Abstract
Background: Laparoscopic resection is increasingly used in colorectal cancer (CRC). It has been suggested to carry short-term benefits in safety, recovery, and preservation on immune function for patients with CRC. However, the impact of laparoscopic resection on natural killer (NK) cells is largely unclear. Methods: A total of 200 patients with CRC across Dukes A/B/C stages were randomly assigned to laparoscopic or open resection. The blood samples were collected before and after the surgery. The total number of NK cells was quantified by flow cytometer. Lytic units 35 toward K562 was used to quantify NK cells activity. The outcomes between the groups across pathological stages were also analyzed. Results: The number and activity of NK cells decreased after the surgery in both groups. The laparoscopic group showed a faster recovery rate of NK cells function than the control group as assessed by cell count and lytic activity. Natural killer cells were impaired in a higher degree in patients at Dukes B/C stages. The recovery of NK cells to baseline level at day 7 postsurgery was observed in the laparoscopic group across all 3 stages. Conclusion: Generally, laparoscopically assisted surgery resulted in a better preservation on NK cells function. A better outcome was observed in patients with CRC at Dukes B/C stages.
Keywords: laparoscopy, natural killer cells, colorectal cancer, immune function, flow cytometry
Introduction
Globally, colorectal cancer (CRC) is the third most common type of cancer. In 2018, about 1.8 million new cases are diagnosed and about 861 000 deaths are associated with CRC. In United States, CRC is the second leading cause of cancer death. The occurrence rate of CRC is much higher in males than in females.1,2 Dukes staging classification is commonly used to access the pathological status of CRC. In Dukes A, tumor invasion is found into the bowel wall. In Dukes B, tumor invades through the bowel wall but not reaches lymph nodes. The 5-year survival rate for Dukes A/B is usually above 50%. In Dukes C, tumor metastases into lymph nodes and the 5-year survival rate drops to 30%, and Dukes D is the most advance stage of CRC with a widespread metastasis. Although radiotherapy and chemotherapy are used increasingly to treat CRC,3,4 the most effective therapeutic approach is surgical resection. More than 90% of patients with CRC undergo surgical treatment. The conventional method is open colonic surgery which was practiced more than a decade ago. It involves a complete removal of the fatty envelope containing around the rectum encompassing the tumor tissues. The 5-year survival rate of surgical treatment for CRC is less than 50%. The combination of surgical resurrection with adjuvant therapies such as chemotherapy can increase survival rates to above 70% in selected patients.5,6
In recent years, laparoscopically assisted surgical resection has been increasingly used to replace open approach. This approach is minimally invasive and causes less blood loss and a reduction in surgical trauma. However, laparoscopic approach involves more complicated technical procedure and longer operation time and requires more sophisticated training for the doctors. There are many debates on the pros and cons of laparoscopically assisted surgery over open resection, and clinical advantages of laparoscopic need to be established by evidence-based study.7,8
Surgical resection for treating CRC results in tissue trauma and blood loss that subsequently causes alterations in body immune function and affect postoperative morbidity. The reduction in trauma by employing minimally invasive approach such as laparoscopic surgery may deliver better outcomes in preserving the normal immune function.9 For instance, randomized clinical studies have compared the acute phase responses after laparoscopic resection with open surgery and observed a reduced inflammatory response such as the lower plasma levels of interleukin (IL) 6 and C-reactive protein (CRP).10 Cellular immunity is a protective immune response involving the activation of immune cells. There are 2 major components in the cell-mediated immune response. The first one involves nonspecific and acute responses of cells from innate immunity such as macrophages and natural killer cells (NK cells). They kill the target cells through phagocytosis or inducing cytotoxicity. The other one is through acquired and specific responses of adaptive immune cells such as antigen-specific cytotoxic T-lymphocytes. They can destroy tumor cells displaying cancer-specific antigen on the cell surface. The preservation of laparoscopic approach on cellular immunity has been shown on animal studies but it is still considered controversial in human studies.11-13
Natural killer cells are an important immune surveillance of malignant transformation. They are innate lymphoid cells and can directly induce death of malignant cells. Cancer cells often lose the expression of major histocompatibility complex I on their surface, which makes them recognizable by NK cells. Upon detection of tumor, NK cells release cytotoxic granules onto the target cells that lead to the lysis event. This makes them important to fight the cancer growth and restrict tumor metastasis.14 In our study, we aimed to compare the effects of the laparoscopic surgery versus open surgical resection on NK cells function. In addition, we would like to study the NK cells response across different CRC pathological stages and evaluate the surgical impact on NK cells in different tumor stages. This study would give the new insight on the benefits of laparoscopic surgery in preserving immune function in a view of NK cells function.
Methods
Clinical Study Design
A total of 200 patients were enrolled into a randomized comparative prospective study trial. The study was approved by the institutional review board in the First Hospital of Quanzhou Affiliated to Fujian Medical University (approval no. FHQA529j04). All patients provided written informed consent prior to enrollment in the study. Patients were assigned with a conventional open approach (n = 100) or a laparoscopically assisted surgery (n = 100) for the curative resection of CRC. Peripheral blood was collected 24 hours preoperatively and 24, 96, and 168 hours after surgery. Inclusion criteria of patient enrolment include (1) clinically validated pathological diagnosis of CRC, (2) receiving nonemergency surgery, (3) no signs of tumor metastasis to liver tissue or other distant internal organs, and (4) no extensive tumor metastasis to the bladder, uterus, or pelvis.
Exclusion criteria include (1) CRC stage at Dukes 4; (2) with a history of major abdominal surgery; (3) admitted to hospital for acute complete intestinal obstruction, hemorrhage, and perforation; (4) with local infiltration of the masses with severely adhesion and experiencing difficult to remove; and (5) with a history of recurrence of CRC or other types of cancers.
Randomization and Study Procedure
Patients were prospectively randomized through a computer-generated randomization process to either laparoscopic or open surgery (Figure 1). Primary short-term outcomes included length of operation, hospital stay, analgesic use, and any postoperative complications. Follow-up visits were at 1 and 3 months after surgery. The end points included complication rates and mortality. We will report long-term outcomes (survival, recurrence, and quality of life) at 4 years. The study design was aimed for an enrolment of 100 patients for each surgical procedure. The surgical operation was carried out in the First Hospital of Quanzhou Affiliated to Fujian Medical University in a 2-year study timeline.
Figure 1.
Trial profile.
Quantification of Natural Killer Cells
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque gradient centrifugation. The total number of NK cells was analyzed by a flow cytometry (FACScan, Becton Dickinson). Briefly, 50 µL PBMCs were incubated with florescence dye-conjugated antibodies against CD3, CD16, and CD56 for 1 hour at 4°C. The antibodies were purchased from Becton Dickinson. The NK cells were gated as CD3−CD56+, CD3−CD16+, or CD3−CD56+CD16+ PBMC. The absolute number of NK cells was determined based on a standard curve established by adding a fixed number of polystyrene microbeads (20 µm) to the samples before the cytometric analysis.
Standard NK Cells Lytic Activity
The assay was modified from the reported literature.15 Briefly, K562 obtained from American Type Culture Collection was used as target cells. They were cultured in Roswell Park Memorial Institute medium (Invitrogen, Waltham, Massachusetts) supplemented with 10% fetal bovine serum without phenol red at 37°C in 5% CO2. Before the assay, the target cells were labelled with 2 mmol/L dioctadecyloxacarbocyanine perchlorate (DiO; Sigma, St Louis, Missouri). The harvested PBMCs were used as effector cells and cultured in R-10 medium without phenol red. Effector and target cells (3 × 105) were mixed with different effector-to-target ratios (E: T) from 40:1, 20:1, 10:1, 5:1, and 2.5:1. The cells were incubated at 37°C in 5% CO2 for 4 hours. The cytotoxic cells were stained with propidum iodide (PI) and analyzed in flow cytometry. The number of lysis cells was calculated as positive events for both DiO and PI staining. Spontaneous lysis baseline was also defined in control consisted of only target cells. Lytic units 35 was defined as the concentration of effector cells (in thousands) achieving 35% cytotoxicity on target cells.
Statistical Analysis
Mann-Whitney U test was used to evaluate the characteristic differences between the comparison groups. For functional assays, the comparison of 2 groups at a particular time point was analyzed by unpaired Student t test. The comparison within the same group across more different time points was calculated by one-way analysis of variance analysis. Chi-square test was used to analyze the responsive pattern of NK cells to the surgeries in different pathological stages. The SPSS software was used for the statistical analysis. Only P < .05 was considered as statistically significant.
Results
In this study, a total of 200 patients were recruited. Half of them were randomly assigned to laparoscopic surgery with the other half assigned to open surgical approach. The 2 groups have comparable distributions in age and genders, and no significant differences between the 2 groups were found with respect to the histological grading and pathological stages (Table 1). The comparison about the lesion sites from the 2 groups was also detailed in Supplementary Table S1. Patients at Dukes D stage were excluded. As expected, the mean operative time in the laparoscopic group (161 minutes) was significantly longer than the open group (115 minutes), but the patients undergoing laparoscopic surgery (400 mL) experienced less blood loss than the open group (420 mL).
Table 1.
Characteristics of Patients With CRC Enrolled in the Study.
| Characteristics | Laparoscopic | Open |
|---|---|---|
| Number | 100 | 100 |
| Age (years)/median (range) | 72 (48-89) | 70 (50-86) |
| Operative time (min)/mean (range) | 161 (90-298) | 115 (75-250)a |
| Estimated blood loss (mL)/mean (range) | 400 (160-780) | 420 (200-810)b |
| Pathological stages (Dukes) | ||
| Dukes A | 31 | 29 |
| Dukes B | 40 | 44 |
| Dukes C | 29 | 27 |
| Histological grading (WHO) | ||
| Well differentiated | 42 | 41 |
| Moderately differentiated | 40 | 39 |
| Poorly differentiated | 18 | 20 |
Abbreviations: CRC, colorectal cancer; WHO, World Health Organization.
a P = .0211, Mann-Whitney U test.
b P = .0623, Mann-Whitney U test.
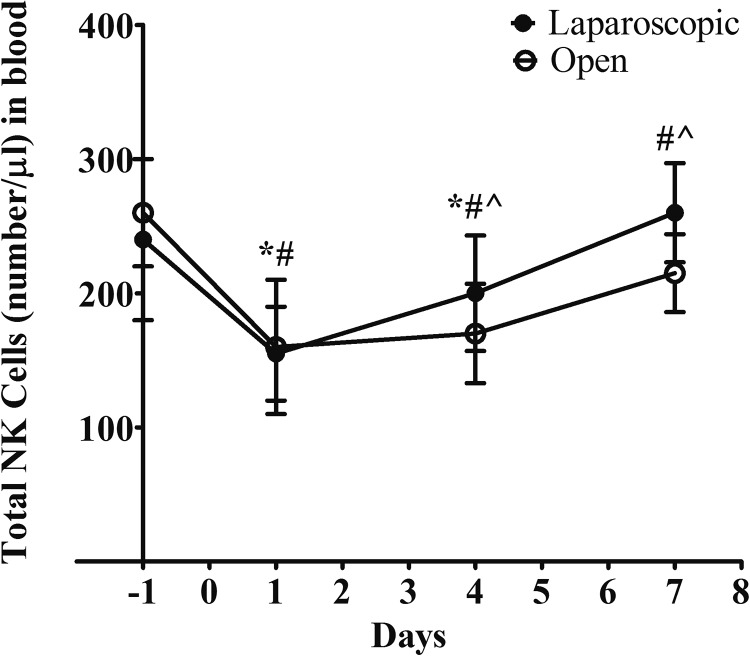
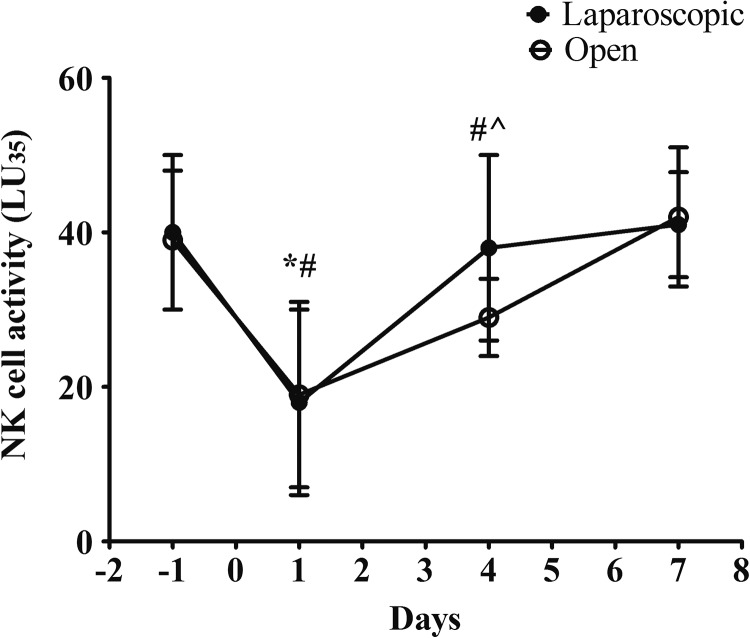
To examine the impact of different surgeries on the numbers and activities of NK cells, we collected the peripheral blood samples from patients at 1 day before the surgery and at days 1, 4, and 7 postoperation. The total number of NK cells in the blood samples were quantified by fluorescence-activated cell sorting. As shown in Figure 2, the total counts of NK cells were depressed markedly after the surgery in both groups. The numbers started to recover as detected at days 4 and 7 after the surgery. Interestingly, the recovery rate of NK cell numbers in blood was faster in the laparoscopic group. The baselines of NK cells count in the 2 groups were similar. At day 1, the changes before and after the surgery were also similar between the 2 groups. At day 4, the total count of NK cells in the laparoscopically assisted group was significantly higher than the control group. At day 7, in the laparoscopic group, the average number of NK cells reached to the baseline level before the surgery. In the control group, the average number of NK cells was still lower than the baseline with a statistical significance. We also proceeded to measure the lytic activity of NK cells. We observed a similar change pattern before and after the surgery in both study groups (Figure 3). The activity of NK cells was largely reduced by 50% after the surgery and started to recover from day 1 after the surgery. In consistent with our observation in terms of the total counts, we found the recovery rate of NK cells was faster in the laparoscopically assisted group. The average lytic activity was returned to the baseline level at day 4; while in the open group, the NK cells activity was still significantly lower than the baseline at day 4 and only recovered until day 7. To be noted, patients with CRC receiving no surgery showed stable activity of NK cells within 8-day time frame (Figure S1), which suggested the change of NK cells was in response to the intervention.
Figure 2.
Comparison of the numbers of natural killer (NK) cells between the surgery groups at different time points. The number of NK cells in blood (absolute counts/µL) was measured by flow cytometer. Values were shown as mean with standard deviation. Statistical analysis: *P < .05 within-group effect in laparoscopic group per analysis of variance (ANOVA); # P < .05 within-group effect in open group per ANOVA. ^P < .05 cross groups at the indicated time point per Student t test.
Figure 3.
Comparison of the activities of natural killer (NK) cells between the surgery groups at different time points. The NK cells in blood were harvested and their lysis activities (LU35) were quantified as described in the Methods section. Statistical analysis: *P < .05 within-group effect in laparoscopic group per analysis of variance (ANOVA); # P < .05 within-group effect in open group per ANOVA. ^P < .05 cross groups at the indicated time point per Student t test.
Next, we sought to understand the impact of pathological stages on NK cells. We first compared the average number of NK cells in the patient blood across 3 different tumor stages, namely Dukes A, Dukes B, and Dukes C. As shown in Figure 4A, the total cell count was higher in the Dukes A group but no difference was observed between Dukes B and C stages. The lytic activity of NK cells showed a similar pattern. The activity score in both Dukes B and C patient groups was significantly lower than Dukes A group (Figure 4B). The comparison study suggested the NK cells quantity and activity may be suppressed in the advance pathological stages of CRC. To be noted, when comparing NK cells in the new tumor-node-metastasis (TNM) classification system, the changes in number and activity of NK cells were observed in patients in TNM 2/3 stages (Figure S2), confirming the correlation of NK cells function with CRC severity.
Figure 4.
Comparison of the total numbers and the activities of natural killer (NK) cells across different pathological stages before surgery. A, The number of NK cells in blood (absolute counts/µL) were measured by flow cytometry. B, Their lysis activities (LU35) were quantified in a standard assay. The data were presented as mean with standard deviation. Statistical analysis: *P < .05 among the pathological stages per analysis of variance (ANOVA).
Taken further, we proceeded to compare the differences of NK cell counts and lytic activities before and after the surgery across 3 different pathological stages of CRC. In the laparoscopic group, patients across the 3 different tumor stages showed no difference with respect to the total cell count and lytic units between day 1 before the surgery and day 7 after the surgery, suggesting the numbers and activities of NK cells recovered fully as described in Tables 2 and 3. Interestingly, the patients at Dukes B and C stages showed less NK cells together with the reduced lytic activity even at day 7 after the open surgery (Tables 2 and 3), but no difference was observed in the patients at Dukes A from the open group. The statistical significance was analyzed by chi-square test. Therefore, the results suggested the conventional open surgery may have larger negative impact on the recovery of NK cells in the patients having CRC with advance pathological stages such as Dukes B and C.
Table 2.
Comparison of Total Numbers of NK Cells Across Different Pathological Stages at Day 1 Before Surgery or at Day 7 After Surgery.a
| Surgery status | Dukes A | Dukes B | Dukes C | P Value by chi-square Test |
|---|---|---|---|---|
| Before laparoscopic | 303 | 244 | 233 | .824 |
| After laparoscopic | 310 | 239 | 251 | |
| Before open surgery | 299 | 250 | 240 | .0089 |
| After open surgery | 291 | 191 | 188 |
Abbreviation: NK cells, natural killer cells.
a The data were presented as mean values (cell numbers/µL in blood) for each group of patients; P value was analyzed by chi-square test within the same surgery groups.
Table 3.
Comparison of the NK Cells Activities in the Blood Across Different Pathological Stages at Day 1 Before Surgery or at Day 7 After Surgery.a
| Surgery status | Dukes A | Dukes B | Dukes C | P Value by Chi-square Test |
|---|---|---|---|---|
| Before laparoscopic | 50.4 | 29.6 | 32.1 | .769 |
| After laparoscopic | 52.1 | 30.2 | 33.2 | |
| Before open surgery | 47.3 | 31.6 | 30.4 | .0124 |
| After open surgery | 49.1 | 24.7 | 23.4 |
Abbreviation: NK cells, natural killer cells.
a The data were presented as mean values (LU35 as described in the Methods section) for each group of patients; P value was analyzed by chi-square test within the same surgery groups.
Discussion
Open surgery for CRC treatment inevitably induces tissue damages that renders significantly systemic immune response of the patients postoperatively. The impaired immune function may be implicated in the recovery of patients after surgical treatment or be associated with postoperative morbidity. These improvements found in laparoscopic resection over conventional surgical approach outweigh the concerns in cost and technical procedure and make the procedure become more accepted to treat CRC. Usually, minimally invasive surgery causes less trauma or blood loss. The reduction in tissue injuries may result in better preservation of body immune function, which in turn improve postoperative morbidity. The immunological consequence of laparoscopic resection on patients with CRC has been intensively accessed in last decade.13,16,17 The first line of immune response in reaction to surgery induced tissue injury is acute-phase response. Acute-phase response is an innate defense against tissue injuries or pathological infections in order to activate healing process and restore body homeostasis. The intensity and duration of acute-phase response are also indications of the severity of the surgical injury and correlate to the occurrence of postoperative complications.18 Proinflammatory cytokines (tumor necrosis factor α [TNF-α], IL-1, and IL-6) are important players during acute-phase response. The production of CRP from liver is also a characteristic feature of acute-phase response. A prospective randomized study has compared postoperative inflammation following either open or laparoscopic CRC surgery. They measured plasma cortisol, insulin, and proinflammatory cytokines including TNF-α, IL-1, and IL-6 at 1 to 6 hours (T1) or 3 to 5 days (T2) postoperatively. No difference was found in cortisol or insulin between the groups, but the serum levels of IL-6, IL-8, and IL-10 were found to be lower in the laparoscopic group at T1, suggesting minimally invasive surgery has less impact on inflammatory mediators.19 Another preliminary report has also described the impact of laparoscopic surgery on both cellular and humoral inflammatory response. Particularly, IL-6 production was preserved better in the laparoscopic group.20 Schwenk et al measured inflammatory responses in 60 patients randomized to receive either open or laparoscopic resection for CRC. Both IL-6 and CRP blood levels were lower after laparoscopic resection, indicating milder surgical trauma inflicted by laparoscopic surgery.17
Cellular immunity consists of 2 arms: one is nonspecific immune response mediated by innate immune cells such as macrophages, neutrophils, and NK cells; the other is adaptive immune response mediated by T cells and B cells. They carry essential roles in the defense against malignancies and inhibiting metastases. The improved preservation of cellular immunity after laparoscopic surgery in patients with CRC has also been reported in several studies. Huang et al studied the change in immune cells of 68 patients who underwent either laparoscopic or open surgery on postoperative days 1, 4, and 7. They found CD4+ T cells, CD8+ T cells, CD45RO+ T cells, and NK cells were significantly higher in the laparoscopic group, especially on day 4.13 The similar observation was reported in another study, in which the authors measured lymphocyte subsets and NK cell cytotoxicity at 24hours, 72 hours, and 8 days after the surgical operation. They found the suppression of T-cell activation was significantly less after laparoscopic resection. However, the difference in NK cell cytotoxicity was not obvious.21 The authors in this study argued the immunologic benefit on NK cells by the laparoscopic resection could be masked by a more advanced state of the patients enrolled into the study. Therefore, in our study, we have enrolled a total of 200 patients across 3 different pathological stages to test the preservation effect of laparoscopic surgery on NK cells. We have confirmed that the number and activity of NK cells were suppressed in both groups, but in the laparoscopic resection group, we observed a better preservation on the immune response of NK cells. In addition, in advanced stages of CRC, NK cells function is suppressed. Given the role of NK cells in tumor surveillance, the suppression of NK cells in CRC can be an indicator of the progression of the cancer. Similarly, surgery-induced trauma elicits greater impairment of the viability and function of NK cells in the patients in Dukes B/C. Interestingly, the laparoscopic resection group demonstrates a faster postoperative recovery of NK cells in the patients in Dukes B/C. Although the patients in Dukes B/C show the lower baseline of NK cells number and lytic activity. The preservation effect on NK cells by laparoscopically assisted surgery is still reproducible in these groups of patients. The tumor surveillance role of NK cells is largely attributed to their ability to recognize and kill tumor cells directly. Low activity of NK cells is known to be a potential risk indicator of CRC progression toward the advanced stages.22 A recent case study also suggests the presence of high number of circulating NK cells with high cytotoxicity toward tumor cells may be associated with a better response to chemotherapies and a longer progression-free survival.23 Another hypothesis is that NK cells are defective typically in filtrating solid tumors. Although the infrequently infiltrating NK cells do not directly regulate tumor progression, they may act together with T cells to trigger antitumor immune response.24 Therefore, the preservation on NK cells function may confer a better protection in controlling tumor progression during postoperative period. The NK cells can interact with many types of immune cells to regulate the outcomes of adaptive immune responses and maintain immune homeostasis.25 It will be also intriguing to investigate the association of NK cells activation level and the adaptive immune response status among the patients receiving different surgeries. Furthermore, our finding suggests laparoscopic resection may deliver more benefits than open surgery in patients from Dukes B/C stages as supported by the recovery rate of NK cells quantity and activation.
Therefore, our study has provided the clinical evidence to support the implication of NK cells in the short-term benefits delivered by laparoscopic resection. The number and activation of NK cells may serve as a prognostic indicator for the recovery of patients with CRC postsurgery. It is also worth examining the preservation on NK cells could translate into long-term clinical benefits for the patients.
Supplemental Material
Supplementary_Materials for Laparoscopic Surgery Versus Open Surgery for Colorectal Cancer: Impacts on Natural Killer Cells by Liangpan Shi, Hailian Guo, Zhihua Zheng, Jiangrui Liu, Yancheng Jiang and Yibin Su in Cancer Control
Footnotes
Authors’ Note: Liangpan Shi and Hailian Guo contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yibin Su  https://orcid.org/0000-0002-3013-5649
https://orcid.org/0000-0002-3013-5649
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cronin KA, Lake AJ, Scott S, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2018;124(13):2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ueno H, Mochizuki H, Hashiguchi Y, et al. Histological grading of colorectal cancer: a simple and objective method. Ann Surg. 2008;247(5):811–818. [DOI] [PubMed] [Google Scholar]
- 4. Barresi V, Reggiani Bonetti L, Ieni A, Caruso RA, Tuccari G. Histological grading in colorectal cancer: new insights and perspectives. Histol Histopathol. 2015;30(9):1059–1067. [DOI] [PubMed] [Google Scholar]
- 5. Aran V, Victorino AP, Thuler LC, Ferreira CG. Colorectal cancer: epidemiology, disease mechanisms and interventions to reduce onset and mortality. Clin Colorectal Cancer. 2016;15(3):195–203. [DOI] [PubMed] [Google Scholar]
- 6. Binefa G, Rodriguez-Moranta F, Teule A, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol. 2014;20(22):6786–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schlottmann F, Patti MG. Laparoscopic versus open surgery still an open debate. J Laparoendosc Adv Surg Tech A. 2017;27(12):1223–1224. [DOI] [PubMed] [Google Scholar]
- 8. Bonjer HJ, Deijen CL, Haglind E, Group CIS. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;373(2):194. [DOI] [PubMed] [Google Scholar]
- 9. Sietses C, Beelen RH, Meijer S, Cuesta MA. Immunological consequences of laparoscopic surgery, speculations on the cause and clinical implications. Langenbecks Arch Surg. 1999;384(3):250–258. [DOI] [PubMed] [Google Scholar]
- 10. Mehigan BJ, Hartley JE, Drew PJ, et al. Changes in T cell subsets, interleukin-6 and C-reactive protein after laparoscopic and open colorectal resection for malignancy. Surg Endosc. 2001;15(11):1289–1293. [DOI] [PubMed] [Google Scholar]
- 11. Ordemann J, Jacobi CA, Schwenk W, Stosslein R, Muller JM. Cellular and humoral inflammatory response after laparoscopic and conventional colorectal resections. Surg Endosc. 2001;15(6):600–608. [DOI] [PubMed] [Google Scholar]
- 12. Karanika S, Karantanos T, Theodoropoulos GE. Immune response after laparoscopic colectomy for cancer: a review. Gastroenterology Rep. 2013;1(2):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C, Huang R, Jiang T, Huang K, Cao J, Qiu Z. Laparoscopic and open resection for colorectal cancer: an evaluation of cellular immunity. BMC Gastroenterology. 2010;10:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pahl J, Cerwenka A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiol. 2017;222(1):11–20. [DOI] [PubMed] [Google Scholar]
- 15. Valiathan R, Lewis JE, Melillo AB, Leonard S, Ali KH, Asthana D. Evaluation of a flow cytometry-based assay for natural killer cell activity in clinical settings. Scand J Immunol. 2012;75(4):455–462. [DOI] [PubMed] [Google Scholar]
- 16. Wichmann MW, Hutt TP, Winter H, et al. Immunological effects of laparoscopic vs open colorectal surgery: a prospective clinical study. Arch Surg. 2005;140(7):692–697. [DOI] [PubMed] [Google Scholar]
- 17. Schwenk W, Jacobi C, Mansmann U, Bohm B, Muller JM. Inflammatory response after laparoscopic and conventional colorectal resections - results of a prospective randomized trial. Langenbecks Arch Surg. 2000;385(1):2–9. [DOI] [PubMed] [Google Scholar]
- 18. Nasser BA, Mesned AR, Tageldein M, Kabbani MS, Sayed NS. Can acute-phase response biomarkers differentiate infection from inflammation postpediatric cardiac surgery? Avicenna J Med. 2017;7(4):182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siekmann W, Eintrei C, Magnuson A, et al. Surgical and not analgesic technique affects postoperative inflammation following colorectal cancer surgery: a prospective, randomized study. Colorectal Dis. 2017;19(6):O186–O195. [DOI] [PubMed] [Google Scholar]
- 20. Laforgia R, D’Elia G, Lattarulo S, Mestice A, Volpi A. Cellular and humoral inflammatory response after laparoscopic and conventional colorectal surgery. Preliminary report. Ann Ital Chir. 2016;87:337–342. [PubMed] [Google Scholar]
- 21. Leung KL, Tsang KS, Ng MH, et al. Lymphocyte subsets and natural killer cell cytotoxicity after laparoscopically assisted resection of rectosigmoid carcinoma. Surg Endosc. 2003;17(8):1305–1310. [DOI] [PubMed] [Google Scholar]
- 22. Jung YS, Kwon MJ, Park DI, Sohn CI, Park JH. Association between natural killer cell activity and the risk of colorectal neoplasia. J Gastroenterol Hepatol. 2018;33(4):831–836. [DOI] [PubMed] [Google Scholar]
- 23. Ottaiano A, Napolitano M, Capozzi M, Tafuto S, Avallone A, Scala S. Natural killer cells activity in a metastatic colorectal cancer patient with complete and long lasting response to therapy. World J Clin Cases. 2017;5(11):390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sconocchia G, Eppenberger S, Spagnoli GC, et al. NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology. 2014;3(8):e952197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flodstrom-Tullberg M, Bryceson YT, Shi FD, Hoglund P, Ljunggren HG. Natural killer cells in human autoimmunity. Curr Opin Immunol. 2009;21(6):634–640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Materials for Laparoscopic Surgery Versus Open Surgery for Colorectal Cancer: Impacts on Natural Killer Cells by Liangpan Shi, Hailian Guo, Zhihua Zheng, Jiangrui Liu, Yancheng Jiang and Yibin Su in Cancer Control