Summary
An outbreak of novel coronavirus (2019-nCoV) that began in Wuhan, China, has spread rapidly, with cases now confirmed in multiple countries. We report the first case of 2019-nCoV infection confirmed in the United States and describe the identification, diagnosis, clinical course, and management of the case, including the patient’s initial mild symptoms at presentation with progression to pneumonia on day 9 of illness. This case highlights the importance of close coordination between clinicians and public health authorities at the local, state, and federal levels, as well as the need for rapid dissemination of clinical information related to the care of patients with this emerging infection.
On December 31, 2019, China reported a cluster of cases of pneumonia in people associated with the Huanan Seafood Wholesale Market in Wuhan, Hubei Province.1 On January 7, 2020, Chinese health authorities confirmed that this cluster was associated with a novel coronavirus, 2019-nCoV.2 Although cases were originally reported to be associated with exposure to the seafood market in Wuhan, current epidemiologic data indicate that person-to-person transmission of 2019-nCoV is occurring.3-6 As of January 30, 2020, a total of 9976 cases had been reported in at least 21 countries,7 including the first confirmed case of 2019-nCoV infection in the United States, reported on January 20, 2020. Investigations are under way worldwide to better understand transmission dynamics and the spectrum of clinical illness. This report describes the epidemiologic and clinical features of the first case of 2019-nCoV infection confirmed in the United States.
Case Report
On January 19, 2020, a 35-year-old man presented to an urgent care clinic in Snohomish County, Washington, with a 4-day history of cough and subjective fever. On checking into the clinic, the patient put on a mask in the waiting room. After waiting approximately 20 minutes, he was taken into an examination room and underwent evaluation by a provider. He disclosed that he had returned to Washington State on January 15 after traveling to visit family in Wuhan, China. The patient stated that he had seen a health alert from the U.S. Centers for Disease Control and Prevention (CDC) about the novel coronavirus outbreak in China and, because of his symptoms and recent travel, decided to see a health care provider.
Apart from a history of hypertriglyceridemia, the patient was an otherwise healthy nonsmoker. The physical examination revealed a body temperature of 37.2°C, blood pressure of 134/87 mm Hg, pulse of 110 beats per minute, respiratory rate of 16 breaths per minute, and oxygen saturation of 96% while the patient was breathing ambient air. Lung auscultation revealed rhonchi, and chest radiography was performed, which was reported as showing no abnormalities (Figure 1). A rapid nucleic acid amplification test (NAAT) for influenza A and B was negative. A nasopharyngeal swab specimen was obtained and sent for detection of viral respiratory pathogens by NAAT; this was reported back within 48 hours as negative for all pathogens tested, including influenza A and B, parainfluenza, respiratory syncytial virus, rhinovirus, adenovirus, and four common coronavirus strains known to cause illness in humans (HKU1, NL63, 229E, and OC43).
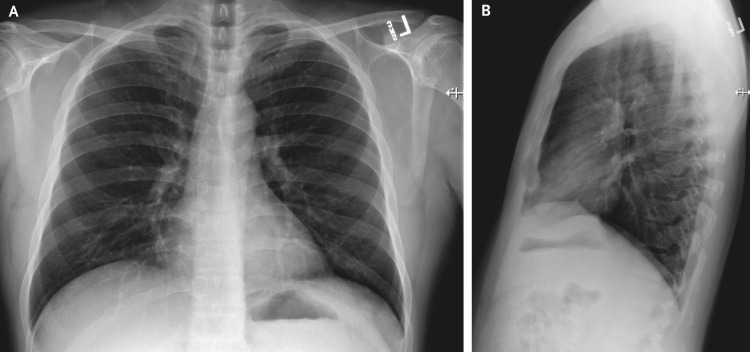
Figure 1. Posteroanterior and Lateral Chest Radiographs, January 19, 2020 (Illness Day 4).
No thoracic abnormalities were noted.
Given the patient’s travel history, the local and state health departments were immediately notified. Together with the urgent care clinician, the Washington Department of Health notified the CDC Emergency Operations Center. Although the patient reported that he had not spent time at the Huanan seafood market and reported no known contact with ill persons during his travel to China, CDC staff concurred with the need to test the patient for 2019-nCoV on the basis of current CDC “persons under investigation” case definitions.8 Specimens were collected in accordance with CDC guidance and included serum and nasopharyngeal and oropharyngeal swab specimens. After specimen collection, the patient was discharged to home isolation with active monitoring by the local health department.
On January 20, 2020, the CDC confirmed that the patient’s nasopharyngeal and oropharyngeal swabs tested positive for 2019-nCoV by real-time reverse-transcriptase–polymerase-chain-reaction (rRT-PCR) assay. In coordination with CDC subject-matter experts, state and local health officials, emergency medical services, and hospital leadership and staff, the patient was admitted to an airborne-isolation unit at Providence Regional Medical Center for clinical observation, with health care workers following CDC recommendations for contact, droplet, and airborne precautions with eye protection.9
On admission, the patient reported persistent dry cough and a 2-day history of nausea and vomiting; he reported that he had no shortness of breath or chest pain. Vital signs were within normal ranges. On physical examination, the patient was found to have dry mucous membranes. The remainder of the examination was generally unremarkable. After admission, the patient received supportive care, including 2 liters of normal saline and ondansetron for nausea.
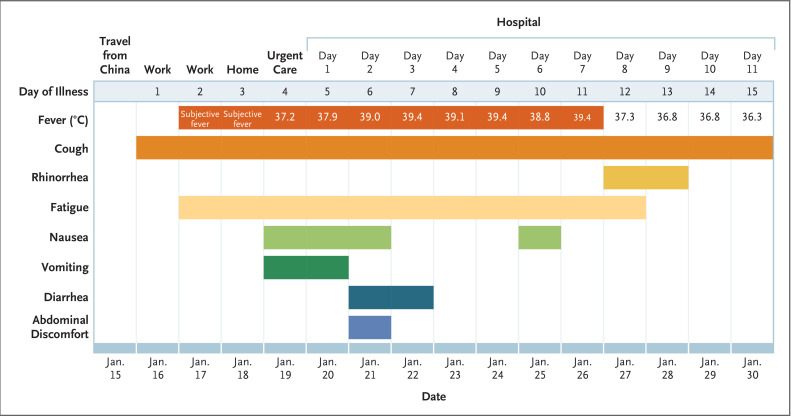
On days 2 through 5 of hospitalization (days 6 through 9 of illness), the patient’s vital signs remained largely stable, apart from the development of intermittent fevers accompanied by periods of tachycardia (Figure 2). The patient continued to report a nonproductive cough and appeared fatigued. On the afternoon of hospital day 2, the patient passed a loose bowel movement and reported abdominal discomfort. A second episode of loose stool was reported overnight; a sample of this stool was collected for rRT-PCR testing, along with additional respiratory specimens (nasopharyngeal and oropharyngeal) and serum. The stool and both respiratory specimens later tested positive by rRT-PCR for 2019-nCoV, whereas the serum remained negative.
Figure 2. Symptoms and Maximum Body Temperatures According to Day of Illness and Day of Hospitalization, January 16 to January 30, 2020.
Treatment during this time was largely supportive. For symptom management, the patient received, as needed, antipyretic therapy consisting of 650 mg of acetaminophen every 4 hours and 600 mg of ibuprofen every 6 hours. He also received 600 mg of guaifenesin for his continued cough and approximately 6 liters of normal saline over the first 6 days of hospitalization.
The nature of the patient isolation unit permitted only point-of-care laboratory testing initially; complete blood counts and serum chemical studies were available starting on hospital day 3. Laboratory results on hospital days 3 and 5 (illness days 7 and 9) reflected leukopenia, mild thrombocytopenia, and elevated levels of creatine kinase (Table 1). In addition, there were alterations in hepatic function measures: levels of alkaline phosphatase (68 U per liter), alanine aminotransferase (105 U per liter), aspartate aminotransferase (77 U per liter), and lactate dehydrogenase (465 U per liter) were all elevated on day 5 of hospitalization. Given the patient’s recurrent fevers, blood cultures were obtained on day 4; these have shown no growth to date.
Table 1. Clinical Laboratory Results.*.
| Measure | Reference Range | Illness Day 6, Hospital Day 2† | Illness Day 7, Hospital Day 3 | Illness Day 9, Hospital Day 5 | Illness Day 11, Hospital Day 7 | Illness Day 13, Hospital Day 9 | Illness Day 14, Hospital Day 10 |
|---|---|---|---|---|---|---|---|
| White-cell count (per μl) | 3800–11,000 | “Slight decrease” | 3120‡ | 3300‡ | 5400 | 5600 | 6500 |
| Red-cell count (per μl) | 4,200,000–5,700,000 | — | 4,870,000 | 5,150,000 | 5,010,000 | 4,650,000 | 5,010,000 |
| Absolute neutrophil count (per μl) | 1900–7400 | — | 1750‡ | 1700‡ | 3700 | 3800 | 3200 |
| Absolute lymphocyte count (per μl) | 1000–3900 | — | 1070 | 1400 | 1400 | 1400 | 2100 |
| Platelet count (per μl) | 150,000–400,000 | “Adequate” | 122,000‡ | 132,000‡ | 151,000 | 150,000 | 239,000 |
| Hemoglobin (g/dl) | 13.2–17.0 | 12.2‡ | 14.2 | 14.8 | 14.8 | 13.5 | 14.2 |
| Hematocrit (%) | 39.0–50.0 | 36.0‡ | 42.0 | 43.0 | 43.0 | 39.3 | 42.0 |
| Sodium (mmol/liter) | 136–145 | 134‡ | 136 | 138 | 138 | 135‡ | 138 |
| Potassium (mmol/liter) | 3.5–5.1 | 3.3‡ | 3.6 | 3.4‡ | 3.6 | 4.1 | 3.9 |
| Chloride (mmol/liter) | 98–107 | 99 | 101 | 105 | 106 | 100 | 103 |
| Calcium (mg/dl) | 8.7–10.4 | — | 8.5‡ | 9.3 | 9.0 | 8.6‡ | 9.3 |
| Carbon dioxide (mmol/liter) | 20–31 | — | 26 | 24 | 25 | 23 | 36§ |
| Anion gap (mmol/liter) | 5–16 | — | 9 | 9 | 7 | 12 | 9 |
| Glucose (mmol/liter) | 65–140 | 104 | 103 | 120 | 96 | 148§ | 104 |
| Blood urea nitrogen (mg/dl) | 9–23 | 15 | 10 | 13 | 13 | 22§ | 18 |
| Creatinine (mg/dl) | 0.7–1.3 | 1.0 | 1.06 | 1.06 | 0.88 | 1.08 | 0.84 |
| Total protein (g/dl) | 5.7–8.2 | — | 6.9 | 7.1 | 6.8 | 6.9 | 6.8 |
| Albumin (g/dl) | 3.2–4.8 | — | 4.2 | 4.7 | 4.5 | 2.9‡ | 4.4 |
| Total bilirubin (mg/dl) | 0.3–1.2 | — | 1.0 | 1.1 | 1.5§ | 0.8 | 1.0 |
| Procalcitonin (ng/ml) | <0.05 | — | — | <0.05 | <0.05 | — | — |
| Alanine aminotransferase (U/liter) | 10–49 | — | 68§ | 105§ | 119§ | 219§ | 203§ |
| Aspartate aminotransferase (U/liter) | ≤33 | — | 37§ | 77§ | 85§ | 129§ | 89§ |
| Alkaline phosphatase (U/liter) | 46–116 | — | 50 | 68§ | 88§ | 137§ | 163§ |
| Fibrinogen (mg/dl) | 150–450 | — | 477§ | — | — | — | — |
| Lactate dehydrogenase (U/liter) | 120–246 | — | 250§ | 465§ | — | — | 388§ |
| Prothrombin time (sec) | 12.2–14.6 | — | 11.9‡ | 11.9‡ | — | — | 12.7 |
| International normalized ratio | 0.9–1.1 | — | 0.9 | 0.9 | — | — | 1.0 |
| Creatine kinase (U/liter) | 62–325 | — | 353§ | 332§ | — | — | — |
| Venous lactate (mmol/liter) | 0.4–2.0 | — | 1.3 | 1.7 | — | — | — |
To convert the values for calcium to millimoles per liter, multiply by 0.250. To convert the values for blood urea nitrogen to millimoles per liter of urea, multiply by 0.357. To convert the values for creatinine to micromoles per liter, multiply by 88.4. To convert the values for total bilirubin to micromoles per liter, multiply by 17.1.
Results are from point-of-care blood analyzer (iStat) testing.
The value in the patient was below normal.
The value in the patient was above normal.
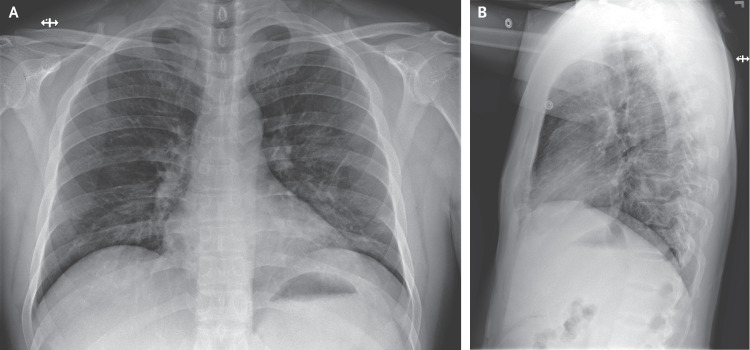
A chest radiograph taken on hospital day 3 (illness day 7) was reported as showing no evidence of infiltrates or abnormalities (Figure 3). However, a second chest radiograph from the night of hospital day 5 (illness day 9) showed evidence of pneumonia in the lower lobe of the left lung (Figure 4). These radiographic findings coincided with a change in respiratory status starting on the evening of hospital day 5, when the patient’s oxygen saturation values as measured by pulse oximetry dropped to as low as 90% while he was breathing ambient air. On day 6, the patient was started on supplemental oxygen, delivered by nasal cannula at 2 liters per minute. Given the changing clinical presentation and concern about hospital-acquired pneumonia, treatment with vancomycin (a 1750-mg loading dose followed by 1 g administered intravenously every 8 hours) and cefepime (administered intravenously every 8 hours) was initiated.
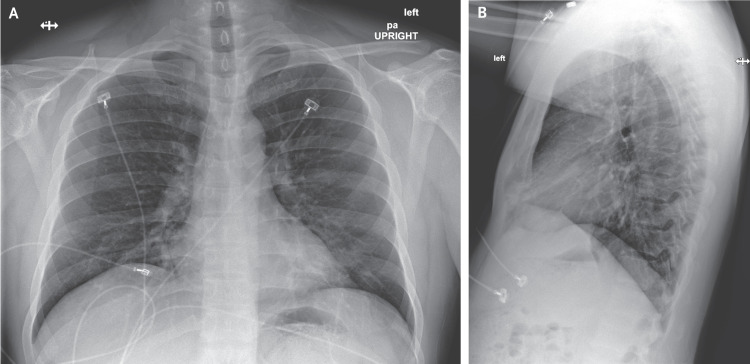
Figure 3. Posteroanterior and Lateral Chest Radiographs, January 22, 2020 (Illness Day 7, Hospital Day 3).
No acute intrathoracic plain-film abnormality was noted.
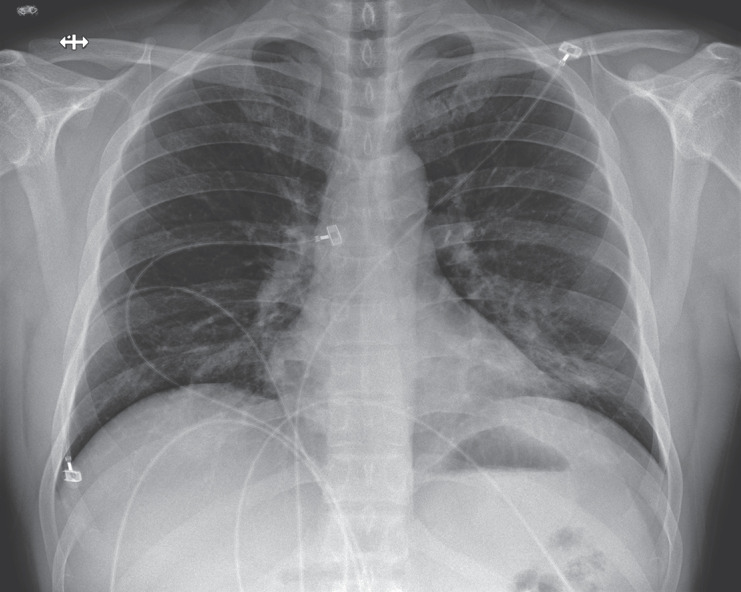
Figure 4. Posteroanterior Chest Radiograph, January 24, 2020 (Illness Day 9, Hospital Day 5).
Increasing left basilar opacity was visible, arousing concern about pneumonia.
On hospital day 6 (illness day 10), a fourth chest radiograph showed basilar streaky opacities in both lungs, a finding consistent with atypical pneumonia (Figure 5), and rales were noted in both lungs on auscultation. Given the radiographic findings, the decision to administer oxygen supplementation, the patient’s ongoing fevers, the persistent positive 2019-nCoV RNA at multiple sites, and published reports of the development of severe pneumonia3,4 at a period consistent with the development of radiographic pneumonia in this patient, clinicians pursued compassionate use of an investigational antiviral therapy. Treatment with intravenous remdesivir (a novel nucleotide analogue prodrug in development10,11) was initiated on the evening of day 7, and no adverse events were observed in association with the infusion. Vancomycin was discontinued on the evening of day 7, and cefepime was discontinued on the following day, after serial negative procalcitonin levels and negative nasal PCR testing for methicillin-resistant Staphylococcus aureus.
Figure 5. Anteroposterior and Lateral Chest Radiographs, January 26, 2020 (Illness Day 10, Hospital Day 6).
Stable streaky opacities in the lung bases were visible, indicating likely atypical pneumonia; the opacities have steadily increased in density over time.
On hospital day 8 (illness day 12), the patient’s clinical condition improved. Supplemental oxygen was discontinued, and his oxygen saturation values improved to 94 to 96% while he was breathing ambient air. The previous bilateral lower-lobe rales were no longer present. His appetite improved, and he was asymptomatic aside from intermittent dry cough and rhinorrhea. As of January 30, 2020, the patient remains hospitalized. He is afebrile, and all symptoms have resolved with the exception of his cough, which is decreasing in severity.
Methods
Specimen Collection
Clinical specimens for 2019-nCoV diagnostic testing were obtained in accordance with CDC guidelines.12 Nasopharyngeal and oropharyngeal swab specimens were collected with synthetic fiber swabs; each swab was inserted into a separate sterile tube containing 2 to 3 ml of viral transport medium. Serum was collected in a serum separator tube and then centrifuged in accordance with CDC guidelines. The urine and stool specimens were each collected in sterile specimen containers. Specimens were stored between 2°C and 8°C until ready for shipment to the CDC. Specimens for repeat 2019-nCoV testing were collected on illness days 7, 11, and 12 and included nasopharyngeal and oropharyngeal swabs, serum, and urine and stool samples.
Diagnostic Testing for 2019-nCoV
Clinical specimens were tested with an rRT-PCR assay that was developed from the publicly released virus sequence. Similar to previous diagnostic assays for severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), it has three nucleocapsid gene targets and a positive control target. A description of this assay13 and sequence information for the rRT-PCR panel primers and probes14 are available on the CDC Laboratory Information website for 2019-nCoV.15
Genetic Sequencing
On January 7, 2020, Chinese researchers shared the full genetic sequence of 2019-nCoV through the National Institutes of Health GenBank database16 and the Global Initiative on Sharing All Influenza Data (GISAID)17 database; a report about the isolation of 2019-nCoV was later published.18 Nucleic acid was extracted from rRT-PCR–positive specimens (oropharyngeal and nasopharyngeal) and used for whole-genome sequencing on both Sanger and next-generation sequencing platforms (Illumina and MinIon). Sequence assembly was completed with the use of Sequencher software, version 5.4.6 (Sanger); minimap software, version 2.17 (MinIon); and freebayes software, version 1.3.1 (MiSeq). Complete genomes were compared with the available 2019-nCoV reference sequence (GenBank accession number NC_045512.2).
Results
Specimen Testing for 2019-nCoV
The initial respiratory specimens (nasopharyngeal and oropharyngeal swabs) obtained from this patient on day 4 of his illness were positive for 2019-nCoV (Table 2). The low cycle threshold (Ct) values (18 to 20 in nasopharyngeal specimens and 21 to 22 in oropharyngeal specimens) on illness day 4 suggest high levels of virus in these specimens, despite the patient’s initial mild symptom presentation. Both upper respiratory specimens obtained on illness day 7 remained positive for 2019-nCoV, including persistent high levels in a nasopharyngeal swab specimen (Ct values, 23 to 24). Stool obtained on illness day 7 was also positive for 2019-nCoV (Ct values, 36 to 38). Serum specimens for both collection dates were negative for 2019-nCoV. Nasopharyngeal and oropharyngeal specimens obtained on illness days 11 and 12 showed a trend toward decreasing levels of virus. The oropharyngeal specimen tested negative for 2019-nCoV on illness day 12. The rRT-PCR results for serum obtained on these dates are still pending.
Table 2. Results of Real-Time Reverse-Transcriptase–Polymerase-Chain-Reaction Testing for the 2019 Novel Coronavirus (2019-nCoV).*.
| Specimen | Illness Day 4 | Illness Day 7 | Illness Day 11 | Illness Day 12 |
|---|---|---|---|---|
| Nasopharyngeal swab | Positive (Ct, 18–20) |
Positive (Ct, 23–24) |
Positive (Ct, 33–34) |
Positive (Ct, 37–40) |
| Oropharyngeal swab | Positive (Ct, 21–22) |
Positive (Ct, 32–33) |
Positive (Ct, 36–40) |
Negative |
| Serum | Negative | Negative | Pending | Pending |
| Urine | NT | Negative | NT | NT |
| Stool | NT | Positive (Ct, 36–38) |
NT | NT |
Lower cycle threshold (Ct) values indicate higher viral loads. NT denotes not tested.
Genetic Sequencing
The full genome sequences from oropharyngeal and nasopharyngeal specimens were identical to one another and were nearly identical to other available 2019-nCoV sequences. There were only 3 nucleotides and 1 amino acid that differed at open reading frame 8 between this patient’s virus and the 2019-nCoV reference sequence (NC_045512.2). The sequence is available through GenBank (accession number MN985325).16
Discussion
Our report of the first confirmed case of 2019-nCoV in the United States illustrates several aspects of this emerging outbreak that are not yet fully understood, including transmission dynamics and the full spectrum of clinical illness. Our case patient had traveled to Wuhan, China, but reported that he had not visited the wholesale seafood market or health care facilities or had any sick contacts during his stay in Wuhan. Although the source of his 2019-nCoV infection is unknown, evidence of person-to-person transmission has been published. Through January 30, 2020, no secondary cases of 2019-nCoV related to this case have been identified, but monitoring of close contacts continues.19
Detection of 2019-nCoV RNA in specimens from the upper respiratory tract with low Ct values on day 4 and day 7 of illness is suggestive of high viral loads and potential for transmissibility. It is notable that we also detected 2019-nCoV RNA in a stool specimen collected on day 7 of the patient’s illness. Although serum specimens from our case patient were repeatedly negative for 2019-nCoV, viral RNA has been detected in blood in severely ill patients in China.4 However, extrapulmonary detection of viral RNA does not necessarily mean that infectious virus is present, and the clinical significance of the detection of viral RNA outside the respiratory tract is unknown at this time.
Currently, our understanding of the clinical spectrum of 2019-nCoV infection is very limited. Complications such as severe pneumonia, respiratory failure, acute respiratory distress syndrome (ARDS), and cardiac injury, including fatal outcomes, have been reported in China.4,18,20 However, it is important to note that these cases were identified on the basis of their pneumonia diagnosis and thus may bias reporting toward more severe outcomes.
Our case patient initially presented with mild cough and low-grade intermittent fevers, without evidence of pneumonia on chest radiography on day 4 of his illness, before having progression to pneumonia by illness day 9. These nonspecific signs and symptoms of mild illness early in the clinical course of 2019-nCoV infection may be indistinguishable clinically from many other common infectious diseases, particularly during the winter respiratory virus season. In addition, the timing of our case patient’s progression to pneumonia on day 9 of illness is consistent with later onset of dyspnea (at a median of 8 days from onset) reported in a recent publication.4 Although a decision to administer remdesivir for compassionate use was based on the case patient’s worsening clinical status, randomized controlled trials are needed to determine the safety and efficacy of remdesivir and any other investigational agents for treatment of patients with 2019-nCoV infection.
We report the clinical features of the first reported patient with 2019-nCoV infection in the United States. Key aspects of this case included the decision made by the patient to seek medical attention after reading public health warnings about the outbreak; recognition of the patient’s recent travel history to Wuhan by local providers, with subsequent coordination among local, state, and federal public health officials; and identification of possible 2019-nCoV infection, which allowed for prompt isolation of the patient and subsequent laboratory confirmation of 2019-nCoV, as well as for admission of the patient for further evaluation and management. This case report highlights the importance of clinicians eliciting a recent history of travel or exposure to sick contacts in any patient presenting for medical care with acute illness symptoms, in order to ensure appropriate identification and prompt isolation of patients who may be at risk for 2019-nCoV infection and to help reduce further transmission. Finally, this report highlights the need to determine the full spectrum and natural history of clinical disease, pathogenesis, and duration of viral shedding associated with 2019-nCoV infection to inform clinical management and public health decision making.
Acknowledgments
We thank the patient; the nurses and clinical staff who are providing care for the patient; staff at the local and state health departments; staff at the Washington State Department of Health Public Health Laboratories and at the Centers for Disease Control and Prevention (CDC) Division of Viral Disease Laboratory; CDC staff at the Emergency Operations Center; and members of the 2019-nCoV response teams at the local, state, and national levels.
Supplementary Appendix
Disclosure Forms
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This article was published on January 31, 2020, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.World Health Organization. Pneumonia of unknown cause — China. 2020. (https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/).
- 2.World Health Organization. Novel coronavirus — China. 2020. (https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/).
- 3.Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020. January 24 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020. January 24 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2019 Novel coronavirus, Wuhan, China: 2019-nCoV situation summary. January 28, 2020. (https://www.cdc.gov/coronavirus/2019-nCoV/summary.html).
- 6.Phan LT, Nguyen TV, Luong QC, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med 2020;382:872-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johns Hopkins University CSSE. Wuhan coronavirus (2019-nCoV) global cases (https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6).
- 8.Centers for Disease Control and Prevention. Interim guidance for healthcare professionals: criteria to guide evaluation of patients under investigation (PUI) for 2019-nCoV. 2020. (https://www.cdc.gov/coronavirus/2019-nCoV/clinical-criteria.html).
- 9.Centers for Disease Control and Prevention. Infection control. 2019 Novel coronavirus, Wuhan, China. 2020. (https://www.cdc.gov/coronavirus/2019-nCoV/infection-control.html).
- 10.Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of ebola virus disease therapeutics. N Engl J Med 2019;381:2293-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 2020;11:222-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from patients under investigation (PUIs) for 2019 novel coronavirus (2019-nCoV). 2020. (https://www.cdc.gov/coronavirus/2019-nCoV/guidelines-clinical-specimens.html).
- 13.Centers for Disease Control and Prevention, Respiratory Viruses Branch, Division of Viral Diseases. Real-time RT-PCR panel for detection 2019-novel coronavirus. 2020. (https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-for-detection-instructions.pdf).
- 14.Centers for Disease Control and Prevention, Respiratory Viruses Branch, Division of Viral Diseases. 2019-novel coronavirus (2019-nCoV) real-time rRT-PCR panel primers and probes. 2020. (https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-primer-probes.pdf).
- 15.Centers for Disease Control and Prevention. Information for laboratories. 2019 novel coronavirus, Wuhan, China. 2020. (https://www.cdc.gov/coronavirus/2019-nCoV/guidance-laboratories.html).
- 16.National Institutes of Health. GenBank overview (https://www.ncbi.nlm.nih.gov/genbank/).
- 17.GISAID (Global Initiative on Sharing All Influenza Data) home page (https://www.gisaid.org/). [DOI] [PMC free article] [PubMed]
- 18.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Washington State Department of Health. Novel coronavirus outbreak 2020 (https://www.doh.wa.gov/Emergencies/Coronavirus).
- 20.Chen N, Zhou M, Dong X Jr, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020. January 30 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.