Abstract
Temporomandibular joint osteoarthritis (TMJOA) is a prevalent source of temporomandibular joint disorder (TMD). Women are more commonly diagnosed with TMD and are more likely to seek care at tertiary orofacial pain clinics. Limited knowledge regarding mechanisms underlying TMJ pain impairs development of improved pain management strategies. In a rat model of unilateral TMJOA, monosodium iodoacetate (MIA) produces joint pathology in a concentration dependent manner. Unilateral MIA produces alterations in meal patterns in males and females without altering overnight time spent eating or weight across 2 weeks. MIA (80 mg/ml) treated males develop ongoing pain within 2 weeks post-MIA injection. Females develop ongoing pain at a 5-fold lower MIA concentration (16.6 mg/m). MIA (80 mg/ml) treated males show spread of tactile hypersensitivity across the face during the first week post-injection and then to the fore- and hind-paws during the second week post-injection indicating development of central sensitization. At the lower dose, female rats demonstrate a similar spread of tactile hypersensitivity whereas male rats do not develop ongoing pain or spread of tactile hypersensitivity outside the area of the ipsilateral TMJ. These observations indicate that females have a higher susceptibility to development of ongoing pain and central sensitization compared to male rats that is not due to differences in MIA-induced joint pathology. This model of TMJOA pain can be used to explore sex differences in pain processes implicated in development of neuropathic pain, ongoing pain and central sensitization allowing for development of individualized strategies for prevention and treatment of TMJD pain.
Women are more commonly affected by temporomandibular joint disorder (TMD), with reported 3:1 female to male diagnoses and 8 or 9:1 female to male seeking care at tertiary orofacial pain clinics [20; 45; 49]. TMD impacts the patients’ daily activity, psychosocial functioning and quality of life with cost of management exceeding $4 billion annually [48]. TMDs are diagnosed based on reports of painful symptoms in the orofacial region that often drive patients to seed medical care from dentists [48]. One subcategory of TMD is osteoarthritis of the temporomandibular joint (TMJOA) which is found in up to 40% of TMD patients [38]. TMJOA is associated with pain, disability and diminished quality of life [10; 57]. The clinical diagnosis of TMJOA is mainly based on the radiographic features of the TMJ condyle and articular eminence, including erosive resorption, sclerosis, attrition, osteophyte formation, and cyst-like changes [10; 21]. TMJOA is characterized by pain in the pre-auricular area with or without associated earache, pain during palpation, coarse crepitus (joint noise) with or without clicking and limited mobility of the jaw [10; 27; 57]. Consistent with the increased prevalence of TMJD in women, TMJOA is approximately twice as prevalent and reported as more severe in women compared to men [60].
The primary goals of treating TMJOA are to decrease joint pain, swelling, and reflex masticatory muscle spasm/pain along with increasing joint function, and prevention of further joint damage, disability and disease related morbidity [10]. Pharmacological therapies for TMJOA are primarily non-steroidal anti-inflammatory drugs (NSAIDs), anxiolytics, muscle relaxants and opioids with no consensus regarding long-term effectiveness of these medications [54]. Nonpharmacological therapies for TMJOA pain include occlusal splinting, reported as effective in up to 60% of patients [27]. Arthrocentesis combined with occlusion splint therapies is used for patients resistant to splinting, but efficacy in patients drops to 66% of the patients at 3 months [27]. Therapeutic exercises designed to increase muscle strength, reduce joint contractures, and maintain a functional range of motion are also used with variable effectiveness [4; 10; 21]. In patients with treatment resistant TMJOA (<20%), joint replacement therapy often effectively alleviates pain, but second joint repair surgeries are commonly necessary [10; 57]. These reports suggest that alternative therapeutics are needed for a significant proportion of these patients. However, limited knowledge of the mechanisms driving TMD-associated pain limit development of improved therapeutic strategies for these patients.
TMD-associated pain is complex with reports of persistent background pain and joint use associated pain. Additionally, patients may experience spread of tactile hypersensitivity to other facial regions and as far as the upper extremities indicating development of central sensitization [16]. Within experimental settings, TMD patients have also been demonstrated to develop signs of central sensitization such as temporal summation [16] and diminished endogenous pain inhibition indicated by reduced conditioned pain modulation [11]. Moreover, some studies indicate that development of these signs of altered pain modulation are sex dependent [45; 49]. We characterized sex differences in development of ongoing pain and central sensitization in a rat model of TMJOA.
Materials and Methods
Animals.
Male and female SAS Sprague-Dawley (Charles River Laboratories) rats weighing 225–275 g (males) and 175–200g (females) were maintained on a 12-hour light/dark cycle with food and water available ad libitum. All experiments were performed in accordance with the policies and recommendations of the Internal Association for the Study of Pain, National Institutes of Health, and the Institutional Animal Care and Use Committee (IACUC) of the University of New England.
Intra-articular MIA Injection.
Injection of MIA is a well-characterized preclinical model of OA that has been used in the knee joint and TMJ [15; 25; 34; 43; 56]. MIA was injected unilaterally into the intraarticular space below the zygoma of the left TMJ. All injections were performed under isoflurane (2% in O2) with the mouth open resulting in expansion of the intra-articular space. Male rats were injected with 80 mg/ml,16.6 mg/ml MIA or equivolume saline (control) in 50 μl volume. Female rats were injected with the lower concentration, 16.6 mg/ml MIA or equivolume saline. Rats were then placed back into their home-cages for videotaping to assess meal patterns overnight (7PM-7AM).
Histology:
Tissue collection to examine joint pathology and cartilage loss occurred 16 days post-MIA. Rats were deeply anesthetized with Beuthanasia-D (100 mg/ml, Merck) followed by intracardiac perfusion with heparinized saline followed by 4% paraformaldehyde. Skulls including the TMJ were post-fixed in 4% paraformaldehyde overnight and then decalcified using 10% EDTA across 10 weeks. Decalcification was verified with x-ray radiographs. The joints were embedded in paraffin and 5 μm sagittal slices were taken for staining. Ipsilateral and contralateral joints were stained using Toluidine blue (0.04%) for imaging of cartilage loss. Sections were stained using hematoxylin and eosin (H&E) to determine joint pathology.
Images were taken using a Keyence BZ-X710 automated brightfield/epifluorescence microscope (Osaka, Japan). Images were acquired using the BZ-X Viewer and the stitching was performed using the plugin Image Merge within the BZ-X analyzer software. Stitched images were scored using a modified Mankin score along with scores of osteophyte development and synovitis. Scores were made for the mandibular fossa and the TMJ condyle using a modified Mankin scale to score changes in the cartilage, synovium, articular disc (meniscus), and bone of the joint [8; 9; 41; 55; 61]. The following 7-point scale was used for structural changes: 0=normal, 1=irregular surface (e.g. thick in some places, thin in others but still clearly organized structure); 2 = pannus or fibrillation (abrasions); 3=superficial cartilage layer being absent; 4=slight disorganization with superficial and deep cell rows absent in places with some small superficial clusters of cells; 5=fissures into calcified cartilage layers and fibrocartilage; 6=disorganized, very irregular surface, with >25% chaotic structure, many cellular clusters and osteoclasts; and 7=distinct tidemark changes with invasion evident and evidence of cysts, pseudocysts or ulcers. Cellular abnormalities were indicated by the following scale: 0=normal; 1=hypercellularity including small superficial clusters; 2=many cell clusters with hypertrophy of chondrocytes and 3=hypocellularity. In addition, scores for synovitis were made using the following scale: 0=normal, 1=hypercellular and slightly thickened, and 2=hypercellular and clearly thickened with loss of space. Osteophytes were also scored according to the following scale, 0=no osteophytes; 1=onset of osteophyte; 2=presence of osteophytes. Ratings were done by an investigator blinded to the treatment group of the images. Ratings were performed on samples collected from 3 rats/group in the low and high dose males and 2 samples collected from the low dose females, 4 sections/animal were rated for the low dose male and female samples and 2 sections/animal were rated for the high dose male samples.
Micro-computed tomography (micro-CT):
Following behavioral analyses (D16 post-MIA), rats were euthanized and TMJ dissected and fixed in 4% paraformaldehyde. They were then transferred to 70% EtOH. To characterize MIA-induced changes in bone, the TMJ condyle was analyzed with a SCANCO VivaCT-40 scanner (Scanco Medical AG, Bassersdorf, Switzerland). Scans were integrated into three-dimensional voxel image using a 10.5 μm isotropic voxel size (2048 × 2048 pixel matrices for trabecular and 1024 × 1024 pixel matrices for all other individual planar stacks). Rat condyles were scanned at 70kVp and 114 μA.
Behavioral Analysis of Pain
Meal Pattern Analysis.
Following assessment of tactile sensory thresholds between 10AM-4PM, rats were placed back into their home-cages for videotaping to assess pre-treatment meal patterns overnight (7PM-7AM). Video capture using webcams was performed in a room lit only using red light (Philips) and video analysis was performed with computer program ANY-maze (Stoelting Co; Wood Dale, Il, USA). Meal pattern analysis was performed in normal weight rats without any caloric restriction using the usual rat chow (Teklad, Envigo, South Easton, MA). Rat cages were placed in a red-lit room with web cameras video recording overnight feeding behavior beginning at 19:00. Rats were housed 3/cage and all rats had access to food and water ad libitum. We measured eating behavior of rats in their home cages overnight rather than in operant chambers as done by others [18; 22–24] to minimize potential stressors of being individually housed and trained to eat outside of the home-cage environment. The rats were kept on a 12 hr light-dark cycle with lights on at 07:00 hr. On the days of experimentation, body weights were measured prior and after overnight video recording. For video analyses of meal patterns, an investigator blinded to cage groups recorded the time each animal began and ended a bout of eating starting when the animal being observed started eating a pellet and ending after 4 seconds of inactivity. This established meal pattern behaviors, i.e. meal duration (sec/meal), total number of meals, and total time spent eating. Since rats were group housed food intake by individual animals was not measured; rather, eating behavior was assessed for each animal within a single cage, with individual animals identified using tail marks. Sample sizes were 6 saline treated males, 7 males treated with 80 mg/ml MIA and 6 females treated with 16.6 mg/ml MIA.
Tactile sensitivity.
Pre-treatment (baseline) assessments of tactile sensory thresholds at the ipsilateral hindpaw, ipsilateral forepaw, contralateral TMJ, periorbital midline, and ipsilateral TMJ was performed, in that order, blind with regard to group assignment using withdrawal behaviors from probing with calibrated von Frey filaments. To test facial tactile sensory thresholds, a two chambered Plexiglas box with two identical chambers (13 3/4” W × 13” D × 13 3/4” H) separated by a solid white wall and an opening (13 3/4” W × 10 1/3” H) for easy access to the facial region of the rat was used. Rats were acclimated for 45 minutes to the chamber, a time-point at which most rats ceased exploration of the chamber. The series of von Frey filaments were applied until buckling of the hair occurred in ascending order beginning with the 1.0 g filament, using the up-down method [6]. The filament was applied only when the rat was stationary and standing on all four paws. A withdrawal response was defined as withdrawal of the head, pressing the head into the filament, grabbing or swatting the filament or grooming following application of the filament. Post-treatment tactile sensory thresholds were measured D1, D3, D7, and D14 post-MIA injection. Sample sizes were 9–11 male rats treated with the 80 mg/ml MIA; 9–12 male rats treated with 16.6 mg/ml MIA; 12 male rats treated with saline (controls); 10–12 female rats treated with 16.6 mg/ml MIA; and 12 female rats treated with saline (controls).
Conditioned place preference (CPP).
The CPP assay has been used extensively to study non-evoked pain across multiple models of chronic pain states including knee joint osteoarthritis [1; 19; 25; 34]. This approach was employed to detect ongoing pain during the second week following injection of MIA into the left TMJ of rats. Rats received the appropriate concentration of intra-articular MIA (80 mg/ml or 16.6 mg/ml in 50 μl volume) or equivolume saline followed 7 days later by verification of development of tactile hypersensitivity in the ipsilateral TMJ. CPP to pain relief induced by systemic administration of duloxetine (30 mg/kg, i.p.) was performed using a 2-day conditioning protocol days 12–15 post intra-articular injection. A three-chambered box equipped with a video camera was used to record behaviors. The box consisted of two end chambers (10 3/8” W × 8 1/8” D×13 1/8” H) with distinct visual (gray vs. stripes), tactile (smooth vs. horizontal grid flooring), and odor cues (vanilla vs. lemonade). The third, central chamber (6 3/4” W × 8 1/8” D × 13 1/8” H) was well lit and had white walls. For the pre-conditioning phase (D12), rats were placed in the center chamber and allowed free access to all 3 chambers for 15 minutes and time spent in each chamber was recorded to verify no pre-conditioning chamber bias. In the morning of Days 13 and 14 rats received vehicle (saline, i.p.) and were immediately confined to the pre-assigned pairing chambers for 30 minutes. Rats were returned to their home cages and 4 hours later they received systemic duloxetine (30 mg/kg, i.p.) and were immediately confined to the opposite chamber for 30 minutes. The chamberv assignments for conditioning were counterbalanced. On Day 15, the rats were allowed free access to all chambers for 15 minutes and the total time spent in each chamber was recorded. Rats were included in the CPP analyses were 14 males treated with saline, 19 males treated with 80 mg/ml MIA, and 11 males treated with 16.6 mg/ml; and 13 female rats treated with 16.6 mg/ml MIA. A total of 6 male and 4 female rats were removed due to adverse reactions to the injections (e.g. diarrhea, broken nail resulting in bleeding, or excessive vocalization following injections); 8 males were removed due to placement into the wrong boxes and 2 males were removed due to failure to move during testing resulting in outlier data (Grubbs analysis). A total of 4 male and 2 female rats were removed due to pre-conditioning chamber bias defined as ≥ 720 or ≤ 180 seconds spent in one of the pairing chambers.
Statistical analysis.
For ratings of the H&E sections, comparisons of pathology between groups for the ipsilateral side to MIA treatment was performed using the nonparametric Kruskal-Wallis ANOVA followed by post-hoc comparisons using the two-stage linear step-up procedure of Benjamin, Kreiger and Yekutieli. Meal pattern measures were analyzed separately in male and female rats using a two-way ANOVA. Post-hoc analyses of time-dependent changes were performed using Dunnett’s multiple comparisons test and post-hoc analysis of between group differences performed using Sidak’s multiple comparisons test. For CPP, the effects of MIA treatment and conditioning chamber were analyzed by a two-way ANOVA. Post-hoc analysis of pre-conditioning (BL) vs post-conditioning values within each treatment group was performed using the Fisher’s LSD test. A significant increase from pre-conditioning time spent in the duloxetine-paired chamber indicates CPP. All analyses were performed using Graphpad Prism 8. Tactile sensory threshold data was analyzed using a mixed-effects analysis with post-hoc analysis of difference from pre-injection baselines performed using Dunnett’s multiple comparisons test. Statistical results are in Table 1. For analysis of tactile hypersensitivity, post-administration means were compared with the baseline values by repeated measures analysis of variance (ANOVA), followed by post hoc analysis of least significant difference for multiple comparisons. A probability level of 0.05 was used to establish significance.
Table 1:
Results from statistical analyses.
| Figure | Analysis | F (DFn, DFd) | p value |
|---|---|---|---|
| Figure 1d | Kruskal-Wallis test | Number of treatments = 3 Number of values = 8 Kruskal-Wallis statistic = 5.556 |
P=0.0250 |
| Figure 5a | 2-way ANOVA | Interaction F (3,41) = 14.24 Day F (3,41) = 37.57 Treatment F (1,41) = 11.59 |
P<0.0001; P<0.0001; P=0.0015 |
| Figure 5b | 2-way ANOVA | Interaction F (3,42) = 10.20 Day F (3,42) = 24.95 Treatment F (1,42) = 9.06 |
P<0.0001; P<0.0001; P=0.0044 |
| Figure 5c | 2-way ANOVA | Interaction F (3, 64) = 1.123 Day F (3, 64) = 14.41 Treatment F (1, 64) = 19.70 |
P=0.3464; P<0.0001; P<0.0001 |
| Figure 5d | 2-way ANOVA | Interaction F (3,64) = 0.8426 Day F (3,64) = 29.38 Treatment F (1,64) = 8.484 |
P=0.4756 P<0.0001; P=0.0049 |
| Figure 5e | 2-way ANOVA | Interaction F (3,42) = 0.5806 Day F (3,42) = 2.139 Treatment F (1,42) = 1.694 |
P=0.6310; P=0.1097 P=0.2002 |
| Figure 5f | 2-way ANOVA | Interaction F (3, 64) = 1.757 Day F (3, 64) = 3.851 Treatment F (1, 64) = 9.981 |
P=0.1643; P=0.0134 P=0.0024 |
| Figure 5g | 2-way ANOVA | Interaction F (28,435) = 0.0747 Day F (14,435) = 2.268 Treatment F (2,435) = 5.488 |
P=0.9999; P=0.0054 P=0.0044 |
| Figure 5h | 2-way ANOVA | Interaction F (14,300) = 0.2686 Day F (14,300) = 7.472 Treatment F (1,300) = 26.96 |
P=0.9965; P<0.0001; P<0.0001 |
| Figure 6a | 2-way RM ANOVA | Interaction F (15, 100) = 36.90; Time F (5, 100) = 42.63; Treatment F (3, 20) = 878.2 Subject F (20, 100) = 1.796 |
P<0.0001; P<0.0001; P<0.0001 P=0.0310 |
| Figure 6b | 2-way RM ANOVA | Interaction F (4, 57) = 5.751; Treatment F (4,57) = 1.987; Time F (1, 57) = 7.250 Subject F (57,57) = 1.670 |
P=0.0006; P=0.1088; P=0.0093 P=0.0276 |
| Figure 7a | Mixed Effect Analysis | Time F (4, 215) = 431.9; Treatment F (3, 215) = 348.0 Interaction F (12, 215) = 62.79 |
P<0.0001; P<0.0001; P<0.0001 |
| Figure 7b | Mixed Effect Analysis | Time F (4, 152) = 69.85; Treatment F (3, 38) = 36.61; Interaction F (12, 152) = 23.49 |
P<0.0001; P<0.0001; P<0.0001 |
| Figure 7c | Mixed Effect Analysis | Time F (4, 150) = 422.3; Treatment F (3, 38) = 393.4; Interaction F (12, 150) = 80.69 |
P<0.0001; P<0.0001; P<0.0001 |
| Figure 7d | Mixed Effect Analysis | Time F (4, 136) = 177.7; Treatment F (3, 34) = 109.3; Interaction F (12, 136) = 58.93 |
P<0.0001; P<0.0001; P<0.0001 |
| Figure 7e | Mixed Effect Analysis | Time F (4, 218) = 57.87; Treatment F (3, 218) = 372.7; Interaction F (12, 218) = 36.80; |
P<0.0001; P<0.0001; P<0.0001 |
| Figure 7f | Mixed Effect Analysis | Time F (4, 152) = 3.062; Treatment F (3, 38) = 0.7153; Interaction F (12, 152) = 2.015 |
P=0.0184 P=0.5490 P=0.0263 |
3. Results
3.1. Injection and verification of OA.
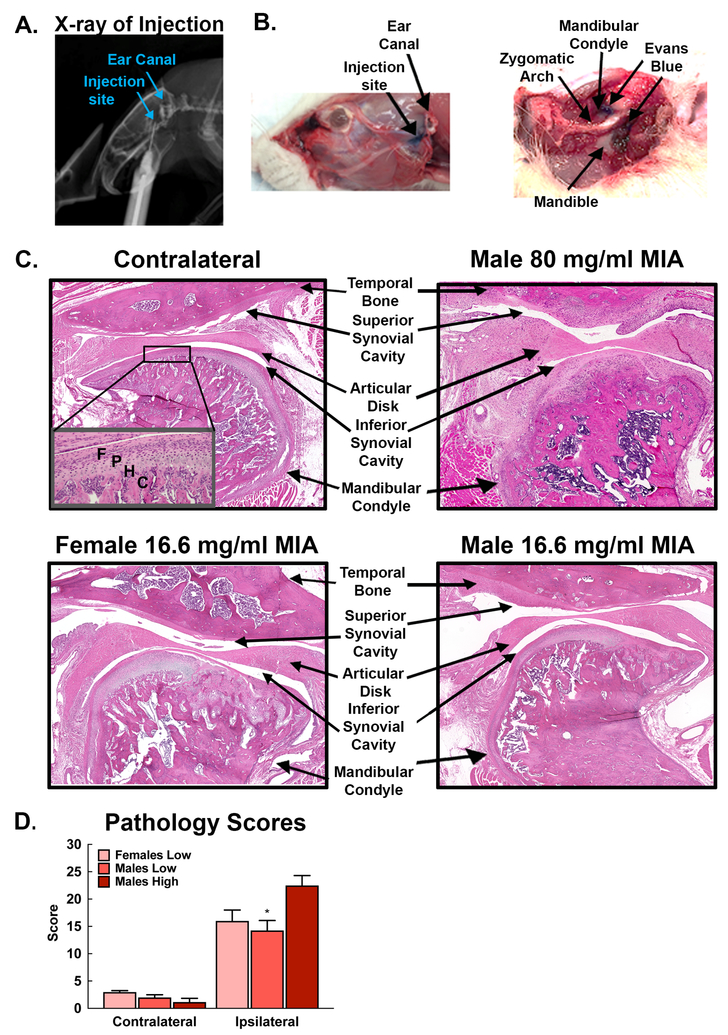
Injections were made in the upper compartment of the left TMJ (Fig 1A). To verify that the injection site in the compartment of the TMJ, Evan’s blue solution was injected at the same volume as MIA (50 μl) into the TMJ of 2 rats prior to behavioral experiments (Fig 1B). Visual inspection of the ink demonstrates that the volume used for MIA injections produces a blue stained region that was visible at the site of the TMJ and some ink observed in surrounding muscle as represented by Fig 1B. This volume was used for all MIA and saline injections.
Figure 1.
A. Radiographic image of rat with needle inserted below root of the zygoma into the intra-articular space. B. Evans blue demonstrates the injection site located below the ear canal. Dissection of the injection site demonstrates Evans blue at the site of the TMJ condyle and mandible. C. H&E demonstrates pathology of the TMJ joint. The contralateral TMJ demonstrates normal structure of the temporal bone, articular disc (meniscus), synovium, and mandibular condyle. The inset demonstrates normal cellular organization in the surface of the condyle. F: fibrous layer; P: proliferative layer; H: hypertrophy layer; C: calcified layer; B: subchondral bone. Male rats treated with 80 mg/ml MIA demonstrate dramatic bone restructuring of both the temporal bone and mandibular condyle. Non-nucleated tissue replacement is apparent in the articular disc indicating formation of scar tissue. Synovitis is apparent and fibrinous exudates are observed in the synovial cavities. There is clear loss of regular alignment and multilayer arrangement of chondrocytes in the mandibular condyle. Female and male rats treated with 15 mg/ml MIA also demonstrate bone restructuring of both the mandibular fossa and condyle, although not to the degree observed in 80 mg/ml MIA treated rats. D) Pathology scores of MIA treated TMJ were significantly elevated, compared to the contralateral TMJ. Pathology scores of the ipsilateral TMJ from males treated with the 80 mg/ml MIA (high) were significantly higher than scores for ipsilateral TMJ from males (*p<0.05 vs Males-high) treated with the lower concentration of 16.6. mg/ml MIA. Scores did not differ between the ipsilateral TMJ from males and females treated with the low MIA concentration.
3.2. MIA-induced joint pathology.
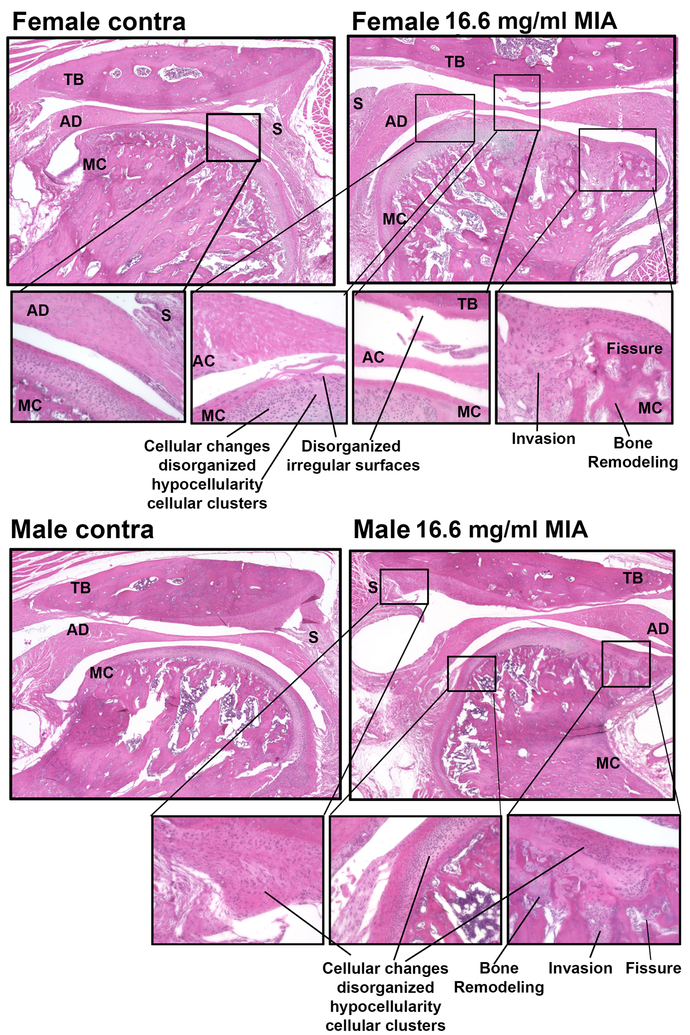
Comparison of H&E stains of the ipsilateral and contralateral TMJ of male rats treated with 80 mg/ml MIA reveals that clear pathologic changes occur within 14 days in the MIA treated joint and surrounding tissue in a concentration-dependent manner (Fig 1C,D). Male and female rats treated with the low dose demonstrate clear evidence of synovitis, with many demonstrating structural changes ranging from fissures into calcified cartilage layers and fibrocartilage corresponding to a ranking of 5 on the modified Mankin scale, disorganized irregular surfaces with chaotic structure, cellular clusters and osteoclasts corresponding to a rating of 6 on the Mankin scale, and some sections demonstrating distinct tidemark changes with cysts and pseudocysts (Fig 1C,D). TMJ sections from male rats treated with 80 mg/ml MIA demonstrated a significantly greater amount of pathology, with clear structural changes in both the temporal bone and the mandibular condyle and synovitis and osteophytes observed on the mandibular fossa, condyle, or both structures (Fig 1C). The pathology score of the high dose MIA treated male TMJ joints was significantly higher compared to males treated with the 16.6 mg/ml MIA (Fig 1D, *p<0.05). Pathology scores for the ipsilateral TMJ for the low dose MIA treated male and female rats did not significantly differ (p>0.05). Notably, rankings from male and female rats were higher compared to the control joints (Fig 1D). Low MIA induced similar levels of pathology in male and female TMJ including hypertrophy of the meniscal disk, fibrinogen in the synovial cavities, disruption of chondrocytes-showing cellular disorganization and invasion into the bone, fissures, and bone remodeling (Fig 2). No acute or chronic inflammatory cells were observed in the ipsilateral joints.
Figure 2.
H&E demonstrates low-dose MIA induced similar levels of pathology of the TMJ joint in female and male rats. The contralateral TMJ demonstrates normal structure of the temporal bone, articular disc (meniscus), synovium, and mandibular condyle which has normal cellular organization in the surface of the condyle. In MIA treated TMJ, there is clear loss of regular alignment and multilayer arrangement of chondrocytes in the mandibular condyle and the temporal bone. The articular surface of both the temporal bone and mandibular condyle demonstrate disorganized irregular surfaces. Female and male rats treated with low dose MIA also demonstrate enlargement of the articular disk and bone remodeling in the mandibular condyle. TB-temporal bone, AD-articular disk, MC-mandibular condyle, S-synovium. Call out images taken with 20x objective.
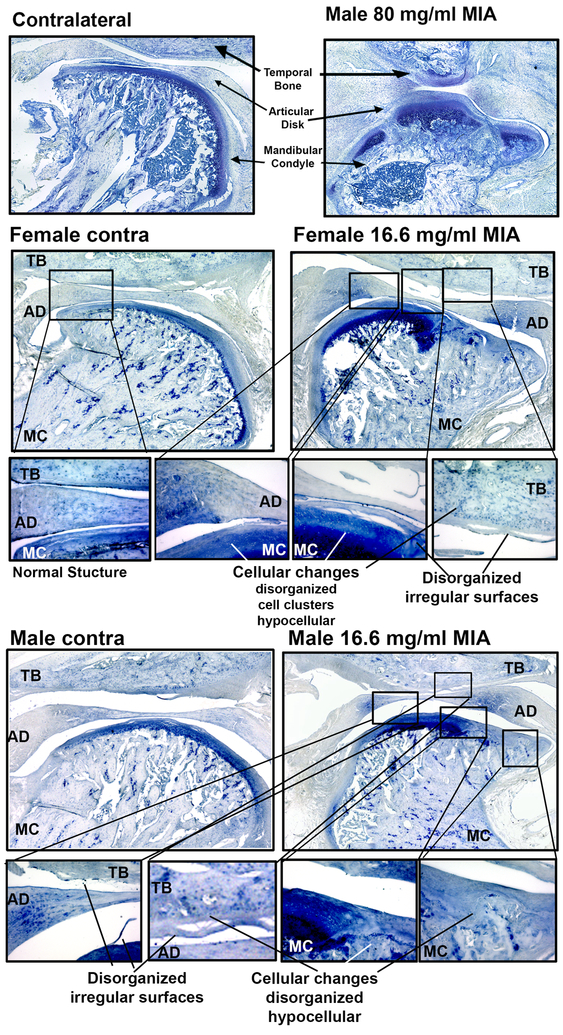
3.3. MIA-induced cartilage loss and subchondral bone changes
Cartilage loss was examined using toluidine blue histology. Staining was performed on contralateral and ipsilateral TMJ of MIA treated rats (Fig 3). Representative images demonstrated that MIA induces histopathologic changes to the cartilage in the rat TMJ consistent with previous reports [56]. Toluidine blue staining of the control tissue shows the hypertrophic layer stained blue indicating abundant proteoglycans in the condylar cartilage. In the ipsilateral TMJ of the high dose (80 mg/ml MIA), there is an absence of blue staining in the hypertrophic layer indicating cartilage loss with complete loss of chondrocytes on the TMJ condyle. Ipsilateral TMJ of the low dose (16.6 mg/ml MIA) also show diminished cartilage staining. Similar cartilage loss is evident in the female and male rats treated with the lower concentration (16.6 mg/ml) of MIA. Similar pathology was observed in the toluidine blue stained sections as observed in the H&E stained sections.
Figure 3.
Toluidine blue stain confirms cartilage loss in the MIA treated TMJ condyle. Male rats treated with the high concentration of MIA demonstrate a loss of cartilage in the ipsilateral mandibular condyle. Low does MIA induced cartilage loss as indicated by diminished blue staining in the hypertrophic layer indicating cartilage loss with loss of chondrocytes on the TMJ condyle in both female and male TMJ. Call-out figures show cellular changes and disorganized irregular articular surfaces of the mandibular condyle and temporal bone in both female and male MIA treated TMJ. TB-temporal bone, AD-articular disk, MC-mandibular condyle, S-synovium. Images taken with 20x objective.
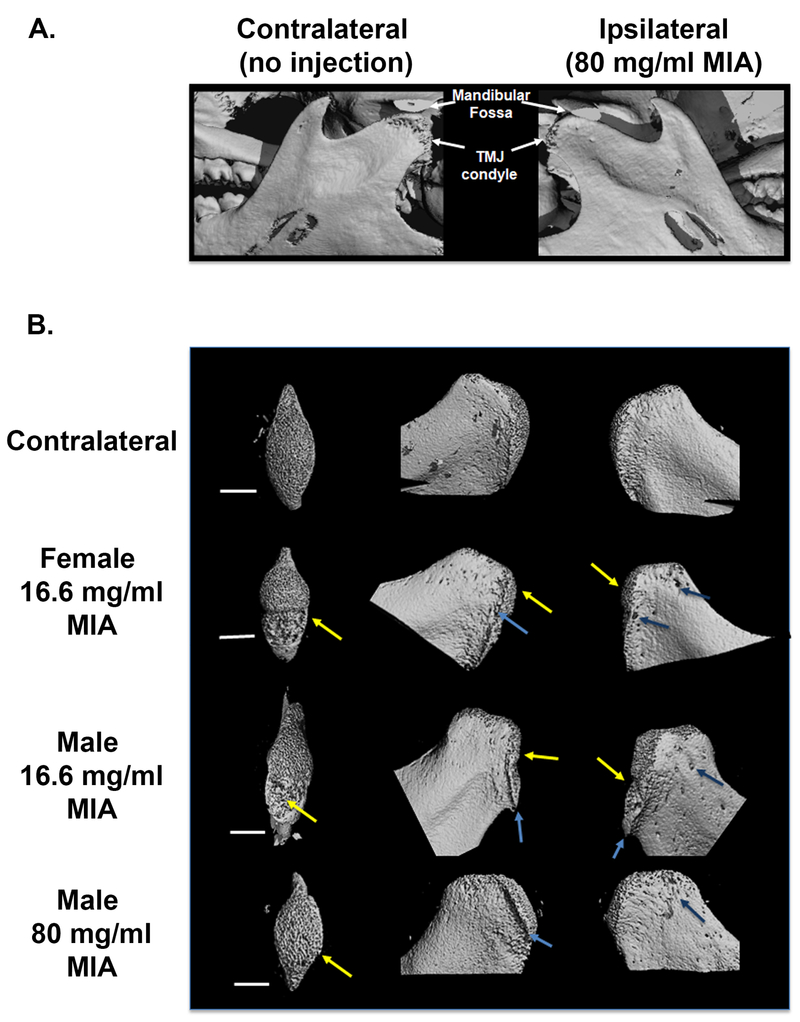
MicroCT analysis of the jaw confirmed that MIA injections induced bone remodeling consistent with previously reports [56]. The 3D remodeling of the joint demonstrates degeneration of the TMJ condoyle in the male rats treated with 80 mg/ml MIA (Fig 4A). Remodeling of the condyle demonstrates subchondral bone remodeling (Fig 4B) including thickening of the trabecular bone with apparent pitting and erosive absorption apparent along the top ridge of the condyle (left column), formation of an osteophyte lipping (arrows, middle column) and degradation and pitting along the edge of the condyle (right column).
Figure 4:
A) MicroCT images demonstrating bone remodeling of the mandibular condyle following 80 mg/ml MIA. B) Contralateral non-MIA treated bone are shown in the top row. Ipsilateral mandibular condyles received MIA. Bone remodeling of the ipsilateral mandibular condyle is apparent in female and male rats treated with 16.6 mg/ml MIA as well as in males treated with 80 mg/ml MIA when compared to the contralateral non-MIA treated bone. Left side of panel shows top down images of the mandibular condyle (transaxial views) illustrating trabecular bone thickening along with areas of bone loss in the condyle (yellow arrows). Similar levels of bone remodeling were observed in the female and male rats treated with 16.6 mg/ml MIA. Middle column of images shows lateral views of the condyles with areas of apparent bone erosion (yellow arrows) as well as formation of osteophytes and lipping (blue arrows) along the top edge. Right column of images of medial views of the mandibular condyle (right column) also demonstrate development of pitting along the condyle (dark blue arrows).
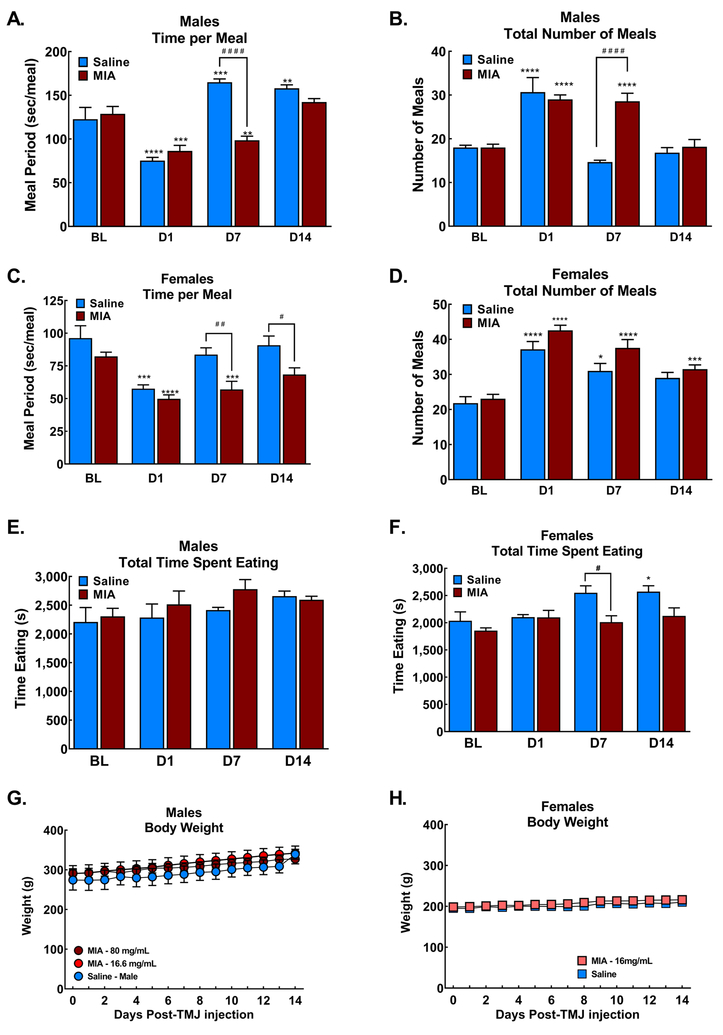
3.4. Sex dependent changes in meal patterns
Saline induced a transient decrease in the time spent eating during each meal and a concurrent increase in the number of meals eaten during the first overnight observation period in males (Fig 5A, 5B respectively). Time spent eating during each meal recovered and the number of meals eaten returned to baseline at 7- and 14-days post-injection in the saline treated male rats. Male rats treated with the MIA (80 mg/ml) demonstrated diminished time spent eating per meal and a concurrent increase in the number of meals eaten during the overnight period both at the 1- and 7-day timepoints (Fig 5A&B, ****p<0.001 vs BL; ####p<0.001 vs saline). The time spent eating and the number of meals eaten overnight returned to baseline patterns by 14 days post-injection (Fig 5A&B). Similarly, females demonstrated a decrease in the number of meals eaten with a concurrent increase in the number of meals during the first overnight observation period (Fig 5C&D respectively). Time spent eating returned to pre-injection baselines within 7 days and the total number of meals diminished at D7 and did not differ from BL 14 days post saline injection. Female rats treated with MIA (16.6 mg/ml) demonstrated similar patterns of diminished time spent eating (Fig 5C, ****p<0.001 vs BL, ##p<0.01 vs saline) and increased number of meals overnight (Fig 5D, ****p<0.001 vs BL) at the D1 and D7 timepoints. At the D14 time-point, the females continued to show diminished time per meal (#p<0.05 vs saline). The number of meals females had overnight remained elevated compared to pre-injection (BL) levels at the D14 time-point (***p<0.05 vs BL), but was equivalent to the saline controls (Fig 5D). The total time spent eating overnight did not change in the males treated with saline or high dose MIA (Fig 5E). Female saline treated animals demonstrated an increase in the time spent eating at D7 and D14 post-injection (Fig 5F). Bodyweight of MIA treated males and females did not differ from saline treated animals across the time-course of the study (Fig 5G and H, respectively) indicating that the shift in meal eating behavior resulted in maintenance of body weight.
Figure 5:
Meal pattern analysis of rats overnight 24 hrs, 7 days and 14 days post-MIA injection in male and female rats. A) Meal eating time for each meal (sec/meal) demonstrates decreased time spent eating in both saline and MIA treated male rats during the first observation period D1 post-injection. Males treated with 80 mg/ml MIA demonstrated reduced time spent eating through 7 days post-injection (***p<0.001 vs BL; ####p<0.0001 vs saline). Time spent eating was equivalent between saline and MIA treated rats 14 days post-injection, with saline rats demonstrating increased time per meal compared to BL (**p<0.01 vs BL). B) Males treated with saline or 80 mg/ml MIA injections increased the total number of meals across the overnight period during the first observation period D1 post-injection. The number of meals returned to BL 7 days post-saline, but not post-MIA. Number of meals were equivalent in saline and MIA treated rats D14 post-MIA, ***p<0.001 vs BL; ####p<0.0001 vs saline). C. Females treated with 16.6 mg/ml MIA or saline demonstrated reduced time per meal during the first observation period (***p<0.001 vs BL). Saline treated females returned to pre-injection time per meal within 7 days of injection. MIA treated females showed persistent decreased time per meal7 and 14 days post injection compared to saline treated controls (#p<0.05; ##p<0.01 vs saline, ***p<0.001 vs BL). D. Females treated with 16.6 mg/ml MIA or saline demonstrated increased number of meals during the first observation period post-injection (****p<0.0001 vs BL). MIA treated females continued to show elevated number of meals compared to BL 7- and 14-days post-injection (***p<0.001 vs BL) but did not differ from the saline treated controls. E. Total time spent eating did not change in males treated with saline or MIA at any time-point. F. Females treated with saline demonstrated elevated time spent eating 7- and 14-days post-injection (#p<0.05 vs saline; *p<0.05 vs BL), MIA treated females did not show any changes in time spent eating. G. Weight gain over 14 days did not differ between saline and MIA treated male rats. H. Weight gain over 14 days did not differ between saline and MIA treated female rats. All graphs represent mean ± SEM, n=6 for all treatment groups.
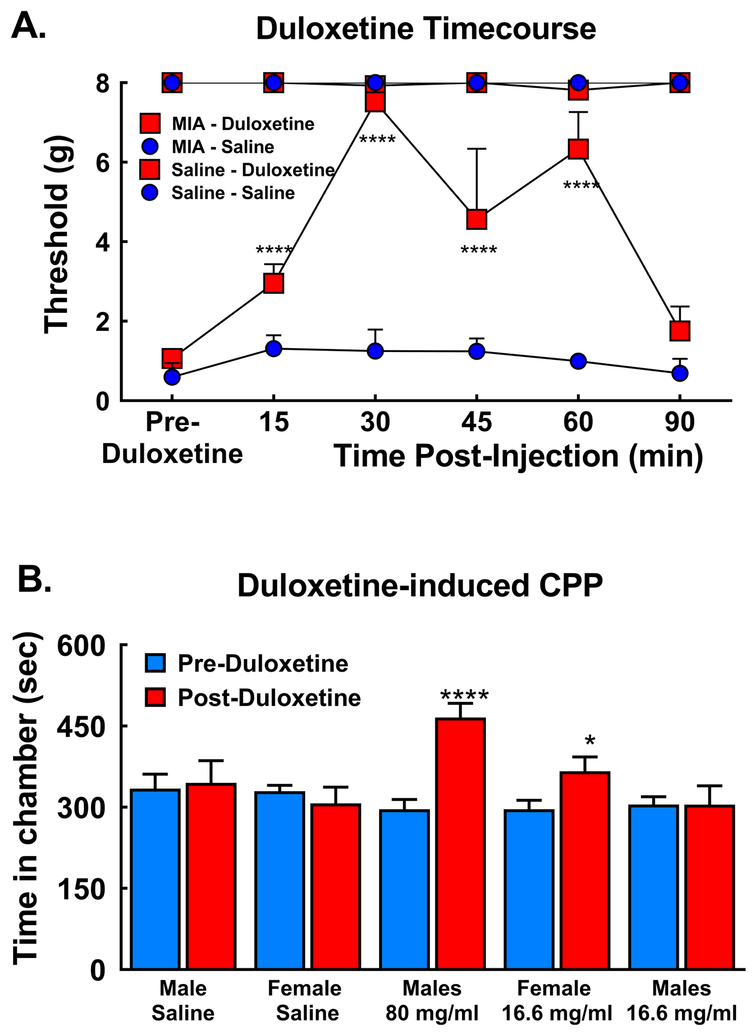
3.5. Duloxetine reverses MIA-induced tactile hypersensitivity and ongoing pain
Duloxetine administered 14 days post-MIA injection attenuated MIA-induced tactile hypersensitivity at the ipsilateral TMJ within 15 min of systemic administration, with full reversal observed 30 min post-injection in male rats treated with MIA (80 mg/ml) (Fig 6A, ****p<0.001 vs pre-duloxetine (BL)). Tactile hypersensitivity returned to pre-duloxetine values 90 min post-duloxetine. As the onset of duloxetine-induced reversal of MIA-induced tactile hypersensitivity occurred across the first 30 min post-administration, this time frame was used for the pairing trials during the CPP trials in both male and female rats.
Figure 6:
A) Systemic duloxetine (30 mg/kg i.p.) attenuated MIA-induced tactile hypersensitivity within 15 min and fully reversed the hypersensitivity by 30 min in male rats treated with 80 mg/ml MIA, ****p<0.0001 vs pre-duloxetine; n=6/treatment group B) Duloxetine significantly increased time spent in the duloxetine paired chamber selectively in male rats treated with 80 mg/ml MIA and female rats treated with 16.6 mg/ml MIA, but not in male rats treated with 16.6 mg/ml MIA (****p<0.001; p<0.05 vs pre-Duloxetine). Duloxetine did not alter time spent in the pairing chamber in male or female rats treated with saline. All graphs represent mean ± SEM, n=14 male saline, 5 female saline; 19 male 80 mg/ml MIA; 13 female 16.6 mg/ml MIA and 11 males 16.6 mg/ml MIA.
Duloxetine also alleviated the MIA-induced ongoing pain in males treated with the high dose (80 mg/ml) and females treated with the lower dose (16.6 mg/ml) MIA, but not males treated with the low dose of MIA (Fig 6B). Comparison of pre- vs post-conditioning time spent in the duloxetine paired chamber demonstrates that males treated with the high dose MIA increased time spent in the duloxetine paired chamber (Fig 6B, ****p<0.0001). Female rats treated with the lower dose also demonstrated increased the time spent in the duloxetine paired chamber (Fig 6B, *p<0.05) indicating that females develop ongoing TMJ pain at a 5-fold lower dose compared to male rats. Males treated with the lower dose did not alter the time spent in the duloxetine paired chamber, with equivalent pre- and post- conditioning time spent in the duloxetine paired chamber (Fig 6B). Saline injection into the TMJ failed to induce ongoing pain in male or female rats as indicated by no change in time spent in the duloxetine paired chamber.
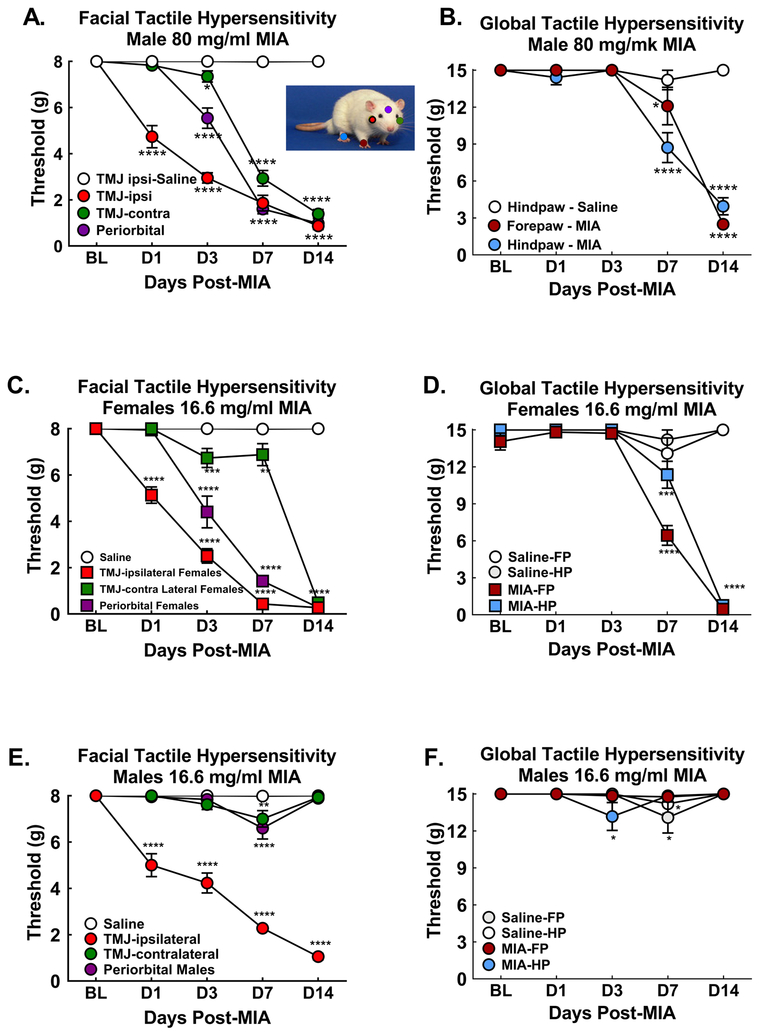
3.6. MIA-induced development of tactile hypersensitivity
Male rats treated with MIA (80 mg/ml) developed tactile hypersensitivity at the ipsilateral TMJ test site within 24 hrs that gradually increased across the 14-day test period (Fig 7A). Tactile hypersensitivity at the periorbital midline site emerged 3 days post-MIA injection and continued to develop across the 14 days (Fig 7A). Tactile hypersensitivity at the contralateral TMJ test site developed between 3- and 7-days post-MIA and remained through D14 post-injection (Fig. 7A). Saline injection did not induce facial tactile hypersensitivity at any of the time-points (Fig 7A). Ipsilateral forepaw and hindpaw hypersensitivity emerged 7 days post-MIA injection t (Fig 7B). Tactile hypersensitivity was equivalent in the forepaw and hindpaw by day 14 post-MIA injection (Fig 7B). Saline injection into the TMJ failed to alter sensory thresholds of the ipsilateral forepaw or hindpaw at any of the time-points (Fig 7B). Female rats treated with a 5-fold lower dose of MIA (16.6 mg/ml) demonstrated similar patterns of tactile hypersensitivity. Tactile hypersensitivity first emerged at the ipsilateral TMJ test site within 24 hrs of injection (Fig 7C) followed by tactile hypersensitivity at the periorbital and contralateral TMJ 3 days post-injection (Fig 7C). Tactile hypersensitivity at the ipsilateral forepaw and hindpaw emerged at 7 days post-injection, with increased hypersensitivity observed D14 at both paws (Fig 7D). As with the males, saline injection did not induce tactile hypersensitivity at any test site (Fig 7C&D). Males treated with the lower dose of MIA developed ipsilateral tactile hypersensitivity at the TMJ test site that persisted through the 14-day post-injection test period (Fig 7E). However, there was no spread of the tactile hypersensitivity across the face (Fig 7E) or to the forepaw or hindpaw (Fig 7F).
Figure 7:
The time-course of MIA-induced tactile hypersensitivity was assessed at 5 sites to determine development of central sensitization: Diagram showing regions von Frey tested on rat (colors match the symbols on the graphs); A) Male rats treated with 80 mg/ml MIA developed tactile hypersensitivity at the ipsilateral TMJ test site within 1 day. Tactile hypersensitivity spread to periorbital test site within 3 days and the contralateral TMJ test site within 7 days post-injection; n=11–12. B) Males treated with 80 mg/ml MIA into the TMJ developed tactile hypersensitivity in the ipsilateral forepaw and hindpaw days 7 and 14 post-injection; n=9–12. Males treated with 80 mg/ml MIA into the TMJ developed tactile hypersensitivity in the ipsilateral forepaw and hindpaw days 7 and 14 post-injection; n=9–12. C. Female rats treated with 16.6 mg/ml MIA developed tactile hypersensitivity at the ipsilateral test site within 1 day. Tactile hypersensitivity spread to the periorbital test site within 3 days and to the contralateral TMJ test site within 14 days post-MIA; n=10–12. D) Females treated with 16.6 mg/ml MIA into the TMJ developed tactile hypersensitivity in the forepaw and hindpaw days 7 and 14 post-injection; n=9–10. E. Male rats treated with 16.6 mg/ml MIA developed hypersensitivity at the ipsilateral TMJ test site within 1 day but failed to show tactile hypersensitivity at the periorbital or contralateral TMJ test sites; n=12/group. F. Male rats treated with 16.6 mg/ml MIA failed to develop tactile hypersensitivity in the forepaw or hindpaw; n=9–12. For all graphs, ****p<0.0001, *p<0.05 vs BL. All graphs represent mean ± SEM.
Discussion
Female rats are more susceptible to development of TMJOA-induced pain and central sensitization compared to male rats. MIA induced joint pathology that is consistent with other descriptions of OA [30; 43; 56]. The lower dose of MIA produced similar levels of joint pathology in males and females indicating that the increased susceptibility to ongoing pain and central sensitization in females is not due to increased pathology compared to males. These observations are consistent with clinical reports that women are more commonly affected by temporomandibular joint disorder (TMJD) than men [20; 45; 49]. MIA injections that produced ongoing pain also resulted in spread of tactile hypersensitivity across the face within the first week and then to fore- and hind-paws during the second week, consistent with development of central sensitization. Moreover, this spread of tactile hypersensitivity is consistent with reports of central sensitization including spread of tactile hypersensitivity that has been reported in some TMJD patients [12; 14; 28; 29; 47; 50].
Our observations of MIA-induced signs of joint pathology and changes to the TMJ condyle are consistent with previous reports of TMJOA. Histological staining demonstrate that MIA induced clear pathologic changes and cartilage loss consistent with previous reports [7; 31]. Synovial hyperplasia observed around both the superior and inferior synovial cavity is consistent with synovial hyperplasia described in the clinical setting and preclinical models [30; 56]. Development of fibrinous exudate within the joint spaces indicates ongoing pathology and repair processes characteristic of chronic OA and the vascular invasion are consistent with other reports in OA joints [30; 32]. MIA-induced pathology has a rapid onset, clearly evident within 15 days post-injection, differing from the clinical setting and rodent models of surgical or naturally occurring OA in which the development of the OA pathology is much slower [33; 57]. However, the histology demonstrates changes that are consistent with previous preclinical models and with histopathological descriptions of OA within the clinical settings [10; 15; 30; 32; 43; 57]. MicroCT imaging demonstrated concentration-dependent MIA-induced bone remodeling of the TMJ condyle. Subchondral bone erosion, sclerosis and formation of osteophyte lipping is consistent with previous reports in rodent models [56] and clinical reports of osteophyte formation in patients with TMJD [2; 54]. Joint pathology and bone remodeling were similar between male and female rats treated with the low concentration of MIA.
Unilateral MIA induced similar alterations in meal eating patterns with shorter meal times and increased number of meals overnight. Both saline and MIA injections altered meal eating behaviors across the first overnight observation period post-injection, with diminished time per meal and increased number of meals. Saline treated rats returned to pre-injection patterns by 7 days post-injection indicating the overnight alteration reflects transient discomfort from the injection. Male rats demonstrated altered meal patterns through day 7 post-injection and returned to pre-injection patterns by day 14 post-injection. Female rats treated with the 5-fold lower dose of MIA demonstrated similar alterations in their eating patterns. Other groups demonstrated that bilateral injection of the complete Freund’s adjuvant (CFA) alters meal patterns for 4 days post-injection while overall food intake was maintained [18; 22–24]. However, changes in meal pattern differed with bilateral CFA treated rats showing increased time spent eating per meal and diminished numbers of meals [18; 22–24]. Several differences in the experimental protocols may account for these differences including group vs individual housing and unilateral vs bilateral injections [18; 22–24]. We demonstrate that both male and female MIA treated rats maintained total time spent eating overnight and maintained weight across the observation period. These observations indicate that the rats adapted their eating behaviors to maintain food intake sufficient to maintain weight. The return to pre-MIA eating patterns in the male rats likely do not reflect a reduced evoked pain but rather adaptation of chewing to diminish joint discomfort. Supporting this interpretation, facial tactile hypersensitivity was maintained and spread across the face and body during the 14-day time-course suggesting development of sensitization rather than diminished hypersensitivity. We hypothesize that the MIA treated rats adapted their mastication to minimize joint discomfort. Future studies analyzing mastication in MIA compared to saline treated rats using well established measures (e.g. [53]) may shed light on potential compensatory changes made in response to TMJ pain.
Female rats developed ongoing pain at a 5-fold lower concentration compared to males. Female rats treated with 16.6 mg/ml MIA showed CPP to pain relief induced by duloxetine whereas male rats treated with that concentration did not indicating that female, but not male rats develop ongoing pain. Indeed, consistent with our previous reports, male rats developed ongoing pain to 80 mg/m MIA, but not lower doses [19; 34]. Systemic administration of duloxetine reversed the MIA-induced tactile hypersensitivity and blocked ongoing pain in the MIA treated rats, similar to our earlier observations with MIA-induced knee joint pain [19]. Notably, duloxetine produced CPP using a 2-pairing protocol previously described as necessary to induce CPP with systemic administration of drugs in rat models of neuropathic pain [39; 40]. Our observations that duloxetine blocks MIA-induced TMJ pain is consistent with our earlier observations that duloxetine blocks ongoing pain in a rat model of knee joint OA pain [19]. Duloxetine is used within the clinical setting to treat neuropathic pain and has been FDA approved for treatment of OA pain [3; 26; 42]. Other studies using similar concentrations of MIA injected into the knee joint have indicated that MIA-induced joint pain is associated with signs of neuropathic pain such as resistance to anti-inflammatory drugs [34; 43] and relief of evoked and ongoing pain in response to drugs such as pregabalin and duloxetine used in treatment of neuropathic pain [19; 43; 51; 52]. Further, the higher concentration of MIA into the knee joint of male rats has been demonstrated to induce ATF-3 expression in cell bodies within the DRG, a marker of nerve damage [51; 52]. It is unclear whether the low dose that produces neuropathic pain differentially in males and females or whether differences in pain modulatory pathways within the brain mediate the sex difference. Future studies are warranted to explore this question.
MIA injection into the TMJ produced local tactile hypersensitivity within 24 hrs followed by a time-dependent development of global tactile hypersensitivity. Female rats developed global tactile hypersensitivity at a 5-fold lower MIA concentration compared to male rats. Spread of hypersensitivity from a local site of injury to regions outside the receptive field has been proposed to indicate development of central sensitization [35–37; 58; 59]. Several studies have demonstrated that central sensitization develops in persistent pain states in which there is a tonic input [35–37; 58; 59]. Supporting this, we have previously demonstrated that spinal sensitization develops in male rats treated with 80 mg/ml MIA that induces ongoing pain, but is absent in male rats treated with lower MIA concentrations that induce weight asymmetry in the absence of ongoing pain [19]. Similarly, male rats treated with the 5-fold lower dose of MIA (16.6 mg/ml in 50 μl) failed to show a spread of tactile hypersensitivity from the ipsilateral TMJ test site. We hypothesize that central sensitization develops in rats that develop persistent ongoing pain in a time-dependent manner indicated by the spread of tactile hypersensitivity across the face and to the fore- and hind-paws across the weeks following injection. This would be consistent with observations in models of nerve injury-induced pain wherein early periods of thermal and tactile hypersensitivity are mediated at the peripheral or spinal sites whereas later periods of hypersensitivity are dependent on descending pain facilitatory pathways from the rostral ventromedial medulla (RVM) [5]. Spread of tactile hypersensitivity and development of central sensitization are also consistent with clinical observations. Several clinical studies have demonstrated that some patients with painful temporomandibular joint disorder (TMJD) develop widespread hyperalgesia [14; 28; 29; 47] and activity in regions of the brain that associated with pain processing and pain modulatory circuits such as the posterior insula and the rostral anterior cingulate cortex [13; 16; 17]. Diminished descending noxious inhibitory control and enhanced temporal summation indicating enhanced net descending facilitation have also been reported in TMJD patients [11; 46]. Notably, sex differences in endogenous pain modulatory systems have been proposed to underlie the susceptibility of females to develop TMD [44; 45; 49]. Further studies examining the role of descending pain modulation may allow for better understanding of development of pain in TMJD patients.
Development of TMJOA-induced pain occurs in a sex dependent manner, with females showing a greater susceptibility to development of persistent ongoing pain and central sensitization compared to males. This development of enhanced pain and central sensitization is not dependent on differences in joint pathology, as the concentration of MIA that induces pain in females but no pain in males did not show differences in joint pathology or bone remodeling. Determination of sex differences in pain processes implicated in development of neuropathic pain, ongoing pain and central sensitization are required to better understand mechanisms contributing to susceptibility to TMJD pain in females and protection from development of TMJD pain in males allowing for development of individualized strategies for prevention and treatment of TMJD pain.
Statement of funding:
This work supported by a COBRE grant (P20GM103643: Project PI: T. King; COBRE PI: I. Meng). Microcomputed tomography was performed within the Mouse Transgenic and In Vivo Imaging Core Facility, partially supported by NIH award U54GM115516 (PI: C. J. Rosen) to Maine Medical Center.
Footnotes
Conflict of interest: The authors have no conflict of interest
References
- [1].Allen J, Imbert I, Havelin J, Henderson T, Stevenson G, Liaw L, King T. Effects of Treadmill Exercise on Advanced Osteoarthritis Pain in Rats. Arthritis Rheumatol 2017;69(7):1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bag AK, Gaddikeri S, Singhal A, Hardin S, Tran BD, Medina JA, Curé JK. Imaging of the temporomandibular joint: An update. World journal of radiology 2014;6(8):567–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Blikman T, Rienstra W, van Raaij TM, ten Hagen AJ, Dijkstra B, Zijlstra WP, Bulstra SK, van den Akker-Scheek I, Stevens M. Duloxetine in OsteoArthritis (DOA) study: study protocol of a pragmatic open-label randomised controlled trial assessing the effect of preoperative pain treatment on postoperative outcome after total hip or knee arthroplasty. BMJ open 2016;6(3):e010343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Borenstein DG, Hassett AL, Pisetsky D. Pain management in rheumatology research, training, and practice. Clin Exp Rheumatol 2017;35 Suppl 107(5):2–7. [PubMed] [Google Scholar]
- [5].Burgess SE, Gardell LR, Ossipov MH, Malan TP Jr., Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci 2002;22(12):5129–5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- [7].Clements KM, Ball AD, Jones HB, Brinckmann S, Read SJ, Murray F. Cellular and histopathological changes in the infrapatellar fat pad in the monoiodoacetate model of osteoarthritis pain. Osteoarthritis and cartilage 2009;17(6):805–812. [DOI] [PubMed] [Google Scholar]
- [8].Coq J-O, Strata F, Russier M, Safadi F, M Merzenich M, Byl N, Barbe M. Impact of neonatal asphyxia and hind limb immobilization on musculoskeletal tissues and S1 map organization: Implications for cerebral palsy, Vol. 210, 2008. [DOI] [PubMed] [Google Scholar]
- [9].Custers RJH, Creemers LB, Verbout AJ, van Rijen MHP, Dhert WJA, Saris DBF. Reliability, reproducibility and variability of the traditional Histologic/Histochemical Grading System vs the new OARSI Osteoarthritis Cartilage Histopathology Assessment System. Osteoarthritis and cartilage 2007;15(11):1241–1248. [DOI] [PubMed] [Google Scholar]
- [10].de Souza RF, Lovato da Silva CH, Nasser M, Fedorowicz Z, Al-Muharraqi MA. Interventions for the management of temporomandibular joint osteoarthritis. Cochrane Database Syst Rev 2012(4):CD007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol 2009;65(2):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fillingim RB, Maixner W, Kincaid S, Sigurdsson A, Harris MB. Pain sensitivity in patients with temporomandibular disorders: relationship to clinical and psychosocial factors. Clin J Pain 1996;12(4):260–269. [DOI] [PubMed] [Google Scholar]
- [13].Gerstner GE, Gracely RH, Deebajah A, Ichesco E, Quintero A, Clauw DJ, Sundgren PC. Posterior insular molecular changes in myofascial pain. J Dent Res 2012;91(5):485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Diatchenko L, Liu Q, Maixner W. Pain Sensitivity and Autonomic Factors Associated with Development of TMD: the OPPERA Prospective Cohort Study. The journal of pain : official journal of the American Pain Society 2013;14(12 0):T63–74.e61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Guzman RE, Evans MG, Bove S, Morenko B, Kilgore K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol 2003;31(6):619–624. [DOI] [PubMed] [Google Scholar]
- [16].Harper DE, Schrepf A, Clauw DJ. Pain Mechanisms and Centralized Pain in Temporomandibular Disorders. J Dent Res 2016;95(10):1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Harper DE, Shah Y, Ichesco E, Gerstner GE, Peltier SJ. Multivariate classification of pain-evoked brain activity in temporomandibular disorder. Pain Rep 2016;1(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Harper RP, Kerins CA, Talwar R, Spears R, Hutchins B, Carlson DS, McIntosh JE, Bellinger LL. Meal pattern analysis in response to temporomandibular joint inflammation in the rat. J Dent Res 2000;79(9):1704–1711. [DOI] [PubMed] [Google Scholar]
- [19].Havelin J, Imbert I, Cormier J, Allen J, Porreca F, King T. Central Sensitization and Neuropathic Features of Ongoing Pain in a Rat Model of Advanced Osteoarthritis. J Pain 2016;17(3):374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Johansson A, Unell L, Carlsson GE, Soderfeldt B, Halling A. Gender difference in symptoms related to temporomandibular disorders in a population of 50-year-old subjects. Journal of orofacial pain 2003;17(1):29–35. [PubMed] [Google Scholar]
- [21].Kalladka M, Quek S, Heir G, Eliav E, Mupparapu M, Viswanath A. Temporomandibular joint osteoarthritis: diagnosis and long-term conservative management: a topic review. J Indian Prosthodont Soc 2014;14(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kerins C, Carlson D, McIntosh J, Bellinger L. A role for cyclooxygenase II inhibitors in modulating temporomandibular joint inflammation from a meal pattern analysis perspective. J Oral Maxillofac Surg 2004;62(8):989–995. [DOI] [PubMed] [Google Scholar]
- [23].Kerins CA, Carlson DS, Hinton RJ, Hutchins B, Grogan DM, Marr K, Kramer PR, Spears RD, Bellinger LL. Specificity of meal pattern analysis as an animal model of determining temporomandibular joint inflammation/pain. Int J Oral Maxillofac Surg 2005;34(4):425–431. [DOI] [PubMed] [Google Scholar]
- [24].Kerins CA, Carlson DS, McIntosh JE, Bellinger LL. Meal pattern changes associated with temporomandibular joint inflammation/pain in rats; analgesic effects. Pharmacol Biochem Behav 2003;75(1):181–189. [DOI] [PubMed] [Google Scholar]
- [25].Liu P, Okun A, Ren J, Guo RC, Ossipov MH, Xie J, King T, Porreca F. Ongoing pain in the MIA model of osteoarthritis. Neuroscience letters 2011;493(3):72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev 2014;1:CD007115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Machon V, Hirjak D, Lukas J. Therapy of the osteoarthritis of the temporomandibular joint. Journal of Cranio-Maxillofacial Surgery 2011;39(2):127–130. [DOI] [PubMed] [Google Scholar]
- [28].Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain 1995;63(3):341–351. [DOI] [PubMed] [Google Scholar]
- [29].Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain 1998;76(1–2):71–81. [DOI] [PubMed] [Google Scholar]
- [30].Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nature reviews Rheumatology 2012;8(7):390–398. [DOI] [PubMed] [Google Scholar]
- [31].Muley MM, Krustev E, Reid AR, McDougall JJ. Prophylactic inhibition of neutrophil elastase prevents the development of chronic neuropathic pain in osteoarthritic mice. Journal of neuroinflammation 2017;14(1):168–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nwosu LN, Mapp PI, Chapman V, Walsh DA. Relationship between structural pathology and pain behaviour in a model of osteoarthritis (OA). Osteoarthritis and Cartilage 2016;24(11):1910–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].O’Brien M, Philpott HT, McDougall JJ. Understanding osteoarthritis pain through animal models. Clin Exp Rheumatol 2017;35 Suppl 107(5):47–52. [PubMed] [Google Scholar]
- [34].Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, Ossipov MH, Xie J, Dussor GO, King T, Porreca F. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain 2012;153(4):924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. The Journal of Clinical Investigation 2010;120(11):3779–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ossipov MH, Lai J, Malan TP, Jr., Porreca F. Spinal and supraspinal mechanisms of neuropathic pain. Ann N Y Acad Sci 2000;909:12–24. [DOI] [PubMed] [Google Scholar]
- [37].Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 2014;8(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Paniagua B, Pascal L, Prieto J, Vimort JB, Gomes L, Yatabe M, Ruellas AC, Budin F, Pieper S, Styner M, Benavides E, Cevidanes L. Diagnostic Index: An open-source tool to classify TMJ OA condyles. Proceedings of SPIE--the International Society for Optical Engineering 2017;10137:101372H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Park HJ, Sandor K, McQueen J, Woller SA, Svensson CI, Corr M, Yaksh TL. The effect of gabapentin and ketorolac on allodynia and conditioned place preference in antibody-induced inflammation. Eur J Pain 2016;20(6):917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Park HJ, Stokes JA, Pirie E, Skahen J, Shtaerman Y, Yaksh TL. Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesth Analg 2013;116(1):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pauli C, Grogan SP, Patil S, Otsuki S, Hasegawa A, Koziol J, Lotz MK, D’Lima DD. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis and cartilage 2011;19(9):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pergolizzi JV Jr., Raffa RB, Taylor R Jr., Rodriguez G, Nalamachu S, Langley P. A review of duloxetine 60 mg once-daily dosing for the management of diabetic peripheral neuropathic pain, fibromyalgia, and chronic musculoskeletal pain due to chronic osteoarthritis pain and low back pain. Pain practice : the official journal of World Institute of Pain 2013;13(3):239–252. [DOI] [PubMed] [Google Scholar]
- [43].Pomonis JD, Boulet JM, Gottshall SL, Phillips S, Sellers R, Bunton T, Walker K. Development and pharmacological characterization of a rat model of osteoarthritis pain. Pain 2005;114(3):339–346. [DOI] [PubMed] [Google Scholar]
- [44].Popescu A, LeResche L, Truelove EL, Drangsholt MT. Gender differences in pain modulation by diffuse noxious inhibitory controls: a systematic review. Pain 2010;150(2):309–318. [DOI] [PubMed] [Google Scholar]
- [45].Sarlani E, Garrett PH, Grace EG, Greenspan JD. Temporal summation of pain characterizes women but not men with temporomandibular disorders. Journal of orofacial pain 2007;21(4):309–317. [PMC free article] [PubMed] [Google Scholar]
- [46].Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Evidence for up-regulated central nociceptive processing in patients with masticatory myofascial pain. Journal of orofacial pain 2004;18(1):41–55. [PubMed] [Google Scholar]
- [47].Sarlani E, Greenspan JD. Why look in the brain for answers to temporomandibular disorder pain? Cells, tissues, organs 2005;180(1):69–75. [DOI] [PubMed] [Google Scholar]
- [48].Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet J-P, List T, Svensson P, Gonzalez Y, Lobbezoo F, Michelotti A, Brooks SL, Ceusters W, Drangsholt M, Ettlin D, Gaul C, Goldberg LJ, Haythornthwaite JA, Hollender L, Jensen R, John MT, De Laat A, de Leeuw R, Maixner W, van der Meulen M, Murray GM, Nixdorf DR, Palla S, Petersson A, Pionchon P, Smith B, Visscher CM, Zakrzewska J, Dworkin SF. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network() and Orofacial Pain Special Interest Group(). Journal of oral & facial pain and headache 2014;28(1):6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shaefer JR, Holland N, Whelan JS, Velly AM. Pain and Temporomandibular Disorders: A Pharmaco-Gender Dilemma. Dental Clinics of North America 2013;57(2):233–262. [DOI] [PubMed] [Google Scholar]
- [50].Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, Dubner R, Diatchenko L, Meloto CB, Smith S, Maixner W. Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies. J Dent Res 2016;95(10):1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Thakur M, Dickenson AH, Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nature reviews Rheumatology 2014;10(6):374–380. [DOI] [PubMed] [Google Scholar]
- [52].Thakur M, Rahman W, Hobbs C, Dickenson AH, Bennett DLH. Characterisation of a Peripheral Neuropathic Component of the Rat Monoiodoacetate Model of Osteoarthritis. PLoS ONE 2012;7(3):e33730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tsuji T, Yamamoto T, Tanaka S, Bakhshishayan S, Kogo M. Analyses of the facilitatory effect of orexin on eating and masticatory muscle activity in rats. Journal of Neurophysiology 2011;106(6):3129–3135. [DOI] [PubMed] [Google Scholar]
- [54].Van Bellinghen X, Idoux-Gillet Y, Pugliano M, Strub M, Bornert F, Clauss F, Schwinté P, Keller L, Benkirane-Jessel N, Kuchler-Bopp S, Lutz JC, Fioretti F. Temporomandibular Joint Regenerative Medicine. International journal of molecular sciences 2018;19(2):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].van der Sluijs JA, Geesink RGT, van der Linden AJ, Bulstra SK, Kuyer R, Drukker J. The reliability of the mankin score for osteoarthritis. Journal of Orthopaedic Research 1992;10(1):58–61. [DOI] [PubMed] [Google Scholar]
- [56].Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, Liu Y, Zhang JN, Gan YH, Zhou YH. Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS One 2012;7(9):e45036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang XD, Zhang JN, Gan YH, Zhou YH. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J Dent Res 2015;94(5):666–673. [DOI] [PubMed] [Google Scholar]
- [58].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152(3 Suppl):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Woolf CJ. What to call the amplification of nociceptive signals in the central nervous system that contribute to widespread pain? Pain 2014;155(10):1911–1912. [DOI] [PubMed] [Google Scholar]
- [60].Xue X-T, Zhang T, Cui S-J, He D-Q, Wang X-D, Yang R-L, Liu D-W, Liu Y, Gan Y-H, Kou X-X, Zhou Y-H. Sexual dimorphism of estrogen-sensitized synoviocytes contributes to gender difference in temporomandibular joint osteoarthritis. Oral Diseases 2018;0(0). [DOI] [PubMed] [Google Scholar]
- [61].Yamamoto K, Shishido T, Masaoka T, Imakiire A. Morphological studies on the ageing and osteoarthritis of the articular cartilage in C57 black mice. Journal of orthopaedic surgery (Hong Kong) 2005;13(1):8–18. [DOI] [PubMed] [Google Scholar]