Abstract
Purpose:
Ocular diurnal rhythms have been implicated in myopia, glaucoma, diabetes, and other ocular pathologies. Ocular rhythms have been well described in adults; however, they have not yet been fully examined in children. The goal of this study was to investigate ocular and systemic diurnal rhythms over 24 h in children.
Methods:
Subjects, ages 5 to 14 years (n = 18), wore a light, sleep, and activity monitor for one week to assess habitual sleep/wake patterns, then underwent diurnal measurements every 4 h for 24 h. Measurements included blood pressure, heart rate, body temperature, intraocular pressure (IOP), ocular biometry, and optical coherence tomography imaging. Saliva was collected for melatonin and cortisol analysis. Mean ocular perfusion pressure was calculated from IOP and blood pressure. Central corneal thickness, corneal power, anterior chamber depth, lens thickness, vitreous chamber depth, and axial length were determined from biometry. Total retinal thickness, retinal pigment epithelium (RPE) + photoreceptor outer segment thickness, photoreceptor inner segment thickness, and choroidal thickness were determined for a 1 mm diameter centred on the fovea. Subjects’ amplitude and acrophase of diurnal variation for each parameter were determined using Fourier analysis, and mean acrophase was calculated using unit vector averaging.
Results:
Repeated measures analysis of variance (ANOVA) showed that all parameters except anterior chamber depth exhibited significant variations over 24 h (p ≤ 0.005 for all). Axial length underwent diurnal variation of 45.25 ± 6.30 μm with an acrophase at 12.92 h, and choroidal thickness underwent diurnal variation of 26.25 ± 2.67 μm with an acrophase at 1.90 h. IOP was approximately in phase with axial length, with a diurnal variation of 4.19 ± 0.50 mmHg and acrophase at 11.37 h. Total retinal thickness underwent a significant diurnal variation of 4.09 ± 0.39 μm with an acrophase at 15.04 h. The RPE + outer segment layer was thickest at 3.25 h, while the inner segment layer was thickest at 14.95 h. Melatonin peaked during the dark period at 2.36 h, and cortisol peaked after light onset at 9.22 h.
Conclusions:
Ocular and systemic diurnal rhythms were robust in children and similar to those previously reported in adult populations. Axial length and IOP were approximately in phase with each other, and in antiphase to choroidal thickness. These findings may have important implications in myopia development in children.
Keywords: diurnal rhythms, circadian rhythms, choroidal thickness, axial length, melatonin, cortisol
Introduction
Circadian rhythms are intrinsic variations in physiological parameters that undergo a cycle of approximately 24 h. Circadian rhythms are entrained to the 24 h solar day by several cues, or zeitgebers, the most potent of which is light.1 Information about light exposure is relayed from the intrinsically photosensitive retinal ganglion cells to the suprachiasmatic nucleus in the hypothalamus, which is considered the master clock of the body.2,3 When circadian rhythms are synchronised to the 24 h solar day, they are considered diurnal rhythms. Advances in non-contact low coherence optical biometry and optical coherence tomography4 have made the evaluation of ocular diurnal changes in humans feasible. Diurnal rhythms have been demonstrated in humans for several ocular parameters, including central corneal thickness,5,6 intraocular pressure,7 axial length,8 and retinal9 and choroidal thicknesses.10
Axial length was first shown to undergo diurnal fluctuations in chicks,11,12 then later demonstrated in humans.13 The majority of studies examining axial length rhythms in humans has been performed in adults, with findings showing that axial length is longest in the morning and shortest during the night.7,8,13 It is now well established that the choroid, the vascular structure providing oxygen and nutrients to the outer retina, also undergoes diurnal variations in thickness in animal models, including the chick14 and marmoset,15 and in humans.10,16–19 Evidence suggests that the choroid relays information from the retina to the sclera, acting as a regulator of scleral growth.20 Diurnal rhythms in axial length and choroidal thickness have been implicated in eye growth control.21,22 In chick eyes that are developing refractive errors, the phase and amplitude of axial length and choroidal thickness rhythms are altered.12,14,21 In humans, the amplitude of choroidal thickness diurnal variation has been shown to be correlated with axial length.10,18 However, studies have shown that the relationship between axial length rhythms and choroidal thickness rhythms are similar in adult emmetropes and myopes.10,16 These 24 h rhythms have not yet been examined in children, which is when myopia typically develops.23 Evaluating diurnal rhythms in axial length and choroidal thickness in children is imperative to understand the potential significance of these factors in myopia development and progression.
Diurnal variations in intraocular pressure (IOP) are known to have an important role in glaucoma,24 and may also be important in myopia.25 Diurnal IOP can be assessed through repeated tonometry in a laboratory,7,26 or via home monitoring using hand held tonometry27 or continuously measuring contact lenses.24,28 IOP is known to be the greatest in the morning, and lowest before bed-time, with healthy adult subjects showing diurnal variations of approximately 4–5 mmHg.26 Recently, hand held portable instruments have been utilised to assess diurnal variations in children in their home, with data collection performed by children’s parents.29–31 Flemmons, et al., reported findings for 11 healthy children, and showed that, similar to adults, children’s IOP was greatest in the morning and lowest at nighttime, when assessed from 6:00 am to 9:00 pm.29 To the best of our knowledge, IOP measurements have not yet been reported for a full 24 h period in children.
Investigations of diurnal variations in corneal parameters have also been performed in adults.6,32 Studies show that the cornea is thickest in the morning, immediately after eye opening, and decreases throughout the day. Daily fluctuations in corneal thickness have implications in IOP measurement, which has been shown to vary with corneal thickness depending on the type of tonometer used.33,34 An understanding of corneal diurnal rhythms is also important when considering the effects of contact lens wear, including soft,35 scleral, and orthokeratology contact lenses,36 on the ocular surface and in ocular perfusion.
Ocular diurnal rhythms have been implicated in myopia,22,37 glaucoma,24 diabetes,38 and other ocular pathologies.39 Ocular rhythms have not yet been fully examined in children. We sought to understand if school-age children demonstrate similar 24 h ocular diurnal rhythms as previously reported in adult populations.
Methods
Healthy subjects ages 5–14 years participated (n = 18). Subjects provided assent and parents provided permission after the purpose of the study and the risks were explained. The study was approved by the Committee for Protection of Human Subjects at the University of Houston and followed the tenets of the Declaration of Helsinki.
The protocol is outlined in Figure 1. Subjects underwent a screening to determine ocular and systemic health. Non-cycloplegic autorefraction (WAM-5500 Binocular Accommodation Auto Ref/Keratometer, www.grandseiko.com) and axial length (LenStar biometer, www.haag-streit.com) were measured in both eyes. All subjects had best corrected visual acuity of 20/25 (0.1 logMAR, 6/7.5, 0.8 decimal) or better. Exclusion criteria were ocular disease, the use of melatonin or other pharmacological sleep aids, or travel outside of two time zones in the month before the experiment. Following screening and enrolment, an Actiwatch Spectrum device (www.actigraphy.com/) was dispensed for subjects to wear for 1 week. The Actiwatch provided continuous, objective measurements of each subject’s habitual light exposure, sleep, and physical activity. Data obtained from the Actiwatch included minutes per day spent exposed to high intensity outdoor light (>1000 lux), mean daily light exposure (lux), activity (counts per minute) and sleep duration (hours), and sleep and wake times.
Figure 1.
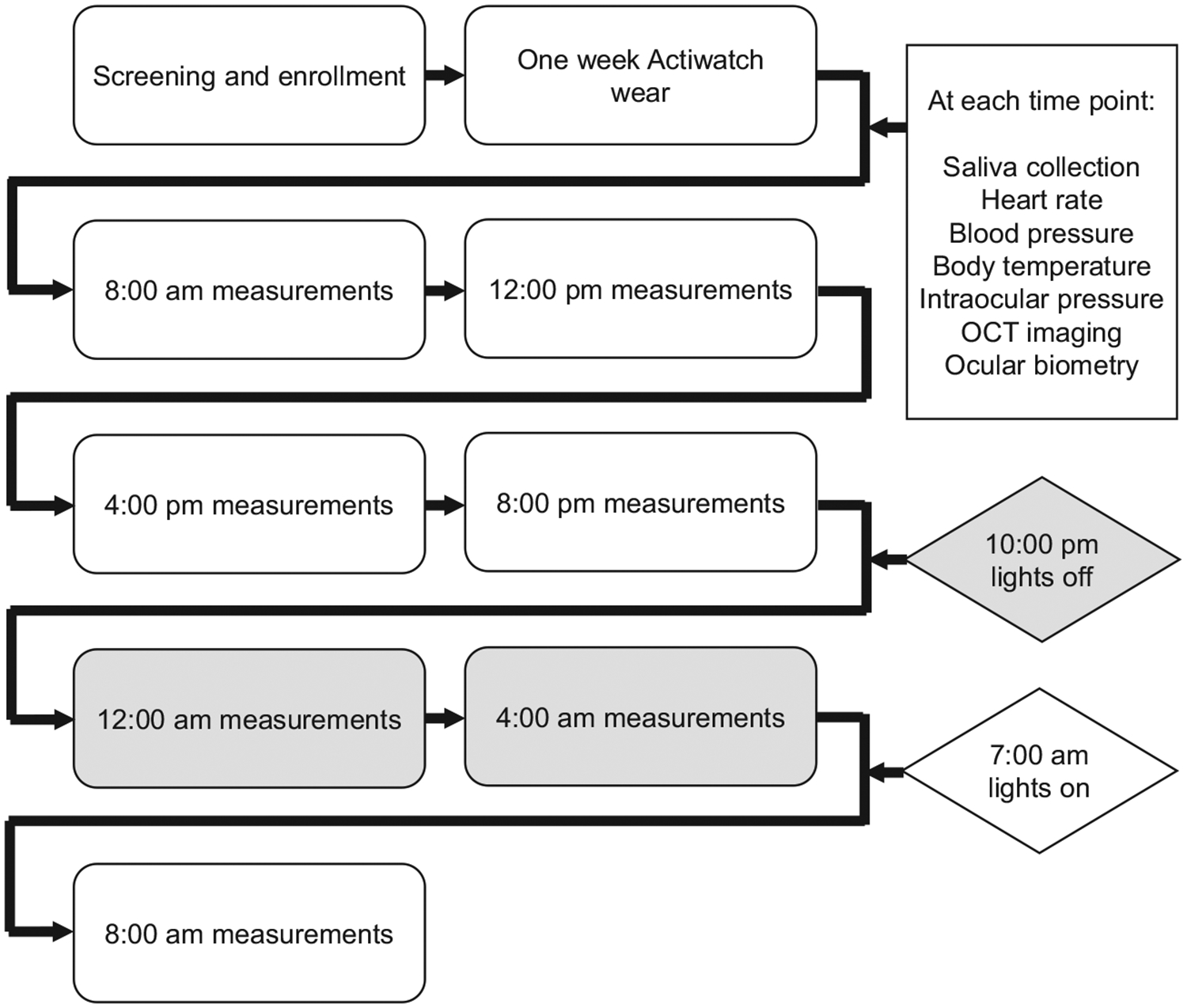
Protocol. Following screening and enrolment, subjects wore an Actiwatch for one week, then underwent ocular and systemic measurements every 4 h for 24 h. Grey areas represent the dark period.
After 1 week, subjects arrived at the lab at 8:00 am for the first set of measurements, which were collected every 4 h for 24 h. From 8:30 am to 10:00 pm, subjects were free to go about their daily activities and return to the lab at each time point for data collection. From 10:00 pm to 7:00 am, subjects slept in the lab with all lights off, along with a parent or guardian, and were woken at 12:00 am and 4:00 am for measurements. Measurements during the night were performed under dim red illumination. Measurements took approximately 20–30 min at each time point.
All measurements were collected with subjects in a seated position. At each time point, except for the two during the dark period, subjects first underwent a distance viewing period of 10 min to help standardise conditions under which measurements were recorded. During this time, subjects sat quietly and viewed a television screen at 4 metres under controlled laboratory illumination of approximately 400 lux (LX1330B Digital Illuminance Light Meter, www.drmeter.com).
At each time point, subjects collected 1 mL of saliva into a vial for melatonin and cortisol analysis. Samples were immediately placed into a −20°C freezer and analysed within 1 month of collection using an ELISA salivary control kit (www.salimetrics.com). Each sample was analysed in duplicate.
Heart rate and blood pressure were measured three times, separated by approximately 1 min each, using an OMRON electronic cuff (www.omronhealthcare.com/). Body temperature was measured three times using a Welch Allyn under-the-tongue digital thermometer with disposable probe cover (www.welchallyn.com/en.html). Intraocular pressure (IOP) was measured in the right eye using an Icare® rebound tonometer (www.icaretonometer.com/). Three readings were recorded, each an average of six measures. From the diastolic (DPB) and systolic (SBP) blood pressure and IOP, mean ocular perfusion pressure (MOPP) was calculated for each time point using Equation 1.
| (1) |
Spectral domain optical coherence tomography (SD-OCT) was performed with a Spectralis OCT (www.heidelbergengineering.com) to assess retinal and choroidal thicknesses. At each time point, two high resolution, six line 30° radial scans, centred on the fovea and with enhanced depth imaging, were acquired. For noise reduction, B-scan averaging was set at 16 frames, and scans with less than 24 dB quality were repeated. The first image at the first time point (8:00 am) was set as the reference for each subject, with the instrument’s tracking function utilised for subsequent imaging. OCT B-scans were exported and analysed with custom written software in MATLAB (www.mathworks.com) using a semi-automated process. Lateral magnification for each scan was determined using a three surface schematic eye, constructed using optical biometry obtained axial length, corneal curvature, anterior chamber depth, and lens thickness, and assuming a spherical retinal surface.40–42 The internal limiting membrane, external limiting membrane, inner segment/outer segment border, and Bruch’s membrane (identified by the retinal pigment epithelium (RPE) and Bruch’s membrane junction) were automatically segmented based on A-scan intensity profiles, and errors made in border detection were manually corrected. OCT images were compensated, and sclera/choroid border was manually segmented. A representative b-scan with segmentation is shown in Figure 2. The distance from Bruch’s membrane to the internal limiting membrane was calculated as the total retinal thickness. The distance from Bruch’s membrane to the inner segment/outer segment border was calculated as the retinal pigment epithelium (RPE) + photoreceptor outer segment thickness. The distance from the inner segment/outer segment border to the external limiting membrane was calculated as the photoreceptor inner segment thickness. The distance from Bruch’s membrane to the posterior choroid was calculated as the choroidal thickness. Axial thickness for each layer was determined for 1536 points along each of the six scan lines. Data are presented as an average for the two images at each time point for the central 1 mm diameter.
Figure 2.
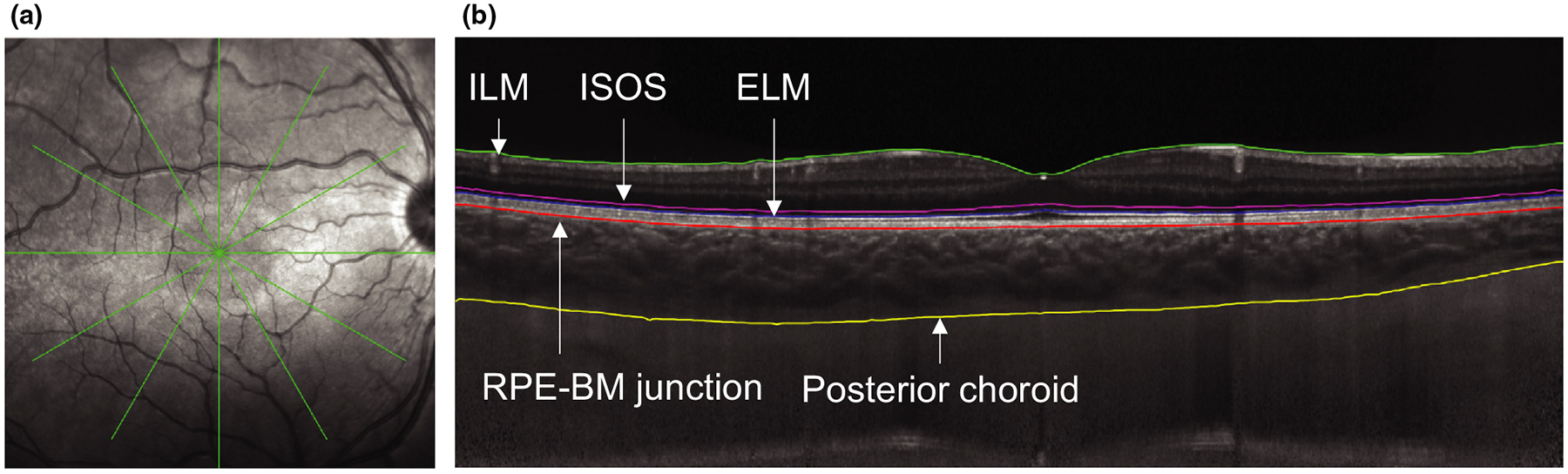
a) Representative infrared image showing the radial scan pattern and b) b-scan with segmentation as follows: inner limiting membrane (ILM, green), inner segment/outer segment border (ISOS, magenta), external limiting membrane (ELM, blue), retinal pigment epithelium/Bruch’s membrane junction (RPE-BM, red), and posterior choroid (yellow).
Lastly, a non-contact low coherence optical biometer (LenStar, https://www.haag-streit.com) was used to measure central corneal thickness, corneal power, anterior chamber depth, lens thickness, vitreous chamber depth, and axial length. Five measurements were recorded from the right eye and averaged at each time point.
Statistical analyses were performed with Microsoft Excel and MedCalc (https://www.medcalc.org). Data are expressed as mean ± standard error unless otherwise noted. A critical value < 0.05 is considered statistically significant. Normality was confirmed with the Shapiro-Wilk test. For analysis of diurnal measurements, data for the first and last time points (8:00 am on the first and second day) were averaged. Diurnal changes for each parameter were normalized to the average measurement across 24 h for each subject. Repeated measures ANOVA was performed for time-of-day (within-subjects factor) to identify significant diurnal variations. For axial length, central 1 mm choroidal and retinal thicknesses, IOP, and MOPP, relationships were evaluated using cosinor analysis,43 which uses the least squares method, in this case minimising least-squared residuals between subjects, to fit a sine wave to a time series, and is often used in the analysis of biologic time series that demonstrate predictable rhythms. Note that amplitudes of the diurnal change appear attenuated in cosinor fits due to variations in individual subject’s acrophase being averaged together.
To estimate the acrophase and amplitude of diurnal variation for each parameter for each subject, Fourier analysis was used.44 The fundamental cosine was determined using equation 2,
| (2) |
where t is time of measurement (on a 24 h clock), Acrophase is the time where the fitted cosine reaches its peak, and Amplitude is the difference between maximum and minimum y values in the fitted cosine. Acrophase was averaged across all subjects using circular statistical methods,45 averaging of the unit vectors on a 24 h clock with the resultant phase to give average acrophase and a circular standard deviation S calculated using equation 3,
| (3) |
where r is the average of the unit vectors. Standard error was calculated as S divided by the square root of the count.
Results
Mean subject age was 10.06 ± 2.53 years (range 5.79 to 14.18) and included 10 females and 8 males. Spherical equivalent refraction of right eyes was +0.35 ± 0.38 D (range +3.44 to −2.38 D) and of left eyes was +0.33 ± 0.35 D (range + 3.13 to −2.12 D). Right and left eyes were not significantly different (p = 0.81), and only right eyes are considered further. Twelve subjects were non-myopic (+3.44 to −0.12 D), and six subjects were myopic (−1.00 to −2.38 D).
All subjects were compliant wearing the Actiwatch and did not take the device off at any time during the week. However, three of the watches underwent an error during the recording period; therefore, actigraph and light exposure data are reported for 15 subjects. Subjects demonstrated regular sleep/wake patterns the week before the experiment, i.e. one sleep period per 24 h occurring during the night. Mean daily sleep duration was 8.39 ± 0.21 h, with a mean wake time of 6:41 am ± 11 min and mean sleep time of 9:30 pm ± 46 min. Objectively measured time spent outdoors per day (minutes exposed to >1000 lux) was 62.51 ± 9.59 min. Daily average white light exposure during the day was 831.58 ± 111.77 lux, and during the night was 0.50 ± 0.26 lux. Light exposure during the night was calculated as average lux during the time the Actiwatch detected that the subject was sleeping. Mean daily physical activity during wake periods was 485.26 ± 27.53 counts per minute.
Ocular rhythms
Repeated measures ANOVA for time-of-day showed that central corneal thickness, corneal power, vitreous chamber depth, and axial length exhibited significant diurnal variation over the 24 h measurement period (Table 1). Mean central corneal thickness was 541.13 ± 8.26 μm and exhibited a diurnal variation of 12.22 ± 1.39 μm with an acrophase during the early morning dark period at 4.27 h (p < 0.001). On the other hand, corneal power (mean 43.86 ± 0.38 D), was greatest in the afternoon at 13.92 h, with a diurnal variation of 0.20 ± 0.03 D (p = 0.005). Mean vitreous chamber depth was 15.16 ± 0.17 mm, with a diurnal variation of 0.086 ± 0.01 mm and acrophase 15.91 h (p = 0.002). Mean axial length was 23.25 ± 0.18 mm. Axial length demonstrated a diurnal variation of 0.045 ± 0.006 mm, with an acrophase in the afternoon at 12.92 h (p < 0.001). Tukey outlier analysis detected that two subjects exhibited extreme values (greater than 10 times the mean) for amplitude of anterior chamber depth and lens thickness diurnal variations. When these two subjects were removed from analysis, the lens thickness exhibited diurnal variation of 0.041 ± 0.006 mm with an acrophase at 0.80 h (p < 0.001), and variations in anterior chamber depth were not significant (p = 0.36). The timing of the minimum vitreous chamber depth corresponded approximately to the timing of the thickest lens and choroid.
Table 1.
Group mean (± standard error), amplitude of diurnal variation, and acrophase for each ocular parameter for all subjects (n = 18); F statistic and p values are reported for repeated measures ANOVA for time-of-day
| Parameter | Group mean | Amplitude | Acrophase | F statistic | p value |
|---|---|---|---|---|---|
| Central corneal thickness (μm) | 541.13 ± 8.26 | 12.22 ± 1.39 | 4.27 ± 0.38 h | 27.15 | <0.001* |
| Corneal power (D) | 43.86 ± 0.38 | 0.20 ± 0.03 | 13.92 ± 0.80 h | 3.63 | 0.005* |
| Anterior chamber depth (mm) | 3.77 ± 0.06 | 0.041 ± 0.005 | 9.43 ± 1.09 h | 0.46 | 0.36† |
| Lens thickness (mm) | 3.55 ± 0.04 | 0.041 ± 0.04 | 0.80 ± 0.64 h | 5.93 | <0.001*,† |
| Vitreous chamber depth (mm) | 15.16 ± 0.17 | 0.086 ± 0.01 | 15.91 ± 3.98 h | 4.26 | 0.002* |
| Axial length (mm) | 23.25 ± 0.18 | 0.045 ± 0.006 | 12.91 ± 0.64 h | 13.51 | <0.001* |
| Retinal thickness (central 1 mm, μm) | 277.49 ± 4.52 | 4.09 ± 0.39 | 15.04 ± 0.53 h | 15.84 | <0.001* |
| RPE + outer segment thickness (μm) | 59.04 ± 0.47 | 1.69 ± 0.18 | 3.25 ± 0.37 h | 18.60 | <0.001* |
| Inner segment thickness (μm) | 28.15 ± 0.37 | 2.62 ± 0.15 | 14.95 ± 0.29 h | 57.36 | <0.001* |
| Choroidal thickness (central 1 mm, μm) | 355.65 ± 13.17 | 26.25 ± 2.67 | 1.90 ± 0.42 h | 23.98 | <0.001* |
| Intraocular pressure (mmHg) | 12.95 ± 0.52 | 4.19 ± 0.50 | 11.37 ± 0.57 h | 17.95 | <0.001* |
| Mean ocular perfusion pressure (mmHg) | 36.24 ± 1.27 | 7.92 ± 0.89 | 23.60 ± 0.58 h | 16.78 | <0.001* |
Significance at p < 0.05.
Two outliers not included.
For the central 1 mm diameter centred on the fovea, total retinal thickness, RPE + outer segment thickness, and inner segment thickness demonstrated significant diurnal variations (p < 0.001 for all). Cosinor analysis for these parameters is shown in Figure 3. Mean total retinal thickness was 277.49 ± 4.52 μm, with a diurnal variation of 4.09 ± 0.39 μm and acrophase at 15.04 h. Mean RPE + outer segment thickness was 59.04 ± 0.47 μm, with a diurnal variation of 1.69 ± 0.18 μm and acrophase at 3.25 h. Mean inner segment thickness was 28.15 ± 0.37 μm, with a diurnal variation of 2.62 ± 0.15 μm and acrophase at 14.95 h.
Figure 3.
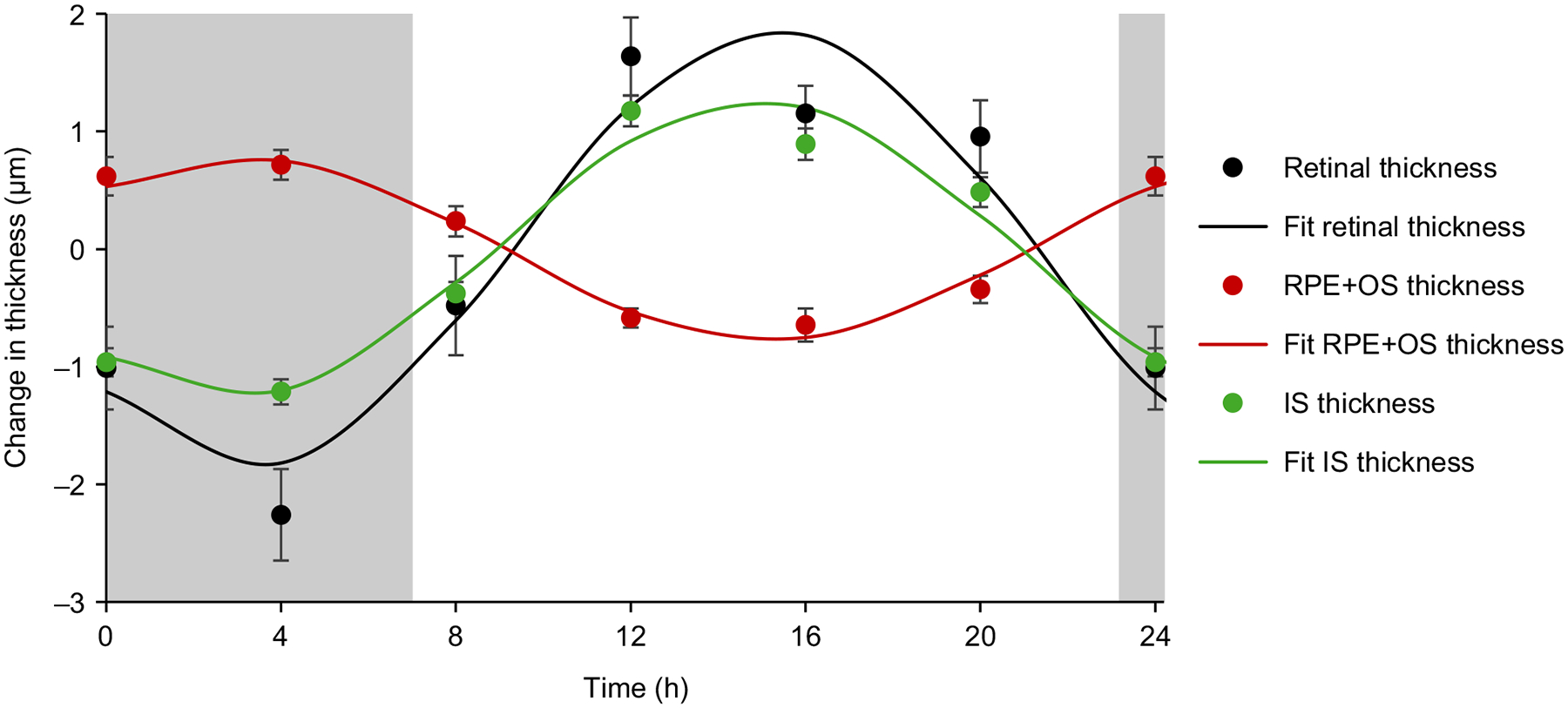
Mean (± standard error) 24 h change in total retinal thickness (μm, black), retinal pigment epithelium + outer segment thickness (RPE+OS μm, red), and inner segment thickness (IS μm, green) for all subjects (n = 18); solid lines are cosinor fits to the data; grey areas represent the dark period.
Mean choroidal thickness over the central 1 mm diameter centred on the fovea was 355.65 ± 13.17 μm. Choroidal thickness demonstrated a significant diurnal variation of 26.25 ± 2.67 μm with an acrophase at 1.90 h (p < 0.001).
Mean intraocular pressure was 12.95 ± 0.52 mmHg, and demonstrated a significant diurnal variation of 4.19 ± 0.50 mmHg with an acrophase at 11.37 h (p < 0.001). Mean MOPP was 36.24 ± 1.27 mmHg, with a diurnal variation of 7.92 ± 0.89 mmHg and acrophase at 23.60 h (p < 0.001). Cosinor analysis showing the relationship between diurnal variations in axial length, choroidal thickness, IOP, and MOPP is shown in Figure 4. Axial length acrophase occurred 1.54 h after IOP acrophase, and 11.01 h after choroidal thickness acrophase. IOP and MOPP were in antiphase with each other, with acrophases 11.77 h apart.
Figure 4.
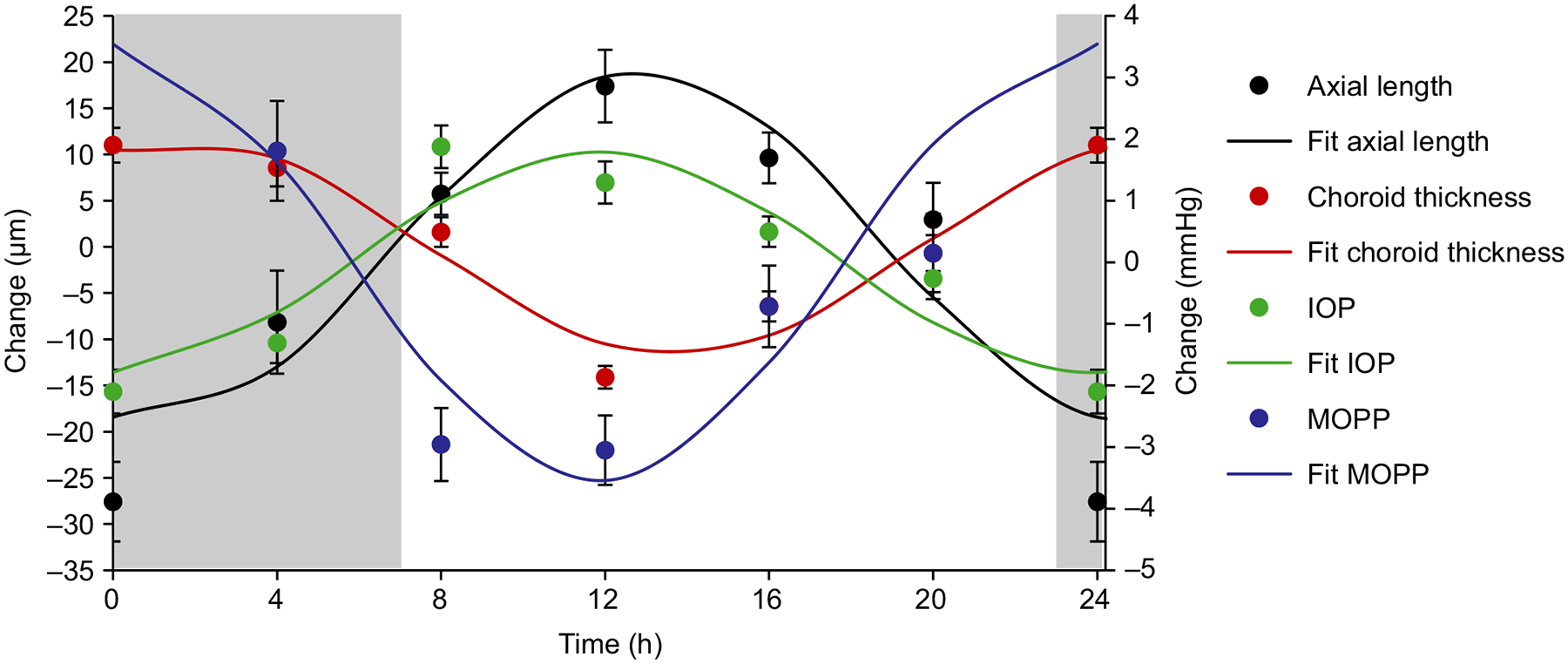
Mean (± standard error) 24 h change in axial length (μm, black), choroidal thickness (μm, red), intraocular pressure (IOP mmHg, green), and mean ocular perfusion pressure (MOPP mmHg, blue) for all subjects (n = 18); solid lines are cosinor fits to the data; grey areas represent the dark period.
Systemic rhythms
Body temperature, heart rate, and mean arterial pressure all demonstrated significant diurnal variations (p ≤ 0.002 for all, Table 2). Mean body temperature was 37.12 ± 0.06°C, with a diurnal variation of 1.07 ± 0.10°C and acrophase minute, with a diurnal variation of 12.53 ± 1.46 beats per minute and acrophase at 19.53 h. Mean arterial pressure was 83.43 ± 0.88 mmHg, with a diurnal variation of 7.17 ± 1.07 mmHg and acrophase at 23.55 h.
Table 2.
Group mean (± standard error), amplitude of diurnal variation and acrophase for each systemic parameter for all subjects (n = 18); F statistic and p values are reported for repeated measures ANOVA for time-of-day
| Parameter | Group mean | Amplitude | Acrophase | F statistic | p value |
|---|---|---|---|---|---|
| Body temperature (°C) | 37.12 ± 0.06 | 1.07 ± 0.10 | 14.65 ± 0.45 | 14.96 | <0.001* |
| Heart rate (bpm) | 88.03 ± 2.38 | 12.53 ± 1.46 | 19.53 ± 0.87 | 4.25 | 0.002* |
| Mean arterial pressure (mmHg) | 83.43 ± 0.88 | 7.17 ± 1.07 | 23.55 ± 0.74 | 5.69 | <0.001* |
| Melatonin (pg mL−1)† | 12.55 ± 1.29 | 29.40 ± 3.15 | 2.36 ± 0.28 | 40.43 | <0.001* |
| Cortisol (μg dL−1)‡ | 0.15 ± 0.03 | 0.26 ± 0.05 | 9.22 ± 0.34 | 15.38 | <0.001* |
Significance at p < 0.05.
n = 16.
n = 17.
One subject (age 7 years) was unable to provide a sufficient saliva sample at the 12:00 am time point; therefore, salivary melatonin and cortisol analyses include only 17 subjects. Additionally, one subject was identified as an extreme outlier for melatonin concentration and was excluded. Melatonin and cortisol concentrations demonstrated significant diurnal variations (p < 0.001 for both, Figure 5). Melatonin maintained a low concentration during the lights on period, and increased during the lights off period. The mean amplitude of melatonin diurnal variation was 29.40 ± 3.15 pg mL−1, with an acrophase at 2.36 h. Cortisol peaked in the morning after lights on, with a mean amplitude of variation of 0.26 ± 0.05 μg dL−1 and an acrophase at 9.22 h.
Figure 5.
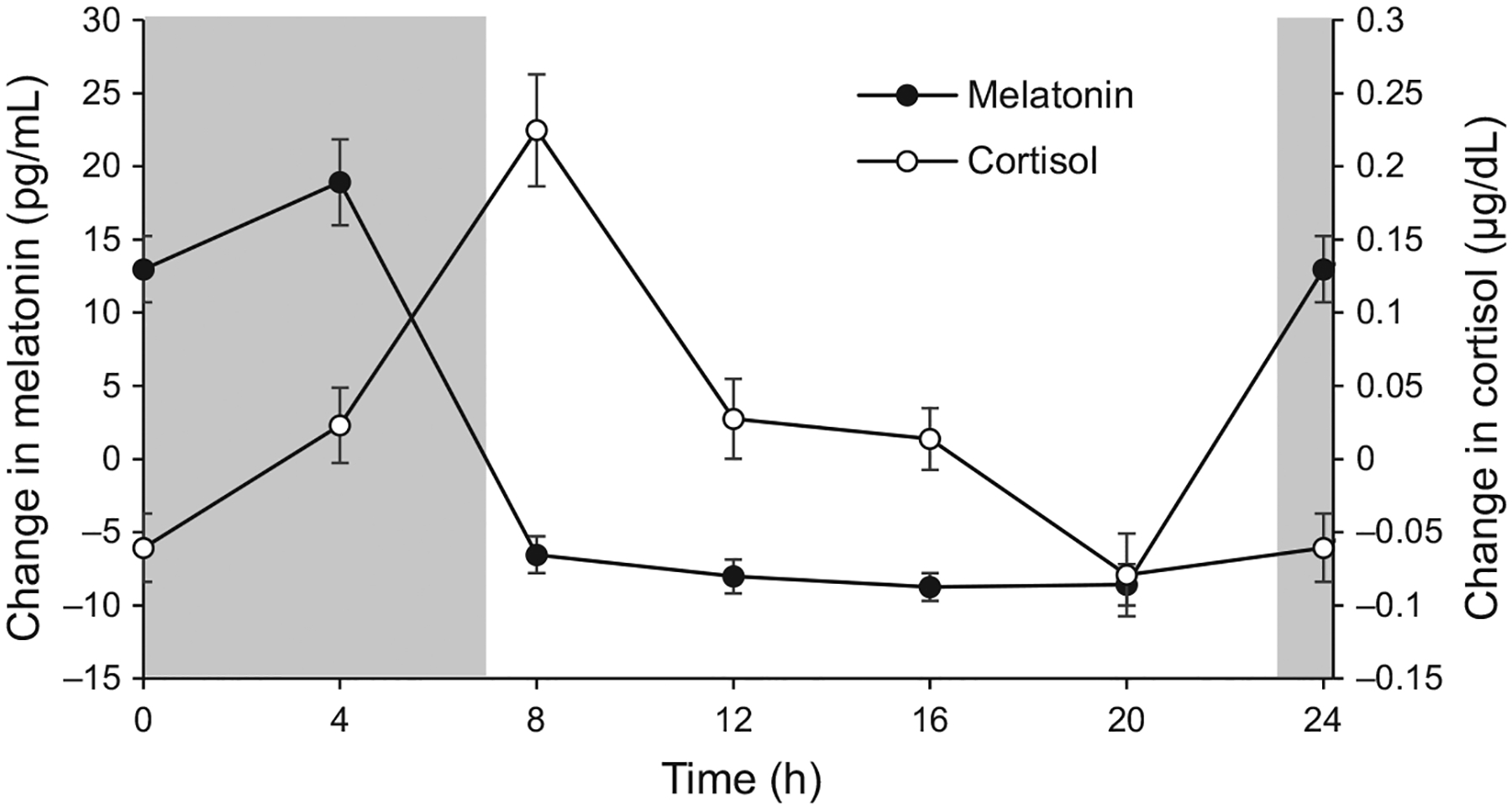
Mean (± standard error) change in melatonin concentration (filled symbols) and cortisol concentration (open symbols) across 24 h for all subjects (melatonin n = 16, cortisol n = 17); grey areas represent the dark period.
Discussion
This study shows that children demonstrate ocular and systemic diurnal rhythms similar to those previously reported in adult populations. Corneal thickness and power, axial length, retinal thickness, choroidal thickness, intraocular pressure, and mean ocular perfusion pressure undergo robust diurnal variations in children over a 24 h period. Diurnal variations in body temperature and melatonin and cortisol concentrations reported here are consistent with previous studies.46–49
Axial length diurnal variations observed here in children are comparable to previous reports in adults. Studies in adults have found amplitudes of diurnal variations of 19–46 lm, with acrophases in the range of 11–13 h.7,10,16 Our findings show that children’s axial length underwent significant diurnal variation of 45.24 ± 0.06 μm, with an acrophase at 12.92 h. Stone, et al., assessed diurnal variations in axial length over 24 h in subjects ages 7 to 53 years old.13 In a re-analysis of their data including only the children (n = 10, ages 7–17 years), six of ten showed significant diurnal variation in axial length, with a mean amplitude of approximately 29 μm, and acrophase at 12.28 h.
In this study, subjects showed a mean choroidal thickness of 355.65 ± 13.17 μm, with a diurnal variation of 26.25 ± 2.67 μm that peaked at 1.90 h. Previous studies examining a pediatric population have shown that the mean subfoveal choroidal thickness ranges from 227 to 359 μm, with thinner choroids in myopic compared to non-myopic children.50–53 The amplitude and acrophase of choroidal thickness variation found here is similar to previous reports in adults, with variations of 26–34 lm, with acrophases ranging from 23.5 to 3.0 h.10,16–18,54,55
Children’s axial length was greatest around noon, then decreased throughout the evening and night. The choroid was thickest during the night, approximately 11 h earlier than axial length acrophase, making the two rhythms in approximate antiphase. In adult subjects, we previously observed a similar pattern, with axial length and choroidal thickness rhythms being out of phase by approximately 10.5 h.10 The relationship between axial length and choroidal thickness diurnal rhythms may play an important role in emmetropization and myopia development and progression.37 Nickla showed that normal chicks exhibit diurnal oscillations in axial length and choroidal thickness that are out of phase.12,14,21 Specifically, axial length and choroidal thickness rhythms in normal emmetropizing chick eyes were out of phase by approximately 9 h. In chick eyes that were growing faster and developing myopia, axial length and choroidal thickness rhythms shifted to 12 h out of phase.12 In chick eyes that were undergoing decreased eye growth rates from prior form deprivation myopia or by wearing of positive spectacle lenses, the rhythms shifted so that axial length and choroidal thickness were in phase with each other.12 In our subject population, children were likely past the emmetropization phase, and at an age when childhood myopia typically onsets and progresses.23 We only measured refraction on one occasion; therefore, we were unable to determine if subjects were undergoing active myopic axial elongation. Future studies examining rhythms in children who are emmetropes versus progressing myopes may help to clarify the relationship between axial length and choroidal thickness rhythms in human eye growth.
Similar to findings in adults,10 we found that the total retinal thickness, in the 1 mm diameter centred on the fovea, underwent diurnal changes of about 4 lm. The RPE + photoreceptor outer segments showed a diurnal variation of approximately 1.7 μm with an acrophase at 3.25 h, and the photoreceptor inner segments showed a diurnal variation of approximately 2.6 μm with an acrophase at 14.95 h. This antiphase relationship could represent differing functions of the inner and outer segments, with the inner segment containing the mitochondria and other organelles of the cell, and the outer segments containing membranous discs. We speculate that the observed diurnal rhythm in the RPE + photoreceptor outer segments was a function of cone outer segment membranous disc shedding and renewal.56 In the 1 mm diameter surrounding the fovea, the primary photoreceptor types are medium and long wavelength cones.57 Disc shedding has been shown to be rhythmically controlled by an intrinsic circadian oscillator that uses endogenous dopamine and melatonin as its light and dark signal, respectively,58 which likely contributed to the observed diurnal variations in the outer retina. Cone outer segment shedding is known to largely occur at night, following light offset, which is in phase with melatonin rhythms.59
Our findings indicate that children’s anterior segments also undergo diurnal biometric variations. We found that the cornea was thickest in the early morning during the dark period, and corneal power was greatest in the afternoon, similar to previous findings in adult populations.6,60 Thickening of the cornea during the night is likely a result of enema caused by a reduced oxygen supply in a closed-eye environment.61 Decreased corneal power in the night and early morning may be due to flattening of the cornea by the closed eyelid. Lens thickness also demonstrated significant diurnal variation, following removal of two subjects that exhibited lens thickness changes that were identified as outliers, while variations in anterior chamber depth did not reach statistical significance. Previous studies in adults have been conflicting with respect to diurnal anterior chamber depth and lens thickness rhythms, with some showing significant variations,7 and others showing no diurnal change.10 It is possible that children did not show significant diurnal variations in anterior chamber depth because younger lenses exhibit greater accommodative range,62 and therefore greater variability in anterior segment measures.
As discussed, several optical components of the eye demonstrated diurnal variation, including corneal power, lens thickness, and axial length, which could potentially influence refraction and visual quality throughout the day. However, taken together, it is unlikely that these factors would result in a clinically significant change in vision. For example, axial length is greatest in the morning by approximately 45 μm, equivalent to an increase in power of approximately 0.1 D, while corneal power is less in the morning (and greatest in the afternoon), by about 0.2 D. Given the depth of focus of the human eye of about 0.3–0.4 D,63 large accommodative amplitude of children which can compensate for defocus,64 and the rhythms of axial length and corneal power being in approximate antiphase, diurnal variations in optical components would not be expected to impact vision.
Intraocular pressure was highest late in the morning and decreased throughout the day and into the night, consistent with previous studies.29,30 Aqueous production is known to decrease at nighttime, which may contribute to a lower IOP.65 Intraocular pressure is also known to be influenced by posture, with higher IOP observed when the body is in a supine position due to increased episcleral venous pressure.66,67 When diurnal IOP is measured in a supine position, IOP is highest during the night with acrophases ranging from 2 to 7.5 h.68,69 Here, all measurements were recorded with subjects in an upright position, and the IOP acrophase was observed at 11.37 h, approximately in phase with axial length rhythms. It is likely that nighttime IOP would have been higher if subjects were in a supine position for the measurement, which would then shift the acrophase to an earlier hour. A previous study noted that IOP diurnal variations are observable for both upright and supine positions, with no significant differences in rhythm based on body position.70 However, future studies should consider capturing nighttime IOP in a supine position to understand natural nocturnal IOP. Speculation exists whether axial length rhythms are a result of rhythms in IOP.71,72 It would be informative to understand if a shift in IOP acrophase in a supine position would dissociate IOP rhythms from axial length rhythms. While evidence suggests that diurnal increases in IOP are not causative for axial length increases, dissociating the rhythms would further prove that axial length rhythms are not a passive, biomechanical consequence of IOP rhythms.
We were interested in measuring body temperature, melatonin, and cortisol concentrations because they are well known markers of systemic circadian rhythms.47,73 Additionally, recent investigations have suggested they may be altered with myopia.10,74 Therefore, establishing these rhythms with respect to ocular rhythms in children, when myopia typically onsets, is important for future studies. Melatonin is a neurohormone synthesised and secreted from the pineal gland in darkness, mediated by input from intrinsically photosensitive retinal ganglion cells and regulated by the suprachiasmatic nucleus.75 Cortisol is a glucocorticoid secreted from the adrenal gland and plays a role in the stress response.48 As expected, body temperature was highest in the afternoon, melatonin peaked during the dark period, and cortisol peaked in the morning, just after the end of the dark period, similar to previous studies in humans.48,49
In conclusion, we have demonstrated that significant diurnal variations in multiple ocular and systemic parameters are observable over a 24 h period in children. Axial length and choroidal thickness rhythms were in approximate antiphase with each other. With increasing evidence that ocular diurnal rhythms play an important role in emmetropization and myopia development,22 these findings may have important implications for eye growth in children.
Acknowledgements
This study was funded by the National Eye Institute, National Institutes of Health (NIH R01EY030193–01).
Footnotes
Conflict of interest
The authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article.
References
- 1.Aschoff J Circadian rhythms: influences of internal and external factors on the period measured in constant conditions. Z Tierpsychol 1979; 49: 225–249. [DOI] [PubMed] [Google Scholar]
- 2.Dacey DM, Liao HW, Peterson BB et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005; 433: 749–754. [DOI] [PubMed] [Google Scholar]
- 3.Gillette MU & Tischkau SA. Suprachiasmatic nucleus: the brain’s circadian clock. Recent Prog Horm Res 1999; 54: 33–58. [PubMed] [Google Scholar]
- 4.Spaide RF, Koizumi H & Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 2008; 146: 496–500. [DOI] [PubMed] [Google Scholar]
- 5.Harper CL, Boulton ME, Bennett D et al. Diurnal variations in human corneal thickness. Br J Ophthalmol 1996; 80: 1068–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Read SA, Collins MJ & Carney LG. The diurnal variation of corneal topography and aberrations. Cornea 2005; 24: 678–687. [DOI] [PubMed] [Google Scholar]
- 7.Read SA, Collins MJ & Iskander DR. Diurnal variation of axial length, intraocular pressure, and anterior eye biometrics. Invest Ophthalmol Vis Sci 2008; 49: 2911–2918. [DOI] [PubMed] [Google Scholar]
- 8.Wilson LB, Quinn GE, Ying GS et al. The relation of axial length and intraocular pressure fluctuations in human eyes. Invest Ophthalmol Vis Sci 2006; 47: 1778–1784. [DOI] [PubMed] [Google Scholar]
- 9.Ashraf H & Nowroozzadeh MH. Diurnal variation of retinal thickness in healthy subjects. Optom Vis Sci 2014; 91: 615–623. [DOI] [PubMed] [Google Scholar]
- 10.Burfield HJ, Patel NB & Ostrin LA. Ocular biometric diurnal rhythms in emmetropic and myopic adults. Invest Oph- thalmol Vis Sci 2018; 59: 5176–5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss S & Schaeffel F. Diurnal growth rhythms in the chicken eye: relation to myopia development and retinal dopamine levels. J Comp Physiol A 1993; 172: 263–270. [DOI] [PubMed] [Google Scholar]
- 12.Nickla DL, Wildsoet C & Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res 1998; 66: 163–181. [DOI] [PubMed] [Google Scholar]
- 13.Stone RA, Quinn GE, Francis EL et al. Diurnal axial length fluctuations in human eyes. Invest Ophthalmol Vis Sci 2004; 45: 63–70. [DOI] [PubMed] [Google Scholar]
- 14.Papastergiou GI, Schmid GF, Riva CE, Mendel MJ, Stone RA & Laties AM. Ocular axial length and choroidal thickness in newly hatched chicks and one-year-old chickens fluctuate in a diurnal pattern that is influenced by visual experience and intraocular pressure changes. Exp Eye Res 1998; 66: 195–205. [DOI] [PubMed] [Google Scholar]
- 15.Nickla DL, Wildsoet CF & Troilo D. Diurnal rhythms in intraocular pressure, axial length, and choroidal thickness in a primate model of eye growth, the common marmoset. Invest Ophthalmol Vis Sci 2002; 43: 2519–2528. [PubMed] [Google Scholar]
- 16.Chakraborty R, Read SA & Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci 2011; 52: 5121–5129. [DOI] [PubMed] [Google Scholar]
- 17.Seidel G, Hausberger S, Herzog SA et al. Circadian macular volume changes in the healthy human choroid. Am J Ophthalmol 2015; 159: 365–371. [DOI] [PubMed] [Google Scholar]
- 18.Tan CS, Ouyang Y, Ruiz H & Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 2012; 53: 261–266. [DOI] [PubMed] [Google Scholar]
- 19.Brown JS, Flitcroft DI, Ying GS et al. In vivo human choroidal thickness measurements: evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci 2009; 50: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summers JA. The choroid as a sclera growth regulator. Exp Eye Res 2013; 114: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nickla DL. The phase relationships between the diurnal rhythms in axial length and choroidal thickness and the association with ocular growth rate in chicks. J Comp Physiol A 2006; 192: 399–407. [DOI] [PubMed] [Google Scholar]
- 22.Chakraborty R, Ostrin LA, Nickla DL, Iuvone PM, Pardue MT & Stone RA. Circadian rhythms, refractive development, and myopia. Ophthalmic Physiol Opt 2018; 38: 217–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goss DA & Winkler RL. Progression of myopia in youth: age of cessation. Am J Optom Physiol Opt 1983; 60: 651–658. [DOI] [PubMed] [Google Scholar]
- 24.Tojo N, Abe S, Ishida M, Yagou T & Hayashi A. The fluctuation of intraocular pressure measured by a contact lens sensor in normal-tension glaucoma patients and nonglaucoma subjects. J Glaucoma 2017; 26: 195–200. [DOI] [PubMed] [Google Scholar]
- 25.Liu JH, Kripke DF, Twa MD et al. Twenty-four-hour pattern of intraocular pressure in young adults with moderate to severe myopia. Invest Ophthalmol Vis Sci 2002; 43: 2351–2355. [PubMed] [Google Scholar]
- 26.Liu JH, Gokhale PA, Loving RT, Kripke DF & Weinreb RN. Laboratory assessment of diurnal and nocturnal ocular perfusion pressures in humans. J Ocul Pharmacol Ther 2003; 19: 291–297. [DOI] [PubMed] [Google Scholar]
- 27.Dabasia PL, Lawrenson JG & Murdoch IE. Evaluation of a new rebound tonometer for self-measurement of intraocular pressure. Br J Ophthalmol 2016; 100: 1139–1143. [DOI] [PubMed] [Google Scholar]
- 28.Ittoop SM, SooHoo JR, Seibold LK, Mansouri K & Kahook MY. Systematic review of current devices for 24-h intraocular pressure monitoring. Adv Ther 2016; 33: 1679–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flemmons MS, Hsiao YC, Dzau J, Asrani S, Jones S & Freedman SF. Home tonometry for management of pediatric glaucoma. Am J Ophthalmol 2011; 152(470–478): e472. [DOI] [PubMed] [Google Scholar]
- 30.Hsiao YC, Dzau JR, Flemmons MS, Asrani S, Jones S & Freedman SF. Home assessment of diurnal intraocular pressure in healthy children using the Icare rebound tonometer. J AAPOS 2012; 16: 58–60. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi NG, Jones SK & Freedman SF. Icare ONE home tonometry in children with and without known glaucoma. J Glaucoma 2016; 25: e66–e69. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton KE, Pye DC, Aggarwala S, Evian S, Khosla J & Perera R. Diurnal variation of central corneal thickness and Goldmann applanation tonometry estimates of intraocular pressure. J Glaucoma 2007; 16: 29–35. [DOI] [PubMed] [Google Scholar]
- 33.Goldmann H & Schmidt T. Applanation tonometry. Ophthalmologica 1957; 134: 221–242. [DOI] [PubMed] [Google Scholar]
- 34.Liu J & Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg 2005; 31: 146–155. [DOI] [PubMed] [Google Scholar]
- 35.Del Aguila-Carrasco AJ, Dominguez-Vicent A, Perez-Vives C, Ferrer-Blasco T & Montes-Mico R. Assessment of modifications in thickness, curvatures, and volume upon the cornea caused by disposable soft contact lens wear. Eur J Ophthalmol 2015; 25: 385–390. [DOI] [PubMed] [Google Scholar]
- 36.Swarbrick HA, Wong G & O’Leary DJ. Corneal response to orthokeratology. Optom Vis Sci 1998; 75: 791–799. [DOI] [PubMed] [Google Scholar]
- 37.Nickla DL. Ocular diurnal rhythms and eye growth regulation: where we are 50 years after Lauber. Exp Eye Res 2013; 114: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitaya N, Ishiko S, Mori F et al. Diurnal variation of corneal autofluorescence in normal and diabetic eyes. Eye (Lond) 1998; 12: 934–937. [DOI] [PubMed] [Google Scholar]
- 39.Vancura P, Csicsely E, Leiser A, Iuvone PM & Spessert R. Rhythmic regulation of photoreceptor and RPE genes important for vision and genetically associated with severe retinal diseases. Invest Ophthalmol Vis Sci 2018; 59: 3789–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett AG & Rabbetts RB. The schematic eye In: Clinical Visual Optics, (Bennett AG & Rabbetts RB, editors), Butterworths: London, UK, 1989; pp. 249–274. [Google Scholar]
- 41.Patel NB, Wheat JL, Rodriguez A, Tran V & Harwerth RS. Agreement between retinal nerve fiber layer measures from Spectralis and Cirrus spectral domain OCT. Optom Vis Sci 2012; 89: E652–E666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel NB, Garcia B & Harwerth RS. Influence of anterior segment power on the scan path and RNFL thickness using SD-OCT. Invest Ophthalmol Vis Sci 2012; 53: 5788–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson W, Tong YL, Lee JK & Halberg F. Methods for cosinor-rhythmometry. Chronobiologia 1979; 6: 305–323. [PubMed] [Google Scholar]
- 44.James JF. A Student’s Guide to Fourier Transforms with Applications in Physics and Engineering, Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- 45.Batschelet E Circular Statistics in Biology, Academic Press: London, UK, 1981. [Google Scholar]
- 46.Touitou Y, Auzeby A, Camus F & Djeridane Y. Daily profiles of salivary and urinary melatonin and steroids in healthy prepubertal boys. J Pediatr Endocrinol Metab 2009; 22: 1009–1015. [DOI] [PubMed] [Google Scholar]
- 47.Hill SD, Wagner EA, Shedlarski JG Jr & Sears SP. Diurnal cortisol and temperature variation of normal and autistic children. Dev Psychobiol 1977; 10: 579–583. [DOI] [PubMed] [Google Scholar]
- 48.Edwards S, Evans P, Hucklebridge F & Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology 2001; 26: 613–622. [DOI] [PubMed] [Google Scholar]
- 49.Lewy AJ. The dim light melatonin onset, melatonin assays and biological rhythm research in humans. Biol Signals Recept 1999; 8: 79–83. [DOI] [PubMed] [Google Scholar]
- 50.Read SA, Collins MJ, Vincent SJ & Alonso-Caneiro D. Choroidal thickness in myopic and nonmyopic children assessed with enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci 2013; 54: 7578–7586. [DOI] [PubMed] [Google Scholar]
- 51.Fontaine M, Gaucher D, Sauer A & Speeg-Schatz C. Choroidal thickness and ametropia in children: a longitudinal study. Eur J Ophthalmol 2017. [DOI] [PubMed] [Google Scholar]
- 52.Jin P, Zou H, Zhu J et al. Choroidal and retinal thickness in children with different refractive status measured by swept-source optical coherence tomography. Am J Ophthalmol 2016; 168: 164–176. [DOI] [PubMed] [Google Scholar]
- 53.Park KA & Oh SY. Choroidal thickness in healthy children. Retina 2013; 33: 1971–1976. [DOI] [PubMed] [Google Scholar]
- 54.Zhao M, Yang XF, Jiao X et al. The diurnal variation pattern of choroidal thickness in macular region of young healthy female individuals using spectral domain optical coherence tomography. Int J Ophthalmol 2016; 9: 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Usui S, Ikuno Y, Akiba M et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci 2012; 53: 2300–2307. [DOI] [PubMed] [Google Scholar]
- 56.Anderson DH, Fisher SK & Steinberg RH. Mammalian cones: disc shedding, phagocytosis, and renewal. Invest Ophthalmol Vis Sci 1978; 17: 117–133. [PubMed] [Google Scholar]
- 57.Curcio CA, Allen KA, Sloan KR et al. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J Comp Neurol 1991; 312: 610–624. [DOI] [PubMed] [Google Scholar]
- 58.Tosini G, Baba K, Hwang CK & Iuvone PM. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp Eye Res 2012; 103: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LaVail MM. Circadian nature of rod outer segment disc shedding in the rat. Invest Ophthalmol Vis Sci 1980; 19: 407–411. [PubMed] [Google Scholar]
- 60.Reynolds DR & Poynter HL 3rd. Diurnal variation in central corneal curvature. Am J Optom Arch Am Acad Optom 1970; 47: 892–899. [DOI] [PubMed] [Google Scholar]
- 61.Mertz GW. Overnight swelling of the living human cornea. J Am Optom Assoc 1980; 51: 211–214. [PubMed] [Google Scholar]
- 62.Anderson HA, Glasser A, Manny RE & Stuebing KK. Age-related changes in accommodative dynamics from preschool to adulthood. Invest Ophthalmol Vis Sci 2010; 51: 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Atchison DA, Charman WN & Woods RL. Subjective depth-of-focus of the eye. Optom Vis Sci 1997; 74: 511–520. [DOI] [PubMed] [Google Scholar]
- 64.Duane A An attempt to determine the normal range of accommodation at various ages, being a revision of Donder’s experiments. Trans Am Ophthalmol Soc 1908; 11: 634–641. [PMC free article] [PubMed] [Google Scholar]
- 65.Koskela T & Brubaker RF. The nocturnal suppression of aqueous humor flow in humans is not blocked by bright light. Invest Ophthalmol Vis Sci 1991; 32: 2504–2506. [PubMed] [Google Scholar]
- 66.Ozcan MS, Praetel C, Bhatti MT, Gravenstein N, Mahla ME & Seubert CN. The effect of body inclination during prone positioning on intraocular pressure in awake volunteers: a comparison of two operating tables. Anesth Analg 2004; 99: 1152–1158. [DOI] [PubMed] [Google Scholar]
- 67.Chiquet C, Custaud MA, Le Traon AP, Millet C, Gharib C & Denis P. Changes in intraocular pressure during prolonged (7-day) head-down tilt bedrest. J Glaucoma 2003; 12: 204–208. [DOI] [PubMed] [Google Scholar]
- 68.Mottet B, Chiquet C, Aptel F et al. 24-hour intraocular pressure of young healthy humans in supine position: rhythm and reproducibility. Invest Ophthalmol Vis Sci 2012; 53: 8186–8191. [DOI] [PubMed] [Google Scholar]
- 69.Liu JH, Kripke DF, Hoffman RE et al. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci 1998; 39: 2707–2712. [PubMed] [Google Scholar]
- 70.Liu JH, Bouligny RP, Kripke DF & Weinreb RN. Nocturnal elevation of intraocular pressure is detectable in the sitting position. Invest Ophthalmol Vis Sci 2003; 44: 4439–4442. [DOI] [PubMed] [Google Scholar]
- 71.Cashwell LF & Martin CA. Axial length decrease accompanying successful glaucoma filtration surgery. Ophthalmology 1999; 106: 2307–2311. [DOI] [PubMed] [Google Scholar]
- 72.Leydolt C, Findl O & Drexler W. Effects of change in intraocular pressure on axial eye length and lens position. Eye (Lond) 2008; 22: 657–661. [DOI] [PubMed] [Google Scholar]
- 73.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L’Hermite-Baleriaux M & Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms 2005; 20: 178–188. [DOI] [PubMed] [Google Scholar]
- 74.Kearney S, O’Donoghue L, Pourshahidi LK, Cobice D & Saunders KJ. Myopes have significantly higher serum melatonin concentrations than non-myopes. Ophthalmic Physiol Opt 2017; 37: 557–567. [DOI] [PubMed] [Google Scholar]
- 75.Ostrin LA. Ocular and systemic melatonin and the influence of light exposure. Clin Exp Optom 2019; 102: 99–108. [DOI] [PubMed] [Google Scholar]


