Abstract
Over 50,000 human West Nile Virus (WNV) clinical disease cases have been reported to the CDC during the 20 years that the virus has been present in the United States. Despite the establishment and expansion of WNV-focused mosquito surveillance and control efforts and a renewed emphasis on applying Integrated Pest Management (IPM) principles to WNV control, periodic local and regional WNV epidemics with case reports exceeding 2,000 cases per year have occurred during 13 of those 20 years in the United States. In this article, we examine the scientific literature for evidence that mosquito control activities directed at either preventing WNV outbreaks or stopping those outbreaks once in progress reduce WNV human disease or have a measurable impact on entomological indicators of human WNV risk. We found that, despite a proliferation of research investigating larval and adult mosquito control effectiveness, few of these studies actually measure epidemiological outcomes or the entomological surrogates of WNV risk. Although many IPM principles (e.g., control decisions based on surveillance, use of multiple control methodologies appropriate for the ecosystem) have been implemented effectively, the use of action thresholds or meaningful public health outcome assessments have not been used routinely. Establishing thresholds for entomological indicators of human risk analogous to the Economic Injury Level and Economic Thresholds utilized in crop IPM programs may result in more effective WNV prevention.
Introduction
The first mosquito control operations targeting West Nile Virus (WNV) in the United States started on September 3, 1999 within hours of the New York City Department of Health receiving confirmation that a cluster of human disease cases with severe neurologic symptoms in the borough of Queens was caused by a mosquito-transmitted virus (GAO 2000). By September 8, 1999, additional cases had been detected outside the initial outbreak area and mosquito control efforts were expanded citywide. Mosquito monitoring indicated that the Culex pipiens L. mosquito population had been reduced substantially by the control operations (CDC 1999) which likely contributed to the outbreak being limited to 62 confirmed human cases (CDC 2019) out of the City’s 7.4 million residents in 1999. Over the next several years, the geographic range of WNV expanded rapidly and reached the west coast of the United States by 2003 (Petersen et al. 2013).
The Nation’s public health and mosquito control communities mounted an aggressive response to the spread of WNV, the results of which are described in several recent review articles (Reisen and Brault 2007, Petersen et al. 2013, Roehrig 2013). There was a flurry of research activity to identify the primary vector mosquitoes and the avian species important in virus amplification. Enzootic and epizootic WNV surveillance was enhanced through development and implementation of new diagnostic tools. New communication and data sharing networks, such as the ArboNet system (Lindsey et al. 2012), were developed to improve information dissemination. By 2005, the Nation’s WNV knowledge base and surveillance systems had vastly improved (Hadler et al. 2015). As a result, mosquito control programs had information essential to their new mission of reducing WNV disease.
Though a comprehensive analysis of changes made by mosquito control programs to address WNV is not available, efforts to adapt to WNV were extensive. These efforts ranged from implementing new arbovirus surveillance protocols, to adopting rapid diagnostic tests for WNV in mosquito pools to quickly obtain mosquito infection rate information, and to refocusing control resources to manage mosquitoes produced in the thousands of urban/suburban storm water catch basins in areas where Culex pipiens and Culex quinquefasciatus Say were important WNV vectors.
Efforts to control the periodic WNV outbreaks have proven to be very expensive. In the first WNV outbreak response, New York State estimated that the state, city, and four counties in the affected area spent more than $14M on protective measures such as mosquito control from late August through October 1999 (GAO 2000). Additional mosquito control activities associated with the 2002 WNV outbreak in St. Tammany Parish (Louisiana) cost the mosquito control district $1.7M over their usual $2M annual budget (Palmisano et al. 2005). The Sacramento-Yolo Mosquito and Vector Control District spent $700,000 on aerial ULV applications alone in response to the 2005 WNV epidemic in Sacramento, CA. The cost of aerial ULV applications in Dallas County, TX, during the 2012 WNV outbreak was approximately $1.7M (Chung et al. 2013). Despite the increased expenditures and extensive modifications and improvements made to mosquito surveillance and control, WNV has caused over 50,000 confirmed human cases from 1999 – 2018 in the United States (CDC 2019). During 13 of those years, transmission was very intense in many areas and the total number of reported cases exceeded 2,000 per year (CDC 2019).
It is widely accepted that Integrated Vector Management (IVM) programs that make evidence-based control decisions with information derived from well-designed surveillance systems, and that utilize a diversity of ecologically-appropriate control tools, can effectively reduce vector abundance and human WNV risk (CDC 2013). Supporting part of this assumption is ample scientific information demonstrating that currently available control methods can reduce larval and adult mosquito abundance. However, research specifically addressing the effectiveness of IVM programs in reducing human WNV disease is lacking (Bellini et al. 2014). Few publications directly measure the effect of IVM on reducing the number of human cases or on reducing the infection rate in vectors or the Vector Index, surveillance indices which are associated with human risk (Bolling et al. 2009, Kwan et al. 2012, Colborn et al. 2013, Chung et al. 2013, Kilpatrick and Pape 2013). Our objective here is to review several publications that measured those direct indicators of human WNV risk in response to the application of IVM to controlling ongoing outbreaks (reactive control, as described by Reisen and Brault 2007), or to preventing outbreaks from developing (proactive control, Reisen and Brault 2007). We also discuss how developing and incorporating action thresholds derived from surveillance programs and based on Integrated Pest Management (IPM) principles may improve the ability to reduce WNV risk.
Reactive Control of WNV Outbreaks
Mosquito control programs in the Unites States frequently have initiated enhanced mosquito abatement activities in response to ongoing vector-borne disease outbreaks. Under these crisis-driven circumstances it is often difficult to develop and execute a robust vector control plan and to simultaneously evaluate its effectiveness. As a result, there are only a few documented accounts of the direct effects of vector control activities implemented during ongoing vector-borne outbreaks in the United States, including the West Nile outbreaks that have occurred since 1999.
In 2002, the St. Tammany Parish Mosquito Abatement District (STPMAD) anticipated the arrival of WNV and implemented an IVM approach targeting the primary WNV vector, Cx. quinquefasciatus. The STPMAD conducted vector surveillance and preventive control activities throughout the winter and spring months (Palmisano et al. 2005). Despite these efforts, human WNV cases were detected in the Parish beginning in June, and a total of 40 cases were detected by the end of 2002 (Balsamo et al. 2003). Intense control activities against both larval and adult mosquitoes were executed by STPMAD. They reported that, compared to control activities during the prior 5 years, aerial ULV adulticiding increased 450%, ground ULV adulticiding by 63% and larviciding by 46% throughout St Tammany Parish during the outbreak period. The result was a 2 to 10-fold reduction in adult mosquito abundance compared to their prior 5-year average (Palmisano et al. 2005) and human WNV cases in the Parish declined from 27 in July to 6 in August. With the exception of a case in late November, human cases in St. Tammany Parish ceased by the end of August (Palmisano et al. 2005). Human WNV cases in the remainder of Louisiana were detected until December 2002 (Balsamo et al. 2003) suggesting continued WNV transmission in the other parts of the state. Subsequent observations showed that 16% of human WNV neuro-invasive disease cases in Louisiana from 2002 – 2016 occurred in July, 46.6% occurred in August, and 24.1% occurred in September (Louisiana Office of Public Health 2016). Taken together these data provided evidence that the IVM efforts by the STPMAD preceding and during the outbreak in 2002 suppressed human WNV infections and likely helped prevent a much bigger outbreak.
In Sacramento County, California, a reactive control approach targeting Cx. pipiens and Cx. tarsalis Coquillett was implemented in 2005 to prevent WNV transmission to humans. Despite early-season larval control and limited truck-based adulticiding efforts, human WNV cases reached outbreak proportions in August 2005 (Carney et al. 2008). In an effort to reduce WNV transmission to humans, the Sacramento-Yolo Mosquito and Vector Control District used aerial adulticide applications of pyrethrin. The applications were conducted on three consecutive nights in two treatment areas. Before and following the adulticide applications, mosquito abundance was measured using CO2-baited traps. Results indicated a 75.0% reduction in the abundance of Cx. pipiens and a 48.7% reduction in the abundance of Cx. tarsalis in the treated area compared to untreated areas (Elnaiem et al. 2008). In addition, they noted that the WNV infection rate in vector mosquitoes fell from 8.2/1000 before treatment to 4.3/1000 after treatment while the infection rate in untreated areas increased from 2.0/1000 to 8.7/1000 over the same time period. Occurrence of new human WNV cases also declined in in the treated areas. Before the treatments there was no difference in the incidence of human WNV cases among the treated and untreated areas. After the aerial adulticide applications, the human WNV case incidence in treated areas was significantly lower than in the untreated areas and the odds of human WNV infections were approximately six times higher in the untreated areas compared to the treated areas (Carney et al. 2008). These studies provided evidence that intensive aerial ULV application of pyrethrin in 2005 reduced the abundance of infected WNV vectors and decreased the number of human cases.
In the summer of 2012, Texas experienced a WNV epidemic, with the most severe outbreak occurring in four north-central counties, Denton, Collin, Tarrant and Dallas, which accounted for 42% (356) of the 844 total cases reported by the state that year (CDC 2013, Chung et al. 2013). Those counties initially responded by increasing the intensity of mosquito control activities primarily through larviciding and limited adulticiding using truck-mounted ULV sprayers. In mid-August, aerial insecticide applications were initiated to try interrupting WNV transmission to humans. Spraying was conducted three times between 8/16/2012 and 9/2/2012. Ruktanochai et al. (2014) evaluated the effect of the aerial adulticiding applications on the incidence of human disease and found that WNV neuroinvasive disease incidence decreased from 7.31/100,000 before treatment to 0.28/100,000 after treatment in the treated area, producing a pre-treatment:post-treatment incidence rate ratio (IRR) of 26.42 (95%, CI: 12.42–56.20). The incidence decreased in untreated areas as well from 4.80/100,000 in the period before the insecticide was applied in the treated area to 0.45/100,000 in the post-treatment period, producing a pre-treatment:post-treatment IRR of 10.57 (95%, CI: 0.98–6.35) (Ruktanochai et al. 2014). By comparing the IRR in the treatment area to the IRR in the untreated area (26.42/10.57) the authors concluded that the decrease in neuroinvasive disease incidence was 2.5 times greater in the treated area, despite the fact that the WNV outbreak was already waning by the time the aerial adult control was conducted (Chung et al. 2013).
In the city of Chicago, ground ULV treatments with sumithrin were used to target WNV vectors, primarily Cx. pipiens, during a WNV outbreak in 2005 (Mutebi et al. 2011). Two treatments applied 7 days apart during the week of July 31 and the week of August 7 reduced adult mosquito abundance 54% in the treated areas. During the same period, mosquito abundance increased by 153% in the untreated areas (Mutebi et al. 2011). A second round of two ULV treatments applied during the weeks of August 21 and August 29 resulted in a 29% reduction in abundance compared to before the treatments. Though there was no detectable change in the WNV infection rate in these mosquito populations following the control activities, the treatments likely reduced human risk by decreasing the overall abundance of infected Culex mosquitoes in the treated areas.
A study of how vector control programs in Cook County, IL addressed the WNV outbreak in 2002 suggested that differences in vector control practices among the mosquito abatement districts (MADs) may have contributed to the higher incidence of WNV human cases in some of the MADs (Tedesco et al. 2010). This study compared human WNV case incidence among MADs in relation to local characteristics such as housing, income levels, physical environment and MAD control activities. They found that MADs that did minimal larval control in catch basins and minimal or no adult mosquito control had higher WNV case incidence rates. Although this study did not compare vector abundance or infection rates across MADs in Cook County, it provided indirect evidence that “vigorous and timely vector control and education policies” enacted by two of the four vector control agencies resulted in less human WNV disease than in the other two agencies with “limited and less cohesive programs”.
The studies discussed above show that reactive vector control activities during WNV outbreaks were effective in reducing mosquito vector abundance and human WNV case numbers if applied intensively, and may be most effective if applied early in the outbreak period (Chung et al. 2013).
Proactive Prevention of WNV outbreaks
A commonly identified principle among the numerous definitions of IPM (Bajwa and Kogan 2002) is that appropriate control procedures should be used to maintain pest populations at levels that do not produce unacceptable amounts of damage. In the case of IVM for WNV, that means applying pre-emptive or proactive measures that will prevent WNV transmission intensity from reaching levels that produce outbreaks of human disease. This is frequently stated as an objective in mosquito control program management plans, and as noted above, there are numerous examples in the literature documenting the effect of control operations on vector abundance. Culex pipiens larval abundance in catch basins can be effectively reduced through a variety of insecticide treatments and is associated with reductions in adult mosquito abundance (Harbison et al. 2014, 2018). Truck-based ULV-application of mosquito adulticides has given variable results depending on habitat structure and weather conditions (Mount 1998, Bonds 2012), may not effectively reduce Cx. pipiens abundance in some settings (Reddy et al. 2006), though it may result in area-wide vector mosquito population suppression in other settings (Lothrop et al. 2007). The effectiveness of aerial ULV application of insecticides for adult mosquito control is also variable, and is similarly influenced by habitat structure and weather (Mount et al. 1996, Bonds 2012), and both truck-based and aerial ULV application effectiveness may be influenced by insecticide resistance, which is known to vary considerably among local vector populations (Zhou et al. 2009, Richards et al, 2017, Richards et al. 2018). However, aerial ULV applications can significantly reduce mosquito abundance for short durations ranging from 5–6 days, over large areas (Andis et al. 1987, Simpson 2006). While these studies demonstrate that vector abundance can be reduced through proactive control measures using insecticides, none demonstrated that the effect on the vector population was sufficient to reduce WNV transmission activity or human WNV risk.
Fortunately, there are a few studies that have monitored WNV transmission indicators such as WNV infection rate in vector populations in response to IVM activities. These efforts to assess the effectiveness of proactive measures on WNV transmission risk are described below.
McMillan et al. (2019) applied larvicides to catch basins in urban park areas of Atlanta, GA over the course of 2 seasons. They documented >90% reductions in larval/pupal production in catch basins, but there was no concurrent reduction in adult Culex abundance or the WNV infection rate in the adult vector population. This was likely due to the relatively small size of the areas treated relative to the large number of vector production sites available.
Lothrop et al. (2008) evaluated the ability of intensive, early-season adult mosquito control operations to limit WNV amplification in an area at the north shore of the Salton Sea and to reduce spread of WNV to other areas of the Coachella Valley in southern California. Wetlands at the north shore of the Salton Sea were known to be foci of early season arbovirus amplification that subsequently spread to adjacent areas with large human populations. Over the course of the first two years of the study, they determined that reactive ground-based ULV applications and limited aerial ULV applications were insufficient to reduce vector mosquito abundance, WNV infection rate in mosquitoes or spread of WNV transmission out of the local area. However, during the third year of the study, intensive, multiple ULV applications of pyrethrin insecticide by air and ground that commenced at the first detection of WNV in mosquitoes and continued weekly for a total of 26 aerial ULV applications and 31 ground ULV applications resulted in an average 61% reduction in vector abundance after each treatment. This decrease in vector abundance was associated with reductions in infection rate in the treated areas, and reduced expansion of WNV to other areas of the Coachella Valley.
Macedo et al. (2010) described the effect of an intensified aerial ULV control effort on WNV transmission activity in Sacramento County, CA. By late July 2007, WNV infection rates in Cx. tarsalis and Cx. pipiens had exceeded levels of concern established by the California Dept. of Health that would warrant an additional control response (California Department of Public Health 2018). The Sacramento-Yolo Mosquito and Vector Control District conducted aerial ULV applications of a piperonyl butoxide synergized pyrethrin to a 215 km2 area each day for 3 successive days. The results showed a 57% decrease Cx. tarsalis abundance and a 40% reduction in Cx. pipiens abundance, and the WNV minimum infection rate in Cx. tarsalis decreased by 77% and by 21% in Cx. pipiens during the 3 days following the control treatments compared to the three days prior to control applications. This suggests that the control activities may have reduced the risk of WNV transmission to humans by effectively reducing the population of infected adult mosquitoes at the target area. Unfortunately, the longer-term effectiveness of the control measures was not reported.
IPM for WNV management
The few studies cited above indicate that reactive control measures implemented to stop an ongoing WNV epidemic can be effective if sufficiently intensive. However, reactive measures are very expensive and, unfortunately, are often initiated after numerous human cases have been reported and many more people have been infected (Chung et al. 2013). As a result, reactive efforts to reduce human WNV risk often start after most of the human infections have already occurred and the epidemic is already starting to decline naturally. The number of cases prevented is lower than if control activities were implemented earlier in the WNV enzootic-epizootic transmission season. This could be remedied by assuring that surveillance systems monitoring vector abundance and WNV infection rate in vectors are sufficiently sensitive to detect increasing WNV transmission, are coupled with response plans that establish evidence-based guidelines for when to intensify control efforts, and have action thresholds that would indicate control efforts are required before epidemic transmission ensues. One example of such a program is the WNV risk assessment system contained in the California Mosquito-Borne Virus Surveillance and Response Plan (California Department of Public Health 2018). This algorithm utilizes data from several WNV surveillance elements: vector surveillance (abundance and infection rate); avian surveillance (seroconversion in sentinel chickens and counts of dead birds); environmental conditions (temperature); and human case surveillance. A value is assigned to each of the elements, which are then combined to provide an estimate of risk based on historical experience and a systematically collected surveillance database. The three categories of increasing risk defined in the plan (Normal Season, Emergency Planning, Epidemic Conditions) are accompanied with response recommendations that scale up intensity of surveillance, control and other prevention activities accordingly. Although the California Mosquito-Borne Virus Surveillance and Response Plan and similar plans provide general guidance for increasing control activities based on evidence of increasing WNV transmission intensity (e.g., Bajwa et al. 2018, CDC 2013), they do not provide the detailed data and explicit guidance essential for an effective proactive WNV prevention plan.
Mosquito control operations charged with implementing IVM programs to reduce WNV risk have adopted most Integrated Pest Management principles. Many programs are based on a good understanding of the biology of the vector species they are targeting; they conduct robust WNV vector and virus monitoring; control decisions are based on surveillance data; they utilize a variety of control protocols (source reduction, larval mosquito control, adult mosquito control); they employ a variety of pesticide formulations and active ingredients; and they monitor the effectiveness of their control activities and adjust procedures as needed. However one of the key elements lacking from WNV IVM programs is determining levels of mosquito abundance and virus transmission activity that must be maintained in order to prevent WNV outbreaks from developing.
Classical approaches to IPM in agricultural systems are based on the concept that some level of pest activity is tolerable as long as it doesn’t result in excessive economic loss, and control actions must be taken at a point before unacceptable losses occur (Stern et al. 1959). Control decisions are based on knowledge of the pest densities associated with unacceptable losses and surveillance systems that can accurately quantify pest densities. Applying that concept to a WNV IVM system is illustrated in Figure 1. The Enzootic Equilibrium is what Stern et al. (1959) refer to as the General Equilibrium Position of the pest population. This is a low level of WNV transmission activity detected by the surveillance system that is usually seen throughout the season during non-outbreak years. The Outbreak Threshold is analogous to the Economic Threshold in agricultural IPM and is the level below which WNV transmission must be maintained by control activities to prevent unacceptable numbers of human cases. The Epidemic Level is analogous to the Economic Injury Level and is the level of WNV activity the surveillance system indicators show are associated with epidemic years, or unacceptable numbers of human cases. During Non-Outbreak years (Fig. 1) when weather and other factors don’t promote the expansion of WNV transmission from enzootic to epizootic and the surveillance indicators vary around the Enzootic Equilibrium Level, no extraordinary control activities are required to keep human WNV risk at acceptable levels. During years when conditions promote early-season amplification and surveillance indicators demonstrate that WNV transmission is approaching levels associated with unacceptable human risk, control activities can be used to maintain transmission and human risk at acceptable levels (Fig. 1: IVM Implemented). If effective, these measures can prevent amplification and human risk from reaching epidemic levels (Fig. 1. WNV Outbreak Year).
Figure 1.
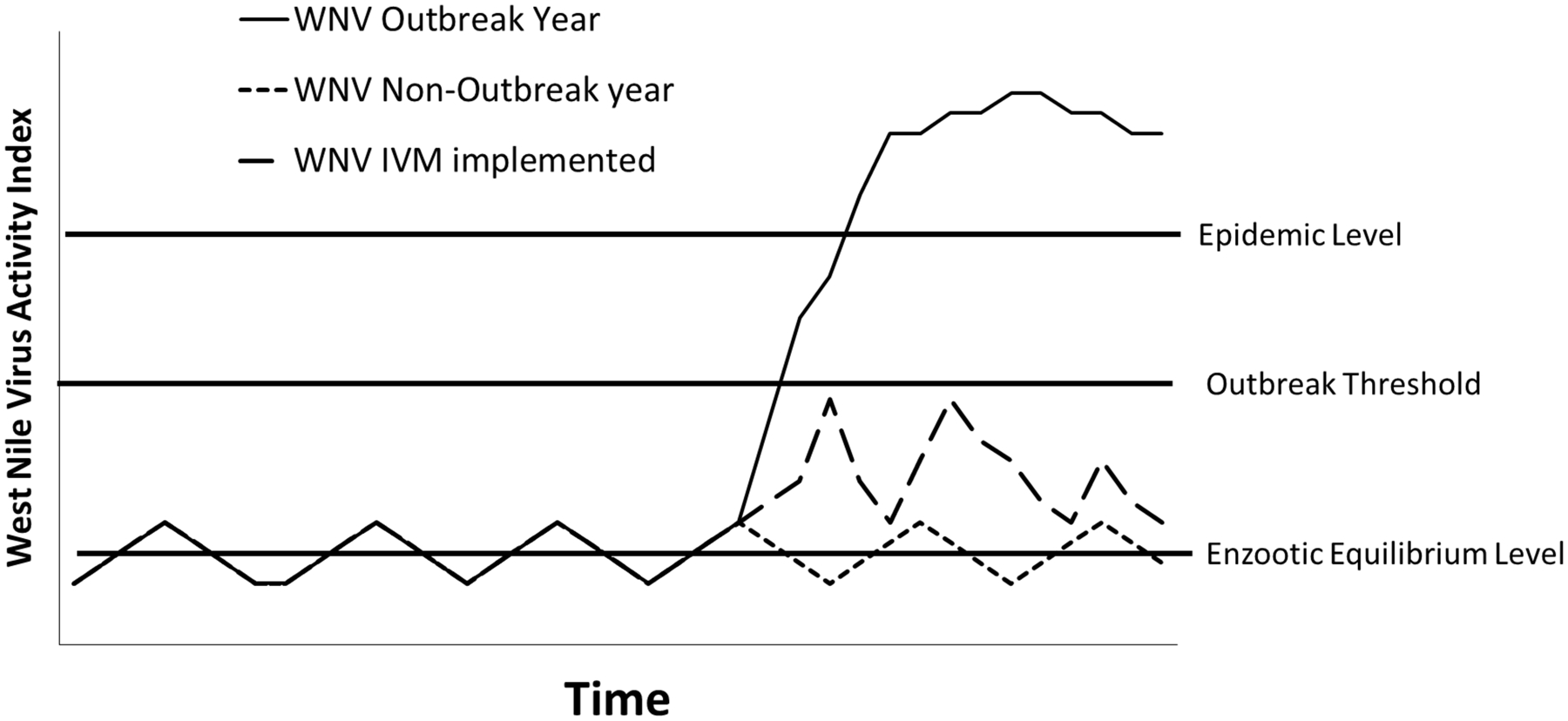
Representation of a WNV Integrated Vector Management system showing the relationships among action thresholds and hypothetical WNV transmission activity levels during a non-outbreak year, an uncontrolled outbreak year, and a year when effective IVM implementation maintains transmission intensity below epidemic levels (based on similar representations of agricultural IPM systems in Stern et al. 1959)
Weather (temperature and precipitation) affects WNV transmission dynamics in the different ecosystems across the United States and can be broadly predictive of WNV transmission levels (e.g., Hahn et al. 2015, Shand et al. 2016, Davis et al. 2018). Mosquito-based surveillance systems also produce good indicators of human risk in the form of the WNV infection rate in the mosquito population or the Vector Index that reflects the abundance of WNV-infected mosquitoes (Bolling et al. 2009, Jones et al. 2011, Kwan et al. 2012, DeFelice et al. 2017). However, we have not combined these factors to determine how low WNV transmission must be kept in an area over the course of a transmission season to prevent epidemic conditions from developing. Action thresholds that quantitatively relate surveillance indicators such as the infection rate or Vector Index to human risk are essential for fully implementing a comprehensive WNV IVM program. Without them, we are unable to determine when to implement intensified control activities, how long they should be kept in place, or, critically, if the available interventions are capable of reducing the risk indicators to the needed levels.
Developing action thresholds that are useful in operational mosquito control programs is likely to be an immensely complex undertaking, given the variation that exists in WNV transmission ecology and in the variation in local WNV surveillance practices. Thresholds for specific WNV eco-regions may be useful, but more local-scale IVM thresholds may be required. Although this may be daunting, there have been recent developments in modeling WNV that evaluate the interactions of weather, landscape structure, vector life history, WNV transmission dynamics, WNV surveillance indicators, and human demography with human cases; and these may provide valuable insight into how these thresholds may be derived and tested. For example: Bouden et al. (2008) included the potential effects of larval control in their model of enzootic-epizootic WNV transmission; Malik (2018) included the effects of both larval control and adult mosquito control in a model of enzootic-epizootic WNV transmission; Pawalek et al. (2014) modeled the effects of adult mosquito control on WNV transmission and provided some insights into how effective control must be to prevent epidemic conditions from developing; and Thomas et al. (2009) speculated in their model evaluation that intensive control of the adult vector population in the Fall may have the greatest potential for reducing WNV amplification the following transmission season. Although these models suggest how WNV risk may be reduced and may lead to developing useful action thresholds, their assumptions must be evaluated in operational IVM programs.
Summary
There is strong evidence that, if implemented with sufficient intensity, reactive adult mosquito control can measurably reduce the entomological indicators of WNV transmission activity, resulting in fewer human WNV cases than if the emergency control measures had not been executed. Unfortunately, there are few published accounts of proactive mosquito control measures maintaining WNV risk indicators below outbreak levels. Although it is generally accepted that routine IVM practices employed by MADs can reduce WNV risk and prevent WNV outbreaks, it is difficult to validate that assumption. It would be experimentally difficult, as well as ethically questionable, to conduct a controlled experiment in which vector control is withheld from a portion of a community at risk for WNV. However, if entomological indicators of WNV risk could be identified and established as IPM-style thresholds, we could evaluate the ability of proactive control measures to maintain WNV risk indicators below those levels during weather conditions that would otherwise promote outbreak-levels of WNV activity.
References
- Andis MD, Sackett SR, Carroll MK, and Bordes ES. 1987. Strategies for the emergency control of arboviral epidemics in New Orleans. J. Am. Mosq. Control Assoc 3: 125–130. [PubMed] [Google Scholar]
- Bajwa WI, and Kogan M. 2002. Compendium of IPM Definitions (CID)- What is IPM and how is it defined in the Worldwide Literature? IPPC Publication No. 998, Integrated Plant Protection Center (IPPC), Oregon State University, Corvallis, OR: 97331, USA. [Google Scholar]
- Bajwa W, Slavinski S, Shah Z, and Zhou L. 2018. Comprehensive Mosquito Surveillance and Control Plan. New York City Department of Health and Mental Hygiene, New York, NY: p. 43 (https://www1.nyc.gov/assets/doh/downloads/pdf/wnv/2018/wnvplan2018.pdf). Accessed 1/15/2019. [Google Scholar]
- Balsamo G, Michaels S, Sokol T, Lees K, Mehta M, Straif-Bourgeois S, Hall S, Krishna N, Talati G, and Ratard R. 2003. West Nile epidemic in Louisiana in 2002. Ochsner J. 5(3):13–5. [PMC free article] [PubMed] [Google Scholar]
- Bellini R, Zeller H, and Van Bortel W. 2014. A review of the vector management methods to prevent and control outbreaks of West Nile virus infection and the challenge for Europe. Parasit. Vectors 7:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling BG, Barker CM, Moore CG,Pape WJ, and Eisen L. 2009. Seasonal patterns for entomological measures of risk for exposure to Culex vectors and West Nile virus in relation to human disease cases in northeastern Colorado. J. Med. Entomol 46(6):1519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds JA 2012. Ultra-low-volume space sprays in mosquito control: a critical review. Med. Vet .Entomol 26(2):121–30. [DOI] [PubMed] [Google Scholar]
- Bouden M, Moulin B, and Gosselin P. 2008. The geosimulation of West Nile virus propagation: a multi-agent and climate sensitive tool for risk management in public health. Int. J. Health Geogr 7;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Department of Public Health. 2018. California Mosquito-borne Virus Surveillance and Response Plan. California: Department of Public Health, Mosquito & Vector Control Association of California, University of California; (http://www.westnile.ca.gov/downloads.php?download_id=4055&filename=2018CAResponsePlan__6-11-18_ADA.pdf). Accessed 1/14/2019. [Google Scholar]
- Carney RM, Husted S, Jean C, Glaser C, and Kramer V. 2005. Efficacy of aerial spraying of mosquito adulticide in reducing incidence of West Nile virus, California, 2005. Emerg. Infect. Dis 14(5):747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 1999. Outbreak of West Nile-like viral encephalitis--New York, 1999. MMWR Morb. Mortal. Wkly. Rep 48(38):845–9. [PubMed] [Google Scholar]
- CDC. 2013. West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control. 4th Edition (https://www.cdc.gov/westnile/resources/pdfs/wnvGuidelines.pdf). Accessed 1/11/2019. [Google Scholar]
- CDC. 2019. West Nile virus disease cases reported to CDC by state of residence, 1999–2017 https://www.cdc.gov/westnile/statsmaps/index.html and West Nile Virus Disease Cases by State 2018. https://www.cdc.gov/westnile/statsmaps/preliminarymapsdata2018/disease-cases-state-2018.html. Accessed 1/8/2019.
- Chung WM, Buseman CM, Joyner SN, Hughes SM, Fomby TB, Luby JP, and Haley RW. 2013. The 2012 West Nile encephalitis epidemic in Dallas, Texas. JAMA. 310(3):297–307 [DOI] [PubMed] [Google Scholar]
- Colborn JM, Smith KA, Townsend J, Damian D, Nasci RS, and Mutebi JP. 2013. West Nile virus outbreak in Phoenix, Arizona−−2010: entomological observations and epidemiological correlations. J. Am. Mosq. Control Assoc 29(2):123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JK, Vincent GP, Hildreth MB, Kightlinger L, Carlson C, and Wimberly MC. 2018. Improving the prediction of arbovirus outbreaks: A comparison of climate-driven models for West Nile virus in an endemic region of the United States. Acta. Trop 185:242–250. [DOI] [PubMed] [Google Scholar]
- DeFelice NB, Little E, Campbell SR, and Shaman J. 2017. Ensemble forecast of human West Nile virus cases and mosquito infection rates. Nat. Commun 8:14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnaiem DEA, Kelley K, Wright S, Laffey R, Yoshimura G, Reed M, Goodman G, Thiemann T, Reimer L, Reisen WK, and Brown D. 2008. Impact of aerial spraying of pyrethrin insecticide on Culex pipiens and Culex tarsalis (Diptera: Culicidae) abundance and West Nile virus infection rates in an urban/suburban area of Sacramento County. California. J. Med. Entomol 45(4):751–757. [DOI] [PubMed] [Google Scholar]
- GAO. 2000. GAO GAO/HEHS-00–180 - West Nile Virus Outbreak: Lessons for Public Health General Accounting Office report issued September 11, 2000. U.S. Government Printing Office; Washington DC: 74 pages. (https://www.gao.gov/products/HEHS-00-180). Accessed 1/9/2019. [Google Scholar]
- Hadler JL, Patel D, Nasci RS, Petersen LR, Hughes JM, Bradley K. Etkind P,Kan L, and Engel J. 2015. Assessment of Arbovirus Surveillance 13 Years after Introduction of West Nile Virus, United States. Emerg. Infect. Dis 21(7):1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Monaghan AJ, Hayden MH, Eisen RJ, Delorey MJ, Lindsey NP, Nasci RS, and Fischer M. 2015. Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am. J. Trop. Med. Hyg 92(5):1013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison JE, Henry M, Xamplas C, and Dugas LR. 2014. Evaluation of Culex pipiens populations in a residential area with a high density of catch basins in a suburb of Chicago, Illinois. J. Am. Mosq. Control Assoc 30(3):228–30. [DOI] [PubMed] [Google Scholar]
- Harbison JE, Nasci RS, Runde A, Henry M, Binnall J, Hulsebosch B, Rutkowski N, Johnson H, Uelmen J, Bradley M, Newton G, Irwin P, Bartlett D, and O’Hara Ruiz M. 2018. Standardized operational evaluations of catch basin larvicides from seven mosquito control programs in the Midwest United States during 2017. J. Amer. Mosq. Control Assoc 34(2):107–116. [DOI] [PubMed] [Google Scholar]
- Jones RC, Weaver KN, Smith S, Blanco C, Flores C, Gibbs K, Markowski D, and Mutebi JP. 2011. Use of the vector index and geographic information system to prospectively inform West Nile virus interventions. J. Am. Mosq. Control Assoc 27(3):315–9. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM and Pape WJ. 2013. Predicting human West Nile virus infections with mosquito surveillance data. Am. J. Epidemiol 178(5):829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan JL, Park BK, Carpenter TE, Ngo V, Civen R, and Reisen WK. 2012. Comparison of enzootic risk measures for predicting West Nile disease, Los Angeles, California, USA, 2004–2010. Emerg. Infect. Dis 18(8):1298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey NP, Brown JA, Kightlinger L, Rosenberg L L, and Fischer M. 2012. State health department perceived utility of and satisfaction with ArboNET, the U.S. National Arboviral Surveillance System. Public Health Rep 127(4):383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothrop H, Lothrop B, Palmer M, Wheeler S, Gutierrez A. Gomsi D, and Reisen WK. 2007. Evaluation of pyrethrin and permethrin ground ultra-low volume applications for adult Culex control in rural and urban environments of the Coachella Valley of California. J. Am. Mosq. Control Assoc 23(2):190–207. [DOI] [PubMed] [Google Scholar]
- Lothrop HD, Lothrop BB, Gomsi DE, and Reisen WK. 2008. Intensive early season adulticide applications decrease arbovirus transmission throughout the Coachella Valley, Riverside County. California. Vector-Borne Zoon. Dis 8(4):475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louisiana Office of Public Health. 2016. Encephalitis WNV Annual Report 2016. (http://ldh.la.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/Annuals/EncephalitisWNV_LaIDAnnual.pdf). Accessed 2/5/2019.
- Macedo PA, Schleier JJ, Reed M, Kelley K, Goodman GW, Brown DA, and D Peterson RK. 2010. Evaluation of efficacy and human health risk of aerial ultra-low volume applications of pyrethrins and piperonyl butoxide for adult mosquito management in response to West Nile virus activity in Sacramento County. California. J. Am. Mosq. Contr. Assoc 26(1):57–66. [DOI] [PubMed] [Google Scholar]
- Malik T 2018. A discrete time West Nile virus transmission model with optimal bird- and vector-specific controls. Math. Biosci 305:60–70. [DOI] [PubMed] [Google Scholar]
- McMillan JR, Blakney RA, Mead DG, Coker SM, Morran LT, Waller LA, Kitron U, and Vazquez-Prokopec GM. 2019. Larviciding Culex spp. (Diptera: Culicidae) populations in catch basins and its impact on West Nile Virus transmission in urban parks in Atlanta, GA. J. Med. Entomol 56(1):222–232. [DOI] [PubMed] [Google Scholar]
- Mount GA 1998. A critical review of ultralow-volume aerosols of insecticide applied with vehicle-mounted generators for adult mosquito control. J. Am. Mosq. Control Assoc 14(3):305–34. [PubMed] [Google Scholar]
- Mount GA, Biery TL, and Haile DG. 1996. A review of ultra low volume aerial sprays of insecticide for mosquito control. J. Am. Mosq. Control Assoc 12(4):601–18. [PubMed] [Google Scholar]
- Mutebi JP, Delorey MJ, Jones RS, Plate DK, Gerber SI, Gibbs KP, Sun GH, Cohen NJ, and Paul WS. 2011. The impact of adulticide applications on mosquito density in Chicago, 2005. J. Am. Mosq. Control Assoc 27(1):69–76. [DOI] [PubMed] [Google Scholar]
- Palmisano CT, Taylor V, Caillouet K, Byrd B, and Wesson DM. 2005. Impact of West Nile virus outbreak upon St. Tammany Parish Mosquito Abatement District. J. Am. Mosq. Control Assoc 21(1):33–38. [DOI] [PubMed] [Google Scholar]
- Pawelek KA, Niehaus P, Salmeron C, Hager EJ, and Hunt GJ. 2014. Modeling dynamics of Culex pipiens complex populations and assessing abatement strategies for West Nile Virus. PLoS ONE 9(9): e108452. doi: 10.1371/journal.pone.0108452 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Petersen LR, Brault AC, and Nasci RS. 2013. West Nile virus: review of the literature. JAMA. 310(3):308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MR, Spielman A, Lepore TJ, Henley D, Kiszewski AE, and Reiter P. 2006. Efficacy of resmethrin aerosols applied from the road for suppressing Culex vectors of West Nile virus. Vector-Borne Zoon. Dis 6(2):117–127. [DOI] [PubMed] [Google Scholar]
- Reisen W and Brault AC. 2007. West Nile virus in North America: perspectives on epidemiology and intervention. Pest Manag. Sci 63(7):641–6. [DOI] [PubMed] [Google Scholar]
- Richards SL, Balanay JAG, Fields M, and Vandock K. 2017. Baseline insecticide susceptibility screening against six active ingredients for Culex and Aedes (Diptera: Culicidae) mosquitoes in the United States. J. Med. Entomol 54(3):682–695 [DOI] [PubMed] [Google Scholar]
- Richards SL, Balanay JAG, White AV, Hope J, Vandock K, Byrd BD, and Reiskind MH. 2018. Insecticide Susceptibility Screening Against Culex and Aedes (Diptera: Culicidae) Mosquitoes From the United States. J. Med. Entomol 55(2):398–407. [DOI] [PubMed] [Google Scholar]
- Roehrig JT 2013. West Nile Virus in the United States - A historical perspective. Viruses. 5(12):3088–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruktanonchai DJ, Stonecipher S, Lindsey N, McAllister J, Pillai SK, Horiuchi K, Delorey M, Biggerstaff BJ, Sidwa T, Zoretic J, Nasci R, Fischer M, and Hills SL. 2014. Effect of aerial insecticide spraying on West Nile virus disease--north-central Texas, 2012. Am. J. Trop. Med. Hyg 91(2):240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand L, Brown WM, Chaves LF, Goldberg TL, Hamer GL, Haramis L, Kitron U, Walker ED, and Ruiz MO. 2016. Predicting West Nile Virus infection risk from the synergistic effects of rainfall and temperature. J. Med. Entomol 53(4):935–944. [DOI] [PubMed] [Google Scholar]
- Simpson JE 2006. Emergency mosquito aerial spray response to the 2004 Florida hurricanes Charley, Frances, Ivan, and Jeanne: an overview of control results. J. Am. Mosq. Control Assoc 22(3):457–463. [DOI] [PubMed] [Google Scholar]
- Stern V, F Smith R, Van Den Bosch R, and Hagen KS. 1959. The integrated control concept. Hilgardia 29, 81–101. [Google Scholar]
- Tedesco C, Ruiz M, and McLafferty S. 2010. Mosquito politics: local vector control policies and the spread of West Nile Virus in the Chicago region. Health and Place. 16(6):1188–95. [DOI] [PubMed] [Google Scholar]
- Thomas D, Weedermann M, Billings L, Hoffacker J, and Washington-Allen RA. 2009. When to spray: a time-scale calculus approach to controlling the impact of West Nile virus. Ecology and Society 14(2): 21. [Google Scholar]
- Zhou L, Lawrence GG, Vineis JH, McAllister JC, Wirtz RA, and Brogdon WG. 2009. Detection of broadly distributed sodium channel alleles characteristic of insect pyrethroid resistance in West Nile virus vector Culex pipiens complex mosquitoes in the United States. J. Med. Entomol 46(2):321–7. [DOI] [PubMed] [Google Scholar]


