Abstract
Objective
Despite previous research that focused on liver transaminases as predictors of cardiovascular disease, there has been limited research evaluating the predictive value of AST/ALT ratio in patients with heart failure. We aimed to investigate AST/ALT ratio as an indicator of the functional severity in chronic heart failure with reduced left ventricular ejection fraction.
Results
Overall, 105 patients previously diagnosed with HFrEF from Buraidah-Al Qassim province, Saudi Arabia were included in this retrospective cross-sectional study. Data on study variables, including demographic data, left ventricular ejection fraction, NYHA class, and AST/ALT ratio, were collected from patients’ records. The patients were divided into two groups, namely group-1 (AST/ALT ratio < 1) and group-2 (AST/ALT ratio ≥ 1), to identify any differences in their cardiac function profiles. NYHA class and NT-proBNP were higher and LVEF was lower in group-2 than in group-1. We found a mild significant correlation between AST/ALT ratio and APRI, FIB-4 score, NYHA-class, and LVEF (r = 0.2, 0.25, 0.26, and − 0.24, respectively; P < 0.05). Multivariate linear regression analysis model and ROC curve showed that AST/ALT ratio could independently predict HFrEF functional severity with a best cut-off value of 0.9, sensitivity of 43.6%, and specificity of 81.4%.
Keywords: AST/ALT ratio, Heart failure, Hepatic fibrosis
Introduction
Medical care has greatly advanced over the last three decades. However; heart failure remains a major cause of morbidity and mortality, with a general prevalence rate of approximately 2% and an abrupt rise to 9% in those aged ≥ 65 years [1].
Additionally, the prevalence of heart failure is increasing due to an increase in cardiovascular risk factors and increased life expectancy [2].
Established cardiovascular biomarkers include N-terminal pro-brain natriuretic peptide (NT-proBNP) [3] and troponin [4], while emerging biomarkers include miRNAs [5], mimecan [6], and orexin [7]. However, none of these biomarkers are routinely measured, indicating the need for new feasible and reproducible biomarkers that help in screening the functional severity in heart failure patients with reduced ejection fraction (HFrEF).
Consequently, research is still continuing to improve risk stratification tools for cardiovascular disease (CVD), including those specific for chronic heart failure.
Multiple theories have attempted to explain the cardiovascular-hepatic relationship that has been documented with abnormal liver function test results in patients with CVD, including heart failure [8], ischemic heart disease [9], and atherosclerosis [10].
Hepatic fibrosis is no longer considered an isolated hepatic disease on the basis of its association with multisystem dysfunction, including CVD [11]. Non-invasive indices were developed for early detection and monitoring of hepatic fibrosis [12]. The validated indices include the Aspartate aminotransferase (AST)/Alanine aminotransferase (ALT) ratio [13], AST to platelet ratio index (APRI) [14], Fibrosis-4 score (FIB-4) [15], and non-alcoholic fatty liver disease (NAFLD) fibrosis score [16].
Previous reports highlighted the value of non-invasive hepatic fibrosis indices as prognostic tools in hepatic patients [17]. However, there is little research evaluating these indices in HFrEF patients.
In our study, we have focused on the role of AST/ALT ratio in predicting the functional severity of HFrEF.
Main text
Methods
Study design and participants
This retrospective cross-sectional study included 105 patients recruited from the heart failure outpatient clinic of Prince Sultan Cardiac Center Qassim (PSCCQ), Buraidah, Saudi Arabia.
All recruited patients had HFrEF according to American College of Cardiology heart failure diagnostic criteria, evidenced by left ventricular ejection fraction (LVEF) ≤ 40% [18]; we excluded those with any of the following: primary liver disease, sepsis, shock, malignancy, pregnancy, or renal failure.
Demographic data
The following variables were collected: age, sex, duration of heart failure, and cardiovascular risk factors including; obesity, diabetes mellitus, smoking, and hypertension.
Laboratory investigations and calculation of liver fibrosis indices
Complete blood count, liver function tests, kidney function tests, thyroid profile, and NT-proBNP levels were recorded.
We calculated the following standard non-invasive hepatic fibrosis indices:
Transthoracic echocardiography
Transthoracic echocardiography was performed according to the recommendations of both the American Society of Echocardiography and European Association of Cardiovascular Imaging.
The modified Simpson’s rule was used to estimate left ventricular (LV) volumes and measure the ejection fraction [19].
Estimation of systolic pulmonary arterial pressure (SPAP)
SPAP was estimated using the modified Bernoulli formula (SPAP [mmHg] = 4 × tricuspid regurgitation velocity2 + right atrial pressure) [20].
Classification of patients according to the AST/ALT ratio
Based on literature review, an AST/ALT ratio ≥ 1 is highly specific and predictive of liver cirrhosis in patients with chronic HCV infection [13]; we used the same cut-off value to classify our patients into two groups:
Group-1: AST/ALT ratio < 1.0
Group-2: AST/ALT ratio ≥ 1.0
We adopted patients’ laboratory values, New York Heart Association (NYHA) functional Class and LVEF measurements that were performed after their first interview in the outpatient clinic.
Statistical methods
The statistical package MedCalc version 19.0.5 was used for all statistical analyses. Quantitative data are presented as mean ± standard deviation, and qualitative data are presented as percentages. Comparisons between groups were made using the Mann–Whitney test. Correlations between variables were analyzed using Spearman’s rank correlation coefficient. Multivariate linear regression was used to investigate the independent predictability of AST/ALT ratio for LVEF percentage [LVEF (%)].
Results
This study included 105 patients (50 ± 14.71, 16–88 years; 68 men). Both study groups showed comparable results; however, Group-2 showed a less favorable cardiac profile. NYHA class and NT-proBNP were higher and LVEF was lower in group-2 than in group-1 (Table 1).
Table 1.
Descriptive data of subjects with AST/ALT ratio < 1 and ≥ 1
| Parameter | Group-1 (58 patients) [AST/ALT ratio < 1] | Group-2 (47 patients) [AST/ALT ratio ≥ 1] | P-value |
|---|---|---|---|
| Age (year) | 50.03 ± 15.25 (16–82) | 50.17 ± 14.18 (21–88) | 0.915 |
| Gender (n, %) | 44 males (75%) | 24 males (51%) | 0.009 |
| Heart failure Duration (year) | 5.43 ± 2.46 (2–12) | 4.55 ± 3.09 (1–13) | 0.021 |
| NYHA–class | 2.12 ± 1.04 (1–4) | 2.49 ± 0.86 (1–4) | 0.042 |
| Diabetes mellitus (n, %) | 37 (63.8%) | 26 (55.32%) | 0.052 |
| Hypertension (n, %) | 33 (56.9%) | 25 (53.2%) | 0.632 |
| Smoking (n, %) | 10 (17.24%) | 11(23.4%) | 0.493 |
| BMI (kg/m2) | 28.72 ± 5.8 (16–40) | 30.33 ± 9.42 (17.6–70) | 0.609 |
| Systolic BP (mmHg) | 123.19 ± 20.19 (90–185) | 127.02 ± 17.5 (100–171) | 0.248 |
| Diastolic BP (mmHg) | 73 ± 11.57 (52–99) | 73.68 ± 10.63 (50–111) | 0.701 |
| Hemoglobin (g/dL) | 13.65 ± 1.76 (9.2–17.7) | 12.86 ± 2.18 (7.5–16.3) | 0.086 |
| Platelets (× 109/L) | 267.68 ± 72.25 (137–422) | 299.71 ± 94.94 (149–588) | 0.180 |
| Fasting blood sugar (mmol/L) | 15.62 ± 27.62 (4.38–140) | 10.24 ± 6.44 (4.82–24.8) | 0.874 |
| Potassium+ (mEq/L) | 4.2 ± 0.52 (3.1–5.8) | 4.1 ± 0.58 (2.8–5.7) | 0.415 |
| Sodium+ (mEq/L) | 136.96 ± 4.43 (127–146) | 135.98 ± 3.83 (127–143) | 0.261 |
| Serum creatinine (mmol/L) | 109.57 ± 80.95 (50–581) | 114.86 ± 97.72 (57–538) | 0.741 |
| Blood urea (mmol/L) | 7 ± 3.36 (2.7–19) | 7.34 ± 5.27 (2.8–30.6) | 0.588 |
| Total cholesterol (mmol/L) | 3.99 ± 1.13 (1.06–5.86) | 4.35 ± 1.64 (1.2–7.8) | 0.373 |
| HDL cholesterol (mmol/L) | 0.93 ± 0.25 (0.53–1.39) | 0.99 ± 0.35 (0.25–1.68) | 0.459 |
| Triglycerides (mmol/L) | 2.08 ± 1.36 (0.51–6.36) | 1.57 ± 0.99 (0.64–4.94) | 0.068 |
| T3 (nmol/L) | 5.25 ± 2.75 (1.11–16.95) | 6.87 ± 4.78 (4.07–17.67) | 0.655 |
| T4 (nmol/L) | 13.64 ± 5.15 (1.62–20.7) | 13.75 ± 4.46 (3.71–19.99) | 0.981 |
| TSH (mIU/L) | 9.15 ± 32.54 (1–193) | 6.6 ± 10.05 (0.19–48) | 0.899 |
| Bilirubin (mg/dL) | 11.88 ± 11.63 (3–67.9) | 12.75 ± 12.2 (0.7–64) | 0.722 |
| Albumin (g/dL) | 36.02 ± 4.51 (24.2–45.4) | 33.68 ± 8.61 (38–44.2) | 0.492 |
| aPTT (s) | 38.08 ± 20.72 (16–126) | 35.38 ± 13.57 (18–84.3) | 0.549 |
| INR | 1.15 ± 0.15 (1–1.6) | 1.22 ± 47 (0.94–3.7) | 0.737 |
| AST (IU/L) | 32.03 ± 50.31 (10–351) | 75.17 ± 163.59 (7–763) | 0.195 |
| ALT (IU/L) | 52.05 ± 71.05 (15–520) | 25.57 ± 24.37 (4–121) | 0.001 |
| GGT (U/L) | 89.67 ± 101.8 (6–203) | 25.48 ± 19.33 (6–52) | 0.479 |
| ALP (U/L) | 102.38 ± 57.81 (48–385) | 98.89 ± 53.14 (34–276) | 0.449 |
| AST/ALT ratio | 0.63 ± 0.19 (0.16–0.94) | 1.3 ± 0.25 (0.95–1.85) | 0.001 |
| APRI index | 0.31 ± 0.41 (0.09–2.8) | 0.74 ± 1.76 (0.07–8.03) | 0.877 |
| FIB-4 score | 0.8 ± 0.43 (0.17–1.96) | 1.1 ± 0.81 (0.3–3.6) | 0.002 |
| NT-proBNP (pg/mL) | 1212.9 ± 1869.2 (8.4–8455) | 1845.6 ± 2271.8 (4.1–9595) | 0.089 |
| LVEF (%) | 28.93 ± 10.26 (16–43) | 23.83 ± 7.04 (14–36) | 0.012 |
| SPAP (mmHg) | 42.33 ± 19.03 (15–63) | 45.25 ± 13.34 (22–70) | 0.344 |
The results showed a mild significant correlation between the AST/ALT ratio and the following variables: APRI, FIB-4 score, NYHA-class, and LVEF (r = 0.2, 0.25, 0.26, and − 0.24;P < 0.05) respectively.
Additionally, LVEF had significant correlations (− 0.26, − 0.46, − 0.29, − 0.25; P < 0.05) with NYHA-class, left ventricular end systolic pressure, SPAP, and NT-proBNP, respectively.
The receiver operating characteristic (ROC) curve showed that the ASL/ALT ratio could predict the functional severity of LV systolic failure measured by LVEF. We used the American Society of Echocardiography cut-off value (LVEF < 30%) for diagnosing patients with severely impaired LVEF [19]. The area under the curve (AUC) was 0.64 (P < 0.05) with a 95% confidence interval of 0.54–0.73 with a best cut-off value of 0.9, sensitivity of 43.6%, and specificity of 81.4% (Fig. 1).
Fig. 1.
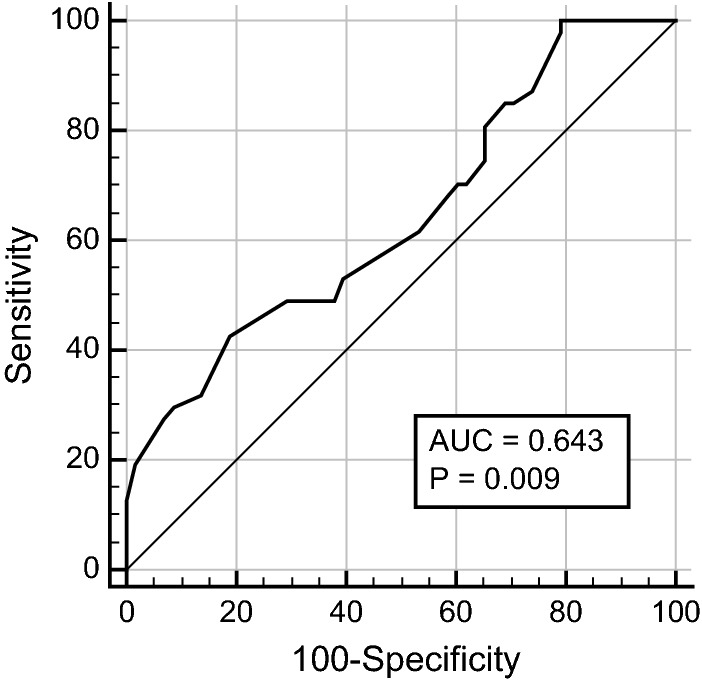
AROC-curve showing ALT/AST ratio prediction of reduced LVEF
In our multivariate linear regression model that included age, body mass index (BMI), diabetes mellitus, hypertension, and NT-proBNP, the AST/ALT ratio was a significant independent predictor of LVEF% (95% confidence interval, 0.85–1.01; P < 0.05, Table 2).
Table 2.
Multivariate linear regression model for prediction of LVEF(%)
| Parameters | Standardized coefficient | 95% CI | P-value |
|---|---|---|---|
| Age | − 0.174 | 47.25–52.94 | 0.102 |
| BMI | 0.061 | 27.80–30.99 | 0.696 |
| Diabetes mellitus | 0.433 | 1.56–1.75 | 0.879 |
| Hypertension | 1.903 | 1.46–1.66 | 0.502 |
| AST/ALT ratio | − 6.796 | 0.85–1.01 | 0.045 |
| NT-proBNP | − 0.001 | 932.91–2007.76 | 0.195 |
Discussion
Our main finding in this study is that an increased AST/ALT ratio could predict functional status decline in HFrEF patients, as shown by the less favorable cardiac profile in Group-2.
Moreover, the predictive value of the AST/ALT ratio is independent after adjustment for age, BMI, hypertension, diabetes mellitus, and NT-proBNP.
Our explanation for this main finding is multi-factorial; it is known that AST is released from many tissues, including the myocardium and the liver, while ALT is only released from the liver. Therefore, more severe myocardial pathology would lead to an anticipated increase in the AST/ALT ratio [21]. Additionally, higher the AST/ALT ratio, greater the probability of hepatic fibrosis, which is associated with cardiovascular disease (CVD) pathogenesis through different mechanisms, including increased plasma inflammatory mediators, insulin resistance, oxidative stress, and metabolic syndrome [22].
Based on their medical records, our patients did not have any primary hepatic disorders to justify the increased AST/ALT ratio. It is known that an increased AST/ALT ratio is also caused by alcoholic liver disease [23]. However, our records did not include those with alcohol drinking habits.
Hypoxic hepatitis is another cause of increased AST/ALT ratio in its early phase; however, our data showed that ALT and AST values were far less than those required for diagnosing classic hypoxic hepatitis [24].
Previous research has focused on the value of hepatic transaminases as CVD predictors. Lazo et al. [25] showed that elevated levels of liver transaminases were significantly correlated with the cardiac biomarkers; troponin T and NT-proBNP. Consequently, they concluded that liver transaminases could be used as predictors in patients at risk of CVD. However, they did not enroll heart failure patients in their study, as they were only concerned with predicting subclinical myocardial injury.
Yokoyama et al. [26] recently concluded that an increased AST/ALT ratio positively correlates with NT-proBNP levels, yet their study was not specific for HFrEF patients.
Zoppini et al. [27] studied patients with type 2 diabetes mellitus and found that the AST/ALT ratio positively correlated with CVD mortality; however, as in the above studies, they did not include HFrEF patients.
On the other hand, there are some studies that did not show significant correlation between ALT and CVD, as seen by both Ruhl et al. [28] and Fraser et al. [29] However, they did not include AST or the AST/ALT ratio in their correlations with CVD, which could be an explanation for the non-significant result.
In our study, we found that 0.9 was the best predictive cut-off value of the AST/ALT ratio when considering functional severity in HFrEF patients. Our finding was in agreement with the finding of Long et al. [30] that 1 was the best cut-off value of the AST/ALT ratio that predicted cardio-metabolic risk in their study.
To our knowledge, our study is the first in Saudi Arabia and other Arabian countries to correlate the AST/ALT ratio with functional severity in HFrEF patients. Consequently, we could not find comparable AST/ALT cut-off values in the Arab population.
Our cut-off value could be very helpful in monitoring the functional status of HFrEF patients in primary healthcare settings and accordingly adjusting their follow-up investigations/management plans.
Conclusion
Our study highlighted the value of the AST/ALT ratio as a simple independent predictor of LV functional status in patients with HFrEF.
Recommendation
Based on our findings, we recommend future prospective studies to establish the potential practical value of AST/ALT ratio in monitoring the functional status of patients with HFrEF.
Limitations
Our study had some limitations, including its retrospective nature; accordingly, we could not follow-up with the patients for detailed morbidity and mortality records in relation to AST/ALT ratio measurements. In addition, our study was a single-center study and prospective multicenter studies could improve our understanding regarding the correlation of AST/ALT ratio with HFrEF functional status.
Acknowledgements
Not applicable.
Abbreviations
- ALT
Alanine aminotransferase
- APRI index
AST to platelet ratio index
- AST
Aspartate aminotransferase
- AUC
Area under the curve
- CVD
Cardiovascular disease
- FIB-4
Fibrosis-4
- HFrEF
Heart failure with reduced ejection fraction
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- LVEF (%)
Left ventricular ejection fraction percentage
- NYHA
New York Heart Association
- NT-proBNP
N-terminal pro-Brain natriuretic peptide
- ROC
Receiver operating characteristic
- SPAP
Systolic pulmonary arterial pressure
Authors’ contributions
All authors, ME, HS, ASA, SAA, DAAR, SAA, RA contributed towards methodology. All authors contributed towards writing of the paper. All authors read and approved the final manuscript.
Funding
The authors received no specific funding for this work.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Ethical approval from the regional research ethics committee was taken prior to commencing the study (Al Qassim providence, Saudi Arabia Ministry of Health, approval number: 20181009).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019 doi: 10.1161/cir.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Naghavi M, Wang H, Lozano R, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhardwaj A, Rehman SU, Mohammed A, Baggish AL, Moore SA, Januzzi JL. Design and methods of the Pro-B Type Natriuretic Peptide Outpatient Tailored Chronic Heart Failure Therapy (PROTECT) Study. Am Heart J. 2010 doi: 10.1016/j.ahj.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Motiwala SR, Gaggin HK, Gandhi PU, et al. Concentrations of highly sensitive cardiac troponin-I predict poor cardiovascular outcomes and adverse remodeling in chronic heart failure. J Cardiovasc Transl Res. 2015;8(3):164–172. doi: 10.1007/s12265-015-9618-4. [DOI] [PubMed] [Google Scholar]
- 5.Motiwala SR, Szymonifka J, Belcher A, et al. Measurement of novel biomarkers to predict chronic heart failure outcomes and left ventricular remodeling. J Cardiovasc Transl Res. 2014;7(2):250–261. doi: 10.1007/s12265-013-9522-8. [DOI] [PubMed] [Google Scholar]
- 6.Kortekaas KA, Hoogslag GE, De Boer RA, et al. Galectin-3 and left ventricular reverse remodelling after surgical mitral valve repair. Eur J Heart Fail. 2013;15(9):1011–1018. doi: 10.1093/eurjhf/hft056. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim NE, Rabideau DJ, Gaggin HK, et al. Circulating concentrations of orexin a predict left ventricular myocardial remodeling. J Am Coll Cardiol. 2016;68(20):2238–2240. doi: 10.1016/j.jacc.2016.08.049. [DOI] [PubMed] [Google Scholar]
- 8.Samsky MD, Patel CB, Dewald TA, et al. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol. 2013;61(24):2397–2405. doi: 10.1016/j.jacc.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Lofthus DM, Stevens SR, Armstrong PW, Granger CB, Mahaffey KW. Pattern of liver enzyme elevations in acute ST-elevation myocardial infarction. Coron Artery Dis. 2012;23(1):22–30. doi: 10.1097/MCA.0b013e32834e4ef1. [DOI] [PubMed] [Google Scholar]
- 10.Alonso A, Misialek JR, Amiin MA, et al. Circulating levels of liver enzymes and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities cohort. Heart. 2014;100(19):1511–1516. doi: 10.1136/heartjnl-2014-305756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 12.Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol. 2013;58(5):1007–1019. doi: 10.1016/j.jhep.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93(1):44–48. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- 14.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 15.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 16.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 17.Angulo P, Bugianesi E, Bjornsson ES, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145(4):782–789.e4. doi: 10.1053/j.gastro.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Victor MA, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Augustine DX, Augustine DX, Coates-bradshaw LD, et al. Echocardiographic assessment of pulmonary hypertension: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2018;5(3):11–24. doi: 10.1530/ERP-17-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glinghammar B, Rafter I, Lindström AK, Hedberg JJ, Andersson HB, Lindblom P, Berg AL, Cotgreave I. Detection of the mitochondrial and catalytically active alanine aminotransferase in human tissues and plasma. Int J Mol Med. 2009;23(5):621–631. doi: 10.3892/ijmm. [DOI] [PubMed] [Google Scholar]
- 22.Targher G, Bertolini L, Rodella S, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity. 2008;16(6):1394–1399. doi: 10.1038/oby.2008.64. [DOI] [PubMed] [Google Scholar]
- 23.Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20(33):11684–11699. doi: 10.3748/wjg.v20.i33.11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aboelsoud MM, Javaid AI, Al-Qadi MO, Lewis JH. Hypoxic hepatitis—its biochemical profile, causes and risk factors of mortality in critically-ill patients: a cohort study of 565 patients. J Crit Care. 2017;41:9–15. doi: 10.1016/j.jcrc.2017.04.040. [DOI] [PubMed] [Google Scholar]
- 25.Lazo M, Rubin J, Clark JM, et al. The association of liver enzymes with biomarkers of subclinical myocardial damage and structural heart disease. J Hepatol. 2015;62(4):841–847. doi: 10.1016/j.jhep.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoyama M, Watanabe T, Otaki Y, et al. Association of the aspartate aminotransferase to alanine aminotransferase ratio with BNP level and cardiovascular mortality in the general population: the Yamagata study 10-year follow-up. Dis Markers. 2016 doi: 10.1155/2016/4857917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zoppini G, Cacciatori V, Negri C, et al. The aspartate aminotransferase-to-alanine aminotransferase ratio predicts all-cause and cardiovascular mortality in patients with type 2 diabetes. Med (United States). 2016;95(43):1–7. doi: 10.1097/MD.0000000000004821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and γ-glutamyltransferase and mortality in the United States Population. Gastroenterology. 2009;136(2):477–485.e11. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 29.Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor DA. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women’s Heart and Health study and meta-analysis. Arterioscler Thromb Vasc Biol. 2007;27(12):2729–2735. doi: 10.1161/ATVBAHA.107.152298. [DOI] [PubMed] [Google Scholar]
- 30.Long MT, Pedley A, Massaro JM, Hoffmann U, Fox CS. The association between non-invasive hepatic fibrosis markers and cardiometabolic risk factors in the Framingham Heart Study. PLoS ONE. 2016;11(6):1–13. doi: 10.1371/journal.pone.0157517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


