Abstract
The post-transplant outcomes of patients with Model for End-stage Liver Disease (MELD) score primarily driven by renal dysfunction are poorly understood. This was a retrospective cohort study of liver transplant (LT) alone recipients between 2005-2017 using the United Network for Organ Sharing (UNOS) database. The proportion of MELD Sodium score attributable to creatinine (“KidneyMELD”) was calculated: (9.57 x ln (creatinine) x 100) / (MELD-Na – 6.43). The association of KidneyMELD with (1) all-cause mortality and (2) estimated glomerular filtration rate (eGFR) ≤30mL/min/1.732 at 1-year post-LT were evaluated. Recipients with KidneyMELD ≥50% had a 52% higher risk of post-LT mortality (adjusted hazard ratio 1.52 vs KidneyMELD 0%, 95% CI: 1.36-1.69; p<0.001). This risk was significantly greater for older patients, particularly when >50 years at LT (interaction p<0.001). KidneyMELD ≥50% was also associated with an 11-fold increase in the odds of advanced renal dysfunction at 1-year post-LT (adjusted odds ratio 11.53 vs KidneyMELD 0%; 95% CI 8.9-14.93; p<0.001). Recipients prioritized for LT primarily on the basis of renal dysfunction have marked post-LT mortality and morbidity independent of MELD Sodium score. The implications of these results in the context of the new UNOS ‘safety net’ kidney transplant policy require further study.
Keywords: liver transplantation, kidney diseases, liver diseases
Introduction
In February 2002, the liver transplant community adopted the Model for End-stage Liver Disease (MELD) score to prioritize candidates awaiting liver transplantation (LT). The original MELD score was developed as a means to differentiate patients at risk for worse outcomes after transjugular intrahepatic portosystemic shunt, and was derived using covariate coefficients from a statistical model in which patients with intrinsic renal disease were excluded(1). Subsequent studies demonstrated good predictive power with regards to short-term waitlist mortality using the original coefficients irrespective of pre-LT renal function (2, 3). However, from the standpoint of resource utilization and transplant equity, the adoption of the MELD additionally led to a significant rise in the prevalence of candidates with renal dysfunction at and after LT(4-6).
The issue of pre-LT renal function, MELD score and outcomes after LT is complex. The MELD score cannot differentiate between acute and chronic kidney dysfunction, which have differing impacts on waitlist and post-LT survival(7-10). Ideally, waitlist prioritization on the basis of creatinine should favor candidates with hepatorenal syndrome (HRS), who are known to have significant waitlist mortality and in whom LT can reverse renal dysfunction. The inclusion of serum sodium into the MELD (MELD-Na) score in 2016 may have improved this due to the relationship between HRS and hyponatremia, though this has not been specifically studied(11, 12). Simultaneous liver-kidney (SLK) transplantation remains an option for candidates with advanced renal dysfunction who are at low likelihood of renal recovery after LT, though the survival benefit of SLK over LT alone in such patients has also been questioned(13, 14).
The decision to pursue LT alone in candidates with advanced renal dysfunction is ultimately at the discretion of the transplant center, with decisions frequently based on limited objective data. The primary aims of this study were to evaluate the risk of post-LT mortality and advanced post-LT renal insufficiency (defined as an estimated glomerular filtration rate (eGFR) of ≤30mL/min/1.732 at 1 year post-LT) in LT alone recipients whose MELD-Na score is primarily driven by elevated creatinine. Secondarily, this study evaluated the post-LT outcomes of recipients ineligible for SLK transplant by current United Network for Organ Sharing (UNOS) guidelines in whom MELD-Na is predominantly driven by renal dysfunction.
Methods
Study population & definitions
This was a retrospective cohort study of adult (≥18 years) initial deceased donor LT (DDLT) alone recipients between 2005-2017 using the UNOS database. Subjects were excluded if: 1) they underwent living donor liver transplantation; 2) they had received exception points at any time during waitlisting; 3) they were listed as Status 1 (i.e., emergent LT). In these candidates, laboratory MELD-Na score does not determine organ allocation.
The proportion of MELD-Na attributable to creatinine, defined as the “KidneyMELD” and expressed as a percentage, was obtained using the following equation: (9.57 x ln(creatinine) x 100) / (MELD-Na – 6.43). Sample KidneyMELD values are shown in Supplementary Table 1. KidneyMELD was evaluated in categorical (0%, 1-24%, 25-49% and ≥50%) and binary (<50% vs ≥50%) forms. The laboratory MELD-Na score was calculated using the equation provided by UNOS(12). Creatinine values and MELD-Na scores were capped at 4mg/dL and 40, respectively, as per UNOS allocation policy. The minimum creatinine value was set at 1mg/dL, as UNOS policy does not distinguish lower values. As per UNOS policy, subjects on dialysis at the time of LT were coded as having a creatinine of 4mg/dL. All laboratory values were obtained at the time of LT.
Among recipients waitlisted at least 90 days, pre-LT chronic kidney disease (CKD) was defined as the presence of at least 2 recorded eGFR values <60mL/min/1.732 at least 90 days apart prior to LT date with all eGFR values recorded in between also <60mL/min/1.732. Recipients’ eGFR was calculated using the Modification of Diet in Renal Disease 4 (MDRD-4) formula(15).
Statistical analysis
Demographic and clinical characteristics of recipients with KidneyMELD <50% and ≥50% were compared using Chi-squared tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Temporal and geographic trends by UNOS region, as well as center variability in the proportion of recipients transplanted with KidneyMELD ≥50% were evaluated.
Post-LT cause of death according to KidneyMELD at LT was evaluated descriptively. Survival analysis was used to study the association between KidneyMELD and all-cause post-LT mortality. Recipients were censored at the last date of follow-up in the UNOS database or at the end of the study period (i.e., December 31, 2017). The proportional hazards (PH) assumption was assessed graphically using scaled Schoenfeld residual plots. Unadjusted post-LT survival according to KidneyMELD as a categorical variable was evaluated using Kaplan-Meier plots and compared using the log-rank test.
Multivariable Cox PH models were used to obtain adjusted hazard ratios (aHR) for mortality according to KidneyMELD. Pre-specified interactions between KidneyMELD and the following covariates were investigated: age, gender, race/ethnicity, non-alcoholic steatohepatitis (NASH) vs non-NASH etiology of liver disease, MELD-Na score and diabetes. Multivariable models were adjusted for the following covariates: age (continuous), gender, race/ethnicity, primary etiology of liver disease, laboratory MELD-Na score (continuous), serum albumin at LT (continuous), ascites (categorical: none, mild, moderate), diabetes (binary), dialysis at LT (binary), location prior to LT (home, hospital ward, hospital intensive care), donor age (continuous) and receipt of organ donated after circulatory determination of death (DCDD).
Advanced renal insufficiency after LT was defined as an eGFR of ≤30mL/min/1.732 by MDRD-4 (binary yes/no). Logistic regression models evaluated the association of KidneyMELD and eGFR of ≤30mL/min/1.732 at 1 year post-LT. Multivariable models were adjusted for the same covariates as those specified above in the post-LT survival Cox PH models. Adjusted odds ratios (aOR) were obtained from these analyses. As a secondary analysis the pursuit of kidney transplantation after LT alone in those with KidneyMELD ≥50% was described.
Exploratory analysis
In an exploratory analysis, the unadjusted post-LT survival of recipients with CKD and KidneyMELD ≥50% who were ineligible for SLK was compared to that of SLK recipients transplanted during the same period (2005-2017). Recipients were defined as eligible for SLK according to UNOS policy if they had CKD as defined above and had an eGFR ≤30mL/min/1.732 or dialysis support at the time of LT(12). Therefore, recipients ineligible for SLK were those waitlisted at least 90 days, with or without CKD, and with eGFR at LT >30mL/min/1.732 without dialysis support. Kaplan-Meier curves and the log-rank test were used.
This study was reviewed by the Institutional Review Board at the University of Pennsylvania and received exempt status.
Results
Between 2005-2017, a total of 34,949 patients underwent initial DDLT alone without prior exception points, of which 1,421 (4.1%) had a KidneyMELD at LT of ≥50%. Basic demographic and clinical characteristics of DDLT recipients with and without KidneyMELD at LT of ≥50% are shown in Table 1. Recipients with KidneyMELD at LT ≥50% were more likely to have NASH (28.7% vs 21%) and less likely to have auto-immune liver disease (6.9% vs 14.2%; p<0.001). These recipients were also more likely to have diabetes pre-LT (39.4% vs 23%; p<0.001) and moderate ascites (51.7% vs 39.9%; p<0.001). Among those waitlisted at least 90 days pre-LT (N=12,640), 68.9% of those with KidneyMELD ≥50% had pre-LT CKD.
Table 1:
Demographic and clinical characteristics of DDLT recipients with and without KidneyMELD at LT ≥50% between 2005-2017 (N=34,920)
| KidneyMELD at LT <50% (N=33,499) |
KidneyMELD at LT ≥50% (N=1,421) |
p-value | |
|---|---|---|---|
| Male gender, % | 65.9 | 66.0 | 0.9 |
| Age at LT, median (IQR) | 55 (48-61) | 58 (53-63) | <0.001 |
| Liver disease, % | <0.001 | ||
| HCV | 32.1 | 31.0 | |
| Alcohol | 24.7 | 27.4 | |
| NASH | 21.0 | 28.7 | |
| AI disease* | 14.2 | 6.9 | |
| HBV | 2.4 | 2.0 | |
| Other | 5.8 | 4.0 | |
| MELD-Na at LT, median (IQR) | 27 (21-34) | 27 (20-31) | <0.001 |
| Albumin at LT, median (IQR) | 2.9 (2.4-3.4) | 3.1 (2.7-3.6) | <0.001 |
| Diabetes, % | 23.0 | 39.4 | <0.001 |
| Ascites, % | <0.001 | ||
| None | 11.7 | 7.5 | |
| Mild | 48.5 | 40.9 | |
| Moderate | 39.9 | 51.7 | |
| Location prior to LT, % | <0.001 | ||
| Home | 58.9 | 52.8 | |
| Inpatient ward | 26.2 | 29.6 | |
| Inpatient intensive care | 15.0 | 17.7 | |
| Dialysis at LT, % | 11.4 | 41.5 | <0.001 |
| Waiting time, median (IQR) | 40 (11-156) | 43 (10-163) | 0.2 |
| Donor age, median (IQR) | 43 (27-55) | 45 (28-57) | <0.001 |
| DCDD organ, % | 5.6% | 6.1% | 0.4 |
Abbreviations: AI – auto-immune; DCDD – donation after circulatory determination of death; DDLT – deceased donor liver transplantation; HBV – hepatitis B virus; HCV – hepatitis C virus; IQR – interquartile range; KidneyMELD – proportion of MELD-Na score attributable to creatinine; LT – liver transplantation; MELD-Na – Model for End-stage Liver Disease Sodium score; NASH – non-alcoholic steatohepatitis
Includes: auto-immune hepatitis, primary sclerosing cholangitis and primary biliary cholangitis
Though statistically significant, there were no clear geographic trends observed in the proportion of DDLT recipients transplanted with KidneyMELD ≥50% during the study period (range: 3.3% in region 3 to 5.3% in region 7; p<0.001). In the largest UNOS region (region 3; N=7,152), the proportion of LT alone recipients with KidneyMELD ≥50% ranged from 0.1% to 10% among the 16 centers transplanting ≥10 patients during the study period. There was a small but significant decrease in the proportion of LT alone recipients with KidneyMELD ≥50% over time, which accounted for 4.8% of the study cohort transplanted between 2005-2008, 4.1% between 2009-2013 and 3.4% between 2014-2017 (p<0.001). This trend was also observed in the subgroup with NASH (6.9% between 2005-2008, 5.4% between 2009-2013 and 4.9% between 2014-2017; p=0.015).
Post-LT outcomes according to KidneyMELD at LT
A total of 7,605 (21.8%) post-LT deaths were observed during follow-up. Among LT recipients with a known cause of death (COD, 88.2%), cardiovascular disease was listed as the primary COD in 20.8% and renal failure was the primary or secondary COD in 5.8%. There was a significant increase in the proportion of post-LT deaths attributable to cardiovascular disease and renal failure with increasing KidneyMELD (Table 2). In recipients with KidneyMELD ≥50%, 25.1% of post-LT deaths were primarily due cardiovascular disease and 11.1% were either primarily or secondarily attributable to renal failure.
Table 2:
Proportion of post-LT deaths attributable to cardiovascular disease or renal failure with increasing KidneyMELD
| KidneyMELD at LT | ||||
|---|---|---|---|---|
| 0% (N=1,754) |
1-24% (N=1,837) |
25-49% (N=2,267) |
≥50% (N=387) |
|
| % deaths due to CVD as primary COD* | 16.5 | 20.5 | 23.5 | 25.1 |
| % deaths due to renal failure as primary or secondary COD* | 3.4 | 5.6 | 7.0 | 11.1 |
p<0.001
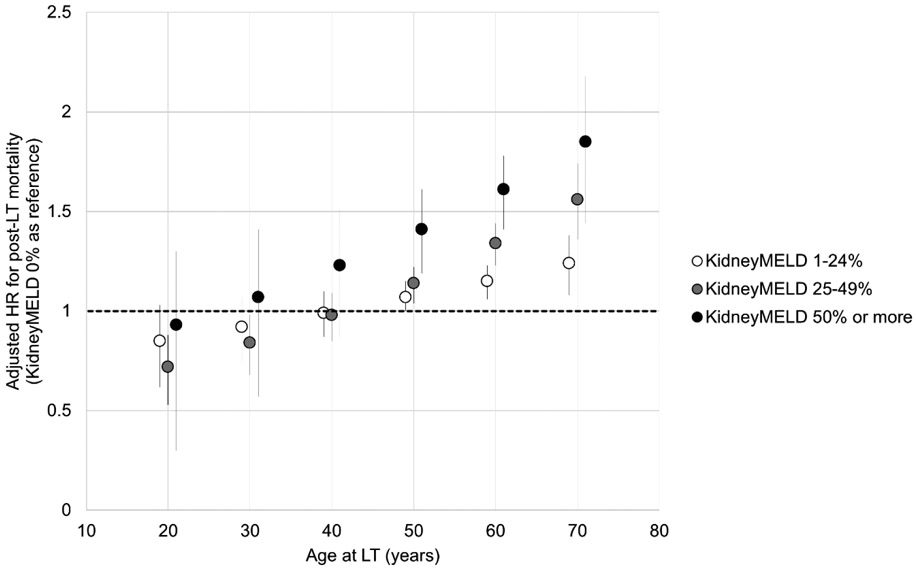
Accounting for recipient and donor factors, there was a stepwise increase in post-LT mortality with increasing KidneyMELD, which reached an aHR of 1.52 (95% CI: 1.36-1.69) for those with KidneyMELD ≥50% (Table 3). The association of increasing KidneyMELD with post-LT mortality was greater with increasing age (interaction p<0.001), particularly for recipients over 50 years at LT (Figure 1). As an example, using KidneyMELD 0% as reference, the aHR with KidneyMELD ≥50% was 1.85 (95% CI: 1.52-2.26) for 70-year old recipients, but not significantly different from KidneyMELD 0% for 40-year-old recipients (aHR 1.22, 95% CI: 0.95-1.59). The other interactions evaluated were not statistically significant, including that of KidneyMELD and MELD-Na, indicating that the effect of increasing KidneyMELD on post-LT mortality was the same across all MELD-Na scores.
Table 3:
Association between increasing KidneyMELD post-LT outcomes
| aHR for death post-LT (95% CI) (N=34,149) |
aOR for eGFR ≤30mL/min/1.732 at 1-year (95% CI) (N=22,433) |
|
|---|---|---|
| KidneyMELD category* | ||
| 0% | Reference | Reference |
| 1-24% | 1.09 (1.02-1.16) | 1.90 (1.51-2.40) |
| 25-49% | 1.24 (1.15-1.33) | 4.83 (3.87-6.04) |
| ≥50% | 1.52 (1.36-1.69) | 11.56 (8.93-14.97) |
p<0.001 for both models
Each model was adjusted for the following covariates at LT: age, gender, race/ethnicity, primary etiology of liver disease, laboratory MELD-Na score, serum albumin at LT, ascites at LT, diabetes, dialysis at LT, location prior to LT, donor age and receipt of DCDD organ.
Figure 1:
Association of KidneyMELD on adjusted post-LT mortality according to recipient age (N=34,149)
Abbreviations: HR – hazard ratio; KidneyMELD – proportion of MELD-Na score attributable to creatinine; LT – liver transplantation
There were 22,811 recipients in the cohort with renal function data at 1-year post-LT, of which 5.5% were noted to have eGFR ≤30mL/min/1.732. Similar to the risk of post-LT mortality, there was a stepwise increase in the risk of advanced post-LT renal insufficiency with increasing KidneyMELD particularly when KidneyMELD reached ≥50% (aOR 11.56, 95% CI: 8.93-14.97; Table 3). As a secondary analysis, the pursuit of kidney transplantation after LT alone in patients with KidneyMELD ≥50% was investigated. Of the 1,421 LT alone recipients with KidneyMELD ≥50%, 185 (13%) were waitlisted for a subsequent kidney transplant at a median of 563 days from LT (IQR: 282-1153). A total of 97 (6.8%) patients with KidneyMELD ≥50% underwent kidney transplant after LT alone (median time 933 days, IQR: 410-1769), of which 25 (25.8%) were from living donors.
KidneyMELD ≥50% and post-LT survival in recipients ineligible for SLK
Of the 1,421 recipients with KidneyMELD ≥50%, 517 (36.4%) were waitlisted at least 90 days allowing for an assessment of CKD status, and therefore SLK eligibility. Among these, 297 (57.5%) were deemed SLK ineligible by current UNOS policy criteria. CKD was present in 45.8% (136/297) of the patients in this group. Recipients with CKD who were transplanted with KidneyMELD ≥50% had a median MELD-Na at LT of 17 (IQR: 14-20). A majority had no or mild ascites at LT (11.2% and 50%, respectively), and no or grade 1-2 encephalopathy at LT (35.3% and 58.9%, respectively).
The unadjusted survival of the 136 LT alone recipients with CKD and KidneyMELD ≥50% who were ineligible for SLK transplantation by current UNOS criteria was compared to that of 2,865 adult SLK recipients transplanted between 2005-2017, and was found to be not significantly different (p=0.2; Supplemental Figure 1). For example, 3-year post-transplant survival was 80% in the group ineligible for SLK and 80.2% in SLK recipients.
Discussion
Candidates waitlisted for LT undergo careful evaluation to ensure that the risks of transplantation surgery and subsequent lifelong immunosuppression do not outweigh the benefits. While the presence of pre-LT renal dysfunction raises the MELD score and subsequently the likelihood of LT, it also significantly increases the risk of post-LT morbidity and mortality(8-10, 16). Due to diagnostic challenges in the identification of recoverable renal dysfunction and the availability of dialysis support after LT, kidney disease often influences LT candidacy to a lesser extent than other chronic co-morbidities, such as cardiovascular disease. This may become even more relevant in the future given the recently implemented UNOS ‘safety net’ pathway for expedited kidney transplantation after LT(17). This study demonstrates that recipients in whom the MELD-Na is primarily driven by creatinine have a markedly increased risk of all-cause mortality and advanced renal dysfunction after LT alone, and caution is particularly warranted for those who are over age 50. While the morbidity and mortality associated with persistent renal dysfunction post-LT may be minimized by early kidney transplantation after LT, further research is needed to better estimate the likelihood of post-LT kidney transplantation eligibility before offering LT alone for such candidates.
KidneyMELD is essentially a weighted estimate of renal dysfunction at any given MELD-Na score, and as such is a more valuable tool than creatinine. Candidates with KidneyMELD ≥50% may actually have lower MELD-Na scores than those with KidneyMELD 0%. This is in contrast to creatinine (or eGFR) which parallels the MELD-Na score: holding other parameters constant, an increase in creatinine from 1mg/dL to 2mg/dL increases the MELD-Na score by approximately 6 points across the MELD-Na spectrum. However, changes in creatinine affect KidneyMELD at lower MELD-Na scores more so than at high MELD-Na scores (i.e., when there is a greater degree of hepatic dysfunction). For example, increasing creatinine from 1mg/dL to 2mg/dL would increase KidneyMELD from 0% to 46% at a baseline MELD-Na score of 15, but to only 26% at a baseline MELD-Na score of 27. The use of KidneyMELD as a parameter of interest therefore not only estimates the relative impact of renal dysfunction on post-LT mortality, but also provides greater understanding of this risk at low MELD-Na scores, an area that remains understudied.
The absence of an interaction between MELD-Na and KidneyMELD, indicates that the risk of increasing KidneyMELD on post-LT mortality is the same across all MELD-Na scores. This is particularly relevant for candidates at low MELD-Na scores, who have lower predicted waitlist mortality and in whom the survival benefit of LT alone may differ appreciably. Conversely, the significant interaction of KidneyMELD with age is an important finding given current trends in recipient age at LT(18, 19). While UNOS provides SLK eligibility guidelines with respect to renal dysfunction, many transplant centers have formal age limits for SLKs that are frequently lower than those for LT alone. This study adds to the available literature guiding the decision of whether to pursue LT alone for older individuals with a predominance of renal dysfunction pre-LT.
The UNOS ‘safety net’ kidney transplant after LT policy is a new mechanism by which the morbidity and mortality associated with persistent renal dysfunction after LT alone can potentially be circumvented. Though not specifically addressed by UNOS, the option of planned living donor kidney transplantation after LT should also be strongly encouraged by centers, as this would avoid disadvantaging candidates awaiting kidney transplant alone. An important concern, however, is whether candidates with high KidneyMELD before LT and persistent renal dysfunction after LT will be medically ‘fit’ enough to undergo early kidney transplantation, particularly if they are also of older age. In the coming years, it will be vital to understand the predictors of successful early kidney transplantation after LT for patients ineligible for SLK, such that the survival benefit of pursuing LT alone in those with high KidneyMELD can be determined upfront. Our exploratory analyses suggested that post-LT survival in patients with CKD and KidneyMELD ≥50% who were ineligible for SLK by UNOS criteria was not significantly different from SLK recipients, suggesting that an expansion of the current UNOS SLK criteria may not be warranted. Therefore, for patients with KidneyMELD ≥50% and CKD ineligible for SLK, a careful estimation of the likelihood of successful early kidney transplantation after LT is paramount to the LT selection process.
This study also highlights the marked practice heterogeneity among centers with regards to candidate selection: within the largest UNOS region, the proportion of patients who underwent LT alone with KidneyMELD ≥50% by center was observed to vary by 100-fold. Center differences in the rates of SLK have been previously described and were a key reason for the development of the recently updated UNOS SLK guidelines(20). However, many candidates with KidneyMELD ≥50% do not meet SLK criteria. Moreover, a majority of the LT alone recipients with KidneyMELD ≥50% and CKD in this study had low MELD-Na scores and either no or mild hepatic decompensations. Thus, the benefit of LT alone in such recipients must be weighed against that of patients with similar waitlist priority, but in whom MELD-Na is primarily driven by liver dysfunction.
This research had several limitations. The cohort of patients with KidneyMELD ≥50% amounted to 1,421 individuals and therefore related subgroup analyses involved small sample sizes, particularly in the assessment of SLK eligibility. The survival curve for candidates with KidneyMELD ≥50% was noted to cross the others, indicating non-proportionality of hazards. Thus, the effect of high KidneyMELD on post-LT survival in the multivariable Cox model should be interpreted as the average aHR over follow-up. In this study, candidates were defined as ineligible for SLK if they were waitlisted for at least 90 days with eGFR at LT >30mL/min/1.732 not on dialysis. This allowed for subjects with CKD to be identified on the basis of two eGFR values <60mL/min/1.732 at least 90 days apart leading up to LT. This method also captured candidates with CKD and low MELD-Na scores, as they only require scheduled MELD score updates every 90 days. It is possible that a small number of recipients with true CKD were misclassified as not having CKD by this method, as laboratory data was not available outside of that available from MELD score updates. Moreover, data from MELD score updates are subject to bias from informative missingness: centers are unlikely to submit a non-scheduled score update if the candidate’s MELD-Na score is lower than that registered during the previous scheduled score update. This could have caused misclassification of patients without CKD as having true CKD, and thus the proportion of patients with KidneyMELD ≥50% and CKD who were ineligible for SLK may be smaller than that reported here.
In conclusion, the proportion of MELD-Na score driven by renal dysfunction is an important and independent predictor of post-LT survival and severe renal dysfunction. Candidates transplanted primarily on the basis of kidney dysfunction (i.e., KidneyMELD ≥50%) had a 52% greater risk of post-LT mortality and an 11-fold increase in the risk of having an eGFR ≤30mL/min/1.732 at 1 year post-LT, compared to those without renal dysfunction at LT. Moreover, the aHR for mortality after LT observed with KidneyMELD ≥50% increased significantly with advancing age, particularly for recipients over 50 years at LT. Such patients should be considered for LT alone with caution, particularly in the context of other co-morbidities that may additionally contribute to their predicted risk of adverse post-LT outcomes and/or a reduced likelihood of kidney transplant candidacy should their renal dysfunction persist. Further research is needed to assess whether improved post-LT outcomes will occur over time for those undergoing LT alone with high KidneyMELD with the implementation of new UNOS ‘safety net’ policy.
Supplementary Material
Acknowledgements
This work was the basis for a National Institutes of Health Loan Repayment Program Award (Principal Investigator: Dr. Therese Bittermann – 2-L30-DK110788-02). In addition, the following authors receive grant funding via the National Institutes of Health for other research activities: Dr. Therese Bittermann (1-K08-DK117013-01), Dr. Rebecca Hubbard (1-R21-CA227613-01A1) and Dr. David Goldberg (1-R01-DK120561-01).
Abbreviations
- aHR
adjusted hazard ratio
- aOR
adjusted odds ratio
- AI
auto-immune
- CKD
chronic kidney disease
- Cr
creatinine
- COD
cause of death
- CVD
cardiovascular disease
- DCDD
donation after circulatory determination of death
- DDLT
deceased donor liver transplantation
- eGFR
estimated glomerular function
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HRS
hepatorenal syndrome
- IQR
interquartile range
- KidneyMELD
proportion of MELD-Na score attributable to creatinine as a percentage
- LT
liver transplantation
- MDRD-4
Modification of Diet in Renal Disease 4
- MELD
Model for End-stage Liver Disease score
- MELD-Na
Model for End-stage Liver Disease Sodium score
- NASH
non-alcoholic steatohepatitis
- PH
proportional hazards
- SLK
simultaneous liver-kidney
- UNOS
United Network for Organ Sharing
Footnotes
Disclosure
All authors declare no direct conflicts of interest related to the contents of this study. Dr. Rebecca Hubbard additionally receives research grant funding from Humana. Dr. James Lewis receives consulting honoraria from Johnson & Johnson Consumer Inc., Takeda, Merck, Celgene, Janssen Pharmaceuticals, AbbVie, Eli Lilly and Company, Samsung Bioepis, Dark Canyon Laboratories, Bridge Biotherapeutics Inc., and Bristol-Myers Squibb. He also serves on the Data Safety Monitoring Board for Pfizer, Gilead and UCB, and receives research grant funding from Takeda and Janssen. Dr. David Goldberg additionally receives research grant funding from Merck, Zydus and Gilead.
References
- 1.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–71. [DOI] [PubMed] [Google Scholar]
- 2.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R, United Network for Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–6. [DOI] [PubMed] [Google Scholar]
- 3.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–70. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Schaubel DE, Guidinger MK, Goodrich NP, Ojo AO, Merion RM. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. American Journal of Transplantation. 2011;11(11):2372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson D, Waisanen L, Wolfe R, Merion RM, McCullough K, Rodgers A. Simulating the allocation of organs for transplantation. Health Care Management Science. 2004;7(4):331–8. [DOI] [PubMed] [Google Scholar]
- 6.Trotter JF. Impact of the Model for Endstage Liver Disease score on liver transplantation. Current Opinion in Organ Transplantation. 2007;12(3):294–7. [DOI] [PubMed] [Google Scholar]
- 7.Cullaro G, Verna EC, Lai JC. Association between renal function pattern and mortality with cirrhosis. Clinical Gastroenterology and Hepatology. 2019;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, Goodrich NP, Zhang M, Guidinger MK, Schaubel DE, Merion RM. Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clinical Journal of the American Society of Nephrology. 2013;8(7):1135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahirwani R, Forde KA, Mu Y, Lin F, Reese P, Goldberg D, Abt P, Reddy KR, Levine M. End-stage renal disease after liver transplantation in patients with pre-transplant chronic kidney disease. Clinical Transplantation. 2014;28(2):205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruebner R, Goldberg D, Abt PL, Bahirwani R, Levine M, Sawinski D, Bloom RD, Reese PP. Risk of end-stage renal disease among liver transplant recipients with pretransplant renal dysfunction. American Journal of Transplantation. 2012;12(11):2958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohanty A, Garcia-Tsao G. Hyponatremia and hepatorenal syndrome. Gastroenterology Hepatology. 2015;11(4):220–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Organ Procurement and Transplantation Network. OPTN Policy 9 - Allocation of livers and liver-intestines: U.S. Department of Health & Human Services; 2019. [March 1, 2019]. Available from: https://optn.transplant.hrsa.gov/governance/policies/. [Google Scholar]
- 13.Sharma P, Shu X, Schaubel DE, Sung RS, Magee JC. Propensity score-based survival benefit of simultaneous liver-kidney transplant over liver transplant alone for recipients with pretransplant renal dysfunction. Liver Transplantation. 2016;22(1):71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan TV, Lunsford KE, Vagefi PA, Bostrom A, Ma M, Feng S. Renal outcomes of simultaneous liver-kidney transplantation compared to liver transplant alone for candidates with renal dysfunction. Clinical Transplantation. 2015;29(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skluzacek PA, Szewc RG, Nolan CR, Riley DJ, Lee S, Pergola PE. Prediction of GFR in liver transplant candidates. American Journal of Kidney Diseases. 2003;42(6):1169–76. [DOI] [PubMed] [Google Scholar]
- 16.Allen AM, Kim WR, Therneau TM, Larson JJ, Heimbach JK, Rule AD. Chronic kidney disease and associated mortality after liver transplantation -- a time-dependent analysis using measured glomerular filtration rate. Journal of Hepatology. 2014;61(2):286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Organ Procurement and Transplantation Network. OPTN Policy 8 - Allocation of kidneys: U.S. Department of Health & Human Services; 2017. [June 15, 2017]. Available from: https://optn.transplant.hrsa.gov/governance/policies/. [Google Scholar]
- 18.Bittermann T, Goldberg DS. Quantifying the effect of transplanting older donor livers into younger recipients: the need for donor-recipient age matching. Transplantation. 2018;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Harper AM, Wainright JL, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2016 Annual Data Report: Liver. American Journal of Transplantation. 2018;18(Suppl 1):172–253. [DOI] [PubMed] [Google Scholar]
- 20.Parajuli S, Foley D, Djamali A, Mandelbrot D. Renal function and transplantation in liver disease. Transplantation. 2015;99(9):1756–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.