Short abstract
Purpose
The aim of this systematic review and meta-analysis is to determine the diagnostic accuracy of computed tomography brain perfusion in the prediction of haemorrhagic transformation and patient outcome in acute ischaemic stroke.
Method
Electronic databases and grey literature published over the last 10 years related to healthcare and radiology were searched using the key terms: ‘computed tomography perfusion’, ‘haemorrhagic transformation’, ‘acute ischaemic stroke’, ‘functional outcome’ and their synonyms using both UK and American spellings. Inclusion criteria were: sample size at least 30 patients, original research, evaluate ability of computed tomography perfusion to predict haemorrhagic transformation, reports diagnostic accuracy or provide relevant data for a 2 × 2 contingency table, use follow-up non-contrast computed tomography (NCCT) or magnetic resonance imaging as reference standard.
Findings
Twelve studies were included in the review; studies cover a total of 808 patients. Haemorrhagic transformation occurred in 30.2% of patients. Pooled sensitivity and specificity were 85.9% (95% CI; 65–97%), 73.9% (95% CI; 45–92%) and accuracy of 79.1% (95% CI; 57–98%). Pooled NPV was 92.9% with a high false positive rate (19.8%), which could be explained in terms of outcome classification, acquisition artefact and computed tomography perfusion processing algorithms.
Discussion
This review evaluated the importance of using pre-defined threshold measurement for optimal prediction of HT, the relevance of patient pre-treatment clinical parameters to HT occurrence, the CTP parameters and the measurements that are independent predictors of HT, the significance of rtPA rather as an exacerbator of HT and the impact of both minor and major HT/PH on patient 20 functional outcome.
Conclusion
Computed tomography perfusion has a high sensitivity and moderately high specificity for prediction of haemorrhagic transformation in acute ischaemic stroke. Pre-treatment clinical decision making requires consideration of clinical factors in addition to imaging findings. This systematic review and meta-analysis highlights that pre-treatment computed tomography perfusion adds to clinical confidence by predicting potential for haemorrhage, both in thrombolysed and un-thrombolysed patients, and also influences decisions about alternative treatments for acute ischaemic stroke patients.
Keywords: Haemorrhagic transformation, acute ischaemic stroke, computed tomography perfusion, sensitivity, specificity, functional outcome
Introduction and literature review
Ischaemic stroke (IS) is associated with multiple risk factors which can be divided into modifiable (e.g. hypertension) and non-modifiable (e.g. race) categories. IS is caused by sudden blockage of the blood supply to a region of brain by a clot or acute stenosis.1
When a patient is admitted with stroke symptoms, diagnostic imaging is performed to answer four critical questions;2 (i) is there haemorrhage or a stroke mimic? (ii) can ischaemia or thrombus be identified? (iii) can an irreversibly damaged infarct core be identified? and (iv) can a salvageable tissue (penumbra) be identified?
Recombinant tissue plasminogen activator (rtPA), a thrombolytic drug, gives significant benefits to IS patients by reducing degree of disability. However, its administration is guided by knowing the interval since symptom onset – up to 4.5 h for intravenous administration (for good benefit and good functional outcome, but there are report of iv use up to 9 h) and up to 6–9 h using an intra-arterial route and measurable neuro-deficits, thus limiting the number of patients who can benefit.3 rtPA also causes fibrinolysis and thus carry the potential to induce or increase haemorrhagic transformation (HT) of ischaemic lesions.4,5 HT occurs in up to 40% of patients and is frequently seen within the first week of stroke6 causing rapid deterioration and poor functional outcome.7
Since about 2007, there has been a shift from the traditional ‘time-based’ approach to intervention to a physiology-based approach. This reflects a better understanding of the haemodynamic changes that occur during arterial occlusion and specifically the identification of two distinct regions in the ischaemic hemisphere – irreversibly damaged tissue (infarct core) and potentially salvageable tissue (Penumbra).8 This was made possible by advancements in neuro-imaging, especially the development of perfusion imaging. Perfusion imaging helps delineate the core-penumbra borders/mismatch.9 The appearance of this penumbra on imaging differs from individual to individual.10 The main aim of penumbra imaging is to identify the patients that are likely to improve following successful reperfusion from those that are at risk of complications, e.g. haemorrhage.9 Penumbra imaging is a useful predictor of HT in acute IS.11
Under normal circumstances, the blood–brain barrier (BBB) is impermeable to large molecules (e.g. blood cells); however, in the presence of pathology, e.g. neoplasm or ischaemia, BBB permeability increases with associated increased diffusion of large molecules into the extravascular space leading to haemorrhage and oedema. Loss of integrity of the BBB may play a role in the pathogenesis of HT in ischaemic lesions and is thought to occur as early as 3.5 h post onset.12
HT is associated with poorer clinical outcome independent of thrombolytic therapy; it occurs 10 times more frequently in thrombolysed patients versus placebo.10 The severity of HT is well correlated with functional outcome at three months,13 patients with larger haemorrhages being at higher risk of more severe disability and death.14 The ability to predict the likelihood of HT prior to thrombolytic intervention thus becomes more important as clinicians attempt to extend the potential benefits of therapeutic intervention across a larger number of patients.
Computed tomography (CT) has few contra-indications. It is relatively quick and cost-effective to perform and is an accessible imaging test for stroke patients. Several studies have shown correlation between CT perfusion (CTP) imaging and magnetic resonance imaging (MRI)8,15–19 and correlation of CTP penumbra appearances and permeability surface area measurements with stroke severity and HT.20,21 This paper presents a systematic review and meta-analysis evaluating the diagnostic accuracy of CTP to predict HT of acute ischaemia.
Methods
A broad and comprehensive search of MEDLINE, EMBASE, CINAHL, DARE, AMED, NICE Evidence, OVID, TRIP, COCHRANE AND Greylit electronic sources was performed. Hand searches of key radiology journals did not yield additional articles. A structured search strategy was adopted using the PICOS framework22 to identify relevant key concepts, search terms and keywords including ‘computed tomography perfusion’, ‘haemorrhagic transformation’, ‘sensitivity’, ‘specificity’, ‘acute ischaemic stroke’, ‘functional outcome’ that were then adapted for each separate database.
A two-stage approach to study selection (initial screening of titles and abstracts against inclusion criteria and then screening of full text to identify relevant articles)23 was undertaken to reduce subjectivity bias and the selection process was conducted using the PRISMA flowchart.24 Inclusion criteria were (1) sample size at least 30 IS patients, (2) admission CTP performed, (3) CT or MRI as reference standard, (4) Diagnostic accuracy values of CTP were reported and (5) primary studies published between 2007 and 2017.
Methodological quality of selected studies was assessed by using CASP diagnostic test checklist25 for methodological adequacy and the validity of results and the QUADAS 2 diagnostic accuracy checklist26 was used to address the risk of bias and applicability. The CASP tool required slight modification with addition of few questions but the QUADAS tool was not modified.
The Cochrane diagnostic accuracy data extraction tool27 was used to extract data in a consistent and standardised manner to eliminate subjectivity bias. Meta-analysis of extracted data was undertaken; combining the results of individual studies in a meta-analysis increases the power and precision in estimating the effects of intervention, narrows the confidence intervals and provide a greater chance of detecting a real effect as statistically significant.23 RevMan v5.3™ and MedCal v17.5™ software were used to synthesise the collated data, for subgroup analysis and to generate forest plots and a summary receiver operating characteristic curve and the final diagnostic accuracy values.
Results
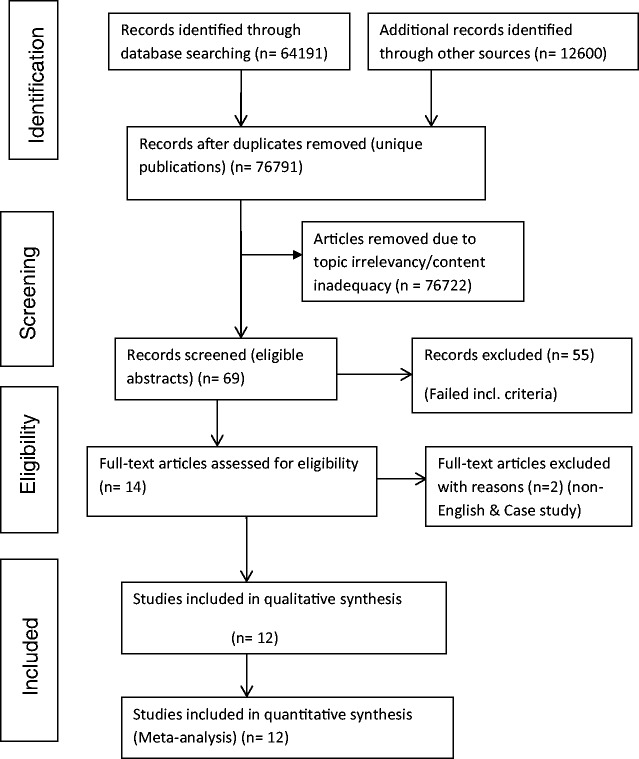
Search and selection ‘results’ – PRISMA chart
A total of 76,791 articles were initially identified and use of the inclusion/exclusion criteria narrowed this down to 12 studies which met the criteria (Figure 1).
Figure 1.
PRISMA (2009) search flow diagram for eligible articles.33
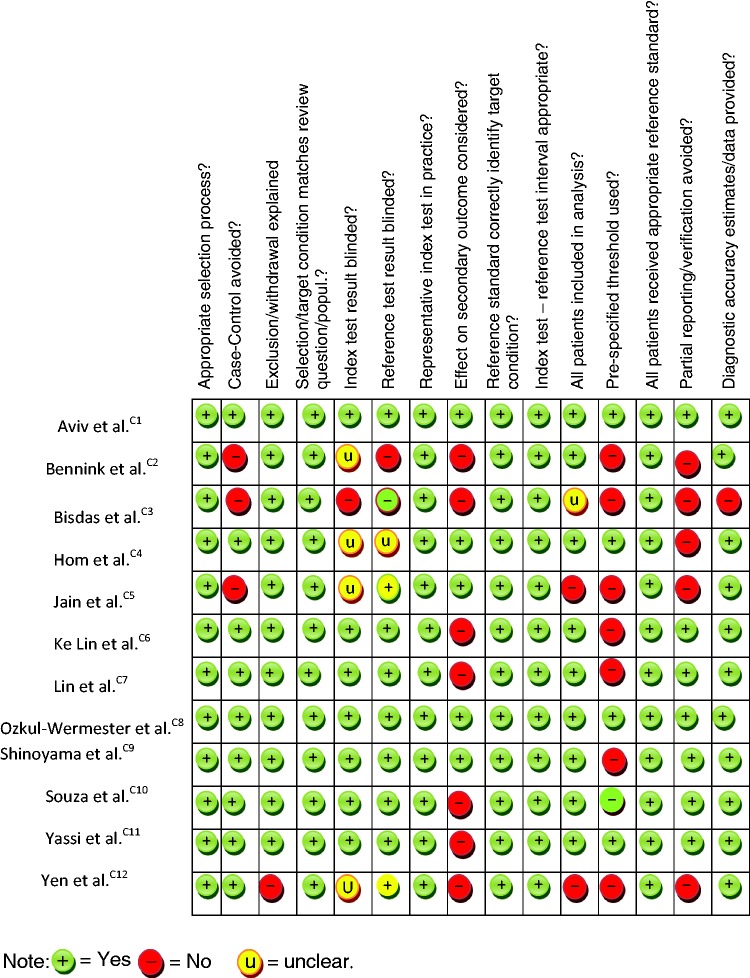
Quality assessment results
Table 1 shows the assessment outcome using QUADAS2 tool. Overall, selected studies were of good methodological quality (See also additional online table i; outcome using CASP tool).
Table 1.
Risk of bias and methodological quality assessment using QUADAS 2 tool.
|
Characteristic of included studies
The 12 selected studies were published between 2007 and 2017; the studies were heterogeneous in terms of design, index test parameters, z-coverage, perfusion image analysis method and reported accuracy values. The characteristics of the 12 studies are summarised in Table 5.
Table 5.
Characteristics of included studies.
| StudyC1–C12 | Year | Design | Pat. population (AIS) | NIHSS Score (Mean or Median.) | Time to CTP (from onset) h | Patients Thrombolysed | Reference Standard | Time to Ref. standard | Outcome (HT) (%) | CTP analysis method | Review /stats. method. | Second. Outcome (mRs) (Disch. or 90 days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bisdas et al.C3 | 2007 | Cohort –retrosp. | 68 | Not stated | 2.5 h ± 2.3 | Non | NCCT brain | 2.1 days ± 1.8 | 8 HT (11.8%) | Distributed tracer kinetics | z-test | Nil stated |
| Hom et al.C4 | 2011 | Cohort – retrospe. | 32 | 14 (IQR10–17) | 2 h (1–12 h) | 6 | NCCT | 24.8 h (range = 4.75–75 h) | 3 SHT & 3 ME (19%) | Patlak model | Univariate and Multivariate regression | NIHSS incr. >4 = 8 pats. |
| Yassi et al.C11 | 2013 | Cohort –Prospec. | 132 | 13 (IQR=9–18) | 2.5 h ± 2 | 70 | NCCT or MRI brain | 23–48 h | 14 PH (6=sICH) (10.6%) Any-HT = 48 (36.6%) | Deconvolution (Patlak) | Mann-Whitney U and Fisher exact tests. Univariate and multivariate regression. | Nil stated |
| Shinoyama et al.C9 | 2013 | Cohort –retrospe. | 68 | 7 (range 0–30) | 3 (range 0–24 h) | 10 | MRI brain | 24 h and 2 weeks | 34HT (18HI & 16PH), (50%) | Deconvolution | Two-tailed X, unpaired student t, and Mann–Whitney U. | mRs 0–2 =11 and mRs 3–6= 23. |
| Bennink et al.C2 | 2015 | Cohort –Retrospe. | 60 | 11 (SD±6) | 1.38 h (±1 h) | 55 (40 no-HT and 15HT) | NCCT brain | 0–72 h | 20HT. (33.3%) | Non-linear regression- (deconvolution-based) | Pearson coeff. Mann-Whitney U, Wilcoxon rank test. | Not considered. |
| Aviv et al.C1 | 2009 | Cohort –prospect. | 41 | 15 (IQR 7–20) | 125 min ±46 min | 22 | CT and MRI | 5–7 days | 23HT (15HI, 8PH), (56.1%) | Deconvolution (Johnson-Wilson model) | Univ. + Multi. Regression. Unpaired t, Wilcoxon, Fisher exact and Pearson | HT mRs median=4 (IQR 2–6). No-HT mRs median=1 (IQR 0–2). |
| Lin et al.C7 | 2007 | Cohort –Retrospe | 50 | Not stated | <3 h | 18 | MRI + NCCT | 3 days (IQR 6 h–15 days) | 6HT (3 rtPA & 3 no-rtPA), (12%) | Deconvolution + Max. Slope | Wilcoxon signed-rank, Mann–Whitney U | Not considered |
| Jain et al.C5 | 2013 | Cohort –Retrospe | 83 (only 49 analysed) | 7 (IQR 3–15) | 124 min (IQR 64–314 min) | 23 | NCCT or MRI | 5 (IQR 3–12 days) | 16HT (19.3%) | Deconvolution | Wilcoxon rank sum and conditional regression. | HT group; median = 5 (IQR = 3–5). No-HT group; median =3.5 (IQR = 1–5). |
| Ozkul-Wermester et al.C8 | 2014 | Cohort –prospect | 86 | 12 (IQR 1–24) | 150 min (IQR 60–360 min) | 31 | MRI (+NCCT) | 5–7 days | 27HT. (31.4%) | Deconvolution | Fisher Exact, Mann–Whitney U, ROC. | HT group median= 3; No-HT group median = 2. |
| Souza et al.C10 | 2012 | Cohort –Retrospe | 96 | Mean 16.5 (HT) and10.9 (no-HT) | Median 3.9 h (IQR 2–5) | 44 | MRI (+NCCT) | 5 days | 22HT (23%) | Deconvolution | Student t, Mann–Whitney U and Chi-square. | Not considered. |
| Ke Lin et al.C6 | 2012 | Cohort –Retrospe | 84 | Median 12 (IQR 2–25) | 3.9 h ±2.0 h | 44 | MRI (+NCCT) | Not stated | 22HT (26.2%) | Deconvolution | Only Univ, + Multiv. Regression and ROC stated | Not considered |
| Yen et al.C12 | 2016 | Cohort –Retrospe | 42 (of 84 selected) | Not stated | 111 min | 38 | NCCT | 24 h + | 15HT (35.7%) | Deconvolution | Student-t | Not considered. |
Patient selection
Three studies selected patients prospectivelyC1,C8,C11 and nine selected them retrospectively.C2–C7,C9,C10,C12 The 12 studies collectively included a total of 874 patients although only 808 patients were accounted for in analysis; Jain et al.C5 excluded 34 patients because no matched-controls were found and Yen et al.C12 excluded 32 patients who had only MRI as follow-up. Average age of patients was 69.9 years (range33–93 years) and median admission NIHSS score was 11.4 (IQR 1–25).
Imaging protocols
Mean time from symptom onset to CTP was 2.5 h (±2.3 h; range 1–24 h). Four studies used NCCT alone as the reference standard, one study used MRI alone and seven studies used both NCCT and MRI. The mean time to reference standard imaging was 2.9 days (range 1–15 days). Thrombolysed patients tended to receive earlier reference standard/confirmatory test imaging.
Seven of the studies used ‘permeability surface area product’ (PS or relative PS) measurements for HT prediction although image acquisition time and technique varied. Souza et al.C10 suggested that for PS to correlate well with HT acquisition, time must be at least 120 s to allow for sufficient contrast leakage into extravascular space, and Lin et al.C7 argued that increased BBB permeability is possible within 2–4 h post ictus and should be detectable on admission CTP performed within this time. A summary of study imaging parameters is included in Tables 6 & ii (online).
Table 6.
CTP outcome ± statistical analysis.
| Study | Study type | Main CTP parameter for diag. | Mean ± SD or median (IQR) | Non-ischaemic (contralateral) region/Control. | Univ. regression or other analysis (predictor) | Multiv. regression (independ. predictor) | TP | TN | FP | FN | PPV % | NPV % | Sens. % | Spec. % | Accuracy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Bisdas et al.C3 | Retrospect. | PS | 71.5 ±150.0 | 13.6 ±61.2 | N/S | N/S | 8 | 60 | 0 | 0 | 100 | 100 | 100 | 100 | 100 |
| 2. Hom et al.C4 | Retrospect | Absolute BBBP threshold | >7 ml/100 g/min | Control (>5 mL/100 g/min) | BBBP, age, NIHSS, tPA, infarct vol. reperfusion score | BBBP + age + rtPA | 3 | 24 | 5 | 0 | 37.5 | 100 | 100 | 79 | 84.4 |
| 3. Yassi et al.C11 | Prospective | Optimal Tmax threshold/vol. | >14 s and vol. >5 ml | rCBF <30% of contralateral mean @ vol. of 12 ml. | Age, NIHSS, rtPA, vol. of Tmax >14 s, vol. of rCBF <30%. | rtPA + vol. of Tmax >14 s | 11 | 80 | 38 | 3 | 22.4 | 96.4 | 79 | 68 | 68.9 |
| 4. Shinoyama et al.C9 | Retrospect. | Initial TTP map defect | TAC time lag (27 s) or lack of peak in ischaemic area. | TTP @ Contralateral = 17 s (on TAC). | N/S | Not done | 34 | 28 | 6 | 0 | 85 | 100 | 100 | 82.4 | 91 |
| 5. Bennink et al.C2 | Retrospect. | NLR rPS infarct core | NLR rPS ; Threshold 1.12 | NLR rPS contralat = 0.89 | NLR; rKtrans, rCBF and rMTT. | NLR rPS | 15 | 30 | 10 | 5 | 60 | 85.7 | 75 | 75 | 75 |
| 6. Aviv et al.C1 | Prospect. | Mean PS ischaemic region | Mean PS (HT) =0.49. Threshold cut-off = 0.23 | Mean PS (no-HT) = 0.09 | PS, NIHSS, rtPA, ASPECT | PS and ASPECT | 18 | 17 | 1 | 5 | 94 | 75 | 77 | 94 | 84 |
| 7. Lin et al.C7 | Retrospect | Mean PS infarct | Mean PS infarct = 3.5±3.1, Threshold PS =5.99 | Mean PS control = 0.28±0.27. | N/S | N/S | 5 | 44 | 0 | 1 | 83.3 | 100 | 83.3 | 100 | 98 |
| 8. Jain et al.C5 | Retrospect | rCBV | Median 0.92 (0.83–1.07) | Median 1.11 (1.0–1.29) | rCBF, rMTT | rCBV | 10 | 26 | 10 | 3 | 50 | 89.6 | 76.9 | 72.2 | 73.5 |
| 9. Ozkul-Wermester et al.C4 | Prospective | PS infarct (HT) | Median 1.41 (0.66–2.75) threshold =0.84 | (No-HT) median PS =1.04 | NIHSS, A. Fib., MTT vol., adm. Glucose, Large vessel occlusion, PS infarct, DM. | PS infarct (HT). A. Fib., MTT vol., | 26 | 24 | 35 | 1 | 36 | 96 | 96 | 41 | 58.1 |
| 10. Souza et al.C10 | Retrospect | rCBF and rMTT | Mean rCBF (HT) =75.2 ±6.9; Mean rMTT = 120.4 ±10.0 | Mean rCBF (no-HT) = 53.6 ±5.0; Mean rMTT (no-HT) =89.5 ±6.3 | NIHSS, rCBF, rMTT (DWI), mechanical thrombectomy, LVO | Threshold rCBF <0.48 and rMTT >1.3 | 19 18 | 3944 | 3530 | 34 | 35.237.5 | 92.991.7 | 8682 | 53 60 | 60.4 64.6 |
| 11. Ke Lin et al.C6 | Retrospect | CBV + ASPECT | Threshold CBV < 0.5 ml/100 g | No-HT group, threshold 0.8 ml/100 g | Serum glucose, NIHSS, proximal occlusion, collateral score, ASPECTs, CBV | CBV for any-HT, ASPECTS for sICH | 19 | 48 | 14 | 3 | 57.6 | 94.1 | 86.4 | 77.4 | 79.8 |
| 12. Yen et al.C12 | Retrospect | rPS | rPS infarct (HT) = 1.71 ± 1.6 | rPS infarct (no-HT) = 1.07 ± 1.30 | Not stated | Overall mean rPS threshold = 1.3 | 11 | 21 | 6 | 4 | 62.5 | 84.6 | 71.4 | 78.6 | 76.2 |
All studies used low-osmolar non-ionic contrast media with similar molecular weights (800 mg/mol ± 22) giving relatively similar diffusion performance (permeability) across studies.28
CTP software and analysis
All studies used deconvolution-based software except Bisdas et al.C3 which used custom-written software. Lin et al.C7 initially used Maximum Slope method to calculate parametric maps but subsequently used deconvolution method to calculate PS for HT prediction. In general, software algorithms were either tracer delay-sensitive, delay-insensitive or delay-corrected. A detailed discussion about these principles could be found in Kontas et al.2,29 and Abels et al.30
The range of statistical tests used by each study is shown in Table 5 and Table 6 displays the regression analysis used to determine the parameters that are independent predictors of HT with a level of significance set at p < 0.05 and the diagnostic accuracy values (see also online table ii for review methods used by each study).
Eleven (92%) of the included studies used a combination of visual assessment and a pre-defined threshold to determine the optimal measurement(s) for predicting HT.
These thresholds are quantitative measurements (numbers) representing the point at which HT is deemed certain by the authors, and the value of which depends on the parameter being used for measurement, e.g. relative permeability surface area, infarct size, etc.
Primary and secondary outcome measures
All studies used HT as primary outcome measure except Yassi et al.C11 which used parenchymal haemorrhage (PH). Hom et al.,C4 Ke Lin et al.C6 and Yen et al.C12 also considered whether HT (or PH) is symptomatic. Five studies (Hom,C4 Shinoyama,C9 Aviv,C1 JainC5 and Ozkul-WermesterC8) considered the effect of HT on patient’s functional (2°) outcome, whether HT was minor, asymptomatic or symptomatic; overall concluding that patients with HT were worse off at discharge or at 90-day follow-up. Example of 2° outcome measures stated in selected studies includes disability (e.g. hemiplegia), dependence and death, and severity of 1° outcome correlates well with degree of 2° outcome. See also Table 2 for pooled numerical results of each 1° outcome. As shown, 30 patients (12.3%) had symptomatic HT/PH, with sICH being defined as a CT evidence of a bleed correlating with patient symptoms and neurological deterioration >4 on NIHSS. Table 3 shows the overall accuracy of CTP to predict symptomatic HT.
Table 2.
Number of HT, PH and symptomatic HT in included studies.
| Study | All HT | PH | Symptomatic HT/PH |
|---|---|---|---|
| Bisdas et al.C3 | 8 | N/S | N/S |
| Hom et al.C4 | 3 | 3 | 3 |
| Yassi et al.C11 | 48 | 14 | 6 |
| Shinoyama et al.C9 | 34 | 16 | 4 |
| Bennink et al.C2 | 20 | N/S | N/S |
| Aviv et al.C1 | 23 | 8 | N/S |
| Lin et al.C7 | 6 | 3 | 3 (All PH) |
| Jain et al.C5 | 16 | 1 | N/S |
| Ozkul-Werm. et al 2014 | 27 | 11 | N/S |
| Souza et al.C10 | 22 | 3 | 6 (4HI, 1PH1, 1PH2) |
| Ke Lin et al.C6 | 22 | 8 | 8 (all PH) |
| Yen et al.C12 | 15 | 7 | N/S |
| Total | 244 | 74 | 30 |
N/S: not specified; PH: parenchymal haematoma; HI: haemorrhagic infarction.
Table 3.
Accuracy of CTP to predict symptomatic HT (from included studies).
Thrombolysis/thrombectomy and HT occurrence
Across the 12 studies, 361 patients (44.7%) were thrombolysed (± thrombectomy), 134 (37.1%) of these developing HT and 227 shows no HT. On the other hand, 447 patients were not thrombolysed but 110 (24.6%) of these developed HT. Thus, HT occurred in 244 patients (30.2%) in total (Table 5).
Diagnostic accuracy analysis
Diagnostic accuracy of CTP to predict HT was given in 11 of the 12 studies. Bisdas et al.C3 stated only odds ratio and p-values; 2 × 2 contingency tables were constructed for each study with estimated values calculated from the ‘Mean (+/− SD)’ data in Bisdas et al.C3 This is an acceptable approach/practice according to CRD28 and pooled values are further displayed via subgroup analysis – see Table 7.
Table 7.
Pooled sub-group analysis.
| Analysis type | Number of studies | Number of patients | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| All studies | 12 | 808 | 85.9 | 73.9 |
| Excluding Bisdas et al.C3 | 11 | 740 | 84.6 | 74.6 |
| Prospective studiesC1,8,11 | 3 | 259 | 84 | 67.7 |
| Retrospective studiesC2,3–7,9,10,12 | 9 | 549 | 86.5 | 79.7 |
| Z-coverage of 4–16 cm | 9 | 579 | 84.6 | 71.5 |
| Delayed/multi-phase >120 s | 4 | 219 | 87 | 72.3 |
| All studies excluding matched case-control studies | 9 | 631 | 86.6 | 74.8 |
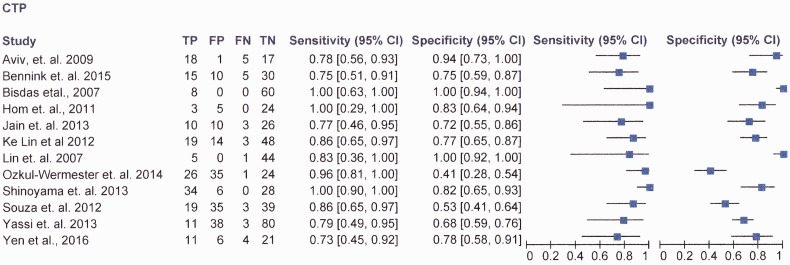
The lowest sensitivity reported was 71.4% (Yen et al.C12), and the highest being 100% (Hom et al.,C4 Shinoyama et al.C9 and Bisdas et al.C3). The lowest specificity was 41%,C8 and (Ozkul-Wermester et al.C8)), the highest was 100% (Lin et al.C7).
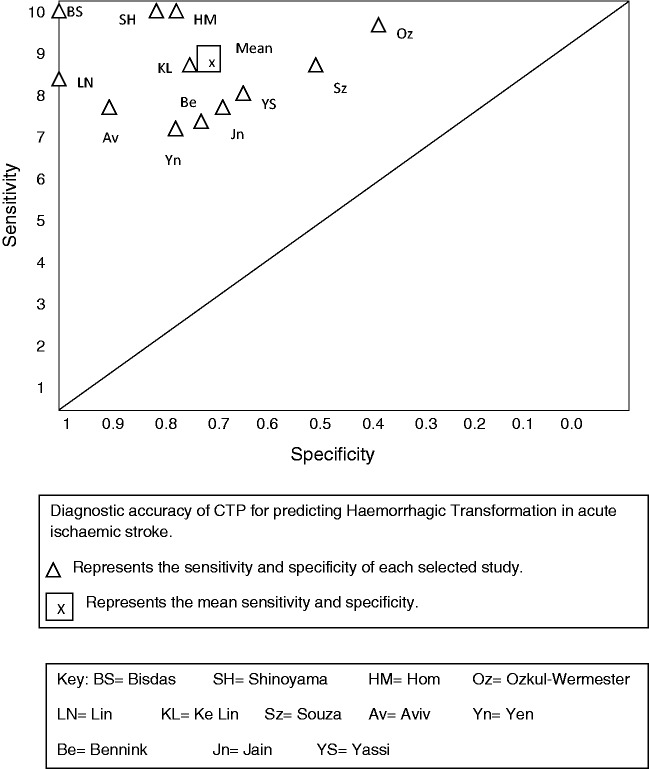
Figure 2 shows the study sensitivity and specificity with 95% confidence intervals and paired forest plots (see also online fig. i MedCal v17.5 software display of diagnostic accuracy). Figure 3 shows pooled estimates of sensitivity and specificity in the receiver operating characteristic curve (ROC) space.
Figure 2.
RevMan v5.3 software data.
Figure 3.
Summary sensitivity and specificity in ROC space.
Table 4 shows the result of Multivariate regression analysis performed by authors to determine independent predictors of HT (or PH) (and their threshold).
Table 4.
Multivariate analysis result.
| CTP imaging parameter; independent predictor of HT | Threshold value for HT |
|---|---|
| High BBB permeability | BBBP >7 ml/100 g/min |
| Mean PS Vol = 0.23 ml/100 g/min | |
| PS Infarct vol = 0.84 ml/100 g/min | |
| rPSCore = 1.12. | |
| Acute severe hypo-perfusion | CBV < 0.5 ml/100 g |
| rCBV = 1.09 | |
| rCBF < 0.48 | |
| Tmax > 14 s | |
| rMTT = 1.3 | |
| TTP = 0.27 s | |
| Infarct size | ASPECTS score < 5 |
| Pre-treatment clinical parameters | Atrial fibrillation |
| Advanced age | |
| High NIHSS score |
Note: The use of rtPA (or mechanical thrombectomy) in addition to any of the above carries a higher risk of HT.
BBBP: blood-brain-barrier permeability; PS: permeability surface area; rPS: relative permeability surface area; CBV: cerebral blood volume; rCBV: relative cerebral blood volume; rCBF: relative cerebral blood flow; Tmax: time of maximum; rMTT: relative mean transit time; TTP: time to Peak; NIHSS: national institute of health stroke score; ASPECTS: Alberta stroke programme early CT score.
From selected studies, overall odds ratio for HT is 2.319 (95%CI; 1.497–3.592; p = 0.0002) and relative risk ratio of HT is 1.938 (95%CI 1.348–2.788; p = 0.0004) (using MedCal v17.5) (OR = Bad outcome TP, FN vs. Good outcome TN, FP and RR = Experimental bad outcome TP, good outcome TN vs. Control bad outcome FN, good outcome FP).
There was no statistically significant difference in patients who had HT whether they received rtPA or did not (37.1% thrombolysed (p = 0.0019) vs. 24.6% un-thrombolysed (p = 0.0104)). However, there was a statistically significant difference between patients who received rtPA and then developed HT compared to those who received rtPA but did not develop HT (37.1% (p = 0.0019) vs. 62.9% (p = 0.998) respectively).
Discussion
This systematic review and meta-analysis demonstrated high sensitivity (85.9%, 95% CI; 65–97%) and moderately high specificity (73.9%, 95% CI; 45–92%) for CTP in predicting HT (overall accuracy of 79.1%). The high NPV (92.9%) and lower PPV (60.3%) suggested that despite high sensitivity, factors such as patient clinical status were also important consideration at thrombolysis decision point. This is reflected by the high false positive rate (19.8%; 160 of the 808 patients). On the other hand, the false negative rate is very low (3.5%; only 28 patients). Thus, the power of this index test lies in its high negative predictive value.
However, the review demonstrated methodological heterogeneity in selected studies including CTP data acquisition and analysis technique, index test measurement, outcome classification and some inappropriate exclusion. CTP parameters varied amongst the studies included in this review but the most commonly used diagnostic measurement was the permeability surface area product (PS or rPS). Eleven of the 12 included studies used a combination of visual assessment and a pre-defined threshold to determine the optimal measurement(s) for predicting HT. In all cases whatever CTP parameter was used, the location of measurement which produced the optimal threshold corresponded to the region of the most acute BBB injury, the highest permeability value or region of severe hypo-perfusion, and thus the region most likely to undergo HT. Another important advantage of the optimal threshold approach was that they represented the point at which HT was deemed certain (by the investigators) irrespective of whether or not the patient received thrombolysis, and thus are important markers for clinicians. There was also heterogeneity in study population, and these include age (range 33–93 years), gender (51% male), pre-treatment clinical factors (HTN, AF, Diabetes, use of antiplatelets, high admission glucose, etc.) and stroke severity measured by admission NIHSS score (Median was 11.4). These factors were given consideration in many of the selected studies because of their impact on risk of HT after rtPA and/or thrombectomy.
The parameters and thresholds used by these selected studies using regression analysis to determine the independent predictors of HT (or PH) include; CTP imaging appearance suggestive of high BBB permeability (i.e. BBBP > 7 ml/100 g/min; mean PSvol. 0.23 ml/100 g/min; PSinfarct vol. 0.84 ml/100 g/min; rPScore = 1.12); acute severe hypoperfusion (i.e. CBV < 0.5 ml/100 g; rCBV = 1.09; rCBF < 0.48; Tmax > 14 s; rMTT.1.3; TTP 0.27 s); infarct size (ASPECTS < 5) and pre-treatment clinical parameters; Atrial Fib., advanced age and high NHISS score. Any of the above plus rtPA or mechanical thrombectomy carries a higher risk for HT occurrence.
In comparison, there is literature evidence of the potential use of MRI to predict HT. MR perfusion maps showing large diffusion-weighted imaging (DWI) lesion or low apparent diffusion coefficient (ADC),15 extreme hypo-perfusion (low CBF, low CBV, high MTT, prolonged Tmax) showing as low signal intensity on T2*W (PWI)31 imaging, and features of microbleeds on T2W–GRE or early contrast enhancement on T1W imaging are useful radiological biomarkers. However, there is lack of consensus on the specific imaging parameter that best predict HT but current trend favours parameters with perfusion imaging and setting a critical threshold beyond which prediction of HT has a high specificity and sensitivity (e.g. CBV > 2 mL/100 g, CBF > 4.9 mL/100 g/min or MTT > 145% of contra-lateral side).
Ten of the 12 studies recorded false positive (FP) and false negative (FN) cases. The remaining two studiesC3,C7 used first pass acquisition data at which time it is possible that significant BBB injury has not occurred. In some studies, FP and FN cases were modified by choice of primary outcome measure. For example, Hom et al.C4 selected symptomatic HT plus ECASS III benchmark of clinical deterioration and NIHSS increase of > 4. Five of the patients in their study who met these criteria had malignant oedema, pneumonia or septicaemia, rather than HT. However, in other studies, it was not possible to determine the reason for classifying cases as FP or FN.
Similarly, in the area of the effect of chosen outcome on patient prognosis, 8 of the 12 studies considered that any HT at all was an important contributor to patient morbidity and mortality and included all patients with HT. Two studies, Ke LinC6 and YenC12 considered only symptomatic or catastrophic HT as important but still included patients with minor HT in their analysis. Two studies, YassiC11 and HomC4 considered only PH2 or symptomatic HT as important and eliminated patients with minor HT from their analysis. The relevance for this review relates to the fact that minor/asymptomatic HT if not predicted in advance has the potential to progress to severe and symptomatic HT if thrombolytic therapy is administered, and the consequent worsening of these patients’ secondary functional outcome. Similarly, as argued by Aviv et al.,C1 Lin et al.C7 and Shinoyama et al.,C9 even asymptomatic HT (without rtPA administration) requires close monitoring of blood pressure and discontinuation of anticoagulant/antiplatelet therapies to prevent development of catastrophic HT.
Although previous studies4,5 have suggested that rtPA increases the risk of HT, in this review and meta-analysis we found no statistically significant difference in patients who had HT whether they received rtPA or not (37.1% thrombolysed vs 24.6% un-thrombolysed had HT). However, there was a statistically significant difference between patients who received rtPA and then developed HT and those who received rtPA but did not develop HT (37.1% vs. 62.9%). This suggests that rather than having a direct causative effect, rtPA is more likely to exacerbate HT – the patients who had HT after rtPA will already have a condition that predispose them to HT (e.g. high BBB permeability) and were either tipped-over the threshold or their condition exacerbated by rtPA administration. CTP imaging is thus useful to determine the pre-treatment perfusion characteristics of an individual patient’s ischaemic lesion.32
This review has generated evidence that CT brain perfusion imaging can augment clinical confidence in stoke intervention decision making because of its ability to predict HT with high sensitivity and high negative predictive value.
We know that the extent of brain perfusion after an ischaemic event is individualistic in nature, that ischaemia induces injury to the BBB and that there is a potential for HT to develop in the most ischaemia-injured portion of the brain. In clinical practice therefore using CTP to both judge the extent of an individual’s perfusion status and predict the possibility of HT is a clinically reasonable approach to inform treatment option(s).
This review shows that both minor and major HT are significant for patients as both impact on functional outcome, and patients experiencing HT being worse-off at discharge or 90-day follow-up. Predicting even minor HT is important to clinicians because it influences their decision to either administer rtPA, or choose an alternative intervention and consider treatment choices for patients on other (co-morbid) medications before the stroke event.
Conclusion, recommendation and implication for practice
In conclusion, CTP is an accurate predictor of HT in acute IS patients and a useful prognostic tool for clinicians at the point of intervention decision making. It is therefore recommended that CTP be included in the initial (admission) imaging protocol of acute stroke patients. The technique could be readily incorporated into existing emergency CT protocols, and can be performed by most multi-slice scanners and adds minimal time (usually less than 10 min) to patient treatment for a clinically significant contribution to prognosis. CTP requires minimal additional resource by way of imaging staff training and/or scanner system upgrade.
Limitations and proposal for future research
This systematic review has some important limitations. First, the potential for a reviewer to erroneously interpret or report studies and for methodological failures. However, measures were taken to minimise these errors and bias by re-reading and double-checking every step of the review process and by conducting minor pilots where appropriate. Also, publication bias cannot be excluded as only English language articles were included. Similarly, majority of the selected studies were retrospective with the well-known inherent bias in such studies which may influence accuracy values. Future research may also consider the cost effectiveness of CTP against other imaging modalities.
Supplemental Material
Supplemental material, ESO883461 Supplemetal Figure1 for Diagnostic accuracy of computed tomography perfusion in the prediction of haemorrhagic transformation and patient outcome in acute ischaemic stroke: A systematic review and meta-analysis by Olushola D Adebayo and Gary Culpan in European Stroke Journal
Supplemental material, ESO883461 Supplemental Tables for Diagnostic accuracy of computed tomography perfusion in the prediction of haemorrhagic transformation and patient outcome in acute ischaemic stroke: A systematic review and meta-analysis by Olushola D Adebayo and Gary Culpan in European Stroke Journal
Acknowledgements
The reviewers wish to thank Dr David Ryan, Dr Cathy Dewhurst and Dr Marie Staunton for reviewing the piloting and extraction/QA tools and checking the accuracy of CTP information. And Dr Lee Crush for clinical mentoring and Mr Andrew Owen for facilitating the study.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Ethical approval was not sought for this article because it is a systematic review and meta-analysis of published research and does not involve the use of human or in vitro subject.
Informed consent
Informed consent was not sought for this article because it is a systematic review and meta-analysis of published research and does not involve the use of human or in vitro subject.
Guarantor
ODA.
Contributorship
ODA and GC conceived the study and researched literature. GC and ODA involved in protocol development, QA tools and data analysis. ODA involved in data extraction and quality assessment. ODA wrote the first draft of the manuscript. Both authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1.Howard R. Ischaemic stroke. Anaesth Intensive Care Med 2010; 11: 340–342. [Google Scholar]
- 2.Kontas AA, Goldmakher GV, Lee TY, et al. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, Part 1: theoretic basis. AJNR Am J Neuroradiol 2009. a; 30: 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schumacher HC, Bateman BT, Boden-Albala B, et al. Use of thrombolysis in acute ischemic stroke; analysis of the nationwide in-patient sample 1999 to 2004. Am Emerg Med 2007; 50: 99–107. [DOI] [PubMed] [Google Scholar]
- 4.NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke 1997; 28: 2109–2118. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Sabin J, Maisterra O, Santamarina E, et al. Factors Influencing haemorrhagic transformation in ischaemic stroke. Lancet Neurol 2013; 12: 689–705. [DOI] [PubMed] [Google Scholar]
- 6.Tong D, Adami A, Moseley ME, et al. Relationship between apparent diffusion coefficient and subsequent hemorrhagic transformation following acute ischemic stroke. Stroke 2000; 31: 2378–2384. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, Ko Y, Kim WJ, et al. Is asymptomatic hemorrhagic transformation really innocuous? Neurology 2012; 78: 421–426. [DOI] [PubMed] [Google Scholar]
- 8.Alves JE, Carneiro A, Xavier J. Reliability of CT perfusion in the evaluation of the ischemic penumbra. Neuroradiol J 2014; 27: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morhard D, Wirth CD, Reiser MF, et al. Optimal sequence timing of CT angiography and perfusion CT in patients with stroke. Eur J Radiol. 2013; 82: e286–e289. [DOI] [PubMed] [Google Scholar]
- 10.Fisher M, Albers GW. Advanced Imaging to extend the therapeutic time window of acute ischemic stroke. Ann Neurol 2013; 73: 4–9. [DOI] [PubMed] [Google Scholar]
- 11.Hom J, Dankbaar JW, Schneider T, et al. Optimal duration of acquisition for dynamic perfusion CT assessment of blood-brain-barrier permeability using the Patlak model. AJNR Am J Neuroradiol 2009; 30: 1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latour LL, Kang DW, Ezzedine MA, et al. Early blood-brain-barrier disruption in human focal brain ischemia. Ann Neurol 2004; 56: 468–477. [DOI] [PubMed] [Google Scholar]
- 13.Van Seeters T, Biessels GJ, van der Schaaf IC, et al. Prediction of outcome in patients with suspected acute ischemic stroke with CT perfusion and CT angiography; the Dutch acute stroke trial (DUST) study protocol. BMC Neurol 2014; 14: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sztriha LK, Manawadu D, Jarosz J, et al. Safety and clinical outcome of thrombolysis in ischaemic stroke using a perfusion CT mismatch between 3 and 6hours. PLoS One 2011; 6: e25796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim EY, Na DG, Kim SS, et al. Prediction of Hemorrhagic transformation in acute ischemic stroke: role of diffusion-weighted imaging and early parenchymal enhancement. Am J Neuroradiol 2005; 26: 1050–1055. [PMC free article] [PubMed] [Google Scholar]
- 16.Scalzo F, Alger JR, Hu X, et al. Multi-center prediction of hemorrhagic transformation of acute ischemic stroke using permeability imaging features. Magn Reson Imag 2013; 31: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer PW, Barak ER, Kamalian S, et al. Quantitative assessment of core/penumbra mismatch in acute stroke: CT and MR perfusion imaging are strongly correlated when sufficient brain volume is imaged. Stroke 2008; 39: 2986–2992. [DOI] [PubMed] [Google Scholar]
- 18.Soares BP, Dankbaar JW, Bredno J, et al. Automatic versus manual post-processing of perfusion-CT data in patients with acute cerebral ischemia: influence on interobserver variability. Neuroradiology 2009; 51: 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Esterre C, D; Roversi G, Padroni M, Bernardoni A, et al. CT perfusion cerebral blood volume does not always predict infarct core in acute ischemic stroke. Neurol Sci 2015; 36: 1777–1783. [DOI] [PubMed] [Google Scholar]
- 20.Zhu G, Michel P, Aghaebrahim A, et al. Perfusion computed tomography adds value compared with clinical evaluation, non-contrast computed tomography and computed tomography angiography in terms of prediction of outcome. Stroke 2013; 44: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 21.Horsch AD, Dankbaar JW, van Seeters T, et al. Relation between stroke severity, patient characteristics and CT-perfusion derived blood-brain-barrier permeability measurements in acute ischemic stroke. Clin Neuroradiol 2016; 26(4): 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall G, Sykes AE. Systematic reviews: a guide for radiographers and other healthcare professionals. Radiography 2010; 17: 158–164. [Google Scholar]
- 23.Centre for Reviews and Dissemination. Guidance for undertaking reviews in healthcare. 2nd ed York: University of York, 2009. [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Critical Appraisal Skills Programme (2013). Diagnostic Test Study Checklist v 3.0, www.casp-uk.et (accessed 11 December 2015).
- 26.Scottish Intercollegiate Guidelines Network (2006). Methodology checklist 5; studies of diagnostic accuracy, www.bris.ac.uk/quadas/ (accessed 12 December 2015).
- 27.Higgins JPT, Green S. (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011www.cochrane-handbook.org (accessed 15 December 2015).
- 28.American College of Radiology. Manual on contrast media, version 10.2. New York, USA: ACR, 2016. [Google Scholar]
- 29.Kontas AA, Goldmakher GV, Lee TY, et al. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 2: technical implementations. AJNR Am J Neuroradiol 2009. b; 30: 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abels B, Klotz E, Tomandl BF, et al. Perfusion CT in acute ischemic stroke; a qualitative and quantitative comparison of deconvolution and maximum slope approach. AJNR Am J Neuroradiol 2010; 31: 1690–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Copen WA, Schaefer PW, Wu O. MR perfusion imaging in acute ischemic stroke. Neuroimaging Clin N Am 2011; 21: 259–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biesbroek JM, Niesten JM, Dankbaar JW, et al. Diagnostic accuracy of CT perfusion imaging for detecting acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis 2013; 35: 493–501. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and MetaAnalyses: The PRISMA Statement. PLoS Med 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed]
List of selected studies
- C1.Aviv R, d’Esterre C, Murphy, et al. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology 2009; 250: 867–877. [DOI] [PubMed] [Google Scholar]
- C2.Bennink E, Horsch A, Dankbaar, et al. CT perfusion analysis by non-linear regression for predicting hemorrhagic transformation in ischemic stroke. Med Phys 2015; 42: 4610–4618. [DOI] [PubMed] [Google Scholar]
- C3.Bisdas S, Hartel M, Cheong, et al. Prediction of subsequent hemorrhage in acute ischemic stroke using permeability CT imaging and a distributed parameter tracer kinetic model. J Neuroradiol 2007; 34: 101–108. [DOI] [PubMed] [Google Scholar]
- C4.Hom J, Dankbaar J, Soares, et al. Blood-brain barrier permeability assessed by perfusion CT predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR Am J Neuroradiol 2011; 32: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C5.Jain A, Jain M, Kanthala, et al. Association of CT perfusion parameters with hemorrhagic transformation in acute ischemic stroke. AJNR Am J Neuroradiol 2013; 34: 1895–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C6.Ke Lin Zink W, Tsiouris J, John, et al. Risk assessment of hemorrhagic transformation of acute middle cerebral artery stroke using multimodal CT. J Neuroimaging 2012; 22: 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C7.Lin K, Kazmi K, Law, et al. Measuring elevated microvascular permeability and predicting hemorrhagic transformation in acute ischemic stroke using first-pass dynamic perfusion CT imaging. Am J Neuroradiol 2007; 28: 1292–1298. Doi.10.3174/ajnr.A0539. Accessed on 14/11/2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C8.Ozkul-Wermester O, Guegan-Massardier E, Triquenot, et al. Increased blood-brain barrier on perfusion computed tomography predicts hemorrhagic transformation in acute ischemic stroke. Eur Neurol 2014; 72: 45–53. [DOI] [PubMed] [Google Scholar]
- C9.Shinoyama M, Nakagarawa J, Yoneda, et al. Initial ‘TTP Map-Defect’ of computed tomography perfusion as a predictor of hemorrhagic transformation in acute ischemic stroke. Cerebrovasc Dis Extra 2013; 3: 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C10.Souza L, Payabvash S, Wang, et al. Admission CT perfusion is an independent predictor of hemorrhagic transformation in acute stroke with similar accuracy to DWI. Cerebrovasc Dis 2012; 33: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C11.Yassi N, Parsons M, Christensen, et al. Prediction of post-stroke hemorrhagic transformation using computed tomography perfusion. Stroke 2013; 44: 3039–3043. [DOI] [PubMed] [Google Scholar]
- C12.Yen P, Cobb A, Shiva Shanka J. Does computed tomography permeability predict hemorrhagic transformation after ischemic stroke? World J Radiol 2016; 8: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, ESO883461 Supplemetal Figure1 for Diagnostic accuracy of computed tomography perfusion in the prediction of haemorrhagic transformation and patient outcome in acute ischaemic stroke: A systematic review and meta-analysis by Olushola D Adebayo and Gary Culpan in European Stroke Journal
Supplemental material, ESO883461 Supplemental Tables for Diagnostic accuracy of computed tomography perfusion in the prediction of haemorrhagic transformation and patient outcome in acute ischaemic stroke: A systematic review and meta-analysis by Olushola D Adebayo and Gary Culpan in European Stroke Journal