Abstract
Objective:
We characterized patterns of preoperative opioid use in patients undergoing elective surgery to identify the relationship between preoperative use and subsequent opioid fill after surgery.
Background:
Preoperative opioid use is common, and varies by dose, recency, duration, and continuity of fills. To date, there is little evidence to guide postoperative prescribing need based on prior opioid use.
Methods:
We analyzed claims data from Clinformatics® DataMart Database for patients aged 18 – 64 years undergoing major and minor surgery between 2008 and 2015. Preoperative use was defined as any opioid prescription filled in the year before surgery. We used cluster analysis to group patients by the dose, recency, duration, and continuity of use. Our primary outcome was second postoperative fill within 30 postoperative days. Our primary explanatory variable was opioid use group. We used logistic regression to examine likelihood of second fill by opioid use group.
Results:
Out of 267,252 patients, 102,748 (38%) filled an opioid prescription in the 12 months before surgery. Cluster analysis yielded 6 groups of preoperative opioid use, ranging from minimal (27.6%) to intermittent (7.7%) to chronic use (2.7%). Preoperative opioid use was the most influential predictor of second fill, with larger effect sizes than other factors even for patients with minimal or intermittent opioid use. Increasing preoperative use was associated with risk-adjusted likelihood of requiring a second opioid fill compared to naïve patients (minimal use: OR 1.49, 95% CI 1.45 – 1.53; recent intermittent use: OR 6.51, 95% CI 6.16 – 6.88; high chronic use: OR 60.79, 95% CI 27.81 – 132.92, all p-values <0.001).
Conclusions:
Preoperative opioid use is common among patients who undergo elective surgery. Although the majority of patients infrequently fill opioids prior to surgery, even minimal use increases the probability of needing additional postoperative prescriptions in the 30-days after surgery when compared to opioid naïve patients. Going forward, identifying preoperative opioid use can inform surgeon prescribing and care coordination for pain management after surgery.
Introduction
Opioid prescribing is higher in the U.S. than in any other country, and 11 – 14 million Americans are estimated to be on chronic opioid therapy.1 However, the number of people who intermittently use opioids may be much higher. The 2015 National Survey on Drug Use and Health found that over 1 in 3 adults (38%) report taking an opioid medication sometime in the past year.2,3 Surgery is one of the most common reasons for opioid prescribing, and opioid prescriptions related to procedural care have risen in recent years in proportion to other types of care.4 Postoperative pain management for patients with existing opioid use can be challenging, as long-term use can lead to dependence, tolerance, and hyperalgesia.5–7 To date, little is known regarding pain medication needs among patients who use opioids at the time of surgery.8–12
Recent opioid prescribing guidelines have focused on either the management of chronic use or surgical prescribing in opioid-naïve patients, and there is little evidence to guide prescribing for patients with previous opioid use.13–15 Preoperative opioid use is highly variable with respect to dose, recency, duration, or continuity of use.12,16 A method of classifying patients by preoperative use could help inform prescribing or trigger preoperative referrals for patients with greatest preoperative use. Furthermore, the need for additional postoperative prescriptions could also guide prescribing protocols. For example, a recent study of opioid-naïve patients demonstrated that roughly 6% will require a refill following surgery whether they receive a small or large initial prescription. These data suggest that prescriptions in naive patients can be reduced without increasing refill needs, but this relationship is unknown for patients with preoperative use.17,18 A better understanding of preoperative opioid use, especially in relationship to outcomes such as postoperative opioid requirements, would be useful for empiric assessment and best practice development.
In this context, we examined data from a national private insurance payer to categorize patients with previous opioid use into groups based on the dose, recency, duration, and continuity of opioid fills, with a goal of understanding different phenotypes of preoperative use. We then compared the likelihood of second postoperative opioid fill between preoperative use groups. We also compared the initial postoperative prescription amount to determine whether surgeon prescribing behavior varied according to preoperative use. We hypothesized that patients with long duration or high-dose opioid use would have a higher likelihood of second postoperative fill than patients with shorter or lower-dose use. We further hypothesized that surgeons infrequently tailor their postoperative prescribing with respect to preoperative use.
Methods
Data source and study population
We used private insurance claims data from the Clinformatics® DataMart Database (OptumInsight, Eden Prairie, MN), which captures health claims across the United States for members of a national managed-care company affiliated with Optum Insight. This study used de-identified patient data and was deemed exempt by the University of Michigan Institutional Review Board.
The study cohort included patients ages 18 – 64 who underwent a surgical procedure between January 1, 2008 and March 31, 2015. We specified that patients were continuously enrolled for 12 months before and 6 months after the operation, to capture preoperative and postoperative opioid use. We excluded patients aged 65 and older as the capture of Medicare Part D prescription claims data may be incomplete. We also excluded patients who had a length of stay of greater than 30 days, patients who were not discharged home and patients who had subsequent surgical procedures in the 6 months after index surgery. We chose 13 common elective procedures, categorized as minor and major procedures as described in prior work.8 Minor surgical procedures included varicose vein removal, laparoscopic cholecystectomy, laparoscopic appendectomy, hemorrhoidectomy, thyroidectomy, transurethral prostate surgery, parathyroidectomy, and carpal tunnel release. Major surgical procedures included ventral incisional hernia repair, colectomy, reflux surgery, bariatric surgery, and hysterectomy. We identified patients undergoing surgery using Current Procedural Terminology or International Statistical Classification of Diseases and Related Health Problems (ICD-9) procedure codes (Appendix 1).
Defining Preoperative Use
We identified patients as preoperative opioid users if they had filled at least one opioid prescription in the year prior to surgery. We used generic drug names matched with National Drug Codes to identify the opioid prescriptions. We then converted prescription amounts into Oral Morphine Equivalents (OMEs) using the morphine conversion factor for each medication type according to CDC guidelines.19 For ease of interpretation, all results describing opioid amount in OMEs are presented as the equivalent number of “pills” of 5/325mg hydrocodone-acetaminophen (one 5mg hydrocodone pill = 5 OME).
We classified patients according to attributes of their opioid use in the year before surgery, including duration, dose, recency, and continuity of their use. Dose was defined as total OMEs filled in the year prior to the operation. Recency was defined as the number of months since the last opioid fill before the operation. For example, if a patient had surgery on December 15th, and received a prescription starting November 1st, their recency would be one month. Duration was the total number of months during which a patient filled an opioid prescription. Continuity was defined as the longest “streak” of months that a patient consecutively filled a prescription. For example, if a patient filled in January, March, April, and September, their duration would be four months, and their continuity would be two months.
To interpret the complex relationships between attributes of preoperative opioid use (dose, recency, duration, and continuity), we used cluster analysis to classify patients into groups of preoperative use based on these attributes. Cluster analysis is a machine learning technique that is used to organize multivariate data into meaningful groups (clusters) based on information found in the data that describes the objects and their relationships.20 We used the Density-Based Spatial Clustering of Applications with Noise (DBSCAN) clustering algorithm, which functions on the idea that clusters are dense groups of data points. It groups together points that are close to each other and marks points that are in low-density regions as outliers.21 The DBSCAN algorithm requires two parameters: the minimum distance between two points and the minimum number of points to form a dense region. The minimum distance between two points was determined as 0.35 using k-distance graph and the minimum number of points in a cluster was chosen as 20, as larger values are better for data sets with noise and will yield more significant clusters. After this analysis yielded “clusters” of patients by attributes of preoperative use, we further grouped clusters with similar patterns of preoperative use into larger groups, based on clinical judgment of their similarities and independent of the group’s relationship to outcome.
Outcome and Explanatory Variables
Our primary outcome was second postoperative fill as a dichotomous variable, with ‘1’ defined as a fill of opioid medication after the initial postoperative prescription up to 30 days after the operation, and ‘0’ as no additional postoperative fill.
Our secondary outcome was the total OMEs in the initial postoperative prescription as a continuous variable. The initial postoperative prescription was defined as the prescription filled closest to surgery within 30 days before to 14 days after the operation. This accounted for some patients receiving a prescription at their preoperative appointment intended for postoperative use.8,11
The explanatory variable of interest included preoperative use group as described above. Other patient covariates included age, sex, race (white, black, Hispanic, or other), education level, and region of residence (out of nine regions in the United States). Comorbidity burden was identified using the Charlson Comorbidity Index. Tobacco use and pain disorders (arthritis, back, neck, and other pain) were captured with ICD-9 diagnosis codes. Mental health disorders (adjustment, personality, or substance use disorders, and suicide or psychosis) were captured using the Clinical Classification System from the Agency of Healthcare Research and Quality, as these conditions are known to be associated with increased opioid use after surgery.8,17,22 Preoperative benzodiazepine use was captured if patients filled a prescription for a benzodiazepine medication in the year prior to surgery.
Statistical Analysis
We used descriptive statistics to detail the clinical and demographic attributes of the study cohort. We then used logistic regression to identify the effect of preoperative use group on likelihood of second postoperative fill while controlling for patient and provider factors. In this model, we controlled for the size of the initial postoperative prescription in OMEs and the probability of second fill. We then used a linear regression model to examine the relationship between preoperative use group and the size of postoperative prescription received using a second linear regression model. Cluster analysis was performed using R version 3.5.1 (Vienna, Austria), and all other analyses were conducted using StataSE version 14 (College Station, TX).
Results
We identified 267,252 patients who underwent major and minor elective surgical procedures during the study period. In this cohort, 61,173 (22.9%) underwent a major procedure, and 206,079 (77.1%) underwent a minor procedure. Additionally, 183,621(68.7%) were female, 191,189 (71.5%) were white, and 28,906 (10.8%) were African American. There were 68,026 (25.2%) patients who filled a benzodiazepine prescription preoperatively. Finally, 165,741 (62%) patients were opioid-naïve, while 102,748 (38%) had filled a prescription in the year before surgery (Table 1).
Table 1.
Patient characteristics.
| Characteristics | N (%) |
|---|---|
| Total Cases | 267252 |
| Age | |
| 18–29 | 27974 (10.5) |
| 30–39 | 51456 (19.3) |
| 40–49 | 79325 (29.7) |
| 50–59 | 77158 (28.9) |
| 60–64 | 31339 (11.7) |
| Gender | |
| Female | 183621 (68.7) |
| Male | 83581 (31.3) |
| Unknown | 50 (0) |
| Race | |
| White | 191189 (71.5) |
| Asian | 6982 (2.6) |
| Black | 28906 (10.8) |
| Hispanic | 29297 (11) |
| Unknown | 10878 (4.1) |
| Education | |
| Less than 12th Grade | 1190 (0.5) |
| High School Diploma | 80072 (30) |
| Bachelor’s Degree | 141446 (52.9) |
| Bachelor’s Degree Plus | 41819 (15.7) |
| Unknown | 2725 (1.0) |
| Geographic Region (ref group: South Atlantic) | |
| East North Central | 40725 (15.2) |
| East South Central | 11880 (4.5) |
| Middle Atlantic | 14668 (5.5) |
| South Atlantic | 79431 (29.7) |
| New England | 7368 (2.8) |
| Mountain | 25352 (9.5) |
| Pacific | 16459 (6.2) |
| West North Central | 23786 (8.9) |
| West South Central | 47293 (17.7) |
| Unknown | 290 (0.1) |
| Charlson comorbidity score, mean (SD) | 0.93 (1.60) |
| History of tobacco use | 75823 (28.4) |
| Surgery Type | |
| Major Surgery | 61173 (22.9) |
| Minor Surgery | 206079 (77.1) |
| Opioid-naïve | 165741 (62.0%) |
| Mental health disordersa | |
| Adjustment | 11671 (4.4) |
| Anxiety | 44631 (16.7) |
| Mood | 50823 (19.0) |
| Suicide or self-harm | 1047 (0.4) |
| Disruptive | 6150 (2.3) |
| Personality | 838 (0.3) |
| Psychosis | 1804 (0.7) |
| Alcohol or substance abuse disorders | 7756 (2.9) |
| Other | 10470 (3.9) |
| Pain disordersa | |
| Arthritis | 140903 (52.7) |
| Back | 82628 (30.9) |
| Neck | 42628 (16.0) |
| Other pain conditions | 125753 (47.1) |
| Preoperative benzodiazepine | 68026 (25.5) |
, not mutually exclusive categories.
Patterns of Preoperative Opioid Use
We identified 15 distinct clusters of preoperative opioid use, which were further collapsed into six larger groups by similarities in dose, recency, duration, and continuity. For each group, continuity of use did not tend to deviate appreciably from the total duration, indicating that overall, patients with long total duration were filling prescriptions consistently, without significant month-long interruptions. Each group was named for the dose, recency, and duration of their use. These are summarized in Table 2, arranged in increasing order of use.
Table 2.
Patient groups and attributes of preoperative opioid exposure.
| Preoperative Exposure | Group Name | N | % | Total Dose in Pills (IQR) | Duration in Months (IQR) | Continuity in Months (IQR) | Recency in Months (IQR) |
|---|---|---|---|---|---|---|---|
| Minimal | 1 - Minimal | 73761 | 27.6% | 36 (40) | 1 (0) | 1 (0) | 3 (6) |
| Intermittent | 2 - Remote | 12974 | 4.9% | 129 (162) | 3 (2) | 2 (0) | 2 (4) |
| 3 - Recent | 7423 | 2.8% | 420 (566) | 6 (3) | 3 (1) | 1 (2) | |
| Chronic | 4 - Low | 3337 | 1.2% | 1262 (1386) | 10 (2) | 7 (3) | 1 (1) |
| 5 - Medium | 3960 | 1.5% | 3028 (4705) | 12 (0) | 12 (0) | 1 (1) | |
| 6 - High | 56 | 0.02% | 18923 (11673) | 12 (1) | 12 (1) | 1 (1) |
Minimal use:
Group 1 patients (n = 73,761) were characterized by low dose, <1-month duration use of varying recency. Median (interquartile range [IQR]) prescription size was 36 (40) pills, and patients received the prescription at a median of 3 (6) months before surgery. We termed these patients “minimal use,” analogous to a patient receiving a short course of opioids for a remote acute condition before surgery.
Intermittent use:
Group 2 (n = 12,974), had a low dose, remote, short-course use (“remote intermittent”): median total dose 129 (238) pills over a median of 3 months, filled 2 months before surgery. Group 3 (n = 7,423) included three clusters and had low dose, recent, medium course use (“recent intermittent”): slightly higher total dose than Group 2, longer duration (4 – 7 months), more recent use (within 1 month), and some discontinuity of fill (median continuity 3 months).
Chronic use:
Group 4 (n = 3,337) included five clusters and had medium total dose, recent, long duration (9 – 11 months), and sustained use (“low chronic”). Although this group’s total dose was higher than that of previous groups (median 1,262 pills over 10 months), it was comparatively lower than the next groups of long duration. Group 5 (n = 3,960) included three clusters and had higher dose use (“medium chronic”). Group 6 (n = 56) included two groups and had the highest dose use (“high chronic”). Patients in this group received a median equivalent of 18,923 (11,673) pills over a 12-month duration, equivalent to 52 pills per day.
Likelihood of Second Postoperative Fill by Use Group
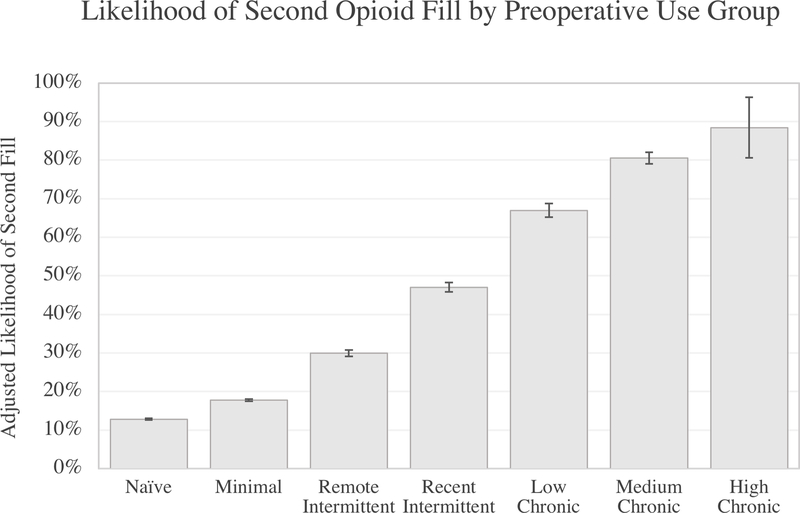
On multivariate analysis, preoperative opioid use was significantly associated with the likelihood of having a second postoperative opioid fill compared to opioid-naïve patients, who had a 13% likelihood (p<0.001) (Figure 1). Preoperative opioid use was the most influential predictor of second fill, with larger effect sizes than other factors even for patients with minimal or intermittent opioid use. Compared with opioid naïve patients, patients with minimal opioid use had an 18% likelihood of second fill. Patients with remote intermittent use had 30% likelihood of second fill, followed by 47% in patients with recent intermittent use. Over half of patients with chronic use were likely to require a second fill following surgery. For example, patients with low chronic use had a 67% likelihood, and patients with moderate and high chronic use were 81% and 88% likely to require second fill, respectively.
Figure 1 Legend.
Likelihood of second postoperative opioid fill (%) by opioid use group.
Table 3 summarizes the patient factors associated with second postoperative fill. Patients undergoing minor surgery were less likely to require second fill compared to those who had major surgery (OR 0.53, 95% CI 0.52 – 0.55, p<0.001). Initial postoperative prescription size had a very small but statistically significant negative association with second fill (in increments of 10 pills, odds ratio [OR] 0.992, 95% confidence interval [CI] 0.990 – 0.994, p<0.001). Patients with history of tobacco use were more likely to require second fill compared to patients without, even after controlling for procedure type (OR 1.45, 95% CI 1.42 – 1.49, p<0.001). Patients with mental health disorders including anxiety, mood, disruptive, or alcohol/substance abuse disorders were also more likely to require second fill, as were patients with arthritis, back pain, or other pain conditions. In addition, patients with a preoperative benzodiazepine prescription were more likely to require second fill (OR 1.34, 95% CI 1.30 – 1.38, p<0.001).
Table 3.
Multivariable analysis examining the likelihood of refill within 30 days of surgery.
| Odds Ratio | p-value | 95% Confidence Interval |
||
|---|---|---|---|---|
| Initial postoperative prescription size (in increments of 10 pills of 5mg hydrocodone) | 0.99 | <0.001 | 0.99 | 0.99 |
| Preoperative Exposure Group (ref: Opioid-Naïve) | ||||
| Minimal | 1.49 | <0.001 | 1.45 | 1.53 |
| Remote Intermittent | 3.02 | <0.002 | 2.88 | 3.16 |
| Recent Intermittent | 6.51 | <0.003 | 6.16 | 6.88 |
| Low Chronic | 15.45 | <0.004 | 14.19 | 16.83 |
| Medium Chronic | 32.31 | <0.005 | 29.28 | 35.66 |
| High Chronic | 60.79 | <0.006 | 27.81 | 132.92 |
| Age category (ref group: 18–29) | ||||
| 30–39 | 1.08 | 0.002 | 1.03 | 1.13 |
| 40–49 | 1.01 | 0.70 | 0.96 | 1.06 |
| 50–59 | 0.87 | <0.001 | 0.83 | 0.91 |
| 60–64 | 0.75 | <0.001 | 0.71 | 0.79 |
| Gender (ref group: Female) | ||||
| Male | 1.19 | <0.001 | 1.15 | 1.22 |
| Unknown | 0.57 | 0.34 | 0.18 | 1.82 |
| Race (ref group: Caucasian) | ||||
| Asian | 0.79 | <0.001 | 0.72 | 0.87 |
| Black | 1.04 | 0.05 | 1.00 | 1.08 |
| Hispanic | 0.87 | <0.001 | 0.84 | 0.91 |
| Unknown | 1.02 | 0.56 | 0.95 | 1.09 |
| Education (ref group: Bachelor’s Degree) | ||||
| Less than 12th Grade | 1.09 | 0.38 | 0.90 | 1.31 |
| High School Diploma | 1.02 | 0.19 | 0.99 | 1.05 |
| Bachelor’s Degree Plus | 0.93 | <0.001 | 0.90 | 0.97 |
| Unknown | 1.13 | 0.08 | 0.99 | 1.29 |
| Charlson comorbidity score | 1.01 | 0.001 | 1.00 | 1.02 |
| History of tobacco use | 1.45 | <0.001 | 1.42 | 1.49 |
| Surgery Type (ref group: Major Surgery) | ||||
| Minor Surgery | 0.54 | <0.001 | 0.52 | 0.55 |
| Mental health disorders | ||||
| Adjustment | 0.97 | 0.22 | 0.91 | 1.02 |
| Anxiety | 1.13 | <0.001 | 1.09 | 1.17 |
| Mood | 1.15 | <0.001 | 1.12 | 1.19 |
| Suicide or self-harm | 0.95 | 0.52 | 0.80 | 1.12 |
| Disruptive | 1.13 | 0.001 | 1.05 | 1.22 |
| Personality | 1.18 | 0.09 | 0.97 | 1.43 |
| Psychosis | 1.02 | 0.83 | 0.89 | 1.16 |
| Alcohol or substance abuse disorders | 1.44 | <0.001 | 1.35 | 1.53 |
| Other | 0.90 | <0.001 | 0.85 | 0.95 |
| Pain disorders | ||||
| Arthritis | 1.07 | <0.001 | 1.04 | 1.10 |
| Back | 1.06 | <0.001 | 1.03 | 1.09 |
| Neck | 0.98 | 0.36 | 0.95 | 1.02 |
| Other pain conditions | 1.16 | <0.001 | 1.13 | 1.19 |
| Preoperative benzodiazepine | 1.34 | <0.001 | 1.30 | 1.38 |
Postoperative Prescription Size by Preoperative Use Group
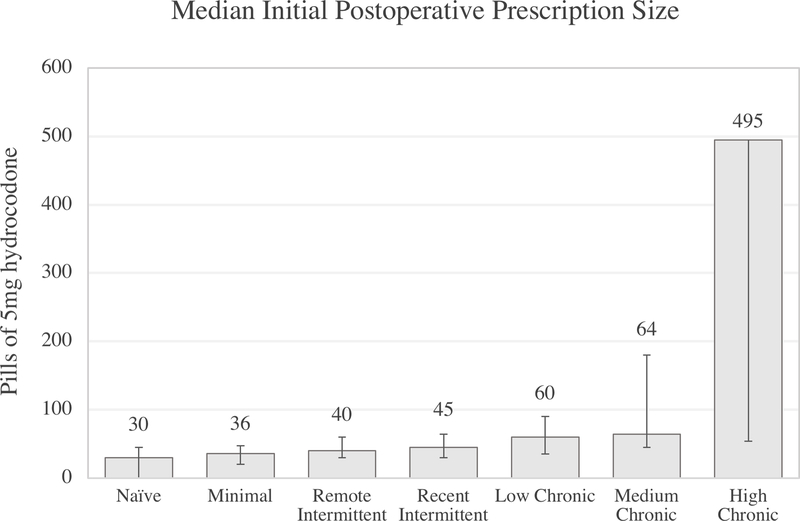
Initial postoperative prescription size was skewed toward larger prescriptions, especially for increasing opioid use. Prescription sizes are thus reported in median (IQR) pills. Opioid-naïve patients received a median of 30 (45) pills, and patients with minimal, remote intermittent, recent intermittent, low chronic, and medium chronic use all received prescriptions under 70 pills, with statistically significant differences between prescription sizes for all groups (p<0.001) (Figure 2). While these amounts are statistically significantly different, the prescription sizes are similar, especially for naïve to recent intermittent use patients. Patients with high chronic use received a median prescription equivalent to 495 (1566) pills. Linear regression analysis adjusting for patient factors confirmed this relationship, with patients with high chronic use receiving much higher prescriptions compared to patients in other groups.
Figure 2 Legend.
Unadjusted prescription size by opioid use group for major and minor surgery. Error bars denote the 25th and 75th percentile of prescriptions for each group. Error bars have been omitted for the 75th percentile of the high chronic group to preserve an interpretable scale. For this group, 75th percentile: 1620 pills.
Discussion
In this national study of adults undergoing elective surgery, we observed that 38% of patients filled an opioid prescription in the year prior to surgery. Patients were grouped by patterns of preoperative opioid use—the majority of these patients had low-dose, intermittent preoperative use, and only 2.7% of patients had continuous, high-dose use. As expected, patients with high use were likely to require a second opioid fill after surgery. However, even for patients with relatively minimal preoperative use, 1 in 5 required a second fill following surgery—1.5 times more likely than opioid-naïve patients. For patients with recent, low-dose use, this likelihood increased to 6.5 times that of naïve patients. Additionally, surgeons rarely tailored initial prescriptions to preoperative opioid use. Taken together, these findings suggest that identifying and quantifying opioid use prior to surgery is an important opportunity to inform postoperative opioid prescribing. In addition, characterizing preoperative use may also facilitate transitions of care for patients with high preoperative use, who may need more personalized pain management in partnership with their primary provider to assure long-term wellness.
For surgeons, there is increasing pressure to reduce opioid prescribing to better match patients’ needs. However, patients with preoperative opioid use present a common and complex challenge. When assessing a patient with preoperative opioid use, it may be unclear whether remote versus recent use should be treated differently, or whether to prioritize duration versus dose. We present a novel method to stratify preoperative opioid use in a clinically meaningful way. The patient groups from cluster analysis allow preoperative use attributes to be understood together rather than in isolation, aiding interpretation and clinical management. These findings may also help surgeons use a patient’s preoperative opioid use to better tailor prescriptions after surgery. There is evidence of wide provider variation in postoperative prescription sizes due to a lack of guidelines.13,23,24 This variation may supply some patients with excess medication while leaving others with poorly controlled pain. Additionally, surgical providers may not be regularly screening for past opioid use as part of the preoperative risk assessment, possibly leading to the uncoordinated prescribing patterns seen in this study.25,26
Simply writing larger prescriptions for patients with existing use should not be the only way forward.27 For a patient with minimal preoperative use, a surgeon should be aware of their potential need for additional prescriptions following surgery and take the opportunity to counsel both the patient and clinicians caring for patients following surgery regarding the expectations for pain. Conversely, a patient with higher dose, daily use for several months can be expected to need ongoing opioid prescriptions after surgery. Characterizing current opioid use provides an opportunity to coordinate perioperative pain management (e.g., regional blockade, multimodal anesthesia) and postoperative pain management with their usual prescriber. Surgeons would also have an opportunity to educate patients about the risks of respiratory depression or other adverse opioid-related effects, as well as discussing opioid weaning if possible.
Unlike other preoperative risk factors, such as tobacco, obesity, hypertension, and diabetes, opioid use thresholds that correspond to clinical outcomes have not been defined. In future work, these groups can be compared in other relevant outcomes, such as complication or readmission rates, to further “validate” thresholds of clinically meaningful risk. Chronic opioid use results in greater morbidity and expenditures following surgery, and opioid use predisposes to complications such as ileus, respiratory depression, and pneumonia.16,28 Risk-stratifying patients based on their pattern of preoperative use could allow surgeons to pursue risk-modifying steps, such as delaying elective surgery until high doses can be tapered. For example, in spine and joint replacement surgery where opioid use is prevalent, preoperative opioid reduction and referrals to pain specialists have improved clinical and patient-reported outcomes.29–31 Future studies may help target preoperative optimization toward patients at highest opioid-related risk.
Patients with preoperative use are highly likely to require additional opioid prescriptions after surgery. In our analysis, we did not attribute these subsequent prescriptions to surgical providers, as we wished to identify all patients who received more than one opioid prescription after surgery. For opioid-naïve patients, these likely represent true refills from the surgeon after the operation, but for patients with preoperative use (especially chronic use), these prescriptions could be surgical refills, additional fills from their usual pain provider, or even prescriptions from a third provider (e.g., from the Emergency Department). Patients with previous or current opioid use are at higher risk for persistent use or opioid-related adverse events after surgery.32–34 Acute-on-chronic opioid consumption may increase the risk of overdose, and duplicate prescriptions potentially supply patients with excess medication for later use or diversion.35 For patients with preoperative opioid use, surgeons should check Prescription Drug Monitoring programs before writing refills and communicate with a patient’s usual prescriber to coordinate which provider will supply continuing prescriptions. The usual prescriber can also offer valuable insight into a patient’s history (such as previous substance use, which could prompt more cautious prescribing and close follow-up) or recommendations for escalating pain control. In return, surgeons should provide estimates of how long postoperative pain should last or whether there were any complications that could reasonably cause more pain.
This study has several limitations to consider. Firstly, its generalizability is limited by the patient sample: continuously enrolled adults with private insurance. This population potentially misses preoperative users without one year of continuous enrollment or those paying for opioid prescriptions out-of-pocket. Also, this sample does not include adults enrolled in Medicare or Medicaid, and thus does not represent an important sociodemographic subset of patients. Additionally, we cannot account for opioid use that occurs outside of prescription fills, such as misuse of unprescribed opioid pills, nonmedical use, and heroin abuse. The data examined include prescriptions filled but do not account for the amount of pills consumed within those prescriptions. Finally, we did not separate second postoperative fills into prescriptions written by surgeons versus other providers, which could help assess the burden of postoperative prescribing that falls on surgeons.
Conclusion
In conclusion, understanding preoperative opioid use is critical for surgeons to provide optimal surgical care for all patients. Opioid use is complex and varies by attributes including dose, recency, duration, and continuity. This study used cluster analysis, a novel method to empirically classify patients by these domains. We found that patients with any preoperative use were more likely to require additional opioid prescriptions. We also found that postoperative prescribing was largely arbitrary across groups except for patients with higher-dose, chronic use, highlighting an opportunity to better tailor postoperative prescribing. These groups of preoperative use can be used to compare patient outcomes, healthcare utilization, and risk of overdose or other opioid-related events. Given the prevalence of preoperative opioid use, this work lays a foundation for using preoperative opioid use to inform risk assessment and develop best practices in surgical prescribing.
Supplementary Material
Acknowledgments
Financial support: Dr. Vu receives funding from the National Institutes of Health Ruth L. Kirschstein National Research Service Award (1F32DK115340-01A1). Drs. Englesbe, Brummett, and Waljee receive funding from the Michigan Department of Health and Human Services, and the Substance Abuse and Mental Health Services Administration (E20180568-001). The content is solely the responsibility of the authors and does not necessarily represent the official views of SAMHSA.
Disclosures: Dr. Brummett reports a patent for Peripheral Perineural Dexmedetomidine licensed to the University of Michigan and has received research funding from Neuros Medical.
References
- 1.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiology and drug safety 2009;18:1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frenk SM, Porter KS, Paulozzi LJ. Prescription opioid analgesic use among adults: United States, 1999–2012. NCHS data brief 2015:1–8. [PubMed] [Google Scholar]
- 3.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Ann Intern Med 2017;167:293–301. [DOI] [PubMed] [Google Scholar]
- 4.Larach DB, Waljee JF, Hu HM, et al. Patterns of Initial Opioid Prescribing to Opioid-Naive Patients. Annals of surgery 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hina N, Fletcher D, Poindessous-Jazat F, Martinez V. Hyperalgesia induced by low-dose opioid treatment before orthopaedic surgery: An observational case-control study. European journal of anaesthesiology 2015;32:255–61. [DOI] [PubMed] [Google Scholar]
- 6.Trang T, Al-Hasani R, Salvemini D, Salter MW, Gutstein H, Cahill CM. Pain and Poppies: The Good, the Bad, and the Ugly of Opioid Analgesics. The Journal of neuroscience : the official journal of the Society for Neuroscience 2015;35:13879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittner R, Arregui ME, Bisgaard T, et al. Guidelines for laparoscopic (TAPP) and endoscopic (TEP) treatment of inguinal Hernia [International Endohernia Society (IEHS)]. Surgical Endoscopy 2011;25:2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg 2017;152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waljee JF, Li L, Brummett CM, Englesbe MJ. Iatrogenic Opioid Dependence in the United States: Are Surgeons the Gatekeepers? Annals of surgery 2017;265:728–30. [DOI] [PubMed] [Google Scholar]
- 10.Harbaugh CM, Lee JS, Hu HM, et al. Persistent Opioid Use Among Pediatric Patients After Surgery. Pediatrics 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JS, Hu HM, Edelman AL, et al. New Persistent Opioid Use Among Patients With Cancer After Curative-Intent Surgery. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017:Jco2017741363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilliard PE, Waljee J, Moser S, et al. Prevalence of Preoperative Opioid Use and Characteristics Associated With Opioid Use Among Patients Presenting for Surgery. JAMA Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard R, Waljee J, Brummett C, Englesbe M, Lee J. Reduction in Opioid Prescribing Through Evidence-Based Prescribing Guidelines. JAMA Surg 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill MV, Stucke RS, McMahon ML, Beeman JL, Barth RJ, Jr. An Educational Intervention Decreases Opioid Prescribing After General Surgical Operations. Annals of surgery 2017. [DOI] [PubMed] [Google Scholar]
- 15.Opioid Prescribing Recommendations for Surgery. 2017. (Accessed 1/4/2018, 2018, at https://opioidprescribing.info.)
- 16.Cron DC, Englesbe MJ, Bolton CJ, et al. Preoperative Opioid Use is Independently Associated With Increased Costs and Worse Outcomes After Major Abdominal Surgery. Annals of surgery 2017;265:695–701. [DOI] [PubMed] [Google Scholar]
- 17.Sekhri S, Arora NS, Cottrell H, et al. Probability of Opioid Prescription Refilling After Surgery: Does Initial Prescription Dose Matter? Annals of surgery 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bateman BT, Cole NM, Maeda A, et al. Patterns of Opioid Prescription and Use After Cesarean Delivery. Obstetrics and gynecology 2017;130:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gammaitoni AR, Fine P, Alvarez N, McPherson ML, Bergmark S. Clinical application of opioid equianalgesic data. Clin J Pain 2003;19:286–97. [DOI] [PubMed] [Google Scholar]
- 20.Wilks DS. Chapter 15 - Cluster Analysis In: Wilks DS, ed. International Geophysics: Academic Press; 2011:603–16. [Google Scholar]
- 21.Ester M, Kriegel H-P, #246, Sander r, Xu X. A density-based algorithm for discovering clusters a density-based algorithm for discovering clusters in large spatial databases with noise. Proceedings of the Second International Conference on Knowledge Discovery and Data Mining Portland, Oregon: AAAI Press; 1996:226–31. [Google Scholar]
- 22.Utter Garth H, Cox Ginger L, Atolagbe Oluseun O, Owens Pamela L, Romano Patrick S. Conversion of the Agency for Healthcare Research and Quality’s Quality Indicators from ICD-9-CM to ICD-10-CM/PCS: The Process, Results, and Implications for Users. Health Services Research 2018;0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blay E Jr., Nooromid MJ, Bilimoria KY, et al. Variation in post-discharge opioid prescriptions among members of a surgical team. American journal of surgery 2018;216:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill MV, McMahon ML, Stucke RS, Barth RJ, Jr. Wide Variation and Excessive Dosage of Opioid Prescriptions for Common General Surgical Procedures. Annals of surgery 2017;265:709–14. [DOI] [PubMed] [Google Scholar]
- 25.Chiu AS, Healy JM, DeWane MP, Longo WE, Yoo PS. Trainees as Agents of Change in the Opioid Epidemic: Optimizing the Opioid Prescription Practices of Surgical Residents. Journal of Surgical Education 2017. [DOI] [PubMed] [Google Scholar]
- 26.Madsen AM, Stark LM, Has P, Emerson JB, Schulkin J, Matteson KA. Opioid Knowledge and Prescribing Practices Among Obstetrician-Gynecologists. Obstetrics and gynecology 2017. [DOI] [PubMed] [Google Scholar]
- 27.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. The Journal of Pain 2016;17:131–57. [DOI] [PubMed] [Google Scholar]
- 28.Waljee JF, Cron DC, Steiger RM, Zhong L, Englesbe MJ, Brummett CM. Effect of Preoperative Opioid Exposure on Healthcare Utilization and Expenditures Following Elective Abdominal Surgery. Annals of surgery 2017;265:715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soffin EM, Waldman SA, Stack RJ, Liguori GA. An Evidence-Based Approach to the Prescription Opioid Epidemic in Orthopedic Surgery. Anesthesia and analgesia 2017;125:1704–13. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen LC, Sing DC, Bozic KJ. Preoperative Reduction of Opioid Use Before Total Joint Arthroplasty. J Arthroplasty 2016;31:282–7. [DOI] [PubMed] [Google Scholar]
- 31.McAnally H. Rationale for and approach to preoperative opioid weaning: a preoperative optimization protocol. Perioperative Medicine 2017;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim KY, Anoushiravani AA, Chen KK, Roof M, Long WJ, Schwarzkopf R. Preoperative Chronic Opioid Users in Total Knee Arthroplasty—Which Patients Persistently Abuse Opiates Following Surgery? The Journal of Arthroplasty 2018;33:107–12. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y, Cortez AR, Wima K, et al. Impact of Preoperative Opioid Use After Emergency General Surgery. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 2018;22:1098–103. [DOI] [PubMed] [Google Scholar]
- 34.Vu JV, Lin LA. Opioid Overdose-the Surgeon’s Role. Annals of surgery 2018;268:32–4. [DOI] [PubMed] [Google Scholar]
- 35.Gomes T, Khuu W, Craiovan D, et al. Comparing the contribution of prescribed opioids to opioid-related hospitalizations across Canada: A multi-jurisdictional cross-sectional study. Drug and Alcohol Dependence 2018;191:86–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.