To the Editor:
Itch is an unpleasant cutaneous sensation that elicits a desire to scratch or scratch reflex. Itch generally serves as a warning and a self-protection mechanism against harmful external agents. However, chronic itch caused by multiple dermatological or systemic diseases (eg, atopic dermatitis) has strongly deleterious effects on the quality of life of millions of people worldwide.1
Sensory information from the peripheral endings of primary afferent sensory neurons in the skin is conveyed through these neurons to the spinal dorsal horn (SDH), where it activates brain-projecting neurons via neural circuits. Neuronal pathways from the periphery to the brain selective for itch are still poorly understood, but recent studies have identified neuronal populations for itch at the levels of primary afferents and the SDH.2 Han et al3 showed that deleting a subpopulation of primary afferent sensory neurons that express Mas-related G protein–coupled receptor A3 (MrgprA3) led to a selective deficit in behaviors related to itch but not to pain, indicating that MrgprA3+ primary sensory neurons are essential for itch sensation. However, little is known about endogenously expressed molecules that control the activity of this population. Because MrgprA3+ neurons are also necessary for chronic itch,3 the identification of such molecules controlling MrgprA3+ neurons will help our understanding of the mechanism for acute and chronic itch and also provide a new therapeutic strategy for chronic itch.
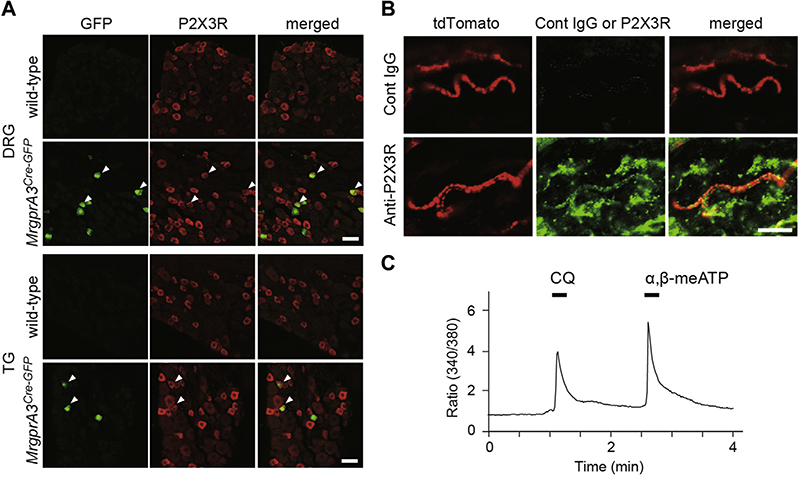
In this study, we focused on P2X3 purinoceptors (P2X3Rs), a subtype of ATP-gated cation channels, because immunoreactivity for P2X3Rs has previously been detected in small-diameter dorsal root ganglion (DRG) and trigeminal (TG) neurons, including the MrgprA3+ population.3 However, it remains unknown whether P2X3Rs are functional in the MrgprA3+ population and, more importantly, whether they play a role in itch sensation. Consistent with previous observations in lumbar DRG neurons,3 we observed P2X3R immunofluorescence in cervical DRG neurons positive for green fluorescent protein (GFP) in Mrgpra3GFP-Cre mice (Fig 1, A) in which GFP is expressed in the MrgprA3+ neuronal population.3 P2X3Rs were also observed in MrgprA3+ fibers (tdTomato+) in the skin of Mrgpra3GFP-Cre;ROSA26tdTomato mice (Fig 1, B), but P2X3R mRNA was extremely low in the skin (see Fig E1 in this article’s Online Repository at www.jacionline.org). Thus, P2X3Rs are expressed in MrgprA3+ nerve endings in the skin. We also found that 61.5% and 55.2% of chloroquine (an MrgprA3 agonist)-responded DRG and TG neurons, respectively, showed intracellular Ca2+ transients after application of α,β-methylene ATP (α,β-meATP), which activates P2X3Rs (Fig 1, C). These percentages were similar to those of DRG and TG neurons with double-immunoreactivity for P2X3R and GFP in Mrgpra3GFP-Cre mice (P2X3R+ DRG and TG neurons: 57.4% and 44.9% of total GFP+ neurons, respectively; see Fig E2 in this article’s Online Repository at www.jacionline.org). These results indicate that approximately half of the MrgprA3+ DRG and TG neurons expressed functional P2X3Rs.
FIG 1.
MrgprA3-positive sensory neurons express functional P2X3Rs. A, Immunofluorescence images of DRG/TG neurons of wild-type and MrgprA3GFP-Cre mice. The MrgprA3+ neurons (GFP+ cells, green) were counterstained for P2X3R immunoreactivity (red). Arrowheads indicate double-positive neurons. B, Immunofluorescence images of the skin of Mrgpra3GFP-Cre;ROSA26tdTomato mice. MrgprA3+ fibers (tdTomato+, red) were counterstained for P2X3R (bottom, green) or isotype control (Cont IgG, top, green). Scale bar, 20 μm. C, Representative intracellular Ca2+ responses to chloroquine (CQ) and α,β-meATP in DRG neurons.
FIG E1.
P2X3R mRNA expression in the skin, DRG, and TG. P2X3R mRNA in the skin, cervical DRG, or TG of C57BL/6J mice (n = 3). Values represent the relative ratio of P2X3R mRNA (normalized to GAPDH mRNA value) to the value of the DRG. P2X3R mRNA expression levels in the skin were very low compared with those in the DRG (<0.02%). GAPDH, Glyceraldehyde 3-phosphate dehydrogenase. Data are shown as means ± SEM.
FIG E2.
Expression profiles of P2X3R and MrgprA3+ neurons. The Venn diagram illustrating the expression profiles of DRG neurons positive for P2X3R, MrgprA3, and/or TRPV1 in MrgprA3Cre-GFP mice. The sizes of the circles represent the proportion of each neuronal population.
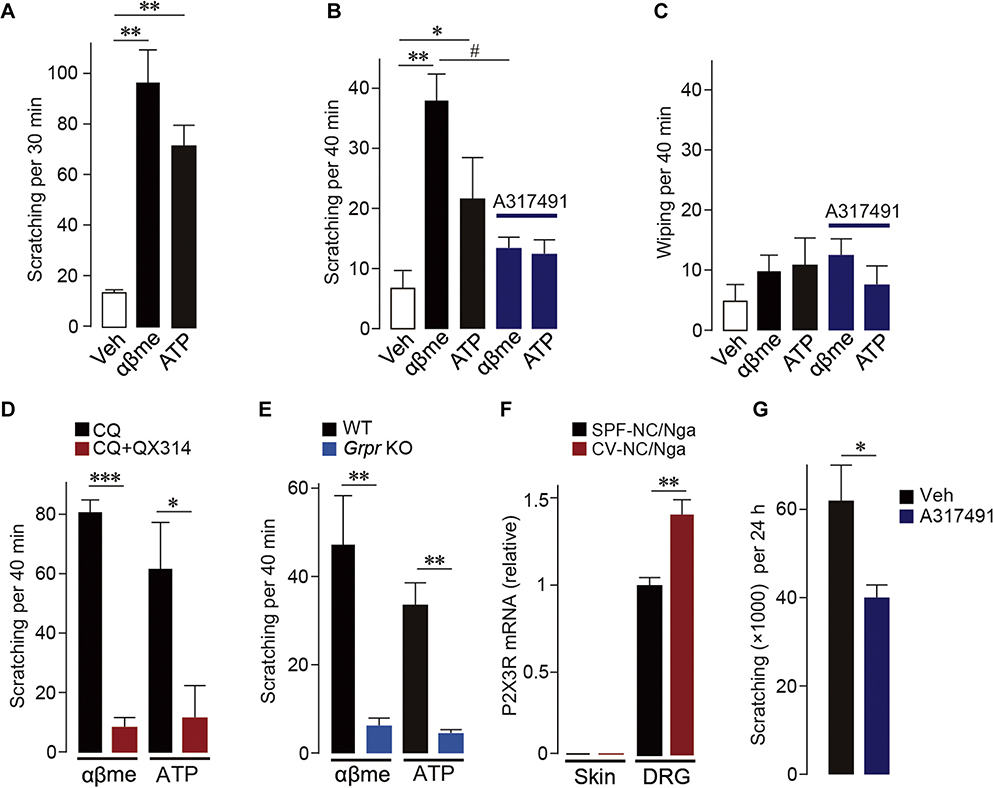
To assess the ability of P2X3R agonists to produce itch, we monitored scratching behavior after intradermal injection of ATP or α,β-meATP to the rostral back in mice. Scratching directed to the injection site was elicited by either ATP or α,β-meATP (Fig 2, A). Similarly, in the cheek model,4 an alternative behavioral assay for itch, these agonists when administered to the cheeks of mice increased scratching with the hindlimb (Fig 2, B; see Fig E3 in this article’s Online Repository at www.jacionline.org). The α,β-meATP–induced scratching was prevented by A317491, a P2X3R-selective antagonist (Fig 2, B; see Fig E4 in this article’s Online Repository at www.jacionline.org). By contrast, ATP and α,β-meATP did not induce wiping, a behavior that reflects pain-related responses in the cheek model (Fig 2, C). These results indicate that activation of P2X3Rs in the skin elicits scratching. To determine whether MrgprA3+ neurons mediate P2X3R-dependent scratching, we intradermally coadministered the lidocaine derivative QX-314 with chloroquine, a treatment that selectively silences MrgprA3+ sensory neurons.5 This treatment markedly suppressed scratching evoked by ATP and α,β-meATP (Fig 2, D). Chloroquine or QX-314 alone had no effect on the α,β-meATP–induced scratching (data not shown). These results indicate that activation of P2X3Rs expressed in MrgprA3+ sensory neurons induces scratching.
FIG 2.
Activation of P2X3Rs in MrgprA3-positive sensory neurons induces scratching behavior mediated via GRPR signaling. A, Scratching induced after intradermal injection of ATP and α,β-meATP (αβme) into the rostral back (n = 6). B and C, Scratching (Fig 2, B) or wiping (Fig 2, C) induced after cheek injection of ATP and α,β-meATP (αβme) with or without A317491, a selective antagonist for P2X3Rs (n = 6–10). D, Effect of coinjection of chloroquine (CQ) and QX-314 on ATP and α,β-meATP (αβme)-induced scratching (n = 5–6). E, Scratching evoked by ATP and α,β-meATP (αβme) in Grpr-knockout (KO) mice (n = 6–7). F, P2X3R mRNA in the skin or DRG of SPF (control, maintained in specific pathogen-free condition) or CV-NC/Nga mice (n = 4). G, Effect of intradermal injection of A317491 on scratching in CV-NC/Nga mice (n = 7–9). Veh, Vehicle; WT, Wild-type. Data are shown as means ± SEM. Mann-Whitney U test (Fig 2, A, E, and G), unpaired t test (Fig 2, D and F), and 1-way ANOVA followed by post hoc Tukey multiple comparisons test (Fig 2, B and C) were used for statistical analyses. *,#P < .05, **P < .01, ***P < .001.
FIG E3.
Dose-dependent increase in scratching induced by P2X3R agonists. Dose-responses for increase in scratching after cheek injection of ATP or α,β-meATP (n = 4–6). Veh, Vehicle. Data are shown as means ± SEM. *P < .05, unpaired t test; **P < .01, Mann-Whitney U test.
FIG E4.
Effect of A317491 on scratching caused by intradermal injection of α,β-meATP into the rostral back. α,β-meATP (αβme: 200 μg/50 μL) was intradermally injected into the rostral back with or without A317491 (A31: 200 μg/50 μL), a selective antagonist for P2X3Rs (n = 6). Scratching behavior was counted for 30 minutes after α,β-meATP injection. Data are shown as means ± SEM. *P < .05, unpaired t test.</_Caption>
Itch information received at the terminals of primary afferents in the skin is transmitted to the SDH. A recent study revealed that gastrin-releasing peptide receptors (GRPRs) are crucial for processing itch information in the SDH.6 We thus next examined whether GRPRs mediate P2X3R-derived scratching using Grpr-knockout mice. The ATP- and α,β-meATP–induced scratching seen in wild-type mice was markedly suppressed in Grpr-knockout mice (Fig 2, E), indicating that GRPRs are required for P2X3R-dependent scratching.
GRPR signaling in the SDH has been implicated not only in acute but also in chronic itch.7 We therefore predicted that P2X3Rs would also participate in chronic pathological itch using NC/Nga mice, a model of atopic dermatitis. These mice are known to develop spontaneous severe dermatitis and show repetitive scratching when maintained under conventional (CV) conditions, mimicking human atopic dermatitis.8 We found that P2X3R mRNA was increased in the DRG (but not skin) of CV-NC/Nga mice (Fig 2, F) and that compared with vehicle, intradermal treatment with A317491 decreased scratching in CV-NC/Nga mice (Fig 2, G) without affecting skin inflammation (see Fig E5 in this article’s Online Repository at www.jacionline.org), suggesting that P2X3Rs play a role in chronic scratching.
FIG E5.
Effect of A317491 on skin inflammation of NC/Nga mice. A, Photographs of hematoxylin-eosin–stained deparaffinized sections of the rostral back skin taken from SPF (control) and CV (chronic scratching)-NC/Nga mice 24 hours after intradermal administration of vehicle (Veh) or A317491 (A31). Scale bar, 50 μm. B, Number of inflammatory cells in the skin of SPF, CV (Veh), and CV (A31)-NC/Nga mice (n = 3). C, Epidermal thickness of SPF, CV (Veh), and CV (A31)-NC/Nga mice (n = 3). SPF, Specific pathogen free. Data are shown as means ± SEM.
The present findings demonstrate that activation of P2X3Rs at the nerve endings of MrgprA3+ primary afferent sensory neurons in the skin produces scratching behavior through GRPR signaling in the SDH. Therefore, it is possible that P2X3Rs are important regulators of MrgprA3+ sensory neurons linked to itch. Reduction of repetitive scratching in atopic dermatitis by pharmacological blockade of P2X3Rs implies that chronic itch related to atopic dermatitis involves ongoing signaling via P2X3Rs, likely activated by extracellular ATP that may be released from mechanically stimulated keratinocytes or from damaged skin tissues.9 In inflammatory skin diseases including atopic dermatitis, skin lesions induced by scratching can lead to more severe itch and further scratching (the itch-scratch vicious cycle). It is thus conceivable that ATP-P2X3R signaling may be involved in this cycle in a pathway via MrgprA3+ sensory neurons and spinal GRPRs, which are also implicated in chronic itch.3,7 Thus, our findings provide the possibility that targeting P2X3Rs may be a novel strategy for treating chronic itch (for additional discussion including the role of P2X3Rs in MrgprA3− neurons, see this article’s Online Repository at www.jacionline.org).
METHODS
Animals
C57BL/6J mice were purchased from Clea Japan (Tokyo, Japan) and Charles River Japan (Kanagawa, Japan). Specific pathogen free and CV-NC/Nga mice (male, 15 weeks old) were purchased from SLC Japan (Hamamatsu, Japan). B6.129X1-Grprtm1Jfb/J (Grpr-knockout) and B6;129S6-Gt(ROSA) 26Sortm14(CAG-tdTomato)Hze/J mice were purchased from the Jackson Laboratory (Bar Harbor, Me). Mrgpra3GFP-Cre mice were kindly provided by Prof. Xinzhong Dong (Johns Hopkins University). All animals were housed at a temperature of 22°C ± 1°C with a 12-hour light-dark cycle (light on 8:00–20:00) and fed food and water ad libitum. All animal experiments were conducted according to relevant national and international guidelines contained in the “Act on Welfare and Management of Animals” (Ministry of Environment of Japan) and “Regulation of Laboratory Animals” (Kyushu University) and under the protocols approved by the Institutional Animal Care and Use Committee review panels at Kyushu University.
Immunohistochemistry
Mice were deeply anesthetized by intraperitoneal injection of pentobarbital (100 mg/kg) and perfused transcardially with 20 mL of PBS, followed by 50 mL ice-cold 4% paraformaldehyde. The third to fifth cervical segments of the DRG, TG, or skin were removed, postfixed in the same fixative at 4°C, and placed in 30% sucrose solution for 48 hours at 4°C. DRG, TG (15 μm), or skin (30 μm) sections were incubated in blocking solution (3% normal goat or donkey serum) for 2 hours at room temperature and then incubated for 48 hours (DRG and TG) or 120 hours (skin) at 4°C with primary antibodies: anti-P2X3R (rabbit polyclonal, 1:4000; Chemicon (Temecula, Calif); guinea pig polyclonal, 1:12000; Neuromics, Edina, Minn), anti-GFP (chicken polyclonal, 1:20000; Aves Labs, Tigard, Ore), anti-RFP (rabbit polyclonal, 1:5000; MBL, Nagoya, Japan), and anti-TRPV1 (goat polyclonal, 1:2000; Neuromics). Following incubation, tissue sections were washed and incubated for 3 hours at room temperature in secondary antibody solution (Alexa Fluor 405, 488, or 546, 1:1000). The tissue sections were washed, slide mounted, and subsequently coverslipped with Vectashield Hardmount with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, Calif). Immunofluorescence images were obtained with confocal laser microscope (LSM510 or LSM700, Carl Zeiss, Thornwood, NY).
Real-time RT-PCR
Mice were anesthetized with pentobarbital and perfused transcardially with PBS. The third to fifth cervical segments of the DRG, TG, and skin were removed immediately. Total RNA was extracted using TRIsure (Bioline, London, United Kingdom) according to the manufacturer’s protocol. The amount of total RNA was quantified by OD260 using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, Mass). For reverse transcription, 250 ng of total RNA was transferred to the reaction with Prime Script reverse transcriptase (Takara, Kusatsu, Japan) and random 6-mer primers. Quantitative PCR was carried out with Fast Start Essential DNA Probes Master (Roche, Mannheim, Germany) using a LightCycler 96 system (Roche) according to the manufacturer’s specifications, and the data were analyzed by LightCycler 96 Software (Roche) using standard curves. All values were normalized to glyceraldehyde 3-phosphate dehydrogenase expression. The TaqMan probe, forward primer, and reverse primer used in this study were as follows: P2X3R, probe, 5′-FAM-TCCTGAAGGCTTTTGGCATCCGCTT-TAMRA-3′; forward primer, 5′-ATCGGCTGGGTGTGTGATCT-3′; reverse primer, 5′-GTTGAACTTGCCAGCGTTCC-3′; glyceraldehyde 3-phosphate dehydrogenase, probe, 5′-FAM-ACCACCAACTGCTTAGCCCCCCTG-TAMRA-3′; forward primer, 5′-TGCCCCCATGTTTGTGATG-3′; reverse primer, 5′-GGCATGGACTGTGGTCATGA-3′.
Acute dissociation of DRG and TG neurons
The DRGs from the third cervical to the second thoracic level or the TGs of 4-week-old mice were collected in cold Dulbecco modified Eagle medium/F-12 medium and treated with collagenase (Worthington, Freehold, NJ) at 37°C. After trituration and centrifugation, cells were resuspended in Dulbecco modified Eagle medium/F-12 and plated at 0.5 to 2.0 × 106 cells/well on poly-L-lysine and laminin-coated glass coverslips surrounded by silicon rubber walls (Flexiperm, Greiner Bio-One GmbH, Frickenhausen, Germany). Cells were maintained in an incubator at 37°C and used within 3 hours.
Intracellular Ca2+ imaging
Ca2+ imaging was performed as previously described.E1 Briefly, neurons were loaded with 2.5 μM Fura 2-AM (Molecular Probes, Eugene, Ore) containing 0.01% pluronic F-127 (Molecular Probes) in a balanced salt solution (150 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1.2 mM MgCl2, 10 mM D-glucose, and 25 mM HEPES, pH 7.4) for 30 minutes in the dark at 37°C. After washing, neurons were imaged at 340- and 380-nm excitation to detect intracellular free Ca2+. Data were calculated using the relative increase ratio (F340/F380) from the basal level before the first application.
Behavioral tests
Injections of chemicals into the rostral back or cheek were performed as previously described.E2 Briefly, mice were shaved at the rostral back or the right cheek at least the day before injection. After intradermal injection into the skin of the back (α,β-meATP [200 μg/50 μL, Sigma, Poole, United Kingdom] with or without A317491 [200 μg/50 μL, Sigma] and ATP [200 μg/50 μL, Sigma]) or the cheek (α,β-meATP [50, 100, and 200 μg/10 μL, Sigma] and ATP [50, 200, and 500 μg/10 μL, Sigma] with or without A317491 [200 μg/10 μL, Sigma]), each mouse was placed in a plastic chamber (11 cm in diameter, 10 cm high). Behavioral responses were video-recorded for 30 (back) or 40 (cheek) minutes.E3,E4 Hindlimb scratching behaviour and, for cheek, wiping with the forepaw, directed toward the injection site, were counted. One scratch was defined as a lifting of the hindlimb toward the injection site and then placing the limb back on the floor, regardless of how many scratching strokes took place between those 2 movements.
For activity-dependent neuronal silencing, N-ethyl-lidocaine (QX-314, Sigma) was used.E5 Mice received cheek injections (conditional injection) of chloroquine with or without QX-314 (2%, 30 μL in PBS) for 30 minutes before injection of the agonists αβ-meATP (100 μg/10 μL) or ATP (200 μg/10 μL) into the same site as the conditional injection (test injection). After the test injection, behavioral responses were video-recorded for 40 minutes and the number of hindlimb scratching behaviors directed toward the injection site was counted.
In chronic itch model using CV-NC/Nga mice, scratching behavior was automatically detected and objectively evaluated using MicroAct (Neuroscience, Tokyo, Japan) in accordance with a method described previously.E2 Briefly, a small Teflon-coated magnet (1 mm in diameter, 3 mm in length; Neuroscience) was implanted subcutaneously into the hindpaws of the mice at least 1 day before the first recording and placed in an observation chamber (11 cm in diameter, 18 cm high) with food and tap water, surrounded by a round coil. Movements of magnets implanted into the hindpaws were recorded for 24 hours after intradermal injection of vehicle or A317491 (200 μg/50 μL in PBS) into the rostral back, and the characteristic waves by the movements of magnets (reflecting scratching behavior) were analyzed by MicroAct software.E6,E7
Skin histology
Hairs on the back skin were shaved off. Skin biopsies were fixed with 10% phosphate-buffered formalin and embedded in paraffin. Deparaffinized sections of 3 μm thickness were stained with hematoxylin and eosin, and analyzed by light microscopy. Inflammatory cells were counted and the epidermal thickness was measured.
Statistical analysis
Statistical analyses of the results were performed using the unpaired t test, the Mann-Whitney U test, and 1-way ANOVA followed by post hoc Tukey multiple comparisons test. Values were considered significantly different at P values of less than .05.
DISCUSSION
Percentage of P2X3R expression in MrgprA3+ DRG neurons
This study demonstrated that approximately half of MrgprA3+ neurons expressed P2X3Rs in the cervical DRG. However, Han et alE8 have previously shown that approximately 80% of MrgprA3+ neurons coexpress P2X3Rs in the lumbar DRG of Mrgpra3GFP-Cre;ROSA26tdTomato mice. The reason for this discrepancy remains unclear, but one possibility may be related to the segment of the DRG used for immunohistochemical analyses; that is, we used cervical segments, whereas Han et al used lumbar segments. In fact, in Mrgpra3GFP-Cre;ROSA26tdTomato mice we found that 54.9% (113 of 206 cells) and 70.2% (106 of 151 cells) of tdTomato+ neurons coexpressed P2X3Rs in the cervical and lumbar DRGs, respectively, suggesting that the percentage of P2X3R expression in MrgprA3+ DRG neurons is slightly higher in the lumbar segment.
Role of P2X3R+ MrgprA3− primary afferent neurons in somatosensory information
We demonstrated that a small population of the DRG neurons expressed both P2X3R and MrgprA3 (3.4% of total neurons), whereas 34.9% of total DRG neurons were P2X3R+ MrgprA3− (Fig E2). In addition, we performed triple-labeling experiments using antibodies to P2X3R, GFP, and TRPV1 in the DRG of Mrgpra3GFP-Cre mice and found that 50% of P2X3R+ MrgprA3+ DRG neurons express TRPV1.
Because P2X3Rs have previously been implicated in pain,E9,E10 P2X3R+ MrgprA3− neurons may have a role in transducing nociceptive information from the skin to the spinal cord. However, our study failed to detect a significant pain-related wiping behavior by α,β-meATP injected into the cheek (Fig 2, C). The cheek model has been reported to assess not only itch but also pain by measuring 2 distinct behaviors: scratching and wiping, respectively.E11 Indeed, pain-evoking substances (such as capsaicin and bradykinin) injected into the cheek produce wiping behavior.E11,E12 However, a previous report showed that formalin, which is well known to produce pain, does not produce wiping behavior when injected into the cheek.E3 From these findings, it is conceivable that not all pain-producing substances robustly elicit wiping behavior. Considering the previous findings demonstrating that the formalin-induced pain behavior involves P2X3R signaling,E9,E10 it is possible that pain behavior caused by P2X3R agonists may be difficult to be detected in the cheek model.
Role of MrgprA3 in itch
Mrgprs are a family of G protein–coupled receptors (GPCRs) that are composed of more than 50 members in the mouse genome.E13 The expression of some members including MrgprA3 is restricted to primary afferent sensory neurons.E14 Furthermore, some of the Mrgpr members are activated not only by mammalian peptide but also by nonmammalian peptide such as molluscan FMRF amide (Phe-Met-Arg-Phe).E14 However, endogenous ligands for MrgprA3 have not been identified to date. Moreover, the role of MrgprA3 itself in chronic itch is yet to be determined, although MrgprA3-expressing sensory neurons are crucial for acute and chronic itch.E8 In addition, our study revealed a subset of sensory neurons coexpressing P2X3R and MrgprA3. However, whether P2X3R and MrgprA3 interact at nerve endings in the skin and whether such interaction contributes to chronic itch remains unknown; these will be important subjects for future studies.
Other factors involved in chronic itch
Intradermal treatment with the selective P2X3R antagonist A317491 significantly reduced spontaneous scratching in atopic dermatitis model mice. However, considering the fact that A317491 reduced scratching by approximately 30%, it seems that ATP-P2X3R signaling contributes partially to chronic scratching in atopic dermatitis. Indeed, previous reports have shown that other factors (eg, T-cell–derived IL-31,E15,E16 substance P,E17 and thymic stromal lymphopoietinE18) contribute to chronic itch associated with atopic dermatitis by acting on nerve endings of primary afferent sensory neurons in the skin.
P2X3R and cough
P2X3Rs have been implicated in cough via activation of pulmonary vagal afferent fibers.E19,E20 A recent study has shown that MrgprC11-expressing vagal sensory neurons innervating the airway mediate cholinergic bronchoconstriction and airway hyperresponsiveness.E21 The MrgprC11+ subset is known to be an almost identical population that expresses MrgprA3,E22 which suggests the possibility that MrgprC11+ neurons could express P2X3Rs. Thus, cough and itch may have a common mechanism in activation of primary afferents (especially the MrgprA3/C11-expressing subset) by P2X3Rs.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS KAKENHI; grant no. JP15H02522 to M.T.), the Core Research for Evolutional Science and Technology program from Japan Agency for Medical Research and Development (AMED; grant no. JP17gm0910006 to M.T.), the Practical Research Project for Allergic Diseases and Immunology (Research on Allergic Diseases and Immunology) from AMED (grant no. JP17ek0410034 to M.T.), the Bilateral Collaborations (Joint Research Projects and Seminars) from JSPS (to M.T.), Astellas Foundation for Research on Metabolic Disorders, and Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research) from AMED (grant no. JP18am0101091 to M.T.).
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Miller G Grasping for clues to the biology of itch. Science 2007;318:188–9. [DOI] [PubMed] [Google Scholar]
- 2.LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci 2014;15:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 2013;16:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain 2008;139:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci 2013;16:910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y-G, Chen Z-F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007;448:700–3. [DOI] [PubMed] [Google Scholar]
- 7.Shiratori-Hayashi M, Koga K, Tozaki-Saitoh H, Kohro Y, Toyonaga H, Yamaguchi C, et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat Med 2015;21:927–31. [DOI] [PubMed] [Google Scholar]
- E1.Yamashita T, Yamamoto S, Zhang J, Kometani M, Tomiyama D, Kohno K, et al. Duloxetine inhibits microglial P2X4 receptor function and alleviates neuropathic pain after peripheral nerve injury. PloS One 2016;11:e0165189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Shiratori-Hayashi M, Koga K, Tozaki-Saitoh H, Kohro Y, Toyonaga H, Yamaguchi C, et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat Med 2015;21:927–31. [DOI] [PubMed] [Google Scholar]
- E3.Akiyama T, Carstens MI, Carstens E. Differential itch- and pain-related behavioral responses and μ-opoid modulation in mice. Acta Derm Venerol 2010;90:575–81. [DOI] [PubMed] [Google Scholar]
- E4.Sun Y-G, Chen Z-F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007;448:700–3. [DOI] [PubMed] [Google Scholar]
- E5.Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci 2013;16:910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Inagaki N, Igeta K, Kim JF, Nagao M, Shiraishi N, Nakamura N, et al. Involvement of unique mechanisms in the induction of scratching behavior in BALB/c mice by compound 48/80. Eur J Pharmaacol 2002;448:175–83. [DOI] [PubMed] [Google Scholar]
- E7.Takano N, Arai I, Kurachi M. Analysis of the spontaneous scratching behavior by NC/Nga mice: a possible approach to evaluate antipruritics for subjects with atopic dermatitis. Eur J Pharmaacol 2002;471:223–8. [DOI] [PubMed] [Google Scholar]
- E8.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 2013;16:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 2000;407:1011–5. [DOI] [PubMed] [Google Scholar]
- E10.Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, et al. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature 2000;407:1015–7. [DOI] [PubMed] [Google Scholar]
- E11.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain 2008;139:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Fu K, Qu L, Shimada SG, Nie H, LaMotte RH. Enhanced scratching elicited by a pruritogen and an algogen in a mouse model of contact hypersensitivity. Neurosci Lett 2014;579:190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Bader M, Alenina N, Andrade-Navarro MA, Santos RA. MAS and its related G protein-coupled receptors. Mrgprs. Pharmacol Rev 2014;66:1080–105. [DOI] [PubMed] [Google Scholar]
- E14.Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 2001;106: 619–32. [DOI] [PubMed] [Google Scholar]
- E15.Grimstad O, Sawanobori Y, Vestergaard C, Bilsborough J, Olsen UB, Grønhøj-Larsen C, et al. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol 2009;18:35–43. [DOI] [PubMed] [Google Scholar]
- E16.Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol 2014;133: 448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E17.Ohmura T, Hayashi T, Satoh Y, Konomi A, Jung B, Satoh H, et al. Involvement of substance P in scratching behaviour in an atopic dermatitis model. Eur J Pharmacol 2004;491:191–4. [DOI] [PubMed] [Google Scholar]
- E18.Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013;155:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E19.Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 2015;385:1198–205. [DOI] [PubMed] [Google Scholar]
- E20.Kamei J, Takahashi Y. Involvement of ionotropic purinergic receptors in the histamine-induced enhancement of the cough reflex sensitivity in guinea pigs. Eur J Pharmacol 2006;547:160–4. [DOI] [PubMed] [Google Scholar]
- E21.Han L, Limjunyawong N, Ru F, Li Z, Hall OJ, Steele H, et al. Mrgprs on vagal sensory neurons contribute to bronchoconstriction and airway hyper-responsiveness. Nat Neurosci 2018;21:324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E22.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 2009;139:1353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]