Abstract
Background
Testing for carcino‐embryonic antigen (CEA) in the blood is a recommended part of follow‐up to detect recurrence of colorectal cancer following primary curative treatment. There is substantial clinical variation in the cut‐off level applied to trigger further investigation.
Objectives
To determine the diagnostic performance of different blood CEA levels in identifying people with colorectal cancer recurrence in order to inform clinical practice.
Search methods
We conducted all searches to January 29 2014. We applied no language limits to the searches, and translated non‐English manuscripts. We searched for relevant reviews in the MEDLINE, EMBASE, MEDION and DARE databases. We searched for primary studies (including conference abstracts) in the Cochrane Central Register of Controlled Trials (CENTRAL), in MEDLINE, EMBASE, and the Science Citation Index & Conference Proceedings Citation Index – Science. We identified ongoing studies by searching WHO ICTRP and the ASCO meeting library.
Selection criteria
We included cross‐sectional diagnostic test accuracy studies, cohort studies, and randomised controlled trials (RCTs) of post‐resection colorectal cancer follow‐up that compared CEA to a reference standard. We included studies only if we could extract 2 x 2 accuracy data. We excluded case‐control studies, as the ratio of cases to controls is determined by the study design, making the data unsuitable for assessing test accuracy.
Data collection and analysis
Two review authors (BDN, IP) assessed the quality of all articles independently, discussing any disagreements. Where we could not reach consensus, a third author (BS) acted as moderator. We assessed methodological quality against QUADAS‐2 criteria. We extracted binary diagnostic accuracy data from all included studies as 2 x 2 tables. We conducted a bivariate meta‐analysis. We used the xtmelogit command in Stata to produce the pooled estimates of sensitivity and specificity and we also produced hierarchical summary ROC plots.
Main results
In the 52 included studies, sensitivity ranged from 41% to 97% and specificity from 52% to 100%. In the seven studies reporting the impact of applying a threshold of 2.5 µg/L, pooled sensitivity was 82% (95% confidence interval (CI) 78% to 86%) and pooled specificity 80% (95% CI 59% to 92%). In the 23 studies reporting the impact of applying a threshold of 5 µg/L, pooled sensitivity was 71% (95% CI 64% to 76%) and pooled specificity 88% (95% CI 84% to 92%). In the seven studies reporting the impact of applying a threshold of 10 µg/L, pooled sensitivity was 68% (95% CI 53% to 79%) and pooled specificity 97% (95% CI 90% to 99%).
Authors' conclusions
CEA is insufficiently sensitive to be used alone, even with a low threshold. It is therefore essential to augment CEA monitoring with another diagnostic modality in order to avoid missed cases. Trying to improve sensitivity by adopting a low threshold is a poor strategy because of the high numbers of false alarms generated. We therefore recommend monitoring for colorectal cancer recurrence with more than one diagnostic modality but applying the highest CEA cut‐off assessed (10 µg/L).
Plain language summary
Detecting recurrent colorectal cancer by testing for blood carcino‐embryonic antigen (CEA).
Background
After surgery for cancer in the colon or rectum (colorectal cancer), most people are intensively followed up for at least five years to monitor for signs of the cancer returning. When this occurs, it usually causes a rise in a blood protein called CEA (carcino‐embryonic antigen). An increased level of CEA can be picked up by a blood test, which is normally done every three to six months after colorectal cancer surgery. Those people with raised CEA levels are further investigated by x‐ray imaging (usually a scan of the chest, abdomen and pelvis). We conducted this review to help decide what level of blood CEA should lead to further investigation.
Key Results
This review shows that setting a low cut‐off point will increase the number of genuine cases of colorectal cancer recurrence that are detected (true positives), but a low cut‐off will also cause unnecessary alarm by incorrectly classifying too many cases that are not actually recurrences (false positives). In addition, this review shows that a rise in CEA does not occur in up to 20% of patients with a true recurrence (false negatives). The current evidence supports using the highest cut‐off point assessed (10 µg/L), but that adding another diagnostic modality (e.g. a single scan of the chest, abdomen and pelvis at 12 to 18 months) is necessary in order to avoid the missed cases.
Summary of findings
Summary of findings 1. Summary of results table: different cut‐offs.
| Review question: What is the accuracy of single‐measurement blood CEA as a triage test to prompt further investigation for colorectal cancer recurrence after curative resection? | ||||
| Population: adults with no detectable residual disease after curative surgery (with or without adjuvant therapy) | ||||
| Studies: cross‐sectional diagnostic test accuracy studies, cohort studies, and RCTs, reporting 2 x 2 data | ||||
| Index test: Blood carcino‐embryonic antigen (CEA) | ||||
| Reference standard: appropriate¹ imaging, histology, or routine clinical follow‐up | ||||
| Setting: primary or hospital care. | ||||
| Subgroup | Number (Studies) | Sensitivity (95% CI) | Specificity (95% CI) |
Interpretation Assuming a constant incidence of 2%² recurrence at each measurement point, testing 1000 people will have the following outcome depending on the CEA threshold applied |
| 2.5 µg/L | 1515 (7) | 82% (78 to 86) | 80% (59 to 92) | 16 cases of recurrence will be detected and 4 cases will be missed. 196 people will be referred unnecessarily for further testing |
| 5 µg/L | 4585 (23) | 71% (64 to 76) | 88% (84 to 92) | 14 cases of recurrence will be detected and 6 cases will be missed. 118 people will be referred unnecessarily for further testing |
| 10 µg/L | 2341 (7) | 68% (53 to 79) | 97% (90 to 99) | 14 cases of recurrence will be detected and 6 cases will be missed. 29 people will be referred unnecessarily for further testing |
1as defined in the Reference standards section of the Methods. 2three‐monthly prevalence is estimated as 2%, as the median prevalence amongst the included studies was 30% and a standard follow‐up schedule will include 14 to 15 CEA tests over five years.
Background
International guidelines recommend that blood carcino‐embryonic antigen (CEA) levels are measured to detect recurrent colorectal cancer (CRC) as part of an intensive follow‐up regimen (Duffy 2013b; Labianca 2010; Locker 2006; NCCN 2013; NICE 2011).
A previous Cochrane review (Jeffery 2007) of eight randomised controlled trials (RCTs) (Kjeldsen 1997; Makela 1995; Ohlsson 1995; Pietra 1998; Rodriguez‐Moranta 2006b; Schoemaker 1998; Secco 2002; Wattchow 2006) evaluated the impact of follow‐up strategy on overall survival and the number of recurrences detected. The analysis included very scant data on CEA; data on overall survival were only available from one trial (odds ratio (OR) 0.57, 95% confidence interval (CI) 0.26 to 1.29) and data on recurrence rate only from two (OR 0.85, 95% CI 0.58 to 1.25).The follow‐up strategies implemented in each study were instead broadly classed as either intensive or minimal and the investigative modalities included in each strategy varied greatly between studies. Compared to minimal follow‐up, it was estimated that an intensive regimen could significantly reduce five‐year all‐cause mortality (OR 0.73, 95% CI 0.59 to 0.91).
The validity of this conclusion has been questioned because the mechanism by which a mortality reduction of this magnitude could be achieved by treating asymptomatic recurrence is unclear. There is evidence from one trial that starting chemotherapy for recurrence at an asymptomatic rather than symptomatic stage increases length of survival by a median of five months (Glimelius 1992). There is also observational evidence that surgical resection of metastases when feasible is associated with over 40% survival at five years (Colibaseanu 2013; Gonzalez 2013; Kanas 2012), and one commentator has suggested that advances in chemotherapy, hepatic resection, and multidisciplinary CRC follow‐up mean that the clinical benefits of intensive follow‐up will be even greater today (Labianca 2010). It is certainly true that there are now a number of well‐tolerated effective chemotherapy regimens for recurrent CRC in older populations (Cunningham 2010; Locker 2006). However, the authors of the CEASL (CEA second‐look) trial argue that identifying and treating asymptomatic recurrence has the potential to increase overall mortality (Treasure 2014), and the FACS (Follow‐up After Colorectal Surgery) trial suggests that the effect of follow‐up on absolute mortality is much smaller than that suggested by the 2007 review (Primrose 2014).
Nevertheless, the FACS trial has re‐awakened interest in CEA follow‐up. It showed that measuring blood CEA three‐ to six‐monthly for five years, augmented by a single CT (computed tomography) scan at 12 to 18 months, leads to earlier diagnosis of recurrence and increases by about three‐fold the proportion of recurrences that can be treated with curative intent (Primrose 2014). As CEA monitoring does not involve x‐rays, it can be done in the community, and is potentially more cost‐effective than CT imaging. The FACS trial result has raised substantial interest in CEA as a first‐line follow‐up modality.
CEA is a glycoprotein involved in cell adhesion produced during foetal development. Production usually ceases at birth, but elevated levels can be detected in people with colorectal, breast, lung and pancreatic cancer, in smokers, and in people with benign conditions such as cirrhosis of the liver, jaundice, diabetes, pancreatitis, chronic renal failure, colitis, diverticulitis, irritable bowel syndrome, pleurisy and pneumonia (Newton 2011; Sturgeon 2009). Prior to first diagnosis, CEA levels may rise between four and eight months before the development of cancer‐related symptoms (Goldstein 2005). Approximately 90% of colorectal cancers produce CEA (Dallas 2012). Predicting those people who do not secrete CEA is a challenge, with conflicting reports regarding whether well‐ or poorly‐differentiated tumours are associated with increased secretion (Davidson 1989). During follow‐up, CEA appears to be most sensitive for detecting hepatic and retroperitoneal metastases, and is least sensitive for local recurrences and peritoneal or pulmonary disease (Scheer 2009; Tsikitis 2009). However, CEA needs to be seen as a triage test (where a rise should lead to further investigation rather than initiation of therapy), as it gives no information about the location and extent of recurrence (Duffy 2013b).
Although serial CEA measurements are taken during follow‐up, the decision to investigate further with imaging is usually based on a single elevated CEA measurement (although a repeat blood test is often done to confirm the raised level). An absolute threshold somewhere between 3 and 7 µg/L is typically used to trigger further investigation. In the FACS trial, the threshold used was based on the difference of the CEA level at a single time point from the postoperative baseline (Primrose 2014).
The most recent systematic review exploring the accuracy of CEA for diagnosing recurrent CRC includes a meta‐analysis of 20 studies (Tan 2009). These studies implemented a wide range of thresholds (3 to 15 µg/L) and measured CEA using a variety of test kits. The pooled estimates of sensitivity and specificity were 64% (95% CI 61% to 67%) and 90% (95% CI 89% to 91%) respectively. The pooled area under the curve (AUC) was 0.79 (standard error = 0.054). A subgroup analysis of four studies that reported accuracy at a threshold of 3 µg/L gave an improved sensitivity of 73% (95% CI 69% to 77%) but at the expense of a reduced specificity of 68% (95% CI 65% to 72%). Based on a metaregression analysis, the authors suggest that a cut‐off of 2.2 µg/L provides the ideal balance between sensitivity and specificity, but this is based on extrapolation beyond the data analysed, as the lowest threshold applied in any included study was 3 µg/L. We were also unable to identify some of the data included in the analysis from the published studies.
Target condition being diagnosed
Colorectal cancer is globally the third most common cancer, accounting for 9.8% of all detected cancers. In 2008, the age‐standardised incidence rate was 17.3 cases per 100,000 (30.1 in high‐income countries and 10.7 in low‐ or middle‐income countries) (Ferlay 2013).
Colorectal adenocarcinoma arises in the colonic mucosa and progressively invades through the layers of bowel wall into surrounding structures, leading to peritoneal, neural, lymphatic and haematological metastasis (Gore 1997). This process provides the basis of the internationally recognised TNM (tumour node metastasis) staging system (Sobin 2009) and the earlier Dukes classification (Dukes 1932). The first site of haematological metastasis is the liver via the portal vein, after which distant metastasis occurs most commonly in the lungs but also in the bones and brain (Guthrie 2002). Prognosis is closely related to stage, with higher‐grade metastatic tumours having a poorer prognosis (Maringe 2013). Approximately two‐thirds of patients will present with a primary CRC amenable to radical surgery (Jeffery 2007).
Following surgery, however, 30% to 50% of patients will develop recurrence (Labianca 2010), although the results of the FACS trials suggest that perhaps half these cases result from inadequate preliminary staging and might have been detectable through more rigorous investigation at the time of primary treatment (Primrose 2014). The most common site for recurrence is the liver, followed by the lungs, but it can also occur in the abdomen and pelvis (Cunningham 2010; Jeffery 2007).
As stated in the Background, the effectiveness of treatment of recurrence is a matter of hot debate (Godlee 2014; Treasure 2014). In the absence of trials of treatment versus no treatment, most estimates of impact are based on observational data. Patients undergoing secondary surgery with curative intent have a median survival time of 35.8 to 84.8 months. Chemotherapy has been estimated to prolong life by one to two years (Arriola 2006; Cunningham 2010; Tsikitis 2009). However, apart from the Nordic trial showing that the initiation of chemotherapy at an asymptomatic stage increases survival (Glimelius 1992), there is no evidence from trials to confirm that treatment of early‐diagnosed asymptomatic recurrence improves survival or other outcomes. There is a need therefore to determine the most accurate means of detecting early‐stage recurrence before the impact of treatment strategies can be further explored.
Index test(s)
CEA is a relatively simple and low‐cost biomarker that can be detected by a blood test. The analysis of CEA in clinical studies utilises the technique of immunoassay in a variety of forms and from a number of different manufacturers. Earlier methods were manual immunoassays, such as radio‐immunoassay, but most laboratories now use fully automated non‐isotopic methods. The reproducibility of these fully automated methods are generally superior to the older manual methods. Unfortunately, the details of the methods used in clinical studies and their analytical performance are often lacking (Wild 2013).
Data from external quality assessment schemes have repeatedly shown good precision for most methods at low CEA concentrations. In 2010, within‐laboratory precision over a 12‐month period at a concentration of 3 µg/L (equivalent to 54 U/L) was less than 9% on average for all major methods. A greater analytical challenge is the difference in method bias (Wild 2013). Despite the availability of an international reference preparation (IRP 73/601) since 1975 and its widespread use in commercial assays since the early 1990s, method bias may still be ± 20%, and the degree of this bias is often sample‐dependent (Bormer 1991; Laurence 1975). CEA has a complex molecular structure and the antibodies used in the immunoassays recognise different epitopes of the molecule, which is considered to be a major source of method bias (Bormer 1991). Consequently, the interpretation of data from clinical studies, especially the use of any particular threshold, needs to take account of the actual method used. Due to the good reproducibility but significant method‐dependent bias, it is advised that the same assay technique should be used throughout any follow‐up period (Duffy 2013b).
Clinical pathway
Following radical surgery (with or without adjuvant therapy), there is wide variation in the recommended intensive follow‐up regimen (Duffy 2013b; Labianca 2010; Locker 2006; NCCN 2013; NICE 2011).
The European Society of Medical Oncology (ESMO) recommend history, physical examination, and CEA determination every three to six months for the first three years, and every six to 12 months in years four and five. A colonoscopy is recommended at one year, then every three to five years looking for metachronous adenomas and cancers. A CT scan of the chest and contrast‐enhanced ultrasound scan (USS) or CT scan of the abdomen is recommended every six to 12 months for the first three years in patients considered to be at higher risk. Other laboratory and radiological examinations are not recommended unless patients have suspicious symptoms (Labianca 2010).
The American Society of Clincal Oncology (ASCO) recommends that CEA is performed every three months for the first three years in patients with stage II or III disease if the patient is a candidate for surgery or systemic therapy, and that raised CEA levels (> 5 µg/L, confirmed by a repeat test) warrant further evaluation for metastatic disease (Locker 2006). Unlike ASCO, ESMO does not specify a threshold nor limit testing to specific tumour stages. The European Group on Tumour Markers (EGTM) specify CEA measurement at baseline and then every two to three months for three years, then six‐monthly for five years in patients with stage II to III disease who would tolerate further surgery or systemic therapy. EGTM recommend that any increase in CEA (confirmed by a repeat test) should trigger further investigations (Duffy 2013b).
The National Institute for Health and Clinical Excellence (NICE) recommended follow‐up from four to six weeks following curative treatment, for all patients who could tolerate and accept the balance of risk and benefits of further treatment, including CEA measurement at least every six months in the first three years, two CT scans of the chest and abdomen in the first three years, and colonoscopy at one year and five years (NICE 2011).
Once recurrence is suspected on the basis of a raised CEA level, patients then undergo further diagnostic testing to confirm recurrence (Duffy 2013a). The modality used to provide a definitive diagnosis is usually either CT or USS, but could also be clinical assessment, colonoscopy, flexible sigmoidoscopy and barium enema, CT colonography, positron emission tomography–computed tomography (PET‐CT), or magnetic resonance imaging (MRI).
Prior test(s)
As detailed above, CEA is often the most frequently undertaken modality within an intensive follow‐up regimen. Prior testing in this context is irrelevant, because CEA is measured routinely within intensive follow‐up programmes.
Role of index test(s)
As a triage test to prompt further investigation for CRC recurrence.
Alternative test(s)
Circulating tumour cells and cytokeratins have been examined as possible biomarkers of CRC recurrence, but the studies are few and limited. Ca125 is regarded as an emerging biomarker for use in postoperative follow‐up, but as yet evidence is limited (Duffy 2013b; Newton 2011). CT imaging is the only other test that meta‐analysis suggests has potential to detect metastatic recurrence amenable to resection, but it is more expensive than measuring blood CEA. CT‐PET is used in some centres, but will only be preferred to standard CT for routine follow‐up if future evidence suggests much superior performance. Endoscopic imaging (colonoscopy) is routinely used as an adjunct to CEA or CT imaging or both in follow‐up care to detect metachronous polyps or cancer (and rarely intraluminal recurrence). Clinical and ultrasound examination lack sensitivity. MRI can realistically be applied only to the liver and lacks strong evidence of effectiveness in detecting recurrence.
Rationale
This diagnostic test accuracy (DTA) review aims to clarify the accuracy of blood CEA as a triage test for CRC recurrence. If found to be sufficiently accurate, CEA could be a cost‐effective means of reducing unnecessary, more expensive investigations.
Objectives
To determine the diagnostic performance of different blood CEA levels in identifying people with colorectal cancer recurrence in order to inform clinical practice.
Secondary objectives
To identify sources of between‐ and within‐study heterogeneity to inform future study designs.
Methods
Criteria for considering studies for this review
Types of studies
We include cross‐sectional diagnostic test accuracy studies, cohort studies, and RCTs that directly compared follow‐up after CRC resection using CEA to a reference standard. We included studies only if we could extract 2 x 2 accuracy data. We excluded case‐control studies, as the ratio of cases to controls is determined by the study design, making the data unsuitable for assessing test accuracy.
Participants
Participants were adults with no detectable residual disease after primary treatment with surgical resection (with or without adjuvant therapy) being followed‐up for recurrence.
Index tests
Blood carcino‐embryonic antigen (CEA).
Target conditions
Recurrence of colorectal cancer following curative resection, including locoregional recurrence and metastatic disease.
Reference standards
Imaging done per protocol or to investigate for suspected recurrence (usually CT, MRI or PET‐CT, but also endoscopy, CT colonography, ultrasound, and barium enema).
The histological confirmation of recurrence following surgery or tissue biopsy.
Routine clinical follow‐up used as a reference standard to confirm negative index test values where imaging is not indicated as part of the follow‐up schedule (standard protocols run for three to five years).
We had hoped to compare the results of using these different reference standards in a sensitivity analysis. However, the majority of studies (73%) reported a composite reference standard, including more than one of the three reference standards listed above, as part of a prespecified clinical pathway and so the specific reference standard applied varied between participants within the same study. Without individual patient data, identifying the exact investigative modality applied as the reference standard was not possible and so we did not conduct the planned sensitivity analysis.
We classified the chosen reference standard (or composite reference standard) used in each study as 'appropriate' (1 to 3 above), 'inappropriate' (a reference standard not included in 1 to 3 above), or 'not stated' for further subgroup analysis.
There were insufficient data available to classify deaths during follow‐up as 'death from CRC', 'death with CRC', 'death from other causes', or 'death unspecified', as detailed in the original protocol.
Search methods for identification of studies
Electronic searches
Our information specialist (NR, trained in Cochrane DTA methodology) designed our search strategy, and conducted all searches to January 29 2014. We applied no language limits to the searches, and translated non‐English manuscripts to assess suitability for inclusion.
We searched for relevant reviews in the MEDION database (www.mediondatabase.nl), using the search terms 'cea' OR 'carcinoembryonic' or 'carcino‐embryonic' and restricting to Malignancy OR Digestive. Using the same terms, we also searched MEDLINE (OvidsSP) [1946 to current, In‐process], and EMBASE (OvidSP) [1974 to current] using the Reviews Clinical Query, and the DARE database (the Cochrane Library, Wiley).
We searched for primary studies (including conference abstracts) in the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Library, Wiley (Appendix 1), MEDLINE (OvidSP) [1946 to current, In‐process] (Appendix 2), EMBASE (OvidSP) [1974 to current] (Appendix 3), , and the Science Citation Index & Conference Proceedings Citation Index ‐ Science (Web of Science, Thomson) [1945 to current] (Appendix 4).
We identified ongoing studies by searching WHO ICTRP (apps.who.int/trialsearch/) using the following search terms: (Condition = (colorectal cancer OR colon cancer OR colorectal neoplas* OR colon neoplas* OR rectal cancer OR rectal neoplas*) AND Intervention = (cea OR Carcinoembryonic Antigen OR carcinoembryonic antibod*)), and by searching ClinicalTrials (clinicaltrials.gov) using the following search terms: (Condition = (colorectal cancer OR colon cancer OR colorectal neoplas* OR colon neoplas* OR rectal cancer OR rectal neoplas*) AND Intervention = (cea OR Carcinoembryonic Antigen OR carcinoembryonic antibod*)).
We conducted an additional search of the ASCO meeting library (meetinglibrary.asco.org/) for conference abstracts using the following search terms: (Title word search: “cea OR "carcinoembryonic antigen" OR "carcinoembryonic antigen").
Searching other resources
Following the search of bibliographic databases, we checked reference lists of retrieved reviews and all included studies. In addition, we performed a 'Related articles' search on PubMed on all included studies.
In the protocol, we stated we would contact the principal investigators of all included studies to identify further relevant literature, clarify methodological queries if they exist and to ask for any unpublished data relevant to this review. Unfortunately, due to time constraints and the large number of studies included in our review, we were not able to do this.
Data collection and analysis
Selection of studies
To identify relevant studies, two review authors (BDN and IP) scanned all titles and excluded those studies clearly not relevant to the topic of CEA for the detection of CRC recurrence. Following this, the same two review authors (BDN, IP) independently assessed both the titles and abstracts of the selected studies and retrieved the full‐text articles for those deemed to be relevant and for those where a decision could not be made on the basis of the title and abstract alone.
We assessed the remaining full‐text articles to see whether 2 x 2 accuracy data were available and, if so, we included the study in the review and implemented a full data extraction. Reasons for exclusions are detailed in Figure 1. A third review author (BS) resolved any disputes over which references should be included.
Data extraction and management
Full data extraction was guided by a background information sheet describing how each item should be interpreted. Two review authors piloted and refined this form, using three initial studies. A third review author resolved any disagreements over extracted data.
We extracted data into an Excel spreadsheet under the following headings: author, year, title, country, study design, setting, dates of data collection, population (n), inclusion criteria, exclusion criteria, included participants (n), age, smoking status, site of primary tumour, stage/grade of primary tumour, investigations done to ensure no residual disease, chemotherapy/radiotherapy, follow‐up schedule, cases of recurrence (n), CEA timing, CEA technique, CEA threshold, reference standard, timing of CEA versus reference standard, true positives (TP), false positives (FP), true negatives (TN), false negatives (FN), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), AUC, QUADAS‐2 items (including CEA laboratory technique, Appendix 5).
In the protocol we stated we would contact authors if data were not available, but due to time constraints we were not able to do this.
Assessment of methodological quality
Assessment of methodological quality
QUADAS‐2 is a generic set of criteria for assessing the quality of diagnostic accuracy studies. It consists of four key domains: patient selection, index test, reference standard, and the flow of patients through the study and timing of the index test in relation to the reference standard. Signalling questions are provided to guide judgement of the risk of bias across these four domains (Whiting 2011).
We modified QUADAS‐2 to exclude items not applicable to this review. A guide to the operational definitions for the modified QUADAS‐2 items can be found in Appendix 5.
We included additional questions regarding index test repetition (4.A.1) and CEA laboratory technique (2.A.2 to 2.A.4). We modified "Was there an appropriate interval between index test(s) and reference standard?" (Yes/No/Unclear) to instead read "4.A.2. Was the timing between index test(s) and reference standard ascertainable?" (Yes/Unclear). We also modified "Did all patients receive a reference standard?" to instead read "Did all included patients who had at least one CEA measurement receive a reference standard?". We removed "Was a case‐control design avoided?" from the original QUADAS‐2 template as we excluded all case‐control studies. We also removed "Were the index test results interpreted without knowledge of the results of the reference standard?" as knowledge of the reference test result would not bias the interpretation of a positive or negative CEA result, as CEA is an objective test using a predetermined dichotomous threshold.
For the index test domain, items were weighted so that the use of a prespecified threshold and a consistent method for CEA measurement had more influence on the overall judgement than the items regarding estimation of method reproducibility and indication of method accuracy. We made this decision as the latter two items were very rarely reported.
For the reference standard domain, items were weighted so that correctly classifying recurrent CRC had more influence on the overall judgement than whether the reference standard was interpreted without the knowledge of the index test. We made this decision as there were no blinded studies included in the review.
For the flow and timing domain, the five items were weighted so that the inclusion of all patients in the final analysis had the most influence and everyone receiving a reference standard was second most influential. Repetition of the index test prior to the reference standard, ascertainable timing between the index test and reference standard, and to all patients receiving the same reference standard were weighted equally lower.
Signalling questions weighted as high priority determined the overall rating within each domain.
Two review authors (BDN, IP) assessed the quality of all articles independently, discussing any disagreements. Where they could not reach consensus, a third author (BS) acted as moderator. We used the results of the quality assessment for descriptive purposes to provide an evaluation of the overall quality of the included studies and to investigate potential sources of heterogeneity.
Statistical analysis and data synthesis
We used descriptive statistics to present summary data for each included study. The Characteristics of included studies tables detail patient sample, study design, CEA technique, follow‐up characteristics and the CEA threshold(s) at which accuracy was reported. We extracted binary diagnostic accuracy data from all included studies as 2 x 2 tables. We present the risk of bias results for each of the four domains of the QUADAS‐2 assessment graphically as described by Whiting 2011.
Inferential statistics were guided by Chapter 10 of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Macaskill 2010).
We used Review Manager 5 to produce forest plots showing the variability of sensitivity and specificity across primary studies, with corresponding 95% confidence intervals for visual comparison. For studies reporting more than one threshold, we extracted 2 x 2 data for all thresholds. We plotted sensitivity and specificity estimates from each study in ROC space, using the inverse standard error of each estimate to adjust the size of each box to represent precision. For both of these graphs, we included sensitivity and specificity at the threshold closest to 5 µg/L (the most commonly reported threshold). We did not conduct a meta‐analysis across all of the included studies, as we had a sufficient number of studies to carry out meta‐analyses at specific thresholds (see next section), which is clinically more informative.
We used the bivariate model to perform meta‐analysis of sensitivity and specificity (Reitsma 2005). We conducted analyses using the xtmelogit command in Stata (Takwoingi 2013).
We estimated the absolute numbers of false alarms (false positives) and missed cases (false negatives) per 1000 patients tested for each three‐monthly testing interval by applying the pooled sensitivity and specificity derived from this review to: 1) the observed median reported prevalence of recurrence divided by 15 (national guidance is to conduct 14 to 15 CEA tests during follow‐up); 2) the incidence of recurrence data per follow‐up period reported by Sargent 2007 (as in reality the proportion developing recurrence between tests is not constant but falls over time).
Investigations of heterogeneity
Based on the results of the quality assessment, we determined the following most likely sources of heterogeneity: effect of CEA threshold, whether a single CEA measurement or serial measurements were evaluated, and the laboratory techniques employed.
For each subgroup analysis, we conducted bivariate meta‐analyses (Reitsma 2005), using the xtmelogit command in Stata to produce pooled estimates of sensitivity and specificity. Summary ROC plots and forest plots are reported to provide a basic picture of between‐study variability in these accuracy estimates.
CEA Threshold
For tests producing a continuous outcome, the threshold at which a positive result is defined directly impacts on the accuracy of the test. The use of different thresholds between studies is therefore a key source of heterogeneity.
We investigated the effect of threshold by carrying out subgroup meta‐analyses for thresholds where sufficient data were available. As some studies reported 2 x 2 data for more than one threshold, this analysis allowed us to include all of the available data. We used Review Manager 5 to produce a forest plot showing the variability of sensitivity and specificity across primary studies at specific thresholds.
Although the original plan was to apply a meta‐analysis method incorporating more than one 2 x 2 table from a single study (Hamza 2009), this method requires data to be reported at consistent thresholds across all included studies, and this was not the case in our review.
Timing of CEA Measurement
Despite sequential CEA measurements being taken in the majority of studies, 2 x 2 data were not reported for each scheduled measurement in any of these studies.
Some studies provided 2 x 2 data for the CEA measurement taken closest to the time point at which recurrence was detected or, for patients who did not experience recurrence, their final follow‐up measurement. Others looked across all of the measurements available for each individual to assess whether any of the sequential measurements had crossed the threshold during the entire follow‐up period. This approach meant the time interval between a rise in CEA and confirmed recurrence was variable across individuals within the same study, but this interval was not reported in any study. Consequently, we classified a patient without confirmed recurrence during the follow‐up period and at least one measurement above the threshold as a false positive in the 2 x 2 table, and a patient with confirmed recurrence but without any CEA rise above the threshold as a false negative.
As this information was not consistently reported in all studies, we could not include this variable in the metaregression analysis. Instead, we explored whether this had a significant impact on accuracy by carrying out a subgroup analysis on those studies that did provide this information. This analysis was also limited to studies reporting accuracy at 5 µg/L (the most commonly reported threshold) to avoid any threshold effects.
Laboratory Technique
The intention was to carry out subgroup analyses on studies using the same laboratory technique in order to assess the effect of technique on accuracy. However, given that so few studies provided sufficient detail regarding the laboratory technique employed, this was not possible. We were interested in exploring whether the implementation of IRP 73/601 reduced between‐study variability in sensitivity and specificity. We therefore used the information provided in each study to assess whether laboratory methods predated the introduction of IRP (e.g. manual Radioimmunoassay (RIA) and Immunoradiometric assay (IRMA) methods) and whether the samples were analysed pre‐1992. We then carried out a subgroup analysis and compared the widths of the 95% confidence intervals for the pooled estimates of sensitivity and specificity. We again limited this analysis to those studies reporting accuracy at 5 µg/L to avoid threshold effects.
Sensitivity analyses
To explore whether study quality biased the sensitivity and specificity of CEA, we planned a subgroup analysis to include those studies which had a low risk of bias across all four domains. We also carried out a metaregression analysis using the 'Metadas' macro in SAS, including all of the four domains as ordinal covariates (low risk, unclear, high risk).
Assessment of reporting bias
As described in the protocol and by Van Roon 2011, investigation of publication bias in DTA studies is known to be problematic, and so we have not included assessment of reporting bias in this review (Deeks 2005; Leeflang 2008; Song 2002).
Results
Results of the search
Figure 1 summarises the studies that we identified, screened and selected for this review. Our search resulted in 6782 hits, including 6571 primary studies, 128 reviews, 46 conference abstracts, and 37 registered trials. We identified 45 additional articles by checking the reference lists of retrieved reviews and by performing a 'Related articles' search in PubMed. We removed duplicates (n = 3016), leaving 3811 records for title and abstract screening. Of these, we requested 268 full‐text articles for review, of which we excluded 216 (see Figure 1 for reasons for exclusion). Fifty‐two studies met our inclusion criteria and are included in the final review.
1.
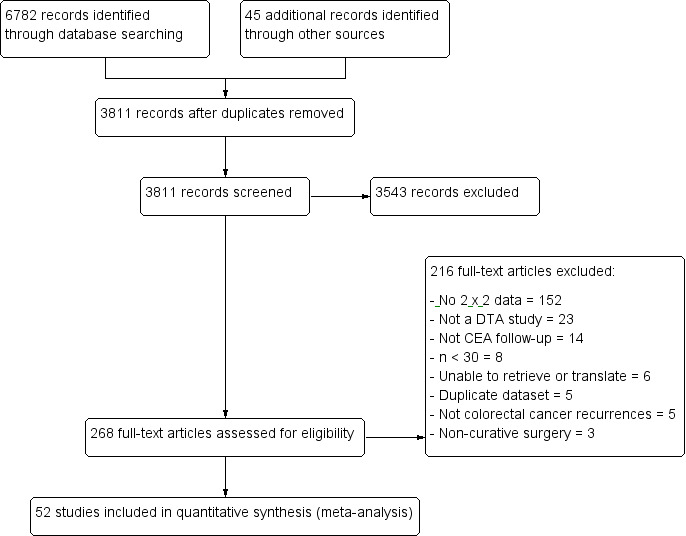
PRISMA flow diagram: results of the search for studies evaluating the diagnostic accuracy of blood CEA to detect recurrent colorectal cancer in patients following curative resection.
Included studies
Prevalence
Included studies were published between 1974 and 2014 and were conducted across 22 countries. All studies were conducted in secondary care, except one Norweigian prospective study (Johnson 1985) in which follow‐up was conducted in both primary and secondary care. In total, 9717 patients were included, and 2951 recurrences detected. The median number of participants in the studies was 139 (interquartile range (IQR): 72 to 247) and the proportion of recurrences detected ranged from 13.5% (Fezoulidis 1987) to 72.3% (Ochoa‐Figueroa 2012) (median: 29.5%, IQR: 24.3 to 36.3%).
Study Design
In 24 studies (46%) a prospective design was used, three of which were randomised controlled trials (RCTs) (McCall 1994; Ohlsson 1995; Steele 1982). One study prospectively followed up a cohort of patients of whom some were identified retrospectively (Tate 1982), while another sampled retrospectively from a prospective cohort (Korner 2007). The remaining 26 studies (50%) used a retrospective design.
Clincal features of included patients
Location of recurrence
The location of recurrence was reported in 25 studies (48%) including local, locoregional, and distant recurrence. However, the description of CRC recurrence was heterogeneous and all studies lacked 2 x 2 tables for the diagnostic accuracy of CEA to detect recurrence at each location (Characteristics of included studies).
Staging of primary colorectal cancer
Apart from the two studies (4%) which included only patients with rectal cancer (Barillari 1992; Fezoulidis 1987), the majority of studies (n = 50, 96%) included patients with both colon and rectal cancer.
Thirty‐three studies (63%) used the Dukes staging to describe the primary CRC. A further 11 studies (21%) used the TNM grading system and one study (2%) used the Astler‐Coller staging. The staging was unclear or not reported in the remaining seven studies (13%) (Carlsson 1983; Kohler 1980; Koizumi 1992; Li Destri 1998; Mittal 2011; Ochoa‐Figueroa 2012; Wood 1980).
Of those using Dukes staging, seven included Dukes A ‐ D (Banaszkiewicz 2011; Carpelan‐Holmström 2004; Jubert 1978; Mach 1978; Mariani 1980; Seregni 1992; Yu 1992); 15 included Dukes A ‐ C (Barillari 1992; Deveney 1984; Farinon 1980; Fezoulidis 1987; Fucini 1987; Graffner 1985; Hine 1984; Irvine 2007; Kato 1980; Korner 2007; Luporini 1979; Mackay 1974; McCall 1994; Ohlsson 1995; Triboulet 1983); three used Dukes B ‐ C (Beart 1981; Steele 1982; Wang 1994); two used Dukes C (Hara 2008; Tobaruela 1997); one used Dukes A ‐ C plus palliative cases (Johnson 1985); one used Dukes A ‐ C plus unknown cases (Tate 1982); and four used Dukes A ‐ D plus unknown cases (Bjerkeset 1988; Engarås 2003; Miles 1995; Minton 1985).
Of the 11 studies using the TNM grading system: five included TNM I ‐ III (Kanellos 2006a; Ohtsuka 2008; Park 2009; Tang 2009; Yakabe 2010); four used TNM I ‐ IV (Carriquiry 1999; Nishida 1988; Peng 2013; Staib 2000); and one included TNM II ‐ III (Kim 2013). Only one study reported 2 x 2 tables by stage, reporting on TNM II and TNM III (Hara 2010).
The study that used Astler‐Coller staging included A ‐ C2 (Lucha 1997).
Smokers
Three studies explicitly excluded smokers (Kanellos 2006a; Mariani 1980; Staib 2000), four studies explicitly included some smokers (but there was no way of identifying these patients in the 2 x 2 tables), and the remaining studies did not report smoking status. In the two studies which gave precise figures for smoking prevalence, it was low at 2% smokers (Fucini 1987) and 9% heavy smokers (Mach 1978).
Investigations for residual disease
In 43 studies (83%) it was not clear which (if any) perioperative investigations were done to ensure there was no residual disease before entering follow‐up. In the nine studies that reported this information, three reported using a persistent postoperative elevation of CEA as evidence of residual disease (Hara 2008; Irvine 2007; Steele 1982); one used "signs" of malignancy at the first follow‐up examination (Tate 1982); one used preoperative colonoscopy to resect any lesions outside the section of bowel planned for resection (Banaszkiewicz 2011); one reported using the intraoperative detection of gross residual disease (Lucha 1997); one specified no gross residual disease and clear resection margins (Bjerkeset 1988); one used preoperative abdominal CT and interoperative palpation to exclude liver metastases (Kanellos 2006a); and one reported using preoperative barium enema (BE), chest x‐ray (CXR), liver function tests (LFTs) and CEA, and postoperative BE and colonoscopy to ensure there was no residual disease (Ohlsson 1995).
Treatment
In 14 studies (27%) some (but not all) patients received chemotherapy, and in no studies was a subgroup analysis performed comparing the diagnostic accuracy of CEA in those receiving chemotherapy compared to those who did not (Characteristics of included studies).
Reference standard
In 38 studies (73%) a composite reference standard was used, the composition of which varied greatly between studies (see Characteristics of included studies). In 12 of these, a predefined multimodal follow‐up schedule was used for each patient (although the composition of these varied across studies) (Banaszkiewicz 2011; Carlsson 1983; Fucini 1987; Hara 2008; Irvine 2007; Jubert 1978; Kanellos 2006a; McCall 1994; Ohlsson 1995; Park 2009; Peng 2013; Steele 1982). In 26 studies (50%) a predefined composite follow‐up schedule was used to trigger further investigations for suspected recurrence.
A single investigation was used in three studies (6%) (Mittal 2011; Ochoa‐Figueroa 2012; Staib 2000), of which one reported 2 x 2 tables separately for PET and for CT (Ochoa‐Figueroa 2012).
In the remaining 11 studies (21%), it was unclear what was used as a reference standard.
CEA measurement
The use of predefined follow‐up schedules resulted in multiple CEA measurements being available for analysis.
Eight studies (15%) reported the accuracy of the CEA measurement closest to the time at which recurrence was detected by the reference standard, whilst nine studies (17%) defined CEA as positive if any CEA measurement crossed the threshold at any time within the follow‐up period. In a subset of studies, the authors stated clearly that a single 'positive' measurement would be followed up by a repeat test to confirm the result.
For the remaining 35 studies (67%), it was impossible to unpick which CEA value had been used, due to limited reporting.
Reporting units
CEA studies have used both ng/mL and µg/L in their publications. Numerically these are the same value and for consistency we have used µg/L throughout the review.
Laboratory technique
Details regarding laboratory methods for CEA analysis were inconsistently reported across the included studies. Based on the available information relating to laboratory technique, we were able to able to group the studies as follows:
Twenty‐two studies (42%) analysed samples before the introduction of the international reference preparation (IRP) using manual RIA and IRMA methods;
Seven studies (13%) used an identifiable laboratory technique following introduction of IRP;
Eight studies (15%) used unfamiliar laboratory techniques after the introduction of IRP;
Fifteen studies (29%) did not report laboratory technique.
For the seven studies reporting an identifiable laboratory technique following IRP introduction, six distinct techniques were used: Autodelfia post‐year 2000 (Carpelan‐Holmström 2004; Engarås 2003); Abbott automated instrumentation (Korner 2007); Bayer Immuno 1 (Irvine 2007); Siemens ADVIA centaur (Kim 2013); Roche elecsys (Mittal 2011); and Diasorin/byk santec liaison (Staib 2000). Across these, four thresholds were reported: 3 µg/L (Staib 2000); 5 µg/L (Carpelan‐Holmström 2004; Kim 2013; Mittal 2011); 5.6 µg/L (Engarås 2003); and 10 µg/L (Irvine 2007; Korner 2007).
Forty‐three studies (83%) did not report an estimation of CEA method reproducibility nor an indication of method accuracy. Of the remaining nine studies, three (6%) reported both an estimation of reproducibility and an indication of method accuracy (Carpelan‐Holmström 2004; Engarås 2003; Steele 1982), four (8%) clearly reported only an estimation of reproducibility (Fucini 1987; Hine 1984; Mach 1978; Mackay 1974), and the remaining two (4%) reported only the indication of method accuracy (Irvine 2007; Miles 1995).
Excluded studies
Of the 216 excluded full‐text articles (Figure 1; Characteristics of excluded studies):
152 studies (70%) did not report complete 2 x 2 data, and 74 (34%) reported no 2 x 2 data at all: 59 (27%) only reported recurrences; 16 (7%) only reported CEA positive cases; and three (1%) only CEA negative);
23 studies (11%) did not conduct a single‐point diagnostic test accuracy study (14 (6%) used alternative analyses (trend, nomogram, slope, or median CEA); five were case‐control studies (2%); three (1%) were review articles; and one was an economic analysis);
14 studies (6%) did not report an analysis of serum CEA measurements taken as part of a follow‐up schedule (seven (5%) reported preoperative CEA measurements; six (3%) reported the prognostic value of one postoperative CEA measurement; and one used intraoperative portal vein sampling);
eight studies (4%) included fewer than 30 patients;
six studies were unavailable or needed translation (five studies (2%) were not retrieved after worldwide search by the British Library, and we were not able to translate the remaining study);
five studies (2%) did not clearly report colorectal cancer recurrence (three (1%) reported on only liver metastases; and two (1%) reported colorectal cancer recurrence together with other cancer types);
five studies (2%) reported datasets already included in the review;
three studies (1%) reported non‐curative surgery.
We have not included two large RCTs in the review: the FACS trial (as 2 x 2 data were not reported in the published paper (Primrose 2014)), and the CEASL trial, which was published following our search and did not report on negative CEA cases (Treasure 2014).
Methodological quality of included studies
We assessed all 52 studies using the QUADAS‐2 framework. Figure 2 shows the summary of overall risk of bias and applicability concerns, and Figure 3 presents the risk of bias and applicability concerns as overall percentages.
2.
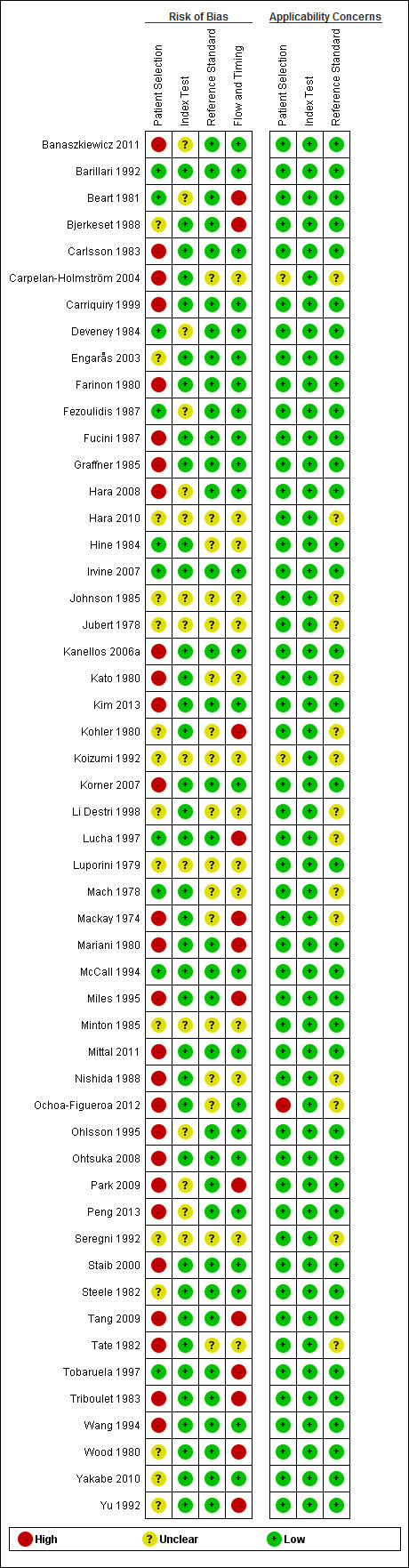
QUADAS‐2 risk of bias and applicability concerns summary including review authors' judgements about each domain for each included study
3.
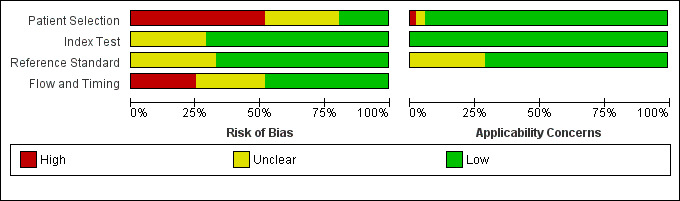
QUADAS‐2 risk of bias and applicability concerns graph including review authors' judgements about each domain presented as percentages across included studies
Three studies (6%), including 516 participants of whom 177 experienced recurrence, were assessed as being at low risk of bias and low concern regarding applicability across all domains (Barillari 1992; Irvine 2007; McCall 1994). Across these studies, each reported a different threshold (3, 10, and 5 µg/L respectively) using CEA test kits from three different manufacturers (with poor description of method accuracy). Each study applied a different but "appropriate" follow‐up schedule to detect recurrence. Consequently, the planned subgroup analysis of high‐quality studies (low risk of bias in all four domains) was not feasible.
Risk of bias
We judged 34 studies (65%) to be at high risk of bias in at least one of the four domains (Figure 3).
For the patient selection domain, items were weighted so that the presence of inappropriate exclusions had more influence on the overall judgement than the presence of a consecutive or random sample. Of the 27 studies judged to be at high risk of bias for patient selection (52%), inappropriate exclusions were based on:
advanced age (Carlsson 1983; Graffner 1985; Korner 2007; Ohlsson 1995);
previous malignancy (Ohtsuka 2008);
poor prognosis for further surgery (Banaszkiewicz 2011; Ohlsson 1995);
preoperative CEA values (Carpelan‐Holmström 2004; Carriquiry 1999; Farinon 1980; Miles 1995; Tang 2009; Wang 1994);
temporary rises in CEA (Mackay 1974);
non‐rising CEA (Mittal 2011);
using a minimum follow‐up period (Carriquiry 1999; Kim 2013; Korner 2007; Mackay 1974);
'incomplete' follow‐up measurements (Carpelan‐Holmström 2004; Carriquiry 1999; Hara 2008; Kato 1980; Korner 2007; Nishida 1988);
factors related to other follow‐up tests (Fucini 1987; Peng 2013; Tang 2009; Triboulet 1983);
early signs of malignancy or death (Carlsson 1983; Tate 1982);
smoking status (Kanellos 2006a; Mariani 1980);
concomitant benign disease or recent surgery (Kanellos 2006a; Mariani 1980; Park 2009; Peng 2013; Staib 2000);
patients not presenting a “diagnostic problem” (Staib 2000).
There were no studies deemed to be at high risk of bias based on the judgements made about the index test.
There were no studies at high risk of bias based on the judgements made about the reference standard, and in 17 (33%) the risk was unclear.
Thirteen studies (25%) were deemed to be at high risk of bias based on flow and timing. In four studies, not all patients were included in the final analysis (Beart 1981; Bjerkeset 1988; Kohler 1980; Park 2009). In the remaining nine studies, a raised CEA value triggered the reference standard which could introduce work‐up bias and result in false negative CEA results being misclassified as true negative results (Lucha 1997; Mackay 1974; Mariani 1980; Miles 1995; Tang 2009; Tobaruela 1997; Triboulet 1983; Wood 1980; Yu 1992).
Applicability concerns
We judged 37 studies (71%) to be at low risk of applicability concerns in all three domains (Figure 3). We rated only one study (Ochoa‐Figueroa 2012) at high risk of applicability concerns in relation to patient selection, as it did not include all patients undergoing postoperative follow‐up, but only those referred with suspected recurrence to the Department of Nuclear Medicine for fluoro‐deoxy‐glucose (FDG) PET‐CT. There were no studies deemed to be at high risk for applicability based on the index test or reference standard.
Unclear risk
Of the 364 domains, we deemed 85 (23%) to be at unclear risk of bias or applicability. For the vast majority of these items poor reporting accounted for the unclear rating.
Findings
Diagnostic accuracy
The forest plot in Figure 4 (Analysis 1) shows the range of sensitivity and specificity of CEA for the detection of recurrent colorectal cancer across all 52 included studies.
4.
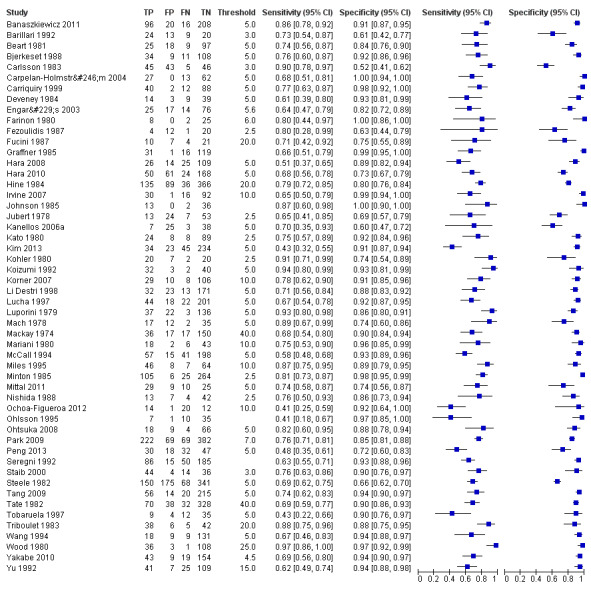
Forest plot for all 52 included studies for the threshold reported closest to 5 µg/L
TP = true positive; FP = false positive; FN = false negative; TN = true negative
The blue square depicts the sensitivity and specificity for each study and the horizontal line represents the corresponding 95% confidence interval for these estimates.
For studies reporting accuracy at more than one threshold, 2 x 2 data at the threshold closest to 5 μg/L are included in the plot (5 μg/L was the most commonly reported threshold).
.Sensitivity ranged from 41% to 97% and specificity from 52% to 100%.
Figure 5 plots each of the 52 studies in ROC space. The size of each box is proportional to the inverse standard error for sensitivity and specificity for each study (a larger box indicates greater precision).
5.
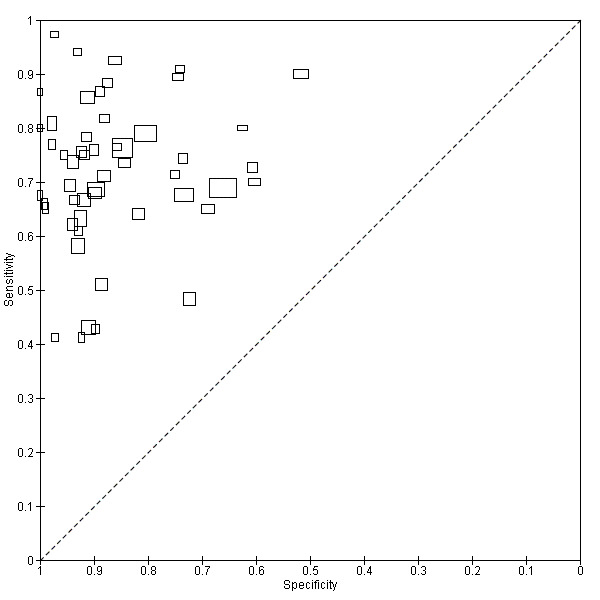
Scatter plot of sensitivity versus specificity for all 52 studies, regardless of threshold.
Each box represents the 2 x 2 data extracted from each study, with the width of the boxes being proportional to the inverse standard error of the specificity and the height of the boxes proportional to the inverse standard error of the sensitivity.
Effect of CEA threshold on diagnostic accuracy
Forty‐one studies (79%) reported accuracy at just a single threshold. A wide range of thresholds were reported (2 to 40 µg/L). Four studies (8%) did not report which threshold they used (Graffner 1985; Johnson 1985; Ohlsson 1995; Seregni 1992).Seven studies (13%) reported 2 x 2 data for more than one threshold:
Banaszkiewicz 2011: 5 and 10 µg/L
Bjerkeset 1988: 3.5 and 5 µg/L
Carlsson 1983: 3 and 7.5 µg/L
Korner 2007: 4 and 10 µg/L
Mittal 2011: 3, 5, 10, 20, and 50 µg/L
Steele 1982: 2.5, 5, 10, and 20 µg/L
Wood 1980: 25 and 40 µg/L
The forest plots in Figure 6 (Analysis 2) show the range of sensitivity and specificity for studies reporting the accuracy of CEA at cut‐off values of 2.5, 5 and 10 µg/L.
6.
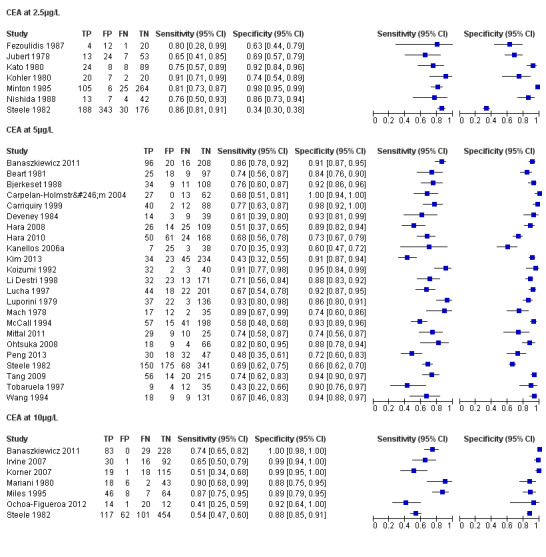
Forest plot broken down by threshold: CEA at 2.5µg/L, CEA at 5µg/L, CEA at 10µg/L.
TP = true positive; FP = false positive; FN = false negative; TN = true negative
The blue square depicts the sensitivity and specificity for each study and the horizontal line represents the corresponding 95% confidence intervals for these estimates.
The summary ROC curves and the summary estimates including confidence ellipses for the threshold values of 2.5, 5, and 10 µg/L (Analyses 3, 4 and 5) can be found in Figure 7, Figure 8 and Figure 9 respectively.
7.
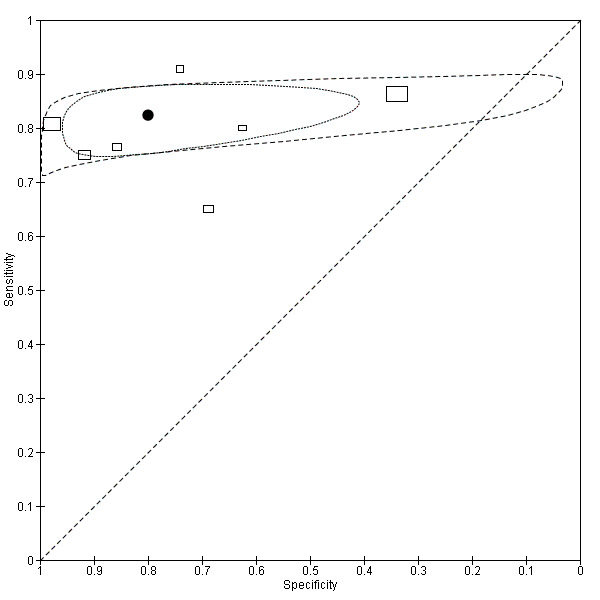
Summary ROC plot of accuracy at a threshold of 2.5 µg/L.
Each box represents the 2 x 2 data extracted from each study. The width of the box is proportional to the number of patients who did not experience recurrence in each study, and the height is proportional to the number of patients that did develop recurrent CRC.
The filled circle is the pooled estimate for sensitivity and specificity and the line running through it is the summary ROC curve.
The smaller dotted ellipse represents the 95% credible region around the summary estimate; the larger dashed ellipse represents the 95% prediction region.
8.
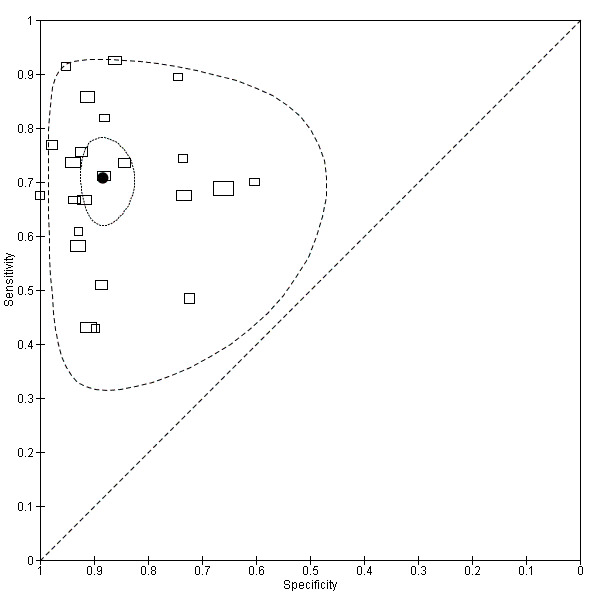
Summary ROC plot of accuracy at a threshold of 5 µg/L.
Each box represents the 2 x 2 data extracted from each study.
The width of the box is proportional to the number of patients who did not experience recurrence in each study, and the height is proportional to the number of patients that did develop recurrent CRC.
The filled circle is the pooled estimate for sensitivity and specificity and the line running through it is the summary ROC curve.
The smaller dotted ellipse represents the 95% credible region around the summary estimate; the larger dashed ellipse represents the 95% prediction region.
9.
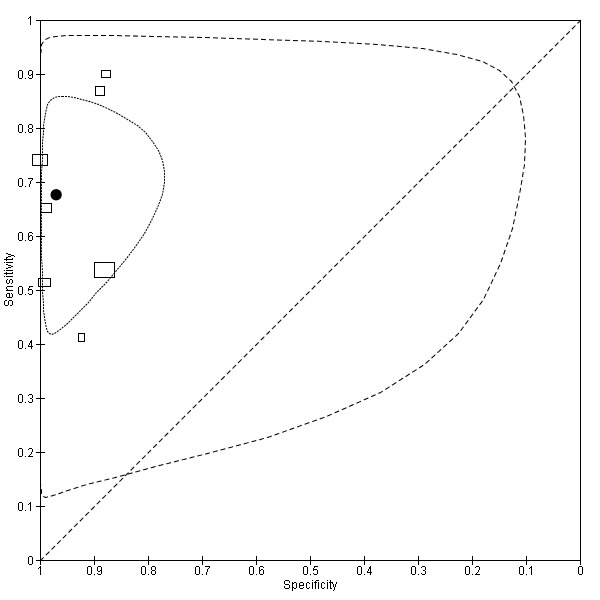
Summary ROC plot of accuracy at a threshold of 10 µg/L.
Each box represents the 2 x 2 data extracted from each study.
The width of the box is proportional to the number of patients who did not experience recurrence in each study, and the height is proportional to the number of patients that did develop recurrent CRC.
The filled circle is the pooled estimate for sensitivity and specificity and the line running through it is the summary ROC curve.
The smaller dotted ellipse represents the 95% credible region around the summary estimate; the larger dashed ellipse represents the 95% prediction region.
In the seven studies reporting a threshold of 2.5 µg/L, the sensitivity ranged from 65% to 91% and specificity from 34% to 98%. The pooled sensitivity of these studies was 82% (95% CI 78% to 86%) and pooled specificity 80% (95% CI 59% to 92%). Assuming that the proportion of patients with recurrence in any single testing period is 2% (based on our observed prevalence of recurrence of 30% and national guidance to conduct 14 to 15 CEA tests during follow‐up), for every 1000 patients tested at a threshold of 2.5 µg/L, 16 cases of recurrence will be detected, four cases will be missed, and there will be 196 false alarms (people referred unnecessarily for further testing). More precise estimates of test performance using the incidence data reported by Sargent 2007 can be found in Table 2.
Summary of findings 2. Outcome of follow‐up testing using a CEA threshold of 2.5 µg/L.
| Month when CEA measured | per 1000 patients tested at a threshold of 2.5 µg/L | False alarm rate | ||||
| Estimated recurrences¹ | Referrals for raised CEA | Cases of recurrence detected | Cases of recurrence missed | False alarms (cases investigated when cancer not present) | ||
| Follow‐up years 1 and 2: 3‐monthly CEA testing | ||||||
| 3 | 19 | 212 | 16 | 3 | 196 | 92% |
| 6 | 19 | 212 | 16 | 3 | 196 | 92% |
| 9 | 39 | 224 | 32 | 7 | 192 | 86% |
| 12 | 39 | 224 | 32 | 7 | 192 | 86% |
| 15 | 37 | 223 | 30 | 7 | 193 | 87% |
| 18 | 37 | 223 | 30 | 7 | 193 | 87% |
| 21 | 31 | 219 | 25 | 6 | 194 | 89% |
| 24 | 31 | 219 | 25 | 6 | 194 | 89% |
| Follow‐up years 3, 4 and 5: 6‐monthly CEA testing | ||||||
| 30 | 46 | 229 | 38 | 8 | 191 | 83% |
| 36 | 36 | 223 | 30 | 6 | 193 | 87% |
| 42 | 27 | 217 | 22 | 5 | 195 | 90% |
| 48 | 25 | 216 | 21 | 4 | 195 | 90% |
| 54 | 17 | 211 | 14 | 3 | 197 | 93% |
| 60 | 14 | 208 | 11 | 3 | 197 | 95% |
1Estimates are based on data reported by Sargent 2007. Three‐monthly data were unavailable, and so constant rates were assumed during each six‐month period for the first two years. Estimates are rounded.
In the 23 studies which reported the impact of applying a threshold of 5 µg/L, sensitivity ranged from 43% to 93% and specificity from 60% to 100%. The pooled sensitivity of these studies was 71% (95% CI 64% to 76%) and pooled specificity 88% (95% CI 84% to 92%). For every 1000 patients tested at a threshold of 5 µg/L, 14 cases of recurrence will be detected, six cases will be missed, and there will be 118 false alarms. More precise estimates of test performance using the incidence data reported by Sargent 2007 can be found in Table 3
Summary of findings 3. Outcome of follow‐up testing using a CEA threshold of 5 µg/L.
| Month when CEA measured | per 1000 patients tested at a threshold of 5 µg/L | False alarm rate | ||||
| Estimated recurrences¹ | Referrals for raised CEA | Cases of recurrence detected | Cases of recurrence missed | False alarms (cases investigated when cancer not present) | ||
| Follow‐up years 1 and 2: 3‐monthly CEA testing | ||||||
| 3 | 19 | 131 | 13 | 6 | 118 | 90% |
| 6 | 19 | 131 | 13 | 6 | 118 | 90% |
| 9 | 39 | 143 | 28 | 11 | 115 | 80% |
| 12 | 39 | 143 | 28 | 11 | 115 | 80% |
| 15 | 37 | 142 | 26 | 11 | 116 | 82% |
| 18 | 37 | 142 | 26 | 11 | 116 | 82% |
| 21 | 31 | 138 | 22 | 9 | 116 | 84% |
| 24 | 31 | 138 | 22 | 9 | 116 | 84% |
| Follow‐up years 3, 4 and 5: 6‐ monthly CEA testing | ||||||
| 30 | 46 | 147 | 33 | 13 | 114 | 78% |
| 36 | 36 | 142 | 26 | 10 | 116 | 82% |
| 42 | 27 | 136 | 19 | 8 | 117 | 86% |
| 48 | 25 | 135 | 18 | 7 | 117 | 87% |
| 54 | 17 | 130 | 12 | 5 | 118 | 91% |
| 60 | 14 | 128 | 10 | 4 | 118 | 92% |
1Estimates are based on data reported by Sargent 2007. Three‐monthly data were unavailable, and so constant rates were assumed during each six‐month period for the first two years. Estimates are rounded.
In the seven studies reporting the impact of applying a threshold of 10 µg/L, sensitivity ranged from 41% to 87% and specificity from 88% to 100%. The pooled sensitivity of these studies was 68% (95% CI 53% to 79%) and pooled specificity 97% (95% CI 90% to 99%). For every 1000 patients tested at a threshold of 10 µg/L, 14 cases of recurrence will be detected, seven cases will be missed, and there will be 29 false alarms. More precise estimates of test performance using the incidence data reported by Sargent 2007 can be found in Table 4.
Summary of findings 4. Outcome of follow‐up testing using a CEA threshold of 10 µg/L.
| Month when CEA measured | per 1000 patients tested at a threshold of 10 µg/L | False alarm rate | ||||
| Estimated recurrences¹ | Referrals for raised CEA | Cases of recurrence detected | Cases of recurrence missed | False alarms (cases investigated when cancer not present) | ||
| Follow‐up years 1 and 2: 3‐ monthly CEA testing | ||||||
| 3 | 19 | 42 | 13 | 6 | 30 | 70% |
| 6 | 19 | 42 | 13 | 6 | 29 | 70% |
| 9 | 39 | 55 | 27 | 13 | 29 | 52% |
| 12 | 39 | 55 | 27 | 13 | 29 | 52% |
| 15 | 37 | 54 | 25 | 12 | 29 | 53% |
| 18 | 37 | 54 | 25 | 12 | 29 | 53% |
| 21 | 31 | 50 | 21 | 10 | 29 | 58% |
| 24 | 31 | 50 | 21 | 10 | 29 | 58% |
| Follow‐up years 3, 4 and 5: 6‐ monthly CEA testing | ||||||
| 30 | 46 | 60 | 31 | 15 | 29 | 48% |
| 36 | 36 | 53 | 24 | 12 | 29 | 54% |
| 42 | 27 | 48 | 19 | 9 | 29 | 61% |
| 48 | 25 | 46 | 17 | 8 | 29 | 63% |
| 54 | 17 | 41 | 11 | 6 | 30 | 72% |
| 60 | 14 | 39 | 10 | 5 | 30 | 75% |
1Estimates are based on data reported by Sargent 2007. Three‐monthly data were unavailable, and so constant rates were assumed during each six‐month period for the first two years. Estimates are rounded.
Effect of the timing of CEA measurement
As previously described, we used two approaches when choosing which CEA measurement to include in the 2 x 2 tables. The first was to evaluate the CEA measurement taken closest to the time point at which recurrence was detected; the second was to look across all measurements to assess whether any had crossed the threshold during the entire follow‐up period.
Including only those studies reporting accuracy at a threshold of 5 µg/L, we carried out a subgroup analysis for these two strategies.
We adopted the first strategy in eight studies, for which the pooled sensitivity and specificity were 69.0% (95% CI 57.3% to 78.7%) and 90.0% (95% CI 77.8% to 95.9%) respectively. We adopted the second strategy in nine studies, for which the pooled sensitivity and specificity were 64.5% (95% CI 55.2% to 72.9%) and 89.5% (95% CI 83.4% to 93.5%) respectively.
Effect of laboratory technique
We were unable to carry out a subgroup analysis based on specific laboratory techniques, as reporting was so limited that it is was difficult to identify groups of studies where we could be confident that they had all used consistent methods.
For those studies reporting accuracy at a threshold of 5 µg/L, we carried out a subgroup analysis comparing the variability in accuracy before and after the introduction of the international reference preparation (IRP 73/601) calibration. We excluded one study (Li Destri 1998) from this analysis, as there was insufficient information about the timing of the sample analysis and laboratory technique. There were 11 studies predating the introduction of the IRP, providing a pooled sensitivity of 73.6% (95% CI 63.2% to 81.8%) and a pooled specificity of 88.5% (95% CI 83.2% to 92.2%), and 11 studies used methods which incorporated the IRP, resulting in a pooled sensitivity of 67.9% (95% CI 58.6% to 75.9%) and a pooled specificity of 88.6% (95% CI 80.0% to 93.7%). These results indicate no significant reduction in variability, and this was confirmed when we added it as a covariate in the metaregression (P = 0.958).
Effect of patient selection on diagnostic accuracy
When restricting the analyses to the 11 studies deemed to be at low risk of bias in the patient selection domain of the QUADAS‐2 assessment, the sensitivity ranged from 43% to 93% and specificity from 61% to 99%.
We added the patient selection risk of bias item as an ordinal covariate (low risk = 6, unclear risk = 6 and high risk = 11) in the metaregression analysis for those studies reporting accuracy at 5 µg/L. The effect of this covariate was not significant (P = 0.771).
Effect of index test on diagnostic accuracy
There were no studies deemed to be at high risk of bias in the index test domain of the QUADAS‐2 assessment. When restricting the analyses to the 37 studies (71%) deemed to be at low risk of bias in the index test domain of the QUADAS‐2 assessment, the sensitivity ranged from 41% to 97% and specificity from 52% to 100%.
We added the index test risk of bias item as a covariate (low risk = 15, unclear risk = 8) in the metaregression analysis for those studies reporting accuracy at 5 µg/L. The effect of this covariate was not significant (P = 0.901).
Effect of the reference standard on diagnostic accuracy
There were also no studies deemed to be at high risk of bias in the reference standard domain of the QUADAS‐2 assessment. When restricting the analyses to the 35 studies (67%) deemed to be at low risk of bias in the reference standard domain of the QUADAS‐2 assessment, the sensitivity ranged from 41% to 97% and specificity from 52% to 100%.
We added the reference standard risk of bias item as a covariate (low risk = 17, unclear risk = 6) in the metaregression analysis for those studies reporting accuracy at 5 µg/L. The effect of this covariate was not significant (P = 0.292).
Effect of flow and timing on diagnostic accuracy
When restricting the analyses to the 25 studies (48%) deemed to be at low risk of bias in the flow and timing domain of the QUADAS‐2 assessment, the sensitivity ranged from 41% to 95% and specificity from 52% to 100%.
We added the flow and timing risk of bias item as an ordinal covariate (low risk = 12, unclear risk = 6 and high risk = 5) in the metaregression analysis for those studies reporting accuracy at 5 µg/L. The effect of this covariate was not significant (P = 0.664).
Discussion
Summary of main results
We include 52 studies in the meta‐analysis, covering 9717 patients (median sample size = 139, IQR: 72 ‐ 247). The median proportion of recurrences in each study was 29% (IQR: 24% ‐ 36%), agreeing with previously reported recurrence rates (Labianca 2010).
The diagnostic accuracy of CEA was reported at 15 different thresholds, ranging from 2 to 40 µg/L. Seven studies (13%) reported accuracy at a threshold of 2.5 µg/L, providing a pooled sensitivity of 82% (95% CI 78% to 86%) and a pooled specificity of 80% (95% CI 59% to 92%). The most commonly reported threshold was 5 µg/L (23 studies, 44%), providing a lower sensitivity of 71% (95% CI 64% to 76%) and an increased specificity of 88% (95% CI 84% to 92%). Seven studies (13%) reported accuracy at a threshold of 10 µg/L. Implementing such a high threshold reduced sensitivity to 68% (95% CI 53% to 79%), but provided high specificity of 97% (95% CI 90% to 99%).
Reporting quality was insufficient in important areas such as laboratory techniques. Insufficient detail about laboratory techniques and the frequent use of composite reference standards made it impossible to conduct desirable subgroup analyses. An individual‐patient data meta‐analysis would be required to fully explore the influence of factors such as preoperative CEA levels, chemotherapy, site of recurrence and smoking status, that are known to impact on CEA levels in follow‐up.
Our results compared with other reports
Tan 2009 carried out a meta‐analysis of 20 studies that reported the accuracy of CEA for the diagnosis of colorectal cancer recurrence using the Moses‐Littenberg Method (Moses 1993). Their pooled estimate for specificity at a threshold of 5 µg/L was the same as ours (88%). Our pooled estimate for sensitivity was higher (71% versus 63%), but this difference is not statistically significant.
The method used by Tan 2009 to identify 2.2 µg/L as the 'optimum' CEA threshold was based on linear extrapolation (the lowest threshold included in their study was 3 µg/L). We instead implement bivariate meta‐analyses (Reitsma 2005), as recommended in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Macaskill 2010). This method is statistically more rigorous than the method implemented in Tan 2009, and directly accounts for the within‐ and between‐study variability in sensitivity and specificity.
We question the Tan 2009 recommendation of 2.2 µg/L (which was based on achieving high sensitivity) not just on the basis of the low specificity (and high false alarm rate), but also because there appears to be a 'ceiling' effect in terms of sensitivity ‐ even at a threshold of 2.5 µg/L, around one in five cases of recurrence would be missed. The failure to exceed a sensitivity of about 80% even with a low threshold or poor specificity reflects the well‐documented fact that some recurrent cancers are not associated with a rise in blood CEA levels.
Strengths and weaknesses of the review
Completeness
A key strength of this review is the comprehensiveness of our searches. We avoided the use of search filters and did not restrict our review to English‐language publications. Two review authors screened all abstracts independently, with a third independently settling any disagreement over inclusion. We retrieved and analysed all full‐text articles that we felt could be potentially relevant based on the title and abstract. We based additional searches on the citation of full‐text articles to reduce the risk of missing relevant studies. Foreign‐language articles were translated or assessed or both by colleagues of the authors proficient in the language in question.
It is not possible to estimate the impact of unpublished studies on our findings, as little is known about the mechanisms of publication bias for diagnostic accuracy studies (Allen 2013). Despite this, our included studies are likely to represent the vast majority of studies that provide evidence on this topic.
Two review authors then extracted data independently, and three authors independently performed QUADAS‐2 assessment of the included studies, with subsequent discussion to reach consensus on overall judgements of risk of bias and applicability. The meta‐analyses followed Cochrane DTA guidelines.
Variability
A major weakness of this review is that we considered many included studies to be at high risk of bias. There was also considerable between‐study variation in the reporting of: 1) stage of primary disease included; 2) approach to ensuring no residual disease; 3) reporting of smoking; 4) reporting of chemotherapy treatment; and 5) the location of recurrence. All of these factors could plausibly have some influence on CEA levels, but corresponding 2 x 2 tables were not presented for these subgroups, and so it was not possible to adjust for this variation in our analyses.
The QUADAS‐2 assessment of methodological quality highlighted the extent of the quality issues in the existing literature. Even the three studies that we assessed as having no risk of bias or applicability concerns were subject to considerable between‐study heterogeneity: they each reported accuracy at different CEA thresholds, implemented different CEA laboratory techniques, and used differing composite reference standards to detect recurrence. The varying thresholds made it unfeasible to provide pooled diagnostic accuracy estimates for these high‐quality studies.
Over half of the included studies (n = 27, 52%) were at high risk of selection bias, mainly due to inappropriate patient exclusions. We deemed a further 15 studies (29%) to be at unclear risk of bias for patient selection, due to poor reporting. This makes our accuracy estimates susceptible to selection bias, particularly if those excluded were at particularly high or low risk of recurrence. To investigate this further, we removed those studies at high and unclear risk of bias for patient selection in a sensitivity analysis. The pooled estimates were not significantly different from the overall pooled results (sensitivity = 73%, 95% CI 64% to 80%; specificity = 87%, 95% CI 79% to 92%).
The methods used to measure CEA were also poorly reported: three studies (6%) did not report the CEA threshold used to determine a positive result, 15 studies (29%) did not report which laboratory technique had been used, and 43 studies (83%) failed to report any indicator of method accuracy or an estimate of CEA reproducibility. It is well known that variability exists between laboratory methods and between laboratories, and without this information it is impossible to adjust for any bias that has been introduced by the differences in method. The IRP calibration (73/601, introduced in 1992) attempts to reduce between‐laboratory and between‐technique variability, so we performed a sensitivity analysis leaving only the studies that were conducted after its introduction. We did not find the pooled accuracy estimates to be significantly different from the overall analysis (sensitivity = 67.9%, 95% CI 58.6% to 75.9%; specificity = 88.6%, 95% CI 80.0% to 93.7%).
A possible source of bias in this review is likely to be the methods used to implement the reference standard. In nine studies, the reference standard was only carried out if a rise in CEA was detected, possibly causing false‐negative results to be misclassified as true‐negative results. Furthermore, most studies implemented a composite reference standard, but failed to consistently reported which investigation (within the composite) actually diagnosed recurrence. In half of the studies (n = 26, 50%), positive results for certain reference tests triggered the use of other reference tests. These concerns over partial and differential verification were considered in the flow and timing domain of QUADAS‐2, explaining why there were no studies deemed to be at high risk of bias in the reference standard domain.
The time between the CEA measurement and the reference test used in the 2 x 2 table was not reported in any of the studies. There is therefore a high chance of misclassification due to disease progression during the time between CEA and the reference test. Understanding this relationship is important in this setting as: a) a high‐grade recurrence will progress more quickly than low‐grade; b) this information is required to estimate lead time. Furthermore, no study reported 2 x 2 data for each three‐ to six‐month period of follow‐up, which would be desirable given that CRC recurrence is known to occur more commonly in the first two years of follow‐up, suggesting that a variable threshold may have greater accuracy (Sargent 2007).
Applicability of findings to the review question
All of the studies identified were carried out in hospital outpatient clinics, except one that followed up patients in both primary and secondary care. As the patient population is so well defined in this review (postoperative curative colorectal cancer resection), it is unlikely that the actual clinical setting in which follow‐up takes place would have any influence on the severity of disease seen or consequently on the accuracy of CEA.
Changing the setting of follow‐up could affect the accuracy of the CEA measurement if transporting blood samples taken in a community setting are stored suboptimally and there are long delays in blood reaching the laboratory. But monitoring CEA in primary care is already common practice in many countries and these potential problems have been successfully addressed. Implementation of the reference standard might also vary if patients being followed up in hospital are more likely to be referred for further investigation for reasons other than a rise in CEA. However, the Australian multicentre RCT investigating GP versus surgical follow‐up reported similar recurrence rates and times to detection, irrespective of place of follow‐up (Wattchow 2006).
For these reasons, we regard the findings of this review as applicable to follow‐up in the primary and specialist care setting.
To make sense of the meta‐analysis results and calculate false‐alarm rates, the pooled estimates of sensitivity and specificity need to be converted into predictive values, taking into account the incidence of disease in the relevant testing interval. In making this conversion, we assumed that sensitivity and specificity are constant during the follow‐up period, which seems reasonable, as we are aware of no evidence that recurrences presenting at different time points have a different propensity to release CEA.
CEA is usually measured about 14 to 15 times during the five years following primary treatment (three‐monthly for two years and then six‐monthly) and so the crudest estimate of the number of recurrences potentially detectable in each testing interval is 2% (the median incidence of recurrence in the included studies of 30% divided by 15). However, in reality incidence is not constant at each testing point, but changes with time and follow‐up interval. So, as some readers will wish to apply the findings of our review to a more precise estimate of incidence from actual clinical practice, we have reported estimates of test performance based on external data from Sargent 2007, which is the best data currently available on the incidence of recurrence at each point during follow‐up.
Authors' conclusions
Implications for practice.
The most important conclusion from this review is that CEA has inadequate sensitivity to be used as the sole method of detecting recurrence. Most national guidelines already recommend that it should be used in conjunction with another mode of diagnosis (such as CT imaging of the thorax, abdomen, and pelvis at 12 to 18 months) to pick up the remaining cases. Our review supports this recommendation. If CEA is used as the sole triage test, a significant number of cases will be missed, whatever threshold is adopted for defining a positive test.
It is important to point out that this review provides no evidence to help choose which diagnostic modality to use for this supplementary testing, nor the frequency with which it should be undertaken. However, current recommendations are consistent with the results of the FACS trial which showed that regular CEA blood testing achieves similar diagnostic performance to regular CT imaging, if supplemented with a single CT scan at 12 to 18 months (Primrose 2014).
Supplementing CEA with another testing modality to improve sensitivity also makes it easier to adopt a threshold for defining a positive test which reduces the number of patients requiring further investigation with CT imaging or other more invasive investigations. This is important for minimising unnecessary anxiety and radiation hazard for patients. It is also important in health economies such as the NHS, because of the expense and limited capacity for investigations such as CT imaging and colonoscopy.
Current standard practice (based on national recommendations) is to apply a threshold 5 µg/L. At this threshold, assuming that the proportion of patients with recurrence in any single testing period is about 2% (based on our observed prevalence of recurrence of 30% and national guidance to conduct 14 to 15 CEA tests during follow‐up), then there would be 118 false alarms and six missed cases for every 1000 patients tested. Increasing the threshold to 10 µg/L reduces the number of false alarms to 29 at a cost of six missed cases (Table 1). It is possible (although beyond the scope of this review to assess) that these missed cases may be avoided by the strategy of supplementary testing with another investigative modality as recommended above. For those interested in reviewing national recommendations on testing frequency, and the optimal threshold to apply at each time point (which need not necessarily be constant), we have included more precise estimates of test performance derived from incidence data reported by Sargent 2007 for the thresholds of 2.5 µg/L (Table 2), 5 µg/L (Table 3), and 10 µg/L (Table 4).
One potential solution to improve the diagnostic performance of CEA that is not addressed by this review is to treat CEA as a monitoring test rather than a one‐off diagnostic test. Studies excluded from this review (Characteristics of excluded studies) for not being DTA studies have investigated the utility of: CEA frequency (Carl 1983), CEA slope (Staab 1985a), CEA doubling time (Ito 2002; Koga 1999) and a CEA nomogram (Minton 1978a; Minton 1978b; Minton 1989). The authors of the FACS trial have more recently pointed out that taking account of the change in CEA results over time and setting a threshold on the basis of the trend in CEA level could have substantially improved CEA performance, with an area under the ROC curve increasing from 0.74 to 0.90 (Shinkins 2014).
Implications for research.
It is clear that measuring blood CEA has insufficient sensitivity to be used alone. Future research needs to explore the optimal timing and extent of supplementary CT imaging. It is also becoming clear that using one‐off CEA measurements is suboptimal. An analysis of the benefits of making decisions to further investigate on the basis of trends over time needs to be done, and to be augmented by cost‐benefit analysis of different strategies for the timing of monitoring tests and the optimal combination of CEA blood testing and CT imaging.
The other clear outcome from this review is the overall poor quality of reporting of diagnostic accuracy studies in this field. This poor reporting is compounded by the considerable between‐study heterogeneity and limitations of study quality. In response to the methodological limitations highlighted in this review, authors of future research investigating the diagnostic accuracy of CEA for CRC recurrence should take care to clearly report: the CEA threshold and technique used, with an indication of method accuracy and of CEA reproducibility; the reference test used in any 2 x 2 table reported; 2 x 2 tables for each time point that the index test is measured; and the timing of the CEA test in relation to the index test (preferably as individual patient data).
The lack of significant improvement in diagnostic accuracy following sensitivity analysis using studies deemed to be at low risk of bias in the QUADAS‐2 assessment also suggests that modifications to QUADAS‐2 may be warranted in assessing the quality of diagnostic tests used for follow‐up monitoring.
Acknowledgements
The review authors would like to thank Professor Paul Glasziou for his input, especially into the development of the modified QUADAS‐2 assessment tool, Dr Clare Davenport for her input into the development of theTitle Registration Form, Copy Edit Support (CES) and Henning Keinke Andersen (ME of the CCCG) for careful revision of the review.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials search strategy
| #1 | MeSH descriptor: [Colorectal Neoplasms] explode all trees |
| #2 | (colorectal near/3 (neoplas* or cancer* or tumour* or tumor* or carcinoma*)):ti,ab,kw (Word variations have been searched) |
| #3 | (colon* near/3 (neoplas* or cancer* or tumour* or tumor* or carcinoma*)):ti,ab,kw (Word variations have been searched) |
| #4 | (bowel near/3 (neoplas* or cancer* or tumour* or tumor* or carcinoma*)):ti,ab,kw (Word variations have been searched) |
| #5 | (rectal near/3 (neoplas* or cancer* or tumour* or tumor* or carcinoma*)):ti,ab,kw (Word variations have been searched) |
| #6 | (rectum near/3 (neoplas* or cancer* or tumour* or tumor* or carcinoma*)):ti,ab,kw (Word variations have been searched) |
| #7 | #1 or #2 or #3 or #4 or #5 or #6 |
| #8 | MeSH descriptor: [Carcinoembryonic Antigen] explode all trees |
| #9 | cea:ti,ab,kw (Word variations have been searched) |
| #10 | (carcinoembryonic near/3 antigen*):ti,ab,kw (Word variations have been searched) |
| #11 | (carcinoembryonic near/3 antibod*):ti,ab,kw (Word variations have been searched) |
| #12 | (carcino‐embryonic near/3 antigen*):ti,ab,kw (Word variations have been searched) |
| #13 | (carcino‐embryonic near/3 antibod*):ti,ab,kw (Word variations have been searched) |
| #14 | #8 or #9 or #10 or #11 or #12 or #13 |
| #15 | #7 and #14 |
Appendix 2. MEDLINE search strategy
| 1 | colorectal neoplasms/ or exp adenomatous polyposis coli/ or exp colonic neoplasms/ or colorectal neoplasms, hereditary nonpolyposis/ or exp rectal neoplasms/ | 142383 |
| 2 | (colorectal adj3 (neoplas* or cancer? or tumour? or tumor? or carcinoma?)).ti,ab. | 69267 |
| 3 | (colon* adj3 (neoplas* or cancer? or tumour? or tumor? or carcinoma?)).ti,ab. | 56720 |
| 4 | (bowel adj3 (neoplas* or cancer? or tumour? or tumor? or carcinoma?)).ti,ab. | 3988 |
| 5 | (rectal adj3 (neoplas* or cancer? or tumour? or tumor? or carcinoma?)).ti,ab. | 18409 |
| 6 | (rectum adj3 (neoplas* or cancer? or tumour? or tumor? or carcinoma?)).ti,ab. | 4598 |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 | 179150 |
| 8 | Carcinoembryonic Antigen/ | 13372 |
| 9 | cea.ti,ab. | 16371 |
| 10 | (carcinoembryonic adj3 antigen?).ti,ab. | 11442 |
| 11 | (carcinoembryonic adj3 antibod*).ti,ab. | 622 |
| 12 | (carcino‐embryonic adj3 antigen?).ti,ab. | 431 |
| 13 | (carcino‐embryonic adj3 antibod*).ti,ab. | 13 |
| 14 | 8 or 9 or 10 or 11 or 12 or 13 | 23958 |
| 15 | Neoplasm Recurrence, Local/ | 79823 |
| 16 | Recurrence/ | 155149 |
| 17 | recur*.ti,ab. | 381384 |
| 18 | relaps*.ti,ab. | 116217 |
| 19 | treatment failure/ | 25585 |
| 20 | Reoperation/ | 63998 |
| 21 | Follow‐Up Studies/ and Postoperative Care/ | 5767 |
| 22 | reoperat*.ti,ab. | 23840 |
| 23 | ((local or distant) adj2 failure).ti,ab. | 3371 |
| 24 | ((therap* or treatment or surg*) adj3 fail*).ti,ab. | 58705 |
| 25 | ((therap* or treatment or surg*) adj3 (respond* or response*)).ti,ab. | 116904 |
| 26 | ((postoperat* or post‐operat* or postsurg* or post‐surg* or posttreat* or post‐treat* or posttherap* or post‐therap*) adj5 follow up).ti,ab. | 16723 |
| 27 | ((postoperat* or post‐operat* or postsurg* or post‐surg* or posttreat* or post‐treat* or posttherap* or post‐therap*) adj5 surveillance).ti,ab. | 1277 |
| 28 | ((postoperat* or post‐operat* or postsurg* or post‐surg* or posttreat* or post‐treat* or posttherap* or post‐therap*) adj5 monitor*).ti,ab. | 3604 |
| 29 | 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 | 802827 |
| 30 | 7 and 14 and 29 | 1993 |
| 31 | 7 and 14 | 6353 |
| 32 | limit 31 to "reviews (maximizes specificity)" | 41 |
| 33 | 30 not 32 | 1966 |
Appendix 3. Embase search strategy
| 1 | exp colon cancer/ or exp rectum cancer/ | 172220 |
| 2 | (colorectal adj3 (neoplas* or cancer? or tumour? or tumor? or carcinoma?)).ti,ab. | 97898 |
| 3 | (colon* adj3 (neoplas* or cancer? or tumour? or tumor? or carcinoma?)).ti,ab. | 75721 |
| 4 | (bowel adj3 (neoplas* or cancer? or tumour? or tumor? or carcinoma?)).ti,ab. | 5761 |
| 5 | (rectal adj3 (neoplas* or cancer? or tumour? or tumor? or carcinoma?)).ti,ab. | 26610 |
| 6 | (rectum adj3 (neoplas* or cancer? or tumour? or tumor? or carcinoma?)).ti,ab. | 5978 |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 | 234787 |
| 8 | carcinoembryonic antigen/ | 25911 |
| 9 | cea.ti,ab. | 22520 |
| 10 | (carcinoembryonic adj3 antigen?).ti,ab. | 13394 |
| 11 | (carcinoembryonic adj3 antibod*).ti,ab. | 657 |
| 12 | (carcino‐embryonic adj3 antigen?).ti,ab. | 617 |
| 13 | (carcino‐embryonic adj3 antibod*).ti,ab. | 21 |
| 14 | 8 or 9 or 10 or 11 or 12 or 13 | 36255 |
| 15 | cancer recurrence/ or tumor recurrence/ | 119064 |
| 16 | recurrent disease/ or relapse/ | 192303 |
| 17 | recur*.ti,ab. | 523223 |
| 18 | relaps*.ti,ab. | 174290 |
| 19 | exp treatment failure/ | 82867 |
| 20 | Reoperation/ | 53394 |
| 21 | follow up/ and (postoperative care/ or postoperative period/) | 38038 |
| 22 | reoperat*.ti,ab. | 31321 |
| 23 | ((local or distant) adj2 failure).ti,ab. | 4986 |
| 24 | ((therap* or treatment or surg*) adj3 fail*).ti,ab. | 83522 |
| 25 | ((therap* or treatment or surg*) adj3 (respond* or response*)).ti,ab. | 167374 |
| 26 | ((postoperat* or post‐operat* or postsurg* or post‐surg* or posttreat* or post‐treat* or posttherap* or post‐therap*) adj5 follow up).ti,ab. | 23063 |
| 27 | ((postoperat* or post‐operat* or postsurg* or post‐surg* or posttreat* or post‐treat* or posttherap* or post‐therap*) adj5 surveillance).ti,ab. | 1797 |
| 28 | ((postoperat* or post‐operat* or postsurg* or post‐surg* or posttreat* or post‐treat* or posttherap* or post‐therap*) adj5 monitor*).ti,ab. | 4961 |
| 29 | 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 | 1107887 |
| 30 | 7 and 14 and 29 | 2994 |
| 31 | (meta‐analysis or systematic review or MEDLINE).tw. | 144743 |
| 32 | 7 and 14 and 31 | 78 |
| 33 | 30 not 32 | 2952 |
Appendix 4. Science Citation Index & Conference Proceedings Citation Index ‐ Science search strategy:
| #1 |
TOPIC: ((colorectal NEAR/3 (neoplas* or cancer* or tumour* or tumor* or carcinoma*))) ORTOPIC: ((colon* NEAR/3 (neoplas* or cancer* or tumour* or tumor* or carcinoma*))) ORTOPIC: ((bowel NEAR/3 (neoplas* or cancer* or tumour* or tumor* or carcinoma*))) ORTOPIC: ((rectal NEAR/3 (neoplas* or cancer* or tumour* or tumor* or carcinoma*))) ORTOPIC: ((rectum NEAR/3 (neoplas* or cancer* or tumour* or tumor* or carcinoma*))) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
189,742 |
| #2 |
TOPIC: (cea) ORTOPIC: ((carcinoembryonic NEAR/3 antigen*)) ORTOPIC: ((carcinoembryonic NEAR/3 antibod*)) ORTOPIC: ((carcino‐embryonic NEAR/3 antigen*)) ORTOPIC: ((carcino‐embryonic NEAR/3 antibod*)) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
23,879 |
| #3 |
TOPIC: (recur*) ORTOPIC: (relaps*) ORTOPIC: (reoperat*) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
511,568 |
| #4 |
TOPIC: (((local or distant) NEAR/2 failure)) ORTOPIC: (((therap* or treatment or surg*) NEAR/3 fail*)) ORTOPIC: (((therap* or treatment or surg*) NEAR/3 (respond* or response*))) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
200,865 |
| #5 |
TOPIC: (((postoperat* or post‐operat* or postsurg* or post‐surg* or posttreat* or post‐treat* or posttherap* or post‐therap*) NEAR/5 "follow up")) ORTOPIC: (((postoperat* or post‐operat* or postsurg* or post‐surg* or posttreat* or post‐treat* or posttherap* or post‐therap*) NEAR/5 surveillance)) ORTOPIC: (((postoperat* or post‐operat* or postsurg* or post‐surg* or posttreat* or post‐treat* or posttherap* or post‐therap*) NEAR/5 monitor*)) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
17,719 |
| #6 | #5 OR #4 OR #3 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
699,223 |
| #7 | #6 AND #2 AND #1 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
1,518 |
Appendix 5. Operational guidance for modified QUADAS‐2 tool
Unless otherwise specified, each item must be explicitly reported to achieve a “yes” answer.
| DOMAIN 1: Patient Selection | ||
| A: Risk of Bias | ||
| 1. Was a consecutive or random sample of patients enrolled? Yes/No/Unclear | ||
| 2. Did the study avoid inappropriate exclusions? | ||
| Yes | Patients are included in follow‐up post radical CRC resection, OR Exclusions was justified in the text and reviewers reached consensus on the appropriateness of any exclusions. Exclusions based on patient characteristics allowing subgroup analysis (e.g. tumour grade) should be deemed appropriate |
|
| No | Criteria for “yes” not achieved. | |
| Unclear | Exclusions not reported clearly. | |
| OVERALL RISK OF BIAS: LOW/HIGH/UNCLEAR | ||
| B: Applicability | ||
| 1. Is there concern that the included patients do not match the review question? | ||
| Yes | Patients are not undergoing follow‐up post radical CRC resection including CEA measurement. | |
| No | Patients are undergoing follow‐up post radical CRC resection including CEA measurement. | |
| Unclear | The included population is not defined. | |
| OVERALL CONCERN REGARDING APPLICABILITY: LOW/HIGH/UNCLEAR | ||
| DOMAIN 2: Index Tests | ||
| A: Risk of Bias | ||
| 1. If a threshold was used, was it pre‐specified? Yes/No/Unclear | ||
| 2. Is the same method and instrument used for all CEA measurements? Yes/No/Unclear | ||
| 3. Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? Yes/No/Unclear | ||
| 4. Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? Yes/No/Unclear | ||
| OVERALL RISK OF BIAS: LOW/HIGH/UNCLEAR | ||
| B: Applicability | ||
| 1. Is there concern that the index test, its conduct, or interpretation differ from the review question? | ||
| Yes | Blood CEA is not interpreted as a stand‐alone test to trigger investigation for CRC recurrence | |
| No | Blood CEA is interpreted as a stand‐alone test to trigger investigation for CRC recurrence | |
| Unclear | It is unclear whether the index test differs from the review question | |
| OVERALL CONCERN REGARDING APPLICABILITY: LOW/HIGH/UNCLEAR | ||
| DOMAIN 3: Reference Standard | ||
| A: Risk of Bias | ||
| 1. Is the reference standard likely to correctly classify the target condition? ‐ can we confidently exclude recurrence on the basis of no clinical detection of recurrence when we are assessing the utility of CEA at detecting asymptomatic recurrence amenable to resection? | ||
| Yes | An appropriate reference standard (as defined in the protocol) is used. | |
| No | An inappropriate reference standard is used | |
| Unclear | The reference standard used is not clearly specified. | |
| 2. Were the reference standard results interpreted without knowledge of the results of the index test? ‐ If tests are done as part of a follow‐up regime it must not be assumed that the interpretation of each test is independent of another. It must be clearly stated when reference test interpretation occurred. | ||
| Yes | The reference standard results were interpreted without knowledge of the index test(s). | |
| No | The reference standard results were interpreted with knowledge of the index test(s). | |
| Unclear | It is not clear whether interpretation was blinded or not. | |
| OVERALL RISK OF BIAS: LOW/HIGH/UNCLEAR | ||
| B: Applicability | ||
| 1. Is there concern that the target condition as defined by the reference standard does not match the review question? Yes/No/Unclear | ||
| OVERALL CONCERN REGARDING APPLICABILITY: LOW/HIGH/UNCLEAR | ||
| DOMAIN 4: Flow and Timing | ||
| A: Risk of Bias | ||
| 1. Was the index test repeated prior to the reference standard? Yes/No/Unclear | ||
| 2. Was the the timing between index test(s) and reference standard ascertainable? | ||
| Yes | The timing was ascertainable. | |
| Unclear | Not reported, variable or could not be clearly determined | |
| 3. Did all included patients who had at least one CEA measurement receive a reference standard? Yes/No/Unclear | ||
| 4. Did patients receive the same reference standard? | ||
| Yes | >95% of patients received the same reference standard regardless of index test results or place within a follow‐up schedule. | |
| No | >95% of patients did not receive the same reference standard regardless of index test results, or place within the follow‐up schedule. | |
| Unclear | It is unclear whether all the included patients received same reference standard regardless of index test results | |
| 5. Were all patients included in the analysis? Yes/No/Unclear | ||
| OVERALL RISK OF BIAS: LOW/HIGH/UNCLEAR | ||
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 CEA ‐ all thresholds | 52 | 9717 |
| 2 CEA at 2.5µg/L | 7 | 1515 |
| 3 CEA at 5µg/L | 23 | 4585 |
| 4 CEA at 10µg/L | 7 | 1607 |
1. Test.
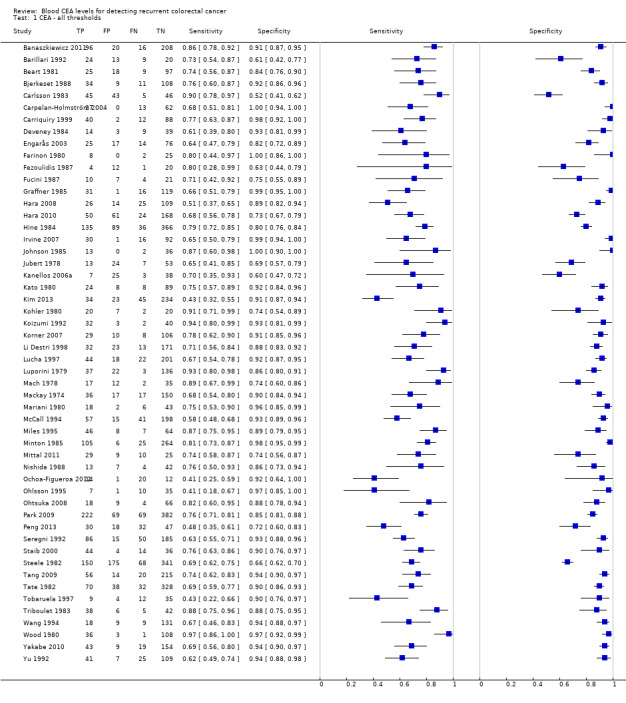
CEA ‐ all thresholds.
2. Test.
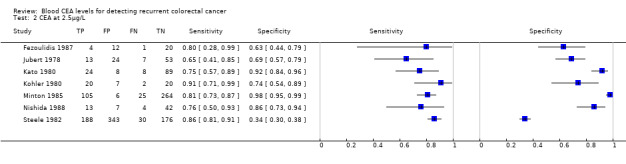
CEA at 2.5µg/L.
3. Test.
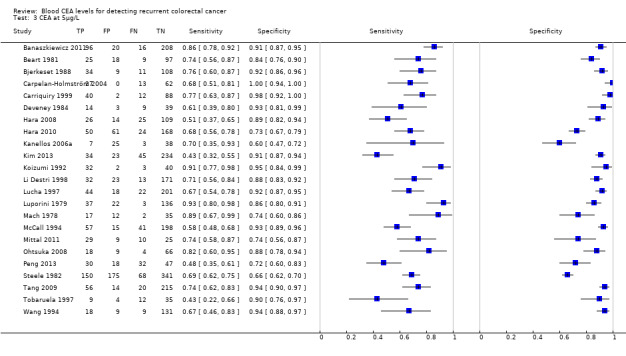
CEA at 5µg/L.
4. Test.
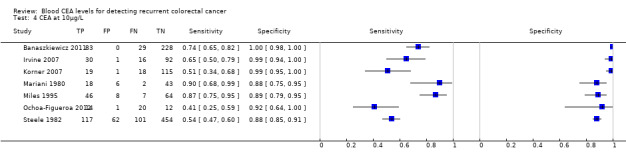
CEA at 10µg/L.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Banaszkiewicz 2011.
| Study characteristics | |||
| Patient sampling |
Country Poland Study design Retrospective casenote review Setting Hospital Dates of data collection N/R Population (n) 965 Inclusion criteria Patients after radical surgery in whom prognosis following a possible second operation was good Exclusion criteria Non‐radical surgery or concomitant disease making survival of a second operation unlikely Participants included (n) 340 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A ‐ D Perioperative Investigations done to ensure no residual disease Endoscopic polypectomy Chemotherapy/radiotherapy? Radical Recurrences (n) 112 Site of recurrences Liver 44, Local 32, Lung 7, Disseminated 12, other 6, 2 sites 11 |
||
| Index tests |
CEA timing CEA 3, 6, 12 months, then once a year up to 5 years CEA technique N/R CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R |
||
| Target condition and reference standard(s) |
Follow‐up schedule Follow‐up visits at 3, 6, 12 months, then once a year up to 5 years. Follow‐up schedule included patient’s history and physical examination, measurement of CEA serum concentration and classic colonoscopy |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Barillari 1992.
| Study characteristics | |||
| Patient sampling |
Country Italy Study design Prospective Setting Hospital Dates of data collection N/R Population (n) 66 Inclusion criteria Rectal cancer treated for cure Exclusion criteria N/R Participants included (n) 66 |
||
| Patient characteristics and setting |
Age range 62.3 yrs (mean) Smoking status N/R Site of primary tumour rectum Stage of primary tumour 6 Stage A, 32 Stage B, 28 Stage C Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 33 Site of recurrences Local 10, Metastatic 25 (Lungs 3, peritoneum 10,bones 2,liver 21, multiple 8) |
||
| Index tests |
CEA timing 3‐monthly CEA CEA technique CEA was analysed using a direct radioimmunologic method (CEA‐PR; Sorin Biomedica) CEA threshold 3 µg/L Definition of positive Any elevation of 1 of the antigen levels greater than the limit defined by the between assay coefficient of variation (calculated on the basis of 2 standard deviations) was defined as significant, and the assay was repeated after 10 days. Which CEA value (s) used? Repeated value. |
||
| Target condition and reference standard(s) |
Follow‐up schedule 3‐monthly to 60 months: blood CEA, TPA, CA19.9 and clinical exam. 6, 18, 30, 42, 54 months: USS Abdomen, CXR, Barium Enema. 12, 24, 36, 48, 60 months: colonoscopy, CT body. 6, 18, 30, 42 months: Bone scan. Reference standard Abdominal or total body CT, a chest x‐ray examination, a bone scan, an endoscopy, and a clinical examination were performed. An exploratory laparotomy was performed when all three markers were elevated, even if recurrence was not confirmed by total body CT scan and clinical examinations |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Yes | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Beart 1981.
| Study characteristics | |||
| Patient sampling |
Country USA Study Design Prospective Setting Department of Surgery and Oncology, Mayo Clinic and Mayo Foundation Dates of data collection 1976 ‐ 1986 Population (n) 149 Inclusion criteria Resection of Dukes' B2 or C colorectal carcinoma was followed from the time of operation until the time of tumour recurrence or writing the published paper Exclusion criteria N/R Participants included (n) 149 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Colon Stage of primary tumour Dukes B or C Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Some got radiotherapy, chemotherapy, and/or immunotherapy. Numbers not specified Recurrences (n) 34 Site of recurrences Liver metastasis 14, Chest 6, Pelvic disease 12 |
||
| Index tests |
CEA Timing At least every 15 week CEA technique N/R CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R |
||
| Target condition and reference standard(s) |
Follow‐up schedule At least every 15 weeks a complete history was taken and physical examination was carried out. A CXR was obtained, and laboratory determinations included complete blood count, alkaline phosphatase, SGOT, SGPT, and CEA. LDH and proctoscopic examinations were done every 6 months. A BE and liver scanning were done annually Reference standard Additional tests including CT, laparoscopy, liver biopsy, and abdominal exploration were ordered as indicated by the history, physical examination, or positive laboratory results. All recurrent tumours were documented histologically |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| High | |||
Bjerkeset 1988.
| Study characteristics | |||
| Patient sampling |
Country Norway Study Design Prospective Setting Hospital Dates of data collection 1976 ‐ 1979 Population (n) 244 Inclusion criteria colorectal cancer resection operated for cure Exclusion criteria Did not survive resection, residual disease, resection margins not clear, pre‐ and post‐op CEA determination Participants included (n) 164 |
||
| Patient characteristics and setting |
Age range N/R Smoking status Some, but not quantified Site of primary tumour Colorectal Stage of primary tumour Dukes A 58, B 76, C 48, D 50, unknown 12 Perioperative Investigations done to ensure no residual disease Clear resection margins, no residual disease Chemotherapy / Radiotherapy? 22 Dukes B ‐ C randomised to 5‐year follow‐up; 21 had preoperative external radiation Recurrences (n) 47 Site of recurrences Liver 12, Lungs 10, Local 8, Local and distant 5, carcinomatosis 6, multiple 6 |
||
| Index tests |
CEA timing 3, 6, 12, 18, 24, then yearly CEA technique Roche Ria test‐ repeat if raised, if repeat raised then test as described in follow‐up CEA threshold 3.5 µg/L Definition of positive Transient Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule 3, 6, 12, 18, 24 months then yearly CEA, clinical, biochemical, immunological (immunoglobulins and complement). CXR. Colonoscopy 6, 18 months. DCBE 1, 3, 5 years. "complimentary radiographic, scintographic, ultrasonographic added if indicated." Reference standard If nothing found investigating for increased CEA, then a second‐look operation was performed (laparotomy and biopsy) in 23, liver imaging 8, autopsy 5, clinical course 6 |
||
| Flow and timing |
Timing of CEA vs reference standard (days) as per follow‐up schedule |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Was the index test repeated prior to the reference standard? | Yes | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| High | |||
Carlsson 1983.
| Study characteristics | |||
| Patient sampling |
Country Sweden Study design Prospective study Setting Hospital Dates of data collection N/R Population (n) 163 Inclusion Criteria Curative operation for colorectal cancer Exclusion Criteria Advanced age, moving away, death 3 months postop Participants Included (n) 139 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour N/R Perioperative Investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 50 Site of recurrences N/R |
||
| Index tests |
CEA timing Blood tests 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 60 months post‐1977‐ blood tests 3, 6, 12, 18, 24, 30, 36, 42, 48, 60 months CEA technique Direct radio immunoassay method developed at the Department of Nuclear Medicine, Malmo General Hospital CEA threshold 3 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Follow‐up schedule Until 1977: Follow‐up exam and rectoscopy 3, 6, 9, 12, 15, 18 ,21, 24, 26, 42, 48, 60 months. Double contrast enema 3, 12, 24, 36, 48, 60 months. CXR and blood tests 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 60 months. From 1977: Physical exam and rectoscopy 3, 12, 24, 36, 48, 60 months. Double contrast enema 3, 12, 24, 36, 48, 60 months. CXR and blood tests 3, 6, 12, 18, 24, 30, 36, 42, 48, 60 months |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Carpelan‐Holmström 2004.
| Study characteristics | |||
| Patient sampling |
Country Finland Study Design Retrospective Setting Hospital Dates of data collection N/R Population (n) 354 Inclusion criteria Curative surgery, but unclear Exclusion criteria Palliative, followed up elsewhere, no preoperative serum samples, no serum at the time of recurrence Participants included (n) 102 |
||
| Patient characteristics and setting |
Age range 29 ‐ 88 yrs Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A ‐ D (16 Dukes A, 45 Dukes B, 34 Dukes C, and 7 Dukes D) Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 40 Site of recurrences Local 17, Liver 10, Various 13 |
||
| Index tests |
CEA timing N/R CEA technique CEA was measured with a time‐resolved immunofluorometric assay (AutoDELFIA®; Wallac, Turku, Finland). The detection limit of the assay is 0.2 µg/L, and the inter‐assay coefficient of variation is 3% in the concentration range 3 – 90 µg/L (total CV 4%) CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Reference standard Clinical follow‐up |
||
| Flow and timing |
Timing of CEA vs reference standard (days) Unclear |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Carriquiry 1999.
| Study characteristics | |||
| Patient sampling |
Country Uruguay Study design Retrospective casenote review. Setting Hospital Dates of data collection 1985 ‐ 1998 Population (n) 209 Inclusion criteria Histologically proven colorectal carcinoma, 3 postoperative CEA measurements, minimum period of follow‐up 24 months Exclusion criteria Postoperative death and Stage IV (unless radical resection of synchronous liver metastases), no preop CEA Participants Included (n) 142 |
||
| Patient characteristics and setting |
Age range 30 ‐ 91 yrs Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour TNM staging system: 32 patients had Stage I, 57 had Stage II, 86 had Stage III, and 27 had Stage IV disease Perioperative Investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 52 Site of recurrences N/R |
||
| Index tests |
CEA timing CEA 3‐monthly for 24 months, 4‐monthly for yrs 3 ‐ 4, and once per year after this (strict adherence in only 42 patients) CEA technique Serum concentrations of CEA were determined by a standard commercially‐available immunoenzymatic assay CEA threshold 5 µg/L Definition of positive 2 consecutive values above 5 regarded abnormal; repeated at 2 ‐ 4 weeks Which CEA value (s) used? Repeated value at the time of recurrence |
||
| Target condition and reference standard(s) |
Follow‐up schedule CEA 3‐monthly for 24 months, 4‐monthly for yrs 3 ‐ 4, and once per year after this (strict adherence in only 42 patients). Clinical follow‐up, rectoscopy and/or colonoscopy at 1 yr and 3 yrs Reference standard USS/MRI/CT indicated on basis of raised CEA or clinical suspicion. CEA second‐look surgery never used |
||
| Flow and timing |
Timing of CEA vs reference standard (days) as per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Yes | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Deveney 1984.
| Study characteristics | |||
| Patient sampling |
Country USA Study design Prospective Setting Hospital Dates of data collection starting in 1978 Population (n) N/R Inclusion criteria Resection for curable adenocarcinoma of the colon or rectum Exclusion criteria Dukes D Participants included (n) 65 |
||
| Patient characteristics and setting |
Age range 67 yrs mean Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour 8 Dukes A tumours, 34 had Dukes B tumours, and 20 had Dukes C tumours Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Some got radio/chemotherapy Recurrences (n) 23 Site of recurrences N/R |
||
| Index tests |
CEA timing 3‐monthly in yr 1, then 6‐monthly to year 5 CEA technique N/R CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule 3‐monthly in year 1, then 6‐monthly to year 5: clinical history, examination, FOBT, LFT, CEA. 6‐monthly CXR, CT abdomen, total colonoscopy. BE at 6 and 12 months then annually Reference standard A positive finding on any test prompted additional confirmatory tests, including laparotomy, thoracotomy,or percutaneous CT‐directed biopsy |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Engarås 2003.
| Study characteristics | |||
| Patient sampling |
Country Sweden Study design Prospective Setting Hospital Dates of data collection 1998 ‐ 1990 Population (n) 151 Inclusion criteria Surgery with curative intent with 5 years follow‐up Exclusion criteria N/R Participants Included (n) 132 |
||
| Patient characteristics and setting |
Age range 27 ‐ 75 Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Duke A 11, B 76, C 43,D 1, Undefined 1 Perioperative investigations done to ensure no residual disease Not specified Chemotherapy/radiotherapy? N/R Recurrences (n) 39 Site of recurrences N/R |
||
| Index tests |
CEA timing Monthly during year 1 and then at 18 and 24 months CEA technique Delfia® test kits (Wallac Oy, Turku, Finland). The accuracy of the assays was assessed by analysis of 2 control samples in each assay and by measurement of the coefficient of variation by duplicate analyses of the samples CEA threshold 5.6 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule Monthly outpatient clinic visit during year 1, serum tests monthly during year 1, then 18 and 24 months. Clinical examinations at 1 year and 2 year with CXR, Sigmoidoscopy, BE, and CT Liver. Reference standard Radiologic and/or endoscopic investigations at surgery or post mortem |
||
| Flow and timing |
Timing of CEA vs reference standard (days) as per follow‐up schedule |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Farinon 1980.
| Study characteristics | |||
| Patient sampling |
Country Italy Study design Retrospective Setting Hospital Dates of data collection N/R Population (n) 87 Inclusion criteria Preoperative CEA test > 6, operated in with end‐to‐end anastomosis Exclusion criteria N/R Participants included (n) 35 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A 3, B 26, C 6 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 10 Site of recurrences N/R |
||
| Index tests |
CEA timing 3 monthly CEA technique CEA radioimmunoassay direct method CEA threshold 6 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule CEA and colonoscopy every 3 months Reference standard Second look surgery if not clear from CEA + colonoscopy. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Fezoulidis 1987.
| Study characteristics | |||
| Patient sampling |
Country Germany Study design Prospective Setting Hospital Dates of data collection 1984 ‐ 1986 Population (n) 48 Inclusion criteria radical surgery Exclusion criteria No exclusion criteria were defined; results from all 48 participants are included in the study Participants Included (n) 48 |
||
| Patient characteristics and setting |
Age range Study does not describe age bands; median age is 64 Smoking status N/R Site of primary tumour Rectum Stage of primary tumour Dukes A 9, Dukes B 16, Dukes C1 19, Dukes C2 4 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 5 Site of recurrences 5 local rectal recurrences |
||
| Index tests |
CEA timing 6 weeks, (then 3‐monthly); main text of the study only mentions that CEA was measured postoperatively; ?Only once; it is not clear if there was a sequence of measurements. Table 4 looks more like a one‐off CEA technique Unknown CEA threshold 2.5 µg/L Definition of positive Unclear Which CEA value (s) used? Probably 4 ‐ 6 weeks postoperatively |
||
| Target condition and reference standard(s) |
Follow‐up schedule 4 ‐ 6 weeks postoperatively, then 3‐monthly clinical examination, CT, and CEA Reference standard 4 patients underwent CT guided biopsy but at unknown stage |
||
| Flow and timing |
Timing of CEA vs reference standard (days) Unclear |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Fucini 1987.
| Study characteristics | |||
| Patient sampling |
Country Italy Study design Retrospective Setting Hospital Dates of data collection 1979 ‐ 1983 Population (n) 64 Inclusion criteria Potentially curative surgery Exclusion criteria Died or demonstrated recurrence before 1982 (introduction of TPA and CA19‐9 assays) Participants Included (n) 52 |
||
| Patient characteristics and setting |
Age range 40 ‐ 77 Smoking status 1 smoker Site of primary tumour Colorectal Stage of primary tumour Dukes A: 28, B 17, C19 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 10 Site of recurrences N/R |
||
| Index tests |
CEA timing As per protocol, then repeated within 2 weeks considered positive CEA technique Double antibody method (CEA‐PR, Sorin Biomedica) CEA threshold 20 (95% control group) Definition of positive 2 consecutive samples Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Follow‐up schedule CEA + TPA + CA19‐9, clinical exam at 3, 7, 14 days then 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 54, 60 months. Blood count at 3, 6, 12, 18, 24, 26, 48, 60 months. Liver USS at 3, 6, 18, 30, 36, 42, 48, 54, 60 months. CXR 3, 6, 12, 18, 24, 30, 36, 48, 60 months. DCBE at 18, 42, 60 months. Colonoscopy 6, 12, 24, 36, 48, 60 months. APCT 12, 24 months. Random perineal percutaneous needle biopsy (rectal cancer) 6, 12, 18, 24, 36, 48, 60 |
||
| Flow and timing |
Timing of CEA vs reference standard (days) Sensitivity uses CEA at the time of recurrence, specificity uses CEA over threshold at any time during follow‐up |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Graffner 1985.
| Study characteristics | |||
| Patient sampling |
Country Sweden Study design Prospective Setting Hospital Dates of data collection N/R Population (n) 190 Inclusion criteria Curative resection, age able to attend follow‐up Exclusion criteria Moved from area, died of intercurrent illness, did not follow the schedule Participants included (n) 167 |
||
| Patient characteristics and setting |
Age range 55 ‐ 74 Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A 24, B 89, C 77 Perioperative Investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 47 Site of recurrences Liver 18, anastomotic 4, perineal 7, lungs 4, skin 5, multiple organs 8, skeleton 1 |
||
| Index tests |
CEA timing CEA every second month during the first 2 years and every third month thereafter CEA technique Radioimmunoassay CEA threshold Abnormal blood values (CEA used same method as Colleen et al 1979 "the reference value was calculated from serum sampled from 89 apparently healthy persons aged 25 to 69 years. It was 10+/‐ 2.5 ug/l (mean+/‐S.D)") or a rise of CEA levels within the normal range of more than 50% Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Follow‐up schedule CEA, ESR, haemoglobin, ALP, glutamyltranspeptidase (GGT), orosomucoid, alpha‐antitrypsin, and haptoglobin every second month during the first 2 years and every third month thereafter. Physical exam and rectoscopy 3, 6, 9, 12, 18, 24, 36, 48, 60 months. DCBE and CXR 12, 36, 60 months Reference standard CXR, CT liver, CT perineum, endoscopic investigation of anastomosis, DCBE, angiography and bone scintography in selected cases |
||
| Flow and timing |
Timing of CEA vs reference standard (days) if abnormal CEA detected reference standard triggered |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Hara 2008.
| Study characteristics | |||
| Patient sampling |
Country Japan Study design Retrospective Setting Hospital Dates of data collection 1990 ‐ 2000 Population (n) 680 Inclusion criteria Curative resection, dukes C Exclusion criteria Multiple cancers, insufficient examinations, persistent post‐op CEA, and SCC, randomised to pretest probability group Participants Included (n) 174 |
||
| Patient characteristics and setting |
Age range 60.6 ± 11.1 Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour All Dukes C‐ Stage 1 18, 2 59, 3 232, 4 39 Perioperative investigations done to ensure no residual disease Persistent CEA elevation excluded Chemotherapy/radiotherapy? No Recurrences (n) 51 Site of recurrences N/R |
||
| Index tests |
CEA timing 3‐monthly CEA technique N/R CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Follow‐up schedule All patients were followed for more than 5 years or until death with routine serum CEA examination every 3 months. USS and/or CT and CXR examinations were performed every 3 ‐ 6 months Reference standard Additional imaging was performed in patients with elevated postoperative CEA levels to determine whether recurrence was present |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Hara 2010.
| Study characteristics | |||
| Patient sampling |
Country Japan Study design Retrospective Setting Hospital Dates of data collection 1990 ‐ 2004 Population (n) 488 Inclusion criteria Stage II or III curative resection Exclusion criteria Patients with squamous cell, carcinoma, more than one cancer, or insufficient follow‐up Participants Included (n) Stage II: 167 Stage III: 136 |
||
| Patient characteristics and setting |
Age range Stage II: 68.3 ± 10.5 (38 – 92) Stage III: 63.4 ± 9.4 (44 – 88) Smoking status N/R Site of primary tumour Stage II: Colon 112, rectum 55 Stage III: Colon 89, rectum 47 Stage of primary tumour Stage II: Depth T1 0, 2 0, 3 142, 4 23 Stage III: Depth T1 3, 2 89, 3 32, 4 12 Perioperative investigations done to ensure no residual disease Not specified Chemotherapy/radiotherapy? No Recurrences (n) Stage II: 23 Stage III: 51 Site of recurrences N/R |
||
| Index tests |
CEA timing Unclear CEA technique N/R CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R |
||
| Target condition and reference standard(s) |
Follow‐up schedule All patients underwent routine serum CEA assays and radiological examination |
||
| Flow and timing |
Timing of CEA vs reference standard (days) unclear |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Hine 1984.
| Study characteristics | |||
| Patient sampling |
Country UK Study design Prospective Setting Hospital Dates of data collection N/R Population (n) 663 Inclusion criteria Radical surgery for colorectal cancer Exclusion criteria SCC anus, tumours in the appendix. 6 were lost to clinical follow‐up and 5 others were removed from the trial. Removal followed the development of unassociated conditions such as alcoholic cirrhosis which interfered with the interpretation of a significant CEA rise (3 patients) and in 2 patients the onset of psychiatric illness made the use of cancer chemotherapy inadvisable Participants Included (n) 626 |
||
| Patient characteristics and setting |
Age range 59 Smoking status Unknown Site of primary tumour 290 rectum, 373 colon Stage of primary tumour A in 38, B in 377 and C in 248 Perioperative investigations done to ensure no residual disease Not specified Chemotherapy/radiotherapy? Patients with at least 2 progressively rising CEA values of > 35 ngml‐1 but no other definite evidence of recurrent malignancy were randomised in a prospective trial of cytotoxic therapy Recurrences (n) 171 Site of recurrences N/R |
||
| Index tests |
CEA Timing At each follow‐up visit CEA Technique CEA was measured in the unextracted serum by a double antibody radio‐immunoassay as developed by Egan et al. (1972) and adapted by Laurence et al. (1972). The inter‐ and intra‐assay variation of the method was found to be < 10%. An upper limit of 15 µg/L will include 99% of a normal population and in the present study a level of > 20 µg/L was regarded as abnormal CEA threshold 20 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule 3‐monthly for for first 2 postoperative years, then 6 ‐ 12‐monthly depending on the surgeon. Full clinical examination including sigmoidoscopy was performed Reference standard recurrence was primarily made on the basis of symptoms and signs of disease confirmed by other investigations when indicated (e.g. liver scan, bone scan, biopsy). Thorough clinical examination including sigmoidoscopy. If this indicated recurrent malignancy, confirmatory investigations were ordered and management was initiated appropriate to the results. When clinical examination failed to reveal malignancy, the subsequent course of events depended on the degree of elevation of the CEA. If the level was >20ngml‐1 but <35ngml‐ 1, the test was repeated at monthly intervals until it fell below 20ngml‐1 or rose above 35ngml‐1. All patients with levels >35ngml‐1 and no clinical evidence of recurrence had a further CEA estimation, full blood count, erythrocyte sedimentation rate, liver function tests, barium enema, chest X‐ray and isotope and/or ultrasound liver scan, together with bone scan and colonoscopy where indicated. If recurrence was diagnosed from the results of these investigations then appropriate management was instituted. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) Raised CEAs were recalled to clinic within 2 months of the date of the first sample for clinical exam and sigmoidoscopoy. If no recurrence found intensified frequency of testing whilst in the 20 ‐ 35 range. If > 35 but no signs of recurrence, then chemotherapy |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Unclear | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Irvine 2007.
| Study characteristics | |||
| Patient sampling |
Country UK Study design Retrospective Setting Hospital Dates of data collection 1996 ‐ 2000 Population (n) 150 Inclusion criteria Curative surgery for colorectal cancer Exclusion criteria Palliative patients, non‐operative patients, 11 who developed metastases or recurrences within 3 months of surgery, persistently elevated CEA postoperatively (deemed non‐curative resection) Participants Included (n) 139 |
||
| Patient characteristics and setting |
Age range 22 ‐ 87 Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A 10, B 82, C 47 Perioperative investigations done to ensure no residual disease Development of metastases or recurrence within 3 months of surgery, persistently elevated CEA postoperatively Chemotherapy/radiotherapy? No Recurrences (n) 46 Site of recurrences N/R |
||
| Index tests |
CEA timing Postoperatively 3‐monthly for 2 yrs, then 6‐monthly to 5 yrs. The CEA measurements for each patient were analysed twice, once looking for a small rise in CEA and again looking for a CEA value that rose above the traditional normal limit (10 µg/L) CEA technique Bayer immunoassay, which at the levels in this study has an error rate of 2.3% CEA threshold 10 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Follow‐up schedule 6‐monthly CT for 2 years, plus CEA 3‐monthly for 2 years, then 6‐monthly to 5 years |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Johnson 1985.
| Study characteristics | |||
| Patient sampling |
Country Norway Study design Propsective Setting Hosptial + primary care Dates of data collection N/R Population (n) 93 Inclusion criteria Radical treatment for colorectal cancer Exclusion criteria Palliative, new cancers, no CEA monitoring Participants included (n) 51 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Colon 49, rectal 44 Stage of primary tumour Dukes A 28, B 27, C 21, palliative 17 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 15 Site of recurrences N/R |
||
| Index tests |
CEA timing Postoperatively, then at 3 ‐ 4‐monthly intervals CEA technique N/R CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R More data available? N/R |
||
| Target condition and reference standard(s) |
Follow‐up schedule Postoperatively, then at 3 ‐ 4 monthly intervals, rising CEA resulted in further investigation, general clinical investigations, angiography of the liver, resection. No fixed schedule |
||
| Flow and timing |
Timing of CEA vs reference standard (days) CEA triggered investigation |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Unclear | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Jubert 1978.
| Study characteristics | |||
| Patient sampling |
Country USA Study design Retrospective Setting Hospital Dates of data collection N/R Population (n) 97 Inclusion criteria Colorectal cancer Exclusion criteria N/R Participants Included (n) 97 |
||
| Patient characteristics and setting |
Age range 65 mean (39 ‐ 89) Smoking status Unknown Site of primary tumour Colon 56, rectum 41 Stage of primary tumour Dukes A 10, B 42, C 34, D 6 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? 5 chemo, 5 immuno Recurrences (n) 20 Site of recurrences 7 liver, 13 non‐liver |
||
| Index tests |
CEA timing At 6‐week intervals postoperatively CEA technique N/R CEA threshold 2.5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Follow‐up schedule CEA is done preoperatively and at six week intervals postoperatively. In addition, patients are evaluated postoperatively at 6 to 8 week intervals by physical examination and the usual laboratory and radiological tests, and where indicated, suspicions of recurrence and/or metastasis are documented histologically for the most part. Reference standard "suspicions of recurrence and/or metastasis are documented histologically for the most part". |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Kanellos 2006a.
| Study characteristics | |||
| Patient sampling |
Country Greece Study design Prospective Setting Hospital Dates of data collection 1991 ‐ 1999 Population (n) N/R Inclusion criteria Histologically proven colorectal cancer, no detectable liver metastasis, curative surgery for colorectal cancer Exclusion criteria Confirmed liver metastasis, peritoneal carcinomatosis, ascites, emergency surgery for obstruction or perforation, smokers, obstructive biliary disease or biliary surgery, or refused consent Participants Included (n) 73 |
||
| Patient characteristics and setting |
Age range 64.2 (SD: 9.7) Smoking status Non‐smokers Site of primary tumour Colorectal Stage of primary tumour Stage I 14, II 37, III 22 Perioperative investigations done to ensure no residual disease Pre‐op abdominal CT, intraoperative liver palpation to exclude liver metastases Chemotherapy/radiotherapy? 22 patients with stage III cancer had adjuvant chemo Recurrences (n) 10 Site of recurrences N/R |
||
| Index tests |
CEA timing 3‐monthly to 3 yrs, the 6‐monthly to 5 yrs CEA technique Monoclonal antibody technique, using a solid‐phase 2‐site mouse monoclonal antibody radioimmunoassay kit CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Follow‐up schedule Every 3 months for the first 3 years and every 6 months thereafter: clinical examination routine biochemical analysis, CXR, and CT. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) Simultaneous, per protocol. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Kato 1980.
| Study characteristics | |||
| Patient sampling |
Country Japan Study design Prospective Setting Hospital Dates of data collection 1977 ‐ 79 Population (n) N/R Inclusion criteria Surgically treated for adenocarcinoma of the colon or rectum with curative intent Exclusion criteria Incomplete CEA dataset Participants Included (n) 129 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A,B,C Perioperative investigations done to ensure no residual disease Not specified Chemotherapy/radiotherapy? No Recurrences (n) 32 Site of recurrences N/R |
||
| Index tests |
CEA timing Unclear CEA technique RIA kit by Dynabot CEA threshold 2.5 and 5 µg/L Definition of positive N/R Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Follow‐up schedule N/R |
||
| Flow and timing |
Timing of CEA vs reference standard (days) Unclear |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Unclear | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Kim 2013.
| Study characteristics | |||
| Patient sampling |
Country Korea Study design Retrospective Setting Hospital Dates of data collection 2005 ‐ 2009 Population (n) N/R Inclusion criteria Radical resection Exclusion criteria Patients with stage 0, I or IV cancer, insufficient follow‐up (less than 3 years), abnormal CEA in the first measurement after surgery (checked within three months after surgery), history of other cancers and/or history of preoperative concurrent chemoradiation therapy were excluded Participants Included (n) 336 |
||
| Patient characteristics and setting |
Age range Stage 111: 29 ‐ 81, Stage 11: 33 ‐ 83 Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Stage II 189, Stage III 147 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 79 Site of recurrences |
||
| Index tests |
CEA timing CEA levels were assayed with a 3‐month interval for the first 2 years and every 6 months thereafter CEA technique Immunoassay method (ADIVA Centaur XP immunoassay system, Siemen AG, Erlangen, Germany) CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule CEA levels were assayed with a 3‐month interval for the first 2 years and every 6 months thereafter. Chest CT and abdomino‐pelvic CT were performed with a 6‐month interval for the first 2 years and every year thereafter Reference standard The diagnosis of a tumour recurrence was confirmed by biopsy and radiologic evidence |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Kohler 1980.
| Study characteristics | |||
| Patient sampling |
Country USA Study design Retrospective casenote review. Setting Hospital Dates of data collection 1971 ‐ 1974 Population (n) 144 Inclusion criteria Surgically confirmed adenocarcinoma of colon or rectum Exclusion criteria N/R Participants Included (n) 49 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour N/R Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 22 Site of recurrences N/R |
||
| Index tests |
CEA timing Not clear CEA technique Hansens radioimmunoassay CEA threshold 2.5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule N/R |
||
| Flow and timing |
Timing of CEA vs reference standard (days) N/R |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Unclear | ||
| High | |||
Koizumi 1992.
| Study characteristics | |||
| Patient sampling |
Country Japan Study design Cross‐sectional with follow‐up of cases Setting Hospital Dates of data collection 1986 ‐ 1990 Population (n) 194 Inclusion criteria Unclear Exclusion criteria Cases undergoing operation later, benign colorectal disease. Participants Included (n) 77 |
||
| Patient characteristics and setting |
Age range 32 ‐ 83 Smoking status Unknown Site of primary tumour Colorectal Stage of primary tumour Unknown Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Follow‐up schedule N/R Recurrences (n) 34 Site of recurrences N/R |
||
| Index tests |
CEA timing N/R CEA technique N/R CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Reference standard Unclear |
||
| Flow and timing |
Timing of CEA vs reference standard (days) N/R |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Korner 2007.
| Study characteristics | |||
| Patient sampling |
Country Norway Study design Prospective cohort with retrospective sampling Setting Hospital Dates of data collection 1996 ‐ 1999 Population (n) 314 Inclusion criteria Surgically treated for adenocarcinoma of the colon or rectum with curative intent, age < 75 yrs, national guidelines followed Exclusion criteria Not systematically followed up for 5 years or until recurrence, incomplete CEA dataset. Dukes D Participants included (n) 153 |
||
| Patient characteristics and setting |
Age range < 75 Smoking status N/R Site of primary tumour Colon 102, rectum 50 Stage of primary tumour Dukes A 31, B 79, C 42 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 37 Site of recurrences N/R |
||
| Index tests |
CEA timing CEA 3, 6, 9, 12, 18, 24, 30, 36, 42, 48, 54, 60 months CEA technique Immunoassay kit from Abbot diagnostic IL, USA CEA threshold 4 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Follow‐up schedule CEA 3, 6, 9, 12, 18, 24, 30, 36, 42, 48, 54, 60 months. USS Liver & CXR 6, 12, 18, 24, 30, 36, 42, 48, 54, 60 months. Colonoscopy 12, 60 months. Reference standard Biopsy and/or imaging studies to confirm recurrence, or disease‐free interval of 60 months without proof of recurrence. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) not specified if different from protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Li Destri 1998.
| Study characteristics | |||
| Patient sampling |
Country Italy Study design Retrospective Setting Hospital Dates of data collection N/R Population (n) 364 Inclusion criteria Radical surgery for colorectal cancer CEA measured postoperatively Exclusion criteria N/R Participants included (n) 239 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour N/R Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 45 Site of recurrences hepatic 18, non‐hepatic 22, mixed 5 |
||
| Index tests |
CEA timing CEA monitoring, conducted every 3 months for years 1, 2, and 3, every 6 months for years 4 and 5, then yearly up to year 10 CEA technique The antigen was determined using the radioimmunoassay method. CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R |
||
| Target condition and reference standard(s) |
Follow‐up schedule CEA monitoring, conducted every 3 months for years 1, 2, and 3, every 6 months for years 4 and 5, then yearly up to year 10. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) N/R |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Lucha 1997.
| Study characteristics | |||
| Patient sampling |
Country USA Study design Retrospective Setting Hospital Dates of data collection 1981 ‐ 1985 Population (n) N/R Inclusion criteria Newly diagnosed colorectal cancer undergoing operative resection for cure (Astler Coller A,B,C) Exclusion criteria Metastatic disease and synchronous cancers Participants Included (n) 285 |
||
| Patient characteristics and setting |
Age range 66.8 (range, 31 ‐ 96) Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Astler‐Coller Stage A 39, B1 57, B2 109, C1 15, C2 60 Perioperative investigations done to ensure no residual disease Intraoperative criteria for curative resection included absence of gross residual disease Chemotherapy/radiotherapy? No Recurrences (n) 66 Site of recurrences N/R |
||
| Index tests |
CEA timing 2‐monthly for 2 years, 3‐monthly for year 3, 6‐monthly for years 4 ‐ 5, annually afterwards. A repeat CEA was performed in patients who had an abnormal rise CEA technique Abbott CEA threshold 5 µg/L Definition of positive 2 consecutive samples Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Follow‐up schedule 2 monthly for 2 years, 3 monthly for year 3, 6 monthly for years 4 and 5, annually afterwards. A detailed history and physical examination was performed, and CEA levels were monitored at each encounter. Reference standard Two successive CEA elevations were investigated with diagnostic imaging and / or endoscopy when indicated. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
Luporini 1979.
| Study characteristics | |||
| Patient sampling |
Country Italy Study design retrospective Setting Hospital Dates of data collection 1974 ‐ 1976 Population (n) 204 Inclusion criteria Large bowel malignancies, radical resection Exclusion criteria N/R Participants Included (n) 198 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Large intestine Stage of primary tumour Dukes A ‐ B 11, C1 39, C2 30, CH (liver involvement) 32 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Yes Recurrences (n) 62 Site of recurrences N/R |
||
| Index tests |
CEA timing N/R CEA technique N/R CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R More data available? N/R |
||
| Target condition and reference standard(s) |
Follow‐up schedule N/R |
||
| Flow and timing |
Timing of CEA vs reference standard (days) Unclear |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Mach 1978.
| Study characteristics | |||
| Patient sampling |
Country Switzerland Study design Retrospective Setting Hospital Dates of data collection 1977 ‐ 1978 Population (n) 200 Inclusion criteria Histologically confirmed diagnosis of adenocarcinoma of colon or rectum Exclusion criteria Incomplete tumour resection Participants Included (n) 66 |
||
| Patient characteristics and setting |
Age range 65 Smoking status 12 patients who had CEA levels fluctuating around the normal limit of 5 ng/ml during the last 2 or 3 years without a definite rise of CEA levels and also without clinical evidence of tumour relapse. Among them were 6 heavy smokers Site of primary tumour Colorectal Stage of primary tumour Dukes ABCD Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? 2 of the recurrences were reported to have chemo Recurrences (n) 19 Site of recurrences N/R |
||
| Index tests |
CEA timing 3‐monthly CEA technique The radioimmunoassay of CEA was performed according to the method of Goldz as modified by Mach el al. The major modification was that duplicates of 1 ml of plasma (10 ml of blood was collected in tubes containing 33 mg of dry E.D.T.A. K3) instead of 5 ml of serum, were extracted in perchloric acid. The sensitivity of the test is 1 µg/L. The normal value determined in 90 nonsmoking blood bank donors, unselected for age and sex, ranged between 0 to 3.5 µg/L. Our CEA assay is similar to the Hansen method,'but our numerical values are slightly higher and should be divided by a factor of 1.5 in order to make a direct comparison. CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow up schedule N/R |
||
| Flow and timing |
Timing of CEA vs reference standard (days) N/R |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Mackay 1974.
| Study characteristics | |||
| Patient sampling |
Country UK Study design Prospective Setting Hospital Dates of data collection Approx 1970 ‐ 1973 Population (n) N/R Inclusion criteria Surgically resected colorectal carcinoma (a) Their operations were considered to be clinically curative. (b) Pathological staging showed the carcinoma to fall into Dukes (1950) A, B, or C category. (c) The participants had been followed up for at least 12 months and most for 24 months either after the operation or after the first plasma CEA assay Exclusion criteria Inadequate follow‐up time or because the plasma CEA values had risen temporarily to or remained at levels between 20 and 40 µg/L Participants included (n) 220 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Duke ABC Perioperative investigations done to ensure no residual disease Unclear Chemotherapy/radiotherapy? N/R Recurrences (n) 53 Site of recurrences Liver 31, lung 3, peritoneum and pelvis 17, bones 2, local 6, skin 2 |
||
| Index tests |
CEA timing 3 monthly CEA technique Double‐antibody radioimmunoassay CEA threshold 40 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule N/R Reference standard Recurrence of tumour was detected clinically or by radioisotope scanning or other radiographic techniques. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) Reference standard triggered by a rise in CEA |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
Mariani 1980.
| Study characteristics | |||
| Patient sampling |
Country Italy Study design Prospective Setting Hospital Dates of data collection N/R Population (n) N/R Inclusion criteria Histologically confirmed adenocarcinoma submitted for resection (included ± pre‐op measurements) Exclusion criteria Heavy smokers (> 15 cigarettes/day) and patients with known, or suspected alcoholic hepatitis Participants included (n) 69 |
||
| Patient characteristics and setting |
Age range 60.2 ± 11.6 yrs Smoking status Excluded Site of primary tumour Colorectal Stage of primary tumour Dukes A 5, B 18, C 14, D 2. Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 24 Site of recurrences N/R |
||
| Index tests |
CEA timing The 4th and 14th day after surgery. Subsequent blood samples were taken at regular intervals (every 2 ‐ 3 months) in the following 12 ‐ 20 months. Moreover, an increased CEA value was always confirmed by repeated assays of the same sample, and by assaying an additional sample obtained from the same patient CEA technique Radioimmunoassay (RIA), using commercial EAK kits (purchased through SORIN Biomedica, Saluggia, Italy) CEA threshold 10 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule All patients had a blood sample taken for CEA assay preoperatively, then at the 4th and 14th day after surgery. Subsequent blood samples were taken at regular intervals (every 2‐3 months) in the following 12‐20 months with follow‐up examinations; the complete work‐up of the patients included physical examination, chest standard X‐ray, recto‐sigmoidoscopy, liver scan, hemogram and liver function tests; barium enema and bone scan were performed when indicated. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) not specified |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
McCall 1994.
| Study characteristics | |||
| Patient sampling |
Country Australia Study design Prospective RCT Setting Hospital Dates of data collection 1984 ‐ 1990 Population (n) 328 Inclusion criteria curative resection of colorectal cancers Exclusion criteria Patients with metastatic disease at presentation and those who for geographic or medical reasons were not able to be followed were excluded from the trial. Less than two years follow‐up completed (16 patients: 10 died of unrelated causes; 6 withdrew consent or were lost to follow‐up) and failure to obtain CEA levels (one patient). Participants Included (n) 311 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes ABC Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 98 Site of recurrences N/R |
||
| Index tests |
CEA timing Patients entered into both arms of the study had serum CEA levels measured for 5 consecutive years: every 3 months for the first 2 years, then every 6 months for the next 3 years CEA technique Enzyme immunoassay method (Abbott Laboratories, North Chicago, IL) CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule Standard follow up: Clinical review plus CEA, Liver function, and fecal occult blood ‐ 3 monthly til 2 years, 6 monthly til 5 years. CXR, Liver CT, Colonoscopy at 0 and 5 years; Aggressive follow up: As for standard follow‐up plus CXR , Liver CT and Colonoscopy annually Reference standard Radiology, histology |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Miles 1995.
| Study characteristics | |||
| Patient sampling |
Country Scotland Study design Retrospective notes review Setting Hospital Dates of data collection 1988 ‐ 1992 Population (n) 265 Inclusion criteria Patients who underwent a resection, with curative intent. Exclusion criteria Patients were excluded where, on inspection of the patients' notes, it was found that primary surgery was palliative, follow‐up was incomplete or there were fewer than 1 preoperative and 2 postoperative carcinoembryonic antigen level estimations Participants included (n) 125 |
||
| Patient characteristics and setting |
Age range 69 (41 ‐ 90) Smoking status Unknown Site of primary tumour Colorectal Stage of primary tumour Dukes A 10, B 27, C 38, D 22, unknown 27 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 53 Site of recurrences N/R |
||
| Index tests |
CEA timing Not clear CEA technique Using international standard International Reference Preparation 73/601, National Institute for Biological Standards and Control CEA threshold 10 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule History is recorded and clinical examination (including rectal examination and rigid sigmoidoscopy), faecal occult blood test and estimation of carcinoembryonic antigen level are undertaken Reference standard The presence of recurrent disease is confirmed by clinical examination, colonoscopy, biopsy, chest radiography, ultrasonography, computerized axial tomography scanning and laparotomy. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol, CEA triggers reference standard. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
Minton 1985.
| Study characteristics | |||
| Patient sampling |
Country USA Study design Prospective Setting Hospital Dates of data collection 1978 ‐ 1983 Population (n) 400 Inclusion criteria post‐colorectal cancer resection Exclusion criteria N/R Participants included (n) 400 |
||
| Patient characteristics and setting |
Age range 58 (18 ‐ 84) Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A 17, B1 91, B2 31, C1 119, C2 122, D 6, unknown 6 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 130 Site of recurrences Liver 49, Anastomosis site or mesentery of bowel 26, peritoneum 7, pelvis 6, para‐aortic nodes 2, mesentric nodes 2, multiple 7, other 28, no disease found 3 |
||
| Index tests |
CEA timing CEA performed every 2 months for the first 2 years, and then every 4 months for the next 3 years. To rule out laboratory variations, a repeat CEA value was required to confirm an abnormal CEA elevation CEA technique N/R CEA threshold 2.5 µg/L Definition of positive Abnormal repeated Which CEA value (s) used? Unclear |
||
| Target condition and reference standard(s) |
Follow‐up schedule Patients were evaluated postoperatively with each surgeon's customary follow‐up procedures and frequency of CEA determinations. Reference standard Second‐look surgery was performed on any potentially resectable recurrent cancer discovered by physical examination or symptoms of bowel or ureteral obstruction, gastrointestinal bleeding, or findings from rectal, vaginal, or colostomy examinations. In addition, second‐look surgery was done when a persistently rising CEA value was detected. Before the second‐look procedure was performed a careful physical examination complemented by chest roentgenogram, bone and brain scans, and appropriate gastrointestinal and genitourinary roentgenograms was done to rule out the possibility of unresectable metastases. A computerized axial tomography (CAT) scan of the abdomen was not required, but was considered appropriate for institutions with that capability. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) not specified |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Mittal 2011.
| Study characteristics | |||
| Patient sampling |
Country India Study design Retrospective Setting Hospital Dates of data collection N/R Population (n) 73 Inclusion criteria Histologically proven postoperative CRC resection undergoing PET/CT and conventional imaging to detect suspected recurrence triggered by a rising CEA Exclusion criteria N/R Participants included (n) 73 |
||
| Patient characteristics and setting |
Age range 25 ‐ 80 Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour N/R Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 38 Site of recurrences N/R |
||
| Index tests |
CEA timing Within 7 ‐ 10 days of imaging CEA technique Electro‐chemiluminescent immunoassay CEA threshold 3 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At point of recurrence |
||
| Target condition and reference standard(s) |
Reference standard PET/CT |
||
| Flow and timing |
Timing of CEA vs reference standard (days) within 7‐10 days of CEA |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Nishida 1988.
| Study characteristics | |||
| Patient sampling |
Country Japan Study design Prospective Setting Hospital Dates of data collection N/R Population (n) N/R Inclusion criteria Surgically treated for adenocarcinoma of the colon or rectum with curative intent and CEA measurements Exclusion criteria incomplete CEA dataset Participants included (n) 66 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Stage I ‐ V Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Follow‐up schedule N/R Recurrences (n) 20 Site of recurrences N/R |
||
| Index tests |
CEA timing CEA 1 month CEA technique RIA kit by Dynabot CEA threshold 2.5 µg/L Definition of positive N/R Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Reference standard N/R |
||
| Flow and timing |
Timing of CEA vs reference standard (days) Unclear |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Unclear | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Ochoa‐Figueroa 2012.
| Study characteristics | |||
| Patient sampling |
Country Spain Study design Retrospective Setting Hospital Dates of data collection 2007 ‐ 2011 Population (n) 54 Inclusion criteria Referred to the Dept of Nuclear Medicine for FDG PET‐CT with suspected CRC recurrence following surgical resection and posterior histological confirmation Exclusion criteria Not possible to follow up, mixed malignancy of the salivary gland Participants Included (n) 47 |
||
| Patient characteristics and setting |
Age range 63 (32 ‐ 87) Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour N/R Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? 38 chemo, 9 chemo and radio Recurrences (n) 34 Site of recurrences N/R |
||
| Index tests |
CEA timing CEA used as a marker of suspected recurrence or measured when recurrence suspected by CT CEA technique Radioimmunoanalysis CEA threshold 10 µg/L Definition of positive 1 elevated value Which CEA value (s) used? Single measurement taken at point of recurrence |
||
| Target condition and reference standard(s) |
Reference standard Histopathology or Clinical evolution, FDG PET‐CT |
||
| Flow and timing |
Timing of CEA vs reference standard (days) CEA prior to Referral; no more clear than this |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Ohlsson 1995.
| Study characteristics | |||
| Patient sampling |
Country Sweden Study design RCT Setting Hospital Dates of data collection 1983 ‐ 1986 Population (n) 107 Inclusion criteria Resection with curative intent, recruited to follow‐up group Exclusion criteria Patients operated with local excision or having demonstrable distant metastases were excluded, as were patients in whom age or severe illness was considered to preclude treatment of recurrent disease. Other exclusion criteria were: Inability to cooperate, ulcerative colitis, Crohn's disease, familial polyposis, and incomplete colonoscopy together with uncertain findings at the barium enema examination Participants Included (n) 53 |
||
| Patient characteristics and setting |
Age range 65.7 (40.6 ‐ 83.3) Smoking status N/R Site of primary tumour Rectum 19, colon 34 Stage of primary tumour Dukes A 10, B 21, C 22 Perioperative investigations done to ensure no residual disease Preoperative investigation included barium enema, pulmonary x‐ray, and blood tests for liver function test, carcinoembryonic antigen and colonoscopy Chemotherapy/radiotherapy? N/R Recurrences (n) 17 Site of recurrences Local 11, liver 3, lung 3, peritoneum 2, ovary 1 |
||
| Index tests |
CEA timing 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 60 months CEA technique Not specified CEA threshold N/R Definition of positive N/R Which CEA value (s) used? N/R |
||
| Target condition and reference standard(s) |
Follow‐up schedule Physical examination, Rigid Proctosigmoidoscopy, Blood tests ‐ CEA, ALP, GGT, Faecal Heamoglobin, CXR: 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 60 months. Endoscopic control of the anastomosis: 9, 21, 42 months. Colonoscopy: 3, 15, 30, 60 months. CT Pelvis: 3, 6, 12, 18, 24 months. Reference standard CT/ Endoscopy/colonoscopy |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol, immediate diagnostic work‐up did not reveal the site of recurrence in 4 asymptomatic patients with raised CEA levels; in these patients the time interval between elevation of CEA and symptoms of tumour recurrence varied between 0.2 and 4.7 (median 0.5) years |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Ohtsuka 2008.
| Study characteristics | |||
| Patient sampling |
Country Japan Study design Retrospective Setting Hospital Dates of data collection 2002 ‐ 2005 Population (n) 138 Inclusion criteria Curative resection, stage 0 – III according to the General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum, and Anus, 7th edition, 2006, no residuals Exclusion criteria History of another malignancy before or after the operation, lost to follow‐up Participants Included (n) 97 |
||
| Patient characteristics and setting |
Age range 70 (37 ‐ 86) Smoking status Chronic benign disease or smoking in 46 cases Site of primary tumour 32 right colon, 32 left colon, 30 rectum, 3 multiple Stage of primary tumour 0 in 8, I in 12, II in 37, and III in 40 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Yes, but not described Recurrences (n) 22 Site of recurrences |
||
| Index tests |
CEA timing Every 1 – 3 months during the initial 6 months after the operation, every 3 – 6 months from 6 months to 2 years, and every 6 – 12 months during 2 – 5 years after the operation CEA technique CEA, a latex immunoassay, Mitsubishi Chemical Ltd., Japan CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R |
||
| Target condition and reference standard(s) |
Follow‐up schedule the follow‐up schedule of the tumour markers and physical examination after the operation were: every 1 – 3 months during the initial 6 months after the operation, every 3 – 6 months from 6 months to 2 years, and every 6 – 12 months during 2 – 5 years after the operation. Radiological examinations including abdominal ultrasonography, computed tomography (CT), chest X‐ray, gastrointestinal series, and/or endoscopic evaluation were performed every 6 – 12 months during the follow‐up period. Marker evaluations and physical/radiological examinations were performed at shorter‐term intervals than those described above in patients with suspected recurrence, those undergoing chemotherapy, or in those demonstrating marker elevations. Reference standard radiological examinations / histology |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol or reference standard triggered by rise in CEA |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Park 2009.
| Study characteristics | |||
| Patient sampling |
Country Korea Study design Prospective Setting Hospital Dates of data collection N/R Population (n) 1707 Inclusion criteria curative resection for colorectal cancer followed by surveillance programme Exclusion criteria Patients with synchronous metastatic disease or patients undergoing palliative resection, and those with carcinoma in situ, inflammatory bowel disease, familial adenomatous polyposis or pathology other than adenocarcinoma were excluded, as were patients with T1 cancer treated by endoscopic mucosal resection or transanal excision. In addition, patients with chronic obstructive lung disease, chronic liver disease, peptic ulcer, and diabetes were excluded. Participants Included (n) 1263 |
||
| Patient characteristics and setting |
Age range 61 (21 ‐ 90) Smoking status N/R Site of primary tumour Colon 631, rectum 632 Stage of primary tumour I 212, II 514, III 537 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Yes, but not specified Recurrences (n) 291 Site of recurrences N/R |
||
| Index tests |
CEA timing per schedule CEA technique N/R CEA threshold 7 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All, although at point of recurrence for 18.8% |
||
| Target condition and reference standard(s) |
Follow‐up schedule 2‐ or 3‐ month intervals for the first 2 years and at 6‐month intervals thereafter. At each visit, CEA levels are assayed, a full history is obtained, and a physical examination is per‐ formed. A serum CEA assay is performed with at least a 2‐ week interval after the administration of chemotherapy. Colonoscopy is performed within 6 months to 1 year following surgery, and every 3years thereafter. Chest radiographs and abdominopelvic computed tomography (CT) are performed 6 months postoperatively and then at yearly intervals. Unscheduled CT or positron emission tomography (PET) scans were performed on patients with increased serum CEA concentrations or patients who were symptomatic. Reference standard diagnosis of a tumour recurrence was confirmed by biopsy or examination of the resected specimen. Other‐ wise, tumour recurrence was documented from the first clinical or radiologic sign of disease that showed an unrelenting course leading to tumour progression and/or death. The criteria for establishment of recurrent disease included histologic confirmation, palpable disease, or radiographic evidence of disease with subsequent clinical progression and supportive biochemical data, particularly an increased CEA level. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| High | |||
Peng 2013.
| Study characteristics | |||
| Patient sampling |
Country China Study design Retrospective comparative diagnostic accuracy study Setting Hospital Dates of data collection 2006 ‐ 2012 Population (n) 128 Inclusion criteria Colorectal cancer with full response to primary surgery ± chemo, undergoing FDG‐PET/CT for either elevated CEA levels or in patients with a suspicion of recurrence without CEA rise Exclusion criteria Unstable, severe DM, severe illness, 1 or more additional tumours, unable to remain supine for 30 mins Participants Included (n) 96 |
||
| Patient characteristics and setting |
Age range 61 (34 ‐ 85) Smoking status N/R Site of primary tumour Colon 53, rectum 42 Stage of primary tumour 0 in 1, I 15, II 31, III39, IV 9, unknown 1 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Yes, but not specified Recurrences (n) 63 Site of recurrences N/R |
||
| Index tests |
CEA timing 3‐monthly CEA technique N/R CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence |
||
| Target condition and reference standard(s) |
Reference standard FDG‐PET/CT +/‐ histology |
||
| Flow and timing |
Timing of CEA vs reference standard (days) Detection of recurrent lesions within 6 months of the FDG‐PET scan/CEA ± histology |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Seregni 1992.
| Study characteristics | |||
| Patient sampling |
Country Italy Study design Retrospective Setting Hospital Dates of data collection 1975 ‐ 1990 Population (n) 431 Inclusion criteria Curative resection Exclusion criteria N/R Participants Included (n) 336 |
||
| Patient characteristics and setting |
Age range 21 ‐ 92 Smoking status N/R Site of primary tumour Colon 247, rectum 184 Stage of primary tumour Dukes A 40, B 186, C 107, D 72 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 136 Site of recurrences 50 local recurrences, 136 distant recurrences |
||
| Index tests |
CEA timing N/R CEA technique N/R CEA threshold N/R Definition of positive Unclear Which CEA value (s) used? Unclear |
||
| Target condition and reference standard(s) |
Reference standard N/R |
||
| Flow and timing |
Timing of CEA vs reference standard (days) N/R |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Staib 2000.
| Study characteristics | |||
| Patient sampling |
Country Germany Study design Prospective Setting Hospital Dates of data collection 1994 ‐ 1998 Population (n) 100 Inclusion Criteria Patients undergoing a whole‐body PET scan for suspected relapse after curative resection of histologically confirmed colorectal cancer and who caused a “diagnostic problem”. The “diagnostic problems” of the patients that led to a PET scan were (1) staging of rest of the body in patients with known recurrence (n = 30); (2) suspected recurrence (n = 32); (3) increasing CEA level (n = 13); (4) unclear finding on pelvic CT (n = 7); and (5) confirmation of liver metastases (n = 12) and lung metastases (n = 6). Exclusion Criteria No CEA evaluation, uncontrolled DM, or acute inflammation Participants Included (n) 98 |
||
| Patient characteristics and setting |
Age range 62 (32 ‐ 80) Smoking status N/R Site of primary tumour Rectal 52, sigmoid 12, colon 22, lung or liver metastases 9, peritoneum 1 Stage of primary tumour I 8, II 25, III 46, IV 21 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Chemo/immunotherapy 25 Recurrences (n) 58 Site of recurrences N/R |
||
| Index tests |
CEA timing N/R CEA technique Liaison Kit (Byk‐Sangtec, Diet‐ zenbach, Germany) CEA threshold 3 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At point of recurrence |
||
| Target condition and reference standard(s) |
Follow‐up schedule Followed up with the department’s established follow‐up program. The indication for a whole body PET scan was given for patient s with suspected relapse after curative resection of colorectal cancer and who caused a “diagnostic problem” Reference standard FDG‐PET/CT |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Steele 1982.
| Study characteristics | |||
| Patient sampling |
Country USA Study design RCT Setting Hospital Dates of data collection 1975 ‐ 1980 Population (n) 770 Inclusion criteria B2 C colon or rectal cancer, 2 treatment arms: GITSG protocol 7175 was designed to evaluate adjuvant therapy (chemotherapy, radiotherapy, both, and none) following curative resection of Dukes' B2,C1,or C2 rectal carcinoma. Protocol 6175 was the study of the potential benefit of adjuvant therapy (chemotherapy, immunotherapy, both, and none) following clinically curative resection of Dukes' B2, C1, or C2 colon cancers. Exclusion criteria CEA not recorded post‐op Participants Included (n) 734 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Rectal 191, colon 543 Stage of primary tumour N/R Perioperative investigations done to ensure no residual disease CEA < 5 Chemotherapy/radiotherapy? Yes, but not described Recurrences (n) 149 Site of recurrences Colon |
||
| Index tests |
CEA timing On active treatment arms CEA values during and after treatment were to be obtained monthly during the first 3 months,every 3 months for the remainder of the first year, and every six months from then on. For control arms were to have CEA values obtained before operation, 1 week after operation, and at weeks 5, 10, 15, 25 after operation,and every 15 weeks thereafter CEA technique Hansen Z‐gel technique. Interassay comparisons among the institutions and intra‐assay analysis performed in the GITSG CEA reference laboratory at the Mallory Gastrointestinal Institute (Boston, Massachusetts) showed excellent reproducibility and acceptable variation among the various laboratories CEA threshold 2.5 µg/L Definition of positive Maximum level of CEA Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule Patients in both protocols were scheduled for regular clinic visits every 5 weeks during the first 6 months after surgery and every 15 weeks for the remainder of the first year. Physical examination, complete blood count, and liver function tests were performed at each visit. Liver/ spleen scan,chest posterio‐anterior,and lateral roentgenograms were obtained every 6 months. Sigmoidoscopic examination and large‐bowel, contrast roentgenograms were performed every year.Histologic evidence of tumor was the fundamental criterion for recurrence. However,roentgenographic evidence was acceptable in cases of lung or bony metastases. In the rectal‐cancer adjuvant study, liver metastases were also accepted on the basis of liver scan, and local recurrence was accepted on the basis of perineal pain occurring acutely after a pain‐free interval. Reference standard Histology, XR for bony or lung mets, liver scan for liver mets in rectal study, or perineal pain occurring acutely after a pain‐free interval. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Tang 2009.
| Study characteristics | |||
| Patient sampling |
Country Taiwan Study design prospective Setting Hospital Dates of data collection 1995 ‐ 2007 Population (n) N/A Inclusion criteria (1) Prior curative resection for histology‐proven primary adenocarcinoma of the colorectum between 1995 and 2002, (2) availability of serial serum samples from before the operation and from after the surgery, and (3) follow‐up with a definitive clinical outcome Exclusion criteria (1) synchronous or metachronous extracolonic cancers, (2) having neoadjuvant therapy for rectal cancer, and (3) fewer than 3 follow‐up samples available for s‐p53Ab analysis Participants Included (n) 305 |
||
| Patient characteristics and setting |
Age range 20 ‐ 90 Smoking status N/R Site of primary tumour Colon 95, rectum 101, both 4 Stage of primary tumour I 45, II 130, III 130. Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 76 Site of recurrences locoregional 7, intra‐abdominal or retro‐peritoneal 18, hepatic 29, pulmonary 17, brain or bone 9 |
||
| Index tests |
CEA timing The CEA test was defined as positive if 2 consecutive postoperative CEA values were greater than 5 µg/L or the elevated preoperative CEA values had not returned to the normal level (5 µg/L) after surgery CEA technique Abbott Architect 2000 (Abbott Laboratories, Abbott Park, IL, USA) CEA threshold 5 µg/L Definition of positive The CEA test was defined as positive if 2 consecutive postoperative CEA values were greater than 5 µg/L or the elevated preoperative CEA values did not returned to the normal level (5 µg/L) after surgery Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule All cases were followed up at the outpatient department every 3 – 6 months until death or until December 2007. All the patients were followed according to the hospital guidelines of care. Briefly, all patients underwent a follow‐up protocol of an outpatient visits every 3 – 6 months. The follow‐up included physical examination and carcinoembryonic antigen tests as well as chest X‐ray, abdominal sonography or abdominal computer‐assisted tomography scan, and colonoscopy every 1 – 3 years after operation. Reference standard Relapse confirmed by histology or by an imaging study |
||
| Flow and timing |
Timing of CEA vs reference standard (days) Triggered by positive CEA |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Unclear | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
Tate 1982.
| Study characteristics | |||
| Patient sampling |
Country UK Study design Prospective study after some retrospective sampling Setting Hospital Dates of data collection 1973 ‐ 1978 Population (n) 520 Inclusion criteria curative resection Exclusion criteria Dukes D, no follow‐up information available, signs of malignancy on first postoperative examination, malignancy of other sites during follow‐up Participants Included (n) 468 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour N/R Stage of primary tumour A 94, B 226, C 128, unknown 20 Perioperative investigations done to ensure no residual disease First postoperative exam Chemotherapy/radiotherapy? Not stated Recurrences (n) 108 Site of recurrences N/R |
||
| Index tests |
CEA timing At each follow‐up visit CEA technique Assayed by a double‐antibody radioimmunoassay system CEA threshold 40 µg/L Definition of positive 1 elevated value Which CEA value (s) used? all |
||
| Target condition and reference standard(s) |
Follow‐up schedule The follow‐up procedure for each patient complied with the normal clinical practice for the hospital concerned and, in addition, at each follow up examination a specimen of plasma was taken for CEA determination. At least 6mly. Reference standard Variable |
||
| Flow and timing |
Timing of CEA vs reference standard (days) Very variable |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Tobaruela 1997.
| Study characteristics | |||
| Patient sampling |
Country Spain Study design Retrospective Setting Hospital Dates of data collection 1988 ‐ 1993 Population (n) N/R Inclusion Criteria Colorectal cancer, curative surgery for Dukes C disease. Exclusion Criteria Dukes A, B, D Participants Included (n) 60 |
||
| Patient characteristics and setting |
Age range < 5 preop 60.9 (34 ‐ 85) + > 5 preop 64.9 (47 ‐ 83) Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes C = 60 Perioperative investigations done to ensure no residual disease No Chemotherapy/radiotherapy? N/R Recurrences (n) 21 Site of recurrences Hepatic 9, locoregional 6, combined 3, pulmonary 3 |
||
| Index tests |
CEA timing As follow‐up schedule CEA technique Enzyme‐linked immunoassay CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule Physical examination and CEA 3 monthly for 2 years, then 6 monthly up to 5 years. USS abdomen twice a year. CT if CEA increased Reference standard CT if CEA increased |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
Triboulet 1983.
| Study characteristics | |||
| Patient sampling |
Country France Study design Prospective cohort study Setting Hospital Dates of data collection 1976 ‐ 1979 Population (n) 91 Inclusion criteria Operated on with curative intent for colorectal cancer Exclusion criteria Conditions which could affect B2 microglobulin level: altered renal function (creatinine > 88.4 umol/l); liver disease: chronic active cirrhosis, primary biliary cirrhosis, acute hepatitis. Metastasis or Dukes D cancers. Patients whose CEA had not returned to normal within 3 months of the operation. |
||
| Patient characteristics and setting |
Participants included (n) 91 Age range 33 ‐ 80 Smoking status N/R Site of primary tumour Colon 65, rectum 26 Stage of primary tumour Dukes A&B = 50; C = 41 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 43 Site of recurrences 12 rectum, 31 colon |
||
| Index tests |
CEA timing Every 3 months CEA technique Radioimmunoassay (sorin) CEA threshold 20 µg/L Definition of positive N/R Which CEA value (s) used? N/R |
||
| Target condition and reference standard(s) |
Follow‐up schedule CEA & B2m every 3 months for at least 2 years. Clinical and laboratory monitoring was ensured by the same physician during the first two years post‐op in a pre‐established protocol with a barium enema and / or an endoscopy during the first two years enema. CXR and Liver USS annually. Further investigations if indicated (CT chest, bone scan) |
||
| Flow and timing |
Timing of CEA vs reference standard (days) Yearly CXR and liver USS; enema and/or endoscopy done at least once in the 2 year follow‐up |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
Wang 1994.
| Study characteristics | |||
| Patient sampling |
Country Study design Retrospective Setting Hospital Dates of data collection 1981 ‐ 1986 Population (n) 352 Inclusion criteria Operated for histologically proven colorectal cancer Exclusion criteria No preoperative CEA or lost to follow‐up, Dukes A, or Dukes D Participants Included (n) 272 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes B 160, C 112 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 27 Site of recurrences N/R |
||
| Index tests |
CEA timing Blood samples for CEA measurement were taken a few days before operation and about 1 month after operation and afterward at intervals of 3 ‐ 4 months, combined with physical examination CEA technique Radioimmunoassay kit manufactured by Abbott Laboratory (Chicago, IL, USA) CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule Blood samples for CEA measurement were taken a. few days before operation and about one month after operation and afterward at intervals of three to four months, combined with physical examination. Other procedures such as colonoscopy, liver sonography, and chest x‐ray were performed annually, Reference standard In the cases where we suspected recurrence the patient underwent additional abdominal computed tomography, bone scanning, or other diagnostic procedures. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Wood 1980.
| Study characteristics | |||
| Patient sampling |
Country UK Study design Retrospective Setting Hospital Dates of data collection 1974 ‐ 1976 Population (n) 148 Inclusion criteria Apparently curative surgery for adenocarcinoma of the colon and rectum without evidence of metastatic disease Exclusion criteria N/R Participants Included (n) 148 |
||
| Patient characteristics and setting |
Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour N/R Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 36 Site of recurrences Local 17, local + liver 2, local + bone 2, local + metachronous primary 1, liver 8, bone 5, lung 2 |
||
| Index tests |
CEA timing Each follow‐up visit, 2 consecutive raised CEA triggered investigation for recurrence CEA technique CEA levels were assayed by a double antibody radioimmunoassay on unextracted serum CEA threshold 25 µg/L Definition of positive 2 consecutively elevated values Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule CEA at 3 ‐ 6 months intervals post‐operative for up to 56 months or until death. Reference standard If CEA positive then CXR, Liver scan, and bone scan. If these are negative, additional BE and/or colonoscopy. |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
Yakabe 2010.
| Study characteristics | |||
| Patient sampling |
Country Japan Study design Prospective Setting Hospital Dates of data collection 1999 ‐ 2003 Population (n) 266 Inclusion criteria Curative resection for colorectal cancer, TNM stages I ‐ III, postoperative examinations according to the follow‐up schedule Exclusion criteria Inappropriate follow‐up Participants Included (n) 227 |
||
| Patient characteristics and setting |
Age range 65.2 (± 10.8) years Smoking status N/R Site of primary tumour Colon 138, rectum 89 Stage of primary tumour I 34, II 94, III 99 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 62 Site of recurrences N/R |
||
| Index tests |
CEA timing 3 months for the first 3 years and every 6 months during years 4 and 5 CEA technique Latex immunoassay, Mitsubishi Chemical Ltd, Japan CEA threshold 4.5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule History was taken and a physical examination and measurement of tumor markers were performed every 3 months for the first 3 years and every 6 months during years 4 and 5. Chest X‐ ray and abdominal computed tomography (CT) were done every 6 months for 5 years, and colonoscopy was performed at 1 and 3 years after surgery. Patients were observed until 5 years after surgery or until recurrence was confirmed. Reference standard Recurrence was confirmed histologically or radiologically |
||
| Flow and timing |
Timing of CEA vs reference standard (days) per protocol |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Yu 1992.
| Study characteristics | |||
| Patient sampling |
Country China Study design Retrospective observational study Setting Teaching hospital in Shanghai Dates of data collection May 1988 ‐ March 1990 Population (n) 216 Inclusion criteria Primary colorectal cancer having curative surgery in the teaching hospital or other hospitals Exclusion Criteria N/R Participants Included (n) 182 |
||
| Patient characteristics and setting |
Age
range N/R Smoking status N/R Site of primary tumour Colorectal cancer 121, colon cancer 95 Stage of primary tumour Only reported Dukes stage data for the 28 before‐ surgery cases (Table 1) Perioperative investigations done to ensure no residual disease N/R Chemotherapy/ radiotherapy? N/R Recurrences (n) 66 Site of recurrences N/R |
||
| Index tests |
CEA
timing N/R CEA technique RIA CEA threshold 15 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All |
||
| Target condition and reference standard(s) |
Follow‐up schedule CEA first measured at 6 weeks after curative surgery; then every 3 months, plus liver ultrasound test and basic health check. Reference standard Positive CEA and CA‐19‐9 triggers ultrasound and CT or colonoscopy |
||
| Flow and timing |
Timing of CEA vs reference standard (days) N/R |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
ACBE= air contrast barium enema ALP: alkaline phosphatase APCT: abdominopelvic computed tomography BE: barium enema CT: computed tomography CXR: chest xray DCBE: double contrast barium enema DM: diabetes mellitus ESR: erythrocyte sedimentation rate FOBT: faecal occult blood test LDH: lactate dehydrogenase LFT: latex fixation test MRI: magnetic resonance imaging N/R: not reported RIA: radioimmunoassay SCC: squamous cell carcinoma. SGOT: serum glutamic oxaloacetic transaminase SGPT: serum glutamate pyruvate transaminase TNM: primary tumour, regional nodes, metastasis TPA: tissue plasminogen activator µg/L = micrograms per litre USS = ultrasound scan
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afsaneh 2012 | 2 x 2 data not ascertainable |
| Ahmed 2013 | Only CEA positive |
| Aitkin 2012 | Only CEA positive |
| Amin 2012 | 2 x 2 data not ascertainable |
| Arnaud 1979 | 2 x 2 data not ascertainable |
| Arnaud 1997 | 2 x 2 data not ascertainable |
| Arriola 2006 | 2 x 2 data not ascertainable |
| Auer 1977 | Stomach and colorectal cancer combined |
| Bakalakos 1999 | Liver metastases only |
| Barrillari 1996 | Only cases of recurrence |
| Beatty 1979 | Only cases of recurrence |
| Beets 1994 | Only cases of recurrence |
| Bhatavedekar 1992 | Alternative analysis ‐ median CEA |
| Bivins 1974 | n < 30 |
| Boey 1984 | Alternative analysis ‐ slope |
| Borie 2004 | Only cases of recurrence |
| Brummendorf 1985 | 2 x 2 data not ascertainable |
| Brummendorf 1986 | Alternative analysis ‐ doubling time |
| Bucci 1994 | 2 x 2 data not ascertainable |
| Camunas 1991 | Only cases of recurrence |
| Cangemi 1984 | n < 30 |
| Cangemi 1987 | Case‐control study |
| Carl 1983 | Alternative analysis ‐ slope |
| Carpelan‐Holmström 1996 | Only cases of recurrence |
| Castells 1998 | 2 x 2 data not ascertainable |
| Catania 1981 | 2 x 2 data not ascertainable |
| Chang 2012 | Only cases of recurrence |
| Chapman 1998 | Pre‐operative CEA |
| Chen 2010 | Only CEA positive cases |
| Cho 2007 | Pre‐operative CEA |
| Choi 1997 | Only CEA positive cases |
| Colombo 1986 | 2 x 2 data not ascertainable |
| Cossu 1984 | Alternative analysis |
| Dalton 2010 | 2 x 2 data not ascertainable |
| Dash 2012 | Only CEA negative cases |
| De Brauw 1987 | 2 x 2 data not ascertainable |
| De Levin 1982 | n<30 |
| De Salvo 1997 | Only cases of recurrence |
| Dhar 1972 | 2 x 2 data not ascertainable |
| Di Cristofaro 2012 | Alter n a tive analysis ‐ economic |
| Engarås 2001 | Only cases of recurrence |
| Farquharson 2012 | Only CEA positive cases |
| Fernandes 2006 | 2 x 2 data not ascertainable |
| Filella 1994 | 2 x 2 data not ascertainable |
| Filiz 2009 | Not follow‐up for recurrence ‐ prognostic value of postoperative CEA |
| Finlay 1983 | Not curative resection |
| Fiocchi 2011 | Not follow‐up for recurrence ‐ includes patients with suspicion of recurrence on CT |
| Florio 1988 | 2 x 2 data not ascertainable |
| Fora 2012 | 2 x 2 data not ascertainable |
| Forones 1997 | Preoperative CEA |
| Forones 1998 | n < 30 |
| Fortner 1988 | Only cases of recurrence |
| Fournier 1999 | 2 x 2 data not ascertainable |
| Fucini 1983 | Duplicated dataset |
| Fucini 1984 | n < 30 |
| Fucini 1985 | 2 x 2 data not ascertainable |
| Gail 1981 | Alteranative analysis ‐ modelling |
| Gajdukevich 2010 | Not curative surgery |
| Gaudagni 1999 | 2 x 2 data not ascertainable |
| Graham 1998 | Only cases of recurrence |
| Gray 1981 | Only cases of recurrence |
| Griesenberg 1999 | Only cases of recurrence |
| Grossetti 1981 | 2 x 2 data not ascertainable |
| Grossmann 2007 | 2 x 2 data not ascertainable |
| Haga 1990 | Only cases of recurrence |
| Hall 1994 | 2 x 2 data not ascertainable |
| Hara 2011 | Duplicate dataset |
| Herrera 1976 | Case‐control study |
| Hida 1996 | 2 x 2 data not ascertainable |
| Hohenberger 1994 | Only cases of recurrence |
| Holt 2010 | Only cases of recurrence |
| Holubec 2000 | 2 x 2 data not ascertainable |
| Holyoke 2975 | n < 30 |
| Houlbec 2001 | 2 x 2 data not ascertainable |
| Humphreys 2011 | Only CEA negative cases |
| Huyghe 1983 | 2 x 2 data not ascertainable |
| Iarumov 1998 | Unable to locate full text |
| Indinnimeo 1999 | Unable to locate full text |
| Ito 2002 | Alternative analysis ‐ doubling time |
| Jaeger 1975 | Only cases of recurrence |
| Jiang 1989 | 2 x 2 data not ascertainable |
| Kanellos 2006b | Not follow‐up ‐ portal CEA sampling |
| Karesen 1980 | 2 x 2 data not ascertainable |
| Kawamura 2010 | Only cases of recurrence |
| Kerr 2012 | 2 x 2 data not ascertainable |
| Khan 2009 | 2 x 2 data not ascertainable |
| Kimura 1986 | Only cases of recurrence |
| Kishimoto 2010 | 2 x 2 data not ascertainable |
| Koch 1977 | 2 x 2 data not ascertainable |
| Koch 1979 | Not follow‐up for recurrence ‐ prognostic value of postoperative CEA |
| Koch 1982 | Not follow‐up for recurrence ‐ prognostic value of postoperative CEA |
| Koga 1999 | Alternative analysis ‐ doubling time |
| Korner 2005 | 2 x 2 data not ascertainable |
| Kumar 2011 | Only cases of recurrence |
| Lagache 1980 | Only cases of recurrence |
| Lauterbach 1987 | 2 x 2 data not ascertainable |
| Lavin 1981 | Case‐control study |
| Lechner 2000 | 2 x 2 data not ascertainable |
| Leventakos 2013 | Only cases of recurrence |
| Levy 2012 | Duplicate dataset |
| Lipska 2007 | Only cases of recurrence |
| Lipska 2010 | Only cases of recurrence |
| Lorenz 1986 | Not follow‐up for recurrence ‐ prognostic value of postoperative CEA |
| Lunde 1982 | Only cases of recurrence |
| Ma 2006 | Not follow‐up for recurrence ‐ prognostic value of postoperative CEA |
| Mach 1974 | Case‐control study |
| Makela 1995 | 2 x 2 data not ascertainable |
| Makis 2013 | 2 x 2 data not ascertainable |
| Mant 2013 | Duplicate dataset |
| Martin 1976 | Only CEA positive case |
| Martin 1979 | Only CEA positive case |
| Martin 1980 | Only CEA positive case |
| Marucci 1983 | Not follow‐up for recurrence ‐ prognostic value of postoperative CEA |
| May 2012 | 2 x 2 data not ascertainable |
| Mazilu 2012 | Unable to locate full text |
| McCarthy 1985 | 2 x 2 data not ascertainable |
| Meling 1992 | 2 x 2 data not ascertainable |
| Mentges 1986 | 2 x 2 data not ascertainable |
| Mentges 1988 | Only cases of recurrence |
| Metzger 1983 | Only cases of recurrence |
| Metzger 1985 | Only cases of recurrence |
| Minton 1978a | Alternative analysis ‐ nomogram |
| Minton 1978b | Only cases of recurrence |
| Minton 1989 | Alternative analysis ‐ nomogram |
| Miwa 1980 | Only cases of recurrence |
| Moertel 1978 | Only cases of recurrence |
| Morelli 1985 | n<30 |
| Moreno Carretero 1998 | 2 x 2 data not ascertainable |
| Moschl 1980 | 2 x 2 data not ascertainable |
| Nicolini 1995 | 2 x 2 data not ascertainable |
| Nicolini 2005 | 2 x 2 data not ascertainable |
| Nicolini 2010 | Only cases of recurrence |
| Northover 1985 | 2 x 2 data not ascertainable |
| Northover 1986 | Review article |
| Northover 2003 | Review article |
| Novis 1986 | Only cases of recurrence |
| Nowacki 1983 | 2 x 2 data not ascertainable |
| Ntinas 2004 | 2 x 2 data not ascertainable |
| O'Dwyer 1987 | 2 x 2 data not ascertainable |
| O'Dwyer 1988 | Only CEA positive cases |
| Obradovic 2011 | 2 x 2 data not ascertainable |
| Odariuk 1989 | Only CEA positive cases |
| Ovaska 1989 | Only cases of recurrence |
| Ozhiganov 1986 | Unable to translate |
| Ozkan 2012a | 2 x 2 data not ascertainable |
| Ozkan 2012b | 2 x 2 data not ascertainable |
| Park 2012 | Only cases of recurrence |
| Park 2013 | 2 x 2 data not ascertainable |
| Pecorella 1996 | 2 x 2 data not ascertainable |
| Peethambaram 1997 | 2 x 2 data not ascertainable |
| Pereira 2004 | Unable to locate |
| Persijin 1981 | 2 x 2 data not ascertainable |
| Pfeiffer 1979 | 2 x 2 data not ascertainable |
| Philips 1984 | 2 x 2 data not ascertainable |
| Pietra 1998 | 2 x 2 data not ascertainable |
| Plebani 1996 | 2 x 2 data not ascertainable |
| Pompecki 1980 | n < 30 |
| Pribelsky 2002 | Only cases of recurrence |
| Primrose 2011 | Duplicate dataset |
| Primrose 2014 | 2 x 2 data not ascertainable |
| Quentmeier 1990 | Only cases of recurrence |
| Reddy 2013 | Only cases of recurrence |
| Revetria 1989 | Case‐control stu dy |
| Rezamansourian 2011 | Review article |
| Rieger 1975 | Only cases of recurrence |
| Rockall 1999 | 2 x 2 data not ascertainable |
| Rocklin 1990 | Only cases of recurrence |
| Rocklin 1991 | 2 x 2 data not ascertainable |
| Rodriguez‐Moranta 2006a | Only cases of recurrence |
| Rognum 1986 | Only cases of recurrence |
| Sagar 1989 | 2 x 2 data not ascertainable |
| Sandelewski 2005 | Only cases of recurrence |
| Sanli 2012 | Only CEA positive cases |
| Sardi 1989 | Only cases of recurrence |
| Sarikaya 2007 | Only CEA negative cases |
| Secco 1989 | 2 x 2 data not ascertainable |
| Secco 2000 | Only cases of recurrence |
| Segol 1977 | Not follow‐up for recurrence ‐ prognostic value of postoperative CEA |
| Shirley 2012 | 2 x 2 data not ascertainable |
| Simo 2002 | Only CEA positive cases |
| Sirisriro 1996 | Only CEA positive cases |
| Song 2010 | Alternative analysis ‐ CEA trend |
| Sorensen 2010 | Only CEA positive cases |
| Staab 1985a | Alternative analysis ‐ slope |
| Staab 1985b | Alternative analysis ‐ slope |
| Stautner‐Brückmann 1990 | Only cases of recurrence |
| Steele 1980 | Only CEA positive cases |
| Stuckle 2000 | 2 x 2 data not ascertainable |
| Su 2012 | Only cases of recurrence |
| Sugarbaker 1976 | Only CEA positive cases |
| Szymendera 1982 a | Only cases of recurrence |
| Szymendera 1982 b | 2 x 2 data not ascertainable |
| Szymendera 1985 | 2 x 2 data not ascertainable |
| Takashima 1982 | Only cases of recurrence |
| Tomoda 1981 | Non‐curative surgery |
| Tsai 2009 | Only cases of recurrence |
| Tsikitis 2009 | Only cases of recurrence |
| Verberne 2013 a | Liver metastases only |
| Verberne 2013 b | Liver metastases only |
| Wan 1994 | 2 x 2 data not ascertainable |
| Wanebo 1978a | Only cases of recurrence |
| Wanebo 1978b | Only cases of recurrence |
| Wang 2007 | Not follow‐up for recurrence ‐ prognostic value of postoperative CEA |
| Wang 2010 | 2 x 2 data not ascertainable |
| Wedell 1981 | Only cases of recurrence |
| Weiss 1998 | 2 x 2 data not ascertainable |
| Wichmann 2000a | Only cases of recurrence |
| Wichmann 2000b | Preoperative CEA |
| Wichmann 2002 | Preoperative CEA |
| Wolf 1997 | Only cases of recurrence |
| Wood 1975 | Unable to locate |
| Yu 2013 | Only cases of recurrence |
| Zeng 1993 | Only cases of recurrence |
| Zervos 2001 | 2 x 2 data not ascertainable |
| Ziegenbein 1980 | Alternative analysis ‐ trend |
| Zuniga 1989 | 2 x 2 data not ascertainable |
Differences between protocol and review
We stated we would contact the principal investigators to clarify methodological queries and ask for any unpublished data relevant to this review. This has not yet been done, and we have stated this in the Methods section.
We were unable to apply the Hamza method which allows data for multiple thresholds from a single study to be incorporated in the meta‐analysis. This method requires 2 x 2 data at consistent thresholds across studies, but in our review accuracy has been reported at a wide range of inconsistent thresholds.
In terms of sensitivity analyses, we did not feel it necessary to remove each study in turn from the analyses as our review includes such a large number of studies, of which none is notably larger than the others, making it high unlikely that one particular study would heavily skew the overall pooled estimates.
Contributions of authors
NWR and BDN devised the search strategy. BDN and IP reviewed titles, abstracts, and full‐text articles, and extracted all data. BS acted as moderator at all stages. BDN, IP, and BS performed the QUADAS‐2 assessment. BS, BDN, and DM devised the statistical analysis. BS conducted statistical analyses in R, Stata, and SAS. BDN and BS wrote the initial draft of the review DM, TJJ, SM, IP, JP, and RP provided comments and edited the draft.
Sources of support
Internal sources
No sources of support supplied
External sources
-
HTA ‐ 11/136/81, UK.
This work is partly funded by the National Institute of Health Research (NIHR) Health Technology Appraisal Programme project grant "What CEA level should trigger further investigation during follow up after curative treatment for colorectal cancer?" (HTA ‐ 11/136/81).
-
National Institute for Health Research (NIHR) School for Primary Care Research (SPCR), UK.
The Nuffield Department of Primary Care Health Sciences receives funding from the National Institute for Health Research (NIHR) School for Primary Care Research (SPCR).
Declarations of interest
None
New
References
References to studies included in this review
Banaszkiewicz 2011 {published data only}
- Banaszkiewicz Z, Jarmocik P, Frasz J, Tojek K, Mrozowski M, Jawien A. Usefulness of CEA concentration measurement and classic colonoscopy in follow‐up after radical treatment of colorectal cancer. Polski Przeglad Chirurgiczny 2011 Jun;83(6):310‐8. [DOI] [PubMed] [Google Scholar]
Barillari 1992 {published data only}
- Barillari P, Bolognese A, Chirletti P, Cardi M, Sammartino P, Stipa V. Role of CEA, TPA, and Ca 19‐9 in the early detection of localized and diffuse recurrent rectal cancer. Diseases of the Colon and Rectum 1992 May;35(5):471‐6. [DOI] [PubMed] [Google Scholar]
Beart 1981 {published data only}
- Beart RW Jr, Metzger PP, O'Connell MJ, Schutt AJ. Postoperative screening of patients with carcinoma of the colon . Diseases of the Colon and Rectum 1981;24(8):585‐8. [DOI] [PubMed] [Google Scholar]
Bjerkeset 1988 {published data only}
- Bjerkeset T, Orjasaeter H, Soreide O. The role of carcinoembryonic antigen (CEA) in routine investigation and postsurgical monitoring in patients with colorectal cancer. Surgical Research Communications 1988;2:205‐12. [Google Scholar]
Carlsson 1983 {published data only}
- Carlsson U, Stewénius J, Ekelund G, Leandoer L, Nosslin B. Is CEA analysis of value in screening for recurrences after surgery for colorectal carcinoma?. Diseases of the Colon and Rectum 1983;26(6):369‐73. [DOI] [PubMed] [Google Scholar]
Carpelan‐Holmström 2004 {published data only}
- Carpelan‐Holmström M, Louhimo J, Stenman UH, Alfthan H, Järvinen H, Haglund C. CEA, CA 242, CA 19‐9, CA 72‐4 and hCGbeta in the diagnosis of recurrent colorectal cancer. Tumour Biology 2004;25(5‐6):228‐34. [DOI] [PubMed] [Google Scholar]
Carriquiry 1999 {published data only}
- Carriquiry LA, Piñeyro A. Should carcinoembryonic antigen be used in the management of patients with colorectal cancer?. Diseases of the Colon and Rectum 1999;42(7):921‐9. [DOI] [PubMed] [Google Scholar]
Deveney 1984 {published data only}
- Deveney KE, Way LW. Follow‐up of patients with colorectal cancer. American Journal of Surgery 1984;148(6):717‐22. [DOI] [PubMed] [Google Scholar]
Engarås 2003 {published data only}
- Engarås B. Individual cutoff levels of carcinoembryonic antigen and CA 242 indicate recurrence of colorectal cancer with high sensitivity. Diseases of the Colon and Rectum 2003;46(3):313‐21. [DOI] [PubMed] [Google Scholar]
Farinon 1980 {published data only}
- Farinon AM, Sivelli R, Sianesi M, Zanella E. Carcinoembryonic antigen (CEA) test associated with colonoscopy: a monitoring method for the early detection of recurrent colorectal carcinoma. Surgery in Italy 1980;20(3):190‐8. [Google Scholar]
Fezoulidis 1987 {published data only}
- Fezoulidis I, Imhof H, Karner‐Hanusch J, Teleky B, Wunderlich M, Schiessel R. The value of computed tomography after operation of carcinoma of the rectum. Digitale Bilddiagnostik 1987;7(4):194‐8. [PubMed] [Google Scholar]
Fucini 1987 {published data only}
- Fucini C, Tommasi SM, Rosi S, Malatantis G, Cardona G, Panichi S, et al. Follow‐up of colorectal cancer resected for cure. An experience with CEA, TPA, Ca 19‐9 analysis and second‐look surgery. Diseases of the Colon and Rectum 1987;30(4):273‐7. [DOI] [PubMed] [Google Scholar]
Graffner 1985 {published data only}
- Graffner H, Hultberg B, Johansson B, Möller T, Petersson BG. Detection of recurrent cancer of the colon and rectum. Journal of Surgical Oncology 1985;28(2):156‐9. [DOI] [PubMed] [Google Scholar]
Hara 2008 {published data only}
- Hara M, Kanemitsu Y, Hirai T, Komori K, Kato T. Negative serum carcinoembryonic antigen has insufficient accuracy for excluding recurrence from patients withDukes C colorectal cancer: analysis with likelihood ratio and posttest probability in a follow‐up study. Diseases of the Colon and Rectum 2008;51(11):1675‐80. [DOI] [PubMed] [Google Scholar]
Hara 2010 {published data only}
- Hara M, Sato M, Takahashi H, Takayama S, Takeyama H. Does serum carcinoembryonic antigen elevation in patients with postoperative stage II colorectal cancer indicate recurrence. Comparison with stage III. Journal of Surgical Oncology 2010;102(2):154‐7. [DOI] [PubMed] [Google Scholar]
Hine 1984 {published data only}
- Hine KR, Dykes PW. Serum CEA testing in the post‐operative surveillance of colorectal carcinoma. British Journal of Cancer 1984;49(6):689‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irvine 2007 {published data only}
- Irvine T, Scott M, Clark CI. A small rise in CEA is sensitive for recurrence after surgery for colorectal cancer. Colorectal Diseases 2007;9(6):527‐31. [DOI] [PubMed] [Google Scholar]
Johnson 1985 {published data only}
- Johnson JA, Giercksky KE. [Colorectal cancer recurrence. Early diagnosis by determination of a carcinoembryonic antigen in the serum]. Tidsskrift for Den Norske Laegeforening 1985;105(29):2044‐6. [PubMed] [Google Scholar]
Jubert 1978 {published data only}
- Jubert AV, Talbott TM, Maycroft TM. Characteristics of adenocarcinomas of the colorectum with low levels of preoperative plasma carcinoembryonic antigen (CEA). Cancer 1978;42(2):635‐9. [DOI] [PubMed] [Google Scholar]
Kanellos 2006a {published data only}
- Kanellos I, Zacharakis E, Demetriades H, Christoforidis E, Kanellos D, Pramateftakis MG, et al. Value of carcinoembryonic antigen assay in predicting hepatic metastases, local recurrence, and survival after curative resection of colorectal cancer . Surgery Today 2006;36(10):879‐84. [DOI] [PubMed] [Google Scholar]
Kato 1980 {published data only}
- Kato K, Morimoto T, Kato T, Yasue M, Takagi H, Kito T, et al. CEA assays in postoperative detection of recurrent colorectal carcinoma (author's transl). Nihin Gan Chyryo Gakkai Shi 1980;15(7):1137‐42. [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim HS, Lee MR. Diagnostic accuracy of elevated serum carcinoembryonic antigen for recurrence in postoperative stage II colorectal cancer patients: comparison with stage III. Annals of Coloproctology 2013;29(4):155‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohler 1980 {published data only}
- Kohler JP, Simonowitz D, Paloyan D. Preoperative CEA level: a prognostic test in patients with colorectal carcinoma. The American Surgeon 1980;46(8):449‐52. [PubMed] [Google Scholar]
Koizumi 1992 {published data only}
- Koizumi F, Odagiri H, Fujimoto H, Kawamura T, Ishimori A. [Clinical evaluation of four tumor markers (CEA, TPA, CA50 and CA72‐4) in colorectal cancer]. Rinsho Byori ‐ Japanese Journal of Clinical Pathology 1992;40(5):523‐8. [PubMed] [Google Scholar]
Korner 2007 {published data only}
- Körner H, Söreide K, Stokkeland PJ, Söreide JA. Diagnostic accuracy of serum‐carcinoembryonic antigen in recurrent colorectal cancer: a receiver operating characteristic curve analysis. Annals of Surgical Oncology 2007 Feb;14(2):417‐23. [DOI] [PubMed] [Google Scholar]
Li Destri 1998 {published data only}
- Li Destri G, Greco S, Rinzivillo C, Racalbuto A, Curreri R, Cataldo A. Monitoring Carcinoembryonic Antigen in Colorectal Cancer: Is it Still Useful?. Surgery Today 1998;28(12):1233‐6. [DOI] [PubMed] [Google Scholar]
Lucha 1997 {published data only}
- Lucha PA Jr, Rosen L, Olenwine JA, Reed JF 3rd, Riether RD, Stasik JJ Jr, et al. Value of carcinoembryonic antigen monitoring in curative surgery for recurrent colorectal carcinoma. Diseases of the Colon and Rectum 1997;40(2):145‐9. [DOI] [PubMed] [Google Scholar]
Luporini 1979 {published data only}
- Luporini G, Mangiarotti F, Fraschini P, Labianca R, Tassi GC, Barbieri A. [Importance of the analysis of the carcinoembryonic antigen in clinical oncology]. Minerva Medica 1979;70(2):127‐34. [PubMed] [Google Scholar]
Mach 1978 {published data only}
- Mach JP, Vienny H, Jaeger P, Haldemann B, Egely R, Pettavel J. Long‐term follow‐up of colorectal carcinoma patients by repeated CEA radioimmunoassay. Cancer 1978;42(3 Suppl):1439‐47. [DOI] [PubMed] [Google Scholar]
Mackay 1974 {published data only}
- Mackay AM, Patel S, Carter S, Stevens U, Laurence DJ, Cooper EH, et al. Role of serial plasma C.E.A. assays in detection of recurrent and metastatic colorectal carcinomas. British Medical Journal 1974 Nov;4(5941):382‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mariani 1980 {published data only}
- Mariani G, Carmellini M, Bonaguidi F, Benelli MA, Toni MG. Serum CEA Monitoring in the follow‐up of Colorectal Cancer Patients with negative preoperative Serum CEA. European Journal of Cancer 1980;16(8):1099‐103. [DOI] [PubMed] [Google Scholar]
McCall 1994 {published data only}
- McCall JL, Black RB, Rich CA, Harvey JR, Baker RA, Watts JM, et al. The Value of serum carcinoembryonic antigen in predicting recurrent disease following curative resection of colorectal cancer. Diseases of the Colon and Rectum 1994;37(9):875‐81. [DOI] [PubMed] [Google Scholar]
Miles 1995 {published data only}
- Miles WF, Greig JD, Seth J, Sturgeon C, Nixon SJ. Raised carcinoembryonic antigen level as an indicator of recurrent disease in follow up of patients with colorectal cancer. British Journal of General Practice 1995;45(395):287‐8. [PMC free article] [PubMed] [Google Scholar]
Minton 1985 {published data only}
- Minton JP, Hoehn JL, Gerber DM, Horsley JS, Connolly DP, Salwan F, et al. Results of a 400‐patient carcinoembryonic antigen second‐look colorectal cancer study. Cancer 1985;55(6):1284‐90. [DOI] [PubMed] [Google Scholar]
Mittal 2011 {published data only}
- Mittal BR, Senthil R, Kashyap R, Bhattacharya A, Singh B, Kapoor R, et al. 18F‐FDG PET‐CT in evaluation of postoperative colorectal cancer patients with rising CEA level. Nuclear Medicine Communications 2011;32(9):789‐93. [DOI] [PubMed] [Google Scholar]
Nishida 1988 {published data only}
- Nishida O, Shiroto H, Satoh N, Nakajima Y. Clnical evaluation of a combination assay of CEA, CA19‐9 and TPA in patients with colorectal cancer. Japanese Journal of Cancer Clinics 1988;34(416):1096‐1100. [PubMed] [Google Scholar]
Ochoa‐Figueroa 2012 {published data only}
- Ochoa‐Figueroa MA, Uña‐Gorospe J, Allende‐Riera A, Cárdenas‐Negro JC, Muñoz‐Iglesias J, Cabello‐García D, et al. Utility of low dose (18)F‐FDG PET‐CT in patients with suspected colorectal carcinoma recurrence in conventional diagnostic methods. Revista Espanola de Medicina Nuclear e Imagen Molecular 2012;31(5):249‐56. [DOI] [PubMed] [Google Scholar]
Ohlsson 1995 {published data only}
- Ohlsson B, Breland U, Ekberg H, Graffner H, Tranberg KG. Follow‐up after curative surgery for colorectal carcinoma. Randomized comparison with no follow‐up. Diseases of the Colon and Rectum 1995;38(6):619‐26. [DOI] [PubMed] [Google Scholar]
Ohtsuka 2008 {published data only}
- Ohtsuka T, Nakafusa Y, Sato S, Kitajima Y, Tanaka M, Miyazaki K. Different roles of tumor marker monitoring after curative resections of gastric and colorectal cancers. Digestive Diseases and Sciences 2008;53(6):1537‐43. [DOI] [PubMed] [Google Scholar]
Park 2009 {published data only}
- Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: clinical significance of the preoperative level. Annals of Surgical Oncology 2009;16(11):3087‐93. [DOI] [PubMed] [Google Scholar]
Peng 2013 {published data only}
- Peng NJ, Hu C, King TM, Chiu YL, Wang JH, Liu RS. Detection of resectable recurrences in colorectal cancer patients with 2‐[18F]fluoro‐2‐deoxy‐D‐glucose‐positron emission tomography/computed tomography. Cancer Biotherapy & Radiopharmaceuticals 2013;28(6):479‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seregni 1992 {published data only}
- Seregni E, Bombardieri E, Bogni A, Crippa F, Jager E, Buraggi GL. The role of serum carcinoembryonic antigen (CEA) in the management of patients with colorectal carcinoma: the experience of the Istituto Tumori of Milan. International Journal of Biological Markers 1992;7(3):167‐70. [DOI] [PubMed] [Google Scholar]
Staib 2000 {published data only}
- Staib L, Schirrmeister H, Reske SN, Beger HG. Is (18)F‐fluorodeoxyglucose positron emission tomography in recurrent colorectal cancer a contribution to surgical decision making?. American Journal of Surgery 2000;180(1):1‐5. [DOI] [PubMed] [Google Scholar]
Steele 1982 {published data only}
- Steele G Jr, Ellenberg S, Ramming K, O'Connell M, Moertel C, Lessner H, et al. CEA monitoring among patients in multi‐institutional adjuvant G.I. therapy protocols. Annals of Surgery 1982;196(2):162‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2009 {published data only}
- Tang R, Yeh CY, Wang JY, Changchien CR, Chen JS, Hsieh LL. Serum p53 antibody as tumor marker for follow‐up of colorectal cancer after curative resection. Annals of Surgical Oncology 2009;16(9):2516‐23. [DOI] [PubMed] [Google Scholar]
Tate 1982 {published data only}
- Tate H. Plasma CEA in the post‐surgical monitoring of colorectal carcinoma. British Journal of Cancer 1982;46(3):323‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tobaruela 1997 {published data only}
- Tobaruela E, Enriquez JM, Diez M, Camunas J, Muguerza J, Granell J. Evaluation of serum carcinoembryonic antigen monitoring in the follow‐up of colorectal cancer patients with metastatic lymph nodes and a normal preoperative serum level. International Journal of Biological Markers 1997;12(1):18‐21. [DOI] [PubMed] [Google Scholar]
Triboulet 1983 {published data only}
- Triboulet JP, Dessaint JP, Lagache G. [Diagnostic value of the assay of beta 2 microglobulin during the monitoring of surgically‐treated colorectal cancers. Comparison with carcinoembryonic antigen]. Gastroenterologie Clinique et Biologique 1983;7(10):808‐11. [PubMed] [Google Scholar]
Wang 1994 {published data only}
- Wang JY, Tang R, Chiang JM. Value of carcinoembryonic antigen in the management of colorectal cancer . Diseases of the Colon and Rectum 1994;37(3):272‐7. [DOI] [PubMed] [Google Scholar]
Wood 1980 {published data only}
- Wood CB, Ratcliffe JG, Burt RW, Malcolm AJ, Blumgart LH. The clinical significance of the pattern of elevated serum carcinoembryonic antigen (CEA) levels in recurrent colorectal cancer . British Journal of Surgery 1980;67(1):46‐8. [DOI] [PubMed] [Google Scholar]
Yakabe 2010 {published data only}
- Yakabe T, Nakafusa Y, Sumi K, Miyoshi A, Kitajima Y, Sato S, et al. Clinical significance of CEA and CA19‐9 in postoperative follow‐up of colorectal cancer. Annals of Surgical Oncology 2010;17(9):2349‐56. [DOI] [PubMed] [Google Scholar]
Yu 1992 {published data only}
- Yu BM. Evaluation of combined CA‐19‐9 and CEA assay in monitoring recurrences and metastases of colorectal cancer. Zhonghua Wai Ke Za Zhi 1992;30(12):707‐9. [PubMed] [Google Scholar]
References to studies excluded from this review
Afsaneh 2012 {published data only}
- Motamed‐Khorasani A, Small‐Howard A, Etemadi H, Beart H. A new strategy for the early detection of colorectal cancer recurrence. Gastrointestinal Cancers Symposium (ASCO). San Francisco, CA, USA, January 19 ‐ 21. 2012.
Ahmed 2013 {published data only}
- Ahmed H, Bashir H, Khalid Nawaz M, Faruqui Z, Saeed Kazmi A, Ali Syed A, et al. Early detection of recurrence of colorectal carcinoma on F18‐FDG PET‐CT and its correlation with other clinicopathological parameters. Nuclear Medicine Communications 2013;34(4):394‐5. [Google Scholar]
Aitkin 2012 {published data only}
- Aitken K, Barbachano Y, Sharma B, Cunningham D, Cook G, Rao S. Elevated CEA level in the asymptomatic patient with normal conventional imaging: How useful is PET‐CT for the detection of colorectal cancer recurrence? Gastrointestinal Cancers Symposium, San Francisco, CA, USA. Journal of Clinical Oncology. 2012.
Amin 2012 {published data only}
- Amin A, Reddy A, Wilson R, Jha M, Miranda S, Amin J. Unnecessary surgery can be avoided by judicious use of PET/CT scanning in colorectal cancer patients. Journal of Gastrointestinal Cancer 2012;43(4):594‐8. [DOI] [PubMed] [Google Scholar]
Arnaud 1979 {published data only}
- Arnaud JP, Simon P, Ollier JC, Koehl C, Adloff M. [Interest and limits of estimation of the carcinoembryonic antigen in colonic and rectal (author's transl)]. Journal de Chirurgie 1979;116(11):633‐6. [PubMed] [Google Scholar]
Arnaud 1997 {published data only}
- Arnaud JP, Cervi C, Bergamaschi R, Tuech JJ. [Value of oncologic follow‐up of patients operated for colorectal cancer. A prospective study of 1000 patients]. Journal de Chirurgie 1997;134(2):45‐50. [PubMed] [Google Scholar]
Arriola 2006 {published data only}
- Arriola E, Navarro M, Parés D, Muñoz M, Pareja L, Figueras J, et al. Imaging techniques contribute to increased surgical rescue of relapse in the follow‐up of colorectal cancer. Diseases of the Colon and Rectum 2006;49(4):478‐84. [DOI] [PubMed] [Google Scholar]
Auer 1977 {published data only}
- Auer IO, Schmid L, Hoecht B. The clinical value of the plasma CEA level for postoperative detection of recurrence and metastases of carcinoma of the gastrointestinal tract [[German] Klinische Wertigkeit Der Plasmakonzentration Des Karzinoembryonalen Antigens Bei Der Postoperativen Verlaufskontrolle Von Karzinomen Des Verdauungstraktes]. Medizinische Klinik 1977;72(21):934‐41. [PubMed] [Google Scholar]
Bakalakos 1999 {published data only}
- Bakalakos EA, Burak WE, Young DC, Martin EW. Is carcino‐embryonic antigen useful in the follow‐up management of patients with colorectal liver metastases?. American Journal of Surgery 1999;177(1):2‐6. [DOI] [PubMed] [Google Scholar]
Barrillari 1996 {published data only}
- Barillari P, Ramacciato G, Manetti G, Bovino A, Sammartino P, Stipa V. Surveillance of colorectal cancer: effectiveness of early detection of intraluminal recurrences on prognosis and survival of patients treated for cure. Diseases of the Colon and Rectum 1996;39(4):388‐93. [DOI] [PubMed] [Google Scholar]
Beatty 1979 {published data only}
- Beatty J, Romero C, Brown PW, Lawrence W, Jr, Terz JJ. Clinical value of carcinoembryonic antigen: diagnosis, prognosis, and follow‐up of patients with cancer. Archives of Surgery 1979;114(5):563‐7. [DOI] [PubMed] [Google Scholar]
Beets 1994 {published data only}
- Beets G, Penninckx F, Schiepers C, Filez L, Mortelmans L, Kerremans R, et al. Clinical value of whole‐body positron emission tomography with [18F] fluorodeoxyglucose in recurrent colorectal cancer. British Journal of Surgery 1994;81(11):1666‐70. [DOI] [PubMed] [Google Scholar]
Bhatavedekar 1992 {published data only}
- Bhatavdekar JM, Patel DD, Giri DD, Karelia NH, Vora HH, Ghosh N, et al. Comparison of plasma prolactin and CEA in monitoring patients with adenocarcinoma of colon and rectum. British Journal of Cancer 1992;66(5):977‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bivins 1974 {published data only}
- Bivins BA, Meeker WR Jr, Griffen WO Jr. CEA levels and prognosis in colon carcinoma. Journal of Surgical Oncology 1974;6(5):413‐21. [DOI] [PubMed] [Google Scholar]
Boey 1984 {published data only}
- Boey J, Cheung HC, Lai CK, Wong J. A prospective evaluation of serum carcinoembryonic antigen (CEA) levels in the management of colorectal carcinoma. World Journal of Surgery 1984;8(3):279‐86. [DOI] [PubMed] [Google Scholar]
Borie 2004 {published data only}
- Borie F, Daurès JP, Millat B, Trétarre B. Cost and effectiveness of follow‐up examinations in patients with colorectal cancer resected for cure in a French population‐based study. Journal of Gastrointestinal Surgery 2004;8(5):552‐8. [DOI] [PubMed] [Google Scholar]
Brummendorf 1985 {published data only}
- Brummendorf T, Anderer FA, Staab HJ, Hornung A, Kieninger G. [Carcinoembryonic antigen: diagnosis and tumor progression in gastrointestinal tumors]. Deutsche Medizinische Wochenschrift 1985;110(51‐52):1963‐8. [DOI] [PubMed] [Google Scholar]
Brummendorf 1986 {published data only}
- Brummendorf T, Anderer FA, Staab HJ, Hornung A, Kieninger G. [The doubling time of circulating CEA as an individual prognostic criterion of recurrence in patients with gastrointestinal cancers]. Klinische Wochenschrift 1986;64(2):63‐9. [DOI] [PubMed] [Google Scholar]
Bucci 1994 {published data only}
- Bucci L, Benassai G, Santoro GA. Second look in colorectal surgery. Diseases of the Colon and Rectum 1994;37(2 Suppl):S123‐6. [DOI] [PubMed] [Google Scholar]
Camunas 1991 {published data only}
- Camunas J, Enriquez JM, Devesa JM, Morales V, Millan I. Value of follow‐up in the management of recurrent colorectal cancer. European Journal of Surgical Oncology 1991;17(5):530‐5. [PubMed] [Google Scholar]
Cangemi 1984 {published data only}
- Cangemi V, Fiori E, Santeusanio G. The clinical usefulness of post‐operative monitoring of plasmatic CEA in colorectal cancer [[Italian] Utilita clinica del monitoraggio post‐operatorio del cea ematico nei cancri del colon e del retto]. Giornale di Chirurgia 1984;5(1):83‐8. [Google Scholar]
Cangemi 1987 {published data only}
- Cangemi V, Volpino P, Fiori E. The role of tumour markers (CEA, TPA, CA 19‐9) in colon and rectum carcinomas. Journal of Nuclear Medicine and Allied Sciences 1987;31(2):189‐93. [PubMed] [Google Scholar]
Carl 1983 {published data only}
- Carl J, Bentzen SM, Norgaard‐Pedersen B, Kronborg O. Modelling of serial carcinoembryonic antigen changes in colorectal cancer. Scandinavian Journal of Clinical & Laboratory Investigation 1993;53(7):751‐5. [DOI] [PubMed] [Google Scholar]
Carpelan‐Holmström 1996 {published data only}
- Carpelan‐Holmström MA, Haglund CH, Järvinen HJ, Roberts PJ. Serum CA 242 and CEA detect different patients with recurrent colorectal cancer. Anticancer Research 1996;16(2):981‐6. [PubMed] [Google Scholar]
Castells 1998 {published data only}
- Castells A, Bessa X, Daniels M, Ascaso C, Lacy AM, García‐Valdecasas JC, et al. Value of postoperative surveillance after radical surgery for colorectal cancer: results of a cohort study. Diseases of the Colon and Rectum 1998;41(6):714‐23. [DOI] [PubMed] [Google Scholar]
Catania 1981 {published data only}
- Catania G, Basile F, Cardi F, Azzarello G, Mazzarino C, Campo M, et al. [Value of carcinoembryonic antigen (CEA) in the diagnosis and postoperative monitoring of patients with colorectal cancer]. Minerva Chirurgica 1981;36(9):569‐80. [PubMed] [Google Scholar]
Chang 2012 {published data only}
- Chang AC, Warrena LR, Barretoa SG, Williams R. Differing serum CEA in primary and recurrent rectal cancer ‐ a reflection of histology?. World Journal of Oncology 2012;3(2):59‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 1998 {published data only}
- Chapman MA, Buckley D, Henson DB, Armitage NC. Preoperative carcinoembryonic antigen is related to tumour stage and long‐term survival in colorectal cancer. British Journal of Cancer 1998;78(10):1346‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2010 {published data only}
- Chen CH, Hsieh MC, Lai CC, Yeh CY, Chen JS, Hsieh PS, et al. Lead time of carcinoembryonic antigen elevation in the postoperative follow‐up of colorectal cancer did not affect the survival rate after recurrence. International Journal of Colorectal Disease 2010;25(5):567‐71. [DOI] [PubMed] [Google Scholar]
Cho 2007 {published data only}
- Cho YB, Chun HK, Yun HR, Lee WS, Yun SH, Lee WY. Clinical and pathologic evaluation of patients with recurrence of colorectal cancer five or more years after curative resection. Diseases of the Colon and Rectum 2007;50(8):1204‐10. [DOI] [PubMed] [Google Scholar]
Choi 1997 {published data only}
- Choi JS, Min JS. Significance of postoperative serum level of carcinoembryonic antigen (CEA) and actual half life of CEA in colorectal cancer patients.. Yonsei Medical Journal 1997 Feb;38(1):1‐7. [DOI] [PubMed] [Google Scholar]
Colombo 1986 {published data only}
- Colombo PL, Lovotti D, Franco F, Coronelli M, Bonacasa R. [Value of CEA in the early diagnosis of colorectal cancer recurrence in the light of second‐look results]. Minerva Medica 1986;77(13):495‐8. [PubMed] [Google Scholar]
Cossu 1984 {published data only}
- Cossu F, Fodde M, Ledda P, Pisano G, Rombi GP, Scintu F, et al. [Prognostic value of CEA in postoperative monitoring of colorectal cancers]. Minerva Medica 1984;75(40):2373‐80. [PubMed] [Google Scholar]
Dalton 2010 {published data only}
- Dalton RSJ, Burn PR, Eyrebrook I. CT surveillance to detect colorectal cancer recurrence is the best option for possible curative resection. Colorectal Disease 2010;12(Suppl I):1‐13. [Google Scholar]
Dash 2012 {published data only}
- S. Dash, A. Gupta. Clinical utility of FDG PET‐CT in surveillance of patients with colorectal malignancy when serum CEA level is within normal range ‐ A prospective study. European Journal of Nuclear Medical Molecular Imaging 2012;39(Suppl 2):S581. [Google Scholar]
De Brauw 1987 {published data only}
- Brauw LM, Velde CJH, Albers GHR, Zwaveling A. The value of follow‐up after surgery for carcinoma of the colon. Nederlands Tijdschrift voor Geneeskunde 1987;131:496‐500. [PubMed] [Google Scholar]
De Levin 1982 {published data only}
- Levin RW, Levin E. Correlation of single and serial CEA determinations with the clinical evolution of cancer patients. Archiv für Geschwulstforschung 1982;52(2):105‐12. [PubMed] [Google Scholar]
De Salvo 1997 {published data only}
- Salvo L, Razzetta F, Arezzo A, Tassone U, Bogliolo G, Bruzzone D, et al. Surveillance after colorectal cancer surgery. European Journal of Surgical Oncology. 1997;23(6):522‐5. [DOI] [PubMed] [Google Scholar]
Dhar 1972 {published data only}
- Dhar P, Moore T, Zamcheck N, Kupchik HZ. Carcinoembryonic antigen (CEA) in colonic cancer. Use in preoperative and postoperative diagnosis and prognosis. JAMA 1972;221(1):31‐5. [PubMed] [Google Scholar]
Di Cristofaro 2012 {published data only}
- Cristofaro L, Scarpa M, Angriman I, Perissinotto E, Ruffolo C, Frego M, et al. Cost‐effectiveness analysis of postoperative surveillance protocols following radical surgery for colorectal cancer. Acta Chirurgica Belgica 2012;112(1):24‐32. [DOI] [PubMed] [Google Scholar]
Engarås 2001 {published data only}
- Engarås B, Kewenter J, Nilsson O, Wedel H, Hafström L. CEA, CA 50 and CA 242 in patients surviving colorectal cancer without recurrent disease. European Journal of Surgical Oncology 2001;27(1):43‐8. [DOI] [PubMed] [Google Scholar]
Farquharson 2012 {published data only}
- Farquharson AL, Genever AV, Belfield J, Hersey N, Amin SN, Noronha R. PET‐CT scan is a specific test for the detection of recurrent colorectal cancer but has limitations for patients with mucinous tumours. Colorectal Disease 2012;14(Suppl I):1‐11. [Google Scholar]
Fernandes 2006 {published data only}
- Fernandes LC, Kim SB, Saad SS, Matos D. Value of carcinoembryonic antigen and cytokeratins for the detection of recurrent disease following curative resection of colorectal cancer. World Journal of Gastroenterololgy 2006;12(24):3891‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Filella 1994 {published data only}
- Filella X, Molina R. Use of CA 19‐9 in the early detection of recurrences in colorectal cancer: comparison with CEA. Tumour Biology 1994;15(1):1‐6. [DOI] [PubMed] [Google Scholar]
Filiz 2009 {published data only}
- Filiz AI, Sucullu I, Kurt Y, Karakas DO, Gulec B, Akin ML. Persistent high postoperative carcinoembryonic antigen in colorectal cancer patients‐‐is it important?. Clinics (Sao Paulo) 2009;64(4):287‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finlay 1983 {published data only}
- Finlay IG, McArdle CS. Role of carcinoembryonic antigen in detection of asymptomatic disseminated disease in colorectal carcinoma. British Medical Journal (Clinical Research Edition) 1983;286(6373):1242‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiocchi 2011 {published data only}
- Fiocchi F, Iotti V, Ligabue G, Malavasi N, Luppi G, Bagni B, et al. Role of carcinoembryonic antigen, magnetic resonance imaging, and positron emission tomography‐computed tomography in the evaluation of patients with suspected local recurrence of colorectal cancer. Clinical Imaging 2011;35(4):266‐73. [DOI] [PubMed] [Google Scholar]
Florio 1988 {published data only}
- Florio MG, Artemisia A, Giorgianni G, Cogliandolo A, Giacobbe G, Manganaro T, et al. Recurrences in patients operated on by colorectal cancer. Diagnostic reliability of two tumour markers. Chirurgia Gastroenterologica 1988;22(4):475‐7. [Google Scholar]
Fora 2012 {published data only}
- Fora AA, Patta AM, Attwood K, Wilding GE, Fakih M. Intensive radiographic and CEA screening and salvage resection in patients with stage II and III colorectal cancer. Journal of Clinical Oncology 2012;30(Supplement 4):abstract 405. [Google Scholar]
Forones 1997 {published data only}
- Forones NM, Tanaka M, Falcão JB. CEA as a prognostic index in colorectal cancer. Sao Paulo Medical Journal 1997 Nov‐Dec;115(6):1589‐92. [DOI] [PubMed] [Google Scholar]
Forones 1998 {published data only}
- Forones NM, Tanaka M, Machado D. Increased carcinoembryonic antigen and absence of recurrence in monitoring colorectal cancer. Arquivos de Gastroenterologia 1998;35(2):100‐3. [PubMed] [Google Scholar]
Fortner 1988 {published data only}
- Fortner JG. Recurrence of colorectal cancer after hepatic resection. America Journal of Surgery 1988;155(3):378‐82. [DOI] [PubMed] [Google Scholar]
Fournier 1999 {published data only}
- Fournier RS, Kilroy K, Alavi A. Correlation between FDG PET imaging results and plasma CEA levels in patients with suspected recurrent colon carcinoma. Journal of Nuclear Medicine 1999;40(5):241P. [Google Scholar]
Fucini 1983 {published data only}
- Fucini C, Tommasi MS, Cardona G, Malatantis G, Panichi S, Bettini U. Limitations of CEA monitoring as a guide to second‐look surgery in colorectal cancer follow‐up. Tumori 1983;69(4):359‐64. [DOI] [PubMed] [Google Scholar]
Fucini 1984 {published data only}
- Fucini C, Malatantis G, D'Elia M, Tommasi MS. CEA monitoring and recurrences in the active follow‐up of rectal cancer operated for cure [[Italian] Monitoraggio del cea e recidive nel follow‐up attivo del cancro del retto operato radicalmente]. Rivista Italiana di Colon‐Proctologia 1984;3(1):37‐45. [Google Scholar]
Fucini 1985 {published data only}
- Fucini C, Rosi S, Herd‐Smith A. Value and limitations of intensive follow‐up after radical surgery for rectocolonic cancer [[Italian] Significato e limiti del follow up intensivo dopo intervento radicale per cancro del colon‐retto]. Minerva Chirurgica 1985;40(11):783‐6. [PubMed] [Google Scholar]
Gail 1981 {published data only}
- Gail MH. Evaluating serial cancer marker studies in patients at risk of recurrent disease. Biometrics 1981;37(1):67‐78. [PubMed] [Google Scholar]
Gajdukevich 2010 {published data only}
- Gajdukevich IV, Kitaev AB, Sharapov GN, Byhovets IV, Turlaj DM. The value of biomolecular tumour markers in abdominal cancer recurrence. Techniques in Coloproctology 2010;14(1):75‐6. [Google Scholar]
Gaudagni 1999 {published data only}
- Gaudagni F. The clinical utility of serum tumor markers in the management of gastrointestinal cancer patients. Journal of Clinical Ligand Assay 1999;22(4):364‐6. [Google Scholar]
Graham 1998 {published data only}
- Graham RA, Wang S, Catalano PJ, Haller DG. Postsurgical surveillance of colon cancer: preliminary cost analysis of physician examination, carcinoembryonic antigen testing, chest x‐ray, and colonoscopy. Annals of Surgery 1998 Jul;228(1):59‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gray 1981 {published data only}
- Gray BN, Walker C, Barnard R. Value of serial carcinoembryonic antigen determinations for early detection of recurrent cancer. Medical Journal of Australia 1981;1(4):177‐8. [DOI] [PubMed] [Google Scholar]
Griesenberg 1999 {published data only}
- Griesenberg D, Nurnberg R, Bahlo M, Klapdor R. CEA, TPS, CA 19‐9 and CA 72‐4 and the fecal occult blood test in the preoperative diagnosis and follow‐up after resective surgery of colorectal cancer. Anticancer Research 1999;19(4A):2443‐50. [PubMed] [Google Scholar]
Grossetti 1981 {published data only}
- Grossetti D, Certaines J, Trebuchet G. The value and limitations of carcinoembryonic antigen levels (CEA) in colorectal cancers [[French] Valeur et limites du dosage de l'antigene carcino‐embryonnaire (Ace) dans les cancers colo‐rectaux]. Annales de Chirurgie 1981;35(19):875‐7. [PubMed] [Google Scholar]
Grossmann 2007 {published data only}
- Grossmann I, Bock GH, Meershoek‐Klein Kranenbarg WM, Velde CJ, Wiggers T. Carcinoembryonic antigen (CEA) measurement during follow‐up for rectal carcinoma is useful even if normal levels exist before surgery. A retrospective study of CEA values in the TME trial. European Journal of Surgical Oncology 2007;33(2):183‐7. [DOI] [PubMed] [Google Scholar]
Haga 1990 {published data only}
- Haga S, Takahashi N, Kato H, Mori M, Umeda H, Azuhata H, et al. Study of serum CEA as an index for forecasting recurrence of colorectal carcinoma. Journal of Tokyo Women's Medical College 1990;60(2):163‐6. [Google Scholar]
Hall 1994 {published data only}
- Hall NR, Finan PJ, Stephenson BM, Purves DA, Cooper EH. The role of CA‐242 and CEA in surveillance following curative resection for colorectal cancer. British Journal of Cancer 1994 Sep;70(3):549‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hara 2011 {published data only}
- Hara M, Sato M, Takahashi H, Takayama S, Takeyama H. Accuracy of monitoring serum carcinoembryonic antigen levels in postoperative stage III colorectal cancer patients is limited to only the first postoperative year. Surgery Today 2011 Oct;41(10):1357‐62. [DOI] [PubMed] [Google Scholar]
Herrera 1976 {published data only}
- Herrera MA, Chu TM, Holyoke ED. Carcinoembryonic antigen (CEA) as a prognostic and monitoring test in clinically complete resection of colorectal carcinoma. Annals of Surgery 1976;183(1):5‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hida 1996 {published data only}
- Hida J, Yasutomi M, Shindoh K, Kitaoka M, Fujimoto K, Ieda S, et al. Second‐look operation for recurrent colorectal cancer based on carcinoembryonic antigen and imaging techniques. Diseases of the Colon and Rectum 1996;39(1):74‐9. [DOI] [PubMed] [Google Scholar]
Hohenberger 1994 {published data only}
- Hohenberger P, Schlag PM, Gerneth T, Herfarth C. Pre‐ and postoperative carcinoembryonic antigen determinations in hepatic resection for colorectal metastases. Predictive value and implications for adjuvant treatment based on multivariate analysis. Annals of Surgery 1994;219(2):135‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holt 2010 {published data only}
- Holt A, Nelson RA, Lai L. Surveillance with serial serum carcinoembryonic levels detect colorectal cancer recurrences in patients who are initial nonsecretors. American Surgeon 2010;76(10):1100‐3. [PubMed] [Google Scholar]
Holubec 2000 {published data only}
- Holubec L Jr, Pikner R, Topolcan O, Finek J, Holubec L Sen, Pecen L. The usefulness of tumor markers in patients with colorectal carcinoma for the detection of local recurrences and distant metastases. Coloproctology 2001;23:26‐31. [Google Scholar]
Holyoke 2975 {published data only}
- Holyoke ED, Chu TM, Murphy GP. CEA as a monitor of gastrointestinal malignancy. Cancer 1975;35(3):830‐6. [DOI] [PubMed] [Google Scholar]
Houlbec 2001 {published data only}
- Holubec Jr L, Pikner R, Topolcan O, Finek J, Holubec Sen L, Pecen L. The usefulness of tumor markers in patients with colorectal carcinoma for the detection of local recurrences and distant metastases. Coloproctology 2001;23(1):26‐31. [Google Scholar]
Humphreys 2011 {published data only}
- Humphreys A, Cornish J, Stevenson J, Corr C, Billings P, Chandran P. Is a normal CEA false reassurance following curative resection for non metastatic colorectal cancer?. Colorectal Disease. Copenhagen: 6th Scientific and Annual Meeting of the European Society of Coloproctology, 2011; Vol. 13:36.
Huyghe 1983 {published data only}
- Huyghe J. CEA radioimmunoassay. Clinical applications in colorectal cancer. Acta Chirurgica Belgica 1983;83(2):77‐88. [PubMed] [Google Scholar]
Iarumov 1998 {published data only}
- Iarumov N, Ignatov A, Viiachki I. [The pre‐ and postoperative monitoring of the immunological indices and tumor markers in colorectal carcinoma]. Khirurgiia 1998;51(3):42‐8. [PubMed] [Google Scholar]
Indinnimeo 1999 {published data only}
- Indinnimeo M, Cicchini C, Stazi A, Ghini C, Alessandrini G, Reale MG. Carcinoembryonic antigen in recurrence of colorectal cancer. 23rd National Congress of the Societa‐Italiana‐di‐Chirurgia‐Oncologica (SICO), Perugia, Italy. 1999.
Ito 2002 {published data only}
- Ito K, Hibi K, Ando H, Hidemura K, Yamazaki T, Akiyama S, et al. Usefulness of analytical CEA doubling time and half‐life time for overlooked synchronous metastases in colorectal carcinoma. Japanese Journal of Clinical Oncology 2002;32(2):54‐8. [DOI] [PubMed] [Google Scholar]
Jaeger 1975 {published data only}
- Jaeger P, Pettavel J, Wuilleret B, Bertholet MM, Mach JP. [Value and limits of the determination of carcinoembryonic antigen (CEA) in postoperative evaluation of patients with colonic and rectal carcinomas]. Schweizerische Medizinische Wochenschrift Journal Suisse de Medecine 1975;105(46):1533‐8. [PubMed] [Google Scholar]
Jiang 1989 {published data only}
- Jiang R. [Clinical significance of serum CEA determination in the diagnosis of colorectal cancer]. Chung‐Hua Chung Liu Tsa Chih [Chinese Journal of Oncology] 1989;11(5):348‐51. [PubMed] [Google Scholar]
Kanellos 2006b {published data only}
- Kanellos I, Zacharakis E, Demetriades H, Christoforidis E, Kanellos D, Pramateftakis MG, et al. Value of carcinoembryonic antigen assay in predicting hepatic metastases, local recurrence, and survival after curative resection of colorectal cancer. Surgery Today 2006;36(10):879‐84. [DOI] [PubMed] [Google Scholar]
Karesen 1980 {published data only}
- Karesen R, Hertzberg J, Johannesen J, Thoresen BO, Orjasaeter H. Carcinoembryonic antigen in the diagnosis and follow‐up of colorectal carcinoma. American Journal of Proctology, Gastroenterology & Colon & Rectal Surgery 1980;31(11):18‐22. [PubMed] [Google Scholar]
Kawamura 2010 {published data only}
- Kawamura YJ, Tokumitsu A, Mizokami K, Sasaki J, Tsujinaka S, Konishi F. First alert for recurrence during follow‐up after potentially curative resection for colorectal carcinoma: CA 19‐9 should be included in surveillance programs. Clinical Colorectal Cancer 2010;9(1):48‐51. [DOI] [PubMed] [Google Scholar]
Kerr 2012 {published data only}
- Kerr NA, Jha B, Edwards T, Karnati G, Mackey PM. The detection of colorectal cancer recurrence following curative resection. Colorectal Disease 2012;14(Suppl 1):12‐40. [Google Scholar]
Khan 2009 {published data only}
- Khan K, Rathore M, Loughlin V, Tham TCK, Bhatti MI, Allen D, et al. Retrospective analysis of resected primary colorectal cancer revealed no correlation between node harvest and node involvement. European Journal of Cancer 2009;7(2‐3):326. [PubMed] [Google Scholar]
Kimura 1986 {published data only}
- Kimura O, Kaibara N, Nishidoi H, Okamoto T, Takebayashi M, Kawasumi H, et al. Carcinoembryonic antigen slope analysis as an early indicator for recurrence of colorectal carcinoma. Japanese Journal of Surgery 1986;16(2):106‐11. [DOI] [PubMed] [Google Scholar]
Kishimoto 2010 {published data only}
- Kishimoto G, Murakami K, Con SA, Yamasaki E, Domeki Y, Tsubaki M, et al. [Follow‐up after curative surgery for colorectal cancer: impact of positron emission tomography ‐ computed tomography (PET/CT)]. Revista de Gastroenterologia del Peru 2010;30(4):328‐33. [PubMed] [Google Scholar]
Koch 1977 {published data only}
- Koch M, McPherson TA, Morrish DW. Carcinoembryonic antigen: 3 years' experience in a cancer clinic.. Canadian Medical Association Journal 1977 Apr 9;116(7):769‐71. [PMC free article] [PubMed] [Google Scholar]
Koch 1979 {published data only}
- Koch M, McPherson TA. Predictive value of plasma CEA in patients with colorectal carcinoma. Journal of Surgical Oncology 1979;12(4):319‐25. [DOI] [PubMed] [Google Scholar]
Koch 1982 {published data only}
- Koch M, Washer G, Gaedke H, McPherson TA. Carcinoembryonic antigen: usefulness as a postsurgical method in the detection of recurrence in Dukes stages B2 and C colorectal cancers. Journal of the National Cancer Institute 1982;69(4):813‐5. [PubMed] [Google Scholar]
Koga 1999 {published data only}
- Koga H, Moriya Y, Akasu T, Fujita S. The relationship between prognosis and CEA‐dt after hepatic resection in patients with colorectal carcinomas. European Journal of Surgical Oncology 1999;25(3):292‐6. [DOI] [PubMed] [Google Scholar]
Korner 2005 {published data only}
- Körner H, Söreide K, Stokkeland PJ, Söreide JA. Systematic follow‐up after curative surgery for colorectal cancer in Norway: a population‐based audit of effectiveness, costs, and compliance. Journal of Gastrointestinal Surgery 2005;9(3):320‐8. [DOI] [PubMed] [Google Scholar]
Kumar 2011 {published data only}
- Kumara K, Aggarwalb D, Ardakanib A, Syedb M, Ingham Clark C. Efficacy of an intensive colorectal cancer follow‐up programme. European Journal of Surgical Oncology 2011;37(11):997. [Google Scholar]
Lagache 1980 {published data only}
- Lagache G, Dessaint JP, Triboulet JP. Indications for repeat operations for recurrence of rectocolic cancer: Contribution of serum carcino‐embryonic antigen (CEA) levels [[French] Valeur du taux serique d'antigene carcino‐embryonnaire (A.C.E.) dans l'indication de reintervention pour recidive de cancer colo‐rectal]. Chirurgie ‐ Memoires de l'Academie de Chirurgie 1980;106(5):322‐34. [PubMed] [Google Scholar]
Lauterbach 1987 {published data only}
- Lauterbach M, Dehne A, Hesse V, Hofig G. [CEA determination in the follow‐up of colorectal cancers]. Zentralblatt fur Chirurgie 1987;112(15):968‐74. [PubMed] [Google Scholar]
Lavin 1981 {published data only}
- Lavin PT, Day J, Holyoke ED, Mittelman A, Chu TM. A statistical evaluation of baseline and follow‐up carcinoembryonic antigen in patients with resectable colorectal carcinoma. Cancer. 1981;47(4):823‐6. [DOI] [PubMed] [Google Scholar]
Lechner 2000 {published data only}
- Lechner P, Lind P, Goldenberg DM. Can postoperative surveillance with serial CEA immunoscintigraphy detect resectable rectal cancer recurrence and potentially improve tumor‐free survival?. Journal of American College of Surgeons 2000;191(5):511‐8. [DOI] [PubMed] [Google Scholar]
Leventakos 2013 {published data only}
- Leventakos K, Lu SS, Perry DJ. Intensive CT scan surveillance for patients who have undergone curative intent treatment for colorectalcancer: The Medstar Washington Hospital Center experience. Journal of Clinical Oncology 2013;31(Suppl):abstract e14675. [Google Scholar]
Levy 2012 {published data only}
- Levy M, Lipska L, Visokai V, Veskrna K, Simsa J. Tumor markers in colorectal cancer relapse. Tumor Biology 2012;33(Suppl I):S15‐S80. [Google Scholar]
Lipska 2007 {published data only}
- Lipska L, Visokai V, Levy M, Svobodova S, Kormunda S, Finek J. Tumor markers in patients with relapse of colorectal carcinoma. Anticancer Research 2007;27(4A):1901‐5. [PubMed] [Google Scholar]
Lipska 2010 {published data only}
- Lipska L, Visokai V, Levy M. Resectability of colorectal cancer relapse. European Journal of Surgical Oncology 2010;36(9):876‐7. [Google Scholar]
Lorenz 1986 {published data only}
- Lorenz M, Happ J, Hottenrott C, Maul FD, Baum RP, Hor G, et al. [Clinical evaluation of the tumor marker CA 19‐9 in comparison with carcinoembryonic antigen (CEA) in surgical pre‐ and postoperative diagnosis]. Nuclear‐Medizin 1986;25(1):9‐14. [PubMed] [Google Scholar]
Lunde 1982 {published data only}
- Lunde OC, Havig O. Clinical significance of carcinoembryonic antigen (CEA) in patients with adenocarcinoma in colon and rectum. Acta Chirurgica Scandinavica 1982;148(2):189‐93. [PubMed] [Google Scholar]
Ma 2006 {published data only}
- Ma CJ, Hsieh JS, Wang WM, Su YC, Huang CJ, Huang TJ, et al. Multivariate analysis of prognostic determinants for colorectal cancer patients with high preoperative serum CEA levels: prognostic value of postoperative serum CEA levels. Kaohsiung Journal of Medical Sciences 2006;22(12):604‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mach 1974 {published data only}
- Mach JP, Jaeger P, Bertholet MM, Ruegsegger CH, Loosli RM, Pettavel J. Detection of recurrence of large‐bowel carcinoma by radioimmunoassay of circulating carcinoembryonic antigen (C.E.A.). Lancet 1974;2(7880):535‐40. [DOI] [PubMed] [Google Scholar]
Makela 1995 {published data only}
- Makela JT, Laitinen SO, Kairaluoma MI. Five‐year follow‐up after radical surgery for colorectal cancer. Results of a prospective randomized trial. Archives of Surgery 1995;130(10):1062‐7. [DOI] [PubMed] [Google Scholar]
Makis 2013 {published data only}
- Makis W, Kurzencwyg D, Hickeson M. 18F‐FDG PET/CT superior to serum CEA in detection of colorectal cancer and its recurrence. Clinical Imaging 2013;37(6):1094‐7. [DOI] [PubMed] [Google Scholar]
Mant 2013 {published data only}
- Mant D, Perera R, Gray A, Rose P, Fuller A, Corkhill A, et al. Effect of 3‐5 years of scheduled CEA and CT follow‐up to detect recurrence of colorectal cancer: FACS randomized controlled trial. Journal of Clinical Oncology. Chicago: Annual Meeting of the American Society of Clinical Oncology, ASCO, 2013; Vol. 20.
Martin 1976 {published data only}
- Martin EW, Jr, Kibbey WE, DiVecchia L, Anderson G, Catalano P, Minton JP. Carcinoembryonic antigen: clinical and historical aspects. Cancer 1976;37(1):62‐81. [DOI] [PubMed] [Google Scholar]
Martin 1979 {published data only}
- Martin EW Jr, Cooperman M, King G, Rinker L, Carey LC, Minton JP. A retrospective and prospective study of serial CEA determinations in the early detection of recurrent colon cancer. American Journal of Surgery 1979;137(2):167‐9. [DOI] [PubMed] [Google Scholar]
Martin 1980 {published data only}
- Martin EW Jr, Cooperman M, Carey LC, Minton JP. Sixty second‐look procedures indicated primarily by rise in serial carcinoembryonic antigen. Journal of Surgical Research 1980;28(5):389‐94. [DOI] [PubMed] [Google Scholar]
Marucci 1983 {published data only}
- Marucci MM, Capussotti L, Molinaro G, Duglio A, Torossian K, Marini C. [Carcinoembryonic antigen in colorectal tumors. Prognostic evaluation and postoperative monitoring]. Minerva Chirurgica 1983;38(11):751‐5. [PubMed] [Google Scholar]
May 2012 {published data only}
- May DJ, Richardson JRC, Saunders BW, Miles AJG. Intensive long term follow‐up after T1 and T2 node negative colorectal cancer is not necessary. Colorectal Disease 2012;14:22‐3. [Google Scholar]
Mazilu 2012 {published data only}
- Mazilu L, Ciufu N, Galan M, Suceveanu AI, Suceveanu AP, Parepa IR, et al. Postherapeutic follow‐up of colorectal cancer patients treated with curative intent. Chirurgia 2012;107(1):55‐8. [PubMed] [Google Scholar]
McCarthy 1985 {published data only}
- McCarthy SM, Barnes D, Deveney K, Moss AA, Goldberg HI. Detection of recurrent rectosigmoid carcinoma: prospective evaluation of CT and clinical factors. American Journal of Roentgenology 1985;144(3):577‐9. [DOI] [PubMed] [Google Scholar]
Meling 1992 {published data only}
- Meling GI, Rognum TO, Clausen OP, Børmer O, Lunde OC, Schlichting E, et al. Serum carcinoembryonic antigen in relation to survival, DNA ploidy pattern, and recurrent disease in 406 colorectal carcinoma patients. Scandinavian Journal of Gastroenterology 1992 Dec;27(12):1061‐8. [DOI] [PubMed] [Google Scholar]
Mentges 1986 {published data only}
- Mentges B, Grussner R, Klotter HJ, Batz W. 10 years experience with CEA in the postoperative monitoring of patients undergoing surgery to cure colorectal carcinomas. Acta Medica Austriaca 1986;13:93‐4. [Google Scholar]
Mentges 1988 {published data only}
- Mentges B. [Effect of serial CEA determination on diagnosis, therapy and prognosis of recurrent colorectal cancer]. Langenbecks Archiv für Chirurgie 1988;373(4):227‐34. [DOI] [PubMed] [Google Scholar]
Metzger 1983 {published data only}
- Metzger U, Decurtins M, Joller H. Carcinoembryonic antigen (CEA) in large bowel cancer treated by surgery [[German] Das karzinoembryonale antigen (Cea) beim operierten dickdarmkarzinom]. schweizerische medizinische wochenschrift 1983;113(15):548‐9. [PubMed] [Google Scholar]
Metzger 1985 {published data only}
- Metzger U, Bronz K, Buhler H, Dolder A, Seefeld U, Hollinger A, et al. [Prospective follow‐up study of radically resected colorectal carcinoma. Status after 5 years]. Schweizerische Medizinische Wochenschrift Journal Suisse de Medecine 1985;115(29):1001‐4. [PubMed] [Google Scholar]
Minton 1978a {published data only}
- Minton JP, James KK, Hurtubise PE, Rinker L, Joyce S, Martin EW, Jr. The use of serial carcinoembryonic antigen determinations to predict recurrence of carcinoma of the colon and the time for a second‐look operation. Surgery, Gynecology & Obstetrics 1978;147(2):208‐10. [PubMed] [Google Scholar]
Minton 1978b {published data only}
- Minton JP, Martin EW Jr. The use of serial CEA determinations to predict recurrence of colon cancer and when to do a second‐look operation. Cancer 1978;42(3 Suppl):1422‐7. [DOI] [PubMed] [Google Scholar]
Minton 1989 {published data only}
- Minton J, Chevinsky AH. CEA directed second‐look surgery for colon and rectal cancer. Chirurgiae et Gynaecologiae 1989;78(1):32‐7. [PubMed] [Google Scholar]
Miwa 1980 {published data only}
- Miwa Y. Serum CEA levels in various gastrointestinal diseases with special reference to colorectal cancer. Japanese Journal of Gastroenterology 1980;77(7):1069‐75. [PubMed] [Google Scholar]
Moertel 1978 {published data only}
- Moertel CG, Schutt AJ, Go VL. Carcinoembryonic antigen test for recurrent colorectal carcinoma. JAMA 1978;239(11):1065‐6. [PubMed] [Google Scholar]
Morelli 1985 {published data only}
- Morelli M, Nardi M, Valle M, Balducci D, Serafini D, Bernardini P. [Experience in the use of the tumor markers CEA, GICA and TPA in the postoperative monitoring of colorectal neoplasms]. Minerva Medica 1985;76:34‐35. [PubMed] [Google Scholar]
Moreno Carretero 1998 {published data only}
- Moreno Carretero G, Cerdan Miguel FJ, Maestro de las Casas ML, Martinez Cortijo S, Ortega MD, Pardo Martinez M, et al. Serum and tissue CEA in colorectal cancer: clinical relevance. Revista Espanola de Enfermedades Digestivas 1998;90(6):391‐401. [PubMed] [Google Scholar]
Moschl 1980 {published data only}
- Moschl P, Staritz C, Keiler A, Kreuzer W, Fasching W. [Carcino‐embryonic antigen as screening protein in the follow‐up of patients with surgically‐treated gastrointestinal cancer (author's transl)]. Wiener Klinische Wochenschrift 1980;92(4):128‐30. [PubMed] [Google Scholar]
Nicolini 1995 {published data only}
- Nicolini A, Caciagli M, Zampieri F, Ciampalini G, Carpi A, Spisni R, et al. Usefulness of CEA, TPA, GICA, CA 72.4, and CA 195 in the Diagnosis of primary colorectal cancer and at its relapse. Cancer Detection & Prevention 1995;19(2):183‐95. [PubMed] [Google Scholar]
Nicolini 2005 {published data only}
- Nicolini A, Ferrari P, Anselmi L, Metelli MR, Carpi A, Spisni R, et al. Recurrences of colorectal cancer: time distribution and diagnostic sensitivity of serum CEA, TPA, CA19.9, CA72.4 tumour markers. Journal of Clinical Oncology 2005;23(16):301S‐S. [Google Scholar]
Nicolini 2010 {published data only}
- Nicolini A, Ferrari P, Duffy MJ, Antonelli A, Rossi G, Metelli MR, et al. Intensive risk‐adjusted follow‐up with the CEA, TPA, CA19.9, and CA72.4 tumor marker panel and abdominal ultrasonography to diagnose operable colorectal cancer recurrences: effect on survival. Archives of Surgery 2010;145(12):1177‐83. [DOI] [PubMed] [Google Scholar]
Northover 1985 {published data only}
- Northover JM. Carcinoembryonic antigen and recurrent colorectal cancer. British Journal of Surgery 1985;Suppl:S44‐6. [DOI] [PubMed] [Google Scholar]
Northover 1986 {published data only}
- Northover J. Carcinoembryonic antigen and recurrent colorectal cancer. Gut 1986;27(2):117‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Northover 2003 {published data only}
- Northover J. Follow‐up after curative surgery for colorectal cancer. Scandinavian Journal of Surgery: SJS 2003;92(1):84‐9. [DOI] [PubMed] [Google Scholar]
Novis 1986 {published data only}
- Novis BH, Gluck E, Thomas P, Steele GD, Zurawski VR Jr, Stewart R, et al. Serial levels of CA 19‐9 and CEA in colonic cancer. Journal of Clinical Oncology 1986;4(6):987‐93. [DOI] [PubMed] [Google Scholar]
Nowacki 1983 {published data only}
- Nowacki M. [Clinical usefulness of the study of serum carcinoembryonic antigen (CEA) for the determination of the degree of tumor progression, prognosis and monitoring of surgical treatment of neoplasms of the large intestine]. Nowotwory 1983;33(1):13‐26. [PubMed] [Google Scholar]
Ntinas 2004 {published data only}
- Ntinas A, Zambas N, Al Mogrambi S, Petras P, Chalvatzoulis E, Frangandreas G, et al. Postoperative follow‐up of patients with colorectal cancer: a combined evaluation of CT scan, colonoscopy and tumour markers. Techniques in Coloproctology 2004;8(Suppl 1):s190‐2. [DOI] [PubMed] [Google Scholar]
O'Dwyer 1987 {published data only}
- O'Dwyer PJ, Mojzisik C, McCabe DP, Sickle‐Santanello BJ, Farrar WB, Martin EW Jr. Variation in recognition of recurrent colonic cancer by different CEA assays. Diseases of the Colon and Rectum 1987;30(2):133‐6. [DOI] [PubMed] [Google Scholar]
O'Dwyer 1988 {published data only}
- O'Dwyer PJ, Mojzisik C, McCabe DP, Farrar WB, Carey LC, Martin EW Jr. Reoperation directed by carcinoembryonic antigen level: the importance of a thorough preoperative evaluation. American Journal of Surgery 1988;155(2):227‐31. [DOI] [PubMed] [Google Scholar]
Obradovic 2011 {published data only}
- Obradovic VB, Artiko V, Sobic D, Petrovic N, Todorovic‐Tirnanic M, Brajkovic L, et al. Diagnosis of recurrences and metastases of colorectal carcinomas using 18FDG PET/CT. European Journal of Nuclear Medicine and Molecular Imaging 2011;38:S274. [Google Scholar]
Odariuk 1989 {published data only}
- Odariuk TS, Sevost'ianov SI, Mit'kov VV, Orlova LP. [A comparative evaluation of methods for diagnosing recurrence of rectal cancer after radical surgery]. Voprosy Onkologii 1989;35(9):1097‐103. [PubMed] [Google Scholar]
Ovaska 1989 {published data only}
- Ovaska JT, Jarvinen HJ, Mecklin JP. The value of a follow‐up programme after radical surgery for colorectal carcinoma. Scandinavian Journal of Gastroenterology 1989;24(4):416‐22. [DOI] [PubMed] [Google Scholar]
Ozhiganov 1986 {published data only}
- Ozhiganov EL, Kuznetsova LF. [Determination of carcinoembryonic and carbohydrate antigens in the diagnosis of recurrences and metastases of rectal cancer]. Meditsinskaia Radiologiia 1986;31(8):40‐4. [PubMed] [Google Scholar]
Ozkan 2012a {published data only}
- Ozkan E, Soydal C, Araz M, Aras G. Serum carcinoembryonic antigen measurement, abdominal contrast‐enhanced computed tomography, and fluorine‐18 fluorodeoxyglucose positron emission tomography/computed tomography in the detection of colorectal cancer recurrence: a correlative study. Nuclear Medicine Communications 2012;33(9):990‐4. [DOI] [PubMed] [Google Scholar]
Ozkan 2012b {published data only}
- Ozkan E, Soydal C, Araz M, Kir KM, Ibis E. The role of 18F‐FDG PET/CT in detecting colorectal cancer recurrence in patients with elevated CEA levels. Nuclear Medicine Communications 21012;33(4):395‐402. [DOI] [PubMed] [Google Scholar]
Park 2012 {published data only}
- Park I, You Y, Skibber JM, Rodriguez‐Bigas MA, Feig B, Nguyen S, et al. Recurrence patterns after multidisciplinary therapy for rectal cancer in 725 patients. Annals of Surgical Oncology 2012;19:S15‐6. [Google Scholar]
Park 2013 {published data only}
- Park JW, Chang HJ, Kim BC, Yeo HY, Kim DY. Clinical validity of tissue carcinoembryonic antigen expression as ancillary to serum carcinoembryonic antigen concentration in patients curatively resected for colorectal cancer. Colorectal Disease 2013;15(9):e503‐11. [DOI] [PubMed] [Google Scholar]
Pecorella 1996 {published data only}
- Pecorella G, Bracchitta S, Petrolito E, Cacciaguerra B, Blanco F, Cirino E. [Follow up in carcinoma of the large intestine]. Annali Italiani di Chirurgia 1996;67(1):41‐7. [PubMed] [Google Scholar]
Peethambaram 1997 {published data only}
- Peethambaram P, Weiss M, Loprinzi CL, Novotny P, O'Fallon JR, Erlichman C, et al. An evaluation of postoperative follow‐up tests in colon cancer patients treated for cure. Oncology 1997;54(4):287‐92. [DOI] [PubMed] [Google Scholar]
Pereira 2004 {published data only}
- Pereira JMC, Martinez C, Pimenta T, Oliveira MC. Carcinoembryonic antigen dosage and colorectal cancer recurrence. Diseases of the Colon and Rectum 2004;47(6):986. [Google Scholar]
Persijin 1981 {published data only}
- Persijn JP, Hart AA. Prognostic significance of CEA in colorectal cancer: a statistical study. Journal of Clinical Chemistry & Clinical Biochemistry 1981;19(11):1117‐23. [DOI] [PubMed] [Google Scholar]
Pfeiffer 1979 {published data only}
- Pfeiffer R, Reis HE, Wittig HD. CEA determination with the Hansen method: Postsurgical follow‐up of patients with colorectal carcinoma [[German] Erfahrungen mit der cea‐bestimmung bei der postoperativen verlaufskontrolle von patienten mit colorectalem carcinom]. Journal of Cancer Research and Clinical Oncology 1979;93(1):85‐92. [Google Scholar]
Philips 1984 {published data only}
- Phillips RK, Hittinger R, Blesovsky L, Fry JS, Fielding LP. Local recurrence following 'curative' surgery for large bowel cancer: I. The overall picture. British Journal of Surgery 1984;71(1):12‐6. [DOI] [PubMed] [Google Scholar]
Pietra 1998 {published data only}
- Pietra N, Sarli L, Costi R, Ouchemi C, Grattarola M, Peracchia A. Role of follow‐up in management of local recurrences of colorectal cancer ‐ A prospective, randomized study. Diseases of the Colon and Rectum 1998;41(9):1127‐33. [DOI] [PubMed] [Google Scholar]
Plebani 1996 {published data only}
- Plebani M, Paoli M, Basso D, Roveroni G, Giacomini A, Galeotti F, et al. Serum tumor markers in colorectal cancer staging, grading, and follow‐up. Journal of Surgical Oncology 1996;62(4):239‐44. [DOI] [PubMed] [Google Scholar]
Pompecki 1980 {published data only}
- Pompecki R, Winkler R. Clinical value of serial serum CEA determinations in postoperative follow‐up of colorectal cancer [[German] Klinische bedeutung der routinemassigen serum‐cea‐bestimmung fur die postoperative kontrolle des kolorektalen karzinoms]. Medizinische Welt 1980;31(49):1780‐3. [PubMed] [Google Scholar]
Pribelsky 2002 {published data only}
- Pribelsky M, Pechan J, Krizan M, Pindak D. CEA and relapse after the operation of colorectal carcinoma. Bratislavske Lekarske Listy 2002;103(11):428‐31. [PubMed] [Google Scholar]
Primrose 2011 {published data only}
- Primrose JN, Fuller A, Rose P, Perera‐Salazar R, Mellor J, Corkhill A, et al. Follow‐up after colorectal cancer surgery: Preliminary observational findings from the UK FACS trial. Journal of Clinical Oncology. Chicago: ASCO Annual Meeting 2011, 2011.
Primrose 2014 {published data only}
- Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow‐up to detect recurrence of colorectal cancer: the FACS randomized clinical trial.. JAMA 2014;311(3):263‐70. [DOI] [PubMed] [Google Scholar]
Quentmeier 1990 {published data only}
- Quentmeier A, Schlag P, Smok M, Herfarth C. Re‐operation for recurrent colorectal cancer: the importance of early diagnosis for resectability and survival. European Journal of Surgical Oncology 1990;16(4):319‐25. [PubMed] [Google Scholar]
Reddy 2013 {published data only}
- Reddy GA, Abrar ML, Rajender K, Anish B, Mittal BR. Role of FDG PET/CT in evaluation of suspected recurrence of disease in patients of colon carcinoma in relation to carcinoembryonic antigen (CEA) levels. European Journal of Nuclear Medicine and Molecular Imaging 2013;40:S474‐S. [Google Scholar]
Revetria 1989 {published data only}
- Revetria P, Repetto L, Perino M, Ciabattoni N, Gambetta G, Ferro A. Postoperative monitoring of carcinoma of the large bowel. Diagnostic and prognostic value of tumor markers CEA, CA 19‐9 and TPA. [Italian]. Chirurgia 1989;2(3):121‐4. [Google Scholar]
Rezamansourian 2011 {published data only}
- Rezamansourian A, Ghaemi E. The prevalence of elevated carcinoembroynic antigen at Gorgan south‐east Caspian sea of Northern Iran. Journal of Clinical and Diagnostic Research 2011;5(1):74‐7. [Google Scholar]
Rieger 1975 {published data only}
- Rieger A, Wahren B. CEA levels at recurrence and metastases; importance for detecting secondary disease. Scandinavian Journal of Gastroenterology 1975;10(8):869‐74. [PubMed] [Google Scholar]
Rockall 1999 {published data only}
- Rockall TA, McDonald PJ. Carcinoembryonic antigen: its value in the follow‐up of patients with colorectal cancer. International Journal of Colorectal Disease 1999;14(1):73‐7. [DOI] [PubMed] [Google Scholar]
Rocklin 1990 {published data only}
- Rocklin MS, Slomski CA, Watne AL. Postoperative surveillance of patients with carcinoma of the colon and rectum. American Surgeon 1990;56(1):22‐7. [PubMed] [Google Scholar]
Rocklin 1991 {published data only}
- Rocklin MS, Senagore AJ, Talbott TM. Role of carcinoembryonic antigen and liver function tests in the detection of recurrent colorectal carcinoma. Diseases of the Colon and Rectum 1991;34(9):794‐7. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Moranta 2006a {published data only}
- Rodriguez‐Moranta F, Salo J, Arcusa A, Boadas J, Pinol V, Bessa X, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. Journal of Clinical Oncology 2006;24(3):386‐93. [DOI] [PubMed] [Google Scholar]
Rognum 1986 {published data only}
- Rognum TO, Heier HE, Orjasaeter H, Thorud E, Brandtzaeg P. Comparison of two CEA assays in primary and recurrent large bowel carcinoma with different DNA ploidy pattern. European Journal of Cancer & Clinical Oncology 1986;22(10):1165‐9. [DOI] [PubMed] [Google Scholar]
Sagar 1989 {published data only}
- Sagar PM, Cooper EH, Finan PJ. A prospective assessment of the tumor‐markers Ca50, Ca195 and CEA in prediction of tumor stage and recurrence in colorectal‐cancer. British Journal of Cancer 1989;60(3):488. [Google Scholar]
Sandelewski 2005 {published data only}
- Sandelewski A, Kokocinska D, Partyka R, Kocot J, Starzewski J, Chanek I, et al. [Usefulness of evaluation of carcinoembryonic antigen (CEA) and soluble fragments of cytokeratin 18‐th (TPS) in postoperative monitoring of patients with colorectal cancer]. Polski Merkuriusz Lekarski 2005;18(108):647‐50. [PubMed] [Google Scholar]
Sanli 2012 {published data only}
- Sanli Y, Kuyumcu S, Ozkan ZG, Kilic L, Balik E, Turkmen C, et al. The utility of FDG‐PET/CT as an effective tool for detecting recurrent colorectal cancer regardless of serum CEA levels. Annals of Nuclear Medicine 2012;26(7):551‐8. [DOI] [PubMed] [Google Scholar]
Sardi 1989 {published data only}
- Sardi A, Agnone CM, Nieroda CA, Mojzisik C, Hinkle G, Ferrara P, et al. Radioimmunoguided surgery in recurrent colorectal cancer: the role of carcinoembryonic antigen, computerized tomography, and physical examination . Southern Medical Journal 1989;82(10):1235‐44. [PubMed] [Google Scholar]
Sarikaya 2007 {published data only}
- Sarikaya I, Bloomston M, Povoski SP, Zhang J, Hall NC, Knopp MV, et al. FDG‐PET scan in patients with clinically and/or radiologically suspicious colorectal cancer recurrence but normal CEA. World Journal of Surgical Oncology 2007;5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Secco 1989 {published data only}
- Secco GB, Fardelli R, Campora E, Rovida S, Onetto M, Marroni P, et al. CEA as a prognostic factor and early indicator of recurrence in colorectal cancer. Journal of Experimental and Clinical Cancer Research 1989;8(3):173‐7. [Google Scholar]
Secco 2000 {published data only}
- Secco GB, Fardelli R, Rovida S, Gianquinto D, Baldi E, Bonfante P, et al. Is intensive follow‐up really able to improve prognosis of patients with local recurrence after curative surgery for rectal cancer?. Annals of Surgical Oncology 2000;7(1):32‐7. [DOI] [PubMed] [Google Scholar]
Segol 1977 {published data only}
- Segol PH, Travert G, Davy A. Serum carcinoembryonic antigen determinations during post operative follow up of colorectal carcinomas [[French] Le dosage de l'antigene carcino embryonnaire dans la surveillance post operatoire des cancers colo rectaux]. Lyon Chirurgical 1977;73(4):289‐92. [Google Scholar]
Shirley 2012 {published data only}
- Shirley LA, McNally M, Huntington J, Jones N, Malhotra L, Bloomston M, et al. Correlation of postoperative CEA trends with survival and patterns of recurrence after hepatectomy for colorectal cancer metastases. Journal of Clinical Oncology. San Francisco, CA United States: 2012 Gastrointestinal Cancers Symposium, 2012.
Simo 2002 {published data only}
- Simo M, Lomena F, Setoain J, Perez G, Castellucci P, Costansa JM, et al. FDG‐PET improves the management of patients with suspected recurrence of colorectal cancer. Nuclear Medicine Communications 2002;23(10):975‐82. [DOI] [PubMed] [Google Scholar]
Sirisriro 1996 {published data only}
- Sirisriro R, Podoloff DA, Patt YZ, Curley SA, Kasi LP, Bhadkamkar VA, et al. 99Tcm‐IMMU4 imaging in recurrent colorectal cancer: efficacy and impact on surgical management. Nuclear Medicine Communications 1996;17(7):568‐76. [DOI] [PubMed] [Google Scholar]
Song 2010 {published data only}
- Song S, Minsky B, Polite B, Liauw S. Post‐treatment CEA values, and CEA trends at the time of uncertain post‐treatment imaging, have prognostic value after combined modality therapy for rectal cancer. International Journal of Radiation Oncology Biology Physics. 52nd Annual Meeting of the American Society for Radiation Oncology, San Diego, CA, USA. 2010:S313.
Sorensen 2010 {published data only}
- Sorensen NF, Jensen AB, Wille‐Jorgensen P, Friberg L, Rordam L, Ingeman L, et al. Strict follow‐up programme including CT and 18F‐FDG‐PET after curative surgery for colorectal cancer. Colorectal Disease 2010;12:e224‐8. [DOI] [PubMed] [Google Scholar]
Staab 1985a {published data only}
- Staab HJ, Anderer FA, Stumpf E, Hornung A, Fischer R, Kieninger G. Eighty‐four potential second‐look operations based on sequential carcinoembryonic antigen determinations and clinical investigations in patients with recurrent gastrointestinal cancer. American Journal of Surgery 1985;149(2):198‐104. [DOI] [PubMed] [Google Scholar]
Staab 1985b {published data only}
- Staab HJ, Brummendorf T, Hornung A, Anderer FA, Kieninger G. The clinical validity of circulating tumor‐associated antigens CEA and CA 19‐9 in primary diagnosis and follow‐up of patients with gastrointestinal malignancies. Klinische Wochenschrift 1985;63(3):106‐15. [DOI] [PubMed] [Google Scholar]
Stautner‐Brückmann 1990 {published data only}
- Stautner‐Brückmann C, Schneider W, Gresser U, Richter‐Turtur M, Eibl‐Eibesfeld B, Zoller WG, et al. Is ultrasound superior to carcinoembryonic antigen (CEA) measurement in recurrent tumor screening of patients with colorectal carcinoma?. Bildgebung 1990;57(1‐2):17‐20. [PubMed] [Google Scholar]
Steele 1980 {published data only}
- Steele G Jr, Zamcheck N, Wilson R. Results of CEA‐initiated second‐look surgery for recurrent colorectal cancer. American Journal of Surgery 1980;139(4):544‐8. [DOI] [PubMed] [Google Scholar]
Stuckle 2000 {published data only}
- Stuckle CA, Ibing HP, Adamietz IA. Value of computed tomography and tumor markers in detection of recurrent rectal carcinoma after surgery and radiotherapy [[German] Wertigkeit der computertomographie und der tumormarker zur beurteilung von lokalrezidiven beim operierten und nachbestrahlten rektumkarzinom]. Tumor Diagnostik und Therapie 2000;21(3):61‐7. [Google Scholar]
Su 2012 {published data only}
- Su BB, Shi H, Wan J. Role of serum carcinoembryonic antigen in the detection of colorectal cancer before and after surgical resection. World Journal of Gastroenterology 2012;18(17):2121‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sugarbaker 1976 {published data only}
- Sugarbaker PH, Zamcheck N, Moore FD. Assessment of serial carcinoembryonic antigen (CEA) assays in postoperative detection of recurrent colorectal cancer. Cancer 1976;38(6):2310‐5. [DOI] [PubMed] [Google Scholar]
Szymendera 1982 a {published data only}
- Szymendera JJ, Nowacki MP, Szawlowski AW, Kaminska JA. Predictive value of plasma CEA levels: preoperative prognosis and postoperative monitoring of patients with colorectal carcinoma. Diseases of the Colon and Rectum 1982;25(1):46‐52. [DOI] [PubMed] [Google Scholar]
Szymendera 1982 b {published data only}
- Szymendera JJ, Wilczynska JE, Nowacki MP, Kaminska JA, Szawowski AW. Serial CEA assays and liver scintigraphy for the detection of hepatic metastases from colorectal carcinoma. Diseases of the Colon and Rectum 1982;25(3):191‐7. [DOI] [PubMed] [Google Scholar]
Szymendera 1985 {published data only}
- Szymendera JJ, Nowacki MP, Kozlowicz‐Gudzinska I, Kowalska M. Value of serum levels of carcinoembryonic antigen, CEA, and gastrointestinal cancer antigen, GICA or CA 19‐9, for preoperative staging and postoperative monitoring of patients with colorectal carcinoma. Diseases of the Colon and Rectum 1985;28(12):895‐9. [DOI] [PubMed] [Google Scholar]
Takashima 1982 {published data only}
- Takashima S, Kosaka T, Uemura T. Clinical meanings of carcinoembryonic antigen (CEA) in patients with colorectal cancer. Journal of the Japan Society of Colo‐Proctology 1981;35(2):137‐46. [Google Scholar]
Tomoda 1981 {published data only}
- Tomoda H, Furusawa M. The usefulness and limitations of CEA assay in the management of colorectal cancer. Japanese Journal of Surgery 1981;11(1):33‐8. [DOI] [PubMed] [Google Scholar]
Tsai 2009 {published data only}
- Tsai HL, Chu KS, Huang YH, Su YC, Wu JY, Kuo CH, et al. Predictive factors of early relapse in UICC stage I‐III colorectal cancer patients after curative resection. Journal of Surgical Oncology 2009;100(8):736‐43. [DOI] [PubMed] [Google Scholar]
Tsikitis 2009 {published data only}
- Tsikitis VL, Malireddy K, Green EA, Christensen B, Whelan R, Hyder J, et al. Postoperative surveillance recommendations for early stage colon cancer based on results from the clinical outcomes of surgical therapy trial. Journal of Clinical Oncology 2009;27(22):3671‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verberne 2013 a {published data only}
- Verberne CJ, Wiggers T, Vermeulen KM, Jong KP. Detection of recurrences during follow‐up after liver surgery for colorectal metastases: both carcinoembryonic antigen (CEA) and imaging are important. Annals of Surgical Oncology 2013;20(2):457‐63. [DOI] [PubMed] [Google Scholar]
Verberne 2013 b {published data only}
- Verberne C, Doornbos PM, Grossmann I, Bock GH, Wiggers T. Intensified follow‐up in colorectal cancer patients using frequent carcino‐embryonic antigen (CEA) measurements and CEA‐triggered imaging. European Cancer Congress 2013, ECC 2013, Amsterdam. European Journal of Cancer. 2013; Vol. 49:S480. [DOI] [PubMed]
Wan 1994 {published data only}
- Wang JY, Tang R, Chiang JM. Value of carcinoembryonic antigen in the management of colorectal cancer. Diseases of the Colon and Rectum 1994 Mar;37(3):272‐7. [DOI] [PubMed] [Google Scholar]
Wanebo 1978a {published data only}
- Wanebo HJ, Rao B, Pinsky CM, Hoffman RG, Stearns M, Schwartz MK, et al. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. New England Journal of Medicine 1978;299(9):448‐51. [DOI] [PubMed] [Google Scholar]
Wanebo 1978b {published data only}
- Wanebo JH, Stearns M, Schwartz MK. Use of CEA as an indicator of early recurrence and as a guide to a selected second‐look procedure in patients with colorectal cancer. Annals of Surgery 1978;188(4):481‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang JY, Lu CY, Chu KS, Ma CJ, Wu DC, Tsai HL, et al. Prognostic significance of pre‐ and postoperative serum carcinoembryonic antigen levels in patients with colorectal cancer. European Surgical Research 2007;39(4):245‐50. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang ZY. Serum p53 antibody as a tumor marker in the follow‐up of colorectal cancer after curative resection. Techniques in Coloproctology 2010;14(1):57‐8. [Google Scholar]
Wedell 1981 {published data only}
- Wedell J, Meier zu Esssen P, Luu TH, Fiedler R, Calker H, Koldowski P, et al. A retrospective study of serial CEA determinations in the early detection of recurrent colorectal cancer. Diseases of the Colon and Rectum 1981;24(8):618‐21. [DOI] [PubMed] [Google Scholar]
Weiss 1998 {published data only}
- Weiss NS, Cook LS. Evaluating the efficacy of screening for recurrence of cancer. Journal of the National Cancer Institute 1998;90(24):1870‐2. [DOI] [PubMed] [Google Scholar]
Wichmann 2000a {published data only}
- Wichmann MW, Lau‐Werner U, Müller C, Hornung HM, Stieber P, Schildberg FW, et al. Carcinoembryonic antigen for the detection of recurrent disease following curative resection of colorectal cancer. Anticancer Research 2000;20(6D):4953‐5. [PubMed] [Google Scholar]
Wichmann 2000b {published data only}
- Wichmann MW, Müller C, Lau‐Werner U, Strauss T, Lang RA, Hornung HM, et al. The role of carcinoembryonic antigen for the detection of recurrent disease following curative resection of large‐bowel cancer. Langenbecks Archives of Surgery 2000;385(4):271‐5. [DOI] [PubMed] [Google Scholar]
Wichmann 2002 {published data only}
- Wichmann MW, Müller C, Hornung HM, Lau‐Werner U, Schildberg FW, Colorectal Cancer Study G. Results of long‐term follow‐up after curative resection of Dukes A colorectal cancer. World Journal of Surgery 2002;26(6):732‐6. [DOI] [PubMed] [Google Scholar]
Wolf 1997 {published data only}
- Wolf RF, Cohen AM. The miniscule benefit of serial carcinoembryonic antigen monitoring after effective curative treatment for primary colorectal cancer. Journal of the American College of Surgeons 1997;185(1):60‐4. [PubMed] [Google Scholar]
Wood 1975 {published data only}
- Wood CB, Malcolm AJH, Burt R. Assessment of patients with elevated carcinoembryonic antigen (CEA) level after surgery for primary colorectal cancer. Bulletin de la Societe Internationale de Chirurgie 11975;34(6):413‐6. [Google Scholar]
Yu 2013 {published data only}
- Yu H, Zhao M, Xing J, Jin H, Li Y. Relationship of the applied value of 18F‐FDG PET/CT in postoperative relapse with metastasis of colorectal cancer and CEA levels during PET/CT scanning. Chinese Journal of Clinical Oncology 2013;40(12):717‐20. [Google Scholar]
Zeng 1993 {published data only}
- Zeng Z, Cohen AM, Urmacher C. Usefulness of carcinoembryonic antigen monitoring despite normal preoperative values in node‐positive colon cancer patients. Diseases of the Colon and Rectum 1993;36(11):1063‐8. [DOI] [PubMed] [Google Scholar]
Zervos 2001 {published data only}
- Zervos EE, Badgwell BD, Burak WE Jr, Arnold MW, Martin EW. Fluorodeoxyglucose positron emission tomography as an adjunct to carcinoembryonic antigen in the management of patients with presumed recurrent colorectal cancer and nondiagnostic radiologic workup. Surgery 2001;130(4):636‐43. [DOI] [PubMed] [Google Scholar]
Ziegenbein 1980 {published data only}
- Ziegenbein R, Jacobasch KH, Pilgrim G, Seifart W. [The CEA‐concentration in plasma of patients with colorectal carcinoma and polyps has been determined by radioimmunoassay (RIA, Z‐gel‐method)]. Archiv für Geschwulstforschung 1980;50(2):165‐8. [PubMed] [Google Scholar]
Zuniga 1989 {published data only}
- Zuniga A, Rahmer A, Guzman S, Llanos O, Lopez F, Herreros R. [Colorectal cancer: follow‐up after curative resection]. Revista Medica de Chile 1989;117(3):273‐8. [PubMed] [Google Scholar]
Additional references
Allen 2013
- Allen VB, Gurusamy KS, Takwoingi Y, Kalia A, Davidson BR. Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD009323.pub2] [DOI] [PubMed] [Google Scholar]
Bormer 1991
- Bormer OP. Standardization, specificity, and diagnostic sensitivity of four immunoassays for carcinoembryonic antigen. Clinical Chemistry 1991;37(2):231‐6. [PubMed] [Google Scholar]
Colibaseanu 2013
- Colibaseanu DT, Mathis KL, Abdelsattar ZM, Larson DW, Haddock MG, Dozois EJ. Is curative resection and long‐term survival possible for locally re‐recurrent colorectal cancer in the pelvis?. Diseases of the Colon and Rectum 2013;56(1):14‐9. [DOI] [PubMed] [Google Scholar]
Cunningham 2010
- Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet 2010;375(9719):1030‐47. [DOI] [PubMed] [Google Scholar]
Dallas 2012
- Dallas MR, Liu G, Chen W, Thomas SN, Wirtz D, Huso DL, et al. Divergent roles of CD44 and carcinoembryonic antigen in colon cancer metastasis. FASEB Journal 2012;26(6):2648‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidson 1989
- Davidson BR, Sams VR, Styles J, Dean C, Boulos PB. Comparative study of carcinoembryonic antigen and epithelial membrane antigen expression in normal colon, adenomas and adenocarcinomas of the colon and rectum . Gut 1989;30(9):1260‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Mackaskil P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58(9):882‐93. [DOI] [PubMed] [Google Scholar]
Duffy 2013a
- Duffy MJ. Tumor markers in clinical practice: a review focusing on common solid cancers. Medical Principles and Practice 2013;22(1):4‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffy 2013b
- Duffy MJ, Lamerz R, Haglund C, NIcolini A, Kalousova M, Holubec L, et al. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European Group on Tumour Markers (EGTM) 2014 Guidelines Update. International Journal of Cancer 2013;134(11):2513‐22. [DOI: 10.1002/ijc.28384] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dukes 1932
- Dukes CE. The classification of cancer of the rectum. Journal of Pathological Bacteriology 1932;35:323‐32. [Google Scholar]
Ferlay 2013
- Ferlay I, Soerjomataram, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on 31 March 2014.
Glimelius 1992
- Glimelius B, Påhlman L, Graf W, Tveit K, Adami HO, Iasarett V, et al. Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal cancer: a randomized trial. Journal of Clinical Oncology 1992;10(6):904‐11. [DOI] [PubMed] [Google Scholar]
Godlee 2014
- Godlee F. Colorectal cancer: a cautionary tale. BMJ 2014;348:g3311. [Google Scholar]
Goldstein 2005
- Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow‐up of patients with colorectal cancer. Cancer Investigations 2005;23(4):339‐51. [DOI] [PubMed] [Google Scholar]
Gonzalez 2013
- Gonzalez M, Poncet A, Combescure C, Robert J, Ris HB, Gervaz P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta‐analysis. Annals of Surgical Oncology 2013;20(2):572‐9. [DOI] [PubMed] [Google Scholar]
Gore 1997
- Gore RM. Colorectal cancer. Clinical and pathologic features. Radiological Clinics of North America 1997;35(2):403‐29. [PubMed] [Google Scholar]
Guthrie 2002
- Guthrie JA. Colorectal cancer: follow‐up and detection of recurrence. Abdominal Imaging 2002;27(5):570‐7. [DOI] [PubMed] [Google Scholar]
Hamza 2009
- Hamza TH, Arends LR, Houwelingen HC, Stijnen T. Multivariate random effects meta‐analysis of diagnostic tests with multiple thresholds. BMC Medical Research Methodology 2009;9(73):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeffery 2007
- Jeffery M, Hickey BE, Hilder PN. Follow‐up strategies for patients treated for non‐metastatic colorectal cancer. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD002200.pub2] [DOI] [PubMed] [Google Scholar]
Kanas 2012
- Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: review and meta‐analysis of prognostic factors . Clinical Epidemiology 201;4:283‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjeldsen 1997
- Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. A prospective randomised study of follow‐up after radical surgery for colorectal cancer. British Journal of Surgery 1997;84:666‐9. [PubMed] [Google Scholar]
Labianca 2010
- Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A. Primary colon cancer: ESMO Clinical Practice Guidelines for Diagnosis, adjuvant treatment and follow‐up. Annals of Oncology 2010;21(Suppl 5):v70‐v77. [DOI] [PubMed] [Google Scholar]
Laurence 1975
- Laurence DJ, Turberville C, Anderson SG, Neville AM. First British standard for carcinoembryonic antigen (CEA). British Journal of Cancer 1975;32(3):295‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leeflang 2008
- Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Systematic review of diagnostic test accuracy. Annals of Internal Medicine 2008;149(12):889‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Locker 2006
- Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, MacDonald JS, et al. ASCO 2006 update of Recommendations for the use of tumour markers in gastrointestinal cancer. Journal of Clinical Oncology 2006;24(33):5313‐27. [DOI] [PubMed] [Google Scholar]
Macaskill 2010
- Macakill P, Gatonis C, Deeks JJ, Harbord RM, Takwoingi Y (editors). Chapter 10: Analysing and presenting results. In: Cochrane Handbook for Systematic Reviews of Test Accuracy Version 1.0, 2010. Available from dta.cochrane.org/handbook‐dta‐reviews. The Cochrane Collaboration.
Maringe 2013
- Maringe C, Walters S, Rachet B, Butler J, Fields T, Finan P, et al. Stage at diagnosis and colorectal cancer survival in six high‐income countries: A population‐based study of patients diagnosed during 2000‐2007. Acta Oncologica 2013;52(5):919‐32. [DOI] [PubMed] [Google Scholar]
Moses 1993
- Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data‐analytic approaches and some additional considerations. Statistics in Medicine 1993;12(14):1293‐316. [DOI] [PubMed] [Google Scholar]
NCCN 2013
- NCCN 2013. NCCN Guidelines Version 3.2013 Colon Cancer. NCCN Clinical Practice Guidelines in Oncology 2013.
Newton 2011
- Newton KF, Newman W, Hill J. Review of biomarkers in colorectal cancer. Colorectal Disease 2011;14(1):3‐17. [DOI] [PubMed] [Google Scholar]
NICE 2011
- NICE clinical guideline [CG131]. Colorectal Cancer; diagnosis and management. www.nice.org.uk/guidance/cg131/chapter/1‐recommendations 2011 (accessed 5th December 2015).
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Moranta 2006b
- Rodriguez‐Moranta F, Salo J, Arcusa A, Boadas J, Pinol V, Bessa X, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: A prospective, multicenter, randomised, controlled trial. Journal of Clinical Oncology 2005;24(1):1‐8. [DOI] [PubMed] [Google Scholar]
Sargent 2007
- Sargent DJ, Patiyil S, Yothers G, Haller DG, Gray R, Benedetti J, et al. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data From 20,898 patients enrolled onto 18 randomized trials from the ACCENT group. Journal of Clinical Oncology 2007;25(29):4569‐74. [DOI] [PubMed] [Google Scholar]
Scheer 2009
- Scheer A, Auer RAC. Surveillance after curative resection of colorectal cancer. Clinics in Colon and Rectal Surgery 2009;22(4):242‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoemaker 1998
- Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5‐year survival of colorectal patients. Gastroenterology 1998;114:7‐14. [DOI] [PubMed] [Google Scholar]
Secco 2002
- Secco GB, Fardelli RM, Gianquinto D, Bonfante P, Baldi E, Ravera G, et al. Efficacy and cost of risk adapted follow‐up in patients after colorectal surgery: a prospective, randomised and controlled trial. European Journal of Surgical Oncology 2002;28:418‐23. [DOI] [PubMed] [Google Scholar]
Shinkins 2014
- Shinkins B, Nicholson BD, James TJ, Primrose JN, Mant D. Carcinoembryonic antigen monitoring to detect recurrence of colorectal cancer: how should we interpret the results?. Clinical Chemistry 2014;60(12):1572‐4. [DOI] [PubMed] [Google Scholar]
Sobin 2009
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7. Wiley‐Blackwell, 2009. [Google Scholar]
Song 2002
- Song F, Khan KS, Dinnes J, Sutton AJ. Asymmetric funnel plots and publication bias in meta‐analysis of diagnostic accuracy. International Journal of Epidemiology 2002;31(1):88‐95. [DOI] [PubMed] [Google Scholar]
Sturgeon 2009
- Sturgeon CM, Lai LC, Duffy MJ. Serum tumour markers: how to order and interpret them. BMJ 2009;339:852‐8. [DOI] [PubMed] [Google Scholar]
Takwoingi 2013
- Takwoingi Y. Meta‐analysis of test accuracy studies in Stata: a bivariate model approach. Available from: srdta.cochrane.org/ November 2013, issue Version 1.0.
Tan 2009
- Tan E, Gouvas N, Nicholls RJ, Ziprin P, Xynos E, Tekkis PP. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surgical Oncology 2009;18(1):15‐24. [DOI] [PubMed] [Google Scholar]
Treasure 2014
- Treasure T, Monson K, Fiorentino F, Russell C. The CEA Second‐Look Trial:a randomised controlled trial of carcinoembryonic antigen prompted reoperation for recurrent colorectal cancer.. BMJ Open 2014;4:e004385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Roon 2011
- Roon AHC, Dam L, Zauber AG, Ballegooijen M, Borsboom GJJM, Steyerberg EW, et al. Guaiac‐based faecal occult blood tests versus faecal immunochemical tests for colorectal cancer screening in average‐risk individuals. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD009276] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wattchow 2006
- Wattchow DA, Weller DP, Esterman A, Pilotto LS, McGorm K, Hammett Z, et al. General practice vs surgical‐based follow‐up for patients with colon cancer: randomised controlled trial. British Journal of Cancer 2006;94(8):1116‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: A revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
Wild 2013
- Wild D. The Immunoassay Handbook: Theory and Applications of Ligand Binding, ELISA and Related Techniques. 4th Edition. Elsevier, 31 Jan 2013. [Google Scholar]


