Short abstract
Introduction
Cerebral small vessel disease is an important cause for both ischaemic stroke and intracranial haemorrhage. To date, knowledge on the impact of small vessel disease on the clinical course in stroke patients treated with oral anticoagulation for atrial fibrillation is limited.
Patients and Methods
Registry-based prospective observational study of 320 patients (aged 78.2 ± 9.2 years) treated with anticoagulation following atrial fibrillation stroke. Patients underwent standardised magnetic-resonance-imaging assessing measures of small vessel disease, including cerebral microbleeds and white matter hyperintensities. Median follow-up was 754 (interquartile range = [708–828]) days. Using adjusted logistic and Cox regression, we assessed the association of imaging measures with clinical outcome including recurrent ischaemic stroke, intracranial haemorrhage and death and assessed disability (modified Rankin Scale).
Results
Overall, recurrent ischaemic stroke was more common than intracranial haemorrhage (22 versus 8, respectively). Cerebral microbleeds were related to an increased risk of the composite endpoint (ischaemic stroke, intracranial haemorrhage, death: odds ratio (OR) 2.05, 95% confidence interval (CI) 1.27–3.31; P = 0.003), as were white matter hyperintensities (OR 2.00, 95%CI 1.23–3.27, P = 0.005). This was also true in time-to-event analysis (cerebral microbleeds: HR 2.31, 95%CI 1.39–3.52; P < 0.001; white matter hyperintensities: HR 1.99, 95%CI 1.20–3.17; P = 0.007). Both measures were associated with an increased risk for recurrent ischaemic stroke (cerebral microbleeds: HR 4.42, 95%CI 1.07–18.20; P = 0.04; white matter hyperintensities: HR 5.27, 95%CI 1.08–25.79, P = 0.04) and intracranial haemorrhage (cerebral microbleeds: HR 2.43, 95%CI 1.04–5.69; P = 0.04; white matter hyperintensities: HR 2.57, 95%CI 1.11–5.98, P = 0.03). Furthermore, confluent white matter hyperintensities were associated with increased disability (OR 4.03; 95%CI 2.16–7.52; P < 0.001) and mortality (HR 1.81, 95%CI 1.04–3.14, P = 0.04).
Discussion and conclusion
In atrial fibrillation stroke patients treated with oral anticoagulation, small vessel disease is associated with an unfavourable outcome. The presence of microbleeds indicated a risk higher for recurrent ischaemic stroke than for intracranial haemorrhage.
Keywords: Stroke, atrial fibrillation, anticoagulation, small vessel disease, microbleeds, magnetic resonance imaging
Introduction
Non-valvular atrial fibrillation (AF) is the most common arrhythmia and is responsible for approximately 25% of all ischaemic strokes by means of cardioembolism.1,2 Oral anticoagulation with either vitamin K antagonists (VKAs) or direct oral anticoagulants (DOACs) is the treatment of choice in secondary prevention of cardioembolic stroke related to AF.3,4 Anticoagulation substantially reduces the risk of recurrent ischaemic stroke (IS) in AF patients but bears the risk of intracranial haemorrhage (ICH).5,6 Due to their more favourable safety profile with regard to ICH and other advantages over the VKAs, DOACs are currently regarded as the standard of care.7,8
The prevalence of AF increases with age,9 as does that of cerebral small vessel disease (SVD), another important cause of IS and ICH, which is also associated with cognitive decline and disability.10 Upon magnetic resonance imaging (MRI), SVD is characterised by the presence of ischaemic white matter hyperintensities (WMH) as well as cerebral microbleeds (CMBs).10,11 It is the same risk factors including age, hypertension and diabetes that are implicated both in the pathogenesis of SVD and in the occurrence of ischaemic stroke and bleeding in AF patients.10,12,13 The frequent coexistence of SVD and AF in elderly stroke patients requiring oral anticoagulation raises the question of the impact of concomitant SVD on the clinical course and outcome in AF patients treated with oral anticoagulants following stroke. Recent studies suggest that the presence of CMBs may be associated with an increased risk of ICH in patients on oral anticoagulation.14–17 However, the majority of patients were treated with VKAs, and DOAC-treated patients were absent or largely underrepresented. Also, recent studies largely included heterogeneous stroke cohorts, with variable stroke mechanisms, different clinical and imaging work-up, limited follow-up and heterogeneous treatments for secondary prevention. Also, the impact of SVD on recurrent ischaemic stroke and disability in stroke patients on oral anticoagulation remains elusive.14,16,18 Therefore, in this observational, prospective cohort study, we aimed to explore the association of CMBs and WMH as markers of SVD on the clinical course and outcome in AF-patients treated with oral anticoagulants – predominantly DOACs – after an acute ischaemic stroke or transient ischaemic attack. Especially, we wanted to address the questions whether CMBs are associated with an increased risk for OAC-related ICH only or also to IS.
Patients and methods
Study design and patient population
This study is based on the prospective, ongoing registry Novel Oral Anticoagulants in Ischaemic Stroke Patients (NOACISP)-LONGTERM conducted at the Stroke Center of the University Hospital of Basel. Since April 2013, the registry enrolls consecutive patients with atrial fibrillation aged ≥18 years, who are treated with DOACs or VKAs after an acute IS, defined as a focal neurological deficit with acute onset and presence of a corresponding lesion on diffusion weighted magnetic resonance imaging (DWI), or transient ischaemic attack (TIA), defined as a transient, acute-onset focal neurological deficit of presumed ischaemic origin. Patients are prospectively followed-up using a standardised form at 3 months with an outpatient visit, and thereafter with regular telephone interviews at 6 and 12 months and a final follow-up at least 24 months after the index event. Follow-up information is obtained from the patient and where applicable, general practitioner and hospital records. Baseline and follow-up data are entered into an electronic database with predefined variables, as previously described.19,20 This includes a detailed risk factor profile applying predefined criteria21 (age, sex, use of antiplatelets, arterial hypertension, diabetes, hypercholesteremia, current smoking, alcohol consumption [more than eight standard drinks weekly]), baseline National Institutes of Health Stroke Scale (NIHSS) score, and the follow-up outcome variables outlined below. For this study, we included all consecutive NOACISP-LONGTERM patients diagnosed and treated at the USB stroke centre up to August 2016 with a minimum follow-up of three months and in whom brain MRI from their index event hospitalisation was available. Our analysis was conducted in accordance with the STROBE criteria for observational studies.22
Cranial MRI
All patients received a standardised MRI-stroke protocol including axial DWI and apparent diffusion coefficient (ADC), axial T2-weighted fluid-attenuated inversion recovery (FLAIR) imaging and susceptibility-weighted imaging (SWI) with whole brain coverage. MRI quality was categorised as good, sufficient with little artefacts and insufficient (e.g. due to motion artefacts). In the latter case, patients were excluded from the study. CMBs were defined as nodular, strongly hypointense SWI lesions and classified as superficial or deep and according to anatomical localisation using a validated rating scale (white matter, basal ganglia, thalamus, cerebellar or brainstem);23,24 furthermore, CMBs were quantified in the following categories: 0, 1, 2–5, 6–10 and >10 CMBs.
WMH were defined as FLAIR hyperintense lesions in the subcortical white matter and rated on the age-related white matter changes (ARWMC) – scale from 0–3 as absent, focal, beginning or diffuse confluent lesions, respectively.25 Given the low numbers of patients with either no WMH (n = 16) and diffuse confluent WMH (n = 15) we performed analyses grouping subjects into two groups: one group with no or focal WMH (ARWMC categories 0 and 1, n = 195) and group two with confluent WMH (ARWMC categories 2 and 3, n = 117), respectively. MRI assessments were performed by three experienced readers (LH, JL and NP), all blinded to the clinical course and outcome of the patients. Initially, a simultaneous consensus reading of the first 10% of the images was performed by all readers. Finally, after completion of reading, again a consensus reading was performed for ambiguous cases, followed by a second confirmatory and blinded consensus reading.
Study outcomes
Primarily, we analysed a clinically relevant composite endpoint including: (1) recurrent IS (defined as a focal neurological deficit with acute onset and presence of a corresponding lesion on DWI or, if no MRI was acquired, signs of early ischaemic injury on CT); (2) ICH (defined as clinically manifest bleeding on head CT or MRI in accordance to the ISTH criteria26); (3) death including its cause where available. In addition, all three outcomes were analysed separately. Furthermore, we assessed physical disability based on the modified Rankin scale (mRS).
Statistical analyses
Descriptive statistics were used to present baseline clinical and MRI characteristics. Analyses were performed by categorising groups by the presence of CMBs and WMH.
For categorical variables, the number and the proportions are presented and the groups were compared using chi-square tests. For continuous variables, the mean and standard deviations are presented and the groups were compared using t-tests or one-way analysis of variance as appropriate. In case of violation of the normal distribution assumption, the Mann–Whitney U-test or the Kruskal–Wallis rank sum test were used as appropriate and the median and the lower and upper quartile are presented.
The association of outcomes with CMBs was assessed using two different approaches: First, the incidence of outcome events during the follow-up period was analysed in a mixed effects logistic model. The presence of CMBs (dichotomised as no or at least one lesion) was included as predictor. Furthermore, age in years, sex, use of antiplatelets and vascular risk factors were the included fixed effects. There were no missing values in any of the baseline variables except for smoking (17.5%) and alcohol consumption (15.9% missing values rate), which were modeled using three categories ‘yes’, ‘no’ and ‘unknown’. The patient identifier and the follow-up time points were included in the model as random effect. Age was included as continuous predictor. In order to further assess linearity of age, the model was refit including age as penalised spline and the models were compared using a likelihood ratio test. There was evidence for a non-linear age effect. The model was repeated including CMBs as a categorical variable (categorised: 0, 1, 2–5, 6–10 and >10). The localisation of CMBs was assessed in a third logistic model including a binary variable (no lesion vs. at least one lesion) for each localisation ‘superficial’, ‘deep’, ‘cerebellum’ and ‘brainstem’ as predictor.
Second, ‘time to first outcome event’ was assessed in a fixed effects Cox-proportional hazards model. Subsequent events were ignored in this analysis and since they occurred in only six patients (two patients with two IS, two patients with IS followed by death and two patients with IS followed by ICH), no repeated measure model was fitted. We used the same fixed effects as described above. Furthermore, a proportional sub-distribution hazards regression model was fitted for the competing events ‘ICH’, ‘IS’ and ‘death (from any cause)’ according to Fine-Gray.27 Due to the overall limited number of outcome events, in particular ICH, no covariates were included in the Fine-Gray models. Cumulative incidence sub-distribution estimates for the competing events were presented graphically; a Kaplan–Meier curve was presented for the composite endpoint.
All analyses described above were performed for the WMH accordingly based on the ARWMC-classification. Age, sex, use of antiplatelet drugs and vascular risk factors were again included as covariates.
The association with the mRS score, measured at 3, 6, 12, and 24 months, was assessed with a mixed effects’ proportional odds model (i.e. ordered logistic regression or shift analysis; mRS treated as ordinal variable). Again, the covariates described above were included and we added NIHSS at baseline.
Ethics and patient consent
The NOACISP-LONGTERM registry has been approved by the local ethics committee (BASEC PB_2016-00662, former EKBB 52/13). Written informed consent was obtained from all patients.
Results
The overall cohort comprised 320 patients (mean age 78.2 ± 9.2 years, 45.6% female, 10%TIA as qualifying event), of which CMBs and WMH could be evaluated for 315 and 316 patients, respectively. The detailed study flowchart is presented in the Supplemental Figure. Median follow-up period was 754 (interquartile range = [708–828]) days with overall 617.6 patient-years, and 74% of patients were treated with a DOAC, with a median CHA2DS2-VASc and HAS-BLED score of 6 and 2, respectively. Overall, there were 22 recurrent IS and 8 ICH as first outcome events during the follow-up period. Fifty-three patients died, with two deaths being related to IS. The other deaths were classified as ‘other vascular’, ‘non-vascular’ or ‘of unknown cause’.
Cerebral microbleeds
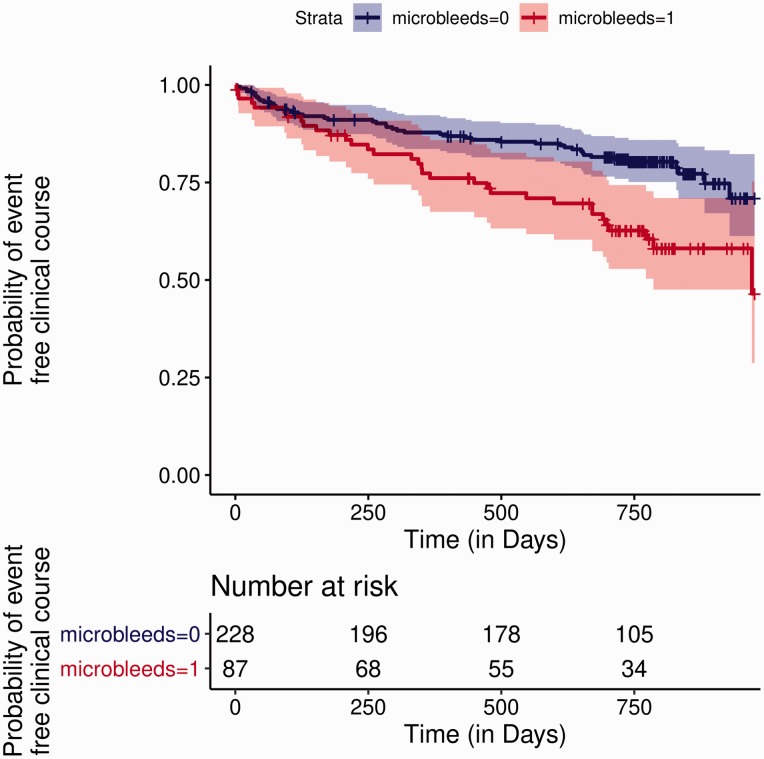
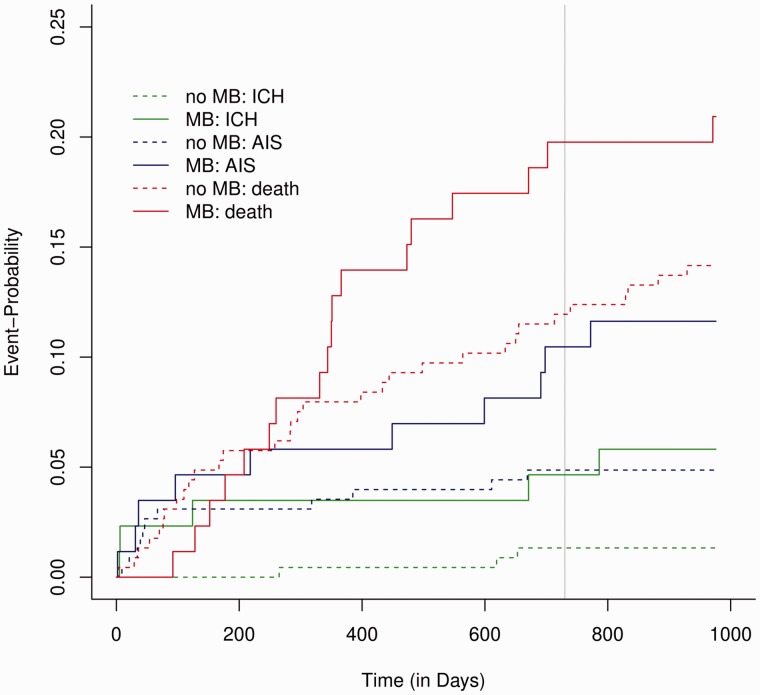
Of the 315 patients included in this analysis, CMBs were present in 87 (28%). Detailed characteristics of the two subgroups with and without CMBs are presented in Table 1. There were no differences regarding age, sex, vascular risk factors, CHA2DS2-VASc and HAS-BLED score, type of anticoagulation as well as follow-up period. The presence of CMBs was associated with an increased risk for the composite outcome (odds ratio (OR) 2.05, 95% confidence interval (CI) 1.27–3.31; P = 0.003) in the adjusted logistic regression analysis. Further predictors of the composite outcome were age (OR 1.04, 95%CI 1.01–1.07; P = 0.01) and concomitant antiplatelet use (OR 2.12, 95%CI 1.14–3.97; P = 0.019). We found no association between the localisation of CMBs and the risk for the composite outcome. Also, there was overall no clear increase in the risk for the composite outcome with increasing number of CMBs: here, an association was observed for two groups, those with only 1 CMB (n = 35; OR 2.15, 95%CI 1.11–4.16; P = 0.024) and those with more than 10 CMBs (n = 11; OR 4.05, 95%CI 1.47–11.12; P = 0.007). In the latter group, we did not observe ICH during the follow-up and death constitutes the majority of events. In accordance with the logistic model, time-to-event analysis also showed an increased hazard for the composite outcome in patients with CMBs compared to those without (hazard ratio (HR) 2.31, 95%CI 1.39–3.52; P < 0.001; Figure 1). Looking at the three outcomes separately, CMBs were associated with a higher hazard for recurrent IS (HR 4.42, 95%CI 1.07–18.20; P = 0.04) and for ICH (HR 2.43, 95% CI 1.04–5.69; P = 0.04), with a trend for association with death (Figure 2). Irrespective of the presence or absence of CMBs the absolute risk of IS was higher than that of ICH, with an annualised event rate of 0.06 for IS versus 0.03 for ICH and 0.02 versus 0.01 with or without presence of CMBs, respectively.
Table 1.
Patient characteristics by the presence of CMB.
| CMB absent (228) | CMB present (87) | P-value | |
|---|---|---|---|
| Demographics | |||
| Age, years (mean, SD) | 78.0 (9.4) | 78.8 (8.7) | 0.498 |
| Male sex, number of participants (%) | 117 (51.3) | 53 (60.9) | 0.161 |
| Qualifying event, n (%) | |||
| TIA | 22 (9.6) | 10 (11.5) | 0.782 |
| Medication, n (%) | 0.355 | ||
| DOAC | 158 (69.3) | 58 (66.7) | |
| DOAC/antiplatelet | 10 (4.4) | 8 (9.2) | |
| VKA | 43 (18.9) | 18 (20.7) | |
| VKA/antiplatelet | 13 (5.7) | 2 (2.3) | |
| Antiplatet | 4 (1.8) | 1 (1.1) | |
| Vascular risk factors, n (%) | |||
| Hypertension | 173 (75.9) | 68 (78.2) | 0.780 |
| Diabetes | 43 (18.9) | 19 (21.8) | 0.663 |
| Hypercholesterolemia | 89 (39.0) | 33 (37.9) | 0.960 |
| Non-smoking | 171 (75.0) | 68 (78.2) | 0.833 |
| No regular alcohol consumption | 173 (75.9) | 69 (79.3) | 0.788 |
| CHA2DS2-VASc-score (median [IQR]) | 5.5 (5–6) | 6 (5–6.5) | 0.187 |
| HAS-BLED score (median [IQR])) | 2.4 (0.9) | 2.4 (0.8) | 0.757 |
| Follow-up time, days (median [IQR]) | 754 [720–830] | 758 [677–825] | 0.877 |
| WMH-ARWMC, n (%) | 0.007 | ||
| 0 | 15 (6.7) | 0 | |
| 1 | 137 (61.2) | 44 (50.6) | |
| 2 | 63 (28.1) | 37 (42.5) | |
| 3 | 9 (4.0) | 6 (6.9) | |
For categorical variables, the number and the proportions are presented. Patients with and without CMBs were compared using chi-square tests. For continuous variables, the mean and standard deviation are presented and groups were compared using t-tests. In case of violation of the normal distribution assumption, the Mann–Whitney U-test was used and the median and the lower and upper quartiles are presented. IQR = interquartile range.
Figure 1.
Presence of cerebral microbleeds is associated with an increased risk of the composite outcome. The Kaplan–Meier curves show the estimates for event-free clinical course stratified by the presence or absence of cerebral microbleeds (shaded areas: 95%CIs).
Figure 2.
Cerebral microbleeds are associated with an increased risk for recurrent ischaemic stroke and intracranial haemorrhage. The Cuminc-plot shows the cumulative incidence sub-distribution estimates for competing events.
White matter hyperintensities
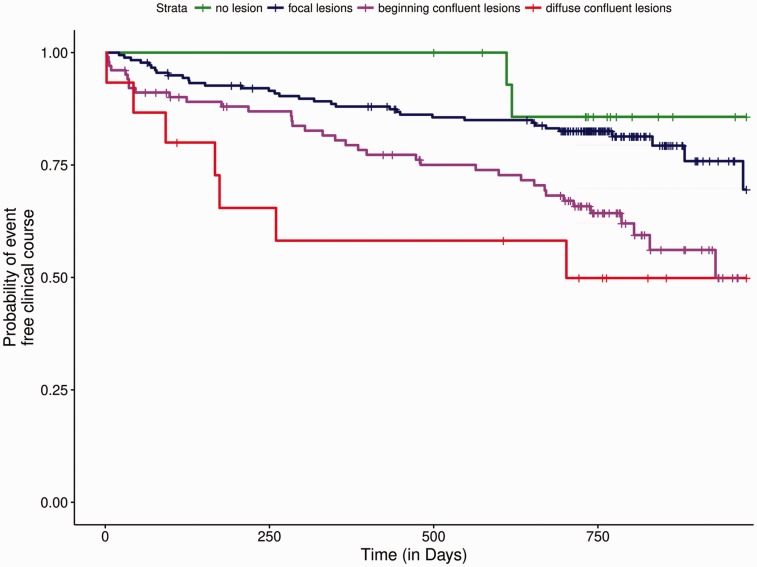
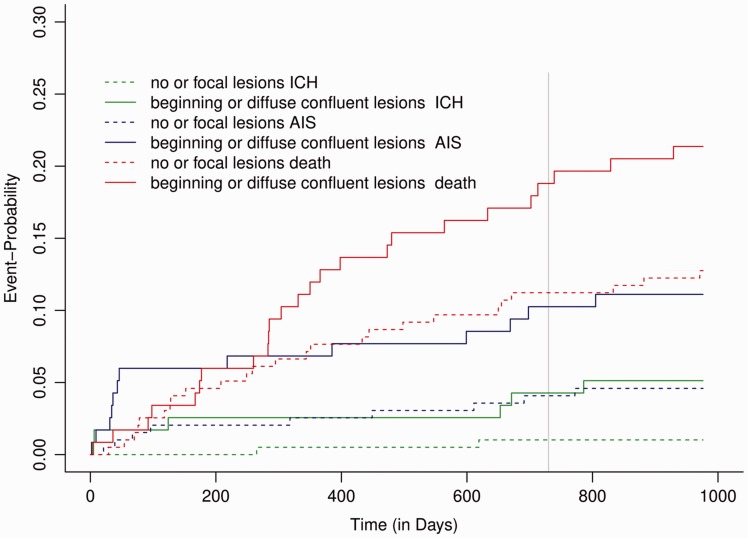
Among 316 patients with evaluable MRI-FLAIR-sequence, the extent of WMH was associated with age and with the presence of CMBs (Table 2). In the adjusted logistic regression analysis, the presence of confluent WMH was associated with a twofold increased risk of the composite outcome (OR 2.00; 95%CI 1.23–3.27, P = 0.005). In the time-to-event analysis, increasing extent of WMH was associated with an increased hazard for the composite outcome (Figure 3). The presence of confluent WMH lead to a twofold hazard for a composite outcome event (HR 1.99; 95%CI 1.20–3.17, P = 0.007). Moreover, confluent WMH were associated with an increased hazard for all outcome measures separately – IS: HR 5.27, 95% CI 1.08–25.79, P = 0.04; ICH: HR 2.57, 95% CI 1.11–5.98, P = 0.03 and death: HR 1.81, 95% CI 1.04–3.14, P = 0.04 (Figure 4).
Table 2.
Patient characteristics by presence of WMH (ARWMC).
| 0 | 1 | 2 | 3 | P-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| n | 16 | 182 | 103 | 15 | |
| Age, years (mean, SD) | 67.6 (12.3) | 76.5 (9.0) | 82.1 (7.1) | 83.1 (4.7) | <0.001 |
| Male sex, nr of participants (%) | 12 (75.0) | 107 (58.8) | 48 (46.6) | 5 (33.3) | 0.025 |
| Qualifying event, n (%) | |||||
| TIA | 3 (18.8) | 16 (8.8) | 10 (9.7) | 2 (13.3) | 0.600 |
| Medication, n (%) | 0.835 | ||||
| DOAC | 13 (81.2) | 129 (70.9) | 64 (62.4) | 9 (60.0) | – |
| DOAC/antiplatelet | 0 | 9 (4.9) | 7 (6.8) | 2 (13.3) | – |
| VKA | 2 (12.5) | 34 (18.7) | 23 (22.3) | 3 (20.0) | – |
| VKA/antiplatelet | 1 (6.2) | 8 (4.4) | 6 (5.8) | 1 (6.7) | – |
| Antiplatet | 0 | 2 (1.1) | 3 (2.9) | 0 | – |
| Vascular risk factors, n (%) | |||||
| Hypertension | 9 (56.2) | 137 (75.3) | 86 (83.5) | 11 (73.3) | 0.081 |
| Diabetes | 1 (6.2) | 36 (19.8) | 21 (20.4) | 3 (20.0) | 0.602 |
| Hypercholesterolemia | 4 (25.0) | 77 (42.3) | 34 (33.0) | 7 (46.7) | 0.254 |
| Non-smoking | 12 (75.0) | 138 (75.8) | 76 (73.8) | 12 (80.0) | 0.453 |
| No regular alcohol consumption | 13 (81.2) | 140/76.9) | 80 (77.7) | 11 (73.3) | 0.990 |
| CHA2DS2-VASc-score (median [IQR]) | 4 [3.8–5.2] | 5 [4.0–6.0] | 6 [5.0–6.0] | 6 [5.5–7.0] | <0.001 |
| HAS-BLED score (median [IQR]) | 2 [1.8–3.0] | 2 [2.0–3.0] | 2 [2.0–3.0] | 3 [2.0–3.0] | 0.070 |
| Follow-up , days (median [IQR]) | 767 [734–812] | 754 [716--830] | 751 [702--820] | 744 [628--847] | 0.785 |
| CMB, n (%) | 0 | 44 (24.3) | 37 (37.0) | 6 (40.0) | 0.007 |
For categorical variables number and proportions are presented. Patients with increasing extent of WMH were compared using chi-square tests. For continuous variables the mean and SD are presented; groups were compared using one-way analysis of variance. In case of violation of the normal distribution assumption, the Kruskal–Wallis rank sum test was used and the median and lower and upper quartiles are presented.IQR = interquartile range.
Figure 3.
Increasing extent of white matter hyperintensities is associated with an increased risk for the composite outcome. The Kaplan–Meier curves show the estimates for event-free clinical course stratified by the increasing extent of white matter hyperintensities.
Figure 4.
Confluent white matter hyperintensities are associated with an increased risk for recurrent ischaemic stroke, intracranial haemorrhage and death. The Cuminc-plot shows the cumulative incidence sub-distribution estimates for competing events.
Impact on disability
For CMBs overall, there was no association with disability, also when looking at the mRS among survivors only (mRS < 6). Only for the small group of patients with a high load of CMBs > 10 (n = 11), the risk for a higher mRS was strongly increased (OR 18.05, 95% CI 3.13–104.2; P = 0.001). In contrast, confluent (ARWMC 2 and 3) WMH were associated with an increased disability as assessed by the mRS in adjusted analyses (OR 4.03; 95% CI 2.16–7.52; P < 0.001). This finding remained significant also when adjusting for baseline stroke severity based on NIHSS-score (OR 3.69; 95% CI 1.89–7.20; P < 0.001). Besides WMH, predictors of a higher mRS were age (OR 1.12, 95%CI 1.09–1.17; P < 0.001), arterial hypertension (OR 2.27, 95%CI 1.02–5.03; P = 0.040) and diabetes (OR 4.43, 95%CI 1.92–10.22; P < 0.001).
Discussion
In the present study, we found markers of cerebral SVD being associated with an unfavourable outcome in AF-patients treated with oral anticoagulation following stroke or TIA. In particular, CMBs as well as WMH were associated with an increased risk for the composite outcome defined as recurrent IS, ICH or death over a follow-up period of more than two years. Both, CMBs and WMH, were related to an increased risk for both IS and ICH. Furthermore, WMH predicted a higher degree of disability and were associated with higher mortality.
Overall, we observed a slightly higher rate of outcome events compared to the recent Cromis-2 study, so far the largest study analysing the impact of CMBs on the clinical course on subjects treated with oral anticoagulation after IS/TIA.16 Yet, with an annual rate of approximately 3.5% for IS and 1.3% for ICH, our rates are low with respect to the predicted rates according to the CHA2DS2-VASc12 and HAS-BLED13 score, respectively. The recently published HERO-study reported a rate of 1.9% ICH during a two-year follow-up period in stroke patients on anticoagulation.17
CMBs were present in 28% of patients, which is well within the range of the known prevalence of CMBs.17,28 While CMBs predicted the occurrence of ICH in the Cromis-2 trial, there was no clear association with recurrent IS.16 In contrast, we found the presence of CMBs to be associated with an increased risk also for recurrent IS, confirming and extending previously reported findings on broader cohorts not focusing on anticoagulation.18,29 In fact, the absolute risk of recurrent IS was higher than that for ICH in our study, which is in line with a very recently published large multicentre study based on a pooled analysis of individual patient data from different stroke cohorts, irrespective of the presence or absence of CMBs.30
We did not observe an influence of the location of CMBs within the brain on the clinical course in our cohort (e.g. deep versus superficial CMBs), nor was there a clear quantitative effect with respect to the number of CMBs. We did observe an increased risk with respect to the composite outcome in the group with a high lesion load with more than 10 CMBs. However, the number of patients in this group was small and the results were driven mostly by death. Of note, no patient within this group experienced ICH during the follow-up period. Overall – in line with Cromis-2 – we did not observe a clear threshold of CMB-burden indicating increased risk for IS or ICH,15 but increasing CMB-burden has been associated with an increased risk for IS and ICH.30
We observed an increased risk of the composite outcome in subjects on additional antiplatelet treatment, which was mostly prescribed due to concomitant coronary artery disease. Most likely, this finding is to be interpreted as a marker for subjects with cardiovascular morbidity associated with increased risk. Yet, given the rather low number of subjects in this subgroup, conclusions from these results must be drawn cautiously.
As expected, the extent of WMH increased with age in our cohort. However, independent of age, we observed an increased risk for the composite outcome with increasing extent of WMH. As for CMBs, this was also true for both IS and ICH, which had not been observed in the Cromis-2 study,16 but in the recently published HERO-study.17 Of note, WMH were also associated with an increased mortality. Only two of these deaths were related to recurrent IS and none to ICH, indicating the relevant impact of concomitant SVD on prognosis in stroke patients with AF independent of recurrent cerebrovascular events.31,32 Our findings indicate that these patients require thorough post-stroke medical guidance and cardiovascular risk factor control.
Furthermore, our finding of the association of the extent of WMH with increased disability at follow-up – independent of stroke severity at baseline – suggests that AF-patients with concomitant SVD are at a higher risk of an unfavourable functional outcome following stroke. In line with this, a high load of CMBs was also associated with an increased risk of a higher mRS; however, numbers in this group were small and confident intervals large. Our findings suggest that intensive rehabilitation measures seem to be warranted in these anticoagulated patients with concomitant SVD, e.g. to avoid falls.31,33
Besides WMH, higher mRS was associated not only with age but also with hypertension and diabetes. SVD is known to be age-related and associated with vascular risk factors, including hypertension and diabetes. Our findings suggest that these risk factors may modify functional outcome not only via SVD but also independently, and underscore the importance of control and treatment of these concomitant risk factors in AF-related stroke patients, not only with respect to lowering cardio- and cerebrovascular risk but also with regard to physical disability.
Our study has some limitations, which should be addressed: we did not perform a controlled trial, thus limiting comparison of the different forms of anticoagulation (i.e. VKAs versus DOACs). Also, we did not perform longitudinal MRI in our cohort; thus, we cannot comment on the course of SVD markers, especially CMBs, during the follow-up period under oral anticoagulation. However, the prospective assessment of meaningful clinical endpoints including cerebrovascular events, mortality and functional outcome allowed us to analyse the prognostic effect of SVD markers in this representative cohort of AF-stroke patients. Furthermore, our analyses were limited due to the rather small number of events (in particular ICH) prohibiting the use of a multistate model. The type of the first outcome event per patient was assessed in a Fine-Gray model, but no risk factors could be included as covariates. Yet, the rather low rate of outcome events also indicates that subjects with CMBs on oral anticoagulation were not exposed to an exceedingly high risk of ICH. The low numbers most likely also account for the finding of a higher relative risk for IS compared to ICH associated with CMBs in our study, in comparison to the recently published multicentre data.30 Yet, the latter study comprised broader, rather heterogeneous stroke populations and differences were observed with respect to IS while increase of risk of ICH associated with CMBs was comparable.
Finally, follow-up comprised a period of approximately two years, but not beyond. However, the distinct clinical course related to presence of SVD as illustrated by the diverging Kaplan–Meier Curves does not suggest a different course beyond the two-year period. Also, functional outcome is not likely to change substantially more than two years after ischaemic stroke.
Our study has several strengths: we performed a prospective cohort study with a comprehensive and standardised clinical assessment and regular follow-ups over the entire period. Patients were treated and enrolled at our comprehensive stroke centre, being the referral centre for the entire north-western region in Switzerland. Therefore, our cohort is likely to be a representative stroke cohort related to AF. Treatment decisions were made on an individual basis by experienced stroke neurologists at our centre according to standard operating procedures, thus reflecting current standard of care of AF-stroke patients. All subjects underwent a standardised stroke MRI with the appropriate sequences and images were evaluated by three experienced readers blinded to treatment and outcome. Besides cerebrovascular events, we included mortality and disability in our analyses, thus extending results from previous studies. Finally, in contrast to previous studies14,16, our cohort included a high rate of subjects treated with DOACs, which nowadays are considered the standard of care for AF.
Conclusion
In conclusion, in our study, we found concomitant SVD in patients treated with oral anticoagulation following stroke or TIA to be associated with an unfavourable outcome and increased disability. Cerebral microbleeds were associated with an increased risk for both ischaemic stroke and intracranial haemorrhage, with a higher risk for ischaemic stroke than haemorrhage in this cohort of patients on oral anticoagulation, irrespective of the presence or absence of CMBs. In accordance with a recent multicentre study on relevance of CMBs on the clinical course following stroke and TIA,30 our data also do not suggest that anticoagulation should in general be withheld in patients solely based on the presence of cerebral microbleeds, being aware that a conclusive answer on this clinically relevant question should be ideally addressed by randomised trials. Patients with concomitant SVD should be guided thoroughly during the post-stroke phase with intensive control and treatment of existing vascular risk factors and effective rehabilitation measures. Future studies are needed to study any potentially differential effects of certain types of anticoagulation in this important group of stroke patients.
Supplemental Material
Supplemental material, ESO888016 Supplementary material for Small vessel disease is associated with an unfavourable outcome in stroke patients on oral anticoagulation by Lisa Hert, Alexandros A. Polymeris, Sabine Schaedelin, Johanna Lieb, David J. Seiffge, Christopher Traenka, Joachim Fladt, Sebastian Thilemann, Henrik Gensicke, Gian Marco De Marchis, Leo Bonati, Philippe Lyrer, Stefan T. Engelter and Nils Peters in European Stroke Journal
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DJS served on scientific advisory boards for Bayer and Pfizer and received compensation for educational efforts by Stago. CT received travel-compensation from Bayer. GMDM has received consultant honoraria by Bayer and speaker honoraria by Medtronic and BMS/Pfizer. LHB has received a research grant from AstraZeneca, and consultancy fees/speaker’s honoraria from Amgen, Bayer, Bristol-Myers Squibb, and Claret Medical, and travel grants from Amgen and Bayer. STE has received travel-compensation and speaker honoraria from Bayer, Boehringer and Daiichi-Sankyo. He has served on advisory boards for Bayer, Boehringer, BMS/Pfizer. PL has served on scientific advisory boards for Bayer, Boehringer, and BMS/Pfizer. NP has served on scientific advisory boards for Bayer, Boehringer, BMS/Pfizer and Daiichi-Sankyo.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Basel Stroke Funds, the Science Funds Rehabilitation Felix-Platter-Hospital Basel and the Neurology Research Pool of the University Hospital Basel. The NOACISP registry was supported by grants from the Science Funds, Bayer AG (Switzerland) and the Stroke Fund of the University Hospital Basel.
Ethical approval
The NOACISP-LONGTERM registry has been approved by the local ethics committee (BASEC PB_2016-00662, former EKBB 52/13).
Informed consent
Written informed consent was obtained from the patient(s) for their anonymised information to be published in this article.
Guarantor
NP.
Contributorship
LH, AP, STE and NP contributed to the conception and design of the study. LH, AP, SS, JL, DS, CT, JF, ST, HG, GMdM, LB, PL, STE and NP contributed to the acquisition of data. LH, AP, SS and NP contributed to analysis of data, drafting the text, and preparing the figures.
Supplemental Material
Supplemental Material for this article is available online.
References
- 1.Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke 2005; 36: 1115–1119. [DOI] [PubMed] [Google Scholar]
- 2.Rockson SG, Albers GW. Comparing the guidelines: anticoagulation therapy to optimize stroke prevention in patients with atrial fibrillation. J Am Coll Cardiol 2004; 43: 929–935. [DOI] [PubMed] [Google Scholar]
- 3.Dogliotti A, Paolasso E, Giugliano RP. Current and new oral antithrombotics in non-valvular atrial fibrillation: a network meta-analysis of 79 808 patients. Heart 2014; 100: 396–405. [DOI] [PubMed] [Google Scholar]
- 4.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146: 857–867. [DOI] [PubMed] [Google Scholar]
- 5.Secondary prevention in non rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European atrial fibrillation trial) study group. Lancet 1993; 342: 1255–1262. [PubMed] [Google Scholar]
- 6.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383: 955–962. [DOI] [PubMed] [Google Scholar]
- 7.Huisman MV, Rothman KJ, Paquette M, et al. The changing landscape for stroke prevention in AF: Findings from the GLORIA-AF registry phase 2. J Am Coll Cardiol 2017; 69: 777–785. [DOI] [PubMed] [Google Scholar]
- 8.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg 2016; 50: e1–e88. [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA 2001; 285: 2370-2375. [DOI] [PubMed] [Google Scholar]
- 10.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet. Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest 2010; 137: 263–272. [DOI] [PubMed] [Google Scholar]
- 13.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (has-bled) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro heart survey. Chest 2010; 138: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 14.Charidimou A, Boulouis G, Shams S, et al. Intracerebral haemorrhage risk in microbleed-positive ischaemic stroke patients with atrial fibrillation: preliminary meta-analysis of cohorts and anticoagulation decision schema. J Neurol Sci 2017; 378: 102–109. [DOI] [PubMed] [Google Scholar]
- 15.Charidimou A, Karayiannis C, Song TJ, et al. Brain microbleeds, anticoagulation, and hemorrhage risk: meta-analysis in stroke patients with AF. Neurology 2017; 89: 2317–2326. [DOI] [PubMed] [Google Scholar]
- 16.Wilson D, Ambler G, Shakeshaft C, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol 2018; 17: 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marti-Fabregas J, Medrano-Martorell S, Merino E, et al. MRI predicts intracranial hemorrhage in patients who receive long-term oral anticoagulation. Neurology 2019; 92: e2432–e2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson D, Charidimou A, Ambler G, et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: a meta-analysis. Neurology 2016; 87: 1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seiffge DJ, Traenka C, Polymeris A, et al. Early start of doac after ischemic stroke: risk of intracranial hemorrhage and recurrent events. Neurology 2016; 87: 1856–1862. [DOI] [PubMed] [Google Scholar]
- 20.Polymeris AA, Traenka C, Hert L, et al. Frequency and determinants of adherence to oral anticoagulants in stroke patients with atrial fibrillation in clinical practice. Eur Neurol 2016; 76: 187–193. [DOI] [PubMed] [Google Scholar]
- 21.Fluri F, Hatz F, Voss B, et al. Restenosis after carotid endarterectomy: Significance of newly acquired risk factors. Eur J Neurol 2010; 17: 493–498. [DOI] [PubMed] [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 23.Gregoire SM, Chaudhary UJ, Brown MM, et al. The microbleed anatomical rating scale (MARS): reliability of a tool to map brain microbleeds. Neurology 2009; 73: 1759–1766. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009; 8: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001; 32: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 26.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thrombosis Haemostasis 2005; 3: 692–694. [DOI] [PubMed] [Google Scholar]
- 27.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 28.Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis 2011; 32: 528–534. [DOI] [PubMed] [Google Scholar]
- 29.Charidimou A, Shams S, Romero JR, et al. Clinical significance of cerebral microbleeds on MRI: a comprehensive meta-analysis of risk of intracerebral hemorrhage, ischemic stroke, mortality, and dementia in cohort studies (v1). Int J Stroke 2018; 13: 454–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson D, Ambler G, Lee KJ, et al. Cerebral microbleeds and stroke risk after ischaemic stroke or transient ischaemic attack: a pooled analysis of individual patient data from cohort studies. Lancet Neurol 2019; 18: 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Holst HM, van Uden IW, Tuladhar AM, et al. Factors associated with 8-year mortality in older patients with cerebral small vessel disease: the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) study. JAMA Neurol 2016; 73: 402–409. [DOI] [PubMed] [Google Scholar]
- 32.Rensma SP, van Sloten TT, Launer LJ, et al. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci Biobehav Rev 2018; 90: 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Holst HM, Tuladhar AM, Zerbi V, et al. White matter changes and gait decline in cerebral small vessel disease. NeuroImage Clin 2017; 17: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, ESO888016 Supplementary material for Small vessel disease is associated with an unfavourable outcome in stroke patients on oral anticoagulation by Lisa Hert, Alexandros A. Polymeris, Sabine Schaedelin, Johanna Lieb, David J. Seiffge, Christopher Traenka, Joachim Fladt, Sebastian Thilemann, Henrik Gensicke, Gian Marco De Marchis, Leo Bonati, Philippe Lyrer, Stefan T. Engelter and Nils Peters in European Stroke Journal