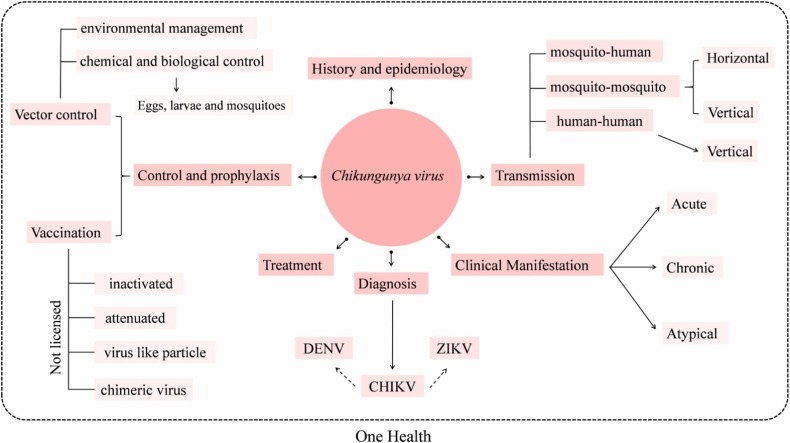
Graphical abstract
Keywords: Chikungunya fever, Arboviruses, Dengue, Zika
Abstract
Chikungunya fever is a mosquito-borne viral illness characterized by a sudden onset of fever associated with joint pains. It was first described in the 1950s during a Chikungunya virus (CHIKV) outbreak in southern Tanzania and has since (re-) emerged and spread to several other geographical areas, reaching large populations and causing massive epidemics. In recent years, CHIKV has gained considerable attention due to its quick spread to the Caribbean and then in the Americas, with many cases reported between 2014 and 2017. CHIKV has further garnered attention due to the clinical diagnostic difficulties when Zika (ZIKV) and dengue (DENV) viruses are simultaneously present. In this review, topical CHIKV-related issues, such as epidemiology and transmission, are examined. The different manifestations of infection (acute, chronic and atypical) are described and a particular focus is placed upon the diagnostic handling in the case of ZIKV and DENV co-circulating. Natural and synthetic compounds under evaluation for treatment of chikungunya disease, including drugs already licensed for other purposes, are also discussed. Finally, previous and current vaccine strategies, as well as the control of the CHIKV transmission through an integrated vector management, are reviewed in some detail.
1. Historical and epidemiological aspects
Chikungunya virus (CHIKV) is the etiological agent of chikungunya fever (CHIKF), an arthropod-borne disease transmitted mainly by Aedes genus species (Weaver, 2006). First described in 1952 in present-day Tanzania, the early CHIKF cases were treated as Dengue virus (DENV) infection (Lumsden, 1955). Following isolation of CHIKV from infected patients’ sera, as well as Ae. (Stegomyia) aegypti (Linnaeus, 1762) and Culex spp. mosquitoes in 1953, the virus was placed in the arbovirus group A (Ross, 1956; Calisher and Karabatsos, 1988). Currently, CHIKV is grouped within the Alphavirus genus, Togaviridae family, and identified as member of the Semliki Forest virus (SFV) antigenic complex (van Duijl-Richter et al., 2015; ICTV, 2017).
Shortly after identification in East Africa (1952), CHIKV was described in both central and southern regions of Africa (Uganda and sub-Saharan region, respectively) (Weinbren et al., 1958). With the advent of nucleic acid sequencing and tools enabling the elucidation of molecular evolution, these CHIKV strains were grouped and named according to geographical location of isolation: East-, Central- and South African lineage (ECSA) (Powers et al., 2000). Phylogenetic analysis of CHIKV isolated during outbreaks from 1958 to 1973 in Asia placed them into another monophyletic group called Asian lineage (Powers et al., 2000; Volk et al., 2010). By the end of the 20th century, phylogenetic inferences based on CHIKV isolated from mosquitoes from West Africa (Senegal) demonstrated still another viral lineage, geographically more restricted, known as West African (WA) (Powers et al., 2000; Volk et al., 2010).
After a large CHIKV outbreak on the coast of Kenya in 2004, the virus started to spread to the islands of the Indian ocean, India and southeast Asia, with more than 6 million probable cases (Powers et al., 2000; Sergon et al., 2007, 2008; Staples et al., 2009; Thiberville et al., 2013). These viruses, which were evolved from the ECSA clade, were then included in the Indian Ocean Lineage (IOL), repeatedly associated with CHIKV outbreaks from 2005 to 2014 (Tsetsarkin et al., 2011; Nunes et al., 2015). In 2005, for instance, a CHIKV outbreak was reported in the Comoros causing infection in approximately 215,000 people (Sergon et al., 2007). Between March 2005 and April 2006, 255,000 people were infected in Reunion Island (CIRE, 2006; Josseran et al., 2006).
In this last context, in Reunion Island, where there is a high density of the vector mosquito Aedes albopictus (Skuse, 1894), a non-synonymous mutation in the E1 glycoprotein of the viral envelope (E1-A226 V) was identified in 90% of the isolates (Schuffenecker et al., 2006). Reverse genetics studies proved the importance of the E1-A226 V mutation for viral fitness in Ae. albopictus (Tsetsarkin et al., 2007). Currently, the E1-A226 V substitution is thought to enhance viral infectivity in Ae. albopictus midgut cells due to the proximity of amino acid 226 to the fusion peptide, which is responsible for viral release from the endosome during early stages of the infection. Notably, this mutation did not compromise viral replication in Ae. aegypti (Tsetsarkin et al., 2007). Mutations in the same (or similar) position on the glycoprotein E1 have been reported in other alphavirus, such as SFV and Sindbis virus (SINV), and also have been associated with an increase of the capacity of infection and viral exit in Ae. albopictus (C6/36) cell line (Vashishtha et al., 1998; Lu et al., 1999).
Concerns over CHIKV increased after 2007, when the virus was found in northern Italy having been presumably introduced by an infected traveler from India (Rezza et al., 2007). In September 2010, autochthonous case of chikungunya disease also was reported in southeastern France (Grandadam et al., 2011). In the same year, CHIKV caused disease in India, Indonesia, Myanmar, Thailand and Maldives, and re-emerged in Reunion Island. In 2010, imported cases from Indonesia, Reunion Island, India and Asia were identified respectively in Taiwan, France, USA and in Brazil (Rezza et al., 2007; Thiberville et al., 2013; Brasil, 2014). In 2013, CHIKV arrived on the American continent, initially spreading in the Caribbean, before reaching Brazil in 2014 (Nunes et al., 2015). Two years later, in 2015, CHIKV was declared a notifiable disease by the CDC (CDC, 2015). The most recent CHIKV outbreak was reported in Mombasa, Kenya, in February 2018 (WHO, 2018).
In summary, these CHIKV epidemics presented a cyclical movement, characterized by remote outbreaks interspersed with periods of epidemiological silence, ranging from years to decades. This epidemiological profile is likely of multi-casual origin and probably includes environmental determinants, mosquito ecology, viral genetics, human behavior and differences in susceptibility to infections in humans and vectors (Josseran et al., 2006; Schuffenecker et al., 2006; Tsetsarkin et al., 2006; Pialoux et al., 2007; Simon et al., 2008; Mohan et al., 2010).
Currently, CHIKV infection has been reported in different countries on all continents, except Antarctica (CDC, 2018a). In some regions, especially in South America, the co-circulation of CHIKV with other arboviruses, such as DENV, ZIKV, Mayaro (MAYV) and yellow fever (YFV), requires rigorous epidemiological surveillance and differential diagnosis strategies (Figueiredo and Figueiredo, 2014; Benelli and Mehlhorn, 2016; CDC, 2018b). Here, we review the main aspects related to transmission, clinical signs and symptoms, diagnosis, treatment and control of CHIKV infections, focusing on factors especially important in the current scenario of the global DENV-CHIKV-ZIKV triad.
2. Transmission
Both urban and sylvatic CHIKV transmission cycles have been described (Caglioti et al., 2013). The sylvatic cycle (especially in Africa) may involve the participation of some Aedes species, such as Aedes furcifer (Edwards, 1913), Aedes taylori (Edwards, 1936), Aedes luteocephalus (Newstead et al., 1907), Aedes vittatus (Bigot, 1861) and Aedes fulgens (Edwards, 1917), and different non-human primate (NHP) species, possible reservoirs or amplifiers hosts for CHIKV [e.g. African green monkeys (Chlorocebus sabaeus) (Linnaeus, 1766), patas monkeys (Erythrocebus patas) (Schreber, 1775), Guinea baboons (Papio papio) (Desmarest, 1820), guenons (Cercopithecus aethiops) (Linnaeus, 1758), bushbabies (Galago senegalensis) (Geoffroy, 1796), mandrills (Mandrillus sphinx) (Linnaeus, 1758), red-tail monkeys (Cercopithecus ascanius schmidti) (Matschie, 1892) and Chacma baboons (Papio ursinus) (Kerr, 1792)] (McIntosh, 1970; McCrae et al., 1971; Diallo et al., 1999; Chevillon et al., 2008; Pruetz et al., 2010 Caglioti et al., 2013; Kading et al., 2013; Althouse et al., 2018). A vector role has also been suggested for Culex and Anopheles mosquitoes, which have been found infected in Senegal from 1972 to 1996 (Diallo et al., 1999).
In the urban cycle, Ae. aegypti and Ae. albopictus are known as the main vectors (Weaver, 2006). Currently, they probably remain as the main vectors for CHIKV transmission in the Americas, Africa, Europe, Asia and Oceania (Horwood et al., 2013; Vega-Rúa et al., 2014, 2015; Zeller et al., 2016 Ngoagouni et al., 2017). Significantly, Ae. aegypti can also be the main vector of other viruses, such as ZIKV and DENV, and co-transmission events may be observed (Carrillo-Hernández et al., 2018). However, it has been demonstrated that simultaneous infections by DENV/CHIKV/ZIKV or DENV/CHIKV in Ae. aegypti does not compromise the vector competence (Le Coupanec et al., 2017; Rückert et al., 2017).
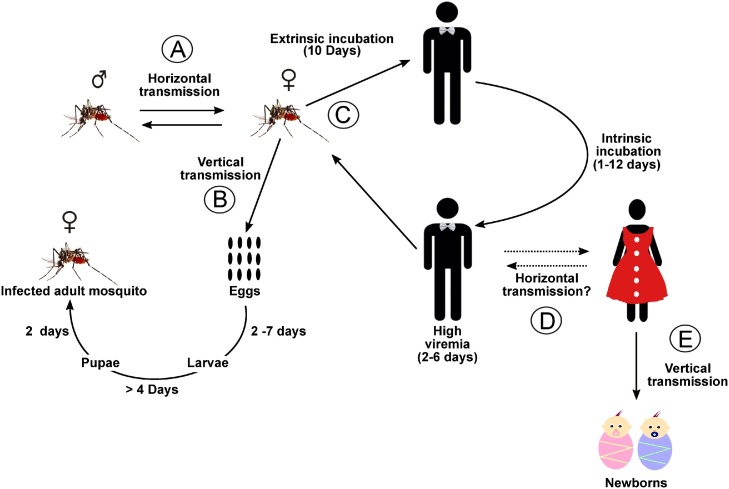
It is known that horizontal transmission of CHIKV in Aedes mosquitoes can occur and act positively in the maintenance of infection cycles (Fig. 1 A) (Mavale et al., 2010). Vertical transmission of CHIKV into Aedes has also been observed under natural and experimental conditions and has been pinpointed as a possible reason for viral persistence under harsh environmental conditions (Fig. 1B) (Agarwal et al., 2014; Chompoosri et al., 2016; Jain et al., 2016).
Fig. 1.
CHIKV transmission diagram. (A) Horizontal transmission between CHIKV vectors; (B) Vertical transmission within vectors; (C) Transmission to a susceptible human; (D) Horizontal transmission in humans; (E) Vertical transmission in humans. Dashed lines represent transmission pathways that have not yet been described.
Once inside the arthropod vector, CHIKV must replicate and reach the mosquitoes’ salivary glands within roughly seven to ten days for transmission to a susceptible human (Lim et al., 2018) (Fig. 1C). In human hosts, the intrinsic incubation time can vary from one to twelve days and infected individuals may present viremia of up to ten days (Kam et al., 2009; Simon et al., 2011; Azevedo et al., 2015).
Maternal-fetal transmission has also been reported in humans (Fig. 1E). Neonatal encephalitis, as consequence of vertical transmission, was observed, for instance, during the Brazilian epidemic in 2016 (Bandeira et al., 2016a; Lyra et al., 2016). However, no breast milk transmission has been evidenced (Patterson et al., 2016). Despite the fact that CHIKV RNA has been detected in semen even after 30 days post symptom onset, indicating possible transmission via sexual intercourse, horizontal transmission between humans has not yet been reported (Fig. 1D) (Bandeira et al., 2016b; Patterson et al., 2016).
3. CHIKV infection manifestation
3.1. Acute
Approximately 50–97% of individuals infected with CHIKV develop clinical disease with fever and arthralgia (Staples et al., 2009; Ayu et al., 2010; Nakkhara et al., 2013). CHIKV infection has been associated with sudden onset of febrile illness (>38.9 °C) (92% of patients), arthralgia (87% of patients), back pain (67% of patients), headache (62% of patients) and fatigue (WHO, 2008; Thiberville et al., 2013). The most common symptom in CHIKF is polyarthralgia, typically of bilateral polyarticular nature, affecting mainly peripheral joints (ankles, wrists and phalanges) and some large joints (knees and elbows) (WHO, 2008; Morrison, 2014).
Cutaneous manifestations are reported in circa 50% of acute cases. The lesions are characterized by macular or transitory maculopapular eruption, which can be edematous or itchy, often occurring in the body extremities, palms, soles of the feet, torso and face (Simon et al., 2011; Thiberville et al., 2013). Gastrointestinal symptoms, such as diarrhea, vomiting, nausea and abdominal pain, occur in 15–47% of cases during the acute phase (Thiberville et al., 2013). Other possible symptoms include erythema, asthenia, conjunctival effusion, persistent conjunctivitis and cervical lymphadenopathy (Staples et al., 2009; Staples and Fischer, 2014; Madariaga et al., 2016). Different studies have demonstrated that CHIKV infection can reach high viral loads, ranging from 105 to 109 copies of viral RNA/mL, which seem to be correlated with the presence and severity of clinical signs and symptoms (Chow et al., 2011; Appassakij et al., 2013).
3.2. Chronic
Polyarthralgia and/or polyarthritis are hallmark symptoms of chronic chikungunya, mostly affecting small joints, such as phalanges and wrists, as well as large joints (e.g., ankles, knees and shoulders). The condition is usually severe and leads to mobility limitations of afflicted patients (Hoarau et al., 2010).
Polyarthralgia has been described to persist for varying periods of time, lasting from weeks to several months and, in some cases, up to five years, depending on the populations evaluated (Borgherini et al., 2008; Sissoko et al., 2009; Manimunda et al., 2010; Simon et al., 2011). The persistence of polyarthralgia in some alphaviruses, such as SFV and SINV, seems to be associated with persistence of viral antigens and immune responses (inflammation) in the joints (Atkinson et al., 1986; Perri et al., 2000; Hoarau et al., 2010; Labadie et al., 2010; Simon et al., 2011; Poo et al., 2014; Silva and Dermody, 2017). About the viral antigens, there is still no consensus whether they have replication competence (perhaps with mutations to promote their persistence) or are only the result of a delayed clearance of non-replicating viral antigen (Atkinson et al., 1986; Perri et al., 2000; Poo et al., 2014; Weaver and Lecuit, 2015).
Precisely on CHIKV, a 2010 study described the presence of macrophages with CHIKV genetic material and viral proteins in the synovial tissue of an 18-month-long chronically infected patient (Hoarau et al., 2010). Experimental studies have shown CHIKV persistence in lymphoid organs, liver, joints, muscles and macrophages from NHPs (Labadie et al., 2010).
In addition, the presence of infiltrating cells, mainly macrophages, monocytes and lymphocytes, and specific proinflammatory mediators, such as IL-6, IL-8, and MCP-1, within the synovial fluid probably also contribute to the chronicity of the inflammation in chikungunya disease (Silva and Dermody, 2017). Moreover, severe cases of chikungunya may be related to age and diverse underlying medical conditions, such as hypertension, respiratory conditions and diabetes mellitus (Borgherini et al., 2007, 2008; Sankari et al., 2008; Economopoulou et al., 2009; Sissoko et al., 2009; Tandale et al., 2009).
The pathogenesis of rheumatoid arthritis in CHIKF is still under debate. While certain studies have suggested that viral infection may trigger initiation of this chronic inflammatory disorder, other studies did not find inflammatory markers in infected individuals with chronic symptoms (Staples et al., 2009; Schilte et al., 2013).
3.3. Atypical
CHIKV infections can also lead to atypical clinical manifestations. Guillain-Barré syndrome (GBS), for instance, has been associated with CHIKV infection (Lebrun et al., 2009; Oehler et al., 2015). GBS comprises an acute inflammatory demyelinating polyneuropathy of global incidence, in which about two-thirds of cases occur after bacterial (e.g. Campylobacter jejuni) (Heikema et al., 2015) or viral infection (Oehler et al., 2015), such as by Dengue- (Simon et al., 2016), West Nile- (Leis and Stokic, 2012), Influenza- (Choi and Yeon, 2016), Cytomegalo- (Steger et al., 2012), Human Immunodeficiency- (Girgin et al., 2014), Epstein-Barr (Phillips, 1973; Kim et al., 2016) and Zika viruses (Rozé et al., 2017).
During the more recent CHIKV outbreaks, total or partial alopecia on the head or body, predominately in female patients, and ophthalmological alterations, such as uveitis and retinitis, were described during the chronic phase of infection (Martínez-Pulgarín et al., 2016; Cunha and Trinta, 2017).
In newborns, congenital infections may be accompanied by varying clinical signs, such as fever, lack of appetite, apnea, skin manifestations, distal and cerebral edema, encephalitis and hemorrhage (Gopakumar and Ramachandran, 2012; Bandeira et al., 2016a; Lyra et al., 2016). Heart- and gastrointestinal disorders and cutaneous lesions are reported to manifest up to two days after the onset of fever in CHIKV-infected newborns and children (Ernould et al., 2008). Bullous lesions associated to CHIKV infection have also been reported in four-month-old babies, who had 20% of their body surface affected on the second day after the onset of fever (Robin et al., 2010).
Deaths from CHIKV infection were previously considered a rare event. This perception, however, has changed since the latest epidemics, which presented a considerably increased mortality rate, probably due to neurological affections, mainly in neonates, immunocompromised and elderly (Rampal et al., 2007; Kee et al., 2010 Chusri et al., 2011; Bandeira et al., 2016a).
4. Differential diagnosis between chikungunya, Zika and dengue diseases in areas of co-circulation
It is challenging to differentiate clinical signs and symptoms of CHIKV infection from other pathologies, especially when ZIKV and DENV are co-circulating in the same geographical region (Hua and Combe, 2017). Individuals infected by these arboviruses can present a wide range of similar clinical manifestations, such as rash, myalgia, exanthema, arthralgia, joint pain, headache, lymph node hypertrophy, neurological impairment and fever (Brito and Cordeiro, 2016). In addition, it is difficult to determine the frequency and intensity of the symptoms and correctly assess pain (mild, moderate and intense) of afflicted patients (Table 1 ). In this context, variations in the clinical presentation of cases can give hints as to the viral etiology; for instance, the salient and prolonged polyarthralgia, often accompanied by rash, is typically more indicative of chikungunya, while hemorrhagic manifestations and myalgia are more commonly observed in DENV infections (Lee et al., 2012)
Table 1.
Signals and symptoms that may contribute in the differential diagnosis between dengue, chikungunya and Zika illnesses.
| Signals/Symptoms | Arboviruses |
||
|---|---|---|---|
| Chikungunya | Dengue | Zika | |
| Fever | >38 °C (2–3da) | >38 °C (4–7d) | ≤38 °C (1-2d) |
| Rash | ++ (d2-d5b) | + (d4c) | +++ (d1-d2d) |
| Pruritus | +/++ | +/+++ | ++/+++ |
| Myalgia | + | +++ | ++ |
| Arthralgia | +++ | + | ++ |
| Retrorbital pain | +/- | +++ | +/- |
| Conjunctivites | + | +/- | ++/+++ |
| Skin Bleeding | +/- | ++ | +/- |
| Joint swelling | ++/+++ | +/- | + |
| Headache | ++ | +++ | ++ |
| Diarrhea | +/- | ++ | +/- |
| Neurological impairment | ++ | + | +++ |
| Lymphadenophaty | ++ | + | +++ |
| Hemorrhagic dyscrasia | + | ++ | – |
References: Brito and Cordeiro (2016); PAHO (2017).
Duration of fever in days.
Onset of rash in 50% of cases.
Onset of rash in 30–50% of cases.
Onset of rash in 100% of cases.
Despite the patients co-infected with CHIKV/DENV, CHIKV/ZIKV and CHIKV/DENV/ZIKV often do not show exacerbation of clinical signs, the co-infection presents as additional obstacle during differential diagnosis (Furuya-Kanamori et al., 2016; Villamil-Gómez et al., 2016; Carrillo-Hernández et al., 2018). Table 1, Table 2 summarize clinical and laboratory features that should be evaluated for efficient differential diagnosis of CHIKV, DENV and ZIKV infections.
Table 2.
Laboratory findings that may contribute in the differential diagnosis between dengue, chikungunya and Zika illnesses.
| Laboratory finding | Arboviruses |
||
|---|---|---|---|
| Chikungunya | Dengue | Zika | |
| Leukopenia | +/++ | ++/+++ | +/++ |
| Lymphopenia | Usual | Unusual | Unusual |
| Thrombocytopenia | +/++ | +++ | – |
| Platelet count | Normal to low | Normal to very low | Normal to low |
| C-reactive protein | Elevated | Normal | Elevated |
| High hematocrit level | Infrequent | Warning sign | Infrequent |
References: Brito and Cordeiro (2016); PAHO (2017).
4.1. Laboratory diagnostic
Since the variety and intensity of symptoms associated to CHIKV, DENV and ZIKV infections are so similar and make clinical diagnosis difficult in areas of co-circulation, laboratory analysis is necessary to confirm the respective viral etiology. Hematology findings associated to CHIKV infection are commonly either unspecific, however, lymphopenia and hypocalcemia were the most frequent observation, and severe thrombocytopenia is rare (Borgherini et al., 2007; Brito and Cordeiro, 2016; PAHO, 2017). Moreover, the C-reactive protein is generally elevated in the acute phase illness (Table 2) (Venugopalan et al., 2014; Brito and Cordeiro, 2016; PAHO, 2017). Elevation of hepatic enzymes, as well as elevated creatinine and creatine phosphokinase levels, have also been reported (Danis-Lozano et al., 2017). The different laboratory patterns observed during CHIKV, DENV and ZIKV infections, added to clinical findings, may be incorporated to support a correct diagnosis (Table 1, Table 2).
Laboratory tests for specific diagnosis of CHIKV infection are based on virus isolation, viral RNA detection and serology (Johnson et al., 2016). Despite not usually employed in routine diagnosis, viral isolation can be performed from sera collected up to seven days after onset of illness and inoculated into mosquito- or mammalian cell lines, in which cytopathic effects can appear between one to three days after inoculation. Confirmation of results is possible via immunofluorescence or RT-PCR assays (Panning et al., 2008; Staples et al., 2009 Dash et al., 2011). Recently, an immunochromatographic assay used anti-CHIKV E1 monoclonal antibodies to detect different CHIKV genotypes in samples from acutely infected patients. This highly specific and sensitive assay may also be an alternative method for CHIKV infection diagnosis (Okabayashi et al., 2015).
Molecular methods of CHIKV diagnosis, such as RT-PCR, RT-LAMP, qRT-PCR, have gained increasing importance. They are more sensitive and faster than viral isolation, and permit RNA detection from all CHIKV lineages with high specificity. Usually, serum samples collected up to seven days of symptom-onset are suitable for CHIKV detection by molecular diagnostic platforms (Edwards et al., 2007; Litzba et al., 2008; Sharma et al., 2010).
In addition, novel multiplex assays are capable of differentiating CHIKV from other infectious agents with a similar clinical spectrum. Among them, RT-LAMP assay has been shown to be capable of differentiating between ZIKV, CHIKV and DENV infections (Yaren et al., 2017). A RT-qPCR capable of successfully differentiating between ZIKV-CHIKV-DENV and CHIKV-DENV-Leptospira infections was also recently described (Pabbaraju et al., 2016; Giry et al., 2017).
In later phases of infection, CHIKV detection is usually based on serological methods, such as ELISA and plaque reduction neutralization testing (PRNT). ELISA techniques are useful to distinguish between acute or convalescent infections via detection of anti-CHIKV IgM or IgG antibodies. IgM can be detected from two/four days up to three months after the onset of illness, while IgG can be detected for several years (Grivard et al., 2007; Pialoux et al., 2007; Reddy et al., 2012). Moreover, ELISA for CHIKV diagnosis are highly specific and have a high accuracy (Johnson et al., 2016). IgM antibody-capture ELISA (MAC-ELISA), via which IgM antibodies can be detected in serum samples collected from four days after onset of symptoms, are the most commonly used tests for laboratory-based diagnoses (Reddy et al., 2012). PRNT, used as a parameter to measure circulating neutralizing antibodies, is useful to establish immunoprotection levels based on the determination of serum antibody titers required to neutralize a known amount of infectious virus particles (Azami et al., 2016). Alternative techniques for anti-CHIKV antibody detection include immunofluorescence and hemagglutination inhibition (Staples et al., 2009).
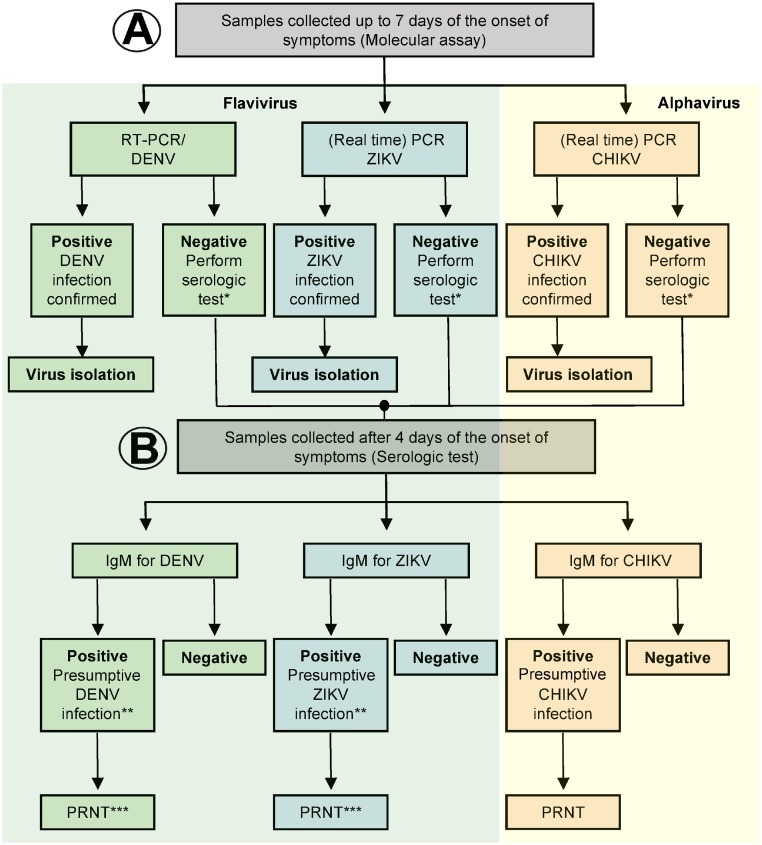
In general, any suitable etiological diagnosis should be reached through combined epidemiological, clinical and laboratory approaches performed by experienced health professionals. An algorithm-guided infographic with specific laboratory key findings to be used for differential diagnosis of CHIKV, DENV and/or ZIKV infections is summarized in Fig. 2 .
Fig. 2.
Algorithm for arbovirus diagnosis in suspect cases of chikungunya, dengue and Zika diseases. (A) Samples should be tested by RT-PCR due to the cross-reaction observed in flavivirus samples. (B) Once the molecular test is negative, serological test must be performed. *perform serological tests for samples collected ≥ 4 days after the onset of clinical signs and symptoms; **PRNT is required due to the cross-reaction between DENV and ZIKV; ***Test also for other flavivirus (e.g. West Nile and Saint Louis encephalitis viruses). Reference: CDC (2016) and PAHO (2017).
5. Treatment
There is no licensed specific antiviral available for the control of CHIKV replication, thus therapeutic strategies must be supportive and symptomatic, including fluid intake (Jain et al., 2008; WHO, 2008; Kaur and Chu, 2013). In this context, non-steroidal anti-inflammatory drugs (NSAIDs), such as paracetamol, are indicated to reduce the fever and relieve arthralgic pain (WHO, 2008). However, NSAIDs that interfere (at secondary level) with platelet aggregation or with other mechanisms of blood clotting (e.g. aspirin) should be avoided (Goupil and Mores, 2016). The co-administration of NSAIDs with low-dose systemic corticosteroids has been recommended to reduce pain and improve quality of life in treating acute chikungunya cases with arthralgia (Padmakumar et al., 2009). Past concerns that corticosteroid treatment may exacerbate alphaviral arthritides appear unjustified, since serodiagnosis has demonstrated antiviral immunity (Mylonas et al., 2004).
A succinct overview of current strategies for inhibition of CHIKV infection has recently been published (Subudhi et al., 2018). Briefly, among the anti-CHIKV drugs under evaluation, there are preparations that target the viral adsorption and fusion, translation of viral protein and genome replication (mainly in viral non-structural protein 2, nsP2), maturation of viral glycoproteins and immunological molecules (Brighton, 1984; Briolant et al., 2004; De Lamballerie et al., 2008; Ozden et al., 2008; Khan et al., 2010; Pohjala et al., 2011; Wintachai et al., 2012; Kaur et al., 2013; Lani et al., 2015; Wintachai et al., 2015; Wichit et al., 2017). Examples of drugs and compounds under evaluation are available on Table 3 .
Table 3.
Drugs and compounds under evaluation for treatment of CHIKV infection.
| Drugs/compounds | Mechanism of action | Licensed (for other purposes) |
Description | References |
|---|---|---|---|---|
| Chloroquine | It impairs endocytosis and/or acidification of the endosome. | Licensed for malaria treatment | Antiviral activity has been already demonstrated against HIVa, SARS-CoVb and SFV. A pilot study shown that chloroquine could be employed in the treatment of arthralgia in chronic chikungunya. However, its use in the acute phase is still debated and some studies have also shown an increase of viral replication of SFV and ECMV after treatment with chloroquine. | (Brighton, 1984; De Lamballerie et al., 2008; Maheshwari, Srikanta, Bhartiyaet, 1991; Khan et al., 2010) |
| Arbidol | Inhibition of the fusion between virus particle and plasma membrane, and between virus particle and the membrane of endosome | Influenza virus antiviral licensed in Russia and China | Evaluation of its activity against CHIKV in vitro show strong inhibition of viral replication in Vero and MRC-5c cell line. | (Wang et al., 2017; Villalain, 2010; Delogu et al., 2011) |
| Prohibitin ligants (sulfonyl amidine 1 m and the flavaglines FL3 and FL23) |
Potential entry inhibitors by competition for binding with prohibitin, one of the probable cellular receptor for CHIKV | No | Inhibition of CHIKV infection in mammalian HEK293T/17 cellsd. | (Wintachai et al., 2012, 2015) |
| Imipramine | Interference with intracellular cholesterol trafficking | Hyperactivity and impulsivity in patients with attention deficit | Able to inhibit CHIKV fusion and replication in human skin fibroblasts and also shown activity against ZIKV, DENV, and WNVe. | (Wichit et al., 2017) |
| Harringtonine | Inhibit expression of viral proteins nsP3 and E2, and formation of positive and negative RNA strain | No | Potent inhibition of CHIKV infection with minimal cytotoxicity; also inhibited the SINV replication. | (Kaur et al., 2013) |
| Silymarin | Reduction of both CHIKV replication efficiency and down-regulating of viral proteins involved in replication. | No | Silymarin interferes with post-entry stages of CHIKV infection, reducing, in a dose dependent manner, the nsP1, nsP3 and E2 proteins production. | (Lani et al., 2015) |
| Ribavirin | Probable inhibition of the viral mRNA polymerase by binding to the nucleotide binding site of the enzyme. | Antiviral licensed for treatment of RSVf and HCVg. | Patients in the drug group reported improvement in the joint pains and the soft tissue swelling also reduced. Together with IFN-alpha2b was able to inhibit CHIKV and SFV replication in Vero cells | (Ravichandran, Manian; 2008; Palumbo, 2011; Turner et al., 2014; Briolant et al., 2004) |
| Decanoyl-RVKR-chloromethyl ketone (dec-RVKR-cmk) |
Inhibition of the maturation of E2 glycoprotein by inhibition of furin. | No | Inhibition of CHIKV infection in human muscle satellite cells. Interestingly, dec-RVKR-cmk induced stronger inhibition of viral infection than chloroquine when added just after infection. Combination of both drugs induces an additive effect, mainly when the drugs were added before infection. | (Ozden et al., 2008) |
Human immunodeficiency virus.
Severe acute respiratory syndrome–associated coronavirus.
Human lung fibroblast cell line.
Human embryonic kidney cell line.
West Nile virus.
Respiratory syncytial virus.
Hepatitis C virus.
6. Control and prophylaxis
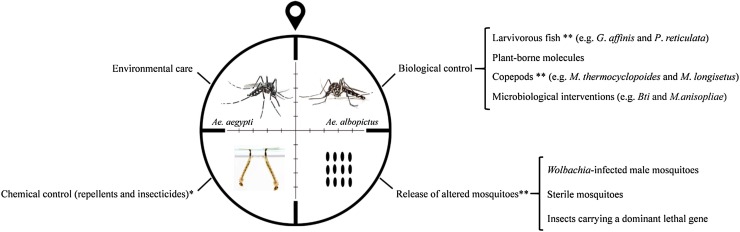
In the absence of therapeutic strategies and licensed vaccines, efficient vector control plays a crucial role in CHIKV prevention (Huang et al., 2017). Unfortunately, uncontrolled urbanization, lack of proper basic sanitation and increasing resistance to various classes of insecticides challenge the true impact of vector control measures for the reduction of arbovirus incidence (Resnik, 2014; Liang et al., 2015 Benelli et al., 2016 WHO, 2016). To overcome these obstacles, integrated anti-virus control is required and should include: a) epidemiological surveillance; b) environmental management focusing on educative actions to eliminate potential mosquito breeding sites and reduce standing water sites; c) chemical control using repellents (mainly for travelers and pregnant women) and insecticides, respecting the vectors resistance; and d) biological control against eggs, larvae and mosquitoes (Fig. 3 ) (Hemingway and Ranson, 2000; Dumont and Chiroleu, 2010; Benelli, 2015; Benelli et al., 2016; Islam et al., 2017; Benelli, 2018).
Fig. 3.
Management for an integrated Aedes vector control. *The use of insecticides and repellents should be carried out taking into account the mosquitoes’ resistance profiles; ** It is important to consider and evaluate the influence of these interventions on ecosystem balance.
In this last context, larvivorous fish belonging to the genus Gambusia (e.g. Gambusia affinis) (Baird and Girard, 1853) and Poecilia (e.g. Poecilia reticulata) (Peter, 1859) have been suggested in several countries and regions for mosquito control, mainly for Ae. aegypti (Hoy, 1985; Das and Prasad, 1991; Cavalcanti et al., 2007; Walton, 2007; Chandra et al., 2008; Seng et al., 2008; Dumont and Chiroleu, 2010; Kweka et al., 2011; Kamareddine, 2012; Shulse et al., 2013; Pereira and Oliveira, 2014; Chobu et al., 2015; Benelli et al., 2016). More natural mosquito predators, including copepods [e.g. Mesocyclops thermocyclopoides (Harada, 1931) and Mesocyclops longisetus (Thiébaud, 1912)] and other invertebrate aquatic organism, have also been implemented successfully to reduce Ae. aegypti and other culicidae populations (Rawlins et al., 1997; Manrique-Saide et al., 1998; Vu et al., 1998; Schaper, 1999; Mahesh Kumar et al., 2012; Benelli, 2015; Pavela, 2015). Plant-borne molecules have shown activity against Aedes, Anopheles and Culex larval instars (Benelli et al., 2016). More recent approaches, based on “green”-synthesized nanoparticles, have been suggested for use against DENV and Ae. aegypti vector (Madhiyazhagan et al., 2015; Sujitha et al., 2015; Benelli et al., 2018).
Microbiological interventions, such as the use of Bacillus thuringiensis var. israelensis (Bti) (Berliner, 1911) and entomopathogenic fungi [e.g. Metarhizium anisopliae (Metschnikoff, 1879) Sorokin, 1883 and Beauveria bassiana (Balsamo) Vuillemin, 1912)], have shown effects on malaria mosquitoes, as well as on Ae. aegypti and Ae. albopictus (Novak et al., 1985; Blanford et al., 2005; Armengol et al., 2006; Knols et al., 2010; Lam et al., 2010; Ritchie et al., 2010; Paula et al., 2011a, b). Recently, Bti was used to prevent ZIKV transmission in the continental United States (Stoddard, 2018).
In another promising approach to arbovirus control, including also ZIKV, the release of Wolbachia-infected male mosquitoes, sterile mosquitoes or insects carrying a dominant lethal gene can be used to reduce Ae. aegypti and Ae. albopictus populations (Fig. 3) (Ferreira et al., 2008; Alphey et al., 2010; Walker et al., 2011; Boyer, 2012; Alphey et al., 2013; Massonnet-Bruneel et al., 2013; Lee et al., 2013; Zhang et al., 2015a, b; Dickens et al., 2016). Despite the theoretical basis for their effectiveness, additional studies are needed to verify the true risks and benefits of programs based on altered mosquitoes. This concern appears to be greater in relation to transgenic mosquitoes, especially on their efficacy, sustainability and impact on the environment and target and non-target species (Wilke et al., 2018).
Although many of these strategies have been initially directed against DENV, their application to transmission control of CHIKV should be considered, mainly within an integrated approach, which rationally combines different measures. Control programs for other insect-borne diseases, such as malaria (Benelli and Beier, 2017), can also serve as model for CHIKV prevention.
In a synergistic way, mass immunization against CHIKV would be an important tool for viral control and prophylaxis. Several distinct vaccine approaches are currently under development, however, no vaccine has been licensed. Anti-CHIKV candidates that have been already tested in humans and/or animals include inactivated-, attenuated-, virus like particle- (VLP), DNA- and chimeric vaccines (Eckels et al., 1970; Levitt et al., 1986; Muthumani et al., 2008; Wang et al., 2008; Tiwari et al., 2009;Sharma et al., 2012 Akahata et al., 2010; Plante et al., 2011; Wang et al., 2011; Gorchakov et al., 2012; Brandler et al., 2013; Chang et al., 2014; García-Arriaza et al., 2014; Tretyakova et al., 2014; van den Doel et al., 2014; Erasmus et al., 2017).
In the past, viral inactivation strategies were the first to be tested in the course of anti-CHIKV vaccine development. CHIKV replicated in cell culture and subsequently inactivated by formalin, ether or 1,5 iodonapthyl azide (INA) was able to stimulate the production of neutralizing antibodies (Eckels et al., 1970; Tiwari et al., 2009; Sharma et al., 2012). Particularly, the use of INA resulted in reduced binding capacity of anti-E2 neutralizing antibodies (Sharma et al., 2012), while formalin inactivation stimulated the cellular immune response with the production of anti- and pro-inflammatory cytokines (Tiwari et al., 2009).
With the same aim of outlining the virulence of CHIKV infection, Brandler et al. (2013) reported a recombinant live-attenuated measles vaccine expressing a VLP composed of the capsid (C) and envelope (E) proteins from the La Réunion 06–46 CHIKV strain (ECSA lineage). In mice susceptible to Measles virus (MV), immunization with MV-CHIKV induced high titers of CHIKV antibodies, specific cellular immune responses and protected all animals from lethal CHIKV challenge (Brandler et al., 2013). In phase I clinical trial (European Clinical Trials Database, 2013-001084-23), a second vaccination resulted in 100% seroconversion for all participants. The immunogenicity of the MV-CHIKV was not affected by pre-existing anti-MV immunity and no vaccination-related serious adverse effects were recorded (Ramsauer et al., 2015).
Also using the vaccine platform VLP-based, Akahata et al. (2010) proposed the use of a vaccinal VLP expressing CHIKV structural proteins. The VLP-based vaccine, VRC-CHKVLP059-00-VP, was obtained by transfection of a plasmid expressing C and E proteins of the 37997 CHIKV strain (WA lineage). The vaccine stimulated the production of neutralizing antibodies against the E protein of different CHIKV strains and was able to protect challenged monkeys. Further tests in humans showed vaccine efficacy and immunogenicity, without reports of arthralgia as side effect (Chang et al., 2014). The VRC-CHKVLP059-00-VP is one of the most developed strategies and is currently undergoing phase II clinical trials (National Clinical Trials, 02562482).
Another approach to CHIKV vaccine development is the use of DNA vaccines. In this context, a plasmid expressing consensus sequences of C, E1 and E2 CHIKV proteins elicited a robust cellular immune response and the production of high antibody titers capable of recognizing wild virus antigens (Muthumani et al., 2008). In another study, mice immunized with a DNA vaccine containing the complete CHIKV genome of the 181/25 strain developed neutralizing antibodies and were protected from neurovirulent CHIKV (Tretyakova et al., 2014).
These strategies involving inactivated viruses, VLP and DNA vaccines often stimulate the humoral immune response, one of the main mechanisms for control and prevention of CHIKV infection (Lum et al., 2013). Empirically- or reverse-engineered attenuated live vaccines, however, have been shown to be capable of inducing both cellular and humoral immune responses and have also been suggested to prevent CHIKV infection (Levitt et al., 1986; Wang et al., 2008, 2011; Plante et al., 2011; Gorchakov et al., 2012; García-Arriaza et al., 2014). An important example of attenuated CHIKV vaccine is the TSI-GSD-218, a vaccine based on an empirically attenuated CHIKV 15561 strain (Asian lineage) isolated from patient sera from the 1962 CHIKV outbreak in Thailand (Levitt et al., 1986). By the end of its phase II clinical trial evaluation, 98% of the vaccinated individuals presented neutralizing antibodies, 85% remained seroconverted after one year and 8.47% presented temporary arthralgia (Edelman et al., 2000).
In addition to classical attenuation via serial viral passage in cells, reverse genetics strategies have been employed as platforms for construction of recombinant attenuated viruses or vaccine chimeras (Wang et al., 2008; Plante et al., 2011; Wang et al., 2011; García-Arriaza et al., 2014; van den Doel et al., 2014; Erasmus et al., 2017). An anti-CHIKV strategy involves the development of an attenuated chimeric vaccine using the Internal Ribosome Entry Site (IRES) of the Encephalomyocarditis virus (ECMV). In this approach, the CHIKV subgenomic promoter from the LR2006-OPY1 strain (ECSA lineage) is knocked via insertion of 13 synonymous mutations and the ECMV IRES sequence is added as new promoter for subgenomic RNA transcription (Plante et al., 2011). Briefly, the addition of the IRES sequence to the attenuated viral genome prevents viral propagation in mosquito cells, thus restricting the target population. This vaccine strategy has been shown to lead to the stimulation of TCD4 and TCD8 responses in mice (Plante et al., 2011). In NHP, CHIKV/IRES vaccine was also showed to be safe and immunogenically effective (Roy et al., 2014). Finally, the preclinical safety of the CHIKV/IRES vaccine was ensured in interferon-α/β receptor-incompetent mice (A129 mice) (Plante et al., 2015).
Other viral chimeras have been proposed in the context of anti-CHIKV vaccine strategies. Notably, attenuated strains of the Eastern equine encephalitis virus (VEEV) or Eastern equine encephalitis virus (EEEV) were used as backbones to express CHIKV structural proteins, creating immunogenic chimeras able to stimulate production of neutralizing antibodies and protect mice against disease and viremia after CHIKV challenge (Wang et al., 2008). Another chimeric construction, the Modified Vaccinia Ankara expressing E2 glycoprotein or E3-E2-6H-E1 proteins was shown to stimulate production of neutralizing antibodies in IFN-I (IFN α/β) and II (IFN-γ) receptor knockout mice (AG129 mice), protecting against lethal infection (van den Doel et al., 2014). A replication-incompetent adenovirus vector also has been used to express the ORF coding CHIKV structural polyproteins. A single dose of the chimera induced high antibodies titers capable of neutralizing Asian and IOL CHIKV lineages, protecting mice against viremia and arthralgia (Wang et al., 2011).
Most recently, a promising vaccine based on a chimera of Eilat virus (EILV) and CHIKV has been reported (Erasmus et al., 2017). The ELIV/CHIKV possesses non-structural proteins of EILV and structural and accessory proteins of 99659 CHIKV strain (Asian lineage). Since the EILV is an alphavirus specific for insects, the EILV/CHIKV was unable to replicate in vertebrate hosts, thereby providing a high degree of safety. In NHP, ELIV/CHIKV stimulated immune response and guaranteed protection against viremia (Erasmus et al., 2017).
7. Conclusions and unresolved questions
In the last decade, there has been an increase in the dissemination and co-circulation of several arboviruses. Currently, areas that were endemic just to DENV, for instance, are with autochthonous cases of chikungunya and Zika diseases. These arboviruses have similar clinical spectrum and require an efficient laboratory diagnosis, especially for a rigorous epidemiological surveillance. In addition, CHIKV infections represent a serious public health problem. The high morbidity of chikungunya often results in absenteeism of afflicted individuals, incurring both psychosocial and economic impacts. In this context, the development of a specific anti-CHIKV drug is certainly an important demand. On the other hand, the ideal control strategy for CHIKV should combine an integrated vector management with mass immunization. Although the different vector biocontrol strategies are promising, mainly within an integrated use, it is necessary to think about their sustainable use, assessing their real impacts on ecosystem equilibrium. The absence of CHIKV serotypes is considered a facilitative aspect in anti-CHIKV vaccine development, since a formulation developed from one strain will likely result in immunity against all CHIKV (Smalley et al., 2016). Despite recent advances in vaccine strategies, the major challenge regarding CHIKV vaccine development remains the establishment of an equilibrium between immunogenicity and safety, notably a reduction of side effects, such as secondary arthralgia following immunization with attenuated virus. Finally, recognizing the competence of vector and arboviruses control measures, we believe that the prevention of CHIKV infections should be planned within a global and multifactorial approach. This interdisciplinary strategy, currently framed within the One Health concept, should thus integrate all aspects of health care for humans, animals and the environment (Benelli and Duggan, 2018).
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Acknowledgments
E.F. Oliveira-Filho and R. Durães-Carvalho are supported by Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) and MCT/CNPq DCR grants. J.V.J. Silva Júnior is supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). T.R.R. Lopes is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- Agarwal A., Dash P.K., Singh A.K., Sharma S., Gopalan N., Rao P.V., et al. Evidence of experimental vertical transmission of emerging novel ECSA genotype of Chikungunya virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2014;8(7):e2990. doi: 10.1371/journal.pntd.0002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akahata W., Yang Z.Y., Andersen H., Sun S., Holdaway H.A., Kong W.P., et al. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat. Med. 2010;16(3):334–338. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey L., Benedict M., Bellini R., Clark G.G., Dame D.A., Service M.W., et al. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis. 2010;10(3):295–311. doi: 10.1089/vbz.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey L., McKemey A., Nimmo D., Neira Oviedo M., Lacroix R., Matzen K., et al. Genetic control of Aedes mosquitoes. Pathog. Glob. Health. 2013;107(4):170–179. doi: 10.1179/2047773213Y.0000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althouse B.M., Guerbois M., Cummings D.A.T., Diop O.M., Faye O., Faye A., et al. Role of monkeys in the sylvatic cycle of chikungunya virus in Senegal. Nat. Commun. 2018;9(1):1046. doi: 10.1038/s41467-018-03332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appassakij H., Khuntikij P., Kemapunmanus M., Wutthanarungsan R., Silpapojakul K. Viremic profiles in asymptomatic and symptomatic chikungunya fever: a blood transfusion threat? Transfusion. 2013;53(10 Pt 2):2567–2574. doi: 10.1111/j.1537-2995.2012.03960.x. [DOI] [PubMed] [Google Scholar]
- Armengol G., Hernandez J., Velez J.G., Orduz S. Long-lasting effects of a Bacillus thuringiensis serovar israelensis experimental tablet formulation for Aedes aegypti (Diptera: culicidae) control. J. Econ. Entomol. 2006;99(5):1590–1595. doi: 10.1603/0022-0493-99.5.1590. [DOI] [PubMed] [Google Scholar]
- Atkinson T., Barrett A.D., Mackenzie A., Dimmock N.J. Persistence of virulent Semliki forest virus in mouse brain following co-inoculation with defective interfering particles. J. Gen. Virol. 1986;67(Pt 6):1189–1194. doi: 10.1099/0022-1317-67-6-1189. [DOI] [PubMed] [Google Scholar]
- Ayu S.M., Lai L.R., Chan Y.F., Hatim A., Hairi N.N., Ayob A., et al. Seroprevalence survey of Chikungunya virus in Bagan Panchor, Malaysia. Am. J. Trop. Med. Hyg. 2010;83(6):1245–1248. doi: 10.4269/ajtmh.2010.10-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azami N.A., Moi M.L., Takasaki T. Neutralization assay for Chikungunya virus infection: plaque reduction neutralization test. Methods Mol. Biol. 2016;1426:273–282. doi: 10.1007/978-1-4939-3618-2_25. [DOI] [PubMed] [Google Scholar]
- Azevedo Ro S., Oliveira C.S., Vasconcelos P.F. Chikungunya risk for Brazil. Rev. Saude Publica. 2015;49:58. doi: 10.1590/S0034-8910.2015049006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira A.C., Campos G.S., Sardi S.I., Rocha V.F., Rocha G.C. Neonatal encephalitis due to Chikungunya vertical transmission: first report in Brazil. IDCases. 2016;5:57–59. doi: 10.1016/j.idcr.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira A.C., Campos G.S., Rocha V.F., Souza B.S., Soares M.B., Oliveira A.A., et al. Prolonged shedding of Chikungunya virus in semen and urine: a new perspective for diagnosis and implications for transmission. IDCases. 2016;6:100–103. doi: 10.1016/j.idcr.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli G. Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: a systematic review. Parasitol. Res. 2015;114(9):3201–3212. doi: 10.1007/s00436-015-4656-z. [DOI] [PubMed] [Google Scholar]
- Benelli G. Managing mosquitoes and ticks in a rapidly changing world – facts and trends. Saudi J. Biol. Sci. 2018 doi: 10.1016/j.sjbs.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli G., Beier J.C. Current vector control challenges in the fight against malaria. Acta Trop. 2017;174:91–96. doi: 10.1016/j.actatropica.2017.06.028. [DOI] [PubMed] [Google Scholar]
- Benelli G., Duggan M.F. Management of arthropod vector data - social and ecological dynamics facing the one health perspective. Acta Trop. 2018;182:80–91. doi: 10.1016/j.actatropica.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Benelli G., Mehlhorn H. Declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitol. Res. 2016;115(5):1747–1754. doi: 10.1007/s00436-016-4971-z. [DOI] [PubMed] [Google Scholar]
- Benelli G., Jeffries C.L., Walker T. Biological control of mosquito vectors: past, present, and future. Insects. 2016;7(4) doi: 10.3390/insects7040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli G., Maggi F., Pavela R., Murugan K., Govindarajan M., Vaseeharan B., et al. Mosquito control with green nanopesticides: towards the one Health approach? A review of non-target effects. Environ. Sci. Pollut. Res. 2018;25:10184–10206. doi: 10.1007/s11356-017-9752-4. [DOI] [PubMed] [Google Scholar]
- Blanford S., Chan B.H., Jenkins N., Sim D., Turner R.J., Read A.F., et al. Fungal pathogen reduces potential for malaria transmission. Science. 2005;308(5728):1638–1641. doi: 10.1126/science.1108423. [DOI] [PubMed] [Google Scholar]
- Borgherini G., Poubeau P., Staikowsky F., Lory M., Le Moullec N., Becquart J.P., et al. Outbreak of chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin. Infect. Dis. 2007;44(11):1401–1407. doi: 10.1086/517537. [DOI] [PubMed] [Google Scholar]
- Borgherini G., Poubeau P., Jossaume A., Gouix A., Cotte L., Michault A., et al. Persistent arthralgia associated with chikungunya virus: a study of 88 adult patients on reunion island. Clin. Infect. Dis. 2008;47(4):469–475. doi: 10.1086/590003. [DOI] [PubMed] [Google Scholar]
- Boyer S. [Sterile insect technique: targeted control without insecticide] Med. Trop. (Mars) 2012;72 Spec No:60-2. [PubMed] [Google Scholar]
- Brandler S., Ruffié C., Combredet C., Brault J.B., Najburg V., Prevost M.C., et al. A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine. 2013;31(36):3718–3725. doi: 10.1016/j.vaccine.2013.05.086. [DOI] [PubMed] [Google Scholar]
- Brasil . 2014. Preparação e Resposta à Introdução do Vírus Chikungunya no Brasil.http://bvsms.saude.gov.br/bvs/publicacoes/preparacao_resposta_virus_chikungunya_brasil.pdf (Accessed 11 July 2018) [Google Scholar]
- Brighton S.W. Chloroquine phosphate treatment of chronic Chikungunya arthritis. An open pilot study. S. Afr. Med. J. 1984;66(6):217–218. [PubMed] [Google Scholar]
- Briolant S., Garin D., Scaramozzino N., Jouan A., Crance J.M. In vitro inhibition of Chikungunya and Semliki forest viruses replication by antiviral compounds: synergistic effect of interferon-alpha and ribavirin combination. Antiviral Res. 2004;61(2):111–117. doi: 10.1016/j.antiviral.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Brito C.A., Cordeiro M.T. One year after the Zika virus outbreak in Brazil: from hypotheses to evidence. Rev. Soc. Bras. Med. Trop. 2016;49(5):537–543. doi: 10.1590/0037-8682-0328-2016. [DOI] [PubMed] [Google Scholar]
- Caglioti C., Lalle E., Castilletti C., Carletti F., Capobianchi M.R., Bordi L. Chikungunya virus infection: an overview. New Microbiol. 2013;36(3):211–227. [PubMed] [Google Scholar]
- Calisher C.H., Karabatsos N. In: Monath T.P., editor. vol. I. CRC Press; Boca Raton, Florida: 1988. Arbovirus serogroups: definition and geographic distribution. (The Arboviruses: Epidemiology and Ecology). [Google Scholar]
- Carrillo-Hernández M.Y., Ruiz-Saenz J., Villamizar L.J., Gómez-Rangel S.Y., Martínez-Gutierrez M. Co-circulation and simultaneous co-infection of dengue, chikungunya, and zika viruses in patients with febrile syndrome at the Colombian-Venezuelan border. BMC Infect. Dis. 2018;18(1):61. doi: 10.1186/s12879-018-2976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti L.P.G., Pontes R.J.S., Regazzi A.C.F., Paula F.J., Jr, Frutuoso R.L., Sousa E.P., et al. Competência de peixes como predadores de larvas de Aedes aegypti, em condições de laboratório. Rev. Saúde Públ. 2007;41(4):638–644. doi: 10.1590/s0034-89102006005000041. [DOI] [PubMed] [Google Scholar]
- CDC . 2015. Clinical Evaluation & Disease.https://www.cdc.gov/chikungunya/hc/clinicalevaluation.html (Accessed 11 July 2018) [Google Scholar]
- CDC . 2016. Revised Diagnostic Testing for Zika, Chikungunya, and Dengue Viruses in US Public Health Laboratories.https://www.cdc.gov/zika/pdfs/denvchikvzikv-testing-algorithm.pdf (Accessed 11 July 2018) [Google Scholar]
- CDC . Geographic Distribution. 2018. Chikungunya Virus.https://www.cdc.gov/chikungunya/geo/index.html (Accessed 21 August 2018) [Google Scholar]
- CDC . 2018. Chapter 3 Infectious Diseases Related to Travel.https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/yellow-fever (Accessed 21 August 2018) [Google Scholar]
- Chandra G., Bhattacharjee I., Chatterjee S.N., Ghosh A. Mosquito control by Larvivorous fish. Indian J. Med. Res. 2008;127(1):13–27. [PubMed] [Google Scholar]
- Chang L.J., Dowd K.A., Mendoza F.H., Saunders J.G., Sitar S., Plummer S.H., et al. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial. Lancet. 2014;384(9959):2046–2052. doi: 10.1016/S0140-6736(14)61185-5. [DOI] [PubMed] [Google Scholar]
- Chevillon C., Briant L., Renaud F., Devaux C. The chikungunya threat: an ecological and evolutionary perspective. Trends Microbiol. 2008;16:80–88. doi: 10.1016/j.tim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Chobu M., Nkwengulila G., Mahande A.M., Mwang’onde B.J., Kweka E.J. Direct and indirect effect of predators on Anopheles gambiae sensu stricto. Acta Trop. 2015;142:131–137. doi: 10.1016/j.actatropica.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Choi H.G., Yeon G.M. Guillain-Barré syndrome caused by influenza virus. Pediatr. Infect. Vaccine. 2016;23(3):236–239. [Google Scholar]
- Chompoosri J., Thavara U., Tawatsin A., Boonserm R., Phumee A., Sangkitporn S., et al. Vertical transmission of Indian ocean lineage of chikungunya virus in Aedes aegypti and Aedes albopictus mosquitoes. Parasit. Vectors. 2016;9:227. doi: 10.1186/s13071-016-1505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A., Her Z., Ong E.K., Chen J.M., Dimatatac F., Kwek D.J., et al. Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J. Infect. Dis. 2011;203(2):149–157. doi: 10.1093/infdis/jiq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chusri S., Siripaitoon P., Hirunpat S., Silpapojakul K. Case reports of neuro-Chikungunya in southern Thailand. Am. J. Trop. Med. Hyg. 2011;85(2):386–389. doi: 10.4269/ajtmh.2011.10-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIRE . 2006. Cire La Reunion-Mayotte. Epidemie de chikungunya a la Reunion: point au 1er juin 2006 pour la semaine 21 allant du 22 au 28 mai 2006.http://www.invs.sante.fr/presse/2006/le_point_sur/chikungunya_reunion_020606/chikungunya_reunion_s21.pdf Accessed 11 July 2018. [Google Scholar]
- Cunha R.V.D., Trinta K.S. Chikungunya virus: clinical aspects and treatment - a review. Mem. Inst. Oswaldo Cruz. 2017;112(8):523–531. doi: 10.1590/0074-02760170044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danis-Lozano R., Díaz-González E.E., Trujillo-Murillo K.D.C., Caballero-Sosa S., Sepúlveda-Delgado J., Malo-García I.R., et al. Clinical characterization of acute and convalescent illness of confirmed chikungunya cases from Chiapas, S. Mexico: a cross sectional study. PLoS One. 2017;12(10):e0186923. doi: 10.1371/journal.pone.0186923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M.K., Prasad R.N. Evaluation of mosquito fish Gambusia affinis in the control of mosquito breeding in rice fields. Indian J. Malariol. 1991;28(3):171–177. [PubMed] [Google Scholar]
- Dash M., Mohanty I., Padhi S. Laboratory diagnosis of chikungunya virus: do we really need it? Indian J. Med. Sci. 2011;65(3):83–91. [PubMed] [Google Scholar]
- De Lamballerie X., Boisson V., Reynier J.C., Enault S., Charrel R.N., Flahault A., et al. On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis. 2008;8(6):837–839. doi: 10.1089/vbz.2008.0049. [DOI] [PubMed] [Google Scholar]
- Diallo M., Thonnon J., Traore-Lamizana M., Fontenille D. Vectors of Chikungunya virus in Senegal: current data and transmission cycles. Am. J. Trop. Med. Hyg. 1999;60(2):281–286. doi: 10.4269/ajtmh.1999.60.281. [DOI] [PubMed] [Google Scholar]
- Dickens B.L., Yang J., Cook A.R., Carrasco L.R. Time to empower release of insects carrying a dominant lethal and Wolbachia against Zika. Open Forum Infect. Dis. 2016;3(2):ofw103. doi: 10.1093/ofid/ofw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont Y., Chiroleu F. Vector control for the Chikungunya disease. Math. Biosci. Eng. 2010;7(2):313–345. doi: 10.3934/mbe.2010.7.313. [DOI] [PubMed] [Google Scholar]
- Eckels K.H., Harrison V.R., Hetrick F.M. Chikungunya virus vaccine prepared by Tween-ether extraction. Appl. Microbiol. 1970;19(2):321–325. doi: 10.1128/am.19.2.321-325.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economopoulou A., Dominguez M., Helynck B., Sissoko D., Wichmann O., Quenel P., et al. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005-2006 outbreak on Réunion. Epidemiol. Infect. 2009;137(4):534–541. doi: 10.1017/S0950268808001167. [DOI] [PubMed] [Google Scholar]
- Edelman R., Tacket C.O., Wasserman S.S., Bodison S.A., Perry J.G., Mangiafico J.A. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am. J. Trop. Med. Hyg. 2000;62(6):681–685. doi: 10.4269/ajtmh.2000.62.681. [DOI] [PubMed] [Google Scholar]
- Edwards C.J., Welch S.R., Chamberlain J., Hewson R., Tolley H., Cane P.A., et al. Molecular diagnosis and analysis of Chikungunya virus. J. Clin. Virol. 2007;39(4):271–275. doi: 10.1016/j.jcv.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Erasmus Jesse H., Auguste Albert J., Kaelber Jason T., Luo Huanle, Rossi Shannan L., Fenton Karla, et al. A Chikungunya fever vaccine utilizing an insect-specific virus platform. Nat. Med. 2017;23(February (2)):192–199. doi: 10.1038/nm.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernould S., Walters H., Alessandri J.L., Llanas B., Jaffar M.C., Robin S., et al. [Chikungunya in paediatrics: epidemic of 2005-2006 in Saint-Denis, Reunion Island] Arch. Pediatr. 2008;15(3):253–262. doi: 10.1016/j.arcped.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Ferreira C.P., Yang H.M., Esteva L. Assessing the suitability of sterile insect technique applied to Aedes aegypti. J. Biol. Syst. 2008;16(4):565–577. [Google Scholar]
- Figueiredo M.L., Figueiredo L.T. Emerging alphaviruses in the Americas: Chikungunya and Mayaro. Rev. Soc. Bras. Med. Trop. 2014;47(6):677–683. doi: 10.1590/0037-8682-0246-2014. [DOI] [PubMed] [Google Scholar]
- Furuya-Kanamori L., Liang S., Milinovich G., Soares Magalhaes R.J., Clements A.C., Hu W., et al. Co-distribution and co-infection of chikungunya and dengue viruses. BMC Infect. Dis. 2016;16:84. doi: 10.1186/s12879-016-1417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Arriaza J., Cepeda V., Hallengärd D., Sorzano C., Kümmerer B.M., Liljeström P., et al. A novel poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and protects mice against chikungunya infection. J. Virol. 2014;88(6):3527–3547. doi: 10.1128/JVI.03418-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgin N.K., İşçimen R., Yılmaz E., Kahveci Ş, Kutlay O. Guillain-Barré syndrome and human immunodeficiency virus. Turk. J. Anaesthesiol. Reanim. 2014;42(2):100–102. doi: 10.5152/TJAR.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giry C., Roquebert B., Li-Pat-Yuen G., Gasque P., Jaffar-Bandjee M.C. Simultaneous detection of chikungunya virus, dengue virus and human pathogenic Leptospira genomes using a multiplex TaqMan® assay. BMC Microbiol. 2017;17(1):105. doi: 10.1186/s12866-017-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopakumar H., Ramachandran S. Congenital chikungunya. J. Clin. Neonatol. 2012;1(3):155–156. doi: 10.4103/2249-4847.101704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchakov R., Wang E., Leal G., Forrester N.L., Plante K., Rossi S.L., et al. Attenuation of Chikungunya virus vaccine strain 181/clone 25 is determined by two amino acid substitutions in the E2 envelope glycoprotein. J. Virol. 2012;86(11):6084–6096. doi: 10.1128/JVI.06449-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupil B.A., Mores C.N. A Review of Chikungunya Virus-induced Arthralgia: Clinical Manifestations, Therapeutics, and Pathogenesis. Open Rheumatol. J. 2016;10:129–140. doi: 10.2174/1874312901610010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandadam M., Caro V., Plumet S., Thiberge J.M., Souarès Y., Failloux A.B., et al. Chikungunya virus, southeastern France. Emerg Infect Dis. 2011;17(5):910–913. doi: 10.3201/eid1705.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivard P., Le Roux K., Laurent P., Fianu A., Perrau J., Gigan J., et al. Molecular and serological diagnosis of Chikungunya virus infection. Pathol. Biol. (Paris) 2007;55(10):490–494. doi: 10.1016/j.patbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Heikema A.P., Islam Z., Horst-Kreft D., Huizinga R., Jacobs B.C., Wagenaar J.A., et al. Campylobacter jejuni capsular genotypes are related to Guillain-Barré syndrome. Clin. Microbiol. Infect. 2015;21(9):852. doi: 10.1016/j.cmi.2015.05.031. e1-9. [DOI] [PubMed] [Google Scholar]
- Hemingway J., Ranson H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- Hoarau J.J., Jaffar Bandjee M.C., Trotot P. Krejbich, Das T., Li-Pat-Yuen G., Dassa B., et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 2010;184(10):5914–5927. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- Horwood P., Bande G., Dagina R., Guillaumot L., Aaskov J., Pavlin B. The threat of chikungunya in Oceania. Western Pac. Surveill. Response J. 2013;4(2):8–10. doi: 10.5365/WPSAR.2013.4.2.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy J.B. Experimental mass-rearing of the mosquitofish, Gambusia affinis. J. Am. Mosq. Control Assoc. 1985;1(3):295–298. [PubMed] [Google Scholar]
- Hua C., Combe B. Chikungunya virus-associated disease. Curr. Rheumatol. Rep. 2017;19(11):69. doi: 10.1007/s11926-017-0694-0. [DOI] [PubMed] [Google Scholar]
- Huang Y.S., Higgs S., Vanlandingham D.L. Biological Control Strategies for Mosquito Vectors of Arboviruses. Insects. 2017;8(1) doi: 10.3390/insects8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICTV . 2017. Virus Taxonomy: 2017 Release.https://talk.ictvonline.org/taxonomy/ (Accessed 11 July 2018) [Google Scholar]
- Islam J., Zaman K., Duarah S., Raju P.S., Chattopadhyay P. Mosquito repellents: an insight into the chronological perspectives and novel discoveries. Acta Trop. 2017;167:216–230. doi: 10.1016/j.actatropica.2016.12.031. [DOI] [PubMed] [Google Scholar]
- Jain M., Rai S., Chakravarti A. Chikungunya: a review. Trop. Doct. 2008;38(2):70–72. doi: 10.1258/td.2007.070019. [DOI] [PubMed] [Google Scholar]
- Jain J., Kushwah R.B.S., Singh S.S., Sharma A., Adak T., Singh O.P., et al. Evidence for natural vertical transmission of chikungunya viruses in field populations of Aedes Aegypti in Delhi and Haryana states in India-a preliminary report. Acta Trop. 2016;162:46–55. doi: 10.1016/j.actatropica.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Johnson B.W., Russell B.J., Goodman C.H. Laboratory diagnosis of chikungunya virus infections and commercial sources for diagnostic assays. J. Infect. Dis. 2016;214(suppl 5):S471–S474. doi: 10.1093/infdis/jiw274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josseran L., Paquet C., Zehgnoun A., Caillere N., Le Tertre A., Solet J.L., et al. Chikungunya disease outbreak, Reunion Island. Emerg. Infect. Dis. 2006;12(12):1994–1995. doi: 10.3201/eid1212.060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kading R.C., Borland E.M., Cranfield M., Powers A.M. Prevalence of antibodies to alphaviruses and flaviviruses in free-ranging game animals and nonhuman primates in the greater Congo basin. J. Wildl. Dis. 2013;49:587–599. doi: 10.7589/2012-08-212. [DOI] [PubMed] [Google Scholar]
- Kam Y.W., Ong E.K., Rénia L., Tong J.C., Ng L.F. Immuno-biology of Chikungunya and implications for disease intervention. Microbes Infect. 2009;11(14-15):1186–1196. doi: 10.1016/j.micinf.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kamareddine L. The biological control of the malaria vector. Toxins (Basel) 2012;4(9):748–767. doi: 10.3390/toxins4090748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P., Chu J.J. Chikungunya virus: an update on antiviral development and challenges. Drug Discov. Today. 2013;18(19-20):969–983. doi: 10.1016/j.drudis.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P., Thiruchelvan M., Lee R.C., Chen H., Chen K.C., Ng M.L., et al. Inhibition of chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob. Agents Chemother. 2013;57(1):155–167. doi: 10.1128/AAC.01467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee A.C., Yang S., Tambyah P. Atypical chikungunya virus infections in immunocompromised patients. Emerg Infect Dis. 2010;16(6):1038–1040. doi: 10.3201/eid1606.091115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Santhosh S.R., Tiwari M., Lakshmana Rao P.V., Parida M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against Chikungunya virus in vero cells. J. Med. Virol. 2010;82(5):817–824. doi: 10.1002/jmv.21663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Choe K.W., Park S., Yoon D., Ock C.Y., Hong S.W., et al. Mild form of Guillain-Barré syndrome in a patient with primary Epstein-Barr virus infection. Korean J. Intern. Med. 2016;31(6):1191–1193. doi: 10.3904/kjim.2015.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knols B.G., Bukhari T., Farenhorst M. Entomopathogenic fungi as the next-generation control agents against malaria mosquitoes. Future Microbiol. 2010;5(3):339–341. doi: 10.2217/fmb.10.11. [DOI] [PubMed] [Google Scholar]
- Kumar P. Mahesh, Murugan K., Kovendan K., Panneerselvam C., Kumar K. Prasanna, Amerasan D., et al. Mosquitocidal activity of Solanum xanthocarpum fruit extract and copepod Mesocyclops thermocyclopoides for the control of dengue vector Aedes aegypti. Parasitol. Res. 2012;111(2):609–618. doi: 10.1007/s00436-012-2876-z. [DOI] [PubMed] [Google Scholar]
- Kweka E.J., Zhou G., Gilbreath T.M., Afrane Y., Nyindo M., Githeko A.K., et al. Predation efficiency of Anopheles gambiae larvae by aquatic predators in western Kenya highlands. Parasit. Vectors. 2011;4:128. doi: 10.1186/1756-3305-4-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labadie K., Larcher T., Joubert C., Mannioui A., Delache B., Brochard P., et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Invest. 2010;120(3):894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam P.H., Boon C.S., Yng N.Y., Benjamin S. Aedes albopictus control with spray application of Bacillus thuringiensis israelensis, strain AM 65-52. Southeast Asian J. Trop. Med. Public Health. 2010;41(5):1071–1081. [PubMed] [Google Scholar]
- Lani R., Hassandarvish P., Chiam C.W., Moghaddam E., Chu J.J., Rausalu K., et al. Antiviral activity of Silymarin against Chikungunya virus. Sci. Rep. 2015;5:11421. doi: 10.1038/srep11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Coupanec A., Tchankouo-Nguetcheu S., Roux P., Khun H., Huerre M., Morales-Vargas R., et al. Co-infection of mosquitoes with Chikungunya and dengue viruses reveals modulation of the replication of both viruses in Midguts and salivary glands of Aedes aegypti mosquitoes. Int. J. Mol. Sci. 2017;18(8) doi: 10.3390/ijms18081708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun G., Chadda K., Reboux A.H., Martinet O., Gaüzère B.A. Guillain-Barré syndrome after chikungunya infection. Emerg. Infect Dis. 2009;15(3):495–496. doi: 10.3201/eid1503.071482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V.J., Chow A., Zheng X., Carrasco L.R., Cook A.R., Lye D.C., et al. Simple clinical and laboratory predictors of Chikungunya versus dengue infections in adults. PLoS Negl. Trop. Dis. 2012;6(9):e1786. doi: 10.1371/journal.pntd.0001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Seirin, Baker R.E., Gaffney E.A., White S.M. Modelling Aedes aegypti mosquito control via transgenic and sterile insect techniques: endemics and emerging outbreaks. J. Theor. Biol. 2013;331:78–90. doi: 10.1016/j.jtbi.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Leis A.A., Stokic D.S. Neuromuscular manifestations of west Nile virus infection. Front. Neurol. 2012;3:37. doi: 10.3389/fneur.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt N.H., Ramsburg H.H., Hasty S.E., Repik P.M., Cole F.E., Lupton H.W. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine. 1986;4(3):157–162. doi: 10.1016/0264-410x(86)90003-4. [DOI] [PubMed] [Google Scholar]
- Liang G., Gao X., Gould E.A. Factors responsible for the emergence of arboviruses; strategies, challenges and limitations for their control. Emerg. Microbes Infect. 2015;4(3):e18. doi: 10.1038/emi.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E.X.Y., Lee W.S., Madzokere E.T., Herrero L.J. Mosquitoes as suitable vectors for alphaviruses. Viruses. 2018;10(2) doi: 10.3390/v10020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litzba N., Schuffenecker I., Zeller H., Drosten C., Emmerich P., Charrel R., et al. Evaluation of the first commercial chikungunya virus indirect immunofluorescence test. J. Virol. Methods. 2008;149(1):175–179. doi: 10.1016/j.jviromet.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Lu Y.E., Cassese T., Kielian M. The cholesterol requirement for sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J. Virol. 1999;73(5):4272–4278. doi: 10.1128/jvi.73.5.4272-4278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum F.M., Teo T.H., Lee W.W., Kam Y.W., Rénia L., Ng L.F. An essential role of antibodies in the control of Chikungunya virus infection. J. Immunol. 2013;190(12):6295–6302. doi: 10.4049/jimmunol.1300304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden W.H. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53. II. General description and epidemiology. Trans. R. Soc. Trop. Med. Hyg. 1955;49(1):33–57. doi: 10.1016/0035-9203(55)90081-x. [DOI] [PubMed] [Google Scholar]
- Lyra P.P., Campos G.S., Bandeira I.D., Sardi S.I., Costa L.F., Santos F.R., et al. Congenital chikungunya virus infection after an outbreak in Salvador, Bahia, Brazil. AJP Rep. 2016;6(3):e299–e300. doi: 10.1055/s-0036-1587323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madariaga M., Ticona E., Resurrecion C. Chikungunya: bending over the Americas and the rest of the world. Braz. J. Infect. Dis. 2016;20(1):91–98. doi: 10.1016/j.bjid.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhiyazhagan P., Murugan K., Kumar A.Naresh, Nataraj T., Dinesh D., Panneerselvam C., et al. Sargassum muticum-synthetized silver nanoparticles: an effective control tool against mosquito vectors and bacterial pathogens. Parasitol. Res. 2015;114:4305–4317. doi: 10.1007/s00436-015-4671-0. [DOI] [PubMed] [Google Scholar]
- Manimunda S.P., Vijayachari P., Uppoor R., Sugunan A.P., Singh S.S., Rai S.K., et al. Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Trans. R. Soc. Trop. Med. Hyg. 2010;104(6):392–399. doi: 10.1016/j.trstmh.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Manrique-Saide P., Ibáñez-Bernal S., Delfín-González H., Tabla V.Parra. Mesocyclops longisetus effects on survivorship of Aedes aegypti immature stages in car tyres. Med. Vet. Entomol. 1998;12(4):386–390. doi: 10.1046/j.1365-2915.1998.00126.x. [DOI] [PubMed] [Google Scholar]
- Martínez-Pulgarín D.F., Chowdhury F.R., Villamil-Gomez W.E., Rodriguez-Morales A.J., Blohm G.M., Paniz-Mondolfi A.E. Ophthalmologic aspects of chikungunya infection. Travel Med. Infect. Dis. 2016;14(5):451–457. doi: 10.1016/j.tmaid.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Massonnet-Bruneel B., Corre-Catelin N., Lacroix R., Lees R.S., Hoang K.P., Nimmo D., et al. Fitness of transgenic mosquito Aedes aegypti males carrying a dominant lethal genetic system. PLoS One. 2013;8(5):e62711. doi: 10.1371/journal.pone.0062711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavale M., Parashar D., Sudeep A., Gokhale M., Ghodke Y., Geevarghese G., et al. Venereal transmission of chikungunya virus by Aedes aegypti mosquitoes (Diptera: culicidae) Am. J. Trop. Med. Hyg. 2010;83(6):1242–1244. doi: 10.4269/ajtmh.2010.09-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae A.W.R., Henderson B.E., Kirya B.G., Sempala S.D.K. Chikungunya virus in the entebbe area of uganda: isolations and epidemiology. Trans. R. Soc. Trop. Med. Hyg. 1971;65:152–168. doi: 10.1016/0035-9203(71)90212-4. [DOI] [PubMed] [Google Scholar]
- McIntosh B.M. Antibody against chikungunya virus in wild primates in Southern Africa. S. Afr. J. Med. Sci. 1970;35:65. [PubMed] [Google Scholar]
- Mohan A., Kiran D.H., Manohar I.C., Kumar D.P. Epidemiology, clinical manifestations, and diagnosis of Chikungunya fever: lessons learned from the re-emerging epidemic. Indian J. Dermatol. 2010;55(1):54–63. doi: 10.4103/0019-5154.60355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T.E. Reemergence of chikungunya virus. J. Virol. 2014;88(20):11644–11647. doi: 10.1128/JVI.01432-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumani K., Lankaraman K.M., Laddy D.J., Sundaram S.G., Chung C.W., Sako E., et al. Immunogenicity of novel consensus-based DNA vaccines against Chikungunya virus. Vaccine. 2008;26(40):5128–5134. doi: 10.1016/j.vaccine.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonas A.D., Harley D., Purdie D.M., Pandeya N., Vecchio P.C., Farmer J.F., et al. Corticosteroid therapy in an Alphaviral arthritis. J. Clin. Rheumatol. 2004;10(6):326–330. doi: 10.1097/01.rhu.0000147052.11190.36. [DOI] [PubMed] [Google Scholar]
- Nakkhara P., Chongsuvivatwong V., Thammapalo S. Risk factors for symptomatic and asymptomatic chikungunya infection. Trans. R. Soc. Trop. Med. Hyg. 2013;107(12):789–796. doi: 10.1093/trstmh/trt083. [DOI] [PubMed] [Google Scholar]
- Ngoagouni C., Kamgang B., Kazanji M., Paupy C., Nakouné E. Potential of Aedes aegypti and Aedes albopictus populations in the Central African Republic to transmit enzootic chikungunya virus strains. Parasit. Vectors. 2017;10(1):164. doi: 10.1186/s13071-017-2101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak R.J., Gubler D.J., Underwood D. Evaluation of slow-release formulations of temephos (Abate) and Bacillus thuringiensis var. israelensis for the control of Aedes aegypti in Puerto Rico. J Am Mosq Control Assoc. 1985;1(4):449–453. [PubMed] [Google Scholar]
- Nunes M.R., Faria N.R., de Vasconcelos J.M., Golding N., Kraemer M.U., de Oliveira L.F., et al. Emergence and potential for spread of Chikungunya virus in Brazil. BMC Med. 2015;13:102. doi: 10.1186/s12916-015-0348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehler E., Fournier E., Leparc-Goffart I., Larre P., Cubizolle S., Sookhareea C., et al. Increase in cases of Guillain-Barré syndrome during a Chikungunya outbreak, French Polynesia, 2014 to 2015. Euro Surveil. 2015;20(48):30079. doi: 10.2807/1560-7917.ES.2015.20.48.30079. [DOI] [PubMed] [Google Scholar]
- Okabayashi T., Sasaki T., Masrinoul P., Chantawat N., Yoksan S., Nitatpattana N., et al. Detection of chikungunya virus antigen by a novel rapid immunochromatographic test. J. Clin. Microbiol. 2015;53(2):382–388. doi: 10.1128/JCM.02033-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozden S., Lucas-Hourani M., Ceccaldi P.E., Basak A., Valentine M., Benjannet S., et al. Inhibition of Chikungunya virus infection in cultured human muscle cells by furin inhibitors: impairment of the maturation of the E2 surface glycoprotein. J. Biol. Chem. 2008;283(32):21899–218908. doi: 10.1074/jbc.M802444200. [DOI] [PubMed] [Google Scholar]
- Pabbaraju K., Wong S., Gill K., Fonseca K., Tipples G.A., Tellier R. Simultaneous detection of Zika, Chikungunya and Dengue viruses by a multiplex real-time RT-PCR assay. J. Clin. Virol. 2016;83:66–71. doi: 10.1016/j.jcv.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Padmakumar B., Jayan J.B., Menon R., Krishnankutty B., Payippallil R., Nishab R.S. Comparative evaluation of four therapeutic regimes in chikungunya arthritis: a prospective randomized parallelgroup study. Indian J. Rheumatol. 2009;4(3):4–101. [Google Scholar]
- PAHO . 2017. Tool for the Diagnosis and Care of Patients with Suspected Arboviral Diseases.http://iris.paho.org/xmlui/bitstream/handle/123456789/33895/9789275119365_eng.pdf?sequence=1&isAllowed=y (Accessed 11 July 2018) [Google Scholar]
- Panning M., Grywna K., van Esbroeck M., Emmerich P., Drosten C. Chikungunya fever in travelers returning to Europe from the Indian Ocean region, 2006. Emerg. Infect Dis. 2008;14(3):416–422. doi: 10.3201/eid1403.070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J., Sammon M., Garg M. Dengue, Zika and Chikungunya: emerging arboviruses in the new world. West. J. Emerg. Med. 2016;17(6):671–679. doi: 10.5811/westjem.2016.9.30904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula A.R., Carolino A.T., Paula C.O., Samuels R.I. The combination of the entomopathogenic fungus Metarhizium anisopliae with the insecticide Imidacloprid increases virulence against the dengue vector Aedes aegypti (Diptera: Culicidae) Parasit. Vectors. 2011;4:8. doi: 10.1186/1756-3305-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula A.R., Carolino A.T., Silva C.P., Samuels R.I. Susceptibility of adult female Aedes aegypti (Diptera: culicidae) to the entomopathogenic fungus Metarhizium anisopliae is modified following blood feeding. Parasit. Vectors. 2011;4:91. doi: 10.1186/1756-3305-4-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavela R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus say larvae. Parasitol. Res. 2015;114(10):3835–3853. doi: 10.1007/s00436-015-4614-9. [DOI] [PubMed] [Google Scholar]
- Pereira B.B., Oliveira E.A. Determinação do potencial larvófago de Poecilia reticulata em condições domésticas de controle biológico. Cad. saúde colet. 2014;22(3):241–245. [Google Scholar]
- Perri S., Driver D.A., Gardner J.P., Sherrill S., Belli B.A., Dubensky T.W., et al. Replicon vectors derived from Sindbis virus and Semliki forest virus that establish persistent replication in host cells. J. Virol. 2000;74(20):9802–9807. doi: 10.1128/jvi.74.20.9802-9807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P.E. Guillain-Barré syndrome and Epstein-Barr virus. Lancet. 1973;1(7805):720. doi: 10.1016/s0140-6736(73)91503-1. [DOI] [PubMed] [Google Scholar]
- Pialoux G., Gaüzère B.A., Jauréguiberry S., Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect. Dis. 2007;7(5):319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- Plante K., Wang E., Partidos C.D., Weger J., Gorchakov R., Tsetsarkin K., et al. Novel chikungunya vaccine candidate with an IRES-based attenuation and host range alteration mechanism. PLoS Pathog. 2011;7(7):e1002142. doi: 10.1371/journal.ppat.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante K.S., Rossi S.L., Bergren N.A., Seymour R.L., Weaver S.C. Extended preclinical safety, efficacy and stability testing of a live-attenuated chikungunya vaccine candidate. PLoS Negl. Trop. Dis. 2015;9(9):e0004007. doi: 10.1371/journal.pntd.0004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjala L., Utt A., Varjak M., Lulla A., Merits A., Ahola T., et al. Inhibitors of alphavirus entry and replication identified with a stable Chikungunya replicon cell line and virus-based assays. PLoS One. 2011;6(12):e28923. doi: 10.1371/journal.pone.0028923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo Y.S., Rudd P.A., Gardner J., Wilson J.A., Larcher T., Colle M.A., et al. Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS Negl. Trop. Dis. 2014;8(12):e3354. doi: 10.1371/journal.pntd.0003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers A.M., Brault A.C., Tesh R.B., Weaver S.C. Re-emergence of Chikungunya and O’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J. Gen. Virol. 2000;81(Pt 2):471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- Pruetz J.D., Socha A., Kante D. New range record for the lesser spot-nosed Guenon (Cercopithecus petaurista) in southeastern Senegal. Afr. Primates. 2010;7:64–66. [Google Scholar]
- Rampal, Sharda M., Meena H. Neurological complications in Chikungunya fever. J. Assoc. Physicians India. 2007;55:765–769l. PMID: 18290551. [PubMed] [Google Scholar]
- Ramsauer K., Schwameis M., Firbas C., Müllner M., Putnak R.J., Thomas S.J., et al. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect. Dis. 2015;15(5):519–527. doi: 10.1016/S1473-3099(15)70043-5. [DOI] [PubMed] [Google Scholar]
- Rawlins S.C., Martinez R., Wiltshire S., Clarke D., Prabhakar P., Spinks M. Evaluation of Caribbean strains of Macrocyclops and Mesocyclops (Cyclopoida:cyclopidae) as biological control tools for the dengue vector Aedes aegypti. J. Am. Mosq. Control Assoc. 1997;13(1):18–23. [PubMed] [Google Scholar]
- Reddy V., Ravi V., Desai A., Parida M., Powers A.M., Johnson B.W. Utility of IgM ELISA, TaqMan real-time PCR, reverse transcription PCR, and RT-LAMP assay for the diagnosis of Chikungunya fever. J. Med. Virol. 2012;84(11):1771–1778. doi: 10.1002/jmv.23406. [DOI] [PubMed] [Google Scholar]
- Resnik D.B. Ethical issues in field trials of genetically modified disease-resistant mosquitoes. Dev. World Bioeth. 2014;14(1):37–46. doi: 10.1111/dewb.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza G., Nicoletti L., Angelini R., Romi R., Finarelli A.C., Panning M., et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370(9602):1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- Ritchie S.A., Rapley L.P., Benjamin S. Bacillus thuringiensis var. Israelensis (Bti) provides residual control of Aedes aegypti in small containers. Am. J. Trop. Med. Hyg. 2010;82(6):1053–1059. doi: 10.4269/ajtmh.2010.09-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin S., Ramful D., Zettor J., Benhamou L., Jaffar-Bandjee M.C., Rivière J.P., et al. Severe bullous skin lesions associated with Chikungunya virus infection in small infants. Eur. J. Pediatr. 2010;169(1):67–72. doi: 10.1007/s00431-009-0986-0. [DOI] [PubMed] [Google Scholar]
- Ross R.W. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J. Hyg. (Lond) 1956;54(2):177–191. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C.J., Adams A.P., Wang E., Plante K., Gorchakov R., Seymour R.L., et al. Chikungunya vaccine candidate is highly attenuated and protects nonhuman primates against telemetrically monitored disease following a single dose. J. Infect. Dis. 2014;209(12):1891–1899. doi: 10.1093/infdis/jiu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozé B., Najioullah F., Fergé J.L., Dorléans F., Apetse K., Barnay J.L., et al. Guillain-Barré syndrome associated with zika virus infection in Martinique in 2016: a prospective study. Clin. Infect. Dis. 2017;65(9):1462–1468. doi: 10.1093/cid/cix588. [DOI] [PubMed] [Google Scholar]
- Rückert C., Weger-Lucarelli J., Garcia-Luna S.M., Young M.C., Byas A.D., Murrieta R.A., et al. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat. Commun. 2017;8:15412. doi: 10.1038/ncomms15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankari T., Hoti S.L., Govindaraj V., Das P.K. Chikungunya and respiratory viral infections. Lancet Infect. Dis. 2008;8(1):3–4. doi: 10.1016/S1473-3099(07)70295-5. [DOI] [PubMed] [Google Scholar]
- Schaper S. Evaluation of Costa Rican copepods (Crustacea: eudecapoda) for larval Aedes aegypti control with special reference to Mesocyclops thermocyclopoides. J. Am. Mosq. Control Assoc. 1999;15(4):510–519. [PubMed] [Google Scholar]
- Schilte C., Staikowsky F., Staikovsky F., Couderc T., Madec Y., Carpentier F., et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl. Trop. Dis. 2013;7(3):e2137. doi: 10.1371/journal.pntd.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuffenecker I., Iteman I., Michault A., Murri S., Frangeul L., Vaney M.C., et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3(7):e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng C.M., Setha T., Nealon J., Socheat D., Chantha N., Nathan M.B. Community-based use of the larvivorous fish Poecilia reticulata to control the dengue vector Aedes aegypti in domestic water storage containers in rural Cambodia. J. Vector Ecol. 2008;33(1):139–144. doi: 10.3376/1081-1710(2008)33[139:cuotlf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sergon K., Yahaya A.A., Brown J., Bedja S.A., Mlindasse M., Agata N., et al. Seroprevalence of Chikungunya virus infection on Grande Comore Island, union of the Comoros, 2005. Am. J. Trop. Med. Hyg. 2007;76(6):1189–1193. [PubMed] [Google Scholar]
- Sergon K., Njuguna C., Kalani R., Ofula V., Onyango C., Konongoi L.S., et al. Seroprevalence of Chikungunya virus (CHIKV) infection on Lamu Island, Kenya, 2004. Am. J. Trop. Med. Hyg. 2008;78(October (2)):333–337. [PubMed] [Google Scholar]
- Sharma S., Dash P.K., Santhosh S.R., Shukla J., Parida M., Rao P.V. Development of a quantitative competitive reverse transcription polymerase chain reaction (QC-RT-PCR) for detection and quantitation of Chikungunya virus. Mol. Biotechnol. 2010;45(1):49–55. doi: 10.1007/s12033-009-9238-9. [DOI] [PubMed] [Google Scholar]
- Sharma A., Gupta P., Maheshwari R.K. Inactivation of Chikungunya virus by 1,5 iodonapthyl azide. Virol. J. 2012;9:301. doi: 10.1186/1743-422X-9-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulse C.D., Semlitsch R.D., Trauth K.M. Mosquitofish dominate amphibian and invertebrate community development in experimental wetlands. J. Appl. Ecol. 2013;50(5):1244–1256. [Google Scholar]
- Silva L.A., Dermody T.S. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J. Clin. Invest. 2017;127(3):737–749. doi: 10.1172/JCI84417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon F., Savini H., Parola P. Chikungunya: a paradigm of emergence and globalization of vector-borne diseases. Med. Clin. North Am. 2008;92(6):1323–1343. doi: 10.1016/j.mcna.2008.07.008. ix. [DOI] [PubMed] [Google Scholar]
- Simon F., Javelle E., Oliver M., Leparc-Goffart I., Marimoutou C. Chikungunya virus infection. Curr. Infect. Dis. Rep. 2011;13(3):218–228. doi: 10.1007/s11908-011-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon O., Billot S., Guyon D., Daures M., Descloux E., Gourinat A.C., et al. Early Guillain-Barré syndrome associated with acute dengue fever. J. Clin. Virol. 2016;77:29–31. doi: 10.1016/j.jcv.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Sissoko D., Malvy D., Ezzedine K., Renault P., Moscetti F., Ledrans M., et al. Post-epidemic Chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl. Trop. Dis. 2009;3(3):e389. doi: 10.1371/journal.pntd.0000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley C., Erasmus J.H., Chesson C.B., Beasley D.W.C. Status of research and development of vaccines for chikungunya. Vaccine. 2016;34(26):2976–2981. doi: 10.1016/j.vaccine.2016.03.076. [DOI] [PubMed] [Google Scholar]
- Staples J.E., Fischer M. Chikungunya virus in the Americas--what a vectorborne pathogen can do. N. Engl. J. Med. 2014;371(10):887–889. doi: 10.1056/NEJMp1407698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples J.E., Breiman R.F., Powers A.M. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin. Infect. Dis. 2009;49(6):942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- Steger C.M., Antretter H., Höfer D. Guillain-Barré syndrome due to CMV reactivation after cardiac transplantation. Case Rep. Cardiol. 2012;2012:506290. doi: 10.1155/2012/506290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard P.K. Managing Aedes aegypti populations in the first Zika transmission zones in the continental United States. Acta Trop. 2018;187:108–118. doi: 10.1016/j.actatropica.2018.07.031. [DOI] [PubMed] [Google Scholar]
- Subudhi B.B., Chattopadhyay S., Mishra P., Kumar A. Current strategies for inhibition of chikungunya infection. Viruses. 2018;10(5) doi: 10.3390/v10050235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sujitha V., Murugan K., Paulpandi M., Panneerselvam C., Suresh U., Roni M., et al. Green synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol. Res. 2015;114:3315–3325. doi: 10.1007/s00436-015-4556-2. [DOI] [PubMed] [Google Scholar]
- Tandale B.V., Sathe P.S., Arankalle V.A., Wadia R.S., Kulkarni R., Shah S.V., et al. Systemic involvements and fatalities during Chikungunya epidemic in India, 2006. J. Clin. Virol. 2009;46(2):145–149. doi: 10.1016/j.jcv.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Thiberville S.D., Moyen N., Dupuis-Maguiraga L., Nougairede A., Gould E.A., Roques P., et al. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013;99(3):345–370. doi: 10.1016/j.antiviral.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari M., Parida M., Santhosh S.R., Khan M., Dash P.K., Rao P.V. Assessment of immunogenic potential of Vero adapted formalin inactivated vaccine derived from novel ECSA genotype of Chikungunya virus. Vaccine. 2009;27(18):2513–2522. doi: 10.1016/j.vaccine.2009.02.062. [DOI] [PubMed] [Google Scholar]
- Tretyakova I., Hearn J., Wang E., Weaver S., Pushko P. DNA vaccine initiates replication of live attenuated chikungunya virus in vitro and elicits protective immune response in mice. J. Infect. Dis. 2014;209(12):1882–1890. doi: 10.1093/infdis/jiu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin K., Higgs S., McGee C.E., De Lamballerie X., Charrel R.N., Vanlandingham D.L. Infectious clones of Chikungunya virus (La Réunion isolate) for vector competence studies. Vector Borne Zoonotic Dis. 2006;6(4):325–337. doi: 10.1089/vbz.2006.6.325. [DOI] [PubMed] [Google Scholar]
- Tsetsarkin K.A., Vanlandingham D.L., McGee C.E., Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin K.A., Chen R., Sherman M.B., Weaver S.C. Chikungunya virus: evolution and genetic determinants of emergence. Curr. Opin. Virol. 2011;1(4):310–317. doi: 10.1016/j.coviro.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Doel P., Volz A., Roose J.M., Sewbalaksing V.D., Pijlman G.P., van Middelkoop I., et al. Recombinant modified vaccinia virus Ankara expressing glycoprotein E2 of Chikungunya virus protects AG129 mice against lethal challenge. PLoS Negl. Trop. Dis. 2014;8(9):e3101. doi: 10.1371/journal.pntd.0003101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijl-Richter M.K., Hoornweg T.E., Rodenhuis-Zybert I.A., Smit J.M. Early events in Chikungunya virus infection-from virus cell binding to membrane fusion. Viruses. 2015;7(7):3647–3674. doi: 10.3390/v7072792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashishtha M., Phalen T., Marquardt M.T., Ryu J.S., Ng A.C., Kielian M. A single point mutation controls the cholesterol dependence of Semliki Forest virus entry and exit. J. Cell Biol. 1998;140(1):91–99. doi: 10.1083/jcb.140.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Rúa A., Zouache K., Girod R., Failloux A.B., Lourenço-de-Oliveira R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J. Virol. 2014;88(11):6294–6306. doi: 10.1128/JVI.00370-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Rúa A., Lourenço-de-Oliveira R., Mousson L., Vazeille M., Fuchs S., Yébakima A., et al. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl. Trop. Dis. 2015;9(5):e0003780. doi: 10.1371/journal.pntd.0003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan A., Ghorpade R.P., Chopra A. Cytokines in acute chikungunya. PLoS One. 2014;9(10):e111305. doi: 10.1371/journal.pone.0111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamil-Gómez W.E., Rodríguez-Morales A.J., Uribe-García A.M., González-Arismendy E., Castellanos J.E., Calvo E.P., et al. Zika, dengue, and chikungunya co-infection in a pregnant woman from Colombia. Int. J. Infect. Dis. 2016;51:135–138. doi: 10.1016/j.ijid.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Volk S.M., Chen R., Tsetsarkin K.A., Adams A.P., Garcia T.I., Sall A.A., et al. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J. Virol. 2010;84(13):6497–6504. doi: 10.1128/JVI.01603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu S.N., Nguyen T.Y., Kay B.H., Marten G.G., Reid J.W. Eradication of Aedes aegypti from a village in Vietnam, using copepods and community participation. Am. J. Trop. Med. Hyg. 1998;59(4):657–660. doi: 10.4269/ajtmh.1998.59.657. [DOI] [PubMed] [Google Scholar]
- Walker T., Johnson P.H., Moreira L.A., Iturbe-Ormaetxe I., Frentiu F.D., McMeniman C.J., et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476(7361):450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- Walton W.E. Larvivorous fish including Gambusia. J. Am. Mosq. Control Assoc. 2007;23(2 Suppl):184–220. doi: 10.2987/8756-971X(2007)23[184:LFIG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Wang E., Volkova E., Adams A.P., Forrester N., Xiao S.Y., Frolov I., et al. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine. 2008;26(39):5030–5039. doi: 10.1016/j.vaccine.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Suhrbier A., Penn-Nicholson A., Woraratanadharm J., Gardner J., Luo M., et al. A complex adenovirus vaccine against chikungunya virus provides complete protection against viraemia and arthritis. Vaccine. 2011;29(15):2803–2809. doi: 10.1016/j.vaccine.2011.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C. Evolutionary influences in arboviral disease. Curr. Top. Microbiol. Immunol. 2006;299:285–314. doi: 10.1007/3-540-26397-7_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C., Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015;372(13):1231–1239. doi: 10.1056/NEJMra1406035. [DOI] [PubMed] [Google Scholar]
- Weinbren M.P., Haddow A.J., Williams M.C. The occurrence of Chikungunya virus in Uganda. I. Isolation from mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 1958;52(3):253–257. doi: 10.1016/0035-9203(58)90084-1. [DOI] [PubMed] [Google Scholar]
- WHO . 2008. Guidelines on Clinical Management of Chikungunya Fever Guidelines on Clinical Management.http://www.wpro.who.int/mvp/topics/ntd/Clinical_Mgnt_Chikungunya_WHO_SEARO.pdf (Accessed 11 July 2018) [Google Scholar]
- WHO . 2016. Monitoring and Managing Insecticide Resistance in Aedes mosquito Populations-Interim Guidance for Entomologists.http://www.who.int/csr/resources/publications/zika/insecticide-resistance/en/ (Accessed 11 July 2018) [Google Scholar]
- WHO . 2018. Chikungunya.http://www.who.int/csr/don/archive/disease/chikungunya/en/ (Accessed 11 July 2018) [Google Scholar]
- Wichit S., Hamel R., Bernard E., Talignani L., Diop F., Ferraris P., et al. Imipramine inhibits Chikungunya virus replication in human skin fibroblasts through interference with Intracellular Cholesterol Trafficking. Sci Rep. 2017;7(1):3145. doi: 10.1038/s41598-017-03316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke A.B.B., Beier J.C., Benelli G. Transgenic mosquitoes - fact or fiction? Trends Parasitol. 2018;34(6):456–465. doi: 10.1016/j.pt.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Wintachai P., Wikan N., Kuadkitkan A., Jaimipuk T., Ubol S., Pulmanausahakul R., et al. Identification of prohibitin as a Chikungunya virus receptor protein. J. Med. Virol. 2012;84(11):1757–1770. doi: 10.1002/jmv.23403. [DOI] [PubMed] [Google Scholar]
- Wintachai P., Thuaud F., Basmadjian C., Roytrakul S., Ubol S., Désaubry L., et al. Assessment of flavaglines as potential chikungunya virus entry inhibitors. Microbiol. Immunol. 2015;59(3):129–141. doi: 10.1111/1348-0421.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaren O., Alto B.W., Gangodkar P.V., Ranade S.R., Patil K.N., Bradley K.M., et al. Point of sampling detection of Zika virus within a multiplexed kit capable of detecting dengue and chikungunya. BMC Infect. Dis. 2017;17(1):293. doi: 10.1186/s12879-017-2382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller H., Van Bortel W., Sudre B. Chikungunya: its history in Africa and Asia and its spread to new regions in 2013-2014. J. Infect. Dis. 2016;214(Suppl. 5):S436–S440. doi: 10.1093/infdis/jiw391. [DOI] [PubMed] [Google Scholar]
- Zhang D., Lees R.S., Xi Z., Gilles J.R., Bourtzis K. Combining the sterile insect technique with wolbachia-based approaches: II--a safer approach to Aedes albopictus population suppression programmes, designed to minimize the consequences of inadvertent female release. PLoS One. 2015;10(8):e0135194. doi: 10.1371/journal.pone.0135194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Zheng X., Xi Z., Bourtzis K., Gilles J.R. Combining the sterile insect technique with the incompatible insect technique: I-impact of wolbachia infection on the fitness of triple- and double-infected strains of Aedes albopictus. PLoS One. 2015;10(4):e0121126. doi: 10.1371/journal.pone.0121126. [DOI] [PMC free article] [PubMed] [Google Scholar]