Abstract
Considering data from in vitro and in vivo studies, cannabidiol (CBD) seems to be a promising candidate for the treatment of both somatic and psychiatric disorders. The aim of this review was to collect dose(s), dosage schemes, efficacy and safety reports of CBD use in adults from clinical studies. A systematic search was performed in PubMed, Embase and Cochrane library for articles published in English between January 1, 2000 and October 25, 2019. The search terms used were related to cannabis and CBD in adults. We identified 25 studies (927 patients; 538 men and 389 women), of which 22 studies were controlled clinical trials (833 patients) and three were observational designs (94 patients) from five countries. Formulations, dose and dosage schemes varied significantly between studies. Varying effects were identified from the randomized controlled trials (RCTs), more apparent effects from non-RCTs and minor safety issues in general. From the controlled trials, we identified anxiolytic effects with acute CBD administration, and therapeutic effects for social anxiety disorder, psychotic disorder and substance use disorders. In general, studies were heterogeneous and showed substantial risks of bias. Although promising results have been identified, considerable variation in dosage schemes and route of administration were employed across studies. There was evidence to support single dose positive effect on social anxiety disorder, short medium-term effects on symptomatic improvement in schizophrenia and lack of effect in the short medium-term on cognitive functioning in psychotic disorders. Overall, the administration was well tolerated with mild side effects.
Keywords: Cannabidiol, Dose, Safety, Efficacy, Cannabis
Introduction
Cannabis sativa L. has a long tradition of medical use. However, its clinical use has been limited due to the effects on the central nervous system and the possibility of drug abuse and addiction. The plant exudes a resin containing a mix of cannabinoids with two principal components, Δ9-tetrahydrocannbinol (THC) and cannabidiol (CBD). The structure and configuration of CBD was discovered in the 60s and has gained particular attention due to the lack of psychotropic activity. Because of its excellent tolerability in humans, the lack of psychoactive action and the low abuse potential, it seems an ideal candidate for use in a clinical context [1].
In addition to its good safety profile and the lack of psychoactive effects, CBD presents also a wide range of therapeutic effects [2]. Possibly for these reasons, CBD is currently one of the most studied cannabinoids [3]. Several experimental in vitro and in vivo studies have shown that CBD has a broad range of therapeutic applications, displaying anti-inflammatory and immunomodulatory properties [4], anti-psychotic [5], analgesic [6] and anti-epileptic [7] effects, among others. Compared to Δ9-THC, CBD shows low affinity for cannabinoid receptor type 1 (CB1) and type 2 (CB2) [8]. CB1 receptors are mainly found in the terminals of central and peripheral neurons and CB2 receptors mainly in immune cells [9]. Several in vitro studies have shown that CBD, at low concentrations, has weak CB1 and CB2 antagonistic effect [10]. It has also been reported that it behaves as a negative allosteric modulator of CB1, meaning that CBD does not activate the receptor directly but alter the potency and efficacy of orthosteric ligands of this receptor: Δ9-THC and 2-arachidonoylglycerol (2-AG) [11]. These preliminary results need further validation, but may explain the ability of CBD to antagonize some of the effects of Δ9-THC reported in in vitro, in vivo and clinical human studies [12]. It has also been suggested that the role of CBD as an allosteric modulator of CB1 can explain its therapeutic role in the treatment of central and peripheral nervous system disorders [2]. CBD has also shown to have a strong inhibition effect of neutrophil chemotaxis and proliferation. In addition, it may induce stimulation of arachidonic acid release, reducing prostaglandin E2 (PGE2), and nitric oxide (NO) production. Furthermore, CBD reduces the expression of specific interleukins (IL-12 while increasing that of IL-10) by macrophages, and decreases the production and release of pro-inflammatory cytokines, such as IL-1, IL-6 and interferon gamma (IFNγ) from lipopolysaccharide (LPS)-activated microglial cells [13]. The role of CBD as an inverse agonist of CB2 receptor may explain its known anti-inflammatory effects but this needs further investigation. There is also evidence of an antagonistic effect of CBD at the novel cannabinoid receptor G protein-coupled receptor 55 (GPR55), emerging from in vitro and in vivo studies. GPR55 has a role in bone physiology via regulating osteoclast function, formation and ultimately bone mass. CBD may affect the endocannabinoid system also indirectly, for example CBD can affect the endocannabinoid tone by increasing availability of anandamide; one possible mechanism is by inhibition of fatty acid amide hydrolase (FAAH), the enzyme that hydrolyzes the endocannabinoid anandamide [14].
However, not all physiological effects of CBD are mediated by cannabinoid receptors. Indeed CBD has numerous targets outside the endocannabinoid system and the cannabinoid receptor independent action is the subject of recent pharmacological studies on CBD [2]. Some of these physiological effects such as anti-inflammatory and immunosuppressive effect are mediated by more than one target [8]. The anti-inflammatory immunosuppressive effects are possibly mediated by activation of adenosine receptors, A1A and A2A and strychnine-sensitive α1 and α1β glycine receptors and the inhibition of the equilibrative nucleoside transporter [8]. The activity of CBD at one defined target may also elicit different physiological effects. For example, anti-inflammatory action and suppression of neuropathic pain are mediated by the same glycine receptors or anxiolytic, panicolytic and anti-depressant effects via serotonin 5HT1A receptor sub-type [8]. Pisano et al (2017) showed an in-depth review of the molecular pharmacology of CBD [2]. Despite the great number of studies published on molecular pharmacology of CBD, the exact pharmacological action of CBD remains not fully characterized and ongoing efforts are directed toward fully elucidating these mechanisms [3].
The clinical studies on the effects of CBD date back to the 80s but recently the number of studies and registered trials evaluating the effectiveness of this compound has risen exponentially. Currently, CBD is commercially available in different formulations and used for several health conditions but could be indicated for several diseases or disorders in addition to or in replacement of medical marijuana or other medical therapy. However, it remains unclear in which form and dose CBD should be administered to assess safety and efficacy across indications. This might further be complected by the nature of altered dose depending on indication of intervention. To highlight administration and dosage for known indications (e.g. diseases and disorders), this systematic review reports the current evidence, literature and experiences of how and in which dose CBD could be administered as well as efficacy and safety reports.
Methods
Study eligibility criteria
One author (CL) systematically searched PubMed, EMBASE and the Cochrane Library for published studies in English. The search was last updated on November 5, 2019. We included human studies of reporting more than 10 adult patients. We restricted our search to studies published after the year of 2000 with no demographic limitation. We included studies with single treatment of CBD, e.g. studies with other cannabinoid add-on therapy or adjuvant regimes were excluded. Studies must report dosage and dosage schemes to be included. There was no limitation regarding study design. Studies on pediatric populations were excluded. Only studies published in English, from 2000 to the date last databases were accessed. Full-text articles were interdependently assessed by two reviewers and eventual disagreement was resolved through consensus.
PubMed, Embase and Cochrane database were searched with all combinations of CBD and dosage in human studies. Reference lists of included studies were evaluated, and if needed, authors were contacted for further information.
Data collection and critical appraisal
We extracted the following data: condition and symptoms, year of publication, country, study population, number of patients, age, gender, weight, outcome measures, effect of treatment, side effects, dosage, administration form (pills, smoking, oil, etc.), length of treatment, doses, product/brand, isolated CBD or full spectrum and funding/sponsors.
The methodological quality of randomized controlled trials (RCTs) was assessed using the Cochrane risk of bias tool [15]. If all domains were judged as being at low risk of bias, the study was considered at low risk of bias. If at least one domain was rated high, the study was considered at high risk of bias. In situations that differ from those described above, the study was considered at unclear rick of bias. Data extraction and risk of bias was assessed by two independent reviewers and disagreements were solved by a third reviewer or with consensus.
Synthesis
Clinical heterogeneity was assessed by grouping studies by indication and outcome (including disease-specific outcomes and if specified adverse events (AEs)) and scheme of drug administration. Data were too heterogeneous to be pooled, so we used a narrative synthesis.
To rate the overall quality of evidence for risk of bias, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) was used for each outcome of interest across all domains: methodological limitations of the studies, indirectness, imprecision, inconsistency and publication bias [16].
Results
Study selection and characteristics
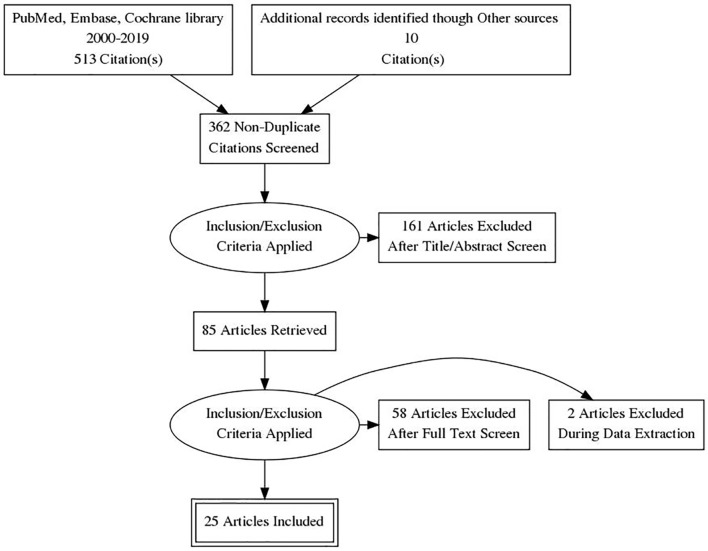
We identified 362 studies of which 85 were assessed for full-text eligibility (Fig. 1). Finally, 25 studies were included: 22 controlled clinical trials and three non-controlled (single arm) trials [10-34]. Among controlled clinical trials, two were non-RCTs and 20 RCTs (14 individually randomized parallel group trial, five individually randomized cross-over trials and one cluster randomized cross-over trial). Studies were conducted in five countries with the majority of studies being conducted in the UK. All studies were reported in full length journal articles. The administration of CBD varied from single doses to chronic administration, up to 48 weeks. The pure form of CBD was used in the majority of the studies except for two studies using seed CBD oil and CBD-rich botanical extracts. The most common form of drug administration was in the form of oral capsules, and other forms were vaporization and sublingual oil. The most common comparator was placebo only one study used an active-control group. Results for controlled clinical trials and non-controlled studies are presented in Table 1 [17-37] and Table 2 [38-40], providing a summary of the included studies and their principal findings.
Figure 1.
PRISMA flow diagram of literature search and selection process. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1. Overview of Included Studies and Principal Findings for Controlled Clinical Trials.
| Author, year of publication | Population (no. of participants) | Study design | CBD dose and scheme | Formulation/route | Primary outcome | Measures | Effect |
|---|---|---|---|---|---|---|---|
| Anxiety | |||||||
| Bergamaschi et al (2011) [17] | Social anxiety disorder patients (24) | IR parallel group trial | 600 mg; single dose | Oral capsules | Anxiety | Visual analogue mood scale (sub-scales; anxiety, cognitive impairment, discomfort and alert) | CBD significantly reduced anxiety, cognitive impairment, discomfort and alert during simulation public speaking test |
| Crippa et al (2011) [22] | Social anxiety disorder patients (10) | IR cross-over trial | 400 mg; single dose | Oral capsules | Anxiety | Visual analogue mood scale | Acute administration reduced subjective anxiety |
| Arndt et al (2017) [25] | Healthy volunteers (38) | IR cross-over trial | 300, 600 and 900 mg; single dose | Oral solution | Reactivity to negative stimuli | Behavioral tasks | Single doses of CBD had little effect on reactions to negative emotional stimuli |
| Crippa et al (2004) [21] | Healthy volunteers (10) | IR cross-over trial | 400 mg; single dose | Oral capsules | Anxiety | Visual analogue mood scale (sub-scales; anxiety, physical sedation, mental sedation and other feelings and attitudes) | CBD significantly decreased subjective anxiety and increased mental sedation |
| Zuardi et al (2017) [19] | Healthy volunteers (60) | IR parallel group trial | 100, 300 and 900 mg; single dose | Oral capsules | Anxiety | Visual analogue mood scale (anxiety and sedation factors) | CBD 300 mg reduced subjective anxiety measures and presented lower sedation level when compared with clonazepam. This was not observed with CBD 100 and 900 mg. |
| Martin-Santos et al (2012) [23] | Healthy volunteers (16) | NR controlled trial | 600 mg; single dose | Oral | Anxiety | Spielberger State Anxiety Inventory, visual analogue mood scale | There was no difference between CBD and placebo on anxiety levels. |
| Bhattacharyya et al (2010) [24] | Healthy volunteers (15) | NR controlled trial | 600 mg; single dose | Oral capsules | Anxiety | Visual analogue mood scale- tranquilization and calming sub-scale. | CBD single dose reduced anxiety levels. |
| Linares et al (2019) [18] | Healthy volunteers (57) | IR parallel group trial | 150, 300 and 600 mg; single dose | Oral capsules | Anxiety | Visual analogue mood scale | 300 mg of CBD significantly reduced anxiety during simulation public speaking test |
| Das et al (2013) [27] | Healthy volunteers (48) | IR parallel group trial | 32 mg; single dose | Inhalation/vaporized | Extinction and consolidation | Skin conductance and shock expectancy measures | CBD enhanced consolidation of extinction learning |
| Hindocha et al (2015) [26] | High and low cannabis use and high and low schizotipy (48) | CR cross-over trial | 16 mg; single dose | Inhalation/vaporized | Emotional processing | Computer-based emotional processing task | CBD improved recognition of emotional facial affect. |
| Hundal et al (2018) [20] | Non-clinical high paranoid group (32) | IR parallel group trial | 600 mg; single dose | Oral capsules | Anxiety | Beck’s anxiety inventory | CBD apparently seemed to increase anxiety levels. |
| Psychotic disorders | |||||||
| Hallak et al (2010) [31] | Schizophrenia patients (28) | IR parallel group trial | 300 or 600 mg; single dose | Oral capsules | Cognitive functioning | Stroop color word test | 300 and 600 mg of CBD do not lead to cognitive improvement |
| Boggs et al (2018) [28] | Chronic schizophrenia patients (42) | IR parallel group trial | 600 mg/day; 6 weeks | Oral capsules | Psychotic symptoms, cognitive functioning | Positive and negative syndrome scale T score of MATRICS consensus cognitive battery | Psychotic symptoms improved in both groups without significant difference, cognitive functioning improved only in placebo group. |
| Leweke et al (2012) [29] | Acutely psychotic patients (32) | IR parallel group triala | 200 mg/day up to 800 mg/day; 4 weeks | Oral capsules | Psychotic symptoms | Positive and negative syndrome scale; brief psychiatric rating scale | CBD is as effective as amisulpride in improving psychotic symptoms |
| McGuire et al (2018) [30] | Schizophrenia patients (88) | IR parallel group trial | 1,000 mg/day; 6 weeksb | Oral solution | Psychotic symptoms | Positive and negative syndrome scale, clinical global impressions scale | Treatment with CBD improved positive psychotic symptoms and clinicians’ impressions of illness improvement. |
| Cognitive functioning, level of functioning | Brief assessment of cognition in schizophrenia; global assessment of functioning scale | Improvement in cognitive performance and in the level of overall functioning with CBD although does not reach statistical significance. | |||||
| Hundal et al (2018) [20] | Non-clinical high paranoid group (32) | IR parallel group trial | 600 mg; single dose | Oral capsules | Persecutory ideation | State social paranoia scale, community assessment of psychic experiences scale | CBD had no effect on precursory thinking and psychotic-like experiences |
| Cannabis use disorder | |||||||
| Haney et al (2016) [32] | Healthy cannabis smokers (32) | IR cross-over trial | 200, 400 and 800 mg; single dose | Oral capsules | Cannabis subjective effects | Subjective mood and drug effects measured with visual analogue scale | CBD did not alter the subjective effects of smoked cannabis |
| Nicotine addiction | |||||||
| Morgan et al (2013) [33] | Tobacco smokers (24) | IR parallel group trial | 400 µg; 1 week | Inhalation/vaporized | Reduction in the number of cigarettes smoked | Number of cigarettes smoked | CBD reduced the number of cigarettes smoked during treatment and at follow-up |
| Hindocha et al (2018) [34] | Tobacco smokers (30) | IR parallel group trial | 800 mg; single dose | Oral capsules | Nicotine withdrawal | Visual probe task and pleasantness rating task | CBD reduced the salience and pleasantness of cigarette cues, compared with placebo but did not influence tobacco craving or withdrawal |
| Dyslipidemia | |||||||
| Jadoon et al (2016) [35] | Patients with type 2 diabetes and dyslipidemia (62) | IR parallel group trial | 200 mg/day; 13 weeks | Oral capsules | HDL cholesterol concentrations | Enzymatic calorimetric assays | CBD did not produce any effect on HDL levels compared to placebo |
| Crohn’s disease | |||||||
| Naftali et al (2017) [36] | Patients with diagnosis of Crohn’s disease (19) | IR parallel group trial | 20 mg/day; 8 weeksb | Sublingual oil | Disease activity | Crohn’s disease activity index | A reduction in disease activity at the end of the study but no significant difference with placebo. |
| Ulcerative colitis | |||||||
| Irving et al (2018) [37] | Patients with mild to moderate ulcerative colitis (60) | IR parallel group trial | 50 mg up to 250 mg/day; 10 weeksa, b, c | Oral capsules | Remission at the end of treatment | Mayo score of ≤ 2 | The primary endpoint was negative but CBD may be beneficial for symptomatic treatment of ulcerative colitis |
aActive-controlled trial, amisulpride. bConcomitant treatment. cCBD-rich botanical extracts capsules contained other compound (up to 4.7% THC). IR: individually randomized; NR: non-randomized; CR: cluster randomized; CBD: cannabidiol; HDL: high-density lipoprotein.
Table 2. Overview of Included Studies and Principal Findings for Non-Controlled Clinical Trials.
| Author/year of publication | Indication | No. of participants | Dose evaluated (administration scheme) | Administration method | Follow-up | Outcome | Measure | Effect |
|---|---|---|---|---|---|---|---|---|
| Szaflarski et al (2018) [38] | Epilepsy | 60 | 5 up to 50 mg/kg/dayd | Oral oil | 48 weeks | Seizure control | Seizure frequency, Chalfont seizure severity scale | Improved compared to baseline |
| Palmieri et al (2019) [39] | Skin disorders | 20 | N/A (two times a day/3 months) | Topical gel | 3 months | Hydration | Hydration probe | Improved compared to baseline |
| Transepidermal water loss | DermaLab® USB instrumenta | Improved compared to baseline | ||||||
| Skin elasticity | ElastiMeter®b | Improved compared to baseline | ||||||
| Severity of atopic dermatitis | Scoring atopic dermatitis | Improved compared to baseline | ||||||
| Severity of acne | Acne disability index | Improved compared to baseline | ||||||
| Severity of psoriasis | Psoriasis area severity index | Improved compared to baseline | ||||||
| Palmieri et al (2017) [40] | ADR following HPV vaccine | 12 | 25 mg/mL, 150 mg/mL (one time/day × 3 months)c | Sublingual oil drops | 3 months | Quality of life | Medical outcome short-form health survey questionnaire | Improved compared to baseline |
aCortex Technologies, Hadsund, Denmark. bDelfin Technologies, Kuopio, Finland. cConcomitant treatment for the condition with anti-oxidant and pain killer. dConcomitant treatment with anti-seizure drugs. N/A: non-available; HPV: human papillomavirus; ADR: adverse drug reaction.
The risk of bias within studies
Among the randomized trials, three were judged at low risk of bias, eight were judged at high risk of bias and nine at uncertain risk. Major potential sources of bias were frequent in the following domains: randomization process and selection of the reported results. Most studies reported were randomized and double-blinded but few reported methods of randomization or participants and outcome assessor blinding. Selective outcome reporting was also a potential source of bias, since some studies did not report data for all outcomes specified in the methods section or trial register or changed the primary outcome from what was specified in the trial register.
Most studies reported outcomes differently and even in cases where the same outcome was reported it was measured differently. Outcome level assessment was performed only for anxiety in social anxiety disorder (SAD) and psychotic symptoms and cognitive function in schizophrenia patients following recommendations applying GRADE approach in narrative synthesis [9]. GRADE rating is presented in Table 3. Two studies assessed anxiety in treatment-naive SAD populations, and three assessed cognitive function and positive and negative symptoms in schizophrenia patients.
Table 3. Cannabidiol Compared to Control for Psychotic and Anxiety Disorders.
| Outcomes | No. of participants (studies) follow-up | Certainty of the evidence (GRADE)a | Impact |
|---|---|---|---|
| Anxiety symptoms assessed with visual analogue mood scale | 44 (two RCTs) | ⨁⨁◯◯ low |
All studies showed a reduction in symptoms compared to placebo. |
| Psychotic symptoms assessed with positive and negative syndrome scale, brief psychiatric rating scale | 171 (three RCTs) | ⨁⨁⨁◯ moderate |
Two studies showed improvement in psychotic symptoms. Some variability in the intervention (doses) and population (stage of illness) was noted. |
| Cognitive function assessed with T score of MATRICS consensus cognitive battery, stroop color word test, composite score on the brief assessment of cognition in schizophrenia | 148 (three RCTs) | ⨁⨁⨁◯ moderate |
Two studies showed no improvement in cognitive function. One showed only small improvements. |
The outcome of interest are: anxiety symptoms, psychotic symptoms and cognitive function. A pooled effect estimate was not available and a narrative synthesis of the evidence was provided. aSymbols are used to describe certainty in evidence profiles. High certainty: ⨁⨁⨁⨁; Moderate certainty: ⨁⨁⨁◯; Low certainty: ⨁⨁◯◯; Very low certainty: ⨁◯◯◯. RCT: randomized controlled trial.
Anxiety disorder
Anxiety was assessed in 11 studies (358 participants) [17-27]. Anxiety was the main outcome in eight studies, while possible anxiolytic effects were evaluated in the remaining three studies, through indirect measures such as emotional processing, reactivity to negative stimuli, etc. Among the studies providing data on anxiety, there were only two restricted analyses to patients with anxiety disorders and another to non-clinic paranoia patients. Nine studies were randomized clinical trials: one of the studies was judged at low risk of bias, four at uncertain risk and four at high risk. Single doses of pure CBD 150 - 900 mg in the form of gelatin capsules were administered in the majority of the cases. Two individually randomized parallel-group trials evaluated the effect of CBD on subjective anxiety on patients with SAD [17] or healthy subjects [18, 19] during simulated public speaking. These studies reported that acute oral administration of 600 mg (SAD subjects) and 300 mg (healthy subjects) of CBD reduced anxiety assessed by the visual analogue mood scale compared to placebo. Linares et al (2018) and Zuardi et al (2017) also tested other doses and observed no effect on anxiety levels after acute administration of 150, 600 and 900 mg of CBD on anxiety levels. No effect was found for the physiological measures such as heart rate and systolic/diastolic blood pressure [17-19]. Another small, individually randomized parallel-group trial, reported that acute administration of oral CBD (600 mg) had a negative effect on anxiety levels, measured with the Beck’s anxiety inventory, compared to placebo [20]. Single oral doses of 400 and 600 mg decreased anxiety and increased mental sedation measured with visual analogue mood scale in two individually randomized cross-over trials [21, 22]. Two small non-RCTs administered 600 mg of oral CBD to health volunteers. One found no difference between placebo and CBD group on anxiety levels [23], while the other found a reduction on anxiety measured with visual analogue mood scale [24].
One randomized cross-over trial measured reactivity to negative stimulus through behavioral tasks and reported no effect at different incremental single doses of CBD (300, 600 and 900 mg). This measure was based on the hypotheses that drug anxiolytic effect should be manifested through changes in responses to negative stimuli, which also occur with single doses for common anxiolytics [25]. A cluster randomized cross-over trial assessed the effect of small doses (16 mg) administered through inhalation on fear extinction and consolidation and found that it was effective when compared to placebo [26]. Das et al (2013) found that acute administration of 32 mg of CBD, before training, enhances extinction of conditioned fear responses [27].
Psychosis
Four randomized parallel-group trials (196 participants) assessed the role of CBD on cognitive impairment and psychotic symptoms in patients with psychotic disorders (schizophrenia) [28-31]. One study was judged as being at high risk of bias and three at uncertain risk of bias. Studies included patients with a confirmed diagnosis of schizophrenia. CBD was administered orally, in the form of oral gelatin capsules with doses ranging from 600 to 1,000 mg/day and the effects were compared to a placebo and active control. Boggs et al (2018) assessed the effects of CBD (600 mg/day for 6 weeks) as an adjunctive treatment in chronic schizophrenia patients, for a period of 6 weeks. No significant difference was observed between placebo and CBD group on cognitive function and psychotic symptoms [28]. Leweke et al (2012) included a small group of acute schizophrenia patients who were administered 200 up to 800 mg/day for 4 weeks or amisulpride, a potent antipsychotic. CBD was as effective as amisulpride in improving psychotic symptoms and associated with marked tolerability and safety, when compared with amisulpride [29]. McGurie et al (2019) administered 1,000 mg/day for 6 weeks to a group of schizophrenia patients as an adjunct to current antipsychotic treatment and found a significant improvement on positive psychotic symptoms and clinicians’ impressions of illness improvement. Despite improvement on cognitive function and overall level of functioning, no significant difference was found compared to placebo [30]. Hallak et al (2010) included a small group of heterogeneous schizophrenia patients and administered a single dose of CBD (300 or 600 mg). They found no effect of CBD on selective attention, measured by Stroop color and word test [31].
Substance use disorder
In a randomized cross-over design trial, Haney et al (2016) tested a range (200, 400 and 800 mg) of oral single doses of pure CBD, on cannabis smokers to assess the reinforcing subjective and psychological effects of smoked cannabis. The authors found no evidence with this treatment and dose scheme, and CBD can reduce the reinforcing or positive effects of smoked cannabis in current smokers [32]. Optional use of inhaled pure CBD (400 µg/dose) over 1 week produced positive effects with regard to nicotine addiction, as measured by a reduction on the number of cigarette smoked in a group of healthy smokers willing to quit the habit. However, CBD did not show effect on carving symptoms [33]. After overnight tobacco abstinence single doses of 800 mg of CBD reduced the salience and pleasantness of cigarette cues which indicate that CBD may have a potential effect on motivational aspects of addiction [34]. Two studies were judged at uncertain risk of bias and one at high risk.
Other conditions
Three RCTs, all individually randomized parallel group, reported on different medical conditions (one study for each category) [28-37]. Doses ranged from 20 to 250 mg/day with treatment duration from 8 to 13 weeks. Isolated compound or plant exacts rich in CBD were administered in the form of hard oral capsules or oil. One study was judged at uncertain risk of bias and two at high risk.
In one trial, 200 mg/day was administered for 13 weeks as an adjunct to current treatment to patients with type 2 diabetes. Compared to placebo, CBD did not have a significant effect on the level of high-density lipoproteins, the primary endpoint for this study [35]. The effect of oral administration of CBD (20 mg/day for 8 weeks) on disease activity assessed by the Crohn’s disease activity index was evaluated in a small group of patients with long-standing Crohn’s disease taking concomitant medications. CBD had no effect on disease activity at the end of treatment and at 2 weeks follow-up [36]. Irvin et al (2018) reported no effects of CBD-rich extracts (100 mg/day up to 250/day for 8 weeks) added to current treatment and administrated in the form of oral capsules to patients diagnosed with ulcerative colitis [37].
Non-controlled intervention studies
A label single arm trial [38] assessed long-term use of isolated CBD (5 mg/kg/day incremental doses in some patients reaching 2,000 mg/day) as an adjunct to anti-epileptic drugs, in patients refractory to conventional anti-seizure drugs and reported a decrease in seizure frequency (144.4 to 52.2, P = 0.01) and severity measured with the Chalfont seizure severity scale (from 80.7 at baseline to 39.2, P < 0.0001) at week 12, with values being stable thereafter (total follow-up 48 weeks). The other two studies [39, 40] included reported on the chronic administration effect of CBD in the form of a topical cream for skin disorders and sublingual oil drops (25 mg/day up to 150 mg/day for 12 weeks) for adverse drugs reaction (ADR) following human papilloma virus (HPV) vaccine, with a 3-month follow-up period each. The topical application produced positive results for serous skin inflammatory conditions such a psoriasis, acne and related scars, with no side effects being reported [39]. The sublingual administration of CBD also improved the quality of life in girls presenting with ADR following HPV vaccine when added to standard treatment. The evidence however arises from two case series and this should be taken into consideration when considering the results from this study [40].
AEs
AEs were reported in 10 studies. In most studies reporting on AEs [20, 28-30, 35-38, 40, 41], CBD was administered chronically, with follow-up ranging from 6 to 48 weeks, except for two studies where AEs were reported after a single dose of CBD (600 and 750 mg, respectively) [20-41]. The total number of reported patients, withdrawing from the study due to experience of side effects, was 16 in the CBD group. One study reported that side effects did not differ between the CBD and placebo groups after 8 weeks of follow-up [36], and another study reported a decrease of AEs 12 weeks after CBD initiation [38]. In another study side effects were monitored by answering question to a questionnaire [37]. The only study comprising CBD effects with an active group receiving an antipsychotic drug, amisulpride, in patients with schizophrenia reported that the CBD group had fewer extrapyramidal symptoms, less weight gain and prolactin release [29]. Results for the remaining studies, by indication and follow-up, for the number of participants experiencing any AEs compared to placebo AEs, classified by primary system organ class are reported in Table 4.
Table 4. Adverse Events (Treatment-Related All Causality) by Primary System Organ Class and Duration of Follow-Up.
| Follow-up < 1 day |
Follow-up 1 week |
Follow-up 10 weeks |
Follow-up 13 weeks |
|||||
|---|---|---|---|---|---|---|---|---|
| Cannabidiol (%) | Placebo (%) | Cannabidiol (%) | Placebo (%) | Cannabidiol (%) | Placebo (%) | Cannabidiol (%) | Placebo (%) | |
| Subjects experiencing any TEAE (%) | 66.7 | 62.5 | 100 | 83.3 | 90 | 48 | 84.6 | 92.9 |
| Nervous system disorders | 33.3 | 37.5 | 66.7 | 33.3 | 83 | 26 | ||
| Gastrointestinal system disorders | 16.7 | 50.0 | 66.7 | 50.0 | 38 | 16 | ||
| Infections and infestations | 22.2 | 0 | 0 | 0 | ||||
| Psychiatric disorders | 0 | 0 | 24 | 3 | ||||
| General disorders and administration site conditions | 22.2 | 50.0 | 21 | 10 | ||||
| Musculoskeletal and connective tissue disorders | 0 | 16.7 | 14 | 0 | ||||
| Skin and subcutaneous tissue disorders | 2.2 | 16.7 | 3 | 0 | ||||
Discussion
This systematic review assessed the dosage schemes, effects and safety issues associated with the use of CBD. We included 20 RCTs, two non-RCTs and three observational studies for all indications of CBD use. In summary, 20 RCTs evaluated efficacy of CBD-use for schizophrenia, anxiety, Crohn’s disease, ulcerative colitis, dyslipidemia, nicotine addiction and cannabis use disorder. Most of these studies reported positive effect of CBD on anxiety, schizophrenia, tobacco addiction and minor effects or no effect on primary outcome measures for Crohn’s disease, ulcerative colitis, dyslipidemia and cannabis use disorder. All observational studies (skin disorders, ADR following HPV vaccine and epilepsy) reported positive effect of CBD when compared to baseline measures.
Regarding safety issues, most studies reported no AEs with acute administration and mild to moderate adverse effects with chronic administration. In comparison to other drugs, a better side effect profile was presented [29, 30]. The chronic administration of CBD for most of the included studies had a duration of few weeks (4 - 6 weeks). There is a need for longer term safety data and systematic/uniform reporting of AEs to better weight benefit and harms in future reviews. At least, two systematic reviews have been published on safety and side effects of CBD. The comprehensive review by Bergamaschi et al included animal and clinical studies reporting a favorable safety profile of CBD in humans [42]. The other review was an update of the previous focusing more on clinical data. The authors reported that most commonly side effects were tiredness, diarrhea and changes of appetite/weight. In comparison to other treatments used for epilepsy and psychotic disorders, CBD administration presented fewer side effects [43].
Most of the studies included in this review showed an improvement of anxiety levels after single doses of oral CBD with doses ranging from 300 to 600 mg [17-19, 21, 22, 24, 26, 27]. It is important to note that only two studies included a clinical population (SAD) while the majority involved healthy subjects. Both studies tested acute administration of CBD and found a reduction in subjective anxiety. A systematic review on specific anxiety conditions which included data from clinical and pre-clinical studies found strong evidence arising from pre-clinical studies to support the anxiolytic effects of CBD, and similarly that evidence from human studies was limited to acute dosing in mostly healthy subjects [44]. There is a need for better designed studies to evaluate the therapeutic potential of CBD in this clinical population, possibly with chronic dosing in a relevant clinical population.
A systematic review providing evidence from clinical studies, mainly randomized clinical trial and case series, on the efficacy of CBD in the treatment of schizophrenia and/or substance abuse disorders observed large differences in study population, doses and administration [45]. In the present review, two principal outcomes were considered for schizophrenia patients, psychotic symptoms and cognitive functioning. CBD had positive effect on psychotic symptoms especially in acutely psychotic patients [29], while it had small or no effect on chronic schizophrenia patients who had been treated with anti-psychotics. The possibility that larger effects may be observed for patients in the early phases of the disease had been suggested [28, 45]. Regarding cognitive function little or no effects were observed after chronic or acute administration. A recent systematic review including 27 RTCs that investigated the effects of CBD on different psychiatric disorders such as psychosis, moods disorders and anxiety found that because of large heterogeneity across studies CBD doses, formulations and the study populations, it was not possible to make definitive conclusion about clinical effects [44]. The authors suggested that large-scale placebo controlled studies are needed to investigate the effects of CBD as an adjunct treatment for psychiatric disorder [46].
No effect was reported in the studies included in this review for the treatment of Crohn’s disease, ulcerative colitis and type 2 diabetes with doses ranging between 20 and 250 mg/day for a treatment duration of 8 - 13 weeks [38-40]. While positive results were reported on seizure improvement with doses ranging from 350 to 2,000 mg/day and on the quality of life for patients with ADRs following HPV vaccine, with doses ranging from 25 to 150 mg/day, after 12 weeks of treatment [31, 33]. No definitive conclusion can be made on doses required for a positive effect since this may depend on the outcome assessed and study population.
In all studies, expect for one that used plant extracts [37], purified CBD was administered. Data derived from pre-clinical animal models indicate that purified CBD may have a bell shape response [47] which was also confirmed in two studies included in this review [25, 26]. It is likely that the use of a single cannabinoid may be inferior to the extract where other components synergize with CBD to obtain the desired effect, known as the “entourage effect” [48]. Further, the main route of administration for the studies included in this review was oral (either in the form of capsules or sublingual oil). Animal studies suggest that oral bioavailability is low [48]; on the other hand, as highlighted in a recent systematic review on the pharmacokinetics of the CBD in humans [49], there is a lack of data in humans [50].
We used the Cochrane risk of bias tool to assess the included RCTs [8]. Overall, several methodological weaknesses were identified, e.g. selective outcome reporting, inadequate randomization and blinding. Further, sample sizes were very small in most studies significantly decreasing strength of detecting differences between study groups. An important finding of this review is the heterogeneous use of doses, dosage schemes and formulations (inhalation, oral capsules and sublingual oil, topical gel) across all indications of CBD. This has several implications. Besides excluding the option of pooling data for a meta-analysis to evaluate efficacy, the consequence of non-consensus of CBD dose is important when evaluating safety issues. Commercially, several online “dose-calculators” are available for dose recommendations (e.g. https://www.mydosage.com/); the data to support such calculators remain unclear on appropriate doses for efficacy although it seems reasonable to guide patients to safe dosage schemes and avoid adverse effects and gathering more data on how CBD is commonly being applied [51].
Although this review followed the recommendations for rigorous systematic reviews, it bears limitations here amongst a language and date restriction applied as well as a search strategy limited to electronic databases. However unlikely, other studies may not have been identified which can limit the applicability of the findings. Most of the disorders/diseases were only evaluated in single studies providing limited experience and no option to pool data, and some studies failed to present specific inclusion criteria meaning no restriction as to study group. The studies identified that evaluated identical conditions, regrettably employed different endpoints or tools of assessment.
RCTs are needed to confirm the effect of CBD on skin disorders, epilepsy and ADR following HPV vaccine. In addition, large and robust RTCs are needed to confirm the effects of CBD particularly on anxiety and psychosis. Studies should adhere to reporting standards for trials and use similar outcomes, standard measurements/tools to assess outcomes, and comparable treatment regimens to allow comparisons in future review studies. International guidelines should be implemented before the justification of further trials.
Conclusions
Studies included in this review evaluated mainly oral administration of purified CBD with placebo group as a comparator. However, there was larger heterogeneity between studies with regard to the population, schemes and doses of CBD, outcomes and tool of measurement. There is some evidence, even though low quality, that supports anxiolytic effect of acute administration of oral CBD. There is moderate-quality evidence that chronic and acute administration of CBD can improve psychotic symptoms in schizophrenia patients. Further, large RCTs are needed to confirm the effect of CBD for the treatment of Crohn’s disease, ulcerative colitis, dyslipidemia and cannabis use disorders. Insufficient data regarding safety issues were provided, but most studies reported no AEs with acute administration and mild to moderate adverse effects with chronic administration.
Acknowledgments
None to declare.
Financial Disclosure
Nordic Cannabis Research Institute (www.ncrinstitute.com).
Conflict of Interest
None to declare.
Author Contributions
CL designed the study. CL and JSH participated in data acquisition, extraction, analysis and drafted the final work. Authors reviewed the final version of the manuscript and approved it for publication.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon request.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- CBD
cannabidiol
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- Δ9-THC
delta-9-tetrahydrocannabinol
- FAAH
fatty acid amide hydrolase
- GPR55
G protein-coupled receptor 55
- IFNγ
interferon gamma
- IL
interleukin
- LPS
lipopolysaccharide
- PGE2
prostaglandin E2
- NO
nitric oxide
References
- 1.Schubart CD, Sommer IE, van Gastel WA, Goetgebuer RL, Kahn RS, Boks MP. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr Res. 2011;130(1-3):216–221. doi: 10.1016/j.schres.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, Abate M. et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol Ther. 2017;175:133–150. doi: 10.1016/j.pharmthera.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Morales P, Hurst DP, Reggio PH. Molecular targets of the phytocannabinoids: a complex picture. Prog Chem Org Nat Prod. 2017;103:103–131. doi: 10.1007/978-3-319-45541-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esposito G, Filippis DD, Cirillo C, Iuvone T, Capoccia E, Scuderi C, Steardo A. et al. Cannabidiol in inflammatory bowel diseases: a brief overview. Phytother Res. 2013;27(5):633–636. doi: 10.1002/ptr.4781. [DOI] [PubMed] [Google Scholar]
- 5.Campos AC, Fogaca MV, Sonego AB, Guimaraes FS. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016;112:119–127. doi: 10.1016/j.phrs.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 6.Boychuk DG, Goddard G, Mauro G, Orellana MF. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7–14. doi: 10.11607/ofph.1274. [DOI] [PubMed] [Google Scholar]
- 7.Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, Miller I. et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270–278. doi: 10.1016/S1474-4422(15)00379-8. [DOI] [PubMed] [Google Scholar]
- 8.Turner SE, Williams CM, Iversen L, Whalley BJ. Molecular pharmacology of phytocannabinoids. Prog Chem Org Nat Prod. 2017;103:61–101. doi: 10.1007/978-3-319-45541-9_3. [DOI] [PubMed] [Google Scholar]
- 9.Cascio MG, Pertwee RG, Marini P. In: Botany and Biotechnology. Cannabis Sativa L, editor. Springer International Publishing; 2017. The pharmacology and therapeutic potential of plant cannabinoids; pp. 207–225. [DOI] [Google Scholar]
- 10.McPartland JM, Glass M, Pertwee RG. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol. 2007;152(5):583–593. doi: 10.1038/sj.bjp.0707399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172(20):4790–4805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66(2):234–246. doi: 10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 13.De Filippis D, Esposito G, Cirillo C, Cipriano M, De Winter BY, Scuderi C, Sarnelli G. et al. Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis. PLoS One. 2011;6(12):e28159. doi: 10.1371/journal.pone.0028159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30(10):515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J. et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murad MH, Mustafa RA, Schunemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid Based Med. 2017;22(3):85–87. doi: 10.1136/ebmed-2017-110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, Quevedo J. et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology. 2011;36(6):1219–1226. doi: 10.1038/npp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linares IM, Zuardi AW, Pereira LC, Queiroz RH, Mechoulam R, Guimaraes FS, Crippa JA. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz J Psychiatry. 2019;41(1):9–14. doi: 10.1590/1516-4446-2017-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuardi AW, Rodrigues NP, Silva AL, Bernardo SA, Hallak JEC, Guimaraes FS, Crippa JAS. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol. 2017;8:259. doi: 10.3389/fphar.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hundal H, Lister R, Evans N, Antley A, Englund A, Murray RM, Freeman D. et al. The effects of cannabidiol on persecutory ideation and anxiety in a high trait paranoid group. J Psychopharmacol. 2018;32(3):276–282. doi: 10.1177/0269881117737400. [DOI] [PubMed] [Google Scholar]
- 21.Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R, Ferrari L, Azevedo-Marques PM. et al. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology. 2004;29(2):417–426. doi: 10.1038/sj.npp.1300340. [DOI] [PubMed] [Google Scholar]
- 22.Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, Simoes MV. et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25(1):121–130. doi: 10.1177/0269881110379283. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Santos R, Crippa JA, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, Allen P. et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des. 2012;18(32):4966–4979. doi: 10.2174/138161212802884780. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C. et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35(3):764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arndt DL, de Wit H. Cannabidiol does not dampen responses to emotional stimuli in healthy adults. Cannabis Cannabinoid Res. 2017;2(1):105–113. doi: 10.1089/can.2017.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJ, Curran HV. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur Neuropsychopharmacol. 2015;25(3):325–334. doi: 10.1016/j.euroneuro.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das RK, Kamboj SK, Ramadas M, Yogan K, Gupta V, Redman E, Curran HV. et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl) 2013;226(4):781–792. doi: 10.1007/s00213-012-2955-y. [DOI] [PubMed] [Google Scholar]
- 28.Boggs DL, Surti T, Gupta A, Gupta S, Niciu M, Pittman B, Schnakenberg Martin AM. et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl) 2018;235(7):1923–1932. doi: 10.1007/s00213-018-4885-9. [DOI] [PubMed] [Google Scholar]
- 29.Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, Klosterkotter J. et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, Taylor A. et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225–231. doi: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]
- 31.Hallak JE, Machado-de-Sousa JP, Crippa JA, Sanches RF, Trzesniak C, Chaves C, Bernardo SA. et al. Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD) Braz J Psychiatry. 2010;32(1):56–61. doi: 10.1590/S1516-44462010000100011. [DOI] [PubMed] [Google Scholar]
- 32.Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, Gray KM. et al. Oral Cannabidiol does not Alter the Subjective, Reinforcing or Cardiovascular Effects of Smoked Cannabis. Neuropsychopharmacology. 2016;41(8):1974–1982. doi: 10.1038/npp.2015.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan CJ, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav. 2013;38(9):2433–2436. doi: 10.1016/j.addbeh.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Hindocha C, Freeman TP, Grabski M, Stroud JB, Crudgington H, Davies AC, Das RK. et al. Cannabidiol reverses attentional bias to cigarette cues in a human experimental model of tobacco withdrawal. Addiction. 2018;113(9):1696–1705. doi: 10.1111/add.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, Bell JD, O'Sullivan SE. et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care. 2016;39(10):1777–1786. doi: 10.2337/dc16-0650. [DOI] [PubMed] [Google Scholar]
- 36.Naftali T, Mechulam R, Marii A, Gabay G, Stein A, Bronshtain M, Laish I. et al. low-dose cannabidiol is safe but not effective in the treatment for crohn's disease, a randomized controlled trial. Dig Dis Sci. 2017;62(6):1615–1620. doi: 10.1007/s10620-017-4540-z. [DOI] [PubMed] [Google Scholar]
- 37.Irving PM, Iqbal T, Nwokolo C, Subramanian S, Bloom S, Prasad N, Hart A. et al. A randomized, double-blind, placebo-controlled, parallel-group, pilot study of cannabidiol-rich botanical extract in the symptomatic treatment of ulcerative colitis. Inflamm Bowel Dis. 2018;24(4):714–724. doi: 10.1093/ibd/izy002. [DOI] [PubMed] [Google Scholar]
- 38.Szaflarski JP, Bebin EM, Cutter G, DeWolfe J, Dure LS, Gaston TE, Kankirawatana P. et al. Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open-label add-on prospective study. Epilepsy Behav. 2018;87:131–136. doi: 10.1016/j.yebeh.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 39.Palmieri B, Laurino C, Vadala M. A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars. Clin Ter. 2019;170(2):e93–e99. doi: 10.7417/CT.2019.2116. [DOI] [PubMed] [Google Scholar]
- 40.Palmieri B, Laurino C, Vadala M. Short-Term Efficacy of CBD-Enriched Hemp Oil in Girls with Dysautonomic Syndrome after Human Papillomavirus Vaccination. Isr Med Assoc J. 2017;19(2):79–84. [PubMed] [Google Scholar]
- 41.Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32(11):1053–1067. doi: 10.1007/s40263-018-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6(4):237–249. doi: 10.2174/157488611798280924. [DOI] [PubMed] [Google Scholar]
- 43.Iffland K, Grotenhermen F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017;2(1):139–154. doi: 10.1089/can.2016.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blessing EM, Steenkamp MM, Manzanares J, Marmar CR. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics. 2015;12(4):825–836. doi: 10.1007/s13311-015-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batalla A, Janssen H, Gangadin SS, Bossong MG. The potential of cannabidiol as a treatment for psychosis and addiction: who benefits most? A systematic review. J Clin Med. 2019;8(7):1058. doi: 10.3390/jcm8071058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonaccorso S, Ricciardi A, Zangani C, Chiappini S, Schifano F. Cannabidiol (CBD) use in psychiatric disorders: A systematic review. Neurotoxicology. 2019;74:282–298. doi: 10.1016/j.neuro.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Gallily R, Yekhtin Z, Hanus LO. Overcoming the bell shaped dose response of cannabidiol by using cannabis extract enriched in cannabidiol. Pharmacology & Pharmacy. 2015;6(2):75–85. doi: 10.4236/pp.2015.62010. [DOI] [Google Scholar]
- 48.Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Millar SA, Stone NL, Yates AS, O'Sullivan SE. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front Pharmacol. 2018;9:1365. doi: 10.3389/fphar.2018.01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Millar SA, Stone NL, Bellman ZD, Yates AS, England TJ, O'Sullivan SE. A systematic review of cannabidiol dosing in clinical populations. Br J Clin Pharmacol. 2019;85(9):1888–1900. doi: 10.1111/bcp.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. https://www.mydosage.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon request.