Dilated cardiomyopathy (DCM) is a substrate for the development of heart failure and arrhythmias. Increasingly, genetic testing is being used to subclassify DCM and to better stratify arrhythmia risk in DCM. With expansion of genetic testing, especially in DCM, genetic mutation status can help better guide who may benefit from more tailored arrhythmia management (1). Furthermore, arrhythmogenic right ventricular cardiomyopathy (ARVC) is now appreciated as having risks for the left ventricle (2), and with this recognition, genetic variants which alter desmosomal proteins are seen as carrying information to help guide arrhythmia management, even in DCM.
Nearly 100 genes have been implicated in the development of genetic forms of DCM. The genes implicated in DCM encode proteins with a diversity of functions for the heart including cardiac contraction, attachment of the sarcomere to the membrane, matrix and ion channel function, as well as nuclear and nuclear membrane function (3). Most mutations linked to DCM are inherited in an autosomal dominant manner with markedly variable phenotypic expression affecting age of onset, progression of ventricular dysfunction, and accompanying arrhythmia risk. In a new report, Gigli et al. describe the long term outcomes of nearly 500 DCM patients followed from both a US and Italian center (4). Patients were screened for genetic mutations, and outcomes over a median follow-up of ten years were correlated with gene mutation status.
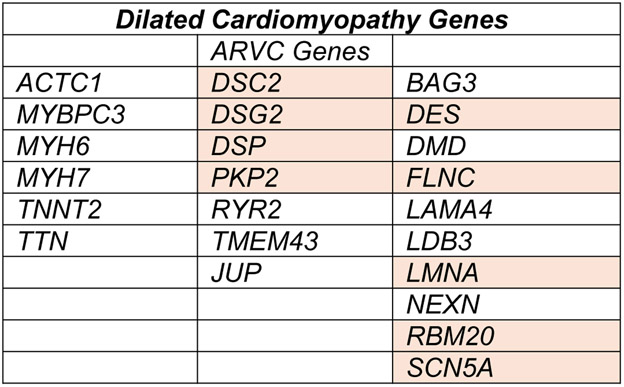
Although current gene panels are more comprehensive, this study focused its analysis on 23 DCM genes, including genes typically considered as ARVC genes (Figure 1). The cohort contained DCM patients with mutations in DSC2, DSG2, DSP, and PKP2, which encode the desmosome, a specialized cell-to-cell adhesion, that is especially important for right ventricular function. Although DCM subjects with ARVC genes were retained in this analysis, those meeting phenotypic diagnostic criteria for ARVC were excluded from the cohort. These findings indicate that desmosomal gene mutations are relevant for DCM and expand the number of genes that increase arrhythmia risk in DCM.
Figure 1.
DCM genes evaluated by Gigli et al. in a DCM cohort. Genes in the center column are also linked to ARVC. Genes with heightened ventricular arrhythmia risk in the setting of DCM are shown in orange.
The cohort had 487 DCM cases (429 unrelated subjects). From the entire cohort, 178 were identified as having a mutation (37%). TTN truncation variants accounted for over 30% of the gene-positive cohort. TTN encodes titin, a molecular ruler for the sarcomere. TTN truncation variants contribute to 10-30% of DCM, and on a population level associate with an increased risk for atrial fibrillation (5). The next most commonly mutated gene in the gene-positive cohort was TNNT2, and specifically 19 of 28 carried TNNT2 Arg173Trp, which was previously well characterized to segregate with disease (6). With the gene-positive group, LMNA (n=19), DSP (n=14), and FLNC (n=12) mutations were found. The remaining genes each had fewer than 10 mutations.
Clinical outcomes included death, heart failure, need for transplant, and major ventricular arrhythmias or sudden cardiac death. Consistent with prior reports, LMNA mutations had an unusually high risk for ventricular arrhythmias and sudden death (7,8). However, the desmosomal gene grouping, largely driven by the observations made in DSP-mutant patients, also emerged as having higher risk for ventricular arrhythmias and sudden death. DSP encodes desmoplakin, a large structural component of the desmosome. Population surveys of gene variation indicated the DSP gene is intolerant of loss of function mutations (https://gnomad.broadinstitute.org/gene/ENSG00000096696), and 11 of the 14 DSP mutations were premature truncations. Since these subjects were excluded for having ARVC, this study underscores the importance of DSP truncation variants as having risk for the left ventricle as well as significant ventricular arrhythmias.
In aggregate, mutation carriers fared worse, and outcomes did not specifically correlate with degree of left ventricular dysfunction, emphasizing primary arrhythmia mechanisms. These findings are similar to an early genetic study describing left dominant ARVC due to desmosomal mutations in which the propensity towards arrhythmia exceeded the degree of left ventricular dysfunction (9). The current study supports desmosomal variants as having arrhythmia risk comparable to LMNA mutations.
A recent study of RBM20 mutation carriers suggested that ventricular arrhythmias risk with RBM20 mutations was similar to LMNA mutations (10). RBM20 encodes an RNA binding protein implicated in splicing while LMNA specifies the nuclear membrane protein lamin A/C. LMNA mutations are recognized by international guidelines as requiring specific management (11,12). It is expected that newer guidelines will incorporate recommendations for using genetic information to tailor management of DCM arrhythmia risk, given available and emerging data (13,14).
The DANISH trial suggested prophylactic ICD placement in nonischemic DCM was not significantly better than medical therapy (15). However, a recent meta-analysis of six randomized trials supported a greater than 20% reduction in all-cause mortality in nearly 3000 patients with nonischemic DCM with ICDs compared to those with only optimal medical therapy (16). Together, these studies support the need for more nuanced decision analysis for nonischemic DCM arrhythmia management. Employing ventricular dysfunction as the principal prognostic factor for ventricular arrhythmias and sudden cardiac death in nonischemic DCM appears to be inadequate. With an expanded era of genotype-phenotype studies, gene mutation data is one means of improving the precision of the device implant decision (17), and specific genes and mutations are now emerging with sufficient support for inclusion in these decisions (18).
There are several limitations of the current study. Only 23 genes were considered in the analysis, and current gene panels typically now have many more genes. Additionally, the study was biased towards those of Caucasian ancestry derived from relatively narrow geographic areas. This latter limitation likely accounts for the number of TNNT2 mutations observed (> 15% of the gene-positive cohort). Larger multi-site studies should improve on these observations and potentially extend the conclusions to encompass more genes and additional types of genetic variation.
Acknowledgments
Support: NIH HL134633, NIH HL128075, NIH U01HL131914, AHA,
Footnotes
COI: EMM serves as a consultant to Invitae, Tenaya Therapeutics, Exonics, and is the founder of Ikaika Therapeutics. These activities are unrelated to the content of this work. APA has no conflicts of interest to disclose.
References
- 1.Wilcox JE, Hershberger RE. Genetic cardiomyopathies. Curr Opin Cardiol 2018;33:354–362. [DOI] [PubMed] [Google Scholar]
- 2.Elliott PM, Anastasakis A, Asimaki A et al. Definition and treatment of arrhythmogenic cardiomyopathy: an updated expert panel report. Eur J Heart Fail 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNally EM, Mestroni L. Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ Res 2017;121:731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gigli M, Merlo M, Graw S et al. Genetic Risks for Arrhythmia Phenotypes in Dilated Cardiomyopathy. J Am Coll Cardiol 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi SH, Weng LC, Roselli C et al. Association Between Titin Loss-of-Function Variants and Early-Onset Atrial Fibrillation. Jama 2018;320:2354–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merlo M, Sinagra G, Carniel E et al. Poor prognosis of rare sarcomeric gene variants in patients with dilated cardiomyopathy. Clin Transl Sci 2013;6:424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Captur G, Arbustini E, Bonne G et al. Lamin and the heart. Heart 2018;104:468–479. [DOI] [PubMed] [Google Scholar]
- 8.Hershberger RE, Morales A. LMNA-Related Dilated Cardiomyopathy In: Adam MP, Ardinger HH, Pagon RA, et al. , editors. GeneReviews((R)). Seattle (WA): University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle; All rights reserved., 1993. [Google Scholar]
- 9.Sen-Chowdhry S, Syrris P, Prasad SK et al. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol 2008;52:2175–87. [DOI] [PubMed] [Google Scholar]
- 10.Parikh VN, Caleshu C, Reuter C et al. Regional Variation in RBM20 Causes a Highly Penetrant Arrhythmogenic Cardiomyopathy. Circ Heart Fail 2019;12:e005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Priori SG, Wilde AA, Horie M et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013;10:1932–63. [DOI] [PubMed] [Google Scholar]
- 12.Priori SG, Blomstrom-Lundqvist C, Mazzanti A et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 13.Schultheiss HP, Fairweather D, Caforio ALP et al. Dilated cardiomyopathy. Nat Rev Dis Primers 2019;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin KM, Trembley MA, Chandler SF et al. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat Rev Cardiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kober L, Thune JJ, Nielsen JC et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med 2016;375:1221–30. [DOI] [PubMed] [Google Scholar]
- 16.Golwala H, Bajaj NS, Arora G, Arora P. Implantable Cardioverter-Defibrillator for Nonischemic Cardiomyopathy: An Updated Meta-Analysis. Circulation 2017;135:201–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halliday BP, Cleland JGF, Goldberger JJ, Prasad SK. Personalizing Risk Stratification for Sudden Death in Dilated Cardiomyopathy: The Past, Present, and Future. Circulation 2017;136:215–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters S, Kumar S, Elliott P, Kalman JM, Fatkin D. Arrhythmic Genotypes in Familial Dilated Cardiomyopathy: Implications for Genetic Testing and Clinical Management. Heart Lung Circ 2019;28:31–38. [DOI] [PubMed] [Google Scholar]