Abstract
Purpose of Review
Osteoporosis is disproportionately common in rheumatology patients. For the past three decades, the diagnosis of osteoporosis has benefited from well-established practice guidelines that emphasized the use of dual x-ray absorptiometry (DXA). Despite these guidelines and the wide availability of DXA, approximately two thirds of eligible patients do not undergo testing. One strategy to improve osteoporosis testing is to employ computed tomography (CT) examinations obtained as part ofroutine patient care to “opportunistically” screen for osteoporosis, without additional cost or radiation exposure to patients. This review examines the role of opportunistic CT in the evaluation of osteoporosis.
Recent Findings
Recent evidence suggests that opportunistic measurement of bone attenuation (radiodensity) using CT has sensitivity comparable to DXA. More importantly, such an approach has been shown to predict osteoporotic fractures.
Summary
The paradigm shift of using CTs obtained for other reasons to opportunistically screen for osteoporosis promises to substantially improve patient care.
Keywords: Computed tomography, Fracture, Opportunistic screening, Osteoporosis
Introduction
Osteoporosis is increasing in prevalence and resulting in a significant public health impact [1]. There is increasing evidence on reduced quality of life and shortened life span in patients with low bone mineral density (BMD), regardless of fracture status [2–4]. Owing to the use of glucocorticoids and other comorbidities, osteoporosis is especially common and has especially dire consequences in the rheumatology patient population [5, 6].
The current standard of care for the diagnosis of osteoporosis relies heavily on the diagnosis of a fragility fracture or the presence of low BMD on dual x-ray absorptiometry (DXA) testing [7, 8]. For over three decades clinical practice guidelines from the American College of Rheumatology (ACRheum),American College of Radiology (ACRad), International Society for Clinical Densitometry (ISCD), National Osteoporosis Foundation (NOF), and many other professional organizations throughout the world have emphasized the utility of DXA in the diagnosis of osteoporosis [9–16]. Unfortunately, in the USA, only about one third of eligible patients undergo DXA testing [17]. Osteoporosis remains largely underdiagnosed and undertreated, and the situation is particularly concerning in chronic glucocorticoid users [18–21].
Novel approaches to finding patients at risk for osteoporotic fracture who are candidates for pharmacologic therapy are long overdue. “Opportunistic CT” is one such approach. The essential insight or “paradigm shift” of this approach is that routine CT scans already performed for standard clinical indications can be used to screen for additional body composition information, such as the attenuation (radiodensity) of the bone. Thus, regardless of their primary indications, CT scans of the chest, abdomen, pelvis, and spine may be used to screen patients for osteoporosis. Importantly, there is no additional burden to the patient in terms of radiation exposure, examination time, or image acquisition cost. Used properly, CT promises to shift the paradigm of care for many rheumatology patients.
The timing for the paradigm shift is critical. Throughout the world, the population is aging and the burden of comorbidities is rapidly increasing [22]. In tandem with progressively aging populations, there has also been a sustained growth in the clinical use of CT scanning, with over 70 million examinations performed each year in the USA [23]. Worldwide, the growth of CT utilization has increased even more sharply due to a growing middle class and generally improved access to advanced imaging.
The ability to opportunistically mine quantitative data from CT images is becoming more feasible. The machine learning revolution, poised to impact all aspects of medical imaging, will eventually allow fully automated measurement of bone and other tissues using CT images. If these trends continue, substantial cost savings to our health care systems and improved care for our patients will follow. This review examines the role of opportunistic CT in the evaluation of osteoporosis.
CT for Osteoporosis: Past, Present, and Future
The Past
The evaluation of osteoporosis using CT has been possible since the 1980s [24]. The traditional approach, called quantitative CT (QCT), was applied to the spine and determined volumetric BMD (vBMD) with the aid of specialized software and a calibration phantom, placed under the patient during scanning [25]. The phantom calibration accounted for the variability in CT scanners by converting CT attenuation (measured in Hounsfield units [HU]) to vBMD (measured in mg/cm3) [25]. To this day, QCT-derived vBMD continues to be a powerful research tool, used to investigate everything from basic bone biology to population health [26–33]. However, its use for directing patient care has been dwarfed by DXA.
Mainly due to its lower cost, wider availability, and lower radiation dose, the vast majority of observational studies and randomized clinical trials have used DXA-derived areal BMD (aBMD), rather than QCT-derived vBMD [34–36]. This, in turn, led to wide clinical acceptance of DXA as well as the proliferation of clinical practice guidelines that amplified the value of DXA in patient management.
The Present
Recently, there has been resurgence in the use of CT for osteoporosis screening. One of the explanations has to do with favorable evolution of CT technology. In the past decade, the CTsystems have become progressively better calibrated, allowing for more consistent and accurate bone measurements. In 2015, the ISCD convened a Position Development Conference, largely devoted to QCT [37, 38]. Shortly after, the most widely used fracture risk instrument, the Fracture Risk Assessment Tool (FRAX™) was modified to allow for fracture risk calculation based on QCT-derived aBMD of the proximal femur.
The use of CT is favored by many because, as a cross-sectional technique, it allows measurements of trabecular bone. By comparison, DXA is a projectional technique that cannot differentiate between trabecular and cortical bone.
Opportunistic CT for BMD evaluation has become increasing popular because patients undergoing clinical CT examinations are often at increased risk for osteoporosis, including rheumatologic and geriatric patients.
The Future
In the future, the use of CT for the evaluation of osteoporosis will undoubtedly increase. However, much of that grow this likely to come not from CTs ordered specifically to assess osteoporosis, but from CTs obtained for other reasons where osteoporosis will be assessed concurrently on a routine basis.
Technical Considerations
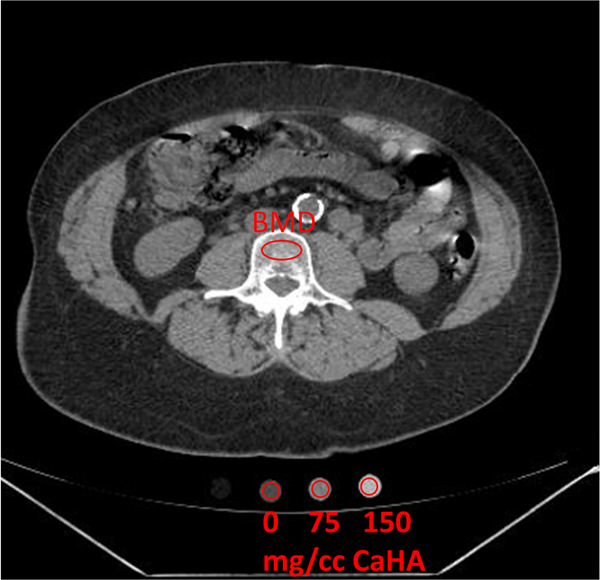
There are many scanner-related factors that influence bone measurement using CT. These include scanner manufacturer and model, scanner calibration, collimated beam width (e.g., 20 mm vs. 40 mm), and scanning protocol (e.g., kV, slice thickness, reconstruction algorithms) [38]. For these reasons, the traditional approach to QCT standardizes vBMD measurements using a calibration phantom, made with known amount of hydroxyapatite (HA) or potassium phosphate (K2HPO4), that is scanned at the same time as the patient—commonly referred to as “synchronous calibration” (Fig. 1).
Fig. 1.
Traditional quantitative CT (QCT) with calcium hydroxyapatite (CaHA) phantom for determination of vertebral bone mineral density (BMD)
While synchronous calibration is still commonly used in research, it is generally not practical for opportunistic screening, since the vast majority of clinical CTs are acquired without simultaneous imaging of a patient with a calibration phantom. For opportunistic screening using clinical CTs, an alternative approach is needed. The three main opportunistic approaches are often referred to as “phantomless” because a calibration phantom is not placed under the patient during scanning: (1) synchronous internal calibration, (2) asynchronous external calibration with BMD phantom, and (3) asynchronous external calibration with the American College of Radiology (ACRad) phantom [39•].
Synchronous Internal Calibration
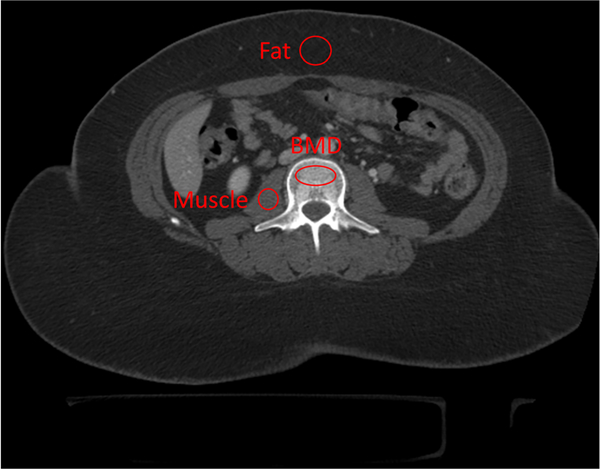
With this phantomless approach, no external calibration phantom is scanned. Instead, the CT attenuation of adjacent internal tissues (blood or fat) and air are used to calibrate attenuation measurements and calculate vBMD [40–43] (Fig. 2). VirtuOst® software (O.N. Diagnostics, Berkeley, CA) uses this approach.
Fig. 2.
Synchronous internal calibration of phantomless CT for determining vertebral BMD using two additional regions of interest: psoas muscle and subcutaneous fat
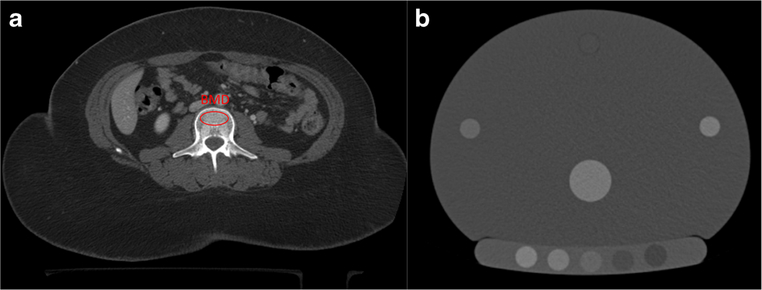
Asynchronous External Calibration with BMD Phantom
With this asynchronous approach, a calibration phantom is scanned at a different time from the patient and specialized software is then used to calibrate the phantom scan and the separate patient scan in order to calculate vBMD [44–47] (Fig. 3). CliniQCT (Mindways, Inc) is a commercially available product that uses this approach. Asynchronous CTof the proximal femur has also been adapted to derive aBMD, from which DXA-equivalent T-scores are obtained [44]. These DXA-equivalent T-scores have a much wider clinical acceptance than traditional QCT T-scores and can be used to classify patients based on the World Health Organization criteria and determine fracture risk based on FRAX™ [38].
Fig. 3.
Asynchronous external calibration of phantomless CT (a) with post-hoc BMD phantom scan (b)
Asynchronous External Calibration with ACRad Phantom
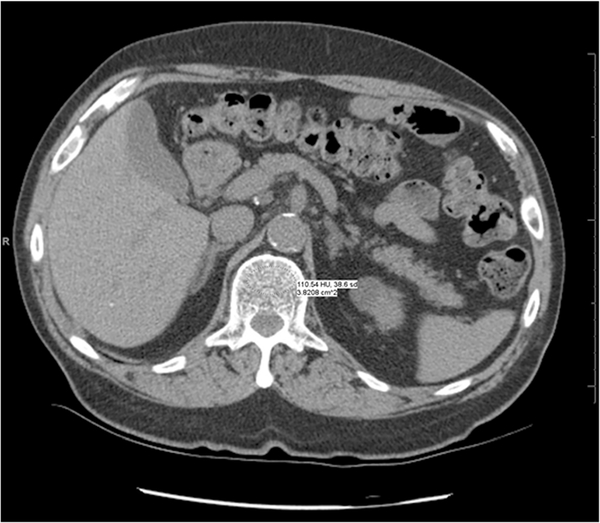
With this approach, direct CT attenuation values (HUs) are used determine trabecular radiodensity without a BMD-specific calibration phantom [48–54]. This approach does not require any specialized software and can be performed on a picture archiving and communication system (PACS) workstation or any computer with standard tools that are used for viewing CT images (Figs. 4 and 5). However, it does require careful attention to standard quality assurance procedures. On CTscanners, HUs are normalized values of the linear attenuation coefficient, where water attenuation is calibrated to 0 HU and air attenuation to − 1000 HU. Reliable results depend on standardized quality assurance measures performed for the ACRad accreditation of CT scanners. The ACRad recommends a vigorous quality control program that checks daily for artifacts, noise, and calibration to water within ± 5 HU [55].
Fig. 4.
Asynchronous external calibration with non-bone (ACRad) phantom. Trabecular attenuation at L1 is measured at 110.5 HU, which is considered within the range of normal.
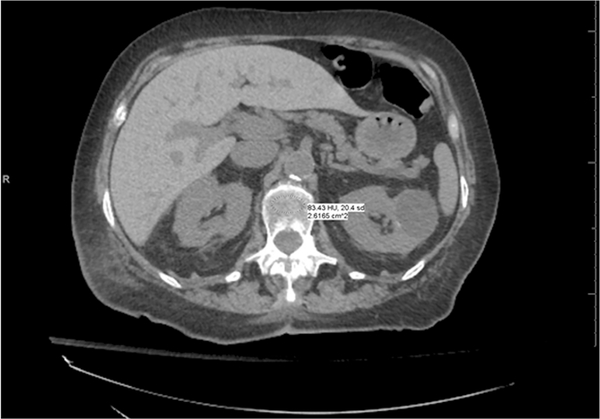
Fig. 5.
Asynchronous external calibration with non-BMD (ACRad) phantom. Trabecular attenuation at L1 is 83.4 HU, which is considered in the osteoporotic range. Further evaluation with DXA is recommended.
Table 1 summarizes the three approaches used for opportunistic CT measurements of bone. An approach that does not require purchase of an additional proprietary phantom or software seems to offer the greatest promise, but only if challenges related to reproducibility can be overcome [56, 57]. Lee et al. [42] reported precision errors for phantomless measurements that are not significantly different than those phantom-based measurements (< 1%). However, there is continuing debate whether routine calibration of CT scanners using ACRad phantoms is sufficient for such opportunistic measurement of bone [58]. One reason for this is that the ACRad phantom is only 20 cm in diameter, far smaller than an adult torso, and therefore the beam hardening and scatter properties are quite different. Furthermore, the clinical utility of opportunistic CT may be influenced by newer technology (e.g., dual-energy CT and ultra-high-resolution CT) that continues to evolve rapidly.
Table 1.
Comparison of CT methods used for evaluation of osteoporosis
| CT method | Additional requirements | Calibration accuracy | Research utility | Clinical utility | Additional cost |
|---|---|---|---|---|---|
| Traditional QCT with synchronous BMD phantom | Proprietary software and phantom | High | High | Medium | High |
| Synchronous internal calibration without BMD phantom | Proprietary software | Medium | Medium | Medium | Low |
| Asynchronous external calibration with BMD phantom | Proprietary software and phantom | Medium | Medium | Medium | Medium |
| Asynchronous external calibration with non-BMD (ACRad) phantom | None | Low | Medium | High | None |
Road to Clinical Implementation
The Past
Because the most important clinical consequence of osteoporosis is fracture, the best way to validate any diagnostic test for osteoporosis is to determine its ability to predict spine and hip fractures. With traditional QCT, this has been shown in several large prospective studies including the Age, Gene, Environment Susceptibility-Reykjavik (AGES-Reykjavik) Study and the Osteoporotic Fractures in Men (MrOS) Study [39•, 59, 60].
The Present
For the three CT approaches best suited for opportunistic screening, the initial studies have focused on comparing new methods to traditional QCT and to DXA. Opportunistic measurements of bone have shown good correlation with measurements of vBMD using traditional QCT [41, 52, 61] and aBMD using DXA [44, 48, 51, 53, 62–66].
More recent studies on opportunistic CT have used fractures as outcomes [67–74]. In patients who had a hip fracture after an abdominopelvic CT examination performed for other reasons, Lee et al. [73] reported significantly lower L1 trabecular attenuation values (98.5 ± 36.8 HU) in patients who fractured, compared to age- and sex-matched controls (129.7 ± 44.9 HU). In a later study, the same investigators applied a 90 HU cut-point at L1 in 507 adults aged ≥ 65 years who underwent chest and/or abdominal CT for any indication, and reported an increased risk for future osteoporotic fracture, even after adjusting for common confounding variables [74].
Perry Pickhardt and colleagues at the University of Wisconsin have led important work validating opportunistic CT [75•]. They have published data on various HU thresholds developed based on Receiver Operating Characteristic (ROC) analyses, using either DXA-derived T-scores or fractures as the standard. Based on their research, they have adapted the following approach to their clinical interpretation of “phantomless” CT exams. At L1 and T12, they have suggested a conservative diagnostic cut-point of < 90 or 100 HU for L1 to recommend further testing with DXA [75•]. At the hip, they use an asynchronous calibration method and report CT-derived T-scores (rather than HU) for the diagnosis of osteoporosis [75•].
How Exactly Is It Done?
Using routine PACS viewing software, the CT measurement of attenuation is performed most commonly at the L1 vertebral body by placing an oval region of interest in the anterior trabecular space on an axial or sagittal CT image of the chest or abdomen. When the L1 attenuation is < 100 HU, this is concerning for osteoporosis and significantly increased fracture risk, and DXA is recommended for these patients. Because bone attenuation is strongly influenced by the kV used for the scan, the threshold value of 100 HU assumes the CT was performed at 120 kV.
Although the thresholds used by the University for Wisconsin seem sensible, other investigators have proposed different thresholds at the same vertebral levels. In a systematic review of opportunistic use of CT for osteoporosis screening, Gausden et al. [76] summarized various skeletal sites and the associated HU-based diagnostic thresholds. Their work makes it clear that, even in research trials, the approach to opportunistic measurement of bone is far from standardized.
The Future
Future research will help direct optimal clinical implantation of opportunistic CT. The research will undoubtedly include refinements in both technical (e.g., CT methodology) and clinical (e.g., appropriate diagnostic thresholds) factors.
Concerns about high variability in bone measurements between and within CT scanners, has led to widespread caution on the use of opportunistic CT. This was the main concern raised by the ISCD Position Development Conference in 2015 [37], the systematic review of opportunistic CT by Gausden et al. in 2017 [76], and the comprehensive review of CT and fracture risk by Johannesdottir et al. in 2018 [39•].
There are several studies that mitigate such concerns about CT scanner variability. In a study of 4126 subjects measured on 14 CT scanner models from 5 CT manufacturers, Budoff et al. [77] reported high correlation between phantomless CT and traditional QCT(r=0.987,p<0.001). Similarly, in a study of 1959 subjects with incident hip fracture and 1979 controls measured on 80 different CT scanners (15 scanner models), Adams et al. [78•] reported that 86% of the CT scans provided valid measurements and that CT-derived femoral bone strength was as effective as DXA-derived aBMD in determining hip fracture risk.
It is also important to consider the concerns about standardization across CT manufacturers in the context of similar concerns about DXA. Although DXA has been widely used for osteoporosis screening for nearly four decades, different DXA manufacturers continue to use quite different methodology for aBMD measurement. In fact, ISCD guidelines encourage patients to have follow-up scans on the same DXA scanner, in order to avoid having to cross-calibrate scanners.
With DXA, it took multiple consensus conferences over many years to settle important standardization issues relating to scan acquisition, scan analysis, and quality assurance. To gain wider acceptance, a similar process will likely be needed for opportunistic CT. Hopefully, with the proactive cooperation of clinicians, radiologists, medical physicists, and industry, the process will proceed faster than it did with DXA. In any case, future practice guidelines should consider how to best standardize opportunistic CT measurements of bone.
Concerns about variability in measurements between various skeletal sites and the lack of standardized diagnostic thresholds have also limited the use of opportunistic CT. Although the technique has been applied mostly to the spine and proximal femur, it also has been used in the proximal humerus, distal radius, distal tibia, talus, and sacrum [76, 79, 80]. Site-specific diagnostic thresholds need to be independently validated before bone measurements at these nonstandard skeletal sites are accepted into clinical practice.
For now, the most robust data for opportunistic bone measurement are at T12 and L1, using a non-contrast CT. While the effect of intravenous contrast on bone attenuation has been investigated in several studies, the results have not been consistent [45, 81]. Similarly, the effect of variable kV on bone attenuation can benefit from further study [82]. In particular, it is important to determine if post-hoc adjustments in HU results should be made to correct for variations introduced by known CT variables such as use of intravenous contrast or variable kV.
Opportunistic CT research is rapidly gaining momentum. Additional clinical trials and meta-analyses are certainly forthcoming. These studies will help resolve some of the outstanding issues, including:
Should opportunistic CT be used only in older adults or are there other high-risk groups that would benefit from testing?
Should opportunistic CT be used only as a screening test, with DXA retaining its established role in the definitive diagnosis of osteoporosis?
Should skeletal sites other than the spine and the hip be used for opportunistic CT screening?
Should opportunistic CT be used to influence the choice of surgical techniques or be limited to post-operative risk stratification?
Should machine learning tools be used to maximize efficiency and consistency of measurements across large populations?
Ultimately, the most important driver in the clinical implementation of opportunistic CT should be based on evidence on improved patient outcomes.
Collateral Benefits of Using CT for Osteoporosis Screening
Current approaches to osteoporosis screening rely on areal BMD (aBMD) measured with DXA. Importantly, over 50% of fractures occur in persons classified as non-osteoporotic based on aBMD [83]. While there are many explanations for this, decreased accuracy of aBMD in obese patients is worth emphasizing [84]. By 2030, the prevalence of obesity in older Americans is expected to increase from 37 to 50%, potentially adding to DXA-based underdiagnosis [85, 86]. Importantly, CT measurements are much less susceptible to error from obesity [84].
CT measurements offer other distinct advantages compared to DXA measurements. CT measurements of bone are less influenced by spine degenerative diseases, vascular calcifications, deformities such as scoliosis and kyphosis, patient positioning errors, and various internal and external artifacts [25].
Another benefit of CT is that opportunistic measurement of bone could be combined with opportunistic measurement of muscle [87]. This would not only help diagnose sarcopenia, but also improve the prediction of fracture risk. Generally defined as decreased skeletal muscle mass and function, sarcopenia has been shown to be an independent predictor of hip fractures and has also been associated with decreased quality of life and increased mortality [88–92]. The term “osteosarcopenia” has been applied to patients who have both conditions, generally indicating a poorer prognosis than either sarcopenia or osteoporosis alone [93].
On CT images, sarcopenia may be diagnosed based on low muscle cross-sectional area (and skeletal muscle index [SMI] which adjusts muscle area for patient height) or low muscle attenuation [94•]. While different approaches have been used to assess muscle area, the most common measures all visualized muscles at the L3 vertebral level and applies the following diagnostic thresholds for sarcopenia: SMI < 52.4 cm2/m2 in men and SMI < 38.5 cm2/m2 in women [94•].
Regardless of the approach, opportunistic CT promises to provide a collateral benefit to patients by helping assess muscles as well as bones.
Finite Element Analysis
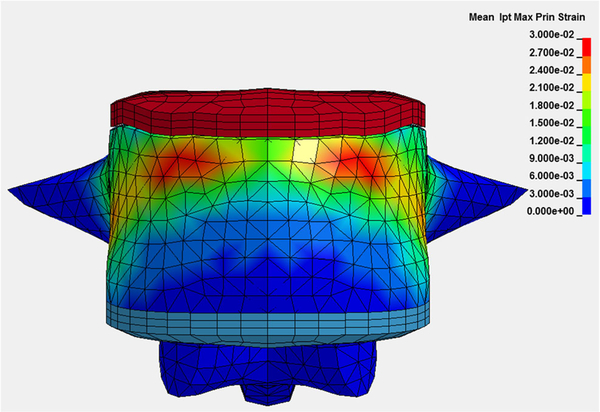
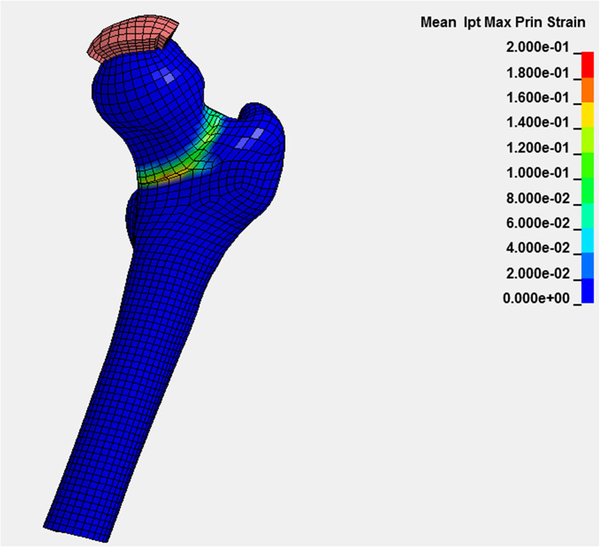
Whether measured with DXA or CT, BMD is used as a surrogate biomarker for bone strength, the key component of fracture risk. Research using CT for finite element analysis (FEA) of bone is becoming progressively more sophisticated, employing volumetric BMD, cortical thickness, computational anatomy, and other methods to assess bone strength (Figs. 6 and 7) [95–101].
Fig. 6.
Subject-specific vBMD, cortical thickness, and bone geometry derived from CT are used in a finite element analysis to predict bone strength in a vertebral compression simulation
Fig. 7.
Subject-specific vBMD, cortical thickness, and bone geometry derived from CT are used in a finite element analysis to predict bone strength in a femoral stance simulation
FEA tools used in research are being translated for clinical implementation with opportunistic CT [78•, 98•]. This is particularly important, since there are many contributors to fracture risk that are not captured by BMD. CT allows for comprehensive assessment of many other phenotypes that increase fracture risk, including decreased cortical thickness, decreased cortical density, and increased bone marrow adipose tissue (BMAT) [95–101].
An increase in BMAT is associated with aging and obesity. BMAT infiltrates medullary cavity spaces that could otherwise be occupied by bone and may compete with osteogenesis because both osteoblasts and adipocytes differentiate from a common mesenchymal stem cell. The increased heterogeneity of the trabecular matrix leads to decreased bone strength [102].
Opportunistic CT could be used to construct patient-specific FEA models. The models would use CT-derived bone geometry, cortical thickness, and material property data to predict bone strength and fracture risk in vivo, at clinically relevant sites including the spine and hip. While such FEA techniques have been widely used in research, the challenge is to adapt them to clinically acquired scans that typically use lower spatial resolution [39•].
The future of opportunistic CT will also be fundamentally changed by further automation in image analysis and tissue segmentation. Quantitative analysis of CTimages now requires manual placement or adjustment of regions of interest in bone or around adjacent tissue (e.g., muscle or fat). In the future, machine learning tools will enable automated CT segmentation of various tissues, including bone. We expect that clinical implementation of fully automated tissue segmentation with measurement of trabecular, cortical, subchondral bone, as well as FEA modeling, is forthcoming. Analogous to the current DXA reports, we also predict that future CT reports will routinely include quantitative results with evidence-based risk stratification that will contribute to improvements in patient care.
Conclusion
Just as imaging methods including ultrasound and magnetic resonance imaging have been useful in the early diagnosis of patients with inflammatory arthritis, leading to early treatment, imaging methods such as DXA and CT are increasingly used to assess for pre-clinical manifestations of another common condition in rheumatology patients: osteoporosis.
Compared to DXA, CT examinations are more expensive and result in higher radiation dose to patients. Both of these disadvantages are avoided when CTs obtained for other reasons are used opportunistically to screen for osteoporosis. Part of the increased cost of traditional QCT was the requirement for a specialized calibration phantom and analysis software. In the past decade, improvements in CT technology have facilitated “phantomless” approaches to CT measurement of bone, thus eliminating the added cost.
For decades, most of the evidence on the relationship between BMD and fracture risk came from studies that used DXA. More recently, fracture data became available for CT; not just traditional QCT, but also opportunistic CT [39•]. These data have paved the way for wider acceptance of measurement of bone on CT scans obtained for other reasons. Outside of the scope of this review, opportunistic CT has also been used to screen for vertebral fractures, providing further benefit to patients at risk for osteoporosis [71].
Importantly, concerns about the higher radiation exposure of CT compared to DXA become moot when BMD measurements are analyzed on CT exams that are already being performed for other indications. Such opportunistic CT evaluation becomes particularly important for patients who would otherwise not undergo DXA testing.
At this time, opportunistic CT is still in its infancy and incorporation into routine clinical workflow is limited. Effective integration of CT-based diagnosis of osteoporosis into patient care will require increased collaboration between clinicians and radiologists. An incidental finding of osteopenia in a recently menopausal woman may have different implications than the same diagnosis in an elderly rheumatology patient with frailty. Despite these challenges, radiologists should be encouraged to begin using CT measurements to help screen for osteoporosis.
Opportunistic CT is a rapid and reproducible method of screening patients for osteoporosis, and may even show significant bone loss before it can be detected with DXA. Furthermore, by identifying patients at risk for osteoporotic fracture who would otherwise not undergo DXA testing or be diagnosed with osteoporosis, opportunistic screening using CT has the potential to shift the existing diagnostic paradigm and ultimately improve patient care.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Curtis EM, Moon RJ, Harvey NC, Cooper C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone. 2017; 104:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenchik L, Register TC, Hsu FC, Xu J, Smith SC, Carr JJ, et al. Bone mineral density of the radius predicts all-cause mortality in patients with type 2 diabetes: diabetes heart study. J Clin Densitom. 2018;21(3):347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenchik L, Register TC, Russell GB, Xu J, Smith SC, Bowden DW, et al. Volumetric bone mineral density of the spine predicts mortality in African-American men with type 2 diabetes. Osteoporos Int. 2018;29(9):2049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckens CF, van der Graaf Y, Verkooijen HM, Mali WP, Isgum I, Mol CP, et al. Osteoporosis markers on low-dose lung cancer screening chest computed tomography scans predict all-cause mortality. Eur Radiol. 2015. January;25(1):132–9. [DOI] [PubMed] [Google Scholar]
- 5.Jin S, Hsieh E, Peng L, Yu C, Wang Y, Wu C, et al. Incidence of fractures among patients with rheumatoid arthritis: a systematic review and meta-analysis. Osteoporos Int. 2018. June;29(6):1263–75. [DOI] [PubMed] [Google Scholar]
- 6.Balasubramanian A, Wade SW, Adler RA, Saag K, Pannacciulli N, Curtis JR. Glucocorticoid exposure and fracture risk in a cohort of US patients with selected conditions. J Bone Miner Res. 2018. 10.1002/jbmr.3523. [DOI] [PubMed] [Google Scholar]
- 7.Lenchik L, Rochmis P, Sartoris DJ. Optimized interpretation and reporting of dual x-ray absorptiometry (DXA) scans. AJR Am J Roentgenol. 1998;171(6):1509–19. [DOI] [PubMed] [Google Scholar]
- 8.Wright NC, Saag KG, Dawson-Hughes B, Khosla S, Siris ES. The impact of the new National Bone Health Alliance (NBHA) diagnostic criteria on the prevalence of osteoporosis in the United States: supplementary presentation. Osteoporos Int. 2017;28(11): 3283–4. [DOI] [PubMed] [Google Scholar]
- 9.Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheum. 2017;69(8):1521–37. [DOI] [PubMed] [Google Scholar]
- 10.Expert Panel on Musculoskeletal Imaging, Ward RJ, Roberts CC, Bencardino JT, Arnold E, Baccei SJ, et al. ACR Appropriateness Criteria® osteoporosis and bone mineral density. J Am Coll Radiol. 2017;14(5S):S189–202. [DOI] [PubMed] [Google Scholar]
- 11.Lenchik L, Leib ES, Hamdy RC, Binkley NC, Miller PD, Watts NB, et al. Executive summary International Society for Clinical Densitometry position development conference Denver, Colorado July 20–22, 2001. J Clin Densitom. 2002;5:S1–3. [DOI] [PubMed] [Google Scholar]
- 12.Schousboe JT, Shepherd JA, Bilezikian JP, Baim S. Executive summary of the 2013 International Society for Clinical Densitometry position development conference on bone densitometry. J Clin Densitom. 2013;16(4):455–66. [DOI] [PubMed] [Google Scholar]
- 13.Kanis JA, Harvey NC, Cooper C, Johansson H, Odén A, Mc Closkey EV, et al. Arch Osteoporos. 2016;11(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(Suppl 4):1–42. [DOI] [PubMed] [Google Scholar]
- 15.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. National Osteoporosis Guideline Group (NOGG). UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messina C, Bignotti B, Bazzocchi A, Phan CM, Tagliafico A, Guglielmi G, et al. A critical appraisal of the quality of adult dual-energy X-ray absorptiometry guidelines in osteoporosis using the AGREE II tool: an EuroAIM initiative. Insights Imaging. 2017;8(3):311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes BL, Curtis JR, Laster A, Saag K, Tanner SB, Liu C, et al. Osteoporosis care in the United States after declines in reimbursements for DXA. J Clin Densitom. 2010;13:352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SC, Kim DH, Mogun H, Eddings W, Polinski JM, Franklin JM, et al. Impact of the U.S. Food and Drug Administration’s safety-related announcements on the use of bisphosphonates after hip fracture. J Bone Miner Res. 2016;31(8):1536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albaum JM, Youn S, Levesque LE, Gershon AS, Cadarette SM. Osteoporosis management among chronic glucocorticoid users: a systematic review. J Popul Ther Clin Pharmacol. 2014;21(3): e486–504. [PubMed] [Google Scholar]
- 20.Seaman AT, Steffen M, Doo T, Healy HS, Solimeo SL. Metasynthesis of patient attitudes toward bone densitometry. J Gen Intern Med. 2018;27:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozen G, Kamen DL, Mikuls TR, England BR, Wolfe F, Michaud K. Trends and determinants of osteoporosis treatment and screening in patients with rheumatoid arthritis compared to osteoarthritis. Arthritis Care Res. 2018;70(5):713–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson A, Dennison E. Measuring the musculoskeletal aging phenotype. Maturitas. 2016;93:13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IMV Medical Information Division. 2017 CT market outlook report. IMV website https://imvinfocom/product/2017-ct-marketoutlook-report Published November 2017. [Accessed August 24, 2018].
- 24.Genant HK, Ettinger B, Cann CE, Reiser U, Gordan GS, Kolb FO. Osteoporosis: assessment by quantitative computed tomography. Orthop Clin North Am. 1985;16(3):557–68. [PubMed] [Google Scholar]
- 25.Link TM, Lang TF. Axial QCT: clinical applications and new developments. J Clin Densitom. 2014;17(4):438–48. [DOI] [PubMed] [Google Scholar]
- 26.Lang T, Keyak J, Heitz M, Augat P, Lu Y, Mathur A, et al. Volumetric quantitative computed tomography of the proximal femur: precision and relation to bone strength. Bone. 1997;21(1):101–8. [DOI] [PubMed] [Google Scholar]
- 27.Lenchik L, Shi R, Register TC, Beck SR, Langefeld CD, Carr JJ. Measurement of trabecular bone mineral density in the thoracic spine using cardiac gated quantitative computed tomography. J Comput Assist Tomogr. 2004;28(1):134–9. [DOI] [PubMed] [Google Scholar]
- 28.Lenchik L, Register TC, Hsu F-C, Nicklas BJ, Freedman BI, Langefeld CD, et al. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone. 2003;33(4):646–51. [DOI] [PubMed] [Google Scholar]
- 29.Lenchik L, Hsu FC, Register TC, Lohman KK, Freedman BI, Langefeld CD, et al. Heritability of spinal trabecular volumetric bone mineral density measured by QCT in the Diabetes Heart Study. Calcif Tissue Int. 2004;75(4):305–12. [DOI] [PubMed] [Google Scholar]
- 30.Freedman BI, Bowden DW, Ziegler JT, Langefeld CD, Lehtinen AB, Rudock ME, et al. Bone morphogenetic protein 7 (BMP7) gene polymorphisms are associated with inverse relationships between vascular calcification and BMD: the Diabetes Heart Study. J Bone Miner Res. 2009;24(10):1719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagenknecht LE, Divers J, Register TC, Russell GB, Bowden DW, Xu J, et al. Bone mineral density and progression of subclinical atherosclerosis in African Americans with type 2 diabetes. J Clin Endocrinol Metab. 2016;101(11):4135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan GC, Divers J, Russell GB, Langefeld CD, Wagenknecht LE, Xu J, et al. Adipose tissue depot volume relationships with spinal trabecular bone mineral density in African Americans with diabetes. PLoS One. 2018;13(1):e0191674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beavers KM, Walkup MP, Weaver AA, Lenchik L, Kritchevsky SB, Nicklas BJ, et al. Effect of exercise modality during weight loss on bone health in older adults with obesity and cardiovascular disease or metabolic syndrome: a randomized controlled trial. J Bone Miner Res. 2018. 10.1002/jbmr.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lenchik L, Sartoris DJ. Current concepts in osteoporosis. AJR Am J Roentgenol. 1997;168(4):905–11. [DOI] [PubMed] [Google Scholar]
- 35.Jackson RD, Mysiw WJ. Insights into the epidemiology of postmenopausal osteoporosis: the Women’s Health Initiative. Semin Reprod Med. 2014;32(6):454–62. [DOI] [PubMed] [Google Scholar]
- 36.Crandall CJ, Newberry SJ, Diamant A, Lim YW, Gellad WF, Booth MJ, et al. Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Ann Intern Med. 2014;161(10):711–23. [DOI] [PubMed] [Google Scholar]
- 37.Shepherd JA, Schousboe JT, Broy SB, Engelke K, Leslie WD. Executive summary of the 2015 ISCD position development conference on advanced measures from DXA and QCT: fracture prediction beyond BMD. J Clin Densitom. 2015;18(3):274–86. [DOI] [PubMed] [Google Scholar]
- 38.Engelke K, Lang T, Khosla S, Qin L, Zysset P, Leslie WD, et al. Clinical use of quantitative computed tomography (QCT) of the hip in the management of osteoporosis in adults: the 2015 ISCD official positions-part I. J Clin Densitom. 2015;18(3):338–58. [DOI] [PubMed] [Google Scholar]
- 39.Johannesdottir F, Allaire B, Bouxsein ML. Fracture prediction by computed tomography and finite element analysis: current and future perspectives. Curr Osteoporos Rep. 2018;16(4):411–22• Comprehensive review of using CT-based methods to predict hip and spine fractures.
- 40.Weaver AA, Beavers KM, Hightower RC, Lynch SK, Miller AN, Stitzel JD. Lumbar bone mineral density phantomless computed tomography measurements and correlation with age and fracture incidence. Traffic Inj Prev. 2015;16(Suppl 2 sup2):S153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saffarzadeh M, Hightower RC, Talton JW, Miller AN, Stitzel JD, Weaver AA. Multicenter analysis of CIREN occupant lumbar bone mineral density and correlation with age and fracture incidence. Traffic Inj Prev. 2016;17(Suppl 1):34–41. [DOI] [PubMed] [Google Scholar]
- 42.Lee DC, Hoffmann PF, Kopperdahl DL, Keaveny TM. Phantomless calibration of CT scans for measurement of BMD and bone strength-inter-operator reanalysis precision. Bone. 2017;103:325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwaiger BJ, Kopperdahl DL, Nardo L, Facchetti L, Gersing AS, Neumann J, et al. Vertebral and femoral bone mineral density and bone strength in prostate cancer patients assessed in phantomless PET/CT examinations. Bone. 2017;101:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziemlewicz TJ, Maciejewski A, Binkley N, Brett AD, Brown JK, Pickhardt PJ. Opportunistic quantitative CT bone mineral density measurement at the proximal femur using routine contrastenhanced scans: direct comparison with DXA in 355 adults. J Bone Miner Res. 2016;31(10):1835–40. [DOI] [PubMed] [Google Scholar]
- 45.Ziemlewicz TJ, Maciejewski A, Binkley N, Brett AD, Brown JK, Pickhardt PJ. Direct comparison of unenhanced and contrastenhanced CT for opportunistic proximal femur bone mineral density measurement: implications for osteoporosis screening. AJR Am J Roentgenol. 2016;206(4):694–8. [DOI] [PubMed] [Google Scholar]
- 46.Brett AD, Brown JK. Quantitative computed tomography and opportunistic bone density screening by dual use of computed tomography scans. J Orthop Translat. 2015;3(4):178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habashy AH, Yan X, Brown JK, Xiong X, Kaste SC. Estimation of bone mineral density in children from diagnostic CT images: a comparison of methods with and without an internal calibration standard. Bone. 2011;48(5):1087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158(8):588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emohare O, Cagan A, Morgan R, Davis R, Asis M, Switzer J, et al. The use of computed tomography attenuation to evaluate osteoporosis following acute fractures of the thoracic and lumbar vertebra. Geriatr Orthop Surg Rehabil. 2014;5(2):50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziemlewicz TJ, Binkley N, Pickhardt PJ. Opportunistic osteoporosis screening: addition of quantitative CT bone mineral density evaluation to CT colonography. J Am Coll Radiol. 2015;12(10): 1036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckens CF, Dijkhuis G, de Keizer B, Verhaar HJ, de Jong PA. Opportunistic screening for osteoporosis on routine computed tomography? An external validation study. Eur Radiol. 2015;25(7): 2074–9. [DOI] [PubMed] [Google Scholar]
- 52.Gerety EL, Hopper MA, Bearcroft PW. The reliability of measuring the density of the L1 vertebral body on CT imaging as a predictor of bone mineral density. Clin Radiol. 2017;72(2): 177.e9–177.e15. [DOI] [PubMed] [Google Scholar]
- 53.Alacreu E, Moratal D, Arana E. Opportunistic screening for osteoporosis by routine CT in southern Europe. Osteoporos Int. 2017;28(3):983–90. [DOI] [PubMed] [Google Scholar]
- 54.Graffy PM, Lee SJ, Ziemlewicz TJ, Pickhardt PJ. Prevalence of vertebral compression fractures on routine CT scans according to L1 trabecular attenuation: determining relevant thresholds for opportunistic osteoporosis screening. AJR Am J Roentgenol. 2017; 22:1–6. [DOI] [PubMed] [Google Scholar]
- 55.Dillon C, Breeden W, Clements J, et al. ACR computed tomography quality control manual. American College of Radiology. 2017. [Google Scholar]
- 56.Therkildsen J, Thygesen J, Winther S, et al. Vertebral bone mineral density measured by quantitative computed tomography with and without a calibration phantom: a comparison between 2 different software solutions. J Clin Densitom. 2018; 21:367–74. [DOI] [PubMed] [Google Scholar]
- 57.Troy KL, Edwards WB. Practical considerations for obtaining high quality quantitative computed tomography data of the skeletal system. Bone. 2018; 110:58–65. [DOI] [PubMed] [Google Scholar]
- 58.Engelke K Quantitative computed tomography-current status and new developments. J Clin Densitom. 2017;20(3):309–21. [DOI] [PubMed] [Google Scholar]
- 59.Carballido-Gamio J, Harnish R, Saeed I, Streeper T, Sigurdsson S, Amin S, et al. Proximal femoral density distribution and structure in relation to age and hip fracture risk in women. J Bone Miner Res. 2013;28(3):537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chalhoub D, Orwoll ES, Cawthon PM, Ensrud KE, Boudreau R, Greenspan S, et al. Areal and volumetric bone mineral density and risk of multiple types of fracture in older men. Bone. 2016;92: 100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mueller DK, Kutscherenko A, Bartel H, Vlassenbroek A, Ourednicek P, Erckenbrecht J. Phantom-less QCT BMD system as screening tool for osteoporosis without additional radiation. Eur J Radiol. 2011;79(3):375–81. [DOI] [PubMed] [Google Scholar]
- 62.Donohue D, Decker S, Ford J, Foley R, Dunbar K, Kumm T, et al. Opportunistic CT screening for osteoporosis in patients with pelvic and acetabular trauma: technique and potential clinical impact. J Orthop Trauma. 2018;32(8):408–13. [DOI] [PubMed] [Google Scholar]
- 63.Kim YW, Kim JH, Yoon SH, Lee JH, Lee CH, Shin CS, et al. Vertebral bone attenuation on low-dose chest CT: quantitative volumetric analysis for bone fragility assessment. Osteoporos Int. 2017;28(1):329–38. [DOI] [PubMed] [Google Scholar]
- 64.Burke CJ, Didolkar MM, Barnhart HX, Vinson EN. The use of routine non density calibrated clinical computed tomography data as a potentially useful screening tool for identifying patients with osteoporosis. Clin Cases Miner Bone Metab. 2016;13(2):135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marinova M, Edon B, Wolter K, Katsimbari B, Schild HH, Strunk HM. Use of routine thoracic and abdominal computed tomography scans for assessing bone mineral density and detecting osteoporosis. Curr Med Res Opin. 2015;31(10):1871–81. [DOI] [PubMed] [Google Scholar]
- 66.Pickhardt PJ, Bodeen G, Brett A, Brown JK, Binkley N. Comparison of femoral neck BMD evaluation obtained using lunar DXA and QCT with asynchronous calibration from CT colonography. J Clin Densitom. 2015;18(1):5–12. [DOI] [PubMed] [Google Scholar]
- 67.Emohare O, Wiggin M, Hemmati P, Switzer J. Assessing bone mineral density following acute hip fractures: the role of computed tomography attenuation. Geriatr Orthop Surg Rehabil. 2015;6(1): 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pickhardt PJ, Lee LJ, del Rio AM, Lauder T, Bruce RJ, Summers RM, et al. Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J Bone Miner Res. 2011;26(9):2194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Graffy PM, Lee SJ, Ziemlewicz TJ, Pickhardt PJ. Prevalence of vertebral compression fractures on routine CT scans according to L1 trabecular attenuation: determining relevant thresholds for opportunistic osteoporosis screening. AJR Am J Roentgenol. 2017;209(3):491–6. [DOI] [PubMed] [Google Scholar]
- 70.Emohare O, Dittmer A, Morgan RA, Switzer JA, Polly DW Jr. Osteoporosis in acute fractures of the cervical spine: the role of opportunistic CT screening. J Neurosurg Spine. 2015;23(1):1–7. [DOI] [PubMed] [Google Scholar]
- 71.de Jong WU, de Jong PA, Vliegenthart R, Isgum I, Lammers JW, Oudkerk M, et al. Association of chronic obstructive pulmonary disease and smoking status with bone density and vertebral fractures in male lung cancer screening participants. J Bone Miner Res. 2014;29(10):2224–9. [DOI] [PubMed] [Google Scholar]
- 72.Fang J, Franconeri A, Boos J, Nimhuircheartaigh J, Zhang Z, Brook A, Brook OR. Opportunistic bone density measurement on abdomen and pelvis computed tomography to predict fracture risk in women aged 50 to 64 years without osteoporosis risk factors. J Comput Assist Tomogr. 2018;42(5):798–806. 10.1097/RCT.0000000000000744. [DOI] [PubMed] [Google Scholar]
- 73.Lee SJ, Anderson PA, Pickhardt PJ. Predicting future hip fractures on routine abdominal CT using opportunistic osteoporosis screening measures: a matched case-control study. AJR Am J Roentgenol. 2017;1:1–8. [DOI] [PubMed] [Google Scholar]
- 74.Lee SJ, Graffy PM, Zea RD, Ziemlewicz TJ, Pickhardt PJ. Future osteoporotic fracture risk related to lumbar vertebral trabecular attenuation measured at routine body CT. J Bone Miner Res. 2018;33(5):860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee SJ, Pickhardt PJ.Opportunistic screening for osteoporosis using body CT scans obtained for other indications: the UW experience. Clinical Reviews in Bone and Mineral Metabolism. 2017;15(3): 128–37• Provides practical approach for implementing opportunistic CTscreening.
- 76.Gausden EB, Nwachukwu BU, Schreiber JJ, Lorich DG, Lane JM. Opportunistic use of CT imaging for osteoporosis screening and bone density assessment: a qualitative systematic review. J Bone Joint Surg Am. 2017;99(18):1580–90. [DOI] [PubMed] [Google Scholar]
- 77.Budoff MJ, Malpeso JM, Zeb I, Gao YL, Li D, Choi TY, et al. Measurement of phantomless thoracic bone mineral density on coronary artery calcium CT scans acquired with various CT scanner models. Radiology. 2013;267(3):830–6. [DOI] [PubMed] [Google Scholar]
- 78.Adams AL, Fischer H, Kopperdahl DL, Lee DC, Black DM, Bouxsein ML, et al. Osteoporosis and hip fracture risk from routine computed tomography scans: the fracture, osteoporosis, and CT utilization study (FOCUS). J Bone Miner Res. 2018;33(7): 1291–301• Largest study comparing clinical CT to DXA for identifying patients at risk for hip fracture.
- 79.Hoel RJ, Ledonio CG, Takahashi T, Polly DW Jr. Sacral bone mineral density (BMD) assessment using opportunistic CT scans. J Orthop Res. 2017. January;35(1):160–6. [DOI] [PubMed] [Google Scholar]
- 80.Schreiber JJ, Gausden EB, Anderson PA, Carlson MG, Weiland AJ. Opportunistic osteoporosis screening - gleaning additional information from diagnostic wrist CT scans. J Bone Joint Surg Am. 2015;97(13):1095–100. [DOI] [PubMed] [Google Scholar]
- 81.Boutin RD, Kaptuch JM, Bateni CP, Chalfant JS, Yao L. Influence of IV contrast administration on CT measures of muscle and bone attenuation: implications for sarcopenia and osteoporosis evaluation. AJR Am J Roentgenol. 2016;207(5):1046–54. [DOI] [PubMed] [Google Scholar]
- 82.Garner HW, Paturzo MM, Gaudier G, Pickhardt PJ, Wessell DE. Variation in attenuation in L1 trabecular bone at different tube voltages: caution is warranted when screening for osteoporosis with the use of opportunistic CT. AJR Am J Roentgenol. 2017;208(1):165–70. [DOI] [PubMed] [Google Scholar]
- 83.Nguyen ND, Eisman JA, Center JR, Nguyen TV. Risk factors for fracture in nonosteoporotic men and women. J Clin Endocrinol Metab. 2007;92(3):955–62. [DOI] [PubMed] [Google Scholar]
- 84.Yu EW, Bouxsein ML, Roy AE, Baldwin C, Cange A, Neer RM, et al. Bone loss after bariatric surgery: discordant results between DXA and QCT bone density. J Bone Miner Res. 2014;29(3):542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319(16):1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2017. [Google Scholar]
- 87.Kaplan SJ, Pham TN, Arbabi S, Gross JA, Damodarasamy M, Bentov I, et al. Association of radiologic indicators of frailty with 1-year mortality in older trauma patients: opportunistic screening for sarcopenia and osteopenia. JAMA Surg. 2017;152(2): e164604. [DOI] [PubMed] [Google Scholar]
- 88.Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: current concepts and imaging implications. AJR Am J Roentgenol. 2015;205(3):W255–66. [DOI] [PubMed] [Google Scholar]
- 89.Boutin RD, Bamrungchart S, Bateni CP, Beavers DP, Beavers KM, Meehan JP, et al. CT of patients with hip fracture: muscle size and attenuation help predict mortality. AJR Am J Roentgenol. 2017;208(6):W208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murea M, Lenchik L, Register TC, Russell GB, Xu J, Smith SC, et al. Psoas and paraspinous muscle index as a predictor of mortality in African American men with type 2 diabetes mellitus. J Diabetes Complicat. 2018;32(6):558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Foster B, Boutin RD, Lenchik L, Gedeon D, Liu Y, Nittur V, Badawi RD, Li CS, Canter RJ, Chaudhari AJ. Skeletal muscle metrics on clinical (18)F-FDG PET/CT predict health outcomes in patients with sarcoma. J Nat Sci. 2018;4(5). [PMC free article] [PubMed] [Google Scholar]
- 92.Lenchik L, Lenoir KM, Tan J, Boutin RD, Callahan KE, Kritchevsky SB, Wells BJ. Opportunistic measurement of skeletal muscle size and muscle attenuation on computed tomography predicts one-year mortality in medicare patients. J Gerontol A Biol Sci Med Sci. 2018. 10.1093/gerona/gly183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paintin J, Cooper C, Dennison E. Osteosarcopenia. Br J Hosp Med (Lond). 2018;79(5):253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lenchik L, Boutin RD. Sarcopenia: beyond muscle atrophy and into the new frontiers of opportunistic imaging, precision medicine, and machine learning.Semin Musculoskelet Radiol.2018;22(3):307–22• Provides a perspective on combining opportunistic screening for osteoporosis with screening for sarcopenia.
- 95.Schoell SL, Weaver AA, Beavers DP, Lenchik L, Marsh AP, Rejeski WJ, Stitzel JD, Beavers KM. Development of subjectspecific proximal femur finite element models of older adults with obesity to evaluate the effects of weight loss on bone strength. J Osteoporos Phys Act. 2018;6(1). 10.4172/2329-9509.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schoell SL, Beavers KM, Beavers DP, Lenchik L, Rejeski WJ, Stitzel JD, Weaver AA. Prediction of lumbar vertebral body compressive strength of older obese adults using morphed subjectspecific finite element models to evaluate the effects of weight loss. Aging Clin Exp Res. 2018. 10.1007/s40520-018-1010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Knowles NK, Reeves JM, Ferreira LM. Quantitative computed tomography (QCT) derived bone mineral density (BMD) in finite element studies: a review of the literature. J Exp Orthop. 2016;3(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fidler JL, Murthy NS, Khosla S, Clarke BL, Bruining DH, Kopperdahl DL, et al. Comprehensive assessment of osteoporosis and bone fragility with CT Colonography. Radiology. 2016;278(1): 172–80• Great example of finite element modeling based on clinically acquired CTscans.
- 99.Keyak J, Sigurdsson S, Karlsdottir GS, Oskarsdottir D, Sigmarsdottir A, et al. Effect of finite element model loading condition on fracture risk assessment in men and women: the AGES-Reykjavik study. Bone. 2013;57:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schileo E, Taddei F, Malandrino A, Cristofolini L, Viceconti M. Subject-specific finite element models can accurately predict strain levels in long bones. J Biomech. 2007; 40:2982–9. [DOI] [PubMed] [Google Scholar]
- 101.Schileo E, Taddei F, Cristofolini L, Viceconti M. Subject-specific finite element models implementing a maximum principal strain criterion are able to estimate failure risk and fracture location on human femurs tested in vitro. J Biomech. 2008; 41:356–67. [DOI] [PubMed] [Google Scholar]
- 102.Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, Rosen CJ, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98(6):2294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]