Abstract
Background
Melanoma is among the most aggressive forms of cancer. Our latest retrospective analysis showed that recombinant human interferon-alpha1b (IFN-α1b) led to significantly prolonged survival with mild toxicity in patients with stage IV melanoma. Based on this clinical finding, the current study sought to investigate the influence of IFN-α1b on the antitumor immunity of melanoma, with interferon-alpha2b (IFN-α2b) used as a control.
Material/Methods
Peripheral blood mononuclear cells were stimulated with culture medium alone, or medium supplemented with IFN-α1b or IFN-α2b. Flow cytometry and lactate dehydrogenase release assays were used to evaluate cytotoxic effects. Flow cytometry and enzyme-linked immunospot assays were used to analyze immunoregulatory effects on natural killer (NK) cells, natural killer T (NKT) cells, CD3+CD8+ T cells, and melanoma cells. Cell Counting Kit-8 assay was performed to measure the effect on proliferation of melanoma cells in vitro.
Results
IFN-α1b enhanced the activity of NK cells, NKT cells, and CD3+CD8+ T cells from melanoma patients. Compared with IFN-α2b, IFN-α1b induced a relatively lower level of programmed cell death-ligand 1 (PD-L1) in melanoma cells without affecting the expression of PD-L1 in CD3+CD8+ T cells. Additionally, IFN-α1b showed a much stronger inhibition of the proliferation of melanoma cells than IFN-α2b.
Conclusions
IFN-α1b has an immunostimulatory activity similar to IFN-α2b and possesses milder adverse effects on immune checkpoints and stronger inhibitory effects on melanoma cell growth than IFN-α2b. Therefore, IFN-α1b is a promising drug for the treatment of melanoma.
MeSH Keywords: Antigens, CD274; Immunomodulation; Interferon-alpha; Melanoma
Background
Melanoma is one of the most aggressive skin cancers, with a high mortality rate. The emergence of immune checkpoint blockers (ICBs) like programmed death-1 (PD-1) neutralizing antibodies and gene-targeted therapy, especially BRAF inhibitors, has led to prolonged survival in patients with advanced melanoma [1]. However, the response rate of melanoma patients who accept ICBs therapy is relatively low [2]. Moreover, BRAF mutant is infrequently seen in Chinese melanoma patients, which hinders the application of gene-targeted therapy [3]. Hence, there is still an unmet need for the development of effective treatments specifically applicable to melanoma patients in China.
Interferon-alpha (IFN-α) proteins are a class of homologous cytokines that belong to the family of type I IFNs. IFN-α proteins can activate janus kinase-signal transducer and activator of transcription 1 (JAK-STAT1) pathways, which ultimately results in the activation of cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, which are not only involved in antiviral response, but are also important to immune surveillance of tumors, including melanoma [4,5]. In 1995, the FDA approved high-dose IFN-α2b (HDI) as adjuvant therapy for high-risk melanoma [6]. However, the clinical application of IFN-α2b has been hampered by its severe toxicity, which prompted exploration of the use of other IFN-α subtypes in the treatment of melanoma.
IFN-α1 is another major subtype of IFN-α, with 80% of its amino acid sequence homologous with IFN-α2 [7,8]. A previous phase I clinical trial reported that, in 18 patients with metastatic malignancies who received recombinant IFN-α1b therapy for over 8 weeks, 6 patients (5 with renal cell carcinoma and 1 with malignant hemangioendothelioma) showed progression-free survival for over 1 year, indicating a potential antitumor effect of IFN-α1b [9]. Encouragingly, our latest retrospective analysis showed that a recombinant product of IFN-α1b, which was developed by a Chinese genetic engineering group and has been approved for treatment of melanoma by the Chinese FDA, led to a median survival of 14.1 months in patients with stage IV melanoma, and only 5.6% of the patients had severe adverse events (unpublished data). These data demonstrate that IFN-α1b is a promising drug for the treatment of Chinese melanoma patients.
Based on our clinical findings, we sought to investigate the effect of IFN-α1b on the amount and the function of antitumor immune cells from melanoma patients, with IFN-α2b used as a control. In addition, the influence of IFN-α1b and IFN-α2b on the immune checkpoint axis of PD-1 and programmed death-ligand 1 (PD-L1) was also evaluated.
Material and Methods
Patients
Peripheral blood samples were collected from 45 patients with histologically verified melanoma in the Department of Dermatology, Xijing Hospital. Blood was drawn at the time of diagnosis, prior to therapy. Patients with current or recent therapy and other ongoing acute medical conditions were excluded. Written informed consent was obtained from all patients in the study. The characteristics of the melanoma patients involved in this study are listed in Supplementary Table 1. The progression stages of all cases were classified according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (seventh edition) [10]. The research protocol was designed and performed according to the principles of the Helsinki Declaration and was approved by the Ethics Review Board of Xijing Hospital, Fourth Military Medical University.
Cell culture and reagents
The FLFMM-34 cell line, derived from a Chinese patient with metastatic melanoma, was established in our laboratory [11]. The A2058 cell line was purchased from the American Type Culture Collection (ATCC). Both cell lines were cultured in Dulbecco’s MEMV F12 medium (Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA). Recombinant human IFN-α1b (Hapgen®, 50 μg/ml, 5×106 U/ml, specific activity 1.41×108 IU/mg in Madin-Darby bovine kidney [MDBK] cells) was obtained from Beijing Tri-Prime Gene Pharmaceutical Co. (Beijing, China). Recombinant human IFN-α2b (INTRON A®, 1.8×107 U/1.2 ml, specific activity of 2×108 IU/mg) was obtained from Merck Sharp & Dohme Limited (Kenilworth, NJ, USA). The following directly-labeled monoclonal antibodies (mAbs) were purchased from BioLegend (San Diego, CA, USA): CD3-FITC (clone HIT3a), CD8-Percp/cyanine5.5 (clone SK1), CD56-PerCP/Cyanine5.5 (clone 5.1H11), CD69-PE (clone FN50), perforin-PE (clone dG9), PD-L1/PE(clone MIH2), PD-1/PE (clone EH12.2H7), and human leukocyte antigen-ABC (HLA-ABC)/FITC (clone W6/32).
Isolation, culture, and stimulation of PBMCs
Peripheral blood mononuclear cells (PBMCs) were separated from peripheral blood by Ficoll-Hypaque (Dakewei, Shenzhen, China). Fresh PBMCs were washed and incubated overnight in RPMI-1640 culture medium (Invitrogen, Carlsbad, CA, USA) at 37°C in 5% CO2. They were then washed twice, and 2×106 cells were stimulated in each well of 6-well plates for 18 h in medium alone (negative control, NC), or in medium supplemented with IFN-α1b (1000 U/ml) or IFN-α2b (1000 U/ml), at 37°C in humid atmosphere with 5% CO2.
Evaluation of cell apoptosis and death
It is reported that the concentration of 1000 U/ml for IFN-α2b for in vitro experiments is equivalent to the average serum concentration (20 MU/m2) of IFN-α2b in melanoma patients who receive intravenous HDI infusion therapy [12], and IFN-α2b at this concentration can induce maximal phosphorylation of STAT1-pY701 in PBMCs [13]. We therefore selected 1000 U/ml as the stimulating dosage for both IFN-α1b and IFN-α2b.
The apoptosis of PBMCs was evaluated by flow cytometry with Annexin-V-FITC and propidium iodide (PI) staining (7 Sea Biotech, Shanghai, China) according to the manufacturer’s instructions. PBMCs (1×106 cells) were treated as indicated for 48 h. At least 10 000 events were collected using a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA).
The toxicity of IFN-α was evaluated by measuring lactate dehydrogenase (LDH) release using a CytoTox96 nonradioactive assay (Promega, Madison, WI, USA) according to the manufacturer’s instructions [14]. PBMCs (1×105 cells) were cultured in 96-well plates and treated with IFN-α1b or IFN-α2b for 48 h. Their absorbance levels at 490 nm were recorded. We used corrected values to calculate the percent cytotoxicity according to the following formula: percent cytotoxicity=100×experimental LDH release (OD490)/maximum LDH release (OD490).
Flow cytometric analysis of cell subsets
PBMCs were stained for different cell surface markers and then were sorted by flow cytometry (BD FACS Calibur) into a CD3–CD56+ NK cell subset, a CD3+CD56+ NKT subset, and a CD3+CD8+ T cells subset (Supplementary Figure 1A–1F). The following combinations of directly-labeled mAbs were also identified: CD3/CD56/CD69, CD3/CD8/CD69, CD3/CD8/PD-L1, and CD3/CD8/PD-1. For perforin evaluation, PBMCs were treated with Brefeldin A (1: 1000, eBioscience, San Diego, CA, USA) for 6 h, followed by fixing and permeabilizing with Cytofix/Cytoperm solution (BD Biosciences, San Diego, CA, USA) according to the manufacturer’s instructions. Cells were first stained for surface antigens with CD3/CD56 or CD3/CD8 antibodies, and subsequently stained with anti-perforin antibody. A total of 10 000–100 000 gated events verified as lymphocytes according to their physical characteristics (FSC and SSC) were collected per sample. Analyses were performed using FlowJo V10 software (TreeStar, Ashland, OR, USA).
IFN-γ ELISPOT assay
The interferon-γ (IFN-γ) release enzyme-linked immune absorbent spot (ELISPOT) assay was performed using a commercial kit (Human IFN-γ ELISPOT, Mabtech, Stockholm, Sweden) according to the manufacturer’s instructions. Briefly, fresh PBMCs were washed and incubated overnight in DAYOU serum-free medium (Dakewe, Beijing, China) at 37°C in 5% CO2. Then, PBMCs (2×105 cells/well) were plated in the IFN-γ ELISPOT plate at 37°C and 5% CO2. Medium was used as a negative control, and the anti-CD3 antibody (1: 1000) was used as a positive control. After 48-h stimulation, cells were removed and plates were washed 5 times with PBS. Biotinylated detection anti-IFN-γ mAb (1 μg/ml) was added into the wells, followed by a 2-h incubation at room temperature, and the plates were then incubated for a further 1 h at room temperature with diluted streptavidin-ALP (1: 1000) in PBS-0.5% FCS at 100 μl per well. The plates were then washed 5 times with PBS, followed by addition of substrate solution BCIP/NBT-plus. Tap water was used to stop the reaction until distinct spots appeared. All plates were evaluated by a computer-assisted ELISPOT reader (Cell Technology Inc., Jessup, MD, USA).
Detection of HLA-ABC and PD-L1 in human melanoma cells
To ascertain the expressions of HLA-ABC and PD-L1 in melanoma cells by flow cytometry (BD FACS Calibur), FLFMM-34 and A2058 cells were seeded at a density of 2.5×105 cells/well in 6-well plates and cultured in Dulbecco’s MEMV F12 medium with or without INF-α1b/INF-α2b (1000 U/ml) for 48 h. Afterwards, the cells were harvested and incubated with HLA-ABC/FITC or PD-L1/PE antibodies for approximately 30 min at 4°C and then washed twice with PBS. The mean fluorescence intensity (MFI) of HLA-ABC expression and the percentage of PD-L1-positive cells were analyzed by flow cytometry with gates set for viable cells. MFI represents the average fluorescence intensity of each event and refers to the expression quantity of the parameter on each event.
Cell viability assay
Cell viability was measured by Cell Counting Kit-8 (CCK8) assay according to the manufacturer’s instructions (7 Sea biotech, Shanghai, China). Briefly, FLFMM-34 cells and A2058 cells were seeded into 96-well plates (5×103 cells/well), cultured for 20 h, and stimulated with either medium (negative control) or medium containing IFN-α1b/IFN-α2b with a concentration gradient (1×105, 2.5×105, 5×105, 1×106 U/ml). After stimulation for 48 h, cells were incubated with CCK8 solution for half an hour, and absorbance (A) at 450 nm was then analyzed for each well using a microplate reader (Model 680, Bio-Rad, Hercules, CA, USA). The data on cell growth were expressed as percent inhibition of control. The experiments were repeated 3 times with 4 wells for each concentration.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 7.0. The nonparametric Wilcoxon matched-pairs signed rank test was used to detect the apoptosis of PBMCs, the cytotoxicity of IFN-α to PBMCs, the effects of IFN-α on NK cells, NKT cells, and CD3+CD8+ T cells, and on the production of IFN-γ in PBMCs. MFI of HLA-ABC and the expression of PD-L1 in melanoma cells were estimated using the Mann-Whitney test. For comparison of parameters with CCK8, an unpaired t test was applied. All experiments were repeated at least 3 times. Median and interquartile range were measured for the Wilcoxon matched-pairs signed rank test and the Mann-Whitney test. For unpaired t test, data were presented as mean±standard deviation (SD). All P-values were two-tailed, and values of P<0.05 were considered statistically significant.
Results
IFN-α1b or IFN-α2b at the concentration of 1000 U/ml has no toxic effect on PBMCs
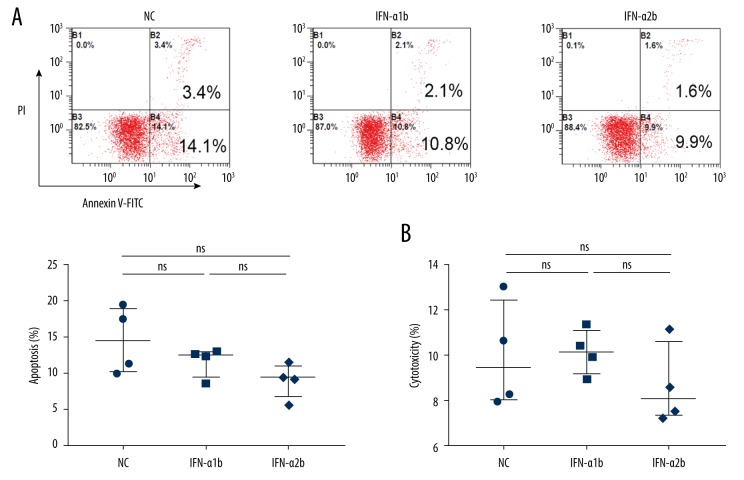
Our initial flow cytometry assay showed that the percentage of apoptotic cells in PBMCs stimulated by IFN-α1b or IFN-α2b at 1000 U/ml was not increased compared with control (Figure 1A). Consistently, subsequent LDH assay demonstrated no change in the death of PBMCs after exposure to IFN-α1b or IFN-α2b at 1000 U/ml for 48 h compared with control (Figure 1B). Therefore, IFN-α1b or IFN-α2b at the concentration of 1000 U/ml has no toxic effect on PBMCs.
Figure 1.
Apoptotic and cytotoxic effects of IFN-α1b/IFN-α2b on PBMCs. (A) Analysis of apoptosis was performed by flow cytometry after IFN-α1b/IFN-α2b (0, 1000 U/ml) treatment for 48 h (n=4). (B) Cytotoxicity was evaluated by LDH release after treatment with IFN-α1b/IFN-α2b (0, 1000 U/ml) for 48 h (n=4). Statistical analyses were performed with the Wilcoxon matched-pairs signed rank test (ns – not significant).
IFN-α1b and IFN-α2b promote the activation of NK and NKT cells from melanoma patients
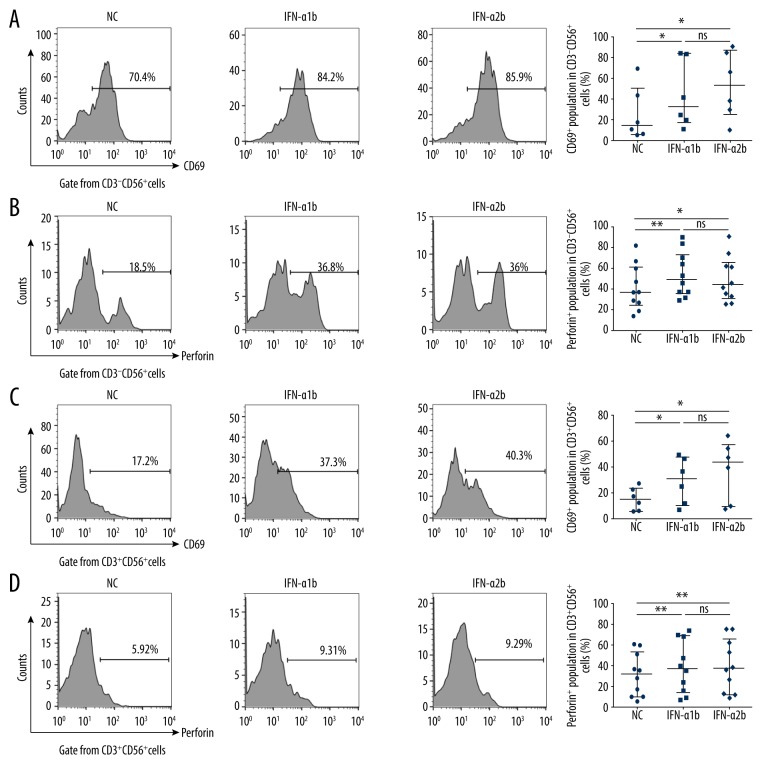
CD3−CD56+ NK cells and CD3+CD56+ NKT cells are critical innate immune cells for first-line host defense and play important roles in antitumor immune response. We thus analyzed the influence of IFN-α1b and IFN-α2b on the population and the activation of NK cells and NKT cells from melanoma patients. Our flow cytometry assay showed that the percentages of CD3−CD56+ NK cells (Supplementary Figure 2A) and CD3+CD56+ NKT (Supplementary Figure 2B) cells were not significantly changed in PBMCs incubated with IFN-α1b or IFN-α2b. However, both IFN-α1b and IFN-α2b could increase the percentage of CD3−CD56bright+ NK cell subset with predominant immunoregulatory function (Supplementary Figure 2C), although the percentage of CD3−CD56dim+ NK cell subset with mainly cytotoxic effect failed to respond (Supplementary Figure 2D). Moreover, IFN-α1b and IFN-α2b enhanced the membranous expression of CD69, which is a classical marker for immune cell activation, and the cytoplasmic level of perforin, which is a vital cytotoxic mediator in NK cells (Figure 2A, 2B) and NKT cells (Figure 2C, 2D) from melanoma patients, whereas the effects of IFN-α1b and IFN-α2b showed no significant difference. Our findings indicate that IFN-α1b and IFN-α2b promotes activation of NK cells and NKT cells to a similar extent.
Figure 2.
Activating effects of IFN-α1b/IFN-α2b on NK and NKT cells. (A, B) Representative flow charts and statistical analysis of CD69 (n=6) and perforin (n=10) in CD3−CD56+ NK cells after IFN-α1b/IFN-α2b (0, 1000 U/ml) treatment for 18 h. (C, D) Representative flow charts and statistical analysis of CD69 (n=6) and perforin (n=10) in CD3+CD56+ NKT cells after IFN-α1b/IFN-α2b (0, 1000 U/ml) treatment for 18 h. Statistical analyses were performed with the Wilcoxon matched-pairs signed rank test (* P<0.05; ** P<0.01; ns – not significant).
IFN-α1b and IFN-α2b have different effects on CD8+ T cells from melanoma patients
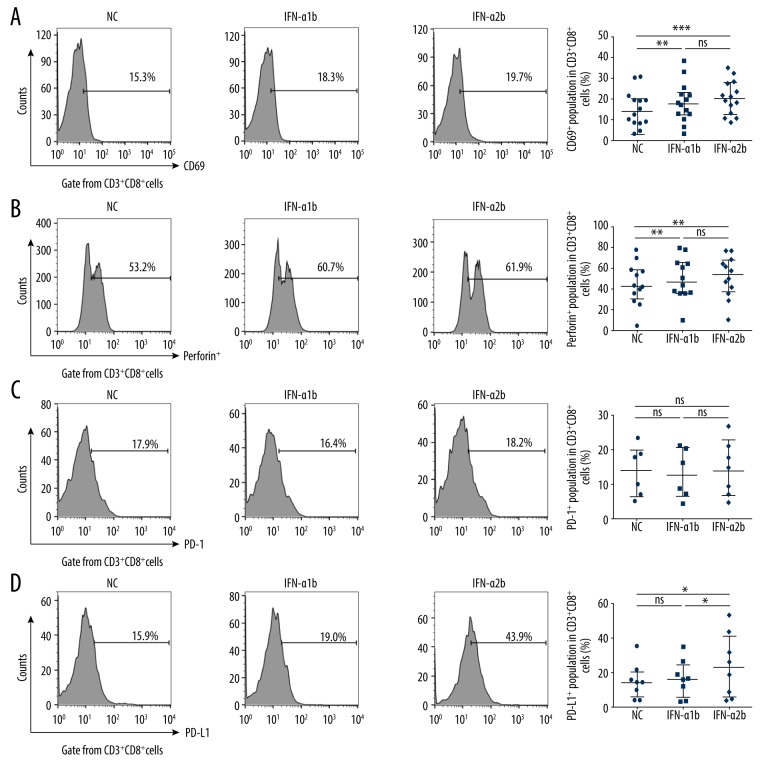
Given that CD8+ T cells play a central role in T cells-mediated cytotoxicity against tumor cells, we went on to analyze the influence of IFN-α1b and IFN-α2b on the immune function of CD8+ T cells. We found that the levels of CD69 (Figure 3A) and perforin (Figure 3B) in CD3+CD8+ T cells from melanoma patients were both increased by treatment with IFN-α1b or IFN-α2b, with no significant difference between the effects of the 2 stimulations.
Figure 3.
Effects of IFN-α1b/IFN-α2b on CD3+CD8+T cells. (A, B) Representative flow charts and statistical analysis of CD69 (n=14) and perforin (n=12) in CD3+CD8+T cells after IFN-α1b/IFN-α2b (0, 1000 U/ml) treatment for 18 h. (C, D) Representative flow charts and statistical analysis of PD-1 (n=6) and PD-L1 (n=8) on CD3+CD8+T cells after IFN-α1b/IFN-α2b (0, 1000 U/ml) treatment for 18 h. Statistical analyses were performed with the Wilcoxon matched-pairs signed rank test (* P<0.05; ** P<0.01; *** P<0.001; ns – not significant).
Then, we analyzed the influence of IFN-α1b and IFN-α2b on the expression of PD-1 and PD-L1, which are negative regulators of antitumor immune response, and subsequently found that neither IFN-α1b nor IFN-α2b influenced the expression of PD-1 in CD3+CD8+ cells (Figure 3C). Notably, IFN-α2b significantly upregulated the expression of PD-L1 in CD3+CD8+ T cells, whereas IFN-α1b did not show this adverse effect (Figure 3D).
IFN-α1b and IFN-α2b prompt the production of IFN-γ in PBMCs from melanoma patients
Next, we analyzed the influence of IFN-α1b and IFN-α2b on the production of IFN-γ, an important antitumor cytokine that can be induced by type I IFN. As expected, our ELISPOT assay showed that the production of IFN-γ was enhanced in PMBCs from melanoma patients after treatment with IFN-α1b or IFN-α2b, and IFN-α2b seemed to be a stronger inducer of IFN-γ than was IFN-α1b (Supplementary Figure 3).
IFN-α1b and IFN-α2b have discrepant influences on the immunogenicity and the growth of melanoma cells
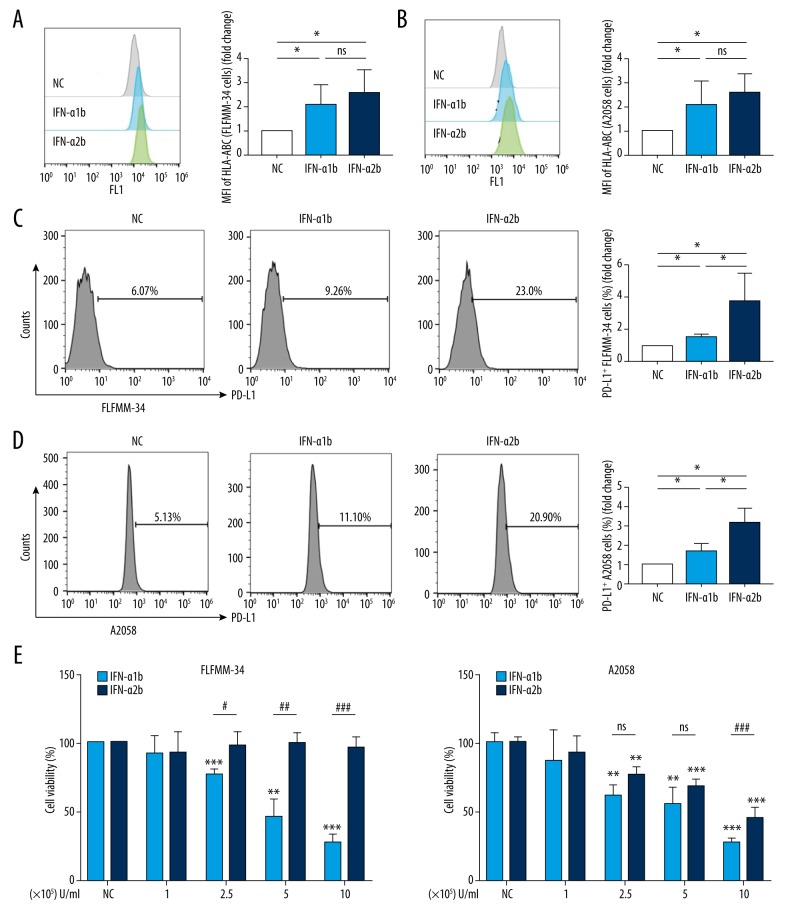
IFN-α can elevate the expression of HLA-ABC in tumor cells, which makes tumor cells more susceptible to immune recognition and destruction. Accordingly, we found that IFN-α1b and IFN-α2b enhanced the expression of HLA-ABC in FLFMM-34 cells (Figure 4A) and A2058 cells (Figure 4B), which are 2 similarly metastatic melanoma cell lines, indicating that both IFN-α1b and IFN-α2b can promote the immunogenicity of melanoma cells. Furthermore, treatment with IFN-α1b or IFN-α2b increased the expression of PD-L1 on the surface of FLFMM-34 cells (Figure 4C) and A2058 cells (Figure 4D), but this unfavorable effect was stronger in the cells treated with IFN-α2b than IFN-α1b.
Figure 4.
Effects of IFN-α1b/IFN-α2b on immunomodulatory activities and growth of melanoma cell lines. (A, B) HLA-ABC levels (fold change of MFI) in FLFMM-34 and A2058 cell lines after IFN-α1b/IFN-α2b (0 and 1000 U/ml) treatment for 48 h (n=4). Statistical analyses were performed with the Mann-Whitney test (* P<0.05; ns – not significant). (C, D) Representative histogram and statistical analysis of the expression of PD-L1 (fold change of percentage) in FLFMM-34 and A2058 cell lines after IFN-α1b/IFN-α2b (0 and 1000 U/ml) treatment for 48 h (n=4). Statistical analyses were performed with the Mann-Whitney test (* P<0.05). (E, F) Cell viability of FLFMM-34 cells and A2058 cells was determined by the CCK8 assay after treatment with IFN-α1b/IFN-α2b at various concentrations (1×105–1×106 U/ml) for 48 h (n=3). Statistical analyses were performed with unpaired t tests (*/# for P<0.05; **/## for P<0.01; ***/### for P<0.001, and ns – not significant). Asterisks (*) show comparisons with untreated controls; hashtags (#) indicate comparisons between treatments.
We compared the influence of IFN-α1b and IFN-α2b on the growth of melanoma cells by using CCK8 assay. We found that IFN-α1b significantly repressed the growth of FLFMM-34 cells at concentrations over 2.5×105 U/ml, whereas IFN-α2b failed to show a parallel effect (Figure 4E). For A2058 cells, IFN-α1b and IFN-α2b showed similar suppression of the growth of A2058 cells at concentrations of 2.5×105 U/ml and 5×105 U/ml. However, IFN-α1b exhibited a superior inhibition of the growth of A2058 cells than did IFN-α2b when the stimulating concentration reached 1×106 U/ml (Figure 4F). Collectively, our results demonstrate that IFN-α1b and IFN-α2b have different influences on the immunogenicity and the growth of melanoma cells.
Discussion
We investigated the mechanism underlying the therapeutic effect of IFN-α1b against melanoma. IFN-α1b enhanced the activity of NK cells, NKT cells, and CD3+CD8+ T cells. Compared with IFN-α2b, IFN-α1b induced a relatively lower level of PD-L1 expression in melanoma cells and did not affect the expression of PD-L1 in CD3+CD8+ T cells, demonstrating that the adverse effect of IFN-α1b on the immune checkpoint axis is weaker than that of IFN-α2b. IFN-α1b showed a much stronger inhibition of the proliferation of melanoma cells than did IFN-α2b.
It is reported that IFN-α promotes the activity and cytotoxicity of NK cells and CTLs [15,16]. In melanoma patients who received IFN-α2b treatment, higher proportions of CD8+ T cells and CD56bright NK cells in circulating blood cells have been observed [17,18]. In this study, we also demonstrated the stimulatory effect of IFN-α1b on immune cell activity, as evidenced by increased levels of CD69 and perforin expression in NK cells, NKT cells and CD3+CD8+ T cells, as well as an elevated proportion of CD3−CD56bright+ NK cells, all of which were equal to the effect of IFN-α2b. In addition, IFN-α1b facilitated the expression of HLA-ABC in melanoma cells, indicating that IFN-α1b enhances the immunogenicity of melanoma cells and overcomes the immune evasion of melanoma caused by HLA-ABC loss.
It has become increasingly clear that IFN-α has an adverse effect of upregulating immunosuppressive molecules such as PD-L1 [19,20]. PD-L1 is expressed on the membrane of immune cells and tumor cells and can inhibit T cell response by binding to PD-1 [21]. Therefore, the upregulation of PD-L1 contributes to tumor immune evasion and correlates with a poor prognosis for patients with cancer [22]. Our study showed that either IFN-α1b or IFN-α2b could induce PD-L1 expression. However, in contrast to IFN-α2b, which showed substantial increase of PD-L1 expression in both melanoma cells and CD3+CD8+ T cells, IFN-α1b did not influence the expression of PD-L1 in CD3+CD8+ T cells and moderately upregulated PD-L1 in melanoma cells. Thus, IFN-α1b appears to be a stronger immune enhancer for melanoma patients due to its weaker adverse effect on the axis of PD-1 and PD-L1 compared to that of IFN-α2b.
Type I IFNs have been observed to stimulate the production of IFN-γ in NK cells and CTLs [15]. Consistently, our study showed an increase of IFN-γ production in PBMCs after treatment with IFN-α1b or IFN-α2b. IFN-γ promotes antitumor immunity via mechanisms similar to type I IFN, such as activating NK cells and CTLs, enhancing tumor immunogenicity, and directly inhibiting tumor cell proliferation [23]. However, a growing body of evidence also indicates an immunosuppressive effect of IFN-γ, which can promote tumor growth. Specifically, IFN-γ can promote the expression of PD-L1 and PD-L2 in tumor cells and even in stromal cells, including immune-infiltrating cells, thus suppressing the effector function of T cells or NK cells [24]. The crosstalk between type I IFN and IFN-γ and the dual role of IFNs in tumor immunity need further study.
Although the mechanism underlying the inhibition of IFN-α in tumor cell growth has not been fully clarified, previous studies indicated that IFN-α can cause cell cycle arrest and cell death in tumor cells [25–27]. A study focusing on the direct antiproliferative effect of IFNs reported that melanoma cell lines differed markedly in their sensitivity to different types of IFNs and even different concentrations of a single subtype [25]. In line with previous work, we also identified different sensitivities of the 2 melanoma cell lines to IFN-α1b and IFN-α2b, and IFN-α1b showed greater inhibition of the growth of melanoma cells. Notably, we observed that the sensitivity of A2058 to IFN-α1b and IFN-α2b was identical at low concentrations, while higher sensitivity to IFN-α1b than IFN-α2b occurred at the highest concentration. These results suggest a better antiproliferative effect of IFN-α1b than IFN-α2b at high concentrations. Considering the mild toxicity of IFN-α1b in clinical application, it may be feasible to utilize high-concentration IFN-α1b in the treatment of melanoma, especially by intratumoral injection, thus bringing about better clinical benefits than achieved with IFN-α2b.
Taken together, our results demonstrate that IFN-α1b can promote antitumor immunity in melanoma via enhancing the activity of NK cells, NKT cells, and CD3+CD8+ T cells. Moreover, IFN-α1b possesses unique characteristics in antitumor response, inducing lower expression of PD-L1 and stronger inhibition on the proliferation of melanoma cells than IFN-α2b. Nevertheless, this study has several limitations. The sample size was relatively small. In addition, the present study performed in vitro may not accurately reflect the conditions in vivo. Thus, our findings need to be verified by experiments in an animal model or in further clinical trials.
Conclusions
Our findings demonstrate that IFN-α1b has an immunostimulatory activity similar to that of IFN-α2b and has milder adverse effect on immune checkpoints and stronger inhibitory effect on melanoma cell growth than IFN-α2b. Our study provides an explanation for the tumor regression following use of IFN-α1b in Chinese melanoma patients, and supports IFN-α1b as a promising medicine for the treatment of melanoma.
Supplementary Data
The gates shown by flow cytometry into NK (CD3−CD56+) cells, NK (CD3+CD56+) T cells, and CD3+CD8+ T cells. (A) Representative FACS plots of PBMCs for membrane cytokine staining. (B) Representative FACS plots of NK cells and NKT cells for membrane cytokine staining. (C) Representative FACS plots of CD3+CD8+ T cells for membrane cytokine staining. (D) Representative FACS plots of PBMCs for intracellular cytokine staining. (E) Representative FACS plots of NK cells and NKT cells for intracellular cytokine staining. (F) Representative FACS plots of CD3+CD8+ T cells for intracellular cytokine staining.
(A, B) Representative flow charts and statistical analysis of the percentage of CD3−CD56+ NK cells and CD3+CD56+ NKT cells in PBMCs after IFN-α1b/IFN-α2b (0, 1000 U/ml) treatment for 18 h (n=6). (C, D) Representative flow charts and statistical analysis of the percentages of CD3−CD56bright+ NK cells and CD3−CD56dim+ NK cells in PBMCs after IFN-α1b/IFN-α2b (0 and 1,000 U/ml) treatment (n=6). Statistical analyses were performed with the Wilcoxon matched-pairs signed rank test (* P<0.05; ns – not significant).
Effects of IFN-α1b/IFN-α2b on levels of IFN-γ in PBMCs. Representative ELISPOT spots and statistical analysis of the secreting IFN-γ in PBMCs after IFN-α1b/IFN-α2b (0, 1000 U/ml) treatment for 48 h (n=7). Statistical analyses were performed with the Wilcoxon matched-pairs signed rank test (* P<0.05).
Supplementary Table 1.
Demographic and disease characteristics of the patients.
| Characteristics | Cases |
|---|---|
| Total number | 45 |
| Age (years), mean (range) | 58 (34–81) |
| Sex ratio (M/F) | 22/23 |
| AJCC stage (seventh edition) | |
| I | 8 |
| II | 23 |
| III | 9 |
| IV | 5 |
| Primary site of melanoma | |
| Acral | 38 |
| Cutaneous | 3 |
| Mucosal | 4 |
Footnotes
Source of support: This work was supported by National Natural Science Foundation of China [No. 81502863, No. 81572672, No. 81602906, No. 81625020, and No. 81902791]
Conflict of interest
None.
References
- 1.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14(8):463–82. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 2.Si L, Zhang X, Shu Y, et al. A Phase Ib study of pembrolizumab as second-line therapy for Chinese patients with advanced or metastatic melanoma (KEYNOTE-151) Transl Oncol. 2019;12(6):828–35. doi: 10.1016/j.tranon.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: Large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48(1):94–100. doi: 10.1016/j.ejca.2011.06.056. [DOI] [PubMed] [Google Scholar]
- 4.Zitvogel L, Galluzzi L, Kepp O, et al. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15(7):405–14. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 5.Snell LM, McGaha TL, Brooks DG. Type I interferon in chronic virus infection and cancer. Trends Immunol. 2017;38(8):542–57. doi: 10.1016/j.it.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarhini AA, Gogas H, Kirkwood JM. IFN-alpha in the treatment of melanoma. J Immunol. 2012;189(8):3789–93. doi: 10.4049/jimmunol.1290060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaritsky LA, Bedsaul JR, Zoon KC. Virus multiplicity of infection affects type I interferon subtype induction profiles and interferon-stimulated genes. J Virol. 2015;89(22):11534–48. doi: 10.1128/JVI.01727-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markusic M, Santak M, Kosutic-Gulija T, et al. Induction of IFN-alpha subtypes and their antiviral activity in mumps virus infection. Viral Immunol. 2014;27(10):497–505. doi: 10.1089/vim.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masci P, Olencki T, Wood L, et al. Gene modulatory effects, pharmacokinetics, and clinical tolerance of interferon-alpha1b: A second member of the interferon-alpha family. Clin Pharmacol Ther. 2007;81(3):354–61. doi: 10.1038/sj.clpt.6100081. [DOI] [PubMed] [Google Scholar]
- 10.Retsas S. Progress but not enough: The 2009 (7th) revision of the American Joint Committee on Cancer (AJCC) for melanoma staging and classification. J BUON. 2011;16(1):38–39. [PubMed] [Google Scholar]
- 11.Yi X, Zhu G, Li Y, et al. Establishment of a novel Chinese metastatic melanoma cell line showing the new cytogenetic and biological properties. Cell Biol Int. 2015;39(4):508–14. doi: 10.1002/cbin.10417. [DOI] [PubMed] [Google Scholar]
- 12.Ismail A, Yusuf N. Type I interferons: Key players in normal skin and select cutaneous malignancies. Dermatol Res Pract. 2014;2014 doi: 10.1155/2014/847545. 847545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suarez-Kelly LP, Levine KM, et al. A pilot study of interferon-alpha-2b dose reduction in the adjuvant therapy of high-risk melanoma. Cancer Immunol Immunother. 2019;68(4):619–29. doi: 10.1007/s00262-019-02308-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirshafiee V, Sun B, Chang CH, et al. Toxicological profiling of metal oxide nanoparticles in liver context reveals pyroptosis in Kupffer cells and macrophages versus apoptosis in hepatocytes. ACS Nano. 2018;12(4):3836–52. doi: 10.1021/acsnano.8b01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker BS, Rautela J, Hertzog PJ. Antitumour actions of interferons: Implications for cancer therapy. Nat Rev Cancer. 2016;16(3):131–44. doi: 10.1038/nrc.2016.14. [DOI] [PubMed] [Google Scholar]
- 16.Konjevic G, Mirjacic MK, Vuletic A, Babovic N. In-vitro IL-2 or IFN-alpha-induced NKG2D and CD161 NK cell receptor expression indicates novel aspects of NK cell activation in metastatic melanoma patients. Melanoma Res. 2010;20(6):459–67. doi: 10.1097/CMR.0b013e32833e3286. [DOI] [PubMed] [Google Scholar]
- 17.Simons DL, Lee G, Kirkwood JM, Lee PP. Interferon signaling patterns in peripheral blood lymphocytes may predict clinical outcome after high-dose interferon therapy in melanoma patients. J Transl Med. 2011;9:52. doi: 10.1186/1479-5876-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vujanovic L, Chuckran C, Lin Y, et al. CD56(dim) CD16(-) natural killer cell profiling in melanoma patients receiving a cancer vaccine and interferon-alpha. Front Immunol. 2019;10:14. doi: 10.3389/fimmu.2019.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bazhin AV, von Ahn K, Fritz J, et al. Interferon-alpha up-regulates the expression of PD-L1 molecules on immune cells through STAT3 and p38 signaling. Front Immunol. 2018;9:2129. doi: 10.3389/fimmu.2018.02129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Diaz A, Shin DS, Moreno BH, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19(6):1189–201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribas A. Adaptive immune resistance: How cancer protects from immune attack. Cancer Discov. 2015;5(9):915–19. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostrand-Rosenberg S, Horn LA, Haile ST. The programmed death-1 immune-suppressive pathway: barrier to antitumor immunity. J Immunol. 2014;193(8):3835–41. doi: 10.4049/jimmunol.1401572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alspach E, Lussier DM, Schreiber RD. Interferon gamma and its important roles in promoting and inhibiting spontaneous and therapeutic cancer immunity. Cold Spring Harb Perspect Biol. 2019;11(3) doi: 10.1101/cshperspect.a028480. pii: a028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mojic M, Takeda K, Hayakawa Y. The dark side of IFN-gamma: Its role in promoting cancer immunoevasion. Int J Mol Sci. 2017;19(1) doi: 10.3390/ijms19010089. pii: E89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johns TG, Mackay IR, Callister KA, et al. Antiproliferative potencies of interferons on melanoma cell lines and xenografts: higher efficacy of interferon beta. J Natl Cancer Inst. 1992;84(15):1185–90. doi: 10.1093/jnci/84.15.1185. [DOI] [PubMed] [Google Scholar]
- 26.Xin XL, Zhang R, Yuan XM, Liu L. Mechanisms of IFNalpha-1a-induced apoptosis in a laryngeal cancer cell line. Med Sci Monit. 2019;25:7100–14. doi: 10.12659/MSM.917097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyake K, Bekisz J, Zhao T, et al. Apoptosis-inducing factor (AIF) is targeted in IFN-alpha2a-induced Bid-mediated apoptosis through Bak activation in ovarian cancer cells. Biochim Biophys Acta. 2012;1823(8):1378–88. doi: 10.1016/j.bbamcr.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The gates shown by flow cytometry into NK (CD3−CD56+) cells, NK (CD3+CD56+) T cells, and CD3+CD8+ T cells. (A) Representative FACS plots of PBMCs for membrane cytokine staining. (B) Representative FACS plots of NK cells and NKT cells for membrane cytokine staining. (C) Representative FACS plots of CD3+CD8+ T cells for membrane cytokine staining. (D) Representative FACS plots of PBMCs for intracellular cytokine staining. (E) Representative FACS plots of NK cells and NKT cells for intracellular cytokine staining. (F) Representative FACS plots of CD3+CD8+ T cells for intracellular cytokine staining.
(A, B) Representative flow charts and statistical analysis of the percentage of CD3−CD56+ NK cells and CD3+CD56+ NKT cells in PBMCs after IFN-α1b/IFN-α2b (0, 1000 U/ml) treatment for 18 h (n=6). (C, D) Representative flow charts and statistical analysis of the percentages of CD3−CD56bright+ NK cells and CD3−CD56dim+ NK cells in PBMCs after IFN-α1b/IFN-α2b (0 and 1,000 U/ml) treatment (n=6). Statistical analyses were performed with the Wilcoxon matched-pairs signed rank test (* P<0.05; ns – not significant).
Effects of IFN-α1b/IFN-α2b on levels of IFN-γ in PBMCs. Representative ELISPOT spots and statistical analysis of the secreting IFN-γ in PBMCs after IFN-α1b/IFN-α2b (0, 1000 U/ml) treatment for 48 h (n=7). Statistical analyses were performed with the Wilcoxon matched-pairs signed rank test (* P<0.05).
Supplementary Table 1.
Demographic and disease characteristics of the patients.
| Characteristics | Cases |
|---|---|
| Total number | 45 |
| Age (years), mean (range) | 58 (34–81) |
| Sex ratio (M/F) | 22/23 |
| AJCC stage (seventh edition) | |
| I | 8 |
| II | 23 |
| III | 9 |
| IV | 5 |
| Primary site of melanoma | |
| Acral | 38 |
| Cutaneous | 3 |
| Mucosal | 4 |