Abstract
Psoriasis lesions are rich in IL-17-producing T-cells as well as neutrophils, which release webs of DNA-protein complexes known as neutrophil extracellular traps (NETs). Because we and others have observed increased NETosis in psoriatic lesions, we hypothesized that NETs contribute to increased Th17 cells in psoriasis. After stimulating peripheral blood mononuclear cells (PBMCs) with anti-CD3/CD28 beads for 7 days, we found significantly higher percentages of CD3+CD4+IL-17+ (Th17) cells in the presence vs. absence of NETs, as assessed by flow cytometry, IL-17 ELISA, and IL17A/F and RORC mRNAs. Memory, but not naïve, T-cells were competent and monocytes were required for CD3/CD28-mediated Th17 induction, with or without NETs. Th17 induction was enhanced by the T allele of rs33980500 (T/C), a psoriasis risk-associated variant in the TRAF3IP2 gene encoding the D10N variant of Act1, a key mediator of IL-17 signal transduction. Global transcriptome analysis of CD3/CD28-stimulated PBMC by RNA-seq confirmed the stimulatory effects of NETs, demonstrated NET-induced enhancement of cytokine gene expression, and verified that the effect of Act1 D10N was greater in the presence of NETs. Collectively, these results implicate NETs and the Act1 D10N variant in human Th17 induction from PBMC, with ramifications for immunogenetic studies of psoriasis and other autoimmune diseases.
Keywords: Psoriasis, Th17, neutrophils, monocytes, RNA-seq
INTRODUCTION
Interleukin-17 (IL-17)-expressing T-cells (T17 cells) are characterized by the production of IL-17 and are considered to have evolved to protect against extracellular bacteria and fungi (Tesmer et al., 2008). However, recent discoveries have shifted focus to the central role of T17 cells in the pathogenesis of autoimmune and inflammatory disorders (Amatya et al., 2017). The role of IL-17 in the pathogenesis of psoriasis is evidenced by genetic (Harden et al., 2015), immunologic (Kryczek et al., 2008, Lowes et al., 2008) and pharmacologic studies (Lowes et al., 2014, Mease, 2015). The clinical effectiveness of drugs targeting IL-23, a cytokine required for the survival and expansion of human T17 cells (Dolgin, 2016, Wilson et al., 2007), further supports the importance of T17 cells as a key target of psoriasis management.
In addition to T17 cells, neutrophils are increased in psoriasis lesions as well, appearing early in developing lesions and accumulating within Munro’s microabscesses and spongiform pustules of Kogoj in the epidermis. Cross-talk between neutrophils and T17 cells has been suggested, with T17-associated cytokines increasing the development, recruitment, and lifespan of neutrophils (Kalyan and Kabelitz, 2014, Pelletier et al., 2010, Reich et al., 2015), and neutrophils signaling to T-cells via DCs (Amulic et al., 2012) and macrophages (Warnatsch et al., 2015). While the precise role of neutrophils in psoriasis pathophysiology is not well understood, considerable clinical data indicate their in vivo relevance. The drug razoxane, which depresses neutrophil counts in a dose-dependent manner, was highly effective in treating cutaneous and arthritic disease in 35 patients, with an initial response rate of 97% in cutaneous disease (Atherton et al., 1980). Similarly, remission was observed in a psoriatic patient with ticlopidine-induced agranulocytosis, followed by the reappearance of plaques upon neutrophil repletion (Toichi et al., 2000). Dimethylfumarate, a well-known psoriasis treatment, inhibits neutrophil function in vitro and in vivo (Muller et al., 2016). Dapsone (an anti-neutrophil drug) has been successfully used to treat pustular psoriasis (Sheu et al., 2016), as has granulocyte/monocyte leukapharesis (Fujisawa et al., 2015, Fujisawa et al., 2014).
Neutrophils undergo a specialized form of cell death termed NETosis, in which protein-binding webs of decondensed chromatin extrude from the neutrophil (Branzk and Papayannopoulos, 2013). It has been proposed that NETs eliminate bacteria, fungi, and parasites by physically trapping them in an environment with high local concentrations of antimicrobials (Brinkmann et al., 2004, Papayannopoulos and Zychlinsky, 2009, Remijsen et al., 2011). Others have emphasized their role as “sentinels” of innate immunity (Sorensen and Borregaard, 2016). Although the mechanism is not yet fully understood, NADPH oxidase, myeloperoxidase, neutrophil elastase and histone citrullination are important for NET formation (Papayannopoulos et al., 2010, Patel et al., 2010, Wang et al., 2009). NETs are prominent within psoriasis lesions (Hu et al., 2016, Lin et al., 2011), and they are markedly increased in neutrophils prepared from blood of psoriasis patients (Hu et al., 2016). NETs are also present in several other autoimmune disorders including systemic lupus erythematosus (Garcia-Romo et al., 2011), small-vessel vasculitis (Kessenbrock et al., 2009), and rheumatoid arthritis (Khandpur et al., 2013). Based on these observations, we sought to address the possible role of NETs in psoriasis pathogenesis. To this end, we developed and characterized a method for induction of helper T17 (Th17) cells from human PBMCs, and used it as a model to explore the role of NETs as modulators of this process. We also used the model to assess the effect of a missense psoriasis-associated genetic variant in the TRAF3IP2 gene.
RESULTS
NETs promote the induction of Th17 cells from PBMC
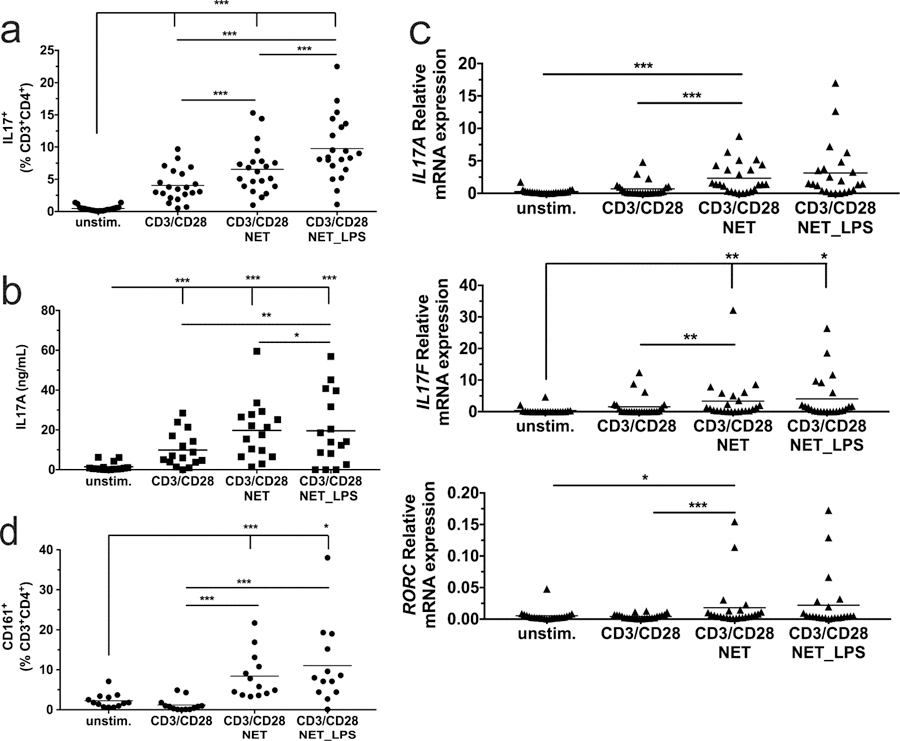
To assess the role of NETs in T17 induction, neutrophils isolated from healthy donors were plated on poly-L-lysine (PLL)-coated wells incubated in the absence or presence of 5ng/ml E. coli lipopolysaccharide (LPS) to induce NETosis. PBMC, isolated from the same blood samples, were seeded on these NET-coated wells, or on PLL-coated wells without NETs, with or without anti-CD3/anti-CD28 beads to effect polyclonal T-cell activation. After 7 days of culture, flow cytometric analysis revealed a significant (p=0.0088), 1.6-fold increase in the percentage of CD3+CD4+IL-17+ cells (i.e. Th17 cells) cells when CD3/CD28-stimulated PBMC were exposed to NETs that formed in the absence of LPS, increasing to 2.4-fold in the presence of LPS (p=4.3x10−7) (Figures 1a and S1a). CD3+CD8+IL-17+ cells (“Tc17”) cells were nearly absent (data not shown). ELISA assays revealed increased secreted IL-17A protein by co-culture with NETs, reaching significance for LPS-treated NETs (1.9-fold, p=0.009) compared to CD3/CD28 stimulation alone (Figures 1b and S1b). We also observed corresponding increases in IL17A, IL17F, and RORC mRNA levels (Figure 1c and S1c). Th17 cells are known to express CD161, also known as the killer cell lectin like receptor B1 (Kleinschek et al., 2009). As shown in Figures 1d and S1d, cell surface CD161 expression in CD3+CD4+ T cells was increased 7-fold in co-cultures with spontaneous NETs (p=8.1x10−5) and 9.2-fold in co-cultures with LPS-induced NETs (p=4x10−4), compared to CD3/CD28 stimulation alone. CD3/CD28 stimulation was also effective in increasing the percentage of CD3+CD4+IFN-γ-expressing Th1 cells. However, unlike Th17 cells, there was no further increase in the percentage of Th1 cells as a function of NET exposure (Figure S1e).
Figure 1. NETs promote CD3/CD28 induction of Th17 cells from PBMCs.
Unstimulated PBMCs, and PBMCs activated with anti-CD3/CD28 beads in presence of NETs or LPS-induced NETs, were incubated for 7 days followed by (a) flow cytometry to determine the percentage of IL-17+T-cells; (b) ELISA to determine secreted IL-17A protein; (c) qPCR to determine IL-17A, IL-17F and RORC mRNA levels; and (d) flow cytometry to determine the percentage of CD161+T-cells. Means are indicated by horizontal lines. Statistics: one-way repeated measures ANOVA was performed on transformed data (A and D, logit; B log (ln); C log2), with all p-values corrected to maintain experimentwise type I error rate for the tested factor. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001. Graphical results for the transformed data and a summary of statistically-significant outcomes are presented in Figure S1.
NETs presented a similar morphology in the presence or absence of LPS (Figure S2a), with the effect of LPS being most evident at early time points. Quantitation of DNA released from NETs revealed that treatment with 5ng/ml LPS for 4 hours did not significantly increase NETosis (Figure S2b). We also generated NETs using phorbol myristate acetate (PMA), another frequently-used stimulus for NET formation (Khan et al., 2017). Interestingly, while 25nM PMA was highly effective in inducing NETosis (Figure S2b), the resulting NETs were completely ineffective in inducing Th17 cells (Figure S2c).
Given that LPS has been reported to enhance Th17 induction from PBMCs (Evans et al., 2007), one concern was that LPS might be retained on the NETs, thereby influencing myeloid cell activation. To address this question, we measured LPS levels after washing of NETs using the Limulus Amebocyte Lysate (LAL) endotoxin assay. We found that LPS was undetectable in media immediately after NET washing, as well as on day 7 (all <0.01ng/mL, n=3). Additionally, we found that addition of 5ng/ml LPS for the duration of the 7-day assay did not increase the percentage of Th17 cells, whether in the presence or absence of NETs (Figure S3a). To ask whether the effects of NETs on Th17 induction might also be observed using live, intact neutrophils as opposed to NETs, we cultured CD3/CD28-stimulated PBMCs in the presence or absence of autologous neutrophils for 7 days. We found no significant difference in comparison to CD3/CD28-stimulated PBMCs cultured without neutrophils (Figure S3b). The purified neutrophils were viable at the time of addition, as demonstrated by Trypan blue exclusion (6.0 +/− 1.2 % of neutrophils were Trypan blue-positive, n=3).
As shown in Figure 2a, the IL-17+ population was limited to 0.4% of the CD4+T-cell population at the beginning of the culture and was not significantly increased after 7 days of culture in the absence of CD3/CD28 stimulation. Moreover, NETs alone did not significantly increase the proportion of Th17 cells in the absence of CD3/CD28 stimulation. We also analyzed Th17 cell divisions after 7 days of culture using a dye dilution assay. There was no significant difference across the three CD3/CD28-stimulated conditions when considered as a group (Figure 2b). On average, fewer than two divisions of IL17+ T cells took place over the culture period, which would not appear to be sufficient to achieve the observed percentages of Th17 cells.
Figure 2. Induction of Th17 by NETs cannot be fully explained by expansion of T-cells.
(a) Percentage of Th17 cells in the starting population (day 0) compared to 7 days of culture, either in the presence of NETs alone, with CD3/CD28 stimulation alone, or both. NETs were prepared with or without 5 ng/ml LPS, as indicated below the figure. Statistics: Asterisks (*) indicate p = 0.05 by one-way repeated measures ANOVA of untransformed data, with Dunnett’s test for multiple comparisons. (b) Th17 proliferation index. PBMC were labeled at the time of seeding with carboxyfluorescein succinimidyl ester (CSFE) and the number of Th17 cell divisions were measured as a proliferation index after 7 days of culture as described in Methods. Statistics: analysis of untransformed data by one-way ANOVA with Tukey’s correction.
NET-induced Th17 induction is monocyte-dependent and requires cell-cell contact
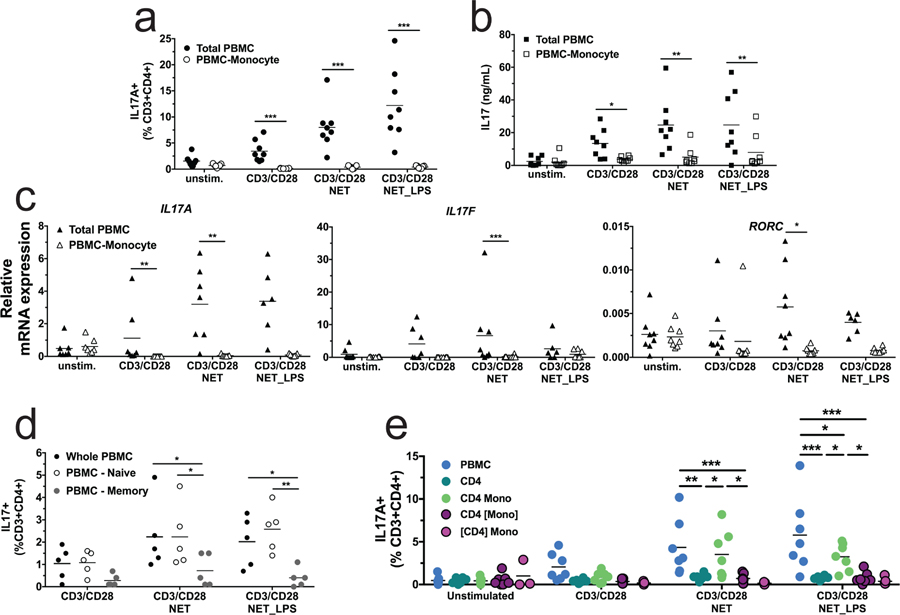
We used immunomagnetic bead purification to characterize the monocyte dependence of Th17 induction by depletion experiments. As shown in Figure 3a, depletion of monocytes from PBMC abrogated the induction of Th17 cells after CD3/CD28 activation, whether in the presence or absence of NETs. This monocyte requirement was also observed in ELISA assays for IL-17 protein (Figure 3b) and qPCR assays for IL-17A, IL-17F and RORC mRNAs (Figure 3c). We performed several experiments to ask whether the observed change in the Th17 population was due to differentiation of naïve T-cells, or the induction or expansion of Th17 cells from an existing population. As shown in Figure 3d, depletion of memory T-cells greatly diminished acquisition of a Th17 phenotype, whereas depletion of naïve T-cells had no effect. We then performed Transwell experiments to ask whether the Th17 induction was due to soluble factors or contact between cells. We found that the Th17 induction did not occur when the CD4+ T cells or the monocytes were not in contact with the other cell types (Figure 3e). Taken together, these results suggest that Th17 cells are being expanded and/or induced from pre-existing memory T-cells, rather than by differentiation of naïve T-cells, and that the presence of monocytes as well as physical contact between the different cell type involved is necessary.
Figure 3. Monocytes, memory CD4+ T cell and cell-cell contact are required for NET-induced Th17 induction.
(a-c), monocyte depletion experiment. (a) Percentage of IL-17+T-cells in whole PBMC (filled squares) or monocyte-depleted PBMC (open squares), as determined by flow cytometry. (b) Secreted IL-17 protein, as determined by ELISA. (c) IL-17A, IL-17F and RORC mRNA levels, as determined by qPCR. Statistics: two-way repeated measures ANOVA of transformed data (a, logit, b, log (ln), c, log2) with all p-values corrected to maintain experimentwise type I error rate for the tested factor. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001. (d) T-cell depletion experiment. Memory or naïve T-cells were removed from PBMC prior to 7 days of CD3/CD28 stimulation. Closed circles represent whole PBMC, open squares indicate depletion of naïve T-cells, and gray squares indicate depletion of memory T-cells. Statistics: analysis of non-transformed data by two-way ANOVA with Tukey’s test for multiple comparisons, * indicates p < 0.05, ** indicates p < 0.01. (e) Cell-cell contact experiment. Percentage of IL-17+T-cells in whole PBMC, CD4 T cell, CD4 T cell with monocytes or CD4 T cell or monocytes seeded in transwell determined by flow cytometry. Brackets ([ ]) indicate the cell type confined to the transwell. Statistics: analysis of non-transformed data by two-way ANOVA with Tukey’s test for multiple comparisons, * indicates p < 0.05, ** indicates p < 0.01, *** indicates p<0.001.
Effect of TRAF3IP2 variation on NET-induced Th17 induction
We and others reported that a coding variant of the TRAF3IP2 gene encoded by SNP rs33980500T/C is associated with increased risk for psoriasis (Ellinghaus et al., 2010, Huffmeier et al., 2010). TRAF3IP2 encodes Act1, an adaptor with ubiquitin ligase activity that connects the IL-17 receptor to downstream signaling pathways (Qian et al., 2007). rs33980500 T causes an amino acid change from aspartic acid to asparagine residue at position 10 (Act1 D10N) that interferes with binding to TRAF2, TRAF3, and TRAF6 (Wang et al., 2013).
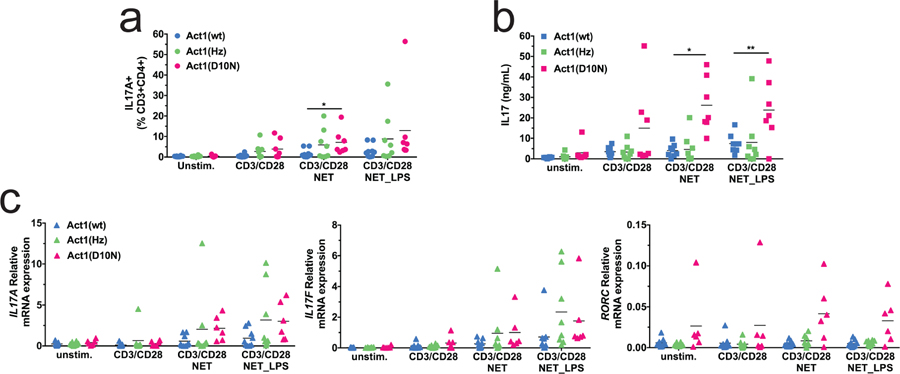
We investigated the effects of NETs on Th17 induction and IL-17 production in donors carrying each of the three genotypes for the Act1 D10N variant. In the presence of spontaneous NETs, both the percentage of IL-17+ T-cells (Figure 4a) and IL-17A release into the culture supernatant (Figure 4b) were significantly more pronounced for Act1 D10N homozygotes compared to wild type (WT) homozygotes, with significant linear trend tests as a function of D10N genotype (0, 1, or 2 alleles) for both the percentage of Th17 cells (p = 0.037) and IL17A secretion (p = 0.0035). There was also a strong main effect of Act1 genotype on IL-17 secretion across all stimulation conditions (p = 0.0045), with a fold-change (FC) of 4.25 for Act1 D10N vs. WT (p = 0.0078) (Figure S4). As shown in Figures 4c and S4c, there was a trend towards increased IL17A, IL17F, and RORC mRNAs as a function of Act1 D10N genotype, with a significant main effect of Act1 genotype for IL17A mRNA.
Figure 4. The Act1 D10N variant promotes Th17 induction.
Unstimulated PBMCs, PBMCs activated with anti-CD3/CD28 beads in presence of NETs or LPS-induced NETs were incubated for 7 days followed by (a) flow cytometry to determine the percentage of IL-17+T-cells; (b) ELISA to determine secreted IL-17 protein; and (c) qPCR to determine IL17A, IL17F and RORC mRNA levels. Means are indicated by horizontal lines. Statistics: two-way repeated measures ANOVA of transformed data (a, logit, b, log, c, log2) with all p-values corrected to maintain experimentwise type I error rate for the tested factor. * indicates p < 0.05, ** indicates p < 0.01. Graphical results for the transformed data and a summary of statistically-significant outcomes are presented in Figure S4.
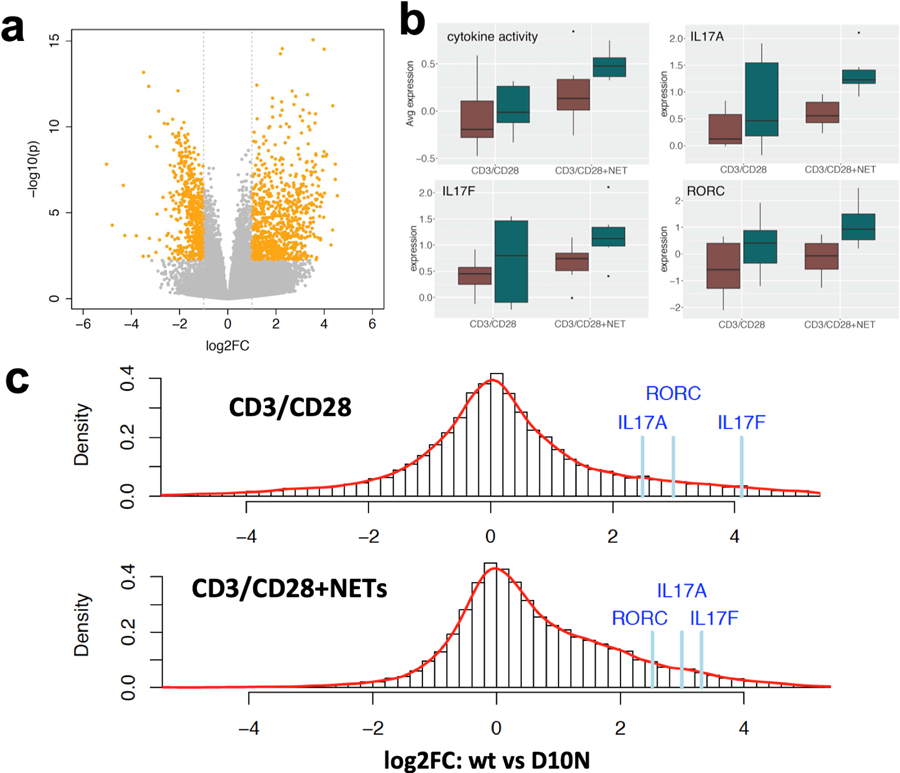
Using a global approach that did not focus exclusively on Th17 cells, we next applied RNA-seq to globally assess differentially expressed genes (DEGs) as a function of NET exposure and Act1 D10N genotype. For this analysis, we compared Act1 D10N homozygotes to WT homozygotes, and focused on “spontaneous” NETs t o avoid confounding due to LPS exposure. As shown in Figure 5a, analysis of DEGs in CD3/CD28-stimulated PBC showed that NETs had a stimulatory effect on global gene expression, with 1,343 genes yielding padj ≤ 0.05 and |log2 FC| ≥ 1 (840 up and 503 down, Table S1). Functional enrichment analysis of this dataset found significant enrichment for the Gene Ontology (GO) term “cytokine activity” (4.3-fold, FDR = 3.3 x 10−19) among others (Table S2). On average, expression of DEGs mapping to this GO term was markedly increased as a function of NET exposure (53 up-regulated genes averaging 5.9-fold, vs. 2 down-regulated genes averaging 0.28-fold, Table S3) with further increases for the Act1 D10N variant (Figure 5b). The Th17-related genes IL17A, IL17F, and RORC analyzed by qPCR in Figures 1, 3, and 4 were among the “cytokine activity” genes whose expression was increased by NETs and further up-regulated in Act1 D10N homozygotes (Figure 5b). Genomewide, Act1 D10N homozygotes presented a skewed distribution of FC values with respect to WT homozygotes under conditions of NET exposure, with the effect of Act1D10N being significantly more prominent in the presence of NETs (p < 1 x 10−16 by Wilcoxon signed-rank test, Figure 5c).
Figure 5. NETs enhance the effect of the Act1 D10N variant on global gene expression in CD3/CD28-stimulated PBMCs.
(a) Volcano plot showing log 2 fold-change values for global gene expression determined by RNA-seq for 7 day CD3/CD28-stimulated PBMCs in the presence or the absence of NETs. Significant DEGs (|log2 FC| ≥ 1, padj < 0.05) are highlighted in orange. 840 genes were significantly up-regulated and 503 genes were significantly down-regulated. (b) Box and whisker plots showing average gene expression across all expressed genes belonging to the GO term “cytokine activity”, as well as individual gene expression values for IL17A, IL17F, and RORC. Red bars indicate values obtained for Act1 WT homozygotes and blue bars indicate values obtained for Act1 D10N homozygotes. The top and the bottom of the box represent 25th and 75th percentiles respectively and the centerline is the 50th percentile; upper and lower whiskers extend to the 1.5 fold interquartile range of the 25th and 75th percentiles, respectively; and dots represent outliers beyond the 1.5-fold interquartile range. (c) Histograms summarizing the distribution of FC values (Act1 D10N/WT) after CD3/CD28 stimulation in the absence of NETs (upper panel) vs. the presence of NETs (lower panel). Individual gene expression ratio values for IL17A, IL17F, and RORC are indicated above the histograms. Note the extended “tail” of positive gene expression ratios in the presence of NETs.
DISCUSSION
Here we describe a method for induction of human Th17 cells from PBMCs, and use it to explore the role of NETs and the Act1D10N variant as modulators of this process. We found that CD3/CD28 stimulation of PBMCs significantly increased the percentage of Th17 cells (Figure 1a) and IL-17A secretion (Figure 1b), both of which were further enhanced by NETs. IL17A, IL17F, and RORC mRNAs were also increased by NETs, relative to CD3/CD28 stimulation alone (Figure 1c). Indicative of a specific requirement for NETosis, the effect of NETs could not be mimicked by intact viable neutrophils (Figure S3b). Moreover, NETs alone could not induce Th17 cells in the absence of CD3/CD28 stimulation (Figure 2a). NET exposure also markedly increased the proportion of Th17 cells expressing CD161, a lectin-like cell surface receptor coupled to STAT3 and mTOR activation (Bai et al., 2014) (Figure 1d). CD161 defines a distinctive transcriptional and functional profile shared across multiple T-cell subsets including mucosal-invariant T (MAIT) cells (Fergusson et al., 2014), suggesting that NETs may promote “innate-like” immune responses.
It is important to ask whether our results reflect differentiation of Th17 cells from naïve T-cells, preferential expansion of existing Th17 cells, or induction of Th17 cells from “precommitted” memory T-cell subsets. Using deplet ion experiments, we confirmed observations (Evans et al., 2007, van Beelen et al., 2007) that the induction of human Th17 cells from CD3/CD28-stimulated PBMC is much more efficient using memory than naïve CD4+ T cells (Figure 3d). Given that only ~0.4% of CD4+ T cells are IL-17+ directly ex vivo (Figure 2a), and that replication-competent IL-17-expressing CD4+ T-cells averaged fewer than two divisions over 7 days as determined by dye dilution assays (Figure 2b), we consider it unlikely that the observed increase in the percentage of Th17 cells can be explained solely by proliferation. T cell polarity has been shown to be flexible in human CD4+ T cells (Cosmi et al., 2014), and emerging data suggest that Th17 cells may be derived from IL-17-negative helper T cells by metabolic reprogramming (Binger et al., 2017). Hence, in this report we utilize the non-specific term “induction”, rather than “differentia tion” or “expansion”, to describe our findings regarding Th17 cells.
We confirmed previous observations (Evans et al., 2007) that monocytes are required for CD3/CD28-mediated Th17 induction from PBMCs (Figure 3). These findings suggest that myeloid cells comprise a “bridge” between NETs on t he one hand, and Th17 cells on the other. This would be consonant with the in vivo environment of psoriatic skin, in which NETs are abundantly present (Hu et al., 2016, Lin et al., 2011) in an environment rich in myeloid cells (Kim and Krueger, 2015) and memory T-cells (Clark, 2015).
We utilized LPS to enhance NET formation, and found that this significantly increased the percentage of Th17 cells (Figure 1). However, LPS has also been reported to enhance the ability of monocytes to induce Th17 from memory T-cells (Evans et al., 2007), raising the possibility that our results might be due to effects of LPS on monocytes, rather than its effects upon NETosis. We consider this to be unlikely for several reasons. First, we found that LPS levels in our cultures were below the limit of sensitivity of the LAL assay (<0.01ng/mL), whether measured immediately after NET washing, or at the end of the culture period on day 7. Thus, LPS levels in our cultures were at least 10,000 times lower than those used by Evans et al. (100ng/ml) (Evans et al., 2007). Moreover, addition of LPS did not stimulate the induction of Th17 cells in our system (Figure S3a), possibly because we used a considerably lower concentration of LPS (5 vs. 100ng/ml).
We utilized 25nM PMA as an alternative stimulus for NETosis, and were interested to find that PMA-induced NETs were completely ineffective for induction of Th17 cells (Figure S2c), despite PMA being a strong stimulus for NETosis (Figures S2a and S2b). Recent studies (Khan et al., 2017) have shown that LPS and PMA utilize different signaling pathways to induce NETosis, with LPS triggering a TLR4-dependent activation of NADPH oxidase to effect NETosis (Khan et al., 2017). While both sets of experiments utilized E. coli 0111:B4 LPS, we used a much lower concentration (5ng/ml vs. 100–25,000ng/ml). Consistent with our findings (Figure S2b), Khan et al. observed very little enhancement of DNA release at 100ng/ml LPS (<10% over control, not significant). These results suggest that LPS may be enhancing Th17 via one or more TLR4-dependent effects on neutrophils other than or in addition to the stimulation of NETosis per se.
A major puzzle in psoriasis genetics is why the rs33980500T variant in TRAF3IP2, encoding Act1 D10N, is associated with increased risk for psoriasis and PsA (Ellinghaus et al., 2010, Huffmeier et al., 2010, Strange et al., 2010, Stuart et al., 2015), given that the Act1 D10N variant is hypofunctional in response to IL-17 in mouse embryo fibroblasts (Wang et al., 2013) and Act1 silencing leads to lower responses to IL-17 in human keratinocytes (Lambert et al., 2017). To ask whether the Act1 D10N variant might promote Th17 induction in human cells, we used our model to study individuals of varying Act1 D10N genotype. We observed a significant stimulatory effect of Act1 D10N genotype on induction of Th17 cells and IL-17A secretion, which was potentiated in the presence of NETs (Figures 4a and 4b).
We next utilized RNA-seq to more globally assess gene expression changes influenced by NETs in the context of CD3/CD28 activation. Volcano plot analysis confirmed a significant effect of NETs in this model, with up-regulated genes exceeding down-regulated genes (Figure 5a). Functional analysis of DEGs revealed enrichment for genes associated with the GO term “cytokine activity” and related terms (Table S1). On average, DEGs associated with “cytokine activity” were increased by NET exposure, with a fu rther increase in Act1 D10N homozygotes vs. nullizygotes (Figure 5b). Moreover, Act1D10N-dependent up-regulation was observed for IL17A, IL17F, and RORC (Figure 5b). By measuring the gene expression ratios for Act1 D10N vs. WT homozygotes, we observed a highly significant (p < 1 x 10−16) global effect of Act1 D10N genotype on gene expression, which was enhanced by NETs (Figure 5c). Together, these results corroborate those of our Th17-focused experiments (Figures 1–4).
Overall, these results are in agreement with studies in mice indicating that the Act1 D10N variant results in a hyperactive Th17 response and increased skin inflammation, despite the fact that it behaves like a loss of function variant in response to IL-17 stimulation (Wang et al., 2013, Wu et al., 2014). While the mechanism underlying this effect requires further investigation, our observations would be consistent with a recent report (Zhang et al., 2018) indicating that Act1 inhibits STAT3, which in turn exerts multifaceted effects on Th17 biology as a downstream mediator of IL-23 signaling (Mathur et al., 2007). Future studies dissecting the constituent cell populations of the PBMC assay using single-cell techniques should prove fruitful in understanding both the effects of the Act1 D10N variant and the role of NETs in Th17 induction in psoriasis.
MATERIALS & METHODS
This study was approved by the University of Michigan’s Institutional Review Board, and all patients provided written informed consent in adherence with the Declaration of Helsinki principles.
PBMC and neutrophils were isolated from blood by centrifugation over Ficoll 1.077D (Sigma-Aldrich, St. Louis MO). NETs were prepared by adding 5x105 neutrophils in 0.5 mL RPMI 1640 to 24-well poly-L-lysine-coated tissue culture plates (Corning BioCoat, Corning, NY), with or without 5ng/mL E. coli-derived LPS (Serotype O111:B4, Sigma Aldrich) or 25 nM PMA and incubated for 4 hours to allow for NETosis, then washed twice with fresh RMPI 1640 lacking phenol red (Thermo Fisher Scientific, Waltham, MA). Th17 cells were induced by cultivating 5x105 PBMC in 0.5 ml RPMI 1640 containing 10% fetal bovine serum with Human T-Activator CD3/CD28 Dynabeads (Thermo Fisher) in 24-well plates. PBMC were suspended in empty wells or wells in which NETs from the same donor had just been generated, with or without LPS or PMA. In other experiments, we added 5ng/ml LPS for the duration of the culture, or we depleted monocytes, memory T-cells, or naïve T-cells from PBMC using immunomagnetic bead kits (Miltenyi Biotec, Auburn, CA). Cultures were fed fresh medium after 4 days. After 7 days, cells were harvested for analysis by flow cytometry or qPCR, and conditioned medium was assayed for IL-17A by ELISA (DuoSet assay, R&D Systems). For flow cytometry experiments cells were stimulated for 6h with PMA (5ng/mL) and ionomycin (1µg/mL) in presence of Brefeldin A (BioLegend). The gating strategy for flow cytometry is shown in Figure S5. We activated cultures with PMA and ionomycin (without Brefeldin A) for 6h to assess mRNA expression (including RNA-seq) and for 24h to assess protein secretion, as these time points revealed the best induction of IL-17 mRNA or protein in preliminary experiments (Figure S6). Details of flow cytometry, RNA-seq, statistical analysis, and other procedures are provided in the Supplementary Information.
RNA-seq data have been submitted to Gene Expression Omnibus, accession number GSE121315.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by awards from the National Institutes of Health (R01AR063611 and R01AR065183 to JTE, and K01-AR064765 to AJ), and by the Dawn and Dudley Holmes Foundation and the Babcock Memorial Trust. JTE is supported by the Ann Arbor Veterans Affairs Hospital.
ABBREVIATIONS:
- ELISA
enzyme-linked immunosorbent assay
- FC
fold-change
- HBSS
Hank’s balanced salt solution
- IFN
interferon
- IL-17
interleukin-17
- LAL
Limulus amebocyte lysate
- LPS
lipopolysaccharide
- NET
neutrophil extracellular trap
- PBMC
peripheral blood mononuclear cell
- PMA
phorbol myristate acetate
- qPCR
quantitative reverse transcription and PCR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- Amatya N, Garg AV, Gaffen SL. IL-17 Signaling: The Yin and the Yang. Trends Immunol 2017;38(5):310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol 2012;30:459–89. [DOI] [PubMed] [Google Scholar]
- Atherton DJ, Wells RS, Laurent MR, Williams YF. Razoxane (ICRF 159) in the treatment of psoriasis. Br J Dermatol 1980;102(3):307–17. [DOI] [PubMed] [Google Scholar]
- Bai A, Moss A, Kokkotou E, Usheva A, Sun X, Cheifetz A, et al. CD39 and CD161 modulate Th17 responses in Crohn’s disease. J Immunol 2014;193(7):3366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binger KJ, Corte-Real BF, Kleinewietfeld M. Immunometabolic Regulation of Interleukin-17-Producing T Helper Cells: Uncoupling New Targets for Autoimmunity. Front Immunol 2017;8:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzk N, Papayannopoulos V. Molecular mechanisms regulating NETosis in infection and disease. Semin Immunopathol 2013;35(4):513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303(5663):1532–5. [DOI] [PubMed] [Google Scholar]
- Clark RA. Resident memory T cells in human health and disease. Sci Transl Med 2015;7(269):269rv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmi L, Santarlasci V, Maggi L, Liotta F, Annunziato F. Th17 plasticity: pathophysiology and treatment of chronic inflammatory disorders. Curr Opin Pharmacol 2014;17:12–6. [DOI] [PubMed] [Google Scholar]
- Dolgin E New anti-IL-23 drugs raise hopes for psoriasis plaque clearance. Nat Biotechnol 2016;34(12):1218–9. [DOI] [PubMed] [Google Scholar]
- Ellinghaus E, Ellinghaus D, Stuart PE, Nair RP, Debrus S, Raelson JV, et al. Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat Genet 2010;42(11):991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci U S A 2007;104(43):17034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson JR, Smith KE, Fleming VM, Rajoriya N, Newell EW, Simmons R, et al. CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell Rep 2014;9(3):1075–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T, Suzuki S, Mizutani Y, Doi T, Yoshida S, Ogura S, et al. Granulocyte and Monocyte Adsorption Apheresis for Generalized Pustular Psoriasis: Therapeutic Outcomes in Three Refractory Patients. Ther Apher Dial 2015;19(4):336–41. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Tawada C, Mizutani Y, Doi T, Yoshida S, Ogura S, et al. Efficacy of granulocyte and monocyte adsorption apheresis for treatment of palmoplantar pustulosis. Ther Apher Dial 2014;18(3):238–43. [DOI] [PubMed] [Google Scholar]
- Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011;3(73):73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden JL, Krueger JG, Bowcock AM. The immunogenetics of Psoriasis: A comprehensive review. J Autoimmun 2015;64:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SC, Yu HS, Yen FL, Lin CL, Chen GS, Lan CC. Neutrophil extracellular trap formation is increased in psoriasis and induces human beta-defensin-2 production in epidermal keratinocytes. Sci Rep 2016;6:31119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffmeier U, Uebe S, Ekici AB, Bowes J, Giardina E, Korendowych E, et al. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat Genet 2010;42(11):996–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyan S, Kabelitz D. When neutrophils meet T cells: beginnings of a tumultuous relationship with underappreciated potential. Eur J Immunol 2014;44(3):627–33. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009;15(6):623–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Farahvash A, Douda DN, Licht JC, Grasemann H, Sweezey N, et al. JNK Activation Turns on LPS- and Gram-Negative Bacteria-Induced NADPH Oxidase-Dependent Suicidal NETosis. Sci Rep 2017;7(1):3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 2013;5(178):178ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Krueger JG. The immunopathogenesis of psoriasis. Dermatologic clinics 2015;33(1):13–23. [DOI] [PubMed] [Google Scholar]
- Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med 2009;206(3):525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol 2008;181(7):4733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Swindell WR, Tsoi LC, Stoll SW, Elder JT. Dual Role of Act1 in Keratinocyte Differentiation and Host Defense: TRAF3IP2 Silencing Alters Keratinocyte Differentiation and Inhibits IL-17 Responses. J Invest Dermatol 2017;137(7):1501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol 2011;187(1):490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol 2008;doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol 2014;32:227–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol 2007;178(8):4901–7. [DOI] [PubMed] [Google Scholar]
- Mease PJ. Inhibition of interleukin-17, interleukin-23 and the TH17 cell pathway in the treatment of psoriatic arthritis and psoriasis. Curr Opin Rheumatol 2015;27(2):127–33. [DOI] [PubMed] [Google Scholar]
- Muller S, Behnen M, Bieber K, Moller S, Hellberg L, Witte M, et al. Dimethylfumarate Impairs Neutrophil Functions. J Invest Dermatol 2016;136(1):117–26. [DOI] [PubMed] [Google Scholar]
- Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 2010;191(3):677–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol 2009;30(11):513–21. [DOI] [PubMed] [Google Scholar]
- Patel S, Kumar S, Jyoti A, Srinag BS, Keshari RS, Saluja R, et al. Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide 2010;22(3):226–34. [DOI] [PubMed] [Google Scholar]
- Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 2010;115(2):335–43. [DOI] [PubMed] [Google Scholar]
- Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol 2007;8(3):247–56. [DOI] [PubMed] [Google Scholar]
- Reich K, Papp KA, Matheson RT, Tu JH, Bissonnette R, Bourcier M, et al. Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp Dermatol 2015;24(7):529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ 2011;18(4):581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu JS, Divito SJ, Enamandram M, Merola JF. Dapsone Therapy for Pustular Psoriasis: Case Series and Review of the Literature. Dermatology 2016;232(1):97–101. [DOI] [PubMed] [Google Scholar]
- Sorensen OE, Borregaard N. Neutrophil extracellular traps - the dark side of neutrophils. J Clin Invest 2016;126(5):1612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange A, Capon F, Spencer CC, Knight J, Weale ME, Allen MH, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet 2010;42(11):985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart PE, Nair RP, Tsoi LC, Tejasvi T, Das S, Kang HM, et al. Genome-wide Association Analysis of Psoriatic Arthritis and Cutaneous Psoriasis Reveals Differences in Their Genetic Architecture. Am J Hum Genet 2015;97(6):816–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev 2008;223:87–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toichi E, Tachibana T, Furukawa F. Rapid improvement of psoriasis vulgaris during drug-induced agranulocytosis. J Am Acad Dermatol 2000;43(2 Pt 2):391–5. [DOI] [PubMed] [Google Scholar]
- van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 2007;27(4):660–9. [DOI] [PubMed] [Google Scholar]
- Wang C, Wu L, Bulek K, Martin BN, Zepp JA, Kang Z, et al. The psoriasis-associated D10N variant of the adaptor Act1 with impaired regulation by the molecular chaperone hsp90. Nat Immunol 2013;14(1):72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009;184(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015;349(6245):316–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 2007;8(9):950–7. [DOI] [PubMed] [Google Scholar]
- Wu L, Wang C, Boisson B, Misra S, Rayman P, Finke JH, et al. The differential regulation of human ACT1 isoforms by Hsp90 in IL-17 signaling. J Immunol 2014;193(4):1590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CJ, Wang C, Jiang M, Gu C, Xiao J, Chen X, et al. Act1 is a negative regulator in T and B cells via direct inhibition of STAT3. Nat Commun 2018;9(1):2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.