Abstract
Background
Excessive weight loss in newborns is associated with neonatal complications such as jaundice and dehydration, which cause renal failure, thrombosis, hypovolemic shock, and seizures. The identification of the risk factors for excessive weight loss will help to discover preventive measures. The aim of this study was to determine the factors associated with excessive weight loss, defined as weight loss of ≥10%, in breastfed full-term newborns in Japan.
Methods
The present retrospective study, which was performed in a tertiary perinatal center accredited as a Baby-Friendly Hospital, included neonates who were born alive with a gestational age of ≥37 weeks. Cases of multiple births, admission to the neonatal intensive care unit (NICU), referral to another facility, or exclusive formula feeding were excluded. Multivariate logistic regression analyses were performed to assess the association between maternal or neonatal characteristics and excessive weight loss.
Results
We studied 399 newborns, of whom 164 (41%) had excessive weight loss. According to the adjusted multiple regression analysis, the factors associated with excessive weight loss were an older maternal age, primiparity, and antepartum Caesarean section, with adjusted odds ratios (95% Confidence Intervals [CIs]) of 1.07 (1.02, 1.11), 2.72 (1.69, 4.38), and 2.00 (1.09, 3.65), respectively.
Conclusions
Close monitoring of infants born to older mothers, primiparous mothers, or infants delivered by antepartum Cesarean section is recommended, and earlier supplementation with artificial milk may be considered.
Keywords: Newborn, Breastfeeding, Birth weight, Weight loss, Caesarean section
Background
Almost all newborns lose weight in the first days of life [1, 2], and this is called physiological weight loss [3]. It is mainly due to fluid reduction [4]. Late cord clamping is related to a higher birth weight, which results in a greater weight change [5]. Neonatal weight loss is also a consequence of the use of adipose tissue as a source of energy by the newborns [3]. Excessive weight loss is associated with complications such as jaundice [6–9], hypoglycemia [10], and dehydration, which cause renal failure, thrombosis, hypovolemic shock, and seizures [11–19].
Breastfeeding undoubtedly provides health advantages to both the infant and the mother. Supplementation with formula should be avoided unless medically indicated as it may discourage the initiation and decrease the duration of breastfeeding [20–22]. The extent of a newborn’s weight loss in the first days of life is used as an indicator of breastfeeding adequacy [23]. The percentage of weight reduction that indicates formula supplementation is controversial [24]. A weight reduction of 10% is frequently applied because it is related to hypernatremic dehydration [25–27]. It is important to determine the risk factors for excessive weight loss to discover preventive measures.
Caesarean section is a known risk factor for excessive weight loss among newborns [1, 28]. However, little is known about the differences in risk according to the type of Caesarean section (antepartum or intrapartum) and the existence of other risk factors, especially in newborns at an accredited Baby-Friendly Hospital. The risk factors identified in the literature differ, depending on the setting [28–33]. Therefore, the aim of this study was to determine the factors associated with excessive weight loss in breastfed full-term newborns at a Baby-Friendly Hospital.
Methods
Study design, population, and setting
This was a retrospective observational study with no patient contact. It was conducted at a tertiary perinatal center (The National Hospital Organization Nagasaki Medical Center) in Japan. We included live babies who were born at ≥37 weeks of gestation between January 1, 2018, and December 31, 2018. We excluded cases of multiple births, cases in which babies were admitted to the neonatal intensive care unit (NICU) or referred to other facilities, and cases of exclusive formula feeding.
Our hospital is accredited as a Baby-Friendly Hospital and promotes the initiation of exclusive breastfeeding [34]. A total of 66 facilities, including 27 perinatal centers, are accredited as Baby-Friendly Hospitals in Japan [35]. In our hospital, early cord clamping is performed after a baby is delivered. In addition, we adhere closely to the “Ten Steps to Successful Breastfeeding” [34]. We do not have any lactation consultants. However, trained midwives or nurses help mothers after delivery to assure adequate breastfeeding. Temporary formula feeding is considered in cases of weight loss ≥10% in relation to the birth weight after a physical examination and blood tests, including the blood levels of glucose, urea nitrogen, and electrolytes, are performed. However, in cases of medical needs such as constant hypoglycemia or dehydration, minimal water or formula is given to infants even before weight loss reaches 10%. Hospital discharge at our facility routinely occurs when the infants are 5 days old in the case of vaginal delivery or 6 days old after a Caesarean section. All newborns are weighed daily between birth and discharge while naked with an electronic scale by a nurse or a midwife. Discharge is postponed if there are any maternal or neonatal complications, and discharge is not permitted until the infant’s weight begins to increase after its nadir.
Data collection and definitions
We retrospectively obtained the demographic and perinatal data from the patients’ medical records. The following maternal and neonatal variables were assessed: maternal age, prepregnancy weight, prepregnancy body mass index (BMI), parity, infertility treatment, maternal weight gain during pregnancy, hypertension, diabetes, gestational week at delivery, use of magnesium sulfate in labor, use of oxytocin in labor, mode of delivery (vaginal delivery, intrapartum Caesarean section, or antepartum Caesarean section), postpartum hemorrhaging, maternal hemoglobin after delivery, birth weight, newborn sex, use of infant formula or water before a weight loss of ≥10% and neonatal jaundice requiring phototherapy. Prepregnancy BMI was calculated with maternal height at the first antenatal visit and self-reported prepregnancy weight. Maternal hypertension was defined as a systolic blood pressure ≥ 140 mmHg or a diastolic blood pressure ≥ 90 mmHg, regardless of the time of diagnosis, including before pregnancy, in pregnancy, and during the hospital stay. It included chronic hypertension, gestational hypertension, preeclampsia, and eclampsia. Diabetes included type 1, type 2, and gestational diabetes. Operative deliveries were classified as ‘antepartum’ if they were performed before the onset of labor and ‘intrapartum’ if they were performed after labor began [36]. The start of labor was defined as regular contractions occurring < 10 min apart and progressive cervical dilation or effacement [37]. All elective Caesarean sections were categorized as antepartum Caesarean sections. Emergency Caesarean sections could be antepartum or intrapartum Caesarean sections. Postpartum hemorrhaging was defined as a cumulative blood loss of ≥1000 mL within 24 h of the birth process, regardless of the mode of delivery [38]. Hemoglobin levels were measured within 2–3 days after delivery.
Outcomes
The outcome was excessive weight loss, which was defined as a weight reduction of ≥10% in the first 5 days of life in relation to the birth weight.
Secondary analysis
We examined whether there were any newborns who required intravenous therapy due to hypoglycemia or dehydration. We also investigated the association between neonatal jaundice requiring phototherapy and a higher weight loss.
We analyzed the blood test results of newborns who experienced a weight reduction of ≥10%. The plasma osmolality was calculated by the following formula: plasma osmolality = (sodium [mEq/L] × 2) + (glucose [mg/dL] / 18) + (blood urea nitrogen [mg/dL] / 2.8). The mean plasma osmolality results were compared among the groups stratified according to the mode of delivery.
Statistical analysis
Data were entered in the Microsoft Excel software program (version 14.1.0; Microsoft®, Redmond, WA, USA) and exported to EZR (version 3.1.2; Saitama Medical Center, Jichi Medical University, Saitama, Japan), which was used to carry out the statistical analyses. Descriptive statistics were performed to determine the means (± standard deviations) and percentages. The Kolmogorov–Smirnov test was used to examine normality. The Student’s t-test or the Mann–Whitney U test was used to analyze 2 continuous variables, as appropriate. The ANOVA test or the Kruskal-Wallis test was used to analyze 3 continuous variables, as appropriate. Pearson’s chi-squared test was used to analyze categorical variables. Univariate logistic regression analysis of these variables was performed to estimate the crude odds ratios and their 95% confidence intervals (CIs). Then, we conducted multivariate logistic regression analysis. Factors with p values < 0.05 according to the univariate analysis were entered into the multivariate analysis. Neonatal jaundice requiring phototherapy was not included in the regression model of the primary outcome because neonatal jaundice is not the cause but the result of weight loss. Statistical interaction was examined between parity and the mode of delivery. P values < 0.05 were considered to indicate statistical significance.
Ethical considerations
This study was approved by the Research Ethics Committee of the National Hospital Organization Nagasaki Medical under protocol number 2019079, approved on October 10, 2019, with opt-out consent to obtain patient data from the medical records. No ethical issues arose during this study as it was retrospective and all data were anonymous.
Results
A total of 452 babies were born alive with a gestational age of ≥37 weeks during the study period, and 399 newborns were included in the current study after excluding 53 cases: 26 for multiple births, 22 for admission to the NICU, 2 for referral to another facility, and 3 for exclusive formula feeding. The mean percentage of weight reduction was 9.4%. Of these, 164 newborns (41%) lost ≥10% of their body weight (Fig. 1). The weight reached its nadir at day 3.0 ± 0.8 (mean ± standard deviation).
Fig. 1.
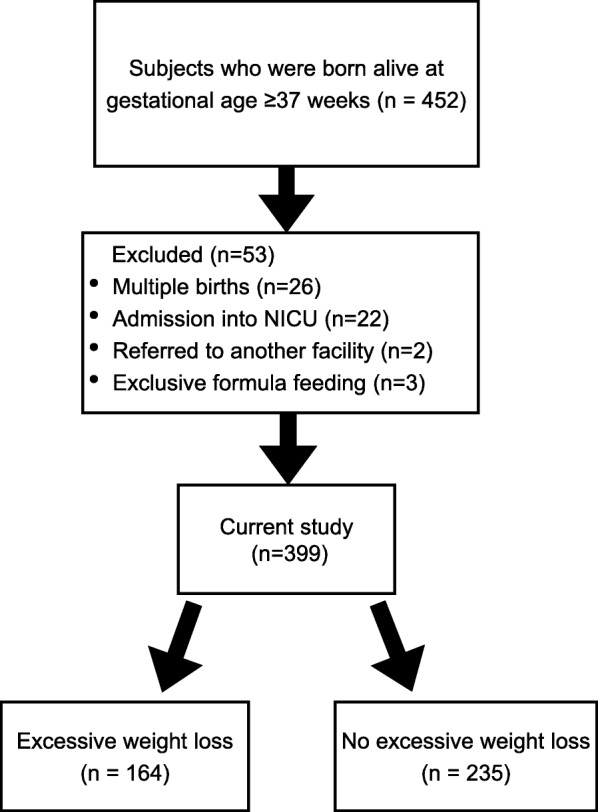
Flow chart of the patients included in this study
Table 1 shows the comparison of the maternal and neonatal characteristics between newborns with and without excessive weight loss. The mothers of newborns with excessive weight loss were older (p = 0.004), had a higher BMI (p = 0.043), were more frequently primiparous (p = 0.001), had more frequently received infertility treatment (p = 0.009), had more frequent postpartum hemorrhaging ≥1000 mL (p = 0.013), and had a lower hemoglobin level after delivery (p = 0.013) than mothers of newborns without excessive weight loss (Table 1).
Table 1.
Comparison of the maternal and neonatal characteristics between newborns with and without excessive weight loss
| Weight loss ≥10% N = 164 |
Weight loss < 10% N = 235 |
Test value or U value | Degree of freedom | p | |
|---|---|---|---|---|---|
| Maternal age, yearsa | 15,989 | NA | 0.004 | ||
| Minimum | 21 | 16 | |||
| 25 percentile | 31 | 28 | |||
| Median | 35 | 32 | |||
| 75 percentile | 37 | 37 | |||
| Maximum | 45 | 43 | |||
| Prepregnancy maternal weighta | 17,068 | NA | |||
| Minimum | 35 | 40 | |||
| 25 percentile | 50 | 48 | |||
| Median | 55 | 53 | |||
| 75 percentile | 63 | 60 | |||
| Maximum | 105 | 111 | |||
| Prepregnancy BMI, kg/m2a | 16,974 | NA | 0.043 | ||
| Minimum | 15.1 | 14.7 | |||
| 25 percentile | 19.8 | 19.2 | |||
| Median | 22.0 | 21.2 | |||
| 75 percentile | 25.2 | 24.0 | |||
| Maximum | 39.3 | 42.9 | |||
| Primiparityb | 85 (52%) | 82 (35%) | 10.7 | 1 | 0.001 |
| Infertility treatmentb | 30 (18.3%) | 21 (8.9%) | 6.8 | 1 | 0.009 |
| Maternal weight gain, kga | 20,860 | NA | 0.16 | ||
| Minimum | −4.0 | −8.4 | |||
| 25 percentile | 6.3 | 6.6 | |||
| Median | 8.7 | 9.5 | |||
| 75 percentile | 11.4 | 12.1 | |||
| Maximum | 24.5 | 25.6 | |||
| Hypertensionb | 14 (8.5%) | 14 (6.0%) | 0.63 | 1 | 0.43 |
| Diabetesb | 42 (26%) | 45 (19%) | 2.0 | 1 | 0.16 |
| Use of MgSO4 in laborb | 2 (1.2%) | 7 (3.0%) | 0.68 | 1 | 0.41 |
| Use of oxytocin in laborb | 52 (32%) | 59 (25%) | 1.8 | 1 | 0.18 |
| Gestational weeks at deliverya | 18,431 | NA | 0.46 | ||
| Minimum | 37.1 | 37.0 | |||
| 25 percentile | 38.7 | 38.4 | |||
| Median | 39.3 | 39.1 | |||
| 75 percentile | 39.9 | 40.0 | |||
| Maximum | 42.1 | 41.7 | |||
| Mode of deliveryb | 5.9 | 2 | 0.052 | ||
| Vaginal delivery | 106 (64.6%) | 178 (75.7%) | |||
| Intrapartum CS | 20 (12.2%) | 21 (8.9%) | |||
| Antepartum CS | 38 (23.2%) | 36 (15.3%) | |||
| Postpartum hemorrhagingb | 40 (24%) | 33 (14%) | 1.6 | 1 | 0.013 |
| Hemoglobin after delivery, g/dLa | 22,098 | NA | 0.013 | ||
| Minimum | 5.9 | 5.5 | |||
| 25 percentile | 9.3 | 9.6 | |||
| Median | 10.1 | 10.4 | |||
| 75 percentile | 11.0 | 11.2 | |||
| Maximum | 12.6 | 13.6 | |||
| Birth weight of infant < 2500 gb | 10 (6.1%) | 23 (9.8%) | 1.3 | 1 | 0.26 |
| Maleb | 80 (49%) | 113 (48%) | 0.0012 | 1 | 0.97 |
| Use of infant formula or water before weight loss ≥10%b | 22 (13%) | 33 (14%) | 0.001 | 1 | 0.98 |
| Neonatal jaundice requiring phototherapyb | 22 (13%) | 19 (8.1%) | 2.4 | 1 | 0.12 |
Abbreviations: BMI body mass index, CS Caesarean section, NA not applicable
a The Mann–Whitney U test was used
b The Pearson’s chi-squared test was used
Table 2 shows the univariate and multivariate logistic analysis results of the associations between the variables and excessive weight loss in newborns. According to the univariate analysis, maternal age, primiparity, infertility treatment, antepartum Caesarean section, postpartum hemorrhaging, and the hemoglobin level after delivery were associated with excessive weight loss in newborns. Meanwhile, according to the adjusted multiple regression analysis, the factors associated with excessive weight loss in newborns were an older maternal age, primiparity, and antepartum Caesarean section, with adjusted odds ratios (95% Confidence Intervals [CIs]) of 1.07 (1.02, 1.11), 2.72 (1.69, 4.38), and 2.00 (1.09, 3.65), respectively (Table 2). No statistical interaction was noted between parity and the mode of delivery, or between maternal age and infertility treatment.
Table 2.
Multivariate logistic analysis of the association between the variables and excessive weight loss
| Crude OR (95% CI) |
p | Adjusteda OR (95% CI) | p | |
|---|---|---|---|---|
| Maternal age | 1.06 (1.02, 1.10) | 0.001 | 1.07 (1.02, 1.11) | 0.002 |
| Prepregnancy BMI, kg/m2 | 1.04 (0.99, 1.09) | 0.13 | 1.04 (0.99, 1.09) | 0.15 |
| Primiparity | 2.01 (1.34, 3.02) | < 0.001 | 2.72 (1.69, 4.38) | < 0.001 |
| Infertility treatment | 2.28 (1.25, 4.15) | 0.007 | 1.27 (0.65, 2.52) | 0.49 |
| Maternal weight gain, kg | 0.99 (0.95, 1.03) | 0.58 | 1.00 (0.96, 1.05) | 0.91 |
| Hypertension | 1.47 (0.68, 3.18) | 0.32 | 1.15 (0.51, 2.59) | 0.74 |
| Diabetes | 1.45 (0.90, 2.34) | 0.14 | 1.40 (0.83, 2.35) | 0.21 |
| Use of MgSO4 in labor | 0.40 (0.08, 1.96) | 0.26 | 0.28 (0.06, 1.42) | 0.13 |
| Use of oxytocin in labor | 1.38 (0.89, 2.15) | 0.15 | 1.13 (0.68, 1.88) | 0.65 |
| Gestational weeks at delivery | 1.05 (0.86, 1.28) | 0.62 | 1.09 (0.87, 1.36) | 0.47 |
| Mode of delivery | ||||
| Vaginal delivery | 1 | 1 | ||
| intrapartum CS | 1.60 (0.83, 3.09) | 0.16 | 0.99 (0.48, 2.05) | 0.98 |
| antepartum CS | 1.77 (1.06, 3.00) | 0.030 | 2.00 (1.09, 3.65) | 0.024 |
| Postpartum hemorrhaging | 1.97 (1.18, 3.30) | 0.009 | 1.31 (0.71, 2.41) | 0.39 |
| Hemoglobin after delivery, g/dL | 0.83 (0.71, 0.97) | 0.019 | 0.93 (0.78, 1.12) | 0.45 |
| Birth weight of infant < 2500 g | 0.60 (0.28, 1.29) | 0.19 | 0.58 (0.26, 1.32) | 0.19 |
| Male | 1.03 (0.69, 1.53) | 0.83 | 1.05 (0.69, 1.60) | 0.82 |
| Use of infant formula or water before weight loss ≥10% | 0.95 (0.53, 1.69) | 0.86 | 0.65 (0.34, 1.23) | 0.18 |
Abbreviations: BMI body mass index, CS Caesarean section
a Adjusted for maternal age, primiparity, infertility treatment, antepartum Caesarean section, postpartum hemorrhaging, and the hemoglobin level after delivery
No full-term newborn included in this study required intravenous therapy due to hypoglycemia or dehydration. However, neonatal jaundice requiring phototherapy was associated with a higher weight loss (percentage) (odds ratio: 1.22; 95% CI: 1.05–1.42).
Table 3 shows the laboratory test results of the newborns who were examined on the day of weight loss ≥10%. The mean plasma osmolalities on the day of weight loss ≥10% were 306, 304, and 303 mOsm/kgH2O in newborns delivered by vaginal delivery, intrapartum Caesarean section, and antepartum Caesarean section, respectively (p = 0.043).
Table 3.
Comparison of the blood test results according to the mode of delivery
| Vaginal deliveryN = 106 | Intrapartum Cesarean sectionN = 20 | Antepartum Cesarean sectionN = 38 | Test value or F value | Degree of freedom | p | |
|---|---|---|---|---|---|---|
| Sodium, mEq/La | 0.96 | 2 | 0.62 | |||
| Minimum | 142 | 141 | 143 | |||
| 25 percentile | 147 | 146.8 | 147 | |||
| Median | 148 | 148 | 148 | |||
| 75 percentile | 150 | 149.5 | 150 | |||
| Maximum | 154 | 152 | 154 | |||
| Potassium, mEq/La | 6.5 | 2 | 0.038 | |||
| Minimum | 3.2 | 3.8 | 3.4 | |||
| 25 percentile | 4 | 4.1 | 3.8 | |||
| Median | 4.3 | 4.3 | 4.1 | |||
| 75 percentile | 4.7 | 4.7 | 4.3 | |||
| Maximum | 7.1 | 5.4 | 5.7 | |||
| Ionized calcium, mmol/Lb | 1.21 ± 0.08 | 1.17 ± 0.07 | 1.21 ± 0.08 | 2.7 | 2 | 0.069 |
| Glucose, mg/dLa | 0.29 | 2 | 0.87 | |||
| Minimum | 31 | 39 | 34 | |||
| 25 percentile | 45 | 46 | 47 | |||
| Median | 53 | 53 | 51 | |||
| 75 percentile | 65 | 58 | 60 | |||
| Maximum | 88 | 84 | 90 | |||
| Blood urea nitrogen, mg/dLa | 18.4 | 2 | < 0.001 | |||
| Minimum | 3 | 5 | 3 | |||
| 25 percentile | 11 | 12 | 7 | |||
| Median | 15 | 14 | 9 | |||
| 75 percentile | 19 | 18 | 12 | |||
| Maximum | 37 | 31 | 22 | |||
| Creatinine, mg/dLa | 3.3 | 2 | 0.19 | |||
| Minimum | 0.3 | 0.3 | 0.4 | |||
| 25 percentile | 0.6 | 0.6 | 0.5 | |||
| Median | 0.7 | 0.8 | 0.6 | |||
| 75 percentile | 0.8 | 0.8 | 0.7 | |||
| Maximum | 1.3 | 1.2 | 1.1 | |||
| Osmotic pressure as osmolality, mOsm/kgH2Ob | 305.7 ± 5.8 | 304.2 ± 4.8 | 303.0 ± 5.3 | 3.2 | 2 | 0.043 |
a The Kruskal-Wallis test was used
b The ANOVA test was used
Discussion
Our data showed a high mean percentage of weight reduction (9.4%) and a high rate (41%) of excessive weight loss in healthy full-term newborns at our center. The mean percentage of weight reduction in our center was much higher than that reported in the literature in other developed countries (2.4–8.6%) [25–27, 31, 39, 40]. This discrepancy may be partly due to the delayed lactogenesis in Asians compared to other ethnic groups [41]. It is also because we check the weight every day and keep a baby in the hospital until we confirm a nadir of the weight. The rate is also higher than that of other perinatal centers accredited as Baby-Friendly Hospitals in Japan. In 2018, the mean weight reduction rates at 27 perinatal centers accredited as Baby-Friendly Hospitals in Japan were 8.2 and 8.9% for vaginal delivery and Caesarean section, respectively [42]. In comparison, the rates were 9.0 and 9.9% at our center, respectively [42]. Our cohort’s baseline characteristics were similar to those of 27 perinatal centers accredited as Baby-Friendly Hospitals in Japan [42]. This difference of the weight reduction is possibly related to the high rate of exclusively breastfed newborns. The same report showed that the mean rate of exclusively breastfed newborns at hospital discharge at 27 perinatal centers was 77% [42], while the rate at our center was 84% [42].
An older maternal age [29] and primiparity [19, 30] also have been shown as risk factors for excessive newborn weight loss in previous studies [29, 30]. An older maternal age [43, 44] and primiparity [30, 45, 46] are associated with excessive weight loss in newborns due to the delayed onset of lactation.
The most notable finding of this study was that there was a significant difference in the frequency of excessive weight loss between the newborns delivered by antepartum Caesarean section and those delivered by vaginal delivery, while no marked difference was noted between infants delivered via intrapartum Caesarean section and those delivered by vaginal delivery. Most of the previous studies on this topic did not distinguish between antepartum Caesarean section and intrapartum Caesarean section. Our finding is consistent with one previous study [47]. In addition, an observational cohort study has demonstrated that primary antepartum Caesarean section is an independent risk factor for the failure of exclusive breastfeeding [48]. However, their study outcome was different from ours, and the study did not distinguish between the experience of never trying to breastfeed and that of trying but not succeeding.
Three hypotheses may explain these findings. First, labor causes hormonal changes in the mother, and these changes promote lactogenesis. Some studies suggest that labor can elevate the plasma concentrations of oxytocin and prolactin [49, 50]. Second, labor causes hormonal changes in newborns, and these changes affect the kidney function and insensible water loss. For example, glucocorticoids are known to reduce the rate of insensible water loss in newborns [51], and oxytocin is an antidiuretic [52]. Third, labor itself or the excessive administration of fluids to the mother before she gives birth may affect the amount of total body water in the newborn at birth. An observational cohort study has shown that the timing and the amount of maternal intravenous fluids are related to the neonatal output and newborn weight loss [53]. The differences of mean plasma osmolality on the day of ≥10% in newborns delivered by vaginal delivery, intrapartum Caesarean section, and antepartum Caesarean section indicated that the newborns delivered by antepartum Caesarean section tended to be born with more body water, and the shedding of this excess water after birth accounts for part of the weight loss. Further research is necessary to determine the mechanisms involved.
The results of the present study suggest that earlier intervention including closer monitoring of mothers and babies as well as earlier initiation of formula feeding in cases with an older maternal age, primiparity, and antepartum Caesarean section may help to prevent complications in newborns due to excessive weight loss.
Several limitations associated with the present study warrant mention. First, because our study population was derived from one hospital in Japan, the generalizability of our study results might be limited to some extent. Second, there might be other factors associated with excessive weight loss in newborns. For example, we did not examine the rate of weight loss or the feeding methods in previous pregnancies. In addition, we did not analyze drug use in labor or pregnancy except for oxytocin and magnesium sulfate use in labor, which might affect infant feeding. Third, prepregnancy BMI is subject to memory bias and the timing of weight measurement is imprecise. Fourth, the accurate amount of formula or water administered before weight loss reaches 10% was not documented. However this had little effect on weight loss because minimal amount was usually given in a single dose.
Conclusions
An older maternal age, primiparity, and antepartum Caesarean section were found to be independent risk factors for excessive weight loss in breastfed full-term newborns in this study. Closer monitoring of these infants is recommended and earlier supplementation with artificial milk may be indicated.
Acknowledgements
We sincerely thank Dr. Ichiro Yasuhi, Dr. Hiroshi Yamashita, and Dr. Sachie Suga (Department of Obstetrics and Gynecology, National Hospital Organization Nagasaki Medical Center, Nagasaki, Japan) for helpful discussions and valuable advice regarding the statistical analyses.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- NICU
Neonatal intensive care unit
Authors’ contributions
YM conceived the study idea, collected data, performed the data analysis, and wrote the manuscript. HS, MA, and ST reviewed and approved the final version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of the National Hospital Organization Nagasaki Medical under protocol number 2019079, approved on October 10, 2019, with opt-out consent to obtain patient data from the medical records.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest in association with the present study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flaherman VJ, Schaefer EW, Kuzniewicz MW, Li SX, Walsh EM, Paul IM. Early weight loss nomograms for exclusively breastfed newborns. Pediatrics. 2015;135(1):e16–e23. doi: 10.1542/peds.2014-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul IM, Schaefer EW, Miller JR, Kuzniewicz MW, Li SX, Walsh EM, et al. Weight change nomograms for the first month after birth. Pediatrics. 2016;138(6):e20162625. [DOI] [PubMed]
- 3.Noel-Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. 2008;2(4):99–110. [PMC free article] [PubMed] [Google Scholar]
- 4.Brace AR. Fluid and electrolyte metabolism. In: Polin AR, Fox W, Abman S, editors. Fetal and neonatal physiology. 3. Philadelphia: Saunders; 2004. pp. 1341–1350. [Google Scholar]
- 5.McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2013;7:CD004074. [DOI] [PMC free article] [PubMed]
- 6.Chang RJ, Chou HC, Chang YH, Chen MH, Chen CY, Hsieh WS, et al. Weight loss percentage prediction of subsequent neonatal hyperbilirubinemia in exclusively breastfed neonates. Pediatr Neonatol. 2012;53(1):41–44. doi: 10.1016/j.pedneo.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Salas AA, Salazar J, Burgoa CV, De-Villegas CA, Quevedo V, Soliz A. Significant weight loss in breastfed term infants readmitted for hyperbilirubinemia. BMC Pediatr. 2009;9:82. doi: 10.1186/1471-2431-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuppa AA, Sindico P, Antichi E, Carducci C, Alighieri G, Cardiello V, et al. Weight loss and jaundice in healthy term newborns in partial and full rooming-in. J Matern Fetal Neonatal Med. 2009;22(9):801–805. doi: 10.3109/14767050902994499. [DOI] [PubMed] [Google Scholar]
- 9.Huang A, Tai BC, Wong LY, Lee J, Yong EL. Differential risk for early breastfeeding jaundice in a multi-ethnic Asian cohort. Ann Acad Med Singap. 2009;38(3):217–224. [PubMed] [Google Scholar]
- 10.Yaseen H, Salem M, Darwich M. Clinical presentation of hypernatremic dehydration in exclusively breastfed neonates. Indian J Pediatr. 2004;71(12):1059–1062. doi: 10.1007/BF02829814. [DOI] [PubMed] [Google Scholar]
- 11.Pelleboer RA, Bontemps ST, Verkerk PH, Van Dommelen P, Pereira RR, Van Wouwe JP. A nationwide study on hospital admissions due to dehydration in exclusively breastfed infants in the Netherlands: its incidence, clinical characteristics, treatment and outcome. Acta Paediatr. 2009;98(5):807–811. doi: 10.1111/j.1651-2227.2009.01230.x. [DOI] [PubMed] [Google Scholar]
- 12.Shroff R, Hignett R, Pierce C, Marks S, van't Hoff W. Life-threatening hypernatraemic dehydration in breastfed babies. Arch Dis Child. 2006;91(12):1025–1026. doi: 10.1136/adc.2006.095497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oddie SJ, Craven V, Deakin K, Westman J, Scally A. Severe neonatal hypernatraemia: a population based study. Arch Dis Child Fetal Neonatal Ed. 2013;98(5):F384–F387. doi: 10.1136/archdischild-2012-302908. [DOI] [PubMed] [Google Scholar]
- 14.Oddie S, Richmond S, Coulthard M. Hypernatraemic dehydration and breast feeding: a population study. Arch Dis Child. 2001;85(4):318–320. doi: 10.1136/adc.85.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke AJ, Sibert JR. Hypernatraemic dehydration and necrotizing enterocolitis. Postgrad Med J. 1985;61(711):65–66. doi: 10.1136/pgmj.61.711.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper WO, Atherton HD, Kahana M, Kotagal UR. Increased incidence of severe breastfeeding malnutrition and hypernatremia in a metropolitan area. Pediatrics. 1995;96(5 Pt 1):957–960. [PubMed] [Google Scholar]
- 17.Kaplan JA, Siegler RW, Schmunk GA. Fatal hypernatremic dehydration in exclusively breastfed newborn infants due to maternal lactation failure. Am J Forensic Med Pathol. 1998;19(1):19–22. doi: 10.1097/00000433-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 18.van Dommelen P, van Wouwe JP, Breuning-Boers JM, van Buuren S, Verkerk PH. Reference chart for relative weight change to detect hypernatraemic dehydration. Arch Dis Child. 2007;92(6):490–494. doi: 10.1136/adc.2006.104331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caglar MK, Ozer I, Altugan FS. Risk factors for excess weight loss and hypernatremia in exclusively breastfed infants. Braz J Med Biol Res. 2006;39(4):539–544. doi: 10.1590/s0100-879x2006000400015. [DOI] [PubMed] [Google Scholar]
- 20.Johnston M, Landers S, Noble L, Szucs K, Viehmann L. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 21.Bass JL, Gartley T, Kleinman R. World Health Organization baby-friendly hospital initiative guideline and 2018 implementation guidance. JAMA Pediatr. 2019;173(1):93–94. doi: 10.1001/jamapediatrics.2018.3808. [DOI] [PubMed] [Google Scholar]
- 22.Becker GE, Remmington T. Early additional food and fluids for healthy breastfed full-term infants. Cochrane Database Syst Rev. 2014;11:CD006462. [DOI] [PubMed]
- 23.Gartner LM, Morton J, Lawrence RA, Naylor AJ, O’Hare D, Schanler RJ, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 24.Simpson E, Goyal NK, Dhepyasuwan N, Flaherman VJ, Chung EK, Von Kohorn I, et al. Prioritizing a research agenda: a Delphi study of the better outcomes through research for newborns (BORN) network. Hosp Pediatr. 2014;4(4):195–202. doi: 10.1542/hpeds.2014-0003. [DOI] [PubMed] [Google Scholar]
- 25.Manganaro R, Mamì C, Marrone T, Marseglia L, Gemelli M. Incidence of dehydration and hypernatremia in exclusively breastfed infants. J Pediatr. 2001;139:673–675. doi: 10.1067/mpd.2001.118880. [DOI] [PubMed] [Google Scholar]
- 26.Konetzny G, Bucher HU, Arlettaz R. Prevention of hypernatraemic dehydration in breastfed newborn infants by daily weighing. Eur J Pediatr. 2009;168:815–818. doi: 10.1007/s00431-008-0841-8. [DOI] [PubMed] [Google Scholar]
- 27.Wright CM, Parkinson KN. Postnatal weight loss in term infants: what is normal and do growth charts allow for it? Arch Dis Child Fetal Neonatal Ed. 2004;89:254–257. doi: 10.1136/adc.2003.026906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mezzacappa MA, Ferreira BG. Excessive weight loss in exclusively breastfed full-term newborns in a baby-friendly hospital. Rev Paul Pediatr. 2016;34(3):281–286. doi: 10.1016/j.rppede.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fonseca MJ, Severo M, Barros H, Santos AC. Determinants of weight changes during the first 96 hours of life in full-term newborns. Birth. 2014;41(2):160–168. doi: 10.1111/birt.12087. [DOI] [PubMed] [Google Scholar]
- 30.Dewey KG, Nommsen-Rivers LA, Heinig MJ, Cohen RJ. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics. 2003;112(3 Pt 1):607–619. doi: 10.1542/peds.112.3.607. [DOI] [PubMed] [Google Scholar]
- 31.Davanzo R, Cannioto Z, Ronfani L, Monasta L, Demarini S. Breastfeeding and neonatal weight loss in healthy term infants. J Hum Lact. 2013;29(1):45–53. doi: 10.1177/0890334412444005. [DOI] [PubMed] [Google Scholar]
- 32.Martens PJ, Romphf L. Factors associated with newborn in-hospital weight loss: comparisons by feeding method, demographics, and birthing procedures. J Hum Lact. 2007;23(3):233–241. doi: 10.1177/0890334407303888. [DOI] [PubMed] [Google Scholar]
- 33.Regnault N, Botton J, Blanc L, Hankard R, Forhan A, Goua V, et al. Determinants of neonatal weight loss in term-infants: specific association with pre-pregnancy maternal body mass index and infant feeding mode. Arch Dis Child Fetal Neonatal Ed. 2011;96(3):F217–F222. doi: 10.1136/adc.2010.185546. [DOI] [PubMed] [Google Scholar]
- 34.Implementation guidance . Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services—The revised Baby-friendly Hospital Initiative. Geneva: World Health Organization; 2018. [PubMed] [Google Scholar]
- 35.Baby Friendly Hospital . Japan Breastfeeding Association. 2019. [Google Scholar]
- 36.Minkoff H, Chervenak FA. Elective primary cesarean delivery. N Engl J Med. 2003;348(10):946–950. doi: 10.1056/NEJMsb022734. [DOI] [PubMed] [Google Scholar]
- 37.Jokic M, Guillois B, Cauquelin B, Giroux JD, Bessis JL, Morello R, et al. Fetal distress increases interleukin-6 and interleukin-8 and decreases tumour necrosis factor-alpha cord blood levels in noninfected full-term neonates. BJOG. 2000;107(3):420–425. doi: 10.1111/j.1471-0528.2000.tb13241.x. [DOI] [PubMed] [Google Scholar]
- 38.Shields LE, Goffman D, Caughey AB. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130(4):e168–e186. doi: 10.1097/AOG.0000000000002351. [DOI] [PubMed] [Google Scholar]
- 39.Grossman X, Chaudhuri JH, Feldman-Winter L, Merewood A. Neonatal weight loss at a US baby-friendly hospital. J Acad Nutr Diet. 2012;112(3):410–413. doi: 10.1016/j.jada.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Marinelli KA, Gill SL, Thulier D. Weighing the facts: a systematic review of expected patterns of weight loss in full-term, breastfed infants. J Hum Lact. 2016;32(1):28–34. doi: 10.1177/0890334415597681. [DOI] [PubMed] [Google Scholar]
- 41.Dewey KG, Nommsen-Rivers L, Cohen RJ, Chantry CJ, Peerson JM. Delayed lactogenesis and excess neonatal weight loss are common across ethnic and socioeconomic categories of primiparous women in northern California. FASEB J. 2009;23(Suppl 1):344–347. [Google Scholar]
- 42.Nishimaki S, Okutani T, Tanimura S. Baby friendly hospital data book 2018. Tokyo: Japan Breast Feeding Association; 2019. [Google Scholar]
- 43.Rocha BO, Machado MP, Bastos LL, Barbosa SL, Santos AP, Santos LC, et al. Risk factors for delayed onset of lactogenesis II among primiparous mothers from a Brazilian baby-friendly hospital. J Hum Lact. 2020;36(1):146–156. doi: 10.1177/0890334419835174. [DOI] [PubMed] [Google Scholar]
- 44.Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr. 2010;92(3):574–584. doi: 10.3945/ajcn.2010.29192. [DOI] [PubMed] [Google Scholar]
- 45.Scott JA, Binns CW, Oddy WH. Predictors of delayed onset of lactation. Matern Child Nutr. 2007;3(3):186–193. doi: 10.1111/j.1740-8709.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurst NM. Recognizing and treating delayed or failed lactogenesis II. J Midwifery Womens Health. 2007;52(6):588–594. doi: 10.1016/j.jmwh.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Jordan S, Emery S, Watkins A, Evans JD, Storey M, Morgan G. Associations of drugs routinely given in labour with breastfeeding at 48 hours: analysis of the Cardiff births survey. BJOG. 2009;116(12):1622–1629. doi: 10.1111/j.1471-0528.2009.02256.x. [DOI] [PubMed] [Google Scholar]
- 48.Zanardo V, Pigozzo A, Wainer G, Marchesoni D, Gasparoni A, Di Fabio S, et al. Early lactation failure and formula adoption after elective caesarean delivery: cohort study. Arch Dis Child Fetal Neonatal Ed. 2013;98(1):F37–F41. doi: 10.1136/archdischild-2011-301218. [DOI] [PubMed] [Google Scholar]
- 49.Heasman L, Spencer JA, Symonds ME. Plasma prolactin concentrations after caesarean section or vaginal delivery. Arch Dis Child Fetal Neonatal Ed. 1997;77(3):F237–F238. doi: 10.1136/fn.77.3.f237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uvnäs Moberg K, Prime DK. Oxytocin effects in mothers and infants during breastfeeding. Infant. 2013;9(6):201–206. [Google Scholar]
- 51.Omar SA, DeCristofaro JD, Agarwal BI, La Gamma EF. Effects of prenatal steroids on water and sodium homeostasis in extremely low birth weight neonates. Pediatrics. 1999;104(3 Pt 1):482–488. doi: 10.1542/peds.104.3.482. [DOI] [PubMed] [Google Scholar]
- 52.Feeney JG. Water intoxication and oxytocin. Br Med J (Clin Res Ed) 1982;285(6337):243. doi: 10.1136/bmj.285.6337.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noel-Weiss J, Woodend AK, Peterson WE, Gibb W, Groll DL. An observational study of associations among maternal fluids during parturition, neonatal output, and breastfed newborn weight loss. Int Breastfeed J. 2011;6:9. doi: 10.1186/1746-4358-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


