Abstract
Background
Exonuclease 1 (Exo1) participates in a variety of DNA damage repair, including mismatch repair, nucleotide excision repair, and homologous recombination. Genetic study in yeast indicates a role of Exo1 in non-homologous end joining (NHEJ), acting as a regulator for accuracy repairing DNA. This study aimed to investigate the effects of human Exo1 in NHEJ and drug resistance in ovarian cells.
Material/Methods
Ectopic expression of Exo1 was carried out using pcDNA3.1-EXO1 plasmid in SKOV3 cells. GST-tagged human Exo1 was purified using pTXB1-gst-EXO1 and the his-tagged-Ku was collected using pET15b.his.Ku. Exo1 and Ku70 proteins expressed in bacteria were harvested and purified. DNA-protein binding was examined using affinity capture assay. The cells were treated using drugs for 72 hours. Then, the viabilities of cells were evaluated with sulforhodamine B cell viability analysis. The protein expression was evaluated using western blot assay.
Results
As expected, human cells that deficient of Exo1 were sensitive to ionizing radiation and DNA damaging drugs (cisplatin and doxorubicin). Cisplatin resistant ovarian cancer cell line and Exo1 deficient cell lines were successfully generated. Exo1 interacts with NHEJ required factor Ku70 and affects NHEJ efficiency. We observed that Exo1 expression level was upregulated in drug resistant cell line and knockdown of Exo1 in drug resistant cells sensitized cells to cisplatin and doxorubicin.
Conclusions
Exo1 participated in mammalian non-homologous end joining and contributed to drug resistance in ovarian cancer.
MeSH Keywords: Cisplatin, DNA End-Joining Repair, Exonucleases, Ovarian Neoplasms
Background
Ovarian cancer is the third most common and the first cause of death among gynecologic cancer [1]. Despite decreased mortality from ovarian cancer by 21.7% and 2.2% in younger women and elderly woman, respectively. Late stage diagnosis and relapse after surgical followed by platinum chemotherapy still contribute to low survival rate with advanced stage ovarian cancer [2–4]. Therefore, more in depth understanding of ovarian cancer treatment resistance and developing new chemotherapy strategies are needed to benefit the patients.
DNA damages, especially DNA double strand breaks (DSBs), are toxic to cell survival [5]. Efficient DNA damage repair is necessary to maintain genome integrity. Failure or to repair DSBs may generate mutations that can lead to cell cycle arrest and apoptosis [6]. Therefore, introducing DSBs in cancer cells to generate cell death via ionizing radiation or chemotherapy is a widely used approach in cancer therapy [7]. However, cells developed multiple DSBs repair pathways to maintain genome integrity. Among DSBs repair (DSBR) signaling pathways, the non-homologous end joining (NHEJ) pathway and the homologous recombination (HR) pathway are responsible for repair majority of DSBs in mammalian cells [8,9].
NHEJ, unlike HR, does not need sister chromatid as template to repair DSBs [10]. Therefore, this allows cells to employ NHEJ throughout cell cycle, especially when sister chromatid is not available during G0, G1, and early-S phase [11]. According to the previous studies [12–19], the classical NHEJ signaling pathway requires several integrants to repair DSBs. The general mechanism of NHEJ is conserved across all species, and the majority of NHEJ factors are conserved from yeast to humans [20]. Because NHEJ repairs DSB independently of homologous DNA template, this pathway is generally considered to be error prone [21]. However, NHEJ pathway is not a simple end-to-end joining. NHEJ pathway is confronted with multiple challenges, such as DSBs detection, DNA ends synapsis, and end processing.
NHEJ repair events show deletions and insertions suggesting a DNA end processing step during repair. Other than cell cycle phase, DNA end can also determine pathway choice between HR and NHEJ [22]. Blunt ends are more favored by NHEJ other than HR, which requires end resection to generate 3′ overhangs [23]. Therefore, several phosphodiesterases that can remove damages from DNA ends, nucleases that delete mismatched nucleotides, and polymerases that subsequent extend the strand have been showed to participate in NHEJ [24–28].
Exonuclease 1 (Exo1) is an XPG family nuclease (Rad2 family of exonuclease) and shows 5′ to 3′ dsDNA exonuclease and 5′ flap endonuclease activities [29]. Exo1 has a conserved N-terminal catalytic domain like other Rad2 family nucleases and the C-terminal is less conserved, however, which is responsible for protein-protein interactions [30]. Exo1 has been identified in Schizosaccharomyces Pombe as a 5′-3′ exonuclease that participates in DNA mismatch repair (MMR) [31]. Exo1 also participates in end resection of homologous recombination via its exonuclease activity [32]. However, Exo1 is not essential for MMR nor homologous recombination, suggesting that Exo1 is redundant with the other end processing factors in these DNA repair pathways. A yeast study [33] showed that Exo1 participates in NHEJ by regulating end joining accuracy. Here, we found that Exo1 contributes to NHEJ efficiency in mammalian cells using in vitro NHEJ assay. Exo1 knockout in ovarian cancer cells sensitize cells to ionizing radiation and DNA damaging agents, cisplatin and doxorubicin. We proposed that Exo1 plays a role in NHEJ via its interaction with NHEJ required factor Ku.
Material and Methods
Cell culture, siRNAs, and transfections
SKOV3 cells, including HTB-77, Exo1-deficient cells, were purchased from ATCC Cell Bank (Manassas, VA, USA) and cultured in 5% CO2 at 37°C. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Gibco BRL. Co. Ltd., Grand Island, New York, USA). The transfections were conducted with Lipofectamine 2000 Transfection Kit (Cat. No. #11668027, Thermo Fisher Scientific, Rockford, IL, USA) based on manufacturer’s instruction. The ectopic expression of Exo1 was carried out using pcDNA3.1-EXO1 plasmid. GST-tagged human Exo1 was purified using pTXB1-gst-EXO1 and his-tagged-Ku was collected using pET15b.his.Ku
Expression of proteins in bacteria and protein purification
Exo1 and Ku70 expressed in bacteria were harvested and purified according to the following procedures: the BL21(DEW) Escherichia coli cell (NEB) was transformed using the heat-shot approach at 42°C and using plasmids. The BL21(DE3) E. coli cell (NEB) was grown in Luria Broth at 37°C until optical density reaching to OD-600 1.0. Cells were incubated at 37°C in a shaker incubator, induced with 1 m misopropyl b-D-thiogalactopyranoside (IPTG) and grown for 7 hours. Cells were collected by centrifuging at speed of 6000 g and 4°C for 0.5 hour. Then, mediums were discarded, and the retained cell pellets were re-suspended in lysis buffer (Beyotime Biotech., Shanghai, China). Subsequently, the lysates were sonicated with the Branson Sonifier 15%, for 10 time (10 seconds per time) to shear the DNA in order to lower the viscosity. Extracts were purified by centrifuging at speed of 20 000 g, for 15 minutes at 4°C.
The clarified supernatant was bound to the GST-tagged protein (with dosage of 0.5 mL, purchasing from Thermo Scientific Pierce (Cat. No. RP-75563, Rockford, IL, USA) or Ni-NTA (his-tagged protein, with dosage of 50 mL, Cat. No. 30410, Qiagen, Hilden, Germany) for 2 hours at 4°C. Then, all beads were packed onto the columns and washed using the washing buffer (Qiagen, Hilden, Germany). His-tagged protein was eluted from beads using the commercial elution buffer (Qiagen, Hilden, Germany). GST-tagged protein was also eluted from beads with the commercial elution buffer (Qiagen, Hilden, Germany). Elute was dialyzed to lower the salt concentration using commercial dialyzing buffer (Thermo Scientific Pierce, Rockford, IL, USA) for overnight at 4°C. The purified proteins were frozen with liquid-nitrogen and stored at temperature of −80°C. Finally, the concentration of the proteins was measured using the Bradford assay.
Affinity capture analysis
Total of 100 μg GST-Exo1 was diluted with the binding-buffer (at final dosage of 25 mM Tris, adjusting to pH 7.9, 150 mM NaCl, 1 mM DTT and 1 mM EDTA,) in a total volume of 400 μL with 100 μL of glutathione beads (50% slurry). Incubate the protein and beads at 4°C with gentle mix for 1 hour. Resin and the bound protein were collected by centrifuging for 1 minute at speed of 1000 g. The resin was washed using binding buffer (0.5 mL) for 3 times (5 minute per time), and then obtained resin was collected by centrifuging for 1 minute at 1000 g. Total of 100 μg of his-Ku70 was incubate with the resin in 500 μL solution undergoing gentle mix at 4°C for 2 hours. Then, resin was washed using commercial binding buffer for 3 times (5 minutes per time). Subsequently, the obtained supernatants were discarded by centrifuging for 1 minute at speed of 1000 g. The bound proteins were eluted from resin through treating resin using wash buffer (50 μL) and glutathione (at final concentration of 10 mM) at 4°C for 15 minutes. Bound proteins were collected by saving the supernatant after centrifugation at 1000 g for 1 minute. The samples were separated using SDS-PAGE gel and then transferred onto the PVDF membrane. Targeting protein was then observed with the western blot assay and anti-Ku antibody (Cat. No. 83501, Abcam Biotech., Cambridge, MA, USA).
CRISPR/Cas9 knockout
Cas9 containing Exo1-guide RNA plasmids was synthesized by ligating the oligonucleotide duplexes and the target sequence 5′-AACGTTAC CATAGCAGTGTC-3′. Then, the ligated Cas9 plasmid was sub-cloned into the BbsI cut pX330-U6-Chimeric-BB-CBh-hSpCas9 plasmid (Cat. No. #42230, Addgene, Cambridge, MA, USA). The plasmids were transfected into SKOV3 cells together with pcDNA3.1.puro plasmid (Invitrogen/Life Technologies, Carlsbad, CA, USA) using lipofectamine 2000 (Invitrogen/Life Technologies) for 48 hours. The cells that transfected with the plasmids, were screened and selected using the 100 μg/mL puromycin (Sigma-Aldrich, St. Louis, MO, USA) for 2 days. Finally, the cells were collected and seeded onto the 96-well plates at final density of 1000 cells/mL medium and treated for 14 days. Individual clones were picked-out and cultured for screening the Exo1 expression.
Sulforhodamine B (SRB) cell viability assay
The cells were seeded at density of 5000 cells/well onto the 96-well plates and cultured overnight to make the cells to adhere. Cells were then incubated using drugs for 72 hours and SRB assay was conducted to evaluated cell viabilities, as the following procedures: The cells fixed together with the 10% trichloroacetic acid (100 μL) and incubated for 1 hour at 4°C. Then, the plates were washed using the running-tap water for 4 times (5 minutes per time) and were air dried at room temperature for 1 hour. The cells were incubated with 1% acetate acid containing 0.02% SRB for 1 hour at room temperature, washed using 1% acetate acid (at dosage of 200 μL/well) for 3 times (5 minutes per time) and air dried. The color was dissolved in tris-HCl (final concentration of 10 mM), adjusting to pH 10.5 and following with shaking for 1 hour on a shaker. Eventually, the absorbance was examined using micro-plate reader (Bio-Tek Inc., Winooski, VT, USA) at wavelength of 510 nm.
Ionizing-radiation followed with clonogenic survival analysis
The cells were re-suspended in the cell culture medium (10 mL) and exposed to the ionizing radiation using Gammator 50 137Cs source irradiator (Radiation Machinery Co., Par-Sippany, NJ, USA). Then, the cells were seeded into 6-well plates and cultured for 14 days. Finally, the obtained colonies were stained using crystal violet (Beyotime Beotech. Shanghai, China), scanned and counted.
Pem1GFP assay
Pem1GFP plasmid is linearized with NheI restriction for 3 hours in a 37°C water bath. Linearized DNA was visualized on agarose gel and purified with Qiagen gel extraction kit. The cells were seeded and cultured at density of 3×105 cells/mL medium in the 6-well plates. 1 μg/well linearized plasmid was transfected into cells with Lipofectamine 2000 (Invitrogen/Life Technologies) and treated for 24 hours. Cells transfecting with plasmid were selected with 0.5 mg/mL geneticin and incubated for a week. Geneticin resistant cells were expanded and harvested for future use.
Pem1 cells were seeded at density of 3×105 cells/mL medium in the 6-well plates and cultured for 24 hours to generate the adhered cells. The I-SceI (at dosage of 2 μg/well) was added into cells using Lipofectamine 2000 and treated for 48 hours. The cells were harvested using the trypsin and re-suspend in the phosphate-buffered saline (PBS) by pipetting for 10 times. The GFP-positive cells were counted using the flow cytometer (Beckman Coulter Inc., Brea, CA, USA).
Western blotting assay
The samples were re-suspend using SDS-PAGE sample buffer (Beyotime Beotech. Shanghai, China), and then heated at 95°C for 10 minutes. The treated samples were loaded onto the 8% SDS-PAGE and separated using an electrophoresis apparatus (Bio-Rad Laboratories, Hercules, CA, USA). The separated samples were then transferred onto the PVDF membrane (Amresco Inc., Solon, OH, USA) on ice for 1 hour. PVDF membranes were blocked using 3% non-fat-dry milk diluting in PBS containing the 0.1% Tween 20 (Beyotime Biotech.). The PVDF membranes were incubated with relevant antibodies and following incubated using the horseradish peroxidase (HRP)-conjugated rabbit secondary antibodies. The western blotting signals were developed using SuperSignal™ west Dura Chemiluminescent Substrate Regent (Cat. No. #34076, Thermo Fisher Scientific) and then detected using the ChemiDoc™ (Bio-Rad Laboratories, Hercules, CA, USA).
Data analysis
Data in this study was represented as mean±standard deviation (SD) and analyzed using SPSS software (version: 18.0, SPSS, Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was employed to analyze the differences between groups. The P value <0.05 was assigned as significant difference.
Results
Cisplatin resistant ovarian cancer cell line was successfully generated
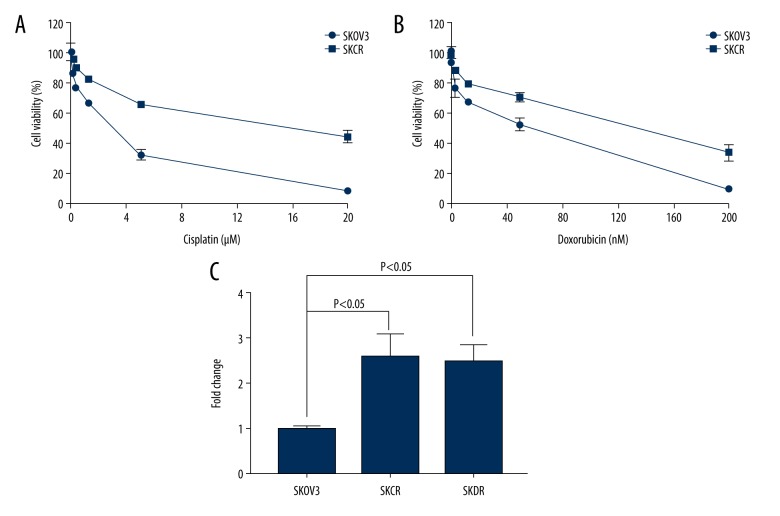
Cisplatin has been the most active and widely used drug for ovarian cancer for more than 4 decades [34]. However, the majority of the patients will develop resistance to cisplatin after front-line treatment [35]. Therefore, improving therapeutic efficiency of cisplatin could be a significant contribution in ovarian cancer treatment. Doxorubicin is FDA approved anti-cancer drugs and is used for advanced recurrent ovarian cancer [36]. Like cisplatin, unfortunately, ovarian cancer patients have also developed resistance to this second-line drug. To understand the resistant mechanism and to determine whether we can sensitize resistant ovarian cancer cells to cisplatin or doxorubicin, we generated SKOV3 cell lines that are resistant to cisplatin (SKCR) and doxorubicin (SKDR) (Figure 1A, 1B). The resistant cell lines we generated showed 5-fold to 10-fold resistance to cisplatin or doxorubicin (Table 1). To determine whether Exo1 is upregulated in these drug-resistant cell lines, we evaluated Exo1 expression and mRNA level by using western blot and real-time polymerase chain reaction (PCR) respectively. Indeed, we observed increased expression and mRNA level of Exo1 in all 4 resistant cells (Figure 1C).
Figure 1.
The generation of the cisplatin resistant ovarian cancer cells. (A) SKOV3 cell line which is resistant to cisplatin is generated. SKCR: SKOV3 cells that are resistant to cisplatin. Cisplatin concentrations are 0, 0.078, 0.31, 1.25, 5, and 20 μM, respectively. (B) SKOV3 cell line which is resistant to doxorubicin is generated. SKDR: SKOV3 cells that are resistant to doxorubicin. Doxorubicin dosages are 0, 0.78, 3.13, 12.5, 50, and 200 μM, respectively. (C) Real-time polymerase chain reaction showed increased Exo1 mRNA level in SKCR and SKDR. Statistically significant differences (P<0.05) in fold change were determined.
Table 1.
IC50 of SKOV3 WT and resistant cell lines to cisplatin and doxorubicin.
| IC50 | WT | SKCR | SKDR | Fold change |
|---|---|---|---|---|
| Cisplatin (μM) | 2.08 | 11.47 | 5.51× | |
| Doxorubicin (nM) | 32.97 | 97.28 | 2.95× |
Exo1 deficient cell lines were generated using CRISPR/Cas9
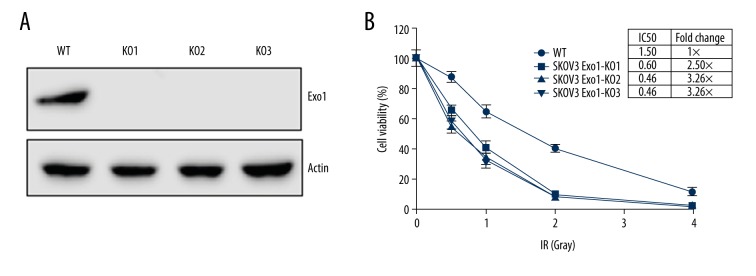
Because Exo1 is upregulated in drug resistant ovarian cancer cell line, we want to determine if Exo1 depletion can sensitize cells to cisplatin or doxorubicin. In yeast, Exo1 play a role in regulating NHEJ fidelity [33], so we also want to evaluate the effect of Exo1 on NHEJ. We utilized the CRISPR/Cas9 to knockout the Exo1 in SKOV3 cells. The guide-RNAs were designed and synthesized to target the exon1 of Exo1. We collected individual clones to evaluate Exo1 expression (Figure 2A).
Figure 2.
The generation for the Exo1 deficient cells using the CRISPR/Cas9. (A) Western blotting assay for the endogenous Exo1 expression in SKOV3 wild type (WT) cells and 3 SKOV3-Exo1 knockout clones (KO1, KO2, and KO3) generated by CRISPR/Cas9. No detectable Exo1 was observed. (B) Exo1 knockout cell lines are sensitive to ionizing radiation (IR). Doses of IR are 0 Gy, 0.5 Gy, 1 Gy, 2 Gy, and 4 Gy. The IC50 of IR for 4 cell lines are indicated in the table next to Figure 2B.
Ionizing radiation can generate DSBs and the majority of the damages are repaired via NHEJ [37,38]. Therefore, cells that are lack of NHEJ required factors will lead to defect of NHEJ and result in increased sensitivity to ionizing radiation. We observed that Exo1 knockout ovarian cancer cells are more sensitive relatively to the ionizing radiation compared to that in the wild type parental cells (Figure 2B). This is consistent with our hypothesis that Exo1 participates in NHEJ in mammalian cells.
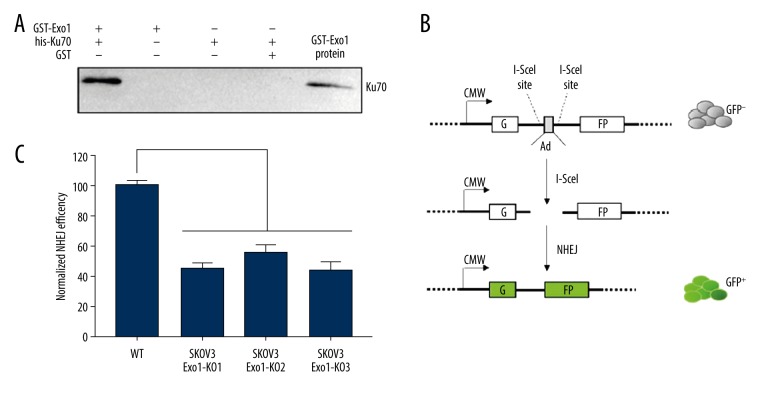
Exo1 interacts with NHEJ required factor Ku70 and affects NHEJ efficiency
In order to clarify how Exo1 is involved in the NHEJ, we carried out affinity-capture assay with purified flag-tagged Exo1 (flag-Exo1) and his-tagged NHEJ factors. We found that Exo1 interacts with Ku70 (Figure 3A) in vitro. We used DNA nuclease and ethidium bromide in assay to eliminate DNA contamination. The interaction between Exo1 and Ku70 should not be tethered by DNA. To further evaluate effect of Exo1 on NHEJ, we used Pem1-GFP reporter system, which employs restriction-endonuclease, I-SceI, to synthesize the DSBs with complementary DNA ends. The Pem1-GFP system is diagramed in Figure 3B. Because I-SceI site only exist in the chromosomally integrated reporter DNA but not in endogenous chromosomal DNA, cell would use NHEJ to join DSBs instead of using HR while template is not available. Successfully repair of DSBs generated by I-SceI in the assay will result in GFP expression, which will be examined using FACS to indicate NHEJ efficiency. As shown in Figure 3C, all 3 Exo1 deficient SKOV3 clones showed more than 50% decrease of NHEJ efficiency. The ectopic expression for the wild-type Exo1 in Exo1 deficient cells rescued NHEJ efficiency to that observed in the wild-type cells. Our result indicate that Exo1 contributes to efficient NHEJ in mammalian cells.
Figure 3.
Exo1 interacts with NHEJ required factor Ku70 and affects NHEJ efficiency. (A) Exo1 and Ku70 interact in vitro. GST-Exo1 or GST was used to capture his-Ku70 on glutathione resin. * Positive control for western blot: 3 ng of purified recombinant his-Ku70. The samples were separated using the SDS-PAGE and the his-Ku70 was examined using anti-Ku70 antibodies. (B) Schematic diagram of NHEJ reporter assay Pem1GFP. GFP coding gene is disrupted by polyadenylation sites (Ad). Ad is flanked by I-SceI sites. DSBs were introduced by transfecting of I-SceI endonuclease. After NHEJ join cohesive DNA ends and delete the Ad site from the reporter to restore GFP expression. (C) Exo1 knockout cell line showed decrease NHEJ efficiency. NHEJ efficiency of SKOV3 WT cell line was normalized to 100%. Three clones of Exo1-KO cell lines showed 45%, 55.33%, and 43.67% NHEJ efficiency. Statistically significant differences (P<0.0001) in NHEJ efficiency were determined.
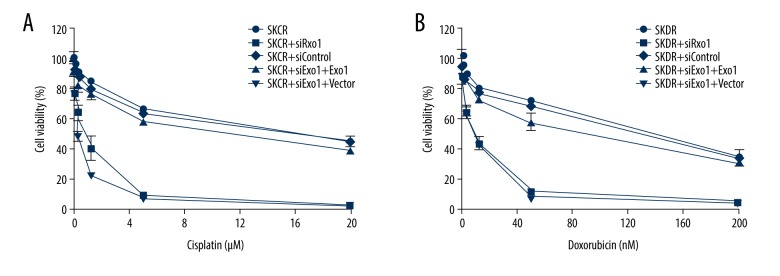
Exo1 deficiency sensitize ovarian cancer resistant cells to cisplatin and doxorubicin
Both cisplatin and doxorubicin can generate DNA damage that will lead to cell death. Therefore, Exo1, which participates in multiple DNA repair pathways could be one of the drug resistant mechanism in cancer therapy. Because we observed that Exo1 is upregulated in cisplatin and doxorubicin resistant ovarian cancer cell line, we hypothesize that knockout Exo1 may sensitize drug resistant cells to cisplatin and doxorubicin. SRB cell viability assay showed that knockdown Exo1 in SKCR and SKDR cell line sensitize drug resistant cells to cisplatin and doxorubicin (Figure 4A, 4B) by more than 10-fold (Table 2). Ectopic expression of wild type Exo1 restored resistance to these drugs. Our result suggests that Exo1 contributes to drug resistance in both first-line and recurred ovarian cancer therapy.
Figure 4.
Exo1 deficiency sensitize ovarian cancer resistant cells to cisplatin and doxorubicin. (A) Exo1 deficiency sensitize SKCR cells to cisplatin. WT Exo1 or Vector was expressed after 24 hours of siExo1 or Control transfection. Cisplatin was added after 24 hours of Exo1 or Vector expression. Cisplatin concentrations: 0, 0.078, 0.31, 1.25, 5, and 20 μM, respectively. (B) Exo1 deficiency sensitize SKDR cells to doxorubicin. Doxorubicin concentrations are 0, 0.78, 3.13, 12.5, 50, and 200 μM, respectively.
Table 2.
Exo1 knockdown sensitizes drug resistant cells to cisplatin and doxorubicin.
| IC50 | SKCR | SKCR+siExo1 | SKDR | SKDR+siExo1 | Fold change |
|---|---|---|---|---|---|
| Cisplatin (μM) | 11.47 | 0.57 | 20.12× | ||
| Doxorubicin (nM) | 97.28 | 7.35 | 13.24× |
Discussion
Exo1 has been well characterized in end processing in MMR, HR, as well as nucleotide excision repair (NER) [39–47]. We identified Exo1 is necessary for efficient NHEJ, possibly via interaction with NHEJ required factor Ku70. A variety of anti-cancer drugs target DNA to generate single- or double-strand DNA damages to result in cell death [48]. Therefore, multiple DNA repair mechanisms would generate drug resistance by maintaining cancer cell genome integrity.
Proteins, like Exo1, which are involved in several DNA repair pathways, should be potential targets for combinational cancer therapy. Mechanism of action of cisplatin is linked to its activity to crosslink with purine bases on the DNA, causing DNA damage that is predominantly repaired by NER [49,50]. Doxorubicin poisons topoisomerase II to stabilize topoisomerase II-DNA covalent complex after topoisomerase II cleaves both strands of DNA, causing DSBs and subsequent cell death. Therefore, repair of DSBs by HR or NHEJ in mammalian cells could be the drug resistance mechanism of doxorubicin. Because Exo1 participates in both NER and NHEJ, we suggest that inhibition of Exo1 along with DNA damaging drugs may improve ovarian cancer therapy. Moreover, the limitation of this study should be emphasized here. In this study, we have not further given photo demonstration for the DNA double-strand breaks. In a follow-up study, we would demonstrate DNA double-strand breaks using the associated morphological methods.
Conclusions
Human cells that were deficient of Exo1 were sensitive to ionizing radiation and DNA damaging drugs-cisplatin and doxorubicin. Exo1 expression level was upregulated in drug resistant cell line and knockdown of Exo1 in drug resistant cells sensitized cells to cisplatin and doxorubicin. In summary, the present study indicated a role of Exo1 in mammalian NHEJ and contribution of drug resistance in ovarian cancer cells.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Tortorella L, Vizzielli G, Fusco D, et al. Ovarians Aging Dis. 2017;8:677–84. doi: 10.14336/AD.2017.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumas L, Ring A, Butler J, et al. Improving outcomes for older women with gynaecological malignancies. Cancer Treat Rev. 2016;50:99–108. doi: 10.1016/j.ctrv.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Q, Zhang Y, Su J, et al. Role of hydroxysteroid dehydrogenase-like 2 (HSDL2) in human ovarian cancer. Med Sci Monit. 2018;24:3997–4008. doi: 10.12659/MSM.909418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich T, Allen RL, Wyllie AH. Defying death after DNA damage. Nature. 2000;407:777–83. doi: 10.1038/35037717. [DOI] [PubMed] [Google Scholar]
- 6.Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 2008;9:297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- 7.Nambiar M, Raghavan SC. How does DNA break during chromosomal translocations? Nucleic Acids Res. 2011;39:5813–25. doi: 10.1093/nar/gkr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haber JE. Partners and pathways repairing a double-strand break. Trends Genet. 2000;16:259–64. doi: 10.1016/s0168-9525(00)02022-9. [DOI] [PubMed] [Google Scholar]
- 9.Chang HHY, Pannunzio NR, Adachi N, et al. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol. 2017;18:495–506. doi: 10.1038/nrm.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deriano L, Roth DB. Modernizing the nonhomologous end-joining repertoire: Alternative and classical NHEJ share the stage. Annu Rev Genet. 2013;47:433–55. doi: 10.1146/annurev-genet-110711-155540. [DOI] [PubMed] [Google Scholar]
- 11.Taleei R, Nikjoo H. The non-homologous end-joining (NHEJ) pathway for the repair of DNA double-strand breaks: I. A mathematical model. Radiat Res. 2013;179:530–39. doi: 10.1667/RR3123.1. [DOI] [PubMed] [Google Scholar]
- 12.Kabotyanski EB, Gomelsky L, Han JO, et al. Double-strand break repair in Ku86- and XRCC4-deficient cells. Nucleic Acids Res. 1998;26:5333–42. doi: 10.1093/nar/26.23.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith J, Baldeyron C, De Oliveira I, et al. The influence of DNA double-strand break structure on end-joining in human cells. Nucleic Acids Res. 2001;29:4783–92. doi: 10.1093/nar/29.23.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith J, Riballo E, Kysela B, et al. Impact of DNA ligase IV on the fidelity of end joining in human cells. Nucleic Acids Res. 2003;31:2157–67. doi: 10.1093/nar/gkg317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leber R, Wise TW, Mizuta R, et al. The XRCC4 gene product is a target for and interacts with the DNA-dependent protein kinase. J Biol Chem. 1998;273:1794–801. doi: 10.1074/jbc.273.3.1794. [DOI] [PubMed] [Google Scholar]
- 16.Mari PO, Florea BI, Persengiev SP, et al. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc Natl Acad Sci USA. 2006;103:18597–602. doi: 10.1073/pnas.0609061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yano K, Chen DJ. Live cell imaging of XLF and XRCC4 reveals a novel view of protein assembly in the non-homologous end-joining pathway. Cell Cycle. 2008;7:1321–25. doi: 10.4161/cc.7.10.5898. [DOI] [PubMed] [Google Scholar]
- 18.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yano K, Morotomi-Yano K, Lee KJ, et al. Functional significance of the interaction with Ku in DNA double-strand break recognition of XLF. FEBS Lett. 2011;585:841–46. doi: 10.1016/j.febslet.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shuman S, Glickman MS. Bacterial DNA repair by non-homologous end joining. Nat Rev Microbiol. 2007;5:852–61. doi: 10.1038/nrmicro1768. [DOI] [PubMed] [Google Scholar]
- 21.Chiruvella KK, Liang Z, Wilson TE. Repair of double-strand breaks by end joining. Cold Spring Harb Perspect Biol. 2013;5:a012757. doi: 10.1101/cshperspect.a012757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Symington LS, Gautier J. Double-strand break end resection and repair pathway choice. Annu Rev Genet. 2011;45:247–71. doi: 10.1146/annurev-genet-110410-132435. [DOI] [PubMed] [Google Scholar]
- 23.Zang Y, Pascal LE, Zhou Y, et al. ELL2 regulates DNA non-homologous end joining (NHEJ) repair in prostate cancer cells. Cancer Lett. 2018;415:198–207. doi: 10.1016/j.canlet.2017.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rass E, Grabarz A, Plo I, et al. Role of Mre11 in chromosomal nonhomologous end joining in mammalian cells. Nat Struct Mol Biol. 2009;16:819–24. doi: 10.1038/nsmb.1641. [DOI] [PubMed] [Google Scholar]
- 25.Heo J, Li J, Summerlin M, et al. TDP1 promotes assembly of non-homologous end joining protein complexes on DNA. DNA Repair (Amst) 2015;30:28–37. doi: 10.1016/j.dnarep.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin MJ, Juarez R, Blanco L. DNA-binding determinants promoting NHEJ by human Polmu. Nucleic Acids Res. 2012;40:11389–403. doi: 10.1093/nar/gks896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bebenek K, Garcia-Diaz M, Zhou RZ, et al. Loop 1 modulates the fidelity of DNA polymerase lambda. Nucleic Acids Res. 2010;38:5419–31. doi: 10.1093/nar/gkq261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Povirk LF, Zhou T, Zhou R, et al. Processing of 3′-phosphoglycolate-terminated DNA double strand breaks by Artemis nuclease. J Biol Chem. 2007;282:3547–58. doi: 10.1074/jbc.M607745200. [DOI] [PubMed] [Google Scholar]
- 29.Tran PT, Erdeniz N, Symington LS, et al. EXO1-A multi-tasking eukaryotic nuclease. DNA Repair (Amst) 2004;3:1549–59. doi: 10.1016/j.dnarep.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Orans J, McSweeney EA, Iyer RR, et al. Structures of human exonuclease 1 DNA complexes suggest a unified mechanism for nuclease family. Cell. 2011;145:212–23. doi: 10.1016/j.cell.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szankasi P, Smith GR. A DNA exonuclease induced during meiosis of Schizosaccharomyces pombe. J Biol Chem. 1992;267:3014–23. [PubMed] [Google Scholar]
- 32.Tsubouchi H, Ogawa H. Exo1 roles for repair of DNA double-strand breaks and meiotic crossing over in Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:2221–33. doi: 10.1091/mbc.11.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bahmed K, Seth A, Nitiss KC, et al. End-processing during non-homologous end-joining: A role for exonuclease 1. Nucleic Acids Res. 2011;39:970–78. doi: 10.1093/nar/gkq886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasey PA, Jayson GC, Gordon A, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96:1682–91. doi: 10.1093/jnci/djh323. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg B, VanCamp L, Trosko JE, et al. Platinum compounds: A new class of potent antitumour agents. Nature. 1969;222:385–86. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 36.Colombo N. When nonplatinum is the answer: The role of trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. Future Oncol. 2017;13:23–29. doi: 10.2217/fon-2017-0319. [DOI] [PubMed] [Google Scholar]
- 37.Biedermann KA, Sun JR, Giaccia AJ, et al. Scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci USA. 1991;88:1394–97. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, Lees-Miller SP. Detection and repair of ionizing radiation-induced DNA double strand breaks: New developments in nonhomologous end joining. Int J Radiat Oncol Biol Phys. 2013;86:440–49. doi: 10.1016/j.ijrobp.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Desai A, Gerson S. Exo1 independent DNA mismatch repair involves multiple compensatory nucleases. DNA Repair (Amst) 2014;21:55–64. doi: 10.1016/j.dnarep.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran PT, Fey JP, Erdeniz N, et al. A mutation in EXO1 defines separable roles in DNA mismatch repair and post-replication repair. DNA Repair (Amst) 2017;6:1572–83. doi: 10.1016/j.dnarep.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amin NS, Nguyen MN, Oh S, et al. Exo1-Dependent mutator mutations: Model system for studying functional interactions in mismatch repair. Mol Cell Biol. 2001;21:5142–55. doi: 10.1128/MCB.21.15.5142-5155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sokolsky T, Alani E. EXO1 and MSH6 are high-copy suppressors of conditional mutations in the MSH2 mismatch repair gene of Saccharomyces cerevisiae. Genetics. 2000;155:589–99. doi: 10.1093/genetics/155.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tishkoff DX, Amin NS, Viars CS, et al. Identification of a human gene encoding a homologue of Saccharomyces cerevisiae EXO1, an exonuclease implicated in mismatch repair and recombination. Cancer Res. 1998;58:5027–31. [PubMed] [Google Scholar]
- 44.Chen CC, Avdievich E, Zhang Y, et al. EXO1 suppresses double-strand break induced homologous recombination between diverged sequences in mammalian cells. DNA Repair (Amst) 2017;57:98–106. doi: 10.1016/j.dnarep.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoa NN, Akagawa R, Yamasaki T, et al. Relative contribution of four nucleases, CtIP, Dna2, Exo1 and Mre11, to the initial step of DNA double-strand break repair by homologous recombination in both the chicken DT40 and human TK6 cell lines. Genes Cells. 2015;20:1059–76. doi: 10.1111/gtc.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsang E, Miyabe I, Iraqui I, et al. The extent of error-prone replication restart by homologous recombination is controlled by Exo1 and checkpoint proteins. J Cell Sci. 2014;127:2983–94. doi: 10.1242/jcs.152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li G, Wang X, Wang X, et al. Polymorphism rs1052536 in base excision repair gene is a risk factor in a high-risk area of neural tube defects in China. Med Sci Monit. 2018;24:5015–26. doi: 10.12659/MSM.907492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bozko M, Bozko A, Scholta T, et al. Editorial: DNA damage as a strategy for anticancer chemotherapy. Curr Med Chem. 24:1487. doi: 10.2174/092986732415170630115722. 201. [DOI] [PubMed] [Google Scholar]
- 49.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helm CW, States JC. Enhancing the efficacy of cisplatin in ovarian cancer treatment – could arsenic have a role. J Ovarian Res. 2009;2:2. doi: 10.1186/1757-2215-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]