endothelial injury and inflammation occur in the HCT setting. However, precise mechanisms remain elusive. Moreover, the Framingham risk score is insensitive in identifying CHD risk in HCT survivors. We utilized a proteomics discovery platform (SOMAscan®, SomaLogic, Inc), previously shown to predict cardiovascular events among patients with stable CHD [Ganz, et al., JAMA2016], to discover markers for CHD in HCT survivors.
Methods: CHD was defined by cardiac computed tomography (CT) imaging in 76 consecutive asymptomatic HCT survivors at the time of their survivorship clinic visits. Using modified DNA-aptamers (SOMAmers) for each target, 1305 proteins were measured in plasma samples and reported in relative fluorescence units. Those protein analytes were simultaneously analyzed and selected using random forests, a machine-learning approach. Receiver operating characteristic (ROC) curves were generated to estimate the optimal thresholds of the protein biomarkers and the area under the curves (C-statistics) for CHD classification. A protein risk score was derived based on multivariate logistic regression and its diagnostic performance was compared with the conventional Framingham risk score.
Results: Of the 76 HCT survivors, 61% were male, 84% had received fully ablative transplants with >95% receiving total body irradiation (TBI), median age was 47 years at study with a median survivorship duration of 8 years (range 2–20 years). Survivors were exhaustively characterized in terms of conventional cardiac risk factors. 10-year Framingham scores were 87% low, 8% intermediate and 5% high risk. 33 (43%) subjects had unequivocal evidence of early CHD by cardiac CT imaging. 94% of the lesions were non-obstructive. Of 1305 protein markers analyzed, six of the ten important biomarkers selected by Random Forests were significantly associated with the early CHD diagnosis (P < 0.005). In multivariate logistic analysis, four of the six biomarkers were independently related to early CHD, with or without adjustment for the Framingham risk score. A 4-protein CHD risk score was derived (range 0 to 6), composed of high levels of cardiac biomarkers [troponin T (TNNT2) and troponin I (TNNI2)] and interleukin-1 receptor accessory protein (IL1RAP) and ectonucleoside triphosphate diphosphohydrolase 5 (ENTPD5). The 4-protein risk score ≥4 was strongly associated with increased risk of early CHD (76% vs. 9% CHD for patients with risk score ≤3, odds ratio = 30.5, 95% CI 8.3–111.9, P = 2.2 x10−9). Adding this 4-protein risk score to Framingham risk score increased the C-statistic by 0.16 (95% CI 0.06–0.27, P = 0.002). C-statistics were 0.89 for the 4-protein risk score, 0.93 for the 4-protein plus Framingham risk score, and 0.77 for Framingham risk score.
Conclusions: In the NHLBI HCT survivorship cohort, we identified six protein biomarkers among more than 1300 analytes from the SOMAscan proteomic platform that were associated with early CHD using random forest machine learning methodology. The 4-protein risk score for early CHD performed better than the Framingham risk score, based on clinical and laboratory variables. Further investigation and validation are needed in larger cohorts and other populations.
Clinical Trial Registry: ClinicalTrials.gov Identifier NCT00106925; NCT01621594
Conflict of interest: Nothing to disclose for all authors
O063 Liver Stiffness Measurements: A useful and accurate non-invasive-tests for SOS/VOD diagnosis: results from a prospective monocentric study (ELASTOVOD) performed both in paediatrics and adults patients
Federico Ravaioli1, Antonio Colecchia1,2, Riccardo Masetti3, Mariarosaria Sessa4, Vanessa Luigina Alemanni1, Elton Dajti1, Giovanni Marasco1, Amanda Vestito1, Arcangelo Prete3, Davide Festi1, Andrea Pession3, Francesca Bonifazi4
1University of Bologna, Gastroenterology Unit, Department of Medical and Surgical Sciences (DIMEC), Bologna, Italy; 2University of Verona and AOVR Borgo Trento, Department of Medicine, Verona, Italy; 3University of Bologna, Pediatric Oncology and Haematology Unit 'Lalla Seràgnoli', Department of Pediatrics, Bologna, Italy; 4University of Bologna, Institute of Hematology 'Seràgnoli', Bologna, Italy
Background: Veno Occlusive Disease (SOS/VOD) is a rare complication affecting patients undergoing hematopoietic stem cells transplantation (HSCT), with mortality rate as high as 80%. Patients developing SOS/VOD present a higher survival rate when earlier SOS/VOD-specific therapy has initiated. In order to get better outcomes, it is necessary to improve early diagnosis of SOS/VOD, overcoming the inadequacy of clinical criteria and finding new non-invasive diagnostic instruments. The aim of this monocentric study is to assess in a large, mixed (adult and paediatric) population undergoing HSCT, the diagnostic role for SOS/VOD with a new non-invasive method, Liver Stiffness Measurement (LSM), assessed by Transient Elastography (TE).
Methods: From November 2014 to November 2017, patients aged between 4 and 70 with indications for allo- and auto-HSCT, were prospectively included. Laboratory tests and LSM were carried out before HSCT and subsequently at day +9/10 (T1), +15/17 (T2) and +22/24 (T3) after HSCT. Modified Seattle/Baltimore criteria were used to establish VOD/SOS diagnosis.
Results: Out of the 98 enrolled patients, 65 were adults and 33 were children. Among them, 9 patients developed SOS/VOD (9.2%) during the period of the study, from +1 until + 26 days after HSCT with a median time of +22 days after HSCT (IQR:19–25). At baseline, median LSM values were 4.2 kPa (IQR: 3.7–5.2) with no significant difference (p = 0.653) between group who developed SOS/VOD (5 kPa; IQR 3.7 -6.1) and who did not develop SOS/VOD (4.2 kPa; IQR 3.7 -5.1).
We observed significantly higher values of LSM in patients who developed SOS/VOD (range from 9.2 kPa up to 59.3 kPa), compared to patients who did not. We observed that during follow up, a sudden increase of LSM values in comparison to the previous time point assessment was found only in patients who developed SOS/VOD. Interestingly, the stiffness increases occurred when the patients were still asymptomatic, anticipating the clinical appearance of SOS/VOD from 1 to 6 days (Graph 1).
In a logistic regression analysis, higher values of LSM were significantly (p-value < 0.0001) associated to SOS/VOD development (OR: 1.994 95%CI: 1.410–2.819). The diagnostic performance of LSM>10 kPa in SOS/VOD diagnosis showed AUROC 0.997 (Sens.100%; Spec. 98.82%; LR+ 84.7; LR- 0.00; PPV 75%; NPV 100%).
Conclusions: These results, obtained in a large cohort of patients with high age range (adults and children) undergoing HSCT, confirm our previous preliminary data on paediatric population showing how a sudden increase of LSM during follow-up after HSCT seems to be related to the occurrence of SOS/VOD. Therefore, LSM could represents a new useful tool to help the clinicians in the diagnosis of SOS/VOD, being an accurate, non-invasive, easily bedside executable test. Further investigations in multicentre studies are needed to confirm and validate the role of LSM in SOS/VOD diagnosis and to assess the role of LSM in differential diagnosis with other liver-related complications after HSCT.
Conflict of interest: None of the authors has anything to disclose
[O063 Figure].
[Variation of LSM values at each determination for all patients.]
.
O064 Pooled Analysis of Defibrotide Studies in Treatment of Adult Patients with Veno-occlusive Disease/Sinusoidal Obstruction Syndrome after Hematopoietic Stem Cell Transplantation (HSCT) or Chemotherapy Without HSCT
Paul G. Richardson1, Enric Carreras2, Antonio Pagliuca3, Robert J. Soiffer4, Joseph H. Antin4, Vincent T. Ho4, Saurabh Aggarwal5, Ozlem Topaloglu5, Kathleen F. Villa6, Mohamad Mohty7
1Jerome Lipper Multiple Myeloma Center, Division of Hematologic Malignancy, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, United States; 2Spanish Bone Marrow Donor Program, Josep Carreras Leukemia Foundation and Leukemia Research Institute, Barcelona, Spain; 3King's College Hospital, Department of Haematology, London, United Kingdom; 4Stem Cell/Bone Marrow Transplantation Program, Division of Hematologic Malignancy, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, United States; 5Novel Health Strategies, Bethesda, MD, United States; 6Jazz Pharmaceuticals, Inc., Palo Alto, CA, United States; 7Hematology Department, Hôpital Saint Antoine, AP-HP, and Université Pierre & Marie Curie, Paris, France
Background: A systematic literature review was previously conducted to identify all published studies of defibrotide for the treatment of patients of all ages with veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS). The objective of the current analysis was to assess Day+100 survival reported in the published literature for adults (>18 or >16 years old, depending on study) with VOD/SOS with and without multi-organ dysfunction (MOD) treated with defibrotide.
Methods: The PubMed and Embase databases were searched for English language papers and conference abstracts published up to July 10, 2017 with the term “defibrotide”. Recent 2017 congress abstracts were also searched directly using conference websites. Duplicates were removed. The following types of studies were selected for inclusion: randomized controlled trials, single-arm studies, cohort studies, case series (with ≥10 cases), and retrospective chart reviews. Case reports with < 10 cases, meta-analyses, reviews, animal studies, modeling studies, pharmacokinetic studies, chromatography studies, child/pediatric studies, guidelines, articles, and letters were excluded. Results from the literature searches (ie, study titles and/or abstracts) were screened to remove irrelevant studies based on the exclusion criteria. Full-text articles were then reviewed for eligibility. Study characteristics (eg, sample size, treatment dose, patient characteristics, clinical outcomes, HSCT/non-HSCT) of selected publications were summarized and publications were categorized by those that reported all VOD/SOS patients, those with VOD/SOS with MOD and those with VOD/SOS without MOD. Patient-level data were evaluated when available. When necessary, additional data tables for these studies were also requested. A random effects model was used for pooling data for efficacy. Inter-study heterogeneity was assessed with Cochran's Q-test. The percentage of total variation across studies due to heterogeneity was evaluated by the I2 measure. Reported adverse events (AEs) were reviewed.
Results: Eleven published studies reported survival outcomes for adult patients with VOD/SOS (n = 1128). Day+100 survival (and 95% confidence interval [CI]) was 45% (0.38–0.51) in the pooled analysis. For those with VOD/SOS with MOD, five published studies were identified (n = 464) with a pooled Day+100 survival of 35% (0.30–0.40). Only one open-label expanded-access study, the treatment-IND (n = 199 for adult patients [>16 years]), reported outcomes separately for adult patients with VOD/SOS without MOD. The Kaplan-Meier estimate for Day+100 survival for those patients was 57% (0.50–0.64). Safety results were not pooled due to differences in reporting methodology; however, results of individual studies were generally consistent with the safety profile found in the phase 3 historically controlled trial in VOD/SOS pts with MOD, in which all but 1 of the 102 defibrotide-treated pts and all 32 controls experienced ≥1 AE. Hypotension was the most frequent AE (39% for defibrotide, 50% for controls), and common hemorrhagic AEs (ie, pulmonary alveolar and gastrointestinal hemorrhage), occurred in 64% of defibrotide-treated pts and 75% of controls.
Conclusions: This systematic literature review and pooled analysis of defibrotide treatment of adult patients with VOD/SOS is the largest of its kind and reports Day+100 survival ranging from 35% in VOD/SOS with MOD to 57% in VOD/SOS without MOD. These data support the use of defibrotide in VOD/SOS with/without MOD and the clinical benefit seen in this setting.
Conflict of interest:
Support: Jazz Pharmaceuticals.
Disclosures:
P. Richardson has served on advisory committees and as a consultant, and received research funding from Jazz Pharmaceuticals.
E. Carreras has served on advisory boards and the speakers bureau for, received research funding from, and provided expert testimony for Gentium.
A. Pagliuca has served on advisory boards and the speakers bureau for and received honoraria from Gentium/Jazz Pharmaceuticals.
R. Soiffer has served on advisory committees with Jazz Pharmaceuticals.
J. Antin served on advisory committees for Gentium SpA/Jazz Pharmaceuticals.
V. Ho has served as a consultant to Jazz Pharmaceuticals.
S. Aggarwal and O. Topaloglu are employees of Novel Health Strategies, which received funding from Jazz Pharmaceuticals for this analysis.
K. Villa is an employee of Jazz Pharmaceuticals, Inc., who in the course of employment has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc.
M. Mohty has received honoraria and research funding from Jazz Pharmaceuticals.
Experimental stem cell transplantation
O065 Motifs of peptides binding in HLA-DP and their relation with T-cell epitope groups relevant in stem cell transplantation
Peter Van Balen1, Michel G.D. Kester1, Pietro Crivello2, Arnoud H. De Ru3, Wendy De Klerk1, Marian Van De Meent1, Inge Jedema1, Katharina Fleischhauer2, J.H.Frederik Falkenburg1, Peter A. Van Veelen3
1Leiden University Medical Center, Hematology, Leiden, Netherlands; 2Essen University Hospital, Institute for Experimental Cellular Therapy, Essen, Germany; 3Leiden University Medical Center, Center for Proteomics and Metabolomics, Leiden, Netherlands
Background: Approximately 80% of matched unrelated donors for allogeneic stem cell transplantations are mismatched from patients for HLA-DP. Although frequently not taken into account in donor selection, mismatched HLA-DP can induce potent immune responses, consisting of graft-versus-leukemia reactivity and graft-versus-host disease. Some HLA-DP mismatches are more permissive than others because they belong to the same T-cell epitope (TCE) group. HLA-DPB1 alleles are categorized into TCE groups based on in vitro experiments using recognition patterns of anti-HLA-DP directed T-cells and peptide sequences of the binding groove. TCE groups 1 and 2 are clearly defined, but TCE group 3 contains the HLA-DPB1 alleles not belonging to group 1 or 2, and may represent a relatively heterogeneous group. To investigate whether peptides binding in HLA-DP can be of influence in the categorization into TCE groups, we analyzed the peptidome of 11 HLA-DP molecules.
Methods: To investigate peptides presented in HLA-DP molecules encoded by DPB1*09:01, 10:01, 17:01 (TCE1), DPB1*03:01, 14:01 (TCE2) and DPB1*01:01, 02:01, 04:01, 04:02, 05:01, 13:01 (TCE3), HLA-DPB1 typed EBV-LCL were expanded to 2-8x10^9 cells and lysed. HLA-DP immunoaffinity chromatography using anti-HLA-DP B7.21 antibody was performed, followed by analysis of eluted peptides using mass spectrometry. Peptides with amino acid length of 12–20 were aligned and clustered using Gibbs sampling to obtain motifs of peptides binding in different HLA-DP molecules.
Results: Elutions were performed from HLA-DP molecules of 17 EBV-LCL and 3124–9438 unique peptides were detected per HLA-DP allele. Using Gibbs clustering, motifs within the peptide pools could be identified. Specific amino acids were found on positions P1, P6 and P9, which interact with amino acids in hypervariable regions (HvR) of the HLA-DP binding groove. All 3 alleles from TCE group 1 had a similar motif KAL at P1, P6 and P9, reflecting their structural similarity in the relevant HvR. This motif was shared also by DPB1*14:01 from TCE2, while the motif from HLA-DPB1*03:01 was different (RAS). TCE3 alleles could be classified into two groups: those with a totally different FFV motif at P1, P6 and P9 (DPB1*02:01, 04:01, 04:02 with high structural similarity), and those with a motif more similar to TCE1 in P1, but different in P6 and P9 (KXX), i.e. DPB1*01:01, 05:01 and 13:01. Interestingly, the latter three share the amino acid sequence DEAV in one HvR with TCE1 and TCE2 alleles, suggesting that they might constitute a fourth, functionally distinct TCE group. These observations may have important consequences for the classification of permissive HLA-DPB1 mismatches according to the TCE model.
Conclusions: The motifs of peptides binding in 11 HLA-DP molecules were determined and these motifs show clear relation with amino acids in the binding groove that are known to be in interaction with the peptide. These results increase the knowledge of peptides binding in HLA-DP and are of importance in the explanation why some HLA-DP mismatches are more permissive than others. The current categorization into TCE groups may need to be adjusted based on these results, especially with regard to potentially permissive or non-permissive mismatches within HLA-DP alleles in TCE group 3.
Conflict of interest: All authors declare not to have conflicts of interest.
O066
Abstract previously published
O067
Abstract previously published
O068
Abstract previously published
O069 HIV status in long-term follow-up after allogeneic stem cell transplantation: “The Granada patients”
Jon Badiola1, Maria Salgado2, Mi Kwon3, Cristina Gálvez2, Monique Nijhuis4, Cristina Vilaplana5, Lucia Moratalla1, Elisa López-Fernández1, Pedro González-Sierra1, Pascual Balsalobre3, Jose Luis Díez-Martín3, Annemarie Wensing4, Javier Martinez-Picado2, Manuel Jurado-Chacón1
1Hospital Virgen de las Nieves, Department of Hematology, Granada, Spain; 2AIDS Research Institute, IrsiCaixa, Badalona, Spain; 3Hospital Gregorio Marañón, Madrid, Spain; 4University Medical Center Utrecht, Utrecht, Netherlands; 5Fundació Institut d'Investigació en Ciències de la Salut Germans Trias i Pujol (IGTP), Badalona, Spain
Background: The success of “the Berlin patient,” the first and only documented HIV cure case to date, has raised the interest in allogeneic stem cell transplantation (SCT) in HIV-infected patients in the last decade. This procedure considerably reduces the viral reservoir, however, the extent of this reduction and the consequences of antiretroviral therapy (ART) withdrawal remains unknown. The goal of this work is to determine the HIV status in our allogeneic stem cell transplantation HIV-patients.
Methods: Clinical data, peripheral blood, leukapheresis, bone marrow, lymph node, cerebrospinal fluid (CSF) and ileum biopsies were collected from 2 HIV-infected subjects that had undergone SCT with CCR5wt donor cells in Granada (Spain), within the IciStem cohort (participant 27 and 28). To determine their HIV status, serological and ultrasensitive viral load studies, quantitative viral outgrowth assays (QVOA) and HIV-DNA measurements were performed in tissue samples. Furthermore, as a potential tool to predict early viral rebound after ART discontinuation, we performed mice viral outgrowth assays (mVOA) infusing leukapheresis products from the patients.
Results: Patient 27: 51-year-old man with HIV infection since 2005 and ART since 2013. An allogeneic SCT was done in 2013 from a matched related donor with non-myeloablative conditioning regimen for gray zone lymphoma.
Patient 28: 52-year-old man diagnosed with HIV in acute phase in 1995 and treated with ART since then. An allogeneic SCT with non-myeloablative conditioning regimen for lymphocyte-depleted classical Hodgkin lymphoma was carried out in 2009 from a matched non-related donor.
Full donor chimera was reached on day +160 and day +28, respectively. Patient 27 had mild cutaneous chronic graft-versus-host disease (GvHD) while patient 28 had an acute, grade 2 GvHD followed by chronic-moderate GvHD. In both patients the ART was maintained with good compliance during and after SCT and both are in complete hematologic remission up to this day.
Ultrasensitive determination of viral load in plasma and CSF was negative (< 1 cop/ml) in both patients. QVOA in the leukopheresis-obtained CD4+T cells (135x106) was also negative (< 5UIx109). HIV-DNA measured in the samples (leukapheresis, bone marrow, lymph node, and ileum biopsies) was also undetectable. Plasma viral load and HIV-DNA in blood and spleen cells were negative up to week 4 in mVOA (picture 1a and 1b).
Western blot serological studies revealed the absence of one band (P18) in patient 27. Notably, no bands were detected in patient 28 after SCT (picture 1c).
Conclusions:
● Allogeneic SCT dramatically reduces the HIV reservoir.
● With current techniques we have not been able to find latent viral reservoirs in either blood or tissues in these patients.
● Patient 28 showed a negative serology, probably indicating the absence of HIV recognition by the immune system.
● The use of ART in the acute phase of HIV, the maintenance of ART during SCT, the achievement of full donor chimera, the alloreactivity (GvHD and graft-versus-HIV effect) and the spontaneous elimination of reservoir by natural cell turnover may be important factors in HIV reservoir eradication after SCT.
Conflict of interest: None of the authors has anything to disclose
[O069 Figure].
Figure 1]
.
Gene therapy
O070
Abstract previously published
O071 Lenti-D Hematopoietic Stem Cell Gene Therapy to Arrest Progression of Cerebral Adrenoleukodystrophy: Interim Results of an International Phase 2/3 Trial
Adrian Thrasher1, Florian Eichler2, Christine Duncan3, Patricia Musolino2, Paul Orchard4, Satiro De Oliveira5, Myriam Armant3, Colleen Dansereau3, Troy Lund4, Weston Miller4, Gerald Raymond4, Raman Sankar5, Ami Shah5, Caroline Sevin6, H. Bobby Gaspar1, Paul Gissen1, Hernan Amartino7, Drago Bratkovic8, Nicholas Smith8, Asif Paker9, Esther Shamir9, Tara O'Meara9, David Davidson9, Patrick Aubourg6, David Williams10
1University College London Great Ormond Street Hospital Institute of Child Health and Great Ormond Street Hospital NHS Foundation Trust, London, United Kingdom; 2Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States; 3Dana Farber and Boston Children's Cancer and Blood Disorders Center, Boston, MA, United States; 4University of Minnesota Children's Hospital, Minneapolis, MN, United States; 5University of California Los Angeles, Los Angeles, CA, United States; 6Hôpital Bicêtre, Hopitaux Universitaires Paris Sud, Le Kremlin Bicêtre, France; 7Fundacion Investigar, Buenos Aires, Argentina; 8Women's and Children's Hospital, North Adelaide, Australia; 9bluebird bio, Cambridge, MA, United States; 10Dana Farber, Boston Children's Cancer and Blood Disorders Center, Harvard Medical School, Harvard Stem Cell Institute, Boston, MA, United States
Background: Adrenoleukodystrophy (ALD) is an X-linked genetic disease caused by mutations in the ABCD1 gene which encodes the peroxisomal membrane half-transporter ALD protein. These mutations result in the toxic accumulation of very long chain fatty acids predominantly in adrenal and nervous system tissues. Cerebral ALD (CALD), affecting roughly 35–40% of boys with ALD, is characterized by inflammatory demyelination leading to progressive loss of neurologic function and death. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) has been shown to have a positive impact on indices of cerebral disease progression, if performed early in the course of the disease, but can be associated with significant risk, especially when performed using cells from a non-matched sibling donor.
Methods: Boys ≤17 years of age with CALD were enrolled in a single-arm, open-label, phase 2/3 study of the safety and efficacy of gene therapy with Lenti-D drug product (DP). Treatment involves infusion of mobilized autologous CD34+ hematopoietic stem cells transduced ex vivo with the elivaldogene tavalentivec (Lenti-D) lentiviral vector to contain functional copies of ABCD1. Patients were required to have active cerebral disease measured by gadolinium enhancement on MRI with low scores for radiologic and clinical measures (MRI Loes score between 0.5 and 9; Neurologic Function Score [NFS] ≤1). Patients received myeloablative conditioning with busulfan and cyclophosphamide followed by intravenous infusion of Lenti-D DP. Efficacy assessments included development of major functional disabilities (MFDs), changes in NFS and Loes score, and mortality. Safety assessments included the proportion of patients who experience either engraftment failure, acute (≥Grade II) graft-versus-host disease (GVHD), or chronic GVHD.
Results: As of August 2017, 21 patients were treated (median follow-up 30.2 months, range 1 to 46 months). Following Lenti-D DP infusion, all subjects with evaluable data demonstrated neutrophil engraftment (N = 4 without G-CSF at a median Day +31, range +20 to +39; and N = 17 with G-CSF at a median Day +12, range +11 to +20) and platelet engraftment (N = 18 at median Day +28.5, range +16 to +55). Measurable ALD protein expression in peripheral blood cells was observed by month 2 in all patients with evaluable data. At 24 months, 15 of the first 17 patients (88%) treated with Lenti-D DP remain alive and MFD-free with minimal clinical symptoms. One patient had rapid neurologic deterioration shortly after transplantation, and succumbed to disease. Another patient with evidence of post-treatment gadolinium enhancement on cerebral MRI was withdrawn by the treating physician for a secondary allo-HSCT and later died of allo-HSCT-related complications. There was no evidence of replication competent lentivirus or insertional oncogenesis. No graft failure, GVHD, or transplant-related mortality were reported. Most adverse events (AEs) were consistent with myeloablative conditioning. Two AEs were considered possibly or probably related to DP: BK-mediated viral cystitis (serious, grade 3), and tachycardia (non-serious, grade 1); one AE, vomiting (non-serious, grade 1), was considered related to DP.
Conclusions: These data suggest that Lenti-D gene therapy may offer an alternative to allo-HSCT in pediatric patients with CALD. Additional follow-up is needed to assess durability of efficacy and long-term safety.
Clinical Trial Registry: ClinicalTrials.gov number, NCT01896102;
ClinicalTrialsRegister.eu number, 2011-001953-10
Conflict of interest:
A. Thrasher has received consulting fees from Orchard Therapeutics and Autolous Ltd;
F. Eichler, T. Lund, and P. Aubourg have received grant support from bluebird bio;
P. Orchard has received grants from bluebirdbio;
C. Duncan has received consulting fees from bluebird bio;
Weston Miller has received grant and travel support from bluebird bio;
Gerald Raymond has received grants and consulting fees from bluebird bio, and consulting fees from Minoryx and Vertex;
H. B. Gaspar holds equity in, has consulted for, and is an author of a patent licensed to Orchard Therapeutics;
N. Smith has received clinical trial funding from bluebird bio;
A. Paker was an employee of bluebird bio at the time of the study;
E. Shamir, T. O’Meara, M. Asmal, and D. Davidson are employees of bluebird bio;
D. Williams has received research funding from bluebird bio and has licensed intellectual property relevant to sickle cell disease to bluebird bio;
P. Musolino, S. De Oliveira, M. Armant, C. Dansereau, R. Sankar, A. Shah, C. Sevin, P. Gissen, H. Amartino, and D. Bratkovic have nothing to disclose.
Graft-versus-host disease – clinical
O072
Abstract previously published
O073 KD025-208: A Phase 2 Open-Label Trial of KD025-208 for Steroid-Dependent Chronic Graft-Versus-Host Disease (cGVHD)
Madan Jagasia1, Aleksandr Lazaryan2, Amandeep Salhotra3, Beyhar Zoghi4, James Essell5, Carlos Bachier6, Emily Skelton7, Olivier Schueller7, David Eiznhamer7, John Ryan8, Stephanie J. Lee9
1Vanderbilt University Medical Center, Nashville, TN, United States; 2University of Minnesota, Minneapolis, MN, United States; 3City of Hope, Duarte, CA, United States; 4Texas Transplant Institute, San Antonio, TX, United States; 5Oncology/Hematology Care, Cincinnati, OH, United States; 6Sarah Cannon Research Institute, Nashville, TN, United States; 7Kadmon Corporation, LLC, Cambridge, MA, United States; 8Kadmon Corporation, LLC, New York, NY, United States; 9Fred Hutchinson Cancer Research Center, Seattle, WA, United States
Background: KD025 is a ROCK2-selective inhibitor in Phase 2 development for chronic graft-versus-host disease (cGVHD). By downregulating Th17 and Tfh cells while upregulating regulatory T cells and decreasing myofibroblast formation and proliferation, KD025 may have a beneficial effect on both the inflammatory and fibrotic components of cGVHD.
Methods: KD025-208 is an open label, Phase 2 study in patients with steroid-dependent or refractory cGVHD. Three cohorts (200mg QD, 200mg BID, and 400mg QD) of 16 patients each are planned, followed by an expansion cohort. The primary endpoint is the overall Complete and Partial Response rate, defined per the 2014 NIH Consensus criteria.
Results: Patients enrolled in Cohorts 1 (n = 17) and 2 (n = 16) had received a median of 3 and 2 prior lines of cGVHD therapy, respectively. The most frequently involved organs in Cohorts 1 and 2, respectively, were eyes (82%, 69%), skin (76%, 75%), mouth (76%, 69%), joints (71%, 69%), and lung (24%, 44%). Forty-seven percent (47%) of patients in Cohort 1 and 69% in Cohort 2 had involvement of ≥ 4 organs. Eight patients remain on treatment with KD025 in each cohort after median treatment duration of 33 and 22 weeks, respectively. As of a data cutoff date of November 20, 2017, the Overall Response Rate was 65% in Cohort 1 and 63% in Cohort 2. Responses were rapid, with 71% of responders achieving a response by the first assessment (at 8 weeks). Seven of 17 patients (7/17; 41%) in Cohort 1 have sustained a response for ≥ 20 weeks. Duration of response data for both cohorts continue to mature. In responders with ≥ 4 organs involved, 75% and 38% in Cohorts 1 and 2, respectively, showed response in ≥ 4 organs. Responses were observed across all affected organ systems, including CRs in upper GI, lower GI, esophagus, mouth, skin, joints, eyes, and liver. The median corticosteroid dose (mg/kg/day) decreased from 0.22 at baseline to 0.14 while on study in Cohort 1, and from 0.20 to 0.09 in Cohort 2. Four patients completely discontinued corticosteroid treatment while receiving KD025. Sixty-five percent (65%) and 38% of patients in Cohorts 1 and 2, respectively, achieved an improvement (≥ 7 point reduction) in the Lee cGVHD Symptom Scale Summary Score. KD025 was well tolerated. Commonly reported AEs were AST/ALT elevations, anemia, nausea, diarrhea and URTI. Grade 3 or higher AEs were reported in 13 patients and SAEs in 8 patients. No apparent increase in incidence of infection was observed. Ten of 33 patients discontinued treatment due to progression of cGVHD, 3 due to adverse events, 2 due to recurrence of underlying malignancy, and 2 due to patient decision.
Conclusions: Treatment with KD025 has resulted in clinically meaningful and durable overall responses across all affected organ systems. Corticosteroid doses were reduced in both responders and non-responders. KD025 treatment was well tolerated with an AE profile consistent with that expected in cGVHD patients receiving corticosteroids. There was no apparent increased risk of infection observed with KD025. Treatment in all cohorts is ongoing.
Clinical Trial Registry: clinicaltrials.gov NCT02841995
Conflict of interest:
M. Jagasia; Janssen, consultancy and research funding; Mallinckrodt, consultancy
A. Salhotra: Kadmon, consultancy
A. Lazaryan, B. Zoghi, C. Bachier: nothing to disclose
E. Skelton, O. Schueller, D. Eiznhamer, J. Ryan: Kadmon, employment and equity ownership
S. Lee: Malinckrodt, Honoraria; Amgen, One-time advisory board member; Bristol-Myers-Squibb, One-time advisory board member; Kadmon, One-time advisory board member
O074
Abstract previously published
O075 A multicenter observational study of chronic and late acute graft-versus-host disease defined by 2005 NIH criteria: a prospective validation of Japanese cohort
Chikako Ohwada1, Emiko Sakaida1, Aiko Igarashi2, Noriko Doki2, Takehiko Mori3, Jun Kato3, Heiwa Kanamori4, Masatsugu Tanaka4, Shin Fujisawa5, Eriko Ogusa5, Masako Toyosaki6, Yasuyuki Aoyama6, Maki Hagihara7, Yoshinobu Kanda8, Hiroaki Shimizu9, Seiko Kato10, Reiko Watanabe11, Katsuhiro Shono12, Rika Sakai13, Takeshi Saito14, Tohru Sakura15, Kensuke Usuki16, Chiaki Nakaseko1,17, Shinichiro Okamoto3,18
1Chiba University, Department of hematology, Chiba, Japan; 2Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital, Hematology Division, Tokyo, Japan; 3Keio University School of Medicine, Division of Hematology, Department of Medicine, Tokyo, Japan; 4Kanagawa Cancer Center, Department of hematology, Yokohama, Japan; 5Yokohama City University Medical Center, Department of Hematology, Yokohama, Japan; 6Tokai University School of Medicine, Division of Hematology/Oncology, Department of Internal Medicine, Isehara, Japan; 7Yokohama City University School of Medicine, Department of Hematology and Clinical Immunology, Yokohama, Japan; 8Saitama Medical Center, Jichi Medical University, Division of Hematology, Omiya, Japan; 9Gunma University Graduate School of Medicine, Department of Haematology, Maebashi, Japan; 10University of Tokyo, Department of Hematology/Oncology, Institute of Medical Science, Tokyo, Japan; 11Saitama Medical Center, Saitama Medical University, Department of Hematology, Kawagoe, Japan; 12Chiba Aoba Municipal Hospital, Division of Hematology, Department of Internal Medicine, Chiba, Japan; 13Kanagawa Cancer Center, Department of Medical Oncology, Yokohama, Japan; 14Jikei University, Division of Clinical Oncology/Hematology, Tokyo, Japan; 15Saiseikai Maebashi Hospital, Division of Hematology, Maebashi, Japan; 16NTT Medical Center, Department of Hematology, Tokyo, Japan; 17International University of Health and Welfare School of Medicine, Department of Hematology, Narita, Japan; 18Kanto Study Group for Cell Therapy (KSGCT), Tokyo, Japan
Background: There has been no prospective validation study of 2005 NIH consensus GVHD criteria in Asian population reported so far.
Methods: We prospectively enrolled 406 allogeneic hematopoietic transplantation (HCT) recipient at 16 centers of Kanto Study Group for Cell Therapy between May 2012 to June 2014, to validate the 2005 NIH consensus GVHD criteria in Japanese cohort.
Results: The median age at HCT was 50 years. 238 (58.6%), 96 (23.7%) and 72 (17.7%) patients received bone marrow, cord blood, and peripheral blood(PB), respectively, from 295 (72.7%) unrelated and 111 (27.3%) related donors.
The 2-year cumulative incidence of chronic GVHD (cGVHD) was 35.4% (145/406) with a median of 8.4 months, and that of late acute GVHD was 3.5% (14/406) with a median of 3.6 months. A higher incidence of cGVHD was seen in recipients of PB (43.1%) than those of other sources, but the difference was not statistically significant.
In 145 cGVHD patients, global severity at the onset was mild in 30.3%, moderate in 43.5%, and severe in 26.2%. The most common affected site was skin (51.7%), followed by mouth (45.5%), liver (44.1%), lung (34.5%), eyes (26.9%), GI (15.9%) and joints (6.2%). 81% of the cGVHD patients received systemic immunosuppressive therapy (IST); all of them received calcineurin inhibitors, and additional agents such as steroids (72%) and MMF (11%) were also given. The 2-year overall complete response (CR) rate, partial response (PR) rate, and cumulative incidence of IST discontinuation were 31.9%, 40.7%, and 30.3%, respectively. Prior acute GVHD (HR 0.42, 95% CI: 0.23–0.76, P = 0.004) and severe global scoring (HR 0.37, 95% CI: 0.16–0.90, P = 0.02) were the negative predictive factors for discontinuation of IST.
The median observation period of the survivors was 29.3 months from the cGVHD onset, and the 2-year overall survival (OS), and GVHD specific survival (GSS) rate was 81.3% and 88.1%, respectively, and the cumulative incidence of disease relapse and non-relapse mortality (NRM) was 12.2% and 13.1%, respectively. “Severe” patients at the onset showed significantly lower disease relapse rate than that of “Mild + Moderate” patients (3.8% vs. 17.4%, P = 0.03), however, there was no significant difference in NRM, OS, and GSS according to their maximum global severity during the first 6 months from the onset.
The multivariate analysis identified prior acute GVHD (HR 2.79, 95% CI: 1.28–5.89, P = 0.01), skin score higher than 2 at the time of onset (HR 2.93, 95% CI: 1.45–5.93, P = 0.003), and thrombocytopenia at the time of onset (HR 3.47, 95% CI: 1.6–7.85, P = 0.001) were associated with inferior OS.
Conclusions: Our analysis provided a detailed profile of late acute and chronic GVHD for the first time in Japanese prospective cohort, especially in regard to organ involvement and treatment outcome. The incidence of late acute and chronic GVHD in our study was lower than that reported from prospective observation in north American cohort (Arora et al. Biol Blood Marrow Transplant, 2016). This discrepancy outlines different characteristics between the cohorts, probably reflecting both graft sources and genetic backgrounds.
Clinical Trial Registry: UMIN000007793
Conflict of interest: None of the authors has anything to disclose.
O076
Abstract previously published
O077 Refractory graft-versus-host disease-free, relapse-free survival: proposal of a simple and accurate endpoint to evaluate the long-term success of allogeneic hematopoietic stem cell transplantation
Koji Kawamura1, Hideki Nakasone1, Saiko Kurosawa2, Kazuki Yoshimura1, Yukiko Misaki1, Ayumi Gomyo1, Jin Hayakawa1, Masaharu Tamaki1, Yu Akahoshi1, Machiko Kusuda1, Kazuaki Kameda1, Hidenori Wada1, Yuko Ishihara1, Miki Sato1, Kiriko Terasako-Saito1, Misato Kikuchi1, Shun-Ichi Kimura1, Aki Tanihara1, Shinichi Kako1, Heiwa Kanamori3, Takehiko Mori4, Satoshi Takahashi5, Shuichi Taniguchi6, Yoshiko Atsuta7, Yoshinobu Kanda1
1Saitama Medical Center, Jichi Medical University, Division of Hematology, Saitama, Japan; 2National Cancer Center Hospital, Tokyo, Japan; 3Kanagawa Cancer Center, Yokohama, Japan; 4Keio University School of Medicine, Tokyo, Japan; 5Institute of Medical Science, University of Tokyo, Tokyo, Japan; 6Toranomon Hospital, Tokyo, Japan; 7Japanese Data Center for Hematopoietic Cell Transplantation, Nagoya, Japan
Background: Graft-versus-host disease-free, relapse-free survival (GRFS), defined as the absence of grade III-IV acute graft-versus-host disease (GVHD), chronic GVHD requiring systemic treatment, relapse, or death, has been proposed as a novel composite endpoint for clinical trials evaluating GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation (allo-HCT). GRFS is currently regarded as one of important measures to evaluate transplant success, since it represents not only disease-free survival but also survival without major morbidity related to GVHD. However, GRFS treats GVHD as a fixed failure event, even though GVHD may be resolved by treatment and thus GRFS apparently overestimates the impact of GVHD on the outcome of allo-HCT. Therefore, we aimed to develop a new composite endpoint that accurately reflects the long-term success of allo-HCT in terms of life expectancy, disease remission, and quality of life (QOL).
Methods: First, we compared QOL after allo-HCT according to the status of chronic GVHD using a nationwide cross-sectional questionnaire study. Next, we developed two new composite endpoints, current GRFS (cGRFS) and refractory GRFS (rGRFS), using a single-center cohort (n = 315). cGRFS was defined as survival without disease relapse/progression or active chronic GVHD at a given time after allo-HCT, calculated using two distinct methods; a linear combination of a Kaplan-Meier estimates approach and a multistate modelling approach. rGRFS was calculated similarly to conventional GRFS treating grade III-IV acute GVHD, chronic GVHD requiring systemic treatment, and disease relapse/progression as events, except that GVHD that resolved and did not require systemic treatment at the last evaluation was excluded as an event.
Results: Only active chronic GVHD had an adverse impact on QOL after allo-HCT, and QOL of patients with resolved chronic GVHD was comparable to that of patients without chronic GVHD. The two cGRFS curves obtained using two different approaches were superimposed and both were superior to that of conventional GRFS, reflecting the proportion of patients with resolved chronic GVHD (Figure 1). Finally, the curves of rGRFS and cGRFS were also superimposed, except for the early post-transplant period (Figure 1). In addition, we confirmed that the difference in the cumulative area under the curve (AUC) between rGRFS and cGRFS became stable 2 years after allo-HCT, whereas the difference between cGRFS and conventional GRFS increased year by year.
Conclusions: We showed that cGRFS and rGRFS more accurately reflect long-term transplant success than conventional GRFS. Especially, rGRFS can be easily calculated and analyzed with widely-used statistical approaches including proportional hazard modeling. Therefore, we propose rGRFS as a more effective and simple endpoint for assessing the long-term transplant success.
Conflict of interest: None of the authors has anything to disclose.
[ [O077 Figure] Comparison of refractory GRFS, current GRFS and conventional GRFS.]
O078 Comparison of the GVHD prophylaxis with single-agent post-transplantation cyclophosphamide or calcineurin inhibitors in matched related bone marrow transplantation
Ivan Moiseev, Olga Pirogova, Elena Babenko, Tatyana Gindina, Elena Darskaya, Elena Morozova, Sergey Bondarenko, Boris Afanasyev
Pavlov First St. Petersburg State Medical University, R.M. Gorbacheva Memorial Institute of Hematology, Oncology and Transplantation, St. Petersburg, Russian Federation
Background: Several studies were published demonstrating efficacy of single-agent graft-versus-host disease prophylaxis (GVHD) with post-transplantation cyclophosphamide (saPTCy) in matched related bone marrow transplantations (BMT), however no comparisons were published between saPTCy and conventional GVHD prophylaxis based on calcineurin inhibitors (CNIs).
Methods: In this single-center study 78 patients grafted with bone marrow from matched related donor (MRD) with saPTCy GVHD prophylaxis were compared to 105 historical control patients also receiving bone marrow from MRD, but with CNI and mycophenolate mofetil/methotrexate prophylaxis. PTCy was administered 50 mg/kg at days +3,+4 without further immunosuppression. In the control group patients received CNI starting day -1 and either short-course methotrexate or mycophenolate mofetil 30 mg/kg. 32% received myeloablative conditioning and 68% reduced-intensity conditioning. Groups were comparable in the other pre-transplant characteristics of patients, except higher prevalence of salvage patients and acute lymphoblastic leukemia in CNIs cohort (36% vs 14%, p = 0.001). Median follow-up was 24 months in the PTCy group and 50 months in CNI group. 2-year outcomes were compared.
Results: There was no difference in the primary graft failure (1.1 vs 1.6%, p = 0.42), but engraftment was slower in the PTCy group (19 vs 24 days, p < 0.001). PTCy was superior to CNIs in prevention of grade II-IV (HR 0.239, 95% CI 0.099–0.58, p = 0.002), grade III-IV acute GVHD (HR 0.192, 95% CI 0.055–0.666, p = 0.009) and reduced relapse incidence (HR 0.519, 95% CI 0.297–0.893, p = 0.023). No difference was observed for moderate and severe chronic GVHD (HR 0.898, 95% CI 0.477–1.69, p = 0.74) and non-relapse mortality (HR 0.384, 95% CI 0.089–1.437, p = 0.1768). Patients after saPTCy had improved overall survival (HR 0.489, 95% CI 0.261–0.917, p = 0.03), event-free-survival (HR 0.571, 95% CI 0.334–0.976, p = 0.04) and GVHD-relapse-free survival (HR 0.493, 95% CI 0.309–0.786, p = 0.003).
The toxicity of BMT was generally comparable, except lower incidence of nephrotoxicity (33% vs 43%, p = 0.008) after PTCy, but with higher incidence of grade 3–4 mucositis in this group (41% vs 34%, p = 0.02).
Conclusions: Despite limitations of the single-center retrospective design and heterogenic cohort of patients, this study demonstrated superiority of saPTCy other CNI-based prophylaxis, but these results should be confirmed in the prospective randomized trials.
Conflict of interest: The authors have no conflicts of interest and have nothing to disclose.
[O078 Figure].
Figure 1. Univariate and multivariate analyses of 2-year transplantation outcomes]
O079
Abstract previously published
O080 Updated Results of a Phase 1b/2 Study of Ibrutinib in Chronic Graft Versus Host Disease After Failure of Prior Therapy
Edmund K. Waller1, David Miklos2, Corey S. Cutler3, Mukta Arora4, Madan Jagasia5, Iskra Pusic6, Mary E. Flowers7, Aaron C. Logan8, Ryotaro Nakamura9, Jason Dubovsky10, Stephen Chang10, Fong Clow10, Indu D. Lal10, Lori Styles10, Samantha Jaglowski11
1Winship Cancer Institute of Emory University, Atlanta, GA, United States; 2Stanford University, Stanford, CA, United States; 3Dana Farber Cancer Institute, Boston, MA, United States; 4University of Minnesota, Minneapolis, MN, United States; 5Vanderbilt-Ingram Cancer Center, Nashville, TN, United States; 6Washington University School of Medicine, St. Louis, MO, United States; 7Fred Hutchinson Cancer Research Center, Seattle, WA, United States; 8University of California San Francisco, San Francisco, CA, United States; 9City of Hope, Duarte, CA, United States; 10Pharmacyclics LLC, an AbbVie Company, Sunnyvale, CA, United States; 11Ohio State University, Columbus, OH, United States
Background: Chronic GVHD (cGVHD) is a life-threatening complication of allogeneic stem cell transplantation. Ibrutinib (ibr), a once-daily inhibitor of Bruton's tyrosine kinase, produced sustained responses in patients (pts) with cGVHD that had failed ≥1 prior treatments (Miklos, Blood 2017). Updated results of this trial are presented.
Methods: Enrolled pts had ≤3 prior regimens for cGVHD and either >25% BSA erythematous rash or a NIH mouth score >4. Pts received daily ibr (420 mg) until cGVHD progression/unacceptable toxicity. The primary endpoint was cGVHD response by 2005 NIH consensus panel criteria. Secondary endpoints included sustained response rate, changes in Lee cGVHD symptom scale scores, steroid doses over time, and safety.
Results: For 42 pts (median, 2 prior regimens [range, 1–3], ORR at a median follow-up of 25.6 mo was 69% (29/42 pts; 13 [31%] CR, 16 [38%] PR). Sustained responses of ≥20, ≥32, and ≥44 weeks were seen in 20 (69%), 18 (62%), and 16 (55%) of the 29 responders, respectively. Of 26 pts with ≥2 involved organs, 19 (73%) showed responses in ≥2 organs; 6/10 pts (60%) with ≥3 involved organs showed responses in ≥3 organs. Of 18 patients with sclerosis at baseline, 11 (61%) showed a sclerotic response (39% CR, 22% PR). By week 52, 26 pts (62%) had reduced steroid dose to < 0.15 mg/kg/d; 8 pts discontinued steroids. Responses were supported by a clinically meaningful (≥7 point) decrease in Lee cGVHD symptom scale scores in 12 (29%) pts on ≥2 consecutive visits; 16/29 responders (55%) compared with 1/13 nonresponders (8%) showed an improvement in Lee cGVHD symptom scale scores at month 12. Common grade ≥3 AEs were pneumonia (n = 6), fatigue (n = 5), and diarrhea (n = 4). Serious AEs (SAEs) were reported in 22 pts (52%); grade ≥3 SAEs occurred in 19 pts (45%) and included 6 pts with pneumonia, 1 of which was fatal. The onset of new grade ≥3 AEs decreased from 71% in the first year of treatment to 25% in Year 2 (n = 12). Primary reasons for treatment discontinuation included AEs (n = 15) and progressive cGVHD (n = 5); 4 pts discontinued ibr after cGVHD resolution.
Conclusions: At a median follow-up of >2 years, ibrutinib continued to produce durable responses in pts with cGVHD that had failed ≥1 prior therapy. Improvements from the previously reported 1-year results were documented in CR rates (21% to 31%), sustained response rates for ≥32 weeks (48% to 62%), and improvement in Lee cGVHD symptom scale scores on ≥2 consecutive visits (24% to 29%). In this pretreated, high-risk population, the observed benefit supports ibr's recent approval in the US for the treatment of adult pts with cGVHD after failure of ≥1 lines of systemic therapy.
Conflict of interest:
E. Waller: equity ownership with Cerus, Chimerix, and Cambium; honoraria from Novartis; consulting or advisory role with Novartis and Amgen; research funding with Celldex and Novartis; patents/royalties/other intellectual property with Cambium; travel expenses with Novartis, Amgen, and Cambium;
D. Miklos: consultancy/advisory role for Pharmacyclics LLC, an AbbVie Company, Sanofi, Adaptive Biotechnologies, Kite, Genentech, Velos; research funding from Pharmacyclics LLC, an AbbVie Company; travel expenses from Pharmacyclics LLC, an AbbVie Company and Sanofi Oncology; patents, royalties, other intellectual property from Pharmacyclics LLC, an AbbVie Company;
C. Cutler: consulting or advisory role with Pfizer, Pharmacyclics LLC, an AbbVie Company, Kite, Bristol-Myers Squibb, Incyte, Astellas;
M. Arora: consultancy/advisory role for Takeda; research funding with Pharmacyclics LLC, an AbbVie Company;
M. Jagasia: consultancy/advisory role and research funding from Theracos and Janssen;
I. Pusic: travel expenses from Pharmacyclics LLC, an AbbVie Company;
M. Flowers: consultancy/advisory role with Pharmacyclics LLC, an AbbVie Company;
A. Logan: consulting or advisory role with Amgen; research funding with Astellas, Novartis, Pharmacyclics LLC, an AbbVie Company; speakers bureau with Amgen;
R. Nakamura: consultancy/advisory role for Pharmacyclics LLC, an AbbVie Company, Molmed, Amgen, Merck; research funding from Pharmacyclics LLC, an AbbVie Company, Helocyte, Alopexx, Generon;
J. Dubovsky: employment with Pharmacyclics LLC, an AbbVie Company; equity ownership with AbbVie;
S. Chang: employment with Pharmacyclics LLC, an AbbVie Company; equity ownership with AbbVie, Johnson & Johnson, and Portola;
F. Clow: employment, leadership, and travel expenses with Pharmacyclics LLC, an AbbVie Company; equity ownership with AbbVie;
I. Lal: employment with The Permanente Medical Group (TPMG), Pharmacyclics LLC, an AbbVie Company; equity ownership with The Permanente Medical Group, Gilead Sciences, Reviva Pharmaceuticals, Clovis, Infinity, and AbbVie;
L. Styles: employment with Pharmacyclics LLC, an AbbVie Company; equity ownership with AbbVie;
S. Jaglowski: consulting/advisory role and research funding from Pharmacyclics LLC, an AbbVie Company
O081 Early termination of a randomised study comparing addition of mesenchymal stromal cells to standard care for steroid-naive grade II-IV acute graft versus host disease
Duncan Purtill1, Janice Fogarty2, Melita Cirillo2, Julian Cooney1, Paul Cannell1, Matthew Wright1, Richard Herrmann2, Marian Sturm2
1Fiona Stanley Hospital, Murdoch, Australia; 2Cell and Tissue Therapies WA, Perth, Australia
Background: We investigated whether the addition of mesenchymal stromal cells (MSC) to standard therapy would improve outcomes for patients developing acute graft versus host disease (GVHD) after allogeneic haemopoietic progenitor cell transplant.
Methods: Patients with grade II - IV acute GVHD were randomised within 72 hours of commencing steroids to receive standard care (2mg/kg methylprednisolone IV daily or oral equivalent) with or without MSC (2 doses of 2x106/kg given 7 days apart). MSC were from third party donors and manufactured in a licensed facility. The study was powered to detect a 40% improvement in overall survival for the MSC arm at 12 months post-randomisation, and planned to recruit 66 participants.
Results: Data from 28 patients recruited between 2012 and 2017 were submitted to the Data Safety and Monitoring Board. All 15 patients randomised to the treatment arm received MSC per protocol. The MSC patients were younger (median age 47 vs 55 years), with lower risk diseases (93% vs 62% low/intermediate revised disease risk index (rDRI)) and less severe aGVHD at randomisation (20% vs 54% grade III-IV) than the control arm. Resolution of GVHD at 28 days post-randomisation was observed in 7 (47%) MSC and 8 (62%) control subjects. At data submission, 12-month follow-up was complete in 25 (89%) patients. There had been 7 deaths in the MSC and 3 in the control arm (47 vs 23%). Causes of death were similar (1 relapse in each arm, all others GVHD) and median time to death was 3 months from randomisation in both arms. Mortality was higher during the early phase of study recruitment: 6/7 (86%) subjects in 2012–2013 vs 4/23 (17%) in 2014–2017. The only treatment change made during this time was to abandon the myeloablative busulphan / fludarabine regimen in late 2014, which 5 participants (3 in MSC and 2 in control arm) had received (all deceased). Study enrolment was terminated on grounds of futility.
Conclusions: A small excess of deaths in the treatment arm at interim analysis meant that the primary endpoint was unlikely to be met, leading to early termination of this randomised study. GVHD response at day 28 post-randomisation was similar in both arms. We postulate that steroid pre-treatment may have inhibited MSC activity. Our results highlight the difficulties involved in conducting interventional studies in GVHD. Recruitment was slow, perhaps due to a reduction in incidence of grade II-IV aGVHD at our centre (43% in 2012–14 vs 30% in 2015–2016). The chosen primary endpoint of 12-month overall survival, while robust, is subject to influence by non-study variables, including conditioning regimen selection. Our results do not support the use of MSC in addition to corticosteroids at diagnosis of acute GVHD. Early clinical studies reporting responses to MSC in steroid-refractory GVHD warrant further investigation.
Clinical Trial Registry: Australian New Zealand Clinical Trials Registry number NCT01589549
Conflict of interest: None of the authors has anything to disclose.
O082
Abstract previously published
O083 Multiparametric Magnetic Resonance Enterography for the Diagnosis and Staging of Intestinal Acute Graft-Versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation
Francesca Maccioni1, Walter Barberi2, Davide Bencardino1, Ursula La Rocca2, Mariangela Lopez1, Michela Ansuinelli2, Davide Vitale1, Luisa Quattrocchi2, Robin Foà2, Anna Paola Iori2
1Department of Radiological Sciences Oncology and Pathology, Policlinico Umberto 1, Sapienza University, Rome, Italy; 2Department of Biomedical Sciences and Hematology, Policlinico Umberto 1, Sapienza University, Rome, Italy
Background: Intestinal Acute graft-versus-host disease (i-aGVHD) is a major complication of allogeneic hematopoietic stem cell transplantation (HSCT). The clinical diagnosis of i-aGVHD is usually based on symptoms and CT findings but a definite diagnosis is made by endoscopic biopsies. To date, there are few data on the use of magnetic resonance enterography (MRE) in this setting. We hereby investigated the value of MRE in the diagnosis of i-aGVHD.
Methods: A retrospective observational study was carried out on 35 patients (16 men, 19 women; age 9–69 years) with hematologic malignancies who underwent a MRE for a suspect of i-aGvHD according to the Glucksberg criteria between 2015 and 2017. MRE examinations were performed with a 1.5 Tesla scanner (Siemens, MagnetomAvanto), equipped with 16-channels phased-array coils. We used a protocol including axial and coronal T2-weighted Half Fourier Acquisition Single Shot Turbo Spin Echo sequences, with and without fat saturation; T2 weighted axial and coronal TrueFISP sequences; DWI (Diffusion Weighted Imaging) sequences with b-values of 0, 500 and 1000; axial and coronal T1 weighted VIBE sequences, before and 70 seconds after the intravenous administration of contrast media (0.1 mmol gadolinium per kilogram of body weight, Gd-DOTA, Dotarem®, Guerbet, Aulnays-sous-Bois, France). In adult patients without contraindications, a 10 ml IV dose of hyoscine butylbromide was administered before contrast injection, in order to reduce motion artefacts. To evaluate the presence and severity of the disease, the following parameters were assessed and qualitatively scored for all intestinal segments (from stomach to rectum): a) MRI inflammation-activity including mural T2 signal (oedema), mesenteric T2 signal (oedema), gadolinium wall enhancement on T1 (vascularity), DWI signal (inflammation); b) morphologic parameters of activity and severity, including maximum wall thickness, increased number and/or size of local mesenteric lymph nodes, comb sign (mesenteric vessels dilation), presence of peritoneal effusion.
Results: Out of 35 patients, 21 had a definite histologic diagnosis of i-aGVHD while in the remaining 14 patients i-aGVHD was excluded. The above MRE parameters were observed in 19 out of 21(90.5%) patients with a definite diagnosis of i-aGVHD and in none of the 14 patients in which a diagnosis of i-aGVHD was excluded. A stratified mucosal wall enhancement after gadolinium injection was found in 90% of patients. Parietal enhancement was associated with high-grade oedema of mesenteric fat tissue and comb sign in 76% of cases. In 52.4% of cases, mesenteric lymph nodes were not found. Free intra-peritoneal fluid was observed in 57.2% of patients. The most commonly involved intestinal segments were distal ileum (85.7%), mean ileum (66.6%) and proximal ileum (57.1%), followed by ascending colon and sigmoid equally (38%). Spearman's test showed a statistically significant correlation between clinical stage of i-aGVHD and mural T2 signal, number of involved intestinal segments, wall thickness, T1 enhancement, peritoneal effusion (p < .001).
Conclusions: In our experience MRE parameters showed a good correlation with a definite i-aGVHD diagnosed and with the stage of disease. Therefore, in patients with a clinical suspect of i-aGVHD MRE examination may be considered in order to replace endoscopic biopsy to confirm the diagnosis.
Conflict of interest: All authors declare no conflict of interest.
O084 Validation of biomarkers of graft versus-host-disease in the multicentric French cohort Cryostem of the SFGM-TC
Etienne Daguindau1,2, Thomas Pagliardini3, Ghislaine Bernard4, Marie-Thérèse Rubio5, Boris Calmels6, Mathilde Blois4, Regis Peffault de Latour7, Pierre-Simon Rohrlich3
1University Hospital of Besançon, Hematology, Besançon, France; 2Univ. Bourgogne Franche-Comté, INSERM, EFS BFC, UMR1098, Interactions Hôte-Greffon-Tumeur/Ingénierie Cellulaire et Génique, Besançon, France; 3University Hospital of Nice, Hematology, Nice, France; 4University Hospital of Nice, Immunology, Nice, France; 5University Hospital of Nancy, hematology, Nancy, France; 6Institut Paoli-Calmettes, Marseille, France; 7Hôpital Saint-Louis, Assistance Publique-Hôpitaux de Paris, hematology, Paris, France
Background: Soluble ST2 has been validated in various transplantation settings as a relevant prognostic, diagnostic, and predictive biomarker for acute and chronic graft-versus host disease (GVHD). Other plasma biomarkers have been validated in independent cohorts such as TIM3 for aGVHD and the panel gathering ST2, CXCL9, matrix metalloproteinase 3 (MMP3), and osteopontin (OPN) in cGVHD.
Methods: We measured by enzyme-linked immunosorbent assay the concentration of ST2, TIM3, OPN, MMP3 and CXCL9 in plasma samples from 250 transplanted patients collected into the multicentric French Prospective Biorepository Cryostem. For each patient, samples were collected at onset of aGVHD, before treatment (n = 90), and 30 days later (n = 90), at onset of cGVHD (n = 70), and 100 days later (n = 70). Patients without GVHD were harvested at day 100 (n = 90) and one year (n = 90) after transplantation. Protein level differences between groups were assessed with unpaired t-test. Area under the curve (AUC) of receiver operating characteristic (ROC) curves were computed nonparametrically.
Results: Median follow-up was 24.3 months and median time for onset of aGVHD was day +30 after transplantation.
ST2 level was significantly higher in patients with aGVHD compared to patients without GVHD collected at D+100, both when measured at onset or day +30 (p < 0.005 for both)(fig 1A). At onset of aGVHD, ST2 and TIM3 were significantly higher in patients with severe GVHD (grade III-IV) compared to low grade aGVHD (respectively p < 0.005 and p = 0.01). Patients who deceased exhibited a higher level of ST2 at onset of aGVHD (p = 0.01). Non relapse mortality stratified by level of ST2 at onset of aGVHD (above or below median ST2 level) highlights a non-significant trend to better NRM for patients with low ST2 (fig 1B). However, neither ST2 nor TIM3 level at onset of aGVHD differed according to steroid response.
CXCL9, MMP3 and ST2 measured before treatment of cGVHD are higher compared to the group without cGVHD (p < 0.005 for each protein)(fig 1A). Individual ROC curves for ST2, CXCL9 and MMP3 are shown in figure 1. The biomarkers get respectively an AUC of 0.62, 0.85 and 0.72 suggesting CXCL9 as the better diagnostic biomarker for cGVHD in our cohort. We identified a statistical difference between alive and deceased patients by the end of follow-up only for ST2 level measured at 100 days after onset of cGVHD (p < 0.005).
Conclusions: We demonstrate in this study the relevant diagnostic and prognostic value of ST2 in biological samples drawn in context of aGVHD in a European homogeneous cohort coming from the CRYOSTEM repository. Diagnostic values of ST2, CXCL9 and MMP3 for cGVHD are also strongly suggested. Those results are consistent with previous published data. However, we didn't find an association between ST2 and TIM3 levels and corticosteroid responsiveness in aGVHD. This is likely explained by the low proportion of aGVHD patients with no response to steroids in this cohort. Finally, all these biomarkers could further support prospective evaluations addressing new tailored treatment, either in acute or chronic GVHD.
Conflict of interest: The authors declare no competing financial interests or conflict of interest that could perceived to bias this work.
This work was supported by grants from the International Research Group on Hematopoietic cells Transplantation and from Journée nationale Contre la Leucémie.
E. Daguindau was a recipient of a Nuovo Soldati fellowship.
[O084 Figure].
Figure 1. A/ Absolute values of proteins B/ CI of NRM stratified by the ST2 level (onset of GVHD)]
O085 Post-transplantation cyclophosphamide compared to graft-versus-host disease prophylaxis with ATG in mismatched unrelated donor hematopoietic stem cell transplantation
Yves Soltermann1, Dominik Heim1, Helen Baldomero1, Michael Medinger1,2, Jörg P. Halter1, Jakob P. Passweg1, Martina Kleber1,2
1University Hospital Basel, Divisions of Hematology, Basel, Switzerland; 2University Hospital Basel, Department of Internal Medicine, Basel, Switzerland
Background: Post-transplantation cyclophosphamide (PTCy) has been shown to be an effective strategy to prevent graft-versus-host disease (GvHD) after haploidentical hematopoietic stem cell transplantation (HCT). Mismatched unrelated HCT carries higher risks of GvHD and may benefit from best possible GvHD prophylaxis.
Methods: This study includes 1-antigen HLA-mismatched unrelated donor (9/10 MUD) transplant patients for a hematological disorder between 2010 and 2017 at the Hematology division of the Basel University Hospital. 78 patients were analysed, 20 patients received a PTCy-based GvHD prophylaxis (40mg/kg, day 3+4 after HCT) with cyclophosphamide, cyclosporine A (CyA) and mycophenolatmofetil (MMF), in 58 patients the GvHD conventional prophylaxis consisted of anti-thymocyte globulin (ATG-Fresenius), cyclosporine or tacrolimus, methotrexate (MTX) or MMF. The primary study aim was to compare the incidence of acute GvHD (aGvHD) at day 100, and chronic GvHD (cGvHD) 6, 12 months and outcome after HCT [1-year overall survival (OS)].
Results: The median age in the PTCy- and ATG group was 55 (IQR 44–68) vs. 50 years (IQR 39–60; p = 0.047), respectively. Median follow-up was 12 months (range 0.8–89 months). Graft source was peripheral blood in the PTCy- (95%) and ATG (91%) groups (p = NS), respectively. Patients in the PTCy group showed less grade II-IV aGvHD at day 100 with 20% vs. 27%, respectively (p = 0.177) and grade III-IV aGvHD (0% vs. 5%, respectively, p = 0.103) compared to the ATG group, but without statistical significance. Moreover, the PTCy group revealed a significantly lower incidence of cGvHD at 6 months with 15% vs. 26%, respectively (p = 0.008) and at 12 months (15% vs. 21%, respectively, p < 0.001) compared to the ATG group (Table 1). The 1-year OS of the PTCy vs. ATG group is depicted in Figure1. Median time to neutrophil engraftment (> 500/µl) was comparable in the PTCy- and ATG group (14 days vs. 16 days, respectively, p = 0.130) and also the median time to platelet engraftment (> 20'000/µl) showed no significant difference in both groups (15 days vs. 13 days, respectively, p = 0.089).
Conclusions: Our results revealed that PTCy-based prophylaxis is an effective and safe strategy to prevent GvHD in 9/10 MUD patients undergoing HCT for hematologic malignancies at day 100 and for cGvHD compared to ATG based GvHD prophylaxis.
Conflict of interest: All authors have nothing to disclose
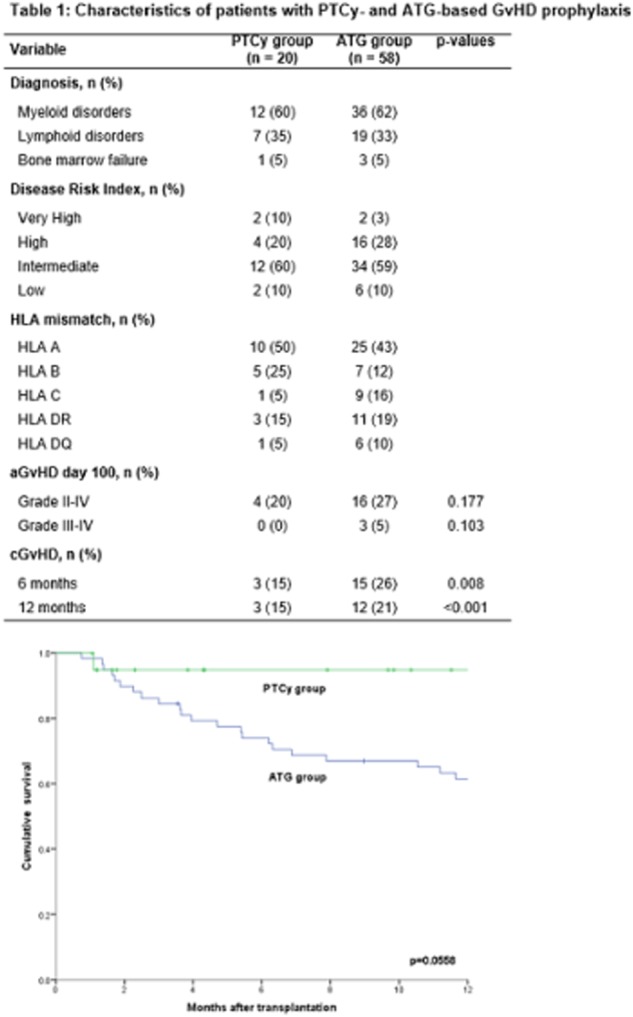
[ [O085 Figure] Graph]
Table 1 Characteristics of patients with PTCy- and ATG-based GvHD prophylaxisTab3
O086
Abstract previously published
O087 Effective treatment and low mortality in patients with therapy-refractory aGvHD after treatment with MSC: post-approval observational data from 69 consecutive patients treated with “MSC-FFM”
Peter Bader1, Zyrafete Kuci1, Shahrzad Bakhtiar1, Oliver Basu2, Gesine Bug3, Michael Dennis4, Johann Greil5, Aniko Barta6, Kristián Kállay7, Peter Lang8, Giovanna Lucchini9, Raj Pol10, Ansgar Schulz11, Karl-Walter Sykora12, Irene von Teichert-Luettichau13, Grit Herter-Sprie14, Mohammad Ashab Uddin15, Philip Jenkins Jenkins15, Abdulrahman Alsultan16, Jochen Buechner17, Jerry Stein18, Agnes Kelemen19, Andrea Jarisch1, Jan Soerensen1, Emilia Salzmann1, Martin Hutter1, Richard Schaefer20, Erhard Seifried20, Thomas Klingebiel1, Halvard Boenig20, Selim Kuci1
1University Hospital Frankfurt, Department for Children and Adolescents; Division for Stem Cell Transplantation, Frankfurt, Germany; 2University Children's Hospital Essen, Essen, Germany; 3University Hospital Frankfurt, Department fo Medicine II, Frankfurt, Germany; 4Christie Hospital, Department of Hematology, Manchester, United Kingdom; 5University Children's Hospital Heidelberg, Heidelberg, Germany; 6St. István and St. László Hospital, Department for Hematology and SCT, Budapest, Hungary; 7St. István and St. László Hospital, Department for Pediatric Hematology and SCT, Budapest, Hungary; 8University Children's Hospital Tuebingen, Tuebingen, Germany; 9Great Ormond Street Hospital, Department of Hematology/Oncology, London, United Kingdom; 10University of Sheffield, Department of Hematology, Sheffield, United Kingdom; 11University Medical Center Ulm, Department of Pediatrics, Ulm, Germany; 12University Children's Hospital Hannover, Hannover, Germany; 13Technical University Munich, Division of Pediatric Hematology/Oncology, Department of Pediatrics, Munich, Germany; 14University Hospital of Cologne, Department I for Internal Medicine, Cologne, Germany; 15NHSBT, Department for Stem Cells & Immunotherapies, Birminham, United Kingdom; 16King Abdullah Specialist Children's Hospital, Department of Pediatric Hematology/Oncology, Riyadh, Saudi Arabia; 17Oslo University Hospital Rikshospitalet, Department of Pediatric Medicine, Section for Pediatric Hematology/Oncology, Oslo, Norway; 18Schneider Children's Medical Center of Israel, Department for Hemato-Oncology, Petach Tikva, Israel; 19B-A-Z County Hospital, Pediatric Hematology and SCT Unit, Miskolc, Hungary; 20German Red Cross Blood Center and Institute of Transfusion Medicine and Immunohematology, Goethe University Medical Center, Frankfurt, Germany
Background: Despite ample clinical evidence suggesting effectiveness, unambiguous demonstration of the true potential of mesenchymal stromal cell (MSC) therapy for refractory acute graft-versus-host disease (aGvHD) is still lacking.
Methods: We developed a novel MSC manufacturing protocol ensuring equipotency of all individual therapeutic doses. The resulting product, MSC-FFM, has since received a national marketing authorization (Number: PEI: A.11748.01.1). We here report outcomes of 69 consecutively treated patients in six countries who received MSC-FFM in routine clinical use. Children (< 18 years, n = 51) and adults (>18 years, n = 18) transplanted for malignant (74%) or non-malignant diseases (26%) and suffering from refractory aGvHD grade II (4%), III (36%) or IV (59%), were enrolled.
Stem cell donors were MSD (n = 14; 20%), MUD (n = 45; 65%) or MMFD (n = 10; 15%). Grafts were derived from BM (n = 35; 51%) or peripheral blood (n = 33; 48%) (cord blood: n = 1).
Patients received MSC infusions in four weekly intervals after having failed to respond to either first-line treatment (steroids) (n = 20, 29%), or 1–5 additional lines of immunosuppressants (n = 49, 71%).
Response was defined as either complete response (CR), i.e. complete resolution of all signs of GvHD, partial response (PR), i.e. GvHD reduction by at least one grade according to the Glucksberg criteria, or non-response (NR) at day 28 after first MSC transfusion.
Results: At day 28, 22 (32%) patients achieved CR, 35 (51%) PR, 10 (14%) NR and for two of patients (3%) no day 28 data were available. This resulted in an overall response (OR) of 83%. At the last follow-up (median follow-up: 8.19 months; range, 0.9–54.02 months), 42 (61%) patients were in CR, 17 (25%) patients in PR, and 10 patients (14%) were NR. These response rates resulted in a predicted six month non-relapse mortality rate (NRM) of 27% (95% CI 16–38) and cumulative relapse mortality incidence of 2% (0–5), for an overall survival rate (OS) of 71% (61–83).
Patients with aGvHD grade III or grade IV at 6 months had an estimated overall survival probability of 75% (59–94) and 67% (54–84). Clinical responsiveness did not differ between children (< 18 years, n = 51) and adults (>18 years, n = 18): Of the 51 children, 13 (25%) and 28 (55%) reached CR or PR by day 28, respectively. Eight (16%) were NR and in 2 (4%) patients no day 28 report was available. Among those 18 patients >18 years of age, 9 (50%) achieved CR, 7 (39%) PR and 2 (11%) patients did not respond. This resulted in an OR of 80% in children and 89% in adults. Thus estimated six-month survival rates in children and adults were 75% (64–88) and 61% (42–88), respectively (p = 0.398). Similarly, non-relapse mortality at six-month was 25% (12–36) or 33% (8–52) in children vs. adults, respectively (p = 0.577). The outcomes of steroid-refractory vs. treatment-refractory patients did not reveal statistically significant differences. The predicted six-month overall survival was 69% (52–93) vs. 72% (60–86) for steroid-refractory vs. treatment-refractory patients (p = 0.925) with a NRM of 31% (7–48) vs. 26% (12–38) (p = 0.763).
Conclusions: MSC-FFM offers highly promising salvage therapy for both steroid and treatment-refractory aGvHD, warranting further clinical evaluation.
Conflict of interest: P. Bader, Z. Kuci, H. Boenig, and S. Kuci own IP for MSC-FFM. All other authors have nothing to disclose.
Graft-versus-host disease – preclinical and animal models
O088 HLA-DM mediates permissiveness of T-cell alloreactivity to HLA-DPB1
Maximilian Metzing1, Pietro Crivello1, Esteban Arrieta-Bolanos1, Michel Kester2, Dominik A. Megger3,4, Thuja Meurer1, Cornelis van Bergen2, Mareike Griffioen2, Peter A. Horn5, Barbara Sitek3, J.H. Frederik Falkenburg2, Katharina Fleischhauer1
1University Hospital Essen, Institute for Experimental Cellular Therapy, Essen, Germany; 2Leiden University Medical Center, Department of Hematology, Leiden, Netherlands; 3Ruhr University Bochum, Department of Clinical Proteomics, Bochum, Germany; 4University Hospital Essen, Institute for Virology, Essen, Germany; 5University Hospital Essen, Institute for Transfusion Medicine, Essen, Germany
Background: Unrelated donor (UD)-recipient HLA-DPB1 mismatches are targets of both graft-versus-host-disease (GvHD) and graft-versus-leukemia (GvL) after stem cell transplantation (SCT). Amino acid variability at peptide-binding positions in the HLA-DP molecule has been shown to determine functionally distinct T-cell epitope (TCE) groups. Clinical risk associations have been found between DPB1 mismatches within the same TCE group (“permissive”) or across different TCE groups (“non-permissive”), eliciting limited and strong donor T-cell alloreactivity, respectively. The non-classical HLA-DM, a class II peptide editor that can be antagonized by HLA-DO in certain subsets of healthy and malignant immune cells, catalyzes dissociation of low-affinity peptides and binding of high-affinity peptides to HLA class II molecules during endosomal processing. We hypothesized that HLA-DM-mediated changes in the peptide repertoire presented by HLA-DP could impact T-cell alloreactivity in the permissive and/or non-permissive context.
Methods: HLA-DPB1*10:01 and *04:02 were used as prototypes of TCE groups 1 and 3, respectively. HeLa cells transduced to express these two alleles as single HLA-DP molecules, together with the invariant chain and co-stimulatory molecules in the presence (HeLa-DP-DM) or absence (HeLa-DP) of HLA-DM were used for immunoaffinity chromatography purification of HLA-DP and peptide identification by liquid chromatography tandem mass spectrometry. HeLa-DP-DM and HeLa-DP were also comparatively used as stimulators in co-cultures with CD4+ T-cells from healthy donors homozygous for HLA-DPB1*04:01 (TCE group 3) as responders, resulting in a permissive (Hela-DPB1*04:02) or non-permissive (Hela-DPB1*10:01) mismatch. Quantification by flow cytometry of T-cells specifically up-regulating the activation marker CD137 was used to measure the strength of the in vitro alloresponse after 14 days of culture. T-cell receptor (TCR) diversity of activated CD137+CD4+ T-cells was measured by flow cytometry of TCR-Vb families and by next-generation sequencing of TCRB CDR3 regions.
Results: The presence of HLA-DM resulted in a 52% and 47% decrease of the number of different peptides eluted from DPB1*04:02 and *10:01, respectively. Moreover, only 27.4% (*04:02) and 25.4% (*10:01) of the peptides eluted in the absence of HLA-DM were also found in its presence, showing similar HLA-DM dependency of both alleles. 84 independent co-cultures showed significantly higher percentages of CD4+CD137+ T-cells in response to non-permissive vs permissive mismatches in the presence of HLA-DM (p < 0.0001). However, the absence of HLA-DM significantly increased the magnitude of alloreactivity to permissive mismatches to levels comparable to non-permissive mismatches (p < 0.0001), while alloreactivity to non-permissive mismatches was not affected (p = n.s.). TCR diversity was higher in T-cells responding to non-permissive compared with permissive mismatches, both by the number of TCR-Vb families of highly reacting T-cells and by the number of clonotypes carrying unique TCRB CDR3 sequences. Importantly, when HLA-DM was not present, TCR diversity to permissive mismatches was also increased to levels comparable with non-permissive mismatches.
Conclusions: HLA-DM regulates allele-specific immunogenicity of allogeneic HLA-DP molecules, thereby mediating permissiveness of HLA-DPB1 TCE group mismatches. This observation suggests that modulation of HLA-DM activity, for instance by the antagonistic effect of HLA-DO, could favor GvL over GvHD in certain hematologic malignancies, and opens new avenues for the exploitation of HLA-DM to regulate T-cell alloreactivity after SCT.
Conflict of interest: The authors have no conflicts of interest to declare.
This work was supported by a grant from the Deutsche José Carreras Leukämie Stiftung (DJCLS R 15/02).
O089 Reductions in oral intake perturb the intestinal microbiome and compromise the colonic mucus barrier
Zaker I. Schwabkey, Diana H. Wiesnoski, Dung Pham, Christopher A. Sanchez, Chia-Chi Chang, Mohamed A. Jamal, Robert R. Jenq
University of Texas MD Anderson Cancer Center, Houston, TX, United States
Background: Reduced oral intake (ROI) is common in allogeneic hematopoietic cell transplantation (allo HCT) recipients following conditioning and with intestinal graft-versus-host disease (GVHD). Here we examine the interplay between nutrition, intestinal bacteria and colonic mucus.
Methods: Mice were subjected to ROI for 7 days (2 grams of chow per mouse per day, or ~ 50% reduction in oral intake) with unlimited water. Intestinal bacterial composition was assayed by 16S deep sequencing. Mucolytic activity of fecal samples or Akkermansia muciniphila (ATCC) was evaluated using porcine gastric mucin (Sigma) and a colorimetric assay that quantifies polysaccharides (Periodic acid-Schiff method, PAS). In 8 allo HCT patients with a 30% or greater reduction in oral intake from pre-HCT (day -8 to day -4) to post-HCT (day +4 to day +10), fecal samples were collected and evaluated with their consent. Colonic mucus layer thickness in mice was measured by PAS staining of histological samples. Supplemental sugars were introduced to the drinking water of mice (2g/L).
Results: In mice we found that after ROI, the intestinal bacterial composition was perturbed with a pronounced increase in Akkermansia muciniphila (Fig A), an intestinal commensal that degrades mucins as a carbohydrate source. ROI also led to increased mucolytic function in fecal samples (Fig B). Histologically, the colonic mucus was thinned following ROI (Fig C). Increases in mucolytic function were seen in 4 of 8 patients undergoing allo HCT who developed mucositis and nausea (Fig D).
We asked how ROI could favor mucolytic bacteria. Hypothesizing that ROI reduced bacterial fermentation, we evaluated the pH of the colonic lumen. We found that ROI led to a higher pH in the colonic lumen (Fig E). Also, Akkermansia muciniphila gains the ability to degrade mucins in vitro when the pH rises from 5.5 to 6.0 (Fig F).
Finally, we asked if supplementation of sugars to mice undergoing ROI could prevent thinning of the colonic mucus layer. We found that 4 different sugars in the drinking water of mice all prevented, to varying degrees, the thinning of the colonic mucus (Fig G).
Conclusions: Reduced oral intake in mice and many patients leads to increased mucolytic activity of the intestinal microbiome and may be an important clinical contributor to impaired intestinal barrier function during allo HCT. A strategy of low-dose oral supplementation with sugars could help suppress mucolytic activity of the intestinal microbiome.
Conflict of interest: RRJ is on the board of directors or an advisory committee for Seres Therapeutics, Inc.; has consulted for Ziopharm Oncology; and holds patents with or receives royalties from Seres Therapeutics, Inc. The other authors declare no conflict of interest.
[[O089 Figure].
Reductions in oral intake increase intestinal bacterial mucolytic activity.]
(A) Effects of ROI on intestinal bacterial composition. NOI indicates normal oral intake. (B) Functional mucolytic assay; remaining mucin levels after 48-hour culture with indicated bacterial sources. (C) Histological thickness of the inner mucus layer in mice. (D) Functional mucolytic assay using patient fecal specimens. Above: 4 patients without increases in mucolytic function. Below: 4 patients with increases in mucolytic function. (E) Effects of ROI on colonic luminal pH. (F) Effects of pH on Akkermansia mucolytic function in vitro. (G) Effects of supplemental sugars on colonic mucus thickness.
O090 Intestinal graft-versus-host disease is driven by BATF-dependent IL-7RhiGM-CSF+ T cells
Evelyn Ullrich1,2, Benjamin Abendroth3, Johanna Rothamer2, Carina Huber3, Maike Büttner-Herold4, Vera Kitowski3, Tina Vogler3, Thomas Longerich5, Sebastian Zundler3, Simon Völkl2, Andreas Beilhack6, Stefan Rose-John7, Stefan Wirtz3, Georg F. Weber8, Sakhila Ghimire9, Marina Kreutz9, Ernst Holler9, Andreas Mackensen2, Markus F. Neurath3, Kai Hildner3
1Goethe University Frankfurt am Main, Division for Stem Cell Transplantation and Immunology, Department for Children and Adolescents, Frankfurt, Germany; 2University Hospital Erlangen, Department of Medicine 5, Erlangen, Germany; 3University Hospital Erlangen, Department of Medicine 1, Erlangen, Germany; 4University Hospital Erlangen, Institute of Pathology, Department of Nephropathology, Erlangen, Germany; 5University Hospital Heidelberg, Institute of Pathology, Heidelberg, Germany; 6University Würzburg, Center for Interdisciplinary Clinical Research, Würzburg, Germany; 7Christian-Albrechts-University, Institute of Biochemistry, Kiel, Germany; 8University Hospital Erlangen, Department of Surgery, Erlangen, Germany; 9University Hospital Regensburg, Department of Hematology and Oncology, Regensburg, Germany
Background: Acute graft-versus-host disease (GVHD) represents a severe, T cell-driven inflammatory complication following allogeneic hematopoietic cell transplantation (allo-HCT). GVHD often affects the intestine and is associated with a poor prognosis. Although frequently detectable, the role of the pro-inflammatory IL-17a-producing T helper lymphocyte subset (Th17) in the pathogenesis of intestinal GVHD is controversially discussed and remains to be fully elucidated.
Methods: We performed gene expression analyses of transcription factors such as BATF in GVHD-affected colonic tissues from mice and men undergoing allo-HCT. Furthermore, Batf-/-, Csf2-/- donor T cells in comparison to WT donor T cells were adoptively transferred in both complete MHC and miHA-mismatched GVHD models. Finally, reconstitution studies with intestinal GVHD-derived IL-7Rhi T cells were performed to induce GVHD-associated colitis in Batf-/- donor T cell receiving mice.
Results: First, we found that BATF expression is strongly induced within GVHD-affected colonic tissues derived from mice and men undergoing allo-HCT. Given that BATF is predominately expressed by lymphoid lineages, we hypothesized that BATF-expressing T cells might contribute to this enhanced expression prompting us to study the functional relevance of donor T cell-intrinsic BATF for GVHD manifestation. Our data demonstrate that BATF is absolutely indispensable for the manifestation of intestinal GVHD. We found initial expansion and homing of allo-reactive T cells into the colonic lamina propria (cLP) compartment to be largely unaffected in the absence of BATF. However, upon GVHD onset the magnitude of the colonic donor T cell population became increasingly BATF-dependent. Interestingly, in addition to Th17 differentiation, we found that T cell-intrinsic GM-CSF- and IL-6-expression of colonic donor T cells were highly dependent on BATF while Th1 differentiation was unaffected.
Functional studies employing cytokine reconstitution experiments of Batf-/- donor T cell-receiving mice and cytokine inactivation studies by using Csf2-/- donor T cells clearly supported that GM-CSF is promoting GVHD-associated colitis manifestation. GVHD was sufficiently suppressed by a combined IL-7R and GM-CSF blockade, thereby suggesting synergistic mechanisms to inhibit the functionality of intestinal IL7RhiGM-CSF+ donor T cells. Importantly, studies employing Csf2-/- T cells confirmed that T cells themselves are the most important source of GM-CSF as reduction of intestinal GVHD equalled results obtained with systemic antibody-mediated GM-CSF neutralization.
Finally, reconstitution studies functionally demonstrated that intestinal GVHD-derived IL-7Rhi cLP T cells were able to reconstitute GVHD-associated colitis in Batf-/- donor T cell receiving mice.
Conclusions: Together, this study provides a crucial example of a BATF-dependent, however RORgt- and hence Th17 fate-independent regulation of a pathogenic effector T cell population with critical relevance for intestinal inflammation in GVHD and broad implications for inflammatory processes previously assumed to be selectively Th17-driven. Hence, therapeutic targeting of the IL-7R/BATF/GM-CSF-axis might represent a novel future option to mitigate acute, life-threatening intestinal GVHD.
Conflict of interest: The authors have nothing to disclose.
O091
Abstract previously published
O092
Abstract previously published
Haemoglobinopathy and inborn errors of metabolism
O093I dentical thalassaemia free survival in fully matched family and unrelated donor transplantation using a treosulfan based protocol
Revathi Raj1, Ramya Uppuluri1, Dhaarani Jayaraman1, Meena Sankaran1, V S Venkateswaran1, Shivani Patel1, Lakshman Vaidyanathan1, Joseph John2
1Apollo Speciality Cancer Hospitals, Paediatric Blood and Marrow Transplantation, Chennai, India; 2Christian Medical College, Clinical Haematology and Oncology, Ludhiana, India
Background: Haematopoietic stem cell transplantation (HSCT) is currently the only curative option for patients with thalassaemia major. With the advent of treosulfan based conditioning regimen, we are able to offer HSCT for children with a matched family and unrelated donor to help achieve thalassaemia free survival.
Methods: The study is a retrospective analysis over an eight year period between 2009 and 2016 of patients transplanted in two HSCT centres in India. Patients with a diagnosis of thalassaemia major were treated with a uniform conditioning regimen consisting of thiotepa, treosulfan and fludarabine with ATG added to the patients undergoing unrelated donor transplantation. The donor was a family or an unrelated donor and the source of stem cells was peripheral blood or bone marrow or cord blood. Graft versus host disease prophylaxis was tacrolimus in one centre and cyclosporine in the other with a short course of methotrexate. The thalassaemia free survival rates were analysed based on the degree of HLA matching of the donor and the stem cell source between family and unrelated donor with a follow up period of 1 to 8 years.
Results: A total of 217 patients with thalassaemia major were analysed and the patients were between the ages of 7 months to 18 years. Matched family donor transplantation was performed in 168 children and unrelated in 37 children. The thalassaemia free survival at 8 years in the 6/6 matched related donor and 10/10 matched unrelated donor groups were almost identical at 89% with a follow up period ranging from 12 to 96 months. In the one antigen mismatched family transplants, there were a total of 9 patients with 3 deaths and 2 rejections with a thalassaemia free survival of 44% and all the three of the single allele/antigen mismatched unrelated transplant children died with a thalassaemia free survival of 0%. Risk stratification based on Pesaro class did not have an impact on the survival in these children and none of the children died of sinusoidal obstruction syndrome.
Conclusions: With the advent of the reduced toxicity conditioning with treosulfan, the significance of Pesaro based risk stratification and donor source between family and unrelated donors in patients with thalassaemia major has now become obsolete. The rate of graft rejection has been reduced dramatically since the initiation of a protocol for close follow up of donor chimerism and the preferential use of peripheral blood stem cells as the graft source. Optimal outcomes can be ensured with accurate HLA matching and this data reinforces the need for early counselling for children with thalassaemia major to undergo HSCT from either donor source.
Clinical Trial Registry: N/A
Conflict of interest: None
Hematopoietic stem cells
O094 Leukemia relapse following unmanipulated haploidentical transplantation: a risk-factors analysis of on behalf of the Acute Leukemia Working Party of the EBMT
Simona Piemontese1, Arianne Boumendil2,3,4, Myriam Labopin2,3,4, Christoph Schmid5, Fabio Ciceri1,6, William Arcese7, Yener Koc8, Zafer Gulbas9, Johanna Tischer10, Bendetto Bruno11, Depei Wu12, Didier Blaise13, Dietrich Beelen14, Giuseppe Irrera15, Annalisa Ruggeri2,3,4, Mohamed Houhou2,3,4, Mohamad Mohty2,3,4, Arnon Nagler2,16
1San Raffaele Scientific Institute, Hematology and Bone Marrow Transplantation Unit, Milan, Italy; 2EBMT Paris Study Office / CEREST-TC, Paris, France; 3Saint Antoine Hospital, Department of Haematology, Paris, France; 4INSERM UMR 938, Paris, France; 5Klinikum Augsburg, Augsburg, Germany; 6University 'Vita-Salute' San Raffaele, Milan, Italy; 7Policlinico Universitario Tor Vergata, Stem Cell Transplant Unit, Rome, Italy; 8Medical Park Hospitals, Stem Cell Transplant Unit, Antalya, Turkey; 9Anadolu Medical Center Hospital, Bone Marrow Transplantation Department, Kocaeli, Turkey; 10Klinikum Grosshadern Med, Munich, Germany; 11A.O.U Citta della Salute e della Scienza di Torino, S.S.C.V.D Trapianto di Cellule Staminali, Turin, Italy; 12First Affiliated Hospital of Soochow University, Department of Hematology, Suzhou, China; 13Centre de Recherche en Cancérologie de Marseille, Institut Paoli Calmettes, Programme de Transplantation & Therapie Cellulaire, Marseille, France; 14University Hospital, Department of Bone Marrow Transplantation, Essen, Germany; 15Azienda Ospedaliera, Centro Unico Regionale Trapianti, Alberto Neri, Reggio Calabria, Italy; 16Chaim Sheba Medical Center, Tel Aviv, Israel
Background: Relapse of acute leukemia remains the leading cause of failure after allogeneic stem cell transplantation. We aimed to identify risk factors for relapse of both AML and ALL post T-replete haploidentical transplantations (haplo-SCT).
Methods: From January 2007 to December 2014, 1660 T-replete haplo-SCT were performed as first allogeneic transplantations for adult patients (pts) with acute leukemia in 186 EBMT centres. The current report is based on analysis of 587 pts (AML-456; ALL-131) for which detailed informations were received from the transplant centers via specific questionnaires.
Results: Median follow-up of surviving pts was 32mo for AML and 25 mo for ALL, respectively. AML and ALL pts differ in several parameters including patient age at haplo-SCT (48y vs 37y, p < 0.0001), time from diagnosis to transplant (13mo vs 20mo, p = 0.003), number of induction courses to achieve first complete remission (CR1) (1 course -65% vs 75%, 2- 25% vs 15% and ≥ 3- 7% vs 10%, p = 0.005), HCT-CI (0 in 38% vs 54%, 1–2 in 35% vs 29% and ≥ 3 in 27% vs 17%, p = 0.003) and conditioning intensity (myeloablative (MAC) in 48% vs 70%, p < 0.0001). Post-transplant cyclophosphamide was used in 63% and 65% of the haplo-SCT for AML and ALL, respectively (p = 0.760). The 3-year (y) progression free survival for AML and ALL was 38% and 26%,respectively (p = 0.081).The 3-y overall survival was 41% and 31% for AML and ALL, respectively (p = 0.151). Relapse incidence (RI) was 31% at 2 and 32% at 3y for AML and 41% and 44% for ALL respectively (p = 0.024). Median time from haplo-SCT to relapse was 7 mo (1–63). In univariate analysis 2y RI for AML was statistically different according to cytogenetics (good 18%, intermediate 30%, poor 45%, p < 0.001), disease status at transplant (CR1 20%, CR2 23%, advanced 45%, p>0.001) and intensity of conditioning regimen (MAC 24%, RIC 37%, p = 0.01). In ALL disease status at transplant was the only factor associated with a higher RI at 2y after haplo-SCT: CR1 22%, CR2 42% (p < 0.001). All but one ALL patient with advanced disease experienced relapse/progression. In multivariate analysis risk factors for a higher RI in AML were disease status at transplant (advanced vs CR1: p < 0.0001; HR 3.95; CI: 2.53–6.17) and HCT-CI (≥ 3 vs 0: p = 0.014; HR 1.75; CI: 1.12–1.75). In addition transplants performed in more recent years were associated with lower RI (p = 0.042; HR 0.91; CI: 0.84–1).In ALL the predictive factors for a higher RI were disease status at transplant (CR2 vs CR1: p = 0.011; HR 2.85; CI: 1.26–6.42; advanced vs CR1: p < 0.0001; HR 14.28; CI: 6.03–33.79) and donor gender (male vs female: p = 0.0002; HR 3.7; CI: 1.87–7.33).
Conclusions: Disease status at transplant, year of transplantation and comorbidities are the factors for prediction of AML relapse post haplo-SCT, while the predictive factors for relapse in ALL are disease status and donor gender. Future strategies for reducing relapse incidence post haplo-SCT should be tailored as per relapse specific predicting factors.
Conflict of interest: None of the authors has anything to disclose.
O095
Abstract previously published
O096 Selective HSC-Ablation using Anti-CD117 Antibody Drug Conjugate Enables Safe and Effective Murine and Human Hematopoietic Stem Cell Transplantation
Agnieszka Czechowicz1,2,3, Rahul Palchaudhuri4,5,6, Amelia Scheck1,3,4, Yu Hu1, Jonathan Hoggatt4,5,7, Borja Saez4,5,8, Michael K. Mansour4,5,9, Florian Winau1, David T. Scadden4,5,7, Derrick J. Rossi1,4,7
1Boston Children's Hospital, Program in Cellular and Molecular Medicine, Department of Medicine, Boston, MA, United States; 2Dana-Farber Cancer Institute and Boston Children's Hospital, Division of Pediatric Hematology/Oncology, Boston, MA, United States; 3Stanford University, School of Medicine, Department of Pediatrics, Division of Stem Cell Transplantation and Regenerative Medicine, Stanford, CA, United States; 4Harvard University, Stem Cell and Regenerative Biology, Cambridge, MA, United States; 5Massachusetts General Hospital, Center for Regenerative Medicine, Boston, MA, United States; 6Magenta Therapeutics, Cambridge, MA, United States; 7Harvard Stem Cell Institute, Cambridge, MA, United States; 8Center For Applied Medical Research, Pamplona, Spain; 9Massachusetts General Hospital, Division of Infectious Diseases, Boston, MA, United States
Background: Hematopoietic stem cell transplantation (HSCT) can be curative for many blood and immune diseases. However, despite its widespread potential, today HSCT is primarily restricted to deadly malignant diseases with few other treatment options. In these situations, the potential benefit of HSCT outweighs the current risks. Although if made safer, HSCT could be applied in many more disease settings. The procedure today results in frequent morbidity/mortality mainly due to graft versus host disease and toxicity from irradiation/chemotherapy conditioning currently employed to enable donor HSC engraftment. Although many efforts have been undertaken to reduce toxic conditioning, most patients still suffer from side effects including organ damage, infertility, secondary malignancies, and cytopenias. Eliminating genotoxic conditioning would dramatically improve HSCT, which would be especially beneficial in gene therapy/gene editing settings where this is the major limitation to expanded use.
Methods: Over the last decade we have pioneered several novel antibody-based strategies to overcome the need for genotoxic conditioning. Specifically, we have previously shown that competition with host HSC limits donor HSC engraftment, and that antagonistic anti-CD117 antibodies depleting host HSC are an effective, safe alternative conditioning approach in immunodeficient mice (Czechowicz Science 2007). These antagonistic anti-CD117 antibodies were subsequently shown to be effective in conditioning wildtype mice, however additional strategies/agents were needed to enable donor engraftment which caused significant cytopenias (Xue Blood 2010, Chhabra Sci Trans Med 2016,). As an alternative, we developed anti-CD45 antibody-drug conjugates, however these induced a temporary lymphopenia which is not desirable in many settings (Palchaudhuri Nature Biotechnology 2016). To overcome these limitations, here we generated an exceptional anti-CD117 antibody-drug conjugate by linking non-antagonistic anti-CD117 antibodies to protein synthesis toxins.
Results: These anti-CD117-saporin antibody-drug conjugates led to >99.9% depletion of host HSCs and subsequently enabled rapid >99.9 ± 0.1% engraftment of donor murine whole bone marrow cells and >69.0 ± 12.8% engraftment of donor murine purified HSCs in a cell dose dependent manner. Importantly and uniquely, these non-genotoxic agents did not cause any significant cytopenias. Rather they grossly spared red blood cells, platelets, and all major immune cells, no transfusions were needed, and immunity remained functionally intact as compared to post traditional conditioning. Additionally, these anti-CD117 antibody-drug conjugates were effective at creating irradiation-free human xenografts in NSG animals. In this setting, depletion of host murine HSCs enabled robust human cord blood HSC engraftment with >48.6 ± 0.28% human total peripheral blood engraftment. Importantly, engraftment was multi-lineage with human myeloid, B-cell and T-cell engraftment which paralleled that of traditional irradiation conditioning, providing the opportunity to study human HSCs in vivo in a non-irradiated setting.
Conclusions: Anti-CD117 antibody-drug conjugates provide the possibility of safe and effective transplantation of both murine and human HSCs without major cytopenias or perturbations to immunity. These agents provide important tools to study murine and human hematopoiesis, but also are likely to become important agents in clinical transplantation. As multiple anti-CD117 antibodies are in development and being tested in clinical trials, such an approach may be rapidly translatable a range of patients with blood and immune diseases including sickle cell anemia, beta thalassemia, immunodeficiencies and HIV.
Conflict of interest:
A. Czechowicz: Magenta Therapeutics (scientific co-founder, stockholder, financial benefit and/or patents), Editas Medicine (stockholder, financial benefit and/or patents), Global Blood Therapeutics (stockholder), Decibel Therapeutics (stockholder) and Third Rock Ventures (consultant).
R. Palchaudhuri: Magenta Therapeutics (scientific co-founder, salary, stockholder, financial benefit and/or patents)
J. Hoggatt: Magenta Therapeutics (scientific co-founder, stockholder, salary, financial benefit and/or patents)
A. Scheck, Y. Hu, B. Saez, M. Mansour, F. Winau: Nothing to disclose
D. Scadden: Magenta Therapeutics (co-founder, stockholder, director, consultant), Fate Therapeutics (stockholder, consultant), and Agio Pharmaceuticals (stockholder, director).
D. Rossi: Moderna Therapeutics (co-founder, stockholder, financial benefit and/or patents), Intellia Therapeutics (co-founder, stockholder, consultant), Vor Biopharma (stockholder, consultant), Magenta Therapeutics (co-founder, stockholder, financial benefit and/or patents), Stelexis Therapeutics (stockholder, consultant, director), Convelo Therapeutics (stockholder, consultant, director).
O097
Abstract previously published
O098
Abstract previously published
O099
Abstract previously published
O100 In vivo Tracking of Hematopoietic Progenitors in Humans Unveils Resilience of Hematopoietic Stem Cells and Long-term Survival of Lymphoid Precursors
Serena Scala1, Luca Basso-Ricci1, Francesca Dionisio1, Danilo Pellin2, Stefania Giannelli1, Federica Andrea Salerio1, Lorena Leonardelli1, Maria Pia Cicalese1,3, Francesca Ferrua1,3, Alessandro Aiuti1,3,4, Luca Biasco1,2
1SR-TIGET, Milan, Italy; 2Dana Farber and Boston Children's Cancer and Blood Disorders Center, Boston, MA, United States; 3San Raffaele Scientific Institute, Pediatric Immunohematology and Stem Cell Programme, Milan, Italy; 4Università Vita-Salute San Raffaele, Milan, Italy
Background: The human Hematopoietic Stem/Progenitor cells (HSPC) population is composed of subtypes with different potentials and is responsible for the maintenance of the hematopoietic system. Transplantation of HSPC from an allogeneic donor or of autologous HSPC corrected by ex vivo gene transfer (gene therapy, GT), can effectively replenish a defective blood cell production due to congenital or acquired disorders. Despite their wide clinical use, there is no information on the in vivo composition and reconstitution of individual human HSPC subtypes after transplantation.
Methods: Combining phenotypic HSPC characterization and clonal tracking of lentiviral integration sites (IS) we studied the in vivo reconstitution dynamics of human HSPC subtypes including hematopoietic stem cells (HSC), multi-potent progenitors (MPP), lymphoid- (MLP, PreB/NK) and myeloid-committed (CMP, GMP and MEP) precursors in 6 Wiskott-Aldrich Syndrome (WAS) patients after ex vivo lentiviral GT . We retrieved about 8,000 IS from HSPC subpopulations sorted at early and steady-state reconstitution phases which were cross-analyzed with IS from bone marrow (BM) progenitors and peripheral blood (PB) mature cells up to 5yrs after GT.
Results: By analyzing HSPC composition during hematopoietic reconstitution we found that early hematopoietic recovery was driven by committed progenitors in WAS-GT patients followed by HSPC stabilization starting from 1–2 years post-GT. Molecular analyses showed that the transduction level was similar in the distinct HSPC subpopulations and consistent among the patients. Mathematical modeling suggests that short-living MPP were more active in the early phases and were replenished around 1 year after GT by long-living HSC, which then remained on top of the hematopoietic hierarchy at steady state. Dissecting the lymphoid/myeloid distribution of the IS detected exclusively at early (EARLY) or at late (LATE) time-points after GT, we showed that only HSC distributed in a balanced fashion in both datasets. Conversely, the EARLY IS detected in the other HSPC populations, including MPP, were found later as mostly marking long-term surviving lymphoid cells, thus suggesting an exhaustion of the original progenitor clones. Interestingly while LATE IS from MPP and myeloid progenitors were balanced, supporting the establishment of a stable HSC multipotent output, MLP and PreB/NK appeared skewed towards the lymphoid compartment, suggesting an HSC-independent survival.
Conclusions: Our findings support a distinct role of primitive HSPC. While MPP are more active in the early phases, long-living HSC are on top of the hematopoietic hierarchy at steady state. Importantly we found that long-term HSC, that were activated in vitro, were capable of homing and resilience upon re-infusion. Moreover, our data suggest that primitive lymphoid-committed progenitors are capable of long-term survival independent from HSC output. This work represents the first in vivo in human analysis of individual HSPC subpopulations dynamics and activity, generating unique information on HSPC subpopulations behavior after transplant.
Clinical Trial Registry: NCT01515462
Conflict of interest: The WAS gene therapy trial (NCT01515462) was originally sponsored by Fondazione Telethon and promoted by San Raffaele Telethon Institute for Gene Therapy (SR-Tiget). GlaxoSmithKline subsequently in-licensed the investigational product (GSK2696275) and became the financial and regulatory sponsor of the study. A. Aiuti is the PI of the clinical trial.
Immunodeficiency diseases and macrophage
O101 HLA-mismatched Donor Hematopoietic Stem Cell Transplantation in Children with Primary Immune Deficiencies: outcome survey of various approaches across the UK
Reem Elfeky1, Mohamed Najib Unni2, Ravi Shah2, Zohreh Nademi2, Waseem Qasim1, Persis Amrolia1, Robert Chiesa1, Kanchan Rao1, Giovanna Lucchini1, Juliana Furtado-Silva1, Austen Worth1, Andrew Cant2, Terence Flood2, Sophie Hambleton2, Mario Abinun2, Andrew Gennery2, Mary Slatter2, Paul Veys1
1Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom; 2Great North Children's Hospital, Newcastle upon Tyne Hospital NHS Foundation Trust, Newcastle upon Tyne, United Kingdom
Background: Mismatched related and unrelated donor (mMUD) stem cell transplantation is associated with high risk of graft loss, graft versus host disease (GvHD) and transplant related mortality (TRM). Here we present graft manipulation strategies employed over the last 10 years to reduce these risks in primary immune deficiency (PID).
Methods: Between 2007–2017, 147 PID patients received 155 haploidentical or mMUD grafts. Patients were divided into 4 groups; Group 1: TCRαβ+/CD19+depleted (n = 30), Group 2: Cord (n = 43), Group 3: CD34+ selection with T cell add-back (n = 17) and Group 4: Unmanipulated graft (n = 65).
Results: Median follow up was 22.6 months(m), 32.7m, 47.8m and 49.6m in groups 1–4 respectively. The estimated 5 year survival of the entire cohort was 79%: 80%, 84%, 71% and 74% in the four respective groups. TRM was 21.7% with the most common cause of death in the first 100 days being viral infection (60%). Among the four groups, there was no difference in graft failure rates, post-transplant autoimmunity and TRM. However, post-transplant viral reactivation rates were significantly higher in group 1 versus other groups (70% versus 30–40%; p = 0.05). There was a trend towards a greater incidence of aGvHD grade II-IV and visceral aGvHD in group 2: 56% and 42% respectively versus 11.5 % and 3.8% respectively in group 1. Chronic GvHD was not recorded in any of group 1 cases while it occurred in 38% of group 3 cases (p = 0.016). Thrombotic microangiopathy (TMA) occurred in 7 patients in group 1 at a median of 5 months post-transplant. Three patients developed TMA after a second transplant. Estimated 5 year event free survival (EFS) was 70.8%, 56%, 50% and 70.9% for the respective groups (p = 0.32). Rapid neutrophil and platelet engraftment was recorded among TCRαβ+/CD19+depleted transplants while T cell immune reconstitution was robust among cord transplants (Table 1, Figure 1). Stable full donor engraftment was significantly higher at 80% among TCRαβ+/CD19+depleted and cord transplants versus 40–60% among the other groups.
Conclusions: Cord transplantation without serotherapy and TCRαβ+/CD19+depleted grafts produced comparable survival rates of around 80% and exhibited higher donor chimerism compared to other strategies of graft manipulation. Early T cell reconstitution with cord transplant reduced the risk of viral reactivation albeit with high incidence of visceral aGvHD. Intensive TCRαβ+ depletion has significantly reduced the risk of visceral and cGvHD but viral reactivation is an ongoing problem that needs to be addressed.
Conflict of interest: All authors have nothing to disclose.
[ [O101 Table] Patients' data]
| Graft type | Group 1 | Group 2 | Group 3 | Group 4 | P value |
|---|---|---|---|---|---|
| SCID (n = 38), Non-SCID (n = 117) | 20/30, 10/30 | 20/43, 23/43 | 4/30, 13/30 | 4/65, 61/65 | P = 0.001 |
| HLA: 9/10, 8/10, ≤7/10 | 0/30, 3/30, 27/30 | 20/43, 14/43, 9/43 | 14/17, 3/17, 0/17 | 57/65, 7/65, 1/65 | P = 0 |
| Main Conditioning regimen(%) | Treosulphan/ Fludarabine/ Thiotepa (83%) | Treosulphan/ Fludarabine (63%) | Fludarabine/ Melphalan (58%) | Busulphan based myeloablative protocol (63%) | P = 0 |
| Serotherpy included(%) | 90% | 27.9% | 100% | 98.4% | P = 0 |
| Median days to Neutrophil/ Platelet engraftment | 13.5/8 | 23/29 | 18/11 | 14/14 | P = 0/0.003 |
| Median CD4 counts at 3m | 73 | 380 | 50 | 88.5 | P = 0 |
| Median CD4 counts at 6m | 494 | 725 | 455 | 276 | P = 0 |
| Median naive CD4 at 6m | 172 | 357 | 275 | 68 | P = 0.05 |
| Time to CD4 ≥ 300 cells/ul | 6 | 2.5 | 7 | 7 | P = 0.007 |
[O101 Figure].
![[O101 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/89c35471d618/41409_2018_318_Figt_HTML.jpg)
Robust CD3 counts recovery in cord transplants]
O102 Risk factors for mixed chimerism after stem cell transplantation with treosulfan or melphalan based conditioning regimens in children and adolescents with primary hemophagocytic lymphohistocytosis
Katharina Wustrau1, Michael H. Albert2, Peter Bader3, Rita Beier4, Birgit Burkhardt5, Martin Chada6, Johann Greil7, Bernd Gruhn8, Jörn-Sven Kühl9, Peter Lang10, Roland Meisel11, Ansgar Schulz12, Markus Seidel13, Carsten Speckmann14, Karl-Walter Sykora15, Angela Wawer16, Wilhelm Wössmann17, Ann-Kathrin Ozga18, Stephan Ehl14, Ingo Müller1, Kai Lehmberg1
1Pediatric Stem Cell Transplantation and Immunology, University Medical Center Hamburg, Hamburg, Germany; 2Pediatric Hematology and Oncology, Dr. von Hauner University Childrens Hospital Munich, Munich, Germany; 3Pediatric Hematology and Oncology, University Hospital Frankfurt, Frankfurt, Germany; 4Pediatric Hematology and Oncology, University Hospital Essen, Essen, Germany; 5Pediatric Hematology and Oncology, University Hospital Münster, Münster, Germany; 6Pediatric Hematology and Oncology, University Hospital Erlangen, Erlangen, Germany; 7Pediatric Hematology and Oncology, University Hospital Heidelberg, Heidelberg, Germany; 8Pediatric Hematology and Oncology, University Hospital Jena, Jena, Germany; 9Pediatric Hematology and Oncology, University Hospital Leipzig, Leipzig, Germany; 10Pediatric Hematology and Oncology, University Hospital Tübingen, Tübingen, Germany; 11Pediatric Hematology and Oncology, University Hospital Düsseldorf, Düsseldorf, Germany; 12Pediatric Hematology and Oncology, University Hospital Ulm, Ulm, Germany; 13Pediatric Hematology-Oncology, Medical University Graz, Austria, Graz, Austria; 14Center of Chronic Immunodeficiency, University Hospital Freiburg, Freiburg, Germany; 15Pediatric Hematology and Oncology, Medical University Hannover, Hannover, Germany; 16Pediatric Hematology and Oncology, University Hospital Munich, Munich, Germany; 17Pediatric Hematology and Oncology, University Hospital Gießen, Gießen, Germany; 18Biometrics and Epidemiology, University Medical Center Hamburg, Hamburg, Germany
Background: Primary hemophagocytic lymphohistiocytosis (HLH) is a genetic hyperinflammatory syndrome that can currently only be cured by stem cell transplantation (SCT). Reduced toxicity conditioning results in favorable survival rates at the expense of higher rates of mixed chimerism. Donor chimerism >25% allows for long-term disease-free survival. Factors predisposing to mixed chimerism remain to be determined.
Methods: The retrospective multicenter analysis included patients from the German and Austrian HLH registry transplanted since 2009 with a treosulfan or melphalan based regimen for hereditary disease predisposing to HLH (familial HLH 2–5, X-linked lymphoproliferative syndrome 1 and 2, Griscelli-Syndrome 2, Chediak-Higashi syndrome). Patients were analyzed for survival, engraftment, donor chimerism, and serious adverse events. Recipient chimerism was considered substantial if additional post-SCT cell therapy was administered at the discretion of the treating physician (secondary HSCT, donor lymphocyte infusion, or stem cell boost) and/or if mixed chimerism decreased to < 25% donor chimerism. A multivariate analysis of 5 potential risk factors for the appearance of substantial recipient chimerism was performed, applying a logistic regression model: (1) donor type (matched related or unrelated vs. mismatched), (2) graft source (peripheral stem cells vs. bone marrow), (3) type of alkylating agent (melphalan vs. treosulfan, both mostly with additional thiotepa), (4) serotherapy (alemtuzumab vs. ATG (Fresenius/Grafalon® or Thymoglobulin®)), and (5) remission (remission vs. non-remission). Haploidentical SCT were excluded for the risk analysis.
Results: In total, 61 consecutive patients were analyzed, with a median follow up of 721 days post SCT (range 4 days - 8.2 years). Overall survival was 77%. Primary engraftment was successful in 95% of patients. Occurrence of donor chimerism < 95% was 46%. Substantial recipient chimerism (i.e. post SCT cell therapy and/or chimerism < 25%) was recorded for 31% of patients. Secondary post-SCT cell therapy was administered in 28% of all patients (of which 75% DLI, 25% boost, 46% 2° SCT). Donor type was the only significant risk factor for the occurrence of substantial recipient chimerism (p = 0,013). A mismatched donor (8 or 9/10; n = 26) increased the risk in comparison to a related or unrelated matched donor (10/10, n = 27) by 6.3 (OR, CI95% 1.6–31). The type of alkylating agent was not a significant risk factor. Mild or moderate veno-occlusive disease occurred in 10 patients. Acute GvHD ≥3° was diagnosed in 11% of patients, and limited chronic GvHD in one patient.
Conclusions: Outcomes after treosulfan or melphalan based conditioning regimens are favorable, however the occurrence of mixed chimerism is frequent. A mismatched donor is a significant and relevant risk factor for the development of substantial mixed chimerism.
Conflict of interest: nothing to disclose
O103 Outcomes of children with primary immunodeficiencies receiving alpha/beta T-cell depleted HLA-haplo-HSCT followed by infusion of lymphocytes transduced with the inducible caspase 9 (iC9) suicide gene
Mattia Algeri1, Mary Slatter2, Waseem Qasim3, Valentina Bertaina1, Daria Pagliara1, Federica Galaverna1, Giuseppina Li Pira1, Letizia Pomponia Brescia1, Francessca Del Bufalo1, Mauro Montanari1, Mohamed Najib Mohamed Unni2, Reem Elfeky3, Alice Bertaina1, Pietro Merli1, Paul Veys3, Andrew R Gennery4, Franco Locatelli1,5
1IRCCS Ospedale Pediatrico Bambino Gesù, Pediatric Hematology and Oncology, Rome, Italy; 2Great North Children's Hospital, Department of Paediatric Oncology and BMT, Newcastle upon Tyne, United Kingdom; 3Great Ormond Street Hospital for Children NHS Foundation Trust, Bone Marrow Transplant Unit and Biomedical Research Centre, London, United Kingdom; 4Great North Children's Hospital, Department of Immunology and BMT, Newcastle upon Tyne, United Kingdom; 5University of Pavia, Pavia, United Kingdom
Background: Allogeneic HSCT is the treatment of choice for many children with primary immunodeficiencies (PIDs), gene-therapy approaches being still reserved to patients with few selected PID variants and treated in a few centers with dedicated programs. In the past, outcome of PID children undergoing haplo-HSCT appeared to be inferior to that of children receiving the allograft from an HLA-matched either related or unrelated donor. We conducted a prospective trial on alpha/beta T-cell and B-cell depleted haplo-HSCT, followed by infusion of donor lymphocytes genetically transduced with the iC9 suicide gene (BPX-501 cells) in children with PIDs treated in 3 European centers.
Methods: Between 11/2014 and 11/2017, 43 patients were recruited. The 3 most frequent diagnoses were SCID (15 patients) WAS (9 patients) and hemophagocytic lymphohistiocytosis (HLH, 5 patients, see Table for details). All children received a conditioning regimen, which varied according to the original disease. To prevent graft rejection, all patients received anti-thymocyte globulins (ATLG Neovii® 4 mg/kg/day on days -4, -3, -2). Details on the numbers of cell infused are shown in the Table.
Results: Thirty-nine patients exhibited sustained donor engraftment; children experiencing graft failure (3 with HLH and one affected by combined immunodeficiency, CID) engrafted after either a second haplo-HSCT or, in one case, following an unrelated cord blood transplant. Median time to neutrophil and platelet recovery was 16 and 10 days, respectively. Twelve patients developed grade I-III acute GvHD, the cumulative incidence of the disease being 28.8%. Among the 38 patients at risk, none developed chronic GvHD. Three patients received the agent (AP1903, Rimiducid) capable of activating iC9; two children obtained a complete response. Two patients (the child with CID who rejected the first allograft and one with WAS) died after transplantation due to pulmonary hemorrhage caused by aspergillus infection (already existing before HSCT) and undefined leukoencephalopathy, respectively. The 2-year probabilities of OS and DFS for the entire cohort of patients are 95%. Considering graft failure and death by any cause as events, the 2-year EFS is 88%. T cells progressively recovered over time; the mean number of CD3+ cells at 1, 3, 6, 12 and 24 months after HSCT was 377, 690, 1563, 3096 and 3300/µl, respectively. The mean number of BPX-501 cells at the same time-points was 11, 148, 211, 385 and 29/µl, respectively.
Conclusions: HLA-haploidentical HSCT performed by selective T-cell depletion of the graft followed by BPX-501 infusion to accelerate recovery of adaptive immunity was found to be a safe and highly effective transplantation strategy in PID. Incidence of both acute and chronic GVHD was remarkably low despite high number of HLA-disparate BPX-501 cells infused.
Clinical Trial Registry: ClinicalTrials.gov Identifier: NCT02065869
EUDRACT number: 2014-000584-41
Conflict of interest: None of the authors has anything to disclose.
Table
[ [O103 Figure] Patient and transplantation characteristics]
Infectious complications
O104 Monitoring the reconstitution of antiviral immunity after allogeneic stem cell transplantation as a tool for viral infection risk stratification and guided therapeutic decision making
Anastasia Papadopoulou1,2, Vasiliki Kalaitzidou3, Kyriakos Koukoulias1, Maria Alvanou1, Ioanna Vallianou1,2, Apostolia Papalexandri3, Zoe Bousiou3, Ioannis Batsis3, Despina Mallouri3, Ioanna Sakellari3, Achilles Anagnostopoulos1, Evangelia Yannaki1,4
1G. Papanicolaou Hospital, Gene and Cell Therapy Center, Hematology-HSCT Unit, Thessaloniki, Greece; 2Aristotle University, School of Biology, Department of Genetics, Development and Molecular Biology, Thessaloniki, Greece; 3G. Papanicolaou Hospital, Hematology-HSCT Unit, Thessaloniki, Greece; 4University of Washington, School of Medicine, Seattle, WA, United States
Background: Despite the introduction of routine post-transplant viral monitoring and preemptive antiviral therapy, viral infections remain a major cause of allogeneic hematopoietic stem cell
transplantation-(allo-HSCT) related mortality. The aim of our IRB approved study was to prospectively assess the kinetics and the magnitude of the cytomegalovirus-(CMV), Epstein Barr virus-(EBV) and BK virus-(BKV) specific T cell (ST) responses post allo-HSCT and to evaluate their role in guiding therapeutic decisions.
Methods: The virus-specific immune recovery was assessed at day +20,+30,+60,+100,+150,+200 post allo-HSCT and in case of viral reactivation/infection weekly for one month, in all patients transplanted between 10/2015–11/2016. CMV- and EBV-STs were measured in blood and BKV-STs following 10-day expansion in culture (due to their low frequency in blood) using interferon-γ (IFN-γ) Elispot. Viral loads were determined by real-time PCR. CMV/EBV reactivation/infection was defined as viremia with >500 copies/ml in two consecutive measurements or >1000 copies/ml in a single screenshot and BKV reactivation/infection as viremia or viruria with >104 and >107 copies/ml, respectively. Virus detection in tissue fluid or sample accompanied by clinical symptoms was defined as viral disease.
Results: A total of 51 patients who received allo-HSCT from matched related (MRD,17/51-33%), matched unrelated (MUD,20/51-39%), mismatched unrelated (MMUD,8/51-16%), haploidentical (PT-CY haplo, 5/51-10%) and mismatched related donor (MMRD, 1/51-2%), were included in the study. Overall, the mean reactivation/disease number/patient from all 3 viruses was 1.74 ± 0.2 (MRD:0.82 ± 0.27, MUD:1.9 ± 0.32, MMUD:2.63 ± 0.73, haplo:1.6 ± 0.24). The infection rate ranged from 20% (MRD) to 63% (MMUD) for CMV, from 35% (MRD) to 88% (MMUD) for EBV and from 6% (MRD) to 60% (haplo) for BKV. The lower infection rate in the MRD-cohort was correlated with an early reconstitution of virus-specific immunity (MRD day+20; mean spot forming cells-SFC/5x105 cells: CMV:783 ± 125, EBV:90 ± 32, BKV:303 ± 148 vs MUD+MMUD+Ηaplo: CMV:84 ± 47, EBV:5 ± 3, BKV:25 ± 5; p≤0.004). In line with this, the mean number of virus-STs at baseline (d+20) in patients with low (< 500 copies/ml in two consecutive measurements) or without viremia was 2–5fold higher than in patients with viral reactivation/disease (mean SFC/5x105 cells CMV-STs:353 ± 68 vs 115 ± 75; EBV-STs:42 ± 17 vs 8 ± 4, BKV-STs:190 ± 24 vs 95 ± 19, respectively; p≤0.05). Likewise, among infected patients, the complete-responders had higher numbers of virus-STs at the onset of reactivation compared to partial- or no-responders (mean SFC/5x105 cells: CMV-STs:250 ± 74 vs 27 ± 19, EBV-STs:55 ± 28 vs 12 ± 6, BKV-STs:1063 ± 223 vs 296 ± 106, respectively), showed a more robust expansion of virus-STs and effectively controlled the viral reactivation/disease (mean copies/ml week4 post reactivation: CMV:762 ± 339 vs 16.338 ± 8.740, EBV:17.366 ± 5.688 vs 96.377 ± 79.477, BKV:0.6 ± 0.3x106 vs 1.084 ± 609x106, respectively; p≤0.05). Three patients with EBV reactivation (max mean copies/ml: 38.833 ± 23.590), who showed expansion of their EBV-STs (mean SFC/5x105 cells: 2.67 ± 4.62®674 ± 493), did not receive antiviral pharmacotherapy and remained under close monitoring. Notably, viremia was effectively controlled by the expanded EBV-STs without disease development.
Conclusions: Immune monitoring of virus-specific T-cell reconstitution in conjunction with virologic monitoring, may serve as an important tool of identifying patients at risk of developing viral infections or/and appropriate candidates for adoptive transfer of virus-STs. This strategy may enable patient-tailored treatment and guide preemptive therapeutic choices based on the actual infectious risk, thus minimizing overtreatment and its associated toxicities.
Clinical Trial Registry: N/A
Conflict of interest: No conflict of interest to declare
O105 Genotypic, Phenotypic and Functional Characterization of KIR-Regulated Natural Killer Cells Responding To Epstein-Barr Virus: Protection against Post-Transplant Lymphoproliferative Disease (PTLD) after Allogeneic HCT
Rehan Faridi1, Brittin Adams1, Poonam Dharmani-Khan1, Victor Lewis2, Noureddine Berka1, Jan Storek3, Faisal Khan1
1University of Calgary, Pathology and Laboratory Medicine, Calgary, Canada; 2University of Calgary, Alberta Children's Hospital, Calgary, Canada; 3University of Calgary, Medicine, Calgary, Canada
Background: A compromised immune system early after allogeneic Hematopoietic Cell Transplantation (HCT) results in vulnerability of transplant recipients to a heightened risk of reactivation of otherwise latent viral infections. One of such major posttransplant viral complication is an uncontrolled reactivation of Epstein-Barr virus (EBV) leading to post-transplant lymphoproliferative disease (PTLD) particularly after T-cell depleted HCT. Recovering within weeks after HCT and being first in the line of defense against viral infections, natural killer (NK) cells are deemed important in the immune surveillance against the reactivation and complications of EBV. The complexity of NK cell response is a function of a series of activating and inhibitory cell surface receptors known as Killer Immunoglobulin-like Receptors (KIR), which sense perturbations in HLA expression after viral transformation of the target cell. Here, we determined how KIR gene repertoire and associated NK cell transcripts and phenotypes influences NK cell against EBV targets and confers protection against PTLD.
Methods: Next generation sequencing based KIR profiling was performed to obtain KIR gene repertoires of 356 HLA-matched donor-recipient pairs of first allo-HCT and 45 healthy individuals. NK cells negatively selected from PBMNCs of healthy individuals were stimulated with EBV-transformed target cells. Flow cytometry based enumeration of NK cells that were either degranulating (CD107a expression) or producing cytokine (IFN-γ), as well as their simultaneous KIR-based phenotypic characterization was performed. Total RNA was extracted before and after co-stimulation of NK cells with EBV targets, on which the transcript analysis was performed using a custom NanoString-based codeset containing probes for 53 NK cell function related genes.
Results: Donor KIR Telomeric A01 motifs (Tel-A01, KIR3DL1+veKIR2DS4+ve), strongly protected against PTLD (p = 0.0002, SHR = 0.21) after allogeneic HCT. The protection of donor Tel-A01 motifs against PTLD was independent of Tel-A01 copy number.
The numbers of EBV induced functional NK cell subsets were significantly higher in healthy individuals carrying Tel-A01 motifs in comparison to individuals not carrying Tel-A01 motifs. Twenty four distinct NK cell phenotypes were identified based on the expression of various combinations of KIR proteins and other NK cell receptors that differentially correlated with response to EBV targets. NK cells expressing KIR3DL1 and KIR2DS4 (Tel-A01 motif genes) were most functional against EBV target cells.
Significant differences in expression of NK cell signaling proteins (DAP10, FYN, PKB), effectors (GM-CSF, IFN-γ, TNF) and receptors in response to EBV were observed.
Conclusions: NK cell responsiveness, a function of KIR gene repertoire has a profound effect on the development of PTLD. Presence of KIR-TelA01 motif confers strong protection against PTLD due to its influence on NK cell responses against EBV. Our findings provide vital mechanistic clues to NK cells' response to EBV. KIR gene profiling of HCT donors and phenotypic and functional reconstitution kinetics of identified subsets and transcripts after HCT can identify patients at a high risk for developing PTLD and help enable a pre-emptive intervention.
Conflict of interest: None of the authors has anything to disclose.
O106 A Phase 2b, Randomized, Double-Blind, Placebo-Controlled Trial of Presatovir (GS-5806) for Treatment of Hematopoietic-Cell Transplantation Patients with an Respiratory Syncytial Virus Upper Respiratory Tract Infection
Michael Boeckh1,2, Sanjeet Dadwal3, Anne Bergeron4, Per Ljungman5, Yae-Jean Kim6, Guang-Shing Cheng1, Sudhakar Pipavath2, Ajit Limaye2, Elodie Blanchard7, Drew Winston8, Patrick Stiff9, Francisco Marty10, Tsila Rosenvald-Zuckerman11, Silvy LaChance12, Galia Rahav13, Catherine Small14, Kathleen Mullane15, Roberto Patron16, Dong-Gun Lee17, Hans Hirsch18, Alpana Waghmare1, Matt McKevitt19, Robert Jordan19, Ying Guo19, Polina German19, Danielle Porter19, David Gossage19, Timothy Watkins19, Jason Chien19, Roy Chemaly20
1Fred Hutchinson Cancer Research Center, Seattle, WA, United States; 2University of Washington School of Medicine, Seattle, WA, United States; 3City of Hope National Medical Center, Duarte, CA, United States; 4Hospital Saint Louis, Paris, France; 5Karolinska Universitetssjukhuset, Stockholm, Sweden; 6Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of; 7CHU de Bordeaux, Bordeaux, France; 8UCLA Ronald Reagan Medical Center, Los Angeles, CA, United States; 9Loyola University Medical Center, Chicago, IL, United States; 10Dana-Farber Cancer Institute, Boston, MA, United States; 11Rambam Medical Center Health Care Campus, Haifa, Israel; 12Hopital Maisonneuve-Rosemont, Montreal, Canada; 13Chaim Sheba Medical Center, Ramat Gan, Israel; 14Weil Cornell Medical Center, New York, NY, United States; 15University of Chicago Medical Center, Chicago, IL, United States; 16Mayo Clinic, Phoenix, AZ, United States; 17College of Medicine, The Catholic University of Korea, Seoul, Korea, Republic of; 18University Hospital Basel, Basel, Switzerland; 19Gilead Sciences Inc., Foster City, CA, United States; 20MD Anderson Cancer Center, Houston, TX, United States
Background: Presatovir significantly reduced nasal viral load and signs and symptoms of RSV infection in a human challenge study. We evaluated whether presatovir is safe and effective in the treatment of HCT patients with RSV URTI.
Methods: HCT patients with new respiratory symptoms for ≤7 days, confirmed RSV URTI within 6 days, and no new chest X-ray abnormalities within 48 hours were randomized (1:1) to receive oral presatovir 200mg every 4 days for 5 doses or placebo, in addition to each center's standard of care. Enrollment was stratified by presence of lymphopenia (≤200 cells/µL) and treatment with ribavirin. The co-primary endpoints were time weighted average change in RSV viral load through Day 9 (DAVG9) measured by nasal sampling (a = 0.01) and development of infectious or noninfectious lower respiratory tract complication ([LRTC], a = 0.05 if p≤0.01 for RSV viral load or a = 0.04 if p>0.01 for viral load) within 28 days, as determined by a blinded endpoint adjudication committee. The secondary endpoint was the proportion of subjects who developed respiratory failure requiring mechanical ventilation or died.
Results: From January 2015 to May 2017, 185 subjects from 42 centers were randomized and dosed (Table). The median age was 57 (interquartile range [IQR] 40,63) and 55 (IQR 50,63) for presatovir and placebo subjects respectively. Most subjects were males (presatovir 21 [70%], placebo 23 [79%]). The median duration of symptoms prior to first dose was similar (presatovir 6 days [IQR 4,8], placebo 5 days [IQR 3,7]), as was mean baseline RSV viral load (presatovir 6.3 log10 copies/mL [standard deviation (SD) 1.9], placebo 6.5 log10 copies/mL [SD 1.4]). Ribavirin treatment was given to 22 (23%, 7 inhaled, 16 oral) presatovir and 22 (24%, 3 inhaled, 20 oral) placebo treated subjects. Mean plasma concentrations after the last dose were maintained above 4-fold paEC95 for at least 5 days. Presatovir treatment reduced the DAVG9 (presatovir -1.10 log10 copies/mL, placebo -0.78 log10 copies/mL; mean treatment difference -0.33 log10 copies/mL, 95% confidence interval [CI] -0.64, -0.02, p = 0.04) and was associated with numerically fewer LRTC cases (presatovir 10 [11%], placebo 17 [20%], p = 0.11). Stratified analysis indicated that presatovir treatment of lymphopenic subjects resulted in an 80% reduction in LRTC events (presatovir 2/15 [13%], placebo 9/14 [64%], nominal p = 0.008). In multivariate time-to-event analysis (adjusted for stratification variables), presatovir treatment was associated with a 49% reduction in the risk of developing LRTC (hazard ratio 0.51, 95% CI 0.23, 1.13, nominal p = 0.09, Figure). The proportion of subjects developing respiratory failure or death due to any cause was similar between presatovir and placebo (presatovir 5 [6%], placebo 5 [6%], p = 0.98). Treatment-emergent adverse events ([TEAEs] presatovir 80%, placebo 87%) and ≥Grade 3 TEAEs (presatovir 23%, placebo 23%) were similar in both arms.
Conclusions: Presatovir treatment was well tolerated in HCT patients with RSV URTI. While the prespecified a thresholds were not met for all endpoints, these results suggest that presatovir treatment of URTI in HCT patients may reduce nasal viral load and risk of developing a LRTC, particularly among lymphopenic patients.
[O106 Figure].
URTI HCT Study and LRTC Cumulative Incidence Curve]
Clinical Trial Registry: NCT02254408
https://clinicaltrials.gov/ct2/show/NCT02254408
Conflict of interest:
M. Boeckh: Consultant for Gilead, Ablynx, Humabs, VirBio; research funding from Gilead and VirBio
S. Dadwal, A. Bergeron, P. Ljungman, Y. Kim, G. Cheng, S. Pipavath, A. Limaye, E. Blanchard, D. Winston, P. Stiff, F. Marty, T. Zuckerman, S. LaChance, G. Rahav, C. Small, K. Mullane, R. Patron, D. Lee, H. Hirsch: Research funding from Gilead
A. Waghmare: Research funding from Gilead and Aviragen Therapeutics
M. McKevitt, R. Jordan, Y. Guo, P. German, D. Porter, D. Gossage, T. Watkins, J. Chien: Employees of Gilead
R. Chemaly: Consultant and research funding from Gilead, Ansun, Ablynx, Janssen, ADMA Biologics
O107 Clinical validation of a novel ELISpot-based in vitro diagnostic assay to monitor CMV-specific cell-mediated immunity in hematopoietic stem cell transplant recipients
Eva Wagner1, Daniel Teschner1, Christine Wolschke2, Dietlinde Jason2, Kerstin Schaefer-Eckart3, Johannes Gaertner3, Stephan Mielke4, Martin Schreder4, Guido Kobbe5, Mustafa Kondakci5, Stefan Klein6, Daniela Heidenreich6, Sebastian Kreil6, Inken Hilgendorf7, Marie von Lilienfeld-Toal7, Mareike Verbeek8, Sandra Grass8, Markus Ditschkowski9, Tanja Gromke9, Martina Koch10, Thomas Huenig11, Monika Lindemann12, Traudel Schmidt13, Anne Rascle13, Sascha Barabas13, Ludwig Deml13, Ralf Wagner13,14, Daniel Wolff15
1University Medical Center Mainz, Hematology, Oncology and Pneumology, Mainz, Germany; 2Clinic for Stem Cell Transplantation, UKE University Medical Center Hamburg-Eppendorf, Hamburg, Germany; 3Klinikum Nord, Oncology, Hematology and Bone Marrow Transplantation Unit, Nuernberg, Germany; 4University Medical Center Wuerzburg, Hematology and Oncology, Wuerzburg, Germany; 5University Medical Center Duesseldorf, Hematology, Oncology and Clinical Immunology, Duesseldorf, Germany; 6UMM University Medical Center Mannheim, University of Heidelberg, Hematology and Oncology, Mannheim, Germany; 7University Hospital Jena, Hematology and Oncology, Jena, Germany; 8Clinic rechts der Isar, Technical University Munich, Hematology and Oncology, Munich, Germany; 9Clinic for Bone Marrow Transplantation, University Hospital Essen, Essen, Germany; 10University Medical Center Hamburg-Eppendorf, Hepatobiliary Surgery and Transplantation, Hamburg, Germany; 11University Medical Center Wuerzburg, Institute of Virology and Immunobiology, Wuerzburg, Germany; 12University Hospital Essen, Institute for Transfusion Medicine, Essen, Germany; 13Lophius Biosciences, Regensburg, Germany; 14University Medical Center Regensburg, Institute of Clinical Microbiology and Hygiene, Regensburg, Germany; 15University Medical Center Regensburg, Hematology and Oncology, Internal Medicine III, Regensburg, Germany
Background: Impaired cytomegalovirus (CMV)-specific cell-mediated immunity (CMV-CMI) is a major cause of uncontrolled CMV reactivations and associated complications in hematopoietic stem cell transplantation (HSCT). Reliably assessing CMV-CMI would improve the risk stratification of patients and allow optimizing and individualizing patient care following HSCT. This study aims to evaluate the suitability of a novel IFN-γ ELISpot assay (T-Track® CMV), based on the stimulation of peripheral blood mononuclear cells with pp65 and IE-1 CMV proteins, to predict protection from recurrent CMV reactivation following the resolution of a treatment-requiring primary CMV reactivation.
Methods: A prospective, longitudinal, observational, multicenter studies is being conducted in 175 intermediate- and high-risk (D+/R+, D+/R-, D-/R+) HSCT recipients (last patient out expected in April 2018). Patients underwent pre-emptive antiviral therapy per institutional guidelines. CMV-CMI was measured at day 45, 60, 80, 100 and 120 post-transplantation, as well as at onset and following the end of treatment of the primary CMV reactivation. Occurrence of recurrent CMV reactivation was monitored up to 7.5 months post-transplantation. CMV viral load, clinical complications (GvHD) and subsequent treatments were also documented.
Results: Interim data analysis showed that out of 45 patients representative of the total population (D+/R+, D+/R-, D-/R+) and who experienced a (treatment-requiring) primary CMV reactivation, 12 (27%) faced a recurrent CMV reactivation during the observational period. Interestingly, 30/33 patients free of recurrent reactivation had a positive pp65-specific ELISpot test result after resolution of the primary CMV reactivation, resulting in a 91% specificity in diagnostic accuracy. Accordingly, interim ROC analyses indicated that pp65-specific response measured following a primary CMV reactivation is a fair predictor of occurrence of recurrent CMV reactivation.
Conclusions: Altogether, this novel standardized IFN-γ ELISpot assay (T-Track® CMV) allows an improved risk stratification of CMV-related clinical complications, and can support clinicians in the identification of patients with increased risk of recurrent CMV reactivation following HSCT.
Clinical Trial Registry: ClinicalTrials.gov identifier: NCT02156479 (https://clinicaltrials.gov/ct2/show/NCT02156479)
Conflict of interest: The participating clinical and measurement centers received research funding from Lophius Biosciences for this study.
L. Deml, S. Barabas, T. Schmidt and A. Rascle are employees of Lophius Biosciences. L. Deml is co-founder and chief scientific officer of Lophius Biosciences.
R. Wagner is Chairman of the Board of Lophius Biosciences. R. Wagner, L. Deml and S. Barabas are shareholders of Lophius Biosciences GmbH.
O108 Fecal microbiota transplantation in immunocompromised patients carrying multidrug-resistance bacteria
Giorgia Battipaglia1,2, Florent Malard1,3,4, Marie Therese Rubio1, Annalisa Ruggeri1, Anne Claire Mamez1, Eolia Brissot1,3,4, Federica Giannotti1, Remy Dulery1, Anne Christine Joly5, Mihn-Tam Baylatry5, Marie-Jeanne Kossmann6, Jacques Tankovic4,7, Lauretìnt Beaugerie4,8, Harry Sokol4,8,9, Mohamad Mohty1,3,4
1Hôpital Saint Antoine, Service d'Hématologie et Thérapie Cellulaire, Hematology Department, Paris, France; 2Federico II University, Hematology, Naples, Italy; 3INSERM UMR 938, Paris, France; 4Université Pierre et Marie Curie, Paris, France; 5Hopital Saint Antoine, Microbiote Transplant Préparations Unit, Pharmacy Department, Paris, France; 6Saint Antoine Hospital, Unité d'Hygiène et de lutte contre les Infections Nosocomiales, Paris, France; 7Saint Antoine Hospital, Department of Bacteriology-Virology, Paris, France; 8Saint Antoine Hospital, Department of Gastroenterology, Paris, France; 9INSERM ERL 1157, UMR CNRS 7203, Paris, France
Background: Fecal microbiota transplantation (FMT) is an effective treatment in recurrent Clostridium difficile infection. Promising results suggest that FMT might be effective to decolonize patients with multidrug-resistant (MDR) bacteria carriage. Safety concerns exist in immunocompromised patients.
Methods: We report the results of FMT performed for MDR-bacteria decolonization before or after allogeneic hematopoietic stem cell transplantation (HSCT) in adults with hematologic malignancies.
Stools were obtained from healthy related or unrelated donors. Fecal material was delivered either by enema or via nasogastric tube. A bowel preparation was performed the day before the FMT by administration of 4 liters of polyethylene glycol (PEG) based solution. Large spectrum antibiotics were discontinued 48–72 hours prior to FMT.
Results: Between 2014 and 2017, 10 patients underwent FMT for gut colonization with carbapenem-resistant bacteria (n = 8) or vancomycin-resistant enterococci (VRE, n = 2). Six patients were contemporarily colonized by extended-spectrum beta-lactamase (ESBL+) enterobacteriaceae. Median age at FMT was 48 (range 16–64) years. Four patients underwent FMT as a decolonization strategy before HSCT. Median interval from FMT to HSCT was 28 (range 9–46) days. Six patients underwent FMT after HSCT. Median time from HSCT to FMT was 163 (range 98–344) days. All patients undergoing FMT after HSCT were still under immunosuppressive therapy, with one of them presenting active grade IV gut graft-versus-host disease (GVHD) when undergoing FMT. A frozen FMT product was used in eight out of ten patients. Enema administration was used in all but one patient. All but one patient had a neutrophil count >1x109/L at time of FMT. Two patients experienced systemic infections due to MDR-bacteria before FMT. Three patients needed a second FMT from the same donor, due to initial failure of the procedure. Reasons for failure were mainly technical, with one patient probably receiving an insufficient quantity of stools from an unrelated donor (< 50 g) and another who kept the FMT material for less than 2 hours. In the third patient a “compassionate” FMT for multiple infections and grade IV GVHD was performed but failed twice.
With a median follow-up of 14 (range 1–46) months, decolonization from ERV or carbapenem-resistant bacteria was achieved after one or two FMT in seven out of ten patients. In patients colonized with ESBL+ Enterobacteriaceae, decolonization from ESBL was achieved in 4 out of 6. In one patient, ERV was undetectable until 3 years after FMT, then colonization was again detectable contemporarily to disease relapse. In all patients, FMT was safe: one patient presented constipation during the first 5 days after FMT and 2 patients had grade I diarrhea. Except for the patient who underwent FMT while having an active infection and grade IV GVHD, no infectious complications related to MDR-bacteria occurred after FMT and no patient experienced gut GVHD after HSCT. At last follow-up, three out of ten patients died, two due to progression of hematological disease and one due to persistent graft failure, GVHD and infections.
Conclusions: In patients carrying or infected by MDR-bacteria, FMT is an effective and safe decolonization strategy, even in the context of hematological malignancies and profound immunosuppression.
Conflict of interest: nothing to disclose
O109 Combination of HCMV-gB-CAR T cells and induced dendritic cells expressing gB generated from seronegative donors to treat HCMV reactivation in post-transplant patients
Henning Olbrich1, Sebastian Theobald1, Maksim Mamonkin2, Renata Stripecke1,3
1Hannover Medical School, Hematology Oncology Stem Cell Transplantation, Hannover, Germany; 2Baylor College of Medicine, Cell and Gene Therapy Center, Houston, TX, United States; 3Hannover Medical School, German Center for Infections Research (DZIF), Hannover, Germany
Background: Human cytomegalovirus (HCMV) can be controlled by immune competent heathy subjects but maintains latent through several mechanisms of immune suppression and escape. Reactivated infection with HCMV is associated with poor outcome in immune compromised hosts after stem cell transplantation (SCT). Adoptive transfer of virus-specific T cells in a SCT-setting has proven efficacious, but the approach is problematic when seropositive donors are not available, such as in the case of cord blood (CB)-SCT. Hence, we designed T cells expressing HCMV-specific chimeric antigen receptors (CARs) from HCMV seronegative donors to control viral lytic reactivation. The HCMV-glycoprotein B (gB) was used as target, because it is a highly conserved surface-bound protein abundantly expressed on cells hosting lytic replication. We further tested if a professional antigen presenting cell expressing gB would provide optimal homeostatic and antigenic stimuli to maintain gB-CAR T cells active.
Methods: gB-CARs were constructed by fusion of single-chain variable fragments of a highly affine gB-specific human mAB (SM5-1) to CAR-backbones comprising CD28/CD3ζ and 4-1BB/CD3ζ domains. Transduction of human T cells from PBMC and CB with γ-retroviral vectors yielded 60–95% CAR-expression. Co-transduction of human monocytes with lentiviral vectors encoding GM-CSF/IFNα and gB directed their self-differentiation into induced dendritic cells with high levels of gB-surface expression (iDCgB) and secreted GM-CSF/IFNα.
Results: For in vitro potency assays gB-CAR T cells we used mesenchymal stem cells (MSCs) permissive to HCMV-infection. MSCs were infected at multiplicity of infection (MOI) 10−2 with the HCMV-strain TB40 expressing a secretable Gaussia luciferase (HCMV-gLuc). Both CD28/CD3ζ and 4-1BB/CD3ζ-containing gB-CAR T cells effectively killed HCMV-infected MSCs, assessed by lower detection of gLuc-luminescence and death of target cells by flow cytometry. gB-CAR T cells co-cultured with HCMV-infected MSCs proliferated and secreted IFN-γ. Neither CD19-CAR T cells nor uninfected MSCs reproduced these effects. A serial killing assay demonstrated persistent killing with less pronounced exhaustion (measured by PD-1 expression) for the 4-1BB/CD3ζ-containing gB-CAR T in comparison with CD28/CD3ζ. Co-culture of gB-CAR T cells with iDCgB boosted their expansion (6-fold increase in cell counts) and stimulated the CAR expression levels (2-fold increase in MFI). A Nod.Rag.Gamma (NRG) mouse model humanized with hematopoietic stem cells injected i.p. with MRC-5 cells carrying HCMV-gLuc and treated with G-CSF for viral reactivation was established. Bio-distribution of HCMV infection could be followed by optical imaging analyses. Pilot results indicated that the treatment of gB-CAR T cells in combination with iDCgB in vivo reduced levels of HCMV infection.
Conclusions: gB-CAR T cells are activated by HCMV-infected cells in vitro and generate cytotoxic effects. Co-culture of gB-CAR T cells with iDCgB improves their performance. Additional experiments to evaluate the effects of gB-CAR T with or without iDCgB to control HCMV infection in humanized mice are currently being completed.
Conflict of interest: No conflict of interest to declare.
O110 Lethal infectious complications after hematopoietic stem cell transplantation: progress and challenges in Infectious Diseases Working Party study
Jan Styczynski1, Gloria Tridello2, Linda Koster3, Simona Iacobelli4, Anja van Biezen3, Steffie van der Werf3, Malgorzata Mikulska5, Lidia Gil6, Catherine Cordonnier7, Per Ljungman8, Diana Averbuch9, Simone Cesaro2, Rafael de la Camara10, Helen Baldomero11, Peter Bader12, Grzegorz Basak13, Chiara Bonini14, Rafael Duarte15, Carlo Dufour16, Jurgen Kuball17, Arjan Lankester18, Silvia Montoto19, John Snowden20, Arnon Nagler21, Nicolaus Kröger22, Mohamad Mohty23, Alois Gratwohl11
1Collegium Medicum UMK, Bydgoszcz, Poland; 2Policlinico G.B. Rossi, Verona, Italy; 3EBMT Data Office, Leiden, Netherlands; 4Università di Roma 'Tor Vergata', Roma, Italy; 5University of Genoa (DISSAL) and Ospedale Policlinico San Martino, Genoa, Italy; 6Medical University, Poznan, Poland; 7Hôpital Henri Mondor, Creteil, France; 8Karolinska University Hospital, Stockholm, Sweden; 9Hadassah University Hospital, Jerusalem, Israel; 10Hospital de la Princesa, Madrid, Spain, Madrid, Spain; 11EBMT Activity Survey Office, Basel, Switzerland; 12Universitätsklinikum Frankfurt, Goethe-Universität, Frankfurt, Germany; 13Medical University, Warszawa, Poland; 14Università Vita-Salute San Raffaele, Milan, Italy; 15Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, Spain; 16G. Gaslini Children's Institute, Genova, Italy; 17University Medical Centre, Utrecht, Netherlands; 18Leiden University Hospital, Leiden, Netherlands; 19St. Bartholomew`s and The Royal London NHS Trust, London, United Kingdom; 20Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom; 21Chaim Sheba Medical Center, Tel Hashomer, Israel; 22University Hospital Eppendorf, Hamburg, Germany; 23Hospital Saint Antoine, Paris, France
Background: Detailed data on incidence and factors associated with lethal complications is warranted to further improve transplant outcome.
Objective: Analysis of incidence and specific causes of deaths after HSCT, with focus on infectious deaths in two time periods, 1980–2002 (cohort1) and 2003–2015 (cohort2).
Methods: All patients with HSCT for ALL, AML, or CML, registered in the EBMT database were included (n = 114,491; 84% allogeneic). The main endpoint was cumulative incidence (CuIn) of overall mortality, at specified time points (early, +30d; intermediate, +100d; late, +1y, and very late, +5y) in a landmark analysis, divided into deaths from relapse, GVHD, infections and "other". Infectious deaths were analysed as bacterial, fungal, viral, parasitic, mixed and unknown infections.
Results: Overall mortality for all patients decreased from cohort1 to cohort2 at +30d (CuIn 5.35;5.14–5.57 vs 3.95;3.81–4.09), at +100d (13.67;13.34–14.01 vs 9.61;9.39–9.83), was not significantly different at +1y (26.06;25.59–26.52 vs 24.93;24.56–25.30), and increased at +5y (CuIn 23.27;22.73–23.81 vs 24.34;23.85–24.83.
There were major differences between autologous and allogeneic HSCT. In auto-HSCT, mortality decreased at each time point (+5y: 31.09;29.99–32.20 vs 24.57;23.07–26.10), for each cause of death, including relapse (+5y: 23.51;22.51–24.52 vs 18.04;16.72–19.40), and infections (+5y: 2.90;2.52–3.31 vs 1.59;1.20–2.07) except for "other" in very late phase, and for all types of infectious deaths, except for "mixed" at +5y. In allo-HSCT mortality from GvHD (+1y: 5.21;4.94–5.50 vs 4.28;4.10–4.46), infections (+1y: 6.45;6.14–6.76 vs 4.34;4.17–4.53) and other causes decreased in early, intermediate and late phases, but increased in very late phase for GvHD (+5y 2.95;2.71–3.22 vs 3.85 (3.62–4.08) and other causes. Mortality from relapse increased in all post-transplant phases (+5y: 10.71;10.25–11.18 vs 13.26;12.85–13.67). As a result, overall mortality decreased in the early (+d30: 6.13;5.87–6.40 vs 4.14;3.98–4.29) and intermediate phase, increased in the very late phase (+5y: 20.14;19.54–20.74 vs 24.31;23.79–24.83).
The majority of lethal infections were of mixed or unknown (64.59%) etiology. Their contribution to deaths increased in early phase (from 1.35% to 1.54%). Mortality from bacterial, viral, fungal and parasitic infections decreased in early, intermediate, and late phases. In the very late phase mortality from bacterial and fungal infections decreased, while mortality from viral or unknown infectious etiology did not change. The pattern was similar for allo- and auto-HSCT, with a distinct and constant lower CuIn for all types of infections at all phases after HSCT for auto-HSCT, reflecting the impact of "allogenicity" on infectious complications. This difference was abolished when allo-HSCT patients with no-GvHD-ever were compared with patients after auto-HSCT (CuIn infectious deaths +5y: 2.16;1.92–2.43 vs 1.59;1.20–2.07).
Conclusions:
Post-transplant mortality has significantly decreased in all phases, from all causes after auto-HSCT; it has decreased in early phases after allo-HSCT, not in the very late phase.
The key specific causes of death differ depending on the time phase after HSCT: infections are the main cause before day +100, with relapse afterwards.
Mortality from infectious deaths significantly improved; the high proportion of "mixed" and "unknown" at any time point remain of concern.
Long-term survival outcomes following allo-HSCT may be improved by focussing research into preventable and/or reversible late and very late complications.
Conflict of interest: None of the authors has anything to disclose.
O111 Immunogenicity and Safety of an Adjuvanted Herpes Zoster Subunit Vaccine in Adult Autologous Hematopoietic Stem Cell Transplant Recipients: Phase 3, Randomized, Placebo-Controlled, ZOE-HSCT Clinical Trial
Keith Sullivan1, Sunil Abhyankar2, Laura Campora3, Claudia Cellini4, Pranatharthi Chandrasekar5, Javier de la Serna6, Mohamed El Idrissi3, Gianluca Gaidano7, Nicolas Issa8, Je-Jung Lee9, Marta López Fauqued3, Javier Lopez Jimenez10, Lidia Oostvogels3, David Pohlreich11, Philippe Quittet12, Stefan Schwartz13, Edward Stadtmauer14, Jeff Szer15, Adriana Bastidas3
1Duke University Medical Center, Durham, NC, United States; 2University of Kansas Cancer Center, Westwood, KS, United States; 3GSK, Wavre, Belgium; 4Hospital Santa Maria delle Croci, Ravenna, Italy; 5Wayne State University, Detroit, MI, United States; 6Hospital Universitario 12 de Octubre, Madrid, Spain; 7University of Eastern Piedmont, Novara, Italy; 8Brigham and Women's Hospital and Dana-Farber Cancer Institute, Boston, MA, United States; 9Chonnam National University Hwasun Hospital, Jellanamdo, Korea, Republic of; 10Ramón Y Cajal Hospital, Madrid, Spain; 11Charles University Hospital, Prague, Czech Republic; 12University Hospital of Montpellier, Montpellier, France; 13Charité University Medical Center, Berlin, Germany; 14University of Pennsylvania, Philadelphia, PA, United States; 15Royal Melbourne Hospital, Melbourne, Australia
Background: Immunocompromised individuals are at increased risk of herpes zoster (HZ). In autologous hematopoietic stem cell transplant (HSCT) recipients the risk is highest in the first year after transplantation, with 8–30% HZ incidence.1,2 The adjuvanted HZ subunit vaccine (HZ/su) demonstrated high vaccine efficacy (VE) against HZ in autologous HSCT recipients ≥18 years of age (YOA) (overall: 68%, 18–49 YOA: 72%, ≥50 YOA: 67%). HZ/su showed 89% overall VE against postherpetic neuralgia and 78% overall VE against other HZ-related complications.3 Here, we report the immunogenicity and safety of HZ/su given early after autologous HSCT.
Methods: In this phase 3, observer-blind, multicenter study (NCT01610414), adults ≥18 YOA were randomized 1:1 to receive 2 intramuscular doses of HZ/su or placebo 1–2 months apart, starting 50–70 days after autologous HSCT. HZ/su contains recombinant varicella-zoster virus glycoprotein E (gE) and the AS01B Adjuvant System. A minimization procedure for age (18–49, ≥50 years) and underlying disease (multiple myeloma, other diseases) was applied. Humoral immune responses including vaccine response rates (VRR) (secondary descriptive objective) were assessed in a study-population subset (according-to-protocol [ATP] cohort for humoral immunogenicity) based on anti-gE concentrations. Cell-mediated immunogenicity (CMI) including VRR were assessed in the ATP cohort for CMI (tertiary descriptive objective), based on the frequency of gE-specific CD4+ T-cells expressing ≥2 activation markers (among interferon-γ, interleukin-2, tumor necrosis factor-α and CD40 ligand). Immune responses were assessed at baseline, 1 month post each dose and 1 and 2 years post-dose 2. Solicited and unsolicited adverse events (AEs) were collected for 7 and 30 days after each dose, respectively. Serious AEs (SAEs) and potential immune-mediated diseases (pIMDs) were recorded up to 1 year post-dose 2. Any fatal SAE, SAEs considered vaccination-related by investigators and underlying disease relapses were recorded until study end. Safety was assessed in the total vaccinated cohort (TVC).
Results: Of the 1846 participants in the TVC (HZ/su: 922, placebo: 924; mean age 54.8, 55.1 years, respectively) 158 (HZ/su: 82, placebo: 76) were included in the ATP cohort for humoral immunogenicity and 114 (HZ/su: 59, placebo: 55) in the ATP cohort for CMI. Humoral and cell-mediated immune responses were higher in the HZ/su group at all analyzed post-vaccination time-points (Table). Solicited AEs were more frequent in the HZ/su group (local: HZ/su: 85.8% [95% confidence interval [CI]: 83.3–88.0], placebo: 10.4% [95%CI: 8.5–12.6]; general: HZ/su: 75.2% [95%CI: 72.3–78.0]; placebo: 50.9% [95%CI: 47.6–54.2]). Nevertheless, dose 2 compliance was comparable between groups (HZ/su: 94.7%, placebo: 93.3%). The most common solicited AEs were injection-site pain (HZ/su: 83.9% [95%CI: 81.3–86.2], placebo: 9.3% [95%CI: 7.5–11.4]) and fatigue (HZ/su: 56.4% [95%CI: 53.1–59.6], placebo: 38.0% [95%CI: 34.8–41.3]). Overall, the incidences of unsolicited AEs, SAEs (including fatal), pIMDs and underlying disease relapses were similar between groups (Table). The median follow-up period in the TVC was approximately 29 months.
Conclusions: HZ/su induced robust immune responses and had a clinically acceptable safety profile when administered to adults ≥18 YOA early after autologous HSCT.
References:
1. Rogers et al, Transpl Infect Dis 2011;13:480–4
2. Berman et al, Bone Marrow Transplant 2006;37:73–80
3. Submitted abstract, BMT Tandem Meetings, US, February 2018.
Clinical Trial Registry: ClinicalTrials.gov: NCT01610414
Conflict of interest: Funding: GlaxoSmithKline Biologicals SA
L. Campora, M. El Idrissi, M. López Fauqued, L. Oostvogels and A. Bastidas are employees of the GSK group of companies (GSK).
M. El Idrissi and L. Oostvogels own shares in the GSK.
J. de la Serna reports advisory boards and honoraria [Roche, AbbVie, Janssen, Gilead, Jazzpharma and Takeda] outside the submitted work
G. Gaidano reports advisory boards, consultancy and honoraria [Janssen, Gilead, AbbVie, Roche and Morphosys] outside the submitted work.
N. Issa reports grants [GSK] during study conduct, grants [Astellas] and personal fees [Akros Pharma] outside the submitted work.
S. Schwartz reports reimbursement for treatment and monitoring of study participants [GSK] received by the employer, without personal benefits.
E. Stadtmauer reports grants [GSK] during study conduct.
K. Sullivan reports Data Monitoring Committee and honoraria [Kiadis Pharmaceutical] and Adjudication Committee and honoraria [Roche Genentech] outside the submitted work.
J. Szer reports advisory board, consultancy and honoraria [Alexion and Shire Pharmaceutical], advisory board and honoraria [Sanofi Genzyme], advisory board and consultancy [Pfizer] and advisory board [Amgen and Novartis] outside the submitted work.
S. Abhyankar, C. Cellini, J. Lee, J. Lopez Jimenez, P. Chandrasekar, D. Pohlreich and P. Quittet have nothing to disclose.
[O111 Figure].
Table presenting humoral and cellular immunogenicity and safety results]
O112 An Immunochip-based approach identifies ARTN2 and CX3CR1 as new risk loci for invasive aspergillosis in high risk hematological patients
Carmen Belén Lupiañez1, Manuel Martínez-Bueno1, Jose Sanchez-Maldonado1, Cristina Cuhna2, Jan Springer3, Michaela Lackner4, Rafael Rios-Tamayo1, Juana Segura-Catena1, Luz Canet1, Laura Alcazar-Fuoli5, Miguel Ángel López-Nevot6, Luana Fianchi7, Jon Badiola8, José María Aguado9, Livio Pagano7, Ana Rodríguez-Ramos1, Ana Comino10, Marta Alarcón-Riquelme11, Leonardo Potenza12, Samuel Martins Gonçalves2, José Juan Jiménez-Moleón13, Mario Luppi12, Carlos Solano14, Antonio Sampedro15, Manuel Cuenca-Estrella5, Katrien Lagrou16, Johan A Maertens17, Cornelia Lass-Flörl4, Hermann Einsele3, Lourdes Vázquez18, Jurgen Loeffler3, Agostinho Carvalho2, Manuel Jurado8, Juan Sainz1
1GENYO. Center for Genomics and Oncological Research, Genomic Oncology, Granada, Spain; 2Life and Health Sciences Research Institute (ICVS), School of Medicine, Braga, Portugal; 3Universitätsklinikum Würzburg, Medizinische Klinik II, Würzburg, Germany; 4Division of Hygiene and Medical Microbiology, Medical University of Innsbruck, Innsbruck, Austria; 5Mycology Reference Laboratory, Centro Nacional de Microbiologia, Instituto de Salud Carlos III, Madrid, Spain; 6Virgen de las Nieves University Hospital, Immunology, Granada, Spain; 7Istituto di Ematologia, Università Cattolica del S. Cuore, Rome, Italy; 8Virgen de las Nieves University Hospital, Hematology, Granada, Spain; 9Hospital Universitario '12 de Octubre', Instituto de Investigación Hospital '12 de Octubre' (i+12), Unit of Infectious Diseases, Madrid, Spain; 10Virgen de las Nieves University Hospital, Experimental Research Unit, Granada, Spain; 11GENYO. Center for Genomics and Oncological Research, Genomic Medicine, Granada, Spain; 12University of Modena and Reggio Emilia, AOU Policlinico, Department of Medical and Surgical Sciences, Modena, Italy; 13CIBER Epidemiology and Public Health (CIBER-ESP), Health Research Institute Carlos III, Madrid, Spain; 14Hospital Clinico Universitario-INCLIVA, University of Valencia, Hematology Department, Valencia, Spain; 15Virgen de las Nieves University Hospital, Department of Microbiology, Granada, Spain; 16KU Leuven – University of Leuven, Department of Microbiology and Immunology, Leuven, Belgium; 17University Hospitals Leuven, Department of Hematology, Leuven, Belgium; 18University Hospital of Salamanca, Hematology Department, Salamanca, Spain
Background: Invasive Aspergillosis (IA) is a life-threatening infection in which Aspergillus spp. colonizes lung or sinus tissues and spreads through the blood stream to other sites in the body. Despite the effective diagnosis and the use of new generation antifungal drugs, IA is increasing in incidence among immunocompromised, post-operative and critically ill and solid-organ transplanted patients. There is evidence that the combination of clinical risk factors and a specific host genetic background may render individuals more vulnerable to IA and increase the risk of infection-related hospitalizations and deaths. Thus, the aim of this study was to evaluate whether 124,093 single nucleotide polymorphisms (SNPs) within 186 host immunity loci influence the risk of IA and whether genotyping of specific markers could improve disease risk prediction.
Methods: The discovery population consisted of 1,251 European subjects: 77 haematological patients diagnosed with proven or probable IA, 387 non-infected and disease matched patients and 787 healthy controls. Haematological patients were allo-transplanted or diagnosed with acute leukaemia receiving intensive remission-induction chemotherapy. Proven and probable IA cases were diagnosed according to the revised EORTC/MSG criteria. The Immunochip® array was used to genotype all subjects. The association of the most relevant markers was then validated in a disease-matched population including 474 subjects (94 IA and 380 non-IA) and functional experiments were conducted to determine their effect on the immune response to Aspergillus. MDR and ROC curve analyses were used to identify sets of SNPs associated with IA risk that could improve the prediction of the infection.
Results: We identified 14 genetic regions encompassing host immunity genes moderately associated with IA risk. The most significant effect was found for SNPs within the ARNT2 and CX3CR1 loci (PMeta = 3.01•10−5 and 6.87•10−5). Mechanistically, we observed that monocyte-derived macrophages (MDM) from subjects carrying the ARNTR2rs1374213G allele or GG genotype showed a significantly impaired fungicidal activity (PAAvs.GA+GG = 0.012 and PAAvs.GG = 0.0176) whereas MDM from carriers of the ARNT2rs1374213G and CX3CR1rs9823718G alleles had a pronounced deregulation of immune responses mediated by IL8, IL1b, TNFa, IFNg and IL6. Importantly, we also found that two 4-SNP sets ([A]-CX3CR1rs7631529-IL18RAPrs116260662-ERAP1rs79682341-SERPINA9rs7149309 and [B]-CX3CR1rs7631529-IL18RAPrs116260662-IL2|IL21rs67143487-SERPINA9rs7149309) were strongly associated with IA risk in both the discovery and replication populations (PMeta = 2.55·10−16 and 6.14·10−16, respectively). Interestingly, we found that the two most common combinations of risk alleles for these sets of SNPs were consistently associated with IA in both populations (PDiscovery_[A]_1_2_0_2 = 0.001 and PReplication_[A]_1_2_0_2 = 0.00096 and PDiscovery_[B]_1_2_0_2 = 0.0012 and PReplication_[B]_1_2_0_2 = 0.0071, respectively). The meta-analysis of both populations confirmed the strong association of these risk allele combinations with an increased risk of IA (PMeta_[A]_1_2_0_2 = 3.20·10−6 and PMeta_[B]_1_2_0_2 = 3.04·10−5). Finally, we found that these 4-SNP sets significantly improved the prediction of IA risk when compared with a reference model (AUCGENETIC_1 = 0.77 and AUCGENETIC_2 = 0.76 vs. AUC_CLINICAL = 0.67; PLR = 4.94·10−4).
Conclusions: These findings highlight the role of ARNT2 and CX3CR1 loci in modulating the risk of IA and provide new insights about the possible role of these loci to modulate innate and adaptive immune responses against AF. This work also confirmed the importance of considering combination of risk alleles within CX3CR1, IL18RAP, SERPINA9, ERAP1 and IL2|IL21 loci when assessing and predicting susceptibility to IA.
On behalf of the PCRAGA Study Group
Conflict of interest: The authors declare that no conflict of interest exists.
O113 Pre-engraftment and post-engraftment Gram-negative rods bacteremia in HSCT patients: Risk factors and association with mortality. Intercontinental study of Infectious Diseases Working Party of EBMT
Diana Averbuch1, Gloria Tridello2, Jennifer Hoek3, Malgorzata Mikulska4, Thomas Pabst5, Lucrecia Yaňez San Segundo6, Hamdi Akan7, Tülay Özçelik8, Irene Donnini9, Galina Klyasova10, Aida Botelho de Sousa11, Tsila Zuckerman12, Depei Wu13, Cristina Tecchio14, Rafael de la Camara15, Sahika Zeynep Aki16, Per Ljungman17, Zafer Gülbas18, Emmanuelle Nicolas19, Elisabetta Calore20, Katia Perruccio21, Ron Ram22, Claudio Annaloro23, Rodrigo Martino24, Batia Avni1, Peter Shaw25, Lidia Gil26, Simona Iacobelli27, Jan Styczynski28, Dan Engelhard1, Simone Cesaro2
1Hadassah University Hospital, Jerusalem, Israel; 2Azienda Ospedaliera Universitaria Integrata Verona, Verona, Italy; 3EBMT Data Office, Leiden, Netherlands; 4Ospedale San Martino, Genova, Italy; 5University Hospital, Collegium Medicum UMK, Bern, Switzerland; 6Hospital U. Marqués de Valdecilla, Santander, Spain; 7Ankara University faculty of Medicine, Ankara, Turkey; 8Florence Nightingale Sisli Hospital, Istanbul, Turkey; 9Azienda Ospedaliera Universitaria Careggi, Firenze, Italy; 10National Research Center for Hematology, Moscow, Russian Federation; 11Hospital dos Capuchos, Lisboa, Portugal; 12Rambam Medical Center, Haifa, Israel; 13First Affiliated Hospital of Soochow University, Suzhou Jiangsu, China; 14Policlinico G.B. Rossi, Verona, Italy; 15Hospital de la Princesa, Madrid, Spain; 16Gazi University Faculty of Medicine, Ankara, Turkey; 17Karolinska University Hospital, Stockholm, Sweden; 18Anadolu Medical Center Hospital, Kocaeli, Turkey; 19Centre Leon Berard, Lyon, France; 20Clinica di Oncoematologia Pediatrica, Padova, Italy; 21Ospedale Santa Maria della Misericordia, Università di Perugia, Perugia, Italy; 22Tel Aviv Sourasky Medical Center, Sourasky Medical School, Tel Aviv University, Tel Aviv, Israel; 23Granda Ospedale Maggiore Policlinico IRCCS, Milano, Italy; 24Hospital Santa Creu I Sant Pau, Barcelona, Spain; 25The Children's Hospital at West Mead, Sydney, Australia; 26University of Medical Sciences, Poznan, Poland; 27Tor Vergata University, Rome, Italy; 28Collegium Medicum, Nicolaus Copernicus University Torun, Bydgoszcz, Poland
Background: Gram-negative rods bacteremia (GNRB) is an important cause of morbidity and mortality after HSCT. Here we present data on risk factors for GNRB and associated mortality.
Methods: Data on GNRB episodes occurring during 6 months after the HSCT were collected prospectively (2.2014–5.2015) in 72 centers from 25 countries (Europe, Asia, Australia). In patients with and without at least one GNRB we retrospectively compared: demography, underlying disease status, Karnofsky/Lansky score, HSCT type, conditioning, fluoroquinolone prophylaxis (FQP), department involvement of infectious control team (ICT); presence of engraftment and GVHD (time dependent). FQP was provided in 87% allo-HSCT and 74% auto-HSCT centers, ICT was operated in 89% allo-HSCT and 80% auto-HSCT centers.
Results: Risk factors analysis was performed for pre-engraftment GNRB in 2640 allo-HSCT (pre-allo-GNRB, median age 41.5 years; range 0.1–74.9; 61% males) and in 2901 auto-HSCT (pre-auto-GNRB, median age 56.4 years; 0.5–79.6; 60% males); for post-engraftment GNRB in 2572 allo-HSCT (post-allo-GNRB, median age 41.2 years; range 0.1–74.9; 61% males) patients.
Among allo-HSCT: 70% received myeloablative conditioning; the stem cells sources were: peripheral blood, 64%; bone marrow, 31%; cord blood, 5%. Donor types were MRD 35%, MUD 48% and MMD 17%. The underlying diseases were high relapse-risk malignancies, 38%; low-relapse risk malignancies, 52% and non-malignant disease, 10%. Neutrophil engraftment was achieved in 96% of patients; within median 16 days (1–102) post-HSCT. 65% of patients and 51% of donors were CMV seropositive. 28% patients developed acute grade II-IV GVHD; 4% received steroids for GVHD prophylaxis.
Among auto-HSCT: the underlying diseases were high relapse-risk malignancies, 91%; low-relapse risk malignancies, 8% and non-malignant disease, 1%.
The cumulative incidence of pre-allo-GNRB was 8.3% (95%CI: 7–9%); pre-auto-GNRB 6.7% (6–8%); while of post-allo-GNRB 5.5% (5–7%) and post-auto-GNRB 0.8% (0.5–1%).
In the univariate analysis, south-east center location, non-malignant and high risk malignant disease, MMD, cord blood, CMV seropositive recipient, FQP, lower Karnofsky/Lansky score, GVHD prophylaxis including steroids and other than cyclosporine/tacrolimus/methotrexate combination and conditioning regimen not based only on busulfan/cyclophosphamide predisposed to pre-allo-GNRB;
older age, non-malignant disease, non-providing FQP and absence of ICT predisposed to pre-auto-GNRB; and south-east location, lower Karnofsky/Lansky score, non-providing FQP and anti-GVHD prophylaxis other than cyclosporine/tacrolimus/methotrexate combination predisposed to post-allo-GNRB.
In the multivariate analysis the following risk factors predisposed for pre-allo-GNRB: south-east location (HR 3.49; 95%CI 2.08–5.86), FQP (2.70; 1.10–6.67), MMD (2.23; 1.38–3.61) and lower Karnofsky/Lansky score (10 points effect 0.81; 0.72–0.91); for pre-auto-GNRB: non-malignant disease (3.45; 1.18–10.07) and no ICT (1.97; 1.27–3.06); and for post-allo-GNRB: south-east (vs. north-west) European location (5.73, 2.90–11.32) and non-providing FQP (5.07, 2.96–8.67).
Overall survival 6-months post-HSCT was 87% (95%CI: 85–88).
There was increased risk of mortality in patients with vs. without GNRB (p < 0.0001 for all), with HR (95% CI) 2.44 (1.88–3.16) for pre-allo-GNRB; 2.82 (1.77–4.50) for pre-auto-GNRB; 4.20 (2.96–5.96) for post-allo-GNRB.
Conclusions: Risk factors for bacteremia differ in pre- and post-engraftment post-HSCT period. The association between fluoroquinolone prophylaxis and increased risk of pre-engraftment GNRB in allo-HSCT patients contradicts the current concepts on benefit of FQP for prevention of bacteremia. Both pre-engraftment and post-engraftment bacteremia are associated with mortality.
Clinical Trial Registry:
ClinicalTrials.gov NCT02257931
Conflict of interest: No conflicts of interest
O114 Frequency, characteristics and outcomes of Lymphoproliferative Disorders After Allogeneic Stem Cell Transplant: results of a multicenter study from the Grupo Español de Trasplante Hematopoyético (GETH)
Irene García Cadenas1, Lucrecia Yañez2, Isidro Jarque3, Jose Antonio Pérez Simón4, Rodrigo Martino5, David Valcárcel6, Jaime Sanz3, Arancha Bermúdez2, Cristina Muñoz7, Cristina Calderón4, Estefania García8, Cristina Díaz de Heredia6, Maria Suárez-Lledó9, Marta González Vicent10, Inmaculada Heras11, Mari Cruz Viguria12, Montse Batlle13, Lourdes Vázquez14, Carlos Solano15
1Hospital de la Santa Creu i Sant Pau, Jose Carreras Leukaemia Research Institute, Hematology Department, Barcelona, Spain; 2Hospital Universitario Marqués de Valdecilla, Santander, Spain; 3Hospital La Fe, Valencia, Spain; 4Hospital Virgen del Rocio, Sevilla, Spain; 5Hospital de la Santa Creu i Sant Pau, Jose Carreras Leukaemia Research Institute, Barcelona, Spain; 6Hospital Vall d´Hebrón, Barcelona, Spain; 7Hospital Gregorio Marañón, Madrid, Spain; 8Hospital Reina Sofía, Córdoba, Spain; 9Hospital Clinic de Barcelona, Barcelona, Spain; 10Hospital Niño Jesús, Madrid, Spain; 11Hospital Morales Meseguer, Murcia, Spain; 12Complejo Hospitalario de Navarra, Navarra, Spain; 13Hospital Germans Trias i Pujol, Barcelona, Spain; 14Hospital Clínico de Salamanca, Salamanca, Spain; 15Hospital Clinico de Valencia, Valencia, Spain
Background: Post-transplant lymphoproliferative disorder (PTLD) is a rare but lifethreatening complication after allogeneic stem cell transplantation (allo-SCT).
Methods: Multicenter, retrospective analysis of allo-SCT performed in 14 pediatric and adult transplant centers in Spain. Centers volunteered the data on each of their patients who was diagnosed with proven or probable PTLD between 2000 and 2015.
Our aim was to investigate the frequency, characteristics and management of PTLD after allo-SCT in Spain and to identify prognostic factors influencing outcomes.
Results: During the study period, 102 PTLD were diagnosed among 12641 allo-SCT performed in the participating centers, leading to an estimated PTLD diagnostic frequency of 0.8%. Eigthy seven cases (85%) were diagnosed between 2006 and 2013 (Figure 1).
A total of 82 patients (81%) received SCT from alternative donors, and 89 of them (87%) included anti-thymocyte globulin before infusion. The indication for allo-SCT was severe aplasia in 20 cases (20%).
PTLD was diagnosed at a median of day +106 after SCT (range: 27–3764). PTLD was biopsy-proven lymphoma in 78 patients (76%), whereas 11 (11%) had probable disease (clinically-defned PTLD + EBV DNAemia). CD20 expression was positive in 51/60 (85%) cases with available information. Forty-eight patients (47%) had undergone weekly EBV DNAemia monitoring (cases > the year 2006). The median EBV DNAemia at PTLD diagnosis was 12591 copies /mL (range: 0–5.580.700)
Seventy-three patients (72%) had new onset lymphadenopathies at diagnosis. B-symptoms were present in 58 cases (57%), and 23 (22%) and 17 patients (17%) had gastrointestinal tract and CNS involvement, respectively. Ann Arbor staging was ≥ III in 54% cases.
Eighty-seven patients (85%) received Rituximab treatment, alone or in combination with reduction of immunosuppression (n = 65, 64%). Although Rituximab was initiated only 5 days after the first EBV DNAemia or immediatly after the diagnosis of PTLD, the overall response-rate (ORR) was only 49%. For Rituximab non-responding patients, additional treatments used were chemoteraphy in 27 cases and EBV-specific T cell therapy in 9 cases, with ORR of 37% and 40%, respectively. Fifteen patients did not receive treatment with Rituximab.
Forty-seven patients died due to PTLD (46%) and 22 due to opportunistic infection(s) after complete resolution of PTLD. The 2-year overall survival was 33% (95%CI: 23–42). The estimated incidence of PTLD-related mortality was 45% (95% CI: 36–57).
In univariate analysis, the variables associated with survival were: age < 40, non-malignant underlying disease, Rituximab treatment, reduction of immunosupression, platelet count >50x109/L and lymphocyte count >0.5x109/L at PTLD diagnosis. In multivariate analysis, the only significant variables associated with better overall survival were platelet count >50x109/L (HR: 2.6, p = 0.005) and non-severe lymphocytopenia (HR :2.7, p: 0.008) at PTLD diagnosis.
Conclusions: Although this retrospective study found that only a small proportion of our allo-SCT recipients developed PTLD, affected patients showed an agressive clinical course and had a dismal overall survival, despite Rituximab-based treatment, due to a very high TRM mainly because of PTLD and opportunistic infections . In this setting, lymphocyte and platelet count at PTLD diagnosis may be predictive factors for survival.
Conflict of interest: Nothing to disclose
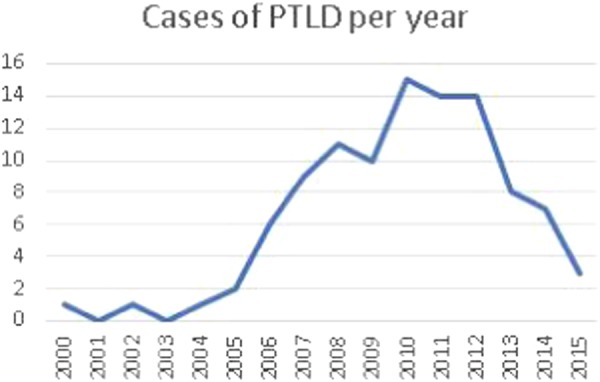
[ [O114 Figure] Cases of PTLD per year]
O115 Incidence of Adenovirus Infections in Pediatric and Adult Allogeneic Hematopoietic Cell Transplant Recipients in Europe
Sebastian Voigt1, Cécile Pochon2, Kanchan Rao3, Antonio Pérez-Martínez4, Marco Zecca5, Enrikas Vainorius6, Tom Brundage6, Artak Khachatryan7, Essy Mozaffari6, Garrett Nichols6
1Charité-Universitätsmedizin Berlin, Department of Pediatric Oncology/Hematology/Stem Cell Transplantation, Berlin, Germany; 2University Hospital of Nancy, Allogeneic Hematopoietic Stem Cell Transplantation Unit, Department of Hematology, Vandoeuvre-lès-Nancy, France; 3Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom; 4Hospital Universitario La Paz, Pediatric Hemato-Oncology, Madrid, Spain; 5Fondazione IRCCS Policlinico San Matteo, Pediatric Hematology / Oncology, Pavia, Italy; 6Chimerix, Durham, NC, United States; 7Analytica-Laser, London, United Kingdom
Background: Allogeneic hematopoietic cell transplant (allo-HCT) recipients are at increased risk of viral infections, including adenovirus (AdV). These infections may lead to life-threatening conditions that further complicate the complex management of allo-HCT recipients. Pediatric allo-HCT patients are at particularly high risk for development of AdV infections. Current evidence regarding AdV epidemiology after allo-HCT is generally limited to single-center studies. In this analysis, we report the incidence of AdV infections in pediatric and adult allo-HCT recipients in AdVance, a multicenter, multinational study conducted in Europe.
Methods: The AdVance study is a retrospective review of medical charts and laboratory records for recipients of an allo-HCT from January 2013 to September 2015; the study was designed to determine the incidence, management and clinical outcomes of AdV infections in adult (≥18 years) and pediatric patients. Quantitative and qualitative data were extracted to determine the incidence of AdV infection, AdV viremia, and AdV viremia ≥1000 copies/mL.
Results: Overall, 3377 patients underwent allo-HCT: 1103 pediatric and 2274 adults. At least one AdV positive assay in blood, stool, urine, or nasal secretions was identified in 26.8% (296/1103) of pediatric and 4.8% (110/2274) of adult allo-HCT recipients within 6 months of transplant (see table). Among the 296 pediatric patients who had an AdV positive test, 211 had viremia, more than half of whom (122/211) had clinically relevant AdV viremia ≥1000 copies/mL. AdV positivity was more frequent in pediatric patients than in adults for all subgroups analyzed, including stratifications for graft types and conditioning regimens. One-year post-HCT mortality was 28.4% (95% CI: 23.6–33.2) in pediatric patients with any AdV infection within 6 months of allo-HCT and 21.8% (95% CI: 19.5–24.1) in those without AdV infection. In adults, one-year post-HCT mortality was 45.5% (95% CI: 36.2–54.7) in patients with any AdV infection within 6 months of allo-HCT and 33.6% (95% CI: 31.7–35.5) in those without AdV infection.
[[O115 Figure] Table].
Table 1 Incidence of adenovirus infection within 6 months following allo-HCT, n (%)
Conclusions: Based on this representative sample of European transplant centers, approximately one in four pediatric allo-HCT recipients developed AdV infection; of these, over two-thirds developed viremia, more than half of whom had clinically relevant viremia of ≥1000 copies/mL. AdV infections and viremia following allo-HCT were more common in pediatric patients than in adults, although screening is less systematic in adults, which may lead to underreporting of the true incidence. One-year post-HCT mortality was higher in patients with AdV infections than in patients without AdV infections.
Conflict of interest:
S. Voigt, C. Pochon, K. Rao, A. Pérez-Martínez, and M. Zecca are investigators in the AdVance study sponsored by Chimerix.
E. Vainorius, T. Brundage, E. Mozaffari, and G. Nichols are employees of the study sponsor, Chimerix.
A. Khachatryan is an employee of Analytica-Laser, a research consultancy who conducted the study on behalf of the sponsor, Chimerix.
O116 Adenovirus Viral Burden is Associated with Mortality in Pediatric Allogeneic Hematopoietic Cell Transplant Recipients: Results from the AdVance Study
Marco Zecca1, Kanchan Rao2, Antonio Pérez-Martínez3, Cécile Pochon4, Sebastian Voigt5, Enrikas Vainorius6, Tom Brundage6, Aastha Chandak7, Essy Mozaffari6, Garrett Nichols6
1Fondazione IRCCS Policlinico San Matteo, Pediatric Hematology / Oncology, Pavia, Italy;2Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom;3Hospital Universitario La Paz, Pediatric Hemato-Oncology, Madrid, Spain;4University Hospital of Nancy, Allogeneic Hematopoietic Stem Cell Transplantation Unit, Department of Hematology, Vandoeuvre-lès-Nancy, France;5Charité-Universitätsmedizin Berlin, Department of Pediatric Oncology/Hematology/Stem Cell Transplantation, Berlin, Germany;6Chimerix, Durham, NC, United States;7Analytica-Laser, New York, NY, United States
Background: Both pediatric and adult allogeneic hematopoietic cell transplant (allo-HCT) recipients are at high risk for adenovirus (AdV) infections, particularly during the period before immune reconstitution. AdV viremia has been strongly correlated with mortality in single-center studies (Lion 2014; Mynarek 2014). There is a critical need for robust data from multicenter studies evaluating the relationship between AdV viral burden and mortality. AdV viral load over time, a quantitative measure in plasma of cytolytic AdV infection in the gastrointestinal tract, lungs, liver and/or kidney, is proposed as a surrogate marker of clinical outcomes in studies of investigational antiviral therapies.
Methods: AdVance is a retrospective multicenter study of the incidence, management and clinical outcomes for AdV infections in European adult and pediatric allo-HCT recipients. In this analysis, pediatric patients who underwent allo-HCT between January 2013 and September 2015 and had at least 12 months' follow-up were included. Reported AdV treatment included reduced immunosuppression, cidofovir, ribavirin, ganciclovir, T-cell therapy, or donor lymphocytes. AdV viral burden was assessed as time-averaged area under the viremia-time curve (AAUC) over 16 weeks. Mortality within 6 months of AdV diagnosis was determined for patients with AdV viremia ≥1000 copies/mL.
Results: The study included 1103 pediatric allo-HCT recipients. Two hundred ninety-six subjects (26.8%) developed an AdV infection in any body fluid within 6 months of allo-HCT; 211 (19.1%) developed AdV viremia, over half of whom (122/211, 57.8%) surpassed a clinically relevant threshold of ≥1000 copies/mL. Twenty-one of these patients died (21/122, 17.2%, 95% CI: 10.7–23.7) of any cause within 6 months of first AdV viremia; two of the 21 died of relapse of their underlying malignancy, while the remainder died of non-relapse related causes. Of those with >1000 copies/mL, mean (SD) AdV AAUC was 3.5 (1.4) log10 copies/mL in those who died vs. 1.5 (1.3) log10 copies/mL in survivors (p < 0.0001). The highest mortality was observed in those with highest AdV viral burden (4th quartile of AAUC of 3.1–6.1 log10 AdV copies/mL), in which 14 of 30 (46.7%) patients died, compared with no deaths reported among 30 patients in the lowest quartile (AAUC of 0.0-< 0.6 log10 AdV copies/mL). Time to death decreased significantly with increasing AAUC (p < 0.0001). The majority of deaths occurred in the first 90 days after AdV viremia ≥1000 copies/mL.
Conclusions: AdVance is the first large retrospective multicenter study to examine the impact of AdV viremia on patient outcomes in the modern allo-HCT setting. Over half of the pediatric allo-HCT recipients who developed AdV viremia surpassed the clinically relevant threshold of ≥1000 copies/mL, previously associated with high short-term mortality. AdV plasma viral burden as measured by AdV AAUC has a strong correlation with both overall and non-relapse related mortality in pediatric allo-HCT recipients, and is therefore an appropriate measure to assess the potential benefits of antiviral therapies.
References:
Lion T. Clin Microbiol Rev. 2014;27:441-62.
Mynarek M, et al. Biol Blood Marrow Transplant. 2014;20:250-6.
Conflict of interest:
M. Zecca, K. Rao, A. Pérez-Martínez, C. Pochon, and S. Voigt are investigators in the AdVance study sponsored by Chimerix.
E. Vainorius, T. Brundage, E. Mozaffari, and G. Nichols are employees of the study sponsor, Chimerix.
A. Chandak is an employee of Analytica-Laser, a research consultancy who conducted the study on behalf of the sponsor, Chimerix.
[[O116 Figure].
![[[O116 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/92b99cefbf50/41409_2018_318_Figz_HTML.jpg)
Fig]
O117 Current antimicrobial practice in febrile neutropenia: Infectious Disease Working Party EBMT Survey
Anke Verlinden1,2, Malgorzata Mikulska3, Nina Knelange4, Dina Averbuch5, Jan Styczynski6
1Antwerp University Hospital, Department of Haematology, Edegem, Belgium; 2University of Antwerp, Vaccine & Infectious Disease Institute, Faculty of Medicine & Health Sciences, Antwerp, Belgium; 3University of Genova (DISSAL) and Ospedale Policlinico San Martino, Division of Infectious Diseases, Genova, Italy; 4EBMT Data Office, Leiden, Netherlands; 5Hadassah-Hebrew University Medical Centre, Pediatric Infectious Diseases, Jerusalem, Israel; 6Collegium Medicum, Nicolaus Copernicus University Torun, Department of Pediatric Hematology and Oncology, Bydgoszcz, Poland
Background: With rising antibiotic resistance and knowledge that disruption of the gut microbiome has a negative impact on patient outcome, antibiotic policies are increasingly important. In contrast to IDSA/ESMO guidelines which recommend continuing antibiotics until neutrophil recovery, ECIL-4 guidelines suggest earlier discontinuation under specific conditions.
The aim of this survey was to summarize current antimicrobial practice in febrile neutropenia and presence of key aspects of antimicrobial stewardship in EBMT centres.
Methods: In August 2017 a questionnaire was e-mailed to all 567 EBMT centres in 57 countries. It sought information on: (i) key aspects of antimicrobial stewardship, (ii) antimicrobial prophylaxis, (iii) empirical therapy, (iv) escalation/de-escalation strategies. European countries were divided in two geographical zones: north/west (NW) and south/east (SE).
Results: Complete responses were obtained from 164 (28.9%) centres in 40 (70%) countries, including 60 (36.6%) from NW and 99 (60.4%) from SE European centres (Figure 1). Five responses (3.0%) from countries outside Europe were excluded from geographical analysis.
Most centres (93.3%) have written local guidelines on antibiotic policy. Infectious disease/microbiology departments are often involved in writing guidelines (56.6%) and deciding on antimicrobial treatment (51.8%). 71.7% of NW versus 89.8% of SE centres (p = 0.003) perform surveillance cultures. 82.8% of centres receive regular updates on local epidemiology and resistance pattern. Positive blood cultures are reported actively in 93.2% and within 24 hours in 93.8%. Antimicrobial resistance profile is reported within 24 hours after positive blood culture in 79.8%.
Fluoroquinolone prophylaxis is used in 46.7% of NW versus 69.8% of SE centres (p = 0.016). In 61.3% first-line empirical therapy is piperacilline/tazobactam, whereas 12.5% use carbapenems routinely. 36.3% of centres use combination therapy (mainly aminoglycosides) in first-line in stable patients without history of resistant pathogens. 35.2% of these centres de-escalate to monotherapy within 3 days and 22.2% after 10 days. 60.4% of centres (48.3% NW versus 66.7% SE; p = 0.024) add a glycopeptide empirically in fever lasting longer than 2–3 days. 73.8% of centres (65.5% NW versus 79.4% SE; p = 0.056) escalate to a broader spectrum agent empirically in fever lasting longer than 3–5 days.
Antibiotics are not frequently discontinued before neutrophil recovery. In case of positive blood cultures with a susceptible pathogen and uncomplicated presentation, 50.9% of NW versus 28.9% of SE centres (p = 0.006) discontinue before neutrophil recovery. When confronted with a clinically documented infection with uncomplicated presentation, 64.9% of NW versus 29.8% of SE centres (p < 0.001) discontinue before neutrophil recovery. In fever of unknown origin with uncomplicated presentation, 65.5% of NW versus 38.1% of SE centres (p = 0.001) discontinue before neutrophil recovery.
Conclusions: A third of centres use empirical combination therapy in first-line, with de-escalation to monotherapy being performed within 3 days in only one third of those centres. Many centres add a glycopeptide and/or escalate to a broader spectrum agent empirically in stable patients, even though all available guidelines advise against it. Despite ECIL guidelines suggesting de-escalation and/or discontinuation of antibiotics prior to neutrophil recovery in specific situations, this has not been widely adopted in clinical practice across Europe.
Conflict of interest: No conflicts of interest.
Lymphoma
O118
Abstract previously published
O119 T-cell replete HLA-haploidentical transplantation using PTCY versus HLA-matched related/unrelated donor transplantation in refractory or relapsed aggressive NHL - results of a single center matched-pair analysis
Sarah Haebe, Alessia Fraccaroli, Dusan Prevalsek, Zoellner Anna-Katharina, Christoph Schulz, Martin Dreyling, Andreas Hausmann, Johanna Tischer
Ludwig-Maximilians-University Hospital Grosshadern, Department of Internal Medicine III, Hematopoietic Stem Cell Transplantation, Munich, Germany
Background: Over the past T-cell replete (TCR) HLA-haploidentical hematopoietic stem cell transplantation (haplo-HSCT) utilizing post-transplantation cyclophosphamide (PTCY) for prophylaxis of GvHD has become a valuable treatment alternative in patients with various hematologic diseases lacking a conventional donor. When compared to patients with myeloid malignancies, in patients with lymphoma a remarkable survival benefit was observed. Recently, we have shown that sequential TCR/PTCY haplo-HSCT is feasible and effective in relapsed and refractory (R/R) NHL (Zoellner AK et al, BMT 2015). To further clearify the role of haplo-HSCT strategy in aggressive lymphoma, we performed a matched-pair analysis comparing outcome of HLA-matched related donor (MRD) or unrelated donor (MURD) transplantation versus PTCY-based haploidentical donor (haplo-D) transplantation at our institution.
Methods: Recipients of TCR/PTCY haplo-HSCT were pair-matched with patients receiving HLA-matched transplantation. Matching variables were
(1) age (± 5years) and
(2) stage at HSCT.
Stage at HSCT was defined as either complete response (CR) or non-CR.
21 patients undergoing TCR/PTCY haplo-HSCT were successfully pair-matched (p = 1.0 for stage and 0.9 for age) with 21 recipients of MRD and 21 patients of MURD transplantation. Within the entire cohort, median age was 48y (range: 20–65 years). At time of conditioning, 81% had not achieved CR. 19% were in CR. All patients received at least two prior treatment lines. In 16 patients (76%) undergoing haplo-HSCT a sequential conditioning regimen, comprising cytoreductive chemotherapy (Clofarabine) shortly before reduced intensity conditioning (RIC) therapy, was performed.
Results: Overall CR rate of the entire cohort at d+30 was 90%, 6 patients died early in aplasia, mostly due to infections.
Median time to engraftment was 16, 18 and 20 days after MRD, MURD and haplo-D transplantation, respectively (p = 0.2).
Small differences were observed among the three groups regarding PFS at 2 years (MRD: 51%, MURD 65% and haplo-D HSC 55%) and OS at 2 years after transplantation (MRD: 43%; MURD 38%, haplo-D 52%)
Occurrence of aGvHD ≥II° was not significantly different among the three groups. Recipients of MURD transplantation showed a slightly higher incidence of aGvHD ≥II° (33% MURD vs 24% MRD and 19% haplo-D, p 0.5). 43% of MURD vs. 24% of MRD vs. 29% of haplo-D recipients died without relapse within in the first year after transplantation. 1-year relapse rates in MRD and haplo-D transplantation was comparable with 33% vs. 38%, respectively.
Conclusions: In aggressive lymphoma, TCR haplo-HSCT using PTCY for GvHD prophylaxis results in similar outcome when compared to HLA-matched transplantation at our institution. Due to the observed trend towards a higher incidence of aGvHD and NRM after MURD transplantation, we suggest that timely limited access to a MURD might force the decision towards the performance of a haplo-D transplantation also in aggressive NHL.
Conflict of interest: nothing to disclose
O120
Abstract previously published
O121
Abstract previously published
O122 Impact of chromosomal abnormality in ATL patients who received allogeneic hematopoietic transplantation. An Analysis on behalf of JSHCT-ATL-WG
Nobuaki Nakano1, Atae Utsunomiya1, Keitaro Matsuo2, Hiroshi Fujiwara3, Sigeo Fuji4, Yoshifusa Takatsuka1, Takahiro Fukuda5, Toshihiro Miyamoto6, Kaname Miyashita7, Hirohisa Nakamae8, Yasushi Sawayama9, Mitsuhiro Yuasa10, Tatsuo Ichinohe11, Yoshiko Atsuta12, Koji Kato6
1Imamura General Hospital, Department of Hematology, Kagoshima, Japan; 2Aichi Cancer Center Research Institute, Division of Molecular and Clinical Epidemiology, Nagoya, Japan; 3Ehime University Hospital., Department of Hematology, Clinical Immunology and Infectious Diseases, Matsuyama, Japan; 4Osaka International Cancer Institute, Department of Hematology, Osaka, Japan; 5National Cancer Center Hospital, Department of Hematopoietic Stem Cell Transplantation, Tokyo, Japan; 6Kyushu University, Medicine and Biosystemic Science, Graduate School of Medical Sciences, Fukuoka, Japan; 7National Kyushu Cancer Center, Department of Hematology, Fukuoka, Japan; 8Osaka City University Hospital, Department of Hematology, Osaka, Japan; 9Nagasaki University Hospital, Department of Hematology, Nagasaki, Japan; 10Federation of National Public Service Personnel Mutual Aid Associations Toranomon Hospital, Department of Hematology, Tokyo, Japan; 11Research Institute for Radiation Biology and Medicine, Hiroshima University, Department of Hematology and Oncology, Hiroshima, Japan; 12Japanese Data Center for Hematopoietic Cell Transplantation, Nagoya, Japan
Background: A previous study described that ATL cells frequently have chromosomal abnormalities including numerical aberrations and structural abnormalities. However, there has been no large study to examine the correlation between chromosomal abnormalities and survival especially in ATL patients received allogeneic HSCT (allo-HSCT). On behalf of the Japan Society for Hematopoietic Cell Transplantation (JSHCT) ATL working group, we here report the impact of chromosomal abnormalities on survival in those with allo-HSCT by using the Transplant Registry Unified Management Program (TRUMP) which is the nationwide survey database of the Japanese Data Center for Hematopoietic Cell Transplantation.
Methods: In this study, 756 ATL patients registered in TRUMP database were enrolled. To collect detailed information of chromosomal analysis and clinical backgrounds being not listed in TRUMP database, we conducted the nationwide secondary survey in those 756 patients. The collection rate was 70%. Patients representing multiploid, without mitosis, no clonality, and insufficient information by G-banding stain were excluded. As a consequence, 300 patients (170 male, 130 female) were analyzed. On the basis of numerical abnormality and structural break point, abnormal karyotypes using G-banding stain were analyzed. The karyotype with 3 or more abnormalities was defined as complex one. We used selected chromosomal abnormalities, whose frequencies were more than 15, as variables for survival analysis. Survival analysis was performed with Cox model (OS) and competing risk regression model (NRM and ATL-related death) and multivariate analysis were done with Backward stepwise method. Finally, we extracted significant abnormalities with Holm method and evaluate their correlations. Separately, survival analysis on complex karyotype was performed.
Results: Median age of these 300 patients was 55 years (24–74). One hundred and eighty-three patients were diagnosed with acute type ATL, 70 lymphoma type, 33 chronic type, and 14 smoldering type, respectively. Myeloablative conditioning regimens were employed for 183 patients, and CBT were conducted for 65 patients. The median level of soluble IL-2 receptor at diagnosis was 17969 U/mL (443.8–1130505). Two hundred and eight patients represented some kinds of karyotypic abnormalities. Specimens for chromosomal analysis were taken from bone marrow (n = 166), lymph nodes (n = 86), peripheral blood (n = 41), and others (n = 7). -14, +3, -13, and -Y were observed frequently as numerical aberrations (n>20), while the structural break points were frequently located at 6q, 9q, 1q, 2q, and 14q (n>40), respectively. In survival analyses, breakpoints at 2q (HR 1.5, 95%CI 1.023–2.198, P = 0.038) and 5q (HR 2.17, 95%CI 1.22–3.85, P = 0.008) negatively contributed to OS with a statistical significance. On the other hand, -14, and breakpoints at 3p, 5q, and 6q were the negative factors in ATL-related death. As a whole, the complex karyotype didn't demonstrate any negative impact on OS, however, some subsets of complex karyotypes including a breakpoint at 5q or 3p-6q coexistence showed a negative impact on OS and ATL related death.
Conclusions: This is the first large study showing the survival impact of chromosomal abnormality in ATL patient received allo-HSCT. It demonstrated that structural break points at 2q and 5q were the independent risk factors for OS.
Conflict of interest: None of the authors has anything to disclose.
O123 Early Events after Allogeneic Stem Cell Transplantation in Patients with Follicular Lymphoma Exposed to Idelalisib: A Survey of the EBMT Lymphoma Working Party
Leopold Sellner1, Peter Dreger1, Linda Koster2, Paul Browne3, Dietrich Beelen4, Marco R. De Groot5, Didier Blaise6, Veronika Valkova7, Jakob Passweg8, Helene Schoemans9, Per Ljungman10, Lutz P. Müller11, Gerald Wulf12, Michel van Gelder13, Christine Wolschke14, Mathilde Hunault-Berger15, Nigel Russell16, Attilio Olivieri17, Johannes Schetelig18, Nicolaus Kröger14, Silvia Montoto19
1University Hospital Heidelberg, Heidelberg, Germany; 2EBMT Data Office, Leiden, Netherlands; 3St. James's Hospital, Dublin, Ireland; 4University Hospital Essen, Essen, Germany; 5University Medical Center Groningen (UMCG), Groningen, Netherlands; 6Institut Paoli Calmettes, Marseille, France; 7Institute of Hematology and Blood Transfusion, Prague, Czech Republic; 8University Hospital Basel, Basel, Switzerland; 9University Hospital Gasthuisberg, Leuven, Belgium; 10Karolinska University Hospital, Stockholm, Sweden; 11University Hospital Halle, Halle, Germany; 12University Hospital Goettingen, Goettingen, Germany; 13University Hospital Maastricht, Maastricht, Netherlands; 14University Hospital Eppendorf, Hamburg, Germany; 15CHRU Limoges, Angers, France; 16Nottingham University, Nottingham, United Kingdom; 17Azienda Ospedali Riuniti di Ancona, Ancona, Italy; 18University Hospital Dresden, Dresden, Germany; 19St. Bartolomew's Hospital, Barts Health NHS Trust, London, United Kingdom
Background: Idelalisib is an approved novel agent for treating relapsed and refractory (R/R) follicular lymphoma (FL) which may be used for bridging patients to allogeneic stem cell transplantation (alloSCT). However, information on the impact of idelalisib on feasibility and safety of a subsequent alloSCT is sparse. We present results of an EBMT registry study on the outcome of patients with FL receiving an alloSCT following exposure to idelalisib.
Methods: Eligible were patients aged >18 years and registered in the EBMT database who had an alloSCT for FL after exposure to idelalisib at any time before transplant. Baseline patient, disease, and transplant data were collected from MED-A forms. Centers were requested to provide additional treatment and follow-up information (MED-B and C).
Results: 38 patients (63% male) met the eligibility criteria. AlloSCT were performed between 2015 and 2017. Median age at alloSCT was 56 (34–71) years and the median number of treatment lines prior to idelalisib was 2 (1–8), including autoSCT in 20 patients (53%). Twenty-five patients (66%) received idelalisib immediately before alloSCT as bridging, 24% in combination with a CD20 antibody, 11% with chemotherapy. Conditioning was total body irradiation (TBI)-based in 11 patients (29%) and alkylator-based in the remainder, and was considered as reduced intensity in 74% of the patients. 24% of donors were matched siblings, 68% matched unrelated, and 8% haploidentical. 81% of all patients were in complete or partial remission at alloSCT. In patients who received idelalisib for bridging to alloSCT, 91% were still responding to the idelalisib-containing regimen at time of alloSCT. Median time to reach neutrophils of >0.5/nl and platelets of >20/nl was 16.5 (2–35) and 16.5 (0–128) days post-transplant, respectively. Two patients failed to engraft, in both cases due to early death at day +10 and +23 because of infections. Acute GvHD grade 2–4 (3–4) was observed in 40% (17%), and overall and extensive chronic GVHD occurred in 21% and 3% of patients at risk with a median follow-up of 150 (100–720) days post alloSCT. There were 7 non-relapse deaths within the first 10 months post alloSCT. Causes of death included infection (n = 3), GvHD (n = 3), GI toxicity (n = 1) and relapse (n = 1). Six-month incidence of non-relapse mortality (NRM), relapse (REL), progression-free survival (PFS), and overall survival (OS) was 11%, 8%, 82%, and 87%. In patients who received idelalisib for bridging, six-month NRM, REL, PFS, and OS was 8%, 4%, 96%, and 96%. Causes of death in this subset were infection (n = 2), GvHD (n = 1) and GI toxicity (n = 1).
Conclusions: Idelalisib is an effective drug for bridging patients with R/R FL to alloSCT as demonstrated by the high percentage of patients in response at the time of transplant. However, and taking into account that this is a heavily pretreated population, the early non-relapse deaths raise concern.
Conflict of interest: Nothing to disclose
O124 Immune checkpoints activation dictates outcome after allogeneic transplant in lymphoma
Enrico Derenzini1, Simona Sammassimo1, Valentina Tabanelli2, Angelica Calleri2, Federica Gigli1, Daniele Avenoso1, Elisabetta Todisco1, Tommaso Radice1, Alberto Agazzi1, Anna Vanazzi1, Safaa Ramadan1, Stefano Pileri2, Rocco Pastano1, Corrado Tarella1
1European Institute of Oncology, Hemato-Oncology, Milan, Italy; 2European Institute of Oncology, Hemolymphopathology Unit, Milan, Italy
Background: Immune checkpoint blockade through inhibition of the programmed death ligand-1(PDL1)/PD1 axis has recently emerged as a promising treatment strategy in lymphoma. Lymphocyte activation gene-3 (LAG3) expression has been shown to cooperate with the PD1/PDL1 axis in favoring T-cell exhaustion and immune escape. The recent demonstration of clinical activity of immune checkpoint inhibition in the post allogeneic hematopoietic transplant (allo-HSCT) setting, prompted us to investigate whether or not the expression of PD1/PDL1 axis and/or LAG3 in pretransplant tumor biopsies predicts outcome following allo-HSCT in lymphoma.
Methods: We retrospectively reviewed clinical and follow-up data of lymphoma patients undergoing allo-HSCT at our Institution between 2003 and 2016. Only patients with available tissue samples from pre-transplant nodal biopsies were considered. Twenty-four patients were considered. Median age was 45 years (range 22–66), 15 patients were male, 17 patients received HLA identical, 7 haploidentical HSCT; 18 patients received reduced intensity conditioning (75%); the diagnosis was: Hodgkin lymphoma (HL) (9 patients), B-cell non Hodgkin lymphoma (NHL) (9 patients, with 3 diffuse large B-cell lymphoma, 3 follicular lymphoma, 3 chronic lymphocytic leukemia), peripheral T-cell lymphoma (PTCL) (6 patients); 17 patients (71%) had prior autologous-HSCT. Median time from biopsy to allo-HSCT was 14 months (range 3–37 months). At the time of HSCT, 8 patients (33%) were in complete or partial response (CR/PR), 16 patients (67%) had stable or progressive disease (SD/PD). The semiquantitative immunohistochemical H-score method, was used to quantify the expression levels of PD1 and LAG3 in T-lymphocytes, and PDL1 in lymphoma cells.
Results: After a median follow-up of 29 months (1.5–166), PFS and overall survival (OS) of the whole cohort were 62% and 38% respectively (Figure 1A,B): 7 patients (29%) died without lymphoma progression because of infections and/or GVHD-related complications. Seven patients (29%) suffered from acute graft versus host disease (GVHD), 9 (37.5%) had chronic GVHD. Neither disease status at transplant nor the incidence of GVHD were significantly associated with outcome. PD1 was expressed (>1% of positive cells) in 10 (42%) patients (6 HL, 4 B-cell NHL), PDL1 in 10 patients (8 HL, 2 PTCL), LAG3 in 13 patients (54%) (7 HL, 3 B-cell NHL, 3 PTCL). Considering the combined H-score ratio (sum of PD1, PDL1, LAG3 H-scores divided by number of patients), those patients progressing or relapsing after allo-HSCT showed a higher combined H-score ratio compared to those who did not relapse (146.3 vs 53.9 respectively, p < 0.01). Patients showing a H-score > 100 in at least one biomarker (PD1, PDL1, LAG3) or a H-score > 50 in 2 of 3 biomarkers had a significantly inferior PFS following allo-SCT compared with patients who did not (18% vs 92% respectively at 5 years, p < 0.01); similar results were obtained when considering only patients with a minimum of 1 year of follow-up (25% vs 100% PFS at 5 years, p < 0.01) (Figure 1C,D).
Conclusions: These data suggest that immunecheckpoints activation could decrease the efficacy of allo-HSCT in lymphoma, and that low expression levels of immunecheckpoints biomarkers could define a subgroup of lymphoma patients who could derive maximal benefit from allo-HSCT.
Conflict of interest: None of the authors has anything to disclose
[O124 Figure].
Figure 1
O125 Thiotepa-based Autologous hematopoietic stem cell transplantation (ASCT) for CNS or non-CNS lymphoma: first results of a prospective, multicenter, non-interventional study
Herbert G. Sayer1, Mathias Witzens-Harig2, Mohammed Wattad3, Agnieszka Korfel4, Inken Hilgendorf5, Georg-Nikolaus Franke6, Gerald Illerhaus7, Michael Heinsch8, Bernd Metzner9, Eva Bettina Zinngrebe10, Nadezda Basara11, Mascha Binder12, Volker Schmidt1, Mark Ringhoffer13, Alexander Röth14, Thomas Geer15, Peter Dreger16
1Helios Klinikum Erfurt, 4. Medizinische Klinik (Hämatologie und Onkologie), Erfurt, Germany; 2Universitätsklinikum Heidelberg, Heidelberg, Germany; 3Kliniken Essen Süd, Essen, Germany; 4Charite - Campus Benjamin Franklin, Hämatologie, Berlin, Germany; 5Universitätsklinikum Jena, Innere Medizin II (Hämatologie/Onkologie), Jena, Germany; 6Universitätsklinikum Leipzig, Abteilung für Hämatologie und Onkologie, Leipzig, Germany; 7Klinikum Stuttgart, Klinik für Hämatologie und Onkologie, Stuttgart, Germany; 8Helios St. Johannes Klinik Duisburg, Klinik für Onkologie und Hämatologie, Duisburg, Germany; 9Klinikum Oldenburg, Universitätsklinik für Innere Medizin - Onkologie und Hämatologie, Oldenburg, Germany; 10Ev. Krankenhaus Bielefeld gGmbH, Klinik für Innere Medizin Hämatologie/Onkologie, Bielefeld, Germany; 11Malteser Krankenhaus St. Franziskus-Hospital, Medizinische Klinik I, Flensburg, Germany; 12Universitätsklinikum Hamburg-Eppendorf, II Medizinische Klinik, Hamburg, Germany; 13Städtisches Klinikum Karlsruhe GmbH, Medizinische Klinik III, Karlsruhe, Germany; 14Universitätsklinik Essen, Klinik für Hämatologie, Essen, Germany; 15Diakonie-Klinikum Schwäbisch Hall, Innere Medizin III, Schwäbisch Hall, Germany; 16Universitätsklinikum Heidelberg, Innere Medizin V, Heidelberg, Germany
Background: Thiotepa-containing high-dose chemotherapy [HDC] followed by ASCT has found special interest in the treatment of primary (PCNSL) or secondary central nervous system (SCNSL) lymphoma for its capacity to penetrate the blood-brain barrier. We initiated a prospective, multicenter, non-interventional study to evaluate thiotepa-based HDC prior ASCT in patients with PCNSL or SCNSL. Primary aims of this study were assessment of safety and efficacy of thiotepa-based high-dose regimens in ASCT for lymphoma.
Methods: Eligible were patients > = 18 years who were assigned to undergo ASCT after HDC with thiotepa 20mg/kg, BCNU 400mg/m² +/- etoposide 450mg/m² (TBE) for PCNSL/SCNSL; or thiotepa 10mg/kg, cytarabine 1600mg/m², etoposide 800mg/m²,melphalan 140mg/m² (TEAM) for non-CNS lymphoma (NHL/HL). Primary endpoints were toxicity and efficacy.
Results: From Oct 2013 to Apr 2017, 80 patients were registered, of whom 66 (43 male/ 23 female, median age 59 years (range: 22–78)) had 12-month follow-up data available and were included in this analysis. TBE and TEAM were used in 38 (58%) and 28 (42%) patients, respectively. Diagnosis was PCNSL in 26 (39%) patients, SCNSL in 12 (18%) patients, and Non-Hodgkin or Hodgkin lymphoma (NHL/HL) in 28 (42%) patients. Main non-hematological grade 3–4 organ toxicities up to day +30 were mucositis, diarrhea, infection, and fever, occurring in 61%, 19%, 46%, and 15%, respectively, of all 66 patients. Hematological recovery for granulocytes (>500/µl) occurred at a median of 10 days, and platelet recovery (>20.000/µl) at a median of 12 days after transplantation. Therapy-related mortality at day +30 was 0 and on day +100 8% corresponding to non-adjusted five fatalities [three sepsis (2x TEAM, 1x TBE), one pneumonia (TBE), one encephalitis (TBE]. On day 100, 59 patients were evaluable for response, here 31 (53%) achieved complete response, 25 (42%) partial response and 3 (5%) patients stable disease. Fourteen (21%) patients experienced relapse/progression, translating into a progression-free survival at one year of 70% (PCNSL: 80%; SCNSL: 58%; NHL/HL: 66%), and an overall survival of 76% (PCNSL: 84%; SCNSL: 67% and NHL/HL: 70%).
Conclusions: Early results of this prospective study suggest that Thiotepa-based high-dose therapy for ASCT for both CNS and non-CNS lymphoma is effective and does not raise safety concerns compared to other HDC regimens commonly used for ASCT of lymphoma.
Conflict of interest: H. Sayer: RIEMSER Pharma GmbH: consultancy, honoraria
Minimal residual disease, tolerance, chimerism and immune reconstitution
O126
Abstract previously published
O127 Prophylactic or preemptive therapy with Sorafenib after allogeneic hematopoietic cell transplantation improves overall survival of FLT3 AML patients
Ali Bazarbachi1,2, Myriam Labopin3,4,5, Giorgia Battipaglia4, Azedine Djabali3,4, Edouard Forcade6, William Arcese7, Gerard Socié8, Didier Blaise9, Jakob Passweg10, Jan J Cornelissen11, Patrice Chevallier12, Johan Maertens13, Nicolaas Schaap14, Khowla Hashaishi3, Jean El Cheikh1, Jordi Esteve3,15, Arnon Nagler3,16, Mohamad Mohty3,5,17
1American University of Beirut Medical Center, Bone Marrow Transplantation Program, Department of Internal Medicine, Beirut, Lebanon; 2American University of Beirut, Department of Cell Biology, Anatomy and Physiological Sciences, Beirut, Lebanon; 3Acute Leukemia Working Party of EBMT, Paris, France; 4Hôpital Saint Antoine, Service d'Hématologie et Thérapie Cellulaire, Hematology Department, Paris, France; 5Hopital Saint Antoine, Universite Pierre & Marie Curie, INSERM, UMRs 938, Paris, France; 6CHU Bordeaux Hôpital Haut-Leveque, Pessac, France; 7Tor Vergata University of Rome, Stem Cell Transplant Unit, Policlinico Universitario Tor Vergata, Rome, Italy; 8Hopital St. Louis, Department.of Hematology - BMT, Paris, France; 9Centre de Recherche en Cancérologie de Marseille, Institut Paoli Calmettes, Programme de Transplantation & Therapie Cellulaire, Marseille, France; 10University Hospital, Hematology, Basel, Switzerland; 11Erasmus MC Cancer Institute, University Medical Center Rotterdam, Department of Hematology, Rotterdam, Netherlands; 12CHU Nantes, Departement d'Hematologie, Nantes, France; 13University Hospital Gasthuisberg, Department of Hematology, Leuven, Belgium; 14Nijmegen Medical Centre, Department of Hematology, Nijmegen, Netherlands; 15Hematology Department, IDIBAPS, Hospital Clinic, Barcelona, Spain; 16Chaim Sheba Medical Center, Department of Bone Marrow Transplantation, Tel-Hashomer, Israel; 17Hematology Department, Hôpital Saint Antoine, Service d'Hématologie et Thérapie Cellulaire, Paris, France
Background: Maintenance therapy with sorafenib after allogeneic HCT (allo-HCT) has shown encouraging results in FLT3 ITD AML. The purpose of this study was to assess the safety and efficacy of sorafenib if given as prophylactic or preemptive treatment for FLT3 mutated AML post allo-HCT.
Methods: We identified 459 adult patients with FLT3 mutated AML (FLT3 ITD = 434; FLT3 TKD = 11; 14 both) allografted between 2012 and 2015 from a matched related (187 patients), matched unrelated (223 patients) or haploidentical donor (49 patients) at EBMT participating centers. The outcome of 28 patients who received post-transplant sorafenib prophylactic (n = 18) or preemptive therapy (n = 9) or both (n = 1) with sorafenib was compared to 431 patients who did not receive sorafenib treatment. Sorafenib treatment was initiated at a median of 55 days post transplant (1–173) at a median dose of 800 (200–800) mg daily. The dose of sorafenib was modified in 12 patients, mainly because of side effects. Median follow-up of alive patients was 39 months (range 1–87)
Results: Patients in the sorafenib group were more recently transplanted (2014 versus 2012), more likely to receive sorafenib during induction (11% versus 1%; p = 0.001), and a myeloablative conditioning (MAC) (75% versus 52%; p = 0.017), but less likely to be MRD negative at the time of allo-HCT (57% versus 79%; p = 0.02). The two groups were comparable in terms of age at transplant, type of FLT3 mutation, patient and donor gender, patient and donor CMV status, karyotype, NPM1 mutation status, median number of induction courses, use of consolidation, disease status at transplant, in vivo T cell depletion, source of stem cells, and rate of acute GVHD. In multivariate Cox analysis with sorafenib administration as time dependent variable, sorafenib significantly reduced relapse incidence (HR = 0.39; p = 0.05), and improved leukemia free survival (LFS; HR = 0.35; p = 0.013), overall survival (OS; HR 0.36; p = 0.03) and GVHD relapse free survival (GRFS; HR = 0.44; p = 0.023), but did not significantly affect non relapse mortality (NRM). Finally, pair matched analysis was performed on 26 patients in the sorafenib group and 26 controls who engrafted and with survival without relapse and without acute GVHD grade II-IV at least equal or superior to time to sorafenib initiation. Matching factors also included conditioning (reduced intensity versus MAC), status at transplant (CR1 versus CR2 versus active disease), NPM1 mutation status, and age at transplant. Two year LFS and OS were 79% and 83% for patients in the sorafenib group versus 54% and 62% for controls (p = 0.002 and 0.007 respectively). Prophylactic or preemptive sorafenib significantly reduced RI (HR = 0.38; p = 0.046) and improved LFS (HR = 0.37; p = 0.024), and OS (HR = 0.32; p = 0.007), without affecting NRM.
Conclusions: Post transplant prophylactic or preemptive sorafenib is a safe and effective therapy for patients with FLT3 mutated AML significantly improving LFS and OS. Based on these results Sorafenib may be considered as standard of care in that setting.
Conflict of interest: nothing to disclose
Arnon Nagler and Mohamad Mohty are equal contributors.
Multiple myeloma
O128 The outcome of Haploidentical Transplantation in Patients with Relapsed Multiple Myeloma. An EBMT/CIBMTR Report
Firoozeh Sahebi1, Laurent Garderet2, Abraham Kanate3, Diderik-Jan Eikema4, Nina Simone Knelange5, Omar F Dávila Alvelo6, Yener Koc7, Didier Blaise8, Qaiser Bashir9, José M Moraleda10, Peter Dreger11, Stefan Ciurea12, Harry Schouten13, Nirav Shah14, Mareike Verbeek15, Wolf Rösler16, Jose L Diez Martin17, Stefan Schoenland11, Anita D'Souza14, Nicolaus Kröger18, Parameswaran Hari14
1City of Hope Medical Center/Southern California Kaiser Permanente Medical Center, Duarte, CA, United States; 2Hospital Saint Antoine, Paris, France; 3Osborn Hematopoietic Malignancy and Transplantation Program, Parkersburg, WV, United States; 4EBMT Statistical Unit, Leiden, Netherlands; 5EBMT Data Office, Leiden, Netherlands; 6CIBMTR Milwaukee Campus, Milwaukee, WI, United States; 7Medical Park Hospitals, Antalya, Turkey; 8Centre de Recherche en Cancérologie de Marseille, Marseille, France; 9University of Texas MD Anderson Cancer Center, Houston, TX, United States; 10Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain; 11University of Heidelberg, Heidelberg, Germany; 12The University of Texas MD Anderson Cancer Center, Houston, TX, United States; 13University Hospital Maastricht, Maastricht, Netherlands; 14Medical College of Wisconsin, Milwaukee, WI, United States; 15Klinikum Rechts der Isar, Munich, Germany; 16University Hospital Erlangen, Erlangen, Germany; 17Hospital Gregorio Marañón, Madrid, Spain; 18University Hospital, Eppendorf, Germany
Background: Allogeneic stem cell transplantation has the potential for long-term disease control in a subset of high-risk multiple myeloma (MM) patients (pts). The limited number of matched donors, easy availability of haploidentical donors, and early encouraging results with lower non relapse mortality (NRM) have expanded haploidentical transplantation as a potentially curative treatment in a variety of hematologic malignancies. Data on the use of haploidentical transplant in MM are limited.
Methods: We conducted a retrospective analysis to examine the outcome of patients with MM who underwent haploidentical stem cell transplantation within EBMT/CIBMTR centers.
Results: A total of 96 pts underwent haploidentical transplant between 2008–2016. Median age 54.9 y/o (36.6–73.3), gender M/F 63/33, ISS stage I-II 43 (44.8%) and III 37 (38.5%), subtype IgG 41 (42.7%), IgA 15 (15.%), light chain 34 (35.4%), others 4 (4,2%) and missing 2(2.1%). Sixty six pts (68.8%) had prior autologous transplant and 30 pts (31.2%) had >1 prior autologous transplant. Disease status: CR/sCR/VGPR 36 (37.5%), PR 30 (31.2%), SD 13 (13.5%) and PD/relapse 17 (17.7%). Time from diagnosis >24 months (mo.) in 79 pts (82%), 18–24 mo. 8 (8.3%) and < 24 mo. in 9 pts (9.4%). Recipient/donor CMV status: both seronegative 13 (13.5%), recipient-/donor+ 6.2 (8.3%), recipient+/donor- 8 (8.3%) and both seropositive 39 (40.6%), missing data in 30. Recipient/donor gender match: M/M 31 (32.3%), M/F donor 31 (32.3%), F/M donor 17 (17.7%) and both females 16 (16.7%). Bone marrow used in 33 pts (34.4%) and peripheral blood in 62 (64.6%). Conditioning: myloablative 18 (18.7%) with total body irradiation (TBI) 5 pts (5.2%) and non TBI 13 (13.5%), reduced intensity withTBI used in 52 pts (54.2%) and without TBI in 25 (26%), and missing 1 pt. GVHD prophylaxis: post-transplant cyclophosphamide (post-Cy) in 73 (76%) and no post-Cy 17 (17.7%). Forty (41.7%) had ATG/Campath, 11 (11.5%) had ATG alone, 1 Campath and 40 pts (41.7%) neither.
With a median follow up of 19.9 mo (9.3–39.1), overall survival (OS) was 48% (36–59%) at 2 years with cumulative risk of relapse 56% (45–67) and NRM of 26% (17–36) at 2 yrs. Incidence of cGVHD 45% (33–57). By univariate analysis, the use of ATG/Campath (p 0.001) and TBI + Cy-based regimens (p < 0.001) were associated with higher relapse rates. Use of ATG (p 0.01) and TBI + other conditioning (p 0.01) were associated with inferior OS, and use of ATG (p 0.012), or TBI based regimens (p 0.005) were associated with higher NRM. CMV status had no impact on NRM or GVHD.
Conclusions: Haploidentical transplantation is feasible for pts with multiply relapsed or high-risk MM, with encouraging 2 yr overall survival of 48% and NRM 26%. These results support further investigation of haploidentical transplantation with post-CY in high-risk MM.
Conflict of interest: The authors declare no potential conflict of interest.
O129 Improved survival after allogeneic stem cell transplantation for light chain amyloidosis: a retrospective analysis in 55 patients of the Chronic Malignancy Working Party (EBMT)
Stefan Schönland1, Simona Iacobelli2, Jennifer Hoek3, Ute Hegenbart1, Tobias Dittrich1, Eefke Petersen4, Olivier Hermine5, Stephanie Nguyen-Quoc6, Dietger Niederwieser7, M.R. de Groot8, Ellen Meijer9, Christine Wolschke10, Jean Henri Bourhis11, Jacob Passweg12, Monserrat Rovira13, Xavier Poire14, Francesca Bonifazi15, Andy Peniket16, Pierre Zachee17, M.R. Schipperus18, Matthias Stelljes19, Nigel Russell20, Giorgio La Nasa21, Pascal Turlure22, Laurent Garderet23, Nicolaus Kröger10
1University and University Hospital of Heidelberg, Heidelberg, Germany; 2Tor Vergata University, Rome, Italy; 3EBMT Data Office, Leiden, Netherlands; 4University Medical Center Utrecht, Utrecht, Netherlands; 5Hopital Necker, Paris, France; 6Hopital la Pitié-Salpêtrière, Paris, France; 7University Hospital Leipzig, Leipzig, Germany; 8University Medical Center Groningen (UMCG), Groningen, Netherlands; 9VU University Medical Center Amsterdam, Amsterdam, Netherlands; 10University Hospital Eppendorf, Hamburg, Germany; 11Gustave Roussy, Villejuif, France; 12University and University Hospital of Basel, Basel, Switzerland; 13Hospital Clínic Barcelona, Barcelona, Spain; 14Clinique Universitaires St. Luc, Bruxelles, Belgium; 15S. Orsola-Malpighi University Hospital, University of Bologna, Bologna, Italy; 16Churchill Hospital, Oxford, United Kingdom; 17ZNA, Antwerpen, Belgium; 18Haga Hospital, The Hague, Netherlands; 19University of Muenster, Department of Medicine / Hematology and Oncology, Münster, Germany; 20Nottingham University Hospital, Nottingham, United Kingdom; 21Centro Trapianti di Modollo Osseo, Cagliari, Italy; 22CHRU Limoges, Limoges, France; 23Hospital Saint Antoine, Paris, France
Background: Systemic light chain amyloidosis (AL) is a rare protein deposition disorder which, in most cases, is caused by a monoclonal plasma cell disorder with a poor prognosis if the heart is severely affected. In a small retrospective EBMT analysis allogeneic stem cell transplantation (allo-SCT) has emerged as an effective treatment but was associated with high mortality (Schönland et al., Blood 2006) especially after myeloablative conditioning. In 2006 our working party has initiated a non-interventional clinical study (NIS) about allo-SCT in AL.
Methods: We performed a retrospective analysis within ProMISe including all patients with AL treated with allo-SCT. Primary endpoint was overall survival (OS) and secondary endpoints were engraftment, acute/chronic Graft-versus-host-disease (a/cGvHD), hematologic remission and relapse. We identified 55 patients (14 from our NIS, 19 from our published cohort and 22 additional patients) transplanted 1987–2015. More than 10 allo-SCTs were performed in Germany (17), Netherlands (12) and France (11). 29 patients were male. Dominant amyloid organ involvement was kidney, heart and soft-tissue. Median interval from first diagnosis to allo-SCT was 21 months. Allo-SCT was the second transplant in 30 patients (22 as first and only 3 patients as third transplant). Peripheral blood was the stem cell source in 42 patients, BM was used in 12 and CB in 1. Conditioning regimen was considered myeloablative in 12 and included TBI in 32 with a median total dose of 2 Gy [2,12], Fludarabine (29), Melphalan (11), Cyclophosphamide (9) and Busulfan (6). HLA matching was syngeneic (7), matched relative (34), mismatched relative (2), matched unrelated (11) and mismatched unrelated (1). Eleven patients received ATG and 3 Campath. Median Karnofsky Index was 90% [50,100], age 51 years [30,67] and serum creatinine 91 µmol/l [52,902].
Results: Median interval between allo-SCT and neutrophils>0.5 was 16 [6,29] and for platelets>50 15 days [1,76]. aGvHD grade 2–4 occurred in 11 patients with a median interval of 1.1 months from allo-SCT [0.5,3.2]. cGvHD was reported in 17 patients (extensive 12,limited 5) with a median interval of 6.2 months [3.2,50]. Best response after allo-SCT was CR in 28 patients with a median interval of 5.7 months from allo-SCT. Relapse was diagnosed in 17 patients with a median interval of 8.1 months [0.9,155]. Median follow-up was 73 months [0,286]. Median OS was 44 months, survival at 1-year was 68% (CI 57%,82%) and at 10-years 44% (CI 32%,61%). At last follow up, 25/55 patients were alive (4/7 syngeneic with OS plateau at 57%) and 16 in CR. When we split all transplants before and after 2006, OS was significantly better for the recent group (median OS not reached vs. 21 months, p = 0.006) with a 63% plateau beginning at 70 months.
Conclusions: This is the largest cohort of AL patients treated with allo-SCT. Allo-SCT was feasible and effective in selected patients in the last 10 years. In opposite to our previous retrospective analysis, we observed a low mortality using mostly RIC. Our updated results revealed a rewarding long-term survival. Therefore, allo-SCT with an HLA-identical donor might be considered in heavily pretreated patients.
Conflict of interest: All authors declare no conflicts of interest.
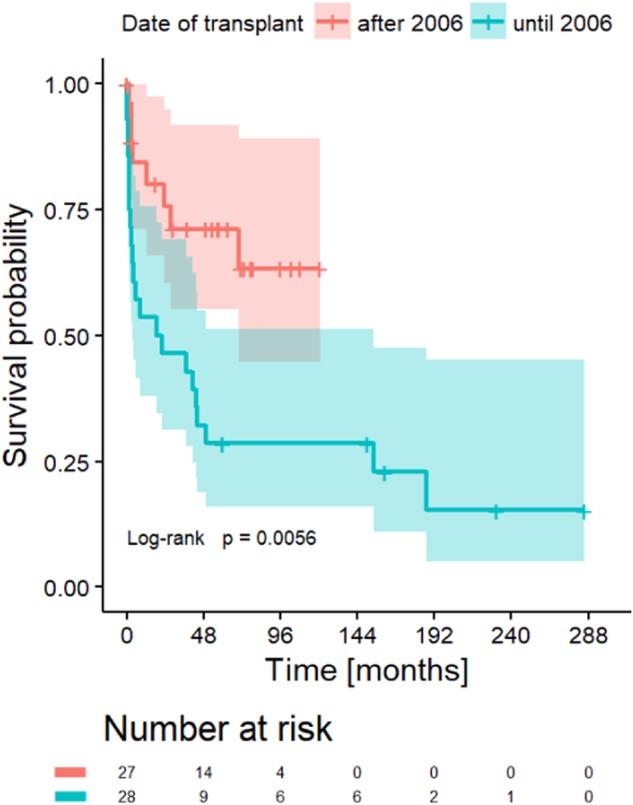
[ [O129 Figure] figure]
O130 European Myeloma Network phase I trial on RIC allogeneic transplantation: an optimized program for high risk relapsed myeloma patients
C Calderón-Cabrera1, T Caballero-Velázquez1, L López-Corral2, MV Mateos2, FJ Márquez-Malaver1, M Cabrero2, J Martín Sánchez1, E Pérez2, ML Martino Galiana1, I Espigado Tocino1, AA Martín2, Jose Antonio Pérez-Simón1
1Hospital Universitario Virgen del Rocío / Instituto de Biomedicina de Sevilla / CSIC / Universidad de Sevilla, Hematology, Sevilla, Spain; 2Hospital Clínico Universitario de Salamanca, Hematology, Salamanca, Spain
Background: The use of reduced intensity conditioning (RIC) has decreased the non-relapse mortality (NRM) in patients diagnosed with multiple myeloma (MM), but with a higher risk of relapse than myeloablative. Graft versus host disease (GVHD) is the main cause of morbidity and mortality after transplantation. According to preclinical studies, the combination of sirolimus (Siro) plus bortezomib (Bz) has a synergistic anti-myeloma effect and could also be effective for GVHD prophylaxis. We proposed the intensification of the RIC by adding Bz to increase the antitumor efficacy of the conditioning regimen and the use of Siro/Bz/tacrolimus (Tk) as prophylaxis of GVHD.
Methods: Patients received RIC based on fludarabine and melphalan (FluMel) plus Bz (days -9 and -2). GVHD prophylaxis consisted on Bz 1.3mg/m2 (days +1,+4,+7), Siro from day -5 and Tk from -3(except the first 5 patients that did not receive Tk). Security was evaluated in terms of graft failure, neuropathy and gastrointestinal toxicity attributed to Bz. Efficacy was evaluated in terms of incidence of acute GVHD. Studies of dendritic cells and T, B and NK cells were performed at +100, +180, +270 and +365.
Results: Twenty-five patients were included, 23 MM and 2 plasma cell leukemia. Median of previous treatment lines was 3(1–8) and 8/25 had received 5 or more. Twenty-four (96%) underwent prior autologous transplantation. Twelve patients presented poor prognosis cytogenetics at diagnosis. Only 7 underwent transplantation in complete remission (CR).
No serious adverse events related to medication were reported. With the Siro/Bz/Tk combination only two patients had grade 3 acute GVHD for a cumulative incidence of grades 2–4 of 43% and 17% for chronic GVHD. Of the 21 patients analyzed at day +100, 14(67%) were in CR, 1(4%) in VGPR and 4(19%) in partial response. NRM at 2 years was 16% whereas relapse-related mortality was 13.5%. Median overall survival (OS) was 30 months and event free survival 16 months. OS at 3 years was 60%. In comparison to a control group, significant differences were found in % of plasmacytoid dendritic cells and B lymphocytes, inversion of CD4/CD8 ratio and naive/memory/effector distribution.
Conclusions: The addition of Bz to the conditioning regimen with FluMel is safe and effective, reaching 71% CR/VGPR at day +100 in very high risk patients; just 2 patients developed grade 3 GVHD with the triple combination Siro/Bz/Tk. This is the first trial that evaluates the addition of Bz to both the conditioning regimen and the GVHD prophylaxis in patients with MM.
Clinical Trial Registry: EudraCT number: 2010-018594-37
In collaboration with the CLWP of the EBMT and the GETH. CIBERON code C CB16/12/00480
Conflict of interest: Nothing to disclose
| N (%) | |
|---|---|
| Pretransplant disease status: CR/VGPR/PR/SD/Progression | 7/14/1/1/2 |
| Donor: MRD/MURD/MMURD | 19/5/1 |
| Source of stem cell (BM/PB) | 2/23 |
| Donor age (median, range) | 49 (19–77) |
| Donor sex (male/female) | 13/12 |
| Nº infused cells (median, range) | 5.8 (2.5–8.7) |
| Graft - Neutrophils (yes/no) - Platelets (yes/no) | 25/0 23/2 |
| Early toxicity (≤100 days): Mucositis (1–3) / Gastrointestinal (1–3) / TMA (1–2) / CNS (neuropathy 1–2) | 8/12/2/5 |
| Acute GVHD (yes/no); chronic GVHD (yes/no) | 19/6 ; 5/19 |
[ [O130 Table] Table 1. Transplant-related data]
O131
Abstract previously published
O132 Clinical Benefit After Galinpepimut-S (GPS), a WT1 Immunotherapeutic, Correlates With Antigen-Specific Immune Responses in High-Risk Multiple Myeloma: Complete Analysis of the Ph2 GPS Maintenance Study
Guenther Koehne1, Sean Devlin2, Neha Korde3, Sham Mailankody3, Heather Landau3, David Chung4, Sergio Giralt4, Nicholas Sarlis5, Ola Landgren3
1Miami Cancer Institute, Bone Marrow Transplantation and Hematologic Oncology, Miami, FL, United States; 2Memorial Sloan Kettering Cancer Center, Dept. of Epidemiology & Biostatistics, New York, NY, United States; 3Memorial Sloan Kettering Cancer Center, Myeloma Service, New York, NY, United States; 4Memorial Sloan Kettering Cancer Center, Bone Marrow Transplant Service, New York, NY, United States; 5Sellas Life Sciences Group, Ltd., New York, NY, United States
Background: Multiple myeloma [MM] patients [pts] with high-risk [HR] cytogenetics [CG] who also remain positive for minimal residual disease [MRD+] post-frontline autologous stem cell transplantation [ASCT] present with poor clinical outcomes, despite immunomodulatory drug maintenance. In a previous report, such pts immunized with the Wilms tumor-1 protein [WT1]-targeting heteroclitic peptide mixture galinpepimut-S [GPS] in a Phase 2 study experienced a median progression-free survival [mPFS] of 23.6 months [mo] and an 18-mo overall survival [OS] of 88%; therapy was well tolerated. We hereby present a correlative analysis between clinical benefit [CB] (complete response/very good partial response [CR/VGPR] per IWM) and antigen-specific immuno-responses [IRs] using the complete dataset from this study.
Methods: All pts (n = 18; median age 61.6 years - range 46–72) were positive for WT1 by immunohistochemistry at diagnosis (>5% of bone marrow plasmacytes). GPS was administered with the oil emulsifier montanide s.c. and low-dose GM-CSF starting 2 weeks [wks] post-ASCT and q2wks thereafter (x 6 doses) followed by boosters q4wks (x 6 additional doses). All pts received lenalidomide (10 mg daily) starting 3 months post-ASCT. GPS consisted of 4 peptides, 2 of the which were mutated (heteroclitic; *): WT1A-1*; 427L [long]; 331L, and 122A1L*, to induce stronger HLA-binding/reduce tolerance. WT1-specific IRs were assessed by intracellular IFN-γ analyses (baseline, post-6 & -12 GPS doses), using PBMC's pulsed with: each of the 4 WT1 peptides in GPS; or, the 2 native counterparts (to the 2 heteroclitic ones); or, a 'total pool' of 113 overlapping 15mers along the full-length WT1.
Results: Highly specific, time-dependent and robust (CD4 and/or CD8) IRs against the 4 WT1 peptides within GPS, as well as the 2 native counterpart peptides, were confirmed in up to 91% of pts across HLA allele types, whereas multifunctional cross-epitope T-cell reactivity, a hallmark of an effective, cytotoxicity-inducing vaccine, was corroborated. In those pts who received all 12 doses of GPS (n = 12), there was a consistent and bidirectional relationship between CB and frequency of CD4/CD8 IRs. Indeed, pts with CB exhibited positive IR rates of 82% to 100%, whereas pts with positive IRs achieved CB at rates of 57–63%. The strongest CB-IR association was found for the 2 heteroclitic peptides (122A1L and WT1A-1); this was mostly 'driven' by high potency, long-term CD4 responses, although several pts with CB also maintained a low level of CD8 IRs. Multivalent IRs were observed in 64% of treated pts, were durable and correlated with higher rates of long-term CB.
Conclusions: These results suggest an immunobiological basis for both a prolonged mPFS and high rate of sustained CB (achievement of CR/VGPR) after GPS (when administered post-ASCT), and mechanistically support a role of key WT1 peptide antigens within the GPS mixture, capable of driving multivalent responses and leading to both long-term CD4 activation and cross-epitopic reactivity. These data offer a unique link between clinical and immune responses, which has not been previously described for a peptide vaccine in MM, and justify further exploration of potential anti-myeloma activity of GPS in this setting in a larger randomized clinical study.
Clinical Trial Registry: NCT01827137; https://clinicaltrials.gov/ct2/show/NCT01827137
Conflict of interest:
Support sources: Funding for this study was provided by Leo A. Guthart and Kathryn Medina Research Fund in Multiple Myeloma, and Sellas Life Sciences Group, Ltd.
Disclosures of potential COI: Nicholas Sarlis: Employment by Sellas Life Sciences Group, Ltd.;
Guenther Koehne: Scientific Adviser for and recipient of research funding from Sellas Life Sciences Group, Ltd. All other authors have nothing to disclose.
O133S emi-ambulatory autologous stem cell transplant for multiple myeloma: a monocentric experience over a period of 17 years - feasibility and medico-economic aspects
Marie-Pierre Ledoux, Nathalie Chevallier, Karin Bilger, Annegret Laplace, Blandine Guffroy, Elise Toussaint, Cécile Fohrer-Sonntag, Luc-Matthieu Fornecker, Bruno Lioure
Hôpitaux Universitaires de Strasbourg, Hematology, Strasbourg, France
Background: Notwithstanding the recent advances in target therapies, the use of high dose melphalan (HDM) followed by autologous stem cell transplantation (ASCT) remains a critical step of the gold standard treatment for fit patients diagnosed with multiple myeloma, the second most common hematological cancer. HDM followed by ASCT is associated with nausea, mucositis, aplasia and its complications and therefore usually consists in a prolonged hospitalization, resulting in a considerable burden both for patients and institutions.
Methods: We report the short-term outcome of patients treated in our institution between 1999 and 2017 with a semi-ambulatory ASCT with early discharge at day 0: patients were hospitalized in a conventional hematology unit from day -3 to day 0, receiving HDM on day -2 (after a premedication by amifostin from 2002 on), and being discharged on the day of the ASCT after an injection of PEGfilgrastim and an erythropoietin analog. Patients were then under prophylaxis by valaciclovir, levofloxacin, fluconazole and aerosolized pentamidine and followed-up in day hospital at days 5, 7, 10, 12 and 14, in a specialized bone marrow transplant recipients day care unit, where transfusions were performed as needed.
Results: During 17 years, 429 patients were treated with semi-ambulatory early-discharge ASCT at our institution, with ages comprised between 27 and 71 years (median 56,3). Patients with significant comorbidities, such as dialyze-requiring renal failure or mental retardation, or with social frailty, were excluded from this management strategy. The mean aplasia output occurred at day 10. With the support of drugs such as amifostin, ondansetron and aprepitant, the rate of grade 3–4 mucositis and grade 3–4 nausea improved over years to less than 20% and 10% respectively. The rate of re-hospitalization was 34%, mainly from day 7 to day 12. Main cause for re-hospitalization was febrile neutropenia. No death related to early discharge among the 429 patients.
As calculated with French economic data, this strategy cost 48,6% less than a standard 15-day hospitalization for ASCT, and remained more cost-effective in case of a re-hospitalization. Moreover, this strategy enabled our institution to perform more ASCT in a constraint setting.
Conclusions: The semi-ambulatory management of ASCT for multiple myeloma with early discharge at day 0 is feasible and safe with a day-hospital dedicated to bone marrow transplant patients. By enabling a shorter hospitalization, it increases the capacity of the institution for treatment of myeloma patients. Although one can assume it is more convenient for patients not to have a prolonged stay in hospital, the quality-of-life associated to this strategy remains to be assessed.
Conflict of interest: None of the authors has anything to disclose.
O134 Outcome of Multiple Myeloma Patients Following Allogeneic Transplantation: A Single Center Experience
Christine Greil1, Monika Engelhardt1, Hartmut Bertz1, Reinhard Marks1, Robert Zeiser1, Hermann Einsele2, Jürgen Finke1, Ralph Wäsch1
1University Medical Center, Hematology/Oncology, Freiburg, Germany; 2Würzburg University Medical Center, Würzburg, Germany
Background: Despite a remarkable increase of effective treatment options, multiple myeloma (MM) still remains mostly incurable. Especially in subgroups with high-risk cytogenetics and higher ISS stage, the outcome is poor with an overall survival (OS) of 24 to 36 months. Allogeneic stem cell transplantation (allo-SCT) offers a potentially curative option but it is discussed controversially because of considerable treatment related mortality and the risk of graft-versus-host disease (GvHD). The few available studies indicate that allo-SCT can individually be considered for younger patients with good performance status and high-risk disease in the initial course of therapy or in the first chemotherapy-sensitive relapse with the potential to achieve a better long-term survival, but should primarily be restricted to clinical trials. Moreover, the current development of effective immunotherapy approaches, including antibodies and CAR-T cells, have re-introduced a larger interest into allo-SCT
Methods: We retrospectively analyzed the outcome of 109 consecutive patients diagnosed with MM who received allo-SCT at the Freiburg University Medical Center between 2000 und 2015 with regard to treatment response, survival and adverse reactions.
Results: The median age in our cohort was 56 years with equal numbers of men and women. 54% of these patients showed high-risk cytogenetics. 35% were treated in terms of individual salvage attempts in relapsed or refractory situations after extensive pre-treatment, 92% received prior auto-SCT. 37% were included in preemptive settings within different clinical trials of the German Myeloma Study Group (DSMM). 51% of allo-SCTs were conducted with a HLA-matched unrelated donor, 8% with a HLA-mismatched unrelated and 41% with a related donor. Reduced-intensity conditioning regimens were used. We observed a median OS of 40.3 months and a median progression free survival of 16.3 months with a median follow up of 71.5 months. The overall response rate was 72% with 43% achieving a complete remission, 8% a very good partial remission and 20% a partial remission. The non-relapse mortality was low with 13%. Acute GvHD II-IV° occurred in 25%, extensive chronic GvHD in 21%.
Conclusions: Allo-SCT has been conducted at our academic center in heavily pretreated young patients with relapsed/refractory or high-risk disease. We could observe a high response rate with a low treatment-related mortality. Thus, our data suggest that allo-SCT in the context of novel agents and immunotherapy approaches may enable long-term survival and even potential cure in a carefully selected subgroup of fit high-risk patients.
Conflict of interest: The authors declare no conflict of interest.
O135 Impact of cytogenetics on outcome after stem-cell transplantation in multiple myeloma with extramedullary disease: an analysis of the CMWP-EBMT
Nico Gagelmann1, Diderik-Jan Eikema2, Linda Koster3, Denis Caillot4, Pietro Pioltelli5, Juan Bargay Lleonart6, Peter Remenyi7, Didier Blaise8, Nicolaas Schaap9, Marek Trneny10, Jakob Passweg11, Rocio Parody Porras12, Jean Yves Cahn13, Maurizio Musso14, Xavier Poire15, Guido Kobbe16, Maija Itälä-Remes17, Vincenzo Pavone18, Loic Fouillard19, Helene Schoemans20, Dominique Bron21, Anastasia Pouli22, Wilfried Schroyens23, Laurent Garderet24, Nicolaus Kröger1
1University Medical Center Hamburg Eppendorf, Hamburg, Germany; 2Ebmt Data Office, Leiden, Netherlands; 3EBMT Data Office, Leiden, Netherlands; 4CHU Dijon, Dijon, France; 5ASST Monza, Monza, Italy; 6Hospital son Llatzer, Palma de Mallorca, Spain; 7St. István & St. Laszlo Hospital, Budapest, Hungary; 8Institut Paoli Calmettes, Marseille, France; 9Radboud University Medical Centre, Nijmegen, Netherlands; 10Charles University Hospital, Prague, Czech Republic; 11University of Basel, Basel, Switzerland; 12ICO - Hospital Duran i Reynals, Barcelona, Spain; 13CHU Grenoble Alpes, Grenoble, France; 14Ospedale La Maddalena, Palermo, Italy; 15Cliniques Universitaires St. Luc, Brussels, Belgium; 16Heinrich Heine University, Düsseldorf, Germany; 17Turku University Hospital, Turku, Finland; 18Hospital C. Panico, Tricase, Italy; 19Grand Hôpital de l`Est Francilien, Meaux, France; 20UZ Leuven, Leuven, Belgium; 21Institut Jules Bordet, Brussels, Belgium; 22St.Savvas Oncology Hospital, Athens, Greece; 23Antwerp University Hospital, Antwerp, Belgium; 24Hopital Saint-Antoine, Paris, France
Background: Extramedullary manifestation of multiple myeloma is associated with poor outcome. Here, we aimed to analyze the impact of cytogenetics on outcome after single-, tandem-autologous (tandem-auto) and autologous/reduced-intensity allogeneic (auto-allo) stem-cell transplantation in newly diagnosed multiple myeloma (NDMM) patients with extramedullary disease (EMD).
Methods: Within the EBMT registry, we identified 488 patients (59% male, 41% female) with available data on extramedullary involvement and cytogenetics at diagnosis who received upfront single-auto (n = 373), tandem-auto (n = 84) or auto-allo (n = 31) between 2003 and 2015. Extramedullary involvement was defined as manifestations resulting from bone lesions (paraskeletal, n = 396) or hematogenous spread into different organs (n = 92). High-risk cytogenetics were defined as presence of at least one of the following abnormalities: del(17p) (n = 66), t(4;14) (n = 83), t(14;16) (n = 10), t(14;20) (n = 6), and abn(1) (n = 25); and was thus detected in 190 (39%) patients. The remaining patients had normal cytogenetics (n = 250) or other (n = 48), including other translocations or deletions, hyper- or hypodiploidy.
Patients receiving auto-allo were younger (median, 49 years) and tended to have more high-risk cytogenetics (52%) versus single-auto (60 years and 37%) and tandem-auto (60 years and 44%; p < 0.001 and p = 0.15). Before transplant, complete remission was achieved by 24% (single-auto), 13% (tandem-auto) and 19% (auto-allo; p = 0.08). Median follow-up was 49.3 months. Primary end points were progression-free survival (PFS) and overall survival (OS) at four years.
Results: In univariate analysis, high-risk cytogenetics showed significantly lower PFS and OS of 28.4% (95% confidence interval, 19.6–37.2) and 48.2% (40.0–56.4) versus 48.5% (41.4–54.8) and 78.0% (72.5–83.5; p < 0.001, respectively). PFS appeared to be better after tandem-auto and auto-allo with 51.5% (39.3–63.7; p = 0.06) and 60.7% (32.7–88.7; p = 0.14) versus 38.3% (32.0–44.6) for single-auto while OS was significantly better for tandem-auto with 77.8% (68.4–87.2) versus single-auto showing 62.1% (56.2–68.0; p = 0.04), and not significant for auto-allo (81.1%, 66.0–96.2) versus single-auto (p = 0.17). Cumulative incidence of relapse and non-relapse mortality at four years was 47.1% and 1.4% for tandem-auto, 37.1% and 8.6% for auto-allo, and 58.8% and 3.0% for single-auto (p = 0.21 and p = 0.14, pooled overall).
In patients with EMD and high-risk cytogenetics, tandem-auto resulted in a significantly improved PFS and OS (p = 0.02 and p = 0.001) in comparison to single-auto while auto-allo showed significantly improved OS versus single-auto (p = 0.05).
In a subgroup analysis, tandem-auto overcame poor prognosis of high-risk versus normal or other cytogenetics in the univariate (PFS at four years: 50.4 % versus 53.6%, p = 0.49; OS: 80.8% versus 80.4%, p = 0.92; Figure 1) as well as in the multivariate analysis in terms of PFS (hazard ratio, 1.17; p = 0.70) and OS (hazard ratio, 0.92; p = 0.90).
Conclusions: High-risk cytogenetic is seen in nearly 40% of NDMM with extramedullary disease and significantly influences PFS and OS. In comparison to single autografting, tandem-autologous transplantation improves survival and overcomes poor prognosis of high-risk cytogenetics.
Conflict of interest: No conflicts of interest related to the abstract.
Myelodysplastic Syndromes
O136 Comparison between Upfront Transplantation and Pretransplant Cytoreductive Treatment in Patients with MDS and secondary AML - a single center analysis of 165 consecutive patients
Thomas Schroeder1, Nadija Wegener1, Michael Lauseker2, Christina Rautenberg1, Kathrin Nachtkamp1, Esther Schuler1, Mustafa Kondakci1, Rainer Haas1, Ulrich Germing1, Guido Kobbe1
1University Hospital Duesseldorf, Hematology, Duesseldorf, Germany; 2Ludwig Maximillian Universität München, München, Germany
Background: In the absence of prospective studies it is still controversial whether cytoreductive treatment either with chemotherapy (CTX) or hypomethylating agents (HMA) before allo-SCT is superior to upfront transplantation without previous therapy.
Methods: To contribute to this debate we retrospectively analysed the outcome of 165 consecutive patients with MDS (n = 132) and sAML (n = 33) with a BM blast count >5% who underwent allo-SCT from a related (29%) or unrelated (71%) donor at our institution between 1999 to 2016 according to their pretransplant strategy.
While 67 pts were directly transplanted (upfront group) including 74% of them receiving a FLAMSA-based sequential conditioning, 98 pts had received cytoreductive treatment prior transplant (induction CTX n = 64, HMA n = 34). At transplant, 59% and 18% of the patients were in remission in the CTX and HMA group respectively. In the upfront group median blast count at transplant was 15% (5–70%)
Results: With a median follow-up of 52 months for surviving pts 4-year overall (OS) and relapse-free survival (RFS) for the entire group were 55% and 39%. The 4-year OS of the upfront group, CTX group and HMA group was 64%, 50% and 45%, respectively (p = 0.204), while RFS was 40%, 41% and 38% (p = 0.995).
Pts who were refractory after induction chemotherapy had a significantly lower 4-year OS (34% vs. 64%, p = 0.035) and RFS (22% vs. 40%, p = 0.015) compared to pts in the upfront group, while the outcome of those pts with CR (OS 60%; RFS 53%) was comparable with the upfront group. Pts not responding to HMA also had lower 4-year OS (42% vs. 64%, p = 0.0718). In patients receiving upfront allo-SCT, no difference in survival was seen with respect to blast count at transplant (>10% vs < 10%). Non-relapse mortality (NRM) did not significantly differ between the different treatment groups.
Of interest, pts in the upfront group had a higher likelihood to respond to HMA salvage therapy in case of relapse (54%) in comparison to relapsing patients in the CTX (5%; p = 0.001) and HMA group (18%; p = 0.0713).
Conclusions: These data suggest that an upfront transplant strategy using a sequential conditioning regimen may be a feasible and promising alternative for patients with MDS and sAML that can be augmented by HMA salvage therapy in case of relapse after allo-SCT.
Conflict of interest: T. Schroeder, G. Kobbe and C. Rautenberg received financial travel support from Celgene GmbH; T. Schroeder and G. Kobbe received lecture fees and research funding from Celgene GmbH; T. Schroeder had an advisory role for Celgene GmbH. T. Schroeder received lecture fees and travel support from Janssen Cilag GmbH.
O137 Transplant-specific EBMT scoring system improves prognostic capability in myelodysplastic syndromes after allogeneic stem-stell transplantation
Nico Gagelmann1, Diderik-Jan Eikema2, Dietrich Beelen3, Matthias Stelljes4, Linda Koster2, Ghulam Mufti5, Dietger Niederwieser6, Gerhard Ehninger7, Henrik Sengeloev8, Arnon Nagler9, Ibrahim Yakoub-Agha10, Ellen Meijer11, Per Ljungman12, Johan Maertens13, Lothar Kanz14, Lucia L Corral15, Arne Brecht16, Charles Craddock17, Jürgen Finke18, Jan J Cornelissen19, Paolo Bernasconi20, Patrice Chevallier21, Jorge Sierra22, Marie Robin23, Nicolaus Kröger1
1University Medical Center Hamburg Eppendorf, Hamburg, Germany; 2EBMT Data Office, Leiden, Netherlands; 3Univeristy Hospital Essen, Essen, Germany; 4University of Münster, Münster, Germany; 5King's College London, London, United Kingdom; 6University of Leipzig, Leipzig, Germany; 7Uniklinikum Dresden, Dresden, Germany; 8Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark; 9Chaim Sheba Medical Center, Tel Aviv, Israel; 10CHU de Lille, INSERM U 995, Lille, France; 11VU University Medical Center, Amsterdam, Netherlands; 12Karolinska institute, Stockholm, Sweden; 13UZ Leuven, Leuven, Belgium; 14University of Tübingen, Tübingen, Germany; 15Hospital Clínic, Salamanca, Spain; 16Helios Kliniken, Wiesbaden, Germany; 17University Hospital of Birmingham, Birmingham, United Kingdom; 18University of Freiburg, Freiburg, Germany; 19Erasmus MC Cancer Institute, University Medical Center, Rotterdam, Netherlands; 20Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; 21CHU Nantes, Nantes, France; 22Hospital Santa Creu i Sant Pau, Barcelona, Spain; 23Hopital St. Louis, Paris, France
Background: Our aim was to investigate recently established systems (from GITMO and CIBMTR) and to optimize the transplant-specific prediction of outcome in patients with myelodysplastic syndromes (MDS) following allogeneic stem-cell transplantation.
Methods: Between 2000 and 2014, we identified 1059 patients with MDS who underwent transplantation from an identical sibling or unrelated donor. On that basis, two sets were created to validate GITMO (n = 480) and CIBMTR (n = 876). The total EBMT cohort was then randomly assigned to a training (n = 662) and validation set (n = 397). Median follow-up in the total cohort was 69 months (95% confidence interval [CI], 62–76). A Cox model detected independent predictors of survival in the training set while the weighted score was tested in the validation set. One point in the scoring system was assigned to hazard ratios (HRs) of 1.25–1.59 while 2 points were assigned to HRs > 1.59. C-statistics and cross-validation (5-fold with 100 iterations) was used for comparison of scores.
Results: First, scores from GITMO and CIBMTR could be validated overall (p = 0.002 and p < 0.001) while within both scores no difference was found between the low and intermediate-risk group (p = 0.20 and p = 0.10). C-indices showed moderate power of 0.555 (95% CI, 0.524–0.586) and 0.558 (95% CI, 0.534–0.582).
Second, a weighted score of 2 was assigned to the following factors associated with survival in the EBMT training set: older age (≥ 50 years), and very poor cytognetics or monosomal karyotype. One point was assigned to unrelated donor, Karnofsky status < 90%, positive cytomegalovirus (CMV) status of the recipient, blood blasts > 1%, and platelet count ≤ 50 x109/L at the time of transplantation. A score consisting of four risk groups was designed: low (score of 0–1), intermediate (score of 2–3), high (score of 4), and very high (score of > 4). In the training set, the HR for death was 1.99 (95% CI, 1.31–3.04; p = 0.001) for the intermediate-risk, 3.01 (95% CI, 1.99–4.56; p < 0.001) for the high-risk, and 5.19 (95% CI, 3.29–8.19; p < 0.001) for the very high-risk group, with reference to the low-risk group. Overall, the score was predictive of OS in the training and validation set (p < 0.001, respectively; Figure 1). The developed score was also predictive of relapse-free survival, non-relapse mortality and relapse (p < 0.001, respectively).
Third, C-statistics showed improvement in prognostic ability being 0.609 (95% CI, 0.588–0.629) versus 0.555 (GITMO) and 0.558 (CIBMTR). Furthermore, by using age as well as blood blasts as continuous variables and by incorporating them into the score, prognositc capability of this personalised system was even higher with C-index being 0.628 (95% CI, 0.616–0.637).
Conclusions: By combining and optimizing GITMO and CIBMTR scores, we could develop an improved EBMT transplant-specific system prognostic of outcome. This optimized system can be readily computed and expanded to a personalised score resulting in an even better prognostic power in MDS patients following stem-cell transplantation.
Conflict of interest: No conflicts of interest in relation to the abstract.
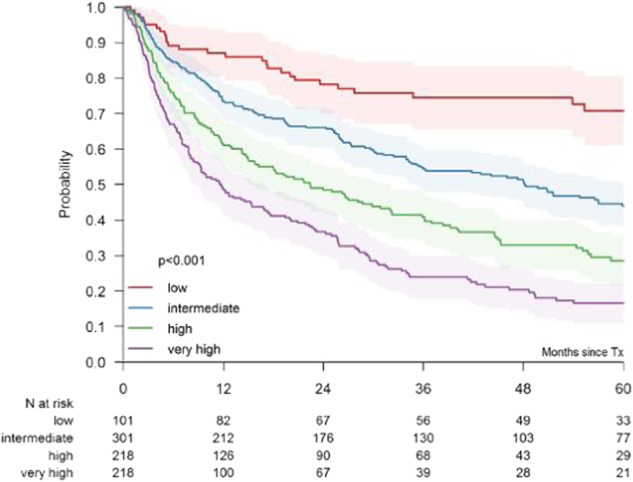
[ [O137 Figure] Figure 1: Overall survival according to risk group of optimized EBMT score]
New drug- and cell-based immune therapies
O138
Abstract previously published
O139
Abstract previously published
O140 CD229 CAR T Cells are an Effective Treatment for Multiple Myeloma
Tim Luetkens, Sabarinath Venniyil Radhakrishnan, Djordje Atanackovic
University of Utah/Huntsman Cancer Institute, Internal Medicine, Salt Lake City, UT, United States
Background: Several chimeric antigen receptor (CAR) T cell approaches have been developed for the treatment of multiple myeloma (MM), and CAR T cells targeting BCMA have shown efficacy in early clinical trials. However, variable expression of BCMA and early relapses in a substantial proportion of patients indicate that additional approaches may be necessary.
Methods: We have generated high-affinity, fully human antibodies against CD229 by antibody phage display and used these to engineer CAR T cells. We performed molecular and cellular screening assays to identify clones with optimal selectivity and activity against CD229-positive MM cells and characterized the efficacy of these cells in vitro and in vivo. In addition, we also assessed phenotype, function, and potential autotargeting by CD229 CAR T cells.
Results: Of the 23 newly generated, fully human CD229-specific antibodies, 15 clones still showed strong surface expression and antigen binding when expressed as CAR constructs. Importantly, none of the clones bound to other SLAM family receptors as determined by surface plasmon resonance. We selected lead candidate 2D3, which had low nanomolar affinity to CD229 and showed high CAR surface expression and stability when expressed as a soluble scFv or scFv-Fc. We engineered primary human CD229 CAR T cells based on 2D3 and compared their in vitro expansion and expression of exhaustion marker PD-1 during production to CD19-specific CAR T cells. We found that CD229 CAR T cells expanded similarly to well established CD19 CAR T cells and showed no sustained upregulation of PD-1 indicating the absence of autotargeting and tonic signaling. In addition, T cell phenotypes mirrored those of CD19 CAR T cells during and after production, further substantiating the absence of selective autotargeting. As CD229 has previously been shown to be expressed on T cells, we performed in vitro cytotoxicity assays using normal T cells and found that, while untreated T cells are susceptible to CD229 CAR T cell killing, T cells previously activated with CD3/CD28 beads were protected explaining the lack of autotargeting during production. We also did not observe any killing of CD34+ hematopoietic stem cells or natural killer cells by CD229 CAR T cells. Importantly, MM cell lines were efficiently and specifically killed by CD229 CAR T cells at very low effector target ratios. In mouse xenograft models, animals treated with 1x106 CD229 CAR T cells showed a significant delay in MM progression compared to animals treated with CAR T cells lacking a binding domain (StopX). In animals treated with 3x106 CAR T cells, we observed complete eradication of MM (Fig. 1). Importantly, we did not observe the emergence of antigen loss variants during treatment with CD229 CAR T cells.
Conclusions: CD229 CAR T cells can be manufactured efficiently, are highly effective against MM in vitro and in vivo, and show limited killing of normal cells. In contrast to other CAR T cell approaches, there is no emergence of antigen loss variants in response to CAR T cell treatment.
Conflict of interest: T. Luetkens, S. V. Radhakrishnan, and D. Atanackovic are inventors on PCT application PCT/US2017/42840 “CD229 CAR T cells for the Treatment of Multiple Myeloma.”
[[O140 Figure].
In vivo efficacy of CD229 CAR T cells against MM.]
O141 Idelalisib-mediated PI3Kδ inhibition for optimized generation of CD19-specific chimeric antigen receptor T cells
Sophia Stock1, Maria-Luisa Schubert1, Jean-Marc Hoffmann1, Lei Wang1, Wenjie Gong1, Brigitte Neuber1, Angela Hueckelhoven1, Ulrike Gern1, Anita Schmitt1, Carsten Mueller-Tidow1,2, Peter Dreger1,2, Michael Schmitt1,2, Leopold Sellner1,2
1Heidelberg University Hospital, Department of Internal Medicine V, Heidelberg, Germany; 2German Cancer Consortium (DKTK), Heidelberg, Germany
Background: Chimeric antigen receptor T (CART) cells are currently among the most promising treatment approaches in cancer immunotherapy. In vivo efficacy and persistence is linked to the proportion of less-differentiated CART cells within the product. The PI3K/AKT/mTOR pathway is one of the main pathways involved in T cell differentiation. In this study, we investigated idelalisib-mediated inhibition of PI3Kδ during CART cell production in order to increase less-differentiated T cell subsets within the final cell product.
Methods: Peripheral blood mononuclear cells (PBMCs) of ten healthy donors (HDs) and six untreated chronic lymphocytic leukemia (CLL) patients were transduced with a CD19-specific 3rd generation (CD19.CAR.CD28.CD137zeta) retroviral CAR vector (kindly provided by Malcolm Brenner, Baylor College of Medicine, Houston, TX, USA). PBMCs were activated with anti-CD3/anti-CD28 antibodies under addition of IL-7/IL-15. Cultivation was performed without or with 1 µM idelalisib over 17 days. Transduction efficiency, immune phenotype and cytokine production were analyzed longitudinally by flow cytometry. Antigen-specific cytotoxicity of CART cells was evaluated via chromium-51 release assay.
Results: Viability of HD-derived CART cells improved significantly with idelalisib (day 14: 94 ± 3% vs 87 ± 9%; p = 0.006). A similar trend was seen in CLL patient samples (day 10: 85 ± 11% vs 78 ± 12%; p = 0.075). Idelalisib did not impair expansion of CART cells. Transduction efficiency of HD (65 ± 13% vs 60 ± 12%; p = 0.001) and CLL patient-derived cells (73 ± 12% vs 67 ± 14%; p = 0.01) was significantly higher with idelalisib on day 14. Idelalisib decreased CD3+/CD4+ cells (day 14: 50 ± 14% vs 71 ± 12%; p = 0.002) and increased CD3+/CD8+ cells (day 14: 45 ± 15% vs 24 ± 11%; p = 0.002) in CLL patient-derived cells. This led to a CD4:CD8 ratio similar to HD-derived CART cell products. This ratio closer to 1:1 is being considered to be most beneficial with regards to cancer eradication. An idelalisib-mediated increase of naïve-like T cells was seen in CD3+ (31 ± 11% vs 23 ± 9%; p < 0.001), CD3+/CD8+ (36 ± 15% vs 27 ± 12%; p < 0.001) and CD3+/CD4+ (25 ± 12% vs 19 ± 10%; p = 0.003) cells of HD-derived cells and in CD3+ (12 ± 9% vs 7 ± 5%; p = 0.06) and CD3+/CD8+ (19 ± 14% vs 8 ± 6%; p = 0.04) cells of CLL patient-derived cells on day 14. Idelalisib significantly reduced expression of the exhaustion markers Tim-3 and PD-1 in CART cells from HDs (66 ± 10% vs 82 ± 5%; p = 0.003 and 4 ± 3% vs 6 ± 3%; p = 0.003) and CLL patients (62 ± 11% vs 72 ± 17%; p = 0.045 and 30 ± 10% vs 41 ± 14%; p = 0.009) on day 14. Moreover, idelalisib increased the expression of homing marker CD62L in HD-derived CART cells (84 ± 9% vs 70 ± 9%; p < 0.001) on day 14. CART cell production with idelalisib significantly decreased intracellular TNF-α (50 ± 8% vs 64 ± 8%; p < 0.001) and IFN-γ production (35 ± 14% vs 46 ± 14%; p < 0.001) after stimulation with CD19+ Daudi cells. In addition, less cytotoxic lysis was seen in chromium-51 release assay with idelalisib. This effect was reversible after overnight resting of CART cells without idelalisib.
Conclusions: PI3Kδ inhibition during CART cell production with idelalisib generated less-differentiated as well as less exhausted CART cells. In addition, a probably more beneficial ratio of CD4+ to CD8+ T cells can be achieved in CLL patient-derived CART cells. Identification of the optimal culturing condition of CART cells will further increase the benefit of this promising treatment approach.
Conflict of interest: There are no relevant conflicts of interest to disclose.
O142 Donor lymphocytes depleted of alloreactive T-cells product (ATIR101) can be administered to haploidentical HSCT without causing severe GVHD: Final 2-year follow-up of Phase 2 study
Denis Claude Roy1, Irwin Walker2, Johan Maertens3, Philippe Lewalle4, Eduardo Olavarria5, Dominik Selleslag6, Silvy Lachance1, Halvard Bönig7, Stephan Mielke8
1Hôpital Maisonneuve-Rosemont, University of Montreal, Montreal, Canada; 2Juravinski Hospital and Cancer Centre, Hamilton, Canada; 3University Hospital Gasthuisberg, Leuven, Belgium; 4Institut Jules Bordet-Université Libre de Bruxelles, Brussels, Belgium; 5Hammersmith Hospital, London, United Kingdom; 6AZ Sint-Jan Brugge-Oostende AV, Brugge, Belgium; 7German Red Cross Blood Center and Institute for Transfusion Medicine and Immunohematology, Frankfurt, Germany; 8Julius-Maximilian-University, Würzburg, Germany
Background: Haploidentical hematopoietic stem cell transplantation (HSCT) using T-cell depleted grafts often suffers from delayed immune reconstitution. To overcome this issue, we have developed a donor lymphocyte infusion (DLI) product that can be administered to HSCT patients in order to reduce infectious complications and prevent disease relapse. This cell product (ATIR101) uses ex vivo photodepletion to selectively eliminate donor cells that react to host cells, while preserving resting T-cells with the ability to fight infectious agents and residual tumor cells.
Methods: In this open-label, multicenter Phase II study, 23 patients with AML (70%) and ALL (30%) in complete remission underwent a T-cell depleted (CD34-selection, Miltenyi Biotec) myeloablative haploidentical HSCT. Patients treated had a median age of 41 years (range 21–64), 57% having a "high risk" Disease-Risk Index assessment. All patients were administered ATIR101 (Kiadis Pharma) at a fixed dose of 2x106 CD3+ cells/kg, a median of 28 days (range: 28–73) post-HSCT. No post-translant GVHD immunosuppressive agents were administered. Primary endpoint was transplant-related mortality (TRM) at 6 months and patients were all followed for 2 years post-HSCT. Data were compared to historical control data (CR-AIR-006) from patients having undergone CD34-selected haploidentical HSCT matching the inclusion/exclusion criteria and from the same hospitals. Follow-up period for the control group was of only one year post-transplant.
Results: Administration of ATIR101 was well tolerated with no observed infusion reactions. Only 5 patients post HSCT developed acute GVHD after ATIR administration. Patients had grade I (8.6 %) or grade II (13%) and no patient developed severe grade III-IV acute GVHD. In addition, three patients developed acute GVHD more than a year post-HSCT, shortly after administration of unmanipulated DLI (15–40 days post-DLI). Only one patient developed chronic GVHD (4.3%). TRM at 6 months (primary study endpoint) was 13.0% in the CD34-selected with ATIR, and 37.1% in the CD34-selected without ATIR control group. The hazard ratio (HR) for TRM at 6 months was 0.21 (95% CI 0.05,0.92) with a p-value of 0.03, indicating that ATIR patients had a lower TRM than the No ATIR group. Relapse-related mortality at 2 years was 25%. Nine patients (39.1%) died within the first 12 months, vs 28 patients (80.0%) in the control group of study CR-AIR-006. The HR for overall survival (OS) was 0.32 (95% CI 0.15, 0.71; p = 0.0035), indicating a statistically significant improvement in OS for ATIR101 patients. Overall survival was 61% at 1 year (Fig.1).
Conclusions: Post-HSCT administration of donor lymphocytes through ATIR101 as immunotherapy is safe and effective. This strategy results in an immunosuppressant-free transplant regimen, with low TRM, low acute and chronic GVHD, and improved overall survival over CD34-selected HSCT without ATIR. The ATIR T-cell product may represent an interesting adjunct to promote anti-infection and anti-leukemia activity after ex vivo or in vivo T-cell depleted haploidentical HSCT approaches. A randomized phase III haploidentical HSCT clinical trial (HATCY study, NCT 02999854) comparing T-cell depleted stem cell graft with ATIR versus T-cell replete graft and post-transplant cyclophosphamide has just opened.
Clinical Trial Registry: ClinicalTrials.gov: CR-AIR-007; NCT01794299
Conflict of interest:
DC Roy is author on a patent held by the Université de Montréal and has received travel awards and research funding from Kiadis Pharma.
The other authors have nothing to disclose.
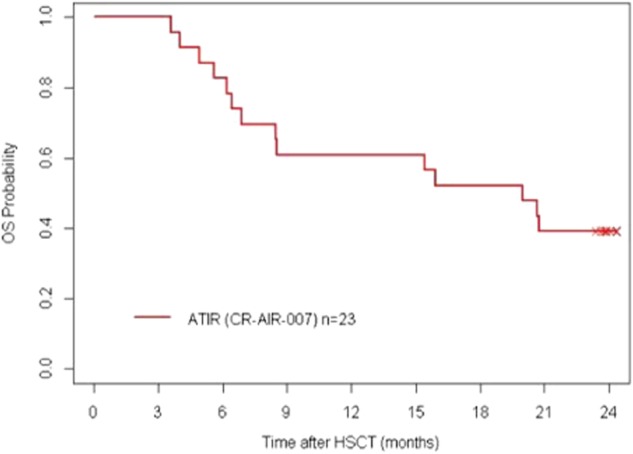
[ [O142 Figure] Figure 1: Overall survival for CD34-selected HSCT + ATIR101]
O143 Trends and Opportunities with CAR-T: a survey on CAR-T clinical trial activity world-wide and in the EU
Vasco Gonçalves, Hermann Einsele, Michael Hudecek
Universitätsklinikum Würzburg, Medizinische Klinik und Poliklinik II, Würzburg, Germany
Background: Immunotherapy with chimeric antigen receptor (CAR)-modified T cells is a breakthrough technology to achieve durable complete remissions in hematologic malignancies. Here, we provide a survey on the CAR-T clinical trial landscape in the EU and on a global scale and examine trends in parameters including indication, target antigen choice and innovation with next-generation CAR technologies.
Methods: Aggregated data was generated from ClinicalTrials.gov (search terms: Chimeric Antigen Receptor + CAR T cell).
Results: As of December 17th 2017, >300 CAR-T clinical trials were registered world-wide representing a steady increase from the 200 and 119 trials registered at by 2016 and 2015, respectively. A total of 213 trials are actively recruiting patients at centers concentrated in the US (n = 73) and China (n = 123), while active trials in the EU approximate 10% (n = 27) of the global effort (considering multicenter trials), with the largest number of trials open in the UK (n = 9).
The focus of clinical trial activities remains with hematology indications and targeting CD19, particularly in Lymphoma (n = 79 active trials), followed by ALL (n = 61), and CLL (n = 40). A considerable number of studies is designed as basket trials that allow inclusion of several entities that express CD19. Of note is the increase in “hit fast, hit hard” combinatorial targeting approaches in response to reports of CD19low/CD19- relapses following CD19 CAR-T therapy: CD20 and CD22 are not only being pursued as individual targets alternative to CD19 (n = 11 trials) but also for simultaneous targeting with bispecific CARs or combination CAR-T products (n = 16 trials in 2017 compared to n = 3 in 2016).
Also worth noticing is the virtual doubling of CAR-T trials in multiple myeloma with 12 new trials opened in 2017, making a total of 23 on the global map, 1 of these in the EU. The majority of trials is pursuing B-cell maturation antigen (BCMA, approx. 70% of trials), accompanied by studies targeting CD19, CD38, CD138 or kappa light. Still the runner up in clinical trial activity is AML with n = 8 active trials pursuing CD33 and CD123 as target antigens, unfortunately none of them open in the EU.
Regarding manufacture, the majority of ongoing trials still opt for transforming PBMC mixtures with the CAR-transgene however, a reduced number of studies use advanced protocols to formulate autologous CAR-T products with defined CD8:CD4 ratio and subset composition based on pre-clinical data showing superior safety and efficacy with this strategy (Sommermeyer 2016). Also, several clinical trials implemented virus-free gene-transfer strategies to increase CAR-T safety profiles and reduce manufacturing cost (Monjezi 2017). The use of third-party allogeneic CAR-T is pursued in n = 2 trials.
Conclusions: There is a continuous and steady increase in CAR-T clinical trial activity, both globally and in the EU. Novel clinical CAR-T indications include myeloma and AML, and there is a trend towards combinatorial antigen targeting to increase efficacy and reduce the risk of relapse. Database surveys provide a means to keep an overview on the increasingly complex CAR-T clinical trial landscape and a means to determine patient access to this innovative treatment.
Conflict of interest: None
O144 Safety and Efficacy of Autologous or Donor-derived CD19 CAR-T Treatment in Relapsed B Acute Lymphocytic Leukemia after Allo-HSCT
Xian Zhang1, Xin-An Lu2, Min Xiong1, Jun-Fang Yang1, Jian-Ping Zhang1, Xiao-Su Zhou1, Ting He2, Pei-Hua Lu1
1Hebei Yanda Ludaopei Hospital, Langfang, China; 2Immuno China Pharmaceutical Co. Ltd, Beijing, China
Background: Although CD19 CAR-T treatment has been approved for treating refractory/relapsed B cell acute lymphocytic leukemia (B-ALL), whether CAR-T is safe and effective for treating post-allogenic hematopoietic stem cell transplantation (allo-HSCT) relapse remains to be investigated.
Methods: Between Dec. 26 2016 and Nov 30, 2017, with a median follow-up of 122 days, eight patients with relapsed B-ALL after allo-HSCT received CD19 CAR-T treatment. 5/8 had prior haploidentical HSCT; 3/8 had matched sibling HSCT. Of the eight patients, 4 had relapsed leukemia < 6 months after allo-HSCT and 6 had prior donor lymphocyte infusion (DLI). The median blast count in bone marrow (BM) before CAR-T treatment was 46.8%. All patients were given conditioning regiment of fludarabine and cyclophosphamide. Six patients had a single infusion of the CAR-T cells ranging from 1x105/kg to 1x106/kg, and 2 received 2 separate CAR-T infusions. CAR transducing efficiency ranged from 29 to 62%.
Results: Seven of 8 patients (87.5%) treated with anti-CD19 CAR-T cells achieved complete remission (CR). Four patients who received their HSCT donor-derived CAR-T cells didn't show significant acute or chronic graft versus host disease (GVHD). Four of eight patients had grade I cytokine release syndrome (CRS), 2 had grade III CRS, and 1 had grade IV CRS.
Conclusions: In conclusion, autologous or donor-derived anti-CD19 CAR-T cells are effective for treating relapsed B-ALL patients after allo-HSCT. The side effects are manageable. CR was achieved even in patients refractory to DLIs. No significant GVHD after CAR-T treatment was observed.
Clinical Trial Registry: Clinical Trials. gov NCT03173417
Conflict of interest: None to declare.
| Age/Sex | 6/M | 10/M | 6/M | 2/F | 26/F | 6/M | 31/F | 14/M |
|---|---|---|---|---|---|---|---|---|
| Post Allo-HSCT Relapse(Months) | 24 | 9 | 29 | 7 | 1 | 2 | 3 | 3.5 |
| Blasts in BM(%) | 68 | 75 | 16 | 69 | 1 | 34.5 | 59 | 7.5 |
| Follow-up Day | 345 | 218 | 167 | 124 | 119 | 99 | 90 | 83 |
| Dose/kg | 1x106 | 1x106 1x105 | 3x105 | 3x105 | 3x105 | 1x105 | 1x105 2x105 | 3x105 |
| CAR-T Cells | Donor | Auto | Auto | Donor | Donor | Donor | Auto | Auto |
| CRS Grade | IV | III | III | I | I | I | II | I |
| GVHD after CAR-T | No | No | No | aGVHD(II) | No | Mild cGVHD | No | No |
| Initial Response | CR | CR | CR | NR | CR | CR | CR | CR |
| Outcome | Relapse on day 167 | 2nd allo-HSCT Remain CR | Relapse on day 98 | Other therapy | Remain CR | Remain CR | MLL-AF4 0.02% on day 75 | Remain CR |
[ [O144 Table] Clinical Characteristics and Treatment of Subjects]
O145 Sorafenib salvage improves overall survival of FLT3 AML patients in relapse after allogeneic hematopoietic cell transplantation: a report of the EBMT acute leukemia Working Party
Ali Bazarbachi1,2, Myriam Labopin3,4,5, Giorgia Battipaglia4, Azedine Djabali3,4, Jakob Passweg6, Gerard Socié7, Edouard Forcade8, Didier Blaise9, Patrice Chevallier10, Sylvie Francois11, Jan J Cornelissen12, William Arcese13, Sylvain Chantepie14, Khowla Hashaishi3, Jean El Cheikh1, Jordi Esteve3,15, Arnon Nagler3,16, Mohamad Mohty3,5,17
1Bone Marrow Transplantation Program, Department of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon; 2American University of Beirut, Department of Cell Biology, Anatomy and Physiological Sciences, Beirut, Lebanon; 3Acute Leukemia Working Party of EBMT, Paris, France; 4Hôpital Saint Antoine, Service d'Hématologie et Thérapie Cellulaire, Hematology Department, Paris, France; 5Hopital Saint Antoine, Universite Pierre & Marie Curie, INSERM, UMRs 938, Paris, France; 6University Hospital, Hematology, Basel, Switzerland; 7Hopital St. Louis, Department of Hematology - BMT, Paris, France; 8CHU Bordeaux Hôpital Haut-leveque, Pessac, France; 9Centre de Recherche en Cancérologie de Marseille, Institut Paoli Calmettes, Programme de Transplantation & Therapie Cellulaire, Marseille, France; 10CHU Nantes, Departement d'Hematologie, Nantes, France; 11CHRU, Service des Maladies du Sang, Angers, France; 12Erasmus MC Cancer Institute, University Medical Center Rotterdam, 12. Department of Hematology, Rotterdam, Netherlands; 13Tor Vergata University of Rome, Stem Cell Transplant Unit, Policlinico Universitario Tor Vergata, Rome, Italy; 14CHU Caen, Institut d'Hématologie de Basse-Normandie, Caen, France; 15IDIBAPS, Hospital Clinic, Hematology Department, Barcelona, Spain; 16Chaim Sheba Medical Center, Department of Bone Marrow Transplantation, Tel Hashomer, Israel; 17Hematology Department, Hôpital Saint Antoine, Service d'Hématologie et Thérapie Cellulaire, Paris, France
Background: Anecdotal reports suggest the efficacy of sorafenib for relapse of FLT3 ITD AML after allogeneic HCT (allo-HCT).
Methods: We identified 158 adult patients with FLT3 mutated AML (156 FLT3 ITD) allografted between 2012 and 2015 from a matched related (MRD) (76 patients), matched unrelated (68 patients) or haploidentical donor (14 patients) at EBMT participating centers and who relapsed or progressed after allo-HCT. Ninety two patients were transplanted in first complete remission (CR1), 18 in CR2, 1 in CR3 and 47 with active disease. Seven patients received pretransplant sorafenib. Conditioning was myeloablative (MAC) in 80 patients and reduced intensity (RIC) in 78 patients. Seven patients received post-transplant sorafenib maintenance. Median time from allo-HCT to relapse was 3 months (range 0.4–58). Thirty four patients received sorafenib as salvage therapy for relapse/progression after allo-HCT (sorafenib group). Sorafenib dose was 800 mg/day in 21 patients; 400 mg /day in 12 patients and 200 mg /day in 1 patient, respectively with duration of 79 days (1–1670). Sorafenib dose was modified in 35% of patients, mostly because of hematological toxicity. These patients were compared with 124 patients who did not receive sorafenib salvage after allo-HCT (no-sorafenib group). Median follow-up after relapse of alive patients was 23 months (range 4–68).
Results: Patients in the sorafenib group were less likely CMV positive but more likely to receive transplant from a MRD, a MAC and post-transplant prophylactic sorafenib (9% versus 0%; p = 0.001). The two groups were comparable in terms of other patients, disease and transplant characteristics. Twenty three patients in the sorafenib group received sorafenib as first treatment for relapse whereas 11 patients received sorafenib after salvage chemotherapy. Conversely, in the no-sorafenib group, first line salvage therapy consisted of chemotherapy in 83 patients. Overall, 33% of patients in the sorafenib group received DLI versus 16% in the no-sorafenib group. A second allo-HCT was performed in 13% and 15% of patients, respectively (NS).
Sorafenib induced complete remission (CR) in 10 (39%) of 26 patients with available data. In multivariate Cox analysis with sorafenib salvage as time dependent variable, sorafenib given for relapse significantly improved OS (HR:0.48; p = 0.006). Similarly, older age, active disease at transplant and use of RIC adversely affected OS. Finally, pair matched analysis was performed on 25 patients in the sorafenib group and 25 controls with survival after relapse at least equal to the delay between relapse and sorafenib administration for each case. Matching factors included time from transplant to relapse, conditioning, status at transplant, and age at transplant. One year and 2 year OS were 41% and 30% for patients in the sorafenib group versus 14% and 14% for controls (p = 0.0015). Comparison using the Cox model confirmed that sorafenib significantly improved OS (HR = 0.36; p = 0.0015) whether given as first treatment for relapse (HR = 0.45; p = 0.011) or when given with or without chemotherapy (HR = 0.45; p = 0.026) compared to controls.
Conclusions: Sorafenib is a safe and effective salvage therapy for patients with FLT3 mutated AML relapsing or progressing after allo-HCT leading to a significant improvement of OS.
Conflict of interest: nothing to disclose
Arnon Nagler and Mohamad Mohty equal contributors
Paediatric issues
O146 The Hematopoietic Cell Transplant Co-Morbidity Index (HCT-CI) predicts overall survival in children with non-malignant diseases treated with allogeneic HCT
Monica Thakar1, Larisa Broglie1, Brent Logan1, Andrew Artz2, David Jacobsohn3, Nancy Bunin4, Lauri Burroughs5, Caridad Martinez6, Adam Nelson7, Ann Woolfrey5, Marcelo Pasquini1, Mohamed Sorror5
1Medical College of Wisconsin, Milwaukee, WI, United States; 2University of Chicago School of Medicine, Chicago, IL, United States; 3Children's National Health System, Washington, DC, United States; 4Children's Hospital of Philadelphia, Philadelphia, PA, United States; 5Fred Hutchinson Cancer Research Center, Seattle, WA, United States; 6Texas Children's Hospital, Houston, TX, United States; 7Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States
Background: The HCT-CI has been shown to predict overall survival (OS) and non-relapse mortality in the adult population, yet evidence on its applicability in children is limited.
Methods: Comorbidities were prospectively scored in children given first allogeneic HCT for non-malignant diseases and reported to CIBMTR between 2007–2014. Primary endpoint was OS. Multivariate Cox regression analysis was performed, adjusting for factors that affected survival, namely age, donor, graft source, recipient CMV status, disease, and performance status. Kaplan-Meier (KM) estimates for survival, stratified by HCT-CI scores, were performed.
Results:
3,195 children ≤20 years of age were identified having immune deficiencies (23%), acquired aplastic anemia (21%), hemoglobinopathies (19%), marrow failure (12%), histiocytic disorders (12%), metabolic diseases (11%), and autoimmune diseases (< 1%). The most frequent comorbidities were infection (8%), mild hepatic disease (7.5%), and moderate pulmonary insufficiency (6%). Overall, patients with HCT-CI scores of 0 (69%), 1–2 (19%), and ≥3 (13%) had 2-year OS of 83%, 83%, and 74%, respectively (p < 0.0001). In multivariate analysis, HCT-CI scores of ≥3 [hazard ratio (HR): 1.61, p < 0.0001] were associated with worse OS compared to scores of 0, while scores of 1–2 were not (HR:1.08, p = 0.459). In subgroup analyses per age groups (Table 1), scores of ≥3 predicted worse OS in children up to age 2 and >2–10 years old. Lack of association between scores ≥3 and survival in patients aged >10–20 years could be partially explained by the unique diagnosis of hemoglobinopathies in most of these patients, who as a group have an OS at 2-years >85%.
Conclusions: This is the largest study to date investigating the impact of comorbidities per the HCT-CI in children. Children up to age 10 years old, who had non-malignant diseases and HCT-CI scores ≥ 3, experience increased risk of death after allogeneic transplantation. Currently, we are studying other measures of health impairments to further refine pediatric risk assessment, and to better understand risks of older children and those with hemoglobinopathies.
Conflict of interest: The authors have nothing to disclose.
| HCT-CI | p value | |||
|---|---|---|---|---|
| 0 | 1–2 | >=3 | ||
| (n) | HR (n) | |||
| 0–2 yrs (n = 906) | 673 | 1.03 (131) | 1.76 (n = 102) | 0.013 |
| 2.01–10 yrs (n = 1319) | 954 | 1.08 (231) | 1.77 (n = 134) | 0.009 |
| 10.01–20 yrs (n = 957) | 559 | 0.89 (228) | 0.95 (n = 170) | 0.825 |
[ [O146 Table] Multivariate analysis of age subgroups]
O147 Stem Cell Transplantation for Patients with Adrenoleukodystrophy in Japan
Koji Kato1, Hiromasa Yabe2, Souichi Adachi3, Mineo Kurokawa4, Nao Yoshida1, Yoshiko Hashii5, Atsushi Sato6, Yoshiko Atsuta7, Tomohiro Morio8
1Japanese Red Cross Nagoya First Hospital, Department of Hematology and Oncology, Children's Medical Center, Nagoya, Japan; 2Tokai University School of Medicine, Isehara, Japan; 3Kyoto University Hospital, Department of Pediatrics, Kyoto, Japan; 4University of Tokyo Hospital, Department of Cell Therapy and Transplantation Medicine, Tokyo, Japan; 5Osaka University Graduate School of Medicine, Department of Pediatrics, osaka, Japan; 6Miyagi Children's Hospital, Hematology and Oncology, Sendai, Japan; 7Japanese Data Center for Hematopoietic Cell Transplantation, Nagoya, Japan; 8Tokyo Medical and Dental University, Department of Pediatrics and Developmental Biology, Tokyo, Japan
Background: Adrenoleukodystrophy (ALD) is an autosomal recessive disorder with progressive neurodegeneration caused by the mutation of ABCD1 gene, and allogeneic stem cell transplantation (SCT) at its early stage is recognized as the only effective treatment modality to stabilize neurological symptoms, even though gene therapy has recently been introduced. We have retrospectively analyzed the transplant outcome of patients with ALD in Japan and tried to clarify the prognostic factors of transplant outcomes based on the TRUMP, database of Japanese Data Center for Hematopoietic Cell Transplantation.
Methods: From 1988 to 2015, 112 male patients have undergone SCT at 1–40years old (median 8) and interval from diagnosis to SCT was 0.7–295 months (median 3.7months). Stem cell source was bone marrow (BM, n = 62, 34 related and 28 unrelated) or cord blood (CB, n = 50, one related and 49 unrelated). Serological HLA disparity was 0/6 (n = 63), 1/6 (n = 36), and 2/6 (n = 10). Conditioning regimen included A: FLU+MEL+low dose TBI (n = 44), B; BU+CY ± others (n = 27), C: MEL+TLI/TAI ± ATG (n = 30), and D: others (n = 11). GVHD prophylaxis was done with MTX+TAC (n = 69), MTX+CSA (n = 30) and others (n = 13). Statistical analysis was done with EZR (Saitama, Japan) and rejection or any death was counted as event.
Results: Sustained engraftment was obtained in 94patients (79.4%) and it was significantly higher in BMT than CBT (90.3% vs 66.0%, P < 0.001). Cumulative incidence of grade II-IV acute GVHD and chronic GVHD was 18.6% and 20.2% respectively. Five-year overall survival (5-yr OS), event-free survival (EFS) and transplant-related mortality (TRM) was 91.1%, 76.0% and 7.9%, with median follow-up of 4.3years, respectively. 5-yr OS according to the conditioning regimen was 100.0% in A, 92.4% in B, 86.0% in C, and 70.7% in D (P = 0.033). 5-yr EFS was 80.6% in A, 81.3% in B, 73.3% in C, and 53.0% in D (P = 0.430), respectively. According to stem cell source, 5-yr OS was 93.1% in BMT and 88.1% in CBT (P = 0.430) and 5-yr EFS was 85.1% in BMT and 65.1% in CBT. TRM was 15.5% before 2005 and it was significantly decreased to 1.9% after 2006 (P = 0.045). 5-yr OS was significantly improved after 2006 than before 2005 (86.0% vs 96.1%, P = 0.035) and it was remarkable after 2006 in CBT (69.8% vs 96.6%, P = 0.005). By multivariate analysis, TBI was identified as the only significant favorable prognostic factor for EFS (HR = 0.420, P = 0.042). Fourteen patients died of ARDS (n = 3), TMA (n = 1), chicken pox (n = 1), interstitial pneumonia (n = 6), and progression of disease (n = 3).
Conclusions: Our results showed that conditioning regimen which includes TBI, even at low dose could provide better transplant outcome and the result of CBT was significantly improved after 2006. Since immediate transplant is essential for symptomatic ALD patients, CBT is suitable for urgent SCT when family donor is not available.
Conflict of interest: I have nothing to disclose.
O148
Abstract previously published
O149 Ongoing recurrent hospitalization, malignancy, and mortality among survivors of childhood hematopoietic stem cell transplantation performed for non-malignant indications
Jason Pole1, Muhammad Ali2, Tony Truong3, Paul Nathan2, Joerg Krueger2, Mark Greenberg2, Maggie Rumantir4, KY Chiang2, Yaron Finkelstein2, Paul Gibson5, Donna Johnston6, Yigal Dror2, Donna Wall2, Tal Schechcter2
1University of Toronto, Pediatric Oncology Group of Ontario, Toronto, Canada; 2Hospital for Sick Children/ University of Toronto, Toronto, Canada; 3Alberta Children's Hospital, Calgary, Canada; 4Hospital for Sick Children, Toronto, Canada; 5Children's Hospital at London Health Sciences Centre, London, Canada; 6Children's Hospital of Eastern Ontario, Ottawa, Canada
Background: Hematopoietic stem cell transplantation (HSCT) has become a standard component of therapy for several pediatric non-malignant indications. As HSCT improves survival and quality of life among those afflicted with these diseases, and supportive care advances diminish the acute toxicities of HSCT, the risk for late complications in survivors is of increasing concern. The burden of late morbidity and mortality after HSCT for a non-malignant indication is not known and potentially under recognized. The frequency of hospitalizations can serve as a proxy measure of severe morbidity but only national / provincial databases can provide accurate information. The province of Ontario has health care utilization data (the Institute for Clinical Evaluative Sciences (ICES)) that can provide information on all hospitalizations, including the development of malignant neoplasms and mortality.
Objectives: To assess ongoing health care utilization as measured by the number and the acuity of hospitalization episodes, late mortality and malignant neoplasms in survivors of pediatric HSCT for a non-malignant indication greater than 2 years post transplant.
Methods: We used record linkage between The Hospital for Sick Children clinical transplant database and provincial health care utilization data housed at ICES. The study population included all survivors of a childhood HSCT for a non-malignant indication between 1992–2014 in Ontario, who had survived more than 2 years from transplant. Hospitalizations, death and malignant neoplasms were captured beginning at 2 years after HSCT (index date) until the end of the follow-up period (Dec 2014) or death.
Results: The cohort consisted of 131 2-year survivors who were followed for a median of 10.6 years from the index date (IQR range: 5.7–15.1). Indications for transplant were inherited bone-marrow failures (BMF) - 14(10.7%); severe aplastic anemia (SAA) - 43(32.8%); and genetic/metabolic diseases and hemoglobinopathies - 74 (56.5%). Of these, 72(55%) underwent a related donor HSCT. At the time of HSCT, 57(43.5%) were 0–4y; 38(29%) were 5–9y; 26(19.8%) were 10–14y; and 10(7.6%) were 15–18y. Eighty-four (63%) patients had at least 1 hospitalization after 2 years post-HSCT (range 1–24 hospitalizations) for a total of 384 hospitalizations. This represents a rate of 0.34 hospitalizations per follow-up year. Mean length of hospital stay was 5.8 days (SD = 11.8). A total of 36 intensive-care unit admissions were documented among 14 patients. The top indications for hospitalization (using ICD major groupings) were infections - 88(23%), orthopedic procedures/fractures - 53(14%), gastrointestinal symptoms 46(12%) and dental procedures 30(8%). Predictive of re-hospitalization were: the diagnosis of inherited BMF (p < 0.01), unrelated-donor transplants (p = 0.028), and history of acute (p = 0.004) or chronic GVHD (p = 0.03). Five patients (4%) developed malignant neoplasms among 124 non-Fanconi anemia patients. In the follow-up period, 11 (8.3%) patients died.
Conclusions: We have identified a high rate of ongoing hospitalization in survivors who underwent HSCT during childhood for a non-malignant indication. A diagnosis of inherited BMF, unrelated donor and any GVHD were associated with increased risk for hospitalization. Our future studies will work on identifying disease- or transplant-related associated causes of late morbidity and mortality and developing mitigation strategies.
Clinical Trial Registry: NA
Conflict of interest: Nothing to disclose
O150 Allogeneic hematopoietic stem cell transplantation in Diamond-Blackfan Anemia: report from the German and French DBA registry
Brigitte Strahm1, Charlotte Niemeyer1, Marcin Wlodarski1, Ina Hainmann2, Friedrich Kapp1, Alexandra Fischer1, Lydie Dacosta3, Regis Peffault de Latour4,5, Thierry Leblanc6, Jean-Hugues Dalle6,7
1Medical Center, University of Freiburg, Faculty of Medicine, Division of Pedaitric Hematology and Oncology, Department of Pediatrics, Freiburg, Germany; 2Pediatric Blood Disorders, Immunodeficiency and Stem Cell Transplantation, Astrid Lindgren Children’s Hospital, Karolinska University HospitalHematology and Oncology, Univerity Childrens Hospital Bonn, Bonn, Germany; 3Robert-Debré Hospital, APHP, Hematology Laboratory, Paris, France; 4Saint-Louis Hospital, APHP, Paris, France; 5University Paris 7-Paris Diderot, Paris, France; 6Robert-Debré Hospital, APHP, Department of Hemato-Immunology, Paris, France; 7Paris 7- Paris Diderot University, Paris, France
Background: Diamond Blackfan anemia (DBA) is a congenital bone marrow failure syndrome caused by mutations in ribosomal genes. Patients usually present with transfusion-dependent anemia in early infancy. A significant proportion of patients has mild neutropenia and may develop additional thrombocytopenia in the course of the disease. Allogeneic stem cell transplantation (HSCT) is a curative treatment for the hematological manifestations. Here we report the outcome following HSCT of patients registered in the German and the French DBA registry.
Methods: Sixty-eight patients (45 males/23 females) were transplanted between 1987 and 2017. Median age at HSCT was 5.2 (range 0.9–16.8) years. In addition to transfusion dependency for red blood cells indications for HSCT were neutropenia or thrombocytopenia(8), steroid intolerance (6) or myelodysplastic syndrome (1). Forty-six patients were transplanted from a matched sibling donor (MSD), while 22 patients were grafted from a matched (13) or mismatched (7 incl. 6 HLA 9/10 and one HLA 8/10) unrelated donor (UD). The recipient-donor HLA match was unknown in 2 UD-HSCT. Stem cell source was bone marrow (56), peripheral blood (5) or cord blood (MSD only) (7). Preparative regimens included bulsulfan/cyclophosphamide (35), other busulfan based regimens (13), a treosulfan based regimen (12) or other regimens (8). All patients transplanted from a UD and 13/46 patients transplanted from a MSD received anti-thymocyte globuline.
Results: Primary engraftment was achieved in all patients. One of two patients with secondary graft failure was rescued with a second allograft from a second MUD whereas one died of infectious complications despite a second allograft from the same sibling donor. Five patients experienced transplant related mortality due to pulmonary toxicity/pneumonitis (4) or systemic adenovirus infection (1). Acute GvHD °II-IV and °III-IV occurred in 16 (23%) and 4(6%) patients, respectively. Seven patients were diagnosed with chronic GvHD being extensive/severe in 3 of them. Overall the probability of overall suvirval (OS) and event free survival (EFS) was 0.91 [0.84–0.98] and 0.89 [0.81–0.97], respectively. While there was no difference according to donor (0.91 [0.83–0.99] for MSD vs 0.85 [0.69–1.00] for UD, p = ns), EFS was superior for patients transplanted at a younger age (0.94 [0.87–1.0] for < 10 yrs vs 0.75 [0.53–0.97] for ≥ 10 yrs, p = 0.04) and after 2000 (0.80 [0.62–0.98] prior to 2000, 0.96 [0.87–1.00] in 2000–2009 and 0.91 [0.79–1.00] after 2010).
Conclusions: In the view of these excellent results for HSCT in patients with DBA including UD-HSCT and the higher risk of transplant related mortality for older patients (≥ 10 yrs) donor availability and indications for HSCT such as transfusion dependency, inefficient chelation, steroid intolerance and additional cytopenias should be carefully evaluated early in the course of disease. International studies are warranted to identify preparative regimens resulting in safe engraftment and a low risk of long-term side effects.
Conflict of interest: Nothing to disclose.
O151 NKregs and CD19+CD21Low B-cells are Prognostic Biomarkers for Chronic and Late Acute GVHD in Children: Applied Biomarkers of Late Effects (ABLE) / PBMTC 1202 Study
Geoff Cuvelier1, Eneida Nemecek2, Justin Whalstrom3, Andrew Harris4, Michael Pulsipher5, Victor Lewis6, Henrique Bittencourt7, Sung Won Choi8, Carrie Kitko9, Emi Caywood10, Monica Bhatia11, Kimberly Kasow12, David Jacobsohn13, Benjamin Oshrine14, Albert Kheradpour15, Sonali Chaudhury16, Joseph Chewning17, Tal Schechter18, Allyson Flower19, Donald Coulter20, Michael Joyce21, Sureyya Savasan22, Anna Pawlowska23, Gail Megason24, David Mitchell25, Alexandra Cheerva26, Amina Kariminia27, Anat Halevy27, Kirk Schultz27
1CancerCare Manitoba, University of Manitoba, Manitoba Blood and Marrow Transplant Program, Winnipeg, Canada; 2Oregon Health & Science University, Portland, OR, United States; 3University of California San Francisco, San Francisco, CA, United States; 4University of Utah, Primary Children's Hospital, Salt Lake City, UT, United States; 5Childrens Hospital Los Angeles, Los Angeles, CA, United States; 6University of Calgary, Alberta Children's Hospital, Calgary, Canada; 7St. Justine University Hospital, Montreal, Canada; 8C.S. Mott Children's Hospital, Ann Arbor, MI, United States; 9Vanderbilt University Medical Center, Nashville, TN, United States; 10Nemours Alfred I. DuPont Hospital for Children, Wilmington, DE, United States; 11Columbia University Medical Center, New York, NY, United States; 12University of North Carolina at Chapel Hill, Chapel Hill, NC, United States; 13George Washington University School of Medicine and Health Sciences, Children's National Health System, Washington, DC, United States; 14Johns Hopkins All Children's Hospital, St. Petersburg, FL, United States; 15Loma Linda University Medical Centre, Loma Linda, CA, United States; 16Northwestern University Feinberg School of Medicine, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; 17University of Alabama at Birmingham, Birmingham, AL, United States; 18University of Toronto, Hospital for Sick Children, Toronto, Canada; 19Maria Fareri Children's Hospital New York Medical College, Valhalla, NY, United States; 20University of Nebraska Medical Center, Omaha, NE, United States; 21Nemours Children's Clinic, Jacsonville, FL, United States; 22Children's Hospital of Michigan, Detroit, MI, United States; 23City of Hope, Duarte, CA, United States; 24University of Mississippi Medical Center, Jackson, MS, United States; 25McGill University, Montreal Children's Hospital, Montreal, Canada; 26University of Louisville, Norton Children's Hospital, Louisville, KY, United States; 27University of British Columbia, B.C. Children's Hospital, Vancouver, Canada
Background: Our group has previously shown that non-cytolytic regulatory NK cells (NKregs; CD56brightCD335+granzymeLow) are proportionally lower in the donor grafts of adults who later develop chronic GVHD [Hematologica 2017]. Greinix et al. [BBMT 2015] have previously documented that CD19+CD21Low B cells at d100 are proportionately higher in adults later developing cGVHD. We sought to understand whether these two cell populations were also prognostic biomarkers at d100 for cGVHD in children in similar directions as seen in adults, given our previous finding that recent thymic emigrants and Treg RTEs were also prognostic biomarkers of cGVHD in children, but in a direction opposite to adults [ASBMT abstract 2018].
Methods: Allo-HCT patients (< 18yrs) with malignant and non-malignant diagnoses were enrolled before HCT and prospectively followed for cGvHD until 1-year post-HCT. Blood was analyzed by 8-color flow cytometry at d100 (+/- 14d) for prognostic cellular cGvHD biomarkers. Mean values are reported. Biomarkers were clinically significant if means were >1.5x or < 0.7x the control and p < 0.05. 212 patients were enrolled before June 30, 2016: 144 were evaluable with 1-year follow up and 68 excluded for relapse, non-engraftment or early death. Of the evaluable patients, 37 (25.7%) developed NIH criteria cGvHD (14 with overlap syndrome), 34 (23.6%) had late aGvHD but no cGvHD, and 73 (50.7%) had no cGvHD. The no cGvHD group included patients both with and without a past history aGvHD before d100, as we found previous aGvHD had no impact on d100 cellular populations in the absence of cGvHD. To determine whether these biomarkers would predict the development of any form of GVHD after d100 (late aGVHD, cGHVD and overlap syndrome), a combined cGVHD and late aGVHD group was created.
Results: Children developing NIH criteria cGVHD had lower proportions of NKregs at d100 compared to those with no cGVHD (p < 0.001), with NKregs being a prognostic biomarker for cGVHD development (ROC AUC 0.71). When patients with late aGVHD were added to the cGVHD group, the relationship was maintained. By comparison, CD19+CD21Low B cells were proportionally higher in the cGVHD group, although this did not reach statistical significance. When late aGVHD individuals were added, CD19+CD21Low B cells were proportionally higher (p = 0.018), suggesting higher proportions are prognostic for any form of GVHD after day 100.
Conclusions: In children, low proportions of NKregs at d100 are associated with development of cGVHD specifically, as well as other forms of GVHD after d100. Higher proportions of CD19+CD21Low B cells at d100 are also associated with GVHD after d100. This data is consistent with adult data and suggests that cellular populations not involving the thymus may have similar prognostic significance for predicting GVHD in children and adults.
Clinical Trial Registry: Registered on ClinicalTrials.gov (NCT02067832)
Conflict of interest: J. Whalstrom: Employed by Pharmacyclics. The other authors have nothing to disclose.
| Cell Population | No cGVHD | cGVHD only | p-value against no cGVHD group (AUC) | cGVHD + Late aGVHD | p-value against no cGVHD group (AUC) |
|---|---|---|---|---|---|
| NK Regs: CD56Bright GranzymeLow (% of CD56 NK cells) | 20.3% | 10.8% | <0.001 (0.71) | 13.7% | 0.004 (0.64) |
| CD19+CD21Low (% of CD19 B cells) | 10.8% | 17.3% | 0.061 (0.59) | 16.8% | 0.018 (0.60) |
[ [O151 Table] Proportion of Cellular Biomarkers at Day 100]
O152 Success of the HR-NBL/SIOPEN local control trial strategy in high risk neuroblastoma patients undergoing high dose therapy with peripheral stem cell transplantation
Ruth Ladenstein1, Ulrike Poetscher2, Roberto Luksch3, Victoria Castel4, Isaac Yaniv5, Vassilios Papadakis6, Geneviève Laureys7, Josef Malis8, Walentyna Balwierz9, Ellen Ruud10, Per Kogner11, Henrik Schroeder12, Ana Forjaz de Lacerda13, Maja Beck-Popovic14, Pavel Bician15, Miklos Garami16, Toby Trahair17, Adela Canete4, Peter Ambros2, Keith Holmes18, Alberto Garaventa19, Jean Michon20, Andrew Pearson21, Dominique Valteau-Couanet22, Mark Gaze23, Tom Botterberg24
1St. Anna Kinderkrebsforschung e.V., Studies & Statistics for Integrated Research and Projects, Vienna, Austria; 2St. Anna Kinderkrebsforschung e.V., Vienna, Austria; 3Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; 4Hospital La Fe, Valencia, Spain; 5Schneider Children's Medical Center of Israel, Petach Tikvah, Israel; 6Agia Sophia Children's Hospital Athens, Athens, Greece; 7University Hospital Ghent, Ghent, Belgium; 8University Hospital Motol, Prague, Czech Republic; 9Jagiellonian University Medical Collage, University Children’s Hospital in Cracow., Krakow, Poland; 10Rikshospitalet University Hospital, Oslo, Norway; 11Karolinska institute, Stockholm, Sweden; 12University Hospital of Aarhus, Aarhus, Denmark; 13Portuguese Institute of Oncology, Lisboa, Portugal; 14University Hospital Lausanne, Lausanne, Switzerland; 15University Children Hospital, Banska Bystrica, Slovakia; 16Semmelweiss University, Budapest, Hungary; 17Sydney Children's Hospital, Randwick, Australia; 18St. Georges Hospital, London, United Kingdom; 19Istituto Giannina Gaslini, Genoa, Italy; 20Institut Curie, Paris, France; 21Institute of Cancer Research, Royal Marsden Hospital, Sutton, United Kingdom; 22Gustave Roussy, Villejuif, Paris, France; 23University College Hospital, London, Austria; 24Ghent University Hospital, Department of Radiation Oncology, Ghent, Belgium
Background: The HR-NBL1/SIOPEN trial investigated the benefit of busulphan and melphalan as HDT/PSCT showing an improved outcome in patients with high-risk neuroblastoma (Ladenstein et al, Lancet Oncol. 2017;500-14). Here we explored the additive value of the HR-NBL1/SIOPEN stringent local control measures. A previous European survey in 2000 revealed a 40% local relapse rate (LRR) in first line HR-NBL submitted to HDT/ABMT or PSCT.
Methods: HR-NBL1/SIOPEN strategy included induction with Rapid Cojec chemotherapy or the modified N7 regimen, two additional courses of TVD in case of inadequate metastatic response and attempt of gross resection of the primary tumour. Further treatments after HDT/SCR were radiotherapy (21 Gray) to the primary site and 13-cis-retinoic acid with additional immunotherapy after 2008 with ch14.18/CHO antibody.
All patients with therapy start before 2016 with HDT that were alive without event after day 30 post ASCR and available radiotherapy data were included in this analysis. The cumulative incidence of local relapses/progression (CIR/P) either isolated or in combination with other sites were calculated for each event type taking into account the competing risks from other causes including isolated distant relapses/progressions, relapses/progressions with unknown sites or deaths or secondary malignancies without preceding progression /relapse.
Results: A total of 1297 patients were eligible for analysis. The compliance to radiotherapy was high: 90% received radiotherapy regardless of the primary tumour status post HDT). Patients with a local CR had radiotherapy in 91% (807/888) and in those with < local CR 90% received it (290/334). In patients receiving radiotherapy with local CR, the isolated local CIR (29/807) was 0.03 ± 0.01 whilst the combined (local and distant) relapse CIR was (77/807) 0.13 ± 0.01 at 5 years each. In patients without radiotherapy with local CR, the isolated local CIR (6/81) was 0.08 ± 0.03 whilst the combined (local and distant) relapse CIR was (10/81) 0.22 ± 0.05 at 5 years each. Local CR patients receiving radiotherapy had an the event free survival (5yr-EFS) at 5 years of 0.55 ± 0.02 but it was only 0.38 ± 0.06 for those without radiotherapy. In patients receiving radiotherapy with < CR, the isolated local CIR (17/290) was 0.06 ± 0.02 whilst the combined (local and distant) relapse CIR was (39/290) 0.21 ± 0.03 at 5 years each. In patients without radiotherapy with local < CR, the isolated local CIR (3/32) was 0.09 ± 0.05 whilst the combined (local and distant) relapse CIR was (9/32) 0.38 ± 0.09 at 5 years each. Patients with local < CR receiving radiotherapy had an 5yr-EFS of 0.47 ± 0.03 but it was only 0.10 ± 0.06 for those without radiotherapy. However one must consider that patient not receiving radiotherapy may differ as the decision not to irradiate was mostly related to large radiotherapy fields based on pre-operative volumes or very young age.
Conclusions: Local radiotherapy with 21 Gy is an important treatment component and has significantly lowered the local relapse rate in local CR and < CR patients irradiated. Most importantly, applied local radiotherapy significantly contributed to improved outcomes.
Clinical Trial Registry: EudraCT: 2006-001489-17
Conflict of interest: none
O153
Abstract previously published
O154 Safety and Efficacy of TCRalpha/beta and CD19 Depleted Haploidentical Stem Cell Transplantation in Children: Results of a Prospective Multicenter Phase I/II Clinical Trial
Peter Lang1, Paul-Gerhardt Schlegel2, Roland Meisel3, Ansgar Schulz4, Johann Greil5, Peter Bader6, Sandra Karitzky7, Michaela Malchow7, Silke Holtkamp7, Christiane Siewert7, Stefanie Pflitsch7, Michael Schumm1, Matthias Eyrich2, Markus Wiesneth8, Halvard Bönig9, Rupert Handgretinger1
1University Children's Hospital, Hematology/Oncology, Tuebingen, Germany; 2University Children's Hospital, Wuerzburg, Germany; 3University Hospital, Pediatric Oncology, Duesseldorf, Germany; 4University Medical Center, Pediatrics, Ulm, Germany; 5University Children's Hospital, Heidelberg, Germany; 6University Hospital Frankfurt, Center for Children and Adolescents, Frankfurt, Germany; 7Miltenyi Biotec GmbH, Bergisch Gladbach, Germany; 8Insitute for Clinical Transfusion Medicine and Immunogenetics, Ulm, Germany; 9Institute for Transfusion Medicine and Immunohematology, Frankfurt, Germany
Background: We report one year follow-up data of a multi-center, open-label, phase I/II trial with TCRalpha/beta and CD19-depleted haploidentical stem cells.
Methods: The CliniMACS plus System (Miltenyi Biotec, Germany) was used for graft manipulation in 4 GMP sites. The conditioning regimen comprised 15 or 30 mg ATG (Grafalon) or 7 Gy total nodal irradiation, 160 mg/m2 fludarabine, 10 mg/kg thiotepa, and 140 mg/m2 melphalan. MMF was given for 30 days. 30 pediatric patients from 6 hospitals were enrolled (ALL, AML n = 18; MDS, n = 3; solid tumors, n = 6; nonmalignant, n = 3). Disease status in leukemias/MDS were: CR1 (n = 4), relapsed/refractory (n = 17).
Results: Median numbers of CD34+ cells, TCRαβ+ cells and CD20+ cells infused per kg bw were 14.6 x 106 (4–54.9), 14 x 103 (0.62–40.6) and 0.55 x 105 (0.04–1.85). Additional components were NK and TCRγδ+ cells (6.67 x 107 and 1.58 x 107 cells/kg bw). Depletion efficacy of TCRαβ+ cells was 4.75log. Final engraftment occurred in 29/30 patients. 97% of patients experienced no or grade I aGvHD. One patient had aGvHD grade II. No severe acute aGvHD grades III - IV was observed. Chronic extensive GvHD occurred in 2/23 evaluable patients (9%).
24 patients were evaluable for assessment of immune reconstitution until 1 year or discontinuation. Figure 1 shows a fast and stable reconstitution of T, B and NK cells. Mean numbers of CD3+ T cells at d14, d100 and d365 were 147, 261 and 1284 cells/µL. TCRαβ+ T cells reached 8.5 and 141 cells/µL at d14 and d100 and increased up to normal levels, whereas TCRγδ+ T cells recovered faster (103 cells/µL at d14) and remained stable after having reached this plateau. TCR repertoire diversity increased slow but steadily. Thymic function (determined by TREC values) started to significantly increase on d100.
ADV reactivation in 16 patients contributed most to infectious complications. Of these 6 patients developed ADV disease. 3 ADV associated deaths occurred. One EBV reactivation was observed but recovered completely. 8 patients experienced CMV reactivation after transplantation. 7/8 patients recovered. Another 2 patients had CMV reactivation prior to transplant, but recovered after engraftment. No CMV disease was observed within the first year. 6 patients had bacterial sepsis but recovered.
At a median of 367 days (range, 14–809) among the 30 recipients, 16 are alive at time of last follow-up, 8 died of relapse, 3 died of ADV infection, 1 died of sepsis after graft failure and 1 died of ARDS. 1 patient is lost to follow-up. 1year OS and DFS for all patients were 64% and 60%, respectively. A cumulative incidence of NRM of 16% at 1 year was observed. 1y DFS and cumulative incidences of relapse in patients with leukemias/MDS at 1year were 60% and 15% (any CR) as well as 45% and 42% (non CR).
Conclusions: TCRαβ+/CD19+ depletion yielded large numbers of CD34+ cells, NK cells and TCRγδ+ cells with minimal risk of GVHD. Immune reconstitution was rapid. Coupled with a reduced-toxicity regimen, NRM was low. However, further efforts are necessary to avoid ADV infections.
Clinical Trial Registry: www.clinicaltrialsregister.org; 2011-005562-38
Conflict of interest:
P. Lang: Miltenyi Biotec, Research Funding
R. Meisel: Miltenyi Biotec, Neovii Biotech, Research Funding,
P. Bader: Novartis, Medac, Amgen, Riemser, Neovii, Consultancy, Honoraria and Research Funding
H. Bönig: Miltenyi Biotec Research Funding and Speakers Bureau
R. Handgretinger: Miltenyi Biotec Patents & Royalties: Co-patent holder of TcR alpha/beta depletion technologies and research funding
S. Karitzky, S. Holtkamp, C. Siewert, S. Pflitsch: Miltenyi Biotec, employment
P. Schlegel, A. Schulz, J. Greil, M. Schumm, M. Eyrich, M. Wiesneth: nothing to disclose
[[O154 Figure].
Immune Reconstitution after transplantation of TCR-ab/CD19 depleted haploidentical stem cell grafts]
O155 Impact of EBMT pediatric criteria on the management of sinusoidal occlusive syndrome in patients with solid tumors treated with busulfan and autologous stem cell rescue
Claudia Pasqualini1, Christelle Dufour1, Kamelia Alexandrova2, Pablo Berlanga1, Morgane Pondrom1, Valérie Lapierre2, Dominique Valteau-Couanet1
1Gustave Roussy Cancer Campus, Pediatric and Adolescent Oncology Department, Villejuif Cedex, France; 2Gustave Roussy Cancer Campus, Cell Therapy, Villejuif Cedex, France
Background: The new EBMT pediatric criteria for diagnosis and severity of sinusoidal occlusive syndrome/veno-occlusive disease (SOS/VOD) aim at improving SOS/VOD management by an earlier diagnosis thanks to a pediatric-specific definition.
Methods: We retrospectively analyzed data from children and adolescents (≤ 18 years) with solid tumors treated with high-dose intravenous busulfan (HD Bu)-containing regimen followed by autologous stem cell rescue (ASCR) in the transplantation unit of the Pediatric and Adolescent Oncology Department at Gustave Roussy from February 2006 to March 2016. SOS/VOD diagnosis was established using modified Seattle and EBMT criteria. Its severity was defined by Bearman and EBMT criteria. Univariate and multivariate analysis of incidence, severity and risk factors were performed using SPSS 20.0.
Results: Data from 123 patients were analysed. Their median age at ASCR was 4.8 years (range 0.8–18.0). The underlying malignancy was neuroblastoma in 87 patients, medulloblastoma in 19 patients, Ewing sarcoma in 15 and rhabdomyosarcoma in 2. All received HD Bu combined with either HD Melphalan (n = 103) or Thiotepa (n = 20). ASCR consisted of peripheral blood stem cells, bone marrow and both in 117, 3 and 3 patients, respectively. Sixty-three patients received a prophylaxis for SOS/VOD with ursodeoxycholic acid, 11 with defibrotide and 49 had no prophylaxis.
The use of EBMT criteria significantly increased the incidence of SOS/VOD in comparison to Seattle criteria, with an incidence of 42.3% (n = 52) and 22.8% (n = 28), respectively (p < 0.001). Moreover, EBMT criteria identified a higher number of severe/very severe diseases than Bearman criteria, with 37 and 4 severe/very severe SOS/VOD, respectively (p = 0.004). Two patients died of SOS/VOD. The 24 patients for whom the diagnosis of SOS/VOD was defined only by EBMT criteria had a mild disease according to Berman criteria. The hospitalization was longer for patients with severe/very severe SOS/VOD (median of 35 days, range: 23–103) vs moderate (31 days, range: 25–44) and mild/no (27 days, range: 22–38) disease (p = 0.004), according to EBMT criteria.
In this selected cohort of patients with high-risk solid tumors receiving high-dose busulfan followed by ASCR, the diagnosis of neuroblastoma and Ewing sarcoma were associated with a higher risk of SOS/VOD in comparison with medulloblastoma in the univariate and multivariate analysis (p = 0.045). The type of prophylaxis (ursodeoxycholic acid vs defibrotide vs no prophylaxis) had no significant impact on the incidence or severity of SOS/VOD.
Conclusions: The use of the new EBMT pediatric criteria might lead to an overestimation of the incidence and severity of SOS/VOD in children and adolescent with solid tumors treated with HD-Bu followed by ASCR. These criteria should be cautiously applied to guide the clinical management of patients in this specific setting. Their impact according to different frameworks should be evaluated in prospective clinical studies.
Conflict of interest: Nothing to disclose.
O156 Second allogeneic hematopoietic stem cell transplantation in children with juvenile myelomonocytic leukaemia
Ayami Yoshimi1, Franco Locatelli2, Peter Nöllke1, Jan Starý3, Henrik Hasle4, Michael Dworzak5, Markus Schmugge6, Barbara De Moerloose7, Ricardo Masetti8, Owen Smith9, Marek Ussowicz10, Albert Catala11, Marco Zecca12, Arjan Lankester13, Peter Lang14, Marc Bierings15, Marry van den Heuvel-Eibrink16, Brigitte Strahm1, Charlotte Niemeyer1
1University of Freiburg, Department of Pediatrics, Faculty of Medicine, Freiburg, Germany; 2Bambino Gesu' Children's Hospital, Department of Pediatric Hematology and Oncology, Rom, Italy; 3Charles University and University Hospital Motol, Department of Pediatric Hematology/ Oncology, Prague, Czech Republic; 4Aarhus University Hospital Skejby, Department of Pediatrics, Aarhus, Denmark; 5St. Anna Children's Hospital, Medical University of Vienna, Vienna, Austria; 6University Children's Hospital, Department of Hematology and Oncology, Zurich, Switzerland; 7Ghent University Hospital, Department of Pediatric Hematology-Oncology and Stem Cell Transplantation, Ghent, Belgium; 8University of Bologna, Department of Pediatric Oncology and Hematology, Bolona, Italy; 9Our Lady´s Children´s Hospital Crumlin, Dublin, Paediatric Oncology and Haematology, Dublin, Ireland; 10Medical University of Wroclaw, Department of Pediatric Hematology, Oncology, and BMT, Wroclaw, Poland; 11Hospital Sant Joan de Déu, Department of Hematology and Oncology, Barcelona, Spain; 12University of Pavia, Department of Pediatrics, IRCCS Policlinic San Matteo, Pavia, Italy; 13Department of Pediatric Hematology, Leiden University Medical Center, Leiden, Netherlands; 14University of Tuebingen, Department of Pediatrics, Tuebingen, Germany; 15University Hospital for Children, Medical Center, Department of Hematology, Utrecht, Netherlands; 16Princess Maxima Center, The Netherlands and DCOG, Utrecht, Netherlands
Background: Hematopoietic stem cell transplantation (HSCT) remains the only curative treatment for most patients with juvenile myelomonocytic leukaemia (JMML), but the incidences of relapse and graft failure (GF) after HSCT remain high. 2nd HSCT is the only curative therapy for such patients. We analyzed the long-term outcome of 79 JMML patients who received 2nd HSCT (63 relapse/16 GF after 1st HSCT).
Methods: The median age of patients at 2nd HSCT was 3.7 (1.0–15.8) years. Fifteen patients had monosomy 7 and 6 patients had other aberrations (normal karyotype: n = 54, no data: n = 4). The JMML mutational subgroup was characterized by PTPN11 (44), NRAS (10), KRAS (3), NF1 (3), CBL (2), no mutation (6) or no data (13). The donor for 1st /2nd HSCT was matched siblings (21/17), haplo-identical donors (7/11), other family members (4/6), or unrelated donors (47/45). Stem cell source for 1st /2nd HSCT was bone marrow (52/36), peripheral blood (14/35), or cord blood (13/8). The conditioning regimen for 1st HSCT was busulfan/ cyclophosphamide/ melphalan in 59 patients and other regimen in 20 patients; that for 2nd HSCT was a radiation-based regimen in 34 patients, treosulfan (Treo)/ fludarabine (Flu)/ thiotepa (TT) in 11 patients, and others in 34 patients.
Results: The median follow-up after 2nd HSCT was 5.4 years for survivors. The evert-free-survival (EFS) at 5 years was 34% with no difference according to indication of 2nd HSCT (relapse/GF after 1st HSCT: 35%/31%). Engraftment was achieved in 68 patients (86%) after 2nd HSCT. The cumulative incidence of grade II-IV acute graft-versus-host-disease (GvHD), chronic GvHD, relapse at 5 years and transplant related mortality (TRM) at 5 years was 46%, 32%, 37% and 29%, respectively. Patients transplanted following a radiation-based regimen showed a better engraftment rate (only 1 GF), but no superior EFS to other regimens. In contrast, 9 of 10 patients given the Treo/Flu/TT regimen (6 relapse and 4 GF after 1st HSCT) are alive in remission. The analysis for the subgroup with relapse after 1st HSCT showed that the longer interval between 1st HSCT and relapse gives rise to a better EFS (≥180 days: 50%, < 180 days: 23%, p = 0.04). The EFS of patients with a PTPN11 mutation who relapsed after 1st HSCT was 32% with the high incidence of 2nd relapse (47%).
Conclusions: 2nd HSCT is a feasible treatment for relapse or GF after 1st HSCT. TRM and relapse contributes equally to treatment failure and further efforts are necessary to improve the outcome. The survival of the group with patients given the Treo/Flu/TT regimen was excellent, although the number of patients is small and further studies are needed to confirm this promising result.
Conflict of interest: nothing to disclose
O157 ATG-Genzyme and ATG-Fresenius differ in pharmacokinetic profiles: impact on T-cell recovery and acute GvHD after pediatric HSCT
Lisa V.E. Oostenbrink1, Katrine Kielsen2, Cornelia M. Jol-van der Zijde1, Anja M. Jansen-Hoogendijk1, Monique M. van Ostaijen-ten Dam1, Astrid G.S. van Halteren1, Marianne Rosenkrantz Segelcke Ifversen2, Robbert G.M. Bredius1, Klaus G Müller2, Arjan C. Lankester1, Marco W. Schilham1, Maarten J.D. van Tol1
1Leiden University Medical Centre, Pediatric HSCT Unit and Laboratory for Immunology, Leiden, Netherlands; 2University Hospital Rigshospitalet, Pediatrics and Adolescent Medicine, Copenhagen, Denmark
Background: Previous studies comparing the effect of different doses of ATG on post-HSCT immune recovery and clinical outcome parameters have mainly been performed in limited and heterogeneous groups of patients, often investigating only one ATG brand.1,2 In the present study, we examined the clearance of the active (T-lymphocyte binding) component of ATG-Genzyme (ATG-G, Thymoglobulin®) and ATG-Fresenius (ATG-F, Grafalon®) and its effect on post-HSCT immune recovery and clinical outcome in a homogenous group of pediatric acute leukemia patients.
Methods: Fifty-eight children (n = 42 ATG-G, n = 16 ATG-F), who received T-cell replete BM or PBSC from unrelated donors for the treatment of ALL or AML in Leiden (n = 38) or Copenhagen (n = 20) were included. ATG-G was given at a total dose of 6–10 mg/kg; ATG-F at a total dose of 45–60 mg/kg. Serum samples (pre-conditioning, day 0; +1; +2; +3; +4 and +6 weeks and +2 and +3 months after graft infusion) were analyzed by quantitative flow cytometry on HUT78 cells for the presence of active ATG. T-lymphocyte (sub)populations were analyzed at +1, +2, +3, +6 and +12 months post-HSCT by flow cytometry. As reference for immune recovery, twenty-two children (Leiden n = 13, Copenhagen n = 9) transplanted for ALL or AML with an HLA-identical sibling donor and not receiving serotherapy were included.
Results: The overall clearance of active ATG-G is slower as compared to the kinetics of active ATG-F, but showed a larger inter-patient variation. Therefore, patients with either slow clearance (GS group) or fast clearance (GF group) can be distinguished. At 3 weeks post-HSCT, the active ATG concentration of the GF group equals the concentration of active ATG-F (F group in Figure, panel A). However, CD4 and CD8 T-cell recovery in the GF group is more rapid than in the ATG-F group and more closely resembles the recovery in the no serotherapy control group. T-cell recovery in the GS group is significantly more delayed as none of the patients from this group displayed >100 CD4+ or CD8+ cells/uL within the first month after HSCT (Figure, panel B). The incidence of acute GvHD (grade 1–4) in the GS group was significantly lower than observed in both the GF and the ATG-F group (p = 0.03, Figure, panel B). Remarkably, severe acute GvHD (grade 3–4) only occurred in the GF group. No significant differences were observed for CMV or EBV infection, relapse and overall survival between the ATG groups.
Conclusions: Within this homogenous cohort of pediatric acute leukemia patients, the different clearance rates of the active component of ATG-G and ATG-F have an impact on immune recovery and occurrence of acute GvHD. In general, patients receiving ATG-F and patients with a low level of ATG-G exposure (GF group), have a more rapid reconstitution of T-cells and a concurrent higher risk of developing acute GvHD. Our results show, that in multicenter studies to immune recovery the brand, dose and clearance of ATG should be used as covariates in multivariate analyses.
References:
1. Admiraal et al. Clin. Pharmacokinet. 2015 Apr;54(4):435–46
2. Locatelli et al. Lancet Oncol. 2017 Aug;18(8):1126–1136
Conflict of interest: The authors have nothing to disclose.
[[O157 Figure].
ATG clearance; Immune recovery and aGvHD]
O158 Continuous positive pressure (CPAP) treatment of acute respiratory failure in children after hematopoietic stem cell transplantation
Fabio Pavan1,2, Attilio Rovelli1,2
1MBBM Foundation/ASST San Gerardo, Monza, Italy; 2University of Milano-Bicocca, Milano, Italy
Background: Acute respiratory failure (ARF) in children is the most frequent cause of PICU admission after HSCT with a high rate of mortality after endotracheal intubation (EI), ranging from 50 to 80%. Non-invasive ventilation (NIV) may allow to avoid intubation and transfer to PICU. Nevertheless indications about the use of NIV in pediatric transplanted patients are still lacking and results are conflicting. In this paper we describe ten events of ARF occurring out of 60 patients who underwent HSCT treated with CPAP in their post-transplant course.
Methods: Clinical charts of 60 patients undergoing HSCT between January 2016 and September 2017 at our Institution were reviewed and clinical characteristics and respiratory parameters of patients developing ARF were collected.
Most patients with ARF were treated in the onco-hematological unit without inserting an arterial line so we could not calculate the Oxygenation Index (OI). Therefore we considered the SpO2/FiO2 ratio and respiratory rate (RR) as respiratory parameters. We evaluated also the rate of intubation, the rate of PICU admission and the outcome of the ARF related events.
Results: Among 60 pediatric patients (age 1–18 years, median 8 years) who underwent allogeneic HSCT in the study period we found 10 events (16.6%) of ARF. Median age of the patients with ARF was 13 years. Conditioning regimen was full intensity in 4 cases. All the events but one occurred in the early post transplant period (range -3 to +22 days post stem cell infusion) while the patients were in aplasia. Gvhd was present in one case. Clinical features, diagnoses and comorbidities are summarized in Table 1. All the patients with ARF started CPAP support with the helmet device in the onco-hematological unit. The positive end expiratory pressure/oxygen fraction settings as well as the SpO2/FiO2 ratio and the RR before and after starting CPAP are summarized in Table 1. Transfer to PICU was necessary in 6 out of 10 events: 2 patients were intubate and the others were transferred for a better monitoring and management of the fluid overload or due to cardiac failure. CPAP was effective in improving and maintaining oxygenation in all patients (range SpO2 pre - post CPAP was 82–100% and 98–100%, respectively), and in reducing the work of breathing (improvement of RR) in all but one case, in whom ARF was refractory to NIV and required PICU admission and intubation.
Two patients required intubation (20%) and three patients died overall (30%), one of whom was one of the two intubated patient. Causes of death were multiorgan failure, brain hemorrhage, intestinal perforation, which were not directly related with the ARF.
Conclusions: ARF is the most frequent cause of transfer to PICU, occurs mainly in the early post-transplant course, particularly among adolescents. The prognosis of those who require EI is still dismal. CPAP is a feasible NIV technique manageable in the onco-hematological unit and can prevent EI and standard intensive care in most cases.
Conflict of interest: The authors declare they do not have any potential conflicts of interest
| DIAGNOSIS | AGE | ARF CAUSE | PEEP/FIO2 (cmH2O/%) | SpO2/FiO2 (%/%) pre-post cpap | SpO2/FiO2 (%/%) 72h post cpap | RR pre - post CPAP | EI | Days BMT to start CPAP/admission to PICU | Death (days BMT - cause) |
|---|---|---|---|---|---|---|---|---|---|
| NHL CR4 | 12 | Sepsis, capillary leakage | 5/40 | 100/21-100/40 | n.a. | 40-n.a. | no | -3/no | -1 sepsis |
| SAA | 13 | Fluid overload, capillary leakage | 8/60 | 88/30 - 100/60 | 99/40 | 48–26 | no | +4/+7 | no |
| AML CR1 | 13 | Cardiac failure, renal failure, fluid overload | 8/50 | 98/30 - 100/50 | 98/30 | 30–22 | no | +10/+10 | no |
| AML CR1 | 15 | Pneumonitis ab ingestis | 7/50 | 93/30 -100/50 | 100/40 | 20–17 | no | -2/-2 | no |
| ALL CR2 (2ND EVENT) | 9 | Candidemia, staphylococcus spp bacteremia, cardiac failure (ARDS, rhinovirus) | 8/50 (8/40) | 99/30 - 100/50 (90/40 - 98/40) | 100/40 (96/60) | 35–38 (34–60) | no (yes) | +22/+26 (+168/+171) | no (no) |
| HL CR3 | 18 | Capillary leakage, TMA | 8/50 | 82/30-100/50 | 100/40 | 30–22 | no | +5/no | +15 - brain hemorrage |
| AML CR1 | 3 | Cardiac failure, abdominal distension by ADV infection | 5/35 | 88/21-100/35 | 100/35 | 60–40 | yes | +6/+13 | +30 intestinal perforation |
| SCN | 2 | Pneumonitis by Aspergillus spp | 5/35 | 99/21-99-35 | 100/35 | 44–32 | no | +7/no | no |
| AML CR2 | 18 | Capillary leakage, sepsis | 8/40 | 95/21 -99/40 | 98/40 | 40–24 | no | +6/no | no |
[ [O158 Table] table 1]
Stem cell donor
O159 Should Donor Selection be Driven by Disease Risk? A Retrospective Analysis of the EBMT Registry on Behalf of the Acute Leukemia Working Party
Roni Shouval1, Joshua Fein1, Myriam Labopin2, Nicolaus Kröger3, Rafael F. Duarte4, Peter Bader5, Chiara Bonini6, Jurgen Kuball7, Grzegorz Basak8, Carlo Dufour9, Arjan Lankester10, Silvia Montoto11, John A Snowden12, Jan Styczynski13, Mohamad Mohty14, Arnon Nagler1,2
1Chaim Sheba Medical Center, Ramat Gan, Israel; 2Acute Leukemia Working Party, Paris Study Office, European Society for Blood and Marrow Transplantation, Paris, France; 3University Hospital Eppendorf, Hamburg, Germany; 4Hospital Universitario Puerta de Hierro, Madrid, Spain; 5University Hospital Frankfurt, Frankfurt, Germany; 6Fondazione San Raffaele del Monte Tabor, Milan, Italy; 7UMC Utrecht, Utrecht, Netherlands; 8Medical University of Warsaw, Warsaw, Poland; 9G. Gaslini Chidlren's Hospital, Genova, Italy; 10Leiden University Medical Centre, Leiden, Netherlands; 11Barts Health NHS Trust, London, United Kingdom; 12Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom; 13Collegium Medicum, Nicolaus Copernicus University Torun, Bydgoszcz, Poland; 14Hopital Saint-Antoine, Université Pierre & Marie Curie, Paris, France
Background: Disease status at the time of an allogeneic hematopoietic stem cell transplantation (HSCT) remains the most important predictor of survival. We set out to characterize the evolution of outcomes in transplantation with different donor types across levels of disease-associated risk.
Methods: This retrospective study included adult patients treated for hematologic malignancies who underwent first allogeneic HSCT between 2001 and 2015 in EBMT centers. Missing values were accounted for by multiple imputations. A three-level disease-risk scheme (low, intermediate, and high) was defined by introducing combinations of diagnosis and disease status into a Cox multivariate model for overall survival (OS). Additional covariates included in the model were reflective of patient, disease, transplant, and center related features.
A variable combining donor type and HSCT year (2001–2005, 2006–2010, 2011–2015) was created. Using a Cox mutlivariable model, adjusted for key transplant covariates, the association of the joint donor type/transplant-year variable with OS, non-relapse mortality (NRM), and relapse, was studied seperately within each disease risk category. Competing risk analysis was used to calculate cumulative incidence of non-relapse mortality (NRM) and relapse.
Results: A total of 103,049 patients were analyzed (Table). A broad spectrum of hematological malignancies were included.
There was an improvement in overall survival across all donor types. Change was driven largely by reduction in NRM, especially among patients transplanted from Haplo donors (2-y NRM @ 2001–2005: 50.9% [95% CI:45.3–57.2] vs. 2011–2015: 29.6% [27.9–31.4], p < 000.1). In the Haplo setting, the improvement over time in NRM was maintained in all disease risk groups. However, in the high-risk group, the reduction in NRM was counteracted by high rates of relapse (Figure A). The risk of overall mortality, NRM, and relapse varied depending on the combination of donor type, disease risk and transplantation year. Among patients with low risk disease transplanted between 2011–2015, MSD was associated with the lowest risk for mortality (MSD [reference], MUD HR = 1.14 [1.07–1.21], MMUD 1.64 [1.50–1.79], Haplo 1.44 [1.28–1.61]). MSD consistently had the lowest risk of NRM, regardless of disease risk and transplant year (Figure B). In contrast, the risk of relapse in transplants performed between 2011–2015, in patients with low disease risk, was lower with HLA discordant donors (MSD [reference], MUD HR = 0.85 [0.78–0.91], MMUD 0.94 [0.83–1.07], Haplo (0.71 [0.60–0.84]) (Figure C).
Conclusions: Survival following of allogeneic HSCT continues to improve. The change is driven by a reduction in NRM, most notably in Haplo transplant. Our findings indicate that donor type interacts with disease risk; relapse risk with unrelated and Haplo donors was lower than that of MSD, primarily in low risk disease.
Conflict of interest: nothing to disclose
| Category | N (%)/ Median (IQR) | |
|---|---|---|
| Patient age | 49.2 (37.1–58.1) | |
| Conditioning | Myeloablative | 54,924 (53.3%) |
| Reduced-Intensity | 48,125 (46.7%) | |
| Donor | Matched Sibling Donor | 45,766 (44.4%) |
| Matched Unrelated 8/8 (MUD) | 41,117 (39.9%) | |
| Mismatched Unrelated 7/8 (MMUD) | 11,964 (11.6%) | |
| Haplo-identical | 4,202 (4.1%) |
[ [O159 Table] Population Characteristics]
[[O159 Figure].
NRM and Relapse incidence and risk]
O160 Early adoptive immunotherapy improves the outcome for patients with advanced myeloid malignancies undergoing PTCy-based haploidentical HSCT: a prospective study of three sequential protocols
Sarita Rani Jaiswal1,2, Prakash Bhakuni2, Priyanka Bharadwaj2, Aby Joy2, Nisha Murli2, Ashoke Rajoreya2, Shahid Akthar2, Ajay Dash2, Paul O'Donnell3, Suparno Chakrabarti1,2
1Manashi Chakrabarti Foundation, Cellular Therapy and Immunology, Noida, India; 2Dharamshila Narayana Superspeciality Hospital, BMT and Hematology, New Delhi, India; 3Fred Hutchinson Cancer Research Institute, Seattle, WA, United States
Background: Advanced myeloid malignancies are associated with extremely poor outcome following allogeneic HSCT and this is often linked to the extent of residual disease pre-transplant.
Methods: We carried out PTCy based Haploidentical PBSC transplantation in 74 patients with advanced myeloid malignancies. The first 30 patients did not receive any early post-transplant adoptive immunotherapy (non-EAI group) and the subsequent 44 patients received early adoptive immunotherapy (EAI group) in the form of prophylactic DLI on days +21, 25 and 60 (n = 21), CD56 enriched DLI on day +7 (n = 10) and CTLA4Ig-primed DLI on days +7, +21 and +35(n = 13). Majority received conditioning with Flu-Bu-Mel (n = 54), 10 each received Flu-Treo-2Gy TBI and Flu-Mel-Cyclo. GVHD prophylaxis consisted of PTCy 50 mg/kg on days +3 and +4, followed by cyclosporine with or without mycophenolate.
Results: The median age of the cohort was 28 years (2–65) with a median donor age of 34 years. All had relapsed/refractory disease with 46 of them having adverse cytogenetics. There was no difference between the EAI and the non-EAI groups in terms of patient or donor age and gender, disease-risk, residual disease at transplant, HCT-CI, ABO or HLA mismatches, graft composition and donor NK alloreactivity. The median time to engraftment was 14 days. Acute GVHD was significantly higher in the non-EAI group (CI 36.7% vs 14.8% in EAI group, p = 0.01), whereas chronic GVHD was more in the EAI group (CI 40.1% vs 7.4% in EAI group, p = 0.01). The overall incidence of NRM was 13% and the disease progression was 45.6%. Disease progression in the EAI group was 24.8 % vs 74.7% in the non-EAI group (p = 0.0001). At a median follow-up of 34 months (9 months-7 years), the overall survival was 54%; 72.6 % in EAI group vs 26.7% in the non-EAI group (p = 0.0001, Fig). Disease progression was favorably influenced by EAI (HR-0.23; p = 0.0001) and development of chronic GVHD (HR-0.24, p = 0.05). None developed severe GVHD and all surviving patients were free of GVHD and progression at 18 months.
The incidences of acute GVHD were lowest in those receiving CD56 enriched DLI or CTLA4-primed DLI in the EAI group. On analysis of immune reconstitution, CD56+16+ NK cell recovery at day 30 was significantly higher in those receiving CD56 enriched DLI or CTLA4-primed DLI, whereas the recovery of CD8 and CD4 cells were more rapid in the DLI group, as compared to the non-EAI group. Rapid recovery of Tregs at day 30 correlated with lack of acute GVHD but had no impact on chronic GVHD.
Conclusions: PTCy based haploidentical PBSC transplantation provides an optimal platform for exploration of early adoptive immunotherapy in patients with advanced myeloid malignancies. Early post-transplant intervention with adoptive immunotherapy with either a T cell or NK cell directed approach results in marked reduction in disease progression with a low incidence of acute GVHD and NRM and improves overall survival in patients with advanced myeloid malignancies irrespective of pre-transplant disease status, as compared to those receiving similar conditioning without post-transplant intervention.
Conflict of interest: No conflict of interest
[O160 Figure].
![[O160 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/acf6d8b35415/41409_2018_318_Fig35_HTML.jpg)
Impact of Early Adoptive Immunotherapy on Survival]
O161 Comparative outcomes for matched and mismatched (haplo) family donors for Myelodysplastic syndromes: Results from the EBMT Chronic Leukaemia Working Party
Kavita Raj1, Dirk-Jan Eikema2, Linda Koster3, Maria Teresa Van Lint4, Didier Blaise5, Arnon Nagler6, Dietrich Beelen7, Gerard Socié8, Yener Koc9, Ibrahim Yakoub-Agha10, Ghulam.J. Mufti11, Johann Maertens12, Ardeshir Ghavamzadeh13, Patrice Chevallier14, Péter Reményi15, Lucia Lopez Corral16, Gerhard Ehninger17, Ellen Meijer18, Thierry Lamy19, Jose Antonio Pérez-Simón20, Depei Wu21, Marie Robin8, Nicolaus Kröger22
1Kings College Hospital and Guys and St Thomas' NHS Foundation Trust, Haematology, London, United Kingdom; 2EBMT Statistical Unit Data Office Leiden, Leiden, Netherlands; 3EBMT Data Office Leiden, Leiden, Netherlands; 4Ospedale San Martino, Haematology, Genova, Italy; 5Institut Paoli Calmettes, Marseille, France; 6Chaim Sheba Medical Center Tel Hashomer, Tel Aviv, Israel; 7University Hospital, Essen, Germany; 8Hopital St. Louis, Paris, France; 9Medical Park Hospitals, Antalya, Turkey; 10CHU de Lille, Lille, France; 11Kings College Hospital and Kings College London, London, United Kingdom; 12University Hospital Gasthuisberg, Leuven, Belgium; 13Shariati Hospital, Teheran, Iran, Islamic Republic of; 14CHU Nantes, Nantes, France; 15St. István & St. Laszlo Hospital, Budapest, Hungary; 16Hospital Clínico, Salamanca, Spain; 17Universitaetsklinikum Dresden, Dresden, Germany; 18VU University Medical Center, Amsterdam, Netherlands; 19Centre Hospitalier Universitaire de Rennes, Rennes, France; 20Hospital Universitario Virgen del Rocío, Sevilla, Spain; 21First Affiliated Hospital of Soochow University, Suzhou Jiangsu, China; 22University Hospital Eppendorf, Hamburg, Germany
Background: Myelodysplastic syndromes (MDS) are the second common indication for an Allo HSCT. We sought to compare the outcomes of sibling (sib) with mismatched family donors (haplo) from the EBMT database.
Methods: Adult patients with MDS transplanted with sib (n = 1815) or haplo (n = 230) donor between 2011 and 2016 (2048) were studied. Patient demographics, disease status and outcomes in terms of engraftment, non relapse mortality, relapse, overall survival, relapse free survival, incidence of aGVHD and cGVHD were studied.
Results: The median age at transplant for sibs was 57 (19–77) haplo 61 (20–77) years, with 61% male recipients. KPS was >90% in 70% of patients. For sibs and haplo WHO RA/RARS/del5q accounted for 8% and 5%; RCMD-(RS) 15% and 10%; RAEB-1 15 and 13%; RAEB-2 32% each and AML 21% and 34% respectively. At transplant 32% sibs and 30% haplo were in CR. The median time from diagnosis to transplant was 9 months (0.1–527). Peripheral blood was the stem cell source in 88% and 51% of sib and haplo recipients.
Conditioning was MA in 58% sib and 54% haplo, TBI was used in 14% sib and 25% haplo. In-vivo T cell depletion was used in 41% sib with ATG (33%) and Alemtuzumab (8%) whereas post transplant cyclophosphamide was used in 100% haplo and 3% sib. Median time to neutrophil engraftment was 17 days (95% CI 16–17) and 19 days (95% CI 19–20) for sibs and haplo respectively. Platelet engraftment was significantly longer for haplo 28 days (95% CI 26–32) compared to 14 days (95% CI 14–15) for sibs (p < 0.001). The CI of aGVHD grade II-IV for sib was 25% (95% CI 23–27%) and for haplo was 22% (95% CI 17–28%) (p = 0.31) and grade III-IV for sib was 11% (95% CI 10–13%) and haplo was 6% (95% CI 3–9%)(p = 0.02). The CI of cGVHD at 1 year for sib was 17% (95% CI 14–19%) and haplo was 10% (95% CI 5–14%) (p = 0.003) and at 2 years was for sib 19% (95% CI 17–22%) and haplo was 13% (95% CI 8–18%). The median follow-up for sib and haplo was18 and 24 months respectively with similar OS, RFS and CIR at 2 years (Table 1).
Causes of death were relapse (37% &23%); GVHD (23% & 16%); Infection (21% & 37%) secondary malignancy or PTLD (2% & 1%); organ failure (6% &7%) HSCT related 11% and 17% for sibling and haplo recipients respectively.
Conclusions: This data set shows that the outcomes for a haplo transplant are similar to those from an identical sib with lower severe aGVHD and chronic GVHD. Further analysis of factors affecting outcomes are necessary.
Conflict of interest: The authors have nothing to disclose.
| Outcomes | Siblings n = 1728 | Haploidentical n = 217 |
|---|---|---|
| 2 yr Overall Survival | 59% (95% CI 56–61%) | 57% (95% CI 50–65%) NS |
| 2 yr Progression Free Survival | 52% (95% CI 50–55%) | 48% (95% CI 40–55%) NS |
| 2 yr Relapse | 28% (95% CI 25–30%) | 24% (95% CI 18–30%) NS |
| 2 yr Non Relapse Mortality | 20% (95% CI 18–22%) | 28% (95% CI 22–35%) p = 0.012 |
[ [O161 Table] Outcomes]
O162 Single Cord Blood Unit Plus Third Party Donor Cells (Haplo-Cord) Transplantation Compared to Adult Unrelated Donors in Patients with Acute Leukemia: A Retrospective Case-Control Study
Guiomar Bautista1, Carmen Canals2, Mi Kwon3, Isabel Sanchez-Ortega4, Carmen Regidor1, Pascual Balsalobre3, Arancha Bermúdez5, Montserrat Rovira6, José Luis Diez-Martin3, Almudena de Laiglesia1, José Antonio Pérez-Simón7, Lucrecia Yañez5, Christelle Ferrá8, Jorge Sierra9, Jose Luis Bello10, Inmaculada Herrera-Arroyo11, Carlos Solano12, Maria Jesús Pascual13, Teresa Zudaire14, Lucía López- Corral15, Angela Figuera16, Inmaculada Heras17, Anna Sureda4, Rafael Cabrera1, Rafael F. Duarte1
1Hospital Universitario Puerta de Hierro Majadahonda, Majadahonda, Spain; 2Banc de Sang i Teixits, Barcelona, Spain; 3Hospital General Universitario Gregorio Marañón, Madrid, Spain; 4Institut Català d'Oncologia Duran i Reynals, L'Hospitalet de Llobregat, Spain; 5Hospital Universitario Marqués de Valdecilla, Santander, Spain; 6Hospital Universitari Clínic, Barcelona, Spain; 7Hospital Universitario Virgen del Rocío, Sevilla, Spain; 8Institut Català d'Oncologia Trias i Pujol, Badalona, Spain; 9Hospital Santa Creu i Sant Pau-Universitat Autònoma, Barcelona, Spain; 10H Clínico Universitario Santiago de Compostela, Santiago de Compostela, Spain; 11Hospital Universitario Reina Sofía, Córdoba, Spain; 12Hospital Universitario Clínico Valencia, Valencia, Spain; 13Hospital Universitario Carlos Haya, Málaga, Spain; 14Complejo Hospitalario de Navarra, Pamplona, Spain; 15Hospital Clínico Universitario de Salamanca, Salamanca, Spain; 16Hospital Universitario de La Princesa, Madrid, Spain; 17Hospital General Universitario Morales Meseguer, Murcia, Spain
Background: The best alternative donor for allogeneic HCT (alloHCT) candidates without a matched sibling remains to be defined. In the absence of prospective randomized trials, additional data are needed to inform donor choice in these patients. Recently, Milano et al (N Engl J Med 2016;375:944–53) showed that cord blood HCT may improve relapse rates and overall survival compared to unrelated donors (UD) in patients with high-risk acute leukemia (AL) and residual disease prior to HCT.
Methods: We present a retrospective case-controlled study by the Spanish HCT group of first alloHCT for high-risk AL including 94 alloHCT recipients of single cord-blood units plus third-party donor CD34+ cells (haplo-cord) compared (1:2) with 188 recipients of UD alloHCT matched for age, gender, WHO diagnosis, disease status at HCT, time from diagnosis to HCT, prior auto-HCT, TBI use in conditioning and year of HCT. Haplo-cord cases included 57 men (61%) and 37 women (39%), median age 34 years (range 16–64), median weight 70 kg (42–111), 51 AML and 43 ALL, 49 in first CR, 16 in CR2 and 29 more advanced, including 23 with detectable disease. Six had a prior autologous HCT. Median time from diagnosis to alloHCT was 8.3 months (range 2–66).
Results: Overall outcomes for the whole series at 6 years are comparable between haplo-cord and UD-controls for non-relapse mortality (33.0%, 95CI: 24.7–44.0 versus 34.4%, 95CI: 28.1–42.0, respectively; n.s.), and show a statistical trend in favour of haplo-cord in relapse rate (24.5%, 95CI:17.2–34.9 in haplo-cord versus 30.7%, 95CI:24.7–38.2 in UD-controls; p = 0.135) and overall survival (47.7%, 95CI: 37.6–57.8 in haplo-cord versus 37.0%, 95CI: 29.9–44.0 in UD-controls; p = 0.079).In addition, grade II-IV acute GvHD was significantly lower in the haplo-cord group (12.1% vs 40.7%, p = 0.001; 35.8% in matched vs 45.6% in mismatched controls) as well as chronic GvHD (29.9% vs 50.0%, p = 0.02; 43.6% in matched vs 55.8% in mismatched controls).
Of note, high-risk AL patients transplanted with advanced disease (CR3 or later, partial remission or refractory disease), had significantly better 6-year outcomes following haplo-cord alloHCT than their UD-controls: relapse rate was 37.9% (95CI: 23.8–60.4) versus 47.8% (95CI: 35.4–64.7), respectively (p = 0.069), progression-free survival was 24.1% (95CI: 8.6–39.7) versus 13.0% (95CI: 3.3–22.8), respectively (p = 0.046), and overall survival was 31% (95CI: 14.2–47.9) versus 13.0% (95CI: 3.3–22.8), respectively (p = 0.046).
Conclusions: With the increase availability and use of alternative donors for alloHCT, data to inform donor choice are needed. It is unlikely that any single type of alternative donor will be the best choice for all patients lacking a matched related donor. Our data with haplo-cord HCT, in line with recent findings by Milano et al, contribute to the evidence to suggest that unrelated CB reduces the incidence of GvHD while controlling the underlying AL, and might be a preferable donor choice with improved overall survival for patients with AL and a high-risk of relapse.
Conflict of interest: nothing to disclose
O163 Comparison of the outcomes of allogeneic hematopoietic stem cell transplantation from HLA-identical siblings versus unmanipulated haploidentical donors in relapsed or refractory acute myeloid leukemia
Giorgia Battipaglia1,2, Ariane Boumendil3, Myriam Labopin1,3,4, Annalisa Ruggeri1, Fabio Ciceri5, Johanna Tischer6, Matthias Steljes7, Gerhard Ehninger8, Dietrich Beelen9, Jurgen Finke10, Maria Teresa Van Lint11, Arnold Ganser12, Boris Afanasyev13, Renato Fanin14, Mohamad Mohty1,3,4, Arnon Nagler3,15
1Hopital Saint Antoine, Paris, France; 2Federico II University, Hematology, Naples, Italy; 3Acute Leukemia Working Party, Paris Study Office, European Society for Blood and Marrow Transplantation, Paris, France; 4Hopital Saint Antoine, Universite Pierre & Marie Curie, INSERM, UMRs 938, Paris, France; 5Hematology and BMT Unit, IRCCS Ospedale San Raffaele; University Vita-Salute San Raffaele, Milan, Italy; 6University Hospital, LMU Munich, Munich, Germany; 7University of Muenster, Department of Medicine / Hematology and Oncology, Muenster, Germany; 8Universitaetsklinikum Dresden, Medizinische Klinik und Poliklinik I, Dresden, Germany; 9West German Cancer Center, University Hospital of Essen, Department of Bone Marrow Transplantation, Essen, Germany; 10University of Freiburg, Department of Medicine -Hematology, Oncology, Freiburg, Germany; 11Ospedale San Martino, Department of Haematology II, Genova, Italy; 12Hannover Medical School, Department of Haematology, Hemostasis, Oncologyand Stem Cell Transplantation, Hannover, Germany; 13First State Pavlov Medical University of St. Petersburg, Lev Tolstoy, St Petersburg, Russian Federation; 14Azienda Ospedaliero Universitaria di Udine, Division of Hematology, Udine, Italy; 15Chaim Sheba Medical Center, Department of Bone Marrow Transplantation, Tel Hashomer, Israel
Background: Refractory or relapsed acute myeloid leukemia (R/R-AML) has very poor prognosis. Allogeneic hematopoietic stem cell transplantation (HSCT) may be the only chance of cure, with need to rapidly find a donor. Haploidentical donors (Haplo) may represent an option in the absence of matched sibling donors (MSD).
Methods: We compared outcomes of patients (pts) with R/R-AML undergoing HSCT from either a MSD (n = 1654) or Haplo (n = 389) in the period 2007–2015. The Haplo group included pts receiving an unmanipulated graft with post-transplant cyclophosphamide (PTCY, n = 278), in vivo T-cell depletion (TCD, n = 95), or both (n = 16).
Results: Median age at HSCT was 52 (range 18–74) years. Median follow-up was 16 and 22 months for MSD and Haplo recipients, respectively (p = 0.11). At time of HSCT, 41% and 56% presented a primary refractory AML in Haplo and MSD groups, respectively (p < 0.01). Compared to MSD, Haplo HSCT were performed more recently (2013 vs 2011, p < 0.01) more pts had a poor Karnofsky PS (22% vs 16%, p < 0.01), a longer interval from diagnosis to HSCT (7 vs 5 months, p < 0.01), a more frequent use of bone marrow as stem cell source (47% vs 8%, p < 0.01) and of a reduced intensity conditioning regimen (50% vs 43%, p < 0.03). Cumulative incidence (CI) of engraftment was higher (93% vs 83%, p < 0.01) with a shorter median time to engraftment (15 vs 18 days, p < 0.01) in MSD recipients. In univariate analysis, Haplo presented lower incidence of cGVHD (27% vs 42%, p < 0.01), but also lower OS (25% vs 32%, p < 0.01), LFS (19% vs 27%, p < 0.01), GRFS (18% vs 26%, p < 0.01) and higher NRM (31% vs 22%, p < 0.01). Relapse incidence was similar (50% vs 51%, p = 0.60). In a multivariate analysis adjusted for the differences between the two groups, Haplo HSCT was associated with lower GRFS (HR 1.19, CI 1.02–1.39; p < 0.03), higher CI of grade II-IV aGVHD (1.31, CI 1.02–1.69; p < 0.04), and higher NRM (1.38, CI 1.07–1.80; p < 0.02), mainly due to a higher incidence of infections (41% vs 25%, p < 0.01). A trend toward a lower OS in Haplo was observed as well (HR 1.16, CI 1.00–1.37; p < 0.06). Regardless of donor type, relapsed or progressive AML was associated with a lower OS, LFS and GRFS and higher RI, as compared to primary refractory pts (p < 0.01). All survival outcomes were worse in pts with poor Karnofsky PS (p < 0.01).The use of PBSC was associated with a higher risk of grade II-IV aGVHD (HR 1.57, CI 1.17–2.10; p < 0.01) and cGVHD (HR 1.64, CI 1.21–2.23; p < 0.01) but was not associated with LFS.
Conclusions: In R/R-AML, when available, HSCT from MSD remains the gold standard, due to the higher NRM associated with Haplo. However, in the absence of MSD, Haplo HSCT may represent a valid and rapidly available alternative, with nearly one quarter of the R/R-AML pts surviving at 2 years from transplant. Future goals in Haplo transplantation include therefore reducing NRM, GVHD and rate of infections in order to further improve outcomes.
Conflict of interest: Nothing to disclose
O164 Outcomes after T-Replete HLA-Haploidentical Transplantation Using Post-Transplant Cyclophosphamide Compared to Matched Unrelated Donor Transplantation for Acute Myeloid Leukemia in Remission in Older Adults
Miguel-Angel Perales1, Benjamin Tomlinson2, Mei-Jie Zhang3, Andrew St. Martin3, Hillard Lazarus2, David Marks4, Rizwan Romee5, Melhem Solh6, John Wagner7, Daniel Weisdorf7, Marcos de Lima2, Mary Eapen3
1Memorial Sloan Kettering Cancer Center, New York, NY, United States; 2Case Western Reserve, Cleveland, OH, United States; 3Medical College of Wisconsin, Milwaukee, WI, United States; 4University Hospital Bristol, Bristol, United Kingdom; 5Washington University School of Medicine, St. Louis, MO, United States; 6Northside Hospital, Atlanta, GA, United States; 7University of Minnesota, Minneapolis, MN, United States
Background: Transplants are increasing performed in patients 50 years and older and it is not known if survival after haploidentical transplantation is comparable to that after transplantation with matched unrelated donors (URD).
Methods: Included are 822 patients with AML in first or second complete remission (CR) aged 50–76 years and transplanted between 2008 and 2015. Cox regression models were built for recipients of haploidentical (N = 192; CR1 76%; CR2 24%) and HLA-matched unrelated (N = 631; CR1 83%; CR2 17%) transplantations to study the effects of donor type on outcomes. Primary endpoint was overall survival.
Results: Characteristics of recipients of haploidentical and URD transplantations were similar except recipients of haploidentical transplantations had better HCT-CI, more likely to have favorable or intermediate risk cytogenetics, and to receive reduced intensity conditioning. The median time to haploidentical transplant for patients in CR1 and CR2 were 5 and 20 months, respectively. The corresponding times for URD transplant were 5 and 18 months. Marrow was the predominant graft for haploidentical and peripheral blood for URD transplantations. All recipients of haploidentical transplants received post-transplant cyclophosphamide with a calcineurin inhibitor and mycophenolate, and recipients of URD transplants, calcineurin inhibitor ± mycophenolate or methotrexate. Haploidentical donors (25% siblings and 75% offspring) were mismatched at ≥2 HLA-loci with a median donor age, 37 years (range: 17–69). Adult unrelated donors were allele-level matched at HLA-A, -B, -C and -DRB1 with a median age 27 years (range 18–40). Unrelated adult donors aged > 40 years were excluded as the median age of unrelated donors is ~30 years for recent URD transplants reported to the Center for International Blood and Marrow Transplant Research. Multivariate models showed lower incidence of grade II-IV acute graft-versus-host disease (GVHD) after haploidentical (hazard ratio [HR] 0.57, p = 0.001) and lower chronic GVHD with haploidentical marrow (HR 0.32, p < 0.001) but not haploidentical peripheral blood transplants (HR 0.92, p = 0.69). Table 1 shows the risk of non-relapse mortality, relapse and overall survival by donor type. The 3-year probability of overall survival adjusted for HCT-CI and cytogenetic risk was 43% and 47% after haploidentical compared to URD transplantations. The effect of chronic GVHD on survival was modeled as time dependent covariate; no significant effect was observed (p = 0.33).
Conclusions: These data support selecting HLA-matched adult unrelated donors aged less than 40 years when such a donor is available for patients aged 50 years or older with AML in remission.
Clinical Trial Registry: Not applicable
Conflict of interest: None to disclose
| Outcomes | Hazard Ratio | 3-year Probability |
|---|---|---|
| Non-relapse mortality | ||
| Matched URD HCT | 1.00 | 25% (95% CI 21–29) |
| Haploidentical HCT | 1.02 (95% CI 0.70–1.48) p = 0.92 | 22% (95% CI 15–29) |
| Relapse | ||
| Matched URD HCT | 1.00 | 40% (95% CI 35–44) |
| Haploidentical HCT | 0.99 (95% CI 0.72–1.37) p = 0.95 | 41% (95% CI 33–49) |
| Survival | ||
| Matched URD HCT | 1.00 | 47% (95% CI 42–51) |
| Haploidentical HCT | 1.27 (95% CI 1.01–1.59) p = 0.04 | 43% (95% CI 34–51) |
[ [O164 Table] Results of Multivariate Analyses]
Stem cell source
O165 Prognostic factors for adult single cord blood transplantation among European and Japanese populations: the Eurocord/ALWP-EBMT and JSHCT/JDCHCT collaborative study
Junya Kanda1, Hiromi Hayashi2,3, Annalisa Ruggeri2,3,4, Fumihiko Kimura5, Fernanda Volt2,3, Satoshi Takahashi6, Myriam Labopin7, Shinichi Kako8, Karina Tozatto-Maio2,3,9, Shingo Yano10, Guillermo Sanz11, Naoyuki Uchida12, Maria Teresa Van Lint13, Seiko Kato14, Mohamad Mohty4,7, Noël Milpied15, Heiwa Kanamori16, Jorge Sierra17, Yuju Ohno18, Riccardo Saccardi19, Takahiro Fukuda20, Tatsuo Ichinohe21, Minoko Takanashi22, Shinichiro Okamoto23, Arnon Nagler7,24, Yoshiko Atsuta25, Eliane Gluckman2,3
1Kyoto University, Department of Hematology and Oncology, Kyoto, Japan; 2Eurocord, Paris, France; 3Monacord, Monaco, Monaco; 4Hôpital Saint-Antoine, Service d'Hématologie Clinique et Thérapie Cellulaire, Paris, France; 5National Defense Medical College, Division of Hematology, Department of Internal Medicine, Tokorozawa, Japan; 6Institute of Medical Science, University of Tokyo, Division of Molecular Therapy, Advanced Clinical Research Center, Tokyo, Japan; 7Faculté de Médicine Saint-Antoine and EBMT Data Office, Paris, France; 8Saitama Medical Center, Jichi Medical University, Division of Hematology, Saitama, Japan; 9Universidade de São Paulo, Faculdade de Medicina de Ribeirão Preto, Ribeirão Preto, Brazil; 10Jikei University School of Medicine, Division of Clinical Oncology and Hematology, Department of Internal Medicine, Tokyo, Japan; 11Hospital Universitari i politècnic La Fe, Hematology Department, Valencia, Spain; 12Toranomon Hospital, Department of Hematology, Tokyo, Japan; 13Ospedale San Martino, Department of Haematology II, Genova, Italy; 14Institute of Medical Science, University of Tokyo, Department of Hematology/Oncology, Tokyo, Japan; 15CHU Bordeaux Hôpital Haut-leveque, Bordeaux, Italy; 16Kanagawa Cancer Center, Department of Hematology, Yokohama, Japan; 17Hospital Santa Creu i Sant Pau, Hematology Department, Barcelona, Spain; 18Kitakyushu Municipal Medical Center, Kitakyushu, Japan; 19Azienda Ospedaliera Universitaria Careggi, Cell Therapy and Transfusion Medicine Unit, Firenze, Italy; 20National Cancer Center Hospital, Hematopoietic Stem Cell Transplantation Division, Tokyo, Japan; 21Research Institute for Radiation Biology and Medicine, Hiroshima University, Department of Hematology and Oncology, Hiroshima, Japan; 22Blood Service Headquarters, Japanese Red Cross Society, Tokyo, Japan; 23Keio University School of Medicine, Division of Hematology, Department of Medicine, Tokyo, Japan; 24Chaim Sheba Medical Center, Division of Hematology and Bone Marrow Transplantation, Tel Hashomer, Israel; 25Japanese Data Center for Hematopoietic Cell Transplantation, Nagoya, Japan
Background: Unrelated cord blood transplantation (CBT) has been actively performed worldwide. Large differences in patient and transplant backgrounds make it difficult to identify consistent prognostic factors among different populations. To address this, we performed a collaborative study between Eurocord/the Acute Leukemia working party (ALWP) of European Society for Blood and Marrow Transplantation (EBMT) and the Japan Society for Hematopoietic Cell Transplantation (JSHCT)/Japanese Data Center for Hematopoietic Cell Transplantation (JDCHCT).
Methods: Adults aged 18–75 years with acute leukemia who received the first single unrelated CBT between 2000 and 2014 were eligible. Patients who received manipulated or multiple CBTs, or CBT combined with other cell sources were excluded. A total of 3764 and 1027 patients in the JSHCT/JDCHCT and Eurocord/ALWP-EBMT registries, respectively, were included. We analyzed the effect of patient, donor, and transplant characteristics on outcomes separately in each registry cohort.
Results: Median follow-up of survivors was 49 and 44 months in the JSHCT/JDCHCT and Eurocord/ALWP-EBMT registries, respectively. Median age of the Japanese and European cohorts was 51 (18–75) and 38 (18–74) years, correspondingly. The JSHCT/JDCHCT cohort consisted of more male patients (56% vs. 49%), had lower median weight (55 vs. 65Kg) and higher refined disease-risk-index (rDRI) (56% vs. 33%) than the European cohort. Three or more HLA mismatches were more frequently observed in the JSHCT/JDCHCT than in Eurocord/ALWP-EBMT (23% vs. 4%) cohort. Median TNC count (×107/kg) was 2.58 and 3.51 in the JSHCT/JDCHCT and Eurocord/ALWP-EBMT cohorts, respectively. Reduced-intensity conditioning was used in 35% and 25% of the Japanese and European cohorts, respectively. Anti-thymocyte globulin was used in only 2% of the JSHCT/JDCHCT cohort in contrast to 65% of the Eurocord/ALWP-EBMT. Calcineurin inhibitor plus mycophenolate mofetil was used as GVHD prophylaxis in 21% of the Japanese patients and in 46% of the Europeans. Calcineurin inhibitor plus methotrexate was used in 60% of the Japanese cohort but only in 5% of the European cohort. Overall survival (OS) at 3-year was 41% in JSHCT/JDCHCT and 33% in Eurocord/ALWP-EBMT.
In the multivariate analysis, TNC dose and HLA matching had no significant effect on OS, in either cohort, whereas transplant year, patient age, and rDRI had an impact on OS in both populations. The impact of center experience on OS was more prominent in the JSHCT/JDCHCT than Eurocord/ALWP-EBMT cohort. HLA matching (≥2 vs. 0–1) was significantly associated with lower risk of relapse and higher risk of non-relapse mortality in the Japanese population, but not in the European. Higher TNC dose (>3.5 vs. 2.5–3.5 × 107/kg) was significantly associated with better neutrophil engraftment only in the JSHCT/JDCHCT, but had no significant impact on other outcomes in either cohort. As primary cause of death, infection and GVHD were more frequently observed in the Eurocord/ALWP-EBMT cohort, whereas graft failure, organ failure, and hemorrhage were more frequent in the Japanese cohort.
Conclusions: Despite considerable differences in patient and transplant characteristics between the Japanese and European cohorts, we observed similar prognostic factors affecting CBT outcomes in adult patients with acute leukemia in both registries.
Clinical Trial Registry: not applicable
Conflict of interest: none
O166
Abstract previously published
O167 Rapid and Robust CD4+ and CD8+ T-, NK-, B- and monocyte cell reconstitution after Nicotinamide-expanded Cord Blood (NiCord) Transplantation
Stefan Nierkens, Coco de Koning, Jaap Jan Boelens
UMC Utrecht, Translational Immunology, Utrecht, Netherlands
Background: Nicotinamide-expanded cord blood (NiCord) is a promising alternative source for allogeneic hematopoietic cell transplantation (HCT) when an HLA-identical donor is lacking. Results from a phase 1/2 trial, with NiCord as standalone HCT, showed rapid neutrophil engraftment (median 11days) and thrombocyte engraftment (median 34days). However, successful CD4+-reconstitution has shown to be crucial for disease and viral control and is associated with favorable survival chances (Admiraal et al. JACI2017). We therefore evaluated early immune reconstitution (IR) after NiCord transplantation.
Methods: In the phase1/2 multicenter trial, patients (n = 36) with hematologic malignancies received a standalone NiCord-transplantation after myeloablative (MA) conditioning (without ATG). Immune monitoring was performed (with harmonized sampling, handling and analyses in a central lab) in a random subgroup. The primary endpoint was probability of achieving CD4+ T-cell IR (>50*106/L within 100 days). Secondary endpoints were IR over time of CD4+, CD8+, naïve and effector/memory T-cell subsets, monocytes, natural killer (NK) and B-cells during the first 6 months after HCT. Data were compared with IR in cohorts of adolescent and young adult (AYA) patients at the UMC Utrecht receiving either unmanipulated cord blood transplantation (unCBT) or T-repleted unrelated bone marrow transplantation (BMT) for hematological malignancy after MA conditioning without ATG. Linear-mixed effects modelling in LOESS-regression curves and two-sided log-rank test for univariate comparisons in cumulative incidence plots were used.
Results: 15 NiCord recipients (median age 41.5; 13.4–61.7 yrs) were included. NiCord cell dose consisted of median 6,4*106 CD34+/kg, and 2.3*106 CD3+ T-cells/kg of the co-infused negative fraction (obtained following CD133+-selection). Over 85% of patients achieved successful CD4+ IR after NiCord and over-time CD4+, monocyte, NK and B-cell reconstitution was rapid and robust (Fig 1, 2). When comparing the NiCord with 20 CBT (median age 15.4; 12.2–22.1 yrs) and 14 BMT (median age 14.3; 12.1–19.7 yrs), no difference in probability of CD4+-IR was noted (p = 0.98: Fig 1). Overall T-cell IR (CD3+; p = 0.57, CD4+; p = 0.53, CD8+; p = 0.26) was similar, while IR of NK-cells (p < 0.001), B-cells (p = 0.017) and monocytes (p < 0.001), was faster after transplantation with NiCord, compared to unCBT and BMT cohorts (Fig 2A-C).
Conclusions: In summary, in addition to NiCord rapid neutrophil and platelets reconstitution, also IR of CD4+T, NK, B and monocyte -cells was rapid and robust and appears favorable even in comparison to the 2 “younger” unCBT and BMT cohorts, expected to achieve faster IR. This may be explained by the higher stem cell dose and higher proliferative capacity of NiCord- expanded product.
Conflict of interest: The authors declare no conflicts of interest.
[O167 Figure].
Figures]
O168 FMS-like receptor tyrosine kinase 3 ligand treatment of bone marrow donors increases survival and GvT in allo-BMT recipients while reducing GvHD in murine transplant model
Mojibade Hassan1, Alina Ulezko1, Edmund Waller1,2
1Emory University, Atlanta, GA, United States; 2Winship Cancer Institute of Emory University, Hematology and Medical Oncology, Atlanta, GA, United States
Background: BMT CTN 0201 data indicate that high numbers of plasmacytoid dendritic cells (pDC) in donor grafts resulted in increased survival and reduced graft-versus-host disease (GvHD) in recipients of bone marrow (BM), but not granulocyte-colony stimulating factor peripheral blood grafts. Our data demonstrated that donor BM pDC modulate post-transplant outcomes through an IL-12 pathway with a T cell dependent IFNy feedback loop resulting in upregulation of IDO in pDC with no loss of graft-versus-tumor (GvT) effect, but a reduction in GvHD. The studies described here examined the effect of FMS-like receptor tyrosine kinase 3 ligand (Flt3L) treated BM (F-BM) and pDC on transplant outcomes in a murine transplant model.
Methods: Mice were treated with 300ug/kg of Flt3L on days -1 and -4. Cells counts of pDC and HSC in murine F-BM grafts were analyzed by flow cytometry. Murine H2b to H2k transplants were performed to compare survival and GVHD of BM versus F-BM donor graft recipients. A lymphoblastic T cell leukemia cell line, LBRM was used to assess GvT and survival in a murine tumor model. Gene expression of human BM and Ftl3L mobilized peripheral blood (F-PB) samples was assessed by Illumina chip. Differential expression analysis was performed using Limma R package.
Results: Flt3L increased pDC content 3-fold and HSC content 1.5-fold in BM grafts. Transplantation of Flt3L treated BM with untreated T cells increased survival to 75% from 45% in recipients of untreated BM with T cells (Figure 1A). Flt3L treated BM grafts with T cells increased survival in a tumor model to 60% as compared to survival of mice transplanted with untreated BM grafts, which had a survival of 30%. Finally, human gene expression data show that BM pDC upregulate innate immunity pathways including toll-like receptor pathways more than F-PB pDC (Figure 1B).
Conclusions: Flt3L increases pDC number in a BM graft. The use of F-BM grafts with or without T cells show a beneficial effect of Flt3L treatment on survival and GvHD due to limited GvHD without deletion of GvT activity of donor T cells. Furthermore, F-BM grafts increase survival in a murine tumor model. The difference in gene expression that results when comparing BM and F-PB samples show that BM pDC activate the innate immune system more than F-PB pDC and this may be the reason why there are differences in survival and GvHD. Ultimately, the use of Flt3L treatment for BM donors may prove to be an effective method to increase survival and GvT while reducing GvHD in recipients post transplant.
(A) Murine transplant recipients received 5 million T cell depleted BM of F-BM cells with or without 4 million untreated T cells as indicated in the legend. Survival curve of murine transplant. *P < .05 represents a log-rank survival curve comparison. (B) Volcano plot of human gene expression of BM compared to Flt3L treated mobilized peripheral blood (F-PB). Toll-like receptor cascades were upregulated in BM samples compared to F-PB (P = 2.48E-04).
Conflict of interest: The authors have nothing to disclose.
[[O168 Figure].
Flt3L treatment increases survival and downregulates innate immune system pathways]
Footnotes
18-21 March 2018 ● Lisbon, Portugal



![[O014 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/3c484f948008/41409_2018_318_Figa_HTML.jpg)
![[O022 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/81a7222e2bc9/41409_2018_318_Figb_HTML.jpg)
![[O026 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/7ebf3266d129/41409_2018_318_Figd_HTML.jpg)
![[O035 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/69116261d5d9/41409_2018_318_Figg_HTML.jpg)
![[[O047 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/d2c3dc9bb1fd/41409_2018_318_Figh_HTML.jpg)
![[O057 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/66157cff8a3f/41409_2018_318_Figj_HTML.jpg)
![[[O059 Figure] table 1]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/8ca32d8f57d8/41409_2018_318_Figk_HTML.jpg)
![[O063 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/107cac99e304/41409_2018_318_Figm_HTML.jpg)
![[O069 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/ed0705e19b8d/41409_2018_318_Fign_HTML.jpg)
![[O078 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/195f740ecd9c/41409_2018_318_Figp_HTML.jpg)
![[O084 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/cabd0268ef02/41409_2018_318_Figq_HTML.jpg)
![[[O089 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/fbb4cab3816a/41409_2018_318_Figs_HTML.jpg)
![[O106 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/28e46f0d0764/41409_2018_318_Figv_HTML.jpg)
![[O111 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/ffbe714a5cc9/41409_2018_318_Figw_HTML.jpg)
![[[O115 Figure] Table]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/7c3f47463e27/41409_2018_318_Figy_HTML.jpg)
![[O124 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/1bc1cf86dffb/41409_2018_318_Fig27_HTML.jpg)
![[[O140 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/e4557f2407f4/41409_2018_318_Fig30_HTML.jpg)
![[[O154 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/ade0fb6f1bfc/41409_2018_318_Fig32_HTML.jpg)
![[[O157 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/f160fe839964/41409_2018_318_Fig33_HTML.jpg)
![[[O159 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/d90e129455b5/41409_2018_318_Fig34_HTML.jpg)
![[O167 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/a4c86234dcc0/41409_2018_318_Fig36_HTML.jpg)
![[[O168 Figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7513/7092343/8038ee0012f8/41409_2018_318_Fig37_HTML.jpg)