Abstract
Uterine glands and their secretions are indispensable for endometrial function and fertility; however, the mechanisms regulating their development and function are not well understood. Forkhead transcription factor box A2 (FOXA2) is uniquely expressed in the glandular epithelial (GE) cells of the uterus, and conditional deletion of Foxa2 after birth impedes uterine gland development. An integrative approach was used here to define the FOXA2 cistrome in the murine uterus. Genome-wide mapping of FOXA2 binding sites was combined with transcriptomic analyses of isolated GE and Foxa2-deleted uteri. ChIP-Seq analyses found the number of FOXA2 target genes was substantially greater in the adult (8893) than neonatal uterus (1101). In the neonatal uterus, FOXA2-bound and GE-expressed genes (469) were enriched for developmentally related processes, including cell cycle, cell junction, focal adhesion, and WNT signaling. In the adult uterus, FOXA2-bound and GE-expressed genes (3730) were enriched for functional processes, including metabolic pathways, focal adhesion, bacterial invasion of epithelial cells, and WNT signaling. Analysis of the uterine FOXA2 cistrome provides novel insights into mechanisms governing endometrial gland development and function, which are important to understand fundamental aspects of uterine differentiation, regeneration and disease.— Filant, J., Lydon, J. P., Spencer, T. E. Integrated chromatin immunoprecipitation sequencing and microarray analysis identifies FOXA2 target genes in the glands of the mouse uterus.
Keywords: ChIP-Seq, development, function, transcription factor
Uterine glands synthesize and secrete or transport substances critical for blastocyst survival, development, and implantation in the uterus (1-5). Sheep that lack uterine glands, known as uterine gland-knockout ewes, are infertile and display recurrent early pregnancy loss due to defects in peri-implantation blastocyst survival and growth (6). Similarly, mice that lack uterine glands are infertile due to defects in blastocyst implantation attributed to defects in blastocyst activation for attachment and endometrial receptivity (3, 7, 8). Available evidence in humans also supports a primary role for uterine glands and their secretions in blastocyst implantation and recurrent early pregnancy loss (9, 10). In addition, uterine gland secretions have an emerging role in stromal cell decidualization that is necessary for establishment of pregnancy in humans and rodents (3, 8). However, we know little about the mechanisms regulating uterine gland differentiation and development in the endometrium as well as their function in the adult uterus, which is important, as uterine glands are essential determinants of the embryotrophic potential and functional capacity of the uterus (1, 3).
In laboratory animals, domestic animals, and humans, uterine gland morphogenesis or adenogenesis is uniquely or primarily a postnatal event (for review, see ref. 1). The newborn mouse uterus lacks uterine glands and consists of a simple luminal epithelium (LE) supported by undifferentiated mesenchyme. Between birth [postnatal day (PD) 0] and PD 6, glandular epithelium (GE) cells differentiate and bud from the LE. By PD 12, GE buds extend from the LE into the surrounding endometrial stroma as they develop toward the inner circular layer of myometrium. By PD 15, the histoarchitecture of the uterus resembles that of the adult, and the glands are well developed. In addition to initial development after birth, GE development is a fundamental aspect of endometrial regeneration after parturition and during the proliferative phase of each menstrual cycle in humans (11). A variety of genes and their signaling pathways, including the canonical wingless-type MMTV integration site family member (WNT) pathway, have been implicated in or found to be essential for uterine adenogenesis in the neonatal mouse uterus (1, 12). Of particular note, a recent study identified forkhead box A2 (FOXA2) as a critical regulator of uterine adenogenesis in mice (8). In the adult mouse uterus, FOXA2 is expressed solely in the GE cells of the endometrium (3, 8). Conditional deletion of Foxa2 after birth in the uterus, using the progesterone receptor Cre (PgrCre) mouse (13), impeded gland development, which thereby rendered the adult mouse infertile due to defects in blastocyst implantation stemming from a lack of endometrial glands and their secretions, such as leukemia inhibitor factor (LIF; ref. 8). FOXA2 belongs to a family of 3 forkhead transcription factors encoded by different genes (14). The FOXA genes have an important role in genesis, differentiation, and function of several organs, including the uterus (1, 14, 15). Forkhead transcription factors may also be involved in uterine dysfunction and disease, as FOXA2 was recently implicated as a factor in development of complex atypical endometrial hyperplasia (16) and is down-regulated in endometriosis (17).
Collective available evidence supports the hypothesis that FOXA2 has a biological role in GE differentiation, development, and function in the mouse uterus after birth and in the adult (3, 8, 12). As a critical first step to begin understanding the FOXA2 function in the endometrial glands of the uterus, genome-wide investigation of in vivo FOXA2 binding target regions was determined in the neonatal and adult uterus by chromatin immunoprecipitation coupled with massively parallel sequencing (ChIP-Seq). Those data were then integrated with laser capture microdissection (LCM) and transcriptomic analyses of GE from neonatal and adult uteri, as well as uteri from conditional Foxa2-deleted adult mice. The results provide novel insights into FOXA2 regulation of uterine gland development and function.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of Washington State University and conducted according to the U.S. National Institutes of Health guide for the care and use of laboratory animals and institutional guidelines. For histology, uteri were collected from CD-1 female mice on PD 5, 10, and 12 or day of pseudopregnancy (DOPP) 2.5 and 3.5 (vaginal plug: DOPP 0.5) after breeding to a vasectomized male, fixed in 4% paraformaldehyde in PBS (pH 7.2), and then embedded in paraffin. For ChIP-Seq, uteri were collected from CD-1 female mice on PD 12, DOPP 2.5, and DOPP 3.5, snap-frozen in liquid nitrogen, and stored at −80°C until analysis. For LCM, uteri were collected from CD-1 female mice on PD 10, DOPP 2.5, and DOPP 3.5 and immediately frozen in Tissue-Tek optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA) using liquid nitrogen and stored at −80°C. For microarray analysis, uteri were collected on DOPP 3.5 from control (Pgr+/+Foxa2flox/flox) and conditionally deleted Foxa2 (PgrCre/+Foxa2flox/flox) female mice using the PgrCre mouse (13) and Foxa2 flox mice generated by mating using described methods (8). Uteri were snap-frozen in liquid nitrogen stored at −80°C until analysis.
Immunohistochemistry
Fixed uteri were embedded in paraffin wax and then sectioned (5 μm). Sections were mounted on slides, deparaffinized in xylene substitute, and rehydrated in a graded alcohol series. Antigen retrieval was performed by incubating sections for 10 min in boiling 10 mM citrate buffer (pH 6.0). After cooling to room temperature, sections were incubated with 10% normal goat serum in PBS (pH 7.5) for 10 min at room temperature and then overnight at 4°C with rabbit anti-FOXA2 IgG (1.2 μg/ml of 1% BSA in PBS, pH 7.5; cat. no. LS-C 138006; LifeSpan Biosciences, Seattle, WA, USA) or normal rabbit IgG (Sigma-Aldrich, St. Louis, MO, USA) at the same final concentration. Sections were washed in PBS and incubated with biotinylated secondary antibody (5 μg/ml; PK-4001; Vector Laboratories, Burlingame, CA, USA) for 1 h at 37°C. Immunoreactive FOXA2 protein was visualized using a Vectastain ABC kit (Vector Laboratories) using diaminobenzidine tetrahydrochloride as the chromagen. Sections were counterstained with hematoxylin, and coverslips were affixed to slides with Permount mounting medium (Fisher Scientific, Fairlawn, NJ, USA).
ChIP and analyses
Genpathway FactorPath ChIP analysis was conducted by Active Motif (Carlsbad, CA, USA) using frozen PD 12 and DOPP 2.5 and 3.5 mouse uteri. Uterine tissue samples (∼ 100 mg) were submersed in PBS + 1% formaldehyde, cut into small pieces, and incubated at room temperature for 15 min. Fixation was stopped by the addition of 0.125 M glycine. The tissue pieces were then treated with a TissueTearor (BioSpec Products, Bartlesville, OK, USA) and finally spun down and washed twice in PBS. Chromatin was isolated by disrupting the cells with a Dounce homogenizer. Lysates were sonicated using a Misonix Sonicator 3000 (Misonix, Vernon Hills, IL, USA) equipped with a microtip in order to shear the DNA to an average length of 300–500 bp. Lysates were cleared by centrifugation and stored at −80°C. Genomic DNA (input) was prepared by treating aliquots of chromatin with RNase, proteinase K, and heat for decrosslinking, followed by phenol/chloroform extraction and ethanol precipitation. Purified DNA was quantified on a NanoDrop spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA). Extrapolation to the original chromatin volume allowed quantitation of the total chromatin yield. For each ChIP reaction, 30 μg of chromatin was precleared with protein A agarose beads (Invitrogen, Carlsbad, CA, USA). Immunoprecipitation was performed using 4 μg of goat anti-FOXA2 polyclonal IgG (sc-6554; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Following overnight incubation at 4°C, protein A agarose was added, and incubation at 4°C continued for another 3 h. Immune complexes were washed 2 times each with a series of buffers consisting of the deoxycholate sonication buffer, high-salt buffer, LiCl buffer, and TE buffer. Immune complexes were eluted from the beads with SDS buffer and subjected to RNase treatment and proteinase K treatment. Crosslinks were reversed by incubation overnight at 65°C, and ChIP DNA was purified by phenol-chloroform extraction and ethanol precipitation.
ChIP-Seq
Input and ChIP DNA were amplified using an Illumina ChIP-Seq DNA Sample Prep Kit (Illumina, San Diego, CA, USA). Briefly, DNA ends were polished and 5′-phosphorylated using T4 DNA polymerase, Klenow polymerase, and T4 polynucleotide kinase. After addition of 3′-adenine to blunt ends using Klenow (3′-5′ exo minus), Illumina genomic adapters were ligated, and the sample was size fractionated to 200–250 bp on a 2% agarose gel. After a PCR amplification (30 s at 98°C; followed by 10 s at 98°C, 30 s at 65°C, and 30 s at 72°C for 18 cycles; followed by 5 min at 72°C) with Phusion High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA, USA), the resulting DNA libraries were tested by reverse transcription quantitative PCR (RT-qPCR) at the same specific genomic regions as the original ChIP DNA to assess quality of the amplification reactions. The DNA libraries were sent to Illumina Sequencing Services for sequencing on an Illumina HiSeq 2000. Sequences (50 bases, single end) were aligned to the mouse genome [U.S. National Center for Biotechnology Information (NCBI) Build 37, July 2007, mm9] using the BWA algorithm. Alignments were extended in silico at their 3′-ends to a length of 150 bp and assigned to 32-nt bins along the genome. The resulting histograms were stored in binary analysis results (BAR) files. Peak locations were determined by applying a threshold of 18 (5 consecutive bins containing 18 aligns) and storing the resulting intervals in browser extensible data (BED) files. Interval locations in BED format are deposited in the NCBI Gene Expression Omnibus (GEO; GSE48341; http://www.ncbi.nlm.nih.gov/geo/).
The BED files were analyzed using GenPathway software (Active Motif) that provides information on genomic annotation, peak metrics, and sample comparisons for all intervals. The model based analysis of ChIP-Seq (MACS; ref. 18) peak-finding algorithm was used to normalize ChIP against the input control (P<10−7; mfold 8,30, bandwidth 150). Sequence conservation to identify phastCons scores, analysis of enriched motifs, and cis-regulatory element annotation system (CEAS) were performed using the Cistrome Analysis Pipeline software (http://cistrome.org.ap/) under default settings (19). For gene classification, the public Database for Annotation, Visualization, and Integrated Discovery (DAVID; http://david.abcc.ncifcrf.gov/) or Web-based Gene Set Analysis Toolkit (WebGestalt; http://bioinfo.vanderbilt.edu/webgestalt/) were utilized (20, 21).
ChIP-qPCR
Primers were designed to amplify regions of FOXA2 binding identified by ChIP-Seq analysis (Table 1). Positive and negative control PCR primer pairs from the ChIP-IT qPCR Analysis Kit (53029; Active Motif) were included in each analysis. qPCR was carried out in triplicate using SsoAdvanced SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) and CFX Connect Real-time PCR Detection System (Bio-Rad). The resulting signals were normalized to input DNA and presented as fold enrichment in relation to negative control.
Table 1.
Primer sequences used for ChIP-qPCR analysis
| RefSeq ID | Gene | Forward primer | Reverse primer |
|---|---|---|---|
| NM_009922 | Cnn1 | CTACCCTCCATGCTCACTCC | TTAAACCCCCTTACCCGTTC |
| NM_007614 | Ctnnb1 | TTTCCCTGTTGCCATTGTTT | GCCCCATTGTTGTCCTTC |
| NM_011339 | Cxcl15 | CATCTCAACCTGCTCTCAAC | CTTCCACTCTCCCCACTGAA |
| NM_053272 | Dhcr24 | GGCGAGGTCAGAGGTTGT | AAAGTGGATGGAGGCTTGG |
| NM_010446 | Foxa2 | ATGGGCGAGCGATAAGAAAC | GGGACAATGGAGACTGAAA |
| NM_010544 | Ihh | CCCATTCCTCCCTCTTCTTC | GTCCTAGTGCCTGCCATGTT |
| NM_008564 | Mcm2 | CCCAGTTACAGCGAAGGAGA | CTACACCCGGCACAGA |
| NM_010835 | Msx1 | TAAGTGTGAGCCCCAAGACC | AGCCCTGCCTATCTCCAT |
| NM_053259 | Prss28 | GGTCCTTTCTCACTGGG | CAAACTGCTGACGACTAATGCT |
| NM_016802 | Rhoa | TGGTGTTGGCTGAGTTGTT | GTGAGTCCCATGTTTGCTT |
| NM_172448 | Rnf43 | TCACCCTTTCCTCTGTCCA | TCCTTCTTCCCCTCAACTCC |
| NM_009258 | Spink3 | CCTCCCTTCATCATCTTT | ACTTTAGCCAGCCACCACTT |
LCM and RNA extraction
Uteri frozen in OCT compound were cryosectioned (12 μm) using a Leica CM1950 cryostat (Leica Microsystems, Wetzlar, Germany). Sections were mounted onto room-temperature RNase-free polyethylene napthalate (PEN)-coated slides (Carl Zeiss, Munich, Germany) and immediately placed on dry ice and fixed and stained on the same day using previously described methods (22). Briefly, slides were transferred from dry ice into ice-cold 95% ethanol for 30 s and incubated in 75% ethanol for 30 s. Sections were briefly stained in a solution of 1% cresyl violet in 75% ethanol. Tissue sections were dehydrated through 75% ethanol (30 s) and 95% ethanol (30 s), followed by two 30-s and one 5-min incubation in anhydrous 100% ethanol. Slides were dried for 5 min at room temperature and stored in vacuum-sealed containers at −80°C until use. LCM was performed for no longer than 60 min using a PALM MicroBeam LCM microscope (Carl Zeiss), and 8–10 slides were processed to capture LE or GE cells. Total RNA was extracted from collected cells using the RNeasy MinElute kit (Qiagen, Valencia, CA, USA) and eluted with 14 μl of RNase-free water. The integrity and concentration of RNA was analyzed with the Agilent RNA 6000 Pico Kit using an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The RNA yield ranged from 100 to 700 ng for each cell type with RNA integrity number (RIN) > 7.
Microarray
For LCM-isolated samples (n=2 pools/cell type), total RNA was amplified using Ovation Pico WTA System V2 (NuGen, San Carlos, CA, USA). For DOPP 3.5 mouse uteri (n=4/genotype), total RNA was extracted from uteri using Trizol reagent (Invitrogen), and on-column DNase treatment and clean-up were performed (Qiagen). Total RNA quality and quantity was determined using the Agilent Bioanalyzer and a NanoDrop 1000 (Thermo Fisher Scientific), respectively. cDNA was biotin labeled using a GeneChip One-Cycle Target Labeling Kit (Affymetrix, Santa Clara, CA, USA) and then hybridized to a Mouse Gene 1.0 ST microarray (Affymetrix). For the hybridization, wash, and staining process, the GeneChip Hybridization, Wash, and Stain Kit (Affymetrix) and a Fluidic Station 450 (Affymetrix) were used. All steps were done according to the manufacturer's protocol. The processed arrays were scanned with a GeneChip Scanner 3000 (Affymetrix). Microarray data are deposited in the NCBI GEO (GSE48239 and GSE48339).
GeneSpring 7.0 software (Agilent Technologies) was used for analysis of microarray data. Array output was normalized via the robust multiarray method (23), and probe sets were filtered based on expression calls. For LCM-derived microarray data, a gene was considered as expressed if the normalized probe intensity value was ≥100. Data analysis was conducted using ANOVA (P=0.05) with a Benjamini and Hochberg false discovery rate (FDR) multiple test correction to determine differentially expressed genes. DAVID 6.7 was used to generate specific functional annotations of biological processes.
Semiquantitative real-time RT-PCR
Microarray results were validated by real-time qPCR using methods described previously (12). Primers used for PCR analysis are provided in Table 2. The qPCR was carried out in triplicate using SsoAdvanced SYBR Green Supermix (Bio-Rad) and a CFX Connect Real-time PCR Detection System (Bio-Rad). Data was subjected to least-squares analyses of variance (ANOVA) using the General Linear Models (GLM) procedures of the Statistical Analysis System (SAS Institute Inc., Cary, NC, USA). In analysis, error terms used in test of significance were identified according to the expectation of the mean squares for error. The Ct values of the target mRNA were analyzed for effects of cell type, with the Gapdh values used as a covariate. Significance (P<0.05) was determined by probability differences of least-squares means (LSM).
Table 2.
Primer information for qPCR analysis
| mRNA | Primers, 5′–3′ | Product size (bp) | GenBank accession number |
|---|---|---|---|
| Abp1 | F: ATCCAATCTCCTGTCGTC | 80 | NM_010195.2 |
| R: GTAAGGCTCGGTTCCCTGT | |||
| Apobec2 | F: ATTCTCAAAACCCTCAGCAA | 88 | NM_009694.3 |
| R: CCTGGACCTCTGGCTCCTC | |||
| Clca3 | F: CCCTCCAGCCTCTCCTCTTT | 67 | NM_013737.5 |
| R: TTCATTTTTGCGATGTCCTTT | |||
| Ctse | F: AGGCACACCCAGTATTCCATCCA | 143 | NM_007799.3 |
| R: GCCATCCACAGTCAACCCTTCCA | |||
| Foxa2 | F: AGCAGAGCCCCAACAAGA | 117 | NM_010446.2 |
| R: AGAGAGAGTGGCGGATGGAG | |||
| Gapdh | F: CAACTACATGGTCTACATGTT | 122 | NM_008084.2 |
| R: CTCGCTCCTGGAAGATG | |||
| Ltf | F: ATGCTGGAGATGTGGCTT | 68 | NM_008522.3 |
| R: CCTTTCGGCTTTATTTGGT | |||
| Muc1 | F: TTGAGCATAACGGGACAAC | 123 | NM_001161848.2 |
| R: CATACAGGACAGCCCCAAGA | |||
| Ptgs2 | F: GAGCACCTTTGGAGGCGA | 131 | NM_011198.3 |
| R: CTGTTTTGGTAGGCTGTGGA | |||
| Prss28 | F: CATCCGACGAGCACAAAG | 89 | NM_053259.2 |
| R: CCCAGAGTCACCAAAACAG | |||
| Serpina3n | F: GACAATGGGACACAACTGGA | 70 | NM_009252.2 |
| R: TGTAGAGGCTGAAGGCAAA | |||
| Sidt1 | F: CTTTGCCTTCTTTGGATTG | 144 | NM_198034.3 |
| R: ACTGGTAGCGGGAGGACTTT | |||
| Spink3 | F: AACGCATAGAGCCTGTCCT | 100 | NM_009258.5 |
| R: ACGAACCCACTTGCCAAA | |||
| Ttr | F: CAGAGTGGACCAACCG | 135 | NM_013697.5 |
| R: CCCAGGGCTTTTGAACATGC | |||
| Wfdc3 | F: CTCTCATAGCCAGCAGGAC | 117 | NM_027961.1 |
| R: CCGGACATTCACCTCTCAA |
F, forward; R, reverse.
RESULTS
An integrated approach combining ChIP-Seq and transcriptomic analysis was used to identify candidate FOXA2-regulated genes and pathways in the endometrial glands of the neonatal and adult uterus. The PD 10 or 12 uterus of the neonatal mouse were used, because distinct differentiated and developing glands are present in the endometrium (Fig. 1). Pseudopregnant mice were used on DOPP 2.5 and 3.5, because the embryo enters the uterus by d 3.5 postmating, and endometrial gland secretions begin to influence uterine receptivity and blastocyst implantation (2). Further, the uteri of pseudopregnant mice exhibit the same gene expression changes as pregnant mice, but uteri can be isolated without blastocysts (24).
Figure 1.
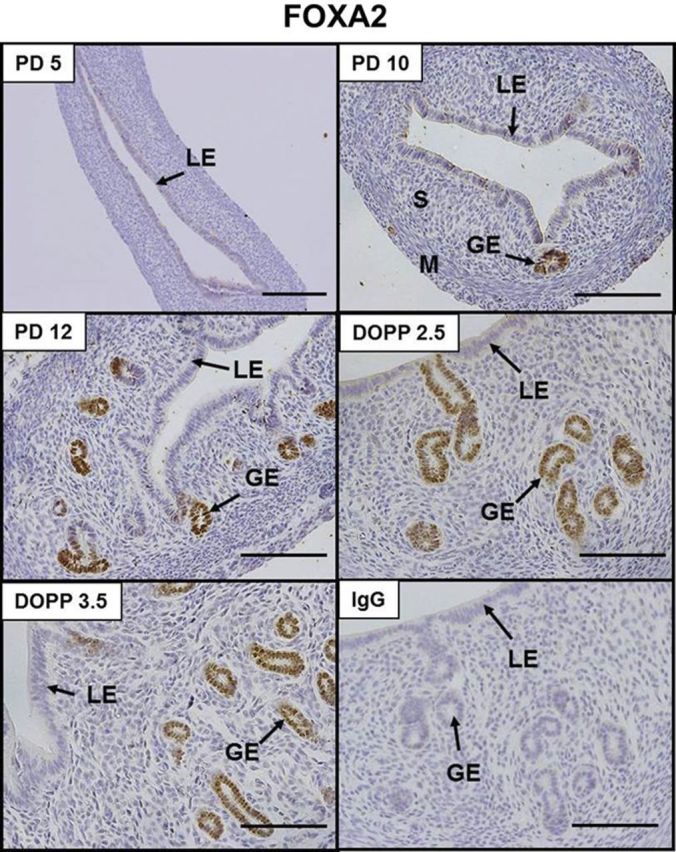
Immunolocalization of FOXA2 in the neonatal and adult mouse uterus. Sections were lightly counterstained with hematoxylin after immunolocalization of FOXA2 protein (brown). S, stroma; M, myometrium. Scale bars = 100 μm.
FOXA2 is expressed in the neonatal and adult mouse uterus
As illustrated in Fig. 1, immunoreactive FOXA2 was found solely in the nuclei of GE cells in the endometrium of both neonatal (PD 10 and 12) and adult pseudopregnant (DOPP 2.5 and 3.5) mouse uteri. Note the absence of detectable FOXA2 in the PD 5 uteri that do not contain endometrial glands and in the LE of PD 12 and DOPP 2.5 and 3.5 uteri.
Identification of FOXA2-binding sites in mouse uterine chromatin
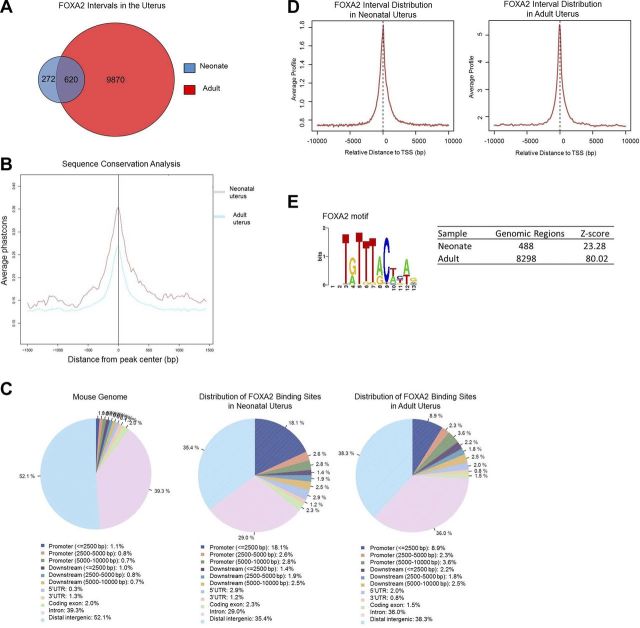
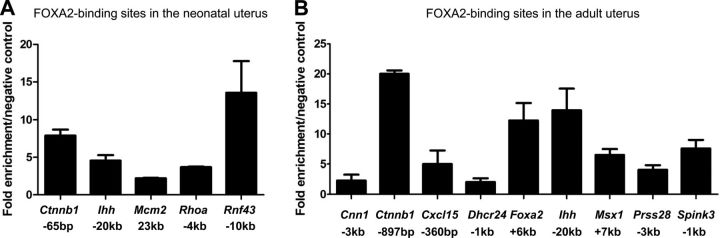
To determine the FOXA2 cistrome in the endometrial glands, ChIP-Seq was conducted and used to create a genome-wide profile of in vivo FOXA2-binding sites in the neonatal (PD 12) and adult (DOPP 2.5 and 3.5) mouse uterus. More than 15 × 106 tags of each sample were mapped to unique locations in the mouse genome. A model-based analysis peak-finding algorithm was used to normalize immunoprecipitated chromatin against input with a value of P = 1 × 10−7 and <1% FDR. This approach identified 892 FOXA2-bound intervals in the neonatal uterus and 10,490 intervals in the adult uterus (Fig. 2A). A total of 620 common FOXA2-bound intervals were found in the neonatal and adult uterus. Several genes identified as FOXA2 bound in ChIP-Seq analyses of the neonatal uterus (Ctnnb1, Ihh, Mcm2, Rhoa, and Rnf43) and adult uterus (Cnn1, Ctnnb1, Dhcr24, Foxa2, Ihh, Msx1, Prss28, and Spink3) were verified by ChIP-qPCR (Fig. 3).
Figure 2.
FOXA2-binding locations in the neonatal and adult mouse uterus. Location of FOXA2 binding in the whole mouse uterus was determined by ChIP-Seq analysis. A) FOXA2 intervals in the neonatal (PD 12) and adult (DOPP 2.5 and 3.5) mouse uterus. B) Sequence conservation analysis. FOXA2 intervals were aligned at their centers and uniformly expanded 1500 bp in each direction, and phastCons scores were retrieved and averaged at each position. C) Distribution of genome-wide FOXA2-binding locations throughout the neonatal and adult mouse uterus. D) Distribution of FOXA2 binding sites near promoter regions. E) FOXA2 consensus binding motif identified in FOXA2-bound intervals using the SeqPos tool of Cistrome. Number of genomic locations containing the FOXA2 consensus motif is indicated.
Figure 3.
ChIP validation of FOXA2 target genes. Validation of FOXA2-binding sites by ChIP-qPCR with the FOXA2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) on uteri isolated from neonatal (PD 12) and adult (DOPP 2.5 and 3.5). Negative control is a gene-deficient chromosomal region. Data are represented as fold enrichment of FOXA2 binding of FOXA2-location sites over that of a negative control region.
Distribution of FOXA2-binding sites
A comparison of sequences from the identified FOXA2 intervals among various genomes of placental mammals demonstrated a high level of evolutionary conservation within the regions of FOXA2 binding but not in surrounding regions (Fig. 2B). The intervals were mapped to reference genes of the NCBI mouse genome database (NCBI37/mm9). The neonatal and adult datasets were analyzed using the CEAS module of the web-based application Cistrome, and FOXA2 interval distribution was analyzed relative to genomic boundaries. Analyses found that the majority of FOXA2-binding sites in the neonatal and adult uterus were located within a gene or enriched near promoter regions of genes (Fig. 2C, D).
As determined by de novo motif analysis, the conserved FOXA consensus binding motif T[A/G]TT[G/T]AC was significantly enriched in FOXA2-bound intervals (Fig. 2E). Scanning of FOXA2 intervals with position-specific scoring matrix (PSSM) for FOXA2 yielded 488 (55%) and 8298 (79%) genomic regions that matched the given motif in the neonatal and adult datasets, respectively. Additional motif analysis on FOXA2 interval sequences using the Cistrome motif database found many transcription factor motifs to be significantly enriched (P<0.001) within ± 300 bp from the center of the interval. In the FOXA2-bound intervals of the neonatal uterus, a total of 42 motifs were enriched including sequences characteristic for numerous Forkhead, ETS, and E2F DNA-binding domains (Table 3). In the FOXA2-bound intervals of the adult uterus, more than 200 enriched motifs were identified (Table 4). Examples of identified motifs were those for other Forkhead family members (e.g., FOXO1 and FOXJ2), SOX transcription factors (e.g., SOX9 and SOX17), MSX2, SP1, and transcription factors with homeodomain DNA binding domains.
Table 3.
Top significantly enriched sequence motifs identified using the SeqPos tool on the web-based application Cistrome for the dataset containing FOXA2 binding in the neonatal uterus
| ID | Factor | DNA-binding domain | P |
|---|---|---|---|
| MC00023 | FOXA2 | Forkhead domain family | 1.00e-30 |
| MC00010 | FOXJ3 | Forkhead domain family | 1.00e-30 |
| MA0157 | FOXO3 | Forkhead domain family | 1.00e-30 |
| MC00039 | RAD21 | None | 1.48e-18 |
| MA0139 | CTCF | ββα-Zinc finger | 1.65e-18 |
| M00473 | FOXO1 | Forkhead domain family | 3.03e-14 |
| EN0433 | WRNIP1 | None | 9.11e-11 |
| MC00016 | ELF1 | Ets domain family | 3.16e-8 |
| EN0196 | E2F1 | E2F domain family | 7.12e-8 |
| hPDI017 | RARG | Hormone-nuclear receptor | 7.53e-8 |
| MC00034 | NRF1 | Leucine zipper (bZIP) | 2.05e-7 |
| UP00418 | Etv6 | Ets domain family | 2.24e-7 |
| MA0161 | NFIC | Nuclear factor I-CCAAT-binding transcription factor | 7.52e-7 |
| UP00415 | ELK4 | Ets domain family | 1.85e-6 |
| UP00401 | SOX4 | High-mobility group (box) | 1.62e-6 |
| UP00230 | DLX5 | Homeodomain | 1.94e-6 |
| M01243 | MTF1 | ββα-Zinc finger | 1.23e-5 |
| UP00403 | EHF | Ets domain family | 4.04e-5 |
| M00422 | FOXJ2 | Forkhead domain family | 4.63e-5 |
| UP00404 | ELF2 | Ets domain family | 7.06e-5 |
Table 4.
Top significantly enriched sequence motifs identified using the SeqPos tool on the web-based application Cistrome for the dataset containing FOXA2 binding in the adult uterus
| ID | Factor | DNA-binding domain | P |
|---|---|---|---|
| MC00023 | FOXA2 | Forkhead domain family | 1.00e-30 |
| MA0157 | FOXO3 | Forkhead domain family | 1.00e-30 |
| M00473 | FOXO1 | Forkhead domain family | 1.00e-30 |
| MA0077 | SOX9 | High-mobility group (box) | 1.00e-30 |
| M00422 | FOXJ2 | Forkhead domain family | 1.00e-30 |
| UP00401 | SOX4 | High-mobility group (box) | 1.00e-30 |
| UP00064 | SOX18 | High-mobility group (box) | 1.00e-30 |
| MA0161 | NFIC | Nuclear factor I-CCAAT-binding transcription factor family | 1.00e-30 |
| M01590 | SMAD1 | MH1 domain family | 1.00e-30 |
| hPDI137 | HMG20A | High-mobility group (box) | 1.00e-30 |
| UP00230 | DLX5 | Homeodomain | 1.00e-30 |
| UP00014 | SOX17 | High-mobility group (box) family | 1.00e-30 |
| MA0139 | CTCF | ββα-Zinc finger family | 1.00e-30 |
| hPDI180 | NFIB | Nuclear factor I-CCAAT-binding transcription factor family | 1.00e-30 |
| MC00022 | FOSL2 | Leucine zipper (bZIP) | 1.00e-30 |
| EN0377 | SREBF2 | Helix-loop-helix (bHLH) | 1.00e-30 |
| MC00039 | RAD21 | None | 1.96e-29 |
| UP00259 | HOXB6 | Homeodomain | 6.69e-22 |
| M00672 | TEF | Leucine zipper (bZIP) | 4.63e-20 |
| MC00026 | IRF3 | Interferon regulatory factor | 1.47e-19 |
| MC00046 | SP2 | ββα-Zinc finger family | 3.58e-18 |
| MC00045 | SP1 | ββα-Zinc finger family | 7.11e-18 |
| hPDI100 | ZFP3 | ββα-Zinc finger family | 4.52e-17 |
| UP00156 | MSX2 | Homeodomain | 1.38e-16 |
| MC00044 | SMARCC1 | None | 2.46e-14 |
| EN0123 | SMARCA4 | None | 1.03e-13 |
Identification of potential FOXA2 target genes in the mouse uterus
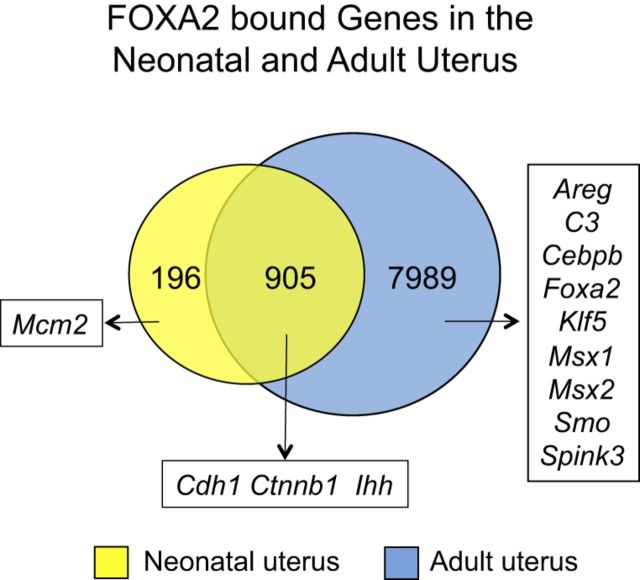
The FOXA transcription factors act as pioneer factors for other transcription factors in several adult organs (25). After mapping the enriched intervals to within 25 kb of transcription start site (TSS) of the nearest RefSeq gene, 1101 and 8893 genes were identified as candidate FOXA2-regulated genes in the neonatal and adult mouse uterus, respectively (Fig. 4). Most (82%) of the candidate FOXA2-bound genes (905 of 1,101) identified in neonatal uteri overlapped with those found in the adult uterus. Pathway analysis of candidate FOXA2-regulated genes in the neonatal uterus revealed quantitatively enriched functional categories related to the WNT signaling pathway (e.g., Rhoa and Ctnnb1), ribosome (e.g., Rps18 and Rpl21), tight junction (e.g., Cdc42 and Tjp3), cell cycle (e.g., Trp53 and Mcm7), N-glycan biosynthesis (e.g., Alg2 and Ddost), adherens junction (e.g., Cdc42 and Smad3), focal adhesion (e.g., Lama4 and Pip5k1c), glycosphingolipid biosynthesis (e.g., Hexa and St8sia5), cell adhesion molecules (e.g., Nlgn1 and Cldn14), and pathways in cancer (e.g., Cdh1 and Egf). In the adult uterus, pathway analysis of candidate FOXA2-regulated genes revealed quantitatively enriched functional categories related to focal adhesion (e.g., Rhoa and Rock2), amino acid degradation (e.g., Aldh6a1 and Bcat2), WNT signaling (e.g., Ctnnb1 and Lef1), mTOR signaling (e.g., Akt1 and Tsc1), cytokine-cytokine receptor interaction (e.g., Il1r2 and Lepr), allograft rejection (e.g., Il4 and H2-Q1), MAPK signaling (e.g., Fgf7 and Cacng2), cell adhesion molecules (e.g., Cdh2 and Cldn14) and arachidonic acid metabolism (e.g., Ptgs2 and Pla2g1b).
Figure 4.
Venn diagram illustrating nonredundant FOXA2-bound genes in the neonatal (PD 12) and adult (DOPP 2.5 and 3.5) mouse uterus. Genes with a FOXA2-binding location within 25 kb of their TSS are shown based on ChIP-Seq analysis. Note that more FOXA2-bound genes (blue) are found in the adult uterus than in the neonatal uterus (yellow). Examples of genes previously studied in the uterus are provided.
Integrated approach to identify FOXA2-regulated genes in the neonatal and adult uterus
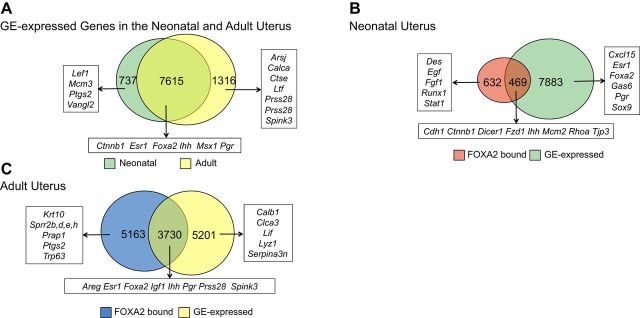
To identify genes expressed in the uterine glands of the neonatal and adult uterus, the GE of PD 10, DOPP 2.5, and 3.5 uteri was isolated by LCM, and transcriptome analysis was conducted using comprehensive microarrays. The mRNAs, identified as expressed in the GE by microarray analysis, were then integrated with the FOXA2 ChIP-Seq analysis to identify candidate FOXA2-regulated genes expressed in the endometrial glands of the neonatal and adult uterus (Fig. 5). Of the >28,000 coding transcripts present in the mouse Affymetrix array, 8352 and 8931 were determined to be expressed in the GE of the neonatal and adult mouse uterus, respectively, using this approach (Fig. 5A). This analysis identified a number of genes already known to be expressed in the GE of the adult mouse uterus, including Foxa2, Lif, and Spink3 (3, 8, 26, 27). Pathway analysis found that cell cycle (e.g., Cdk6 and Cdkn1b), WNT signaling (e.g., Ctnnb1 and Lef1), mTOR signaling (e.g., Mtor and Rictor), and adherens junctions (e.g., Erbb2, Cdc42, and Ctnna1) were the most enriched in the glands of the neonatal mouse uterus. In contrast, genes important for metabolism (e.g., Asns and Man1b1), protein processing in endoplasmic reticulum (e.g., Pdia3 and Hsp90ab1), amino acid degradation (e.g., Bcat1 and Acat2), mTOR signaling (e.g., Mtor and Rictor) and ErbB signaling pathways (e.g., Erbb2 and Hbegf) were most enriched in the glands of the adult mouse uterus. In the neonatal uterus, 469 GE-expressed genes were also bound by FOXA2 (Fig. 5B). In contrast, 3730 GE-expressed genes in the adult uterus were bound by FOXA2 (Fig. 5C).
Figure 5.
Venn diagrams illustrating nonredundant genes expressed in the endometrial glandular epithelia (GE) and bound by FOXA2 in the mouse uterus. Gene expression in the endometrial GE of the neonatal (PD 12) and adult (DOPP 2.5 and 3.5) mouse uterus was determined by microarray analysis of laser capture microdissected GE. Genes with a FOXA2-binding location within 25 kb of their TSS are shown based on ChIP-Seq analysis. A) Comparison of GE-expressed genes in neonatal (green) and adult uterus (yellow). B) Comparison of genes bound by FOXA2 and expressed in the GE of the neonatal uterus. C) Comparison of genes bound by FOXA2 and expressed in the GE of the adult uterus. Examples of genes previously implicated in uterine biology are provided.
Functional annotation analysis was done to determine candidate FOXA2-regulated pathways in GE cells of the neonatal and adult uterus. In the neonatal uterus (Supplemental Table S1), candidate FOXA2-activated pathways were involved in WNT signaling (e.g., Sfrp1 and Ctnnb1), adherens junction (e.g., Cdc42 and Cdh1), tight junction (e.g., Pard3 and Tjp3), ribosome (e.g., Rps18 and Rpl31), cell cycle (e.g., Mcm2 and Cdk6), and focal adhesion (e.g., Tln2 and Ctnnb1). As summarized in Supplemental Table S2, candidate FOXA2-repressed pathways were involved in glycosphingolipid biosynthesis (e.g., St6galnac4 and B3galt4), cancer (e.g., Ctnna3, Fos, Wnt5b, and Egf) and diverse immune responses (e.g., Rasgrp, Map3k14, and Pik3cg). In the adult uterus, candidate FOXA2-activated genes were involved in metabolism, pathways in cancer (e.g., Fgfr2 and Egfr), protein processing (e.g., Pdia3 and Calr), lysosome (e.g., Ctsc and Ctse), adherens junction (e.g., Smad3 and Ctnna1), bacterial invasion of epithelial cells (e.g., Wasf2 and Cbl), focal adhesion (e.g., Ctnnb1 and Rock2), and WNT signaling (e.g., Ctnnb1 and Gsk3b). In contrast, the candidate FOXA2-bound repressed genes in the uterine glands of the adult were not enriched for any particular signaling pathway.
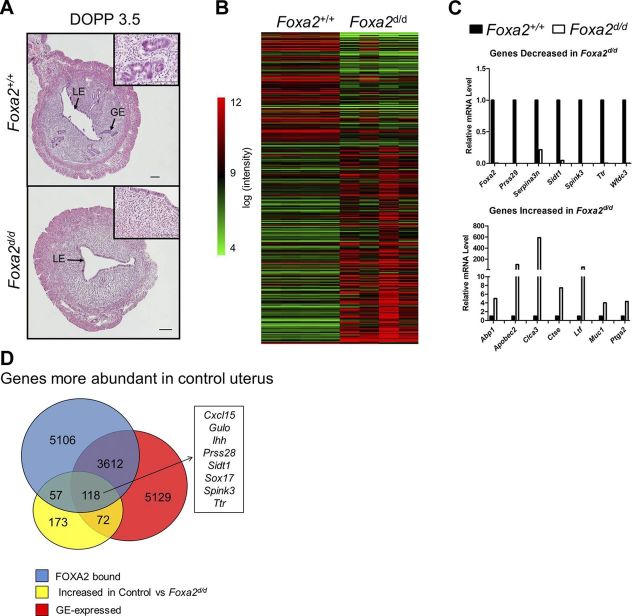
The effect of FOXA2 deletion after birth on gene expression in the adult uterus was determined by transcriptome profiling of uteri from control (Foxa2+/+ or Pgr+/+Foxa2+/+) and Foxa2-deleted (Foxa2d/d or PgrCre/+Foxa2flox/flox) mice on DOPP 3.5. This analysis was expected to identify genes expressed in the endometrial GE or affected by the loss of endometrial GE. Uteri from DOPP 3.5 was chosen for analysis, because the uterine glands secrete essential factors on d 3.5 postmating that affects blastocyst activation, attachment and implantation in the mouse (2). As expected, uterine glands were not present in the uterus of Foxa2-deleted mice (Fig. 6A). Conditional deletion of Foxa2 in the uterus after birth affected the expression of many genes in the DOPP 3.5 uterus (Fig. 6B). Several differentially expressed genes from the array data were verified by qPCR (Fig. 6C and Table 5). Expression of 400 genes was lower (P<0.05, >1.5 fold change) in Foxa2-deleted than control uteri (Fig. 6D). Of those genes, 175 were bound by FOXA2 and 118 were expressed in the GE, as determined by LCM and microarray analysis. Functional annotation analysis implicated those genes in organic acid biosynthesis (e.g., Gulo and Hpgds), steroid biosynthesis (e.g., Cyp1b1 and Soat1), hedgehog signaling pathway (e.g., Ptch1 and Ihh), and cell adhesion (e.g., Cdh5 and Pecam1). Several of the genes more abundant in the control uteri are known to be expressed in the endometrial GE of the adult mouse uterus, e.g., Prss28 and Spink3 (26, 28). Interestingly, 515 genes were higher (P<0.05, <−1.5 fold change) in the Foxa2-deleted than control uteri.
Figure 6.
Effect of Foxa2 deletion on gene expression in the adult uterus. A) Histological analysis of hematoxylin- and eosin-stained control and Foxa2-deleted uteri on DOPP 3.5. Scale bars = 100 μm. B) Heatmap illustrating differentially expressed genes (P<0.05, 1.5-fold change) in control as compared to Foxa2-deleted uteri. Gene expression was determined by microarray analysis of uteri from control and Foxa2-deleted mice on DOPP 3.5. C) Relative mRNA levels of selected differentially expressed genes in the control and Foxa2-deleted uteri on DOPP 3.5 as determined by real-time qPCR analysis. D) Venn diagram comparing FOXA2-bound genes and GE-expressed genes with genes more abundant in control as compared to Foxa2-deleted uteri on DOPP 3.5.
Table 5.
Selected genes differentially expressed in uteri of control and Foxa2-deleted adult (DOPP 3.5) mice, determined by microarray analysis (FDR 5%, fold change cutoff 1)
| Probe set ID | Gene symbol | Gene description | GeneBank | Fold change | P adj |
|---|---|---|---|---|---|
| 10458704 | Spink3 | Serine peptidase inhibitor, Kazal type 3 | NM_009258 | 19.4 | 2.5E-06 |
| 10523145 | Cxcl15 | Chemokine (C-X-C motif) ligand 15 | NM_011339 | 15.8 | 5.8E-09 |
| 10531100 | Sult1d1 | Sulfotransferase family 1D, member 1 | NM_016771 | 11.6 | 3.0E-03 |
| 10547641 | Slc2a3 | Solute carrier family 2 (facilitated glucose transporter), member 3 | NM_011401 | 3.6 | 1.4E-02 |
| 10401109 | Gpx2 | Glutathione peroxidase 2 | NM_030677 | 2.9 | 9.2E-04 |
| 10571444 | Slc7a2 | Solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 | NM_007514 | 2.9 | 4.2E-05 |
| 10462542 | Lipf | Lipase, gastric | NM_026334 | 2.7 | 6.3E-04 |
| 10412260 | Fst | Follistatin | NM_008046 | 2.6 | 6.0E-03 |
| 10565627 | Aqp11 | Aquaporin 11 | NM_175105 | 2.6 | 1.7E-02 |
| 10454192 | Ttr | Transthyretin | NM_013697 | 2.3 | 1.0E-04 |
| 10493474 | Muc1 | Mucin 1, transmembrane | NM_013605 | –3.0 | 2.0E-03 |
| 10558590 | Prap1 | Proline-rich acidic protein 1 | NM_009475 | –3.5 | 7.0E-03 |
| 10523717 | Spp1 | Secreted phosphoprotein 1 | NM_009263 | –3.9 | 2.0E-03 |
| 10350516 | Ptgs2 | Prostaglandin-endoperoxide synthase 2 | NM_011198 | –5.0 | 8.0E-03 |
| 10536563 | Cftr | Cystic fibrosis transmembrane conductance regulator | NM_021050 | –5.4 | 4.9E-04 |
| 10493870 | Sprr2f | Small proline-rich protein 2F | NM_011472 | –10.7 | 2.6E-05 |
| 10493864 | Sprr2d | Small proline-rich protein 2D | NM_011470 | –10.9 | 4.7E-04 |
| 10435112 | Muc4 | Mucin 4 | NM_080457 | –13.1 | 1.3E-05 |
| 10589703 | Ltf | Lactotransferrin | NM_008522 | –18.0 | 2.6E-06 |
| 10502622 | Clca3 | Chloride channel calcium activated 3 | NM_017474 | –23.4 | 8.3E-06 |
DISCUSSION
This study represents the first comprehensive analysis of the FOXA2 cistrome in the uterus, which is important, as FOXA2 has a biological role in differentiation and development, as well as, perhaps, function of endometrial glands in the mouse uterus (1, 3, 8). As has been noted in genome-wide mapping of nuclear receptors (29), a large number of FOXA2 binding sites occur in distal intergenic regions, whereas those located within genes are found predominantly within introns. Of those binding sites located in designated gene promoter regions, FOXA2 binding was highly distributed in proximity to the TSSs. It will be of interest to determine what functional role, if any, those FOXA2 binding locations have in gene transcription, possibly through the mechanism of DNA looping in which distal enhancer sites interact with transcriptional complexes located near gene boundaries through verified DNA-looping structures (30). Indeed, FOXA transcription factors are proposed to act as pioneer transcription factors, displacing linker histones from compacted chromatin in ATP-independent manner and facilitating the binding of other transcription factors to the gene enhancer (for review, see ref. 25). Further, FOXA proteins have an N-terminal transactivation domain that recruits coregulators, which, in turn, can facilitate other factors to enter chromatin (25).
Results of the present study support the emerging concept that FOXA2 can activate, as well as repress, transcription of genes within cells, tissues, and organs (31, 32). The corepressors Gro, TLE, and Grg have been shown to interact with FOXA1 (33), and Aes, Tle1, and Tl3 are expressed in the endometrial GE of the neonatal and adult mouse uterus, based on the present study. The present study also identified candidate FOXA2-repressed genes in the developing uterine glands by identifying FOXA2-bound genes in the neonatal uterus that were not expressed based on microarray analysis of LCM-isolated GE. For example, Ihh is bound by FOXA2 in both the neonatal and adult uterus, but Ihh is not active in the neonatal uterus, based on microarray analysis of isolated GE and real-time PCR analysis (12). Pathway analysis using DAVID indicated that molecules related to glycosphingolipid biosynthesis and pathways in cancer were enriched in the set of inactive FOXA2-bound genes in the neonatal uterus. This result supports the idea that FOXA2 represses genes to ensure maintenance of GE cell phenotype. In support of this idea, FOXA2 regulates polarity and epithelialization in the endoderm layer of the mouse embryo by suppressing a mesenchymal cell fate (33). Studies in lung cancer cells indicate that FOXA2 expression is positively correlated with epithelial cell identity (Cdh1 and Tjp1) and negatively correlated with mesenchymal identity (Vim) (32). In the present study, Cdh1 and Tjp3 were both identified as FOXA2-bound and GE-expressed genes in the neonatal uterus. Interestingly, FOXA2 functions as suppressor of epithelial to mesenchymal transition (EMT), one of the major mechanisms of cancer invasion and metastasis (32). Thus, FOXA2 positively regulates signaling pathways that are essential for cell fate determination, proliferation and adhesion, whereas it suppresses pathways that could trigger EMT and carcinogenesis.
In the neonatal uterus, uterine adenogenesis involves differentiation of the GE from LE and subsequent GE cell proliferation and migration as the glands grow in a coiling fashion to the myometrium (for review, see ref. 1). To date, a limited number of genes and their signaling pathways have been implicated in uterine gland development in mouse uterus (1, 3, 8, 12). The genes expressed in the developing glands of the neonatal uterus, identified by microarray analysis of LCM-isolated GE in the present study, will undoubtedly provide significant insights into key developmental pathways regulating gland morphogenesis. Among the most highly enriched biological pathways, metabolic pathways, cell cycle, WNT signaling, mTOR signaling, and adherens junctions were enriched in the neonatal uterine GE. The components of the WNT signaling pathway that were GE expressed included Sfrp1, Fzd1, Dvl1, Cdh1, Ctnnb1, Lef1, Rho, and Rock1. Indeed, the canonical WNT/β-catenin (CTNNB1) signaling pathway is of paramount importance for endometrial GE differentiation and development, since conditional deletion of Wnt7a, Ctnnb1, or Lef1 abrogates endometrial adenogenesis in mice (34–36), whereas expression of a dominant stabilized CTNNB1 resulted in endometrial gland hyperplasia (35). Indeed, one possible signaling pathway that either regulates Foxa2 gene transcription or is regulated by FOXA2 is the WNT signaling pathway. In other cells, FOXA2 was found to regulate the expression of multiple WNTs, including Wnt3a, Wnt8a, and Wnt7b (37), and a reciprocal interaction between FOXA2 and WNT signaling has been noted in that CTNNB1, a downstream effector of canonical WNT signaling, can promote Foxa2 expression (38). Whether the canonical WNT signaling cascade is upstream or downstream of FOXA2 in the regulatory cascade governing endometrial adenogenesis remains to be determined.
The integrated ChIP-Seq and GE transcriptome analyses presented here define pathways governing endometrial gland development and function, particularly since FOXA2 is expressed exclusively in the developing GE in the neonatal uterus and essential for GE differentiation in the postnatal uterus (8). The present study found many candidate genes that are likely positively regulated by FOXA2 in the developing GE of the neonatal uterus. Future mechanistic studies will be needed to understand their biological roles in endometrial gland differentiation and development. The candidate FOXA2 target genes are involved WNT signaling pathway, tight junction, cell cycle, adherens junction, and focal adhesion pathways. Indeed, all of those pathways are important for morphogenesis of epithelial tubes in the uterus and other organs (1, 39). In the present study, several transcription factors (e.g., NRF1) were expressed in glands of the neonatal uterus and their motifs enriched in FOXA2-binding intervals. Null mutation of those genes result in embryonic or early postnatal lethality; therefore, examination of their functional role in uterine development can only be accomplished via conditional gene deletion in the postnatal uterus (40).
In the present study, the number of genes expressed in the GE and bound by FOXA2 was greater in the adult (DOPP 2.5 and 3.5) than neonatal uterus, which is likely a consequence of organ maturity and onset of differentiated gland function in the adult uterus that involves action of steroid hormones, estrogen and progesterone, from the ovary (2, 41). Indeed, endometrial glands synthesize or transport substances critical for blastocyst survival, development, and implantation in mice and other mammals (1, 3, 5, 6, 8). Although endometrial glands are unequivocally required for the uterus to support blastocyst implantation and establishment of pregnancy, few studies have defined genes expressed in the GE during any stage of pregnancy (1, 42). Prior to the present study, only a few genes were known to be expressed in the endometrial glands of the mouse uterus, including Foxa2, Lef1, Prss28, and Spink3 (8, 26, 28, 36). In contrast, the present study identified a large number of genes (8931) expressed in the endometrial GE of DOPP 2.5 and 3.5 mouse uteri. Analysis revealed functionally related groups of genes important for metabolism, protein processing in endoplasmic reticulum, amino acid degradation, and mTOR and ErbB signaling pathways. Genes expressed in the endometrial glands have important roles in uterine receptivity, blastocyst implantation, and stromal cell decidualization (2, 3, 8, 41). For instance, LIF is expressed in GE in response to nidatory surge of ovarian estrogen on d 3.5 (27). Lif-null mice exhibit implantation failure, and supplementation with LIF rescues this defect (27). Although implantation and decidualization can be induced in hormone-primed ovariectomized mice by substituting LIF for nidatory estrogen (27), endometrial glands secrete other factors involved in blastocyst implantation and stromal cell decidualization, as revealed by recent studies with uterine gland-knockout mice (3). The discovery of genes expressed in uterine glands of adult mice here provides an important resource to understand the biological roles of endometrial glands and their secretions in early pregnancy. This information is essential to ensure investigations into conserved genes and pathways involved in endometrial function in mice and humans and may facilitate the discovery of biomarkers to diagnose and treat uterine-based infertility and enhance outcomes in assisted reproductive technologies (1).
In addition to a fundamental role of FOXA2 in organogenesis, FOXA2 is an important factor regulating homeostasis and function of adult organs (14). The integrated approach used in the present study identified a large number of candidate FOXA2 regulated genes in the endometrial glands of the adult mouse uterus. Those genes are likely involved in uterine homeostasis and function because they are functionally related to focal adhesion, metabolism, and protein processing in endoplasmic reticulum. Notably, uterine glands synthesize and secrete a number of essential bioactive molecule,s such as amino acids and glucose (43). Indeed, amino acids play an important role in trophectoderm expansion, motility, and outgrowth that is required for implantation of the mouse blastocyst (4). Interestingly, a number of estrogen (e.g., Cebpb, Cyr61, Errfi1, Igf1, and Muc1) and progesterone regulated genes (e.g., Areg, Ihh, Pgr, Per1, Sox17, and Clock) were found to be FOXA2 target genes in the present study (29, 44). Of note, pioneer transcription factors can regulate organ homeostasis by passive means, where their presence at a regulatory sequence could reduce the number of subsequent events required for transcriptional activation (25). In this case, target genes can be more rapidly activated when the final rate-limiting factors appear (25). Thus, it is likely that the presence of FOXA2 binding sites near regulatory sequences of steroid hormone responsive genes in adult uterine glands can help confer rapid endometrial responses to hormonal induction. The motifs of other transcription factors found to be overrepresented in the FOXA2-bound intervals and expressed in the glands of the mouse uterus included MSX2, SOX17, and a number of other FOX transcription factors. MSX2 is a nonclassical homeobox transcription factor transiently expressed in the uterine LE and GE on the morning of d 4 of pregnancy, but its expression is suppressed on the termination of uterine receptivity (2, 41). Notably, Msx1/Msx2 mutant mice show implantation failure with altered LE cell polarity and impaired stromal-epithelial communication (2, 41). Recently, SOX motifs were found to overlap with PGR binding sites on known progesterone-regulated genes, suggesting that SOX17 may act as a mediator of progesterone signaling in the adult mouse uterus (44). The remaining transcription factors that were identified near FOXA2-bound genomic locations are embryonically lethal, and their role in adult uterine function remains to be determined using a conditional gene targeting approach.
In the present study, gene expression was profiled in control and Foxa2-deleted mice on DOPP 3.5 in order to determine the effect of FOXA2 deletion after birth on gene expression in adult uterus. A set of 118 genes was identified by comparing candidate FOXA2-activated and expressed target GE genes with those lower in Foxa2-deficient than control DOPP 3.5 uteri. Those genes are involved in steroid biosynthesis (Lipa and Soat1), hedgehog signaling (Ptch1, Ihh, and Lrp2), cell adhesion (e.g., Cdh5, Jam2, and Pecam1), and biosynthesis of unsaturated fatty acids (Acot1 and Acot7). Further, a number of those genes encode secreted (e.g., Arsj, Cxcl15, Ctgf, Prss28, Spink3, and Spock2) and transport proteins (e.g., Aqp11, Slc1a1, Slco4c1, and Ttr), which could affect blastocyst implantation and endometrial cell function. Thus, one function of FOXA2 may be to positively regulate secretory and transport functions of glands during early pregnancy. One of the difficulties of using the Foxa2-deficient uteri to identify direct FOXA2 target genes is the fact that Foxa2 mutant mice also lack uterine glands. Thus, it is difficult to distinguish between genes differentially expressed due to the absence of the uterine glands vs. those regulated by FOXA2. A conditional inducible deletion mouse model will be necessary to unravel the biological role of FOXA2 in the adult uterus with respect to transcriptional regulation of target genes identified by ChIP-Seq analysis in the present study. Indeed, the gland-specific genes identified in the present study should enable the acquisition or generation of mouse models useful to conditionally delete or overexpress genes specifically in the developing glands of the neonatal uterus or functioning glands of the adult uterus.
Interestingly, many genes were also increased in the uteri of Foxa2-deleted as compared with control DOPP 3.5 mice. Many of the genes increased in expression in the Foxa2-deleted uteri are expressed in the endometrial LE in the adult uterus, including Clca3, Prap1, and Sprr2 family (45, 46). Intriguingly, small proline-rich proteins (SPRRs) construct the cornified cell envelope in the stratified squamous epithelial cells of tissues such as the vagina, but are also expressed in the uterine LE and GE (47). Thus, the up-regulation of the Sprr2 genes and other keratin genes may render the LE unable to mediate blastocyst attachment for implantation, which could be part of the implantation defect observed in conditional Foxa2-deleted mice as well as progesterone-induced uterine gland knockout mice. Collectively, these results support the idea that endometrial glands and their products play a biological role in the development of other cell types in the uterus, particularly the endometrial LE and stroma, and may be necessary for overall homeostasis of the uterus. Thus, the lack of proper endometrial gland generation and regeneration may have multiple effects on uterine function and early pregnancy. Future mechanistic studies are needed to understand Foxa2 gene regulation and the complex FOXA2-dependent genetic regulatory network governing endometrial gland development and function, which could provide significant insights into fundamental aspects of endometrial function and regeneration as well as uterine dysfunction underlying fertility problems and uterine disease including endometriosis and endometrial cancer.
Acknowledgments
The authors thank Dr. Paul Labhart (Active Motif, Carlsbad, CA, USA) for Performing the ChIP-seq and Derek Pouchnik (Molecular Biology and Genomics Core, Washington State University, Pullman, WA, USA) for performing microarray gene expression analyses.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ANOVA
- analysis of variance
- BED
- browser extensible data
- CEAS
- cis-regulatory element annotation system
- ChIP
- chromatin immunoprecipitation
- ChIP-Seq
- chromatin immunoprecipitation coupled with massively parallel sequencing
- DOPP
- day of pseudopregnancy
- FDR
- false discovery rate
- FOXA2
- forkhead transcription factor box A2
- GE
- glandular epithelium
- GEO
- Gene Expression Omnibus
- LCM
- laser capture microdissection
- LE
- luminal epithelium
- LIF
- leukemia inhibitor factor
- OCT
- optimal cutting temperature
- PD
- postnatal day
- PGR
- progesterone receptor
- qPCR
- quantitative PCR
- RT-qPCR
- reverse transcription quantitative PCR
- TSS
- transcription start site
REFERENCES
- 1.Spencer T. E., Dunlap K. A., Filant J. (2012) Comparative Developmental Biology of the uterus: insights into mechanisms and developmental disruption. Mol. Cell. Endocrinol. , 34–53 [DOI] [PubMed] [Google Scholar]
- 2.Cha J., Sun X., Dey S. K. (2012) Mechanisms of implantation: strategies for successful pregnancy. Nat. Med. , 1754–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filant J., Spencer T. E. (2013) Endometrial glands are essential for blastocyst implantation and decidualization in the mouse uterus. Biol. Reprod. , 93. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez I. M., Martin P. M., Burdsal C., Sloan J. L., Mager S., Harris T., Sutherland A. E. (2012) Leucine and arginine regulate trophoblast motility through mTOR-dependent and independent pathways in the preimplantation mouse embryo. Dev. Biol. , 286–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooke P. S., Spencer T. E., Bartol F. F., Hayashi K. (2013) Uterine glands: development, function and experimental model systems. Mol. Hum. Reprod. , 547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray C. A., Burghardt R. C., Johnson G. A., Bazer F. W., Spencer T. E. (2002) Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction , 289–300 [PubMed] [Google Scholar]
- 7.Cooke P. S., Ekman G. C., Kaur J., Davila J., Bagchi I. C., Clark S. G., Dziuk P. J., Hayashi K., Bartol F. F. (2012) Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice. Biol. Reprod. , 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong J. W., Kwak I., Lee K. Y., Kim T. H., Large M. J., Stewart C. L., Kaestner K. H., Lydon J. P., DeMayo F. J. (2010) Foxa2 is essential for mouse endometrial gland development and fertility. Biol. Reprod. , 396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton G. J., Scioscia M., Rademacher T. W. (2011) Endometrial secretions: creating a stimulatory microenvironment within the human early placenta and implications for the aetiopathogenesis of preeclampsia. J. Reprod. Immunol. , 118–125 [DOI] [PubMed] [Google Scholar]
- 10.Dimitriadis E., Stoikos C., Stafford-Bell M., Clark I., Paiva P., Kovacs G., Salamonsen L. A. (2006) Interleukin-11, IL-11 receptoralpha and leukemia inhibitory factor are dysregulated in endometrium of infertile women with endometriosis during the implantation window. J. Reprod. Immunol. , 53–64 [DOI] [PubMed] [Google Scholar]
- 11.Ludwig H., Spornitz U. M. (1991) Microarchitecture of the human endometrium by scanning electron microscopy: menstrual desquamation and remodeling. Ann. N. Y. Acad. Sci. , 28–46 [DOI] [PubMed] [Google Scholar]
- 12.Filant J., Zhou H., Spencer T. E. (2012) Progesterone inhibits uterine gland development in the neonatal mouse uterus. Biol. Reprod. , 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soyal S. M., Mukherjee A., Lee K. Y., Li J., Li H., DeMayo F. J., Lydon J. P. (2005) Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis , 58–66 [DOI] [PubMed] [Google Scholar]
- 14.Friedman J. R., Kaestner K. H. (2006) The Foxa family of transcription factors in development and metabolism. Cell. Mol. Life Sci. , 2317–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaestner K. H. (2010) The FoxA factors in organogenesis and differentiation. Curr. Opin. Genet. Dev. , 527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villacorte M., Suzuki K., Hirasawa A., Ohkawa Y., Suyama M., Maruyama T., Aoki D., Ogino Y., Miyagawa S., Terabayashi T., Tomooka Y., Nakagata N., Yamada G. (2013) beta-Catenin signaling regulates Foxa2 expression during endometrial hyperplasia formation. Oncogene , 3477–3482 [DOI] [PubMed] [Google Scholar]
- 17.Hawkins S. M., Creighton C. J., Han D. Y., Zariff A., Anderson M. L., Gunaratne P. H., Matzuk M. M. (2011) Functional microRNA involved in endometriosis. Mol. Endocrinol. , 821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y., Liu T., Meyer C. A., Eeckhoute J., Johnson D. S., Bernstein B. E., Nusbaum C., Myers R. M., Brown M., Li W., Liu X. S. (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol. , R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T., Ortiz J. A., Taing L., Meyer C. A., Lee B., Zhang Y., Shin H., Wong S. S., Ma J., Lei Y., Pape U. J., Poidinger M., Chen Y., Yeung K., Brown M., Turpaz Y., Liu X. S. (2011) Cistrome: an integrative platform for transcriptional regulation studies. Genome Biol. , R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang da W., Sherman B. T., Lempicki R. A. (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. , 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., Duncan D., Shi Z., Zhang B. (2013) WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res. (Web Server issue), W77–W83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummings M., McGinley C. V., Wilkinson N., Field S. L., Duffy S. R., Orsi N. M. (2011) A robust RNA integrity-preserving staining protocol for laser capture microdissection of endometrial cancer tissue. Anal. Biochem. , 123–125 [DOI] [PubMed] [Google Scholar]
- 23.Irizarry R. A., Hobbs B., Collin F., Beazer-Barclay Y. D., Antonellis K. J., Scherf U., Speed T. P. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics , 249–264 [DOI] [PubMed] [Google Scholar]
- 24.Deb K., Reese J., Paria B. C. (2005) Placenta and Trophoblast: Methods and Protocols, , Humana Press, Totowa, NJ, USA [Google Scholar]
- 25.Zaret K. S., Carroll J. S. (2011) Pioneer transcription factors: establishing competence for gene expression. Genes Dev. , 2227–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W., Han B. C., Wang R. C., Xiong G. F., Peng J. P. (2010) Role of secretory protease inhibitor SPINK3 in mouse uterus during early pregnancy. Cell Tissue Res. , 441–451 [DOI] [PubMed] [Google Scholar]
- 27.Stewart C. L. (1994) The role of leukemia inhibitory factor (LIF) and other cytokines in regulating implantation in mammals. Ann. N. Y. Acad. Sci. , 157–165 [DOI] [PubMed] [Google Scholar]
- 28.O'Sullivan C. M., Liu S. Y., Karpinka J. B., Rancourt D. E. (2002) Embryonic hatching enzyme strypsin/ISP1 is expressed with ISP2 in endometrial glands during implantation. Mol. Reprod. Dev. , 328–334 [DOI] [PubMed] [Google Scholar]
- 29.Hewitt S. C., Li L., Grimm S. A., Chen Y., Liu L., Li Y., Bushel P. R., Fargo D., Korach K. S. (2012) Research resource: whole-genome estrogen receptor alpha binding in mouse uterine tissue revealed by ChIP-seq. Mol. Endocrinol. , 887–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bulger M., Groudine M. (2011) Functional and mechanistic diversity of distal transcription enhancers. Cell , 327–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burtscher I., Lickert H. (2009) Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development , 1029–1038 [DOI] [PubMed] [Google Scholar]
- 32.Tang Y., Shu G., Yuan X., Jing N., Song J. (2011) FOXA2 functions as a suppressor of tumor metastasis by inhibition of epithelial-to-mesenchymal transition in human lung cancers. Cell Res. , 316–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekiya T., Zaret K. S. (2007) Repression by Groucho/TLE/Grg proteins: genomic site recruitment generates compacted chromatin in vitro and impairs activator binding in vivo. Mol. Cell , 291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunlap K. A., Filant J., Hayashi K., Rucker E. B., Song G., Deng J. M., Behringer R. R., DeMayo F. J., Lydon J., Jeong J. W., Spencer T. E. (2011) Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol. Reprod. , 386–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong J. W., Lee H. S., Franco H. L., Broaddus R. R., Taketo M. M., Tsai S. Y., Lydon J. P., DeMayo F. J. (2009) beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene , 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shelton D. N., Fornalik H., Neff T., Park S. Y., Bender D., DeGeest K., Liu X., Xie W., Meyerholz D. K., Engelhardt J. F., Goodheart M. J. (2012) The role of LEF1 in endometrial gland formation and carcinogenesis. PLoS One , e40312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mucenski M. L., Wert S. E., Nation J. M., Loudy D. E., Huelsken J., Birchmeier W., Morrisey E. E., Whitsett J. A. (2003) beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J. Biol. Chem. , 40231–40238 [DOI] [PubMed] [Google Scholar]
- 38.Yu X., Wang Y., Jiang M., Bierie B., Roy-Burman P., Shen M. M., Taketo M. M., Wills M., Matusik R. J. (2009) Activation of beta-Catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. Prostate , 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrew D. J., Ewald A. J. (2010) Morphogenesis of epithelial tubes: insights into tube formation, elongation, and elaboration. Dev. Biol. , 34–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huo L., Scarpulla R. C. (2001) Mitochondrial DNA instability and peri-implantation lethality associated with targeted disruption of nuclear respiratory factor 1 in mice. Mol. Cell. Biol. , 644–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S., Lin H., Kong S., Wang S., Wang H., Wang H., Armant D. R. (2013) Physiological and molecular determinants of embryo implantation. Mol. Aspects Med. , 939–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niklaus A. L., Pollard J. W. (2006) Mining the mouse transcriptome of receptive endometrium reveals distinct molecular signatures for the luminal and glandular epithelium. Endocrinology , 3375–3390 [DOI] [PubMed] [Google Scholar]
- 43.Bazer F. W., Kim J., Ka H., Johnson G. A., Wu G., Song G. (2012) Select nutrients in the uterine lumen of sheep and pigs affect conceptus development. J. Reprod. Dev. , 180–188 [DOI] [PubMed] [Google Scholar]
- 44.Rubel C. A., Lanz R. B., Kommagani R., Franco H. L., Lydon J. P., DeMayo F. J. (2012) Research resource: genome-wide profiling of progesterone receptor binding in the mouse uterus. Mol. Endocrinol. , 1428–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeong J. W., Lee K. Y., Lydon J. P., DeMayo F. J. (2006) Steroid hormone regulation of Clca3 expression in the murine uterus. J. Endocrinol. , 473–484 [DOI] [PubMed] [Google Scholar]
- 46.Diao H., Xiao S., Zhao F., Ye X. (2010) Uterine luminal epithelium-specific proline-rich acidic protein 1 (PRAP1) as a marker for successful embryo implantation. Fertil. Steril. , 2808–2811 e2801 [DOI] [PubMed] [Google Scholar]
- 47.Tan Y. F., Sun X. Y., Li F. X., Tang S., Piao Y. S., Wang Y. L. (2006) Gene expression pattern and hormonal regulation of small proline-rich protein 2 family members in the female mouse reproductive system during the estrous cycle and pregnancy. Reprod. Nutr. Dev. , 641–655 [DOI] [PubMed] [Google Scholar]