Abstract
In recent years, different classifications for muscle injuries have been proposed based on the topographic location of the injury within the bone-tendon-muscle chain. We hereby propose that in addition to the topographic classification of muscle injuries, a histoarchitectonic (description of the damage to connective tissue structures) definition of the injury be included within the nomenclature. Thus, the nomenclature should focus not only on the macroscopic anatomy but also on the histoarchitectonic features of the injury.
Keywords: muscle injuries, sport injuries, extracellular matrix, connective tissue, muscle injury anatomy
Skeletal muscle is composed of skeletal muscle cells and the surrounding connective tissue distributed in a highly organized manner. Naturally, this organ is supplied by small vessels, capillaries, and nerves and also contains a variable number of cells from the immune system.14,17 Skeletal muscle cells are cylindrical and striated multinucleated cells called myofibers due to their elongated shape, and their main function is to generate contractile forces for locomotion.30 Connective tissue has its own cells: fibroblasts. Depending on where it is located in the muscle, this connective tissue shows marked differences in the composition and specific distribution of the extracellular matrix molecules. These variable features make it more or less organized with variable flexibility, thereby carrying out different yet highly specific functions within the muscle.15,17 Thus, the extracellular matrix plays a key role in maintaining the structure and organization of muscle fibers, is highly integral to force transmission, and is essential for the proper regeneration following injury.14,22,24,38 Furthermore, the extracellular matrix generates biochemical signals that regulate myogenesis and modulate various growth factors.6,13,39
In terms of cell biology, the extracellular matrix of muscle consists of 3-dimensional networks made of different collagen molecules, proteoglycans, and noncollagenous extracellular matrix proteins such as laminin, fibronectin, and other adhesion molecules.37 For clinical applications, the concept of the extracellular matrix of skeletal muscle is usually simplified; it is considered simply as the connective tissue structure that surrounds the muscle and is crucial for the mechanical integrity of the tissue. This connective structure is also known by the global term fascia.1
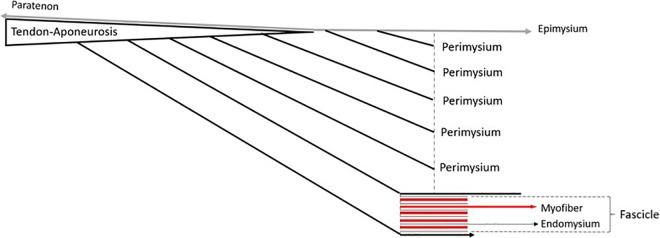
The fibrous collagen networks are arranged in 3 differentiated layers in the muscle belly: endomysium, perimysium, and epimysium.1,14 The endomysium surrounds each individual myofiber and is composed mainly of collagen types I, III, and V.16 The perimysium surrounds several fascicles of myofibers and is composed mainly of collagen types I and III. The epimysium surrounds the entire muscle and is also composed mainly of collagen types I and III. Beyond these 3 structures, type IV collagen is mainly expressed in the basal membrane of the myofiber.19 These 3 structures make up what is known as the myofascial junction (MFJ) between muscle and fascia or the myotendinous junction (MTJ) between muscle and tendon, which mainly has structural function but may also have a mechanical function.5
The aponeuroses have a direct connective continuum with the MFJ via the perimysium.14 Histologically, the perimysium joins the aponeurosis at the ends of the muscle fascicles, facilitating the overall functional unity of the skeletal muscle between the MFJ and the tendons themselves (Figure 1).
Figure 1.
Outline of the global functional unit of skeletal muscle. A connective continuum is seen from the endomysium to the tendon-aponeurosis via the union of the perimysium to the aponeurosis.
In a muscle injury, a myoconnective junction (MCJ) is always involved. This MCJ can be located in a myotendinous junction (MTJ) when the lesion affects an aponeurosis or a tendinous expansion attached to muscle fiber or when it involves an MFJ, that is, when the muscle fiber is attached to the epimysium or perimysium.
Nomenclature of Muscle Injuries Based on Anatomy: Optimizing the Topographic Level of Injury
Different classifications for muscle lesions have been proposed based on the topographic location of the lesion7,26,28,34 but without accurate identification of its level. All indirect muscle injuries occur in MCJs, either at the tendinous aponeurotic level in the MTJ or in the MFJ (epimysium, perimysium). To distinguish injuries to the MCJ, we divide the injuries into 2 main categories: MTJ and MFJ injuries (Table 1). We propose that an MCJ is always involved in a skeletal muscle injury. The affected MCJ can be located either in an MTJ (the lesion affects an aponeurosis or a tendinous expansion attached to muscle fibers) or in the MFJ, when the injury involves muscle fiber and their perimysium and/or epimysium.
Table 1.
Injuries to the Myoconnective Junctiona
| Injury of the peripheral MCJ (MTJ-MFJ continuum) |
| Injury of the MTJ |
| Tendinous (or aponeurotic) injury |
| Myotendinous (or myoaponeurotic) injury |
| Muscle (or intramuscular) injury |
| Injury of the MFJ |
| Without rupture of the epimysium |
| With rupture of the epimysium |
| Injury of the internal or central MCJ |
| Tendinous (or aponeurotic) injury |
| Myotendinous (or myoaponeurotic) injury |
| Muscle (or intramuscular) injury |
aMCJ, myoconnective junction; MFJ, myofascial junction; MTJ, musculotendinous junction.
Although MCJs may be similar to the MTJ from a histological point of view, their macroscopic appearance is determined by their thicknesses (tendon > aponeurosis > myofascia).5 Thus, the 2 opposite end components of the continuum between an MTJ and the MFJ can be clearly differentiated at the level of gross anatomy (in the dissection room or in the operating theater).5 The weakest part of the bone-tendon-muscle chain in adults is the MCJ. Injuries can be located at 2 levels (the peripheral MCJ and the internal or central MCJ) if the muscle contains internal fascia structure.
Peripheral MCJ Injuries
Peripheral MCJ injuries mainly affect the MTJ (ie, the muscle-aponeurosis [or tendon] interface). These are the most common types of injury in the hamstring tendon, the posterior aponeurosis of the rectus femoris, and the medial head of the gastrocnemius (ie, tennis leg). In addition, the areas adjacent to the MTJ can be affected at varying distances, specifically in the perimysium and epimysium (ie, in the nearby MFJ). This type of injury can occur proximal to the posterior aponeurosis of the rectus femoris, at the level of its epimysium.
Classically, injuries that occur in the periphery of a muscle tend to be called myofascial injuries. Indirect MFJ injuries are infrequent because myotendinous injuries in the muscle periphery are often erroneously interpreted as myofascial injuries. Myofascial injuries are usually produced by traction of the epimysium-perimysium from a proximal aponeurosis, which can break it or damage its connection to the muscle fiber.
Central MCJ Injuries
Internal or central MCJ injuries are those affecting the central tendon. These injuries are the intramuscular homolog of peripheral MCJ injuries except that they are surrounded on all surfaces by muscle fascicles and the corresponding perimysium. The main difference between the 2 is that the internal or central MCJ lacks the characteristic external lining of the muscle (epimysium). Typical examples of this type of injury are those of the indirect tendon of the rectus femoris and those of the central tendon of the soleus.
Histological Nomenclature of Muscle Injuries: Optimizing the Histoarchitecture of the Injury
To understand the behavior of a muscle injury, provide an accurate prognosis, and understand why some injuries recur, one needs take into account not only the anatomic and topographic location but also the type and level of histoarchitectural involvement (ie, degree of damage to connective tissue structure) (Table 1). Two injuries in exactly the same topographic location and with the same treatment can heal differently depending on the degree of the damage to the connective tissue component (ie, the extent of damage to its extracellular matrix).5
Injuries of either MCJ—peripheral or central-internal—may have different degrees of histoarchitectural involvement. In this work, we have updated the nomenclature with respect to that in a previous report5 through broad discussions and have ultimately reached a consensus with more authors. Thus, we can define different types of injury depending on their histoarchitecture.
Tendinous (Aponeurotic) Injuries
Tendinous injuries have direct aponeurotic involvement, with an observable gap in the tendinous tissue. In turn, this gap can have different orientations, such as transversal, longitudinal (in splits), or mixed orientation. Retraction of the muscle fibers is observed only when a transverse gap is present. Similarly, fibrous repair secondary to a transverse rupture is greater than that following longitudinal rupture, and thus, these injuries have a greater chance of reinjury. In short, it is essential to assess the type of rupture to guide prognosis and assess the risk of reinjury (Figure 2).
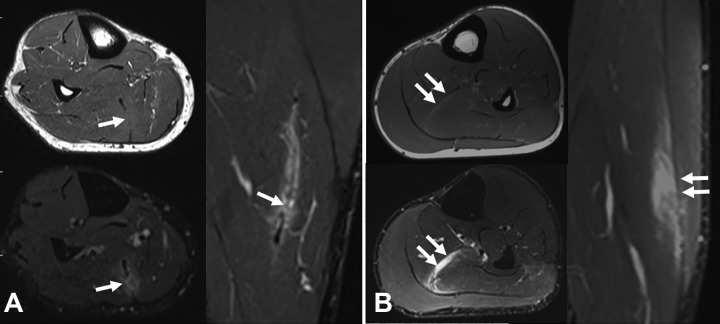
Figure 2.
Three different central tendon injuries of the rectus femoris according to their tendinous gap. Sagittal and axial T2-weighted fat-saturated magnetic resonance imaging scans of (A) a transversal tendon gap (arrows), (B) a longitudinal tendon gap (split, arrows), and (C) a mixed tendon gap: longitudinal (arrows) and transversal (arrowheads).
Myotendinous (Myoaponeurotic) Injuries
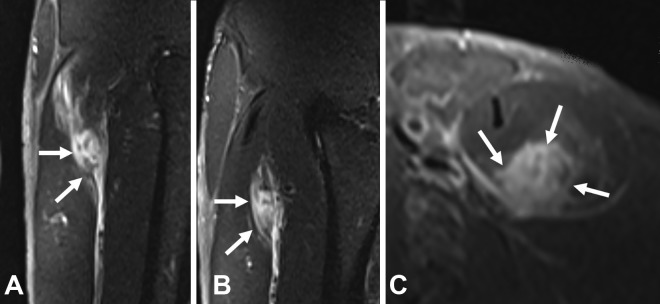
Myotendinous injuries are located at the interface between the aponeurosis and the fiber or muscle fasciculus (and its corresponding perimysium). In this type of injury, the tendon or aponeurosis is affected either focally by small muscle fiber tears or by major tears that produce a muscle gap but not a tendinous gap (Figure 3).
Figure 3.
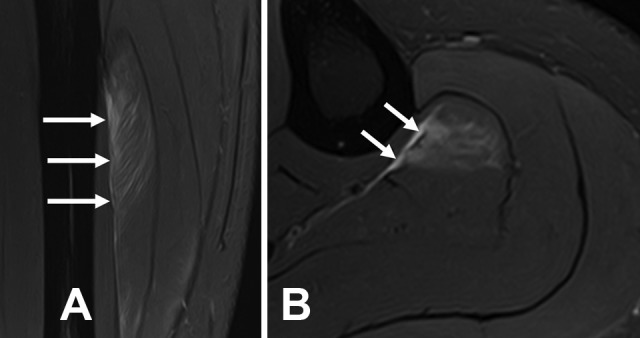
Myotendinous injury located in the anterior aponeurosis of the soleus (arrows). (A) Sagittal and (B) axial T2-weighted fat-saturated magnetic resonance imaging scans. The aponeurosis is affected focally by small retraction of the muscle fiber.
Muscle (Intramuscular) Injuries
Muscle injuries occur in the interface between the perimysium and the fiber or muscle fascicle to which it is attached but with no direct involvement of the tendon or aponeurosis, as the rupture occurs at a distance from them. These injuries are generally secondary to traction at a distance from an aponeurosis or tendon. In these cases, the injury is usually called intramuscular, although, in fact, the MCJ is also involved (Figure 4).
Figure 4.
A case of intramuscular injury in the rectus femoris (arrows). (A) Coronal and (B) sagittal T2-weighted fat-saturated magnetic resonance imaging scans. This type of injury entails no direct or indirect involvement of the tendon or aponeurosis, even though the injury has actually been caused by the traction of a myoconnective junction, in this case by the central tendon of the rectus femoris.
Myofascial Injuries (Injuries in the MFJ)
The affected MFJ is located in the peripheral MCJ and may or may not damage the epimysium. If the epimysium is intact, bleeding occurs in the muscle and is limited by the epimysial fascia (Figure 5). If the epimysium breaks, the edema and fluid migrate along the intermuscular planes; because the epimysium is composed of a variable amount of loose connective tissue20 that has marked distensibility, it is deformed by the bleeding itself. Clinically, these injuries are very well tolerated and even may go unnoticed.
Figure 5.
A case of myofascial injury close to the proximal part of the posterior aponeurosis of the rectus femoris (arrows). (A) Coronal, (B) sagittal, and (C) axial T2-weighted fat-saturated magnetic resonance imaging scans. In this case, since the epimysium is intact, bleeding occurs in the muscle but is limited by the epimysial fascia.
The healing time of an injury is always longer if the injury affects the tendon-aponeurotic structures. Although magnetic resonance imaging (MRI) assessment of the muscle fiber may predict regeneration in approximately 3 weeks,23,25 the repair takes considerably longer when the injury involves the tendon or aponeurosis.8,28,29 This highlights the need to account for connective tissue or fascial involvement in the clinical assessment of the injury. Furthermore, injury can cause damage to all 3 levels of histoarchitectonic structures, leading to an even more uncertain prognosis.
In previous reports, some authors have proposed that injuries in the internal or central MCJ do not have a worse prognosis than those in the peripheral MCJ,36,37 which is justified if the level and degree of histoarchitectural involvement are taken into account rather than just the location (central or peripheral). Thus, some injuries of the central MCJ without connective tissue damage or involvement (muscle injury or intramuscular injury) may have a better prognosis than some peripheral injuries that do have connective tissue damage or involvement.
Proposal to Standardize the Nomenclature
To standardize the nomenclature of muscle injuries according to the model presented here, which we hope will facilitate communication between professionals and ultimately lead to more accurate clinical diagnoses, we propose the following requirements for describing muscle injuries:
Histoarchitectonic level: tendinous (or aponeurotic), myotendinous (myoaponeurotic), muscle (or intramuscular), or myofascial injury
Topographical anatomic level: peripheral MCJ or internal (or central) MCJ
Nominal anatomic level: specific name of the injured structure according to international anatomic terminology
In addition to this nomenclature, which is not a classification, other important clinical details should be added, such as the presence or absence of hematoma or the distance from the tendon insertion, which for some muscles is known to be an important contributing factor for management and prognosis.
Analysis of Typical Injuries According to the Model
Hamstring Tendons
The role of intramuscular tendon involvement is controversial.35 Of the various prognostic factors implicated in injuries of the common hamstring tendon, involvement of the “free tendon” (distance from bony origin or insertion to the point where muscle tissue starts) is particularly important regarding return-to-play (RTP) time and risk of reinjury.2,3 Another important factor is the distance from the injury to the origin of the hamstring, with shorter distances being associated with longer RTP.3,12,32 This is consistent with our proposed model for understanding muscle injuries. Tendon injury has the worst prognosis21,28; the closer the injury occurs to the tendon origin or insertion, the larger the connective tissue lesion. Other important prognostic factors, although not as important as the ones mentioned above, are the length of edema,9,31 its cross-sectional area,2,9,31,33 its volume,2 and the degree of injury.11 These factors, combined with information on location and histoarchitectural involvement, could help to determine the true prognosis of proximal hamstring injuries more accurately.
Injuries to the distal MTJ of the biceps femoris present a special clinical challenge due to its complex anatomic structure and dual innervation to the short and long head of the muscle.11 As the long and short head of the biceps femoris merge together at the distal MTJ, they form a T-shaped aponeurosis. Injuries occurring in this region are demanding to treat; despite long rehabilitation times, the recurrence rates are the highest among skeletal muscle injuries reported to date, as more than 50% of patients sustain reinjury.11 Because the maturation of scar tissue is a slow process, it has been recommended that MRI be used to determine proper scar maturation before RTP can be considered.11
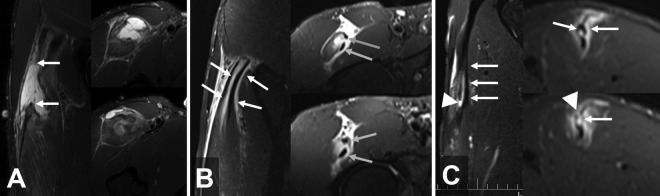
We can observe all 3 types of histoarchitectonic involvement in the common hamstring tendon in daily clinical practice: direct tendon involvement with gap (Figure 6A), purely myotendinous injury without gap (Figure 6B), and muscle injury produced by traction of the common tendon but without direct contact with it (Figure 6C). Comin et al8 used MRI to study gaps or fiber retractions in the central tendon and found these factors to be associated with significantly longer RTP times than injuries without these factors. In this sense, an injury with a tendinous gap leads to the retraction of the affected myofibers, and the time required to repair the injured connective tissue results in a longer duration before RTP.
Figure 6.
Three different cases of common tendon injury of the biceps femoris. Coronal and axial T2-weighted fat-saturated magnetic resonance imaging scans of (A) a tendinous rupture (arrow), (B) a myotendinous rupture (arrow), and (C) an intramuscular rupture (arrow).
Next, we provide a few representative examples from common and clinically demanding skeletal muscle injuries.
Central Tendon of the Soleus
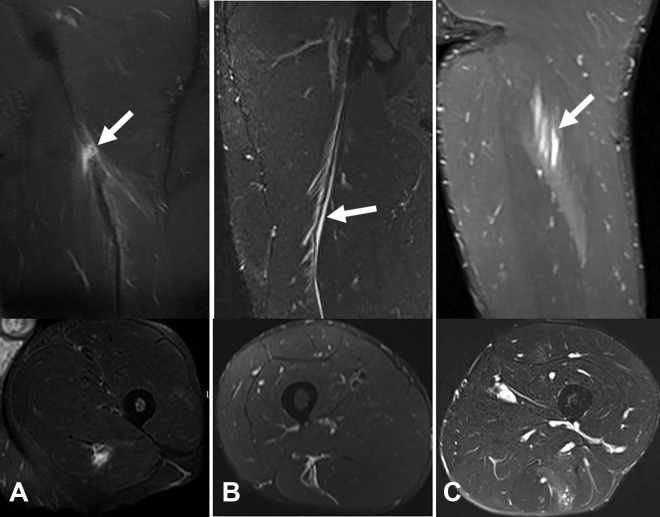
In a series of 100 calf injuries, Prakash et al29 observed that interruption of the muscle connective tissue resulted in a longer recovery time and a longer RTP time than injuries that did not affect the central tendon of the soleus. Although no reported studies have focused only on injuries of the central tendon of the soleus, it seems likely that a central tendon injury with tendon gap (Figure 7A) will have a more complex progression than an injury in which the connective structure is not damaged (Figure 7B). This should be taken into account when one is evaluating an injury to this muscle. In general, injuries affecting the tendon of the central MCJ entail a longer RTP time than those in the peripheral MCJ.27,29 This rule also applies for the central tendon of the rectus femoris.4,10
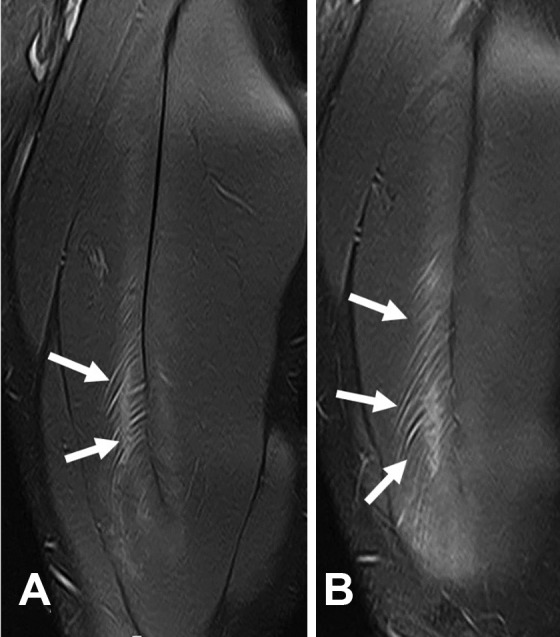
Figure 7.
Two different cases of central tendon injury of the soleus. (A) Axial T1-weighted and axial and sagittal T2-weighted fat-saturated magnetic resonance imaging (MRI) scans of tendinous rupture. The arrows indicate the tendon gap in both T1-weighted and T2-weighted images. (B) Axial T1-weighted and axial and sagittal T2-weighted fat-saturated MRI scans of myotendinous rupture. The arrows indicate the integrity of the central tendon in both T1-weighted and T2-weighted images.
Posterior Aponeurosis of the Rectus Femoris
According to Cross et al,10 injuries in the posterior peripheral MCJ of the rectus femoris have a faster RTP than those in the central MCJ of the central tendon. If the injury occurs in the distal part of the posterior aponeurosis, one may observe a tendinous, myotendinous, or intramuscular injury (Figure 8, A-C, respectively). If the injury is located in the proximal part of the peripheral MCJ, this may affect the posterior aponeurosis itself (myoaponeurotic injury) (Figure 8D) and/or the epimysium (myofascial injury) proximal to it (Figure 8E). True myofascial injuries are those that affect the epimysium only. In the peripheral MCJ of the rectus femoris, pure myofascial injuries are usually located in the posterolateral area18 and are accompanied by interfascial hematoma. In routine clinical practice, this injury is very well-tolerated and can be a casual finding.
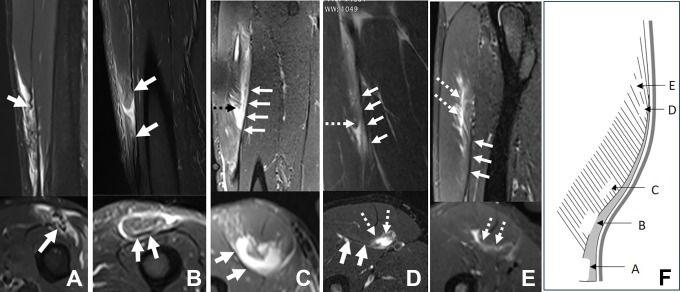
Figure 8.
Five different cases of posterior aponeurosis injury. Sagittal and axial T2-weighted fat-saturated magnetic resonance imaging scans of (A) a tendinous rupture (with posterior aponeurosis retracted), (B) a myotendinous rupture, (C) an intramuscular rupture, (D) a myoaponeurotic rupture, and (E) a myofascial injury. (F) Scheme in relation to these injuries. The white solid arrows indicate the posterior aponeurosis; the white and black dashed arrows indicate the injury.
Conclusion
We propose that an exact description of the affected MCJ, together with knowledge of any histoarchitectural damage to the connective tissue structures, improves our understanding of the clinical characteristics of the injured skeletal muscle. These rules, in turn, can be applied to all skeletal muscle injuries. Considering clinical experience and evidence from published studies, we propose that when an injury in the MCJ has a tendon gap that is detectable by MRI, the injury will heal more slowly and pose a greater risk of reinjury than would an injury without such a connective tissue lesion. Similarly, the injury has a better prognosis if it is purely muscular or if it is myofascial but without clear involvement of the neighboring tendon (or aponeurosis). Moreover, all 3 types of histoarchitectonic involvement can co-occur in the same injury, and it is very difficult to make an accurate prognosis in these cases. However, the determination of scar tissue maturity by MRI might be helpful during follow-up to ascertain readiness to RTP.
Footnotes
Final revision submitted January 10, 2020; accepted January 10, 2020.
References
- 1. Adstrum S, Hedley G, Schleip R, et al. Defining the fascial system. J Bodyw Mov Ther. 2017;21:173–177. [DOI] [PubMed] [Google Scholar]
- 2. Askling CM, Tengvar M, Saartok T, et al. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35:197–206. [DOI] [PubMed] [Google Scholar]
- 3. Askling CM, Tengvar M, Saartok T, et al. Acute hamstring injuries in Swedish elite football: a prospective randomized controlled clinical trial comparing two rehabilitation protocols. Br J Sports Med. 2013;47:953–959. [DOI] [PubMed] [Google Scholar]
- 4. Balius R, Maestro A, Pedret C, et al. Central aponeurosis tears of the rectus femoris: practical sonographic prognosis. Br J Sports Med. 2009;43:818–824. [DOI] [PubMed] [Google Scholar]
- 5. Balius R, Alomar X, Pedret C, et al. Role of the extracellular matrix in muscle injuries: histoarchitectural considerations for muscle injuries. Orthop J Sports Med. 2018;6:2325967118795863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blasi M, Blasi J, Domingo T, et al. Anatomical and histological study of human deep fasciae development. Surg Radiol Anat. 2015;37:571–578. [DOI] [PubMed] [Google Scholar]
- 7. Chan O, Del Buono A, Best TM, et al. Acute muscle strain injuries: a proposed new classification system. Knee Surg Sports Traumatol Arthrosc. 2012;20:2356–2362. [DOI] [PubMed] [Google Scholar]
- 8. Comin J, Malliaras P, Baquie P, et al. Return to competitive play after hamstring injuries involving disruption of the central tendon. Am J Sports Med. 2013;41:111–115. [DOI] [PubMed] [Google Scholar]
- 9. Connell DA, Schneider-Kolsky ME, Hoving JL, et al. Longitudinal study comparing sonographic and MRI assessments of acute and healing hamstring injuries. AJR Am J Roentgenol. 2004;183:975–984. [DOI] [PubMed] [Google Scholar]
- 10. Cross TM, Gibbs N, Houang MT, et al. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32:710–719. [DOI] [PubMed] [Google Scholar]
- 11. Entwisle T, Ling Y, Splatt A, Brukner P, Connell D. Distal musculotendinous T junction injuries of the biceps femoris: an MRI case review. Orthop J Sports Med. 2017;5(7):2325967117714998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ekstrand J, Healy JC, Walden M, et al. Hamstring muscle injuries in professional football: the correlation of MRI findings with return to play. Br J Sports Med. 2012;46:112–117. [DOI] [PubMed] [Google Scholar]
- 13. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44:318–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grounds MD. Complexity of Extracellular Matrix and Skeletal Muscle Regeneration: Skeletal Muscle Repair and Regeneration. Dordrecht, the Netherlands: Springer Netherlands; 2008:269–302. [Google Scholar]
- 16. Jakobsen JR, Mackey AL, Knudsen AB, et al. Composition and adaptation of human myotendinous junction and neighboring muscle fibers to heavy resistance training. Scand J Med Sci Sports. 2017;12:1547–1559. [DOI] [PubMed] [Google Scholar]
- 17. Jarvinen TA, Jarvinen TL, Kaariainen M, et al. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745–764. [DOI] [PubMed] [Google Scholar]
- 18. Kassarjian A, Rodrigo RM, Santisteban JM. Current concepts in MRI of rectus femoris musculotendinous (myotendinous) and myofascial injuries in elite athletes. Eur J Radiol. 2012;81:3763–3771. [DOI] [PubMed] [Google Scholar]
- 19. Kovanen V. Intramuscular extracellular matrix: complex environment of muscle cells. Exerc Sport Sci Rev. 2002;30:20–25. [DOI] [PubMed] [Google Scholar]
- 20. Langevin HM, Huijing PA. Communicating about fascia: history, pitfalls, and recommendations. Int J Ther Massage Bodywork. 2009;2:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Macdonald B, McAleer S, Kelly S, Chakraverty R, Johnston M, Pollock N. Hamstring rehabilitation in elite track and field athletes: applying the British Athletics muscle injury classification in clinical practice. Br J Sports Med. 2019;53(23):1464–1473. [DOI] [PubMed] [Google Scholar]
- 22. Mackey AL, Kjaer M. The breaking and making of healthy adult human skeletal muscle in vivo. Skelet Muscle. 2017;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mackey AL, Magnan M, Chazaud B, et al. Human skeletal muscle fibroblasts stimulate in vitro myogenesis and in vivo muscle regeneration. J Physiol. 2017;595:5115–5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahdy MAA. Skeletal muscle fibrosis: an overview. Cell Tissue Res. 2019;375(3):575–588. [DOI] [PubMed] [Google Scholar]
- 25. Marotta M, Sarria Y, Ruiz-Roig C, et al. Laser microdissection-based expression analysis of key genes involved in muscle regeneration in mdx mice. Neuromuscul Disord. 2007;17:707–718. [DOI] [PubMed] [Google Scholar]
- 26. Mueller-Wohlfahrt H-W, Haensel L, Mithoefer K, et al. Terminology and classification of muscle injuries in sport: the Munich consensus statement. Br J Sports Med. 2013;47:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pedret C, Rodas G, Balius R, et al. Return to play after soleus muscle injuries. Orthop J Sports Med. 2015;3:2325967115595802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pollock N, James SLJ, Lee JC, et al. British athletics muscle injury classification: a new grading system. Br J Sports Med. 2014;48:1347–1351. [DOI] [PubMed] [Google Scholar]
- 29. Prakash A, Entwisle T, Schneider M, et al. Connective tissue injury in calf muscle tears and return to play: MRI correlation. Br J Sports Med. 2018;52:929–933. [DOI] [PubMed] [Google Scholar]
- 30. Sambasivan R, Tajbakhsh S. Adult skeletal muscle stem cells. Results Probl Cell Differ. 2015;56:191–213. [DOI] [PubMed] [Google Scholar]
- 31. Schneider-Kolsky ME, Hoving JL, Warren P, et al. A comparison between clinical assessment and magnetic resonance imaging of acute hamstring injuries. Am J Sports Med. 2006;34:1008–1015. [DOI] [PubMed] [Google Scholar]
- 32. Silder A, Sherry MA, Sanfilippo J, et al. Clinical and morphological changes following 2 rehabilitation programs for acute hamstring strain injuries: a randomized clinical trial. J Orthop Sports Phys Ther. 2013;43:284–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slavotinek JP, Verrall GM, Fon GT. Hamstring injury in athletes: using MR imaging measurements to compare extent of muscle injury with amount of time lost from competition. AJR Am J Roentgenol. 2002;179:1621–1628. [DOI] [PubMed] [Google Scholar]
- 34. Valle X, Alentorn-Geli E, Tol JL, et al. Muscle injuries in sports: a new evidence-informed and expert consensus-based classification with clinical application. Sports Med. 2017;47(7):1241–1253. [DOI] [PubMed] [Google Scholar]
- 35. Van der Made AD, Almusa E, Whiteley R, et al. Intramuscular tendon involvement on MRI has limited value for predicting time to return to play following acute hamstring injury. Br J Sports Med. 2018;52:83–88. [DOI] [PubMed] [Google Scholar]
- 36. Van der Made AD, Almusa E, Reurink G, et al. Intramuscular tendon injury is not associated with an increased hamstring reinjury rate within 12 months after return to play. Br J Sports Med. 2018;52:1261–1266. [DOI] [PubMed] [Google Scholar]
- 37. Velleman SG, Shin J, Li X, Song Y. Review: the skeletal muscle extracellular matrix: possible roles in the regulation of muscle development and growth. Can J Anim Sci. 2012;92:1–10. [Google Scholar]
- 38. Wang Z, Tang Z. Composition and function of extracellular matrix in development of skeletal muscle In: Travascio F, ed. Composition and Function of the Extracellular Matrix in the Human Body. Rijeka, Croatia: InTech; 2016:25–43. [Google Scholar]
- 39. Yue B. Biology of the extracellular matrix: an overview. J Glaucoma. 2014;23:20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]