Abstract
Despite a critical need for a respiratory syncytial virus (RSV) vaccine and decades of development efforts, a vaccine to protect infants, elderly, and other at-risk populations from RSV infection remains elusive. We have previously generated a new, live-attenuated vaccine candidate against RSV using rational, computer-aided gene design and chemical synthesis through a process termed viral gene “deoptimization.” In this study, we assessed the attenuation, immunogenicity, and efficacy of this synthetic, live-attenuated RSV vaccine candidate, RSV-MinL4.0, in African Green Monkeys. RSV-MinL4.0 was produced under good-manufacturing-practice (GMP) in Vero cells. Vaccination with RSV-MinL4.0 resulted in minimal virus shedding after vaccination, generation of robust humoral and cellular immune responses (despite the presence of baseline RSV neutralizing antibodies in one animal) that were comparable to a wildtype infection, and protection from virus shedding post-challenge with wildtype RSV. These findings demonstrate the promise of RSV-MinL4.0 as a live-attenuated vaccine which will undergo clinical trials to test its ability to safely and effectively protect pediatric and elderly populations from infection with RSV.
Keywords: Vaccine, respiratory syncytial virus, non-human primates, respiratory illness, live-attenuated virus, codon-pair-bias, codon usage, African green monkeys
Background
Respiratory syncytial virus (RSV) infections occur worldwide; RSV is the most common cause of lower respiratory tract infection in children [1–4]. Approximately 34 million RSV infections occur globally each year in children under the age 5, about 10% of which warrant hospitalization. In the US an estimated 4,500 – 11,000 deaths per year (mainly among the elderly) are being attributed to RSV infection [2], making RSV the second deadliest respiratory virus after influenza. Significantly, the relative impact of RSV infections is twice that of influenza in children 0-7 years, and six times that of influenza in infants less than 2 years of age, as measured by frequency of emergency room and doctor’s office visits [5]. Despite these health impacts, there are no licensed vaccines against RSV to date.
The development of an RSV vaccine was hampered by the disastrous outcome of a clinical trial in the 1960’s which used a formalin inactivated RSV (FI-RSV) vaccine. In this trial, vaccinated infants subsequently experienced enhanced RSV disease (ERD) when they became naturally infected with circulating wild type RSV, resulting in more hospitalizations and even two deaths in the vaccine cohort [6]. It is thought that ERD results from the absence of cytotoxic CD8+ T-cell response from killed vaccines, and an insufficient neutralizing antibody response which not only fails to prevent reinfection but also prime for an over-exuberant TH2 response upon re-exposure [7]. Inactivated vaccines are therefore not considered safe in serologically RSV-naïve infants.
Several iterations of live attenuated RSV vaccine candidates have been developed over the decades. Most of them are based on a cold-passaged RSV/A2 backbone [8–10] which was subsequently mutagenized to screen for further attenuating mutations. A second-generation vaccine candidate, cpts-248/404, was immunogenic in young children, but was insufficiently attenuated in the youngest vaccine cohort (1-2 months) such that the risk/benefit profile did not warrant further development [11]. Another candidate, rA2cp248/404/1030 Delta SH (equivalent to Medimmune’s MEDI-559 candidate), was sufficiently attenuated in infants [12] but not genetically stable enough. Further attempts have been made to genetically stabilize the temperature sensitive (ts) mutations and prevent reversion to wildtype phenotype by using more “reversion-resistant” codons at the 1321 ts locus resulting in cps2. It was then discovered that a compensatory mutation at position 1313 could overcome the newly “stabilized” ts phenotype, prompting the deletion of that position (Δ1313) to yield a more stable vaccine [13]. In a subsequent iteration, the Δ1313 deletion was paired with an NS2 gene deletion besides the other mutations present in the rA2cp248/404/1030 Delta SH backbone, only to find yet another compensatory mutation I1314T. The investigators further attempted to prevent the I1314T mutation by changing the codon to a leucine in a modified vaccine candidate (ΔNS2/Δ1313/1314L) [14], which is currently being tested in clinical trials. [15] In looking at the boneyard of past live attenuated RSV vaccines it has become evident that there is a fine line between virus attenuation and immunogenicity and striking the right balance has proven a nearly intractable obstacle with conventional approaches. The empirical nature of past RSV vaccine development illustrates the pressing need for a more rational approach to the problem of RSV attenuation.
Codon pair deoptimization of large segments of viral genomes generates highly attenuated viruses that are inherently stable and have a formidable barrier to wildtype reversion [16,17], while retaining 100% amino acid sequence identity to the parent virus [18]. The level of virus attenuation can be custom-tailored on a sliding scale, akin to a “brake pedal” on a car or the “volume” on a radio. More deoptimization results in greater attenuation, reduced deoptimization results in less attenuation [18–21]. Viral attenuation can be designed to any set point between non-viable and wild-type-like.
RSV-MinL4.0 is a codon pair deoptimized, phenotypically and genetically stable RSV vaccine candidate, derived by repeated stress passaging in vitro and reverse engineering [17] of a codon pair deoptimized RSV, originally termed “RSV-MinL” (16). Generated by codon-pair deoptimization (CPD) of the L gene, the parental RSV-MinL vaccine candidate was shown to be ts in cell culture and attenuated in mice and hamsters (16). When compared to two attenuated RSV strains currently in clinical trials, the original MinL vaccine was comparably restricted and immunogenic in African Green Monkeys (AGMs) [22]. Iterative serial passage of RSV-MinL under stress conditions (elevated temperatures) yielded mutations that overcame the ts phenotype with compensatory amino acid mutations. All four of these compensatory mutations were combined and reverse-engineered into MinL, yielding RSV-MinL4.0. When all four amino acid changes were inserted into MinL, the resulting RSV-MinL4.0 had a simultaneous increase in in vivo attenuation combined with improved immunogenicity in small animals [17]. In this study we show that RSV-MinL4.0 induces robust activation of both humoral and cellular immunity in African Green Monkeys (AGM) and protects AGMs from challenge with wild type RSV.
Methods
cGMP Vero Master Cell Bank
To manufacture RSV-MinL4.0 we prepared a Vero Master Cell Bank (Vero MCB) under cGMP at Charles River Laboratories (CRL, Malvern, PA). The Vero MCB was derived from Vero WHO-Seed Lot 10-87, which was prepared by Institut Merieux (France) on behalf of WHO in October 1987. One of the last 6 original vials available in the world (vial No. 1090) of this lot, frozen in 1987, was gifted to Codagenix by permission of the US FDA, who administers the lot. This lot has been extensively release tested by WHO in the past and been used for vaccine production by many manufacturers throughout the world.
Upon receipt of Vero WHO-Seed Lot 10-87 vial No. 1090, it was used to prepare a pre-Master Cell Bank at Codagenix in a laboratory, biosafety cabinet, and cell incubators specifically dedicated to this task. Cells from the original vial were recovered and grown under animal-origin free conditions, using OptiPro-SFM (Thermo Fisher), supplemented with 1x Glutamax (Thermo Fisher). Cells were trypsinized with recombinant Trypsin TrypLE Select (Thermo Fisher). Thus, the cells were never exposed to animal origin substance (including serum) since 1987. The Codagenix pre-Master Seed Lot was prepared after 2 passages from the original WHO vial. Vials of pre-Master Seed Lot were transferred to CRL where they were GMP-tested for sterility and mycoplasma before being admitted to GMP manufacturing. After 5 animal-origin free passages under GMP at CRL on T225 flasks (in OptiPro/Glutamax) the Vero Master Cell Bank “Vero WHO 10-87 +7P AOF” Lot 563173-MCB1 was prepared by CRL. The cells are cryo- preserved in OptiPro containing 5% DMSO (serum-free, animal origin-free). The Vero Master Cell Bank Lot 563173-MCB1 was extensively release tested under cGMP and passed all tests as per FDA and EMA guidance.
Viruses
RSV-MinL4.0 has been described previously as MinL NPM2-1[N88K]L[17] . Both RSV-MinL4.0 and the parental WT-RSV/A2 were grown in the Vero MCB (described above) under animal-origin free conditions in a serum-free medium consisting of equal parts of OptiPro SFM and DMEM. Virus was stabilized for storage at −80C by addition of 25% sucrose. Virus infectivity titers were determined by immune-enhanced focus forming assay on Vero cells. Infected foci were visualized after 5 days infection at 33C under a semisolid overlay medium (0.3% Tragacanth gum/MEM/5% FBS) by staining with a anti RSV F monoclonal antibody 2F7 (Novus Biologicals), anti-mouse IgG-HRP (Jackson Immuno Research) and developed with HRP substrate kit Vector VIP (Vector Laboratories)
Vaccine studies in African Green Monkeys
Animals were single-housed during the quarantine and study phases in stainless steel cages that meet requirements as set forth in the Animal Welfare Act (Public Law 99-198) and the Guide for the Care and Use of Laboratory Animals (8th Edition, Institute of Animal Resources, Commission on Life Sciences, National Research Council; National Academy Press; Washington D.C.; 2011). This study design was reviewed by the IACUC at Southern Research Institute and was approved on 04/10/2018; it was assigned IACUC tracking number 18-03-009F. A total of eight (7 males and 1 female) AGMs seronegative for RSV (reciprocal titer of <11, in enzyme immunoassay for RSV antigens in HEp2 cells infected with 100 TCID50 virus followed by staining for RSV antigens) were divided into two test groups of N=4 (Groups 1 and 2). Group 1 was inoculated on Day 0 with 106 plaque-forming units (PFU) of WT-RSV delivered by intranasal and the intratracheal route (total dose of 2 x 106 PFU). Group 2 was inoculated the same way on Days 0 and 28 with 2 x 106 PFU of MinL4.0 delivered by the intranasal and intratracheal. Samples generated in AGMs were evaluated by RSV neutralization focus formation assay, RSV specific IgG, RSV RT-qPCR (virus quantitation), and an RSV IFNγ ELISPOT Assay for RSV attenuation and immunogenicity. RSV shedding after infection was measured in OP swabs and TOTL by RT-qPCR. RSV neutralizing antibody titers were tested against RSV/A2 (NR-12149, BEI Resources) using an FRNT50 on serum from days 0, 21, 28, and 49 post infection/vaccination. Cell mediated immunity against RSV was measured by IFNγ ELISPOT at baseline (Day 0) and days 14, 21, 28 and 49. Phytohemagglutinin (PHA)-stimulated and unstimulated PBMCs were used as positive and negative controls respectively. Clarified RSV/A2 (NR-12149, BEI Resources) infected HEp2 cell lysates were used to stimulate IFNγ response in the PBMCs from infected/vaccinated NHPs. Previously vaccinated AGMs were challenged with RSV/A2 wild type on day 104, followed by evaluation of virus shedding in OP and TOTL by RT-qPCR.
Results
Attenuation of virus shedding in vaccinated monkeys
Initially, we sought to confirm that RSV-MinL4.0 retained the attenuated phenotype we had observed previously in small animal models using non-human primates [17]. Following infection/vaccination of AGM with either 2 x 106 PFU of wild type (WT) RSV/A2 or RSV-MinL4.0, viral attenuation was measured using viral load in the trans-oro tracheal lavage (TOTL) and oropharyngeal (OP) swabs at various time points post-infection/vaccination by RT-qPCR. In the TOTL samples, WT RSV was detectable at day 2 post-infection, increasing to peak level at day 6, and plateauing up to day 8 (Fig 1A). These levels gradually decreased to baseline by day 14. In RSV-MinL4.0 vaccinated animals, the peak viral levels at day 6 were over 100-fold lower than WT (Fig 1A). This viral load peak was also short lived, as virus could not be detected in RSV-MinL4.0 vaccinated animals at time points beyond Day 8 (Fig 1A).
Fig 1. Attenuation of RSV-MinL4.0 compared to WT RSV/A2 in African Green Monkeys.
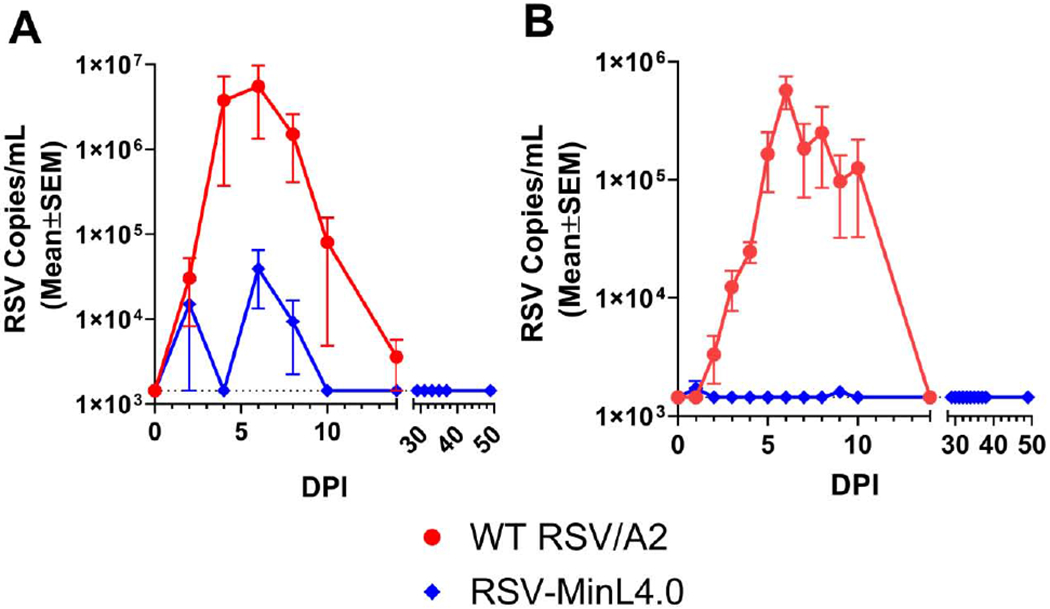
Four AGMs per group were infected with 2 x 106 PFU of WT RSV or RSV-MinL4.0 on day 0. Animals RSV-MinL4.0 received a second identical dose on day 28. Virus shedding was measured in (A) trans-oro tracheal lavage (TOTL) and (B) oropharyngeal (OP) samples at the indicated time point. The limit of detection in RT-qPCR was 1444.8 copies/mL. DPI: days post-infection.
OP swabs were collected to detect potential shedding of our vaccine candidate in the upper respiratory tracts of vaccinated monkeys. In the OP samples (Fig 1B), similar to TOTL samples, WT RSV was detectable by day 2 post-infection. RSV viral load increased exponentially to a peak level at day 6 and remained steady up to day 8. These levels gradually decreased to baseline at Day 14. However, in RSV MinL4.0 vaccinated animals, virus loads remained at or below the threshold of detection at all days, or at least a 330-fold reduction to WT RSV/A2 infected animals. Comparison of both TOTL and OP swab data suggested higher viral loads (1 log higher) in TOTL compared to OP, which is not surprising as RSV is a lower respiratory tract pathogen. The magnitude and time to peak viral shedding observed in this study is similar to what was seen in previous studies [23]. Taken together, viral shedding data from TOTL and OP suggest that RSV MinL4.0 virus is significantly attenuated (>100-fold lower peak viral titers and shorter duration of shedding), compared to WT RSV/A2 (Fig 1A and B).
Immunogenicity of RSV-MinL4.0 vaccine
Although highly attenuated in vivo, CPD vaccine candidates can be very immunogenic as seen with SAVE derived influenza vaccines [20,24]. The use of a live-attenuated vaccine, as opposed to inactivated or subunit vaccines, should engender cellular immunity as well as antibody responses. To measure immunogenicity of the RSV-MinL4.0 vaccine in AGMs, blood was collected at predetermined time points from vaccinated AGMs to test for markers of antibody responses (RSV specific IgG and neutralizing antibodies) and cellular immunity (IFNγ production by PBMCs). RSV-specific IgG in the serum was measured at day 0 (baseline) and days 14, 21, 28 and 49. At baseline, most of the animals did not have detectable levels of RSV specific IgG, as the animals were pre-screened to exclude sero-positives. However, one animal in the RSV-MinL4.0 group (#5615) escaped detection at the screening stage, and entered the study with an IgG titer of 398.2 at baseline (compared to other animals with IgG titers below the level of detection of 200), suggesting that this animal had a previous exposure to natural RSV infection.
We followed the IgG response to RSV in to determine the timeline of each animal’s IgG response in the context of WT infection compared to vaccination with RSV-MinL4.0. Time-dependent increase in RSV IgG titer was observed in animals infected with WT RSV/A2 (Fig 2A). In these animals, RSV IgG titers were detectable at low levels on day 21 post-infection, and increased at day 28. In contrast, animals vaccinated with RSV-MinL4.0 had detectable IgG titers at day 14, albeit with high variability, that remained at similar levels between day 14 and 28. After the RSV-MinL4.0 boost on day 28, RSV IgG titers increased at day 49. The animal with preexisting RSV-specific IgG (#5615) also experienced a boosting in IgG levels upon prime vaccination at day 14. Similar levels of RSV IgG (>3,500 U/mL) were observed at later time points day 21, 28 and 49 (Supplementary Table 3).
Fig 2. Immunogenicity of RSV-MinL4.0 vaccination as measured by specific-IgG and neutralizing antibodies.
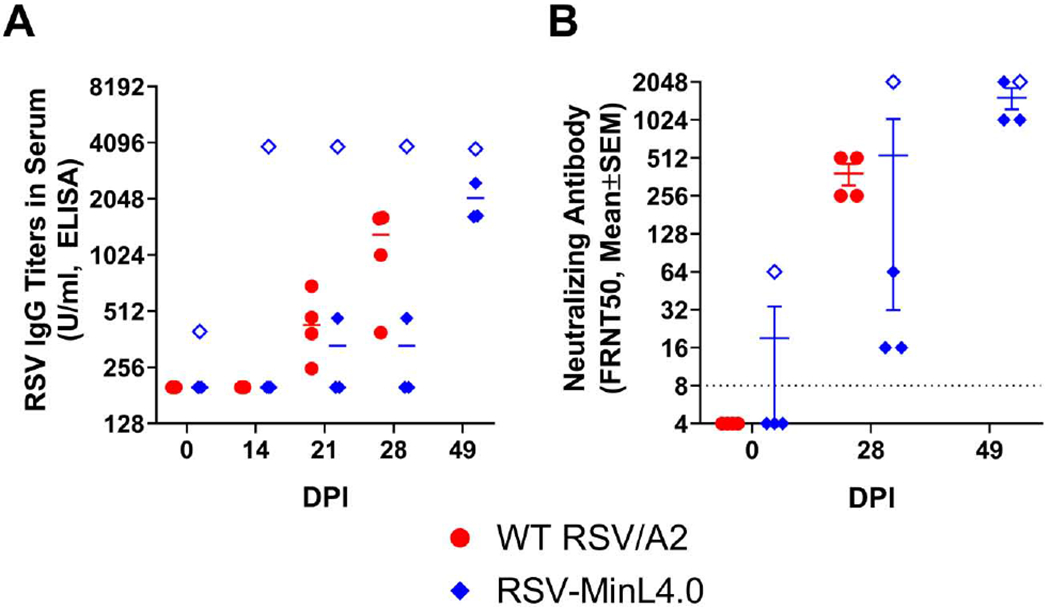
Sera collected from WT RSV infected or RSV-MinL4.0 vaccinated AGMs were tested for RSV F protein specific IgG (A) or neutralizing antibodies by FRNT50 assay (B). The limit of detection (LOD) in ELISA was 200 Units/mL. The limit of detection (LOD) in the FRNT50 assay was 8. DPI: days post-infection. Hollow diamonds represent data points from animal #5615.
At baseline most of the animals showed a neutralizing antibody titer of FRNT50 of <8. In animals infected with WT RSV/A2, FRNT50 titers increased at day 28 post-infection. A similar trend in time-dependent increase in FRNT50 titers was observed in animals vaccinated with RSV-MinL4.0. For RSV-MinL4.0 vaccinated animals, the FRNT50 increased compared to baseline at day 28 and 49 (Fig 2B). Animal #5615 in the RSV-MinL4.0 group, which had also tested positive for RSV IgG, had a significantly elevated FRNT50 titer at baseline (1:64) compared to the other animals (Fig 2B). Despite pre-existing RSV neutralizing antibodies, this animal experience a potent 32-fold boost in neutralization titer as a result of the first dose of RSV-MinL4.0 vaccine. The great increase of neutralizing antibodies in this animal suggest that vaccine “take” of RSV-MinL4.0 was not prevented by pre-existing immunity against RSV.
To assess cell-mediated immunity against RSV/A2 in AGM, ELISPOT was performed with PBMCs collected at baseline (Day 0), after primary vaccination (d 28) and after boost (d 49) (Fig 3). Background IFNγ production was observed with group averages of up to 57.2 ± 13.5 spots per million (Fig 3). One AGM (#5615), which also had higher baseline serum IgG and neutralizing antibodies against RSV (Fig 2A), showed higher IFNγ spots at 145 ± 52.2 spots per million at baseline (Day 0), compared to other animals which ranged from 3.3 to 31.7 IFNγ spots per million. In animals infected with WT RSV/A2, ELISPOT titers increased post-infection (mean ELISPOT titers are presented in Fig 3). A similar trend was observed for animals vaccinated with RSV-MinL4.0 (Fig 3).
Fig 3. Cell-mediated immunogenicity of RSV MinL4.0 vaccination.

PBMCs collected from WT RSV/A2 infected or RSV-MinL4.0 vaccinated AGMs on days 0, 14, 21, 28, and 49 were tested for IFNγ expression by ELISPOT, after stimulation with whole inactivated RSV virus. Hollow diamonds represent data points from animal #5615.
Protection from infection with wildtype RSV/A2
In addition to measuring the cellular and antibody-mediated immune responses to RSV-MinL4.0 vaccination, we also tested for protection against challenge with WT RSV/A2 using prevention of viral load in TOTL and shedding in OP swabs as metrics of efficacy. To measure the protective efficacy of RSV-MinL4.0, vaccinated animals were challenged with 2 x 106 PFU WT RSV/A2 on day 104 post-vaccination followed by measurement of virus shedding by RT-qPCR of TOTL and OP swabs up to 10 days post-challenge (Fig 4). Virus shedding in immunized, challenged animals was compared to the initial shedding seen in naïve animals during the vaccination phase of the experiment. TOTL samples from RSV-MinL4.0 vaccinated animals did not show any detectable challenge viral RNA (3,847-fold lower compared to peak viral titers observed in the naïve animals after WT RSV/A2 infection). OP swabs from day 4 to day 9 showed low levels of RSV with peak virus shedding observed at day 4, which was 44-fold lower than the peak viral titers observed in naïve animals after infection (see Fig 1). These results suggest that RSV vaccine candidate MinL4.0 confers protection against challenge with WT RSV/A2, even at >100 days post-primary vaccination.
Fig 4. Protection from wildtype RSV/A2 challenge.

RSV-MinL4.0 vaccinated AGMs were challenged with 2 x 106 FFU RSV/A2 delivered by IT inoculation and protective efficacy was measured by viral load in TOTL (A) and OP (B) samples at 0-10 days post-challenge (DPC). Note: Data points from mock vaccinated/wt RSV/A2 challenged animals were collected on days 0-10 of the study (during vaccination phase; see also Fig. 1). Limit of detection was 1,444.8 RSV copies/ml.
Discussion
Live-attenuated vaccines against RSV would provide several advantages over killed or protein-based vaccines for the immunization of young children and the elderly: induction of innate, humoral (neutralizing antibodies) and cellular (CD8 T-cell) immunity that is analogous to the natural infection, reduced risk of vaccine-associated enhanced RSV disease, the ability of the vaccine to replicate in the upper respiratory tract even in the presence of neutralizing antibodies (which are mainly circulatory), and convenient intranasal delivery.
Cellular immunity is critical to protection against RSV infection [25] in both neonates and the elderly, however, many RSV vaccines in clinical trials, especially those intended for the elderly, are focused on solely generating neutralizing antibody responses (antigen based vaccine candidates). RSV-MinL4.0, as a live attenuated vaccine, is also able to induce cellular immunity as shown by elevated IFNγ expression comparable to WT RSV/A2.
Of particular interest, one animal (#5615) included in this study had elevated levels of serum IgG and neutralizing antibodies against RSV at baseline that escaped detection during the pre-screening. Interestingly, vaccination triggered a potent immune response in this animal, with serum IgG levels (days 14, 21, and 28) and neutralizing antibody titers (days 21 and 28) after a single dose greatly exceeding the values seen in other animals, even those infected with WT RSV/A2. After the boost delivered on day 28, FRNT50 and RSV IgG titers in RSV-MinL4.0 vaccinated animals increased to the levels of the AGM with pre-existing RSV immunity by day 49. Animal #5615 derived no further boosting of any of the tested immune readouts after the second dose, suggesting that it achieved sterilizing immunity from the first vaccine dose, even in light (or because) of the pre-existing partial RSV immunity. Boosting of RSV specific neutralizing antibody titers and cellular immunity (IFNγ production in PBMCs) in the pre-immune AGMs by RSV-MinL4.0 is a promising observation that may emulate the situation likely to be encountered in the seropositive human population. While this animal was intriguing, conclusions could not be drawn based upon a single animal and further studies are needed to explore RSV-MinL4.0 immunogenicity and protection in the context of previous exposure, as would be likely in a clinical setting.
Any live-attenuated vaccine for RSV needs to be safe (attenuated), phenotypically stable, and able to induce a protective immune response even in the presence of pre-existing immunity. The results presented in this study suggest that RSV-MinL4.0 was sufficiently attenuated in AGMs yet able to generate sufficiently robust humoral and cellular immunity to support further clinical development. MinL4.0 delivered by the combined intranasal and intratracheal route resulted in 100% seroconversion after prime-boost immunization. The intratracheal co-administration was chosen for a more rigorous safety readout of the MinL4.0 vaccine in comparison to the WT RSV/A2 virus in this semi-permissive primate model. The challenge studies in RSV-MinL4.0 vaccinated animals after more than 100 days demonstrated significantly reduced virus shedding in OP swabs (>3 logs lower than WT RSV infection) and TOTL samples (>1 log lower than WT RSV/A2 infection), suggesting that RSV-MinL4.0 also conferred robust protection against WT RSV/A2 challenge. The immunogenicity of RSV-MinL4.0 in the context of pre-existing immunity is especially important for those who may have high levels of neutralizing antibody but with an inadequate cellular immune response. Interestingly, we did not observe an exacerbated virus shedding in the animal (#5615) with pre-existing baseline immunity. In fact, no vaccine virus shedding was detectable in this animal after the first vaccine dose on any day (S1 Table; S2 Table), underscoring the safety and immunogenicity of RSV-MinL4.0. Clinical studies will reveal whether RSV-MinL4.0 will be efficacious in pre-immune recipients of the vaccine, such as the elderly and high-risk adults, who have proven recalcitrant to all previous RSV vaccination approaches.
Supplementary Material
Acknowledgments:
The authors would like to thank the Collins lab at NIH/NIAID for the transfer of RSV MinL4.0 to Codagenix under MTA and all of the assistance they have rendered in the development of SAVE RSV. We are gratefully acknowledging the gift from the US Food and Drug Administration of one of the last remaining original vials of WHO Vero Cell Bank 10-87.
Funding: This work was supported by the National Institute for Allergy and Infectious Diseases at the National Institutes of Health grant number 1 R44 AI131756-01 to SM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentations at Meetings: This work was presented as a poster at ID Week, Oct 2-6, 2019 in Washington DC, poster number 2777
Conflicts of interest: SM, CS, AK, ST, and JRC are paid employees of Codagenix, Inc. SM, JRC and CBS are also shareholders. SM is an inventor on patent US14/766,620.
References
- 1.Ogra PL. Respiratory syncytial virus: The virus, the disease and the immune response. Paediatric Respiratory Reviews. 2004; 5:S119–S126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang Y-W, Crowe JE. Respiratory Syncytial Virus and Human Metapneumovirus. Manual of Clinical Microbiology, 10th Edition. 2011; :1357–1371. [Google Scholar]
- 3.Tregoning JS, Pribul PK, Pennycook AMJ, et al. The Chemokine MIP1α/CCL3 Determines Pathology in Primary RSV Infection by Regulating the Balance of T Cell Populations in the Murine Lung. PLOS ONE. 2010; 5(2):e9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tregoning JS, Schwarze J. Respiratory Viral Infections in Infants: Causes, Clinical Symptoms, Virology, and Immunology. Clinical Microbiology Reviews. 2010; 23(1):74–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourgeois FT, Valim C, McAdam AJ, Mandl KD. Relative Impact of Influenza and Respiratory Syncytial Virus in Young Children. Pediatrics. 2009; 124(6):e1072–e1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HW, Canchola JG, Brandt CD, et al. RESPIRATORY SYNCYTIAL VIRUS DISEASE IN INFANTS DESPITE PRIOR ADMINISTRATION OF ANTIGENIC INACTIVATED VACCINE. Am J Epidemiol. 1969; 89(4):422–434. [DOI] [PubMed] [Google Scholar]
- 7.Durbin AP, Karron RA. Progress in the Development of Respiratory Syncytial Virus and Parainfluenza Virus Vaccines. Clin Infect Dis. 2003; 37(12):1668–1677. [DOI] [PubMed] [Google Scholar]
- 8.Connors M, Crowe JE, Firestone C-Y, Murphy BR, Collins PL. A Cold-Passaged, Attenuated Strain of Human Respiratory Syncytial Virus Contains Mutations in the F and L Genes. Virology. 1995; 208(2):478–484. [DOI] [PubMed] [Google Scholar]
- 9.Friedewald WT, Forsyth BR, Smith CB, Gharpure MA, Chanock RM. Low-Temperature-Grown RS Virus in Adult Volunteers. JAMA. 1968; 204(8):690–694. [PubMed] [Google Scholar]
- 10.Kim HW, Arrobio JO, Pyles G, et al. Clinical and Immunological Response of Infants and Children to Administration of Low-Temperature Adapted Respiratory Syncytial Virus. Pediatrics. 1971; 48(5):745–755. [PubMed] [Google Scholar]
- 11.Wright PF, Karron RA, Belshe RB, et al. Evaluation of a Live, Cold-Passaged, Temperature-Sensitive, Respiratory Syncytial Virus Vaccine Candidate in Infancy. J Infect Dis. 2000; 182(5):1331–1342. [DOI] [PubMed] [Google Scholar]
- 12.Karron RA, Wright PF, Belshe RB, et al. Identification of a Recombinant Live Attenuated Respiratory Syncytial Virus Vaccine Candidate That Is Highly Attenuated in Infants. The Journal of Infectious Diseases. 2005; 191(7):1093–1104. [DOI] [PubMed] [Google Scholar]
- 13.Luongo C, Winter CC, Collins PL, Buchholz UJ. Increased Genetic and Phenotypic Stability of a Promising Live-Attenuated Respiratory Syncytial Virus Vaccine Candidate by Reverse Genetics. J Virol. 2012; 86(19):10792–10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luongo C, Winter CC, Collins PL, Buchholz UJ. Respiratory Syncytial Virus Modified by Deletions of the NS2 Gene and Amino Acid S1313 of the L Polymerase Protein Is a Temperature-Sensitive, Live-Attenuated Vaccine Candidate That Is Phenotypically Stable at Physiological Temperature. J Virol. 2013; 87(4):1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchholz UJ, Cunningham CK, Muresan P, et al. Live Respiratory Syncytial Virus (RSV) Vaccine Candidate Containing Stabilized Temperature-Sensitivity Mutations Is Highly Attenuated in RSV-Seronegative Infants and Children. The Journal of Infectious Diseases. 2018; 217(9):1338–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffin JM. Attenuation by a thousand cuts. N Engl J Med. 2008; 359(21):2283–2285. [DOI] [PubMed] [Google Scholar]
- 17.Nouën CL, McCarty T, Brown M, et al. Genetic stability of genome-scale deoptimized RNA virus vaccine candidates under selective pressure. PNAS. 2017; 114(3):E386–E395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman JR, Papamichail D, Skiena S, Futcher B, Wimmer E, Mueller S. Virus attenuation by genome-scale changes in codon pair bias. Science. 2008; 320(5884):1784–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller S, Coleman JR, Papamichail D, et al. Live attenuated influenza virus vaccines by computer-aided rational design. Nat Biotechnol. 2010; 28(7):723–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang C, Skiena S, Futcher B, Mueller S, Wimmer E. Deliberate reduction of hemagglutinin and neuraminidase expression of influenza virus leads to an ultraprotective live vaccine in mice. Proc Natl Acad Sci USA. 2013; 110(23):9481–9486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stauft CB, Song Y, Gorbatsevych O, et al. Extensive genomic recoding by codon-pair deoptimization selective for mammals is a flexible tool to generate attenuated vaccine candidates for dengue virus 2. Virology. 2019; 537:237–245. [DOI] [PubMed] [Google Scholar]
- 22.Le Nouen C, Brock LG, Luongo C, et al. Attenuation of human respiratory syncytial virus by genome-scale codon-pair deoptimization. Proceedings of the National Academy of Sciences. 2014; 111(36):13169–13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ispas G, Koul A, Verbeeck J, et al. Antiviral Activity of TMC353121, a Respiratory Syncytial Virus (RSV) Fusion Inhibitor, in a Non-Human Primate Model. PLOS ONE. 2015; 10(5):e0126959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan BS, Souza CK, Gauger PC, et al. Vaccination of pigs with a codon-pair bias de-optimized live attenuated influenza vaccine protects from homologous challenge. Vaccine. 2018; 36(8):1101–1107. [DOI] [PubMed] [Google Scholar]
- 25.Openshaw PJ, Chiu C. Protective and dysregulated T cell immunity in RSV infection. Current Opinion in Virology. 2013; 3(4):468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


