Abstract
Background
Anaemia is a frequent condition during pregnancy, particularly among women in low‐ and middle‐income countries. Traditionally, gestational anaemia has been prevented with daily iron supplements throughout pregnancy, but adherence to this regimen due to side effects, interrupted supply of the supplements, and concerns about safety among women with an adequate iron intake, have limited the use of this intervention. Intermittent (i.e. two or three times a week on non‐consecutive days) supplementation has been proposed as an alternative to daily supplementation.
Objectives
To assess the benefits and harms of intermittent supplementation with iron alone or in combination with folic acid or other vitamins and minerals to pregnant women on neonatal and pregnancy outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 July 2015), the WHO International Clinical Trials Registry Platform (ICTRP) (31 July 2015) and contacted relevant organisations for the identification of ongoing and unpublished studies (31 July 2015).
Selection criteria
Randomised or quasi‐randomised trials.
Data collection and analysis
We assessed the methodological quality of trials using standard Cochrane criteria. Two review authors independently assessed trial eligibility, extracted data and conducted checks for accuracy.
Main results
This review includes 27 trials from 15 countries, but only 21 trials (with 5490 women) contributed data to the review. All studies compared daily versus intermittent iron supplementation. The methodological quality of included studies was mixed and most had high levels of attrition.The overall assessment of the quality of the evidence for primary infant outcomes was low and for maternal outcomes very low.
Of the 21 trials contributing data, three studies provided intermittent iron alone, 14 intermittent iron + folic acid and four intermittent iron plus multiple vitamins and minerals in comparison with the same composition of supplements provided in a daily regimen.
Overall, for women receiving any intermittent iron regimen (with or without other vitamins and minerals) compared with a daily regimen there was no clear evidence of differences between groups for any infant primary outcomes: low birthweight (average risk ratio (RR) 0.82; 95% confidence interval (CI) 0.55 to 1.22; participants = 1898; studies = eight; low quality evidence), infant birthweight (mean difference (MD) 5.13 g; 95% CI ‐29.46 to 39.72; participants = 1939; studies = nine; low quality evidence), premature birth (average RR 1.03; 95% CI 0.76 to 1.39; participants = 1177; studies = five; low quality evidence), or neonatal death (average RR 0.49; 95% CI 0.04 to 5.42; participants = 795; studies = one; very low quality). None of the studies reported congenital anomalies.
For maternal outcomes, there was no clear evidence of differences between groups for anaemia at term (average RR 1.22; 95% CI 0.84 to 1.80; participants = 676; studies = four; I² = 10%; very low quality). Women receiving intermittent supplementation had fewer side effects (average RR 0.56; 95% CI 0.37 to 0.84; participants = 1777; studies = 11; I² = 87%; very low quality) and were at lower risk of having high haemoglobin (Hb) concentrations (greater than 130 g/L) during the second or third trimester of pregnancy (average RR 0.53; 95% CI 0.38 to 0.74; participants = 2616; studies = 15; I² = 52%; (this was not a primary outcome)) compared with women receiving daily supplements. There were no significant differences in iron‐deficiency anaemia at term between women receiving intermittent or daily iron + folic acid supplementation (average RR 0.71; 95% CI 0.08 to 6.63; participants = 156; studies = one). There were no maternal deaths (six studies) or women with severe anaemia in pregnancy (six studies). None of the studies reported on iron deficiency at term or infections during pregnancy.
Most of the studies included in the review (14/21 contributing data) compared intermittent oral iron + folic acid supplementation compared with daily oral iron + folic acid supplementation (4653 women) and findings for this comparison broadly reflect findings for the main comparison (any intermittent versus any daily regimen).
Three studies with 464 women examined supplementation with intermittent oral iron alone compared with daily oral iron alone. There were no clear differences between groups for mean birthweight, preterm birth, maternal anaemia or maternal side effects. Other primary outcomes were not reported.
Four studies with a combined sample size of 412 women compared intermittent oral iron + vitamins and minerals supplementation with daily oral iron + vitamins and minerals supplementation. Results were not reported for any of the review's infant primary outcomes. One study reported fewer maternal side effects in the intermittent iron group, and two studies that more women were anaemic at term compared with those receiving daily supplementation.
Where sufficient data were available for primary outcomes, we set up subgroups to look for possible differences between studies in terms of earlier or later supplementation; women's anaemia status at the start of supplementation; higher and lower weekly doses of iron; and the malarial status of the region in which the trials were conducted. There was no clear effect of these variables on results.
Authors' conclusions
This review is the most comprehensive summary of the evidence assessing the benefits and harms of intermittent iron supplementation in pregnant women on haematological and pregnancy outcomes. Findings suggest that intermittent regimens produced similar maternal and infant outcomes as daily supplementation but were associated with fewer side effects and reduced the risk of high levels of Hb in mid and late pregnancy, although the risk of mild anaemia near term was increased. While the quality of the evidence was assessed as low or very low, intermittent may be a feasible alternative to daily iron supplementation among those pregnant women who are not anaemic and have adequate antenatal care.
Plain language summary
Intermittent regimens of iron supplementation during pregnancy
Anaemia is a frequent condition during pregnancy, particularly among women from low‐ and middle‐income countries who have insufficient iron intake to meet increased iron needs. Traditionally, pregnancy anaemia has been prevented with the provision of daily iron supplements, however, it has recently been proposed that if women take supplements less often, such as once or twice weekly rather than daily, this might reduce side effects and increase acceptance and adherence to supplementation. In this review we assess the benefits and harms of intermittent (i.e. two or three times a week on non‐consecutive days) oral supplementation with iron or iron + folic acid or iron + vitamins and minerals for pregnant women.
We included 27 randomised controlled trials, but only 21 trials involving 5490 women, had information on the outcomes we evaluated. Three studies looked at intermittent iron alone versus daily iron alone, the other studies included in the review compared intermittent iron combined with folic acid or other vitamins and minerals compared with the same supplements provided daily. Looking at all the studies together (any intermittent regimen including iron versus a daily regimen), there was no clear evidence of differences between groups for most of the outcomes we examined including infant birthweight, premature birth, and perinatal death, or anaemia, haemoglobin concentration and iron deficiency in women at the end of pregnancy. However, women receiving intermittent rather than daily iron supplements were less likely to report side effects (such as constipation and nausea). In addition, intermittent supplementation appeared to decrease the number of women with high haemoglobin concentrations during mid and late pregnancy compared with daily regimens. High haemoglobin concentrations may be harmful as they may be associated with an increased risk of having a premature birth and low birthweight baby. There were no other clear differences between groups for other outcomes examined.
Summary of findings
Background
This review is an update of the published Cochrane review on intermittent oral iron supplementation which suggested that women receiving intermittent iron supplements have similar pregnancy and birth outcomes as those women receiving supplements daily but intermittent supplements are associated with fewer side effects (Peña‐Rosas 2012).
Description of the condition
It is estimated that 38.2% of pregnant women are anaemic worldwide (WHO 2015c), which translates to 32.4 million pregnant women. Half of the global anaemia prevalence is assumed to be due to iron deficiency in non‐malaria areas (Stevens 2013). Other conditions, such as folate, vitamin B12 and vitamin A deficiencies, chronic inflammation, parasitic infections, and inherited disorders can all cause anaemia (WHO 2001). Globally, the prevalence of anaemia has fallen by 12% between 1995 and 2011 (from 33% to 29% in non‐pregnant women and from 43% to 38% in pregnant women), indicating that progress is possible (WHO 2014a).
Anaemia is a condition in which the number of red blood cells or their oxygen‐carrying capacity is insufficient to meet physiological needs, which vary by age, sex, altitude, smoking, and pregnancy status (WHO 2011a). It is associated with impaired cognitive and motor development leading to loss of productivity from impaired work capacity, and also increased susceptibility to infection (Balarajan 2011).
Among all the populations, women are the most susceptible to iron deficiency because of their physiological vulnerability (Masukume 2015). Women of reproductive age are at higher risk of iron‐deficiency anaemia because of physiological processes such as frequent blood loss (due to menstruation), and their increased iron requirements due to pregnancy and breastfeeding. Along with these physiological changes, the presence of parasitic infections and an inadequate iron intake, typical of populations consuming diets that are limited in meat sources, and high in iron absorption inhibitors such as cereals (e.g. wheat, rice or maize), will result in an impairment of the production of red blood cells, resulting in iron‐deficiency anaemia (Suominen 1998). Anaemia during pregnancy is diagnosed if a woman's haemoglobin (Hb) concentration at sea level is lower than 110 g/L, although it is recognised that during the second trimester of pregnancy, Hb concentrations diminish by approximately 5 g/L (WHO 2011a). When anaemia is accompanied by an indicator of iron deficiency (e.g.low concentrations of serum transferrin receptor, or low ferritin concentrations), it is referred to as iron‐deficiency anaemia (Walsh 2011; WHO 2011a; WHO 2011b). However, the use of a ferritin cut‐off in various populations, particularly those living in settings where inflammation is common is being updated (Garcia‐Casal 2014). Ferritin concentration is usually measured, but it is not a sensible indicator during pregnancy (WHO 2011b). Iron deficiency alone is almost 2.5 times higher than iron‐deficiency anaemia and iron deficiency alone can lead to impairment of tissue development (Goonewardene 2012).
Pregnant women have augmented iron requirements because of rapid tissue growth, the expansion of red cell mass and increasing fetal needs. During the second half of the pregnancy there is a notable increase of iron requirements due to the expansion of the red blood cell mass and the transfer of increasing amounts of iron to both the growing fetus and the placental structure. The amount of iron needed during pregnancy depends on the iron stores before pregnancy, i.e. women may develop iron‐deficiency anaemia due to the fact that iron stores were not sufficient to meet increased needs (Bothwell 2000; Viteri 2005).
It is estimated that most pregnant women would need additional iron in their diets as well as sufficient iron stores (500 mg of iron or more) to prevent iron deficiency (Bothwell 2000; IOM 2001). Low Hb levels during pregnancy, indicative of moderate or severe anaemia, are associated with increased risk of low birthweight, maternal and child mortality, and infectious diseases (INACG 2002). Children born to anaemic mothers are more likely to be anaemic early in life and it has been reported that iron deficiency may irreversibly affect the cognitive performance and development and physical growth of infants (WHO 2001) even in the long term (Gleason 2007; Lozoff 2006; Lozoff 2007; Burke 2014). During pregnancy, the growing fetus is in a vulnerable state and is entirely dependent on the mother and the maternal environment for its nutritional requirements, and it has been suggested that the consequences of inappropriate nutrition in utero can extend into adulthood, a phenomenon known as fetal programming (Andersen 2006). Studies with rats have suggested that iron deficiency during the fetal period resulted in smaller offspring, with smaller kidneys, both in absolute and proportional terms, and an enlarged heart, all which may be associated with hypertension later in life (Andersen 2006; McArdle 2006). The plausibility of this theory, however, needs to be confirmed by epidemiological studies.
There appears to be a U‐shape optimal range for Hb levels during pregnancy, as high Hb concentrations (greater than 130 g/L at sea level) also increase the risk of non‐desirable pregnancy outcomes, including low birthweight and premature birth (Casanueva 2003b; Hytten 1964; Hytten 1971; Murphy 1986; Scholl 1997; Steer 2000). Although the mechanisms for this are far from being elucidated, a low plasma volume appears to precede late pregnancy hypertension, which in turn is associated with low birthweight small‐for‐gestational‐age babies (Gallery 1979; Goodlin 1981; Huisman 1986; Koller 1979; Silver 1998). However, these findings are still inconsistent (Gallery 1979; Hytten 1971; Hytten 1985; Koller 1979; Letsky 1991; Poulsen 1990), and it has been hypothesised that high Hb concentrations increase blood viscosity, with or without a change in the plasma volume, and reduce placental perfusion, leading possibly to placental/fetal hypoxia (Erslev 2001; LeVeen 1980).
Description of the intervention
Intermittent oral iron supplementation (i.e. one, two or three times a week on non‐consecutive days) is recommended as an alternative to daily iron supplementation during pregnancy in non‐anaemic pregnant women (WHO 2012). The rationale for intermittent iron administration is based on two lines of evidence: the first one is related to the concept that exposing intestinal cells to supplemental iron less frequently, (e.g. every week in synchrony with the human mucosal turnover that occurs every five to six days) may improve the efficiency of absorption since the mucosal cells are not "blocked" by large amounts of iron as may occur with daily iron intake (Anderson 2005; Frazer 2003a; Frazer 2003b). The second line is related to the fact that daily iron supplementation, by maintaining an iron‐rich environment in the gut lumen and in the intestinal mucosal cells, produces oxidative stress and is prone to increasing the severity and frequency of undesirable side effects (Srigiridhar 1998; Srigiridhar 2001; Viteri 1997; Viteri 1999a).The side effects are probably caused by the challenges of having to cope with a large non‐physiologic bolus dose of iron, which may also contribute to adverse interactions with infectious diseases including malaria. Ideally, less side effects would lead to a higher adherence to supplementation (Viteri 1995; Viteri 1999b), however, some authors have questioned this belief, indicating that the main reason for the poor compliance with programmes is the unavailability of iron supplements for the targeted women (Galloway 1994). The intervention could be an effective strategy in addressing the problem of forgetfulness and to improve supplementation compliance (Goonewardene 2012).
An important consideration when providing supplemental iron is the presence of malaria. Approximately 40% of the world population is exposed to the parasite and it is endemic in over 100 countries (WHO 2011d) and more than 85 million pregnancies occur in areas with some degree of Plasmodium falciparum transmission (Dellicor 2010). It is estimated that about 15 million pregnant women remain without access to preventive treatment for malaria (WHO 2014d). Of all the complications associated with this disease, anaemia is the most common and causes the highest number of malaria‐related deaths. This parasite causes anaemia by the haemolysis of red blood cells and the compounding suppression of erythropoiesis (Darnton‐Hill 2007). Malaria in pregnant woman and placental malaria increases the risk of maternal death, miscarriage, stillbirth and low birthweight with associated risk of neonatal death (WHO 2011d; WHO 2011e). Intermittent preventive treatment in pregnancy (IPTp) (i.e. administration of sulfadoxine‐pyrimethamine during the second and third trimester of pregnancy) can reduce severe maternal anaemia (WHO 2014d) and is recommended in malaria‐endemic areas at each scheduled antenatal care (ANC) visit for protection against malaria. There is evidence that high doses of folic acid (i.e. 5000 μg or more) may interfere with the efficacy of sulfadoxine‐pyrimethamine as an antimalarial (Roll Back Malaria Partnership 2015).
Provision of iron in malaria‐endemic areas has been a long‐standing controversy due to concerns that iron therapy may exacerbate infections, in particular malaria (Oppenheimer 2001). Although the mechanisms by which additional iron can benefit the parasite are far from clear (Prentice 2007), the use of daily iron supplementation has been limited in programme settings, possibly due to a lack of compliance, concerns about the safety of the intervention among women with an adequate iron intake, and variable availability of the supplements at community level.
How the intervention might work
Screening for iron stores and anaemia in pregnant women usually start in early or mid pregnancy (approximately < 20 weeks of gestational age) (Bencaiova 2012), and provision of daily oral iron with folic acid supplements for pregnant women has been used extensively in prenatal care programmes in low‐ and middle‐income countries as an intervention to prevent and correct iron deficiency and anaemia during pregnancy (Beard 2000; Haider 2013; Villar 1997). Although iron supplementation with or without folic acid has been used in a variety of doses and regimens, current recommendations for pregnant women include the provision of a standard daily dose of 30 to 60 mg of elemental iron and 400 μg (0.4 mg) of folic acid starting as early as possible (WHO 2012a). If at six months of treatment, the ideal Hb concentrations cannot be achieved during pregnancy, either continued supplementation during the postpartum period or increased dosage to 120 mg iron daily during pregnancy should be given (WHO 2012a). Optimal concentrations of red cell folate concentrations have been also proposed with an minimum serum concentration 400 mg/mal or 906 nmol/L) in order to reduce the risk of neural tube defects on children (WHO 2015a).
Currently, the World Health Organization has made recommendations for intermittent iron supplementation intake in non pregnant women of reproductive age and pregnant women. For women in reproductive age, weekly supplementation with 60 mg of elemental iron plus 2800 μg (2.8 mg) of folic acid (WHO 2011c) in populations where the prevalence of anaemia is above 20%. In addition to increasing iron stores, this intervention represents an opportunity to improve folate status before pregnancy and in the very early stages of pregnancy, particularly for those women who may become pregnant or do not know they are already pregnant and are not covered by other programmes as many pregnancies are not planned (WHO 2011c). For non‐anaemic pregnant women, WHO recommends a weekly supplementation of 120 mg of elemental iron plus 2800 μg (2.8 mg) of folic acid when anaemia is lower than 20% in order to prevent anaemia and improve gestational outcomes (WHO 2012).
Why it is important to do this review
Anaemia in pregnancy is a major public health and economic problem (Pasricha 2013). Daily oral supplementation in pregnant women has been a long‐standing, cost‐effective recommended intervention both in the public health and clinical fields (WHO 2012a). However, adherence to daily iron and folic acid supplementation still faces challenges. Data from national surveys from 46 countries (2003 to 2009) indicate that about 52% to 75% of mothers receive any iron tablets during pregnancy, and the duration of supplementation is usually short (Lutter 2011).This may be due to poor distribution of pills, distressing side effects experienced by women or safety concerns related to the routine use of iron supplements in areas where anaemia is not of public health problem or by women who are not anaemic.
The findings of this review will update the previous version of intermittent oral iron supplementation during pregnancy (Peña‐Rosas 2012), which suggested that women receiving intermittent iron supplements have similar pregnancy and birth outcomes as those women receiving supplements daily. This review also suggested that women receiving daily supplements had increased risk of developing high levels of Hb in mid and late pregnancy but were less likely to present mild anaemia near term.
The present review will complement those from other Cochrane reviews assessing the effects and safety of daily iron and iron plus folic acid supplementation during pregnancy (Peña‐Rosas 2015). Other reviews assessing the effects of supplementing pregnant women with different vitamins and minerals include: the effectiveness of different iron therapies for pregnant women with a diagnosis of anaemia attributed to iron deficiency (Reveiz 2011), the effects of supplementation with vitamin A during pregnancy (van den Broek 2010), zinc supplementation in pregnancy (Ota 2015), vitamin C supplementation in pregnancy (Rumbold 2015). The effectiveness of oral folate supplementation alone during pregnancy on haematological and biochemical parameters and on pregnancy outcomes (Lassi 2013), the effects and safety of periconceptional folate supplementation for preventing congenital anomalies (De‐Regil 2010), the effects of multiple vitamin and mineral supplements during pregnancy (Haider 2012), the effects of point‐of‐use fortification with micronutrient powders for women in pregnancy (Suchdev 2011; Suchdev 2015), are evaluated in related reviews.
Objectives
To assess the benefits and harms of intermittent oral supplementation with iron alone or in combination with folic acid or other vitamins and minerals to pregnant women on neonatal and pregnancy outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and quasi‐randomised trials with randomisation either at individual or cluster level. We did not include cross‐over trials or any observational study designs (for example, cohort or case‐control studies) in the meta‐analysis, but we have considered such evidence in the discussion where relevant.
Types of participants
Pregnant women of any gestational age and parity with confirmed pregnancy at the moment of randomisation. Studies specifically targeting women with diagnosed health problems, for example HIV or tuberculosis were excluded.
Types of interventions
Oral supplements of iron, or iron + folic acid, or iron + vitamins and minerals, given as a public health strategy on an intermittent basis and compared with a placebo or no supplementation, or compared with the same supplements provided daily. We excluded studies dealing specifically with iron therapies for anaemic women as a part of clinical practice.
Oral iron supplementation refers to the delivery of iron compounds directly to the oral cavity, either as a tablet (dispersible or not), capsule, or liquid. For the purpose of this review, intermittent supplementation is defined as the provision of iron supplements one, two or three times a week on non‐consecutive days.
We performed the following comparisons.
Any intermittent iron regimen (with or without other vitamins and minerals) compared with no supplementation or placebo.
Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals).
Intermittent oral iron alone supplementation compared with no supplementation or placebo.
Intermittent oral iron + folic acid supplementation compared with no supplementation or placebo.
Intermittent oral iron + vitamins and minerals supplementation compared with no supplementation or placebo.
Intermittent oral iron alone supplementation compared with daily oral iron supplementation.
Intermittent oral iron + folic acid supplementation compared with daily oral iron + folic acid supplementation.
Intermittent oral iron + vitamins and minerals supplementation compared with daily oral iron + vitamins and minerals supplementation.
Interventions that combined iron supplementation with co‐interventions such as education or other approaches were included only if the other co‐interventions were the same in both the intervention and comparison groups. We excluded studies examining tube feeding, parenteral nutrition or supplementary food‐based interventions such as mass fortification of staple or complementary foods, point‐of‐use fortification with micronutrient powders, lipid‐based supplements or Foodlets tablets, or biofortification.
Types of outcome measures
Maternal, perinatal and postpartum clinical and laboratory outcomes and infant clinical and laboratory outcomes as described below.
Primary outcomes
Infant
Low birthweight (less than 2500 g).*
Birthweight (g).*
Premature birth (less than 37 weeks' gestation).*
Neonatal death (within 28 days after delivery).*
Congenital anomalies, including neural tube defects (as defined by trialists).*
Maternal
Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more).*
Maternal iron deficiency at term (as defined by trialists, based on any indicator of iron status at 37 weeks' gestation or more).*
Maternal iron‐deficiency anaemia at term ((Hb less than 110 g/L and at least one additional laboratory indicator at 37 weeks' gestation or more).*
Maternal death (death while pregnant or within 42 days of termination of pregnancy).*
Side effects (any reported throughout intervention period).*
Severe anaemia at any time during second or third trimesters (Hb less than 70 g/L).*
Clinical malaria (as defined by trialists).*
Infection during pregnancy (including urinary tract infections and others as specified by trialists).*
* Outcomes that are included in the 'Summary of findings' tables.
Secondary outcomes
Infant
Very low birthweight (less than 1500 g).
Very premature birth (less than 34 weeks' gestation).
Hb concentration within the first six months (in g/L, counting the last reported measure after birth within this period).
Ferritin concentration within the first six months (in μg/L, counting the last reported measure after birth within this period).
Development and motor skills (as defined by trialists).
Admission to special care unit.
Maternal
Maternal anaemia at or near term (Hb less than 110 g/L at 34 weeks' gestation or more).
Maternal iron deficiency at or near term (as defined by trialists, based on any indicator of iron status at 34 weeks' gestation or more).
Maternal iron‐deficiency anaemia at or near term ((Hb less than 110 g/L and at least one additional laboratory indicator at 34 weeks' gestation or more).
Maternal Hb concentration at or near term (in g/L, at 34 weeks' gestation or more).
Maternal Hb concentration within one month postpartum in g/L.
Maternal high Hb concentrations at any time during second or third trimester (defined as Hb greater than 130 g/L).
Moderate anaemia at postpartum (Hb between 80 and 109 g/L).
Severe anaemia at term (Hb less than 70 g/L at 37 weeks' gestation or more).
Severe anaemia at or near term (Hb less than 70 g/L at 34 weeks' gestation or more).
Severe anaemia postpartum (Hb less than 80 g/L).
Puerperal infection (as defined by trialists).
Antepartum haemorrhage (as defined by trialists).
Postpartum haemorrhage (intrapartum and postnatal, as defined by trialists).
Transfusion given (as defined by trialists).
Diarrhoea (as defined by trialists).
Constipation (as defined by trialists).
Nausea (as defined by trialists).
Heartburn (as defined by trialists).
Vomiting (as defined by trialists).
Maternal well being/satisfaction (as defined by trialists).
Placental abruption (as defined by trialists).
Premature rupture of membranes (as defined by trialists).
Pre‐eclampsia (as defined by trialists).
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 July 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We also searched the WHO International Clinical Trials Registry Platform (ICTRP) for any ongoing or planned trials on (31 July 2015) using the search terms described in Appendix 1.
Searching other resources
For assistance in identifying ongoing or unpublished studies, we also contacted the Departments of Reproductive Health and Research and Nutrition for Health and Development from the World Health Organization (WHO) and the nutrition sections of the United Nations Children's Fund (UNICEF), the World Food Programme (WFP), the Division of Nutrition, Physical Activity and Obesity at the U.S. Centers for Disease Control and Prevention (CDC), the Micronutrient Initiative (MI), the Global Alliance for Improved Nutrition (GAIN), Hellen Keller International (HKI), and Sight and Life (31 July 2015).
We did not apply any date or language restrictions.
Data collection and analysis
For methods used in the previous version of this review, seePeña‐Rosas 2012.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
In this update, two review authors (Heber Gomez Malave (HGM) and Monica Flores Urrutia (MFU)) independently assessed and selected the trials for inclusion in the review. Any disagreement on trial eligibility was resolved by discussion or Juan Pablo Peña‐Rosas (JPPR) served as arbiter.
It was not possible for us to assess the relevance of the trials in a blinded manner because we knew the authors' names, institution, journal of publication and results when we applied the inclusion criteria.
Data extraction and management
We designed a form to facilitate the process of data extraction and to request additional (unpublished) information from the authors of the original reports. We resolved any disagreements by discussion, and, if necessary, sought clarification from the authors of the original reports.
We entered data into Review Manager software (RevMan 2014) and checked them for accuracy.
Assessment of risk of bias in included studies
Review authors LMD, TD or JPPR independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Each trial was assessed by two review authors. We resolved any disagreement by discussion.
For this update, 2015, we have assessed blinding of participants and personnel (performance bias) and blinding of outcome assessors (detection bias) separately, as specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
(1) Sequence generation (checking for possible selection bias)
We have described for each included study the method used to generate the allocation sequence. We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear.
(2) Allocation concealment (checking for possible selection bias)
We have described for each included study the method used to conceal the allocation sequence and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes);
unclear.
(3.1) Blinding of participants and personnel (checking for possible performance)
We have described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. For this type of intervention, where different regimens were compared, it would be theoretically possible to blind study participants and staff by providing both active and placebo tablets to women allocated to intermittent regimens and placebo tablets to women in no supplementation arms of trials.
Blinding was assessed separately for different outcomes or classes of outcomes and we have noted where there was partial blinding.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We have described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed losses to follow‐up and post‐randomisation exclusions systematically for each trial.
We have described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We have noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as:
low, high or unclear risk of bias.
We considered follow‐up to be adequate (low risk of bias) if at least 80% of participants initially randomised in a trial were included in the analysis and any loss was balanced across groups, unclear if the percentage of initially randomised participants included in the analysis was unclear or not stated, and high risk of bias if less than 80% of those initially randomised were included in the analysis.
(5) Selective reporting bias
We have described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; or the study failed to include results of a key outcome that we would have been expected to have been reported);
unclear.
(6) Other sources of bias
We have noted for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low, high or unclear risk for other possible sources of bias.
(7) Overall risk of bias
We summarised the risk of bias at two levels: within studies (across domains) and across studies.
For the first, we made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. Attrition, lack of blinding and losses to follow‐up may be particular problems in studies looking at different regimens of iron supplementation and where women are followed up over time. We explored the impact of the level of bias by undertaking sensitivity analyses, seeSensitivity analysis below.
Measures of treatment effect
For dichotomous data, we present results as average risk ratio (RR) with 95% confidence intervals (95%CI).
For continuous outcomes we present the results as mean difference (MD) with 95% CIs. There was no need to use the standardised mean difference to combine trials as these outcomes were measured with the same methods.
Unit of analysis issues
Cluster‐randomised trials
We included cluster‐randomised trials in the analyses along with individually‐randomised trials. Cluster‐randomised trials are labelled with a (C). We estimated the intracluster correlation co‐efficient (ICC) from trials' original data sets and reported the design effect (Higgins 2011). We estimated the ICCs for Hb from Ridwan 1996 (C) (ICC 0.05; average cluster size 23.1; design effect 2.1) and Winichagoon 2003 (C) (ICC 0.03; average cluster size 31.6; design effect 2.09). In the trial by Ekstrom 2002 (C), trial authors reported that they had adjusted the results by initial Hb measurements as well as by clustering effect within participants and thus we did not carry out any additional adjustment. For the Hanieh 2013 (C) trial, ICCs for all outcomes reported were provided by the authors, and we used these to calculate the cluster design effect for each outcome.
We considered that it was reasonable to combine the results from both cluster‐randomised trials and individually‐randomised trials as there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
Cross‐over trials
The study by (Viteri 2012) was included but only for the period of 20 to 28 weeks. Women in this study were changed to the other group and thus not useful for our study purposes. The characteristics of this study are described in the Characteristics of included studies.
Dealing with missing data
For included studies, levels of attrition have been noted in the Characteristics of included studies tables. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by carrying out sensitivity analysis (these same trials were assessed as being at high risk of bias, seeSensitivity analysis below).
Where possible, we conducted an available case analysis and reinstated previously excluded cases, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial being the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We examined the forest plots from the analyses visually to assess any obvious heterogeneity in terms of the size or direction of treatment effect between studies. We used the I², and Tau² statistics and the P value of the Chi² test for heterogeneity to quantify heterogeneity among the trials in each analysis. The I² statistic quantifies inconsistency and describes the percentage of the variability in effect estimate that is due to heterogeneity rather than sampling error (chance). We considered that heterogeneity was substantial or high if the I² exceeded 50%.
Assessment of reporting biases
We generated funnel plots in RevMan 2014 for those few outcomes with 10 trials or more. We did not find a clear indication of asymmetry.
Where we suspected reporting bias (seeSelective reporting (reporting bias) above), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014).
Because of our experience in conducting other reviews in this area, we anticipated high heterogeneity among trials, and we pooled trial results using a random‐effects model and were cautious in our interpretation of the pooled results. In the text, for statistically significant results, we have given the values of I², Tau² and the P value of the Chi² test for heterogeneity, and have indicated that the random‐effects model gives the average treatment effect. For analyses where there are high levels of heterogeneity, we have provided an estimate of the 95% range of underlying intervention effects (prediction interval).
Assessment of the quality of the evidence using GRADE
For the assessment across studies, we used the GRADE approach to interpret findings as outlined in the GRADE handbook, and the GRADEpro Guideline Development Tool allowed us to import data from Review Manager 5.3 (RevMan 2014) to create 'Summary of findings' tables set out in Table 1 and Table 2 (SoF). The primary outcomes for the main comparison have been listed with estimates of relative effects along with the number of participants and studies contributing data for those outcomes. These tables provide outcome‐specific information concerning the overall quality of evidence from studies included in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes we considered. For each individual outcome, the quality of the evidence was assessed independently by two review authors using the GRADE approach (Balshem 2010).
Summary of findings for the main comparison. Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals)‐infant outcomes.
| Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals)‐ infant outcomes | ||||||
|
Patient or population: Women receiving supplements during pregnancy
Settings: Community settings
Intervention: Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals) Comparison: Daily regimen (with same vitamins and minerals) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Daily regimen (with same vitamins and minerals) | Any intermittent iron regimen (with or without other vitamins and minerals) | |||||
| Low birthweight (less than 2500 g) | Study population | RR 0.82 (0.55 to 1.22) | 1898 (8 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | ||
| 58 per 1000 | 48 per 1000 (32 to 71) | |||||
| Moderate | ||||||
| 131 per 1000 | 108 per 1000 (72 to 160) | |||||
| Birthweight (g) | The mean birthweight (g) in the intervention group was 5.13 g more (29.46 g less to 39.72 g more) | MD 5.13 (‐29.46 to 39.72) | 1939 (9 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | ||
| Premature birth (less than 37 weeks of gestation) | Study population | RR 1.03 (0.76 to 1.39) | 1177 (5 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | ||
| 128 per 1000 | 131 per 1000 (97 to 177) | |||||
| Moderate | ||||||
| 162 per 1000 | 167 per 1000 (123 to 225) | |||||
| Neonatal death (within 28 days after delivery) | Study population | RR 0.49 (0.04 to 5.42) | 795 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3,4 | ||
| 5 per 1000 | 2 per 1000 (0 to 27) | |||||
| Moderate | ||||||
| 5 per 1000 | 2 per 1000 (0 to 28) | |||||
| Congenital anomalies (including neural tube defects) | Study population | (0 studies) | Not reported | |||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Several studies contributing data had serious design limitations
2Wide 95% CI crossing the line of no effect
3Single study with some design limitations contributing data
4Wide 95% CI crossing the line of no effect, few events.
Summary of findings 2. Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals)‐maternal outcomes.
| Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals)‐ maternal outcomes | ||||||
|
Patient or population: Women receiving supplements in pregnancy
Settings: Community settings
Intervention: Any intermittent iron regimen (with or without other vitamins and minerals) Comparison: Daily regimen (with same vitamins and minerals) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Daily regimen (with same vitamins and minerals) | Any intermittent iron regimen (with or without other vitamins and minerals) | |||||
| Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) | Study population | RR 1.22 (0.84 to 1.80) | 676 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,2 | ||
| 169 per 1000 | 206 per 1000 (142 to 304) | |||||
| Moderate | ||||||
| 162 per 1000 | 198 per 1000 (136 to 292) | |||||
| Maternal iron deficiency at term (based on any indicator of iron status at 37 weeks' gestation or more) | Study population | (0 studies) | Not reported | |||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Maternal iron‐deficiency anaemia at term (Hb less than 110 g/L and at least 1 additional laboratory indicators at 37 weeks' gestation or more) | Study population | RR 0.71 (0.08 to 6.63) | 156 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3,4 | ||
| 28 per 1000 | 20 per 1000 (2 to 188) | |||||
| Maternal death (death while pregnant or within 42 days of termination of pregnancy) | Study population | RR 0 (0 to 0) | 00 (0 study) | Not reported | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Side effects (any reported throughout intervention period) | Study population | RR 0.56 (0.37 to 0.84) | 1777 (11 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,5 | ||
| 330 per 1000 | 185 per 1000 (122 to 277) | |||||
| Moderate | ||||||
| 400 per 1000 | 224 per 1000 (148 to 336) | |||||
| Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) | Study population | 1240 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,6 | No events | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Maternal clinical malaria or other infection in pregnancy | Study population | RR 0 (0 to 0) | 00 (0 study) | Not reported | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Studies contributing data had serious design limitations
2Wide 95% CI crossing the line of no effect
3Single study contributing data had design limitations
4Wide 95% CI crossing the line of no effect and few events
5High heterogeneity I² > 80%
6No events
For assessments of the overall quality of evidence for each outcome that included pooled data from included trials, we downgraded the evidence from 'high quality' by one level for serious (or by two for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias. This assessment was limited only to the trials included in this review and, as we did not consider there was a serious risk of indirectness or publication bias, we did not downgrade in these domains.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analysis on the primary outcomes based on the following criteria.
-
By gestational age at start of supplementation:
early (supplementation started before 20 weeks' gestation or prior to pregnancy);
late gestational age (supplementation started at 20 weeks of gestation or later);
unspecified gestational age or mixed gestational ages at the start of supplementation.
-
By anaemia status at baseline:
anaemic (Hb below 110 g/L during first and third trimesters or below 105 g/L in second trimester) at start of supplementation;
non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation;
unspecified/mixed anaemia status at start of supplementation.
-
By weekly iron dose in the group receiving intermittent supplementation:
low weekly dose of iron in the intermittent group (120 mg elemental iron or less per week);
high weekly dose of iron in the intermittent group (more than 120 mg elemental iron per week).
-
By release speed of iron supplements:
slow release iron supplement (as indicated by trialists);
normal release iron supplement;
not specified/unreported/unknown.
-
By bioavailability of the iron compound relative to ferrous sulphate:
higher: NaFeEDTA (sodium iron ethylenediaminetetraacetate);
equivalent or lower: ferrous sulphate, ferrous fumarate, ferrous gluconate, other.
not specified/unreported/unknown.
-
By intermittent iron supplementation regimen:
once a week;
other intermittent regimens.
-
By malaria endemicity of the area in which the trial was conducted:
malaria risk‐free area;
malaria risk area;
not specified/unreported/unknown.
We carried out formal subgroup analysis applying interaction texts as described in the Handbook (Higgins 2011) and have provided both subgroup and overall totals.
Sensitivity analysis
We planned to conduct a sensitivity analysis based on the quality of the studies. We considered a study to be of high quality if it was judged as having low risk of bias for both sequence generation and allocation concealment and in either blinding or loss to follow‐up. All of the trials contributing data to the review were considered at high or unclear risk of bias and none would have been retained in the analysis for sensitivity analysis. We will carry out planned sensitivity analysis by study quality if data from studies at low risk of bias are available for updates.
Results
Description of studies
Results of the search
A single search was carried out for the last version of this review (Peña‐Rosas 2012) and a related review examining daily iron and iron plus folic acid supplementation in pregnancy (Peña‐Rosas 2015). An updated search was carried out looking for additional evidence. The study flow is depicted in Figure 1. We have included 27 trials (in total); six of them (Alaoddolehei 2013; Bouzari 2011; Goshtasebi 2012; Hashim 2012; Mumtaz 2000; Quintero 2004), which were otherwise eligible for inclusion, did not provide outcome data that we were able to use for the meta‐analysis. Of these, four studies comparing intermittent iron alone (Alaoddolehei 2013; Bouzari 2011; Hashim 2012; Quintero 2004), and two studies comparing intermittent iron + folic acid (Goshtasebi 2012; Mumtaz 2000), versus daily regimens were not included in the quantitative analysis. We excluded 139 studies. We identified five ongoing or unpublished trials (Agrawal 2012; Gies 2010; Goonewardene 2014; Kumar 2014; Sherbaf 2012). Details of all studies are provided in Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
1.
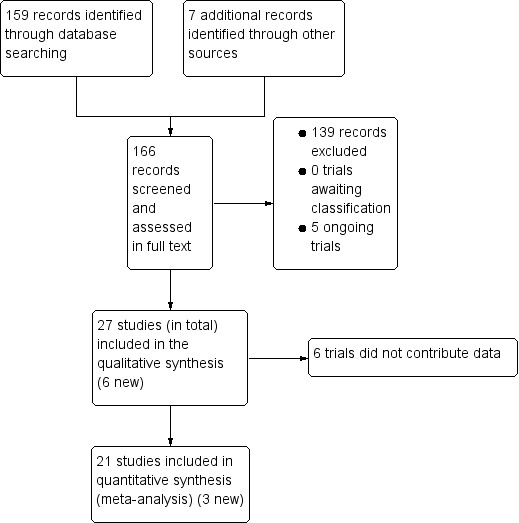
Study flow diagram for 2015 update
In addition to the published papers, abstracts and reports identified by the search, several trial authors provided additional, unpublished information for inclusion in the review (Casanueva 2006; Chew 2004b; Chew 2004a; Ekstrom 2002 (C); Hanieh 2013 (C); Liu 2003; Pita Martin 1999; Quintero 2004; Ridwan 1996 (C); Robinson 1999; Yu 1998. For the (Winichagoon 2003 (C)) trial we have unpublished data only.
We have treated a trial in Guatemala, that included two sub studies, as two separate trials: one with supervised intake (Chew 2004b), and one with unsupervised intake (Chew 2004a). One trial in China (Liu 2003), involved three comparison groups: one receiving weekly doses of iron, one receiving daily doses of iron and a control group. Since the allocation of the control group was not randomised, we included this study in our comparisons of the effects of intermittent versus daily iron supplementation, but have not used the control group in any comparison. Similarly, a three‐arm trial by Pita Martin 1999 included a control group receiving no supplementation, but again participants in the control arm were not selected randomly, and we have not included data for this group in the review (Pita Martin 1999).
Included studies
Settings
The studies included in the review were carried out over the last two decades in countries across the globe: Argentina (Pita Martin 1999), Bangladesh (Ekstrom 2002 (C)), China (Liu 2003), Guatemala (Chew 2004b; Chew 2004a), India (Bhatla 2009; Grover 1998; Mukhopadhyay 2004; Singh 2011), Indonesia (Ridwan 1996 (C); Robinson 1999), Iran (Alaoddolehei 2013; Bouzari 2011; Goshtasebi 2012; Yekta 2011; Zamani 2008), Malawi (Young 2000), Malaysia (Hashim 2012), Mexico (Casanueva 2006; Quintero 2004; Viteri 2012), Pakistan (Mumtaz 2000; Rukhsana 2006), South Korea (Yu 1998), Sri Lanka (Goonewardene 2001),Thailand (Winichagoon 2003 (C)), and Vietnam (Hanieh 2013 (C)).
According to the WHO Global Malaria Report 2014 (WHO 2014d) and WHO international travel and health (WHO 2011d), all the included studies took place in countries with some malaria risks of diverse characteristics. All the study sites were located in countries that in 2014 had some malaria risk in parts of the country (Alaoddolehei 2013; Bouzari 2011; Casanueva 2006; Chew 2004b; Chew 2004a; Goshtasebi 2012; Hanieh 2013 (C); Hashim 2012; Liu 2003, Pita Martin 1999; Quintero 2004;Viteri 2012; Yekta 2011; Yu 1998; Zamani 2008), or in locations with malaria risk locations (Bhatla 2009; Ekstrom 2002 (C); Grover 1998; Goonewardene 2001; Mukhopadhyay 2004; Mumtaz 2000; Ridwan 1996 (C); Robinson 1999; Rukhsana 2006; Singh 2011; Winichagoon 2003 (C); Young 2000). Only one of the trials, carried out in Indonesia, specifically reported that it was conducted in a malaria‐endemic area (Robinson 1999).
In some of these countries/territories, malaria is present only in certain areas or up to a particular altitude. In many countries, malaria has a seasonal pattern (WHO 2014d; WHO 2011e). These details as well as information on the predominant malaria species, status of resistance to antimalarial drugs for each country where an included study was conducted were extracted for 2011 (WHO 2014d, (WHO 2011d; WHO 2011e) and provided in the notes section Characteristics of included studies section.
Participants
In all of the included studies, women known to have severe anaemia at recruitment were excluded, In the trials by Alaoddolehei 2013; Bhatla 2009; Bouzari 2011; Casanueva 2006; Goshtasebi 2012; Mukhopadhyay 2004; Singh 2011; Yekta 2011 and Zamani 2008, none of the women were anaemic; in the trial by Mumtaz 2000 women were anaemic at baseline while in the remaining trials, samples may have included some women with moderate or mild anaemia at baseline.
In nine of the studies, women were recruited and supplementation started before 20 weeks of gestation ( Bhatla 2009; Bouzari 2011; Goshtasebi 2012; Hanieh 2013 (C); Hashim 2012; Mukhopadhyay 2004; Winichagoon 2003 (C); Yekta 2011; Zamani 2008); in the remaining studies, gestational age at the start of supplementation was mixed or unclear.
Interventions
Intermittent regimens
Most of the intermittent regimens involved women taking supplements on one day each week (usually two tablets on the same day each week). Nine trials examined different types of intermittent regimens; in the trials by Goshtasebi 2012; Hanieh 2013 (C); Mumtaz 2000; Rukhsana 2006; Yekta 2011, one of the study arms received iron two times a week; in the trial by Grover 1998 women in the intermittent group took supplements on alternate days, and in that by Pita Martin 1999 and Alaoddolehei 2013 every three days. In the trials by Bouzari 2011 and Goonewardene 2001 one study arm received iron once weekly, another iron three times a week, and a third group received daily iron; and in the trial by Rukhsana 2006, one study arm received iron once weekly, another two times a week and a third group received daily iron.
Weekly dose of iron in the arm receiving intermittent supplements
The weekly dose of iron ranged between 80 mg elemental iron per week and 300 mg of iron. In one trial, the weekly dose was 80 mg elemental iron per week (Mumtaz 2000), while another provided 90 mg elemental iron per week (Zamani 2008). Five studies provided 100 mg elemental iron weekly in the intermittent regimen (Goonewardene 2001; Goshtasebi 2012;Grover 1998; Singh 2011; Yekta 2011); in 12 trials women received 120 mg elemental iron per week (Casanueva 2006; Ekstrom 2002 (C); Hanieh 2013 (C); Hashim 2012; Liu 2003; Pita Martin 1999; Ridwan 1996 (C); Robinson 1999; Rukhsana 2006; Quintero 2004; Viteri 2012; Young 2000); in one study the weekly iron dose was 160 mg elemental iron (Yu 1998); in two trials women received in total a weekly dose of 180 mg elemental iron (Chew 2004b; Chew 2004a); three trials provided 200 mg elemental iron (Bhatla 2009; Mukhopadhyay 2004; Singh 2011). Two studies tested two different intermittent doses of iron, once a week: 100 and 150 mg elemental iron per week in the study by Bouzari 2011, and 120 and 180 mg elemental iron per week in the study by Winichagoon 2003 (C).
The dose of iron in the daily supplementation comparison groups ranged from 40 mg elemental iron daily (Mumtaz 2000); 45 mg elemental iron daily (Zamani 2008); 50 mg elemental iron daily (Alaoddolehei 2013; Bouzari 2011; Goshtasebi 2012; Yekta 2011); 60 mg elemental iron daily (Casanueva 2006; Chew 2004b; Chew 2004a; Ekstrom 2002 (C); Hanieh 2013 (C); Hashim 2012; Liu 2003; Pita Martin 1999; Ridwan 1996 (C); Rukhsana 2006; Robinson 1999; Viteri 2012; Winichagoon 2003 (C); Young 2000); 80 mg elemental iron daily (Yu 1998); 100 mg elemental iron daily (Bhatla 2009; Goonewardene 2001; Mukhopadhyay 2004; Quintero 2004; Singh 2011) to 120 mg elemental iron daily (Liu 2003).
Weekly dose of folic acid in the arm receiving intermittent supplements
For trials providing also folic acid intermittently as part of the intervention, the doses were: 400 μg (0.4 mg) folic acid per week (Casanueva 2006; Viteri 2012); 500 μg (0.5 mg) folic acid (Ekstrom 2002 (C); Liu 2003; Young 2000); 1000 μg (1 mg) folic acid per week (Alaoddolehei 2013; Bhatla 2009; Goshtasebi 2012; Mukhopadhyay 2004); 1500 μg (1.5 mg) folic acid a week (Grover 1998; Singh 2011); 2000 μg (2.0 mg) folic acid per week (Mumtaz 2000); 3000 μg (3 mg) folic acid per week (Hanieh 2013 (C)); and 3500 μg (3.5 mg) folic acid per week (Chew 2004b; Chew 2004a; Winichagoon 2003 (C)).
Type of iron compounds
All supplements used in trials were equivalent or lower, rather than high relative bioavailability iron compounds (ferrous sulphate and ferrous fumarate) and appeared to be standard, rather than slow‐release, preparations. Bioavailability of iron compounds is assessed in comparison (relative) to ferrous sulphate.
Laboratory method for ferritin concentration
Three laboratory methods were reported by the trials: enzyme‐linked‐immuno‐absorben‐assay (ELISA) (Casanueva 2006; Mukhopadhyay 2004; Mumtaz 2000; Pita Martin 1999; Ridwan 1996 (C); Alaoddolehei 2013; Singh 2011; Viteri 2012; Winichagoon 2003 (C)); radioimmunoassay (RIA) (Ekstrom 2002 (C); Goonewardene 2001; Goshtasebi 2012; Yekta 2011); and chemiluminescence‐immunoassay (CLIA) (Hanieh 2013 (C)). In the remaining trials, the ferritin was not measured or the laboratory method was not reported.
Supervision and co‐interventions
In most of the studies women took the supplements without supervision; in the Chew 2004b study women in both the intermittent and daily supplementation groups took supplements under supervision; in the Robinson 1999 trial women in the daily supplementation group were unsupervised, whereas the weekly group was supervised. Some studies included co‐interventions in addition to the nutritional supplement. For example, in the study by Bhatla 2009 the intervention included health education on diet and nutrition. Women in one study (Singh 2011), received deworming treatment at the start of the study. In most studies women received advice on when to take supplements (e.g. before meals).
Setting and health worker cadre
Most (98%) of the trials reported the type of healthcare facility where the trial was conducted, most frequently this was an antenatal clinic. Although the information about the health worker cadre that delivered the intervention was less explicit, in most of the cases it could be reasonably deduced from other details in the report. In three studies iron supplements were supplied by lay workers (Ekstrom 2002 (C); Hanieh 2013 (C); Mukhopadhyay 2004; Winichagoon 2003 (C)), in two trials by midwives (Ridwan 1996 (C);Young 2000), in one by traditional birth attendants (Robinson 1999), and in the rest of the cases by physicians, obstetricians or haematologists.
Comparisons
1. Any intermittent iron regimen (with or without other vitamins and minerals) compared with no supplementation or placebo
No studies contributed data.
2. Any intermittent iron regimen (with or without other vitamins and minerals) compared with any daily iron regimen (with same vitamins and minerals)
Twenty‐one studies contributed data and compared any intermittent iron regimen (with or without other vitamins and minerals) versus any daily regimen (with the same vitamins and minerals) (Bhatla 2009; Casanueva 2006; Chew 2004b; Chew 2004a; Ekstrom 2002 (C); Goonewardene 2001; Grover 1998; Hanieh 2013 (C); Liu 2003; Mukhopadhyay 2004; Pita Martin 1999; Ridwan 1996 (C); Robinson 1999; Rukhsana 2006; Singh 2011; Viteri 2012; Winichagoon 2003 (C); Yekta 2011; Young 2000; Yu 1998; Zamani 2008).
3. Intermittent oral iron alone supplementation compared with no supplementation or placebo
One study examining the provision of intermittent iron alone included a control group receiving no supplementation (Pita Martin 1999). However, as the control group was not selected randomly we have not included these data in this comparison. No other studies compared intermittent iron alone with no supplementation or placebo.
4. Intermittent oral iron + folic acid supplementation compared with no supplementation or placebo, and, 5. Intermittent oral iron + vitamins and minerals supplementation compared with no supplementation or placebo
No studies compared intermittent iron + folic acid with or without other vitamins and minerals with the effects of no supplementation or placebo.
6. Intermittent oral iron alone supplementation compared with daily oral iron alone supplementation
Three studies contributed data to this comparison (Pita Martin 1999; Yekta 2011; Yu 1998).
7. Intermittent oral iron + folic acid supplementation compared with daily oral iron + folic acid supplementation
Fourteen trials reporting on the outcomes included in the review compared the effects of intermittent iron + folic acid supplementation with the effects of daily iron + folic acid supplementation (Bhatla 2009; Chew 2004b; Chew 2004a; Ekstrom 2002 (C); Grover 1998; Hanieh 2013 (C); Liu 2003; Mukhopadhyay 2004; Ridwan 1996 (C); Robinson 1999; Rukhsana 2006; Winichagoon 2003 (C); Young 2000; Zamani 2008).
8. Intermittent oral iron + vitamins and minerals supplementation compared with daily oral iron + vitamins and minerals supplementation
Four studies contributed data to this comparison. Three (Casanueva 2006; Singh 2011; Viteri 2012) compared intermittent supplementation with iron + folic acid + vitamin B12 with the effects of daily supplementation with iron + folic acid + vitamin B12, while another study (Goonewardene 2001) compared the effects of daily, once weekly and three times weekly supplementation with a dose of iron + folic acid + vitamin B12, vitamin B6, vitamin B1, niacinamide and vitamin C.
See the table of Characteristics of included studies for a detailed description of the studies, including iron doses used. All included studies met the pre‐stated inclusion criteria.
Excluded studies
We excluded 139 studies. The main reason for excluding trials was that they did not compare intermittent versus daily regimens or no supplementation/placebo. Trials comparing daily iron supplementation (with or without folic acid and, or other vitamins and minerals) with placebo or no supplementation are included in a related review (Peña‐Rosas 2015). Descriptions of excluded studies along with the reasons for exclusion are set out in the Characteristics of excluded studies tables.
Risk of bias in included studies
For the cluster‐randomised trials we have used the same domains to assess risk of bias as those used where there was individual‐randomisation, however, we have noted in the 'Risk of bias' tables where there are additional possible sources of bias associated with cluster design (e.g. whether the data were adjusted to take account of cluster design effect).
Allocation
Sequence generation
Most of the included trials used computer‐generated random number sequences or random number tables to randomly allocate the intervention groups (Bhatla 2009; Chew 2004b; Chew 2004a; Ekstrom 2002 (C); Hanieh 2013 (C); Mukhopadhyay 2004; Mumtaz 2000; Quintero 2004; Ridwan 1996 (C); Viteri 2012; Young 2000; Zamani 2008). Casanueva 2006 used a method involving drawing lots with a 50% probability of participants being allocated to intervention or control groups. Ten trials did not report, or did not state clearly, the randomisation method used (Bouzari 2011; Goonewardene 2001; Grover 1998; Hashim 2012; Liu 2003; Robinson 1999; Rukhsana 2006; Singh 2011; Winichagoon 2003 (C); Yekta 2011), although in Liu 2003 the non‐supplemented group was self‐selected and so we have assessed this as being at high risk. Four trials were quasi‐randomised using alternation or other non random sequences (Alaoddolehei 2013; Goshtasebi 2012; Pita Martin 1999; Yu 1998).
Allocation concealment
Three trials reported using sealed envelopes when allocating women to treatment groups (Chew 1996a; Chew 1996b; Liu 1996). In the study by Zamani 2008, it was reported that coded vials were used; it was not clear whether the person allocating women to treatment groups also distributed the supplements. If so, then group allocation could possibly be anticipated, as while women in both groups received a supply for a month, one group received eight tablets and the other 30; the vials were likely to have felt different to experienced staff. In 13 other trials there was no description of the methods used to conceal allocation or the description was brief or unclear (Bouzari 2011; Casanueva 2003a; Goonewardene 2001; Grover 1998; Hashim 2012; Mukhopadhyay 2004; Mumtaz 2000; Robinson 1998; Rukhsana 2006; Singh 2011; Viteri 2010; Yekta 2011; Young 2000). In the study by Hanieh 2013 (C), the supplements were in blister packs with codes A, B and C. However, the study was open for the daily supplementation group. Nine other trials were assessed as being at high risk of bias for this domain as they used quasi‐randomisation methods including alternate allocation or allocation by day of the week, so that those carrying out randomisation would be aware of allocation at the point of randomisation (Alaoddolehei 2013; Alizadeh 2010; Bhatla 2009; Ekstrom 2002 (C); Pita Martin 1999; Quintero 2004; Ridwan 1996 (C); Winichagoon 2003 (C); Yu 1998).
Blinding
Blinding of participants, staff and outcome assessors
Clearly, trialists comparing the effects of intermittent supplementation regimens with the effects of daily supplementation regimens would have had difficulty keeping participants blinded as to what treatment they were receiving as this would have required that participants on an intermittent regimen receive placebo for some days. The study conducted by Mumtaz 2000 was the only one that provided placebos during the days that women did not consume the iron supplements. In the rest of the included studies there was no attempt to blind participants by providing inactive supplements to women in the intermittent arms of trials. Similarly, staff providing care were unlikely to have been blinded to group allocation. In several studies it was stated that outcome assessment (at least for laboratory measurements) was carried out by technicians blinded to the study arms (Bhatla 2009; Hanieh 2013 (C); Liu 2003; Mukhopadhyay 2004; Pita Martin 1999; Robinson 1999; Viteri 2012; Yu 1998; Zamani 2008). At the same time, we considered that lack of blinding would be unlikely to affect laboratory outcomes as these are usually done elsewhere. This would be the case for all but two of the remaining studies (Alaoddolehei 2013; Bouzari 2011; Casanueva 2006; Chew 2004b; Chew 2004a; Ekstrom 2002 (C); Goonewardene 2001; Goshtasebi 2012; Grover 1998; Mumtaz 2000; Quintero 2004; Ridwan 1996 (C); Singh 2011; Winichagoon 2003 (C); Yekta 2011; Young 2000). Two studies were assessed as high risk as they did not even mention the laboratory analysis (Hashim 2012; Rukhsana 2006).
While lack of blinding may not represent a serious source of bias for some outcomes (e.g. serum indicators of anaemia) other outcomes (reporting of side effects) may have been affected by knowledge of treatment group.
Incomplete outcome data
Loss of participants to follow‐up, missing data and lack of intention‐to‐treat analyses were serious problems with almost all of the included studies. Avoiding sample attrition in this type of study would not be simple; women who became anaemic were withdrawn from some trials so that they could receive treatment; and withdrawals for this reason may not have been balanced across study arms. In some studies women withdrew because they experienced side effects and there was no intention‐to‐treat analyses. High levels of sample attrition mean that studies are at serious risk of bias, and that results are more difficult to interpret.
In all studies, women were followed up over several months and so we anticipated some attrition and set a cut‐off of 20% as being a reasonable level of loss to follow‐up. In nine studies the attrition was less than 20% or nil and any loss was balanced across groups (Alaoddolehei 2013; Casanueva 2006; Goonewardene 2001; Goshtasebi 2012; Hanieh 2013 (C); Liu 2003; Rukhsana 2006; Singh 2011; Viteri 2012); these studies were assessed as being at low risk of attrition bias. In three studies (Bhatla 2009;Bouzari 2011; Yekta 2011) loss was not balanced across groups and post‐randomisation exclusions may have related to the interventions; for example, if women were unable to tolerate the iron supplements they were excluded. One study did not provide information about sample attrition (Hashim 2012). In all other studies attrition exceeded 20% (Chew 2004b; Chew 2004a; Ekstrom 2002 (C); Mukhopadhyay 2004; Mumtaz 2000; Quintero 2004; Ridwan 1996 (C); Robinson 1999; Winichagoon 2003 (C); Zamani 2008). In five studies a third or more of the women randomised were lost to follow‐up: in the study by Ekstrom 2002 (C) a third of the sample was not followed up, and losses were even greater in the trials by Grover 1998 (40%), Young 2000 (47%), Yu 1998 (47%) and Pita Martin 1999 (57%).
Selective reporting
We had access to trial registrations or protocols for three studies and these studies were assessed as being at low risk of outcome reporting bias (Alaoddolehei 2013; Goshtasebi 2012; Hanieh 2013 (C)). One study reported outcomes only for those women where compliance was known to be high and this study was therefore assessed as being at high risk of bias for this domain (Yu 1998). In the study by Zamani 2008, it was stated that data were collected on outcomes at delivery, which were not reported in the results. However, it is possible these data will be published in future papers. For the remaining studies we did not have access to study protocols and therefore, formally assessing reporting bias was not possible (all assessed as unclear). Insufficient studies contributed data to allow us to carry out exploration of possible publication bias.
Other potential sources of bias
In three studies there was some evidence of baseline imbalance between groups: in the Ridwan 1996 (C) and Yekta 2011 trials women in the weekly supplementation group had lower Hb levels at baseline, and in the Bhatla 2009 trial the daily group started supplements at earlier gestational ages. In the study by (Goshtasebi 2012), the authors reported that due to financial constraints the trial was open and baseline ferritin values were not evaluated for comparability among the groups; and the supervision of adherence was not done. In the trials by Pita Martin 1999 and Robinson 1999, there were changes in protocol during the trial. All of these studies were assessed as high risk of other bias.
In one study (Grover 1998), background data were only provided for those women completing the study, so it was not clear whether women who remained at follow‐up had the same characteristics as those that dropped out or were excluded. In other studies it was not clear from the methods whether studies were likely to be at risk of other sources of bias (Bouzari 2011; Hashim 2012; Zamani 2008).
The remaining studies were all assessed as low risk of bias for this domain. Groups appeared balanced at baseline and other sources of bias were not apparent.
Cluster‐randomised trials
Four trials used cluster‐randomisation (Ekstrom 2002 (C); Hanieh 2013 (C); Ridwan 1996 (C); Winichagoon 2003 (C)). In the trials by Ridwan 1996 (C); Hanieh 2013 (C) and Winichagoon 2003 (C), we were able to adjust the sample sizes to take account of the cluster design effect using data provided by the trial authors. Cluster design effect was estimated but not taken into account in the analysis of Ekstrom 2002 (C).
None of the studies included in this review were rated as high quality. Full details of 'Risk of bias' assessments are included in Characteristics of included studies tables. We have also included figures which summarise our 'Risk of bias' assessments (Figure 2; Figure 3), which we used to help us judge study quality in the Summary of Findings tables (seeTable 1; Table 2).
2.
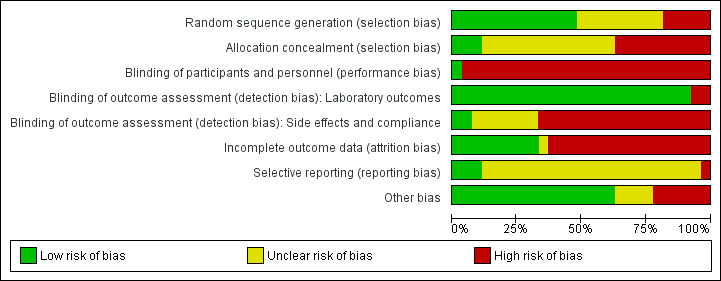
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
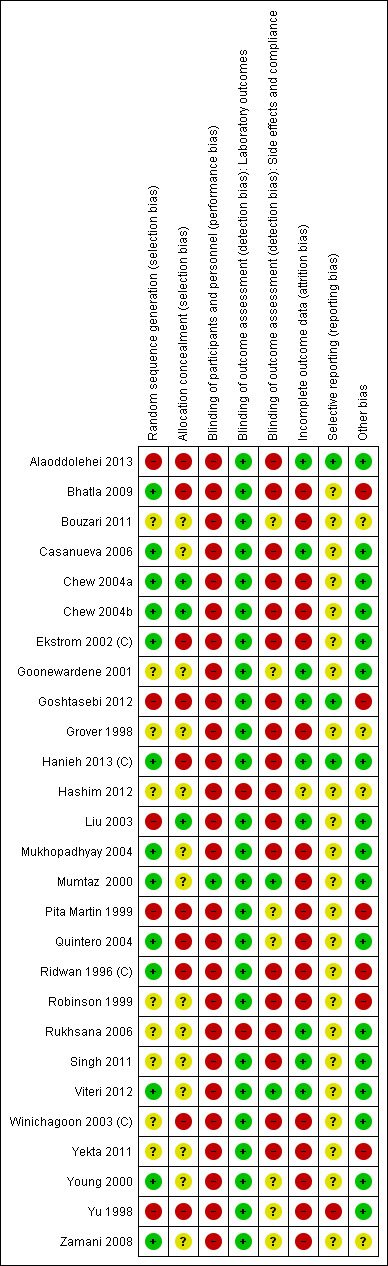
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
We have included data from 21 trials, involving 5490 women (this figure represents the number of women recruited to studies; in some studies we have not included data for all arms of trials in the comparisons; in the cluster‐randomised trials, sample sizes were adjusted to take account of design effect, and in most studies there were high levels of attrition). Overall, outcome data were available for approximately 60% of the original samples. We have organised the summary of results by supplementation regimens compared, and by primary and secondary outcomes for infants and women. Most of the included studies focused on haematological indicators and few reported on any of the other outcomes prespecified in the review protocol. Because some results showed heterogeneity that could not be explained by standard sensitivity analyses including quality assessment, we used a random‐effects model to analyse the results.
In the data and analyses tables we set up all eight prespecified comparisons but outcome data were only available for four of these. We have not added outcomes to those comparisons with no data (comparisons one, three, four and five). For the comparisons with data, we set up tables for all primary outcomes (even where no data were available) not only to highlight gaps in the current research evidence, but also so that we can add any data that become available in future updates.
For each of the comparisons we have indicated the number of studies contributing data and the total number of women recruited in these studies. However, for some outcomes only one or two studies provided data and due to loss to follow‐up, denominators for particular outcomes may have been considerably less than the randomised sample. Therefore, for primary outcomes and for results that were statistically significant, or that we considered to be clinically important, we have indicated the number of studies contributing data and the number of women included in that analysis (if cluster trials were included in the analysis this number represents the effective sample size rather than the actual number of women included in studies).
Subgroup analysis. Where sufficient data were available for primary outcomes, we set up subgroups to look for possible differences between studies in terms of earlier or later supplementation; women's anaemia status at the start of supplementation; higher and lower weekly doses of iron; and the malarial status of the region in which the trials were conducted. We made the pragmatic decision not to include subgroup analysis where the number of trials was sparse (two or less) and did not carry out planned subgroup analysis for the type of iron compound examined, release speed of iron supplements, or malaria setting as all the trials were in the same subgroup category. In other words, all the trials supplemented women with ferrous sulphate, which releases normally, and were conducted in settings with some degree of malaria risk, although only the trial by Robinson 1999 reported clearly that it was carried out in a setting where malaria was endemic. We have indicated in the text whether there was any evidence of subgroup differences. As more data become available, in updates of the review we hope to extend subgroup analysis as a means of exploring heterogeneity between trials.
See the Data and analyses section for detailed results on primary and secondary outcomes and subgroup analysis.
(1) Any intermittent iron regimen (with or without other vitamins and minerals) compared with no supplementation or placebo (no studies)
No studies were included in this comparison.
(2) Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals) (21 studies with 5490 women)
All studies contributing data to the review are included in this comparison (Bhatla 2009; Casanueva 2006; Chew 2004b; Chew 2004a; Ekstrom 2002 (C); Goonewardene 2001; Grover 1998; Hanieh 2013 (C); Liu 2003; Mukhopadhyay 2004; Pita Martin 1999; Ridwan 1996 (C); Robinson 1999; Rukhsana 2006; Singh 2011; Viteri 2012; Winichagoon 2003 (C); Young 2000; Yekta 2011; Yu 1998; Zamani 2008).
Primary outcomes
Infant outcomes
Low birthweight (less than 2500 g)
The data from eight trials (Bhatla 2009; Chew 2004b; Chew 2004a; Grover 1998; Hanieh 2013 (C); Mukhopadhyay 2004; Winichagoon 2003 (C); Yekta 2011) involving 1898 women show that similar numbers of women taking intermittent iron (with or without other vitamins and minerals) had a baby with birthweight below 2500 g compared with those taking daily supplements (4.3% versus 5.8%; average risk ratio (RR) 0.82; 95% confidence interval (CI) 0.55 to 1.22, low quality evidence) (Analysis 2.1) No subgroup differences were identified.
2.1. Analysis.
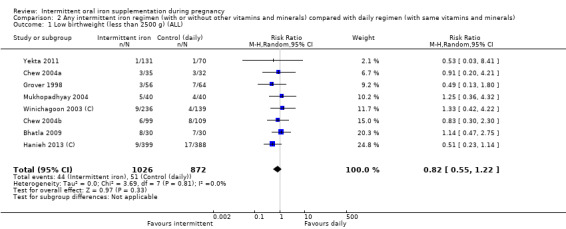
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 1 Low birthweight (less than 2500 g) (ALL).
Birthweight (g)
The data from nine trials (Bhatla 2009; Chew 2004b; Chew 2004a; Grover 1998; Hanieh 2013 (C); Mukhopadhyay 2004; Pita Martin 1999; Winichagoon 2003 (C); Yekta 2011) involving 1939 women show there are no statistically significant differences between groups in terms of mean infant birthweight mean difference (MD) 5.13 g; 95% CI ‐29.46 to 39.72 g, low quality evidence) (Analysis 2.6). No subgroup differences were identified.
2.6. Analysis.
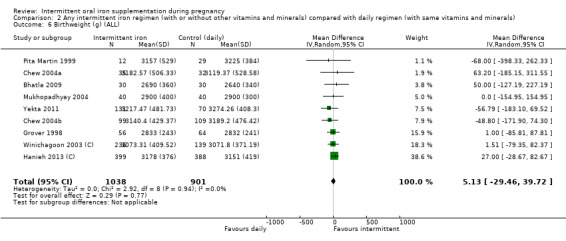
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 6 Birthweight (g) (ALL).
Premature birth (before 37 weeks' gestation)
Five studies including 1177 women reported the number of babies born prematurely (Bhatla 2009; Hanieh 2013 (C); Mukhopadhyay 2004; Pita Martin 1999; Yekta 2011); there was no evidence of a significant difference for premature birth between women receiving daily and intermittent supplements (average RR 1.03; 95% CI 0.76 to 1.39, low quality evidence) (Analysis 2.11). There was no evidence of subgroup differences.
2.11. Analysis.
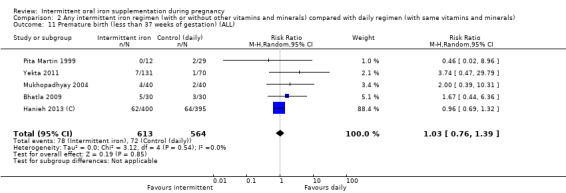
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 11 Premature birth (less than 37 weeks of gestation) (ALL).
Neonatal death
One cluster‐randomised trial reported on neonatal death and there was no evidence of a significant difference between groups (RR 0.49; 95% CI 0.04 to 5.42, very low quality evidence) (Analysis 2.16).
2.16. Analysis.
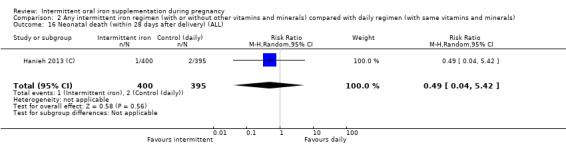
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 16 Neonatal death (within 28 days after delivery) (ALL).
Other primary outcomes
No trials reported on the remaining infant primary outcome, congenital anomalies.
Maternal outcomes
Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more)
Anaemia at term was reported in four trials with 676 women (Chew 2004b; Chew 2004a; Liu 2003; Yekta 2011); there was no evidence of a significant differences between groups (average RR 1.22; 95% CI 0.84 to 1.80, very low quality evidence) (Analysis 2.18). There was no evidence of differences between subgroups.
2.18. Analysis.
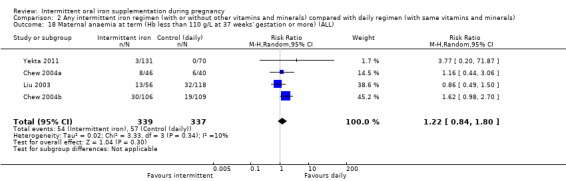
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 18 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) (ALL).
Maternal iron deficiency at term (as defined by trialists, based on any indicator of iron status at 37 weeks' gestation or more
There were no estimable data for this outcome.
Maternal iron‐deficiency anaemia at term (Hb less than 110 g/L and at least one additional laboratory indicator at 37 weeks' gestation or more)
No evidence of significant differences was found between groups in the one trial with data for 156 women (Liu 2003) that reported this outcome (average RR 0.71; 95% CI 0.08 to 6.63, very low quality evidence) (Analysis 2.24).
2.24. Analysis.
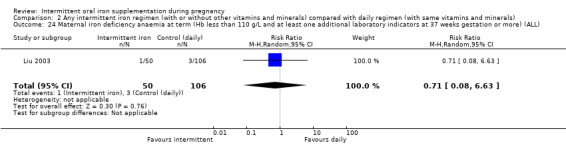
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 24 Maternal iron deficiency anaemia at term (Hb less than 110 g/L and at least one additional laboratory indicators at 37 weeks gestation or more) (ALL).
Maternal death (death while pregnant or within 42 days of termination pf pregnancy)
There were no estimable data for this outcome.
Side effects (any reported throughout intervention period)
Eleven trials with 1777 women described the number of women reporting side effects. Women receiving intermittent iron supplements (with or without other vitamins and minerals) were less likely to report side effects compared with women receiving daily supplements (average RR 0.56; 95% CI 0.37 to 0.84, intermittent 25.2%, daily 35.9% reported side effects; very low quality evidence) (Analysis 2.26). There were high levels of heterogeneity for this outcome and results should be interpreted with caution (I² = 87%, Tau² = 0.30 and P < 0.00001 for the Chi² test for heterogeneity). Subgroup interaction tests suggested that there were some differences between subgroups in terms of gestational age and women's anaemia status at the start of supplementation; however, as in most trials women were recruited at unspecified or mixed gestational ages and with unspecified or mixed anaemia status any possible differences between subgroups are difficult to interpret.
2.26. Analysis.
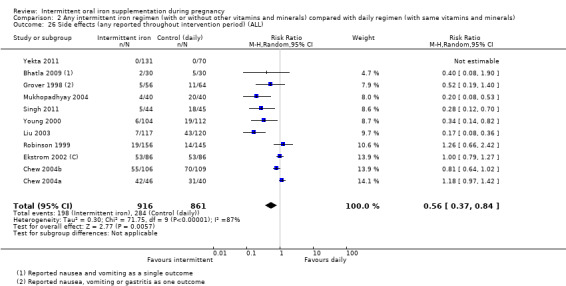
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 26 Side effects (any reported throughout intervention period) (ALL).
Severe anaemia at any time during second or third trimesters (Hb less than 70 g/L)
Six trials involving 1240 women reported no cases of severe anaemia at any time during second or third trimesters.
Other outcomes
No trials reported on the remaining maternal primary outcomes: infection or clinical malaria.
Secondary outcomes
Infant
Very low birthweight (less than 1500 g)
Five studies reported this outcome involving 1524 women (Chew 2004b; Chew 2004a; Hanieh 2013 (C);Winichagoon 2003 (C); Mukhopadhyay 2004); there was evidence of no significant differences between groups (average RR 0.19; 95% CI 0.01 to 4.04) (Analysis 2.38).
2.38. Analysis.
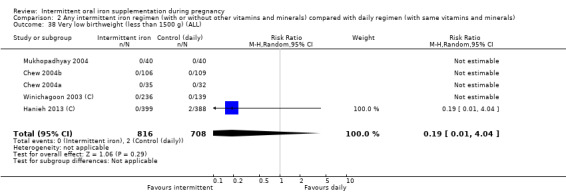
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 38 Very low birthweight (less than 1500 g) (ALL).
Very premature birth (less than 34 weeks' gestation)
Two studies reported this outcome (Casanueva 2006; Mukhopadhyay 2004). There was one very premature birth in each group in one of the trials (Mukhopadhyay 2004), and none in the other (Casanueva 2006) (Analysis 2.39).
2.39. Analysis.
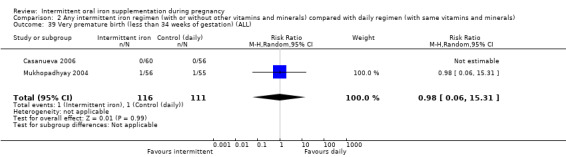
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 39 Very premature birth (less than 34 weeks of gestation) (ALL).
Infant Hb concentration within the first six months (in g/L, counting the last reported measure after birth within this period)
One study (Hanieh 2013 (C)) including 518 women (adjusted sample size) reported this outcome; there was evidence of no significant differences between groups (RR ‐0.50; 95% CI ‐2.44 to 1.44) (Analysis 2.40).
2.40. Analysis.
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 40 Infant Hb concentration within the first six months (in g/L, counting the last reported measure after birth within this period)..
Infant ferritin concentration within the first six months (in μg/L, counting the last reported measure after birth within this period)
A single study (Winichagoon 2003 (C)) including 88 participants (adjusted) reported this outcome. The data from this trial suggest that the infants of women receiving intermittent iron + folic acid supplementation have a higher concentration of serum ferritin at six months (MD 0.09 μg/L; 95% CI 0.05 μg/L to 0.13 μg/L) (Analysis 2.41), but given the scarcity of data for this outcome no firm conclusions can be drawn.
2.41. Analysis.
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 41 Infant ferritin concentration in the first 6 months (μg/L, counting the last reported measure after birth within this period) (ALL).
Other infant secondary outcomes
No trials reported on the remaining infant secondary outcomes.
Maternal
Maternal anaemia at or near term (Hb less than 110 g/L at 34 weeks' gestation or more)
Anaemia at or near term was reported in eight trials with 1385 women (Casanueva 2006; Chew 2004b; Chew 2004a; Goonewardene 2001; Liu 2003; Zamani 2008; Winichagoon 2003 (C); Yekta 2011); for this outcome there was evidence of significant differences between groups with results favouring women in the daily iron group (average RR 1.66; 95% CI 1.09 to 2.53) (Analysis 2.47).
2.47. Analysis.
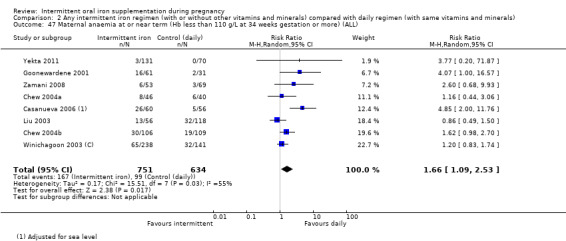
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 47 Maternal anaemia at or near term (Hb less than 110 g/L at 34 weeks gestation or more) (ALL).
Maternal iron deficiency at or near term (as defined by trialists, based on any indicator of iron status at 34 weeks' gestation or more)
Results from three studies with 587 women (Casanueva 2006; Goonewardene 2001; Winichagoon 2003 (C)) suggest that women receiving intermittent iron were more likely than those receiving daily iron to be iron deficient at or near term (RR 2.38; 95% CI 1.30 to 4.36) (Analysis 2.48).
2.48. Analysis.
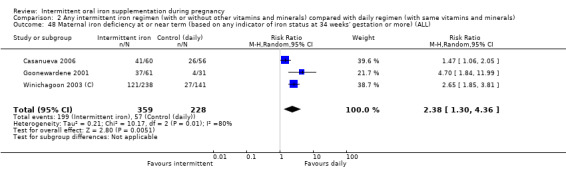
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 48 Maternal iron deficiency at or near term (based on any indicator of iron status at 34 weeks' gestation or more) (ALL).
Maternal iron‐deficiency anaemia at or near term (Hb less than 110 g/L and at least one additional laboratory indicator of iron status at 34 weeks' gestation or more)
Data from two trials involving 278 women suggest that women receiving intermittent iron supplementation were as likely as to have iron‐deficiency anaemia at or near term than those on the daily iron supplementation regimen (RR 2.06; 95% CI 0.65 to 6.61) (Analysis 2.49)
2.49. Analysis.
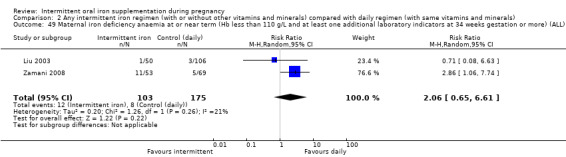
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 49 Maternal iron deficiency anaemia at or near term (Hb less than 110 g/L and at least one additional laboratory indicators at 34 weeks gestation or more) (ALL).
Maternal Hb concentration at or near term (g/L, at 34 weeks' gestation or more)
Eight studies (involving 1306 women) reported on this outcome. We found evidence of significant differences between these groups of women for mean Hb concentration at term (MD ‐ 2.57 g/L; 95% CI ‐5.18 g/L to 0.04 g/L) (Analysis 2.50) There was high heterogeneity for this outcome and results should be interpreted with caution; the size and direction of treatment effects varied considerably in the studies contributing data to this outcome.
2.50. Analysis.
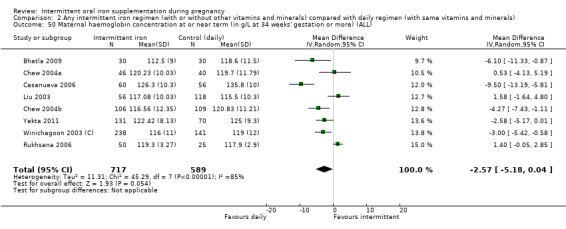
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 50 Maternal haemoglobin concentration at or near term (in g/L at 34 weeks' gestation or more) (ALL).
Maternal high Hb concentrations at any time during second or third trimesters (defined as Hb greater than 130 g/L)
Fifteen trials involving 2616 women (Casanueva 2006; Chew 2004b; Chew 2004a; Ekstrom 2002 (C); Hanieh 2013 (C); Liu 2003; Mukhopadhyay 2004; Pita Martin 1999; Ridwan 1996 (C); Robinson 1999; Viteri 2012; Winichagoon 2003 (C); Yekta 2011; Yu 1998, Zamani 2008) reported on the number of women with high Hb concentrations during the second or third trimesters of pregnancy. Results suggest that women who routinely received intermittent iron supplementation during pregnancy were less likely to have high Hb concentrations during mid and late pregnancy compared with those receiving daily supplements (8.2% versus 17.8%; RR 0.53; 95% CI 0.38 to 0.74) (Analysis 2.51). There were moderate levels of heterogeneity for this outcome.
2.51. Analysis.
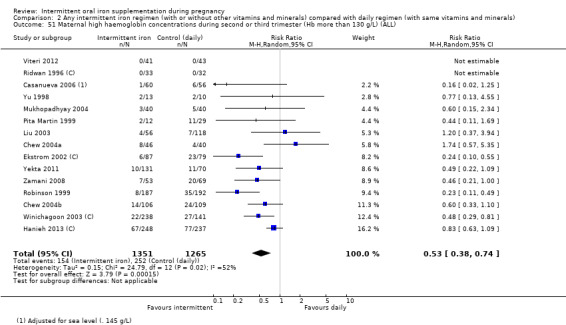
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 51 Maternal high haemoglobin concentrations during second or third trimester (Hb more than 130 g/L) (ALL).
Severe anaemia at term (Hb less than 70 g/L at 37 weeks' gestation or more)
There were no estimable data for this outcome; in the three trials reporting this outcome no women had severe anaemia in either group (Analysis 2.53).
2.53. Analysis.
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 53 Severe anaemia at term (Hb less than 70 g/L at 37 weeks' gestation or more) (ALL).
Severe anaemia at or near term (Hb less than 70 g/L at 34 weeks' gestation or more)
There were no estimable data for this outcome; in the six trials reporting this outcome no women had severe anaemia in either group (Analysis 2.54).
2.54. Analysis.
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 54 Severe anaemia at or near term (Hb less than 70 g/L at 34 weeks' gestation or more) (ALL).
Nausea
Women receiving daily supplements were more likely to report nausea compared with those receiving intermittent supplements (average RR 0.60; 95% CI 0.37 to 0.97; seven trials, 1034 women) (Analysis 2.59).
2.59. Analysis.
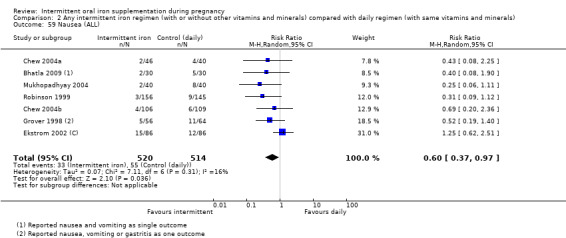
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 59 Nausea (ALL).
Other outcomes
There was no evidence of significant differences between the comparison groups in the following secondary outcomes: severe anaemia at postpartum (Analysis 2.55), antepartum haemorrhage (Analysis 2.56), or individual side effects (diarrhoea, constipation, heartburn, vomiting) (Analysis 2.57; Analysis 2.58; Analysis 2.60; Analysis 2.61), placental abruption (Analysis 2.62), or premature rupture of membranes (Analysis 2.63). No trials reported on the remaining secondary outcomes.
2.55. Analysis.
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 55 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL).
2.56. Analysis.
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 56 Antepartum haemorrhage (ALL).
2.57. Analysis.
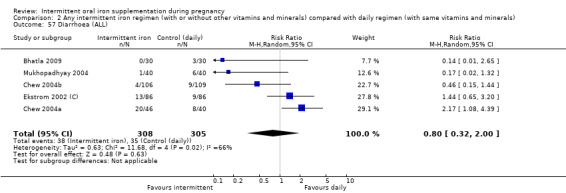
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 57 Diarrhoea (ALL).
2.58. Analysis.
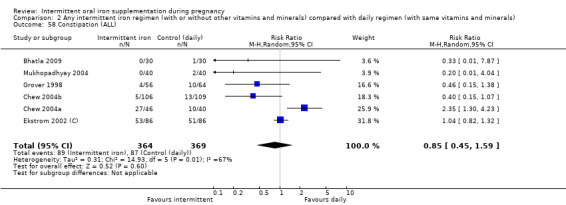
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 58 Constipation (ALL).
2.60. Analysis.
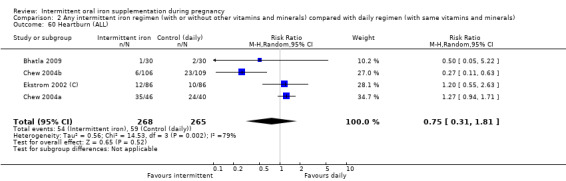
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 60 Heartburn (ALL).
2.61. Analysis.
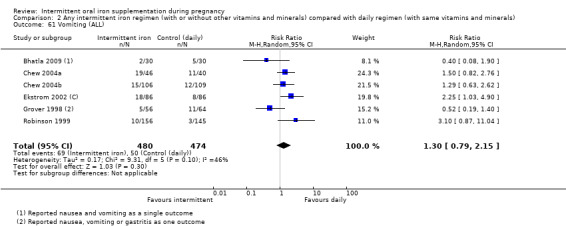
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 61 Vomiting (ALL).
2.62. Analysis.
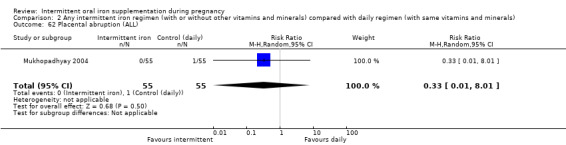
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 62 Placental abruption (ALL).
2.63. Analysis.
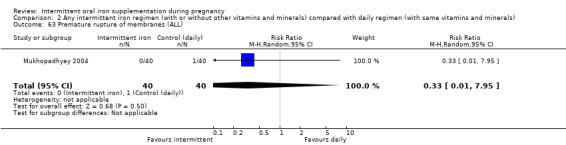
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 63 Premature rupture of membranes (ALL).
(3) Intermittent oral iron alone supplementation compared with no supplementation/placebo (no studies)
No studies were included in this comparison.
(4) Intermittent oral iron + folic acid supplementation compared with no supplementation/placebo (no studies)
No studies were included in this comparison.
(5) Intermittent oral iron + vitamins and minerals supplementation compared with no supplementation/placebo (no studies)
No studies were included in this comparison.
(6) Intermittent oral iron alone supplementation compared with daily oral iron alone supplementation (three studies: 464 women)
Primary outcomes
Infant outcomes
Low birthweight (less than 2500 g)
Only the study by Yekta 2011 (with data for 201 women) reported on this outcome and found one case of low birthweight per group (Analysis 6.1).
6.1. Analysis.
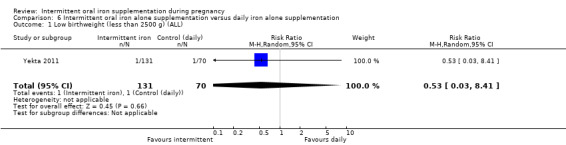
Comparison 6 Intermittent oral iron alone supplementation versus daily iron alone supplementation, Outcome 1 Low birthweight (less than 2500 g) (ALL).
Birthweight (g)
We found no evidence of significant differences between these groups of infants in birthweight although only two studies with 242 women reported on this outcome (Pita Martin 1999; Yekta 2011). SeeAnalysis 6.2.
6.2. Analysis.
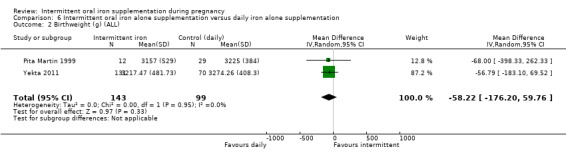
Comparison 6 Intermittent oral iron alone supplementation versus daily iron alone supplementation, Outcome 2 Birthweight (g) (ALL).
Premature birth (less than 37 weeks' gestation)
Two studies with 242 women reported on this outcome (Pita Martin 1999; Yekta 2011); there is no evidence of significant differences between groups for this outcome (Analysis 6.3).
6.3. Analysis.
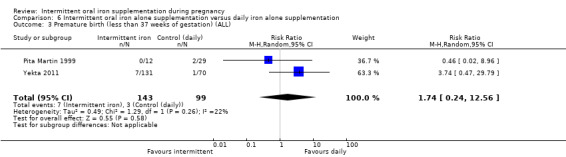
Comparison 6 Intermittent oral iron alone supplementation versus daily iron alone supplementation, Outcome 3 Premature birth (less than 37 weeks of gestation) (ALL).
No trials reported on the remaining primary infant outcomes: low birthweight, neonatal death or congenital anomalies.
Maternal outcomes
Only Yekta 2011 reported on anaemia at or near term (Hb less than 110 g/L at 37 weeks' gestation or more) and side effects (any reported throughout the intervention period) and found no differences between the groups receiving daily or intermittent supplementation for either outcome (Analysis 6.6; Analysis 6.10; Analysis 6.14).
6.6. Analysis.
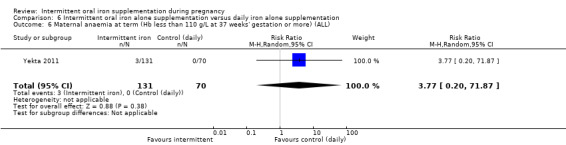
Comparison 6 Intermittent oral iron alone supplementation versus daily iron alone supplementation, Outcome 6 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) (ALL).
6.10. Analysis.
Comparison 6 Intermittent oral iron alone supplementation versus daily iron alone supplementation, Outcome 10 Side effects (any reported throughout intervention period) (ALL).
6.14. Analysis.
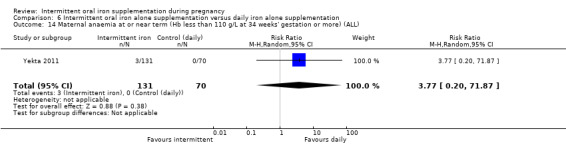
Comparison 6 Intermittent oral iron alone supplementation versus daily iron alone supplementation, Outcome 14 Maternal anaemia at or near term (Hb less than 110 g/L at 34 weeks' gestation or more) (ALL).
The effect of the intervention on severe anaemia at any time during second or third trimesters could not be estimated (Analysis 6.11).
6.11. Analysis.
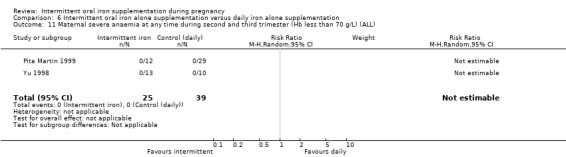
Comparison 6 Intermittent oral iron alone supplementation versus daily iron alone supplementation, Outcome 11 Maternal severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL).
The number of women with high Hb concentrations at or near term was reported in two trials and there was no clear difference between groups (MD ‐2.58, 95% CI ‐5.17 to 0.01) (Analysis 6.15).
6.15. Analysis.
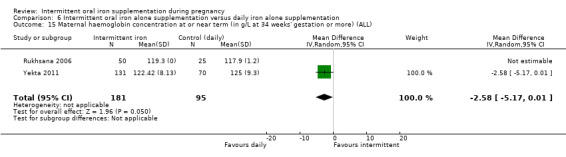
Comparison 6 Intermittent oral iron alone supplementation versus daily iron alone supplementation, Outcome 15 Maternal haemoglobin concentration at or near term (in g/L at 34 weeks' gestation or more) (ALL).
None of the trials reported on any of the remaining prespecified primary outcomes: iron deficiency at term (as defined by trialists, based on any indicator of iron status at 37 weeks' gestation or more), iron‐deficiency anaemia at term (Hb less than 110 g/L and at least one additional laboratory indicator of iron status at 37 weeks' gestation or more); maternal deaths; severe anaemia at any time during second or third trimesters; clinical malaria or infection during pregnancy.
Secondary outcomes
Infant
No trials with this comparison reported on infant secondary outcomes: very low birthweight (less than 1500 g); Hb concentration in the first six months; ferritin concentration in the first six months; development and motor skills or admission to special care unit.
Maternal
Maternal high Hb concentrations during second or third trimester (defined as Hb greater than 130 g/L)
Three trials (Pita Martin 1999; Yekta 2011; Yu 1998) contributed data to this outcome, but there was no significant evidence of differences between women receiving supplements intermittently versus daily (Analysis 6.16).
6.16. Analysis.
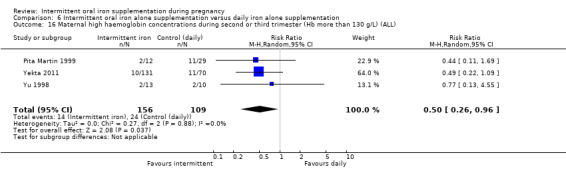
Comparison 6 Intermittent oral iron alone supplementation versus daily iron alone supplementation, Outcome 16 Maternal high haemoglobin concentrations during second or third trimester (Hb more than 130 g/L) (ALL).
No trials reported on the remaining maternal secondary outcomes: anaemia at term; iron deficiency at term; iron‐deficiency anaemia at term; Hb concentration at or near term; Hb concentration within one month postpartum; high Hb concentrations at or near term; moderate anaemia at postpartum; severe anaemia at term; severe anaemia at or near term; severe anaemia postpartum; puerperal infection; antepartum haemorrhage; postpartum haemorrhage; transfusion given; diarrhoea; constipation; nausea; heartburn; vomiting; maternal well being/satisfaction; placental abruption; premature rupture of membranes or pre‐eclampsia.
(7) Intermittent oral iron + folic acid supplementation compared with daily oral iron + folic acid supplementation (14 studies: 4653 women)
Fourteen trials reporting on the outcomes included in the review compared the effects of intermittent iron + folic acid supplementation with the effects of daily iron + folic acid supplementation (Bhatla 2009; Chew 2004b; Chew 2004a; Ekstrom 2002 (C); Grover 1998; Hanieh 2013 (C); Liu 2003; Mukhopadhyay 2004; Ridwan 1996 (C); Robinson 1999; Rukhsana 2006; Winichagoon 2003 (C); Young 2000; Zamani 2008).
Primary outcomes
Infant outcomes
Low birthweight (less than 2500 g)
The data from seven trials (Bhatla 2009; Chew 2004b; Chew 2004a; Grover 1998; Hanieh 2013 (C); Mukhopadhyay 2004; Winichagoon 2003 (C)) involving 1697 women suggest that women who take intermittent iron + folic acid supplementation during pregnancy are as likely as those taking daily supplements to have a baby with birthweight below 2500 g (4.8% versus 6.2%; average RR 0.83; 95% CI 0.56 to 1.24) (Analysis 7.1). We did not find evidence of subgroup differences.
7.1. Analysis.
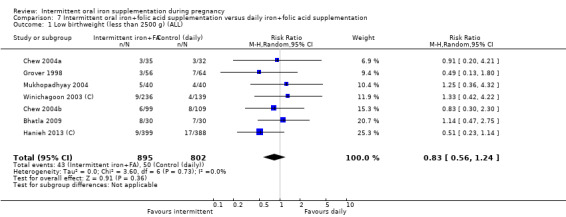
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 1 Low birthweight (less than 2500 g) (ALL).
Birthweight (g)
The data from seven trials (Bhatla 2009; Chew 2004b; Chew 2004a; Grover 1998; Hanieh 2013 (C); Mukhopadhyay 2004; Winichagoon 2003 (C)) involving 1697 women suggest that there is no significant effect in birthweight of infants born from women who had taken daily supplementation with iron + folic acid during pregnancy compared with those being supplemented intermittently (MD 11.09 g; 95% CI ‐25.09 to 47.27 g) (Analysis 7.6). No subgroup differences were identified.
7.6. Analysis.
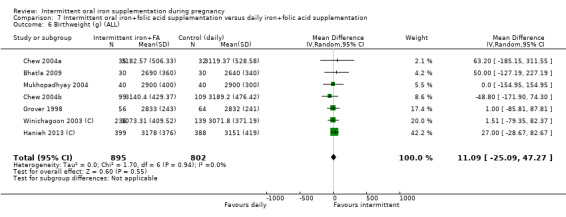
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 6 Birthweight (g) (ALL).
Premature birth (before 37 weeks' gestation)
Three studies including 935 women reported the number of babies born prematurely (Bhatla 2009; Hanieh 2013 (C); Mukhopadhyay 2004); overall 140 babies were born before 37 weeks' gestation, and there was no significant difference between women receiving daily and intermittent supplements (average RR 1.01; 95% CI 0.74 to 1.37) (Analysis 7.11).
7.11. Analysis.
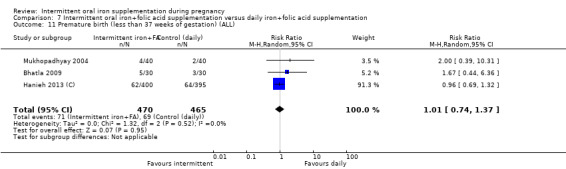
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 11 Premature birth (less than 37 weeks of gestation) (ALL).
Hanieh 2013 (C) reported neonatal death up to 28 days. There were no significant differences between groups (RR 0.49, 95% CI 0.04 to 5.42) (Analysis 7.12).
7.12. Analysis.
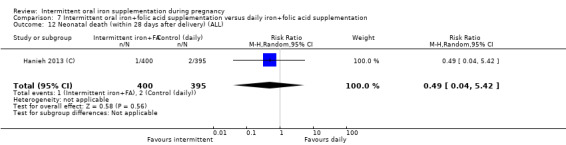
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 12 Neonatal death (within 28 days after delivery) (ALL).
Other primary outcomes
No trials reported on the remaining primary infant outcome: congenital anomalies.
Maternal outcomes
Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more)
The number of women with anaemia at term was reported in three trials with 475 women (Chew 2004b; Chew 2004a; Liu 2003); there was no clear evidence of differences between groups (average RR 1.20; 95% CI 0.78 to 1.83) (Analysis 7.14). There was no evidence of differences between subgroups.
7.14. Analysis.
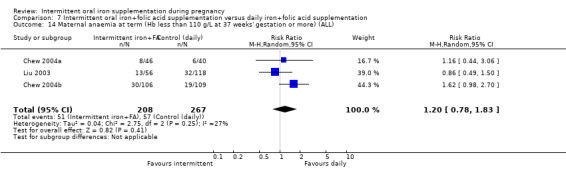
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 14 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) (ALL).
Maternal iron‐deficiency anaemia at term (Hb less than 110 g/L and at least one additional laboratory indicator at 37 weeks' gestation or more)
No evidence of significant differences was found between these groups of women in the one trial (Liu 2003) that reported this outcome (average RR 0.71; 95% CI 0.08 to 6.63) (Analysis 7.20).
7.20. Analysis.
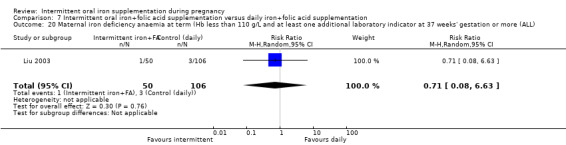
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 20 Maternal iron deficiency anaemia at term (Hb less than 110 g/L and at least one additional laboratory indicator at 37 weeks' gestation or more (ALL).
Maternal death (death while pregnant or within 42 days of termination pf pregnancy)
There were no estimable data for this outcome.
Side effects (any reported throughout intervention period)
Nine trials with 1487 women contributed data to this outcome. Women receiving intermittent iron and folic acid were less likely to report side effects compared with women receiving daily supplements (average RR 0.60; 95% CI 0.40 to 0.91, intermittent 26%, daily 36% reported side effects (Analysis 7.22)). There were high levels of heterogeneity for this outcome (I² = 87%, Tau² = 0.27 and P < 0.00001 for the Chi² test for heterogeneity; 95% prediction interval: 0.16 to 2.26). There appeared to be subgroup differences trends but differences in the subgroups were not significant overall.
7.22. Analysis.
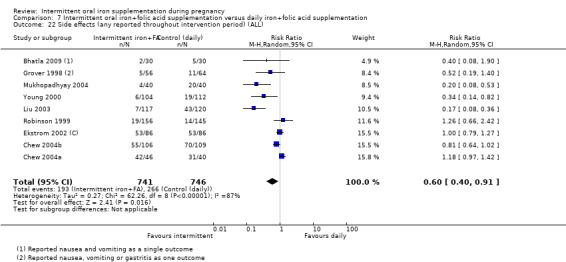
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 22 Side effects (any reported throughout intervention period) (ALL).
Severe anaemia at any time during 2nd or 3rd trimesters (Hb less than 70 g/L)
Six trials reported on severe anaemia at any time during second or third trimesters but there were no estimable data for this outcome (Analysis 7.27).
7.27. Analysis.
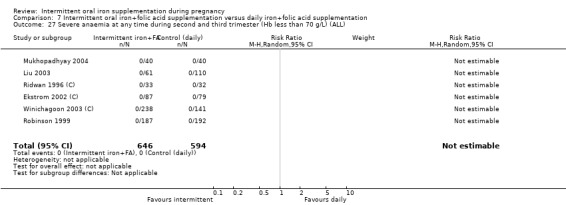
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 27 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL).
Other outcomes
No trials reported on the remaining maternal primary outcomes: clinical malaria or infection during pregnancy.
Secondary outcomes
Infant
Very low birthweight (less than 1500 g)
Five studies reported this outcome (Chew 2004b; Chew 2004a; Hanieh 2013 (C); Mukhopadhyay 2004; Winichagoon 2003 (C)) involving 1524 women. There was evidence of no significant differences between groups (average RR 0.19; 95% CI 0.01 to 4.04) (Analysis 7.34).
7.34. Analysis.
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 34 Very low birthweight (less than 1500 g) (ALL).
Very premature birth (less than 34 weeks' gestation)
One study reported this outcome (Mukhopadhyay 2004). There was one very premature birth in each group (seeAnalysis 7.35).
7.35. Analysis.
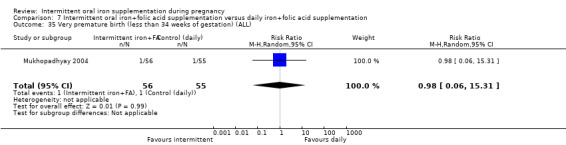
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 35 Very premature birth (less than 34 weeks of gestation) (ALL).
Infant Hb concentration within the first six months (in g/L, counting the last reported measured after birth within this period)
One study (Hanieh 2013 (C)) involving 518 women reported this outcome. There was evidence of no significant difference between the groups (average RR ‐0.50; 95% CI ‐2.44 to 1.44). (Analysis 7.36).
7.36. Analysis.
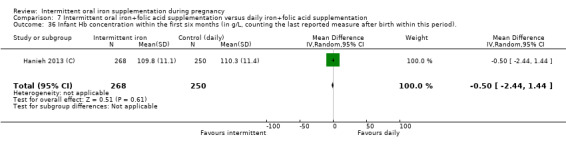
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 36 Infant Hb concentration within the first six months (in g/L, counting the last reported measure after birth within this period)..
Infant ferritin concentration within the first six months (in μg/L, counting the last reported measure after birth within this period)
One study (Winichagoon 2003 (C)) including 88 women reported this outcome. The data from this trial suggest that the infants of women receiving intermittent iron + folic acid supplementation have a higher concentration of serum ferritin at six months (MD 0.09 μg/L; 95% CI 0.05 μg/L to 0.13 μg/L) (Analysis 7.37), but no firm conclusions can be made given the scarcity of the data.
7.37. Analysis.
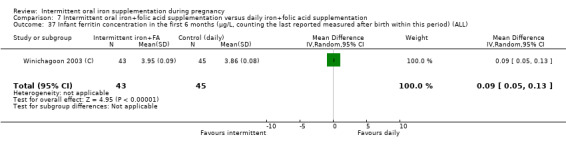
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 37 Infant ferritin concentration in the first 6 months (μg/L, counting the last reported measured after birth within this period) (ALL).
Development and motor skills
One study (Hanieh 2013 (C)) including 713 women reported this outcome. There was no evidence of significant difference between groups (MD 1.90; 95% CI 0.26 to 3.54) and there were no differences between subgroups (Analysis 7.38).
7.38. Analysis.
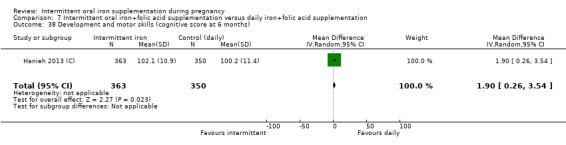
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 38 Development and motor skills (cognitive score at 6 months).
No trials reported on the remaining infant secondary outcomes: admission to special care unit.
Maternal
Maternal anaemia at or near term (Hb less than 110 g/L at 34 weeks' gestation or more)
This outcome was reported in five trials with 976 women (Chew 2004b; Chew 2004a; Liu 2003; Winichagoon 2003 (C); Zamani 2008); there was no evidence of significant differences between groups (average RR 1.24; 95% CI 0.97 to 1.59) (Analysis 7.43).
7.43. Analysis.
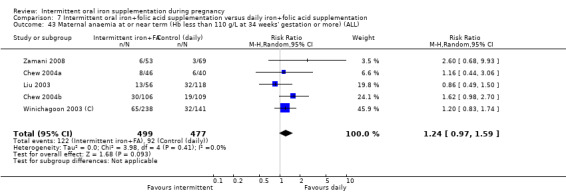
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 43 Maternal anaemia at or near term (Hb less than 110 g/L at 34 weeks' gestation or more) (ALL).
Maternal iron‐deficiency anaemia at or near term (Hb less than 110 g/L and at least one additional laboratory indicator at 34 weeks' gestation or more)
No evidence of significant differences was found between these groups of women in the one trial (Liu 2003) that reported this outcome (average RR 0.71; 95% CI 0.08 to 6.63) (Analysis 7.44).
7.44. Analysis.
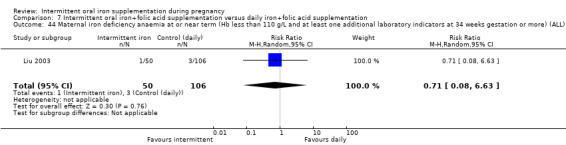
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 44 Maternal iron deficiency anaemia at or near term (Hb less than 110 g/L and at least one additional laboratory indicators at 34 weeks gestation or more) (ALL).
Maternal Hb concentration at or near term (in g/L, at 34 weeks' gestation or more)
We found no evidence of significant differences between these groups of women (MD ‐ 1.02 g/L; 95% CI ‐3.92 g/L to 1.88 g/L; five studies, 610 women) (Analysis 7.45). There was high heterogeneity for this outcome and results should be interpreted with caution (heterogeneity: Tau² = 7.74, I² = 76%, Chi² test for heterogeneity P = 0.002) (Analysis 7.45).
7.45. Analysis.
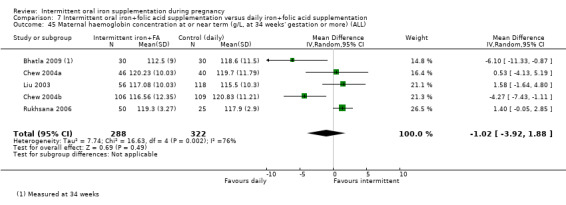
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 45 Maternal haemoglobin concentration at or near term (g/L, at 34 weeks' gestation or more) (ALL).
Maternal high Hb concentrations during second or third trimester (defined as Hb greater than 130 g/L)
Women receiving intermittent supplements were less likely to have Hb concentrations above 130 g/L during the second or third trimester compared to those receiving daily iron (average RR 0.56; 95% CI 0.37 to 0.84; 10 studies, 1942 women (Analysis 7.46)).
7.46. Analysis.
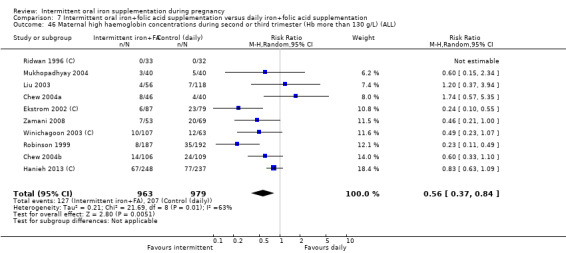
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 46 Maternal high haemoglobin concentrations during second or third trimester (Hb more than 130 g/L) (ALL).
Severe anaemia at term (Hb less than 70 g/Lat 37 weeks' gestation or more)
There was no estimable data for this outcome; in the trials reporting this outcome no women had severe anaemia in either group.
Other outcomes
Women receiving daily supplements were more likely to report nausea compared to those receiving intermittent supplements (average RR 0.60; 95% CI 0.37 to 0.97, seven trials, 1034 women) (Analysis 7.54).
7.54. Analysis.
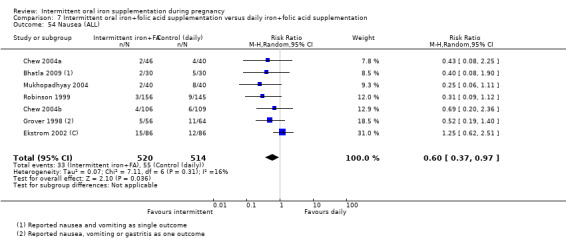
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 54 Nausea (ALL).
There was no evidence of significant differences between the comparison groups in the following secondary outcomes: antepartum haemorrhage (Analysis 7.51), severe anaemia at postpartum (Analysis 7.50), moderate anaemia at postpartum (Analysis 7.48), diarrhoea (Analysis 7.52), constipation (Analysis 7.53), heartburn (Analysis 7.55), vomiting (Analysis 7.56), placental abruption (Analysis 7.57), or premature rupture of membranes (Analysis 7.58). No trials reported on the remaining secondary outcomes: maternal iron deficiency at or near term.
7.51. Analysis.
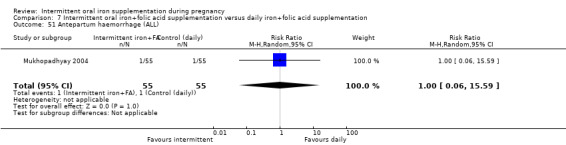
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 51 Antepartum haemorrhage (ALL).
7.50. Analysis.
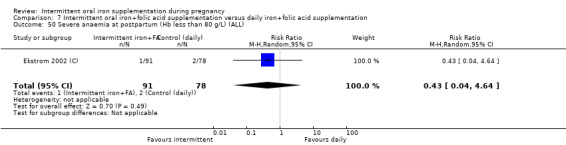
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 50 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL).
7.48. Analysis.
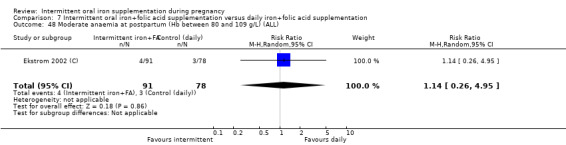
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 48 Moderate anaemia at postpartum (Hb between 80 and 109 g/L) (ALL).
7.52. Analysis.
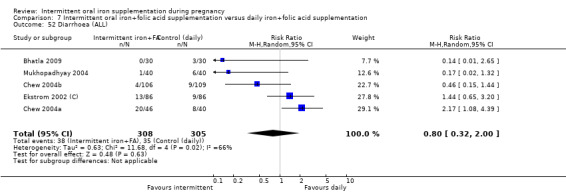
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 52 Diarrhoea (ALL).
7.53. Analysis.
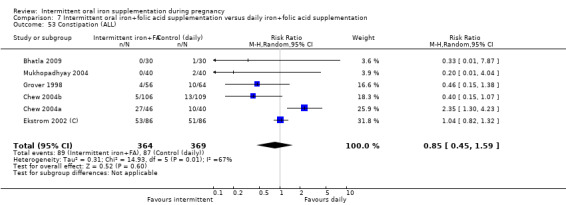
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 53 Constipation (ALL).
7.55. Analysis.
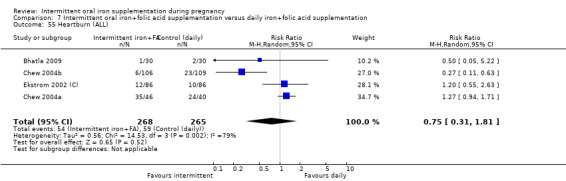
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 55 Heartburn (ALL).
7.56. Analysis.
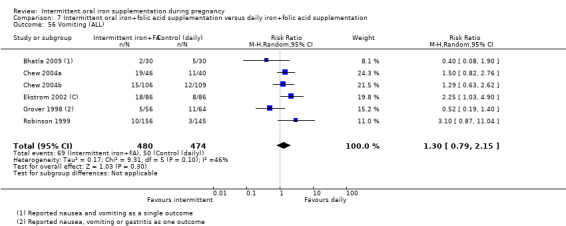
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 56 Vomiting (ALL).
7.57. Analysis.
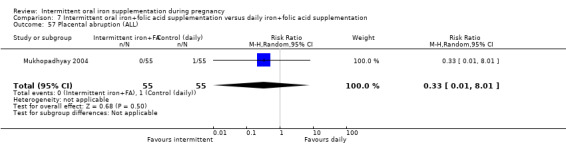
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 57 Placental abruption (ALL).
7.58. Analysis.
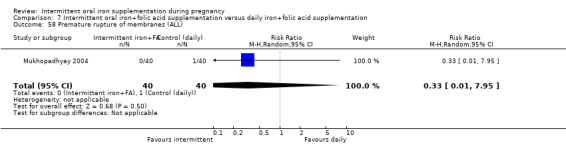
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 58 Premature rupture of membranes (ALL).
(8) Intermittent oral iron + vitamins and minerals supplementation compared with daily oral iron + vitamins and minerals supplementation (three studies: 312 women)
One trial with 120 women (116 followed up), one trial with 92 participants and one with 100 women (89 followed up) contributed to this comparison (Casanueva 2006; Goonewardene 2001; Singh 2011).
Primary outcomes
Infant outcomes
Results were not reported for any of the review's infant primary outcomes.
Maternal outcomes
Side effects (any reported throughout intervention period)
Only one study reported on this outcome (Singh 2011) and found that women receiving supplements intermittently presented side effects less frequently (Analysis 8.10).
8.10. Analysis.
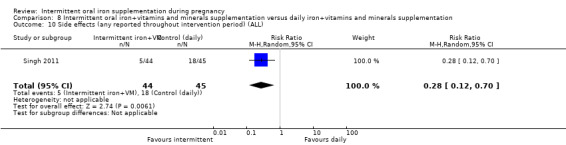
Comparison 8 Intermittent oral iron+vitamins and minerals supplementation versus daily iron+vitamins and minerals supplementation, Outcome 10 Side effects (any reported throughout intervention period) (ALL).
Maternal anaemia at term or near term (Hb less than 110 g/L at 37 weeks' gestation or more)
Maternal anaemia at or near term was reported in two trials contributing data to this comparison (Casanueva 2006; Goonewardene 2001); more women receiving intermittent supplements were anaemic at term or near term compared with those receiving daily supplementation (average RR 4.62; 95% CI 2.18 to 9.76; 208 women) (Analysis 8.6; Analysis 8.14).
8.6. Analysis.
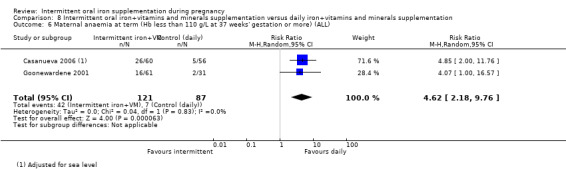
Comparison 8 Intermittent oral iron+vitamins and minerals supplementation versus daily iron+vitamins and minerals supplementation, Outcome 6 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) (ALL).
8.14. Analysis.
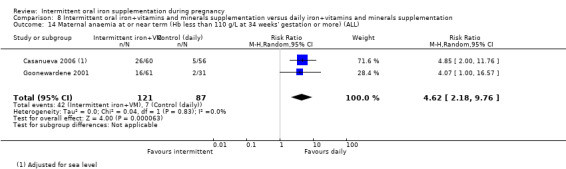
Comparison 8 Intermittent oral iron+vitamins and minerals supplementation versus daily iron+vitamins and minerals supplementation, Outcome 14 Maternal anaemia at or near term (Hb less than 110 g/L at 34 weeks' gestation or more) (ALL).
Other outcomes
The trials did not report results for the remaining maternal primary outcomes: infection; all‐cause mortality, or malaria.
Secondary outcomes
Infant outcomes
Very premature birth (less than 34 weeks' gestation)
There were no estimable data for this outcome.
Other infant secondary outcomes
The trial did not report on the remaining infant secondary outcomes.
Maternal
Maternal iron deficiency at or near term (as defined by trialists, based on any indicator of iron status at 37 weeks' gestation or more)
This outcome was reported in two trials and there was no clear evidence of differences between groups (average RR 2.45; 95% CI 0.70 to 8.66; 208 women) (Analysis 8.15).
8.15. Analysis.
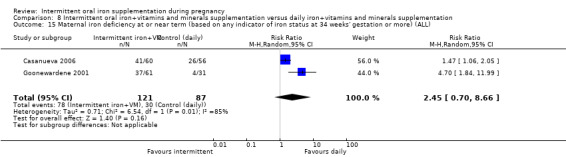
Comparison 8 Intermittent oral iron+vitamins and minerals supplementation versus daily iron+vitamins and minerals supplementation, Outcome 15 Maternal iron deficiency at or near term (based on any indicator of iron status at 34 weeks' gestation or more) (ALL).
Mean Hb concentrations at or near term
Women receiving daily iron had higher mean Hb concentrations at term in the single study reporting this outcome (MD ‐9.50 g/L; 95% CI ‐13.19 g/L to ‐5.81 g/L) (Analysis 8.16).
8.16. Analysis.
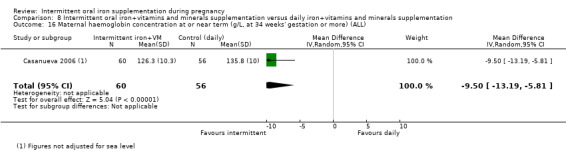
Comparison 8 Intermittent oral iron+vitamins and minerals supplementation versus daily iron+vitamins and minerals supplementation, Outcome 16 Maternal haemoglobin concentration at or near term (g/L, at 34 weeks' gestation or more) (ALL).
High Hb concentrations any time during second or third trimesters (defined as Hb greater than 130 g/L)
There was no significant evidence of differences between women receiving intermittent versus daily supplementation for this outcome (Analysis 8.17).
8.17. Analysis.
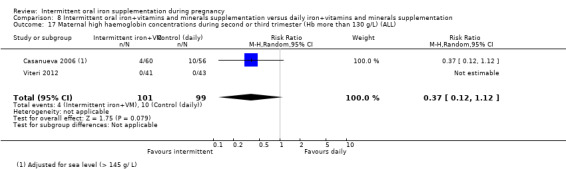
Comparison 8 Intermittent oral iron+vitamins and minerals supplementation versus daily iron+vitamins and minerals supplementation, Outcome 17 Maternal high haemoglobin concentrations during second or third trimester (Hb more than 130 g/L) (ALL).
Severe anaemia at term (Hb less than 70 g/L at 37 weeks' gestation or more)
There were no estimable data for this outcome; no women had severe anaemia in either group (Analysis 8.18).
8.18. Analysis.
Comparison 8 Intermittent oral iron+vitamins and minerals supplementation versus daily iron+vitamins and minerals supplementation, Outcome 18 Severe anaemia at term (Hb less than 70 g/L at 37 weeks' gestation or more) (ALL).
Severe (Hb less than 70 g/L) or moderate (Hb between 70 and 99 g/L) anaemia at any time during the second or third trimester
The effect of the intervention on severe or moderate anaemia at any time during second or third trimesters could not be estimated (Analysis 8.19).
8.19. Analysis.
Comparison 8 Intermittent oral iron+vitamins and minerals supplementation versus daily iron+vitamins and minerals supplementation, Outcome 19 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL).
Other outcomes
The trial did not report on the remaining maternal secondary outcomes.
Discussion
Summary of main results
None of the 27 studies included in this review compared the effects of intermittent iron supplementation with the effects of no iron supplementation, very likely because all the studies involving intermittent supplementation were carried out in developing countries whose legislatures require all pregnant women to be given iron supplements. Only 21 studies with 5490 pregnant women contributed data to the results.
Of the studies contributing data to the review three studies provided iron alone, 14 iron + folic acid and four more iron + multiple vitamins and minerals. Their methodological quality was mixed and most had high levels of attrition. Overall, there was no clear evidence of differences between groups for infant primary outcomes: low birthweight, infant birthweight or premature birth. Only one of the studies reported neonatal death and none reported congenital anomalies, anaemia during the first six months of life, or iron deficiency during the first six months of life.
Regarding maternal outcomes, there was no clear evidence of differences between groups on anaemia at term and women receiving intermittent supplementation had less side effects than those receiving daily supplements. Women receiving intermittent supplements were also at lower risk of having high haemoglobin (Hb) concentrations (greater than 130 g/L) during the second or third trimester of pregnancy. There were no maternal deaths (six studies) or women with severe anaemia (six studies). None of the studies reported on iron deficiency at term, or infections during pregnancy. One study found no significant differences in iron‐deficiency anaemia between women receiving intermittent or daily iron + folic acid supplementation.
There was no clear effects of women's anaemia status at the start of supplementation; higher and lower weekly doses of iron; and the malarial status of the region in which the trials were conducted on the results of the review.
Overall completeness and applicability of evidence
This review included 27 randomised controlled trials conducted since 1996 in a variety of settings, reflecting the growing interest in finding alternatives to daily iron supplementation. We included data from 21 trials. Their results suggest that women receiving iron supplements have similar pregnancy and birth outcomes as those women receiving supplements daily. Although the statistical non difference between regimens was a common pattern across most of the outcomes, the confidence on the applicability and generalisability of these findings may be limited by the reduced number of trials reporting on primary outcomes and probably by the small sample size of many of the trials. It seems wise to limit this intervention to women at low risk of developing anaemia while the body of evidence builds up.
Side effects are a clear drawback to daily supplementation with iron. The results of this review suggest that intermittent iron or iron plus folic acid doses are associated with a lower risk of side effects when compared to the daily regimen. All the trials evaluated in this review provided in a single intermittent dose more than 45 mg of elemental iron, which is considered the upper tolerable limit per day by the Institute of Medicine based on side effects (IOM 2001), although the same institution recognises that the effects of intermittent dosing on gastrointestinal side effects has not been studied adequately. From our results, it is clear that women receiving intermittent supplementation still reported side effects, however, they may had been perceived less frequently as they were only experienced once or twice per week and not on a daily basis. There is no stated upper limit for intermittent iron supplementation, but it has been suggested that 2400 mg elemental iron may be sufficient to produce a maximal Hb response over a period of 12 weeks (Ekstrom 2002 (C)). In this review, most of the trials provided 120 mg of elemental iron once a week and started the supplementation during the second trimester of pregnancy; this dose and duration seem tolerable and feasible to implement during pregnancy in various settings.
Quality of the evidence
The overall quality of the evidence in this review is relatively poor, with many studies being at high risk of bias. For our primary outcomes evidence was graded as low or very low quality; evidence was mainly downgraded due to the risk of bias in studies contributing data and imprecision of effect estimates.
In most of the included trials the methods used to conceal allocation were not described. Blinding of women, care providers and outcome assessors was not generally attempted, although in some studies, technical staff carrying out laboratory investigations were reported to be unaware of group allocation. While for some outcomes (e.g. infant birthweight), the lack of blinding may have been unlikely to have had any impact on results, for others (e.g. maternal reports of side effects to care providers), lack of blinding may represent a potentially serious source of bias. Attrition was a problem in most of these studies where women were followed up over time, and drop‐out rates were high.
Women were frequently excluded from studies or withdrew for outcome‐related reasons. Women who became anaemic were withdrawn from studies to receive treatment, and it was not always clear that loss for this reason was balanced across groups. Similarly, women may have withdrawn from studies, or may have had poor compliance with supplementation regimens (and were sometimes excluded for this reason) because they experienced side effects; again, it was not always clear that loss due to side effects was balanced across groups. Loss of women to follow‐up may mean that the possible advantages and disadvantages of intermittent regimens are less apparent, and more generally high attrition and other sources of bias mean that the results of the review are more difficult to interpret.
Overall assessments of the quality of the evidence for primary outcomes for infants and mothers are presented in Table 1 and Table 2. The quality of the evidence for low birthweight, birthweight and premature birth was assessed as low, and for maternal anaemia at term, iron deficiency at term, and side effects was very low.
Potential biases in the review process
We were aware of the possibility of introducing bias at every stage of the reviewing process. In this updated review, we tried to minimise bias in a number of ways; the eligibility for inclusion of the trials was assessed in duplicate, and two review authors carried out data extraction and assessed risk of bias. Each worked independently. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements. Further, the process of reviewing research studies is known to be affected by prior beliefs and attitudes. It is difficult to control for this type of bias in the reviewing process.
While we attempted to be as inclusive as possible in the search strategy, the literature identified was predominantly written in English and published in North American and European journals. Although we did attempt to assess reporting bias, constraints of time meant that this assessment largely relied on information available in the published trial reports and thus, reporting bias was not usually apparent.
In this updated version of the review we have included 'Summary of findings' tables. Assessing the quality of the evidence relating to specific outcomes is a difficult process, but we attempted to produce the table using a transparent process. Two review authors independently assessed the evidence for each outcome for each quality domain and discussed any disagreements.
Agreements and disagreements with other studies or reviews
Intermittent iron supplementation trials have been described by some authors as an attempt to improve existing supplementation programmes. These same authors have cautioned, however, against changing policies until the results of pending studies were published and scrutinised by the international community and found to be efficacious, proving substantial improvement over current practices (Galloway 1996).
Intermittent iron supplementation regimens were evaluated in the late 1990s as an alternative to daily regimens in various age groups as a way to improve the delivering of supplements (Beaton 1999). There has been extensive controversy in the scientific community about the biological rationale related to absorption of iron provided daily and the potential benefits of the intermittent regimen in terms of efficacy and effectiveness. The review of the literature conducted by Beaton 1999 and referred by the authors as an "inter‐project analysis of experience" and not a selection and tabulation of acceptable studies to estimate a weighted mean result, identified and collected information on many of the completed and ongoing projects to establish whether or not intermittent iron dosing was efficacious in improving and maintaining iron status in various target groups. For pregnant women, relative efficacy was examined but many of the trials were unpublished or ongoing at the time of the review, which did not allow the authors to draw any clear conclusions. Authors mentioned that in their judgement it would be unwise, and perhaps detrimental, to replace existing daily supplementation approaches with weekly supplementation during pregnancy. Interestingly, the authors questioned the use of anaemia as the main outcome being sought to measure the efficacy and effectiveness of the weekly iron supplementation regimen instead of other functional outcomes.
A systematic review comparing the effects of preventive prenatal oral iron or iron + folic acid supplements among pregnant women versus no treatment/placebo in both developed and developing countries (Yakoob 2011) included four randomised trials that evaluated the efficacy of intermittent supplementation only on haematological outcomes. The included trials are also included in this review (Casanueva 2006; Chew 2004b; Chew 2004a; Liu 2003) and their results are in agreement with our review.
The present systematic review is the most comprehensive summary of the evidence assessing the benefits and harms of intermittent regimens on haematological and pregnancy outcomes. The findings suggest that intermittent iron + folic acid regimens produce similar maternal and infant outcomes at birth as daily supplementation but are associated with fewer side effects. Women receiving daily supplements had increased risk of developing high levels of Hb in mid and late pregnancy but were less likely to present mild anaemia near term. Although the evidence is limited and the quality of the trials was low or very low, intermittent may be a feasible alternative to daily iron supplementation among those pregnant women who are not anaemic and have adequate antenatal care.
Authors' conclusions
Implications for practice.
Available data indicate that in comparison with women receiving daily supplements, women receiving iron intermittently:
had a similar risk of anaemia at term;
had similar haemoglobin (Hb) concentrations at term;
had a similar risk of premature infants;
had the same risk of delivering low birthweight infants
had fewer side effects;
had a reduced risk of high Hb concentrations throughout pregnancy.
Given all the above, intermittent supplementation with iron could be considered as a feasible strategy to prevent gestational anaemia.
In order to improve the success of this intervention in public health, it is important to encourage continued monitoring of Hb concentrations during pregnancy and establish logistic procedures that facilitate and improve accessibility to supplements and foster compliance.
Implications for research.
On the basis of the results of this review, we suggest that researchers investigating the effects of intermittent supplementation with iron, iron + folic acid, and iron + vitamins and minerals among pregnant women attempt to undertake the following:
establish effective and safe doses of intermittent supplemental iron with folic acid, and possibly other nutrients, for pregnant women considering gestational age and initial Hb concentration. It is advisable that trials evaluate newborn’s and infant’s health and development for six months or more, and maternal health prior to conception, during gestation and for six months postpartum;
find effective, safe, and affordable iron compounds and dosages that can be used with different diets, producing fewer undesirable effects than current iron supplements for use in intermittent public health supplementation programmes prior to, during and after pregnancy;
assess the safety of intermittent iron supplementation regimens for pregnant women on malaria outcomes;
determine the impact of genetic structure of populations on the effectiveness of the supplementation programmes (e.g. thalassemias);
determine health implications of haemoconcentration during pregnancy and the mechanisms in play of undesirable health and pregnancy outcomes in relation to iron, folate and Hb levels, and how to control them (e.g. increasing intake of antioxidant nutrients, prevention of pre‐eclampsia, measurement of placental perfusion);
understand the factors associated with non compliance to intermittent iron supplementation regimens and the causes of attrition.
What's new
| Date | Event | Description |
|---|---|---|
| 31 July 2015 | New search has been performed | Search updated. |
| 31 July 2015 | New citation required but conclusions have not changed | 27 trials included overall, with 21 trials contributing data. Conclusions remain the same. |
Notes
This review updates part of Peña‐Rosas 2012. Specific changes to the previous version are described in the section Differences between protocol and review above.
Acknowledgements
We would like to thank the trial authors who have contributed additional data for this review and the staff at the editorial office of the Cochrane Pregnancy and Childbirth Group in Liverpool for their support in the preparation of this review and, in particular, Professor Zarko Alfirevic.
We would like to thank Fernando Viteri for his contribution to co‐writing the initial protocol and first two versions of the review, for providing technical support and guidance on earlier versions, and for commenting on the current update (2015).
The World Health Organization and Luz Maria De‐Regil, Heber Gomez Malave, and Therese Dowswell retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms used for additional author searching
Review authors searched the WHO International Clinical Trials Registry Platform (ICTRP) for any ongoing or planned trials on 31 July 2015 using the terms "iron supplementation and pregnancy"; "weekly iron and pregnancy"; "intermittent iron and pregnancy"; "iron and pregnancy"; "iron supplements and pregnancy"; "weekly supplements and pregnancy"; "weekly supplementation and pregnancy"; and "anaemia and pregnancy". Duplicates were removed.
Data and analyses
Comparison 2. Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Low birthweight (less than 2500 g) (ALL) | 8 | 1898 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.55, 1.22] |
| 2 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by weekly dose of iron | 8 | 1898 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.55, 1.22] |
| 2.1 Low weekly dose of iron in the weekly group (120 mg elemental iron or less per week) | 3 | 1184 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.18] |
| 2.2 High weekly dose of iron in the weekly group (more than 120 mg elemental iron per week) | 6 | 714 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.60, 1.57] |
| 3 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by anaemia status | 8 | 1898 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.55, 1.22] |
| 3.1 Non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation | 3 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.56, 2.24] |
| 3.2 Unspecified/mixed anaemia status at start of supplementation | 5 | 1557 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.44, 1.15] |
| 4 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by gestational age | 8 | 1898 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.55, 1.22] |
| 4.1 Early gestational age (supplementation started before 20 weeks' gestation or prior to pregnancy) | 5 | 1503 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.54, 1.40] |
| 4.2 Late gestational age (supplementation started at 20 weeks of gestation or later); | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Unspecified gestational age or mixed gestational ages at the start of supplementation | 3 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.35, 1.47] |
| 5 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by intermittent regimen | 8 | 1968 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.56, 1.22] |
| 5.1 Provision of iron once a week | 7 | 1709 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.58, 1.34] |
| 5.2 Other intermittent regimens | 2 | 259 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.14, 1.55] |
| 6 Birthweight (g) (ALL) | 9 | 1939 | Mean Difference (IV, Random, 95% CI) | 5.13 [‐29.46, 39.72] |
| 7 Birthweight (g) SUBGROUP ANALYSIS by gestational age | 9 | 1939 | Mean Difference (IV, Random, 95% CI) | 5.13 [‐29.46, 39.72] |
| 7.1 Early gestational age (supplementation started before 20 weeks' gestation or prior to pregnancy) | 5 | 1503 | Mean Difference (IV, Random, 95% CI) | 11.40 [‐29.03, 51.83] |
| 7.2 Unspecified gestational age or mixed gestational ages at the start of supplementation | 4 | 436 | Mean Difference (IV, Random, 95% CI) | ‐11.99 [‐78.80, 54.82] |
| 8 Birthweight (g) SUBGROUP ANALYSIS by anaemia status | 9 | 1939 | Mean Difference (IV, Random, 95% CI) | 5.13 [‐29.46, 39.72] |
| 8.1 Non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation | 3 | 341 | Mean Difference (IV, Random, 95% CI) | ‐14.45 [‐100.14, 71.25] |
| 8.2 Unspecified/mixed anaemia status at start of supplementation | 6 | 1598 | Mean Difference (IV, Random, 95% CI) | 8.94 [‐28.86, 46.75] |
| 9 Birthweight (g) SUBGROUP ANALYSIS by weekly dose of iron | 9 | 1939 | Mean Difference (IV, Random, 95% CI) | 5.16 [‐29.42, 39.74] |
| 9.1 Low weekly dose of iron in the weekly group (120 mg elemental iron or less per week) | 5 | 1459 | Mean Difference (IV, Random, 95% CI) | 5.30 [‐37.61, 48.21] |
| 9.2 High weekly dose of iron in the weekly group (more than 120 mg elemental iron per week) | 5 | 480 | Mean Difference (IV, Random, 95% CI) | 4.90 [‐53.51, 63.31] |
| 10 Birthweight (g) SUBGROUP ANALYSIS by intermittent regimen | 9 | 2009 | Mean Difference (IV, Random, 95% CI) | ‐1.46 [‐35.54, 32.61] |
| 10.1 Provision of iron once a week | 7 | 1709 | Mean Difference (IV, Random, 95% CI) | 1.62 [‐37.56, 40.80] |
| 10.2 Other intermittent regimens | 3 | 300 | Mean Difference (IV, Random, 95% CI) | ‐14.79 [‐88.27, 58.69] |
| 11 Premature birth (less than 37 weeks of gestation) (ALL) | 5 | 1177 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.76, 1.39] |
| 12 Premature birth (less than 37 weeks' gestation) SUBGROUP ANALYSIS by gestational age | 5 | 1177 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.76, 1.39] |
| 12.1 Early gestational age (supplementation started before 20 weeks' gestation or prior to pregnancy) | 4 | 1136 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.77, 1.41] |
| 12.2 Unspecified gestational age or mixed gestational ages at the start of supplementation | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.02, 8.96] |
| 13 Premature birth (less than 37 weeks' gestation) SUBGROUP ANALYSIS by anaemia status | 5 | 1177 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.76, 1.39] |
| 13.1 Non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation | 3 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.82, 5.25] |
| 13.2 Unspecified/mixed anaemia status at start of supplementation | 2 | 836 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.69, 1.30] |
| 14 Premature birth (less than 37 weeks' gestation) SUBGROUP ANALYSIS by weekly dose of iron | 5 | 1177 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.76, 1.39] |
| 14.1 Low weekly dose of iron in the weekly group (120 mg elemental iron or less per week) | 3 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.71, 1.34] |
| 14.2 High weekly dose of iron in the weekly group (more than 120 mg elemental iron per week) | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.64, 5.06] |
| 15 Premature birth (less than 37 weeks' gestation) SUBGROUP ANALYSIS by intermittent regimen | 5 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.79, 1.43] |
| 15.1 Provision of iron once a week | 4 | 1067 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.75, 1.58] |
| 15.2 Other intermittent regimens | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.34, 11.85] |
| 16 Neonatal death (within 28 days after delivery) (ALL) | 1 | 795 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.04, 5.42] |
| 17 Congenital anomalies (including neural tube defects) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) (ALL) | 4 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.80] |
| 19 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by gestational age | 4 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.80] |
| 19.1 Early gestational age (supplementation started before 20 weeks' gestation or prior to pregnancy) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 3.77 [0.20, 71.87] |
| 19.2 Late gestational age (supplementation started at 20 weeks of gestation or later) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Unspecified gestational age or mixed gestational ages at the start of supplementation | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.83] |
| 20 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by anaemia status | 4 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.80] |
| 20.1 Non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 3.77 [0.20, 71.87] |
| 20.2 Unspecified/mixed anaemia status at start of supplementation | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.83] |
| 21 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by weekly dose of iron | 4 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.80] |
| 21.1 Low weekly dose of iron in the weekly group (120 mg elemental iron or less per week) | 2 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.52, 1.56] |
| 21.2 High weekly dose of iron in the weekly group (more than 120 mg elemental iron per week) | 2 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.96, 2.37] |
| 22 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by intermittent regimen | 4 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.78, 1.99] |
| 22.1 Provision of iron once a week | 4 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.78, 1.99] |
| 22.2 Other intermittent regimens | 1 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Maternal iron deficiency at term (based on any indicator of iron status at 37 weeks' gestation or more) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Maternal iron deficiency anaemia at term (Hb less than 110 g/L and at least one additional laboratory indicators at 37 weeks gestation or more) (ALL) | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.08, 6.63] |
| 25 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Side effects (any reported throughout intervention period) (ALL) | 11 | 1777 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.84] |
| 27 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by gestational age | 11 | 1777 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.84] |
| 27.1 Early gestational age (supplementation started before 20 weeks' gestation or prior to pregnancy) | 3 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.11, 0.56] |
| 27.2 Late gestational age (supplementation started at 20 weeks of gestation or later) | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.79, 1.27] |
| 27.3 Unspecified gestational age or mixed gestational ages at the start of supplementation | 7 | 1264 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.32, 0.97] |
| 28 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by anaemia status | 11 | 1777 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.84] |
| 28.1 Non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation | 4 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.14, 0.48] |
| 28.2 Unspecified/mixed anaemia status at start of supplementation | 7 | 1347 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.05] |
| 29 Side effects (any reported throughout intervention period)SUBGROUP ANALYSIS by weekly dose of iron | 11 | 1777 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.81] |
| 29.1 Low weekly dose of iron in the weekly group (120 mg elemental iron or less per week) | 6 | 1101 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.32, 1.10] |
| 29.2 High weekly dose of iron in the weekly group (more than 120 mg elemental iron per week) | 6 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.23, 0.90] |
| 30 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by intermittent regimen | 11 | 1978 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.84] |
| 30.1 Provision of iron once a week | 10 | 1657 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.86] |
| 30.2 Other intermittent regimens | 2 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.19, 1.40] |
| 31 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL) | 6 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Severe anaemia at any time during the 2nd and 3rd trimesters SUBGROUP ANALYSIS by gestational age | 6 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.1 Early gestational age (supplementation started before 20 weeks' gestation or prior to pregnancy) | 2 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Unspecified gestational age or mixed gestational ages at the start of supplementation | 4 | 781 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Severe anaemia at any time during the 2nd and 3rd trimesters SUBGROUP ANALYSIS by anaemia status | 6 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.1 Non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Unspecified/mixed anaemia status at start of supplementation | 5 | 1160 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Severe anaemia at any time during the 2nd and 3rd trimesters SUBGROUP ANALYSIS by weekly dose of iron | 6 | 1381 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.1 Low weekly dose of iron in the weekly group (120 mg elemental iron or less per week) | 5 | 1049 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.2 High weekly dose of iron in the weekly group (more than 120 mg elemental iron per week) | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Severe anaemia at any time during the 2nd and 3rd trimesters SUBGROUP ANALYSIS by intermittent regimen | 6 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 Provision of iron once a week | 6 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Other intermittent regimens | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Maternal clinical malaria | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Maternal infection during pregnancy (including urinary tract infections and others) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Very low birthweight (less than 1500 g) (ALL) | 5 | 1524 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 4.04] |
| 39 Very premature birth (less than 34 weeks of gestation) (ALL) | 2 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.06, 15.31] |
| 40 Infant Hb concentration within the first six months (in g/L, counting the last reported measure after birth within this period). | 1 | 518 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐2.44, 1.44] |
| 41 Infant ferritin concentration in the first 6 months (μg/L, counting the last reported measure after birth within this period) (ALL) | 1 | 88 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.05, 0.13] |
| 42 Development and motor skills (cognitive score at 6 months) | 1 | 713 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.26, 3.54] |
| 43 Development and motor skills (language at 6 months) | 1 | 640 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐86.26, 87.86] |
| 44 Development and motor skills (motor at 6 months) | 1 | 709 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐1.64, 3.84] |
| 45 Development and motor skills (socioemotional at 6 months) | 1 | 532 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.21, 2.61] |
| 46 Development and motor skills (adaptive behaviour at 6 months) | 1 | 581 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.39, 1.79] |
| 47 Maternal anaemia at or near term (Hb less than 110 g/L at 34 weeks gestation or more) (ALL) | 8 | 1385 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.09, 2.53] |
| 48 Maternal iron deficiency at or near term (based on any indicator of iron status at 34 weeks' gestation or more) (ALL) | 3 | 587 | Risk Ratio (M‐H, Random, 95% CI) | 2.38 [1.30, 4.36] |
| 49 Maternal iron deficiency anaemia at or near term (Hb less than 110 g/L and at least one additional laboratory indicators at 34 weeks gestation or more) (ALL) | 2 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.65, 6.61] |
| 50 Maternal haemoglobin concentration at or near term (in g/L at 34 weeks' gestation or more) (ALL) | 8 | 1306 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐5.18, 0.04] |
| 51 Maternal high haemoglobin concentrations during second or third trimester (Hb more than 130 g/L) (ALL) | 15 | 2616 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.38, 0.74] |
| 52 Moderate anaemia at any time during second or third trimester (Hb between 70 and 99 g/L)(ALL) | 9 | 1291 | Risk Ratio (M‐H, Random, 95% CI) | 2.50 [0.84, 7.48] |
| 53 Severe anaemia at term (Hb less than 70 g/L at 37 weeks' gestation or more) (ALL) | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 54 Severe anaemia at or near term (Hb less than 70 g/L at 34 weeks' gestation or more) (ALL) | 6 | 1050 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 55 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL) | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.04, 4.64] |
| 56 Antepartum haemorrhage (ALL) | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.59] |
| 57 Diarrhoea (ALL) | 5 | 613 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.32, 2.00] |
| 58 Constipation (ALL) | 6 | 733 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.45, 1.59] |
| 59 Nausea (ALL) | 7 | 1034 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.37, 0.97] |
| 60 Heartburn (ALL) | 4 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.31, 1.81] |
| 61 Vomiting (ALL) | 6 | 954 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.79, 2.15] |
| 62 Placental abruption (ALL) | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.01] |
| 63 Premature rupture of membranes (ALL) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.95] |
2.2. Analysis.
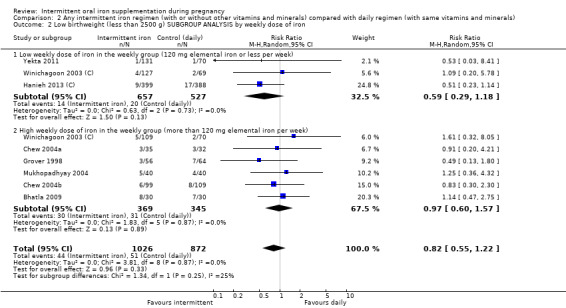
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 2 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by weekly dose of iron.
2.3. Analysis.
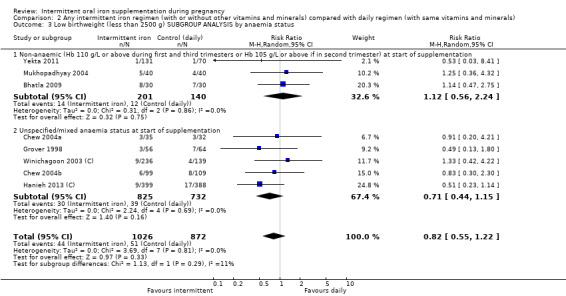
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 3 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by anaemia status.
2.4. Analysis.
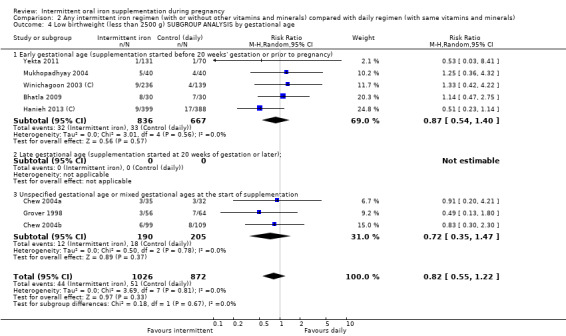
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 4 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by gestational age.
2.5. Analysis.
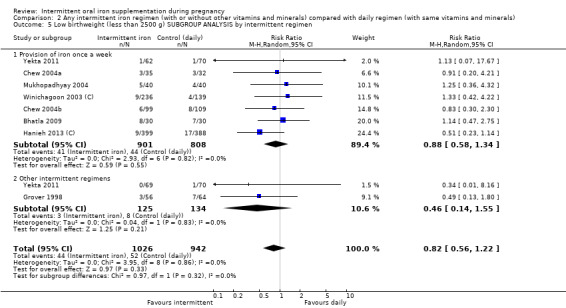
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 5 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by intermittent regimen.
2.7. Analysis.
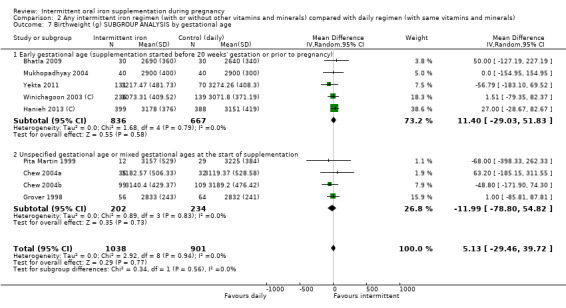
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 7 Birthweight (g) SUBGROUP ANALYSIS by gestational age.
2.8. Analysis.
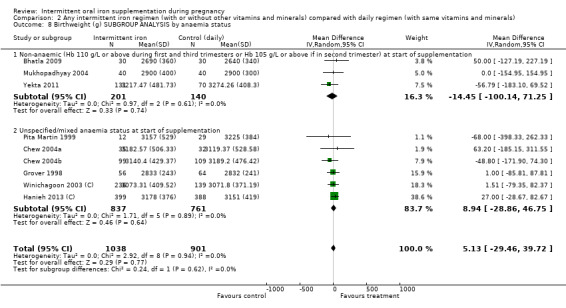
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 8 Birthweight (g) SUBGROUP ANALYSIS by anaemia status.
2.9. Analysis.
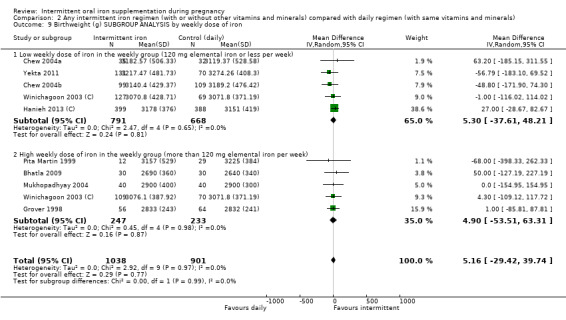
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 9 Birthweight (g) SUBGROUP ANALYSIS by weekly dose of iron.
2.10. Analysis.
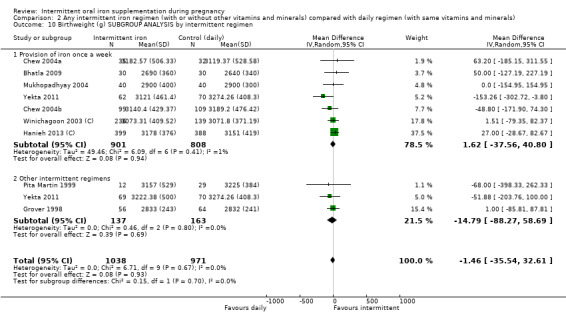
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 10 Birthweight (g) SUBGROUP ANALYSIS by intermittent regimen.
2.12. Analysis.
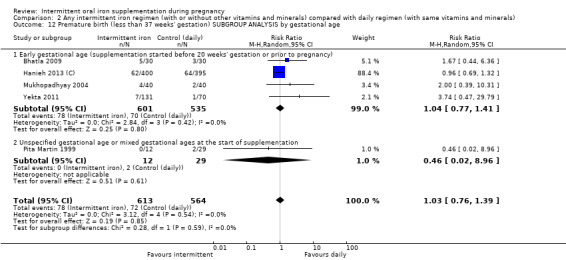
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 12 Premature birth (less than 37 weeks' gestation) SUBGROUP ANALYSIS by gestational age.
2.13. Analysis.
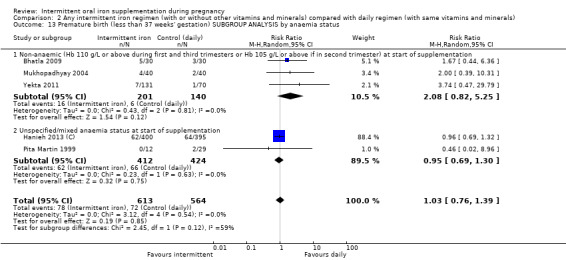
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 13 Premature birth (less than 37 weeks' gestation) SUBGROUP ANALYSIS by anaemia status.
2.14. Analysis.
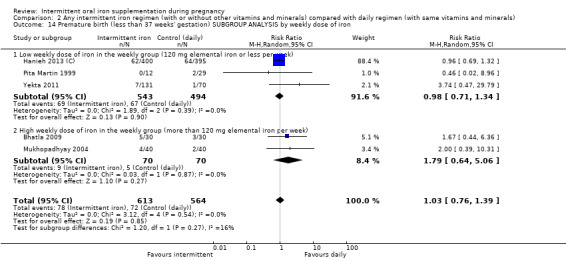
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 14 Premature birth (less than 37 weeks' gestation) SUBGROUP ANALYSIS by weekly dose of iron.
2.15. Analysis.
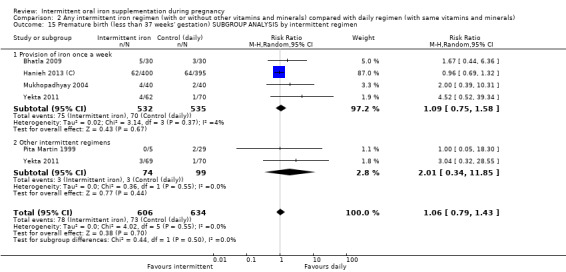
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 15 Premature birth (less than 37 weeks' gestation) SUBGROUP ANALYSIS by intermittent regimen.
2.19. Analysis.
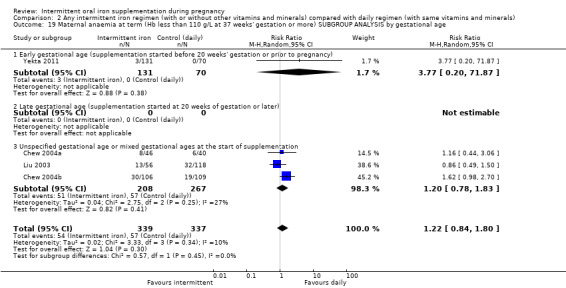
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 19 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by gestational age.
2.20. Analysis.
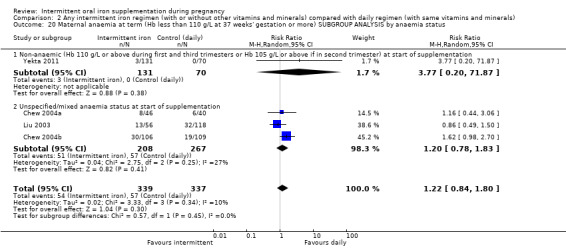
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 20 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by anaemia status.
2.21. Analysis.
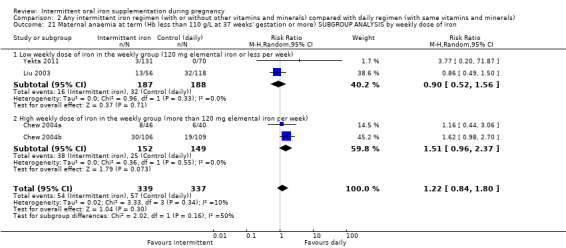
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 21 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by weekly dose of iron.
2.22. Analysis.
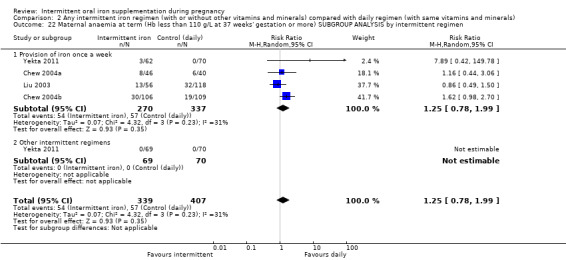
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 22 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by intermittent regimen.
2.27. Analysis.
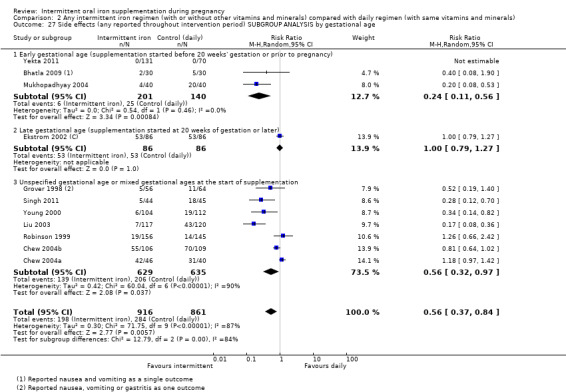
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 27 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by gestational age.
2.28. Analysis.
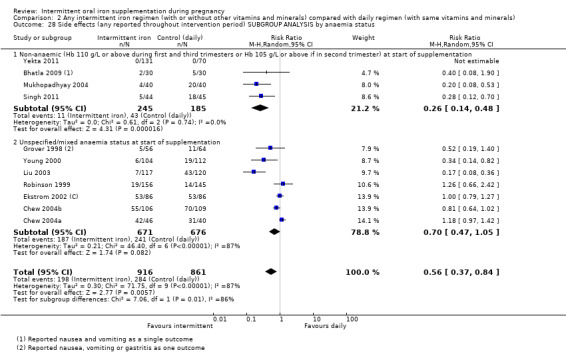
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 28 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by anaemia status.
2.29. Analysis.
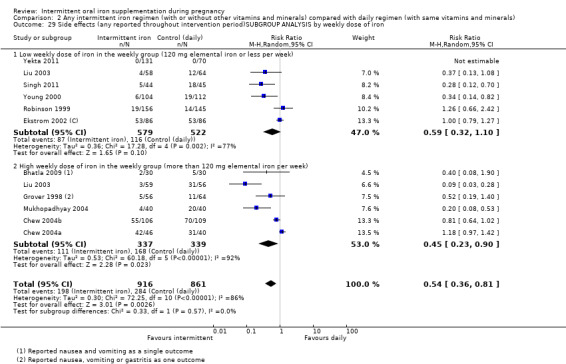
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 29 Side effects (any reported throughout intervention period)SUBGROUP ANALYSIS by weekly dose of iron.
2.30. Analysis.
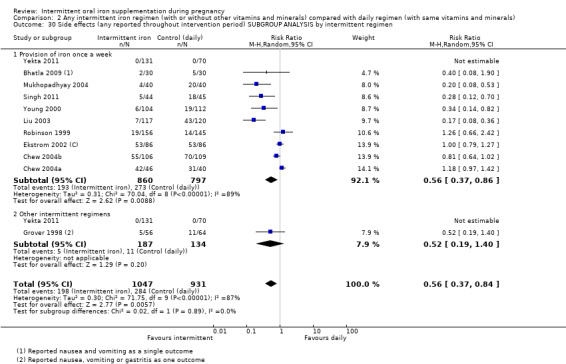
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 30 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by intermittent regimen.
2.31. Analysis.
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 31 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL).
2.32. Analysis.
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 32 Severe anaemia at any time during the 2nd and 3rd trimesters SUBGROUP ANALYSIS by gestational age.
2.33. Analysis.
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 33 Severe anaemia at any time during the 2nd and 3rd trimesters SUBGROUP ANALYSIS by anaemia status.
2.34. Analysis.
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 34 Severe anaemia at any time during the 2nd and 3rd trimesters SUBGROUP ANALYSIS by weekly dose of iron.
2.35. Analysis.
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 35 Severe anaemia at any time during the 2nd and 3rd trimesters SUBGROUP ANALYSIS by intermittent regimen.
2.42. Analysis.
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 42 Development and motor skills (cognitive score at 6 months).
2.43. Analysis.
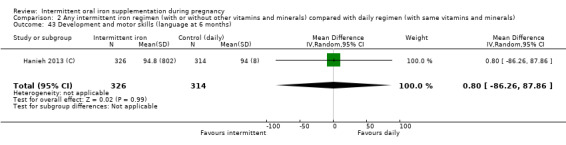
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 43 Development and motor skills (language at 6 months).
2.44. Analysis.
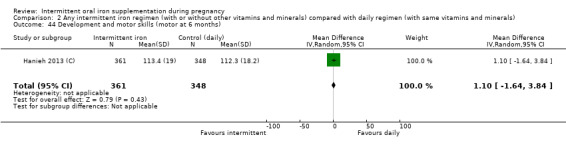
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 44 Development and motor skills (motor at 6 months).
2.45. Analysis.
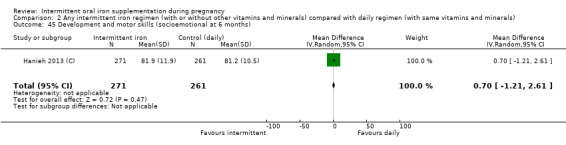
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 45 Development and motor skills (socioemotional at 6 months).
2.46. Analysis.
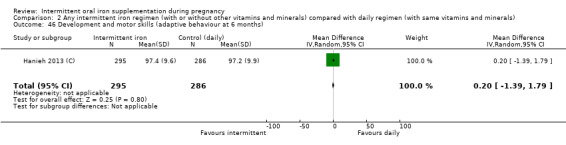
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 46 Development and motor skills (adaptive behaviour at 6 months).
2.52. Analysis.
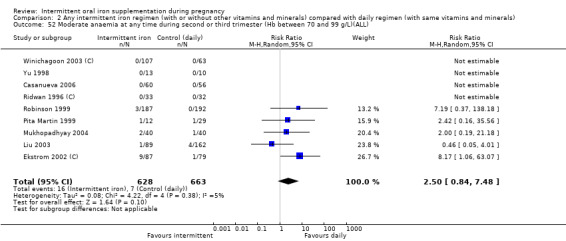
Comparison 2 Any intermittent iron regimen (with or without other vitamins and minerals) compared with daily regimen (with same vitamins and minerals), Outcome 52 Moderate anaemia at any time during second or third trimester (Hb between 70 and 99 g/L)(ALL).
Comparison 6. Intermittent oral iron alone supplementation versus daily iron alone supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Low birthweight (less than 2500 g) (ALL) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.03, 8.41] |
| 2 Birthweight (g) (ALL) | 2 | 242 | Mean Difference (IV, Random, 95% CI) | ‐58.22 [‐176.20, 59.76] |
| 3 Premature birth (less than 37 weeks of gestation) (ALL) | 2 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.24, 12.56] |
| 4 Neonatal death (within 28 days after delivery) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Congenital anomalies (including neural tube defects) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) (ALL) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 3.77 [0.20, 71.87] |
| 7 Maternal iron deficiency at term (based on any indicator of iron status at 37 weeks' gestation or more) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal iron deficiency anaemia at term (Hb less than 110 g/L and at least one additional laboratory indicator at 37 weeks' gestation or more)(ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Side effects (any reported throughout intervention period) (ALL) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL) | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal clinical malaria | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal infection during pregnancy (including urinary tract infections and others) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal anaemia at or near term (Hb less than 110 g/L at 34 weeks' gestation or more) (ALL) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 3.77 [0.20, 71.87] |
| 15 Maternal haemoglobin concentration at or near term (in g/L at 34 weeks' gestation or more) (ALL) | 2 | 276 | Mean Difference (IV, Random, 95% CI) | ‐2.58 [‐5.17, 0.01] |
| 16 Maternal high haemoglobin concentrations during second or third trimester (Hb more than 130 g/L) (ALL) | 3 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.26, 0.96] |
Comparison 7. Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Low birthweight (less than 2500 g) (ALL) | 7 | 1697 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.56, 1.24] |
| 2 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by gestational age | 7 | 1697 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.56, 1.24] |
| 2.1 Early gestational age (supplementation started before 20 weeks' gestation or prior to pregnancy) | 4 | 1302 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.55, 1.44] |
| 2.2 Unspecified gestational age or mixed gestational ages at the start of supplementation | 3 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.35, 1.47] |
| 3 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by anaemia status | 7 | 1697 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.56, 1.24] |
| 3.1 Non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.57, 2.41] |
| 3.2 Unspecified/mixed anaemia status at start of supplementation | 5 | 1557 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.44, 1.15] |
| 4 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by weekly dose of iron | 7 | 1697 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.56, 1.24] |
| 4.1 Low weekly dose of iron in the weekly group (120 mg elemental iron or less per week) | 2 | 984 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.22] |
| 4.2 High weekly dose of iron in the weekly group (more than 120 mg elemental iron per week) | 6 | 713 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.60, 1.57] |
| 5 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by intermittent regimen | 7 | 1697 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.56, 1.24] |
| 5.1 Provision of iron once a week | 6 | 1577 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.58, 1.34] |
| 5.2 Other intermittent regimens | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.13, 1.80] |
| 6 Birthweight (g) (ALL) | 7 | 1697 | Mean Difference (IV, Random, 95% CI) | 11.09 [‐25.09, 47.27] |
| 7 Birthweight (g) SUBGROUP ANALYSIS by gestational age | 7 | 1697 | Mean Difference (IV, Random, 95% CI) | 11.09 [‐25.09, 47.27] |
| 7.1 Early gestational age (supplementation started before 20 weeks' gestation or prior to pregnancy) | 4 | 1302 | Mean Difference (IV, Random, 95% CI) | 19.19 [‐23.49, 61.86] |
| 7.2 Unspecified gestational age or mixed gestational ages at the start of supplementation | 3 | 395 | Mean Difference (IV, Random, 95% CI) | ‐9.60 [‐77.82, 58.62] |
| 8 Birthweight (g) SUBGROUP ANALYSIS by anaemia status | 7 | 1697 | Mean Difference (IV, Random, 95% CI) | 11.09 [‐25.09, 47.27] |
| 8.1 Non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation | 2 | 140 | Mean Difference (IV, Random, 95% CI) | 21.67 [‐94.98, 138.31] |
| 8.2 Unspecified/mixed anaemia status at start of supplementation | 5 | 1557 | Mean Difference (IV, Random, 95% CI) | 9.96 [‐28.09, 48.02] |
| 9 Birthweight (g) SUBGROUP ANALYSIS by weekly dose of iron | 7 | 1697 | Mean Difference (IV, Random, 95% CI) | 11.12 [‐25.06, 47.29] |
| 9.1 Low weekly dose of iron in the weekly group (120 mg elemental iron or less per week) | 4 | 1259 | Mean Difference (IV, Random, 95% CI) | 13.39 [‐32.21, 58.98] |
| 9.2 High weekly dose of iron in the weekly group (more than 120 mg elemental iron per week) | 4 | 438 | Mean Difference (IV, Random, 95% CI) | 7.26 [‐52.16, 66.68] |
| 10 Birthweight (g) SUBGROUP ANALYSIS by intermittent regimen | 7 | 1697 | Mean Difference (IV, Random, 95% CI) | 11.09 [‐25.09, 47.27] |
| 10.1 Provision of iron once a week | 6 | 1577 | Mean Difference (IV, Random, 95% CI) | 13.21 [‐26.59, 53.01] |
| 10.2 Provision of iron by three times on non‐consecutive days or other intermittent regimens | 1 | 120 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐85.81, 87.81] |
| 11 Premature birth (less than 37 weeks of gestation) (ALL) | 3 | 935 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.74, 1.37] |
| 12 Neonatal death (within 28 days after delivery) (ALL) | 1 | 795 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.04, 5.42] |
| 13 Congenital anomalies (including neural tube defects) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) (ALL) | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.83] |
| 15 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by gestational age | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.83] |
| 15.1 Early gestational age (supplementation started before 20 weeks' gestation or prior to pregnancy) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Late gestational age (supplementation started at 20 weeks of gestation or later) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Unspecified gestational age or mixed gestational ages at the start of supplementation | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.83] |
| 16 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by anaemia status | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.83] |
| 16.1 Non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Unspecified/mixed anaemia status at start of supplementation | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.83] |
| 17 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by weekly dose of iron | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.83] |
| 17.1 Low weekly dose of iron in the weekly group (120 mg elemental iron or less per week) | 1 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.49, 1.50] |
| 17.2 High weekly dose of iron in the weekly group (more than 120 mg elemental iron per week) | 2 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.96, 2.37] |
| 18 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by intermittent regimen | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.83] |
| 18.1 Provision of iron once a week | 3 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.83] |
| 18.2 Other intermittent regimens | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal iron deficiency at term (based on any indicator of iron status at 37 weeks' gestation or more) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Maternal iron deficiency anaemia at term (Hb less than 110 g/L and at least one additional laboratory indicator at 37 weeks' gestation or more (ALL) | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.08, 6.63] |
| 21 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Side effects (any reported throughout intervention period) (ALL) | 9 | 1487 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.40, 0.91] |
| 23 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by gestational age | 9 | 1487 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.40, 0.91] |
| 23.1 Early gestational age (supplementation started before 20 weeks' gestation or prior to pregnancy) | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.11, 0.56] |
| 23.2 Late gestational age (supplementation started at 20 weeks of gestation or later) | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.79, 1.27] |
| 23.3 Unspecified gestational age or mixed gestational ages at the start of supplementation | 6 | 1175 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.36, 1.08] |
| 24 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by anaemia status | 9 | 1487 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.40, 0.91] |
| 24.1 Non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.11, 0.56] |
| 24.2 Unspecified/mixed anaemia status at start of supplementation | 7 | 1347 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.05] |
| 25 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by weekly dose of iron | 9 | 1487 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.39, 0.87] |
| 25.1 Low weekly dose of iron in the weekly group (120 mg elemental iron or less per week) | 4 | 812 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.40, 1.29] |
| 25.2 High weekly dose of iron in the weekly group (more than 120 mg elemental iron per week) | 6 | 675 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.23, 0.90] |
| 26 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by intermittent regimen | 9 | 1487 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.40, 0.91] |
| 26.1 Provision of iron once a week | 8 | 1367 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.40, 0.94] |
| 26.2 Other intermittent regimens | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.19, 1.40] |
| 27 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL) | 6 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Severe anaemia at any time during the 2nd and 3rd trimesters (Hb less than 70 g/L) SUBGROUP ANALYSIS by gestational age | 6 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.1 Early gestational age (supplementation started before 20 weeks' gestation or prior to pregnancy) | 2 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 Unspecified gestational age or mixed gestational ages at the start of supplementation | 4 | 781 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Severe anaemia at any time during the 2nd and 3rd trimesters (Hb less than 70 g/L) SUBGROUP ANALYSIS by anaemia status | 6 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.1 Non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 Unspecified/mixed anaemia status at start of supplementation | 5 | 1160 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Severe anaemia at any time during the 2nd and 3rd trimesters (Hb less than 70 g/L) SUBGROUP ANALYSIS by intermittent regimen | 6 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.1 Provision of iron once a week | 6 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 Other intermittent regimens | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Severe anaemia at any time during the 2nd and 3rd trimesters (Hb less than 70 g/L) SUBGROUP ANALYSIS by weekly dose of iron | 6 | 1381 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.1 Low weekly dose of iron in the weekly group (120 mg elemental iron or less per week) | 5 | 1049 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 High weekly dose of iron in the weekly group (more than 120 mg elemental iron per week) | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Maternal clinical malaria | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Maternal infection during pregnancy (including urinary tract infections or others) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Very low birthweight (less than 1500 g) (ALL) | 5 | 1524 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 4.04] |
| 35 Very premature birth (less than 34 weeks of gestation) (ALL) | 1 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.06, 15.31] |
| 36 Infant Hb concentration within the first six months (in g/L, counting the last reported measure after birth within this period). | 1 | 518 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐2.44, 1.44] |
| 37 Infant ferritin concentration in the first 6 months (μg/L, counting the last reported measured after birth within this period) (ALL) | 1 | 88 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.05, 0.13] |
| 38 Development and motor skills (cognitive score at 6 months) | 1 | 713 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.26, 3.54] |
| 39 Development and motor skills (language at 6 months) | 1 | 640 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐86.26, 87.86] |
| 40 Development and motor skills (motor at 6 months) | 1 | 709 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐1.64, 3.84] |
| 41 Development and motor skills (socioemotional at 6 months) | 1 | 532 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.21, 2.61] |
| 42 Development and motor skills (adaptive behaviour at 6 months) | 1 | 581 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.39, 1.79] |
| 43 Maternal anaemia at or near term (Hb less than 110 g/L at 34 weeks' gestation or more) (ALL) | 5 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.97, 1.59] |
| 44 Maternal iron deficiency anaemia at or near term (Hb less than 110 g/L and at least one additional laboratory indicators at 34 weeks gestation or more) (ALL) | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.08, 6.63] |
| 45 Maternal haemoglobin concentration at or near term (g/L, at 34 weeks' gestation or more) (ALL) | 5 | 610 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐3.92, 1.88] |
| 46 Maternal high haemoglobin concentrations during second or third trimester (Hb more than 130 g/L) (ALL) | 10 | 1942 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.84] |
| 47 Moderate anaemia at any time during second or third trimester (Hb between 70 and 99 g/L)(ALL) | 6 | 1111 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [0.63, 10.17] |
| 48 Moderate anaemia at postpartum (Hb between 80 and 109 g/L) (ALL) | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.26, 4.95] |
| 49 Severe anaemia at term (Hb less than 70 g/L at 37 weeks' gestation or more) (ALL) | 4 | 555 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 50 Severe anaemia at postpartum (Hb less than 80 g/L) (ALL) | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.04, 4.64] |
| 51 Antepartum haemorrhage (ALL) | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.59] |
| 52 Diarrhoea (ALL) | 5 | 613 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.32, 2.00] |
| 53 Constipation (ALL) | 6 | 733 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.45, 1.59] |
| 54 Nausea (ALL) | 7 | 1034 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.37, 0.97] |
| 55 Heartburn (ALL) | 4 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.31, 1.81] |
| 56 Vomiting (ALL) | 6 | 954 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.79, 2.15] |
| 57 Placental abruption (ALL) | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.01] |
| 58 Premature rupture of membranes (ALL) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.95] |
7.2. Analysis.
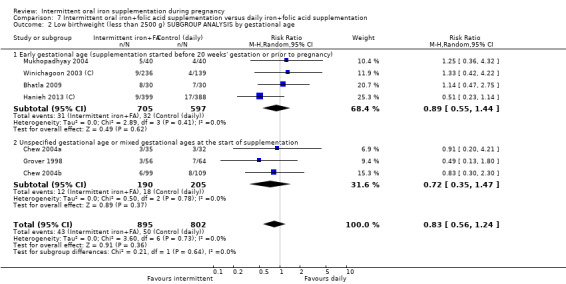
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 2 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by gestational age.
7.3. Analysis.
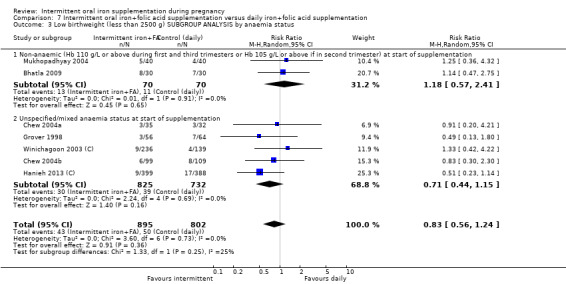
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 3 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by anaemia status.
7.4. Analysis.
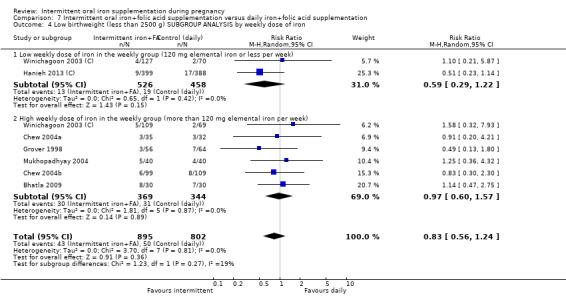
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 4 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by weekly dose of iron.
7.5. Analysis.
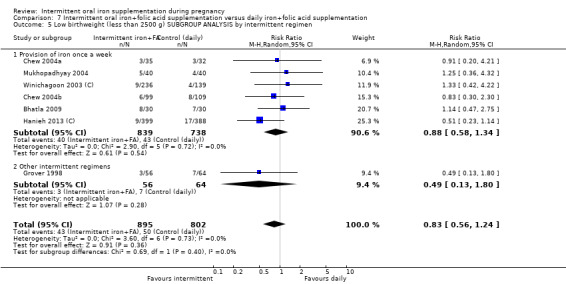
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 5 Low birthweight (less than 2500 g) SUBGROUP ANALYSIS by intermittent regimen.
7.7. Analysis.
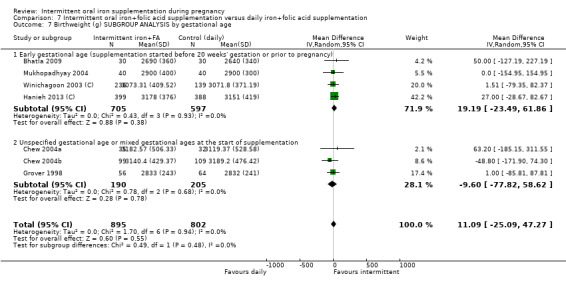
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 7 Birthweight (g) SUBGROUP ANALYSIS by gestational age.
7.8. Analysis.
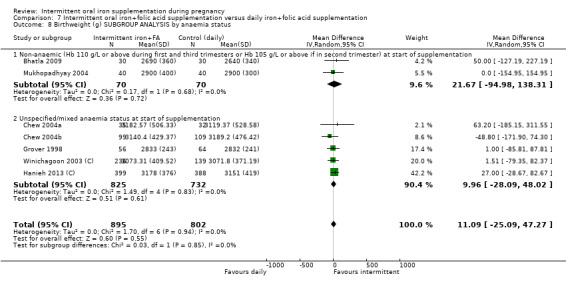
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 8 Birthweight (g) SUBGROUP ANALYSIS by anaemia status.
7.9. Analysis.
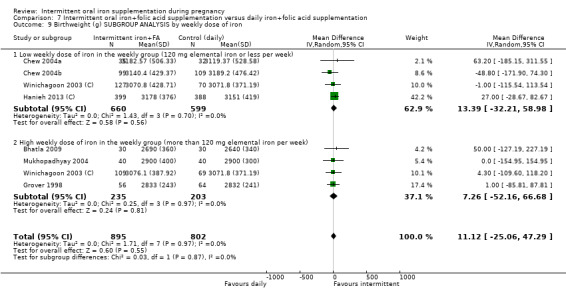
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 9 Birthweight (g) SUBGROUP ANALYSIS by weekly dose of iron.
7.10. Analysis.
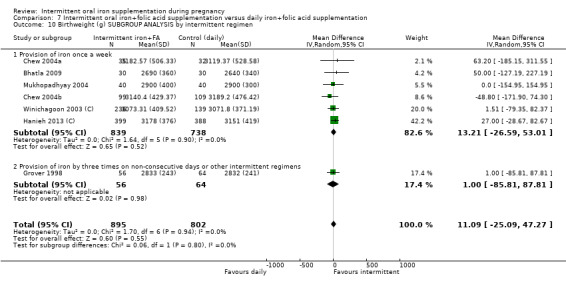
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 10 Birthweight (g) SUBGROUP ANALYSIS by intermittent regimen.
7.15. Analysis.
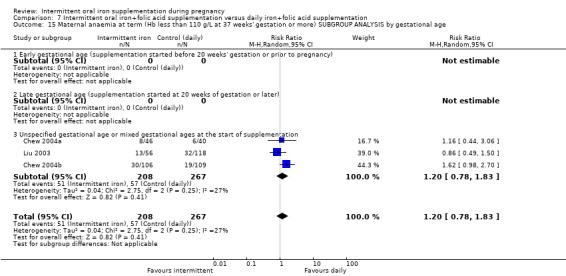
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 15 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by gestational age.
7.16. Analysis.
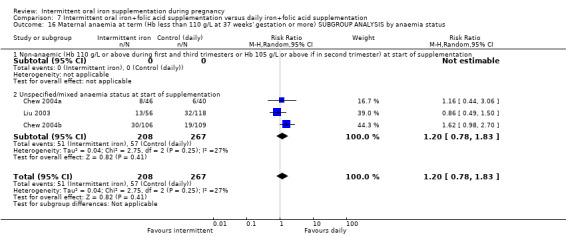
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 16 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by anaemia status.
7.17. Analysis.
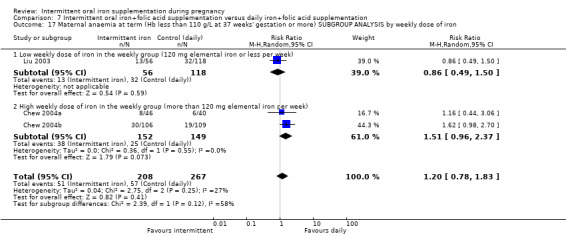
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 17 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by weekly dose of iron.
7.18. Analysis.
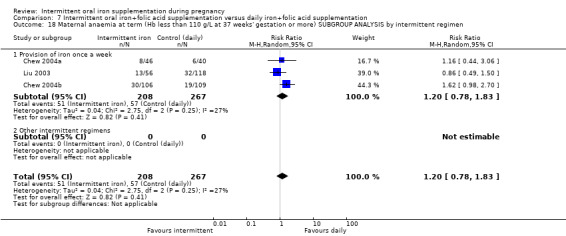
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 18 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) SUBGROUP ANALYSIS by intermittent regimen.
7.23. Analysis.
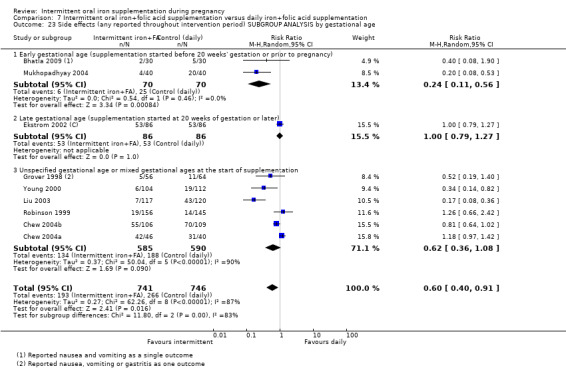
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 23 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by gestational age.
7.24. Analysis.
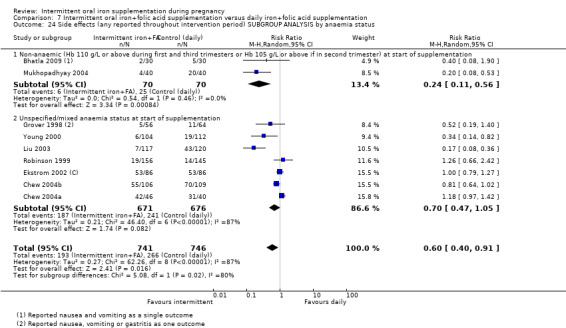
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 24 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by anaemia status.
7.25. Analysis.
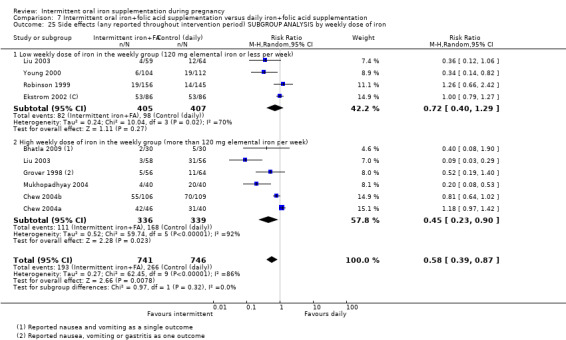
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 25 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by weekly dose of iron.
7.26. Analysis.
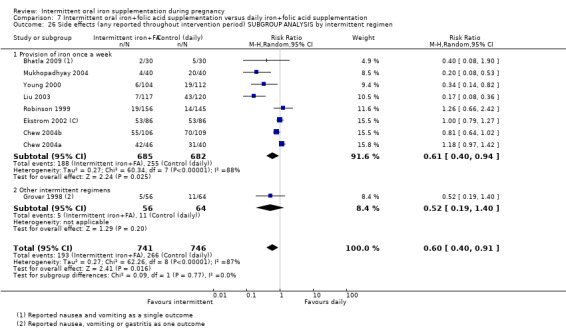
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 26 Side effects (any reported throughout intervention period) SUBGROUP ANALYSIS by intermittent regimen.
7.28. Analysis.
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 28 Severe anaemia at any time during the 2nd and 3rd trimesters (Hb less than 70 g/L) SUBGROUP ANALYSIS by gestational age.
7.29. Analysis.
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 29 Severe anaemia at any time during the 2nd and 3rd trimesters (Hb less than 70 g/L) SUBGROUP ANALYSIS by anaemia status.
7.30. Analysis.
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 30 Severe anaemia at any time during the 2nd and 3rd trimesters (Hb less than 70 g/L) SUBGROUP ANALYSIS by intermittent regimen.
7.31. Analysis.
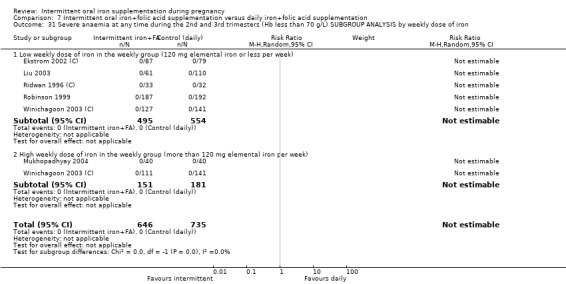
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 31 Severe anaemia at any time during the 2nd and 3rd trimesters (Hb less than 70 g/L) SUBGROUP ANALYSIS by weekly dose of iron.
7.39. Analysis.
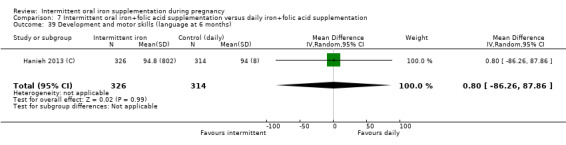
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 39 Development and motor skills (language at 6 months).
7.40. Analysis.
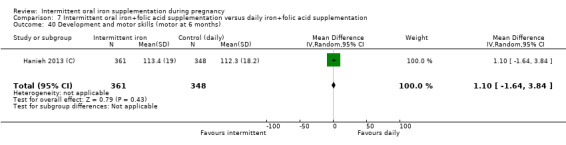
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 40 Development and motor skills (motor at 6 months).
7.41. Analysis.
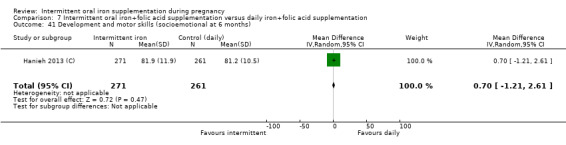
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 41 Development and motor skills (socioemotional at 6 months).
7.42. Analysis.
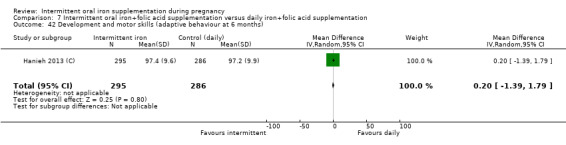
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 42 Development and motor skills (adaptive behaviour at 6 months).
7.47. Analysis.
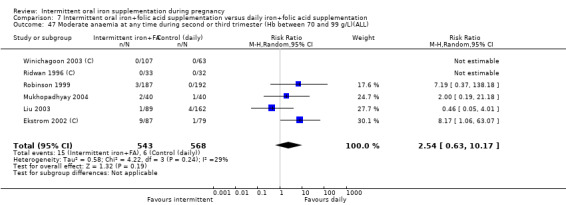
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 47 Moderate anaemia at any time during second or third trimester (Hb between 70 and 99 g/L)(ALL).
7.49. Analysis.
Comparison 7 Intermittent oral iron+folic acid supplementation versus daily iron+folic acid supplementation, Outcome 49 Severe anaemia at term (Hb less than 70 g/L at 37 weeks' gestation or more) (ALL).
Comparison 8. Intermittent oral iron+vitamins and minerals supplementation versus daily iron+vitamins and minerals supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Low birthweight (less than 2500 g) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Birthweight (g) (ALL) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Premature birth (less than 37 weeks of gestation) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Neonatal death (within 28 days after delivery) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Congenital anomalies (including neural tube defects) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Maternal anaemia at term (Hb less than 110 g/L at 37 weeks' gestation or more) (ALL) | 2 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 4.62 [2.18, 9.76] |
| 7 Maternal iron deficiency at term (based on any indicator of iron status at 37 weeks' gestation or more) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal iron deficiency anaemia at or near term (Hb less than 110 g/L and at least one additional laboratory indicator at 34 weeks' gestation or more (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Maternal death (death while pregnant or within 42 days of termination of pregnancy) (ALL) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Side effects (any reported throughout intervention period) (ALL) | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.12, 0.70] |
| 11 Maternal clinical malaria | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Maternal infection during pregnancy (including urinary tract infections and others) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Very premature birth (less than 34 weeks of gestation) (ALL) | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Maternal anaemia at or near term (Hb less than 110 g/L at 34 weeks' gestation or more) (ALL) | 2 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 4.62 [2.18, 9.76] |
| 15 Maternal iron deficiency at or near term (based on any indicator of iron status at 34 weeks' gestation or more) (ALL) | 2 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [0.70, 8.66] |
| 16 Maternal haemoglobin concentration at or near term (g/L, at 34 weeks' gestation or more) (ALL) | 1 | 116 | Mean Difference (IV, Random, 95% CI) | ‐9.50 [‐13.19, ‐5.81] |
| 17 Maternal high haemoglobin concentrations during second or third trimester (Hb more than 130 g/L) (ALL) | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.12, 1.12] |
| 18 Severe anaemia at term (Hb less than 70 g/L at 37 weeks' gestation or more) (ALL) | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Severe anaemia at any time during second and third trimester (Hb less than 70 g/L) (ALL) | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
8.13. Analysis.
Comparison 8 Intermittent oral iron+vitamins and minerals supplementation versus daily iron+vitamins and minerals supplementation, Outcome 13 Very premature birth (less than 34 weeks of gestation) (ALL).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alaoddolehei 2013.
| Methods | Quasi‐randomised trial with 2 arms from October 2002 to September 2005. | |
| Participants | 145 non‐anaemic healthy pregnant women, 20 to 40 years of age with Hb 110 g/L or higher, at 20 wks' gestation, in the Gynecology and Obstetrics Clinic, Babol, Iran, from October 2002 to September 2005. Participants with β minor thalassaemia, Hb less than 11 g/dL, more than 1 delivery and diagnosed with internal and infectious diseases were excluded. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 (n = 72) received intermittent dose of 50 mg elemental iron supplement 3 times/wk; group 2 (n = 73) received 50 mg elemental iron daily. Supplementation started from the 20th wk of pregnancy for both groups. Setting and health worker cadre: all participants were enrolled in the Gynecology and Obstetrics Clinic, Babol, Iran. The health worker cadre is not described in the report. |
|
| Outcomes | Consumption of tablets, side effects. Iron, zinc and haematological examination, including complete blood count and ferritin, at enrolment, 26‐28 and 34‐37 wks of gestation. Laboratory method for ferritin concentration: ELISA (ORG5Fe, Bngomtak, Germany). |
|
| Notes | 1. By gestational age: late gestational age (supplementation started at 20 wks of gestation or later). 2. By anaemia status at baseline: non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: high weekly dose of iron in the intermittent group (more than 120 mg elemental iron per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: not specified/unreported/unknown. 6. By intermittent iron supplementation regimen: other intermittent regimens (3 times a wk). 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Source of funding: Research chancellery of Babol University of Medical Sciences, Iran. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomly divided in to 2 groups on the basis of number given them at first visit. |
| Allocation concealment (selection bias) | High risk | Open random allocation by number assignment. Even numbers entered in a group (group1 = 73 cases) received daily dose of iron supplement. Odd numbers entered in another group (group 2 = 72 cases) received intermittent dose of iron supplement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding described. Open to participants and care providers. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Lack of blinding would be unlikely to affect laboratory outcomes. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Assessment of compliance may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were not losses to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | Not apparent. This clinical trial was registered in the Iranian Registry of Clinical Trials (www.irct.ir) with registration number ID: 2012082810682N1. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Bhatla 2009.
| Methods | RCT, 3‐arm trial with individual randomisation. The study was conducted from April 2002 through April 2004. | |
| Participants | 109 pregnant non‐anaemic women between 14 and 18 wks (49% vegetarian) with no prior intake of iron supplements in the Department of Obstetrics and Gynaecology of the All India Institute of Medical Sciences in New Delhi, India were invited to participate in the study. Exclusion criteria were: Hb < 110 g/L, PCV < 30; cigarette smoking; pre‐existing hypertension or diabetes; history of chronic illness, e.g. liver or renal disease, tuberculosis, heart disease, malaria; history of bleeding disorders, bleeding piles, chronic peptic ulcer; thalassaemia or other haemoglobinopathies; intake of drugs such as antiepileptics, NSAIDs, antithyroid medication, vitamins, antioxidants; multiple pregnancy; and prior history of blood transfusion. |
|
| Interventions | Participants were randomly allocated into 1 of 3 different groups: group 1 (n = 37) received the standard Government of India supply of Irofol® tablets containing 100 mg of elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) folic acid (Nestor Pharmaceuticals Ltd., Faridabad, Haryana, India) to be taken once daily; group 2 (n = 36) received the standard Government of India supply of Irofol® tablets containing 100 mg of elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) folic acid and were instructed to take 2 tablets on any 1 day of the wk; 1 before lunch and the other before dinner (total 200 mg elemental iron and 1000 μg (1 mg) folic acid per wk) with no tablets taken during the rest of the wk; and group 3 (n = 36) received Ferium® tablets iron (III)‐hydroxide poly maltose complex tablets daily containing Iron (III) Hydroxide Polymaltose containing 100 mg elemental iron and 350 μg (0.35 mg) folic acid to be taken 1 tablet daily (Emcure Pharmaceuticals Ltd., Pune). All groups received health education regarding the importance of diet in pregnancy, iron‐rich foods and appropriate dietary practices and were instructed to take the tablets 30 min before meals and not with tea, coffee or milk. All women were also advised to take calcium supplements after meals. Setting and health worker cadre: the intervention was performed by obstetricians and hematologists at the All India Institute of Medical Sciences in New Delhi, India. |
|
| Outcomes | Maternal: miscarriage, intrauterine demise, Hb, HCT, MCV and MCHC, thiobarbituric acid reactive substances (TBARS) and glutathione levels at baseline (14‐16 wks) and at 30‐34 wks, compliance, side effects, nausea, vomiting, diarrhoea, constipation, metallic taste, epigastric discomfort, premature birth, hypertension during pregnancy, preeclampsia, C‐section. Infant: birthweight, low birthweight (LBW), placental weight, 1‐minute Apgar score and incidence of meconium. Laboratory method for ferritin concentration: ferritin not measured. |
|
| Notes | Mean gestation at which supplementation was started was 16.1 1.3 wks and mean duration of iron supplementation before final sampling was 17.9 1.4 wks. Overall, 22.2% of women were non‐compliant: 12 (40%) women in the IFA daily (group 1) and 4 (13.3%) women in the IFA weekly (group 2) did not comply with the prescribed schedule (P = 0.016). 1. By gestational age: early (supplementation started before 20 wks' gestation or prior to pregnancy). 2. By anaemia status at baseline: non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: high weekly dose of iron in the weekly group (more than 120 mg elemental iron per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: study carried out in malaria risk locations. As of 2011: malaria risk exists throughout the year in the whole country at altitudes below 2000 m, with overall 40% to 50% of cases due to Plasmodium falciparum and the remainder due to Plasmodium vivax. Source of funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | High risk | Patient and doctor were both aware of the allotted groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open to participants and care providers. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | The “technician who performed the blood tests was not aware of the group to which the patient was allocated”. It is not clear whether outcome assessment for other outcomes was blinded |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Reporting of side effects and compliance may have been affected by lack of blinding and some women changed or stopped medication. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 109 women were enrolled, 90 were included in the analysis (rate of attrition 17%). Loss to follow‐up and post randomisation exclusions were not balanced across groups and women were excluded for reasons likely to introduce bias. 5 women were excluded (4 in the daily iron group and 1 in the weekly iron group) because they could not tolerate the supplementation and were given alternative treatment. 2 women were lost to follow‐up because of adverse foetal outcome (1 miscarriage and 1 intrauterine death). |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | There was some baseline imbalance between groups, e.g. in the daily iron sulphate group the supplementation was started earlier. |
Bouzari 2011.
| Methods | RCT with 3 arms. individual randomisation. 6 health centres were selected from 40 centres affiliated to the Babol University of Medical Sciences. | |
| Participants | 150 healthy non‐anaemic pregnant women with 16th wk of gestation attending 6 health centres affiliated to Babol University of Medical Sciences, northern Iran. Exclusion criteria: women with Hb less than 11g/dL. with chronic haematological conditions (e.g. thalassaemia) or with multiple pregnancy. |
|
| Interventions | Participants were randomly assigned to 1 of 3 groups: group 1 (n = 50) received 50 mg iron (presumably 50 mg elemental iron as ferrous sulphate); group 2 received 50 mg ferrous sulphate 3 times per wk; group 3 received 100 mg (2 50 mg tablets) of ferrous sulphate weekly. Setting and health worker cadre: it was not clear how the intervention was delivered; the setting was antenatal clinics so iron was probably provided by obstetricians or midwives. |
|
| Outcomes | Hb, serum ferritin and serum at the beginning (wk 16) and the end of treatment (wk 28). Laboratory method for ferritin concentration: RIA technique (Genesis factory, USA). |
|
| Notes | 1. By gestational age at start of supplementation:early (supplementation started before 20 wks' gestation or prior to pregnancy); 2. By anaemia status at baseline: non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: high weekly dose of iron in the 3 times weekly (more than 120 mg elemental iron per wk) (group 2) and lower weekly dose of iron in the once weekly (120 mg elemental iron or less per wk) (group 3). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk (group 3); and 3 times a wk (group 2). 7. By malaria endemicity of the area in which the trial was conducted: study carried out in malaria risk‐free parts of countries that has malaria risk in other parts. As of 2011: malaria risk due to Plasmodium vivax and Plasmodium falciparum exists from March to November inclusive in rural areas of the provinces of Hormozgan and Kerman (tropical part) and the southern part of Sistan‐Baluchestan. Source of funding: Research’s Vice chancellor of the Babol University of Medical Sciences. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described but it was stated that women were "divided into three equal groups". |
| Allocation concealment (selection bias) | Unclear risk | Method not described. The abstract states that women were "simply randomised". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding. Different regimens were compared. Women and staff would be aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory tests were unlikely to have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 150 women were randomised. 8 women were excluded after randomisation (7 of these women were in the 3 times weekly group). |
| Selective reporting (reporting bias) | Unclear risk | Assessment was from a brief trial report and we did not have access to the study protocol. |
| Other bias | Unclear risk | The methods were not well described. It was stated that the 3 randomised groups "were matched in terms of age, numbers of pregnant, income and education". This may mean that background characteristics in the 3 randomised groups were similar, but this was not clear. |
Casanueva 2006.
| Methods | RCT 2‐arm trial with individual randomisation. | |
| Participants | 120 singleton pregnant women attending the Instituto Nacional de Perinatologia in Mexico City, Mexico with Hb concentrations higher than 115 g/L at 20 wks of gestation (equivalent to 105 g/L at sea level). | |
| Interventions | Participants were randomly assigned to 1 of 2 groups, group 1: 1 tablet containing 60 mg of elemental iron (as ferrous sulphate), 200 μg (0.2 mg) folic acid and 1 μg vitamin B12 given daily, and group 2: 2 tablets (total 120 mg of elemental iron (as ferrous sulphate), 400 μg (0.4 mg) folic acid, and 2 μg vitamin B12) to be taken once weekly. The groups received either daily supplementation or weekly supplementation at no cost. Supplement tablets were identical in content and were to be ingested from the 20th wk of pregnancy until delivery. The 60 women in the daily group were supplied monthly with 30‐31 tablets and were instructed to ingest 1 tablet daily. The women in the weekly group were supplied monthly with 8‐10 tablets and were instructed to consume 2 tablets once each wk. If the scheduled day was missed, they were instructed to take the tablets the next day and not to wait until the following wk before ingesting them. Both groups were told that the tablets were to be ingested with water at least 1 h after a meal and preferably before going to bed at night to decrease gastrointestinal side effects. Setting and health worker cadre: the intervention was performed by obstetricians at the Instituto Nacional de Perinatologia Isidro Espinosa de los Reyes (INPerIER), a teaching and research centre in Mexico City, Mexico. |
|
| Outcomes | Maternal: Hb and serum ferritin concentrations every 4 wks from wks 20 until 36, side effects, compliance, birthweight, gestational age at birth, anaemia, iron deficiency, haemoconcentration (defined as Hb level > 145 g/L, which considers an adjustment by altitude of Mexico city by the addition of 10 g/L, making it equivalent to sea level value of 135 g Hb/L. Infant: weight. Laboratory method for ferritin concentration: ELISA (Opus, Dade Behringer, Newark, USA). |
|
| Notes | Non‐compliance with allocated intervention (stopped/started) reported as adherence 50th percentile (i.e. positive outcome) was 93% in the weekly regimen and 90% in the daily regimen. 1. By gestational age at start of supplementation: late gestational age (supplementation started at 20 wk of gestation or later). 2. By anaemia status at baseline: non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk‐free parts of countries that has malaria risk in other parts. As of 2011: malaria risk due almost exclusively to Plasmodium vivax exists throughout the year in some rural areas. There is moderate risk in some localities in the states of Chiapas and Oaxaca; very low‐risk localities are also found in the states of Chihuahua, Durango, Nayarit, Quintana Roo and Sinaloa. Source of funding: Instituto Nacional de Perinatologia Isidro Espinoza de los Reyes, Mexico city, Mexico; Children's Hospital Oakland Research Institute, Oakland, USA; Department of Nutritional Sciences and Toxicology, University of California, Berkeley, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by drawing lots without replacement; there was a 50% probability of being placed in either group. |
| Allocation concealment (selection bias) | Unclear risk | There is insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although tablets were reported to be identical, different regimens (different number of tablets) were compared. It was reported that the study was not totally blinded as the nutritionist was aware of groups to dispense medication and assess compliance. It was stated that attending doctors were not aware of group allocation. However, women would be aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were unlikely to have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Reporting of side effects and compliance may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 120 women were randomised, only 4 were lost to follow‐up (all in the daily group). Reasons for loss were explained and did not appear to relate to supplementation. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | Group appeared similar in terms of baseline characteristics. |
Chew 2004a.
| Methods | RCT, 2‐arm trial. | |
| Participants | 120 clinically healthy pregnant women attending 1 antenatal care clinic in Guatemala City, Guatemala with Hb > 80 g/L were recruited. Women were from low SES. City of Guatemala is 1500 m above sea level, so values were adjusted by altitude subtracting 5 g/L in Hb. | |
| Interventions | Participants from low SES were randomly assigned to 1 of 2 groups: group 3: daily unsupervised intake of 60 mg elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) folic acid; or group 4: weekly unsupervised intake of 180 mg of elemental iron (as ferrous sulphate) and 3500 μg (3.5 mg) of folic acid in 1 intake once a wk.
Supplementation started at an average of 20.5 wks of gestation until 38th wk. Setting and health worker cadre: the intervention was performed by physicians and researchers at 1 antenatal care clinic in Guatemala City, Guatemala. |
|
| Outcomes | Maternal: Hb concentration at baseline and at term (38th wk of gestation); side effects and total iron intake.
Infant: birthweight. Laboratory method for ferritin concentration: ferritin not measured. |
|
| Notes | Unsupervised. 1. By gestational age at start of supplementation: unspecified gestational age or mixed gestational ages at the start of supplementation. 2. By anaemia status at baseline: unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: high weekly dose of iron in the intermittent group (more than 120 mg elemental iron per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk‐free parts of countries that has malaria risk in other parts. As of 2011: malaria risk due predominantly to Plasmodium vivax exists throughout the year below 1500 m. There is moderate risk in the departments of Escuintla and Izabal, and low risk in Alta Verapaz, Baja Verapaz, Chiquimala, Petén, Suchitepéquez and Zacapa. Source of funding: Institute of Nutrition of Central America and Panama (INCAP), Guatemala city, Guatemala and Department of Nutritional Sciences, University of California, Berkeley, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different regimens compared. Women and staff would be aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were unlikely to have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Reporting of side effects and compliance may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 20% lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Chew 2004b.
| Methods | RCT 2‐arm trial. | |
| Participants | 256 clinically healthy pregnant women from low SES attending 1 antenatal care clinic in Guatemala City, Guatemala and Hb > 80 g/L were recruited. City of Guatemala is at 1500 m above sea level, so values were adjusted by altitude subtracting 5 g/L in Hb. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1: daily supervised intake of 60 mg elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) folic acid; group 2: weekly supervised intake of 180 mg of elemental iron (as ferrous sulphate) and 3500 μg (3.5 mg) of folic acid in 1 intake once a wk.
Supplementation started at different gestational age for each participant. Average gestational age at start was 20.5 wks until 38th wk. Setting and health worker cadre: the intervention was performed by physicians and researchers at 1 antenatal care clinic in Guatemala City, Guatemala. |
|
| Outcomes | Maternal: Hb concentration at baseline and at term (38th wk of gestation); side effects and total iron intake.
Infant: birthweight. Laboratory method for ferritin concentration: ferritin not measured. |
|
| Notes | Supervised. 1. By gestational age at start of supplementation: unspecified gestational age or mixed gestational ages at the start of supplementation. 2. By anaemia status at baseline: unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: high weekly dose of iron in the intermittent group (more than 120 mg elemental iron per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk‐free parts of countries that has malaria risk in other parts. As of 2011: malaria risk due predominantly to Plasmodium vivax exists throughout the year below 1500 m. There is moderate risk in the departments of Escuintla and Izabal, and low risk in Alta Verapaz, Baja Verapaz, Chiquimala, Petén, Suchitepéquez and Zacapa. Source of funding: Institute of Nutrition of Central America and Panama (INCAP), Guatemala city, Guatemala and Department of Nutritional Sciences, University of California, Berkeley, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computerised random numbers. |
| Allocation concealment (selection bias) | Low risk | Authors used sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different regimens compared. Women and staff would be aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were unlikely to have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Reporting of side effects and compliance may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 20% lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Ekstrom 2002 (C).
| Methods | Cluster‐randomised trial. 50 antenatal clinics randomised. | |
| Participants | 209 apparently healthy women attending antenatal care clinics in rural areas of Mymemsingh thana, Bangladesh, with fundal height of 14‐22 cm (18‐24 wks of gestation), who had not used iron supplements prior to the study. Exclusion criteria: women with Hb concentrations < 80 g/L. | |
| Interventions | Each clinic was randomly assigned to 1 of 2 interventions: 60 mg of elemental iron (as ferrous sulphate) and 250 μg (0.25 mg) folic acid given in 1 tablet daily, or 120 mg of elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) folic acid once a wk (given in 2 tablets 1 day of the wk). Supplementation continued until 6 wks postpartum.
Supplementation started at baseline for 12 wks. Setting and health worker cadre: the intervention was performed by lay health workers and health centre staff at rural community antenatal centres run by an NGO among primarily illiterate women of lower SES in Bengladesh. |
|
| Outcomes | Maternal: Hb concentration at baseline and after 12 wks of supplementation. Compliance, side effects, serum ferritin and serum transferrin receptors at 6 wks postpartum. Laboratory method for ferritin concentration: RIA (Diagnostic Products, San Diego,CA, USA) |
|
| Notes | Unsupervised.
Cluster randomisation used among 52 antenatal clinics: n = 25 to daily supplementation and n = 25 to weekly supplementation. 2 antenatal care units ceased operation during the trial period. Compliance was 104% and 68% for weekly and daily groups respectively. The compliance above 100% for the weekly means that more tablets that were indicated to be taken were ingested in the period of time. Cluster design effect was not taken into account in the analysis. 1. By gestational age at start of supplementation: early (supplementation started before 20 wks' gestation or prior to pregnancy). 2. By anaemia status at baseline: unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements:not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate:equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk locations. As of 2011: malaria risk exists throughout the year in the whole country excluding Dhaka city, with highest risk in Chittagong Division, the districts of Mymensingh, Netrakona and Sherpur in Dhaka Division, and Kurigram district in Rajshahi Division. Source of funding: World Health Organization, Government of Tanzania, Hoffmann‐La Roche, Basel, Switzerland donated the gastric delivery system iron supplement. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster by random list numbers. |
| Allocation concealment (selection bias) | High risk | "On the day of the scheduled antenatal service, the first 4 women who booked for service at each centre
and who fulfilled the primary inclusion criteria were invited to participate." As the randomisation was per centre the allocation was known. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Cluster trial so women may not have been affected by contamination bias, but will have been aware of treatment regimen. Women who developed anaemia were excluded. Staff were likely to have been aware of regimen. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were unlikely to have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Reporting of side effects may have related to knowledge of the regimen. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 20% lost to follow‐up. 209 women in 50 centres randomised. 140 followed up, therefore attrition 33%. Attrition was not balanced across groups with women in the weekly arm being more likely to have incomplete data and 2 from this group were withdrawn with anaemia (vs 0 in daily group). |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | Results adjusted by initial Hb measurements as well as by clustering effect within participants. |
Goonewardene 2001.
| Methods | RCT, 3‐arm trial with individual randomisation. | |
| Participants | 92 pregnant women from 14‐24 wks of gestation attending the university antenatal clinic, in Galle, Sri Lanka. | |
| Interventions | Participants were randomly assigned to 1 of 3 regimens: group 1 (n = 26) received a tablet containing 100 mg of elemental iron (as ferrous fumarate) 500 μg (0.5 mg) folic acid, 10 mg vitamin B12, 100 mg vitamin C, 10 mg vitamin B6 and 50 mg niacinamide once a wk; group 2 (n = 35) received the same tablet but 3 times a wk; and group 3 (n = 31) received the same supplement in a daily fashion. All groups received 100 mg mebendazole twice daily for 3 days before they were randomly allocated to the groups. Women were advised to take the supplement with water at 11:00 a.m. (approximately 1 hour before lunch). Setting and health worker cadre: the intervention was performed by obstetricians at a prenatal clinic in Galle, Sri Lanka. |
|
| Outcomes | Hb, serum ferritin, hematocrit at entry and at 34‐36 wks' gestation, before the onset of labour. Laboratory method for ferritin concentration: Immunoradiometric assay (IRMA) (Diagnostic Products Corporation, Los Angeles, USA). |
|
| Notes | Unsupervised.
Compliance was described as "good" in all 3 groups and no serious side effects were reported. 1. By gestational age at start of supplementation: unspecified gestational age or mixed gestational ages at the start of supplementation. 2. By anaemia status at baseline: unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous fumarate. 6. By intermittent iron supplementation regimen: other intermittent regimens (3 times a wk). 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk locations. As of 2011: limited malaria risk – Plasmodium vivax (88%), Plasmodium falciparum (12%) – exists throughout the year, except in the districts of Colombo, Galle, Gampaha, Kalutara, Matara and Nuwara Eliya. Source of funding: International Atomic Energy Agency, Vienna, Austria. The supplement was provided by Astron Ltd, Sri Lanka. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomly assigned but method of sequence generation is unclear. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different regimens compared. Women and staff were likely to be aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were unlikely to have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | Unclear risk | Not reported. (Information was collected by interview but results for side effects and compliance were not reported in the published paper.) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 88 out of 92 provided blood sample for the analysis. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | No baseline imbalance apparent but baseline data were only provided for those women available to follow‐up. There were not significant differences in income, educational level, age and parity. |
Goshtasebi 2012.
| Methods | Quasi‐RCT, 2‐arm trial. | |
| Participants | 370 non‐anaemic pregnant women aged 18‐35 with singleton pregnancy, parity < 4 and normal BMI. gestational age 14–20 wks, and no history of high‐risk pregnancy, smoking or drug abuse, in Teheran, Iran between February and November 2009. Exclusion criteria: Hb less than 105 g/L, preterm delivery before wk 34, any condition that needs medical or surgical intervention. |
|
| Interventions | Participants were randomly assigned to 1 of 2 groups: .group 1 received 1 tablet containing 50 mg elemental iron (as ferrous sulphate) and 1 tablet containing 1000 µg (1 mg) folic acid tablet per day, since 20th gestational wk until delivery; group 2 received 1 tablet containing 50 mg elemental iron (as ferrous sulphate) and 1 tablet containing 1000 µg (1 mg) folic acid twice a wk on Mondays and Thursdays, since 20th gestational wk until delivery. All mothers took also part in an educational programme on nutrition in pregnancy as co‐intervention. Setting and health worker cadre: prenatal clinic of the Imam Hospital, Sari, Islamic Republic of Iran. Mothers of both groups received routine care and were followed up until delivery. The health worker who provided the tablets is not specified. |
|
| Outcomes | Hb and ferritin in cord blood, birthweight, anaemia indices and side effects in mother during pregnancy. Laboratory method for ferritin concentration: RIA (Gamma Counter System, Kontron). |
|
| Notes | 1. By gestational age: early (supplementation started before 20 wks' gestation or prior to pregnancy). 2. By anaemia status at baseline: non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: other intermittent regimens (twice a wk). 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Source of funding: Tarbiat Modares University research office, Iran as part of a Midwifery Master of Science dissertation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Reported as random but method not reported. |
| Allocation concealment (selection bias) | High risk | Without blinding, random allocation was done according to the day of wk a pregnant woman attended the clinic: clients on even days were assigned to the daily group and attendees on odd days were allocated to the twice‐weekly group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported as open trial. Participants and personnel knew which intervention a participant received. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Lack of blinding would be unlikely to affect laboratory outcomes. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Reporting of side effects may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available for all participants in the study. |
| Selective reporting (reporting bias) | Low risk | Not apparent. This clinical trial was registered in the Iranian Registry of Clinical Trials (www.irct.ir) with registration number ID: IRCT138802131641N4. |
| Other bias | High risk | Authors report that due to financial constraints the trial was open and baseline ferritin values were not evaluated for comparability among the groups. Also the supervision on adherence was not done. Supervised directly. |
Grover 1998.
| Methods | RCT, 2‐arm trial with individual randomisation. | |
| Participants | 200 pregnant women with gestation 16‐24 wks attending for care in rural health centre in Gazipur village in East Delhi, India from Jan‐Dec 1994 with Hb 70 g/L or more and no tuberculosis, chronic diseases, "toxaemia", bleeding piles. Participants: 9.2% > 30 years, nearly 60% were illiterate and 23.4% primigravid. Baseline Hb in the daily iron group was 96 g/L and in the intermittent group 97 g/L. 200 women randomised but data available for 120. |
|
| Interventions | From recruitment (16‐24 wks) until delivery (not clear). Participants were randomly assigned to 1 of 2 groups: group 1: women received 100 mg of elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) of folic acid on alternate days: (data available for 56 women); group 2: women received 100 mg of elemental iron daily (as ferrous sulphate) and 500 μg (0.5 mg) of folic acid (data available for 64 women). It is not clear how the doses were supplied. Setting and health worker cadre: the intervention was performed by physicians and medical social workers at a weekly, rural antenatal health centre run by the Department of Preventative and Social Medicine of the University College of Medical Sciences in New Delhi, India. |
|
| Outcomes | Birthweight at 48‐72 hours, side effects and compliance. Laboratory method for ferritin concentration: ferritin not measured. |
|
| Notes | Malarial status of the study area not stated. Setting prevalence of anaemia, haemoglobinopathies, and hookworm not mentioned. 1. By gestational age at start of supplementation: unspecified gestational age or mixed gestational ages at the start of supplementation. 2. By anaemia status at baseline: unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: high weekly dose of iron in the intermittent group (more than 120 mg elemental iron per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: other intermittent regimens (alternate days). 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk locations. As of 2011: malaria risk exists throughout the year in the whole country at altitudes below 2000 m, with overall 40% to 50% of cases due to Plasmodium falciparum and the remainder due to Plasmodium vivax. There is no transmission in parts of the states of Himachal Pradesh, Jammu and Kashmir, and Sikkim. Source of funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “assigned to the two different groups randomly.” |
| Allocation concealment (selection bias) | Unclear risk | There is insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different regimens compared. Women and staff would be aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were unlikely to have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Reporting of side effects and compliance may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “200 pregnant women were enrolled for the study out of which only 120 could complete the course of therapy”. It was not clear why women were lost to follow‐up or did not complete the course of therapy, nor was it clear whether the loss from the 2 groups was at the same level or for the same reasons. This level of attrition (40%) means this study is at high risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | No baseline imbalance apparent but baseline data were only provided for those women available to follow‐up. |
Hanieh 2013 (C).
| Methods | Cluster‐randomised trial with 3‐arms. "Robust standard errors were calculated using the Huber‐White Sandwich estimator to for study design (i.e. clustering at commune level). The ICC was calculated for continuous and binary outcome measures using one way analysis of variance with commune as the group variable". | |
| Participants | 1258 pregnant women older than 16 years of age, 16 wks of gestation and living in 104 communes in Ha Nam, a malaria‐free province, 60 km from Hanoi, in northern Viet Nam. Women with a multiple pregnancy, a significant medical condition or severe anaemia (Hb concentration below 80 g/L) at enrolment were excluded. Women with a significant medical condition or severe anaemia were also referred to the commune health station for further management. | |
| Interventions | Communes (clusters) were randomly assigned to 1 of 3 groups: group 1 (n = 34 communes, 426 participants) received 1 tablet daily containing 60 mg elemental iron (as ferrous sulphate) plus 400 µg (0.4 mg) folic acid, administered as 7 tablets/wk; group 2 (n = 35 communes, 425 participants) received twice weekly supplements containing 60 mg elemental iron (as ferrous sulphate) plus 1500 µg (1.5 mg) folic acid per capsule; administered as 2 capsules/wk); group 3 (n‐35 communes, 407 participants) received 1 capsule containing 60 mg elemental iron (as ferrous sulphate) plus 1500 µg (1.5 mg) folic acid plus 20 mg zinc, 300 mg iodine, 4 mg copper, 130 mg selenium, 1.6 mg vitamin A, 2.8 mg thiamine, 2.8 mg riboflavin, 36 mg niacin, 3.8 mg vitamin B6, 5.2 mg vitamin B12, 140 mg vitamin C, 400 IU vitamin D, and 20 mg vitamin E twice weekly . The supplementation started from enrolment an continued until 3 months postpartum. Setting and health worker cadre: communes from 5 rural districts of Ha Nam, Viet Nam. Trained project officers recruited women, in conjunction with village and commune health station workers. Maternal and infant outcomes were recorded by trained commune health station. Communes health workers provide medical treatment for women in the trial. Participants were visited every 6 wks at home, to receive the supplements along with instructions for the next 6 wks, and collect outcome data. Stillbirths and early neonatal deaths were documented by trained commune health station midwives. |
|
| Outcomes | Primary: infant birthweight. Secondary: maternal Hb, ferritin, anaemia, iron deficiency, iron‐deficiency anaemia, high ferritin, haemoconcentration at 32 wk, and 6 mo postpartum, serum folate, vitamin B12 concentrations, vitamin B12 deficiency, folate deficiency at 32 wk gestation; serum 25(OH) vitamin D concentration, vitamin D insufficiency and urinary iodine concentration at 32 wks' gestation. Infant length‐for‐age Z‐scores, infant Hb, ferritin concentrations, anaemia, iron deficiency and cognitive development at 6 mo of age (Bayley Scales of Infant and Toddler Development, 3rd edition (BSID III). Stillbirths (fetal deaths at or after 28 wk of pregnancy) and early neonatal deaths, low birthweight; very low birthweight, and preterm birth. Laboratory method for ferritin concentration: chemiluminescent microparticle assay. |
|
| Notes | Data entered have been adjusted per ICC following standard Cochrane methods. Only data from group 2 (weekly iron‐folic acid) vs group 1 (daily iron‐folic acid) are included in the analysis. Intake was not supervised. 1. By gestational age at start of supplementation: early (supplementation started before 20 wks' gestation or prior to pregnancy). 2. By anaemia status at baseline:unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: not specified/unreported/unknown. 6. By intermittent iron supplementation regimen: other intermittent regimens (twice a wk). 7. By malaria endemicity of the area in which the trial was conducted: extremely low risk malaria area but no data (as per author communication). Source of funding: National Health and Medical Research Coucil of Australia (Grant number 628751). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed by an independent statistician not involved in the study and blinded to the identity of the communes, using ‘ralloc’ in Stata (StataCorp)." |
| Allocation concealment (selection bias) | High risk | "Supplements were received from the manufacturing company in blister packs, with a code A, B, or C embossed on each blister pack." However the study was open for the daily supplementation group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The investigators, field staff, and participants were blinded to the codes of the intermittent supplement groups throughout the study and during data analysis." "It was not possible to blind the field team to the daily supplementation arm". |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | "Laboratory staff were unaware of the intervention groups". |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | "The investigators, field staff, and participants were blinded to the codes of the intermittent supplement groups throughout the study and during data analysis." "It was not possible to blind the field team to the daily supplementation arm". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was 8.9% (38/426) in the group 1, 6.1% (26/425) in the group 2, and 6.4% (26/407) in the group 3, for birthweight. |
| Selective reporting (reporting bias) | Low risk | The study’s prespecified outcomes and all expected outcomes of interest to the review were reported as per registered protocol in the Australia New Zealand Clinical Trials Registry: 12610000944033. |
| Other bias | Low risk | Baseline characteristics of women at enrolment were balanced. |
Hashim 2012.
| Methods | 2‐arm randomised control trial. | |
| Participants | 70 pregnant women at 12‐20 wks of gestation with Hb level of 90‐110 g/L attending antenatal clinic at a Maternal and Child Health Clinic at Klinik Kesihatan Bandar Kota Bharu, Kelantan, Malaysia. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 (n = 35) received iron (as 200 mg of ferrous fumarate) to be ingested daily; group 2 (n = 35) 2 tablets containing iron (as 200 mg of ferrous fumarate) once a wk. The supplementation period was 6 months. Setting and health worker cadre: antenatal clinic at a Maternal and Child Health Clinic at Klinik Kesihatan Bandar Kota Bharu, Kelantan, Malaysia. The iron tablets were distributed by the investigator at the clinic for a month supply. |
|
| Outcomes | Hb, ferritin, maternal anaemia during second trimester of pregnancy. Laboratory method for ferritin concentration: unreported/unknown. |
|
| Notes | Abstract does not specify if the iron tablets were taken before the study. 1. By gestational age at start of supplementation: Early (supplementation started before 20 wks' gestation or prior to pregnancy). 2. By anaemia status at baseline: anaemic (Hb below 110 g/L during first and third trimesters or below 105 g/L in second trimester) at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk);high weekly dose of iron in the intermittent group (more than 120 mg elemental iron per wk). 4. By release speed of iron supplements: specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate equivalent or lower: ferrous fumarate. 6. By intermittent iron supplementation regimen once a wk. 7. By malaria endemicity of the area in which the trial was conducted: specified/unreported/unknown. Source of funding: Universiti Sains Malaysia; Kuban Kerian, Malaysia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random method not specified in the abstract. |
| Allocation concealment (selection bias) | Unclear risk | Information not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | High risk | Not reported. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Unclear risk | Characteristics of groups at baseline not reported. |
Liu 2003.
| Methods | RCT. 3‐arm trial with additional non‐random control group. | |
| Participants | 395 healthy, anaemic and non‐anaemic, pregnant women attending prenatal care at 2 outpatient clinics at Changji Hospital and Shihezi Maternal and Child Health Station in Xianjiang, China. Women with Hb < 80 g/L were excluded. Maternal age was 25.15 ± 2.28 years. | |
| Interventions | Participants were randomly assigned to 1 of 3 groups: group 1: 60 mg elemental iron as ferrous sulphate and 250 μg (0.25 mg) of folic acid daily; group 2: 120 mg of elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) of folic acid daily; group 3: 120 mg elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) of folic acid once weekly. A control group that received no iron was composed of women who did not want to participate in the study and did not receive any iron supplements. Since the allocation of the control group was not randomised, we included this study in our comparisons of the effects of intermittent vs daily iron supplementation. Setting and health worker cadre: the intervention was performed by physicians and researchers in 2 outpatient clinics of the department of Obstetrics in Changji Hospital and Shihezi Maternal and Child Health Station in Xianjiang, China. |
|
| Outcomes | Maternal: Hb concentration at 3, 5, 8 months and at term; serum ferritin concentrations at 3 months and at term in a subgroup; side effects.
Weight at entry and at term (not used in the review). Laboratory method for ferritin concentration: not reported. |
|
| Notes | Unsupervised.
Iron supplementation is not mandatory for women in China, if they have a Hb concentration > 80 g/L. Compliance for group 1 (daily 60 mg Fe), group 2 (daily 120 mg Fe) and group 3 (weekly 120 mg Fe) were 77%, 75% and 86% respectively. 1. By gestational age at start of supplementation: unspecified gestational age or mixed gestational ages at the start of supplementation. 2. By anaemia status at baseline: unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk‐free parts of countries that has malaria risk in other parts. As of 2011: malaria risk, including Plasmodium falciparum malaria, exists in Yunnan and to a lesser extent in Hainan. There is no malaria risk in urban areas. Source of funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method unclear. Non‐supplemented group was self‐selected. |
| Allocation concealment (selection bias) | Low risk | Sealed closed envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different regimens compared. Women and staff would be aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Outcome assessor reported to be blinded. Laboratory outcomes unlikely to be affected by blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Women's reporting of side effects and compliance may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 20% lost to follow‐up but there were missing data for some outcomes (only 2 arms of this trial included in the analyses). |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Mukhopadhyay 2004.
| Methods | RCT 2‐arm trial. | |
| Participants | 111 apparently healthy pregnant women with less than 20 wks and no prior intake of iron supplements during this pregnancy with Hb equal or higher than 100 g/L and singleton pregnancy in New Delhi, India. Women who were taking anti‐epileptics or anti‐thyroid medications, had history of menorrhagia, bleeding disorders, chronic peptic ulcers, bleeding piles, thalassaemia or other haemoglobinopathies, or history of haemorrhage in present or past pregnancies were excluded. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 received 2 tablets of 100 mg elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) folic acid each (total 200 mg elemental iron and 1000 μg (1 mg) folic acid), to be taken only once a wk, 1 tablet before lunch and another tablet before dinner; group 2 received 1 tablet of 100 mg elemental iron and 500 μg (0.5 mg) folic acid daily. Women were advised to take the supplements 30 minutes before the meals and not with tea, coffee or milk. Also, women were advised to take calcium supplements after meals (500 mg elemental calcium twice daily). Iron supplementation started between 14 and 20 wks until delivery. Deworming, if required, was carried out with Mebendazole 100 mg twice a day for 3 days in the second trimester. Setting and health worker cadre: the intervention was performed by physicians and lay health workers at the All India Institute of Medical Sciences in New Delhi, India. |
|
| Outcomes | Maternal: Hb, serum ferritin concentrations at baseline and at 32‐34 wks, prevalence of anaemia, compliance to treatment, presence of intestinal parasites.
Infant: birthweight. Laboratory method for ferritin concentration: ELISA (Bioplus, South San Francisco, CA, USA). |
|
| Notes | Unsupervised.
Compliance measured by pill count and interview. compliance was 85% in group 1 (intermittent) and 40% in group 2 (daily). 1. By gestational age at start of supplementation: early (supplementation started before 20 wks' gestation or prior to pregnancy). 2. By anaemia status at baseline: non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: high weekly dose of iron in the intermittent group (more than 120 mg elemental iron per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk locations. As of 2011: malaria risk exists throughout the year in the whole country at altitudes below 2000 m, with overall 40% to 50% of cases due to Plasmodium falciparum and the remainder due to Plasmodium vivax. There is no transmission in parts of the states of Himachal Pradesh, Jammu and Kashmir, and Sikkim. Source of funding: Dean’s Research Grant, All India Institute of Medical Sciences, New Delhi, India. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. Block randomisation (block size = 10). |
| Allocation concealment (selection bias) | Unclear risk | There is insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different regimens compared. Women and staff would be aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Some outcome data (laboratory measures) were reported to have been collected and analysed by blinded technicians. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Reporting of side effects and compliance may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number randomised: 111 women. Only 80 were available for follow‐up (40 in each group) (20% attrition) and reasons for loss to follow‐up were not balanced across groups. 7 women in the daily supplementation group compared with 2 in the intermittent group were not included in the analysis because the "complained of gastrointestinal intolerance and changed to a different iron preparation". There was no ITT analysis. More than 20% excluded. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Mumtaz 2000.
| Methods | Double blinded RCT, 2‐arm trial with individual randomisation. | |
| Participants | 191 anaemic pregnant women between the ages of 17‐35 years of age, and uneventful obstetric history attending the Maternity wing of the Federal Government Services Hospital in Islamabad and the Maternal & Child Health Clinic at the Christian Mission Hospital in Taxila, Pakistan. | |
| Interventions | Participants were randomly assigned to 1 of 2 interventions: group 1 received 40 mg elemental iron (as 200 mg of ferrous sulphate) with 1000 μg (1 mg) of folic acid once daily; and group 2 received 40 mg elemental iron (as 200 mg of ferrous sulphate) with 1000 μg (1 mg) of folic acid on 2 days of the wk and placebo the rest of the days. Setting and health worker cadre: the intervention was performed by physicians at the Maternity Wing of the Federal Government Services Hospital, an urban, tertiary care hospital in Islamabad, and the Maternal & Child Health Clinic of the Christian Mission Hospital, a peri‐urban mission hospital in Taxila, Pakistan. |
|
| Outcomes | Outcomes measured included Hb concentration and serum ferritin at baseline and during the 3 following consecutive visits as well as compliance and weight. Change in Hb Z‐scores after supplementation was the main outcome variable, in women recruited at different gestational ages and duration of intervention varied. Data were not reported on outcomes prespecified in this review. Laboratory method for ferritin concentration: ELISA (Roche Diagnostics GmbH, Mannheim, Germany). |
|
| Notes | Data from this study have not been included in the analyses. 1. By gestational age at start of supplementation: unspecified gestational age or mixed gestational ages at the start of supplementation. 2. By anaemia status at baseline: unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Malaria risk throughout the country including urban areas. As of 2011: malaria risk – Plasmodium vivax and Plasmodium falciparum – exists throughout the year in the whole country below 2000 m. Source of funding: Pakistan Medical Research Council (Project No.P/61), Pakistan. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Not described clearly but women were provided with placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a placebo‐controlled trial. Women and staff would not be aware of treatment allocation. It was stated that the randomisation coded was not revealed until after follow‐up. It was reported that packs appeared identical. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Placebo‐controlled trial. It was reported that the randomisation code was not revealed until after follow‐up. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | Low risk | Placebo‐controlled trial. It was reported that the randomisation code was not revealed until after follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 191 women recruited and 160 followed up for 4 wks but there was further drop out at subsequent visits with 55% completing 4 scheduled follow‐up visits. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Pita Martin 1999.
| Methods | RCT 3‐arm trial. | |
| Participants | 203 healthy pregnant women with normal blood pressure at first visit, attending antenatal care clinic at Diego Paroissien Hospital in the Province of Buenos Aires, Argentina were included in the study, but in this review only 41 women who were randomised and completed the study were included in the analysis. | |
| Interventions | Participants were assigned to 1 of 3 groups: group 1 received 60 mg of elemental iron (as ferrous fumarate) daily; group 2 received 60 mg elemental iron (as ferrous fumarate) every 3 days; and group 3 received no treatment. Supplementation started at 8‐28 wks until 34‐37 wks of gestation. Setting and health worker cadre: the intervention was performed by physicians at the Diego Paroissien Hospital in the Matanza Provincia of Buenos Aires, Argentina. |
|
| Outcomes | Maternal: Hb, HCT, erythroporphyrin, serum ferritin concentration at baseline and at 34‐37 wks' gestation, premature birth.
Infant: birthweight. Laboratory method for ferritin concentration: ELISA (Boehringer Lab). |
|
| Notes | Unsupervised.
Women from control group (group 3) were not assigned randomly. These women were recruited but due to delays in the acquisition of the iron tablets and the progression of their pregnancies without supplementation they were left as controls in the study.
This study is used only for comparison between intermittent and daily iron supplementation (group 2 vs group 1).
Compliance not reported. 1. By gestational age at start of supplementation: unspecified gestational age or mixed gestational ages at the start of supplementation. 2. By anaemia status at baseline:unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. by bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous fumarate; 6. By intermittent iron supplementation regimen: other intermittent regimens (every 3 days). 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk‐free parts of countries that has malaria risk in other parts. As of 2011: malaria risk due exclusively to Plasmodium vivax is very low and is confined to rural areas along the borders with Plurinational State of Bolivia (lowlands of Salta province) and with Paraguay (lowlands of Chacoand Misiones provinces). Source of funding: Universidad de Buenos Aires, Argentina (subsidio BA 086). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Inadequate ‐ quasi‐randomised. Uneven group size. |
| Allocation concealment (selection bias) | High risk | The medical doctor had the list of the assignments for the coming participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and medical staff would be aware of treatment allocation (personal communication). |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were unlikely to have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | Unclear risk | Side effects not reported (not clear if this information was collected). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Inadequate. More than 20% lost to follow‐up. 203 randomised but analysis was for only 88 women who completed the trial (57% attrition). It was not clear how many women were randomised to each group, at follow‐up the size of the control group was much larger than the intervention groups: control n = 47, daily group n = 29 and intermittent group n = 12. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | The participants were all added to the control group as the iron supplements had not arrived at the time of the study initiation. |
Quintero 2004.
| Methods | RCT, 2‐arm trial with individual randomisation | |
| Participants | 107 healthy pregnant women with 6‐20 wks of gestation who had not received iron supplements during the current pregnancy attending 19 health units in the State of Morelos, Mexico. | |
| Interventions | Participants were randomly assigned by block pairs (anaemic and not anaemic) to receive either 120 mg of elemental iron (as ferrous sulphate) in a single dose daily or once weekly for 10 wks. Setting and health worker cadre: the intervention was performed by physicians at primary healthcare clinics in Morelos, Mexico. |
|
| Outcomes | Hb concentration, prevalence of anaemia and nutrient consumption at baseline and after 10 wks of supplementation were measured. Data on none of the prespecified outcomes of this review were available. Gestational ages at recruitment and follow‐up were very variable among the participants and results are therefore difficult to interpret. Laboratory method for ferritin concentration: ferritin not measured. |
|
| Notes | Data from this study have not been included in the analyses. 1. By gestational age at start of supplementation: early (supplementation started before 20 wks' gestation or prior to pregnancy). 2. By anaemia status at baseline: unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk‐free parts of countries that has malaria risk in other parts. As of 2011: malaria risk due almost exclusively to Plasmodium vivax exists throughout the year in some rural areas. There is moderate risk in some localities in the states of Chiapas and Oaxaca; very low‐risk localities are also found in the states of Chihuahua, Durango, Nayarit, Quintana Roo and Sinaloa. Source of funding: Health Department State of Morelos, Mexico; National Institute of Public Health, Curenavaca, Mexico. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer‐generated random numbers. |
| Allocation concealment (selection bias) | High risk | Participants were allocated to the groups consecutively by pairs. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different regimens compared. Women and staff would be aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were unlikely to have been affected by lack of blinding (not reported in the review). |
| Blinding of outcome assessment (detection bias) Side effects and compliance | Unclear risk | Not clear if data on side effects were collected (not reported). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 107 women recruited and complete data available for 77 women. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Ridwan 1996 (C).
| Methods | Cluster‐randomised trial from January to May 1995. | |
| Participants | 176 pregnant women with 8‐24 wks of gestation attending antenatal care at 6 health centres in West Java, Indonesia. | |
| Interventions | Health centres were randomised to 1 of 2 interventions: weekly regimen, where women received 120 mg of elemental iron (as ferrous sulphate) with 500 μg (0.5 mg) of folic acid; or daily regimen where women received 60 mg of elemental iron (as ferrous sulphate) with 250 μg (0.25 mg) of folic acid daily until wk 28‐32 of gestation.
Supplementation started at 8‐24 wks until 28‐32 wks of gestation. Setting and health worker cadre: the intervention was performed by midwives at rural health centres in Tangeran, West Java, Indonesia. |
|
| Outcomes | Maternal: Hb concentration, serum ferritin, weight at baseline and at 28‐32 wks of gestation; compliance to treatment and prevalence of parasitic infections. Laboratory method for ferritin concentration: ELISA (IMXSystem; Abbott, Abbott Park, IL). |
|
| Notes | Unsupervised but frequent contact with participants.
Randomisation was made by health centres.
Compliance measured by stool tests was 54.3% in the daily group and 62.2% in the weekly group.
Adjustment by ICC to show effective sample size taking into account cluster randomisation and unit of analysis. 1. By gestational age at start of supplementation: unspecified gestational age or mixed gestational ages at the start of supplementation. 2. By anaemia status at baseline: unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk locations. As of 2011: malaria risk exists throughout the year in all areas of the 5 eastern provinces of East Nusa Tenggara, Maluku, North Maluku, Papua and West Papua. In other parts of the country, there is malaria risk in some districts, except in Jakarta Municipality and in big cities. Source of funding: Abbott laboratories, Kimia Pharma, Jakarta, Indonesia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised using randomised numbers table. |
| Allocation concealment (selection bias) | High risk | As the assignment was per health centre, it was known which intervention the participants from the centre would get. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was a cluster‐randomised trial so contamination between groups may have been avoided. Staff and women would be aware of treatment allocation (tablets were distributed by midwives). |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were not aware of the groups. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Reported compliance and side effects may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 20% lost to follow‐up. 176 women randomised, full data for 139. Dropouts were described as similar to those remaining to follow‐up and reasons for attrition were balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | Some differences at baseline; women in the weekly group had significantly lower Hb, and serum ferritin levels (NS). |
Robinson 1999.
| Methods | RCT. 3 arms. | |
| Participants | 580 pregnant women served by 11 health centres from 5 sub‐districts on or near the western end of the island of Seram in the Province of Maluku, Indonesia between October 1996 and September 1997. | |
| Interventions | Participants were assigned to 1 of 3 interventions: group 1 (n = 200) received 60 mg of elemental iron (as ferrous sulphate) with 250 μg (0.25 mg) of folic acid daily by a traditional birth attendant; group 2 (n = 199) received 120 mg of elemental iron (as ferrous sulphate) with 500 μg (0.5 mg) of folic acid once a wk (two tablets) by the traditional home visiting birth attendants group 3 (n = 181) control group was formed by participants receiving traditional iron supplements (60 mg elemental iron) with folic acid from health centres in a usual manner, self‐administered without incentive. Each of the group was further assigned alternatively by registration number to receive 500 mg of mebendazole or a placebo at the second trimester of pregnancy. Setting and health worker cadre: the intervention was performed by traditional birth attendants in villages greater than 10 km from a health centre in Maluku, Indonesia. |
|
| Outcomes | Maternal: Hb concentration at baseline and after 12 and 20 wks of supplementation; serum ferritin at baseline and after 12 wks of supplementation; compliance. Laboratory method for ferritin concentration: not reported. |
|
| Notes | Daily group and control unsupervised. Weekly group supervised.
Only groups 1 and 2 are used in this analysis. Compliance was 69.6%, 96.2% and 46.9% for groups 1, 2 and control respectively. The study area is endemic to malaria. 1. By gestational age at start of supplementation: unspecified gestational age or mixed gestational ages at the start of supplementation. 2. By anaemia status at baseline: unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. As of 2011: malaria risk exists throughout the year in all areas of the 5 eastern provinces of East Nusa Tenggara, Maluku, North Maluku, Papua and West Papua. In other parts of the country, there is malaria risk in some districts, except in Jakarta Municipality and in big cities. Source of funding: World Health Organization, Geneva, Switzerland. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different regimens compared. Women and staff would be aware of treatment allocation. Supplements distributed by traditional birth attendants. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were unlikely to have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Compliance may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 20% lost to follow‐up and missing data for some outcomes. "Women with missing or bizarre data were not included in the analysis. In addition, women who did not carry their pregnancy the whole study period... were not included." |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | High risk | The study was a follow‐up of a previous study that included 200 women who were either receiving daily iron supplements distributed by traditional birth attendants and 100 women receiving usual care as of Ministry of Health provides in this country. Several months after the study was initiated, the daily group was further divided into daily or weekly regimens. The methodologies for assessing Hb and ferritin were also changed during the course of the study. The sample size also increased after the study design was changed. |
Rukhsana 2006.
| Methods | RCT with 3 arms. | |
| Participants | 90 pregnant clinically anaemic women (Hb < 110 g/L) with singleton pregnancy at 20‐24 wks of gestation and without previous intake of iron supplements attending antenatal care in the Department of Physiology, Basic Medical Sciences Institute with the collaboration of Deparment of Obstetrics and Gynaecology in Karachi, Pakistan. Women having severe anaemia, history of chronic illness, multiple pregnancy, known haemoglobinopathies, any prior blood transfusion and other high‐risk pregnancy were excluded. |
|
| Interventions | Participants were randomly allocated to 1 of the 3 groups: group 1 (n = 30) received 60 mg ferrous sulphate daily; group 2 (n = 30) received 60 mg ferrous sulphate twice weekly; and group 3 (n = 30) received 120 mg ferrous sulphate once weekly for 12 wks. Women were followed up during 12 wks of supplementation at 4 wks interval. Setting and health worker cadre: the study was carried out in Department of Physiology, Basic Medical Sciences Institute with the collaboration of Deparment of Obstetrics and Gynaecology in Karachi, Pakistan. It is not reported who conducted the measurements and who provided the supplements. |
|
| Outcomes | Hb concentration, RBC count, reticulocyte count, red cell indices, anaemia, severity of anaemia, MCV, MCH, MCHC, at baseline and after 12 wks of supplementation (approximately 34‐36 wks of gestation). Laboratory method for ferritin concentration: ferritin not measured. |
|
| Notes | 1. By gestational age at start of supplementation: late gestational age (supplementation started at 20 wks of gestation or later). 2. By anaemia status at baseline: anaemic (Hb below 110 g/L during first and third trimesters or below 105 g/L in second trimester) at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: twice a wk (group 2) and once a wk (group 3). 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Source of funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not mentioned in the study. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | High risk | Blinding of outcome assessment is not mentioned in the report. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Blinding of outcome assessment is not mentioned in the report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 82/90 participants were followed until 4 wks of the intervention and 75 completed the full duration of the study. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | All 3 groups were comparable in maternal age, parity, blood pressure, weight, height, BMI, and gestational age were similar at baseline. |
Singh 2011.
| Methods | RCT; 2‐arms | |
| Participants | 100 apparently healthy women with a singleton pregnancy between 18‐22 wks' gestation attending routine antenatal care at the Department of Obstetrics and Gynaecology of the Medical University, Lucknow in Uttar Pradesh, India. Women with less than 90 g/L or more than 110 g/L Hb and any chronic systemic disorder or with high‐risk pregnancy were excluded. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 (n = 50) received 1 table daily containing 100 mg elemental iron, 1500 μg (1.5 mg) folic acid and 15 μg vitamin B12; group 2 (n = 50) received 2 tablets to be taken once a wk containing a total of 200 mg elemental iron, 1500 μg (1.5 mg) folic acid and 15 μg vitamin B12). All participants were de‐wormed using a single dose tablet containing 400 mg of albendazole. The intervention lasted 14 wks. Setting and health worker cadre: antenatal care at the Department of Obstetrics and Gynaecology of the Medical University, Lucknow in Uttar Pradesh, India. the intake of the supplements was reported to have been supervised but it is not indicated by who. However they report these women received routine antenatal care. |
|
| Outcomes | Hb, PCV after 4, 8 and 14 wks of the intervention, side effects, serum iron, total iron binding capacity, serum ferritin at 14 wks after supplementation. Laboratory method for ferritin concentration: enzyme immunoassay method. |
|
| Notes | Participants in the weekly group received supervised intake on the tablets. Participants in the iron daily group reported every wk to show the empty blister before getting the iron for the next wk in an effort to improve compliance. 1. By gestational age at start of supplementation: unspecified gestational age or mixed gestational ages at the start of supplementation. 2. By anaemia status at baseline: non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: high weekly dose of iron in the intermittent group (more than 120 mg elemental iron per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: not specified/unreported/unknown. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk locations. As of 2011: malaria risk exists throughout the year in the whole country at altitudes below 2000 m, with overall 40% to 50% of cases due to Plasmodium falciparum and the remainder due to Plasmodium vivax. Source of funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As patients had to report the blisters weekly to the care providers to receive the next wk dose, and those participants in the weekly group received supervised intake, it is implied that this was an open trial. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were unlikely to have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Reporting of side effects and compliance may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was 11%. Data for 89/100 were included in the trial. There were 5 losses in the daily group and 6 in the weekly iron supplemented group. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Viteri 2012.
| Methods | Cross‐over design with 2 interventions. | |
| Participants | 100 apparently healthy pregnant women with no disease or addictions (including tobacco), no nutritional supplements taken before gestational wk 20, single‐fetus pregnancy, and blood Hb > 115 g/L (equivalent to 105 g/L at sea level) at gestational wk 20 were recruited during their first visit for prenatal care at the Instituto Nacional de Perinatología Isidro Espinosa de los Reyes (INPerIER) in Mexico City, Mexico. | |
| Interventions | Participants were randomly assigned, without replacement, to 1 of 2 groups: group 1 received daily supplementation from gestational wk 20 to wk 28 with a tablet containing 60 mg elemental iron, 200 μg (0.2 mg) of folic acid, and 1 mg of vitamin B12, and then, weekly supplementation with 2 tablets (providing 120 mg of elemental iron, 400 μg (0.4 mg) of folic acid, and 2 mg of vitamin B12) from wk 28 to wk 36; group 2 received the supplementation scheme inverted, that is the weekly regimen from gestational wk 20 to wk 28, followed by daily supplementation up to wk 36. Setting and health worker cadre: the study was performed at the Instituto Nacional de Perinatología (INPer) in Mexico City (Mexico), located at an altitude of 2240 m (7350 feet) above sea level. The intervention and care were provided by attending physicians, laboratory personnel, and researchers including a nutritionist. |
|
| Outcomes | Hb values at different gestational ages, serum ferritin, serum iron concentrations, serum malon‐di‐aldehyde (MDA) equivalent values (µmoles/L) as Thio‐Barbituric‐Acid‐Reacting‐Substances (TBARS), abortion, birthweight, gestational age at birth and maternal iron status. Laboratory method for ferritin concentration: ELISA (Opus, Dade Behringer, Newark, USA). |
|
| Notes | Only data from 20 up at 28 wks were included in the analysis. 1. By gestational age at start of supplementation: late gestational age (supplementation started at 20 wks of gestation or later). 2. By anaemia status at baseline: non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: not specified/unreported/unknown. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk‐free parts of countries that has malaria risk in other parts. As of 2011: malaria risk due almost exclusively to Plasmodium vivax exists throughout the year in some rural areas. There is moderate risk in some localities in the states of Chiapas and Oaxaca; very low‐risk localities are also found in the states of Chihuahua, Durango, Nayarit, Quintana Roo and Sinaloa. Source of funding: CONACYT, Mexico (grant 212250‐49381) and by funds from an unrestricted donation of funds for the author at Children's Hospital Oakland research Institute, Oakland, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From a computer‐generated randomised list. |
| Allocation concealment (selection bias) | Unclear risk | There is no description of any method used to conceal the allocation sequence or assess whether the intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Of the whole research team, only the nutritionist knew the subject’s assigned group in order to dispense the tablets and evaluate the subject’s adherence to supplementation. However, the rest of the personnel, including the attending physicians, laboratory personnel, and researchers were blinded." |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory personnel were blinded to the intervention groups. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | Low risk | Attending physicians and researchers were blinded to the groups of the participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 84% completed the study including all required antenatal care. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | Age, parity, literacy, and Hb and ferritin concentrations at baseline did not differ between the women who completed the study and those who did not. "No differences were observed between supplementation groups at baseline. Both groups showed an adequate adherence to the supplementation programme (86%)." |
Winichagoon 2003 (C).
| Methods | Cluster‐randomised trial. A total of 61 villages in six sub‐districts were stratified by size and then cluster randomised at village level. | |
| Participants | 484 apparently healthy pregnant women with 13‐17 wks of gestation who had not received iron supplements before enrolling in the study, and who had a Hb concentration > 80 g/L attending antenatal care clinics at the district hospital and 7 health centres from 54 villages in the Province of Khon‐Kaen in northeast Thailand. | |
| Interventions | The 61 villages were grouped according to size and then randomised in 4 clusters to 1 of 3 interventions: group 1 received a daily regimen providing 60 mg of elemental iron (as ferrous sulphate) with 250 μg (0.25 mg) of folic acid daily; group 2 received 120 mg of elemental iron (as ferrous sulphate) with 3500 μg (3.5 mg) of folic acid once a wk; and group 3 received 180 mg of elemental iron (as ferrous sulphate) with 3500 μg (3.5 mg) of folic acid once a wk.
Supplementation started at 15 +/‐ 2 wks until delivery. Setting and health worker cadre: the intervention was performed by lay health workers in rural villages in Ubolrat district of Khon‐Kaen Province in Thailand. |
|
| Outcomes | Maternal: Hb concentration, serum ferritin, free erythrocyte protoporphyrin at baseline and at 35 +/‐ 2 wks of gestation, and 4‐6 months postpartum; HCT prior to delivery; weight at baseline and at 35 wks of gestation; compliance, Hb type, and hookworm prevalence.
Infant: birthweight, Hb concentration and serum ferritin at 4‐6 months. Laboratory method for ferritin concentration: immunofluorescence (ELISA kit (Opus, Dade Behringer, Newark, DE, USA). |
|
| Notes | Unsupervised.
Compliance not reported.
Values adjusted to reflect effective sample size for cluster randomisation. 1. By gestational age at start of supplementation: early (supplementation started before 20 wks' gestation or prior to pregnancy). 2. By anaemia status at baseline: unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk) (group 2) and high weekly dose of iron in the intermittent group (more than 120 mg elemental iron per wk) (group 3). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk locations.As of 2011: malaria risk exists throughout the year in rural, especially forested and hilly, areas of the whole country, mainly towards the international borders, including the southernmost provinces. There is no risk in cities (e.g. Bangkok, Chiang Mai city, Pattaya), Samui island and Phuket island. However, there is a risk in some other areas and islands. Source of funding: UNICEF Thailand. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomisation but method unclear. |
| Allocation concealment (selection bias) | High risk | The villages were stratified by size and then assigned the group at village level. All participants from that village would receive a predetermined intervention. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was a cluster‐randomised trial which may have meant contamination was reduced. Different regimens compared. Women and staff would be aware of treatment allocation although it was stated that the tablets had the same appearance so the comparison of different doses of weekly supplementation may have been blinded for women. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were unlikely to have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Reporting of side effects and compliance may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 20% lost to follow‐up. "... the analysis of data was performed only on women who ingested more than 75% of the iron tablets provided and whose haemoglobin never fell < 80 g/L". 484 women randomised, 379 completed study (22% attrition). 11 excluded from the weekly group for poor compliance or because they received other supplements. Reasons for attrition across groups were not balanced. |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Yekta 2011.
| Methods | RCT 3‐arms with individual randomisation. | |
| Participants | 210 pregnant women with 17–20 wks' gestation and singleton pregnancies, no known disease, and Hb levels higher than 110 g/L attending local public healthcare centres at 7 prenatal healthcare clinics between September 2007 and February 2009 in the urban regions of Urmia city North West Iran. | |
| Interventions | Participants were randomly assigned to 1 of 3 groups: group 1 (n = 70) received 2 iron supplementation tablets once weekly providing 100 mg elemental iron per wk (as ferrous sulphate); group 2 (n = 70) received 1 tablet twice weekly providing 100 mg elemental iron per wk (as ferrous sulphate); and group 3 (n = 70) received 1 tablet daily containing 50 mg elemental iron per day (as ferrous sulphate). No additional micronutrients were supplied. Setting and health worker cadre: the intervention was performed by an assigned healthcare provider in local public healthcare centres at 7 prenatal healthcare clinics between September 2007 and February 2009 in the urban regions of Urmia city North West Iran. |
|
| Outcomes | Hb and serum ferritin levels were measured at 20, 28, and 38 wk; anaemia and haemoconcentration were calculated. Pregnancy and birth outcomes (pregnancy termination, method of delivery, birthweight, stillbirth) were reported. Adherence and side effects were also recorded. Laboratory method for ferritin concentration: RIA (Kavoshyar ferritin IRMA [I125] kit, Kavoshyar Iran Co, Tehran, Iran). |
|
| Notes | 1. By gestational age at start of supplementation: early (supplementation started before 20 wks' gestation or prior to pregnancy). 2. By anaemia status at baseline: non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk (group 1) and other intermittent regimens (twice a wk) (group 2). 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk‐free parts of countries that has malaria risk in other parts. As of 2011: malaria risk due to Plasmodium vivax and Plasmodium falciparum exists from March to November inclusive in rural areas of the provinces of Hormozgan and Kerman (tropical part) and the southern part of Sistan‐Baluchestan. Source of funding: Student Research Committee, Urmia University of Medical Sciences, Iran. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The assignment to the groups is described as random but the method of randomisation used is not described. |
| Allocation concealment (selection bias) | Unclear risk | There is insufficient information in the article to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, care providers and outcome assessor were not blinded to the interventions. It was mentioned in the paper that some women decided to change to daily regimens based on the recommendations of their private physicians. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were unlikely to have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | High risk | Reporting of side effects and compliance may have been affected by lack of blinding (although it was reported that compliance was 100% and there were no side effects reported). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 201 women completed the study out of 210 randomised (less than 5% lost to follow‐up). The exclusions were mostly in the group 1 that had 8 losses (11%), compared to 1 participant in group 2 (1/70 = 1%) and none in the daily regimen. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess. |
| Other bias | High risk | Participants were similar in age, body weight, parity, and height at baseline. Women in the weekly group had significantly lower Hb at baseline. |
Young 2000.
| Methods | RCT 2‐arm trial. | |
| Participants | 413 healthy non‐severely anaemic pregnant women attending antenatal care at Ekwendeni Hospital or its mobile clinics in northern Malawi with less than 30 wks of gestation at their first visit, stratified by initial Hb concentration before randomisation. Supplementation starting time variable (22.2 +/‐ 4.8 wks) and ending time variable (32.2 +/‐ 4.4 wks of gestation). | |
| Interventions | Participants were randomly assigned within each anaemia grade category to 1 of 2 interventions: group 1 received 120 mg of elemental iron (as ferrous sulphate) with 500 μg (0.5 mg) of folic acid once a wk; group 2 received 60 mg of elemental iron (as ferrous sulphate) with 250 μg (0.25 mg) of folic acid daily. Setting and health worker cadre: the intervention was performed by midwives and public health workers at rural, weekly antenatal clinics and monthly mobile maternal and child health clinics in northern Malawi. |
|
| Outcomes | Maternal: Hb concentration at baseline and after 8 wks of supplementation; compliance, presence of side effects, and prevalence of anaemia. Laboratory method for ferritin concentration: unreported/unknown. |
|
| Notes | Unsupervised.
Average gestational age at start was 22.2 +/‐ 4.8 wk and 32.2 +/‐ 4.4 wk at the end of study.
Compliance estimated by self reporting was 76% and 60% in the weekly and daily groups respectively. 1. By gestational age at start of supplementation: unspecified gestational age or mixed gestational ages at the start of supplementation. 2. By anaemia status at baseline: unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. study carried out in malaria risk locations. As of 2011: malaria risk due predominantly to Plasmodium falciparum exists throughout the year in the whole country. Source of funding: UNICEF Malawi and World Vision Malawi. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate by computer‐generated random number table. |
| Allocation concealment (selection bias) | Unclear risk | There is insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different regimens compared. Women and staff would be aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes were unlikely to have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | Unclear risk | Reporting of side effects and compliance may have been affected by lack of blinding (compliance also objectively tested by stool test).Compliance estimated by self‐reporting was 76% and 60% in the weekly and daily groups respectively. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 47% lost to follow‐up.Stated that dropouts had similar baseline characteristics as those remaining for follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Yu 1998.
| Methods | Quasi‐randomised trial. | |
| Participants | 51 healthy pregnant women with 18‐22 wks of gestation who had not taken supplements or medication in the previous 6 months attending public health centre in Ulsan, South Korea. | |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 received 160 mg of elemental iron (as ferrous sulphate) in 1 intake once a wk; group 2 received 80 mg of elemental iron (as ferrous sulphate) daily. Women with low Hb were assigned by the trialists to daily regimen. Supplementation started at 20.1 wks and 20.2 wks of gestation for groups 1 and 2 respectively. Setting and health worker cadre: the intervention was performed by physicians at a public health centre in Ulsan, Korea. |
|
| Outcomes | Maternal: Hb concentration, serum ferritin, red blood cell count, hematocrit, MCV, MCH, MCHC, serum iron, total iron binding capacity, transferrin saturation at baseline and after intervention; zinc status before and after intervention, weight gain, nutrient intake before and after intervention.
Infant: birthweight. Laboratory method for ferritin concentration: unreported/unknown. |
|
| Notes | Unsupervised.
No compliance reported for all the groups. Analysis reported on high compliers only. 1. By gestational age at start of supplementation: late gestational age (supplementation started at 20 wks of gestation or later). 2. By anaemia status at baseline: unspecified/mixed anaemia status at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: high weekly dose of iron in the intermittent group (more than 120 mg elemental iron per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk; 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. Study carried out in malaria risk‐free parts of countries that has malaria risk in other parts. As of 2011: limited malaria risk due exclusively to Plasmodium vivax exists mainly in the northern areas of Gangwon‐do and Gyeonggi‐do Provinces and Incheon City (towards the Demilitarized Zone or DMZ). Source of funding: Korean Science and Engineering Foundation (project number 971‐0603‐019‐1. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised. |
| Allocation concealment (selection bias) | High risk | "The study subjects were divided to two groups randomly but the subjects whose Hb level was very low were allocated to the daily group ethically because the effect of weekly supplementation was not yet established as safety". It was not stated how many women this involved or whether these women were included in the analysis. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different regimens compared. (Assessment from brief abstract) |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Laboratory outcomes blinded. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | Unclear risk | Side effects not reported. No compliance reported for all the groups. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 54% lost to follow‐up. The number randomised was not clear. 14 were lost from the daily group for various reasons including wanting to change supplementation or low compliance. 10 were included in the analysis in the daily group and 13 in the weekly group. |
| Selective reporting (reporting bias) | High risk | Analysis reported on high compliers only. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Zamani 2008.
| Methods | Described as a “field‐based randomised trial”. 2 study arms. Individual randomisation. | |
| Participants | 152 healthy, non‐anaemic pregnant women aged 18‐38 years, 15‐16 wks’ gestation (gestation estimated by menstrual dates and ultrasound) attending 2 clinics for prenatal care in Isfahan, Iran. ("In Iran, it is mandatory to prescribe iron (1 tablet containing 45 mg elemental iron (as ferrous sulphate) per day) and folic acid supplements to pregnant women after the 15th‐18th week of gestation".) Exclusion criteria: current anaemia (Hb < 110 g/L), past history of anaemia, thalassaemia, or other blood disorders, history of previous obstetric problems (haemorrhage, pregnancy‐induced hypertension, diabetes) or any other chronic systemic disorder. |
|
| Interventions | Participants were assigned to 1 of 2 groups: group 1 (experimental group) received 2 tablets of 45 mg elemental iron (as ferrous sulphate) taken on a single day each wk. “Women in the trial group were instructed to choose any day of the wk and to take 2 tablets of 45 mg elemental iron (as ferrous sulphate) each on the same day every week, one in the morning and one before dinner” i.e. 90 mg of “elemental iron (as ferrous sulphate) one day per week in two takes”. (Supplied as 8 tablets every 4 wks) for 16 wks (from recruitment at 16‐18 wks); group 2 (control group) were to take 1 tablet containing 45 mg elemental iron (as ferrous sulphate) daily for 16 wks (from recruitment at 16‐18 wks). Supplied as 28 tablets every 4 wks. Women were followed up 4 weekly until 16 wks of supplementation. It was not clear whether or not women received additional nutritional supplements, but according to local protocol women in both groups probably received folate supplements but the length and dose of supplements were not mentioned. Setting and health worker cadre: the intervention was performed by obstetricians at a prenatal clinic in Isfahan, Iran. |
|
| Outcomes | Compliance and side effects recorded by women on diary card. Compliance was also assessed through interview. Hb concentration (g/L) before and after intervention (with breakdown of results at various baseline Hb levels). Serum ferritin (g/L) before and after intervention. In the introduction it was stated that gestation at delivery and mode of delivery were noted but results were not reported for these outcomes Laboratory method for ferritin concentration: unreported/unknown. |
|
| Notes | Setting re anaemia, haemoglobinopathies, hookworm prevalence: not mentioned. 1. By gestational age at start of supplementation: early (supplementation started before 20 wks' gestation or prior to pregnancy). 2. By anaemia status at baseline: non‐anaemic (Hb 110 g/L or above during first and third trimesters or Hb 105 g/L or above if in second trimester) at start of supplementation. 3. By weekly iron dose in the group receiving intermittent supplementation: low weekly dose of iron in the intermittent group (120 mg elemental iron or less per wk). 4. By release speed of iron supplements: not specified/unreported/unknown. 5. By bioavailability of the iron compound relative to ferrous sulphate: equivalent or lower: ferrous sulphate. 6. By intermittent iron supplementation regimen: once a wk. 7. By malaria endemicity of the area in which the trial was conducted: not specified/unreported/unknown. study carried out in malaria risk‐free parts of countries that has malaria risk in other parts. As of 2011: malaria risk due to Plasmodium vivax and Plasmodium falciparum exists from March to November inclusive in rural areas of the provinces of Hormozgan and Kerman (tropical part) and the southern part of Sistan‐Baluchestan. Source of funding: Isfahan University of Medical Sciences, Isfahan, Iran. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers. |
| Allocation concealment (selection bias) | Unclear risk | Described the use of coded vials but these would contain different numbers of tablets. Not clear whether those carrying out recruitment distributed tablets. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women would be aware that they were taking tablets every day vs 1 day each wk. Not clear whether blinding would be convincing to staff. It was stated that the tablets were provided in identical coded vials. “The investigators were blind to allocation of the treatment group ... at initial recruitment. Laboratory technicians were not aware of the group..”. However, the vials would feel different: 1 contained 8 and 1, 28 tablets. |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | It was stated that laboratory technicians were blinded. |
| Blinding of outcome assessment (detection bias) Side effects and compliance | Unclear risk | It was stated that women recorded side effects on cards that did not reveal allocation but it was not clear whether recall bias might be different in women taking iron daily or intermittently. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 152 women recruited, 76 in each group, 30 women were lost to follow‐up ‐ 20% attrition, but loss was not balanced across groups: 7 were lost from the daily supplementation group and 23 from the intermittent group. |
| Selective reporting (reporting bias) | Unclear risk | It was stated that gestation at delivery and mode of delivery were noted but results were not reported for these outcomes. |
| Other bias | Unclear risk | Baseline information was only provided for those women completing the study and loss to follow‐up was not balanced across groups ‐ although it was stated in the text that the baseline characteristics of women that dropped out were similar to those that completed the study. |
BMI: body mass index ELISA: enzyme‐linked immunosorbent assay Fe: iron Hb: haemoglobin HCT: hematocrit (same as PCV) ICC: intra class correlation coefficient ITT: intention‐to‐treat IU: international unit MCH: mean corpuscular haemoglobin MCHC: mean corpuscular haemoglobin concentration MCV: mean corpuscular volume NSAIDs: non‐steroidal anti‐inflammatory drugs PCV: packed cell volume (same as HCT) RBC: red blood cell RCT: randomised controlled trial RIA: radioimmuno assay SES: socio‐economic status vs: versus wk(s): week(s)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aaseth 2001 | 67 non‐anaemic pregnant women attending prenatal care clinics in Kingsvinger Hospital, in Kingsvinger, Norway were allocated to a regimen of either 100 mg elemental iron daily or 15 mg elemental iron daily. Both groups received iron at different doses to be taken daily. The types of interventions do not allow for comparisons within the scope of this review. |
| Abel 2000 | Community‐based study in Vellore district, India using a pre‐post experimental design measuring the impact of an iron supplementation program, helminthic treatment and education intervention in the prevalence of anaemia in the different trimesters of pregnancy. The same pregnant women were not followed. |
| Adhikari 2009 | 320 pregnant women attending the Tribhuvan University Teaching Hospital, Nepal for antenatal care were randomised to 1 of 4 groups: group 1: 60 mg elemental iron daily (as ferrous sulphate); group 2: 60 mg elemental iron daily (as ferrous sulphate) with a count of unused pills at antenatal appointments; group 3: 60 mg elemental iron daily (as ferrous sulphate) with education (direct counselling and colour brochure) on iron and anaemia; group 4: 60 mg elemental iron daily (as ferrous sulphate) with pill count and education (direct counselling and colour brochure) on iron and anaemia. In this randomised trial the aim of the intervention was to increase compliance and all 4 intervention groups received daily iron supplements. The types of interventions do not allow for comparisons within the scope of this review. |
| Afifi 1978 | 260 pregnant women from Cairo, Egypt (formerly part of United Arab Republic) were randomly allocated to 1 of 2 groups: group 1 received 130 mg elemental iron daily (a slow release ferrous sulphate preparation, Plexafer‐F®) and 360 μg (0.36 mg) folic acid; group 2 received iron (as ferrous sulphate, no dose reported) in addition to 5000 μg (5 mg) folic acid. Both groups received daily iron supplementation in different preparations. The types of interventions do not allow for comparisons within the scope of this review. |
| Ahn 2006 | 209 pregnant women between 18 and 45 years of age, attending outpatient obstetric clinics at North York General Hospital and the Hospital for Sick Children in Toronto, Canada were randomly assigned to receive multiple micronutrient supplements containing 60 mg of elemental iron (as ferrous fumarate) (Materna®) or another supplement (PregVit®) to be taken twice daily with the morning dose containing 35 mg of elemental iron (as ferrous fumarate) and the evening dose containing 300 mg calcium, and other vitamins and minerals. Both groups received daily iron in different doses as well as other vitamins and minerals. The types of interventions do not allow for comparisons within the scope of this review. |
| Angeles‐Agdeppa 2005 | 744 apparently healthy pregnant (with less than 20 wks) and non‐pregnant women of reproductive age (15‐49 years) from the municipalities of Calasiao, Binmaley and Santa Barbara, Philippines who were pregnant or most likely to become pregnant within the 12‐month duration of the study, and who volunteered to participate in the study were provided 2 preparations of iron + folic acid supplements. Women with severe anaemia or history of malaria were excluded. Non‐pregnant women were prescribed 4 capsules monthly each containing 60 mg of elemental iron and 3500 μg (3.5 mg) folic acid to be taken once weekly before bedtime (to be purchased by the women in local drugstores). Pregnant women received free of cost 4 capsules monthly each containing 120 mg of elemental iron and 3500 μg (3.5 mg) of folic acid to be taken once a wk before bedtime until delivery and for 3 months thereafter. Pregnant women seen at the health centres with 20 wks or more of gestation were advised to take their usual daily dose of iron+folic acid tablets containing 60 mg of elemental iron and 500 μg (0.5 mg) of folic acid. Women were followed for 12 months. Hb, HCT, MCV, MCHC, serum ferritin, transferrin receptors, prevalence of iron deficiency and anaemia, compliance were assessed at baseline, 4.5, 9 and 12 months. There was not randomisation and the control group was not appropriate for comparisons. |
| Babior 1985 | 15 healthy pregnant women 22‐32 years old, in the first trimester of pregnancy from Boston, Massachusetts, USA were randomly assigned to 3 different multiple micronutrient preparations to assess absorption of iron. |
| Barton 1994 | 97 healthy women attending prenatal care at National Maternity Hospital, Dublin, Ireland with singleton pregnancy, during their first trimester of pregnancy, and with Hb equal or higher than 140 g/L were randomly assigned to 1 of 2 groups: group 1: received 1 tablet containing 60 mg elemental iron and 500 μg (0.5 mg) of folic acid and to be taken by mouth twice daily (total 120 mg elemental iron daily and 1000 μg (1 mg) folic acid); group 2: placebo tablets also to be taken by mouth twice daily. Supplementation started at 12 wks until delivery. No postpartum supplementation. The types of interventions do not allow for comparisons within the scope of this review. |
| Batu 1976 | 133 women attending an antenatal clinic for the fist time in Yangoon (also known as Rangoon), Myanmar (Burma). Women with severe anaemia were excluded. Women were randomly assigned to 1 of 4 groups starting at 22‐25 wks: group 1: 1 tablet containing 60 mg of elemental iron (as ferrous sulphate), and 1 placebo tablet twice daily; group 2: 1 tablet containing 60 mg of elemental iron (as ferrous sulphate), and 1 tablet containing 500 μg (0.5 mg) of folic acid twice daily; group 3: 2 placebo tablets twice daily; group 4: 1 placebo tablet and 1 tablet containing 500 μg (0.5 mg) of folic acid twice daily. Administration of the treatments was carefully supervised. Supplementation started at 22‐25 wks until term. The types of interventions do not allow for comparisons within the scope of this review. |
| Bencaiova 2007 | 260 women with singleton pregnancy in Zurich, Germany, were randomised at 21‐24 wks of gestation to receive either intravenous iron group (further divided into 2 doses of 200 mg iron saccharate or 3 doses of 200 mg iron) or 80 mg elemental iron (as ferrous sulphate) daily. Both groups received iron in different routes of administration. No comparisons allowed within the scope of this review. |
| Berger 2005 | 864 apparently healthy married pregnant and non‐pregnant nulliparous women of reproductive age planning to have a child soon from 19 rural communes of the Thanh Mien district in Hai Duong province, Vietnam were invited to participate and assigned to 1 of the following interventions according to their pregnancy status at baseline: women who were pregnant received free of charge UNICEF tablets containing 60 mg of elemental iron and 250 μg (0.25 mg) of folic acid to be taken daily and women who were non‐pregnant were prescribed pink packs of tablets containing 60 mg of elemental iron and 3500 μg (3.5 mg) of folic acid that they could buy at their village from the Women's Union, to be taken once weekly. If these women became pregnant, women received red packs of tablets containing 120 mg of elemental iron and 3500 μg (3.5 mg) of folic acid free of charge to be taken once weekly. After delivery women were given tablets containing 60 mg of elemental iron and 500 μg (0.5 mg) of folic acid free of charge for 3 months to be taken weekly. Hb concentration, serum ferritin, and serum ferritin receptors, prevalence of anaemia and iron deficiency and compliance were measured at baseline, at 4.5, 9 and 12 months. This is not a randomised study and no comparisons can be made for the aims of this review. |
| Bergsjo 1987 | Planned study registered at the Oxford Database of Perinatal Trials. Author contacted and informed the project was not completed. |
| Blot 1980 | 203 pregnant women attending prenatal care clinics during their 6th month visit were randomly allocated to either 105 mg of elemental iron with 500 mg of ascorbic acid or a placebo. Both groups received iron. No comparisons allowed within the scope of this review. |
| Brown 1972 | 109 pregnant women attending prenatal care clinics in Manchester, England were randomly allocated to 1 of 3 groups: group A: 1 tablet daily given in 'reminder packs', group B: 1 tablet daily given in loose forms, or group C 2 tablets daily given in loose form. Tablets contained 50 mg of elemental iron (as slow release ferrous sulphate) and 400 μg (0.4 mg) of folic acid. All groups received iron daily. The types of interventions do not allow for comparisons within the scope of this review. |
| Burslem 1968 | 472 pregnant women attending the booking clinic in Manchester, England were alternatively allocated to 2 forms of iron: group 1 received 105 mg elemental iron (as a slow release ferrous sulphate preparation) and a tablet containing 5000 μg (5 mg) folic acid; group 2 received 3 tablets of combined conventional 60 mg elemental iron (as ferrous sulphate) and 1 tablet containing 5000 μg (5 mg) folic acid for a total of 180 mg elemental iron daily. Both groups received daily iron supplementation in different preparations. The types of interventions do not allow for comparisons within the scope of this review. |
| Buss 1981 | 18 pregnant women were randomly assigned to receive either a tablet containing 80 mg of elemental iron with a new mucous membrane vaccine (Tardyferon®) or a tablet containing 80 mg elemental iron with 350 μg (0.35 mg) folic acid (Tardyferon‐Fol®) for a period of 3 months. All women received daily iron. The types of interventions do not allow for comparisons within the scope of this review. |
| Butler 1968 | 200 women before 20th wk of gestation and Hb above 100 g/L attending antenatal clinic in Cardiff, United Kingdom were studied. Women were randomly allocated to 3 groups: group 1 received 122 mg of elemental iron (as ferrous sulphate) daily; group 2: received 122 mg of elemental iron (as ferrous sulphate) plus 3400 μg (3.4 mg) of folic acid daily; group 3: no treatment. A group 4 was formed as some participants (n = 38) from group 3 received iron supplements for treatment of anaemia in the course of the intervention. Women were supplemented from wk 20 to wk 40 of gestation. The types of interventions do not allow for comparisons within the scope of this review. |
| Buytaert 1983 | 45 non‐anaemic women with singleton pregnancy and no major illnesses attending the University Hospital Obstetric and Gynaecologic Clinic in Antwerp, Belgium. Women were randomly assigned to 1 of 2 groups: group 1 received 105 mg of elemental iron (as sustained released ferrous sulphate) daily; group 2 received no iron supplement. Supplementation started at 14‐16th wk of gestation and continued until delivery. The types of interventions do not allow for comparisons within the scope of this review. |
| Cantlie 1971 | 27 non‐anaemic pregnant women 17‐35 years of age from 4 participating obstetricians' private practice clinics from Montreal, Canada in their 1‐5th month of pregnancy with Hb 12 g/dL or higher in first trimester and 11 g/dL or higher in second trimester. Women were randomly assigned to 2 groups: group 1 received 39 mg elemental iron to be taken twice daily with meals (total daily 78 mg elemental iron); group 2 who received no iron tablets. Both groups received 1 tablet of multiple micronutrients supplement daily containing: copper citrate 2 mg, magnesium stearate 6 mg, manganese carbonate 0.3 mg, vitamin A 1000 IU, vitamin D 500 IU, bone flour 130 mg, vitamin B1 1 mg, vitamin B2 1 mg, brewer yeast concentrate 50 mg, niacinamide 5 mg, vitamin C 25 mg, sodium iodide 0.2 mg and folate 0.049 μg (naturally occurring). Duration of supplementation unclear. The types of interventions do not allow for comparisons within the scope of this review. |
| Carrasco 1962 | 2 liquid preparations were used in this study: 1 with D‐sorbitol and the other without. Both preparations contained vitamin B12, vitamin B6, ferric pyrophosphate and folic acid. |
| Chan 2009 | 1164 women with singleton pregnancies, a gestational age of 16 wks or less and able to understand English or Chinese attending first antenatal booking visit at single regional hospital in Hong Kong, China between April 2005 and March 2007 were randomly assigned to receive 60 mg elemental iron daily (as ferrous sulphate) or daily placebo indistinguishable in appearance from the active treatment. Main maternal outcomes are development of gestational diabetes at 28 or 36 wks, Hb (g/dL, serum transferrin (g/L), serum ferritin (pmol/L), compliance, glucose level, mode of delivery. Neonatal outcomes: gestational age at delivery, preterm delivery, birthweight, Apgar score at 1 and 5 minutes, arterial blood pH, Hb of cord blood (g/dL), ferritin of cord blood (pmol/L), jaundice, birth trauma, infection, congenital abnormality or metabolic disorder. The types of interventions do not allow for comparisons within the scope of this review. |
| Chanarin 1965 | 190 pregnant women attending antenatal clinic in St Mary's Hospital in London, England were invited to participate in the study and 189 accepted and were randomly assigned to 1 of 3 groups: group 1 received 3 tablets containing 100 mg of ferrous fumarate to be taken daily (total 300 mg ferrous fumarate daily); group 2 received 3 tablets containing 100 mg of ferrous fumarate with 10 μg (0.01 mg) folic acid (total 300 mg ferrous fumarate and 30 μg (0.03 mg) folic acid daily, or placebo (containing lactose). The outcomes measured include full blood count at 20th, 30th, 35th and 39th wk of gestation and 6th day after delivery. The paper does not report standard deviations in the variables measured and no data can be extracted. The types of interventions do not allow for comparisons within the scope of this review. |
| Chanarin 1971 | 251 women attending antenatal clinic at a London hospital, United Kingdom before 20th wk of gestation. Women were allocated by sequence to 1 of 5 groups: group 1: oral dose of 30 mg of elemental iron daily (as ferrous fumarate); group 2: oral dose of 60 mg of elemental iron (as ferrous fumarate) daily; group 3: oral dose of 120 mg of elemental iron (as ferrous fumarate) daily; group 4: placebo; group 5: 1 g of iron (Imferon, 4 x 250 mg) intravenously before wk 20, and thereafter oral 60 mg of elemental iron (as ferrous fumarate) daily. Supplementation started at 20th wk until 37th wk. The types of interventions do not allow for comparisons within the scope of this review. |
| Charoenlarp 1988 | 325 pregnant women with Hb (AA) and 232 pregnant women with Hb (AE) attending midwife centres in 80 villages in the Ubon Province, Thailand. Chronic illness, complicated pregnancy, severe anaemia (Hb < 80 g/L), haemoglobinopathies Hb (EE) and (EF), and unwillingness to co‐operate were reason for exclusion. Individuals with Hb (AA) have normal haemoglobin genes. Women were divided into 2 groups according to Hb (AA) and Hb (AE) and studied separately. Women from each group were randomly assigned to 1 of the following interventions: group 1: placebo, supervised; group 2, 120 mg of elemental iron (as ferrous sulphate) and 5000 μg (5 mg) folic acid daily supervised; group 3, 240 mg of elemental iron (as ferrous sulphate) daily supervised; group 4: 240 mg of elemental iron (as ferrous sulphate) daily supervised; group 5: 120 mg elemental iron (as ferrous sulphate) and 5000 μg (5 mg) of folic acid, motivated but unsupervised; and group 6: 240 mg of elemental iron (as ferrous sulphate) and 5000 μg (5 mg) of folic acid daily, motivated but unsupervised. For the Hb (AE) group, women were randomly assigned to 1 of the following groups: group 7: placebo, supervised; group 8: 240 mg elemental iron (as ferrous sulphate) and 5000 μg (5 mg) of folic acid daily, supervised; group 9: 240 mg of elemental iron (as ferrous sulphate) daily, supervised; group 10: 120 mg of elemental iron (as ferrous sulphate) and 5000 μg (5 mg) of folic acid daily, motivated but unsupervised, and group 11: 240 mg of elemental iron (as ferrous sulphate) and 5000 μg (5 mg) of folic acid daily, motivated but unsupervised. Starting and ending time of supplementation not stated. The types of interventions do not allow for comparisons within the scope of this review. |
| Chawla 1995 | 81 pregnant women with 20 +/‐ wks of gestation from Ludhiana City, India were divided into 1 of 3 groups: group 1 received 60 mg of elemental iron (as ferrous sulphate) and 500 µg (0.5 mg) of folic acid daily; group 2, 60 mg of elemental iron (as ferrous sulphate) and 2,000,000 IU of vitamin A, or group 3, who did not receive any supplements. Supplementation was for a period of 15 wks. Outcomes measured included Hb, red blood cell count, total iron binding capacity, transferrin saturation, serum iron, serum vitamin A at baseline and at 36 +/‐ 2 wks of gestation. Poor methodological quality. None of the outcomes pre‐specified in our protocol were recorded due to the varied time of final measurements. The types of interventions do not allow for comparisons within the scope of this review. |
| Chisholm 1966 | 360 non‐anaemic women attending antenatal clinic in Oxford, United Kingdom before 28th wk of gestation, who had not taken iron supplements in the preceding 8 wks and with Hb >= 102 g/L or a normal serum iron reading were randomly assigned to 6 groups as follows:group 1: 900 mg elemental iron (as ferrous gluconate) alone daily; group 2: 900 mg elemental iron (as ferrous gluconate) and 500 μg (0.5 mg) folic acid daily; group 3: 900 mg elemental iron (as ferrous gluconate) and 5000 µg (5 mg) folic acid daily; group 4: placebo; group 5: 500 μg (0.5 mg) folic acid daily; group 6: 5000 μg (5 mg) of folic acid daily. Iron and folic acid placebos were used. Supplementation started at 28th wk until 40th wk. The types of interventions do not allow for comparisons within the scope of this review. |
| Christian 2003 | Cluster‐randomised trial. 4998 married pregnant women living in the south eastern plains district of Sarlahi, Nepal. Women were randomly assigned to 1 of 5 groups: group 1 received 1000 μg retinol equivalents vitamin A (control) daily; group 2 received 1000 μg retinol equivalents vitamin A and 400 μg (0.4 mg) folic acid daily; group 3 received 1000 μg retinol equivalents vitamin A, 400 µg (0.4 mg) folic acid and 60 mg elemental iron (as ferrous fumarate) daily; group 4 received 1000 μg retinol equivalents vitamin A, 400 μg (0.4 mg) folic acid, 60 mg of elemental iron (as ferrous fumarate) and 30 mg of zinc sulphate daily; and group 5 received 1000 μg retinol equivalents vitamin A, 400 μg (0.4 mg) folic acid, 60 mg elemental iron (as ferrous fumarate), 30 mg of zinc, 10 μg vitamin D, 10 mg vitamin E, 1.6 mg thiamine, 1.8 mg riboflavin, 20 mg niacin, 2.2 mg vitamin B6, 2.6 μg vitamin B12, 100 mg vitamin C, 65 μg vitamin K, 2 mg cooper, and 100 mg magnesium daily. Supplementation started at recruitment and continued until 3 month postpartum in the case of live births of 5 wks or more after a miscarriage or stillbirth. All participating women were offered deworming treatment (albendazole 400 mg single dose) in the second and third trimester. The types of interventions do not allow for comparisons within the scope of this review. |
| Coelho 2000 | 100 pregnant women with 20‐34 wks of gestation attending the antenatal clinic at The Bandra Holy Family Hospital, Bandra, Mumbai India were randomly assigned to 1 of 2 groups: group 1 received 30 mg elemental iron + other essential vitamins and minerals daily; groups 2 received 116 mg elemental iron, folic acid, zinc and vitamin C daily. Outcomes included Hb concentration, maternal weight gain, infant birth weight and maternal compliance and side effects Both groups received iron supplementation. Both groups received daily iron supplementation. The types of interventions do not allow for comparisons within the scope of this review. |
| Cogswell 2003 | 275 non‐anaemic, low‐income pregnant women at < 20 wks of gestation with ferritin levels above 20 µg/L enrolled at the Cuyahoga County, Supplemental Nutrition Program for Women, Infants and Children in Cleveland, Ohio, USA were randomly assigned to 1 of 2 groups: group 1 received 1 gelatin capsule containing 30 mg of elemental iron (as ferrous sulphate) daily; group 2 received 1 placebo soft gelatin capsule daily for 119 days. Supplementation started at an average of 11 wks of gestation until delivery. The types of interventions do not allow for comparisons within the scope of this review. |
| Cogswell 2006 | Randomised controlled trial. Pregnant women 20 years or older who live in 1 of the study counties (Laoting, Mancheng, Fengrun, Xianghe, Yuanshi) in China., and who can follow instructions and can swallow pills were randomly assigned to receive a daily supplement containing 30 mg elemental iron and 400 μg (0.4 mg) folic acid; group 2 received a daily supplement containing 30 mg elemental iron, 400 μg (0.4 mg) folic acid and other vitamins and minerals (UNICEF formulation) and group 3 received a daily supplement containing 400 μg (0.4 mg) folic acid. Outcomes measured were mortality, morbidity, and complications during pregnancy, labour, and delivery. The types of interventions do not allow for comparisons within the scope of this review. |
| Cook 1990 | 200 women were randomly assigned to receive 50 mg iron daily given either as Gastric Delivery System (GDS) or conventional ferrous sulphate. Gastrointestinal side effects were evaluated. The participants were non‐pregnant women. |
| Corrigan 1936 | In this quasi‐randomised trial, 200 pregnant women voluntarily attending prenatal care clinic in at Boston City Hospital, Boston, USA between 3 and 7 months gestation were assigned a number according to the order they presented. Participants in an alternate fashion received either oral coated tablets containing 200 mg (0.2 g) of ferrous sulphate 3 times daily (those with odd numbers assigned) or a placebo of similar appearance containing only lactose (those with even number assigned). Women received daily iron or placebo. The types of interventions do not allow for comparisons within the scope of this review. |
| Dawson 1987 | 42 healthy women with less than 16 wks of pregnancy were randomly assigned to receive either a daily multiple micronutrient supplement containing 65 mg of elemental iron or 1 daily multiple micronutrient supplement with no iron, calcium, zinc and copper and pantothenic acid. Both groups received different multivitamin/multi mineral supplement formulations. The types of interventions do not allow for comparisons within the scope of this review. |
| De Benaze 1989 | 191 non‐anaemic pregnant women 12‐18 wks of gestation attending an hospital antenatal clinic, Paris, France. Supplementation started at 12‐18 wks until delivery. Women were randomly allocated to 1 of 2 groups: group 1: daily intake of 45 mg of elemental iron (as ferrous betainate hydrochloride) (15 mg elemental iron per tablet) and group 2: placebo tablet. The types of interventions do not allow for comparisons within the scope of this review. |
| Dijkhuizen 2004 | 170 pregnant women with less than 20 wks' gestation from 13 adjacent villages in a rural area in Bogor District, West Java, Indonesia were randomly assigned to receive daily supplementation with B‐carotene (4.5 mg), zinc (30 mg), both, or placebo containing 30 mg elemental iron and 400 μg (0.4 mg) folic acid. Both groups received daily iron and folic acid. The types of interventions do not allow for comparisons within the scope of this review. |
| Dommisse 1983 | 146 pregnant women with less than 20 wks of gestation were randomly allocated to receive either a multivitamin tablet twice a day or a multivitamin tablet in conjunction with a standard ferrous sulphate tablet twice a day providing a total of 120 mg of elemental iron daily. Both groups received a multivitamin supplement. No data can be extracted from the published article. |
| Edgar 1956 | 179 pregnant women with Hb levels below 105 g/L and more than 16 wks of gestation volunteered for this study and were divided into 4 supplementation groups according to the stage of pregnancy: 16th wk, 20th wk, 24th wk, and non‐supplemented controls. 37% of these women were lost to follow‐up and were excluded from the final analysis. Data are presented without standard deviation. No data can be extracted from the publication for this review. |
| Ekstrom 1996 | 176 pregnant women attending Ilula Lutheran Health Center's antenatal service in Iringa region, Tanzania with 21‐26 wks of gestational age and Hb > 80 g/L were randomly assigned to receive 120 mg elemental iron (as ferrous sulphate in conventional form) daily or 50 mg elemental iron as gastric delivery system (GDS) daily. Both groups received daily iron supplementation in different preparations. The types of interventions do not allow for comparisons within the scope of this review. |
| Eskeland 1997 | 90 healthy non‐anaemic pregnant women with singleton pregnancy of less than 13 wks, attending an inner city maternity centre in Bergen, Norway. Women were randomly allocated to 1 of the following: group 1: 3 tablets containing 1.2 mg heme iron from porcine blood and 9 mg of elemental iron (as ferrous fumarate) (Hemofer®) and 1 placebo tablet (total 27 mg elemental iron a day); group 2: 1 tablet containing 27 mg elemental iron (as iron fumarate) with 100 mg vitamin C (Collet®) and 3 placebo tablets; or group 3: 4 placebo tablets. Supplementation started at 20th wk until 38‐40th wk. Women received daily iron supplementation or placebo. The types of interventions do not allow for comparisons within the scope of this review. |
| Fenton 1977 | 154 pregnant women with less than 14 wks of gestation, and who had not received or were receiving treatment for a blood disorder were divided into 2 groups according to the day in which they attended the clinic in Cardiff: group 1 received 60 mg of elemental iron (as ferrous sulphate) daily and group 2 received placebo. Hb concentration, mean corpuscular volume (MCV), serum ferritin, serum iron and total iron binding capacity were measured at 10‐14 wk and at term. The types of interventions do not allow for comparisons within the scope of this review. |
| Fleming 1974 | 146 consecutive pregnant women attending a public antenatal clinic in Western Australia before the 20th wk of gestation who had not received iron supplements and were willing to participate were randomly assigned in sequences of 50 to 1 of the 5 interventions groups: group 1 received placebo; group 2 received 60 mg of elemental iron (as ferrous sulphate); group 3 received 500 μg (0.5 mg) of folic acid; group 4 received 60 mg of elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) of folic acid; and group 5 received 60 mg of elemental iron (as ferrous sulphate) and 5000 μg (5 mg) of folic acid. Supplementation with iron was from 20th wk of gestation until delivery. All women had received 50 mg of ascorbic acid daily from the first visit until wk 20th. More than 20% of the women were lost to follow‐up. No data can be extracted from the publication for this review. |
| Fleming 1986 | 200 apparently healthy primigravidae Hausa women living in Zaria, Nigeria and planning to deliver in Zaria, with less than 24 wks of gestation, who had not taken any antimalarial treatment or iron supplements in current pregnancy were randomly assigned to 1 of 5 groups: group 1: received no active treatment; group 2: received chloroquine 600 mg base once, followed by proguanil 100 mg per day; group 3 received in addition to chloroquine and proguanil, 60 mg elemental iron daily; group 4 received in addition to chloroquine and proguanil, 1000 μg (1 mg) of folic acid daily, and group 5: in addition to chloroquine and proguanil received 60 mg of elemental iron and 1000 μg (1 mg) of folic acid daily. 89 out of 200 women delivered in the hospital and no other complete clear data can be extracted for the outcomes of interest in this review. |
| Fletcher 1971 | 643 pregnant women attending antenatal clinic in London were randomly assigned to 1 of 2 groups: group 1 received 200 mg of ferrous sulphate daily; group 2 received 200 mg of ferrous sulphate with 5000 μg (5 mg) of folic acid daily. Both groups received iron. No comparisons allowed within the scope of this review. |
| Foulkes 1982 | 568 apparently healthy pregnant women with less than 20 wks of pregnancy and no prior iron supplementation were allocated alternatively to receive 100 mg of elemental iron and 350 μg (0.35 mg) folic acid daily or no treatment. Ferritin and Hb concentrations were measured at baseline and at 28 and 36 wks of gestation and 2 days postpartum. MCV and MCH were measured at 2 days postpartum. Only means and median are presented. No standard deviation is shown and for ferritin concentrations no ln‐transformed data are presented. No data were extractable from the paper and subsequent communication with author. |
| Freire 1989 | 412 non‐black pregnant women with 26 ± 2 wks of gestation, who had not received iron supplements in the previous 6 months, from low SES using the prenatal unit of Quito's public obstetric hospital, Ecuador were randomly assigned to receive 2 tablets containing 78 mg of elemental iron (as ferrous sulphate) daily or placebo during a period of 2 months. Overall loss to follow‐up rate was 41.7%. Hb, PCV, red cell indices, serum ferritin, total iron binding capacity, serum folate, serum vitamin B12 at baseline and after 2 months. Prevalence of iron deficiency was estimated by response to therapy. No prespecified outcomes from this review are presented in the paper. No further data were available. |
| Gomber 2002 | 40 apparently healthy women with singleton pregnancy in their second trimester (between 16‐24 wks of gestation), living in urban slums, from low socio‐economic status attending Guru Teg Bahadur Hospital, Delhi, India were randomly assigned to receive 1 tablet containing 100 mg of elemental iron as ferrous sulphate with 500 μg (0.5 mg) folic acid daily or once a wk. Weekly intake was supervised. Duration of supplementation was 100 days. Hb and HCT concentrations at baseline, at 4 wks, 8 wks and 14 wks of supplementation, serum ferritin concentration, at baseline, at 14 wks of supplementation and at delivery. No prespecified outcomes in this review are reported. Serum ferritin values is reported as log transformed values but no standard deviations are presented. |
| Gopalan 2004 | 900 pregnant women of poor socio‐economic status females attending government antenatal care clinics were grouped in 3 groups: group 1 (n = 300) received routine antenatal care; group 2 (n = 300) received 100 mg of elemental iron and 500 μg (0.5 mg) folic acid daily from the 20th wk of gestation and group 3 (n = 300) received 100 mg of elemental iron and 500 μg (0.5 mg) folic acid daily from the 20th wk of gestation and additionally 900 mg of alpha linolenic acid from the 22nd wk of gestation. Outcomes assessed included birth weight, low birth weigh, premature delivery. The study is not reported as randomised and is excluded in the first screening for eligibility. |
| Gringras 1982 | 40 pregnant women attending antenatal care clinic were given a tablet containing 47 mg of elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) of folic acid daily or a tablet containing 100 mg of elemental iron (as ferrous glycine sulphate) daily. Both groups received iron. No comparisons allowed within the scope of this review. |
| Groner 1986 | 40 pregnant women attending antenatal care at the Adolescent Pregnancy Clinic and Obstetrics Clinics at the John Hopkins and Sinai Hospital in Baltimore, Maryland, USA at or before 16 wks of pregnancy with hematocrit equal or above 31% were randomly assigned to 1 of 2 groups: group 1 (n = 16) received 60 mg of elemental iron (as ferrous fumarate) and prenatal vitamins daily; or group 2 (n = 9) received only the prenatal vitamins with no iron. 2 women objected to the randomisation and 13 dropped out of the study. Both groups received multiple micronutrients. Supplementation lasted a month. Psychometric tests (arithmetic, total digit span, digit symbol, vocabulary and others) were performed and hematologic status was measured at baseline and after a month. Haematological outcomes cannot be extracted from the paper. None of the other outcomes were sought. |
| Guldholt 1991 | 192 pregnant women were consecutively randomised to receive 1 of 2 treatments: group 1: received a daily vitamin‐mineral tablet containing 15 mg of elemental iron or group 2: received a daily vitamin‐mineral tablet containing 100 mg of elemental iron. Both groups received iron in different doses. No comparisons allowed within the scope of this review. |
| Hampel 1974 | 65 untreated and 54 treated pregnant women in West Berlin, Germany were assessed during pregnancy for Hb concentrations, iron and folate levels, total iron binding capacity, and red cell count. No data are presented for outcomes prespecified in the review. Women were of different gestational age. No outcomes can be extracted from the paper. |
| Han 2011 | 153 anaemic pregnant women, with 80 g/L or more but less than 110 g/L in China were randomly allocated to 1 of 3 groups: group 1 (n = 51) received placebo, group 2 (n = 51) was supplemented daily with 60 mg elemental iron (as ferrous sulphate), and group 3 (n = 51) was supplemented daily with 60 mg elemental iron (as NaFeEDTA). The intervention lasted 2 months. There is not an intermittent iron supplementation group. The comparisons in this trial are outside the scope of this review. |
| Hankin 1962 | 174 primigravidae or secundigravidae at their first visit at the antenatal Clinic of Queen Elizabeth Hospital in Woodville, Australia with ability to write and speak English. Women were divided into a supplemented group receiving a daily dose of 100 mg of elemental iron (as ferrous gluconate) or a control group that was unsupplemented. Supplementation started during 2nd trimester and ending time is unclear. |
| Hartman‐Craven 2009 | In this cross‐over study 2 types of multivitamin supplements were compared: 18 healthy pregnant women 24‐32 wks' gestation attending a Toronto hospital were recruited and received 2 different supplements in a random order and followed up over 8 hours. Both preparations contained iron and folic acid (although in different doses). the aim of the study was to see whether absorption was improved with a powdered preparation. |
| Harvey 2007 | 13 healthy non‐anaemic pregnant women aged 18‐40 years and < 14 wks of gestation with singleton pregnancy recruited through local medical practitioners and the Maternity Department of the Norfolk and Norwich University Hospital, England, United Kingdom. Women were randomly assigned to 1 of 2 groups: group 1 received 100 mg elemental iron (as ferrous gluconate) daily after food and group 2 received a placebo. Supplementation started at 16th wk of gestation until delivery. |
| Hawkins 1987 | No report available of the study results. |
| Hemminki 1995 | 2994 pregnant women less than 16 wks' gestation attending 15 communal maternity centres and 12 centres in 5 neighbouring communities in Tampere, Finland. Women were randomly assigned to 1 of 2 groups: group 1 (routine) were recommended to take 100 mg elemental iron alone (iron compounds and dosage varied as per midwife recommendation) daily after the 16th wks' gestation; or group 2 (selective) who received no iron supplements unless required. |
| Hermsdorf 1986 | 120 unselected pregnant women were given 114 mg of elemental iron daily from wk 15 until delivery, or not treatment. Only an abstract with insufficient data available. |
| Hoa 2005 | 202 apparently healthy pregnant women 20‐32 years of age attending health clinics from 12 communes in Dong HungDistrict, Thai Binh Province, Vietnam with 14‐18 wks of gestation who agreed to participate in the study were selected to participate. Women were assigned through block randomisation, randomly assigned to 1 of 4 interventions: group 1 (n = 44) received 400 mL fortified milk with iron (ferrous fumarate), vitamin C and folic acid daily; group 2 (n = 41) received 400 mL of milk fortified with vitamin C and folic acid but no iron daily; group 3 (n = 40) received 1 tablet containing 60 mg of elemental iron (as ferrous sulphate) and 250 μg (0.25 mg) folic acid daily and group 4 (n = 43) received 1 placebo tablet daily. This study looked at daily supplementation regimens. |
| Holly 1955 | 207 pregnant women less than 26 wks of gestation and Hb > 100 g/L attending antenatal care clinic in Nebraska, USA. Women were randomly assigned to 1 of 3 groups: group 1 received 1 g of an iron salt daily; group 2 received 0.8‐1.2 g of ferrous sulphate and 60‐90 mg of cobalt chloride daily, and group 3 received no treatment. Supplementation started at various times before 26th wk of gestation until delivery. |
| Hood 1960 | 75 pregnant women 32‐34 wks of gestation attending a hospital maternity clinic in Oklahoma, USA. Women were randomly divided into 3 groups: group 1: no treatment; group 2 : 220 mg elemental iron (as ferrous sulphate) daily; and group 3: 55 mg elemental iron (as sustained release ferrous sulphate) daily. Supplementation started at 32‐34 wk of gestation until delivery. |
| Horgan 1966 | 42 apparently healthy pregnant women attending 2 antenatal care clinics in London, England were assigned to 1 of 3 interventions: group 1 received 200 mg ferrous sulphate with 5000 μg (5 mg) of folic acid 3 times a day; group 2 received 350 mg of ferrous aminoate with 50 μg (0.05 mg) folic acid 3 times a day; and group 3 received 200 mg of ferrous sulphate with 500 μg (0.5 mg) folic acid once a day. Intervention period was 3 wks. All groups received daily iron and folic acid. No comparisons allowed within the scope of this review. |
| Hosokawa 1989 | 84 anaemic women seeking antenatal care in the Department of Obstetrics and Gynaecology of the Fukui School of Medicine Hospital, Japan were randomly assigned to receive 100 mg of elemental iron (as ferrous sulphate) daily after the evening meal, or the same dose + vitamin C for 4 wks. Both groups received daily iron. No comparisons allowed within the scope of this review. |
| Iyengar 1970 | 800 pregnant women with less than 24 wks of gestation and Hb > 85 g/L in India were assigned by rotation to 1 of 4 groups: group 1 received placebo tablets; group 2 received 30 mg of elemental iron as ferrous fumarate in a single tablet daily; group 3 received 30 mg of elemental iron (as ferrous fumarate) with 500 μg (0.5 mg) folic acid in a single tablet; and group 4 received in addition to iron and folic acid, 2 μg of vitamin B12 in a single tablet. Loss to follow‐up was 65%. None of the pre‐specified outcomes in the protocol was reported and no data were extractable from the paper. |
| Kaestel 2005 | 2100 pregnant women (22 +/‐ 7 wks' gestation at entry) attending antenatal clinics in Bissau, Guinea‐Bissau or who were identified by The Bandim Health project were randomly assigned to receive daily multi micronutrient tablet containing 1 Recommended Dietary Allowance (RDA) of 15 micronutrients, or daily multi micronutrients containing 2 times the RDA except for iron that was maintained at 1 RDA or a conventional prenatal daily iron (60 mg elemental iron) and 400 μg (0.4 mg) folic acid supplement. All groups receive iron and folic acid daily. No comparisons allowed within the scope of this review. |
| Kann 1988 | 36 healthy non‐anaemic pregnant women in second or third trimesters of gestation were randomly assigned to receive 1 of 4 prenatal commercial multivitamin/multi mineral preparations daily: Stuartnatal 1+1; Stuart Prenatal; Materna; and Natalins Rx. All participants received multiple micronutrients. No comparisons allowed within the scope of this review. |
| Kerr 1958 | 430 women with 24‐25 wks of singleton pregnancy and Hb equal or above 104 g/L attending antenatal clinic in Edinburgh, Scotland, United Kingdom. Women were randomly allocated to 1 of 4 groups: group 1: 35 mg of elemental iron (as ferrous sulphate) 3 times a day; group 2: 35 mg of elemental iron (as ferrous gluconate) 3 times a day; group 3: 35 mg of elemental iron (as ferrous gluconate) with 25 mg of ascorbic acid, 3 times a day; group 4: placebo. Supplementation started at 24‐25th wk of gestation until term. |
| Khambalia 2009 | In this randomised trial carried out in Bangladesh childless, non‐pregnant married women under 40 were randomised to receive food supplements (sprinkles) containing either iron and folic acid or folic acid alone. 272 women were randomised and were followed up for 9 months. If women became pregnant they were withdrawn from the study and ALL pregnant women received both iron and folic acid. The study was excluded as it focused on a non‐pregnant population. |
| Kuizon 1979 | 679 pregnant women attending for antenatal care in public health clinics in Manila, Philippines were randomised to 4 groups. Group 1 received placebo only. Group 2 received 325 mg ferrous sulphate oral tablets (1 or 3 tablets daily). Group 3 100 mg ascorbic acid (1 or 3 tablets) and Group 4 received iron plus ascorbic acid (1 or 3 tablets). Anaemic and non anaemic women were included and received different doses. All supplements were daily. |
| Kumar 2005 | 220 pregnant women with a singleton pregnancy and Hb between 80‐110 g/L at 16‐24 wks' gestation from New Delhi, India were randomly allocated to receive daily oral iron therapy of 100 mg elemental iron (as ferrous sulphate) with 500 μg (0.5 mg) folic acid or 250 mg of iron sorbitol intramuscularly and repeated at an interval of 4‐6 wks. This trial compares the effects of daily oral iron with 2 injections of high dose parenteral iron. No comparisons allowed within the scope of this review. |
| Lee 2005 | 154 apparently healthy pregnant women seeking prenatal care in Gwangju, South Korea during first trimester of pregnancy who did not receive other supplements or medications throughout pregnancy and who were willing to participate were recruited. Women were randomly allocated to 1 of 5 groups: group 1 received 30 mg elemental iron (as ferrous sulphate) and 175 μg (0.17 mg) folic acid daily from first trimester until delivery; group 2 received 60 mg of elemental iron (as ferrous sulphate) with 350 μg (0.35 mg) folic acid from first trimester until delivery; group 3 received 30 mg elemental iron (as ferrous sulphate) and 175 μg (0.17 mg) folic acid from 20th wk of gestation until delivery; group 4 received 60 mg elemental iron (as ferrous sulphate) and 350 μg (0.35 mg) folic acid from 20th wk of gestation until delivery; or control group with no supplement. |
| Madan 1999 | 109 apparently healthy pregnant women with 16‐24 wks of gestation who had not received iron supplements were randomly assigned to 1 of 3 groups: group 1 received 60 mg of elemental iron + 500 μg (0.5 mg) folic acid once daily; group 2 received 120 mg of elemental iron + 500 μg (0.5 mg) folic acid once daily; group 3 received 120 mg of elemental iron twice daily + 500 μg (0.5 mg) folic acid. Duration of supplementation was 12‐14 wks. All participants received iron and folic acid daily. No comparisons allowed within the scope of this review. |
| Makrides 2003 | 430 non‐anaemic pregnant women attending antenatal clinics in Adelaide, Australia. Exclusion criteria: diagnosis of thalassaemia, history of drug or alcohol abuse and history of vitamin and mineral preparations containing iron prior to enrolment in study. Women were randomly assigned to receive 1 tablet containing 20 mg of elemental iron daily between meals from wk 20 until delivery or a placebo tablet. |
| Mbaye 2006 | 1035 pregnant women attending mother and child health clinics near the town of Farafenni, Gambia were randomised to receive either folic acid (500‐1500 μg/day) together with oral iron (47 mg of ferrous sulphate per tablet) or oral iron alone (60 mg of ferrous sulphate per tablet) daily for 14 days. All women received treatment with 3 tablets of SP (25 mg of pyrimethamine and 500 mg of sulphadoxine). Both groups received iron daily. No comparisons allowed within the scope of this review. |
| McKenna 2003 | 102 healthy pregnant women attending antenatal clinics at the Royal Jubilee Maternity Hospital in Belfast, Ireland with a singleton pregnancy and Hb > 104 g/L and known gestational age of less than 20 wks who were non‐compliers with routine prescription of 200 mg of ferrous sulphate daily, were randomly assigned to receive 2 sachets of 24 mL each of Spatone® water containing 10 mg of elemental iron or placebo. Participants were instructed to take the 2 sachets daily half an hour before breakfast diluting it in orange juice. Primary outcomes were compliance and side effects. Duration of intervention was from wk 22 to wk 28 of gestation. |
| Meier 2003 | 144 non‐iron deficient adolescents 15‐18 years old in their first pregnancy and adult women attending prenatal care at Marshfield Clinic, Wisconsin, USA. Women were randomly assigned to receive once daily 60 mg of elemental iron (as ferrous sulphate) 1000 μg (1 mg) folic acid daily or a placebo and 1000 μg (1 mg) folic acid daily. |
| Menendez 1994 | 550 multi gravidae pregnant women were recruited with less than 34 wks of gestation attending antenatal care clinics in 18 villages near the town of Farafenni, in North Bank Division, Gambia where malaria is endemic with high transmission during 4‐5 months a year. Women were allocated randomly by compound of residence to receive 60 mg of elemental iron (as ferrous sulphate) daily and a weekly tablet of 5000 μg (5 mg) of folic acid or placebo daily and a weekly tablet of 5000 μg (5 mg) of folic acid. The women received no antimalarial chemoprophylaxis. Supplementation started at 23‐24 wks until delivery. |
| Menon 1962 | 273 healthy pregnant women with 16‐24 wks of gestation and Hb concentrations at or above 105 g/L attending antenatal care clinics were divided in order in which they were registered in 3 groups: group 1 was given 5 g of ferrous sulphate daily; group 2 received 5000 μg (5 mg) of folic acid daily; and group 3 received 5 g of ferrous sulphate and 5000 μg (5 mg) of folic acid daily. All participants were given 3 multivitamin tablets daily containing vitamin A, vitamin B, C and D. The study was not randomised. |
| Milman 1991 | 248 women attending Birth Clinic in Copenhagen, Denmark within 9‐18 wks of gestation and normal pregnancy. Women were randomly assigned to receive 66 mg of elemental iron (as ferrous fumarate) daily (n = 121) or placebo (n = 127) until delivery. Supplementation started at 8‐9th wk until delivery |
| Milman 2005 | 427 healthy Danish pregnant women living in the north eastern part of Copenhagen County, Denmark were randomly allocated to receive iron (as ferrous fumarate) in daily doses of 20 mg (n = 105), 40 mg (n = 108), 60 mg (n = 106), and 80 mg (n = 108) from 18 wks of gestation. Hb, serum ferritin, and serum soluble transferrin receptor concentrations were measured at 18 wks (inclusion), 32 wks, and 39 wks of gestation and 8 wks postpartum. All women received iron daily. No comparisons allowed within the scope of this review. |
| Morgan 1987 | 356 pregnant women attending 2 different antenatal care clinics at the King Edward Memorial Hospital for Women in Subiaco, Australia received according to the clinic they visited, either no treatment or 100 mg of elemental iron as ferrous gluconate daily. No systematic allocation was used in this open trial. |
| Morrison 1977 | 105 pregnant women attending the University Unit, Mater Misericordiae Mothers' Hospital, South Brisbane, Australia, with normal height, weight and nutrition for the Australian population and with no previous adverse medical, surgical or obstetrical history were allotted by random selection to 1 of 4 types of supplements: group 1 received 50 mg of elemental iron as dried ferrous sulphate daily; group 2 received 80 mg elemental iron as dried ferrous sulphate with 300 μg (0.3 mg) folic acid daily; group 3 received 105 mg elemental iron as ferrous sulphate and group 4 received 105 mg of elemental iron (as ferrous sulphate) with 300 μg (0.3 mg) folic acid. All groups received iron daily. No comparisons allowed within the scope of this review. |
| Muslimatun 2001 | 143 women 17‐35 years of age with 16‐20 wk gestational age, parity < 6 and Hb 80‐140 g/L recruited from 9 villages in the Leuwiliang sub district, Bogor district, West Java, Indonesia, were randomly assigned to 1 of 2 groups: group 1 received weekly supplements containing 120 mg elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) folic acid; group 2 received weekly supplements containing 120 mg elemental iron (as ferrous sulphate); 500 μg (0.5 mg) folic acid and 4800 retinol equivalents (RE) of vitamin A. A group of 123 pregnant women from other 4 villages participating in the ongoing national government daily iron plus folic acid supplementation program, were reported as control although they were not randomly assigned to the daily intervention. This daily supplementation programme consists of providing women with 90‐120 mg elemental iron plus folic acid tablets daily throughout pregnancy distributed through medical services. Only the women in the weekly regimens were randomly assigned. The type of study and interventions do not allow for comparisons within the scope of this review. |
| Nguyen 2008 | 167 pregnant women with less than 20 wks of gestation who called either Motherisk General Information line or the Motherisk Nausea and Vomiting of Pregnancy (NVP) Helpline (Hospital for Sick Children, Toronto) and had not started taking or had discontinued any multivitamin due to adverse events were randomly assigned to 1 of 2 groups: group 1 were provided, PregVit® (a small‐size, containing 35 mg elemental iron as ferrous fumarate and multivitamins; or group 2 who received Orifer F® (high iron content, small size) containing 60 mg elemental iron (as ferrous sulphate) and multivitamins. Follow‐up interviews documented pill intake and adverse events. Participants from both groups received iron in different amounts and compounds. |
| Nogueira 2002 | 74 low‐income pregnant adolescents ranging from 13‐18 years of age attending antenatal care at the Evangelina Rosa Maternity Hospital in Teresina, Piaui State, Brazil were distributed into 5 groups: group 1 received 120 mg elemental iron as ferrous sulphate and 250 µg (0.25 mg) of folic acid daily; group 2 received 80 mg elemental iron as ferrous sulphate and 250 μg (0.25 mg) folic acid daily; group 3 received 120 mg of elemental iron, with 5 mg of zinc sulphate and 250 μg (0.25 mg) folic acid daily; and group 4 received 80 mg of elemental iron as ferrous sulphate, with 5 mg of zinc sulphate and 250 μg (0.25 mg) folic acid daily. All groups received iron and 2 groups received zinc in addition to iron and folic acid daily. No comparisons allowed within the scope of this review. |
| Ogunbode 1984 | 80 apparently healthy non‐anaemic pregnant women attending University College Hospital and Inalende Maternity Hospital in Ibadan, Nigeria during the first and second trimesters of pregnancy were randomly allocated to 1 of 2 groups: group 1 (n = 39) received 1 tablet Ferrograd Folic 500 Plus® daily, a sustained‐released formulation containing ferrous sulphate and folic acid (composition is not available); or group 2 (n = 41) received a capsule containing 200 mg ferrous sulphate and 5000 μg (5 mg) of folic acid daily. All patients were also provided 25 mg weekly of pyrimethamine throughout pregnancy as an anti‐malarial agent. Patients who became anaemic during pregnancy were excluded of the study and analysis. Outcomes measured included reticulocyte count, HCT, anaemia, side effects. Both groups received iron and folic acid supplements, thus making the comparisons not suitable for this review. |
| Ogunbode 1992 | 315 apparently healthy pregnant women attending 4 prenatal care clinics in 4 geographical areas of Nigeria with mild to moderate anaemia (as defined by hematocrit between 26% to 34%) and 18‐28 wks of gestation, single pregnancies, no complications and who consented to participate in the study were randomly allocated to 1 of 2 groups: group 1 (n = 159) received 1 daily capsule of a multiple micronutrient supplement Chemiron® containing 300 mg of ferrous fumarate, 5000 µg (5 mg) folic acid, 10 μg vitamin B12, 25 mg of vitamin C, 0.3 mg magnesium sulphate and 0.3 mg of zinc sulphate; group 2 (n = 156) received a capsule containing 200 mg ferrous sulphate and 5000 μg (5 mg) of folic acid. All patients were also provided 600 mg of chloroquine to be taken under supervision and 25 mg weekly of pyrimethamine throughout pregnancy. Patients who became anaemic during pregnancy were excluded of the study and analysis. Outcomes measured included blood Hb, anaemia, HCT, serum ferritin levels, side effects. A second published study followed these same women and their infants. Both groups received iron and folic acid supplements, thus making the comparisons not suitable for this review. |
| Ortega‐Soler 1998 | 41 healthy pregnant women, attending prenatal care clinics at Hospital Diego Paroissien in La Matanza, Province of Buenos Aires, Argentina with serum ferritin below 50 mg/mL. Women were assigned to 1 of 2 groups: group 1 received 100 mg of elemental iron daily (as ferric maltosate), and group 2 received no treatment. Supplementation started at 21 +/‐ 7 wks of gestation until birth. Maternal outcomes measured included: Hb, erythrocyte protoporphyrin, serum ferritin at baseline and term, dietary intake. The iron intake was unsupervised and compliance was not reported. The trial is not randomised nor quasi‐randomised so it does not fill the inclusion criteria for this review. |
| Osrin 2005 | 1200 healthy pregnant women with a singleton pregnancy and less than 20 wks' gestation attending an antenatal clinic at Janakpur zonal hospital in Nepal, were randomly assigned to receive routine 60 mg elemental iron and 400 μg (0.4 mg) folic acid supplements daily or a multiple micronutrient supplement containing 15 vitamins and minerals including 30 mg elemental iron and 400 μg (0.4 mg) folic acid daily. Both groups received iron and folic acid daily. No comparisons allowed within the scope of this review. |
| Paintin 1966 | 180 primigravidae women with less than 20 wks' gestation and Hb > 100 g/L attending antenatal clinic in Aberdeen Maternity Hospital, Scotland, United Kingdom. Women were randomly assigned to 1 of 3 groups: group 1 received 3 tablets containing 4 mg elemental iron each (total 12 mg daily); group 2 received 3 tablets containing 35 mg elemental iron (total 105 mg elemental iron daily) and group 3 received placebo. Intervention was from wk 20 to wk 36 of gestation. |
| Payne 1968 | 200 pregnant women attending antenatal clinics in Glasgow, Scotland with less than 20 wks' gestation, whose antenatal care was undertaken wholly by the hospital antenatal clinic and who subsequently had a normal delivery, were randomly allocated to receive 200 mg of ferrous sulphate daily or 200 mg of ferrous sulphate with 1700 μg (1.7 mg) folic acid daily throughout pregnancy. Both groups received daily iron. No comparisons allowed within the scope of this review. |
| Pena‐Rosas 2003 | 116 pregnant women of 10‐30 wk of gestational age attended antenatal care clinics in Trujillo, Venezuela were randomly allocated to receive a 120 mg oral dose of elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) folic acid weekly (n = 52) or 60 mg elemental iron and 250 μg (0.25 mg) folic acid and a placebo twice weekly (n = 44). Hb, HCT, serum ferritin and transferrin saturation were estimated at baseline and at 36‐39 wks of gestation. All groups received iron and folic acid in 2 intermittent regimens with no control group. No comparisons allowed within the scope of this review. |
| Picha 1975 | In a randomised double‐blind study the new effervescent iron tablet Loesferron® was tested in 57 postpartum women. The participants were not pregnant women. |
| Preziosi 1997 | 197 healthy pregnant women 17‐40 years of age, with 28 +/‐ 3 wks of gestation attending antenatal care clinic in a Mother‐Child Health Centre in Niamey, Niger. Women were randomly assigned to 1 of 2 groups: group 1 received 100 mg of elemental iron (as ferrous betainate) daily; group 2 received placebo. Supplementation was from 28 +/‐ 3 wks of gestation until delivery. |
| Pritchard 1958 | 172 pregnant women in the second trimester of pregnancy attending a hospital antenatal care clinic in Dallas, Texas, USA. Women were randomly assigned to 1 of 3 interventions: group 1 received 1000 mg of iron intramuscularly as iron‐dextran; group 2 received 112 mg of elemental iron (as ferrous gluconate) daily in 3 tablets; group 3 received placebo tablets. Supplementation started during 2nd trimester until delivery. |
| Puolakka 1980 | 32 healthy non‐anaemic pregnant women attending antenatal care at maternity centres of Oulu University Central Hospital, Finland with uncomplicated pregnancy of less than 16 wks, and no earlier haematological problems.Women were randomly assigned to 1 of 2 groups: group 1 received 200 mg of elemental iron (as ferrous sulphate) daily; group 2 received no treatment. Supplementation started at 16th wk of gestation until 1 month postpartum. |
| Ramakrishnan 2003 | 873 pregnant women living near Cuernavaca, Morelos, Mexico with less than 13 wks of gestation who did not use micronutrient supplements were randomly assigned to receive a daily multiple micronutrient supplement or a daily iron‐only supplement. Both supplements contained 60 mg of elemental iron (as ferrous sulphate). Supplement intake was supervised by trained workers from registration until delivery by home visits 6 days a wk. No comparison allowed within the scope of this review. |
| Rayado 1997 | 394 healthy non‐anaemic adult pregnant women with 24‐32 wks of gestation and singleton pregnancy from Fuentalabra, Spain were randomly assigned to 1 of 2 groups: group 1 received 40 mg of elemental iron (as iron mannitol albumin) daily; and group 2 received 40 mg elemental iron (as iron protein succinylate) daily. Both groups received iron daily. No comparisons allowed within the scope of this review. |
| Reddaiah 1989 | 110 pregnant women attending the antenatal clinic at Comprehensive Rura Health Services Project Hospital, Ballabgarh, India, with 16‐24 wks of gestation were randomly assigned to 1 of 3 groups: group 1 received 60 mg elemental iron (as ferrous sulphate) and 500 μg (0.5 mg) folic acid daily; group 2 received 120 mg elemental iron (as ferrous sulphate) with 500 μg (0.5 mg) of folic acid daily; and group 3 received 240 mg elemental iron (as ferrous sulphate) and 0.5 mg of folic acid daily. All groups received iron daily. No comparisons allowed within the scope of this review. |
| Romslo 1983 | 52 healthy pregnant women attending outpatient Women's clinic at Haukeland Hospital, Bergen, Norway. Women were randomly assigned to 1 of 2 groups: group 1 received 200 mg of elemental iron (as ferrous sulphate) daily; group 2 received placebo. Supplementation started at 10 wks of gestation. |
| Roztocil 1994 | 84 non‐anaemic pregnant women at Mazarik University Brno in Czech Republic were treated from 20‐24 wks with 1 capsule of Actiferrin Compositum®, and from 36 wks to delivery with 2 capsules. The group was compared with 57 non‐anaemic pregnant women who received no supplements. The supplement contained 34.5 mg of elemental iron (as ferrous sulphate), 500 μg (0.5 mg) of folic acid, and 0.3 mg of cyanocobalamin. No comparisons allowed within the scope of this review. |
| Rybo 1971 | 117 pregnant women between 20‐29 wks of gestation were alternatively assigned during 3 consecutive 2 wks periods to receive daily tablets containing 200 mg of elemental iron (as ferrous sulphate), 200 mg of elemental iron (as a sustained released iron) or placebo. After each 2‐wk treatment period women were questioned about possible side effects. No side effects are reported by group assigned. No comparisons are allowed within the scope of this review. |
| Sachdeva 1993 | In this study carried out in rural India 66 pregnant women from low‐ and middle‐income groups in an area where Government practice recommends the provision of iron and folic acid for the last 100 days of pregnancy. In addition to iron and folic acid supplements, women in the experimental group received a calcium supplement, individual and group counselling and a booklet about nutrition in pregnancy. "Nutrient supplements in the form of Folifer (iron and folic acid) and Calcium Sandoz tablets were supplied regularly to the subjects of E group from the fifth month of pregnancy till delivery. On the other hand, some of the subjects of the C group who visited the subsidiary health centre.. consumed only Folifer tablets as these are supplied free of cost." This study was not included as all women received iron and folic acid supplements (the dose and regimen were not clear); the focus of the study was on nutritional education and extra contacts with women in the experimental group. It was not clear that allocation to groups was random, "Sixty six pregnant women were equally divided into two groups". |
| Sandstad 2003 | 233 pregnant women attending their second antenatal care visit at the University Health Services of Oslo, Norway with serum ferritin concentration < 60 µg/L were randomised to 2 different iron preparations, group 1 received 1 tablet containing 60 mg of elemental iron (as ferrous sulphate) daily; group 2 received 3 tablets each containing 1.2 mg of heme iron from porcine blood plus 8 mg of elemental iron (as ferrous fumarate) per tablet (total 3.6 heme iron and 24 mg elemental iron) daily. A third group (n = 93) of pregnant women who had been given advice to take or not the iron supplements according to the centre recommendations were enrolled in the trial at 6 wks postpartum and served as control. The study groups were not randomised to the interventions and no comparisons can be made within the scope of this review. |
| Seck 2008 | 221 apparently healthy pregnant women, had not used iron supplements prior to enrolment, who were 12 to 16 wks were recruited from 6 health centres in Dakar, Senegal during their first prenatal visit, and randomly assigned to receive either a prescription to purchase iron/folic acid tablets to be taken daily, according to official policy, or to receive free tablets. Compliance was assessed 20 wks after enrolment through interviews and pill count. the study compares prescribed iron daily to free tablets to be taken daily. No comparisons allowed within the scope of this review. |
| Shatrugna 1999 | 115 healthy pregnant women with 20‐28 wks of gestation attending the antenatal clinic of the National Institute of Nutrition, Government Maternity Hospital, India were randomly assigned to 1 of 11 different formulations and doses of iron and then underwent iron tolerance tests. They received ferrous sulphate tablets containing 60 mg, 12 mg, and 180 mg of elemental iron; formulations containing 60 mg of elemental iron as pure ferrous sulphate salt, ferrous fumarate tablets, ferrous fumarate syrup, excipients added to pure ferrous sulphate salts; powdered ferrous sulphate tablets, iron tablets distributed by the National Nutritional Anaemia Prophylaxis Programme and pure ferrous salt in gelatin capsules. |
| Siega‐Riz 2006 | 429 non‐anaemic iron replete women with less than 20 wks of gestation attending the prenatal clinic at the Wake County Human services in Raleigh, North Carolina, USA. Women were randomly assigned to 1 of 2 groups: group 1 received multivitamin/mineral supplements containing 30 mg of iron (as ferrous sulphate) daily or group 2 received multivitamin/mineral supplements containing 0 mg of iron (no iron) until 29 wks of gestation. Supplementation started on average at 12 wks. Folic acid supplements were prescribed for all women who had received the positive pregnancy test until the first prenatal visit. |
| Simmons 1993 | 376 pregnant women with ages between 16‐35 years, with mild anaemia (Hb concentrations between 80‐110 g/L) attending 8 maternal and child health centres in Kingston, St. Andrews and Spanish Town, Jamaica, with gestational age between 14‐22 wks were randomly assigned to 1 of 3 groups: group 1 received 1 placebo tablet daily; group 2 received 100 mg of elemental iron (as ferrous sulphate) daily; group 3 received 50 mg elemental iron (as gastric delivery system capsule) daily. All women received 400 μg (0.4 mg) folic acid. Outcomes measure included Hb, HCT, MCV, white cell count, serum iron, total iron binding capacity, serum ferritin, serum transferrin receptor, at baseline, at 6 wks and at 12 wks after start of supplementation as well as side effects. No prespecified outcomes are presented in the paper as gestational ages differed in the participants. |
| Sinha 2011 | 50 pregnant women between 16‐20 wks of gestation with Hb equal or greater than 100 g/L in Allahabad, in the north Indian state of Uttar Pradesh, India were randomly assigned to 1 of 2 groups: group 1 (n = 22): women received 2 doses of 400 mg iron sucrose infusion, 1 at 16‐20 wks' gestation and a second infusion at 28‐32 wks' gestation; group 2 (n = 28): women received 100 mg oral ferrous ascorbate daily starting at 16‐20 wks' gestation. The type of intervention is outside the scope of this review. |
| Sjostedt 1977 | 300 pregnant women attending the Maternity Welfare Center, in Oulu, Finland before the 5th month of pregnancy were randomly assigned to 1 of 3 interventions: group 1 received 100 mg of elemental iron daily as sustained‐release tablets daily; group 2 received 200 mg of elemental iron daily (as sustained‐release tablets) and group 3 received 200 mg of elemental iron daily (as rapidly disintegrating ferrous sulphate tablets). All groups received daily iron in different doses and formulations. |
| Sood 1979 | 151 healthy pregnant women with Hb > 50 g/L who had not received iron supplements during the last 6 months from Delhi and Vellore, India were divided in 1 of 3 strata according to Hb concentration (50‐79 g/L; 80‐109 g/L;110 g/L and above) and within each strata were allocated randomly to 1 of 5 interventions: group 1 received 120 mg of elemental iron (as ferrous sulphate) 6 days a wk; group 2 received 100 mg of elemental iron (as iron dextran complex) intramuscular twice per wk; group 3 received iron as group 1 + pteroylmonoglutamic acid 5 mg/day 6 days a wk + cyanocobalamin 100 μg intramuscular once per 14 day; group 4 received 100 mg of elemental iron intramuscular + pteroylmonoglutamic acid + cyanocobalamin 100 µg intramuscular; and group 5 received iron dextran complex intramuscular in a single total dose infusion + 5 mg/day pteroylmonoglutamic acid + 100 μg intramuscular cyanocobalamin once per 14 days. All groups received iron at different doses and routes. No comparisons allowed within the scope of this review. |
| Steer 1992 | Trial abandoned. No data available. |
| Stone 1975 | 248 healthy pregnant women attending hospital antenatal clinic in London, England, were allocated randomly to receive a slow‐release dose of 105 mg of elemental iron (as ferrous sulphate) and 350 μg (0.35 mg) folic acid daily or 80 mg of elemental iron (as ferrous fumarate) and 400 μg (0.4 mg) folic acid daily in a standard preparation. Both groups received daily iron and folic acid in different doses and preparations. No comparisons allowed within the scope of this review. |
| Suharno 1993 | 251 pregnant women aged 17‐35 years, parity 0‐4 and Hb concentrations between 80 and 109 g/L were randomly allocated to 1 of 4 groups: group 1 received 2.4 mg of retinol and 1 placebo iron tablet daily; group 2 received 60 mg of elemental iron (as ferrous sulphate) and a placebo vitamin A tablet daily; group 3 received 2.4 mg of retinol and 60 mg of elemental iron (as ferrous sulphate); and group 4 received 2 placebos for 8 wks. Outcomes measured include: Hb, HCT, serum ferritin, serum iron, total iron binding capacity, serum retinol, transferrin saturation, at baseline and after 8 wks of supplementation. None of the pre‐specified outcomes in this review can be extracted from this paper. |
| Svanberg 1975 | 60 healthy primiparous women attending antenatal care clinic in Goteborg, Sweden and less than 14 wks of gestation and with Hb concentrations above 120 g/L who had not received iron supplements in the previous 6 months. Women whose Hb concentration fell below 100 g/L during the study period were excluded and received immediate therapy. Women were randomly allocated to receive 200 mg of elemental iron (as a sustained release preparation of ferrous sulphate) daily or placebo from 12 wks of gestation until 9 wks post delivery. |
| Swain 2011 | 100 women with uncomplicated pregnancy were assigned to received either injectable iron sucrose (400 mg diluted in 400 mL of normal saline) over 2‐3 hours or to receive oral dose of 100 mg elemental iron daily. The interventions in this trial are outside of the scope of this review. |
| Tampakoudis 1996 | 82 pregnant women with Hb concentrations 140 g/L or above attending clinic in Thessaloniki, Greece were randomised to receive 80 mg iron protein succinylate daily or a placebo. Serial Hb, HCT and serum erythropoietin were measured from maternal blood and cord blood on delivery; serum ferritin measured in frequent intervals. Abstract only available. Insufficient information to assess characteristics of the trial. |
| Tan 1995 | 285 healthy middle‐class pregnant women with Hb concentration above 100 g/L attending antenatal clinic at the University Hospital at Kuala Lumpur, Malaysia were assigned to receive daily iron supplements or no treatment. Abstract only available. No additional information was available, including doses, regimens and other characteristics of the trial. |
| Tange 1993 | 128 anaemic and non‐anaemic pregnant females aged 10‐19 years old, with an average gestation of 16 wks, were grouped for 3 levels of iron supplementation: group 1 (n = 42 non‐anaemic participants) received placebo (no iron); group 2 (n = 41 anaemic and non‐anaemic participants) received 22 mg of elemental iron daily and group 3 (n = 45 anaemic and non‐anaemic participants) received 55 mg elemental iron daily. Women were supplemented from 16 wks until delivery. Outcomes assessed included Hb, HCT, red cell count, MCV, serum iron, serum transferring and serum, ferritin measured every 4 wks. The study is not reported as randomised and is excluded in the first screening for eligibility. |
| Taylor 1982 | 48 healthy pregnant women with no adverse medical or obstetric history attending antenatal care clinic in Newcastle, England, United Kingdom before 12 wks of gestation.Women were randomly allocated to 1 of 2 groups: group 1 receive 65 mg elemental iron (as 325 mg of ferrous sulphate) and 350 μg (0.35 mg) folic acid daily from 12 wks until delivery and group 2 received no supplements. |
| Thane‐Toe 1982 | 135 healthy pregnant women between 22‐28 wks of gestation attending antenatal clinic in Burma, were randomly assigned to receive a daily dose of 60 mg, 120 mg or 240 mg of elemental iron (as ferrous sulphate). A control group was composed by 47 apparently healthy adults (17 males and 30 single women). Control groups are not appropriate. No comparisons allowed within the scope of this review. |
| Tholin 1993 | 83 healthy nulliparous non vegetarian, non‐anaemic pregnant women with serum ferritin concentrations above 10 µg/L were randomly assigned to 1 of 3 groups: group 1 received 100 mg of elemental iron (as ferrous sulphate) daily; group 2 received placebo, and group 3 received dietary advice only. Blood Hb, serum ferritin and blood manganese were determined at baseline before 15th wk of gestation, between 25‐28 wks, and between 35‐40 wks of gestation. Median and ranges are presented. No outcomes were extractable from this report for this review. |
| Thomsen 1993 | 52 healthy non‐anaemic nulliparous women with normal singleton pregnancy and serum ferritin levels above 15 mg/L at 16th wk in Herlev, Denmark were randomly assigned to receive either a daily tablet containing 18 mg elemental iron and also a tablet containing 300 μg (0.3 mg) of folic acid daily or a daily tablet containing 100 mg of elemental iron from 16 wks until delivery and 300 μg (0.3 mg) of folic acid daily. All women received daily iron in different doses. This comparison is not within the scope of this review. |
| Tura 1989 | 254 non‐anaemic non‐iron deficient healthy pregnant women from multiple centres in Italy. Women were randomly assigned to receive 40 mg of elemental iron containing 250 g of ferritin in a microgranulated gastric resistant capsule daily or no treatment from 12‐16 wks of gestation until the end of puerperium. |
| Van Eijk 1978 | 30 pregnant women with uncomplicated pregnancies attending antenatal care clinic at the University Hospital Obstetric Unit in Rotterdam, The Netherlands. Women received 100 mg of elemental iron (as ferrous sulphate) daily or no treatment from the third month of gestation until delivery. Follow‐up was until 12 wks after delivery. |
| Vogel 1963 | 191 consecutive pregnant when attending antenatal care clinics and at 32 wks of gestation were divided in 2 groups by alternate allocation by clinic: group 1 received 140 mg of elemental iron daily (as ferrous gluconate) in 4 tablets; group 2 received 150 mg elemental iron daily (as ferrous glutamate) in 3 tablets. All women received iron in different dose and number of tablets. No comparisons allowed within the scope of this review. |
| Wali 2002 | 60 iron‐deficiency anaemic pregnant women with the gestational age of 12‐34 wks were randomly assigned to 1 of 3 groups: group 1 (n = 15) received intravenous 500 mg of iron sucrose for storage; group 2 (n = 20) received intravenous iron sucrose according to deficit calculated as per formula with 200 mg of iron was given for storage and group 3 received intra muscular iron Sorbitol in the dose used as practice. All groups received iron intravenous or intramuscular. |
| Wallenburg 1983 | 44 non‐anaemic women with singleton pregnancy attending the University Hospital Obstetrical Clinic of the Erasmus University in Rotterdam who had not received iron supplementation during their first visit. Women were randomly assigned to 1 of 2 groups:group 1: received 105 mg of elemental iron (as ferrous sulphate in a sustained release preparation ) daily and group 2: received no iron supplement. Supplementation started at 14‐16th wk of gestation until delivery. |
| Willoughby 1966 | 350 consecutive pregnant women attending antenatal care clinic were allocated to 1 of 5 groups: group 1 received no haematinic supplements; group 2 received 105 mg of elemental iron daily (as iron chelate aminoates); group 3 received 105 mg of elemental iron daily with 100 μg (0.1 mg) folic acid; group 4 received 105 mg of elemental iron daily with 300 μg (0.3 mg) folic acid; and group 5 received 105 mg of elemental iron daily th 450 μg (0.45 mg) folic acid. All women received a multivitamin preparation (Vivatel) free of folic acid. |
| Willoughby 1967 | 3599 pregnant women with Hb above 100 g/L at their antenatal care clinic visit at Queen's Mother's Hospital in Glasgow, Scotland, United Kingdom. Women were randomly allocated to 1 of 5 interventions: group 1 received no prophylactic supplements; group 2 received 105 mg of elemental iron daily (as chelated iron aminoates); group 3 received 105 mg of elemental iron with 100 μg (0.1 mg) folic acid; group 4 received 105 mg of elemental iron daily with 300 μg (0.3 mg) folic acid; and group 5 received 105 mg elemental iron daily with 450 μg (0.45 mg) folic acid. Starting and ending time of supplementation variable. |
| Willoughby 1968 | 68 pregnant women attending antenatal care clinic in Queen Mother's Hospital in Scotland, were randomly allocated to receive 195 mg of elemental iron alone daily or 195 mg of elemental iron in conjunction with 300 μg (0.3 mg) folic acid daily. |
| Wills 1947 | 500 pregnant women attending antenatal care clinic at the Royal Free Hospital in London, England, United Kingdom during wartime, with ages between 18‐43 years. Women with severe anaemic or rheumatoid arthritis were excluded. Women were alternatively allocated to receive 580 mg of elemental iron (as ferrous gluconate) daily or placebo from their first visit. Supplementation starting variable and ending time unclear. |
| Wu 1998 | 369 pregnant women attending antenatal care at Beijing Hospital, China were divided into 2 groups according to their initial Hb concentrations. Women with Hb 110 g/L or above were randomly assigned to 1 of 2 groups: group 1 (n = 96) received 1 daily tablet of maternal supplement containing 60 mg of elemental iron in addition to other micronutrients including calcium and magnesium ; group 2 (n = 95) served as control and received no supplements. Another group of women with Hb < 110 g/L (treatment group) were randomly assigned to 1 of 3 groups: group 1 received 1 tablet of maternal supplement daily; group 2 received 0.9 g of ferrous sulphate daily; and group 3 received 1 tablet of Ferroids, a sustained released preparation daily. In the preventive group, women entered the study from 20‐24 gestational wks. In the treatment groups, women less than 36 gestational wks were accepted. No comparisons allowed due to the addition of other micronutrients in the treatment. |
| Zeng 2008 | In this cluster‐randomised trial 5828 pregnant women in rural north west China were randomised to 1 of 3 groups: group 1 received daily supplements of 400 μg (0.4 mg) folic acid alone; group 2 received 60 mg elemental iron and 400 μg (0.4 mg) folic acid; and group 3 received a multi‐micronutrient (containing both iron and folic acid). |
| Zhou 2009 | 180 anaemic women (Hb < 110 g/L) attending antenatal care at the Children, Youth and Women's Health Service, Adelaide, Australia with 24‐32 wks of gestation and a singleton pregnancy. Women were excluded if they were taking iron or vitamin and mineral supplements, had presumptive diagnosis of non iron deficiency‐related anaemia, history of thalassaemia, drug or alcohol abuse and/or diabetes requiring insulin or a known fetal abnormality. Women were randomly assigned to receive a daily dose of 20, 40 or 80 mg of elemental iron (as ferrous sulphate) for 8 wks or until birth. The primary outcomes measured were Hb levels, anaemia at the end of the intervention and gastrointestinal side effects during treatment. All women received iron at different doses. No comparisons allowed within the scope of this review. |
| Ziaei 2007 | 750 non‐anaemic (with Hb higher or equal to 132 g/L) pregnant women in early stage of second trimester attending prenatal care in Tehran, Iran. Women were randomly assigned to 1 of 2 groups: group 1 received 50 mg of elemental iron (as ferrous sulphate) with 1000 μg (1 mg) folic acid daily and group 2 received placebo and 1000 μg (1 mg) of folic acid daily. |
| Ziaei 2008 | 244 pregnant women attending prenatal care in Tehran, Iran, 13‐18 wks of gestation and non‐anaemic (Hb 132 g/L or higher) and normal serum ferritin (15 µg/L or higher). Women were randomly assigned to 1 of 2 groups: group 1 received 50 mg of elemental iron (as ferrous sulphate) daily and group 2 received placebo from 20th wk of gestation until delivery. All women received 50 mg elemental iron (as ferrous sulphate) after delivery for 6 wks. |
| Zittoun 1983 | 203 pregnant women attending antenatal clinic in Paris, France, with 28 +/‐ 2 wks of gestation were studied. Women with Hb below 110 g/L (n = 48) were provided 105 mg of elemental iron and 500 mg of ascorbic acid daily. Women with Hb concentration above 110 g/L were randomly assigned to receive 105 mg of elemental iron and 500 mg of ascorbic acid daily until delivery or placebo. Iron was provided in conjunction with vitamin C. No comparisons allowed within the scope of this review. |
| Zutshi 2004 | 200 apparently pregnant women with 24‐26 wks of gestation, with singleton pregnancy with moderate anaemia (Hb > 80 g/L and < 110 g/L) were randomly assigned to receive injectable iron‐sorbitol‐citrate in 3 intramuscular doses of 150 mg each at 4 wks intervals or 100 mg of elemental iron daily. Hb concentrations were measured at baseline, every 4 wks and at delivery. The study compares 2 routes of iron administration. Both groups receive iron. No comparisons allowed within the scope of this review. |
Hb: haemoglobin HCT: haematocrit (same as PCV) IU: international units MCH: mean corpuscular haemoglobin MCHC: mean corpuscular haemoglobin concentration MCV: mean corpuscular volume PCV: packed cell volume (same as HCT) SES: socio‐economic status wk(s): week(s)
Characteristics of ongoing studies [ordered by study ID]
Agrawal 2012.
| Trial name or title | Impact of 2 oral iron supplementation regimens (intermittently and continuously/daily) for prevention of anaemia in pregnancy in women with normal haemoglobin levels. |
| Methods | Randomised controlled trial. Stratified block randomisation with sequentially numbered, sealed, opaque envelopes. |
| Participants | 150 apparently healthy pregnant women 19‐40 years of age with normal Hb levels between 13‐16 weeks' gestation attending antenatal care clinic, Department of Obstetrics and Gynecology, Kasturba Medical College, Maniopal, India. Exclusion criteria: suspected acute infection (upper respiratory tract and urinary tract infections, gastroenteritis), pre‐existing chronic illness, like chronic renal disease, hepatic cirrhosis, viral hepatitis, Inflammatory bowel disease, recent blood transfusion, beta‐thalassaemia and other haemoglobinopathies. |
| Interventions | Participants will be randomised to 1 of 2 groups: group 1 will receive an oral intake with water of a capsule of Autrin® daily containing 98.6 mg elemental iron (as ferrous fumarate); group 2 will receive oral intake with water of the same capsule Autrin® only during 4 days a week (Monday‐Thursday). Oral iron in both the groups will be continued throughout pregnancy. |
| Outcomes | Hb (more than 105 g/L), serum ferritin at 28 and 38 weeks' gestation, side effects of oral iron: nausea, vomiting, constipation, heartburn, diarrhoea, metallic taste, pre‐eclampsia, IUGR in 3rd trimester, preterm labor, birthweight, placenta weight, compliance. |
| Starting date | Date of first enrolment: 01/01/2010. Recruitment ongoing. |
| Contact information | Nimisha Agrawal Junior Resident Kasturba Medical College, Manipal, Department of Obstetrics and Gynecology, Kasturba Hospital, ManipalL‐576104, Udupi District, Karnataka, India 576104 Phone 0820‐2932600 Email: nimisha_4u@yahoo.co.in |
| Notes | Sponsors: Manipal University, Manipal, India and Kasturba Medical College, Manipal, India. Trial registered retrospectively. |
Gies 2010.
| Trial name or title | Malaria risk prior to and during early pregnancy in nulliparous women receiving long‐term weekly iron and folic acid supplementation (WIFS): a non‐inferiority randomised controlled trial. |
| Methods | Randomised double‐blind trial. |
| Participants | 1800 nulliparous women 15‐24 years of age at enrolment, residing within the Demographic Surveillance System are in Burkina Faso. Women will be followed up weekly up to 18 months. Women who become pregnant will be followed up until delivery. Women with no menses for more than 3 months and/or palpable uterus or positive pregnancy test if history unclear; concurrent enrolment in another study; intention to move out of the study area for more than 2 months within the next 18 months; any significant illness at the time of screening that requires hospitalisation, including clinical signs of severe anaemia (conjunctival or mucosal pallor, tachycardia, respiratory distress); history or presence of major clinical disease likely to influence pregnancy outcome (sickle cell disease, diabetes mellitus, severe renal or heart disease, open tuberculosis, epilepsy) will be excluded. |
| Interventions | Participants will be randomly assigned to 1 of 2 groups: group 1: 60 mg elemental iron and 2800 μg (2.8 mg) folic acid weekly for 18 months; group 2: 2800 μg (2.8 mg) folic acid weekly for 18 months. Women who become pregnant will change to 60 mg elemental iron and 400 μg (0.4 mg) folic acid daily after the first antenatal care visit at 13‐16 weeks of gestation. |
| Outcomes | Primary: Prevalence of peripheral parasitaemia at first antenatal clinic visit (13‐16 weeks' gestation). Secondary: In the pregnant cohort: incidence of adverse pregnancy outcomes, incidence of clinical malaria during the first and subsequent trimesters, mean birthweight and prevalence of low birthweight (less than 2500 g), mean gestational age at delivery, prevalence of anaemia at first antenatal clinic visit, prevalence of iron deficiency at first antenatal visit, prevalence of placental malaria. |
| Starting date | January 2011. |
| Contact information | Dr Sabine Gies Liverpool School of Tropical Medicine Tel: +22670700738 Email: sgies@itg.be |
| Notes | Sponsors: Centre Muraz; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD); Institut de Recherche en Sciences de la Sante‐Direction Regionale de l'Ouest; Institute of Tropical Medicine, Belgium. National Institutes of Health (NIH) and University of Manchester. |
Goonewardene 2014.
| Trial name or title | Randomised control trial comparing efficacy of weekly versus daily antenatal oral iron supplementation in preventing anaemia during pregnancy. |
| Methods | Randomised controlled trial with 2 arms. |
| Participants | 291 pregnant women between 12 to 20 weeks' gestation and Hb higher than 110 g/L or more, irrespective of the serum ferritin concentrations attending for antenatal care in the Academic Obstetric Unit, Teaching Hospital Mahamodara, Galle, Sri Lanka. Pregnant women with diagnosis of haematological disorders or any chronic diseases were excluded. |
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 (n = 142) women will receive 60 mg elemental iron (as 200 mg ferrous sulphate), 1000 µg (1 mg) folic acid and 100 mg ascorbic acid daily from recruitment until 3 months postpartum; group 2 (n = 149) women will receive 60 mg elemental iron (as 200 mg ferrous sulphate), 1000 µg (1 mg) folic acid and 100 mg ascorbic acid once weekly from recruitment until 3 months postpartum. The anti–helminthic Mebendazole 100 mg was given twice daily was given to all the women before recruitment as per current practice in Sri Lanka. |
| Outcomes | Primary: Hb concentrations, hematocrit or packed cell volume (PCV) and serum ferritin at 34‐36 weeks of gestation. Secondary: compliance (assessed by pill count and questionnaire) and infant birthweight. |
| Starting date | 1 December 2014 (Data collection completed.Results being analysed). |
| Contact information | Professor Malik Goonewardene Senior Professor & Head Department of Obstetrics & Gynaecology, Faculty of Medicine, PO Box 70, Galle, Sri Lanka. Tel: +94 91 2246878 Email: malikg@eureka.lk |
| Notes | Sponsors: Department of Obstetrics and Gynecology of the Faculty of Medicine, University of Ruhuna, Sri Lanka. |
Kumar 2014.
| Trial name or title | Estimation of oxidative stress in pregnant women on daily versus weekly iron supplementation (an ICMR task force study). |
| Methods | Randomised, parallel group trial. |
| Participants | 650 non‐anaemic (Hb above 110 g/L) healthy primiparous women 20‐30 years of age with 14‐16 weeks of gestation, from middle socio‐economic income group attending antenatal care at Human Reproductive Research Centre, Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences in New Delhi, India. Women with history of chronic diseases or who reported taking any iron supplements at the time of enrolment will be excluded. |
| Interventions | Participants will be randomly assigned to 1 of 2 groups: group 1 will receive a daily dose of 100 mg elemental iron folic acid tablet starting from 13‐16 weeks of gestation until 6 weeks postpartum as per recommendation of National Anaemia Control Programme; group 2: will receive 200 mg elemental iron and folic acid tablet once weekly dose from recruitment until 6 weeks postpartum. |
| Outcomes | Primary: oxidative stress status in plasma at baseline (14‐16 weeks of gestation), thirst trimester and 6 weeks postpartum. Secondary: birth outcomes, but not specified in the registry form. |
| Starting date | 30/03/2009 |
| Contact information | Dr Neeta Kumar Scientist, Division of Human and Reproductive Health Centre, Indian Council of Medical Research (ICMR), All India Institute of Medical Sciences, New Delhi, DELHI 110029, India Tel: +9313195247 Email: neeta@icmr.org.in |
| Notes | Sponsor: Indian Council of Medical Research, New Delhi, India. Trial registered retrospectively. |
Sherbaf 2012.
| Trial name or title | Comparison the effect of daily versus twice weekly usage of iron supplementation on pregnancy outcomes in nonanaemic pregnant women. |
| Methods | Randomised controlled trial with 2 arms. |
| Participants | 508 singleton non‐iron deficient non‐anaemic pregnant women (Hb 110‐138 g/L and ferritin 10‐300 ng/mL) with less than 18 weeks of gestation, no history of smoking or use of glucocorticoid and sympathomimetic drugs, and no history of asthma or chronic hypertension, gestational diabetes, pre‐eclampsia or overt diabetes in previous pregnancies attending The Great Women Hospital in Tehran, Islamic Republic of Iran. Women with Hb concentrations less than 105 g/L in second trimester or less than 110 g/L in third trimester will be excluded from the study. |
| Interventions | Participants will be randomly assigned to 1 of 2 groups: group 1 (n = 254) will receive 50 mg elemental iron (as ferrous sulphate) daily from 20 weeks of gestation until delivery; group 2 (n = 254) will receive 50 mg elemental iron (as ferrous sulphate) twice a week from 20th week of gestation until delivery. |
| Outcomes | Primary: gestational diabetes at gestational age of 24‐28 weeks and at 32‐36 weeks of gestation, pre‐eclampsia, birthweight of neonate, low birthweight. Secondary: any adverse effects, nausea,vomiting, constipation, metallic oral taste, Hb concentrations, ferritin concentrations at 24‐28 weeks and at 32‐36 weeks of gestation. |
| Starting date | 10 February 2012. |
| Contact information | Dr Fateme Rahimi Sherbaf Mirza Kuchack Khan Hospital (Women Hospital), North Nejatolahi Street, Tehran 15978, Islamic Republic of Iran Tel: +98 21 88900002 Email: rahimish@tums.ac.ir |
| Notes | Sponsor: Tehran University of Medical Sciences. |
Hb: haemoglobin IUGR: intrauterine growth restriction
Differences between protocol and review
This review updates Peña‐Rosas 2012 and aims to evaluate only the effects of intermittent (i.e. one, two or three times a week on non‐consecutive days) supplement intake versus the standard daily regimens and the effects of these interventions on pregnancy outcomes. In this update we have included 27 trials; 21 of these studies were included in the previous version of the review.
Outcomes
We have added a description of the ferritin laboratory methods for each trial that measured this outcome (Garcia‐Casal 2014).
Methods
We have divided the blinding of outcome assessment (detection bias) into two categories: one addressing laboratory outcomes and the other addressing side effects and compliance.
GRADE methods for incorporating 'Summary of findings' tables have been added to the review (2012 update).
Contributions of authors
In this updated version of the review HGM and MFU assessed eligibility for included trials and carried out additional data extraction. JPPR and TD completed the description of included and excluded trials. TD and LMD produced the GRADE evidence profiles for the critical outcomes. All the authors actively participated in the discussions and drafted the manuscript.
Sources of support
Internal sources
-
Evidence and Programme Guidance, Department of Nutrition for Health and Development, World Health Organization (WHO), Switzerland.
Juan Pablo Pena‐Rosas is full‐time staff of the World Health Organization. Monica Flores Urrutia was an intern during her involvement in this update version of the review.
University of Liverpool, UK.
-
The Micronutrient Initiative, Canada.
Luz Maria De‐Regil is full‐time staff if The Micronutrient Initiative.
External sources
-
Centers for Disease Control and Prevention (CDC), USA.
The International Micronutrient Prevention and Control Programme (IMMPaCt) provides technical and financial support to the Department of Nutrition for Health and Development for the update of systematic reviews in nutrition.
-
The Bill & Melinda Gates Foundation, USA.
The World Health Organization gratefully acknowledges the financial contribution of the Bill & Mellinda Gates Foundation for the work on systematic reviews of the evidence in nutrition.
-
International Life Sciences Institute (ILSI), USA.
ILSI provided partial financial support for the internship of Monica Flores Urrutia in the Evidence and Programme Guidance unit, Department of Nutrition for Health and Development in Geneva, Switzerland from November 2014 until June 2015.
Declarations of interest
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (e.g. employment, consultancy, stock ownership, honoraria, expert testimony).
Juan Pablo Peña‐Rosas was author of an excluded study on iron and folic acid intermittent supplementation (Pena‐Rosas 2003). Juan Pablo Peña‐Rosas and Luz Maria De‐Regil provided technical input in the development of the protocol of one included study (Hanieh 2013 (C)). Neither of these authors was involved in assessing study eligibility.
Juan Pablo Peña‐Rosas is currently a staff member of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization.
Monica C Flores‐Urrutia worked on the review during an internship at the World Health Organization and was financially supported by the International Life Sciences Institute (ILSI).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Alaoddolehei 2013 {published data only}
- Alaoddolehei H, Sadighian F. Comparison effect of daily versus intermittent dose of iron supplement on the blood indices, serum level of iron, ferritin and zinc in healthy pregnant women ‐ iron supplementation and pregnancy. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 2 July 2015) 2015.
- Alaoddolehei H, Samiei H, Sadighian F, Kalantari N. Efficacy of daily versus intermittent administration of iron supplementation in anemia or blood indices during pregnancy. Caspian Journal of Internal Medicine 2013;4(1):569‐73. [PMC free article] [PubMed] [Google Scholar]
Bhatla 2009 {published data only}
- Bhatla N, Kaul N, Lal N, Kriplani A, Agarwal N, Saxena R, et al. Comparison of effect of daily versus weekly iron supplementation during pregnancy on lipid peroxidation. Journal of Obstetrics and Gynaecology Research 2009;35(3):438‐45. [DOI] [PubMed] [Google Scholar]
Bouzari 2011 {published data only}
- Bouzari Z, Basirat Z, Zeinal Zadeh M, Yazdani Cherati S, Didehdar Ardebil M, Mohammadnetaj M, et al. Daily versus intermittent iron supplementation in pregnant women. BMC Research Notes 2011;4(1):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casanueva 2006 {published and unpublished data}
- Casanueva E. Weekly iron‐folate (Fe‐fol) supplementation during pregnancy in Mexican women. Personal communication 2003.
- Casanueva E, Viteri FE, Mares‐Galindo M, Meza‐Camacho C, Loria A, Schnaas L, et al. Weekly iron as a safe alternative to daily supplementation for nonanemic pregnant women. Archives of Medical Research 2006;37(5):674‐82. [DOI] [PubMed] [Google Scholar]
Chew 2004a {published and unpublished data}
- Chew F, Torun B, Viteri FE. Comparison of weekly and daily iron supplementation to pregnant women in Guatemala (supervised and unsupervised). FASEB Journal 1996;10:A4221. [Google Scholar]
- Chew F, Torun B, Viteri FE. Individual patient data (as supplied 15 January 2004). Data on file.
- Chew F, Torún B, Viteri FE. Comparison of daily and weekly iron supplementation in pregnant women with and without direct supervision [Comparación de la suplementación diaria o semanal de hierro en mujeres embarazadas con y sin supervisión directa]. XI Congreso Latino Americano de Nutrición, Libro de Resumenes. Guatemala: SLAN, 1997:94.
Chew 2004b {published and unpublished data}
- Chew F, Torun B, Viteri FE. Comparison of weekly and daily iron supplementation to pregnant women in Guatemala (supervised and unsupervised). FASEB Journal 1996;10:A4221. [Google Scholar]
- Chew F, Torun B, Viteri FE. Individual patient data (as supplied 15 January 2004). Data on file.
- Chew F, Torún B, Viteri FE. Comparison of daily and weekly iron supplementation in pregnant women with and without direct supervision [Comparación de la suplementación diaria o semanal de hierro en mujeres embarazadas con y sin supervisión directa]. XI Congreso Latino Americano de Nutrición, Libro de Resumenes. Guatemala: SLAN, 1997:94.
Ekstrom 2002 (C) {published and unpublished data}
- Ekstrom EC. Personal communication 2004 April 12.
- Ekstrom EC, Hyder SM, Chowdhury AM, Chowdhury SA, Lonnerdal B, Habicht JP, et al. Efficacy and trial effectiveness of weekly and daily iron supplementation among pregnant women in rural Bangladesh: disentangling the issues. American Journal of Clinical Nutrition 2002;76(6):1392‐400. [DOI] [PubMed] [Google Scholar]
- Hyder SM, Persson LA, Chowdhury AM, Ekstrom EC. Do side‐effects reduce compliance to iron supplementation? A study of daily‐ and weekly‐dose regimens in pregnancy. Journal of Health, Population and Nutrition 2002;2:175‐9. [PubMed] [Google Scholar]
- Hyder SM, Persson LA, Chowdhury R, Lonnerdal B, Ekstrom EC. Impact of daily and weekly iron supplementation to women in pregnancy and puerperium on haemoglobin and iron status six weeks postpartum: results from a community‐based study in Bangladesh. Scandinavian Journal of Nutrition 2003;47(1):19‐25. [Google Scholar]
Goonewardene 2001 {published data only}
- Goonewardene M, Liyanage C, Fernando R. Intermittent oral iron supplementation during pregnancy. Ceylon Medical Journal 2001;46(4):132‐5. [DOI] [PubMed] [Google Scholar]
Goshtasebi 2012 {published data only}
- Alizadeh M, Goshtasebi A, Kazemnejad A. The impact of twice weekly versus daily iron supplementation on cord blood hemoglobin and ferritin levels: a randomized clinical trial. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23 Suppl 1:140. [Google Scholar]
- Goshtasebi A. To compare daily and twice weekly iron supplementation on the anemia indices and side effects in mother and neonate (cord) during pregnancy. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 March 2011) 2010.
- Goshtasebi A, Alizadeh M. Impact of twice weekly versus daily iron supplementation during pregnancy on maternal and fetal haematological indices: A randomized clinical trial [Impact d'une prise de complement en fer bihebdomadaire par rapport a une prise quotidienne pendant la grossesse sur des indices hematologiques maternels et foetaux: Une etude clinique randomisee]. Eastern Mediterranean Health Journal 2012;18(6):561‐6. [DOI] [PubMed] [Google Scholar]
Grover 1998 {published data only}
- Grover V, Aggarwal OP, Gupta A, Kumar P, Tiwari RS. Effect of daily and alternate day iron and folic acid supplementation to pregnant females on the weight of the newborn. Indian Journal of Community Medicine 1998;23(4):165‐8. [Google Scholar]
Hanieh 2013 (C) {published and unpublished data}
- Biggs BA. A randomised controlled trial to compare the impact on birth weight of daily iron‐folic acid, twice weekly iron‐folic acid and twice weekly multiple micronutrient supplementation for pregnant women in Ha Nam province, Vietnam. http://apps.who.int/trialsearch/Trial.aspx?TrialID=ACTRN12610000944033 (accessed 17 January 2011).
- Hanieh S, Ha TT, Simpson JA, Casey GC, Thuy T, Khuong NC, et al. The effect of intermittent antenatal iron supplementation on infant outcomes in rural Vietnam: a cluster randomised trial. Annals of Nutrition and Metabolism 2013;63(Suppl 1):778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanieh S, Ha TT, Simpson JA, Casey GJ, Khuong NC, Thoang DD, et al. The effect of intermittent antenatal iron supplementation on maternal and infant outcomes in rural Vietnam: a cluster randomised trial. Plos Medicine 2013;10(6):e1001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TD, Fisher J, Hanieh S, Tran T, Simpson JA, Tran H, et al. Antenatal iron supplementation regimens for pregnant women in rural Vietnam and subsequent haemoglobin concentration and anaemia among their Infants. PLoS One 2015;10(4):e0125740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hashim 2012 {published data only}
- Hashim H, Ismail AH, Shaaban J. The effectiveness of weekly versus daily iron supplementation among mild anemic pregnant women. BJOG: an international journal of obstetrics and gynaecology 2012;119(Suppl 1):94‐5. [Google Scholar]
Liu 2003 {published and unpublished data}
- Liu XN, Liu PY. The effectiveness of weekly iron supplementation regimen in improving the iron status of Chinese children and pregnant women. Biomedical and Environmental Sciences 1996;9:341‐7. [PubMed] [Google Scholar]
- Liu XN, Liu PY, Viteri FE. Individual patient data (as supplied December 2003). Data on file.
- Liu XN, Yang W, Zhang J, Ying H, Gen Y, Xie J, et al. Weekly iron supplementation is effective and safe in pregnant women. FASEB Journal 1995;9:A658. [Google Scholar]
Mukhopadhyay 2004 {published data only}
- Mukhopadhyay A, Bhatla N, Kriplani A, Agarwal N, Saxena R. Erythrocyte indices in pregnancy: effect of intermittent iron supplementation. National Medical Journal of India 2004;17(3):135‐7. [PubMed] [Google Scholar]
- Mukhopadhyay A, Bhatla N, Kriplani A, Pandey RM, Saxena R. Daily versus intermittent iron supplementation in pregnant women: hematological and pregnancy outcome. Journal of Obstetrics and Gynaecology Research 2004;30(6):409‐17. [DOI] [PubMed] [Google Scholar]
Mumtaz 2000 {published data only}
- Mumtaz Z, Shahab S, Butt N, Rab MA, DeMuynck A. Daily iron supplementation is more effective than twice weekly iron supplementation in pregnant women in Pakistan in a randomized double‐blind clinical trial. Journal of Nutrition 2000;130(11):2697‐702. [DOI] [PubMed] [Google Scholar]
Pita Martin 1999 {published and unpublished data}
- Pita Martin de Portela ML. Personal communication 2004 March 22.
- Pita Martin de Portela ML, Langini SH, Fleischman S, Garcia M, Lopez LB, Guntin R, et al. Effect of iron supplementation and its frequency during pregnancy. Medicina 1999;59:430‐6. [PubMed] [Google Scholar]
Quintero 2004 {unpublished data only}
- Quintero Gutierrez AG, Gonzalez Rosendo G, Cedillo Espana F, Rivera‐Dommarco J. Single weekly iron supplementation in pregnant women. Personal communication 2004 February 17.
Ridwan 1996 (C) {published and unpublished data}
- Ridwan E, Schultink W, Dillon D, Gross R. Effects of weekly iron supplementation on pregnant Indonesian women are similar to those of daily supplementation. American Journal of Clinical Nutrition 1996;63(6):884‐90. [DOI] [PubMed] [Google Scholar]
- Schultink W, Ridwan E, Dillon D, Gross R. Individual patient data (as supplied 12 January 2004). Data on file.
Robinson 1999 {published and unpublished data}
- Robinson JS. Individual patient data (as supplied 11 March 2004). Data on file.
- Robinson JS. Working with traditional birth attendants to improve iron tablet utilization by pregnant women. MotherCare Technical Working Paper #7. Arlington, VA 1998.
- Robinson JS, Sopacua J, Napitapulu J. Using traditional birth attendants to improve iron tablet utilization by pregnant women. Maluku Province, Indonesia. Draft paper. Mother Care Project. Project Concern International San Diego CA 1999.
- Robinson JS, Yip R. Weekly versus daily iron tablet supplementation in pregnant women in Indonesia. Draft paper 2000.
Rukhsana 2006 {published data only}
- Rukhsana N, Bano H, Gurbakhshani AL, Dudani AL, Jafri TK, Mannan A. Changes in hemoglobin, red cell count, red cell indices and reticulocyte count in response to daily versus intermittent oral iron supplementation during pregnancy in local population. Medical Channel 2006;12(3):7‐10. [Google Scholar]
Singh 2011 {published data only}
- Singh U, Sinha S, Gupta HP, Natu SM, Deo S, Bagchi S. Once a week (200 mg) elemental iron: An effective option for prophylaxis in non‐anaemic pregnant women. Journal of the Indian Medical Association 2011;109(9):654‐6. [PubMed] [Google Scholar]
Viteri 2012 {published and unpublished data}
- Viteri FE, Casanueva E, Tolentino M, Rosales X, Erazo B. Oxidative stress in pregnant women receiving daily but not weekly iron supplements. Is daily iron toxic?. Personal communication, 10 Jul 2010.
- Viteri FE, Casanueva E, Tolentino MC, Diaz‐Frances J, Erazo AB. Antenatal iron supplements consumed daily produce oxidative stress in contrast to weekly supplementation in Mexican non‐anemic women. Reproductive Toxicology 2012;34(1):125‐32. [DOI] [PubMed] [Google Scholar]
Winichagoon 2003 (C) {unpublished data only}
- Winichagoon P, Lertmullikaporn N, Chitcumroonchokechai C, Thamrongwarangkul T. Daily versus weekly iron supplementation to pregnant women in rural northeast Thailand. Personal communication 2003.
Yekta 2011 {published data only}
- Yekta Z, Pourali R, Mladkova N, Ghasemi‐rad M, Boromand F, Hazrati Tappeh K. Role of iron supplementation in promoting maternal and fetal outcome. Therapeutics and Clinical Risk Management 2011;7:421‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2000 {published data only}
- Young MW, Lupafya E, Kapenda E, Bobrow EA. The effectiveness of weekly iron supplementation in pregnant women of rural northern Malawi. Tropical Doctor 2000;30(2):84‐8. [DOI] [PubMed] [Google Scholar]
Yu 1998 {published and unpublished data}
- Yu KH, Yoon JS. The effect of weekly iron supplementation on iron and zinc nutritional status in pregnant women. Korean Journal of Nutrition 1998;31(8):1270‐82. [Google Scholar]
- Yu KH, Yoon, JS. Individual patient data (as supplied 11 March 2004). Data on file.
Zamani 2008 {published data only}
- Zamani AR, Farajzadegan Z, Ghahiri A, Khademloo M, Golshiri P. Effectiveness of twice weekly iron supplementation compared with daily regimen in reducing anemia and iron deficiency during pregnancy: a randomized trial in Iran. Journal of Research in Medical Sciences 2008;13(5):230‐9. [Google Scholar]
References to studies excluded from this review
Aaseth 2001 {published data only}
- Aaseth J, Thomassen Y, Ellingsen DG, Stoa‐Birketvedt G. Prophylactic iron supplementation in pregnant women in Norway. Journal of Trace Elements in Medicine & Biology 2001;15(2‐3):167‐74. [DOI] [PubMed] [Google Scholar]
Abel 2000 {published data only}
- Abel R, Rajaratnam J, Kalaimani A, Kirubakaran S. Can iron status be improved in each of the three trimesters? A community base study. European Journal of Clinical Nutrition 2000;54:490‐3. [DOI] [PubMed] [Google Scholar]
Adhikari 2009 {published data only}
- Adhikari K, Liabsuetrakul T, Pradhan N. Effect of education and pill count on hemoglobin status during prenatal care in Nepalese women: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2009;35(3):459‐66. [DOI] [PubMed] [Google Scholar]
Afifi 1978 {published data only}
- Afifi AM. Plexafer‐F in the management of latent iron deficiency in pregnancy. Journal of International Medical Research 1978;6:34‐40. [DOI] [PubMed] [Google Scholar]
Ahn 2006 {published data only}
- Ahn E, Pairaudeau N, Pairaudeau N, Cerat Y, Couturier B, Fortier A, et al. A randomized cross over trial of tolerability and compliance of a micronutrient supplement with low iron separated from calcium vs high iron combined with calcium in pregnant women. BMC Pregnancy and Childbirth 2006;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Angeles‐Agdeppa 2005 {published data only (unpublished sought but not used)}
- Angeles‐Agdeppa I. The effects of a community‐based weekly iron‐folate supplementation on hemoglobin and iron status of pregnant and non‐pregnant women in Philippines. Meeting on weekly iron/folic acid supplementation for preventing anaemia in women of reproductive age in the Western Pacific Region Report. Manila, Philippines, February 2004.
- Angeles‐Agdeppa I, Paulino LS, Ramos AC, Etorma UM, Cavalli‐Sforza T, Milani S. Government‐industry partnership in weekly iron‐folic acid supplementation for women of reproductive age in the Philippines: impact on iron status. Nutrition Reviews 2005;63(12 Pt 2):S116‐S125. [DOI] [PubMed] [Google Scholar]
- Paulino LS, Angeles‐Agdeppa I, Etorma UM, Ramos AC, Cavalli‐Sforza T. Weekly iron‐folic acid supplementation to improve iron status and prevent pregnancy anemia in Filipino women of reproductive age: the Philippine experience through government and private partnership. Nutrition Reviews 2005;63(12 Pt 2):S109‐S115. [DOI] [PubMed] [Google Scholar]
Babior 1985 {published data only}
- Babior BM, Peters WA, Briden PM, Cetrulo CL. Pregnant women's absorption of iron from prenatal supplements. Journal of Reproductive Medicine 1985;30:355‐7. [PubMed] [Google Scholar]
Barton 1994 {published data only}
- Barton DPJ, Joy MT, Lappin TRJ, Afrasiabi M, Morel JG, O'Riordan J, et al. Maternal erythropoietin in singleton pregnancies: a randomized trial on the effect of oral hematinic supplementation. American Journal of Obstetrics and Gynecology 1994;170:896‐901. [DOI] [PubMed] [Google Scholar]
- Murphy JF. Randomised double blind control trial of iron/placebo administration to pregnant women with high booking Hb concentrations (Hb>149/dl). Personal communication 1990.
Batu 1976 {published data only}
- Batu AT, Toe T, Pe H, Nyunt KK. A prophylactic trial of iron and folic acid supplements in pregnant Burmese women. Israel Journal of Medical Sciences 1976;12:1410‐7. [PubMed] [Google Scholar]
Bencaiova 2007 {unpublished data only}
- Bencaiova G, Mandach U, Zimmerman R. Optimal prophylaxis of a lack of iron and iron‐deficiency anemia in the pregnancy [Optimale Prophylaxe e Ines Elsenmangels und elner Eisenmangelanamie In der Schwangerschaft: eine randomisierte Studie]. Gynakologisch‐Geburtshilfliche Rundschau 2007;47:140. [Google Scholar]
- Bencaiova G, Mandach U, Zimmermann R. Iron prophylaxis in pregnancy: intravenous route versus oral route. European Journal Obstetrics & Gynecology and Reproductive Biology 2009;144(2):135‐9. [DOI] [PubMed] [Google Scholar]
Berger 2005 {published data only (unpublished sought but not used)}
- Berger J. Effectiveness of weekly iron/folate supplementation on anaemia and iron status in women of reproductive age in rural Viet Nam. Meeting on weekly iron/folic acid supplementation for preventing anaemia in women of reproductive age in the Western Pacific Region Report. Manila, Philippines, February 2004.
- Berger J, Thanh HT, Cavalli‐Sforza T, Smitasiri S, Khan NC, Milani S, et al. Community mobilization and social marketing to promote weekly iron‐folic acid supplementation in women of reproductive age in Vietnam: impact on anemia and iron status. Nutrition Reviews 2005;63(12 Pt 2):S95‐S108. [DOI] [PubMed] [Google Scholar]
- Hoa PT, Berger J, Paliakara N, Nhien NV, Morestin‐Cadet S, Quyen DT, et al. Weekly iron‐folate supplementation in women in reproductive age in Vietnam: a new approach to control iron deficiency anemia during pregnancy. INACG Symposium; February 2001; Hanoi, Vietnam. 2001:45, Abstract No: 18.
- Khan NC, Thanh HT, Berger J, Hoa PT, Quang ND, Smitasiri S, et al. Community mobilization and social marketing to promote weekly iron‐folic acid supplementation: a new approach toward controlling anemia among women of reproductive age in Vietnam. Nutrition Reviews 2005;63(12 Pt 2):S87‐S94. [DOI] [PubMed] [Google Scholar]
Bergsjo 1987 {published data only}
- Bergsjo P. The effects of iron supplementation in pregnancy. Personal communication 1987.
Blot 1980 {published data only}
- Blot I, Tchernia G, Chenayer M, Hill C, Hajeri H, Leluc R. Iron deficiency in the pregnant woman. Its repercussions on the newborn. The influence of systematic iron treatment. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1980;9:489‐95. [PubMed] [Google Scholar]
Brown 1972 {published data only}
- Brown GM, Dawson DW. Prevention of anaemia in pregnancy. Current Medical Research and Opinion 1972;1:93‐9. [DOI] [PubMed] [Google Scholar]
Burslem 1968 {published data only}
- Burslem RW, Poller L, Wacks H. A trial of slow release ferrous sulphate (Ferrogradumet) in prevention of iron deficiency in pregnancy. Acta Haematologica 1968;40:200‐4. [DOI] [PubMed] [Google Scholar]
Buss 1981 {published data only}
- Buss M. Therapy of iron‐folic acid deficiency in pregnancy [Therapie des Eisen‐Folsauremangels in der Schwangerschaft]. Zeitschrift fur Allgemeinmedizin 1981;57(22):1526‐32. [PubMed] [Google Scholar]
Butler 1968 {published data only}
- Butler EB. The effect of iron and folic acid on red cell and plasma volume in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1968;75:497‐510. [DOI] [PubMed] [Google Scholar]
Buytaert 1983 {published data only}
- Buytaert G, Wallenburg HCS, Eijk HG, Buytaert P. Iron supplementation during pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology 1983;15:11‐6. [DOI] [PubMed] [Google Scholar]
Cantlie 1971 {published data only}
- Cantlie GSD, Leeuw NKM, Lowenstein L. Iron and folate nutrition in a group of private obstetrical patients. American Journal of Clinical Nutrition 1971;24:637‐41. [DOI] [PubMed] [Google Scholar]
Carrasco 1962 {published data only}
- Carrasco E, Jose F, Samson G, Germar E, Padilla B. Effect of D‐sorbitol on the absorption and transfer of nutrients from mother to fetus. American Journal of Clinical Nutrition 1962;11:533‐6. [DOI] [PubMed] [Google Scholar]
Chan 2009 {published data only}
- Chan KK, Chan BC, Lam KF, Tam S, Lao TT. Iron supplement in pregnancy and development of gestational diabetes‐‐a randomised placebo‐controlled trial. BJOG: an international journal of obstetrics and gynaecology 2009;116(6):789‐98. [DOI] [PubMed] [Google Scholar]
Chanarin 1965 {published data only}
- Chanarin I, Rothman D, Berry V. Iron deficiency and its relation to folic acid status in pregnancy: results of a clinical trial. British Medical Journal 1965;1:480‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chanarin 1971 {published data only}
- Chanarin I, Rothman D. Further observations on the relation between iron and folate status in pregnancy. British Medical Journal 1971;2:81‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charoenlarp 1988 {published data only}
- Charoenlarp P, Dhanamitta S, Kaewvichit R, Silprasert A, Suwanaradd C, Na‐Nakorn S, et al. A WHO collaborative study on iron supplementation in Burma and in Thailand. American Journal of Clinical Nutrition 1988;47(2):280‐97. [DOI] [PubMed] [Google Scholar]
Chawla 1995 {published data only}
- Chawla PK, Puri R. Impact of nutritional supplements on hematological profile of pregnant women. Indian Pediatrics 1995;32:876‐80. [PubMed] [Google Scholar]
Chisholm 1966 {published data only}
- Chisholm M. A controlled clinical trial of prophylactic folic acid and iron in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1966;73:191‐6. [DOI] [PubMed] [Google Scholar]
Christian 2003 {published and unpublished data}
- Christian P. Personal communication 2007 September 28.
- Christian P, Darmstadt GL, Wu L, Khatry SK, LeClerq SC, Katz J, et al. The effect of maternal micronutrient supplementation on early neonatal morbidity in rural Nepal: a randomised, controlled, community trial. Archives of Disease in Childhood 2008;93(8):660‐4. [DOI] [PubMed] [Google Scholar]
- Christian P, Jiang T, Khatry SK, LeClerq SC, Shrestha SR, West Jr KP. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. American Journal of Clinical Nutrition 2006;83:788‐94. [DOI] [PubMed] [Google Scholar]
- Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ 2003;326(7389):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P, Khatry SK, LeClerq SC, Dali SM. Effects of prenatal micronutrient supplementation on complications of labor and delivery and puerperal morbidity in rural Nepal. International Journal of Gynecology & Obstetrics 2009;106(1):3‐7. [DOI] [PubMed] [Google Scholar]
- Christian P, Shrestha J, LeClerq SC, Khatry SK, Jiang T, Wagner T, et al. Supplementation with micronutrients in addition to iron and folic acid does not further improve the hematologic status of pregnant women in rural Nepal. Journal of Nutrition 2003;133(11):3492‐8. [DOI] [PubMed] [Google Scholar]
- Christian P, Stewart CP, LeClerq SC, Wu L, Katz J, West KP Jr, et al. Antenatal and postnatal iron supplementation and childhood mortality in rural Nepal: a prospective follow‐up in a randomized, controlled community trial. American Journal of Epidemiology 2009;170(9):1127‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P, West KP, Khatry SK, Leclerq SC, Pradhan EK, Katz J, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster‐randomized trial in Nepal. American Journal of Clinical Nutrition 2003;78(6):1194‐202. [DOI] [PubMed] [Google Scholar]
- Christian PS, Darmstadt GL, Wu L, Khatry SK, Leclerq SC, Katz J, et al. The impact of maternal micronutrient supplementation on early neonatal morbidity in rural Nepal: a randomized, controlled, community trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2007 Aug 3. [Epub ahead of print]. [DOI] [PubMed]
- Katz J, Christian P, Dominici F, Zeger SL. Treatment effects of maternal micronutrient supplementation vary by percentiles of the birth weight distribution in rural Nepal. Journal of Nutrition 2006;136(5):1389‐94. [DOI] [PubMed] [Google Scholar]
- Kulkarni B, Christian P, LeClerq SC, Khatry SK. Determinants of compliance to antenatal micronutrient supplementation and women's perceptions of supplement use in rural Nepal. Public Health Nutrition 2010;13(1):82‐90. [DOI] [PubMed] [Google Scholar]
- Stewart CP, Christian P, LeClerq SC, West KP Jr, Khatry SK. Antenatal supplementation with folic acid + iron + zinc improves linear growth and reduces peripheral adiposity in school‐age children in rural Nepal. American Journal of Clinical Nutrition 2009;90(1):132‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP Jr, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6‐ to 8‐year‐old children in rural Nepal. Journal of Nutrition 2009;139(8):1575‐81. [DOI] [PubMed] [Google Scholar]
- Stewart CP, Katz J, Khatry SK, LeClerq SC, Shrestha SR, West KP Jr, et al. Preterm delivery but not intrauterine growth retardation is associated with young maternal age among primiparae in rural Nepal. Maternal & Child Nutrition 2007;3(3):174‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coelho 2000 {published data only}
- Coelho K, Ramdas S, Pillai S. A comparative study of changes in haemoglobin with high and low dose iron preparations in pregnant women. Journal of Obstetrics and Gynecology of India 2000;50(2):37‐9. [Google Scholar]
Cogswell 2003 {published and unpublished data}
- Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM. Individual patient data (as supplied 4 February 2004). Data on file.
- Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM. Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. American Journal of Clinical Nutrition 2003;78(4):773‐81. [DOI] [PubMed] [Google Scholar]
- Cogswell ME, Parvanta I, Yip R, Brittenham GM. Iron supplementation during pregnancy for initially non‐anemic, iron replete women ‐ decreased prevalence of low birth weight infants. Report of the 2001 INACG Symposium. Why iron is important and what to do about it: a new perspective. Washington D.C.: ILSI Human Nutrition Institute, 2002; Vol. 1:42, Abstract no: 7.
- Cogswell ME, Parvanta I, Yip R, Brittenham GM. Low iron during pregnancy increases the risk of delivering preterm or small infants. Report of the 2001 INACG Symposium. Why iron is important and what to do about it: a new perspective. Washington DC: ILSI Human Nutrition Institute, 2002; Vol. 1:42, Abstract no: 8.
Cogswell 2006 {published data only}
- Cogswell ME. Impact of prenatal vitamin/mineral supplements on perinatal mortality. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006).
Cook 1990 {published data only}
- Cook JD, Carriaga M, Kahn SG, Schalch W, Skikne BS. Gastric delivery system for iron supplementation. Lancet 1990;335(8698):1136‐9. [DOI] [PubMed] [Google Scholar]
Corrigan 1936 {published data only}
- Corrigan JC, Strauss MB. The prevention of hypochromic anemia in pregnancy. Journal of the American Medical Association 1936;106(13):1088‐90. [Google Scholar]
Dawson 1987 {published data only}
- Dawson EB, McGanity WJ. Protection of maternal iron stores in pregnancy. Journal of Reproductive Medicine 1987;32(6 Suppl):478‐87. [PubMed] [Google Scholar]
De Benaze 1989 {published data only}
- Benaze C, Galan P, Wainer R, Hercberg S. Prevention of iron deficient anemia during pregnancy by early iron supplementation: a controlled trial. Revue d Epidemiologie et de Sante Publique 1989;37:109‐18. [PubMed] [Google Scholar]
Dijkhuizen 2004 {published data only}
- Dijkhuizen MA, Wieringa FT, West CE, Muhilal. Zinc plus beta‐carotene supplementation of pregnant women is superior to beta‐carotene supplementation alone in improving vitamin a status in both mothers and infants. American Journal of Clinical Nutrition 2004;80(5):1299‐307. [DOI] [PubMed] [Google Scholar]
Dommisse 1983 {published data only}
- Dommisse J, Bell DJH, Du Toit ED, Midgley V, Cohen M. Iron‐storage deficiency and iron supplementation in pregnancy. South African Medical Journal 1983;64:1047‐51. [PubMed] [Google Scholar]
Edgar 1956 {published data only}
- Edgar W, Rice HM. Administration of iron in antenatal clinics. Lancet 1956;1:599‐602. [DOI] [PubMed] [Google Scholar]
Ekstrom 1996 {published data only}
- Ekstrom EM, Kavishe FP, Habicht J, Frongillo EA, Rasmussen KM, Hemed L. Adherence to iron supplementation during pregnancy in Tanzania: determinants and hematologic consequences. American Journal of Clinical Nutrition 1996;64:368‐74. [DOI] [PubMed] [Google Scholar]
Eskeland 1997 {published and unpublished data}
- Eskeland B. Database provided by authors (as supplied 22 February 2004). Data on file.
- Eskeland B, Malterud K, Ulvik RJ, Hunskaar S. Iron supplementation in pregnancy: is less enough? A randomized, placebo controlled trial of low dose iron supplementation with and without heme iron. Acta Obstetricia et Gynecologica Scandinavica 1997;76(9):822‐8. [DOI] [PubMed] [Google Scholar]
Fenton 1977 {published data only}
- Fenton V, Cavill I, Fisher J. Iron stores in pregnancy. British Journal of Haematology 1977;37:145‐9. [PubMed] [Google Scholar]
Fleming 1974 {published data only}
- Fleming AF, Martin JD, Hahnel R, Westlake AJ. Effects of iron and folic acid antenatal supplements on maternal haematology and fetal wellbeing. Medical Journal of Australia 1974;2:429‐36. [DOI] [PubMed] [Google Scholar]
Fleming 1986 {published data only}
- Fleming AF. Anaemia in pregnancy in the Guinea Savanna of Nigeria. In: Ludwig H, Thomsen K editor(s). Gynecology and Obstetrics. Berlin: Springer‐Verlag, 1986:122‐4. [Google Scholar]
- Fleming AF, Ghatoura GBS, Harrison KA, Briggs ND, Dunn DT. The prevention of anaemia in pregnancy in primigravidae in the guinea savanna of Nigeria. Annals of Tropical Medicine and Parasitology 1986;80:211‐33. [DOI] [PubMed] [Google Scholar]
- Harrison KA, Fleming AF, Briggs ND, Rossiter CE. Child‐bearing, health and social priorities: a survey of 22,774 consecutive hospital births in Zaria, Northern Nigeria. 5. Growth during pregnancy in Nigerian teenage primigravidae. British Journal of Obstetrics and Gynaecology 1985;92(5):32‐9. [PubMed] [Google Scholar]
Fletcher 1971 {published data only}
- Fletcher J, Gurr A, Fellingham F, Prankerd T, Brant H, Menzies D. The value of folic acid supplements in pregnancy. Journal of Obstetrics and Gynaecology of the British Empire 1971;78:781‐5. [DOI] [PubMed] [Google Scholar]
Foulkes 1982 {published data only}
- Foulkes J, Goldie DJ. The use of ferritin to assess the need for iron supplements in pregnancy. Journal of Obstetrics and Gynaecology 1982;3:11‐6. [Google Scholar]
Freire 1989 {published data only}
- Freire WB. Hemoglobin as a predictor of response to iron therapy and its use in screening and prevalence estimates. American Journal of Clinical Nutrition 1989;50:1442‐9. [DOI] [PubMed] [Google Scholar]
Gomber 2002 {published data only}
- Gomber S, Agarwal KN, Mahajan C, Agarwal N. Impact of daily versus weekly hematinic supplementation on anemia in pregnant women. Indian Pediatrics 2002;39(4):339‐46. [PubMed] [Google Scholar]
Gopalan 2004 {published data only}
- Gopalan S, Patnaik R, Ganesh K. Feasible strategies to combat low birth weight and intra‐uterine growth retardation. Journal of Pediatric Gastroenterology and Nutrition 2004;39(Suppl 1):S37. [Google Scholar]
Gringras 1982 {published data only}
- Gringras M. A comparison of two combined iron‐folic acid preparations in the prevention of anaemia in pregnancy. Journal of International Medical Research 1982;10:268‐70. [DOI] [PubMed] [Google Scholar]
Groner 1986 {published data only}
- Groner JA, Holtzman NA, Charney E, Mellits ED. A randomized trial of oral iron on tests of short‐term memory and attention span in young pregnant women. Journal of Adolescent Health Care 1986;7:44‐8. [DOI] [PubMed] [Google Scholar]
Guldholt 1991 {published data only}
- Guldholt IS, Trolle BG, Hvidman LE. Iron supplementation during pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1991;70:9‐12. [DOI] [PubMed] [Google Scholar]
Hampel 1974 {published data only}
- Hampel K, Roetz R. Influence of a long‐time substitution with a folate‐iron combination in pregnancy on serum folate and serum iron and on hematological parameters. Geburtshilfe und Frauenheilkunde 1974;34:409‐17. [PubMed] [Google Scholar]
Han 2011 {published data only}
- Han XX, Sun YY, Ma AG, Yang F, Zhang FZ, Jiang DC, et al. Moderate NaFeEDTA and ferrous sulfate supplementation can improve both hematologic status and oxidative stress in anemic pregnant women. Asia Pacific Journal of Clinical Nutrition 2011;20(4):514‐20. [PubMed] [Google Scholar]
Hankin 1962 {published data only}
- Hankin ME. The value of iron supplementation during pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology 1963;3:111‐8. [DOI] [PubMed] [Google Scholar]
- Hankin ME, Symonds EM. Body weight, diet and pre‐eclamptic toxaemia in pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology 1962;4:156‐60. [Google Scholar]
Hartman‐Craven 2009 {published data only}
- Hartman‐Craven B, Christofides A, O'Connor DL, Zlotkin S. Relative bioavailability of iron and folic acid from a new powdered supplement compared to a traditional tablet in pregnant women. BMC Pregnancy and Childbirth 2009;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harvey 2007 {published and unpublished data}
- Fairweather‐Tait S. Personal communication. 2007 September 5.
- Harvey LJ, Dainty JR, Hollands WJ, Bull VJ, Hoogewerff JA, Foxall RJ, et al. Effect of high‐dose iron supplements on fractional zinc absorption and status in pregnant women. American Journal of Clinical Nutrition 2007;85:131‐6. [DOI] [PubMed] [Google Scholar]
Hawkins 1987 {published data only}
- Hawkins DF. Relative efficacy of sustained release iron and iron with folic acid treatment in pregnancy. Personal communication 1987.
Hemminki 1995 {published and unpublished data}
- Hemminki E, Merilainen J. Long‐term effects of iron prophylaxis during pregnancy. International Journal of Gynecology & Obstetrics 1994;46:3. [Google Scholar]
- Hemminki E, Merilainen J. Long‐term follow‐up of mothers and their infants in a randomized trial on iron prophylaxis during pregnancy. American Journal of Obstetrics and Gynecology 1995;173:205‐9. [DOI] [PubMed] [Google Scholar]
- Hemminki E, Rimpela U. A randomized comparison of routine vs selective iron supplementation during pregnancy. Journal of the American College of Nutrition 1991;10:3‐10. [DOI] [PubMed] [Google Scholar]
- Hemminki E, Rimpela U. Iron supplementation, maternal packed cell volume, and fetal growth. Archives of Disease in Childhood 1991;66:422‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki E, Rimpela U, Yla‐Outinen A. Iron prophylaxis during pregnancy and infections. International Journal of Vitamin and Nutrition Research 1991;61:370‐1. [PubMed] [Google Scholar]
- Hemminki E, Uski A, Koponen P, Rimpela U. Iron supplementation during pregnancy ‐ experiences of a randomized trial relying on health service personnel. Controlled Clinical Trials 1989;10:290‐8. [DOI] [PubMed] [Google Scholar]
Hermsdorf 1986 {published data only}
- Hermsdorf J, Ring D, Retzke U, Bruschke G. Oral iron prophylaxis during pregnancy. A longitudinal study about hematologic and clinical parameters in treated and non‐treated pregnant women. Proceedings of 10th European Congress of Perinatal Medicine; 1986 August 12‐16; Leipzig, Germany. 1986:84.
Hoa 2005 {published data only}
- Hoa PT, Khan NC, Beusekom C, Gross R, Conde WL, Khoi HD. Milk fortified with iron or iron supplementation to improve nutritional status of pregnant women: an intervention trial from rural Vietnam. Food & Nutrition Bulletin 2005;26(1):32‐8. [DOI] [PubMed] [Google Scholar]
Holly 1955 {published data only}
- Holly RG. Anemia in pregnancy. Obstetrics & Gynecology 1955;5:562‐9. [PubMed] [Google Scholar]
Hood 1960 {published data only}
- Hood WE, Bond WL. Iron deficiency prophylaxis during pregnancy. Obstetrics & Gynecology 1960;16:82‐4. [PubMed] [Google Scholar]
Horgan 1966 {published data only}
- Horgan M, Woodliff M, Mangion J. A combined iron and folic‐acid preparation in the prophylaxis of anaemia of pregnancy. Practitioner 1966;197:683‐6. [PubMed] [Google Scholar]
Hosokawa 1989 {published data only}
- Hosokawa K. Studies on anemia in pregnant women: therapeutic efficacy of iron monotherapy vs. combination therapy with iron and vitamin C. Rinsho to Kenkyu (Japanese Journal of Clinical and Experimental Medicine) 1989;66(10):3329‐35. [Google Scholar]
Iyengar 1970 {published data only}
- Iyengar L, Apte SV. Prophylaxis of anemia in pregnancy. American Journal of Clinical Nutrition 1970;23:725‐30. [DOI] [PubMed] [Google Scholar]
Kaestel 2005 {published data only}
- Kaestel P, Michaelsen KF, Aaby P, Friis H. Effects of prenatal multimicronutrient supplements on birth weight and perinatal mortality: a randomised, controlled trial in Guinea‐Bissau. European Journal of Clinical Nutrition 2005;59(9):1081‐9. [DOI] [PubMed] [Google Scholar]
Kann 1988 {published data only}
- Kann J, Lyon JA, Bon C. Availability of iron from four prenatal multivitamin/multimineral products. Clinical Therapeutics 1988;10:287‐93. [PubMed] [Google Scholar]
Kerr 1958 {published data only}
- Kerr DNS, Davidson S. The prophylaxis of iron‐deficiency anemia in pregnancy. Lancet 1958;2:483‐8. [DOI] [PubMed] [Google Scholar]
Khambalia 2009 {published data only}
- Khambalia AZ, O'Connor DL, Macarthur C, Dupuis A, Zlotkin SH. Periconceptional iron supplementation does not reduce anemia or improve iron status among pregnant women in rural Bangladesh. American Journal of Clinical Nutrition 2009;90(5):1295‐302. [DOI] [PubMed] [Google Scholar]
Kuizon 1979 {published data only}
- Kuizon MD, Platon TP, Ancheta LP, Angeles JC, Nunez CB, Macapinlac MP. Iron supplementation studies among pregnant women. Southeast Asian Journal of Tropical Medicine and Public Health 1979;10(4):520‐7. [PubMed] [Google Scholar]
Kumar 2005 {published data only}
- Kumar A, Jain S, Singh NP, Singh T. Oral versus high dose parenteral iron supplementation in pregnancy. International Journal of Gynecology & Obstetrics 2005;89:7‐13. [DOI] [PubMed] [Google Scholar]
Lee 2005 {published and unpublished data}
- Lee JI, Lee JA, Lim HS. Effect of time of initiation and dose of prenatal iron and folic acid supplementation on iron and folate nutriture of Korean women during pregnancy. American Journal of Clinical Nutrition 2005;82(4):843‐9. [DOI] [PubMed] [Google Scholar]
- Lim HS. Personal communication 2007 September 27.
Madan 1999 {published data only}
- Madan N, Prasannaraj P, Rusia U, Sundaram KR, Nath LM, Sood SK. Monitoring oral iron therapy with protoporphyrin/heme ratios in pregnant women. Annals of Hematology 1999;78(6):279‐83. [DOI] [PubMed] [Google Scholar]
Makrides 2003 {published and unpublished data}
- Makrides M. Personal communication 2004 April 12.
- Makrides M, Crowther CA, Gibson RA, Gibson RS, Skeaff CM. Efficacy and tolerability of low‐dose iron supplements during pregnancy: a randomised controlled trial. American Journal of Clinical Nutrition 2003;78:145‐53. [DOI] [PubMed] [Google Scholar]
- Makrides M, Crowther CA, Gibson RA, Gibson RS, Skeaff CM. Low‐dose iron supplements in pregnancy prevent iron deficiency at the end of pregnancy and during the post‐partum period: the results of a randomised controlled trial. Perinatal Society of Australia and New Zealand 7th Annual Congress; 2003 March 9‐12; Tasmania, Australia. 2003:P99.
- Parsons AG, Zhou SJ, Spurrier NJ, Makrides M. Effect of iron supplementation during pregnancy on the behaviour of children at early school age: long‐term follow‐up of a randomised controlled trial. British Journal of Nutrition 2008;99(5):1133‐9. [DOI] [PubMed] [Google Scholar]
- Zhou SH, Gibson RA, Crowther CA, Baghurst P, Makrides M. Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 years of age: long term follow‐up of a randomized controlled trial. American Journal of Clinical Nutrition 2006;83(5):1112‐7. [DOI] [PubMed] [Google Scholar]
- Zhou SJ, Gibson RA, Makrides M. Routine iron supplementation in pregnancy has no effect on iron status of children at six months and four years of age. Journal of Pediatrics 2007;151(4):438‐40. [DOI] [PubMed] [Google Scholar]
Mbaye 2006 {published data only}
- Mbaye A, Richardson K, Balajo B, Dunyo S, Shulman C, Milligan P, et al. Lack of inhibition of the anti‐malarial action of sulfadoxine‐pyrimethamine by folic acid supplementation when used for intermittent preventive treatment in Gambian primigravidae. American Journal of Tropical Medicine & Hygiene 2006;74(6):960‐4. [PubMed] [Google Scholar]
McKenna 2003 {published data only (unpublished sought but not used)}
- McKenna D, Spence D, Dornan J. A randomised, double‐blind, placebo‐controlled trial investigating the place of spatone‐iron plus as a prophylaxis against iron deficiency in pregnancy [abstract]. Journal of Obstetrics and Gynaecology 2002;22(2 Suppl):S45. [Google Scholar]
- McKenna D, Spence D, Haggan SE, McCrum E, Dornan JC, Lappin TR. A randomized trial investigating an iron‐rich natural mineral water as a prophlylaxis against iron deficiency in pregnancy. Clinical and Laboratory Haematology 2003;25:99‐103. [DOI] [PubMed] [Google Scholar]
Meier 2003 {published data only}
- Meier PR, Nickerson HJ, Olson KA, Berg RL, Meyer JA. Prevention of iron deficiency anemia in adolescent and adult pregnancies. Clinical Medicine and Research 2003;1(1):29‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menendez 1994 {published data only}
- Menendez C, Todd J, Alonso PL, Francis N, Lulat S, Ceesay S, et al. The effects or iron supplementation during pregnancy, given by traditional birth attendants, on the prevalence of anaemia and malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88:590‐3. [DOI] [PubMed] [Google Scholar]
- Menendez C, Todd J, Alonso PL, Francis N, Lulat S, Ceesay S, et al. The response to iron supplementation of pregnant women with the haemoglobin genotype AA or AS. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(3):289‐92. [DOI] [PubMed] [Google Scholar]
Menon 1962 {published data only}
- Menon MKK, Rajan L. Prophylaxis of anaemia in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1962;12:382‐9. [Google Scholar]
Milman 1991 {published data only}
- Milman N, Agger AO, Nielsen OJ. Iron supplementation during pregnancy. Effect on iron status markers, serum erythropoietin and human placental lactogen. A placebo controlled study in 207 Danish women. Danish Medical Bulletin 1991;38(6):471‐6. [PubMed] [Google Scholar]
- Milman N, Agger AO, Nielson OJ. Iron status markers and serum erythropoietin in 120 mothers and newborn infants: effect of iron supplementation in normal pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1994;73:200‐4. [DOI] [PubMed] [Google Scholar]
- Milman N, Byg KE, Agger AO. Hemoglobin and erythrocyte indices during normal pregnancy and postpartum in 206 women with and without iron supplementation. Acta Obstetricia et Gynecologica Scandinavica 2000;79(2):89‐98. [DOI] [PubMed] [Google Scholar]
- Milman N, Graudal N, Agger AO. Iron status markers during pregnancy. No relationship between levels at the beginning of the second trimester, prior to delivery and post partum. Journal of Internal Medicine 1995;237:261‐7. [DOI] [PubMed] [Google Scholar]
- Milman N, Graudal N, Nielsen OJ, Agger AO. Serum erythropoietin during normal pregnancy: relationship to hemoglobin and iron status markers and impact of iron supplementation in a longitudinal, placebo‐controlled study on 118 women. International Journal of Hematology 1997;66(2):159‐68. [DOI] [PubMed] [Google Scholar]
- Milman N, Graudal NA, Agger AO. Iron status markers during normal pregnancy in 120 women. No clinically useful relationship between levels in the second trimester, later in pregnancy, and post partum. Ugeskrift for Laeger 1995;157:6571‐5. [PubMed] [Google Scholar]
Milman 2005 {published data only}
- Milman N, Bergholt T, Eriksen L, Byg KE, Graudal N, Pedersen P, et al. Iron prophylaxis during pregnancy ‐ how much iron is needed? A randomized dose‐response study of 20‐80 mg ferrous iron daily in pregnant women. Acta Obstetricia et Gynecologica Scandinavica 2005;84:238‐47. [DOI] [PubMed] [Google Scholar]
- Milman N, Byg KE, Bergholt T, Eriksen L. Side effects of oral iron prophylaxis in pregnancy ‐ myth or reality?. Acta Haematologica 2006;115(1‐2):53‐7. [DOI] [PubMed] [Google Scholar]
- Milman N, Byg KE, Bergholt T, Eriksen L, Hvas AM. Body iron and individual iron prophylaxis in pregnancy ‐ should the iron dose be adjusted according to serum ferritin?. Annals of Hematology 2006;85(9):567‐73. [DOI] [PubMed] [Google Scholar]
Morgan 1987 {published data only}
- Morgan EH. Plasma‐iron and haemoglobin levels in pregnancy. Lancet 1961;1:9‐12. [DOI] [PubMed] [Google Scholar]
- Morgan EH. Plasma‐iron and haemoglobin levels in pregnancy. Personal communication 1987 January 19.
Morrison 1977 {published data only}
- Morrison J, Bell J, Chang AMZ, Larkin PK. A comparative trial of haematinic supplements in pregnancy. Medical Journal of Australia 1977;1:482‐4. [DOI] [PubMed] [Google Scholar]
Muslimatun 2001 {published data only}
- Muslimatun S, Schmidt MK, Schultink W, West CE, Hautvast JA, Gross R, Muhilal. Weekly supplementation with iron and vitamin A during pregnancy increases hemoglobin concentration but decreases serum ferritin concentration in Indonesian pregnant women. Journal of Nutrition 2001;131(1):85‐90. [DOI] [PubMed] [Google Scholar]
Nguyen 2008 {published data only}
- Gill SK, Nguyen P, Koren G. Adherence and tolerability of iron‐containing prenatal multivitamins in pregnant women with pre‐existing gastrointestinal conditions. Journal of Obstetrics and Gynaecology 2009;29(7):594‐8. [DOI] [PubMed] [Google Scholar]
- Nguyen P, Nava‐Ocampo A, Levy A, O'Connor DL, Einarson TR, Taddio A, et al. Effect of iron content on the tolerability of prenatal multivitamins in pregnancy. BMC Pregnancy and Childbirth 2008;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nogueira 2002 {published data only}
- Nogueira NDN, Macedo ADS, Parente JV, Cozzolino SMF. Nutritional profile of newborns of adolescent mothers supplemented with iron, in different concentrations, zinc and pholic acid. Revista de Nutricao 2002;15:193‐200. [Google Scholar]
- Nogueira Ndo N, Parente JV, Cozzolino SM. Changes in plasma zinc and folic acid concentrations in pregnant adolescents submitted to different supplementation regimens. Cadernos de Saude Publica 2003;19(1):155‐60. [DOI] [PubMed] [Google Scholar]
Ogunbode 1984 {published data only}
- Ogunbode O, Damole IO. Prophylaxis of anaemia in obstetric patients: administration of Ferrograd Folic 500 Plus compared with conventional iron and folic supplementation. Current Therapeutic Research, Clinical and Experimental 1984;35:1043‐8. [Google Scholar]
Ogunbode 1992 {published data only}
- Ogunbode O, Otubu JAM, Akeredolu OO, Akintunde EA, Olatunji PO, Jolayemi ET. The effect of Chemiron capsules on maternal and fetal hematologic indices, including birth weight. Current Therapeutic Research, Clinical and Experimental 1992;51:634‐46. [Google Scholar]
- Ogunbode O, Otubu JAM, Briggs ND, Adeleye JA. Chemiron ‐ A new hematinic preparation. How effective during pregnancy?. Current Therapeutic Research, Clinical & Experimental 1992;51:163‐73. [Google Scholar]
Ortega‐Soler 1998 {unpublished data only}
- Ortega‐Soler CR, Langini SH, Fleishman S, Lopez LB, Garcia M, Guntin R, et al. Iron nutritional status in pregnant women with and without iron supplementation [Estado nutricional con respecto al hierro (Fe) en gestantes con y sin suplementacion]. Personal communication 1998.
Osrin 2005 {published data only}
- Adhikari R, Manandhar D, Costello A, Tompkins A, Filteau S, Osrin D, et al. The effects of antenatal multiple micronutrient supplementation on birthweight, gestation and infection: a double blind, randomised controlled trial conducted in Nepal: study protocol. MIRA Janakpur Multiple Micronutrient Supplementation 2003.
- Osrin D, Vaidya A, Shrestha Y, Baniya RB, Manandhar DS, Adhikari RK, et al. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double‐blind, randomised controlled trial. Lancet 2005;365:955‐62. [DOI] [PubMed] [Google Scholar]
Paintin 1966 {published and unpublished data}
- Paintin DB, Thompson AM, Hytten FE. Personal communication 1986.
- Paintin DB, Thomson AM, Hytten FE. Iron and haemoglobin level in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1966;73:181‐90. [Google Scholar]
Payne 1968 {published data only}
- Payne RW. Prophylaxis of anaemia in pregnancy. Journal of the Royal College of General Practitioners 1968;16:353‐8. [PMC free article] [PubMed] [Google Scholar]
Pena‐Rosas 2003 {published data only}
- Pena‐Rosas JP, Nesheim M, Garcia‐Casal MN, Crompton DWT, Sanjur D, Viteri FE, et al. Intermittent iron supplementation regimens are able to maintain safe maternal hemoglobin concentrations during pregnancy in Venezuela. Journal of Nutrition 2004;134(5):1099‐104. [DOI] [PubMed] [Google Scholar]
Picha 1975 {published data only}
- Picha E. Iron treatment by effervescent tablets [Ein neuer Weg der Eisentherapie]. Geburtshilfe und Frauenheilkunde 1975;35(10):792‐5. [PubMed] [Google Scholar]
Preziosi 1997 {published data only}
- Preziosi P, Prual A, Galan P, Daouda H, Boureima H, Hercberg S. Effect of iron supplementation on the iron status of pregnant women: consequences for newborns. American Journal of Clinical Nutrition 1997;66:1178‐82. [DOI] [PubMed] [Google Scholar]
Pritchard 1958 {published data only}
- Pritchard J, Hunt C. A comparison of the hematologic responses following the routine prenatal administration of intramuscular and oral iron. Surgery, Gynecology and Obstetrics 1958;106:516‐8. [PubMed] [Google Scholar]
Puolakka 1980 {published data only}
- Puolakka J, Janne O, Pakarinen A, Jarvinen PA, Vihko R. Serum ferritin as a measure of iron stores during and after normal pregnancy with and without iron supplement. Acta Obstetricia et Gynecologica Scandinavica 1980;95:43‐51. [DOI] [PubMed] [Google Scholar]
Ramakrishnan 2003 {published data only}
- Ramakrishnan U, Gonzalez‐Cossio T, Neufeld LM, Rivera J, Martorell R. Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron‐only supplementation: a randomized controlled trial in a semirural community in Mexico. American Journal of Clinical Nutrition 2003;77(3):720‐5. [DOI] [PubMed] [Google Scholar]
Rayado 1997 {published data only}
- Rayado B, Carrillo JA, Fernandez‐Esteban JA, Gomez‐Cedillo A, Martin M, Coronel P. A comparative study of 2 ferrous proteins in the prevention of iron deficiency anaemia during pregnancy. Clinica e Investigacion en Ginecologia y Obstetricia 1997;24:46‐50. [Google Scholar]
Reddaiah 1989 {published data only}
- Reddaiah VP, Raj PP, Ramachandran K, Nath LM, Sood SK, Madan N, et al. Supplementary iron dose in pregnancy anemia prophylaxis. Indian Journal of Pediatrics 1989;56:109‐14. [DOI] [PubMed] [Google Scholar]
Romslo 1983 {published data only}
- Romslo I, Haram K, Sagen N, Augensen K. Iron requirements in normal pregnancy as assessed by serum ferritin, serum transferrin saturation and erythrocyte protoporphyrin determinations. British Journal of Obstetrics and Gynaecology 1983;90:101‐7. [DOI] [PubMed] [Google Scholar]
Roztocil 1994 {published data only}
- Roztocil A, Charvatova M, Harastova L, Zahradkova J, Studenik P, Sochorova V, et al. Anti‐anemia therapy with prophylactic administration of fe2+ in normal pregnancy and its effect on prepartum hematologic parameters in the mother and neonate. Ceska Gynekologie 1994;59(3):130‐3. [PubMed] [Google Scholar]
Rybo 1971 {published data only}
- Rybo G, Solvell L. Side‐effect studies on a new sustained release iron preparation. Scandinavian Journal of Hematology 1971;8(4):257‐64. [DOI] [PubMed] [Google Scholar]
Sachdeva 1993 {published data only}
- Sachdeva R, Mann SK. Impact of nutrition education and medical supervision on pregnancy outcome. Indian Pediatrics 1993;30(11):1309‐14. [PubMed] [Google Scholar]
Sandstad 2003 {published data only}
- Sandstad B, Borch‐Iohnson B, Andersen GM, Dahl‐Jorgensen B, Froysa I, Leslie C, et al. Selective iron supplementation based on serum ferritin values early in pregnancy: are the Norwegian recommendations satisfactory?. Acta Obstetricia et Gynecologica Scandinavica 2003;82:537‐42. [DOI] [PubMed] [Google Scholar]
Seck 2008 {published data only}
- Seck BC, Jackson RT. Determinants of compliance with iron supplementation among pregnant women in Senegal. Public Health Nutrition 2008;11(6):596‐605. [DOI] [PubMed] [Google Scholar]
- Seck BC, Jackson RT. Providing iron/folic acid tablets free of charge improves compliance in pregnant women in Senegal. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(5):485‐92. [DOI] [PubMed] [Google Scholar]
Shatrugna 1999 {published data only}
- Shatrugna V, Raman L, Kailash U, Balakrishna N, Rao KV. Effect of dose and formulation on iron tolerance in pregnancy. National Medical Journal of India 1999;12(1):18‐20. [PubMed] [Google Scholar]
Siega‐Riz 2006 {published and unpublished data}
- Bodnar LM, Davidian M, Siega‐Riz AM, Tsiatis AA. Marginal structural models for analyzing causal effects of time‐dependent treatments: an application in perinatal epidemiology. American Journal of Epidemiology 2004;159(10):926‐34. [DOI] [PubMed] [Google Scholar]
- Jasti S, Siega‐Riz AM, Cogswell ME, Hartzema AG. Correction for errors in measuring adherence to prenatal multivitamin/mineral supplement use among low‐income women. Journal of Nutrition 2006;136(2):479‐83. [DOI] [PubMed] [Google Scholar]
- Jasti S, Siega‐Riz AM, Cogswell ME, Hartzema AG, Bentley ME. Pill count adherence to prenatal multivitamin/mineral supplement use among low‐income women. Journal of Nutrition 2005;135(5):1093‐101. [DOI] [PubMed] [Google Scholar]
- Siega‐Riz A, Hartzema A, Turnbull C, Thorp JJ, McDonald T. A trial of selective versus routine iron supplementation to prevent third trimester anemia during pregnancy. American Journal of Obstetrics and Gynecology 2001; Vol. 185, issue 6 Suppl:S119.
- Siega‐Riz AM, Hartzema AG, Turnbull C, Thorp J, McDonald T, Cogswell ME. The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2006;194(2):512‐9. [DOI] [PubMed] [Google Scholar]
Simmons 1993 {published data only}
- Simmons WK, Cook JD, Bingham KC, Thomas M, Jackson J, Jackson M, et al. Evaluation of a gastric delivery system for iron supplementation in pregnancy. American Journal of Clinical Nutrition 1993;58:622‐6. [DOI] [PubMed] [Google Scholar]
Sinha 2011 {published data only}
- Sinha V, Dayal M, Mehrotra R, Mishra V. Intravenous iron sucrose versus oral ferrous ascorbate in the prevention of anaemia in pregnant women. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:72.
Sjostedt 1977 {published data only}
- Sjostedt JE, Manner P, Nummi S, Ekenved G. Oral iron prophylaxis during pregnancy ‐ a comparative study on different dosage regimens. Acta Obstetricia et Gynecologica Scandinavica 1977;66:3‐9. [DOI] [PubMed] [Google Scholar]
Sood 1979 {published data only}
- Sood SK, Ramachandran K, Rani K, Ramalingaswami V, Mathan VI, Ponniah J, et al. WHO sponsored collaborative studies on nutritional anaemia in India. The effect of parenteral iron administration in the control of anaemia of pregnancy. British Journal of Nutrition 1979;42:399‐406. [DOI] [PubMed] [Google Scholar]
Steer 1992 {published data only}
- Steer PJ. Trial to assess the effects of iron and folate supplementation on pregnancy outcome [trial abandoned]. Personal communication 1992.
Stone 1975 {published data only}
- Stone M, Elder MG. The relative merits of a slow‐release and a standard iron preparation during pregnancy. Current Medical Research and Opinion 1975;3:469‐72. [Google Scholar]
Suharno 1993 {published data only}
- Suharno D, West CE, Karyadi D, Hautvast JGA. Supplementation with vitamin A and iron for nutritional anaemia in pregnant women in West Java, Indonesia. Lancet 1993;342:1325‐8. [DOI] [PubMed] [Google Scholar]
Svanberg 1975 {published data only}
- Svanberg B, Arvidsson B, Norrby A, Rybo G, Solvell L. Absorption of supplemental iron during pregnancy ‐ a longitudinal study with repeated bone marrow studies and absorption measurements. Acta Obstetricia et Gynecologica Scandinavica 1975;48:87‐108. [DOI] [PubMed] [Google Scholar]
Swain 2011 {published data only}
- Swain S, Mahapatra PC, Majhi C, Das TK. Two doses of parenteral iron sucrose injection as anaemia prophylaxis in pregnancy. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:181.
Tampakoudis 1996 {published data only}
- Tampakoudis P, Tantanassis T, Tsatalas K, Lazaridis E, Tsalikis T, Venetis C, et al. A randomized trial on the effect of oral supplementation with iron protein succinylate in singleton pregnancies. The role of maternal erythropoietin as a marker. Prenatal and Neonatal Medicine 1996;1 Suppl 1:181. [Google Scholar]
Tan 1995 {published data only}
- Tan CH, Ng KB. The effect of oral iron on the haemoglobin concentration during the second half of pregnancy. 27th British Congress of Obstetrics and Gynaecology 1995 July 4‐7; Dublin, Ireland. Royal College of Obstetricians & Gynaecologists, 1995:101.
Tange 1993 {published data only}
- Tange E, Weigand E, Mbofung CM. Effect of iron supplementation on anemic and non‐anemic pregnant teenagers in Cameroon. TEMA 8: Proceedings of the Eighth International Symposium on Trace Elements in Man and Animals; 1993 May 16‐22; Dresden, Germany. 1993:220‐3.
Taylor 1982 {published data only}
- Taylor DJ, Mallen C, McDougall N, Lind T. Personal communication 1982.
- Taylor DJ, Mallen C, McDougall N, Lind T. Effect of iron supplementation on serum ferritin levels during and after pregnancy. British Journal of Obstetrics and Gynaecology 1982;89:1011‐7. [DOI] [PubMed] [Google Scholar]
Thane‐Toe 1982 {published data only}
- Thane‐Toe, Thein‐Than. The effects of oral iron supplementation on ferritin levels in pregnant Burmese women. American Journal of Clinical Nutrition 1982;35(1):95‐9. [DOI] [PubMed] [Google Scholar]
Tholin 1993 {published data only}
- Tholin K, Hallmans G, Sandstrom B, Goop M, Palm R, Abrahamsson M. Serum zinc, iron supplementation and pregnancy outcome. TEMA 8: Proceedings of the Eighth International Symposium on Trace Elements in Man and Animals; 1993 May 16‐22; Dresden, Germany. 1993:894‐5.
- Tholin K, Sandstrom B, Palm R, Hallmans G. Changes in blood manganese levels during pregnancy in iron supplemented and non supplemented women. Journal of Trace Elements in Medicine and Biology 1995;9(1):13‐7. [DOI] [PubMed] [Google Scholar]
Thomsen 1993 {published data only}
- Thomsen JK, Prien‐Larsen JC, Devantier A, Fogh‐Andersen N. Low dose iron supplementation does not cover the need for iron during pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1993;72:93‐8. [DOI] [PubMed] [Google Scholar]
Tura 1989 {published data only}
- Tura S, Carenza L, Baccarani M, Bagnara M, Bocci A, Bottone P, et al. Therapy and iron supplements with ferritin during pregnancy. A randomized prospective study of 458 cases. Recenti Progessi in Medicina 1989;80:607‐14. [PubMed] [Google Scholar]
Van Eijk 1978 {published data only}
- Eijk HG, Kroos MJ, Hoogendoorn GA, Wallenburg HC. Serum ferritin and iron stores during pregnancy. Clinica Chimica Acta 1978;83(1‐2):81‐91. [DOI] [PubMed] [Google Scholar]
Vogel 1963 {published data only}
- Vogel L, Steingold L, Suchet J. Iron therapy in the treatment of anaemia in pregnancy. Lancet 1963;1:1296‐9. [DOI] [PubMed] [Google Scholar]
Wali 2002 {published data only}
- Wali A, Mushtaq A, Nilofer. Comparative study‐‐efficacy, safety and compliance of intravenous iron sucrose and intramuscular iron sorbitol in iron deficiency anemia of pregnancy. Journal of the Pakistan Medical Association 2002;52(9):392‐5. [PubMed] [Google Scholar]
Wallenburg 1983 {published data only}
- Buytaert G, Wallenburg HCS, Eijk HG, Buytaert P. Iron supplementation during pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology 1983;15:11‐6. [DOI] [PubMed] [Google Scholar]
- Wallenburg HCS, Eijk HG. Effect of oral iron supplementation during pregnancy on maternal and fetal iron status. Journal of Perinatal Medicine 1984;12:7‐12. [DOI] [PubMed] [Google Scholar]
Willoughby 1966 {published data only}
- Willoughby M, Jewell F. Investigation of folic acid requirements in pregnancy. British Medical Journal 1966;2:1568‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Willoughby 1967 {published data only}
- Willoughby MLN. An investigation of folic acid requirements in pregnancy. II. British Journal of Haematology 1967;13:503‐9. [DOI] [PubMed] [Google Scholar]
Willoughby 1968 {published data only}
- Willoughby MLN, Jewell FG. Folate status throughout pregnancy and in postpartum period. British Medical Journal 1968;4:356‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wills 1947 {published data only}
- Wills L, Hill G, Bingham K, Miall M, Wrigley J. Haemoglobin levels in pregnancy: the effect of the rationing scheme and routine administration of iron. British Journal of Nutrition 1947;1:126‐38. [DOI] [PubMed] [Google Scholar]
Wu 1998 {published data only}
- Wu Y, Weng L, Wu L. Clinical experience with iron supplementation in pregnancy. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 1998;33(4):206‐8. [PubMed] [Google Scholar]
Zeng 2008 {published data only}
- Chang S, Zeng L, Brouwer ID, Kok FJ, Yan H. Effect of iron deficiency anemia in pregnancy on child mental development in rural china. Pediatrics 2013;131(3):e755‐63. [DOI] [PubMed] [Google Scholar]
- Li Q, Yan H, Zeng L, Cheng Y, Liang W, Dang S, et al. Effects of maternal multimicronutrient supplementation on the mental development of infants in rural western China: follow‐up evaluation of a double‐blind, randomized, controlled trial. Pediatrics 2009;123(4):e685‐e692. [DOI] [PubMed] [Google Scholar]
- Yan H. Impact of iron/folate versus multi‐micronutrient supplementation during pregnancy on birth weight: a randomised controlled trial in rural Western China. Current Controlled Trials (www.controlled‐trials.com) (accessed 15 February 2007) 2007.
- Zeng L, Dibley MJ, Cheng Y, Dang S, Chang S, Kong L, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ 2008;337:a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2009 {published data only}
- Zhou SJ, Gibson RA, Crowther CA, Makrides M. Should we lower the dose of iron when treating anaemia in pregnancy? A randomized dose‐response trial. European Journal of Clinical Nutrition 2009;63(2):183‐90. [DOI] [PubMed] [Google Scholar]
Ziaei 2007 {published and unpublished data}
- Ziaei S. Personal communication 2007 October 1.
- Ziaei S, Norrozi M, Faghihzadeh S, Jafarbegloo E. A randomised placebo‐controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin > or = 13.2 g/dl. BJOG: an international journal of obstetrics and gynaecology 2007;114(6):684‐8. [DOI] [PubMed] [Google Scholar]
Ziaei 2008 {published and unpublished data}
- Janghorban R, Ziaei S, Faghihzade S. Evaluation of serum copper level in pregnant women with high hemoglobin. Iranian Journal of Medical Sciences 2006;31(3):170‐2. [Google Scholar]
- Ziaei S. Iron status markers in non‐anaemic pregnant women with or without iron supplementation in pregnancy. Personal communication 2007 October 9.
- Ziaei S, Janghorban R, Shariatdoust S, Faghihzadeh S. The effects of iron supplementation on serum copper and zinc levels in pregnant women with high‐normal hemoglobin. International Journal of Gynecology & Obstetrics 2008;100:133‐5. [DOI] [PubMed] [Google Scholar]
- Ziaei S, Mehrnia M, Faghihzadeh S. Iron status markers in nonanemic pregnant women with and without iron supplementation. International Journal of Gynecology & Obstetrics 2008;100:130‐2. [DOI] [PubMed] [Google Scholar]
Zittoun 1983 {published data only}
- Zittoun J, Blot I, Hill C, Zittoun R, Papiernik E, Tchernia G. Iron supplements vs placebo during pregnancy: its effects on iron and folate status on mothers and newborns. Annals of Nutrition and Metabolism 1983;27:320‐7. [DOI] [PubMed] [Google Scholar]
Zutshi 2004 {published data only}
- Zutshi V, Batra S, Ahmad SS, Khera N, Chauhan G, Gandhi G, et al. Injectable iron supplementation instead of oral therapy for antenatal care. Journal of Obstetrics and Gynecology of India 2004;54(1):37‐8. [Google Scholar]
References to ongoing studies
Agrawal 2012 {published data only}
- Agrawal N, Rai L. Impact of two oral iron supplementation regimens (intermittently and continuously/daily) for prevention of anaemia in pregnancy in women with normal haemoglobin levels. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=3536 (accessed 23 March 2012).
Gies 2010 {published data only}
- Gies S. Malaria risk prior to and during early pregnancy in nulliparous women receiving long‐term weekly iron and folic acid supplementation (WIFS): a non‐inferiority randomized controlled trial. http://apps.who.int/trialsearch/Trial.aspx?TrialID=NCT01210040 (accessed 11 March 2011).
Goonewardene 2014 {published data only}
Kumar 2014 {published data only}
- Kumar N. Estimation of oxidative stress in pregnant women on daily versus weekly iron supplementation (An ICMR Task Force Study). Clinical Trials Registry ‐ India (http://ctri.nic.in) [accessed 20 March 2015] 2014.
Sherbaf 2012 {published data only}
- Sherbaf FR. The single blind clinical trial study of comparing the effect of daily usage of iron complementary to usage of twice a week of it in nonanemic pregnant women on the prenatal and pregnancy outcome (gestational diabetes, preeclampsia, low birth weight). IRCT Iranian Registry of Clinical Trials (www.irct.ir) [accessed 20 March 2015].
Additional references
Andersen 2006
- Andersen HS, Gambling L, Holtrop G, McArdle HJ. Maternal iron deficiency identifies critical windows for growth and cardiovascular development in the rat postimplantation embryo. Journal of Nutrition 2006;136(5):1171‐7. [DOI] [PubMed] [Google Scholar]
Anderson 2005
- Anderson GJ, Frazer DM, McKie AT, Vulpe CD, Smith A. Mechanisms of haem and non‐haem iron absorption: lessons from inherited disorders of iron metabolism. Biometals 2005;18(4):339‐48. [DOI] [PubMed] [Google Scholar]
Balarajan 2011
- Balarajan Y, Ramakrishnan U, Özaltin E, Shankar AH, Subramanian SV. Anaemia in low‐income and middle‐income countries. Lancet 2011;378(9809):2123‐35. [DOI] [PubMed] [Google Scholar]
Balshem 2010
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Beard 2000
- Beard J. Effectiveness and strategies of iron supplementation during pregnancy. American Journal of Clinical Nutrition 2000;71(5):1288S‐1294S. [DOI] [PubMed] [Google Scholar]
Beaton 1999
- Beaton GH, McCabe GP. Efficacy of intermittent iron supplementation in the control of iron deficiency anaemia in developing countries. an analysis of experience: final report to the Micronutrient Initiative. http://idl‐bnc.idrc.ca/dspace/bitstream/10625/29662/3/117067.pdf (accessed 2012). Ottawa: The Micronutrient Initiative, 1999.
Bencaiova 2012
- Bencaiova G, Burkhardt T, Breymann C. Anemia—prevalence and risk factors in pregnancy. European Journal of Internal Medicine 2012;23(6):529‐33. [DOI] [PubMed] [Google Scholar]
Bothwell 2000
- Bothwell TH. Iron requirements in pregnancy and strategies to meet them. American Journal of Clinical Nutrition 2000;72(1 Suppl):257S‐264S. [DOI] [PubMed] [Google Scholar]
Burke 2014
- Burke RM, Leon JS, Suchdev PS. Identification, prevention and treatment of iron deficiency during the first 1000 days. Nutrients 2014;6(10):4093‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casanueva 2003b
- Casanueva E, Viteri FE. Iron and oxidative stress in pregnancy. Journal of Nutrition 2003;133(5):1700S‐1708S. [DOI] [PubMed] [Google Scholar]
Darnton‐Hill 2007
- Darnton‐Hill I, Paragas N, Cavalli‐Sforza T. Global perspectives: accelerating progress on preventing and controlling nutritional anaemia. Nutritional Anemia. Basel, Switzerland: Sight and Life Press, 2007:359‐82. [Google Scholar]
De‐Regil 2010
- Regil LM, Fernández‐Gaxiola AC, Dowswell T, Peña‐Rosas JP. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007950.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dellicor 2010
- Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Medicine 2010;26(1):e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erslev 2001
- Erslev AJ. Clinical manifestations and classification of erythrocyte disorders. In: Beutler E, Litchman MA, Coller BS, Kipps TJ, Seligsohn U editor(s). William's Hematology. 6th Edition. New York, NY, USA: McGraw Hill, 2001:369‐74. [Google Scholar]
Frazer 2003a
- Frazer DM, Anderson GJ. The orchestration of body iron intake: how and where do enterocytes receive their cues?. Blood Cells Molecules and Disease 2003;30(3):288‐97. [DOI] [PubMed] [Google Scholar]
Frazer 2003b
- Frazer DM, Wilkins SJ, Becker EM, Murphy TL, Vulpe CD, McKie AT, et al. A rapid decrease in the expression of DMT1 and Dcytb but not Ireg1 or hephaestin explains the mucosal block phenomenon of iron absorption. Gut 2003;52(3):340‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallery 1979
- Gallery EDM, Hunyor SN, Gyory AZ. Plasma volume contraction: a significant factor in both pregnancy‐associated hypertension (pre‐eclampsia) and chronic hypertension in pregnancy. Quarterly Journal of Medicine 1979;48(192):593‐602. [PubMed] [Google Scholar]
Galloway 1994
- Galloway R, McGuire J. Determinants of compliance with iron supplementation: supplies, side effects, or psychology?. Social Science and Medicine 1994;39(3):381‐90. [DOI] [PubMed] [Google Scholar]
Galloway 1996
- Galloway R, McGuire J. Daily vs weekly: how many iron pills do pregnant women need?. Nutrition reviews 1996;54(10):318‐23. [DOI] [PubMed] [Google Scholar]
Garcia‐Casal 2014
- Garcia‐Casal MN, Peña‐Rosas JP, Pasricha SR. Rethinking ferritin cutoffs for iron deficiency and overload. Lancet Haematology 2014;1(3):e92‐e94. [DOI] [PubMed] [Google Scholar]
Gleason 2007
- Gleason G, Scrimshaw NS. An overview of the functional significance if iron deficiency. In: Kraemer K, Zimmermann MB editor(s). Nutritional Anemia. Vol. 1, Basel, Switzerland: Sight and Life, 2007:45‐58. [Google Scholar]
Goodlin 1981
- Goodlin RC, Quaife MA, Dirksen JW. The significance, diagnosis, and treatment of maternal hypovolemia as associated with fetal/maternal illness. Seminars in Perinatology 1981;5(2):163‐74. [PubMed] [Google Scholar]
Goonewardene 2012
- Goonewardene M, Shehata M, Hamad A. Anaemia in pregnancy. Best Practice & Research Clinical Obstetrics & Gynaecology 2012;26(1):3‐24. [DOI] [PubMed] [Google Scholar]
Haider 2012
- Haider BA, Bhutta ZA. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD004905.pub3] [DOI] [PubMed] [Google Scholar]
Haider 2013
- Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta‐analysis. BMJ 2013;346:f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huisman 1986
- Huisman A, Aarnoudse JG. Increased 2nd trimester hemoglobin concentration in pregnancies later complicated by hypertension and growth retardation. Early evidence of a reduced plasma volume. Acta Obstetricia et Gynecologica Scandinavica 1986;65(6):605‐8. [DOI] [PubMed] [Google Scholar]
Hytten 1964
- Hytten FE, Leitch I. The Physiology of Human Pregnancy. Oxford: Blackwell Scientific Publications, 1964:14. [Google Scholar]
Hytten 1971
- Hytten FE, Leitch I, Baird D. The Physiology of Human Pregnancy. 2nd Edition. Oxford: Blackwell Scientific Publications, 1971:1‐43. [Google Scholar]
Hytten 1985
- Hytten F. Blood volume changes in normal pregnancy. Clinics in Haematology 1985;14(3):601‐12. [PubMed] [Google Scholar]
INACG 2002
- International Anemia Consultative Group (INACG). Why is iron important and what to do about it: a new perspective. Report of the 2001 INACG Symposium; 2001 February 15‐16; Hanoi, Vietnam. 2002:1‐50.
IOM 2001
- Institute of Medicine. Iron. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington DC: National Academy Press, 2001:290‐393. [PubMed] [Google Scholar]
Koller 1979
- Koller O, Sagen N, Ulstein M, Vaula D. Fetal growth retardation associated with inadequate haemodilution in otherwise uncomplicated pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1979;58(1):9‐13. [DOI] [PubMed] [Google Scholar]
Lassi 2013
- Lassi ZS, Salam RA, Haider BA, Bhutta ZA. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD006896.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Letsky 1991
- Letsky E. The haematological system. In: Hytten F, Chamberlain G editor(s). Clinical Physiology in Obstetrics. 2nd Edition. Oxford UK: Blackwell Scientific Publications, 1991:39‐86. [Google Scholar]
LeVeen 1980
- LeVeen HH, Ip M, Ahmed N, Mascardo T, Guinto RB, Falk G, et al. Lowering blood viscosity to overcome vascular resistance. Surgery, Gynecology and Obstetrics 1980;150(2):139‐49. [PubMed] [Google Scholar]
Lozoff 2006
- Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Archives of Pediatrics & Adolescent Medicine 2006;160(11):1108‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lozoff 2007
- Lozoff B. Iron deficiency and child development. Food and Nutrition Bulletin 2007;28(4 Suppl 1):S560‐S571. [DOI] [PubMed] [Google Scholar]
Lutter 2011
- Lutter CK, Daelmans BM, Onis M, Kothari MT, Ruel MT, Arimond M, Deitchler M, et al. Undernutrition, poor feeding practices, and low coverage of key nutrition interventions. Pediatrics 2011;128(6):e1418‐e1427. [DOI] [PubMed] [Google Scholar]
Masukume 2015
- Masukume G, Khashan AS, Kenny LC, Baker PN, Nelson G, Consortium Scope. Risk factors and birth outcomes of anaemia in early pregnancy in a nulliparous cohort. PLoS ONE 2015;10:e0122729. [DOI] [PMC free article] [PubMed] [Google Scholar]
McArdle 2006
- McArdle HJ, Andersen HS, Jones H, Gambling L. Fetal programming: causes and consequences as revealed by studies of dietary manipulation in rats ‐‐ a review. Placenta 2006;27 Suppl A:S56‐S60. [DOI] [PubMed] [Google Scholar]
Murphy 1986
- Murphy JF, O'Riordan J, Newcombe RG, Coles EC, Pearson JF. Relation of haemoglobin levels in first and second trimesters to outcome. Lancet 1986;3(1):992‐5. [DOI] [PubMed] [Google Scholar]
Oppenheimer 2001
- Oppenheimer SJ. Iron and its relation to immunity and infectious disease. Journal of Nutrition 2001;131:616S‐635S. [DOI] [PubMed] [Google Scholar]
Ota 2015
- Ota E, Mori R, Middleton P, Tobe‐Gai R, Mahomed K, Miyazaki C, et al. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD000230.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasricha 2013
- Pasricha SR, Drakesmith H, Black J, Hipgrave D, Biggs BA. Control of iron deficiency anemia in low‐ and middle‐income countries. Blood 2013;121(14):2607‐17. [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2015
- Peña‐Rosas JP, De‐Regil LM, Garcia‐Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD004736.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poulsen 1990
- Poulsen HF, Mortensen PE. Hemoglobin concentration prior to the 20th week of pregnancy correlated with complications in the third trimester. Ugeskrift for Laeger 1990;152(14):1010‐1. [PubMed] [Google Scholar]
Prentice 2007
- Prentice AM, Ghattas H, Doherty C, Cox SE. Iron metabolism and malaria. Food and Nutrition Bulletin 2007;28(4):S524‐S539. [DOI] [PubMed] [Google Scholar]
Reveiz 2011
- Reveiz L, Gyte GM, Cuervo LG, Casasbuenas A. Treatments for iron‐deficiency anaemia in pregnancy. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD003094.pub3] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roll Back Malaria Partnership 2015
- Roll Back Malaria Partnership. Malaria in Pregnancy Working Group. Consensus statement on folic acid supplementation during pregnancy. http://www.rollbackmalaria.org/files/files/partnership/1_FOLIC%20ACID_EN.PDF, accessed 2 July 2015 2015.
Rumbold 2015
- Rumbold A, Ota E, Nagata C, Shahrook S, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD004072.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scholl 1997
- Scholl TO, Hediger ML, Bendich A, Schall JI, Smith WK, Krueger PM. Use of multivitamin/mineral prenatal supplements: influence on the outcome of pregnancy. American Journal of Epidemiology 1997;146(2):134‐41. [DOI] [PubMed] [Google Scholar]
Silver 1998
- Silver HM, Seebeck MA, Carlson R. Comparison of total blood volume in normal, preeclamptic, and nonproteinuric gestational hypertensive pregnancy by simultaneous measurements of red blood cell and plasma volumes. American Journal of Obstetrics and Gynecology 1998;179(1):87‐93. [DOI] [PubMed] [Google Scholar]
Srigiridhar 1998
- Srigiridhar K, Nair KM. Iron‐deficient intestine is more susceptible to peroxidative damage during iron supplementation in rats. Journal of Free Radicals in Biology & Medicine 1998;25(6):660‐5. [DOI] [PubMed] [Google Scholar]
Srigiridhar 2001
- Srigiridhar K, Nair KM, Subramanian R, Singotamu L. Oral repletion of iron induces free radical mediated alterations in the gastrointestinal tract of rat. Molecular and Cellular Biochemistry 2001;219(1‐2):91‐8. [DOI] [PubMed] [Google Scholar]
Steer 2000
- Steer PJ. Maternal hemoglobin concentration and birth weight. American Journal of Clinical Nutrition 2000;71(5 Suppl):1285S‐1287S. [DOI] [PubMed] [Google Scholar]
Stevens 2013
- Stevens GA, Finucane MM, De‐Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non‐pregnant women for 1995‐2011: a systematic analysis of population‐representative data. Lancet Global Health 2013;1:e16‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suchdev 2011
- Suchdev PS, De‐Regil LM, Walleser S, Vist GE, Peña‐Rosas JP. Multiple micronutrient powders for home (point‐of‐use) fortification of foods in pregnant women: a systematic review.. Vol. WHO e‐Library of Evidence for Nutrition Actions, Geneva: World Health Organization, 2011. [Google Scholar]
Suchdev 2015
- Suchdev PS, Peña‐Rosas JP, De‐Regil LM. Multiple micronutrient powders for home (point‐of‐use) fortification of foods in pregnant women. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011158.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suominen 1998
- Suominen P, Punnonen K, Rajamaki A, Irjala K. Serum transferrin receptor and transferrin receptor‐ferritin index identify healthy subjects with subclinical iron deficits. Blood 1998;92(8):2934. [PubMed] [Google Scholar]
van den Broek 2010
- Broek Nynke, Dou Lixia, Othman Mohammad, Neilson James P, Gates Simon, Gülmezoglu A Metin. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD008666.pub2] [DOI] [PubMed] [Google Scholar]
Villar 1997
- Villar J, Bergsjo P. Scientific basis for the content of routine antenatal care. I. Philosophy, recent studies and power to eliminate or alleviate adverse maternal outcomes. Acta Obstetricia et Gynecologica Scandinavica 1997;76(1):1‐14. [DOI] [PubMed] [Google Scholar]
Viteri 1995
- Viteri FE, Liu XN, Martin A, Tolomei K. True absorption and retention of supplemental iron is more efficient when administered every three days rather than daily to iron‐normal and iron‐deficient rats. Journal of Nutrition 1995;125(1):82‐91. [DOI] [PubMed] [Google Scholar]
Viteri 1997
- Viteri FE. Iron supplementation for the control of iron deficiency in populations at risk. Nutrition Reviews 1997;55(6):195‐209. [DOI] [PubMed] [Google Scholar]
Viteri 1999a
- Viteri FE, Mendoza C, Guiro A, Hercberg S, Galan P. Daily and weekly supplementation and reference‐dose iron (Fe) absorption in Berkeley, Ca. and Dakar, Senegal. FASEB Journal 1999;13:A536.4. [Google Scholar]
Viteri 1999b
- Viteri FE. Iron supplementation as a strategy for the control of iron deficiency and ferropenic anemia. Archivos Latinoamericanos de Nutricion 1999;49 Suppl:S15‐S22. [PubMed] [Google Scholar]
Viteri 2005
- Viteri FE, Berger J. Importance of pre‐pregnancy and pregnancy iron status: can long‐term weekly preventive iron and folic acid supplementation achieve desirable and safe status?. Nutrition Reviews 2005;63(12):S65‐S76. [DOI] [PubMed] [Google Scholar]
Walsh 2011
- Walsh T, O'Broin SD, Cooley S, Donnelly J, Kennedy J, Harrison RF, et al. Laboratory assessment of iron status in pregnancy. Clinical Chemistry and Laboratory Medicine 2011;49(7):1225‐30. [DOI] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. Iron deficiency anaemia assessment prevention and control: a guide for program managers. Geneva: World Health Organization, 2001:132. [Google Scholar]
WHO 2011a
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/MNM/11.1) (http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed April 29 2011). Geneva: World Health Organization, 2011.
WHO 2011b
- World Health Organization. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization, 2011. [Google Scholar]
WHO 2011c
- World Health Organization. Intermittent iron and folic acid supplementation in menstruating women. Geneva: World Health Organization, 2011. [PubMed] [Google Scholar]
WHO 2011d
- World Health Organization. E‐book International Travel and Health 2011 (PDF format). Geneva: World Health Organization, 2011. [Google Scholar]
WHO 2011e
- World Health Organization. Global malaria report 2011 (http://whqlibdoc.who.int/publications/2010/9789241564106_eng.pdf). Geneva: World Health Organization, 2011. [Google Scholar]
WHO 2012
- WHO. Guideline: Intermittent iron and folic acid supplementation in non‐anaemic pregnant women. Geneva: World Health Organization. Geneva: World Health Organization,, 2012. [PubMed]
WHO 2012a
- WHO. Guideline: Daily iron and folic acid supplementation in pregnant women. Geneva:World Health Organization 2012. [PubMed]
WHO 2014a
- WHO. Global nutrition targets 2025: anaemia policy brief (WHO/NMH/NHD/14.4). Geneva: World Health Organization. Geneva: World Health Organization, 2014.
WHO 2014d
- WHO. World Malaria Report 2014. Geneva, World Health Organization (http://www.who.int/malaria/publications/world_malaria_report_2014/report/en/, accessed 28 May 2015), 2014. [Google Scholar]
WHO 2015a
- WHO. Guideline: Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects. Geneva: World Health Organization. Geneva: World Health Organization, 2015. [PubMed]
WHO 2015c
- World Health Organization. The global prevalence of anaemia in 2011. Geneva: World Health Organization, 2015. [Google Scholar]
Yakoob 2011
- Yakoob MY, Bhutta ZA. Effect of routine iron supplementation with or without folic acid on anemia during pregnancy. BMC Public Health 2011;11 Suppl 3:S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Mahomed 1998b
- Mahomed K. Iron and folate supplementation in pregnancy. Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI: 10.1002/14651858.CD001135] [DOI] [PubMed] [Google Scholar]
Mahomed 2000b
- Mahomed K. Iron supplementation in pregnancy. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD000117] [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2006
- Peña‐Rosas JP, Viteri FE. Effects of routine oral iron supplementation with or without folic acid for women during pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004736.pub2] [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2009
- Peña‐Rosas JP, Viteri FE. Effects and safety of preventive oral iron or iron+folic acid supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004736.pub3] [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2012
- Peña‐Rosas JP, De‐Regil LM, Dowswell T, Viteri FE. Intermittent oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009997] [DOI] [PMC free article] [PubMed] [Google Scholar]


