Short abstract
Background
There are limited data on the safety of intravenous recombinant tissue plasminogen activator (rtPA) for treating acute ischemic stroke in patients with gastrointestinal malignancy or recent gastrointestinal bleeding within 21 days of their index stroke.
Aims
To evaluate clinical outcomes in patients treated with rtPA for acute ischemic stroke who had gastrointestinal malignancy or recent gastrointestinal bleeding
Methods
We identified patients who were treated with rtPA for acute ischemic stroke between 2/2009 and 12/2015 from the Get With The Guidelines-Stroke linked to Medicare claims data. Gastrointestinal malignancy and recent gastrointestinal bleeding were defined as any gastrointestinal malignancy hospitalisation within one year prior to acute ischemic stroke and gastrointestinal bleeding hospitalisation within 21 days prior to acute ischemic stroke, respectively. Outcomes of interest included in-hospital mortality and bleeding complications.
Results
Among 40,396 patients aged 65 years or older treated with rtPA for acute ischemic stroke from 1522 sites (mean age [SD] 81.0 [8.1] years; 41.9% women), 136 (0.3%) had gastrointestinal malignancy (n = 96) or recent gastrointestinal bleeding (n = 43). Patients with gastrointestinal malignancy or bleeding had more severe stroke than those without (median NIHSS [interquartile range]: 14.0 [8.0–19.0] vs. 11.0 [6.0–18.0]). The rates of in-hospital mortality and life-threatening systemic haemorrhage were not significantly different between those with and without gastrointestinal malignancy or bleeding (mortality: 10.3% vs. 9.0%, adjusted odds ratio [aOR] 1.01, 95%CI 0.58–1.75; bleeding: 2.3% vs. 1.2%, aOR 1.72, 95%CI 0.58–5.11).
Conclusions
In this observational cohort, we did not find increased risk of in-hospital mortality and bleeding in rtPA-treated patients with gastrointestinal malignancy or recent gastrointestinal bleeding.
Keywords: Recombinant tissue plasminogen activator, thrombolysis, stroke, contraindication, eligibility criteria
Introduction
The clinical benefit of thrombolysis therapy with intravenous recombinant tissue plasminogen activator (rtPA) for patients with a diagnosis of acute ischemic stroke (AIS) has been established. Yet, owing to the concern for exacerbating risk of bleeding, use of rtPA in AIS patients with structural gastrointestinal (GI) malignancy or recent history of GI bleeding event within 21 days of the indexed stroke event is contraindicated in the 2018 American Heart Association (AHA)/American Stroke Association (ASA) guideline based on consensus of expert opinion.1 However, there are limited data to support or refute the safety of rtPA for this specific population.2
To address the sparse evidence, the goals of the study were to evaluate the characteristics and clinical outcomes in patients treated with rtPA for AIS who had GI malignancy or recent history of GI bleeding event compared with those treated with rtPA but have no medical history of GI malignancy or bleeding.
Methods
Data source
The cohort for this analysis was derived from the AHA/ASA Get With The Guidelines-Stroke (GTWG-Stroke). The GWTG-Stroke registry is an ongoing, voluntary, continuous registry sponsored by the AHA/ASA.3 The in-hospital data from the GWTG-Stroke registry were linked to the Centers for Medicare & Medicaid Services (CMS) claims data to identify structural GI malignancy or recent GI bleeding prior to the index stroke event. Because GWTG-Stroke is an inpatient registry, we link the GWTG-Stroke to CMS claims among Medicare fee-for-services patients 65 years or older and determine the timing of the previous GI malignancy or bleeding event. This probabilistic linkage has previously been validated and was done by matching on a series of indirect identifiers, including admission date, discharge date, patient age or date of birth, and sex.4 Prior work has shown that patients in the linked GWTG-Stroke/CMS database are representative of the national Medicare AIS population.5,6 IQVIA serves as the data collection and coordination centre for GWTG-Stroke.
Study population
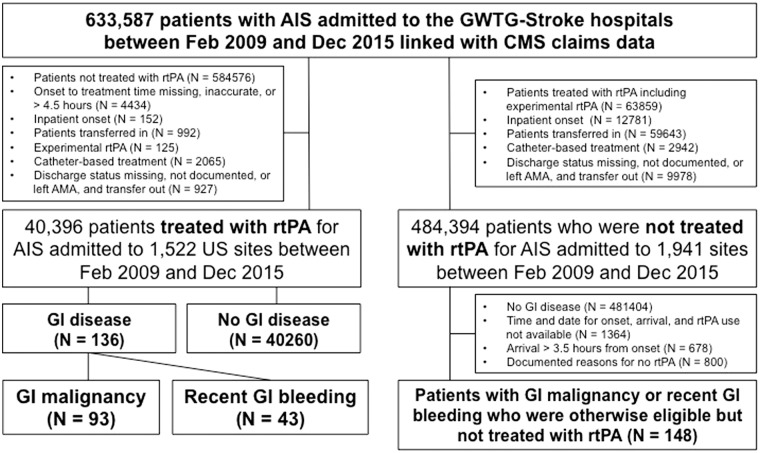
For the purpose of this analysis, we identified all patients aged 65 years or older with a diagnosis of AIS treated with rtPA within 4.5 h from the symptom onset from the GWTG-Stroke/CMS linked dataset between February 2009 and December 2015. In this study, only patients aged 65 years or older were included to enable to link to Medicare database, since it provides health insurance for Americans aged 65 and older. We excluded (1) inpatient onset of AIS, (2) patients transferred in, (3) patients treated with experimental rtPA, (4) discharge status missing, not documented, or discharge against medical advice, and transfer out, and (5) patients who underwent catheter-based treatment (Figure 1). Patients treated with experimental rtPA refer to those participating in clinical trials. Then, we classified patients according to the presence or absence of either GI malignancy or recent GI bleeding or both. GI malignancy and recent GI bleeding were identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) primary diagnosis codes (eAppendix in the Supplement). GI malignancy was defined as any GI malignancy hospitalisation within one year prior to AIS. Recent GI bleeding was defined as GI bleeding hospitalisation within 21 days prior to AIS.
Figure 1.
Study cohort creation.
AIS: acute ischemic stroke; CMS: Centers for Medicare & Medicaid Services; FFS: fee-for-service; rtPA: recombinant tissue plasminogen activator; AMA: against medical advice; GI: gastrointestinal.
To evaluate potential treatment selection in patients with GI malignancy or bleeding who received rtPA and those not received rtPA, we also identified a separate cohort of AIS patients with GI malignancy or recent GI bleeding who were otherwise eligible but not treated with rtPA. Along with our primary study population, baseline characteristics as well as outcomes of AIS patients with GI malignancy or bleeding who were treated with rtPA were compared with those of AIS patients with GI malignancy or bleeding who were otherwise eligible but not treated with tPA. Patients were considered eligible if they arrived within 3.5 h from symptom onset (potentially eligible for the 0–4.5 h treatment window) without any documented reasons for no rtPA use except for GI malignancy or recent GI bleeding (Figure 1).
Outcome measures
The primary outcomes were in-hospital mortality and life-threatening or serious systemic haemorrhage within 36 h after rtPA use (yes vs. no). Other outcomes included discharge disposition (home vs. other), in-hospital mortality or discharge to hospice, ambulatory status at discharge (able to ambulate independently vs. not), and modified Rankin Scale (mRS) at discharge (range of 0 [no symptoms] to 6 [death], with 0–2 as functional independence) and complications related to rtPA, including symptomatic intracranial haemorrhage within 36 h and any serious complications.
Statistical analysis
We reported the medians with interquartile ranges (IQRs) for continuous and counts with percentages for categorical variables. An absolute standardised difference was used to compare baseline differences among groups. An absolute standardised difference greater than 10 indicates significant imbalance. Multivariable logistic regression models were performed to assess the relationship between a history of GI malignancy or bleeding with each clinical outcome. These analyses adjusted for all variables are listed in Table 1. Generalised estimation equations modelling approach was used to account for within-hospital clustering of patients. As a sub-group analysis, GI malignancy or recent GI bleeding was separately analysed. All variables in the multivariable analyses had less than 5% missingness except for arrival via EMS (6%), preadmission medications (6% for antiplatelet and anticoagulation, 15% for antihypertensive and diabetic medications), and systolic blood pressure (11%). Given that missing data were uncommon, single imputation methods were used for multivariable analyses; female for sex, non-Hispanic white for race/ethnicity, and “No” for others.
Table 1.
Baseline characteristics between AIS patients treated with rtPA with vs. without GI malignancy or recent GI bleeding.
| Characteristics | GI malignancy or Recent GI bleeding | No GI disease | Absolute standardised difference (%) |
|---|---|---|---|
| (N = 136) | (N = 40,260) | ||
| Age, median (IQR), y | 80 (76–86) | 81 (74–87) | 5.67 |
| Women, no. (%) | 72 (52.9) | 23,370 (58.1) | 10.3 |
| Race, no. (%) | 21.6 | ||
| Non-Hispanic white | 120 (88.2) | 33,205 (82.6) | |
| Non-Hispanic black | 8 (5.9) | 3337 (8.3) | |
| Hispanic | 3 (2.2) | 1526 (3.8) | |
| Asian | 4 (2.9) | 829 (2.1) | |
| Other | 1 (0.7) | 1298 (3.2) | |
| Medical history, No. (%) | |||
| AF or atrial flutter | 69 (51.5) | 12,227 (30.6) | 43.6 |
| Previous stroke | 26 (19.4) | 8123 (20.3) | 2.3 |
| Previous transient ischemic attack | 14 (10.5) | 4122 (10.3) | 0.5 |
| Carotid stenosis | 8 (6.0) | 1394 (3.5) | 11.7 |
| CAD including prior myocardial infarction | 45 (33.6) | 12,078 (30.2) | 7.3 |
| Diabetes mellitus | 41 (30.6) | 10,429 (26.1) | 10.1 |
| Peripheral vascular disease | 7 (5.2) | 1787 (4.5) | 3.5 |
| Hypertension | 105 (78.4) | 31,803 (79.5) | 2.8 |
| Smoker | 9 (6.7) | 3052 (7.6) | 3.5 |
| Dyslipidaemia | 69 (51.5) | 18,775 (46.9) | 9.1 |
| Heart failure | 20 (14.9) | 4803 (12.1) | 8.6 |
| Laboratory data | |||
| Creatinine, median (IQR), mg/dL | 1.0 (0.9–1.4) | 1.0 (0.8–1.3) | 1.5 |
| Arrival and admission information, No. (%) | |||
| EMS arrival | 109 (89.3) | 32,914 (87.3) | 6.3 |
| Arrived off-hours | 72 (52.9) | 19,019 (47.2) | 11.4 |
| NIHSS at presentation, median (IQR) | 14 (8–19) | 11 (6–18) | 23.2 |
| Preadmission medication, no. (%) | |||
| Antiplatelet | 46 (37.4) | 20,382 (54.2) | 34.1 |
| Anticoagulant | 16 (13.1) | 3105 (8.3) | 15.8 |
| Antihypertensive | 94 (82.5) | 26,306 (76.8) | 14.1 |
| Cholesterol reducer | 58 (43.9) | 19,063 (47.6) | 7.3 |
| Diabetic medications | 22 (19.6) | 6711 (20.1) | 1.11 |
| Vital signs | |||
| Heart rate, median (IQR), bpm | 79 (69–92) | 78 (68–90) | 6.5 |
| sBP, median (IQR), mmHg | 154 (136–171) | 157 (140–177) | 18.1 |
| dBP, median (IQR), mmHg | 79 (67–89) | 81 (70–93) | 24.3 |
| Hospital characteristics | |||
| Bed size, median (IQR), No. | 361 (260–538) | 378 (258–585) | 8 |
| Academic centre, No. (%) | 105 (79.0) | 30,794 (77.5) | 3.5 |
| Primary stroke centre, No. (%) | 29 (21.3) | 9253 (23.0) | 4 |
| Rural hospital, No. (%) | 5 (3.7) | 1251 (3.1) | 3.1 |
| Annual IV rtPA cases, median (IQR) | 23.0 (16.6–38.2) | 26.0 (15.9–38.6) | 8.6 |
GI: gastrointestinal; IQR: interquartile range; EMS: emergency medical services; sBP: systolic blood pressure; dPB: diastolic blood pressure; rtPA: recombinant tissue plasminogen activator.
Ethical approval for this study was obtained from the institutional review board of Duke University. Each participating hospital received either human research approval to enrol patients without individual patient consent under the Common Rule or a waiver of authorisation and exemption from subsequent review by their institutional review board. All statistical analyses were performed by the Duke Clinical Research Institute using SAS software version 9.4 (SAS Institute, Inc., Cary, NC). All P-values are two-sided, and P < 0.05 was considered statistically significant and 95% confidence interval was reported.
Results
Baseline characteristics
Our study included a total of 40,396 AIS patients who were treated with rtPA (mean age [SD] 81.0 [8.1] years; 41.9% women) at 1522 US sites between February 2009 and December 2015 (Figure 1). Of these, 136 patients (0.3%) had GI malignancy (n = 93) within one year or recent GI bleeding (n = 43) within 21 days. The median time interval from recent GI bleeding event to stroke onset was 15 days (25–75 percentiles 10–18) with a minimum of 3 days and maximum of 21 days. Baseline characteristics by GI conditions are shown in Table 1. Patients with GI malignancy or bleeding were more likely to be male and non-Hispanic white, and have a higher prevalence of atrial fibrillation or flutter and diabetes. The severity of AIS at admission as measured by NIHSS was greater in patients with GI malignancy or bleeding than those without (median [IQR]: 14.0 [8.0–19.0] vs. 11.0 [6.0–18.0]).
Among AIS patients with GI malignancy or bleeding who arrived within the 3.5 h time window, 148 were otherwise eligible but did not receive rtPA (Figure 1). Therefore, in the GWTG-Stroke/CMS linked dataset between February 2009 and December 2015, there were a total of 284 AIS patients with GI malignancy or bleeding otherwise eligible for rtPA, and of these, 47.9% (n = 136) was actually treated with rtPA. When compared with those treated with rtPA, patients not receiving rtPA were more likely to be older and female, have a higher prevalence of comorbidities, arrive later (median [IQR] onset to arrival time: 65.5 [41.5–94.0] min vs. 55.0 [35.0–89.0] min), be on oral anticoagulants prior to AIS, have less severe AIS (median [IQR]: 10.0 [4.0–18.0] vs. 14.0 [8.0–19.0]), and be treated in less experienced institutes for rtPA use (eTable 1 in the Supplement).
Clinical outcomes
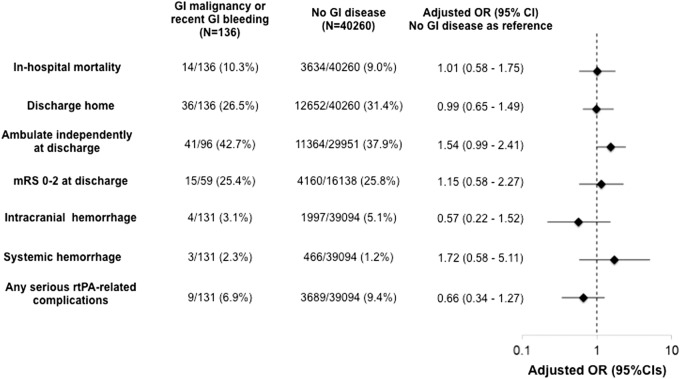
Clinical outcomes by the presence or absence of GI malignancy or bleeding are summarised in Figure 2. The unadjusted in-hospital mortality rates were 10.3% for those with GI malignancy or bleeding and 9.0% for those without. After the adjustment for confounders, there was no difference in in-hospital mortality between groups (adjusted odds ratio [aOR] 1.01, 95%CI 0.58–1.75, P = 0.97). Although the unadjusted rates of life-threatening systemic haemorrhage were numerically higher in those with GI malignancy or bleeding than those without, the difference was not significant after the risk adjustment (2.3% vs. 1.2%, aOR 1.72, 95%CI 0.58–5.11, P = 0.33).
Figure 2.
Clinical outcomes by the presence or absence of GI malignancy or recent GI bleeding.
GI: gastrointestinal; OR: odds ratio; CI: confidence interval; mRS: modified Rankin scale; rtPA: recombinant tissue plasminogen activator.
Sub-group analyses by GI condition
Among a total of 136 AIS patients with GI disease, 93 patients had GI malignancy and 43 patients had recent GI bleeding. Clinical outcomes by GI malignancy or recent GI bleeding are summarised in Table 2. Overall, even when GI malignancy or recent GI bleeding was separately analysed, findings were consistent with the main analysis.
Table 2.
Clinical outcomes in sub-group analyses.
| GI malignancy in past 1 year | No GI malignancy in past 1 year | Adjusted OR (95% CI) | P | Recent GI bleeding | No GI bleeding in past 21 days | Adjusted OR (95% CI) | P | |
|---|---|---|---|---|---|---|---|---|
| N = 93 | N = 40,303 | N = 43 | N = 39,781 | |||||
| In-hospital outcomes | ||||||||
| In-hospital mortality | 10/93 (10.8%) | 3638/40,303 (9.0%) | 1.14 (0.57, 2.30) | 0.71 | 4/43 (9.3%) | 3572/39,781 (9.0%) | 0.79 (0.29, 2.12) | 0.64 |
| In-hospital death or discharge to hospice | 21/93 (22.6%) | 7141/40,303 (17.7%) | 1.36 (0.76, 2.44) | 0.3 | 7/43 (16.3%) | 7011/39,781 (17.6%) | 0.57 (0.26, 1.25) | 0.16 |
| Able to ambulate independently at discharge | 30/65 (46.2%) | 11,375/29,982 (37.9%) | 1.49 (0.86, 2.57) | 0.15 | 11/31 (35.5%) | 11,273/29,621 (38.1%) | 1.68 (0.82, 3.46) | 0.16 |
| Discharge home | 27/93 (29.0%) | 12,661/40,303 (31.4%) | 0.95 (0.59, 1.50) | 0.81 | 9/43 (20.9%) | 12,539/39,781 (31.5%) | 1.10 (0.46, 2.61) | 0.83 |
| Modified Rankin scale 0–2 | 13/41 (31.7%) | 4162/16,156 (25.8%) | 1.32 (0.61, 2.88) | 0.48 | 2/18 (11.1%) | 4137/15,937 (26.0%) | 0.65 (0.11, 3.75) | 0.63 |
| rtPA related complication | ||||||||
| Symptomatic intracranial haemorrhage | 4/89 (4.5%) | 1997/39,136 (5.1%) | 0.88 (0.32, 2.40) | 0.81 | 0/42 (0%) | 1968/39,628 (5.1%) | NA | NA |
| Life-threatening systemic haemorrhage | 2/89 (2.3%) | 467/39,136 (1.2%) | 1.52 (0.42, 5.56) | 0.52 | 1/42 (2.4%) | 456/38,628 (1.2%) | 2.33 (0.33, 16.3) | 0.4 |
| Any serious complication related to rtPA | 8/89 (9.0%) | 3690/39,136 (9.4%) | 0.92 (0.44, 1.89) | 0.81 | 1/42 (2.4%) | 3632/38,628 (9.4%) | 0.20 (0.03, 1.29) | 0.09 |
GI: gastrointestinal; OR: odds ratio; rtPA: recombinant tissue plasminogen activator; NA: not applicable.
Discussion
In this registry-based retrospective cohort study, among >40,000 AIS patients who were treated with rtPA, 0.3% of patients had GI malignancy or recent GI bleeding. After the risk adjustment, GI malignancy or recent GI bleeding was not associated with increased risks of in-hospital mortality and bleeding complications compared with no history of GI malignancy or bleeding.
Owing to the concern for exacerbating risk of bleeding, GI malignancy and recent GI bleeding were excluded from the landmark clinical trials evaluating the efficacy and safety of rtPA in patients with a diagnosis of AIS, such as NINDS and ECASS 2 trials.7,8 As a result, these conditions are considered to be contraindicated for the use of rtPA in the AHA/ASA guidelines.1 Reflecting the lack of evidence, the relevant statement in the 2018 guideline is not identical to that in the 2013 guideline. In the 2013 guideline, “active internal bleeding” is listed as a contraindication and differentiated from “recent GI haemorrhage within previous 21 days” that is considered as a warning,9 whereas, in the 2018 guideline, they are unified into “recent GI bleeding event within 21 days of their stroke event” and considered as a contraindication.1 In addition, a structural GI malignancy is newly listed as a contraindication.1 However, to date, there is no study to support or refute this statement. In our analysis, the rates of mortality and bleeding complications in patients with GI malignancy or recent GI bleeding were not significantly higher than those in patients without GI malignancy or bleeding, suggesting that use of rtPA for patients with GI malignancy or recent GI bleeding may be considered in carefully selected patients.
The safety of thrombolytic therapy for treating AIS in patients with cancer is still controversial;10–18 however, the majority of previous studies suggest that rtPA may be able to be used safely in selected patients. Murthy et al.10 reported the largest study from the US Nationwide Inpatient Sample evaluating in-hospital outcomes after rtPA use in AIS patients with cancer (n = 807) compared with non-cancer patients (n = 31,769) and found that there was no difference in rates of in-hospital mortality and intracerebral hemorrhage.10 To the contrary, Nam et al.17 retrospectively reviewed 12 AIS patients with active cancer treated with rtPA and showed their poor clinical outcomes. These discrepancies may be due to the difference in the definition of malignancy and severity of the indexed stroke among studies. Importantly, none of these prior works were specific to GI malignancy. To our knowledge, our present study analysed the largest scale of AIS patients with GI malignancy and suggested the potential safety of rtPA use for this population.
No data are available with respect to the safety of rtPA use in patients with recent history of GI bleeding event within 21 days of the indexed stroke event. To date, two studies assessed the safety of off-label use of rtPA for treating AIS and showed the off-label thrombolysis therapy was not associated with poor clinical outcomes.19,20 In these two studies, a total of nine patients with “systemic disease with risk of bleeding” were included; however, there were no patients with recent GI bleeding. Given no difference in subsequent bleeding events after rtPA use, our study suggests that thrombolysis therapy may be considered, once a bleeding site is well controlled.
Limitation
There are several limitations in our study. First, our study does not have any information regarding the severity of underlying GI malignancy and recent GI bleeding, which may affect mortality and subsequent bleeding events. Second, despite the use of the largest stroke registry in the US, sample size may not be sufficient to analyse the safety of rtPA use in patients with GI malignancy or recent GI bleeding. However, we would like to emphasise that this is the first report to evaluate the safety of rtPA use in this specific population with this large-scale database. Third, rtPA was used only for selected subset in patients with GI malignancy or bleeding, which may have skewed the results. This selection bias may have resulted in either underestimated or overestimated risks in patients with GI malignancy or bleeding. Fourth, we were unable to evaluate the benefits of rtPA vs. no rtPA in patients with GI malignancy or bleeding because 90-day mRS and other post discharge functional outcomes are not collected in the registry. Fifth, we did not have data on concomitant use of proton pump inhibitors despite their potential effects on reducing the risk of GI bleeding.21,22 However, their use would likely be considered in patients at known high risk of upper GI bleeding.
Conclusions
In this observational cohort, we did not find statistically significant increased risk of mortality and bleeding complications in rtPA-treated patients with structural GI malignancy or recent GI bleeding within 21 days of their index stroke.
Supplemental Material
Supplemental Material for Thrombolytic therapy in older acute ischemic stroke patients with gastrointestinal malignancy or recent bleeding by Taku Inohara, Li Liang, Andrzej S Kosinski, Eric E Smith, Lee H Schwamm, Adrian F Hernandez, Deepak L Bhatt, Gregg C Fonarow, Eric D Peterson and Ying Xian in European Stroke Journal
Acknowledgements
None.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: T Inohara: Research Grant: JSPS Overseas Research fellowship and Boston Scientific.
L Liang: No relevant disclosures to report.
A Kosinski: No relevant disclosures to report.
EE Smith: Member of the Get With the Guidelines Steering Committee; Consultant: Portola and Alnylam Pharmaceuticals.
LH Schwamm: Principal investigator of an investigator-initiated study of extended-window intravenous thrombolysis funded by the National Institutes of Neurological Disorders and Stroke (clinicaltrials.gov/show/NCT01282242) for which Genentech provided alteplase free of charge to Massachusetts General Hospital as well as supplemental per-patient payments to participating sites; serving as chair of the American Stroke Association Advisory Committee, GWTG-Stroke Systems of Care advisory group, stroke systems consultant to the Massachusetts Department of Public Health; and serving as a scientific consultant to LifeImage regarding user interface design and usability, and regarding trial design and conduct to Penumbra (data and safety monitoring committee, Separator 3D and MIND trials) and Medtronic (Victory AF and Stroke AF trials).
AF Hernandez: Research Grant: AstraZeneca, Bristol-Myers Squibb, Daiichi, GlaxoSmithKline, Janssen, Merck, Novartis and Verily Honoraria: AstraZeneca, Bayer, Boehringer Ingelheim Merck, and Novartis.
DL Bhatt reports the following financial disclosures: Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, Takeda.
GC Fonarow: Member of GWTG Steering Committee; Grant funding from Patient Centred Outcome Research Institute; Employee of the University of California which has a patent on an endovascular therapy device, Consultant: Janssen.
ED Peterson: Research Grant: Janssen, Genetech, AHA Get With The Guidelines (GWTG)-Stroke Analytic. Consultant/Advisory Board: Janssen, Boehringer Ingelhiem, Sanofi, Bayer, Merck, Astra Zeneca, Signal Path, and Venable.
Y Xian: Research Grant: Genentech, Janssen, and Daiichi Sankyo.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by awards from the American Heart Association (13CRP14410024 and 14SDG20460081) awarded to Dr. Ying Xian. The Get With The Guidelines-Stroke (GWTG-Stroke) program is provided by the American Heart Association/American Stroke Association (AHA/ASA). GWTG-Stroke is sponsored, in part, by Medtronic and has been funded in the past through support from Boeringher-Ingelheim, Merck, Bristol-Myers Squib/Sanofi Pharmaceutical Partnership, Janseen Pharmaceutical Companies of Johnson & Johnson, and the American Heart Association Pharmaceutical Roundtable. The funding organisation had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Informed consent
Each participating hospital received either human research approval to enrol patients without individual patient consent under the Common Rule or a waiver of authorisation and exemption from subsequent review by their institutional review board.
Ethical approval
Ethical approval for this study was obtained from the institutional review board of Duke University.
Guarantor
YX.
Trial registration
ClinicalTrials.gov Identifier: NCT02693223.
Authors’ contributions
Dr. Taku Inohara and Dr. Ying Xian had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Dr. Taku Inohara and Dr. Ying Xian.
Acquisition, analysis, or interpretation of data: Dr. Taku Inohara, Dr. Li Liang, Dr. Andrzej Kosinski, Dr. Eric E. Smith, Dr. Lee H. Schwamm, Dr. Adrian F. Hernandez, Dr. Deepak L. Bhatt, Dr. Gregg C. Fonarow, Dr. Eric D. Peterson, and Dr. Ying Xian.
Drafting of the manuscript: Dr. Taku Inohara and Dr. Ying Xian.
Critical revision of the manuscript for important intellectual content: Dr. Li Liang, Dr. Andrzej Kosinski, Dr. Eric E. Smith, Dr. Lee H. Schwamm, Dr. Adrian F. Hernandez, Dr. Deepak L. Bhatt, Dr. Gregg C. Fonarow, Dr. Eric D. Peterson, and Dr. Ying Xian.
Statistical analysis: Dr. Li Liang and Dr. Andrzej Kosinski.
Obtained funding: Dr. Ying Xian, Dr. Adrian F. Hernandez, Dr. Eric D. Peterson, and Dr. Gregg C. Fonarow.
Administrative, technical, or material support: Dr. Ying Xian, Dr. Adrian F. Hernandez, and Dr. Eric D. Peterson.
Study supervision: Dr. Ying Xian and Dr. Eric D. Peterson.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 2.Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016; 47: 581–641. [DOI] [PubMed] [Google Scholar]
- 3.Fonarow GC, Reeves MJ, Smith EE, et al. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in Get With The Guidelines-Stroke. Circ Cardiovasc Qual Outcomes 2010; 3: 291–302. [DOI] [PubMed] [Google Scholar]
- 4.Hammill BG, Hernandez AF, Peterson ED, et al. Linking inpatient clinical registry data to medicare claims data using indirect identifiers. Am Heart J 2009; 157: 995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reeves MJ, Fonarow GC, Smith EE, et al. Representativeness of the Get with The Guidelines-Stroke registry: comparison of patient and hospital characteristics among medicare beneficiaries hospitalized with ischemic stroke. Stroke 2012; 43: 44–49. [DOI] [PubMed] [Google Scholar]
- 6.Xian Y, Fonarow GC, Reeves MJ, et al. Data quality in the American Heart Association Get With The Guidelines-stroke (GWTG-Stroke): Results from a national data validation audit. Am Heart J 2012; 163: 392–398. [DOI] [PubMed] [Google Scholar]
- 7. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 8.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS ii). Second European-Australasian acute stroke study investigators. Lancet 1998; 352: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 9.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 10.Murthy SB, Karanth S, Shah S, et al. Thrombolysis for acute ischemic stroke in patients with cancer: a population study. Stroke 2013; 44: 3573–3576. [DOI] [PubMed] [Google Scholar]
- 11.Selvik HA, Naess H, Kvistad CE. Intravenous thrombolysis in ischemic stroke patients with active cancer. Front Neurol 2018; 9: 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masrur S, Abdullah AR, Smith EE, et al. Risk of thrombolytic therapy for acute ischemic stroke in patients with current malignancy. J Stroke Cerebrovasc Dis 2011; 20: 124–130. [DOI] [PubMed] [Google Scholar]
- 13.Sobolewski P, Brola W, Szczuchniak W, et al. Safety of intravenous thrombolysis for acute ischaemic stroke including concomitant neoplastic disease sufferers - experience from poland. Int J Clin Pract 2015; 69: 666–673. [DOI] [PubMed] [Google Scholar]
- 14.Graber JJ, Nayak L, Deangelis LM. Use of recombinant tissue plasminogen activator in cancer patients with acute stroke. J Neurooncol 2012; 107: 571–573. [DOI] [PubMed] [Google Scholar]
- 15.Casado-Naranjo I, Calle ML, Falcon A, et al. Intravenous thrombolysis for acute stroke in patients with cancer. J Neurol Neurosurg Psychiatry 2011; 82: 1404–1405. [DOI] [PubMed] [Google Scholar]
- 16.Cappellari M, Carletti M, Micheletti N, et al. Intravenous alteplase for acute ischemic stroke in patients with current malignant neoplasm. J Neurol Sci 2013; 325: 100–102. [DOI] [PubMed] [Google Scholar]
- 17.Nam KW, Kim CK, Kim TJ, et al. Intravenous thrombolysis in acute ischemic stroke with active cancer. BioMed Res Int 2017; 2017: 4635829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weeda ER, Bohm N. Association between comorbid cancer and outcomes among admissions for acute ischemic stroke receiving systemic thrombolysis. Int J Stroke 2019; 14: 48–52. [DOI] [PubMed] [Google Scholar]
- 19.Meretoja A, Putaala J, Tatlisumak T, et al. Off-label thrombolysis is not associated with poor outcome in patients with stroke. Stroke 2010; 41: 1450–1458. [DOI] [PubMed] [Google Scholar]
- 20.Guillan M, Alonso-Canovas A, Garcia-Caldentey J, et al. Off-label intravenous thrombolysis in acute stroke. Eur J Neurol 2012; 19: 390–394. [DOI] [PubMed] [Google Scholar]
- 21.Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363: 1909–1917. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118: 1894–1909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Thrombolytic therapy in older acute ischemic stroke patients with gastrointestinal malignancy or recent bleeding by Taku Inohara, Li Liang, Andrzej S Kosinski, Eric E Smith, Lee H Schwamm, Adrian F Hernandez, Deepak L Bhatt, Gregg C Fonarow, Eric D Peterson and Ying Xian in European Stroke Journal