Abstract
Background
Patients with type 2 diabetes mellitus (T2DM) are at increased risk for impairments in diastolic relaxation and heart failure with preserved ejection fraction (EF). Recent clinical data suggest that several sodium glucose transporter-2 (SGLT2) inhibitors are found to reduce cardiovascular disease (CVD) events in elderly diabetic patients, but the effect of tofogliflozin, one of the SGLT2 inhibitors, on CVD is unknown. We retrospectively investigated the effect of tofogliflozin on cardiac function in elderly patients with T2DM.
Methods
Patients received 20 mg of tofogliflozin daily for 1 month. EF, ratio of early filling to atrial filling (E/A), a change in mitral inflow E and mitral e' annular velocities (E/e'), left atrial dimension (LAD) and maximal diameter of inferior vena cava (IVCmax) were measured between baseline and 1 month after the administration of tofogliflozin.
Results
Body weight, systolic and diastolic blood pressures significantly decreased, while renin and aldosterone level significantly increased after 1 month of tofogliflozin treatment. Most of the physiological parameters and the level of serum electrolyte did not change significantly. E/A, E/e' and LAD significantly decreased, while no significant changes were observed in EF and IVCmax. The interactions of E/e' between time, gender and age were not significant.
Conclusion
The present study suggested that tofogliflozin improved left ventricular diastolic function irrespective of gender and age, while preserving IVC, renal function and electrolyte balance.
Keywords: Tofogliflozin, SGLT2 inhibitor, Type 2 diabetes mellitus, Elderly, Left ventricular diastolic function
Introduction
Type 2 diabetes mellitus (T2DM) is one of the important causes of heart failure (HF), as well as cardiovascular disease (CVD) [1, 2]. Sodium glucose co-transporter 2 (SGLT2) inhibitors are a new class of antidiabetic drugs that inhibit glucose reabsorption in the renal proximal tubules followed by excretion of glucose into the urine [3]. Six SGTL2 inhibitors (ipragliflozin, dapagliflozin, tofogliflozin, canagliflozin, empagliflozin and luseogliflozin) have been currently available in Japan [4]. Recent cardiovascular studies have demonstrated that SGLT2 inhibitors significantly decreased major adverse cardiovascular events, death and hospitalizations for HF during the treatment with canagliflozin, empagliflozin and dapagliflozin in T2DM patients with complication of CVD [5-8]. Furthermore, empagliflozin and canagliflozin have known to reduce cardiovascular risk, including weight loss as well as blood pressure lowering [6, 8-10]. A prospective study has also found that treatment with dapagliflozin improved left ventricular (LV) diastolic functional parameters in T2DM patients with HF [11]. LV diastolic dysfunction has been known to associate strongly with HF [12, 13]. Thus, the use of SGLT2 inhibitors which improve cardiovascular outcomes in patients with T2DM has been attracting attention. However, little is known concerning the effect of tofogliflozin, one of the SGLT2 inhibitors, on the LV diastolic function of T2DM patients with HF. The purpose of this study was to investigate the effect of tofogliflozin on the cardiac functions, including LV diastolic function in patients with T2DM.
Patients and Methods
The present study was a retrospective study to investigate the effect of tofogliflozin, one of the SGLT2 inhibitors, on LV diastolic functional parameters in elderly patients with T2DM. This study included elderly patients with a diagnosis of T2DM who attended clinics at the Kanazawa Medical University Himi Municipal Hospital from April 2017 to March 2018. Elderly was defined as aged ≥ 65 years. All patients received a single 20 mg dose of tofogliflozin daily for 1 month. Patients with significant comorbid conditions were excluded. Demographic and baseline characteristics and data that were collected throughout the 1-month treatment period were extracted from patients’ medical records. The data for patient characteristics were collected, gender (male/female), age (years), NYHA classification, co-administered drugs, serum glycated hemoglobin (HbA1c), levels of hematocrit, brain natriuretic peptide (BNP), estimated glomerular filtration rate (eGFR), blood urea nitrogen (BUN), serum electrolyte (i.e. sodium, potassium and chloride), serum renin and aldosterone were measured at baseline and 1 month.
Echocardiographic examination was performed with commercially available ultrasound systems, Hitachi Ultrasonic Diagnostic Apparatus Hivision Perius. Standard echocardiographic measurements were obtained in accordance with the current guidelines of the American Society of Echocardiography/European Association of Cardiovascular Imaging and the Guideline from Japanese Society of Echocardiography [13-15]. The maximal diameter of the inferior vena cava (IVCmax) was defined by the following procedure. Below the costal arch in parallel with the trunk and in the recumbent position before the inferior vein transitions to the right atrium at end-expiration. The largest diameter was measured between 0.5 and 3 cm from the inflow to the right atrium in the IVC long-axis cross-section at supine position. Maximum diameter was measured at expiration at the proximal hepatic vein junction. The left atrial dimension (LAD) was also measured. In parallel, ejection fraction (EF) and the ratio of early filling to atrial filling (E/A) were obtained by the early diastolic (E) and atrial wave (A) velocities, and the E-wave deceleration time was measured by means of pulsed wave Doppler recording from the apical four-chamber view. Spectral pulsed-wave Doppler-derived early diastolic velocity (e') was obtained by averaging the septal and lateral mitral annulus, and the mitral e' annular velocities (E/e') were calculated.
Statistical analysis
To examine differences in characteristics of the patients between baseline and 1 month after tofogliflozin treatment, paired t-test was conducted for each variable, HbA1c, body weight, systolic blood pressure, BNP, eGFR, BUN, serum electrolytes, blood osmotic pressure, renin, aldosterone, EF, E/A, E/e', LAD and diameter of IVC. In addition, to investigate the source of variation, the fixed effect of treatment time period, gender, age and their interactions (time × gender, time × age) as well as the random effect of subjects were assessed using analysis of variance with a mixed effect model. All statistical analyses were two-tailed, and P < 0.05 was regarded as statistically significant. The data were analyzed using the freely available EZR (Easy R) software (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [16].
Ethical considerations
The study was conducted in accordance with the guidelines of the Declaration of Helsinki. The study was formally approved by the Clinical Research Ethics Committee of Kanazawa Medical University Himi Municipal Hospital (receipt no. 107).
Results
The baseline characteristics of 42 elderly patients with T2DM (male: 18, female: 24) are summarized in Table 1. The total number of patients included was 42. Their mean age was 83.0 ± 7.6 (mean ± standard deviation (SD)), with range of 65 - 97 years of age. NYHA I/II was 33/9. Co-administered drugs were dipeptidyl peptidase-4 (DPP-4) (n = 33), calcium channel blocker (CCB) (n = 22), diuretics (n = 14), etc. During the study period, myocardial infarction, stress cardiomyopathy and exacerbation of HF were not observed.
Table 1. Characteristics of the Patients.
| Variables | Number of patients |
|---|---|
| Gender (N, male/female) | 18/24 |
| Age (years) | 83.0 ± 7.6 |
| NYHA I/II (III, IV: none) | 33/9 |
| Co-administered drug | |
| DPP-4 | 33 |
| BG | 12 |
| SU | 10 |
| Insulin | 2 |
| ARB | 10 |
| β-blocker | 5 |
| CCB | 22 |
| Diuretics | 14 |
DDP-4: dipeptidyl peptidase-4; BG: biguanides; SU: sulphonylureas; ARB: angiotensin receptor blocker; CCB: calcium channel blocker.
Comparison of patient characteristics at baseline and 1 month after administration of tofogliflozin is summarized in Table 2. HbA1c tended to decrease 1 month after administration of tofogliflozin from 7.33% at baseline to 7.08% (P = 0.056). Body weight significantly decreased from 55.1 to 52.7 kg (P < 0.01). Systolic and diastolic blood pressures also showed significant decreases from 137.4 to 124.1 mm Hg and from 74.5 to 69.3 mm Hg, respectively (P < 0.01). Serum renin and aldosterone showed significant increase from 3.45 to 6.97 ng/mL (P < 0.01) and from 89.3 to 104.4 pg/mL (P < 0.05), respectively. No significant changes were observed on hematocrit, BNP, eGFR, BUN, serum electrolytes (sodium, potassium and chloride) and blood osmotic pressure during the treatment with tofogliflozin.
Table 2. Characteristics of Laboratory Test Results.
| Variables | Baseline | 1 month | P-value* |
|---|---|---|---|
| HbA1c (%) | 7.3 ± 1.1 | 7.1 ± 0.9 | 0.06 |
| Body weight (kg) | 55.1 ± 12.2 | 52.7 ± 11.6 | < 0.01 |
| Systolic blood pressure (mm Hg) | 137.4 ± 27.0 | 124.1 ± 18.8 | < 0.01 |
| Diastolic blood pressure (mm Hg) | 74.5 ± 13.5 | 69.3 ± 11.4 | < 0.01 |
| Hematocrit | 37.9 ± 6.9 | 38.4 ± 5.9 | 0.3 |
| BNP (pg/mL) | 321.5 ± 805.7 | 191.0 ± 371.0 | 0.08 |
| eGFR | 63.1 ± 23.2 | 60.9 ± 30.5 | 0.4 |
| BUN (mg/dL) | 19.1 ± 8.6 | 18.5 ± 7.7 | 0.6 |
| Na+ (mEq/L) | 139.1 ± 4.0 | 139.2 ± 3.5 | 0.9 |
| K+ (mEq/L) | 4.2 ± 0.7 | 4.1 ± 0.6 | 0.4 |
| Cl- (mEq/L) | 103.7 ± 4.1 | 103.8 ± 5.5 | 0.9 |
| Blood osmotic pressure (mOsm/L) | 293.0 ± 9.3 | 291.7 ± 7.5 | 0.3 |
| Renin (ng/mL) | 3.5 ± 5.2 | 7.0 ± 9.2 | < 0.01 |
| Aldosterone (pg/mL) | 89.3 ± 48.6 | 104.4 ± 59.7 | < 0.05 |
*Paired t-test. HbA1c: glycated hemoglobin; BNP: brain natriuretic peptide; eGFR: estimated glomerular filtration rate; BUN: blood urea nitrogen.
Changes in echocardiographic endpoints are summarized in Table 3. After 1 month’s administration of tofogliflozin, the E/A showed significant decrease from 0.69 to 0.60 (P < 0.05). The E/e' also showed significant decrease from 12.6 to 9.6 (P < 0.01). The LAD also showed significant decrease from 39.7 to 36.8 (P < 0.01). EF and IVCmax were not significantly changed during the treatment with tofogliflozin.
Table 3. Characteristics of Cardiac Function.
| Variables | Baseline | 1 month | P-value |
|---|---|---|---|
| EF | 63.6 ± 10.9 | 62.9 ± 11.0 | 0.1 |
| E/A | 0.7 ± 0.3 | 0.6 ± 0.2 | < 0.05 |
| E/e' | 12.6 ± 5.0 | 9.6 ± 3.2 | < 0.01 |
| LAD (mm) | 39.7 ± 7.4 | 36.8 ± 7.3 | < 0.01 |
| IVCmax (mm) | 13.7 ± 4.5 | 13.6 ± 4.2 | 0.6 |
*Paired t-test. EF: ejection fraction; E/A: early filling/atrial filling; E/e': change in inflow E and mitral e' annular velocities; LAD: left atrial dimension; IVCmax: maximal diameter of inferior vena cava.
A 3-month interim analysis using a small number of samples (n = 18) has also found that E/e' (mean ± SD) on baseline, 1 month and 3 months were 12.13 ± 4.70, 9.50 ± 3.23 and 9.71 ± 4.09, respectively (baseline vs. 1 month, P < 0.01, baseline vs. 3 months, P < 0.01, by paired t-test).
The results of analysis of variance of E/e' with a mixed effect model were summarized in Table 4, showing that fixed effect (treatment time) and random effect (subjects) were significant (P < 0.05), whereas the interactions (time × gender, time × age) were not significant. Figure 1 shows an interaction plot likely to exist between time and gender, which was not significant (P = 0.09).
Table 4. Results of Two-Factor Mixed Effect Model to Evaluate Effects and Interaction of E/e'.
| SOV | df | Sum sq. | Mean sq. | F | Prob > F | Significance |
|---|---|---|---|---|---|---|
| Time | 1 | 76.6 | 76.6 | 8.0 | 0.01 | * |
| Subject | 23 | 574.2 | 25.0 | 2.6 | 0.01 | * |
| Gender | 1 | 43.5 | 43.5 | 1.7 | 0.2 | |
| Time × gender | 1 | 30.3 | 30.3 | 3.2 | 0.09 | |
| Error | 23 | 221.2 | 9.6 | |||
| Total | 49 | 984.1 | 20.1 | |||
| Time | 1 | 81.2 | 81.2 | 7.7 | 0.01 | * |
| Subject | 23 | 539.9 | 23.5 | 2.2 | 0.03 | * |
| Age (< 80 years) | 1 | 77.9 | 77.9 | 3.3 | 0.08 | |
| Time × age | 1 | 7.5 | 7.5 | 0.7 | 0.4 | |
| Error | 23 | 243.9 | 10.6 | |||
| Total | 49 | 984.1 | 20.1 |
Analysis of variance table for the mixed effects model. SOV: source of variance; Sum sq.: sum of squares; df: degree of freedom; Mean sq.: mean squares; F: F statistic; Prob: probability. *P < 0.05; **P < 0.01.
Figure 1.
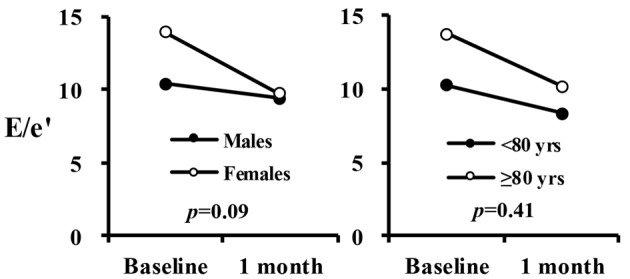
Interaction plot in E/e′ between time, gender and age.
Discussion
The present study found that LV diastolic function as measured by E/A, E/e' and LAD for patients with T2DM under stable HF had significantly improved 1 month after administration of tofogliflozin in elderly patients with T2DM. Other cardiac functional parameters such as EF and diameter of IVC did not change 1 month after administration of tofogliflozin. The results also suggested that body weight and blood pressure significantly decreased, consistent with the previous results [17]. The results of analysis of variance of cardiac parameter E/e' with a mixed effect model found that the observed significant decrease of E/e' by tofogliflozin was independent of the underlying source of variation, such as gender and age. Random effect was also significant, indicating that the effect of tofogliflozin varied between subjects. The mechanism still remains unclear, but it is suggested that SGLT2 inhibitor in reducing the risk of adverse cardiovascular outcomes in patients with T2DM including anti-atherosclerotic effect is considered unlikely, given the speed of the observed decrease in cardiovascular mortality. Hemodynamic effects, such as reductions in blood pressure and intravascular volume, involving osmotic diuresis, may provide a more plausible explanation. Metabolic effects, such as cardiac fuel energetics, and hormonal effects, such as increased glucagon release, may also be included in the mechanism [18].
T2DM is one of the most crucial comorbidities associated with CVD that attributes to end organ damage. T2DM also increases the risk for developing HF [19] and its associated complications, including death [20]. SGLT2 inhibitors have been known to improve glycemia and promote weight loss and body fat mass reduction in humans and animals with diabetes, in part, due to caloric loss by high excretion of urinary glucose and an enhancement of oxidative metabolism from carbohydrate to fatty acid [21, 22]. The effect of tofogliflozin on cardiac function has been known to increase EF from 48% to 61%, in parallel with increase of ketone bodies (beta-hydroxybutyrate and acetoacetate) levels in a T2DM case [23]. Although we did not measure ketone bodies, the mechanism of the effect may underlie some metabolic shift. Tofogliflozin has been known to be a most selective SGLT2 inhibitor. Compared to SGLT1, the selectivity of tofogliflozin is highest, approximately 3,000-fold [24]. SGLT2 transporter is located mostly in the kidney, whereas SGLT1 is located in various organs, i.e. small intestine, kidney, parotid glands, submandibular glands and in the heart [25]. These results suggest that the effect of SGLT2 inhibitors on cardiac functions commonly observed by both tofogliflozin and canagliflozin may not be mediated via SGLT1.
LV diastolic dysfunction is the most frequently observed early LV functional abnormality in patients with T2DM [26]. The LV diastolic dysfunction is thought to be due to the underlying pathophysiological abnormality of cardiac function, and thus its assessment plays an important role for diagnosis of patients with HF. The LV diastolic dysfunction is known to be independently relevant to outcomes in patients with HF [12, 13]. SGLT2 inhibitors have a multimodal effect on cardiac function including improvement in endothelial dysfunction and aortic stiffness [27]. Short-term treatment with empagliflozin is known to reduce LV diastolic function as assessed in terms of e' [28]. The effects of canagliflozin on LV diastolic function as assessed by E/e' and left ventricular mass index (LVMI) had significantly improved by 3 months treatment of canagliflozin while maintaining EF [29]. Soga et al also showed that 6-month treatment with dapagliflozin improved LV diastolic functional parameters including E/e', left atrial volume index (LAVI) and LVMI [11]. Although the precise mechanism of the effect of SGLT2 inhibitors on LV diastolic function remains uncertain, significant correlation was reported between E/e' and pulmonary capillary wedge pressure [30, 31], indicating that decrease of E/e' would be beneficial in T2DM patients with HF.
The present study also showed significant increase in renin and aldosterone. The renin-angiotensin-aldosterone system is a hormonal system known to regulate blood pressure and fluid and electrolyte balance, as well as systemic vascular resistance [32]. We have already revealed that the electrolyte level was stable between before and after tofogliflozin treatment [33]. A previous study demonstrated that canagliflozin increased plasma renin activity while decreased plasma atrial natriuretic peptide and N-terminal pro-b-type natriuretic peptide levels, presumably via compensatory mechanism for sodium retention, leading to subsequent urine output recovery [34]. SGLT2 inhibitor is also known to reduce urinary angiotensinogen/creatinine ratio [35]. Renin-angiotensin blocker also showed improvement in LV function [36]. These results suggest that LV diastolic function is relevant to the function of renin-angiotensin system.
The present study also showed significant decrease in systolic blood pressure during 1-month treatment with tofogliflozin, consistent with the Japanese post-marketing study [17]. Reductions in blood pressure are known to correlate with the amelioration of albuminuria in patients with T2DM [37]. Several clinical trials have also shown that SGLT2 inhibitors exert blood pressure-lowering effects through their diuretic actions, which may partially contribute to the amelioration of albuminuria in diabetic patients [38]. On the other hand, in a rodent study high-dose ipragliflozin reduced the LV chamber size while maintaining stably in blood pressure [39]. The effect of SGLT2 inhibitor on blood pressure still remains controversial, which may be partially caused by the time at which blood pressure was measured. Tofogliflozin has also been known to improve insulin resistance in both animals and humans [40-42].
Study limitations
The results of our study should be considered in the context of several potential limitations. First, due to the observational nature of the study, and despite robust statistical techniques, a possibility of residual, unmeasured confounding cannot be excluded. Specifically, certain patient and physician factors may not be adequately captured. Second, the present study evaluated only 1 month of drug treatment period, which could be insufficient to detect change in physiological parameters. However, the 3-month interim analysis using a small number of samples has found that E/e' significantly decreased both 1 month and 3 months period, suggesting that the effect on E/e' could exhibit in relatively short term and 1 month could be enough to become stable. Longer-term follow-up will be needed to clarify the effects of tofogliflozin on various physiological parameters.
Conclusions
The present study found that tofogliflozin improved LV diastolic function regardless of gender and age, under preserving IVC, renal function and electrolyte balance. Tofogliflozin could improve LV diastolic function in elderly patients with T2DM. SGLT2 inhibition may emerge as an effective and safe adjunctive therapy for HF that promotes hemodynamic stability and helps correct volume overload, while avoiding the risks of volume depletion, independent of effects on hyperglycemia. Nevertheless, the mechanisms responsible for the acute cardioprotective effects of SGLT2 inhibition, such as sodium and water homeostasis and plasma volume regulation remain unknown in patients with T2DM and in those with HF. Further studies will be expected to elucidate these mechanisms.
Acknowledgments
None to declare.
Financial Disclosure
The present study was financially supported by kissoykai (2019-11).
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Patients were not required to give informed consent to the study because the retrospective analysis used anonymous clinical data that were obtained after each patient agreed to the treatment schedule by written consent.
Author Contributions
Toshihiro Higashikawa wrote the manuscript; Daisuke Usuda and Susumu Takagi collected data for review; all authors read and approved the final version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241(19):2035–2038. doi: 10.1001/jama.1979.03290450033020. [DOI] [PubMed] [Google Scholar]
- 2.Vazquez-Benitez G, Desai JR, Xu S, Goodrich GK, Schroeder EB, Nichols GA, Segal J. et al. Preventable major cardiovascular events associated with uncontrolled glucose, blood pressure, and lipids and active smoking in adults with diabetes with and without cardiovascular disease: a contemporary analysis. Diabetes Care. 2015;38(5):905–912. doi: 10.2337/dc14-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair S, Wilding JP. Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab. 2010;95(1):34–42. doi: 10.1210/jc.2009-0473. [DOI] [PubMed] [Google Scholar]
- 4.Tahara A, Takasu T, Yokono M, Imamura M, Kurosaki E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors in pharmacokinetics, pharmacodynamics, and pharmacologic effects. J Pharmacol Sci. 2016;130(3):159–169. doi: 10.1016/j.jphs.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W. et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study) Circulation. 2018;137(4):323–334. doi: 10.1161/CIRCULATIONAHA.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W. et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 7.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 8.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 9.Haring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, Broedl UC. et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36(11):3396–3404. doi: 10.2337/dc12-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, Broedl UC. et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37(7):1815–1823. doi: 10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
- 11.Soga F, Tanaka H, Tatsumi K, Mochizuki Y, Sano H, Toki H, Matsumoto K. et al. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol. 2018;17(1):132. doi: 10.1186/s12933-018-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, Sato K. et al. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol. 2017;69(15):1937–1948. doi: 10.1016/j.jacc.2017.01.058. [DOI] [PubMed] [Google Scholar]
- 13.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 14.Daimon M, Akaishi M, Asanuma T, Hashimoto S, Izumi C, Iwanaga S, Kawai H. et al. Guideline from Japanese Society of Echocardiography: 2018 focused update incorporated into guidance for the management and maintenance of echocardiography equipment. J Echocardiogr. 2018;16(1):1–5. doi: 10.1007/s12574-018-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102(15):1788–1794. doi: 10.1161/01.CIR.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 16.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Utsunomiya K, Shimmoto N, Senda M, Kurihara Y, Gunji R, Fujii S, Kakiuchi S. et al. Safety and effectiveness of tofogliflozin in elderly Japanese patients with type 2 diabetes mellitus: A post-marketing study (J-STEP/EL Study) J Diabetes Investig. 2017;8(6):766–775. doi: 10.1111/jdi.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staels B. Cardiovascular Protection by Sodium Glucose Cotransporter 2 Inhibitors: Potential Mechanisms. Am J Med. 2017;130(6S):S30–S39. doi: 10.1016/j.amjmed.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, Kjeldsen K. et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64(21):2281–2293. doi: 10.1016/j.jacc.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34(1):29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 21.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217–2224. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimura R, Tanaka Y, Koiwai K, Inoue K, Hach T, Salsali A, Lund SS. et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11. doi: 10.1186/s12933-014-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutoh E, Hayashi J. Effect of Canagliflozin on Heart Function Involving Ketone Bodies in Patients with Type 2 Diabetes. Drug Res (Stuttg) 2019;69(5):297–300. doi: 10.1055/a-0748-5745. [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes. 2013;62(10):3324–3328. doi: 10.2337/db13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madunic IV, Breljak D, Karaica D, Koepsell H, Sabolic I. Expression profiling and immunolocalization of Na(+)-D-glucose-cotransporter 1 in mice employing knockout mice as specificity control indicate novel locations and differences between mice and rats. Pflugers Arch. 2017;469(12):1545–1565. doi: 10.1007/s00424-017-2056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25(4):543–567. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- 27.Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, Bruno RM. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16(1):138. doi: 10.1186/s12933-017-0621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma S, Garg A, Yan AT, Gupta AK, Al-Omran M, Sabongui A, Teoh H. et al. Effect of Empagliflozin on Left Ventricular Mass and Diastolic Function in Individuals With Diabetes: An Important Clue to the EMPA-REG OUTCOME Trial? Diabetes Care. 2016;39(12):e212–e213. doi: 10.2337/dc16-1312. [DOI] [PubMed] [Google Scholar]
- 29.Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):73. doi: 10.1186/s12933-018-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushita K, Minamishima T, Goda A, Ishiguro H, Kosho H, Sakata K, Satoh T. et al. Comparison of the reliability of E/E' to estimate pulmonary capillary wedge pressure in heart failure patients with preserved ejection fraction versus those with reduced ejection fraction. Int J Cardiovasc Imaging. 2015;31(8):1497–1502. doi: 10.1007/s10554-015-0718-7. [DOI] [PubMed] [Google Scholar]
- 32.Vaidya A, Brown JM, Williams JS. The renin-angiotensin-aldosterone system and calcium-regulatory hormones. J Hum Hypertens. 2015;29(9):515–521. doi: 10.1038/jhh.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higashikawa T, Ito T, Mizuno T, Ishigami K, Kohori M, Mae K, Sangen R. et al. The effects of 12-month administration of tofogliflozin on electrolytes and dehydration in mainly elderly Japanese patients with type 2 diabetes mellitus. J Int Med Res. 2018;46(12):5117–5126. doi: 10.1177/0300060518790870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka H, Takano K, Iijima H, Kubo H, Maruyama N, Hashimoto T, Arakawa K. et al. Factors Affecting Canagliflozin-Induced Transient Urine Volume Increase in Patients with Type 2 Diabetes Mellitus. Adv Ther. 2017;34(2):436–451. doi: 10.1007/s12325-016-0457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimoto T, Furuki T, Kobori H, Miyakawa M, Imachi H, Murao K, Nishiyama A. Effects of sodium-glucose cotransporter 2 inhibitors on urinary excretion of intact and total angiotensinogen in patients with type 2 diabetes. J Investig Med. 2017;65(7):1057–1061. doi: 10.1136/jim-2017-000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito H, Ishii K, Kihara H, Kasayuki N, Nakamura F, Shimada K, Fukuda S. et al. Adding thiazide to a renin-angiotensin blocker improves left ventricular relaxation and improves heart failure in patients with hypertension. Hypertens Res. 2012;35(1):93–99. doi: 10.1038/hr.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603–615. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 38.Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8(4):262–275. doi: 10.1016/j.jash.2014.01.007. e269. [DOI] [PubMed] [Google Scholar]
- 39.Kamezaki M, Kusaba T, Komaki K, Fushimura Y, Watanabe N, Ikeda K, Kitani T. et al. Comprehensive renoprotective effects of ipragliflozin on early diabetic nephropathy in mice. Sci Rep. 2018;8(1):4029. doi: 10.1038/s41598-018-22229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M. et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13(3):294–307. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 41.Obata A, Kubota N, Kubota T, Iwamoto M, Sato H, Sakurai Y, Takamoto I. et al. Tofogliflozin Improves Insulin Resistance in Skeletal Muscle and Accelerates Lipolysis in Adipose Tissue in Male Mice. Endocrinology. 2016;157(3):1029–1042. doi: 10.1210/en.2015-1588. [DOI] [PubMed] [Google Scholar]
- 42.Tobe K, Suganami H, Kaku K. Sodium-glucose cotransporter 2 inhibitor, tofogliflozin, shows better improvements of blood glucose and insulin secretion in patients with high insulin levels at baseline. J Diabetes Investig. 2018;9(4):862–869. doi: 10.1111/jdi.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.


